Note: Descriptions are shown in the official language in which they were submitted.
CA 02496096 2005-02-16
- 1 -
DESCRIPTION
MODIFIED SUBSTRATE AND METHOD FOR PRODUCING MODIFIED
SUBSTRATE
Technical Field
The present invention relates to a modified substrate
wherein the surface thereof is subjected to a
hydrophilization treatment. The modified substrate of the
present invention can be preferably used in medical devices.
Preferably, the modified substrate of the present invention
can also be used as, for example, separation membranes for
water treatment, separation membranes of biogenic substances,
instruments used for biological experiments, bioreactors,
molecular motors, drug delivery systems (DDS), protein chips,
DNA chips, biosensors, or components of analytical
instruments. In particular, the modified substrate of the
present invention is preferably used for applications in
which the substrate is brought into contact with a biogenic
substance, for example, a module for blood purification such
as an artificial kidney.
Background Art
In medical devices that are in contact with a body
fluid, for example, an artificial blood vessel, a catheter,
CA 02496096 2005-02-16
- 2 -
a blood bag, a contact lens, an intraocular lens, and an
artificial kidney, biocompatibility, in particular,
hematologic compatibility is an important problem. For
example, in separation membranes used for blood purification,
adhesion of proteins, or adhesion or activation of blood
platelets causes blood clotting. It is known that
performing a hydrophilization treatment on the surface of a
substrate is effective in remedying such a problem of
hematologic compatibility. For example, polysulfone
polymers are used as a material for the separation membranes
for blood purification. In order to provide a polysulfone
with hematologic compatibility, a hydrophilic polymer such
as polyvinylpyrrolidone is mixed in the stock solution for
preparation of the membrane. Although this method provides
hematologic compatibility to some degree, the hematologic
compatibility is not sufficient.
In a method disclosed in Japanese Unexamined Patent
Application Publication No. 10-118472, in order to improve
hematologic compatibility on the surface of a substrate, a
polysulfone separation membrane is brought into. contact with
a solution of a hydrophilic polymer such as
polyvinylpyrrolidone. Thus, the separation membrane
physically adsorbs the hydrophilic polymer. However, in
this method, the hydrophilic polymer is only adsorbed on the
surface. Therefore, when the separation membrane is in
CA 02496096 2005-02-16
- 3 -
contact with blood, the hydrophilic polymer may be dissolved
into the blood. In a method disclosed in Japanese
Unexamined Patent Application Publication No. 6-238139, a
polysulfone separation membrane is brought into contact with
a solution of a hydrophilic polymer such as
polyvinylpyrrolidone. In this method, an insolubilized
hydrophilic polymer layer is formed on the surface of the
membrane utilizing radiation crosslinking. This method
suppresses the dissolution of the hydrophilic polymer.
However, when the membrane is in contact with blood, the
insolubilized hydrophilic polymer activates the blood
platelets. As a result, hematologic compatibility is
deteriorated rather than improved.
Disclosure of Invention
It is an object of the present invention to provide a
modified substrate having high hematologic compatibility
wherein a hydrophilic polymer is immobilized on the surface
of the substrate, and a method for producing the same.
As a result of intensive study, the present inventors
have found a method for immobilizing a hydrophilic polymer
on a substrate without excessive crosslinking or degrading
the hydrophilic polymer, and have accomplished the present
invention.
The present invention provides a modified substrate
CA 02496096 2005-02-16
- 4 -
including a hydrophilic polymer, wherein the soluble
hydrophilic polymer ratio is 15 weight percent or less and
the number of adhered human blood platelets is 10/4.3x103 ~tm2
or less.
In addition, according to the modified substrate of the
present invention, the substrate is obtainable by
irradiating with radiation while the substrate is brought
into contact with an aqueous solution containing the
hydrophilic polymer and an antioxidant.
The present invention also includes a separation
membrane using the modified substrate.
The present invention also includes a system including
a plurality of the modified substrates.
The present invention also provides a method for
producing a modified substrate including a step of
irradiating the substrate with radiation while the substrate
is brought into contact with an aqueous solution containing
a hydrophilic polymer and an antioxidant.
The present invention also provides a method for
producing a system including a step of irradiating a
plurality of substrates with radiation at the same time
while the system including the plurality of substrates is
brought into contact with an aqueous solution containing a
hydrophilic polymer and an antioxidant.
CA 02496096 2005-02-16
- 5 -
Brief Description of the Drawing
Fig. 1 is a view showing an example of the basic
structure of an artificial kidney system.
Best Mode for Carrying Out the Invention
In the present invention, a substrate is irradiated
with radiation while the substrate is brought into contact
with an aqueous solution of a hydrophilic polymer, thus
producing a modified substrate wherein the hydrophilic
polymer is immobilized on the surface of the substrate.
Hematologic compatibility of a substrate depends on the
surface state of areas that are in contact with blood. In
general, the higher the hydrophilicity of the surface and
the higher the mobility of the hydrophilic polymer
immobilized on the surface, the higher the hematologic
compatibility of the substrate is. This is because a
hydrophilic polymer having high mobility eliminates proteins
or blood platelets due to its molecular motion.
Herein, the term immobilization refers to a state in
which a hydrophilic polymer is bonded with a substrate. In
the present invention, it is necessary for the soluble
hydrophilic polymer ratio to be 15 weight percent or less,
and preferably, 10 weight percent or less. Herein, the term
soluble hydrophilic polymer refers to a hydrophilic polymer
that is neither crosslinked nor insolubilized due to
CA 02496096 2005-02-16
- 6 -
immobilization on the substrate. The soluble hydrophilic
polymer ratio is defined as a ratio of the soluble
hydrophilic polymer to the total of the hydrophilic polymer
in the modified substrate. A detailed method for measuring
the soluble hydrophilic polymer ratio will be described
later. When the soluble hydrophilic polymer ratio exceeds
weight percent, bonding of the hydrophilic polymer with
the substrate is insufficient. Therefore, when the modified
substrate is brought into contact with blood, the
10 hydrophilic polymer may be dissolved into the blood.
The amount of dissolution of the hydrophilic polymer is
preferably 0.5 mg/m2 or less, more preferably, 0.3 mg/m2 or
less. Herein, the amount of dissolution of the hydrophilic
polymer is defined as follows: A substrate is brought into
15 contact with purified water at 37°C for 4 hours. The amount
of the hydrophilic polymer that is dissolved into the
purified water is converted to an amount per unit area of
the measured substrate. A detailed method for measuring the
amount of dissolution will be described later. When the
amount of dissolution of the hydrophilic polymer exceeds the
above range, there is a concern that, in medical devices
that are in contact with blood, the dissolved hydrophilic
polymer is accumulated in the body of patients. When the
molecular weight of the hydrophilic polymer exceeds 50,000,
the polymer is not filtered by the kidneys and is not
CA 02496096 2005-02-16
_ 7
excreted from the body. Therefore, such accumulation is a
particular concern. Furthermore, when the substrate is used
as an artificial kidney, the artificial kidney is used for
patients who have poor or no their renal function.
Therefore, even when the molecular weight of the hydrophilic
polymer is 50,000 or less, the accumulation in the body of
patients is a concern. In addition, when the substrate is
used as analytical instruments such as a protein chip or a
biosensor, there is a concern that the dissolved hydrophilic
polymer becomes an inhibiting factor in the analysis.
The condition for irradiating with radiation is
preferably controlled as follows. In an aqueous solution of
a hydrophilic polymer being in contact with a substrate, the
maximum increasing value of ultraviolet absorption value in
the wavelength range of 260 to 300 nm, the increase being
caused by irradiating with radiation, is preferably 1 or
less, more preferably 0.5 or less. Herein, the maximum
increasing value of ultraviolet absorption value is defined
as follows: Values are calculated by subtracting the
ultraviolet absorption values of the aqueous solution of the
hydrophilic polymer in the range of 260 to 300 nm before
irradiating with radiation from the ultraviolet absorption
values of the aqueous solution of the hydrophilic polymer in
the same wavelength range after irradiating with radiation.
Among the above values, the maximum value in the above
CA 02496096 2005-02-16
_ g _
wavelength range is defined as the maximum increasing value
of the ultraviolet absorption value. Under some conditions
for irradiating with radiation, the hydrophilic polymer is
degraded to generate a substance absorbing light in the
wavelength range of 260 to 300 nm and having a relatively
high reactivity. In particular, in medical devices, the
amount of such a substance is preferably small in terms of
safety.
In the modified substrate of the present invention, a
surface hydrophilic polymer ratio is preferably at least 20
weight percent. Herein, the surface hydrophilic polymer
ratio is defined as a ratio represented by A/(A+B), wherein
(A) is the weight of the monomer unit of the hydrophilic
polymer on the surface of the modified substrate (the number
of moles of the monomer unit x the molecular weight of the
monomer unit) and (B) is the weight of the monomer unit of
the polymer forming the substrate on the surface of the
modified substrate (the number of moles of the monomer unit
x the molecular weight of the monomer unit). This surface
hydrophilic polymer ratio is a parameter representing the
degree of hydrophilicity on the surface of the modified
substrate.
The surface hydrophilic polymer ratio is measured by
analyzing only the surface of the modified substrate, i.e.,
the depth profile of about 10 nm from the surface, by X-ray
CA 02496096 2005-02-16
- 9 -
photoelectron spectrometry (ESCA). The surface hydrophilic
polymer ratio is preferably at least 20 weight percent, more
..
preferably, at least 32 weight percent. When the surface
hydrophilic polymer ratio is less than 20 weight percent,
the effect at suppressing the adhesion of organic matter
such as proteins, or biogenic substances is decreased. This
is because the hydrophilic polymer cannot cover the surface
of the substrate, and therefore, the ratio of the substrate
exposed on the surface of the modified substrate is
increased.
In the modified substrate of the present invention, a
hydrophilic polymer is immobilized on the surface of the
substrate, and in addition, for example, excessive
crosslinking or degradation of the hydrophilic polymer is
prevented. As a result, the adhesion of organic matter such
as proteins, or biogenic substances can be suppressed. The
modified substrate of the present invention particularly has
high hematologic compatibility. Specifically, in the
modified substrate of the present invention, the number of
adhered human blood platelets is 10/4.3x103 ~,m2 or less. The
number of adhered blood platelets is defined as follows: A
modified substrate is brought into contact with blood for
one hour. The number of blood platelets adhered on the
surface of the modified substrate is represented as the
number per 4.3x103 ~m2 of the surface area of the modified
CA 02496096 2005-02-16
- 10 -
substrate. Detailed methods for measuring the number of
adhered blood platelets will be described later. When the
number of adhered human blood platelets exceeds 10/4.3x103
~.m2, hematologic compatibility is insufficient, and in
addition, the effect at suppressing the adhesion of organic
matter such as proteins, or biogenic substances is also
insufficient.
Because of its high hematologic compatibility, the
modified substrate of the present invention can be
preferably used as medical substrates. The medical
substrates used in the present invention include substrates
used in an artificial blood vessel, a catheter, a blood bag,
a contact lens, an intraocular lens, auxiliary instruments
for surgical operation, and a module for blood purification.
In particular, the modified substrate of the present
invention is suitable for applications in which the
substrate is brought into contact with a biogenic substance,
for example, a module for blood purification such as an
artificial kidney. Herein, the module for blood
purification refers to a module having a function of
circulating the blood in order to remove waste products or
harmful substances from the blood in order to excrete them
from the body. Examples of the module for blood
purification include an artificial kidney and an adsorption
column for exotoxins. The module for an artificial kidney
CA 02496096 2005-02-16
- 11 -
includes a coil type, a flat plate type, and a hollow fiber
membrane type. In terms of, for example, high processing
efficiency, the hollow fiber membrane type is preferable.
Furthermore, medical substrates used for adsorbing and
removing substances such as a cytokine, e.g., interleukin-6
(hereinafter abbreviated as IL-6), substances having an
adverse effect on the living body, are known. Preferably,
such medical substrates also have high hematologic
compatibility. As a result of hydrophilization treatment
performed on the surface of the substrate, the adhesion on
the substrate of blood platelets or proteins related to
clotting is suppressed. However, at the same time, the
adsorption on the substrate of target substances to be
removed such as IL-6 is also suppressed. The modified
substrate of the present invention can achieve high
hematologic compatibility while maintaining the adsorption
of a cytokine such as IL-6. Specifically, a modified
substrate having high hematologic compatibility can be
produced, while the adsorptivity to cytokine of the modified
substrate is maintained so as to be at least 90~ of the
adsorptivity to cytokine of the substrate before
modification. In the modified substrate of the present
invention, the adsorptivity to IL-6 is preferably at least
0.1 ng/cm2. When the adsorptivity to IL-6 is within this
range, the modified substrate can be preferably used as an
CA 02496096 2005-02-16
- 12 -
adsorption column for IL-6.
Preferably, the modified substrate of the present
invention can also be used as, for example, separation
membranes for water treatment, separation membranes of
biogenic substances, instruments used for biological
experiments, bioreactors, molecular motors, DDS, protein
chips, DNA chips, biosensors, or components of analytical
instruments, utilizing the feature in which the modified
substrate suppresses the adhesion of biogenic substances.
In addition, since the modified substrate of the present
invention includes a hydrophilic polymer having a low degree
of three-dimensional crosslinking thereon, the modified
substrate can be applied to a material that requires low
frictionality.
In the present invention, the substrate represents a
material to which hydrophilicity is provided. The substrate
is preferably composed of a polymeric material. Examples of
the polymeric material include polysulfones, polystyrene,
polyurethanes, polycarbonate, polymethylmethacrylate,
polyethylene, polypropylene, polyvinylidene fluoride,
polyacrylonitrile, polyesters, and polyamides. These
polymeric materials may be used as a copolymer. Furthermore,
carbon materials such as carbon fibers; carbon plates e.g.,
a glassy carbon plate and a carbon sheet; carbon nanotube;
and fullerene; and composite materials including these
CA 02496096 2005-02-16
- 13 -
carbon materials and a resin may also be used. Materials
prepared by substituting a part of these materials with a
functional group can also be applied as the substrate. The
reaction mechanism to provide hydrophilicity using the
carbon materials is not known exactly. It is not known
whether the carbon materials directly react or a trace of
impurities physically contained in the carbon materials
reacts. However, the carbon materials can also make the
substrate hydrophilic as in the polymeric materials.
Examples of the form of the substrate include a fiber, a
film, a resin, and a separation membrane. The form of the
substrate is not limited to the above.
When the substrate is used as a medical substrate, the
substrate is preferably composed of, for example, polyvinyl
chloride; cellulose polymers; polystyrene;
polymethylmethacrylate; polycarbonate; polysulfone polymers
such as polysulfones and polyethersulfones; polyurethanes;
polyacrylonitrile; and polyvinylidene fluoride. In
particular, among these polymers, polysulfone polymers are
preferably used because polysulfone polymers are readily
formed and separation membranes composed of polysulfone
polymers have an excellent performance in terms of the
permeation of a substance.
Polysulfone polymers include aromatic rings, a sulfonyl
group, and an ether group in the main chain. For example,
CA 02496096 2005-02-16
- 14 -
polysulfones represented by the following chemical formula
(1) and/or (2) are preferably used. Symbol n in the
formulae is preferably 50 to 80.
W
CH, o~ S ~- O-
~~ y-
n
CH3 Q n
(2)
I I ~
$ d-~
O n
Examples of the polysulfones include Udel (registered
trademark) polysulfone P-1700, P-3500 (from Teijin Amoco
Engineering Plastics Limited); Ultrason (registered
trademark) 53010 and S6010 (from BASF); Victrex (registered
trademark) (from 5umitomo Chemical Co., Ltd.); Radel
(registered trademark) A (from Teijin Amoco Engineering
Plastics Limited); and Ultrason (registered trademark) E
(from BASF). Although the polysulfones used in the present
invention preferably include only the repeating unit
represented by the above formula (1) and/or (2), the
polysulfones may be copolymerized with other monomers so
CA 02496096 2005-02-16
- 15 -
long as the advantage of the present invention is not
impaired. The amount of the other copolymerization monomers
is preferably 10 weight percent or less.
When the substrate is used as a medical substrate for
adsorbing and removing a cytokine such as IL-6, the
substrate is preferably composed of a hydrophobic polymer
because such a polymer has a high adsorbing performance.
Because of its high adsorbing performance,
polymethylmethacrylate is particularly preferable.
In the present invention, a hydrophilic polymer refers
to a polymer including a hydrophilic functional group in the
main chain or the side chain of the polymer. Hydrophilic
polymers having solubility in water at 25°C of, preferably,
at least 0.001 weight percent, more preferably, at least
0.01 weight percent, and most preferably, at least 0.1
weight percent, are readily applied to the present
technology. Examples of the hydrophilic polymer include
polyvinylpyrrolidone, polyethylene glycol, polypropylene
glycol, polyvinyl alcohol, polyethyleneimine,
polyallylamines, polyvinylamine, polyvinyl acetate,
polyacrylic acid, polyacrylamide, and copolymers and graft
polymers of these and other monomers. Nonionic hydrophilic
polymers such as polyalkylene glycols and
polyvinylpyrrolidone provide an inhibiting effect of
nonspecific adsorption. Cationic hydrophilic polymers such
CA 02496096 2005-02-16
- 16 -
as polyethyleneimine provide an excellent inhibiting effect
of adsorption of acidic substances such as an oxidized low-
density lipoprotein (LDL). Anionic polymers such as dextran
sulfate and polyvinyl sulfate provide an excellent
inhibiting effect of adsorption of basic substances such as
lysozyme. In terms of a high inhibiting effect of
adsorption, polyalkylene glycols such as polyethylene glycol
and polypropylene glycol or polyvinylpyrrolidone is
particularly preferable. In particular,
polyvinylpyrrolidone provides a high inhibiting effect of
adsorption. Polyalkylene glycols advantageously provide a
high inhibiting effect of adsorption without adding an
antioxidant, which will be described later.
When a polyalkylene glycol is used as the hydrophilic
polymer, the immobilization density of the polyalkylene
glycol is preferably at least 150 mg/m2, more preferably, at
least 200 mg/m2. In addition, the immobilization density of
the polyalkylene glycol is preferably 3,000 mg/m2 or less.
Herein, the immobilization density of polyalkylene glycol
represents the amount of polyalkylene glycol immobilized on
the surface of a substrate. An excessively low
immobilization density of polyalkylene glycol decreases the
antithrombogenicity of the substrate. On the other hand,
when the substrate is used for adsorbing and removing
cytokines, an excessively high immobilization density of
CA 02496096 2005-02-16
- 17 -
polyalkylene glycol decreases the adsorption capacity of
cytokines. The method for measuring the amount of
hydrophilic polymer immobilized on the surface of the
substrate is different depending on the kinds of substrate
and hydrophilic polymer and the method is appropriately
selected. Preferably, the amount of the hydrophilic polymer
bonded on the modified substrate is directly measured.
However, more simple methods may also be used. For example,
the concentration of the hydrophilic polymer in an aqueous
solution before irradiating with radiation may be compared
with that in the aqueous solution after irradiating with
radiation. Thus, the amount of decrease in the hydrophilic
polymer in the aqueous solution is calculated. This amount
may be defined as the amount of the immobilized hydrophilic
polymer. In another simple method, the contact angle of the
surface may be measured to estimate the amount of the
immobilized hydrophilic polymer.
Also, polymers derived from the living body, for
example, proteins are preferably used as the hydrophilic
polymer. Immobilization on the substrate of such a polymer
derived from the living body can provide the substrate with
a function of the polymer derived from the living body.
Examples of the polymer derived from the living body include
polymers having a sugar chain structure such as dextran and
dextran sulfate, peptides, proteins, lipids, and composites
CA 02496096 2005-02-16
- 18 -
such as polysaccharides.
The use of a plurality of hydrophilic polymers is also
preferable. For example, when a nonionic hydrophilic
polymer and a cationic hydrophilic polymer are used, the
nonionic hydrophilic polymer provides an inhibiting effect
of nonspecific adsorption, and in addition, the cationic
hydrophilic polymer provides an excellent inhibiting effect
of adsorption of acidic substances such as an oxidized low-
density lipoprotein (hereinafter referred to as oxidized
LDL). Thus, both advantages in the two hydrophilic polymers
can be provided. When a nonionic hydrophilic polymer and an
anionic polymer are used, the nonionic hydrophilic polymer
provides the inhibiting effect of nonspecific adsorption,
and in addition, the anionic polymer provides an efficient
inhibiting effect of adsorption of basic substances such as
lysozyme. When a synthetic hydrophilic polymer and a
hydrophilic polymer derived from the living body are used at
the same time, a modified substrate having high hematologic
compatibility and a function of the biopolymer can be
provided. In order to immobilize a plurality of hydrophilic
polymers, the hydrophilic polymers may be immobilized one
after another. Alternatively, a mixture of a plurality of
hydrophilic polymers may be immobilized at one time. This
method is simple and more preferable.
The molecular weight of the hydrophilic polymer is
CA 02496096 2005-02-16
- 19 -
preferably at least 100, more preferably, at least 500, and
most preferably at least 1,000. The molecular weight of the
hydrophilic polymer is preferably 50,000 or less.
Examples of the radiation used include a-ray, (i-ray, y-
ray, X-ray, ultraviolet rays, and electron beams. Medical
devices such as an artificial kidney require sterilization.
In terms of low residual toxicity and convenience, recently,
radiosterilization using y-ray or an electron beam is often
used. In other words, when the method of the present
invention is used in medical substrates, sterilization and
modification of a substrate can be preferably achieved at
the same time. In particular, the method of the present
invention is preferably applied to an artificial kidney. In
the artificial kidney, a wet type is mainly used in which
the separation membrane is in a state containing water.
Accordingly, the method of the present invention can be
conveniently used by only replacing the water with an
aqueous solution containing a hydrophilic polymer solution.
When sterilization and modification of a substrate are
performed at the same time, the substrate is preferably
irradiated with radiation with an absorbed dose of at least
20 kGy. This is because an absorbed dose of at least 20 kGy
is effective in order to sterilize, for example, a module
for blood purification with y-ray. However, when the
absorbed dose is 20 kGy or more, the hydrophilic polymer is
CA 02496096 2005-02-16
- 20 -
subjected to three-dimensional crosslinking or degraded,
thereby decreasing hematologic compatibility. Therefore, in
the present invention, an antioxidant is preferably added.
Specifically, the substrate is irradiated with radiation
while the substrate is brought into contact with an aqueous
solution containing a hydrophilic polymer and an antioxidant.
The addition of the antioxidant provides the following
features: Excessive crosslinking or degradation of the
hydrophilic polymer can be prevented, while the hydrophilic
polymer is immobilized, furthermore, sterilization can be
performed at the same time. However, when the substrate is
used in applications that do not require sterilization, the
absorbed dose need not be limited to the above. In such a
case, the substrate can be modified by irradiating with
radiation with an absorbed dose of 15 kGy or less, and
without adding the antioxidant.
The antioxidant according to the present invention
refers to molecules that readily provide other molecules
with electrons. When a hydrophilic polymer such as
polyvinylpyrrolidone is subjected to a radical reaction with
radiation, the antioxidant inhibits the reaction. Examples
of the antioxidant include water-soluble vitamins such as
vitamin C; polyphenols; alcohols such as methanol, ethanol,
propanol, ethylene glycol, and glycerin; saccharides such as
glucose, galactose, mannose, and trehalose; inorganic salts
CA 02496096 2005-02-16
- 21 -
such as sodium hydrosulfite, sodium pyrosulfite, and sodium
dithionate; uric acid; cysteine; glutathione; and oxygen.
These antioxidants may be used alone or in combination of
two or more. When the method of the present invention is
used in medical devices, the safety must be considered.
Therefore, antioxidants having low toxicity are preferably
used in such a case. In particular, alcohols, saccharides,
and inorganic salts are preferably used.
The concentration of antioxidant in an aqueous solution
is different depending on, for example, the kind of
antioxidant and the exposure dose of radiation. An
excessively low concentration of antioxidant causes three-
dimensional crosslinking or degradation of the hydrophilic
polymer to decrease hematologic compatibility. On the other
hand, the addition of an excessive amount of antioxidant
decreases the immobilization efficiency on the substrate.
Therefore, sufficient hematologic compatibility is not
achieved.
A method for producing a modified substrate of the
present invention will now be described in detail with
reference to an example using an antioxidant.
In a method for modifying the substrate, the substrate
is irradiated with radiation while the substrate is brought
into contact with an aqueous solution containing a
hydrophilic polymer and an antioxidant. For example, when
CA 02496096 2005-02-16
- 22 -
the substrate is a film, preferably, the substrate is
irradiated with radiation while the substrate is immersed in
an aqueous solution containing a hydrophilic polymer and an
antioxidant. When the substrate is a hollow substrate such
as a hollow fiber membrane and hydrophilicity should be
provided on the inner surface of the hollow part, the
aqueous solution is filled inside of the hollow part and
then the substrate is preferably irradiated with radiation.
Furthermore, when the substrate is disposed in a module, the
aqueous solution is filled in the module and then the whole
module is preferably irradiated with radiation. For example,
in an artificial kidney, separation membranes are disposed
in a module case. In such a case, an aqueous solution
containing a hydrophilic polymer and an antioxidant is
filled in the module and then the whole module may be
irradiated with radiation. Alternatively, only the
separation membranes may be irradiated with radiation while
the separation membranes are immersed in the aqueous
solution containing the hydrophilic polymer and the
antioxidant. Subsequently, the separation membranes may be
fitted in the module. Since modification and sterilization
can be performed at the same time, more preferably, the
aqueous solution containing the hydrophilic polymer and the
antioxidant is filled in the module and then the whole
module is irradiated with radiation.
CA 02496096 2005-02-16
- 23 -
Preferably, the substrate may be irradiated with
radiation while the substrate is in a wet state with an
aqueous solution containing a hydrophilic polymer and an
antioxidant. Herein, the wet state refers to a state in
which the aqueous solution used for immersing the substrate
is removed but the substrate is not dried. Although the
water content is not particularly limited, the substrate
preferably contains at least one weight percent of water
relative to the dry substrate. In other words, the
substrate is immersed in the aqueous solution and is then
removed from the aqueous solution. Subsequently, the
substrate may be irradiated with radiation. Alternatively,
the aqueous solution is filled in the module including the
substrate and most of the aqueous solution is then
discharged from the module with, for example, a nitrogen gas
jet. Subsequently, the module may be irradiated with
radiation.
In another method, the substrate is immersed in an
aqueous solution of a hydrophilic polymer in advance such
that the surface of the substrate is coated with the
hydrophilic polymer. Subsequently, the substrate may be
irradiated with y-ray while the substrate is immersed in a
solution containing an antioxidant. This method can also
make the surface of the substrate hydrophilic efficiently.
The area to which the hydrophilic polymer is provided
CA 02496096 2005-02-16
- 24 -
can be variously controlled according to the kind of
substrate and the method of modification. For example, in a
substrate used as a hollow fiber membrane, an aqueous
solution containing a hydrophilic polymer is introduced to
the inside of the hollow fiber membrane and the hollow fiber
membrane is then irradiated with radiation. In such a case,
the hydrophilic polymer can be immobilized on the inner
surface of the hollow fiber membrane. For example, this
method is preferably applied to an artificial kidney in
which the substrate is used such that blood flows only on
the inner surface thereof. In addition to the inner surface,
when hydrophilization needs to be performed on the outer
surface of the hollow fiber membrane, the aqueous solution
containing the hydrophilic polymer is brought into contact
with the outer surface of the hollow fiber membrane. For
example, when hollow fiber membranes are disposed in a
module case, the aqueous solution containing the hydrophilic
polymer is filled in the clearance formed between the hollow
fiber membranes and the module case.
In a substrate used as a separation membrane, an
aqueous solution containing a hydrophilic polymer is filled
while the solution is filtered through the membrane. Since
the hydrophilic polymer is concentrated on the surface of
the membrane, this method is effective at making the surface
more hydrophilic. In such a case, when a polymer that does
CA 02496096 2005-02-16
- 25 -
not readily permeate through the membrane, for example, a
high-molecular weight hydrophilic polymer, is used as the
hydrophilic polymer, the hydrophilic polymer is further
concentrated on the surface of the membrane to provide a
higher effect.
In contrast, when a low-molecular weight hydrophilic
polymer is used, hydrophilization treatment can be performed
on the inside of the membrane. For example, in a membrane
used for separating biogenic substances and recovering a
part of the substances by filtering or dialysis, i.e., a
separation membrane of biogenic substances, even when only
the surface of the membrane is subjected to hydrophilization,
the adsorption of the biogenic substances at the inside of
the membrane cannot be suppressed. Accordingly, in an
embodiment of the separation membrane of biogenic substances,
hydrophilization treatment is preferably performed on the
inside of the membrane.
In the present invention, a plurality of substrates are
irradiated with radiation at the same time, while a system
including the plurality of the substrates is brought into
contact with an aqueous solution containing a hydrophilic
polymer and an antioxidant. Thus, a plurality of substrates
can be modified at one time. In particular, when the
plurality of substrates are composed of different materials,
this method provides a significant effect. In a known
CA 02496096 2005-02-16
- 26 -
method for modification, it is difficult to modify a
plurality of substrates composed of different materials at
the same time because the conditions for modifying each
substrate significantly depend on the kinds of the
substrates.
Herein, the system including a plurality of substrates
refers to, for example, a separation membrane system
including port elements, separation membranes, and a circuit.
For example, modules for blood purification such as an
artificial kidney and an adsorption column for exotoxins
include a plurality of substrates such as a catheter, a
blood circuit, a chamber, an inlet port element and an
outlet port element of a module, and separation membranes,
the substrates being composed of different materials. In
the present invention, all or a part of the substrates can
be modified at the same time. Preferably, at least a part
of the port elements, the separation membranes, and the
circuit is modified. For example, in an artificial kidney
system, an inlet port element of a module, an outlet port
element of the module, and a blood circuit are connected to
a hollow fiber membrane module. An aqueous solution of a
hydrophilic polymer is then introduced from the blood
circuit to fill the entire system with the solution.
Subsequently, the entire system is irradiated with radiation
in this state.
CA 02496096 2005-02-16
- 27 -
Various methods for producing a module for blood
purification are known depending on the application. The
methods are broadly divided into the steps of producing
separation membranes for blood purification and the steps of
fitting the separation membranes in the module.
An example of a method for producing a hollow fiber
membrane module used in an artificial kidney will now be
described. A method for producing a hollow fiber membrane
fitted in the artificial kidney includes the following
method. A stock solution is prepared by dissolving a
polysulfone and polyvinylpyrrolidone in a good solvent or a
mixed solvent containing a good solvent. The concentration
of the polymer is preferably 10 to 30 weight percent, more
preferably, 15 to 25 weight percent. The ratio by weight of
the polysulfone to the polyvinylpyrrolidone is preferably
20:1 to 1:5, more preferably, 5:1 to 1:1. N,N-
dimethylacetamide, dimethylsulfoxide, dimethylformamide, and
N-methylpyrrolidone, and dioxane are preferably used as the
good solvent. The stock solution is discharged from an
outer tube of a double-annular spinneret to run through a
dry step. Subsequently, the stock solution is led to a
coagulation bath. An injection liquid or a gas to form a
hollow part is discharged from an inner tube of the double-
annular spinneret. In this process, the humidity in the dry
step affects the characteristics of the membrane. Therefore,
CA 02496096 2005-02-16
- 28 -
moisture may be supplied from the outer surface of the
membrane while the stock solution runs through the dry step
in order to accelerate a phase separation behavior in the
vicinity of the outer surface. As a result, the diameter of
the opening is increased. Thus, permeation resistance and
diffusion resistance when used for dialysis can be decreased.
However, when the relative humidity is excessively high,
coagulation of the stock solution at the outer surface
becomes dominant. As a result, the diameter of the opening
is decreased. Accordingly, permeation resistance and
diffusion resistance when used for dialysis are increased.
Therefore, the relative humidity is preferably 60~ to 900.
In terms of process suitability, the composition of the
injection liquid preferably includes the solvent used to
prepare the stock solution as a basic component. Regarding
the concentration of the injection liquid, for example, when
dimethylacetamide is used, an aqueous solution having a
concentration of preferably 45 to 80 weight percent, more
preferably, 60 to 75 weight percent is used.
Although a method for fitting hollow fiber membranes in
a module is not particularly limited, an example of the
method is as follows. Firstly, hollow fiber membranes are
cut so as to have a desired length. A required number of
the hollow fiber membranes are bundled to put in a
cylindrical case. Subsequently, both ends are closed with
CA 02496096 2005-02-16
- 29 -
temporal caps. A potting agent is added in both ends of the
hollow fiber membranes. Preferably, the potting agent is
added while the module is rotated with a centrifuge because
the potting agent can be filled uniformly. After the
potting agent is solidified, both ends are cut such that
both ends of the hollow fiber membranes are opened, thus
producing a hollow fiber membrane module.
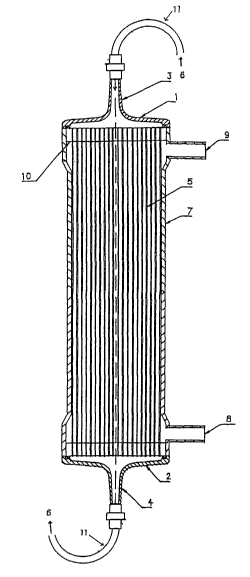
Fig. 1 shows an example of the basic structure of an
artificial kidney system using a hollow fiber membrane
module produced by the above method. A bundle of hollow
fiber membranes 5 is inserted in a cylindrical plastic case
7. A resin 10 seals both ends of the hollow fibers. The
case 7 includes an inlet 8 and an outlet 9 for dialysate.
For example, dialysate, physiological saline, or filtered
water flows in the outside of the hollow fiber membranes 5.
An inlet port element 1 and an outlet port element 2 are
disposed at the ends of the case 7. Blood 6 is introduced
from a blood inlet 3 disposed in the inlet port element 1,
and is introduced to the inside of the hollow fiber
membranes 5 by the port element 1 having a funnel shape.
The blood 6 filtered with the hollow fiber membranes 5 is
collected by the outlet port element 2 to discharge from a
blood outlet 4. The blood inlet 3 and the blood outlet 4
are connected to a blood circuit 11.
The present invention will now be described with
CA 02496096 2005-02-16
- 30 -
reference to Examples. The present invention is not limited
by the Examples.
1. Methods for preparing substrates
(Preparation of polysulfone film 1)
Polysulfone (Udel (registered trademark) P-3500 from
Teijin Amoco Engineering Plastics Limited) (10 parts by
weight) was added to N,N'-dimethylacetamide (80 parts by
weight) and allowed to dissolve at room temperature. Thus,
a membrane stock solution was prepared. A glass plate was
heated with a hot-plate such that the surface temperature of
the glass plate was 100°C. The membrane stock solution was
cast such that the thickness was 203 dun. The surface
temperature was measured with a contact type thermometer.
After the casting, the membrane was left to stand for 5
minutes on the hot-plate to evaporate the solvent.
Subsequently, the whole glass plate was immersed in a water
bath to prepare a polysulfone film 1. The purpose of the
immersion in the water bath is to allow the polysulfone film
to be peeled readily from the glass plate.
(Preparation of hollow fiber membrane module 1)
Polysulfone (Udel (registered trademark) P-3500 from
Teijin Amoco Engineering Plastics Limited) (18 parts by
weight) and polyvinylpyrrolidone (K30 from BASF) (9 parts by
weight) were added to a mixed solvent containing N,N'-
dimethylacetamide (72 parts by weight) and water (1 part by
CA 02496096 2005-02-16
- 31 -
weight). The mixture was heated at 90°C for 14 hours to
dissolve the polymers. Thus, a membrane stock solution was
prepared. The membrane stock solution was discharged from
an outer tube of an orifice type double-cylindrical
spinneret having an outer diameter of 0.3 mm and an inner
diameter of 0.2 mm. A core liquid containing N,N'-
dimethylacetamide (58 parts by weight) and water (42 parts
by weight) was discharged from an inner tube. The
discharged membrane stock solution was passed through a dry
step having a length of 350 mm and was then introduced in a
1000 water coagulation bath. Thus, a hollow fiber was
prepared.
The resultant 10,000 hollow fibers were inserted in a
cylindrical plastic case as shown in Fig. 1, which includes
an inlet and an outlet for dialysate. Both ends of the
membranes were sealed with a resin to prepare a hollow fiber
membrane module 1 for an artificial kidney having an
effective membrane area of 1.6 m2.
(Preparation of hollow fiber membrane module 2)
Isotactic-polymethylmethacrylate (5 parts by weight)
and syndiotactic-polymethylmethacrylate (20 parts by weight)
were added to dimethylsulfoxide (75 parts by weight). The
mixture was heated to dissolve the polymers. Thus, a
membrane stock solution was prepared. The membrane stock
solution was discharged from an outer tube of an orifice
CA 02496096 2005-02-16
- 32 -
type double-cylindrical spinneret. The discharged membrane
stock solution was passed through air for 200 mm and was
then introduced in a 1000 water coagulation bath. Thus, a
hollow fiber was prepared. In this process, dry nitrogen
was discharged from an inner tube as an inside injection gas.
The resultant hollow fiber had an inner diameter of 0.2 mm
and a thickness of 0.03 mm. A hollow fiber membrane module
2 having an effective membrane area of 1.6 m2 was prepared
using the resultant 10,000 hollow fibers, as in the hollow
fiber membrane module 1.
2. Measuring method
(1) Measurement of the soluble hydrophilic polymer ratio
A measurement sample was dried and the dry weight was
measured. Subsequently, the sample was dissolved in a
solvent that can dissolve both the substrate and the
hydrophilic polymer. A solvent that dissolves the
hydrophilic polymer but does not dissolve the substrate was
added to the resultant solution. As a result of this
operation, the substrate and the hydrophilic polymer
immobilized on the substrate-were precipitated, whereas a
soluble hydrophilic polymer remained dissolved. The amount
of hydrophilic polymer in the supernatant was quantitatively
determined by high performance liquid chromatography (HPLC).
Thus, the weight of soluble hydrophilic polymer per unit
weight of the measurement sample could be calculated. On
CA 02496096 2005-02-16
- 33 -
the other hand; the elemental analysis of the measurement
sample provided the weight of total hydrophilic polymer per
unit weight of the measurement sample. The soluble
hydrophilic polymer ratio was calculated by dividing the
weight of soluble hydrophilic polymer per unit weight of the
measurement sample by the weight of total hydrophilic
polymer per unit weight of the measurement sample.
When polyvinylpyrrolidone was used as the hydrophilic
polymer and Udel (registered trademark) P-3500 was used as
the substrate, the soluble hydrophilic polymer ratio was
measured as follows. A dry measurement sample was dissolved
in N-methyl-2-pyrrolidone such that the concentration of the
solution was 2.5 weight percent. Water (1.7 fold by volume)
was added dropwise to the solution while the solution was
stirred, thereby precipitating the substrate polymer. In
this process, the water should not be added at once because
the polysulfone is precipitated while the polysulfone
becomes entangled with soluble polyvinylpyrrolidone.
Attention should be paid because an accurate measurement may
be impossible in such a case. The soluble
polyvinylpyrrolidone was included in the solution with the
dispersed fine polysulfone particles. The solution was
filtered with a nonaqueous filter (from Tosoh Corporation,
diameter 2.5 dun) for HPLC to remove the fine polysulfone
particles in the solution. Subsequently,
CA 02496096 2005-02-16
- 34 -
polyvinylpyrrolidone in the filtrate was quantitatively
determined by HPLC under the following conditions.
Apparatus: Waters, GPC-244
Column: TSK-gel GMPWXL, 2 columns
Solvent : Water-based, 0 . 1 M ammonium chloride, 0 . 1 N ammonia,
pH 9.5
Flow rate: 1.0 mL/min.
Temperature: 23°C
The weight of soluble polyvinylpyrrolidone per unit
weight of the measurement sample was calculated from the
amount of polyvinylpyrrolidone in the filtrate. This weight
was divided by the weight of total polyvinylpyrrolidone per
unit weight of the measurement sample, which was determined
by elemental analysis. Thus, the soluble
polyvinylpyrrolidone ratio was determined.
(2) Dissolution test of hydrophilic polymer
An aqueous solution of a hydrophilic polymer in which a
measurement sample was immersed was removed. Subsequently,
the measurement sample was immersed in water at 37°C for 4
hours. The volume of water was 0.25 mL/cmz relative to the
area of the surface of the modified substrate. Thus, the
amount of dissolved hydrophilic polymer was quantitatively
determined.
When the hollow fiber membrane module 1 was used as the
measurement sample, the amount of dissolution was measured
CA 02496096 2005-02-16
- 35 -
as follows. The blood side of the hollow fiber membrane
module 1 was washed with 700 mL of ultrapure water at room
temperature, and the dialysate side thereof was washed with
2,500 mL of ultrapure water at room temperature. The blood
side was then washed again with 300 mL of ultrapure water at
room temperature to wash away hydrophilic polymers
originally included in the filling fluid. Subsequently, the
blood side was perfused with 4,000 mL of ultrapure water
heated at 37°C for 4 hours at a flow rate of 200 mL/min.
Subsequently, the perfusate was concentrated by 200 fold to
measure by gel permeation chromatography (GPC). The total
amount of hydrophilic polymer dissolved in the perfusate was
calculated from the analytical value. When the hydrophilic
polymer was polyvinylpyrrolidone, the measurement conditions
for GPC were as follows. A GMPWXL column was used, the flow
rate was 0.5 mL/min., a mixed solvent of methanol containing
0.1 N lithium nitrate . water = 1 . 1 (volume ratio) was
used as the solvent, and the column temperature was 40°C.
Polyvinylpyrrolidone K90 (from BASF) was used for a
calibration curve of the concentration of
polyvinylpyrrolidone.
(3) Measurement of maximum increasing value of ultraviolet
absorption value
An ultraviolet absorption value of an aqueous solution
of a hydrophilic polymer being in contact with a measurement
CA 02496096 2005-02-16
- 36 -
sample was measured before and after irradiating with
radiation. The ultraviolet absorption value was measured in
a wavelength range of 260 to 300 nm. An aqueous solution
(about 3 mL) for measurement was prepared in a quartz cell
having an optical path length of 1 cm. The ultraviolet
absorption value was measured with a spectrophotometer U-
2000 (from Hitachi, Ltd.) at room temperature. The
increasing value of ultraviolet absorption value was
calculated by subtracting the ultraviolet absorption value
measured before irradiating with radiation from the
ultraviolet absorption value measured after irradiating with
radiation. The maximum increasing value in the wavelength
range of 260 to 300 nm was defined as the maximum increasing
value of ultraviolet absorption value.
When a hollow fiber membrane module was used as the
measurement sample and an aqueous solution of a hydrophilic
polymer was filled in the blood side, after irradiating with
radiation, only the aqueous solution dripping by free fall
was sampled. However, when the aqueous solution of the
hydrophilic polymer was filled in the blood side, the
solution was then discharged by, for example, blowing, and
the substrate was irradiated with radiation in a wet state,
the aqueous solution might not drip by free fall. In such a
case, water is filled in the module again, and the module is
left to stand at room temperature for at least one hour.
CA 02496096 2005-02-16
- 37 -
Subsequently, water at the blood side dripping by free fall
may be sampled.
When a substrate other than a hollow fiber membrane
module is irradiated with radiation in a wet state, the
substrate is immersed in water of 0.1 mL/cm2 at room
temperature for one hour. Subsequently, the measurement is
performed using the water, and the measured value is
multiplied by 20. The resultant value is used. In the
above hollow fiber membrane module, the volume of filling
fluid at the blood side relative to the inner surface area,
that is, the bath ratio, is 0.005 mL/cm2. The above
calculation indicates that the bath ratio is converted so as
to correspond with the above value. If the substrate cannot
be immersed in the water volume of 0.1 mL/cm2, water may be
appropriately added to perform the measurement.
Subsequently, the bath ratio is converted so as to
correspond with 0.005 mL/cm2.
(4) Measurement of surface hydrophilic polymer ratio
The hydrophilic polymer ratio on the surface was
measured by X-ray photoelectron spectrometry (ESCA). A
measurement apparatus ESCALAB220iXL was used and a sample
was prepared in the apparatus. In the measurement, the
angle of a detector to the angle of incidence of X-ray was
90 degrees. In a film sample, the surface of the film on
the glass used for casting was measured. In a hollow fiber
CA 02496096 2005-02-16
- 38 -
membrane sample, the hollow fiber membrane was cut with a
single edged knife to form a semicylindrical shape and the
inner surface of the hollow fiber membrane was measured.
The measurement sample was rinsed with ultrapure water and
was then dried at room temperature and at 0.5 Torr for 10
hours. Subsequently, the sample was used for the
measurement.
When polyvinylpyrrolidone was used as the hydrophilic
polymer and Udel (registered trademark) P-3500 was used as
the substrate, the surface polyvinylpyrrolidone ratio was
calculated as follows. The amount of nitrogen (a) on the
surface and the amount of sulfur (b) on the surface were
calculated from the integrated intensity of Cls, Nls, and
S2p spectra, which were obtained by ESCA, using a relative
sensitivity coefficient provided from the apparatus. The
surface polyvinylpyrrolidone ratio was calculated by the
following formula:
Surface polyvinylpyrrolidone ratio (weight percent) -
ax100/(axlll+bx442)
(5) Measurement of immobilization density of polyethylene
glycol
A hollow fiber after irradiating with radiation was
immersed in distilled water at 37°C for one hour. The
volume of the distilled water was 1 L per 1 m2 of the
surface area of the substrate. The hollow fiber was washed
CA 02496096 2005-02-16
- 39 -
while distilled water was changed until the amount of
polyethylene glycol dissolved into the distilled water was 1
mg or less. Thus, polyethylene glycol that is not
immobilized on the substrate was removed. The washed
substrate was dried at 50°C and at 0.5 Torr for 10 hours.
In a test tube, 10 to 100 mg of the dry substrate was
prepared. A mixed solution (2 mL) containing acetic
anhydride and para-toluenesulfonic acid was added to the
substrate to acetylate the mixture at 120°C for about one
hour. After cooling, the wall was washed with 2 mL of
purified water. Subsequently, 20o sodium hydrogencarbonate
was added to the mixture to neutralize. The neutralized
solution was extracted with trichloromethane (5 mL). The
extract was analyzed by gas chromatography (hereinafter
abbreviated as GC). The analytical conditions for GC were
as follows. The amount of polyethylene glycol immobilized
on the substrate was determined using a calibration curve
prepared in advance.
(Analytical conditions for GC)
Apparatus: Shimadzu GC-9A
Column: Supelcowax-10, 60 m x 0.75 mm I.D.
Carrier gas: Helium
Detector: Flame-ionization detector (FID) (H2 inlet: 0.7
kg/cm2, Air inlet: 0.6 kg/cm2, Temperature: 200°C)
Column temperature: 80°C, holding for 5 min.-(20 min.)-
CA 02496096 2005-02-16
- 40 -
200°C, holding for 5 min.
Injector temperature: 200°C
(6) Measurement of contact angle
The contact angle was measured with a contact angle
meter CA-D from Kyowa Interface Science Co., Ltd. The
measurement was performed in a room where the room
temperature was controlled at 25°C.
(7) Method of adhering test of rabbit blood platelets on
film
A film for measurement was disposed on the bottom of a
cylindrical polystyrene tube having a diameter of 18 mm.
The cylindrical tube was filled with physiological saline.
If contaminations, flaws, fold lines, or the like are
disposed on the surface of the film, blood platelets are
adhered on such areas. Attention should be paid because an
accurate evaluation may be impossible in such a case. A
blood sample containing an aqueous solution of 3.20
trisodium citrate dehydrate and fresh rabbit blood at a
volume ratio of 1:9 was subjected to centrifugal separation
at 1,000 rpm for 10 minutes to recover the supernatant
(referred to as blood plasma 1). After the supernatant was
recovered, the resultant blood was subjected to centrifugal
separation again at 3,000 rpm for 10 minutes to recover the
supernatant (referred to as blood plasma 2). The blood
plasma 1 was diluted by adding the blood plasma 2 (the
CA 02496096 2005-02-16
- 41 -
concentration of blood platelets in the blood plasma 2 was
lower than that in the blood plasma 1) to prepare a
platelet-rich plasma (hereinafter referred to as PRP) having
20x106 /mL of blood platelets. The physiological saline
prepared in the cylindrical tube was removed and 1.0 mL of
the PRP was then added in the cylindrical tube. The
cylindrical tube was shaken at 37°C for one hour.
Subsequently, the measurement film was washed three times
with physiological saline. The blood component was fixed
with an aqueous solution of 3~ glutaraldehyde. The film was
washed with distilled water and was then dried under a
reduced pressure for at least 5 hours.
The film was adhered on a specimen support for a
scanning electron microscope with a double-sided adhesive
tape. A thin film composed of Pt-Pd was deposited on the
surface of the film by sputtering to prepare a sample. The
surface of the sample was observed with a scanning electron
microscope (5800 from Hitachi, Ltd.). Since the blood
readily retained in the portions of the film being in
contact with the cylindrical tube, the central part of the
film was mainly observed at a magnification ratio of 3,000
to count the number of adhered blood platelets found per one
field of view (1.12x103 ~.un2) . The average number of adhered
blood platelets in 10 different fields of view in the
vicinity of the center of the film was calculated. The
CA 02496096 2005-02-16
- 42 -
number of adhered blood platelets (number/l.Ox103 ~m2) was
calculated by dividing the above average number of adhered
blood platelets by 1.12.
(8) Method of adhering test of human blood platelets on film
A film for measurement was fixed on a polystyrene
circular plate having a diameter of 18 mm with a double-
sided adhesive tape. If contaminations, flaws, fold lines,
or the like are disposed on the surface of the film, blood
platelets are adhered on such areas. Attention should be
paid because an accurate evaluation may be impossible in
such a case. The circular plate was fitted in a Falcon
(registered trademark) tube (18 mm in diameter, No. 2051),
which was cut in a tubular shape, such that the surface
having the film thereon was disposed at the inside of the
cylinder. The clearance was filled with Parafilm. The
inside of this cylindrical tube was washed with
physiological saline and was then filled with physiological
saline. Human venous blood was collected and heparin was
then added to the blood immediately so as to have a
concentration of 50 U/mL. The physiological saline in the
cylindrical tube was removed. Subsequently, 1.0 mL of the
blood was filled in the cylindrical tube within 10 minutes
from the collection. The cylindrical tube was shaken at
37°C for one hour. Subsequently, the measurement film was
washed with 10 mL of physiological saline. The blood
CA 02496096 2005-02-16
- 43 -
component was fixed with physiological saline containing
2.5o glutaraldehyde. The film was washed with 20 mL of
distilled water. The washed film was then dried at room
temperature under a reduced pressure of 0.5 Torr for 10
hours. A thin film composed of Pt-Pd was then deposited on
the surface of the film by sputtering to prepare a sample.
The surface of the sample was observed with a field emission
scanning electron microscope (5800 from Hitachi, Ltd.) at a
magnification ratio of 1,500 to count the number of adhered
blood platelets found per one field of view (4.3x103 ~.m2) .
The average number of adhered blood platelets in 10
different fields of view in the vicinity of the center of
the film was calculated. The average number of adhered
blood platelets was defined as the number of adhered blood
platelets (number/4.3x103 ~.m2) .
(9) Method of adhering test of rabbit blood platelets on
hollow fiber membrane
Thirty hollow fiber separation membranes were bundled.
Both ends of the membranes were fixed in a glass tube module
case with an epoxy-based potting agent such that the hollow
parts of the hollow fibers were not clogged. Thus, a mini
module having a diameter of about 7 mm and a length of about
10 cm was prepared. A blood inlet of the mini module was
connected to a dialysate outlet thereof with a silicone tube.
In order to wash the hollow fibers and the inside of the
CA 02496096 2005-02-16
- 44 -
module, 100 mL of distilled water was allowed to flow from a
blood outlet at a flow rate of 10 mL/min. Physiological
saline was then filled, and a dialysate inlet and the outlet
were closed with caps. Subsequently, physiological saline
was supplied from the blood inlet at a flow rate of 0.59
mL/min. for two hours to perform priming. A blood sample
containing an aqueous solution of 3.2o trisodium citrate
dehydrate and fresh rabbit blood at a volume ratio of 1:9
was prepared. Seven milliliters of the blood sample was
perfused at a flow rate of 0.59 mL/min. for one hour.
Subsequently, the membranes were washed with physiological
saline using a 10-mL syringe. An aqueous solution of 30
glutaraldehyde was filled in the inside of the hollow fibers
and the dialysate side. The module was left to stand at
least one night to perform glutaraldehyde fixation.
Subsequently, the glutaraldehyde was washed with distilled
water. A hollow fiber membrane was cut out from the mini
module and was dried under a reduced pressure for at least 5
hours. The hollow fiber membrane was adhered on a specimen
support for a scanning electron microscope with a double-
sided adhesive tape. The membrane was then sliced in the
longitudinal direction so as to expose the inner surface. A
thin film composed of Pt-Pd was deposited on the sample by
sputtering. The inner surface of the sample was observed
with a scanning electron microscope (5800 from Hitachi,
CA 02496096 2005-02-16
- 45 -
Ltd.) at a magnification ratio of 3,000 to count the number
of adhered blood platelets found per one field of view
(1.12x103 ~m2). The average number of adhered blood
platelets in 10 different fields of view was calculated.
The number of adhered blood platelets (number/l.Ox103 ~.un2)
was calculated by dividing the above average number of
adhered blood platelets by 1.12.
(10) Method of adhering test of human blood platelets on
hollow fiber membrane
A hollow fiber membrane was fixed on a polystyrene
circular plate having a diameter of 18 mm with a double-
sided adhesive tape. The adhered hollow fiber membrane was
cut with a single edged knife to form a semicylindrical
shape, thereby exposing the inner surface of the hollow
fiber membrane. If contaminations, flaws, fold lines, or
the like are disposed on the inner surface of the hollow
fiber, blood platelets are adhered on such areas. Attention
should be paid because an accurate evaluation may be
impossible in such a case. The circular plate was fitted in
a Falcon (registered trademark) tube (18 mm in diameter, No.
2051), which was cut in a tubular shape, such that the
surface having the hollow fiber membrane thereon was
disposed at the inside of the cylinder. The clearance was
filled with Parafilm. The inside of this cylindrical tube
was washed with physiological saline and was then filled
CA 02496096 2005-02-16
- 46 -
with physiological saline. Human venous blood was collected
and heparin was then added to the blood immediately so as to
have a concentration of 50 U/mL. The physiological saline
in the cylindrical tube was removed. Subsequently, 1.0 mL
of the blood was filled in the cylindrical tube within 10
minutes from the collection. The cylindrical tube was
shaken at 37°C for one hour. Subsequently, the hollow fiber
membrane was washed with 10 mL of physiological saline. The
blood component was fixed with physiological saline
containing 2.5o glutaraldehyde. The hollow fiber membrane
was washed with 20 mL of distilled water. The washed hollow
fiber membrane was then dried at room temperature under a
reduced pressure of 0.5 Torr for 10 hours. The film was
adhered on a specimen support for a scanning electron
microscope with a double-sided adhesive tape. A thin film
composed of Pt-Pd was then deposited on the surface of the
hollow fiber membrane by sputtering to prepare a sample.
The inner surface of the hollow fiber membrane was observed
with a field emission scanning electron microscope (5800
from Hitachi, Ltd.) at a magnification ratio of 1,500 to
count the number of adhered blood platelets found per one
field of view (4.3x103 ~m2). The average number of adhered
blood platelets in 10 different fields of view in the
vicinity of the center of the hollow fiber in the
longitudinal direction was calculated. The average number
CA 02496096 2005-02-16
- 47 -
of adhered blood platelets was defined as the number of
adhered blood platelets (number/4.3x103 ~.m2) . This was
because the blood readily retained at the end portions of
the hollow fiber in the longitudinal direction.
(11) Method of adhering test of human blood platelets in
blood circuit for artificial kidney
A blood circuit for an artificial kidney was finely cut
into small pieces of about 0.1 g. (If a mesh part was used,
the weight was about 0.01 g.) An adhering test of human
blood platelets was performed using the small pieces as in
the above item (9).
In the adhering tests of blood platelets described in
the above items (7) to (11), in order to confirm whether the
tests are adequately performed or not, a positive control
and a negative control were added in each test as a
benchmark. The positive control was a known sample in which
a large amount of blood platelets can be adhered. In
contrast, the negative control was a known sample in which a
small amount of blood platelets is adhered. In the adhering
tests of human blood platelets, a sample having a number of
adhered blood platelets of at least 40 (/4.3x103 ~unz) under
the above experimental conditions was used as the positive
control. In addition, a sample having a number of adhered
blood platelets of up to 5 (/4.3x103 ~,m2) was used as the
negative control. In the adhering tests of rabbit blood
CA 02496096 2005-02-16
- 48 -
platelets, a sample having a number of adhered blood
platelets of at least 30 (/1.0x103 ~m2) was used as the
positive control. In addition, a sample having a number of
adhered blood platelets of up to 5 (/1.0x103 ~.m2) was used as
the negative control. In the following Examples, a hollow
fiber membrane used in an artificial kidney Filtryzer BG-
1.6U from Toray Industries, Inc, was used as the positive
control. A hollow fiber membrane used in an artificial
kidney PS-1.6UW from Kawasumi Laboratories, Inc. was used as
the negative control. After a test, when the number of
blood platelets adhered on the positive control was the
above value or more, and in addition, the number of blood
platelets adhered on the negative control was the above
value or less, the measurement values could be used. When
the number of blood platelets adhered on the controls was
not within the above ranges, the test was performed again.
In such a case, the freshness of the blood might be
insufficient or the blood might be excessively activated.
(12) Adsorption test of IL-6
The same thirty hollow fiber separation membranes as
used in the above hollow fiber membrane module 2 were
bundled. Both ends of the membranes were fixed in a glass
tube module case with an epoxy-based potting agent such that
the hollow parts of the hollow fibers were not clogged.
Thus, a mini module having a diameter of about 7 mm and a
CA 02496096 2005-02-16
- 49 -
length of about 10 cm was prepared. A blood inlet of the
mini module was connected to a dialysate outlet thereof with
a silicone tube. In order to wash the hollow fibers and the
inside of the module, 100 mL of distilled water was allowed
to flow from a blood outlet at a flow rate of 10 mL/min.
Subsequently, an aqueous solution of PBS (Dulbecco PBS (-)
from Nissui Pharmaceutical Co., Ltd.) was filled, and a
dialysate inlet and the outlet were closed with caps.
IL-6 was added to 10 mL of human plasma so as to have a
concentration of 1 ng/mL (referred to as liquid 1). The
dialysate inlet and the dialysate outlet were closed with
the caps, and the inlet of the blood side was connected to
the outlet of the blood side with a silicone tube.
Perfusion was performed at 37°C for 4 hours with the liquid
1 at a flow rate of 1 mL/min. The IL-6 was quantitatively
determined before and after the perfusion. The adsorptivity
on the substrate was calculated from the decrease in the IL-
6.
(13) Method of adsorptive removal test of oxidized LDL
(a) Preparation of antioxidized LDL antibody
Antioxidized LDL antibody specimens prepared by Itabe
et al. (H. Itabe et al., J. Biol. Chem. Vol. 269: p. 15274,
1994) were used. Specifically, mice were immunized by
injecting a human atherosclerotic lesion homogenate. The
hybridomas were prepared from the spleen cells of the mice,
CA 02496096 2005-02-16
- 50 -
followed by screening those that were allowed to react with
LDL that had been treated with copper sulfate. Thus, the
antioxidized LDL antibody was prepared. The resultant
antibody was classified as mouse IgM, and was not allowed to
react with native LDL, acetylated LDL, or malondialdehyde-
treated LDL. On the other hand, the antioxidized LDL
antibody was allowed to react with some peroxidation
products of phosphatidylcholine, including aldehyde
derivatives and hydroperoxides of phosphatidylcholine. The
antioxidized LDL antibody was dissolved in a 10 mM borate
buffer solution (pH 8.5) containing 150 mM NaCl. The
solution (protein concentration 0.60 mg/mL) was used as
specimens.
(b) Preparation of oxidized LDL
A commercial LDL (from Funakoshi Co., Ltd.) was
desalinated and was then diluted with a phosphate buffer
solution (hereinafter abbreviated as PBS) so as to have a
concentration of 0.2 mg/mL. Subsequently, 2 weight percent
of a 0.5 mM aqueous solution of copper sulfate was added to
the solution. The solution was allowed to react at 37°C for
5 hours. A 25 mM ethylenediaminetetraacetic acid (EDTA)
solution and 10 weight percent sodium azide were added to
the resultant solution such that the concentration of the
EDTA was 1 weight percent and the concentration of the
sodium azide was 0.02 weight percent. This solution was
CA 02496096 2005-02-16
- 51 -
used as an oxidized LDL specimen.
(c) Determination of the concentration of oxidized LDL
The above antioxidized LDL antibody was diluted with
PB5 so as to have a concentration of 5 ~,g/mL. The solution
was dispensed to a 96-well plate at a rate of 100 ~,L/well.
The plate was shaken at room temperature for two hours.
Subsequently the plate was left to stand at 4°C for at least
one night to allow the antibody to be adsorbed on the walls.
The antibody solution was removed from the wells. A
tris-HC1 buffer solution (pH 8.0) containing 1o bovine serum
albumin (BSA, Fraction V from Seikagaku Corporation) was
dispensed at a rate of 200 ~,L/well. The plate was shaken at
room temperature for two hours to block the walls. The BSA
solution was then removed from the wells. Blood plasma
containing the oxidized LDL was dispensed at a rate of 100
~L/well. Standard solutions used for plotting a calibration
curve were dispensed at a rate of 100 ~.L/well. The plate
was shaken at room temperature for 30 minutes and was then
left to stand at 4°C for one night.
The temperature of the specimens was increased to room
temperature and the solution was removed from the wells.
The wells were washed three times with a tris-HC1 buffer
solution (pH 8.0) containing 0.050 Tween (registered
trademark)-20. A solution of sheep anti-apoB antibody
diluted with a 2,000-fold volume of PBS was. dispensed in
CA 02496096 2005-02-16
- 52 -
each washed well at a rate of 100 ~.L/well. The plate was
shaken at room temperature for two hours and the anti-apoB
antibody was removed from the wells. The wells were washed
three times with a tris-HC1 buffer solution (pH 8.0)
containing 0.05% Tween-20. Subsequently, alkaline
phosphatase-conjugated donkey anti-sheep IgG antibody
diluted with a 2,000-fold volume of a tris-HC1 buffer
solution (pH 8.0) containing 2% Block Ace (from Dainippon
Pharmaceutical Co., Ltd.) was dispensed in each washed well
at a rate of 100 ~.L/well. The plate was shaken at room
temperature for two hours. Subsequently, the conjugated
antibody was removed from the wells. The wells were washed
three times with a tris-HC1 buffer solution (pH 8.0)
containing 0.05% Tween-20. The wells were further washed
two times with a tris-HC1 buffer solution (pH 8.0).
Subsequently, a solution (0.0005 M MgCl2, 1 M diethanolamine
buffer solution, pH 9.8) of p-nitrophenyl phosphate (1
mg/mL) was dispensed at a rate of 100 ~,L/well. The plate
was allowed to react at room temperature for an adequate
period of time. Subsequently, the absorbance at the
wavelength of 415 nm was measured with a plate reader. The
calibration curve was plotted using the results with the
standard solutions to determine the concentration of the
oxidized LDL.
(d) Measurement of adsorptive removal ratio of oxidized LDL
CA 02496096 2005-02-16
- 53 -
The above oxidized LDL was added to blood plasma of a
normal healthy subject (30-years old Japanese, LDL ((3
lipoprotein) concentration 275 mg/dL, HDL-cholesterol
concentration 70 mg/dL) so as to have a concentration of 2
~g/mL.
Seventy hollow fiber membranes were bundled and were
inserted in a glass tube module case having a diameter of
about 7 mm and a length of 12 cm. Both ends of the hollow
fiber membranes were fixed with an epoxy-based potting agent
such that the hollow parts of the hollow fiber membranes
were not clogged. Thus, a mini module (inner surface area
53 cm2) was prepared. The mini module was washed with
ultrapure water at 37°C for 30 minutes. Subsequently a
silicone tube (product name ARAM (registered trademark),
inner diameter: 0.8 mm, outer diameter: 1 mm, length: 37 cm)
was connected to both ends of the mini module through
silicone tubes (product name ARAM (registered trademark),
inner diameter: 7 mm, outer diameter: 10 mm, length: 2 cm)
and irregular shaped connectors. The above blood plasma
(1.5 mL) was perfused in the hollow fiber membranes under a
nitrogen atmosphere at 25°C for 4 hours with a flow rate of
0.5 mL/min. The volume of blood plasma per 1 m2 of the
surface area of hollow fiber membranes was 2.8x102 mL/m2. In
addition, the same perfusion procedure was performed for the
silicone tubes alone without using the mini module. The
CA 02496096 2005-02-16
- 54 -
concentration of oxidized LDL in the blood plasma was
quantitatively determined before and after the perfusion
procedure. The adsorptive removal ratio was calculated by
the following formulae.
Adsorptive removal ratio of oxidized LDL (o) -
adsorptive removal ratio of oxidized LDL (o) in mini module
- adsorptive removal ratio of oxidized LDL (o) in silicone
tubes alone
Adsorptive removal ratio of oxidized LDL (%) - 100 x
(concentration before perfusing - concentration after
perfusing) / concentration before perfusing
(EXAMPLE 1)
The above polysulfone film 1 was used as a substrate.
Polyvinylpyrrolidone (K90 from BASF) was used as a
hydrophilic polymer and ethanol was used as an antioxidant.
The substrate was immersed in an aqueous solution containing
the polyvinylpyrrolidone (0.1 weight percent) and ethanol
(0.5 weight percent) and was irradiated with y-ray. The
absorbed dose of the y-ray was 27 kGy. The film was rinsed
with purified water. Subsequently, the film was placed in
purified water at 80°C and the purified water was stirred
for 60 minutes. The purified water was replaced with fresh
purified water and was stirred again at 80°C for 60 minutes.
Furthermore, the purified water was replaced with fresh
purified water and was stirred at 80°C for 60 minutes to
. CA 02496096 2005-02-16
- 55 -
remove the adsorbed polyvinylpyrrolidone. The measurement
of the surface polyvinylpyrrolidone ratio, the measurement
of the contact angle of the surface, the adhering tests of
blood platelets, and the measurement of the soluble
hydrophilic polymer ratio were performed using the film. As
a result, as shown in Table l, a polysulfone film having a
low soluble hydrophilic polymer ratio, high hydrophilicity,
small numbers of adhered blood platelets, and high
hematologic compatibility was provided.
(COMPARATIVE EXAMPLE 1)
The above polysulfone film 1 was irradiated with y-ray
in purified water. The absorbed dose of the y-ray was 28
kGy. The film was rinsed with purified water. Subsequently,
the film was placed in purified water at 80°C and the
purified water was stirred for 60 minutes. The purified
water was replaced with fresh purified water and was stirred
again at 80°C for 60 minutes. Furthermore, the purified
water was replaced with fresh purified water and was stirred
at 80°C for 60 minutes. The measurement of the surface
polyvinylpyrrolidone ratio, the measurement of the contact
angle of the surface, the adhering tests of blood platelets,
and the measurement of the soluble hydrophilic polymer ratio
were performed using the film. As a result, as shown in
Table l, the numbers of adhered blood platelets of this film
were larger than those of the film in Example 1. Thus, a
CA 02496096 2005-02-16
- 56 -
polysulfone film having low hematologic compatibility was
provided.
(COMPARATIVE EXAMPLE 2)
Polyvinylpyrrolidone (K90 from BASF) was used as a
hydrophilic polymer and ethanol was used as an antioxidant.
The polysulfone film 1 was immersed in an aqueous solution
containing the polyvinylpyrrolidone (0.1 weight percent) and
ethanol (0.5 weight percent) and was left to stand for three
days at room temperature. Subsequently, the film was rinsed
with purified water. The film was placed in purified water
at 80°C and the purified water was stirred for 60 minutes.
The purified water was replaced with fresh purified water
and was stirred again at 80°C for 60 minutes. Furthermore,
the purified water was replaced with fresh purified water
and was stirred at 80°C for 60 minutes. The measurement of
the surface polyvinylpyrrolidone ratio, the measurement of
the contact angle of the surface, the adhering tests of
blood platelets, and the measurement of the soluble
hydrophilic polymer ratio were performed using the film. As
a result, as shown in Table 1, the contact angle and the
numbers of adhered blood platelets of this film were larger
than those of the film in Example 1. Thus, a polysulfone
film having low hydrophilicity and low hematologic
compatibility was provided.
(COMPARATIVE EXAMPLE 3)
CA 02496096 2005-02-16
- 57 -
The polysulfone film 1 without irradiating with y-ray
was rinsed with purified water. The film was placed in
purified water at 80°C and the purified water was stirred
for 60 minutes. The purified water was replaced with fresh
purified water and was stirred again at 80°C for 60 minutes.
Furthermore, the purified water was replaced with fresh
purified water and was stirred at 80°C for 60 minutes. The
measurement of the surface polyvinylpyrrolidone ratio, the
measurement of the contact angle of the surface, the
adhering tests of blood platelets, and the measurement of
the soluble hydrophilic polymer ratio were performed using
the film. As a result, as shown in Table l, the contact
angle and the numbers of adhered blood platelets of this
film were larger than those of the film in Example 1. Thus,
a polysulfone film having low hydrophilicity and low
hematologic compatibility was provided.
Table 1-1
Absorbed Surface
Hydrophilic Antioxidant polyvinylpyrrolidone
polymer
dose of ratio
~y-ray
Example 27 kGy Polyvinylpy Ethanol 0.5 21 wt%
1 oolidone wt%
0.1 wt /o
Comparative2g kGy None None < 2 wt%
Exam le
1
omparative0 kGy PolyvinylpyroolidoneEthanol 0.5 5 wt%
wt%
Exam le 0.1 wt /
2
omparativep kGy None None < 2 wt%
Exam le
3
CA 02496096 2005-02-16
- 58 -
Table 1-2
Number of
Number of adhered Soluble
human
Contact blood platelets adhered rabbithydrophilic
angle (number14.3x103~m2)blood plateletspolymer ratio
number110
m
Example 41 0.1 0.1 0.2
1
omparative43 83 60 0
Exam le
1
omparative80 78 50 0.1
Exam le
2
omparative82 77 58 0
Exam le
3
(EXAMPLE 2)
Polyvinylpyrrolidone (K90 from BASF) was used as a
hydrophilic polymer and ethanol was used as an antioxidant.
An aqueous solution containing the polyvinylpyrrolidone (0.1
weight percent) and ethanol (0.5 weight percent) was
prepared. One thousand milliliters of the aqueous solution
was introduced in the blood side and a further 1,000 mL was
introduced in the dialysate side of the above hollow fiber
membrane module 1 so that the module was filled with the
aqueous solution. Subsequently, the module was irradiated
with y-ray. The absorbed dose of the 'y-ray was 29 kGy. The
dissolution test of polyvinylpyrrolidone was performed using
this module. As a result, the amount of dissolution of
polyvinylpyrrolidone was 0.15 mg/m2. A hollow fiber in the
module was cut into pieces to evaluate the surface
polyvinylpyrrolidone ratio, the soluble hydrophilic polymer
CA 02496096 2005-02-16
- 59 -
ratio, and the numbers of adhered blood platelets. Table 2
shows the results.
(EXAMPLE 3)
Polyvinyl~yrrolidone (K90 from BASF) was used as a
hydrophilic polymer and sodium pyrosulfite was used as an
antioxidant. An aqueous solution containing the
polyvinylpyrrolidone (0.1 weight percent) and sodium
pyrosulfite (500 ppm) was prepared. One thousand
milliliters of the aqueous solution was introduced in the
blood side and a further 1,000 mL was introduced in the
dialysate side of the hollow fiber membrane module 1 so that
the module was filled with the aqueous solution.
Subsequently, the module was irradiated with y-ray. The
absorbed dose of the y-ray was 29 kGy. A hollow fiber in
the module was cut into pieces to evaluate the surface
polyvinylpyrrolidone ratio, the soluble hydrophilic polymer
ratio, and the numbers of adhered blood platelets. Table 2
shows the results.
(COMPAR.ATIVE EXAMPLE 4)
One thousand milliliters of purified water was
introduced in the blood side and a further 1,000 mL was
introduced in the dialysate side of the hollow fiber
membrane module 1 so that the module was filled with the
purified water. Subsequently, the module was irradiated
with y-ray. The absorbed dose of the y-ray was 28 kGy. A
CA 02496096 2005-02-16
- 60 -
hollow fiber in the module was cut into pieces to evaluate
the surface polyvinylpyrrolidone ratio, the soluble
hydrophilic polymer ratio, and the numbers of adhered blood
platelets. As a result, as shown in Table 2, the numbers of
adhered blood platelets of this membrane were larger than
those of the membranes in Examples 2 and 3. In the filling
fluid in the blood side of the module, the maximum
increasing value of ultraviolet absorption value in the
wavelength range of 260 to 300 nm, the increase being caused
by irradiating with y-ray, was also measured. Furthermore,
a mini module was prepared using the same hollow fiber
membranes as used in the hollow fiber membrane module 1.
The mini module was used for the adsorption test of the
oxidized LDL. As shown in Table 3, the adsorptive removal
ratio of oxidized LDL of this membrane was lower than that
of a hollow fiber membrane on which a cationic hydrophilic
polymer was immobilized.
( COMPAR.AT IVE EXAMPLE 5 )
Polyvinylpyrrolidone (K90 from BASF) was used as a
hydrophilic polymer. An aqueous solution containing the
polyvinylpyrrolidone (0.1 weight percent) was prepared. One
thousand milliliters of the aqueous solution was introduced
in the blood side and a further 1,000 mL was introduced in
the dialysate side of the hollow fiber membrane module 1 so
that the module was filled with the aqueous solution.
CA 02496096 2005-02-16
- 61 -
Subsequently, the module was irradiated with y-ray. The
absorbed dose of the y-ray was 29 kGy. A hollow fiber in
the module was cut into pieces to evaluate the surface
polyvinylpyrrolidone ratio, the soluble hydrophilic polymer
ratio, and the numbers of adhered blood platelets. As a
result, as shown in Table 2, the numbers of adhered blood
platelets of this membrane were larger than those of the
membranes in Examples 2 and 3.
(COMPARATIVE EXAMPLE 6)
Ethanol was used as an antioxidant. An aqueous
solution containing ethanol (0.5 weight percent) was
prepared. One thousand milliliters of the aqueous solution
was introduced in the blood side and a further 1,000 mL was
introduced in the dialysate side of the hollow fiber
membrane module 1 so that the module was filled with the
aqueous solution. Subsequently, the module was irradiated
with y-ray. The absorbed dose of the y-ray was 29 kGy. A
hollow fiber in the module was cut into pieces to evaluate
the surface polyvinylpyrrolidone ratio, the soluble
hydrophilic polymer ratio, and the numbers of adhered blood
platelets. As a result, as shown in Table 2, the numbers of
adhered blood platelets of this membrane were larger than
those of the membranes in Examples 2 and 3.
(COMPARATIVE EXAMPLE 7)
Sodium pyrosulfite was used as an antioxidant. An
CA 02496096 2005-02-16
- 62 -
aqueous solution containing sodium pyrosulfite (500 ppm) was
prepared. One thousand milliliters of the aqueous solution
was introduced in the blood side and a further 1,000 mL was
introduced in the dialysate side of the hollow fiber
membrane module 1 so that the module was filled with the
aqueous solution. Subsequently, the module was irradiated
with y-ray. The absorbed dose of the y-ray was 29 kGy. A
hollow fiber in the module was cut into pieces to evaluate
the surface polyvinylpyrrolidone ratio, the soluble
hydrophilic polymer ratio, and the numbers of adhered blood
platelets. As a result, as shown in Table 2, the numbers of
adhered blood platelets of this membrane were larger than
those of the membranes in Examples 2 and 3.
(COMPARATIVE EXAMPLE 8)
Polyvinylpyrrolidone (K90 from BASF) was used as a
hydrophilic polymer and ethanol was used as an antioxidant.
An aqueous solution containing the polyvinylpyrrolidone (0.1
weight percent) and ethanol (0.5 weight percent) was
prepared. One thousand milliliters of the aqueous solution
was introduced in the blood side and a further 1,000 mL was
introduced in the dialysate side of the hollow fiber
membrane module 1 so that the module was filled with the
aqueous solution. Subsequently, the module was left to
stand for three days at room temperature. The dissolution
test of polyvinylpyrrolidone was performed using this module.
CA 02496096 2005-02-16
- 63 -
As a result, the amount of dissolution of
polyvinylpyrrolidone was 0.68 mg/m2, which was larger than
that of the membrane in Example 2. A hollow fiber in the
module was cut into pieces to evaluate the surface
polyvinylpyrrolidone ratio, the soluble hydrophilic polymer
ratio, and the numbers of adhered blood platelets. Table 2
shows the results. This membrane was not irradiated with y-
ray. Therefore, the numbers of adhered blood platelets were
small. However, the amount of dissolution of
polyvinylpyrrolidone was large because a grafting reaction
or a crosslinking of the polyvinylpyrrolidone was not
performed.
Table 2-1
Absorbed Hydrophilic polymerAntioxidant
dose of
-ra
Example 29 kGy Polyvinylp~rolidoneEthanol 0.5
2 0.1 wt%
PolyvinylpyrrolidoneSodium
0.1
Example 29 kGy wt% pyrosulfite
3
500 m
omparative2g kGy None None
Exam le
4
omparative2g kG PolyvinylpyrrolidoneNone
Y 0.1
Exam le wt%
5
omparative2g kGy None Ethanol 0.5
wt%
Exam le
6
omparative Sodium
Example 29 kGy None pyrosulfite
7
500 m
a 0 kGy PolYvinylp~rolidoneEthanol 0.5
a 0.1 wt%
Exam
le 8
, CA 02496096 2005-02-16
- 64 -
Table 2-2
Number of adheredNumber of Soluble
adhered
human blood plateletsrabbit blood hydrophilic
(number14.3x103~,mz)platelets polymer ratio
3 (%)
numberl10
m
Example 0.1 0.1 9
2
Example 0.1 0.1 8.5
3
omparative65 48 3.5
Exam le
4
omparative30 25 3
6
Exam le .
Comparative25 22 9.7
Exam le
6
Comparative31 18 9
5
Exam le .
7
Comparative0 1 73
5 3
Exam le . .
8
(EXAMPLE 4)
Polyvinylpyrrolidone (K90 from BASF) was used as a
5 nonionic hydrophilic polymer and polyethyleneimine (weight-
average molecular weight: 1,000,000, from BASF) was used as
a cationic hydrophilic polymer. An aqueous solution
containing the polyvinylpyrrolidone (0.1 weight percent) and
the polyethyleneimine (0.1 weight percent) was prepared.
One thousand milliliters of the aqueous solution was
introduced in the blood side and a further 1,000 mL was
introduced in the dialysate side of the hollow fiber
membrane module 1 so that the module was filled with the
aqueous solution. Subsequently, the module was irradiated
with y-ray. The absorbed dose of the y-ray was 27 kGy. In
, CA 02496096 2005-02-16
- 65 -
the filling fluid in the blood side of the module, the
maximum increasing value of ultraviolet absorption value in
the wavelength range of 260 to 300 nm, the increase being
caused by irradiating with y-ray, was measured. A hollow
fiber in the module was cut into pieces to evaluate the
number of adhered blood platelets. Furthermore, a mini
module was prepared using the same hollow fiber membranes as
used in the hollow fiber membrane module 1. The mini module
was used for the adsorption test of the oxidized LDL.
Results shown in Table 3 were obtained. Table 3 shows the
results.
(EXAMPLE 5)
Polyethyleneimine (weight-average molecular weight:
1,000,000, from BASF) was used as a cationic hydrophilic
polymer and ethanol was used as an antioxidant. An aqueous
solution containing the polyethyleneimine (0.1 weight
percent) and ethanol was prepared. One thousand milliliters
of the aqueous solution was introduced in the blood side and
a further 1,000 mL was introduced in the dialysate side of
the hollow fiber membrane module 1 so that the module was
filled with the aqueous solution. Subsequently, the module
was irradiated with y-ray. The absorbed dose of the y-ray
was 29 kGy. In the filling fluid in the blood side of the
module, the maximum increasing value of ultraviolet
absorption value in the wavelength range of 260 to 300 nm,
CA 02496096 2005-02-16
- 66 -
the increase being caused by irradiating with y-ray, was
measured. As a result, as shown in Table 3, the maximum
increasing value of ultraviolet absorption value of this
membrane is lower than that of the membrane in Comparative
Example 9. A hollow fiber in the module was cut into pieces
to evaluate the number of adhered blood platelets.
Furthermore, a mini module was prepared using the same
hollow fiber membranes as used in the hollow fiber membrane
module 1. The mini module was used for the adsorption test
of the oxidized LDL. Table 3 shows the results.
(COMPARATIVE EXAMPLE 9)
Polyethyleneimine (weight-average molecular weight:
1,000,000, from BASF) was used as a cationic hydrophilic
polymer. An aqueous solution containing the
polyethyleneimine (0.1 weight percent) was prepared. One
thousand milliliters of the aqueous solution was introduced
in the blood side and a further 1,000 mL was introduced in
the dialysate side of the hollow fiber membrane module 1 so
that the module was filled with the aqueous solution.
Subsequently, the module was irradiated with y-ray. The
absorbed dose of the y-ray was 28 kGy. In the filling fluid
in the blood side of the module, the maximum increasing
value of ultraviolet absorption value in the wavelength
range of 260 to 300 nm, the increase being caused by
irradiating with y-ray, was measured. A hollow fiber in the
CA 02496096 2005-02-16
- 67 -
module was cut into pieces to evaluate the number of adhered
blood platelets. Furthermore, a mini module was prepared
using the same hollow fiber membranes as used in the hollow
fiber membrane module 1. The mini module was used for the
adsorption test of the oxidized LDL. As a result, as shown
in Table 3, the number of adhered blood platelets of this
membrane was larger than that of the membrane in Example 4.
Table 3-1
Absorbed Nonionic hydrophilicCationic hydrophilicAntioxidant
dose of of mer of mer
-ra
Example 27 kGy PolyvinylpyroolidonePolyethyle None
4 oeimine
0.1 wt /0 0.1 wt /o
Example 29 kGy None Polyethyle Ethanol 0.5
5 oeimine wt%
0.1 Wt/o
omparative2g kGy None PolyethyleneimineNone
Exam le 0.1 wt /
9
omparative2g kGy None None None
Exam le
4
Table 3-2
Maximum
Number of adheredSoluble Adsorptive
human blood hydrophilic increasing removal ratio
platelets value of
(number14.3x103~m2)polymer ratioof ultravioletoxidized LDL
(%) (%)
absor tion
value
Example 0.2 10 0.60 26
4
Example 12 12 0.25 27
5
omparative14 8.7 0.61 30
Exam le
9
Comparative65 3.5 0.15 10
Exam le
4
EXAMPLE 6 )
CA 02496096 2005-02-16
- 68 -
Five thousand milliliters of ultrapure water at 40°C
was introduced in the blood side and a further 5,000 mL of
ultrapure water at 40°C was introduced in the dialysate side
of the above hollow fiber membrane module 2 in order to wash
the module. Polyethylene glycol (Macrogol (registered
trademark) 6000 from NOF Corporation) was used as a
hydrophilic polymer. An aqueous solution containing the
polyethylene glycol (0.075 weight percent) was prepared.
One thousand milliliters of the aqueous solution was
introduced in the blood side and a further 1,000 mL was
introduced in the dialysate side of the module so that the
module was filled with the aqueous solution. Subsequently,
the module was irradiated with y-ray. The absorbed dose of
the y-ray was 28 kGy. The measurement of the immobilization
density of polyethylene glycol, the adhering test of blood
platelets, and the adsorption test of IL-6 were performed
with the module. Table 4 shows the results.
(EXAMPLE 7)
Five thousand milliliters of ultrapure water at 40°C
was introduced in the blood side and a further 5,000 mL of
ultrapure water at 40°C was introduced in the dialysate side
of the hollow fiber membrane module 2 in order to wash the
module. Polyethylene glycol (Macrogol (registered
trademark) 6000 from NOF Corporation) was used as a
hydrophilic polymer. An aqueous solution containing the
~
CA 02496096 2005-02-16
- 69 -
polyethylene glycol (0.100 weight percent) was prepared.
One thousand milliliters of the aqueous solution was
introduced in the blood side and a further 1,000 mL was
introduced in the dialysate side of the module so that the
module was filled with the aqueous solution. Subsequently,
the module was irradiated with y-ray. The absorbed dose of
the y-ray was 28 kGy. The measurement of the immobilization
density of polyethylene glycol, the adhering test of blood
platelets, and the adsorption test of IL-6 were performed
with the module. Table 4 shows the results.
(EXAMPLE 8)
Five thousand milliliters of ultrapure water at 40°C
was introduced in the blood side and a further 5,000 mL of
ultrapure water at 40°C was introduced in the dialysate side
of the hollow fiber membrane module 2 in order to wash the
module. Polyvinylpyrrolidone (K90 from ISP) was used as a
hydrophilic polymer. An aqueous solution containing the
polyvinylpyrrolidone (0.100 weight percent) was prepared.
One thousand milliliters of the aqueous solution was
introduced in the blood side and a further 1,000 mL was
introduced in the dialysate side of the module so that the
module was filled with the aqueous solution. Subsequently,
the module was irradiated with y-ray. The absorbed dose of
the y-ray was 28 kGy. The adhering test of blood platelets
and the adsorption test of IL-6 were performed with the
CA 02496096 2005-02-16
- 70 -
module. Table 4 shows the results.
(COMPARATIVE EXAMPLE 10)
Five thousand milliliters of ultrapure water at 40°C
was introduced in the blood side and a further 5,000 mL of
ultrapure water at 40°C was introduced in the dialysate side
of the hollow fiber membrane module 2 in order to wash the
module. Polyethylene glycol (Macrogol (registered
trademark) 6000 from NOF Corporation) was used as a
hydrophilic polymer. An aqueous solution containing the
polyethylene glycol (0.010 weight percent) was prepared.
One thousand milliliters of the aqueous solution was
introduced in the blood side and a further 1,000 mL was
introduced in the dialysate side of the module so that the
module was filled with the aqueous solution. Subsequently,
the module was irradiated with y-ray. The absorbed dose of
the y-ray was 28 kGy. The measurement of the immobilization
density of polyethylene glycol, the adhering test of blood
platelets, and the adsorption test of IL-6 were performed
with the module. Table 4 shows the results.
(COMPARATIVE EXAMPLE 11)
Five thousand milliliters of ultrapure water at 40°C
was introduced in the blood side and a further 5,000 mL of
ultrapure water at 40°C was introduced in the dialysate side
of the hollow fiber membrane module 2 in order to wash the
module. Polyethylene glycol (polyethylene glycol #200 from
CA 02496096 2005-02-16
- 71 -
Nacalai Tesque, Inc.) was used as a hydrophilic polymer. An
aqueous solution containing the polyethylene glycol (0.100
weight percent) was prepared. One thousand milliliters of
the aqueous solution was introduced in the blood side and a
further 1,000 mL was introduced in the dialysate side of the
module so that the module was filled with the aqueous
solution. Subsequently, the module was irradiated with g-
ray. The absorbed dose of the y-ray was 28 kGy. The
measurement of the immobilization density of polyethylene
glycol, the adhering test of blood platelets, and the
adsorption test of IL-6 were performed with the module.
Table 4 shows the results.
(COMPAR.ATIVE EXAMPLE 12)
Five thousand milliliters of ultrapure water at 40°C
was introduced in the blood side and a further 5,000 mL of
ultrapure water at 40°C was introduced in the dialysate side
of the hollow fiber membrane module 2 in order to wash the
module. Polyethylene glycol (Mw 900,000 from Scientific
Polymers Products, Inc.) was used as a hydrophilic polymer.
An aqueous solution containing the polyethylene glycol
(0.100 weight percent) was prepared. One thousand
milliliters of the aqueous solution was introduced in the
blood side and a further 1,000 mL was introduced in the
dialysate side of the module so that the module was filled
with the aqueous solution. Subsequently, the module was
CA 02496096 2005-02-16
- 72 -
irradiated with y-ray. The absorbed dose of the y-ray was 28
kGy. The measurement of the immobilization density of
polyethylene glycol, the adhering test of blood platelets,
and the adsorption test of IL-6 were performed with the
module. Table 4 shows the results.
(COMPARATIVE EXAMPLE 13)
Five thousand milliliters of ultrapure water at 40°C
was introduced in the blood side and a further 5,000 mL of
ultrapure water at 40°C was introduced in the dialysate side
of the hollow fiber membrane module 2 in order to wash the
module. Subsequently, the module was filled with ultrapure
water and was irradiated with y-ray. The absorbed dose of
the y-ray was 28 kGy. The adhering test of blood platelets
and the adsorption test of IL-6 were performed with the
module. Table 4 shows the results.
CA 02496096 2005-02-16
- 73 -
Table 4
Number of adheredAdsorptivity Immobilization
to IL-6 density of
human blood plateletsn Icm2 polyethylene glycol
~ g ~ 2
number14.3x103 m Im
m2
Example 0.56 0.209 384
6
Example 0.43 0.180 353
7
Example 0.99 0.163 -
8
Comparative3.23 0.032 137
Exam le
Comparative48 0.282 172
59
Exam le ,
11
omparative0.56 0.053 239
Exam le
12
omparative100 or more 0.162 0
Exam le
13
( EXAMPLE 9 )
5 A connector part at the blood side of an artificial
kidney module of a commercial blood circuit for an
artificial kidney (artificial kidney blood circuit H-102-KTS
from Toray Medical Co., Ltd) was cut into small pieces to
prepare a measurement sample of 1 g. Polyvinylpyrrolidone
10 (K90 from ISP) was used as a hydrophilic polymer and ethanol
was used as an antioxidant. The measurement sample was
immersed in an aqueous solution (60 mL) containing the
polyvinylpyrrolidone (0.100 weight percent) and ethanol
(0.100 weight percent), and was irradiated with Y-ray. The
adhering test of blood platelets was performed. Table 5
shows the result.
CA 02496096 2005-02-16
- 74 -
( EXAMPLE 10 )
A blood tube part of a commercial blood circuit for an
artificial kidney (artificial kidney blood circuit H-102-KTS
from Toray Medical Co., Ltd) was cut into small pieces to
prepare a measurement sample of 1 g. Polyvinylpyrrolidone
(K90 from ISP) was used as a hydrophilic polymer and ethanol
was used as an antioxidant. The measurement sample was
immersed in an aqueous solution (60 mL) containing the
polyvinylpyrrolidone (0.100 weight percent) and ethanol
(0.100 weight percent), and was irradiated with y-ray. The
adhering test of blood platelets was performed. Table 5
shows the result.
(EXAMPLE 11)
A blood chamber part of a commercial blood circuit for
an artificial kidney (artificial kidney blood circuit H-102-
KTS from Toray Medical Co., Ltd) was cut into small pieces
to prepare a measurement sample of 1 g.
Polyvinylpyrrolidone (K90 from ISP) was used as a
hydrophilic polymer and ethanol was used as an antioxidant.
The measurement sample was immersed in an aqueous solution
(60 mL) containing the polyvinylpyrrolidone (0.100 weight
percent) and ethanol (0.100 weight percent), and was
irradiated with y-ray. The adhering test of blood platelets
was performed. Table 5 shows the result.
(EXAMPLE 12)
CA 02496096 2005-02-16
- 75 -
A mesh part of a commercial blood circuit for an
artificial kidney (artificial kidney blood circuit H-102-KTS
from Toray Medical Co., Ltd) was cut into small pieces to
prepare a measurement sample of 1 g. Polyvinylpyrrolidone
(Ii90 from ISP) was used as a hydrophilic polymer and ethanol
was used as an antioxidant. The measurement sample was
immersed in an aqueous solution (60 mL) containing the
polyvinylpyrrolidone (0.100 weight percent) and ethanol
(0.100 weight percent), and was irradiated with y-ray. The
adhering test of blood platelets was performed. Table 5
shows the result.
(COMPARATIVE EXAMPLE 14)
A connector part at the blood side of an artificial
kidney module of a commercial blood circuit for an
artificial kidney (artificial kidney blood circuit H-102-KTS
from Toray Medical Co., Ltd) was cut into small pieces to
perform the adhering test of blood platelets. Table 5 shows
the result.
(COMPARATIVE EXAMPLE 15)
A blood tube part of a commercial blood circuit for an
artificial kidney (artificial kidney blood circuit H-102-KTS
from Toray Medical Co., Ltd) was cut into small pieces to
perform the adhering test of blood platelets. Table 5 shows
the result.
(COMPARATIVE EXAMPLE 16)
CA 02496096 2005-02-16
- 76 -
A blood chamber part of a commercial blood circuit for
an artificial kidney (artificial kidney blood circuit H-102-
KTS from Toray Medical Co., Ltd) was cut into small pieces
to perform the adhering test of blood platelets. As a
result, as shown in Table 5, the number of adhered blood
platelets was 7.0 (/4.3x103 ~un2) .
(COMPARATIVE EXAMPLE 17)
A mesh part of a commercial blood circuit for an
artificial kidney (artificial kidney blood circuit H-102-KTS
from Toray Medical Co., Ltd) was cut into small pieces to
perform the adhering test of blood platelets. Table 5 shows
the result.
Table 5
Number of adhered human blood platelets(number14.3x103~,m2)
Example 0.67
9
Example 0.67
10
Example 0.33
11
Example 29.00
12
Comparative
Exam le 5.67
14
Comparative
Exam le 3.33
Comparative7.00
Exam le
16
Comparative100 or more
Exam le
17
- CA 02496096 2005-02-16
_ 77 _
(EXAMPLE 13)
A commercial glassy carbon plate (from Toyo Tanso Co.,
Ltd.) was used as a substrate. Polyvinylpyrrolidone (K90
from BASF) was used as a hydrophilic polymer and ethanol was
used as an antioxidant. The substrate was immersed in an
aqueous solution containing the polyvinylpyrrolidone (0.01
weight percent) and ethanol (0.1 weight percent) and was
irradiated with y-ray. The absorbed dose of the y-ray was 27
kGy. The film was rinsed with purified water. Subsequently,
the film was placed in purified water at 80°C and the
purified water was stirred for 60 minutes. The purified
water was replaced with fresh purified water and was stirred
again at 80°C for 60 minutes. Furthermore, the purified
water was replaced with fresh purified water and was stirred
at 80°C for 60 minutes to remove the adsorbed
polyvinylpyrrolidone. The contact.angle of the surface of
the film was measured. The contact angle of the film was 39
degrees, whereas that of an untreated film was 98 degrees.
This result showed that the film was significantly subjected
to hydrophilization.
(EXAMPLE 14)
A commercial glassy carbon plate (from Toyo Tanso Co.,
Ltd.) was used as a substrate. Polyvinylpyrrolidone (K90
from BASF) was used as a hydrophilic polymer. The substrate
was immersed in an aqueous solution containing the
CA 02496096 2005-02-16
polyvinylpyrrolidone (0.01 weight percent) and was
irradiated with y-ray. The absorbed dose of the 'y-ray was 27
kGy. The film was rinsed with purified water. Subsequently,
the film was placed in purified'water at 80°C and the
purified water was stirred for 60 minutes. The purified
water was replaced with fresh purified water and was stirred
again at 80°C for 60 minutes. Furthermore, the purified
water was replaced with fresh purified water and was stirred
at 80°C for 60 minutes to remove the adsorbed
polyvinylpyrrolidone. The contact angle of the surface of
the film was measured. The contact angle of the film was 52
degrees, whereas that of the untreated film was 98 degrees.
This result showed that the film was significantly subjected
to hydrophilization.
(COMPAR.ATIVE EXAMPLE 18)
The glassy carbon plate used in Example 13 was
irradiated with y-ray in purified water. The absorbed dose
of the 'y-ray was 28 kGy. The film was rinsed with purified
water. Subsequently, the film was placed in purified water
at 80°C and the purified water was stirred for 60 minutes.
The purified water was replaced with fresh purified water
and was stirred again at 80°C for 60 minutes. Furthermore,
the purified water was replaced with fresh purified water
and was stirred at 80°C for 60 minutes. The contact angle
of the surface of the film was 98 degrees, which was the
CA 02496096 2005-02-16
- 79 -
same as the 98 degrees of the untreated film.
(EXAMPLE 15)
A commercial carbon sheet (from Toray Industries, Inc.)
was used as a substrate. Polyvinylpyrrolidone (K90 from
BASF) was used as a hydrophilic polymer and ethanol was used
as an antioxidant. The substrate was immersed in an aqueous
solution containing the polyvinylpyrrolidone (0.1 weight
percent) and ethanol (0.1 weight percent) and was irradiated
with y-ray. The absorbed dose of the y-ray was 27 kGy. The
film was rinsed with purified water. Subsequently, the film
was placed in purified water at 80°C and the purified water
was stirred for 60 minutes. The purified water was replaced
with fresh purified water and was stirred again at 80°C for
60 minutes. Furthermore, the purified water was replaced
with fresh purified water and was stirred at 80°C for 60
minutes to remove the adsorbed polyvinylpyrrolidone. The
contact angle of the surface of the film was measured. The
contact angle of the film was 30 degrees, whereas that of an
untreated film was 131 degrees. This result showed that the
film was significantly subjected to hydrophilization.
Industrial Applicability
According to a modified substrate of the present
invention, a hydrophilic polymer is immobilized on the
surface, and in addition, excessive crosslinking,
CA 02496096 2005-02-16
degradation or the like of the hydrophilic polymer is
prevented. Accordingly, the adhesion of organic matter such
as proteins, or biogenic substances can be suppressed. In
particular, the modified substrate of the present invention
has high hematologic compatibility. Furthermore, the high
hematologic compatibility can be achieved while the
adsorption of a cytokine is maintained.
The modified substrate of the present invention can be
widely used for applications that require hydrophilicity on
the surface. For example, the modified substrate of the
present invention can be preferably used in medical devices
such as an artificial blood vessel, a catheter, a blood bag,
a blood filter, a contact lens, an intraocular lens, an
artificial kidney, an artificial lung, and auxiliary
instruments for surgical operation. The modified substrate
of the present invention can be preferably used in
separation membranes of biogenic substances such as amino
acids, peptides, saccharides, proteins, and composites
thereof. The modified substrate of the present invention
can be preferably used in instruments used for biological
experiments such as pipette tips, tubes, Petri dishes, and
sample collection tubes; bioreactors; molecular motors; DDS;
protein chips; DNA chips; biosensors; and components of
analytical instruments such as an atomic force microscope
(AFM), a scanning near-field optical microscope (SNOM), and
CA 02496096 2005-02-16
- 81 -
a surface plasmon resonance (SPR) sensor. In addition, the
modified substrate of the present invention can be
preferably used in separation membranes for water treatment
such as membranes for a water purifier, membranes for
purifying clean water, membranes for purifying sewage, and
reverse osmosis (RO) membranes. In particular, the modified
substrate of the present invention is preferably used for
applications in which the substrate is brought into contact
with a biogenic substance, for example, a module for blood
purification such as an artificial kidney.