Note: Descriptions are shown in the official language in which they were submitted.
CA 02496249 2010-12-14
25771-1004
1
8-[3-AMINO-PIPERIDIN-1-YL]-XANTHINES, THE PRODUCTION THEREOF
AND THE USE OF THE SAME AS MEDICAMENTS
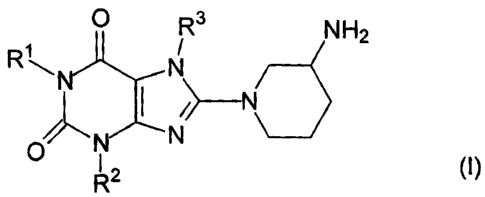
The present invention relates to new substituted xanthines of general formula
0 3 NH2
R'`
N
om (I),
NN
R2
the tautomers, the stereoisomers, the mixtures, the prodrugs thereof and the
salts thereof, particularly the physiologically acceptable salts thereof with
inorganic or organic acids or bases which have valuable pharmacological
properties, particularly an inhibiting effect on the activity of the enzyme
dipeptidylpeptidase-IV (DPP-IV), the preparation thereof, the use thereof for
the prevention or treatment of diseases or conditions associated with an
increased DPP-IV activity or capable of being prevented or alleviated by
reducing the DPP-IV activity, particularly. type I or type II diabetes
mellitus, the
pharmaceutical compositions containing a compound of general formula (I) or
a physiologically acceptable salt thereof as well as processes for the
preparation thereof.
In the above formula I
R' denotes a methyl group,
a methyl group which is substituted by a dimethylaminocarbonyl, pyrrolidin-1-
ylcarbonyl, piperidin-1-ylcarbonyl, tert.-butylcarbonyl or a
cyclohexylcarbonyl-
group,
CA 02496249 2005-02-18
2
a methyl group which is substituted by a naphthyl, methylnaphthyl,
methoxynaphthyl, nitronaphthyl or dimethylaminonaphthyl group,
a methyl group which is substituted by a 2-phenylethenyl or a biphenylyl
group,
a methyl group which is substituted by a phenyloxadiazolyl, 5-methyl-3-
phenyl-isoxazolyl, phenylpyridinyl, indolyl, benzothiophenyl, quinolinyl,
isoquinolinyl, methylisoquinolinyl, (methoxycarbonylmethylamino)-
isoquinolinyl, cinnolinyl, quinazolinyl, methylquinazolinyl, 1,2-dihydro-1-
methyl-2-oxo-quinolinyl, 1,2-dihydro-2-methyl-1-oxo-isoquinolinyl, 3,4-dihydro-
4-oxo-phthalazinyl, 3,4-dihydro-3-methyl-4-oxo-phthalazinyl, 3,4-dihydro-4-
oxo-quinazolinyl, 3,4-dihydro-3-methyl-4-oxo-quinazolinyl or a 2-oxo-2H-
chromenyl group,
a 2-methoxyethyl, 2-phenyloxyethyl or 2-cyanoethyl group,
a phenylcarbonylmethyl or a 1-(phenylcarbonyl)-ethyl group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by an
amino, cyanomethylamino, methylcarbonylamino, ethylcarbonylamino,
isopropylcarbonylamino, methoxycarbonylamino, (ethyloxycarbonylamino)-
carbonylamino or a 2-oxo-imidazolidin-1-yl group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by a
carboxy, methoxycarbonyl, ethyloxycarbonyl, aminocarbonyl,
methylaminocarbonyl, dimethylaminocarbonyl or morpholin-4-ylcarbonyl
group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by a
methylsuiphanyl, methylsulphinyl or methylsulphonyl group,
CA 02496249 2005-02-18
3
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by a
carboxymethoxy, ethyloxycarbonylmethoxy, isopropyloxycarbonylmethoxy,
aminocarbonylmethoxy, methylaminocarbonylmethoxy,
ethyl aminocarbonylmethoxy, isopropylaminocarbonylmethoxy,
dimethylaminocarbonylmethoxy, pyrrolidin-1-ylcarbonylmethoxy or morpholin-
4-ylcarbonylmethoxy group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by a
1-(methoxycarbonyl)-ethyloxy or a 1-(aminocarbonyl)-ethyloxy group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by a
methylsulphinylmethoxy group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by two
methoxy groups or
a phenylcarbonylmethyl group wherein in the phenyl moiety two adjacent
hydrogen atoms are replaced by a -O-CH2-O, -O-CH2-CH2-O or a -N(CH3)-
CO-O group,
R2 denotes a hydrogen atom,
a methyl, isopropyl, 2-propen-1-yl, 2-propyn-1-yl or phenyl group or
a cyanomethyl or methoxycarbonylmethyl group and
R3 denotes a 2-cyanobenzyl or 2,6-dicyanobenzyl group,
a 2-methyl-2-propen-1-yl, 2-chloro-2-propen-1-yl or 3-bromo-2-propen-1-yl
group
a 2-buten-1-yl, 3-methyl-2-buten-1-yl or 2,3-dimethyl-2-buten-1-yl group,
a 2-butyn-1-yl group,
CA 02496249 2005-02-18
4
a 1-cyclopenten-1-ylmethyl group or
a 2-furanylmethyl group.
The carboxy groups mentioned in the definition of the above mentioned
groups may be replaced by a group which can be converted into a carboxy
group in vivo or by a group which is negatively charged under physiological
conditions,
and furthermore the amino and imino groups mentioned in the definition of the
above mentioned groups may be substituted by a group which can be cleaved
in vivo. Such groups are described for example in WO 98/46576 and by N.M.
Nielsen et al. in International Journal of Pharmaceutics 39, 75-85 (1987).
Compounds which contain a group that can be cleaved in vivo are prodrugs of
the corresponding compounds wherein this group that can be cleaved in vivo
has been cleaved.
By a group which can be converted in vivo into a carboxy group is meant, for
example, a hydroxymethyl group, a carboxy group esterified with an alcohol
wherein the alcohol moiety is preferably a C1_6-alkanol, a phenyl-C1.3-
alkanol,
a C3.9-cycloalkanol, while a C5-8-cycloalkanol may additionally be substituted
by one or two C1_3-alkyl groups, a C5.5-cycloalkanol wherein a methylene
group in the 3 or 4 position is replaced by an oxygen atom or by an imino
group optionally substituted by a C1_3-alkyl, phenyl-C1.3-alkyl, phenyl-
C1_3-alkyloxycarbonyl or C2_6-alkanoyl group and the cycloalkanol moiety may
additionally be substituted by one or two C1_3-alkyl groups, a C4_7-
cycloalkenol,
a C3.5-alkenol, a phenyl-C3.5-alkenol, a C3.5-alkynol or phenyl-C3.5-alkynol
with
the proviso that no bonds to the oxygen atom start from a carbon atom which
carries a double or triple bond, a C3.8-cycloalkyl-C1.3-alkanol, a
bicycloalkanol
with a total of 8 to 10 carbon atoms which may additionally be substituted in
the bicycloalkyl moiety by one or two C1.3-alkyl groups, a 1,3-dihydro-3-oxo-1-
isobenzofuranol or an alcohol of formula
CA 02496249 2005-02-18
Rp-CO-O-(RgCRr)-OH,
wherein
Rp denotes a C,_8-alkyl, C5_7-cycloalkyl, C1_8-alkyloxy, C5.7-
cycloalkyloxy, phenyl or phenyl- C1_3-alkyl group,
Rq denotes a hydrogen atom, a C,_3-alkyl, C5.7-cycloalkyl or phenyl
group and
Rr denotes a hydrogen atom or a C1_3-alkyl group,
by a group which is negatively charged under physiological conditions is
meant, for example, a tetrazol-5-yl, phenylcarbonylaminocarbonyl,
trifluoromethylcarbonylaminocarbonyl, C1_6-alkylsulphonylamino,
phenylsulphonylamino, benzylsulphonylamino, trifluoromethylsulphonylamino,
C1_6-alkylsulphonylaminocarbonyl, phenylsulphonylaminocarbonyl,
benzylsulphonylaminocarbonyl or perfluoro-C1.6-alkylsulphonylaminocarbonyl
group
and by a group which can be cleaved in vivo from an imino or amino group is
meant, for example, a hydroxy group, an acyl group such as a phenylcarbonyl
group optionally mono- or disubstituted by fluorine, chlorine, bromine or
iodine
atoms, by C1.3-alkyl or C1.3-alkoxy groups, while the substituents may be
identical or different, a pyridinoyl group or a C1_16-alkanoyl group such as
the
formyl, acetyl, propionyl, butanoyl, pentanoyl or hexanoyl group, a 3,3,3-
trichloropropionyl or allyloxycarbonyl group, a C1.16-alkoxycarbonyl or
C1.16-alkylcarbonyloxy group, wherein hydrogen atoms may be wholly or
partially replaced by fluorine or chlorine atoms such as the methoxycarbonyl,
ethoxycarbonyl, propoxycarbonyl, isopropoxycarbonyl, butoxycarbonyl,
tert.butoxycarbonyl, pentoxycarbonyl, hexoxycarbonyl, octyloxycarbonyl,
nonyloxycarbonyl, decyloxycarbonyl, undecyloxycarbonyl,
dodecyloxycarbonyl, hexadecyloxycarbonyl, methylcarbonyloxy,
CA 02496249 2010-12-14
25771-1004
6
ethylcarbonyloxy, 2,2,2-trichloroethylcarbonyloxy, propylcarbonyloxy,
isopropylcarbonyloxy, butylcarbonyloxy, tert.butylcarbonyloxy,
pentylcarbonyloxy, hexylcarbonyloxy, octylcarbonyloxy, nonylcarbonyloxy,
decylcarbonyloxy, undecylcarbonyloxy, dodecylcarbonyloxy or
hexadecylcarbonyloxy group, a phenyl-C,.6-alkoxycarbonyl group such as the
benzyloxycarbonyl, phenylethoxycarbonyl or phenylpropoxycarbonyl group, a
3-amino-propionyl group wherein the amino group may be mono- or
disubstituted by C,_6-alkyl or C3.7-cycloalkyl groups and the substituents may
be identical or different, a C1.3-alkylsulphonyl-C2.4-alkoxycarbonyi, C1.3-
alkoxy-
C2.4-alkoxy-C2-4-alkoxycarbonyl, Rp-CO-O-(RqCRr)-O-CO, C14-alkyl-CO-NH-
(RSCRt)-O-CO- or C1.6-alkyl-CO-O-(R3CRt)-(RSCRt)-O-CO- group, wherein Rp
to Rr are as hereinbefore defined,
Ra and Rt, which may be identical or different, denote hydrogen atoms or
C1.3-alkyl groups.
A first aspect of the invention relates to compounds of general formula (1)
wherein
R1 denotes a methyl group which is substituted by a dimethylaminocarbonyl,
pyrrolidin-1-ylcarbonyl, piperidin-1-ylcarbonyl, tert.-butylcarbonyl or a
cyclohexylcarbonyl group,
a methyl group which is substituted by a naphthyl, methylnaphthyl,
methoxynaphthyl, nitronaphthyl or (dimethylamino)-naphthyl group,
a methyl group which is substituted by a 2-phenylethenyl or a biphenylyl
group,
a methyl group which is substituted by a phenyl-oxadiazolyl, 5-methyl-3-
phenyl-isoxazolyl, phenyl-pyridinyl, indolyl, benzothiophenyl, quinolinyl,
isoquinolinyl, methylisoquinolinyl, (methoxycarbonylmethylamino)-
isoquinolinyl, cinnolinyl, quinazolinyl, methyiquinazolinyl, 1,2-dihydro-1-
CA 02496249 2005-02-18
7
methyl-2-oxo-quinolinyl, 1,2-dihydro-2-methyl-1-oxo-isoquinolinyl, 3,4-dihydro-
4-oxo-phthalazinyl, 3,4-dihydro-3-methyl-4-oxo-phthalazinyl, 3,4-dihydro-4-
oxo-quinazolinyl, 3,4-dihydro-3-methyl-4-oxo-quinazolinyl or a 2-oxo-2H-
chromenyl group,
a 2-methoxyethyl, 2-phenyloxyethyl or 2-cyanoethyl group,
a phenylcarbonylmethyl or a 1-(phenylcarbonyl)-ethyl group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by an
amino, cyanomethylamino, methylcarbonylamino, ethylcarbonylamino,
isopropylcarbonylamino, methoxycarbonylamino, (ethyloxycarbonylamino)-
carbonylamino or a 2-oxo-imidazolidin-1-yl group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by a
carboxy, methoxycarbonyl, ethyloxycarbonyl, aminocarbonyl,
methylaminocarbonyl, dimethylaminocarbonyl or morpholin-4-ylcarbonyl
group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by a
methylsulphanyl, methylsulphinyl or methylsulphonyl group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by a
carboxymethoxy, ethyloxycarbonylmethoxy, isopropyloxycarbonylmethoxy,
aminocarbonylmethoxy, methylaminocarbonylmethoxy,
ethylaminocarbonylmethoxy, isopropylaminocarbonylmethoxy,
dimethylaminocarbonylmethoxy, pyrrolidin-1-ylcarbonylmethoxy or morpholin-
4-ylcarbonylmethoxy group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by a
1-(methoxycarbonyl)-ethyloxy or a 1-(aminocarbonyl)-ethyloxy group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by a
methylsulphinylmethoxy group,
8
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by two
methoxy groups or
a phenylcarbonylmethyl group wherein in the phenyl moiety two adjacent
hydrogen atoms are replaced by a -O-CH2-O, -O-CH2-CH2-O or a -N(CH3)-
CO-O group,
R2 denotes a methyl, isopropyl or phenyl group
and
R3 denotes a 2-methyl -2-propen-1-yl, 2-chloro-2-propen-1-yl or 3-bromo-2-
propen-1-yl group
a 2-buten-1-yl or 2,3-dimethyl-2-buten-1-yl group,
a 2-butyn-1-yl group,
a 1-cyclopenten-1 -ylmethyl group or
a 2-furanylmethyl group,
as well as the compounds
1-(2-cyano-ethyl)-3-methyl -7-(2-cyano-be nzyl)-8-(3-amino-pi peridin-1-yl)-
xanthine,
1-(2-{2-[(ethoxycarbonyl )methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-methyl-
2-buten-I -yl)-8-(3-amino-piperidin-1-yl)-xanthine,
1-(2-{2-[(aminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-methyl-
2-buten-I -yl)-8-(3-amino-piperidin-1-yl)-xanthine,
CA 02496249 2005-02-18
9
1-(2-{3-[(methanesulphinyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-
methyl-2-buten-l-yl)-8-(3-amino-piperidin-l-yl)-xanthine,
1-(1-methyl-2-oxo-2-phenyl-ethyl)-3-methyl-7-(3-methyl-2-buten-1-yl)-8-(3-
amino-piperidin-1-yi)-xanthine,
1 -(2-p h enoxy-ethyl)-3-methyl-7-(2-cyano-benzyl)-8-(3-am ino-pi pe rid i n-
1 -yl)-
xanthine,
1-(2-phenyl-2-oxo-ethyl)-7-(3-methyl-2-buten-1 -yl)-8-(3-amino-piperidin-1 -
yl)-
xanthine,
1-(2-{3-[(ethoxycarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-methyl-
2-buten-1-yl)-8-(3-amino-piperidin-l-yl)-xanthine,
1-(2-{2-[(methylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-
methyl-2-buten-l-yl)-8-(3-amino-piperidin-l-yl)-xanthine,
1-(2-{2-[(dimethylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-(3-amino-piperidin-l-yl)-xanthine,
1-(2-methoxy-ethyl)-3-methyl-7-(2-cyano-benzyl)-8-(3-amino-piperidin-1-yl)-
xanthine,
1-methyl-3-[(methoxycarbonyl)methyl]-7-(2-cyano-benzyl)-8-(3-amino-
piperidin-l -yl)-xanthine,
1 -methyl-3-cyanomethyl-7-(2-cyano-benzyl)-8-(3-amino-piperidin-1 -yl)-
xanthine,
1-methyl-3-(2- propyn-1-yl)-7-(2-cyano-benzyl)-8-(3-amino-piperidin-1-yl)-
xanthine,
CA 02496249 2005-02-18
10
1-{2-[3-(2-oxo-imidazolidin-1-yl)-phenyl]-2-oxo-ethyl}-3-methyl-7-(3-methyl-2-
buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine,
1-methyl-3-(2-propen-1-yl)-7-(2-cyano-benzyl)-8-(3-amino-piperidin-1-yl)-
xanthine,
1-(2-{2-[(ethylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-methyl-2-
buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine,
1-methyl-3-phenyl-7-(2-cyano-benzyl)-8-(3-amino-piperidin-1-yl)-xanthine,
1-[2-(2-amino-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-((S)-
3-amino-piperidin-1-yl)-xanthine,
1-(2-phenyl-2-oxo-ethyl)-3-cyanomethyl-7-(3-methyl-2-buten-1-yi)-8-(3-amino-
piperidin-1-yl)-xanthine,
1-[(quinolin-2-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-(3-amino-
piperidin-1-yl)-xanthine,
1-[(2-oxo-2H-chromen-4-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine,
1-[(cinnolin-4-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-(3-amino-
piperidin-1-yl)-xanthine,
1-[(1-methyl-2-oxo-1,2-dihydro-quinolin-4-yl)methyl]-3-methyl -7-(3-methyl-2-
buten-1-yi)-8-(3-amino-piperidin-1-yl)-xanthine,
1-[(4-oxo-3,4-dihydro-phthalazin-1-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-
yl)-8-(3-amino-piperidin-1-yl)-xanthine,
1-[(quinazolin-4-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-(3-amino-
piperidin-1-yl)-xanthine,
CA 02496249 2005-02-18
11
1-[(5-methyl-3-phenyl-isoxazol-4-yl)methyl]-3-methyl-7-(3-methyl-2-buten-l-
yl)-8-(3-amino-piperidin-1-yl)-xanthine,
1-[(isoquinolin-3-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-(3-amino-
piperidin-1-yl)-xanthine,
1-[(3-phenyl-[1,2,4]oxadiazol-5-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-
8-(3-amino-piperidin-1-yl)-xanthine,
1-[(4-phenyl-pyridin-2-yl)meth yl]-3-methyl -7-(3-methyl-2-buten-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine,
1-[(5-phenyl-pyridin-2-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine,
1-[(3-methyl-4-oxo-3,4-dihydro-phthalazin-1 -yl)methyl]-3-methyl-7-(3-methyl-
2-buten-1 -yl)-8-(3-amino-piperidin-1 -yl)-xanthine,
1 - [2-(3- m ethyl s u I ph anyl-phenyl)-2-oxo-ethyl]-3-methyl -7-(3-methyl-2-
buten-1-
yl)-8-(3-amino-piperidin-1-yl)-xanthine,
1-[2-(3-methanesulphinyl-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-
1-yl)-8-(3-amino-piperidin-1-yl )-xanthine,
1-[2-(3-methanesulphonyl-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-
1-yl)-8-(3-amino-piperidin-1-yl)-xanthine,
1-[2-(3-carboxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine,
1-[2-(3-methoxycarbonyl-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-
yl)-8-(3-amino-piperidin-1-yl)-xanthine,
CA 02496249 2005-02-18
12
1-{2-[3-(methylaminocarbonyl)-phenyl]-2-oxo-ethyl}-3-methyl-7-(3-methyl-2-
buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine,
1-{2-[3-(dimethylaminocarbonyl)-phenyl]-2-oxo-ethyl}-3-methyl-7-(3-methyl-2-
buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine,
1-{2-[3-(morpholin-4-yl-carbonyl)-phenyl]-2-oxo-ethyl}-3-methyl-7-(3-methyl-2-
buten-1-yl)-8-(3-amino-piperidin-1-yi)-xanthine,
1-[2-(2-carboxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine,
1-[2-(2-ethoxycarbonyl-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-
yl)-8-(3-amino-piperidin-1-yl)-xanthine,
1-{2-[2-(d imethylaminocarbonyl)-phenyl]-2-oxo-ethyl}-3-methyl-7-(3-methyl-2-
buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine,
1-{2-[2-(mo rp hol i n-4-yl-ca rbo nyl)-phenyl]-2-oxo-ethyl}-3-methyl-7-(3-
methyl-2-
buten-1-yl)-8-(3-amino-piperidin-1-yi)-xanthine,
1-[2-(2,6-dimethoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-
8-(3-amino-piperidin-1-yl)-xanthine,
1-((E)-3-phenyl-alIyl)-3-methyl -7-(3-methyl-2-buten-1-yl)-8-(3-amino-
piperidin-
1-yl)-xanthine,
1-[(benzo[b]thiophen-3-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine,
1-[(1 H-indol-3-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-(3-amino-
piperidin-1-yi)-xanthine,
CA 02496249 2005-02-18
13
1-[(biphenyl-4-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-(3-amino-
piperidin-1-yl)-xanthine,
1-(2-cyclohexyl-2-oxo-ethyl)-3-methyl-7-(3-methyl-2-buten-1-yl)-8-(3-amino-
piperidin-1-yl)-xanthine,
1-(3,3-dimethyl-2-oxo-butyl)-3-methyl-7-(3-methyl-2-buten-1 -yl)-8-(3-amino-
piperidin-1 -yl)-xanthine,
1-({5-[(methoxycarbonyl)methylamino]-isoquinolin-1-yl}methyl)-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine,
1-(2-dimethylamino-2-oxo-ethyl)-3-methyl-7-(3-methyl-2-buten-1 -yl)-8-(3-
amino-piperidin-1 -yi)-xanthine,
1-[2-(piperidin-1-yl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine,
1-[(2-methyl-1 -oxo-1,2-dihydro-isoquinolin-4-yl)methyl]-7-(3-methyl-2-buten-1-
yl)-8-(3-amino-piperidin-1-yl)-xanthine,
1-[2-(2,3-dimethoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-
8-(3-amino-piperidin-1-yl)-xanthine,
1-[2-(pyrrolidin-1-yl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine,
1-[2-(2,3-dihydro-benzo[1,4]dioxi n-5-yl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-
buten-1-yi)-8-(3-amino-piperidin-1-yl)-xanthine,
1-[2-(3-methyl -2-oxo-2,3-d ihyd ro-benzooxazol-7-yl)-2-oxo-ethyl] -3- m ethyl
-7-
(3-methyl-2-buten-1 -yl)-8-(3-amino-piperidin-1 -yl)-xanthine,
CA 02496249 2005-02-18
14
1-[2-(benzo[1, 3]dioxol-4-yl)-2-oxo-ethyl]-3-methyl -7-(3-methyl -2-buten-1-
yl)-8-
(3-amino-piperidin-1-yl)-xanthine,
1-methyl-3-isopropyl-7-(2-cyano-benzyl)-8-(3-amino-piperidin-1-yl)-xanthine,
1-[2-(2-cyanomethylamino-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-
1-yl)-8-(3-amino-piperidin-1-yl)-xanthine,
1 -[(isoquinolin-1 -yl)methyl]-3-[(methoxycarbonyl)methyl]-7-(3-methyl-2-buten-
1-yl)-8-(3-amino-piperidin-1-yl)-xanthine,
1-(2-{2-[(isopropyloxycarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine,
1-[2-(2-{[(ethoxycarbonylamino)carbonyl]amino}-phenyl)-2-oxo-ethyl]-3-
methyl-7-(3-methyl-2-buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine,
1-[2-(2-acetylamino-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-
((S)-3-amino-piperidin-1-yl)-xanthine,
1-(2-{2-[(methyl aminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-((S)-3-amino-piperid in-1-yl)-xanthine,
1-methyl-7-(2-cyano-benzyl)-8-(3-amino-pi perid i n- 1 -yl)-xanthine,
1-(2-{2-[(isopropyl carbon yl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-methyl-
2-buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine,
1-(2-{2-[(methoxycarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-methyl-
2-buten-I -yl)-8-(3-amino-piperid in-1-yl)-xanthine,
1-[2-(3-methyl-2-oxo-2, 3-d ihyd ro-benzooxazol-4-yl)-2-oxo-ethyl]-3-methyl-7-
(3-methyl-2-buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine,
CA 02496249 2005-02-18
CA 02496249 2010-12-14
25771-1004
1-[2-(2-nitro-3-methoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-
yl)-8-(3-amino-pipe(din-l -yl)-xanthine,
1-[2-(2-amino-3-methoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-
1-yl)-8-(3-amino-piperidin-1-yl)-xanthine,
1-[2-(2-oxo-2,3-dihydro-benzooxazol-7-yl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-
2-buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine,
1-[(3-methyl-isoquinolin-1-yl)methyl]-3-methyl-7-(3-methyl-1-buten-1-yl)-8-
((R)-3-amino-piperidin-1-yl)-xanthine,
1-[(3-methyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-cyano-benzyl)-8-(3-amino-
piperidin-1-yl)-xanthine,
1-[2-(3-carboxymethoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-l -
yl)-8-(3-amino-piperidin-1-yl)-xanthine and
1-[2-(2-carboxymethoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-
yi)-8-(3-amino-piperidin-1-yl)-xanthine,
the tautomers, enantiomers, diastereomers, the mixtures thereof, the
prodrugs thereof and the salts thereof.
A first preferred sub-group of the first aspect of the invention comprises
compounds of general formula I wherein
R' denotes a 4-methoxy-1-naphthylmethyl group,
a 2-quinolinylmethyl, 4-quinolinylmethyl or a 6-quinolinylmethyl group,
a 1-isoquinolinylmethyl, 3-methyl-1-isoquinolinylmethyl, 4-methyl-1-
isoquinolinylmethyl or a 3-isoquinolinylmethyl group or
CA 02496249 2011-07-04
25771-1004(S)
16
a 2-quinazolinylmethyl, 4-methyl-2-quinazolinylmethyl or a
4-quinazolinylmethyl group,
R2 denotes a methyl group and
R3 denotes a 2-buten-1-yl or a 2-butyn-1-yl group,
the tautomers, enantiomers, diastereomers, the mixtures thereof and the
salts thereof.
A further sub-group of the above embodiment comprises a
compound of general formula I, wherein
R1 denotes a 2-quinazolinylmethyl, 4-methyl-2-quinazolinylmethyl or
4-quinazolinylmethyl group,
R2 denotes a methyl group and
R3 denotes a 2-buten-1-yl or a 2-butyn-1-yl group,
a tautomer, enantiomer, diastereomer, mixture thereof or salt thereof.
In the above embodiment, R3 as 2-butyn-1-yl is exemplary.
A second preferred sub-group of the first aspect of the invention
comprises compounds of general formula I, wherein
R1 denotes a [2-(methylcarbonylamino)-phenyl]-carbonylmethyl group,
a [2-(ethylcarbonylamino)-phenyl]-carbonylmethyl group or
a [2-(isopropylcarbonylamino)-phenyl]-carbonylmethyl group,
R2 denotes a methyl group and
CA 02496249 2011-07-04
25771-1004(S)
16a
R3 denotes a 2-buten-1-yl or a 2-butyn-1-yl group,
the tautomers, enantiomers, diastereomers, the mixtures thereof and the
salts thereof.
A third preferred sub-group of the first aspect of the invention comprises
compounds of general formula 1, wherein
R' denotes a [2-(aminocarbonylmethoxy)-phenyl]-carbonylmethyl
group,
[2-(methylaminocarbonylmethoxy)-phenyl]-carbonylmethyl group,
CA 02496249 2010-12-14
25771-1004
17
a [2-(ethylaminocarbonylmethoxy)-phenyl]-carbonylmethyl group or
a [2-(isopropylaminocarbonylmethoxy)-phenyl]-carbonylmethyi group,
R2 denotes a methyl group and
R3 denotes a 2-buten-1-yl group,
a 2-butyn-1-yl group or
a 1-cyclopenten-1-ylmethyl group,
the tautomers, enantiomers, diastereomers, the mixtures thereof and the salts
thereof.
A second aspect of the invention relates to compounds of general formula 1,
wherein
R1 denotes a methyl group which is substituted by a naphthyl, fluoronaphthyl,
methyinaphthyl, methoxynaphthyl, (difluoromethoxy)-naphthyl, cyanonaphthyl,
nitronaphthyl or (dimethylamino)-naphthyl group,
a methyl group which is substituted by a phenanthrenyl group,
a methyl group which is,substituted by a 2-phenylethenyl, 2-[(trifluoromethyl)-
phenyl]-ethenyl, 2-(nitrophenyl)ethenyl, 2-(pentafluorophenyl)ethenyl or a
biphenylyl group,
a methyl group which is substituted by a phenyloxadiazolyl, phenylpyridinyl,
indolyl, methylindolyl, dimethyl-6,7-dihydro-5H-[2]pyrindinyl, benzimidazolyl,
methylbenzimidazolyl, (cyanoethyl)-benzimidazolyi, (methylamino-
carbonylmethyl)benzimidazolyl, benzylbenzimidazolyl, benzofuranyl,
acetylbenzofuranyl, cyanobenzofuranyl, benzoxazolyl, nitrobenzoxazolyl,
benzothiophenyl, methylbenzothiazolyl, quinolinyl, methoxyquinolinyl,
CA 02496249 2005-02-18
18
isoquinolinyl, methylisoquinolinyl, (difluoromethyl)-isoquinolinyl,
(trifluoromethyl)-isoquinolinyl, dimethylisoquinolinyl, (1-cyano-1-methyl-
ethyl)isoquinolinyl, phenylisoquinolinyl, methoxyisoquinolinyl, methoxy-chloro-
isoquinolinyl, methoxy-bromo-isoquinolinyl, (methoxycarbonylmethylamino)-
isoquinolinyl, dimethyl-5,6,7,8-tetrahydroisoquinolinyl, 1,2,3,4-tetrahydro-
phenanthridinyl, cinnolinyl, quinazolinyl, methylquinazolinyl,
isopropylquinazolinyl, cyclopropylquinazolinyl, phenylquinazolinyl,
aminoquinazolinyl, (dimethylamino)-quinazolinyl, pyrrolidin-1-ylquinazolinyl,
piperidin-1-ylquinazolinyl, piperazin-l-ylquinazolinyl, morpholin-4-
ylquinazolinyl, ethoxyquinazolinyl, isopropyloxyquinazolinyl, phenyl-
oxyquinazolinyl, imidazo[1,2-a]pyridinyl, methylimidazo[1,2-a]pyridinyl,
phenyl-
imidazo[1,2-a]pyridinyl, benzylimidazo[I,2-a]pyridinyl, pyrazolo[1,5-
a]pyridinyl,
quinoxalinyl, methylquinoxalinyl, dimethylquinoxalinyl, trimethylquinoxalinyl,
phenylquinoxalinyl, methylphthalazinyl, naphthyridinyl, 2,3-dihydro-benzo[1,4]-
dioxinyl, 1,2-dihydro-2-oxo-quinolinyl, 1,2-dihydro-1-methyl-2-oxo-quinolinyl,
1,2-dihydro-2-methyl-1-oxo-isoquinolinyl, 3,4-dihydro-4-oxo-phthalazinyl, 3,4-
dihydro-3-methyl-4-oxo-phthalazinyl, 3,4-dihydro-4-oxo-quinazolinyl, 3,4-
dihydro-3-methyl-4-oxo-quinazolinyl or a 2-oxo-2H-chromenyl group,
a phenylcarbonylmethyl group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by an
amino, cyanomethylamino, (ethyloxycarbonylmethyl)amino,
(methylaminocarbonyl)methylamino, methylcarbonylamino,
ethylcarbonylamino, isopropylcarbonylamino, phenylcarbonylamino,
methoxycarbonylamino, (ethyloxycarbonylamino)-carbonylamino or a 2-oxo-
imidazolidin-1-yl group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by a
phenyl group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by a
carboxy, methoxycarbonyl, ethyloxycarbonyl, aminocarbonyl,
CA 02496249 2005-02-18
19
methylaminocarbonyl, dimethylaminocarbonyl or morpholin-4-ylcarbonyl
group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by a
methylsulphanyl, methylsuiphinyl or methylsulphonyl group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by a
methoxy, difluoromethoxy, trifluoromethoxy, ethyloxy, isopropyloxy or
phenyloxy group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by a
methylsulphinylmethoxy, carboxymethoxy, ethyloxycarbonylmethoxy,
isopropyloxycarbonylmethoxy, aminocarbonylmethoxy,
methylaminocarbonylmethoxy, ethylaminocarbonylmethoxy, isopropylamino-
carbonylmethoxy, dimethylaminocarbonylmethoxy, pyrrolidin-1-
ylcarbonylmethoxy or morpholin-4-ylcarbonylmethoxy group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by a
1 -(ethyl oxyca rbo nyl)- 1 -methyl-ethyloxy, 1 -(methoxycarbonyl)-ethyloxy or
a 1-
(aminocarbonyl)-ethyloxy group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by two
methoxy groups,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by a
methoxy group and a nitro group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by a
methoxy group and an amino group,
a phenylcarbonylmethyl group wherein in the phenyl moiety two adjacent
hydrogen atoms are replaced by a -O-CH2-O, -O-CF2-O, -O-CH2-CH2-O, -NH-
CO-NH, -N(CH3)-CO-NH, -N(CH3)-CO-N(CH3), -NH-CO-O- or a -N(CH3)-
CO-O group,
CA 02496249 2010-12-14
25771-1004
a (2-phenylethyl)carbonylmethyl group,
a naphthylcarbonylmethyl, indolylcarbonylmethyl or quinolinylcarbonylmethyl
group or
a 2-cyanimino-2-phenyl-ethyl group,
R2 denotes a methyl, isopropyl, cyclopropyl, phenyl or fluorophenyl group and
R3 denotes a 2-methyl-2-propen-1-yl, 2-chloro-2-propen-1-yl or 3-bromo-2-
propen-1-yl group
a 1-buten-1-yl, 3-methyl-1-buten-1-yl, 2-buten-l-yl, 2-methyl-2-buten-1-yl-
or 2,3-dimethyl-2-buten-1-yl group,
a 2-butyn-1-yl group,
a 1-cyclopenten-l-ylmethyl group or
a 2-furanylmethyl group,
the tautomers, enantiomers, diastereomers, the mixtures thereof, the
prodrugs thereof and the salts thereof.
A preferred sub-group of the second aspect of the invention comprises
compounds of general formula I wherein
R1 and R2 are as hereinbefore defined and
R3 denotes a 1-buten-1-yl, 2-buten-1-yl or 2-butyn-l-yl group,
the tautomers, enantiomers, diastereomers, the mixtures thereof and the salts
thereof.
CA 02496249 2010-12-14
25771-1004
21
A particularly preferred sub-group of the second aspect of the invention
comprises compounds of general formula I wherein
R1 denotes a methyl group which is substituted by a naphthyl, fluoronaphthyl,
methylnaphthyl, methoxynaphthyl, (difluoromethoxy)-naphthyl, cyanonaphthyl
or nitronaphthyl group,
a methyl group which is substituted by a 2-(pentafluorophenyl)ethenyl group,
a methyl group which is substituted by a benzofuranyl, methylbenzothiazolyl,
quinolinyl, methoxyquinotinyl, isoquinolinyl, methylisoquinolinyl,
(difluoromethyl)-isoquinolinyl, (trifluoromethyl)-isoquinolinyl,
dimethylisoquinolinyl, (1-cyano-1-methyl-ethyl)isoquinolinyl, phenyl-
isoquinolinyl, methoxyisoquinolinyl, 1,2,3,4-tetrahydrophenanthridinyl,
quinazolinyl, methylquinazolinyl, isopropytquinazolinyl, cyclopropyl-
quinazolinyl, phenyiquinazolinyl, aminoquinazolinyl, (dimethylamino)-
quinazolinyl, pyrrolidin-1-ylquinazolinyl, piperidin-I-ylquinazolinyl,
piperazin-1-
ylquinazolinyl, morpholin-4-ylquinazolinyl, ethoxyquinazolinyl,
isopropyloxyquinazolinyl, quinoxalinyl, methylquinoxalinyl,
dimethylquinoxalinyl, trimethylquinoxalinyi, phenylquinoxalinyl,
[I,5]naphthyridinyl, [I,6]naphthyridinyl, [I,8]naphthyridinyl or a 1,2-dihydro-
1-
methyl-2-oxo-quinolinyl group,
a phenylcarbonylmethyl group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by a
phenyl group,
a phenylcarbonylmethyl group wherein the phenyl moiety is substituted by a
methoxy, difluoromethoxy, trifluoromethoxy, ethyloxy, isopropyloxy or
phenyloxy group,
CA 02496249 2010-12-14
25771-1004
22
a phenylcarbonylmethyl group wherein in the phenyl moiety two adjacent
hydrogen atoms are replaced by a -0-CH2-O, -O-CF2-O, -O-CH2-CH2-O, -
N(CH3)-CO-N(CH3) or a -N(CH3)-CO-O group,
a naphthylcarbonylmethyl, indolylcarbonylmethyl or quinolinylcarbonylmethyl
group or
a 2-cyanimino-2-phenyl-ethyl group,
R2 denotes a methyl, isopropyl, cyclopropyl, phenyl or 4-fluorophenyl group
and
R3 denotes a 1-buten-1-yl, 2-buten-1-yl or a 2-butyn-1-yl group,
the tautomers, enantiomers, diastereomers, the mixtures thereof and the salts
thereof.
A second preferred sub-group of the second aspect of the invention comprises
compounds of general formula I, wherein R' and R2 are defined as
immediately above and R3 denotes a 1-buten-1-yl group,
the tautomers, enantiomers, diastereomers, the mixtures thereof and the salts
thereof.
A third preferred sub-group of the second aspect of the invention comprises
compounds of general formula I wherein R' and R2 are defined as
immediately above and R3 denotes a 2-buten-1-yl group,
the tautomers, enantiomers, diastereomers, the mixtures thereof and the salts
thereof.
A fourth preferred sub-group of the second aspect of the invention comprises
compounds of general formula I wherein R' and R2 are defined as
immediately above and R3 denotes a 2-butyn-1-yl group,
the tautomers, enantiomers, diastereomers, the mixtures thereof and the salts
thereof.
CA 02496249 2010-12-14
25771-1004
23
A third aspect of the invention relates to compounds of general formula I
wherein
R1 denotes a methyl group which is substituted by a naphthyl, fluoronaphthyl,
methylnaphthyl, methoxynaphthyl, (difluoromethoxy)-naphthyl, cyanonaphthyl
or nitronaphthyl- group,
a methyl group which is substituted by a 2-(pentafluorophenyl)ethenyl group,
or
a methyl group which is substituted by a benzofuranyl, methylbenzothiazolyl,
quinolinyl, methoxyquinolinyl, isoquinolinyl, methylisoquinolinyl,
(difluoromethyl)-isoquinolinyl, (trifluoromethyl)-isoquinolinyl,
dimethylisoquinolinyl, (1-cyano-1-methyl-ethyl)isoquinolinyl, phenyl-
isoquinolinyl, methoxyisoquinolinyl, 1,2,3,4-tetrahydrophenanthridinyl,
quinazolinyl, methylquinazolinyl, isopropylquinazolinyl, cyclopropyl-
quinazolinyl, phenylquinazolinyl, aminoquinazolinyl, (dimethylamino)-
quinazolinyl, pyrrolidin-l-ylquinazolinyl, piperidin-l-ylquinazolinyl,
piperazin-l-
ylquinazolinyl, morpholin-4-ylquinazolinyl, ethoxyquinazolinyl,
isopropyloxyquinazolinyl, quinoxalinyl, methylquinoxalinyl,
dimethylquinoxalinyl, trimethylquinoxalinyl, phenylquinoxalinyl,
[1,5]naphthyridinyl, [1,6]naphthyridinyl, [1,8]naphthyridinyl or a 1,2-dihydro-
1-
methyl-2-oxo-quinolinyl group,
R2 denotes a methyl, isopropyl, cyclopropyl or phenyl group and
R3 denotes a 2-chlorobenzyl, 2-bromobenzyl, 2-ethynylbenzyl or 2-
cyanobenzyl group,
the tautomers, enantiomers, diastereomers, the mixtures thereof and the salts
thereof.
CA 02496249 2010-12-14
25771-1004
24
A first preferred sub-group of the third aspect of the invention comprises
compounds of general formula I wherein
R1 denotes a (3-methyl-isoquinolin-1-yl)methyl group,
R2 denotes a methyl group and
R3 denotes a 2-chlorobenzyl, 2-bromobenzyl, 2-ethynylbenzyl or 2-
cyanobenzyl group,
the tautomers, enantiomers, diastereomers, the mixtures thereof and the salts
thereof.
A second preferred sub-group of the third aspect of the invention comprises
compounds of general formula I wherein R' and R2 are as hereinbefore
defined and R3 denotes a 2-chlorobenzyl group, the tautomers, enantiomers,
diastereomers, the mixtures thereof and the salts thereof.
A third preferred sub-group of the third aspect of the invention comprises
compounds of general formula I wherein R' and R2 are as hereinbefore
defined and R3 denotes a 2-bromobenzyl group, the tautomers, enantiomers,
diastereomers, the mixtures thereof and the salts thereof.
A fourth preferred sub-group of the third aspect of the invention comprises
compounds of general formula I wherein R' and R2 are as hereinbefore
defined and R3 denotes a 2-ethynylbenzyl group, the tautomers, enantiomers,
diastereomers, the mixtures thereof and the salts thereof.
A fifth preferred sub-group of the third aspect of the invention comprises
compounds of general formula I wherein R' and R2 are as hereinbefore
defined and R3 denotes a 2-cyanobenzyl group, the tautomers, enantiomers,
diastereomers, the mixtures thereof and the salts thereof.
25
CA 02496249 2005-02-18
Most particularly preferred are the following compounds of general formula I:
(1) 1-[(quinazolin-2-yl)methyl]-3-methyl -7-(2-butyn-1-yl)-8-((R)-3-amino-
piperidin-1-yl)-xanthine,
(2) 1-(2-{2-[(ethylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(2-
butyn-1-yl)-8-((R)-3-amino-piperidin-1-yl)-xanthine,
(3) 1-(2-{2-[(methylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
(2-butyn-I -yl)-8-((R)-3-amino-piperidin-1 -yl)-xanthine,
(4) 1-(2-phenyl-2-oxo-ethyl)-3-methyl -7-((E)-2-buten-1-yl)-8-((R)-3-amino-
piperidin-1-yl)-xanthine,
(5) 1-[(3-methyl-isoquinolin-1-yl)methyl]-3-methyl -7-(2-butyn-1-yl)-8-((S)-3-
amino-piperidin-1-yl)-xanthine,
(6) 1-[(3-methyl-isoquinolin-1-yl)methyl]-3-methyl -7-(2-butyn-1-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine,
(7) 1-[(4-methyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-((S)-3-
amino-piperidin-1-yl)-xanthine,
(8) 1-[(4-methyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine,
(9) 1-[2-(2,3-dihydro-benzo[1,4]dioxin-5-yl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-
1-yl)-8-(3-(R)-amino-piperidin-1-yl)-xanthine,
(10) 1-[(4-methoxy-naphthalen-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-((S)-3-
amino-piperidin-1-yl)-xanthine,
(11) 1-[(4-methoxy-naphthalen-1-yl)methyl]-3-methyl -7-(2-butyn-1-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine,
26
(12) 1-[2-(benzo[1,3]dioxol-4-yl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-
((R)-
3-amino-piperidin-1-yl)-xanthine,
(13) 1-[(4-methyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-(R)-
amino-piperidin-1-yl)-xanthine,
(14) 1-(2-{2-[(iso propylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-((E)-
2-buten-1-yl)-8-((S)-3-amino-piperidin-1-yl)-xanthine,
(15) 1-(2-{2-[(methylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
((E)-2-buten-1-yl)-8-((R)-3-amino-piperidin-1-yl)-xanthine,
(16) 1-(2-{2-[(methylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
((E)-2-buten-1-yl)-8-((S)-3-amino-piperidin-1-yl)-xanthine,
(17) 1-(2-{2-[(isopropylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-(2-
butyn-1-yl)-8-((R)-3-amino-piperidin-1-yl)-xanthine,
(18) 1-(2-{2-[(isopropylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-((E)-
2-buten-1-yl)-8-((R)-3-amino-piperidin-1-yl)-xanthine,
(19) 1-[(4-cyano-naphthalen-1-yl)methyl]-3-methyl -7-(2-butyn-1-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine,
(20) 1-[(4-phenyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yi)-8-((R)-3-
amino-piperidin-1-yl)-xanthine,
(21) 1-[(8-methyl-quinoxalin-6-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine,
(22) 1-[(4-fluoro-naphthalen-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine ,
CA 02496249 2005-02-18
27
(23) 1-((E)-3-pentafluorophenyl-allyl)-3-methyl-7-(2-butyn-1-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine ,
(24) 1-[(3-trifluoromethyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-
8-
((R)-3-amino-piperidin-1-yl)-xanthine,
(25) 1-[(3-difluoromethyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-
((R)-3-amino-piperidin-1-yl)-xanthine,
(26) 1-[2-(biphenyl-2-yl)-2-oxo-ethyl]-3-methyl -7-(2-butyn-1-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine,
(27) 1-[(3-methyl-isoquinolin-1-yl)methyl]-3-cyclopropyl-7-(2-butyn-1-yl)-8-
((R)-3-amino-piperidin-1-yl)-xanthine,
(28) 1-[2-(3-methoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine,
(29) 1-[(3-methyl -isoquinolin-1-yl)methyl]-3-methyl-7-(2-chloro-benzyl)-8-
((R)-
3-amino-piperidin-1-yl)-xanthine and
(30) 1-[(3-methyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-bromo-benzyl)-8-((R)-
3-amino-piperidin-1-yl)-xanthine
as well as the tautomers, enantiomers, diastereomers, the mixtures thereof
and the salts thereof.
According to the invention the compounds of general formula I are obtained
by methods known per se, for example by the following methods:
a) reacting a compound of general formula
CA 02496249 2005-02-18
CA 02496249 2005-02-18
28
0 R3
RI N
Z1 ~II)r
O N N
I
Rs
wherein
R1 to R3 are as hereinbefore defined and
Z' denotes a leaving group such as a halogen atom, a substituted hydroxy,
mercapto, sulphinyl, sulphonyl or sulphonyloxy group such as a chlorine or
bromine atom, a methanesulphonyl or methanesulphonyloxy group,
with 3-aminopiperidine, the enantiomers thereof or the salts thereof.
The reaction is expediently carried out in a solvent such as isopropanol,
butanol, tetrahydrofuran, dioxane, dimethylformamide, dimethylsulphoxide,
ethyleneglycol monomethylether, ethyleneglycol diethylether or sulpholane,
optionally in the presence of an inorganic or tertiary organic base, e.g.
sodium
carbonate, potassium carbonate or potassium hydroxide, a tertiary organic
base, e.g. triethylamine, or in the presence of N-ethyl-diisopropylamine
(Hunig
base), while these organic bases may simultaneously also serve as solvent,
and optionally in the presence of a reaction accelerator such as an alkali
metal halide or a palladium-based catalyst at temperatures between -20 and
180 C, but preferably at temperatures between -10 and 120 C. The reaction
may, however, also be carried out without a solvent or in an excess of the 3-
aminopiperidine.
CA 02496249 2005-02-18
29
b) deprotecting a compound of general formula
O R3 N H Ox
\\
R1N N 0
'~'~ J N>- (III),
O N
I
R2
wherein R1, R2 and R3 are as hereinbefore defined.
The tert.-butyloxycarbonyl group is preferably cleaved by treatment with an
acid such as trifluoroacetic acid or hydrochloric acid or by treatment with
bromotrimethylsilane or iodotrimethylsilane, optionally using a solvent such
as
methylene chloride, ethyl acetate, dioxane, methanol, isopropanol or diethyl
ether at temperatures between 0 and 80 C.
c) In order to prepare a compound of general formula I wherein R1 according
to the definition provided hereinbefore contains a carboxy group:
deprotecting a compound of general formula
O R3 NH2
R1~N N
N (IV),
O N
I
R2
wherein R2 and R3 are as hereinbefore defined and R"contains a carboxy
group protected by a C1_4-alkyl group.
The protecting group is cleaved by hydrolysis, for example, using an acid
such as hydrochloric acid or sulphuric acid or an alkali metal hydroxide such
CA 02496249 2005-02-18
as lithium hydroxide, sodium hydroxide or potassium hydroxide in a solvent
such as methanol, ethanol, isopropanol, tetrahydrofuran or dioxane in the
presence of water.
In the reactions described hereinbefore, any reactive groups present such as
carboxy, amino, alkylamino or imino groups may be protected during the
reaction by conventional protecting groups which are cleaved again after the
reaction.
For example, a protecting group for a carboxy group may be a trimethylsilyl,
methyl, ethyl, tert.butyl, benzyl or tetrahydropyranyl group and
protecting groups for an amino, alkylamino or imino group may be a formyl,
acetyl, trifluoroacetyl, ethoxycarbonyl, tert.butoxycarbonyl,
benzyloxycarbonyl,
benzyl, methoxybenzyl or 2,4-dimethoxybenzyl group and additionally, for the
amino group, a phthalyl group.
Any protecting group used is optionally subsequently cleaved for example by
hydrolysis in an aqueous solvent, e.g. in water, isopropanol/water, acetic
acid/water, tetrahydrofuran/water or dioxane/water, in the presence of an acid
such as trifluoroacetic acid, hydrochloric acid or sulphuric acid or in the
presence of an alkali metal base such as sodium hydroxide or potassium
hydroxide or aprotically, e.g. in the presence of iodotrimethylsilane, at
temperatures between 0 and 120 C, preferably at temperatures between 10
and 100 C.
However, a benzyl, methoxybenzyl or benzyloxycarbonyl group is cleaved, for
example, hydrogenolytically, e.g. with hydrogen in the presence of a catalyst
such as palladium/charcoal in a suitable solvent such as methanol, ethanol,
ethyl acetate or glacial acetic acid, optionally with the addition of an acid
such
as hydrochloric acid at temperatures between 0 and 100 C, but preferably at
temperatures between 20 and 60 C, and at a hydrogen pressure of 1 to 7 bar,
but preferably 3 to 5 bar. A 2,4-dimethoxybenzyl group, however, is preferably
cleaved in trifluoroacetic acid in the presence of anisol.
CA 02496249 2005-02-18
31
A tert.butyl or tert.butyloxycarbonyl group is preferably cleaved by treating
with
an acid such as trifluoroacetic acid or hydrochloric acid or by treating with
iodotrimethylsilane, optionally using a solvent such as methylene chloride,
dioxan, methanol or diethylether.
A trifluoroacetyl group is preferably cleaved by treating with an acid such as
hydrochloric acid, optionally in the presence of a solvent such as acetic acid
at
temperatures between 50 and 120 C, or by treating with sodium hydroxide
solution, optionally in the presence of a solvent such as tetrahydrofuran at
temperatures between 0 and 50 C.
A phthalyl group is preferably cleaved in the presence of hydrazine or a
primary amine such as methylamine, ethylamine or n-butylamine in a solvent
such as methanol, ethanol, isopropanol, toluene/water or dioxan at
temperatures between 20 and 50 C.
Moreover, the compounds of general formula I obtained may be resolved into
their enantiomers and/or diastereomers, as mentioned hereinbefore. Thus, for
example, cis/trans mixtures may be resolved into their cis and trans isomers,
and compounds with at least one optically active carbon atom may be
separated into their enantiomers.
Thus, for example, the cis/trans mixtures may be resolved by chromatography
into the cis and trans isomers thereof, the compounds of general formula I
obtained which occur as racemates may be separated by methods known per
se (cf. Allinger N. L. and Eliel E. L. in "Topics in Stereochemistry", Vol. 6,
Wiley
Interscience, 1971) into their optical antipodes and compounds of general
formula I with at least 2 asymmetric carbon atoms may be resolved into their
diastereomers on the basis of their physical-chemical differences using
methods known per se, e.g. by chromatography and/or fractional
crystallisation, and, if these compounds are obtained in racemic form, they
may subsequently be resolved into the enantiomers as mentioned above.
CA 02496249 2005-02-18
32
The enantiomers are preferably separated by column separation on chiral
phases or by recrystallisation from an optically active solvent or by reacting
with an optically active substance which forms salts or derivatives such as
e.g.
esters or amides with the racemic compound, particularly acids and the
activated derivatives or alcohols thereof, and separating the diastereomeric
mixture of salts or derivatives thus obtained, e.g. on the basis of their
differences in solubility, whilst the free antipodes may be released from the
pure diastereomeric salts or derivatives by the action of suitable agents.
Optically active acids in common use are e.g. the D- and L-forms of tartaric
acid or dibenzoyltartaric acid, di-o-tolyltartaric acid, malic acid, mandelic
acid,
camphorsulphonic acid, glutamic acid, aspartic acid or quinic acid. An
optically
active alcohol may be for example (+) or (-)-menthol and an optically active
acyl group in amides, for example, may be a (+)-or (-)-menthyloxycarbonyl.
Furthermore, the compounds of formula I may be converted into the salts
thereof, particularly for pharmaceutical use into the physiologically
acceptable
salts with inorganic or organic acids. Acids which may be used for this
purpose
include for example hydrochloric acid, hydrobromic acid, sulphuric acid,
phosphoric acid, fumaric acid, succinic acid, lactic acid, citric acid,
tartaric acid
or maleic acid.
Moreover, if the new compounds of formula I thus obtained contain a carboxy
group, they may subsequently, if desired, be converted into the salts thereof
with inorganic or organic bases, particularly for pharmaceutical use into the
physiologically acceptable salts thereof. Suitable bases for this purpose
include for example sodium hydroxide, potassium hydroxide, arginine,
cyclohexylamine, ethanolamine, diethanolamine and triethanolamine.
The compounds of general formulae II to IV used as starting materials are
either known from the literature or may be obtained by methods known from
the literature (cf. Examples I to LXXI).
As already mentioned hereinbefore, the compounds of general formula I
according to the invention and the physiologically acceptable salts thereof
CA 02496249 2010-12-14
25771-1004
33
have valuable pharmacological properties, particularly an inhibiting effect on
the enzyme DPP-IV.
The biological properties of the new compounds were investigated as follows:
The ability of the substances and their corresponding salts to inhibit the DPP-
IV activity can be demonstrated in a test set-up in which an extract of human
colon carcinoma cell line Caco-2 is used as the DPP-IV source. The
differentiation of the cells in order to induce the DPP-IV expression was
carried out as described by Reiher et al. in an article entitled "Increased
expression of intestinal cell line Caco-2", which appeared in Proc: Natl.
Acad.
Sci. Vol. 90, pages 5757-5761 (1993). The cell extract was obtained from
cells solubilised in a buffer (10mM Tris HCI, 0.15 M NaCl, 0.04 t.i.u.
aprotinin,
TN
0.5% Nonidet-P40, pH 8.0) by centrifuging at 35,000 g of for 30 minutes at
4 C (to remove cell debris).
The DPP-IV assay was carried out as follows:
50 pl substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final
concentration 100 NM, were placed in black microtitre plates. 20 NI of assay
buffer (final concentrations 50 mM Tris HCI pH 7.8, 50 mM NaCl, 1 % DMSO)
was pipetted in. The reaction was started by adding 30 NI of solubilised Caco-
2 protein (final concentration 0.14 pg of protein per well). The test
substances
.to be investigated were typically added prediluted in 20 ill, and the volume
of
assay buffer was then reduced accordingly. The reaction was carried out at
ambient temperature, incubating for 60 minutes. Then the fluorescence was
measured in a Victor 1420 Multilabel Counter, the excitation wavelength being
405 nm and the emission wavelength being 535 nm. Blank readings
(corresponding to 0 % activity) were obtained in mixtures without any Caco-2
protein (volume replaced by assay buffer), control values (corresponding to
100 % activity) were obtained in mixtures with no substance added. The
potency of the test substances in question, expressed as IC50 values, was
calculated from dosage/activity curves consisting of 11 measuring points in
each case. The following results were obtained:
CA 02496249 2005-02-18
34
Compound DPP-IV inhibition
(Example no.) IC5o [nMJ
2(3) 2160
2(9) 264
2(12) 16
2(17) 32
2(20) 12
2(25) 4
2(27) 9
2(35) 5
2(37) 5
2(43) 6
2(51) 6
2(52) 9
2(59) 250
2(66) 22
2(80) 1
2(86) 2
2(96) 2
2(99) 1
2(100) 3
2(108) 3
2(129) 3
2(130) 3
2(131) 3
2(132) 1
2(135) 3
2(137) 13
2(138) 8
2(139) 4
2(142) 1
2(145) 4
2(148) 1
CA 02496249 2005-02-18
2(150) 1
2(151) 3
2(152) 4
2(185) 3
2(217) 4
2(247) 2
2(251) 12
2(256) 8
2(260) 13
2(264) 6
2(277) 6
2(280) 5
2(285) 3
2(287) 11
2(288) 14
The compounds prepared according to the invention are well tolerated, as for
example when 10 mg/kg of the compound of Example 2(80) were
administered to rats by oral route no changes in the animals' behaviour could
be detected.
In view of their ability to inhibit DPP-IV activity, the compounds of general
formula I according to the invention and the corresponding pharmaceutically
acceptable salts thereof are suitable for treating all those conditions or
illnesses which can be influenced by the inhibition of the DPP-IV activity. It
is
therefore to be expected that the compounds according to the invention will
be suitable for the prevention or treatment of diseases or conditions such as
type 1 and type 2 diabetes mellitus, diabetic complications (such as e.g.
retinopathy, nephropathy or neuropathies), metabolic acidosis or ketosis,
reactive hypoglycaemia, insulin resistance, metabolic syndrome,
dyslipidaemias of various origins, arthritis, atherosclerosis and related
diseases, obesity, allograft transplantation and calcitonin-induced
osteoporosis. In addition these substances are capable of preventing B-cell
degeneration such as e.g. apoptosis or necrosis of pancreatic B-cells. The
CA 02496249 2005-02-18
36
substances are also suitable for improving or restoring the function of
pancreatic cells and also increasing the number and size of pancreatic B-
cells. Additionally, and on the basis of the role of the Glucagon-Like
Peptides,
such as e.g. GLP-1 and GLP-2 and their link with DPP-IV inhibition, it is
likely
that the compounds according to the invention are suitable for achieving,
inter
alia, a sedative or anxiety-relieving effect and also for favourably affecting
catabolic states after operations or hormonal stress responses or reducing
mortality or morbidity after myocardial infarct. They are also suitable for
treating all conditions which are connected with the above mentioned effects
and which are mediated by GLP-1 or GLP-2. The compounds according to the
invention may also be used as diuretics or antihypertensives and are suitable
for preventing and treating acute renal failure. Furthermore, the compounds
according to the invention may be used to treat inflammatory diseases of the
respiratory tract. They are also suitable for the prevention and treatment of
chronic inflammatory intestinal diseases such as e.g. irritable bowel syndrome
(IBS), Crohn's disease or ulcerative colitis and also pancreatitis. It is also
likely that they can be used for all kinds of damage to or impairment of the
gastrointestinal tract such as colitis and enteritis, for example. It is also
expected that DPP-IV inhibitors and hence also the compounds according to
the invention may be used to treat infertility or to improve fertility in
humans or
mammals, particularly when the infertility is connected with insulin
resistance
or polycystic ovary syndrome. On the other hand these substances are
suitable for affecting sperm motility and can thus be used as male
contraceptives. The substances are also suitable for treating deficiencies of
growth hormone which are associated with reduced stature, and may also be
used to advantage in any indications in which growth hormone may be used.
The compounds according to the invention are also suitable, on the basis of
their inhibitory effect on DPP-IV, for treating various autoimmune diseases
such as e.g. rheumatoid arthritis, multiple sclerosis, thyroiditis and
Basedow's
disease, etc. They may also be used to treat viral diseases and also, for
example, in HIV infections, for stimulating blood production, in benign
prostatic hyperplasia, gingivitis, as well as for the treatment of neuronal
defects and neurodegenerative diseases such as Alzheimer's disease, for
example. The compounds described may also be used for the treatment of
CA 02496249 2005-02-18
37
tumours, particularly for modifying tumour invasion and also metastasisation;
examples here are their use in treating T-cell lymphomas, acute lymphoblastic
leukaemia, cell-based pancreatic carcinomas, basal cell carcinomas or breast
cancers. Other indications are stroke, ischaemia of various origins, Parkin-
son's disease and migraine. In addition, further indications include
follicular
and epidermal hyperkeratoses, increased keratinocyte proliferation, psoriasis,
encephalomyelitis, glomerulonephritis, lipodystrophies, as well as psycho-
somatic, depressive and neuropsychiatric diseases of all kinds.
The compounds according to the invention may also be used in conjunction
with other active substances. Therapeutic agents which are suitable for such
combinations include, for example, antidiabetics, such as metformin,
sulphonylureas (e.g. glibenclamide, tolbutamide, glimepiride), nateglinide,
repaglinide, thiazolidinedione (e.g. rosiglitazone, pioglitazone), PPAR-gamma
agonists (e.g. GI 262570) and antagonists, PPAR-gamma/alpha modulators
(e.g. KRP 297), alpha-glucosidase inhibitors (e.g. acarbose, voglibose), other
DPPIV inhibitors, alpha2 antagonists, insulin and insulin analogues, GLP-1
and GLP-1 analogues (e.g. exendin-4) or amylin. Also, SGLT2 inhibitors such
as T-1095, inhibitors of protein tyrosine phosphatase 1, substances which
influence deregulated glucose production in the liver, such as e.g. inhibitors
of
glucose-6-phosphatase, or fructose-l,6-bisphosphatase, glycogen
phosphorylase, glucagon receptor antagonists and inhibitors of phosphoenol -
pyruvate carboxykinase, glycogen synthase kinase or pyruvate
dehydrokinase, lipid lowering agents, such as HMG-CoA-reductase inhibitors
(e.g. simvastatin, atorvastatin), fibrates (e.g. bezafibrate, fenofibrate),
nicotinic
acid and its derivatives, PPAR-alpha agonists, PPAR-delta agonists, ACAT
inhibitors (e.g. avasimibe) or cholesterol resorption inhibitors such as for
example ezetimibe, bile acid-binding substances such as for example
cholestyramine, inhibitors of ileac bile acid transport, HDL-raising compounds
such as for example inhibitors of CETP or regulators of ABC1 or active
substances for the treatment of obesity, such as e.g. sibutramine or
tetrahydrolipostatin, dexfenfluramine, axokine, antagonists of the
cannabinoidl receptor, MCH-1 receptor antagonists, MC4 receptor agonists,
CA 02496249 2005-02-18
38
NPY5 or NPY2 antagonists or R3-agonists such as SB-418790 or AD-9677 as
well as agonists of the 5HT2c receptor.
It is also possible to combine the compounds with drugs for treating high
blood pressure such as e.g. All antagonists or ACE inhibitors, diuretics,
13-blockers, Ca-antagonists, etc., or combinations thereof.
The dosage required to achieve such an effect is expediently, by intravenous
route, 1 to 100 mg, preferably 1 to 30 mg, and by oral route 1 to 1000 mg,
preferably 1 to 100 mg, in each case 1 to 4 times a day. For this purpose, the
compounds of formula I prepared according to the invention, optionally
combined with other active substances, may be incorporated together with
one or more inert conventional carriers and/or diluents, e.g. with corn
starch,
lactose, glucose, microcrystalline cellulose, magnesium stearate,
polyvinylpyrrolidone, citric acid, tartaric acid, water, water/ethanol,
water/glycerol, water/sorbitol, water/polyethylene glycol, propylene glycol,
cetylstearyl alcohol, carboxymethyl ce I lu lose or fatty substances such as
hard
fat or suitable mixtures thereof into conventional galenic preparations such
as
plain or coated tablets, capsules, powders, suspensions or suppositories.
The Examples that follow are intended to illustrate the invention:
CA 02496249 2005-02-18
39
Preparation of the starting compounds:
Example I
1 , 3-d i methyl-7-(2,6-dicyano-benzyl)-8-bromo-xanthine
A mixture of 555 mg of 8-bromotheophyllin and 0.39 ml of Hunig base in 9 ml
N,N-dimethylformamide is combined with 600 mg of 2-bromomethyl-
isophthalonitrile and stirred overnight at ambient temperature. For working up
the reaction mixture is poured onto water. The precipitate formed is suction
filtered, washed with water and dried.
Yield: 686 mg (83 % of theory)
Rf value: 0.56 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 399, 401 [M+H]+
The following compounds are obtained analogously to Example I:
(1) 3-methyl-7-(3-methyl-2-buten-1-yl)-8-chloro-(anthine
Mass spectrum (ESI+): m/z = 269, 271 [M+H]+
(2) 3-methyl-7-(2-cyano-benzyl)-8-chloro-xanthine
Mass spectrum (ESI+): m/z = 316, 318 [M+H]+
(3) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-(2-butyn-1-yl)-8-bromo-xanthine
Mass spectrum (ESI+): m/z = 415, 417 [M+H]+
(4) 3-methyl-7-[(2-trimethylsilanyl-ethoxy)methyl]-8-bromo-xanthine
(Carried out in the presence of potassium carbonate)
Mass spectrum (ESI+): m/z = 375, 377 [M+H]+
(5) 3-methyl-7-(3-methyl-2-buten-1-yl)-8-bromo-xanthine
Mass spectrum (ESI+): m/z = 313, 315 [M+H]+
(6) 3-methyl-7-(2,3-d imethyl-2-buten-1-yl)-8-bromo-xanthine
Rf value: 0.43 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 327, 329 [M+H]+
CA 02496249 2005-02-18
(7) 3-methyl-7-(2-butyn- 1 -yl)-8- bro m o-xa nth ine
Rf value: 0.72 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 297/299 [M+H]+
(8) 3-methyl-7-((E)-2-buten-1-yl)-8-bromo-xanthine
(The product is contaminated with approx. 10-20 % of Z compound)
Rf value: 0.55 (silica gel, cyclohexane/ethyl acetate/methanol = 6:3:1)
Mass spectrum (ESI+): m/z = 299, 301 [M+H]+
(9) 3-methyl-7-[(1-cyclopenten-1-yl)methyl]-8-bromo-xanthine
Mass spectrum (ESI+): m/z = 325, 327 [M+H]+
(10) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-[(1-cyclopenten-l-yl)methyl]-8-
bromo-xanthine
Mass spectrum (ESI+): m/z = 443, 445 [M+H]+
(11) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-((E)-2-buten-1-yl)-8-bromo-xanthine
(product contains approx. 25 % of Z isomer)
Mass spectrum (ESI+): m/z = 417, 419 [M+H]+
(12) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-(2-methyl-al lyl)-8-bromo-xanthine
Rf value: 0.71 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 417, 419 [M+H]+
(13) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-(3-bromo-allyl)-8-bromo-xanthine
Rf value: 0.68 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 481, 483, 485 [M+H]+
(14) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-[(furan-2-yl)methyl]-8-bromo-
xanthine
Rf value: 0.60 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 443, 445 [M+H]+
CA 02496249 2005-02-18
41
(15) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-(2-chloro-allyl)-8-bromo-xanthine
Rf value: 0.77 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 437, 439, 441 [M+H]+
(16) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-((Z)-2-methyl-2-buten-1-yl)-8-bromo-
xanthine
Rf value: 0.77 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 431, 433 [M+H]+
(17) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-((E)-2-methyl-2-buten-1-yl)-8-bromo-
xanthine
Rf value: 0.77 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 431, 433 [M+H]+
(18) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-(1-phenylsulphanyl-butyl)-8-bromo-
xanthine
Rf value: 0.83 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 527, 529 [M+H]+
(19) 3-methyl-7-(3-methyl-1-phenylsulphanyl-butyl)-8-bromo-xanthine
(The [(1-chloro-3-methyl-butyl)sulphanyl]-benzene used as starting material
for the reaction is obtained by chlorination of [(3-methyl-butyl)sulphanyl]-
benzene with N-chloro-succinimide in carbon tetrachloride)
Rf value: 0.38 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 423, 425 [M+H]+
(20) 1,3-dimethyl-7-(2-bromo-benzyl)-8-chloro-xanthine
Rf value: 0.50 (silica gel, cyclohexane/ethyl acetate = 1:1)
(21) 1,3-dimethyl-7-(2-chloro-benzyl)-8-chloro-xanthine
Rf value: 0.50 (silica gel, cyclohexane/ethyl acetate = 1:1)
(22) 3-cyclopropyl-7-(2-butyn-1-yl)-8-bromo-xanthine
CA 02496249 2005-02-18
42
Rf value: 0.45 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 223/225 [M+H]+
Example II
1-(2-{2-[(ethoxycarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-methyl-
2-buten-1-vl)-8-[3-(tert.-butvloxvcarbonvlami no)-piperidin-1-vll-xanth ine
63 mg of ethyl bromoacetate are added to a mixture of 200 mg of 1-[2-(2-
hyd roxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-bute n-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine and 63 mg of potassium
carbonate in 3 ml N,N-dimethylformamide. The reaction mixture is stirred for
five hours at ambient temperature. For working up it is combined with water
and the precipitate formed is suction filtered, washed with water and dried
for
three hours at 80 C in the drying cupboard.
Yield: 216 mg (94 % of theory)
Mass spectrum (ESI+): m/z = 653 [M+H]+
The following compounds are obtained analogously to Example II:
(1) 1-(2-{2-[(aminocarbo nyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yi]-
xanthine
Mass spectrum (ESI+): m/z = 624 [M+H]+
(2) 1-(2-{3-[(methylsulphanyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-
xanthine
Rf value: 0.20 (silica gel, cyclohexane/ethyl acetate = 6:4)
Mass spectrum (ESI+): m/z = 627 [M+H]+
(3) 1-(2-{3-[(ethoxycarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-
methyl-2-buten-l-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-l -yl]-
xanthine
Rf value: 0.50 (silica gel, cyclohexane/ethyl acetate = 3:7)
............. .
CA 02496249 2005-02-18
43
(4) 1-(2-{2-[(methylaminocarbonyl)meth oxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
(3-methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-l -yl]-
xanthine
Mass spectrum (ESI+): m/z = 638 [M+H]+
(5) 1-(2-{2-[(dimethylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-
7-(3-methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylami no)-piperidin-1-yl]-
xanthine
Mass spectrum (ESI+): m/z = 652 [M+H]+
(6) 1-(2-{3-[(methoxycarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin- l -yl]-
xanthine
Mass spectrum (ESI+): m/z = 639 [M+H]+
(7) 1-(2-{2-[(ethylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(2-
butyn-1-yl)-8-[(R)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 636 [M+H]+
(8) 1-(2-{2-[(methylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
[(1-cyclopenten-1-yl)methyl]-8-[3-(tert.-butyloxycarbonylamino)-piperidin-l-
yl]-
xanthine
Mass spectrum (ESI+): m/z = 650 [M+H]+
(9) 1-(2-{2-[(methyl am inocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
(2-butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 622 [M+H]+
(10) 1-(2-{2-[(aminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(2-
butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Mass spectrum (ESI+): m/z = 608 [M+H]+
CA 02496249 2005-02-18
44
(11) 1-(2-{2-[(methoxycarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(2-
butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 623 [M+H]+
(12) 1-(2-{2-[(isopropyloxycarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
(3-methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-
xanthine
Mass spectrum (ESI+): m/z = 667 [M+H]+
(13) 1-(2-{2-[(methylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
(2-butyn-1-yl)-8-[(R)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 622 [M+H]+
(14) 1-(2-{2-[(methylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
((E)-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(product contains some Z isomer)
Rf value: 0.35 (silica gel, cyclohexane/ethyl acetate/methanol = 5:4:1)
Mass spectrum (ESI+): m/z = 624 [M+H]+
(15) 1-(2-{2-[(ethylaminocarbonyl)meth oxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
(2-butyn-1-yl)-8-[(S)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 636 [M+H]+
(16) 1-(2-{2-[(methylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
(2-butyn-1-yl)-8-[(S)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 622 [M+H]+
(17) 1-(2-{2-[(meth oxycarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-
xanthine
Mass spectrum (ESI+): m/z = 639 [M+H]+
CA 02496249 2005-02-18
(18) 1-(2-{2-[(methylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
(3-methyl-2-buten-1-yl)-8-[(S)-3-(tert.-butyloxycarbonylami no)-piperidin-1-
yl]-
xanthine
Mass spectrum (ESI+): m/z = 638 [M+H]+
(19) 2-(2-acetyl-phenoxy)-N-ethyl-acetamide
Mass spectrum (ESI+): m/z = 222 [M+H]+
(20) 1-{2-[2-(1-methoxycarbonyl-ethoxy)-phenyl]-2-oxo-ethyl}-3-methyl-7-(2-
butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 637 [M+H]+
(21) 1-{2-[2-(1-aminocarbonyl-ethoxy)-phenyl]-2-oxo-ethyl}-3-methyl-7-(2-
butyn-1-yl)-8-[3-(tert.-butyloxycarbonylami no)-piperidin-l-yl]-xanthine
Mass spectrum (ESI+): m/z = 622 [M+H]+
(22) 2-(2-acetyl-phenoxy)-N-methyl-acetamide
Mass spectrum (ESI+): m/z = 208 [M+H]+
(23) 1-{2-[2-(2-oxo-propoxy)-phenyl]-2-oxo-ethyl}-3-methyl-7-(2-butyn-1-yl)-8-
[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 607 [M+H]+
(24) 1-{2-[2-(1-ethoxycarbonyl-1-methyl-ethoxy)-phenyl]-2-oxo-ethyl}-3-
methyl-7-(2-butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-
xanthine
Mass spectrum (ESI+): m/z = 665 [M+H]+
(25) 1-{2-[2-cyanomethoxy-phenyl]-2-oxo-ethyl}-3-methyl-7-(2-butyn-l-yl)-8-
[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 590 [M+H]+
(26) 1-(2-{2-[(methylsulphanyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(2-
butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
CA 02496249 2005-02-18
46
Mass spectrum (ESI+): m/z = 611 [M+H]+
(27) 1-{[2-(tert.-butylcarbonyl)-benzofuran-3-yl]methyl}-3-methyl-7-(2-butyn-1-
yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(Formed as main product when 1-[2-(2-hydroxy-phenyl)-2-oxo-ethyl]-3-methyl-
7-(2-butyn-1-yl)-8-[3-(tent.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
is
reacted with 1-chloro-3,3-dimethyl-butan-2-one)
Mass spectrum (ESI+): m/z = 631 [M+H]+
Example III
1-[2-(2-hydroxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-[3:
(tert.-butyloxycarbonvlam ino)-piperidin-1-yll-xanthine
1.30 g of 3-tert.-butyloxycarbonylamino-piperidine are added to a mixture of
2.51 g of 1-[2-(2-hydroxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-
1-yl)-8-chloro-xanthine and 880 mg of sodium carbonate in 8 ml of
dimethylsulphoxide. The reaction mixture is stirred for 18 hours at 60 C. For
working up it is combined with water and the precipitate formed is suction
filtered. The solid crude product is dissolved in ethyl acetate, the solution
is
dried over magnesium sulphate and evaporated down. The flask residue is
chromatographed through a silica gel column with cyclohexane/ethyl acetate
(10:1 to 1:1) as eluant.
Yield: 2.56 g (91 % of theory)
Mass spectrum (ESI+): m/z = 567 [M+H]+
The following compounds are obtained analogously to Example III:
(1) 3-methyl-7-(3-methyl-2-buten-l-yl)-8-[3-(tert.-butyloxycarbonylamino)-
piperidin-l-yl]-xanthine
Mass spectrum (ESI+): m/z = 433 [M+H]+
(2) 1-(1-methyl-2-oxo-2-phenyl-ethyl)-3-methyl-7-(3-methyl-2-buten-l-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Mass spectrum (ESI+): m/z = 565 [M+H]+
CA 02496249 2005-02-18
47
(3) 3-methyl-7-(2-cyano-benzyl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-
1-yl]-xanthine
Rf value: 0.90 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI"): m/z = 478 [M-H]"
(4) 1-methyl-3-[(methoxycarbonyl)methyl]-7-(2-cyano-benzyl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Mass spectrum (ESI+): m/z = 552 [M+H]+
(5) 1-methyl-3-cyanomethyl-7-(2-cyano-benzyl)-8-[3-(tert.-butyloxycarbonyl-
amino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 519 [M+H]+
(6) 1-methyl-3-(2-propyn-1-yl)-7-(2-cyano-benzyl)-8-[3-(tert.-butyloxycarbonyl-
amino)-piperidin-l-yl]-xanthine
Mass spectrum (ESI+): m/z = 518 [M+H]+
(7) 1-[2-(3-nitro-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.25 (silica gel, cyclohexane/ethyl acetate/methanol = 7:2:1)
Mass spectrum (ESI+): m/z = 596 [M+H]+
(8) 1-methyl-3-(2-propen-1-yl)-7-(2-cyano-benzyl)-8-[3-(tert.-butyloxycarbonyl-
amino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 520 [M+H]+
(9) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Mass spectrum (ESI+): m/z = 535 [M+H]+
(10) 1-[2-(2-nitro-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-
[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.52 (silica gel, cyclohexane/ethyl acetate = 3:7)
Mass spectrum (ESI+): m/z = 596 [M+H]+
CA 02496249 2005-02-18
48
(11) 1-methyl-3-phenyl-7-(2-cyano-benzyl)-8-[3-(tert.-butyloxycarbonylamino)-
p i peri d i n-1-yl]-xanth i n e
Mass spectrum (ESI+): m/z = 556 [M+H]+
(12) 1-[2-(2-nitro-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-l-yl)-8-
[(S)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 596 [M+H]+
(13) 1-[(cinnolin-4-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine mixed with 1 -[(1,4-dihydro-
cinnolin-4-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1 -yl]-xanthine
Rf value: 0.62 (silica gel, ethyl acetate)
(14) 1-({4-oxo-3-[(2-trimethylsilanyl-ethoxy)methyl]-3,4-dihydro-phthalazin-1-
yl}methyl)-3-methyl-7-(3-methyl-2-buten-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(Carried out with potassium carbonate in the presence of Hunig base)
Rf value: 0.27 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 720 [M+H]+
(15) 1-[(isoquinolin-3-yl)methyl]-3-methyl-7-(3-methyl-2-buten-l-yl)-8-[3-
(tert.-
butyloxycarbonylami no)-piperidin-l-yl]-xanthine
Rf value: 0.31 (silica gel, ethyl acetate/petroleum ether = 7:3)
Mass spectrum (ESI+): m/z = 574 [M+H]+
(16) 1-[(3-methyl-4-oxo-3,4-dihydro-phthalazin-1-yl)methyl]-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-
xanthine
Rf value: 0.45 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5)
Mass spectrum (ESI+): m/z = 605 [M+H]+
CA 02496249 2005-02-18
49
(17) 3-methyl-7-(2,3-dimethyl-2-buten-l-yl)-8-[3-(tert.-butyloxycarbonylamino)-
piperid in-1-yl]-xanthine
(Carried out with potassium carbonate)
Rf value: 0.42 (silica gel, methylene chloride/methanol = 20:1)
Mass spectrum (ESI+): m/z = 447 [M+H]+
(18) 3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-
yl]-xanthine
(Carried out with potassium carbonate)
Melting point: 235-237 C
Mass spectrum (ESI+): m/z = 417 [M+H]+
(19) 1-[(quinolin-4-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxycarbonylami no)-piperidin-l-yl]-xanthine
(Carried out with potassium carbonate)
Rf value: 0.36 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 558 [M+H]+
(20) 1-[(isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(Carried out with potassium carbonate)
Rf value: 0.71 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 558 [M+H]+
(21) 1-[(isoquinolin-l-yl)methyl]-3-methyl-7-((E)-2-buten-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(Carried out with potassium carbonate; the product contains approx. 20 % of
Z isomer)
Rf value: 0.24 (silica gel, ethyl acetate/petroleum ether = 1:1)
Mass spectrum (ESI+): m/z = 560 [M+H]+
(22) 3-methyl-7-(2-butyn-1-yl)-8-[(R)-3-(tert.-butyloxycarbonylamino)-
piperidin-
1-yl]-xanthine
(Carried out with potassium carbonate)
CA 02496249 2005-02-18
Rf value: 0.64 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 417 [M+H]+
(23) 3-methyl-7-(2-butyn-1-yl)-8-[(S)-3-(tert.-butyloxycarbonylamino)-
piperidin-
1-yl]-xanthine
(Carried out with potassium carbonate)
Rf value: 0.64 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 417 [M+H]+
(24) 3-methyl-7-((E)-2-buten-1-yl)-8-[(S)-3-(tert.-butyloxycarbonylamino)-
piperidin-l-yl]-xanthine
(product contains approx. 15 % of Z isomer)
Rf value: 0.35 (silica gel, cyclohexane/ ethyl acetate = 3:7)
Mass spectrum (ESI+): m/z = 419 [M+H]+
(25) 3-methyl-7-((E)-2-buten-1-yl)-8-[(R)-3-(tert.-butyloxycarbonylamino)-
piperidin-1-yl]-xanthine
(product contains approx. 15 % of Z isomer)
Rf value: 0.35 (silica gel, cyclohexane/ ethyl acetate = 3:7)
Mass spectrum (ESI+): m/z = 419 [M+H]+
(26) 1-[2-(2-hydroxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-l-yl)-8-[(R)-3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 551 [M+H]+
(27) 1-[2-(2-amino-phenyl)-2-oxo-ethyl]-3-methyl-7-[(1-cyclopenten-1-
yl)methyl]-8-[3-(tert.-butyloxycarbonylamino)-piperidin-l-yi]-xanthine
Mass spectrum (ESI+): m/z = 578 [M+H]+
(28) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-[(1-cyclopenten-l-yl)methyl]-8-[3-
(tent.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Mass spectrum (ESI+): m/z = 563 [M+H]+
51
CA 02496249 2005-02-18
(29) 1-[2-(2-hydroxy-phenyl)-2-oxo-ethyl]-3-methyl -7-[(1-cyclopenten-1-
yl)methyl]-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 579 [M+H]+
(30) 1-methyl-3-isopropyl-7-(2-cyano-benzyl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Mass spectrum (ESI+): m/z = 522 [M+H]+
(31) 1-[2-(2-hydroxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 551 [M+H]+
(32) 1-[2-(2-amino-phenyl)-2-oxo-ethyl]-3-methyl -7-((E)-2-buten-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(product contains approx. 10 % of Z isomer)
Rf value: 0.20 (silica gel, cyclohexane/ethyl acetate =1:1)
Mass spectrum (ESI+): m/z = 552 [M+H]+
(33) 1-(2-phenyl-2-oxo-ethyl)-3-methyl -7-((E)-2-buten- 1-yl)-8-[3-(tert.-
butyl oxy-
carbonylamino)-piperidin-l-yl]-xanthine
(product contains approx. 25 % of Z isomer)
Mass spectrum (ESI+): m/z = 537 [M+H]+
(34) 1-[2-(2-hydroxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-l-yl)-8-[(R)-3-
(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
(35) 1-[2-(2-hydroxy-phenyl)-2-oxo-ethyl]-3-methyl-7-((E)-2-buten-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperid in-l-yl]-xanthine
(product contains some Z isomer)
Rf value: 0.30 (silica gel, cyclohexane/ethyl acetate = 4:6)
Mass spectrum (ESI+): m/z = 553 [M+H]+
(36) 1-[2-(2-hydroxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-[(S)-3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
CA 02496249 2005-02-18
52
Mass spectrum (ESI+): m/z = 551 [M+H]+
(37) 1-[2-(2-amino-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-l-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.50 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 550 [M+H]+
(38) 1-[2-(2-hydroxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-
8-[(S)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 567 [M+H]+
(39) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-(2-butyn-l-yl)-8-[(R)-3-(tert.-
butyloxy-
carbonylamino)-piperidin-l-yl]-xanthine
Mass spectrum (ESI+): m/z = 535 [M+H]+
(40) 1-methyl-3-[(2-trimethylsilanyl-ethoxy)methyl]-7-(2-cyano-benzyl)-8-[3-
(tert: butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 610 [M+H]+
(41) 3-methyl-7-(2-butyn-1-yl)-8-[(S)- 3-(tert.-butyloxycarbonylamino)-
piperidin-1-yl]-xanth ine
(Carried out with potassium carbonate)
Rf value: 0.52 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 417 [M+H]+
(42) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-(2-methyl-al lyl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.46 (silica gel, methylene chloride/methanol = 95:5)
(43) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-(3-bromo-allyl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.22 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 601, 603 [M+H]+
CA 02496249 2005-02-18
53
(44) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-[(furan-2-yl)methyl]-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.41 (silica gel, methylene chloride/methanol = 95:5)
(45) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-(2-chloro-allyl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.49 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 557, 559 [M+H]+
(46) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-(2-butyn-1-yl)-8-[(S)-3-(tert.-
butyloxy-
carbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 535 [M+H]+
(47) 1-[2-(2-amino-phenyl)-2-oxo-ethyl]-3-methyl-7-((E)-2-buten-1-yl)-8-[(S)-3-
(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Rf value: 0.40 (silica gel, cyclohexane/ethyl acetate = 4:6)
Mass spectrum (ESI+): m/z = 552 [M+H]+
(48) 1-[2-(2-nitro-phenyl)-2-oxo-ethyl]-3-methyl-7-((E)-2-buten-1-yl)-8-[(R)-3-
(tert.-butyloxycarbonylam ino)-pi peridin-1-yl]-xanthine
Rf value: 0.50 (silica gel, cyclohexane/ethyl acetate = 1:2)
(49) 1-[2-(2-nitro-phenyl)-2-oxo-ethyl]-3-methyl-7-((E)-2-buten-1-yl)-8-[(S)-3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 582 [M+H]+
(50) 1-[2-(2-nitro-3-methoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-
buten-l-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 626 [M+H]+
(51) 1-(2-{2-oxo-3-[(2-trimethylsilanyl-ethoxy)methyl]-2,3-dihydro-
benzooxazol-7-yl}-2-oxo-ethyl)-3-methyl-7-(3-methyl-2-buten-l-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 738 [M+H]+
CA 02496249 2005-02-18
54
(52) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-((Z)-2-methyl-2-buten-l-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.48 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 551 [M+H]+
(53) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-((E)-2-methyl-2-buten-l-yl)-8-[3-
(tert.-butyloxycarbonylamino)-pi peridin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 551 [M+H]+
(54) 1-(2-{2-oxo-3-[(2-trimethylsilanyl-ethoxy)methyl]-2,3-dihydro-
benzooxazo l-4-yl}-2-oxo-ethyl)-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.40 (silica gel, petroleum ether/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 722 [M+H]+
(55) 1-[2-(2,2-difluoro-benzo[I,3]dioxol-4-yl)-2-oxo-ethyl]-3-methyl-7-(2-
butyn-
1-yl)-8-[(R)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 615 [M+H]+
(56) 1-[2-(2,2-difluoro-benzo[1,3]dioxol-4-yl)-2-oxo-ethyl]-3-methyl-7-(2-
butyn-
1-yl)-8-[(S)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 615 [M+H]+
(57) 1-[(1-methyl-1H-indol-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxy-carbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.80 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 560 [M+H]+
(58) 1-[(quinolin-3-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-[3-(tert.-
butyloxycarbonyl-amino)-piperidin-1-yl]-xanthine
Rf value: 0.50 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 558 [M+H]+
CA 02496249 2005-02-18
(59) 1-{[1-(tert.-butyloxycarbonylamino)-1H-indol-2-yl]methyl}-3-methyl-7-(2-
butyn-l-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rfvalue: 0.40 (silica gel, petroleum ether/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 646 [M+H]+
(60) 1-[(2-methyl-1-[(2-trimethylsilanyl-ethoxy)methyl]-1 H-benzoimidazol-5-
yI)methyl]-3-methyl-7-(2-butyn- l -yl)-8-[3-(tert.-butyloxycarbonylamino)-
piperidin- 1-yl]-xanthine (mixed with 1-[(2-methyl-3-[(2-trimethylsilanyl-
ethoxy)methyl]-3H-benzoimidazol-5-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine)
Rf value: 0.15 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 691 [M+H]+
(61) 1-[2-(quinolin-8-yl-]-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxy-carbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.35 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 586 [M+H]+
(62) 1-[(1-[(2-trimethylsilanyl-ethoxy)methyl]-1 H-benzoimidazol-5-yl)methyl]-
3-
methyl-7-(2-butyn-l -yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1 -yl]-
xanthine (mixed with 1-[(3-[(2-trimethylsilanyl-ethoxy)methyl]-3H-
benzoi midazol-5-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-l-yl]-xanthine)
Rf value: 0.23 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 677 [M+H]+
(63) 1-[(pyrazolo[1,5-a]pyridin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert:butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.46 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 547 [M+H]+
(64) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-((E)-1-buten-l-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Rf value: 0.48 (silica gel, methylene chloride/methanol = 95:5)
CA 02496249 2005-02-18
56
Mass spectrum (ESI+): m/z = 537 [M+H]+
(65) 1-{2-[1-(tert.-butyloxycarbonyl)-1H-indol-7-yl]-2-oxo-ethyl}-3-methyl-7-
(2-
butyn-l-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.38 (silica gel, petroleum ether/ethyl acetate = 1:1)
(66) 1-[(3-methyl-isoquinolin-1-yl)methyl]-3-methyl-7-(3-methyl-1-buten-1-yl)-
8-[(R)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.40 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 588 [M+H]+
(67) 1,3-dimethyl-7-(2-bromo-benzyl)-8-[3-(tert.-butyloxycarbonylamino)-
piperidin-1-yl]-xanthine
Rf value: 0.40 (silica gel, cyclohexane/ethyl acetate = 1:1)
(68) 1,3-dimethyl-7-(2-chloro-benzyl)-8-[3-(tert.-butyloxycarbonylamino)-
piperidin- 1-yl]-xanthine
Rf value: 0.42 (silica gel, cyclohexane/ethyl acetate = 1:1)
(69) 1-[(3-methyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-cyano-benzyl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 635 [M+H]+
Rf value: 0.40 (silica gel, cyclohexane/ethyl acetate = 3:7)
(70) 3-cyclopropyl-7-(2-butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-
piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 443 [M+H]+
Rf value: 0.70 (silica gel, ethyl acetate)
(71) 3-cyclopropyl-7-(2-butyn-1-yl)-8-[(R)-3-(tert.-butyloxycarbonylamino)-
piperidin-1-yl]-xanthine
Rf value: 0.35 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 443 [M+H]+
CA 02496249 2005-02-18
57
(72) 1-[(3-methyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-chloro-benzyl)-8-[(R)-
3-(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Mass spectrum (ESI+): m/z = 644, 646 [M+H]+
Rf value: 0.39 (silica gel, cyclohexane/ethyl acetate = 1:1)
(73) 1-[(3-methyl-isoquinolin-l-yl)methyl]-3-methyl-7-(2-bromo-benzyl)-8-[(R)-
3-(tert.-butyloxycarbonylamino)-piperidi n-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 644, 646 [M+H]+
(74) 1-[(4-methyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-chloro-benzyl)-8-[(R)-
3-(tert.-butyloxycarbonylami no)- pi perid i n- 1 -yl]-xa nth i ne
Prepared by reacting (4-methyl-quinazolin-2-yl)-methylchloride and 3-methyl-
7-(2-chlorobenzyl)-8-bromo-xanthine and subsequently reacting with (R)-3-
(tert.-butyloxycarbonylamino)-piperidine
Mass spectrum (ESI+): m/z = 645, 647 [M+H]+
(75) 1-[(4-phenyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-chloro-benzyl)-8-[(R)-
3-(tert.-butyloxycarbonylami no)-piperidin-1-yl]-xanthine
Prepared by reacting (4-phenyl-quinazolin-2-yl)-methylchloride and 3-methyl-
7-(2-chlorobenzyl)-8-bromo-xanthine and subsequently reacting with (R)-3-
(tert.-butyloxycarbonylamino)-piperidine
Mass spectrum (ESI+): m/z = 707, 709 [M+H]+
Example IV
1-[2-(2-hydroxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-
chloro-xanthine
Prepared by treating 1-[2-(2-methoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-chloro-xanthine with boron tribromide in methylene
chloride. The desired product is contaminated with approx. 20 % 1-[2-(2-
hydroxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-bromo-3-methyl-butyl)-8-chloro-
xanthine.
Mass spectrum (ESI+): m/z = 403, 405 [M+H]+
CA 02496249 2005-02-18
58
The following compounds are obtained analogously to Example IV:
(1) 1-[2-(2-hydroxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-bromo-
xanthine
(product is contaminated with approx. 20 % 1-[2-(2-hydroxy-phenyl)-2-oxo-
ethyl]-3-methyl-7-(3-bromo-2-buten-1-yl)-8-bromo-xanthine)
Mass spectrum (ESI+): mlz = 431, 433 [M+H]+
(2) 1-[2-(2-hydroxy-phenyl)-2-oxo-ethyl]-3-methyl-7-[(1-cyclopenten-1-
yl)methyl]-8-bromo-xanthine
Mass spectrum (ESI+): m/z = 459, 461 [M+H]+
(3) 1-[2-(2-hydroxy-phenyl)-2-oxo-ethyl]-3-methyl-7-((E)-2-buten-l-yl)-8-
bromo-xanthine
(product contains some Z isomer)
Rf value: 0.60 (silica gel, cyclohexane/ethyl acetate = 4:6)
Mass spectrum (ESI+): m/z = 433, 435 [M+H]+
(4) 1-[2-(2-hydroxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-
bromo-xanthine
Mass spectrum (ESI+): m/z = 447, 449 [M+H]+
Example V
1-[2-(2-methoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-
chloro-xanthine
1.71 g of 2-bromo-1-(2-methoxy-phenyl)-ethanone are added to a mixture of
2.00 g of 3-methyl-7-(3-methyl-2-buten-1-yl)-8-chloro-xanthine and 1.38 mg of
potassium carbonate in 15 ml of N,N-dimethylformamide. The reaction
mixture is stirred for eight hours at ambient temperature. After aqueous
working up the crude product is purified by chromatography through a silica
gel column with cyclohexane/ethyl acetate (8:1 to 8:1) as eluant.
Yield: 2.61 g (84 % of theory)
Mass spectrum (ESI+): m/z = 417, 419 [M+H]+
CA 02496249 2005-02-18
59
The following compounds are obtained analogously to Example V:
(1) 1-[2-(3-hydroxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-
[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(The reaction is carried out with 2-bromo-1-[3-(tert.-butyldimethylsilanyloxy)-
phenyl]-ethanone)
Rf value: 0.40 (silica gel, cyclohexane/ethyl acetate = 3:7)
Mass spectrum (ESI+): m/z = 567 [M+H]+
(2) 1-(1-methyl-2-oxo-2-phenyl-ethyl)-3-methyl-7-(3-methyl-2-buten-1-yl)-8-
chloro-xanthine
Mass spectrum (ESI+): m/z = 401, 403 [M+H]+
(3) 1-(2-cyano-ethyl)-3-methyl-7-(2-cyano-benzyl)-8-chloro-xanthine
Mass spectrum (ESI+): m/z = 391, 393 [M+Na]+
(4) 1-(2-phenoxy-ethyl)-3-methyl-7-(2-cyano-benzyl)-8-[3-(tert.-
butyloxycarbonylami no)-pi peridin-1-yl]-xanthine
Rf value: 0.90 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 600 [M+H]+
(5) 1-(2-phenyl-2-oxo-ethyl)-3-[(2-trimethylsilanyl-ethoxy)methyl]-7-(3-methyl-
2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Mass spectrum (ESI+): m/z = 667 [M+H]+
(6) 1-(2-methoxy-ethyl)-3-methyl-7-(2-cyano-benzyl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.90 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 538 [M+H]+
(7) 1-methyl-3-[(2-trimethylsilanyl-ethoxy)methyl]-7-(2-cyano-benzyl)-xanthine
Rf value: 0.60 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 412 [M+H]+
CA 02496249 2005-02-18
(8) 1-[2-(3-nitro-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-l-yl)-8-
chloro-xanthine
Rf value: 0.40 (silica gel, cyclohexane/ethyl acetate/methanol = 7:2:1)
Mass spectrum (ESI+): m/z = 432, 434 [M+H]+
(9) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-[(2-trimethylsi lanyl-ethoxy)methyl]-8-
bromo-xanthine
Mass spectrum (ESI+): m/z = 493, 495 [M+H]+
(10) 1-[2-(2-nitro-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-
chloro-xanthine
Rf value: 0.64 (silica gel, cyclohexane/ethyl acetate = 3:7)
Mass spectrum (ESI+): m/z = 432, 434 [M+H]+
(11) 1-[2-(2-nitro-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-
bromo-xanthine
Mass spectrum (ESI+): m/z = 476, 478 [M+HJ+
(12) 1-[(quinolin-2-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.45 (silica gel, ethyl acetate/petroleum ether = 7:3)
Mass spectrum (ESI+): m/z = 574 [M+H]+
(13) 1-[(2-oxo-2H-chromen-4-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-
[3-(tert.-butyloxycarbonylami no)-piperidin-1-yl]-xanthine
(The starting material 4-bromomethyl-chromen-2-one is prepared analogously
to Kimura et al., Chem. Pharm. Bull. 1982, 30, 552-558.)
Rf value: 0.52 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 591 [M+H]+
(14) 1-[(1-methyl-2-oxo-1,2-dihydro-quinolin-4-yl)methyl]-3-methyl-7-(3-
methyl-2-buten-l-yl)-8-[3-(tert.-butyloxycarbonylami no)-piperidin-1-yl]-
xanthine
Rf value: 0.54 (silica gel, methylene chloride/methanol = 95:5)
CA 02496249 2005-02-18
61
Mass spectrum (ESI+): m/z = 604 [M+H]+
(15) 1-[(quinazolin-4-yl)methyl]-3-methyl-7-(3-methyl-2-buten-l-yl)-8-[3-
(tert.-
butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Melting point: 195-197 C
Mass spectrum (ESI+): m/z = 575 [M+H]+
(16) 1-[(5-methyl-3-phenyl-isoxazol-4-yl)methyl]-3-methyl-7-(3-methyl-2-
buten-1-yl)-8-[3-(tert.-butyloxycarbonylam ino)-piperidin-1-yl]-xanthine
Rf value: 0.40 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 604 [M+H]+
(17) 1-[(3-phenyl-[1,2,4]oxadiazol-5-yl)methyl]-3-methyl-7-(3-methyl-2-buten-
1-yI)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.18 (silica gel, petroleum ether/ethyl acetate = 2:1)
Mass spectrum (ESI+): m/z = 591 [M+H]+
(18) 1-[(4-phenyl-pyridin-2-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-[3-
(tert.-butyloxycarbonylami no)-piperidin-1-yl]-xanthine
Rf value: 0.53 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 600 [M+H]+
(19) 1-[(5-phenyl-pyridin-2-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.73 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 600 [M+H]+
(20) 1-[2-(3-methylsulphanyl-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-
buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.43 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 597 [M+H]+
(21) 1-[2-(3-methoxycarbonyl-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-
buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
CA 02496249 2005-02-18
62
(Carried out in N-methylpyrrolidin-2-one at 60 C)
Rf value: 0.27 (silica gel, methylene chloride/methanol = 20:1)
Mass spectrum (ESI+): m/z = 609 [M+H]+
(22) 1-[2-(2- ethoxycarbonyl-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-
buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(Carried out in N-methylpyrrolidin-2-one at 60 C)
Rf value: 0.35 (silica gel, methylene chloride/methanol = 20:1)
Mass spectrum (ESI+): m/z = 623 [M+H]+
(23) 1-[2-(2,6-d imethoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-
yl)-8-[3-(tert.-butyloxycarbo nylami no)-pi peridin-1-yl]-xanthine
(Carried out in N-methylpyrrolidin-2-one at 60 C)
Rf value: 0.53 (silica gel, methylene chloride/methanol = 20:1)
Mass spectrum (ESI+): m/z = 611 [M+H]+
(24) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-(2,3-dimethyl-2-buten-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
(Carried out in N-methylpyrrolidin-2-one at 60 C)
Rf value: 0.38 (silica gel, methylene chloride/methanol = 20:1)
Mass spectrum (ESI+): m/z = 565 [M+H]+
(25) 1-((E)-3-phenyl-al lyl)-3-methyl-7-(3-methyl-2-buten-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.54 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:0.1)
Mass spectrum (ESI+): m/z = 549 [M+H]+
(26) 1-[(1-benzo[b]thiophen-3-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-
[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.75 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 579 [M+H]+
CA 02496249 2005-02-18
63
(27) 1-{[1-(tert.-butyloxycarbonyl)-indol-3-yl]methyl}-3-methyl-7-(3-methyl-2-
buten-1-yl)-8-[3-(tert.-butyloxycarbonyaam ino)-piperidin-1-yl]-xanthine
Rf value: 0.61 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 662 [M+H]+
(28) 1-[(biphenyl-4-yl)methyl]-3-methyl-7-(3-methyl-2-buten-l-yl)-8-[3-(tert.-
butyloxycarbonylami no)-piperidin-1-yl]-xanthine
Rf value: 0.68 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 599 [M+H]+
(29) 1-[(1-naphthyl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxycarbonylami no)-piperidin-l-yl]-xanthine
Rf value: 0.83 (silica gel, ethyl acetate/petroleum ether = 4:1)
Mass spectrum (ESI+): m/z = 557 [M+H]+
(30) 1-[(1-methyl-2-oxo-l,2-dihydro-quinolin-4-yl)methyl]-3-methyl-7-(2-butyn-
1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.25 (silica gel, ethyl acetate/petroleum ether = 4:1)
Mass spectrum (ESI+): m/z = 588 [M+H]+
(31) 1-(2-cyclohexyl-2-oxo-ethyl)-3-methyl-7-(3-methyl-2-buten-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Melting point: 163-165 C
Mass spectrum (ESI+): m/z = 557 [M+H]+
(32) 1-(3,3-dimethyl-2-oxo-butyl)-3-methyl-7-(3-methyl-2-buten-l-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.95 (silica gel, ethyl acetate/petroleum ether = 4:1)
Mass spectrum (ESI+): m/z = 531 [M+H]+
(33) 1-[(quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.40 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 559 [M+H]+
CA 02496249 2005-02-18
64
(34) 1-[(2-methyl-naphthalen-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-
butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Rf value: 0.80 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 571 [M+H]+
(35) 1-[(5-nitro-isoquinolin-1-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-
[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.54 (silica gel, methylene chloride/methanol = 95:5)
(36) 1-(2-dimethylamino-2-oxo-ethyl)-3-methyl-7-(3-methyl-2-buten-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.23 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 518 [M+H]+
(37) 1-[2-(piperidin-1-yl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-
[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.44 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 558 [M+H]+
(38) 1-[(2-methyl-1-oxo-l,2-dihydro-isoquinolin-4-yl)methyl]-7-(2-butyn-1-yl)-
8-
[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.25 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 588 [M+H]+
(39) 1-[(2-methyl-1-oxo-l,2-dihydro-isoquinolin-4-yl)methyl]-7-(3-methyl-2-
buten-l-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Rf value: 0.30 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 604 [M+H]+
(40) 1-[(2-methoxy-naphthalen-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Rf value: 0.75 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 587 [M+H]+
CA 02496249 2005-02-18
(41) 1-[(4-methoxy-naphthalen-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.80 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 587 [M+H]+
(42) 1-[(3-methyl-isoquinolin-l-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.56 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 572 [M+H]+
(43) 1-[2-(2,3-dimethoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-
yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.83 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 611 [M+H]+
(44) 1-[(5-nitro-naphthalen-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.78 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 602 [M+H]+
(45) 1-[2-(pyrrolidin-1-yl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-
[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.39 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 544 [M+H]+
(46) 1-[(4-methyl-isoquinolin-l-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-
butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Rf value: 0.56 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 572 [M+H]+
(47) 1-[(2-naphthyl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.78 (silica gel, ethyl acetate)
CA 02496249 2005-02-18
66
Mass spectrum (ESI+): m/z = 557 [M+H]+
(48) 1-cyanomethyl-3-methyl-7-(2-butyn-l-yl)-8-[3-(tert.-butyloxycarbonyl-
amino)-piperidin-1-yl]-xanthine
Rf value: 0.80 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 456 [M+H]+
(49) 1-[(quinolin-6-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.40 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 558 [M+H]+
(50) 1-[(3-methoxy-naphthalen-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.83 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 587 [M+H]+
(51) 1-[2-(2,3-dihydro-benzo[ 1,4]dioxin-5-yl)-2-oxo-ethyl]-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylami no)-piperidin-1-yl]-
xanthine
Rf value: 0.38 (silica gel, ethyl acetate/petroleum ether = 1:1)
Mass spectrum (ESI+): m/z = 609 [M+H]+
(52) 1-[2-(3-methyl-2-oxo-2,3-dihydro-benzooxazol-7-yl)-2-oxo-ethyl]-3-
methyl-7-(3-methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-
1-yl]-xanthine
(Carried out with potassium-tert. butoxide in dimethylsulphoxide)
Rf value: 0.48 (silica gel, ethyl acetate/petroleum ether = 2:1)
Mass spectrum (ESI+): m/z = 622 [M+H]+
(53) 1-[2-(benzo[ 1, 3]dioxol-4-yl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-
1-
yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 595 [M+H]+
CA 02496249 2005-02-18
67
(54) 1-[(quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yi)-8-[(R)-3-(tert.-
butyloxycarbonylami no)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 559 [M+H]+
(55) 1-[(quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[(S)-3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 559 [M+H]+
(56) 1-[(quinazolin-2-yl)methyl]-3-methyl-7-((E)-2-buten-1-yl)-8-[(S)-3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(product contains approx. 15 % of Z isomer)
Rf value: 0.30 (silica gel, ethyl acetate/cyclohexane = 8:2)
Mass spectrum (ESI+): m/z = 561 [M+H]+
(57) 1-[(quinazolin-2-yl)methyl]-3-methyl-7-((E)-2-buten-1-yl)-8-[(R)-3-(tert.-
butyloxycarbonylamino)-piperidin-l-yl]-xanthine
(product contains approx. 15 % of Z isomer)
Rf value: 0.30 (silica gel, ethyl acetate/cyclohexane = 8:2)
Mass spectrum (ESI+): m/z = 561 [M+H]+
(58) 1-[(3-methyl-isoquinolin-1-yl)methyl]-3-methyl-7-((E)-2-buten-1-yl)-8-
[(R)-
3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(product contains approx. 17 % of Z isomer)
Rf value: 0.58 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 574 [M+H]+
(59) 1-[(3-methyl-isoquinolin-l-yl)methyl]-3-methyl-7-((E)-2-buten-1-yl)-8-
[(S)-
3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(product contains approx. 17 % of Z isomer)
Rf value: 0.58 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 574 [M+H]+
(60) 1-[2-(2-methoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn- l -yl)-8-bromo-
xanthine
CA 02496249 2005-02-18
68
Mass spectrum (ESI+): m/z = 445, 447 [M+H]+
(61) 1-[2-(2-nitro-phenyl)-2-oxo-ethyl]-3-methyl-7-[(1-cyclopenten-1-
yl)methyl]-
8-bromo-xanthine
Mass spectrum (ESI+): m/z = 488, 490 [M+H]+
(62) 1-[2-(2-methoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-[(1-cyclopenten-1-
yl)methyl]-8-bromo-xanthine
Mass spectrum (ESI+): m/z = 473, 475 [M+H]+
(63) 1-[(isoquinolin-1-yl)methyl]-3-[(methoxycarbonyl)methyl]-7-(3-methyl-2-
buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.35 (silica gel, methylene chloride/methanol = 95:5)
(64) 1-[2-(2-nitro-phenyl)-2-oxo-ethyl]-3-methyl-7-((E)-2-buten-1-yl)-8-bromo-
xanthine
(product contains approx. 10 % of Z isomer)
Rf value: 0.60 (silica gel, cyclohexane/ethyl acetate = 4:6)
Mass spectrum (ESI+): m/z = 462, 464 [M+H]+
(65) 1-[2-(2-methoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-((E)-2-buten-1-yl)-8-
bromo-xanthine
(product contains some Z isomer)
Rf value: 0.30 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 447, 449 [M+H]+
(66) 1-[2-(2-nitro-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-bromo-
xanthine
Rf value: 0.77 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 460, 462 [M+H]+
(67) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-((E)-2-buten-1-yl)-8-[(R)-3-(tert.-
butyloxycarbonylami no)-piperid in-1-yl]-xanthine
(product contains approx. 20 % of Z isomer)
CA 02496249 2005-02-18
69
Mass spectrum (ESI+): m/z = 537 [M+H]+
(68) 1-[2-(2-methoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-
8-bromo-xanthine
Mass spectrum (ESI+): m/z = 461, 463 [M+H]+
(69) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-(2-butyn-1-yl)-8-bromo-xanthine
Rf value: 0.61 (silica gel, cyclohexane/ethyl acetate = 4:6)
(70) 1-(2-{2-[(ethylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
((E)-2-buten-1-yl)-8-[(R)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-
xanthine
(product contains approx. 17 % of Z isomer)
Mass spectrum (ESI+): m/z = 638 [M+H]+
(71) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-((E)-2-buten-1-yl)-8-[(S)-3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(product contains approx. 18 % of Z isomer)
Rf value: 0.35 (silica gel, cyclohexane/ethyl acetate = 6:4)
Mass spectrum (ESI+): m/z = 537 [M+H]+
(72) 1-[2-(2-nitro-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-[(S)-3-
(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.60 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 580 [M+H]+
(73) 1-[(3-methyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[(S)-3-
(tert.-butyloxycarbonylami no)-piperidin-1-yl]-xanthine
Rf value: 0.52 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 572 [M+H]+
(74) 1-[(3-methyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[(R)-3-
(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Mass spectrum (ESI+): m/z = 572 [M+H]+
CA 02496249 2005-02-18
(75) 1-[(4-methyl-isoquinolin-l-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[(S)-3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 572 [M+H]+
(76) 1-[(4-methyl-isoquinolin-l-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[(R)-3-
(tert.-butyloxycarbonylamino)-piperidin-1-yi]-xanthine
Mass spectrum (ESI+): m/z = 572 [M+H]+
(77) 1-[(4-methyl-isoquinolin-1-yl)methyl]-3-methyl-7-((E)-2-buten-1-yl)-8-
[(S)-
3-(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Rf value: 0.52 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 574 [M+H]+
(78) 1-[(4-methyl-isoquinolin-l-yl)methyl]-3-methyl-7-((E)-2-buten-1-yl)-8-
[(R)-
3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.52 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 574 [M+H]+
(79) 1-[2-(2,3-dihydro-benzo[1,4]dioxin-5-yl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-
1-yl)-8-[(R)-3-(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Rf value: 0.18 (silica gel, ethyl acetate/petroleum ether = 1:1)
Mass spectrum (ESI+): m/z = 593 [M+H]+
(80) 1-[2-(2,3-dihydro-benzo[I ,4]dioxin-5-yl)-2-oxo-ethyl]-3-methyl-7-(2-
butyn-
1-yl)-8-[(S)-3-(tert.-butyloxycarbonylami no)- pi perid i n- 1 -yl]-xa nth ine
Mass spectrum (ESI+): m/z = 593 [M+H]+
(81) 1-[(4-methoxy-naphthalen-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[(S)-3-
(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Rf value: 0.56 (silica gel, petroleum ether/ethyl acetate = 1:2)
Mass spectrum (ESI+): m/z = 587 [M+H]+
CA 02496249 2005-02-18
71
(82) 1-[(4-methoxy-naphthalen-l-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-[(R)-3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 587 [M+H]+
(83) 1-[2-(benzo[1,3]dioxol-4-yl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-
[(R)-
3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.86 (silica gel, ethyl acetate/petroleum ether = 4:1)
Mass spectrum (ESI+): m/z = 579 [M+H]+
(84) 1-[2-(benzo[1,3]dioxol-4-yl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-l-yl)-8-
[(S)-
3-(tert.-butyloxycarbonylamino)-piperidi n- 1 -yl]-xa nth i ne
Rf value: 0.86 (silica gel, ethyl acetate/petroleum ether = 4:1)
Mass spectrum (ESI+): m/z = 579 [M+H]+
(85) 1-[(4-methyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[(S)-3-
(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Rf value: 0.48 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 573 [M+H]+
(86) 1-[(4-methyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-[(R)-3-
(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Rf value: 0.48 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 573 [M+H]+
(87) 1-[2-(3-methyl-2-oxo-2,3-dihydro-benzooxazol-4-yl)-2-oxo-ethyl]-3-
methyl-7-(3-methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-
1-yl]-xanthine
Rf value: 0.50 (silica gel, petroleum ether/ethyl acetate = 1:2)
Mass spectrum (ESI+): m/z = 622 [M+H]+
(88) 1-(2-{2-[(ethylaminocarbonyl)meth oxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
((E)-2-buten-l -yl)-8-[(S)-3-(tert.-butyloxycarbonylami no)-piperidin-1-yl]-
xanthine
Mass spectrum (ESI+): m/z = 638 [M+H]+
CA 02496249 2005-02-18
72
(89) 1-(2-{2-[(methylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
((E)-2-buten-1-yl)-8-[(R)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-
xanthine
Mass spectrum (ESI+): m/z = 624 [M+H]+
(90) 1-(2-{2-[(methylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
((E)-2-buten-1-yl)-8-[(S)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-
xanthine
Mass spectrum (ESI+): m/z = 624 [M+H]+
(91) 1-[2-(2-nitro-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-[(R)-3-
(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.60 (silica gel, methylene chloride/methanol = 95:5)
(92) 1-[2-(2-nitro-3-methoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-
buten-1-yl)-8-bromo-xanthine
Mass spectrum (ESI+): m/z = 506, 508 [M+H]+
(93) 1-[(4-dimethylamino-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-
[3-(tert.-butyloxycarbonylami no)-piperidin-1-yl]-xanthine
(Carried out in the presence of caesium carbonate)
Rf value: 0.40 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 602 [M+H]+
(94) 1-(2-{2-oxo-3-[(2-trimethylsilanyl-ethoxy)methyl]-2,3-dihydro-
benzooxazol-7-yl}-2-oxo-ethyl)-3-methyl-7-(3-methyl-2-buten-l-yl)-8-bromo-
xanthine
Rf value: 0.75 (silica gel, ethyl acetate/petroleum ether = 1:1)
Mass spectrum (ESI+): m/z = 618, 620 [M+H]+
(95) 1-[(imidazo[1,2-a]pyridin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.44 (silica gel, methylene chloride/methanol = 95:5)
CA 02496249 2005-02-18
73
Mass spectrum (ESI+): m/z = 547 [M+H]+
(96) 1-[(quinoxalin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxycarbonyl-amino)-piperidin-1-yl]-xanthine
Rf value: 0.50 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 559 [M+H]+
(97) 1-[2-(1,3-dimethyl-2-oxo-2,3-dihydro-1 H-benzoimidazol-4-yl)-2-oxo-ethyl]-
3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-l-yl]-
xanthine
Rf value: 0.50 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 619 [M+H]+
(98) 1-[(quinoxalin-6-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.35 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 559 [M+H]+
(99) 1-(2-{2-oxo-3-[(2-trimethylsilanyl-ethoxy)methyl]-2,3-dihydro-
benzooxazol-4-yl}-2-oxo-ethyl)-3-methyl-7-(2-butyn-1-yl)-8-bromo-xanth ine
Rf value: 0.30 (silica gel, petroleum ether/ethyl acetate = 2:1)
Mass spectrum (ESI-): m/z = 600, 602 [M-H]-
(100) 1-[(3-methyl-quinoxalin-2-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-[3-
(tert.-
butyloxycarbonylamino)-piperid in-1-yl]-xanthine
Rf value: 0.44 (silica gel, petroleum ether/ethyl acetate = 1:2)
Mass spectrum (ESI+): m/z = 573 [M+H]+
(101) 1-[(3-phenyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-butyloxycarbonylam ino)-piperidin-1-yl]-xanthine
Rf value: 0.85 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 634 [M+H]+
CA 02496249 2005-02-18
74
(102) 1-[(3,4-dimethyl-isoquinolin-l-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.60 (silica gel, ethyl acetate/methanol = 3:1)
Mass spectrum (ESI+): m/z = 586 [M+H]+
(103) 1-[(benzofuran-2-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-[(R)-3-(tert.-
butyl-
oxycarbonylamino)-piperidin-l-yl]-xanthine
Mass spectrum (ESI+): m/z = 547 [M+H]+
(104) 1-{[4-(morpholin-4-yl)-quinazolin-2-yl]methyl}-3-methyl-7-(2-butyn-l-yl)-
8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(Carried out in the presence of caesium carbonate)
Rf value: 0.28 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 644 [M+H]+
(105) 1-{[4-(piperidin-1-yl)-quinazolin-2-yl]methyl}-3-methyl-7-(2-butyn-l-yl)-
8-
[3-(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
(Carried out in the presence of caesium carbonate)
Rf value: 0.35 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 642 [M+H]+
(106) 1-({4-[4-(tert.-butyloxycarbonyl)-piperazin-l-yl]-quinazolin-2-
yl}methyl)-
3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-pi peridin-l-yl]-
xanthine
(Carried out in the presence of caesium carbonate)
Rf value: 0.50 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 743 [M+H]+
(107) 1-{[4-(pyrrolidin-l-yl)-quinazolin-2-yl]methyl}-3-methyl-7-(2-butyn-1-
yl)-8-
[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(Carried out in the presence of caesium carbonate)
Rf value: 0.59 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
95:5:0.1)
Mass spectrum (ESI+): m/z = 628 [M+H]+
CA 02496249 2005-02-18
(108) 1-[2-(1-ethoxycarbonyl-3-methyl-2-oxo-2,3-dihydro- 1 H-benzoimidazol-4-
yl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-l-yl)-8-[3-(tert.-butyloxycarbonylamino)-
piperidin-1-yl]-xanthine
Rf value: 0.25 (silica gel, petroleum ether/ethyl acetate = 1:2)
Mass spectrum (ESI+): m/z = 677 [M+H]+
(109) 1-[(4-cyano-naphthalen-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[(R)-3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.77 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 582 [M+H]+
(110) 1-[(imidazo[1,2-a]pyridine-3-yl)methyl]-3-methyl-7-(2-butyn-1 -yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin- l-yl]-xanthine
Mass spectrum (ESI+): m/z = 547 [M+H]+
(111) 1-[(8-methyl-imidazo[1,2-a]pyridin-2-yl)methyl]-3-methyl-7-(2-butyn-l-
yl)-8-[3-(tent.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Rf value: 0.25 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 561 [M+H]+
(112) 1-[(8-methoxy-quinolin-5-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-[3-
(tert.-
butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Rf value: 0.60 (silica gel, ethyl acetate/methanol = 9:1)
Mass spectrum (ESI+): m/z = 588 [M+H]+
(113) 1-[(5-methoxy-quinolin-8-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-[3-
(tert.-
butyloxycarbonylamino)-piperid in-l-yl]-xanthine
Rf value: 0.40 (silica gel, methylene chloride/methanol = 20:1)
Mass spectrum (ESI+): m/z = 588 [M+H]+
(114) 1-[(4-phenyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-[3-
(tert.-
butyloxycarbonylami no)-piperidin-l-yl]-xanthine
CA 02496249 2005-02-18
76
Mass spectrum (ESI+): m/z = 635 [M+H]+
(115) 1-[(7-methyl-imidazo[1,2-a]pyridin-2-yl)methyl]-3-methyl-7-(2-butyn-1-
yl)-8-[3-(tert.-butyloxycarbonylamino)-piperid i n- 1 -yl]-xa nth i ne
Rf value: 0.50 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 561 [M+H]+
(116) 1-(2-oxo-4-phenyl-butyl)-3-methyl-7-(2-butyn-1-yl)-8-[(R)-3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 563 [M+H]+
(117) 1-(2-{2-oxo-1,3-bis-[(2-trimethylsilanyl-ethoxy)methyl]-2,3-dihydro-1 H-
benzoimidazol-4-yl}-2-oxo-ethyl)-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxy-
carbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.50 (silica gel, ethyl acetate/petroleum ether = 1:1)
Mass spectrum (ESI+): m/z = 851 [M+H]+
(118) 1-[(3-difluoromethyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-
8-
[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(By-product of the reaction of 3-methyl-7-(2-butyn-l-yl)-8-[3-(tert.-butyloxy-
carbonylamino)-piperidin-1-yl]-xanthine with 1-chloromethyl-3-trifluoromethyl-
3,4-dihydro-isoquinoline)
Rf value: 0.75 (aluminium oxide, petroleum ether/ethyl acetate = 1:2)
Mass spectrum (ESI+): m/z = 608 [M+H]+
(119) 1-[2-(2,2-difluoro-benzo[1, 3]dioxol-4-yl)-2-oxo-ethyl]-3-methyl-7-(2-
butyn-1-yl)-8-bromo-xanthine
Mass spectrum (ESI+): m/z = 495, 497 [M+H]+
(120) 1-[(3-methyl-imidazo[1,2-a]pyridin-2-yl)methyl]-3-methyl-7-(2-butyn-1-
yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.50 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 561 [M+H]+
CA 02496249 2005-02-18
77
(121) 1-[(5-methyl-imidazo[1,2-a]pyridin-2-yl)methyl]-3-methyl-7-(2-butyn-1-
yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.50 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 561 [M+H]+
(122) 1-[(6-methyl-imidazo[I ,2-a]pyridin-2-yl)methyl]-3-methyl-7-(2-butyn-1-
yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.10 (silica gel, ethyl acetate/methanol = 98:2)
Mass spectrum (ESI+): m/z = 561 [M+H]+
(123) 1-[(3-benzyl-imidazo[1,2-a]pyridin-2-yl)methyl]-3-methyl-7-(2-butyn-1-
yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.60 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 637 [M+H]+
(124) 1-[(4-isopropyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.50 (silica gel, ethyl acetate/petroleum ether = 8:2)
Mass spectrum (ESI+): m/z = 601 [M+H]+
(125) 1-[(2,3-dihydro-benzo[1,4]dioxin-6-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-
8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.53 (silica gel, ethyl acetate/petroleum ether = 3:2)
(126) 1-[(3-phenyl-imidazo[1,2-a]pyridin-2-yl)methyl]-3-methyl-7-(2-butyn-1-
yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.50 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 623 [M+H]+
(127) 1-[2-(naphthalen-1-yl]-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.54 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 585 [M+H]+
CA 02496249 2005-02-18
78
(128) 1-[(5-methoxy-isoquinolin-8-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.50 (silica gel, ethyl acetate/methanol = 24:1)
Mass spectrum (ESI+): m/z = 588 [M+H]+
(129) 1-[(3-difluoromethyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-
8-
[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(By-product of the reaction of 3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-butyloxy-
carbonylamino)-piperidin-1-yl]-xanthine with 1-chloromethyl-3-trifluoromethyl-
3,4-dihydro-isoquinoline)
Rf value: 0.75 (aluminium oxide, petroleum ether/ethyl acetate = 1:2)
Mass spectrum (ESI+): m/z = 608 [M+H]+
(130) 1-{[1-(1-cyano-1-methyl-ethyl)-isoquinolin-3-yl]methyl}-3-methyl-7-(2-
butyn-1-yl)-8-[3-(tert. -butyl oxyca rbonyl am i no)-pi perid i n-1-yl]-xa nth
i ne
Rf value: 0.75 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 625 [M+H]+
(132) 1-methoxycarbonylmethyl-3-methyl-7-(2-butyn-l-yl)-8-[3-(tert.-
butyloxycarbonyl-amino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 489 [M+H]+
(133) 1-[(4-phenyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[(R)-3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 635 [M+H]+
(134) 1-[(2,3-dimethyl-quinoxalin-6-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin- l-yl]-xanthine
(Carried out in the presence of caesium carbonate)
Rf value: 0.40 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 587 [M+H]+
CA 02496249 2005-02-18
79
(135) 1-[(4-phenyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-[(S)-3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.55 (silica gel, ethyl acetate/petroleum ether = 8:2)
Mass spectrum (ESI+): m/z = 635 [M+H]+
(136) 1-[2-(quinol in-8-yl-]-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-bromo-
xanthine
Rf value: 0.55 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 466, 468 [M+H]+
(137) 1-[(3,4-dimethyl-6,7-dihydro-5H-[2]pyrindin-l-yl)methyl]-3-methyl-7-(2-
butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.65 (aluminium oxide, ethyl acetate/petroleum ether = 3:1)
Mass spectrum (ESI+): m/z = 576 [M+H]+
(138) 1-[(3,4-dimethyl-5,6,7,8-tetrahydro-isoquinolin-1-yl)methyl]-3-methyl-7-
(2-butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.40 (aluminium oxide, methylene chloride/methanol = 20:1)
Mass spectrum (ESI+): m/z = 590 [M+H]+
(139) 1-{2-[1-(tert.-butyloxycarbonyl)-1 H-indol-4-yl]-2-oxo-ethyl}-3-methyl-7-
(2-
butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Rf value: 0.55 (silica gel, petroleum ether/ethyl acetate = 1:2)
Mass spectrum (ESI+): m/z = 674 [M+H]+
(140) 1-[(1-methyl-2-oxo-l,2-dihydro-quinolin-6-yl)methyl]-3-methyl-7-(2-
butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (El): m/z = 587 [M]+
(141) 1-({1-[(2-trimethylsilanyl-ethoxy)methyl]-2-oxo-1,2-dihydro-quinolin-6-
yl}methyl)-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-
piperidin- 1-yl]-xanthine
Mass spectrum (ESI+): m/z = 704 [M+H]+
CA 02496249 2005-02-18
(142) 1-[(2,3,8-trimethyl-quinoxalin-6-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-
[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 601 [M+H]+
(143) 1-[(8-methyl-quinoxalin-6-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 573 [M+H]+
(144) 1-[(4-methyl-phthalazin-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.65 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 573 [M+H]+
(145) 1-[(4-bromo-3-methoxy-isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-1-
yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.65 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 666, 668 [M+H]+
(146) 1-[(4-difluoromethoxy-naphthalen-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-
8-[(R)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.80 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 623 [M+H]+
(147) 1-{2-[1-(tert.-butyloxycarbonyl)-1H-indol-7-yl]-2-oxo-ethyl}-3-methyl-7-
(2-
butyn-1-yl)-8-bromo-xanthine
Rf value: 0.83 (silica gel, methylene chloride/methanol = 95:5)
(148) 1-[(E)-3-(2-nitro-phenyl)-2-propen-l-yl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Mass spectrum (ESI+): m/z = 578 [M+H]+
(149) 1 -((E)-3- pentafl uorophenyl-2-propen- 1-yl)-3-methyl-7-(2-butyn-1-yl)-
8-
[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
CA 02496249 2005-02-18
81
Mass spectrum (ESI+): m/z = 623 [M+H]+
(150) 1-[(4-nitro-naphthalen-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[(R)-3-
(tert.-butyloxycarbonylami no)- pi peri di n- 1 -yl]-xa nth i ne
Rf value: 0.41 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 602 [M+H]+
(151) 1-[(benzooxazol-2-y)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxycarbo-nylamino)-piperidin-1-yl]-xanthine
Rf value: 0.40 (silica gel, cyclohexane/ethyl acetate = 3:7)
Mass spectrum (ESI+): m/z = 548 [M+H]+
(152) 1-[(5-nitro-benzooxazol-2-y)methyl]-3-methyl-7-(2-butyn-l-yl)-8-[3-
(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.50 (silica gel, cyclohexane/ethyl acetate/methanol = 5:4:1)
Mass spectrum (ESI+): m/z = 593 [M+H]+
(153) 1-[(3-methyl-isoquinolin-l-yl)methyl]-3-methyl-7-(3-methyl-1-buten-1-yl)-
8-bromo-xanthine
Rf value: 0.65 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 468, 470 [M+H]+
(154) 1-[(quinolin-7-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxycarbonyl-amino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 558 [M+H]+
(155) 1-[([1,5]naphthyridin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 559 [M+H]+
(156) 1-[(8-methyl-quinoxalin-6-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[(R)-3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.45 (silica gel, methylene chloride/methanol = 19:1)
Mass spectrum (ESI+): m/z = 573 [M+H]+
CA 02496249 2005-02-18
82
(157) 1-[(2,3,8-trimethyl-quinoxalin-6-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-
[(R)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.32 (silica gel, methylene chloride/methanol = 96:4)
Mass spectrum (ESI+): m/z = 601 [M+H]+
(158) 1-[([1,6]naphthyridin-5-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.20 (silica gel, ethyl acetate/methanol = 98:2)
Mass spectrum (ESI+): m/z = 559 [M+H]+
(159) 1-[([1,8]naphthyridin-2-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.12 (silica gel, ethyl acetate/methanol = 98:2)
Mass spectrum (ESI+): m/z = 559 [M+H]+
(160) 1-[(4-fluoro-naphthalen-1-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-[(R)-3-
(tert.-butyloxycarbonyl a mi no)-pi peri di n- 1 -yl]-xa nth i ne
Rf value: 0.47 (silica gel, petroleum ether/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 575 [M+H]+
(161) 1-[([1,5]naphthyridin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[(R)-3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.39 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 559 [M+H]+
(162) 1-[2-(3-methyl-2-oxo-2,3-dihydro-benzooxazol-4-yl)-2-oxo-ethyl]-3-
methyl-7-(2-butyn-1-yl)-8-[(R)-3-(tert.-butyloxycarbonyla mino)-piperidin-1-
yl]-
xanthine
Rf value: 0.60 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 606 [M+H]+
(163) 1-[(8-phenyl-quinoxalin-6-yl)methyl]-3-methyl-7-(2-butyn-l -yl)-8-[(R)-3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
CA 02496249 2005-02-18
83
Rf value: 0.48 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 356 [M+H]+
(164) 1-[([1,5]naphthyridin-4-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-[(R)-3-
(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Rf value: 0.25 (silica gel, ethyl acetate/petroleum ether = 4:1)
Mass spectrum (ESI+): m/z = 559 [M+H]+
(165) 1-((E)-3-pentafluorophenyl-allyl)-3-methyl-7-(2-butyn-l-yl)-8-[(R)-3-
(tert.-
butyloxycarbonylami no)- pi perid i n- 1 -yl]-xa nth i ne
Mass spectrum (ESI+): m/z = 623 [M+H]+
(166) 1-[(E)-3-(2-trifluoromethyl-phenyl)-allyl]-3-methyl-7-(2-butyn-l-yl)-8-
[(R)-
3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 601 [M+H]+
(167) 1-[(E)-3-(3-trifluoromethyl-phenyl)-allyl]-3-methyl-7-(2-butyn-1-yl)-8-
[(R)-
3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 601 [M+H]+
(168) 1-[(E)-3-(4-trifluoromethyl-phenyl)-aIIyI]-3-methyl-7-(2-butyn-1-yl)-8-
[(R)-
3-(tert.-butyloxycarbonylam ino)-piperidin-l-yl]-xanthine
Mass spectrum (ESI+): m/z = 601 [M+H]+
(169) 1-[(3-trifluoromethyl-isoquinolin-l-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-
8-
[(R)-3-(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Rf value: 0.68 (silica gel, cyclohexane/ethyl acetate = 3:7)
Mass spectrum (ESI+): m/z = 626 [M+H]+
(170) 1-[(3-methyl-isoquinolin-l-yl)methyl]-3-methyl-7-(2-cyano-benzyl)-8-
chloro-xanthine
(171) 1-[(3-difluoromethyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-
8-
[(R)-3-(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
CA 02496249 2005-02-18
84
Rf value: 0.38 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 608 [M+H]+
(172) 1-[(4-chloro-3-methoxy-isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-1-
yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Rf value: 0.65 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 622, 624 [M+H]+
(173) 1-[(4-ethoxy-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.25 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 603 [M+H]+
(174) 1-[(4-isopropyloxy-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-
[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.40 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 617 [M+H]+
(175) 1-[(2-methyl-benzothiazol-6-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.56 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 578 [M+H]+
(176) 1-[(3-phenyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-[(R)-3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.75 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 634 [M+H]+
(177) 1-[(4-phenyloxy-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.35 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 651 [M+H]+
CA 02496249 2005-02-18
(178) 1-[(4-phenyl-quinazolin-2-yl)methyl]-3-cyclopropyl-7-(2-butyn-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.45 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 661 [M+H]+
(179) 1-[(3-methyl-isoquinolin-l-yl)methyl]-3-cyclopropyl-7-(2-butyn-1-yl)-8-
[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.50 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 598 [M+H]+
(180) 1-[2-(3-difluoromethoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-
8-[(R)-3-(tert.-butyloxycarbonylami no)-piperidin-1-yl]-xanthine
Rf value: 0.77 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 601 [M+H]+
(181) 1-[(2-phenyl-quinazolin-4-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-
butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Rf value: 0.65 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 635 [M+H]+
(182) 1-[2-(2-methoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.57 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 565 [M+H]+
(183) 1-[2-(3-methoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidi n-1-yl]-xanthine
Rf value: 0.63 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 565 [M+H]+
(184) 1-[2-(3-trifluoromethoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-
8-[(R)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.64 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 619 [M+H]+
CA 02496249 2005-02-18
86
(185) 1-[2-(biphenyl-2-yl)-2-oxo-ethyl]-3-methyl -7-(2-butyn-1-yl)-8-[(R)-3-
(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.70 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 611 [M+H]+
(186) 1-[2-(biphenyl-3-yl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-[(R)-3-
(tert.-
butyloxycarbonylamino)-piperid in-l-yl]-xanthine
Rf value: 0.75 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 611 [M+H]+
(187) 1-[2-(3-isopropyloxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-l-yl)-8-
[(R)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.66 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 593 [M+H]+
(188) 1-[(3-methyl-isoquinolin-1-yl)methyl]-3-cyclopropyl-7-(2-butyn-1-yl)-8-
[(R)-3-(tert.-butyloxycarbonylam ino)-piperidin-1-yl]-xanthine
Rf value: 0.50 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 598 [M+H]+
(189) 1-[(4-phenyl-quinazolin-2-yl)methyl]-3-cyclopropyl-7-(2-butyn-l-yl)-8-
[(R)-3-(tert.-butyloxycarbonylam ino)-piperidin-l-yl]-xanthine
Rf value: 0.50 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 661 [M+H]+
(190) 1-[(4-cyano-naphthalen-l-yl)methyl]-3-cyclopropyl-7-(2-butyn-1-yl)-8-
[(R)-3-(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Rf value: 0.75 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 608 [M+H]+
(191) 1-[2-(2-phenyloxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-l -yl)-8-[(R)-
3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.85 (silica gel, methylene chloride/ethyl acetate = 1:1)
CA 02496249 2005-02-18
87
Mass spectrum (ESI+): m/z = 627 [M+H]+
(192) 1-[2-(3-ethoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-[(R)-3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.72 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 579 [M+H]+
(193) 1-[2-(3-methoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-[(R)-3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.67 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 565 [M+H]+
(194) 1-[2-(2-methoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-l-yl)-8-[(R)-3-
(tert.-butyloxycarbonylamino)-pi peridin-1-yl)-xanthine
Rf value: 0.57 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 565 [M+H]+
(195) 1-[(3-methyl-isoquinolin-l-yl)methyl]-3-methyl-7-(2-chloro-benzyl)-8-
bromo-xanthine
(196) 1-[(3-methyl-isoquinolin-l-yl)methyl]-3-methyl-7-(2-bromo-benzyl)-8-
[(R)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(197) 1-[(1,2,3,4-tetrahydro-phenanthridin-6-yl)methyl]-3-methyl-7-(2-butyn-1-
yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.55 (silica gel, ethyl acetate/ petroleum ether = 2:1)
Mass spectrum (ESI+): mlz = 612 [M+H]+
Example VI
1-(2-{3-[(methanesulphi nyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-
methvl-2-buten-1-yl)-8-[3-(tert.-butyloxyca rbonvlami no)-piperid in-1-yll-
xanthine
CA 02496249 2005-02-18
88
To a solution of 402 mg of 1-(2-{3-[(methylsulphanyl)methoxy]-phenyl}-2-oxo-
ethyl)-3-methyl-7-(3-methyl-2-buten-l-yl)-8-[3-(tert.-butyloxycarbonylamino)-
piperidin-1-yl]-xanthine in 10 ml hexafluoroisopropanol are added 0.15 ml of a
35 % hydrogen peroxide solution. The reaction mixture is stirred for half an
hour at ambient temperature.Then 5 ml of a 10 % sodium thiosulphate
solution are added. The aqueous phase is extracted twice with 5 ml of
methylene chloride. The combined extracts are dried over sodium sulphate
and evaporated down. The yellow residue is purified by chromatography
through a silica gel column with cyclohexane/ ethyl acetate/methanol (5:4:1)
as eluant.
Yield: 299 mg (73 % of theory)
Rf value: 0.28 (silica gel, cyclohexane/ethyl acetate/methanol = 5:4:1)
Mass spectrum (ESI+): m/z = 643 [M+H]+
The following compounds are obtained analogously to Example VI:
(1) 1-[2-(3-methanesulphinyl-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-
buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.05 (silica gel, ethyl acetate/cyclohexane = 3:1)
Mass spectrum (ESI+): m/z = 613 [M+H]+
(2) 1-(2-{2-[(methanesu[phi nyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(2-
butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 627 [M+H]+
Example VII
3-[(2-trimethylsilanyl-ethoxy)methyl]-7-(3-methyl-2-buten-l-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yll-xanthine
236 pl of 1,8-diazabicyclo[5.4.0]undec-7-ene are added dropwise to 630 mg of
7-(3-methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-
xanthine in 11 ml of acetonitrile. The solution is stirred for two hours at
ambient temperature, then the acetonitrile is distilled off in vacuo. The
flask
residue is taken up in 11 ml of N,N-dimethylformamide and combined with
258 mg of (2-trimethylsilanyl-ethoxy)methyl chloride. The reaction mixture is
CA 02496249 2005-02-18
89
stirred for three hours at 120 C. For working up water is added, the
precipitate formed is filtered off and taken up in ethyl acetate. The solution
is
dried over magnesium sulphate, evaporated down and chromatographed
through a silica gel column with cyclohexane/ethyl acetate/methanol (6:1:0 to
0:5:1) as eluant.
Yield: 435 mg (53 % of theory)
Mass spectrum (ESI+): m/z = 549 [M+H]+
The following compounds are obtained analogously to Example VII:
(1) 3-[(2-tri methylsi lanyl-ethoxy) m ethyl]-7-(2-cyano- benzyl)-xa nth i ne
Mass spectrum (ESI"): m/z = 396 [M-H]-
(2) 3-[(methoxycarbonyl)methyl]-7-(3-methyl-2-buten-l-yl)-8-[3-(tert.-butyloxy-
carbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.31 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 491 [M+H]+
Example VIII
7-(3-methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylami no)-piperidin-1-yll-
xanthine
510 mg of potassium-tert. butoxide are added to 2.32 g of 2-[3-(tert.-
butyloxycarbonylami no)-piperidin-1-yl]-3-(3-methyl-2-buten-1-yl)-4-
ethoxycarbonyl-5-{[(ethoxycarbonylamino)carbonyl]amino}-3H-imidazole in 35
ml of ethanol. The yellow solution is refluxed for five hours. After cooling
to
ambient temperature it is diluted with methylene chloride. The organic phase
is washed with saturated ammonium chloride solution and saturated sodium
chloride solution, dried over magnesium sulphate and evaporated down. The
crude product is purified by chromatography through a silica gel column with
methylene chloride/methanol/conc. methanolic ammonia (95:5:1 to 90:10:1)
as eluant.
Yield: 630 mg (35 % of theory)
CA 02496249 2005-02-18
Rf value: 0.24 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 419 [M+H]+
Example IX
2-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-3-(3-methyl-2-buten-1-yl)-4-
ethoxycarbonyl-5-{f(ethoxycarbonylamino)carbonyllamino}-3H-imidazole
2.97 ml of ethyl isocanatoformate are added to 4.00 g of 2-[3-(tert.-
butyloxycarbonylami no)-piperidin-1-yl]-3-(3-methyl-2-buten-1-yl)-4-
ethoxycarbonyl-5-amino-3H-imidazole in 90 ml of 1,2-dimethoxyethane and
the light brown solution is heated overnight at 120 C in an oil bath. Then a
further 0.6 ml of ethyl isocyanatoformate is added and heating is continued
for
a further four hours. For working up the reaction mixture is combined with
saturated potassium carbonate solution and extracted with ethyl acetate. The
organic phase is dried over magnesium sulphate, evaporated down and
purified through a silica gel column with methylene chloride/methanol/conc.
methanolic ammonia (98:2:1 to 90:10:1) as eluant.
Yield: 2.27 g (45 % of theory)
Rf value: 0.29 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 537 [M+H]+
Example X
2-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-3-(3-methyl-2-buten-1-yl)-4-
ethoxycarbonvl-5-amino-3H-imidazole
Prepared by refluxing cyanimino-[N-(3-methyl-2-buten-1-yl)-N-(ethoxy-
carbonylmethyl)-amino]-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-
methane with sodium in ethanol.
Rf value: 0.26 (aluminium oxide, ethyl acetate/petroleum ether = 8:2)
Mass spectrum (ESI+): m/z = 422 [M+H]+
Example XI
Cyanimino-[N-(3-methyl-2-buten-1-yl)-N-(ethoxycarbonylmethyl)-amino]-[3-
(tert.-butyloxycarbonylamino)-piperidin-1-vll-methane
CA 02496249 2005-02-18
91
Prepared by reacting cyanimino-[N-(3-methyl-2-buten-1-yl)-N-(ethoxy-
carbonylmethyl)-amino]-phenyloxy-methane with 3-(tert.-
butyloxycarbonylamino)-piperidine in the presence of potassium carbonate in
N,N-dimethylformamide at ambient temperature.
Rf value: 0.10 (silica gel, petroleum ether/ethyl acetate = 6:4)
Mass spectrum (ESI+): m/z = 422 [M+H]+
Example XII
cyan imino-[N-(3-methyl-2-buten-1-yl)-N-(ethoxycarbonylmethyl)-amino]-
phenyloxy-methane
Prepared by reacting cyanimino-[(ethoxycarbonylmethyl)amino]-phenyloxy-
methane with 1-bromo-3-methyl-2-butene in the presence of potassium
carbonate in acetone at ambient temperature.
Rf value: 0.70 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 316 [M+H]+
Example XIII
cyanimino-t(ethoxycarbonylmethyl)ami nol-phenyloxy-methan
Prepared by reacting diphenylcyanocarbonimidate with ethyl aminoacetate-
hydrochloride in the presence of triethylamine in isopropanol at ambient
temperature (analogously to R. Besse et al., Tetrahedron 1990, 46, 7803-
7812).
Rf value: 0.73 (silica gel, petroleum ether/ethyl acetate = 8:2)
Mass spectrum (ESI+): m/z = 248 [M+H]+
Example XIV
1-methyl-3-[(methoxycarbonyl)methyll-7-(2-cyano-benzvl)-8-chloro-xanthine
Prepared by reacting 1 -methyl-7-(2-cya n o-benzyl)-8-ch lo ro-xa nth i ne
with
methyl bromoacetate in the presence of potassium carbonate in N,N-
dimethylformamide at ambient temperature.
CA 02496249 2005-02-18
92
Rf value: 0.80 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 388, 390 [M+H]+
The following compounds are obtained analogously to Example XIV:
(1) 1-methyl-3-cyanomethyl-7-(2-cyano-benzyl)-8-chloro-xanthine
Mass spectrum (ESI+): mlz = 355, 357 [M+H]+
(2) 1-methyl-3-(2-propyn-1-yl)-7-(2-cyano-benzyl)-8-chloro-xanthine
Rf value: 0.80 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 354, 356 [M+H]+
(3) 1 -methyl-3-(2- propen- 1 -yl)-7-(2-cya no-benzyl)-8-ch loro-xa nth i ne
Rf value: 0.90 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 356, 358 [M+H]+
(4) 1-(2-phenyl-2-oxo-ethyl)-3-cyanomethyl-7-(3-methyl-2-buten-1-yl)-8-[3-
(tert.-butyloxycarbonylami no)-piperidin-1-yl]-xanthine
Rf value: 0.78 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 576 [M+H]+
(5) 1-methyl-3-isopropyl-7-(2-cyano-benzyl)-8-chloro-xanthine
Mass spectrum (ESI+): m/z = 358, 360 [M+H]+
Example XV
1-methyl-7-(2-cyano-benzyl)-8-chloro-xanthine
Prepared by treating 1-methyl-3-[(2-trimethylsilanyl-ethoxy)methyl]-7-(2-
cyano-benzyl)-8-chloro-xanthine with trifluoroacetic acid in methylene
chloride
at ambient temperature.
Rf value: 0.50 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 316, 318 [M+H]+
CA 02496249 2005-02-18
93
The following compounds are obtained analogously to Example XV:
(1) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-8-bromo-xanthine
Rf value: 0.26 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI"): m/z = 361, 363 [M-H]-
(2) 1-[(4-oxo-3,4-dihydro-phthalazin-l-yl)methyl]-3-methyl-7-(3-methyl-2-
buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine
(As the compound still contains impurities which cannot be removed by
chromatography, the material is again converted into the BOC-protected
derivative and then purified by chromatography, cf. Ex. XXV(1).)
Mass spectrum (ESI+): m/z = 491 [M+H]+
Example XVI
1-methyl-3-f(2-trimethylsilanyl-ethoxy)methyll-7-(2-cvano-benzyl)-8-chloro-
xanthine
Prepared by chlorination of 1-methyl-3-[(2-trimethyl siIanyl-ethoxy)methyl]-7-
(2-cyano-benzyl)-xanthine with N-chlorosuccinimide in dichloroethane while
refluxing.
Mass spectrum (El): m/z = 445, 447 [M]+
Example XVII
7-(2-cvano-benzyl)-xanthine
Prepared by treating 16.68 g of 2-amino-7-(2-cyano-benzyl)-1,7-dihydro-purin-
6-one with 17.00 g of sodium nitrite in a mixture of 375 ml of conc. acetic
acid,
84 ml of water and 5.2 ml of conc. hydrochloric acid at 50 C.
Yield: 8.46 g (50 % of theory)
Mass spectrum (ESI+): m/z = 268 [M+H]+
Example XVIII
2-amino-7-(2-cvano-benzyl)-1,7-dihydro-purin-6-one
Prepared by reacting 20.00 g of guanosine-hydrate with 22.54 g of 2-cyano-
benzylbromide in dimethylsulphoxide at 60 C and subsequent treatment with
57 ml of conc. hydrochloric acid.
CA 02496249 2005-02-18
94
Yield: 18.00 g (97% of theory)
Mass spectrum (ESI+): m/z = 267 [M+H]+
Example XIX
1 -{2-[3-(2-oxo-imidazolidin-1-yl)-phenyl]-2-oxo-ethyl}-3-methyl-7-(3-methyl-2-
buten-1-vl)-8-f3-(tert.-butyloxycarbonylamino)-piperidin-1-vll-xanthine
Prepared by treating 1-[2-(3-{[(2-chloro-ethylamino)carbonyl]amino}-phenyl)-
2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-[3-(tert.-butyloxy-
carbonylamino)-piperidin-1-yl]-xanthine with potassium-tert. butoxide in N,N-
dimethylformamide at ambient temperature.
Rf value: 0.50 (silica gel, methylene chloride/methanol = 9:1)
Example XX
1-[2-(3-{[(2-chloro-ethylamino)carbonyl]amino}-phenyl)-2-oxo-ethyl]-3-methyl-
7-(3-methyl-2-buten-1-vl)-8-f3-(tert.-butyloxvcarbonylamino)-piperidin-l-vll-
xanthine
Prepared by reacting 221 mg of 1-[2-(3-amino-phenyl)-2-oxo-ethyl]-3-methyl-
7-(3-methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-
xanthine with 60 pl of 2-chloroethyl isocyanate in 3 ml methylene chloride at
ambient temperature.
Yield: 163 mg (64 % of theory)
Rf value: 0.20 (silica gel, cyclohexane/ethyl acetate/methanol = 6:3:1)
Mass spectrum (ESI+): m/z = 671, 673 [M+H]+
The following compounds are obtained analogously to Example XX:
(1) 1-[2-(2-{[(ethoxycarbonylamino)carbonyl]amino}-phenyl)-2-oxo-ethyl]-3-
methyl-7-(3-methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-
1-yl]-xanthine
(Carried out in N,N-dimethylformamide at 30 C)
Rf value: 0.26 (silica gel, cyclohexane/ethyl acetate = 4:6)
Mass spectrum (ESI+): m/z = 681 [M+H]+
Example XXI
CA 02496249 2005-02-18
1-[2-(3-am i no-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-[3_
(tert.-butvloxycarbonvlamino)-piperidin-1-vll-xanthine
Prepared by treating 1-[2-(3-nitro-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-
buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine with
iron
powder in a mixture of ethanol, water and glacial acetic acid (80:25:10) at
100 C.
Rf value: 0.55 (silica gel, cyclohexane/ethyl acetate/methanol/conc. aqueous
ammonia = 50:30:20:1)
Mass spectrum (ESI+): m/z = 566 [M+H]+
The following compounds are obtained analogously to Example XXI:
(1) 1-[2-(2-amino-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-
[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 566 [M+H]+
(2) 1-[2-(2-amino-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-
[(S)-3-(tert.-butyloxycarbonylami no)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 566 [M+H]+
(3) 1-[(5-amino-isoquinolin-1-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-
[3-(tert.-butyloxycarbonyl ami no)-piperidin-1-yl]-xanthine
Rf value: 0.22 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 589 [M+H]+
(4) 1-[2-(2-amino-phenyl)-2-oxo-ethyl]-3-methyl-7-[(1-cyclopenten-1-
yl)methyl]-8-bromo-xanthine
Mass spectrum (ESI+): m/z = 458, 460 [M+H]+
(5) 1-[2-(2-amino-phenyl)-2-oxo-ethyl]-3-methyl-7-((E)-2-buten-l-yl)-8-bromo-
xanthine
(product contains approx. 10 % of Z isomer)
Rf value: 0.55 (silica gel, cyclohexane/ethyl acetate = 4:6)
Mass spectrum (ESI+): m/z = 432, 434 [M+H]+
CA 02496249 2005-02-18
96
(6) 1-[2-(2-amino-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-bromo-
xanthine
Rf value: 0.50 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 430, 432 [M+H]+
(7) 1-[2-(2-amino-phenyl)-2-oxo-ethyl]-3-methyl-7-((E)-2-buten-l-yl)-8-[(R)-3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 552 [M+H]+
(8) 1-[2-(2-amino-phenyl)-2-oxo-ethyl]-3-methyl-7-((E)-2-buten-1-yl)-8-[(S)-3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 552 [M+H]+
(9) 1-[2-(2-amino-3-methoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-
buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.82 (silica gel, ethyl acetate/petroleum ether = 4:1)
Mass spectrum (ESI+): m/z = 596 [M+H]+
Example XXII
1-(2-{2-[(ethylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-methyl-2-
buten-1-yl)-8-[3-(tert.-butyloxycarbonylam ino)-Piperid in-1-yll-xanthine
Prepared by reacting 248 mg of 1-[2-(2-amino-phenyl)-2-oxo-ethyl]-3-methyl-
7-(3-methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-
xanthine with 40 pi of propionic acid chloride in the presence of 60 pl of
pyridine in N,N-dimethylformamide at 80 C.
Yield: 168 mg (62 % of theory)
Rf value: 0.55 (silica gel, cyclohexane/ethyl acetate = 3:7)
Mass spectrum (ESI+): m/z = 622 [M+H]+
The following compounds are obtained analogously to Example XXII:
CA 02496249 2005-02-18
97
(1) 1-({5-[(methoxycarbonyl)methylamino]-isoquinolin-1-yl}methyl)-3-methyl-7-
(3-methyl-2-buten-l-yl)-8-[3-(tert.-butyloxycarbonylami no)-piperidin-l-yl]-
xanthine
(Carried out with methyl bromoacetate and potassium carbonate)
Rf value: 0.42 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 661 [M+H]+
(2) 1-[2-(2-acetylamino-phenyl)-2-oxo-ethyl]-3-methyl-7-((E)-2-buten-1-yl)-8-
[3-(tert.-butyloxycarbonylami no)-piperidin-1-yl]-xanthine
(product contains approx. 10 % of Z isomer)
Mass spectrum (ESI+): m/z = 594 [M+H]+
(3) 1-(2-{2-[(isopropylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-((E)-2-
buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(product contains approx. 10 % of Z isomer)
Mass spectrum (ESI+): m/z = 622 [M+H]+
(4) 1-[2-(2-acetylamino-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-
yl)-8-[(S)- 3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.50 (silica gel, cyclohexane/ethyl acetate = 3:7)
Mass spectrum (ESI+): m/z = 608 [M+H]+
(5) 1-[2-(2-acetylamino-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-l-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.34 (silica gel, cyclohexane/ethyl acetate = 3:7)
Mass spectrum (ESI+): m/z = 592 [M+H]+
(6) 1-(2-{2-[(isopropylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-
xanthine
Rf value: 0.25 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 636 [M+H]+
CA 02496249 2005-02-18
98
(7) 1-(2-{2-[(isopropylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-(2-
butyn-1-yl)-8-[3-(tert.-butyloxycarbonylami no)-piperidin-1-yl]-xanthine
Rf value: 0.44 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 620 [M+H]+
(8) 1-[2-(2-acetylamino-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-l-yl)-8-[(S)-
3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.34 (silica gel, cyclohexane/ethyl acetate = 3:7)
Mass spectrum (ESI+): m/z = 592 [M+H]+
(9) 1-(2-{2-[(isopropylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-(2-
butyn-1-yl)-8-[(S)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.44 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 620 [M+H]+
(10) 1-(2-{2-[(methoxycarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin- l -yl]-
xanthine
(Carried out in acetonitrile at 55 C)
Rf value: 0.25 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 624 [M+H]+
(11) 1-(2-{2-[(isopropylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-((E)-
2-buten-1-yl)-8-[(S)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(Carried out in acetonitrile at 65 C)
Rf value: 0.50 (silica gel, cyclohexane/ethyl acetate/isopropanol = 14:3:3)
Mass spectrum (ESI+): m/z = 622 [M+H]+
(12) 1-(2-{2-[(ethylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-((E)-2-
buten-1-yl)-8-[(R)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 608 [M+H]+
(13) 1-[2-(2-acetylamino-phenyl)-2-oxo-ethyl]-3-methyl-7-((E)-2-buten-1-yl)-8-
[(R)-3-(tert.-butyloxycarbonylamino)-piperid i n-1-yl]-xanthine
CA 02496249 2005-02-18
99
Mass spectrum (ESI+): m/z = 594 [M+H]+
(14) 1-[2-(2-acetyl amino-phenyl)-2-oxo-ethyl]-3-methyl-7-((E)-2-buten-1-yl)-8-
[(S)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.28 (silica gel, cyclohexane/ethyl acetate/isopropanol = 8:1:1)
Mass spectrum (ESI+): mlz = 594 [M+H]+
(15) 1-(2-{2-[(isopropylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-(2-
butyn-1-yl)-8-[(R)-3-(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Rf value: 0.90 (silica gel, methylene chloride/methanol = 9:1)
(16) 1-(2-{2-[(isopropylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-((E)-
2-buten-1-yl)-8-[(R)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl])-xanthine
(Carried out in 1,2-dichloroethane at 45 C)
Rf value: 0.30 (silica gel, cyclohexane/ethyl acetate/isopropanol = 8:1:1)
Mass spectrum (ESI+): m/z = 622 [M+H]+
(17) 1-(2-{2-[(ethylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-((E)-2-
buten-1-yl)-8-[(S)-3-(tert.-butyloxycarbonylamino)-pi peridi n-1-yl]-xanthine
Rf value: 0.48 (silica gel, cyclohexane/ethyl acetate/isopropanol = 14:3:3)
Mass spectrum (ESI+): m/z = 608 [M+H]+
(18) 1-(2-{2-[(ethylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-(2-butyn-
1-yl)-8-[(R)-3-(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Mass spectrum (ESI+): m/z = 606 [M+H]+
(19) 1-(2-{2-[(ethylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-(2-butyn-
1-yl)-8-[(S)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.22 (silica gel, methylene chloride/methanol = 95:5)
(20) 1-(2-{2-[(phenylcarbonyl)aminoj-phenyl}-2-oxo-ethyl)-3-methyl-7-((E)-2-
buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.55 (silica gel, cyclohexane/ethyl acetate/isopropanol = 14:3:3)
Mass spectrum (ESI+): m/z = 656 [M+H]+
CA 02496249 2005-02-18
100
(21) 1-(2-{2-[(cyclopropylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-[(1-
cyclopenten-1-yl)methyl]-8-[3-(tert.-butyloxycarbonylamino)-piperidin-l-yl]-
xanthine
(Carried out with Hunig base and 4-dimethylamino-pyridine in methylene
chloride)
Rf value: 0.60 (silica gel, methylene chloride/methanol = 18:1)
Example XXI II
1-(2-{3-[(methoxycarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-
methyl-2- buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine
Prepared by treating 1-(2-{3-[(methoxycarbonyl)methoxy]-phenyl}-2-oxo-
ethyl)-3-methyl-7-(3-methyl-2-buten-1-yl)-8-j3-(tert.-butyloxycarbonylamino)-
piperidin-1-yl]-xanthine with trifluoroacetic acid in methylene chloride at
ambient temperature.
Mass spectrum (ESI+): m/z = 539 [M+H]+
The following compounds are obtained analogously to Example XXIII:
(1) 1-(2-{2-[(methoxycarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-(3-amino-piperidin-l-yl)-xanthine
Mass spectrum (ESI+): m/z = 539 [M+H]+
Example XXIV
1 -methyl-3-phenyl-7-(2-cyano-benzyl)-8-chloro-xanthine
A mixture of 829 mg of 1-methyl-7-(2-cyano-benzyl)-8-chloro-xanthine, 640
mg of phenylboric acid, 509 mg of anhydrous copper acetate and 0.43 ml of
pyridine in 20 ml methylene chloride is stirred for four days at ambient
temperature in the presence of 100 mg of 4A molecular sieves.Then another
320 mg of phenylboric acid are added and the reaction mixture is stirred for
another day at ambient temperature. For working up the mixture is filtered
through talc and washed with ethyl acetate. The filtrate is evaporated down
and chromatographed through a silica gel column with cyclohexane/ethyl
acetate (7:3 to 1:1) as eluant.
CA 02496249 2005-02-18
101
Yield: 142 mg (14 % of theory)
Mass spectrum (ESI+): m/z = 392, 394 [M+H]+
Example XXV
1-(2-phenyl-2-oxo-ethyl)-7-(3-methyl-2-buten-1-yl)-8-[3-(tert.-
butyloxycarbonyl-amino)-piperidin-1-vll-xanthine
Prepared by reacting 1-(2-phenyl-2-oxo-ethyl)-7-(3-methyl-2-buten-l-yl)-8-(3-
amino-piperidin-1-yl)-xanthine with di-tert.butyl pyrocarbonate in the
presence
of HUnig base in methylene chloride at ambient temperature.
Rf value: 0.27 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
The following compounds are obtained analogously to Example XXV:
(1) 1-[(4-oxo-3,4-dihydro-phthalazin-1-yl)methyl]-3-methyl-7-(3-methyl-2-
buten-1-yl)-8-[3-(tert.-butyloxycarbonyl-amino)-piperidin-1-yl]-xanthine
Rf value: 0.27 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 591 [M+H]+
(2) 7-acetyl-1-(tert.-butyloxycarbonyl)-1H-indole
Rf value: 0.82 (silica gel, methylene chloride/petroleum ether/ethyl acetate=
5:4:1)
Mass spectrum (ESI+): m/z = 260 [M+H]+
Example XXVI
1-[(cinnolin-4-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-chloro-xanthine
and 1-1(1,4-dihydro-cinnolin-4-vl)methyll-3-methyl-7-(3-methyl-2-buten-l-vl)-8-
chloro-xanthine
510 mg of a mixture of (cinnolin-4-yl)-methanol and (1,4-dihydro-cinnolin-4-
yl)-
methanol (see Ex. XXVII) are added to 830 mg of 3-methyl-7-(3-methyl-2-
buten-l-yl)-8-chloro-xanthine and 1.25 g of triphenylphosphine in 25 ml of
tetrahydrofuran. The reaction mixture is combined with 0.92 ml diethyl
azodicarboxylate and stirred overnight at ambient temperature.Then it is
evaporated down and chromatographed through a silica gel column with ethyl
CA 02496249 2005-02-18
102
acetate/petroleum ether (7:3 to 0:1) as eluant. A mixture of cinnoline and 1,4-
dihydro-cinnoline compound is obtained.
Yield: 660 mg (52 % of theory)
Rf value: 0.60 (silica gel, ethyl acetate/petroleum ether = 7:3)
The following compounds are obtained analogously to Example XXVI:
(1) 1-({4-oxo-3-[(2-trimethylsilanyl-ethoxy)methyl]-3,4-dihydro-phthalazin-1-
yl}methyl)-3-methyl-7-(3-methyl-2-buten-1-yl)-8-chloro-xanthine
Rf value: 0.85 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 557, 559 [M+H]+
(2) 1-[(isoquinolin-3-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-chloro-
xanthine
Melting point: 194-195 C
Mass spectrum (ESI+): m/z = 410, 412 [M+H]+
(3) 1-[(3-methyl -4-oxo-3,4-dihydro-phthalazin-1-yl)methyl]-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-chloro-xanthine
Rf value: 0.66 (silica gel, ethyl acetate)
Mass spectrum (ESI`): m/z = 441, 443 [M+H]+
(4) 1 -[(q u ino I in-4-yl)methyl]-3-methyl-7-(2-buty n- 1 -yl)-8-bromo-xa nth
i ne
(Carried out with potassium carbonate)
Rf value: 0.45 (silica gel, ethyl acetate)
Mass spectrum (ESI+): mlz = 438, 440 [M+H]+
(5) 1-[(isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-bromo-xanthine
Rf value: 0.78 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 438, 440 [M+H]+
(6) 1-[(4-dimethyl amino-naphtha len-1-yl)methyl]-3-methyl-7-(2-butyn-1-yI)-8-
[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.80 (silica gel, ethyl acetate)
CA 02496249 2005-02-18
103
Mass spectrum (ESI+): m/z = 600 [M+H]+
(7) 1-[(isoquinolin-1-yl)methyl]-3-methyl-7-((E)-2-buten-1-yl)-8-bromo-
xanthine
(The product contains approx. 20 % of Z isomer)
Rf value: 0.71 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 440, 442 [M+H]+
(8) 1-[(1-methyl-1 H-indol-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-bromo-
xanthine
Rf value: 0.95 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 440, 442 [M+H]+
(9) 1-[(quinolin-3-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-bromo-xanthine
Rf value: 0.55 (silica gel, ethyl acetate/petroleum ether = 8:2)
Mass spectrum (ESI+): m/z = 438, 440 [M+H]+
(10) 1-{[1-(tert.-butyloxycarbonylamino)-1H-indol-2-yl]methyl}-3-methyl-7-(2-
butyn-l-yl)-8-bromo-xanthine
Rf value: 0.74 (silica gel, petroleum ether/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 526, 528 [M+H]+
(11) 1-({2-methyl-1-[(2-trimethyl silanyl-ethoxy)methyl]-1 H- benzoi m i dazol-
5-
yl}methyl)-3-methyl-7-(2- butyn- 1 -yl)-8- bro mo-xa nth i ne (mixed with 1-
({2-
methyl-3-[(2-trimethylsilanyl-ethoxy)methyl]-3H-benzoimidazol-5-yl}methyl)-3-
methyl-7-(2-butyn-l-yl)-8-bromo-xanthine)
Mass spectrum (ESI+): m/z = 571, 573 [M+H]+
(12) 1-[(1-[(2-trimethylsilanyl-ethoxy)methyl]-1 H-benzoimidazol-5-yl)methyl]-
3-
methyl-7-(2-butyn-1-yl)-8-bromo-xanthine (mixed with 1-[(3-[(2-trimethylsila
nyl-
ethoxy)methyl]-3H-benzoimidazol-5-yl) methyl]-3-methyl-7-(2-butyn-1-yl)-8-
bromo-xanthine)
Rf value: 0.50 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 557, 559 [M+H]+
CA 02496249 2005-02-18
104
(13) 1-[(pyrazolo[1,5-a]pyridin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-bromo-
xanthine
Rf value: 0.35 (silica gel, petroleum ether/ethyl acetate = 1:2)
Mass spectrum (ESI+): m/z = 427, 429 [M+H]+
Example XXVII
(cinnolin-4-vl)-methanol and (1,4-dihydro-cinnolin-4-vl)-methanol
A solution of 1.00 g of methyl cinnolin-4-carboxylate in 15 ml diethyl ether
is
added dropwise at 0 C to a suspension of 222 mg of lithium aluminium
hydride in 5 ml of diethyl ether. After 1.5 hours water is carefully added
dropwise to the reaction mixture, this is stirred with methylene chloride and
suction filtered through a glass fibre filter. The aqueous phase is extracted
with methylene chloride and the combined organic phases are dried over
magnesium sulphate and evaporated down. According to 'H-NMR a mixture
of cinnoline and 1,4-dihydro-cinnoline compound is obtained as a yellow oil
which is reacted further without any more purification.
Yield: 530 mg (62 % of theory)
Rf value: 0.63 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 161 [M1+H]+ and 163 [M2+H]+
The following compounds are obtained analogously to Example XXVII:
(1) {2-methyl-1-[(2-trimethylsilanyl-ethoxy)methyl]-1 H-benzoimidazol-5-yl}-
methanol
(mixed with {2-methyl-3-[(2-trimethylsilanyl-ethoxy)methyl]-3H-benzoimidazol-
5-yi}-methanol)
Mass spectrum (ESI+): m/z = 293 [M+H]+
(2) (2,3,8-trimethyl-quinoxalin-6-yl)-methanol
Rf value: 0.45 (silica gel, petroleum ether/ethyl acetate = 1:2)
Mass spectrum (ESI+): m/z = 203 [M+H]+
(3) (8-methyl-quinoxalin-6-yl)-methanol
Rf value: 0.18 (silica gel, cyclohexane/ethyl acetate = 1:1)
CA 02496249 2005-02-18
105
Mass spectrum (ESI+): m/z = 175 [M+H]+
(4) (E)-3-pentafluorophenyl-2-propen-1-ol
(Carried out with diisobutylaluminium hydride in toluene)
Mass spectrum (El): m/z = 224 [M]+
(5) (E)-3-(2-trifl uoromethyl-phenyl)-2- pro pen- 1 -ol
(Carried out with diisobutylaluminium hydride in toluene)
(6) (E)-3-(3-trifluoromethyl-phenyl)-2-propen-1-ol
(Carried out with diisobutylaluminium hydride in toluene)
Mass spectrum (El): m/z = 202 [M]+
(7) (E)-3-(4-trifluoromethyl-phenyl)-2-propen-1-ol
(Carried out with diisobutylaluminium hydride in toluene)
Example XXVII I
4-hvdroxvmethvl-2-f(2-trimethylsilanyl-ethoxy)methyll-2H-phthalazin-l-one
Prepared by treating methyl 4-oxo-3-[(2-trimethylsilanyl-ethoxy)methyl]-3,4-
dihydro-phthalazin-1-carboxylate with sodium borohydride in tetrahydrofuran
at 40 C.
Rfvalue: 0.55 (silica gel, cyclohexane/ethyl acetate 1:1)
Mass spectrum (ESI+): m/z = 307 [M+H]+
The following compounds are obtained analogously to Example XXVIII:
(1) (3,4-dimethyl-isoquinolin-1-yl)-methanol
(Carried out with lithium borohydride in tetrahydrofuran)
Rf value: 0.35 (silica gel, petroleum ether/ethyl acetate = 2:1)
Mass spectrum (ESI+): m/z = 188 [M+H]+
(2) (3-methyl-imidazo[1,2-a]pyridin-2-yl)-methanol
(Carried out with lithium borohydride in tetrahydrofuran)
CA 02496249 2005-02-18
106
Rf value: 0.48 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 163 [M+H]+
(3) (3,4-dimethyl-6,7-dihydro-5H-[2]pyrindin-1-yl)-methanol
(Carried out with lithium borohydride in tetrahydrofuran)
Rf value: 0.40 (aluminium oxide, petroleum ether/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 178 [M+H]+
(4) (3,4-dimethyl-5,6,7,8-tetrahydro-isoquinolin-1-yl)-methanol
(Carried out with lithium borohydride in tetrahydrofuran)
Rf value: 0.45 (aluminium oxide, petroleum ether/ethyl acetate = 3:1)
Mass spectrum (ESI+): m/z = 192 [M+H]+
(5) 6-hydroxymethyl-1,2,3,4-tetrahydro-phenanthridine
(Carried out with lithium borohydride in tetrahydrofuran at ambient
temperature)
Rf value: 0.40 (silica gel, petroleum ether/ethyl acetate = 2:1)
Mass spectrum (ESI+): m/z = 214 [M+H]+
Example XXIX
Methyl 4-oxo-3-[(2-trimethylsilanyl-ethoxy)methyl]-3,4-dihydro-phthalazin-1-
carboxylate
Prepared by reacting methyl 4-oxo-3,4-dihydro-phthalazin-1-carboxylate with
(2-trimethyl silanyl-ethoxy)meth ylchIoride in the presence of Hunig base in
methylene chloride at ambient temperature.
Rf value: 0.75 (silica gel, cyclohexane/ethyl acetate 6:4)
Mass spectrum (ESI+): m/z = 335 [M+H]+
The following compounds are obtained analogously to Example XXIX:
(1) 7-acetyl-3-[(2-trimethylsilanyl-ethoxy)methyl]-3H-benzooxazol-2-one
Mass spectrum (ESI+): m/z = 308 [M+H]+
CA 02496249 2005-02-18
107
(2) 4-acetyl-3-[(2-trimethylsilanyl-ethoxy)methyl]-3H-benzooxazol-2-one
Rf value: 0.87 (silica gel, methylene chloride/methanol = 99:1)
(3) 4-acetyl-1,3-bis-[(2-trimethylsilanyl-ethoxy)methyl]-1,3-dihydro-
benzoimidazol-2-one
(Carried out with potassium-tert. butoxide in N,N-dimethylformamide)
Rf value: 0.90 (silica gel, petroleum ether/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 437 [M+H]+
(4) 6-methyl- 1-[(2-trimethylsilanyl-ethoxy)methyl]-1 H-quinolin-2-one
Rf value: 0.78 (silica gel, petroleum ether/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 290 [M+H]+
(5) methyl {2-methyl-1-[(2-trimethylsilanyl-ethoxy)methyl]-1 H-benzoimidazol-5-
yl}-carboxylate (mixed with methyl {2-methyl-3-[(2-trimethylsilanyl-
ethoxy)methyl]-3H-benzoimidazol-5-yl}-carboxylate)
Mass spectrum (ESI+): m/z = 321 [M+H]+
Example XXX
1-[2-(3-methanesulphonyl-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-
1-YI)-8-f 3-(tert.-butyloxycarbonylamino)-piperidin-1-vll-xanthi ne
0.22 ml of a 35 % hydrogen peroxide solution and 20 mg of sodium tungstate
are added to 500 mg of 1-[2-(3-methylsulphanyl-phenyl)-2-oxo-ethyl]-3-
methyl-7-(3-methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-
1-yl]-xanthine in 5 ml methylene chloride. The reaction mixture is stirred
overnight at ambient temperature, then 1 ml of methanol is added. After
another 48 hours a further 1.5 ml of 35 % hydrogen peroxide solution, a
spatula tip of sodium tungstate and two drops of water are added. The next
morning, the oxidation is complete according to thin layer chromatography
and the reaction mixture is diluted with 50 ml methylene chloride and washed
twice with 30 ml of 10 % sodium thiosulphate solution. The organic phase is
dried over magnesium sulphate and evaporated down, leaving a viscous resin
which is reacted further without any more purification.
Yield: 530 mg (100 % of theory)
CA 02496249 2005-02-18
108
Rf value: 0.72 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 629 [M+H]+
Example XXXI
1-[2-(3-carboxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-[3_
(tert.-butyloxycarbonvlamino)-piperidin-1-yll-xanthine
Prepared by treating 1-[2-(3-methoxycarbonyl-phenyl)-2-oxo-ethyl]-3-methyl-
7-(3-methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylami no)-piperidin-l -yl]-
xanthine with 3 M sodium hydroxide solution in methanol at ambient
temperature:
Rf value: 0.34 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 595 [M+H]+
The following compounds are obtained analogously to Example XXXI:
(1) 1-[2-(2-carboxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-
[3-(tert.-butyloxycarbonylami no)-piperidin-1-yi]-xanthine
Rf value: 0.49 (silica gel, methylene chloride/methanol = 9:1)
(2) 1-[2-(2-carboxymethoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-l-yl)-8-
[3-(tert.-butyloxycarbonylami no)-pi peridin-1-yl]-xanthine
(Carried out with 4 M potassiuim hydroxide solution in tetrahydrofuran)
Mass spectrum (ESI+): m/z = 609 [M+H]+
(3) 1-[2-(2-carboxymethylamino-phenyl)-2-oxo-ethyl]-3-methyl-7-((E)-2-buten-
1-yl)-8-[(R)-3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(Carried out with 4 M potassium hydroxide solution in tetrahydrofuran)
Rf value: 0.65 (silica gel, cyclohexane/ethyl acetate = 3:7)
Mass spectrum (ESI+): m/z = 610 [M+H]+
(4) 1-carboxymethyl-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Mass spectrum (ESI+): m/z = 475 [M+H]+
CA 02496249 2005-02-18
109
Example XXXII
1-{2-[3-(methylaminocarbonyl)-phenyl]-2-oxo-ethyl}-3-methyl-7-(3-methyl-2-
buten-1-vl)-8-[3-(tert.-butyloxycarbonvlamino)-piperidin-1-yll-xanthine
A mixture of 190 mg of 1-[2-(3-carboxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-[3-(tent.-butyloxycarbonylamino)-piperidin-1-yl]-
xanthine, 43 pl of a 40 % aqueous methylamine solution, 103 mg of 0-
(benzotriazol- 1 -yl)-N, N, N',N'-tetramethyl uronium tetrafluoroborate, 43 mg
of
N-hydroxybenzotriazole and 45 pl of triethylamine in 3 ml of tetrahydrofuran
is
stirred for eight hours at ambient temperature. For working up the reaction
mixture is diluted with ethyl acetate and washed with water, 10 % citric acid
solution, 10 % potassium carbonate solution and saturated sodium chloride
solution. The organic phase is evaporated down and chromatographed
through a silica gel column with methylene chloride/methanol (98:2 to 80:20)
as eluant.
Yield: 173 mg (89 % of theory)
Rf value: 0.30 (silica gel, methylene chloride/methanol = 20:1)
Mass spectrum (ESI+): m/z = 608 [M+H]+
The following compounds are obtained analogously to Example XXXII:
(1) 1-{2-[3-(dimethylaminocarbonyl)-phenyl]-2-oxo-ethyl}-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylami no)-piperidin-1-yl]-
xanthine
Rfvalue: 0.28 (silica gel, methylene chloride/methanol = 20:1)
Mass spectrum (ESI+): m/z = 622 [M+H]+
(2) 1-{2-[3-(morpholin-4-yl-carbonyl)-phenyl]-2-oxo-ethyl}-3-methyl-7-(3-
methyl-2-buten- l -yl)-8-[3-(tent.-butyloxycarbonylami no)-piperidin-1-yl]-
xanthine
Rf value: 0.30 (silica gel, methylene chloride/methanol = 20:1)
Mass spectrum (ESI+): m/z = 664 [M+H]+
CA 02496249 2005-02-18
110
(3) 1-{2-[2-(dim ethyl aminocarbonyl)-phenyl]-2-oxo-ethyl}-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-
xanthine
Rf value: 0.30 (silica gel, methylene chloride/methanol = 20:1)
Mass spectrum (ESI+): m/z = 622 [M+H]+
(4) 1-{2-[2-(morpholin-4-yl-carbonyl)-phenyl]-2-oxo-ethyl}-3-methyl-7-(3-
methyl-2-buten-l-yi)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-
xanthine
Rf value: 0.30 (silica gel, methylene chloride/methanol = 20:1)
Mass spectrum (ESI+): m/z = 664 [M+H]+
(5) 1-(2-{2-[(isopropylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-
7-(2-butyn-1-yl)-8-[3-(tert.-butyloxycarbonyl amino)-piperidin-1-yl]-xanthine
(Carried out with Hunig base in N,N-dimethylformamide)
Mass spectrum (ESI+): m/z = 650 [M+H]+
(6) 1-(2-{2-[(ethylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(2-
butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(Carried out with Honig base in N,N-dimethylformamide)
Mass spectrum (ESI+): m/z = 636 [M+H]+
(7) 1-(2-{2-[2-oxo-2-(pyrrolidin-1-yl)-ethoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
(2-butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(Carried out with Hunig base in N,N-dimethylformamide)
Mass spectrum (ESI+): m/z = 662 [M+H]+
(8) 1-(2-{2-[2-(morpholin-4-yl)-2-oxo-ethoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
(2-butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
(Carried out with Hunig base in N,N-dimethylformamide)
Mass spectrum (ESI+): m/z = 678 [M+H]+
CA 02496249 2005-02-18
111
(9) 1-(2-{2-[(methylaminocarbonyl)methylamino]-phenyl}-2-oxo-ethyl)-3-
methyl-7-((E)-2-buten-1-yl)-8-[(R)-3-(tert.-butyloxycarbonylamino)-piperidin-l
-
yl]-xanthine
Rf value: 0.40 (silica gel, cyclohexane/ethyl acetate/methanol = 5:4:1)
Mass spectrum (ESI+): m/z = 623 [M+H]+
(10) 1-[(2-amino-phenylaminocarbonyl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.40 (silica gel, cyclohexane/ethyl acetate/methanol = 5:4:1)
Mass spectrum (ESI+): m/z = 565 [M+H]+
Example XXXIII
1-chloromethyl-4-methyl-isoguinoline-hydrochloride
Prepared by treating (4-methyl-isoquinolin-1-yl)-methanol with thionyl
chloride
in methylene chloride.
Rf value: 0.76 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:0.1)
Mass spectrum (ESI+): m/z = 192, 194 [M+H]+
The following compounds are obtained analogously to Example XXXIII:
(1) 1-chloromethyl-3,4-dimethyl-isoquinoline-hydrochloride
Rf value: 0.65 (silica gel, petroleum ether/ethyl acetate = 2:1)
Mass spectrum (ESI+): mlz = 206, 208 [M+H]+
(2) 5-chloromethyl-8-methoxy-quinoline-hydrochloride
Mass spectrum (ESI+): m/z = 208, 210 [M+H]+
(3) 8-chloromethyl-5-methoxy-quinoline-hydrochloride
Mass spectrum (El): m/z = 207, 209 [M]+
(4) 2-chloromethyl-3-methyl-imidazo[I,2-a]pyridine-hydrochloride
Rf value: 0.55 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
CA 02496249 2005-02-18
112
Mass spectrum (ESI+): mlz = 181, 183 [M+H]+
(5) 8-chloromethyl-5-methoxy-isoquinoline-hydrochloride
Mass spectrum (ESI+): m/z = 208, 210 [M+H]+
(6) 1-chloromethyl-3,4-dimethyl-6,7-dihydro-5H-[2]pyridine-hydrochloride
Rf value: 0.50 (aluminium oxide, petroleum ether/ethyl acetate = 10:1)
Mass spectrum (ESI+): m/z = 196, 198 [M+H]+
(7) 1-chloromethyl-3,4-dimethyl-5,6,7,8-tetrahydro-isoquinoline-hydrochloride
Rf value: 0.50 (aluminium oxide, petroleum ether/ethyl acetate = 10:1)
Mass spectrum (ESI+): m/z = 210, 212 [M+H]+
(8) 6-chloromethyl-2,3,8-trimethyl-quinoxaline-hydrochloride
Mass spectrum (ESI+): m/z = 221, 223 [M+H]+
(9) 6-chloromethyl-8-methyl-quinoxaline-hydrochloride
Mass spectrum (ESI+): m/z = 193,195 [M+H]+
(10) 6-chloromethyl-1,2,3,4-tetrahydro-phenanth ridine-hydrochloride
Rf value: 0.50 (silica gel, petroleum ether/ethyl acetate = 5:1)
Mass spectrum (ESI+): m/z = 232, 234 [M+H]+
Example XXXIV
1-[(4-oxo-3,4-dihydro-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3_
(tert.-butyloxycarbonylamino)-piperidin-1-yll-xanthine
0.5 ml of a 1 M sodium methoxide solution in methanol is added dropwise to a
solution of 428 mg of 1-cyanomethyl-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine in 3 ml of methanol at
ambient temperature. After about 20 minutes the thick suspension formed is
heated gently in a water bath and diluted with 2 ml of methanol. As soon as
the reaction to form the iminoester is complete according to thin layer
chromatography, the reaction mixture is neutralised with 0.5 ml 1 M glacial
acetic acid solution in methanol and combined with a solution of 130 mg of
CA 02496249 2005-02-18
113
anthranilic acid in 2 ml of methanol. Gentle heating produces a clear
solution,
which is stirred for 2.5 hours at ambient temperature. Then the reaction
mixture is gently refluxed for about 3.5 hours. After standing overnight at
ambient temperature the methanol is distilled off and the residue is stirred
with
cold water, suction filtered and dried. The crude product is suspended in 5 ml
of methanol, gently heated and after cooling suction filtered, washed with
methanol and dried in the desiccator.
Yield: 302 mg (56 % of theory)
Rf value: 0.55 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 575 [M+H]+
The following compounds are obtained analogously to Example XXXV:
(1) (4-difluoromethoxy-naphthalen-1-yl)-methanol
Rf value: 0.33 (silica gel, cyclohexane/ethyl acetate = 6:4)
Mass spectrum (ESI"): m/z = 223 [M-H]-
Example XXXV
(4-dimethylamino-naphthalen-1-yl)-methanol
prepared by reduction of 4-dimethylamino-naphthalene-1-carbaldehyde with
sodium borohydride in aqueous tetrahydrofuran.
Rf value: 0.67 (silica gel, cyclohexane/ethyl acetate = 1:1)
Example XXXVI
2-bromo-1-(2,3-dihydro-benzo[1,41dioxin-5-vl)-ethanone
prepared by bromination of 1-(2,3-dihydro-benzo[1,4]dioxin-5-yl)-ethanone in
methylene chloride while cooling gently with an ice bath. The dibromo
compound formed as a by-product is separated off by column
chromatography.
Mass spectrum (ESI+): m/z = 257, 259 [M+H]+
Rf value: 0.92 (silica gel, methylene chloride)
The following compounds are obtained analogously to Example XXXVI:
CA 02496249 2005-02-18
114
(1) 7-(2-bromo-acetyl)-3-methyl-3H-benzooxazol-2-one
(bromination is carried out in dioxane at 40 C; the product is contaminated
with approx. 20 % dibromo compound)
Rf value: 0.44 (silica gel, petroleum ether/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 270, 272 [M+H]+
(2) 1-benzo[1,3]dioxol-4-yl-2-bromo-ethanone
Mass spectrum (ESI+): m/z = 243, 245 [M+H]+
Rf value: 0.94 (silica gel, methylene chloride)
(3) 2-[2-(2-bromo-acetyl)-phenoxy]-N-ethyl-acetamide
(bromination is carried out with copper(II)bromide in dioxane)
Mass spectrum (ESI+): m/z = 300, 302 [M+H]+
(4) 4-(2-bromo-acetyl)-3-methyl-3H-benzooxazol-2-one
Rfvalue: 0.67 (silica gel, methylene chloride/methanol = 99:1)
Mass spectrum (ESI+): m/z = 270, 272 [M+H]+
(5) 2-[2-(2-bromo-acetyl)-phenoxy]-N-methyl-acetamide
Mass spectrum (ESI+): m/z = 386, 388 [M+H]+
(6) 7-(2-bromo-acetyl)-3-[(2-trimethylsilanyl-ethoxy)methyl]-3H-benzooxazol-
2-one
Rfvalue: 0.84 (silica gel, methylene chloride/methanol = 99:1)
Mass spectrum (ESI+): m/z = 384, 386 [M+H]+
(7) 4-(2-bromo-acetyl)-1,3-dimethyl-1,3-dihydro-benzoimidazol-2-one
Rf value: 0.38 (silica gel, ethyl acetate/petroleum ether = 1:1)
Mass spectrum (ESI+): m/z = 283, 285 [M+H]+
(8) 4-(2-bromo-acetyl)-3-[(2-trimethylsilanyl-ethoxy)methyl]-3H-benzooxazol-
2-one
Rf value: 0.82 (silica gel, methylene chloride/methanol = 99:1)
CA 02496249 2005-02-18
115
(9) 4-(2-bromo-acetyl)-1-ethoxycarbonyl-3-methyl-l,3-dihydro-benzoimidazol-
2-one
Rf value: 0.39 (silica gel, petroleum ether/ethyl acetate = 2:1)
Mass spectrum (ESI+): m/z = 341, 343 [M+H]+
(10) 2-bromo-1-(2,2-difluoro-benzo[1,3]dioxol-4-yl)-ethanone
Mass spectrum (ESI"): m/z = 277, 279 [M-H]-
Example XXXVII
(2 3-dihydro-benzof 1,41dioxin-5-vl)-ethanone
Prepared by reacting 1-(2,3-dihydroxy-phenyl)-ethanone with 1,2-
dibromoethane in the presence of potassium carbonate in N,N-
dimethylformamide at 100 C.
Rf value: 0.43 (silica gel, ethyl acetate/petroleum ether = 1:4)
Mass spectrum (ESI+): m/z = 179 [M+H]+
Example XXXVIII
1-[(3-methyl-4-oxo-3,4-dihydro-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-
yl)-8-f3-(tert.-butyloxycarbonylami no)-piperidin-1-yll-xanthine
Prepared by reacting 1-[(4-oxo-3,4-dihydro-quinazolin-2-yl)methyl]-3-methyl-
7-(2-butyn-1-yi)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
with methyl iodide in the presence of potassium carbonate in N,N-
dimethylformamide at ambient temperature.
Rf value: 0.50 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 589 [M+H]+
The following compounds are obtained analogously to Example XXXVIII:
(1) 7-acetyl-3-methyl-3H-benzooxazol-2-one
(The methylation is carried out in the presence of sodium carbonate in
methanol)
Rf value: 0.46 (silica gel, petroleum ether/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 192 [M+H]+
CA 02496249 2005-02-18
116
(2) 4-acetyl-3-methyl-3H-benzooxazol-2-one
(The methylation is carried out in the presence of sodium carbonate in
methanol while refluxing)
Rf value: 0.67 (silica gel, methylene chloride/methanol = 99:1)
Mass spectrum (ESI+): m/z = 192 [M+H]+
(3) 4-acetyl-1,3-dimethyl-1,3-dihydro-benzoimidazol-2-one
(Carried out in the presence of potassium-tert. butoxide)
Rfvalue: 0.40 (silica gel, ethyl acetate/petroleum ether = 2:1)
Mass spectrum (ESI+): m/z = 205 [M+H]+
(4) 4-acetyl-1-ethoxycarbonyl-3-methyl-1,3-dihydro-benzoimidazol-2-one
Rfvalue: 0.23 (silica gel, petroleum ether/ethyl acetate = 2:1)
Mass spectrum (ESI+): m/z = 263 [M+H]+
(5) 1-[(1-methyl-1 H-benzoimidazol-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Mass spectrum (ESI+): m/z = 561 [M+H]+
(6) 1-{[1-(2-cyano-ethyl)-1 H-benzoimidazol-2-yl]methyl}-3-methyl-7-(2-butyn-
1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.50 (silica gel, cyclohexane/ethyl acetate/methanol = 5:4:1)
Mass spectrum (ESI+): m/z = 600 [M+H]+
(7) 1-({1-[(methylaminocarbonyl)methyl]-1 H-benzoimidazol-2-yl}methyl)-3-
methyl-7-(2-butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-l-yl]-
xanthine
Rf value: 0.45 (silica gel, cyclohexane/ethyl acetate/methanol = 5:4:1)
Mass spectrum (ESI+): m/z = 618 [M+H]+
(8) 1-[(1-benzyl-1 H-benzoimidazol-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-
(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
Rf value: 0.50 (silica gel, cyclohexane/ethyl acetate/methanol = 5:4:1)
Mass spectrum (ESI+): m/z = 637 [M+H]+
CA 02496249 2005-02-18
117
Example XXXIX
1-[2-(2-cyanomethylamino-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-
1-yI'-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yil-xanthine
Prepared by reacting 1-[2-(2-amino-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylami no)-piperidin- l -yl]-
xanthine with paraformaldehyde and potassium cyanide in the presence of
zinc chloride in glacial acetic acid at 40 C.
Rf value: 0.45 (silica gel, cyclohexane/ethyl acetate = 3:7)
Mass spectrum (ESI+): m/z = 605 [M+H]+
Example XL
1-[2-(2-amino-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-[(S)- 3-(tert.-
butyloxycarbonylamino)-piperidin-1 -vll-xanthine
prepared by reduction of 1-[2-(2-nitro-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-
butyn-l-yl)-8-[(S)- 3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
with
sodium dithionite in a mixture of methylglycol and water (2:1) at 100 C.
Rf value: 0.34 (silica gel, methylene chloride/methanol = 95:5)
The following compounds are obtained analogously to Example XL:
(1) 1-[2-(2-amino-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yi)-8-[(R)-3-
(tert.-
butyloxycarbonylamino)-piperidin-l-yl]-xanthine
Rf value: 0.50 (silica gel, cyclohexane/ethyl acetate = 4:6)
Example XLI
2-ch l oro methyl-4-methyl-g u i nazol i n e
prepared by treatment of 2.95 g of 2-chloromethyl-4-methyl-quinazoline-3-
oxide with 6 ml phosphorus trichloride in 150 ml chloroform while refluxing.
Yield: 1.75 g (57 % of theory)
Rf value: 0.81 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 193, 195 [M+H]+
Example XLII
CA 02496249 2005-02-18
118
2-chloromethyl-4-dimethylamino-quinazoline
A freshly prepared solution of 202 mg of dimethylamine in 3.2 ml of
tetrahydrofuran is added dropwise to 500 mg of 4-chloro-2-chloromethyl-
quinazoline in 5 ml of tetrahydrofuran while cooling with an ice bath. Then
the
reaction mixture is stirred for another 3.5 hours while cooling with an ice
bath
and then for a further 30 minutes at ambient temperature.
The solvent is then gently distilled off using a rotary evaporator and the
residue is taken up in methylene chloride. The solution is washed with
saturated sodium hydrogen carbonate solution and with water, dried over
magnesium sulphate and evaporated down. The solid residue is stirred with a
little tert.-butylmethylether, suction filtered, washed with petroleum ether
and
dried in vacuo.
Yield: 323 mg (62 % of theory)
Rf value: 0.60 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 222, 224 [M+H]+
The following compounds are obtained analogously to Example XLII:
(1) 2-chloromethyl-4-(morpholine-4-yl)-quinazoline
Rf value: 0.50 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 264, 266 [M+H]+
(2) 2-chloromethyl-4-(piperidin-1-yl)-quinazoline
Rf value: 0.70 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 262, 264 [M+H]+
(3) 4-[4-(tert.-butyloxycarbonyl)-piperazin-l-yl]-2-chloromethyl-quinazoline
Rf value: 0.57 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 363, 365 [M+H]+
(4) 2-chloromethyl-4-(pyrrolidin-1-yl)-quinazoline
Rf value: 0.50 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 248, 250 [M+H]+
CA 02496249 2010-12-14
25771-1004
119
(5) 2-chloromethyl-4-ethoxy-quinazoline
(The reaction is carried out with sodium ethoxide in ethanol at ambient
temperature.)
Rf value: 0.50 (silica gel, cyclohexanelethyl acetate = 3:1)
Mass spectrum (ESi+): m/z = 223, 225 [M+H]+
(6) 2-chloromethyl-4-isopropyloxy-quinazoline
(The reaction is carried out with sodium isopropoxide in isopropanol at
ambient temperature.)
Rf value: 0.70 (silica gel, cyclohexane/ethyl acetate = 3:1)
Mass spectrum (ESI+): m/z = 237, 239 [M+H]+
(7) 2-chloromethyl-4-phenyloxy-quinazoline
(The reaction is carried out with sodium hydride and phenol in tetrahydrofuran
at ambient temperature.)
Rf value: 0.65 (silica gel, cyclohexane/ethyl acetate = 3:1)
Mass spectrum (ESI+): m/z = 271, 273 [M+H]+
.Example XLIII
1-(2-{2-[(ethoxycarbonyl)methylam ino]-phenyl}-2-oxo-ethyl)-3-methyl-7-((E)-2-
buten-1-vl)-8-I3-(tert.-butyloxycarbonvlamino)-piperidin-l-yll-xanthine
A solution of 110 pL of ethyl diazoacetate in 0.5 ml of toluene is added
dropwise to 531 mg of 1-[2-(2-amino-phenyl)-2-oxo-ethyl]-3-methyl-7-((E)-2-
buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-pipendin-1-yl]-xanthine and 10
mg of methyltrioxorhenium in 4.5 ml of toluene at ambient temperature under
an argon atmosphere. The reaction mixture is stirred for 15 hours at ambient
temperature.Then approx. another 5 mg of methyltrioxorhenium and 20 pL
ethyl diazoacetate are added and the reaction mixture is heated to 50 C for
two hours. After cooling to ambient temperature another 5 mg of
methyltrioxorhenium and 20 pL ethyl diazoacetate are added. After another 16
hours at ambient temperature the reaction mixture is combined with 5 ml of
TM
conc. aqueous ammonia, shaken thoroughly and added to an Extrelut pack.
After 15 min it is rinsed with 200 ml methylene chloride. The methylene
chloride solution is evaporated down and chromatographed through a silica
CA 02496249 2005-02-18
120
gel column with cyclohexane/ethyl acetate/isopropanol (8:2:0 to 8:1:1) as
eluant.
Yield: 220 mg (36 % of theory)
Rf value: 0.40 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 638 [M+H]+
Example XLIV
1-[(2-cyano-benzofuran-3-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yll-xanthine
A mixture of 215 mg of 1-{2-[2-cyanomethoxy-phenyl]-2-oxo-ethyl}-3-methyl-
7-(2-butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
and
244 mg of caesium carbonate in 4 ml of N,N-dimethylformamide is stirred for
two hours at 50 C, then a further three hours at 70 C. For working up the
reaction mixture is combined with water and the precipitate formed is suction
filtered and dried.
Yield: 130 mg (62 % of theory)
Mass spectrum (ESI+): m/z = 572 [M+H]+
Example XLV
1 -[2-(3-methyl-2-oxo-2,3-d ihyd ro-1 H-benzoimidazol-4-yl)-2-oxo-ethyl]-3-
methyl-7-(2-butyn-1-yl)-8-f 3-(tert.-butyloxycarbonyla mino)-piperidin-1-yll-
xanthine
Prepared by treating 1-[2-(1-ethoxycarbonyl-3-methyl-2-oxo-2,3-dihydro-1H-
benzoim idazol-4-yl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-l-yl)-8-[3-(tert.-
butyloxy-
carbonylamino)-piperidin-l-yl]-xanthine with 1 N sodium hydroxide solution in
methanol at ambient temperature.
Rf value: 0.36 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 605 [M+H]+
Example XLVI
4-acetyl-1-ethoxycarbonyl-1,3-dihydro-benzoimidazol-2-one
5.29 g of diethyldicarbonat and 611 mg of dimethylaminopyridine are added
to 1.50 g of 1-(2,3-diamino-phenyl)-ethanone in 75 ml methylene chloride. The
reaction mixture is stirred for three hours at ambient temperature, then
CA 02496249 2005-02-18
121
another 100 mg of dimethylaminopyridine and 1 ml of diethyldicarbonate are
added and the mixture is stirred for a further 20 hours at ambient
temperature.
For working up the reaction mixture is diluted with methylene chloride,
washed with 2 N citric acid solution as well as saturated sodium hydrogen
carbonate solution and saturated sodium chloride solution, dried over
magnesium sulphate and evaporated down. The residue is chromatographed
through a silica gel column with petroleum etherlethyl acetate (3:1 to 1:2) as
eluant. The desired product is stirred with a little tert.-butylmethylether,
suction
filtered, nachwashed with a little ethyl acetate and tert.-butylmethylether
and
dried.
Yield: 900 mg (36 % of theory)
Rf value: 0.15 (silica gel, petroleum ether/ethyl acetate = 2:1)
Mass spectrum (ESI+): m/z = 249 [M+H]+
Example XLVII
I -[(4-amino-quinazol in-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperid in-1 -yl]-xanthine
501 mg of 1-cyanomethyl-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine are added to a mixture of 17
mg of potassium-tert. butoxide in 10 ml of methanol. After brief heating with
stirring a clear solution is formed and after about 20 minutes the nitrile has
largely reacted to form the iminoester according to thin layer chromatography.
206 mg of 2-amino-benzamidine-hydrochloride are then added and the
reaction mixture is refluxed for four hours. After cooling to ambient
temperature the precipitate formed is suction filtered, washed with methanol
and dried.
Yield: 143 mg (23 % of theory)
Rf value: 0.15 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 574 [M+H]+
Example XLVIII
1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-((Z)-2-buten-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl-xanthine
CA 02496249 2005-02-18
122
150 mg of 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxycarbonylamino)-piperidin-1-yl]-xanthine are hydrogenated in a mixture
of 5 ml of tetrahydrofuran and 5 ml of methanol in the presence of 30 mg of 5
%m palladium on activated charcoal (contaminated with quinoline) at ambient
temperature, until the calculated amount of hydrogen has been taken up.
Then a spatula tip of activated charcoal is added and the mixture is suction
filtered. The filtrate is evaporated down and the crude product is purified by
chromatography over a silica gel column with cyclohexane/ethyl acetate (7:3
to 4:6).
Yield: 120 mg (85 % of theory)
Rf value: 0.40 (silica gel, cyclohexane/ethyl acetate = 4:6)
Mass spectrum (ESI+): m/z = 537 [M+H]+
Example XLIX
8- hvd roxvmeth v l-5-meth oxy-a u i n o l i n e
148 mg of sodium hydride (approx. 60 % in mineral oil) are added batchwise
to a solution of 640 mg of 8-hydroxymethyl-quinolin-5-ol in N,N-
dimethylformamide while cooling with an ice bath and the reaction mixture is
slowly heated to ambient temperature. After the development of gas has
ended, 230 pI methyl iodide are added dropwise while cooling with an ice
bath, then the reaction mixture is stirred for approx. another two hours at
ambient temperature. For working up it is poured onto ice water, saturated
with sodium chloride and extracted with a mixture of diethyl ether and ethyl
acetate. The combined extracts are washed with saturated sodium chloride
solution, dried over magnesium sulphate and evaporated down. The flask
residue is triturated with petroleum ether and the supernatant is decanted.
The crude product is purified through a silica gel column with ethyl acetate
as
eluant.
Yield: 470 mg (68 % of theory)
Rf value: 0.70 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 190 [M+H]+
The following compounds are obtained analogously to Example XLIX:
CA 02496249 2005-02-18
123
(1) 8-hydroxymethyl-5-methoxy-isoquinoline
Rf value: 0.40 (silica gel, methylene chloride/methanol = 10:1)
Mass spectrum (ESI+): m/z = 190 [M+H]+
Example L
8-hydroxymethyl-quinolin-5-ol
3.40 g of quinolin-5-ol is combined with 8 ml of conc. hydrochloric acid and 8
ml of 37 % formalin solution while cooling with an ice bath. Then hydrogen
chloride gas is piped through the reaction mixture for about two hours, while
the temperature slowly rises. The reaction mixture is stirred first overnight
while cooling with an ice bath, then at ambient temperature and then
evaporated down in vacuo. The flask residue is taken up in water, covered
with a layer of diethyl ether and adjusted to pH 10 while cooling with an ice
bath and vigorously stirring with dilute ammonia solution. After another two
hours' vigorous stirring at ambient temperature the organic phase is
separated off and the aqueous phase is extracted with diethyl ether. The
combined organic phases are washed with water and saturated sodium
chloride solution, dried over magnesium sulphate and evaporated down. The
flask residue is chromatographed through a silica gel column with methylene
chloride/methanol (20:1) as eluant.
Yield: 660 mg (16 % of theory)
Mass spectrum (ESI+): m/z = 176 [M+H]+
The following compounds are obtained analogously to Example L:
(1) 8-hydroxymethyl-isoquinolin-5-ol
Rf value: 0.50 (silica gel, methylene chloride/methanol = 5:1)
Mass spectrum (ESI+): m/z = 176 [M+H]+
Example LI
1-[(2-cyclopropyl-quinazolin-4-yl)methyl]-3-methyl-7-[(1-cyclopenten-1-
yl)meth yll-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yll-xanthine
A mixture of 250 mg of 1-(2-{2-[(cyclopropylcarbonyl)amino]-phenyl}-2-oxo-
ethyl)-3-methyl-7-[(1-cyclopenten-1-yl)methyl]-8-[3-(tert.-
CA 02496249 2005-02-18
124
butyloxycarbonylamino)-piperidin-1-yl]-xanthine and 7.5 ml of ethanolic
ammonia solution (6 M) is heated to 150 C for seven hours in a bomb. For
working up the reaction mixture is evaporated down and chromatographed
through a silica gel column with methylene chloride/methanol (100:0 to 70:30)
as eluant. The contaminated product fraction is evaporated down and again
purified through a reversed phase HPLC column with water/
acetonitrile/trifluoroacetic acid (65:15:0.08 to 0:100:0.1) as eluant. The
product fractions are evaporated down, made alkaline with dilute sodium
hydroxide solution and extracted with methylene chloride. The combined
extracts are dried over magnesium sulphate and evaporated down.
Yield: 40 mg (14 % of theory)
Rf value: 0.40 (silica gel, methylene chloride /ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 627 [M+H]+
Example LII
4-(2-bromo-acetyl)-1,3-bis-[(2-trimethylsilanyl-ethoxy)methyl]-1,3-dihydro-
benzoimidazol-2-one
520 mg of 2-pyrrolidinone-hydrotribromide and 89 mg of 2-pyrrolidinone are
added to 420 mg of 4-acetyl-1,3-bis-[(2-trimethylsilanyl-ethoxy)methyl]-1,3-
dihydro-benzoimidazol-2-one in 5 ml of tetrahydrofuran under an argon
atmosphere. The reaction mixture is refluxed for two hours and then suction
filtered while still warm. The filter cake is washed with tetrahydrofuran and
the
filtrate is evaporated down, leaving 660 mg of a yelllowish-brown solid. This
is stirred with a little methanol, suction filtered, washed with some methanol
and dried. The crude product is reacted further without any more purification.
Yield: 430 mg (87 % of theory)
Rf value: 0.23 (silica gel, petroleum ether/ethyl acetate = 9:1)
Mass spectrum (El): m/z = 514, 516 [M]+
The following compounds are obtained analogously to Example LII:
(1) 7-(2-bromo-acetyl)-1-(tert.-butyloxycarbonyl)-1 H-indole
Rf value: 0.33 (silica gel, petroleum ether/ethyl acetate = 9:1)
Mass spectrum (ESI+): m/z = 338, 340 [M+H]+
CA 02496249 2005-02-18
125
(2) 2-bromo-1-(3-isopropyloxy-phenyl)-ethanone
(Carried out with phenyltrimethylammonium tribromide in methylene chloride)
Rf value: 0.39 (silica gel, cyclohexane/ethyl acetate = 9:1)
(3) 2-bromo-1-(3-difluoromethoxy-phenyl)-ethanone
(Carried out with phenyltrimethylammonium tribromide in methylene chloride)
Rf value: 0.24 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Example LIII
methyl 3-methyl-imidazof 1.2-alpyridine-2-carboxylate
A mixture of 1.91 g of 2-aminopyridine and 4.40 g of methyl 3-bromo-2-oxo-
butyrate in 40 ml of ethanol is refluxed for 6 hours and then left to stand
for 2
days at ambient temperature. The solvent is distilled off using the rotary
evaporator and the crude product is purified by chromatography over a silica
gel column with methylene chloride/methanol/methanolic ammonia solution
(95:4:1 to 90:9:1) as eluant. 560 mg of the ethyl ester are isolated as the by-
product.
Yield: 2.09 g (54 % of theory)
Rf value: 0.20 (silica gel, methylene chloride/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 191 [M+H]+
Example LIV
2-chloromethyl-4-isopropyl-auinazoline
Dry hydrogen chloride gas is piped through a solution of 2.86 g of 1-(2-amino-
phenyl)-2-methyl-propan-1-one and 1.33 ml of chloroacetonitrile in 14 ml
dioxane with stirring at ambient temperature for approx. five hours. Then the
dioxane is largely distilled off in a water jet vacuum. The honey-like residue
is
combined with ice water and the resulting suspension is made alkaline with
saturated potassium carbonate solution while cooling with an ice bath. The
precipitate is suction filtered, washed with water and dried. The crude
product
is purified by chromatography over a silica gel column with petroleum
ether/methylene chloride (8:2 to 0:1) as eluant.
CA 02496249 2005-02-18
126
Yield: 1.80 g (58 % of theory)
Rf value: 0.30 (silica gel, methylene chloride/petroleum ether = 1:1)
Mass spectrum (ESI+): m/z = 221, 223 [M+H]+
Example LV
1-chloromethyl-3-trifluoromethyl-3,4-dihydro-isopuinoline
530 mg of N-(1-benzyl-2,2,2-trifluoro-ethyl)-2-chloro-acetamide (prepared by
reacting 1-benzyl-2,2,2-trifluoro-ethylamine with chloroacetyl chloride in the
presence of triethylamine) and 0.74 ml of phosphorus oxychloride are added
to 4.00 g of polyphosphoric acid. The viscous mixture is stirred for 1.5 hours
at 130 C. For working up the reaction mixture is cooled and combined with
ice water, stirred vigorously for ten minutes and suction filtered. The filter
cake
is dissolved in ethyl acetate and the solution is dried over magnesium
sulphate and evaporated down, leaving a white solid.
Yield: 415 mg (84 % of theory)
Rf value: 0.55 (aluminium oxide, petroleum ether/ethyl acetate = 10:1)
Mass spectrum (ESI+): m/z = 248, 250 [M+H]+
The following compound is obtained analogously to Example LV:
(1) 1-methyl-3-trifluoromethyl-3,4-dihydro-isoquinoline
(The starting material N-(1-benzyl-2,2,2-trifluoro-ethyl)-acetamide is
obtained
by reacting 1-benzyl-2,2,2-trifluoro-ethylamine with acetic anhydride.)
Example LVI
3-bromomethyl-1-(1-cyano-1-methyl-ethyl)-isopuinoline
A mixture of 375 mg of 1-(1-cyano-1-methyl-ethyl)-3-methyl-isoquinoline and
321 mg of N-bromosuccinimide in 5 ml carbon tetrachloride is combined with
a spatula tip of 2,2-azoisobutyric acid dinitrile and refluxed for about six
hours. The cooled reaction mixture is filtered and evaporated down. The flask
residue is reacted further without any more purification.
Rf value: 0.70 (silica gel, cyclohexane/ethyl acetate = 3:1)
The following compounds are obtained analogously to Example LVI:
CA 02496249 2005-02-18
127
(1) 6-bromomethyl-1-[(2-trimethylsilanyl-ethoxy)methyl]-1 H-quinolin-2-one
(2) 1-bromomethyl-4-bromo-3-methoxy-isoquinoline
(3) 2-bromomethyl-[1,5]naphthyridine
Mass spectrum (ESI+): m/z = 223, 225 [M+H]+
(4) 5-bromomethyl-[1,6]naphthyridine
Rf value: 0.48 (silica gel, ethyl acetate/methanol = 98:2)
(5) 7-bromomethyl-5-phenyl-quinoxaline
Rf value: 0.85 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 299, 301 [M+H]+
(6) 4-bromomethyl-[1,5]naphthyridine
Rf value: 0.56 (silica gel, methylene chloride/ethyl acetate = 7:3)
Mass spectrum (ESI+): m/z = 223, 225 [M+H]+
(7) 1-bromomethyl-3-trifluoromethyl-isoquinoline
Mass spectrum (ESI+): m/z = 290, 292 [M+H]+
(8) 1-bromomethyl-3-difuoromethyl-isoquinoline
Mass spectrum (ESI+): m/z = 272, 274 [M+H]+
(9) 1-bromomethyl-4-chloro-3-methoxy-isoquinoline
Example LVII
1-(1-cyano-1-methyl-ethyl)-3-methyl-isoq uinoline
3.30 g of 2,2-azoisobutyric acid dinitrile are added to 1.60 g of 3-methyl-
isoquinoline-N-oxide in 30 ml of toluene. The reaction mixture is stirred for
six
hours at 85 C and then left to stand for two days at ambient temperature. For
working up the reaction mixture is extracted with 20% hydrochloric acid. The
combined aqueous phases are diluted with methylene chloride, made alkaline
CA 02496249 2005-02-18
128
with saturated potassium carbonate solution while cooling with an ice bath
and extracted with methylene chloride. The combined methylene chloride
extracts are dried over magnesium sulphate and evaporated down. The
residue is chromatographed through a silica gel column with cyclohexane as
eluant.
Yield: 375 mg (18 % of theory)
Mass spectrum (ESI+): m/z = 211 [M+H]+
Rf value: 0.75 (silica gel, cyclohexane/ethyl acetate = 3:1)
Example LVIII
1-(2-cyanoimino-2-phenyl-ethyl)-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butvloxvcarbonvlamino)-piperidin-1-yll-xanthine (E/Z-mixture)
0.48 ml of a 1 M solution of titaniium tetrachloride in methylene chloride are
added dropwise to 244 mg of 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-(2-butyn-1-
yl)-6-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine in 7 ml of
methylene chloride. Then 88 pl of 1,3-bis(trimethylsilyl)carbodiimide are
added and the mixture is stirred for four hours at ambient temperature. For
working up the reaction mixture is diluted with methylene chloride and poured
onto ice water. The organic phase is washed with 0.5 N citric acid, dried over
magnesium sulphate and evaporated down. The crude product is purified by
chromatography over a silica gel column with methylene chloride/methanol
(98:2 to 95:5) as eluant.
Yield: 206 mg (97 % of theory)
Mass spectrum (ESI-): m/z = 557 [M-H]-
Rf value: 0.16 (silica gel, cyclohexane/ethyl acetate = 1:1)
Example LIX
1-[(1 H-benzoimidazol-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-
butyloxvcarbonvlamino)-piperidin-1-yll-xanthine
350 mg of 1-[(2-amino-phenylaminocarbonyl)methyl]-3-methyl-7-(2-butyn-1-
yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine are refluxed
in 3
ml glacial acetic acid for two hours. Then the reaction mixture is evaporated
down, the flask residue is combined with 5 ml of 1 M sodium hydroxide
solution and washed with methylene chloride. Then the aqueous phase is
CA 02496249 2005-02-18
129
acidified with 1 M hydrochloric acid and extracted with methylene chloride.
The combined extracts are evaporated down and chromatographed through a
silica gel column with cyclohexane/ethyl acetate/methanol (6:4:0 to 5:4:1) as
eluant.
Yield: 250 mg of (74 % of theory)
Mass spectrum (ESI+): m/z = 547 [M+H]+
Example LX
Ethyl 3,4-dimethyl-6,7-dihydro-5H42lpyrindin-1-carboxylate
Prepared by treating 1.16 g of ethyl 3,4-dimethyl-4a-(pyrrolidin-1-yl)-
5,6,7,7a-
tetrahydro-4aH-[2]pyrindin- 1-carboxylate with 1.08 g of 70 % 3-chloro-
perbenzoic acid in 50 ml methylene chloride at ambient temperature.
Yield: 850 mg (97 % of theory)
Rf value: 0.30 (aluminium oxide, petroleum ether/ethyl acetate = 5:1)
Mass spectrum (ESI+): m/z = 220 [M+H]+
The following compounds are obtained analogously to Example LX:
(1) ethyl 3,4-dimethyl-5,6,7,8-tetrahydro-isoquinoline-1-carboxyl ate
Rf value: 0.35 (aluminium oxide, petroleum ether/ethyl acetate = 5:1)
Mass spectrum (ESI+): m/z = 234 [M+H]+
Example LXI
Ethyl 3,4-dimethyl-4a-(pyrrolidin-1-yl)-5,6,7,7a-tetrahydro-4aH-[2]pyrindin-l -
carboxylate
Prepared by reacting 2.50 g of ethyl 5,6-di methyl-[1,2,4]triazin-3-
carboxylate
with 2.74 g of 1-(cyclopenten-1-yl)-pyrrolidine in 25 ml chloroform at ambient
temperature.
Yield: 3.00 g (75 % of theory)
Rf value: 0.60 (aluminium oxide, petroleum ether/ethyl acetate = 5:1)
Mass spectrum (ESI+): m/z = 291 [M+H]+
The following compounds are obtained analogously to Example LXI:
CA 02496249 2005-02-18
130
(1) ethyl 3,4-dimethyl-4a-(pyrrolidin-l-yl)-4a,5,6,7,8,8a-hexahydro-
isoquinoline-1-carboxylate
Rf value: 0.60 (aluminium oxide, petroleum ether/ethyl acetate = 5:1)
Mass spectrum (ESI+): mlz = 305 [M+H]+
Example LXII
Methyl 2 , 3, 8-trimethyl-quinoxalin-6-carboxylate
Prepared by reacting 1.60 g of methyl 3,4-diamino-5-methyl-benzoate with
0.86 ml diacetyl in a mixture of water and ethanol while refluxing.
Yield: 1.53 g (80 % of theory)
Rf value: 0.63 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 231 [M+H]+
The following compounds are obtained analogously to Example LXII:
(1) methyl 8-methyl-quinoxalin-6-carboxylate
(reaction is carried out with glyoxal in water.)
Rf value: 0.55 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 203 [M+H]+
(2) 5-bromo-7-methyl-quinoxaline
(reaction is carried out with glyoxal in a water/ethanol mixture.)
Rf value: 0.75 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 223, 225 [M+H]+
Example LXIII
Methyl 3,4-diamino-5-methyl-benzoate
Prepared by reduction of methyl 3-nitro-4-amino-5-methyl-benzoate at a
partial hydrogen pressure of 50 psi in the presence of Raney nickel in
methanol at ambient temperature.
Rf value: 0.40 (silica gel, tert.-butylmethylether)
Example LXIV
CA 02496249 2005-02-18
131
Methyl 3-nitro-4-amino-5-methyl-benzoate
Prepared by treating 3-nitro-4-acetylamino-5-methyl-benzoic acid with
hydrogen chloride gas in methanol at ambient temperature and subsequently
heating while refluxing.
Mass spectrum (ESI+): m/z = 211 [M+H]+
Rf value: 0.75 (silica gel, tert.-butylmethylether/acetic acid = 99:1)
Example LXV
1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-((E)-1-buten-1-yl)-8-bromo-xanthin e
0.13 ml 35 % hydrogen peroxide solution are added to 290 mg of 1-(2-phenyl-
2-oxo-ethyl)-3-methyl-7-(1-phenylsulphanyl-butyl)-8-bromo-xanthine in 6 ml
hexafluoroisopropanol. The reaction mixture is stirred for one hour at ambient
temperature, diluted with methylene chloride and washed with sodium
thiosuiphate solution. The organic phase is dried over magnesium sulphate
and evaporated down. The flask residue is taken up in 6 ml of toluene and
refluxed for eight hours. Then the toluene is distilled off in vacuo and the
crude product is purified through a silica gel column with methylene
chloride/methanol (100:0 to 95:5) as eluant.
Yield: 104 mg (45 % of theory)
Rf value: 0.61 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 417, 419 [M+H]+
The following compounds are obtained analogously to Example LXV:
(1) 3-methyl-7-(3-methyl-1-buten-1-yl)-8-bromo-xanthine
Rf value: 0.24 (silica gel, methylene chloride/methanol = 95:5)
Mass spectrum (ESI+): m/z = 313, 315 [M+H]+
Example LXVI
1-methanesulphonyloxymethyl-4-difluoromethoxy-naphthalene
Prepared by reacting (4-difluoromethoxy-naphthalen-1-yl)-methanol with
methanesulphonic acid chloride in methylene chloride in the presence of
triethylamine.
CA 02496249 2005-02-18
132
The following compounds are obtained analogously to Example LXVI:
(1) (E)-1-methanesulphonyloxy-3-(2-nitro-phenyl)-2-propene
(2) (E)-1-methanesulphonyloxy-3-pentafluorophenyl-2-propene
(3) (E)-1-methanesulphonyloxy-3-(2-trifluoromethyl-phenyl)-2-propene
(4) (E)-1-methanesulphonyloxy-3-(3-trifluoromethyl-phenyl)-2-propene
(5) (E)-1-methanesulphonyloxy-3-(4-trifluoromethyl-phenyl)-2-propene
Example LXVII
7-methyl-5phenyl-quinoxaline
A mixture of 400 mg of 5-bromo-7-methyl-quinoxaline, 244 mg of phenylboric
acid and 100 mg of tetrakis(triphenylphosphine)palladium in 12 ml dioxane, 4
ml of methanol and 3.6 ml 1 M aqueous sodium carbonate solution is refluxed
for three hours under an argon atmosphere. Then the reaction mixture is
evaporated down and the residue is distributed between ethyl acetate and
water. The ethyl acetate phase is separated off, dried over magnesium
sulphate and evaporated down. The crude product is purified by
chromatography over a silica gel column with cyclohexane/ethyl acetate
(85:15 to 70:30) as eluant.
Yield: 390 mg (66% of theory)
Rf value: 0.36 (silica gel, petroleum ether/ethyl acetate = 5:1)
Mass spectrum (ESI+): m/z = 221 [M+H]+
Example LXVIII
1-methyl-3-trifluoromethyl-isogui noline
CA 02496249 2005-02-18
133
Prepared by treating 905 mg of 1-chloromethyl-3-trifluoromethyl-3,4-dihydro-
isoquinoline with 420 mg of potassium-tert. butoxide in 10 ml of
tetrahydrofuran at ambient temperature.
Yield: 755 mg of (98% of theory)
Mass spectrum (ESI+): m/z = 212 [M+H]+
The following compounds are obtained analogously to Example LXVIII:
(1) 1-methyl-3-difluoromethyl-isoquinoline
(Prepared from 1-methyl-3-trifluoromethyl-3,4-dihydro-isoquinoline)
Mass spectrum (ESI+): m/z = 194 [M+H]+
Example LXIX
4-chloro-3-methoxy-1-methyl-isoguinoline
Prepared by treating 3-methoxy-1-methyl-isoquinoline with sulphuryl chloride
in methylene chloride.
Rf value: 0.30 (silica gel, cyclohexane)
Mass spectrum (ESI+): m/z = 208, 210 [M+H]+
Example LXX
3-cyclopropyl-8-bromo-xanthine
Prepared by reacting 3-cyclopropyl-xanthine with bromine in the presence of
potassium carbonate in acetonitrile at 60 C.
Rf value: 0.65 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 271, 273 [M+H]+
Example LXXI
Ethyl 1,2, 3,4-tetra hydro-phenanth ridi n-6-yl-ca rboxylate
Analogously to the method described by Gonsalves et al. (Tetrahedron 1992,
48, 6821) a solution of 3.90 g of ethyl 5,6,7,8-tetrahydro-benzo[1,2,4]triazin-
3-
carboxylate (Sagi et al., Heterocycles 1989, 29, 2253) in 20 ml dioxane is
refluxed. Then 8.22 g of anthranilic acid and 7.02 g of isoamylnitrite, in
each
case dissolved in 20 ml dioxane, are simultaneously added dropwise within 25
CA 02496249 2005-02-18
134
minutes by means of two dropping funnels. The reaction mixture is refluxed
for a further 30 minutes. For working up the cooled deep-brown reaction
solution is diluted with 150 ml diethyl ether, washed with 100 ml of 2 N
sodium
hydroxide solution and with water, dried over magnesium sulphate and
evaporated down. The brown, oily flask residue is chromatographed through a
silica gel column with ethyl acetate/petroleum ether (20:80 to 50:50) as
eluant.
The product obtained is still somewhat contaminated, but is reacted further
without any more purification.
Yield: 380 mg (8 % of theory)
Rf value: 0.55 (silica gel, petroleum ether/ethyl acetate = 2:1)
Mass spectrum (ESI+): m/z = 256 [M+H]+
CA 02496249 2005-02-18
135
Preparation of the final compounds:
Example 1
1,3-dimethyl-7-(2.6-dicyano-benzyl)-8-(3-amino-piperidin-1-yl)-xanthine
129 mg of 3-amino-piperidine-dihydrochioride are added to a mixture of 298
mg of 1,3-dimethyl -7-(2,6-dicyano-benzyl)-8-bromo-xanthine and 420 mg of
potassium carbonate in 9 ml of N,N-dimethylformamide. The reaction mixture
is stirred for three hours at 80 C. For working up the mixture is diluted with
methylene chloride and washed with saturated sodium chloride solution. The
organic phase is dried over magnesium sulphate and evaporated down. The
crude product is purified by chromatography through a silica gel column with
methylene chloride/methanol/conc. methanolic ammonia (95:5:1 to 80:20:1)
as eluant.
Yield: 43 mg (14 % of theory)
Rf value: 0.67 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 80:20:1)
Mass spectrum (ESI+): m/z = 419 [M+H]+
The following compounds are obtained analogously to Example 1:
(1) 1-(2-cyano-ethyl)-3-methyl -7-(2-cyano-benzyl)-8-(3-amino-pi peridin-1-yl)-
xanthine
Rf value: 0.35 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 433 [M+H]+
Example 2
1-(2-{2-[(ethoxycarbonyl )methoxy]-ph enyl}-2-oxo-ethyl)-3-methyl-7-(3-methyl-
2-buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine
A solution of 209 mg of 1-(2-{2-[(ethoxycarbonyl)methoxy]-phenyl}-2-oxo-
ethyl)-3-methyl-7-(3-methyl-2-buten-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-
piperidin-1-yl]-xanthine in 4 ml methylene chloride is combined with 1 ml of
trifluoroacetic acid and stirred for half an hour at ambient temperature. For
working up the reaction mixture is diluted with methylene chloride and washed
with saturated potassium carbonate solution. The organic phase is dried,
136
evaporated down and chromatographed through a silica gel column with
methylene chloride/methanol (1:0 to 4:1) as eluant.
Yield: 153 mg of (87 % of theory)
Mass spectrum (ESI+): m/z = 553 [M+H]+
The following compounds are obtained analogously to Example 2:
(1) 1-(2-{2-[(aminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-(3-amino-piperidin-l-yl)-xanthine
Mass spectrum (ESI+): m/z = 524 [M+H]+
(2) 1-(2-{3-[(methanesulphinyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine
Rf value: 0.58 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 100:100:0.1)
Mass spectrum (ESI+): m/z = 543 [M+H]+
(3) 1-(1-methyl-2-oxo-2-phenyl-ethyl)-3-methyl-7-(3-methyl-2-buten-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 465 [M+H]+
(4) 1-(2-phenoxy-ethyl)-3-methyl-7-(2-cyano-benzyl)-8-(3-amino-piperidin-1-
yl)-xanthine
Rf value: 0.50 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 500 [M+H]+
(5) 1-(2-phenyl-2-oxo-ethyl)-7-(3-methyl-2-buten-1-yl)-8-(3-amino-piperidin-1-
yl)-xanthine
Rf value: 0.58 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 80:20:1)
Mass spectrum (ESI-): m/z = 435 [M-H]-
(6) 1-(2-{3-[(ethoxycarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-
methyl-2-buten-l-yl)-8-(3-amino-piperidin-l-yl)-xanthine
CA 02496249 2005-02-18
137
CA 02496249 2005-02-18
Mass spectrum (ESI+): m/z = 553 [M+H]+
(7) 1-(2-{2-[(methylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
(3-methyl-2-buten-l-yl)-8-(3-amino-piperidin-l-yl)-xanthine
Mass spectrum (ESI+): m/z = 538 [M+H]+
(8) 1-(2-{2-[(dimethylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-
7-(3-methyl-2-buten-l-yl)-8-(3-amino-piperidin-l-yl)-xanthine
Mass spectrum (ESI+): m/z = 552 [M+H]+
(9) 1-(2-methoxy-ethyl)-3-methyl -7-(2-cyano-benzyl)-8-(3-amino-piperidin-l-
yl)-xanthine
Rf value: 0.40 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 438 [M+H]+
(10) 1-methyl-3-[(methoxycarbonyl)methyl]-7-(2-cyano-benzyl)-8-(3-amino-
piperidin-1-yl)-xanthine
Rf value: 0.40 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 452 [M+H]+
(11) 1-methyl-3-cyanomethyl-7-(2-cyano-benzyl)-8-(3-amino-pi peridin-l-yl)-
xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.20 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 419 [M+H]+
(12) 1-methyl-3-(2-propyn-1-yl)-7-(2-cyano-benzyl)-8-(3-amino-pipe ridin-1-yl)-
xanthine
Rf value: 0.40 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 418 [M+H]+
(13) 1-{2-[3-(2-oxo-imidazolidin-1-yl)-phenyl]-2-oxo-ethyl}-3-methyl-7-(3-
methyl-2-buten-1 -yl)-8-(3-amino-piperidin-1-yl)-xanthine
138
Rf value: 0.54 (ready-made reversed phase TLC plate (E. Merck),
aceton itrile/water/ trifluoroacetic acid = 100:100:0.1)
Mass spectrum (ESI+): m/z = 535 [M+H]+
(14) 1-methyl-3-(2-propen-1-yl)-7-(2-cyano-benzyl)-8-(3-amino-piperidin-l-yl)-
xanthine
Rf value: 0.40 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 420 [M+H]+
(15) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-(2-butyn-1-yl)-8-(3-amino-piperidin-1-
yl)-xanthine
Mass spectrum (ESI+): m/z = 435 [M+H]+
(16) 1-(2-{2-[(ethylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-methyl-
2-buten-1-yl)-8-(3-amino-piperidin-1 -yl)-xanthine
Rf value: 0.50 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/trifluoroacetic acid = 100:100:0.1)
Mass spectrum (ESI+): m/z = 522 [M+H]+
(17) 1-methyl-3-phenyl-7-(2-cyano-benzyl)-8-(3-amino-piperidin-l-yl)-xanthine
Rf value: 0.40 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 456 [M+H]+
(18) 1-[2-(2-amino-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-
((S)-3-amino-piperidin-1-yl)-xanthine
Rf value: 0.50 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:0.1)
Mass spectrum (ESI+): m/z = 466 [M+H]+
(19) 1-(2-phenyl-2-oxo-ethyl)-3-cyanomethyl-7-(3-methyl-2-buten-l-yl)-8-(3-
amino-piperidin-l-yl)-xanthine
Rf value: 0.07 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 476 [M+H]+
CA 02496249 2005-02-18
139
(20) 1-[(quinolin-2-yl)methyl]-3-methyl -7-(3-methyl-2-buten-1-yl)-8-(3-amino-
piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.50 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 474 [M+H]+
(21) 1-[(2-oxo-2H-chromen-4-yl)methyl]-3-methyl -7-(3-methyl -2-buten-1-yl)-8-
(3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Mass spectrum (ESI+): m/z = 491 [M+H]+
Rf value: 0.16 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:0.1)
(22) 1-[(cinnolin-4-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-(3-amino-
piperidin-1 -yl)-xanthine (1:1 mixture with 1-[(1,4-dihydro-cinnolin-4-
yl)methyl]-
3-methyl -7-(3-methyl-2-buten-1-yl)-8-(3-amino-piperidin-l-yl)-xanthine)
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.49 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 475 [M+H]+
(23) 1-[(1-methyl-2-oxo-1,2-dihydro-quinolin-4-yl)methyl]-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-(3-amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Melting point: 178-181 C
Mass spectrum (ESI+): m/z = 504 [M+H]+
(24) 1-[(4-oxo-3,4-dihydro-phthalazin-1-yl)methyl]-3-methyl-7-(3-methyl-2-
buten-l-yl)-8-(3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.06 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:0.1)
CA 02496249 2005-02-18
140
Mass spectrum (ESI+): m/z = 491 [M+H]+
(25) 1-[(quinazolin-4-yl)meth yl]-3-methyl-7-(3-methyl -2-buten-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.48 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 475 [M+H]+
(26) 1-[(5-methyl-3-phenyl-isoxazol-4-yl)methyl]-3-methyl-7-(3-methyl-2-
buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.40 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:0.1)
Mass spectrum (ESI+): m/z = 504 [M+H]+
(27) 1-[(isoquinoline-3-yl)methyl]-3-methyl-7-(3-methyl -2-buten-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.51 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 474 [M+H]+
(28) 1-[(3-phenyl-[1,2,4]oxadiazol-5-yl)methyl]-3-methyl-7-(3-methyl-2-buten-
1-yl)-8-(3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.23 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:1)
Mass spectrum (ESI+): m/z = 491 [M+H]+
(29) 1-[(4-phenyl-pyridin-2-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
CA 02496249 2005-02-18
141
Rf value: 0.51 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 500 [M+H]+
(30) 1-[(5-phenyl-pyridin-2-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.58 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 500 [M+H]+
(31) 1-[(3-methyl-4-oxo-3,4-dihydro-phthalazin-1-yl)methyl]-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine-hydrochloride
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride;
product precipitated as the hydrochloride)
Rf value: 0.55 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:0.1)
Mass spectrum (ESI+): m/z = 505 [M+H]+
(32) 1-[2-(3-methylsulphanyl-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-
buten-l-yl)-8-(3-amino-piperidin-1-yl)-xanthine
Rf value: 0.34 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 497 [M+H]+
(33) 1-[2-(3-methanesulphinyl-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-
buten-l-yl)-8-(3-amino-piperidin-1-yl)-xanthine
Rf value: 0.21 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 513 [M+H]+
(34) 1-[2-(3-methanesulphonyl-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-
buten-1-yl)-8-(3-amino-piperidin-l-yl)-xanthine
CA 02496249 2005-02-18
CA 02496249 2005-02-18
142
Rf value: 0.66 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 529 [M+H]+
(35) 1-[2-(3-carboxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-
8-(3-amino-piperidin-1-yl)-xanthine-hydrochloride
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride;
product precipitated as the hydrochloride)
Rf value: 0.54 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 495 [M+H]+
(36) 1-[2-(3-methoxycarbonyl-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl -2-
buten-1-yl)-8-(3-amino-pi peridin-1-yl)-xanthine-hydrochloride
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride;
product precipitated as the hydrochloride)
Rf value: 0.47 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 509 [M+H]+
(37) 1-{2-[3-(methyl aminocarbonyl)-phenyl]-2-oxo-ethyl}-3-methyl-7-(3-methyl-
2-buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine-hydrochloride
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride;
product obtained as the hydrochloride)
Rf value: 0.53 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 508 [M+H]+
(38) 1-{2-[3-(dim ethyl aminocarbonyl)-phenyl]-2-oxo-ethyl}-3-methyl -7-(3-
methyl-2-buten-1-yl)-8-(3-amino-piperidin-l-yl)-xanthine-hydrochloride
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride;
product obtained as the hydrochloride)
Rf value: 0.53 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
143
Mass spectrum (ESI+): m/z = 522 [M+H]+
(39) 1-{2-[3-(morpholin-4-yl-carbonyl)-phenyl]-2-oxo-ethyl}-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-(3-amino-piperidin-l-yl)-xanthine-hydrochloride
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride;
product obtained as the hydrochloride)
Rf value: 0.53 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 564 [M+H]+
(40) 1-[2-(2-carboxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-l-yl)-
8-(3-amino-piperidin-1-yl)-xanthine-hydrochloride
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride;
product obtained as the hydrochloride)
Rf value: 0.53 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 495 [M+H]+
(41) 1-[2-(2-ethoxycarbonyl-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-
buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine-hydrochloride
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride;
product obtained as the hydrochloride)
Rf value: 0.41 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 523 [M+H]+
(42) 1-{2-[2-(dimethylaminocarbonyl)-phenyl]-2-oxo-ethyl}-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-(3-amino-piperidin-l-yl)-xanthine-hydrochloride
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride;
product obtained as the hydrochloride)
Rf value: 0.53 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 522 [M+H]+
CA 02496249 2005-02-18
144
(43) 1-{2-[2-(morpholin-4-yl-carbonyl)-phenyl]-2-oxo-ethyl}-3-methyl-7-(3-
methyl-2-buten-1-yi)-8-(3-amino-piperidin-1-yl)-xanthine-hydrochloride
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride;
product obtained as the hydrochloride)
Rf value: 0.53 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 564 [M+H]+
(44) 1-[2-(2,6-dimethoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-l -
yl)-8-(3-amino-piperidin-1-yl)-xanthine-hydrochloride
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride;
product obtained as the hydrochloride)
Rf value: 0.44 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 511 [M+H]+
(45) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-(2,3-dimethyl-2-buten-l-yl)-8-(3-
amino-piperidin-1-yl)-xanthine-hydrochloride
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride;
product obtained as the hydrochloride)
Rf value: 0.68 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 465 [M+H]+
(46) 1-((E)-3-phenyl-allyl)-3-methyl-7-(3-methyl -2-buten-1-yl)-8-(3-amino-
piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.38 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:0.1)
Mass spectrum (ESI+): m/z = 449 [M+H]+
(47) 1-[(benzo[b]thiophen-3-yl)methyl]-3-methyl -7-(3-methyl-2-buten-1-yl)-8-
(3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
CA 02496249 2005-02-18
145
Rf value: 0.51 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 479 [M+H]+
(48) 1-[(1 H-indol-3-yl)methyl]-3-methyl-7-(3-methyl-2-buten-l-yl)-8-(3-amino-
piperidin-1-yl)-xanthine
Rf value: 0.48 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 462 [M+H]+
(49) 1-[(biphenyl-4-yl)methyl]-3-methyl-7-(3-methyl-2-buten-1-yi)-8-(3-amino-
piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.30 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:0.1)
Mass spectrum (ESI+): m/z = 499 [M+H]+
(50) 1-[(1-naphthyl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-amino-piperidin-1-
yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.56 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 457 [M+H]+
(51) 1-[(1-methyl-2-oxo-l,2-dihydro-quinolin-4-yl)methyl]-3-methyl-7-(2-butyn-
1-yl)-8-(3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.40 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 488 [M+H]+
(52) 1-[(quinolin-4-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-amino-piperidin-
1-
yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
CA 02496249 2005-02-18
146
Rf value: 0.52 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 458 [M+H]+
(53) 1-(2-cyclohexyl-2-oxo-ethyl)-3-methyl-7-(3-methyl-2-buten-1 -yl)-8-(3-
amino-piperidin-1 -yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.48 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 457 [M+H]+
(54) 1-(3,3-dimethyl-2-oxo-butyl)-3-methyl -7-(3-methyl-2-buten-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.49 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 431 [M+H]+
(55) 1-[(quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-amino-
piperidin-
1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.20 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:0.1)
Mass spectrum (ESI+): m/z = 459 [M+H]+
(56) 1-[(2-methyl-naphthalen-1-yl)methyl]-3-methyl -7-(2-butyn-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.25 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:0.1)
Mass spectrum (ESI+): m/z = 471 [M+H]+
(57) 1-({5-[(methoxycarbonyl)methylamino]-isoquinolin-1-yl}methyl)-3-methyl-
7-(3-methyl-2-buten-1-yl)-8-(3-amino-piperidin-l-yl)-xanthine
CA 02496249 2005-02-18
147
CA 02496249 2005-02-18
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.43 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 561 [M+H]+
(58) 1-(2-dimethylamino-2-oxo-ethyl)-3-methyl -7-(3-methyl-2-buten-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.38 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 418 [M+H]+
(59) 1-[2-(piperidin-1-yl)-2-oxo-ethyl]-3-methyl-7-(3-meth yl-2-buten-1-yl)-8-
(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.35 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 458 [M+H]+
(60) 1-[(2-methyl-1-oxo-l,2-dihydro-isoquinoline-4-yl)methyl]-7-(2-butyn-l-yl)-
8-(3-amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.17 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:0.1)
Mass spectrum (ESI+): m/z = 488 [M+H]+
(61) 1-[(2-methyl-1-oxo-1,2-dihydro-isoquinoline-4-yl)methyl]-7-(3-methyl-2-
buten-l -yl)-8-(3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.13 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:0.1)
Mass spectrum (ESI+): m/z = 504 [M+H]+
148
(62) 1-[(2-methoxy-naphthalen-l-yl)methyl]-3-methyl-7-(2-butyn-1-yi)-8-(3-
amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.17 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:0.1)
Mass spectrum (ESI+): m/z = 487 [M+H]+
(63) 1-[(isoquinolin-l-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-amino-
piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.42 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 458 [M+H]+
(64) 1-[(4-methoxy-naphthalen-1-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.14 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:0.1)
Mass spectrum (ESI+): m/z = 487 [M+H]+
(65) 1-[(3-methyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Melting point: 155-158 C
Mass spectrum (ESI+): m/z = 472 [M+H]+
(66) 1-[2-(2,3-dimethoxy-phenyl)-2-oxo-ethyl]-3-meth yl-7-(3-methyl -2-buten-1-
yl)-8-(3-amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.40 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 511 [M+H]+
CA 02496249 2005-02-18
149
(67) 1-[(5-nitro-naphthalen-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-amino-
piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.15 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:0.1)
Mass spectrum (ESI+): m/z = 502 [M+H]+
(68) 1-[2-(pyrrolidin-1-yl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-yl)-8-
(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.56 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 444 [M+H]+
(69) 1-[(4-methyl-isoquinoIin-1-yl)methyl]-3-methyl -7-(2-butyn-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.46 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 472 [M+H]+
(70) 1-[(2-naphthyl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-amino-piperidin-1-
yl )-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.20 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:0.1)
Mass spectrum (ESI+): m/z = 457 [M+H]+
(71) 1-[(4-oxo-3,4-dihydro-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-
(3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.38 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:0.1)
Mass spectrum (ESI+): m/z = 475 [M+H]+
CA 02496249 2005-02-18
150
CA 02496249 2005-02-18
(72) 1-[(quinolin-6-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-amino-piperidin-
1-
yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.15 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:0.1)
Mass spectrum (ESI+): m/z = 458 [M+H]+
(73) 1-[(4-dimethylamino-naphthalen-l-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-
(3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.18 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:0.1)
Mass spectrum (ESI+): m/z = 500 [M+H]+
(74) 1-[(isoquinolin-1-yl)methyl]-3-methyl-7-((E)-2-buten-1-yl)-8-(3-amino-
piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride;
the product still contains approx. 20 % of Z isomer)
Rf value: 0.66 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:0.1)
Mass spectrum (ESI+): m/z = 460 [M+H]+
(75) 1-[(3-methoxy-naphthalen-2-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.25 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:0.1)
Mass spectrum (ESI+): m/z = 487 [M+H]+
(76) 1-[2-(2,3-dihydro-benzo[1,4]dioxin-5-yl)-2-oxo-ethyl]-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Mass spectrum (ESI+): m/z = 509 [M+H]+
151
(77) 1-[(3-methyl-4-oxo-3,4-dihydro-quinazolin-2-yl)methyl]-3-methyl-7-(2-
butyn-1-yl)-8-(3-amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.20 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:0.1)
Mass spectrum (ESI+): m/z = 489 [M+H]+
(78) 1-[2-(3-methyl-2-oxo-2,3-dihydro-benzooxazol-7-yl)-2-oxo-ethyl]-3-
methyl-7-(3-methyl-2-buten-l-yi)-8-(3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.47 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 522 [M+H]+
(79) 1-[2-(benzo[1,3]dioxoI-4-yl)-2-oxo-ethyl]-3-methyl -7-(3-methyl-2-buten-1-
yl)-8-(3-amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Mass spectrum (ESI+): m/z = 495 [M+H]+
(80) 1-[(quinazolin-2-yl)methyl]-3-methyl -7-(2-butyn-1-yl)-8-((R)-3-amino-
piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Mass spectrum (ESI+): m/z = 459 [M+H]+
(81) 1-[(quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-((S)-3-amino-
piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Mass spectrum (ESI+): m/z = 459 [M+H]+
(82) 1-[(quinazolin-2-yl)methyl]-3-methyl-7-((E)-2-buten-l-yl)-8-((S)-3-amino-
piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride;
the product still contains approx. 20 % of Z isomer)
CA 02496249 2005-02-18
152
Rf value: 0.12 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:0.1)
Mass spectrum (ESI+): m/z = 461 [M+H]+
(83) 1-[(quinazolin-2-yl)methyl]-3-methyl-7-((E)-2-buten-l-yl)-8-((R)-3-amino-
piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride;
the product still contains approx. 15 % of Z isomer)
Rf value: 0.12 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:0.1)
Mass spectrum (ESI+): m/z = 461 [M+H]+
(84) 1-[(3-methyl-isoquinolin-1-yl)methyl]-3-methyl -7-((E)-2-buten-1-yl)-8-
((R)-
3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride;
the product still contains approx. 17 % of Z isomer)
Rf value: 0.54 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 474 [M+H]+
(85) 1-[(3-methyl-isoquinolin-l-yl)methyl]-3-methyl-7-((E)-2-buten-1-yl)-8-
((S)-
3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride;
the product still contains approx. 17 % of Z isomer)
Rf value: 0.54 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 474 [M+H]+
(86) 1-(2-{2-[(ethylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
(2-butyn-l-yl)-8-((R)-3-amino-piperidin-l-yl)-xanthine
Mass spectrum (ESI+): m/z = 536 [M+H]+
(87) 1-[2-(2-amino-phenyl)-2-oxo-ethyl]-3-methyl-7-[(1-cyclopenten-1-
yl)methyl]-8-(3-amino-piperidin-l -yl)-xanthine
CA 02496249 2005-02-18
153
CA 02496249 2005-02-18
Mass spectrum (ESI+): m/z = 478 [M+H]+
(88) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-[(1-cyclopenten-1-yl)methyl]-8-(3-
amino-piperidin-l-yl)-xanthine
Mass spectrum (ESI+): m/z = 463 [M+H]+
(89) 1-(2-{2-[(methyl aminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
[(1-cyclopenten-1-yl)methyl]-8-(3-amino-piperidin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 550 [M+H]+
(90) 1-methyl-3-isopropyl-7-(2-cyano-be nzyl)-8-(3-amino-piperidin-l-yl)-
xanthine
Rf value: 0.40 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 422 [M+H]+
(91) 1-(2-{2-[(methylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
(2-butyn-1-yl)-8-(3-amino-piperidin-l-yl)-xanthine
Mass spectrum (ESI+): m/z = 522 [M+H]+
(92) 1-(2-{2-[(ami nocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(2-
butyn-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 508 [M+H]+
(93) 1-[2-(2-cyanomethylamino-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-
buten-1-yl)-8-(3-amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.50 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 505 [M+H]+
(94) 1-(2-{2-[(isopropylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-
methyl-7-(2-butyn-1-yl)-8-(3-amino-piperidin-l-yl)-xanthine
Rf value: 0.30 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 550 [M+H]+
154
CA 02496249 2005-02-18
(95) 1-[(isoquinolin-1-yl)methyl]-3-[(methoxycarbonyl)methyl]-7-(3-methyl-2-
buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine
Rf value: 0.21 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 532 [M+H]+
(96) 1-[2-(2-acetylamino-phenyl)-2-oxo-ethyl]-3-methyl-7-((E)-2-buten-1-yl)-8-
(3-amino-piperidin-1-yl)-xanthine
(product contains approx. 10 % of Z isomer)
Mass spectrum (ESI+): m/z = 494 [M+H]+
(97) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-((E)-2-buten-1-yl)-8-(3-amino-
piperidin-1-yl)-xanthine
(product contains approx. 25 % of Z isomer)
Rf value: 0.30 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 437 [M+H]+
(98) 1-(2-{2-[(isopropyloxycarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
(3-methyl-2-buten-1-yl)-8-(3-amino-piperidin-l-yl)-xanthine
Mass spectrum (ESI+): m/z = 567 [M+H]+
(99) 1-(2-{2-[(methyl aminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
(2-butyn-1-yl)-8-((R)-3-amino-piperidin-l-yl)-xanthine
Mass spectrum (ESI+): m/z = 522 [M+H]+
(100) 1-(2-{2-[(isopropylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-((E)-
2-buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine
(product contains approx. 10 % of Z isomer)
Mass spectrum (ESI+): m/z = 522 [M+H]+
(101) 1-(2-{2-[(methyl aminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-
7-((E)-2-buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine
(product contains approx. 8 % of Z isomer)
155
CA 02496249 2005-02-18
Rf value: 0.51 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/trifluoroacetic acid = 50:50:0.1)
Mass spectrum (ESI+): m/z = 524 [M+H]+
(102) 1-(2-{2-[(ethylaminocarbonyl)methoxy]-phenyl)-2-oxo-ethyl)-3-methyl-7-
(2-butyn-1-yl)-8-((S)-3-amino-piperidin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 536 [M+H]+
(103) 1-[2-(2-{[(ethoxycarbonylamino)carbonyl]amino}-phenyl)-2-oxo-ethyl]-3-
methyl-7-(3-methyl-2-buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 581 [M+H]+
(104) 1-[2-(2-amino-phenyl)-2-oxo-ethyl]-3-methyl-7-((E)-2-buten-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
Rf value: 0.54 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:0.1)
Mass spectrum (ESI+): m/z = 452 [M+H]+
(105) 1-[2-(2-acetylamino-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-
1-yl)-8-((S)-3-amino-piperidin-l-yl)-xanthine
Rf value: 0.48 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:0.1)
Mass spectrum (ESI+): m/z = 508 [M+H]+
(106) 1-[2-(2-amino-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-l-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
Rf value: 0.31 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 450 [M+H]+
(107) 1-(2-{2-[(methyl aminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-
7-(2-butyn-1-yl)-8-((S)-3-amino-piperidin-l-yl)-xanthine
Mass spectrum (ESI+): m/z = 522 [M+H]+
156
CA 02496249 2005-02-18
(108) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-((E)-2-buten-l-yl)-8-((R)-3-amino-
piperidin-l-yl)-xanthine
(product contains approx. 22 % of Z isomer)
Mass spectrum (ESI+): m/z = 437 [M+H]+
(109) 1-(2-{2-[(ethylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
(2-butyn-1-yl)-8-(3-amino-piperidin-l-yl)-xanthine
Rf value: 0.40 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 536 [M+H]+
(110) 1-[2-(2-acetylamino-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1 -yl)-8-(3-
amino-piperidin-1-yl)-xanthine
Rf value: 0.23 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 492 [M+H]+
(111) 1-(2-{2-[2-oxo-2-(pyrrolidin-1 -yl)-ethoxy]-phenyl}-2-oxo-ethyl)-3-
methyl-
7-(2-butyn-1 -yl)-8-(3-amino-piperidin-1 -yl )-xanthine
Rf value: 0.20 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 562 [M+H]+
(112) 1-(2-{2-[(methyl am inocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-
7-(3-methyl-2-buten-l-yl)-8-((S)-3-amino-piperidin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 538 [M+H]+
(113) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-(2-butyn-1 -yl)-8-((R)-3-amino-
piperidin-1 -yl)-xanthine
Mass spectrum (ESI+): m/z = 435 [M+H]+
(114) 1-(2-{2-[(ethylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
((E)-2-buten-1-yl)-8-((R)-3-amino-piperidin-l-yl)-xanthine
(product contains approx. 30 % of Z isomer)
Mass spectrum (ESI+): m/z = 538 [M+H]+
- -------- ---
157
CA 02496249 2005-02-18
(115) 1-methyl-7-(2-cyano-be nzyl)-8-(3-amino-piperidin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 380 [M+H]+
(116) 1-(2-{2-[(isopropylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine
Rf value: 0.40 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:0.1)
Mass spectrum (ESI+): m/z = 536 [M+H]+
(117) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-((E)-2-buten-1 -yl)-8-((S)-3-amino-
piperidin-1-yl)-xanthine
(product contains approx. 23 % of Z isomer)
Rf value: 0.42 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:0.1)
Mass spectrum (ESI+): m/z = 437 [M+H]+
(118) 1-(2-{2-[(isopropylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-(2-
butyn-1-yl)-8-(3-amino-piperidin-l-yl)-xanthine
Rf value: 0.20 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 520 [M+H]+
(119) 1-[2-(2-acetylamino-ph enyl)-2-oxo-ethyl]-3-methyl -7-(2-butyn-1-yl)-8-
((S)-3-amino-piperidin-1-yl)-xanthine
Rf value: 0.15 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 492 [M+H]+
(120) 1 -(2-{2-[(i so propylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl -7-
(2-
butyn-l-yl)-8-((S)-3-amino-piperidin-l-yl)-xanthine
Mass spectrum (ESI+): m/z = 520 [M+H]+
(121) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-(2-methyl-allyl)-8-(3-amino-
piperidin-1-yl)-xanthine
158
Rf value: 0.21 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 437 [M+H]+
(122) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-(3-bromo-allyi)-8-(3-amino-
piperidin-1-yl)-xanthine
Rf value: 0.14 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 501, 503 [M+H]+
(123) 1-(2-{2-[(methoxycarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-(3-amino-piperidin-l-yl)-xanthine
Rf value: 0.42 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:0.1)
Mass spectrum (ESI+): m/z = 524 [M+H]+
(124) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-[(furan-2-yl)methyl]-8-(3-amino-
piperidin-1-yl)-xanthine
Rf value: 0.23 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 463 [M+H]+
(125) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-(2-chloro-allyl)-8-(3-amino-
piperidin-1-yl)-xanthine
Rf value: 0.18 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
(126) 1-{2-[2-(1-methoxycarbonyl-ethoxy)-phenyl]-2-oxo-ethyl}-3-methyl-7-(2-
butyn-l-yl)-8-(3-amino-piperidin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 537 [M+H]+
(127) 1-{2-[2-(1-aminocarbonyl-ethoxy)-phenyl]-2-oxo-ethyl}-3-methyl-7-(2-
butyn-1-yl)-8-(3-amino-piperidin-l-yl)-xanthine
Mass spectrum (ESI+): m/z = 522 [M+H]+
CA 02496249 2005-02-18
159
(128) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-(2-butyn-l-yl)-8-((S)-3-amino-
piperidin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 435 [M+H]+
(129) 1-[(3-methyl -isoquinolin-1-yl)methyl]-3-meth yl-7-(2-butyn-1-yl)-8-((S)-
3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Melting point: 155-156.5 C
Mass spectrum (ESI+): m/z = 472 [M+H]+
(130) 1-[(3-methyl- isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-((R)-
3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.52 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 472 [M+H]+
(131) 1-[(4-methyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-((S)-3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.46 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 472 [M+H]+
(132) 1-[(4-methyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.46 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 472 [M+H]+
(133) 1-[(4-methyl-isoquinolin-1-yl)methyl]-3-methyl-7-((E)-2-buten-l-yl)-8-
((S)-3-amino-piperidin-l-yl)-xanthine
CA 02496249 2005-02-18
160
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.48 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 474 [M+H]+
(134) 1-[(4-methyl-isoquinolin-1-yl)methyl]-3-methyl-7-((E)-2-buten-1-yl)-8-
((R)-3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Melting point: 167.5-172 C
Mass spectrum (ESI+): m/z = 474 [M+H]+
(135) 1-[2-(2,3-dihydro-benzo[1,4]dioxin-5-yl)-2-oxo-ethyl]-3-methyl -7-(2-
butyn-1-yl)-8-(3-(R)-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.34 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 493 [M+H]+
(136) 1-[2-(2,3-dihydro-benzo[1,4]dioxin-5-yl)-2-oxo-ethyl]-3-methyl-7-(2-
butyn-l-yl)-8-(3-(S)-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Mass spectrum (ESI+): m/z = 493 [M+H]+
(137) 1-[(4-methoxy-naphthalen-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-((S)-
3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.52 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 487 [M+H]+
(138) 1-[(4-methoxy-naphthalen-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-((R)-
3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
CA 02496249 2005-02-18
161
CA 02496249 2005-02-18
Rf value: 0.52 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 487 [M+H]+
(139) 1-[2-(benzo[1, 3]dioxol-4-yl)-2-oxo-ethyl]-3-methyl -7-(2-butyn-1-yl)-8-
((R)-3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.41 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 479 [M+H]+
(140) 1-[2-(benzo[1,3]dioxol-4-yl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-
((S)-3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Mass spectrum (ESI+): m/z = 479 [M+H]+
(141) 1-[(4-methyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-(S)-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.51 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 473 [M+H]+
(142) 1-[(4-methyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-(3-(R)-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Melting point: 198-202 C
Mass spectrum (ESI+): m/z = 473 [M+H]+
(143) 1-[2-(3-methyl-2-oxo-2,3-dihydro-benzooxazol-4-yl)-2-oxo-ethyl]-3-
methyl-7-(3-methyl-2-buten-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.53 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
162
CA 02496249 2005-02-18
Mass spectrum (ESI+): m/z = 522 [M+H]+
(144) 1-(2-{2-[(ethylaminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-
((E)-2-buten-1-yl)-8-((S)-3-amino-piperidin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 538 [M+H]+
(145) 1-(2-{2-[(isopropylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-((E)-
2-buten-l-yl)-8-((S)-3-amino-piperidin-l-yl)-xanthine
Rf value: 0.49 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:0.1)
Mass spectrum (ESI+): m/z = 522 [M+H]+
(146) 1-(2-{2-[(ethylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-((E)-2-
buten-1 -yl)-8-((R)-3-amino-piperidin-1 -yl)-xanthine
Mass spectrum (ESI+): m/z = 508 [M+H]+
(147) 1 - [2-(2-acetyl am i no-phenyl)-2-oxo-ethyl]-3-methyl -7-((E)-2-buten-1-
yl)-
8-((R)-3-amino-pi peridin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 494 [M+H]+
(148) 1-(2-{2-[(methyl aminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-
7-((E)-2-buten-1-yl)-8-((R)-3-amino-piperidin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 524 [M+H]+
(149) 1-[2-(2-acetylamino-phenyl)-2-oxo-ethyl]-3-methyl-7-((E)-2-buten-1-yl)-
8-((S)-3-amino-piperidin-l-yl)-xanthine
Rf value: 0.49 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:0.1)
Mass spectrum (ESI+): m/z = 494 [M+H]+
(150) 1-(2-{2-[(methyl aminocarbonyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-
7-((E)-2-buten-1-yl)-8-((S)-3-amino-piperidin-l-yl)-xanthine
Mass spectrum (ESI+): m/z = 524 [M+H]+
163
CA 02496249 2005-02-18
(151) 1-(2-{2-[(isopropylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-(2-
butyn-1-yl)-8-((R)-3-amino-piperidin-l-yl)-xanthine
Mass spectrum (ESI+): m/z = 520 [M+H]+
(152) 1-(2-{2-[(isopropylcarbonyl)amino]-phenyl)-2-oxo-ethyl)-3-methyl-7-((E)-
2-buten-l-yl)-8-((R)-3-amino-piperidin-l-yl)-xanthine
Mass spectrum (ESI+): m/z = 522 [M+H]+
(153) 1-(2-{2-[2-(morpholin-4-yl)-2-oxo-ethoxy]-phenyl}-2-oxo-ethyl)-3-methyl-
7-(2-butyn-l-yl)-8-(3-amino-piperidin-l-yl)-xanthine
Mass spectrum (ESI+): m/z = 578 [M+H]+
(154) 1-(2-{2-[(ethylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-((E)-2-
buten-l-yl)-8-((S)-3-amino-piperidin-l-yl)-xanthine
Rf value: 0.50 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:0.1)
Mass spectrum (ESI+): m/z = 508 [M+H]+
(155) 1-(2-{2-[(ethylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-(2-butyn-
1-yl)-8-((R)-3-amino-piperidin-l-yl)-xanthine
Mass spectrum (ESI+): m/z = 506 [M+H]+
(156) 1-(2-{2-[(ethylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-(2-butyn-
1-yl)-8-((S)-3-amino-piperidin-1-yl)-xanthine
Rf value: 0.20 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 506 [M+H]+
(157) 1-[2-(2-acetylamino-ph enyl)-2-oxo-ethyl]-3-methyl -7-(2-butyn-l-yl)-8-
((R)-3-a m i n o-p i pe ri d i n-1-yl)-xanth i n e
Mass spectrum (ESI+): m/z = 492 [M+H]+
(158) 1-[2-(2-nitro-3-methoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-
buten-l-yl)-8-(3-amino-piperidin-l-yl)-xanthine
164
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.49 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 526 [M+H]+
(159) 1-(2-{2-[(phenylcarbonyl)amino]-phenyl}-2-oxo-ethyl)-3-methyl-7-((E)-2-
buten-1 -yl)-8-(3-amino-piperidin-1-yl)-xanthine
Rf value: 0.49 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/trifluoroacetic acid = 50:50:0.1)
Mass spectrum (ESI+): m/z = 556 [M+H]+
(160) 1-[(2-acetyl-benzofuran-3-yl)methyl]-3-methyl-7-(2-butyn-l-yi)-8-(3-
amino-piperidin-1-yl)-xanthine
(Obtained as main product when 1-{2-[2-(2-oxo-propoxy)-phenyl]-2-oxo-ethyl}-
3-methyl-7-(2-butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1 -yl]-
xanthine is treated with trifluoroacetic acid in methylene chloride)
Mass spectrum (ESI+): m/z = 489 [M+H]+
(161) 1-{2-[2-(1-ethoxycarbonyl-1-methyl-ethoxy)-phenyl]-2-oxo-ethyl}-3-
methyl-7-(2-butyn-l-yl)-8-(3-amino-piperidin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 565 [M+H]+
(162) 1-[2-(2-amino-3-methoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-
buten-l-yl)-8-(3-amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.38 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 496 [M+H]+
(163) 1-[(4-dimethylamino-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-
(3-amino-piperidin-l-yl)-xanthine
Rf value: 0.30 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:0.1)
Mass spectrum (ESI+): m/z = 502 [M+H]+
CA 02496249 2005-02-18
165
CA 02496249 2005-02-18
(164) 1-[2-(2-oxo-2,3-dihydro-benzooxazol-7-yl)-2-oxo-ethyl]-3-methyl-7-(3-
methyl-2-buten-1-yl)-8-(3-amino-piperidin-l-yl)-xanthine
Rf value: 0.42 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 508 [M+H]+
(165) 1-(2-{2-[(ethoxycarbonyl)methylamino]-phenyl}-2-oxo-ethyl)-3-methyl-7-
((E)-2-buten-1-yl)-8-((R)-3-amino-piperidin-l-yl)-xanthine
Rf value: 0.51 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:0.1)
Mass spectrum (ESI+): m/z = 538 [M+H]+
(166) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-((Z)-2-methyl-2-buten-1-yl)-8-(3-
amino-piperidin-1-yi)-xanthine
Rf value: 0.29 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 451 [M+H]+
(167) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-((E)-2-methyl-2-buten-l-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
Rf value: 0.59 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 80:20:1)
Mass spectrum (ESI+): m/z = 451 [M+H]+
(168) 1-[(imidazo[1,2-a]pyridin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.47 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 447 [M+H]+
(169) 1-[(quinoxalin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-amino-
piperidin-1-yl)-xanthine
166
CA 02496249 2005-02-18
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.50 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 459 [M+H]+
(170) 1-[2-(1,3-dimethyl-2-oxo-2,3-dihydro-1 H-benzoimidazol-4-yl)-2-oxo-
ethyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.55 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 519 [M+H]+
(171) 1-[(quinoxalin-6-yl)methyl]-3-methyl -7-(2-butyn-1-yl)-8-(3-amino-
piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Mass spectrum (ESI+): m/z = 459 [M+H]+
(172) 1-[(2-cyano-benzofuran-3-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 472 [M+H]+
(173) 1-[2-(2-oxo-2,3-dihydro-benzooxazol-4-yl)-2-oxo-ethyl]-3-methyl-7-(2-
butyn-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 492 [M+H]+
Rf value: 0.47 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
(174) 1-[(3-methyl-quinoxalin-2-yl)methyl]-3-methyl -7-(2-butyn-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
Melting point: 188.5-191 C
Mass spectrum (ESI+): m/z = 473 [M+H]+
(175) 1-[(3-phenyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
167
CA 02496249 2005-02-18
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.45 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 534 [M+H]+
(176) 1-(2-{2-[(methanesulphinyl)methoxy]-phenyl}-2-oxo-ethyl)-3-methyl-7-(2-
butyn-l-yl)-8-(3-amino-piperidin-l-yl)-xanthine x trifluoroacetic acid
Mass spectrum (ESI+): m/z = 527 [M+H]+
(177) 1-[(benzofuran-3-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-amino-
piperidin-l-yl)-xanthine
(Produced when 1-{[2-(tert.-butylcarbonyl)-benzofuran-3-yl]methyl}-3-methyl-
7-(2-butyn-1-yl)-8-[3-(tert.-butyloxycarbonylamino)-piperidin-1-yl]-xanthine
is
treated with trifluoroacetic acid in methylene chloride)
Mass spectrum (ESI+): m/z = 447 [M+H]+
(178) 1-[(3,4-dimethyl-isoquinolin-l-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine-hydrochloride
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.75 (aluminium oxide, methylene chloride/methanol = 10:1)
Mass spectrum (ESI+): m/z = 486 [M+H]+
(179) 1-[(benzofuran-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-((R)-3-amino-
piperidin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 447 [M+H]+
(180) 1-{[4-(morpholin-4-yl)-quinazolin-2-yl]methyl}-3-methyl-7-(2-butyn-1-yl)-
8-(3-amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.45 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:0.1)
Mass spectrum (ESI+): m/z = 544 [M+H]+
168
CA 02496249 2005-02-18
(181) 1-{[4-(piperidin-1-yl)-quinazolin-2-yl]methyl}-3-methyl-7-(2-butyn-1-yl)-
8-
(3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.55 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:0.1)
Mass spectrum (ESI+): m/z = 542 [M+H]+
(182) 1-{[4-(piperazin-1-yl)-quinazolin-2-yl]methyl}-3-methyl-7-(2-butyn-l-yl)-
8-
(3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.23 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:0.1)
Mass spectrum (ESI+): m/z = 543 [M+H]+
(183) 1-{[4-(pyrrolidin-1-yl)-quinazolin-2-yl]methyl}-3-methyl-7-(2-butyn-1-
yl)-8-
(3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.50 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:0.1)
Mass spectrum (ESI+): m/z = 528 [M+H]+
(184) 1-[2-(3-methyl-2-oxo-2,3-dihydro-1 H-benzoimidazol-4-yl)-2-oxo-ethyl]-3-
methyl-7-(2-butyn-l-yl)-8-(3-amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.43 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 505 [M+H]+
(185) 1-[(4-cyano-naphthalen-1-yl)methyl]-3-methyl -7-(2-butyn-1-yl)-8-((R)-3-
amino-piperidin-l-yl)-xanthine
Rf value: 0.27 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 482 [M+H]+
169
CA 02496249 2005-02-18
(186) 1-[(imidazo[1,2-a]pyridine-3-yl)methyl] -3-methyl-7-(2-butyn-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.37 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 447 [M+H]+
(187) 1-[(8-methyl-imidazo[1,2-a]pyridin-2-yl)methyl]-3-methyl -7-(2-butyn-1-
yl)-8-(3-amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.46 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 461 [M+H]+
(188) 1-[(4-amino-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-l -yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.40 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:0.1)
Mass spectrum (ESI+): m/z = 474 [M+H]+
(189) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-((Z)-2-buten-1-yl)-8-(3-amino-
piperidin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 437 [M+H]+
(190) 1-[(8-methoxy-quinolin-5-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine x 2 trifluoroacetic acid
Rf value: 0.45 (silica gel, methylene chloride/methanol = 5:1)
Mass spectrum (ESI+): m/z = 488 [M+H]+
(191) 1-[(5-methoxy-quinolin-8-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-(3-
amino-piperidin-l-yl)-xanthine x trifluoroacetic acid
Rf value: 0.20 (silica gel, ethyl acetate/methanol = 1:1)
Mass spectrum (ESI+): m/z = 488 [M+H]+
170
CA 02496249 2005-02-18
(192) 1-[(4-phenyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-l-yi)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.60 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 535 [M+H]+
(193) 1-[(7-methyl-imidazo[1,2-a]pyridin-2-yl)methyl] -3-methyl-7-(2-butyn-1-
yl)-8-(3-amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.48 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 461 [M+H]+
(194) 1-[(2-cyclopropyl-quinazolin-4-yl)methyl]-3-methyl-7-[(1-cyclopenten-1-
yl)methyl]-8-(3-amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.55 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 527 [M+H]+
(195) 1-(2-oxo-4-phenyl-butyl)-3-methyl-7-(2-butyn-l-yl)-8-((R)-3-amino-
piperidin-l-yl)-xanthine x trifluoroacetic acid
Mass spectrum (ESI+): m/z = 463 [M+H]+
(196) 1-(2-{2-[(methylaminocarbonyl)methylamino]-phenyl}-2-oxo-ethyl)-3-
methyl-7-((E)-2-buten-l-yl)-8-((R)-3-amino-piperidin-l-yl)-xanthine
Rf value: 0.52 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:0.1)
Mass spectrum (ESI+): m/z = 523 [M+H]+
(197) 1-[2-(2-oxo-2,3-dihydro-1 H-benzoimidazol-4-yl)-2-oxo-ethyl]-3-methyl-7-
(2-butyn-l-yl)-8-(3-amino-piperidin-l-yl)-xanthine
171
Rf value: 0.40 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 491 [M+H]+
(198) 1-[(3-difluoromethyl-isoquinolin-1-yl)methyl] -3-methyl-7-(2-butyn-l-yl)-
8-
(3-amino-piperidin-l-yl)-xanthine x trifluoroacetic acid
Rf value: 0.75 (silica gel, methylene chloride/methanol = 10:1)
Mass spectrum (ESI+): m/z = 508 [M+H]+
(199) 1-[2-(2,2-difluoro-benzo[1,3]dioxol-4-yl)-2-oxo-ethyl]-3-methyl-7-(2-
butyn-l-yl)-8-((R)-3-amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.80 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 96:4:0.5)
Mass spectrum (ESI+): m/z = 515 [M+H]+
(200) 1-[(3-methyl-imidazo[1,2-a]pyridin-2-yl)methyl]-3-methyl-7-(2-butyn-1-
yl)-8-(3-amino-piperidin-1-yl)-xanthine
Rf-Wet: 0.45 (silica gel, methylene chloride/methanol/conc. aqueous ammonia
= 90:10:1)
Mass spectrum (ESI+): m/z = 461 [M+H]+
(201) 1-[2-(2,2-difluoro-benzo[1,3]dioxol-4-yl)-2-oxo-ethyl]-3-methyl-7-(2-
butyn-1-yl)-8-((S)-3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Mass spectrum (ESI+): m/z = 515 [M+H]+
(202) 1-[(5-methyl-imidazo[1,2-a]pyridin-2-yl)methyl]-3-methyl-7-(2-butyn-l -
yl)-8-(3-amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.53 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 461 [M+H]+
CA 02496249 2005-02-18
172
(203) 1-[(6-methyl-imidazo[1,2-a]pyridin-2-yl)methyl]-3-methyl -7-(2-butyn-1-
yl)-8-(3-amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Melting point: 176.5-178 C
Mass spectrum (ESI+): m/z = 461 [M+H]+
(204) 1-[(3-benzyl-imidazo[1,2-a]pyridin-2-yl)methyl]-3-methyl-7-(2-butyn-1-
yl)-8-(3-amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Melting point: 201-204 C
Mass spectrum (ESI+): m/z = 537 [M+H]+
(205) 1-[(4-isopropyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-(3-
amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.50 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:0.1)
Mass spectrum (ESI+): m/z = 501 [M+H]+
(206) 1-[(2,3-dihydro-benzo[1,4]dioxin-6-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-
8-(3-amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.65 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 465 [M+H]+
(207) 1-[(1-methyl-1 H-indol-2-yl)methyl]-3-methyl-7-(2-butyn-1 -yl)-8-(3-
amino-
piperidin-1-yl)-xanthine-hydrochloride
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.60 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 460 [M+H]+
CA 02496249 2005-02-18
173
(208) 1-[(quinolin-3-yl)methyl] -3-methyl-7-(2-butyn-l-yl)-8-(3-amino-pi
peridin-
1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.50 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:0.1)
Mass spectrum (ESI+): m/z = 458 [M+H]+
(209) 1-[(3-phenyl-imidazo[1,2-a]pyridin-2-yl)methyl]-3-methyl-7-(2-butyn-1-
yl)-8-(3-amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.50 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 523 [M+H]+
(210) 1-[(1 H-indol-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-amino-
piperidin-
1-yl)-xanthine-hydrochloride
Rf value: 0.55 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 446 [M+H]+
(211) 1 -[2 -(naphtha le n - 1 -yl)-2-oxo-ethyl]-3-m ethyl -7-(2-butyn- 1 -yl)-
8-(3-
amino-piperidin-1 -yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.60 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 485 [M+H]+
(212) 1-[(5-methoxy-isoquinolin-8-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-
amino-piperidin-l-yl)-xanthine x trifluoroacetic acid
Rf value: 0.50 (silica gel, methylene chloride/methanol = 5:1)
Mass spectrum (ESI+): m/z = 488 [M+H]+
(213) 1-{[1-(1-cyano-1-methyl-ethyl)-isoquinolin-3-yl]methyl}-3-methyl-7-(2-
butyn-1-yl)-8-(3-amino-piperidin-l-yl)-xanthine
CA 02496249 2005-02-18
174
CA 02496249 2005-02-18
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.25 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 525 [M+H]+
(214) 1-(2-cyanoimino-2-phenyl-ethyl)-3-methyl -7-(2-butyn-1-yl)-8-(3-amino-
piperidin-l-yl)-xanthine x trifluoroacetic acid (E/Z-mixture)
Mass spectrum (ESI+): m/z = 459 [M+H]+
(215) 1-[(1 H-benzoimidazol-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-amino-
piperidin-1-yl)-xanthine x trifluoroacetic acid
Mass spectrum (ESI+): m/z = 447 [M+H]+
(216) 1-[(1-methyl-1 H-benzoimidazol-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-
(3-amino-piperidin-l-yl)-xanthine
Mass spectrum (ESI+): m/z = 461 [M+H]+
(217) 1-[(4-phenyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Mass spectrum (ESI+): m/z = 535 [M+H]+
(218) 1-[(2,3-dimethyl-quinoxaIin-6-yl)methyl]-3-methyl -7-(2-butyn-l -yl)-8-
(3-
amino-piperidin-1 -yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.50 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:0.1)
Mass spectrum (ESI+): m/z = 487 [M+H]+
(219) 1-[(2-methyl-1 H-benzoimidazol-5-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-
(3-amino-piperidin-1 -yl)-xanthine
Rf value: 0.35 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:0.1)
Mass spectrum (ESI+): m/z = 461 [M+H]+
175
CA 02496249 2005-02-18
(220) 1-[(4-phenyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-((S)-3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Mass spectrum (ESI+): m/z = 535 [M+H]+
(221) 1-[2-(quinolin-8-yl-]-2-oxo-ethyl]-3-methyl-7-(2-butyn-l-yl)-8-(3-amino-
piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.48 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 486 [M+H]+
(222) 1-[(3,4-dimethyl-6,7-dihydro-5H-[2]pyridin-l-yl)methyl]-3-methyl-7-(2-
butyn-1 -yl)-8-(3-amino-piperidin-1 -yl)-xanthine x trifluoroacetic acid
Rf value: 0.25 (aluminium oxide, methylene chloride/methanol = 20:1)
Mass spectrum (ESI+): m/z = 476 [M+H]+
(223) 1-[(3,4-dimethyl-5,6,7,8-tetrahydro-isoquinolin-1-yl)methyl]-3-methyl-7-
(2-butyn-l-yl)-8-(3-amino-piperidin-l-yl)-xanthine x trifluoroacetic acid
Rf value: 0.50 (aluminium oxide, methylene chloride/methanol = 20:1)
Mass spectrum (ESI+): m/z = 490 [M+H]+
(224) 1-[2-(1 H-indol-4-yl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1 -yl)-8-(3-amino-
piperidin-1 -yl)-xanthine
Rf value: 0.52 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 474 [M+H]+
(225) 1-[(1 H-benzoimidazol-5-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-amino-
piperidin-l-yl)-xanthine
Rf value: 0.50 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 447 [M+H]+
176
CA 02496249 2005-02-18
(226) 1-[(pyrazolo[1,5-a]pyridin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.47 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 447 [M+H]+
(227) 1-[(1-methyl-2-oxo-1,2-dihydro-quinolin-6-yl)methyl] -3-meth yl-7-(2-
butyn-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.40 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 488 [M+H]+
(228) 1-[(2-oxo-1,2-dihydro-quinolin-6-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-
(3-amino-piperidin-1-yl)-xanthine
Rf value: 0.23 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 474 [M+H]+
(229) 1-[(2,3,8-trimethyl-quinoxalin-6-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-
(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.37 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 501 [M+H]+
(230) 1-[(8-methyl-quinoxalin-6-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.35 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 473 [M+H]+
177
CA 02496249 2005-02-18
(231) 1-[(4-methyl-phthalazin-1-yl)methyl] -3-methyl-7-(2-butyn-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.55 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 473 [M+H]+
(232) 1-[(4-bromo-3-methoxy-isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-1-
yl)-8-(3-amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.40 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 566, 568 [M+H]+
(233) 1-(2-phenyl-2-oxo-ethyl)-3-methyl-7-((E)-1-buten-l-yl)-8-(3-amino-
piperidin-1-yl)-xanthine
Rf value: 0.31 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 437 [M+H]+
(234) 1-[(4-d ifluoromethoxy-naphthalen-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-
8-((R)-3-amino-piperidin-1-yl)-xanthine
Rf value: 0.08 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:1)
Mass spectrum (ESI+): m/z = 523 [M+H]+
(235) 1-[2-(1 H-indol-7-yl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-amino-
piperidin-1-yl)-xanthine x trifluoroacetic acid
Rf value: 0.46 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 474 [M+H]+
178
CA 02496249 2005-02-18
(236) 1-[(E)-3-(2-nitro-phenyl)-2-propen-1-yl]-3-methyl-7-(2-butyn-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 478 [M+H]+
(237) 1-((E)-3-pentafluorophenyl-2-propen-1-yl)-3-methyl-7-(2-butyn-1-yl)-8-
(3-amino-piperidin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 523 [M+H]+
(238) 1-[(4-nitro-naphthalen-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine
Rf value: 0.38 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 502 [M+H]+
(239) 1-{[1-(2-cyano-ethyl)-1 H-benzoimidazol-2-yl]methyl}-3-methyl-7-(2-
butyn-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine-hydrochloride
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.55 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:0.1)
Mass spectrum (ESI+): m/z = 500 [M+H]+
(240) 1-({1-[(methylaminocarbonyl)methyl]-1 H-benzoimidazol-2-yl}methyl)-3-
methyl-7-(2-butyn-1-yl)-8-(3-amino-piperidin-1-yl)-xanthine x trifluoroacetic
acid
Mass spectrum (ESI+): m/z = 518 [M+H]+
(241) 1-[(1-benzyl-1 H-benzoimidazol-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-
(3-amino-piperidin-1-yl)-xanthine
Rf value: 0.47 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:0.1)
Mass spectrum (ESI+): m/z = 537 [M+H]+
(242) 1-[(benzooxazol-2-y)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-amino-
piperidin-l-yl)-xanthine x trifluoroacetic acid
179
CA 02496249 2005-02-18
Rf value: 0.50 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:0.1)
Mass spectrum (ESI+): m/z = 448 [M+H]+
(243) 1-[(5-nitro-benzooxazol-2-y)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
Rf value: 0.49 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:0.1)
Mass spectrum (ESI+): m/z = 493 [M+H]+
(244) 1-[(3-methyl-isoquinolin-1-yl)methyl]-3-methyl-7-(3-methyl-1-buten-1-yl)-
8-((R)-3-amino-piperidin-l-yl)-xanthine
Rf value: 0.21 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 488 [M+H]+
(245) 1-[(quinolin-7-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-amino-piperidin-
1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.55 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 458 [M+H]+
(246) 1-[([1,5]naphthyridin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-amino-
piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.51 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 459 [M+H]+
(247) 1-[(8-methyl-quinoxalin-6-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
180
Rf value: 0.49 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 473 [M+H]+
(248) 1-[(2,3,8-trimethyl-quinoxalin-6-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-
((R)-3-amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.46 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 501 [M+H]+
(249) 1-[([1,6]naphthyridin-5-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-(3-amino-
piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.50 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 459 [M+H]+
(250) 1-[([1,8]naphthyridin-2-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-(3-amino-
piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.45 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 459 [M+H]+
(251) 1-[(4-fluoro-naphthalen-1-yl)methyl]-3-methyl-7-(2-butyn-l-yl)-8-((R)-3-
amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.50 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 475 [M+H]+
(252) 1-[([1,5]naphthyridin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine
CA 02496249 2005-02-18
181
CA 02496249 2005-02-18
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Mass spectrum (ESI+): m/z = 459 [M+H]+
(253) 1-[2-(3-methyl-2-oxo-2,3-dihydro-benzooxazol-4-yl)-2-oxo-ethyl]-3-
methyl-7-(2-butyn-1-yl)-8-((R)-3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Melting point: 187-189 C
Mass spectrum (ESI+): m/z = 506 [M+H]+
(254) 1-[(8-phenyl-quinoxalin-6-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.50 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 535 [M+H]+
(255) 1-[([1,5]naphthyridine-4-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.52 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 459 [M+H]+
(256) 1-((E)-3-pentafluorophenyl-allyl)-3-methyl-7-(2-butyn-1-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 523 [M+H]+
(257) 1-[(E)-3-(2-trifluoromethyl-phenyl)-allyl]-3-methyl-7-(2-butyn-1-yl)-8-
((R)-
3-amino-piperidin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 501 [M+H]+
(258) 1-[(E)-3-(3-trifluoromethyl-phenyl)-allyl]-3-methyl-7-(2-butyn-l -yl)-8-
((R)-
3-amino-piperidin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 501 [M+H]+
182
CA 02496249 2005-02-18
(259) 1-[(E)-3-(4-trifluoromethyl-phenyl)-allyl]-3-methyl-7-(2-butyn-1-yl)-8-
((R)-
3-amino-piperidin-1-yl)-xanthine
Mass spectrum (ESI+): m/z = 501 [M+H]+
(260) 1-[(3-trifluoromethyl-isoquinolin-l-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-
8-
((R)-3-amino-piperidin-l-yl)-xanthine x trifluoroacetic acid
Mass spectrum (ESI+): m/z = 526 [M+H]+
(261) 1-[(3-methyl-isoquinolin-1-yl)methyl]-3-isopropyl-7-(2-butyn-1-yl)-8-
((R)-
3-amino-piperidin-1-yl)-xanthine
(262) 1-[(3-methyl-isoquinolin-1-yl)methyl] -3-(4-fluorophenyl)-7-(2-butyn-1-
yl)-
8-((R)-3-amino-piperidin-1-yl)-xanthine
(263) 1-[(3-methyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-cyano-benzyl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.51 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 535 [M+H]+
(264) 1-[(3-d ifluoromethyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-
8-
((R)-3-amino-piperidin-l-yl)-xanthine
Mass spectrum (ESI+): m/z = 508 [M+H]+
(265) 1-[(4-chloro-3-methoxy-isoquinolin-l-yl)methyl]-3-methyl-7-(2-butyn-1-
yl)-8-(3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Mass spectrum (ESI+): m/z = 522, 524 [M+H]+
Rf value: 0.40 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
183
CA 02496249 2005-02-18
(266) 1-[(4-ethoxy-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 1 M ethereal hydrochloric acid)
Rf value: 0.60 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 503 [M+H]+
(267) 1-[(4-isopropyloxy-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-
(3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.55 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 517 [M+H]+
(268) 1-[(2-methyl-benzothiazol-6-yl)methyl]-3-methyl-7-(2-butyn-l -yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Melting point: 167 C
Mass spectrum (ESI+): m/z = 478 [M+H]+
(269) 1-[(3-phenyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.45 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 534 [M+H]+
(270) 1-[(4-phenyloxy-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.60 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 551 [M+H]+
184
CA 02496249 2005-02-18
(271) 1-[(4-phenyl-quinazolin-2-yl)methyl]-3-cyclopropyl-7-(2-butyn-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.45 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 561 [M+H]+
(272) 1-[(3-methyl-isoquinolin-1-yl)methyl]-3-cyclopropyl-7-(2-butyn-1-yl)-8-
(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.55 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 498 [M+H]+
(273) 1-[(2-phenyl-quinazolin-4-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.50 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 535 [M+H]+
(274) 1-[2-(2-methoxy-phenyl)-2-oxo-ethyl]-3-methyl -7-(2-butyn-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.29 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 465 [M+H]+
(275) 1-[2-(3-methoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-(3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.27 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 465 [M+H]+
185
CA 02496249 2005-02-18
(276) 1-[2-(3-trifluoromethoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-
8-((R)-3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.29 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 519 [M+H]+
(277) 1-[2-(biphenyl-2-yl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.35 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 511 [M+H]+
(278) 1-[2-(biphenyl-3-yl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.35 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 511 [M+H]+
(279) 1-[2-(3-isopropyloxy-phenyl)-2-oxo-ethyl]-3-methyl -7-(2-butyn-1-yl)-8-
((R)-3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.20 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 493 [M+H]+
(280) 1-[(3-methyl-isoquinolin-1-yl)methyl]-3-cyclopropyl-7-(2-butyn-1-yl)-8-
((R)-3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.50 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
186
CA 02496249 2005-02-18
Mass spectrum (ESI+): m/z = 498 [M+H]+
(281) 1-[(4-phenyl-quinazolin-2-yl)methyl]-3-cyclopropyl-7-(2-butyn-1-yl)-8-
((R)-3-amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.45 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 561 [M+H]+
(282) 1-[(4-cyano-naphthalen-1-yl)methyl]-3-cyclopropyl-7-(2-butyn-1-yl)-8-
((R)-3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Melting point: 191 C
Mass spectrum (ESI+): m/z = 508 [M+H]+
(283) 1-[2-(2-phenyloxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-((R)-
3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.40 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 527 [M+H]+
(284) 1-[2-(3-ethoxy-phenyl)-2-oxo-ethyl]-3-methyl -7-(2-butyn-l-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.29 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 479 [M+H]+
(285) 1-[2-(3-methoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.28 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
187
CA 02496249 2005-02-18
Mass spectrum (ESI+): m/z = 465 [M+H]+
(286) 1-[2-(2-methoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-8-((R)-3-
amino-piperidin-l-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.34 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 465 [M+H]+
(287) 1-[(3-methyl-isoquinolin-1-yl)methyl]-3-methyl -7-(2-chloro-benzyl)-8-
((R)-3-amino-piperidin-l-yl)-xanthine
Mass spectrum (ESI+): m/z = 544, 546 [M+H]+
(288) 1-[(3-methyl-isoquinolin-l-yl)methyl]-3-methyl-7-(2-bromo-benzyl)-8-
((R)-3-amino-piperidin-l-yl)-xanthine
Mass spectrum (ESI+): m/z = 588, 590 [M+H]+
(289) 1-[(1,2,3,4-tetrahydro-phenanthrid in-6-yl)methyl] -3-methyl-7-(2-butyn-
1-
yl)-8-(3-amino-piperidin-1-yl)-xanthine x trifluoroacetic acid
Rf value: 0.75 (aluminium oxide, methylene chloride/methanol = 10:1)
Mass spectrum (ESI+): m/z = 512 [M+H]+
(290) 1-[2-(3-difluoromethoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(2-butyn-1-yl)-
8-((R)-3-amino-piperidin-1-yl)-xanthine
(Carried out with 5-6 M isopropanolic hydrochloric acid in methylene chloride)
Rf value: 0.28 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 501 [M+H]+
(291) 1-[(3-methyl-isoquinolin-1-yl)methyl]-3-methyl-7-(2-ethynyl-benzyl)-8-
((R)-3-amino-piperidin-l-yl)-xanthine
(292) 1-[(3-methyl-isoquinolin-l-yl)methyl]-3-phenyl-7-(2-butyn-l-yl)-8-((R)-3-
amino-piperidin-1-yl)-xanthine
188
(293) 1-[(phenanthren-9-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-((R)-3-amino-
piperidin-1-yl)-xanthine
(294) 1-[(4-methyl-quinazolin-2-yl)methyl] -3-methyl-7-(2-chloro-benzyl)-8-
((R)-
3-amino-piperidin-1-yl)-xanthine
Rf value: 0.35 (silica gel, methylene chloride/methanol/triethylamine =
90:10:1)
Mass spectrum (ESI+): m/z = 545, 547 [M+H]+
(295) 1-[(4-phenyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-chloro-benzyl)-8-
((R)-3-amino-piperidin-l-yl)-xanthine
Rf value: 0.40 (silica gel, methylene chloride/methanol/triethylamine
90:10:1)
Mass spectrum (ESI+): m/z = 607, 609 [M+H]+
Example 3
1-[2-(3-carboxymethoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-buten-1-
vl)-8-(3-amino-piperidin-1-yl)-xanthine
Prepared by saponifying 70 mg of 1-(2-{3-[(methoxycarbonyl)methoxy]-
phenyl}-2-oxo-ethyl)-3-methyl -7-(3-methyl-2-buten-1-yl)-8-(3-amino-piperid in-
1-yl)-xanthine with 0.10 ml of 4 M potassium hydroxide solution in a mixture
of
1 ml of tetrahydrofuran and 0.5 ml of methanol at ambient temperature.
Yield: 57 mg (84 % of theory)
Rf value: 0.55 (ready-made reversed phase TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:0.1)
Mass spectrum (ESI+): m/z = 525 [M+H]+
The following compounds are obtained analogously to Example 3:
(1) 1-[2-(2-carboxymethoxy-phenyl)-2-oxo-ethyl]-3-methyl-7-(3-methyl-2-
buten-l-yl)-8-(3-amino-piperidin-1-yl)-xanthine
(Carried out with sodium hydroxide solution)
Mass spectrum (ESI-): m/z = 523 [M-H]-
CA 02496249 2005-02-18
CA 02496249 2005-02-18
189
Example 4
Coated tablets containing 75 mg of active substance
1 tablet core contains:
active substance 75.0 mg
calcium phosphate 93.0 mg
corn starch 35.5 mg
polyvinylpyrrolidone 10.0 mg
hydroxypropylmethylcellulose 15.0 mg
magnesium stearate 1.5 mg
230.0 mg
Preparation:
The active substance is mixed with calcium phosphate, corn starch, polyvin-
ylpyrrolidone, hydroxypropylmethylcellulose and half the specified amount of
magnesium stearate. Blanks 13 mm in diameter are produced in a tablet-
making machine and these are then rubbed through a screen with a mesh size
of 1.5 mm using a suitable machine and mixed with the rest of the magnesium
stearate. This granulate is compressed in a tablet-making machine to form
tablets of the desired shape.
Weight of core: 230 mg
die: 9 mm, convex
The tablet cores thus produced are coated with a film consisting essentially
of
hydroxypropylmethylcellulose. The finished film-coated tablets are polished
with beeswax.
Weight of coated tablet: 245 mg.
Example 5
Tablets containing 100 mg of active substance
Composition:
1 tablet contains:
active substance 100.0 mg
lactose 80.0 mg
CA 02496249 2005-02-18
190
corn starch 34.0 mg
polyvinylpyrrolidone 4.0 mg
magnesium stearate 2.0 mg
220.0 mg
Method of Preparation:
The active substance, lactose and starch are mixed together and uniformly
moistened with an aqueous solution of the polyvinylpyrrolidone. After the
moist
composition has been screened (2.0 mm mesh size) and dried in a rack-type
drier at 50 C it is screened again (1.5 mm mesh size) and the lubricant is
added. The finished mixture is compressed to form tablets.
Weight of tablet: 220 mg
Diameter: 10 mm, biplanar, facetted on both sides and notched on one
side.
Example 6
Tablets containing 150 mg of active substance
Composition:
1 tablet contains:
active substance 150.0 mg
powdered lactose 89.0 mg
corn starch 40.0 mg
colloidal silica 10.0 mg
polyvinylpyrrolidone 10.0 mg
magnesium stearate 1.0 mg
300.0 mg
Preparation:
The active substance mixed with lactose, corn starch and silica is moistened
with a 20% aqueous polyvinylpyrrolidone solution and passed through a
screen with a mesh size of 1.5 mm. The granules, dried at 45 C, are passed
through the same screen again and mixed with the specified amount of
magnesium stearate. Tablets are pressed from the mixture.
CA 02496249 2005-02-18
191
Weight of tablet: 300 mg
die: 10 mm, flat
Example 7
Hard gelatine capsules containing 150 mg of active substance
1 capsule contains:
active substance 150.0 mg
corn starch (dried approx. 80.0 mg
lactose (powdered) approx. 87.0 mg
magnesium stearate 3.0 mg
approx. 420.0 mg
Preparation:
The active substance is mixed with the excipients, passed through a screen
with a mesh size of 0.75 mm and homogeneously mixed using a suitable
apparatus. The finished mixture is packed into size 1 hard gelatine capsules.
Capsule filling: approx. 320 mg
Capsule shell: size 1 hard gelatine capsule.
Example 8
Suppositories containing 150 mg of active substance
1 suppository contains:
active substance 150.0 mg
polyethyleneglycol 1500 550.0 mg
polyethyleneglycol 6000 460.0 mg
polyoxyethylene sorbitan monostearate 840.0 mg
2,000.0 mg
Preparation:
After the suppository mass has been melted the active substance is
homogeneously distributed therein and the melt is poured into chilled moulds.
Example 9
CA 02496249 2005-02-18
192
Suspension containing 50 mg
of active substance
100 ml of suspension contain:
active substance 1.00 g
carboxymethylcellulose-Na-salt 0.10 g
methyl p-hydroxybenzoate 0.05 g
propyl p-hydroxybenzoate 0.01 g
glucose 10.00 g
glycerol 5.00 g
70% sorbitol solution 20.00 g
flavouring 0.30 g
dist. water ad 100 ml
Preparation:
The distilled water is heated to 70 C. The methyl and propyl
p-hydroxybenzoates together with the glycerol and sodium salt of
carboxymethylcellulose are dissolved therein with stirring. The solution is
cooled to ambient temperature and the active substance is added and
homogeneously dispersed therein with stirring. After the sugar, the sorbitol
solution and the flavouring have been added and dissolved, the suspension is
evacuated with stirring to eliminate air.
ml of suspension contain 50 mg of active substance.
Example 10
Ampoules containing 10 mq active substance
Composition:
active substance 10.0 mg
0.01 N hydrochloric acid q.s.
double-distilled water ad 2.0 ml
Preparation:
CA 02496249 2005-02-18
193
The active substance is dissolved in the necessary amount of 0.01 N HCl,
made isotonic with common salt, filtered sterile and transferred into 2 ml
ampoules.
Example 11
Ampoules containing 50 mg of active substance
Composition:
active substance 50.0 mg
0.01 N hydrochloric acid q.s.
double-distilled water ad 10.0 ml
Preparation:
The active substance is dissolved in the necessary amount of 0.01 N HCI,
made isotonic with common salt, filtered sterile and transferred into 10 ml
ampoules.