Note: Descriptions are shown in the official language in which they were submitted.
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
AZABICYCLIC COMPOUNDS ARE CENTRAL NERVOUS SYSTEM ACTIVE AGENTS
FIELD OF THE INVENTION
The present invention is directed to a series of azabicyclic compounds and a
method for
treating pain in mammals.
BACKGROUND OF THE INVENTION
Three major groups of drugs currently used for the treatment ofpain include
opioids,
non-steroidal anti-inflammatory drugs (NSAIDs), and analgesic adjuvants.
Opioids such as, but
not limited to, morphine act at opioid receptors in the brain and spinal cord
to block the
transmission of pain signals. However, clinical use of opioids is commonly
associated with
potential abuse and addiction liabilities, the development of tolerance, and
other side effects such
as constipation, nausea, and cognitive impairments. NSAIDs typically, but not
exclusively,
block the production of prostaglandins to prevent sensitization of nerve
endings that facilitate the
pain signal to the brain. NSAIDs effectively treat mild-to-moderate pain with
an inflammatory
component, but they have a ceiling effect and are not particularly effective
in relieving severe or
chronic neuropathic pain. Many commonly prescribed over-the-counter NSAIDs
cause gastric
distress and bleeding, although the newer COX-2 selective NSAIDs may address
these side
effects liabilities. Analgesic adjuvants, including certain antidepressants,
local anesthetics and
anticonvulsants, have been shown to be effective in treating some chromic pain
states that have
not responded to NSAID or opioid therapy.
A substantial number of medical disorders and conditions produce pain as part
of the
disorder or condition. Relief of this pain is a major aspect of ameliorating
or treating the overall
disease or condition. One class of pain reliever may not be effective for a
particular patient or
group of patients. Therefore, a need exists for novel compounds that treat
pain through
mechanisms different from the established analgesics.
The compounds of the present invention are novel analgesic compounds that bind
to
nicotinic acetylcholine receptors. In particular, these compounds axe active
at one or more of the
subtypes of neuronal nicotinic receptors including, but not limited to,
alpha4beta2, alpha7, and
alpha3beta4. The compounds of the present invention have utility in treating
pain and can be
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
administered in combination with an opioid such as, but not limited to,
morphine, a non-steroid
anti-inflammatory agent such as, but not limited to, aspirin, a tricyclic
antidepressant, or an
anticonvulsant such as, but not limited to, gabapentin or pregabalin for
treating pain. The
compounds of the present invention have utility for treating disorders
associated with the
cholinergic system.
SUMMARY OF THE INVENTION
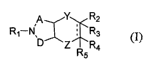
The present invention discloses novel azabicyclic compounds and a method for
treating
pain in mammals. More particularly, the present invention is directed to
compounds of formula
(I):
q Y R2
R~-N, ~ ~ R3
Z R R4
(I),
or a pharmaceutically acceptable salt, amide, ester and prodrug thereof,
wherein
represents a single bond or a double bond;
A is selected from a covalent bond or CH2;
D is selected from CHZ, CHzCH2 or CHzCH2CH2, provided that when D is
CH2CHzCH2,
then A is a covalent bond;
Y is selected from a covalent bond, CH2, or CH2CI~;
Z is selected from a covalent bond, CH2, or CHzCH2, provided that when Z is
CH2CH2,
then Y is a covalent bond, and further provided that when Y is CH2CHa then Z
is a covalent
bond;
Rl is selected from hydrogen, alkoxycarbonyl, alkyl, benzyloxycarbonyl,
cyanoalkyl,
dihydro-3-pyridinylcarbonyl, hydroxy, hydroxyalkyl, phenoxycarbonyl, NR1oR11,
(NRIORn)alkyl or (NRloRll)carbonylalkyl wherein Rio and Rll are independently
selected from
hydrogen, alkyl or alkylcarbonyl;
R2 and R4 are independently selected from hydrogen, aryl or heterocycle,
provided that
one of R2 or R4 is hydrogen; and
R3 and RS are both absent or are independently selected from hydrogen, alkoxy
or
hydroxy;
2
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
provided that when A is a covalent bond, D is CIA and Y is a covalent bond,
then Z is
other than a covalent bond; and
further provided that when A is a covalent bond, D is CHZ and Z is a covalent
bond, then
Y is other than a covalent bond.
DETAILED DESCRIPTION OF THE INVENTION
All patents, patent applications, and literature references cited in the
specification are
herein incorporated by reference in their entirety.
The present invention is directed to compounds of formula (I):
A Y R2
R~ N, ~ ~R3
Z R R4
(I),
or a pharmaceutically acceptable salt, amide, ester and prodrug thereof,
wherein
represents a single bond or a double bond;
A is selected from a covalent bond or CH2;
D is selected from CH2, CH2CH2 or CHzCH2CH2, provided that when D is
CH2CHzCH2,
then A is a covalent bond;
Y is selected from a covalent bond, CH2, or CH2CHz;
Z is selected from a covalent bond, CHZ, or CHzCH2, provided that when Z is
CHZCH2,
then Y is a covalent bond, and fiu-ther provided that when Y is CH2CHa then Z
is a covalent
bond;
Rl is selected from hydrogen, alkoxycarbonyl, alkyl, benzyloxycarbonyl,
cyanoalkyl,
dihydro-3-pyridinylcarbonyl, hydroxy, hydroxyalkyl, phenoxycarbonyl, -NR1oR11,
(NRIOR~ 1)alkyl or (NRIORn)carbonylalkyl wherein Rlo and Rl1 are independently
selected from
hydrogen, alkyl or alkylcarbonyl;
R2 and R4 are independently selected from hydrogen, aryl or heterocycle,
provided that
one of R2 or R4. is hydrogen; and
R3 and RS are both absent or are independently selected from hydrogen, alkoxy
or
hydroxy;
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
provided that when A is a covalent bond, D is CHI and Y is a covalent bond,
then Z is
other than a covalent bond; and
further provided that when A is a covalent bond, D is CH2 and Z is a covalent
bond, then
Y is other than a covalent bond.
In another embodiment of the present invention, compounds of formula (II) are
disclosed
R~.N Y ~ R2
L~ '1 Rs.
Z/IR\R4
(II),
or a pharmaceutically acceptable salt, amide, ester or prodrug thereof wherein
Y, Z, RI, R2, R3,
R4 and RS are as defined in formula (I).
In another embodiment of the pxesent invention, compounds of formula (II) are
disclosed
wherein Y is a covalent bond; Z is CH2; and RI, R~, R3, R4, and RS are as
defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein =- represents a double bond; Y is a covalent bond; Z is CH2; R2 is
hydrogen; R3 and
RS are absent; R4 is heterocycle; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein =- represents a double bond; Y is a covalent bond; Z is CH2; R2 is
hydrogen; R3 and
RS are absent; R4 is heterocycle whexein the heterocycle is selected from
furyl, imidazolyl,
isothiazolyl, isoxazolyl, oxadiazolyl, oxazolyl, pyrazinyl, pyrazolyl,
pyridinyl, pyrimidinyl,
pyridazinyl, pyrrolyl, tetrazinyl, tetrazolyl, thiadiazolyl, thiazolyl,
thienyl, triazinyl, triazolyl,
benzimidazolyl, benzothiazolyl, benzotluenyl, benzoxazolyl, benzofuranyl,
cinnolinyl, faro[2,3-
c]pyridine, faro[3,2-c]pyridine, faro[3,2-b]pyridinyl, faro[2,3-b]pyridinyl,
imidazo[1,2-
a]pyridinyl, indazolyl, indolyl, indolizinyl, naphthyridinyl, isobenzofuxanyl,
isobenzothienyl,
isoindolyl, isoquinolinyl, phthalazinyl, quinolinyl, quinolizinyl,
quinoxalinyl, quinazolinyl,
thieno[2,3-c]pyridine, thieno[3,2-c]pyridine, thieno[3,2-b]pyridinyl or
thieno[2,3-b]pyridinyl
wherein the heterocycle is substituted with 0, 1, 2, or 3 substituents
independently selected from
alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl,
alkoxycaxbonylalkyl, alkyl,
alkylcarbonyl, alkylcarbonyloxy, alkylthio, alkynyl, carboxy, carboxyalkyl,
cyano, cyanoalkyl,
formyl, formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl,
mercapto,
mercaptoalkyl, nitro, triphenylmethyl (trityl), -C(NH)NR1oR11, -NRloR1 i,
(NRioRl1)alkyl,
4
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(NR1oR11)carbonyl, (NR1oR11)carbonylalkyl, (NR1oR11)sulfonyl, NR12S(O~R13,
-C~12)NR13R14WCHZC(NRI2)NR13R14~ -C~ORIa)R13> "CCNCI~R12~ -C~Ri2Ris)R14~
-S(O)20R12, or-S(O)ZR12; R12, Ru, and R14 are independently selected from
hydrogen, alkyl,
aryl, or arylalkyl; and Rl, Rlo, and Rl1 are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein - represents a double bond; Y is a covalent bond; Z is CH2; R2 is
hydrogen; R~ and
RS are absent; R4 is heterocycle wherein the heterocycle is selected from
imidazolyl, isoxazolyl,
pyridinyl, tetrazolyl, thiadiazolyl, thiazolyl, thienyl, imidazo[1,2-
a]pyridinyl, thieno[3,2-
b]pyridinyl or thieno[2,3-b]pyridinyl wherein the heterocycle is substituted
with 0, l, or 2
substituents independently selected from alkenyl, alkenyloxy, alkoxy, alkyl,
alkynyl, cyano,
halogen, vitro, (NR1oR11)sulfonyl, and -C(NI~NR1oR11; and RI, Rlo, and Rll are
as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (II) axe
disclosed
wherein -- represents a double bond; Y is a covalent bond; Z is CHa; RZ is
hydrogen; R3 and
RS are absent; R4 is heterocycle wherein the heterocycle is pyridinyl
substituted with 0, l, or 2
substituents independently selected from alkenyl, allcenyloxy, alkoxy, alkyl,
alkynyl, cyano,
halogen, and vitro; and RI is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein -- represents a double bond; Y is a covalent bond; Z is CH2; R2 is
hydro~n; R3 and
RS are absent; R4 is heterocycle wherein the heterocycle is selected from 3-
pyridinyl, 6-bromo-3-
pyridinyl, 6-chloro-3-pyridinyl, 6-fluoro-3 pyridinyl, 6-methyl-3-pyridinyl, 5-
cyano-3-pyridinyl,
5-methyl-3-pyridinyl, 5-chloro-3-pyridinyl, 5-fluoro-3-pyridinyl, 5-bromo-3-
pyridinyl, 5-vinyl-
3-pyridinyl, 5-methoxy-3-pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-3-
pyridinyl, 5,6-dichloro-
3-pyridinyl, 6-bromo-5-chloro-3-pyridinyl, 5-cyano-6-methyl-3-pyridinyl, 6-
chloro-5-cyano-3-
pyridinyl, 6-bromo-5-cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl, 6-bromo-
5-methyl-3-
pyridinyl, 5-methoxy-6-methyl-3-pyridinyl, 6-chloro-5-methoxy-3-pyridinyl, or
6-bromo-5-
methoxy-3-pyridinyl; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein - represents a double bond; Y is a covalent bond; Z is CH2; R2 is
heterocycle; R3 and
R$ are absent; R4 is hydrogen; and Rl is as defined in formula (I).
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
In another embodiment.of the present invention, compounds of formula (II) are
disclosed
wherein = _ = represents a double bond; Y is a covalent bond; Z is CH2; R2 is
heterocycle wherein
the heterocycle is selected from fiuyl, imidazolyl, isothiazolyl, isoxazolyl,
oxadiazolyl, oxazolyl,
pyrazinyl, pyrazolyl, pyridinyl, pyrimidinyl, pyridazinyl, pyrrolyl,
tetrazinyl, tetrazolyl,
thiadiazolyl, thiazolyl, thienyl, triazinyl, triazolyl, benzimidazolyl,
benzothiazolyl, benzothienyl,
benzoxazolyl, benzofuranyl, ciimolinyl, faro[2,3-c]pyridine, faro[3,2-
c]pyridine, faro[3,2-
b]pyridinyl, faro[2,3-b]pyridinyl, imidazo[1,2-a]pyridinyl, indazolyl,
indolyl, indolizinyl,
naphthyridinyl, isobenzofuranyl, isobenzothienyl, isoindolyl, isoquinolinyl,
phthalazinyl,
quinolinyl, quinolizinyl, quinoxalinyl, quinazolinyl, thieno[2,3-c]pyridine,
thieno[3,2-c]pyridine,
thieno[3,2-b]pyridinyl or thieno[2,3-b]pyridinyl wherein the heterocycle is
substituted with 0, l,
2, or 3 substituents independently selected from alkenyl, alkoxy,
alkoxyalkoxy, alkoxyalkyl,
alkoxycarbonyl, alkoxycarbonylalkyl, alkyl, alkylcarbonyl, alkylcarbonyloxy,
alkylthio, alkynyl,
carboxy, carboxyalkyl, cyano, cyanoalkyl, formyl, formylalkyl, haloalkoxy,
haloalkyl, halogen,
hydroxy, hydroxyalkyl, mercapto, mercaptoalkyl, nitro, triphenylmethyl
(trityl), -C(NH)NRI ORI I,
-NRIORII~ (NRIORII)alkyl, (NRIORII)carbonyl, (NRIORII)carbonylalkyl,
(NRIORlI)sulfonyl,
-~125(0)2R13~ -C~12)~13R14, -CHZC(NRIZ)~ISRi4~ -C(NORIZ)R13, -C(NCI~R12,
-C(NNR12R13)R14, -s(4)z~Rla~ or-S(~)ZRIZ; R3 and RS are absent; R4 is
hydrogen; R12, R13, and
Rl~ are independently selected from hydrogen, alkyl, aryl, or arylalkyl; and
Ru Rlo, and Rl l are
as defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein =- represents a double bond; Y is a covalent bond; Z is CH2; Rz is
heterocycle wherein
the heterocycle is selected from imidazolyl, isoxazolyl, pyridinyl,
tetrazolyl, thiadiazolyl,
thiazolyl, thienyl, imidazo[I,2-a]pyridinyl, thieno[3,2-b]pyridinyl or
thieno[2,3-b]pyridinyl
wherein the heterocycle is substituted with 0, l, or 2 substituents
independently selected from
alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen,nitro,
(NKIORlI)sulfonyl, and
-C(NH)NRIORlI; R3 and RS are absent; R4 is hydrogen; and Rl, Rlo, and Rl l are
as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein -'== represents a double bond; Y is a covalent bond; Z is CH2; R2 is
heterocycle wherein
the heterocycle is pyridinyl substituted with 0, I, or 2 substituents
independently selected from
6
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen, and vitro; R3 and
RS are absent; R-0 is
hydrogen; and Rj is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein ~ represents a double bond; Y is a covalent bond; Z is CH2; R2 is
heterocycle wherein
the heterocycle is selected from 3-pyridinyl, 6-bromo-3-pyridinyl, 6-chloro-3-
pyridinyl, 6-
fluoro-3 pyridinyl, 6-methyl-3-pyridinyl, 5-cyano-3-pyridinyl, 5-methyl-3-
pyridinyl, 5-chloro-3-
pyridinyl, 5-fluoro-3-pyridinyl, 5-bromo-3-pyridinyl, 5-vinyl-3-pyridinyl, 5-
methoxy-3-
pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-3-pyridinyl, 5,6-dichloro-3-
pyridinyl, 6-bromo-5-
chloro-3-pyridinyl, 5-cyano-6-methyl-3-pyridinyl, 6-chloro-5-cyano-3-
pyridinyl, 6-bromo-5-
cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl, 6-bromo-5-methyl-3-
pyridinyl, 5-methoxy-6-
methyl-3-pyridinyl, 6-chloro-5-methoxy-3-pyridinyl, or 6-bromo-5-methoxy-3-
pyridinyl; R3 and
RS are absent; R4 is hydrogen; and Rz is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein Y is CH2; Z is a covalent bond; and RI, R2, R3, R4, and RS are as
defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein - represents a double bond; Y is CH2; Z is a covalent bond; 8215
heterocycle; R3 and
RS are absent; R4 is hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein -- represents a double bond; Y is CH2; Z is a covalent bond; R2 is
heterocycle wherein
the heterocycle is selected from furyl, imidazolyl, isothiazolyl, isoxazolyl,
oxadiazolyl, oxazolyl,
pyrazinyl, pyrazolyl, pyridinyl, pyrimidinyl, pyridazinyl, pyrrolyl,
tetrazinyl, tetrazolyl,
thiadiazolyl, thiazolyl, thienyl, triazinyl, triazolyl, benzimidazolyl,
benzothiazolyl, benzothienyl,
benzoxazolyl, benzofuranyl, cinnolinyl, faro[2,3-c]pyridine, faro[3,2-
c]pyridine, faro[3,2-
b]pyridinyl, faro[2,3-b]pyridinyl, imidazo[1,2-a]pyridinyl, indazolyl,
indolyl, indolizinyl,
naphthyridinyl, isobenzofuranyl, isobenzothienyl, isoindolyl, isoquinolinyl,
phthalazinyl,
quinolinyl, quinolizinyl, quinoxalinyl, quinazolinyl, thieno[2,3-c]pyridine,
thieno[3,2-c]pyridine,
thieno[3,2-b]pyridinyl or thieno[2,3-b]pyridinyl wherein the heterocycle is
substituted with 0, l,
2, or 3 substituents independently selected from alkenyl, alkoxy,
alkoxyalkoxy, alkoxyalkyl,
alkoxycarbonyl, alkoxycarbonylalkyl, alkyl, alkylcarbonyl, alkylcarbonyloxy,
alkylthio, alkynyl,
carboxy, carboxyalkyl, cyano, cyanoalkyl, formyl, formylalkyl, haloalkoxy,
haloalkyl, halogen,
7
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
hydroxy, hydroxyalkyl, mercapto, mercaptoalkyl, nitro, triphenylmethyl
(trityl), -C(NH)NR1oR11,
-NRIORn, (NR1oR11)alkyl, (NR1oR11)c~bonyl, (NR1oR11)carbonylalkyl,
(NR1oR11)sulfonyl,
~125(0)2R13~ 'C~12)~13R14, -CH2~~12)~13R14~ -C(NORIZ)R13~ -C(NCN)R12,
-~~12RI3)R14~ -S(O)20R12, or-S(O)2R12; R3 and RS are absent; R4 is hydrogen;
R12, R13, and
R14 are independently selected from hydrogen, alkyl, aryl, or arylalkyl; and
Ru Rlo, and Rll are
as defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein - = represents a double bond; Y is CH2; Z is a covalent bond; R2 is
heterocycle wherein
the heterocycle is selected from imidazolyl, isoxazolyl, pyridinyl,
tetrazolyl, thiadiazolyl,
thiazolyl, thienyl, imidazo[1,2-a]pyridinyl, thieno[3,2-b]pyridinyl or
thieno[2,3-b]pyridinyl
wherein the heterocycle is substituted with 0, 1, or 2 substituents
independently selected from
alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen, nitro,
(NRIORI)sulfonyl, and
-C(NH)NR1oR11; R3 and RS are absent; R4 is hydrogen; and Rl, Rlo, and Rl1 are
as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein -- represents a double bond; Y is CH2; Z is a covalent bond; R2 is
heterocycle wherein
the heterocycle is pyridinyl substituted with 0, 1, ox 2 substituents
independently selected from
alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen, and nitro; R3 and
RS are absent; Ri is
hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein -- represents a double bond; Y is CH2; Z is a covalent bond; R2 is
heterocycle wherein
the heterocycle is selected from 3-pyridinyl, 6-bromo-3-pyridinyl, 6-chloro-3-
pyridinyl, 6-
fluoro-3 pyridinyl, 6-methyl-3-pyridinyl, S-cyano-3-pyridinyl, 5-methyl-3-
pyxidinyl, 5-chloro-3-
pyridinyl, 5-fluoro-3-pyridinyl, 5-bromo-3-pyridinyl, 5-vinyl-3-pyridinyl, 5-
methoxy-3-
pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-3-pyridinyl, 5,6-dichloro-3-
pyridinyl, 6-bromo-5-
chloro-3-pyridinyl, 5-cyano-6-methyl-3-pyridinyl, 6-chloro-5-cyano-3-
pyridinyl, 6-bromo-5-
cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl, 6-bromo-5-methyl-3-
pyridinyl, S-methoxy-6-
methyl-3-pyridinyl, 6-chloro-5-methoxy-3-pyridinyl, or 6-bromo-5-methoxy-3-
pyridinyl; R3 and
RS are absent; R4 is hydrogen; and Rl is as defined in formula (I).
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
In another embodiment of the present invention, compounds of formula (II) are
disclosed
whereinY is a covalent bond; Z is CH2CH2; and Iy, R2, R3, R4, and RS are as
defined in formula
(I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein - represents a double bond; Y is a covalent bond; Z is CH2CH2; R2 is
hydrogen; R3
and RS are absent; R4 is heterocycle; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein =~ represents a double bond; Y is a covalent bond; Z is CH2CH2; R2 is
hydrogen; R3
and RS axe absent; R4 is heterocycle wherein the heterocycle is selected from
furyl, imidazolyl,
isothiazolyl, isoxazolyl, oxadiazolyl, oxazolyl, pyrazinyl, pyrazolyl,
pyridinyl, pyrimidinyl,
pyridazinyl, pyrrolyl, tetrazinyl, tetrazolyl, thiadiazolyl, thiazolyl,
thienyl, triazinyl, triazolyl,
benzimidazolyl, benzothiazolyl, benzothienyl, benzoxazolyl, benzofuranyl,
cinnolinyl, faro[2,3-
c]pyridine, faro[3,2-c]pyridine, faro[3,2-b]pyridinyl, faro[2,3-b]pyridinyl,
imidazo[1,2-
a]pyridinyl, indazolyl, indolyl, indolizinyl, naphthyridinyl, isobenzofuranyl,
isobenzothienyl,
isoindolyl, isoquinolinyl, phthalazinyl, quinolinyl, quinolizinyl,
quinoxalinyl, quinazolinyl,
thieno[2,3-c]pyridine, thieno[3,2-c]pyridine, thieno[3,2-b]pyridinyl or
thieno[2,3-b]pyridinyl
wherein the heterocycle is substituted with 0, 1, 2, or 3 substituents
independently selected from
alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl,
alkoxycarbonylalkyl, alkyl,
alkylcarbonyl, alkylcarbonyloxy, alkylthio, alkynyl, carboxy, carboxyalkyl,
cyano, cyanoalkyl,
formyl, formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl,
mercapto,
mercaptoalkyl, vitro, triphenylmethyl (trityl), -C(NH)NR1oR11, -NRioRI,
(NRloRl1)~Yl,
(IVR1oR11)carbonyl, (NRIORI)carbonylalkyl, (NR1oR11)sulfonyl, NR12S(O~R13,
-C~12)~13R14~ -CH2C(NR12)~13R14WC~OR12)R-13~ -C~C~R12~ "~~12R13)R14~
-S(O)20R12, or-S(O)2R12; R12, Rls, and R14 are independently selected from
hydrogen, alkyl,
aryl, or arylalkyl; and Rl, Rlo, and Rl1 are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein - = represents a double bond; Y is a covalent bond; Z is CH2CH2; R2 is
hydrogen; R3
and RS are absent; R4 is heterocycle wherein the heterocycle is selected from
imidazolyl,
isoxazolyl, pyridinyl, tetrazolyl, thiadiazolyl, thiazolyl, thienyl,
imidazo[1,2 a]pyridinyl,
thieno[3,2-b]pyridinyl or thieno[2,3-b]pyridinyl wherein the heterocycle is
substituted with 0, I,
9
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
or 2 substituents independently selected from alkenyl, alkenyloxy, alkoxy,
alkyl, alkynyl, cyano,
halogen, nitro, (NRIORr 1)sulfonyl, and -C(NH)NR1oR11; and Rl, Rio, and RI1
are as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein - represents a double bond; Y is a covalent bond; Z is CH2CH2; Rz is
hydrogen; R3
and RS are absent; R4 is heterocycle wherein the heterocycle is pyridinyl
substituted with 0, 1, or
2 substituents independently selected from alkenyl, alkenyloxy, alkoxy, alkyl,
alkynyl, cyano,
halogen, and nitro; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein - represents a double bond; Y is a covalent bond; Z is CHZCH2; R2 is
hydrogen; R3
and RS are absent; R4 is heterocycle wherein the heterocycle is selected from
3-pyridinyl, 6-
bromo-3-pyridinyl, 6-chloro-3-pyridinyl, 6-fluoro-3 pyridinyl, 6-methyl-3-
pyridinyl, 5-cyano-3-
pyridinyl, 5-methyl-3-pyridinyl, 5-chloro-3-pyridinyl, 5-fluoro-3-pyridinyl, 5-
bromo-3-pyridinyl,
5-vinyl-3-pyridinyl, 5-methoxy-3-pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-
3-pyridinyl, 5,6-
dichloro-3-pyridinyl, 6-bromo-5-chloro-3-pyridinyl, 5-cyano-6-methyl-3-
pyridinyl, 6-chloro-5-
cyano-3-pyridinyl, 6-bromo-5-cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl,
6-bromo-5-
methyl-3-pyridinyl, 5-methoxy-6-methyl-3-pyridinyl, 6-chloro-5-methoxy-3-
pyridinyl, or 6-
bromo-5-methoxy-3-pyridinyl; and Ri is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein -- represents a double bond; Y is a covalent bond; Z is CHZCH2; RZ is
heterocycle; R3
and RS are absent; R4 is hydrogen; and RI is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein -- represents a double bond; Y is a covalent bond; Z is CHZCH2; R2 is
heterocycle
wherein the heterocycle is selected from furyl, imidazolyl, isothiazolyl,
isoxazolyl, oxadiazolyl,
oxazolyl, pyrazinyl, pyrazolyl, pyridinyl, pyrimidinyl, pyridazinyl, pyrrolyl,
tetrazinyl, tetrazolyl,
thiadiazolyl, thiazolyl, thienyl, triazinyl, triazolyl, benzimidazolyl,
benzothiazolyl, benzothienyl,
benzoxazolyl, benzofuranyl, cinnolinyl, faro[2,3-c]pyridine, faro[3,2-
c]pyridine, faro[3,2-
b]pyridinyl, faro[2,3-b]pyridinyl, imidazo[1,2-a]pyridinyl, indazolyl,
indolyl, indolizinyl,
naphthyridinyl, isobenzofuranyl, isobenzothienyl, isoindolyl, isoquinolinyl,
phthalazinyl,
quinolinyl, quinolizinyl, quinoxalinyl, quinazolinyl, thieno[2,3-c]pyridine,
thieno[3,2-c]pyridine,
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
thieno[3,2-b]pyridinyl or thieno[2,3-b]pyridinyl wherein the heterocycle is
substituted with 0, 1,
2, or 3 substituents independently selected from alkenyl, alkoxy,
alkoxyallcoxy, alkoxyalkyl,
alkoxycarbonyl, alkoxycarbonylalkyl, alkyl, alkylcarbonyl, alkylcarbonyloxy,
alkylthio, alkynyl,
carboxy, carboxyalkyl, cyano, cyanoalkyl, formyl, formylalkyl, haloalkoxy,
haloalkyl, halogen,
hydroxy, hydroxyalkyl, mercapto, mercaptoalkyl, vitro, triphenylmethyl
(trityl), -C(NH)NR1oR11,
-NRIORI, (NR~oRI)alkyl, (NR1oR11)carbonyl, (NR1oR11)carbonylalkyl,
(NR1oR11)sulfonyl,
-NR12S(~)2R13~ -C~12)~13R14~ -~H2C~12)~13R14~ -C(NORIZ)R~3, -C(NCN)Rla,
-C(NNR~~R13)R14, 'S(~)2ORI2, or-S(O)2Rlz; R3 and RS are absent; R4 is
hydrogen; R12, R13, and
R14 are independently selected from hydrogen, alkyl, aryl, or arylalkyl; and
Rv R10, and R11 are
as defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein -- represents a double bond; Y is a covalent bond; Z is CH2CH2; R2 is
heteracycle
wherein the heterocycle is selected from imidazolyl, isoxazolyl, pyridinyl,
tetrazolyl,
thiadiazolyl, thiazolyl, thienyl, imidazo[1,2-a]pyridinyl, thieno[3,2
b)pyxidinyl or thieno[2,3-
b]pyridinyl wherein the heterocycle is substituted with 0, 1, or 2
substituents independently
selected from alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen,
vitro,
(NR1oR11)sulfonyl, and-C(NH)NR1oR11; R3 and RS are absent; R4 is hydrogen; and
Rl, Rlo, and
Rl l are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein -- represents a double bond; Y is a covalent bond; Z is CH2CH2; R2 is
heterocycle
wherein the heterocycle is pyridinyl substituted with 0, l, or 2 substituents
independently
selected from alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen, and
vitro; R3 and RS
are absent; R4 is hydrogen; and Ri is as defined in formula (I).
In another embodiment of the pxesent invention, compounds of formula (II) are
disclosed
wherein - represents a double bond; Y is a covalent bond; Z is CHZCH2; Rz is
heterocycle
wherein the heterocycle is selected from 3-pyridinyl, 6-bromo-3-pyridinyl, 6-
chloro-3-pyridinyl,
6-fluoro-3 pyridinyl, 6-methyl-3-pyridinyl, 5-cyano-3-pyridinyl, 5-methyl-3-
pyridinyl, 5-chloro-
3-pyridinyl, 5-fluoro-3-pyridinyl, 5-bromo-3-pyridinyl, 5-vinyl-3-pyridinyl, 5-
methoxy-3-
pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-3-pyridinyl, 5,6-dichloro-3-
pyridinyl, 6-bromo-5-
chloro-3-pyridinyl, 5-cyano-6-methyl-3-pyridinyl, 6-chloro-5-cyano-3-
pyridinyl, 6-bromo-5-
11
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl, 6-bromo-5-methyl-3-
pyridinyl, 5-rnethoxy-6-
methyl-3-pyridinyl, 6-chloro-5-methoxy-3-pyridinyl, or 6-bromo-5-methoxy-3-
pyridinyl; R3 and
RS are absent; R4 is hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein Y is CH2; Z is CH2; and Rl, R2, R3, R4, and RS are as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein ' represents a double bond; Y is CH2; Z is CH2; R2 is heterocycle; R3
and RS are
absent; R4 is hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein - represents a double bond; Y is CH2; Z is CHZ; R2 is heterocycle
wherein the
heterocycle is selected from furyl, imidazolyl, isothiazolyl, isoxazolyl,
oxadiazolyl, oxazolyl,
pyrazinyl, pyrazolyl, pyridinyl, pyrimidinyl, pyridazinyl, pyrrolyl,
tetrazinyl, tetrazolyl,
thiadiazolyl, thiazolyl, thienyl, triazinyl, triazolyl, benzimidazolyl,
benzothiazolyl, benzothienyl,
benzoxazolyl, benzofuranyl, cinnolinyl, faro[2,3-c]pyridine, faro[3,2-
c]pyridine, faro[3,2-
b]pyridinyl, faro[2,3-b]pyridinyl, imidazo[1,2-a]pyridinyl, indazolyl,
indolyl, indolizinyl,
naphthyridinyl, isobenzofuranyl, isobenzothienyl, isoindolyl, isoquinolinyl,
phthalazinyl,
quinolinyl, quinolizinyl, quinoxalinyl, quinazolinyl, thieno[2,3-c]pyridine,
thieno[3,2-c]pyridine,
thieno[3,2-b]pyridinyl or thieno[2,3-b]pyridinyl wherein the heterocycle is
substituted with 0, 1,
2, or 3 substituents independently selected from alkenyl, alkoxy,
alkoxyalkoxy, alkoxyalkyl,
alkoxycarbonyl, alkoxycarbonylalkyl, alkyl, alkylcarbonyl, alkylcarbonyloxy,
alkylthio, alkynyl,
carboxy, carboxyalkyl, cyano, cyanoalkyl, formyl, formylalkyl, haloalkoxy,
haloalkyl, halogen,
hydroxy, hydroxyalkyl, mercapto, mercaptoalkyl, nitro, triphenylmethyl
(trityl),-C(NH)NRIORn,
NRIORII, (NR1oR11)alkyl, (NR1oR11)carbonyl, (NR1oR11)carbonylalkyl,
(NRIORn)sulfonyl,
-~125(~)2R13~ -C~12)~13RI4~ -~H2~~12)~13R14, -C(NCRiz)Ris~ -C(NCN)Rlz,
-C(~RI2R13)R14~ -S(C)zCRia, or-S(O)2R12; R3 and RS are absent; R4 is hydrogen;
R12, R~3, and
RI4 are independently selected from hydrogen, alkyl, aryl, or arylalkyl; and
Rv Rlo, and R11 are
as defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein -- represents a double bond; Y is CH2; Z is CH2; R2 is heterocycle
wherein the
heterocycle is selected from imidazolyl, isoxazolyl, pyridinyl, tetrazolyl,
thiadiazolyl, thiazolyl,
12
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
thienyl, imidazo[1,2-a]pyridinyl, thieno[3,2-b]pyridinyl or thieno[2,3-
b]pyridinyl wherein the
heterocycle is substituted with 0, 1, or 2 substituents independently selected
from alkenyl,
alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen, nitro, (NRloRi1)sulfonyl,
and
-C(NH)NRloR1 z; R3 and Rs are absent; R4 is hydrogen; and Rl, Rlo, and Rl1 are
as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (TI) are
disclosed
wherein -- represents a double bond; Y is CH2; Z is CH2; R2 is heterocycle
wherein the
heterocycle is pyridinyl substituted with 0, 1, or 2 substituents
independently selected from
alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen, and nitra; R3 and
RS are absent; R4 is
hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein - = represents a double bond; Y is CH2; Z is CH2; RZ is heterocycle
wherein the
heterocycle is selected from 3-pyridinyl, 6-bromo-3-pyridinyl, 6-chloro-3-
pyridinyl, 6-fluoro-3
pyridinyl, 6-methyl-3-pyridinyl, 5-cyano-3-pyridinyl, 5-methyl-3-pyridinyl, 5-
chloro-3-
pyridinyl, 5-fluoro-3-pyridinyl, 5-bromo-3-pyridinyl, 5-vinyl-3-pyridinyl, 5-
methoxy-3-
pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-3-pyridinyl, 5,6-dichloro-3-
pyridinyl, 6-bromo-5-
chloro-3-pyridinyl, 5-cyano-6-methyl-3-pyridinyl, 6-chloro-5-cyano-3-
pyridinyl, 6-bromo-5-
cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl, 6-bromo-5-methyl-3-
pyridinyl, 5-methoxy-6-
methyl-3-pyridinyl, 6-chloro-5-methoxy-3-pyridinyl, or 6-bromo-5-methoxy-3-
pyridinyl; R3 and
Rs are absent; R4 is hydrogen; and R~ is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (II) are
disclosed
wherein Y is CHZCHZ; Z is a covalent bond; and Rl, RZ, R3, R4, and Rs are as
defined in formula
(I).
Representative compounds of formula (II) include, but are not limited to:
(cis)-4-(3-pyridinyl)-7-azabicyclo [4.2.0] oct-4-ene;
(cis)-4-(6-chloro-3-pyridinyl)-7-azabicyclo [4.2. 0] oct-4-ene;
(cis)-4-(6-bromo-3-pyridinyl)-7-azabicyclo [4.2.0] oct-4-ene;
(cis)-4-(6-fluoro-3-pyridinyl)-7-azabicyclo[4.2.0]oct-4-ene;
(cis)-4-(6-methyl-3-pyridinyl)-7-azabicyclo[4.2.0]oct-4-ene;
13
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-5-(7-azabicyclo [4.2.0] oct-4-en-4-yl)nicotinonitrile;
(cis)-4-(5-methyl-3-pyridinyl)-7-azabicyclo[4.2.0]oct-4-ene;
(cis)-4-(5-chloro-3-pyridinyl)-7-azabicyclo[4.2.0]oct-4-ene;
(cis)-4-(5-fluoro-3-pyridinyl)-7-azabicyclo[4.2.0]oct-4-ene;
(cis)-4-(5-bromo-3-pyridinyl)-7-azabicyclo [4.2.0] oct-4-ene;
(cis)-4-(5-vinyl-3-pyridinyl)-7-azabicyclo [4.2.0] oct-4-ene;
(cis)-4-(5-methoxy-3-pyridiriyl)-7-azabicyclo[4.2.0]oct-4-ene;
(cis)-4-[5-(vinyloxy)-3-pyridinyl]-7-azabicyclo [4.2.0] oct-4-ene;
(cis)-4-(5-ethynyl-3-pyridinyl)-7-azabicyclo [4.2.0] oct-4-ene;
(cis)-4-(5,6-dichloro-3-pyridinyl)-7-azabicyclo[4.2.0]oct-4-ene;
(cis)-4-(6-bromo-5-chloro-3-pyridinyl)-7-azabicyclo[4.2.0]oct-4-ene;
(cis)-5-(7-azabicyclo [4.2.0] oct-4-en-4-yl)-2-methylnicotinonitrile;
(cis)-5-(7-azabicyclo [4.2.0] oct-4-en-4-yl)-2-chloronicotinonitrile;
(cis)-5-(7-azabicyclo [4.2.0] oct-4-en-4-yl)-2-bromonicotinonitrile;
(cis)-4-(6-chloro-5-methyl-3-pyridinyl)-7-azabicyclo[4.2.0]oct-4-ene;
(cis)-4-(6-bromo-5-methyl-3-pyridinyl)-7-azabicyclo[4.2.0] oct-4-ene;
(cis)-4-(5-methoxy-6-methyl-3-pyridinyl)-7-azabicyclo [4.2.0] oct-4-ene;
(cis)-4-(6-chloro-5-methoxy-3-pyridinyl)-7-azabicyclo [4.2.0] oct-4-ene;
(cis)-4-(6-bromo-5-methoxy-3-pyridinyl)-7-azabicyclo [4.2.0] oct-4-ene;
(cis)-4-(3-methyl-5-isoxazolyl)-7-azabicyclo[4.2.0]oct-4-ene;
(cis)-2-(7-azabicyclo [4.2.0] oct-4-en-4-yl)furo [3,2-b]pyridine;
(cis)-3-(6-chloro-3-pyridinyl)-6-azabicyclo [3 .2.0]hept-3-ene;
(cis)-3-(6-bromo-3-pyridinyl)-6-azabicyclo[3.2.0]kept-3-ene;
(cis)-3-(6-fluoro-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-3-(6-methyl-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-5-(6-azabicyclo[3.2.0]kept-3-en-3-yl)nicotinonitrile;
(cis)-3-(5-methyl-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-3-(5-chlaro-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-3-(5-fluoro-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
14
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-3-(5-bromo-3-pyridinyl)-6-azabicyclo [3.2.0]hept-3-ene;
(cis)-3-(5-vinyl-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-3-(5-methoxy-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-3-(5-vinyloxy-3-pyridinyl)-6-azabicyclo [3 .2.0]kept-3-ene;
(cis)-3-(5-ethynyl-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-3-(5,6-dichloro-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-3-(6-bromo-5-chloro-3-pyridinyl)-6-azabicyclo [3.2.0]hept-3-ene;
(cis)-5-(6-azabicyclo [3 .2.0]kept-3-en-3-yl)-2-methylnicotinonitrile;
(cis)-S-(6-azabicyclo[3.2.0]hept-3-en-3-yl)-2-chloronicotinonitrile;
(cis)-5-(6-azabicyclo[3.2.0]hept-3-en-3-yl)-2-bromonicotinonitrile;
(cis)-3-(6-chloro-S-methyl-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-3-(6-bromo-5-methyl-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-3-(5-methoxy-6-methyl-3-pyridinyl)-6-azabicyclo [3.2.0]hept-3-ene;
(cis)-3-(6-chloro-5-methoxy-3-pyridinyl)-6-azabicyclo [3.2. 0]hept-3-ene;
(cis)-3-(6-bromo-5-methoxy-3-pyridinyl)-6-azabicyclo [3.2.0]hept-3-ene;
(cis)-3-(3-methyl-5-isoxazolyl)-6-azabicyclo[3.2.0]hept-3-ene
(cis)-2-(6-azabicyclo [3 .2.0] hept-3-en-3-yl)furo [3,2-b]pyridine;
(cis)-4-(6-chloro-3-pyridinyl)-7-azabicyclo[4.2.0]oct-3-ene;
(cis)-4-(6-bromo-3-pyridinyl)-7-azabicyclo[4.2.0]oct-3-ene;
(cis)-4-(6-fluoro-3-pyridinyl)-7-azabicyclo[4.2.0]oct-3-ene;
(cis)-4-(6-methyl-3-pyridinyl)-7-azabicyclo[4.2.0]oct-3-ene;
(cis)-5-(7-azabicyclo [4.2.0] oct-3-en-4-yl)nicotinonitrile;
(cis)-4-(5-methyl-3-pyridinyl)-7-azabicyclo[4.2.0]oct-3-ene;
(cis)-4-(5-chloro-3-pyridinyl)-7-azabicyclo[4.2.0]oct-3-ene;
(cis)-4-(5-fluoro-3-pyridinyl)-7-azabicyclo[4.2.0]oct-3-ene;
(cis)-4-(5-bromo-3-pyridinyl)-7-azabicyclo[4.2.0] oct-3-ene;
(cis)-4-(5-vinyl-3-pyridinyl)-7-azabicyclo[4.2.0]oct-3-ene;
(cis)-4-(5-methoxy-3-pyridinyl)-7-azabicyclo[4.2.0]oct-3-ene;
(cis)-4-(5-vinyloxy-3-pyridinyl)-7-azabicyclo[4.2.0]oct-3-ene;
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-4-(5-ethynyl-3-pyridinyl)-7-azabicyclo[4.2.0]oct-3-ene;
(cis)-4-(5,6-dichloro-3-pyridinyl)-7-azabicyclo[4.2.0]oct-3-ene;
(cis)-4-(6-bromo-5-chloro-3-pyridinyl)-7-azabicyclo[4.2.0]oct-3-ene;
(cis)-5-(7-azabicyclo[4.2.0] oct-3-en-4-yl)-2-methylnicotinonitrile;
(cis)-5-(7-azabicyclo [4.2.0] oct-3-en-4-yl)-2-chloronicotinonitrile;
(cis)-5-(7-azabicyclo [4.2.0] oct-3-en-4-yl)-2-bromonicotinonitrile;
(cis)-4-(6-chloro-5-methyl-3-pyridinyl)-7-azabicyclo[4.2.0]oct-3-ene;
(cis)-4-(6-bromo-5-methyl-3-pyridinyl)-7-azabicyclo[4.2.0]oct-3-ene;
(cis)-4-(5-methoxy-6-methyl-3-pyridinyl)-7-azabicyclo[4.2.0]oct-3-ene;
(cis)-4-(6-chloro-5-methoxy-3-pyridinyl)-7-azabicyclo[4.2.0]oct-3-ene;
(cis)-4-(6-bromo-5-methoxy-3-pyridinyl)-7-azabicyclo[4.2.0]oct-3-ene;
(cis)-4-(3-methyl-5-isoxazolyl)-7-azabicyclo [4.2.0] oct-3-ene;
(cis)-2-(7-azabicyclo[4.2.0}oct-3-en-4-yl)furo[3,2-b]pyridine;
(cis)-3-(3-pyridinyl)-6-azabicyclo[3.2.0]hept-2-ene;
(cis)-3-(6-chloro-3-pyridinyl)-6-azabicyclo[3.2.0]kept-2-ene;
(cis)-3-(6-bromo-3-pyridinyl)-6-azabicyclo[3.2.0]hept-2-ene;
(cis)-3-(6-fluoro-3-pyridinyl)-6-azabicyclo[3.2.0]hept-2-ene;
(cis)-3-(6-methyl-3-pyridinyl)-6-azabicyclo [3 .2.0}hept-2-ene;
(cis)-5-(6-azabicyclo[3.2.0]kept-2-en-3-yl)nicotinonitrile;
(cis)-3-(5-methyl-3-pyridinyl)-6-azabicyclo [3 .2.0]hept-2-ene;
(cis)-3-(5-chloro-3-pyridinyl)-6-azabicyclo [3 .2.0]hept-2-ene;
(cis)-3-(5-fluoro-3-pyridinyl)-6-azabicyclo[3.2.0]hept-2-ene;
(cis)-3-(5-bromo-3-pyridinyl)-6-azabicyclo [3.2.0]hept-2-ene;
(cis)-3-(5-vinyl-3-pyridinyl)-6-azabicyclo[3.2.0]hept-2-ene;
(cis)-3-(5-methoxy-3-pyridinyl)-6-azabicyclo[3.2.0]hept-2-ene;
(cis)-3-(5-vinyloxy-3-pyridinyl)-6-azabicyclo[3.2.0]kept-2-ene;
(cis)-3-(5-ethynyl-3-pyridinyl)-6-azabicyclo[3.2.0]hept-2-ene;
(cis)-3-(5,6-dichloro-3-pyridinyl)-6-azabicyclo[3.2.0]kept-2-ene;
(cis)-3-(6-bromo-5-chloro-3-pyridinyl)-6-azabicyclo[3.2.0]hept-2-ene;
16
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-5-(6-azabicyclo[3.2.0]hept-2-en-3-yl)-2-methylnicotinonitrile;
(cis)-5-(6-azabicyclo [3.2.0]hept-2-en-3-yl)-2-chloronicotinonitrile;
(cis)-5-(6-azabicyclo [3.2.0]hept-2-en-3-yl)-2-bromonicotinonitrile;
(cis)-3-(6-chloro-5-methyl-3-pyridinyl)-6-azabicyclo[3.2.0]hept-2-ene;
(cis)-3-(6-bromo-5-methyl-3-pyridinyl)-6-azabicyclo [3.2.0]hept-2-ene;
(cis)-3-(5-methoxy-6-methyl-3-pyridinyl)-6-azabicyclo [3 .2.0]hept-2-ene;
(cis)-3-(6-chloro-5-methoxy-3-pyridinyl)-6-azabicyclo [3 .2.0]hept-2-ene;
(cis)-3-(6-bromo-5-methoxy-3-pyridinyl)-6-azabicyclo[3.2.0]hept-2-ene;
(cis)-3-(3-methyl-5-isoxazolyl)-6-azabicyclo[3.2.0]hept-2-ene;
(cis)-2-(6-azabicyclo[3.2.0]hept-2-en-3-yl)furo[3,2-b]pyridine;
(cis)-5-(3-pyridinyl)-7-azabicyclo[4.2.0]oct-4-ene;
(cis)-5-(6-chloro-3-pyridinyl)-7-azabicyclo[4.2.0]oct-4-ene;
(cis)-5-(6-bromo-3-pyridinyl)-7-azabicyclo[4.2.0]oct-4-ene;
(cis)-5-(6-fluoro-3-pyridinyl)-7-azabicyclo[4.2.0]oct-4-ene;
(cis)-5-(6-methyl-3-pyridinyl)-7-azabicyclo[4.2.0] oct-4-ene;
(cis)-5-(7-azabicyclo [4.2.0] oct-4-en-5-yl)nicotinonitrile;
(cis)-5-(5-methyl-3-pyridinyl)-7-azabicyclo [4.2.0] oct-4-ene;
(cis)-5-(5-chloro-3-pyridinyl)-7-azabicyclo [4.2.0] oct-4-ene;
(cis)-5-(5-fluoro-3-pyridinyl)-7-azabicyclo[4.2.0]oct-4-ene;
(cis)-5-(5-bromo-3-pyridinyl)-7-azabicyclo [4.2.0]oct-4-ene;
(cis)-5-(5-vinyl-3-pyridinyl)-7-azabicyclo[4.2.0]oct-4-ene;
(cis)-5-(5-methoxy-3-pyridinyl)-7-azabicyclo[4.2.0]oct-4-ene;
(cis)-5-(5-vinyloxy-3-pyridinyl)-7-azabicyclo[4.2.0]oct-4-ene;
(cis)-5-(5-ethynyl-3-pyridinyl)-7-azabicyclo[4.2.0]oct-4-ene;
(cis)-5-(5,6-dichloro-3-pyridinyl)-7-azabicyclo[4.2.0]oct-4-ene;
(cis)-5-(6-bromo-5-chloro-3-pyridinyl)-7-azabicyclo [4.2.0] oct-4-ene;
(cis)-5-(7-azabicyclo [4.2.0] oct-4-en-5-yl)-2-methylnicotinonitrile;
(cis)-5-(7-azabicyclo [4.2. 0] oct-4-en-5-yl)-2-bromonicotinonitrile;
(cis)-5-(7-azabicyclo[4.2.0]oct-4-en-5-yl)-2-chloronicotinonitrile;
17
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-5-(6-chloro-5-methyl-3-pyridinyl)-7-azabicyclo [4.2. 0] oct-4-ene;
(cis)-5-(6-bromo-5-methyl-3-pyridinyl)-7-azabicyclo [4.2.0] oct-4-ene;
(cis)-5-(5-methoxy-6-methyl-3-pyridinyl)-7-azabicyclo [4.2.0] oct-4-ene;
(cis)-5-(6-chloro-5-methoxy-3-pyridinyl)-7-azabicyclo [4.2. 0] oct-4-ene;
(cis)-5-(6-bromo-5-methoxy-3-pyridinyl)-7-azabicyclo [4.2.0] oct-4-ene;
(cis)-2-(7-azabicyclo[4.2.0]oct-4-en-5-yl)furo[3,2-b]pyridine;
(cis)-5-(3-methyl-5-isoxazolyl)-7-azabicyclo [4.2.0] oct-4-ene;
(cis)-4-(3-pyridinyl)-6-azabicyclo[3.2.0}hept-3-ene;
(cis)-4-(6-chloro-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-4-(6-bromo-3-pyridinyl)-6-azabicyclo [3.2.0]hept-3-ene;
(cis)-4-(6-fluoro-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-4-(6-methyl-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-5-(6-azabicyclo [3.2.0}hept-3-en-4-yl)nicotinonitrile;
(cis)-4-(5-methyl-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-4-(5-chloro-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-4-(5-fluoro-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-4-(5-bromo-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-4-(5-vinyl-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-4-(5-methoxy-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-4-(5-vinyloxy-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-4-(5-ethynyl-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-4-(5,6-dichloro-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-4-(6-bromo-5-chloro-3-pyridinyl)-6-azabicyclo [3.2.0]hept-3-ene;
(cis)-5-(6-azabicyclo[3.2.0]hept-3-en-4-yl)-2-methylnicotinonitrile;
(cis)-5-(6-azabicyclo[3.2.0]hept-3-en-4-yl)-2-chloronicotinonitrile;
(cis)-5-(6-azabicyclo [3.2. 0] hept-3-en-4-yl)-2-bromonicotinonitrile;
(cis)-4-(6-chloro-5-methyl-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
(cis)-4-(6-bromo-5-methyl-3-pyridinyl)-6-azabicyclo [3.2.0]hept-3-ene;
(cis)-4-(5-methoxy-6-methyl-3-pyridinyl)-6-azabicyclo[3.2.0]hept-3-ene;
18
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-4-(6-chloro-5-methoxy-3-pyridinyl)-6-azabicyclo [3.2.0]hept-3-ene;
(cis)-4-(6-bromo-5-methoxy-3-pyridinyl)-6-azabicyclo[3.2.0]kept-3-ene;
(cis)-4-(3-methyl-5-isoxazolyl)-6-azabicyclo [3 .2.0]hept-3-ene;
(cis)-2-(6-azabicyclo[3.2.0]hept-3-en-4-yl)furo[3,2-b]pyridine; or a
pharmaceutically
acceptable salt, amide, ester or prodrug thereof.
In another embodiment of the present invention, compounds of formula (III) are
disclosed
R~
Y R2
~~ ~R
3
Z R Ra
(III),
or a pharmaceutically acceptable salt, amide, ester or prodrug thereof wherein
Y,Z, Rl, R2, R3,
R4, and R$ are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein Y is a covalent bond; Z is a covalent bond; and Ri, R2, R3,
R4, and RS are as
defined in formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein Y is a covalent bond; Z is CH2; and Rl, RZ, R3, R4, and R~
are as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein - = represents a double bond; Y is a covalent bond; Z is
Cliz; R2 is hydrogen;
R3 and RS are absent; R4 is heterocycle; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein -' represents a double bond; Y is a covalent bond; Z is
CT~b_; RZ is hydrogen;
R3 and RS are absent; R4 is heterocycle wherein the heterocycle is selected
fr~n furyl,
imidazolyl, isothiazolyl, isoxazolyl, oxadiazolyl, oxazolyl, pyrazinyl,
pyrazolyl, pyridinyl,
pyrimidinyl, pyridazinyl, pyrrolyl, tetrazinyl, tetrazolyl, thiadiazolyl,
thiazolyl, thienyl, triazinyl,
triazolyl, benzimidazolyl, benzothiazolyl, benzothienyl, benzoxazolyl,
benzofuranyl, cinnolinyl,
faro[2,3-c]pyridine, faro[3,2-c]pyridine, faro[3,2-b]pyridinyl, faro[2,3-
b]pyridinyl, imidazo[1,2-
a]pyridinyl, indazolyl, indolyl, indolizinyl, naphthyridinyl, isobenzofuranyl,
isobenzothienyl,
19
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
isoindolyl, isoquinolinyl, phthalazinyl, quinolinyl, quinolizinyl,
quinoxalinyl, quinazolinyl,
thieno[2,3-c]pyridine, thieno[3,2-c]pyridine, thieno[3,2-b]pyridinyl or
thieno[2,3-b]pyridinyl
wherein the heterocycle is substituted with 0, 1, 2, or 3 substituents
independently selected from
alkenyl, alkoxy, alkoxyalkoxy, allcoxyalkyl, alkoxycarbonyl,
alkoxycarbonylalkyl, alkyl,
alkylcarbonyl, alkylcarbonyloxy, alkylthio, alkynyl, carboxy, carboxyalkyl,
cyano, cyanoalkyl,
formyl, formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl,
mercapto,
mercaptoalkyl, vitro, triphenylmethyl (trityl), -C(NH)NR1oR11, -NR1oR11,
(NR1oR11)alkyl,
(NK1oR11)carbonyl, (NR1oR11)carbonylalkyl, (NR1oR11)sulfonyl, NR12S(O)2R13,
-C~12)~13R14, -CH2C(1VRI2)~13RI4, -C(NOR12)Rls~ -C(1~C~R12~ -C~12R13)R14~
-S(O)20R12, or -S(O)2R12; R12, Rls, and R14 are independently selected from
hydrogen, alkyl,
aryl, or arylalkyl; and Rl, Rlo, and Rl1 are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein - represents a double bond; Y is a covalent bond; Z is CH2;
R2 is hydrogen;
R3 and RS are absent; R4 is heterocycle wherein the heterocycle is selected
from imidazolyl,
isoxazolyl, pyridinyl, tetrazolyl, thiadiazolyl, thiazolyl, thienyl,
imidazo[1,2 a]pyridinyl,
thieno[3,2-b]pyridinyl or thieno[2,3-b]pyridinyl wherein the heterocycle is
substituted with 0, 1,
or 2 substituents independently selected from alkenyl, alkenyloxy, alkoxy,
alkyl, alkynyl, cyano,
halogen, vitro, (NR1oR11)sulfonyl, or-C(N~)]NR1oR11; and Rl, Rlo, and Rll are
as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein - represents a double bond; Y is a covalent bond; Z is CI-
Iz; R2 is hydrogen;
R3 and RS are absent; R4 is heterocycle wherein the heterocycle is pyridinyl
substituted with 0, 1,
or 2 substituents independently selected from alkenyl, alkenyloxy, alkoxy,
alkyl, alkynyl, cyano,
halogen, or vitro; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein - repxesents a double bond; Y is a covalent bond; Z is CH2;
R2 is hydrogen;
R3 and RS are absent; Rø is heterocycle wherein the heterocycle is selected
from 3-pyridinyl, 6-
bromo-3-pyridinyl, 6-chloro-3-pyridinyl, 6-fluoro-3 pyridinyl, 6-methyl-3-
pyridinyl, 5-cyano-3-
pyridinyl, S-methyl-3-pyridinyl, 5-chloro-3-pyridinyl, 5-fluoro-3-pyridinyl, 5-
bromo-3-pyridinyl,
5-vinyl-3-pyridinyl, 5-methoxy-3-pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-
3-pyridinyl, 5,6-
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
dichloro-3-pyridinyl, 6-bromo-5-chloro-3-pyridinyl, 5-cyano-6-methyl-3-
pyridinyl, 6-chloro-5-
cyano-3-pyridinyl, 6-bromo-5-cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl,
6-bromo-5-
methyl-3-pyridinyl, 5-methoxy-6-methyl-3-pyridinyl, 6-chloro-5-methoxy-3-
pyridinyl, or 6-
bromo-5-methoxy-3-pyridinyl; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein =- represents a double bond; Y is a covalent bond; Z is CH2;
R2 is
heterocycle; R3 and RS are absent; R4 is hydrogen; and Rl is as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein -- represents a double bond; Y is a covalent bond; Z is CHz;
R2 is
heterocycle wherein the heterocycle is selected firm fiuyl, imidazolyl,
isothiazolyl, isoxazolyl,
oxadiazolyl, oxazolyl, pyrazinyl, pyrazolyl, pyridinyl, pyrimidinyl,
pyridazinyl, pyrrolyl,
tetrazinyl, tetrazolyl, thiadiazolyl, thiazolyl, thienyl, triazinyl,
triazolyl, benzimidazolyl,
benzothiazolyl, benzothienyl, benzoxazolyl, benzofuranyl, cinnolinyl, faro[2,3-
c]pyridine,
faro[3,2-c]pyridine, faro[3,2-b]pyridinyl, faro[2,3-b]pyridinyl, imidazo[1,2-
a]pyridinyl,
indazolyl, indolyl, indolizinyl, naphthyridinyl, isobenzofuranyl,
isobenzothienyl, isoindolyl,
isoquinolinyl, phthalazinyl, quinolinyl, quinolizinyl, quinoxalinyl,
quinazolinyl, thieno[2,3-
c]pyridine, thieno[3,2-c]pyridine, thieno[3,2-b]pyridinyl or thieno[2,3-
b]pyridinyl wherein the
heterocycle is substituted with 0, 1, 2, or 3 substituents independently
selected from alkenyl,
alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl, alkoxycarbonylalkyl, alkyl,
alkylcarbonyl,
alkylcarbonyloxy, alkylthio, alkynyl, carboxy, carboxyalkyl, cyano,
cyanoalkyl, formyl,
formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl, mercapto,
mercaptoalkyl,
vitro, triphenylmethyl (trityl), -C(NH)NR1oR11, NR1oR11, (NR1oR11)alkyl,
(NR1oR11)carbonyl,
(NR1oR11)carbonylalkyl, (NR1oR11)sulfonyl, NR12S(O)2R13, -C(NR12)NR1~14~
-CHZC(NRi2)~13R14~ -C~OR12)Rls~ '~~~Ri2~ -C~1zR13)Rla~ -s(~)20R12~ ~r
-s(p)2R12; R3 and RS are absent; R4 is hydrogen; R12, Rls, and R14 are
independently selected
from hydrogen, alkyl, aryl, or arylalkyl; and Rl, Rlo, and Rll are as defined
in formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein - = represents a double bond; Y is a covalent bond; Z is
CIA; R2 is
heterocycle wherein the heterocycle is selected from imidazolyl, isoxazolyl,
pyridinyl, tetrazolyl,
thiadiazolyl, thiazolyl, thienyl, imidazo[1,2-a]pyridinyl, thieno[3,2
b]pyridinyl or thieno[2,3-
21
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
b]pyridinyl wherein the heterocycle is substituted with 0, 1, or 2
substituents independently
selected from alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen,
vitro,
(NR1oR11)sulfonyl, or-C(NH)NRIOR11; R3 and Rs are absent; R4 is hydrogen; and
Rl, Rro, and
RI i are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein -- represents a double bond; Y is a covalent bond; Z is CI-
Iy; R2 is
heterocycle wherein the heterocycle is pyridinyl substituted with 0, 1, or 2
substituents
independently selected from alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl,
cyano, halogen, or vitro;
R3 and Rs are absent; R4 is hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein -'- represents a double bond; Y is a covalent bond; Z is
CH2; RZ is
heterocycle wherein the heterocycle is selected from 3-pyridinyl, 6-bromo-3-
pyridinyl, 6-chloro-
3-pyridinyl, 6-fluoro-3 pyridinyl, 6-methyl-3-pyridinyl, 5-cyano-3-pyridinyl,
5-methyl-3-
pyridinyl, 5-chloro-3-pyridinyl, 5-fluoro-3-pyridinyl, 5-bromo-3-pyridinyl, 5-
vinyl-3-pyridinyl,
5-methoxy-3-pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-3-pyridinyl, 5,6-
dichloro-3-pyridinyl,
6-bromo-5-chloro-3-pyridinyl, 5-cyano-6-methyl-3-pyridinyl, 6-chloro-5-cyano-3-
pyridinyl, 6-
bromo-5-cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl, 6-bromo-5-methyl-3-
pyridinyl, 5-
methoxy-6-methyl-3-pyridinyl, 6-chloro-5-methoxy-3-pyridinyl, or 6-bromo-S-
methoxy-3-
pyridinyl; R3 and Rs are absent; R4 is hydrogen; and Rl is as defined
informula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein Y is CH2; Z is a covalent bond; and Rl, R2, R3, R4, and Rs
axe as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein - represents a double bond; Y is CH2; Z is a covalent bond;
R2 is
heterocycle; R3 and Rs are absent; R4 is hydrogen; and Rl is as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein -- represents a double bond; Y is CH2; Z is a covalent bond;
Ra is
heterocycle wherein the heterocycle is selected from furyl, imidazolyl,
isothiazolyl, isoxazolyl,
oxadiazolyl, oxazolyl, pyrazinyl, pyrazolyl, pyridinyl, pyrimidinyl,
pyridazinyl, pyrrolyl,
tetrazinyl, tetrazolyl, thiadiazolyl, thiazolyl, thienyl, triazinyl,
triazolyl, benzimidazolyl,
22
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
benzothiazolyl, benzothienyl, benzoxazolyl, benzofuranyl, cinnolinyl, faro[2,3-
c]pyridine,
faro[3,2-c]pyridine, faro[3,2-b]pyridinyl, faro[2,3 b]pyridinyl, imidazo[1,2-
a]pyridinyl,
indazolyl, indolyl, indolizinyl, naphthyridinyl, isobenzofuranyl,
isobenzothienyl, isoindolyl,
isoquinolinyl, phthalazinyl, quinolinyl, quinolizinyl, quinoxalinyl,
quinazolinyl, thieno[2;3-
c]pyridine, thieno[3,2-c]pyridine, thieno[3,2-b]pyridinyl or thieno[2,3-
b]pyridinyl wherein the
heterocycle is substituted with 0, 1, 2, or 3 substituents independently
selected from alkenyl,
alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl, alkoxycarbonylalkyl, alkyl,
alkylcarbonyl,
alkylcarbonyloxy, alkylthio, alkynyl, carboxy, carboxyalkyl, cyano,
cyanoalkyl, formyl,
formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl, mercapto,
mercaptoalkyl,
nitro, triphenylmethyl (trityl), -C(NH)NR1oR11, NRIORm (NW oRn)alkyl,
(NR1oR11)carbonyl,
(NR1oR11)carbonylalkyl, (NR1oR11)sulfonyl, NR12S(O)2R13, -C(NR12)NRl3Rla,
-CHaCWa)NRi3Ria~ -C~ORIZ)Ris~ -C~C~~2~ -CWaRis)Ria~ -S(C)24R1z~ or
-S(O)2R12; R3 and Rs are absent; R4 is hydrogen; Rlz, Ri3, and R14 are
independently selected
from hydrogen, alkyl, aryl, or arylalkyl; and Rl, Rlo, and Rll are as defined
in formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein -- represents a double bond; Y is CH2; Z is a covalent bond;
R2 is
heterocycle wherein the heterocycle is selected from imidazolyl, isoxazolyl,
pyridinyl, tetrazolyl,
thiadiazolyl, thiazolyl, thienyl, imidazo[1,2-a]pyridinyl, thieno[3,2
b]pyridinyl or thieno[2,3-
b]pyridinyl wherein the heterocycle is substituted with 0, 1, or 2
substituents independently
selected from alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen,
nitre
(NR1oR11)sulfonyl, or -C(NH)NRIORW R3 and Rs are absent; R4 is hydrogen; and
Rl, Rlo, and
Rl l are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein -- represents a double bond; Y is CH2; Z is a covalent bond;
R2 is
heterocycle wherein the heterocycle is pyridinyl substituted with 0, l, or 2
substituents
independently selected from alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl,
cyano, halogen, or niLro;
R3 and Rs are absent; R4 is hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein - represents a double bond; Y is CH2; Z is a covalent bond;
R2 is
heterocycle wherein the heterocycle is selected from 3-pyridinyl, 6-bromo-3-
pyridinyl, 6-chloro-
23
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
3-pyridinyl, 6-fluoro-3 pyridinyl, 6-methyl-3-pyridinyl, 5-cyano-3-pyridinyl,
5-methyl-3-
pyridinyl, 5-chloro-3-pyridinyl, 5-fluoro-3-pyridinyl, 5-bromo-3-pyridinyl, 5-
vinyl-3-pyridinyl,
5-methoxy-3-pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-3-pyridinyl, 5,6-
dichloro-3-pyridinyl,
6-bromo-5-chloro-3-pyridinyl, 5-cyano-6-methyl-3-pyridinyl, 6-chloro-5-cyano-3-
pyridinyl, 6-
bromo-5-cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl, 6-bromo-5-methyl-3-
pyridinyl, 5-
methoxy-6-methyl-3-pyridinyl, 6-chloro-5-methoxy-3-pyridinyl, or 6-bromo-5-
methoxy-3-
pyridinyl; R3 and RS are absent; R4 is hydrogen; and Rl is as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein Y is a covalent bond; Z is CH2CH2; and Rl, R2, R3, R4 and RS
are as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein - represents a double bond; Y is a covalent bond; Z is
C~bCH2; R2 is
hydrogen; R3 and RS are absent; R4 is heterocycle; and Rl is as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein - represents a double bond; Y is a covalent bond; Z is
CHzCH2; RZ is
hydrogen; R3 and RS are absent; R4 is heterocycle wherein the heterocycle is
selected from furyl,
imidazolyl, isothiazolyl, isoxazolyl, oxadiazolyl, oxazolyl, pyrazinyl,
pyrazolyl, pyridinyl,
pyrimidinyl, pyridazinyl, pyrrolyl, tetrazinyl, tetrazolyl, thiadiazolyl,
thiazolyl, thienyl, triazinyl,
triazolyl, benzimidazolyl, benzothiazolyl, benzothienyl, benzoxazolyl,
benzofuranyl, cinnolinyl,
faro[2,3-c]pyridine, faro[3,2-c]pyridine, faro[3,2 b]pyridinyl, faro[2,3-
b]pyridinyl, imidazo[1,2-
a]pyridinyl, indazolyl, indolyl, indolizinyl, naphthyridinyl, isobenzofuranyl,
isobenzothienyl,
isoindolyl, isoquinolinyl, phthalazinyl, quinolinyl, quinolizinyl,
quinoxalinyl, quinazolinyl,
thieno[2,3-c]pyridine, thieno[3,2-c]pyridine, thieno[3,2-b]pyridinyl or
thieno[2,3-b]pyridinyl
wherein the heterocycle is substituted with 0, 1, 2, or 3 substituents
independently selected from
alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl,
alkoxycarbonylalkyl, alkyl,
alkylcarbonyl, alkylcarbonyloxy, alkylthio, alkynyl, carboxy, carboxyalkyl,
cyano, cyanoalkyl,
formyl, formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl,
mercapto,
mercaptoalkyl, nitro, triphenylmethyl (trityl), -C~NRIORn, -NRIORI,
(NRioRI)alkyl,
(NRIORII)carbonyl, (NR1oR11)carbonylalkyl, (NRloRii)sulfonyl, NR12S(O~R13,
-C(~12y13R14~ -CH2C(I~R12y13R14~ -C(NOR12)R13, -C(NCN)R12, -C(NNRI2R13)R14~
24
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
-S(O)zORlz, or -S(O)zRlz; Rlz, R13, and Rr4 are independently selected from
hydrogen, alkyl,
aryl, or arylalkyl; and Rl, Rin, and Rll are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein -= represents a double bond; Y is a covalent bond; Z is
C~bCHz; Rz is
hydrogen; R3 and RS are absent; R4 is heterocycle wherein the heterocycle is
selected from
imidazolyl, isoxazolyl, pyridinyl, tefirazolyl, thiadiazolyl, thiazolyl,
thienyl, imidazo[1,2
a]pyridinyl, thieno[3,2-b]pyridinyl or thieno[2,3-b]pyridinyl wherein the
heterocycle is
substituted with 0, l, or 2 substituents independently selected from alkenyl,
alkenyloxy, alkoxy,
alkyl, alkynyl, cyano, halogen, nitro, (NRloR~1)sulfonyl, or -C~NRIORI; and
Rl, Rlg and Rll
are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein - represents a double bond; Y is a covalent bond; Z is
CHzCHz; Rz is
hydrogen; R3 and RS are absent; R4 is heterocycle wherein the heterocycle is
pyridinyl
substituted with 0, 1, or 2 substituents independently selected from alkenyl,
alkenyloxy, alkoxy,
alkyl, alkynyl, cyano, halogen, or nitro; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein -- represents a double bond; Y is a covalent bond; Z is
CHzCHz; Rz is
hydrogen; R3 and RS are absent; R4 is heterocycle wherein the heterocycle is
selected from 3-
pyridinyl, 6-bromo-3-pyridinyl, 6-chloro-3-pyridinyl, 6-fluoro-3 pyridinyl, 6-
methyl-3-pyridinyl,
S-cyano-3-pyridinyl, 5-methyl-3-pyridinyl, 5-chloro-3-pyridinyl, 5-fluoro-3-
pyridinyl, 5-bromo-
3-pyridinyl, 5-vinyl-3-pyridinyl, 5-methoxy-3-pyridinyl, 5-vinyloxy-3-
pyridinyl, 5-ethynyl-3-
pyridinyl, 5,6-dichloro-3-pyridinyl, 6-bromo-5-chloro-3-pyridinyl, 5-cyano-6-
methyl-3-
pyridinyl, 6-chloro-5-cyano-3-pyridinyl, 6-bromo-5-cyano-3-pyridinyl, 6-chloro-
5-methyl-3-
pyridinyl, 6-bromo-5-methyl-3-pyridinyl, 5-methoxy-6-methyl-3-pyridinyl, 6-
chloro-5-methoxy-
3-pyridinyl, or 6-bromo-5-methoxy-3-pyridinyl; and RI is as defined in formula
(I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein -- represents a double bond; Y is a covalent bond; Z is
CHzCHz; Rz is
heterocycle; R3 and RS are absent; R4 is hydrogen; and Rl is as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein --= represents a double bond; Y is a covalent bond; Z is
CHzCHz; Rz is
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
heterocycle wherein the heterocycle is selected from furyl, imidazolyl,
isothiazolyl, isoxazolyl,
oxadiazolyl, oxazolyl, pyrazinyl, pyrazolyl, pyridinyl, pyrimidinyl,
pyridazinyl, pyrrolyl,
tetrazinyl, tetrazolyl, thiadiazolyl, thiazolyl, thienyl, triazinyl,
triazolyl, benzimidazolyl,
benzothiazolyl, benzothienyl, benzoxazolyl, benzofuranyl, cinnolinyl, faro[2,3-
c]pyridine,
faro[3,2-c]pyridine, faro[3,2-b]pyridinyl, faro[2,3 b]pyridinyl, imidazo[1,2-
a]pyridinyl,
indazolyl, indolyl, indolizinyl, naphthyridinyl, isobenzofuranyl,
isobenzothienyl, isoindolyl,
isoquinolinyl, phthalazinyl, quinolinyl, quinolizinyl, quinoxalinyl,
quinazolinyl, thieno[2,3-
c]pyridine, thieno[3,2-c]pyridine, thieno[3,2-b]pyridinyl or thieno[2,3-
b]pyridinyl wherein the
heterocycle is substituted with 0, 1, 2, or 3 substituents independently
selected from alkenyl,
alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl, alkoxycarbonylalkyl, alkyl,
alkylcarbonyl,
alkylcarbonyloxy, alkylthio, alkynyl, carboxy, carboxyalkyl, cyano,
cyanoalkyl, formyl,
formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl, mercapto,
mercaptoalkyl,
vitro, triphenylmethyl (trityl), -C(NH)NR1oR11, NR1oR11, (NRIORn)alkyl,
(NRzoRrl)carbonyl,
(NR1oR11)carbonylalkyl, (NRIORn)sulfonyl, NR12S(O)2R13, -C(NRm)NR~3Ri4,
-CH2C~12)~13R14~ -C~ORIZ)Ri3~ -C(NCI~~z~ -C~Ri2Ri3)Ria~ -S(C)z4Riz~ or
-S(O)2R12; R3 and RS are absent; R4 is hydrogen; RI2, R13, and R14 are
independently selected
from hydrogen, alkyl, aryl, or arylalkyl; and Rl, Rlo, and R11 are as defned
in formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein -- represents a double bond; Y is a covalent bond; Z is
CI~CH2; RZ is
heterocycle wherein the heterocycle is selected from imidazolyl, isoxazolyl,
pyridinyl, tetrazolyl,
thiadiazolyl, thiazolyl, thienyl, imidazo[1,2-a]pyridinyl, thieno[3,2
b]pyridinyl or thieno[2,3-
b]pyridinyl wherein the heterocycle is substituted with 0, 1, or 2
substituents independently
selected from alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen,
vitro,
(NR1oR11)sulfonyl, or-C(NH)NR1oR11; R3 and RS are absent; R4 is hydrogen; and
Rl, Rlo, and
Rl l are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein -- represents a double bond; Y is a covalent bond; Z is
CI~CH2; R2 is
heterocycle wherein the heterocycle is pyridinyl substituted with 0, 1, or 2
substituents
independently selected from alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl,
cyano, halogen, or vitro;
R3 and RS are absent; R4 is hydrogen; and Rl is as defined in formula (I).
26
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein - represents a double bond; Y is a covalent bond; Z is
CHzCH2; R2 is
heterocycle wherein the heterocycle is selected from 3-pyridinyl, 6-bromo-3-
pyridinyl, 6-chloro-
3-pyridinyl, 6-fluoro-3 pyridinyl, 6-methyl-3-pyridinyl, 5-cyano-3-pyridinyl,
5-methyl-3-
pyridinyl, 5-chloro-3-pyridinyl, 5-fluoro-3-pyridinyl, 5-bromo-3-pyridinyl, 5-
vinyl-3-pyridinyl,
5-methoxy-3-pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-3-pyridinyl, 5,6-
dichloro-3-pyridinyl,
6-bromo-5-chloro-3-pyridinyl, 5-cyano-6-methyl-3-pyridinyl, 6-chloro-5-cyano-3-
pyridinyl, 6-
bromo-5-cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl, 6-bromo-5-methyl-3-
pyridinyl, 5-
methoxy-6-methyl-3-pyridinyl, 6-chloro-5-methoxy-3-pyridinyl, or 6-bromo-5-
methoxy-3-
pyridinyl; R3 and RS are absent; R4 is hydrogen; and RI is as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein Y is CH2; Z is CH2; and RI, R2, R3, R4, and RS are as
defined in formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein -- represents a double bond; Y is CH2; Z is CH2; R2 is
heterocycle; R3 and
RS are absent; R4 is hydrogen; and Rl is as defined in formula (I).
In another embodiment of the pxesent invention, compounds of formula (III) are
disclosed wherein -= represents a double bond; Y is CH2; Z is CH2; R2 is
heterocycle wherein
the heterocycle is selected from furyl, imidazolyl, isothiazolyl, isoxazolyl,
oxadiazolyl, oxazolyl,
pyrazinyl, pyrazolyl, pyridinyl, pyrimidinyl, pyridazinyl, pyrrolyl,
tetrazinyl, tetrazolyl,
thiadiazolyl, thiazolyl, thienyl, triazinyl, triazolyl, benzimidazolyl,
benzothiazolyl, benzothienyl,
benzoxazolyl, benzofuranyl, cinnolinyl, faro[2,3-c]pyridine, faro[3,2-
c]pyridine, fuxo[3,2-
b]pyridinyl, faro[2,3-b]pyridinyl, imidazo[1,2-a]pyridinyl, indazolyl,
indolyl, indolizinyl,
naphthyridinyl, isobenzofuranyl, isobenzothienyl, isoindolyl, isoquinolinyl,
phthalazinyl,
quinolinyl, quinolizinyl, quinoxalinyl, quinazolinyl, thieno[2,3-c]pyridine,
thieno[3,2-c]pyridine,
thieno[3,2-b]pyridinyl or thieno[2,3-b]pyridinyl wherein the heterocycle is
substituted with 0, 1,
2, or 3 substituents independently selected from alkenyl, alkoxy,
alkoxyalkoxy, alkoxyalkyl,
alkoxycarbonyl, alkoxycarbonylalkyl, alkyl, alkylcarbonyl, alkylcarbonyloxy,
alkylthio, alkynyl,
carboxy, carboxyalkyl, cyano, cyanoalkyl, formyl, formylalkyl, haloalkoxy,
haloalkyl, halogen,
hydroxy, hydroxyalkyl, mexcapto, mercaptoalkyl, vitro, triphenylmethyl
(trityl), -C(NH)NRloR1 n
-NRIORm (NRioRll)alkyl, (NR1oR11)carbonyl, (NR1oR11)carbonylalkyl,
(NRjoRll)sulfonyl,
27
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
-~125(~)2R13~ 'C~12WI3R14~ -CH2C(~RI2W13R14~ -C(NORIa)Ri3, -C(NCN)RI2,
-C~12R13)R14~ 'S(~)2OR12, or -S(O)2R12; R3 and RS are absent; R4 is hydrogen;
R12, R13, and
R14 are independently selected from hydrogen, alkyl, aryl, or arylalkyl; and
Rb Rlo, and Rl ~ are
as defined in formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein - represents a double bond; Y is CH2; Z is CH2; R2 is
heterocycle wherein
the heterocycle is selected from imidazolyl, isoxazolyl, pyridinyl,
tetrazolyl, thiadiazolyl,
thiazolyl, thienyl, imidazo[1,2-a]pyridinyl, thieno[3,2-b]pyridinyl or
thieno[2,~b]pyridinyl
wherein the heterocycle is substituted with 0, 1, or 2 substituents
independently selected from
alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen, nitro,
(NRioRl1)sulfonyl, or
-C(NH)NRIORI l; R3 and RS are absent; R4 is hydrogen; and Rl, RIO, and Rl1 are
as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein =~ represents a double bond; Y is CH2; Z is CH2; R~ is
heterocycle wherein
the heterocycle is pyridinyl substituted with 0, l, or 2 substituents
independently selected from
alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen, or nitro; R3 and
RS are absent; R4 is
hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein --= represents a double bond; Y is CH2; Z is CH2; R2 is
heterocycle wherein
the heterocycle is selected from 3-pyridinyl, 6-bromo-3-pyridinyl, 6-chloro-3-
pyridinyl, 6-
fluoro-3 pyridinyl, 6-methyl-3-pyridinyl, 5-cyano-3-pyridinyl, 5-methyl-3-
pyridinyl, 5-chloro-3-
pyridinyl, 5-fluoro-3-pyridinyl, 5-bromo-3-pyridinyl, 5-vinyl-3-pyridinyl, 5-
methoxy-3-
pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-3-pyridinyl, 5,6-dichloro-3-
pyridinyl, 6-bromo-5-
chloro-3-pyridinyl, 5-cyano-6-methyl-3-pyridinyl, 6-chloro-5-cyano-3-
pyridinyl, 6-bromo-5-
cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl, 6-bromo-5-methyl-3-
pyridinyl, 5-methoxy-6-
methyl-3-pyridinyl, 6-chloro-5-methoxy-3-pyridinyl, or 6-bromo-5-methoxy-3-
pyridinyl; R3 and
RS are absent; R4 is hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein Y is CH2CH2; Z is a covalent bond; and Rl, R2, R3, R4, and
R5 are as defined
in formula (I).
28
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Representative compounds of formula (III) include, but are not limited to:
(cis)-S-(3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-S-(3-pyr idinyl)-1,2,3,3 a,6,6a-hexahydrocyclopenta[b]pyrxole;
(cis)-6-(3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H indole;
(cis)-6-(6-chloro-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H indole;
(cis)-6-(6-bromo-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H-indole;
(cis)-6-(6-fluoro-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H indole;
(cis)-6-(6-methyl-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H-indole;
(cis)-S-(2,3,3a,4,S,7a-hexahydro- 1H-indol-6-yl)nicotinonitrile;
(cis)-6-(S-methyl-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H indole;
(cis)-6-(S-chloro-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H indole;
(cis)-6-(S-fluoro-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-IH-indole;
(cis)-6-(S-bromo-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H indole;
(cis)-6-(S-vinyl-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H indole;
(cis)-6-(S-methoxy-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-IH-indole;
(cis)-6-(S-vinyloxy-3-pyridinyl)-2,3,3 a,4, S,7a-hexahydro-1 H-indole;
(cis)-6-(S-ethynyl-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H-indole;
(cis)-6-(S,6-dichloro-3-pyridinyl)-2,3,3 a,4, S,7a-hexahydro-1 H-indole;
(cis)-6-(6-bromo-S-chloro-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H-indole;
(cis)-S-(2,3,3a,4,S,7a-hexahydro-lH-indol-6-yl)-2-methylnicotinonitrile;
(cis)-2-chloro-S-(2,3,3a,4,S,7a-hexahydro-1H-indol-6-yl)nicotinonitrile;
(cis)-2-bromo-S-(2,3,3a,4,S,7a-hexahydro- 1H-indol-6-yl)nicotinonitrile;
(cis)-6-(6-chloro-S-methyl-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H-indole ;
(cis)-6-(6-bromo-S-methyl-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H indole;
(cis)-6-(S-methoxy-6-methyl-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-IH-indole;
(cis)-6-(6-chloro-S-methoxy-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H-indole;
(cis)-6-(6-bromo-S-methoxy-3-pyridinyl)-2,3,3 a,4, S,7a-hexahydro-1 H-indole;
(cis)-6-(3-methyl-S-isoxazolyl)-2,3,3a,4,S,7a-hexahydro-1H-indole;
(cis)-2-(2,3,3a,4,S,7a-hexahydro-lH-indol-6-yl)furo[3,2-b]pyridine;
29
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-5-(6-chloro-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-5-(6-bromo-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-5-(6-fluoro-3-pyridinyl)-I,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-5-(6-methyl-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-S-( I ,2,3,3 a,4, 6a-hexahydrocyclopenta[b] pyrxol-5-yl)nicotinonitrile
(cis)-5-(S-methyl-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-S-(5-chloro-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-5-(5-fluoro-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-5-(5-bromo-3-pyridinyl)-I,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-S-(S-vinyl-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-S-(S-methoxy-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-S-(S-vinyloxy-3-pyxidinyl)-1,2,3,3 a,4,6a-hexahydrocyclopenta
[b]pyrrole;
(cis)-5-(5-ethynyl-3-pyridinyl)-1,2,3,3 a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-S-(5,6-dichloro-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[b]pyrrole;
(cis)-5-(6-bromo-5-chloro-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[b]pyrrole;
(cis)-S-(I,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrol-5-yl)-2-
methylnicotinonitrile
(cis)-2-chloro-5-(1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrol-5-
yl)nicotiilonitrile
(cis)-2-bromo-5-(1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrol-5-
yl)nicotinonitrile
(cis)-S-(6-chloro-5-methyl-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[b]pyrrole;
(cis)-5-(6-bromo-5-methyl-3-pyxidinyl)-I,2,3,3a,4,6a-
hexahydrocyclopenta[b]pyrrole;
(cis)-5-(5-methoxy-6-methyl-3-pyridinyl)- I,2,3,3a,4,6a-
hexahydrocyclopenta[b]pyrrole;
(cis)-5-(6-chloro-5-methoxy-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[b]pyrrole;
(cis)-5-(6-bromo-5-methoxy-3-pyridinyl)-I,2,3,3a,4,6a-
hexahydrocyclopenta[b]pyrrole;
(cis)-5-(3-methyl-5-isoxazolyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-2-(1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrol-5-yl)furo[3,2-b]pyridine;
(cis)-6-(3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H indole;
(cis)-6-(6-chloro-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-IH indole;
(cis)-6-(6-bromo-3-pyridinyl)-2,3,3 a,4,7,7a-hexahydro-1 H-indole;
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-6-(6-fluoro-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-IH indole;
(cis)-6-(6-methyl-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-IH-indole;
(cis)-5-(2,3,3a,4,7,7a-hexahydro- 1H-indol-6-yl)zucotinonitrile;
(cis)-6-(5-methyl-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H-indole;
(cis)-6-(S-chloro-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-IH-indole;
(cis)-6-(5-fluoro-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H-indole;
(cis)-6-(5-bromo-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H-indole;
(cis)-6-(5-vinyl-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H-indole;
(cis)-6-(5-methoxy-3-pyridinyl)-2,3,3a,4,7,7a hexahydro-IH-indole;
(cis)-6-(5-vinyloxy-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H-indole;
(cis)-6-(5-ethynyl-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H indole;
(cis)-6-(5,6-dichloro-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-IH-indole;
(cis)-6-(6-bromo-5-chloro-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H-indole;
(cis)-5-(2,3,3a,4,7,7a-hexahydro- IH-indol-6-yl)-2-methylnicotinozutrile;
(cis)-2-chloro-S-(2, 3,3 a,4,7,7a-hexahydro-1 H-indol-6-yl)nicotinonitrile;
(cis)-2-bromo-5-(2,3,3a,4,7,7a-hexahydro-lH-indol-6-yl)nicotinonitrile;
(cis)-6-(6-chloro-5-methyl-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H-indole;
(cis)-6-(6-bromo-5-methyl-3-pyridinyl)-2, 3,3 a,4,7,7a-hexahydro- I H-indole;
(cis)-6-(5-methoxy-6-methyl-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H-indole;
(cis)-6-(6-chloro-5-methoxy-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-IH-indole;
(cis)-6-(6-brozno-S-methoxy-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H-indole;
(cis)-6-(3-methyl-5-isoxazolyl)-2,3,3a,4,7,7a-hexahydro-IH-indole;
(cis)-2-(2,3,3a,4,7,7a-hexahydro- 1H-indol-6-y1)furo[3,2-b]pyridine;
(cis)-5-(3-pyridinyl)-1,2,3, 3 a,6,6a-hexahydrocyclopenta[b]pyrroIe;
(cis)-5-(6-chloro-3-pyridinyl)-1,2,3,3a,6,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-5-(6-bromo-3-pyridinyl)-1,2,3,3a,6,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-5-(6-fluoro-3-pyridinyl)- I ,2,3, 3 a, 6, 6a-
hexahydrocyclopenta[b]pyrrole;
(cis)-5-(6-methyl-3-pyridinyl)-I,2,3,3a,6,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-5-(I,2,3,3a,6,6a-hexahydrocyclopenta[b]pyrrol-5-yl)nicotinonitrile;
31
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-5-(5-methyl-3-pyridinyl)-1,2,3,3a,6,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-5-(5-chloro-3-pyridinyl)-1,2,3,3a,6,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-5-(5-fluoro-3-pyridinyl)-1,2,3,3a,6,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-5-(5-bromo-3-pyridinyl)-1,2,3,3 a,6,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-5-(5-vinyl-3-pyridinyl)-1,2,3,3a,6,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-5-(5-methoxy-3-pyridinyl)-1,2,3,3a,6,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-5-(5-vinyloxy-3-pyridinyl)-1,2,3,3 a, 6, 6a-
hexahydrocyclopenta[b]pyrrole;
(cis)-5-(5-ethynyl-3-pyridinyl)-1,2,3,3a,6,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-5-(5,6-dichloro-3-pyridinyl)-1,2,3,3a,6,6a-
hexahydrocyclopenta[b]pyrrole;
(cis)-5-(6-bromo-5-chloro-3-pyridinyl)-1,2,3,3a,6,6a-
hexahydrocyclopenta[b]pyrrole;
(cis)-5-( I ,2,3, 3 a, 6, 6a-hexahydrocyclopenta[b]pyrrol-5-yl)-2-
methylnicotinonitrile;
(cis)-2-chloro-5-(1,2,3,3a,6,6a-hexahydrocyclopenta[b]pyrrol-5-
yl)nicotinonitrile;
(cis)-2-bromo-5-( 1,2,3,3a,6,6a-hexahydrocyclopenta[b]pyrrol 5-
yl)nicotinonitrile;
(cis)-5-(6-chloro-5-methyl-3-pyridinyl)-I,2,3,3a,6,6a
hexahydrocyclopenta[b]pyrrole;
(cis)-5-(6-bromo-5-methyl-3-pyridinyl)-1,2,3,3a,6,6a-
hexahydrocyclopenta[b]pyrrole;
(cis)-5-(5-methoxy-6-methyl-3-pyridinyl)-1,2,3,3a,6,6a-
hexahydrocyclopenta[b]pyrrole;
(cis)-5-(6-chloro-5-methoxy-3-pyridinyl)-1,2,3,3a,6,6a
hexahydrocyclopenta[b]pyrrole;
(cis)-5-(6-bromo-5-methoxy-3-pyridinyl)-1,2,3,3a,6,6a-
hexahydrocyclopenta[b]pyrrole;
(cis)-5-(3-methyl-5-isoxazolyl)-1,2,3,3a,6,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-2-(1,2,3,3a,6,6a-hexahydrocyclopenta[b]pyrrol-5-yl)furo[3,2-b]pyridine;
(cis)-7-(3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H-indole;
(cis)-7-(6-chloro-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H indole;
(cis)-7-(6-bromo-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-IH-indole;
(cis)-7-(6-fluoro-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H-indole;
(cis)-7-(6-methyl-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-IH indole;
(cis)-5-(2,3,3a,4,5,7a-hexahydro- IH-indol-7-yl)nicotinonitrile;
(cis)-7-(5-methyl-3-pyridinyl)-2,3, 3 a,4, 5,7a-hexahydro- I H-indole;
(cis)-7-(5-chloro-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H-indole;
(cis)-7-(5-fluoro-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H indole;
32
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-7-(5-bromo-3-pyridinyl)-2,3,3 a,4, 5,7a-hexahydro-1 H-indole;
(cis)-7-(5-vinyl-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H-indole;
(cis)-7-(5-methoxy-3-pyridinyl)-2,3, 3 a,4, 5,7a-hexahydro-1 H-indole;
(cis)-7-(5-vinyloxy-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H indole;
(cis)-7-(5-ethynyl-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H-indole;
(cis)-7-(5,6-dichloro-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H-indole;
(cis)-7-(6-bromo-5-chloro-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H-indole;
(cis)-S-(2,3,3a,4,5,7a-hexahydro- 1H-indol-7-yI)-2-methylnicotinonitrile;
(cis)-2-chloro-5-(2,3,3a,4,5,7a-hexahydro-1H-indol-7-yl)nicotinonitrile;
(cis)-2-bromo-5-(2,3,3a,4,5,7a-hexahydro- 1H-indol-7-yl)nicotinonitrile;
(cis)-7-(6-chloro-5-methyl-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H-indole;
(cis)-7-(6-bromo-5-methyl-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H-indole;
(cis)-7-(5-methoxy-6-methyl-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H-indole;
(cis)-7-(6-chloro-5-methoxy-3-pyridinyl)-2,3,3a,4,5,7a hexahydro-1H-indole;
(cis)-7-(6-bromo-5-methxoy-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H-indole;
(cis)-2-(2,3,3a,4,5,7a-hexahydro- 1H-indol-7-yl)furo[3,2-b]pyridine;
(cis)-7-(3-methyl-5-isoxazolyl)-2,3,3a,4,5,7a-hexahydro-1H indole;
(cis)-6-(3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-6-(6-chloro-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-6-(6-bromo-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-6-(6-fluoro-3-pyridinyl)-1,2,3,3 a,4, 6a-hexahydrocyclopenta[b]pyrrole;
(cis)-6-(6-methyl-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-5-( 1,2,3, 3 a,4,6a-hexahydrocyclopenta[b] pyrrol-6-yl)nicotinonitrile;
(cis)-6-(5-methyl-3-pyridinyl)-1,2,3, 3 a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-6-(5-chloro-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-6-(5-fluoro-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-6-(5-bromo-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-6-(5-vinyl-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-6-(5-vinyloxy-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrole;
33
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-6-(5-ethynyl-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[b}pyrrole;
(cis)-6-(5,6-dichloro-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[b]pyrrole;
(cis)-6-(6-bromo-5-chloro-3-pyridinyl)-1,2,3,3a,4,da-
hexahydrocyclopenta[b]pyrrole;
(cis)-S-(1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrol-6-yl)-2-
methylnicotinonitrile;
(cis)-2-chloro-5-( 1,2, 3, 3 a,4,6a-hexahydrocyclopenta[b]pyrrol-6-
yl)nicotinonitrile;
(cis)-2-bromo-5-( 1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrol-6-
yl)nicotinonitrile;
(cis)-6-(6-chloro-5-methyl-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[b]pyrrole;
(cis)-6-(6-bxomo-5-methyl-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[b}pyrrole;
(cis)-6-(5-methoxy-6-methyl-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[b}pyrrole;
(cis)-6-(6-chloro-5-methoxy-3-pyridinyl)-1,2,3,3a,4,6a
hexahydrocyclopenta[b]pyrrole;
(cis)-6-(6-bromo-5-methoxy-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[b]pyrrole;
(cis)-6-(3-methyl-5-isoxazolyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrole;
(cis)-2-(1,2,3,3a,4,6a-hexahydrocyclopenta[b]pyrrol-6-yl)furo[3,2-b]pyridine;
or a
pharmaceutically acceptable salt, amide, ester and prodrugs thereof.
In another embodiment of the present invention, compounds of formula (IV) are
disclosed
Y R2
R~_N~ ~ R3
Z R Rq
(IV),
or a pharmaceutically acceptable salt, amide, ester or prodrugs thereof
wherein Y, Z, Rl, R2, R3,
R4 and RS are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein Y is a covalent bond; Z is a covalent bond; and Ri, RZ, R3,
R4, and RS are as
defined in formula (1].
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein - represents a double bond; Y is a covalent bond; Z is a
covalent bond; R2
is hydrogen; R3 and RS are absent; R4 is heteroeycle; and Rl is as defined in
formula (I).
Tn another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein =-= represents a double bond; Y is a covalent bond; Z is a
covalent bond; R2
is hydrogen; R3 and RS are absent; R4 is heterocycle wherein the heterocycle
is selected from
34
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
furyl, imidazolyl, isothiazolyl, isoxazolyl, oxadiazolyl, oxazolyl, pyrazinyl,
pyrazolyl, pyridinyl,
pyrimidinyl, pyridazinyl, pyrrolyl, tetrazinyl, tetrazolyl, thiadiazolyl,
thiazolyl, thienyl, triazinyl,
triazolyl, benzimidazolyl, benzothiazolyl, benzothienyl, benzoxazolyl,
benzofuranyl, cinnolinyl,
faro[2,3-c]pyridine, faro[3,2-c]pyridine, faro[3,2 b]pyridinyl, faro[2,3-
b]pyridinyl, imidazo[I,2-
a]pyridinyl, indazolyl, indolyl, indolizinyl, naphthyridinyl, isobenzofuranyl,
isobenzothienyl,
isoindolyl, isoquinolinyl, phthalazinyl, quinolinyl, quinolizinyl,
quinoxalinyl, quinazolinyl,
thieno[2,3-c]pyridine, thieno[3,2-c]pyridine, thieno[3,2-b]pyridinyl or
thieno[2,3-b]pyridinyl
wherein the heterocycle is substituted with 0, 1, 2, or 3 substituents
independently selected from
alkenyl, alkoxy, allcoxyalkoxy, alkoxyalkyl, alkoxycarbonyl,
alkoxycarbonylalkyl, alkyl,
alkylcarbonyl, alkylcarbonyloxy, alkylthio, alkynyl, carboxy, carboxyalkyl,
cyano, cyanoalkyl,
formyl, formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl,
mercapto,
mercaptoalkyl, nitro, triphenylmethyl (trityl),-C(NH)NRloRln -NR1oR11,
(NR1oR11)alkyl,
(NR1oR11)carbonyl, (NR1oR11)carbonylalkyl, (NR1oR11)sulfonyl, NR12S(O~R13,
-~~12)~13RI4~ -~H2C~12)~13R14~ -C~~R12)RI3~ -C~C~R12~ 'C~12R13)R14,
-S(O)20R12, or -S(O)2R12; R12, R13, and R14 are independently selected from
hydrogen, alkyl,
aryl, or arylalkyl; and Rl, Rlo, and Rl1 are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein -'- represents a double bond; Y is a covalent bond; Z is a
covalent bond; R2
is hydrogen; R3 and RS are absent; R4 is heterocycle wherein the heterocycle
is selected from
imidazolyl, isoxazolyl, tetrazolyl, thiadiazolyl, thiazolyl, thienyl,
imidazo[1,2 a]pyridinyl,
thieno[3,2-b]pyridinyl or thieno[2,3-b]pyridinyl wherein the heterocycle is
selected from
imidazolyl, isoxazolyl, pyridinyl, tetrazolyl, thiadiazolyl, thiazolyl,
thienyl, imidazo[1,2
a]pyridinyl, thieno[3,2-b]pyridinyl or thieno[2,3-b]pyridinyl wherein the
heterocycle is
substituted with 0, 1, or 2 substituents independently selected from alkenyl,
alkenyloxy, alkoxy,
alkyl, alkynyl, cyano, halogen, vitro, (NR1oR11)sulfonyl, or -C(NH)NR1oR11;
and Rl, Rl~ and Rll
are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein -= represents a double bond; Y is a covalent bond; Z is a
covalent bond; R2
is hydrogen; R3 and RS are absent; R4 is heterocycle wherein the heterocycle
is pyridinyl
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
substituted with 0, 1, or 2 substituents independently selected from alkenyl,
alkenyloxy, alkoxy,
alkyl, alkynyl, cyano, halogen, or nitro; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein =- represents a double bond; Y is a covalent bond; Z is a
covalent bond; R2
is hydrogen; R3 and RS are absent; R4 is heterocycle wherein the heterocycle
is selected from 3-
pyridinyl, 6-bromo-3-pyridinyl, 6-chloro-3-pyridinyl, 6-fluoro-3 pyridinyl, 6-
methyl-3-pyridinyl,
5-cyano-3-pyridinyl, 5-methyl-3-pyridinyl, 5-chloro-3-pyridinyl, 5-fluoro-3-
pyridinyl, 5-bromo-
3-pyridinyl, 5-vinyl-3-pyridinyl, 5-methoxy-3-pyridinyl, 5-vinyloxy-3-
pyridinyl, 5-ethynyl-3-
pyridinyl, 5,6-dichloro-3-pyridinyl, 6-bromo-5-chlaro-3-pyridinyl, 5-cyano-6-
methyl-3-
pyridinyl, 6-chloro-5-cyano-3-pyridinyl, 6-bromo-5-cyano-3-pyridinyl, 6-chloro-
5-methyl-3-
pyridinyl, 6-bromo-5-methyl-3-pyridinyl, 5-methoxy-6-methyl-3-pyridinyl, 6-
chloro-5-methoxy-
3-pyridinyl, or 6-bromo-5-methoxy-3-pyridinyl; and Rl is as defined in formula
(I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein --' represents a single bond; Y is a covalent bond; Z is a
covalent bond; R2,
R3 and RS are hydrogen; R4 is heterocycle; and Rl is as defined in formula
(I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein -- represents a single bond; Y is a covalent bond; Z is a
covalent bond; R2,
R3 and RS are hydrogen; R4 is heterocycle wherein the heterocycle is selected
from furyl,
imidazolyl, isothiazolyl, isoxazolyl, oxadiazolyl, oxazolyl, pyrazinyl,
pyrazolyl, pyridinyl,
pyrimidinyl, pyridazinyl, pyrrolyl, tetrazinyl, tetrazolyl, thiadiazolyl,
thiazolyl, thienyl, triazinyl,
triazolyl, benzimidazolyl, benzothiazolyl, benzothienyl, benzoxazolyl,
benzofuranyl, cinnolinyl,
faro[2,3-c]pyridine, faro[3,2-c]pyridine, faro[3,2 b]pyridinyl, faro[2,3-
b]pyridinyl, imidazo[1,2-
a]pyridinyl, indazolyl, indolyl, indolizinyl, naphthyridinyl, isobenzofuranyl,
isobenzothienyl,
isoindolyl, isoquinolinyl, phthalazinyl, quinolinyl, quinolizinyl,
quinoxalinyl, quinazolinyl,
thieno[2,3-c]pyridine, thieno[3,2-c]pyridine, thieno[3,2-b]pyridinyl or
thieno[2,3-b]pyridinyl
wherein the heterocycle is substituted with 0, 1, 2, or 3 substituents
independently selected from
alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl,
alkoxycarbonylalkyl, alkyl,
alkylcarbonyl, alkylcarbonyloxy, alkylthio, alkynyl, carboxy, carboxyalkyl,
cyano, cyanoalkyl,
formyl, formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl,
mercapto,
mercaptoalkyl, nitro, triphenylmethyl (trityl), -C(NH)NR1oR11, -NR1oR11,
(NRioRll)alkyl,
36
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(NR1~R11)carbonyl, (NR1oR11)carbonylalkyl, (NR1oR11)sulfonyl, NR12S(O~R13,
-C~12)~I3R14~ -CH2C(~R12)NR13R14~ -C~OR12)R13~ -C~C~R12W~~12RI3)R14~
-S(O)20R12, or -S(O)2R12; R12, R13, and R14 are independently selected from
hydrogen, allcyl,
aryl, or arylalkyl; and Rl, Rl o, and Rl 1 are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein - represents a single bond; Y is a covalent bond; Z is a
covalent bond; R2,
R3 and RS are hydrogen; R4 is heterocycle wherein the heterocycle is selected
from imidazolyl,
isoxazolyl, tetrazolyl, thiadiazolyl, thiazolyl, thienyl, imidazo[1,2
a]pyridinyl, thieno[3,2-
b]pyridinyl or thieno[2,3-b]pyridinyl wherein the heterocycle is substituted
with 0, l, or 2
substituents independently selected from alkenyl, alkenyloxy, alkoxy, alkyl,
alkynyl, cyano,
halogen, utro, (NR1oR11)sulfonyl, or-C(NH)NR1oR11; and Rl, Rlo, and Rll are as
defined in
formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein -- represents a single bond; Y is a covalent bond; Z is a
covalent bond; R2,
R3, and RS are hydrogen; R4 is heterocycle wherein the heterocycle is
pyridinyl substituted with
0, 1, or 2 substituents independently selected from alkenyl, alkenyloxy,
alkoxy, alkyl, alkynyl,
cyano, halogen, or nitro; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein -- represents a single bond; Y is a covalent bond; Z is a
covalent; R2, R3,
and RS are hydrogen; R4 is heterocycle wherein the heterocycle is selected
from 3-pyridinyl, 6-
bromo-3-pyridinyl, 6-chloro-3-pyridinyl, 6-fluoro-3 pyridinyl, 6-methyl-3-
pyridinyl, 5-cyano-3-
pyridinyl, 5-methyl-3-pyridinyl, 5-chloro-3-pyridinyl, 5-fluoro-3-pyridinyl, S-
bromo-3-pyridinyl,
5-vinyl-3-pyridinyl, 5-methoxy-3-pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-
3-pyridinyl, 5,6-
dichloro-3-pyridinyl, 6-bromo-5-chloro-3-pyridinyl, 5-cyano-6-methyl-3-
pyridinyl, 6-chloro-5-
cyano-3-pyridinyl, 6-bromo-5-cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl,
6-bromo-5-
methyl-3-pyridinyl, 5-methoxy-6-methyl-3-pyridinyl, 6-chloro-5-methoxy-3-
pyridinyl, or 6-
bromo-5-methoxy-3-pyridinyl; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein Y is a covalent bond; Z is CH2; and Rl, R2, R3, R4 and Rsare
as defined in
formula (I).
37
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein - represents a double bond; Y is a covalent bond; Z is CIA;
R2 is hydrogen;
R3 and RS are absent; R4 is heterocycle; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein - represents a double bond; Y is a covalent bond; Z is CI-~;
R2 is hydrogen;
R3 and RS are absent; R4 is heterocycle wherein the heterocycle is selected
from furyl,
imidazolyl, isothiazolyl, isoxazolyl, oxadiazolyl, oxazolyl, pyrazinyl,
pyrazolyl, pyridinyl,
pyrimidinyl, pyridazinyl, pyrrolyl, tetrazinyl, tetrazolyl, thiadiazolyl,
thiazolyl, thienyl, triazinyl,
triazolyl, benzimidazolyl, benzothiazolyl, benzothienyl, benzoxazolyl,
benzofuranyl, cinnolinyl,
faro[2,3-c]pyridine, faro[3,2-c]pyridine, faro[3,2 b]pyridinyl, faro[2,3-
b]pyridinyl, imidazo[1,2-
a]pyridinyl, indazolyl, indolyl, indolizinyl, naphthyridinyl, isobenzofuranyl,
isobenzothienyl,
isoindolyl, isoquinolinyl, phthalazinyl, quinolinyl, quinolizinyl,
quinoxalinyl, quinazolinyl,
thieno[2,3-c]pyridine, thieno[3,2-c]pyridine, thieno[3,2-b]pyridinyl or
thieno[2,3-b]pyridinyl
wherein the heterocycle is substituted with 0, 1, 2, or 3 substituents
independently selected from
alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl,
alkoxycarbonylalkyl, alkyl,
alkylcarbonyl, alkylcarbonyloxy, alkylthio, alkynyl, carboxy, carboxyalkyl,
cyano, cyanoalkyl,
formyl, formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl,
mercapto,
mercaptoalkyl, nitro, phenyl, triphenylmethyl (trityl), -C(NH)NR1oR11, -
NR1oR11,
(NR1oR11)alkyl, (NR1oR11)carbonyl, (NR1oR11)carbonylalkyl, (NR1oR11)sulfonyl, -
NR12S(O)2R13,
-C~12)~13R14~'CH2C(1~R12)NRI3R14~ -C~OR12)Ris~ -C~C~R12~ 'L~12R13)R14,
-S(O)20R12, or -S(O)2R12; R12, R13, and R14 are independently selected from
hydrogen, alkyl,
aryl, or arylalkyl; and Rl, Rlo, and Rl1 are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein - represents a double bond; Y is a covalent bond; Z is CH2;
R2 is hydrogen;
R3 and RS are absent; R4 is heterocycle wherein the heterocycle is selected
from imidazolyl,
isoxazolyl, pyridazinyl, tetrazolyl, thiadiazolyl, thiazolyl, thienyl,
imidazo[I,2-a]pyridinyl,
thieno[3,2-b]pyridinyl or thieno[2,3-b]pyridinyl wherein the heterocycle is
substituted with 0, 1,
or 2 substituents independently selected from alkenyl, alkenyloxy, alkoxy,
alkyl, alkynyl, cyano,
halogen, nitro, phenyl, (NK1oR11)sulfonyl, or -C(NH)NR1oR11; and Rv Rlo, and
Rll are as
defined in formula (I).
38
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein - represents a double bond; Y is a covalent bond; Z is CHz;
R2 is hydrogen;
R3 and RS are absent; R4 is heterocycle wherein the heterocycle is pyridinyl
substituted with 0, 1,
or 2 substituents independently selected from alkenyl, alkenyloxy, alkoxy,
alkyl, alkynyl, cyano,
halogen, or nitro; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein ' represents a double bond; Y is a covalent bond; Z is CHz;
R2 is hydrogen;
R3 and RS are absent; R4 is heterocycle whexein the heterocycle is selected
from 3-pyridinyl, 6-
bromo-3-pyridinyl, 6-chloro-3-pyridinyl, 6-fluoro-3 pyridinyl, 6-methyl-3-
pyridinyl, 5-cyano-3-
pyridinyl, 5-methyl-3-pyridinyl, 5-chloro-3-pyridinyl, 5-fluoro-3-pyridinyl, 5-
bromo-3-pyridinyl,
5-vinyl-3-pyridinyl, 5-methoxy-3-pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-
3-pyridinyl, 5,6-
dichloro-3-pyridinyl, 6-bromo-5-chloro-3-pyridinyl, 5-cyano-6-methyl-3-
pyridinyl, 6-chloro-5-
cyano-3-pyridinyl, 6-bromo-S-cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl,
6-bromo-5-
methyl-3-pyridinyl, 5-methoxy-6-methyl-3-pyridinyl, 6-chloro-5-methoxy-3-
pyridinyl, or 6-
bromo-5-methoxy-3-pyridinyl; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein -- represents a double bond; Y is a covalent bond; Z is CI-
~; R2 is hydrogen;
R3 and RS are absent; R4 is heterocycle wherein the heterocycle is pyridazinyl
substituted with
phenyl; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein - repxesents a double bond; Y is a covalent bond; Z is CI-
b_; RZ is hydrogen;
R3 and RS are absent; R4 is heterocycle wherein the heterocycle is 4-
phenylpyridazinyl; and Rl is
hydrogen or methyl.
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein -'- represents a single bond; Y is a covalent bond; Z is
CH2; R~ and R3 axe
hydrogen; RS is selected from hydrogen or hydroxy; R4 is heterocycle; and Rl
is as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein --= represents a single bond; Y is a covalent bond; Z is
CH2; R2 and R3 are
hydrogen; RS is selected from hydxogen ox hydroxy; R4 is selected from
heterocycle wherein the
39
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
heterocycle is selected from furyl, imidazolyl, isothiazolyl, isoxazolyl,
oxadiazolyl, oxazolyl,
pyrazinyl, pyrazolyl, pyridinyl, pyrimidinyl, pyridazinyl, pyrrolyl,
tetrazinyl, tetrazolyl,
thiadiazolyl, thiazolyl, thienyl, triazinyl, triazolyl, benzimidazolyl,
benzothiazolyl, benzothienyl,
benzoxazolyl, benzofuranyl, cinnolinyl, faro[2,3-c]pyridine, faro[3,2-
c]pyridine, faro[3,2-
b]pyridinyl, faro[2,3-b]pyridinyl, imidazo[1,2-a]pyridinyl, indazolyl,
indolyl, indolizinyl,
naphthyridinyl, isobenzofuranyl, isobenzothienyl, isoindolyl, isoquinolinyl,
phthalazinyl,
quinolinyl, quinolizinyl, quinoxalinyl, quinazolinyl, thieno[2,3-c]pyridine,
thieno[3,2-c]pyridine,
thieno[3,2-b]pyridinyl or thieno[2,3-h]pyridinyl wherein the heterocycle is
substituted with 0, 1,
2, or 3 substituents independently selected from alkenyl, alkoxy,
alkoxyalkoxy, alkoxyalkyl,
alkoxycarbonyl, alkoxycarbonylalkyl, alkyl, alkylcarbonyl, alkylcarbonyloxy,
alkylthio, alkynyl,
carboxy, carboxyalkyl, cyano, cyanoalkyl, formyl, formylalkyl, haloalkoxy,
haloalkyl, halogen,
hydroxy, hydroxyalkyl, mercapto, mercaptoalkyl, nitro, triphenylmethyl
(trityl),-C(NH)NRIORI,
-NR1oR11, (NR1oR11)alkyl, (NRIOR~ 1)carbonyl, (NR1oR11)carbonylalkyl, (NRIORi
1)sulfonyl,
-~12s(~)2R13~ -C~12)~13R14~ -CHZC(NR12)~13R14~ -C~ORIa)Ris~ -C(NCN)RI2~
-C~12Rt3)R14~ -~(0)aORl2, or -S(O)2R12; R12, R13, and R14 are independently
selected from
hydrogen, alkyl, aryl, or arylalkyl; and Rl, Rlo, and Rl1 are as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein -~ represents a single bond; Y is a covalent bond; Z is CH2;
R2 and R3 are
hydrogen; RS is selected from hydrogen or hydroxy; R4 is selected from
heterocycle wherein the
heterocycle is selected from imidazolyl, isoxazolyl, pyridinyl, tetrazolyl,
thiadiazolyl, thiazolyl,
thienyl, imidazo[1,2-a]pyridinyl, thieno[3,2-b]pyridinyl or thieno[2,3-
b]pyridinyl wherein the
heterocycle is substituted with 0, 1, or 2 substituents independently selected
from alkenyl,
alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen, nitro, (NRloRj1)sulfonyl,
or-C(Nf-I)NR1oR11;
and Rl, Rio, and R11 are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein - represents a single bond; Y is a covalent bond; Z is CH2;
RZ and R3 are
hydrogen; RS is selected from hydrogen or hydroxy; R4 is heterocycle wherein
the heterocycle is
pyridinyl substituted with 0, 1, or 2 substituents independently selected from
alkenyl,
alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen, or nitro; and Rl is as
defined in formula (I).
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
In another embodiment of the pxesent invention, compounds of formula (IV) are
disclosed wherein - represents a single bond; Y is a covalent bond; Z is CH2;
R2 and R3 are
hydrogen; RS is selected from hydrogen or hydroxy; R4 is heterocycle wherein
the heterocycle is
selected from 3-pyridinyl, 6-bromo-3-pyxidinyl, 6-chloro-3-pyridinyl, 6-fluoro-
3 pyridinyl, 6-
methyl-3-pyridinyl, S-cyano-3-pyridinyl, S-methyl-3-pyridinyl, 5-chloro-3-
pyridinyl, S-fluoro-3-
pyridinyl, S-bromo-3-pyridinyl, S-vinyl-3-pyxidinyl, S-methoxy-3-pyridinyl, S-
vinyloxy-3-
pyridinyl, S-ethynyl-3-pyridinyl, S,6-dichloro-3-pyridinyl, 6-bromo-S-chloro-3-
pyridinyl, S-
cyano-6-methyl-3-pyridinyl, 6-chloro-S-cyano-3-pyridinyl, 6-bromo-S-cyano-3-
pyridinyl, 6-
chloro-S-methyl-3-pyridinyl, 6-bromo-S-methyl-3-pyridinyl, S-methoxy-6-methyl-
3-pyridinyl, 6-
chloro-S-methoxy-3-pyridinyl, or 6-bromo-S-methoxy-3-pyridinyl; and Rl is as
defined in
formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein ~ represents a double bond; Y is a covalent bond; Z is CHI;
R2 is
heterocycle; R3 and RS are absent; R4 is hydrogen; and Rl is as defined in
formula (1).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein - xepresents a double bond; Y is a covalent bond; Z is Cbb_;
R~ is selected
from heterocycle wherein the heterocycle is selected from furyl, imidazolyl,
isothiazolyl,
isoxazolyl, oxadiazolyl, oxazolyl, pyrazinyl, pyrazolyl, pyridinyl,
pyrimidinyl, pyridazinyl,
pyrrolyl, tetrazinyl, tetrazolyl, thiadiazolyl, thiazolyl, thienyl, triazinyl,
triazolyl, benzimidazolyl,
benzothiazolyl, benzothienyl, benzoxazolyl, benzofuxanyl, cinnolinyl, faro[2,3-
c]pyridine,
faro[3,2-c]pyridine, faro[3,2-b]pyridinyl, faro[2,3-b]pyridinyl, imidazo[1,2-
a]pyridinyl,
indazolyl, indolyl, indolizinyl, naphthyridinyl, isobenzofuranyl,
isobenzothienyl, isoindolyl,
isoquinolinyl, phthalazinyl, quinolinyl, quinolizinyl, quinoxalinyl,
quinazolinyl, thieno[2,3-
c]pyridine, thieno[3,2-c]pyridine, thieno[3,2-b]pyridinyl or thieno[2,3-
b]pyridinyl wherein the
heterocycle is substituted with 0, 1, 2, or 3 substituents independently
selected from alkenyl,
alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl, alkoxycarbonylalkyl, alkyl,
alkylcarbonyl,
alkylcaxbonyloxy, alkylthio, alkynyl, carboxy, carboxyalkyl, cyano,
cyanoalkyl, formyl,
formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl, mercapto,
mercaptoalkyl,
nitro, triphenylmethyl (trityl), -C(NH)NRIORII, NR1oR11, (NR1oR11)alkyl,
(NRIORn)carbonyl,
(NK1oR11)carbonylalkyl, (NRloRr1)sulfonyl, NR1~S(O)2R13, -C(NRl2)NRl3Ria,
41
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
-CH2C~12)NRi3R14~ -C~OR12)Ri3~ -C~C~R12~ -C~12R13)R14~ -S(~)2~Ri2~ Or
-S(O)2R12; R3 and RS are absent; R4 is hydrogen; R12, R13, and R14 are
independently selected
from hydrogen, alkyl, aryl, or arylalkyl; and Rl, Rlo, and Rll are as defined
in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein =- represents a double bond; Y is a covalent bond; Z is
CII2; R2 is selected
from heterocycle wherein the heterocycle is selected from imidazolyl,
isoxazolyl, pyridinyl,
tetrazolyl, thiadiazolyl, thiazolyl, thienyl, imidazo[1,2-a]pyridinyl,
thieno[3,2-b]pyridinyl or
thieno[2,3-b]pyridinyl wherein the heterocycle is substituted with 0, 1, or 2
substituents
independently selected from alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl,
cyano, halogen, nitro,
(NR1oR11)sulfonyl, or-C(NH)NR1oR11; R3 and RS are absent; R4 is hydrogen; and
Rl, Rlo, and
Rl1 are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (TV) are
disclosed wherein -- represents a double bond; Y is a covalent bond; Z is
CII2; R2 is
heterocycle wherein the heterocycle is pyridinyl substituted with 0, 1, or 2
substituents
independently selected from alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl,
cyano, halogen, or vitro;
R3 and RS are absent; R4 is hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein -'- represents a double bond; Y is a covalent bond; Z is
CIA; R2 is
heterocycle wherein the heterocycle is selected from 3-pyridinyl, frbromo-3-
pyridinyl, 6-chloro-
3-pyridinyl, 6-fluoro-3 pyridinyl, 6-methyl-3-pyridinyl, S-cyano-3-pyridinyl,
S-methyl-3-
pyridinyl, S-chloro-3-pyridinyl, S-fluoro-3-pyridinyl, S-bromo-3-pyridinyl, S-
vinyl-3-pyridinyl,
S-methoxy-3-pyridinyl, S-vinyloxy-3-pyridinyl, S-ethynyl-3-pyridinyl, S,6-
dichloro-3-pyridinyl,
6-bromo-S-chloro-3-pyridinyl, S-cyano-6-methyl-3-pyridinyl, 6-chloro-S-cyano-3-
pyridinyl, 6-
bromo-S-cyano-3-pyridinyl, 6-chloro-S-methyl-3-pyridinyl, 6-bromo-S-methyl-3-
pyridinyl, S-
methoxy-6-methyl-3-pyridinyl, 6-chloro-S-methoxy-3-pyridinyl, or 6-bromo-S-
methoxy-3-
pyridinyl; R3 and RS are absent; R4 is hydrogen; and RI is as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (IV) axe
disclosed wherein - represents a single bond; Y is a covalent bond; Z is CH2;
R2 is
heterocycle; R3 is selected from hydrogen or hydroxy; R4 and RS are hydrogen;
and Rl is as
defined in formula (I).
42
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein =-= represents a single bond; Y is a covalent bond; Z is
CHz; Rz 15
heterocycle wherein the heterocycle is selected from furyl, imidazolyl,
isothiazolyl, isoxazolyl,
oxadiazolyl, oxazolyl, pyrazinyl, pyrazolyl, pyridinyl, pyrimidinyl,
pyridazinyl, pyrrolyl,
tetrazinyl, tetrazolyl, thiadiazolyl, thiazolyl, thienyl, triazinyl,
triazolyl, benzimidazolyl,
benzothiazolyl, benzothienyl, benzoxazolyl, benzofuranyl, cinnolinyl, faro[2,3-
c]pyridine,
faro[3,2-c]pyridine, faro[3,2-b]pyridinyl, faro[2,3 b]pyridinyl, imidazo[1,2-
a]pyridinyl,
indazolyl, indolyl, indolizinyl, naphthyridinyl, isobenzofuranyl,
isobenzothienyl, isoindolyl,
isoquinolinyl, phthalazinyl, quinolinyl, quinolizinyl, quinoxalinyl,
quinazolinyl, thieno[2,3-
c]pyridine, thieno[3,2-c]pyridine, thieno[3,2-b]pyridinyl or thieno[2,3-
b]pyridinyl wherein the
heterocycle is substituted with 0, l, 2, or 3 substituents independently
selected from alkenyl,
alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl, alkoxycarbonylalkyl, alkyl,
alkylcarbonyl,
alkylcarbonyloxy, alkylthio, alkynyl, carboxy, carboxyalkyl, cyano,
cyanoalkyl, formyl,
formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl, mercapto,
mercaptoalkyl,
nitro, triphenylmethyl (trityl), -C(NH)NRIORIr, NR1oR11, (NRIORI)alkyl,
(NRIORn)carbonyl,
(NRIORI)carbonylalkyl, (NR1oR11)sulfonyl, NRIZS(O)zRl3, -C(NRIZ)NRI3RIa,
-CH2~~R12)~13R14~ -C~ORIZ)Ris~ -C~C~~z~ -CWzRi3)Rra~ -S(C)zCRlz~ or
-S(O)zRlz; R3 is selected from hydrogen or hydroxy; R~ and RS are hydrogen;
Rlz, R13, and Ri4
are independently selected from hydrogen, alkyl, aryl, or arylalkyl; and Ri,
Rlo, and RI1 are as
defined in formula (I).
In another embodiment of the present invention, compotmds of formula (IV) are
disclosed wherein =- represents a single bond; Y is a covalent bond; Z is CHz;
Rz is
heterocycle wherein the heterocycle is selected from imidazolyl, isoxazolyl,
pyridinyl, tetxazolyl,
thiadiazolyl, thiazolyl, thienyl, imidazojl,2-a]pyridinyl, thienoj3,2
b]pyridinyl or thieno[2,3-
b]pyridinyl wherein the heterocycle is substituted with 0, 1, or 2
substituents independently
selected from alkenyl, alkenyloxy, allcoxy, alkyl, alkynyl, cyano, halogen,
nitro,
(NRIORn)sulfonyl, or-C(NH)NRIORW R3 is selected from hydrogen or hydroxy; R4
and RS are
hydrogen; and Rl, Rlo, and Rll are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein -- represents a single bond; Y is a covalent bond; Z is CHz;
Rz is
43
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
heterocycle wherein the heterocycle is pyridinyl substituted with 0, 1, or 2
substituents
independently selected from alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl,
cyano, halogen, or nitro;
R3 is selected from hydrogen or hydroxy; R4 and RS are hydrogen; and Rl is as
defined in
formula (I).
In another embodiment of the present invention, compounds of formula (III) are
disclosed wherein - represents a single bond; Y is a covalent bond; Z is CH2;
RZ is
heterocycle wherein the heterocycle is selected from 3-pyridinyl, 6-bromo-3-
pyridinyl, 6-chloro-
3-pyridinyl, 6-fluoro-3 pyridinyl, 6-methyl-3-pyridinyl, S-cyano-3-pyridinyl,
S-methyl-3-
pyridinyl, S-chloro-3-pyridinyl, S-fluoro-3-pyridinyl, S-bromo-3-pyridinyl, S-
vinyl-3-pyridinyl,
S-methoxy-3-pyridinyl, S-vinyloxy-3-pyridinyl, S-ethynyl-3-pyridinyl, S,6-
dichloro-3-pyridinyl,
6-bromo-S-chloro-3-pyridinyl, S-cyano-6-methyl-3-pyridinyl, 6-chloro-S-cyano-3-
pyridinyl, 6-
bromo-S-cyano-3-pyridinyl, 6-chloro-S-methyl-3-pyridinyl, 6-bromo-5-methyl-3-
pyridinyl, S-
methoxy-6-methyl-3-pyridinyl, 6-chloro-S-methoxy-3-pyridinyl, or 6-bromo-S-
methoxy-3-
pyridinyl; R3 is selected from hydrogen or hydroxy; R4 and RS are hydrogen;
and Rl is as defined
in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein Y is a covalent bond; Z is CHzCHz; and Rl, R2, R3, R4, and
R~ are as defined
in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein -- represents a double bond; Y is a covalent bond; Z is
CHzCH2; R2 is
hydrogen; R3 and RS are absent; R4 is heterocycle; and Rl is as def ned in
formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein -~ represents a double bond; Y is a covalent bond; Z is
CIb_CH2; R2 is
hydrogen; R3 and RS are absent; R4 is heterocycle wherein the heterocycle is
selected from furyl,
imidazolyl, isothiazolyl, isoxazolyl, oxadiazolyl, oxazolyl, pyrazinyl,
pyrazolyl, pyridinyl,
pyrirnidinyl, pyridazinyl, pyrrolyl, tetrazinyl, tetrazolyl, thiadiazolyl,
thiazolyl, thienyl, triazinyl,
triazolyl, benzimidazolyl, benzothiazolyl, benzothienyl, benzoxazolyl,
benzofuranyl, cinnolinyl,
faro[2,3-c]pyridine, faro[3,2-c]pyridine, faro[3,2 b]pyridinyl, faro[2,3-
b]pyridinyl, imidazo[1,2-
a]pyridinyl, indazolyl, indolyl, indolizinyl, naphthyridinyl, isobenzofuranyl,
isobenzothienyl,
isoindolyl, isoquinolinyl, phthalazinyl, quinolinyl, quinolizinyl,
quinoxalinyl, quinazolinyl,
44
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
thieno[2,3-c]pyridine, thieno[3,2-c]pyridine, thieno[3,2-b]pyridinyl or
thieno[2,3-b]pyridinyl
wherein the heterocycle is substituted with 0, l, 2, or 3 substituents
independently selected from
alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl,
alkoxycarbonylalkyl, alkyl,
alkylcarbonyl, alkylcarbonyloxy, alkylthio, alkynyl, carboxy, carboxyalkyl,
cyano, cyanoalkyl,
formyl, formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl,
mercapto,
mercaptoalkyl, vitro, triphenylmethyl (trityl), -C(NH)NR1oR11, -NR1oR11,
(NR1oR11)alkyl,
(NR1oR11)caxbonyl, (NR1oR11)carbonylalkyl, (NR1oR11)sulfonyl, NR12S(O)2R13a
-C~12)~13R14a -CH2C~12)~13R-14a -C~OR12)Rl3a 'c~~~Rl2W~~12R13)RI4a
-S(O)20R12, or -S(O)2R12; Rl2a Rl3a and Rl~ are independently selected from
hydrogen, alkyl,
aryl, or arylalkyl; and Rl, Rlo, and Rl1 are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein -=- represents a double bond; Y is a covalent bond; Z is
CHzCH2; R2 is
hydrogen; R3 and RS are absent; R4 is heterocycle wherein the heterocycle is
selected from
imidazolyl, isoxazolyl, pyridinyl, tetrazolyl, thiadia~olyl, thiazolyl,
thienyl, imidazo[1,2
a]pyridinyl, thieno[3,2-b]pyridinyl or thieno[2,3-b]pyridinyl wherein the
heterocycle is
substituted with 0, 1, or 2 substituents independently selected from alkenyl,
allcenyloxy, alkoxy,
alkyl, alkynyl, cyano, halogen, vitro, (NR1oR11)sulfonyl, or -C(NH)NR1oR11;
and Rl, Rlg and Rll
are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein -- represents a double bond; Y is a covalent bond; Z is
C~bCH2; R2 is
hydrogen; R3 and RS are absent; R4 is heterocycle wherein the heterocycle is
pyridinyl
substituted with 0, 1, or 2 substituents independently selected from alkenyl,
alkenyloxy, alkoxy,
alkyl, alkynyl, cyano, halogen, or vitro; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein -= represents a double bond; Y is a covalent bond; Z is
CI~CH2; R2 is
hydrogen; R3 and RS are absent; R4 is heterocycle wherein the heterocycle is
selected from 3-
pyridinyl, 6-bromo-3-pyridinyl, 6-chloro-3-pyridinyl, 6-fluoro-3 pyridinyl, 6-
methyl-3-pyridinyl,
5-cyano-3-pyridinyl, 5-methyl-3-pyridinyl, 5-chloro-3-pyridinyl, 5-fluoro-3-
pyridinyl, 5-bromo-
3-pyridinyl, 5-vinyl-3-pyridinyl, 5-methoxy-3-pyridinyl, 5-vinyloxy-3-
pyridinyl, 5-ethynyl-3-
pyridinyl, 5,6-dichloro-3-pyridinyl, 6-bromo-5-chloro-3-pyridinyl, 5-cyano-6-
methyl-3-
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
pyridinyl, 6-chloro-S-cyano-3-pyridinyl, 6-bromo-S-cyano-3-pyridinyl, 6-chloro-
S-methyl-3-
pyridinyl, 6-bromo-S-methyl-3-pyridinyl, S-methoxy-6-methyl-3-pyridinyl, 6-
chloro-S-methoxy-
3-pyridinyl, or 6-bromo-S-methoxy-3-pyridinyl; and Rl is as defined in formula
(I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein ' represents a double bond; Y is a covalent bond; Z is CI-
~CHZ; R2 is
heterocycle; R3 and RS are absent; R4 is hydrogen; and Rl is as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein - represents a double bond; Y is a covalent bond; Z is
CH~,CHz; R2 is
heterocycle wherein the heterocycle is selected from furyl, imidazolyl,
isothiazolyl, isoxazolyl,
oxadiazolyl, oxazolyl, pyrazinyl, pyrazolyl, pyridinyl, pyrimidinyl,
pyridazinyl, pyrrolyl,
tetrazinyl, tetrazolyl, thiadiazolyl, thiazolyl, thienyl, triazinyl,
triazolyl, benzimidazolyl,
benzothiazolyl, benzothienyl, benzoxazolyl, benzofuranyl, cinnolinyl, faro[2,3-
c]pyridine,
faro[3,2-c]pyridine, faro[3,2-b]pyridinyl, faro[2,3 b]pyridinyl, imidazo[1,2-
a]pyridinyl,
indazolyl, indolyl, indolizinyl, naphthyridinyl, isobenzofuranyl,
isobenzothienyl, isoindolyl,
isoquinolinyl, phthalazinyl, quinolinyl, quinolizinyl, quinoxalinyl,
quinazolinyl, thieno[2,3-
c]pyridine, thieno[3,2-c]pyridine, thieno[3,2-b]pyridinyl or thieno[2,3-
b]pyridinyl wherein the
heterocycle is substituted with 0, 1, 2, or 3 substituents independently
selected from alkenyl,
alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl, alkoxycarbonylalkyl, alkyl,
alkylcarbonyl,
alkylcarbonyloxy, alkylthio, alkynyl, carboxy, carboxyalkyl, cyano,
cyanoalkyl, formyl,
formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl, mercapto,
mercaptoalkyl,
vitro, triphenylmethyl (trityl), -C(NH)NR1oR11, NR1oR11, (NR1oR11)alkyl,
(NRIOR11)carbonyl,
(NR1oR11)carbonylalkyl, (NR1oR11)sulfonyl, NR12S(O)ZR13, -C(NR12)NR13RI4~
-CHzC~Rla)W3Ria~ -C~OR12)Ri3~ "C~C~RI2~ -C~12RI3)RI4~ ~(~)2~R12~ Or
-S(O)ZR12; R3 and RS are absent; R4 is hydrogen; R12, R13, and R14 are
independently selected
from hydrogen, alkyl, aryl, or arylalkyl; and Rl, Rlo, and Rl1 are as defined
in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein --= represents a double bond; Y is a covalent bond; Z is
CH~CH2; R2 is
heterocycle wherein the heterocycle is selected from imidazolyl, isoxazolyl,
pyridinyl, tetrazolyl,
thiadiazolyl, thiazolyl, thienyl, imidazo[1,2-a]pyridinyl, thieno[3,2
b]pyridinyl or thieno[2,3-
b]pyridinyl wherein the heterocycle is substituted with 0, 1, or 2
substituents independently
46
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
selected from alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen,
vitro,
(NRIORI1)sulfonyl, or -C(NH)NR1oR11; R3 and RS are absent; R4 is hydrogen; and
Rl, Rlo, and
Rl l are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein =- represents a double bond; Y is a covalent bond; Z is
CI~CHZ; R2 is
heterocycle wherein the heterocycle is pyridinyl substituted with 0, 1, or 2
substituents
independently selected from alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl,
cyano, halogen, or vitro;
R3 and RS are absent; R4 is hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein -- represents a double bond; Y is a covalent bond; Z is
CH~CH2; RZ is
heterocycle wherein the heterocycle is selected from 3-pyridinyl, 6-bromo-3-
pyridinyl, 6-chloro-
3-pyridinyl, 6-fluoro-3 pyridinyl, 6-methyl-3-pyridinyl, 5-cyano-3-pyridinyl,
5-methyl-3-
pyridinyl, 5-chloro-3-pyridinyl, 5-fluoro-3-pyridinyl, 5-bromo-3-pyridinyl, 5-
vinyl-3-pyridinyl,
5-methoxy-3-pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-3-pyridinyl, 5,6-
dichloro-3-pyridinyl,
6-bromo-5-chloro-3-pyridinyl, 5-cyano-6-methyl-3-pyridinyl, 6-chloro-5-cyano-3-
pyridinyl, 6-
bromo-5-cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl, 6-bromo-5-methyl-3-
pyridinyl, 5-
methoxy-6-methyl-3-pyridinyl, 6-chloro-5-methoxy-3-pyridinyl, or 6-bromo-5-
methoxy-3-
pyridinyl; R3 and RS are absent; R4 is hydrogen; and Rl is as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein Y is CHZ; Z is CH2; and Rl, Rz, R3, R4, and R5 are as
defined in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein = - represents a double bond; Y is CHZ; Z is CH2; R2 is
heterocycle; R3 and
RS are absent; R4 is hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein - represents a double bond; Y is CH2; Z is CH2; R2 is
heterocycle wherein
the heterocycle is selected from furyl, imidazolyl, isothiazolyl, isoxazolyl,
oxadiazolyl, oxazolyl,
pyrazinyl, pyrazolyl, pyridinyl, pyrimidinyl, pyridazinyl, pyrrolyl,
tetrazinyl, tetrazolyl,
thiadiazolyl, thiazolyl, thienyl, triazinyl, triazolyl, benzimidazolyl,
benzothiazolyl, benzothienyl,
benzoxazolyl, benzofuranyl, cinnolinyl, faro[2,3-c]pyridine, faro[3,2-
c]pyridine, faro[3,2-
b]pyridinyl, faro[2,3-b]pyridinyl, imidazo[1,2,-a]pyridinyl, indazolyl,
indolyl, indolizinyl,
47
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
naphthyridinyl, isobenzofuranyl, isobenzothienyl, isoindolyl, isoquinolinyl,
phthalazinyl,
quinolinyl, quinolizinyl, quinoxalinyl, quinazolinyl, thieno[2,3-c]pyridine,
thieno[3,2-c]pyridine,
thieno[3,2-b]pyxidinyl or thieno[2,3-b]pyridinyl wherein the heterocycle is
substituted with 0, I,
2, or 3 substituents independently selected from alkenyl, alkoxy,
alkoxyalkoxy, alkoxyalkyl,
alkoxycarbonyl, alkoxycaxbonylalkyl, alkyl, alkylcarbonyl, alkylcarbonyloxy,
alkylthio, alkynyl,
carboxy, carboxyalkyl, cyano, cyanoalkyl, formyl, formylalkyl, haloalkoxy,
haloalkyl, halogen,
hydroxy, hydroxyalkyl, mercapto, mercaptoalkyl, vitro, triphenylmethyl
(trityl),-C(NH)NR1oR11,
-~loRl, (NR1oR11)alkyl, (NR1oR11)carbonyl, (NR1oR11)carbonylalkyl,
(NR1oR11)sulfonyl,
-~12~(~)2R13~ -C~12)~13R14~ -CH2C(NR12)~13R14~ -C~ORIa)R13, -C~C~R12~
-C~12R13)R14, -S(~)2~R12, or -S(O)2R12; R3 and Rs are absent; R4 is hydrogen;
R12, R13, and
R14 are independently selected from hydrogen, alkyl, aryl, or arylalkyl; and
Rl, Rlo, and Rl1 are
as defined in formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein =- represents a double bond; Y is CH2; Z is CH2; R2 is
heterocycle wherein
the heterocycle is selected from imidazolyl, isoxazolyl, pyridinyl,
tetrazolyl, thiadiazolyl,
thiazolyl, thienyl, imidazo[1,2-a]pyridinyl, thieno[3,2-b]pyridinyl or
thieno[2,3-b]pyridinyl
wherein the heterocycle is substituted with 0, 1, or 2 substituents
independently selected from
alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen, vitro,
(NR1oR11)sulfonyl, or
-C(NH)NR1oR11; Rs and Rs are absent; R4 is hydrogen; and Rl, Rlo, and Rll are
as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (IV) are
disclosed wherein - = represents a double bond; Y is CH2; Z is CH2; R2 is
heterocycle wherein
the heterocycle is pyxidinyl substituted with 0, 1, or 2 substituents
independently selected from
alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen, or vitro; R3 and
Rs are absent; R4 is
hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (TV) are
disclosed wherein -- represents a double bond; Y is CH2; Z is CH2; R2 is
heterocycle wherein
the heterocycle is selected from 3-pyridinyl, 6-bromo-3-pyridinyl, 6-chloro-3-
pyridinyl, 6-
fluoro-3 pyridinyl, 6-methyl-3-pyridinyl, 5-cyano-3-pyridinyl, 5-methyl-3-
pyridinyl, S-chloro-3-
pyridinyl, 5-fluoro-3-pyridinyl, 5-bromo-3-pyxidinyl, 5-vinyl-3-pyridinyl, 5-
methoxy-3-
48
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
pyridinyl, 5-vinyloxy-3-pyridinyl, S-ethynyl-3-pyridinyl, 5,6-dichloro-3-
pyridinyl, 6-bromo-S-
chloro-3-pyridinyl, S-cyano-6-methyl-3-pyridinyl, 6-chloro-S-cyano-3-
pyridinyl, 6-bromo-5-
cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl, 6-bromo-5-methyl-3-
pyridinyl, 5-methoxy-6-
methyl-3-pyridinyl, 6-chloro-5-methoxy-3-pyridinyl, or 6-bromo-5-methoxy-3-
pyridinyl; R3 and
RS are absent; R4 is hydrogen; and Rl is as defined in formula (I).
Representative compounds of formula (IV) include, but are not limited to:
(cis)-5-(6-chloro-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-5-(5-methoxy-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-5-(6-chloro-5-methyl-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-5-(3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-S-(3-methyl-S-isoxazolyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-S-(5,6-dichloro-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-2-methyl-S-(3-methyl-5-isoxazolyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-5-(S-chloro-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-S-(3-bromo-1,2,4-thiadiazol-5-yl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-5-(1,3-thiazol-2-yl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-5-(3,5-dimethyl-4-isoxazolyl)-1,2,3,3a,4,6a
hexahydrocyclopenta[c]pyrrole;
(cis)-5-( 1 H-imidazol-4-yl)-1,2,3, 3a,4, 6a-hexahydrocyclopenta[c]pyrrole;
(cis)-S-(1,3-thiazol-5-yl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-S-(imidazo[1,2-a]pyridin-3-yl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-5-(imidazo[1,2-a]pyridin-6-yI)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-5-(thieno[3,2-b]pyridin-2-yl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-5-( 1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrol-5-yl)-2-
thiophenesulfonamide;
(cis)-S-(6-methyl-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-5-(2-methyl-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-5-(6-chloro-5-fluoro-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-S-(6-fluoro-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-S-(1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrol-S-yl)-2-
thiophenecarboximidarnide;
(cis)-5-(2-methyl-2H-tetrazol-5-yl)-1,2,3,3a,4,6a
hexahydrocyclopenta[c]pyrrole;
(cis)-5-(thieno[2,3-b]pyridin-5-yl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
49
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-5-(imidazo[I,2-a]pyridin-7-yl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-5-(2-pyridinyl)-I,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-5-(4-pyridinyl)-I,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-5-(5-vitro-1,3-thiazol-2-yl)-1,2, 3, 3 a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-5-(6-methyl-2-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-5-( 1,3,4-thiadiazol-2-yl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(endo)-5-(3-pyridinyl)octahydrocyclopenta[c]pyrrole;
(cis)-5-(6-chloro-3-pyridinyl)octahydrocyclopenta[c]pyrrol-5-0l; -
(endo)-5-(6-chloro-3-pyridinyl)octahydrocyclopenta[c]pyrrole;
(exo)-5-(6-chloro-3-pyridinyl)octahydrocyclopenta[c]pyrrole;
(cis)-5-(5-bromo-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-5-(5-vinyl-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-5-( 1,2, 3,3 a,4,6a-hexahydrocyclopenta[c]pyrrol-5-yl)nicotinonitrile;
(cis)-5-(6-chloro-3-pyridinyl)-I,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-5-(6-chloro-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-6-(6-chloro-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-4-(3-pyridinyl)octahydrocyclopenta[c]pyrrol-4-0l;
(endo)-4-(6-chloro-3-pyridinyl)octahydrocyclopenta[c]pyrxole;
(cis)-6-(6-methyl-3-pyridinyl)-3-azabicyclo[3.2.0]hept-6-eve;
(endo)-6-(6-chloro-3-pyridinyl)-3-azabicyclo[3.2.0]heptane;
(exo)-6-(6-chloro-3-pyridinyl)-3-azabicyclo[3.2.0]heptane;
(cis)-6-(5,6-dichloro-3-pyridinyl)-3-azabicyclo [3 .2.0]hept-6-eve;
(endo)-6-(5,6-dichloro-3-pyridinyl)-3-azabicyclo[3.2.0]heptane;
(cis)-5-(6-phenylpyridazin-3-yI)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-2-methyl-5-(6-phenylpyridazin-3-yl)-1,2,3, 3 a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-5-(6-bromo-3-pyridinyl)-1,2, 3,3 a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-5-(5-methyl-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-5-(6-chloro-5-methoxy-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-5-(6-bromo-5-methoxy-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-5-(5-methoxy-6-methyl-3-pyridinyl)- I,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-2-chloro-5-(I,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrol-5-
yl)nicotinonitrile;
(cis)-2-bromo-5-(1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrol-5-
yl)nicotinonitrile;
(cis)-5-( 1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrol-5-yl)-2-
methylnicotinonitrile;
(cis)-5-(5-bromo-6-chloro-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-5-(5,6-dibromo-3-pyridinyl)-I,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-5-(6-bromo-5-chloro-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-5-(5-chloro-6-methyl-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-5-(5-bromo-6-methyl-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-5-(6-bromo-5-methyl-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-2-(I,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrol-5-yl)furo[3,2-b]pyridine;
(cis)-7-(3-pyridinyl)-2, 3,3 a,4, 5,7a-hexahydro-1 H-isoindole;
(cis)-7-(6-chloro-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H isoindole;
(cis)-7-(6-bromo-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H-isoindole;
(cis)-7-(6-fluoro-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-IH-isoindole;
(cis)-7-{6-methyl-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H-isoindole;
(cis)-5-(2,3,3a,6,7,7a-hexahydro- 1H-isoindol-4-yl)nicotinonitrile;
(cis)-7-(5-methyl-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-IH-isoindole;
(cis)-7-(5-chloro-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-IH isoindole;
(cis)-7-(5-bromo-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H isoindole;
(cis)-7-(5-fluoro-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-IH isoindole;
(cis)-7-(5-vinyl-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H isoindole;
(cis)-7-(5-methoxy-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H-isoindole;
(cis)-7-(5-vinyloxy-3-pyridinyl)-2,3,3 a,4, 5, 7a-hexahydro-1 H-isoindole;
(cis)-7-(5-ethynyl-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-IH-isoindole;
(cis)-7-(5,6-dichloro-3-pyridinyl)-2,3, 3 a,4, 5,7a-hexahydro- I H-isoindole;
(cis)-7-(6-bromo-5-chloro-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-IH-isoindole;
(cis)-5-(2,3,3 a, 6,7,7a-hexahydro- 1 H-isoindol-4-yl)-2-
methylnicotinonitrile;
(cis)-2-chloro-5-(2,3,3a,6,7,7a-hexahydro-1H-isoindol-4-yl)nicotinonitrile;
S1
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-2-bromo-5-(2,3,3a,6,7,7a-hexahydro- IH-isoindol-4-yl)nicotinonitrile;
(cis)-7-(6-chloro-5-methyl-3-pyridinyl)-2,3,3a,4,5,7a hexahydro-IH-isoindole;
(cis)-7-(6-bromo-5-methyl-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H-isoindole;
(cis)-7-(5-methoxy-6-methyl-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H-isoindole;
(cis)-7-(6-chloro-5-methoxy-3-pyridinyl)-2,3,3a,4,5,7a-hexahydro-1H-isoindole;
(cis)-7-(6-bromo-5-methoxy-3-pyridinyl)-2,3,3 a,4, 5, 7a-hexahydro- 1 H-
isoindole;
(cis)-7-(3-methyl-5-isoxazolyl)-2,3,3a,4,5,7a-hexahydro-1H-isoindole;
(cis)-2-(2,3,3a,6,7,7a-hexahydro- IH-isoindol-4-yl)furo[3,2-b]pyridine;
(cis)-6-(3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-6-(6-chloro-3-pyridinyl)-I,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-6-(6-bromo-3-pyridinyl)- I ,2, 3,3 a,4,6a-hexahydrocyclopenta[c]
pyrrole;
(cis)-6-(6-methyl-3-pyridinyl)-1,2,3,3 a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-6-(6-fluoro-3-pyxidinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-S-(I,2,3,3a,6,6a-hexahydrocyclopenta[c]pyrrol-4-yl)nicotinonitrile;
(cis)-6-(5-methyl-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-6-(S-chloro-3-pyridinyl)-1,2,3,3a,4,6a-hexahydxocyclopenta[c]pyrrole;
(cis)-6-(5-fluoro-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-6-(5-bromo-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-6-(5-vinyl-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-6-(5-methoxy-3-pyridinyl)-1,2,3,3 a,4,6a-hexahydrocyclopenta[c] pyrrole;
(cis)-6-(5-vinyloxy-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-6-(S-ethynyl-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole;
(cis)-6-(5,6-dichloro-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-6-(6-bromo-5-chloro-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-5-( I ,2,3,3 a,6,6a-hexahydrocyclopenta[c] pyrrol-4-yl)-2-
methylnicotinonitrile;
(cis)-2-chloro-5-(1,2,3,3a,6,6a-hexahydrocyclopenta[c]pyrrol-4-
yl)nicotinonitrile;
(cis)-2-bromo-5-(1,2,3,3a,6,6a-hexahydrocyclopenta[c]pyrrol-4-
yl)nicotinonitrile;
(cis)-6-(6-chloro-5-methyl-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-6-(6-bromo-5-methyl-3-pyridinyl)-1,2,3,3 a,4, 6a-
hexahydrocyclopenta[c]pyrrole;
52
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-6-(S-methoxy-6-methyl-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-6-(6-chloro-S-methoxy-3-pyridinyl)-1,2,3,3a,4,6a
hexahydrocyclopenta[c]pyrrole;
(cis)-6-(6-bromo-S-methoxy-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-6-(3-methyl-S-isoxazolyl)-1,2, 3, 3 a,4, 6a-
hexahydrocyclopenta[c]pyrrole;
(cis)-2-( 1,2, 3,3 a,6,6a-hexahydrocyclopenta[c]pyrrol-4-yl)furo [3,2-
b]pyridine;
(cis)-6-(3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H-isoindole;
(cis)-6-(6-methyl-3-pyridinyl)-2, 3,3 a,4, S,7a-hexahydro- I H-isoindole;
(cis)-6-(6-bromo-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H-isoindole;
(cis)-6-(6-chloro-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H-isoindole;
(cis)-6-(6-fluoro-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H-isoindole;
(cis)-S-(2,3, 3 a,6,7,7a-hexahydro- I H-isoindol-S-yl)nicotinonitrile;
(cis)-6-(S-methyl-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H isoindole;
(cis)-6-(S-chloro-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-IH isoindole;
(cis)-6-(S-fluoro-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-IH-isoindole;
(cis)-6-(S-bromo-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H-isoindole;
(cis)-6-(S-vinyl-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H-isoindole;
(cis)-6-(S-methoxy-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H-isoindole;
(cis)-6-(S-vinyloxy-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-IH-isoindole;
(cis)-6-(S-ethynyl-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H isoindole;
(cis)-6-(S,6-dichloro-3-pyridinyl)-2,3,3 a,4, S,7a-hexahydro-1 H-isoindole;
(cis)-6-(6-bromo-S-chloro-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H-isoindole;
(cis)-S-(2,3,3a,6,7,7a-hexahydro- 1H-isoindol-S-yl)-2-methylnicotinonitrile;
(cis)-2-chloro-S-(2,3,3a,6,7,7a-hexahydro-1H-isoindol-S-yl)nicotinonitrile;
(cis)-2-bromo-S-(2,3,3a,6,7,7a-hexahydro- 1H-isoindol-S-yl)nicotinonitrile;
(cis)-6-(6-chloro-S-methyl-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H-isoindole;
(cis)-6-(6-bromo-S-methyl-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H-isoindole;
(cis)-6-(S-methoxy-6-methyl-3-pyridinyl)-2,3,3a,4,S,7a-hexahydro-1H-isoindole;
(cis)-6-(6-chloro-S-methoxy-3-pyridinyl)-2,3,3a,4,S,7a hexahydro-1H-isoindole;
(cis)-6-(6-bromo-S-methoxy-3-pyridinyl)-2,3,3 a,4, S,7a-hexahydro-1 H-
isoindole;
S3
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-6-(3-methyl-S-isoxazolyl)-2,3,3a,4,S,7a-hexahydro-1H isoindole;
(cis)-2-(2,3,3a,6,7,7a-hexahydro- 1H-isoindol-S-yl)furo[3,2-b]pyridine;
(cis)-S-(3-pyridinyl)-2,3,3 a,4,7,7a-hexahydro-1 H-isoindole;
(cis)-S-(6-methyl-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H-isoindole;
(cis)-S-(6-chloro-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H-isoindole;
(cis)-S-(6-bromo-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H-isoindole;
(cis)-S-(6-fluoro-3-pyridinyl)-2,3,3 a,4,7,7a-hexahydro-1 H-isoindole;
(cis)-S-(2,3,3a,4,7,7a-hexahydro- 1H-isoindol-S-yl)nicotinonitrile;
(cis)-S-(S-methyl-3-pyridinyl)-2,3,3 a,4,7,7a-hexahydro-1 H-isoindole;
(cis)-S-(S-chloro-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H-isoindole;
(cis)-S-(S-fluoro-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H isoindole;
(cis)-S-(S-bromo-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H isoindole;
(cis)-S-(S-vinyl-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H-isoindole;
(cis)-S-(S-methoxy-3-pyridinyl)-2,3,3a,4,7,7a hexahydro-1H-isoindole;
(cis)-S-(S-vinyloxy-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H-isoindole;
(cis)-S-(S-ethynyl-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H-isoindole;
(cis)-S-(S,6-dichloro-3-pyridinyl)-2,3,3a,4,7,7x-hexahydro-1H-isoindole;
(cis)-S-(6-bromo-S-chloro-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H-isoindole;
(cis)-S-(2,3,3a,4,7,7a-hexahydro- 1H-isoindol-5-yl)-2-methylnicotinonitrile;
(cis)-2-chloro-S-(2,3,3 a,4,7,7a-hexahydro-1 H-isoindol-S-yl)nicotinonitrile;
(cis)-2-bromo-5-(2,3,3a,4,7,7a-hexahydro- 1H-isoindol-S-yl)nicotinonitrile;
(cis)-S-(6-chloro-S-methyl-3-pyridinyl)-2,3,3a,4,7,7a hexahydro-1H-isoindole;
(cis)-S-(6-bromo-S-methyl-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H-isoindole;
(cis)-S-(S-methoxy-6-methyl-3-pyridinyl)-2,3,3a,4,7,7a-hexahydro-1H-isoindole;
(cis)-S-(6-chloro-S-methoxy-3-pyridinyl)-2,3,3a,4,7,7a hexahydro-1H-isoindole;
(cis)-S-(6-bromo-S-methoxy-3-pyridinyl)-2,3,3a,4,7,7x-hexahydro-1H-isoindole;
(cis)-2-(2,3,3a,4,7,7a-hexahydro-lH-isoindol-S-yl)furo[3,2-b]pyridine;
(cis)-S-(3-methyl-S-isoxazolyl)-2,3,3a,4,7,7a-hexahydro-1H-isoindole;
S4
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-6-(3-pyridinyl)-3-azabicyclo [3.2.0]hept-6-ene;
(cis)-6-(6-bromo-3-pyridinyl)-3-azabicyclo[3.2.0]hept-6-ene;
(cis)-6-(6-fluoro-3-pyridinyl)-3-azabicyclo[3.2.0]hept-6-ene;
(cis)-6-(6-methyl-3-pyridinyl)-3-azabicyclo [3.2.0]hept-6-ene;
(cis)-5-(3-azabicyclo[3.2.0]hept-6-en-6-yl)nicotinonitrile;
(cis)-6-(5-chloro-3-pyridinyl)-3-azabicyclo[3.2.0]kept-6-ene;
(cis)-6-(5-bromo-3-pyridinyl)-3-azabicyclo [3.2.0]kept-6-ene;
(cis)-6-(5-fluoro-3-pyridinyl)-3-azabicyclo[3.2.0]hept-6-ene;
(cis)-6-(5-methyl-3-pyridinyl)-3-azabicyclo[3.2.0]kept-6-ene;
(cis)-6-(5-vinyl-3-pyridinyl)-3-azabicyclo[3.2.0]kept-6-ene;
(cis)-6-(5-methoxy-3-pyridinyl)-3-azabicyclo [3.2.0]hept-6-ene;
(cis)-6-(5-vinyloxy-3-pyridinyl)-3-azabicyclo[3.2.0]hept-6-ene;
(cis)-6-(5-ethynyl-3-pyridinyl)-3-azabicyclo [3 .2.0]hept-6-ene;
(cis)-6-(6-bromo-5-chloro-3-pyridinyl)-3-azabicyclo [3.2.0]hept-6-ene;
(cis)-5-(3-azabicyclo[3.2.0]hept-6-en-6-yl)-2-methylnicotinonitrile;
(cis)-5-(3-azabicyclo [3.2.0]hept-6-en-6-yl)-2-bromonicotinonitrile;
(cis)-5-(3-azabicyclo[3.2.0]hept-6-en-6-yl)-2-chloronicotinonitrile;
(cis)-6-(6-chloro-5-methyl-3-pyridinyl)-3-azabicyclo[3.2.0]hept-6-ene;
(cis)-6-(6-bromo-5-methyl-3-pyridinyl)-3-azabicyclo[3.2.0]hept-6-ene;
(cis)-6-(5-methoxy-6-methyl-3-pyridinyl)-3-azabicyclo[3.2.0]hept-6-ene;
(cis)-6-(6-bromo-5-methoxy-3-pyridinyl)-3-azabicyclo[3.2.0]hept-6-ene;
(cis)-6-(6-chloro-5-methoxy-3-pyridinyl)-3-azabicyclo[3.2.0]hept-6-ene; or a
pharmaceutically acceptable salt, amide, ester or prodrugs thereof.
In another embodiment of the present invention, compounds of formula (V) are
disclosed
R~
i
N Y R2
i R3
Z R R4
(V),
or a pharmaceutically acceptable salt, amide, ester or prodrug thereof wherein
Y, Z, Rl, RZ, R3,
R4, and RS are as defined in formula (I).
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein Y is a covalent bond; Z is a covalent bond; and Rl, R2, R3, R4, and R~
are as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein Y is a covalent bond; Z is CH2; and Rl, R2, R3, R4, and RS are as
defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein -- represents a double bond; Y is a covalent bond; Z is CH2; R2 is
hydrogen; R3 and
RS are absent; R4 is heterocycle; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein - represents a double bond; Y is a covalent bond; Z is CH2; R2 is
hydrogen; R3 and
RS are absent; R4 is heterocycle wherein the heterocycle is selected from
furyl, imidazolyl,
isothiazolyl, isoxazolyl, oxadiazolyl, oxazolyl, pyrazinyl, pyrazolyl,
pyridinyl, pyrimidinyl,
pyridazinyl, pyrrolyl, tetrazinyl, tetrazolyl, thiadiazolyl, thiazolyl,
thienyl, triazinyl, triazolyl,
benzimidazolyl, benzothiazolyl, benzothienyl, benzoxazolyl, benzofuranyl,
cinnolinyl, faro[2,3-
c]pyridine, faro[3,2-c]pyridine, faro[3,2-b]pyridinyl, faro[2,3-b]pyridinyl,
imidazo[1,2-
a]pyridinyl, indazolyl, indolyl, indolizinyl, naphthyridinyl, isobenzofuranyl,
isobenzothienyl,
isoindolyl, isoquinolinyl, phthalazinyl, quinolinyl, quinolizinyl,
quinoxalinyl, quinazolinyl,
thieno[2,3-c]pyridine, thieno[3,2-c]pyridine, thieno[3,2-b]pyridinyl or
thieno[2,3-b]pyridinyl
wherein the heterocycle is substituted with 0, 1, 2, or 3 substituents
independently selected from
alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl,
alkoxycarbonylalkyl, alkyl,
alkylcarbonyl, alkylcarbonyloxy, alkylthio, alkynyl, carboxy, carboxyalkyl,
cyano, cyanoalkyl,
formyl, formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl,
mercapto,
mercaptoalkyl, nitro, triphenylrnethyl (trityl), -C(NH)NR1oR11, -NR1oR11,
(NR1oR11)alkyl,
(NR1oR11)carbonyl, (NR1oR11)carbonylalkyl, (NK1oR11)sulfonyl, NR12S(O~R13~
-C~12)~13R14WCH2C(NR12)~13R14WC~OR12)Rls~ -C~C~RI2wC~12Rls)Ri4~
-S(O)20R12, or -S(O)2R12; Rlz, R13, and R14 are independently selected from
hydrogen, alkyl,
aryl, or arylalkyl; and Rl, Rlo, and Rl1 are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein --= represents a double bond; Y is a covalent bond; Z is CH2; R2 is
hydrogen; R3 and
RS are absent; R4 is heterocycle wherein the heterocycle is selected from
imidazolyl, isoxazolyl,
56
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
pyridinyl, tetrazolyl, thiadiazolyl, thiazolyl, thienyl, imidazo[1,2
a]pyridinyl, thieno[3,2-
b]pyridinyl or thieno[2,3-b]pyridinyl wherein the heterocycle is substituted
with 0, l, or 2
substituents independently selected from alkenyl, alkenyloxy, alkoxy, alkyl,
alkynyl, cyano,
halogen, vitro, (NR1oR11)sulfonyl, or-C(NH)NR1oR11; and Rl, Rlo, and Ril are
as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein ' represents a double bond; Y is a covalent bond; Z is CH2; R2 is
hydrogen; R3 and
RS are absent; R4 is heterocycle wherein the heterocycle is pyridinyl
substituted with 0, l, or 2
substituents independently selected from alkenyl, alkenyloxy, alkoxy, alkyl,
alkynyl, cyano,
halogen, or vitro; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein =- represents a double bond; Y is a covalent bond; 2 is CH2; RZ is
hydrogen; R3 and
RS are absent; R4 is heterocycle wherein the heterocycle is selected from 3-
pyridinyl, 6-bromo-3-
pyridinyl, 6-chloro-3-pyridinyl, 6-fluoro-3 pyridinyl, 6-methyl-3-pyridinyl, 5-
cyano-3-pyxidinyl,
5-methyl-3-pyridinyl, 5-chloro-3-pyridinyl, 5-fluoro-3-pyridinyl, 5-bromo-3-
pyridinyl, 5-vinyl-
3-pyridinyl, 5-methoxy-3-pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-3-
pyridinyl, 5,6-dichloro-
3-pyridinyl, 6-bromo-5-chloro-3-pyridinyl, 5-cyano-6-methyl-3-pyridinyl, 6-
chloro-5-cyano-3-
pyridinyl, 6-bromo-5-cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl, 6-bromo-
5-methyl-3-
pyridinyl, 5-methoxy-6-methyl-3-pyridinyl, 6-chloro-5-methoxy-3-pyridinyl, or
6-bromo-5-
methoxy-3-pyridinyl; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein -- represents a double bond; Y is a covalent bond; Z is CH2; R2 is
heterocycle; R3 and
RS are absent; R4 is hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein =- represents a double bond; Y is a covalent band; Z is CHZ; R~ is
heterocycle wherein
the heterocycle is selected from furyl, imidazolyl, isothiazolyl, isoxazolyl,
oxadiazolyl, oxazolyl,
pyrazinyl, pyrazolyl, pyridinyl, pyrimidinyl, pyridazinyl, pyrrolyl,
tetrazinyl, tetrazolyl,
thiadiazolyl, thiazolyl, thienyl, triazinyl, triazolyl, benzimidazolyl,
benzothiazolyl, benzothienyl,
benzoxazolyl, benzofuranyl, cinnolinyl, faro[2,3-c]pyridine, faro[3,2-
c]pyridine, faro[3,2-
b]pyridinyl, faro[2,3-b]pyridinyl, imidazo[1,2-a]pyridinyl, indazolyl,
indolyl, indolizinyl,
57
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
naphthyridinyl, isobenzofuranyl, isobenzothienyl, isoindolyl, isoquinolinyl,
phthalazinyl,
quinolinyl, quinolizinyl, quinoxalinyl, quinazolinyl, thieno[2,3-c]pyridine,
thieno[3,2-c]pyridine,
thieno[3,2-b]pyridinyl or thieno[2,3-b]pyridinyl wherein the heterocycle is
substituted with 0, 1,
2, or 3 substituents independently selected from alkenyl, alkoxy,
alkoxyalkoxy, alkoxyalkyl,
alkoxycarbonyl, alkoxycarbonylalkyl, alkyl, alkylcarbonyl, alkylcarbonyloxy,
alkylthio, alkynyl,
carboxy, carboxyalkyl, cyano, cyanoalkyl, formyl, formylalkyl, haloalkoxy,
haloalkyl, halogen,
hydroxy, hydroxyallcyl, mercapto, mercaptoalkyl, vitro, triphenylmethyl
(trityl),-C(NH)NR1oR11,
-NRioRtl, (NRIORn)alk3'1, (NR1oR11)carbonyl, (NRIORn)carbonylalkyl,
(NR1oR11)sulfonyl,
-~12s(O)2R13~ -C~12)~13RI4~ -CH2C(1~R12)~13R14~ -C~OR12)R13, -C~C~R12~
-C~12R13)R14~ -S(O)zORl2, or -S(O)2R12; R3 and RS axe absent; IZ4 is hydrogen;
R12, R13, and
R14 are independently selected from hydrogen, alkyl, aryl, or arylalkyl; and
Rv Rl o, and Rl 1 are
as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein -- represents a double bond; Y is a covalent bond; Z is CH2; R2 is
heterocycle wherein
the heterocycle is selected from imidazolyl, isoxazolyl, pyridinyl,
tetrazolyl, thiadiazolyl,
thiazolyl, thienyl, imidazo[1,2-a]pyridinyl, thieno[3,2-b]pyridinyl or
thieno[2,3-b]pyridinyl
wherein the heterocycle is substituted with 0, 1, or 2 substituents
independently selected from
alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen, vitro,
(NR1oR11)sulfonyl, or
-C(NH)NR1oR11; Rs and RS are absent; R4 is hydrogen; and Rl, Rlo, and Rl1 are
as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein -- represents a double bond; Y is a covalent bond; Z is CH2; R2 is
heterocycle wherein
the heterocycle is pyridinyl substituted with 0, 1, or 2 substituents
independently selected from
alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen, or vitro; R3 and
RS are absent; R4 is
hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
whexein -- represents a double bond; Y is a covalent bond; Z is CH2; R2 is
heterocycle wherein
the heterocycle is selected from 3-pyridinyl, 6-bromo-3-pyridinyl, 6-chloro-3-
pyridinyl, 6-
fluoro-3 pyridinyl, 6-methyl-3-pyridinyl, 5-cyano-3-pyridinyl, 5-methyl-3-
pyridinyl, 5-chloro-3-
pyridinyl, 5-fluoro-3-pyridinyl, 5-bromo-3-pyridinyl, 5-vinyl-3-pyridinyl, 5-
methoxy-3-
58
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-3-pyxidinyl, 5,6-dichloro-3-
pyridinyl, 6-bromo-5-
chloro-3-pyridinyl, 5-cyano-6-methyl-3-pyridinyl, 6-chloro-5-cyano-3-
pyridinyl, 6-bxomo-5-
cyano-3-pyridinyl, 6-chloro-S-methyl-3-pyridinyl, 6-bromo-5-methyl-3-
pyridinyl, 5-methoxy-6-
methyl-3-pyridinyl, 6-chloro-5-methoxy-3-pyridinyl, or 6-bromo-5-methoxy-3-
pyridinyl; R3 and
RS are absent; R~ is hydrogen; and R~ is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
whexein Y is CH2; Z is a covalent bond; and RI, RZ, R3, R4, and RS are as
defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein - represents a double bond; Y is CH2; Z is a covalent bond; R2 is
heterocycle; R3 and
RS are absent; R4 is hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein -' represents a double bond; Y is CH2; Z is a covalent bond; RZ is
heterocycle wherein
the heterocycle is selected from furyl, imidazolyl, isothiazolyl, isoxazolyl,
oxadiazolyl, oxazolyl,
pyrazinyl, pyxazolyl, pyridinyl, pyrimidinyl, pyridazinyl, pyrrolyl,
tetrazinyl, tetrazolyl,
thiadiazolyl, thiazolyl, tluenyl, triazinyl, triazolyl, benzimidazolyl,
benzothiazolyl, benzothienyl,
benzoxazolyl, benzofuranyl, cinnolinyl, faro[2,3-c]pyridine, faro[3,2-
c]pyridine, faro[3,2-
b]pyridinyl, faro[2,3-b]pyridinyl, imidazo[1,2-a]pyridinyl, indazolyl,
indolyl, indolizinyl,
naphthyridinyl, isobenzofuranyl, isobenzothienyl, isoindolyl, isoquinolinyl,
phthalazinyl,
quinolinyl, quinolizinyl, quinoxalinyl, quinazolinyl, thieno[2,3-c]pyridine,
thieno[3,2-c]pyridine,
thieno[3,2-bJpyridinyl or thieno[2,3-b)pyxidinyl wherein the heterocycle is
substituted with 0, 1,
2, or 3 substituents independently selected from alkenyl, alkoxy,
alkoxyalkoxy, alkoxyalkyl,
alkoxycaxbonyl, alkoxycarbonylalkyl, alkyl, alkylcarbonyl, alkylcarbonyloxy,
alkylthio, alkynyl,
carboxy, carboxyalkyl, cyano, cyanoalkyl, formyl, formylalkyl, haloalkoxy,
haloalkyl, halogen,
hydroxy, hydroxyalkyl, mercapto, mercaptoalkyl, nitro, triphenylmethyl
(trityl), -C(NH)NRI oRi ~,
-NR1oR11, (NRIORn)alkyl, (NRloRlr)carbonyl, (NR1oR11)carbonylalkyl,
(NRIORu)sulfonyl,
-NRizS(~)zR~s~ 'C~12)NR13R14~ -CH2C(NR12)~RI3RI4~ -C~ORIZ)R13, -C~CN)Rla,
-C(~12R13)RI4~ -S(o)2~R12~ or -S(O)ZR12; R3 and RS are absent; R4 is hydrogen;
R12, RI3, and
Rl~ are independently selected from hydrogen, alkyl, aryl, or arylalkyl; and
Rv Rlo, and Rll are
as defined in formula (I).
59
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein - represents a double bond; Y is CH2; Z is a covalent bond; RZ is
heterocycle wherein
the heterocycle is selected from imidazolyl, isoxazolyl, pyridinyl,
tetrazolyl, thiadiazolyl,
thiazolyl, thienyl, imidazo[1,2-a]pyridinyl, thieno[3,2-b]pyridinyl or
thieno[2,3-b]pyridinyl
wherein the heterocycle is substituted with 0, 1, or 2 substituents
independently selected from
alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen, nitro,
(NR1oR11)sulfonyl, or
-C(NH)NRIORW R3 and RS are absent; R4 is hydrogen; and Rl, Rlo, and Rl1 are as
defined in
formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein -- represents a double bond; Y is CH2; Z is a covalent bond; R2 is
heterocycle wherein
the heterocycle is pyridinyl substituted with 0, 1, or 2 substituents
independently selected from
alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen, or vitro; R3 and
RS are absent; R~ is
hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein -- represents a double bond; Y is CH2; Z is a covalent bond; R2 is
heterocycle wherein
the heterocycle is selected from 3-pyridinyl, 6-bromo-3-pyridinyl, 6-chloro-3-
pyridinyl, 6-
fluoro-3 pyridinyl, 6-methyl-3-pyridinyl, 5-cyano-3-pyridinyl, 5-methyl-3-
pyridinyl, 5-chloro-3-
pyridinyl, 5-fluoro-3-pyridinyl, 5-bromo-3-pyridinyl, 5-vinyl-3-pyridinyl, 5-
methoxy-3-
pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-3-pyridinyl, 5,6-dichloro-3-
pyridinyl, 6-bromo-5-
chloro-3-pyridinyl, 5-cyano-6-methyl-3-pyridinyl, 6-chloro-5-cyano-3-
pyridinyl, 6-bromo-5-
cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl, 6-bromo-5-methyl-3-
pyridinyl, 5-methoxy-6-
methyl-3-pyridinyl, 6-chloro-5-methoxy-3-pyridinyl, or 6-bromo-5-methoxy-3-
pyridinyl; R3 and
RS are absent; R4 is hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein Y is a covalent bond; Z is CH2CH2; and Ri, R2, R3, R4, and RS are as
defined in formula
(I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein =- represents a double bond; Y is a covalent bond; Z is CH2CH2; R2 is
hydrogen; R3
and RS are absent; R4 is heterocycle; and Rl is as defined in formula (I).
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein -- represents a double bond; Y is a covalent bond; Z is CH2CH2; R2 is
hydrogen; R3
and RS are absent; R4 is heterocycle Wherein the heterocycle is selected from
furyl, imidazolyl,
isothiazolyl, isoxazolyl, oxadiazolyl, oxazolyl, pyrazinyl, pyrazolyl,
pyridinyl, pyrimidinyl,
pyridazinyl, pyrrolyl, tetrazinyl, tetrazolyl, thiadiazolyl, thiazolyl,
thienyl, triazinyl, triazolyl,
benzimidazolyl, benzothiazolyl, benzothienyl, benzoxazolyl, benzofuranyl,
cinnolinyl, faro[2,3-
c]pyridine, faro[3,2-c]pyridine, faro[3,2-b]pyridinyl, faro[2,3-b]pyridinyl,
imidazo[1,2-
a]pyridinyl, indazolyl, indolyl, indolizinyl, naphthyridinyl, isobenzofuranyl,
isobenzothienyl,
isoindolyl, isoquinolinyl, phthalazinyl, quinolinyl, quinolizinyl,
quinoxalinyl, quinazolinyl,
thieno[2,3-c]pyridine, thieno[3,2-c]pyridine, thieno[3,2-b]pyridinyl or
thieno[2,3-b]pyridinyl
wherein the heterocycle is substituted with 0, 1, 2, or 3 substituents
independently selected from
alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl,
alkoxycarbonylalkyl, alkyl,
alkylcarbonyl, alkylcarbonyloxy, alkylthio, alkynyl, carboxy, carboxyalkyl,
cyano, cyanoalkyl,
formyl, formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl,
mercapto,
mercaptoalkyl, nitro, triphenylinethyl (trityl), -C(NH)NR1oR11, -NR1oR11,
(NR1oR11)alkyl,
(NR1oR11)carbonyl, (NR1oR11)carbonylalkyl, (NR1oR11)sulfonyl, NR12S(O~R13,
-C(NRiz)~13R14~ -CH2C(1~R12)~13R14~ -C~~R12)R13~ -C~C~R12WL~12Ri3)R14~
-S(O)20R12, or -S(O)2R12; R12, R13, and R14 are independently selected from
hydrogen, alkyl,
aryl, or arylalkyl; and Rl, Rlo, and Rl1 are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein - represents a double bond; Y is a covalent bond; Z is CH2CH2; R2 is
hydrogen; R3
and RS are absent; R4 is heterocycle wherein the heterocycle is selected from
imidazolyl,
isoxazolyl, pyridinyl, tetrazolyl, thiadiazolyl, thiazolyl, thienyl,
imidazo[1,2 a]pyridinyl,
thieno[3,2-b]pyridinyl or thieno[2,3-b]pyridinyl wherein the heterocycle is
substituted with 0, 1,
or 2 substituents independently selected from alkenyl, alkenyloxy, alkoxy,
alkyl, alkynyl, cyano,
halogen, nitro, (NR1oR11)sulfonyl, or -C(N~I)NR1oR11; and Rl, Rlo, and Rl1 are
as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein -= represents a double bond; Y is a covalent bond; Z is CH2CH2; R2 is
hydrogen; R3
and RS are absent; R4 is heterocycle wherein the heterocycle is pyridinyl
substituted with 0, 1, or
61
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
2 substituents independently selected from alkenyl, alkenyloxy, alkoxy, alkyl,
alkynyl, cyano,
halogen, or vitro; and RI is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein - represents a double bond; Y is a covalent bond; Z is CHZCH2; R2 is
hydrogen; R3
and RS are absent; R4 is heterocycle wherein the heterocycle is selected from
3-pyridinyl, 6-
bromo-3-pyridinyl, 6-chloro-3-pyridinyl, 6-fluoro-3 pyridinyl, 6-methyl-3-
pyridinyl, S-cyano-3-
pyridinyl, S-methyl-3-pyridinyl, S-chloro-3-pyridinyl, S-fluoro-3-pyridinyl, S-
bromo-3-pyridinyl,
S-vinyl-3-pyridinyl, S-methoxy-3-pyridinyl, S-vinyloxy-3-pyridinyl, S-ethynyl-
3-pyridinyl, S,6-
dichloro-3-pyridinyl, 6-bromo-S-chloro-3-pyridinyl, S-cyano-6-methyl-3-
pyridinyl, 6-chloro-S-
cyano-3-pyridinyl, 6-bromo-S-cyano-3-pyridinyl, 6-chloro-S-methyl-3-pyridinyl,
6-bromo-S-
methyl-3-pyridinyl, S-methoxy-6-methyl-3-pyridinyl, 6-chloxo-S-methoxy-3-
pyridinyl, or 6-
bromo-S-methoxy-3-pyridinyl; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein -- represents a double bond; Y is a covalent bond; Z is CH2CH2; R2 is
heterocycle; R3
and RS are absent; R4 is hydrogen; and RI is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein -- represents a double bond; Y is a covalent bond; Z is CH2CH2; R2 is
heterocycle
wherein the heterocycle is selected from furyl, imidazolyl, isothiazolyl,
isoxazolyl, oxadiazolyl,
oxazolyl, pyrazinyl, pyrazolyl, pyridinyl, pyrimidinyl, pyridazinyl, pyrrolyl,
tetrazinyl, tetrazolyl,
thiadiazolyl, thiazolyl, thienyl, triazinyl, triazolyl, benzimidazolyl,
benzothiazolyl, benzothienyl,
benzoxazolyl, benzofuranyl, cinnolinyl, faro[2,3-c]pyridine, faro[3,2-
c]pyridine, faro[3,2-
b]pyridinyl, faro[2,3-b]pyridinyl, imidazo[1,2-a]pyridinyl, indazolyl,
indolyl, indolizinyl,
naphthyridinyl, isobenzofuranyl, isobenzothienyl, isoindolyl, isoquinolinyl,
phthalazinyl,
quinolinyl, quinolizinyl, quinoxalinyl, quinazolinyl, thieno[2,3-c]pyridine,
thieno[3,2-c]pyridine,
thieno[3,2-b]pyridinyl or thieno[2,3-b]pyridinyl wherein the heterocycle is
substituted with 0, 1,
2, or 3 substituents independently selected from alkenyl, alkoxy,
alkoxyalkoxy, alkoxyalkyl,
alkoxycarbonyl, alkoxycarbonylalkyl, alkyl, alkylcarbonyl, alkylcarbonyloxy,
alkylthio, alkynyl,
carboxy, carboxyalkyl, cyano, cyanoalkyl, formyl, formylalkyl, haloalkoxy,
haloalkyl, halogen,
hydroxy, hydroxyalkyl, mercapto, mercaptoalkyl, vitro, triphenylmethyl
(trityl),-C(NH)NR1oR11,
-NRIORm (NW oRu)alkyl, (NR1oR11)carbonyl, (NRIORII)carbonylalkyl,
(NK1oR11)sulfonyl,
62
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
-~125(~)2R13a -C~12)~13R14a -CH2~~12)~13R14, -C(NORIZ)Rl3a -C(NCI~RI2,
-C(NNR12R13)Rl4a 'S(~)2ORla, or -S(O)2R12; R3 and RS are absent; R4 is
hydrogen; R12, R13, and
R14 are independently selected from hydrogen, alkyl, aryl, or arylalkyl; and
Rv Rlo, and Rl1 are
as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein -= represents a double bond; Y is a covalent bond; Z is CH2CH2; R2 is
heterocycle
wherein the heterocycle is selected from imidazolyl, isoxazolyl, pyridinyl,
tetrazolyl,
thiadiazolyl, thiazolyl, thienyl, imidazo[1,2-a]pyridinyl, thieno[3,2
b]pyridinyl or thieno[2,3-
b]pyridinyl wherein the heterocycle is substituted with 0, 1, or 2
substituents independently
selected from alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen,
vitro,
(NR1oR11)sulfonyl, or-C(NH)NR1oR11; R3 and RS are absent; R4 is hydrogen; and
Rl, Rlo, and
Rl1 are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein - represents a double bond; Y is a covalent bond; Z is CHZCH2; R2 is
heterocycle
wherein the heterocycle is pyridinyl substituted with 0, 1, or 2 substituents
independently
selected from alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen, or
vitro; R3 and R5 are
absent; R4 is hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein - represents a double bond; Y is a covalent bond; Z is CHZCH~; R2 is
heterocycle
wherein the heterocycle is selected from 3-pyridinyl, 6-bromo-3-pyridinyl, 6-
chloro-3-pyridinyl,
6-fluoro-3 pyridinyl, 6-methyl-3-pyridinyl, 5-cyana-3-pyridinyl, 5-methyl-3-
pyridinyl, 5-chloro-
3-pyridinyl, S-fluoxo-3-pyridinyl, 5-bromo-3-pyridinyl, 5-vinyl-3-pyridinyl, 5-
methoxy-3-
pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-3-pyridinyl, 5,6-dichloro-3-
pyridinyl, 6-bromo-5-
chloro-3-pyridinyl, 5-cyano-6-methyl-3-pyridinyl, 6-chloro-5-cyano-3-
pyridinyl, 6-bromo-5-
cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl, 6-bromo-5-methyl-3-
pyridinyl, 5-methoxy-6-
methyl-3-pyridinyl, 6-chloro-5-methoxy-3-pyridinyl, or 6-bromo-5-methoxy-3-
pyridinyl; R3 and
RS are absent; R4 is hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein Y is CH2; Z is CH2; and Rl, R2, R3, R4, and RS are as defined in
formula (I).
63
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein - represents a double bond; Y is CH2; Z is CH2; R2 is heterocycle; R3
and RS are
absent; R4 is hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein - represents a double bond; Y is CH2; Z is CH2; R2 is heterocycle
wherein the
heterocycle is selected from fiuryl, imidazolyl, isothiazolyl, isoxazolyl,
oxadiazolyl, oxazolyl,
pyrazinyl, pyrazolyl, pyridinyl, pyrimidinyl, pyridazinyl, pyrrolyl,
tetrazinyl, tetrazolyl,
thiadiazolyl, thiazolyl, thienyl, triazinyl, triazolyl, benzimidazolyl,
benzothiazolyl, benzothienyl,
benzoxazolyl, benzofuranyl, cinnolinyl, faro[2,3-c]pyridine, faro[3,2-
c]pyridine, faro[3,2-
b]pyridinyl, faro[2,3-b]pyridinyl, imidazo[1,2-a]pyridinyl, indazolyl,
indolyl, indolizinyl,
naphthyridinyl, isobenzofuranyl, isobenzothienyl, isoindolyl, isoquinolinyl,
phthalazinyl,
quinolinyl, quinolizinyl, quinoxalinyl, quinazolinyl, thieno[2,3-c]pyridine,
thieno[3,2-c]pyridine,
thieno[3,2-b]pyridinyl or thieno[2,3-b]pyridinyl wherein the heterocycle is
substituted with 0, l,
2, or 3 substituents independently selected from alkenyl, alkoxy,
alkoxyalkoxy, alkoxyalkyl,
alkoxycarbonyl, alkoxycarbonylalkyl, alkyl, alkylcarbonyl, alkylcarbonyloxy,
alkylthio, alkynyl,
carboxy, carboxyalkyl, cyano, cyanoalkyl, formyl, formylalkyl, haloalkoxy,
haloalkyl, halogen,
hydroxy, hydroxyalkyl, mercapto, mercaptoalkyl, vitro, triphenylmethyl
(trityl),-C(NH)NR1oR11,
-NR1oR11~ (NR1oR11)alkyl, (NRIORI)carbonyl, (NK1oR11)carbonylalkyl,
(NR1oR11)sulfonyl,
-~125(W2R13~ -G~12y13R14~ -CH2G(1~R12)~13R14, -C(NOR12)R13~ -C(NCI~R12,
-C(~R12R13~RI4~ -s(~)2OR12, or -S(O)2R12; R3 and RS are absent; R4 is
hydrogen; R12, R13, and
R14 are independently selected from hydrogen, alkyl, aryl, or arylalkyl; and
Rv Rlo, and Rl 1 are
as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein - represents a double bond; Y is CH2; Z is CH2; R2 is heterocycle
wherein the
heterocycle is selected from imidazolyl, isoxazolyl, pyridinyl, tetrazolyl,
thiadiazolyl, thiazolyl,
thienyl, imidazo[1,2-a]pyridinyl, thieno[3,2-b]pyridinyl or thieno[2,3-
b]pyridinyl wherein the
heterocycle is substituted with 0, 1, or 2 substituents independently selected
from alkenyl,
alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen, vitro, (NR1oR11)sulfonyl,
or-C(NH)NR1oR11;
R3 and RS are absent; R4 is hydrogen; and Rl, Rlo, and Rl1 are as defined in
formula (I).
64
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein - represents a double bond; Y is CH2; Z is CHZ; R2 is heterocycle
wherein the
heterocycle is pyridinyl substituted with 0, 1, or 2 substituents
independently selected from
alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen, or nitro; R3 and
RS are absent; R4 is
hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein -- represents a double bond; Y is CH2; Z is CH2; R2 is heterocycle
wherein the
heterocycle is selected from 3-pyridinyl, 6-bromo-3-pyridinyl, 6-chloro-3-
pyridinyl, 6-fluoro-3
pyridinyl, 6-methyl-3-pyridinyl, S-cyano-3-pyridinyl, S-methyl-3-pyridinyl, S-
chloro-3-
pyridinyl, S-fluoro-3-pyridinyl, S-bromo-3-pyridinyl, 5-vinyl-3-pyridinyl, S-
methoxy-3-
pyridinyl, S-vinyloxy-3-pyridinyl, S-ethynyl-3-pyridinyl, S,6-dichloro-3-
pyridinyl, 6-bromo-S-
chloro-3-pyridinyl, S-cyano-6-methyl-3-pyridinyl, 6-chloro-S-cyano-3-
pyridinyl, 6-bromo-S-
cyano-3-pyridinyl, 6-chloro-S-methyl-3-pyridinyl, 6-bromo-S-methyl-3-
pyridinyl, S-methoxy-6-
methyl-3-pyridinyl, 6-chloro-S-methoxy-3-pyridinyl, or 6-bromo-5-methoxy-3-
pyridinyl; R3 and
RS are absent; R4 is hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (V) are
disclosed
wherein Y is CH2CH2; Z is a covalent bond; and Rl, RZ, R3, R4, and RS are as
defined in formula
(I).
Representative compounds of formula (V) include, but are not limited to:
(cis)-8-(3-pyridinyl)-I,2,3,4,4a,S,6,8a-octahydroquinoline;
(cis)-8-(6-chloro-3-pyridinyl)-1,2,3,4,4a,S,6,8a-octahydroquinoline;
(cis)-8-(6-bromo-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-8-(6-fluoro-3-pyridinyl)-1,2,3,4,4a, S, 6, 8a-octahydroquinoline;
(cis)-8-(6-methyl-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-S-(1,2,3,4,4a,S,6,8a-octahydro-8-quinolinyl)nicotinonitrile;
(cis)-8-(S-methyl-3-pyridinyl)-1,2,3,4,4a,S,6,8a-octahydroquinoline;
(cis)-8-(S-chloro-3-pyridinyl)-1,2,3,4,4a,S,6,8a-octahydroquinoline;
(cis)-8-(S-fluoro-3-pyridinyl)-1,2,3,4,4a,S,6,8a-octahydroquinoline;
(cis)-8-(S-bromo-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroquinoline;
6S
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-8-(5-vinyl-3-pyridinyl)-1,2,3,4,4a, 5, 6, 8a-octahydroquinoline;
(cis)-8-(S-methoxy-3-pyridinyl)-1,2,3,4,4a,5,6,8a octahydroquinoline;
(cis)-8-(5-vinyloxy-3-pyridinyl)-I,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-8-(5-ethynyl-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-8-(5,6-dichloro-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-8-(6-bromo-5-chloro-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-2-methyl-5-(1,2,3,4,4a,5,6,8a-octahydro-8-quinolinyl)nicotinonitrile;
(cis)-2-bromo--5-(1,2,3,4,4a,5,6,8a-octahydro-8-quinolinyl)nicotinonitrile;
(cis)-2-chloro-5-(1,2,3,4,4a,5,6,8a-octahydro-8-quinolinyl)nicotinonitrile;
(cis)-8-(6-chloro-5-methyl-3-pyridinyl)-1,2,3,4,4a,5,6,8a octahydroquinoline;
(cis)-8-(5-methoxy-6-methyl-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-8-(6-chloro-5-methoxy-3-pyridinyl)-1,2,3,4,4a,5,6,8a octahydroquinoline;
(cis)-8-(6-bromo-5-methoxy-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-8-(3-methyl-5-isoxazolyl)-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-8-faro[3,2-b]pyridin-2-yl-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-7-(3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-cyclopenta[b]pyridine;
(cis)-7-(6-chloro-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-7-(6-bromo-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-7-(6-fluoro-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-7-(6-methyl-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H
cyclopenta[b]pyridine;
(cis)-5-(2,3,4,4a,5,7a-hexahydro- 1H-cyclopenta[b]pyridin-7-
yl)nicotinonitrile;
(cis)-7-(5-methyl-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-IH
cyclopenta[b]pyridine;
(cis)-7-(5-chloro-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-IH-
cyclopenta[b]pyridine;
(cis)-7-(5-bromo-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H
cyclopenta[b]pyridine;
(cis)-7-(5-fluoro-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-7-(5-vinyl-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-7-(5-methoxy-3-pyridinyl)-2,3,4,4a, 5,7a-hexahydro-1 H-
cyclopenta[b]pyridine;
(cis)-7-(5-vinyloxy-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-IH-
cyclopenta[b]pyridine;
(cis)-7-(5-ethynyl-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-IH
cyclopenta[b]pyridine;
66
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-7-(5,6-dichloro-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-7-(6-bromo-5-chloro-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-5-(2,3,4,4a,5,7a-hexahydro- 1H-cyclopenta[b]pyridin-7-yl)-2-
methylnicotinonitrile;
(cis)-2-bromo-5-(2,3,4,4a,5,7a-hexahydro- 1 H-cyclopenta[b]pyridin-7-
yl)nicotinonitrile;
(cis)-2-chloro-5-(2,3,4,4a,5,7a-hexahydro-1H-cyclopenta[b]pyridin 7-
yl)nicotinonitrile;
(cis)-7-(6-chloro-5-methyl-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-7-(6-bromo-5-methyl-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-7-(5-methoxy-6-methyl-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H
cyclopenta[b]pyridine;
(cis)-7-(6-chloro-5-methoxy-3-pyridinyl)-2,3,4,4a, 5,7a-hexahydro-1 H-
cyclopenta[b]pyridine;
(cis)-7-(6-bromo-5-methoxy-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-7-(3-methyl-5-isoxazolyl)-2,3,4,4a,5,7a-hexahydro-1H
cyclopenta[b]pyridine;
(cis)-2-(2,3,4,4a,5,7a-hexahydro- 1H-cyclopenta[b]pyridin-7-yl)furo[3,2-
b]pyridine;
(cis)-7-(3-pyridinyl)-1,2,3,4,4a, 5, 6, 8 a-octahydroquinoline;
(cis)-7-(6-chloro-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-7-(6-bromo-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-7-(6-fluoro-3-pyridinyl)-1,2,3,4,4a,5,6, 8a-octahydroquinoline;
(cis)-7-(6-methyl-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-5-(1,2,3,4,4a,5,6,8a octahydro-7-quinolinyl)nicotinonitrile;
(cis)-7-(5-methyl-3-pyridinyl)-1,2,3,4,4a,5,6, 8a-octahydroquinoline;
(cis)-7-(5-chloro-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-7-(5-fluoro-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-7-(5-bromo-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-7-(5-vinyl-3-pyridinyl)-1,2, 3,4,4a,5,6, 8a-octahydroquinoline;
67
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-7-(5-methoxy-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-7-(5-vinyloxy-3-pyridinyl)-1,2,3,4,4a, 5, 6, 8a-octahydroquinoline;
(cis)-7-(5-ethynyl-3-pyridinyl)-1,2,3,4,4a, 5,6, 8 a-octahydroquinoline;
(cis)-2-methyl-5-(1,2,3,4,4a,5,6,8a-octahydro-7-quinolinyl)nicotinonitrile;
(cis)-2-bromo-5-(I,2,3,4,4a,5,6,8a-octahydro-7-quinolinyl)nicotinonitrile;
(cis)-2-chloro-5-(1,2,3,4,4a,5,6,8a-octahydro-7-quinolinyl)nicotinonitrile;
(cis)-7-(S,6-dichloro-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-7-(6-bromo-5-chloro-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-7-(6-chloro-5-methyl-3-pyridinyl)-1,2,3,4,4a,5,6,8a octahydroquinoline;
(cis)-7-(6-bromo-5-methyl-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-7-(5-methoxy-6-methyl-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-7-(6-chloro-5-methoxy-3-pyridinyl)-1,2,3,4,4a,5,6,8a octahydroquinoline;
(cis)-7-(6-bromo-5-methoxy-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-7-faro[3,2-b]pyridin-2-yl-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-7-(3-methyl-5-isoxazolyl)-1,2,3,4,4a,5,6,8a-octahydroquinoline;
(cis)-6-(3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-cyclopenta[b]pyridine;
(cis)-6-(5-chloro-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[bJpyridine;
(cis)-6-(5-bromo-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-IH
cyclopenta[b]pyridine;
(cis)-6-(5-fluoro-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-6-(5-methyl-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H
cyclopenta[b]pyridine;
(cis)-5-(2,3,4,4a,5,7a-hexahydro-1H-cyclopenta[bJpyridin-6-yl)nicotinonitrile;
(cis)-6-(5-methyl-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-6-(5-chloro-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-6-(5-fluoro-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-6-(5-bromo-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-6-(5-vinyl-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-6-(5-methoxy-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-IH-
cyclopenta[b)pyridine;
(cis)-6-(5-vinyloxy-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-6-(5-ethynyl-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-IH-
cyclopenta[b]pyridine;
68
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-6-(5,6-dichloro-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-6-(6-bromo-5-chloro-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-5-(2,3,4,4a,5,7a-hexahydro- 1H-cyclopenta[b]pyridin-6-yl)-2-
methylnicotinonitrile;
(cis)-2-bromo-5-(2,3,4,4a,5,7a-hexahydro- I H-cyclopenta[b]pyridin-6-
yl)nicotinonitrile;
(cis)-2-chloro-5-(2,3,4,4a,5,7a-hexahydro-1H-cyclopenta[b]pyridin 6-
yl)nicotinonitrile;
(cis)-6-(6-chloro-5-methyl-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-6-(6-bromo-5-methyl-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-6-(5-methoxy-6-methyl-3-pyridinyl)-2, 3,4,4a,5,7a-hexahydro- I H
cyclopenta[b]pyridine;
(cis)-6-(6-chloro-5-methoxy-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-6-(6-bromo-5-methoxy-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-6-(3-methyl-5-isoxazolyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-2-(2,3,4,4a,5,7a-hexahydro-1H-cyclopenta[b]pyridin-6-yl)furo[3,2-
b]pyridine;
(cis)-7-(3-pyridinyl)-1,2,3,4,4a,5,8,8a-octahydroquinoline;
(cis)-7-(6-bromo-3-pyridinyl)-I,2,3,4,4a,5,8,8a-octahydroquinoline;
(cis)-7-(6-chloro-3-pyridinyl)-1,2,3,4,4a,5,8,8a-octahydroquinoline;
(cis)-7-(6-fluoro-3-pyridinyl)-1,2, 3,4,4a, 5, 8, 8a-octahydroquinoline;
(cis)-7-(6-methyl-3-pyridinyl)-1,2,3,4,4a,5,8,8a-octahydroquinoline;
(cis)-5-(1,2,3,4,4a,5,8,8a-octahydro-7-quinolinyl)nicotinonitrile;
(cis)-7-(5-methyl-3-pyridinyl)-I,2,3,4,4a,5,8,8a-octahydroquinoline;
(cis)-7-(5-chloro-3-pyridinyl)-I,2,3,4,4a,5,8,8a-octahydroquinoline;
(cis)-7-(5-fluoro-3-pyridinyl)-1,2,3,4,4a,5,8,8a-octahydroquinoline;
(cis)-7-(5-bromo-3-pyridinyl)-1,2,3,4,4a,5,8,8a-octahydroquinoline;
(cis)-7-(5-vinyl-3-pyridinyl)-1,2,3,4,4a,5,8,8a-octahydroquinoline;
69
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-7-(5-methoxy-3-pyridinyl)-1,2,3,4,4a,5,8,8a-octahydroquinoline;
(cis)-7-(5-vinyloxy-3-pyridinyl)-1,2,3,4,4a, 5, 8, 8 a-octahydroquinoline;
(cis)-7-(5-ethynyl-3-pyridinyl)-1,2,3,4,4a,5,8,8a-octahydroquinoline;
(cis)-7-(5,6-dichloro-3-pyridinyl)-1,2,3,4,4a,5,8,8a-octahydroquinoline;
(cis)-7-(6-bromo-5-chloro-3-pyridinyl)-1,2, 3,4,4a, 5, 8, 8 a-
octahydroquinoline;
(cis)-2-methyl-5-(1,2,3,4,4a,5,8,8a-octahydro-7-quinolinyl)nicotinonitrile;
(cis)-2-bromo-5-(1,2,3,4,4a,5,8,8a-octahydro-7-quinolinyl)nicotinonitrile;
(cis)-2-chloro-5-(1,2,3,4,4a,5,8,8a-octahydro-7-quinolinyl)nicotinonitrile;
(cis)-7-(6-chloro-5-methyl-3-pyridinyl)-1,2,3,4,4a,5,8,8a octahydroquinoline;
(cis)-7-(6-bromo-5-methyl-3-pyridinyl)-1,2,3,4,4a,5,8,8a-octahydroquinoline;
(cis)-7-(5-methoxy-6-methyl-3-pyridinyl)-1,2,3,4,4a,5,8,8a-octahydroquinoline;
(cis)-7-(6-chloro-5-methoxy-3-pyridinyl)-1,2,3,4,4a,5,8,8a octahydroquinoline;
(cis)-7-(6-bromo-5-methoxy-3-pyridinyl)-1,2, 3,4,4a, 5, 8, 8a-octahydro
quinoline;
(cis)-7-(3-methyl-5-isoxazolyl)-1,2,3,4,4a,5,8,8a-octahydroquinoline;
(cis)-7-faro[3,2-b]pyridin-2-yl-1,2,3,4,4a,5,8,8a-octahydroquinoline;
(cis)-6-(3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H-cyclopenta[b]pyridine;
(cis)-6-(6-chloro-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-6-(6-fluoro-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1 H-
cyclopenta[b]pyridine;
(cis)-6-(6-bromo-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H
cyclopenta[b]pyridine;
(cis)-6-(6-methyl-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H
cyclopenta[b]pyridine;
(cis)-5-(2,3,4,4a,7,7a-hexahydro-lH-cyclopenta[b]pyridin-6-yl)nicotinonitrile;
(cis)-6-(5-chloro-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H
cyclopenta[b]pyridine;
(cis)-6-(5-methyl-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-6-(5-bromo-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-6-(5-fluoro-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H
cyclopenta[b]pyridine;
(cis)-6-(5-vinyl-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-6-(5-methoxy-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-6-(5-vinyloxy-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-6-(5-ethynyl-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H
cyclopenta[b]pyridine;
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-6-(5,6-dichloro-3-pyridinyl)-2,3,4,4a, 7,7a-hexahydro-1 H-
cyclopenta[b]pyridine;
(cis)-6-(6-brorno-5-chloro-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-5-(2,3,4,4a,7,7a-hexahydro- 1H-cyclopenta[b]pyridin-6-yl)-2-
methylnicotinonitrile;
(cis)-2-brorno-5-(2,3,4,4a,7,7a-hexahydro- 1 H-cyclopenta[b]pyridin-6-
yl)nicotinonitrile;
(cis)-2-chloro-5-(2,3,4,4a,7,7a-hexahydro-1H-cyclopenta[b]pyridin 6-
yl)nicotinonitrile;
(cis)-6-(6-chloro-5-methyl-3-pyridinyl)-2,3,4,4a,7,7a hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-6-(6-bromo-5-methyl-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-6-(5-methoxy-6-methyl-3-pyridinyl)-2, 3,4,4a,7,7a-hexahydro-1 H-
cyclopenta[b]pyridine;
(cis)-6-(6-chloro-5-methoxy-3-pyridinyl)-2, 3,4,4a,7,7a-hexahydro-1 H-
cyclopenta[b]pyridine;
(cis)-6-(6-bromo-5-methoxy-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H-
cyclopenta[b]pyridine;
(cis)-6-(3-methyl-5-isoxazolyl)-2, 3,4,4a,7,7a-hexahydro-1 H-
cyclopenta[b]pyridine;
(cis)-2-(2,3,4,4a,7,7a-hexahydro-lH-cyclopenta[b]pyridin-6-yl)furo[3,2-
b]pyridine; or a
pharmaceutically acceptable salt, amide, ester and prodrug thereof.
In another embodiment of the present invention, compounds of formula (VI) are
disclosed
R~~N Y ~ R2
~ Ra
Z R R4
(VI),
or a pharmaceutically acceptable salt, amide, ester and prodrug thereof
wherein Y, Z, Ri, R2, R3,
R4 and R$ are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein Y is a covalent bond; Z is a covalent bond; and Ri, Ra, R3,
R4, and RS are as
defined in formula (I).
71
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein Y is a covalent bond; Z is CH2; and Rl, R2, R3, R4, and R5
are as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein - represents a double bond; Y is a covalent bond; Z is CHz;
R2 is hydrogen;
R3 and RS are absent; R4 is heterocycle; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein =- represents a double bond; Y is a covalent bond; Z is CH2;
R2 is hydrogen;
R3 and RS are absent; R4 is heterocycle wherein the heterocycle is selected
from furyl,
imidazolyl, isothiazolyl, isoxazolyl, oxadiazolyl, oxazolyl, pyrazinyl,
pyrazolyl, pyridinyl,
pyrimidinyl, pyridazinyl, pyrrolyl, tetrazinyl, tetrazolyl, thiadiazolyl,
thiazolyl, thienyl, triazinyl,
triazolyl,~ benzimidazolyl, benzothiazolyl, benzothienyl, benzoxazolyl,
benzofuranyl, cinnolinyl,
faro[2,3-c]pyridine, faro[3,2-c]pyridine, faro[3,2 b]pyridinyl, furo[2,3-
b]pyridinyl, imidazo[1,2-
a]pyridinyl, indazolyl, indolyl, indolizinyl, naphthyridinyl, isobenzofuranyl,
isobenzothienyl,
isoindolyl, isoquinolinyl, phthalazinyl, quinolinyl, quinolizinyl,
quinoxalinyl, quinazolinyl,
thieno[2,3-c]pyridine, thieno[3,2-c]pyridine, thieno[3,2-b]pyridinyl or
thieno[2,3-b]pyridinyl
wherein the heterocycle is substituted with 0, 1, 2, or 3 substituents
independently selected from
alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl,
alkoxycarbonylalkyl, alkyl,
alkylcarbonyl, alkylcarbonyloxy, alkylthio, alkynyl, carboxy, carboxyalkyl,
cyano, cyanoalkyl,
formyl, formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl,
mercapto,
mercaptoalkyl, vitro, triphenylmethyl (trityl), -C(NH)NR1oR11, -NR1oR11,
(NRioRn)alkyl,
(NRIORII)carbonyl, (NRIOR1I)carbonylalkyl, (NRloRi1)sulfonyl, NR12S(O~RI3,
-C~12)~13R14~ -CIH2C(NR12)~13RI4~ -~~ORIa)Rlsa -C~C~R12~ -C~12RI3)RI4~
-S(O)20R12, or -S(O)2R12; R12, R13, and R14 are independently selected from
hydrogen, alkyl,
aryl, or arylalkyl; and RI, Rlo, and Rll are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein - represents a double bond; Y is a covalent bond; Z is CHz;
R2 is hydrogen;
R3 and RS are absent; R4 is heterocycle wherein the heterocycle is selected
from imidazolyl,
isoxazolyl, pyridinyl, tetrazolyl, thiadiazolyl, thiazolyl, thienyl,
imidazo[1,2 a]pyridinyl,
thieno[3,2-b]pyridinyl or thieno[2,3-b]pyridinyl wherein the heterocycle is
substituted with 0, l,
72
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
or 2 substituents independently selected from alkenyl, alkenyloxy, alkoxy,
alkyl, alkynyl, cyano,
halogen, nitro, (NRIOR~ ~)sulfonyl, or -C(NH)NR1oR11; and Rl, Rl~, and Rl1 are
as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein - represents a double bond; Y is a covalent bond; Z is CHI;
R~ is hydrogen;
R3 and RS are absent; R4 is heterocycle wherein the heterocycle is pyridinyl
substituted with 0, 1,
or 2 substituents independently selected from alkenyl, alkenyloxy, alkoxy,
alkyl, alkynyl, cyano,
halogen, or nitro; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein - represents a double bond; Y is a covalent bond; Z is CHI;
R2 is hydrogen;
R3 and RS are absent; R4 is heterocycle wherein the heterocycle is selected
from 3-pyridinyl, 6-
bromo-3-pyridinyl, 6-chloro-3-pyridinyl, 6-fluoro-3 pyridinyl, 6-methyl-3-
pyridinyl, 5-cyano-3-
pyridinyl, 5-methyl-3-pyridinyl, 5-chloro-3-pyridinyl, 5-fluoro-3-pyridinyl, 5-
bromo-3-pyridinyl,
5-vinyl-3-pyridinyl, 5-methoxy-3-pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-
3-pyridinyl, 5,6-
dichloro-3-pyridinyl, 6-bromo-5-chloro-3-pyridinyl, 5-cyano-6-methyl-3-
pyridinyl, 6-chloro-5-
cyano-3-pyridinyl, 6-bromo-5-cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl,
6-bromo-5-
methyl-3-pyridinyl, 5-methoxy-6-methyl-3-pyridinyl, 6-chloro-5-methoxy-3-
pyridinyl, or 6-
bromo-5-methoxy-3-pyridinyl; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein - represents a double bond; Y is a covalent bond; Z is CHz,;
R2 is
heterocycle; R3 and RS are absent; R4 is hydrogen; and Rl is as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein - represents a double bond; Y is a covalent bond; Z is CI-~;
Rz is
heterocycle wherein the heterocycle is selected from furyl, imidazolyl,
isothiazolyl, isoxazolyl,
oxadiazolyl, oxazolyl, pyrazinyl, pyrazolyl, pyridinyl, pyrimidinyl,
pyridazinyl, pyrrolyl,
tetrazinyl, tetrazolyl, thiadiazolyl, thiazolyl, thienyl, triazinyl,
triazolyl, benzimidazolyl,
benzothiazolyl, benzothienyl, benzoxazolyl, benzofuranyl, cinnolinyl, faro[2,3-
c]pyridine,
faro[3,2-c]pyridine, faro[3,2-b]pyridinyl, faro[2,3 b]pyridinyl, imidazo[1,2-
a]pyridinyl,
indazolyl, indolyl, indolizinyl, naphthyridinyl, isobenzofuranyl,
isobenzothienyl, isoindolyl,
isoquinolinyl, phthalazinyl, quinolinyl, quinolizinyl, quinoxalinyl,
quinazolinyl, thieno[2,3-
73
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
c]pyridine, thieno[3,2-c]pyridine, thieno[3,2-b]pyridinyl or thieno[2,3-
b]pyridinyl wherein the
heterocycle is substituted with 0, 1, 2, or 3 substituents independently
selected from alkenyl,
alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl, alkoxycarbonylalkyl, alkyl,
alkylcarbonyl,
alkylcarbonyloxy, alkylthio, alkynyl, carboxy, carboxyalkyl, cyano,
cyanoalkyl, formyl,
formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl, mercapto,
mercaptoalkyl,
vitro, triphenylinethyl (trityl), -C(NH)NR1OR11, NR1OR11, (NR1OR11)alkyl,
(NRIORn)carbonyl,
(NR1OR11)carbonylalkyl, (NRIORII)sulfonyl, NR12S(O)ZRI3, -C(NR12)NR1sR14~
-CHaC~Riz)NRi3Ria~ -C~ORla)Ri3~ -C~~~R12~ -C~12R13)R14~ 'S(~)2~R12~ ~r
-S(O)2R12; R3 and RS are absent; R4 is hydrogen; R12, Rna and R14 are
independently selected
from hydrogen, alkyl, aryl, or arylalkyl; and Rl, RIO, and Rl1 are as defined
in formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein - represents a double bond; Y is a covalent bond; Z is CHI;
RZ 1S
heterocycle wherein the heterocycle is selected from imidazolyl, isoxazolyl,
pyridinyl, tetrazolyl,
thiadiazolyl, thiazolyl, thienyl, imidazo[1,2-a]pyridinyl, thieno[3,2
b]pyridinyl or thieno[2,3-
b]pyridinyl wherein the heterocycle is substituted with 0, 1, or 2
substituents independently
selected from alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen,
vitro,
(NRIORn)sulfonyl, or -C(NH)NR1ORW R3 and RS are absent; R4 is hydrogen; and
Rl, RIO, and
Rl1 are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein --= represents a double bond; Y is a covalent bond; Z is
CIA; RZ is
heterocycle wherein the heterocycle is pyridinyl substituted with 0, 1, or 2
substituents
independently selected from allcenyl, alkenyloxy, alkoxy, alkyl, alkynyl,
cyano, halogen, or vitro;
R3 and RS are absent; R4 is hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein -- represents a double bond; Y is a covalent bond; Z is CIA;
R2 is
heterocycle wherein the heterocycle is selected from 3-pyridinyl, 6-bromo-3-
pyridinyl, 6-chloro-
3-pyridinyl, 6-fluoro-3 pyridinyl, 6-methyl-3-pyridinyl, 5-cyano-3-pyridinyl,
5-methyl-3-
pyridinyl, 5-chloro-3-pyridinyl, 5-fluoro-3-pyridinyl, 5-bromo-3-pyridinyl, 5-
vinyl-3-pyridinyl,
5-methoxy-3-pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-3-pyridinyl, 5,6-
dichloro-3-pyridinyl,
6-bromo-5-chloro-3-pyridinyl, 5-cyano-6-methyl-3-pyridinyl, 6-chloro-5-cyano-3-
pyridinyl, 6-
74
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
bromo-5-cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl, 6-bromo-5-methyl-3-
pyridinyl, 5-
methoxy-6-methyl-3-pyridinyl, 6-chloro-5-methoxy-3-pyridinyl, or 6-bromo-5-
methoxy-3-
pyridinyl; R3 and RS are absent; R4 is hydrogen; and Rl is as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein Y is CHZ; Z is a covalent bond; and Rl, RZ, R3, R4, and RS
are as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein -'- represents a double bond; Y is CH2; Z is a covalent
bond; R2 is
heterocycle; R3 and RS are absent; R4 is hydrogen; and Rl is as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein -'- represents a double bond; Y is CH2; Z is a covalent
bond; RZ is
heterocycle wherein the heterocycle is selected from fiuyl, imidazolyl,
isothiazolyl, isoxazolyl,
oxadiazolyl, oxazolyl, pyrazinyl, pyrazolyl, pyridinyl, pyrimidinyl,
pyridazinyl, pyrrolyl,
tetrazinyl, tetrazolyl, thiadiazolyl, thiazolyl, thienyl, triazinyl,
triazolyl, benzimidazolyl,
benzothiazolyl, benzothienyl, benzoxazolyl, benzofuranyl, cinnolinyl, faro[2,3-
c]pyridine,
faro[3,2-c]pyridine, faro[3,2-b]pyridinyl, faro[2,3-b]pyridinyl, imidazo[1,2-
a]pyridinyl,
indazolyl, indolyl, indolizinyl, naphthyridinyl, isobenzofuranyl,
isobenzothienyl, isoindolyl,
isoquinolinyl, phthalazinyl, quinolinyl, quinolizinyl, quinoxalinyl,
quinazolinyl, thieno[2,3-
c]pyridine, thieno[3,2-c]pyridine, thieno[3,2-b]pyridinyl or thieno[2,3-
b]pyridinyl wherein the
heterocycle is substituted with 0, 1, 2, or 3 substituents independently
selected from alkenyl,
alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl, alkoxycarbonylalkyl, alkyl,
alkylcarbonyl,
alkylcarbonyloxy, alkylthio, alkynyl, carboxy, carboxyalkyl, cyano,
cyanoalkyl, formyl,
formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl, mercapto,
mercaptoalkyl,
nitro, triphenylmethyl (trityl), -C(NH)NR~oRII, NR1oR11, (NRIORn)alkyl,
(NRIORn)carbonyl,
(NRIORn)carbonylalkyl, (NRIORn)sulfonyl, NR12S(O)2RI3, -C(NRIZ)NR13R14,
-CH2C~12)~13R14~ -C~OR12)Rls~ 'C~C~~2~ 'CWaRis)Ri4~ -S(C)2CR12~ or
-S(O)2Rlz; R3 and RS are absent; R4 is hydrogen; Ri2, RI3, and R14 are
independently selected
from hydrogen, alkyl, aryl, or arylalkyl; and RI, Rlo, and Rll are as defined
in formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein -- represents a double bond; Y is CH2; Z is a covalent bond;
R2 is
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
heterocycle wherein the heterocycle is selected from imidazolyl, isoxazolyl,
pyridinyl, tetrazolyl,
thiadiazolyl, thiazolyl, thienyl, imidazo[1,2-a]pyridinyl, thieno[3,2
b]pyridinyl or thieno[2,3-
b]pyridinyl wherein the heterocycle is substituted with 0, l, or 2
substituents independently
selected from alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen,
nitro,
(NR1oR11)sulfonyl, or-C(NH)NR1oR11; R3 and RS are absent; R4 is hydrogen; and
R~, Rlo, and
RI1 are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein ~ represents a double bond; Y is CH2; Z is a covalent bond;
RZ is
heterocycle wherein the heterocycle is pyridinyl substituted with 0, 1, or 2
substituents
independently selected from alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl,
cyano, halogen, or nitro;
R3 and RS are absent; R4 is hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein - represents a double bond; Y is CH2; Z is a covalent bond;
RZ is
heterocycle wherein the heterocycle is selected from 3-pyridinyl, 6-bromo-3-
pyridinyl, 6-chloro-
3-pyridinyl, 6-fluoro-3 pyridinyl, 6-methyl-3-pyridinyl, 5-cyano-3-pyridinyl,
5-methyl-3-
pyridinyl, 5-chloro-3-pyridinyl, 5-fluoro-3-pyridinyl, 5-bromo-3-pyridinyl, 5-
vinyl-3-pyridinyl,
5-methoxy-3-pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-3-pyridinyl, 5,6-
dichloro-3-pyridinyl,
6-bromo-5-chloro-3-pyridinyl, 5-cyano-6-methyl-3-pyridinyl, 6-chloro-5-cyano-3-
pyridinyl, 6-
bromo-5-cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl, 6-bromo-5-methyl-3-
pyridinyl, 5-
methoxy-6-methyl-3-pyridinyl, 6-chloro-5-methoxy-3-pyridinyl, or 6-bromo-5-
methoxy-3-
pyridinyl; R3 and RS are absent; R4 is hydrogen; and Rl is as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein Y is a covalent bond; Z is CHZCHZ; and Rl, R2, R3, R4, and
I~ are as defined
in formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein -= represents a double bond; Y is a covalent bond; Z is
CI~CH2; R2 is
hydrogen; R3 and RS are absent; R4 is heterocycle; and Rl is as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein --= represents a double bond; Y is a covalent bond; Z is
CI~CH2; R2 is
hydrogen; R3 and RS are absent; R4 is heterocycle wherein the heterocycle is
selected from furyl,
76
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
imidazolyl, isothiazolyl, isoxazolyl, oxadiazolyl, oxazolyl, pyrazinyl,
pyrazolyl, pyridinyl,
pyrimidinyl, pyridazinyl, pyrrolyl, tetrazinyl, tetrazolyl, thiadiazolyl,
thiazolyl, thienyl, triazinyl,
triazolyl, benzimidazolyl, benzothiazolyl, benzothienyl, benzoxazolyl,
benzofuranyl, cinnolinyl,
faro[2,3-c]pyridine, faro[3,2-c]pyridine, faro[3,2 b]pyridinyl, faro[2,3-
b]pyridinyl, imidazo[1,2-
a]pyridinyl, indazolyl, indolyl, indolizinyl, naphthyridinyl, isobenzofuranyl,
isobenzothienyl,
isoindolyl, isoquinolinyl, phthalazinyl, quinolinyl, quinolizinyl,
quinoxalinyl, quinazolinyl,
thieno[2,3-c]pyridine, thieno[3,2-c]pyridine, thieno[3,2-b]pyridinyl or
thieno[2,3-b]pyridinyl
wherein the heterocycle is substituted with 0, 1, 2, or 3 substituents
independently selected from
alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl,
alkoxycarbonylalkyl, alkyl,
alkylcarbonyl, alkylcarbonyloxy, alkylthio, alkynyl, carboxy, carboxyalkyl,
cyano, cyanoalkyl,
formyl, formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl,
mercapto,
mercaptoalkyl, nitro, triphenylmethyl (trityl), -C(NH)NR1oR11, -NR1oR11,
(NRioRn)alkyl,
(NRIORI I)carbonyl, (NRIOR~ 1)carbonylalkyl, (NR~oR~ 1)sulfonyl, NR12S(O~R13,
-~~RI2WI3R14~ -CH2C(1~R12)~13R14~ -C(NORIa)Ri3, -C(NCI~RI2~ 'C(NNR12R13)R14~
-S(O)20R12, or -S(O)2R~~; R12, R~3, and R14 are independently selected from
hydrogen, alkyl,
aryl, or arylalkyl; and RI, Rlo, and Rll are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (VT) are
disclosed wherein --= represents a double bond; Y is a covalent bond; Z is
CI~CH2; R2 is
hydrogen; R3 and RS are absent; R4 is heterocycle wherein the heterocycle is
selected from
imidazolyl, isoxazolyl, pyridinyl, tetrazolyl, thiadiazolyl, thiazolyl,
thienyl, imidazo[1,2
a]pyridinyl, thieno[3,2-b]pyridinyl or thieno[2,3-b]pyridinyl wherein the
heterocycle is
substituted with 0, l, or 2 substituents independently selected from alkenyl,
alkenyloxy, alkoxy,
alkyl, alkynyl, cyano, halogen, nitro, (NRIORn)sulfonyl, or -C(N~NRIORn; and
Rl, Rlg and Rl
are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (VT) are
disclosed wherein -- represents a double bond; Y is a covalent bond; Z is
CI~CHZ; R2 is
hydrogen; R3 and RS are absent; R4 is heterocycle wherein the heterocycle is
pyridinyl
substituted with 0, 1, or 2 substituents independently selected from alkenyl,
alkenyloxy, alkoxy,
alkyl, alkynyl, cyano, halogen, or nitro; and Ri is as defined in formula (I).
77
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein =-' represents a double bond; Y is a covalent bond; Z is
CH~CHZ; R2 is
hydrogen; R3 and RS are absent; R4 is heterocycle wherein the heterocycle is
selected from 3-
pyridinyl, 6-bromo-3-pyridinyl, 6-chloro-3-pyridinyl, 6-fluoro-3 pyridinyl, 6-
methyl-3-pyridinyl,
5-cyano-3-pyridinyl, 5-methyl-3-pyridinyl, 5-chloro-3-pyridinyl, 5-fluoro-3-
pyridinyl, 5-bromo-
3-pyridinyl, 5-vinyl-3-pyridinyl, 5-methoxy-3-pyridinyl, 5-vinyloxy-3-
pyridinyl, 5-ethynyl-3-
pyridinyl, 5,6-dichloro-3-pyridinyl, 6-bromo-5-chloro-3-pyridinyl, 5-cyano-6-
methyl-3-
pyridinyl, 6-chloro-5-cyano-3-pyridinyl, 6-bromo-5-cyano-3-pyridinyl, 6-chloro-
5-methyl-3-
pyridinyl, 6-bromo-5-methyl-3-pyridinyl, 5-methoxy-6-methyl-3-pyridinyl, 6-
chloro-5-methoxy-
3-pyridinyl, or 6-bromo-5-methoxy-3-pyridinyl; and RI is as defined in formula
(I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein - represents a double bond; Y is a covalent bond; Z is
CH2CH2; RZ is
heterocycle; R3 and RS are absent; Rø is hydrogen; and Rl is as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein -- represents a double bond; Y is a covalent bond; Z is CI-
I2CH2; R2 is
heterocycle wherein the heterocycle is selected from furyl, imidazolyl,
isothiazolyl, isoxazolyl,
oxadiazolyl, oxazolyl, pyrazinyl, pyrazolyl, pyridinyl, pyrimidinyl,
pyridazinyl, pyrrolyl,
tetrazinyl, tetrazolyl, thiadiazolyl, thiazolyl, thienyl, triazinyl,
triazolyl, benzimidazolyl,
benzothiazolyl, benzothienyl, benzoxazolyl, benzofuranyl, cinnolinyl, faro[2,3-
c)pyridine,
faro[3,2-c]pyridine, faro[3,2-b]pyridinyl, faro[2,3 b]pyridinyl, imidazo[1,2-
a]pyridinyl,
indazolyl, indolyl, indolizinyl, naphthyridinyl, isobenzofuranyl,
isobenzothienyl, isoindolyl,
isoquinolinyl, phthalazinyl, quinolinyl, quinolizinyl, quinoxalinyl,
quinazolinyl, thieno[2,3-
c]pyridine, thieno(3,2-c]pyridine, thieno[3,2-b]pyridinyl or thieno[2,3-
b]pyridinyl wherein the
heterocycle is substituted with 0, 1, 2, or 3 substituents independently
selected from alkenyl,
alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl, alkoxycarbonylalkyl, alkyl,
alkylcarbonyl,
alkylcarbonyloxy, alkylthio, alkynyl, carboxy, carboxyalkyl, cyano,
cyanoalkyl, formyl,
formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl, mercapto,
mercaptoalkyl,
vitro, triphenylmethyl (trityl), -C(NH)NR1oR11, NR1oR11, (NRIORn)alkyl,
(NR1oR11)carbonyl,
(NR1oR11)carbonylalkyl, (NR1oR11)sulfonyl, NR12S(O)2RI3, -C(NR12)NRmR~4,
-CHaCWz)NR13Ri4~ -C~OR12)R13~ -C~C~~2~ -C~12R13)R14~ -S(~)2CRIa~ or
7g
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
-S(O)zRl2; R3 and RS are absent; R4 is hydrogen; R12, RI3, and R14 are
independently selected
from hydrogen, alkyl, aryl, or arylalkyl; and Ri, Rio, and Rl1 are as defined
in formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein - represents a double bond; Y is a covalent bond; Z is
CI~CH2; R2 is
heterocycle wherein the heterocycle is selected from imidazolyl, isoxazolyl,
pyridinyl, tetrazolyl,
thiadiazolyl, thiazolyl, thienyl, imidazo[1,2,-a]pyridinyl, thieno[3,2
b]pyridinyl or thieno[2,3-
b]pyridinyl wherein the heterocycle is substituted with 0, l, or 2
substituents independently
selected from alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen,
nitro,
(NRIORn)sulfonyl, or-C(NH)NR1oR11; R3 and RS are absent; R4 is hydrogen; and
Rl, Rlo, and
Rl l are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein -'- represents a double bond; Y is a covalent bond; Z is
CI~CH2; RZ is
heterocycle wherein the heterocycle is pyridinyl substituted with 0, 1, or 2
substituents
independently selected from alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl,
cyano, halogen, or nitro;
R3 and RS are absent; R4 is hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein - represents a double bond; Y is a covalent bond; Z is
CH~CH2; R2 is
heterocycle wherein the heterocycle is selected from 3-pyridinyl, 6-bromo-3-
pyridinyl, 6-chloro-
3-pyridinyl, 6-fluoro-3 pyridinyl, 6-methyl-3-pyridinyl, 5-cyano-3-pyridinyl,
5-methyl-3-
pyridinyl, 5-chloro-3-pyridinyl, 5-fluoro-3-pyridinyl, 5-bromo-3-pyridinyl, 5-
vinyl-3-pyridinyl,
5-methoxy-3-pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-3-pyridinyl, 5,6-
dichloro-3-pyridinyl,
6-bromo-5-chloro-3-pyridinyl, 5-cyano-6-methyl-3-pyridinyl, 6-chloro-5-cyano-3-
pyridinyl, 6-
bromo-5-cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl, 6-brorno-5-methyl-3-
pyridinyl, 5-
methoxy-6-methyl-3-pyridinyl, 6-chloro-5-methoxy-3-pyridinyl, or 6-bromo-5-
methoxy-3-
pyridinyl; R3 and RS are absent; R4 is hydrogen; and Rl is as defined in
formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein Y is CH2; Z is CH2; and Rl, R2, R3, R4, and R5 are as
defined in formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein -- represents a double bond; Y is CH2; Z is CH2; R2 is
heterocycle; R3 and
Rs are absent; R4 is hydrogen; and Rl is as defined in formula (I).
79
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein = - represents a double bond; Y is CH2; Z is CH2; R2 is
heterocycle wherein
the heterocycle is selected from futyl, imidazolyl, isothiazolyl, isoxazolyl,
oxadiazolyl, oxazolyl,
pyrazinyl, pyrazolyl, pyridinyl, pyrimidinyl, pyridazinyl, pyrrolyl,
tetrazinyl, tetrazolyl,
thiadiazolyl, thiazolyl, thienyl, triazinyl, triazolyl, benzimidazolyl,
benzothiazolyl, benzothienyl,
benzoxazolyl, benzofuranyl, cinnolinyl, faro[2,3-c]pyridine, faro[3,2-
c]pyridine, faro[3,2-
b]pyridinyl, faro[2,3-b]pyridinyl, imidazo[1,2-a]pyridinyl, indazolyl,
indolyl, indolizinyl,
naphthyridinyl, isobenzofuranyl, isobenzothienyl, isoindolyl, isoquinolinyl,
phthalazinyl,
quinolinyl, quinolizinyl, quinoxalinyl, quinazolinyl, thieno[2,3-c]pyridine,
thieno[3,2-c]pyridine,
thieno[3,2-b]pyridinyl or thieno[2,3-b]pyridinyl wherein the heterocycle is
substituted with 0, 1,
2, or 3 substituents independently selected from alkenyl, alkoxy,
alkoxyalkoxy, alkoxyalkyl,
alkoxycarbonyl, alkoxycarbonylalkyl, alkyl, alkylcarbonyl, alkylcarbonyloxy,
alkylthio, alkynyl,
carboxy, carboxyalkyl, cyano, cyanoalkyl, formyl, formylalkyl, haloalkoxy,
haloalkyl, halogen,
hydroxy, hydroxyalkyl, mercapto, mercaptoalkyl, vitro, triphenylmethyl
(trityl),-C(NH)NRIORI l,
-NR~oRn, (NRIORII)alkyl, (NR1oR11)carbonyl, (NR1oR11)carbonylalkyl,
(NK1oR11)sulfonyl,
-~12s(O)2R13~ -~~12)~13RI4~ -~H2C~12)~13R14~ -C(NOR12)R13, -C(NCN)R12,
-C~12R13)R14~ -S(O)aORl2, or -S(O)2R12; R3 and Rs are absent; R4 is hydrogen;
RI2, Ri3, and
R14 are independently selected from hydrogen, alkyl, aryl, or arylalkyl; and
Rv Rlo, and Rl1 are
as defined in formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein ~ represents a double bond; Y is CHz; Z is CH2; R2 is
heterocycle wherein
the heterocycle is selected from imidazolyl, isoxazolyl, pyridinyl,
tetrazolyl, thiadiazolyl,
thiazolyl, thienyl, imidazo[1,2-a]pyridinyl, thieno[3,2-b]pyridinyl or
thieno[2,3-b]pyridinyl
wherein the heterocycle is substituted with 0, I, or 2 substituents
independently selected from
alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen, vitro,
(NRioRn)sulfonyl, or
-C(NH)NR1oR11; R3 and Rs are absent; R4 is hydrogen; and Rl, Rzo, and Rll are
as defined in
formula (T).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein - represents a double bond; Y is CH2; Z is CH2; R2 is
heterocycle wherein
the heterocycle is pyridinyl substituted with 0, I, or 2 substituents
independently selected from
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
alkenyl, alkenyloxy, alkoxy, alkyl, alkynyl, cyano, halogen, or nitro; R3 and
RS are absent; R4 is
hydrogen; and Rl is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (VI) are
disclosed wherein = - represents a double bond; Y is CHZ; Z is CH2; RZ is
heterocycle wherein
the heterocycle is selected from 3-pyridinyl, 6-bromo-3-pyridinyl, 6-chloro-3-
pyridinyl, 6-
fluoxo-3 pyridinyl, 6-methyl-3-pyridinyl, 5-cyano-3-pyridinyl, 5-methyl-3-
pyridinyl, 5-chloro-3-
pyridinyl, 5-fluoro-3-pyridinyl, 5-bromo-3-pyridinyl, 5-vinyl-3-pyridinyl, 5-
methoxy-3-
pyridinyl, 5-vinyloxy-3-pyridinyl, 5-ethynyl-3-pyridinyl, 5,6-dichloro-3-
pyridinyl, 6-bromo-5-
chloro-3-pyridinyl, 5-cyano-6-methyl-3-pyridinyl, 6-chloro-5-cyano-3-
pyridinyl, 6-bromo-5-
cyano-3-pyridinyl, 6-chloro-5-methyl-3-pyridinyl, 6-bromo-5-methyl-3-
pyridinyl, 5-methoxy-6-
methyl-3-pyridinyl, 6-chloro-5-methoxy-3-pyridinyl, or 6-bxomo-5-methoxy-3-
pyridinyl; R3 and
RS are absent; R4 is hydrogen; and Rl is as defined in formula (I).
In another embodiment of the pxesent invention, compounds of formula (VI) are
disclosed wherein Y is CH2CH2; Z is a covalent bond; and Rl, RZ, R3, R4, and
R~ are as defined
in formula (I).
Representative compounds of formula (VI) include, but are not limited to:
(cis)-8-(3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroisoquinoline;
(cis)-8-(6-chloro-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroisoquinoline;
(cis)-8-(6-bromo-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroisoquinoline;
(cis)-8-(6-fluoro-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroisoquinoline;
(cis)-8-(6-methyl-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroisoquinoline;
(cis)-5-(1,2,3,4,4a,5,6,8a-octahydro-8-isoquinolinyl)nicotinonitrile;
(cis)-8-(5-chloro-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroisoquinoline;
(cis)-8-(5-bromo-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroisoquinoline;
(cis)-8-(5-fluoro-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroisoquinoline;
(cis)-8-(5-methyl-3-pyridinyl)-1,2, 3,4,4a, 5, 6, 8a-octahydroisoquinoline;
(cis)-8-(5-vinyl-3-pyridinyl)-1,2,3,4,4a,5,6,8a-.octahydroisoquinoline;
(cis)-8-(5-methoxy-3-pyridinyl)-1,2,3,4,4a,5,6,8a octahydroisoquinoline;
(cis)-8-(5-vinyloxy-3-pyridinyl)-1,2,3,4,4a; 5, 6, 8a-octahydroisoquinoline;
81
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-8-(5-ethynyl-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroisoquinoline;
(cis)-8-(5,6-dichloro-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroisoquinoline;
(cis)-8-(6-bromo-5-chloxo-3-pyridinyl)-1,2,3,4,4a,5,6, 8 a-
octahydroisoquinoline;
(cis)-2-methyl-5-(1,2,3,4,4a,5,6,8a-octahydro-8-isoquinolinyl)nicotinonitrile;
(cis)-2-bromo-S-(1,2,3,4,4a,5,6,8a-octahydro-8 isoquinolinyl)nicotinonitrile;
(cis)-2-chloro-5-(1,2,3,4,4a,5,6,8a-octahydxo-8-isoquinolinyl)nicotinonitrile;
(cis)-8-(6-chloro-5-methyl-3-pyridinyl)-1,2,3,4,4a,5,6,8x-
octahydroisoquinoline;
(cis)-8-(6-bromo-5-methyl-3-pyridinyl)-1,2,3,4,4a,5,6,8a-
octahydroisoquinoline;
(cis)-8-(S-methoxy-6-methyl-3-pyridinyl)-1,2,3,4,4a,5,6,8a-
octahydroisoquinoline;
(cis)-8-(6-bromo-5-methoxy-3-pyridinyl)-1,2,3,4,4a,5,6,8a-
octahydroisoquinoline;
(cis)-8-(6-chloro-5-methoxy-3-pyridinyl)-1,2,3,4,4a,5,6,8a-
octahydroisoquinoline;
(cis)-8-(3-methyl-5-isoxazolyl)-1,2,3,4,4a,5,6,8a-octahydroisoquinoline;
(cis)-8-faro[3,2-b)pyridin-2-yl-1,2,3,4,4a,5,6,8a-octahydroisoquinoline;
(cis)-7-(3-pyridinyl)-2,3,4,4a, 5, 7a-hexahydro-1 H-cyclopenta[c]pyridine;
{cis)-7-(6-chloro-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-7-{6-bromo-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-7-(6-fluoro-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H
cyclopenta[c]pyridine;
(cis)-7-(6-methyl-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-5-(2,3,4,4a,5,7a-hexahydxo-lH-cyclopenta[c]pyridin 7-yl)nicotinonitrile;
(cis)-7-(5-methyl-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-7-(5-chloro-3-pyridinyl)-2,3,4,4a,S,7a-hexahydro-1H
cyclopenta[c]pyridine;
(cis)-7-(5-bromo-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H
cyclopenta[c]pyridine;
(cis)-7-(S-fluoro-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H
cyclopenta[c]pyridine;
(cis)-7-(5-vinyl-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-7-(5-methoxy-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-7-(5-vinyloxy-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-7-(5-ethynyl-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H
cyclopenta[c]pyridine;
(cis)-5-(2,3,4,4a,5,7a-hexahydro-1H-cyclopenta[c]pyridin-7-yl)-2-
rnethylnicotinonitrile;
(cis)-2-bromo-5-(2,3,4,4a,5,7a-hexahydro-1H-cyclopenta[c]pyridin-7-
yl)nicotinonitrile;
82
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-2-chloro-S-(2,3,4,4a,5,7a-hexahydro-IH-cyclopenta[c]pyridin 7-
yl)nicotinonitrile;
(cis)-7-(S,6-dichloro-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-7-(6-bromo-S-chloro-3-pyridinyl)-2,3,4,4a,S,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-7-(6-chloro-S-methyl-3-pyridinyl)-2,3,4,4a,S,7a.-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-7-(6-bromo-S-methyl-3-pyridinyl)-2,3,4,4a,S,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-7-(S-methoxy-6-methyl-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-7-(6-chloro-S-methoxy-3-pyridinyl)-2,3,4,4a,S,7a hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-7-(6-bromo-S-methoxy-3-pyridinyl)-2,3,4,4a, S, 7a-hexahydro- I H-
cyclopenta[c]pyridine;
(cis)-2-(2,3,4,4a,S,7a-hexahydro- 1H-cyclopenta[c]pyridin-7-yl)furo [3,2-
b]pyridine;
(cis)-7-(3-methyl-S-isoxazolyl)-2,3,4,4a,S,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-7-(3-pyridinyl)-I,2,3,4,4a,S,6,8a-octahydroisoquinoline;
(cis)-7-(6-chloro-3-pyridinyl)-1,2,3,4,4a,S,6,8a-octahydroisoquinoline;
(cis)-7-(6-bromo-3-pyridinyl)-1,2,3,4,4a,S,6,8a-octahydroisoquinoline;
(cis)-7-(6-fluoro-3-pyridinyl)- I ,2, 3,4,4a, 5,6, 8a-octahydroisoquinoline;
(cis)-7-(6-methyl-3-pyridinyl)-1,2,3,4,4a,S,6,8a-octahydroisoquinoline;
(cis)-S-(1,2,3,4,4a,S,6,8a octahydro-7-isoquinolinyl)nicotinonitrile;
(cis)-7-(S-methyl-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroisoquinoline;
(cis)-7-(S-chloro-3-pyridinyl)-1,2,3,4,4a,S,6,8a-octahydroisoquinoline;
(cis)-7-(S-bromo-3-pyridinyl)-1,2,3,4,4a,S,6,8a-octahydroisoquinoline;
(cis)-7-(S-fluoro-3-pyridinyl)-1,2,3,4,4a,S,6,8a-octahydroisoquinoline;
(cis)-7-(S-vinyl-3-pyridinyl)-1,2,3,4,4a,5,6,8a-octahydroisoquinoline;
(cis)-7-(S-methoxy-3-pyridinyl)-1,2,3,4,4a,S,6,8a octahydroisoquinoline;
(cis)-7-(S-vinyloxy-3-pyridinyl)-1,2,3,4,4a,S,6,8a-actahydroisoquinoline;
83
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-7-(S-ethynyl-3-pyridinyl)-1,2,3,4,4a, 5,6, 8 a-octahydroisoquinoline;
(cis)-7-(S,6-dichloro-3-pyridinyl)-1,2,3,4,4a,S,6,8a-octahydroisoquinoline;
(cis)-7-(6-bromo-S-chloro-3-pyridinyl)-1,2,3,4,4a,S,6,8a-
octahydroisoquinoline;
(cis)-2-methyl-S-(1,2,3,4,4a,5,6,8a-octahydro-7-isoquinolinyl)nicotinonitrile;
(cis)-2-bromo-S-(1,2,3,4,4a,5,6,8a-octahydro-7 isoquinolinyl)nicotinonitrile;
(cis)-2-chloro-S-(1,2,3,4,4a,S,6,8a-octahydro-7 isoquinolinyl)nicotinonitrile;
(cis)-7-(6-chloro-S-methyl-3-pyridinyl)-1,2, 3,4,4a, S, 6, 8 a-
octahydroisoquinoline;
(cis)-7-(6-bromo-S-methyl-3-pyridinyl)-1,2,3,4,4a,S,6,8a-
octahydroisoquinoline;
(cis)-7-(S-methoxy-6-methyl-3-pyridinyl)-1,2,3,4,4a,S,6,8a-
octahydroisoquinoline;
(cis)-7-(6-chloro-S-methoxy-3-pyridinyl)-1,2,3,4,4a,S,6,8a
octahydroisoquinoline;
(cis)-7-(6-bromo-S-methoxy-3-pyridinyl)-1,2,3,4,4a, 5,6, 8a-
octahydroisoquinoline;
(cis)-8-(3-methyl-S-isoxazolyl)-1,2,3,4,4a,S,6,8a-octahydroisoquinoline;
(cis)-8-faro[3,2-b]pyridin-2-yl-1,2,3,4,4a,S,6,8a-octahydroisoquinoline;
(cis)-6-(3-pyridinyl)-2,3,4,4a,S,7a-hexahydro-1 H-cyclopenta[c]pyridine;
(cis)-6-(6-chloro-3-pyridinyl)-2,3,4,4a,S,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-6-(6-bromo-3-pyridinyl)-2,3,4,4a,S,7a-hexahydro-1H
cyclopenta[c]pyridine;
(cis)-6-(6-fluoro-3-pyridinyl)-2,3,4,4a,S,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-6-(6-methyl-3-pyridinyl)-2,3,4,4a,S,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-S-(2,3,4,4a,S,7a-hexahydro- 1H-cyclopenta[c]pyridin-6-
yl)nicotinonitrile;
(cis)-6-(S-chloro-3-pyridinyl)-2,3,4,4a,S,7a-hexahydro-lIH
cyclopenta[c]pyridine;
(cis)-6-(S-bromo-3-pyridinyl)-2,3,4,4a,S,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-6-(5,-fluoro-3-pyridinyl)-2,3,4,4a, S, 7a-hexahydro-1 H-
cyclopenta[c]pyridine;
(cis)-6-(S-methyl-3-pyridinyl)-2,3,4,4a,S,7a-hexahydro-1H
cyclopenta[c]pyridine;
(cis)-6-(S-vinyl-3-pyridinyl)-2,3,4,4a,S,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-6-(S-methoxy-3-pyridinyl)-2,3,4,4a,S,7a hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-6-(S-vinyloxy-3-pyridinyl)-2,3,4,4a,S,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-6-(S-ethynyl-3-pyridinyl)-2,3,4,4a,S,7a-hexahydro-1H
cyclopenta[c]pyridine;
(cis)-6-(S,6-dichloro-3-pyridinyl)-2,3,4,4a,S,7a-hexahydro-1H-
cyclopenta[c]pyridine;
84
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-6-(6-bromo-S-chloro-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-5-(2,3,4,4a,5,7a-hexahydro- 1H-cyclopenta[c]pyridin-6-yl)-2-
methylnicotinonitrile;
(cis)-2-chloro-5-(2,3,4,4a,5,7a-hexahydro-1H-cyclopenta[c]pyridin 6-
yl)nicotinonitrile;
(cis)-2-bromo-5-(2, 3,4,4a, 5,7a-hexahydro- 1 H-cyclopenta[c]pyridin-6-
yl)nicotinonitrile;
(cis)-6-(6-chloro-5-methyl-3-pyridinyl)-2,3,4,4a,5,7a hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-6-(6-bromo-5-methyl-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-6-(5-methoxy-6-methyl-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1 H
cyclopenta[c]pyridine;
(cis)-6-(6-bromo-5-methoxy-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro- 1H-
cyclopenta[c]pyridine;
(cis)-6-(6-chloro-5-methoxy-3-pyridinyl)-2,3,4,4a,5,7a-hexahydro-1 H-
cyclopenta[c]pyridine;
(cis)-6-(3-methyl-5-isoxazolyl)-2, 3,4,4a, 5, 7a-hexahydro-1 H-
cyclopenta[c]pyridine;
(cis)-2-(2,3,4,4a,5,7a-hexahydro- 1H-cyclopenta[c]pyridin-6-yl)furo[3,2-
b]pyridine;
(cis)-7-(3-pyridinyl)-1,2,3,4,4a,5,8,8a-octahydroisoquinoline;
(cis)-7-(6-methyl-3-pyridinyl)-1,2,3,4,4a, 5, 8, 8a-octahydroisoquinoline;
(cis)-7-(6-chloro-3-pyridinyl)-1,2,3,4,4a,5,8,8a-octahydroisoquinoline;
(cis)-7-(6-bromo-3-pyridinyl)-1,2,3,4,4a,5,8,8a-octahydroisoquinoline;
(cis)-7-(6-fluoro-3-pyridinyl)-1,2,3,4,4a, 5, 8, 8a-octahydroisoquinoline;
(cis)-5-(1,2,3,4,4a,5,8,8a octahydro-7-isoquinolinyl)nicotinonitrile;
(cis)-7-(5-methyl-3-pyridinyl)-1,2,3,4,4a, 5, 8, 8a-octahydroisoquinoline;
(cis)-7-(5-chloro-3-pyridinyl)-1,2,3,4,4a,5,8,8a-octahydroisoquinoline;
(cis)-7-(5-bromo-3-pyridinyl)-1,2,3,4,4a,5,8,8a-octahydroisoquinoline;
(cis)-7-(5-fluoro-3-pyridinyl)-1,2,3,4,4a, 5, 8, 8a-octahydroisoquinoline;
(cis)-7-(5,6-dichloro-3-pyridinyl)-1,2,3,4,4a, 5, 8, 8a-octahydroisoquinoline;
(cis)-7-(6-bromo-5-chloro-3-pyridinyl)-1,2,3,4,4a,5,8,8a-
octahydroisoquinoline;
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-2-methyl-5-(1,2,3,4,4a,5,8,8a-octahydro-7 isoquinolinyl)nicotinonitrile;
(cis)-2-chloro-5-(1,2,3,4,4a,5,8,8a-octahydro-7 isoquinolinyl)nicotinonitrile;
(cis)-2-bromo-5-(I,2,3,4,4a,5,8,8a-octahydro-7-isoquinolinyl)nicotinonitrile;
(cis)-7-(6-chloro-5-methyl-3-pyridinyl)-1,2,3,4,4a,5,8,8a
octahydroisoquinoline;
(cis)-7-(6-bromo-5-methyl-3-pyridinyl)-1,2,3,4,4a, 5, 8, 8a-
octahydroisoquinoline;
(cis)-7-(5-methoxy-6-methyl-3-pyridinyl)-1,2,3,4,4a,5,8,8a-
octahydroisoquinoline;
(cis)-7-(6-chloro-5-methoxy-3-pyridinyl)-1,2,3,4,4a,5,8,8a-
octahydroisoquinoline;
(cis)-7-(6-bromo-5-methoxy-3-pyridinyl)- I ,2,3,4,4a, 5, 8, 8a-
octahydroisoquinoline;
(cis)-7-(3-methyl-5-isoxazolyl)-1,2,3,4,4a,5, 8, 8a-octahydroisoquinoline;
(cis)-7-faro [3,2-b] pyridin-2-yl-1,2,3,4,4a, 5, 8, 8 a-octahydroisoquinoline;
(cis)-6-(3-pyridinyl)-2, 3,4,4a,7,7a-hexahydro-1 H-cyclopenta[c] pyridine;
(cis)-6-(6-chloro-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-6-(6-bromo-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-IH
cyclopenta[c]pyridine;
(cis)-6-(6-fluoro-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-6-(6-methyl-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-5-(2,3,4,4a,7,7a-hexahydro- 1H-cyclopenta[c]pyridin-6-
yl)nicotinonitrile;
(cis)-6-(5-chloro-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-6-(5-bromo-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1 H-
cyclopenta[c]pyridine;
(cis)-6-(5-fluoro-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H
cyclopenta[c]pyridine;
(cis)-6-(5-methyl-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-6-(5-vinyl-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1 H-
cyclopenta[c]pyridine;
(cis)-6-(5-methoxy-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-IH-
cyclopenta[c]pyridine;
(cis)-6-(5-vinyloxy-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-6-(5-ethynyl-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-6-(5,6-dichJ.oro-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-6-(6-bromo-5-chloro-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-5-(2,3,4,4a,7,7a-hexahydro-lH-cyclopenta[c]pyridin-6-yl)-2-
methylnicotinonitrile;
(cis)-2-chloro-5-(2,3,4,4a,7,7a-hexahydro-IH-cyclopenta[c]pyridin 6-
yl)nicotinonitrile;
86
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-2-bromo-5-(2,3,4,4a,7,7a-hexahydro- 1 H-cyclopenta[c]pyridin-6-
yl)nicotinonitrile;
(cis)-6-(6-bromo-5-methyl-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-6-(6-chloro-5-methyl-3-pyridinyl)-~,3,4,4a,7,7a hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-6-(5-methoxy-6-methyl-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1H
cyclopenta[c]pyridine;
(cis)-6-(6-chloro-5-methoxy-3-pyridinyl)-2,3,4,4a,7,7a hexahydro-1H-
cyclopenta[c]pyridine;
(cis)-6-(6-bromo-5-methoxy-3-pyridinyl)-2,3,4,4a,7,7a-hexahydro-1 H-
cyclopenta[c]pyridine;
(cis)-6-(3-methyl-5-isoxazolyl)-2,3,4,4a,7,7a-hexahydro-1 H-
cyclopenta[c]pyridine;
(cis)-2-(2,3,4,4a,7,7a-hexahydro-lH-cyclopenta[c]pyridin-6-yl)furo[3,2-
b]pyridine; or a
pharmaceutically acceptable salt, amide, ester and prodrug thereof.
Another embodiment of the present invention relates to pharmaceutical
compositions
comprising a therapeutically effective amount of a compound of formula (I VI)
or a
pharmaceutically acceptable salt thereof. The compostion may be in combination
with a
pharmaceutically acceptable carrier.
Another embodiment of the present invention relates to a method of treating a
disorder,
such as Alzheimer's disease, Parkinson's disease, memory dysfunction,
Tourette's syndrome,
sleep disorders, attention deficit hyperactivity disorder, neurodegeneration,
inflammation,
neuroprotection, amyotrophic lateral sclerosis, anxiety, depression, mania,
schizophrenia,
nicotinic withdrawal syndrome, anorexia and other eating disorders,
AID~induced dementia,
epilepsy, urinary incontinence, substance abuse, smoking cessation and
inflammatory bowel
syndrome, in a mammal in need of such treatment comprising administering to
the mammal a
therapeutically effective amount of a compound of formula (I-VI) or a
pharmaceutically
acceptable salt, amide, ester or prodrug thereof.
Another embodiment of the present invention relates to a method for treating
pain in a
mammal in need of such treatment comprising administering to the mammal a
therapeutically
87
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
effective amount of a compound of formula (I-VI) or a pharmaceutically
acceptable salt, amide,
ester or prodrug thereof.
Another embodiment of the present invention relates to a method for treating
pain in a
mammal in need of such treatment comprising administering to the mammal a
therapeutically
effective amount of a compound of formula (I-VI) or a pharmaceutically
acceptable salt, amide,
ester or prodrug thereof in combination with a pharmaceutically acceptable
carrier.
Another embodiment of the present invention relates to a method for treating
pain in a
mammal in need of such treatment comprising administering to the mammal a
therapeutically
effective amount of a compound of formula (I VI) or a pharmaceutically
acceptable salt, amide,
ester or prodrug thereof in combination with an opioid.
Another embodiment of the present invention relates to a method for treating
pain in a
mammal in need of such treatment comprising administering to the mammal a
therapeutically
effective amount of a compound of formula (I VI) or a pharmaceutically
acceptable salt, amide,
ester or prodrug thereof in combination with a non-steroid anti-inflammatory
agent.
Another embodiment of the present invention relates to a method for treating
pain in a
mammal in need of such treatment comprising administering to the mammal a
therapeutically
effective amount of a compound of formula (I VI) or a pharmaceutically
acceptable salt, amide,
ester or prodrug thereof in combination with a tricyclic antidepressant.
Another embodiment of the present invention relates to a method for treating
pain in a
mammal in need of such treatment comprising administering to the mammal a
therapeutically
effective amount of a compound of formula (I-VI) or a pharmaceutically
acceptable salt, amide,
ester or prodrug thereof in combination with an anticonvulsant such as
gabapentin or pregabalin.
Definition of Terms
As used throughout this specification and the appended claims, the following
terms have
the following meanings.
The term "alkenyl" as used herein, means a straight or branched chain
hydrocarbon
containing from 2 to 10 carbons, preferably 2 to 6 carbon atoms, preferably in
a straight chain,
and containing at least one carbon-carbon double bond formed by the removal of
two hydrogens.
Representative examples of alkenyl include, but are not limited to, ethenyl, 2-
propenyl, 2
88
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
methyl-2-propenyl, 3-butenyl, 4-pentenyl, 5-hexenyl, 2-heptenyl, 2 methyl-1-
heptenyl, and 3-
decenyl.
The term "alkoxy" as used herein, means an alkyl group, as defined herein,
appended to
the parent molecular moiety through an oxy moiety, as defined herein.
Representative examples
of alkoxy include, but are not limited to, methoxy, ethoxy, propoxy, 2-
propoxy, butoxy, tern
butoxy, pentyloxy, and hexyloxy.
The term "alkoxyalkoxy" as used herein, means an alkoxy group, as defined
herein,
appended to the parent molecular moiety through another alkoxy group, as
defined herein.
Representative examples of alkoxyalkoxy include, but are not limited to,
ter~butoxymethoxy, 2-
ethoxyethoxy, 2-methoxyethoxy, and methoxymethoxy.
The term "alkoxyalkyl" as used herein, means an allcoxy group, as defined
herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of alkoxyalkyl include, but are not limited to, tern-
butoxymethyl, 2-
ethoxyethyl, 2-methoxyethyl, and methoxymethyl.
The term "alkoxycarbonyl" as used herein, means an alkoxy group, as defined
herein,
appended to the parent molecular moiety through a carbonyl group, as defined
herein.
Representative examples of alkoxycarbonyl include, but are not limited to,
methoxycarbonyl,
ethoxycarbonyl, and tert-butoxycarbonyl.
The term "alkoxycarbonylalkyl" as used herein, means an alkoxycarbonyl group,
as
defined herein, appended to the parent molecular moiety through an alkyl
group, as defined
herein. Representative examples of alkoxycarbonylalkyl include, but are not
limited to, 3-
methoxycarbonylpropyl, 4-ethoxycarbonylbutyl, 2-(sec-butylcarbonyl)ethyl, 2-
(isopropoxycarbonyl)ethyl, and 2-(tert-butoxycarbonyl)ethyl.
The term "alkyl" as used herein, means a straight or branched chain
hydrocarbon
containing from I to 10 carbon atoms, preferably 1 to 6 carbon atoms.
Representative examples
of alkyl include, but are not limited to, methyl, ethyl, rrpropyl, iso-propyl,
n-butyl, sec-butyl,
iso-butyl, tert-butyl, n pentyl, isopentyl, neopentyl, n hexyl, 3-methylhexyl,
2,2-dimethylpentyl,
2,3-dimethylpentyl, n-heptyl, n-octyl, n-nonyl, and n-decyl.
The term "alkylcarbonyl" as used herein, means an alkyl group, as defined
herein,
appended to the parent molecular moiety through a carbonyl group, as defined
herein.
89
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Representative examples of alkylcarbonyl include, but are not limited to,
acetyl, 1-oxopropyl,
2,2-dimethyl-1-oxopropyl, 1-oxobutyl, and 1-oxopentyl.
The term "alkylcarbonyloxy" as used herein, means an alkylcarbonyl group, as
defined
herein, appended to the parent molecular moiety through an oxy moiety, as
defined herein.
Representative examples of alkylcarbonyloxy include, but are not limited to,
acetyloxy,
ethylcarbonyloxy, and tert butylcarbonyloxy.
The term "alkylthio" as used herein, means an alkyl group, as defined herein,
appended to
the parent molecular moiety through a sulfur atom. Representative examples of
alkylthio
include, but are not limited, methylthio, ethylthio, tert butylthio, and
hexylthio.
The term "alkynyl" as used herein, means a straight or branched chain
hydrocarbon group
containing from 2 to 10 carbon atoms, preferably 2 to 6 carbon atoms,
preferably in a straight
chain, and containing at least one carbon-carbon triple bond. Representative
examples of
alkynyl include, but are not limited, to acetylenyl, 1-propynyl, 2-propynyl, 3-
butynyl, 2-
pentynyl, 3-methyl-1-pentynyl, 3,4-dimethyl-1-pentynyl, and 1-butynyl.
The term "aryl" as used herein, means a monocyclic-ring system, or a fused
bicyclic-ring
system wherein one or more of the fused rings are aromatic. Representative
examples of aryl
include, but are not limited to, azulenyl, indanyl, indenyl, naphthyl, phenyl,
and
tetrahydronaphthyl.
The aryl groups of this invention are substituted with 0, 1, 2, 3, 4 or 5
substituents
independently selected from alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl,
alkoxycarbonyl,
alkoxycarbonylalkyl, alkyl, alkylcarbonyl, alkylcarbonyloxy, alkylthio,
alkynyl, carboxy,
carboxyalkyl, cyano, cyanoalkyl, formyl, formylalkyl, haloalkoxy, haloalkyl,
halogen, hydroxy,
hydroxyalkyl, mercapto, mercaptoalkyl, vitro, triphenylmethyl (trityl), -
C(NH)NRIORt i,
-NR1oR11, (NRioRl1)alkyl, (NRIORn)carbonyl, (NRIORn)carbonylalkyl,
(NR1oR11)sulfonyl,
-~125(~)2R13~ -C~12)~13R14~ -CH2C(~R12)~13RI4, -~~OR12)R13~ -C(NCN)R12,
-C~12R13)R14, -S(O)20R~2 and -S(O)2R12 wherein R12, R13 and R14 are
independently
selected from hydrogen, alkyl, alkylcarbonyl, aryl, and arylalkyl, as defined
herein. The aryl
groups of this invention can be further substituted with an additional aryl
group, as defined
herein, or an additional heterocycle, as defined herein, wherein the
additional aryl group and the
additional heterocycle are substituted with 0, 1, 2 or 3 substituents
independently selected from
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl,
alkoxycarbonylalkyl, alkyl,
alkylcarbonyl, alkylcarbonyloxy, alkylthio, alkynyl, carboxy, carboxyalkyl,
cyano, cyanoalkyl,
formyl, formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl,
mercapto,
mercaptoalkyl, vitro, triphenylmethyl (trityl), -C(NH)NR1oR11, -NR1oR11,
(NR1oR11)alkyl,
(NR1oR11)carbonyl, (NR1oR11)carbonylalkyl, (NR1oR11)sulfonyl, NR12S(O~R13,
-G~12W13R14~ 'CH2C(NR12)~13R14~ -C(NORl2)R13, -C(NCN)Rl2wC~12R1~)Rla,
-S(O)20R12 and -S(O)2R12 wherein R12, R13 and R14 are independently selected
from hydrogen,
alkyl, alkylcarbonyl, aryl, and arylalkyl, as defined herein.
The term "carbonyl" as used herein, means a-C(O)- group.
The term "carboxy" as used herein, means a -C02H group.
The term "carboxyalkyl" as used herein, means a carboxy group, as defined
herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of carboxyalkyl include, but are not limited to,
carboxymethyl, 2-
carboxyethyl, and 3-carboxypropyl.
The term "cyano" as used herein, means a-CN group.
The term "cyanoalkyl" as used herein, means a cyano group, as defined herein,
appended
to the parent molecular moiety through an alkyl group, as defined herein.
Representative
examples of cyanoalkyl include, but are not limited to, cyanomethyl, 2-
cyanoethyl, and 3-
cyanopropyl.
The term "formyl" as used herein, means a -G(O)H group.
The term "formylalkyl" as used herein, means a formyl group, as defined
herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of formylalkyl include, but are not limited to,
formylmethyl and 2-
fonnylethyl.
The term "halo" or "halogen" as used herein, means -Cl, -Br, -I or -F.
The term "haloalkoxy" as used herein, means at least one halogen, as defined
herein,
appended to the parent molecular moiety through an alkoxy group, as defined
herein.
Representative examples of haloalkoxy include, but axe not limited to,
chloromethoxy, 2-
fluoroethoxy, trifluoromethoxy, 1,2-difluoroethoxy, and pentafluoroethoxy.
91
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
The term "haloalkyl" as used herein, means at least one halogen, as defined
herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of haloalkyl include, but are not limited to,
chloromethyl, 2~7uoroethyl,
trifluoromethyl, pentafluoroethyl, and 2-chloro-3-fluoropentyl.
The term "heterocycle" or "heterocyclic" as used herein, means a monocyclic,
bicyclic, or
tricyclic ring system. Monocyclic ring systems are exemplified by any 3- or 4-
membexed ring
containing a heteroatom independently selected from oxygen, nitrogen and
sulfur; or a 5-, 6- or
7-membered ring containing one, two or three heteroatoms wherein the
heteroatoms are
independently selected from nitrogen, oxygen and sulfur. The 5-membered ring
has from 0-2
double bonds and the 6- and 7-membered ring have from 0-3 double bonds.
Representative
examples of monocyclic ring systems include, but are not limited to,
azetidinyl, azepinyl,
aziridinyl, diazepinyl, 1,3-dioxolanyl, dioxanyl, dithianyl, fiuyl,
irnidazolyl, imidazolinyl,
imidazolidinyl, isothiazolyl, isothiazolinyl, isothiazolidinyl, isoxazolyl,
isoxazolinyl,
isoxazolidinyl, morpholinyl, oxadiazolyl, oxadiazolinyl, oxadiazolidinyl,
oxazolyl, oxazolinyl,
oxazolidinyl, piperazinyl, piperidinyl, pyranyl, pyrazinyl, pyrazolyl,
pyrazolinyl, pyrazolidinyl,
pyridinyl, pyrimidinyl, pyridazinyl, pyrrolyl, pyrrolinyl, pyrrolidinyl,
tetrahydrofuranyl,
tetrahydrothienyl, tetrazinyl, tetrazolyl, thiadiazolyl, thiadiazolinyl,
thiadiazolidinyl, thiazolyl,
thiazolinyl, thiazolidinyl, thienyl, thiomorpholinyl, l,l-
dioxidothiomorpholinyl (thiomorpholine
sulfone), thiopyranyl, triazinyl, triazolyl, and trithianyl. Bicyclic ring
systems are exemplified
by any of the above heterocyclic ring systems fused to an aryl group as
defined herein, a
cycloalkyl group as defined herein, or another heterocyclic ring system.
Representative
examples of bicyclic ring systems include but are not limited to, for example,
benzimidazolyl,
benzothiazolyl, benzothienyl, benzoxazolyl, benzofuranyl, benzopyranyl,
benzothiopyranyl,
benzodioxinyl, cinnolinyl, furopyridinyl, imidazo[1,2-a]pyridinyl, indazolyl,
indolyl, indolizinyl,
naphthyridinyl, isobenzofuranyl, isobenzothienyl, isoindolyl, isoquinolinyl,
phthalazinyl,
pyranopyridyl, quinolinyl, quinolizinyl, quinoxalinyl, quinazolinyl,
tetrahydroisoquinolinyl,
tetrahydroquinolinyl, thienopyridinyl, thieno[3,2-b]pyridinyl, thieno[2,3-
b]pyridinyl and
thiopyranopyridyl. Tricyclic rings systems are exemplified by any of the above
bicyclic ring
systems fused to an aryl group as defined herein, a cycloalkyl group as
defined herein, or a
heterocyclic ring system. Representative examples of tricyclic ring systems
include, but are not
92
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
limited to, acridinyl, carbazolyl, carbolinyl, dibenzo[b,d)furanyl,
dibenzo[b,d]thienyl,
naphtho[2,3-b]furan, naphtho[2,3-b]thienyl, phenazinyl, phenothiazinyl,
phenoxazinyl,
thianthrenyl, thioxanthenyl and xanthenyl.
The heterocycles of this invention are substituted with 0, l, 2,or 3
substituents
independently selected from alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl,
alkoxycarbonyl,
alkoxycarbonylalkyl, alkyl, alkylcarbonyl, alkylcarbonyloxy, alkylthio,
alkynyl, carboxy,
carboxyalkyl, cyano, cyanoalkyl, formyl, formylalkyl, haloalkoxy, haloalkyl,
halogen, hydroxy,
hydroxyalkyl, mercapto, mercaptoalkyl, vitro, triphenylmethyl (trityl),-
C(NH)NR1oR11,
-NR1oR11~ (NR1oR11)alkyl, (NR1oR11)carbonyl, (NR1oR11)carbonylalkyl,
(NR1oR11)sulfonyl,
'~12s(~)2Ri3~ -C~12)~I3Ri4~ -CH2C(NR12)NR13R14, -C(NOR12)R13, -C(NCI~R12,
-C~12R13)R14~ -S(O)aORlz~ and -S(O)2R12 wherein R12, Rls, and R14 are
independently
selected from hydrogen, alkyl, alkylcarbonyl, aryl, and arylalkyl as defined
herein. The
heterocycles of this invention can be further substituted with an additional
aryl group, as defined
herein, or an additional heterocycle, as defined herein, wherein the
additional aryl group and the
additional heterocycle can be substituted with 1, 2 or 3 substituents
independently selected from
alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl,
alkoxycarbonylalkyl, alkyl,
alkylcarbonyl, alkylcarbonyloxy, alkylthzo, alkynyl, carboxy, carboxyalkyl,
cyano, cyanoalkyl,
formyl, formylalkyl, haloalkoxy, haloalkyl, halogen, hydroxy, hydroxyalkyl,
mercapto,
mercaptoalkyl, vitro, triphenylmethyl (trityl), -C(NH)NR1oR11, -NR1oR11,
(N~oRll)alkyl,
(NR1oR11)carbonyl, (NR1oR11)carbonylalkyl, (NRIO~ 1)sulfonyl, NR12S(O)2R13,
'C~12~~13RI4, -CHZC(NR12)NR13R14, -C(NOR12)Rl3a -C(NCl~Rl2, -C(I~1NR12R13)R14,
-S(O)20R12, and -S(O)2R12 wherein R12, R13, and R14 are independently selected
from hydrogen,
alkyl, aryl, and arylalkyl, as defined herein. Representative examples
include, but are not limited
to, 5-[amino(imino)methyl]thien-2-yl, 5-(aminosulfonyl)thien-2-yl, 5-bromo-3-
pyridinyl, 3-
bromo-1,2,4-thiadiazol-5-yl, 6-chloro-3-pyridinyl, 5-chloro-3-pyridinyl, 6-
chloro-5-fluoro-3-
pyridinyl, 6-chloro-5-methyl-3-pyridinyl, 5-eyano-3-pyridinyl, 5,6-dichloro-3-
pyridinyl, 3,5-
dimethyl-4-isoxazolyl, 6-fluoro-3-pyridinyl, 5-methoxy-3-pyridinyl, 3-methyl-5-
isoxazolyl, 2-
methyl-3-pyridinyl, 6-methyl-3-pyridinyl, 6-methyl-2-pyridinyl, 2-methyl-2H-
tetrazol-5-yl, 5-
nitro-I,3-thiazol-2-yl, 6-phenyl-3-pyridazinyl, and 5-vinyl-3-pyridinyl.
The term "hydroxy" as used herein, means an -OH group.
93
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
The term "hydroxyalkyl" as used herein, means at least one hydroxy group, as
defined
herein, appended to the parent molecular moiety through an alkyl group, as
defined herein.
Representative examples of hydroxyalkyl include, but are not limited to,
hydroxymethyl, 2
hydroxyethyl, 3-hydroxypropyl, 2,3-dihydroxypropyl, and 2-ethyl-4-
hydroxyheptyl.
The term "mercapto" as used herein, means a -SH group.
The term "mercaptoalkyl" as used herein, means at least one mercapto group, as
defined
herein, appended to the parent molecular moiety through an alkyl group, as
defined herein.
Representative examples of mercaptoalkyl include, but are not limited to,
thiornethyl, 2-thioethyl
and 3-thiopropyl.
The term " NR1oR11" as used herein, means two groups, Rlo and R11, which are
appended
to the parent molecular moiety through a nitrogen atom. Rlo and Rl l are
independently sel~ted
from hydrogen, alkyl, allcylcarbonyl, aryl, and arylalkyl as defined herein.
Representative
examples of -NRIORi 1 include, but are not limited to, acetylamino, amino,
benzylamino,
methylamino, dimethylamino, ethylamino, phenylamino, and methylcarbonylamino.
The term "(NRIORlI)alkyl" as used herein, means a -NR1oR11, as defined herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of (NR1oR11)alkyl include, but are not limited,
aminomethyl,
(methylamino)methyl, 2-aminoethyl, and (dimethylamino)methyl.
The term "(NR1oR11)carbonyl" as used herein, means a -NRI~lZl1 group, as
defined
herein, appended to the parent molecular moiety through a carbonyl group, as
defined herein.
Representative examples of (NR1oR11)carbonyl include, but are not limited to,
aminocarbonyl,
dimethylaminocarbonyl, methylaminocarbonyl, and ethylaminocarbonyl.
The term "(NRIORI~)carbonylalkyl" as used herein, means a (NR1oR11)carbonyl
group, as
defined herein, appended to the parent molecular moiety through an alkyl
group, as defined
herein. Representative examples of (NRIOR11)carbonylalkyl include, but are not
limited to, 2-
amino-2-oxoethyl, 2-(methylamino)-2-oxoethyl, 4-amino-4-oxobutyl, and 4-
(dimethylamino)-4-
oxobutyl.
The term "(NR1oR11)sulfonyl" as used herein, means a-NRIORn group, as defined
herein,
appended to the parent molecular moiety through a sulfonyl group, as defined
herein.
Representative examples of (NR1oR11)sulfonyl include, but are not limited to,
aminosulfonyl,
94
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
dimethylaminosulfonyl, methylaminosulfonyl, ethylaminosulfonyl,
phenylaminosulfonyl and
benzylaminosulfonyl.
The term "nitrogen protecting group" or "N-protecting group" as used herein,
means
those groups intended to protect an amino group against undesirable reactions
during synthetic
procedures. Nitrogen protecting groups comprise caxbamates, amides, N-benzyl
derivatives, and
imine derivatives. Preferred nitrogen protecting groups are acetyl, benzoyl,
benzyl,
benzyloxycarbonyl (Cbz), formyl, phenylsulfonyl, pivaloyl, tart butoxycarbonyl
(Boc),
trifluoroacetyl, and triphenylmethyl (trityl).
The term "nitro" as used herein, means a NOZ group.
The term "oxo" as used herein, means a =O moiety.
The term "oxy" as used herein, means a -O- moiety.
The term "sulfonyl" as used herein, means a -S02- group.
Compounds of the present invention were named by ACD/ChemSketch version 5.0
(developed by Advanced Chemistry Development, Inc., Toronto, ON, Canada) or
were given
names which appeared to be consistent with ACD nomenclature.
It is to be understood that compounds of the present invention can be either
cis or trans
and that the cis and trans arrangements are included within the scope of the
present invention.
The present invention contemplates stereoisomers and mixtures thereof which
are
specifically included within the scope of this invention. Stereoisomers
include enantiomers,
diastereomers, and mixtures of enantiomers or diastereomers. Individual
stereoisomers of
compounds of the present invention may be prepared synthetically from
commercially available
starting materials which contain asymmetric or chiral centers or by
preparation of racemic
mixtures followed by resolution well-known to those of ordinary skill in the
art. These methods
of resolution are exemplified by (1) attachment of a mixture of enantiomers to
a chiral auxiliary,
separation of the resulting mixture of diastereomers by recrystallization or
chromatography and
liberation of the optically pure product from the auxiliary or (2) direct
separation of the mixture
of optical enantiomers on chiral chromatographic columns.
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Determination of Biological Actiyity
In Vitro Data
Determination of Nicotinic Acetylcholine Receptor Binding Potencies
Compounds of the present invention were subjected to an in vitro assay against
the
nicotinic acetylcholine receptor as described below and were found to be
effective binders to the
receptor. The In Vitro protocols for determination of nicotinic acetylcholine
channel receptor
binding potencies of ligands were determined as follows.
Binding of [3H]-cytisine ([3H]-CYT) to neuronal nicotinic acetylcholine
receptors was
accomplished using crude synaptic membrane preparations from whole rat brain
(Pabreza et al.,
Molecular Pharmacol., 1990, 39:9). Washed membranes were stored at -80
°C prior to use.
Frozen aliquots were slowly thawed and resuspended in 20 volumes of buffer
(containing: 120
mM NaCI, 5 rnM KCI, 2 mM MgCl2, 2 mM CaCl2 and 50 mM Tris-Cl, pH 7.4 a~4
°C). After
centrifuging at 20,OOOx g for 15 minutes, the pellets were resuspended in 30
volumes of buffer.
The test compounds were dissolved in water to make 10 mM stock solutions. This
solution was then diluted (1:100) with buffer (as above) and further taken
through seven serial
log dilutions to produce test solutions from 10-5 to 10-~ 1 M.
Homogenate (containing 125-150 pg protein) was added to triplicate tubes
containing the
range of concentrations of test compound described above and [3H]-CYT (1.25
nM) in a final
volume of 500 p,L. Samples were incubated for 60 minutes at 4 °C, then
rapidly filtered through
Whatman GFB filters presoaked in 0.5% polyethyleneimine using 3 x 4 mL of ice-
cold buffer.
The filters are counted in 4 mL of Ecolume~ (ICN). Nonspecific binding was
determined in the
presence of 10 wM (-)-nicotine and values were expressed as a percentage of
total binding. ICso
values were determined with the RS-1 (BBN) nonlinear least squares curve-
fitting program arid
ICSO values were converted to Ki values using the Cheng and Prusoff correction
(K;=ICso/(1+[ligand]/Kd of ligand).
Representative compounds of the present invention bound to nicotinic
acetylcholine
receptors with binding affinities from 2300 nM to 0.029 nM. Preferred
compounds of the
present invention bound to nicotinic acetylcholine receptors with binding
affinities less than or
equal to 100 nM.
96
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Tn Viva T)ata
Determination of Effectiveness of Nicotinic Acetylcholine Receptor Ligands as
Analgesic
Agents in the Mouse Hot Plate Paradi~n
An in vivo protocol was utilized to determine the effectiveness of nicotinic
acetylcholine
receptor ligands as analgesic agents in the mouse hot plate paradigm.
Separate groups of mice, (n=8/group) were utilized for dose group. AlI drugs
were
administered by the intraperitoneal route of administration. Test drugs were
dissolved in water
to make a 6.2 mM stock solution. Animals were dosed with this solution (10
mL/kg body
weight) for a 62 micromollkg dose. Lower doses were administered similarly,
following serial
dilution of the stock solution in half log increments. Animals were dosed 30
minutes prior to
testing in the hot plate. The hot-plate utilized was an automated analgesia
monitor (Model
#AHP16AN, Omnitech Electronics, Inc. of Columbus, Ohio). The temperature of
the hot plate
was maintained at 55 °C and a cut-off time of 180 seconds was utilized.
Latency until the tenth
jump was recorded as the dependent measure. An increase in the tenth jump
latency relative to
the control was considered an effect.
Representative compounds of the present invention showed an antinociceptive
effect in
the mouse hot plate paradigm at doses ranging from 62 ~.mol/kg to 6.2 pmol/kg.
Preferred
compounds of the present invention showed an antinociceptive effect in the
mouse hot plate
paradigm at doses less than or equal to 62 p,mol/kg.
Determination of Effectiveness of Nicotinic Acetylcholine Receptor Ligands as
Analgesic
Agents in the Rat Formalin Test
Another in vivo protocol utilized to determine the effectiveness of nicotinic
acetylcholine
receptor ligands as analgesic agents was the rat formalin test.
Male Sprague-Dawley rats (Charles River, Portage, MI) weighing 200 to 400
grams were
used for all experiments. After a 20 minute period of acclimation to
individual cages, 50 pL of a
5% formalin solution was injected subcutaneous into the dorsal aspect of one
of the rear paws
and the rats were then returned to the clear observation cages suspended above
mirror panels.
Rats were observed during phase 2 of the formalin test which was defined as
the 20 minute
period from 30 to 50 minutes after formalin injection. The investigator
recorded nocifensive
97
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
behaviors in the injected paw of four animals during the session by observing
the animals for one
15 second observation period during each 1 minute interval. Nocifensive
behaviors recorded
included flinching, licking or biting the injected paw. In dose~esponse
studies, the test
compound (or saline) was administered intraperitoneally 5 minutes before
injection of formalin.
Representative compounds of the present invention showed an antinociceptive
effect in
the rat formalin test at doses ranging from 62 l,unol/kg to 1.9 ~.mol/kg.
Preferred compounds of
the present invention showed an antinociceptive effect in the rat formalin
test at doses less than
or equal to 62 pmol/kg.
The in vitro and in vivo data demonstrates that compounds of the present
invention bind
to the nicotinic acetylcholine receptor and are useful for treating pain.
The ability of the compounds of the present invention, including but not
limited to those
specified in the examples, to treat pain via the nicotinic acetylcholine
receptors andthe
cholinergic system can be further demonstrated by Williams, M.; Arneric, S.
P.: Beyond the
Tobacco Debate: dissecting out the therapeutic potential of nicotine. Exp.
Opin. Invest. Drugs
(1996)5(8): 1035-1045; and Arneric, S. P.; Sullivan, J. P.; Williams, W.:
Neuronal nicotinic
acetylcholine receptors. Novel targets for central nervous system theraputics.
in:
Psychopharmacology: The Fourth Generation of Progress. Bloom FE, Kupfer DJ
(Eds.), Raven
Press, New York (1995): 95-109.
Additionally, compounds of the present invention are useful for ameliorating
or
preventing disorders affected by nicotinic acetylcholine receptors and the
cholinergic system,
such as Alzheimer's disease, Parkinson's disease, memory dysfunction,
Tourette's syndrome,
sleep disorders, attention deficit hyperactivity disorder, neurodegeneration,
inflammation,
neuroprotection, anxiety, depression, mania, schizophrenia, anorexia and other
eating disorders,
AIDS-induced dementia, epilepsy, urinary incontinence, substance abuse,
smoking cessation and
inflammatory bowel syndrome.
The ability of the compounds of the present invention, including but not
limited to those
specified in the examples, to treat Alzheimer's disease can be demonstrated by
Williams, M.;
Arneric, S. P.: Beyond the Tobacco Debate: dissecting out the therapeutic
potential of nicotine.
Exp. Opin. Invest. Drugs (1996)5(8): 1035-1045; Arneric, S. P.; Sullivan, J.
P.; Williams, W.:
Neuronal nicotinic acetylcholine receptors. Novel targets for central nervous
system theraputics.
98
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
in: Psychopharmacology: The Fourth Generation of Progress. Bloom FE, Kupfer DJ
(Eds.),
Raven Press, New York (1995): 95-109; Arneric, S. P.; Holladay, M. W.;
Sullivan, J. P.:
Cholinergic channel modulators as a novel therapeutic strategy fox Alzheimer's
disease. Exp.
Opin. Invest. Drugs (1996) 5(1): 79-100; Lindstrom, J.: Nicotinic
Acetylchloline Receptors in
Health and Disease. Molecular Neurobiology (1997) 15: 193-222; and Lloyd, G K;
Menzaghi, F;
Bontempi B; Suto, C; Siegel, R; Akong, M; Stauc~rman, K; Velicelebi, G;
Johnson, E; Harpold,
M M; Rao, T S; Sacaan, A I; Chavez-Noriega, L E; Washburn, M S; Vernier, J M;
Cosford, N D
P; McDonald, L A: The potential of subtype selective neuronal nicotinic
acetylcholine receptor
agonists as therapeutic agents. Life Sciences (1998)62(17/18): 1601-1606.
The ability of the compounds of the present invention, including but not
limited to those
specified in the examples, to treat Parkinson's disease can be demonstrated by
Williams, M.;
Arneric, S. P.: Beyond the Tobacco Debate: dissecting out the therapeutic
potential of nicotine.
Exp. Opin. Invest. Drugs (1996)5(8): 1035-1045; Lindstrom, J.: Nicotinic
Acetylchloline
Receptors in Health and Disease. Molecular Neurobiology (1997) 15: 193-222;
and Lloyd, G K;
Menzaghi, F; Bontempi B; Suto, C; Siegel, R; Akong, M; Stauderman, K;
Velicelebi, G;
Johnson, E; Haxpold, M M; Rao, T S; Sacaan, A I; Chave~Noriega, L E; Washburn,
M S;
Vernier, J M; Cosford, N D P; McDonald, L A: The potential of subtype
selective neuronal
nicotinic acetylcholine receptor agonists as therapeutic agents. Life Sciences
(1998)62(17/18):
1601-1606.
The ability of the compounds of the present invention, including but not
limited to those
specified in the examples, to treat memory dysfunction can be demonstrated by
Williams, M.;
Arneric, S. P.: Beyond the Tobacco Debate: dissecting out the therapeutic
potential of nicotine.
Exp. Opin. Invest. Drugs (1996)5(8): 1035-1045; Arneric, S. P.; Sullivan, J.
P.; Williams, W.:
Neuronal nicotinic acetylcholine receptors. Novel targets for central nervous
system theraputics.
in: Psychopharmacology: The Fourth Generation of Progress. Bloom FE, Kupfer DJ
(Eds.),
Raven Press, New York (1995): 95-109; and Lindstrom, J.: Nicotinic
Acetylchloline Receptors
in Health and Disease. .Molecular Neurobiology (1997) 15: 193-222.
The ability of the compounds of the present invention, including but not
limited to those
specified in the examples, to treat Tourette's syndrome can be demonstrated by
Williams, M.;
Arneric, S. P.: Beyond the Tobacco Debate: dissecting out the therapeutic
potential of nicotine.
99
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Exp. Opin. Invest. Drugs (1996)S(8): 1035-1045; Arneric, S. P.; Sullivan, J.
P.; Williams, W.:
Neuronal nicotinic acetylcholine receptors. Novel targets for central nervous
system theraputics.
in: Psychopharmacology: The Fourth Generation of Progress. Bloom FE, Kupfer DJ
(Eds.),
Raven Press, New York (1995): 9S-109; and Lindstrom, J.: Nicotinic
Acetylchloline Receptors
in Health and Disease. Molecular Neurobiology (1997) 1S: 193-222.
The ability of the compounds of the present invention, including but not
limited to those
specified in the examples, to treat sleeping disorders can be demonstrated by
Williams, M.;
Arneric, S. P.: Beyond the Tobacco Debate: dissecting out the therapeutic
potential of nicotine.
Exp. Opin. Invest. Drugs (1996)5(8): 1035-1045.
The ability of the compounds of the present invention, including but not
limited to those
specified in the examples, to treat attention deficit hyperactivity disorder
can be demonstrated by
Williams, M.; Arneric, S. P.: Beyond the Tobacco Debate: dissecting out the
therapeutic
potential of nicotine. Exp. Opin. Invest. Drugs (1996)S(8): 1035-1045; and
Arneric, S. P.;
Holladay, M. W.; Sullivan, J. P.: Cholinergic channel modulators as a novel
therapeutic strategy
for Alzheimer's disease. Exp. Opin. Invest. Drugs (1996) S(1): 79-100.
The ability of the compounds of the present invention, including but not
limited to those
specified in the examples, to treat neurodegeneration and to provide
neuroprotection can be
demonstrated by Arneric, S. P.; Sullivan, J. P.; Williams, W.: Neuronal
nicotinic acetylcholine
receptors. Novel targets for central nervous system theraputics, in:
Psychopharmacology: The
Fourth Generation of Progress. Bloom FE, Kupfer DJ (Eds.), Raven Press, New
York (1995):
9S-109; and Arneric, S. P.; Holladay, M. W.; Sullivan, J. P.: Cholinergic
channel modulators as
a novel therapeutic strategy for Alzheimer's disease. Exp. Opin. Invest. Drugs
(1996) S(1): 79-
100.
The ability of the compounds of the present invention, including but not
limited to those
specified in the examples, to treat inflammation can be demonstrated by
Arneric, S. P.; Sullivan,
J. P.; Williams, W.: Neuronal nicotinic acetylcholine receptors. Novel targets
for central nervous
system theraputics. in: Psychopharmacology: The Fourth Generation of Progress.
Bloom FE,
Kupfer DJ (Eds.), Raven Press, New York (1995): 9S-109; and Arneric, S. P.;
Holladay, M. W.;
Sullivan, J. P.: Cholinergic channel modulators as a novel therapeutic
strategy for Alzheimer's
disease. Exp. Opin. Invest. Drugs (1996) S(1): 79-100.
100
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
The ability of the compounds of the present invention, including but not
limited to those
specified in the examples, to treat amyotrophic lateral sclerosis can be
demonstrated by
Williams, M.; Arneric, S. P.: Beyond the Tobacco Debate: dissecting out the
therapeutic
potential of nicotine. Exp. Opin. Invest. Drugs (1996)5(8): 1035-1045;
Arneric, S. P.; Sullivan,
J. P.; Williams, W.: Neuronal nicotinic acetylcholine receptors. Novel targets
for central nervous
system theraputics. in: Psychopharmacology: The Fourth Generation of Progress.
Bloom FE,
Kupfer DJ (Eds.), Raven Press, New York (1995): 95-109; and Arneric, S. P.;
Holladay, M. W.;
Sullivan, J. P.: Cholinergic channel modulators as a novel therapeutic
strategy for Alzheimer's
disease. Exp. Opin. Invest. Drugs (1996) 5(1): 79-100.
The ability of the compounds of the present invention, including but not
limited to those
specified in the examples, to treat anxiety can be demonstrated by Williams,
M.; Arneric, S. P.:
Beyond the Tobacco Debate: dissecting out the therapeutic potential of
nicotine. Exp. Opin.
Invest. Drugs (1996)5(8): 1035-1045; Arneric, S. P.; Sullivan, J. P.;
Williams, W.: Neuronal
nicotinic acetylcholine receptors. Novel targets for central nervous system
theraputics. in:
Psychopharmacology: The Fourth Generation of Progress. Bloom FE, Kupfer DJ
(Eds.), Raven
Press, New York (1995): 95-109; and Arneric, S. P.; Holladay, M. W.; Sullivan,
J. P.:
Cholinergic channel modulators as a novel therapeutic strategy for Alzheimer's
disease. Exp.
Opin. Invest. Drugs (1996) 5(1): 79-100.
The ability of the compounds of the present invention, including but not
limited to those
specified in the examples, to treat depression can be demonstrated by Arneric,
S. P.; Sullivan, J.
P.; Williams, W.: Neuronal nicotinic acetylcholine receptors. Novel targets
for central nervous
system theraputics. in: Psychopharmacology: The Fourth Generation of Progress.
Bloom FE,
Kupfer DJ (Eds.), Raven Press, New York (1995): 95-109.
The ability of the compounds of the present invention, including but not
limited to those
specified in the examples, to treat mania and schizophrenia can be
demonstrated by Williams,
M.; Arneric, S. P.: Beyond the Tobacco Debate: dissecting out the therapeutic
potential of
nicotine. Exp. Opin. Invest. Drugs (1996)5(8): 1035-1045; Arneric, S. P.;
Sullivan, J. P.;
Williams, W.: Neuronal nicotinic acetylcholine receptors. Novel targets for
central nervous
system theraputics. in: Psychopharmacology: The Fourth Generation of Progress.
Bloom FE,
Kupfer DJ (Eds.), Raven Press, New York (1995): 95-109; and Lindstrom, J.:
Nicotinic
101
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Acetylchloline Receptors in Health and Disease. Molecular Neurobiology (1997)
15: 193-222.
The ability of the compounds of the present invention, including but not
limited to those
specified in the examples, to treat anorexia and other eating disorders can be
demonstrated by
Williams, M.; Arneric, S. P.: Beyond the Tobacco Debate: dissecting out the
therapeutic
potential of nicotine. Exp. Opin. Invest. Drugs (1996)5(8): 1035-1045;
Arneric, S. P.; Sullivan,
J. P.; Williams, W.: Neuronal nicotinic acetylcholine receptors. Novel targets
for central nervous
system theraputics. in: Psychopharmacology: The Fourth Generation of Progress.
Bloom FE,
Kupfer DJ (Eds.), Raven Press, New York (1995): 95-109; and Lindstrom, J.:
Nicotinic
Acetylchloline Receptors in Health and Disease. Molecular Neurobiology (1997)
15: 193-222.
The ability of the compounds of the present invention, including but not
limited to those
specified in the examples, to treat AIDS-induced dementia can be demonstrated
by Williams, M.;
Arneric, S. P.: Beyond the Tobacco Debate: dissecting out the therapeutic
potential of nicotine.
Exp. Opin. Invest. Drugs (1996)5(8): 1035-1045; Arneric, S. P.; Sullivan, J.
P.; Williams, W.:
Neuronal nicotinic acetylcholine receptors. Novel targets for central nervous
system theraputics.
in: Psychopharmacology: The Fourth Generation of Progress. Bloom FE, Kupfer DJ
(Eds.),
Raven Press, New York (1995): 95-109; and Lindstrom, J.: Nicotinic
Acetylchloline Receptors
in Health and Disease. Molecular Neurobiology (1997) 15: 193-222.
The ability of the compounds of the present invention, including but not
limited to those
specified in the examples, to treat epilepsy can be demonstrated by Williams,
M.; Arneric, S. P.:
Beyond the Tobacco Debate: dissecting out the therapeutic potential of
nicotine. Exp. Opin.
Invest. Drugs (1996)5(8): 1035-1045; Arneric, S. P.; Sullivan, J. P.;
Williams, W.: Neuronal
nicotinic acetylcholine receptors. Novel targets for central nervous system
theraputics. in:
Psychopharmacology: The Fourth Generation of Progress. Bloom FE, Kupfer DJ
(Eds.), Raven
Press, New York (1995): 95-109; and Lindstrom, J.: Nicotinic Acetylchloline
Receptors in
Health and Disease. Molecular Neurobiology (1997) 15: 193-222.
The ability of the compounds of the present invention, including but not
limited to those
specified in the examples, to treat urinary incontinence can be demonstrated
by Williams, M.;
Arneric, S. P.: Beyond the Tobacco Debate: dissecting out the therapeutic
potential of nicotine.
Exp. Opin. Invest. Drugs (1996)5(8): 1035-1045.
The ability of the compounds of the present invention, including but not
limited to those
102
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
specified in the examples, to treat premenstrual syndrome can be demonstrated
by Williams, M.;
Arneric, S. P.: Beyond the Tobacco Debate: dissecting out the therapeutic
potential of nicotine.
Exp. Opin. Invest. Drugs (1996)5(8): 1035-1045; and Arneric, S. P.; Sullivan,
J. P.; Williams,
W.: Neuronal nicotinic acetylcholine receptors. Novel targets for central
nervous system
theraputics. in: Psychopharmacology: The Fourth Generation of Progress. Bloom
FE, Kupfer DJ
(Eds.), Raven Press, New York (1995): 9S-109.
The ability of the compounds of the present invention, including but not
limited to those
specified in the examples, to treat substance abuse can be demonstrated by
Williams, M.;
Arneric, S. P.: Beyond the Tobacco Debate: dissecting out the therapeutic
potential of nicotine.
Exp. Opin. Invest. Drugs (1996)5(8): 1035-1045; and Arneric, S. P.; Sullivan,
J. P.; Williams,
W.: Neuronal nicotinic acetylcholine receptors. Novel targets for central
nervous system
theraputics. in: Psychopharmacology: The Fourth Generation of Progress. Bloom
FE, Kupfer DJ
(Eds.), Raven Press, New York (1995): 95-109.
The ability of the compounds of the present invention, including but not
limited to those
specified in the examples, to treat smoking cessation can be demonstrated by
Williams, M.;
Arneric, S. P.: Beyond the Tobacco Debate: dissecting out the therapeutic
potential of nicotine.
Exp. Opin. Invest. Drugs (1996)5(8): 1035-1045; and Arneric, S. P.; Sullivan,
J. P.; Williams,
W.: Neuronal nicotinic acetylcholine receptors. Novel targets for central
nervous system
theraputics. in: Psychopharmacology: The Fourth Generation of Progress. Bloom
FE, Kupfer DJ
(Eds.), Raven Press, New York (1995): 95-109.
The ability of the compounds of the present invention, including but not
limited to those
specified in the examples, to treat inflammatory bowel syndrome can be
demonstrated by
Williams, M.; Arneric, S. P.: Beyond the Tobacco Debate: dissecting out the
therapeutic
potential of nicotine. Exp. Opin. Invest. Drugs (1996)5(8): 1035-1045; and
Lindstrom, J.:
Nicotinic Acetylchloline Receptors in Health and Disease. Molecular
Neurobiology (1997) 15:
193-222.
The term "pharmaceutically acceptable carrier," as used herein, means a
nontoxic, inert
solid, semi-solid or liquid filler, diluent, encapsulating material or
formulation auxiliary of any
type. Some examples of materials which can serve as pharmaceutically
acceptable carriers are
sugars such as lactose, glucose and sucrose; starches such as corn starch and
potato starch;
103
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
cellulose and its derivatives such as sodium carboxymethyl cellulose, ethyl
cellulose and
cellulose acetate; powdered tragacanth; malt; gelatin; talc; excipients such
as cocoa butter and
suppository waxes; oils such as peanut oil, cottonseed oil, safflower oil,
sesame oil, olive oil,
corn oil and soybean oil; glycols, such a propylene glycol; esters such as
ethyl oleate and ethyl
laurate; agar; buffering agents such as magnesium hydroxide and aluminum
hydroxide; alginic
acid; pyrogen-free water; isotonic saline; Ringer's solution; ethyl alcohol,
and phosphate buffer
solutions, as well as other non-toxic compatible lubricants such as sodium
lauryl sulfate and
magnesium stearate, as well as coloring agents, releasing agents, coating
agents, sweetening,
flavoring and perfuming agents, preservatives and antioxidants can also be
present in the
composition, according to the judgment of the formulator. The present
invention provides
pharmaceutical compositions which comprise compounds of the present invention
formulated
together with one or more non-toxic pharmaceutically acceptable carriers. The
pharmaceutical
compositions can be formulated for oral administration in solid or liquid
form, for parenteral
injection or for rectal administration.
Dosage forms for topical administration of a compound of the present invention
include
powders, sprays, ointments and inhalants. The active compound is mixed under
sterile
conditions with a pharmaceutically acceptable carrier and any needed
preservatives, buffers or
propellants which can be required. Opthalmic formulations, eye ointments,
powders and
solutions are also contemplated as being within the scope of this invention.
Actual dosage levels of active ingredients in the pharmaceutical compositions
of this
invention can be varied so as to obtain an amount of the active compounds)
which is effective to
achieve the desired therapeutic response for a particular patient,
compositions, and mode of
administration.
When used in the above or other treatments, a therapeutically effective amount
of one of
the compounds of the present invention can be employed in pure form or, where
such forms
exist, in pharmaceutically acceptable salt, ester, amide, or prodrug form.
Alternatively, the
compound can be administered as a pharmaceutical composition containing the
compound of
interest in combination with one or more pharmaceutically acceptable carriers.
The phrase
"therapeutically effective amount" of the compound of the present invention
means a sufficient
amount of the compound to treat disorders, at a reasonable benefit/risk ratio
applicable to any
104
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
medical treatment. The specific therapeutically effective dose level for any
particular patient
will depend upon a variety of factors including the disorder being treated and
the severity of the
disorder; activity of the specific compound employed; the specific composition
employed; the
age, body weight, general health, sex and diet of the patient; the time of
administration, route of
administration, and rate of excretion of the specific compound employed; the
duration of the
treatment; drugs used in combination or coincidental with the specific
compound employed; and
like factors well known in the medical arts.
The total daily dose of the compounds of the present invention administered to
a
mammal, and particularly a human, may range from about 0.01 to about 50
mg/kg/day. More
preferable doses can be in the range of from about 0.01 to about 5 mg/kg/day.
If desired, the
effective daily dose can be divided into multiple doses for purposes of
administration;
consequently, single dose compositions may contain such amounts or
submultiples thereof to
make up the daily dose.
The present invention also provides pharmaceutical compositions that comprise
compounds of the present invention formulated together with one or more
noirtoxic
pharmaceutically acceptable carriers. The pharmaceutical compositions can be
specially
formulated for oral administration in solid or liquid form, for parenteral
injection or for rectal
administration.
The pharmaceutical compositions of this invention can be administered to
humans and
other mammals orally, rectally, parenterally, intracisternally,
intravaginally, intraperitoneally,
topically (as by powders, ointments or drops), bucally or as an oral or nasal
spray. The term
"parenterally," as used herein, refers to modes of administration which
include intravenous,
intramuscular, intraperitoneal, intrasternal, subcutaneous and intraarticular
injection and
infusion.
Pharmaceutical compositions of this invention for parenteral injection
comprise
pharmaceutically acceptable sterile aqueous or nonaqueous solutions,
dispersions, suspensions or
emulsions as well as sterile powders for reconstitution into sterile
injectable solutions or
dispersions just prior to use. Examples of suitable aqueous and nonaqueous
carriers, diluents,
solvents or vehicles include water, ethanol, polyols (such as glycerol,
propylene glycol,
polyethylene glycol and the like), vegetable oils (such as olive oil),
injectable organic esters
105
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(such as ethyl oleate) and suitable mixtures thereof. Proper fluidity can be
maintained, for
example, by the use of coating materials such as lecithin, by the maintenance
of the required
particle size in the case of dispersions and by the use of surfactants.
These compositions may also contain adjuvants such as preservatives, wetting
agents,
emulsifying agents and dispersing agents. Prevention of the action of
microorganisms can be
ensured by the inclusion of various antibacterial and antifungal agents, for
example, paraben,
chlorobutanol, phenol sorbic acid and the like. It may also be desirable to
include isotonic agents
such as sugars, sodium chloride and the like. Prolonged absorption of the
injectable
pharmaceutical form can be brought about by the inclusion of agents which
delay absorption
such as aluminum monostearate and gelatin.
In some cases, in order to prolong the effect of the drug, it is desirable to
slow the
absorption of the drug from subcutaneous or intramuscular injection. This can
be accomplished
by the use of a liquid suspension of crystalline or amorphous material with
poor water solubility.
The rate of absorption of the drug then depends upon its rate of dissolution
which, in turn, may
depend upon crystal size and crystalline form. Alternatively, delayed
absorption of a
parenterally administered drug forni is accomplished by dissolving or
suspending the drug in an
oil vehicle.
Injectable depot forms are made by forming microencapsule matrices of the drug
in
biodegradable polymers such as polylactide-polyglycolide. Depending upon the
ratio of drug to
polymer and the nature of the particular polymer employed, the rate of drug
release can be
controlled. Examples of other biodegradable polymers include poly(orthoesters)
and
poly(anhydrides). Depot injectable formulations are also prepared by
entrapping the drug in
liposomes or microemulsions which are compatible with body tissues.
The injectable formulations can be sterilized, for example, by filtration
through a
bacterial-retaining filter or by incorporating sterilizing agents in the form
of sterile solid
compositions which can be dissolved or dispersed in sterile water or other
sterile injectable
medium just prior to use.
Solid dosage forms for oral administration include capsules, tablets, pills,
powders and
granules. In such solid dosage forms, the active compound may be mixed with at
least one inert,
pharmaceutically acceptable excipient or carrier, such as sodium citrate or
dicalcium phosphate
106
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
andlor a) fillers or extenders such as starches, lactose, sucrose, glucose,
mannitol and silicic acid;
b) binders such as carboxymethylcellulose, alginates, gelatin,
polyvinylpyrrolidone, sucrose and
acacia; c) humectants such as glycerol; d) disintegrating agents such as agar-
agar, calcium
carbonate, potato or tapioca starch, alginic acid, certain silicates and
sodium carbonate; e)
solution retarding agents such as paraffin; f) absorption accelerators such as
quaternary
ammonium compounds; g) wetting agents such as cetyl alcohol and glycerol
monosteaxate; h)
absorbents such as kaolin and bentonite clay and i) lubricants such as talc,
calcium stearate,
magnesium stearate, solid polyethylene glycols, sodium lauryl sulfate and
mixtures thereof. In
the case of capsules, tablets and pills, the dosage form may also comprise
buffering agents.
Solid compositions of a similar type may also be employed as fillers in soft
and hard-
filled gelatin capsules using such excipients as lactose or milk sugar as well
as high molecular
weight polyethylene glycols and the like.
The solid dosage forms of tablets, capsules, pills and granules can be
prepared with
coatings and shells such as enteric coatings and other coatings well-known in
the pharmaceutical
formulating art. They may optionally contain opacifying agents and may also be
of a
composition such that they release the active ingredients) only, or
preferentially, in a certain part
of the intestinal tract, optionally, in a delayed manner. Examples of
embedding compositions
which can be used include polymeric substances and waxes.
The active compounds can also be in micro-encapsulated form, if appropriate,
with one
or more of the above-mentioned excipients.
Liquid dosage forms for oral administration include pharmaceutically
acceptable
emulsions, solutions, suspensions, syrups and elixirs. In addition to the
active compounds, the
liquid dosage forms may contain inert diluents commouy used in the art such
as, for example,
water or other solvents, solubilizing agents and emulsifiers such as ethyl
alcohol, isopropyl
alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate,
propylene glycol, 1,3-
butylene glycol, dimethyl formamide, oils (in particular, cottonseed,
groundnut, corn, germ,
olive, castor and sesame oils), glycerol, tetrahydrofurfuryl alcohol,
polyethylene glycols and
fatty acid esters of sorbitan and mixtures thereof.
Besides inert diluents, the oral compositions may also include adjuvants such
as wetting
agents, emulsifying and suspending agents, sweetening, flavoring and perfuming
agents.
107
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Suspensions, in addition to the active compounds, may contain suspending
agents as, for
example, ethoxylated isostearyl alcohols, polyoxyethylene sorbitol and
sorbitan esters,
microcrystalline cellulose, aluminum metahydroxide, bentonite, agar-agar,
tragacanth and
mixtures thereof.
Compositions for rectal or vaginal administration are preferably suppositories
which can
be prepared by mixing the compounds of the present invention with suitable non-
irritating
excipients or carriers such as cocoa butter, polyethylene glycol or a
suppository wax which are
solid at room temperature but liquid at body temperature and therefore melt in
the rectum or
vaginal cavity and release the active compound.
Compounds of the present invention can also be administered in the form of
liposomes.
As is known in the art, liposomes are generally derived from phospholipids or
other lipid
substances. Liposomes are formed by mono- or multi-lamellar hydrated liquid
crystals which are
dispersed in an aqueous medium. Any non-toxic, physiologically acceptable and
metabolizable
lipid capable of forming liposomes can be used. The present compositions in
liposome form can
contain, in addition to a compound of the present invention, stabilizers,
preservatives, excipients
and the like. The preferred lipids are natural and synthetic phospholipids and
phosphatidyl
cholines (lecithins) used separately or together.
Methods to form liposomes are known in the art. See, for example, Prescott,
Ed.,
Methods in Cell Biology, Volume XIV, Academic Press, New York, N.Y. (1976), p.
33 et seq.
The present invention contemplates pharmaceutically active compounds either
chemically synthesized or formed by in vivo biotransformation to compounds of
formula (I VI).
The compounds of the invention can exist in unsolvated as well as solvated
forms,
including hydrated forms, such as hemi-hydrates. In general, the solvated
forms, with
pharmaceutically acceptable solvents such as water and ethanol among others
are equivalent to
the unsolvated forms for the purposes of the invention.
The term "pharmaceutically acceptable salt, ester, amide, and prodrug," as
used herein,
refers to carboxylate salts, amino acid addition salts, zwitterions, esters,
amides, and prodrugs of
compounds of formula (I-VI) which are within the scope of sound medical
judgement, suitable
for use in contact with the tissues of humans and lower animals without undue
toxicity, irritation,
allergic response, and the like, are commensurate with a reasonable
benefit/risk ratio, and are
108
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
effective for their intended use. Representative examples include, but are not
limited to, 5-
(1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrol-S-yl)-3-pyridinyl acetate, S-
(1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrol-S-yl)-3-pyridinyl benzoate, 2-chloro-S-
(1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrol-S-yl)-3-pyridinyl benzoate, 2-chloro-S-
(1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrol-S-yl)-3-pyridinyl acetate, [2-chloro-S-
(1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrol-S-yl)-3-pyridinyl]methyl benzoate, [2-chloro-S-
(1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrol-S-yl)-3-pyridinyl]methyl acetate, [S-
(1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrol-S-yl)-3-pyridinyl]methyl acetate, [S-
(1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrol-S-yl)-3-pyridinyl]methyl benzoate, 2-acetyl-S-(3-
pyridinyl)-
1,2,3,3a,4,6a-hexahydrocyclopenta[c]pyrrole, 2-acetyl-S-(6-chloro-3-pyridinyl)-
1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole, 5-(6-chloro-3-pyridinyl)-2-(trifluoroacetyl)-
1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole, S-(3-pyridinyl)-2-(trifluoroacetyl)-
1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole, 2-benzoyl-S-(3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole, 2-benzoyl-S-(6-chloro-3-pyridinyl)-
1,2,3,3a,4,6a-
hexahydrocyclopenta[c]pyrrole, phenyl S-(6-chloro-3-pyridinyl)-3,3a,4,6a-
tetrahydrocyclopenta[c]pyrrole-2(1H)-carboxylate, and phenyl 5-(3-pyridinyl)-
3,3a,4,6a-
tetrahydrocyclopenta[c]pyrrole-2(1H)-carboxylate.
The compounds of the present invention can be used in the form of
pharmaceutically
acceptable salts derived from inorganic or organic acids. The term
"pharmaceutically acceptable
salt" means those salts which are, within the scope of sound medical
judgement, suitable for use
in contact with the tissues of humans and Iower animals without undue
toxicity, irritation,
allergic response and the like and are commensurate with a reasonable
benefit/risk ratio.
Pharmaceutically acceptable salts are well-known in the art. The salts can be
prepared in situ
during the final isolation and purification of the compounds of the present
invention or separately
by reacting a free base function with a suitable organic acid. Representative
acid addition salts
include, but are not limited to acetate, adipate, alginate, citrate,
aspartate, benzoate,
benzenesulfonate, bisulfate, butyrate, camphorate, camphorsufonate,
digluconate,
glycerophosphate, hemisulfate, heptanoate, hexanoate, fumarate, hydrochloride,
hydrobromide,
hydroiodide, 2-hydroxyethansulfonate (isethionate), lactate, maleate,
methanesulfonate,
nicotinate, 2-naphthalenesulfonate, oxalate, pamoate, pectinate, persulfate, 3-
phenylpropionate,
109
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
picrate, pivalate, propionate, succinate, tartrate, thiocyanate, phosphate,
glutamate, bicarbonate,
p-toluenesulfonate and undecanoate. Also, the basic nitrogen.containing groups
can be
quateriuzed with such agents as lower alkyl halides such as methyl, ethyl,
propyl, and butyl
chlorides, bromides and iodides; dialkyl sulfates like dimethyl, diethyl,
dibutyl and diamyl
sulfates; long chain halides such as decyl, lauryl, myristyl and stearyl
chlorides, bromides and
iodides; arylalkyl halides like benzyl and phenethyl bromides and others.
Water or oi~soluble or
dispersible products are thereby obtained. Examples of acids which can be
employed to form
pharmaceutically acceptable acid addition salts include such inorganic acids
as hydrochloric
s
acid, hydrobromic acid, sulphuric acid and phosphoric acid and such organic
acids as malefic
acid, fumaric acid, succinic acid and citric acid.
Basic addition salts can be prepared in situ during the final isolation and
purification of
compounds of this invention by reacting a carboxylic acid-containing moiety
with a suitable base
such as the hydroxide, carbonate or bicarbonate of a pharmaceutically
acceptable metal cation or
with ammonia or an organic primary, secondary or tertiary amine.
Pharmaceutically acceptable
salts include, but are not limited to, cations based on alkali metals or
alkaline earth metals such
as lithium, sodium, potassium, calcium, magnesium and aluminum salts and the
like and
nontoxic quaternary ammonia and amine cations including ammonium,
tetramethylammonium,
tetraethylammonium, methylamine, dimethylamine, trimethylamine, triethylamine,
diethylamine,
ethylamine and the like. Other representative organic amines useful for the
formation of base
addition salts include ethylenediamine, ethanolamine, diethanolamine,
piperidine, piperazine and
the like. Preferred salts of the compounds of the present invention include
phosphate, tris and
acetate.
The term "pharmaceutically acceptable prodrug" or "prodrug, "as used herein,
represents
those prodrugs of the compounds of the present invention which are, within the
scope of sound
medical judgement, suitable for use in contact with the tissues of humans and
lower animals
without undue toxicity, irritation, allergic response, and the Like,
commensurate with a
reasonable benefit/risk ratio, and effective for their intended use. Prodrugs
of the present
invention may be rapidly transformed in vivo to compounds of formula (I-VI),
for example, by
hydrolysis in blood.
The term "pharmaceutically acceptable ester" or "ester," as used herein,
refers to esters of
110
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
compounds of the present invention which hydrolyze in vivo and include those
that break down
readily in the human body to leave the parent compound or a salt thereof.
Examples of
pharmaceutically acceptable, non-toxic esters of the present invention include
Cl-to-C6 alkyl
esters and CS-to-C~ cycloalkyl esters, although C1 to-C4 alkyl esters are
preferred. Esters of the
compounds of formula (I-VI) may be prepared according to conventional methods.
The term "pharmaceutically acceptable amide" or "amide," as used herein,
refers to non-
toxic amides of the present invention derived from ammonia, primary C1 to-C6
alkyl amines and
secondary C1-to-C6 dialkyl amines. In the case of secondary amines, the amine
may also be in
the form of a 5- or 6-membered heterocycle containing one nitrogen atom.
Amides derived from
ammonia, C1-to-C3 alkyl primary amides and C1 to-C2 dialkyl secondary amides
are preferred.
Amides of the compounds of formula (I-VI) may be prepared according to
conventional
methods.
Abbreviations
Abbreviations which have been used in the descriptions of the Schemes and the
Examples that follow are: Ac for acetyl; acyl for alkylcarbonyl; AIBN for 2,2'-
azobis(2
methylpropionitrile) N,N-dimethylformamide; Bn for benzyl; dppf for 1,1'-
bis(diphenylphosphino)ferrocene; DMAP for dimethylaminopyridine; DME for
1,2-dimethoxyethane; DMSO for dimethylsulfoxide; EtOAc for ethyl acetate; EtOH
for ethanol;
formalin for a solution of formaldehyde (37% by weight) in water; HPLC for
high pressure
liquid chromatography; LAH for lithium aluminum hydride; LDA for lithium
diisopropylamine;
MeOH for methanol; Ms for mesylate -S02CH3; Tf for (trifluoromethyl)sulfonyl (-
S02CF3);
TFA for trifluoroacetic acid; THF for tetrahydrofuran; TMS for trimethylsilyl;
Ts or tosyl for p~
CH3(C6H4)S02-; and TsOH for para-toluenesulfonic acid.
Preparation of Compounds of The Present Invention
The compounds and processes of the present invention will be better understood
in
connection with the following synthetic schemes and methods which illustrate a
means by which
the compounds of the invention can be prepared.
111
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
The compounds of this invention can be prepared by a variety of procedures and
synthetic routes. Representative procedures and synthetic routes are shown in,
but are not
limited to, Schemes 1-49.
Scheme 1
O
LiAIH4 H-N\~ Boc20 or PCI or (P)20 P-N\
HN
O (1)
(1) Ru02~xH20, NalO4 P N C02H Ac20, NaOAc P_N\~O
\~~C02H
(2) (3)
Boc-N~ 1) Co2(CO)6 -
2) SiO~, N2, 70°C P N O
(3)
Nitrogen-protected (cis)-hexahydrocyclopenta[c]pyrrol-5(1H)-ones of general
formula
(3), wherein P is a suitable nitrogen protecting group such as, but not
limited to, alkyl, benzyl,
triphenylmethyl (trityl), acyl, p-toluenesulfonyl (Ts), benzyloxy carbonyl
(Cbz) or tern
butoxycarbonyl (Boc), may be prepared as described in Scheme 1. (cis)-
3a,4,7,7a-Tetrahydro-
1H-isoindole-1,3(2H)-dione, purchased commexcially or prepared as described in
Helv. Chim.
Acta (1996) 79(3), 875-894 and J. Org. Chem. (1951) 16, SOI-505, may be
treated with lithium
aluminum hydride to provide (cis)-2,3,3a,4,7,7a-hexahydro-1H isoindole as
described in J. Am.
Chem. Soc. (1980) 102(6), 2005-2010. (cis)-2,3,3a,4,7,7a-Hexahydro-1H
isoindole may be
treated with a nitrogen protecting group precursor such as, but not limited
to, di-tert-butyl
Bicarbonate or benzyl chloroformate to provide nitrogen protected isoindoles
of general formula
(1). Nitrogen protected isoindoles of general formula (1) may be oxidatively
cleaved inthe
presence of ruthenium(IV) oxide hydrate and sodium pexiodate as desribed in J.
Org. Chem.
(1981) 46(19), 3936-3938 and Chem. Pharm. Bull. (1995) 43(8), 1318-1324) to
provide diacids
of general formula (2). Diacids of general formula (2) may be treated with
acetic anhydride and
sodium acetate as described in J. Org. Chem. (1989) 54, 5115-5122 to provide
nitrogen-protected
(cis)-hexahydrocyclopenta[c]pynrol-5(1H)-ones of general formula (3).
112
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Alternatively, tert-butyl (cis)-5-oxohexahydrocyclopenta[c]pyrrole-2(1H)-
carboxylate
may be prepared by treating tert-butyl allyl(2-propynyl)carbamate to a
reductive Pauson-Khand
cyclocarbonylation as described in Tetrahedron (1993) 49(23), 5047-5054. An
asymmetric
cyclocarbonylation has also been reported in J. Org. Chem. (1999) 64, 5547-
5550 that can allow
access to analogs in high enantiomeric excess.
Scheme 2
LDA Pd(PPh3)a
P-N~O Ph--~ P-N~OTf (Me3Sn)2, LiCI P-N~SnMe3
(3) (4) (5)
Br2 or 12 /~
(5) P-N~X X = Br, I
(6)
PdCl2dppf, dppf
(4) + O B_B O KOAc, 1,4-dioxane P N BO
O ~O _ ~ O
(7)
Vinyl triflates of general formula (4), vinyl stannanes of general formula
(5), vinyl
halides of general formula (6) and vinyl boronates of general formula (7),
wherein P is a suitable
nitrogen protecting group such as, but not limited to, alkyl, benzyl, trityl,
acyl, p~toluenesulfonyl
(Ts), benzyloxy carbonyl (Cbz) or tert-butoxycarbonyl (Boc), may be prepared
as described in
Scheme 2. Nitrogen protected (cis)-hexahydrocyclopenta[c]pyrrol-5(1H)-ones of
general
formula (3) may be treated with a base such as, but not limited to, lithium
diisopropylamide or
sodium bis(trimethylsilyl)amide or potassium bis(trimethylsilyl)amide and N-
phenyltrifluormethanesulfonimide to provide vinyl triflates of general formula
(4) as described in
Tetrahedron Lett. (1983) 24(10), 979-282. Vinyl triflates of general formula
(4) may be further
elaborated into vinyl stannanes of general formula (5) as described in J. Org.
Chem. (1986) 51,
277-279. Vinyl stannanes of general formula (5) may be treated with bromine or
iodine to
provide vinyl halides of general formula (6) as described in J. Org. Chem.
(1985) 50, 243&2443.
Vinyl triflates of general formula (4) may also be converted into vinyl
boronates of general
formula (7) as described in Tetrahedron Lett. (2000) 41(19), 3705-3708.
113
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Scheme 3
Pd(0)
~ RB(OH)2 or ~
P-N~~X RSnR"s P-N\~R
X =--OT~~fi, ''//Br, I R = aryl, heterocycl ~e
(4) or (6) R" = alkyl (10)
Pd(0)
P-N~SnMe3 ~ P-N~R
X = I, Br, CI, OT ~f
(5) R = aryl, heterocycle (10)
~ O Pd(0?
P-N\~B ~ P-N~R
p X = I, Br, CI, OT ~f
(7) R = aryl, heterocycle (10)
Azabicyclic compounds of general formula (10), wherein P is a suitable
nitrogen
protecting group such as, but not limited to, alkyl, benzyl, trityl, acyl, p-
toluenesulfonyl (Ts) and
R is aryl or heterocycle, may be prepared as described in Scheme 3. Vinyl
triflates of general
formula (4) or vinyl halides of general formula (6) may be treated with a
palladium catalyst and
an aryl or heterocyclic boronic acid (or an analogous aryl or heterocyclic
stannane) to provide
azabicyclic compounds of general formula (10). Vinyl stannanes of general
formula (5) may be
treated with a palladium catalyst and an aryl halide or a heterocyclic halide
(or triflate) to provide
azabicyclic compounds of general formula (10). Vinyl boronates of general
formula (7) may be
treated with a palladium catalyst and an aryl halide or a heterocyclic halide
(or triflate) to provide
azabicyclic compounds of general formula (10).
114
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Scheme 4
H
p-N\i/~yOH
,l~~R
H MsCI
BuLi, RX (11) major isomer Et3N ~
P N C R = aryl, heterocycle H ~ P-N~R
X ' Br' I p_N~,.~~~CH (10)
~'R
H
(12) minor isomer
An alternative method of preparing azabicyclic compounds of general formula
(10),
wherein P is a suitable nitrogen protecting group such as, but not limited to,
alkyl, benzyl, trityl,
acyl, p-toluenesulfonyl (Ts) and R is aryl or heterocycle, may be used as
described in Scheme 4.
Nitrogen-protected (cis)-hexahydrocyclopenta[c]pyrrol-5(1H)-ones of general
formula (3) may
be treated with n-butyllithium or tent butyllithium and an aryl halide or a
heterocyclic halide to
provide alcohols of general formula (1 I) and general formula (12). In
general, the major
diasteroemer produced in additions of aryl or heterocyclic anions to fused
azabicyclic ketones
such as, but not limited to, (3) and other related fused bicyclic aminoketones
described herein is
expected to result from nucleophilic addition to the exo (or convex) face of
the azabicyclic
ketone as illustrated in Scheme 4. The diastereomers (11) and (12) can be
separated with
standard chromatographic techniques used by those skilled in the art of
organic chemistry.
Alcohols of general formula (I I) and general formula (12) may be treated with
methanesulfonyl
chloride and triethylamine to provide azabicyclic compounds of general formula
(10).
Scheme 5
P-N~R deprotect HN~R
w>
(10) (14)
R~X or /~
(14) (R~)2CC R~-N~R
X=Ci, Br or I (15)
Azabicycles of general formula (14) and azabicycles of general formula (15),
wherein R
is aryl or heterocycle and Rl is as defined in formula (I), may be prepared as
described in
115
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Scheme 5. The nitrogen protecting group in azabicycles of general formula (10)
may be
removed under conditions known to those of skill in the art of organic
chemistry to provide
azabicycles of general formula (I4). Azabicycles of general formula (14.) may
be treated with
alkylating or acylating reagents and a base such as, but not limited to,
triethylamine to provide
azabicycles of general formula (15).
Scheme 6
P-N~~CH Scheme 5 R~-N\~CH
R ' R
(11) or (12) (16)
base, R'X ~ ' , ,
(11) X=CI, Br or I P-N\~OR Scheme 5 R~-N~~OR
or rr~~// ~'R ~/ 'R
(12) (17) (18)
Azabicycles of general formula (16) and azabicycles of general formula (18),
wherein R
is aryl or heterocycle, R' is alkyl and Rl is as defined in formula (I), may
be prepared as
described in Scheme 6. Alcohols of general formula (11) or (12) may be
processed as described
in Scheme 5 to provide azabicycles of general formula (16). Alcohols of
general formula (11) or
(12) may also be treated with a base such as, but not limited to, sodium
hydride and an alkylating
reagent to provide alkoxy compounds of general formula (17). Alkoxy compounds
of general
formula (17) may be processed as described in Scheme 5 to provide azabicycles
of general
formula ( 18).
116
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Scheme 7
H H
1) CICOC02Me, DMAP P_N'~",~R p-N~R
P-N'~OH 2) nBu3SnH, AIB ~N
'R H H
(11) or (12) (endo) major isomer (exo) minor isomer
( 19) (20)
H H
R'-N~w~~R R~-N R
Scheme 5 ~~
(20) H H
(endo) major isomer (exo) minor isomer
(21 ) (22)
Azabicycles of general formula (21) and (22), wherein R is aryl or heterocycle
and Rl is
as defined in formula (I), may be prepared as described in Scheme 7. Alcohols
of general
formula (11) or (12) may be deoxygenated by treatment with methyl
chlorooxoacetate followed
by tributyltin hydride as described in J. Org. Chem. ( 1996) 61 (20), 7189-
7191 to provide
azabicyclics of general formula (19) and (20). In general, the major
diastereomer produced in
deoxygenation reactions performed on compounds (11) or (12) and xelated fused
azabicyclic
systems described herein is expected to be the endo-substituted isomer.
Azabicyclics of general
formula (19) and (20) may be processed as described in Scheme S to provide
azabicyclics of
general formula (21) and (22). The diastereomers (21) and (22) can be
separated with standard
chromatographic techniques used by those skilled in the art of organic
chemistry.
117
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Scheme 8
H H
~ H2 p_N\~.",R p-N~R
P-N\ I /}-R Pd/ .~/ ~/C
,' H H
(10) (endo) major isomer (exo) minor isomer
(24) (25)
N H
Scheme 5
(24) R -N .~nR R~-N\~ i~R
or
(25) ~ H H
(endo) major isomer (exo) minor isomer
(21 ) (22)
Alternatively, azabicyclics of general formula (21) and (22), wherein R is
aryl or
heterocycle and Rl is as defined in formula (I), may be prepared as described
in Scheme 8.
Azabicycles of general formula (10) may be treated with a catalyst such as,
but not limited to,
palladium on carbon under an atmosphere of hydrogen to provide azabicycles of
general formula
(24) and (2S). In general, the major diastereomer produced in hydrogenation
reactions
performed on compounds of general formula (10) and related azabicyclic olefins
described
herein is expected to be the endo-substituted isomer. Azabicycles of general
formula (24) and
(2S) may be processed as described in Scheme S to provide azabicycles of
general formula (21)
and (22).
Scheme 9
1 ) H2, Pd/C
Bn-N 2) Boc20 Boc-N Schemes 2-8
O O
R~-N\,~ R~-N R~-N R~-N
OH '
(28) R (29) R (30) ROR (31) R
Azabicycles of general formula (28), (29), (30) and (31), wherein R is aryl or
heterocycle,
R' is alkyl and Rl is as defined in formula (I), rnay be prepared as described
in Scheme 9. (cis)-
2-Benzylhexahydrocyclopenta[c]pyrrol-4(1H)-one, prepared as described in Chem.
Pharm. Bull.
(1985) 33(7), 2762-2766, may be treated with a transition metal catalyst such
as, but not limited
118
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
to, palladium on carbon under an atmosphere of hydrogen in the presence of di-
tert-butyl
dicaxbonate to provide tert-butyl (cis)-4-oxohexahydrocyclopenta[c]pyrrole-
2(1H)-carboxylate.
tert-Butyl (cis)-4-oxohexahydrocyclopenta[c]pyrrole-2(1H)-carboxylate may be
processed as
described in Schemes 2-8 to provide azabicycles of general formula (28), (29),
(30) and (31).
Scheme 10
O 1) H2, Pd/C O
2) BoczO Schemes 2-8
N N
Bri Boc
R R R R
OH OR'
N N N~ N
R~ (33) R~ (34) R~ (35) R~ (36)
Azabicycles of general formula (33), (34), (35) and (36), wherein R is aryl or
heterocycle,
R' is alkyl and Rl is as defined in foxmula (I), may be prepared as described
in Scheme 10. (cis)-
1-Benzylhexahydrocyclopenta[b]pyxrol-4(1H)-one, prepared as described in J.
Org.
Chem.(1985) 50, 2403-2405, may be treated with a transition metal catalyst
such as, but not
limited to, palladium on carbon under an atmosphere of hydrogen in the
pxesence of di-tert-butyl
dicarbonate to provide tert-butyl (cis)-4-oxohexahydrocyclopenta[b]pyrrole-
I(2H)-carboxylate.
tert-Butyl (cis)-4-oxohexahydrocyclopenta[b]pyrrole-1(2H)-carboxylate may be
processed as
described in Schemes 2-8 to provide azabicycles of general formula (33), (34),
(35) and (36).
Scheme 11
1) H2, Pd/C
N 1 , 2) Boc20 N 1 , Schemes 2-8
Bn ' ~O ' Boc '~ ~O
R ~OH R ~OR
~ (38) ~ (39) ~ (40) ~ (41 )
Azabicycles of general foxmula (38), (39), (40) and (41), wherein R is aryl or
heterocycle,
R' is alkyl and Rl is as defined in formula (I), may be prepared as described
in Scheme 1 I. (cis)-
119
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
1-Benzylhexahydrocyclopenta[b]pyrrol-6(1H)-one, prepared as described in
Tetrahedron Lett.
(1989) 30(41), 5547-SSSO, may be treated with a transition metal catalyst such
as, but not limited
to, palladium on carbon under an atmosphere of hydrogen in the presence of
drtert-butyl
dicarbonate to provide tert-butyl (cis)-6-oxohexahydrocyclopenta[b]pyrrole-
1(2H)-carboxylate.
tert-Butyl (cis)-6-oxohexahydrocyclopenta[b]pyrrole-1(2H)-carboxylate may be
processed as
described in Schemes 2-8 to provide azabicycles of general formula (38), (39),
(40) and (41).
Scheme 12
Zn dust
NHS C~C Schemes 2-8
N /~ J~/
Cbz CI CI CbzN
~~R ~~R ~R ~~Rf ~~R
N~ N N~ ~N
R~ X43) R~ X44) R~ X45) R~ X46) R~ X47)
Azabicycles of general formula (43), (44), (45), (46) and (47), wherein R is
aryl or
heterocycle, R' is alkyl and Rl is as defined in formula (I), may be prepared
as described in
Scheme 12. Benzyl (cis)-6,6-dichloro-5-oxohexahydrocyclopenta[b]pyrrole-1(2H)-
carboxylate,
prepared as described in Tetrahedron Lett. (1997) 38(11), 1869-1872, may be
treated with zinc
dust and ammonium chloride to provide benzyl (cis)-5-
oxohexahydrocyclopenta[b]pyrrole-
1(2H)-carboxylate. Benzyl (cis)-5-oxohexahydrocyclopenta[b]pyrrole-1(2H)-
carboxylate may
be processed as described in Schemes 2-8 to provide azabicycles of general
formula (43), (44),
(45), (46) and (47).
120
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Scheme 13
O O
1 ) H2, Pd/C
N 2) BocaO N Schemes 2-8
Bri Boc
R R OH R OR' R
N N N N
R~ R~ R~ R~
(49) (50) (51 ) (52)
Azabicycles of general formula (49), (50), (51) and (52), wherein R is aryl or
heterocycle,
R' is alkyl and Rl is as defined in formula (I), may be prepared as described
in Scheme 13. (cis)-
1-Benzyloctahydro-4H-indol-4-one, prepared as described in J. Am. Chem. Soc.
(1983) 105(22),
6629-6637, may be treated with a transition metal catalyst such as, but not
limited to, palladium
on carbon under an atmosphere of hydrogen in the presence of di tert-butyl
Bicarbonate to
provide tent-butyl (cis)-4-oxooctahydro-1H-indole-1-carboxylate. tert-Butyl
(cis)-4-
oxooctahydro-1H-indole-1-carboxylate may be processed as described in Schemes
2-8 to provide
azabicycles of general formula (49), (50), (51) and (52).
Scheme 14
O
Schemes 2-8
N
Boc
R R HO R R'O R R
N N N N
i i r i i
Ry R~ R~ R~ R~
(54) (55) (56) (57) (58)
Azabicycles of general formula (54), (55), (56), (57) and (58), wherein R is
aryl or
heterocycle, R' is alkyl and Rl is as defined in formula (I), may be prepared
as described in
Scheme 14. tert Butyl (cis)-5-oxooctahydro-1H-indole-1-carboxylate, prepared
as described in
J. Med. Chem. (1992) 35(19), 3547 3560, may be processed as described in
Schemes 2-8 to
provide azabicycles of general formula (54), (55), (56), (57) and (58).
121
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Scheme 15
Schemes 2-8
N
Boc
N N
R R~ R R~ HO'R R~ R'p'R R~ R
(60) (61) (62) (63) (64)
Azabicycles of general formula (60), (61), (62), (63) and (64), wherein R is
aryl or
heterocycle, R' is alkyl and Rl is as defined in formula (I), may be prepared
as described in
Scheme 15. tert-Butyl (cis)-6-oxooctahydro-1H-indole-1-carboxylate, prepared
as described in
J. Org. Chem. (1996) 61(20), 7106-7115, may be processed as described in
Schemes 2-8 to
provide azabicycles of general formula (60), (61), (62), (63) and (64).
Scheme 16
Schemes 2-8
N
H3C0~0 O
/N~OH NOR' N
R~ R R~ R R~ R R~ IR
(66) (67) (68) (69)
Azabicycles of general formula (66), (67), (68) and (69), wherein R is aryl or
heterocycle,
R' is alkyl and Rl is as defined in formula (I), may be prepared as described
in Scheme 16.
Methyl (cis)-7-oxooctahydro-1H-indole-1-carboxylate, prepared as described in
J. Chem. Soc.
Perkin Trans. I (1995) 2671-2672, may be processed as described in Schemes 2-8
to provide
azabicycles of general formula (66), (67), (68) and (69).
Scheme 17
122
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Cbz-N Schemes 2-8
O
R~-N' I R~-N, I I R~-N R~-N
~I\OH OR'
(71) R (72) R (73) R (74) R
Azabicycles of general formula (71), (72), (73) and (74), wherein R is aryl or
heterocycle,
R' is alkyl and Rl is as defined in formula (I), may be prepared as described
in Scheme 17.
Benzyl (cis)-4-oxooctahydro-2H-isoindole-2-carboxylate, prepared as described
in Eur. J. Med.
Chem. (1991) 26, 889-906, may be processed as described in Schemes 2-8 to
provide
azabicycles of general formula (71), (72), (73) and (74).
Scheme 18
Boc-N\,~ Schemes 2-8
O
R~-N\~R~ -N\~ R~- N R~ -N\ I I R~- N\
R R H~~R ~R'O R R
(76) (77) (78) (79) (80)
Azabicycles of general formula (76), (77), (78), (79) and (80), wherein R is
aryl or
heterocycle, R' is alkyl and Rl is as defined in formula (I), may be prepared
as described in
Scheme 18. tert-Butyl (cis)-5-oxooctahydro-2H-isoindole-2-carboxylate,
prepared as described
in WO 9806720, may be processed as described in Schemes 2-8 to provide
azabicycles of
general formula (76), (77), (78), (79) and (80).
123
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Scheme 19
OBn OBn O
1) BH3 or LAH 1) H2, PD/C
O N 2) Boc20 N 2) Swern N
Boc Boc
O
Schemes 2-8
N
i
Boc
R R R R
OH OR'
N N ~ N
i i N
i
R~ X82) R~ X83) R~ X84) R~ X85)
Azabicycles of general formula (82), (83), (84) and (85), wherein R is aryl or
heterocycle,
R' is alkyl and Rl is as defined in formula (I), may be prepared as described
in Scheme 19. (cis)-
5-(Benzyloxy)octahydro-2H-cyclopenta[b]pyridin-2-one, prepared as described in
WO 9526187,
may be treated with a reducing agent such as, but not limited to, borane-
tetrahydrofuran complex
or lithium aluminum hydride and then treated with di-tert-butyl dicarbonate
(another suitable
nitrogen protecting reagent may also be used such as, but not limited to,
benzyl chloroformate) to
provide tert-butyl (cis)-5-(benzyloxy)octahydro-1H-cyclopenta[b]pyridine-1-
carboxylate. tert-
Butyl (cis)-5-(benzyloxy)octahydro-1H-cyclopenta[b]pyridine-1-carboxylate may
be treated with
a transition metal catalyst such as, but not limited to, palladium on carbon
under a hydrogen
atmosphere and then treated with an oxidizing agent to provide tent butyl
(cis)-5-oxooctahydro-
1H-cyclopenta[b]pyridine-1-carboxylate. tert-Butyl (cis)-5-oxooctahydro-1H-
cyclopenta[b]pyridine-1-carboxylate may be processed as described in Schemes 2-
8 to provide
azabicycles of general formula (82), (83), (84) and (85).
124
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Scheme 21
1) 9-BBN ~
2) H202, NaOH ~~OH
N N~/ + N
Ts Ts Ts 4H
C~OH C~OH C~O
1 ) Na*ICaH~o~l N Swern
Ts Boc ~- Boc
2) Boc20
Ts OH Boc OH Boc ~O
O Schemes 2-8
N
i
Boc
C~R ~~R ~~R ~~ ~R C~ ~R~
N N N N OH N OR
i i i i i
R~ (88) R~ (89) R~ (90) R1 (91 ) R~ (92)
Schemes 2-8
N
Boc O
N' ~ N OOH N FOR' N
i R i R i R R R
R~ (gg) R~ (94) R~ (95) ~ (96)
Azabicycles of general formula (88), (89), (90), (91), (92), (93), (94), (95)
and (96),
wherein R is aryl or heterocycle, R' is alkyl and Rl is as defined in formula
(I), may be prepared
as described in Scheme 21. (cis)-1-[(4-Methylphenyl)sulfonyl]-2,3,4,4a,5,7a-
hexahydro-1H-
cyclopenta[b]pyridine, prepared as described in J. Org. Chem. (1996) 61(11),
3584-3585, maybe
treated with a hydroborating reagent such as, but not limited to, diborane or
9-
borabicyclo[3.3.1]nonane followed by treatment with hydrogen peroxide and
aqueous sodium
125
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
hydroxide to provide stereoisomeric alcohols, (cis)-1-[(4-
methylphenyl)sulfonyl]octahydro-1H-
cyclopenta[b]pyridin-6-of and (cis)-1-[(4-methylphenyl)sulfonyl]octahydro-1H-
cyclopenta[b]pyridin-7-ol. The alcohols may be separated using chromatographic
methods
known to those of skill in the art of organic chemistry. The alcohols may be
treated with sodium
naphthalenide and then treated with di-tert-butyl Bicarbonate to provide tert-
butyl (cis~6-
hydroxyoctahydro-1H-cyclopenta[b]pyridine-1-carboxylate or tert-butyl (cis)-7-
hydroxyoctahydro-1H-cyclopenta[b]pyridine-1-carboxylate. The alcohols may be
oxidized
under Swern conditions (DMSO/oxalyl chloride/triethylamine) to provide the
corresponding
ketones, tert-butyl (cis)-6-oxooctahydro-1H-cyclopenta[b]pyridine-1-
carboxylate or tert-butyl
(cis)-7-oxooctahydro-1H-cyclopenta[b]pyridine-1-carboxylate. tert Butyl (cis)-
6-oxooctahydro-
1H-cyclopenta[b]pyridine-1-carboxylate may be processed as described in
Schemes 2-8 to
provide azabicycles of general formula (88), (89), (90), (91) and (92).
tert.Butyl (cis)-7-
oxooctahydro-1H-cyclopenta[b]pyridine-1-carboxylate may be processed as
described in
Schemes 2-8 to provide azabicycles of general formula (93), (94), (95) and
(96).
Scheme 22
1 ) H2, PdIC
O 2) NaBH4 C~OH 1) Na+[C$H~o~l C~OH
N -~ N N
7s Ts 2) Boc20 Boc
C~OH SWern C~O Schemes 2-8
N~ ~ N
i i
Boc Boc
C~R C~R C~R C\ ~R C\ ~R
N~ N~ N~ N OH N OR
i i i i i
R~ X88) R~ t89) R~ X90) R~ X91) R~ X92)
An alternative method of preparing azabicycles of general formula (88), (89),
(90), (91)
and (92), wherein R is aryl or heterocycle, R' is alkyl and R; is as defined
in formula (I), may be
used as described in Scheme 22. 1-[(4-Methylphenyl)sulfonyl]-1,2,3,4,4a,5-
hexahydro-6H-
cyclopenta[b]pyridin-6-one, prepared as described in J. Org. Chem. (1996)
61(16), 5540-5552,
may be treated with a transition metal catalyst such as, but not limited to,
palladium on carbon
126
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
under a hydrogen atmosphere and then treated with a reducing agent such as,
but not limited to,
sodium borohydride to provide stereoisomeric alcohols. The stereoisomeric
alcohols may be
treated with sodium naphthalenide and then treated with di tert-butyl
Bicarbonate to provide the
N-boc protected stereoisomeric alcohols. The N boc protected stereoisomeric
alcohols may be
oxidized under Swern conditions (DMSO/oxalyl chloride/triethylamine) to
provide tert butyl
(cis)-6-oxooctahydro-1H-cyclopenta[b]pyridine-1-carboxylate. tent-Butyl (cis)-
6-oxooctahydro-
1H-cyclopenta[b]pyridine-1-carboxylate may be processed as described in
Schemes 2-8 to
provide azabicycles of general formula (88), (89), (90), (91) and (92).
Scheme 23
H O
Schemes 2-8
Cbz'N H
H R R H R
R H OH H OR'
R1~N\~Ri IV~ R1 N~ R1 IV
H H
(94) (95) (96) (97)
Azabicycles of general formula (94), (95), (96) and (97), wherein R is aryl or
heterocycle,
R' is alkyl and Rl is as defined in formula (I), may be prepared as described
in Scheme 23.
Benzyl (trans)-5-oxooctahydro-2H-cyclopenta[c]pyridine-2-carboxylate, prepared
as described
in Eur. J. Med. Chem. (1991) 26, 889-906, maybe processed as described in
Schemes 2-8 to
provide azabicycles of general formula (94), (95), (96) and (97).
127
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Scheme 24
H O H O
Et3N Schemes 2-8
Cbz'N H Cbz'N
H R H R H R H R
OH OR'
~N R ,N - R ~N R~ N
R~ H 1 Fi 1 Fi H
(98) (99) ( 100) ( 101 )
Azabicycles of general formula (98), (99), (100) and (101), wherein R is aryl
or
heterocycle, R' is alkyl and Rl is as defined in formula (I), may be prepared
as described in
Scheme 24. Benzyl (trans)-5-oxooctahydro-2H-cyclopenta[c]pyridine-2-
carboxylate, prepared
as described in Eur. J. Med. Chem. (1991) 26, 889-906, may be epimerized with
a base such as,
but not limited to, triethylamine to provide benzyl (cis}-5-oxooctahydro-2H-
cyclopenta[c]pyridine-2-carboxylate. Benzyl (cis)-5-oxooctahydro-2H-
cyclopenta[c]pyridine-2-
carboxylate may be processed as described in Schemes 2 8 to provide
azabicycles of general
formula (98), (99), ( 100) and ( 1 O 1 ).
Scheme 25
1) LAH
0 2) aq~acid ' r\~0 Schemes 2-8
HN p~ 3) BoczO Boc ..//~~N
rNr.~R ~.rlr.~R ..rlr,~R ,~Nr..~H ~Nr~,,~R~
R~ R~ R~ R~ R~
(103) (104) (105) (106) (107)
Azabicycles of general formula (103), (104), (105), (106) and (107), wherein R
is aryl or
heterocycle, R' is alkyl and Rl is as defined in formula (I), may be prepared
as described in
Scheme 25. (cis)-Hexahydro-spiro[6H-cyclopenta[c]pyridine-6,2'-[1,3]dioxolan]-
3(2H)-one,
prepared as described in Tetrahedron: Asymmetry (1997) 8(17), 2893-2903 may be
treated with
a reducing agent such as, but not limited to, borane tetrahydrofuran complex
or lithium
aluminum hydride, treated with aqueous acid and then treated with di-tert-
butyl dicarbonate to
provide tert-butyl (cis)-6-oxooctahydro-2H-cyclopenta[c]pyridine-2-
carboxylate. tert-Butyl
128
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-6-oxooctahydro-2H-cyclopenta[c]pyridine-2-carboxylate may be processed
as described in
Schemes 2-8 to provide azabicycles of general formula (103), (104), (105),
(106) and (107).
Scheme 26
H
Schemes 2-8
Cbz'N H
O
H H H H
R~ N~ R~ N~OH Ri NOR' R~ N
(109) (110) (111 ) (112)
Azabicycles of general formula (109), (110), (111) and (112), wherein R is
aryl or
heterocycle, R' is alkyl and RI is as defined in formula (I), may be prepared
as described in
Scheme 26. Benzyl (trans)-7-oxooctahydro-2H-cyclopenta[c]pyridine-2-
carboxylate, prepared
as described in Eur. J. Med. Chem. (1991) 26, 889-906, may be processed as
described in
Schemes 2-8 to provide azabicycles of general formula (109), (110), (111) and
(112).
Scheme 27
H H
Ef3N _ Schemes 2-8
Cbz'N H Cbz'N hi
O O
H H H H
R~,'N~ R~ N~OH Ri N - OR' R~ N
H R H R ti R H R
(114) (115) (116) (117)
Azabicycles of general formula (114), (115), (116) and (117), wherein R is
aryl or
heterocycle, R' is alkyl and Rl is as defined in formula (I), may be prepared
as described in
Scheme 27. Benzyl (trans)-7-oxooctahydro-2H-cyclopenta[c]pyridine-2-
carboxylate, prepared
as described in Eur. J. Med. Chem. (1991) 26, 889-906, may be epimerized with
a base such as,
but not limited to, triethylamine to provide benzyl (cis)-7-oxooctahydro-2H-
cyclopenta[c]pyridine-2-carboxylate. Benzyl (cis)-7-oxooctahydro-2H-
cyclopenta[c]pyridine-2-
129
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
carboxylate may be processed as described in Schemes 2 8 to provide
azabicycles of general
formula ( 114), ( 115), ( 116) and ( 117).
Scheme 28
HO
Schemes 2-8
NI= v
Boc
H R H R OH H R OR' H R
N~~ N~~ N~~ N
i H i H i H ~ H
R~ R~ R~ R~
(119) (120) (121) (122)
Azabicycles of general formula (119), (120), (121) and (122), wherein R is
aryl or
heterocycle, R' is alkyl and Rl is as defined in formula (I), may be prepared
as described in
Scheme 28. tert-Butyl (trans)-5-oxooctahydro-1(2H)-quinolinecarboxylate,
prepared as
described in J. Med. Chem. (1991) 34(9), 2736-2746, rnay be processed as
described in Schemes
2-8 to provide azabicycles of general formula (119), (120), (121) and (122).
Scheme 29
HO
Schemes 2-8
N H
Boc
H R H R OH H R OR' H R
N ~-~ N ~_~ N ~-~ N
i H i H i hi ~ hi
R~ R~ R~ R~
(124) (125) (126) (127)
Azabicycles of general formula (124), (125), (126) and (127), wherein R is
aryl or
heterocycle, R' is alkyl and Rl is as defined in formula (I), may be prepared
as described in
130
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Scheme 29. tert-Butyl (cis)-5-oxooctahydro-1(2H)-quinolinecarboxylate,
prepared as described
in J. Med. Chem. (1991) 34(9), 2736-2746, may be processed as described in
Schemes 2-8 to
provide azabicycles of general formula (124), (125), (126) and (127).
Scheme 30
H H H O H
Boc20 %~ Scheme 21
H H Boc Boc + Boc O
H O
Schemes 2-8
N
Boc
H ~ R H R H R H R H R
OH C\~~~OR'
N Fi N Fi N Fi N Fi N' Fi
R~ R~ R~ R~ R~
(129) (130) (131) (132) (133)
Schemes 2-8
Boc
H N H H H
OH ~OR'
N H ~ R N H R N ~ R N HER N H R
R~ R~ R~ R~ R~
(134) (135) (136) (137) (138)
Azabicycles of general formula (129), (130), (131), (132), (133), (134),
(135), (136),
(137) and (138), wherein R is aryl ox heterocycle, R' is alkyl and Rl is as
defined in formula (I),
may be prepared as described in Scheme 30. (trans)-
1,2,3,4,4a,5,8,8a~Octahydroquinoline,
prepared as described in J. Med. Chem. (1999) 42(15), 2862 2869, may be
treated with di-tert-
butyl dicarbonate in a solvent such as, but not limited to, THF or 1,4-dioxane
to provide tert
butyl (traps)-3,4,4a,5,8,8a-hexahydro-1(2H)-quinolinecarboxylate. tert-Butyl
(trans)-
3,4,4a,5,8,8a hexahydro-1(2H)-quinolinecarboxylate may be processed as
described in Scheme
H
N ~O
131
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
21 to provide ketones tert-butyl (trans~6-oxooctahydro-1(2H)-
quinolinecarboxylate and tert-
butyl (trans)-7-oxooctahydro-1(2H)-quinolinecarboxylate. The ketones may be
separated using
methods known to those of skill in the art of organic chemistry such as, but
not limited to,
chromatography. tert-Butyl (trans)-6-oxooctahydro-1(2H)-quinolinecarboxylate
may be
processed as described in Schemes 2-8 to provideazabicycles of general formula
(129), (130),
(131), (132) and (133). tert Butyl (trans)-7-oxooctahydro-1(2H)-
quinolinecarboxylate may be
processed as described in Schemes 2-8 to provideazabicycles of general formula
(134), (135),
(136), (137) and (138).
Scheme 31
H H H O H
B°c20 I Scheme 21
H hi N H N hi + N H O
Boc Boc Boc
H O
Schemes 2-8
N H
Boc
R H R H R H R H R
~\ ~ ~~O H ~\ ~ ~~O R' C,
N hi N ti N H N H N H
R~ R~ R~ R~ R~
(139) (140) (141) (142) (143)
Schemes 2-8
Boc
H H H H H
C\~~~OH C\~~~OR' C
N H R N H R N H R N N R N H R
R~ R~ R~ R~ R~
(144) (145) (146) (147) (148)
Azabicycles of general formula (139), (140), (141), (142), (143), (144),
(145), (146),
(147) and (148), wherein R is aryl or heterocycle, R' is alkyl and Rl is as
defined in formula (I),
H
N - O
132
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
may be prepared as described in Scheme 31. (cis)-1,2,3,4,4a,5,8,8a-
Octahydroquinoline,
prepared as described in J. Med. Chem. (1999) 42(15), 2862 2869, may be
treated with di-tert-
butyl dicarbonate in a solvent such as, but not limited to, THF or 1,4-dioxane
to provide tert
butyl (cis)-3,4,4a,5,8,8a hexahydro-1(2H)-quinolinecarboxylate. tert-Butyl
(cis)-3,4,4a,5,8,8x
hexahydro-1(2H)-quinolinecarboxylate may be processed as described in Scheme
21 to provide
ketones tert-butyl (cis)-6-oxooctahydro-1(2H)-quinolinecarboxylate and tert-
butyl (cis)-7-
oxooctahydro-1(2H)-quinolinecarboxylate. The ketones may be separated using
methods known
to those of skill in the art of organic chemistry such as, but not limited to,
chromatography. tert-
Butyl (cis)-6-oxooctahydro-1(2H)-quinolinecarboxylate may be processed as
described in
Schemes 2-8 to provide azabicycles of general formula (139), (140), (141),
(142) and (143). tern
Butyl (cis)-7-oxooctahydro-1(2H)-quinolinecarboxylate may be processed as
described in
Schemes 2-8 to provide azabicycles of general formula (144), (145), (146),
(147) and (148).
Scheme 32
1. Boc20
Pt/C, H2 2. (COCI)2, DMSO,
N Et3 N N
OH H OH Boc O
Schemes 2-8
N
i
Boc O
N~ N N~ , N
R R1 R OH R1 R OR R1 R
(150) (151) (152) (153)
Azabicycles of general formula (150), (151), (152) and (153), wherein R is
aryl or
heterocycle, R' is alkyl and Rl is as defined in formula (I), may be prepared
as described in
Scheme 32. 8-Quinolinol, purchased from Aldrich, may be treated witha platinum
catalyst such
as, but not limited to, platinum on carbon under a hydrogen atmosphere to
provide (cis}~
decahydro-8-quinolinol. (cis)-Decahydro-8-quinolinol may be treated with a
nitrogen protecting
group reagent such as, but not limited to, di tert-butyl dicarbonate and then
oxidized under
Swern conditions to provided tert-butyl (cis)-8-oxooctahydro-1(2H)-
quinolinecarboxylate. tert-
133
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Butyl (cis)-8-oxooctahydro-I(2H)-quinolinecarboxylate may be processed as
described in
Schemes 2-8 to provide azabicycles of general formula (150), (151), (152) aryl
(153).
Scheme 33
H O H2, Pd/C H O
_Boc20 Schemes 2-8
Bri N H Boc~N H
H R H R OH H R OR' H R
R ~ N ~~%~ R ~ N ~.~%~ R ~ N ~,.~ R ~ N ~,.
H ~ Fi ' H ~ Fi
(155) (156) (157) (158)
Azabicycles of general formula ( 1 S 5), ( 156), ( 157) and ( 15 8), wherein R
is aryl or
heterocycle, R' is alkyl and Rl is as defined in formula (I), may be prepared
as described in
Scheme 33. (trans~2-Benzyloctahydro-5(1H)-isoquinolinone, prepared as
described in J. Am.
Chem. Soc. (1991) I 13(23), 8863-8878, may be treated witha metal catalyst
such as, but not
limited to, palladium on carbon under a hydrogen atmosphere in the presence of
di-tert-butyl
dicarbonate to provide tent butyl (trans)-5-oxooctahydro-2(1H)-
isoquinolinecarboxylate. tert-
Butyl (trans)-5-oxooctahydro-2(1H)-isoquinolinecarboxylate may be processed as
described in
Schemes 2-8 to provide azabicycles of general formula (155), (156), (157) and
(I58).
Scheme 34
H OH 1. BoczO H O
2. (COCI)2, DMSO
HN Et3N ~N~J~~ Schemes 2-8
HBr H Boc Fi
H R H R OH H R OR' H R
,N , ,N , ,N ~ ,N .
R~ Fi R~ H R~ H R~ H
(160) (161) (162) (163)
Azabicycles of general formula (160), (161), (162) and (163), wherein R is
aryl or
heterocycle, R' is alkyl and Rl is as defined in formula (I), may be prepared
as described in
134
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Scheme 34. (cis)-Decahydro-5-isoquinolinol hydrobromide, prepared as described
in J. Med.
Chem. (1968) 11(5), 997-100, may be treated with di-tert-butyl dicarbonate and
then oxidized
under Swern conditions to provide tert butyl (cis)-5-oxooctahydro-2(1H)-
isoquinolinecarboxylate. tert-Butyl (cis)-5-oxooctahydro-2(1H)-
isoquinolinecarboxylate may be
processed as described in Schemes 2-8 to provide azabicycles of general
formula (160), (161),
(162) and (163).
Scheme 35
H H2, Pd/C
O _ Bcc20 O Schemes 2-8
Boc' N~~~
Bn H Fi
~ R H R H R H R H R
R s N~~~ R ~ N~~~ R ~ Nr~~~O H R ~ N~~~~OR' R ~ N~~
ti ~ H ~ Fi ~ H ~ H
(165) (166) (167) (168) (169)
Azabicycles of general formula (165), (166), (167), (168) and (169), wherein R
is aryl or
heterocycle, R' is alkyl and Rl is as defined in formula (I), may be prepared
as described in
Scheme 35. (cis)-2-Benzyloctahydro-6(2H)-isoquinolinone, prepared as described
in J. Med.
Chem. (1987) 30(7), 1210-1214, may be treated with a transition metal catalyst
such as, but not
limited to, palladium on carbon under an atmosphere of hydrogen in the
presence of drtert-butyl
dicarbonate to provide tent butyl (cis)-6-oxooctahydro-2(1H)-
isoquinolinecarboxylate. tert-Butyl
(cis)-6-oxooctahydro-2(1H)-isoquinolinecarboxylate may be processed as
described in Schemes
2-8 to provide azabicycles of general formula (165), (166), (167), (168) and
(169).
135
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Scheme 36
H HZ, Pd/C H
/Nr\J~O - Boc20 /Nr\~~O Schemes 2-8
Bn H Boc
H y R H R H R H R H R
~N~~~ ~N~~~~ ~Nr~J~.~~OH ~N OR ,N
R~ R R
H ~ H ~ 11 R~ Fi R~ Fi
(171 ) (172) (173) (174) (175)
Azabicycles of general formula (171), (172), (173), (174) and (175), wherein R
is aryl or
heterocycle, R' is alkyl and Rr is as defined in formula (I), rnay be prepared
as described in
Scheme 36. (trans~2-Benzyloctahydro-6(2H)-isoquinolinone, prepared as
described in J. Med.
Chem. (1987) 30(7), 1210-1214, may be treated with a transition metal catalyst
such as, but not
limited to, palladium on carbon under an atmosphere of hydrogen in the
presence of d~tert-butyl
dicarbonate to provide tert-butyl (trans)-6-oxooctahydro-2(1H)-
isoquinolinecarboxylate. tert-
Butyl (trans)-6-oxooctahydro-2(1H)-isoquinolinecarboxylate may be processed as
described in
Schemes 2-8 to provide azabicycles of general formula (171), (172), (173),
(174) and (175).
Scheme 37
H
Schemes 2-8
EtO2C'N H~O
H H H H
R .-Nr\ I I R ~Nr~~ ~N~~~~OH ~Nr~~~OR' ~Nrs
Fi~ R1 hi R R~ H R R~ H R R~ Fi R
(177) (178)
(179) (180)
(181)
Azabicycles of general formula (177), (178), (179), (180) and (181), wherein R
is aryl or
heterocycle, R' is alkyl and Rl is as defined in formula (I), may be prepared
as described in
Scheme 37. Ethyl (cis~7-oxooctahydro-2(1H)-isoquinolinecarboxylate, prepared
as described in
J. Am. Chem. Soc. (1998) 120(41), 10676-10686, may be processed as described
in Schemes 2-8
to provide azabicycles of general formula (177), (178), (179), (180) and
(181).
136
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Scheme 38
H
Schemes 2-8
EtO2C'N H O
H H H H H
~Nr~~ ~N~~~~ ~Nr~,~~OH ~N OR' .-N
R~ R R R
R~ H R R~ H R R~ H R
(182) (183) (184) (185) (186)
Azabicycles of general formula (182), (183), (184), (185) and (186), wherein R
is aryl or
heterocycle, R' is alkyl and Rl is as defined in formula (1), may be prepared
as described in
Scheme 38. Ethyl (trans}-7-oxooctahydro-2(1H)-isoquinolinecarboxylate,
prepared as described
in J. Am. Chem. Soc. (1998) 120(41), 10676~10686, may be processed as
described in Schemes
2-8 to provide azabicycles of general formula (182), (183), (184), (185) and
(186).
Scheme 39
H H
1. MeCH(CI)OCOCI
2. MeOH
Me'N~ 3. Soc2O Boc~N~ Schemes 2-8
O O
H H H H
,N _ / ,N , ,N ,N
R~ H R R~ Fi R OH R~ ~OR' R~
(188) (189) (190) (191) R
Azabicycles of general formula (188), (189), (190) and (191), wherein R is
aryl or
heterocycle, R' is alkyl and Rl is as defined in formula (I), may be prepared
as described in
Scheme 39. (trans~2-Methyloctahydro-8(2H)-isoquinolinone, prepared as
described in J. Org.
Chem. (1974) 39(22), 3210-3215, may be treated with 1-chloroethyl
chloroformate followed by
methanol and then treatment with di-tert-butyl dicarbonate to provide tert-
butyl (trans~8-
oxooctahydro-2(1H)-isoquinolinecarboxylate. tert-Butyl (trans)-8-oxooctahydro-
2(1H)-
isoquinolinecarboxylate may be processed as described in Schemes 2-8 to
provide azabicycles of
general formula (188), (189), (190) and (191).
137
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Scheme 40
1. H2, Pd/C, Boc20
2. PDC
Bn~N~ Boc~N
OH O
H H H H
~N~ ~N~ ~N~ ,N ,
R~ '' H ~ R~ '' H ~OH R~ H OR' R~ H
(192) R (193) R (194) R (195) R
Azabicycles of general formula (192), (193), (194) and (195), wherein R is
aryl or
heterocycle, R' is alkyl and Rl is as defined in formula (I), may be prepared
as described in
Scheme 40. (cis)-2-Benzyldecahydro-8-isoquinolinol, prepared as described in
Tetrahedron Lett.
(1998) 39(29), 5185-5188, may be treated with a metal catalyst such as, but
not limited to,
palladium on carbon under a hydrogen atmosphere in the presence of di tert-
butyl dicarbonate
and then oxidized with pyridinium dichromate (PDC) to provide tert-butyl (cis)-
8-oxooctahydro-
2(1H)-isoquinolinecarboxylate. tert-Butyl (cis)-8-oxooctahydro-2(1H)-
isoquinolinecarboxylate
may be processed as described in Schemes 2-8 to provide azabicycles of general
formula (192),
(193), (194) and (195).
Scheme 41
O OH 1) Na+[C~oHs~]- OH
Ts-N~ NaBH4 Ts-N~ 2) Boc20 /~,--~
Boc-N
OH O
Boc-N~ SW~ Boc- N\~ Schemes 2-8
R R R R
R~-N' a R~-N~OH R~ -NOR' R~-N
(197) (198) (1 gg) (200)
Azabicycles of general formula (197), (198), (199) and (200), wherein R is
aryl or
heterocycle, R' is alkyl and Rl is as defined in formula (I), may be prepared
as described in
Scheme 41. (cis)-3-[(4-Methylphenyl)sulfonyl]-3-azabicyclo[3.2.0]heptan-6-one,
prepared as
138
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
described in Heterocycles (1989) 28(1), 29-32, may be treated with a reducing
agent such as, but
not limited to, sodium borohydride to provide stereoisomeric alcohols. The
stereoisomeric
alcohols may be treated with sodium naphthalenide and then treated with di-
tert-butyl
Bicarbonate to provide N-boc protected stereoisomeric alchols. The N-boc
protected
stereoisomeric alcohols may be oxidized agent under Swern conditions
(DMSO/oxalyl
chloride/triethylamine) to provide tert-butyl (cis)-6-oxo-3-
azabicyclo[3.2.0]heptane-3-
carboxylate. tert-Butyl (cis)-6-oxo-3-azabicyclo[3.2.0]heptane-3-carboxylate
may be processed
as described in Schemes 2-8 to provide azabicycles of general formula (197),
(198), (199) and
(200).
Scheme 42
Scheme 41 ~ Schemes 2-8
O ~ O
Ts Boc
~OH ~OR'
N R N R N R N R
Ry R~ R~ R~
(201 (202) (203) (204)
)
Azabicycles of general formula (201), (202), (203) and (204), wherein R is
aryl or
heterocycle, R' is alkyl and RI is as defined in formula (I), may be prepared
as described in
Scheme 42. (cis)-2-[(4-Methylphenyl)sulfonyl]-2-azabicyclo[3.2.0]heptan-7-one,
prepared as
described in Heterocycles (1989) 28(1), 29-32, may be processed as described
in Scheme 41 to
provide tert-butyl (cis)-7-oxo-2-azabicyclo[3.2.0]heptane-2-carboxylate. tent-
Butyl (cis)-7-oxo-
2-azabicyclo[3.2.0]heptane-2-carboxylate may be processed as described in
Schemes 2 8 to
provide azabicycles of general formula (201), (202), (203) and (204).
139
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Scheme 43
O ~~O
Scheme 41 Schemes 2-8
N N
Ts Boc
R R R R
~OH ~OR'
R~ R~ R~ R~
(205) (206) (20~) (208)
Azabicycles of general formula (205), (206), (207) and (208), wherein R is
aryl or
heterocycle, R' is alkyl and Rl is as defined in formula (I), may be prepared
as described in
Scheme 43. (cis)-2-[(4-Methylphenyl)sulfonyl]-2-azabicyclo[3.2.0]heptan-6-one,
prepared as
described in Tetrahedron Lett. (1993) 34(1), 27-30, may be processed as
described in Scheme 41
to provide tent-butyl (cis)-6-oxo-2-azabicyclo[3.2.0]heptane-2-carboxylate.
tert-Butyl (cis)-6-
oxo-2-azabicyclo[3.2.0]heptane-2-carboxylate may be processed as described in
Schemes 2 8 to
provide azabicycles of general formula (205), (206), (207) and (208).
Scheme 44
OFi (COCI)2, DMSO O
Et3N
Y
1. LAH sN Boc°N~
O \ 2. Boc20 Boc
3. borane reagent/H202
(COCI)2, DMSO
N~OH Et3N Y oN~O
Boc Boc
O
Schemes 2-8
N ---
Boc
R R R R
ON OR'
N N N N
s o 0 0
R~ (210) R~ (211) R~ (212) R~ (213)
140
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Schemes 2-8
Boc ~'N
sN~R rN~R N~R N~R~ N~R
R~ (214) R~ (215) R~ (216) R~ (217) R~ (218)
Azabicycles of general formula (210), (211), (212), (213), (214), (215),
(216), (217) and
(218), wherein R is aryl or heterocycle, R' is alkyl and Rl is as defined in
formula (I), may be
prepared as described in Scheme 44. (cis)-6-Azabicyclo[3.2.0]hept-2-en-7-one,
prepared as
described in Tetrahedron Lett. (1999) 40(15), 4857-4860, may be treated with
lithium aluminum
hydride (LAH) followed by di-tert-butyl Bicarbonate and then treatment with a
hydroborating
agent such as, but not limited to, 9-borabicyclo[3.3.1]nonane to provide a
stereoisomeric mixture
of alcohols, tert butyl (cis)-2-hydroxy-6-azabicyclo[3.2.0]heptane-6-
carboxylate and tert-butyl
(cis)-3-hydroxy-6-azabicyclo[3.2.0]heptane-6-carboxylate. The alcohols may be
separated using
methods known to those of skill in the art of organic chemistry such as, but
not limited to,
chromatography and then oxidized under Swern conditions to provide the
corresponding ketones.
tert-Butyl (cis)-2-oxo-6-azabicyclo[3.2.0]heptane-6-carboxylate may be
processed as described
in Schemes 2-8 to provide azabicycles of general formula (210), (211), (212)
and (213). tern
Butyl (cis)-3-oxo-6-azabicyclo[3.2.0]heptane-6-carboxylate may be processed as
described in
Schemes 2-8 to provide azabicycles of general formula (214), (215), (216),
(217) and (218).
141
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Scheme 45
(COCI)2, DMSO
~ ~ Et3N
N--U N
1. LAH 8oc~ \OH Boc' O
O 2. Boc20
HN / 3. borane reagent/H202
N~OH
Boc ~'
Schemes 2-8
N
Boc O
Ri N i Ri N OH Ri NOR, Ri N
(220) R (221 ) R (222) R (223) R
Azabicycles of general formula (220), (221), (222) and (223), wherein R is
aryl or
heterocycle, R' is alkyl and RI is as defined in formula (I), may be prepared
as described in
Scheme 45. (cis)-6-Azabicyclo[3.2.0]hept-3-en-7-one, prepared as described in
Tetrahedron
Lett. (1985) 26(16), 1907-1910, may be treated with lithium aluminum hydride
(LAH) followed
by di-tert-butyl dicarbonate and then treatment with a hydroborating agent
such as, but not
limited to, 9-borabicyclo[3.3.1]nonane to provide a stereoisomeric mixture of
alcohols, tert butyl
(cis)-3-hydroxy-6-azabicyclo[3.2.0]heptane-6-carboxylate and tert-butyl (cis)-
4-hydroxy-6-
azabicyclo[3.2.0]heptane-6-carboxylate. The alcohols may be separated using
methods known
to those of skill in the art of organic chemistry such as, but not limited to,
chromatography and
then oxidized under Swern conditions to provide the corresponding ketones.
tert~utyl (cis)-4-
oxo-6-azabicyclo[3.2.0]heptane-6-carboxylate may be processed as described in
Schemes 2 8 to
provide azabicycles of general formula (220), (221), (222) and (223).
142
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Scheme 46
(COCI)2, DMSO O
OH Et3N
1. LAH
O N ~N~
2. Boc O
2 Boc Boc
HN ~ 3. borane reagent/H202
(COCI)2, DMSO
Et3N
N iN
O
Boc OH Boc
O
Schemes 2-8
N
Boc~
R R R R R
iN~ N~ iN~OH N~ORt N
i
R~ (225) R~ (226) R1 (227) R~ (228) R~ (229)
Schemes 2-8
N O
Boc
~N~ N~ ~N~OH NOR' N
R i R R ~ R ~ R
R~ (230) R~ (237) R1 (232) R~ (233) R~ (234)
Azabicycles of general formula (225), (226), (227), (228), (229), (230),
(231), (232),
(233) and (234), wherein R is aryl or heterocycle, R' is alkyl and Rl is as
defined in formula (I),
may be prepared as described in Scheme 46. (cis)-7 Azabicyclo[4.2.0]oct 3-en-8-
one, purchased
from Maybridge or prepared as described in J. Am. Chem. Soc. (1968) 90(14),
3897-3898, may
be treated with lithium aluminum hydride followed by di-tert-butyl dicarbonate
and then
treatment with a hydroborating agent such as, but not limited to, 9-
borabicyclo[3.3.1]nonane to
provide a stereoisomeric mixture of alcohols, tent butyl (cis)-3-hydroxy-7-
azabicyclo[4.2.0]octane-7-carboxylate and tert-butyl (cis)-4-hydroxy-7-
azabicyclo[4.2.0]octane-
7-carboxylate. The alcohols may be separated using methods known to those of
skill in the art of
organic chemistry such as, but not limited to, chromatography and then
oxidized under Swern
conditions to provide the corresponding ketones. tent Butyl (cis)-3-oxo-7-
azabicyclo[4.2.0]octane-7-carboxylate may be processed as described in Schemes
2-8 to provide
143
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
azabicycles of general formula (225), (226), (227), (228) and (229). tert-
Butyl (cis)-4-oxo-7-
azabicyclo[4.2.0]octane-7-carboxylate may be processed as described in Schemes
2-8 to provide
azabicycles of general formula (230), (231), (232), (233) and (234).
Scheme 47
(COCI)2, DMSO
Et3N
1. LAH N sN~
O 2. Boc20 Boc' Bo ~c
3. borane reagent/H202 OH O
HN
s N
Boc OH
N-[ J Schemes 2-8
Bo ~c
O
oN / ~N oN ~N
R~ R~ OH R~ ~ pR~ R~
R R R R
(234) (235) (236) (237)
Azabicycles of general formula (234), (235), (236) and (237), wherein R is
aryl or
heterocycle, R' is alkyl and Rl is as defined in formula (I), may be prepared
as described in
Scheme 47. (cis)-7-Azabicyclo[4.2.0]oct 4-en-8-one, prepared as described in
J. Chem. Soc.,
Perkin Trans. I (1977) 874-884, may be treated with lithium aluminum hydride
followed by dr
tert-butyl dicarbonate and then treatment with a hydroborating agent such as,
but not limited to,
9-borabicyclo[3.3.1]nonane to provide a stereoisomeric mixture of alcohols,
tent-butyl (cis~4-
hydroxy-7-azabicyclo[4.2.0]octane-7-carboxylate and tert-butyl (cis)-5-hydroxy-
7-
azabicyclo[4.2.0]octane-7-carboxylate. The alcohols may be separated using
methods known to
those of skill in the art of organic chemistry such as, but not limited to,
chromatography and then
oxidized under Swern conditions to provide the corresponding ketones.
ter~Butyl (cis)-5-oxo-7-
azabicyclo[4.2.0]octane-7-carboxylate may be processed as described in Schemes
2-8 to provide
azabicycles of general formula (234), (235), (236) and (237).
144
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Scheme 48
O O 1. HS(CH2)2SH,
1. hv, acetone BF3~OEt2
2. aluminum oxide 2. RaNi
N OBn N OBn 3~ H2, Pd/C N OH
' ' 4. Boc20 '
Cbz Cbz Boc
(COCI)2, DMSO
Et3N ~~ Schemes 2-8
OH N O
Boc Boc
C~OH C~OR'
N R NCR NCR N R
R1 R1 R1 R1
(239) (240) (241 ) (242)
Azabicycles of rmula (239),
general fo (240),
(241) and
(242),
wherein
R is aryl
or
heterocycle, R' is alkyl and Rl is as defined in formula (I), may be prepared
as described in
Scheme 48. Benzyl 4-oxo-3,4-dihydro-l(2H)-pyridinecarboxylate, prepared as
described in J.
Chem. Soc., Perkin Trans. I (1977) 874-884, may be treated with benzyl vinyl
ether in a
photochemical [2+2] cycloaddition to provide benzyl (cis~8-(benzyloxy)-5-oxo-2-
azabicyclo[4.2.0]octane-2-carboxylate using methodology described in Helv.
Chim. Acta (1991)
74, 163-177. Benzyl (cis)-8-(benzyloxy)-5-oxo-2-azabicyclo[4.2.0]octane-2-
carboxylate, may
be treated with 1,2-ethanediol and boron trifluoride diethyl etherate and then
treated with Raney
nickel to provide (cis)-2-azabicyclo[4.2.0]octan-8-ol. (cis)-2-
Azabicyclo[4.2.0]octan-8-of may
be treated with a metal catalyst such as, but not limited to, palladium on
carbon under a hydrogen
atmosphere in the presence of di-tert-butyl dicarbonate and then oxidized
under Swern
conditions to provide tert-butyl (cis)-8-oxo-2-azabicyclo[4.2.0]octane-2-
carboxylate. tert-Butyl
(cis)-8-oxo-2-azabicyclo[4.2.0]octane-2-carboxylate may be processed as
described in Schemes
2-8 to provide azabicycles of general formula (239), (240), (241) and (242).
145
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Scheme 49
1. MsCI, Et3N
2. borane reagent H
3. H202, NaOH
(163)
N OH OH (COCI)2, DMSO O
Boc Et3N
N
N i
Boc Boc
O
Schemes 2-8
N
i
Boc
R R R R
~~OH ~~OR'
N \Nr ~NJ-' N
R~ R~ R~ Rq
(244) (245) (246) (247)
Azabicycles of general formula (244), (245), (246) and (247), wherein R is
aryl or
heterocycle, R' is alkyl and Rl is as defined in formula (I), may be prepared
as described in
Scheme 49. tert-Butyl (cis)-8-hydroxy-2-azabicyclo[4.2.0]octane-2-carboxylate,
prepared as
described in Scheme 48, may be treated with methanesulfonyl chloride and a
base such as, but
not limited to, triethylamine to provide tert butyl (cis)-2-
azabicyclo[4.2.0]oct-7-ene-2-
carboxylate. tert-Butyl (cis)-2-azabicyclo[4.2.0]oct-7-ene-2-carboxylate may
be treated with a
borane reagent such as, but not limited to, 9-borabicyclo[3.3.1]nonane and
then basic hydrogen
peroxide to provide a mixture of stereoisomeric alcohols. The alcohols may be
separated using
methods known to those of skill in the art of organic chemistry such as, but
not limited to,
chromatography and then oxidized under Swern conditions to provide the
corresponding ketones.
tert-Butyl (cis)-7-oxo-2-azabicyclo[4.2.0]octane-2-carboxylate may be
processed as described in
Schemes 2-8 to provide azabicycles of general formula (244), (245), (246) and
(247).
N O
i
Boc
146
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Scheme 50
1. NaBH4
2. Na+(C~oH$'l
Ts~N O 3. BoczO Boc~N O
4. Swern ~\~ Schemes 2-8
Rw R R~~ R R~~ R R~~ R
N'~ N'~~OH N~~~OR' N
(249) (250) (251 ) (252)
Azabicycles of general formula (249), (250), (251) and (252), wherein R is
aryl or
heterocycle, R' is alkyl and Rl is as defined in formula (I), may be prepared
as described in
Scheme 50. 3-[(4-Methylphenyl)sulfonyl]-3-azabicyclo[4.2.0]octan-8-one,
prepared as
described in Heterocycles (1989) 28(1), 29-32, may be treated with a reducing
agent such as, but
not limited to, sodium borohydride followed by sodium naphthalenide to remove
the N-tosyl
protecting group. The amine may be treated with di tert-Bicarbonate and then
oxidized under
Swern conditions (DMSO/oxalyl chloride/triethylamine) to provide to provide
tert-butyl (cis)-8-
oxo-3-azabicyclo[4.2.0]octane-3-carboxylate. tert-Butyl (cis)-8-oxo-3-
azabicyclo[4.2.0]octane-
3-carboxylate may be processed as described in Schemes 2-8 to provide
azabicycles of general
formula (249), (250), (251) and (252).
147
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Scheme 51
1. TsCI
2. iBuOCOCI, N-methylmorpholine, Tf20, collidine
H2N~C02H then pyrrolidine ~ then H O
N 2 '
3. NaH, allyl bromide N
i
Ts O
O OH OH
/N~\~ NaBH4 /N~~ Scheme 41 /N~~
Ts ~ Ts ~ Bo ~---~c
OH O
/N~~ Swern /Nr\~ Schemes 2-8
Boc Boc
R R R R
/Nr~ /Nr\~OH /Nr\~OR' /Nr~
R1 (254) R1 (255) R~ (,,25~~6) '' R~ ~(2/57~')
Azabicycles of general formula (254), (255), (256) and (257), wherein R is
aryl or
heterocycle, R' is alkyl and RI is as defined in formula (I), may be prepared
as described in
Scheme 51. 3-[(4-Methylphenyl)sulfonyl]-3-azabicyclo[4.2.0]octan-7-one may be
prepared
from commercially available 4-aminobutyric acid utilizing synthetic
methodology described in
Heterocycles (1989) 28(1), 29-32. 4-Aminobutyric acid may be treated with 4-
methyltoluenesulfonyl chloride to provide the N-protected butyric acid. The N-
protected butyric
acid may be treated with pyrrolidine and a chloroformate such as, but not
limited to,
isobutylchloroformate to provide the amide. The amide may be treate with a
strong base such as,
but not limited to, sodium hydride and allyl bromide to provide I~allyl-4-
methyl-N-[4-oxo-4-(1-
pyrrolidinyl)butyl]benzenesulfonamide. N-Allyl-4-methyl-N-[4-oxo-4-(1-
pyrrolidinyl)butyl]benzenesulfonamide may be treated with
trifluoromethanesulfonyl anhydride
and a base such as, but not limited to, collidine to provide the iminium 2+2
product which may
be treated with water to provide (cis}~3-[(4-methylphenyl)sulfonyl]-3-
azabicyclo[4.2.0]octan-7-
one. (cis)-3-[(4-Methylphenyl)sulfonyl]-3-azabicyclo[4.2.0]octan-7-one may be
treated with a
reducing agent such as, but not limited to, sodium borohydride to provide
stereoisomeric
alcohols. The stereoisomeric alcohols may be processed as described in Scheme
41 to provide
the N-boc protected stereoisomeric alcohols. The N-boc protected
stereoisomeric alcohols may
148
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
be oxidized under Swern conditions (DMSO/oxalyl chloride/triethylamine) to
provide tert butyl
(cis)-7-oxo-3-azabicyclo[4.2.0]octane-3-carboxylate. tent-Butyl (cis)-7-oxo-3-
azabicyclo[4.2.0]octane-3-carboxylate may be processed as described in Scheme
2-8 to provide
azabicycles of general formula (254), (255), (256) and (257).
The compounds and processes of the present invention will be better understood
by
reference to the following Examples, which are intended as an illustration of
and not a limitation
upon the scope of the invention.
Example 1
cis)-5-(6-chloro-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~pyrrole
hydrochloride
Example 1 A
(cis)-2,3,3a,4,7,7a-hexahydro-1H-isoindole
A suspension of lithium aluminum hydride (21.4 g, 0.562 mol) in THF (700 mL)
at room
temperature was treated with cis-1,2,3,6-tetrahydrophthalimide (Aldrich; 37 g,
0.245 mol) in
small portions. The reaction mixture was stirred at 60 °C overnight
then cooled to room
temperature and quenched carefully by the sequential addition of water (22
mL), THF (22 mL),
15% aqueous KOH (22 mL), and water (80 mL). The mixture was diluted with
diethyl ether
(100 mL), stirred at room temperature for 1 hour, and then filtered (methylene
chloride wash).
The filtrate was concentrated under reduced pressure to provide the title
compound as an oil
(26.1g). 1H NMR (CDC13, 300 MHz) 8 1.76-2.02 (m, 2H), 2.10-2.33 (m, 3H), 2.34-
2.56 (m,
2H), 2.61-2.79 (m, 2H), 2.87-3.17 (m, 2H), 5.53-6.03 (m, 2H); MS (DCI/NH3) m/z
124 (M+H)+.
Example 1B
tert-butyl (cis)-1,3,3a,4,7,7a-hexahydro-2H-isoindole-2-carboxylate
The product from Example lA (26 g, 0.21 mol) in methylene chloride (250 mL) at
0 °C
was treated with di-tert-butyl dicarbonate (46.1 g, 0.21 mol). The solution
was stirred for 30
minutes at 0 °C and then 2 hours at ambient temperature. The reaction
mixture was partitioned
between methylene chloride and saturated aqueous sodium bicarbonate. The
organic extract was
washed with saturated aqueous sodium bicarbonate and brine, dried over Na2SO4,
filtered and
149
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
the filtrcate was concentrated under reduced pressure. The residue was
purified by
chromatography (Si02, 15% ethyl acetate/hexane) to provide the title compound
(36 g). 'H
NMR (CDCl3, 300 MHz) 8 1.45 (s, 9 H), 1.84-1.99 (m, 2H), 2. I I-2.40 (m, 4H),
3.07 (m, 1H),
3.18 (m, 1H), 3.33-3.53 (m, 2H), 5.60 (bs, 2H); MS (DCI/NH3) m/z 224 (M+H)+.
Example 1 C
tent-butyl (cis)-3,4-bis(carboxymethyl)-I-pyrrolidinecarboxylate
The product from Example 1B (36 g, 0.16 mol) in carbon tetrachloride (360 mL),
acetonitrile (360 mL), and wafer (540 xnL) was treated with sodium periodate
(I38 g, 0.645 mol)
followed by catalytic ruthenium (IV) oxide hydrate (0.885 g, 0.0066 mol). The
mixture was
stirred vigorously for 24 hours at ambient temperature then diluted with
methylene chloride (500
mL) and water (100 mL), and filtered through diatomaceous earth. The filtrate
was pass through
a small plug of Si02 (methylene chloride wash), then evaporated under reduced
pressure. The
solid was crystallized from diethyl ether to provide the title compound (23.3
g). 1H NMR
(CD30H, 300 MHz) 8 1.45 (s, 9H), 2. I8-2.32 (m, 2H), 2.37 2.52 (m, 2H), 2.52-
2.76 (m, 2H),
3.05-3.21(m, 2H), 3.35-3.56 (m, 2H), 3.79-3.89 (m, IH), 6.1I (m, IH); MS
(DCI/NH3) rn/z 288
(M+H)+.
Example 1 D
tert-butyl (cis)-5-oxohexahydrocyclopenta~c~pyrrole-2(1H)-carboxylate
The product from Example 1C (23 g) in acetic anhydride (140mL) was treated
with
sodium acetate (5.3 g, 0.065 mol). The reaction mixture was stirred at 120
°C for 3 hours then
cooled to ambient temperature, filtered (diethyl ether wash) and the filtrate
was evaporated under
reduced pressure. The residue was purified by chromatography (Si02, 30% ethyl
acetate/hexane)
to provide the title compound (11.7 g). 1H NMR (CDC13, 300 MHz) 8 1.45 (s,
9H), 2.12 (m,lH),
2.19 (m, IH), 2.44 (m, 1H), 2.51 (m, IH), 2.93 (m, 2H), 3.22 (m, 2H), 3.65 (m,
2H); MS
(DCI/NH3) m/z 226 (M+H)+.
Example lE
I50
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
tert-butyl 5-hydroxy-5-(3-pyridinyl)hexahydrocyclopenta~c~pyrrole-2( 1H)-
carboxylate
2-Chloro-S-iodopyridine (1.38 g, 5.75 mmol), prepared as described in
Tetrahedron
Letters, 34, 7493-7496 (1993), in THF (7 mL) was treated with n-butyl lithium
(2.SM solution in
hexanes, 2.30 mL, 5.75 mmol) dropwise over 20 minutes at-78 °C. After
stirring at-78 °C for
40 minutes, the mixture was treated dropwise with the product from Example 1D
(0.259 g, 1.15
mmol) in diethyl ether (3 mL) dropwise. The reaction mixture was stirred at -
78 °C for 30
minutes and then allowed to warm to 0 °C. The mixture was quenched with
saturated aqueous
ammonium chloride and allowed to warm to room temperature. The mixture was
partitioned
between ethyl acetate and saturated aqueous ammonium chloride. The organic
extract was
separated, washed with saturated ammonium chloride, water, brine, dried over
Na2S04, filtered
and the filtrate was concentrated under reduced pressure. The residue was
purified by
chromatography (SiO2, 50% ethyl acetate/hexane) to provide the title compound
(0.255 g). MS
(DCIM3) m/z 339 (M+H)+.
Example 1 F
tert-butyl (cis)-5-(6-chloro-3-pyridinyl)-3,3a,4,6a tetrahydroc
clopenta~c~pyrrole-2(1H)
carboxylate
The product from Example lE (0.098 g, 0.290 mmol) in methylene chloride (4 mL)
was
treated with methanesulfonyl chloride (0.045 mL, 0.58 mmol), triethylamine
(0.080 mL, 0.609
mmol) and catalytic DMAP (0.001 g). After stirring at ambient temperature for
16 hours, the
volatiles were evaporated under reduced pressure. The residue was purified by
chromatography
(Si02, 50% ethyl acetate/hexane) to provide the title compound (0.036 g, 39%).
MS (DCI/NH3)
mlz 321 (M+H)+.
Example 1 G
(cis)-S-(6-chloro-3-pyridin 1)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~pyrrole
hydrochloride
The product from Example 1F (0.036 g, 0.112 mmol) in methanol (1 mL) was
treated
with a 1M solution of HCl in diethyl ether (2 mL). After stirring at ambient
temperature for 2
151
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
hours, the volatiles were evaporated under reduced pressure. The residue was
crystallized from
ethanol and diethyl ether to provide the title compound (0.034 g, 100%). 1H
NMR (CD30D, 300
MHz) 8 2.75 (m, 1H), 3.15 (m, 2H), 3.32 3.55 (m, 4H), 3.82 (m, 1H), 6.24 (m,
1H), 7.46 (d,
J=8.46 Hz, 1H), 7.95 (dd, J=2.57, 8.46 Hz, 1H), 8.47 (d, J=2.21 Hz, 1H); MS
(DCI/NH3) m/z
221 (M+H)+; Anal. calculated for C12H13C1N2~1.8HC1: C, 50.34; H, 5.21; N,
9.78. Found: C,
50.40; H, 5.35; N, 9.61.
Example 2
(cis)-5-(5-methoxy-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~pyrrole
hydrochloride
Example 2A
tert-butyl (cis)-5-hydroxy-5-(5-methoxy-3-
pyridinyl)hexahydrocyclopenta~c~pyrrole-2(1H)-
carboxylate
n-Butyl lithium (2.SM solution in hexanes, 1.5 mL, 3.81 mmol) was added
dropwise over
20 minutes to 3-bromo-5-methoxypyridine (0.717 g, 3.81 mmol), prepared as
described in WO
00/44755 in THF (4 mL) at -78 °C. After stirring at-78 °C for 40
minutes, the product from
Example 1D (0.286 g, 1.27 mmol) in diethyl ether (4 mL) was added dropwise to
the reaction
mixture and stirred at -78 °C for an additional 30 minutes. The
reaction mixture was then
allowed to warm to 0 °C and then quenched with saturated aqueous
ammonium chloride and
allowed to warm to room temperature. The mixture was partitioned between ethyl
acetate and
saturated aqueous ammonium chloride. The organic layer was separated, washed
with saturated
ammonium chloride, water, brine, dried over Na~S04, filtered and the filtrate
was concentrated
under reduced pressure. The residue was purified by chromatography (SiOi,, 50%
ethyl
acetate/hexane) to provide the title compound (0.142 g, 33%). MS (DCI/NH3) m/z
335 (M+H)+.
Example 2B
tert-butyl (cis)-5-(5-methoxy-3-pyridinyl)-3,3a,4,6a-
tetrahydrocyclopenta~c~pyrrole-2(1H)
carboxylate
152
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
The product from Example 2A (0.207 g, 0.619 mmol) in methylene chloride (6 mL)
was
treated with methanesulfonyl chloride (0.144 mL, 1.86 mmol), triethylamine
(0.27 mL, 1.92
mmol) and catalytic DMAP (0.002 g). After stirring at ambient temperature for
16 hours, the
volatiles were evaporated under reduced pressure. The residue was purified by
chromatography
(Si02, 50% ethyl acetate/hexane) to provide the title compound (0.038 g, 19%).
MS (DCI/NH3)
m/z 317 (M+H)+.
Example 2C
his)-5-(5-methoxy-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~pyrrole
hydrochloride
The product from Example 2B (0.038 g, 0.120 mmol) in methanol (1 mL) was
treated
with 1M solution of HCl in diethyl ether (2 mL) and stirred at ambient
temperature for 2 hours.
The volatiles were evaporated under reduced pressure. The residue was
crystallized from
ethanol and diethyl ether to provide the title compound (0.022 g, 64%). 1H NMR
(CD30D, 300
MHz) 8 2.82 (m, 1H), 3.17 3.27 (m, 2H), 3.33-3.61 (m, 4H), 3.88 (m, 1H), 4.07
(s, 3H), 6.54 (m,
1H), 8.17 (bs, 1H), 8.49 (bs, 1H), 8.57 (bs, 1H); MS (DCI/NH3) m/z 217 (M+H)+;
Anal.
calculated for C13Hi60N2'2.2HC1: C, 52.66; H, 6.19; N, 9.45. Found: C, 52.45;
H, 5.99; N,
9.17.
Example 3
(cis)-5-(6-chloro-5-methyl-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta~c~pyrrole
hydrochloride
Example 3A
tert-butyl 5-(6-chloro-5-methyl-3-pyridinyl)-5-hydroxyhexahydrocyclopenta~c~
pyrrole-2( 1 H)-
carboxylate
n-Butyl lithium (2.5 M solution in hexanes, 1.31 mL, 3.27 mmol) was added
dropwise
over 20 minutes to a -78 °C solution of 5-bromo-2-chloro-3-
methylpyridine (0.675 g, 3.27
mmol) in THF (4 mL). After stirring at -78 °C for 40 minutes, the
product from Example 1D
(0.368 g, 1.64 mmol) in THF (3 mL) was added dropwise to the reaction mixture.
After stirring
at -78 °C for 30 minutes, the reaction mixture was allowed to warm to 0
°C and then was
153
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
quenched with saturated aqueous ammonium chloride and allowed to warm to room
temperature.
The mixture was partitioned between ethyl acetate and saturated aqueous
ammonium chloride.
The organic layer was separated, washed with saturated ammonium chloride,
water, brine, dried
over Na2S04, filtered and the filtrate was concentrated under reduced
pressure. The residue was
purified by chromatography (Si02, SO% ethyl acetate/hexane) to provide the
title compound
(0.300 g, S2%). MS (DCI/NH3) m/z 3S3 (M+H)+.
O
O~N
CH3
~N~CI
Example 3B
tert-butyl (cis)-S-(6-chloro-S-methyl-3-pyridinyl)-3,3a,4 6a-tetrah droc
clopenta~c~pyrrole
2( 1 H)-carboxylate
The product from Example 3A (0.300 g, 0.852 mmol) inmethylene chloride (S mL)
was
treated with methanesulfonyl chloride (0.198 mL, 2.56 mmol), triethylamine
(0.38 mL, 2.73
mmol) and catalytic DMAP (0.003 g). The reaction mixture was stirred at
ambient temperature
for 16 hours and then the volatiles were evaporated under reduced pressure.
The residue was
purified by chromatography (Si02, SO% ethyl acetate/hexane) to provide the
title compound
(0.167 g, S9%). MS (DCI/NH3) m/z 33S (M+H)+,
Example 3 C
(cis)-5-(6-chloro-S-methyl-3-p ridinyl)-1,2,3,3a,4,6a-hexahydroc
clopentafc~pyrrole
hydrochloride
The product from Example 3B (0.167 g, 0.500 mmol) in methanol (1 mL) was
treated
with 1M solution of HCl in diethyl ether (3 mL). After stirring at ambient
temperature for 2
hours, the volatiles were evaporated under reduced pressure. The residue was
crystallized from
ethanol and diethyl ether to provide the title compound (0.095 g, 70%). ~H NMR
(CD3OD, 300
MHz) ~ 2.40 (s, 3H), 2.74 (m, 1H), 3.113.20 (m, 2H), 3.36-3.54 (m, 4H), 3.81
(m, 1H), 6.2 (m,
1H), 7.87 (d, J=2.4 Hz, 1H), 8.30 (d, J=2.6 Hz, 1H); MS (DCI/NH3) m/z 23S
(M+H)~; Anal.
1S4
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
calculated for Cl3HisC1N2~1.lHCl: C, 56.81; H, 5.90; N, 10.19. Found: C,
56.52; H, 5.90; N,
9.97.
Example 4
(cis)-5-(3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c~pyrrole
hydrochloride
Example 4A
tert-butyl (cis)-5-f [(trifluoromethyl)sulfon l~oxy~,-3,3a,4,6a-tetrahydroc
clopenta[c~pyrrole
2( 1 H)-carboxylate
The product from Example 1 D (3.0 g, 13.3 mmol) in THF (80 mL) was treated
with
lithium bis(trimethylsilyl)amide ( 1.OM solution in THF, 17.3 mL, 17.3 mmol)
at 78 °C. The
reaction mixture was stirred at -78 °C for 30 minutes and then a
solution of
N-phenyltrifluoromethanesulfonimide (6.65 g, 18.6 mmol) in THF (20 mL) was
added slowly.
The reaction mixture was stirred at -78 °C for 1 hour and then warmed
to 10 °C and stirred for
an additional hour. The volatiles were removed under reduced pressure, and the
residue was
purified by chromatography (Si02, 10% ethyl acetate/hexane) to provide the
title compound as a
white solid (4.44 g, 94%). MS (DCI/NH3) m/z 358 (M+H)~.
Example 4B
tert-butyl (cis)-5-(trimethylstannyl)-3,3a,4,6a-tetrah drocyclopenta[c~pyrrole-
2(1H)-carbox late
The product from Example 4A (1.00 g, 2.80 mmol) in THF (15 mL) was treated
with
hexamethylditin (0.826 g, 2.52 mmol), lithium chloride (0.712 g, 16.8 mmol)
and
tetrakis(triphenylphosphine)palladium(0) (0.065 g, 2 mol %) and stirred at 60
°C for 16 hours.
The reaction mixture was allowed to cool to ambient temperature, diluted with
diethyl ether,
washed with 1N aqueous sodium hydroxide, water, brine, dried over Na2SQ~,
filtered and the
filtrate was concentrated under reduced pressure. The residue was purified by
chromatography
(Si02, 10% ethyl acetate/hexane) to provide the title compound as a white
solid (0.769 g, 74%).
MS (DCI/NH3) mJz 372, 374 (M+H)t.
Example 4C
155
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
tent-butyl (cis)-5-(3-pyridinyl)-3,3a,4,6a-tetrahydrocyclopenta(c~pyrrole-
2(1H)-carboxylate
3-Bromopyridine (0.129 g, 0.82 mmol), triphenylarsine (0.020 g, 0.065 mmol)
and
tris(dibenzylideneacetone)dipalladium(0) (0.015 g, 2 mol%) in anhydrous 1-
methyl-2-
pyrrolidinone (4 mL) were treated with the product from Example 4B (0.305 g,
0.82 mmol) in 1-
methyl-2-pyrrolidinone (2 mL). The reaction mixture was stirred at 60
°C for 16 hoirs, allowed
to cool to ambient temperature and partitioned between ethyl acetate and 1N
aqueous sodium
hydroxide. The organic portion was separated, washed with 1N aqueous sodium
hydroxide,
water, brine, dried over Na2S04, filtered and the filtrate was evaporated
under reduced pressure.
The residue was purified by chromatography (SiOZ, 50% ethyl acetate/hexane) to
provide the
title compound (0.163 g, 69%). MS (DCI/NH3) m/z 287 (M+H)+.
Example 4D
(cis)-5-(3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~pyrrole
hydrochloride hydrochloride
The product from Example 4C (0.163 g, 0.570 mmol) in methanol (1 mL) was
treated
with a 1M solution of HCl in diethyl ether (2 mL). After stirring at ambient
temperature for 2
hours, the volatiles were evaporated under reduced pressure. The residue was
crystallized from
ethanol and diethyl ether to provide the title compound (0.138 g, 93%). 1H NMR
(CD30D, 300
MHz) ~ 2.85 (m, 1H), 3.19-3.25 (m, 2H), 3.35-3.59 (m, 4H), 3.89 (m, 1H), 6.56
(m, 1H), 8.09
(dd, J=5.8, 8.5 Hz, 1H), 8.75 (m, 1H), 8.97 (d, J=1.4 Hz, 1H); MS (DCI/NH3)
m/z 187 (M+I~;
Anal. calculated for C12Hi4Na'2HCl~0.2H20: C, 54.85; H, 6.29; N, 10.66. Found:
C, 54.73; H,
6.66; N, 10.58.
Example 5
(cis)-5-(3-methyl-5-isoxazolyl)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~pyrrole
hydrochloride
Example SA
tert-butyl 5-(3-methyl-5-isoxazolyl)-3,3a,4,6a-tetrahydrocyclopenta~c~pyrrole-
2(1H)-carboxylate
The product from Example 4A (411 mg, 1.15 mmol), triphenylarsine (28 mg, 0.092
mmol) and tris(dibenzylideneacetone)dipalladium(0) (21 mg, 0.023 mmol) in
anhydrous 1-
methyl-2-pyrrolidinone (6 mL) were treated with 3-methyl-5-
(tributylstannyl)isoxazole (448 mg,
156
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
1.2 mmol, prepared according to Salcamoto, T.; et. al., Tetrahedon, 1991, 47,
5111) in pmethyl-
2-pyrrolidinone (2 mL). The reaction mixture was stirred at 60 °C
overnight, allowed to cool to
ambient temperature and 1M aqueous KF (1.5 mL) was added. The mixture was
stirred for 0.5
hours, diluted with ethyl acetate and filtered through Celite. The filtrate
was washed with water
(SX), brine, dried over Na2S04, filtered and the filtrate was concentrated
under reduced pressure.
The residue was purified by chromatography (silica gel, gradient from hexane
to 30%
EtOAc/hexane) to provide 220 mg (66%) of the title compound. MS (DCI/NH3) m/z
291
(M+H)+.
Example SB
(cis)-5-(3-methyl-5-isoxazolyl)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~pyrrole
hydrochloride
The product from Example SA (220 mg, 0.759 mmol) in ethyl acetate (5 mL) was
treated
with a 4M solution of HCl in 1,4-dioxane (1 mL). After stirring at ambient
temperature for 1
hour, a few drops of diethyl ether were added resulting in precipitation of a
white solid that was
collected by filtration and dried at 60 °C to provide 126 mg (73%) of
the hydrochloride salt. 1H
NMR (CD3OD, 300 MHz) 8 2.27 (s, 3H), 2.70 (m, 1H), 3.0'3.20 (m, 2H), 3.35-3.55
(m, 4H),
3.83 (m, 1H), 6.26 (d, J=2.0 Hz, 1 H), 6.31 (s, 1H); MS (DCI/NH3) m/z 191
(M+H)+; Anal.
calculated for C11H14NZO~HCI: C, 58.28; H, 6.67; N, 12.36. Found: C, 58.18; H,
6.55; N,
12.27.
Example 6
~cis)-5-(5,6-dichloro-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~pyrrole
hydrochloride
Example 6A
tert-butyl 5-(5,6-dichloro-3-pyridinyl)-3,3a,4,6a-
tetrahydrocyclopenta~c~pyrrole-2(1H)-
carboxylate
2,3-Dichloro-5-iodopyridine (0.288 g, 1.05 mmol), triphenylarsine (0.026 g,
0.084
mmol), and tris(dibenzylideneacetone)dipalladium(0) (0.019 g, 2 mol%) in
anhydrous 1-methyl-
2-pyrrolidinone (5 mL) were treated with the product from Example 4B (0.392 g,
1.05 mmol) in
1-methyl-2-pyrrolidinone (2 mL). After stirring at 60 °C for 5 hours,
the reaction mixture was
157
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
allowed to cool to ambient temperature and partitioned between ethyl acetate
and 1N aqueous
sodium hydroxide. The organic extract was separated, washed with 1N aqueous
sodium
hydroxide, water, brine, dried over Na2S04, filtered and the filtrate was
concentrated under
reduced pressure. The residue was purified by chromatography (Si02, 50% ethyl
acetate/hexanes) to provide the title compound (0.228 g, 61%). MS (DCI/NH3)
m/z 355
(M+H)+.
Example 6B
(cis)-5-(5,6-dichloro-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~pyrrole
hydrochloride
The product from Example 6A (0.228 g, 0.642 mmol) in methanol (1 mL) was
treated
with a 1M solution of HCl in diethyl ether (2 mL). After stirring at ambient
temperature for 2
hours, the volatiles were evaporated under reduced pressure. The residue was
crystallized from
ethanol and diethyl ether to provide the title compound (0.120 g, 73%). 1H NMR
(CD30D, 300
MHz) 8 2.75 (m, 1H), 3.17 (m, 2H), 3.31-3.55 (m, 4H), 3.83 (m, 1H), 6.30 (m,
1H), 8.10 (d,
J=2.0 Hz, 1H), 8.44 (d, J=2.4 Hz, 1H); MS (DCI/NH3) m/z 255 (M+H)+; Anal.
calculated for
C12H12C12N2~HCI: C, 49.43; H, 4.49; N, 9.61. Found: C, 49.32; H, 4.60; N,
9.38.
Example 7
(cis)-2-methyl-5-(3-methyl-5-isoxazolyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta~c~pyrrole
hydrochloride
The product from Example SB (22 mg, 0.11 mmol), 37% formaldehyde in water and
formic acid were stirred at reflux for 2 hours, allowed to cool to room
tempreature and
concentrated under reduced pressure. The residue was purified by
chromatography (silica gel,
98:2:0.1 CHZC12/MeOH/NH4OH) to provide 13 mg (54%) of the free base that was
processed as
described in Example SB to provide the title compound as the hydrochloride
salt. 1H NMR
(CD30D, 300 MHz) ~ 2.28 (s, 3H), 2.70 (m, 1H), 2.90 (s, 3H), 3.0'3.22 (m, 2H),
3.30-3.60 (m,
4H), 3.84 (m, 1H), 6.25 (br s, 1 H), 6.32 (s, 1H); MS (DCI/NH3) m/z 205
(M+H)+; Anal.
calculated for C11H14N2O~HCl~0.25H20: C, 58.77; H, 7.19; N, 11.42. Found: C,
59.09; H,
6.91; N, 11.49.
158
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Example 8
(cis)-5-(5-chloro-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~pyrrole
dihydrochloride
Example 8A
(cis)-5-(5-chloro-3-pyridinyl)-1,2,3, 3 a,4, 6a-hexahydrocyclopenta~c~ pyrrole
The product from Example 4B and 5-chloro-3-pyridinyl
trifluoromethanesulfonate,
prepared as described in (WO 9740012), were processed as described in Example
4C to provide
the title compound (42% yield) as a light yellow oil: MS (DCI/NH3) m/z 321,
323 (M+H)+.
Example SB
(cis)-5-(5-chloro-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~pyrrole
dihydrochloride
The product from Example 8A (190 mg, 0.59 mmol) in 10 mL of 1:1 EtOAc/EtOH was
treated with a 4M solution of HCl in 1,4-dioxane (1.5 mL, 3.8 mmol). The
mixture was refluxed
for 1 hour and then allowed to cool to room temperature and stirred overnight.
The white solid
was collected by filtration to provide 170 mg of the title compound as the
dihydrochloride salt.
1H NMR (CD3OD, 400 MHz) ~ 2.84 (m, lI~, 3.15-3.24 (m, 2H), 3.3Q3.60 (m, 4H),
3.88 (m,
1H), 6.54 (m, 1H), 8.62 (d, J=1.8 Hz, 1H), 8.868.88 (m, 2H); MS (DCI/NH3) m/z
221,223
(M+H)~; Anal. calculated for C12Hi3C1N2~2HC1: C, 49.09; H, 5.15; N, 9.54.
Found: C, 48.83;
H, 4.89; N, 9.35.
Example 9
(cis)-5-(3-bromo-1,2,4-thiadiazol-5-yl)-1,2,3,3a,4,6a-
hexahydrocyclopenta~c~pyrrole
hydrochloride
3-Bromo-5-chloro-1,2,4-thiadiazole (Across 19.7 mg, 0.099 mmol) in 1-methyl-2
pyrrolidinone (0.2 mL) was treated with 0.1 mL of a solution of
tris(dibenzylideneacetone)dipalladium(0) (8.6 mg, 0.02 mmol), triphenylarsine
(11.5 mg, 0.08
mmol) and copper(I) iodide (1.8 mg, 0.02 mmol) in 1-methyl-2 pyrrolidinone
(0.5 mL). The
reaction mixture was then treated with the product from Example 4B (35 mg,
0.094 mmol) in 1
methyl-2-pyrrolidinone (0.1 mL). The reaction mixture was warmed to 60
°C, stirred over night
and then allowed to cool to room temperature. The mixture was diluted with
CFb_Cl2 and washed
159
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
with 0.5 mL of 1.ON aqueous NaOH. The aqueous phase was extracted twice with 5
mL of
CH2Cl2. All the organic phases were combined and filtered through a 900 mg
silica gel cartridge
(Alltech). The solvent was removed under reduced pressure and the residue was
purified by
reverse phase preparative HPLC (Waters Nova-Pak HR C18 6 m 60 25x100 mm, 50-
95%
MeCN/1 OmM NH40Ac over 10 minutes at 40 mL/min) to provide the free base. The
free base
was dissolved in MeOH (0.3 mL) and treated with a 1.OM solution of HCl in
diethyl ether (0.5
mL, 0.5 mmol). The solution was agitated at 40 °C for 16 hours
resulting in the formation of a
precipitate. The precipitate was triturated with diethyl ether (2X) and dried
under reduced
pressure to provide 20.9 mg (82%) of the title compound. 1H NMR (CD30D) b 2.82
(ddd, J=2.3,
4.2, 16.9 Hz,IH), 3.18 (dd, J=5.2, 12.0 Hz, 1H), 3.2p3.26 (m, 1H), 3.34 (m,
1H), 3.40 (dd,
J=3.1, 12.2 Hz, 1H), 3.47 (dd, J=8.3, 12.0 Hz, 1H), 3.53 (dd, J=8.7, 12.1,
1H), 3.89 (tt, J=2.7, 8.1
Hz, 1H), 6.69 (dd, J=2.3, 4.2 Hz, 1H); MS (ESI) m/z 272, 274 (M+H)+.
Example 10
(cis)-5-(1,3-thiazol-2-yl)-1,2,3,3a,4,6a-hexah droc clopenta~c~pyrrole h
drochloride
The product from Example 4B and 2-bromothiazole (purchased from Aldrich) were
processed as described in Example 9 to provide the title compound. 1H NMR
(CD30D) 8 2.86
(ddd, J=2.2, 4.2, 16.7 Hz,IH), 3.16 (dd, J=5.0, 11.6 Hz, 1H), 3.2>- 3.26 (m,
1H), 3.37 (dd, J=3.1,
11.6 Hz, 1 H), 3.43 (dd, J=8.1, 11.6 Hz, 1 H), 3 .51 (dd, J=8.7, 11.6 Hz, 1
H), 3 . 84 (tt, J=2.6, 8. 0
Hz, 1H), 6.37 (dd, J=2.2, 4.1 Hz, 1H), 7.56 (d, J=3.4 Hz, 1H), 7.80 (d, J=3.4
Hz, 1H); MS (ESI)
m/z 193 (M+H)+.
Example 11
(cis)-5-(3,5-dimethyl-4-isoxazolyl)-1,2,3,3a,4,6a-hexah droc
clopenta~c~pyrrole h drochloride
The product from Example 4B and 3,5-dimethyl-4-iodoisoxazole (purchased from
Avocado) were processed as described in Example 9 to provide the title
compound. 1H NMR
(CD30D) 8 2.30 (s, 3H), 2.46 (s, 3H), 2.67 (ddd, J=1.8, 4.2, 16.5 Hz, 1H),
3.08-3.14 (m, 2H),
3 .21 (m, 1 H), 3 .3 8 (dd, J=7. 8, 11.6 Hz, 1 H), 3 .47 (dd, J=8.7, 11.6 Hz,
1 H), 3.73 (m, 1 H), 5.73
(dd, J=2.0, 3.9 Hz, 1H); MS (ESI) m/z 205 (M+H)+.
160
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Example 12
(cis)-5-(1H-imidazol-4-yl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c~pyrrole
hydrochloride
The product from Example 4B and 4-iodoimidazole ( purchased from Combi-Blocks)
were processed as described in Example 9 to provide the title compound. 1H NMR
(CD3OD) 8
2.71 (dd, J=1.9, 16.5 Hz, 1H), 3.10 (tt, J=2.1, 8.4 Hz, 1H), 3.15 (dd, J=5.5,
12.0 Hz, 1H), 3.35
(dd, J=2.8, 11.5 Hz, 1H), 3.46 (dd, J=8.1, 11.6 Hz, 1H), 3.53 (dd, J=8.7, 11.6
Hz, 1H), 3.84 (m,
1H), 6.19 (dd, J=2.0, 3.9 Hz, 1H), 7.58 (s, 1H), 8.91 (s, 1H); MS (ESI) m/z
176 (M+H)+.
Example 13
(cis)-5-(1,3-thiazol-5-yl)-1,2,3,3a,4,6a-hexahydrocyclopenta[c~pyrrole
hydrochloride
The product from Example 4B and 5-bromothiazole, prepared as described in
(Recl.
Trav. Chim. Pays-Bas, (1954), 73, 325), were processed as described in Example
9 to provide
the title compound. 1H NMR (CD30D) S 2.79 (d, J=16.5 Hz, 1H), 3.16-3.22 (m,
2H), 3.323.38
(m, 2H), 3.43 (dd, J=8.0, 11.7 Hz, 1H), 3.52 (dd, J=8.7, 11.5 Hz, 1H), 3.83
(m, 1H), 6.18 (s, 1H),
8.14 (s, 1H), 9.51 (s, 1H); MS (ESI) m/z 193 (M+H)+.
Example 14
(cis)-5-(imidazo[1,2-a~pyridin-3-yl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c~pyrrole
I~drochloride
The product from Example 4B and 3-bromoimidazo[1,2-a]pyridine, prepared as
described in J. Org. Chem. (1965), 30, 4085-4090, were processed as described
in Example 9 to
provide the title compound. 1H NMR (CD30D) 82.80 (dd, J=1.7, 16.5 Hz, 1H),
3.18 (dd, J=5.5,
11.7 Hz, 1H), 3.21 (m, 1H), 3.38 (dd, J=3.0, 11.7 Hz, 1H), 3.47 (dd, J=8.1,
11.6 Hz, 1H), 3.55
(dd, J=8.7, 11.6 Hz, 1H), 3.86 (m, 1H), 6.35 (d, J=1.6 Hz, 1H), 7.81 (d, J=9.4
Hz, 1H), 7.92 (s,
1H), 8.10 (m, 2H), 8.73 (s, 1H); MS (ESI) m/z 226 (M+H)+.
Example 15
(cis)-5-(imidazo[1,2-a~pyridin-6-yl)-1,2,3,3a,4,6a-
hexahydrocyclopenta[c~pyrrole hydrochloride
The product from Example 4B and 6-bromoimidazo[1,2-a]pyridine, prepared as
described in J. Org. Chem. (1978), 43, 2900-2906, were processed as described
in Example 9 to
161
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
provide the title compound. 1H NMR (CD3OD) 82.90 (d, J=14.0 Hz, 1H), 3.25 (dd,
J=4.4, 11.6
Hz, 1H), 3.32-3.36 (m, 1H), 3.51 (d, J=5.3 Hz, 1H), 3.57 (dd, J=8.1, 11.5 Hz,
1H), 3.98 (m, 1H),
6.44 (s, 1H), 7.59 (dd, J=5.9, 6.7 Hz, 1H), 7.9'8.04 (m, 2H), 8.14 (s, 1H),
9.05 (d, J=7.2 Hz,
1H); MS (ESI) m/z 226 (M+H)+.
Example 16
(cis)-5-(thieno~3,2-b~pyridin-2- 1)-1,2,3,3a,4,6a-hexah droc
clopenta~c~pyrrole h drochloride
The product from Example 4B and 2-iodothieno[3,2-b]pyridine, prepared as
described in
J. Heterocycl. Chem. (1984), 21, 785-790, were processed as described in
Example 9 to provide
the title compound. 1H NMR (CD30D) 8 2.94 (ddd, J=2.2, 3.9, 16.4 Hz, 1H), 3.22
(dd, J=5.2,
12.0 Hz, 1H), 3.26-3.34 (m, 1H), 3.36-3.44 (m, 2H), 3.49 (dd, J=8.1, 11.6 Hz,
1H), 3.57 (dd,
J=8.6, 11.7 Hz, 1H), 3.92 (tt, J=2.7, 8.1 Hz, 1H), 6.52 (dd, J=2.2, 4.1 Hz,
1H), 7.65 (s, 1H), 7.85
(dd, J=5.6, 8.1 Hz, 1H), 8.84 (dd, J=1.0, 5.6 Hz, 1H), 9.04 (d, J=8.1 Hz, 1H);
MS (ESI) m/z 243
(M+H)+.
Example 18
cis)-5-(1,2,3,3a,4,6a-hexahydroc clopenta~c~pyrrol-5- 1)-2-
thiophenesulfonamide
hydrochloride
The product from Example 4B and 5-bromothiophene-2-sulfonamide (purchased from
Fluorochem USA) were processed as described in Example 9 to provide the title
compound. 1H
NMR (CD30D) 8 2.74 (m, 1H), 3.10-3.19 (m, 2H), 3.22-3.35 (m, 2H), 3.38 (dd,
J=8.5, 11.7 Hz,
1H), 3.50 (dd, J=8.7, 11.8 Hz, 1H), 3.79 (m, 1H), 6.04 (br s, 1H), 7.06 (d,
J=3.7 Hz, 1H), 7.48 (d,
J=3.8 Hz, 1H); MS (ESI) m/z 271 (M+H)+.
Example 19
(cis)-5-(6-methyl-3-pyridinyl)-1,2,3,3a,4,6a-hexah droc clopenta~c~pyrrole
dihydrochloride
Example 19A
tert-butyl (cis)-5-(6-methyl-3-p ridinyl)-3,3a,4,6a tetrahydrocyclopenta[cep
mole-2(1H)
carboxylate
162
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
The product from Example 4B and 5-bromo-2-methylpyridine, prepared as
described in
WO 0044755, were processed as described in Example 4C to provide the title
compound (50%
yield). MS (DCI/NH3) m/z 301 (M+H)+.
Example 19B
(cis)-5-(6-methyl-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~pyrrole
dihydrochloride
The product from Example 19A was processed as described in Example 8B to
provide
the title compound (88% yield). 1H NMR (CD30D, 400 MHz) & 2.78 (s, 3H), 2.82
(m, 1H),
3.15-3.27 (m, 2H), 3.29-3.60 (m, 4H), 3.87 (m, 1H), 6.48 (m, 1H), 7.90 (d,
J=8.5 Hz, 1H), 8.60
(dd, J=2.0,8.4 Hz, 1H), 8.75 (d, J=2.1 Hz, 1H); MS (DCI/NH3) m/z 201 (M+H)+;
Anal.
calculated for C13Hi6N2~2HC1: C, 57.15; H, 6.64; N, 10.25. Found: C, 56.88; H,
6.65; N, 10.23.
Example 20
(cis)-5-(2-methyl-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~p rrole h
drochloride
Example 20A
tert-butyl (cis)-5-(2-methyl-3-pyridinyl)-3,3a,4,6a-tetrahydroc
clopenta~c~pyrrole-2(1H)-
carboxylate
The product from Example 4B and 3-bromo-2-methylpyridine were processed as
described in Example 4C to provide the title compound (19% yield). MS
(DCI/NH3) m/z 301
(M+H)+.
Example 20B
(cis)-5-(2-methyl-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~p rrole h
drochloride
The product from Example 20A was processed as described in Example 8B to
provide
the title compound (53% yield). 1H NMR (CD30D, 400 MHz) 8 2.78 (m, 1H), 2.85
(s, 3H),
3.20-3.60 (m, 6H), 3.90 (m, 1H), 6.08 (m, 1H), 7.90 (dd, J=5.7, 7.8 Hz, 1H),
8.49 (d, J=8.1 Hz,
1H), 8.62 (dd, J=1.7, 7.5 Hz, 1H); MS (DCI/NH3) m/z 201 (M+H)+; Anal.
calculated for
C13H16N2~2HC1~0.25 H20: C, 56.23; H, 6.71;N, 10.09. Found: C, 56.40; H, 6.57;
N, 9.96.
163
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Example 21
(cis)-5-(6-chloro-5-fluoro-3-pyridinyl)-1,2,3,3a,4,6a-
hexahydrocyclopenta~c~pyrrole
hydrochloride
Example 21A
tert-butyl (cis)-5-(6-chloro-5-fluoro-3-pyridinyl)-3,3a,4,6a-
tetrahydrocyclopenta~c~pyrrole-
2( 1 H)-carboxylate
2-Chloro-3-fluoro-5-iodopyridine (0.400 g, 1.08 mmol), prepared as described
in J. Org.
Chem. (1993), 58, 7832, triphenylarsine (0.026 g, 0.084 mmol) and
tris(dibenzylideneacetone)dipalladium(0) (0.019 g, 2 mol%) in anhydrous 1-
methyl-2-
pyrrolidinone (5 mL) were treated with the product from Example 4B (0.310 g,
1.20 mmol) in 1-
methyl-2-pyrrolidinone (2 mL). After stirring at 60 °C for 5 hours, the
reaction mixture was
allowed to cool to ambient temperature and partitioned between ethyl acetate
and 1N aqueous
sodium hydroxide. The organic extract was separated, washed with 1N aqueous
sodium
hydroxide, water, brine, dried over NaZS04, filtered and the filtrate
concentrated under reduced
pressure. The residue was purified by chromatography (Si02, 50% ethyl
acetate/hexanes) to
provide the title compound (0.226 g, 62°/~). MS (DCI/NH3) m/z 339
(M+H)+.
Example 21 B
(cis)-5-(6-chloro-5-fluoro-3-pyridinyl)-1,2, 3,3 a,4,6a-hexahydrocyclopenta~c~
pyrrole
hydrochloride
The product from Example 21A (215 mg, 0.60 mmole) in CHZCIz (1 mL) and TFA (1
mL) was stirred at ambient temperature far 1 hour and then concentrated under
reduced pressure.
The residue was purified by chromatography (silica gel; CHCl3/MeOH/NH40H
90:10:1) to
provide the free base of the title compound. The free base was dissolved in
MeOH and treated
with a solution of 1M HCl in diethyl ether to provide 137 mg (77%) of the
title compound. 1H
NMR (CD30D, 300 MHz) S 2.76 (m, 1H), 3.18 (m, 2H), 3.30-3.55 (m, 4H), 3.83 (m,
1H), 6.30
(m, 1H), 7.86 (dd, J=2.0, 9.9 Hz, 1H), 8.35 (d, J=2.0 Hz, 1H); MS (DCI/NI~)
m/z 239 (M+H)+;
Anal. calculated for C12H12C1FN~~l.5HC1~0.2CH30H: C, 48.88; H, 4.81; N, 9.34.
Found: C,
49.00; H, 4.53; N, 8.95.
164
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Example 23
(cis)-5-(6-fluoro-3-pyridinyl)-1,2,3,3a,4,6a-hexahydroc clopenta~c~pyrrole
hydrochloride
Example 23A
tert-butyl5-(6-fluoro-3-pyridin 1)-3,3a,4,6a-tetrah drocyclopenta~c~pyrrole-
2(1H)-carbo late
5-Bromo-2-flouropyridine (0.200 g, 0.538 mmol), triphenylarsine (0.013 g,
0.043 mmol),
and tris(dibenzylideneacetone)dipalladium(0) (0.010 g, 2 mol%) in anhydrous 1-
methyl-2-
pyrrolidinone (2 mL) were treated with the product from Example 4B (0.392 g,
1.05 mmol) in 1-
methyl-2-pyrrolidinone (1 mL). The reaction mixture was stirred at 60
°C for 16 hours, allowed
to cool to ambient temperature and partitioned between ethyl acetate and 1N
aqueous sodium
hydroxide. The organic portion was separated, washed with 1N aqueous sodium
hydroxide,
water, brine, dried over Na2S04, filtered and the filtrate concentrated under
reduced pressure.
The residue was purified by chromatography (SiOz, 50% ethyl acetate/hexanes)
to provide the
title compound (0.126 g, 77%). MS (DCI/NH3) m/z 305 (M+H)+.
Example 23B
(cis)-5-(6-fluoro-3-pyridinyl)-1,2,3,3a,4,6a-hexah droc clopenta~c~p mole h
drochloride
The product from Example 23A (0.126 g, 0.414 mmol) in methanol (1 mL) was
treated
with a 1M solution of HCl in diethyl ether (2 mL). After stirring at ambient
temperature for 2
hours, the volatiles were evaporated under reduced pressure. The residue was
crystallized from
ethanol and diethyl ether to provide the title compound (0.067 g, 67%). 1H NMR
(CD3OD, 300
MHz) & 2.76 (m, 1H), 3.13-3.21 (m, 2H), 3.36-3.55 (m, 4H), 3.81 (m, 1H), 6.16
(m, 1H), 7.07
(ddd, J=0.7, 2.7, 8.8 Hz, 1H), 8.09 (ddd, J=2.7, 7.8, 8.6 Hz, 1H); MS
(DCI/NH3) m/z 205
(M+H)+.
Example 24
(cis)-5-(1,2,3,3a,4,6a-hexah drocyclopenta~c~pyrrol-5-yl)-2-
thiophenecarboximidamide
hydrochloride
165
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
The product from Example 4B and the free base of 5-chloro-2-
thiophenecarboximidamide monohydrochloride, prepared as descibed in EP 819690,
were
processed as described in Example 9 to provide the title compound. 1H NMR
(CD30D} S 2.74
(br d, J=12. 6 Hz, 1 H), 3 .14-3 .24 (m, 2H), 3 .2'~ 3 .41 (m, 2H), 3 .3 8 (m,
1 H), 3 . 84 (m, 1 H), 6.21
(br s, 1H), 7.29 (d, J=4.1 Hz, 1H), 7.88 (d, J=4.0 Hz, 1H); MS (ESI) m/z 234
(M+H)+.
Example 25
(cis)-5-(2-methyl-2H-tetraazol-5-yl)-1,2,3,3a,4,6a-
hexahydrocyclopenta~c~pyrrole hydrochloride
The product from Example 4B and 5-bromo-2-methyl-2H-tetrazole, prepared as
described in Can. J. Chem., (1973), 51, 2315-2322, were processed as described
in Example 9 to
provide the title compound. 1H NMR (CD30D) 82.94 (ddd, J=2.2, 4.1, 17.2 Hz,
1H), 3.15 (dd,
J=5.3, 11.9 Hz, 1H), 3.18-3.24 (m, 1H), 3.37 (dd, J=3.0, 12.0 Hz, 1H}, 3.45
(dd, J=8.0, 12.0 Hz,
1H), 3.52 (dd, J=8.7, 11.9 Hz, 1H), 3.85 (tt, J=2.6, 7.9 Hz, 1H), 4.35 (s,
3H), 6.51 (dd, J=2.2, 4.1
Hz, 1H); MS (ESI) m/z 192 (M+H)+.
Example 26
(cis)-5-(thieno~2,3-b~pyridin-5-yl)-1,2,3,3a,4,6a-
hexahydrocyclopenta~c~pyrrole hydrochloride
The product from Example 4B and 5-bromothieno[2,3-b]pyridine, prepared as
described
in J. Heterocycl. Chem, (1968), 5, 773-778, were processed as described in
Example 9 to provide
the title compound. 1H NMR (CD30D) b 2.87 (dd, J=2.9, 16.4 Hz, 1H), 3.20 (dd,
J=5.0, 11.9
Hz, 1H), 3.26 (tt, J=2.2, 8.3 Hz, 1H), 3.323.37 (m, 1H), 3.42 (dd, J=2.7, 11.7
Hz, 1H), 3.46 (dd,
J=8.0, 11.7 Hz, 1H), 3.55 (dd, J=8.9, 11.7 Hz, 1H), 3.87 (m, 1H), 6.36 (d,
J=1.9 Hz, 1H), 7.50 (d,
J=5.9 Hz, 1H), 7.87 (d, J=5.9 Hz, 1H), 8.56 (d, J=1.9 Hz, 1H), 8.86 (d, J=1.9
Hz, 1H); MS (ESI)
m/z 243 (M+H)+.
Example 27
(cis)-5-(imidazo~l,2-a~pyridin-7-yl)-1,2,3,3a,4,6a
hexahydrocyclopenta~c~pyrrole hydrochloride
The product from Example 4B and 7-chloroimidazo[1,2-a]pyridine, prepared as
described in J. Heterocycl. Chem, (1965), 2, 53-62, were processed as
described in Example 9 to
provide the title compound. IH NMR (CD30D) 82.85 (dd, J=1.6, 16.5 Hz, 1H),
3.19 (dd, J=5.5,
166
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
12.0 Hz, 1H), 3.22-3.28 (m, 1H), 3.35-3.37 (m, 1H), 3.40 (dd, J=3.1, 12.2 Hz,
1H), 3.50 (dd,
J=8.3, 11.7 Hz, 1H), 3.57 (dd, J=9.1, 11.6 Hz, 1H), 3.90 (m, 1H), 6.61 (d,
J=1.6 Hz, 1H), 7.71
7.74 (m, 2H), 8.00 (d, J=2.2 Hz, 1H), 8.17 (d, J=1.6 Hz, 1H), 8.71 (d, J=7.2
Hz, 1H); MS (ESI)
m/z 226 (M+H)+.
Example 28
(cis)-5-(2-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~pyrrole
hydrochloride
The product from Example 4B and 2-bromopyridine (purchased from Aldrich) were
processed as described in Example 9 to provide the title compound. 1H NMR
(CD30D) 8 2.92
(dd, J=1.9, 16.5 Hz, 1H), 3.22 (dd, J=5.5, 12.0 Hz, 1H), 3.263.29 (m, 1H),
3.38-3.41 (m, 1H),
3.44 (dd, J=3.3, 12.3 Hz, 1H), 3.53 (dd, J=8.6, 12.0 Hz, 1H), 3.57 (dd, J=8.6,
12.0 Hz, 1H), 3.96
(tt, J=2.7, 8.2 Hz, 1H), 6.87 (d, J=1.6 Hz, 1H), 7.92 (t, J=6.7 Hz, 1H), 8.14
(d, J=8.4 Hz, 1H),
8.53 (dt, J=4.0, 11.1 Hz, 1H), 8.71 (dd, J=0.9, 5.9 Hz, 1H); MS (ESI) m/z 187
(M+H)+.
HN' I ~ ~ ~N
Example 29
(cis)-5-(4-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~pyrrole
hydrochloride
The product from Example 4B and the free base of 4-bromopyridine hydrochloride
(purchased from Aldrich) were processed as described in Example 9 to provide
the title
compound. 1H NMR (CD30D) 8 2.86 (dd, J=1.7, 16.7 Hz, 1H), 3.19 (dd, J=5.3,
11.6 Hz, 1H),
3.22-3.27 (m, 1H), 3.35-3.40 (m, 1H), 3.42 (dd, J=3.1, 11.6 Hz, 1H), 3.52 (dd,
J=8.6, 12.0 Hz,
1H), 3.56 (dd, J=8.7, 11.6 Hz, 1H), 3.93 (m, 1H), 6.87 (dd, J=2.2, 3.7 Hz,
1H), 8.08 (d, J=6.6 Hz,
2H), 8.75 (d, J=6.4 Hz, 2H); MS (ESI) m/z 187 (M+H)+.
Example 30
(cis)-5-(5-vitro-1,3-thiazol-2-yl)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~pyrrole
hydrochloride
The product from Example 4B and 2-bromo-5-nitrothiazole (purchased from
Aldrich)
were processed as described in Example 9 to provide the title compound. 1H NMR
(CD3OD) 8
2.85 (dd, J=1.9, 16.9 Hz, 1H), 3.16 (dd, J=5.2, 12.0 Hz, 1H), 3.243.27 (m,
1H), 3.33-3.35 (m,
167
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
1H), 3.38 (dd, J=3.1, 11.9 Hz, 1H), 3.45 (dd, J=8.3, 12.0 Hz, 1H), 3.51 (dd,
J=8.4, 11.6 Hz, 1H),
3.87 (m, 1H), 6.67 (dd, J=2.2, 4.1 Hz, 1H), 8.61 (s, 1H); MS (ESI) m/z 238
(M+H)+.
Example 31
(cis)-5-(6-methyl-2-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~pyrrole
hydrochloride
The product from Example 4B and 2-bromo-6-methylpyridine (purchased from
Aldrich)
were processed as described in Example 9 to provide the title compound. IH NMR
(CD30D) b
2.59 (s, 3H), 2.92 (d, J=16.2 Hz, 1H), 3.30-3.42 (m, 3H), 3.47-3.65 (m, 3H),
3.96 (m, 1H), 6.40
(d, J=1.6 Hz, 1H), 7.92 (t, J=6.9 Hz, 1H), 8.49 (d, J=7.8 Hz, 1H), 8.64 (d,
J=5.7 Hz, 1H); MS
(ESI) m/z 201 (M+H)+.
Example 32
his)-5-(1,3,4-thiadiazol-2-yl)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~pyrrole
hydrochloride
The product from Example 4B and 2-bromo-l,3,4-thiadiazole, prepared as
described in
Chem. Ber., (1956), 56, 1534-1543, were processed as described in Example 9 to
provide the
title compound. 1H NMR (CD30D) 8 2.86 (m,lH), 3.20 (dd, J=4.3, 11.8 Hz, 1H),
3.3Q 3.60 (m,
SH), 3.84 (m, lI~, 6.52 (br s, 1H), 9.40 (s, 1H); MS (ESI) m/z 194 (M+H)+.
Example 34
(endo)-5-(3-pyridinyl)octahydrocyclopenta~c~pyrrole bis(4-
methylbenzenesulfonate)
Example 34A
(endo)-5-(3-pyridinyl)octahydrocyclopenta~c~pyrrole
The product from Example 1 G (0.077 g, 0.263 mmol) in ethanol was treated with
hydrogen gas (1 atm) in the presence of Pd/C (0.008 g, 10 wt.%) and stirred at
ambient
temperature for 4 hours. The reaction vessel was evacuated, purged with
nitrogen and the
catalyst was removed by filtration through diatomaceous earth. The filtrate
was concentrated
under reduced pressure and the residue was purified by chromatography (SiOz,
CHC13/MeOH/NH40H, 90:10:1) to provide the title compound (0.036 g, 39%). MS
(DCI/NH3)
m/z 189 (M+H)+.
168
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Example 34B
(endo)-5-(3-pyridinyl)octahydrocyclopenta~c~pyrrole bis(4-
methylbenzenesulfonate)
The product from Example 34A (0.020 g, 0.0795 mmol) in methanol was treated
with p~
toluenesulfonic acid (.030 g, 0.159 mmol) and stirred at ambient temperature
for 5 minutes. The
volatiles were evaporated under reduced pressure and the residue was
crystallized from ethanol
and diethyl ether to provide the title compound (0.015 g, 30%). ~I NMR (CD30D,
300 MHz) 8
1.67 (m, 2H), 2.37 (s, 6H), 2.52 (m, 2H), 3.11 (m, 2H), 3.293.40 (m, 4H), 7.23
(d, J=8.1 Hz,
4H), 7.70 (d, J=8.1 Hz, 4H), 8.03 (dd, J=5.7, 8.1 Hz, 1 H), 8.63 (d, J=8.5 Hz,
1 H), 8.72 (d, J=5.4
Hz, 1H), 8.82 (s, 1H); MS (DCI/NH3) m/z 189 (M+H)+.
Example 35
(cis)-5-(6-chloro-3-pyridinyl)octahydrocyclopenta(c~ pyrrol-5-0l
The product from Example lE (0.044 g, 0.130 mmol) in methanol (1 mL) was
treated
with a 1M solution of HCl in diethyl ether (1 mL). After stirring at ambient
temperature for 2
hours, the volatiles were evaporated under reduced pressure. The residue was
crystallized from
ethanol and diethyl ether to provide the title compound (0.031 g, 87%). 1H NMR
(CD30D, 300
MHz) b 2.08 (d, J=14.0 Hz, 2H), 2.38 (m, 1H), 2.41 (m, 1H), 3.24 (m, 2H), 3.41
(d, J=5.5 Hz,
4H), 7.44 (d, J=8.5 Hz, 1H), 7.93 (dd, J=2.6, 8.5 Hz, 1H), 8.49 (d, J=2.6
Hz,lH); MS (DCI/NH3)
m/z 239 (M+H)+.
Example 36
(endo)-5-(6-chloro-3-pyridinyl)octahydrocyclopenta~c~pyrrole hydrochloride
Example 36A
~endo)-5-(6-chloro-3-pyridinyl)octahydrocyclopenta~c~pyrrole
(exo)-5-(6-chloro-3-pyridinyl)octahydrocyclopenta~c~pyrrole
The product from Example lE (0.336 g, 0.994 mmol) in acetonitrile (5 mL) at 0
°C was
treated with 4-(dimethylamino)pyridine (0.672 g, 1.49 mmol) and methyl oxalyl
chloride (0.32
mL, 1.19 mmol). The reaction mixture was allowed to warm to room temperature,
diluted with
169
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
ethyl acetate, washed with saturated aqueous sodium bicarbonate (20 mL), water
(20 mL), brine
(20 mL), dried over Na2S04, filtered and the filtrate was concentrated under
reduced pressure.
The residue was dissolved in toluene (5 mL) and treated with tributyltin
hydride (0.401 mL, 1.49
mmol) and AIBN (0.024 g, 0.149 mmol). The reaction mixture was stirred at 100
°C for 3 hours,
allowed to cool to room temperature and concentrated under reduced pressure.
The residue was
purified by chromatography (Si02, 30% ethyl acetate/hexane) to provide 0.019 g
(6%) of the
exo-isomer and 0.094 g (29%) of the endo-isomer. exo-isomer: iH NMR (CDC13,
300 MHz) 8
1.47 (s, 9H), 1.86 (m, 2H), 1.99 (m, 2H), 2.90 (m, 2H), 3.143.30 (m, 3H), 3.65
(m, 2H), 7.24 (d,
J=8.6 Hz, 1H), 7.48 (dd, J=2.4, 8.1 Hz, 1H), 8.26 (d, J=2.5 Hz, 1H); MS
(DCI/NH3) m/z 323,
325 (M+H)~. endo-isomer: 1H NMR (CDC13, 300 MHz) 8 1.47 (s, 9H), 1.48 (m, 2H),
2.33 (m,
2H), 2.75 (m, 2H), 3.09 (m, 1H), 3.35 (m, 2H), 3.45 (m, 2H), 7.24 (d, J=8.1
Hz, 1H), 7.51 (dd,
J=2.4, 8.1 Hz, 1H). 8.25 (d, J=2.4 Hz); MS (DCI/NH3) m/z 323, 325 (M+H)+.
Example 36B
(endo)-5-(6-chloro-3-pyridinyl)octahydrocyclopenta[c~pyrrole hydrochloride
The endo-isomer from Example 36A (0.094 g, 0.292 mmol) in methanol (1 mL) was
treated with a 1M solution of HCl in diethyl ether (1 mL). After stirring at
ambient temperature
for 2 hours, the volatiles were evaporated under reduced pressure. The residue
was crystallized
from ethanol and diethyl ether to provide the title compound (0.062 g, 32%).
1H NMR (CD30D,
300 MHz) 8 1.51 (m, 2H), 2.47 (m, 2H), 3.07 (m, 2H), 3.133.38 (m, 5H), 7.40
(d, J=8.1 Hz,
1H), 7.77 (dd, J=2.7, 8.1 Hz, 1H), 8.28 (d, J=2.7, 1H); MS (DCI/NH~ m/z 223
(M+H)+.
Example 37
(exo)-5-(6-chloro-3-pyridinyl)octahydrocyclopenta[c~pyrrole hydrochloride
The exo-isomer from Example 36A (0.019 g, 0.059 mmol) in methanol (1 mL) was
treated with a 1M solution of HCl in diethyl ether (1 mL). After stirring at
ambient temperature
for 2 hours, the volatiles were evaporated under reduced pressure. The residue
was crystallized
from ethanol and diethyl ether to provide the title compound (0.011 g, 72%).
1H NMR (CD30D,
170
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
300 MHz) 8 1.99 (m, 4H), 2.95 (m, 2H), 3.06 (m, 2H), 3.40 (m, 1H), 3.61 (m,
2H), 7.41 (d, J=8.5
Hz, 1H), 7.77 (dd, J=2.6, 8.5 Hz, 1H), 8.29 (d, J=2.6 Hz, 1H); MS (DCI/NH3)
m/z 223 (M+H)+.
Example 38
(cis)-5-(3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta~b~p rrole fumarate
~cis)-5-(3-pyridinyl)-1,2,3,3a,6,6a-hexahydrocyclopenta~b~pyrrole fiunarate
Example 38A
benzyl 5-oxohexahydrocyclopenta(b~pyrrole-1 (2H)-carboxylate
Benzyl 6,6-dichloro-5-oxohexahydrocyclopenta[b]pyrrole-I(2H)-carboxylate (3.87
g,
11.8 mmol), prepared as descibed in Tetrahedron Lett. (1997), 38, 1869-1872,
in MeOH (60 mL)
at 0 °C was treated with solid ammonium chloride (6.32 g, 118 mmol)
followed by zinc duct
(Aldrich; 3.09 g, 47.2 mmol). After stirring at room temperature for 1 hour,
the reaction mixture
was filtered and the filtrate concentrated under reduced pressure. The residue
was purified by
chromatography (Si02, 40% EtOAc/hexanes) to provide 2.57 g (84%) of the title
compound.
MS (DCI/NH3) m/z 260 (M+H)+.
Example 38B
benzyl 5-~ [(trifluoromethyl)sulfonyl~ oxy~-3,3a,6,6a-
tetrahydrocyclopenta(b~pyrrole-1 (2H)-
carboxylate
benzyl 5-{~(trifluorometh 1)sulfonyl~oxy)-3,3a,4,6a
tetrahydrocyclopenta[b~pyrrole-1(2H)
carboxylate
The product from Example 38A (1.56 g, 6.02 mmol) in THF (30 mL) at-78
°C was
treated with potassium bis(trimethylsilyl)amide (O.SM solution in toluene,
14.4 mL, 7.22 mmol).
The reaction mixture was stirred at -78 °C for 30 minutes and then a
solution of
N-phenyltrifluoromethanesulfonimide (2.58 g, 7.22 mmol) in THF (5 mL) was
added dropwise.
The reaction mixture was stirred at -78 °C for 1 hour and then quenched
by the addition of water.
The mixture was warmed to ambient temperature and extracated with CHzCl2. The
organic
extract was washed with saturated aqueous NH4Cl, brine, dried (Na2SO4),
filtered and the filtrate
concentrated under reduced pressure. The residue was purified by
chromatography (SiO2, 20%
171
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
EtOAc/hexanes) to provide 1.84 g (78%) of a 4:1 mixture of the title
compounds. MS
(DCI/NH3) m/z 392 (M+H)+.
Example 38C
benzyl 5-(trimethylstannyl)-3,3a,6,6a-tetrahydrocyclopenta~b~pyrrole-1 (2H)-
carboxylate
benzyl 5-(trimethylstannyl)-3,3 a,4,6a-tetrahydrocyclopenta~b~ pyrrole-1 (2H)-
carboxylate
The product mixture of Example 38B (260 mg, 0.664 mmol) in THF (3.3 mL) was
treated with hexamethylditin (218 mg, 0.664 mmol), lithium chloride (84 mg,
2.0 mmol) and
tetrakis(triphenylphosphine)palladium(0) (15 mg, 0.0013 mmol). The resulting
mixture was
stirred at 60 °C for 14 hours, allowed to cool to room temperature and
filtered through Celite
(diethyl ether wash). The filtrate was washed with 1N aqueous sodium hydroxide
(2X), water,
brine, dried (Na2S04), filtered and the filtrate concentrated under reduced
pressure. The residue
was purified by chromatography (Si02, 15% ethyl acetate/hexanes) to provide
160 mg (57%) of
a 4:1 mixture of the title compounds. MS (DCI/NH3) m/z 423, 425 (M+H)+.
Example 38D
benzyl 5-(3-pyridinyl)-3,3a,6,6a-tetrahydrocyclopenta(b~pyrrole-1 (2H)-
carboxylate
benzyl 5-(3-pyridinyl)-3,3a,4,6a-tetrahydrocyclopenta~b~pyrrole-1 (2H)-
carboxylate
The mixture of Example 38C (140 mg, 0.332 mmol) and 3-bromopyridine (0.042 mL,
0.43 mmol) were processed as described in Example 4C. Purification by
chromatography (Si02,
30% EtOAc/hexane) provided 90 mg (85%) of a 4:1 mixture of the title
compounds. MS
(DCI/NH3) m/z 321 (M+H)+.
Example 38E
(cis)-5-(3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta~b)pyrrole fumarate
(cis)-5-(3-pyridinyl)-1,2,3,3a,6,6a-liexahydrocyclopenta~b~pyrrole fumarate
Iodotrimethylsilane (43 ~L, 0.30 mmol) was added to a 0 °C solution of
the mixture from
Example 38D (80 mg, 0.25 mmol) in 2.5 mL of acetonitrile. The solution was
stirred fox 3 hours
at 0 °C then concentrated under reduced pressure. The residue was
passed through a plug of
silica gel (10% MeOH/CH2Cl2 wash) to provide 38 mg (82%) of a mixture of the
free amines.
172
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
The free amines were dissolved in MeOH and fumaric acid was added (23 mg, 0.19
mmol).
After 30 minutes, a few drops of diethyl ether were added causing formation of
a precipitate that
was then collected by filtration. The solid was dried under reduced pressure
to provide 42 mg of
a 4:1 mixture of the title compounds. 1H NMR for major isomer (D20, 300 MHz)8
1.95 (m,
1H), 2.30 (m, 1H), 2.77 (m, 1H), 3.13-3.49 (m, SH), 6.36 (m, 1H), 6.60 (s,
2H), 7.77 (dd, J=2.6,
8.5 Hz, 1H), 8.34 (m, 1H), 8.60 (m, 1H), 8.83 (m, 1H); MS (DCI/NH~ m/z 187
(M+H)+. Peaks
for the minor isomer in the'H NMR are obscured by the major isomer, except for
the olefinic
proton (multiplet at 6.45 ppm) that allows for a rough determination of the
product ratios.
Example 39
(cis)-5-(5-bromo-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~pyrrole
fiunarate
Example 39A
tert-butyl (cis)-5-(5-bromo-3-pyridinyl)-3,3a,4,6a-
tetrahydrocyclopenta~c~pyrrole-2(1H)-
carboxylate
3,5-Dibromopyridine (0.656 g, 2.77 mmol) and the product from Example 4B (1.03
g,
2.77 mmol) were processed as described in Example 4C to provide 477 mg (47%)
of the title
compound. MS (DCIM3) m/z 365, 367 (M+H)+.
Example 39B
(cis)-5-(5-bromo-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~pyrrole
fumarate
The product from Example 39A (0.110 g, 0.302 mmol) in CH2CIz (mL) was treated
with
TFA (1 mL). After stirring for 30 minutes, the solution was concentrated and
then diluted with
10% NH40H/MeOH. This was concentrated under reduced pressure and the residue
passed
through a pad of silica gel (90:10:1 CHCI3:MeOH:NH40H eluent). The filtrate
was concentrated
to afford the free base of the title compound which was diluted with MeOH and
treated with
fumaric acid (0.033 g 0.28 mmol) to afford the title compound (77 mg, 67%). 'H
NMR
(CD30D, 300 MHz) 8 2.75 (m, 1H), 3.11-3.21 (m, 2H), 3.35-3.59 (m, 4H), 3.89
(m, 1H), 6.29
(m, 1H), 6.65 (s, 2H), 8.12 (dd, J=2.0, 2.1 Hz, 1H), 8.55 (d, J=2.4 Hz, 1H),
8.63 (d, J=2.4 Hz,
173
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
1H); MS (DCI/NH3) m/z 265, 267 (M+H)+; Anal. calculated for Cl2HiaNa~CaHaOa:
C, 50.41; H,
4.49; N, 7.35. Found: C, 50.51; H, 4.53; N, 7.33.
Example 40
(cis)-5-(5-vinyl-3-pyridin 1)-1,2,3,3a,4,6a-hexahydrocyclopenta(c~pyrrole
fumarate
Example 40A
tert-butyl (cis)-5-(5-vinyl-3-pyridinyl)-3,3a,4,6a-tefirah
drocyclopenta(c~pyrrole-2(1H)
carboxylate
Tributyl(vinyl)tin (0.656 g, 2.77 mmol),
tetrakis(triphenylphosphine)palladium(0) (0.026
g, 0.23 mmol), in anhydrous toluene were treated with the product from Example
39A (1.03 g,
2.77 mmol). The reaction mixture was stirred for 3 days at 100 °C.
After cooling to ambient
temperature, the volatiles were removed under reduced pressure and the residue
was purified by
flash chromatography to afford the title compound (279 mg, 93%). MS (DCI/NH3)
m/z 312
(M+H)+.
Example 40B
(cis)-5-(5-vinyl-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta(c~pyrrole
fumarate
The product from Example 40A (0.279 g, 0.894 mmol) was processed as described
in
Example 39B to provide the title compound (0.168 g, 57%). IH NMR (CD30D, 300
MHz) 8
2.80 (m, 1H), 3.14-3.54 (m, 6H), 3.83 (m, 1H), 5.45 (d, J=11.2 Hz, 1H), 5.98
(d, J=18.0 Hz, 1H),
6.27 (m, 1H), 6.66 (s, 2H), 6.80 (dd, J=11.2,18.0 Hz, 1H), 7.99 (dd, J=20, 2.0
Hz, 1H), 8.49 (d,
J=2.0 Hz, 1H), 8.55 (d, J=2.0 Hz, 1H); MS (DCI/NI-~) m/z 213 (M+H)''~; Anal.
calculated for
CiaHisNz~C4Ha0a: C, 65.84; H, 6.14; N, 8.53. Found: C, 65.64; H, 6.07; N,
8.30.
Example 41
(cis)-5-(1,2,3,3a,4,6a-hexahydroc clopenta~c~pyrrol-5-yl)nicotinonitrile
Example 41 A
174
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
tert-butyl (cis)-S-(S-cyano-3-pyridinyl)-3,3a,4,6a-tetrahydrocyclopenta[c)p
rrole-2(1H~
carboxylate
3-Bromo-S-cyanopyridine (0.251 g, 1.37 mmol) and the product from Example 4B
(0.510
g, 137 mmol) were processed as described in Example 4C to provide 232 mg (S4%)
of the title
compound. MS (DCI/NH3) m/z 312 (M+H)+.
Example 41 B
(cis)-S-(I,2,3,3a,4,6a-hexah droc clopenta~c~pyrrol-S- 1)nicotinonitrile
fumarate
The product from Example 41A (0.091 g, 0.29 mmol) was processed as described
in
Example 39B to provide the title compound (O.OS9 g, 62%). 1H NMR (CD30D, 300
MHz) 8
2.77 (m, 1H), 3.12-3.33 (m, 2H), 3.28-3.54 (m, 4H), 3.83 (m, 1H), 6.37 (m,
1H), 6.65 (s, 2H),
8.28 (dd, J=2.0, 2.0 Hz, 1H), 8.80 (d, J=1.70 Hz, 1H), 8.83 (d, J=2.0 Hz, 1H);
MS (DCI/NH3)
m/z 212 (M+H)+; Anal. calculated for C14HI6N2'GH404~ C, 62.38; H, 5.23; N,
12.84. Found:
C, 62.52; H, 5.27; N, 13.01.
Example 42
(cis)-S-(6-chloro-3-pyridinyl)-1 2,3,3a,4,6a-hexah droc clopenta(c~pyrrole h
drochloride
Example 42A
(+)-tert-butyl (cis)-S-(6-chloro-3-pyridin 1)-3,3a,4,6a-tetrah droc
clopenta~c~p mole-2(1H)
carboxylate
and
(-)-tent-butyl (cis)-S-(6-chloro-3-p ridinyl)-3 3a,4,6a-
tetrahydrocyclopenta[c~pyrrole-2(1H)-
carboxylate
The racemic product from Example 1F was separated into its individual
enantiomers on a
Chiralpak AS column (4.6 mm X 2S0 mm) using 98:2 hexane:ethanol as eluent. The
more
mobile enantiomer, retention time = 12.54 minutes, [a]D -46.1 (c 2.0, CH~Cl~),
The less mobile
enantiomer, retention time = 17.66 minutes [a]D 48.8 (c 2.0, CH2Cl2).
Example 42B
175
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(cis)-5-(6-chloro-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~[pyrrole
hydrochloride
The more mobile enantiomer, (-)-tert-Butyl (cis)-5-(6-chloro-3-pyridinyl)-
3,3a,4,6a-
tetrahydrocyclopenta[c]pyrrole-2(1H)-carboxylate, from Example 42A was
processed as
described in Example 1 G to afford the title compound. IH NMR (CD30D, 300 MHz)
8 2.75 (m,
1H), 3.I2-3.22 (m, 2H), 3.32-3.55 (m, 4H), 3.82 (m, 1H), 6.24 (m, 1H), 7.45
(d, J=85 Hz, 1H),
7.94 (dd, J=2.4, 8.5 Hz, 1H), 8.47 (d, J=2.1 Hz, 1H); MS (DCI/NH3) m/z 22I,
223 (M+H)+.
Example 43
(cis)-5-(6-chloro-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta(c]pyrrole
hydrochloride
The less mobile enantiomer, (+)-tert-Butyl (cis)-5-(6-chloro-3-pyridinyl)-
3,3a,4,6a-
tetrahydrocyclopenta[c]pyrrole-2(1H)-carboxylate, from Example 42A was
processed as
described in Example 1 G to afford the title compound. 1H NMR (CD30D, 300 MHz)
~ 2.75 (m,
1H), 3.12-3.22 (m, 2H), 3.32-3.55 (m, 4H), 3.82 (m, 1H), 6.24 (m, 1H), 7.45
(d, J=8.5 Hz, 1H),
7.94 (dd, J=2.5, 8.5 Hz, 1H), 8.47 (d, J=2.4 Hz, 1H); MS (DCI/NH3) m/z 221,
223 (M+H)+.
Example 44
(cis)-6-(6-chloro-3-pyridinyl)-1,2,3,3a,4,6a-hexahydrocyclopenta~c~pyrrole
fumarate
Example 44A
tert-butyl (cis)-4-oxohexahydrocyclopenta~c~pyrrole-2(1H)-carboxylate
(cis)-2-Benzylhexahydrocyclopenta[c]pyrrol-4(1H)-one, prepared as described in
Heterocycles (1988), 27(3), 643-644, and di tert-butyl-dicarbonate in methanol
were treated with
hydrogen gas in the presence of palladium hydroxide (20 wt. %). After
completion of the
reaction, the reaction vessel was purged with nitrogen and the catalyst
removed by filtration.
The filtrate was concentrated under reduced pressure to provide the title
compound. 1H NMR
(CDCI3, 300 MHz) 8 1.45 (s, 9H), 1.87 (m, 1H), 2.16 (m, 1H), 2.342.39 (m, 2H),
2.74 (m, 1H),
3.03 (m, 1H), 3.15 (br m, 1H), 3.42 3.74 (m, 3H).
Example 44B
176
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
tert-butyl (cis)-6-f ~(trifluoromethyl)sulfon lox )-3,3a,4,6a-
tetrahydrocyclopenta~c)pyrrole
2( 1 H)-carboxylate
The product from Example 44A (1.78 g, 7.88 mmol) was processed as described in
Example 4A to afford the title compound (1.57 g, 56%). MS (DCI/NH3) m/z 358
(M+H)+, 375
(M+NH~)+.
Example 44C
tert-butyl (cis)-6-(trirnethylstann 1)-3,3a,4,6a-tetrah
drocyclopenta~c~pyrrole-2(1H)-carbox late
The product from Example 44B (0.303 g, 0.848 mmol) was processed as described
in
Example 4B to afford the title compound (0.225 g, 71 %). MS (DCI/NH3) m/z 372,
374 (M+H)+.
Example 44D
tert-butyl (cis)-6-(6-chloro-3-p~ridinyl)-3,3a,4,6a tetrahydroc clopenta~c)p
rrole 2(1H)
carboxylate
2-Chloro-5-iodopyridine and the product from Example 44C were processed as
described
in Example 4C to afford the title compound. MS (DCI/NH3) m/z 321, 323 (M+H)~.
Example 44E
(cis)-6-(6-chloro-3-pyridin 1)-1,2,3,3a,4,6a-hexah droc clopenta~c~p mole
fumarate
The product from Example 44D was processed as described in Example 39B to
afford the
title compound: 1H NMR (CD30D, 300 MHz) 8 2.50 (m, 1H), 2.96 (m, 1H), 3.07-
3.18 (m, 2H),
3.49-3.62 (m, 2H), 4.10 (m, 1H), 6.39 (m, 1H), 7.46 (dd, J=0.7, 8.1 Hz, 1H),
7.91 (dd, J=2.5,
8.SHz, 1H), 8.44 (d, J=2.4 Hz, 1H); MS (DCI/M~) m/z 221, 223 (M+H)+; Anal.
calculated fox
Ci2HisClNz'C4H404: C, 57.06; H, 5.09; N, 8.32. Found: C, 57.15; H, 5.33; N,
8.36.
Example 45
(cis)-4-(3-pyridinyl)-octah droc clopenta~c]pyrrol-4-0l dihydrochloride
Example 45A
tert-butyl (cis)-4-hydroxy-4-(3-pyridin 1)-hexahydrocyclopenta~c~p mole-2(1H)-
carbox late
177
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
3-Bromopyridine (4.36 g, 27.6 mmol) and the product from Example 44A (2.07 g,
9.19
mmol) were processed as described in Example lE to afford the title compound
(0.743 g, 27%).
MS (DCI/NH3) m/z 305 (M+I~+
Example 45B
(cis)-4-(3-pyridinyl)-octahydrocyclopenta~c~pyrrol-4-0l dihydrochloride
The product from Example 45A was processed as described in Example 35 to
afford the
title compound. IH NMR (CD3OD, 300 MHz) 8 1.92 (m, 1H), 2.17 (m, 1H), 2.322.51
(m, 2H),
2.98-3.14 (m, 2H), 3.23-3.43 (m, 3H), 3.49 (d, J=11.2 Hz, 1H), 8.11 (dd,
J=5.9, 8.4 Hz, 1H),
8.79-8.82 (m, 2H), 9.02 (d, J=1.4 Hz, 1H); MS (DCI/NH3) m/z 205 (M+H~.
Example 45
(cis)-4-(3-pyridinyl)-octahydrocyclopenta~c~pyrrol-4-0l dihydrochloride
Example 45A
tert-butyl-4-hydroxy-4-(3-pyridinyl)-hexahydrocyclopenta~c~pyrrole-2( 1H)-
carboxylate
3-Bromopyridine (4.36 g, 27.6 mmol) and the product from Example 44A (2.07 g,
9.19
mmol) were processed as described in Example lE to afford the title compound
(0.743 g, 27%).
MS (DCI/NH3) m/z 305 (M+H)+.
Example 45B
(cis)-4-(3-pyridinyl)-octahydrocyclopenta~c~pyrrol-4-0l dihydrochloride
The product from Example 45A was processed as described in Example 35 to
afford the
title compound. 1H NMR (CD30D, 300 MHz) 8 1.92 (m, 1H), 2.17 (m, 1H), 2.322.51
(m, 2H),
2.98-3.14 (m, 2H), 3.23-3.43 (m, 3H), 3.49 (d, J=11.2 Hz, 1H), 8.11 (dd,
J=5.9, 8.4 Hz, 1H),
8.79-8.82 (m, 2H), 9.02 (d, J=1.4 Hz, 1H); MS (DCI/NH3) m/z 205 (M+H~.
Example 46
(endo)-4-(6-chloro-3-pyridinyl)octahydrocyclopenta~cLpyrrole 4-
methylbenzenesulfonate
178
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Example 46A
his)-tert-butyl-4-hydroxy-4-(6-chloro-3-pyridinyl)-
hexahydrocyclopenta~c~pyrrole-2( 1H)-
carboxylate
5-Bromo-2-chloropyridine (1.82 g, 9.46 mmol) and the product from Example 44A
(1.64
g, 7.28 mmol) were processed as described in Example lE to afford the title
compound (1.25 g,
51%). MS (DCI/NH3) m/z 339,341 (M+H)+.
Example 46B
(endo)-tert-butyl-4-(6-chloro-3-pyridinyl)octahydrocyclopenta~c~pyrrole
carboxylate
(exo)-tert-butyl -4-(6-chloro-3-pyridinyl)octahydrocyclopenta~c~pyrrole
carboxylate
The product from Example 46A (0.250 g, 0.738 mmol) was processed as described
in
Example 36A. The residue was purified by chromatography (Si02, 10-30% ethyl
acetate/hexane
gradient) to provide 0.044 g (18%) of the faster eluting exo-isomer and 0.145
g (61%) of the
slower eluting endo-isomer. MS (DCI/NH3) m/z 323, 325 (M+H)+.
Example 46C
(endo)-4-(6-chloro-3-pyridinyl)octahydrocyclopenta~c~pyrrole 4-
methylbenzenesulfonate
The endo-isomer from Example 46B (0.094 g, 0.292 mmol) in EtOAc (5 mL) was
treated
with p-toluenesulfonic acid monohydrate (0.074 g, 0.39 mmol). The solution was
warmed to
reflux for 4 hours and then allowed to cool to ambient temperature. The solid
was collected by
filtration (EtOAc wash) and dried under high vacuum to afford the title
compound as a white
solid (107 mg, 71%). 1H NMR (CD3OD, 300 MHz) 8 1.76 (m, 1H), 1.9Q2.10 (m, 3H),
2.37 (s,
3H), 2.50 (dd, J=9.7, 11.5 Hz, 1H), 2.86 (dd, J=7.8, 11.6 Hz, 1H), 2.93312 (m,
2H), 3.27 (m,
1H), 3.41 (m, 1H), 3.64 (dd, J=8.4, 11.0 Hz, 1H), 7.23 (d, J=7.8 Hz, 2H), 7.42
(d, J=8.3 Hz, 1H),
7.70 (d, J=8.1 Hz, 2H), 7.76 (m, 1H), 8.32 (m, 1H); MS (DCI/NH3) m/z 223, 225
(M+H)+; Anal.
calculated for Cl2HisC1N2~1.2C~H803S: C, 57.07; H, 5.77; N, 6.52. Found: C,
56.92; H, 5.79;
N, 6.57.
Example 47
(cis)-6-(6-chloro-3-pyridinyl)-3-azabicyclo~3.2.0~hept-6-ene 4-
methylbenzenesulfonate
179
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Example 47A
(cis)-3-~(4-methylphenyl)sulfonyl~-3-azabicyclo~3.2.0~heptan-6-of
(cis)-3-[(4-Methylphenyl)sulfonyl]-3-azabicyclo[3.2.0]heptan-6-one (8.55 g,
32.2 mmol),
prepared according to Gobeaux and Ghosez, Heterocyles, (1989) 28(1), 29-32, in
2:1
THF/CH2Cl2 (150 mL) at 0 °C was treated with a 2M solution of LiBH4
(19.3 mL, 38.7 mmol).
After 0.5 hours, the reaction mixture was carefully quenched with 2-propanol
(10 mL), allowed
to warm to ambient temperature and the volatiles removed under reduced
pressure. The residue
was diluted with water and extracted twice with CH2C12. The combined organic
extracts were
washed with brine, dried over NaZS04, filtered, and the filtrate concentrated
under reduced
pressure to afford the title compound as a white solid (8.20 g, 95%).
Example 47B
(cis)-tert-butyl 6-hydroxy-3-azabicyclo~3.2.0~heptane-3-carboxylate
A solution of sodium napthalenide was prepared according to the procedure
described by
Heathcock et. al., J. Org. Chem. (1989), 54, 1548-1562 by adding naphthalene
(15.4 g, 120
mmol) to a suspension of finely cut sodium metal (2.30 g, 100 mmol) in
dimethoxyethane (100
mL), and stirring the resulting dark green mixture at room temperature for 2
hours. The product
from Example 47A (8.10 g, 30.3 mmol) in 100 mL of DME at -78 °C was
treated slowly with
the sodium napthalenide solution (~65 mL) until a light green color persisted.
The reaction
mixture was then quenched at-78 °C by the addition of 200 mL of water.
The mixture was
allowed to warm to 0 °C and di-tert-butyl dicarbonate (6.94 g, 31.8
mmol) was added. After 0:5
hours the mixture was diluted with EtOAc and the layers were separated. The
organic extract
was washed with brine, dried over Na2S04, filtered and the filtrate
concentrated under reduced
pressure. The residue was purified by chromatography (5 to 30% EtOAc/CI~C12
gradient) to
afford the title compound as a white solid (5.26 g, 81%). MS (DCI/NH3) m/z 214
(M+H)+.
Example 47C
(cis)-tent-butyl 6-oxo-3-azabicyclo~3.2.0~heptane-3-carboxylate
180
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
DMSO (4.15 mL, 58.5 mmol) was added dropwise to a solution of oxalyl chloride
(2.55
mL, 29.3 mmol) in CH2C12 ( 100 mL) at -78 °C (gas evolution). After 15
minutes, a solution of
the product from Example 47B (5.20 g, 24.4 mmol) in CH2Clz (20 mL) was added
dropwise.
After 30 minutes, triethylamine was added dropwise and the white mixture was
stirred at-78 °C
for 6 hours then allowed to warm to -40 °C for an additional hour. The
mixture was then diluted
with water and allowed to warm to room temperature. The layers were separated
and the organic
extract was washed with water and brine, dried over Na2S04 filtered and and
the filtrate
concentrated under reduced pressure. The residue was purified by
chromatography ( 10 to 20%
EtOAc/CH2C12 gradient) to afford the title compound as a viscous oil (4.05 g,
79%). MS
(DCI/NH3) m/z 212 (M+H)+.
Example 47D
(cis)-tert-butyl6-(6-chloro-3-pyridinyl)-6-hydroxy-3-azabicyclo~3.2.0~heptane-
3-carbo late
5-Bromo-2-chloropyridine (0.888 g, 4.62 mmol) and the product from Example 47C
(0.750 g, 3.55 mmol) were processed as described in Example lE to afford the
title compound
(1.15 g, 66%). MS (DCI/NH3) m/z 325, 327 (M+H)+.
Example 47E
cis)-tert-butyl6-(6-chloro-3-pyridinyl)-3-azabicyclo~3.2.0~hept-6-ene-3-carbox
late
The product from Example 47D (0.400 g, 1.23 mmol), triethylamine (0.36 mL, 2.6
mmol), methanesulfonyl chloride (0.19 mL, 2.5 mmol), and 4-
dimethylaminopyridine (3 mg)
were combined in THF and heated at reflux for 16 hours. The solvent was
removed under
reduced pressure and the residue was purified by chromatography (10%
EtOAc/CH2C12) to
afford the title compound as a white foam (0.280 mg,74%). MS (DCI/NI~) m/z
307, 309
(M+H)+.
Example 47F
~cis)-6-(6-chloro-3-pyridinyl)-3-azabicyclo~3.2.0~hept-6-ene 4-
methylbenzenesulfonate
The product from Example 47E (0.160 g, 0.522 mmol) was processed as described
in
Example 46C to provide the title compound as a white solid (0.122 mg, 62%). 'H
NMR
181
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(CD30D, 300 MHz) 8 2.36 (s, 3H), 3.00-3.15 (m, 2H), 3.40 (d, J=11.9 Hz, 1H),
3.51 (d, J=12.5
Hz, 1H), 3.67 (dd, J=3.4, 6.4 Hz, 1H), 4.03 (dd, X3.7, 6.5 Hz, 1H), 6.48 (s,
1H), 7.23 (d, J=7.8
Hz, 2H), 7.42 (dd, J=0.7, 8.5 Hz, 1H), 7.70 (d, J=8.1 Hz, 2H), 7.89 (dd,
J=2.4, 8.5 Hz, 1H), 8.47
(d, J=2.4 Hz, 1H); MS (DCI/NH3) m/z 207, 209 (M+H)+; Anal. Calculated for
CIIHlClN2~C~H803S: C, 57.06; H, 5.05; N 7.39. Found: C, 56.79; H, 4.93; N,
7.24.
Example 48
(endo)-6-(6-chloro-3-pyridinyl)-3-azabicyclo~3.2.0~heptane 4-
methylbenzenesulfonate
Example 48A
(endo)-tert-butyl 6-(6-chloro-3-pyridinyl)-3-azabicyclo(3.2.O~heptane-3-
carboxylate
(exo)-tert-butyl 6-(6-chloro-3-pyridinyl)-3-azabicyclo~3.2.0~heptane-3-
carboxylate
The product from Example 47D (0.250 g, 0.770 mmol) was processed as described
in
Example 36A. The residue was purified by chromatography (Si02, 5-20% ethyl
acetate/CH2Cl2
gradient) to provide 0.035 g (15%) of the faster eluting exo-isomer and 0.131
g (55%) of the
slower eluting endo-isomer. exo-isomer: 1H NMR (CDC13, 300 MHz) ~ 1.51 (s,
9H), 2.18-2.36
(m, 2H), 2.84-3.02 (m, 2H), 3.24-3.33 (m, 2H), 3.44 (m, 1H), 3.60-3.74 (m,
2H), 7.29 (d, J=8.1
Hz, 1H), 7.53 (dd, J=2.2, 8.4 Hz, 1H), 8.25 (d, J=2.0 Hz, 1H); MS (DCI/NH3)
m/z 309, 311
(M+H)~. endo-isomer: 1H NMR (CDC13, 300 MHz) 8 1.42 (s, 9H), 2.07 (m, 1H),
2.58 (m, 1H),
3.01 (m, 1H), 3.01-3.32 (m, 4H), 3.60 (br m, 1H), 3.70 (m, 1H), 7.28 (d, J=8.5
Hz, 1H), 7.43 (br
d, J=7.4 Hz, 1H), 8.25 (br s, 1H); MS (DCI/NH~ m/z 309, 311 (M+H)+.
Example 48B
(endo)-6-(6-chloro-3-pyridinyl)-3-azabicyclo~3.2.0~heptane 4-
methylbenzenesulfonate
The endo-isomer from Example 48A (0.130 g, 0.421 mmol) was processed as
described
in Example 46C to afford the title compound as a white solid (0.135 g, 84%).
'H NMR (CD30D,
300 MHz) & 2.19 (m, 1H), 2.37 (s, 3H), 2.68, (m, 1H), 3.08 (dd, J=3.9, 13.1
Hz, 1H), 3.25 (m,
1H), 3.30-3.41 (m, 3H), 3.52 (m, 1H), 3.96 (br q, J=9.5 Hz, 1H), 7.23 (d,
J=7.8 Hz, 2H), 7.44 (d,
J=8.3 Hz, 1H), 7.63 (dd, J=3.7, 8.1 Hz, 1H), 7.69 (d, J=8.2 Hz, 2H), 8.17 (d,
J=2.4 Hz, 1H); MS
182
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
(DCI/NH3) m/z 209, 211 (M+H)+; Anal. Calculated for C11H13C1N2~C~Hg03S: C,
56.76; H,
5.56; N 7.35. Found: C, 56.75; H, 5.49; N, 7.26.
Example 49
(exo)-6-(6-chloro-3-pyridinyl)-3-azabicyclo~3.2.0~heptane 4-
methylbenzenesulfonate
The exo-isomer from Example 48A (0.035 g, 0.125 mmol) was processed as
described in
Example 46C to afford the title compound as a white solid (0.026 g, 60%). 1H
NMR (CD30D,
300 MHz) S 2.25-2.50 (m, 3H), 2.36 (s, 3H), 3.26-3.36 (m, 4H), 3.61 (br d,
J=11.2 Hz, 1H), 3.61
(br d, J=11.2 Hz, 1H), 7.23 (d, J=7.8 Hz, 2H), 7.46 (d, J=8.4 Hz, 1H), 7.70
(d, J=8.2 Hz, 2H),
7.84 (m, 1H), 8.27 (d, J=2.4 Hz, 1H); MS (DCI/NH3) m/z 209, 211 (M+H); Anal.
Calculated
for C11Hi3C1N2~C~H803S: C, 56.76; H, 5.56; N 7.35. Found: C, 56.45; H, 5.52;
N, 7.11.
Example 50
(cis)-6-(5,6-dichloro-3-pyridinyl)-3-azabicyclo~3.2.0~hept-6-ene 4-
methylbenzenesulfonate
Example S OA
(cis)-tert-butyl 6-(5,6-dichloro-3-pyridinyl)-6-hydroxy-3-
azabicyclo~3.2.0~heptane-3-carboxylate
2,3-Dichloro-5-bromopyridine (0.758 g, 2.77 mmol) and the product from Example
47A
(0.450 g, 2.13 mmol) were processed as described in Example lE to afford the
title compound
(0.565 g, 74%). MS (DCI/NH3) m/z 359, 361 (M+H)+.
Example SOB
(cis)-tert-butyl 6-(5, 6-dichloro-3-pyridinyl)-3-azabicyclo ~3 .2.0~ hept-6-
ene-3-carboxylate
The product from Example SOA (0.359 g, 1.00 mmol) was processed as described
in
Example 47E to afford the title compound as a white solid (0.131 g, 38%). MS
(DCI/NH3) m/z
341, 343 (M+H)+.
Example SOC
(cis)-6-(5,6-dichloro-3-pyridinyl)-3-azabicyclo~3.2.0~hept-6-ene 4-
methylbenzenesulfonate
183
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
The product from Example SOB (0.125 g, 1.00 mmol) was processed as described
in
Example 46C to afford the title compound as a white solid (0.108 g, 72%). 1H
NMR (CD30D,
300 MHz) b 2.37 (s, 3H), 3.02-3.13 (nn, 2H), 3.41 (d, J=12.2 Hz, 1H), 3.52 (d,
J=12.6 Hz, 1H),
3.68 (dd, J=3.7, 6.4 Hz, 1H), 4.03 (dd, J=3.8, 6.S Hz, 1H), 6.54 (s, 1H), 7.23
(d, J=8.1 Hz, 2H),
7.68 (d, J=8.1 Hz, 2H), 8.08 (d, J=2.0 Hz, 1H), 8.42 (d, J=2.0 Hz, 1H); MS
(DCI/NH3) m/z 241,
243 (M+~+; Anal. Calculated for C11H1oC12Nz'C~H803S: C, 52.31; H, 4.39; N,
6.78. Found:
C, S2.2S; H, 4.52; N, 6.62.
- Example S 1
endo)-6-(S,6-dichloro-3-pyridin 1)-3-azabic clo(3.2.O~heptane 4-
methylbenzenesulfonate
Example S lA
(endo)-tert-butyl 6-(S,6-dichloro-3-pyridinyl)-3-azabic clo~3 2 O~heptane-3-
carboxylate
~exo)-tert-butyl6-(5,6-dichloro-3-p ridin 1)-3-azabicyclo~3.2.0~heptane-3-
carboxylate
The product from Example SOA (0.185 g, O.S1S mmol) was processed as described
in
Example 36A. The residue was purified by chromatography (Si02, S-20% ethyl
acetate/CH2C12
gradient) to provide 0.018 g (10%) of the faster eluting exo-isomer and 0.80 g
(4S%) of the
slower eluting endo-isomer. Data for the exo-isomer: 1H NMR (CDCl3, 300 MHz) 8
1.S 1 (s,
9H), 2.18-2.36 (m, 2H), 2.90-3.02 (m, 2H), 3.24-3.33 (m, 2H), 3.44 (m, IH),
3.60-3.72 (m, 2H),
7.65 (d, J=2.2 Hz, 1H), 8.15 (d, J=2.0 Hz, 1H); MS (DCI/NH3) xn/z 343,345
(M+H)+. Data for
the endo-isomer: 1H NMR (CDCl3, 300 MHz) ~ 1.44 (br s, 9H), 2.07 (m, IH), 2.59
(m, 1H),
3.02 (m, 1H), 3.03-3.32 (m, 4H), 3.58 (br s, 1H), 3.70 (br q, J=9.8 Hz, IH),
7.57 (br s, 1H), 8.03
(br s, 1H); MS (DCI/NH3) m/z 343, 345 (M+H)+.
Example S 1B
(endo)-6-(S,6-dichloro-3-p ridin 1)-3-azabicyclo~3.2 O~heptane 4-meth
lbenzenesulfonate
The endo-isomer from Example SlA (0.077 g, 0.22 mmol) was processed as
described in
Example 46C to afford 0.066 g (71%) of the title compound as a white solid. 1H
NMR (CD30D,
300 MHz) ~ 2.18 (m, IH), 2.37 (s, 3H), 2.68, (m, IH), 3.08 (dd, J=3.7, 13.2
Hz, IH), 3.22 (m,
1 H), 3.3 0-3 .42 (m, 3H), 3 . S 3 (m, 1 H), 3.97 (br q, J=9. S Hz, 1 H), 7.23
(d, J=7. 8 Hz, 2H), 7.70 (d,
184
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
J=8.5 Hz, 2H), 7.82 (d, J=2.1 Hz, 1H), 8.14 (d, J=2.0 Hz, 1H); MS (DCI/1'H3)
m/z 243, 245
(M+H)+; Anal. Calculated for CllHizCl2Nz'C~Hfi03S: C, 52.05; H, 4.85; N, 6.74.
Found: C,
52.11; H, 4.70; N, 6.63.
Example 52
(cis)-5-(6-phenylpyridazin-3-yl)-1,2,3,3a,4,6a-hexahydroc clopenta[c~pyrrole 4-
methylbenzene
sulfonate
Example 52A
tert-butyl5-(6-phenylpyridazin-3-yl)-3,3a,4,6a-tetrah drocyclopenta[cep mole-
2(1H)-
carboxylate
The product from Example 4B (0.200 g, 0.538 mmol), 3-chloro-6-phenylpyridazine
(0.205 g, 1.08 mmol), bis(tri-t-butylphosphine)palladium(0) (0.00275 g, 0.0538
mmol,
commercially available from Strem) and cesium fluoride (0.180 g, 1.18 mmol)
were combined in
1,4-dioxane (1 mL), according to the procedure reported by G. C. Fu and
coworkers in J. Am.
Chem. Soc. (2002) 124, 6343-6348, and stirred at 85 °C for 36 hours.
The mixture was cooled to
ambient temperature, diluted with ethyl acetate and filtered through of plug
of Si02 (ethyl acetate
wash). The filtrate was concentrated and the residue was purified by
chromatography (Si02,
20°1o ethyl acetate/hexanes) to afford the title compound. MS (DCI/NH3)
m/z 364 (M+H)+.
Example 52B
(cis)-5-(6-phenylpyridazin-3-yl)-1,2,3,3a,4,6a-hexah drocyclopenta[cep mole 4-
meth lbenzene
sulfonate
The product from Example S l A (0.075 g, 0.28 mmol) was processed as described
in
Example 46C to afford the title compound. 1H NMR (CD30D, 300 MHz) & 2.36 (s,
3H), 2.98-
308 (m, 1H), 3.20 (dd, J=4.4, 11.9 Hz, 1H), 3.3Q3.60 (m, SH), 3.91 (m, 1H),
6.62 (m, 1H), 7.50~
7.60 (m, 3H), 7.69 (d, J=8.0 Hz, 2H), 8.02 (d, J=9.2 Hz, 1H), 8.10 (m, 2H),
8.14 (d, J=8.8 Hz,
1H; MS (DCI/NH3) m/z 264 (M+I~+; Anal. calculated for C1~H1~T3~C~H803S: C,
66.18; H,
5.79; N, 9.65. Found: C, 65.94; H, 5.62; N, 9.54.
185
CA 02495589 2005-02-09
WO 2004/016604 PCT/US2003/025471
Example 53
(cis)-2-methyl-5-(6-phenylpyridazin-3-yl)-1,2,3,3a,4,6a-
hexahydrocyclopenta~c~pyrrole
The product from Example 52B (0.090 g, 0.21 mmol) was processed as described
in
Example 7 to afford the title compound. 1H NMR (CD30D, 300 MHz) 8 2.33 (s,
3H), 2.58 (m,
2H), 2.74 (m, 2H), 2.85 (m, 1H), 3.14 (m, 1H), 3.23 (m, 1H), 3.62 (m, 1H),
6.63 (m, 1H), 7.47
7.59 (m, 3H), 7.98 (d, J=8.8 Hz, 1H), 8.07 8.10 (m, 3H); MS (DCI/NH3) m/z 278
(M+H)+.
It is understood that the foregoing detailed description and Examples are
merely
illustrative and are not to be taken as limitations upon the scope of the
invention, which is
defined by the appended claims. Various changes and modifications to the
disclosed
embodiments will be apparent to those skilled in the art. Such changes and
modifications,
including without limitation those relating to the chemical structures,
substituents, derivatives,
intermediates, syntheses, formulations and/or methods of use of the invention,
may be made
without departing from the spirit and scope thereof.
186