Note: Descriptions are shown in the official language in which they were submitted.
CA 02496211 2005-02-18
1
81581 fft
Boehringer Ingelheim Pharma GmbH & Co. KG Case 1/1385
D-55216 IngelheimlRhein Foreign filing text
New purine derivatives, the preparation thereof and their use as
pharmaceutical compositions
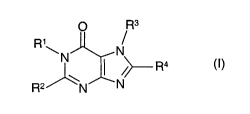
The present invention relates to substituted purines of general formula
p Rs
R~~N N
~\ /~ R4
R2/ \N N
(I),
the tautomers, the stereoisomers, the mixtures, the prodrugs thereof and the
salts thereof, particularly the physiologically acceptable salts thereof with
inorganic or organic acids or bases which have valuable pharmacological
properties, particularly an inhibiting effect on the activity of the enzyme
dipeptidylpeptidase-IV (DPP-IV), the preparation thereof, the use thereof for
the prevention or treatment of diseases or conditions associated with an
increased DPP-IV activity or capable of being prevented or alleviated by
reducing the DPP-IV activity, particularly type I or type II diabetes
mellitus, the
pharmaceutical compositions containing a compound of general formula (I) or
a physiologically acceptable salt thereof as well as processes for the
preparation thereof.
In the above formula I
R' denotes a hydrogen atom,
a C,_$-alkyl group,
a C3_a-alkenyl group,
CA 02496211 2005-02-18
2
a C3_d-alkenyl group which is substituted by a C,_2-alkyloxy-carbonyl,
aminocarbonyl, C,_3-alkylamino-carbonyl, di-(C,_3-alkyl)-amino-carbonyl,
pyrrolidin-1-ylcarbonyl, piperidin-1-ylcarbonyl or morpholin-4-ylcarbonyl
group,
a C3_s-alkynyl group,
a C,_s-alkyl group substituted by a group Ra, where
Ra denotes a C3_~-cycloalkyl, heteroaryl, cyano, carboxy, C,_3-alkyloxy-
carbonyl, aminocarbonyl, C,_3-alkylamino-carbonyl, di-(C,_3-alkyl)-amino-
carbonyl, pyrrolidin-1-ylcarbonyl, piperidin-1-ylcarbonyl, morpholin-4-
ylcarbonyl, piperazin-1-ylcarbonyl, 4-methylpiperazin-1-ylcarbonyl or 4-
ethylpiperazin-1-ylcarbonyl group,
a C,_s-alkyl group substituted by a phenyl group, where the phenyl ring is
substituted by the groups R'° to R'4 and
R'° denotes a hydrogen atom,
a fluorine, chlorine, bromine or iodine atom,
a C,_4-alkyl, hydroxy or C,_4-alkyloxy group,
a nitro, amino, C,_3-alkylamino, di-(C,_s-alkyl)-amino, cyan-C,_3-alkylamino,
N-(cyan-C,_3-alkyl)-N-(C,_3-alkyl)-amino, C,_3-alkyloxy-carbonyl-C,_3-
alkylamino, pyrrolidin-1-yl, piperidin-1-yl, morpholin-4-yl, piperazin-1-yl or
4-(C,_3-alkyl)-piperazin-1-yl group,
a formylamino, C,_3-alkyl-carbonylamino, Cs-s-cycloalkyl-carbonylamino,
Cs-s-cycloalkyl-C,_3-alkyl-carbonylamino, arylcarbonylamino, aryl-C,_3-
alkyl-carbonylamino, C,_3-alkyloxy-carbonylamino, aminocarbonylamino,
C,_3-alkyl-aminocarbonylamino, di-(C,_3-alkyl)-aminocarbonylamino,
pyrrolidin-1-yl-carbonylamino, piperidin-1-yl-carbonylamino, morpholin-4-
CA 02496211 2005-02-18
yl-carbonylamino, piperazin-1-yl-carbonylamino or 4-(C,.3-alkyl)-piperazin-
1-yl-carbonylamino, C~_3-alkyl-sulphonylamino, bis-(C~_3-alkylsulphonyl)-
amino, aminosulphonylamino, C~_3-alkylamino-sulphonylamino, di-(C,_3-
alkyl)-amino-sulphonylamino, pyrrolidin-1-yl-sulphonylamino, piperidin-1-
yl-sulphonylamino, morpholin-4-yl-sulphonylamino, piperazin-1-yl-
sulphonylamino or 4-(C,_3-alkyl)-piperazin-1-yl-sulphonylamino, (C,_3-
alkylamino)-thiocarbonylamino, (C~_3-alkyloxy-carbonylamino)-carbonyl-
amino, arylsulphonylamino or aryl-C,_3-alkyl-sulphonylamino group,
an N-(C~_3-alkyl)-formylamino, N-(C,_3-alkyl)-N (C~_3-alkyl-carbonyl)-amino,
N-(C~_3-alkyl)-N-(C3_s-cycloalkyl-carbonyl)-amino, N-(C~_3-alkyl)-N-(C3_s-
cycloalkyl-C,_3-alkyl-carbonyl)-amino, N-(C,_3-alkyl)-N-(arylcarbonyl)-
amino, N-(C~_3-alkyl)-N-(aryl-C~_3-alkyl-carbonyl)-amino, N-(C,_3-alkyl)-N-
(C~_3-alkyloxy-carbonyl)-amino, N-(aminocarbonyl)-N-(C~_3-alkyl)-amino,
N-(C~_3-alkyl-aminocarbonyl)-N-(C,_3-alkyl)-amino, N-[di-(C,_3-
alkyl)aminocarbonyl]-N-(C~_3-alkyl)-amino, N-(C,_3-alkyl)-N-(C,_3-alkyl-
sulphonyl)-amino, N-(C~_3-alkyl)-N (arylsulphonyl)-amino or N-(C~_3-alkyl)-
N (aryl-C,_3-alkyl-sulphonyl)-amino group,
a 2-oxo-imidazolidin-1-yl, 2,4-dioxo-imidazolidin-1-yl, 2,5-dioxo-imidazo-
lidin-1-yl or 2-oxo-hexahydropyrimidin-1-yl group wherein the nitrogen
atom in the 3 position may be substituted in each case by a methyl or
ethyl group,
a cyano, carboxy, C~_3-alkyloxy-carbonyl, aminocarbonyl, C,_3-alkyl-amino-
carbonyl, di-(C~_3-alkyl)-aminocarbonyl, pyrrolidin-1-yl-carbonyl, piperidin-
1-yl-carbonyl, morpholin-4-yl-carbonyl, piperazin-1-yl-carbonyl or 4-(C~_3-
alkyl)-piperazin-1-yl-carbonyl group,
a C,_3-alkyl-carbonyl or an arylcarbonyl group,
a carboxy-C~_3-alkyl, C~_3-alkyloxy-carbonyl-C~_3-alkyl, cyano-C~_3-alkyl,
aminocarbonyl-C~_3-alkyl, C~_3-alkyl-aminocarbonyl-C~_3-alkyl, di-(C~_3-
alkyl)-aminocarbonyl-C~_3-alkyl, pyrrolidin-1-yl-carbonyl-C,_3-alkyl,
CA 02496211 2005-02-18
piperidin-1-yl-carbonyl-C,_3-alkyl, morpholin-4-yl-carbonyl-C~_3-alkyl,
piperazin-1-yl-carbonyl-C,_3-alkyl or 4-(C~_3-alkyl)-piperazin-1-yl-carbonyl-
C~_3-alkyl group,
a carboxy-C,_3-alkyloxy, C~_3-alkyloxy-carbonyl-C~_3-alkyloxy, cyano-C,_3-
alkyloxy, aminocarbonyl-C~_3-alkyloxy, C~_3-alkyl-aminocarbonyl-C~_3-
alkyloxy, di-(C~_3-alkyl)-aminocarbonyl-C,_3-alkyloxy, pyrrolidin-1-yl-
carbonyl-C,_3-alkyl-oxy, piperidin-1-yl-carbonyl-C~_3-alkyloxy, morpholin-4-
yl-carbonyl-C~_3-alkyl-oxy, piperazin-1-yl-carbonyl-C~_3-alkyloxy or 4-(C~_3-
alkyl)-piperazin-1-yl-carbonyl-C,_3-alkyloxy group,
a hydroxy-C,_3-alkyl, C~_3-alkyloxy-C,_3-alkyl, amino-C,_3-alkyl, C,_3-alkyl-
amino-C,_3-alkyl, di-(C,_3-alkyl)-amino-C~_3-alkyl, pyrrolidin-1-yl-C,_3-
alkyl,
piperidin-1-yl-C~_3-alkyl, morpholin-4-yl-C~_3-alkyl, piperazin-1-yl-C~_3-
alkyl,
4-(C~_3-alkyl)-piperazin-1-yl-C~_3-alkyl group,
a hydroxy-C,_3-alkyloxy, C,_3-alkyloxy-C,_3-alkyloxy, C,_3-alkylsulphanyl-
C~_3-alkyloxy, C,_3-alkylsulphinyl-C,_3-alkyloxy, C~_3-alkylsulphonyl-C~_3-
alkyloxy, amino-C~_3-alkyloxy, C~_3-alkylamino-C~_3-alkyloxy, di-(C~_3-alkyl)-
amino-C,_3-alkyloxy, pyrrolidin-1-yl-C~_3-alkyloxy, piperidin-1-yl-C~_3-
alkyloxy, morpholin-4-yl-C~_3-alkyloxy, piperazin-1-yl-C~_3-alkyloxy, 4-(C~_3'
alkyl)-piperazin-1-yl-C,_3-alkyloxy group,
a mercapto, C,_3-alkylsulphanyl, C,_3-alkysulphinyl, C~_3-alkylsulphonyl,
C,_3-alkylsulphonyloxy, arylsulphonyloxy, trifluoromethylsulphanyl,
trifluoromethylsulphinyl or trifluoromethylsulphonyl group,
a sulpho, aminosulphonyl, C,_3-alkyl-aminosulphonyl, di-(C~_3-alkyl)-
aminosulphonyl, pyrrolidin-1-yl-sulphonyl, piperidin-1-yl-sulphonyl,
morpholin-4-yl-sulphonyl, piperazin-1-yl-sulphonyl or 4-(C,_3-alkyl)-
piperazin-1-yl-sulphonyl group,
a methyl or methoxy group substituted by 1 to 3 fluorine atoms,
CA 02496211 2005-02-18
an ethyl or ethyloxy group substituted by 1 to 5 fluorine atoms,
a Cz_4-alkenyl or C2_4-alkynyl group,
a C3_4-alkenyloxy or C3_4-alkynyloxy group,
a C3_6-cycloalkyl or C3_6-cycloalkyloxy group,
a C3_6-cycloalkyl-C~_3-alkyl or C3_6-cycloalkyl-C~_3-alkyloxy group or
an aryl, aryloxy, aryl-C~_3-alkyl or aryl-C~_3-alkyloxy group,
R" and R'2, which may be identical or different, in each case represent a
hydrogen atom, a fluorine, chlorine, bromine or iodine atom, a C~_3-alkyl,
trifluoromethyl, hydroxy, C~_3-alkyloxy or cyano group, or
R" together with R'2, if they are bound to adjacent carbon atoms, also
represent a methylenedioxy, difluoromethylenedioxy or a straight-chain
C3_5-alkylene group and
R'3 and R'4, which may be identical or different, in each case represent a
hydrogen atom, a fluorine, chlorine or bromine atom, a trifluoromethyl,
C,_3-alkyl or C~_3-alkyloxy group,
a phenyl-C,_4-alkyl group wherein the alkyl moiety is substituted by a cyano,
carboxy, C,_3-alkyloxy-carbonyl, aminocarbonyl, C~_3-alkyl-aminocarbonyl,
di-(C,_3-alkyl)-aminocarbonyl, pyrrolidin-1-yl-carbonyl, piperidin-1-yl-
carbonyl,
morpholin-4-yl-carbonyl-group and the phenyl moiety is substituted by the
groups R'° to R'4, while R'° to R'4 are as hereinbefore defined,
a phenyl group substituted by the groups R'° to R'4, where R'°
to R'" are as
hereinbefore defined,
CA 02496211 2005-02-18
6
a phenyl-CZ_3-alkenyl group wherein the phenyl moiety is substituted by the
groups R'° to R'4, where R'° to R'4 are as hereinbefore defined,
a phenyl-(CHZ)m-A-(CH2)~-group wherein the phenyl moiety is substituted by
R'° to R'4, where R'° to R'4 are as hereinbefore defined
and
A represents a carbonyl group, m represents the number 0, 1 or 2 and
n represents the number 1, 2 or 3,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by
R'°
to R'4, where R'° to R'4 are as hereinbefore defined and the methyl
moiety is
substituted by a C,-3-alkyl group,
a phenyl-(CH2)m-B-(CH2)~-group wherein the phenyl moiety is substituted by
R'° to R'4, where R'° to R'4, m and n are as hereinbefore
defined and
B denotes a methylene group which is substituted by a hydroxy, C~_3-
alkyloxy, amino, C,_3-alkylamino, di-(C,_3-alkyl)-amino, mercapto, C~-a-
alkylsulphanyl, C,_3-alkylsulphinyl or C,_3-alkylsulphonyl group and is
optionally additionally substituted by a methyl or ethyl group,
a naphthyl-C~_3-alkyl group wherein the naphthyl moiety is substituted by the
groups R'° to R'4, where R'° to R'4 are as hereinbefore defined,
a naphthyl-(CH2)m-A-(CH2)~-group wherein the naphthyl moiety is substituted
by R'° to R'°, where R'° to R'4, A, m and n are as
hereinbefore defined,
a naphthyl-(CH2)m-B-(CHZ)~-group wherein the naphthyl moiety is substituted
by R'° to R'4, where R'° to R'4, B, m and n are as hereinbefore
defined,
a [1,4]naphthoquinon-2-yl, chromen-4-on-3-yl, 1-oxoindan-2-yl, 1,3-dioxo-
indan-2-yl or 2,3-dihydro-3-oxo-benzofuran-2-yl group,
CA 02496211 2005-02-18
7
a heteroaryl-(CH2)m-A-(CH2)~ group where A, m and n are as hereinbefore
defined,
a heteroaryl-(CHZ)m-B-(CH2)~ group where B, m and n are as hereinbefore
defined,
a C~_6-alkyl-A-(CHZ)~ group where A and n are as hereinbefore defined,
a C3_~-cycloalkyl-(CH2)m-A-(CHZ)" group where A, m and n are as hereinbefore
defined,
a C3_7-cycloalkyl-(CH2)m-B-(CHz)~ group where B, m and n are as hereinbefore
defined,
an RZ'-A-(CHZ)~-group wherein R2' denotes a C,_3-alkyloxycarbonyl,
aminocarbonyl, C~_3-alkylaminocarbonyl, di-(C,_3-alkyl)aminocarbonyl,
pyrrolidin-1-yl-carbonyl, piperidin-1-yl-carbonyl or morpholin-4-yl-carbonyl,
piperazin-1-yl-carbonyl, 4-methylpiperazin-1-yl-carbonyl or 4-ethylpiperazin-1-
yl-carbonyl group and A and n are as hereinbefore defined,
a phenyl-(CHz)m-D-C,_3-alkyl group wherein the phenyl moiety is substituted
by the groups R'° to R'4, where R'° to R'4 and m are as
mentioned
hereinbefore and D denotes an oxygen or sulphur atom, an imino, C~_3-
alkylimino, sulphinyl or sulphonyl group,
a naphthyl-(CH2)m-D-C~_3-alkyl group wherein the naphthyl moiety is
substituted by the groups R'° to R'4, where R'° to R'4, D and m
are as
mentioned hereinbefore,
a CZ_6-alkyl group substituted by a group Rb, where
Rb is isolated from the cyclic nitrogen atom in the 1 position of the purine
skeleton by at least two carbon atoms and
CA 02496211 2005-02-18
Rb denotes a hydroxy, C~_3-alkyloxy, mercapto, C,_3-alkylsulphanyl, C~_3-
alkylsulphinyl, C~_3-alkylsulphonyl, amino, C~_3-alkyl-carbonylamino, C3_s-
cycloalkyl-carbonyl-amino, arylcarbonylamino, C~_3-alkylamino, di-(C~_3-
alkyl)-amino, pyrrolidin-1-yl, piperidin-1-yl, morpholin-4-yl, piperazin-1-yl
or 4-(C~_3-alkyl)-piperazin-1-yl group,
a C3_6-cycloalkyl group,
or an amino or arylcarbonylamino group,
R2 denotes a hydrogen atom,
a C,_e-alkyl group,
a C3_e-alkenyl group,
a C3_4-alkenyl group which is substituted by a C~_2-alkyloxy-carbonyl,
aminocarbonyl, C,_3-alkylamino-carbonyl, di-(C,_3-alkyl)-amino-carbonyl,
pyrrolidin-1-ylcarbonyl, piperidin-1-ylcarbonyl or morpholin-4-ylcarbonyl
group,
a C3_8-alkynyl group,
a C3_6-cycloalkyl group,
a C~_6-alkyl group substituted by a group Ra, where Ra is as hereinbefore
defined,
a phenyl group which is substituted by R'° to R'4, where R'° to
R'4 are as
hereinbefore defined,
a C~_6-alkyl group substituted by a phenyl group, wherein the phenyl ring is
substituted by the groups R'° to R'4 and R'° to R'4 are as
hereinbefore
defined,
CA 02496211 2005-02-18
a phenyl-C~_4-alkyl group wherein the alkyl moiety is substituted by a cyano,
carboxy, C,_3-alkyloxy-carbonyl, aminocarbonyl, C~_3-alkyl-aminocarbonyl, di-
(C,_3-alkyl)-aminocarbonyl, pyrrolidin-1-yl-carbonyl, piperidin-1-yl-carbonyl,
morpholin-4-yl-carbonyl group and the phenyl moiety is substituted by the
groups R'° to R'4, where R'° to R'4 are as hereinbefore defined,
a phenyl-C2_3-alkenyl group wherein the phenyl moiety is substituted by
R'° to
R'4, where R'° to R'4 are as hereinbefore defined,
a heteroaryl group,
a phenyl-(CH2)m-A or phenyl-(CHZ)m-A-(CHz)~ group wherein the phenyl
moiety is substituted in each case by R'° to R'4, while A, R'°
to R'4, m and n
are as hereinbefore defined,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by
R'°
to R'4, where R'° to R'4 are as hereinbefore defined and the methyl
moiety is
substituted by a C,_3-alkyl group,
a phenyl-(CH2)m-B or phenyl-(CH2)m-B-(CHz)~ group wherein the phenyl
moiety is substituted in each case by R'° to R'4, while B, R'°
to R'4, m and n
are as hereinbefore defined,
a naphthyl-C,_3-alkyl group wherein the naphthyl moiety is substituted by the
groups R'° to R'4, where R'° to R'4 are as hereinbefore defined,
a naphthyl-(CHZ)m A or naphthyl-(CHZ)m A-(CHZ)~ group wherein the naphthyl
moiety is substituted in each case by R'° to R'4, where R'° to
R'4, A, m and n
are as hereinbefore defined,
a naphthyl-(CH2)m B or naphthyl-(CHZ)m-B-(CH2)~ group wherein the naphthyl
moiety is substituted in each case by R'° to R'4, where R'° to
R'4, B, m and n
are as hereinbefore defined,
CA 02496211 2005-02-18
a heteroaryl-(CHz)m-A or heteroaryl-(CH2)m-A-(CH2)~ group where A, m and n
are as hereinbefore defined,
a heteroaryl-(CH2)m-B or heteroaryl-(CH2)m-B-(CHZ)~ group where B, m and n
are as hereinbefore defined,
a C~_6-alkyl-A or C~_6-alkyl-A-(CH2)~ group where A and n are as hereinbefore
defined,
a C3_~-cycloalkyl-(CH2)m-A or C3_~-cycloalkyl-(CHZ)m-A-(CH2)~ group where A,
m and n are as hereinbefore defined,
a C3_~-cycloalkyl-(CH2)m-B or C3_~-cycloalkyl-(CH2)m-B-(CH2)~ group where B,
m and n are as hereinbefore defined,
an RZ'-A-(CH2)~ group wherein R2', A and n are as hereinbefore defined,
a phenyl-(CH2)m-D-C,_3-alkyl group wherein the phenyl moiety is substituted
by the groups R'° to R'4, where R'° to R'4, D and m are as
mentioned
hereinbefore,
a naphthyl-(CH2)m-D-C,_3-alkyl group wherein the naphthyl moiety is
substituted by the groups R'° to R'4, where R'° to R'4, D and m
are as
mentioned hereinbefore,
a C~_6-alkyl group substituted by a group Rb, where Rb is as hereinbefore
defined,
a cyano, carboxy, C~_3-alkyloxy-carbonyl, aminocarbonyl, C,.3-alkylamino-
carbonyl, di-(C,_3-alkyl)-amino-carbonyl, pyrrolidin-1-ylcarbonyl, piperidin-1-
ylcarbonyl, morpholin-4-ylcarbonyl, piperazin-1-ylcarbonyl, 4-methylpiperazin-
1-ylcarbonyl or 4-ethylpiperazin-1-ylcarbonyl group,
CA 02496211 2005-02-18
11
an amino, C~_s-alkylamino or di-(C,_s-alkyl)-amino group,
an amino group substituted by the groups R'S and R's wherein
R'S denotes a hydrogen atom or a C,_s-alkyl group and
R's denotes a C~_s-alkyl group which is substituted by Ra, where Ra is
as hereinbefore defined,
an amino group substituted by the groups R'S and R" wherein
R'S is as hereinbefore defined and
R" denotes a C2_s-alkyl group which is substituted by a hydroxy, C~_3-
alkyloxy, aryloxy, mercapto, C,_3-alkylsulphanyl, C~_3-alkylsulphinyl,
C,_3-alkylsulphonyl, C,_3-alkylsulphonylamino, arylsulphanyl,
arylsulphinyl, arylsulphonyl, arylsulphonylamino, C,_3-alkyl-
carbonylamino, C3_s-cycloalkyl-carbonylamino, arylcarbonylamino,
C,_3-alkyl-oxycarbonylamino, aminocarbonylamino, C,_3-alkyl-amino-
carbonylamino, di-(C~_3-alkyl)-aminocarbonylamino, amino, C~_3-alkyl-
amino, di-(C~_3-alkyl)-amino, pyrrolidin-1-yl, piperidin-1-yl, morpholin-4-
yl, piperazin-1-yl or 4-(C,_3-alkyl)-piperazin-1-yl group,
a C3_s-cycloalkylamino or N-(C3_s-cycloalkyl)-N-(C,_3-alkyl)-amino group,
a phenylamino or N-(phenyl)-N-(C,_3-alkyl)-amino group wherein the phenyl
moiety is substituted in each case by R'° to R'4, where R'° to
R'4 are as
hereinbefore defined,
a phenyl-C,_s-alkylamino or N-(phenyl-C~_s-alkyl)-N-(C~_3-alkyl)-amino group
wherein the phenyl moiety is substituted in each case by R'° to R'4,
where R'°
to R'4 are as hereinbefore defined,
a naphthylamino or N-(naphthyl)-N-(C~_3-alkyl)-amino group,
CA 02496211 2005-02-18
12
a naphthyl-C,_6-alkylamino or N-(naphthyl-C,_6-alkyl)-N (C,_3-alkyl)-amino
group,
a heteroarylamino or N-(heteroaryl)-N-(C,_3-alkyl)-amino group,
a pyrrolidin-1-yl, piperidin-1-yl, homopiperidin-1-yl, morpholin-4-yl, homo-
morpholin-4-yl, piperazin-1-yl, 4-(C,_3-alkyl)-piperazin-1-yl, homopiperazin-1-
yl
or 4-(C,_3-alkyl)-homopiperazin-1-yl group, or
a C~_6-alkyloxy, C3_6-cycloalkyloxy or C3_6-cycloalkyl-C,_6-alkyloxy group,
a C,_s-alkylsulphanyl, C3_s-cycloalkylsulphanyl or C3_6-cycloalkyl-C,_6-alkyl-
sulphanyl group,
a phenyloxy or phenylsulphanyl group wherein the phenyl moiety is
substituted in each case by R'° to R'4, where R'° to R'4 are as
hereinbefore
defined,
a phenyl-C,_6-alkyloxy or phenyl-C,_6-alkylsulphanyl group wherein the phenyl
moiety is substituted in each case by R'° to R'4, where R'° to
R'4 are as
hereinbefore defined,
a naphthyloxy or a naphthylsulphanyl group wherein the naphthyl moiety is
substituted in each case by R'° to R'4, where R'° to R'4 are as
hereinbefore
defined,
a naphthyl-C~_6-alkyloxy or naphthyl-C,_s-alkylsulphanyl group wherein the
naphthyl moiety is substituted in each case by R'° to R'4, where
R'° to R'4 are
as hereinbefore defined,
a heteroaryloxy or heteroarylsulphanyl group or
a heteroaryl-C,_6-alkyloxy or heteroaryl-C,_6-alkylsulphanyl group,
CA 02496211 2005-02-18
13
R3 denotes a C~_S-alkyl group,
a C~_4-alkyl group substituted by the group R~, where
R~ denotes a C3_~-cycloalkyl group optionally substituted by one or two
C,-3-alkyl groups,
a C5_~-cycloalkenyl group optionally substituted by one or two C,_3-alkyl
groups,
an aryl group or
a furanyl, thienyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, pyridyl,
pyridazinyl, pyrimidyl or pyrazinyl group, while the above-mentioned
heterocyclic groups may each be substituted by one or two C~_3-alkyl
groups or by a fluorine, chlorine, bromine or iodine atom or by a
trifluoromethyl, cyano or C,_3-alkyloxy group,
a C3_e-alkenyl group,
a C3_6-alkenyl group substituted by a fluorine, chlorine or bromine atom or a
trifluoromethyl group,
a C3_S-alkynyl group,
an aryl group or
an aryl-CZ_4-alkenyl group,
and
CA 02496211 2005-02-18
14
R4 denotes an azetidin-1-yl or pyrrolidin-1-yl group which is substituted in
the
3 position by an amino, C,_3-alkylamino or di-(C1_3-alkyl)-amino group and may
additionally be substituted by one or two C~_3-alkyl groups,
a piperidin-1-yl or hexahydroazepin-1-yl group which is substituted in the 3
position or in the 4 position by an amino, C,_3-alkylamino or di-(C~_3-
alkyl)amino group and may additionally be substituted by one or two C1_3-alkyl
groups,
a 3-amino-piperidin-1-yl group wherein the piperidin-1-yl-moiety is
additionally
substituted by an aminocarbonyl, C,_z-alkyl-aminocarbonyl, di-(C,_z-alkyl)-
aminocarbonyl, pyrrolidin-1-yl-carbonyl, (2-cyano-pyrrolidin-1-yl-)carbonyl,
thiazolidin-3-yl-carbonyl, (4-cyano-thiazolidin-3-yl)carbonyl, piperidin-1-
ylcarbonyl or morpholin-4-ylcarbonyl group,
a 3-amino-piperidin-1-yl group wherein the piperidin-1-yl moiety is
additionally
substituted in the 4 position or 5 position by a hydroxy or methoxy group,
a 3-amino-piperidin-1-yl group wherein the methylene group is replaced in the
2 position or 6 position by a carbonyl group,
a piperidin-1-yl or hexahydroazepin-1-yl- group substituted in the 3 position
by
an amino, C,_3-alkylamino or di-(C,_3-alkyl)-amino group, wherein two
hydrogen atoms on the carbon skeleton of the piperidin-1-yl or
hexahydroazepin-1-yl group are each replaced by a straight-chain alkylene
bridge, this bridge containing 2 to 5 carbon atoms if the two hydrogen atoms
are on the same carbon atom, or 1 to 4 carbon atoms if the hydrogen atoms
are on adjacent carbon atoms, or 1 to 4 carbon atoms if the hydrogen atoms
are on carbon atoms which are separated by one atom, or 1 to 3 carbon
atoms if the hydrogen atoms are on carbon atoms which are separated by two
atoms,
CA 02496211 2005-02-18
an azetidin-1-yl, pyrrolidin-1-yl, piperidin-1-yl or hexahydroazepin-1-yl
group
which is substituted by an amino-C~_3-alkyl, C~_3-alkylamino-C~_3-alkyl or a
di-
(C~_3-alkyl)amino-C~_3-alkyl group,
a piperazin-1-yl or [1,4]diazepan-1-yl group optionally substituted on the
carbon skeleton by one or two C,_3-alkyl groups,
a 3-imino-piperazin-1-yl, 3-imino-[1,4]diazepan-1-yl or5-imino-[1,4]diazepan-
1-yl group optionally substituted on the carbon skeleton by one or two C,_3-
alkyl groups,
a [1,4]diazepan-1-yl group optionally substituted by one or two C~_3-alkyl
groups, which is substituted in the 6 position by an amino group,
a C3_~-cycloalkyl group which is substituted by an amino, C,_3-alkylamino or
di-(C,_3-alkyl)-amino group,
a C3_~-cycloalkyl group which is substituted by an amino-C~_3-alkyl,
C~_3-alkylamino-C~_3-alkyl or a di-(C,_3-alkyl)amino-C,_3-alkyl group,
a C3_~-cycloalkyl-C,_2-alkyl group wherein the cycloalkyl moiety is
substituted
by an amino, C,_3-alkylamino or di-(C~_3-alkyl)-amino group,
a C3_~-cycloalkyl-C,_Z-alkyl group wherein the cycloalkyl moiety is
substituted
by an amino-C~_3-alkyl, C~_3-alkylamino-C,_3-alkyl or a di-(C~_3-alkyl)amino-
C,.3-
alkyl group,
a C3_~-cycloalkylamino group wherein the cycloalkyl moiety is substituted by
an amino, C,_a-alkylamino or di-(C,_3-alkyl)-amino group, while the two
nitrogen atoms on the cycloalkyl moiety are separated from one another by at
least two carbon atoms,
an N-(C3_,-cycloalkyl)-!V-(C~_3-alkyl)-amino group wherein the cycloalkyl
moiety
is substituted by an amino, C~_3-alkylamino or di-(C~_3-alkyl)-amino group,
CA 02496211 2005-02-18
16
while the two nitrogen atoms on the cycloalkyl moiety are separated from one
another by at least two carbon atoms,
a C3_~-cycloalkylamino group wherein the cycloalkyl moiety is substituted by
an amino-C,_3-alkyl, C~_3-alkylamino-C,_3-alkyl or a di-(C~_3-alkyl)amino-C~_3-
alkyl group,
an N-(C3_~-cycloalkyl)-N-(C~_3-alkyl)-amino group wherein the cycloalkyl
moiety
is substituted by an amino-C~_3-alkyl, C,_3-alkylamino-C,_3-alkyl or a di-
(C~_3-
alkyl)amino-C~_3-alkyl group,
a C3_~-cycloalkyl-C,_2-alkyl-amino group wherein the cycloalkyl moiety is
substituted by an amino, C,_3-alkylamino or di-(C~_3-alkyl)-amino group,
an N-(C3_~-cycloalkyl-C~_2-alkyl)-N-(C,_2-alkyl)-amino group wherein the
cycloalkyl moiety is substituted by an amino, C,_3-alkylamino or di-(C~_3-
alkyl)-
amino group,
a C3_~-cycloalkyl-C~_2-alkyl-amino group wherein the cycloalkyl moiety is
substituted by an amino-C~_3-alkyl, C,_3-alkylamino-Ci_3-alkyl or a di-(C~_3-
alkyl)amino-C,_3-alkyl group,
an N-(C3_~-cycloalkyl-C,_2-alkyl)-N-(C,_2-alkyl)-amino group wherein the
cycloalkyl moiety is substituted by an amino-C,_3-alkyl, C~_3-alkylamino-C,_3-
alkyl or a di-(C,_3-alkyl)amino-C~_3-alkyl group,
an R'9-CZ_4-alkylamino group wherein R'9 is separated from the nitrogen atom
of the C2_4-alkylamino moiety by at least two carbon atoms and
R'9 denotes an amino, C~_3-alkylamino or di-(C,_3-alkyl)-amino group,
an R'9-C2_4-alkylamino group wherein the nitrogen atom of the C2_4-alkylamino
moiety is substituted by a C~_3-alkyl group and R'9 is separated from the
CA 02496211 2005-02-18
17
nitrogen atom of the C2_4-alkylamino moiety by at least two carbon atoms,
where R'9 is as hereinbefore defined,
an amino group substituted by the group Rz° wherein
R2° denotes an azetidin-3-yl, azetidin-2-ylmethyl, azetidin-3-
ylmethyl,
pyrrolidin-3-yl, pyrrolidin-2-ylmethyl, pyrrolidin-3-ylmethyl, piperidin-3-yl,
piperidin-4-yl, piperidin-2-ylmethyl, piperidin-3-ylmethyl or piperidin-4-
ylmethyl group, while the groups mentioned for R2° may each be
substituted by one or two C,_3-alkyl groups,
an amino group substituted by the group R2° and a C,_3-alkyl group
wherein
RZ° is as hereinbefore defined, while the groups mentioned for
R2° may each
be substituted by one or two C~_3-alkyl groups,
an R'9-C3_4-alkyl group wherein the C3_4-alkyl moiety is straight-chained and
may additionally be substituted by one or two C,_3-alkyl groups, where R'9 is
as hereinbefore defined,
a 3-amino-2-oxo-piperidin-5-yl or 3-amino-2-oxo-1-methyl-piperidin-5-yl group,
a pyrrolidin-3-yl, piperidin-3-yl, piperidin-4-yl, hexahydroazepin-3-yl or
hexahydroazepin-4-yl group which is substituted in the 1 position by an
amino, C~_3-alkylamino or di-(C,_3-alkyl)amino group,
or an azetidin-2-yl-C~_Z-alkyl, azetidin-3-yl-C~_2-alkyl, pyrrolidin-2-yl-C,_2-
alkyl,
pyrrolidin-3-yl, pyrrolidin-3-yl-C~_2-alkyl, piperidin-2-yl-C~_Z-alkyl,
piperidin-3-yl,
piperidin-3-yl-C~_2-alkyl, piperidin-4-yl or piperidin-4-yl-C,_2-alkyl group,
while
the above-mentioned groups may each be substituted by one or two C~_3-alkyl
groups,
while by the aryl groups mentioned in the definition of the above groups are
meant phenyl or naphthyl groups, which may be mono- or disubstituted by Rh
independently of one another, where the substituents are identical or
different
CA 02496211 2005-02-18
18
and Rh denotes a fluorine, chlorine, bromine or iodine atom, a
trifluoromethyl,
cyano, nitro, amino, aminocarbonyl, aminosulphonyl, methylsulphonyl,
acetylamino, methylsulphonylamino, C~_3-alkyl, cyclopropyl, ethenyl, ethynyl,
hydroxy, C~_3-alkyloxy, difluoromethoxy or trifluoromethoxy group,
by the heteroaryl groups mentioned in the definitions of the above-mentioned
groups are meant a pyrrolyl, furanyl, thienyl, pyridyl, indolyl, benzofuranyl,
benzothiophenyl, quinolinyl or isoquinolinyl group,
or a pyrrolyl, furanyl, thienyl or pyridyl group wherein one or two methyne
groups are replaced by nitrogen atoms,
or an indolyl, benzofuranyl, benzothiophenyl, quinolinyl or isoquinolinyl
group
wherein one to three methyne groups are replaced by nitrogen atoms,
ora 1,2-dihydro-2-oxo-pyridinyl, 1,4-dihydro-4-oxo-pyridinyl, 2,3-dihydro-3-
oxo-pyridazinyl, 1,2,3,6-tetrahydro-3,6-dioxo-pyridazinyl, 1,2-dihydro-2-oxo-
pyrimidinyl, 3,4-dihydro-4-oxo-pyrimidinyl, 1,2,3,4-tetrahydro-2,4-dioxo-
pyrimidinyl, 1,2-dihydro-2-oxo-pyrazinyl, 1,2,3,4-tetrahydro-2,3-dioxo-
pyrazinyl, 2,3-dihydro-2-oxo-indolyl, 2,3-dihydrobenzofuranyl, 2,3-dihydro-2-
oxo-1H-benzimidazolyl, 2,3-dihydro-2-oxo-benzoxazolyl, 1,2-dihydro-2-oxo-
quinolinyl, 1,4-dihydro-4-oxo-quinolinyl, 1,2-dihydro-1-oxo-isoquinolinyl, 1,4-
dihydro-4-oxo-cinnolinyl, 1,2-dihydro-2-oxo-quinazolinyl, 3,4-dihydro-4-oxo-
quinazolinyl, 1,2,3,4-tetrahydro-2,4-dioxo-quinazolinyl, 1,2-dihydro-2-
oxoquinoxalinyl, 1,2,3,4-tetrahydro-2,3-dioxo-quinoxalinyl, 1,2-dihydro-1-oxo-
phthalazinyl, 1,2,3,4-tetrahydro-1,4-dioxo-phthalazinyl, chromanyl, cumarinyl,
2,3-dihydro-benzo[1,4]dioxinyl or 3,4-dihydro-3-oxo-2H-benzo[1,4]oxazinyl
group,
while the above-mentioned heteroaryl groups may be substituted by
R'° to R", where R'° to R'4 are as hereinbefore defined,
and, unless othenrvise stated, the above-mentioned alkyl, alkenyl and alkynyl
groups may be straight-chain or branched,
CA 02496211 2005-02-18
19
as well as the derivatives which are N-oxidised or methylated or ethylated at
the cyclic nitrogen atom in the 3 position or 9 position of the hypoxanthine
skeleton,
as well as the derivatives wherein the 6-oxo group of the hypoxanthine
skeleton is replaced by a thioxo group,
with the proviso that the compounds
8-(piperidin-4-ylmethyl)-7-(4-fluorobenzyl)-1,7-dihydro-purin-6-one and
8-(1-methyl-piperidin-4-ylmethyl)-7-(4-fluorobenzyl)-1,7-dihydro-purin-6-one
are excluded,
the tautomers, enantiomers, diastereomers, the mixtures thereof, the
prodrugs thereof and the salts thereof.
Compounds which contain a group that can be cleaved in vivo are prodrugs of
the corresponding compounds wherein this group that can be cleaved in vivo
has been cleaved.
The carboxy groups mentioned in the definition of the above-mentioned
groups may be replaced by a group which can be converted into a carboxy
group in vivo or by a group which is negatively charged under physiological
conditions,
and furthermore the amino and imino groups mentioned in the definition of the
above-mentioned groups may be substituted by a group which can be cleaved
in vivo. Such groups are described for example in WO 98146576 and by N.M.
Nielsen et al. in International Journal of Pharmaceutics 39, 75-85 (1987).
CA 02496211 2005-02-18
By a group which can be converted in vivo into a carboxy group is meant, for
example, a hydroxymethyl group, a carboxy group esterified with an alcohol
wherein the alcohol moiety is preferably a C,_6-alkanol, a phenyl-C,_3-
alkanol,
a C3_9-cycloalkanol, while a C5_$-cycloalkanol may additionally be substituted
by one or two C,_3-alkyl groups, a C5_s-cycloalkanol wherein a methylene
group in the 3 or 4 position is replaced by an oxygen atom or by an imino
group optionally substituted by a C,_3-alkyl, phenyl-C~_3-alkyl, phenyl-
C~_3-alkyloxycarbonyl or CZ_6-alkanoyl group and the cycloalkanol moiety may
additionally be substituted by one or two C~_3-alkyl groups, a C4_~-
cycloalkenol,
a C3_5-alkenol, a phenyl-C3_5-alkenol, a C3_5-alkynol or phenyl-C3_5-alkynol
with
the proviso that no bonds to the oxygen atom start from a carbon atom which
carries a double or triple bond, a C3_a-cycloalkyl-C,_3-alkanol, a
bicycloalkanol
with a total of 8 to 10 carbon atoms which may additionally be substituted in
the bicycloalkyl moiety by one or two C~_3-alkyl groups, a 1,3-dihydro-3-oxo-1-
isobenzofuranol or an alcohol of formula
RP-CO-O-(RqCR~)-OH,
wherein
RP denotes a C,_$-alkyl, C5_~-cycloalkyl, C,_8-alkyloxy, C5_~-
cycloalkyloxy, phenyl or phenyl-C,_3-alkyl group,
Rq denotes a hydrogen atom, a C,_3-alkyl, C5_~-cycloalkyl or phenyl
group and
R~ denotes a hydrogen atom or a C~_3-alkyl group,
by a group which is negatively charged under physiological conditions is
meant, for example, a tetrazol-5-yl, phenylcarbonylaminocarbonyl,
trifluoromethylcarbonylaminocarbonyl, C~_6-alkylsulphonylamino,
phenylsulphonylamino, benzylsulphonylamino, trifluoromethylsulphonylamino,
C,_6-alkylsulphonylaminocarbonyl, phenylsulphonylaminocarbonyl,
CA 02496211 2005-02-18
21
benzylsulphonylaminocarbonyl or perfluoro-C,_6-alkylsulphonylaminocarbonyl
group
and by a group which can be cleaved in vivo from an imino or amino group is
meant, for example, a hydroxy group, an acyl group such as a phenylcarbonyl
group optionally mono- or disubstituted by fluorine, chlorine, bromine or
iodine
atoms, by C~_3-alkyl or C~_3-alkyloxy groups, while the substituents may be
identical or different, a pyridinoyl group or a C,_,6-alkanoyl group such as
the
formyl, acetyl, propionyl, butanoyl, pentanoyl or hexanoyl group, a 3,3,3-
trichloropropionyl or allyloxycarbonyl group, a C~_~s-alkyloxycarbonyl or
C,_,6-alkylcarbonyloxy group, wherein hydrogen atoms may be wholly or
partially replaced by fluorine or chlorine atoms such as the methoxycarbonyl,
ethoxycarbonyl, propoxycarbonyl, isopropoxycarbonyl, butoxycarbonyl,
tert.butoxycarbonyl, pentoxycarbonyl, hexoxycarbonyl, octyloxycarbonyl,
nonyloxycarbonyl, decyloxycarbonyl, undecyloxycarbonyl,
dodecyloxycarbonyl, hexadecyloxycarbonyl, methylcarbonyloxy,
ethylcarbonyloxy, 2,2,2-trichloroethylcarbonyloxy, propylcarbonyloxy,
isopropylcarbonyloxy, butylcarbonyloxy, tert.butylcarbonyloxy,
pentylcarbonyloxy, hexylcarbonyloxy, octylcarbonyloxy, nonylcarbonyloxy,
decylcarbonyloxy, undecylcarbonyloxy, dodecylcarbonyloxy or
hexadecylcarbonyloxy group, a phenyl-C~_s-alkyloxycarbonyl group such as
the benzyloxycarbonyl, phenylethoxycarbonyl or phenylpropoxycarbonyl
group, a 3-amino-propionyl group wherein the amino group may be mono- or
disubstituted by C,_6-alkyl or C3_~-cycloalkyl groups and the substituents may
be identical or different, a C~_3-alkylsulphonyl-C2_4-alkyloxycarbonyl,
C,_3-alkyloxy-C2_4-alkyloxy-CZ_4-alkyloxycarbonyl, RP CO-O-(RqCR~)-O-CO,
C~_6-alkyl-CO-NH-(RSCRt)-O-CO- or C,_6-alkyl-CO-O-(RSCR,)-(RSCRt)-O-CO-
group, wherein RP to R~ are as hereinbefore defined,
RS and R~, which may be identical or different, denote hydrogen atoms or
C~_3-alkyl groups.
Moreover, the saturated alkyl and alkyloxy moieties which contain more than
2 carbon atoms mentioned in the foregoing definitions and those that follow,
CA 02496211 2005-02-18
22
unless otherwise stated, also include the branched isomers thereof such as,
for example, the isopropyl, tert.butyl, isobutyl group, etc.
One sub-group deserving special mention relates to those compounds of
general formula I wherein R', R2 and R3 are as hereinbefore defined and
R4 denotes a pyrrolidin-1-yl group which is substituted in the 3 position by
an
amino group,
a piperidin-1-yl group which is substituted in the 3 position by an amino
group,
a piperidin-3-yl or piperidin-4-yl group,
a hexahydroazepin-1-yl group which is substituted in the 3 position or 4
position by an amino group,
a piperazin-1-yl or [1,4]diazepan-1-yl group,
a (2-aminocyclohexyl)amino group,
a cyclohexyl group which is substituted in the 3 position by an amino group,
or an N-(2-aminoethyl)-methylamino or an N-(2-aminoethyl)-ethylamino group,
the tautomers, enantiomers, diastereomers, the mixtures thereof, the
prodrugs and the salts thereof.
Preferred compounds of the above general formula I are those wherein
R' denotes a hydrogen atom,
a C~_6-alkyl group,
CA 02496211 2005-02-18
23
a C3_6-alkenyl group,
a C3_4-alkynyl group,
a C3_6-cycloalkylmethyl group,
a phenyl-C~_3-alkyl group wherein the phenyl moiety is substituted by
R'° and
R" , where
R'° denotes a hydrogen atom, a fluorine, chlorine or bromine atom,
a methyl or trifluoromethyl group,
a cyano, aminocarbonyl, dimethylaminocarbonyl or methylsulphonyl
group,
an amino, acetylamino or methylsulphonylamino group,
a hydroxy, methoxy, difluoromethoxy, trifluoromethoxy,
carboxymethoxy, methoxycarbonylmethoxy, ethyloxycarbonylmethoxy,
aminocarbonylmethoxy, methylaminocarbonylmethoxy, ethylamino-
carbonylmethoxy or dimethylaminocarbonylmethoxy group and
R" denotes a hydrogen atom, a fluorine or chlorine atom,
or a methyl or methoxy group,
a naphthylmethyl group wherein the naphthyl moiety is substituted by
R'° and
R", where R'° and R" are as hereinbefore defined,
a heteroarylmethyl group where the term
heteroaryl denotes a furanyl, thienyl, oxazolyl, isoxazolyl, thiazolyl,
pyridyl, pyrimidinyl, pyrazinyl, quinolinyl, isoquinolinyl or quinazolinyl
CA 02496211 2005-02-18
24
group and the above-mentioned heteroaryl groups are substituted by
R'° and R", where R'° and R" are as hereinbefore defined,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by
R'°
and R", where R'° and R" are as hereinbefore defined,
a furanylcarbonylmethyl, thienylcarbonylmethyl or pyridylcarbonylmethyl
group,
or a 2-oxo-propyl or cyclohexylcarbonylmethyl group,
Rz denotes a hydrogen atom,
a C~_6-alkyl group,
a C3_6-alkenyl group,
a C3_4-alkynyl group,
a C3_6-cycloalkyl or C3_6-cycloalkyl-C,_3-alkyl group,
a phenyl group which is substituted by R'° and R", where R'° and
R" are as
hereinbefore defined,
a phenyl-C,_3-alkyl group wherein the phenyl moiety is substituted by
R'° and
R", where R'° and R" are as hereinbefore defined,
a phenyl-C2_3-alkenyl group wherein the phenyl moiety is substituted by R'o
and R", where R'° and R" are as hereinbefore defined,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by
R'°
and R", where R'° and R" are as hereinbefore defined,
a furanyl, thienyl or pyridyl group,
CA 02496211 2005-02-18
a furanyl-C~_3-alkyl, thienyl-C~_3-alkyl or pyridyl-C~_3-alkyl group,
a cyano group,
an amino, C,_4-alkylamino or di-(C,_4-alkyl)-amino group,
an amino group substituted by the groups R'S and R'6 wherein
R'S denotes a hydrogen atom or a methyl or ethyl group and
R'6 denotes a C~_4-alkyl group which is substituted by a cyano,
carboxy, methoxycarbonyl, ethyloxycarbonyl, aminocarbonyl,
methylaminocarbonyl, dimethylaminocarbonyl, ethylaminocarbonyl,
diethylaminocarbonyl, pyrrolidin-1-ylcarbonyl or morpholin-4-ylcarbonyl
group,
an amino group substituted by the groups R'S and R" wherein
R'S is as hereinbefore defined and
R" denotes a straight-chain C2_4-alkyl group which is terminally
substituted in each case by an amino, methylamino, dimethylamino,
acetylamino, ethyloxycarbonylamino, phenylcarbonylamino,
methylsulphonylamino, phenylsulphonylamino, hydroxy, methoxy,
phenyloxy, methylsulphanyl or phenylsulphanyl group,
a pyrrolidin-1-yl, piperidin-1-yl, morpholin-4-yl, piperazin-1-yl or 4-methyl-
piperazin-1-yl group,
a C3_s-cycloalkylamino or C3_6-cycloalkyl-C~_3-alkylamino group,
a phenylamino group,
CA 02496211 2005-02-18
26
a phenyl-C~_3-alkylamino group wherein the phenyl moiety is substituted by
R'° and R", where R'° and R" are as hereinbefore defined,
a naphthylmethylamino group,
a heteroaryl-C~_2-alkylamino group, where the term heteroaryl is as
hereinbefore defined, or
a methylsulphanyl, benzylsulphanyl or (2-phenylethyl)sulphanyl group,
R3 denotes a C4_6-alkenyl group,
a C3_a-alkenyl group which is substituted by a fluorine, chlorine or bromine
atom or a trifluoromethyl group,
a 2-butyn-1-yl group or
a methyl group substituted by the group R~, where
R~ denotes a 1-cyclopenten-1-yl-or 1-cyclohexen-1-yl group,
a phenyl group optionally substituted by a fluorine, chlorine, bromine or
iodine atom, by a methyl, trifluoromethyl, cyano, methoxy,
difluoromethoxy or trifluoromethoxy group,
a phenyl group which is substituted by two fluorine atoms,
a naphthyl group or
a furanyl, thienyl, or pyridyl group,
and
CA 02496211 2005-02-18
27
R° denotes a piperidin-1-yl group which is substituted in the 3
position by an
amino group,
a hexahydroazepin-1-yl group which is substituted in the 3 position or 4
position by an amino group,
a (2-aminocyclohexyl)amino group,
a cyclohexyl group which is substituted in the 3 position by an amino group,
or
an N-(2-aminoethyl)-methylamino or an N-(2-aminoethyl)-ethylamino group,
while unless otherwise stated, the above-mentioned alkyl, alkenyl and alkynyl
groups may be straight-chain or branched,
the tautomers, enantiomers, diastereomers, the mixtures thereof and the salts
thereof.
Particularly preferred compounds of the above general formula I are those
wherein
R' denotes a hydrogen atom,
a methyl, benzyl or 2-phenylethyl group,
a naphthylmethyl or methoxynaphthylmethyl group or
a phenylcarbonylmethyl group,
R2 denotes a hydrogen atom,
a methyl or 2-phenylethyl group,
a phenylcarbonylmethyl group,
CA 02496211 2005-02-18
28
a cyano group,
an amino, methylamino, dimethylamino, isopropylamino, cyclohexylamino-
or (cyclohexylmethyl)amino group,
a benzylamino, fluorobenzylamino or (2-phenylethyl)amino group or
a piperidin-1-yl group,
R3 denotes a benzyl or 3-methyl-but-2-en-1-yl group
and
R4 denotes a (3-amino-piperidin-1-yl) group,
the tautomers, enantiomers, diastereomers, the mixtures thereof and the salts
thereof,
but particularly the compounds
(1) 8-(3-amino-piperidin-1-yl)-7-benzyl-2-benzylamino-1-methyl-1,7-
dihydro-purin-6-one,
(2) 8-(3-amino-piperidin-1-yl)-7-benzyl-2-(4-fluoro-benzylamino)-1-methyl-
1,7-dihydro-purin-6-one,
(3) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(2-phenylethyl)aminoJ-
1,7-dihydro-purin-6-one,
(4) 8-(3-amino-piperidin-1-yl)-7-benzyl-2-isopropylamino-1-methyl-1,7-
dihydro-purin-6-one,
CA 02496211 2005-02-18
29
(5) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-methylamino-1,7-
dihydro-purin-6-one,
(6) 8-(3-amino-piperidin-1-yl)-7-benzyl-2-cyclohexylamino-1-methyl-1,7-
dihydro-purin-6-one,
(7) 8-(3-amino-piperidin-1-yl)-7-benzyl-2-[(cyclohexylmethyl)amino]-1-
methyl-1,7-dihydro-purin-6-one,
(8) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(piperidin-1-yl)-1,7-
dihydro-purin-6-one,
(9) 8-(3-amino-piperidin-1-yl)-7-benzyl-2-dimethylamino-1-methyl-1,7-
dihydro-purin-6-one,
(10) 2-amino-8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-1,7-dihydro-
purin-6-one,
(11) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(3-methyl-but-2-
en-1-yl)-1,7-dihydro-purin-6-one,
(12) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-methyl-1,7-dihydro-
purin-6-one,
(13) 8-(3-amino-piperidin-1-yl)-1-methyl-7-(3-methyl-but-2-en-1-yl)-2-(2-
phenylethyl)-1,7-dihydro-purin-6-one,
(14) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-1,7-dihydro-purin-6-one,
(15) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(2-oxo-2-phenyl-ethyl)-1,7-
dihydro-purin-6-one,
(16) 8-(3-amino-piperidin-1-yl)-2-methyl-7-(3-methyl-but-2-en-1-yl)-1-
[(naphthalen-1-yl)methyl]-1,7-dihydro-purin-6-one,
30
(17) 8-(3-amino-piperidin-1-yl)-7-(3-methyl-but-2-en-1-yl)-1-[(naphthalen-1-
yl)methyl]-1,7-dihydro-purin-6-one and
(18) 8-(3-amino-piperidin-1-yl)-7-(3-methyl-but-2-en-1-yl)-1-[(4-methoxy-
naphthalen-1-yl)methyl]-1,7-dihydro-purin-6-one
as well as the tautomers, enantiomers, diastereomers, the mixtures thereof
and the salts thereof.
The compounds of general formula I can be prepared by deprotecting a
compound of general formula
O Rs
R1 ~ N N
R4'
N
R2 N
(II),
wherein R', R2 and R3 are as hereinbefore defined and
R4' denotes one of the groups mentioned hereinbefore for R4 which contain
an imino, amino or alkylamino group, while the imino, amino or alkylamino
group is substituted by a protective group, optionally followed by subsequent
alkylation of the imino, amino or C~_3-alkylamino group.
The liberating of an amino group from a protected precursor is a standard
reaction in synthetic organic chemistry. There are many examples of suitable
protective groups. A summary of the chemistry of protective groups can be
found in Theodora W.Greene and Peter G.M.Wuts, Protective Groups in
Organic Synthesis, Second Edition, 1991, published by John Wiley and Sons,
and in Philip J. Kocienski, Protecting Groups, published by Georg Thieme,
1994.
The following are examples of protective groups:
CA 02496211 2005-02-18
CA 02496211 2005-02-18
31
the tert.-butyloxycarbonyl group which can be cleaved by treating with an acid
such as for example trifluoroacetic acid or hydrogen chloride in the presence
of a solvent such as for example methylene chloride, ethyl acetate or dioxane
at temperatures between 0°C and the boiling temperature of the solvent
used,
the 2,2,2-trichloroethoxycarbonyl group which can be cleaved by treating with
metals such as for example zinc or cadmium in a solvent such as acetic acid
or a mixture of tetrahydrofuran and a weak aqueous acid at temperatures
between 0°C and the boiling temperature of the solvent used and
the carbobenzyloxycarbonyl group which can be cleaved for example by
hydrogenolysis in the presence of a noble metal catalyst such as for example
palladium-charcoal and a solvent such as for example alcohols, ethyl acetate,
dioxane, tetrahydrofuran or mixtures of these solvents at temperatures
between 0°C and the boiling point of the solvent, by treating with
boron
tribromide in methylene chloride at temperatures between -20°C and
ambient
temperature, or by treating with aluminium chloridelanisol at temperatures
between 0°C and ambient temperature.
The optional subsequent introduction of a C,_3-alkyl group may be done by
alkylation or reductive alkylation.
The subsequent alkylation is optionally carried out in a solvent or mixture of
solvents such as methylene chloride, dimethylformamide, benzene, toluene,
chlorobenzene, tetrahydrofuran, benzeneltetrahydrofuran or dioxane with an
alkylating agent such as a corresponding halide or sulphonic acid ester, e.g.
with methyl iodide, ethyl bromide, dimethyl sulphate, optionally in the
presence of a tertiary organic base or in the presence of an inorganic base,
conveniently at temperatures between 0 and 150°C, preferably at
temperatures between 0 and 100°C.
The subsequent reductive alkylation is carried out with a corresponding
carbonyl compound such as formaldehyde, acetaldehyde, propionaldehyde or
CA 02496211 2005-02-18
32
acetone in the presence of a complex metal hydride such as sodium
borohydride, lithium borohydride, sodium triacetoxyborohydride or sodium
cyanoborohydride, conveniently at a pH of 6-7 and at ambient temperature or
in the presence of a hydrogenation catalyst, e.g. with hydrogen in the
presence of palladiumlcharcoal, under a hydrogen pressure of 1 to 5 bar. The
methylation may also be carried out in the presence of formic acid as reducing
agent at elevated temperatures, e.g. at temperatures between 60 and
120°C.
The compounds of general formula I obtained may be resolved into their
enantiomers andlor diastereomers. Thus, for example, cisltrans mixtures may
be resolved into their cis and trans isomers, and compounds with at least one
optically active carbon atom may be separated into their enantiomers.
Thus, for example, the cisltrans mixtures may be resolved by chromatography
into the cis and trans isomers thereof, the compounds of general formula I
obtained which occur as racemates may be separated by methods known per
se (cf. Allinger N. L. and Eliel E. L. in "Topics in Stereochemistry", Vol. 6,
Wiley Interscience, 1971) into their optical antipodes and compounds of
general formula I with at least 2 asymmetric carbon atoms may be resolved
into their diastereomers on the basis of their physical-chemical differences
using methods known per se, e.g. by chromatography andlor fractional
crystallisation, and, if these compounds are obtained in racemic form, they
may subsequently be resolved into the enantiomers as mentioned above.
The enantiomers are preferably separated by column separation on chiral
phases or by recrystallisation from an optically active solvent or by reacting
with an optically active substance which forms salts or derivatives such as
e.g. esters or amides with the racemic compound, particularly acids and the
activated derivatives or alcohols thereof, and separating the diastereomeric
mixture of salts or derivatives thus obtained, e.g. on the basis of their
differences in solubility, whilst the free antipodes may be released from the
pure diastereomeric salts or derivatives by the action of suitable agents.
Optically active acids in common use are e.g. the D- and L-forms of tartaric
acid or dibenzoyltartaric acid, di-o-tolyltartaric acid, malic acid, mandelic
acid,
CA 02496211 2005-02-18
33
camphorsulphonic acid, glutamic acid, aspartic acid or quinic acid. An
optically active alcohol may be for example (+) or (-)-menthol and an
optically
active acyl group in amides, for example, may be a (+)-or
(-)-menthyloxycarbonyl.
Furthermore, the compounds of formula I obtained may be converted into the
salts thereof, particularly for pharmaceutical use into the physiologically
acceptable salts with inorganic or organic acids. Acids which may be used for
this purpose include for example hydrochloric acid, hydrobromic acid,
sulphuric acid, methane sulphonic acid, phosphoric acid, fumaric acid,
succinic acid, lactic acid, citric acid, tartaric acid or malefic acid.
Moreover, if the new compounds of formula I thus obtained contain a carboxy
group, they may subsequently, if desired, be converted into the salts thereof
with inorganic or organic bases, particularly for pharmaceutical use into the
physiologically acceptable salts thereof. Suitable bases for this purpose
include for example sodium hydroxide, potassium hydroxide, arginine,
cyclohexylamine, ethanolamine, diethanolamine and triethanolamine.
The starting compounds of general formula I I may be prepared by generally
known methods and according to processes described for example in
Examples I to XIV.
Thus, compounds of general formula II wherein R', R3 and R4~ are as
hereinbefore defined and R2 denotes a hydrogen atom, may be prepared for
example by reacting a compound of formula
O R3
C~_3 Alkyl ~
O N
~>-- R4,
H2N N
(III),
CA 02496211 2005-02-18
34
with a formimido-C,_3-alkyl ester, optionally followed by alkylation at N-1
with
a suitable alkylating agent.
Compounds of general formula II wherein R', R3 and R4~ are as hereinbefore
defined and RZ denotes an alkylsulphanyl, an alkenylsulphanyl or alkynyl-
sulphanyl group may be obtained for example by reacting compounds of
general formula III with a suitable isothiocyanate and subsequently cyclising
them to form compounds of general formula
R3
RAN ,j 'N
~~ R4,
S N N
(IV).
followed by alkylation of the sulphur atom.
If an acyl mustard oil is used, such as for example
ethoxycarbonylisothiocyanate, first of all compounds of general formula IV,
wherein R' denotes a hydrogen atom are obtained, which can be converted
into the desired compounds by subsequent alkylation at the sulphur atom and
at N-1.
Compounds of general formula II wherein R', R3 and R4~ are as hereinbefore
defined and R~ denotes an alkylsulphanyl group can be oxidised to form
compounds of formula II' wherein RZ' denotes an alkylsulphinyl or an
alkylsulphonyl group.
The above-mentioned compounds of general formula II' are starting materials
for preparing the following compounds of formula II.
Reaction with alcohols and phenols yields compounds wherein the group R2
is linked to the purine system via an oxygen atom,
CA 02496211 2005-02-18
Reaction with thiols and thiophenols leads to compounds wherein R2 is linked
to the purine system via a sulphur atom,
Reaction with amines leads to compounds wherein R2 is bound to the purine
system via a nitrogen atom and
Reaction with organometallic compounds such as for example Grignard
reagents, alkyl- or aryl-lithium compounds or reaction with CH-acid
compounds such as for example esters, nitrites or ketones leads to
compounds wherein R2 is linked to the purine system via a carbon atom.
Another method of obtaining compounds of general formula I I consists, for
example, in converting compounds of general formula
3
RAN NH
z ~N NHZ
R
(V),
wherein R', RZ and R3 are as hereinbefore defined, into compounds of
general formula II.
For example, compounds of general formula V are converted into compounds
of general formula II wherein the group R4~ is bound to the purine system via
a
nitrogen atom, by reacting with an orthoformate, subsequently brominating the
resulting purine at C-8 and then reacting with a corresponding amine.
For example, compounds of general formula V are converted into compounds
of general formula II wherein the group R4~ is bound to the C-8 atom of the
purine via a C-atom by reaction with a reactive derivative of a carboxylic
acid R4~-COOH, where R4' is as hereinbefore defined, and subsequent
cyclisation.
CA 02496211 2005-02-18
36
As already mentioned hereinbefore, the compounds of general formula I
according to the invention and the physiologically acceptable salts thereof
have valuable pharmacological properties, particularly an inhibiting effect on
the enzyme DPP-IV.
The biological properties of the new compounds were investigated as follows:
The ability of the substances and their corresponding salts to inhibit the DPP-
IV activity can be demonstrated in a test set-up in which an extract of human
colon carcinoma cell line Caco-2 is used as the DPP IV source. The
differentiation of the cells in order to induce the DPP-IV expression was
carried out as described by Reiher et al. in an article entitled "Increased
expression of intestinal cell line Caco-2" , which appeared in Proc. Natl.
Acad.
Sci. Vol. 90, pages 5757-5761 (1993). The cell extract was obtained from
cells solubilised in a buffer (10mM Tris HCI, 0.15 M NaCI, 0.04 t.i.u.
aprotinin,
0.5% Nonidet-P40, pH 8.0) by centrifuging at 35,000 g for 30 minutes at
4°C
(to remove cell debris).
The DPP-IV assay was carried out as follows:
50 pl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final
concentration 100 NM, were placed in black microtitre plates. 20 NI of assay
buffer (final concentrations 50 mM Tris HCI pH 7.8, 50 mM NaCI, 1 % DMSO)
was pipetted in. The reaction was started by adding 30 pl of solubilised Caco-
2 protein (final concentration 0.14 pg of protein per well). The test
substances
to be investigated were typically added prediluted in 20 pl, and the volume of
assay buffer was then reduced accordingly. The reaction was carried out at
ambient temperature, incubating for 60 minutes. Then the fluorescence was
measured in a Victor 1420 Multilabel Counter, the excitation wavelength being
405 nm and the emission wavelength being 535 nm. Blank readings
(corresponding to 0 % activity) were obtained in mixtures without any Caco-2
protein (volume replaced by assay buffer), control values (corresponding to
100 % activity) were obtained in mixtures with no substance added. The
potency of the test substances in question, expressed as ICSO values, was
CA 02496211 2005-02-18
37
calculated from dosagelactivity curves consisting of 11 measuring points in
each case.
The results obtained are shown in the following Table:
Compound DPP IV inhibition
(Example No.) ICso (nM)
1 11
1(1) 24
1 (2) 42
1(3) 110
1 (4) 58
1 (5) 134
1 (6) 48
1 (7) 434
1 (8) 213
1(9) 61
1(11) 54
1(12) 18
1(13) 152
1(14) 158
1 ( 15) 58
1 (22) 48
1 (23) 157
1(24) 113
1 (25) 275
1 (26) 40
1 (27) 19
1 (28) 57
The compounds prepared according to the invention are well tolerated, as for
example when 30 mg/kg of the compound of Example 1 were administered to
CA 02496211 2005-02-18
38
rats by oral route no toxic side effects or changes in the animals' behaviour
could be detected.
In view of their ability to inhibit DPP-IV activity, the compounds of general
formula I according to the invention and the corresponding pharmaceutically
acceptable salts thereof are suitable for treating all those conditions or
illnesses which can be influenced by the inhibition of the DPP-IV activity. It
is
therefore to be expected that the compounds according to the invention will
be suitable for the prevention or treatment of diseases or conditions such as
type 1 and type 2 diabetes mellitus, diabetic complications (such as e.g.
retinopathy, nephropathy or neuropathies), metabolic acidosis or ketosis,
reactive hypoglycaemia, insulin resistance, metabolic syndrome,
dyslipidaemias of various origins, arthritis, atherosclerosis and related
diseases, obesity, allograft transplantation and calcitonin-induced
osteoporosis. In addition these substances are capable of preventing B-cell
degeneration such as e.g. apoptosis or necrosis of pancreatic B-cells. The
substances are also suitable for improving or restoring the function of
pancreatic cells and also increasing the number and size of pancreatic B-
cells. Additionally, and on the basis of the role of the Glucagon-Like
Peptides,
such as e.g. GLP-1 and GLP-2 and their link with DPP-IV inhibition, it is
likely
that the compounds according to the invention are suitable for achieving,
inter
alia, a sedative or anxiety-relieving effect and also of favourably affecting
catabolic states after operations or hormonal stress responses or of reducing
mortality or morbidity after myocardial infarct. They are also suitable for
treating all conditions which are connected with the above-mentioned effects
and which are mediated by GLP-1 or GLP-2. The compounds according to the
invention may also be used as diuretics or antihypertensives and are suitable
for preventing and treating acute renal failure. Furthermore, the compounds
according to the invention may be used to treat inflammatory diseases of the
respiratory tract. They are also suitable for the prevention and treatment of
chronic inflammatory intestinal diseases such as e.g. irritable bowel syndrome
(IBS), Crohn's disease or ulcerative colitis and also pancreatitis. It is also
likely that they can be used for all kinds of damage to or impairment of the
gastrointestinal tract such as colitis and enteritis, for example. It is also
CA 02496211 2005-02-18
39
expected that DPP-IV inhibitors and hence also the compounds according to
the invention may be used to treat infertility or to improve fertility in
humans or
mammals, particularly when the infertility is connected with insulin
resistance
or polycystic ovary syndrome. On the other hand these substances are
suitable for affecting sperm motility and can thus be used as male
contraceptives. The substances are also suitable for treating deficiencies of
growth hormone which are associated with reduced stature, and may also be
used to advantage in any indications in which growth hormone may be used.
The compounds according to the invention are also suitable, on the basis of
their inhibitory effect on DPP IV, for treating various autoimmune diseases
such as e.g. rheumatoid arthritis, multiple sclerosis, thyroiditis and
Basedow's
disease, etc. They may also be used to treat viral diseases and also, for
example, in HIV infections, for stimulating blood production, in benign
prostatic hyperplasia, gingivitis, as well as for the treatment of neuronal
defects and neurodegenerative diseases such as Alzheimer's disease, for
example. The compounds described may also be used for the treatment of
tumours, particularly for modifying tumour invasion and also metastasisation;
examples here are their use in treating T-cell lymphomas, acute lymphoblastic
leukaemia, cell-based pancreatic carcinomas, basal cell carcinomas or breast
cancers. Other indications are stroke, ischaemia of various origins, Parkin-
son's disease and migraine. In addition, further indications include
follicular
and epidermal hyperkeratoses, increased keratinocyte proliferation, psoriasis,
encephalomyelitis, glomerulonephritis, lipodystrophies, as well as psycho-
somatic, depressive and neuropsychiatric diseases of all kinds.
The compounds according to the invention may also be used in conjunction
with other active substances. Therapeutic agents which are suitable for such
combinations include, for example, antidiabetics, such as metformin,
sulphonylureas (e.g. glibenclamid, tolbutamide, glimepiride), nateglinide,
repaglinide, thiazolidinedione (e.g. rosiglitazone, pioglitazone), PPAR-gamma
agonists (e.g. GI 262570) and antagonists, PPAR-gammalalpha modulators
(e.g. KRP 297), alpha-glucosidase inhibitors (e.g. acarbose, voglibose), other
DPPIV inhibitors, alpha2 antagonists, insulin and insulin analogues, GLP-1
and GLP-1 analogues (e.g. exendin-4) or amylin. Also, inhibitors of protein
CA 02496211 2005-02-18
tyrosine phosphatase 1, substances which influence deregulated glucose
production in the liver, such as e.g. inhibitors of glucose-6-phosphatase, or
fructose-1,6-bisphosphatase, glycogen phosphorylase, glucagon receptor
antagonists and inhibitors of phosphoenol pyruvate carboxykinase, glycogen
synthase kinase or pyruvate dehydrokinase, lipid lowering agents, such as
HMG-CoA-reductase inhibitors (e.g. simvastatin, atonrastatin), fibrates (e.g.
bezafibrate, fenofibrate), nicotinic acid and its derivatives, PPAR-alpha
agonists, PPAR-delta agonists, ACAT inhibitors (e.g. avasimibe) or
cholesterol resorption inhibitors such as for example ezetimibe, bile acid-
binding substances such as for example cholestyramine, inhibitors of ileac
bile acid transport, HDL-raising compounds such as for example inhibitors of
CETP or regulators of ABC1 or active substances for the treatment of obesity
such as e.g. sibutramine or tetrahydrolipostatin, dexfenfluramine, axokine,
antagonists of the cannabinoid1 receptor, MCH-1 receptor antagonists, MC4
receptor agonists, NPY5 or NPY2 antagonists or f33-agonists such as SB-
418790 or AD-9677 as well as agonists of the 5HT2c receptor.
It is also possible to combine the compounds with drugs for treating high
blood pressure such as e.g. All antagonists or ACE inhibitors, diuretics,
f3-blockers, Ca-antagonists, etc., or combinations thereof.
The dosage required to achieve such an effect is expediently, by intravenous
route, 1 to 100 mg, preferably 1 to 30 mg, and by oral route 1 to 1000 mg,
preferably 1 to 100 mg, in each case 1 to 4 a day. For this purpose, the
compounds of formula I prepared according to the invention, optionally
combined with other active substances, may be incorporated together with
one or more inert conventional carriers andlor diluents, e.g. with corn
starch,
lactose, glucose, microcrystalline cellulose, magnesium stearate,
polyvinylpyrrolidone, citric acid, tartaric acid, water, waterlethanol,
wateNglycerol, waterlsorbitol, waterlpolyethylene glycol, propylene glycol,
cetylstearyl alcohol, carboxymethylcellulose or fatty substances such as hard
fat or suitable mixtures thereof into conventional galenic preparations such
as
plain or coated tablets, capsules, powders, suspensions or suppositories.
CA 02496211 2005-02-18
41
The Examples that follow are intended to illustrate the invention:
Preparation of the starting compounds:
Example I
Ethvl 5-amino-3-benzyl-2-! 3-tert.-butyloxycarbonylamino-piperidin-1-yl)-3H-
imidazol-4-carboxylate
50 g (0.199 mol) of N-benzyl-N'-cyano-O-phenyl-isourea and 40.056 g (0.2
mol) of 3-tert.-butyloxycarbonylamino-piperidine are heated to 80°C in
50 ml
of dimethylformamide (DMF) for 4 hours. After standing overnight 250 ml of
ethyl acetate are added, the precipitate is suction filtered, washed with
ethyl
acetate and ether and dried. More product is obtained from the mother liquor
after evaporation and treatment of the residue with ethyl acetate and ether.
Total yield: 48.0 g (67.5% of theory) of N-benzyl-N'-cyano-(3-tert.-butyloxy-
carbonylamino-piperidin )-1-carboxamidine.
R, value: 0.56 (aluminium oxide, methylene chloridelmethanol = 40:1)
10.008 g (28 mmol) of this substance are dissolved in 15 ml DMF. After the
addition of 4.256 g (30.8 mmol) of potassium carbonate the mixture is treated
with ultrasound for three minutes, then 3.416 ml (30.8 mmol) of ethyl
bromoacetate are added in one go and the mixture is stirred for 36 hours at
ambient temperature, adding another 10 ml of DMF after 8 hours for ease of
stirring. The reaction mixture is stirred with water, extracted with ethyl
acetate,
the organic phase is dried and concentrated by evaporation. The resin
obtained is purified by column chromatography (silica gel, ethyl
acetatelpetroleum ether = 3:1 to 9:1 )
5.4 g (43.5 % of theory) of N-benzyl-N'-cyano-N-ethoxycarbonylmethyl-(3-
tert.-butyloxy-carbonylamino-piperidin)-1-carboxamidine are obtained.
Rf value: 0.7 (silica gel, ethyl acetatelpetroleum ether = 4:1 )
5.1 g (11.498 mmol) of this compound are added batchwise to a solution of
0.785 g (11.536 mmol) of sodium ethoxide in 25 ml of ethanol. The mixture is
CA 02496211 2005-02-18
42
stirred for 40 minutes at 60°C, then combined with 50 ml of ethanol and
10 ml
of water and cooled. The precipitate is suction filtered and dissolved in
methylene chloride. After drying and evaporation of the solvent, 4.8 g (94.1
of theory) of the title compound is obtained.
Rf value: 0.4 (silica gel, ethyl acetatelpetroleum ether = 4:1 )
The following was obtained analogously to Example I:
(1) ethyl 5-amino-3-(3-methyl-but-2-enyl)-2-(3-tert.-butyloxycarbonylamino-
piperidin-1-yl)-3H-imidazol-4-carboxylate
prepared from diphenyl-N-cyanocarbonimidate, glycinethylester, (3-tert.-
butyloxy-carbonylamino)-piperidine and 3-methyl-but-2-en-1-yl-bromide.
Rf value: 0.1-0.2 (silica get, ethyl acetatelpetroleum ether = 1:1)
Example II
tert. butyl l1-(7-benzyl-6-oxo-6,7-dihydro-1 HJ~urin-8-yl)-piperidin-3-
y]carbaminate
360 mg (0.812 mmol) of ethyl 5-amino-3-benzyl-2-(3-tert.-butyloxy-
carbonylamino-piperidin-1-yl)-3H-imidazol-4-carboxylate and 131.472 (1.2
mmol) of ethylformidate hydrochloride are placed in 1.3 g phenol. A solution
of 141.667 mg (2mmol) of sodiumethoxide in 2 ml of tetrahydrofuran (THF) is
added dropwise with stirring, the THF is evaporated off and the mixture is
kept
for 2 hours at 150°C. The brown reaction mixture is purified through a
silica
gel column. 50 mg (15.7 % of theory) of the title compound were obtained.
melting point: 208°C.
R, value: 0.15 (silica gel, methylene chloridelmethanol = 15:1)
Example Ill
tert. buyl f1-(7-benzvl-1-methyl-6-oxo-6.7-di~dro-1H-aurin-8-yl)-pJ~eridin-3-
y)carbaminate
19 mg (0.138 mmol) of potassium carbonate and then 16 mg (0.113 mmol) of
methyl iodide are added to a solution of 42 mg (0.099 mmol) of the
compound of Example II in 0.3 ml DMF. The mixture is stirred for 2 hours at
CA 02496211 2005-02-18
43
ambient temperature, then triturated with water and extracted with ethyl
acetate. The organic phase is washed with water and dried with activated
charcoal and magnesium sulphate. After evaporation 29 mg (66.8 % of
theory) of the title compound is obtained.
R, value: 0.3 (silica gel, methylene chloridelmethanol = 15:1 )
The following was obtained analogously to Example III:
(1) tert. butyl [1-(7-benzyl-1-{2-oxo-2-phenyl-ethyl}-6-oxo-6,7-dihydro-1H-
purin-8-yl)-piperidin-3-yl]-carbaminate
prepared from Example II and phenacylbromide.
Rf value: 0.4 (silica gel, methylene chloridelmethanol = 15:1)
Example IV
tert. butyl f1-(7-benzyl-2-methylsulphanyl-6-oxo-6 7-dihydro-1H-purin-8-yl)-
piperidin-3-yll-carbaminate
3.186 ml (27 mmol) of ethyloxycarbonyl isothiocyanate are added dropwise to
a solution of 10.8 g (24.345 mmol) of the compound of Example I in 45 ml
THF. The mixture is heated to boiling for one hour, concentrated by
evaporation and the residue is brought to crystallisation by treating with
diisopropylether.
13.5 g (96.5 % of theory) of ethyl 3-benzyl-2-(3-tert.-butyloxycarbonylamino-
piperidin-1-yl)-5-(N'-ethyloxycarbonyl-thioureido)-3H-imidazol-4-carboxylate
are obtained.
13 g (22.620 mmol) of this compound are placed in 21 ml of n-butanol. After
the addition of 2.536 (22.6 mmol) of potassium-tert.-butoxide the mixture is
stirred for 45 minutes at 100°C, during which time a precipitate
settles out.
After standing overnight at ambient temperature the mixture is combined with
ether, suction filtered and dried. 10.6 g (94.7 % of theory) of tert. butyl [1-
(7-
benzyl-2-mercapto-6-oxo-6,7-dihydro-1 H-purin-8-yl)-piperidin-3-yl]-
carbaminate potassium salt are obtained.
CA 02496211 2005-02-18
44
10.5 g (21.225 mmol) of this compound are suspended in 25 ml of water, and
ethanol is added to the solution. After the addition of 2.135 ml (21.515 mmol)
of dimethylsulphate the mixture is stirred for 4 hours at ambient temperature.
The precipitate is suction filtered, washed with cold ethanol and dried. 8.8 g
(88.1 % of theory) of the title compound are obtained.
Rf value: 0.3 (silica gel, methylene chloridelmethanol = 20:1 )
The following compounds were obtained analogously to Example IV:
(1) tert. butyl [1-(7-benzyl-2-benzylsulphanyl-6-oxo-6,7-dihydro-1H-purin-8-
yl)-piperidin-3-yl]-carbaminate
prepared from compound I, ethyloxycarbonyl isothiocyanate and
benzylbromide.
R, value: 0.65 (silica gel, ethyl acetatelpetroleum ether = 2:1 )
(2) tent. butyl [1-(7-allyl-2-methylsulphanyl-6-oxo-6,7-dihydro-1H-purin-8-yl)-
piperidin-3-yl]-carbaminate
prepared from compound 1.1, ethyloxycarbonyl isothiocyanate and methyl
iodide.
Rf value: 0.6 (silica gel, methylene chloridelmethanol = 10:1)
(3) tert. butyl [1-(7-benzyl-2-[2-phenylethyl]sulphanyl-6-oxo-6,7-dihydro-1H-
purin-8-yl)-piperidin-3-yl]-carbaminate
prepared from compound I, ethyloxycarbonyl isothiocyanate and 2-
phenylethylbromide.
Rf value: 0.65 (silica gel, ethyl acetatelpetroleum ether = 2:1)
Example V
tert-butyl [1-(7-benzvl-1-methyl-2-methylsulphanvi-6-oxo-6.7-dihvdro-1H-
puri n-8-yl)-pi perid in-3-yll-carbami nate
A suspension of 2.5 g (5.312 mmol) of compound IV in 15 ml DMF is
combined with 645.15 mg (5.75mmol) of potassium-tert.-butoxide. 922.605
mg (6.5 mmol) of methyl iodide are added to the resulting solution and the
CA 02496211 2005-02-18
mixture is stirred overnight at ambient temperature. Water is added and the
mixture is extracted with methylene chloride. The organic phase is washed
with water, dried and concentrated by evaporation. The residue is crystallised
with diisopropylether. 2 g (77.7 % of theory) of the title compound are
obtained.
Rf value: 0.55 (silica gel, methylene chloridelmethanol = 20:1)
The following compounds were obtained analogously to Example V:
(1) tert. butyl [1-(7-benzyl-2-methylsulphanyl-1-phenacyl-6-oxo-6,7-dihydro-
1 H-purin-8-yl)-piperidin-3-yl]-carbaminate
prepared from compound IV and phenacylbromide.
Rf value: 0.7 (aluminium oxide, methylene chloride/methanol = 40:1)
(2) tert. butyl [1-(1-methyl-7-(3-methyl-butenyl)-2-methylsulphanyl-6-oxo-6,7-
dihydro-1 H-purin-8-yl)-piperidin-3-yl]-carbaminate
prepared from compound IV.1 and methyl iodide.
Rf value: 0.4 (silica gel, methylene chloridelmethanol = 20:1)
(3) tert. butyl [1-(1-benzyl-7-benzyl-2-methylsulphanyl-6-oxo-6,7-dihydro-1H-
purin-8-yl)-piperidin-3-yl]-carbaminate
prepared from compound IV and benzylbromide.
Rf value: 0.75 (silica gel, ethyl acetatelpetroleum ether = 4:1 )
(4) tent. butyl [1-(7-benzyl-2-methylsulphanyl-1-(2-phenylethyl)-6-oxo-6,7-
dihydro-1 H-purin-8-yl)-piperidin-3-yl]-carbaminate
prepared from compound IV and 2-phenylethylbromide.
Rf value: 0.75 (silica gel, ethyl acetatelpetroleum ether = 4:1)
(5) tert. butyl [1-(7-benzyl-2-benzylsulphanyl-1-methyl-6-oxo-6,7-dihydro-1H-
purin-8-yl)-piperidin-3-yl]-carbaminate
prepared from compound IV.1 and methyl iodide.
Rf value: 0.85 (silica gel, ethyl acetatelpetroleum ether = 1:2)
CA 02496211 2005-02-18
46
(6) tert. butyl [1-(7-benzyl-2-[2-phenylethyl]sulphanyl-1-methyl-6-oxo-6,7-
dihydro-1 H-purin-8-yl)-piperidin-3-yl]-carbaminate
prepared from compound IV.3 and methyl iodide.
Rf value: 0.65 (silica gel, ethyl acetatelpetroleum ether = 1:2)
Example VI
tert. butyl j1-(7-benzyl-1-meth~il-2-methylsulphinvl-6-oxo-6.7-dihydro-1 H-
aurin-
8-yl)-piperidin-3-yll-carbaminate and tert. butyl f1-(7-benzyl-1-methyl-2-
methylsulphonyl-6-oxo-6,7-dih~dro-1 H-aurin-8-yl)-piperidin-3-yll-carbaminate
A solution of 250 mg (0.516 mmol) of compound V in 5 ml of dichloromethane
and 0.5 ml of methanol is combined with 120.789 mg (0.7 mmol) of m-chloro-
perbenzoic acid with stirring and cooling with ice. After 30 minutes the ice
bath is removed and the mixture is stirred overnight at ambient temperature.
50 ml of methylene chloride are added and the mixture is extracted with 10
soda solution. The organic phase is washed with water, dried and
concentrated by evaporation. 220 mg of the two title compounds are obtained
in the ratio 45 : 55.
Rf value: 0.1 (sulphoxide) and 0.8 (sulphone) (silica gel, ethyl acetate)
The following compounds were obtained analogously to Example VI:
(1) tert. butyl [1-methyl-7-(3-methyl-but-2-enyl)-2-methylsulphinyl-6-oxo-6,7-
dihydro-1H-purin-8-yl]-piperidin-3-yl)-carbaminate and tert.butyl [1-methyl-
7-(3-methyl-but-2-enyl)-2-methylsulphonyl-6-oxo-6,7-dihydro-1 H-purin-8-
yl-]-piperidin-3-yl)-carbaminate
prepared from compound V.2 and m-chloro-perbenzoic acid. The product
obtained after a reaction time of 80 minutes is the sulphoxide, which contains
a maximum of 10% sulphone.
Rf value: 0.5 (silica gel, methylene chloride/methanol = 10:1 )
(2) tert. butyl [1-(7-benzyl-2-methanesulphonyl-1-methyl-6-oxo-6,7-dihydro-
1 H-purin-8-yl)- piperidin-3-yl]-carbaminate
prepared from compound V.
CA 02496211 2005-02-18
47
Rf value: 0.75 (silica gel, ethyl acetate)
Mass spectrum (ESI+): mlz = 517 [M+H]+
Example VII
Tert. butyl f 1-(7-benzyl -2-benzylamino-1-methyl-6-oxo-6.7-dihydro-1 H-purin-
8-yl)-pi peridi n-3 yll-carbam inate
258.312 mg of the mixture obtained in Example VI and 214.313 mg of
benzylamine are stirred for 16 hours at ambient temperature. The mixture is
triturated with 20 ml of diisopropylether, the precipitate is suction
filtered,
dissolved in a little methylene chloride and crystallised with
diisopropylether.
250 mg of the title compound are obtained.
Rf value: 0.55 (silica gel, ethyl acetate)
The following compounds were prepared analogously to Example VII:
(1) tert. butyl [1-(7-benzyl-2-[4-fluoro-benzyl]amino-1-methyl-6-oxo-6,7-
dihydro-1 H-purin-8-yl)-piperidin-3-yl]-carbaminate
prepared from Example VI and 4-fluoro-benzylamine
Rf value: 0.49 (silica gel, ethyl acetate)
(2) tert. butyl [1-( 7-benzyl-1-methyl-2-(2-phenylethyl)amino-6-oxo-6,7-
dihydro-1 H-purin-8-yl)-piperidin-3-yl]-carbaminate
prepared from Example VI and 2-(2-phenylethyl)amine.
Rr value: 0.5 (silica gel, ethyl acetate)
(3) tert. butyl [1-(7-benzyl-2-isopropylamino-1-methyl-6-oxo-6,7-dihydro-1H-
purin-8-yl)-piperidin-3-yl-]-carbaminate
prepared from Example VI and isopropylamine.
Rf value: 0.6 (silica gel, methylene chloridelmethanol = 9:1)
(4) tert. butyl [1-(7-benzyl-1-methyl-2-methylamino-6-oxo-6,7-dihydro-1 H-
purin-8-yl)-piperidin-3-yl]-carbaminate
prepared from Example VI and methylamine gas.
CA 02496211 2005-02-18
48
Rf value: 0.27 (silica gel, methylene chloridelmethanol = 19:1)
(5) tert. butyl [1-(7-benzyl-2-cyclohexylamino-1-methyl-6-oxo-6,7-dihydro-1 H-
purin-8-yl)-piperidin-3-yl]-carbaminate
prepared from Example VI and cyclohexylamine
Rf value: 0.65 (silica gel, methylene chloridelmethanol = 9:1)
(6) tert. butyl [1-(7-benzyl-2-cyclohexylmethylamino-1-methyl-6-oxo-6,7-
dihydro-1 H-purin-8-yl)-piperidin-3-yl]-carbaminate
prepared from Example VI and cyclohexylmethylamine.
Rf value: 0.54 (silica gel, ethyl acetate)
(7) tert. butyl [1-(7-benzyl-1-methyl-2-piperidino-6-oxo-6,7-dihydro-1H-purin-
8-
yl)-piperidin-3-yl]-carbaminate
prepared from Example VI and piperidine.
Rf value: 0.45 (silica gel, methylene chloridelmethanol = 20:1)
(8) tert. butyl [1-(7-benzyl-2-dimethylamino-1-methyl-6-oxo-6,7-dihydro-1 H-
purin-8-yl)-piperidin-3-yl]-carbaminate
prepared from Example VI and dimethylamine
Rf value: 0.65 (silica gel, methylene chloride/methanol = 9:1)
(9) tert. butyl [1-(2-amino-7-benzyl-1-methyl-6-oxo-6,7-dihydro-1H-purin-8-yl)-
piperidin-3-yl]-carbaminate
prepared from Example VI and ammonia gas.
Rf value: 0.4 (silica gel, methylene chloridelmethanol = 10:1)
(10) tert. butyl [1-(2-benzylamino-1-methyl-7-[3-methylbut-2-enyl]-6-oxo-6,7-
dihydro-1 H-purin-8-yl)-piperidino-3-yl]-carbaminate
prepared from Example VL1 and benzylamine
Rf value: 0.6 (silica gel, ethyl acetate)
CA 02496211 2005-02-18
49
Example VIII
Tert. butyl f1-(7-benzyl-1-methyl-2-methyl-6-oxo-6 7-dihydro-1H-purin-8-yl)-
piperidin-3-yll-carbaminate
A solution of 258.32 mg of Example VI in 3 ml of THF is combined with 0.2 ml
of a 3-molar solution of methylmagnesium bromide in ether and the mixture is
stirred for 48 hours at ambient temperature. 50 ml of ether were added and
the mixture was extracted with water at pH 4. The organic phase was dried
and concentrated by evaporation. The product obtained was purified through
a silica gel column. 55 mg of the title compound are obtained.
Rf value: 0.55 (silica gel, methylene chloridelmethanol = 10:1)
The following compound was obtained analogously to Example VIII:
(1) tert. butyl [1-(1-methyl-7-[3-methyl-but-2-enylJ-2-[2-phenylethyl]-6-oxo-
6,7-
dihydro-1 H-purin-8-yl)-piperidin-3-yl]-carbaminate
prepared from Example VL1 and (2-phenylethyl)magnesium bromide.
Rf value: 0.5 (silica gel; methylene chloridelmethanol = 10:1)
Example IX
Tert. butyl f1-f7-benzyl-1-methyl-6-oxo-2-(2-oxo-2-phenyl-ethyl)-6 7-dihydro-
1 H-purin-8-yll-piperidin-3-yl}-carbaminate
A solution of 132 mg of acetophenone in 1 ml of tetrahydrofuran is added
dropwise at 0°C to a solution of 0.63 ml of n-butyllithium (1.6 M in n-
hexane)
and 119 mg diisopropylamine in 2 ml of tetrahydrofuran. After 15 minutes a
solution of 500 mg of tert. butyl [1-(7-benzyl-2-methanesulphonyl-1-methyl-6-
oxo-6,7-dihydro-1 H-purin-8-yl)-piperidin-3-ylJ-carbaminate in 2 ml of
tetrahydrofuran is added dropwise. Then the cooling bath is removed and the
reaction mixture is stirred overnight at ambient temperature. As the thin
layer
chromatograph shows that there is still some starting material present, a
further 0.94 ml of n-butyllithium (1.6 M in n-hexane) are added. After another
24 hours the reaction solution is diluted with 50 ml of water, adjusted to pH
6
with 2 N hydrochloric acid and extracted with ethyl acetate. The combined
organic phases are washed with water, dried over magnesium sulphate and
CA 02496211 2005-02-18
evaporated down. The crude product is purified by chromatography over a
silica gel column with methylene chloride as eluant. 54 mg of the title
compound are obtained.
Rf value: 0.55 (aluminium oxide, methylene chloride)
Mass spectrum (ESI+): mlz = 557 [M+H]+
Example X
Tert. butyl f 1-(7-benzyl-2-cyano-1-methyl-6-oxo-6 7-dihydro-1 H-aurin-8-yl)-
piaeridin-3-yll-carbaminate
268 mg of tetrabutylammonium cyanide are added to 258 mg of tert, butyl [1-
(7-benzyl-2-methanesulphonyl-1-methyl-6-oxo-6,7-dihydro-1 H-purin-8-yl)-
piperidin-3-yl]-carbaminate in 1 ml of methylene chloride and the reaction
mixture is stirred for two days at ambient temperature. The reaction solution
is
chromatographed through a silica gel column with methylene
chloride/methanol (97:3 to 90:1 ) as eluant. The product thus obtained is
crystallised from diisopropylether.
126 mg of the title compound are obtained.
Rf value: 0.75 (silica gel, methylene chloridelmethanol = 10:1)
Mass spectrum (ESI+): mlz = 464 [M+H]+
Example XI
Tert. butyl f1-f1-benzvl-2-methyl-7-(3-methyl-but-2-enyl)-6-oxo-6 7-dihydro-1
H-
purin-8-yll-piperidin-3-yl~-carbaminate
A mixture of 105 mg of tert. butyl {1-[5-methyl-1-(3-methyl-but-2-enyl)-7-oxo-
1,7-dihydro-imidazo[4,5-d][1,3]oxazin-2-yl]-piperidin-3-yl}-carbaminate and 40
NI benzylamine in 1.5 ml of methylene chloride is stirred for two days at
40°C.
Then 57 NI triethylamine and 37 NI phosphorus oxychloride are added and the
reaction mixture is stirred for a further six hours at 40°C. For
working up the
reaction mixture is combined with aqueous potassium carbonate solution and
extracted with ethyl acetate. The combined organic phases are dried over
magnesium sulphate and evaporated down. The crude product is purified by
CA 02496211 2005-02-18
51
chromatography over a silica gel column with methylene chloride/methanol
(1:0 to 20:1) as eluant. 30 mg of the title compound are obtained.
Mass spectrum (ESI+): mlz = 507 [M+H]+
The following is obtained analogously to Example XI:
(1) tert. butyl (1-{1-[(naphthalen-1-yl)methyl]-2-methyl-7-(3-methyl-but-2-
enyl)-
6-oxo-6,7-dihydro-1 H-purin-8-yl}-piperidin-3-yl)-carbaminate
prepared from Example XII and 1-aminomethyl-naphthalene.
Mass spectrum (ESI+): mlz = 557 [M+H]+
Example XII
Tert. butyl {1-f5-methyl-1-t3-methyi-but-2-enyl)-7-oxo-1,7-dihvdro-imidazo14,5-
dlf 1,3loxazin-2- rl -piperidin-3-yl)-carbaminate
A mixture of 1.0 g of ethyl 5-acetylamino-2-(3-tert.-butyloxycarbonylamino-
piperidin-1-yl)-3-(3-methyl-but-2-enyl)-3H-imidazol-4-carboxylate, 566 mg of
triphenylphosphine and 0.97 ml of triethylamine in 16 ml of toluene is heated
to 80°C and combined with a solution of 702 mg of 1,2-dibromo-
tetrachloroethane in 8 m1 of toluene. The reaction mixture is stirred for four
hours at 80°C, then the precipitate formed is filtered off and washed
with
toluene. The filtrate is evaporated down in vacuo and chromatographed
through a silica gel column with cyclohexanelethyl acetate as eluant. 804 mg
of the title compound are obtained.
Mass spectrum (ESI+): mlz = 418 [M+H]+
The following compound is obtained from Example X111.1 under the same
reaction conditions:
(1) ethyl 2 -(3-tert.-butyloxycarbonylamino-piperidin-1-yl)-5-isocyano-3-(3-
methyl-but-2-enyl)-3H-imidazol-4-carboxylate
Mass spectrum (ESI+): m/z = 432 [M+H]+
CA 02496211 2005-02-18
52
Example XIII
Ethyl 5-acetylamino-2-(3-tert.-butyloxycarbonylamino-piaeridin-1-yl)-3-(3-
methvl-but-2-enyl)-3H-imidazol-4-carboxylate
prepared from Example 1.1 by reaction with acetylchloride in the presence of
pyridine in methylene chloride.
Mass spectrum (ESI+): mlz = 464 [M+H]+
The following compound is obtained analogously to Example XIII:
(1) ethyl 2-(3-tert.-butyloxycarbonylamino-piperidin-1-yl)-5-formylamino-3-(3-
methyl-but-2-enyl)-3H-imidazol-4-carboxylate
prepared from Example 1.1 by reaction with formic acid and acetic anhydride in
the presence of pyridine in methylene chloride.
Mass spectrum (ESI+): mlz = 450 [M+H]+
Example XIV
Tert. butyl (1-(1-f(naahthalen-1-yl)methyll-7-(3-methyl-but-2-enyl)-6-oxo-6 7-
dihydro-1 H-purin-8-yl]~-piperidin-3-yl)-carbaminate
210 mg of 1-aminomethyl-naphthalene and 30 mg of copper(I)oxide are
added to 295 mg of ethyl 2-(3-tert.-butyloxycarbonylamino-piperidin-1-yl)-5-
isocyano-3-(3-methyl-but-2-enyl)-3H-imidazol-4-carboxylate in 7 ml of
toluene. The reaction mixture is stirred for 10 hours at 120°C. After
cooling to
ambient temperature it is diluted with ethyl acetate and filtered through
Celite.
The filtrate is combined with water and extracted with ethyl acetate. The
combined extracts are dried over magnesium sulphate and evaporated down.
The crude product is chromatographed through a silica gel column with
methylene chloride/methanol (1:0 to 10:1) as eluant. 306 mg of the title
compound are obtained, contaminated with ethyl 5-amino-2-(3-tert.-
butoxycarbonylamino-piperidin-1-yl)-3-(3-methyl-but-2-enyl)-3H-imidazol-4-
.ca rboxylate.
Mass spectrum (ESI+): mlz = 543 [M+H]+
53
The following compound is obtained analogously to Example XIV:
(1 ) tert. butyl (1-~1-[(4-methoxy-naphthalen-1-yl)methyl]-7-(3-methyl-but-2-
enyl)-6-oxo-6,7-dihydro-1 H-purin-8-yl}-piperidin-3-yl)-carbaminate
prepared from Example XI1.1 and 1-aminomethyl-4-methoxy-naphthalene.
Rf value: 0.17 (silica gel, cyclohexanelethyl acetate = 1:9)
Mass spectrum (ESI+): mlz = 573 [M+H]+
Examples of the preparation of the end products:
Example 1
8-(3-amino-piperidin-1-yl)-7-benzyl-2-benzylamino-1-methyl-1,7-dihydro-purin-
6-one
A solution of 200 mg of Example VII in 2 ml of dichloromethane is combined
with 3 ml of trifluoroacetic acid with stirring and cooling with ice. After 30
minutes the ice bath is removed and stirring is continued for 2 hours. The
solvent is evaporated at low temperature, the residue is triturated with
ether,
suction filtered and dried in vacuo. 150 mg (73.1 % of theory) of the
trifluoroacetate of the title compound are obtained.
Rf value: 0.45 (aluminium oxide, methylene chloride/methanol = 20:1 )
'H-NMR spectrum (400 MHz, DMSO-ds):
1.5 (m,2H), 1,7 (m,1 H), 1.95 (m,1 H), 2.8 (m,1 H), 3.0 (m,1 H), 3.15 (d,1 H),
3.4
(s+m,4H), 3.5(d,1 H), 4.55 (d,2H), 5.35(s,2H), 7.1-7.5 (m,12H), 8.0 (s,3H),
8.1-
8.3 (m,1 H)
The following compounds were obtained analogously to Example 1:
(1) 8-(3-amino-piperidin-1-yl)-7-benzyl-2-(4-fluoro-benzylamino)-1-methyl-1,7-
dihydro-purin-6-one trifluoroacetate
prepared from Example VI1.1 and trifluoroacetic acid
Rf value: 0.69 (aluminium oxide, methylene chloridelmethanol = 9:1 )
CA 02496211 2005-02-18
54
(2) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(2-phenylethyl)amino]-1,7-
dihydro-purin-6-one trifluoroacetate
prepared from Example VI1.2 and trifluoroacetic acid.
Rf value: 0.75 (aluminium oxide, methylene chloridelmethanol = 9:1 )
(3) 8-(3-amino-piperidin-1-yl)-7-benzyl-2-isopropylamino-1-methyl-1,7-
dihydro-purin-6-one trifluoroacetate
prepared from Example VI1.3 and trifluoroacetic acid.
Rf value: 0.68 (aluminium oxide, methylene chloridelmethanol = 9:1 )
(4) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-methylamino-1,7-dihydro-
purin-6-one trifluoroacetate
prepared from Example VI1.4 and trifluoroacetic acid.
Rf value: 0.26 (aluminium oxide, methylene chloridelmethanol = 9:1 )
(5) 8-(3-amino-piperidin-1-yl)-7-benzyl-2-cyclohexylamino-1-methyl-1,7-
dihydro-purin-6-one trifluoroacetate
prepared from Example VI1.5 and trifluoroacetic acid.
Rr value: 0.65 (aluminium oxide, methylene chloridelmethanol = 9:1 )
(6) 8-(3-amino-piperidin-1-yl)-7-benzyl-2-cyclohexylmethylamino-1-methyl-
1,7-dihydro-purin-6-one trifluoroacetate
prepared from Example V11.6 and trifluoroacetic acid.
Rf value: 0.69 (aluminium oxide, methylene chloridelmethanol = 9:1 )
(7) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(piperidin-1-yl)-1,7-
dihydro-
purin-6-one hydrochloride
prepared from Example V11.7 and hydrogen chloride in dioxane.
Rf value: 0.3-0.5 (aluminium oxide, methylene chloridelmethanol = 20:1 )
(8) 8-(3-amino-piperidin-1-yl)-7-benzyl-2-dimethylamino-1-methyl-1,7-
dihydro-purin-6-one trifluoroacetate
prepared from Example VI1.8 and trifluoroacetic acid.
Rf value: 0.69 (aluminium oxide, methylene chloride/methanol = 9:1 )
CA 02496211 2005-02-18
55
(9) 2-amino-8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-1,7-dihydro-purin-6-
one trifluoroacetate
prepared from Example VI1.9 and trifluoroacetic acid.
Rf value: 0.2 (aluminium oxide, methylene chloridelmethanol = 10:1 )
(10) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(3-methyl-but-2-en-
1-yl)-1,7-dihydro-purin-6-one trifluoroacetate
prepared from Example V11.10 and trifluoroacetic acid.
Rf value: 0.55 (aluminium oxide, methylene chloridelmethanol = 10:1 )
(11) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-methyl-1,7-dihydro-purin-
6-one trifluoroacetate
prepared from Example VIII and trifluoroacetic acid.
Rf value: 0.6 (aluminium oxide, methylene chloridelmethanol = 10:1 )
(12) 8-(3-amino-piperidin-1-yl)-1-methyl-7-(3-methyl-but-2-en-1-yl)-2-(2-
phenylethyl)-1,7-dihydro-purin-6-one trifluoroacetate
prepared from Example V111.1 and trifluoroacetic acid.
Rf value: 0.4 (aluminium oxide, methylene chloridelmethanol = 20:1 )
(13) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-1,7-dihydro-purin-6-one
hydrochloride
prepared from Example III and hydrogen chloride in dioxane.
Rf value: 0.2-0.5 (aluminium oxide, methylene chloridelmethanol = 20:1 )
(14) 8-(3-amino-piperidin-1-yl)-7-benzyl-2-methylsulphanyl-1,7-dihydro-purin-
6-one hydrochloride
prepared from Example IV and hydrogen chloride in dioxane.
Rf value: 0.5 (aluminium oxide, methylene chloridelmethanol = 10:1 )
(15) 8-(3-amino-piperidin-1-yl)-7-benzyl-2-benzylsulphanyl-1,7-dihydro-purin-
6-one trifluoroacetate
prepared from Example IV.1 and trifluoroacetic acid.
CA 02496211 2005-02-18
56
CA 02496211 2005-02-18
Rf value: 0.5-0.6 (aluminium oxide, methylene chloridelmethanol = 10:1 )
(16) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-methylsulphanyl-1,7-
dihydro-purin-6-one hydrochloride
prepared from Example V and hydrogen chloride in dioxane.
Rf value: 0.5 (aluminium oxide, methylene chloridelmethanol = 20:1 )
(17) 8-(3-amino-piperidin-1-yl)-7-benzyl-2-methylsulphanyl-1-(2-phenyl-2-oxo-
ethyl)-1,7-dihydro-purin-6-one hydrochloride
prepared from Example V.1 and hydrogen chloride in dioxane.
Rf value: 0.4 (aluminium oxide, methylene chloride/methanol = 20:1 )
(18) 8-(3-amino-piperidin-1-yl)-1-benzyl-7-benzyl-2-methylsulphanyl-1,7-
dihydro-purin-6-one hydrochloride
prepared from Example V.3 and hydrogen chloride.
Rf value: 0.4-0.5 (aluminium oxide, methylene chloridelmethanol = 20:1 )
(19) 8-(3-amino-piperidin-1-yl)-7-benzyl-2-methylsulphanyl-1-(2-phenylethyl)-
1,7-dihydro-purin-6-one hydrochloride
prepared from Example V.4 and hydrogen chloride.
Rf value: 0.4 (aluminium oxide, methylene chloridelmethanol = 20:1 )
(20) 8-(3-amino-piperidin-1-yl)-7-benzyl-2-benzylsulphanyl-1-methyl-1,7-
dihydro-purin-6-one
prepared from Example V.5 and hydrogen chloride.
Rf value: 0.5 (aluminium oxide, methylene chloridelmethanol = 20:1 )
(21 ) 8-(3-amino-piperidin-1-yl)-7-benzyl-2-[(2-phenylethyl)sulphanyl]-1-
methyl-
1, 7-d i hyd ro-pu ri n-6-one
prepared from Example V.6 and hydrogen chloride.
Rf value: 0.55 (aluminium oxide, methylene chloridelmethanol = 20:1 )
(22) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(2-oxo-2-phenyl-ethyl)-1,7-dihydro-
purin-6-one trifluoroacetate
57
CA 02496211 2005-02-18
prepared from Example 111.1 and trifluoroacetic acid.
Rf value: 0.65 (aluminium oxide, methylene chloridelmethanol = 10:1 )
(23) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(2-oxo-2-phenyl-ethyl)-1,7-
d i hyd ro-pu rin-6-one
prepared from Example IX and trifluoroacetic acid.
Rf value: 0.20 (silica gel, methylene chloridelmethanol = 10:1 )
Mass spectrum (ESI+): m/z = 457 [M+H]+
(24) 8-(3-amino-piperidin-1-yl)-7-benzyl-2-cyano-1-methyl-1,7-dihydro-purin-6-
one trifluoroacetate
prepared from Example X and trifluoroacetic acid.
Rf value: 0.50 (aluminium oxide, methylene chloridelmethanol = 20:1 )
Mass spectrum (ESI+): mlz = 364 [M+H]+
(25) 8-(3-amino-piperidin-1-yl)-1-benzyl-2-methyl-7-(3-methyl-but-2-en-1-yl)-
1,7-dihydro-purin-6-one
prepared from Example XI and trifluoroacetic acid.
Mass spectrum (ESI+): mlz = 407 [M+H]+
(26) 8-(3-amino-piperidin-1-yl)-2-methyl-7-(3-methyl-but-2-en-1-yl)-1-
[(naphthalen-1-yl)methyl]-1,7-dihydro-purin-6-one
prepared from Example XI.1 and trifluoroacetic acid.
Mass spectrum (ESI+): mlz = 457 [M+H]+
(27) 8-(3-amino-piperidin-1-yl)-7-(3-methyl-but-2-en-1-yl)-1-[(naphthalen-1-
yl)methyl]-1,7-dihydro-purin-6-one
prepared from Example XIV and trifluoroacetic acid.
Mass spectrum (ESI+): mlz = 443 [M+H]+
(28) 8-(3-amino-piperidin-1-yl)-7-(3-methyl-but-2-en-1-yl)-1-[(4-methoxy-
naphthalen-1-yl)methyl]-1,7-dihydro-purin-6-one
prepared from Example XIV.1 and trifluoroacetic acid.
Mass spectrum (ESI+): mlz = 473 [M+H]+
CA 02496211 2005-02-18
58
The following compounds may also be obtained analogously to the preceding
Examples and other methods known from the literature:
(1) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-ethyl-2-benzylamino-1,7-dihydro-
puri n-6-one
(2) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-propyl-2-benzylamino-1,7-dihydro-
purin-6-one
(3) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-isopropyl-2-benzylamino-1,7-dihydro-
purin-6-one
(4) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-butyl-2-benzylamino-1,7-dihydro-
puri n-6-one
(5) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(2-methyl-propyl)-2-benzylamino-1,7-
dihydro-purin-6-one
(6) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-pentyl-2-benzylamino-1,7-dihydro-
purin-6-one
(7) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(3-methyl-butyl)-2-benzylamino-1,7-
dihydro-purin-6-one
(8) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-hexyl-2-benzylamino-1,7-dihydro-
purin-6-one
(9) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(4-methyl-pentyl)-2-benzylamino-1,7-
dihydro-purin-6-one
(10) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-allyl-2-benzylamino-1,7-dihydro-
puri n-6-one
CA 02496211 2005-02-18
59
(11) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(3-methyl-but-2-en-1-yl)-2-
benzylamino-1,7-dihydro-purin-6-one
(12) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(prop-2-in-1-yl)-2-benzylamino-1,7-
dihydro-purin-6-one
(13) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-cyclopropylmethyl-2-benzylamino-
1,7-dihydro-purin-6-one
(14) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-cyclohexylmethyl-2-benzylamino-
1,7-dihydro-purin-6-one
(15) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(4-chlorobenzyl)-2-benzylamino-
1,7-dihydro-purin-6-one
(16) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(2-fluorobenzyl)-2-benzylamino-1,7-
dihydro-purin-6-one
(17) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(4-methylbenzyl)-2-benzylamino-
1,7-dihydro-purin-6-one
(18) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(2,4-dimethylbenzyl)-2-
benzylamino-1,7-dihydro-purin-6-one
(19) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(2,4-dimethoxybenzyl)-2-
benzylamino-1,7-dihydro-purin-6-one
(20) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(3-trifluoromethyl-benzyl)-2-
benzylamino-1,7-dihydro-purin-6-one
(21) 8-(3-amino-piperidin-1-yl)-7-benzyt-1-(3-trifluoromethoxy-benzyl)-2-
benzylamino-1,7-dihydro-purin-6-one
CA 02496211 2005-02-18
(22) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(3-difluoromethoxy-benzyl)-2-
benzylamino-1,7-dihydro-purin-6-one
(23) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(furan-2-yl-methyl)-2-benzylamino-
1,7-dihydro-purin-6-one
(24) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(thien-2-yl-methyl)-2-benzylamino-
1,7-dihydro-purin-6-one
(25) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(3-methyl-isoxazol-5-yl-methyl)-2-
benzylamino-1,7-dihydro-purin-6-one
(26) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(pyridin-4-yl-methyl)-2-
benzylamino-1,7-dihydro-purin-6-one
(27) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(4-methyl-phenyl)-2-oxo-ethyl]-2-
benzylamino-1,7-dihydro-purin-6-one
(28) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(2,4-dichloro-phenyl)-2-oxo-
ethyl]-2-benzylamino-1,7-dihydro-purin-6-one
(29) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(4-methoxy-phenyl)-2-oxo-ethyl]-
2-benzylamino-1,7-dihydro-purin-6-one
(30) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(4-trifluoromethyl-phenyl)-2-oxo-
ethyl]-2-benzylamino-1,7-dihydro-purin-6-one
(31) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(4-trifluoromethoxy-phenyl)-2-
oxo-ethyl]-2-benzylamino-1,7-dihydro-purin-6-one
(32) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(4-difluoromethoxy-phenyl)-2-
oxo-ethyl]-2-benzylamino-1,7-dihydro-purin-6-one
CA 02496211 2005-02-18
61
(33) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(furan-2-yl)-2-oxo-ethyl]-2-
benzylamino-1,7-dihydro-purin-6-one
(34) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(thien-2-yl)-2-oxo-ethyl]-2-
benzylamino-1,7-dihydro-purin-6-one
(35) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(pyridin-3-yl)-2-oxo-ethyl]-2-
benzylamino-1,7-dihydro-purin-6-one
(36) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(2-oxopropyl)-2-benzylamino-1,7-
dihydro-purin-6-one
(37) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(2-cyclohexyl-2-oxo-ethyl)-2-
benzylamino-1,7-dihydro-purin-6-one
(38) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-ethyl-2-(2-phenylethyl)-1,7-dihydro-
purin-6-one
(39) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-propyl-2-(2-phenylethyl)-1,7-
dihydro-purin-6-one
(40) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-isopropyl-2-(2-phenylethyl)-1,7-
dihydro-purin-6-one
(41) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-butyl-2-(2-phenylethyl)-1,7-dihydro-
purin-6-one
(42) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(2-methyl-propyl)-2-(2-phenylethyl)-
1,7-dihydro-purin-6-one
(43) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-pentyl-2-(2-phenylethyl)-1,7-
dihydro-purin-6-one
CA 02496211 2005-02-18
62
(44) 8-(3-amino-piperidin-1-yl)-7-benzyt-1-(3-methyl-butyl)-2-(2-phenylethyl)-
1,7-dihydro-purin-6-one
(45) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-hexyl-2-(2-phenylethyl)-1,7-dihydro-
purin-6-one
(46) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(4-methyl-pentyl)-2-(2-phenylethyl)-
1,7-dihydro-purin-6-one
(47) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-allyl-2-(2-phenylethyl)-1,7-dihydro-
purin-6-one
(48) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(3-methyl-but-2-en-1-yl)-2-(2-
phenylethyl)-1,7-dihydro-purin-6-one
(49) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(prop-2-in-1-yl)-2-(2-phenylethyl)-
1,7-dihydro-purin-6-one
(50) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-cyclopropylmethyl-2-(2-
phenylethyl)-1,7-dihydro-purin-6-one
(51) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-cyclohexylmethyl-2-(2-phenylethyl)-
1,7-dihydro-purin-6-one
(52) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(4-chlorobenzyl)-2-(2-phenylethyl)-
1,7-dihydro-purin-6-one
(53) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(2-fluorobenzyl)-2-(2-phenylethyl)-
1,7-dihydro-purin-6-one
(54) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(4-methylbenzyl)-2-(2-phenylethyl)-
1,7-dihydro-purin-6-one
CA 02496211 2005-02-18
63
(55) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(2,4-dimethylbenzyl)-2-(2-
pheny!ethyl)-1,7-dihydro-purin-6-one
(56) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(2,4-dimethoxybenzyl)-2-(2-
pheny!ethyl)-1,7-dihydro-purin-6-one
(57) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(3-trifluoromethyl-benzyl)-2-(2-
pheny!ethyl)-1,7-dihydro-purin-6-one
(58) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(3-trifluoromethoxy-benzyl)-2-(2-
pheny!ethyl)-1,7-dihydro-purin-6-one
(59) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(3-difluoromethoxy-benzyl)-2-(2-
pheny!ethyl)-1,7-dihydro-purin-6-one
(60) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(furan-2-yl-methyl)-2-(2-
pheny!ethyl)-7-dihydro-purin-6-one
(61) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(thien-2-yl-methyl)-2-(2-
pheny!ethyl)-1,7-dihydro-purin-6-one
(62) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(3-methyl-isoxazol-5-yl-methyl)-2-
(2-pheny!ethyl)-1,7-dihydro-purin-6-one
(63) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(pyridin-4-yl-methyl)-2-(2-
pheny!ethyl)-1,7-dihydro-purin-6-one
(64) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(4-methyl-phenyl)-2-oxo-ethyl]-2-
(2-pheny!ethyl)-1,7-dihydro-purin-6-one
(65) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(2,4-dichloro-phenyl)-2-oxo-
ethyl]-2-(2-pheny!ethyl)-1,7-dihydro-purin-6-one
CA 02496211 2005-02-18
64
(66) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(4-methoxy-phenyl)-2-oxo-ethyl]-
2-(2-phenylethyl)-1,7-dihydro-purin-6-one
(67) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(4-trifluoromethyl-phenyl)-2-oxo-
ethyl]-2-(2-phenylethyl)-1,7-dihydro-purin-6-one
(68) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(4-trifluoromethoxy-phenyl)-2-
oxo-ethyl]-2-(2-phenylethyl)-1,7-dihydro-purin-6-one
(69) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(4-difluoromethoxy-phenyl)-2-
oxo-ethyl]-2-(2-phenylethyl)-1,7-dihydro-purin-6-one
(70) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(furan-2-yl)-2-oxo-ethyl]-2-(2-
phenylethyl)-1,7-dihydro-purin-6-one
(71) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(thien-2-yl)-2-oxo-ethyl]-2-(2-
phenylethyl)-1,7-dihydro-purin-6-one
(72) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(pyridin-3-yl)-2-oxo-ethyl]-2-(2-
phenylethyl)-1,7-dihydro-purin-6-one
(73) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(2-oxo-propyl)-2-(2-phenylethyl)-
1,7-dihydro-purin-6-one
(74) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-{2-cyclohexyl-2-oxo-ethyl)-2-(2-
phenylethyl)-1,7-dihydro-purin-6-one
(75) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-ethylamino-1,7-dihydro-
purin-6-one
(76) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-propylamino-1,7-dihydro-
purin-6-one
CA 02496211 2005-02-18
(77) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-butylamino-1,7-dihydro-
purin-6-one
(78) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(2-methyl-propylamino)-
1,7-dihydro-purin-6-one
(79) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(N-ethyl-N-methyl-amino)-
1,7-dihydro-purin-6-one
(80) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-diethylamino-1,7-dihydro-
purin-6-one
(81) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(N-methyl-N-propyl-
amino)-1,7-dihydro-purin-6-one
(82) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(N-butyl-N-methyl-amino)-
1,7-dihydro-purin-6-one
(83) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(2-hydroxyethyl-amino)-
1,7-dihydro-purin-6-one
(84) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[N-methyl-N-(2-
hydroxyethyl)-amino]-1,7-dihydro-purin-6-one
(85) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(2-methoxy-ethylamino)-
1,7-dihydro-purin-6-one
(86) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(3-methoxy-propylamino)-
1,7-dihydro-purin-6-one
(87) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[N-methyl-N-(2-methoxy-
ethyl)-amino]-1,7-dihydro-purin-6-one
CA 02496211 2005-02-18
66
(88) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(2-phenoxyethyl)-amino]-
1,7-dihydro-purin-6-one
(89) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(2-aminoethyl)-amino]-
1,7-dihydro-purin-6-one
(90) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-((3-aminopropyl)-amino]-
1,7-dihydro-purin-6-one
(91) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(2-methylamino-ethyl)-
amino]-1,7-dihydro-purin-6-one
(92) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(3-dimethylamino-
propyl)amino]-1,7-dihydro-purin-6-one
(93) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(2-acetylamino-
ethyl)amino]-1,7-dihydro-purin-6-one
(94) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(2-benzoylamino-
ethyl)amino]-1,7-dihydro-purin-6-one
(95) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(2-ethoxycarbonylamino-
ethyl)amino]-1,7-dihydro-purin-6-one
(96) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(2-methylsulphanyl-
ethyl)amino]-1,7-dihydro-purin-6-one
(97) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(2-phenylsulphanyl-
ethyl)amino]-1,7-dihydro-purin-6-one
(98) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(2-cyano-ethyl)amino]-
1,7-dihydro-purin-6-one
CA 02496211 2005-02-18
67
(99) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(carboxymethyl)amino]-
1,7-dihydro-purin-6-one
(100) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-
[(ethoxycarbonylmethyl)amino]-1,7-dihydro-purin-6-one
(101) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2
[(aminocarbonylmethyl)amino]-1,7-dihydro-purin-6-one
(102) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(ethylaminocarbonyl-
methyl)amino]-1,7-dihydro-purin-6-one
(103) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(dimethylaminocarbonyl-
methyl)amino]-1,7-dihydro-purin-6-one
(104) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(pyrrolidin-1-yl-
carbonyl-
methyl)amino]-1,7-dihydro-purin-6-one
(105) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(piperidin-1-yl-carbonyl-
methyl)amino]-1,7-dihydro-purin-6-one
(106) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(morpholin-4-
ylcarbonylmethyl)amino]-1,7-dihydro-purin-6-one
(107) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-
[(cyclopentylmethyl)ami no]-1,7-dihydro-purin-6-one
(108) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-
[(cyclobutylmethyl)amino]-1,7-dihydro-purin-6-one
(109) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-
[(cyclopropylmethyl)amino]-1,7-dihydro-purin-6-one
CA 02496211 2005-02-18
68
(110) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(2-cyclohexyl-
ethyl)amino]-1,7-dihydro-purin-6-one
(111) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-((3-cyclohexyl-
propyl)amino]-1,7-dihydro-purin-6-one
(112) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(cyclobutylamino)-1,7-
dihydro-purin-6-one
(113) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(cyclopentylamino)-1,7-
dihydro-purin-6-one
(114) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(phenylamino)-1,7-
dihydro-purin-6-one
(115) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(3,4-dichlorobenzyl-
amino)-1,7-dihydro-purin-6-one
(116) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(4-methylbenzyl-amino)-
1,7-dihydro-purin-6-one
(117) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(4-methoxybenzyl-
amino)-1,7-dihydro-purin-6-one
(118) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(4-trifluoromethylbenzyl-
amino)-1,7-dihydro-purin-6-one
(119) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(4-
trifluoromethoxybenzyl-amino)-1,7-dihydro-purin-6-one
(120) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(4-
difluoromethoxybenzyl-amino)-1,7-dihydro-purin-6-one
CA 02496211 2005-02-18
69
(121) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(3,4-
methylenedioxybenzyl-amino)-1,7-dihydro-purin-6-one
(122) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(furan-2-yl-
methyl)amino]-1,7-dihydro-purin-6-one
(123) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(thien-2-yl-
methyl)amino]-1,7-dihydro-purin-6-one
(124) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(pyridine-2-yl-
methyl)amino]-1,7-dihydro-purin-6-one
(125) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(4-methylthiazol-2-
ylmethyl)amino]-1,7-dihydro-purin-6-one
(126) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-{[2-(pyridine-2-
yl)ethyl]amino}-1,7-dihydro-purin-8-one
(127) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(pyrrolidin-1-yl)-1,7-
dihydro-purin-6-one
(128) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(morpholin-4-yl)-1,7-
dihydro-purin-6-one
(129) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(piperazin-1-yl)-1,7-
dihydro-purin-6-one
(130) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(4-methyl-piperazin-1-yl)-
1,7-dihydro-purin-8-one
(131) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-ethyl-1,7-dihydro-purin-6-
one
CA 02496211 2005-02-18
(132) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-propyl-1,7-dihydro-purin-
6-one
(133) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-isopropyl-1,7-dihydro-
purin-6-one
(134) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-butyl-1,7-dihydro-purin-6-
one
(135) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(2-methyl-propyl)-1,7-
dihydro-purin-6-one
(136) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-pentyl-1,7-dihydro-purin-
6-one
(137) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(3-methyl-butyl)-1,7-
dihydro-purin-6-one
(138) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-hexyl-1,7-dihydro-purin-
6-one
(139) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(4-methyl-pentyl)-1,7-
dihydro-purin-6-one
(140) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-allyl-1,7-dihydro-purin-6-
one
(141) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(3-methyl-but-2-en-1-yl)-
1,7-dihydro-purin-6-one
(142) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(hex-5-en-1-yl)-1,7-
dihydro-purin-6-one
CA 02496211 2005-02-18
71
(143) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(pent-4-en-1-yl)-1,7-
dihydro-purin-6-one
(144) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(prop-2-yn-1-yl)-1,7-
dihydro-purin-6-one
(145) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(2-phenylethenyl)-1,7-
dihydro-purin-6-one
(146) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[2-(4-methyl-
phenyl)ethenyl]-1,7-dihydro-purin-6-one
(147) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[2-(2-chloro-
phenyl)ethenyl]-1,7-dihydro-purin-6-one
(148) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[2-(3-fluoro-
phenyl)ethenyl]-1,7-dihydro-purin-6-one
(149) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[2-(3,4-dimethoxy-
phenyl)ethenyl]-1,7-dihydro-purin-6-one
(150) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[2-(3-chloro-
phenyl)ethenyl]-1,7-dihydro-purin-6-one
(151) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[2-(3-trifluoromethyl-
phenyl)ethenyl]-1,7-dihydro-purin-6-one
(152) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[2-(3-trifluoromethoxy-
phenyl)ethenyl]-1,7-dihydro-purin-6-one
(153) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(3-phenyl-prop-2-en-1-
yl)-1,7-dihydro-purin-6-one
CA 02496211 2005-02-18
72
(154) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-benzyl-1,7-dihydro-purin-
6-one
(155) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-phenyl-1,7-dihydro-purin-
6-one
(156) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(3-phenyl-propyl)-1,7-
dihydro-purin-6-one
(157) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(4-fluoro-benzyl)-1,7-
dihydro-purin-6-one
(158) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(3-trifluoromethyl-
benzyl)-1,7-dihydro-purin-6-one
(159) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(3-chloro-benzyl)-1,7-
dihydro-purin-6-one
(160) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(3-bromo-benzyl)-1,7-
dihydro-purin-6-one
(161) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(3-trifluoromethoxy-
benzyl)-1,7-dihydro-purin-6-one
(162) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(3-methoxy-benzyl)-1,7-
dihydro-purin-6-one
(163) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[2-(3-chloro-
phenyl)ethyl]-1,7-dihydro-purin-6-one
(164) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[2-(2-fluoro-
phenyl)ethyl]-
1,7-dihydro-purin-6-one
CA 02496211 2005-02-18
73
(165) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[2-(4-methyl-
phenyl)ethyl]-1,7-dihydro-purin-6-one
(166) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[2-(4-fluoro-
phenyl)ethyl]-
1,7-dihydro-purin-6-one
(167) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[2-(3-trifluoromethyl-
phenyl)ethyl]-1,7-dihydro-purin-6-one
(168) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-cyclopentyl-1,7-dihydro-
purin-6-one
(169) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-cyclohexyl-1,7-dihydro-
purin-6-one
(170) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(cyclohexylmethyl)-1,7-
dihydro-purin-6-one
(171) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(cyclopentylmethyl)-1,7-
dihydro-purin-6-one
(172) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(2-cyclohexyl-ethyl)-1,7-
dihydro-purin-6-one
(173) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(furan-2-yl)-1,7-dihydro-
purin-6-one
(174) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(thien-2-yl)-1,7-dihydro-
purin-6-one
(175) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(pyridin-2-yl)-1,7-
dihydro-
purin-6-one
CA 02496211 2005-02-18
74
(176) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(pyridin-4-yl)-1,7-
dihydro-
purin-6-one
(177) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(furan-2-yl-methyl)-1,7-
dihydro-purin-6-one
(178) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(thien-2-yl-methyl)-1,7-
dihydro-purin-6-one
(179) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-(pyridin-2-yl-methyl)-1,7-
dihydro-purin-6-one
(180) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[2-(furan-2-yl)ethyl]-1,7-
dihydro-purin-6-one
(181) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[2-(pyridin-2-yl)ethyl]-
1,7-
dihydro-purin-6-one
(182) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[3-(furan-2-yl)propyl]-
1,7-
dihydro-purin-6-one
(183) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[3-(pyridin-2-yl)propyl]-
1,7-dihydro-purin-6-one
(184) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(3-chloro-but-2-en-
1-y1)-1,7-dihydro-purin-6-one
(185) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(3-chloro-prop-2-
en-1-yl)-1,7-dihydro-purin-6-one
(186) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(3-trifluoromethyl-
3-chloro-prop-2-en-1-yl)-1,7-dihydro-purin-6-one
CA 02496211 2005-02-18
(187) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(3,3-dichloro-prop-
2-en-1-yl)-1,7-d ihydro-purin-6-one
(188) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(but-2-yn-1-yl)-1,7-
dihydro-purin-6-one
(189) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(cyclohex-1-en-1-
yl-methyl)-1,7-dihydro-purin-6-one
(190) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-
(cyclohexylmethyl)-1,7-dihydro-purin-6-one
(191) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(4-fluoro-benzyl)-
1,7-dihydro-purin-6-one
(192) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(2-chloro-benzyl)-
1,7-dihydro-purin-6-one
(193) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(3-bromo-benzyl)-
1,7-dihydro-purin-6-one
(194) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(3-methyl-benzyl)-
1,7-dihydro-purin-6-one
(195) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(3-tritluoromethyl-
benzyl)-1,7-dihydro-purin-6-one
(196) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(2-cyano-benzyl)-
1,7-dihydro-purin-6-one
(197) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(2-methoxy-
benzyl)-1,7-dihydro-purin-6-one
CA 02496211 2005-02-18
76
(198) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(3-
trifluoromethoxy-benzyl)-1,7-dihydro-purin-6-one
(199) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(3-
difluoromethoxy-benzyl)-1,7-dihydro-purin-6-one
(200) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(3,4-difluoro-
benzyl)-1,7-dihydro-purin-6-one
(201) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(naphth-1-yl-
methyl)-1,7-dihydro-purin-6-one
(202) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(furan-2-yl-methyl)-
1,7-dihydro-purin-6-one
(203) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(thien-2-yl-methyl)-
1,7-dihydro-purin-6-one
(204) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(pyridin-2-yl-
methyl)-1,7-dihydro-purin-6-one
(205) 8-(3-amino-piperidin-1-yl)-2-(2-phenylethyl)-1-methyl-7-(3-chloro-but-2-
en-1-yl)-1,7-dihydro-purin-6-one
(206) 8-(3-amino-piperidin-1-yl)-2-(2-phenylethyl)-1-methyl-7-(3-chloro-prop-2-
en-1-yl)-1,7-dihydro-purin-6-one
(207) 8-(3-amino-piperidin-1-yl)-2-(2-phenylethyl)-1-methyl-7-(3-
trifluoromethyl-3-chloro-prop-2-en-1-yl)-1,7-dihydro-purin-6-one
(208) 8-(3-amino-piperidin-1-yl)-2-(2-phenylethyl)-1-methyl-7-(3,3-dichloro-
prop-2-en-1-yl)-1,7-dihydro-purin-6-one
CA 02496211 2005-02-18
77
(209) 8-(3-amino-piperidin-1-yl)-2-(2-phenylethyl)-1-methyl-7-(but-2-yn-1-yl)-
1,7-dihydro-purin-6-one
(210) 8-(3-amino-piperidin-1-yl)-2-(2-phenylethyl)-1-methyl-7-(cyclohex-1-en-
1-yl-methyl)-1,7-dihydro-purin-6-one
(211) 8-(3-amino-piperidin-1-yl)-2-(2-phenylethyl)-1-methyl-7-
(cyclohexylmethyl)-1,7-dihydro-purin-6-one
(212) 8-(3-amino-piperidin-1-yl)-2-(2-phenylethyl) -1-methyl-7-(4-fluoro-
benzyl)-1,7-dihydro-purin-6-one
(213) 8-(3-amino-piperidin-1-yl)-2-(2-phenylethyl)-1-methyl-7-(2-chloro-
benzyl)-1,7-dihydro-purin-6-one
(214) 8-(3-amino-piperidin-1-yl)-2-(2-phenylethyl)-1-methyl-7-(3-bromo-
benzyl)-1,7-dihydro-purin-6-one
(215) 8-(3-amino-piperidin-1-yl)-2-(2-phenylethyl)-1-methyl-7-(3-methyl-
benzyl)-1,7-dihydro-purin-6-one
(216) 8-(3-amino-piperidin-1-yl)-2-(2-phenylethyl)-1-methyl-7-(3-
trifluoromethyl-benzyl)-1,7-dihydro-purin-6-one
(217) 8-(3-amino-piperidin-1-yl)-2-(2-phenylethyl)-1-methyl-7-(2-cyano-
benzyl)-1,7-dihydro-purin-6-one
(218) 8-(3-amino-piperidin-1-yl)-2-(2-phenylethyl)-1-methyl-7-(2-methoxy-
benzyl)-1,7-dihydro-purin-6-one
(219) 8-(3-amino-piperidin-1-yl)-2-(2-phenylethyl)-1-methyl-7-(3-
trifluoromethoxy-benzyl)-1,7-dihydro-purin-6-one
CA 02496211 2005-02-18
78
(220) 8-(3-amino-piperidin-1-yl)-2-(2-phenylethyl)-1-methyl-7-(3-
difluoromethoxy-benzyl)-1,7-dihydro-purin-6-one
(221) 8-(3-amino-piperidin-1-yl)-2-(2-phenylethyl)-1-methyl-7-(3,4-difluoro-
benzyl)-1,7-dihydro-purin-6-one
(222) 8-(3-amino-piperidin-1-yl)-2-(2-phenylethyl)-1-methyl-7-(naphth-1-yl-
methyl)-1,7-dihydro-purin-6-one
(223) 8-(3-amino-piperidin-1-yl)-2-(2-phenylethyl)-1-methyl-7-(furan-2-yl-
methyl)-1,7-dihydro-purin-6-one
(224) 8-(3-amino-piperidin-1-yl)-2-(2-phenylethyl)-1-methyl-7-(thien-2-yl-
methyl)-1,7-dihydro-purin-6-one
(225) 8-(3-amino-piperidin-1-yl)-2-(2-phenylethyl)-1-methyl-7-(pyridin-2-yl-
methyl)-1,7-dihydro-purin-6-one
(226) 8-(3-amino-piperidin-1-yl)-2-(2-phenylethyl)-1-methyl-7-benzyl-1,7-
dihydro-purin-6-one
(227) 8-(3-amino-pyrrolidin-1-yl)-2-benzylamino-1-methyl-7-benzyl-1,7-
dihydro-purin-6-one
(228) 8-(piperidin-3-yl)-2-benzylamino-1-methyl-7-benzyl-1,7-dihydro-purin-6-
one
(229) 8-(piperidin-4-yl)-2-benzylamino-1-methyl-7-benzyl-1,7-dihydro-purin-6-
one
(230) 8-(3-amino-hexahydroazepin-1-yl)-2-benzylamino-1-methyl-7-benzyl-
1,7-dihydro-purin-6-one
CA 02496211 2005-02-18
79
(231) 8-(4-amino-hexahydroazepin-1-yl)-2-benzylamino-1-methyl-7-benzyl-
1, 7-d i hyd ro-p a ri n-6-one
(232) 8-(piperazin-1-yl)-2-benzylamino-1-methyl-7-benzyl-1,7-dihydro-purin-6-
one
(233) 8-(1,4-diazepan-1-yl)-2-benzylamino-1-methyl-7-benzyl-1,7-dihydro-
purin-6-one
(234) 8-(2-amino-cyclohexylamino)-2-benzylamino-1-methyl-7-benzyl-1,7-
dihydro-purin-6-one
(235) 8-(3-amino-cyclohexyl)-2-benzylamino-1-methyl-7-benzyl-1,7-dihydro-
puri n-6-one
(236) 8-(3-amino-pyrrolidin-1-yl)-2-(2-phenylethyl)-1-methyl-7-benzyl-1,7-
dihydro-purin-6-one
(237) 8-(piperidin-3-yl)-2-(2-phenylethyl)-1-methyl-7-benzyl-1,7-dihydro-purin-
6-one
(238) 8-(piperidin-4-yl)-2-(2-phenylethyl)-1-methyl-7-benzyl-1,7-dihydro-purin-
6-one
(239) 8-(3-amino-hexahydroazepin-1-yl)-2-(2-phenylethyl)-1-methyl-7-benzyl-
1,7-dihydro-purin-6-one
(240) 8-(4-amino-hexahydroazepin-1-yl)-2-(2-phenylethyl)-1-methyl-7-benzyl-
1,7-dihydro-purin-6-one
(241) 8-(piperazin-1-yl)-2-(2-phenylethyl)-1-methyl-7-benzyl-1,7-dihydro-purin-
6-one
80
(242) 8-(1,4-diazepan-1-yl)-2-(2-phenylethyl)-1-methyl-7-benzyl-1,7-dihydro-
purin-6-one
(243) 8-(2-amino-cyclohexylamino)-2-(2-phenylethyl)-1-methyl-7-benzyl-1,7-
dihydro-purin-6-one
(244) 8-(3-amino-cyclohexyl)-2-(2-phenylethyl)-1-methyl-7-benzyl-1,7-dihydro-
purin-6-one
(245) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(but-2-en-1-yl)-1,7-
dihydro-purin-6-one
(246) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(2-methyl-but-2-
en-1-yl)-1,7-d ihydro-pu rin-6-one
(247) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(2,3-dimethyl-but-
2-en-1-yl)-1,7-dihydro-purin-6-one
(248) 8-(3-amino-piperidin-1-yl)-2-benzylamino-1-methyl-7-(cyclopent-1-en-1-
yl-methyl)-1,7-dihydro-purin-6-one
(249) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(2-phenyl-2-oxo-ethyl)-1,7-
d ihyd ro-pu ri n-6-one
(250) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(2-amino-phenyl)-2-oxo-ethyl]-
1,7-dihydro-purin-6-one
(251 ) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(2-acetylamino-phenyl)-2-oxo-
ethyl]-1,7-dihydro-purin-6-one
(252) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(2-methylsulphonylamino-
phenyl)-2-oxo-ethyl]-1,7-dihydro-purin-6-one
CA 02496211 2005-02-18
CA 02496211 2005-02-18
$1
(253) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(2-hydroxy-phenyl)-2-oxo-ethyl]-
1,7-dihydro-purin-6-one
(254) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-[2-(2-methoxy-phenyl)-2-oxo-
ethyl]-1,7-dihydro-purin-6-one
(255) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-{2-[2-(ethoxycarbonylmethoxy)-
phenyl]-2-oxo-ethyl}-1,7-dihydro-purin-6-one
(256) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-{2-[2-(aminocarbonylmethoxy)-
phenyl]-2-oxo-ethyl}-1,7-dihydro-purin-6-one
(257) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-{2-[2-(methylaminocarbonyl-
methoxy)-phenyl]-2-oxo-ethyl}-1,7-dihydro-purin-6-one
(258) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-{2-[2-(ethylaminocarbonyl-
methoxy)-phenyl]-2-oxo-ethyl}-1,7-dihydro-purin-6-one
(259) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-{2-[2-(dimethylaminocarbonyl-
methoxy)-phenyl]-2-oxo-ethyl}-1,7-dihydro-purin-6-one
(260) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(naphth-1-yl-methyl)-1,7-dihydro-
purin-6-one
(261) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(isoquinolin-1-yl-methyl)-1,7-
dihydro-purin-6-one
(262) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(quinazolin-2-yl-methyl)-1,7-
dihydro-purin-6-one
(263) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(quinolin-4-yl-methyl)-1,7-dihydro-
purin-6-one
CA 02496211 2005-02-18
82
(264) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(quinazolin-4-yl-methyl)-1,7-
dihydro-purin-6-one
(265) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(2-phenylethyl)-1,7-dihydro-purin-
6-one
(266) 8-[(2-aminoethyl)amino]-7-benzyl-1-methyl-2-benzylamino-1,7-dihydro-
puri n-6-one
(267) 8-[N-methyl-N-(2-aminoethyl)-amino]-7-benzyl-1-methyl-2-benzylamino-
1,7-dihydro-purin-6-one
(268) 8-[N-ethyl-N-(2-aminoethyl)-amino]-7-benzyl-1-methyl-2-benzylamino-
1,7-dihydro-purin-6-one
(269) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[2-(2-cyanophenyl)ethyl]-
1, 7-d i hyd ro-pu ri n-6-one
(270) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[2-(2-aminocarbonyl-
phenyl)ethyl]-1,7-dihydro-purin-6-one
(271) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[2-(2-
dimethylaminocarbonyl-phenyl)ethyl]-1,7-dihydro-purin-6-one
(272) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[2-(2-methylsulphonyl-
phenyl)ethyl]-1,7-dihydro-purin-6-one
(273) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[2-(2-
methylsulphonylamino-phenyl)ethyl]-1,7-dihydro-purin-6-one
(274) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(2-
methylsulphonylamino-ethyl)amino]-1,7-dihydro-purin-6-one
CA 02496211 2005-02-18
83
(275) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(2-
phenylsulphonylamino-ethyl)amino]-1,7-dihydro-purin-6-one
(276) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(naphth-1-yl-
methyl)amino]-1,7-dihydro-purin-6-one
(277) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(quinazolin-2-yl-
methyl)ami no]-1,7-dihydro-purin-6-one
(278) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(isoquinolin-1-yl-
methyl)-
amino]-1,7-dihydro-purin-6-one
(279) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(4,5-dimethyl-oxazol-2-yl-methyl)-
1,7-dihydro-purin-6-one
(280) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(4,5-dimethyl-thiazol-2-yl-methyl)-
1,7-dihydro-purin-6-one
(281) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(4,6-dimethyl-pyrimidin-2-yl-
methyl)-1,7-dihydro-purin-6-one
(282) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-(pyrazin-2-yl-methyl)-1,7-dihydro-
purin-6-one
(283) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(3-methyl-isoxazol-5-yl-
methyl)-amino]-1,7-dihydro-purin-6-one
(284) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(4,5-dimethyl-oxazol-2-
yl-methyl)-amino]-1,7-dihydro-purin-6-one
(285) 8-(3-amino-piperidin-1-yl)-7-benzyl-1-methyl-2-[(pyrazin-2-yl-methyl)-
amino]-1,7-dihydro-purin-6-one
CA 02496211 2005-02-18
84
Example 2
Coated tablets containin4 75 ma of active substance
1 tablet core contains:
active substance 75.0 mg
calcium phosphate 93.0 mg
corn starch 35.5 mg
polyvinylpyrrolidone 10.0 mg
hydroxypropylmethylcellulose15.0 mg
magnesium stearate 1.5 mg
230.0 mg
Preparation:
The active substance is mixed with calcium phosphate, corn starch, polyvin-
ylpyrrolidone, hydroxypropylmethylcellulose and half the specified amount of
magnesium stearate. Blanks 13 mm in diameter are produced in a tablet-
making machine and these are then rubbed through a screen with a mesh size
of 1.5 mm using a suitable machine and mixed with the rest of the magnesium
stearate. This granulate is compressed in a tablet-making machine to form
tablets of the desired shape.
Weight of core: 230 mg
die: 9 mm, convex
The tablet cores thus produced are coated with a film consisting essentially
of
hydroxypropylmethylcellulose. The finished film-coated tablets are polished
with beeswax.
Weight of coated tablet: 245 mg.
CA 02496211 2005-02-18
Example 3
Tablets containing 100 ma of active substance
Composition:
1 tablet contains:
active substance 100.0 mg
lactose 80.0 mg
corn starch 34.0 mg
polyvinylpyrrolidone4.0 mg
magnesium stearate 2.0 ma
220.0 mg
Method of Preparation:
The active substance, lactose and starch are mixed together and uniformly
moistened with an aqueous solution of the polyvinylpyrrolidone. After the
moist
composition has been screened (2.0 mm mesh size) and dried in a rack-type
drier at 50°C it is screened again (1.5 mm mesh size) and the lubricant
is
added. The finished mixture is compressed to form tablets.
Weight of tablet: 220 mg
Diameter: 10 mm, biplanar, facetted on both sides and notched on one side.
Example 4
Tablets containing 150 ma of active substance
Composition:
1 tablet contains:
active substance 150.0 mg
powdered lactose 89.0 mg
corn starch 40.0 mg
colloidal silica 10.0 mg
polyvinylpyrrolidone10.0 mg
magnesium stearate1.0 ma
300.0 mg
CA 02496211 2005-02-18
86
Preparation:
The active substance mixed with lactose, corn starch and silica is moistened
with a 20% aqueous polyvinylpyrrolidone solution and passed through a
screen with a mesh size of 1.5 mm. The granules, dried at 45°C, are
passed
through the same screen again and mixed with the specified amount of
magnesium stearate. Tablets are pressed from the mixture.
Weight of tablet: 300 mg
die: 10 mm, flat
Example 5
Hard gelatine capsules containing 150 mg of active substance
1 capsule contains:
active substance 150.0 mg
corn starch (dried) approx. 80.0 mg
lactose (powdered) approx. 87.0 mg
magnesium stearate 3.0 ma
approx. 420.0 mg
Preparation:
The active substance is mixed with the excipients, passed through a screen
with a mesh size of 0.75 mm and homogeneously mixed using a suitable
apparatus. The finished mixture is packed into size 1 hard gelatine capsules.
Capsule filling: approx. 320 mg
Capsule shell: size 1 hard gelatine capsule.
CA 02496211 2005-02-18
87
Example 6
Suppositories containing 150 ma of active substance
1 suppository contains:
active substance 150.0 mg
polyethyleneglycol 1500 550.0 mg
polyethyleneglycol 6000 460.0 mg
polyoxyethylene sorbitan monostearate840.0 ma
2,000.0 mg
Preparation:
After the suppository mass has been melted the active substance is
homogeneously distributed therein and the melt is poured into chilled moulds.
Example 7
Suspension containing 50 mg of active substance
100 ml of suspension contain:
active substance 1.00 g
carboxymethylcellulose-Na-salt0.10 g
methyl p-hydroxybenzoate 0.05 g
propyl p-hydroxybenzoate 0.01 g
glucose 10.00 g
glycerol
5.00 g
70% sorbitol solution 20.00 g
flavouring 0.30 g
dist. water ad 100 ml
Preparation:
The distilled water is heated to 70°C. The methyl and propyl
p-hydroxybenzoates together with the glycerol and sodium salt of
carboxymethylcellulose are dissolved therein with stirring. The solution is
cooled to ambient temperature and the active substance is added and
homogeneously dispersed therein with stirring. After the sugar, the sorbitol
CA 02496211 2005-02-18
solution and the flavouring have been added and dissolved, the suspension is
evacuated with stirring to eliminate air.
ml of suspension contain 50 mg of active substance.
Example 8
Ampoules containing 10 mg active substance
Composition:
active substance 10.0 mg
0.01 N hydrochloric acid q.s.
double-distilled water ad 2.0 ml
Preparation:
The active substance is dissolved in the necessary amount of 0.01 N HCI,
made isotonic with common salt, filtered sterile and transferred into 2 ml
ampoules.
Example 9
Ampoules containing 50 mp of active substance
Composition:
active substance 50.0 mg
0.01 N hydrochloric acid q.s.
double-distilled water ad 10.0 ml
Preparation:
The active substance is dissolved in the necessary amount of 0.01 N HCI,
made isotonic with common salt, filtered sterile and transferred into 10 ml
ampoules.