Note: Descriptions are shown in the official language in which they were submitted.
CA 02493891 2005-O1-17
WO 2004/009743 PCT/EP2003/008061
USE OF A YELLOW FLAME BURNER
The invention is directed to an improved use of a
yellow flame burner.
Conventional designs of oil burner assemblies for
home heating fuel oils employ a traditional fuel/air
mixing process in which the evaporation and combustion of
the fuel oil take place simultaneously. In one form of
oil burner assembly for home heating fuel oils the fuel
oil is sprayed as a hollow cone and air is weakly swirled
along a path which is parallel to the axis of a burner
blast tube and which passes into the hollow cone so that
the trajectories of the fuel oil droplets cross the air
flow streamlines. This leads to a rapid evaporation
giving fuel oil rich regions, which in turn ignite under
local sub-stoichioinetric conditions producing soot, and
results in air pollution as well as well as a waste of a
fossil fuel.
The general pattern of the flame of such an oil
burner assembly is one of heterogeneity in terms of fuel
concentrations; the pockets of fuel lean mixture give
rise to high nitric oxide concentrations from both the
fuel nitrogen and the atmospheric nitrogen, while the
pockets of fuel rich mixture give rise to soot.
The visible flame when using an Industrial Gas Oil fuel
'from such a system is yellow. The yellow colour is the
visible radiation from the high temperature soot
particles and this completely masks other visible
radiations as far as the human eye is concerned.
These soot particles result from non-burnt carbon.
CA 02493891 2005-O1-17
WO 2004/009743 PCT/EP2003/008061
- .~ _
For complete combustion of the carbon; that is
soot-free combustion, the step-wise combustion of carbon
to carbon dioxide via the intermediate carbon monoxide
stage gives rise to a visible radiation 'in the blue
region of the light spectrum. When this occurs the blue
radiation becomes visible in a soot-free or
low-luminosity flame, and oil burners for such soot-free
flames are known as blue flame burners.
Blue flame burners are known to have a desirable low
NOx emission as compared to yellow flame burners.
Nevertheless yellow flame burners are still widely
applied and there is thus a need to reduce the NOx of
such yellow flame burners.
This object is achieved by the following use. Use of
a Fischer-Tropsch derived fuel in a yellow flame burner.
Applicants have found that the low NOx emissions of a
yellow flame burner can be reduced when a Fischer-Tropsch
derived fuel is used. Applicants have even found that the
NOx emission can be reduced to below the level of a blue
flame burner using conventional Industrial Gas Oil as
fuel. An even further advantage is that the carbon
monoxide emission is reduced. A next advantage is that
less odour during start and extinction of the yellow
flame burner has been observed when using this fuel. This
is very advantageous, especially when such a burner is
used in a domestic environment, wherein frequent start
and stops of the burner are common. A next advantage is
that the carbon monoxide and hydrocarbon emissions at the
cold or hot start of the yellow flame burner are less as
compared to when state of the art oil is used. This is
also very advantageous when the burner is used in for
example a domestic heating application wherein frequently
the burner has to start and stop.
CA 02493891 2005-O1-17
WO 2004/009743 PCT/EP2003/008061
- 3 -
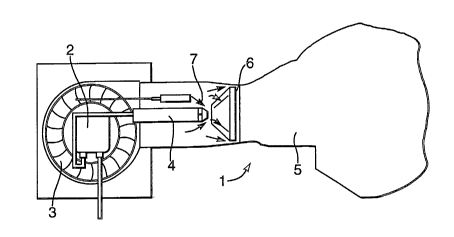
Figure 1 shows a schematic representation of such a
yellow flame burner.
Figure 1 shows a yellow flame burner 1 having pumping
means 2 to supply a liquid fuel and a van 3 to supply an
oxygen containing gas. The oxygen containing gas is
usually air. The fuel is dispersed in a nozzle 4 and
mixed with the air to form a combustible mixture, which
is fed to a combustion space 5 via a conical shaped'
nozzle 6. Figure 1 also shows means 7 to ignite the
mixture.
The operating conditions of the yellow flame burner
may be the same as the operating. conditions used for the
. state of the art fuels. The proportion of air in excess
of that required for stoichiometric combustion is known
as the excess air ratio or "lambda", which is defined as
the ratio of total air available for combustion to that
required to burn all of the fuel. Preferably the lambda
is between 1 and 2 and more preferably between 1 and 1.6.
Applicants found that by using a Fischer-Tropsch derived
fuel a very low lambda of between 1.05 and l.2 could be
applied without large emissions of carbon monoxide as
would be the case when Industrial Gas Oil would be used.
The yellow flame burner using the Fischer-Tropsch
fuels is preferably applied for domestic heating, wherein
the heat of combustion is used. to heat water by indirect
heat exchange in so-called boilers. The heated water may
be used to warm up the house or consumed in for example-
showers and the like. More preferably the yellow-flame
burner is used in (domestic) application wherein more
than 3 starts of the burner per hour takes place. The use
of the present invention is especially suited for such
applications because low hydrocarbon and carbon monoxide
s_
CA 02493891 2005-O1-17
WO 2004/009743 PCT/EP2003/008061
- 4 -
emissions have been found at the start of the burner
running on the Fischer-Tropsch derived fuel.
The yellow flame, burner using the Fischer-Tropsch
- fuels may advantageously be further used for direct
heating of large spaces. Such applications are
characterized in that the flue gasses are directly
supplied to said space to heat up said space. Spaces such
as tents and halls are often heated up with such an
apparatus. Normally gaseous fuels for example natural
gas, ZPG and the like, are used for this application
because the associated flue gasses can be safely~supplied
to said space. A disadvantage of the use of gaseous fuels
is however that handling of 'the pressurized gas
containers and combustion equipment requires professional
skills in order to operate such an apparatus safely.
By using a Fischer-Tropsch derived liquid fuel a
comparable flue gas is obtained in the yellow flame
burner as when a gaseous fuel is used. Thus a method is
provided wherein a liquid fuel can be applied for direct
heating of spaces. The application of the liquid Fischer-
Tropsch derived fuel makes~the use of the apparatus for
direct heating much more simple and safe.
The Fischer-Tropsch derived fuel will comprise a
Fischer-Tropsch product which may be any fraction of the
middle distillate fuel range, which cari be isolated from
the (hydrocracked) Fischer-Tropsch synthesis product.
Typical fractions will boil in the naphtha, kerosene or.
gas oil range. Preferably a Fischer-Tropsch product in
the kerosene or gas oil range is used because these
fractions are easier to handle in for example domestic
environments. Such products will suitably comprise a
fraction larger than 90 wto which boils between
160 and 400 °C, preferably to about 370 °C.
CA 02493891 2005-O1-17
WO 2004/009743 PCT/EP2003/008061
- 5 -
Examples of Fischer-Tropsch derived kerosene and gas oils
are described in EP-A-583836, WO-A-9714768, WO-A-9714769,
WO-A-011116; WO-A-011117, WO-A-0183406, WO-A-0183648,
WO-A-0183647, WO-A-0183641, WO-A-0020535, WO-A-0020534,
EP-A-1101813, US-A-5766274, US-A-5378348, US-A-5888376
and US-A-6204426.
The Fischer-Tropsch derived product will suitably
contain more than 80 wto, preferably more than 90 wto iso
and normal paraffins and less than 1 wto aromatics, the
balance being naphthenics compounds. The content of
sulphur and nitrogen will be very low and normally below
the detection limits-for such compounds. This low content
of these elements is due to the specific process wherein
the Fischer-Tropsch reaction is performed. The content of
sulphur will therefore be below 5 ppm and the content of
nitrogen will be below 1 ppm. As a result of the low
contents of aromatics and nap.hthenics compounds the
density of the Fischer-Tropsch product will be lower than
the conventional mineral derived fuels. The density will
be between 0.65 and 0.8 g/cm3 at'15 °C.
The fuel used in the process of the present invention
may also comprise fuel fractions other than the
Fischer-Tropsch derived fuel components. Examples of such
components may be the kerosene or~gas oil fractions as
obtained in'traditional refinery processes, which upgrade
crude petroleum feedstock to useful products. Preferred
non-Fischer-Tropsch fuel fractions are the ultra low
sulphur (e.g. less than 50 ppm sulphur) kerosene or
diesel fractions, which are currently on the market.
Optionally non-mineral oil based fuels, such as
bio-fuels, may also be present in the fuel composition.
The content of the Fischer-Tropsch derived product in the
fuel will be preferably be above 40 wto, more preferably
CA 02493891 2005-O1-17
WO 2004/009743 PCT/EP2003/008061
above 60 wto and most preferably above 80 wto. It should
be understood that the content of such, currently less
available, Fischer-Tropsch products will be optimised, '
wherein pricing of the total fuel will be balanced with
the advantages of the present invention. For some
applications fuels fully based on a Fischer-Tropsch
product plus optionally some additives may be
advantageously used.
Yellow flame burners are often provided with a flame
detector. Most detectors, which are used today, detect a
particular wavelength associated with the yellow colour
of the flame. Applicants have now found that when a
Fischer-Tropsch derived fuel is used the commonly known
detectors fail to observe the resulting blue coloured
flame. For this reason the yellow flame burner is
preferably provided with a detector, which can detect
this blue flame. Examples of suitable detectors are the
detectors that are used in blue flame burners. Examples
of suitable detectors are the UV sensors and IR sensors.
A more preferred detector is the so-called ionisation
sensor. An ionisation sensor is suitable to monitor
burners with intermittent operation as well as continuous
operation. The principle of operation of the ionisation
flame monitor is based on the rectifying effect of a
flame. If a flame is present, a current~flows between the
burner an the ionisation electrode. This ionisation
current is evaluated by the flame monitor to determine if
a flame is present. In some prior art applications
ionisation sensors could not be used in combination with
a liquid fuel because deposits in the sensor led to false
currents in the sensor. Because use of the Fischer-
Tropsch derived fuel, especially a fuel composition not
containing a metal based combustion improver additive,
CA 02493891 2005-O1-17
WO 2004/009743 PCT/EP2003/008061
- 7 _
results.in less deposits ionisation sensors can be
applied. This is an advantage because these sensors are
more readily available than the IR or UV sensors.
Alternatively additives may be added to the
Fischer-Tropsch derived fuel which result in a flame
which can be detected by the above standard yellow flame
burner detector. Examples of possible additives are azo
dyes and alkali metal=based additives, for example based
on Na or K.
The fuel may also comprise one or~more of the
following additives. Detergents, for example OMA 350 as
obtained from Octel OY; stabilizers, for example Keropon
ES 3500 as obtained from BASF Aktiengesellchaft, FOA 528A
as obtained from OCTEL OY; metal-deactivators, for
example IRGAMET 30 (as obtained from Speciality Chemicals
Inc; (ashless) dispersants, for example as included in
the FOA 528 A package as obtained. from Octel OY; anti-
oxidants; IRGANOX L57 as obtained from Specialtiy
Chemicals Inc; cold flow improvers, for example Keroflux
3283 as obtained from BASF Aktiengesellschaft,
8433 or 8474 as obtained from Infineum UK Ltd;
anti-corrosion: Additin RC 4801 as obtained from Rhein
Chemie GmbH, Kerocorr 3232 as obtained from BASF,
SARKOSYL 0 as obtained from Ciba; re-odorants,
.for example Compensol as obtained from Haarman & Refiner;
biocides, for~example GROTA MAR 71 as obtained from
Schuelke & Mayr; lubricity enhancers,
for example OLI 9000 as obtained from Octel; dehazers,
for example T-9318 from Petrolite; antistatic agents,
for~example Stadis 450 from Octel; and foam reducers,
for example TEGO 2079 from Goldschmidt.
Applicants found that metal-based combustion
improvers, which typically are added to the fuel
CA 02493891 2005-O1-17
WO 2004/009743 PCT/EP2003/008061
- g -
composition used in the prior art method, can be left out
of, the fuel. This is advantageous because as explained
above ionisation sensors may then be advantageously
applied. Metal.-based combustion improvers are for example
5~ ferrocene, methylcyclopentadienylmanganese-tricarbonyl
( MMT ) .
The Fischer-Tropsch derived product is colourless and
odourless. For safety reasons an odour marker, a~s-for
example applied in natural gas for domestic consumption,
may be present in the Fischer-Tropsch derived fuel.
Also a colour marker may be present to distinguish the
fuel from other non-Fischer-Tropsch derived fuels.
The total content of the additives may be suitably
between 0 and 1 wto and preferably below 0.5 wto.
The invention will now be illustrated with the
following non-limiting examples.
Example 1
To a yellow flame burner of Type 800 UZV-S
(Shell Direct GmbH) as placed in a PKR-140 boiler
(Oertli Rohleder Waermetechnik GmbH) a Fischer-Tropsch
derived kerosene (Oil A), a Fischer-Tropsch gas oil
(Oil B), an ultra low sulphur gas oil (Oil D) and
a standard industrial gas oil (Oil C) having the
properties as listed in Table 1 was fed at different'
lambda. The oils contained the same standard additive
package.
CA 02493891 2005-O1-17
WO 2004/009743 PCT/EP2003/008061
_ g _
Table 1
Fischer- Fischer- Reference Reference
Tropsch Tropsch oil-1 (C) oil-2 (D)
kerosene gas oil
(A) (B)
Density (at 734.8 785.2 854.3 846.3
15 C in kg/m3
Sulphur content <0.0005 < 0.0005 0.142 0.061
(wto)
Kinematic 1.246 6.444 3.842 4.621
V1SCOSIty
at 20 C
(mm2/s)
Flash point 43 92 64 66
(C) '
During the experiment the NOx content was measured by
chemoluminescence. In Figure 2 the NOx emission relative
to the energy input is shown at .different Lambda values
for the. fuels A-D. The energy in kWh is calculated from
the amount of~fuel fed to the burner and its caloric
value. It is clear that'the NOx emissions are lower for
the Fischer-Tropsch derived fuels as compared to when a
normal gas oil or an ultra lob sulphur gas oil is used.
The, carbon monoxide emission was also measured.
In Figure 3 the CO emission relative to the energy is
, presented for different values of lambda for oils A-D.
Example 2
To a blue flame burner of type Gulliver BLU BGI
(Riello) as placed in a Vitola 200 Boiler (Viessmann
Werke Gmbh&Co) a conventional gas oil D was~supplied.
The NOx emissions and power were measured at different
lambda. The NOx values were found to be larger
CA 02493891 2005-O1-17
WO 2004/009743 PCT/EP2003/008061
- 10 -
than 140 mg/kWh. These, values are higher than when using
a yellow flame burner and the Fischer-Tropsch derived
fuel as illustrated in Figure 2.
Example 3
Example 1 was repeated for oils A, B and D.
The hydrocarbon and carbon. monoxide emissions were
measured at a warm start up..With a warm start up is here
meant that the boiler temperature was kept constant at
its operating temperature. In Figures 4 and 5 the carbon
monoxide and hydrocarbon emissions are shown as a
function of time. It can be observed that both the CO and
hydrocarbon emissions are less when a Fischer-Tropsch
derived fuel is used when compared to when conventional
gas oil is used.
Example 4
Example 1 with the Fischer kerosene was repeated
using a flame detector. The flame detector was a so-
called photo-element, which delivers amperes (mA) as an
output signal. A high output signal is desirable to make
correct detection'of .a flame. possible. The output signal
of the neat Fischer Tropsch kerosene was 52.7 mA.-
In order to increase the output signal to a higher
level O,lwto of a cyclo-hexane butanoic acid sodium salt
was added. The output signal when the additivated fuel
was used was 57.4 mA. Such higher signal outputs are
beneficiary to make the flame sensor system less
sensitive for system fluctuations.