Note: Descriptions are shown in the official language in which they were submitted.
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
PROCESS FOR POLYMERIZING
CATIONICALLY POLYMERIZABLE MONOMERS
FIELD OF INVENTION
The present invention relates to an improved method for production of
isobutylene-based polymers useful in rubber compounds.
BACKGROUND
Isobutylene-isoprene polymers, generally termed "butyl rubbers", have
Io been well known since the 1930s and their synthesis and properties are
described
by Kresge and Wang in g KIRK-OTHMER ENCYCLOPEDIA OF CHEMICAL
TECHNOLOGY, pp. 934-955 (4th ed. 1993). These butyl rubber polymers have
good impermeability to air and a high level of damping when stretched or
compressed, and are used extensively throughout the tire and pharmaceutical
industries. The copolymers are made by a cationic slurry polymerization
process
at approximately -95°C using a catalyst comprising a Lewis Acid and an
initiator.
Initiators such as water and anhydrous HCl are used extensively. Related
patents
axe EP 0 279 456; WO 00/40624; U.S. 4,35,560, 5,169,914, and 5,506,316,
herein incorporated by reference.
Other background references include US Patent Nos. 4,146,692,
4,171,414, and 4,269,955.
The commercial reactors used to make these rubbers are well mixed
vessels of greater than 10 to 30 liters in volume with a high circulation rate
provided by a pump impeller. The polymerization and the pump both generate
heat and, in order to keep the slurry cold, the reactor contains a heat
exchanger.
One embodiment of such a continuous flow stirred tank reactor ("CFSTR") is
found in U.S. Patent No. 5,417,930, incorporated by reference, hereinafter
3o referred to in general as a "reactor" or "butyl reactor". In these
reactors, slurry
(reacted monomers) is circulated through tubes of a heat exchanger by a pump,
while boiling ethylene on the shell side provides cooling, the slurry
temperature
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
2
being determined by the boiling ethylene temperature, the required heat flux
and
the overall resistance to heat transfer. On the slurry side, the heat
exchanger
surfaces progressively foul, often referred to as film fouling, which causes
the
slurry temperature to rise. This often limits the practical slurry
concentration that
can be used in most reactors from 21 to 2g wt% relative to the total weight of
the
slurry, diluent, and unreacted monomers.
As the slurry temperature increases, there is evidence that the slurry
viscosity rises, causing a measurable reduction in the heat transfer
coefficient and
1o a further increase in slurry temperature. The increase in tempexatuxe will
cause a
further increase in viscosity and the progression continues until the slurry
becomes unstable and starts to agglomerate which can lead to reactor plugging.
Consequently, reactors experiencing rapid warm up, often referred to as run
away,
are taken out of service quickly to avoid fouling and plugging, and subsequent
plant upsets.
Reactor "warm-up" then refers to the gradual rise in the temperature of the
reactor as a polymerization run progresses. At a constant polymerization rate,
the
warm-up is the result of a progressive loss of heat removal capability in the
2o reactor. The heat removed from the reactor can be represented
mathematically by
the following equation (1):
(~ _ (~) (A) (Tslurry - Tethylene~ (1)
where "Q" is the heat removed, "A" is the surface area of the reactor, "U" is
the
overall heat txansfer coefficient, which is a composite of several heat
transfer
coefficients for the slurry itself, the walls of the reactor, the film formed
on the
reactor wall, and the boiling ethylene used to draw heat from the exothermic
polymerization reaction. The "T" values are the temperatures of the slurry and
3o ethylene, respectively.
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
3
In the polymerization process, the temperature difference driving force for
heat transfer must increase if (a) the overall heat transfer coefficient U
decreases,
and/or (b) the heat transfer area is lost during a reactor run, such as by
plugged
tubes. Both can occur as a result of film formation and mass fouling of the
reactor. Also, U will decrease if the reactor circulation rate drops or the
slurry
viscosity increases. Although not wishing to be bound by the following
mathematical relationship, the slurry side heat transfer coefficient can be
related to
the viscosity of the slurry by the Sieder-Tate equation for turbulent fluid
flow as
shown below in equation (2):
to
0.8 0.4 0.167
~SruY~D - 0.023 D v'°
where hs~°ry is the slurry side heat transfer coefficient, D is the
diameter of the
reactor heat transfer tube, k is the thermal conductivity of the reactor
polymerizing
slurry, v is the average velocity of the slurry inside the tube, p is the
average
density of the slurry, ~,b is the average bulk viscosity of the polymerizing
slurry, cp
is the specific heat of the polymerizing slurry, and ~,W is the average wall
viscosity
of the polymerizing slurry. Therefore, hs~°,.,.5, is proportional to
(1/~b)o.4 in equation
(2).
Operating problems associated with using these reactors vary depending
upon the specific reaction taking place and the specific location within the
reactor.
One problem with these reactors is the presence of non-homogenous zones
beneath (or above) the pump impeller where feed is introduced. The monomer-
rich zone adjacent the pump can be particularly troublesome because feed may
be
introduced with as high as 40% monomer concentration, whereas the steady-state
monomer level in the reactor is much lower, typically from 1% to 10%. The
inventors have found that, surprisingly, if an initiator such as a CS or
greater
tertiary halo-alkyl is added to the system, the reactor heat transfer
efficiency
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
4
improves, consistent with a reduction in viscosity of the slurry. This is
unexpected for at least two reasons.
First, the use of the initiator 2-chloro-2,4,4-trimethyl-pentane (TMPCI) has
been demonstrated in the polymerization of an olefin and the highly reactive
para-
alkylstyrene, as disclosed in U.S. Ser. No. 09/684,713, filed on October 6,
2000
(assigned to the assignee of the present application). However, conjugated
dimes,
such as used in butyl rubber production, are known to act as retarding
monomers
in polymerizations. This observation would tend to teach away from using a
to TMPCI or other CS or larger initiators in the polymerization of butyl
rubber.
Second, certain tertiary alkyl halide initiators such as tert-butylchloride (a
C4 tertiary halide) have been shown by Kennedy et al. in U.S. Patent No.
3,560,458 to improve isobutylene polymerization in small scale, batch
experiments when compared to HCI. Yet, there is little to no improvement when
comparing tert-butylchloride and TMPCI in small scale batch experiments.
Further, the lack of steady state conditions in the small batch process means
that
heat transfer and viscosity changes would not be apparent when going to a
continuous, slurry process, nor would the associated problem of reactor
fouling.
The inventors have unexpectedly found that certain alkyl halide
compounds greater than C4 significantly reduces reactor fouling associated
with
using HCl as an initiator for butyl rubber polymerization in continuous slurry
reactors. The present invention enables a higher slurry concentration and/or
longer run lengths than would otherwise be practical in most commercial
reactors.
SUMMARY OF THE INVENTION
Thus, an object of the present invention is to provide a method of
improving heat transfer within a butyl reactor by employing an improved
catalyst
3o system for the polymerization of an isoolefin with a conjugated dime to
form
butyl rubber.
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
S
The improved catalyst system comprises a Lewis Acid and an initiator that
improves heat transfer from the polymerizing slurry to the heat exchanging
system
built into the reactor by lowering the heat transfer coefficient of the
slurry. This
will ultimately lower the fouling rate, and allow higher concentrations of
monomer to be injected into the reactor and higher slurry concentrations to be
maintained, andlor allow the reactor to run for a longer period of time before
washing, thus improving the commercial value of the product and process.
An embodiment of the present invention is a method of improving the heat
to transfer capability within a continuous slurry polymerization reactor in
preparing
random copolymers of one or more isoolefin monomers and one or more
conjugated dime monomers, the reacted monomers forming a slurry within the
reactor. The method comprises reacting in a polar diluent the isoolefin and
dime
monomers, a Lewis acid, and an initiator, wherein the initiator has the
formula:
Ri
RZ C X CA)
R3
wherein X is a halogen; R1 is selected from the group consisting of C1 to Cg
alkyl,
and C2 to C8 alkenyl; R3 is selected from the group consisting of C1 to Cg
alkyl, CZ
to C8 alkenyl and phenylalkyl; and R2 is selected from the group consisting of
C4
2o to CZOO alkyl, CZ to C8 alkenyl, phenyl phenylalkyl, alkylphenyl, C3 to Clo
cycloalkyl, and
Rs
X C Ra
R6
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
6
wherein X is a halogen; RS is selected from the group consisting of C1 to Cg
alkyl,
and C2 to C8 alkenyl; R6 is selected from the group consisting of C1 to C8
alkyl, CZ
to C8 alkenyl and phenylalkyl; and R4 is selected from the group consisting of
phenylene, biphenyl, a,c~-diphenylalkane and --(CH2)n -, wherein n is an
integer
from 1 to 10; and wherein Rl, R2, and R3 can also form adamantyl or bornyl
ring
systems, the X group being in a tertiary carbon position; wherein the Lewis
acid
and the initiator are contacted with a contact time of from less than 60s
prior to
contacting with the isoolefin and the dime monomers. Further, the slurry
within
the reactor is in a concentration of 50 wt% or less in one embodiment.
l0
In another embodiment, the invention provides for a catalyst system and
process for production of isoolefin copolymers containing a pare-alkylstyrene
comonomers. An improved catalyst system and process has been discovered
which affords many unexpected advantages for commercial slurry polymerization
of these copolymers generally, and in particular isobutylene pare-
methylsytrene
(IPMS) copolymers. The invention is particularly useful in production of
isoolefin-pare-alkylstyrene (IPAS) copolymers having a higher PAS content,
particularly isobutylene-pare-methylstyrene (IPMS) copolymers having a higher
PMS content (e.g. 10-20 weight percent PMS).
In a particularly preferred embodiment, the copolymers produced contain
isobutylene as the isoolefin and pare-methylstyrene as the pare-alkylstyrene
comonomer. Discussion of these preferred embodiments should not be construed
so as to limit the broad invention, which is applicable generally to
copolymers of
one or more isoolefin and one or more pare-alkylstyrene (PAS) monomers.
In accordance with the present invention applicants have discovered an
improved polymerization system for copolymerizing an iso-mono-olefin having
from 4 to 7 carbon atoms and pare-alkylstyrene monomers. In accordance with a
3o preferred embodiment of the invention, the process produces copolymers
containing between about ~0 and 99.5 wt% of the isoolefin such as isobutylene
and between about 0.5 and 20 wt% of the pare-alkylstyrene such as para-
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
7
methylstyrene. In accordance with another embodiment, however, where glassy
or plastic materials are being produced as well, the copolymers comprise
between
about 10 and 99.5 wt% of the isoolefin, or isobutylene, and about 0.5 and 90
wt%
of the para-alkylstyrene, or para-methylstyrene.
In a particularly preferred embodiment, the invention provides for a
continuous slurry polymerization process for preparing random copolymers of
one
or more isoolefin monomers and one or more para-alkylstyrene monomers
comprising reacting in an anhydrous polymerization system of said monomers, a
l0 polar solvent, a Lewis acid, and an initiator, said polymerization system
being
capable of forming an in-situ electron pair donor initiator having the
formula:
R2
R~ I x
R3
h
wherein:
Rl is an alkyl, alkenyl, aryl, aralkyl, or aralkenyl group containing up to 30
carbon
atoms but not less than 3 carbon atoms unless Rl contains at least one
olefinic
unsaturation,
R2 and R3 are alkyl, aryl, or aralkyl groups containing up to 30 carbon atoms
and
2o can be the same or different,
x is a halogen or a carboxy, hydroxyl, or alkoxyl group, and
n is a positive whole number; and
wherein the Lewis acid and the initiator are contacted with a contact time of
from
less than 60s prior to contacting with the isoolefin and the para-alkylstyrene
monomers.
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
8
In another embodiment, the invention provides for the production of a
polyisoolefm rubber. It is produced by the polymerization reaction between
isoolefin monomers. The olefin polymerization feeds employed in the present
invention are those olefinic compounds conventionally used in the preparation
of
isobutylene-type rubber polymers. Preferably, the polyisoolefin rubber are
prepared by reacting monomers of a C4 to C6 isoolefin monomer component such
as isobutene.
In one embodiment, the isoolefin is a C4 to C6 compound such as
l0 isobutylene, isobutene, 2-methyl-1-butene, 3-methyl-1-butene, 2-methyl-2-
butene,
and 4-methyl-1-pentene. Desirably, the isoolefin is isobutylene.
In a particularly preferred embodiment, the invention provides for a
polymerization method for use in a continuous slurry polymerization reactor in
preparing a homopolymer of an isoolefin, the reacted monomers forming the
slurry within the reactor, a Lewis acid, and an initiator, wherein the
initiator has
the formula:
R1
R2 C X
R3
wherein X is a halogen; R1 is selected from the group consisting of Cl to C8
alkyl,
2o and C2 to C8 alkenyl; R3 is selected from the group consisting of, C1 to C8
alkyl,
C2 to C8 alkenyl and phenylalkyl; and R2 is selected from the group consisting
of
C4 to C2oo alkyl, C2 to Cg alkenyl, phenyl, phenylalkyl, alkylphenyl, C3 to
Clo
cycloalkyl, and
R5
X C Ra
R6
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
9
wherein X is a halogen; RS is selected from the group consisting of, C1 to C8
alkyl,
and C2 to C8 alkenyl; R6 is selected from the group consisting of, C1 to C8
alkyl,
C2 to Cg alkenyl and phenylalkyl; and R4 is selected from the group consisting
of
phenylene, biphenyl, a,e~-diphenylalkane and --(CH2)n -, wherein n is an
integer
from 1 to 10; and wherein Rl, R2, and R3 can also form adamantyl or bornyl
ring
systems, the X group being in a tertiary carbon position; and
wherein the Lewis acid anc~ the initiator are contacted with a contact time of
from
less than 60s and prior to contacting with the isoolefm.
to
In any of the previous embodiments denoting contact times, alternative
contact times may be from less than 60s, less than 30s, less than 25s, less
than 20s,
less than 15s, less than l Os, or less than Ss.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a graphical representation of data showing butyl polymerization
conditions in an embodiment of the invention, the data plotted as the reactor
slurry
side heat transfer coefficient as a function of reactor turnover;
Figure 2 is a graphical representation of data showing butyl polymerization
conditions in an embodiment of the invention, the data plotted as the reactor
slurry
side heat transfer coefficient as a function of reactor turnover;
Figure 3 is a graphical representation of data showing butyl polymerization
conditions in an embodiment of the invention, the data plotted as the
percentage
isobutylene conversion within the reactor as a function of the reactor
residence
time;
Figure 4A is a graphical representation of data showing butyl
3o polymerization conditions in an embodiment of the invention, the data
plotted as
the reactor pressure as a function of reactor residence time with TMPCI
initiator
present;
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
Figure 4B is a graphical representation of data showing butyl
polymerization conditions in an embodiment of the invention, the data plotted
as
reactor pressure as a function of reactor residence time with HCl as the
initiator;
5
Figure 5 is a graphical representation of data showing butyl polymerization
conditions in an embodiment of the invention, the data plotted as the amperage
drawn to power the reactor pump impeller as a function of reactor residence
time,
wherein TMPCI is present during the first part of the reaction, and HCl is
present
l0 in the second part of the reaction; and
Figure 6 is a graphical representation of data showing butyl polymerization
conditions in an embodiment of the invention, the data plotted as the slurry
temperature as a function of reactor residence time, wherein TMPCI is present
during the first part of the reaction, and HCl is present in the second part
of the
reaction.
Figure 7 is a graphical representation of data showing catalyst efficiency
plotted as a function of TMPCI/EADC contact times.
DETAILED DESCRIPTION
The invention concerns a catalyst system and process for production of
isoolefin copolymers containing a conjugated dime comonomer. An improved
catalyst system and process has been discovered which affords many unexpected
advantages for commercial slurry polymerization processes. The discussion and
examples below are focused on embodiments of the broad invention. To the
extent that the description is specific, this is done solely for the purpose
of
illustrating exemplifying embodiments and should not be taken as restricting
the
invention to these embodiments.
The polymerization system of the invention contains a mixture of at least
two monomers, a Lewis acid catalyst, an initiator, and a polar diluent. The
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
11
copolymerization reactor is maintained substantially free of impurities which
can
complex with the catalyst, the initiator, or the monomers, and the
polymerization
reaction is conducted under conditions to limit or avoid chain transfer and
termination of the growing polymer chains. Anhydrous conditions are highly
preferred and reactive impurities, such as components containing active
hydrogen
atoms (water, alcohol and the like) must be removed from both the monomer and
diluents by techniques well-known in the art.
Definition of Terms
to As used herein, the term "catalyst system" refers to and includes any Lewis
Acid or other metal complex used to catalyze the polymerization of the
olefinic
monomers of the invention, as well as the initiator described below, and other
minor catalyst components.
As used herein, the "polymerization system" is the catalyst system and the
monomers and reacted monomers within the butyl-type reactor.
As used herein, the term "slurry" refers to reacted monomers that have
polymerized to a stage that they have precipitated from the diluent. The
slurry
"concentration" is the weight percent of these reacted monomers--the weight
percent of the reacted monomers by total weight of the slurry, diluent,
unreacted
monomers, and catalyst system.
As used herein, the new numbering scheme for the Periodic Table Groups
are used as in HAWLEY'S CONDENSED CHEMICAL DICTIONARY 852 (13th ed.
1997).
As used herein the term "butyl rubber" is defined to mean a polymer
predominately comprised of repeat units derived from isobutylene but including
repeat units derived from a conjugated dime.
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
12
Isobutylene-Based Polymers
Butyl rubber is produced by the polymerization reaction between isoolefin
and a conjugated dime comonomers, thus containing isoolefin-derived units and
conjugated dime-derived units. The olefin polymerization feeds employed in
connection with the catalyst and initiator system (described in more detail
below)
are those olefinic compounds, the polymerization of which are known to be
cationically initiated, and are free of aromatic monomers such as para-
alkylstyrene
monomers. Preferably, the olefin polymerization feeds employed in the present
to invention are those olefmic compounds conventionally used in the
preparation of
butyl-type rubber polymers. The butyl polymers are prepared by reacting a
comonomer mixture, the mixture having at least (1) a C4 to C6 isoolefin
monomer
component such as isobutene with (2) a multiolefin, or conjugated dime,
monomer component. The isoolefin is in a range from 70 to 99.5 wt% by weight
of the total comonomer mixture in one embodiment, and 85 to 99.5 wt% in
another embodiment. The conjugated dime component in one embodiment is
present in the comonomer mixture from 30 to 0.5 wt% in one embodiment, and
from 15 to 0.5 wt% in another embodiment. In yet another embodiment, from 8 to
0.5 wt% of the comonomer mixture is conjugated dime.
The isoolefin is a C4 to C6 compound such as isobutene or 2-methyl-1-
butene, 3-methyl-1-butene, 2-methyl-2-butene, and 4-methyl-1-pentene. The
multiolefin is a C4 to C14 conjugated dime such as isoprene, butadiene, 2,3-
dimethyl-1,3-butadiene, myrcene, 6,6-dimethyl-fulvene, hexadiene and
piperylene. One embodiment of the butyl rubber polymer of the invention is
obtained by reacting 95 to 99.5 wt% of isobutylene with 0.5 to 8 wt% isoprene,
or
from 0.5 wt% to 5.0 wt% isoprene in yet another embodiment.
In another embodiment, the invention provides for a catalyst system and
3o process for production of isoolefin copolymers containing a para-
alkylstyrene
comonomers. An improved catalyst system and process has been discovered
which affords many unexpected advantages for commercial slurry polymerization
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
13
of these copolymers generally, and in particular isobutylene pa~~a-
methylsytrene
(IPMS) copolymers. The invention is particularly useful in production of
isoolefin-pare-alkylstyrene (IPAS) copolymers having a higher PAS content,
particularly isobutylene-pare-methylstyrene (IPMS) copolymers having a higher
PMS content (e.g. 10-20 weight percent PMS).
In a particularly preferred embodiment, the copolymers produced contain
isobutylene as the isoolefin and pare-methylstyrene as the pare-alkylstyrene
comonomer. Discussion of these preferred embodiments should not be construed
to so as to limit the broad invention, which is applicable generally to
copolymers of
one or more isoolefin and one or more pare-alkylstyrene (PAS) monomers.
In accordance with the present invention applicants have discovered an
improved polymerization system for copolymerizing an iso-mono-olefin having
from 4 to 7 carbon atoms and pare-alkylstyrene monomers. In accordance with a
preferred embodiment of the invention, the process produces copolymers
containing between about 80 and 99.5 wt% of the isoolefin such as isobutylene
and between about 0.5 and 20 wt% of the pare-alkylstyrene such as para-
methylstyrene. In accordance with another embodiment, however, where glassy
or plastic materials are being produced as well, the copolymers comprise
between
about 10 and 99.5 wt% of the isoolefin, or isobutylene, and about 0.5 and 90
wt%
of the pare-alkylstyrene, or pare-methylstyrene.
In another embodiment, the invention provides for the production of a
polyisoolefin rubber. It is produced by the polymerization reaction between
isoolefin monomers. The olefin polymerization feeds employed in the present
invention are those olefinic compounds conventionally used in the preparation
of
isobutylene-type rubber polymers. Preferably, the polyisoolefin rubber are
prepared by reacting monomers of a C4 to C6 isoolefin monomer component such
3o as isobutene.
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
14
In one embodiment, the isoolefin is a C4 to C6 compound such as
isobutylene, isobutene, 2-methyl-1-butene, 3-methyl-1-butene, 2-methyl-2-
butene,
and 4-methyl-1-pentene. Desirably, the isoolefm is isobutylene.
Lewis Acid
Isomonoolefm and conjugated dime, particularly isobutylene and
isoprene, can be copolymerized under cationic conditions. The copolymerization
is carried out by means of a Lewis Acid catalyst. Embodiments of the invention
include Lewis Acid catalysts (including Friedel-Crafts catalysts) which show
good
to polymerization activity. Desirable catalysts are Lewis Acids based on
metals
from Group 4, 13 and 15 of the Periodic Table of the Elements, including
boron,
aluminum, gallium, indium, titanium, zirconium, tin, vanadium, arsenic,
antimony, and bismuth. In one embodiment, the metals are aluminum, boron and
titanium, with aluminum being desirable. In the practice of the method of this
invention, weaker acids are preferred as they lead to less alkylation and
branching
and higher monomer conversion rates.
The Group 13 Lewis Acids have the general formula RnMX3_n, wherein
"M" is a Group 13 metal, R is a monovalent hydrocarbon radical selected from
the
group consisting of C1 to C12 alkyl, aryl, arylalkyl, alkylaryl and cycloalkyl
radicals; and n is an integer from 0 to 3; X is a halogen independently
selected
from the group consisting of fluorine, chlorine, bromine, and iodine,
preferably
chlorine. The term "arylalkyl" refers to a radical containing both aliphatic
and
aromatic structures, the radical being at an alkyl position. The term
"alkylaryl"
refers to a radical containing both aliphatic and aromatic structures, the
radical
being at an aryl position. Nonlimiting examples of these Lewis acids include
aluminum chloride, aluminum bromide, boron trifluoride, boron trichloride,
ethyl
aluminum dichloride (EtAlCl2 or EADC), diethyl aluminum chloride (Et2A1C1 or
DEAC), ethyl aluminum sesquichloride (Etl_SA1C11,5 or EASC), trimethyl
aluminum, and triethyl aluminum.
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
The Group 4 Lewis Acids have the general formula MX4, wherein M is a
Group 4 metal and X is a ligand, preferably a halogen. Nonlimiting examples
include titanium tetrachloride, zirconium tetrachloride, or tin tetrachloride.
5 The Group 15 Lewis Acids have the general formula MXy, wherein M is a
Group 15 metal, X is a ligand, preferably a halogen, and y is an integer from
3 to
5. Nonlimiting examples include vanadium tetrachloride and antimony
pentafluoride.
l0 Particularly preferred Lewis acids may be any of those useful in cationic
polymerization of isobutylene copolymers including: AICl3, EADC, EASC,
DEAL, BF3, TiCl4, etc. with EASC and EADC being especially preferred.
Catalyst efficiency (based on Lewis Acid) in the reactor is maintained
15 between 10000 lb. of polymer/lb. of catalyst and 300 lb. of polymer/lb. of
catalyst
and desirably in the range of 4000 lb. of polymer/lb. of catalyst to 1000 lb.
of
polymer/lb. of catalyst by controlling the molar ratio of Lewis Acid to
initiator.
Initiator
2o According to one embodiment of the invention, the Lewis Acid catalyst is
used in combination with an initiator. The initiators are those initiators
which are
capable of being precomplexed in a suitable diluent with the chosen Lewis Acid
to
yield a complex which is in equilibrium with a carbenium ion pair which
rapidly
forms a propagating polymer chain in the reactor. These initiators yield a
fast,
simple initiation of polymerization in the reactor as opposed to the slow
stepwise
initiations involving several polar complexes in equilibrium characteristic of
the
catalyst systems such as water or HCl initiators conventionally used in
commercial cationic slurry polymerization of isobutylene copolymers. The
initiator is a tertiary halide greater than C4, wherein the initiator has the
formula
(A):
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
16
R1
R2 C X (A)
R3
wherein X is a halogen; R1 is selected from the group consisting of C1 to C8
alkyl,
and CZ to C8 alkenyl; R3 is selected from the group consisting of Cl to Cg
alkyl,
CZ to Cg alkenyl and phenylalkyl; and R2 is selected from the group consisting
of
C4 to C2oo alkyl, C2 to C8 alkenyl, phenyl, phenylalkyl, alkylphenyl, C3 to
Cio
cycloalkyl, and
Rs
X C R4 (B)
wherein X is a halogen; RS is selected from the group consisting of C1 to C8
alkyl,
and CZ to C8 alkenyl; R6 is selected from the group consisting of C1 to C8
alkyl,
l0 C2 to C8 alkenyl and phenylalkyl; and R4 is selected from the group
consisting of
phenylene, biphenyl, a,co-diphenylalkane and --(CH2)p -, wherein n is an
integer
from 1 to 10; and wherein Rl, R2, and R3 can also form adamantyl or bornyl
ring
systems, the X group being in a tertiary carbon position.
Substitution of the above structural formula radical (B) for R2 in formula
(A) results in the following formula (C):
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
17
Rs R1
X C R4 C X (C)
R6 R3
wherein X, R1, R3, R4, Rs and R6 are as defined above. The compounds
represented by structural formula (C) contain two dissociable halides and may
be
considered as merely multiples of those compounds represented by structural
formula (A).
Multifunctional initiators are employed where the production of branched
copolymers is desired, while mono- and di-functional initiators are preferred
for
the production of substantially linear copolymers.
l0
In one desirable embodiment of structure (A), the initiator is an oligomer
of isobutylene as in structure (D):
H3
H HOC ~ X (D)
CH3
i m
wherein X is a halogen, and the value of m is from 1 to 60, and mixtures
thereof.
In another embodiment, m is from 2 to 40. This structure is also described as
a
tertiary alkyl chloride-terminated polyisobutylene having a Mn up to 2500 in
one
embodiment, and up to 1200 in another embodiment.
Non-limiting examples of suitable initiators are cumyl esters of
2o hydrocarbon acids, and alkyl cumyl ethers. Representative initiators, for
example,
comprise compounds such as 2-acetyl-2-phenylpropane, i.e., cumyl acetate; 2-
methoxy-2-phenyl propane, i.e., cumylmethyl-ether; 1,4-di(2-methoxy-2-
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
18
propyl)benzene, i.e., di(cumylmethyl ether); the cumyl halides, particularly
the
chlorides, i.e., 2-chloro-2-phenylpropane, i.e., cumyl chloride (1-chloro-1-
methylethyl)benzene; 1,4-di(2-chloro-2-propyl)benzene, i.e.,
di(cumylchloride);
1,3,5-tri(2-chloro-2-propyl)benzene, i.e., tri(cumylchloride); the aliphatic
halides,
particularly the chlorides, i.e., 2-chloro-2,4,4-trimethylpentane (TMPCI), 2-
bromo-2,4,4-trimethylpentane (TMPBr), 2,6-dichloro-2,4,4,6-tetramethylheptane;
cumyl and aliphatic hydroxyls such as 1,4-di((2-hydroxyl-2-propyl)-benzene),
2,6-dihydroxyl-2,4,4,6-tetramethyl-heptane, 1-chloroadamantane and 1-
chlorobornane, 5-tert-butyl-1,3-di(1-chloro-1-methyl ethyl) benzene and
similar
to compounds. Other suitable initiators may be found in U.S. Patent No.
4,946,899,
herein incorporated by reference for purposes of U.S. patent practice. These
initiators are generally CS or greater tertiary or allylic alkyl or benzylic
halides and
may include polyfunctional initiators. Desirable examples of these initiators
include: TMPCI, TMPBr, 2,6-dichloro-2,4,4,6-tetramethylheptane, cumyl
chloride as well as 'di-' and 'tri ' cumyl chloride or bromide. In another
embodiment, the initiator is a tertiary alkyl chloride-terminated
polyisobutylene
with a Mn (number average molecular weight) up to 2500.
In one embodiment, the TMPCI is made by dissolving isobutylene dimer
in methylchloride and then adding anhydrous HCl to form the alkyl chloride.
Excess HCl is then purged by nitrogen and the resulting solution of TMPCI in
methylchloride is used as the initiator stream in a continuous plant to make
butyl
polymers. In one embodiment of the commercial-type process, the TMPCI stream
is mixed with a cold methylchloride (chloromethane) stream and an aluminum
alkyl stream to form the catalyst system. This stream is then injected into
the
continuous flow stirred tank reactor ("CFSTR") used to produce butyl polymers
under much more controllable and economic conditions than has previously been
possible. In another embodiment, isobutylene dimers are reacted with HCl
inline
and then fed directly into the reactor.
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
19
Polymerization Reaction Conditions
The selected diluent or diluent mixture should provide a diluent medium
having some degree of polarity in order for the polymerization to proceed at a
reasonable rate. To fulfill this requirement, a mixture of nonpolar and polar
diluents can be used. In the alternative, a mixture of, or a single polar
diluent, is
more desirable. Suitable nonpolar diluent components includes hydrocarbons and
preferably aromatic or cyclic hydrocarbons or mixtures thereof. Such compounds
include, for instance, methylcyclohexane, cyclohexane, toluene, carbon
disulfide
and others. Appropriate polar diluents include halogenated hydrocarbons,
normal,
l0 branched chain or cyclic hydrocarbons. Specific compounds include the
preferred
liquid diluents such as ethyl chloride, methylene chloride (dichloromethane,
CH2C12), methylchloride (chloromethane, CH3Cl), C02, CHCl3, CC14, n-butyl
chloride, chlorobenzene, and other chlorinated hydrocarbons. Methylchloride is
desirably used in an embodiment of the invention. To achieve suitable polarity
and solubility, it has been found that if the diluent, or diluents, is mixed,
the
mixture is preferably at least 70 % polar diluent, on a volume basis.
As is normally the case, product molecular weights are determined by
reaction time, temperature, concentration, the nature of the reactants, and
similar
factors. Consequently, different reaction conditions will produce products of
different molecular weights. Synthesis of the desired reaction product will be
achieved, therefore, through monitoring the course of the reaction by the
examination of samples taken periodically during the reaction, a technique
widely
employed in the art and shown in the examples or by sampling the effluent of a
continuous reactor.
The reactors that may be utilized in the practice of the present invention
include any conventional reactors and equivalents thereof capable of
performing a
continuous slurry process, such as disclosed in U.S. 5,417,930, herein
incorporated by reference. The reactor pump impeller can be of the up-pumping
variety or the down-pumping variety. The reactor will contain sufficient
amounts
of the catalyst system of the present invention effective to catalyze the
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
polymerization of the monomer containing feed-stream such that a sufficient
amount of polymer having desired characteristics is produced. The feed-stream
in
one embodiment contains a total monomer concentration greater than 30 wt%
(based on the total weight of the monomers, diluent, and catalyst system),
greater
5 than 35 wt% in another embodiment. In yet another embodiment, the feed-
stream
will contain from 35 wt% to 50 wt% monomer concentration based on the total
weight of monomer, diluent, and catalyst system.
The feed-stream is substantially free from silica ration producing species.
to By substantially free of silica ration producing species, it is meant that
there is no
more than 0.0005 wt% based on the total weight of the monomers of these silica
species in the feed stream. Typical examples of silica ration producing
species
. are halo-alkyl silica compounds having the formula R1R2R3SiX or RlR2SiX2,
etc.,
wherein "R" is an alkyl and "X" is a halogen. Finally, the feed stream should
be
15 free of aromatic-containing monomers such as para-alkylstyrene.
The reaction conditions will be such that desirable temperature, pressure
and residence time are effective to maintain the reaction medium in the liquid
state and to produce the desired polymers having the desired characteristics.
The
2o monomer feed-stream is typically substantially free of any impurity which
is
adversely reactive with the catalyst under the polymerization conditions. For
example, the monomer feed preferably should be substantially free of bases
(such
as caustic), sulfur-containing compounds (such as H2S, COS, and organo-
mercaptans, e.g., methyl mercaptan, ethyl mercaptan), N-containing compounds,
oxygen containing bases such as alcohols and the like.
The polymerization reaction temperature is conveniently selected based on
the target polymer molecular weight and the monomer to be polymerized as well
as standard process variable and economic considerations, e.g., rate,
temperature
control, etc. The temperature for the polymerization is between -10°C
and the
freezing point of the polymerization system in one embodiment, and from -
25°C
to -120°C in another embodiment. In yet another embodiment, the
polymerization
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
21
temperature is from -40°C to -100°C, and from -70°C to -
100°C in yet another
embodiment. In yet another desirable embodiment, the temperature range is from
-80°C to -100°C. The temperature is chosen such that the desired
polymer
molecular weight is achieved. The reaction pressure will be from 200 kPa to
1600
kPa in one embodiment, from 300 kPa to 1200 kPa in another embodiment, and
from 400 kPa to 1000 kPa in yet another embodiment.
The catalyst (Lewis Acid) to monomer ratio utilized will be those
conventional in this art for carbocationic polymerization processes. In one
l0 embodiment of the invention, the catalyst to monomer mole ratios will be
from
0.10 to 20, and in the range of 0.5 to 10 in another embodiment. In yet
another
desirable embodiment, the ratio of Lewis Acid to initiator is from 0.75 to
2.5, or
from 1.25 to 1.5 in yet another desirable embodiment. The overall
concentration
of the initiator is from 50 to 300 ppm within the reactor in one embodiment,
and
from 100 to 250 ppm in another embodiment. The concentration of the initiator
in
the catalyst feed stream is from 500 to 3000 ppm in one embodiment, and from
1000 to 2500 in another embodiment. Another way to describe the amount of
initiator in the reactor is by its amount relative to the polymer. In one
embodiment, there is from 0.25 to 5.0 moles polymer/mole initiator, and from
0.5
2o to 3.0 mole polymer/mole initiator in another embodiment.
The reacted monomers within the reactor form a slurry. The term "slurry"
refers to reacted monomers that have polymerized to a stage that they have
precipitated from the diluent. The slurry "concentration" is the weight
percent of
these reacted monomers--the weight percent of the reacted monomers by total
weight of the slurry, diluent, unreacted monomers, and catalyst system. In one
embodiment, the concentration of the slurry is equal to or greater than 10
wt%. In
another embodiment, the slurry is present in the reactor in a concentration
equal to
or greater than 25 wt%. In yet another embodiment, the slurry concentration in
the reactor is less than or equal to 50 wt%. In yet another embodiment, the
slurry
is present in the reactor from 20 to 50 wt%. And in yet another embodiment,
the
slurry concentration is present in the reactor from 30 to 40 wt%.
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
22
The slurry is characterized by having a heat transfer coefficient (hsurry) as
defined above in equation (2). In one embodiment of the invention, the heat
transfer coefficient of the slurry is from 200 to 500 Btu/hr~ft2°F. In
another
embodiment of the invention, the heat transfer coefficient of the slurry is
from 300
to 450 Btu/hr~ft2°F.
The order of contacting the monomer feed-stream, catalyst, initiator, and
diluent is not critical to this invention. In one embodiment, the initiator
and Lewis
to Acid are pre-complexed by mixing together in cold methylchloride or other
suitable cold polar diluent, immediately before injection into the continuous
reactor through a catalyst nozzle in the standard way. Other methods may also
be
employed that will inject the initiator into the reactor. Desirably, the
monomer is
not contacted with the Lewis Acid and initiator at before entering the
reactor. In
yet another embodiment, Lewis Acid and the initiator are added to the reactor
separately.
In another embodiment of the invention, the stabilizing initiator and Lewis
Acid are allowed to precomplex by mixing together with varying contact times.
2o Depending upon the catalyst efficiency desired, contact times may vary from
O.OOls, 0.002s, 0.003s, 0.004s, O.OOSs, 0.006s, 0.007s, 0.008s, 0.009s,
O.OlOs,
0.020s, 0.030s, 0.040s, O.OSOs, 0.060s, 0.070s, 0.080s, 0.090s, O.100s,
0.200s,
0.300s, 0.400s, O.SOOs, 0.600s, 0.700s, 0.800s, 0.900s, ls, 2s, 3s, 4s, Ss,
6s, 7s, 8s,
9s, to lOs. Preferable ranges include 20s or less, 30s or less, 40s or less,
SOs or
less, 60s or less, 70s or less, 80s or less, 90s or less, 100s or less, 1 l Os
or less, and
120s or less. Other preferable ranges include from 1 ~,s to 120s, 1 OO~,s to
60s, 1 ~,s
to 30s, 0.45s to 25s, O.Ols to 20s, O.OSs to lOs, and O.lOs to Ss.
In another embodiment of the invention, the stabilizing initiator and Lewis
3o Acid are precomplexed and injected into the reactor through a single
reactor
nozzle. In yet another embodiment of the invention, the stabilizing initiator
and
Lewis Acid, when fed into the reactor separately, are precomplexed in a mixing
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
23
zone within the reactor. While not wishing to be bound to theory, it is
believed
that the Lewis Acid and the stabilizing initiator combine to yield a stable
complex
which is in equilibrium with a carbenium ion pair that directly forms a
propagating polymer chain in the reactor. It has been discovered that shorter
contact times have an unexpected beneficial effect on catalyst efficiency.
In a particularly preferred embodiment, TMPCI and EADC are combined
at contact times in the ranges from l~,s to 120s, 100p,s to 60s, l~s to 30s,
0.45s to
25s, O.Ols to 20s, O.OSs to lOs, or O.lOs to Ss prior to combining with
isobutylene
to as the isoolefin and isoprene as the conjugated dime comonomer.
In yet another particularly preferred embodiment, TMPCI and EASC are
combined at contact times in the ranges from 1 ~,s to 120s, 100p.s to 60s, 1
~,s to
30s, 0.45s to 25s, O.Ols to 20s, O.OSs to lOs, or O.lOs to Ss prior to
combining with
isobutylene as the isoolefin and isoprene as the conjugated dime comonomer.
In one embodiment, the polymerization of isobutylene and isoprene to
form butyl rubber comprises several steps. First, a reactor having a pump
impeller
capable of up-pumping or down-pumping is provided. The pump impeller is
typically driven by an electric motor with a measurable amperage. The reactor
typically is equipped with parallel vertical reaction tubes within a jacket
containing liquid ethylene. The total internal volume, including the tubes, is
greater than 30 to 50 liters, thus capable of large scale volume
polymerization
reactions. The reactor typically uses liquid ethylene to draw the heat of the
polymerization reaction away from the forming slurry. The pump impeller keeps
a constant flow of slurry, diluent, catalyst system and unreacted monomers
through the reaction tubes. A feed-stream of the isoprene and isobutylene in a
polar diluent is charged into the reactor, the feed-stream containing less
than
0.0005 wt% of cation producing silica compounds, and typically free of
aromatic
monomers. The catalyst system is then charged into the feed-stream, the
catalyst
system having a Lewis acid and an initiator present in a molar ratio of from
0.50
to 10Ø Within the reactor, the feed-stream of monomers and catalyst system
are
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
24
allowed to contact one another, the reaction thus forming a slurry of butyl
rubber,
wherein the slurry has a concentration of from 25 wt% to 50 wt%. Finally, the
thus formed butyl rubber is allowed to exit the reactor through an outlet or
outflow
line while simultaneously allowing the feed-stream charging to continue, thus
constituting the continuous slurry polymerization. Advantageously, the present
invention improves this process in a number of ways, ultimately reducing the
amount of clogging that occurs in the exit port which is measured by pressure
inconsistencies or "jumps".
to The overall residence time in the reactor can vary, depending upon, e.g.,
catalyst activity and concentration, monomer concentration, feed injection
rate,
production rate, reaction temperature, and desired molecular weight, and
generally
will be between about one minute and five hours, and preferably between about
10
and 60 minutes. The principle variable controlling residence time is the
monomer
feed injection rate. The resultant polymer from one embodiment of the
invention
is a polyisobutylene/isoprene polymer (butyl rubber) that has a molecular
weight
distribution of from about 2 to 5, and an unsaturation of from 0.5 to 2.5 mole
per
100 mole of monomer. This product may be subjected to subsequent halogenation
to afford a halogenated butyl rubber.
The new catalyst system and process affords many unexpected advantages
for commercial slurry polymerization of isoolefins and conjugated dimes. The
improvements obtained with this new initiator are demonstrated in commercial
plant scale tests. The following examples reflect embodiments of the invention
and are by no means intended to be limiting of the scope of the invention.
Examples
Laboratory Experiments. The laboratory scale experiments highlight the
unexpected nature of the invention exemplified in the plant scale experiments
3o below. In these laboratory experiments, a feed blend of isobutylene (9.7
wt%) and
isoprene (0.3 wt%) in methylchloride was chilled to -93°C in a glass
reactor
contained in an inert atmosphere and polymerized in separate experiments by
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
adding various amounts of Lewis acid and initiator as shown in Table 1. The
initiator and Lewis acid concentrations present in each experiment are shown
in
the Table 1 as ppm. The Lewis acid and initiator was added to such a degree to
the batch reactions as to achieve a 30 to 45% conversion of monomers as
5 determined by the gravimetric yield. In all examples, the molecular weights
(Mw)
were determined by Gel Permeation Chromatography using a Waters
Chromatograph operating at ambient temperature (30°C). The HCl
(Matheson)
was used as a 260 ppm solution, and the tent-butylchloride (t-BuCI, Aldrich
Chemical Company) was used as a 710 ppm solution. The TMPCI was made by
l0 ExxonMobil Chemical Company from isobutylene dimers and HCl by methods
common in the art. The monomers are manufactured by ExxonMobil Chemical
Company (Houston, Texas). The molecular weights in Table 1 are an average of
three runs for each experiment.
15 TABLE 1. Laboratory Experiment Data
Example Initiator Lewis Acid ( ppm)yield Mw
(ppm)
1 HCl (22) EADC (230) 47 242,000
2 t-BuCI (55) EADC (210) 55 440,000
3 TMPCI (70) EADC (1S0) 33 510,000
4 TMPCI (130) EADC (350) 43 400,000
5 TMPCI (105) EASC (260) 34 465,000
These data show that, while both tert-butylchloride and TMPCI increase
the molecular weight of the resultant butyl polymer in a batch laboratory
scale
20 experiment, there was no significant difference between the two. Therefore,
from
the laboratory data one would expect that both TMPCI and tert-butylchloride
would behave similarly in the continuous slurry plant reactors. Surprisingly,
TMPCI initiated continuous slurry polymerization resulted in significant heat
transfer and viscosity benefits, whereas tert-butylchloride initiated
polymerization
25 did not.
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
26
Plant Reactor Experiments. The examples are discussed below in relation
to the figures in which the data is represented. Typical reaction conditions
within
the reactor are first outlined in Table 2, which are conditions for the
continuous
slurry reactor plant test examples with initiators of the invention and
comparative
examples using HCl and tert-butylchloride as initiators.
TABLE 2. Properties and Reaction Conditions in Plant Scale Tests
Property/Ingredient Amount/Value
Production Rate 6000 lb/hr (2,727 kg/hr)
Feed Rate 22,000 lb/hr (10,000 kg/hr)
Isobutylene (in feed-stream) 30.7 wt% to 39 wt%
Isoprene 2.65 wt%
TMPCI (20% solution) 35 lb/hr (15.9 kg/hr)
EADC/TMPCI (mole/mole) 1.5 to 1.25
Slurry Concentration 25 wt% to 32.5 wt%
Isobutylene Conversion 85% to 88 wt%
Diluent Methylchloride
Initial Reactor Temperature -98.3C (-145F)
l0 The conditions in Table 2 correspond to TMPCI initiated reactions as well
as the HCl and tert-butylchloride initiated reactions except for the
following: in
the case of the TMPCI initiated reaction, the feed blends were increased from
30.7
wt% to 39 wt%. In the comparative examples, the feed blend was constant at
30.7
wt%. The monomers are manufactured by ExxonMobil Chemical Company
(Houston, Texas). The methylchloride (Dow Chemical Company), EADC
(Albemarle), and HCl (Matheson) were used as received, and the TMPCI was
made by ExxonMobil Chemical Company by reacting isobutylene dimers and HCl
from methods common in the art.
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
27
Figuy~e 1. Figure 1 is a graphical representation of data showing butyl
polymerization conditions in an embodiment of the invention, the data plotted
as
the reactor slurry side heat transfer coefficient as a function of reactor
turnover.
The slurry side heat transfer coefficient (hs~"n.y) is the heat transfer
coefficient (h)
of the slurry within the butyl reactor tubes, as opposed to the heat transfer
resistance of the reactor tube walls and/or the heat transfer coefficient of
the
boiling ethylene used to remove heat from the reactor. The value of "h"
(Btu/hr~ft2~°F) is a function of the viscosity of the slurry (fib), and
is related as such
by the well known Sieder-Tate equation (2) discussed above.
to
In these examples, the reactor was operated with a frozen methylchloride
(the diluent) film coating the tubes. The difference between the bulk slurry
temperature and the frozen ice film was then measured. The temperature of the
frozen ice film can be calculated from the monomer concentration in the
reactor
and the correlation with its freezing point. Using the equation (3) below, the
value
for the slurry side heat transfer coefficient hs~u~.y was obtained:
slurry - ~_ (3)
A Tslurry TttTeClice
where Q is the heat removed from the reactor during polymerization, A is the
heat
transfer area of the reactor, Tsturry is the average bulk temperature of the
reactor
2o slurry, and TMe~u~e (MeCI is methylchloride) is the average temperature of
the
frozen ice film, as defined in equation (4) below:
TMeClice - 143.8 -' x.75 .M~ (4)
where -143.8 is the freezing point temperature (in °F) of pure
methylchloride and
M is the wt% concentration of isobutylene in the slurry.
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
28
The data in Figure 1 shows comparative data between HC1 initiated butyl
polymerization and TMPCI initiated butyl polymerization at various slurry
concentrations. A best fit line (linear regression) is drawn through the
linear
portion of the data, which takes into account the time for the slurry
concentration
in the reactor to build up to its steady state value of three turnovers, and
hence the
initially large hsl°,.ry values. In the comparative HCl initiated
reaction, the slurry
concentration is 25.3 wt% by total weight of slurry, diluent, monomers, and
other
reactor components. At the same slurry concentration, the TMPCI initiated
reaction has a higher hs~",.,~, value, thus translating by equation (1) to a
lower
to viscosity. When the slurry concentration for the TMPCI initiated reaction
is
increased to 29 wt%, the hs~""y value, and hence the viscosity, does not
change
appreciably. When the slurry concentration is increased to 32.5 wt% for the
TMPCI initiated reaction, the value of hsl°"y is above that of the HCl
initiated
reaction.
These data show that the heat transfer coefficient of the slurry is from 375
to 450 Btu/hr~ft2~°F when the slurry concentration is from 25 to 30
wt%; and the
heat transfer coefficient of the slurry is from 200 to 350
Btu/hr~ft2~°F when the
slurry concentration is from 30 to 35 wt%. These data indicate that the TMPCI
initiator raises the heat transfer coefficient and thus allowing a higher
slurry
concentration to be run in the butyl reactor and/or longer reactor run
lengths.
Figure 2. Figure 2 is a graphical representation of data showing butyl
polymerization conditions in an embodiment of the invention, the data plotted
as
the reactor slurry side heat transfer coefficient as a function of reactor
turnover. A
best fit line (linear regression) is drawn through the linear portion of the
data,
which takes into account the time for the slurry concentration in the reactor
to
build up to its steady state value of three turnovers, and hence the initially
large
hsnrry values. In this Example, HCI, tent-butylchloride (Aldrich Chemical
Company), and TMPCI are used as initiators in separate butyl reactions and
compared.
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
29
The values for hsurry for the HCl initiated reaction are around 300
Btu/hr~ftz°F (1.7 kW/mzK) after about 4 to 6 reaction turnovers at a
slurry level of
25.6 wt% and production rate of 6.0 Klb/hr (2.72 T/hr). When tert-
butylchloride
is the initiator at a slurry level of 25.4 wt% and production rate of 6.3
Klb/hr (2.86
T/hr), the hs~",.,y values decrease, thus indicated that the slurry viscosity
increases
slightly. When TMPCI is the initiator at a slurry level of 26.1 wt% and
production
rate of 6.3 Klb/hr (2.86 T/hr), the hsi",ry values increase to above 400
Btu/hr~ftz~°F
(2.27 kW/mzK). Thus, when compared to tent-butylchloride and HCI, the TMPCI
initiated polymerization efficiency is improved.
l0
Figure 3. The present example shows how the conversion of monomers
within the reactor increases when an embodiment of the invention is used.
Specifically, Figure 3 is a graphical representation of data showing butyl
polymerization conditions in an embodiment of the invention, the data plotted
as
the percentage isobutylene conversion within the reactor as a function of the
reactor residence time.
In the HCl initiated butyl polymerization reaction, the isobutylene
conversion increases from about 86.5 to about 87.5% over a residence time of
0.85 hours. The conversion for tent-butylchloride initiated polymerization is
about
87.5%. For the TMPCI initiated reaction, the conversion is from about 88.5% to
about 89.5% isobutylene over a time period of from 0.55 hours to about 0.7
hours.
This embodiment of the invention shows a 15% decrease of the amount of
unreacted monomer remaining, i.e., a significant improvement in monomer
conversion. These data show that the TMPCI initiated reaction improves the
conversion of monomer, thus improving the overall butyl polymerization process
and allowing a higher slurry concentration to be run and/or longer run
lengths.
Figure 4. The present example in Figure 4 highlights the lowered
3o agglomeration tendency due to the lowered viscosity of the butyl slurry
when
using embodiments of the invention. Specifically, Figure 4A is a graphical
representation of data showing butyl polymerization conditions in an
embodiment
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
of the invention, the data plotted as the reactor pressure as a function of
reactor
residence time with TMPCI initiator present. Figure 4B is a graphical
representation of data showing butyl polymerization conditions in an
embodiment
of the invention, the data plotted as reactor pressure as a function of
reactor
5 residence time with HCl as the initiator. The pressure is measured at the
feed inlet
to the reactor, and is representative of the internal pressure within the
reactor
itself. When the internal reactor pressure rises, this is indicative of
agglomeration
of the slurry in the outlet or overflow line, and is detected as a clogging
and,
hence, pressure increase, at that point in the reactor.
l0
The data in Figure 4A is with a slurry concentration of 32.5 wt%, while the
slurry concentration for the HCl initiated reaction in Figure 4B is 30 wt%.
Note
the differences in the y-axis scale between the two graphs. The data show that
the
baseline pressure level at about 41 psia is relatively constant until the
reactor is
15 turned off after about a 20 hour run. However, when HCl is used as the
initiator,
even at the lower slurry level, there are significant pressure buildups after
8 hours
of reaction time, the pressure buildups or "kickings" are indications of
agglomeration and clogging of the butyl reactor.
2o Figure S. The data in this example are consistent with a lowering of the
viscosity of the butyl slurry when embodiments of the invention are used.
Specifically, Figure 5 is a graphical representation of data showing butyl
polymerization conditions in an embodiment of the invention, the data plotted
as
the amperage drawn to power the reactor pump impeller as a function of reactor
25 residence time, wherein TMPCI is present during the first part of the
reaction, and
HCl is present in the second part of the reaction.
In this example, the butyl polymerization reaction is run for about 22 hours
at a slurry concentration of 25 wt% using TMPCI as the initiator in a
3o concentration of 2000 ppm in the catalyst stream entering the reactor, and
200
ppm in the reactor. Then, HCl is added to the reactor, while the TMPCI-laden
slurry is allowed to exit the reactor. Specifically, the concentration of HCl
in the
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
31
catalyst stream entering the reactor is normally in the range of from 100 to
200
ppm, and the concentration in the reactor is between from 10 to 20 ppm. The
transition period from HCl to TMPCI initiator is about 2 hours. As HCl
replaces
the TMPCI as the initiator, the motor that drives the reactor pump impeller
must
work haxder to stir the slurry, as indicated by the increased power draw. This
is
consistent with the lower viscosity with TMPCI as the initiator. While not
wishing to be bound by an equation, the results in Figure 5 axe consistent
with
what equation (2) would predict. Specifically, the hsl°"y values when
HCl is the
initiator range from about 411 to 592 Btu/hr~ft2~°F (2.33 to 3.36 kW/m2
K), while
to that of the TMPCI initiated reaction varies from 241 to 261
Btu/hr~ft2~°F (1.37 to
1.48 kWlm2 K).
Figure 6. In this example, the temperature of the slurry in the example of
Figure 5 was measured directly, beginning with the TMPCI initiated reaction,
and
followed by injection of HCl as the initiator. As seen in the graph, the
temperature is steady at -98.3°C (-145°F), but steadily rises as
HCl is added as the
initiator. These data show how the embodiment of the invention improves heat
transfer in the butyl reactor, thus improving the polymerization efficiency.
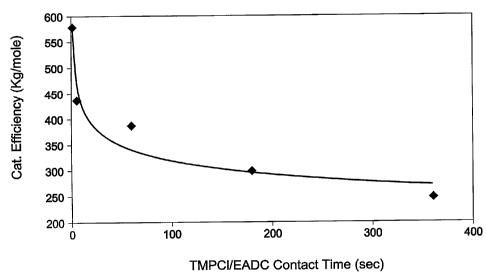
Figure 7. In these examples, the polymerization reactions were carried out
in the cold bath of a dry box using 3-neck round bottom flask reactors with
mechanical stirring at ~-95°C. The initiator (2-chloro-2,4,4-trimethyl
pentane
(TMPCI)) for the polymerization was vacuum distilled at room temperature to
remove excess HCl gas. The co-initiator for the polymerization (25.3 wt% ethyl
aluminum dichloride in Heptane (EADC)) was purchased from AKZO NOBEL
and used directly. The monomer (Isobutylene) and the comonomer (Isoprene) for
the polymerization were passed through a drying column or vacuum distilled
before use. The diluent, methyl chloride, was passed through a drying column
before reaction. The feed blend (10 wt% of monomer solution) was prepared
from the mixture of monomers) and diluent before reaction and stored at -~-
95°C
in the cold bath. The initiator and coinitiator stock solutions were prepared
by
mixing methyl chloride with distilled TMPCI and methyl chloride with 25 wt%
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
32
EADC. The glassware used for the polymerizations was cleaned with 150m1
methyl chloride and l5p,l of 25.3 wt% EADC and prechilled to ~-95°C in
the cold
bath right before reaction. 300m1 of feed blend was transferred into the clean
glass
reactor and chilled to ~-95°C in cold bath before reaction. For shorter
contact
times, i.e., (0 to 1 sec) experiments the initiator was mixed with the feed
blend at
-95°C in cold bath and then the coinitiator was added. For other
contact times,
the initiator and coinitiator were mixed in a glass vial with different
contact times
(2 sec, 5 sec, 60 sec, 180 sec, and 360 sec) before being added to the
reactor.
When the reactor temperature had stopped rising, 25m1 Isopropanol with BHT as
to a stabilizer was added to quench the reaction. After the solvent had boiled
off, the
polymer was dried in a vacuum oven at 45°C overnight. The polymer was
then
removed and weighed to calculate the catalyst efficiency.
TABLE 3. TMPCI/EADC Contact Times and Catalyst Efficiency
RXNl RXN2 RxN3 RxN4 RXNS
Catalyst Efficient 579 437 388 300 248
(Kg/mole)
TMPCI/EADC Contact 0 to 1 5 60 180 360
Time (sec)
As can be see, shorter contact times of TMPCI and EADC unexpectedly
resulted in improved catalyst efficiency. The results are also graphically
depicted
in Figure 7.
Discussion
The present invention has several advantages. Because of the rapid reactor
mass fouling rate that typically occurs, reactors had to be operated at very
low
slurry concentrations and heat loads to achieve the run lengths required to
allow
washing and turnaround to be accomplished in the time available before the
fouled
reactor had to be put back into production to replace another fouled reactor.
The
present invention, as shown in the examples, will allow the butyl reactors to
be
run at higher slurry concentrations and/or run at a lower concentration for a
longer
period of time before fouling. In one embodiment of the invention, the run
length
CA 02493729 2005-O1-26
WO 2004/014968 PCT/US2003/023284
33
is increased from 30% to 200% relative to the run length when HC1 or C4 or
smaller initiators are used in the catalyst system.
Embodiments of the invention improve the heat transfer within the reactor.
The improved heat transfer can allow either higher slurry concentrations, or
longer
run length. The heat transfer coefficient is thus higher due to the lower
viscosity
of the slurry as would be predicted using the Sieder-Tate equation for
turbulent
flow. Not only are higher slurry concentrations possible due to the improved
heat
transfer, but a higher monomer conversion rate is also achieved. Further,
there is
l0 a lower overflow line plugging rate with embodiments of the present
invention
and steadier reactor operation due to the lower pump power consumption. Also,
since heat transfer is improved, longer run lengths at relatively low slurry
concentrations (below 25 wt%) are possible since it is known that the reactor
will
stay cold (liquid ethylene temperatures) for longer when the heat transfer
coefficient of the slurry is low. The cooler temperatures lower the fouling
rate of
the heat transfer surfaces, thus allowing the reactor to stay on line without
stoppage for cleaning for a longer period of time that was previously
possible.
All patents, applications and publications cited herein, including those
2o relied upon for priority, are herein incorporated by reference for purposes
of U.S.
patent practice.