Note: Descriptions are shown in the official language in which they were submitted.
CA 02492255 2005-O1-11
1
P27762US/T1
URSAPHARM Arzneimittel GmbH & Co. KG
Fluid dispenser
TECHNICAL FIELD OF THE INVENTION
The invention relates to a fluid dispenser for germ-free fluids.
DESCRIPTION OF RELATED ART
In the Pharmazeutische Zeitung, 124, No. 20, of 17th May 1979, on pages 949
and
950, a fluid dispenser is described that has the form of a dropping pipette
and is
attached to a container containing eye-drops. Inside the dropping pipette a
silver
deposit consisting of a layer of silver or a difficultly-soluble silver salt
is disposed so
that airborne germs drawn in with the drops that run back into the container
have to
pass an antimicrobial (oligodynamical) active silver layer before they enter
the
container. It is also stated that ceramic rings with silver chloride embedded
and
having a diameter of 9 mm have been found to be suitable. These ceramic rings
can
be firmly installed in the droppers of all the usual kinds of pharmaceuticals,
eye-
dropper bottles simply by pushing them in. This method of introducing the
silver
deposit into the droppers has the disadvantage that only the drops running
back along
the walls of the dropper come into contact with the silver deposit, but not
the
portions of the liquid in the interior of the column of fluid which flows back
into the
container from the dropper after use in the usual way with the dropper facing
downwards. Each use of the eye-drop container thus leads to contamination of
the
eye-drops. A further disadvantage is that the interior of the container is in
contact
with the ambient air through the dropper, so that even while it is not being
used
germs constantly find their way in and lead to contamination of the eye-drops
in the
container.
From DE 40 27 320 C2 a fluid dispenser for germ-free fluid is known which
comprises a through passage connecting an inlet opening for fluid and a
delivery
opening for said fluid and having therein an oligodynamically antimicrobial
active
substance. The device includes a metering pump and inlet and outlet valves.
The
oligodynamical germicidal active substance is present in the region of the
inlet valve
and/or the outlet valve. According to Fig. 1 of this document the springs are
shown
f~iahlaE;fe\piteit27000\277f2\us-ti127762uuO.doc
CA 02492255 2005-O1-11
2
which can be coated with silver. Likewise, the valve ball functioning as the
inlet
valve consists of corundum having embedded therein a silver material as an
oligodynamically effective substance. A disadvantage of this device is that
often
computability problems occur due to the presence of silver and oxidation
processes
which produce undesired by-products, which often results in a limited choice
of
appropriate formulation.
SUMMARY OF THE INVENTION
One aspect of the present invention provides a fluid dispenser of the kind as
referred
to in DE 40 27 320 C2 which does not cause compatibility problems and prevents
the formation of by-products while simultaneously an adequate and comparable
microbiological safety (i.e. germ-free application) of the system is
maintained.
The present invention relates to a fluid dispenser for germ-free fluid
comprising a
through passage connecting an inlet opening for fluid contained in a supply
container
made of flexible material and a delivery opening for dispensing said fluid and
having
therein at least one oligodynamically active substance that is in contact with
the
fluid; a metering pump operating without air pressure compensation, whereby no
pressure compensation takes place in the container through the inflow of air
during
the operation of said metering pump, said pump having a spring means being in
contact with the t7uid, an inlet valve for closing said inlet opening, and an
outlet
valve; and an outlet passage being part of said through passage leading from
said
outlet valve to the delivery opening, wherein a decontamination means is
provided in
the upper part of said outlet passage said decontamination means comprising a
material capable of interacting with germs via an oligodynamical substance
selected
from the group consisting of silver, silver salts, other silver compounds,
alloys and
nanomers thereof in either metallic or salt form or as a chemical compound
thereof.
The present invention relates further to the use of the fluid dispenser of the
invention. The fluid dispenser of the present invention is suitable for
dispensing
minute amounts of a liquid in various fields such as pharmaceutics, cosmetics
and
medical devices. The liquids are usually topically applied. Preferred liquids
are
ophthalmic and nasal compositions.
The term "interacting" should be defined in the context of the present
invention as a
type of a surface reaction. The theory is that the interaction takes place
close to or
preferably on the surface of the material capable of interacting with the
germs
contained in the liquid. The germs may hereby derive from a contamination of
the
CA 02492255 2005-O1-11
3
unprotected outer part of the delivery opening that comes in contact with the
environment. The germs hereby may be contained in the fluid, or in other
substances
coming into contact with the fluid dispenser, such as air, lachrymal liquor,
mucosa
or the like. One possible mechanism could be that the contaminated liquid
comes
into contact with ions derived from metal oxides which has been formed
directly on
the surface of the material. This contact results in an antimicrobial effect.
A general
rule can be seen in the relationship of the material surface and its size: the
larger the
surface is, the better the decontamination effect is. Different levels of
interaction
with the germs are hereby possible. For example, the interaction could result
in a
slowing down or stopping of the growth of the germs in the fluid. A strong
level of
interaction is e. g. the oligodynamic effect in which an oligodynamically
active
substance actually kills germs in the fluid.
According to the fluid dispenser of the invention, the decontamination means
is
provided in the outlet passage and preferably in the upper part of the outlet
passage.
The term "upper part" comprises the region of the outlet passage where still
an
optimum decontamination can be ensured.
According to the invention, a particularly intensive germicidal action results
from
the position of the outlet valve and the decontamination means. Due to the
specific
technical construction the moveable outlet valve does not come into direct
contact
with the environment, which results in a reduction of the risk of a
contamination
during the movement of the outlet valve. As a results an oligodynamically
active
substance has to be provided on the outside of the outlet valve, which is
realised by
the decontamination means. Further, with this construction the fluid in the
container
does not come constantly into contact with the oligodynamically active
substances,
which reduces the above mentioned unwanted reactions of the fluid with the
oligodynamically substance. The metering pump operates without air pressure
compensation, so that contamination of the fluid supply through the air flows
into the
container to effect the pressure compensation in the operation of conventional
metering pumps is prevented. The fluid dispenser of the invention ensures that
the
fluid in the supply container is kept germ-free even during use, so that it is
not
necessary either to add preservatives or to introduce the oligodynamically
active
substance in other regions of the container.
'The oligodynamically active substance is located at or near to the outlet
passage to
prevent microbiological contamination by reducing count of potential arising
germs
from the environment.
CA 02492255 2005-O1-11
4
The materials and elements of the metering pump and the container which are in
contact with the fluid could be any kind of elements and materials which are
compatible with the respective fluid. In some applications, it is not
necessary to
provide any material capable of interacting with germs inside the metering
pump and
the container. However, in other applications it might be advantageous to use
materials capable of interacting with germs within the metering pump and the
container. For example, it might be advantageous if the inlet valve and/or the
spring
means comprise a material capable of interacting with germs. Hereby, the
material
could be selected from the group consisting of silver, silver salts, other
silver
compounds, stainless steel and nanomers thereof in either metallic or salt
form or as
a chemical compound thereof. In this case, the stainless steel could contain
at least
one element selected from the group consisting of chromium, nickel,
molybdenium,
copper, tungsten, aluminium, titanium, niob and tantal, the remainder being
iron as
the main component. Among the above materials, all materials comprising
silver,
silver salts or other silver compounds usually are oligodynamically active.
Stainless
steel materials are believed to be usually not oligodynamically active or, if
they are,
only to a very small extend. However, the stainless steel materials are
believed to be
able to interact with the germs by slowing down or stopping their growth.
Advantageously, said through passage is constantly filled, at least in the
region of
said inlet valve with said fluid. Further advantageously, the oligodynamically
active
substance is provided on the inner side of a cap that can be fitted onto said
fluid
dispenser to cover said delivery opening. Hereby, the cap may be provided with
a
pin and a hole. Further, the pin may fit in the delivery opening located in
the head.
Further advantageously, said inner valve further includes a valve seat
cooperating
with the closure member wherein said valve seat is provided with said
oligodynamically active substance. Further advantageously, the outlet valve
further
includes a valve seat cooperating with the closure member. Further
advantageously,
the inlet valve is a ball valve and a valve housing cooperating with a closure
member
of the inlet valve is provided, said valve housing being provided with said
oligodynamically active substance. Advantageously, the outlet valve is a
piston valve
and a valve housing cooperating with a closure member of said outlet valve.
Further
advantageously, the decontamination means is of a material having a circular
shape.
Hereby, the decontamination means may be a ring, a spiral or a coating. The
material may be corundum having embedded therein the oligodynamically active
compound. Alternatively, the material can be silver.
CA 02492255 2005-O1-11
S
BRIEF DESCRIPTION OF THE DRAWINGS
The invention will now be described in more detail, by way of example, with
reference to the single Figure of the drawings, which shows in longitudinal
section
an embodiment of the invention.
DETAILED DESCRIPTION OF THE INVENTION
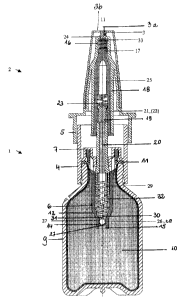
As shown in the Figure, the device comprises a metering pump consisting of a
cylindrical pump body 1, an operating plunger 2 and a cap 3.
The pump body 1 comprises a first hollow cylindrical body part 4, shown in the
drawing as open at the bottom, a second hollow cylindrical body part 5 of
bigger
diameter (part 5 is part of the operating plunger 2), open at the top in the
drawing,
and a hollow cylinder 6 that is open at both ends and is fixed centrally on an
inwardly directed annular flange 7 in the transition region between the two
parts 4,5
of the pump body. The first body part 4 may have an internal screw thread into
which a container 9 filled with a germ-free fluid and indicated only
generally, can be
screwed. As an alternative, instead of the internal screw thread, a snap on
closure
can be used as shown in the Figure. A seal I1 is provided on the underside (in
the
drawing) of the annular flange 7 to ensure an air-tight seal between the
container 9
and the pump body 4. In the neighborhood of the outlet from the first body
part 4 of
the pump the hollow cylinder 6 has a conically tapered-down transition part 12
that
connects with a cylindrical valve section 14 of smaller diameter leading to a
rising
tube, if available. The open bottom end of the rising tube forms the inlet
opening 15
of the metering pump. As an alternative, the rising tube may be omitted, as
shown in
the Figure.
The operating plunger 2 comprises an outer hollow cylindrical part 17, shown
in the
drawing as open at the bottom and closed at the top by a head 16, and a hollow
inner
cylindrical part 18 extending centrally downwards from the head 16. The
diameter of
the hollow outer cylindrical part 17 is smaller than that of the first pump
body part
4.
A piston 19 that fits inside the hollow cylinder 6 and has a through bore 20
is fixed
at its top end in the inner hollow cylinder part 18. A piston valve 21 of an
outlet
valve 22 that fits inside the hollow cylindrical part 18 is supported between
the end
part of the piston 19 at one end and at the other end on the head 16 via a
spring 23.
CA 02492255 2005-O1-11
6
An outlet passage 25 leading to a delivery opening 24 on the head 16 is
connected to
the interior of the inner hollow cylindrical part 18 at the level of the
piston valve 21.
In the upper part of the outlet passage 25 or preferably in the upper part of
the outer
hollow cylindrical part 17 a decontamination means 33 is provided which
comprises
a material capable of interacting via an an oligodynamically active substance
selected
from the group consisting of silver, silver salts, other silver compounds and
alloys
thereof or nanomers in either metallic or salt form or chemical compounds
thereof
close to the surface thereof. The decontamination means 33 may hereby be
provided
at the inner and/or the outer wall of the outlet passage 25.
Silver exhibits the most favourable therapeutically index in terms of
concentration in
parts per billion. Depending on economical considerations, the means can be
made
of silver, of another metal coated with silver or of a material having
embedded
therein the oligodynamically germicidally active substance. In a preferred
embodiment of the invention, the means decontamination 33 has a circular shape
such as a ring or a spiral. It has been shown that corundum can be one of the
convenient materials, when the oligodynamically active substance is embedded
in a
carrier material.
Depending on the construction of the fluid dispenser and its intended use, the
decontamination means 33 can be also provided as a coating. As an example, the
coating can be disposed on the outer hollow cylindrical part 17 in the upper
part of
the outlet passage 25. It is possible to provide a coating made of silver or a
coating
of a suitable material having embedded therein silver or a silver compound.
It has been shown that in the case of using a coating in the upper part of the
outlet
passage 25, the silver coating may be suitably a nanocoating comprised of
nanomeres. For example, a desired nanocoating comprising silver colloids is
described in DE O1 128 625 A 1.
As already explained the piston valve 21 which functions as outlet valve is
not
located directly at the delivery opening 24. Instead the piston valve 21 is
located in
the inner hollow cylindrical part 18 and an outlet passage 25 is provided
leading
from the piston valve 21 to the delivery opening 24. The through bore 20 and
the
outlet passage 25 are thereby separated by the piston valve 21. The function
of the
piston valve 21 is hereby to allow a delivery of the fluid 10 from the
container 9
through the inner space 32, the through bore 20 and the outlet passage 25 to
the
CA 02492255 2005-O1-11
delivery opening 24 but to prevent a flowing back of the fluid 10 from the
outlet
passage 25 to the through bore 20.
With the piston valve 21 a closed system is established, i.e. a system into
which no
fluid flows back once the fluid 10 has left the system. Thereby, the intrusion
of
germs and bacteria into the closed system is effectively prevented. This
results in the
possibility to use any suitable material for the components within the closed
system
as the necessity of using materials capable of interacting with germs or
oligodynamically active substances is not present due to the fact that the
intrusion of
germs is prevented. However, it might be advantageous to use materials which
are
able to interact with germs by stopping or slowing down their growth or even
to use
oligodynamically active substances.
The outlet passage 25 is provided as a very thin and small capillary thereby
reducing
the dead volume, i.e. the volume of the fluid outside the closed system and
coming
into contact with the decontamination means.
According to embodiments of the invention it is possible to provide
antimicrobial
coatings on parts of the inlet valve 26 and on parts of the pump housing. Said
coatings may be applied directly to plastic elements and steel components of
the
pump .
An inlet valve 26 comprising a ball 28 cooperating with a valve seat 27 is
formed in
the valve part 14. A spring 29 fixed to the piston 19 is supported on a
projection 30
on the valve part 14 and supports the pumping action. The space inside the
hollow
cylinder 6 between the piston 19 and the valve part 14 is indicated by the
reference
numeral 32.
The valve ball 28 can comprise a material capable of interacting with germs
eventually even via an oligodynarnically active substance. In addition the
valve seat
27 and the inner side of the inner hollow cylinder part 18 in the region of
the piston
valve 21 may be coated with a material capable of interacting with germs
eventually
even via an oligodynamically active substance. The piston valve 21 can be made
of
any inert material such as plastic.
The spring means 29 may also comprise a material capable of interacting with
germs
eventually even via an oligodynamically active substance. In principle, any
suitable
material may be used, as long as the material is compatible with the
formulation.
CA 02492255 2005-O1-11
8
It has been shown that a preferred material for the above device components is
a
stainless steel. Generally, a stainless steel contains relatively high amounts
of alloy
elements such as chromium, nickel, molybdenium, copper, tungsten, aluminium,
tantal, niob and titanium, while iron being the remainder representing the
major part
of the alloy.
It is known that stainless steels are corrosion-resistent. The corrosion
resistance is
due to an extremely thin and very tough chromium oxide layer on the surface of
the
steel. Chromium as well as other heavy metals in very small amounts can act as
an
oligodynamically active substance which may also reduce microbial growth. For
example, useful stainless steel materials include such as materials 1.4034 and
1.4401. In various embodiments of the invention, an effective killing of germs
may
be achieved when a suitable steel such as stainless steel chromium is used as
an
oligodynamically active substance for the spiral 29 and the inlet valve 26. As
the
upper spring 23 does not come into contact with the fluid to be filled, the
upper
spring 23 may be made of a stainless steel material.
From the viewpoint of compatibility of the stainless steels, especially under
consideration of possible allergic reactions, a nickel-free stainless steel or
a stainless
steel comprising very low amounts of nickel should be used.
It is to be noted, that within the closed system particularly for the inlet
valve 26, the
ball 28, the valve seat 27, the inner part of the hollow cylindrical part 18,
the spring
means 19 and for every part of the fluid dispenser that comes into contact
with the
fluid 10, any material capable of interacting with germs can be used such as
silver,
silver salts, other silver compounds, stainless steel and nanomers thereof in
either
metallic or salt form or as a chemical compound thereof or plastic.
On the other hand, the material and elements used in the closed system can be
free
of any oligodynamically active substances.
The metering pump of the invention operates without air pressure compensation,
that
is to say, no pressure compensation takes place in the container 9 through the
inflow
of air during its operation. Thereby the intrusion of germs or bacteria into
the
container 9 or the closed system over the air is prevented.
The metering pump of the invention operates as follows: when the user removes
the
cap 3 and depresses the operating plunger 2 so as to push it into the second
pump
body part 5 a corresponding movement of the piston 19 against the force of the
CA 02492255 2005-O1-11
9
spring 29 simultaneously takes place. This presses the ball 28 harder against
the
valve seat 27 and applies pressure to the liquid 10 that has been sucked into
the inner
space 32 and the through bore 20 during the previous operation of the metering
pump. This pressure displaces the piston valve 21 of the outlet valve 22
against the
force of the spring 23, so that the connection to the outlet passage 25 is
opened and a
precisely measured quantity of the liquid 10 is delivered through the delivery
opening 24. As soon as the piston 19 reaches its dead centre position, the
pressure in
the inner space 32 and in the through bore 20 drops so far that the outlet
valve 22
closes and the inlet valve 26 opens, so that liquid 10 is sucked out of the
container 9.
The inlet valve 26 then closes again. Thereupon the user replaces the cap 3 on
the
plunger 2 and thereby closes the delivery opening 24.
Liquid remaining at the delivery opening 24, in the outlet passage 25, and in
the
through bore 20, as well as in the inner space 32 and in the inlet valve 29,
come into
contact with the various locations where the oligodynamically germicidally
substances are in contact with the fluid.
The container 9 filled with a germ-free fluid may be made of a flexible
material such
as a plastic material. In some cases depending on the final use of the device,
the
container 9 may be composed of an at least two bag system comprising an
external
part and an internal bag as the main reservoir for the germ-free fluid.
In a preferred embodiment the container 9 consists of an outer container and
of an
inner container containing the fluid 10. The inner container is made of a
flexible
material and with every operation of the metering pump the inner flexible
container
contracts in order to compensate the pressure within the flexible container
when the
fluid 10 is sucked out. Thereby a pressure compensation within the flexible
container
is achieved without the inflow of air into the inner flexible container. The
outer
container preferably is made of an unflexible material in order to allow the
user of
the fluid dispenser to hold the fluid dispenser properly and to operate the
metering
pump. Further, with the outer container the inner flexible container can be
protected
from destruction. In order to allow the inner flexible container to contract
during the
operation of the metering pump and to avoid a negative pressure between the
two
containers at least one small opening in the outer container is provided.
With the above explained system an inflow of air into the container is
prevented.
Further, the inner flexible container contracts, i. e. reduces in volume, with
every
operation of the metering pump. This results in a constant contact of the
fluid 10
with the inlet opening l~ of the metering pump. Thereby, fluid 10 can be
delivered
CA 02492255 2005-O1-11
through the inlet opening 15 independent of the orientation of the fluid
dispenser,
i.e. independent of the way the user holds the fluid dispenser. This allows a
360°-
application of the fluid dispenser, i.e. an operation of the fluid dispenser
in upright,
head first or any other position.
5
In addition, most of the components contained within the container 9, other
than the
decontamination means 33, and including the operating plunger 2 and pump body
4,
may be formed of flexible material such as plastic material due to its
recognised cost
and manufacturing advantages. For other strength or load bearing components,
such
10 as the springs 23, 29, the plastic material should be strong enough to
maintain spring
integrity throughout the lifetime of use of the container 9. Further, in the
case of
wearable components such as the valve ball 28, inlet valve 26, and outlet
valve 22,
the plastic material should be a wear resistant plastic material. Also, as
stated above,
the decontamination means 33 may be formed of plastic material coated with the
oligodynamically active substance.
One embodiment of the present invention provides a fluid dispenser that
includes a
cap 3 to cover and to seal the delivery opening 23. The cap 3 is provided with
a pin
3a and a hole 3b. 'The pin 3a fits in the delivery opening 24 located in the
head 16.
The hole 3b functions as an aeration means. By passing air through this hole
3b, the
excess fluid remaining after use is allowed to evaporate, thus giving still
more
protection against contamination.
The fluid dispenser according to the invention is perfectly for dispensing
minute
amounts of liquids of any kinds, preferably a liquid pharmaceutical
composition. In a
preferred embodiment of the invention the fluid dispenser may be used for
suspensing Liquid pharmaceutical compositions, such as an ophthalmicum or
nasalium. Further administrations are fluids applied as medical devices or
cosmetics.
The fluid dispenser according to the invention may be available in any size
depending on the end use.
While the invention has been described in connection with one or more
embodiments, it is to be understood that the specific mechanisms and
techniques
which have been described are merely illustrative of the principles of the
invention,
numerous modifications may be made to the methods and apparatus described
without departing from the spirit and scope of the invention as defined by the
appended claims.
CA 02492255 2005-O1-11
11
Example 1 Microbiological Test: The microbiological safety of the fluid
dispenser
has been confirmed by the Media Fill Test and the Dye Test. These tests
focused on
evaluating the tightness of the system and the protection of the opening of
the fluid
dispenser. The opening of the fluid dispenser was protected from
microbiological
growth by the design of the area of the opening. It is believed that the
geometry and
the small diameter of the tip area as well as the length of the capillary tube
increases
the difficulty for microbes to enter the fluid dispenser. The antimicrobial
effect is
especially achieved by the location of the outlet valve and the construction
of the
dead volume at the outlet part, which had been designed to be difficult to
reach for
microbial contamination. There may be a hole in the covering cap of the fluid
dispenser through which humidity evaporates. Additionally, to reduce any
residual
risk, a silver spiral was positioned directly behind the opening of the fluid
dispenser.
The metallic silver exerted an oligodynamic effect.
Example 2 In-Use Test: A simulated daily use microbial challenge study to
simulate
the In-Use application of the fluid dispenser was conducted. The objective was
to
determine if microbes would be introduced into the fluid dispenser after
rugged
usage. Microbes which are typically encountered by the consumer were tested by
dispensing drops from the fluid dispenser. The drops were also placed at the
tip of
the fluid dispenser. At the conclusion of the testing period, sterility of the
reservoir
was conducted. The results of the In-Use study indicated that there was no
ingress of
the test microorganisms into the reservoir of the fluid dispenser during the
simulated
daily use of the dispenser.