Note: Descriptions are shown in the official language in which they were submitted.
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-1-
TITLE: METHODS FOR DETECTING PROSTATE CANCER
FIELD OF THE INVENTION
The invention relates to kallikrein 11, prostate-specific antigen, free
prostate-specific antigen and
their use in detecting prostate cancer.
S BACKGROUND OF THE INVENTION
Prostate cancer (CaP) is the most frequently diagnosed cancer in men in North
America and its
mortality rate is second only to lung cancer. Therefore, early diagnosis and
monitoring of prostate cancer is
an important priority. Prostate-specific antigen (PSA) is widely used as the
most reliable tumor marker
established so far~'z. However, non-malignant prostatic diseases, especially
benign prostatic hyperplasia
(BPH) and acute prostatitis also cause serum PSA elevation, thus complicating
the diagnosis of prostate
cancer by PSA measurements alone. Analysis of the molecular forms of PSA
improves specificity for
prostate cancer3'4, The determination of free PSA and its ratio to total PSA
is now clinically established and
is used to reduce the number of unnecessary prostate biopsies5. Despite
numerous efforts to further reduce
unnecessary biopsies, false negative and false positive results still occur
with high frequency.
The human kallikrein 11 gene (KLKll) was originally isolated from human
hippocampus as
trypsin-like serine protease (TLSP)6. Two alternative splice variants of this
gene, also known as hippostasin
have also been identified''9 . With the official nomenclature,
TLSP/hippostasin is now known as human
lcallikrein 11 (hKl1). This protein is encoded by the KLKl l gene that belongs
to the human kallikrein family
along with PSA (hK3) and other kallikreinsl°. hKl l protein has been
found to be highly expressed in the
prostate$'~i.
The citation of any reference herein is not an admission that such reference
is available as prior art
to the instant invention.
SUMMARY OF THE INVENTION
The invention relates to the application of kallikrein 11, free PSA, and total
PSA in the detection of
prostate cancer. These markers may be used for the diagnosis, monitoring,
staging, progression, prevention,
treatment, and prognosis of prostate cancer, and as indicators before surgery
or after relapse,
Kallikrein 11, and PSA, and agents that bind to kallikrein 11 and PSA, may be
used to detect
prostate cancer and they can be used in the diagnostic evaluation of prostate
cancer, and the identification of
subjects with a predisposition to such disorders. Methods for detecting
kallikrein 11 and PSA can be used to
monitor prostate cancer.
The presence of kallikrein 11 and PSA in a sample can be assessed, for
example, by detecting the
presence in the sample of (a) kallikrein 11 and PSA or fragments thereof; or
(b) metabolites which are
produced directly or indirectly by a kallikrein 11 or PSA.
In an aspect, the invention provides a method for detecting prostate cancer in
a subject comprising
measuring kallikrein 11 and prostate specific antigen (PSA) in a sample from
the subject.
In an embodiment, the invention provides a method for detecting kallikrein 11
and PSA comprising
(a) obtaining a sample from a patient; (b) detecting or identifying in the
sample leallikrein 11 and PSA; and
(c) comparing the detected amounts with amounts detected for a standard.
In an aspect of the invention, a method for screening a subject for prostate
cancer is provided
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-2-
comprising (a) obtaining a biological sample from a subject; (b) detecting the
amounts of kallikrein 11, free
PSA, and total PSA in said sample; and (c) comparing said amounts of
kallikrein 11, free PSA, and total
PSA detected to predetermined standards, where detection of different levels
of kallikrein 11, free PSA, and
total PSA compared with that of a standard indicates disease.
The terms "detect", "detecting" and "detection" include assaying or otherwise
establishing the
presence or absence of kallikrein 11, a combination of kallikrein 11 and PSA,
subunits thereof, or
combinations of reagent bound targets, and the like, or assaying for, imaging,
ascertaining, establishing, or
otherwise determining one or more factual characteristics of prostate cancer,
metastasis, stage, or similar
conditions. The term encompasses diagnostic, prognostic, and monitoring
applications.
According to a method involving kallikrein 11 and PSA, the levels in a sample
from a patient are
compared with the normal levels of kallikrein 11 and PSA in samples of the
same type obtained from
controls (e.g. samples from individuals not afflicted with disease).
Significantly altered levels in the sample
of kallikrein 11 and PSA relative to the normal levels in a control is
indicative of disease. Thus, a method is
provided of assessing whether a patient is afflicted with or has a pre-
disposition to prostate cancer,
comprising comparing (a) levels of kallila~ein 11 and PSA in a sample from the
patient; and (b) normal levels
of kallikrein 11 and PSA in samples of the same type obtained from control
patients not afflicted with
prostate cancer, wherein significantly altered levels of kallikrein 11 and PSA
relative to the corresponding
normal levels of kallikrein 11 and, PSA, is an indication that the patient is
afflicted with or has a pre-
disposition to prostate cancer.
A significant difference between the levels of kallikrein 11 and PSA in the
patient and the normal
levels (e.g. lower levels in the patient) is an indication that the patient is
afflicted with or has a predisposition
to prostate cancer.
The present invention also relates to a method for diagnosing and monitoring
prostate cancer in a
subject comprising detecting or measuring kallikrein 11 and PSA in a sample
from the subject using a
reagent that detects kallikrein 11 and PSA, in particular antibodies
specifically reactive with kallikrein 11
and PSA or a part thereof. In an embodiment, the sample is serum.
In an embodiment, the invention relates to a method for diagnosing and
monitoring prostate cancer
in a subject by quantitating leallikrein 11 and PSA in a biological sample
from the subject comprising (a)
reacting the biological sample with antibodies specific for kallila-ein 11 and
PSA which is directly or
indirectly labelled with a detectable substance; and (b) detecting the
detectable substance.
In another aspect the invention provides a method for using antibodies to
detect expression of a
kallilcrein 11 and PSA in a sample, the method comprising: (a) combining
antibodies specific for kallikrein
11 and PSA with a sample under conditions which allow the formation of
antibody:protein complexes; and
(b) detecting complex formation, wherein complex formation indicates
expression of kallila~ein 11 and PSA
in the sample. Expression may be compared with standards and is diagnostic of
prostate cancer.
Embodiments of the methods of the invention involve (a) reacting a biological
sample from a
subject with antibodies specific for kallikrein 11 and PSA which are directly
or indirectly labelled with an
enzyme; (b) adding a substrate for the enzyme wherein the substrate is
selected so that the substrate, or a
reaction product of the enzyme and substrate forms fluorescent complexes; (c)
quantitating kallikrein 11 and
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-3-
PSA in the sample by measuring fluorescence of the fluorescent complexes; and
(d) comparing the
quantitated levels to levels obtained for other samples from the subject
patient, or control subjects. In an
embodiment the quantitated levels are compared to levels quantitated for
control subjects without prostate
cancer wherein lower levels of kallikrein 11 and PSA compared with the control
subjects is indicative of
prostate cancer.
A particular embodiment of the invention comprises the following steps
(a) incubating a biological sample with first antibodies specific for
kallikrein 11 and PSA
which are directly or indirectly labeled with detectable substances, and
second antibodies
specific for kallikrein 11 and PSA which are immobilized;
(b) detecting the detectable substances thereby quantitating kallikrein 11 and
PSA in the
biological sample; and
(c) comparing the quantitated kallikrein 11 and PSA with levels for a
predetermined standard.
The standard may correspond to levels quantitated for samples from control
subjects without
prostate cancer, with a different disease stage, or from other samples of the
subject. Lower levels of
kallikrein 11 and PSA as compared to the standard is indicative of prostate
cancer.
The invention also contemplates the methods described herein using multiple
markers for prostate
cancer. Therefore, the invention contemplates a method for anaylzing a
biological sample for the presence of
lcallikrein 11 and PSA and other markers that are specific indicators of
prostate cancer. The methods
described herein may be modified by including reagents to detect the markers,
or nucleic acids for the
markers.
The invention further relates to a method of assessing the efficacy of a
therapy for inhibiting
prostate cancer in a patient. A method of the invention comprises comparing:
(a) levels of kallikrein 11 and
PSA in a sample from the patient obtained from the patient prior to providing
at least a portion of the therapy
to the patient; and (b) levels of kallikrein 11 and PSA in a second sample
obtained from the patient following
therapy.
A significant difference between the levels of kallikrein 11 and PSA in the
second sample relative
to the first sample (e.g. higher levels of kallikrein 11 and PSA) is an
indication that the therapy is efficacious
for inhibiting prostate cancer.
The "therapy" may be any therapy for treating prostate cancer including but
not limited to
therapeutics, radiation, immunotherapy, gene therapy, and surgical removal of
tissue. Therefore, the method
can be used to evaluate a patient before, during, and after therapy.
In an aspect, the invention provides a method for monitoring the progression
of prostate cancer in a
patient the method comprising:
(a) detecting kallikrein 11 and PSA in a sample from the patient at a first
time point;
(b) repeating step (a) at a subsequent point in time; and
(c) comparing the levels detected in (a) and (b), and therefrom monitoring the
progression of
the prostate cancer.
The invention also provides a method for assessing the potential efficacy of a
test agent for
inhibiting prostate cancer, and a method of selecting an agent for inhibiting
prostate cancer.
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-4-
The invention also contemplates a method of assessing the potential of a test
compound to
contribute to prostate cancer comprising:
(a) maintaining separate aliquots of prostate cancer diseased cells in the
presence and absence
of the test compound; and
(b) comparing the levels of kallikrein 11 and PSA in each of the aliquots.
A significant difference between the levels of kallikrein 11 and PSA in the
aliquot maintained in the
presence of (or exposed to) the test compound relative to the aliquot
maintained in the absence of the test
compound, indicates that the test compound potentially contributes to prostate
cancer.
In the methods of the invention the amount of lcallikrein 11 and the amount of
PSA may be
mathematically combined. In an embodiment of the invention, a method is
provided for detecting or
identifying prostate cancer in a subject comprising:
(a) determining the amount of kallila~ein I 1 in a sample from the subject;
(b) determining the amount of PSA in the sample;
(c) mathematically combining the results of step (a) and step (b); and
(d) relating the combination to the presence of prostate cancer.
In aspects of the invention, the combination is a ratio of kallikrein 11 to
total PSA, or the inverse
thereof. The combination is preferably compared to a mathematical combination
for a predetermined
standard.
In particular, the invention provides a method for screening or identifying
prostate cancer by
determining the ratio between kallikrein llaotal PSA in a sample from a
subject, preferably a serum
sample.
A method of the invention, in particular a method for detecting or identifying
prostate cancer, may
further comprise determining the % free PSA and relating the combination and %
free PSA to the presence
of prostate cancer.
In an aspect the invention provides a method for distinguishing prostate
cancer from benign
prostatic hyperplasia (BPH) in a subject comprising determining the amount of
kallikrein 11 contained in a
sample from the subject, and relating the amount to the presence of prostate
cancer or BPH in the subject.
The amount of kallilaein 11 in the sample may be compared to an amount
determined for a standard. A
standard may be an amount of kallila~ein 11 associated with prostate cancer,
and a higher amount of
kallila-ein 11 in the sample compared to the standard may be indicative of
BPH. A standard may be an
amount of kallikrein 11 associated with BPH, and a lower amount of kallilcrein
11 in the sample compared
to the standard may be indicative of prostate cancer.
In a further aspect, the invention provides a method for distinguishing
prostate cancer from benign
prostatic hyperplasia (BPH) in a subject comprising:
(a) determining the amount of kallikrein 11 contained in a sample from the
subject;
(b) determining the amount of total PSA contained in the sample;
(c) mathematically combining the results of (a) and (b);
(d) relating the combination to the presence of BPH or prostate cancer.
The combination may be a ratio of lcallikrein 11 to total PSA, or the inverse
thereof.
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-5-
Therefore, the invention provides a method for differentiation between BPH or
prostate cancer by
determining the ratio between kallikrein l l aotal PSA in a subject's serum.
The combination (e.g. ratio) may be compared to a combination for a standard
associated with
prostate cancer or BPH. A standard may be a combination (e.g. ratio)
determined for prostate cancer, and a
higher combination (e.g. ratio) determined for the sample compared to the
standard may be indicative of
BPH. A standard may be a combination (e.g. ratio) determined for BPH, and a
lower combination
determined for the sample compared to the standard may be indicative of
prostate cancer.
The methods of the invention may further comprise determining the percentage
of free PSA and
correlating the percentage free PSA and the combination to the presence of
prostate cancer or BPH in the
subject.
In the methods of the invention kallikrein 11 and PSA may be measured using
agents that bind
lcallikrein 11 and PSA, respectively. The binding agents may be directly or
indirectly labeled with a
detectable substance.
In particular, the invention provides a diagnostic method for determining the
presence of BPH or
prostate cancer in a subject comprising;
(a) contacting a sample from the subject with a first binding agent that binds
to kallikrein 11
and a second binding agent that binds to PSA;
(b) determining the presence or amount of first complexes comprising the first
agent and
kallikrein 11 and second complexes comprising the second agent and PSA;
(c) mathematically combining the amount of the first and second complexes; and
(d) relating the combination to the presence of prostate cancer.
In particular methods of the invention, patients with BPH or prostate cancer
can be identified in
patients with total PSA between about 4 to 10 ng/ml.
In still other particular methods of the invention, patients with BPH or
prostate cancer can be
identified in patients with total PSA less than 4 nglml.
The invention also provides a method of improving the accuracy of a diagnosis
of prostate cancer
comprising the steps of: a) performing a method of the invention; and b)
performing at least one of a test for
free PSA and a digital rectal examination.
The invention also relates to kits for carrying out the methods of the
invention. In an embodiment,
the kit is for assessing whether a patient is afflicted with prostate cancer,
and it comprises reagents for
assessing kallikrein 11 and PSA.
In another aspect, the invention relates to a kit for assessing the
suitability of each of a plurality of
test compounds for inhibiting prostate cancer in a patient. The kit comprises
reagents for assessing kallila-ein
11 and PSA and optionally a plurality of test agents or compounds.
The invention contemplates a kit for assessing the presence of prostate cancer
cells, wherein the Icit
comprises antibodies specific for kallikrein 11 and PSA, and optionally
antibodies specific for other markers
associated with prostate cancer.
Additionally the invention provides a lcit for assessing the potential of a
test compound to contribute
to prostate cancer. The kit comprises prostate cancer cells and reagents for
assessing Icallikrein 11 and PSA,
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-G-
and optionally other markers associated with prostate cancer.
The invention also provides a diagnostic composition comprising kallilaein 11
and PSA or agents
that bind to kallikrein 11 and PSA or parts thereof. Agents can be labelled
with detectable substances.
The invention also contemplates the methods, compositions, and kits described
herein using
additional markers associated with prostate cancer. The methods described
herein may be modified by
including reagents to detect the additional markers, or nucleic acids for the
markers.
Other objects, features and advantages of the present invention will become
apparent from the
following detailed description. It should be understood, however, that the
detailed description and the
specific examples while indicating preferred embodiments of the invention are
given by way of illustration
only, since various changes and modifications within the spirit and scope of
the invention will become
apparent to those skilled in the art from this detailed description.
DESCRIPTION OF THE DRAWINGS
The above-mentioned and other features of this invention and the manner of
obtaining them will
become more apparent, and will be best understood, by reference to the
following description, taken in
conjunction with the accompanying drawings. These drawings depict only a
typical embodiment of the
invention and do not therefore limit its scope. They serve to add specificity
and detail, in which:
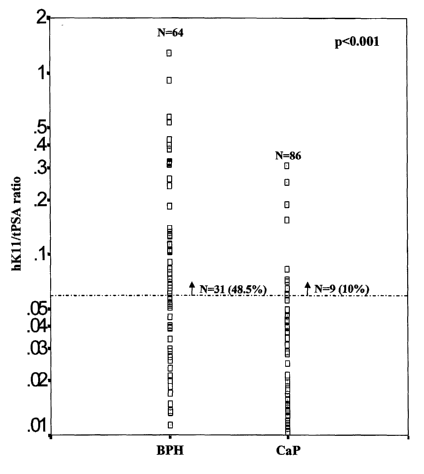
Figure 1 shows the distribution of hKll/total PSA ratio in BPH and CaP
patients. At 90
sensitivity (hKl1/total PSA ratio of 0.06) the specificity is 48.5%. P value
was determined by the Mann-
Whitney U test.
Figure 2 shows the distribution of hKl1/total PSA ratio in the subgroup of
patients with % free PSA
< 20. At 90 % sensitivity (hKl1/total PSA ratio of 0.05)the specificity is
54%. P value was determined by
the Mann-Whitney U test.
Figure 3 shows receiver operating characteristic (ROC) curves for total PSA
(tPSA), % free PSA
and hKl1/total PSA ratio, demonstrating the relative potential of each
variable in the discrimination of BPH
from CaP. AUC, area under the ROC curve; CI, confidence interval
Figure 4 shows the percentage of potentially avoidable biopsies by various
biochemical parameters.
Eight % of BPH patients would have avoided biopsies by combining of % free PSA
and the hKl1/tPSA
ratio (at 90 % sensitivity).
Figure 5 shows receiver operating characteristic (ROC) curves for total PSA, %
free PSA/Total
PSA ratio, and hKll/total PSA ratio, demonstrating the relative potential of
each variable in the
discrimination of BPH from CaP. AUC, area under the ROC curve; CI, confidence
interval
Figure 6 S110WS the distribution of hKllltPSA ratio in BPH and CaP patients.
Figure 7 shows the distribution of hKl 1/tPSA ratio in BPH and CaP patients
where total PSA = 4-
l Op g/L.
Figure 8 shows the distribution of hKl 1/tPSA ratio in BPH and CaP patients
where total PSA = >
10~/L.
Figure 9 shows the distribution of hKl1/tPSA ratio in BPH and CaP patients
where total PSA = <
4~1L.
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
_7_
Figure 10 shows the distribution of hKl 1/tPSA ratio in BPH and CaP patients
where total PSA = <
17.8Et/L (90% sensitivity).
DETAILED DESCRIPTION OF THE INVENTION
The invention relates to newly discovered correlations between expression of
kallikrein 11 in
combination with PSA and prostate cancer. The methods described herein provide
sensitive methods for
detecting prostate cancer. The level of expression of kallikrein 11 and PSA
correlates with the presence of
prostate cancer. The invention is also based on the finding that kallikrein 11
levels and kallikreinll:PSA
ration are significantly lower in prostate cancer patients then in benign
prostatic hyperplasia.
Methods are provided for detecting the presence of prostate cancer in a
sample, the absence of
prostate cancer in a sample, assessing the histology of tissues associated
with prostate cancer, and other
characteristics of prostate cancer that are relevant to prevention, diagnosis,
characterization, and therapy of
prostate cancer in a patient. Methods are also provided for assessing the
efficacy of one or more test agents
for inhibiting kallikrein 11 and PSA that affect prostate cancer, assessing
the efficacy of a therapy for
prostate cancer, monitoring the progression of prostate cancer, selecting an
agent or therapy for inhibiting
prostate cancer, treating a patient afflicted with prostate cancer, inhibiting
prostate cancer in a patient, and
assessing the potential of a test compound to contribute to prostate cancer.
The invention particularly provides methods and kits for detecting prostate
cancer and
differentiating prostate cancer and benign prostatic hyperplasia in a subject
by determining amounts of
lcallikrein 11 alone, or kallikrein 11 and PSA in samples from the subjects
Glossary
Samples that may be analyzed using the methods of the invention include those
which are known or
suspected to express kallikrein 11 and PSA or contain kallilcrein 11 and PSA.
The terms "sample",
"biological sample", and the like mean a material known or suspected of
expressing or containing kallikrein
11 and PSA, in particular kallikrein 11 and PSA associated with prostate
cancer. The test sample can be used
directly as obtained from the source or following a pretreatment to modify the
character of the sample. The
sample can be derived from any biological source, such as tissues, extracts,
or cell cultures, including cells
(e.g. tumor cells), cell lysates, and physiological fluids, such as, for
example, whole blood, plasma, serum,
saliva, ocular lens fluid, cerebral spinal fluid, sweat, urine, milk, ascites
fluid, synovial fluid, peritoneal fluid
and the like.
The sample can be obtained from animals, preferably mammals, most preferably
humans. The
sample can be treated prior to use, such as preparing plasma from blood,
diluting viscous fluids, and the like.
Methods of treatment can involve filtration, distillation, extraction,
concentration, inactivation of interfering
components, the addition of reagents, and the like. Proteins may be isolated
from the samples and utilized in
the methods of the invention.
In embodiments of the invention the sample is a mammalian tissue sample. In
another embodiment
the sample is a human physiological fluid. In a particular embodiment, the
sample is human serum, seminal
plasma, urine, or plasma, most preferably serum.
The terms "subject", "individual" or "patient" refer to a warm-blooded animal
such as a mammal,
which is afflicted with or suspected of having or being pre-disposed to
prostate cancer or condition as
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
_g_
described herein. In particular, the terms refer to a human.
The term "kallikrein 11", "kallikrein 11 polypeptide" or "kallikrein 11
protein" includes human
lcallikrein 11("hKll"), in particular the native-sequence polypeptide,
isoforms, chimeric polypeptides, all
homologs, fragments, and precursors of human kallikrein 11. The amino acid
sequence for native hKll
include the sequences of GenBank Accession Nos. BAA33404 and AB012917 and
shown in SEQ ID NO. 1.
A "native-sequence polypeptide" comprises a polypeptide having the same amino
acid sequence of
a polypeptide derived from nature. Such native-sequence polypeptides can be
isolated from nature or can be
produced by recombinant or synthetic means. The term specifically encompasses
naturally occurring
truncated or secreted forms of a polypeptide, polypeptide variants including
naturally occurring variant
forms (e.g. alternatively spliced forms or splice variants), and naturally
occurring allelic variants.
The term "polypeptide variant" means a polypeptide having at least about 70-
80%, preferably at
least about 85%, more preferably at least about 90%, most preferably at least
about 95% amino acid
sequence identity with a native-sequence polypeptide, in particular having at
least 70-80%, 85%, 90%, 95%
amino acid sequence identity to the sequences identified in the GenBank
Accession Nos. BAA33404 and
AB012917, and shown in SEQ ID NO. 1. Such variants include, for instance,
polypeptides wherein one or
more amino acid residues are added to, or deleted from, the N- or C-terminus
of the full-length or mature
sequences of SEQ ID NO: l, including variants from other species, but excludes
a native-sequence
polypeptide.
An allelic variant may also be created by introducing substitutions,
additions, or deletions into a
nucleic acid encoding a native polypeptide sequence such that one or more
amino acid substitutions,
additions, or deletions are introduced into the encoded protein. Mutations may
be introduced by standard
methods, such as site-directed mutagenesis and PCR mediated mutagenesis. In an
embodiment, conservative
substitutions are made at one or more predicted non-essential amino acid
residues. A "conservative amino
acid substitution" is one in which an amino acid residue is replaced with an
amino acid residue with a similar
side chain. Amino acids with similar side chains are laiown in the art and
include amino acids with basic side
chains (e.g. Lys, Arg, His), acidic. side chains (e.g. Asp, Glu), uncharged
polar side chains (e.g. Gly, Asp,
Glu, Ser, Thr, Tyr and Cys), nonpolar side chains (e.g. Ala, Val, Leu, Iso,
Pro, Trp), beta-branched side
chains (e.g. Thr, Val, Iso), and aromatic side chains (e.g. Tyr, Phe, Trp,
His). Mutations can also be
introduced randomly along part or all of the native sequence, for example, by
saturation mutagenesis.
Following mutagenesis the variant polypeptide can be recombinantly expressed
and the activity of the
polypeptide may be determined.
Polypeptide variants include polypeptides comprising amino acid sequences
sufficiently identical to
or derived from the amino acid sequence of a native polypeptide which include
fewer amino acids than the
full length polypeptides. A portion of a polypeptide can be a polypeptide
which is for example, 10, 15, 20,
25, 30, 35, 40, 45, 50, 60, 70, 80, 90, 100 or more amino acids in length.
Portions in which regions of a
polypeptide are deleted can be prepared by recombinant techniques and can be
evaluated for one or more
functional activities such as the ability to form antibodies specific for a
polypeptide.
A naturally occurring allelic variant may contain conservative amino acid
substitutions from the
native polypeptide sequence or it may contain a substitution of an amino acid
from a corresponding position
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
_9_
in a lcallikrein polypeptide homolog, for example, the murine kallikrein
polypeptide.
The invention also includes polypeptides that are substantially identical to
the sequences of
GenBanlc Accession Nos. BAA33404 and AB012917and shown in SEQ ID NO. 1 ( e.g.
at least about 45%,
preferably 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 97%, 98%, or 99%
sequence identity),
and in particular polypeptides that retain the immunogenic activity of the
corresponding native-sequence
polypeptide.
Percent identity of two amino acid sequences, or of two nucleic acid sequences
identified herein is
defined as the percentage of amino acid residues or nucleotides in a candidate
sequence that are identical
with the amino acid residues in a polypeptide or nucleic acid sequence, after
aligning the sequences and
introducing gaps, if necessary, to achieve the maximum percent sequence
identity, and not considering any
conservative substitutions as part of the sequence identity. Alignment for
purposes of determining percent
amino acid or nucleic acid sequence identity can be achieved in various
conventional ways, for instance,
using publicly available computer software including the GCG program package
(Devereux J. et al., Nucleic
Acids Research 12(1): 387, 1984); BLASTP, BLASTN, and FASTA (Atschul, S.F. et
al. J. Molec. Biol. 215:
403-410, 1990). The BLAST X program is publicly available from NCBI and other
sources (BLAST
Manual, Altschul, S. et al. NCBI NLM NIH Bethesda, Md. 20894; Altschul, S. et
al. J. Mol. Biol. 215: 403-
410, 1990). Skilled artisans can determine appropriate parameters for
measuring alignment, including any
algorithms needed to achieve maximal alignment over the full length of the
sequences being compared.
Methods to determine identity and similarity are codified in publicly
available computer programs.
Kallikrein 11 proteins include chimeric or fusion proteins. A "chimeric
protein" or "fusion protein"
comprises all or part (preferably biologically active) of a kallikrein 11
polypeptide operably linked to a
heterologous polypeptide (i.e., a polypeptide other than a kallila~ein 11
polypeptide). Within the fusion
protein, the term "operably linked" is intended to indicate that a kallilcrein
11 polypeptide and the
heterologous polypeptide are fused in-frame to each other. The heterologous
polypeptide can be fused to the
N-terminus or C-terminus of a kallilcrein 11 polypeptide. A useful fusion
protein is a GST fusion protein in
which a kallikrein l lpolypeptide is fused to the C-terminus of GST sequences.
Another example of a fusion
protein is an immunoglobulin fusion protein in which all or part of a
kallilcrein 11 polypeptide is fused to
sequences derived from a member of the immunoglobulin protein family. Chimeric
and fusion proteins can
be produced by standard recombinant DNA techniques.
Kallikrein polypeptides may be isolated from a variety of sources, such as
from human tissue types
or from another source, or prepared by recombinant or synthetic methods, or by
any combination of these
and similar techniques.
The terms "KLKll" or "ICLKlI nucleic acid(s)" are intended to include DNA and
RNA (e.g.
mRNA) and can be either double stranded or single stranded. The terms include
but are not limited to nucleic
acids that encode a native-sequence polypeptide, a polypeptide variant
including a portion of a kallikrein
polypeptide, an isoform, precursor, and chimeric polypeptide. The nucleic acid
sequences encoding native
kallilcrein polypeptides employed in the present invention include the nucleic
acid sequences of GenBank
Accession No. Accession Nos. AF164623 and AB012917 and in SEQ ID NO: 2 and 3,
or fragments thereof.
Prostate specific antigen (PSA) is a 33-kDa glycosylated single chain serine
protease (Lilja, J Clin
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
- 10-
Invest 1985; Watt et al. Proc Natl Acad Sci (USA) 1986). PSA has been
predicted to be produced as an
inactive zymogen (Lundwall et al. FEBS Lett 1987). Active PSA is secreted into
the seminal plasma (Lilja, J
Clin Invest 1985) and it is one of the most abundant proteins of the prostate
(Lilja et al. The Prostate 1988;
Dub et al. J Androl 1987). PSA is capable of forming complexes with serum
protease inhibitors. Human
serum contains high levels of a,_ antichymotrypsin (ACT) and az-macroglobulin,
both of which form
complexes with PSA. About 70-95% of the PSA in serum that can be detected by
immunoassay is
complexed with ACT. About 30% of serum PSA does not form a complex with ACT.
This non-complexed
fraction of PSA contains an internal peptide bond cleavage at Lys145 which
renders it inactive. PSA in a
complex with a protease inhibitor is referred to "complexed PSA", and PSA that
is not complexed with a
protease inhibitor is referred to herein as "free PSA".
The term "total PSA" in reference to a sample refers to the total complexed
PSA and free PSA in
the sample.
"Statistically different levels" or "significant difference" in levels of
markers in a patient sample
compared to a control or standard (e.g. normal levels or levels in other
samples from a patient) may represent
levels that are higher or lower, in particular lower, than the standard error
of the detection assay.
"Binding agent" refers to a substance such as a polypeptide or antibody that
specifically binds to a
kallikrein 11 or PSA. A substance "specifically binds" to kallikrein 11 or PSA
if it reacts at a detectable level
with a kallila~ein 11 or PSA, and does not react detectably with peptides
containing unrelated sequences or
sequences of different polypeptides. Binding properties may be assessed using
an ELISA, which may be
readily performed by those skilled in the art (see for example, Newton et al ,
Develop. Dynamics 197: 1-13,
1993).
A binding agent may be a ribosome, with or without a peptide component, an RNA
molecule, or a
polypeptide. A binding agent may be a polypeptide that comprises a kallikrein
11 or PSA polypeptide
sequence, a peptide variant thereof, or a non-peptide mimetic of such a
sequence. By way of example a
kallikrein 11 or PSA sequence may be a peptide portion of a kallikrein 11 or
PSA that is capable of
modulating a function mediated by the kallikrein 11 or PSA.
Antibodies for use in the present invention include but are not limited to
monoclonal or polyclonal
antibodies, immunologically active fragments (e.g. a Fab or (Fab)~ fragments),
antibody heavy chains,
humanized antibodies, antibody light chains, genetically engineered single
chain F~ molecules (Ladner et al,
U.S. Pat. No. 4,946,778), chimeric antibodies, for example, antibodies which
contain the binding specificity
of murine antibodies, but in which the remaining portions are of human origin,
or derivatives, such as
enzyme conjugates or labeled derivatives.
Antibodies including monoclonal and polyclonal antibodies, fragments and
chimeras, may be
prepared using methods Irnown to those skilled in the art. Isolated native or
recombinant kallikrein 11 and
PSA may be utilized to prepare antibodies. See, for example, ICohler et al.
(1975) Nature 256:495-497;
ICozbor et al. (1985) J. Immunol Methods 81:31-42; Cote et al. (1983) Proc
Natl Acad Sci 80:2026-2030;
and Cole et al. (1984) Mol Cell Biol 62:109-120 for the preparation of
monoclonal antibodies; Huse et al.
(1989) Science.24G:1275-1281 for the preparation of monoclonal Fab fragments;
and, Pound (1998)
Immunochemical Protocols, Humana Press, Totowa, N.J for the preparation of
phagemid or B-lymphocyte
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-11-
immunoglobulin libraries to identify antibodies. Antibodies specific for
kallikrein 11 and PSA may also be
obtained from scientific or commercial sources.
In an embodiment of the invention, antibodies are reactive against lcallikrein
11 and PSA if they
bind with a Ka of greater than or equal to 10-~ M.
Methods for Detecting Kallikrein 11 and PSA
A variety of methods can be employed for the diagnostic and prognostic
evaluation of prostate
cancer involving kallikrein 11 and PSA, and the identification of subjects
with a predisposition to such
disorders. Such methods may, for example, utilize binding agents (e.g.
antibodies) directed against kallikrein
11 and PSA, including peptide fragments. In particular, antibodies may be
used, for example, for the
detection of either an over- or an under-abundance of kallikrein 11 and PSA
relative to a non-disorder state
or the presence of a modified (e.g., less than full length) kallikrein 11 and
PSA which correlates with a
disorder state, or a progression toward a disorder state.
The invention also contemplates a method for detecting prostate cancer
comprising producing a
profile of levels of kallikrein 11 and PSA and other markers associated with
prostate cancer in cells from a
patient, and comparing the profile with a reference to identify a profile for
the test cells indicative of disease.
The methods described herein may be used to evaluate the probability of the
presence of malignant
or pre-malignant cells, for example, in a group of cells freshly removed from
a host. Such methods can be
used to detect tumors, quantitate their growth, and help in the diagnosis and
prognosis of disease. For
example, lower levels of lcallila~ein 11 and PSA may be indicative of advanced
disease, e.g. advanced
prostate cancer. The methods can be used to detect the presence of cancer
metastasis, as well as confirm the
absence or removal of all tumor tissue following surgery, cancer chemotherapy,
and/or radiation therapy.
They can further be used to monitor cancer chemotherapy and tumor
reappearance.
The methods described herein can be adapted for diagnosing and monitoring
prostate cancer by
detecting kallilcrein 11 and PSA in biological samples from a subject. These
applications require that the
amount of kallikrein 11 and PSA quantitated in a sample from a subject being
tested be compared to levels
quantitated for another sample or an earlier sample from the subject, or
levels quantitated for a control
sample. Levels for control samples from healthy subjects or prostate cancer
subjects may be established by
prospective and/or reh~ospective statistical studies. Healthy subjects who
have no clinically evident disease or
abnormalities may be selected for statistical studies. Diagnosis may be made
by a finding of statistically
different levels of kallikrein 11 and PSA compared to a control sample or
previous levels quantitated for the
same subject.
Binding agents specific for kallikrein 11 and PSA may be used for a variety of
diagnostic and assay
applications. There are a variety of assay formats known to the skilled
artisan for using a binding agent to
detect a target molecule in a sample. (For example, see Harlow and Lane,
Antibodies: A Laboratory Manual,
Cold Spring Harbor Laboratory, 1988). In general, the presence or absence of
prostate cancer in a subject
may be determined by (a) contacting a sample from the subject with binding
agents for kallikrein 11 and
PSA; (b) detecting in the sample levels of Icallikrein 11 and PSA that bind to
the binding agents; and (c)
comparing the levels of kallikrein 11 and PSA with predetermined standards or
cut-off values.
In particular embodiments, the binding agent is an antibody.
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-12-
In an aspect, the invention provides a diagnostic method for monitoring or
diagnosing prostate
cancer in a subject by quantitating kallikrein 11 and PSA in a biological
sample from the subject comprising
reacting the sample with antibodies specific for kallikrein 11 and PSA, which
are directly or indirectly
labeled with detectable substances and detecting the detectable substances.
In an aspect of the invention, a method for detecting prostate cancer is
provided comprising:
(a) obtaining a sample suspected of containing kallikrein 11 and PSA
associated with prostate
cancer;
(b) contacting said sample with antibodies that specifically bind kallikrein
11 and PSA under
conditions effective to bind the antibodies and form complexes;
(c) measuring the amount of kallikrein 11 and PSA present in the sample by
quantitating the
amount of the complexes; and
(d) comparing the amount of kallikrein 11 and PSA present in the samples with
the amount of
kallikrein 11 and PSA in a control, wherein a change or significant difference
in the
amount of kallila~ein 11 and PSA in the sample compared with the amount in the
control is
indicative of prostate cancer.
In an embodiment, the invention contemplates a method for monitoring the
progression of prostate
cancer in an individual, comprising:
(a) contacting antibodies which bind to kallikrein 11 and PSA with a sample
from the
individual so as to form complexes comprising the antibodies and kallikrein 11
or PSA in
the sample;
(b) determining or detecting the presence or amount of complex formation in
the sample;
(c) repeating steps (a) and (b) at a point later in time; and
(d) comparing the result of step (b) with the result of step (c), wherein a
difference in the
amount of complex formation is indicative of disease, disease stage, and/or
progression of
the cancer in said individual.
The amount of complexes may also be compared to a value representative of the
amount of the
complexes from an individual not at risk of, or afflicted with, prostate
cancer at different stages. An decrease
in complex formation may be indicative of advanced disease e.g. advanced
prostate cancer, or an
unfavourable prognosis.
Antibodies specifically reactive with kallikrein 11 and PSA, or derivatives,
such as enzyme
conjugates or labeled derivatives, may be used to detect kallila~ein 11 and
PSA proteins in various samples
(e.g. biological materials). They may be used as diagnostic or prognostic
reagents and they may be used to
detect abnormalities in the level of kallikrein 11 and PSA expression, or
abnormalities in the structure,
and/or temporal, tissue, cellular, or subcellular location of kallikrein 11
and PSA. Antibodies may also be
used to screen potentially therapeutic compounds in vitro to determine their
effects on disorders (e.g.
prostate cancer) involving a kallilcrein 11 and PSA protein, and other
conditions. In vitro immunoassays may
also be used to assess or monitor the efficacy of particular therapies.
Antibodies may be used in any known immunoassays that rely on the binding
interaction between
antigenic determinants of kallikrein 11 and PSA protein and the antibodies.
Immunoassay procedures for in
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-13-
vitro detection of antigens in fluid samples are also well lmown in the art.
[See for example, Paterson et al.,
Int. J. Can. 37:659 (1986) and Burchell et al., Int. J. Can. 34:763 (1984) for
a general description of
immunoassay procedures]. Qualitative and/or quantitative determinations of
kallikrein 11 and PSA in a
sample may be accomplished by competitive or non-competitive immunoassay
procedures in either a direct
or indirect format. Detection of Icallikrein 11 and PSA using antibodies can
be done utilizing immunoassays
which are run in either the forward, reverse or simultaneous modes. Examples
of immunoassays are
radioimmunoassays (RIA), enzyme immunoassays (e.g. ELISA), immunofluorescence,
immunoprecipitation, latex agglutination, hemagglutination, histochemical
tests, and sandwich
(immunometric) assays. These terms are well understood by those skilled in the
art. A person skilled in the
art will know, or can readily discern, other immunoassay formats without undue
experimentation. The
antibodies may be used to detect and quantify kallikrein 11 and PSA in a
sample in order to diagnose and
treat pathological states.
In particular, the antibodies may be used in immunohistochemical analyses, for
example, at the
cellular and sub-subcellular level, to detect kallikrein 11 and PSA proteins,
to localize them to particular
prostate tumor cells and tissues, and to specific subcellular locations, and
to quantitate the level of
expression.
Immunohistochemical methods for the detection of antigens in tissue samples
are well known in the
art. For example, immunohistochemical methods are described in Taylor, Arch.
Pathol. Lab. Med. 102:112
(1978). Briefly, in the context of the present invention, a tissue sample
obtained from a subject suspected of
having a prostate-related problem is contacted with antibodies, preferably
monoclonal antibodies recognizing
kallikrein 11. The site at which the antibodies are bound is determined by
selective staining of the sample by
standard immunohistochemical procedures. The same procedure may be repeated on
the same sample using
other antibodies that recognize kallilcrein 11. Alternatively, a sample may be
contacted with antibodies
against kallikrein 11 and antibodies against PSA simultaneously, provided that
the antibodies are labeled
differently or are able to bind to a different label. In one embodiment of the
present invention, the tissue
sample is obtained from the prostate of a patient. The prostate tissue sample
may be a normal prostate tissue,
a cancer prostate tissue or a benign prostatic hyperplasia tissue.
In a sandwich immunoassay of the invention mouse polyclonal/monoclonal
antibodies specific for
kallikrein 11 and PSA and rabbit polyclonal/monoclonal antibodies specific for
kallikrein 11 and PSA are
utilized.
Antibodies specific for lcallilerein 11 and PSA may be labelled with a
detectable substance and
localised in biological samples based upon the presence of the detectable
substance. Examples of detectable
substances include, but are not limited to, the following: radioisotopes
(e.g., 3H, 14C, ssS ~zsl istl)
fluorescent labels (e.g., FITC, rhodamine, lanthanide phosphors), luminescent
labels such as luminol;
enzymatic labels (e.g., horseradish peroxidase, beta-galactosidase,
luciferase, alkaline phosphatase,
acetylcholinesterase), biotinyl groups (which can be detected by marked avidin
e.g., streptavidin containing a
fluorescent marker or enzymatic activity that can be detected by optical or
calorimetric methods),
predetermined polypeptide epitopes recognized by a secondary reporter (e.g.,
leucine zipper pair sequences,
binding sites for secondary antibodies, metal binding domains, epitope tags).
In some embodiments, labels
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-14-
are attached via spacer arms of various lengths to reduce potential steric
hindrance. Antibodies may also be
coupled to electron dense substances, such as ferritin or colloidal gold,
which are readily visualised by
electron microscopy.
One of the ways an antibody can be detectably labeled is to link it directly
to an enzyme. The
enzyme when later exposed to its substrate will produce a product that can be
detected. Examples of
detectable substances that are enzymes are horseradish peroxidase, beta-
galactosidase, luciferase, alkaline
phosphatase, acetylcholinesterase, malate dehydrogenase, ribonuclease, urease,
catalase, glucose-6
phosphate, staphylococcal nuclease, delta-5-steriod isomerase, yeast alcohol
dehydrogenase, alpha
glycerophosphate, triose phosphate isomerase, asparaginase, glucose oxidase,
and acetylcholine esterase.
For increased sensitivity in an immunoassay system a fluorescence-emitting
metal atom such as Eu
(europium) and other lanthanides can be used. These can be attached to the
desired molecule by means of
metal-chelating groups such as DTPA or EDTA.
A bioluminescent compound may also be used as a detectable substance.
Bioluminescence is a type
of chemiluminescence found in biological systems where a catalytic protein
increases the efficiency of the
chemiluminescent reaction. The presence of a bioluminescent molecule is
determined by detecting the
presence of luminescence. Examples of bioluminescent detectable substances are
luciferin, luciferase and
aequorin.
Indirect methods may also be employed in which the primary antigen-antibody
reaction is amplified
by the introduction of a second antibody, having specificity for the antibody
reactive against kallikrein 11
and PSA. By way of example, if the antibody having specificity against
kallikrein 11 and PSA is a rabbit IgG
antibody, the second antibody may be goat anti-rabbit IgG, Fc fragment
specific antibody labelled with a
detectable substance as described herein.
Methods for conjugating or labelling the antibodies discussed above may be
readily accomplished
by one of ordinary skill in the art. (See for example Inman, Methods In
Enzymology, Vol. 34, Affinity
Techniques, Enzyme Purification: Part B, Jakoby and Wichek (eds.), Academic
Press, New York, p. 30,
1974; and Wilchek and Bayer, "The Avidin-Biotin Complex in Bioanalytical
Applications,"Anal. Biochem.
171:1-32, 1988 re methods for conjugating or labelling the antibodies with
enzyme or ligand binding
partner).
Cytochemical techniques lmown in the art for localizing antigens using light
and electron
microscopy may be used to detect a kalhikrein 11 and PSA protein. Generally,
an antibody may be labeled
with a detectable substance and a kallilcrein 11 and PSA protein may be
localised in tissues and cells based
upon the presence of the detectable substance.
In the context of the methods of the invention, the sample, binding agents
(e.g. antibodies) or
lcallilcrein 11 and PSA may be immobilized on a carrier or support. Examples
of suitable carriers or supports
are agarose, cellulose, nitrocellulose, dextran, Sephadex, Sepharose,
liposomes, carboxymethyl cellulose,
polyacrylamides, polystyrene, gabbros, filter paper, magnetite, ion-exchange
resin, plastic film, plastic tube,
glass, polyamine-methyl vinyl-ether-malefic acid copolymer, amino acid
copolymer, ethylene-malefic acid
copolymer, nylon, silk, etc. The support material may have any possible
configuration including spherical
(e.g. bead), cylindrical (e.g. inside surface of a test tube or well, or the
external surface of a rod), or flat (e.g.
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-15-
sheet, test strip). Thus, the carrier may be in the shape of, for example, a
tube, test plate, well, beads, disc,
sphere, etc. The immobilized material may be prepared by reacting the material
with a suitable insoluble
carrier using known chemical or physical methods, for example, cyanogen
bromide coupling. Binding agents
(e.g. antibodies) may be indirectly immobilized using second binding agents
specific for the first binding
agent. For example, mouse antibodies specific for kallikrein 11 and PSA may be
immobilized using sheep
anti-mouse IgG Fc fragment specific antibody coated on the carrier or support.
Where a radioactive label is used as a detectable substance, a kallikrein 11
and PSA protein may be
localized by radioautography. The results of radioautography may be
quantitated by determining the density
of particles in the radioautographs by various optical methods, or by counting
the grains.
Time-resolved fluorometry may be used to detect a fluorescent signal, label,
or detectable
substance. For example, the method described in Christopoulos TK and Diamandis
EP Anal. Chem.,
1992:64:342-346 may be used with a conventional time-resolved fluorometer.
According to an embodiment of the invention, an immunoassay for detecting
kallikrein 11 in a
biological sample comprises contacting an amount of a first agent that
specifically binds to kallikrein 11 in
the sample under a condition that allows the formation of a first complex
comprising the first agent and
kallikrein 11 and determining the presence or amount of the complex as a
measure of the amount of the
kallikrein 11 contained in the sample. In another embodiment, an immunoassay
is provided for detecting
kallikrein 11 and total PSA in a sample comprising the additional step of
contacting an amount of a second
agent which specifically binds to PSA with the sample under a condition that
allows the formation of a
second complex comprising the second agent and PSA, and determining the
presence or amount of the
second complex as a measure of the amount of the PSA contained in the sample.
In an alternate method, a
sample may be contacted by the first and the second agents simultaneously,
provided that the agents are
labeled differently or are capable of binding to different labels.
In accordance with an embodiment of the invention, a method is provided
wherein kallikrein 11 and
PSA antibodies are directly or indirectly labelled with enzymes, substrates
for the enzymes are added
wherein the substrates are selected so that the substrates, or a reaction
product of an enzyme and substrate,
form fluorescent complexes with a lanthanide metal (e.g. europium, terbium,
samarium, and dysprosium,
preferably europium and terbium). A lanthanide metal is added and kallikrein
11 and PSA are quantitated in
the sample by measuring fluorescence of the fluorescent complexes. Enzymes are
selected based on the
ability of a substrate of the enzyme, or a reaction product of the enzyme and
substrate, to complex with
lanthanide metals such as europium and terbium. Suitable enzymes and
substrates that provide fluorescent
complexes are described in U. S. Patent No. 5,3112,922 to Diamandis. Examples
of suitable enzymes include
alkaline phosphatase and (3-galactosidase. Preferably, the enzyme is alkaline
phosphatase.
Examples of enzymes and substrates for enzymes that provide such fluorescent
complexes are
described in U.S. Patent No. 5,312,922 to Diamandis. By way of example, when
the antibody is directly or
indirectly labelled with alkaline phosphatase the substrate employed in the
method may be 4
methylumbelliferyl phosphate, S-fluorosalicyl phosphate, or diflunisal
phosphate. The fluorescence intensity
of the complexes is typically measured using a time-resolved fluorometer e.g.
a CyberFluor 615
Imunoanalyzer (Nordion International, ISanata, Ontario).
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
- 1G -
Antibodies specific for kallikrein 11 and PSA may also be indirectly labelled
with enzymes. For
example, an antibody may be conjugated to one partner of a ligand binding
pair, and the enzyme may be
coupled to the other partner of the ligand binding pair. Representative
examples include avidin-biotin, and
riboflavin-riboflavin binding protein. In an embodiment, an antibody specific
for the anti-Icallikrein 11 and
PSA antibody is labelled with an enzyme.
In accordance with an embodiment, the present invention provides means for
determining kallikrein
11 and PSA in a serum sample by measuring kallikrein 11 and PSA by
immunoassay. It will be evident to a
skilled artisan that a variety of immunoassay methods can be used to measure
kallikrein 11 and PSA in
serum. In general, a kallikrein 11 and PSA immunoassay method may be
competitive or noncompetitive.
Competitive methods typically employ an immobilized or immobilizable antibody
to kallikrein 11 and PSA
(anti-kallikrein 11 and anti-PSA) and a labeled form of kallikrein 11 and PSA.
Sample kallila~ein 11 and PSA
and labeled kallikrein 11 and PSA compete for binding to anti-kallikrein 11
and PSA. Afrer separation of the
resulting labeled kallikrein 11 and PSA that has become bound to anti-
kallikrein 11 and PSA (bound
fraction) from that which has remained unbound (unbound fraction), the amount
of the label in either bound
or unbound fraction is measured and may be correlated with the amount of
kallikrein 11 and PSA in the test
sample in any conventional manner, e.g., by comparison to a standard curve.
In an aspect, a non-competitive method is used for the determination of
kallikrein 11 and PSA, with
the most common method being the "sandwich" method. In this assay, two anti-
kallikrein 11 and two PSA
antibodies are employed. One of the anti- leallikrein 11 and one or the PSA
antibodies is directly or indirectly
labeled (sometimes referred to as the "detection antibody") and the other is
immobilized or immobilizable
(sometimes referred to as the "capture antibody"). The capture and detection
antibodies can be contacted
simultaneously or sequentially with the test sample. Sequential methods can be
accomplished by incubating
the capture antibody with the sample, and adding the detection antibody at a
predetermined time thereafter
(sometimes referred to as the "forward" method); or the detection antibody can
be incubated with the sample
first and then the capture antibody added (sometimes referred to as the
"reverse" method). After the
necessary incubations) have occurred, to complete the assay, the capture
antibody may be separated from
the liquid test mixture, and the label may be measured in at least a portion
of the separated capture antibody
phase or the remainder of the liquid test mixture. Generally it is measured in
the capture antibody phase
since it comprises kallikrein 11 and PSA bound by ("sandwiched" between) the
capture and detection
antibodies. In another embodiment, the label may be measured without
separating the capture antibody and
liquid test mixture.
In a typical two-site immunometric assay for kallikrein 11 and PSA, one or
both of the capture and
detection antibodies are polyclonal antibodies or one or both of the capture
and detection antibodies are
monoclonal antibodies (i.e. polyclonal/polyclonal, monoclonal/monoclonal, or
monoclonal/polyclonal). The
label used in the detection antibody can be selected from any of those known
conventionally in the art. The
label may be an enzyme or a chemiluminescent moiety, but it can also be a
radioactive isotope, a fluorophor,
a detectable ligand (e.g., detectable by a secondary binding by a labeled
binding partner for the ligand), and
the like. Preferably the antibody is labelled with an enzyme which is detected
by adding a substrate that is
selected so that a reaction product of the enzyme and substrate forms
fluorescent complexes. The capture
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
- 17-
antibody may be selected so that it provides a means for being separated from
the remainder of the test
mixture. Accordingly, the capture antibody can be introduced to the assay in
an already immobilized or
insoluble form, or can be in an immobilizable form, that is, a form which
enables immobilization to be
accomplished subsequent to introduction of the capture antibody to the assay.
An immobilized capture
antibody may comprise an antibody covalently or noncovalently attached to a
solid phase such as a magnetic
particle, a latex particle, a microtiter plate well, a bead, a cuvette, or
other reaction vessel. An example of an
immobilizable capture antibody is antibody which has been chemically modified
with a ligand moiety, e.g., a
hapten, biotin, or the like, and which can be subsequently immobilized by
contact with an immobilized form
of a binding partner for the ligand, e.g., an antibody, avidin, or the like.
In an embodiment, the capture
antibody may be immobilized using a species specific antibody for the capture
antibody that is bound to the
solid phase.
A particular sandwich immunoassay method of the invention employs antibodies
reactive against
kallikrein 11 and PSA, antibodies having specificity against antibodies
reactive against Icallikrein 11 and
PSA labelled with an enzymatic label, and a fluorogenic substrate for the
enzyme. In an embodiment, the
enzyme is alkaline phosphatase (ALP) and the substrate is 5-fluorosalicyl
phosphate. ALP cleaves phosphate
out of the fluorogenic substrate, 5-fluorosalicyl phosphate, to produce 5-
fluorosalicylic acid (FSA). 5-
Fluorosalicylic acid can then form a highly fluorescent ternary complex of the
form FSA-Tb(3+)-EDTA,
which can be quantified by measuring the Tb3+ fluorescence in a time-resolved
mode. Fluorescence intensity
is measured using a time-resolved fluorometer as described herein.
The above-described immunoassay methods and formats are intended to be
exemplary and are not
limiting.
The invention also contemplates the methods described herein using multiple
markers for prostate
cancer. Therefore, the invention contemplates a method for anaylzing a
biological sample for the presence of
kallikrein 11 and PSA and other markers that are specific indicators of
prostate cancer. The methods
described herein may be modified by including reagents to detect the markers,
or nucleic acids for the
markers. The methods described herein may also include reagents to detect hLKl
1. Techniques for detecting
nucleic acid such as polymerase chain reaction (PCR) and hybridization assays
are well known in the art.
The use of kallikrein 11 alone, and kallikrein 11 in combination with PSA may
lead to improved
discrimination between BPH and prostate cancer. The mathematical combination
of the amount of kallikrein
11 and PSA may be used as a serum marker or as an immunohistological marker to
help distinguish prostate
cancer from BPH. Alternatively, kallikrein 11 alone may be used as a serum
marker or as an
immunohistological marker for distinguishing BPH from prostate cancer. The
term "mathematical
combination" as used herein refers to any mathematical calculation of the
amount of kallikrein 11 and PSA.
In an aspect of the invention, the mathematical combination is a ratio. The
ratio of lcallikrein 11 and PSA in a
sample may be determined by comparing the amount of kallikrein 11 to the
amount of total PSA in the
sample. Thus, the mathematical combination may be the ratio of kallikrein 11:
total PSA or the inverse
thereof.
The mathematical combination may be compared to the mathematical combination
of a standard in
order to determine the presence of BPH or prostate cancer in the subject. In a
particular embodiment of the
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-18-
invention a kallikrein 11: total PSA ratio greater than 0.02, 0.03, 0.04,
0.05, 0.06, 0.07, 0.08, 0.09, 0.10, 0.11,
or 0.12 is potentially indicative of BPH while a kallikrein 11: total PSA
ratio less than 0.02, 0.03, 0.04, 0.05,
0.06, 0.07, 0.08, 0.09, 0.10, 0.11, or 0.12 is potentially indicative of
prostate cancer. In a more particular
embodiment, a kallilcrein 11: total PSA ratio greater than 0.05 is potentially
indicative of BPH while a
Icallila~ein 11: total PSA ratio less than 0.05 is potentially indicative of
prostate cancer.
Accordingly, another aspect of the present invention provides a method for
determining the
presence of BPH or prostate cancer in a subject comprising the steps of:
(a) providing a first binding agent that specifically binds to kallikrein 11;
(b) providing a second binding agent that specifically binds to PSA;
(c) contacting the first agent and the second agent With a sample from the
subject under a
condition that allows the formation of a first complex comprising the first
agent and
kallikrein 11 and a second complex comprising the second agent and the PSA;
(d) detecting or determining the presence or amount of the first and second
complexes;
(e) mathematically combining the amount of the first and the second complexes
or the amount
of kallikrein 11; and
(f) relating the combination to the presence of BPH or prostate cancer in the
subject.
In accordance with embodiments of the present invention, the agents comprise
antibodies.
Preferably, the first and the second agents are directly or indirectly labeled
with different detectable
substances to form respective complexes that may be detected separately.
Alternatively, the sample may be
contacted with one agent first so that either kallikrein 11 may be detected
first, then the same sample may be
contacted with another agent in order to detect PSA.
In one embodiment of the present invention, the sample used in the diagnostic
method may be a
sample of human physiological fluid such as, but not limited to, serum,
seminal plasma, urine and plasma,
preferably serum. In another embodiment, the sample may be tissue specimen
from the prostate of a patient.
In a particular embodiment, the combination is a ratio of the first complex to
the second complex,
or the inverse thereof.
In particular methods of the invention, patients with BPH or prostate cancer
can be identified in
patients with total PSA between about 4-lOng/ml.
In particular methods of the invention, patients with BPH or prostate cancer
can be identified in
patients with total PSA less than about 4 ng/ml.
The methods of the invention may utilize commercially available kits for
detecting or quantifying
PSA in a sample. For example the Immulite PSA (Diagnostic Products
Corporation, Cal, USA) assay may be
used to measure PSA in a sample.
Since kallikrein 11 alone may be used as a serum marker or as an
immunohistological marker for
distinguishing BPH from prostate cancer, the present invention also provides a
diagnostic method for
distinguishing BPH from prostate cancer by detecting and determining the
amount of kallikrein 11 in a
sample. The amount of kallilcrein 11 may be determined in tissue samples by
immunohistochemical methods
andlor in patient fluid samples by ira vitro immunoassay procedures described
herein.
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-19-
Computer Systems
Computer readable media comprising kallikrein 11 and PSA and optionally other
markers of
prostate cancer is also provided. "Computer readable media" refers to any
medium that can be read and
accessed directly by a computer, including hut not limited to magnetic storage
media, such as floppy discs,
hard disc storage medium, and magnetic tape; optical storage media such as CD-
ROM; electrical storage
media such as RAM and ROM; and hybrids of these categories such as
magnetic/optical storage media.
Thus, the invention contemplates computer readable medium having recorded
thereon markers identified for
patients and controls.
"Recorded" refers to a process for storing information on computer readable
medium. The skilled
artisan can readily adopt any of the presently known methods for recording
information on computer
readable medium to generate manufactures comprising information on kallikrein
11 and PSA and optionally
other prostate cancer markers.
A variety of data processor programs and formats can be used to store
information on kallikrein 11
and PSA and other prostate cancer markers on computer readable medium. For
example, the information can
be represented in a word processing text file, formatted in commercially-
available software such as
WordPerfect and Microsoft Word, or represented in the form of an ASCII file,
stored in a database
application, such as DB2, Sybase, Oracle, or the like. Any number of
dataprocessor structuring formats (e.g.,
text file or database) may be adapted in order to obtain computer readable
medium having recorded thereon
the marker information.
By providing the marker information in computer readable form, one can
routinely access the
information for a variety of purposes. For example, one skilled in the art can
use the information in computer
readable form to compare marker information obtained during or following
therapy with the information
stored within the data storage means.
The invention provides a medium for holding instructions for performing a
method for determining
whether a patient has prostate cancer or a pre-disposition to prostate cancer,
comprising determining the
presence, absence, or amount of kallikrein 11 and PSA and optionally other
prostate cancer markers, and
based on the presence, absence or amount of kallikrein 11 and PSA and
optionally other markers,
determining whether the patient has prostate cancer, BPH, or a pre-disposition
to prostate cancer, and
optionally recommending treatment for the condition.
The invention also provides in an electronic system and/or in a network, a
method for determining
whether a subject has prostate cancer or a pre-disposition to prostate cancer,
comprising determining the
presence, absence, or amount of lcallikrein 11 and PSA and optionally other
prostate cancer markers, and
based on the presence, absence, or amount of the kallilcrein 11 and PSA and
optionally other markers,
determining whether the subject has prostate cancer, BPH, or a pre-disposition
to prostate cancer, and
optionally recommending treatment for the condition.
The invention further provides in a network, a method for determining whether
a subject has
prostate cancer or a pre-disposition to prostate cancer comprising: (a)
receiving phenotypic information on
the subject and information on kallikrein 11 and PSA and optionally other
prostate cancer markers associated
with samples from the subject; (b) acquiring information from the network
corresponding to the kallila-ein
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-20-
11 and PSA and optionally other markers; and (c) based on the phenotypic
information and information on
the kallikrein 11 and PSA and optionally other markers determining whether the
subject has prostate cancer,
BPH or a pre-disposition to prostate cancer; and (d) optionally recommending
treatment for the condition.
The invention still further provides a system for identifying selected records
that identify a prostate
cancer cell or tissue. A system of the invention generally comprises a digital
computer; a database server
coupled to the computer; a database coupled to the database server having data
stored therein, the data
comprising records of data comprising kallikrein 11 and PSA and optionally
other prostate cancer marleers,
or nucleic acids encoding same, and a code mechanism for applying queries
based upon a desired selection
criteria to the data ale in the database to produce reports of records which
match the desired selection
criteria.
In an aspect of the invention a method is provided for detecting prostate
cancer tissue or cells using
a computer having a processor, memory, display, and input/output devices, the
method comprising the steps
of:
(a) creating records of kallikrein 11 and PSA including mathematical
combinations thereof,
and optionally other prostate cancer markers isolated from a sample suspected
of
containing prostate cancer cells or tissue;
(b) providing a database comprising records of data comprising kallikrein 11
and PSA
including mathematical combinations thereof, and optionally other prostate
cancer
markers; and
(c) using a code mechanism for applying queries based upon a desired selection
criteria to the
data file in the database to produce reports of records of step (a) which
provide a match of
the desired selection criteria of the database of step (b) the presence of a
match being a
positive indication that the markers of step (a) have been isolated from cells
or tissue that
are prostate cancer cells or tissue.
The invention contemplates a business method for determining whether a subject
has prostate
cancer or a pre-disposition to prostate cancer comprising: (a) receiving
phenotypic information on the subject
and information on kallilerein 11 and PSA and optionally other prostate cancer
markers associated with
samples from the subject; (b) acquiring information from a network
corresponding to kallikrein 11 and PSA
and optionally other markers; and (c) based on the phenotypic information,
information on kallikrein 11 and
PSA and optionally other markers, and acquired information, determining
whether the subject has prostate
cancer or a pre-disposition to prostate cancer; and (d) optionally
recommending treatment for the prostate
cancer or pre-condition.
In an aspect of the invention, the computer systems, components, and methods
described herein are
used to monitor disease or determine the stage of disease.
Screening Methods
The invention also contemplates methods for evaluating test agents or
compounds for their ability to
inhibit prostate cancer or potentially contribute to prostate cancer. Test
agents and compounds include but
are not limited to peptides such as soluble peptides including Ig-tailed
fusion peptides, members of random
peptide libraries and combinatorial chemistry-derived molecular libraries made
of D- and/or L-configuration
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-21-
amino acids, phosphopeptides (including members of random or partially
degenerate, directed
phosphopeptide libraries), antibodies [e.g. polyclonal, monoclonal, humanized,
anti-idiotypic, chimeric,
single chain antibodies, fragments, (e.g. Fab, F(ab)z, and Fab expression
library fragments, and epitope-
binding fragments thereof)], and small organic or inorganic molecules. The
agents or compounds may be
endogenous physiological compounds or natural or synthetic compounds.
The invention provides a method for assessing the potential efficacy of a test
agent for inhibiting
prostate cancer in a patient, the method comprising comparing:
(a) levels of kallikrein 11 and PSA including mathematical combinations
thereof, and
optionally other prostate cancer markers in a first sample obtained from a
patient and
exposed to the test agent; and
(b) levels of kallikrein 11 and PSA including mathematical combinations
thereof, and
optionally other markers in a second sample obtained from the patient, wherein
the sample
is not exposed to the test agent, wherein a significant difference in the
levels of expression
of kallikrein 11 and PSA including mathematical combinations thereof, and
optionally the
other markers in the first sample, relative to the second sample, is an
indication that the test
agent is potentially efficacious for inhibiting prostate cancer in the
patient.
The first and second samples may be portions of a single sample obtained from
a patient or portions
of pooled samples obtained from a patient.
In an embodiment, the levels of expression of kallikrein 11 and PSA including
mathematical
combinations thereof, in the first sample are significantly higher relative to
the second sample.
In an aspect, the invention provides a method of selecting an agent for
inhibiting prostate cancer in
a patient comprising:
(a) obtaining a sample from the patient;
(b) separately maintaining aliquots of the sample in the presence of a
plurality of test agents;
(c) comparing kallikrein 11 and PSA including mathematical combinations
thereof and
optionally other prostate cancer markers, in each of the aliquots; and
(d) selecting one of the test agents which alters the levels of kallikrein 11
and PSA including
mathematical combinations thereof and optionally other prostate cancer markers
in the
aliquot containing that test agent, relative to other test agents.
In an embodiment, the levels of Icallikrein 11 and PSA including mathematical
combinations thereof
are significantly higher in the presence of the selected test agent.
Still another aspect of the present invention provides a method of conducting
a drug discovery
business comprising:
(a) providing one or more methods or assay systems for identifying agents that
inhibit prostate
cancer in a patient;
(b) conducting therapeutic profiling of agents identified in step (a), or
further analogs thereof,
for efficacy and toxicity in animals; and
(c) formulating a pharmaceutical preparation including one or more agents
identified in step
(b) as having an acceptable therapeutic profile.
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-22-
In certain embodiments, the subject method can also include a step of
establishing a distribution
system for distributing the pharmaceutical preparation for sale, and may
optionally include establishing a
sales group for marketing the pharmaceutical preparation.
The invention also contemplates a method of assessing the potential of a test
compound to
contribute to prostate cancer comprising:
(a) maintaining separate aliquots of cells or tissues from a patient with
prostate cancer in the
presence and absence of the test compound; and
(b) comparing kallikrein 11 and PSA including mathematical combinations
thereof and
optionally other prostate cancer markers in each of the aliquots.
A significant difference between the levels of the markers in the aliquot
maintained in the presence
of (or exposed to) the test compound relative to the aliquot maintained in the
absence of the test compound,
indicates that the test compound possesses the potential to contribute to
prostate cancer. In an embodiment,
the levels of lcallikrein 11 and PSA including mathematical combinations
thereof are lower in the presence of
the test compound.
In an aspect the invention provides a method of inhibiting prostate cancer in
a patient, the method
comprising (a) obtaining a sample comprising cells affected by prostate cancer
from the patient; (b)
separately maintaining aliquots of the sample in the presence of a plurality
of test agents; (c) comparing
levels of kallikrein 11 and PSA or a mathematical combination thereof in each
of the aliquots; and (d)
administering to the patient at least one of the test agents which alters
kallikrein 11 and PSA or a
mathematical combination thereof in the aliquot containing that test agent,
relative to other test agents.
Kits
The invention contemplates kits for carrying out the methods of the invention.
Such kits typically
comprise two or more components required for performing a diagnostic assay.
Components include but are
not limited to compounds, reagents, containers, andlor equipment.
The methods described herein may be performed by utilizing pre-packaged
diagnostic kits
comprising at least binding agents (e.g. antibodies) described herein, which
may be conveniently used, e.g.
in clinical settings, to screen and diagnose patients, and to screen and
identify those individuals afflicted with
prostate cancer or BPH or exhibiting a predisposition to such condition.
In an embodiment, a container with a kit comprises binding agents as described
herein. By way of
example, the lcit may contain antibodies specific for kallikrein 11 and PSA
and optionally other prostate
cancer markers, antibodies against the antibodies labelled with enzymes, and
substrates for the enzymes. The
kit may also contain microtiter plate wells, standards, assay diluent, wash
buffer, adhesive plate covers,
and/or instructions for carrying out a method of the invention using the kit.
In an aspect of the invention, the kit includes antibodies or antibody
fragments which bind
specifically to epitopes of kallikrein 11 and PSA and optionally other
prostate cancer markers, and means for
detecting binding of the antibodies to epitopes associated with prostate
cancer, either as concentrates
(including lyophilized compositions), which may be further diluted prior to
use or at the concentration of
use, where the vials may include one or more dosages. Where the kits are
intended for in vivo use, single
dosages may be provided in sterilized containers, having the desired amount
and concentration of agents.
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-23-
Containers that provide a formulation for direct use, usually do not require
other reagents.
In particular, the invention provides a kit for determining the presence of
BPH or prostate
comprising a known amount of a first binding agent that specifically binds to
kallikrein 11 wherein the first
binding agent comprises a detectable substance, or it binds directly or
indirectly to a detectable substance.
Another aspect of the present invention also provides a kit for determining
the presence of BPH or
prostate cancer in a sample. The kit includes:
(a) a Imown amount of a first agent which specifically binds to kallikrein 11,
and
(b) a known amount of a second agent which specifically binds to a PSA,
wherein the first and the second agents, respectively, comprise a detectable
label or bind to a detectable
label.
The agents may be antibodies, particularly monoclonal antibodies. Preferably,
the first and the
second agents, respectively, comprise a different detectable substance or bind
to a different detectable
substance.
The reagents suitable for applying the screening methods of the invention to
evaluate compounds
may be packaged into convenient kits described herein providing the necessary
materials packaged into
suitable containers.
Thus, the invention relates to a kit for assessing the suitability of each of
a plurality of test
compounds for inhibiting prostate cancer in a patient. The kit comprises
reagents for assessing kallila-ein 11
and PSA and optionally a plurality of test agents or compounds.
The invention contemplates a kit for assessing the presence of prostate cancer
cells, wherein the kit
comprises antibodies specific for kallikrein 11 and PSA, and optionally
antibodies specific for other markers
associated with prostate cancer.
Additionally the invention provides a lcit for assessing the potential of a
test compound to contribute
to prostate cancer. The kit comprises prostate cancer cells and reagents for
assessing kallikrein 11 and PSA,
and optionally other markers associated with endocrine cancer.
The following examples are intended to illustrate, but not to limit, the scope
of the invention. While
such examples are typical of those that might be used, other procedures known
to those skilled in the art may
alternatively be utilized. Indeed, those of ordinary skill in the art can
readily envision and produce further
embodiments, based on the teachings herein, without undue experimentation.
Example 1
hKl l, total PSA and % free PSA, was analyzed in a total of 150 serum samples
from men with
histologically confirmed benign prostatic hyperplasia (BPH; N = 64) or
prostatic cancer (CaP; N = 86). Total
and free PSA levels were measured by the Immulite PSA assay and hICl l levels
were measured by an
immunofluorometric assay. Serum hKl l levels and the hKl lltotal PSA ratio
were both significantly lower
in CaP patients than in BPH. In the subgroup of patients with % free PSA less
than 20, an additional 54 % of
BPH patients could have avoided biopsies using the hKl1/total PSA ratio. ROC
curve analysis demonstrated
that the hKl 1J total PSA ratio (AUC = 0.81) and % free PSA (AUC =0.82) were
much stronger predictors of
prostate cancer than total PSA (AUC = 0.69). Thus, the hKl 1/total PSA ratio
is a useful tumor marker for
prostate cancer and may be combined with % free PSA to further significantly
reduce the number of
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-24-
unnecessary prostatic biopsies.
Materials and Methods
Study copulation
Included in this study were serum samples from 150 male patients, 64 with
benign prostate
hyperplasia (BPH) (median age 65) and 86 with prostate cancer (CaP) (median
age 62), all histologically
confirmed by biopsy. All patients were from the Department of Urology at
University Hospital Charite,
Humboldt University, Berlin, Germany. Total and %free PSA, measured by the
Immulite method
(Diagnostic Products Crop., San Diego, CA) were available for all samples. All
sera were stored at -80° C
until use.
The samples were collected with informed consent and the study was approved by
the Institutional
Review Board of the University Hospital Charite, Humboldt University, Berlin,
Germany.
Production of monoclonal and nolyclonal antibodies against hKll
Purified recombinant hKl l protein was used to immunize rabbits and mice, to
raise polyclonal and
monoclonal antibodies as described elsewhere 1'. hKll was produced by
expression in Pichia Pastoris
(Invitrogen) and purified by SP Sepharose cation exchange column and reverse
phase liquid
chromatography.
Development of immunofluorometric assay for hKll
In this study, the hKl l assay described previously was used, with minor
modificationsj'. In brief,
sheep anti-mouse IgG (Jackson Immunoresearch) diluted in coating buffer
(containing SOmM Tris, pH 7.8)
was dispensed into a 96-well white polystyrene microtiter plate .(500 ng/ 100
pl/well) and incubated
overnight at room temperature. The plates were then washed three times with
washing buffer that contains 9
g/L NaCI and 0.5 g/L Tween 20 in lOmM Tris, pH 7.8. One hundred IZI of anti-
hKl l monoclonal antibody
(100ng) diluted in 6% BSA were added to each well and incubated with orbital
shaking for 2 hours at room
temperature. The plates were washed 6 times with wash buffer. Fifty pl of hKl
l calibrators (recombinant
hKll in 6°!° BSA) or samples were applied into each well along
with 50 pl of 6% BSA. The plates were
incubated for 2 hours on an orbital shaker. After this step, the previously
described procedure was usedl l, All
samples were analyzed in triplicate.
Statistical analysis
The analysis of differences between measured or calculated parameters, in the
two groups, were
performed with the non-parametric Mann-Whitney U test. Relationships between
different variables were
assessed by Spearman correlation coefficient. Receiver operating
characteristic (ROC) curves were
constructed for total PSA, % free PSA and hKllltotal PSA ratio, by plotting
sensitivity versus (1-
specificity) and the area under the ROC curves (AUC) were analyzed by the
Hanley and McNeil method.
Results
hKll levels were measured in 150 serum samples from 86 patients with prostate
cancer and G4
patients with benign prostate hyperplasia with known total and % free PSA
values. Descriptive statistics are
summarized in Tables 1 and 2.
Total PSA values ranged from 0.17 to 26.2 pg lL in BPH patients, with a mean ~
SE of 5.610.63
pg /L and from 0.35 to 48.0 pg /L in CaP patients, with the mean ~ SE of
9.340.82 pg /L. Percent free PSA
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-25-
levels ranged from 0.85 to 64.0 (mean ~ SE = 22.3 ~ 0.16) and from 1.9 to 47.9
(mean ~ SE = 10.9 ~ 0.8)
in patients with BPH and CaP, respectively. hKl l concentrations ranged from
0.0 to 2.33 ~g /L (mean ~ SE
of 0.410.05 pg /L) in BPH patients and from 0.0 to 0.72 ~g /L (mean ~ SE of
O.15t0.02 pg /L) in CaP
patients. The distributions of hKll between the two groups were signiFcantly
different (p < 0.001). The
mean value ~ SE of hKl1/total PSA ratio was 0.14 ~ 0.03 (range 0.00 - 1.28) in
patients with BPH and
0.028 ~ 0.005 (range 0.00 - 0.31) in patients with CaP. The distribution of
hKll/total PSA ratio was
significantly lower in CaP patients than in BPH patients (p<0.001). At 90
°!° sensitivity (hKllJtotal PSA
ratio of 0.06) the specificity is 48.5% (Figure l). Furthermore, the hKl
1/total PSA ratio was analyzed in the
subgroup of patients with % free PSA < 20 (these patients would have undergone
biopsy based on % free
PSA). At a cutoff point of 0.05(90% sensitivity), specificity was 54%.
Nineteen of these 35 patients could
have avoided biopsy based on this criterion (Figure 2).
A weak positive correlation was found between hKl l and total PSA levels in
the group of BPH
patients (Spearman correlation coefficient rs = 0.29, p = 0.017). However, in
CaP patients, a significant
correlation between hKl l and other variables was not observed.
The discriminatory value of the hKl 1/total PSA ratio was investigated in
relation to the total PSA
and % free PSA values, by ROC curve analysis. The hKl1/total PSA ratio,
overall, had about the same
discriminatory potential as % free PSA (AUC = 0.81, 95% CI = 0.74 - 0.88 for
the hICl 1/total PSA ratio
and AUC = 0.82, 95% CI = 0.76 - 0.89 for the % free PSA) (Figure 3).
Discussion
PSA is widely used as a tumor marker for prostate cancer, but the
discrimination between BPH and
CaP with this test is not possible due to the lack of specificity. It has been
reported that PSA molecular
forms, including free PSA, can contribute to better discrimination4,iz-ia, hK2
is also useful for the
differential diagnosis for prostate cancer in the PSA range of 2.5 to 10
~g/L4es
Recently, an immunoassay was developed for hKll and relatively large amounts
of hKll was
found in seminal plasma and prostatic tissues ~ 1.
In this study, it is demonstrated for the first time that hICl l levels in
serum are significantly lower in
CaP patients than in BPH patients. The hKl 1/total PSA ratio is also
significantly lower in CaP than in BPH.
Using the hKl1/total PSA ratio, approximately 50% of biopsies could be avoided
that could have not been
avoided by the % free PSA test (% free PSA <20). These results demonstrate for
the first time that the
combination of % free PSA and the hKl lltotal PSA ratio could contribute to
better discrimination between
BPH and CaP patients. Out of 64 patients with BPH, and at 90 % sensitivity, 29
patients (45%) would have
avoided biopsy by % free PSA testing (> 20 % free PSA). For the remaining 35
patients, another 19 could
have avoided biopsy by the hKll/total PSA ratio (> 0.05). When the two tests
are combined, 48 BPH
patients (80 %) would have avoided biopsy at about 90 % sensitivity (Figure
4).
ROC curve analysis demonstrated that the hKl lltotal PSA ratio had about the
same discriminatory
value as % free PSA, suggesting that hKl l could be an additional marker for
prostate cancer.
Conclusions
The potential clinical utility of serum hKl l analysis for the differential
diagnosis of prostate cancer
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-26-
was investigated and it was determined that the hKl 1/total PSA ratio could be
a useful novel tumor marker.
Example 2
Human kallilcrein 11, total PSA, and %free PSA were analyzed using the
procedure described in
Example 1 in serum samples from 405 male patients, 247 with benign prostatic
hyperplasia (median age 66)
and 157 with prostate cancer (median age 64), histologically confirmed by
biopsy in all cases. Descriptive
statistics are described in Table 3. The logistic regression analysis of BPH
and CaP patients for predicting
the presence of prostate cancer is shown in Table 4. A comparison of the
sensitivity and specificity at
selected cut-off points of the study variables is shown in Table 5, and a
comparison of the sensitivity and
specificity at selected cut-off points in subgoups according to total PSA
value is shown in Table 6. Table 7
shows serum hKl l concentration classified by tumor grade, stage of disease,
and Gleasson score.
A positive correlation was found between hKl l and patient age in the group of
patients with benign
prostatic hyperplasia (Spearman correlation coefficient rs 0.242, p<p.001) as
well as in the group of patients
with prostate cancer (Spearman correlation coefficient rs 0.208, p=0.009).
The distribution of hKll/total PSA ratio was significantly lower in CaP
patients than in BPH
patients (p<0.001) (Figure 6).
The discriminatory value of the hKl1/total PSA ratio was investigated in
relation to the total PSA
and % free PSA values, by ROC curve analysis (Figure 5). The hKl1/total PSA
ratio had about the same
discriminatory potential as Total PSA.
The hKl1/total PSA ratio was analyzed in the subgroup of patients with total
PSA = 4-10 pg/L,
total PSA >10 pg/L, total PSA < 4 pg/L and %free PSA < 17.8 (Figures 7 to 10).
The ratio of hICl1/total
PSA was also shown to differentiate BPH from prostate cancer in a population
of patients with total PSA less
than 4ng/ml (Figure 9). In the range of 4-lOng/ml total PSA, the combination
of kallikrein 11, ttotal PSA,
and % free PSA was especially useful for differentiating BPH from prostate
cancer.
The present invention is not to be limited in scope by the specific
embodiments described herein,
since such embodiments are intended as but single illustrations of one aspect
of the invention and any
functionally equivalent embodiments are within the scope of this invention.
Indeed, various modifications of
the invention in addition to those shown and described herein will become
apparent to those skilled in the art
from the foregoing description and accompanying drawings. Such modifications
are intended to fall within
the scope of the appended claims.
All publications, patents and patent applications referred to herein are
incorporated by reference in
their entirety to the same extent as if each individual publication, patent or
patent application was
specifically and individually indicated to be incorporated by reference in its
entirety. All publications,
patents and patent applications mentioned herein are incorporated herein by
reference for the purpose of
describing and disclosing the domains, cell lines, vectors, methodologies etc.
which are reported therein
which might be used in connection with the invention. Nothing herein is to be
construed as an admission that
the invention is not entitled to antedate such disclosure by virtue of prior
invention.
It must be noted that as used herein and in the appended claims, the singular
forms "a", "an", and
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-27-
"the" include plural reference unless the context clearly dictates otherwise.
Thus, for example, reference to "a
host cell" includes a plurality of such host cells, reference to the
"antibody" is a reference to one or more
antibodies and equivalents thereof known to those skilled in the art, and so
forth.
Below full citations are set out for the references referred to in the
specification.
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-28-
Table
1
Descriptive BPH patients
statistics
of various
variables
in serum
of 64
Percentiles
Variables Mean Range 10 25 50 75 90
SEa
(median)
hKl l (~g/L)0.41 0.050.00-2.330.060.11 0.24 O.G20.95
Total PSA 5.61 0.630.17 - 1.051.63 4.17 8.0212.95
(~glL) 26.2
Free PSA 1.22 0.04 - 0.220.39 0.64 1.412.01
(~g/L) 0.26 15.0
Free/Total 22.3 0.85 - 10.013.4 18.5 28.238.4
PSA 0.1.56 64.0
hKllltPSA 0.14 0.00 - 0.0130.0290.063 0.130.39
0.03 1.28
a Standard error
Table 2
Descriptive
statistics
of various
variables
in serum
of 86 CaP
patients
Percentiles
Variables Mean * Range 10 25 50 75 90
SE
(median)
hKl l (~glL)0.15 0.020.00-0.720.00 0.05 0.08 0.250.38
Total PSA 9.34 0.820.35 2.86 4.79 7.23 11.5018.72
(~g/L) - 48.0
Free PSA 1.03 0.10 0.27 0.42 0.61 0.971.55
(wglL) 0.26 - 23.0
Free/Total 10.9 1.9 - 3.9 6.6 8.6 13.321.7
PSA 0.8 47.9
hKl l/tPSA 0.028 0.00 0.00 0.0050.014 0.0340.061
0.005 - 0.31
a Standard error
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
_ 29 .
Table 3
Descriptive statistics of various variables in serum of BPH and CaP patients
,~ ~_ ............. Percentiles
Variables Mean's Range 10 25 50 75 90
SEa
(median)
BPH (N=247)
HKl l (~,g/L) 0.19 0.008~ 0.01 0.062 0.110.17 0.220.32
Ob-1.12
Total PSA (~glL)5.37 0.420.58 1.18 1.904.00 6.5011.62
- 82.9
t FreelTotal 17.8 2.1 - 6.1 11.717.1 22.327.8
PSA 0.58 80.0
hKl1/tPSA 6.53 0.085 0.84 2.034.44 8.8015.23
0.43 -51.14
CaP (N=157)
HK11 (~g/L) 0.14 0.006O.OlOb-0.460.056 0.0910.14 0.180.24
Total PSA (~g/L)13.241 0.61- 3.85 5.729.50 14.8725.25
1.07 90.7
Free/Total PSA 9.87 0.531.90 4.05 56.628.35 11.8717.90
- 56.6
hKll/tPSA 1.90 0.140.02-9.380.31 0.731.30 2.324.33
Standard error
6 Half of
the assay detection
limit
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-30-
Table 4
Logistic regression analysis of BPH and CaP patients for
predicting the presence of prostate cancer.
T Univariate Multivariate analysis
Analysis
Covariate Crude Crude
Odds Ratiop-value*Odds Ratio p-value*
Total PSA 1.17 <0.001 1.05 0.031
% Free/Total PSA 0.85 <0.001 0.87 0.001
Ratio
% hKl1/Total PSA 0.66 <0.001 0.76 <0.001
Ratio
*Test for trend
Table 5
Comparison of sensitivity and specificity
at selected cut-off points of the study
variables
Parameter Cut-off Specificity (%) Sensitivity
(%)
All Patients (N=404)
Total PSA 14.92 95 25
11.62 90 40
3.86 49 90
2.89 38 95
Free/Total PSA 5.95 95 30
8.35 90 51
17.8 46 90
19.2 39 95
hKl l/ Total PSA 0.49 95 19
0.93 90 33
3.99 54 90
5.97 38 95
Ratio Combinations 1.50 95 54
1.82 90 G7
3.19 56 90
3.69 46 95
a Ratio Combination function = 0.142*%
FreelTotal PSA +-0.213*% hKl1/
Total PSA as it was formulated from the
logistic regression analysis.
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-31-
Table 6
Comparison of sensitivity and specificity at selected cut-off points
in subgroups according to total PSA value
Parameter Cut-off Specificity (%) Sensitivity (%)
Total PSA <4ug/L (N=139
Free/Total PSA 7.05 95 6
8.85 90 18
19.5 45 90
20.0 44 95
hKl1/ Total PSA 0.73 95 6
2.92 90 35
7.52 57 90
9.19 43 95
Ratio Combinations 2.12 95 18
2.53 90 31
4.12 S8 90
4.31 56 95
Total PSA 4-lOu~JL (N=158)
Free/Total PSA 5.55 95 25
8.35 90 53
15.1 52 90
18.2 42 95
hKl l/ Total PSA 0.79 95 10
1.25 90 22
3.99 28 90
5.24 16 95
Ratio Combination a 1.61 95 47
1.77 90 S8
2.71 56 90
3.13 48 95
Total PSA >10u~/L (N=1071
Free/Total PSA 4.65 95 20
6.35 90 40
14.6 39 90
25.8 10 9S
hKl l/ Total PSA 0.22 95 13
0.30 90 1S
1.44 29 90
1.66 26 95
Ratio Combinations 0.83 9S 20
1.33 90 57
2.22 42 90
2.88 26 95
a Ratio Combination function2*% +0.213*% hKl
= 0.14 Free/Total l/ Total PSA
PSA
as it was formulated from analysis.
the logistic regression
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-32-
Table 7
Serum hkll concentration classified by tumor grade,
stage of the disease and Gleasson score
TotalMean Standard Medianspvalue
Error"
Grade
Gl 8 0.171 0.016 0.166 0.036
G2 90 0.152 0.009 0.145
G3 48 0.120 0.008 0.104
Stage
I 13 0.176 0.021 0.174
II 88 0.141 0.078 0.133 0.038
III/IV 41 0.120 0.011 0.110
Gleason
score
_< G 55 0.151 0.117 0.146
>6 72 0.131 0.008 0.125 0.176
a uglL
~' Calculated by the Mann-Whitney U Test
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-33-
FULL CITATIONS FOR REFERENCES REFERRED TO IN THE SPECIFICATION
1. Oesterling JE: Prostate speciEc antigen: a critical assessment of the most
useful tumor marker for
S adenocarcinoma of the prostate. J Urol 145: 907-923, 1991.
2. Barry MJ: Clinical practice. Prostate-specific-antigen testing for early
diagnosis of prostate cancer.
N Engl J Med 344: 1373-1377, 2001
3. Oesterling JE, Jacobsen SJ, Klee GG, et al: Free, complexed and total serum
prostate specific
antigen: the establishment of appropriate reference ranges for their
concentrations and ratios. J Urol
154: 1090-1095, 1995.
4. Stephan C, Jung K, Lein M, et al: Molecular forms of prostate-specific
antigen and human
kallikrein 2 as promising tools for early diagnosis of prostate cancer. Cancer
Epidemiol Biomarlcers
Prev 9: 1133-1147, 2000.
5. Catalona WJ, Partin AW, Slawin KM, et al: Use of the percentage of free
prostate-specific antigen
1S to enhance differentiation of prostate cancer from benign pxostatic
disease: a prospective
multicenter clinical trial. JAMA 279: 1542-1547, 1998.
6. Yoshida S, Taniguchi M, Suemoto T, et al: cDNA cloning and expression of a
novel serine
protease, TLSP. Biochim Biophys Acta 1399: 225-228, 1998.
7. Mitsui S, Yamada T, Okui A, et al: A novel isoform of a kallilcrein-like
protease, TLSPIIiippostasin,
(PRSS20), is expressed in the human brain and prostate. Biochem Biophys Res
Commun 272: 205
211, 2000.
8. Nakamura T, Mitsui S, Okui A, et al: Alternative splicing isoforms of
hippostasin
(PRSS20/KLKl l) in prostate cancer cell lines. Prostate 49: 72-78, 2001.
9. Yousef GM, Scorilas A, and Diamandis EP: Genomic organization, mapping,
tissue expression, and
hormonal regulation of trypsin-like serine protease (TLSP PRSS20), a new
member of the human
lcallikrein gene family. Genomics 63: 88-96, 2000.
10. Diamandis EP, Yousef GM, Clements J, et al: New nomenclature for the human
tissue kallikrein
gene family. Clin Chem 46: 1855-1858, 2000.
11. Diamandis EP, Okui A, Mitsui S, et al: Human Kallikrein 11: A new
biomarker of prostate and
ovarian carcinoma. Cancer Res 62: 295-300, 2002.
12. Catalona WJ, Partin AW, Finlay JA, et al: Use of percentage of free
prostate-specific antigen to
identify men at high risk of prostate cancer when PSA levels are 2.51 to 4
nglmL and digital rectal
examination is not suspicious for prostate cancer: an alternative model.
Urology 54: 220-224, 1999.
13. Jung IC, Elgeti U, Lein M, et al: Ratio of free or complexed prostate-
specific antigen (PSA) to total
PSA: which ratio improves differentiation between benign prostatic hyperplasia
and prostate
cancer? Clin Chem 46: 55-62, 2000.
14. Oesterling 3B, 3acobsen SJ, and Cooner WH: The use of age-specific
reference ranges for serum
prostate specific antigen in men 60 years old or older. J Urol 153: 1160-1163,
1995.
15. Magklara A, Scorilas A, Catalona WJ, et al: The combination of human
glandular leallikrein and
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-34-
free prostate- specific antigen (PSA) enhances discrimination between prostate
cancer and benign
prostatic hyperplasia in patients with moderately increased total PSA. Clin
Chem 45: 1960-1966,
1999.
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-1-
SEQUENCE LISTING
<110> Mount Sinai Hospital
<120> Methods of Detecting Prostate Cancer
<130> T01114-0017-WO
<140>
<141>
<150> US 60/414,314
<151> 2002-09-26
<160>3
<170> PatentIn version 3.2
<210> 1
<211>282
<2l2> PRT
<213> homo sapiens
<300>
<308>Genbank/AB012917
<309> 1998-04-10
<313> (1)..(282)
<400> 1
Met Gl n Arg Leu Arg Trp Leu Arg Asp Trp Lys Ser
Ser Gly Arg Gly
1 5 10 15
Leu Thr Ala Ala Lys Glu Pro Gly Ala Arg Ser Ser Pro Leu Gln Ala
20 25 30
Met Arg Ile Leu Gln Leu Ile Leu Leu Ala Leu Ala Thr Gly Leu Val
35 40 45
50
Gly Gly Glu Thr Arg Ile Tle Lys Gly Phe Glu Cys Lys Pro His Ser
55 60
Gln Pro Trp Gln Ala Ala Leu Phe Glu Lys Thr Arg Leu Leu Cys Gly
65 70 75 80
A1a Thr Leu Ile Ala Pro Arg Trp Leu Leu Thr Ala Ala His Cys Leu
85 90 95
Lys Pro Arg Tyr Ile Val His Leu Gly Gln His Asn Leu Gln Lys Glu
100 105 110
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-2-
Glu Gly Cys Glu Gln Thr Arg Thr Ala Thr Glu 5er Phe Pro His Pro
115 120 125
Gly Phe Asn Asn Ser Leu Pro Asn Lys Asp His Arg Asn Asp Ile Met
130 135 140
Leu Val Lys Met Ala Ser Pro Val Ser Ile Thr Trp Ala Val Arg Pro
145 150 155 160
20
Leu Thr Leu 5er Ser Arg Cys Val Thr Ala Gly Thr Ser Cys Leu I1e
165 170 175
Ser Gly Trp Gly Ser Thr Ser Ser Pro Gln Leu Arg Leu Pro His Thr
180 185 190
Leu Arg Cys Ala Asn Ile Thr Ile Ile Glu His Gln Lys Cys Glu Asn
195 200 205
Ala Tyr Pro Gly Asn Ile Thr Asp Thr Met Val Cys Ala Ser Val Gln
210 215 220
Glu Gly Gly Lys Asp Ser Cys Gln Gly Asp Ser Gly Gly Pro Leu Val
225 . 230 235 240
40
Cys Asn Gln Ser Leu Gln Gly Ile Ile Ser Trp Gly Gln Asp Pro Cys
245 250 255
Ala Ile Thr Arg Lys Pro Gly Val Tyr Thr Lys Val Cys Lys Tyr Val
260 265 270
Asp Trp Ile Gln Glu Thr Met Lys Asn Asn
275 280
<210> 2
<211> 9120
<212> DNA
<213> homo Sapiens
<300>
<308> Genbank/AF164623.
<309> 1999-07-01
<313> (1)..(9120)
<400> 2
tgataatagt gttctctctc ctcattggtc agggccccag ccattgtcct tgagagaatg 60
ctcgactctt tatgttgtct tgacagcctc ccctgagatt ggtcattaat gactgtgctc 120
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-3-
tctctcctcattggtcagggccccagccattgtccttgagagaacctctgtcctttatgg180
agttccacccttcttccctgggattggcccctagagacagtggttcttctcttttggtta240
gccattgccattgtcctccgggaaagtgattatactcttttgtctaatgaccagacttgg300
agccctccccaaggcccaggactgggttgaagggttggggaggaaaacagaaataagatg360
tctcccttgttcagacagtacttctcttcccttccagggtgattctgggggccccctggt420
gtgtgggggagtccttcaaggtctggtgtcctgggggtctgtggggccctgtggacaaga480
tggcatccctggagtctacacctatatttgcaagtatgtggactggatccggatgatcat540
15gaggaacaactgacctgtttcctccacctccacccccaccccttaacttgggtacccctc600
tggccctcagagcaccaatatctcctccatcacttcccctagctccactcttgttggcct660
gggaacttcttggaactttaactcctgccagcccttctaagacccacgagcggggtgaga720
gaagtgtgcaatagtctggaataaatataaatgaaggaggggccatgtctgtccatttga780
agtcctcatgctggttgagactggaagaaggactcagcagtttccctatctcataggagt840
25agaaacagagctcaaataaggccaggcacagtggctcacacctgtaatcccatcactttg900
ggaagctgaggcaggtggatcacctgaggtcaggaactcgggaccagcctggtcaacata960
gtgaaaccccaactctactaaaaatgcaaaaattagccaggcatggtggcgcatgcctgt1020
aatcccagctactcaggaggctgagacaggagaatagcatgaacccgtgaggcagaggct1080
gcagcgagccgagattgaaccattacactccagcctgggcgacagagcgagactccatct1140
35caaaaacaaacaaacaaaaaacccagtgctcaaataggatgagggtcttccctgagtagt1200
tactcagaaatggagtagaaaaagttacttttaataatataggccgggtgcagtggccca1260
cgcctgtaatcccagcactttgggaggccgaggtgggaggatggcttgagctcagatttc1320
gagatcagcctggcaacacagtgaaatcttgtcactacaaaaacacaaaaaattagctgg1380
gtgtggtggtgcgtgcctgtagtcccagctacttgggaagctgaggtgggaggatcaccc1440
45gagccggggaggtggaggctgcaaagagccgagatcatgccactgcactccagcctgggc1500
aataaagtgagaccttgtctcaaaaacaaaaacccagcaatataaataagacacatgttt1560
cttcatctggcataatagaaatagtgcccagagcttataagcttttcaagagtccacaaa1620
agacccgaaaaagaaaaagaaaattgttagctccaaaataccagatgaaagctgcaaagt1680
caacatttatgaccatttaatccaatgtccataaaacgtagcattctttccactagccaa1740
55ctgcagtttactttcttgtaatgaagcatacattgtatctttaatgtgggacgtggcttt1800
gttctaataagacgaagggtggagtgcaggcttggaaagcaggagagctcagcctacgtc1860
tttaatcctcctgcccaccccttggattctgtctccactgggactcaagaggtgaggaga1920
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
_4_
gaccatctccccaaatgcactgaagggaaactggaggagggagggagtgaggggtgatca1980
taccagcggaggcacatttgctgagcccccccgcagtctgctctttccaagtggaccctc2040
ctggaagcctgatcccaacctcccctgcaagcaggtctgtcacccccatctctcagatga2100
agaaactgagccttgcaggggtggagtcccttgtccccacgtcataagggtagtcatagt2160
10agtaggaagaggaagcacctaggtttgaggccagggctggctgctgtcagaacctaggcc2220
ctcccctgccttgctccacacctggtcaggggagagaggggaggaaagccaagggaaggg2280
acctaactgaaaacaaacaagctgggagaagcaggaatctgcgctcgggttccgcagatg2340
cagaggttgaggtggctgcgggactggaagtcatcgggcagaggtctcacagcagccagt2400
aagtgaacagctggactcgggctgcctgggcggcagggagaagcgggcaggggaagggtc2460
20agcagaggagcgaggccccagaggagccctggggtggagcacagccaagggctctgttcc2520
ctttcctggactcggcttccacaggccctgacctgcctcccccaccctccggtcctgccc2580
ctgtgcctggcagcagccccacctgtgtgacatcccagcacaccccccctctccttgcaa2640
aggagaagggagcggcctaggggaggccaggggcccacctgggctggggctgtggagagg2700
gagtggctgggacgggaggaaaaagagagacggagattagatggaagaagagggatttca2760
30agacaaattgccagagatgcagtcagagagactgactgagagacacaaagatagaaggaa2820
ttagagaaagggccacacagagccagacagagagagaagagtggagatggagacagggac2880
gaggacagagaaaggcagacagacacatagggacagaaagagaaaaatcacacaaagtca2940
gaattactgaatgacagggaatgacacatagaacgagacacagattcagagactcagggc3000
agggaaaggaaggctgcagacagacagacagacagagggaggctgagacacagggagaag3060
40aggggcttggagaggtggcacaggcaggcagccagtgcctcagaggcctccggggagggc3120
cctcacacacaccccgccccggggcattaaggcagggcttggaggccagtcatcctgggc3180
ccgcccagggccgcccccctgccagcccgcctgcctggtgcctggcacctggcgctccaa3240
cccagcctacctgctgtagctgccgccactgccgtctccgccgccactgggcccccagag3300
ccccagccccagagcctgtgagtccaggaggaaagggaagctgcccctccccgtccaggt3360
50gtcagccctccccaaggacacctgtcccactcgggcacccatttctccctctgctctgtc3420
cttctctgcttgggtgggggttcctggcctctctctacacctctcacctccgatggctgt3480
ccgcagcctcagttacctctaatctccatggcttcagctgctgagctggccctctgctcc3540
cacccccgctggccagggcagcggagggcactggccctcccctcgaccagccccgcccag3600
ctttgcttggctgtccttcaaaagggcaggggtttggcggacagggcttcagcaagccgg3660
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-5-
gtgatgggggtcccagacattgtctggggctgagccccctactcccctccagcagacctc3720
aaaggctccatatcgctctgctgcgaagacaatgaaaaaggggtggctacggaacggtgt3780
ctggttccccttgtccttccaccccaagctgctggggcctggccagctctcaaggcaaga3840
aggaaaacatcctctgacatgtgccggggaggtcccatggctgacttgaacagggccgaa3900
ccatggcttgacagctcaaagcccctcccaacgacttccacatggttcttggtatctcgg3960
aagcttctagctgtgaccaggccctctccaaggccaccctagacacctaagatatatttt4020
aagtgtttggagatctgagtgctgtgagaaacaggggatttccccaaccttgtttctccc4080
15aagtggggagcgggagcaggtgagggagagaggagagggcatgagccagcccccccctcc4140
cgatttccccgtaaagtgatgcggccccatgtccctccttgttcccagaggaacctgggg4200
cccgctcctcccccctccaggccatgaggattctgcagttaatcctgcttgctctggcaa4260
caggtacgcaggggatgggggcagggcaggatcctccctcttgaatctctgggatcccct4320
aaccctctgtgtctggacagtgacagggctgattccaaattacagaacaacccataaggc4380
25acctgaactggagcagtggtcatgagggcctggatgcccttctagataatccctttaaat4440
gccaaaggaggagaggtcaagggggtcgtaaagggtcccgtggaggggctgaggaagctg4500
gagttgggggagcagtcactcaaagcgcccaggacaggggctactgaccaaccagtatgg4560
aagtatttccttttttttttttcccagagacaaagtcttgctctattgtccaggctggag4620
tgccgtggtgccaacacggctcactgcagtcttgacttcccgggcttaagtgatccttaa4680
35gccatctcagcttccccggtagctgggaccacaggcacctgccaccaagccaggctaatt4740
gtttaattgtttgtagagatgggggaggaggtctcactatgtttgcctaggctgatctag4800
aactcctgggctcaagtaatcctcccaccttagcctctcaaagtgctgggattacaggca4860
tgagccactgcatttgaccttatggaagtattttcatcctttaatacccgaccccagcat4920
ccagggcaacccagagggacaccagaccagggcccagaccacccactctctttctctcct4980
45ccccacccccatttctgggagtcctcctggtctaccacctctccttcctgagccccttct5040
tttgctctca ccccctccagggcttgtagggggagagaccaggatcatcaaggggttcga5100
gtgcaagcct cactcccagccctggcaggcagccctgttcgagaagacgcggctactctg5160
tggggcgacg ctcatcgcccccagatggctcctgacagcagcccactgcctcaagccgtg5220
ggtgcggggg ctggggcggtgccggggtggggggctgggaatggggagatggatggagag5280
aagctcagggataggggtgctggtaaggggattagagatggggatgggtagtgtcagcaa5340
ggttgatggg ctcgagttggtattgaaggtggggggatgaatggggttgggatggggcta5400
tggctgggaa gggggcttcggtgggagacgtggaagaggttggaagcagagcgatgtttc5460
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
-G-
ttcatcctcaaaggtgtcactcacctctcccacccatgtctcccccgacctttcctcctc5520
caactactgtctctcccacctcagccgctacatagttcacctggggcagcacaacctcca5580
gaaggaggagggctgtgagcagacccggacagccactgagtccttcccccaccccggctt5640
caacaacagcctccccaacaaagaccaccgcaatgacatcatgctggtgaagatggcatc5700
10gccagtctccatcacctgggctgtgcgacccctcaccctctcctcacgctgtgtcactgc5760
tggcaccagctgcctcatttccggctggggcagcacgtccagcccccagtgtaggagcac5820
cagaggggaacctggcagggggtggtgaggagggagtggtcaggattgtggaagggttca5880
gggcatcagagatgcggttcacagtgacgatgtgggataagttgagaggatgtgtggaaa5940
acgtcaggataggggggtggggacaaaagttggggccttggagtcagacggacgggatat6000
20gcaatcatacatccataacctcctggttgtaagaccttaggcaagcagcttcacctctct6060
gaatcttgattttcttctctataaaatgagaatgattatacccacctgtcaggattggat6120
tagagataatgtatatcaagcaactgacataaatcatttattggatagcaggctgggcac6180
cgtggctcacgcctgtaatcccagcactttgggaggccgaggtgggaagatcacctgagg6240
tcaggactttgataccagcctggccaacgtggtgaaatcccatctctactaaaaatgtga6300
30aaattagttgggcgtggttgtgtgcgcctgtaatcccagctactcgggaggttgaggcag6360
gagaatcgcttaaacttgggagacggaggttgcagtgagccaagatcacgccactgcact6420
ccagcctgggcaacagagcaagactctgtctcgaaaaaaaaaaaaaaaaagctggatagc6480
attgctgttgctattgttacaagaagagaggtgagttggctgcgtctaaggacagggatt6540
cccccaggggcgggatcacagcaagcactgcattaggggaggtggcagggggctcattcc6600
40cacagcccctcacgctgtttccacagtacgcctgcctcacaccttgcgatgcgccaacat6660
caccatcattgagcaccagaagtgtgagaacgcctaccccggcaacatcacagacaccat6720
ggtgtgtgccagcgtgcaggaagggggcaaggactcctgccaggtcagtgtggtctccaa6780
ccacagccccatccccatccccagcttcaatgacatctttaccgacatccacaatttcat6840
ccccaacctc aacccgccga cccctgcaac tcccaatcca tctcttcccc tgttcccgtt 6900
SO tctgacctcagcacaaacttcagctccatccccgtttccacaccatttccagctccaacc6960
atccccaaac tcgtttttgagcctaaccccatcctttatcccacccataatcccagcttt7020
atcgctaaac ctatcacctttcccagtgcctacccatcctgtctcggccccactcctaag7080
caccgtcccc acctcctccctggctaacaccatgctcaacgctttctctgaccgacattc7140
tctctccccg tgcccagggtgactccgggggccctctggtctgtaaccagtctcttcaag7200
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
_7_
gcattatctcctggggccaggatccgtgtgcgatcacccgaaagcctggtgtctacacga7260
aagtctgcaaatatgtggactggatccaggagacgatgaagaacaattagactggaccca7320
cccaccacagcccatcaccctccatttccacttggtgtttggttcctgttcactctgtta7380
ataagaaaccctaagccaagaccctctacgaacattctttgggcctcctggactacagga7440
gatgctgtcacttaataatcaacctggggttcgaaatcagtgagacctggattcaaattc7500
tgccttgaaatattgtgactctgggaatgacaacacctggtttgttctctgttgtatccc7560
cagccccaaagacagctcctggccatatatcaaggtttcaataaatatttgctaaatgag7620
15tgaatctactgagtgcttactatgtgctagaccctgatccaatggcttttattttatttt7680
attttttgacagagtctcgctctgtcacccaggctggagtacagtggtgctatctctgct7740
cactgcaacctccacctcctgggttcaagcaattctcctgcctcagcctcctgaatagct7800
gggattacaggtgcctaccaccacatccggctaatttttgtattttttagtagagatggg7860
gcttcaccatgttggccaggctggtctcgaactcctgacctcagatgatctgccctcctt7920
25ggcctcccaaactcctgggattacagacgtgagccaccgcgcccgcccggctttcattta7980
ttaattaaaagaaattaaattaattaatctatttaggagacagtcttgctctgttgccca8040
ggctggagtgcagtaacaatcacagctcacggcaatctcaatttcctggggtcaagtgat8100
tgtcctccctcagcctccagagtagctgggactacaggcacatgccacgaagcccagcta8160
atttttgtatttttcgtagagacagaggtctcagtatgttgccccggctagtctcaaact8220
35cctgggctcaagcagtctgtcctcctcagcctccaaaagtggtgagattacaggcatgag8280
tcgctgtgcctggcctccaagcactttcaaatgtatcaacttaatcctcacaaaaccctg8340
tgaggtcggtactgttttcatacctattttatagttgaagaaacagacacagagaagcaa8400
agtcacttgctcacagtcacgtggctaggagagcaaggatctgaagcaaggcgatctctt8460
aattaccaagtgatgttcctggagtaaggctctgtttgtttcctttcctgtaaaatgctg8520
45catgcaaaagtataacacagtaagtaaagaagtcagttagcctgcacatactaagaccta8580
accaaaggagcttattgtttttctccaacttccatgataggtaattagatagtggagacc8640
tctgctggccaatatggtagccactaaccgcagctggctcttccaattaaaattacataa8700
agccagaaatgtaactcctctgtctcacttgttatatctccaaggctggatagccacatg8760
tgactggtggtggctggattagctagtgcatataaaacatcactgcagaaagttcagctg8820
55agcagcactgagttagatggcctctgaagaggatgtcccacggagagaatccagaactca8880
ggatctttttttttttttttctttgcgacagagtcttgctctgtcacccaggctggagtg8940
cagtggcgtgatctcggctcactgcaacttctgcctcccaggttcaagcaattctcctgc9000
CA 02498147 2005-03-08
WO 2004/029616 PCT/CA2003/001479
_g_
ctcagcctcc ctagtagctg ggactacagg cctgtgccaa catccccagc taatttttgt 9060
gtctttttag tagagatggg gtttcactat gttggccagg ctggtctcga actcctgacc 9120
<210> 3
<211> 1186
<212> DNA
10<213> homoSapiens
<400> 3
aggaatctgcgctcgggttccgcagatgcagaggttgaggtggctgcgggactggaagtc60
15atcgggcagaggtctcacagcagccaaggaacctggggcccgctcctcccccctccaggc120
catgaggattctgcagttaatcctgcttgctctggcaacagggcttgtagggggagagac180
caggatcatcaaggggttcgagtgcaagcctcactcccagccctggcaggcagccctgtt240
20
cgagaagacgcggctactctgtggggcgacgctcatcgcccccagatggctcctgacagc300
agcccactgcctcaagccccgctacatagttcacctggggcagcacaacctccagaagga360
25ggagggctgtgagcagacccggacagccactgagtccttcccccaccccggcttcaacaa420
cagcctccccaacaaagaccaccgcaatgacatcatgctggtgaagatggcatcgccagt480
ctccatcacctgggctgtgcgacccctcaccctctcctcacgctgtgtcactgctggcac540
30
cagctgcctcatttccggctggggcagcacgtccagcccccagttacgcctgcctcacac600
cttgcgatgcgccaacatcaccatcattgagcaccagaagtgtgagaacgcctaccccgg660
35caacatcacagacaccatggtgtgtgccagcgtgcaggaagggggcaaggactcctgcca720
gggtgactccgggggccctctggtctgtaaccagtctcttcaaggcattatctcctgggg780
ccaggatccgtgtgcgatcacccgaaagcctggtgtctacacgaaagtctgcaaatatgt840
40
ggactggatccaggagacgatgaagaacaattagactggacccacccaccacagcccatc900
accctccatttccacttggtgtttggttcctgttcactctgttaataagaaaccctaagc960
45caagaccctctgcgaacattctttgggcctcctggactacaggagatgctgtcacttaat1020
aatcaacctggggttcgaaatcagtgagacctggattcaaattctgccttgaaatattgt1080
gactctgggaatgacaacacctggtttgttctctgttgtatccccagccccaaagacagc1140
50
tcctggccatatatcaaggtttcaataaatatttgctaaatgagtg 1186