Note: Descriptions are shown in the official language in which they were submitted.
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
ELECTROSTATIC TONER COMPOSITION TO ENHANCE COPY QUALITY BY
IMPROVED FUSING AND METHOD OF MANUFACTURING SAME
BACKGROUND OF THE INVENTION
Field of the Invention:
The present invention relates to a developer for developing electrostatic
latent images in
electrophotography, electrostatic recording and electrostatic printing. More
specifically, the
present invention is directed to a composition and method that provides a
developer which
comprises toner particles and a wax to ensure the reduction and potential
elimination of
image offsetting by providing proper fixing or fusing during the
electrophotographic process
and maintaining a stable, high quality image, during extended use.
Description of the Background:
Visible image forming methods associated with toners using electrophotographic
systems
have been extensively studied and are currently widely used. Typical examples
of these
techniques are dual-component developing methods, which use image-forming
particles and
often larger carrier particles, and mono-component developing methods, which
use a toner
comprising only magnetic or non-magnetic image-forming particles. Details of
such
developing methods are described in Kirk-Othmer, Encyclopedia of Chemical
Technology, 4th
ed., 9:261-275 (1994).
An image forming apparatus utilizing an electrophotographic method with toner
is well
known. In the image forming apparatus utilizing the electrophotographic
method, images axe
generally formed onto a sheet of copy paper through the following processes.
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
After uniformly charging a photoconductor that serves as an image-holding
body, images are
exposed onto the surface of the charged photoconductor. Attenuating
electrostatic charges
during the exposure of light forms a latent image. Then the electrostatic
latent images are
visualized by developing with toner to form a toner image. The toner images
are.transferred
onto the aforementioned medium and thereafter fixed on it by heating, pressure
or solvent
vapor.
In recent years, accompanying the rapid growth of computer technology, digital
copiers and
printers have been developed and become widely used. In these machines, mono-
component
developing methods have been applied more often to reduce the number of supply
parts and
ease of customer maintenance requirements compared with that of the dual
component
method.
In the mono-component systems, toner is generally required to have good
fluidity and
uniform chargeability in order to form a good quality visible image as
described in U.S
5,802,284 incorporated herein by reference. The use of silica powder additives
for toner
particles to impart fluidity and chargeability properties has been widely
studied and is one
conventionally accepted method. Many US and international patents exist and
are known that
include the use of silica or silicon dioxide with toner of various
compositions. A subset of
these patents relates to surface treatments of silica or silicon dioxide for
specific purposes to
somehow enhance image quality characteristics relating to electrophotography.
Examples of
the use of hydrophobic silica particles for toner includes JP 46-5782 A, JP 48-
47345A, and JP
48-47346A.
U.S. patents 5,464,722, 5,447,815, 4,868,084 5,702,858, 5,561,019, 4,902,570,
4,618,556,
5,695,902, and 6,004,711 all disclose the manufacture of toners using a form
of silicone oils,
varnishes, silicon dioxide particles or hydrophobic silica fine powder as some
with surface
treatments used as additives to enhance toner images.
The most common toner image fixing system for office and personal use printers
and copiers
uses a heat fixing method in which a heated device contacts a toner image on
the substrate
2
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
under an applied pressure. Offsetting in such a system often describes the
soiling or improper
marking on the imaged substrate by the toner. Cold offsetting is the term
usually used to
describe the soiling that occurs when the temperature of the fixing device is
lower than the
suitable toner fixing temperature range. In this case, insufficiently melted
toner adheres to the
surface of the fixing device and is subsequently deposited incorrectly onto
the substrate. In
contrast, when the fixing device temperature is higher than the suitable
fixing range, the
overly melted toner can adhere incompletely to the substrate due to a loss of
elasticity
resulting in adherence of toner to the fixing device. The subsequent soiling
of the substrate is
usually termed hot offsetting. Actual offsetting is a complex phenomenon and
frequently
related to many factors including surface properties of the substrate and the
fixing device
material, chemical and physical properties of the toner, and toner particle
size.
One method which has been found to reduce or eliminate offsetting includes the
use of wax
additives that have low softening temperatures so the resultant
electrostatically transferred
toner images are fixed without smearing, improper spacing between lines and/or
characters or
margin offset.
In a recent JP filing, JP 10-73952, a color toner formulation with a wax with
a number
average molecular weight (Mn) between 1500 and 7000 is claimed to provide
better results
regarding fusing characteristics. Normally, lower number average molecular
weight (Mn)
waxes have been associated with a phenomenon known as "bleeding". In addition,
this
I~okai patent claims that the ratio of branched carbons to the total carbons
in the wax is
between 0.5 and 20.
Sakashita, I1.S. Pat. No. 5051331, discloses a toner comprising a binding
resin and a low
molecular weight olefin copolymer. Sakashita teaches the low-molecular weight
olefin
copolymer has at least two olefin monomer repeating units and has two or more
peaks of
melting at temperatures between 90 C and 170 C.
Tanikawa et al. U.S. Pat. No. 5,364,722, disclose a toner comprising a binder
resin and a
hydrocarbon wax, and heat-fixing methods using the toner. Tanikawa et al.
teach that the
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
binder resin may be composed of homopolymers of styrene and derivatives
thereof., and
styrene copolymers, such as styrene-acrylate copolymer. Tanikawa et al.
further teach the
hydrocarbon wax provides a differential scanning calorimeter curve showing an
onset
temperature of heat of absorption in the range of 50 to 100 C, and at least
one heat absorption
peak in the range of 70-130 C.
Hagiwara et al., U.S. Pat. No. 5,389,484, disclose a toner having a binding
resin having an
acid component with an acid value of from 0.5 mg KOH/g to 100 mg KOH/g, a
colorant, and
defined aromatic amines. Hagiwara et al. teach that the acid component of the
resin interacts
with the amino group of the aromatic compounds to form an amide bond thereby
cross-
linking the polymer chains. Hagiwara et al. further teach that this can impart
a rubber
elasticity to the toner, so that its anti-offset properties can be improved.
Suzuki et al., U.S. Pat. No. 5,538,828, disclose a toner resin composition
comprising a binder
primarily composed of vinyl copolymer and an ethylene copolymer. Suzuki et al.
further
teach the ethylene copolymer is prepared by copolymerizing ethylene and at
least one alpha-
or beta- derivative of acrylic acid or an unsaturated dicarbonic acid
derivative. Suzuki et. al.
further teach toner-separating agents such as a low molecular weight polyester
or
polypropylene wax may be added.
Taguchi et al., U.S. Pat. No. 5,466,555, disclose a releasing composition for
a toner
comprising a low molecular weight polypropylene and at least one.modified
polyolefin.
Taguchi et al. teach that suitable polypropylenes include polypropylene
homopolymers, and
copolymers of polypropylene with one or more other monomers copolymerizable
therewith,
for example, ethylenes and olefins. The releasing composition may be used in
toner which
comprises the releasing agent, colorant, and binder resin. Taguchi et al.
teach suitable binder
resins include styrenic and/or acrylic resins.
Sawai et al., U.S. Pat. No. 5,565,294, disclose a toner containing a colorant,
a binding resin,
and a polyethylene having a melt viscosity of 22000 to 26800 mPa-s at 140 C.
Sawai et al.
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
teach that when the melt viscosity of the polyethylene is less than 2200 mPa-s
at 140 C, toner
components are not evenly dispersed in the kneading step in the production
process of toner.
moue et al., U.S. Pat. No. 5,658,999, disclose production of propylene waxes
by polymerizing
propylene with a solid catalyst formed of a transition metal compound or a
reaction product
between the transition metal compound and an organometallic compound, an
aluminoxane
and a fine particulate carrier. moue et al. further discloses a toner
composition composed
essentially of a binder resin, a colorant, and as a releasing agent, a
propylene wax.
Akimoto et al. U.S. Pat. No. 5,707,772, disclose a toner comprised of a resin,
a colorant, and
a releasing agent. Akimoto et. al. teach the releasing agent is a low
molecular weight
polyolefiri polymer synthesized using a metallocene catalyst. Akimoto et al.
further teach the
number average molecular weight of the polyolefin is from 2000 to 10000, and
the ratio of
weight average molecular weight to number average molecular weight (Mw/Mn) is
1.6 to 3.5.
Osterhoudt et al., U.S. Pat. No. 5,811,214, disclose a developer comprising
negatively
charged toner particles comprising a polymeric binder, magnetic material, and
a charge
control agent wherein he toner particle surface contains particles of cerium
dioxide,
dimethyldichlorosilane treated silica, and dimethylsiloxane treated silica.
Osterhoudt et al.
teach the polymeric binder may comprise styrene and an acrylate and/or
methacrylate.
Osterhoudt et al. further teach that useful additives include release agents
such as waxes,
including copolymers of ethylene and propylene having a molecular weight of
1000 to 5000
g/mole.
Eguchi et al. U.S. Pat. No. 5928825, disclose a toner comprising a binder
resin, a colorant,
and a lubricant. Eguchi et al. teach the lubricant comprises a modified
polyethylene wax
obtained by grafting a monomer selected from the group consisting of styrene
and unsaturated
carboxylic acid onto an ethylene homo- or copolymer.
Hashimoto et al., U.S. Pat. No. 5948584, disclose a toner comprising toner
particles
containing at least a binder resin, a colorant and a wax. Hashimoto et al.
teach that the binder
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
resin comprises a hybrid component comprising a vinyl polymer component and a
unit of
polyester component bonded to each other.
Kuwashima et al., U.S. Pat. No. 5952138, disclose a magnetic developer
comprising a
magnetic toner containing at least a binder resin, a magnetic material, and a
hydrocarbon wax
which is synthesized by reaction of carbon monoxide with hydrogen or by
polymerizing
ethylene and which has a number average molecular weight of from 600 to 1000.
Kuwashima et al., teach the wax should have an acid value of less than 2.0 rng
KOH/g, and
that if the acid value is higher than 2.0 mg KOH/g, the wax's interfacial
adhesion to the
binder resin may become so large that smearing of characters results.
Urashima, et al., U.S. Pat. No. 5955233, disclose a toner comprising a polymer
obtained by
suspension polymerization in an aqueous medium of a polymerizable monomer
composition,
a coloring agent, and optionally, a magnetic powder in the presence of an
epoxy resin and a
crystalline (meth) acrylic ester type polymer. Urashima et al. further teach
an offset-
preventing agent may be incorporated, and that suitable offset-preventing
agent may include
polyolefin wax which has a weight average molecular weight in the approximate
range of
1000 to 4500, preferably 2000 to 6000, such as homopolymers of polyethylene,
polypropylene and polybutylene, or olefin copolymers such as ethylene-
propylene copolymer.
Livengood, et. al. U.S. Pat. No. 6,331,372, disclose a toner particulates
including a wax
comprising an ethylene polypropylene copolymer with a non-crosslinked
copolymer other
than the wax also comprising an ethylene propylene copolymer. The
U.S. patent 5,707,771 and related U.S. patent 5,955,234 disclose a toner for
developing an
electrostatic image comprised of a binder resin, a colorant and a wax where
the toner has
specific rheological characteristics based on elastic storage modulus at
specific frequencies
where the toner shows a good fixability even at a high colorant content and
shows an
improved fixability during fusing that occurs immediately after power is
supplied to a fixing
device in a cold environment. The binder resin may preferably include a low-
modulus
component and a high-modulus component. The waxes may preferably include both
a high-
melting point wax component and a low melting point wax component.
6
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
U.S. patent 5,635,325 discloses.a toner for developing electrostatic images
that includes at
least a binder resin, a colorant and an ester wax. The ester wax is contained
in 3-40 wt. parts
per 1 OOwt. parts of the binder resin. The ester wax includes ester compounds
represented by
a formula of ;
RI - COO -R2
wherein Rland RZ independently denote a hydrocarbon group of 15-45 carbon
atoms. The
ester wax contains 50-95 wt. °1o thereof of ester compounds having an
identical number of
total carbon atoms. The toner is especially characterized by low-temperature
fixability, wide
non-offset temperature range, good color mixing characteristics and
transparency.
U.S. patent 5,741,617 describes a toner for developing electrostatic images,
which comprises
a binder resin, a colorant and a wax composition, characterized in that the
wax composition
has a molecular weight distribution as measured by GPC containing an ester wax
with a
weight average molecular weight (Mw) of from 350 to 4000 and a number average
molecular
weight of from 200 to .4000.
U.S, patent 5,747,213 details a method of forming a color toner image where
the color toner
contains at least a binder resin, a colorant and wax, the wax having a
molecular weight
distribution measured by GPC.
Finally, U.S. patent number 5,840,457 summarizes many of the solid toner wax
properties
found to be useful for toner resin with magnetic black toner particles that
are used to help
control the degree of gloss, leave little residual toner, provide a high
transfer efficiency, cause
little abrasion of the OPC and other cartridge components, and results in less
image defects
due to soiling of the members pressed against the bearing member. Several wax
characteristics are summarized in USP 5,840,457 and it is indicated in this
patent that low-
molecular weight hydrocarbon waxes, as well as polyethylene waxes and long-
chain alkyl
alcohol waxes are best suited to providing efficient transfer and good gloss
and with the
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
waxes that possess physical characteristics comprising a Mw/Mn = 1.0-2.0 and a
DSC heat
absorption main peak between 60 and 120 C.
Many references exist regarding toner compositions including wax combinations
to enhance
fusing performance. The use of the proper combination for each specific
composition is,
however, unique and complex and therefore the need for proper wax agents in
current specific
formulations still exists.
SUMMARY OF THE INVENTION
It is an object of the present invention to provide an electrostatic developer
composition and
method of using such a composition wherein toner performance is controlled
such that the
toner is suitable for the specific development system, and is capable of
stable high image
quality as determined by image density, background, resolution and other
general
requirements during long term use.
Another object of the present invention is to provide a toner that reduces or
eliminates the
phenomena known as offsetting, smearing, or other print imperfections. Use of
a specific
wax has been shown to reduce the build-up of toner onto the fixing devices.
There are
essentially three basic features of the present invention that are unique:
first, development of a
toner composition with better fusing properties as measured by tape peeling
and rubbing that
characterizes the prevention of smudging or fusing as a result of the fusing
roller and/or
fusing belt apparatus remaining free from contamination or toner buildup;
second, the
prevention of this toner buildup also helps reduce build-up around the picker-
finger; third, the
wax of the present invention also reduces friction between the paper and the
picker-finger
allowing for easier release of the picker-finger from the paper reducing the
propensity for
scratches. In all three cases the use of the wax in the toner composition of
the present
invention leads to better image quality including higher image density (ID),
lower background
(BG), etc.
In addition, the toner allows for a wider temperature operating range of the
fixing device
without offsetting.
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
Another object of the present invention is to provide a toner which is capable
of stable long-
term performance without any undesired toner contamination of the
electrophotographic
system including the photoconductor, the direct photoconductor charging
apparatus, the
fusing roller or fixing system.
These and other objects of the present invention have been satisfied by the
discovery of an
electrostatic developer comprising toner-containing image-forming particles
and an
uncrosslinked, linear hydrocarbon based homopolymer wax, wherein said wax has
a total
number of branches in each of one or more chains that is less than 0.5%,
relative to a total
number of carbons in said wax, wherein said wax is further characterized by
having a set of
endotherms as determined by differential scanning calorimetry (DSC) run at a
maximum rate
of 10°C per minute, said endotherms characterized by a primary
endotherm and at least a
secondary endotherm, said primary endotherm exhibiting a temperature range of
between
70°C and 90°C and said secondary endotherm exhibiting a.
temperature range of between
95°C and 110°C;
and
wherein said wax has a crystallinity of from 75% to 90% as determined by small
angle X-ray
diffraction analysis.
BRIEF DESCRIPTION OF THE FIGURE
Figure 1 provides a sample DSC (differential scanning calorimetry) scan of a
most
preferred wax used in the toner of the present invention.
Figure 2 provides a GPC (gel permeation chromatography) plot comparing a most
preferred wax used in the toner of the present invention with PE 130.
DETAILED DESCRIPTION OF THE INVENTION
The toner components of the present invention comprises a mixture of (1) toner
components
containing image-forming particles, (2) a low softening temperature, nearly
linear
hydrocarbon based homopolymer wax with a specific molecular weight and
crystallinity.
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
The above combination of components allows for high image density and clear
images
without offsetting formed during the electrophotography process. The
development of this
process is at high-resolution power and indicates improved electrostatic
recording is
obtainable.
In electrophotography, electrostatic recording, or the like, in which the
developing method
and the toner according to the present invention are used, the image-forming
particles do not
transfer to the non-image area and proper, non-offset images can be formed,
thereby
providing great industrial merit.
In the present invention, it is shown that a specific number average molecular
weight wax
with little or no branching, provides for better fusing. In addition, and in
contrast to
conventional systems, it is shown that image density (ID) can be maintained
using this
specific wax, whereas conventional waxes with specific molecular weights
sometimes result
in toner compositions that also result in lower ID's. Finally, it has also
been shown by further
analysis, that the wax of the present invention would include less than 0.5%
branching based
on the total number of carbon atoms in the wax molecule.
Here the branching or branch carbons preferably include those carbons
contained in the main
chain.
It is desirable that the toner not accumulate excessively on the fuser roller,
for excessive
accumulation can result in mechanical failure of the fuser. The inventors have
found that
toner particles comprising a low softening temperature non-crosslinked linear
hydrocarbon
based homopolymer wax with a specific molecular weight and crystallinity
provides very
good print quality without accumulating on the fuser roller to a degree which
is likely to
cause mechanical failure. Moreover, this special wax additive reduces or
eliminates the
phenomenon known as offsetting and provides for proper fusing of the toner
particles.
Toner particles in accordance with the present invention comprise a resin, a
wax comprising a
low softening temperature non-crosslinked linear hydrocarbon based homopolymer
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
with a specific molecular weight and crystallinity, and optionally ingredients
such as
magnetic components, colorants and charge control agent.
Another embodiment of the invention includes an electrostatic developer which
comprises a
toner containing image-forming particles and an uncrosslinked, linear
hydrocarbon based
homopolymer wax component; wherein said wax has the total number of branching
carbon
atoms present in each of one or more chain branches that is less than 0.5%,
relative to the
total number of carbons in said wax,
and;
said wax is further characterized by having a set of endotherms as determined
by
differential scanning calorimetry (DSC) run at a rate of 10°C per
minute, said endotherms
characterized by a primary endotherm and at least a secondary endotherm, said
primary
endotherm exhibiting a temperature range of between 70°C and
90°C, and said secondary
endotherm exhibiting a temperature range of between 95°C and
110°C,
and;
wherein said wax has a crystallinity of from 75% to 90% as determined by X-ray
diffraction analysis.
and;
wherein said wax is also characterized by having a molecular weight
polydispersity (Mw/Mn)
in the range of l .l-1.3, where the number average molecular weight is in the
range of 700-790
and the weight average molecular weight is in the range of 890-1000.
Toner components
The toner in the present invention can be prepared by any of the generally
known methods in
the art and various known toner constituent ingredients can be used.
Binder resins for the toner can be selected from a wide variety of materials
including known
thermoplastic resins. There can be mentioned, for example, styrene resin (homo-
or
copolymer containing styrene or substituted styrene) such as a polystyrene,
polychlorostyrene,
poly-methylstyrene, styrene-chlorostyrene polymer, styrene-propylene
copolymer, styrene-
11
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
butadiene copolymer, styrene-vinyl chloride copolymer, styrene-vinyl acetate
copolymer,
styrene-malefic acid copolymer, styrene-acrylate copolymer (for example,
styrene-methyl
acrylate copolymer, styrene-ethyl acrylate copolymer, styrene-butyl acrylate
copolymer,
styrene-octyl acrylate copolymer and styrene-phenyl acrylate copolymer),
styrene-
methacrylate copolymer (for example, styrene-methyl methacrylate copolymer,
styrene-ethyl
methacrylate copolymer, styrene-butyl methacrylate copolymer and stryene-
phenyl
methacrylate copolymer), styrene-methyl a-chloroacrylate copolymer and styrene-
acrylonitrile-acrylate copolymer, vinyl chloride resin, resin modified malefic
acid resin,
phenolic resin, epoxy resin, saturated or unsaturated polyester resin, low
molecular weight
polyethylene, low molecular weight polypropylene, ionomer resin, polyurethane
resin,
silicone resin, ketone resin, ethylene-ethyl acrylate copolymer, xylene resin
and polyvinyl
butyral resin. Preferred resins include styrene resins, and saturated or
unsaturated polyester
resins. Further, the above-mentioned resins may be used not only alone, but
also as a
combination of two or more of them.
Resins typically exhibit a softening temperature and a flow temperature. As
used herein
"softening temperature" is intended to refer to the temperature at which
particle collapse
begins, and "flow temperature" is intended to refer to the temperature at
which the resin
achieves sufficient liquidity to be extruded in a capillary rheometer. The
softening
temperature can be determined using rheometers such as the SHIMADZU~ capillary
rheometer.
The resins for use in toner particulate may include a crosslinking agent in an
amount of from
about 0.01 to about 5 parts by weight per 100 parts by weight of the monomers
employed
therein. Conventional crosslinking agents may be used. In one embodiment, the
toner
comprises a resin which is free of crosslinking agents. .
Toner particles may comprise more than one resin. Generally, the resins will
have a glass
transition temperature of no less than 50 °C. In one embodiment the
particulate comprises a
first resin and a second resin, each resin having a glass transition
temperature of no less than
12
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
about 50 °C, preferably no less than about 55 °C. Generally the
resins will have molecular
weight greater than about 2000:
In order to use the toner of the present invention in the form of a magnetic
toner, magnetic
powder generally known in the art may also be incorporated therein. The
magnetic powder
for the toner of the present invention is preferably chosen from the
ferromagnetic materials
exhibiting ferromagnetism including ferrimagnetism in a working circumstance
temperature
(around 0 to 60°C) for office business machines, plain paper copiers,
printers, etc. For
example, there can be mentioned magnetic powder showing ferromagnetism or
ferrimagnetism in a temperature range of about 0 to 60 °C, selected
from magnetite (Fe304),
maghemite ( - Fea03), a complex of magnetite and maghemite, spinal ferrite
such as ferrite
(MXFe3_X04 in which M represents Mn, Fe, Co, Ni, Cu, Mg, Zn, Cd or mixed
crystal materials
thereof), hexagonal ferrites such as Ba0.6Fe203, garnet-type oxide such as Y3
Fe5012, retile-
type oxide such as Cr02, metal such as Fe, Mn, Ni, Co, and Cr, as well as
other ferromagnetic
alloys. Among them, a powder of magnetite, maghemite or a complex product of
magnetite
and maghemite with an average particle size of not more than 3 Vim, about 0.01
to 1 ~,m are
preferred in view of the performance and the cost. The above-mentioned
magnetic powder
may be used not only alone but also as a combination of two or more of them.
As an example of manufacture of mono-component magnetic toner, the blending
weight ratio
of the binder resin to the magnetic powder can be selected within a range from
1:3 to 7:1,
while taking the fixing property to a transfer material into consideration.
As a colorant used for the toner, any of known dyes and pigments such as
carbon black, lamp
black, ultramarine, nigrosine dye, aniline blue, phthalocyanine.blue,
phthalocyanine green,
hanza yellow G, rhodamine type dye and pigment, chrome yellow, quinacridone,
benzidine
yellow, rose bengale, triallylmethane dyes, monoazo and disazo dyes and
pigments may be
used alone or in admixture. The addition amount of the colorant into the toner
is preferably
from 0.1 to 30 parts by weight, more preferably 0.5 to 10 parts by weight,
based on 100 parts
by weight of the binder resin. The fixing properties become poor if the amount
is excessive,
thus showing tendencies in property performance that is undesirable.
13
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
The charging property of the toner in the present invention may be controlled
by the binder
resin or the dye and pigment per se and, if required, a charge control agent
causing no
problem in view of color reproduction may also be used together. It is also
possible to
include charge control resins.
Examples of the charge controller are well known by way of reference for
example, U.S.
4,957,840, incorporated herein by reference. For positive charge control
agents, examples
may include: nigrosine and its modification products modified by a fatty acid
metal salt;
quaternary ammonium salts, such as tributylbenzyl-ammonium-1 hydroxy-4-
naphthosulfonic
acid salt, and tetrabutylammonium tetrafluoroborate; diorganotin oxides, such
as dibutyltin
oxide, dioctyltin oxide, and dicyclohexyltin oxide; and diorganotin borates,
such as dibutyltin
borate, dioctyltin borate, and dicyclo-hexyltin borate; and triphenylmethane
compound.
These positive charge controllers may be used singularly or as a mixture of
two or more
species. As another type of positive charge controller, there may be use of a
homopolymer of
a monomer having an amino group represented by the formula:
R,
CHz = C
Rz
C00CzH4N'
\ R3
wherein R1 represents H or CH3;
and R2 and R3 each represent a substituted or unsubstituted alkyl group
(preferably C1- C~); or
a copolymer of the monomer having an amine group with another polymerizable
monomer
such as styrene, acrylates, and methacrylates as described above. In this
case, the positive
charge controller may also function as a binder.
Examples of negative charge control agents include: metal complexes or salts
of monoazo
dyes, salicylic acid, alkylsalicylic acid, dialkylsalicylic acid, naphthonic
acid, or
14
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
acetylacetone. It is preferred that the above-mentioned charge controller is
used in the form
of fine powder: In such a case; the number-average particle size thereof may
preferably be 4
microns or smaller, more preferably 3 microns or smaller.
In the case of internal addition, such charge controller may preferably be
used in an amount of
0.1-20 wt. parts, more preferably 0.2-10 wt. parts, per 100 wt. parts of a
binder resin by
taking into consideration the conditions for the manufacturing method
including the
chargeability of the binder resin, the addition amount of the colorant and the
dispersion
method, as well as the chargeability of the other additives.
The toner in the present invention may preferably have a volume median
particle size from 4
to 20 ~,m, more preferably from 5 to 15 ~,m, and most preferably from 6 to 12
~,m, where the
volume median particle size is obtained by using a Coulter counter Model
Multisizer with a
100 micron aperture.
The toner of the present invention may contain one or more of the following
external
ingredients in small amounts, preferably 5% or less, more preferably 2% or
less, still more
preferably 1% or less,.based on total amount of toner: straight chain
saturated fatty acids such
as palmitic acid, stearic acid and montanic acid; unsaturated fatty acids such
as brassidic acid,
eleostearic acid and parinnaric acid; saturated alcohols such as stearyl
alcohol aralkyl alcohol,
behenyl alcohol, carnaubyl alcohol, ceryl alcohol and melissyl alcohol;
polyhydric alcohols
such as sorbitol; fatty acid amides such as linolic acid amide, oleic acid
amide, and lauric acid
amide; saturated fatty acid bisamides such as methylenebisstearic acid amide,
ethylenebiscapric acid amide, ethylenebislauric acid amide, and
hexamethylenebisstearic acid
amide; unsaturated fatty amides such as ethylenebisoleic acid amide,
hexamethylenebisoleic
acid amide, N,N'-bis-dioleyladipic acid amide and N,N'-bis-dioleylsebacic acid
amide;
aromatic bisamides such as xylene-bis-stearic acid amide and N,N'-
distearylisophthalic acid
amide; fatty acid metal: salts (what are commonly called metal soaps) such as
calcium
stearate, calcium laurate, zinc stearate, and magnesium stearate; waxes
obtained by grafting
vinyl monomers such as styrene and acrylic acid onto aliphatic hydrocarbon
waxes; partially
esterified products of fatty acids such as behenic acid monoglyceride with
polyhydric
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
alcohols; and methyl ester compounds having a hydroxyl group, obtained by
hydrogenation of
vegetable fats and oils.
Organic particles for charge control that are employed in the toner
compositions may be
chosen separately from resinous materials. Examples of such resinous materials
are
exemplified by, but not limited to, thermoplastic resins such as polystyrenes,
poly(meth)
acrylic resins, polyolefin resins, polyamide resins, polycarbonate resins,
polyether resins, poly
(sulfine acid) resins, polyester resins, epoxy resins, polybutyral resins,
urea resins,
urethane/urea resins, silicon resins, polyethytlene resins, Teflon resins and
the like
(fluoropolymer resins), thermosetting resins, a mixture thereof, block
copolymers thereof,
graft copolymers thereof, a blend thereof, and the like.
The inorganic oxide particles that are employed for toner compositions may
also be prepared
by any methods known in the art and are preferably selected from the group
consisting of
Si02, A1203, W2 03, Zr02, SeO, TiOz, ZnO and MgO. The particles preferably
have a BET
measurement value of not less than 1 ma/g, more preferably not less than 30
m~/g and even
more preferably not less than 100 ma/g.
The toner of the present invention can be used in conventional
electrophotography processes
using conventional toner cartridges. Such electrophotography processes and
toner cartridges
are well known in the art. Some have been described in various patents cited
herein and
incorporated by reference. Others are detailed in IJ.S. Patents 6,391,510 and
5,520,229, the
relevant portions of which are hereby incorporated by reference.
Toner wax
The toner used in the present invention contains the linear wax which is an
uncrosslinked,
linear hydrocarbon based homopolymer, wherein the wax has a total number of
branches
present in each of one or more chains that is less than 0.5% relative to the
total number of
carbons in said wax. The wax is further characterized by having a set of
endotherms as
determined by differential scanning calorimetry (DSC) It consists of the
primary endotherm
16
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
and at least a secondary endotherm: the primary endotherm exhibits a
temperature range of
between 70°C and 90°C and the secondary endotherm exhibits a
temperature range of
between 95°C and 110°C.
DSC test results were determined by running one heating followed by one
cooling followed
by one heating cycle each at 10°C per minute, with an initial heating
and cooling cycle prior
to the second heating during which test measurements were made.
The wax further has a crystallinity of from 75% to 90% as determined by X-ray
diffraction
analysis.
X-ray diffraction analysis was performed in a URTRAX 18 (manufactured by
Rigaku) X-ray
device utilizing a 40kV-200 mA (8kW) source. A sample wax powder was prepared
for a
cell 1 mm in thickness. The sample was heated to 130 C and held at that
temperature for 5
minutes. Next, the sample was cooled to 60 C at 0.91 C/min and then left to
continue cooling
at ambient temperature for 2 hours.
The detection comprised;
A Cu Ira line monochromated by a graphite monochromater
A collimator 1 mm in diameter
A Slit of O.Smm x O.Smm (height x width)
A detector scintillation counter and;
A step scan: 2 theta = 10-20 degrees, at 0.1 deg/step, 20 seconds/step.
Crystallinity was calculated utilizing Igor Pro Software from Wavemetrics
Corporation to
separate and identify the peaks to crystal faces.
The wax is also characterized by having a molecular weight polydispersity
(Mw/Mn) in the
range of 1.1-1.3, with a number average molecular weight in the range of 700-
790 and a
weight average molecular weight in the range of 890-1000.
17
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
Molecular Weight test results were determined by GPC analysis using PL-210
instrument
from Polymer Laboratories, using a 30 cm x 4 column of TSGgeI GMH-HT (Toso)
and
ODCB (o-dichlorobenzene) as eluent, and a refractive index (RI) detector. The
flow rate was
1.0 mL/min. The sample was injected as a 0.1 wt% solution in the eluent and in
an amount of
500 ~,L. The column temperature was maintained at 135°C. The results
were determined
against a polystyrene standard with a cubic fit calibration curve, along with
a universal
calibration curve with the viscosity equation of polystyrene standard and
polyethylene of
Kps = 1.3 ~E-4, a ps= 0.70, I~pe = 4.77E-4, a pe = 0.70.
The wax of the present invention has branching that has been further
characterized by NMR
as;
0 - .20 methyl branches per 100 carbon atoms,
0 - .10 ethyl branches per 100 carbon atoms and
0 - .10 butyl branches per 100 carbon atoms.
i3C NMR spectrum was quantitatively analyzed to determine the numbers of
branching
carbons in the molecule. The NMR instrumentation included a Varian UnityPlus
400 with
sample preparation utilizing the proton complete decoupling method as follows;
Resonance frequency: 100.56 MHz
Temperature: 130 C
Pulse Angle: 45 degrees
Pulse Interval: 20 seconds
Solvent: o-DCB/p-DCB d4
(DCB = dichlorobenzene, "d4" means that all of the four hydrogens were
substituted with
Deuterium)
The wax of the present invention preferably has a mean particle size of from 1
to 10 ~,m,
more preferably from 4-7 ~,m. The particle size for the wax has been
determined by use of a
Beckman Coulter Multisizer 3.33.
18
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
The wax of the present invention is also preferably prepared by a process
comprising;
gasifying and subsequent liquifying of coal resulting in residual wax residue
forming in a reactor vessel, the reactor vessel containing sides with interior
surfaces, wherein
wax residue forms specifically on the surfaces of the sides of the vessel, the
vessel used for
coal gasification and liquification followed by milling of the wax,
substantially by a jet mill to
accomplish micronizing and classifying of the linear hydrocarbon wax .
The wax is to provide an electrostatic developer comprising a toner of thermal
fixing type
which is capable of providing a reprographic image which exhibits good release
properties
upon thermal fixing, a reduced adhesion to the heated roller or the heated
film, no offset or
contamination, and good fixing properties of the fixed image, and is capable
of preventing the
heated roll or the heated film from undergoing contamination.
Two test methods have been used to determine fusing strength for fusing
properties where
fusing strength is descriptive of the bond strength between the paper and the
toner subsequent
to the electrophotographic process. Once toner has been deposited on the paper
and exposed
to the fusing conditions, the toner has a fusing strength.
One test method, known as the "tape peeling test" includes the use of an
adhesive tape,
specifically Scotch 3M Magic Tape (3/4 inch width). The image density (ID) is
measured
before and after the tape is applied and removed.
The second test method, known as the "Rub Test Reference" uses the back side
(non-adhesive
side) of a Self Stick Removable Notes Pad manufactured by either 3M or
Highland. Non-
adhesive paper side is placed over the fused toner image and is rubbed or
moved from the top
to the bottom of the paper (usually 2 7/8" X 2 7/8"). After the "rub" is
complete, the paper is
examined and a number is associated with the "smudge" if there is one. The
numbering
system is as follows;
1: No smudge - acceptable
2: Very slight and no smear - acceptable
19
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
3: Slight: some smudging - marginal
4: Smear after rubbing is very apparent - unacceptable
5: Bad and lots of smear after rubbing - very unacceptable
It is permissible that the specific wax of the invention is used with one or
more of those wax
components such as polypropylene (PP) or polyethylene (PE) as listed in USP
5,840,457
and/or with one or more auxiliary agents such as various kinds of plasticizers
and releasing
agents for adjusting thermal properties, physical properties, etc. The
addition amount thereof
is preferably from 0.1 to 20 parts by weight based on 100 parts by weight of
the binder resin,
and even more preferably from 1-6 parts, by weight based on 100 parts by
weight of the
binder resin.
Having generally described this invention, a further understanding can be
obtained by
reference to certain specific examples that axe provided herein for purposes
of illustration
only and are not intended to be limiting unless otherwise specified. These
examples describe,
but are not limited to, the preparation of toner by conventional process means
as below.
Examples
In the following Examples and Comparative Examples, "parts by weight" is
merely written as
"parts".
Example 1:
One hundred parts of a styrene-butyl acrylate copolymer (Mw = 15.5 x 104, 90
parts of
magnetite, 4 parts of Wax-A, which was nearly linear hydrocarbon based
homopolymer wax
with a specific molecular weight (Mn=750 and Mw = 966) and crystallinity
(80.4%), and 1
part of a chromium based organic metal complex were all well blended and
kneaded by
means of a twin screw extruder. The kneaded product was cooled, coarsely
crushed by
hammer mill, finally pulverized and classified to obtain black particulate
having a volume
median particle size of 9.0 microns.
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
Then 100 parts of the above black particulate were mixed with 1 part of TG308F
(Cabot)
negatively charged silica, which was fumed and post-treated with
polydimethylsiloxane
(PDMS). The resultant mixture was passed through a 100-mesh sieve.
Comparative example C1 was the same as Example 1 except for using PE130
(Clariant) in
place of Wax-A. Another comparative example C2 was the same as Example 1
except for
using NP505 (Mitsui Chemical) in place of Wax-A.
Those toner samples were subjected to print tests using a.50 (prints/minute)
speed laser
printer machine. The approximate fuser temperature was 180 C. The fusing
apparatus was
visually inspected for contamination.
Example 1 toner (Ex-1) showed good fusing properties as measured by tape
peeling and
rubbing. The prints with Ex-1 had no fusing picker finger scratches. The
fusing unit was free
from contamination after running with the Ex-1 toner.
Table 1
Working and Comparative Examples of Electrostatic Developer with Varying
Amounts of
Low Softening Temperature, Nearly Linear Hydrocarbon Based Homopolymer Wax
SampleWax ID Tape Rub TestPicker Contamination
(4 parts) Test ReferenceFinger
Ex-1 Wax- 1.43 O O O No contamination
A
C.1 PE130 1.33 X O D No contamination
C.2 NP505 1.32 X X X Contamination
* 1: Evaluation was carried out under normal temperature and humidity (N,N=20-
25 degree C,
40-70%RH) conditions.
*2: ID was measured by a Macbeth RD914 Colorimeter
*3: Tape test was conducted using Highland tape and observed resultant image
smudging
*4: Paper rubbing was conducted by using Post-ItR and observed resultant image
smudging
21
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
*5: Pickerfmger soil was observed on black page print.
*6: Contamination of fusing apparatus was observed after 30,000 pages.
In Table 1, the results follow the order of best to worst as shown:
Best: O > O > x : Worst
The toner samples were tested using several different fusing conditions,
changing fusing
temperature and fusing speed to confirm the non-offsetting range. Unfused
prints using the
toner samples were taken from a modified laser printer (HP LaserJet4Plus). The
fusing
apparatus was a modified fusing unit of HP LaserJetSSi for this fusing test.
This test indicated
Ex-1 toner had a wider non-offsetting fusing range than C1 or C2.
Table 2
Offsetting Performance for Example Toners Under Various Fusing Conditions
Fusing n~/S 78 70 62 50 42 42 42 42 42 42 42 42 34 25 17 8
Speed
Fuser Deg. 130130 130130 130140 150 160170 180190 200 200200 200200
Temp. C
Example E~,1 4 3 2 2 1 1 1 1 1 1 1 1 1 1 1 1
No.
ComparativeC-1 5 3 2 2 2 1 1 1 1 1 1 1 1 1 1 4
Example
No.
ComparativeC_2 g 5 3 3 2 3 3 2 2 2 2 1 1 1 1 1
Example
No.
1: No offsetting - acceptable
2: Very slight offsetting - acceptable
3: Slight: some offsetting - marginal
4: Offsetting is very apparent - unacceptable
5: Severe offsetting - very unacceptable
For characterization and evaluation of Wax-A;
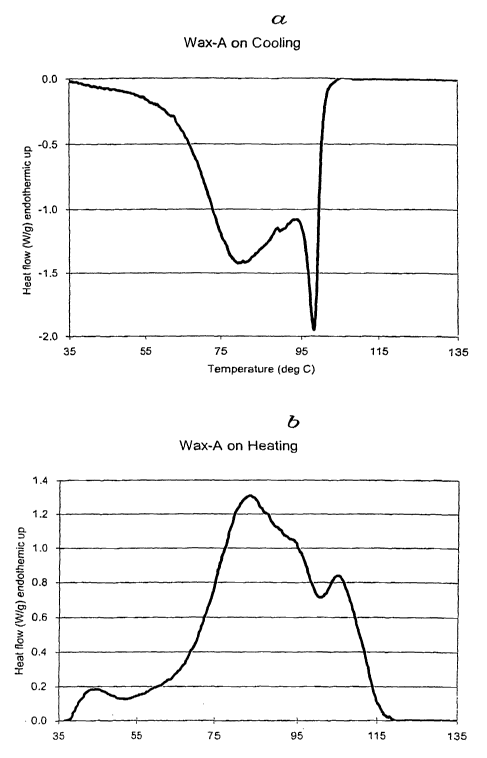
DSC indicated that Wax-A had the primary endotherm at 82 - 83 C and the
secondary
endotherm at 104 C.
DSC test results were determined by running one heating followed by one
cooling followed
by one heating cycle each at 10°C per minute, with an initial heating
and cooling cycle prior
22
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
to the second heating during which test measurements were made. Fig. 1
represents a sample
DSC measurement for Wax-A.'
Wax-A was further characterized by 13C-NMR as;
0.13 methyl branches per 100 carbon atoms,
0.05 ethyl branches per 100 carbon atoms and
0.05 butyl branches per 100 carbon atoms.
i3C NMR spectrum was quantitatively analyzed to determine the numbers of
branching
carbons in the molecule. The NMR instrumentation included a Varian UnityPlus
400 with
sample preparation utilizing the proton complete decoupling method as follows
having;
a resonance frequency: 100.56 MHz
a temperature: 130 C
a pulse angle: 45 degrees
a pulse interval: 20 seconds
and the solvent: o-DCB/p-DCB d4
(DCB = dichlorobenzene, "d4" means that all of the four hydrogens were
substituted with
Deuterium)
Wax-A had a crystallinity of 80.4 - 80.6 °1° as determined by X-
ray diffraction analysis.
Crystallinity test results were determined by X-ray diffraction analysis was
performed in a
URTRAX 18 (manufactured by Rigaku) X-ray device utilizing a 40kV-200 mA (8kW)
source. A sample wax powder was prepared for a cell 1 mm in thickness. The
sample was
heated to 130 C and held at that temperature for 5 minutes. Next, the sample
was cooled to
60 C at 0.91 C/min and then left to continue cooling at ambient temperature
for 2 hours.
The detection comprised;
a Cu Ka line monochromated by a graphite monochromater
a collimator 1 mm in diameter
a Slit of O.Smm x O.Smm (height x width)
a detector scintillation counter and;
a step scan: 2 theta = 10-20 degrees, at 0.1 deg/step, 20 seconds/step.
23
CA 02495898 2005-02-18
WO 2004/023215 PCT/US2003/025852
Crystallinity was calculated utilizing Igor Pro Software from Wavemetrics
Corporation to
separate and identify the peaks to crystal faces.
Wax-A was also characterized by having a molecular weight polydispersity
(Mw/Mn) of
1.3, with the number average molecular weight Mn of 750 and the weight average
molecular
weight Mw of 965.
Molecular Weight test results were determined by GPC analysis using PL-210
instrument
from Polymer Laboratories, using a 30 cm x 4 column of TSGgeI GMH-HT (Toso)
and
ODCB (o-dichlorobenzene) as eluent, and a refractive index (RI) detector. The
flow rate was
1.0 mL/min. The sample was injected as a 0.1 wt% solution in the eluent and in
an amount of
500 ~L. The column temperature was maintained at 135°C. The results
were determined
against a polystyrene standard with a cubic fit calibration curve, along with
a universal
calibration curve with the viscosity equation of polystyrene standard and
polyethylene of
I~ps = 1.38E-4, a ps= 0.70, I~pe = 4.77E-4, a pe = 0.70.
The sample was prepared by dissolving the wax in ODCB with heating at
135°C in an oil
bath, followed by filtering the hot solution with a 3 um PTFE filter. Figure 2
illustrates the
drastic differences in molecular weight distributions between PE 130 and Wax-
A.
The composition of the present invention (containing Wax-A) showed
significantly higher ID
than any of the other formulations tested, which only differed in the type of
wax that was
used.
This application is based on US provisional application serial no. 60/408,878,
filed
September 9, 2002, the entire contents of which are hereby incorporated by
reference.
Obviously, additional modifications and variations of the present invention
are possible in
light of the above teachings. It is therefore to be understood that within the
scope of the
appended claims, the invention may be practiced otherwise than as specifically
described
24
herein.