Note: Descriptions are shown in the official language in which they were submitted.
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
2,4,5-TRISUBSTITUTED IMIDAZOLES AND THEIR USE
AS ANTI-MICROBIAL AGENTS
FIELD OF THE INVENTION
This invention pertains to the field of anti-microbial compounds and, in
particular, to
the use of 2,4,5-trisubstituted imidazole compounds in the treatment of
microbial
infections.
BACKGROUND OF THE INVENTION
There is currently an urgent need for compounds with broad-spectrum anti-
microbial
activity for the preparation of new anti-microbial agents. The increasing
incidence of
infectious disease caused by microbial pathogens in both communities and
hospitals is
a worldwide health concern. Severe invasive infections are reported as the
main
complication in cancer therapies, as well as bone marrow transplantation and
major
surgeries. Infection is also a major concern for immuno-compromised patients
with
haematological malignancy and/or AIDS.
Amongst bacterial pathogens, there has recently been a significant increase of
multi-
2o drug resistance. For example, strains of Staphylococcus aureus (methicillin-
resistant
or MRSA) and coagulase-negative Staphylococci (CoNS) have become resistant to
the most commonly used antibiotics, such that the only available antibiotics
uniformly
active against them are the glycopeptides, vancomycin and teicoplanin. S.
aureus is
one of the leading causes of hospital-acquired bacteremia capable of causing a
wide
range of diseases ranging from superficial skin infections to potentially
fatal illnesses
such as bloodstream infection, endocarditis and pneumonia (Diekema et al.
Clin.
Infect. Dis. 2001, 32:S114-132). Other human pathogens that have begun to
develop
resistance to multiple antibiotics include Streptococcus pneunaoniae (the
leading
cause of nosocomial infections) and Pseudoinonas aeruginosa, Haenzophilus
1
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
influenzae and Moraxella catarrhalis (the most common community-acquired
respiratory pathogens; Hoban et al. Clin. Infect. Dis. 2001, 32:581-93).
Fungal infections are also becoming a major health concern for a number of
reasons,
including the limited number of anti-fungal agents currently available, the
increasing
incidence of species resistant to older anti-fungal agents, and the growing
population
of
immuno-compromised patients at risk for opportunistic fungal infections. The
most
common clinical fungal isolate is Candida albicans (comprising about 19% of
all
isolates). In one study, nearly 40% of all deaths from hospital-acquired
infections
were due to fungi (Sternberg, Science, 1994, 266:1632-1634).
Thus, new classes of anti-microbial agents are needed to address both the
growing
resistance amongst microbes to present therapies and the general lack of
efficacy of
existing antibiotics against slow-growing organisms.
Heterocyclic compounds, especially heterocyclic azole derivatives, have been
shown
to have a wide spectrum of biological activities. One class of compounds with
interesting biological activities is the imidazoles (derivatives containing a
five-
membered heterocyclic azole). A variety of biological activities have been
reported
for imidazole derivatives with different substitution patterns (Lee et al.
Nature 1994
327:739-745; Abdel-Meguid et al. Biochemistry, 1994, 33:11671; Heerding et al.
Bioorg. Med. Chem. Lett. 2001, 11:2061-2065; Bu et al. Tetrahedron Lett. 1996,
37:7331-7334; Lewis JR. Nat. Prod. Rep. 1999, 16:389-418; Lewis JR. Nat. Prod.
Rep. 1998, 15:417-437 and 371-395).
Biological activities have also been reported for aryl-imidazole derivatives,
for
example, these compounds can act as modulators of multi-drug resistance in
cancer
cells (Zhang et al. Bioorg. Med. Chem. Lett. 2000, 10:2603-2605), inhibitors
of p38
MAP kinase (Adams et al. Bioorg. Med. Chem. Lett. 2001, 11:867-2870, McLay et.
al. Bioorg. Med. Chem. 2001, 9:537-554) and of cytokines (U.S. Patent Nos.
2
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
5,656,644; 5,686,455; 5,916,891; 5,945,418; and 6,268,370), and inhibitors of
bacterial growth (Antolini et al. Bioorg. Med. Chem. Lett. 1999, 9:1023-1028).
Recent reports have indicated that triaryl-imidazole compounds can act as
inhibitors
of p38 MAP kinase (for example, see LoGrasso et al. Biochemistry. 1997,
36:10422-
10427) and as modulators of multi-drug resistance in cancer cells (Sarshar et
al.
Bioorg. Med. Chem. Lett. 2000, 10:2599-2601), however, these compounds have
found use mainly as colour producing reagents (U.S. Patent Nos. 4,089,747;
5,024,935; 5,047,318; 5,496,702; 5,514,550; and 5,693,589) and as
photopolymerization initiators (U.S. Patent Nos. 6,117,609 and 6,060,216),
generally
in dimeric form.
This background information is provided for the purpose of making known
information believed by the applicant to be of possible relevance to the
present
invention. No admission is necessarily intended, nor should be construed, that
any of
the preceding information constitutes prior art against the present invention.
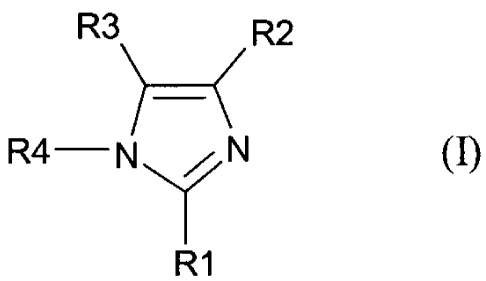
SUMMARY OF THE INVENTION
It is an object of the present invention to provide a class of compounds which
are
2,4,5-trisubstituted imidazole derivatives that have anti-microbial activity.
In
accordance with an aspect of the present invention there is provided a use of
a
compound having structural formula (I), or a salt thereof, as an anti-
microbial agent,
R3\ / R2
R4-N /I N (I)
R1
wherein:
Rl is aryl, substituted aryl, heterocycle, substituted heterocycle,
heteroaryl, or
substituted heteroaryl;
3
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
R2 and R3 are independently aryl, substituted aryl, heterocycle, heteroaryl,
substituted
heterocycle, or substituted heteroaryl or R2 and R3 when taken together along
with
the carbon atoms they are attached to, form aryl or substituted aryl, and
R4 is hydrogen, halogen, hydroxyl, thiol, lower alkyl, substituted lower
alkyl, lower
alkenyl, substituted lower alkenyl, lower alkynyl, substituted lower alkynyl,
alkylalkenyl, alkyl alkynyl, alkoxy, alkylthio, acyl, aryloxy, amino, amido,
carboxyl,
aryl, substituted aryl, heterocycle, heteroaryl, substituted heterocycle,
heteroalkyl,
cycloalkyl, substituted cycloalkyl, alkylcycloalkyl, alkylcycloheteroalkyl,
nitro, or
cyano.
In accordance with another aspect of the present invention, there is provided
a use of a
compound having structural formula (I), or a salt thereof, in the treatment or
prevention of a microbial infection, or a disease or disorder associated
therewith, in an
animal in need of such therapy.
In accordance with another aspect of the present invention, there is provided
a use of a
compound having structural formula (I), or a salt thereof, in the preparation
of an anti-
microbial composition.
In accordance with another aspect of the present invention, there is provided
a method
of inhibiting the growth and/or proliferation of a microbial cell comprising
contacting
said microbial cell with an effective amount of a compound having general
formula
(I), or a salt thereof.
In accordance with another aspect of the present invention, there is provided
an anti-
microbial composition comprising an effective amount of a compound having
strucural formula (I), or a salt thereof, and a carrier, diluent or excipient.
In accordance with another aspect of the present invention, there is provided
a
compound having the structural formula:
4
CA 02496241 2011-03-14
R3 R2
R4-N iN R5
R6 II
I
R10 R8
or a salt thereof, wherein:
R2 and R3 are independently aryl, substituted aryl, heterocycle, heteroaryl,
substituted
heterocycle, or substituted heteroaryl or R2 and R3 when taken together along
with
the carbon atoms they are attached form aryl or substituted aryl, heterocycle,
heteroaryl, substituted heterocycle, or substituted heteroaryl;
R4, R5, R6, R7, R8 and R9 are independently selected from hydrogen, halogen,
hydroxyl, thiol, lower alkyl, substituted lower alkyl, lower alkenyl,
substituted lower
io alkenyl, lower alkynyl, substituted lower alkynyl, alkylalkenyl, alkyl
alkynyl, alkoxy,
alkylthio, acyl, aryloxy, amino, amido, carboxyl, aryl, substituted aryl,
heterocycle,
heteroaryl, substituted heterocycle, heteroalkyl, cycloalkyl, substituted
cycloalkyl,
alkylcycloalkyl, alkylcycloheteroalkyl, nitro, or cyano;
RIO is H, alkyl, substituted alkyl, alkenyl, substituted alkenyl, alkynyl,
substituted
alkynyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl, acyl, -
CH2-aryl, or -
CH2-heteroaryl.
In accordance with another aspect of the present invention, there is provided
a
compound having the structural formula:
Phl Ph2
R4_N N R5 III
R6
R9 N R6
R10 R8
or a salt thereof, wherein:
Phi and Ph2 are independently selected from phenyl and substituted phenyl;
5
CA 02496241 2011-03-14
R4, R5, R6, R7, R8 and R9 are independently selected from hydrogen, halogen,
hydroxyl, thiol, lower alkyl, substituted lower alkyl, lower alkenyl,
substituted lower
alkenyl, lower alkynyl, substituted lower alkynyl, alkylalkenyl, alkyl
alkynyl, alkoxy,
alkylthio, acyl, aryloxy, amino, amido, carboxyl, aryl, substituted aryl,
heterocycle,
heteroaryl, substituted heterocycle, heteroalkyl, cycloalkyl, substituted
cycloalkyl,
alkylcycloalkyl, alkylcycloheteroalkyl, nitro, or cyano;
RIO is H, alkyl, substituted alkyl, alkenyl, substituted alkenyl, alkynyl,
substituted
alkynyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl, acyl, -
CH2-aryl, or -
CH2-heteroaryl;
1o with the proviso that the compounds are other than:
3-(4,5 -diphenyl-1 H-imidazol-2-yl)-1-methyl-1 H-indole;
3-[4-(4-chlorophenyl)-5-phenyl-1 H-imidazol-2-yl]-1-methyl-I H-indole;
3-[4-(4-bromophenyl)-5-phenyl-1 H-imidazol-2-yl]-1-methyl-1 H-indole;
3-[4-(4-methylphenyl)-5-phenyl-1 H-imidazol-2-yl]-1-methyl-I H-indole;
3-[4-(4-methoxyphenyl)-5-phenyl-lH-imidazol-2-yl]-1-methyl-iH-indole;
3-[4-(4-ethoxyphenyl)-5-phenyl-1 H-imidazol-2-yl]-1-methyl-1 H-indole;
3-[4,5-bis (4-methoxydiphenyl)-1H-imidazol-2-yl]-1-methyl-IH-indole;
4,4'-[2-(2-phenyl-1 H-indol-3-yl)-1 H-imidazole-4,5-diyl]bis[N,N-
dimethyl]benzenamine;
4,4'-[2-(5-chloro-lH-indol-3-yl)-1H-imidazole-4,5-diyl]bis[N,N-
dimethyl] benzenamine;
2-(3-indolyl)-4,5-bis[4-(dimethylamino)phenyl] imidazole;
2-(3-indolyl)-4,5-bis[4-(diethylamino)phenyl] imidazole;
2-(2-phenyl-3 -indo lyl)-4, 5 -bis [4-(dimethylam ino)phenyl] im idazole;
2-(2-chloro-3-indolyl)-4,5-bis[4-(dimethylamino)phenyl]imidazole;
2-(2-ethylcarboxylate-3-indolyl)-4,5-bis [4-(dimethylamino)phenyl] imidazole;
2-(5-chloro-3-indolyl)-4,5-bis[4-(dimethylamino)phenyl] imidazole;
2-(5-cyano-3-indolyl)-4,5-bis[4-(dimethylamino)phenyl] imidazole;
2-(5-nitro-3-indolyl)-4,5-bis[4-(dimethylamino)phenyl] imidazole.
In accordance with another aspect of the present invention, there is provided
a
compound having the structural formula:
6
CA 02496241 2011-03-14
z'=r' r=z
Y ,/Y
x x
VI
R4_N N R5
R6
R9 N R7
R10 R8
or a salt thereof, wherein:
R4, R5, R6, R7, R8 and R9 are independently selected from hydrogen, halogen,
hydroxyl, thiol, lower alkyl, substituted lower alkyl, lower alkenyl,
substituted lower
alkenyl, lower alkynyl, substituted lower alkynyl, alkylalkenyl, alkyl
alkynyl, alkoxy,
alkylthio, acyl, aryloxy, amino, amido, carboxyl, aryl, substituted aryl,
heterocycle,
heteroaryl, substituted heterocycle, heteroalkyl, cycloalkyl, substituted
cycloalkyl,
alkylcycloalkyl, alkylcycloheteroalkyl, nitro, or cyano;
RIO is H, alkyl, substituted alkyl, alkenyl, substituted alkenyl, alkynyl,
substituted
alkynyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl, acyl, -
CH2-aryl, or -
CH2-heteroaryl;
x is CRI 1 or N;
y is CR12 or N;
z is CR13 or N;
r is CR14 or N;
x' is CR15 or N;
y' is CR16 or N;
z' is CRI7 or N;
r' is CR18 or N;
R11, R12, R13, R14, R15, R16, R17 and R18 are independently selected from
hydrogen, halogen, hydroxyl, thiol, lower alkyl, substituted lower alkyl,
alkenyl,
alkylalkenyl, alkyl alkynyl, alkoxy, alkylthio, acyl, aryloxy, amino, amido,
carboxyl,
aryl, substituted aryl, heterocycle, heteroaryl, substituted heterocycle,
heteroalkyl,
cycloalkyl, substituted cycloalkyl, alkylcycloalkyl, alkylcycloheteroalkyl,
nitro, or
cyano.
7
CA 02496241 2011-03-14
In accordance with another aspect of the present invention, there is provided
a
compound having the structural formula:
R17 R18 R14 R13
R16 R12
VII
R15 R11
R4_NN N R5
LR6
R9 N R7
R10 R8
or a salt thereof, wherein:
R4, R5, R6, R7, R8 and R9 are independently selected from hydrogen, halogen,
hydroxyl, thiol, lower alkyl, substituted lower alkyl, lower alkenyl,
substituted lower
1o alkenyl, lower alkynyl, substituted lower alkynyl, alkylalkenyl, alkyl
alkynyl, alkoxy,
alkylthio, acyl, aryloxy, amino, amido, carboxyl, aryl, substituted aryl,
heterocycle,
heteroaryl, substituted heterocycle, heteroalkyl, cycloalkyl, substituted
cycloalkyl,
alkylcycloalkyl, alkylcycloheteroalkyl, nitro, or cyano;
RiO is H, alkyl, substituted alkyl, alkenyl, substituted alkenyl, alkynyl,
substituted
alkynyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl, acyl, -
CH2-aryl, or -
CH2-heteroaryl;
RI I, R12, R13, R14, R15, R16, R17 and R18 are independently selected from
hydrogen, halogen, hydroxyl, thiol, lower alkyl, substituted lower alkyl,
alkenyl,
alkenyl, alkylalkenyl, alkyl alkynyl, alkoxy, alkylthio, acyl, aryloxy, amino,
amido,
carboxyl, aryl, substituted aryl, heterocycle, heteroaryl, substituted
heterocycle,
heteroalkyl, cycloalkyl, substituted cycloalkyl, alkylcycloalkyl,
alkylcycloheteroalkyl,
nitro, or cyano.
BRIEF DESCRIPTION OF THE FIGURES
Figure 1 depicts the bactericidal effect of compounds of Formula I against
multi-drug
resistant Staphylococcus aureus (CMRSA-1 B).
8
CA 02496241 2011-03-14
DETAILED DESCRIPTION OF THE INVENTION
The present invention provides a class of 2,4,5-trisubstituted imidazole
compounds
and for their use as anti-microbial agents. In the context of the present
invention, the
term "anti-microbial" refers to the inhibition, prevention or eradication of
the growth
or proliferation of bacteria and/or fungi and to the inhibition, prevention or
eradication of the growth or proliferation of microbially-infected cells.
Definitions
Unless defined otherwise, all technical and scientific terms used herein have
the same
meaning as commonly understood by one of ordinary skill in the art to which
this
invention pertains.
The terms are defined as follows:
The term "halogen" refers to fluorine, bromine, chlorine, and iodine atoms.
The term "hydroxyl" refers to the group -OH.
The term "thiol" or "mercapto" refers to the group -SH, and -S(0)0-2-
The term "lower alkyl" refers to a straight chain or branched, or cyclic,
alkyl group of
one to ten carbon atoms. This term is further exemplified by such groups as
methyl,
ethyl, n-propyl, i-propyl, n-butyl, t-butyl, i-butyl (or 2-methylpropyl),
cyclopropylmethyl, i-amyl, n-amyl, hexyl and the like.
The term "substituted lower alkyl" refers to lower alkyl as just described
including
one or more groups such as hydroxyl, thiol, alkylthiol, halogen, alkoxy,
amino,
amido, carboxyl, cycloalkyl, substituted cycloalkyl, heterocycle,
cycloheteroalkyl,
substituted cycloheteroalkyl, acyl, carboxyl, aryl, substituted aryl, aryloxy,
hetaryl,
9
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
substituted hetaryl, aralkyl, heteroaralkyl, alkyl alkenyl, alkyl alkynyl,
alkyl
cycloalkyl, alkyl cycloheteroalkyl, cyano. These groups may be attached to any
carbon atom of the lower alkyl moiety.
The term "lower alkenyl" refers to a straight chain or branched, alkenyl group
of two
to ten carbon atoms.
The term "substituted lower alkenyl" refers to lower alkenyl as just described
including one or more groups such as hydroxyl, thiol, alkylthiol, halogen,
alkoxy,
amino, amido, carboxyl, cycloalkyl, substituted cycloalkyl, heterocycle,
cycloheteroalkyl, substituted cycloheteroalkyl, acyl, carboxyl, aryl,
substituted aryl,
aryloxy, hetaryl, substituted hetaryl, aralkyl, heteroaralkyl, alkyl, alkenyl,
alkynyl,
alkyl alkenyl, alkyl alkynyl, alkyl cycloalkyl, alkyl cycloheteroalkyl, cyano.
These
groups may be attached to any carbon atom of the lower alkyl moiety.
The term "alkenyl" refers to a group -CR'=CR"R"' where R', R", R"' are each
independently selected from hydrogen, halogen, lower alkyl, substituted lower
alkyl,
acyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl or the like
as defined.
The term "lower alkynyl" refers to a straight chain or branched, alkynyl group
of two
to ten carbon atoms.
The term "substituted lower allcynyl" refers to lower alkynyl as just
described
including one or more groups such as hydroxyl, thiol, alkylthiol, halogen,
alkoxy,
amino, amido, carboxyl, cycloalkyl, substituted cycloalkyl, heterocycle,
cycloheteroalkyl, substituted cycloheteroalkyl, acyl, carboxyl, aryl,
substituted aryl,
aryloxy, hetaryl, substituted hetaryl, aralkyl, heteroaralkyl, alkyl, alkenyl,
alkynyl,
alkyl alkenyl, alkyl alkynyl, alkyl cycloalkyl, alkyl cycloheteroalkyl, cyano.
These
groups may be attached to any carbon atom of the lower alkyl moiety.
CA 02496241 2011-03-14
The term "alkynyl" refers to a group -C=-C-R'; where R' is selected from
hydrogen,
halogen, lower alkyl, substituted lower alkyl, acyl, aryl, substituted aryl,
heteroaryl,
substituted heteroaryl or the like as defined.
The term "alkyl alkenyl" refers to a group -R-CR'=CR"'R"", where R is lower
alkyl,
or substituted lower alkyl, -(CR'=CR")n or -(C=C)n , wherein n is 1-8, R"',
R"" are
each independently selected from hydrogen, halogen, lower alkyl, substituted
lower
alkyl, acyl, aryl, substituted aryl, hetaryl, or substituted hetaryl as
defined below.
The term "alkyl alkynyl" refers to a group -R-C=CR' where R is lower alkyl or
substituted lower alkyl, R' is hydrogen, lower alkyl, substituted lower alkyl,
acyl, aryl,
substituted aryl, hetaryl, or substituted hetaryl as defined below.
The term "alkoxy" refers to the group -OR, where R is lower alkyl, substituted
lower
alkyl, acyl, aryl, substituted aryl, aralkyl, substituted aralkyl,
heteroalkyl,
heteroarylalkyl, cycloalkyl, substituted cycloalkyl, cycloheteroalkyl, or
substituted
cycloheteroalkyl as defined below.
The term "alkylthio" denotes the group -SR, -S(O)õ=1.2 -R, where R is lower
alkyl,
substituted lower alkyl, aryl, substituted aryl, aralkyl or substituted
aralkyl as defined
below.
The term "acyl" refers to groups -C(O)R, where R is hydrogen, lower alkyl,
substituted lower alkyl, aryl, substituted aryl.
The term "aryloxy" refers to groups -OAr, where Ar is an aryl, substituted
aryl,
heteroaryl, or substituted heteroaryl group as defined below.
The term "amino" refers to the group NRR', where R and R' may independently be
3o hydrogen, lower alkyl, substituted lower alkyl, aryl, substituted aryl,
hetaryl,
cycloalkyl, or substituted hetaryl as defined below or acyl.
11
CA 02496241 2011-03-14
The term "amido" refers to the group -C(O)NRR', where R and R' may
independently
be hydrogen, lower alkyl, substituted lower alkyl, aryl, substituted aryl,
hetaryl,
substituted hetaryl as defined below.
The term "carboxyl" refers to the group -C(O)OR, where R may independently be
hydrogen, lower alkyl, substituted lower alkyl, aryl, substituted aryl,
hetaryl,
substituted hetaryl and the like as defined.
The terms "aryl" or "Ar" refer to an aromatic carbocyclic group having at
least one
to aromatic ring (e.g., phenyl or biphenyl) or multiple condensed rings in
which at least
one ring is aromatic (e.g., 1,2,3,4-tetrahydronaphthyl, naphthyl, anthryl, or
phenanthryl, 9-fluorenyl etc.).
The term "substituted aryl" refers to aryl optionally substituted with one or
more
functional groups, e.g., halogen, hydroxyl, thiol, lower alkyl, substituted
lower alkyl,
trifluoromethyl, lower alkenyl, substituted lower alkenyl, lower alkynyl,
substituted
lower alkynyl, alkylalkenyl, alkyl alkynyl, alkoxy, alkylthio, acyl, aryloxy,
amino,
amido, carboxyl, aryl, substituted aryl, heterocycle, substituted heterocycle,
heteroaryl, substituted heteroaryl, heteroalkyl, substituted heteroalkyl,
cycloalkyl,
substituted cycloalkyl, alkylcycloalkyl, alkylcycloheteroalkyl, nitro,
sulfamido or
cyano.
The term "heterocycle" refers to a saturated, unsaturated, or aromatic
carbocyclic
group having a single ring (e.g., morpholino, pyridyl or furyl) or multiple
condensed
rings (e.g., naphthpyridyl, quinoxalyl, quinolinyl, indolizinyl, indanyl or
benzo[b]thienyl) and having at least one hetero atom, such as N, 0 or S,
within the
ring.
The term "substituted heterocycle" refers to heterocycle optionally
substituted with,
halogen, hydroxyl, thiol, lower alkyl, substituted lower alkyl,
trifluoromethyl, lower
alkenyl, substituted lower alkenyl, lower alkynyl, substituted lower alkynyl,
alkylalkenyl, alkyl alkynyl, alkoxy, alkylthio, acyl, aryloxy, amino, amido,
carboxyl,
12
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
aryl, substituted aryl, heterocycle, substituted heterocycle, heteroaryl,
substituted
heteroaryl, heteroalkyl, substituted heteroalkyl, cycloalkyl, substituted
cycloalkyl,
alkylcycloalkyl, alkylcycloheteroalkyl, nitro, sulfamido or cyano and the
like.
The terms "heteroaryl" or "hetaryl" refer to a heterocycle in which at least
one
heterocyclic ring is aromatic.
The term "substituted heteroaryl" refers to a heterocycle optionally mono or
poly
substituted with one or more functional groups, e.g., halogen, hydroxyl,
thiol, lower
alkyl, substituted lower alkyl, trifluoromethyl, lower alkenyl, substituted
lower
alkenyl, lower alkynyl, substituted lower alkynyl, alkylalkenyl, alkyl
alkynyl, alkoxy,
alkylthio, acyl, aryloxy, amino, amido, carboxyl, aryl, substituted aryl,
heterocycle,
substituted heterocycle, heteroaryl, substituted heteroaryl, heteroalkyl,
substituted
heteroalkyl, cycloalkyl, substituted cycloalkyl, alkylcycloalkyl,
alkylcycloheteroalkyl,
nitro, sulfamido or cyano and the like.
The term "aralkyl" refers to the group -R-Ar where Ar is an aryl group and R
is lower
alkyl or substituted lower alkyl group. Aryl groups can optionally be
unsubstituted or
substituted with, e.g., halogen, lower alkyl, alkoxy, alkyl thio,
trifluoromethyl, amino,
amido, carboxyl, hydroxyl, aryl, aryloxy, heterocycle, hetaryl, substituted
hetaryl,
nitro, cyano, alkylthio, thiol, sulfamido and the like.
The term "heteroalkyl" refers to the group -R-Het where Het is a heterocycle
group
and R is a lower alkyl group. Heteroalkyl groups can optionally be
unsubstituted or
substituted with e.g., halogen, lower alkyl, lower alkoxy, lower alkylthio,
trifluoromethyl, amino, amido, carboxyl, hydroxyl, aryl, aryloxy, heterocycle,
hetaryl,
substituted hetaryl, nitro, cyano, alkylthio, thiol, sulfamido and the like.
The term "heteroarylalkyl" refers to the group -R-HetAr where HetAr is an
heteroaryl
group and R lower alkyl or substituted loweralkyl. Heteroarylalkyl groups can
optionally be unsubstituted or substituted with, e.g., halogen, lower alkyl,
substituted
13
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
lower alkyl, alkoxy, alkylthio, aryl, aryloxy, heterocycle, hetaryl,
substituted hetaryl,
nitro, cyano, alkylthio, thiol, sulfamido and the like.
The term "cycloalkyl" refers to a cyclic or polycyclic alkyl group containing
3 to 15
carbon. For polycyclic groups, these may be multiple condensed rings in which
one
of the distal rings may be aromatic (e.g. tetrahydronaphthalene, etc.).
The term "substituted cycloalkyl" refers to a cycloalkyl group comprising one
or more
substituents with, e.g halogen, hydroxyl, thiol, lower alkyl, substituted
lower alkyl,
trifluoromethyl, lower alkenyl, substituted lower alkenyl, lower alkynyl,
substituted
lower alkynyl, alkylalkenyl, alkyl alkynyl, alkoxy, alkylthio, acyl, aryloxy,
amino,
amido, carboxyl, aryl, substituted aryl, heterocycle, heteroaryl, substituted
heterocycle, heteroalkyl, cycloalkyl, substituted cycloalkyl, alkylcycloalkyl,
alkylcycloheteroalkyl, nitro, sulfamido or cyano and the like.
The term "cycloheteroalkyl" refers to a cycloalkyl group wherein one or more
of the
ring carbon atoms is replaced with a heteroatom (e.g., N, 0, S or P).
The term "substituted cycloheteroalkyl" refers to a cycloheteroalkyl group as
herein
defined which contains one or more substituents, such as halogen, lower alkyl,
lower
alkoxy, lower alkylthio, trifluoromethyl, amino, amido, carboxyl, hydroxyl,
aryl,
aryloxy, heterocycle, hetaryl, substituted hetaryl, nitro, cyano, alkylthio,
thiol,
sulfamido and the like.
The term "alkyl cycloalkyl" refers to the group -R-cycloalkyl where cycloalkyl
is a
cycloalkyl group and R is a lower alkyl or substituted lower alkyl. Cycloalkyl
groups
can optionally be unsubstituted or substituted with e.g. halogen, lower alkyl,
lower
alkoxy, lower alkylthio, trifluoromethyl, amino, amido, carboxyl, hydroxyl,
aryl,
aryloxy, heterocycle, hetaryl, substituted hetaryl, nitro, cyano, alkylthio,
thiol,
sulfamido and the like.
I. 2,4,5-Trisubstituted Inzidazole Compounds
14
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
The present invention provides compounds of the general formula (I):
R3 R2
)-( R4 N N (I)
R,
or a salt thereof, wherein:
R1 is aryl, substituted aryl, heterocycle, substituted heterocycle,
heteroaryl, or
substituted heteroaryl;
R2 and R3 are independently aryl, substituted aryl, heterocycle, heteroaryl,
substituted
heterocycle, or substituted heteroaryl or R2 and R3 when taken together along
with
the carbon atoms they are attached to, form aryl or substituted aryl,
heterocycle,
substituted heterocycle, heteroaryl, or substituted heteroaryl;
R4 is hydrogen, halogen, hydroxyl, thiol, lower alkyl, substituted lower
alkyl, lower
alkenyl, substituted lower alkenyl, lower alkynyl, substituted lower alkynyl,
alkylalkenyl, alkyl alkynyl, alkoxy, alkylthio, acyl, aryloxy, amino, amido,
carboxyl,
aryl, substituted aryl, heterocycle, heteroaryl, substituted heterocycle,
heteroalkyl,
cycloalkyl, substituted cycloalkyl, alkylcycloalkyl, alkylcycloheteroalkyl,
nitro, or
cyano.
In another embodiment of the present invention, the compound of Formula I
include
the compound of the structural formula:
Rs` R2
R4- Nei N R5
R6 II
R9 N R7
RIO R8
or a salt thereof, wherein:
CA 02496241 2011-03-14
R2 and R3 are independently aryl, substituted aryl, heterocycle, heteroaryl,
substituted
heterocycle, or substituted heteroaryl or R2 and R3 when taken together along
with
the carbon atoms they are attached form aryl or substituted aryl, heterocycle,
heteroaryl, substituted heterocycle, or substituted heteroaryl;
R4, R5, R6, R7, R8 and R9 are independently selected from hydrogen, halogen,
hydroxyl, thiol, lower alkyl, substituted lower alkyl, lower alkenyl,
substituted lower
alkenyl, lower alkynyl, substituted lower alkynyl, alkylalkenyl, alkyl
alkynyl, alkoxy,
alkylthio, acyl, aryloxy, amino, amido, carboxyl, aryl, substituted aryl,
heterocycle,
heteroaryl, substituted heterocycle, heteroalkyl, cycloalkyl, substituted
cycloalkyl,
1o alkylcycloalkyl, alkylcycloheteroalkyl, nitro, or cyano;
RiO is H, alkyl, substituted alkyl, alkenyl, substituted alkenyl, alkynyl,
substituted
alkynyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl, acyl, -
CH2-aryl, or -
CH2-heteroaryl.
In another embodiment the compound of formula II is other than 3,3'-[5-(4-
methoxyphenyl)-1 H-imidazole-2,4-diyl]bis-1 H-indole.
In another embodiment of the present invention, in the compound of formula II,
when
R2 is selected from phenyl and substituted phenyl then R3 is selected from
heterocycle, heteroaryl, substituted heterocycle, or substituted heteroaryl,
aryl other
than phenyl and substituted aryl other than substituted phenyl or vice versa.
In another embodiment of the present invention, in the compound of formula II,
when
R2 is selected from phenyl and substituted phenyl then R3 is selected from
heterocycle, heteroaryl, substituted heterocycle, or substituted heteroaryl or
vice
versa.
In another embodiment of the present invention, in the compound of formula II,
when
R2 and R3 are independently selected from phenyl and substituted phenyl, then,
i) R2
and R3 are not phenyl at the same time; or ii) R2 and R3 do not have the same
substituents on the same position.
16
CA 02496241 2011-03-14
In another embodiment of the present invention, in the compound of formula II,
when
R2 is selected from phenyl and phenyl substituted with halo, alkyl or alkoxy
then R3
is selected from a phenyl substituted with a substitutent other than halo,
alkyl or
alkoxy.
In another embodiment of the present invention, the compound of Formula II
includes
the compound of the structural formula III:
Ph ;Ph2
R4 _N N R5 III
R6
R9 N R6
R10 RB
or a salt thereof, wherein:
Ph 1 and Ph2 are independently selected from phenyl and substituted phenyl;
R4, R5, R6, R7, R8 and R9 are independently selected from hydrogen, halogen,
hydroxyl, thiol, lower alkyl, substituted lower alkyl, lower alkenyl,
substituted lower
alkenyl, lower alkynyl, substituted lower alkynyl, alkylalkenyl, alkyl
alkynyl, alkoxy,
alkylthio, acyl, aryloxy, amino, amido, carboxyl, aryl, substituted aryl,
heterocycle,
heteroaryl, substituted heterocycle, heteroalkyl, cycloalkyl, substituted
cycloalkyl,
alkylcycloalkyl, alkylcycloheteroalkyl, nitro, or cyano;
RIO is H, alkyl, substituted alkyl, alkenyl, substituted alkenyl, alkynyl,
substituted
alkynyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl, or acyl.
with the proviso that the compounds are other than:
3-(4,5-diphenyl-1 H-imidazol-2-yl)-1-methyl-1 H-indole;
3-[4-(4-chlorophenyl)-5-phenyl-1 H-imidazol-2-yl]-1-methyl-1 H-indole;
3-[4-(4-bromophenyl)-5-phenyl-1 H-imidazol-2-yl]-1-methyl-1 H-indole;
3-[4-(4-methylphenyl)-5-phenyl-IH-imidazol-2-yl]-1-methyl-iH-indole;
3-[4-(4-methoxyphenyl)-5-phenyl-1 H-imidazol-2-yl]-1-methyl-1 H-indole;
3-[4-(4-ethoxyphenyl)-5-phenyl-1 H-imidazol-2-yl]-1-methyl-1 H-indole;
3-[4,5-bis (4-methoxydiphenyl)-IH-imidazol-2-yl]-1-methyl-iH-indole;
17
CA 02496241 2011-03-14
4,4'-[2-(2-phenyl-1 H-indol-3-yl)-1 H-imidazole-4,5-diyl]bis[N,N-
dimethyl]benzenamine;
4,4'-[2-(5-chloro-1 H-indol-3-yl)-1 H-imidazole-4,5-diyl]bis[N,N-
dimethyl] benzenam ine;
2-(3-indolyl)-4,5-bis[4-(dimethylamino)phenyl]imidazole;
2-(3-indolyl)-4,5 -bis[4-(diethylamino)phenyl] imidazole;
2-(2-phenyl-3-indolyl)-4, 5-bis[4-(dimethylamino)phenyl] imidazole;
2-(2-chloro-3-indolyl)-4,5-bis[4-(dimethylamino)phenyl] imidazole;
2-(2-ethylcarboxylate-3-indolyl)-4, 5-bis [4-(dimethylamino)phenyl] imidazole;
2-(5-chloro-3-indolyl)-4,5-bis[4-(dimethylamino)phenyl]imidazole;
2-(5-cyano-3-indolyl)-4, 5-bis[4-(dimethylamino)phenyl] imidazole;
2-(5-nitro-3 -indo lyl)-4, 5 -bis [4-(dimethylamino)phenyl] im idazole;
In another embodiment of the invention, the compound of Formula III is
selected
from:
R13 R11
R13 \ / \ R11
R4
N" NH
R12 R6 R12
R5 / N I R9 N R9
R10 R10
IV V
or a salt thereof, wherein:
R5, R6, R9, RI1, R12 and R13 are independently selected from hydrogen,
halogen,
hydroxyl, thiol, lower alkyl, substituted lower alkyl, lower alkenyl,
substituted lower
alkenyl, lower alkynyl, substituted lower alkynyl, alkylalkenyl, alkyl
alkynyl, alkoxy,
alkylthio, acyl, aryloxy, amino, amido, carboxyl, aryl, substituted aryl,
heterocycle,
heteroaryl, substituted heterocycle, heteroalkyl, cycloalkyl, substituted
cycloalkyl,
alkylcycloalkyl, alkylcycloheteroalkyl, nitro, or cyano;
RIO is H, alkyl, substituted alkyl, alkenyl, substituted alkenyl, alkynyl,
substituted
alkynyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl, or acyl.
18
CA 02496241 2011-03-14
In another embodiment of the present invention, the compound of Formula I
includes
the compound of the structural formula:
z=r' r=z
Y. Y
xX
VI
R4_N~N R5
LR6
R9 N R7
R10 R8
or a salt thereof, wherein:
R4, R5, R6, R7, R8 and R9 are independently selected from hydrogen, halogen,
hydroxyl, thiol, lower alkyl, substituted lower alkyl, lower alkenyl,
substituted lower
alkenyl, lower alkynyl, substituted lower alkynyl, alkylalkenyl, alkyl
alkynyl, alkoxy,
alkylthio, acyl, aryloxy, amino, amido, carboxyl, aryl, substituted aryl,
heterocycle,
heteroaryl, substituted heterocycle, heteroalkyl, cycloalkyl, substituted
cycloalkyl,
alkylcycloalkyl, alkylcycloheteroalkyl, nitro, or cyano;
xis CR11 or N;
yisCR12orN;
z is CR13 or N;
r is CR14 or N;
x' is CR15 or N;
y' is CR16 or N;
z' is CR17 or N;
r' is CR18 or N;
RI I, R12, R13, R14, R15, R16, R17 and R18 are independently selected from
hydrogen, halogen, hydroxyl, thiol, lower alkyl, substituted lower alkyl,
lower
alkenyl, substituted lower alkenyl, lower alkynyl, substituted lower alkynyl,
alkylalkenyl, alkyl alkynyl, alkoxy, alkylthio, acyl, aryloxy, amino, amido,
carboxyl,
19
CA 02496241 2011-03-14
aryl, substituted aryl, heterocycle, heteroaryl, substituted heterocycle,
heteroalkyl,
cycloalkyl, substituted cycloalkyl, alkylcycloalkyl, alkylcycloheteroalkyl,
nitro, or
cyano.
In another embodiment of the present invention, the compound of Formula I
includes
the compound of the structural formula:
R17 R18 R14 R13
R16 R12
VII
R15 R11
R4'"N N R5
R6
R9 N R7
R10 R8
or a salt thereof, wherein:
R4, R5, R6, R7, R8 and R9 are independently selected from hydrogen, halogen,
hydroxyl, thiol, lower alkyl, substituted lower alkyl, lower alkenyl,
substituted lower
alkenyl, lower alkynyl, substituted lower alkynyl, alkylalkenyl, alkyl
alkynyl, alkoxy,
alkylthio, acyl, aryloxy, amino, amido, carboxyl, aryl, substituted aryl,
heterocycle,
heteroaryl, substituted heterocycle, heteroalkyl, cycloalkyl, substituted
cycloalkyl,
alkylcycloalkyl, alkylcycloheteroalkyl, nitro, or cyano;
RIO is H, alkyl, substituted alkyl, alkenyl, substituted alkenyl, alkynyl,
substituted
alkynyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl, or acyl;
RII, R12, R13, R14, R15, R16, R17 and R18 are independently selected from
hydrogen, halogen, hydroxyl, thiol, lower alkyl, substituted lower alkyl,
lower
alkenyl, substituted lower alkenyl, lower alkynyl, substituted lower alkynyl,
alkylalkenyl, alkyl alkynyl, alkoxy, alkylthio, acyl, aryloxy, amino, amido,
carboxyl,
aryl, substituted aryl, heterocycle, heteroaryl, substituted heterocycle,
heteroalkyl,
cycloalkyl, substituted cycloalkyl, alkylcycloalkyl, alkylcycloheteroalkyl,
nitro, or
cyano.
CA 02496241 2011-03-14
In another embodiment of the present invention, the compound of Formula I is
selected from the compound of formula:
R2R3 R2 R3 R2~ R3
II
R4_N - N R4,-N i N R4_N N
R9 R5 or or
I R7 O R7 NH
R8 R6 R5 R5
R7 R6 R6
VIII IX x
wherein:
R2 and R3 are independently aryl, substituted aryl, heterocycle, heteroaryl,
substituted
heterocycle, or substituted heteroaryl or R2 and R3 when taken together along
with
the carbon atoms they are attached to, form a aryl, substituted aryl,
heterocycle,
heteroaryl, substituted heterocycle, or substituted heteroaryl;
R4, R5, R6, R7, R8 and R9 are independently selected from hydrogen, halogen,
1o hydroxyl, thiol, lower alkyl, substituted lower alkyl, lower alkenyl,
substituted lower
alkenyl, lower alkynyl, substituted lower alkynyl, alkylalkenyl, alkyl
alkynyl, alkoxy,
alkylthio, acyl, aryloxy, amino, amido, carboxyl, aryl, substituted aryl,
heterocycle,
heteroaryl, substituted heterocycle, heteroalkyl, cycloalkyl, substituted
cycloalkyl,
alkylcycloalkyl, alkylcycloheteroalkyl, nitro, or cyano.
In another embodiment of the present invention, the compound of Formula I
includes
the compound of the structural formula:
z'=r' z
~ z'=r' r=z\ z'=r' r=z\
Y zY Y\X XY Y\X XY
R4-N i N
Or R4-N N R4-N ,N
::Iiirc: or
R7 NR5 R5
R7 R6 R6
XI XII XIII
21
CA 02496241 2011-03-14
or a salt thereof, wherein:
R4, R5, R6, R7, R8 and R9 are independently selected from hydrogen, halogen,
hydroxyl, thiol, lower alkyl, substituted lower alkyl, lower alkenyl,
substituted lower
alkenyl, lower alkynyl, substituted lower alkynyl, alkylalkenyl, alkyl
alkynyl, alkoxy,
alkylthio, acyl, aryloxy, amino, amido, carboxyl, aryl, substituted aryl,
heterocycle,
heteroaryl, substituted heterocycle, heteroalkyl, cycloalkyl, substituted
cycloalkyl,
alkylcycloalkyl, alkylcycloheteroalkyl, nitro, or cyano;
xis CR11 or N;
y is CR12 or N;
zisCR13orN;
risCR14orN;
x' is CR15 or N;
y' is CR16 or N;
z' is CR17 or N;
r' is CR18 or N;
R11, R12, R13, R14, R15, R16, R17 and R18 are independently selected from
hydrogen, halogen, hydroxyl, thiol, lower alkyl, substituted lower alkyl,
lower
alkenyl, substituted lower alkenyl, lower alkynyl, substituted lower alkynyl,
alkylalkenyl, alkyl alkynyl, alkoxy, alkylthio, acyl, aryloxy, amino, amido,
carboxyl,
aryl, substituted aryl, heterocycle, heteroaryl, substituted heterocycle,
heteroalkyl,
cycloalkyl, substituted cycloalkyl, alkylcycloalkyl, alkylcycloheteroalkyl,
nitro, or
cyano.
In another embodiment of the invention, the compound of Formula I is selected
from:
R12
/
/ \ \ R11 R\ R11
N, R4 N N 'R4
R9 R5 O R9 / R5
R6 R6 R6 R6
R7 R7
xlv xv
22
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
R11
R12 R13 R1 \ R11
N\ N-R\ N N\R4
R9 R5 O
R8 R6 R5
R7
XVI XVII
wherein:
R4, R5, R6, R7, R8, R9, R11, R12 and R13 are independently selected from
hydrogen, halogen, hydroxyl, thiol, lower alkyl, substituted lower alkyl,
lower
alkenyl, substituted lower alkenyl, lower alkynyl, substituted lower alkynyl,
alkylalkenyl, alkyl alkynyl, alkoxy, alkylthio, acyl, aryloxy, amino, amino,
carboxyl,
aryl, substituted aryl, heterocycle, heteroaryl, substituted heterocycle,
heteroalkyl,
cycloalkyl, substituted cycloalkyl, alkylcycloalkyl, alkylcycloheteroalkyl,
nitro, or
cyano.
In another embodiment of the invention the compound of Formula I is selected
from:
R11
N NH R12 / \ R11 R12 R11
R5 NH N NH
R8 \ I ~ I RS
Y R6 R8
R7 R7 R6 R5
XVIII XIX XX
23
CA 02496241 2011-03-14
N NH - R12 N NH R11
R12
p R5
R7 R6 RS R6
XXI R7 XXII
R12
N NH
R6
R7
XXIII
wherein:
R5, R6, R7, R8, RI1 and R12 are independently selected from hydrogen, halogen,
hydroxyl, thiol, lower alkyl, substituted lower alkyl, lower alkenyl,
substituted lower
alkenyl, lower alkynyl, substituted lower alkynyl, alkylalkenyl, alkyl
alkynyl, alkoxy,
alkylthio, acyl, aryloxy, amino, amido, carboxyl, aryl, substituted aryl,
heterocycle,
heteroaryl, substituted heterocycle, heteroalkyl, cycloalkyl, substituted
cycloalkyl,
alkylcycloalkyl, alkylcycloheteroalkyl, nitro, or cyano.
24
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
Compounds of the present invention include, but are not limited to the
following
exemplary compounds:
N NH - N NH
\ I \ N CH3
H
1 HO O
Br / / \ Br / \
N NH
Br N Z NH
Br
HO O O O
3 4
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
Ac-N N HN ~N
Br
CH3
N N
Ac H
6
F F H3CO
F F \ / / I OCH3
HN N HN N HN N
Br
N CH3 j N N CH3
H H H
7 8 9
MeO (H3C)2N (H3C)2N
OMe N(CH3)2 N(CH;
HN N HN N HN
Br Br
\ \ i \ \ CH3
o N N / N
H H H
11 12
BE Br
\ / I Br \ / I Br N
HN N HN N HN c
N Br
CH3 \ CH3
N N N
H H H
13 14 15
26
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
N \ H3CO H3CO
N
HN CN HN HN
N OCH3 Br -N OCH3
Br a
N N 3 N
H H H
16 17 18
ci ci
'IPCi c ci
ci
HN ~N HN N HN -N
Br
N CH3 I/ N N CH3
H H H,
19 20 21
ci \/ ~I krl sci HN N N HN -N HN N
Br . I \ I \ \ CH3 Br I \ I \ \ CH3
N N N N
H
22 H 23 H 24 H 25
F F
02N
N
HN ~N S HN ~N HN HN X\CH3
g\ CH3 Br N N N
H H H H
26 27 28 29
27
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
O2N CI CI
BHN N
N
HN N NO2 HN CgN
Br
N
30 H 31 H 32 H
O2N 02N F / I
HN i N Br HN i N HN N
N N N
H
33 H 34 H 35
F/ l F/ I PhO OPh
Br HN ,,N HN iN HN sN
N N N
36 H 37 H 38 H
PhO OPh Ph Ph Ph / Ph
Br HN r N HN / N Br HN ,N
t~ c
N N
39H 40H 41 H
Br \ I \ I Br \ I \ I \ I - \
HN "I N Br HN /N HN /N
N N N
42 H 43 H 44 H
28
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
Br HN N HN / N HN r N
gN N N
45 H 46 H 47
ON
Br HN iN HN /IN Br HN iN
N N
48 H 49 H 50 H
\I - \I ci
Br HN e N Br HN e N HN /IN
N N N
51 H 52 H 53 H
HN CgN\ N I HN / N HN ~ N
N N
54 H 55 H 56 H
29
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
MeO Me Br Br
HN N HN N HN N HN N
OH OH CO2H CO2H
57 58 59 60
E
~-p
HN &OH N HN &OH N HN ,N Br HN N
OH
OH
61 62 63 64
Br Br Cl CI
\ \ / 02N -I\ 02N
HN /N HN 7N HN &OH N HN N
\I MeO Me OI
CO2H OH OH
65 66 67 68
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
HN /N CI HN N CI HN ,N
OH OH OH
OH OMe
69 70 71
0
F
HN HN /N HN N HN N
OMe OMe
\ I \ I \ I \
CO2H CO2H OMe OMe
72 73 74
F F F
H2N HN /N HN /N 02N HN /N
N N
H H
HN
75 76 77
31
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
N HN N HN /N
HN &OH
NH
CO2H
78 79 80
-N N- - -
HN /N HN N
N N
H H
81 82
OMe
OMe
HN /N
Br HN /-N
MeO \ / \
O N
H N
H
83 84
The present invention includes pharmaceutically acceptable salts of the
compounds
defined by Formula I. Compounds according to the present invention can possess
a
sufficiently acidic, a sufficiently basic, or both functional groups, and
accordingly
32
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
react with a number of organic and inorganic bases, and organic and inorganic
acids,
to form pharmaceutically acceptable salts.
The term "pharmaceutically acceptable salt" as used herein, refers to a salt
of a
compound of Formula I, which is substantially non-toxic to living organisms.
Typical
pharmaceutically acceptable salts include those salts prepared by reaction of
the
compound of the present invention with a pharmaceutically acceptable mineral
or
organic acid or an organic or inorganic base. Such salts are known as acid
addition
and base addition salts.
Acids commonly employed to form acid addition salts are inorganic acids such
as
hydrochloric acid, hydrobromic acid, hydroiodic acid, sulphuric acid,
phosphoric acid,
and the like, and organic acids such as p-toluenesulphonic acid,
methanesulphonic
acid, oxalic acid, p-bromophenylsulphonic acid, carbonic acid, succinic acid,
citric
acid, benzoic acid, acetic acid, and the like. Examples of such
pharmaceutically
acceptable salts are the sulphate, pyrosulphate, bisulphate, sulphite,
phosphate,
monohydrogenphosphate, dihydrogenphosphate, metaphosphate, pyrophosphate,
bromide, iodide, acetate, propionate, decanoate, caprylate, acrylate, formate,
hydrochloride, dihydrochloride, isobutyrate, caproate, heptanoate, propiolate,
oxalate,
malonate, succinate, suberate, sebacate, fumarate, maleate, butyne-1,4-dioate,
hexyne-
1,6-dioate, benzoate, chlorobenzoate, methylbenzoate, hydroxybenzoate,
methoxybenzoate, phthalate, xylenesulphonate, phenylacetate, phenylpropionate,
phenylbutyrate, citrate, lactate, gamma-hydroxybutyrate, glycolate, tartrate,
methanesulphonate, propanesulphonate, naphthalene- 1 -sulfonate, napththalene-
2-
sulfonate, mandelate and the like. Preferred pharmaceutically acceptable acid
addition salts are those formed with mineral acids such as hydrochloric acid
and
hydrobromic acid, and those formed with organic acids such as maleic acid and
methanesulphonic acid.
Salts of amine groups may also comprise quarternary ammonium salts in which
the
amino nitrogen carries a suitable organic group such as an alkyl, lower
alkenyl,
substituted lower allcenyl, lower alkynyl, substituted lower alkynyl, or
aralkyl moiety.
33
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
Base addition salts include those derived from inorganic bases, such as
ammonium or
alkali or alkaline earth metal hydroxides, carbonates, bicarbonates, and the
like. Bases
useful in preparing the salts of this invention thus include sodium hydroxide,
potassium hydroxide, ammonium hydroxide, potassium carbonate, sodium
carbonate,
sodium bicarbonate, potassium bicarbonate, calcium hydroxide, calcium
carbonate,
and the like.
One skilled in the art will understand that the particular counterion forming
a part of a
salt of this invention is usually not of a critical nature, so long as the
salt as a whole is
pharmacologically acceptable and as long as the counterion does not contribute
undesired qualities to the salt as a whole. The present invention further
encompasses
the pharmaceutically acceptable solvates of a compound of Formula I. Many of
the
compounds of Formula I can combine with solvents such as water, methanol,
ethanol
and acetonitrile to form pharmaceutically acceptable solvates such as the
corresponding hydrate, methanolate, ethanolate and acetonitrilate.
The compounds of the present invention may have multiple asymmetric (chiral)
centres. As a consequence of these chiral centres, the compounds of the
present
invention occur as racemates, mixtures of enantiomers and as individual
enantiomers,
as well as diastereomers and mixtures of diastereomers. All asymmetric forms,
individual isomers and combinations thereof, are within the scope of the
present
invention.
It will be readily understood by one skilled in the art that if the
stereochemistry of a
compound of Formula I is critical to its activity, then the relative
stereochemistry of
the compound is established early during synthesis to avoid subsequent
stereoisomer
separation problems. Further manipulation of the molecule will then employ
stereospecific procedures so as to maintain the desired chirality.
Non-toxic metabolically-labile esters or amides of a compound of Formula I are
those
that are hydrolysed in vivo to afford the compound of Formula I and a
34
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
pharmaceutically acceptable alcohol or amine. Examples of metabolically-labile
esters include esters formed with (1-6C) alkanols, in which the alkanol moiety
may be
optionally substituted by a (1-8C) alkoxy group, for example methanol,
ethanol,
propanol and methoxyethanol. Examples of metabolically-labile amides include
amides formed with amines such as methylamine.
II. Preparation of Compounds of Formula I
As is known in the art, triaryl imidazole compounds can be prepared by a
number of
standard techniques. Compounds of Formula I, therefore, can be prepared by
several
general synthetic methods, for example, as described by Grimmett, (Grimmett,
M.R.,
Comprehensive Heterocyclic Chemistry: The Structure, Reaction, Synthesis and
Uses
of Heterocyclic Compounds, A. R. Katrizky and C. W. Rees, eds., Vol. 5,
Pergamon
Press. Oxford, 1984, pp. 457-498; Grimmett, M. R., Imidazole and Benzimidazole
Synthesis, Academic Press, San Diego CA, 1997).
In one embodiment of the present invention, compounds of Formula I are
prepared via
solution or solid phase synthesis, by reacting a dione of Formula II with the
aldehyde
(III) at elevated temperature in the presence of ammonium acetate in acetic
acid (see,
for example, Krieg et al., Naturforsch. 1967, 22b:132; Sarshar et al.,
Tetrahedron
Lett. 1996, 37:835-838).
R3 R2 O H R3 R2 R3 R2
NH4OAc/HOAc
0 O R1
R1 R1
(II) (III)
The compounds of Formula (II) and (III) are either commercially available or
may be
prepared using standard procedures known to a person skilled in the relevant
art.
Compounds of Formula II, therefore, can be prepared by several general
synthetic
methods, for example, as described by: Fischer et. al J. Am. Chem. Soc. 1961,
83,
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
4208-4210); Guijarro et al. (J. Am. Chem. Soc. 1999, 121, 4155-4157); Chi et.
al.
(Synth. Comm. 1994, 24(15), 2119-2122) and Armesto et. al. (Synthesis, 1988,
799-
801).
Compounds of formula II can also be prepared:
i) by oxidizing a compound of formula (IV). Compounds of formula (IV), in
turn can be prepared by reacting a compounds of formula (V) with sodium
cyanide in the presence of a solvent as shown below, wherein R2 is as defined
above:
OH O OH O O
NaCN/solvent oxidation
Y >- )L-~
R2 R2 R2 31
R2 R2
(V) (IV) (I I)
or,
ii) by oxidizing a compound of formula (VI). Compounds of formula (VI), in
turn can be prepared by treating a compound of formula (V) and a compound
of formula (VII) with sodium cyanide in the presence of a solvent as shown
below, wherein R2 and R3 are as defined above:
0 H 0 H
O O
DY I NaCN/solvent O OH oxidation
R2 R3 R2 R3 R2 R3
(V) (VII) NO
(II)
1s
or,
iii) by oxidizing a compound of formula (VIII). Compounds of formula (VIII) in
turn can be prepared by oxidizing a compound of formula (IX) or (X) as
shown below, wherein R2 and R3 are as defined above:
R2 R3
(IX) ~ oxidation O O
oxidation OH OH
or R2 R3 R2 R3
R2 R3 36
(X) (VIII) (II)
CA 02496241 2011-03-14
or,
iv) by oxidizing a compound of formula (X) using PdC12 in DMSO,
or,
v) by deprotecting and oxidizing a compound of formula (XI). Compounds of
formula (XI) in turn can be prepared by reacting a compound of formula (XII)
with a compound of formula (XIII) in the presence of a suitable base:
Base 0 OTBDMS deprotection, O O
R3-**'\OTBDMS II oxidation II "
(XII) ~ RZ R3 R2 / R3
R2 N (XI) (II)
(XIII) \OMe
wherein R2 and R3 are independently aryl, substituted aryl, heteroaryl or
substituted heteroaryl,
or,
vi) by reacting a compound of formula (XIV) with a substituted or
unsubstituted
aryl or substituted or unsubstituted heteroaryl under Friedel Crafts acylation
conditions or by nuecleophilic displacement of the chloride in compound of
formula XIV. Compounds of formula (XIV) in turn can be prepared by
reacting a substituted or unsubstituted aryl or substituted or unsubstituted
heteroaryl with oxalyl chloride under Friedel Crafts acylation conditions:
Substituted/unsubstituted aryl;
or
Substituted/unsubstituted aryl; ~ Substituted/unsubstituted heteroaryl
or 30-
Substituted/unsubstituted heteroaryl R2 CI R2 R3
(XIV) (II)
37
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
wherein R2 and R3 are independently aryl, substituted aryl, heteroaryl or
substituted heteroaryl;.
or
vii) by oxidising a compound of formula (XV). Compounds of formula (XV) in
turn
is prepared by reacting a compound of formula (XVI) with thionyl chloride in
benzene with catalytic dimethylformaride to form an intermediate (XVII). This
intermediate (XVII) is then used directly without purification in a freidel-
Crafts
reaction to produce the Ketone (XV).
O O O O
Are
Ar ~ SSOC Ar ~ A1ci ~ Oxidation
j`v 'OH j`v 'CI A `v 'Ar2 Are
O
XVI XVII XV II
III. Anti Microbial Activity of Compounds of Formula I
The anti-microbial activity of a candidate compound of Formula I can be tested
using
standard techniques known in the art. In accordance with the present
invention, the
anti-microbial activity exhibited by a candidate compound may be anti-
bacterial
activity, anti-fungal activity, or both anti-bacterial and anti-fungal
activity. As is
known in the art, anti-microbial activity of a compound may result in the
killing of
microbial cells (i.e. bacteriocidal and/or fungicidal activity), or it may
result in the
slowing or arrest of the growth of microbial cells (i.e. bacteriostatic or
fungistatic
activity). Thus the compounds of Formula I may be bacteriocidal and/or
fungicidal or
they may be bacteriostatic and/or fungistatic. Compounds of the present
invention that
slow or arrest microbial cell growth may be useful in combination treatments
with
other known anti-microbial agents.
38
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
A. In vitro Testing
In vitro methods of determining the ability of candidate compounds to inhibit,
prevent
or eradicate the growth of microbial cells are well-known in the art. In
general, these
methods involve contacting a culture of the cells of interest with various
concentrations of the candidate compound and monitoring the growth of the cell
culture relative to an untreated control culture. A second control culture
comprising
cells contacted with a known anti-microbial agent may also be included in such
tests,
if desired.
For example, the ability of a candidate compound of Formula I to inhibit the
growth
of microbial cells can readily be determined by measurement of the minimum
inhibitory concentration (MIC) for the compound. The MIC is defined as the
lowest
concentration that inhibits growth of the organism to a pre-determined extent.
For
example, a MIC100 value is defined as the lowest concentration that completely
inhibits growth of the organism, whereas a MIC90 value is defined as the
lowest
concentration that inhibits growth by 90% and a MIC50 value is defined as the
lowest
concentration that inhibits growth by 50%. MIC values are sometimes expressed
as
ranges, for example, the MIC100 for a compound may be expressed as the
concentration at which no growth is observed or as a range between the
concentration
at which no growth is observed and the concentration of the dilution which
immediately follows.
Typically, anti-bacterial MICs for candidate compounds are measured using a
broth
macro- or microdilution assay (see Amsterdam, D. (1996) "Susceptibility
testing of
antimicrobials in liquid media," pp.52-111. In Loman, V., ed. Antibiotics in
Laboratory Medicine, 4th ed. Williams and Wilkins, Baltimore, MD). A
standardised
anti-bacterial susceptibility test is provided by the National Committee for
Clinical
Laboratory Standards (NCCLS) as NCCLS, 2000; document M7-A58.
In the classical broth microdilution method, the candidate anti-bacterial-
compound is
diluted in culture medium in a sterile, covered 96-well microtiter plate. An
overnight
culture of a single bacterial colony is diluted in sterile medium such that,
after
39
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
inoculation, each well in the microtiter plate contains an appropriate number
of
colony forming units (CFU)/ml (typically, approximately 5 x 105 CFU/ml).
Culture
medium only (containing no bacteria) is also included as a negative control
for each
plate and known antibiotics are often included as positive controls. The
inoculated
microtiter plate is subsequently incubated at an appropriate temperature (for
example,
35 C - 37 C for 16-48 hours). The turbidity of each well is then determined by
visual
inspection and/or by measuring the absorbance, or optical density (OD), at
595nm or
600nm using a microplate reader and is used as an indication of the extent of
bacterial
growth.
Techniques for determining anti-fungal MIC values for candidate compounds are
similar to those outlined above for anti-bacterial MICs and include both
macrodilution
and microdilution methods (see, for example, Pfaller, M.A., Rex, J.H.,
Rinaldi, M.G.,
Clin. Infect. Dis., (1997) 24:776-84). A standardised anti-fungal
susceptibility test
method, NCCLS M27-T, has been proposed by the NCCLS (see, Ghannoum, M.A.,
Rex, J.H. and Galgiani J.N., J. Clin. Microbiol., (1996) 34:489-495; Pfaller,
M.A. and
Yu, W.L., Infect. Dis. Clin. North Amer., (2001) 15:1227-1261).
In accordance with one embodiment of the present invention, a compound of
Formula
I is considered to have an anti-microbial effect against a given micro-
organism when
used alone when the MIC of the compound for complete inhibition of growth of
the
organism is less than about 75 g/ml. In one embodiment, the compound has a
MIC
less than about 50 ghul for the relevant micro-organism. In another
embodiment, the
compound has a MIC of less than about 35 g/ml. In other embodiments, the
compound has a MIC of less than about 25 g/ml, less than about 16 g/ml and
less
than about 12.5 g/ml for the relevant micro-organism.
Anti-microbial effects may also be expressed as the percentage (%) inhibition
of
growth of a given micro-organism over a pre-determined period of time by
treatment
with a single concentration of a candidate compound. This method provides a
rapid
method of assessing the ability of a compound to inhibit microbial growth, for
example, prior to conducting more in-depth tests, such as MIC determinations
or in
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
vivo testing. In one embodiment of the present invention, a candidate compound
is
considered to be a potential anti-microbial agent when it is capable of
inhibiting the
growth of a given micro-organism by about 25% when used at a concentration of
about 25 g/ml, with growth of the micro-organism being assessed over a 48
hour
period.
One skilled in the art will appreciate that compounds that exhibit poor anti-
microbial
activity when used alone (for example, a compound that has a MIC of >128
g/ml)
may still be capable of good anti-microbial activity when used in combination
with
one or more known anti-microbial agents. For example, the compound may
sensitise
the microbial cells to the action of the other agent(s), it may act in synergy
with
agent(s), or it may otherwise potentiate the activity of the agent(s).
As such, many anti-microbial compounds show maximal effects when used in
combination with a second drug. The effects can be simply additive, or they
can be
synergistic. For example, a compound that exhibits only bacteriostatic effects
when
used in isolation can become bacteriocidal when used in combination with a
second
anti-bacterial compound. Thus, the present invention contemplates that the
anti-
microbial activity of a compound of Formula I may be enhanced by the presence
of
another compound of Formula I, or by the presence of another known anti-
microbial
agent. Alternatively, the compound of Formula I may enhance the anti-microbial
effect of other anti-microbial agents. Methods of testing for synergistic
and/or
additive effects between two or more compounds are well-known in the art.
For example, the fractional inhibitory concentration (FIC) can be used to
assess the
presence or absence of synergy between two anti-bacterial compounds (see, for
example, U.S. Patent No. 6,288,212). FICs are determined in microtiter plates
in a
similar manner to MICs, except that FICs are performed using a checkerboard
titration of, for example, candidate compounds in one dimension and known
antibiotics in the other dimension. The FIC is calculated by evaluating the
impact of
one antibiotic on the MIC of the other and vice versa. As used herein, FIC can
be
determined as follows:
41
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
MIC (candidate compound in combination) MIC (known antibiotic in combination)
FIC +
MIC (candidate compound alone) MIC (known antibiotic alone)
An FIC value equal to one indicates that the influence of the compounds is
additive
and an FIC value of less than one indicates synergy. An FIC value of less than
0.5 is
typically obtained for synergism.
B. In vivo ' Testing
The ability of a compound of Formula I to act as an anti-microbial agent can
also be
tested in vivo using standard techniques. A number of animal models are known
in the
art that are suitable for testing the activity of anti-microbial compounds and
are
readily available.
Representative examples of animal models suitable for testing the anti-
bacterial
activity of a compound of Formula I in vivo include, but are not limited to,
the
immunosuppressed mouse as a model of acute Staphylococcus aureus infection,
the
burnt mouse or neutropenic mouse as a model for Pseudomonas aeruginosa
infections
and the suckling mouse for Vibrio cholerae infection of the intestine (Klose,
et al.,
(2000), Trends Microbiol., 8:189-91).
Other examples of suitable models and procedures for in vivo testing of anti-
bacterial
compounds are described in Iwahi, T., et al., J. Med. Microbial., (1982)
15:303-316;
Michie, H.R., J Antimicrob. Chemother., (1998) 41:47-49; Yanke, S.J., et al.,
Can. J.
Microbiol., (2000) 46:920-926; Shibata, K., et al., J. Antimicrob. Chemother.,
(2000)
45:379-82; Totsuka, K., et al., I Antimicrob. Chemother., (1999) 44:455-60;
Goto,
Y., et al., Int. J. Antimicrob. Agents., (1999) 11:39-46.
Representative examples of animal models suitable for testing the anti-fungal
activity
of a compound of Formula I in vivo include, but are not limited to, the severe
combined immunodeficiency (SCID) mouse model and a colostrum-deprived SPF
42
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
piglet model for Cryptosporidium parvum infection, a granulocytopenic rabbit
model
of disseminated Candidiasis (see, for example, Walsh, et al., J. Infect. Dis.,
1990,
161:755-760; Thaler, et al., J. Infect. Dis., 1988, 158:80), a mouse model of
disseminated Aspergillosis (see, for example, Arroyo, et al., Antimicrob.
Agents
Cheinoth., 1977, pp. 21-25) and a neutropenic rat model of disseminated
Candidiasis
(see, for example, Lechner, et al., Am. J. Physiol. (Lung Cell. Mol. Physiol.)
1994,
10:1-8).
Methods for conducting in vivo tests to determine the activity of anti-
microbial
compounds are well-known in the art. Typically, in vivo testing comprises
introducing
a selected micro-organism into the appropriate animal model in a sufficient
amount to
cause infection, followed by administration of one or more doses of the test
compound of Formula I. Methods of administration will vary depending on the
compound being employed, but can be, for example, by way of bolus infusion
into a
suitable vein (such as the tail vein of mice or rats), or by oral
administration. Animals
treated with a known anti-microbial agent and/or with a saline or buffer
control
solution serve as controls. Repeat doses of the test compound may be
administered to
the animal, if necessary, at appropriate time intervals. The animals are
subsequently
monitored daily for mortality.
In accordance with the present invention, a compound of Formula I is
considered to
exert an in vivo anti-microbial effect if it results in a decrease in
mortality of at least
about 15% in a treated animal compared to a test animal. In one embodiment of
the
present invention, the compound of Formula I results in a decrease in
mortality of at
least about 25% in the treated animal. In another embodiment, the compound
results
in a decrease in mortality of at least about 40%. In other embodiments, the
compound
results in a decrease in mortality of at least about 50%, 60%, 70%, 80% and
90% in
the treated animal.
IV. Toxicity Testing
43
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
It is important that the anti-microbial compounds of the present invention
exhibit low
toxicity in vivo. Toxicity tests for potential drugs are well-known in the art
(see, for
example, Hayes, A.W., ed., (1994), Principles and Methods of Toxicology, 3rd
ed.,
Raven Press, NY; Maines, M., ed., Current Protocols in Toxicology, John Wiley
&
Sons, Inc., NY).
In vitro acute toxicity testing of a compound of Formula I can be performed
using
mammalian cell lines (see, for example, Ekwall, B., Anti. N.Y. Acad. Sci.,
(1983)
407:64-77). Selection of an appropriate cell line is dependent on the
potential
application of the candidate compound and can be readily determined by one
skilled
in the art.
In vivo toxicity testing can be performed by standard methodology. For
example, by
injecting varying concentrations of the candidate compound into an appropriate
animal model. The compound can be injected once, or administration can be
repeated
over several days. The toxic effects of the compound can be evaluated over an
appropriate time period by monitoring the general health and body weight of
the
animals. After the completion of the period of assessment, the animals can be
sacrificed and the appearance and weight of the relevant organs determined.
In accordance with one embodiment of the present invention, a compound of
Formula
I for use in vivo shows both good anti-microbial activity and low or no
toxicity at the
concentration at which it would be administered as an anti-microbial agent.
V. Uses of the Anti-Microbial Compounds of Formula I
The present invention provides for the use of one or more compounds of Formula
I
for the inhibition, prevention or eradication of the growth and/or
proliferation of
bacteria and/or fungi, either alone or in combination with one or more other
compounds of Formula I or known anti-microbial agents.
Thus, in one embodiment, the present invention provides a method of inhibiting
bacterial growth by contacting a bacterium with an effective amount of one or
more
44
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
compounds of Formula I. The compounds of Formula I may have broad spectrum
anti-bacterial activity, in which case they may be used against gram-positive
or gram-
negative bacteria. Representative examples of bacteria that may be inhibited
by
compounds of Formula I include, but are not limited to, Corynebacterium
xerosis,
Chlamydia pneumoniae, Chlamydia trachomatis, Enterobacter cloacae,
Enterobacter
faecalis, Enterococcus faecium, Escherichia coli, Escherichia coli 0157:H7,
Haemophilus influenzae, Helicobacter pylori, Listeria monocytogenes, Moraxella
catarrhalis, Neisseria gonorrhoae, Neisseria meningitidis, Pseudomonas
aeruginosa,
Pneumococci species, Salmonella enterica, Salmonella typhimurium,
Staphylococcus
aureus, Staphylococcus aureus K147, Staphylococcus epidermidis, Staphylococcus
typhimurium, Streptococcus mitis, Streptococcus pneumoniae, Streptococcus
pyogenes, Vibrio cholerae, Mycobacterium tuberculosis and other acid-fast
staining
bacteria (i.e. M. africanum, M avium-intracellulare, M. pneumoniae, M. bovis,
M.
leprae, M. phlei), Bacillus anthracis and other endospore-forming rods and
cocci. In
one embodiment of the present invention, the compounds are used against gram-
positive bacteria.
It is well-established in the field of microbiology that many multidrug-
resistant strains
of bacteria have emerged in the recent past and will continue to emerge with
the
continued use of standard antibiotics. Examples of currently known resistant
strains of
bacteria include methicillin-resistant Staphylococcus aureus (MRSA),
methicillin-
resistant coagulase-negative staphylococci (MRCNS), penicillin-resistant
Streptococcus pneumoniae, penicillin-resistant pneumococci and multidrug-
resistant
Enterococcus faecium. The present invention, therefore, contemplates the use
of
compounds of Formula I in the inhibition of growth of such multidrug-resistant
strains. In one embodiment of the present invention, compounds of Formula I
are used
to inhibit the growth of multidrug-resistant strains of bacteria. In another
embodiment,
the compounds are used to inhibit the growth of MRSA.
In accordance with one embodiment of the present invention, one or more
compounds
of Formula I can be administered in a therapeutically effective amount alone
or in
combination with one or more other anti-bacterial agents to a subject with a
bacterial
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
disorder. Thus, the present invention provides the use of one or more of the
compounds of Formula I in the treatment of bacterial infections and
bacterially-
related disorders and diseases. Examples of bacterially-related disorders and
diseases
that may be treated with the compounds of the present invention include, but
are not
limited to, tuberculosis, meningitis, ulcers, septicaemia, bacteremia, cystic
fibrosis,
pneumonia, typhoid fever, bacterial conjunctivitis, gonorrhoea, impetigo,
bacterial
eye or ear infections, bacterial diarrhoea, cystitis, bacterial vaginitis,
bacterial
endocarditis, bacterial pericarditis, peliosis, superficial skin infections,
toxic shock,
food poisoning, hemolytic uremic syndrome, botulism, leprosy, gangrene,
tetanus,
lyme disease, plague, anthrax and chancroid.
In another embodiment, the present invention provides a method of inhibiting
fungal
growth by contacting a fungus with an effective amount of one or more
compounds of
Formula I either alone or in combination with one or more other anti-fungal
agents.
Representative examples of fungi that may be inhibited with compounds of
Formula I
include, but are not limited to, Histoplasma (e.g. H. capsulstum),
Coccidioides,
Blastomyces, Paracoccidioides, Cryptococcus (e.g. C. neoformans), Aspergillus
(e.g.
A. fumigatus, A. flaws, A. niger, A. nidulans, A. terreus, A. sydowi, A.
flavatus, and A.
glaucus), Zygomycetes (e.g. Basidiobolus, Conidiobolus, Rhizopus, Mucor,
Absidia,
Mortierella, Cunninghamella, and Saksenaea), Candida (e.g. C. albicans, C.
tropicalis, C. parapsilosis, C. stellatoidea, C. krusei, C. parakrusei, C
lusitaniae, C.
pseudorropicalis, C. guilliermondi and C. glabrata), Cryptosporidium parvum,
Sporothrix schenckii, Piedraia hortae, Trichosporon beigelii, Malassezia
furfur,
Phialophora verrucosa, Fonsecae pedrosoi, Madurella mycetomatis and
Pneumocystis carinii.
In accordance with another embodiment of the present invention, one or more
anti-
microbial compounds of Formula I can be administered in a therapeutically
effective
amount either alone or in combination with other anti-fungal agents to a
subject with a
fungal infection or fungally-related disorder or disease. Examples of fungally-
related
disorders and diseases that may be treated with the compounds of Formula I
include,
but are not limited to, Candidiasis; endemic mycoses (such as Histoplasmosis,
46
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
Coccidioidoinycosis, Blastomycosis, Paracoccidioidomycosis, Cryptococcosis,
Aspergillosis, Mucormycosis), associated disseminated infections and
progressive
pulmonary disease; cryptococcal meningitis; narcotising patchy
bronchopneumonia;
haemorrhagic pulmonary infarction; rhinocerebral disease; neutropenia, black
piedra;
white piedra; tinea (versicolor, capitis, corporis, etc.); Pneumocystis
pneumonia;
chromoblastoinycosis, and maduromycosis.
In accordance with a further embodiment of the present invention, one or more
compounds of Formula I may be used as therapeutic agents in combination with
one
or more known drugs in combination or synergistic therapy for the treatment of
microbial infection, or disorders or diseases associated therewith. Such
therapy is
known in the art and selection of the appropriate drug(s) to be administered
with
compound(s) of Formula I is' readily discernible by one of skill in the art.
For
example, in the treatment of bacterial infections and related diseases, useful
classes of
antibiotics for combination or synergistic therapy include, but are not
limited to,
aminoglycosides, penicillins, cephalosporins, fluoroquinolones, quinolones,
carbapenems, tetracyclines, glycopeptides and macrolides,,and other
antibiotics such
as chloramphenicol, clindamycin, trimethoprim, sulphamethoxazole, nitro
ftirantoin,
rifampin and mupirocin. For the treatment of fungal infections and fungally-
related
diseases, candidate antimicrobial compounds for combination therapy include,
but are
not limited to, araphotericin B and the structurally related compounds
nystatin and
pimaricin; flucytosine; azole derivatives such as ketoconazole, clotrimazole,
miconazole, econazole, butoconazole, oxiconazole, sulconazole, terconazole,
fluconazole and itraconazole; allylamines-thiocarbamates, such as tolnaftate
and
naftifine, and griseofulvin.
The present invention also contemplates the use of compounds of Formula I as
the
active ingredient in anti-microbial cleansers, polishes, paints, sprays,
soaps, or
detergents. The compounds can also be included as an anti-microbial agent in
cosmetic, personal care, household and industrial products, for example, to
improve
shelf-life by inhibiting the growth of microbes within the products. The
compounds
may be formulated for application to surfaces to inhibit the growth of a
microbial
47
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
species thereon, for example, surfaces such as countertops, desks, chairs,
laboratory
benches, tables, floors, sinks, showers, toilets, bathtubs, bed stands, tools
or
equipment, doorknobs and windows. Alternatively, . the compounds may be
formulated for laundry applications, for example, for washing clothes, towels,
sheets
and other bedlinen, washcloths or other cleaning articles. The antimicrobial
cleansers,
polishes, paints, sprays, soaps, or detergents according to the present
invention can
optionally contain suitable solvent(s), carrier(s), thickeners, pigments,
fragrances,
deodorisers, emulsifiers, surfactants, wetting agents, waxes, or oils. In one
embodiment, the present invention provides a formulation containing one or
more
compounds of Formula I for external use as a pharmaceutically acceptable skin
cleanser. The cleansers, polishes, paints, sprays, soaps, and detergents
according to
the present invention are' useful institutions, such as in hospital settings
for the
prevention of nosocomial infections, as well as in home settings.
In addition, the invention contemplates the use of compounds of Formula I in
formulations to kill or inhibit the growth of microbial species in food
preparations, or
to sterilise surgical and other medical equipment and implantable devices,
including
prosthetic joints. The compounds can also be formulated for use in the in situ
sterilisation of indwelling invasive devices such as intravenous lines and
catheters,
which are often foci of infection.
The present invention further contemplates the use of the compounds of Formula
I as
the active ingredient in personal care items, such as soaps, deodorants,
shampoos,
mouthwashes, toothpastes, and the like. Many compositions used in personal
care
applications are susceptible to microbial growth and it is thus desirable to
incorporate
into these compositions an effective anti-microbial material. The anti-
microbial agent
may be incorporated into the personal care formulation using techniques known
in the
art. Thus, the anti-microbial agent may be added to the personal care
formulation as a
solution, emulsion or dispersion in a suitable liquid medium. Alternatively,
the anti-
microbial agent may be added, undiluted, to the personal care formulation or
may be
added with a solid carrier or diluent. The anti-microbial agent may be added
to the
pre-formed personal care formulation or may be added during the formation of
the
48
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
personal care formulation, either separately or premixed with one of the other
components of the formulation.
VI. Pharmaceutical Formulations and Administration of Anti-Microbial
Compounds of Formula I
For use as therapeutic agents in the treatment of microbial infections, or
disorders or
diseases associated therewith in a subject, the anti-microbial compounds of
the
present invention are typically formulated prior to administration. Therefore,
the
present invention provides pharmaceutical formulations comprising one or more
compounds of Formula I and a pharmaceutically-acceptable carrier, diluent, or
excipient. The present pharmaceutical formulations are prepared by standard
procedures using well-known and readily available ingredients. In making the
compositions of the present invention, the active ingredient will usually be
mixed
with a carrier, or diluted by a carrier, or enclosed within a carrier, and may
be in the
form of a capsule, sachet, paper, or other container. When the carrier serves
as a
diluent, it may be a solid, semi-solid, or liquid material that acts as a
vehicle,
excipient, or medium for the active ingredient.
The pharmaceutical compositions comprising the anti-microbial compounds
according to the present invention may be administered in a number of ways
depending upon whether local or systemic treatment is desired and upon the
area to be
treated. Administration may be topical (including ophthalmic and to mucous
membranes including vaginal and rectal delivery), pulmonary, e.g. by
inhalation or
insufflation of powders or aerosols, including by nebulizer; intratracheal,
intranasal,
epidermal and transdermal, oral or parenteral. Parenteral administration
includes
intravenous, intraarterial, subcutaneous, intraperitoneal or intramuscular
injection or
infusion; or intracranial, e.g. intrathecal or intraventricular,
administration.
The anti-microbial compounds of the present invention may be delivered alone
or in
combination, and may be delivered along with a pharmaceutically acceptable
vehicle.
Ideally, such a vehicle would enhance the stability and/or delivery
properties. The
49
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
present invention also.provides for administration of pharmaceutical
compositions
comprising one or more of the compounds of Formula I using a suitable vehicle,
such
as an artificial membrane vesicle (including a liposome, niosome and the
like),
microparticle or microcapsule. The use of such vehicles may be beneficial in
achieving sustained release of the anti-microbial compound(s).
For administration to an individual for the treatment of an infection or
disease, the
present invention also contemplates the formulation of the pharmaceutical
compositions comprising the anti-microbial compounds into oral dosage forms
such
as tablets, capsules and the like. For this purpose, the compounds can be
combined
with conventional carriers, such as magnesium carbonate, magnesium stearate,
talc,
sugar, lactose, pectin, dextrin, starch, gelatine, tragacanth,
methylcellulose, sodium
carboxymethyl-cellulose, low melting wax, cocoa butter and the like. Diluents,
flavouring agents, solubilizers, lubricants, suspending agents, binders,
tablet-disintegrating agents and the like can also be employed, if required.
The anti-
microbial compounds can be encapsulated with or without other carriers. In
accordance with the present invention, the proportion of anti-microbial
compound(s)
in any solid and liquid composition will be at least sufficient to impart the
desired
activity to the individual being treated upon oral administration. The present
invention
further contemplates parenteral injection of the anti-microbial compounds, in
which
case the compounds are formulated as a sterile solution containing other
solutes, for
example, enough saline or glucose to make the solution isotonic.
For administration by inhalation or insufflation, the anti-microbial compounds
can be
formulated into an aqueous or partially aqueous solution, which can then be
utilized in
the form of an aerosol. Aqueous formulations of the anti-microbial compounds
of the
present invention may also be used in the form of ear or eye drops, or
ophthalmic
solutions. The present invention further contemplates topical use of the anti-
microbial
compounds. For this purpose they can be formulated as dusting powders, creams
or
lotions in pharmaceutically acceptable vehicles, which are applied to affected
portions
of the skin.
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
Compositions intended for oral use may be prepared according to procedures
known
in the art for the manufacture of pharmaceutical compositions and such
compositions
may further contain one or more sweetening agents, flavouring agents,
colouring
agents, preserving agents, or a combination thereof, in order to provide
pharmaceutically elegant and palatable preparations. Tablets typically contain
the
anti-microbial compound(s) in admixture with non-toxic pharmaceutically
acceptable
excipients suitable for the manufacture of tablets, such as inert diluents,
for example,
calcium carbonate, sodium carbonate, lactose, calcium phosphate or sodium
phosphate; granulating and disintegrating agents, for example, corn starch, or
alginic
acid; binding agents, for example, starch, gelatine or acacia, and lubricating
agents,
for example, magnesium stearate, stearic acid or talc. The tablets may be
uncoated or
they may be coated by known techniques to delay disintegration and absorption
in the
gastrointestinal tract and thereby provide a sustained action over a longer
period. For
example, a time delay material such as glyceryl monostearate or glyceryl
distearate
may be employed.
Formulations for oral use may also be presented as hard gelatine capsules
wherein the
anti-microbial compound(s) is mixed with an inert solid diluent, for example,
calcium
carbonate, calcium phosphate or kaolin, or as soft gelatine capsules wherein
the active
ingredient is mixed with water or an oil medium, for example, peanut oil,
liquid
paraffin or olive oil.
Aqueous suspensions typically contain the anti-microbial compound(s) in
admixture
with excipients suitable for the manufacture of aqueous suspensions, such as
suspending agents (for example, sodium carboxylmethylcellulose, methyl
cellulose,
hydropropylmethyl cellulose, sodium alginate, polyvinylpyrrolidone, gum
tragacanth
and gum acacia); dispersing or wetting agents such as a naturally-occurring
phosphatide (for example, lecithin), or condensation products of an alkylene
oxide
with fatty acids (for example, polyoxyethylene stearate), or condensation
products of
ethylene oxide with long chain aliphatic alcohols (for example, hepta-
decaethyleneoxycetanol), or condensation products of ethylene oxide with
partial
esters derived from fatty acids and a hexitol (for example, polyoxyethylene
sorbitol
51
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
monooleate), or condensation products of ethylene oxide with partial esters
derived
from fatty acids and hexitol anhydrides (for example, polyethylene sorbitan
monooleate). The aqueous suspensions may further contain one or more
preservatives,
for example, ethyl, or n-propyl-p-hydroxy benzoate; one or more colouring
agents;
one or more flavouring agents, or one or more sweetening agents, such as
sucrose or
saccharin, or a combination thereof.
Oily suspensions maybe formulated by suspending the anti-microbial compound(s)
in
a vegetable oil, for example, peanut oil, olive oil, sesame oil or coconut
oil, or in a
mineral oil such as liquid paraffin. The oily suspensions may contain a
thickening
agent, for example beeswax, hard paraffin or cetyl alcohol. Sweetening agents
such as
those set forth above, and flavouring agents may be added to provide palatable
oral
preparations. These compositions may be preserved by the addition of an anti-
oxidant
such as ascorbic acid.
Dispersible powders and granules suitable for preparation of an aqueous
suspension
by the addition of water provide the anti-microbial compound in admixture with
a
dispersing or wetting agent, suspending agent and one or more preservatives.
Suitable
dispersing or wetting agents and suspending agents are exemplified by those
mentioned above. Additional excipients, for example, sweetening, flavouring
and
colouring agents, may also be present.
Pharmaceutical compositions of the present invention may also be in the form
of oil-
in-water emulsions. The oil phase may be a vegetable oil, for example, olive
oil or
peanut oil, or a mineral oil, for example, liquid paraffin, or mixtures
thereof Suitable
emulsifying agents may be naturally-occurring gums (for example, gum acacia or
gum tragacanth); naturally-occurring phosphatides (for example, soy bean
lecithin),
and esters or partial esters derived from fatty acids and hexitol anhydrides
(for
example, sorbitan monooleate), and condensation products of the partial esters
with
ethylene oxide (for example, polyoxyethylene sorbitan monooleate). The
emulsions
may also contain sweetening and flavouring agents.
52
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
Syrups and elixirs may be formulated with sweetening agents, for example,
glycerol,
propylene glycol, sorbitol or sucrose. Such formulations may also contain one
or
more demulcents, preservatives or flavouring and colouring agents, or
combinations
thereof.
The pharmaceutical compositions may be in the form of a sterile injectable
aqueous or
oleaginous suspension. This suspension may be formulated according to known
art
using suitable dispersing or wetting agents and suspending agents as described
above.
The sterile injectable preparation may also be a solution or a suspension in a
non-
toxic, parentally acceptable diluent or solvent, for example, as a solution in
1,3-
butanediol. Among the acceptable vehicles and solvents that may be employed
are
water, Ringer's solution and isotonic sodium chloride solution. In addition,
sterile,
fixed oils are conventionally employed as a solvent or suspending medium.
Typically, a bland fixed oil is employed for this purpose such as a synthetic
mono- or
diglyceride. In addition, fatty acids such as oleic acid find use in the
preparation of
injectables. Adjuvants, such as local anaesthetics, preservatives and
buffering agents,
may also be included in the injectable formulation.
The compound(s) of Formula I may be administered, together or separately, in
the
form of suppositories for rectal or vaginal administration of the compound.
These
compositions can be prepared by mixing the compound with a suitable non-
irritating
excipient which is solid at ordinary temperatures but liquid at the
rectal/vaginal
temperature and will therefore melt to release the compound. Examples of such
materials include cocoa butter and polyethylene glycols.
Another formulation of the present invention employs transdermal delivery
devices
("patches"). Such transdermal patches may be used to provide continuous or
discontinuous infusion of the anti-microbial compounds of the present
invention in
controlled amounts. The construction and use of transdermal patches for the
delivery
of pharmaceutical agents is well known in the art (see, for example, U.S.
Patent No.
5,023,252; issued Jun. 11, 1991). Such patches may be constructed for
continuous,
pulsatile, or on demand delivery of pharmaceutical agents.
53
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
It may be desirable or necessary to introduce the pharmaceutical composition
to the
brain, either directly or indirectly. Direct techniques usually involve
placement of a
drug delivery catheter into the host's ventricular system to bypass the blood-
brain
barrier. An example of such an implantable delivery system, used for the
transport of
biological factors to specific anatomical regions of the body, is described in
U.S.
Patent No. 5,011,472.
The dosage of the anti-microbial compound to be administered is not subject to
defined limits, but will usually be an effective amount. In general, the
dosage will be
the equivalent, on a molar basis, of the pharmacologically active free form
produced
from a dosage formulation upon the metabolic release of the active free drug
to
achieve its desired pharmacological and physiological effects. The
pharmaceutical
compositions are typically formulated in a unit dosage form, each dosage
containing
from, for example, about 0.05 to about 100 mg of the anti-microbial compound.
The
term "unit dosage form" refers to physically discrete units suitable as
unitary dosages
for administration to human subjects and other animals, each unit containing a
predetermined quantity of anti-microbial compound calculated to produce the
desired
therapeutic effect, in association with a suitable pharmaceutical excipient.
Typical daily dosages of the anti-microbial compounds fall within the range of
about
0.01 to about 200 mg/kg of body weight in single or divided dose. However, it
will
be understood that the amount of the compound actually administered will be
determined by a physician, in the light of the relevant circumstances,
including the
condition to be treated, the chosen route of administration, the actual
compound
administered, the age, weight, and response of the individual patient, and the
severity
of the patient's symptoms, and therefore the above dosage ranges are not
intended to
limit the scope of the invention in any way. In some instances dosage levels
below
the lower limit of the aforesaid range may be more than adequate, while in
other cases
still larger doses may be employed without causing any harmful side effect,
for
example, by. first dividing larger doses into several smaller doses for
administration
throughout the day.
54
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
VII. Kits
The present invention additionally provides for therapeutic kits containing
one or
more compounds of Formula I in pharmaceutical compositions, alone or in
combination with one or more other anti-microbial agents, for use in the
treatment of
infections and disease. Individual components of the kit would be packaged in
separate containers and, associated with such containers, can be a notice in
the form
prescribed by a governmental agency regulating the manufacture, use or sale of
pharmaceuticals or biological products, which notice reflects approval by the
agency
of manufacture, use or sale for human or animal administration.
When the components of the kit are provided in one or more liquid solutions,
the
liquid solution can be an aqueous solution, for example a sterile aqueous
solution. For
in vivo use, the anti-microbial compound may be, formulated into a
pharmaceutically
acceptable syringeable formulation. In this case the container means may
itself be an
inhalant, syringe, pipette, eye dropper, or other such like apparatus, from
which the
formulation may be applied to an infected area of the animal, such as the
lungs,
injected into an animal, or even applied to and mixed with the other
components of
the kit.
The components of the kit may also be provided in dried or lyophilised forms.
When
reagents or components are provided as a dried form, reconstitution generally
is by
the addition of a suitable solvent. It is envisioned that the solvent also may
be
provided in another container means. Irrespective of the number or type of
containers, the kits of the invention also may comprise, or be packaged with,
an
instrument for assisting with the injection/administration or placement of the
ultimate
composition within the body of an animal. Such an instrument may be an
inhalant,
syringe, pipette, forceps, measured spoon, eye dropper or any such medically
approved delivery vehicle.
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
To gain a better understanding of the invention described herein, the
following
examples are set forth. It should be understood that these examples are for
illustrative
purposes only. Therefore they should not limit the scope of the invention in
any way.
EXAMPLES
Preparation of compounds:
All reactions have been carried out according to the scheme shown below;
O O Arl ,Ar2
Art + I NH4Ac/AcOH
Are N`\ /NH
O Indole Y
Indole
In a typical experimental procedure 1 mmol (1 equiv.) of the indole
carboxyaldehyde
was combined with 1.05 - 1.10 mmole (1.05 - 1.1 equiv.) of the benzil and 20
mmole
(20 equiv.) of ammonium acetate and 5 ml of acetic acid. The mixture was
magnetically stirred and heated to reflux for 3-5 hr. The reaction process was
monitored by TLC, until complete consumption of the indole was achieved. The
reaction mixture was cooled to room temperature and added drop-wise into well-
stirred ice-water. The suspension solid was then filtered and the crude solid
was
dissolved in ethyl acetate, dried over sodium sulfate and filtered, the
organic solvent
was removed by vacuum. The products was then either recrystalized with alcohol
or
separated by column chromatography using petroleum ether-Ethyl acetate as an
eluant.
It is noteworthy that, the TLC of the products shows a characteristic blue
florescent
color under the UV (wave length a, = 254nm), a property used as an additional
characterization feature. Melting points were recorded using a MEL-TEMP
capillary
melting point apparatus, the melting point are uncorrected. 1H-NMR was
performed
in a 500 MHz Brucker instrument at room temperature using a suitable
dueterated
solvent.
56
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
EXAMPLE 1: Preparation of compound 2:
O
O H \ / I
Ph-~A +. NH4Ac/AcOH
Ph CH3 HN
O N N
H
C C
CH3
N
H
2
1H-NMR: 8 (DMSO-d6), 12.10 (s, 1H), 11.30 (s, 1H), 7.98 (d, 1H), 7.62 (d, 2H),
7.56
(d, 2H), 7.45 (t, 2H), 7.28-7.40 (m, 4H), 7.24 (t, 1H), 7.03-7.14 (m, 2H),
2.70 (s, 3H).
HRMS m/z for C24H19N3 calc. Is 349.157898, found 349.157897. M.p.= decomposed
at 260-264.
Example 2: Compound 5
1H-NMR (CDC13): 8 = 8.02 (d, 2H), 7.53 (d, 1H), 7.43 - 7.52 (m, 6H), 7.41 (d,
1H),
7.21 - 7.34 (m, 6H), 2.81 (s, 3H), 2.75 (s, 3H). EIMS [M+.] m/z for C28H23N302
is
433. M.p.= 224-227.
Example 3: Compound 11
'H-NMR (CDC13): 8 = 7.70 (d, 1H), 7.41 (d, 4H), 7.32 (d, 1H), 7.09 (q, 2H),
6.77 (d,
4H), 2.95 (s, 12H), 2.67 (s, 3H). ElMS [M+'] m/z for C28H29N5 is 435, M.p.=
decomposed at 236-238.
Example 4: Compound 13
1H-NMR (CDC13): 8 = 7.47 (d, 4H), 7.44 (d, 4H), 7.30-7.34 (1n, 1H), 7.14 -
7.19 (m,
3H), 2.68 (bs, 3H), EIMS [M+'] m/z for C24H17N3Br2 is 507. M.p.= 240-245.
Example 5: Compound 19
57
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
1H-NMR (DMSO-d6): 6 = 12.13 (s, 1H), 11.33 (s, 1H), 7.94 (d, 2H), 7.57 (d,
2H),
7.52 (bd, 2H), 7.39 (bd, 2H), 7.35 (d, 1H), 7.05-7.12 (m, 3H), 2.50 (s, 3H).
EIMS
[M+'] m/z for C24H17N3C12 is 418. M.p.= 165-167.
Example 6: Compound 29
1H-NMR (DMSO-d6): b = 8.83 (q, 2H), 8.73 (m, 1H), 8.68 (d, 1H), 8.46 (d, 1H),
8.24
(s, 1H), 7.74 (t, 2H), 7.62 (t, 2H), 7.51 - 7.56 (m, 1H), 7.23 - 7.27 (m, 2H),
2.71 (s,
3H). EIMS [M+'] m/z for C23H15N3 is 303. M.p.= 135-137.
Example 7: Compound 31
'H-NMR (CDC13): 8 = 8.90 (bs, 1H), 7.62 (bs, 1H), 7.48 (bd, 4H), 7.34 (m, 4H),
7.21
(m, 1H), 7.13 (m, 2H), 2.43 (bs, 3H). ELMS [M+'] m/z for C23H15N3C1Br is 448.
M.p.=
decomposed at 218-220.
Example 8: Compound 35
1H-NMR (CDC13): 8 = 7.78 (bs, 1H), 7.59 (d, 2H), 7.54 (d, 2H), 7.35 - 7.39 (m,
2H),
7.28 - 7.34 (m,2H), 7.13 - 7.18 (m, 2H), 7.01 - 7.05 (m, 2H), 2.72 (bs, 3H).
EIMS
[M+'] m/z for C24H18N3F is 367. M.p.= decomposed at 247-250.
Example 9: Compound 38
1H-NMR (Acetone-d6): 6 = 11.12 (bs, 1H), 10.46 (bs, 1H), 8.12 (d, 1H), 7.80
(bd,
2H), 7.62 (bd, 2H), 7.38-7.48 (m, 5H), 6.98 - 7.22 (m, 12H), 2.84 (bs, 3H).
EIMS
[M+'] m/z for C36H27N302 is 533. M.p.= decomposed at 128-130.
Example 10: Compound 40
1H-NMR (CDC13): 8 = 8.12 (dd, 2H), 7.60 (m, 6H), 7.24-7.53 (m, 1OH), 6.87 (bd,
2H), 6.61 (bd, 2H), 2.08 (s, 3H). M.p.= decomposed at 142.
Example 11: Compound 42
'H-NMR (CDC13): b = 9.39 (bs,1H), 7.39 - 7.50 (m, 4H), 7.28 - 7.38 (m, 6H),
7.06
(bs, 1H), 6.94 (bs, 2H), 2.08 (bs, 3H). EIMS [M+'] m/z for C24H18N3Br is 428.
M.p.=
decomposed at 155-157.
58
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
Example 12: Compound 44
1H-NMR (CDC13): 8 = 8.78 (dd, 2H), 8.19 (dd, 1H), 7.96 (bs, 1H), 7.80 (dd,
1H), 7.80
(dd, 1H), 7.55-7.77 (m, 6H), 7.16-7.42 (m, 2H), 2.87 (bs, 3H). EIMS [M+'] m/z
for
C24H17N3 is 347. M.p.= decomposed at 167.
Example 13: Compound 10
1H-NMR (CDC13): S = 10.68 (bs, 1H), 7.73 (bs, 1H), 7.22 (d, 4H), 6.99 (bs,
1H), 6.92
(bd, 2H), 6.85 (bd, 2H), 6.611 (d, 4H), 3.70 (s, 6H). EIMS [M+'] m/z for
C25H2ON3BrO2 is 474. M.p.=135.
Example 14: Compound 26
1H-NMR (DMSO-d6): 6 = 12.60 (s, 1H), 11.70 (s, 1H), 8.60 (d, 1H), 8.17 (s,
1H),
7.68 (bs, 1H), 7.46 (d, 2H), 7.33 (d, 2H), 7.25 (bs, 2H), 7.09 (bs, 1H). EIMS
[M+']
m/z for C19H12N3BrS2 is 426.
Example 15: Compound 28
1H-NMR (DMSO-d6): 8 = 12.60 (s, 1H), 11.65 (s, 1H), 8.44- 8.64 (m, 3H), 8.01-
8.14
(m, 1H), 7.22-7.66 (m, 8H). EIMS [M+'] m/z for C22H14N4BrF is 433. M.P.=
decomposed at 343.
Example 16: Compound 32
1H-NMR (CDC13): 8 = 8.12 (bs, 1H), 7.48 (d, 2H), 7.46 (d, 2H), 7.23-7.34 (m,
8H).
M.p.=230-232.
Example 17: Compound 34
'H-NMR (DMSO-d6): 8 = 12.63 (s, 1H), 11.67 (s, 1H), 8.62 (d, 1H), 8.21 (d,
2H),
8.08 (d, 1H), 7.86 (d, 2H), 7.39-7.64 (m, 6H), 7.32 (dd, 1H). EIMS [M+'] m/z
for
C23H15N4BrFO2 is 459. M.p.= decomposed at 250-253.
59
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
Example 18: Compound 36
'H-NMR (CDC13): 8 = 10.42 (bs,1H), 7.86 (s, 1H), 7.16-7.33 (m, 6H), 7.04 (dd,
2H),
6.95 (dd, 2H), 6.88 (t, 3H). EIMS [M+'] m/z for C23H15N3BrF is 432. M.p.=
decomposed at 83-86.
Example 19: Compound 41
1H-NMR (CDC13): 8 = 8.08 (d, 4H), 8.07 (bs, 1H), 7.75 (d, 4H), 7.28-7.50 (m,
10H),
7.12 (bd, 2H), 6.97 (bs, 1H). M.P.= 155-158.
Example 20: Compound 43
1H-NMR (CDCl3): 6 = 9.75 (bs, 1H), 7.83 (bs, 1H), 7.36 (m, 3H), 7.25 - 7.29
(m, 5H),
7.12 (m, 3H), 7.10 (bd, 1H). EIMS [M+'] m/z for C23H15N3Br2 is 493. M.P.=
decomposed at 230.
Example 21: Compound 45
'H-NMR (DMSO-d6): 6 = 13.30 (bs, 1H), 11.62 (d, 1H), 8.87 (bd, 2H), 8.64 (bs,
1H),
8.44 (bs, 1H), 8.29 (t, 1H), 7.76 (t, 2H), 7.62 (t, 2H), 7.52 (d, 1H), 7.35 -
7.41 (m,
2H). EIMS [M+'] mlz for C23H14N3Br is 412.
Example 22: Compound 84
1H-NMR (DMSO-d6): 6 = 8.64 (d, 1H), 8.17 (d, 1H), 7.47 (d, 1H), 7.39 (t, 1H),
7.33
(dd, 1H), 7.20-7.31 (m, 2H), 7.12 (bd, 2H), 6.97 (bd, 1H), 6.84 (bd, 1H). 3.77
(s, 3H),
3.72 (s, 3H), EIMS [M+'] m/z for C25H2ON3BrO2 is 474. M.p.= decomposed at 250-
253.
Example 23: Compound 37
'H-NMR (CDC13): 8 = 9.92 (bs, 1H), 8.17 (bs, 1H), 7.87 (t, 1H), 7.55 (bs, 1H),
7.21-
7.33 (m, 6H), 7.15-7.2 (m, 1H), 7.04-7.07 (m, 2H), 6.90 (t, 2H). ). EIMS [M+']
m/z
for C23H16N3F is 353. M.p.= 51.
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
Example 24: Compound 46
1H-NMR (DMSO-d6): 8 = 13.09 (s, 1H), 11.61 (d, 1H), 8.83 (q, 2H), 8.73 (m,
1H),
8.68 (d, 1H), 8.46 (d, 1H), 8.24 (s, 1H), 7.74 (t, 211), 7.62 (t, 2H), 7.51 -
7.56 (m, 1H),
7.23 - 7.27 (m, 2H). EMS [M+'] m/z for C23H15N3 is 333. M.p.=135-137.
Example 25: Compound 83
1H-NMR (DMSO-d6): 8 = 12.40 (s, 1H), 11.80 (s, 1H), 8.56 (d, 1H), 8.24 (s,
1H),
8.15 (s, 1H), 7.78 (d, 1H), 7.65 (d, 2H), 7.54 (d, 2H), 7.47 (t, 2H), 7.32 -
7.42 (m, 3H),
7.24 (t, 1H), 3.90 (s, 3H). EIMS [M+.] m/z for C25H19N3O2 is 393. M.p.= 293-
295.
Example 26: Compound 74
'H-NMR (DMSO-d6): 8 = 11.74 (d, 1H), 7.95 (dd, 1H), 7.32-7.57 (m, 6H), 7.17-
7.31
(m, 2H), 7.12 (t, 1H), 6.70 (d, 1H), 6.66 (bd, 1H). EIMS [M+'] m/z for
C23H19N2FO2
is 374.
Example 27: In vitro Inhibition of Methicillin-Resistant Staphylococcus aureus
(MRSA)
CMRSA-1B is an epidemic multi-drug resistant strain of S. aureus which
accounts for
49% and 70% of S. aureus strains isolated in hospitals in Canada and in
Ontario
respectively. CMRSA-1B was cultured in Tryptic Soy Broth (TSB) at 37 C and
used
for the inhibition assays during the log phase of growth (OD600 of 0.1). 10 l
of each
candidate compound were placed in duplicate into the wells of a 96-microtitre
plate
followed by the addition of 90 l of the CMRSA-1B culture suspensions. The
candidate compounds were dissolved at a concentration of 250 g/ml in 50 %
DMSO,
and diluted to a final concentration of 25 g/ml in 5 % DMSO in the culture
suspension. Bacterial growth was monitored by measuring the absorbance at 600
run
in an ELISA reader. The level of growth inhibition was estimated as percentage
of the
OD600 value with respect to a control consisting of an aliquot of the same
bacterial
suspension in the presence of 5 % DMSO.
61
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
A. Determination of Minimal Inhibitory Concentration (MIC)
The lowest concentration of triaryl-imidazole derivative that completely
inhibited
growth of the micro-organisms in vitro (MIC) was determined by the sequential
macrodilution (tube) broth method. (Nat. Committee for Clinical Laboratory
Standards. Document M7-A5 2000, 20;1-25). Bacterial suspensions containing 5 x
105 colony-forming units (CFU) were incubated with serial two-fold dilutions
of each
drug at 37 C overnight, and growth was monitored visually. The range of MIC
values
among the triaryl-imidazole derivatives was 12.5 - 50 g/ml. Table 1 shows the
MIC
value for some of the 2,4,5-triarylimidazole-derivatives against MRSA.
Table 1
Conc. g/mi Compound 1 Compound 2 Compound 3 = Compound 4
0 + + + +
3.12 + + + +
6.25 + + + +
12.50 - - + +
25.00 - - - +
50.00 - - - -
100.00 - - - -
(+): Positive visual bacterial growth
(- ): Negative visual bacterial growth
62
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
QQ N NH N NH
N CH3
H
1 HO O 2
Br f Br
N, NH
'77
Br N NH
Br
HO O
O O
3 4
B. Bactericidal Effect against MRSA
The bactericidal effect of compounds of Formula I against MRSA was determined
using the same concentrations of compounds and growth conditions used to
determine
the MICs (see above). Serial dilutions of liquid cultures were incubated with
the
compounds at 37 C overnight and then plated on Tryptic Soy Agar (TSA) plates.
After incubation for 17 hours at 37 C, the number of live bacteria was
calculated from
the number of colonies grown per milliliter of culture and expressed as colony-
forming units (CFU). A bactericidal effect was observed at the corresponding
MIC
for derivatives 1 and 2, while derivatives 3 and 4 exhibited a bacteriostatic
effect at
these concentrations (Figure 1).
Example 28: In vivo Inhibition of Methicillin-Resistant Staphylococcus aureus
(MRSA)
Triaryl-imidazole compounds were tested in vivo for antibacterial activity
against
MRSA in a model of acute infection using immunosupressed mice. Suspensions of
400 L containing 5 x 106 MRSA-1B387 bacteria in 5 % mucin were injected into
63
CA 02496241 2011-03-14
groups of 5-10 female, 8-14 weeks CB17-SCID mice. Under these conditions
bacterial infection produced 80-100 % lethality in less than 48 hours. Each
group of
inoculated mice was treated with 50 mg/kg of the respective compound
intraperitoneally (I.P.) immediately and 3 hours after bacterial inoculation.
The results
of the in vivo experiments are shown in Table 2.
Table 2
F~ .;'ia7~r f
Co~ppou Sal
1 1 40
2 1 80
3 2 15
4 3 50
Vancomycin 2 100
Control 4 24
Example 29: In vivo Toxicity Tests
In vivo acute toxicity tests were conducted using compounds 1, 2, 3 and 4.
Mice were
injected with each of the above compounds at a concentration of 200 mg/kg per
day.
No symptoms of sickness, change of total weight, organ weight and appearance
was
observed in any of the mice challenged, indicating that these compounds
exhibit no
toxic effects in mice.
Further in vivo GLP toxicology studies are conducted using different animal
species.
Example 30: In vitro Anti-Bacterial Activity
A standard microdilution method (NCCLS, 2000; Document M7-A5) was used to
determine the minimum inhibitory concentrations (MICs) against S. aureus,
64
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
methicillin-resistant strains (MRSA); 1A-218, 1A-318, 1B-374, 1B-315, 1B-185,
1B387 (Simor et al. 1999 Can Conimun Dis Rep; 25:105-108) and methicillin-
sensitive strains (MSSA); ATCC-6538 and ATCC-29213. The MICs of some
compounds were also determined against other Gram-positive bacteria:
Enterococcus
faecium (ATCC 51559), Enterococcus faecalis (ATCC-51299, ATCC-29212) and
Staphylococcus epidermidis (ATCC-35983).
Mueller-Hinton broth (MHB) is recommended as the medium of choice for
susceptibility testing of commonly isolated, rapidly growing aerobic organisms
such
as the Gram-positive bacteria included in this study. A "cation-adjusted
Mueller-
Hinton broth" (Ca-MHB) was prepared by first preparing standard MHB from a
dehydrated base as recommended by the manufacturer. After the broth was
autoclaved at 121 C for 20 min, it was allowed to cool down to 25 C or lower
before
aseptically adding Ca + and Mg++ (20 mg of Ca++/L and 10 mg of Mgr/L).
Single colonies from fresh agar plates of the different bacterial cultures
were sub-
cultured overnight in 3 ml of Tryptic soy broth (TSB) at 37 C with shaking at
250
RPM. After 18- 20 hours of incubation, the absorbance at X = 600 nm (OD600)
were
determined for each culture and adjusted to a final OD600 = 0.1 (4.2 x 101
cfu/ml).
Bacterial suspensions were then diluted 1:200 in sterile Ca-MHB for the in
vitro
susceptibility test, with each well of the microtitre plate containing
approximately 2 x
105 cfu/ml.
Stock solutions of the different test compounds were prepared at
concentrations of
1,280 g/ml, 640 g/ml, or 320 g/ml in 50% DMSO (dimethyl sulfoxide). Serial
two-fold dilutions were then prepared in 50% DMSO (the working concentrations
were 1:10 of the stock concentrations, in 100 l culture suspensions). The
filled
microtitre plates were sealed in plastic bags and incubated at 35 C for 24 hr
in an
ambient air incubator with shaking at 250 RPM. MIC values were determined as
the
lowest concentration where complete inhibition of the visible bacterial growth
was
observed by the unaided eye and confirmed by the measurement of the optical
density.
(OD600). Table 3 shows the MIC values in g/ml of different compounds, the MIC
CA 02496241 2005-02-18
Printed: 30-11-2004
DESCPAMD' C CA0301229
value provided is against all 8 strains of S_ aureus tested except where
indicated
otherwise. Table 4 shows the MIC values of 3 compounds selected as examples
against other graze positive bacteria, including 2 strains resistant to the
first line
antibiotic vancomycin.
Table 3
2 8-16
5 >128
.7 4-16
9 8
11 8
13 2-4
>128
17 16'
19 4
21 8-16
23 4-8
32-64
27 32-64
29 >128
31 4
33 4-8
8
38 >128
>128
42 4
44 =8
6 4
8 2
10 4
Empf.zeit:20/08/2004 21:20 EmPf.nr,:200 P.001
1 ' AMENDED SHEET `20_08-2004
Printed: 30-11-2004 DESCPAMD' C CA0301229
20 2-4
26
28
32 8
34 >128
36 2
39 >128
41 >128
43 4
45 0.5
48 4-8 .
50 >64
51 16
.52 >64
53 1
54 2-4 .
55 2
56
37 >128
46 4
49 4
83 >128
57- 16
58 4
59 32
60 64
61 $
62 2
63 2
Empf.zeit:20/08/2004 21:20 Empf.nr.:290 P.002
CA 02496241 2005-02-18
2,' AMENDED SHEET 120-08-2004
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
Compound MIC(lu,gtml)
64 4
65 16
66 2
67 16
68 32
69 16
70 8
71 8
72 0.5-2
73 >128
74 >128
75 32
47 >128
76 >128
77 >128
84 2
1 MIC was >128 against strain IA-318
2 MIC was 4 against strain IB-315
Table 4
Compound.
Strain 2 13 19 Vancomycin
Enterococcus faecium 1 32 8 >128
(ATCC-51559)
Enterococcusfaecalis 16 32 8 32
(ATCC-51299)
Enterococcus faecalis 16 n.d. 8 4
(ATCC-29212)
Staphylococcus epidermidis 16 n.d. 4 2
(ATCC-35983)
n.d. Not determined
68
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
Example 31: In Vivo Anti-Bacterial Activity
For in vivo studies, groups of 5-10 female ICR mice (6-7 weeks) were
inoculated
intraperitonially with 3-8 x 107 CFU MRSA 1B-387 per mouse in 400 l of 5 %
mucin. The test compounds at concentrations of 50 or 100 mg/kg were
administered
orally immediately after the bacterial inoculation and again 3 hours later.
The selected
dosages were administered twice daily throughout the experiment. The efficacy
of the
treatment was evaluated by comparison of mortality between the experimental
and
control groups (Table 5).
Table 5
Challenge MRSA Survival
Compound (cfulml/route) Treatment schedule # mice
10 3.0 x 10 /LP 100 mg/Kg/d 5/5 100
32 3.0 x 10 /I.P 100 mg/Kg/d 2/5 40
34 3.0 x 10 /I.P 100 mg/Kg/d 5/5 100
36 3.0 x 10 /I.P 100 mg/Kg/d 4/5 80
43 3.0 x 10 /I.P 100 mg/Kg/d 4/5 80
Control 3.0 x 10 /I.P ----------------- 2/5 40
5 6.8 x 10 /I.P 100 mg/Kg/d 1/5 20
11 6.8 x 10 /I.P 100 mg/Kg/d 0/5 0
13 6.8 x 10 /I.P 100 mg/Kg/d 1/5 20
44 6.8 x 10 /I.P 100 mg/Kg/d 3/5 60
8 6.8 x 10 /I.P 100 mg/Kg/d 1/5 20
46 6.8 x 10 /I.P 100 mg/Kg/d 2/5 40
83 6.8 x 10 /I.P 100 mg/Kg/d 1/5 20
Control 6.8 x 10 /I.P -------------- 0/5 0
45 8.0 x 10 /I.P 200mg/Kg/d 4/10 40
45 8.0 x 10 /I.P 100 mg/Kg/d 1/10 20
69
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
Challenge MRSA Survival
Compound (cfu/mllroute) Treatment schedule # mice %
Control 8.0 x 10 /I.P --------------- .0/10 0
29 7.6 x 10 /I.P 100 mg/Kg/d 1/5 20
33 7.6 x 10 /I.P 100 mg/Kg/d 0/5 0
35 7.6 x 10 /I.P 100 mg/Kg/d 2/50 40
42 7.6 x 10 /I.P 100 mg/Kg/d 0/5 0
6 7.6 x 107/I.P 100 mg/Kg/d 2/5 40
Control 7.6 x 10 /I.P ---------------- 0/5 0
Example 32: In Vitro Anti-fungal Activity
A microdilution method was used to determine the anti-fungal activity of
different test
compounds against Candida albicans (ATCC 24433) at 25 g/ml. RPMI-1640 broth
is recommended as the medium of choice for susceptibility testing of C
albicans
(NCCLS, 2002; Document M27-A2) and was used in this study. The broth was
buffered to pH 7.0 and then sterilized using a 250 ml vacuum driven disposable
0.45um filtration system.
C. albicans was sub-cultured from a -80 C stock onto Sabouraud dextrose agar
and
incubated for 24 - 48 hrs at 37 C. The fungi inoculum was prepared by picking
five
colonies of 1 mm in diameter from 24 - 48 hr old cultures. The colonies were
then
suspended in 5 ml of sterile normal saline (8.5g/L NaCl; 0.85% saline) diluted
1:50
and further diluted 1:20 with medium to obtain two-fold inoculum (1 - 5 x 103
cfu/ml). For MIC determinations, aliquots of this suspension placed in
microtitre
wells were diluted 1:1 with serial dilutions of the test compounds (the final
inoculum
size was 0.5 - 2.5 x 103 cfu/ml). The microtitre plates were then sealed in
plastic bags
and incubated at 35 C for 24 - 48 hrs in an ambient air incubator with shaking
at 250
RPM.
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
Inhibition of visible growth of C. albicans in the microtitre plate wells was
assessed
by the unaided eye and confirmed by OD600 reading. Table 6 shows the anti-
fungal
activity of some derivatives against C. albicans (ATCC 24433) at a single
concentration of 25 g/ml. The MIC values of compounds with significant
activity are
also included.
Table 6
Compound Inhibition ( ,/a) 'Inhibition ( !e) MIC (p /mi)
(25 pg/mi) after 24 h after 48 h
5 0 0
11 27 28
13 0 0
29 0 0
33 0 0
35 100 73
42 77 43
44 0 0
6 0 0
8 100 100 8
0 0
32 93 100 8
34 0 0
36 86 97 16
43 87 59 8
46 33 0
83 0 0
The embodiments of the invention being thus described, it will be obvious that
the
10 same may be varied in many ways. Such variations are not to be regarded as
a
departure from the spirit and scope of the invention, and all such
modifications as
71
CA 02496241 2005-02-18
WO 2004/016086 PCT/CA2003/001229
would be obvious to one skilled in the art are intended to be included within
the scope
of the following claims.
72