Note: Descriptions are shown in the official language in which they were submitted.
CA 02499304 1999-06-10
WO 00/12012 PCT/US99/13249
TITLE OF THE INVENTION
SEL~EXPANDING DEFECT CLOSURE DEVICE
RELATED APPLICATIONS
The present application bears relation to United States Patent
No.6,080,182 which bears relation to United States Patent No.5,879,366.
FIELD OF THE INVENTION
The present invention relates to closure devices, their manufacture and
use to occlude a defect in a tissue or muscle of a animal, such as a human
being, or a defect in a wall of a structure, such as a container or filter.
More
specifically, the present invention relates to a self-expanding closure device
having a membrane that is supported by a structure having elastic properties,
which is capable of being compacted and inserted through a defect, and
thereafter returned to an enlarged configuration to cover or seal the defect.
BACKGROUND OF THE INVENTION
A wall defect is generally a hole in the wall of the tissue of an animal,
such as humans, or a hole in the.wall of a container, tank, bag filter, or
planar
filter, tent, inflatable device, etc. In muscles or tissues of animals,
repairs have
been accomplished by inserting an occlusion or septal closure device into the
aperture or defect. Such devices include those taught by US Patents
5,334,217 to Das and 5,108,420 to Marks.
The Das patent describes a septal defect closure device, its use and
method of assembly, where individual disks of a thin flexible material are
supported by a super-elastic material and are used to occlude a wall defect.
' The disks are conjointly attached to one another at the center of the disk.
The
thin flexible material used in the Das patent can include nylon, polyester, '
polypropylene and polytetrafiuoroethylene (PTFE) polymers. The super-elastic
material is a NT alloy (or "nitinol".)
CA 02499304 1999-06-10
WO 00/12012 PCT/US99/13249
The super-elastic material of the Das patent is formed into a frame
having several legs and can assume geometrical configurations such as
triangles, hexagons, circles, and stars. A membrane is wrapped around the
legs of the frame. The loops between adjacent legs bias the legs outwardly, to
form a concave membrane surface, which is maintained in a highly tensioned
fashion.
The Marks patent describes an occlusion device that can be transported
via a catheter in a compressed state. Once through an aperture to be
occluded, the device is released and wires supporting two membranes are
positioned on each side of the aperture. A domed or umbrella shaped
configuration is formed and the support wires urge the membranes towards one
another and the wall where the aperture is located.
These prior devices have numerous drawbacks. The support frames of
the Das patent include resilient wire loops where leg ends of the frame meet
and are attached to one another. The loops generally extend beyond the
periphery of the membrane and can irritate or damage adjacent muscle or
tissue.
Similarly, the exposed wires of the Marks device act as an irritant to
tissue or muscle adjacent the aperture or septum. Here the bare sharp ends of
the wire structure can further cause tissue erosion.
The Das and Marks patent devices use a membrane of conventional
thickness that when folded over a wire add undesired thickness to the device.
Additionally, the patents rely on peripheral membrane support which leaves the
central occlusion covering portion of the membrane vulnerable.
In the Das patent design, each leg is provided with a bend at the middle
of its length. This bend rnay tend to fold the device when the frame is
sitting
against a very flexible tissue and the membrane is pressurized by the blood.
This may be the potential mechanism of failure as reported in Agacwal, S.K.,
Ghosh, P.K. and Mittal, P.K., "Failure of Devices Used for Closure of Atrial
Septal Defects: Mechanisms and Management," The Journal of Thoracic and
Cardiovascular Surgery, Vol. 112, No. 1, 1996.
Finally, it is believed that none of the previously available devices have
provided a sufficiently small enough insertion diameter andlor collapsed
2
CA 02499304 1999-06-10
WO 00/12012 PCT/US99/I3249
flexibility. This limitation has restricted the utility and ease of use of
such
devices.
Thus, in view of the above, it is desirable to provide a closure device
that eliminates or significantly minimizes the traumatizing effect of existing
5 closure devices. Further, it is desirable to provide such a device to be
stable
under physiological loading conditions when situated against the anatomical
tissue structure. It is additionally desirable to provide a defect closure
device
that is collapsible or compressible so that it may fit into a 9F (9 French),
preferably 5F or 41= or smaller catheter for deployment in a defect.
These drawbacks and disadvantages of the prior devices have been
addressed and overcome by the inventions described in the two co-pending
patent applications (now United States Patent Nos.6,080,182 and
5,879,3666). These prior inventive devices employ unique nitinol frames
combined with thin expanded PTFE membranes to create defect closure
devices that function better than any previous defect closure devices. Of
particular benefit of these devices is the fact that they are capable of being
compacted into very small delivery tools and then deploy to fully operational
diameters. Additionally, unlike some previous defect closure devices, these
inventive devices provide excellent protection of tissue from damage by the
frame, which is protected under the expanded PTFE cover. Further, these
inventive devices also are capable of being relatively easily withdrawn
remotely
from the defect site in the case they need to be retrieved.
Despite the excellent properties of the defect closure devices disclosed
in the parent applications, ongoing development work has revealed that further
improvements may be possible on these devices. First, it has been discovered
that with devices with helical frames the device must be carefully deployed to
assure that the helical frame initially starts to expand with the correct
spiral. If
the helix starts unwrapping in the wrong direction, the operator must withdraw
the device back into the delivery tool and attempt deployment a second time to
assure that the device expands con-ectly. Accordingly, a frame which
consistently deploys with the correct bias in the helical frame is believed
desirable.
Second, the use of the parent devices within tortuous anatomy
generally requires the use of separate guidewire catheters or similar devices
to
3
CA 02499304 1999-06-10
WO 00/12012 PC'T/US99/13249
fielp negotiate the device delivery apparatus to the deployment site. This
requires both the use of additional equipment and often taxes space
limitations
at a surgical site. Thus, it is further believed desirable to incorporate a
simpler
and more compact system that can assist in negotiating the deployment _ ,
apparatus to the defect site.
SUMMARY OF THE INVENTION
The present invention provides significant improvements to deployment
and use of the self-expanding defect closure device described in the co
pending parent applications. The defect closure device of the present
invention
comprises a helical shape periphery supporting a membrane. The helical
shape periphery is formed from an elastic wire. Specific improvements have
been made to the elastic wire frame and the delivery apparatus to assure
consistent correct delivery of the device at the operative site. Additionally,
the
deployment apparatus has been modified to aid in the negotiation of the device
to the site of deployment.
Specifically, the wire frame now includes at least one eyelet having a
non-circular or °asymmetric" shape. A guiding mandrel is similarly
shaped'to
have a complementary non-circular or asymmetric shape which allows the
eyelet to slide linearly along the mandrel. The asymmetric shapes of the wire
. eyelet and the guiding mandrel prevent rotation of the eyelet relative to
the
mandrel. This anti-rotation feature of the present invention provides a bias
or
constraint to the formed elastic support, particularly for a helix shaped
support.
Without this applied bias, a helical wire might assume various deployed
shapes. The addition of the bias means of the present invention insures a
consistent deployed state or configuration.
Additionally, to deliver and place a septa! defect closure device
intravenously, the delivery catheter often must be guided through a tortuous
path. It is thus another aspect of the present invention to provide a self
articulating catheter tip which facilitates and enhances the ease of delivery.
To
this end, the catheter is provided with a hooked end adapted to assist in
4
CA 02499304 1999-06-10
WO 00/12012 PCT/US99/13249
guiding the catheter tube through passageways in a body, much in the same
way that a guidewire catheter can be "snaked" through tortuous anatomy using
its hooked end to "steer" the guidewire into place.
In the present invention, the hooked tip is integrally incorporated into the
deployment apparatus to assist in steering the deployment apparatus to the
deployment site. Once in place, however, the deployment apparatus is
adapted to eliminate the hooked tip to provide accurate defect closure device
placement. This is accomplished by providing a self articulating catheter tip
that can be bent to a variety of angles by advancing or retracting the closure
device from the proximal end of the delivery catheter. In other words, as the
defect closure device is advanced through the tip of the catheter, the tip
will
straighten out to provide linear deployment of the defect closure device.
Thus,
the catheter tip is adapted to assume at least two different positions, an
angular
or bent guiding position and a straightened device deployment position. The
catheter tip actuates from the guiding position to the device deployment
position when the defect closure device is moved through the distal tip of
.the
catheter. To accomplish this, the catheter tube comprises a flexible material
adapted both to maintain a bent orientation while being negotiated into
position
and to flex so as to straighten the hooked end when a device is deployed
through the tube.
These and other aspects and advantages will become more apparent
when considered with the following detailed description, drawings and
appended claims.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 (A) shows the helical shaped elastic wire of the present
invention.
Figure 1 (B) shows a cross section of the helical shaped wire with the
~ 30 bonding adhesive on the outer diameter of the wire.
Figure 1 (C) shows a cross section of the helical shaped wire with the
bonding adhesive on the perimeter of a non-circular wire or ribbon.
5
CA 02499304 1999-06-10
WO 00/12012 PCTNS99/13249
Figures 2 {A) through (C) show a mufti-ply laminate being folded over a
heat resistant tube during the helical device fabrication process.
Figures 2 (Q) and (E) shows the final laminate sheet, cutting pattern and
guiding mandrel hole pattern for the helical closure device.
Figures 3 (A) through (C) show an alternate wrapped film tube process
for fabricating the mufti-ply film laminate. .,
Figures 4 (A) through (C) show the process for threading the guiding
mandrel through the eyelets and pre-cut mandrel holes.
Figures 5 (A) through (C) show a helical defect closure device according
to the present invention being positioned and deployed in a heart defect.
Figures 5 (17) and (E) show a helical defect closure device according to
the present invention with and integral latch and sealing membrane to sealing
membrane securing means.
Figures 6 (A) and (B) show detailed shapes of the helical formed wire in
a linearly constrained and in a unconstrained state.
Figures 7 (A) and (B) show details and alternate configurations of the
integral latching and securing means.
Figures 8 (A) and {B) show details of a biasing or anti-rotation feature of
the wire eyelets and guiding mandrel.
Figures 9 (A) and (B) show the helical shaped wire, the integral biasing
anti-rotation feature, with the wire in relaxed and linearly constrained
states.
Figure 10 shows a low friction coating on the guiding mandrel.
Figures 11 (A) through (G) show the delivery and deployment sequence
of a helical closure device of the present invention along with an integral
latch
and securing means for sealing member securing and sealing member to
sealing member securing. Also shown is a guiding mandrel sealing member
central edge converging means.
Figures 12 (A) through (C) show a suture retrieval means.
Figure 13 shows the proximal end of the delivery catheter with the
coaxial arrangement of the guiding mandrel, the push tube and the delivery
catheter. Also shown is the proximal end of the retrieval suture.
Figures 14 (A) through (C) show the articulating tip of the distal end of
the delivery catheter.
6
CA 02499304 1999-06-10
WO 00/12012 PCT/US99/13249
Figure 15 (A) shows a side view of a closure device of the present
invention in a deployed or large diameter state.
Figure 15 {B) shows a side view of the closure device, in a fully
. collapsed or small diameter state.
DETAILED DESCRIPTION OF THE INVENTION
The defect closure devices of the present invention are composite
assemblies of support structures and membranes. For biological applications,
the membranes may be made from biocompatible materials such as expanded
polytetrafluoroethylene (PTFE). Such membranes block the defect, for
example a septal defect, in an animal and occlude the blood flow. This device
can also be used to repair a variety of wall defects, either by remote or
direct
deployment.
A wall defect can be remotely repaired in a fluid containing vessel
without draining the fluid. Other wall defects in contact with hazardous
materials or environments can be remotely repaired. In addition, those defects
where access is limited due to confined spaces or submersion, can also be
remotely repaired. Direct deployment can be used to repair wall defects in
those cases where access is non-restricted or limited by the immediate
environment.
The supporting wire structures that are used in the devices according to
the present invention have elastic properties that allow for them to be
collapsed
for catheter based delivery or thoracoscopic delivery, and self-expand to a
"memory" induced configuration once positioned in a wall defect. The elastic
wire may be a spring wire, or a shape memory NiTi alloy wire or a super-
elastic
NiTi alloy wire (generally referred to herein as "nitinol"). Upon deployment,
the
. wire structure resumes its deployed shape without permanent deformation.
The supporting structures of the present invention are formed from
elastic wire materials that have diameters between about 0.12 and 0.4 mm. In
a preferred embodiment of the present invention, the wire is about 0.3 mm in
diameter and formed from nitinol metal.
7
CA 02499304 1999-06-10
WO 00/12012 PCT/US99/13249
The membrane that is used in the defect closure devices to occlude the
flow of blood can be manufactured from a variety of materials, such as
DACRON~ polyester, polyethylene, polypropylene; fluoropolymers,
polyurethane foamed films, silicone, nylon, silk, thin sheets of super-elastic
materials, woven materials, polyethylene terephthalate (PET), collagen,
pericardium tissue or any other biocompatible material. In one embodiment of
the present invention, the membrane material is a fluoropolymer, in
particular,
expanded polytetrafluoroethylene (PTFE) having a node-fibril structure, such
as
that described in United States Patents 3,953,566, 4,962,153, 4,096,227,
4,187,390 and 4,902,423. The membrane used
in the present invention is manufactured from thin films of expanded PTFE that
are each approximately 0.0025 to 0.025 mm thick. Thus, the films could be
about 0.0025, 0.005, 0.0075, 0.01, 0.0125, 0.015, 0.175, 0.02, 0.0225 and
0.025 mm or more thick. -
From 1 to about 200 plys (layers) of expanded PTFE film are stacked
up and laminated to one another to obtain a membrane with the desired
mechanical and structural properties. An even number of layers are preferably
stacked together (e.g., 2, 4, 6, 8, 10, etc.), with approximately 2 to 20
layers
being desirable. Crass-lamination occurs by placing superimposed sheets on
one another such that the film drawing direction, or stretching direction, of
each
sheet is angularly offset by angles between 0 and 180 degrees from adjacent
layers or plies. Because the base expanded PTFE is thin, as thin as about
0.0025 mm or less in thickness, superimposed films can be rotated relative to
one another to improve the mechanical properties of the membrane. In one
embodiment of the present invention the membrane is manufactured by
laminating together 8 plies of expanded PTFE film, each film ply being about
0.0125 mm thick. In another embodiment of the present invention the
membrane is manufactured by laminating together 4 plies of expanded PTFE
film; each film ply being about 0.0125 mm thick. The laminated expanded
PTFE sheets are then sintered together at temperatures of about
370°C, for
about 15 minutes under vacuum to adhere the film layers to one another. The
resultant 8 ply laminate structure is typically about 0.04 mm thick.
8
CA 02499304 1999-06-10
WO 00/12012 PCT/US99/13249
The invention will now be described by reference to the figures and non-
limiting embodiments. One embodiment for closing an aperture or defect
according to the present invention is a helical design. As shown in Figure 1
(A), a helical shaped wire frame 2 for a defect closure device is prepared
from
a super-elastic wire material 4. A wire 4 of nitinol is fixtured in a jig (not
shown)
into a shape of a helix 2, eyelets 44, 46, 48, and latching and securing
portions
50. As is explained in greater detail below, one or more of the eyelets 44,
46,
and 48 are preferably formed in a non-circular shape.
The helix 2 shape can include any shape that forms at least a partial
outer periphery and has a longitudinal length. For example, a helical shape
can include a coil with varying or consistent diameters and angles. The outer
periphery of the helical shape can include straight as well as arced segments.
Each helix shape 2 is preferably formed from a single wire that is configured
to
be helical in shape, although multiple wires may be used.
The helical shaped wire 2 is constrained in a jig (not shown) and the
combination is placed into an oven, heated for at least two minutes, up to
about
one-half hour, at about 400° to 600°C, e.g., about 500°C.
The helical shaped
wire 2 is cooled by immersing in approximately 25°C water, and removed
from
the restraining jig. As the result of the 500°C, 30 minute heat
treatment, the
nitinol wire 4 obtains a memory induced configuration, which in this case is
the
shape of a helix. The helical shaped wire 2 exhibits super-elastic properties,
which act to return the wire to the helical shape even after extreme
deformation, such as the straightened orientation shown in Figures 4 (A)
through 4 (C).
As shown in cross section Figure 1 (B), the helical shaped wire 2 is
coated with a bonding agent 6, for example fluorinated ethylene propylene
(FEP) or other suitable adhesive. The adhesive may be applied through
contact coating, powder coating, dip coating, spray coating, or any other
appropriate means. As shown in Figure 1 (C), the cross section of the wire 2
is not limited to circular forms and may include rectangular shaped ribbons,
square forms, other polygones, or other shapes.
' In a preferred embodiment, the FEP adhesive is applied by electrostatic
powder coating per the following process. The formed helical shaped wire is
9
CA 02499304 1999-06-10
WO 00/12012 PCT/US99/13249
first pre-cleaned with isopropyl alcohol and de-ionized water. The formed wire
is then placed onto a spreading and holding fixture to avoid wire to wire
contact
in the formed periphery area. The fixtured wire is then grounded and placed,
for approximately 10 seconds, into the electrostatically charged FEP cloud. .
,
The FEP powder can be procured from Daikin Industries, Ltd., Osaka, Japan,
as part number NCX-1. The FEP powder is then removed from the eyelets 44,
46, 48 and the latching and securing portions 50, by brushing or by the use of
vacuum. The FEP coated wire frame and support fixture is then placed into a
convection oven and heated to approximately 330°C for approximately 1
to 2
minutes, in order to melt and adhere the FEP powder to the formed wire. The
FEP coating can alsa be applied by dipping, spraying, laminating between
sheets, wrapping f=EP film, fitting FEP tubes over the formed wires, or any
other means. If more than one wire is used to form the helical shape the two
ends of the formed wire are attached together at a termination point, by
welding, by crimping a sleeve onto the wire ends, or any other means.
Figure 2 (A) shows a mufti-ply laminate 8 prepared from, for example,
four film layers (plies) of expanded PTFE. The film layers are placed onto a
porous vacuum chuck (not shown) with each film layer being rotated about 90
degrees relative to one another. The four ply laminate 8 can be disk shaped or
any other shape. A high temperature tube 10 is placed on the center line of
the
four ply laminate 8.
Figure 2 (B) shows the multi-ply laminate 8 being folded over the high
temperature tube 10, forming a folded laminate which surrounds the tube.
Figure 2 (C) shows the folded laminate. Since the four ply laminate has
been folded once, the membrane 12 now has formed an eight ply laminate.
This laminate assembly, with the embedded tube, is capped with a KAPTON~
sheet and placed into a sintering press. The edges of the laminate are
constrained, vacuum is applied to the assembly through a porous chuck, and .
the assembly heated to sintering temperatures. The times and temperatures
for this sintering process are as previously described. The sintered assembly
is
cooled and the KAPTONC~? sheet is removed and discarded.
CA 02499304 1999-06-10
WO 00/12012 PCTNS99/13249 -
Figure 2 (D) shows the laminate assembly, or membrane 12, high
temperature tube 10, outline cutting 14 and mandrel hole patterns 16. The -
outline and mandrel hates are cut by laser, steel rule die, or any other
means.
As shown in Figure 2 (E), after the cutting operation, a laminated
assembly 18 is formed having membranes 12, mandrel holes 16 and a heat
resistant tube 10.
In a preferred or alternate method, the cross laminated membranes
may be manufactured by cross wrapping film around a mandrel, thereby
creating a cross ply laminated film tube. As shown in Figure 3 (A), the film
20,
as previously described, is wrapped around a mandrel 22. The film 20 is
wrapped at an angle 26, referenced from a vertical or shortest circumference
line 24 about the mandrel. A clockwise angle from the vertical reference is
described as positive angle while a counterclockwise angle is described as a
negative angle. Shown is a preferred angle 26 of positive 47 degrees from
vertical. The film 20 is preferably wrapped with a pitch 28 of approximately
1.75" (44.45 mm), the film width 30 is preferably about 1.0" (25.4 mm) and the
mandrel diameter 34 is preferably about 1.0" (25.4 mm). The film is wrapped
accordingly to an approximate 24" (610.0 mm) length, traversing from left to
right, completing one pass. The second pass, traversing from right to left
maintains the same pitch 28 and film width 30, but the wrap angle 26 is
changed to negative 47 degrees from vertical. As shown in Figure 3 (B), a
total
of eight passes are used to produce a cross ply laminated film tube 32. While
still on the wrapping mandrel, the film laminate is sintered at approximately
370°C for approximately 45 minutes. The sintered film tube is then
removed
from the wrapping mandrel. The heat resistant tube 10 can then be inserted
into the tube lumen as shown. This assembly, with the embedded tube, is then
capped with a KAPTON~ sheet and placed into a sintering press. The edges
of the laminate are constrained, vacuum is applied to the assembly through a
porous chuck, and the assembly heated to sintering temperatures. The times
and temperatures for this sintering process are as previously described. The
sintered assembly is cooled and the KAPTON~ sheet is removed and
discarded. Shown in Figure 3 (C) is an end view of the laminated assembly,
showing the heat resistant tube 10 and the folded and laminated film tube 32.
11
CA 02499304 1999-06-10
WO 00/12012 PCT/US99/13249
This assembly can then be fixtured onto a laser chuck to allow the cutting of
the
mandrel holes 16 (Figure 2 (D)) and device outline 14 (Figure 2(D)). As
previously shown in Figure 2 (E), after the cutting operation, a laminated
assembly 18 is formed having membranes 12, mandrel holes 16 and a heat
resistant tube 10.
The helical wire 2 (Fig. 1 (B)), with the FEP coating 6 (Fig. 1 (B)), is
then tensioned into an approximate linear shape and inserted into the high
temperature tube 10, such as one constructed from stainless steel. The high
temperature tube 10 is removed from the laminated assembly 18, leaving the
FEP coated wire captured within the laminated assembly. The latch and
securing portion of the helical formed wire is threaded through the pre-cut
mandrel holes to temporarily hold the device in the approximate deployed or
expanded state. By threading the latch and securing portion of the helical
formed wire through the pre-cut guiding mandrel holes, the central edges of
the
seal members or membranes are forced to radialiy converge in upon
themselves, thus assuming the final deployed configuration. The device is then
air heated at 330°C for about 15 minutes causing the FEP coating on the
wire
to bond to the expanded PTFE laminate. The device is then allowed to cool.
The resulting device, when linearly tensioned, is depicted in Figure 4 (A).
As shown in Figure 4 (A), the device is linearly tensioned by applying
loads 40. The helical shaped wire 2 has been formed as previously described.
Formed into the wire 2 is a proximal eyelet 44, an intermediate eyelet 46, a
distal eyelet 48 and a latching and securing means 50. The sealing
membranes 12 have pre-cut mandrel holes 16. As shown in Figure 4 (B) a
guiding mandrel 42 is threaded through the proximal eyelet 44 and sequentially
through the pre-cut mandrel holes 16. The guiding or gathering mandrel forces
the pre-cut holes to be approximately aligned to a common axis thus gathering
the inner edges of the sealing membranes. The guiding mandrel 42 is
essentially straight ar nearly linear, thus the sealing membranes 12 should be
folded or compressed to feed the guiding mandrel sequentially through the pre-
cut holes 16.
The guiding mandrel is essentially tubular and can have inner diameters
ranging from about 0.005" to 0.300" (0.13 mm to 7.62 mm), with a preferred
12
CA 02499304 1999-06-10
WO 00/12012 PCT/U599113249
range of about 0.031'" to 0.033" (0.79 mm to 0.83 mm). The guiding mandrel
can have an outer diameter ranging from about 0.007" to 0.500" (0.18 mm-to
12.7 mm), with a preferred range of about 0.039" to 0.041" (0.99 mm to 1.04
mm).
The guiding mandrel can be fabricated from any suitable bio-compatible
material including polymers or metals. A preferred guiding mandrel material is
nitinol, procured from Memry Corp., Melno Park, CA. The guiding mandrel can
be surface treated or coated to enhance the material's bio-compatibility or
alter
or enhance the surface friction.
As shown in Figure 4 (C), the guiding mandrel 42 is further threaded
through the intermediate eyelet 46, through the remaining pre-cut holes 16 and
through the distal eyelet 48. The sealing membranes 12 are typically folded or
wrinkled as depicted by wrinkles 56. The latching and securing means
50°-
which was pre-formed into the helical shaped wire 2, is resiliently deformed
and
positioned into the open distal end of the guiding mandrel 42. The helical
wire
is then tensioned into a linear shape by sliding the proximal eyelet along the
guiding mandrel away from the distal end of the guiding mandrel. The device is
then loaded into a delivery catheter.
The general deployment of the completed helical closure device is
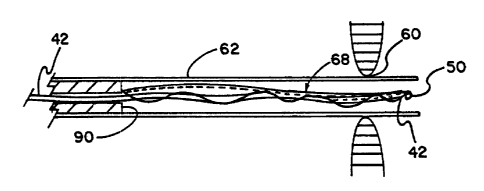
shown in Figures 5 (A) - (C). The delivery,catheter 62 is initially positioned
through a wall defect 60 in a heart. As shown in Figure 5 (A) the distal
sealing
membrane 64 of the helical closure device 68 is forced out of the delivery
catheter 62, allowing the distal side 64 to expand to the deployed shape. As
shown in Figure 5 (B), the catheter 62 is withdrawn out of the wall defect,
the
closure device is forced further out of the catheter, allowing the proximal
sealing membrane 70 to expand to the deployed shape. As shown in Figure 5
(C), when the guiding mandrel and catheter 62 are withdrawn, the latch and
securing portion of the formed wire captures and secures the distal eyelet to
the proximal eyelet, thereby securing the distal sealing membrane 64 to the
proximal sealing membrane 70. The latching and securing means is further
clarified in Figures 5 (D) and (E). As shown in Figure 5D, the latching and
securing means 50 is constrained within the inner lumen of the guiding mandrel
42. The guiding mandrel is also threaded through the three eyelets 44, 46 and
13
CA 02499304 1999-06-10
WO 00/12012 PCT/US99/13249
48 and through the pre-cut mandrel holes. When the proximal eyelet 44 is
fiorced towards or forced to be in close proximity to the distal eyelet 48,
the
latching means will protrude through the proximal eyelet 44, the medial eyelet
46, and the distal eyelet 48. As shown in Figure 5 (E), when the guiding
mandrel 42 is withdrawn, the latching and securing means 50 will be positioned
within the three eyelets and will return to the deployed state on the proximal
side of the closure device 68. Thus by securing the eyelets together, the
proximal sealing membrane 70 is secured to the distal sealing membrane 64,
completing the closure of the wall defect.
As shown in Figure 6 (A), the helical shaped wire can have more than
one loop forming any eyelet. For example, the proximal eyelet 44 can have two
loops as shown. The intermediate eyelets 46 and the proximal eyelets 48 can
similarly have more than one loop, forming an eyelet. The number of loops
forming any eyelet can include approximately 2, 3, 4, 5, 6 or more loops or
approximate partial fractions thereof, for example 1.5, 2.5, 3.5, 4.5, 5.5. A
preferred method uses approximately two loops to form the eyelets 44, 46, and
48. Figure 6 (A) depicts the approximate configuration of the helical wire 2
while under light tension 72, applied along the longitudinal axis of the
helical
wire 2. The latch portion 50 of the helical wire 2 can also include multiple
loops
or any other suitable configuration. Shown is a preferred latch portion 50
configuration having approximately one loop.
As shown in Figure 6 (B), the helical wire 2 expands into the deployed
shape when the longitudinal or linear tension (72, Figure 6 (A)) is removed.
The approximate dimensions of the eyelets 44, 46 and 48 are maintained after
removal of the longitudinal or linear tension. The latch portion 50 of the
helical
wire 2 is also approximately maintained after the longitudinal or linear
tension is
removed.
As shown in Figure 7 (A), a preferred embodiment or configuration of .
the latch portion 50 of the helical shaped wire 2, has at least one loop. The
latch portion 50 may also be formed to deploy inward relative to the deployed
device or towards the distal eyelet 48 to minimize the protrusion of the latch
portion 50 after full deployment. Figure 7 (B) shows an alternate
configuration
of the latch portion 50 of the helical shaped wire 2, wherein the latch
portion
14
CA 02499304 1999-06-10
WO 00/12012 PCT/US99/13249
has approximately two full rotational loops. in addition the terminal end of
the
latch portion 50 can be formed into a small loop.
A bias may be applied to the wire to encourage the deployment
rotational direction and the longitudinal deployment direction in order to
assure
a specific deployed configuration. A preferred method of applying this bias is
shown in Figure 8 (A) and (B): The helical shaped wire 2, is formed to create
at
least one eyelet, for example the proximal eyelet 44. This eyelet is formed
into
a non-circular or "asymmetric" shape. The guiding mandrel 42 is similarly
formed into a corresponding non-circular or "asymmetric" shape. Thus the
eyelet engages the mandrel. As the teens "asymmetric and "non-circular" are
used herein, each is intended to encompass any non-circular shape that will
prevent the eyelet from rotating on a con-espondingly shaped guiding mandrel.
Such shapes may include without limitation: ovals, squareslrectangles,
triangles, and various random shapes, and other shapes that allow linear
progression of the eyelet along the mandrel while preventing uncontrolled
twisting of the eyelet on the mandrel.
The non-circular or asymmetric form of the eyelet 44, along with the
non-circular or asymmetric form of the guiding mandrel 42, prevents rotation
or
twisting of the mandrel 42 relative to the eyelet 44. The bias applied to the
wire
is thus an anti-rotation constraint which can assure a specific shape or
deployment configuration. Figure 8 (B) shows a side and end view of a
preferred guiding mandrel 42 configuration, where the distal end 80 of the
guiding mandrel has been formed into ~ non-circular or asymmetric shape.
The asymmetric shape has a width 76 which is substantially greater {hereby
defined as more than 10%) than the height 78. In a preferred embodiment all
three eyelets (44, 46, 48 Figure 6 (B)) have non-circular or asymmetric forms,
such as an oblate rectangle, and a similarly shaped mandrel which allows the
eyelets to slide over the mandrel while also preventing relative rotation of
the
mandrel to the eyelets. With the incorporation of this anti-rotation feature,
the
eyelets should be, in a preferred embodiment, aligned and not rotated relative
to each other after the guiding mandrel has been threaded through the eyelets
and the pre-cut mandrel holes, as described in Figures 4 (A) through (C).
CA 02499304 1999-06-10
CVO OO/I2012 PGT/US99/13249
As shown in Figure 9 (A) the eyelets are not rotated and are in a
significantly relaxed and unconstrained state while in the deployed
configuration. As shown in Figure 9 (B), the helical formed wire 2 is wrapped
around the guiding mandrel 42, when the eyelets for example 48 and 46, ate
forced apart, as during the mandrel threading operation described in Figures 4
(A) through (C). Thus during the mandrel threading operation, the eyeiets
should be properly aligned so they are configured as shown in Figure 9 (A)
when in the expanded or deployed state. A preferred non-circular or
asymmetric tube has a width between about 0.048" to 0.052" (1.22 mm to 1.32
mm), and a height of about 0.023° to 0.027" (0.58 mm to 0.69 mm).
Preferred
eyelet configurations are sized to allow the eyelets to slide along the
asymmetric mandrel yet prevent rotation of the eyelets relative to the
mandrel.
As shown in Figure 10, a tow friction coating 82 may, in a preferred
embodiment, be applied to the anti-rotation segment of the distal end 80 of
the
guiding mandrel 42. This coating may be sprayed, vapor coated, or thermally
applied. In a preferred embodiment this coating is Paralyne, applied by
Specialty Coating Systems, Indianapolis, IN.
Deployment of the helical closure device with the latch securing means
and the guiding mandrel means is shown in figures 11 (A) through (G). As
shown in Figure 11 (A), the catheter 62 is aligned to and pushed through the
defect 60. The catheter 62 contains the helical closure device 68, a device
push tube 90, the guiding mandrel 42 and the latch and securing means 50.
The guiding mandrel and the latch and securing portion 50 of the helical
shaped wire are then advanced out of the catheter as shown in Figure 11 (A).
As shown in Figure 11 (B), the pusher tube 90 is then advanced, driving
the helical closure device 68 out of the catheter 62. The distal side 64 of
the
helical closure device 68 then assumes the memory induced shape.
As shown in Figure 11 (C), the catheter 62 is withdrawn away from the
defect, forcing the distal side 64 of the closure device 68 against the defect
60.
The pusher tube 90 is then advanced toward the defect, driving the helical
closure device 68 further out of the catheter 62. The proximal side 70 of the
helical closure device 68 then assumes the memory induced shape. The
guiding mandrel 42 forces the pre-cut holes 16 (Figure 2 (E)) to become
16
CA 02499304 1999-06-10
WO 00/12012 PCTNS99/13249
aligned to a common axis and thus provides a sealing member central edge
convergence means. ,
The release of the latch and securing portion 50 is accomplished by
advancing the catheter 62 and the pusher tube 90 toward the defect, as shown
in Figure 11 (D). As shown in Figure 11 (E), the guiding mandrel 42 can then
be drawn away from the helical closure device 68. The pre-cut holes 16
(Figure 2 (E)) are pre-threaded over the guiding mandrel 42 and are thus
aligned to a common axis by the guiding mandrel 42. In this configuration the
latch and securing portion 50 will spring open towards its memory induced
shape, and conform the inner diameter 92 of the pusher tube 90.
As shown in Figure 11 (F), the pusher tube 90 may then be withdrawn
away ftom the helical closure device 68, allowing the latch and securing
portion
50 to further spring open towards the unconstrained memory induced shape
and conform to the inner diameter 94 of the catheter 62.
~ As shown in Figure 11 (G), the catheter 62 can then be withdrawn from
the helical closure device, fully releasing the latch and securing portion 50,
which will then spring open to its memory induced shape. The latch and
securing portion 50 now provides a means to secure the distal side membrane
64 to the proximal side membrane 70 of the helical closure device 68 and also
provide a sealing member securing means. Attemate methods for deploying
the latch include withdrawing the guiding mandrel 42 and the catheter 62
simultaneously away from the helical closure device 68, or withdrawing the
guiding mandrel 42, the pusher tube 90 and the catheter 62 simultaneously
away from the helical closure device 68.
A means for allowing device retrieval during the deployment is
desirable. A device retrieval means is defined as a means to allow the defect
closure device to be reinserted into the delivery catheter after partial
deployment of the defect closure device. If the closure device is
inadvertently
mispositioned during deployment, a suture with one end embedded or attached
to the pusher tube, allows retrieval of the partially deployed device. Thus
the
device can be withdrawn back into the delivery catheter and redeployed to
correct the positioning error
17
CA 02499304 1999-06-10
WO 00/12012 PCTIUS99/13249
Figures 12 (A) through (C) show a preferred embodiment of a suture
retrieval means. As shown in Figure 12 (A), a suture 96 is pre-threaded
through the proximal eyelet 44. One end of the suture 96 is embedded or
attached to the push tube 90 and the other end of the suture 96 is pre
threaded
through the proximal eyelet 44 and pre-threaded through the inside lumen of
the push tube 90. The suture 96 is pre-threaded or routed between the outside
of the guiding mandrel 42 and the inside lumen of the push tube 90 and
extends out of the proximal end of the delivery catheter 62. By tensioning the
exposed end of the suture at the proximal end of the delivery catheter, the
closure device can be drawn hack into the deiivery catheter 62 if re-
deployment
is required: As shown in Figure 12 (B), the push tube 90 has the suture 96
attached of embedded into the push tube 90. As shown in Figure 12 (C),
deployment of the closure device 68 can be completed by releasing the suture
from the proximal end of the delivery catheter 62 and as the catheter 62 is
withdrawn, the suture 96 is allowed to slip through the proximal eyelet 44,
thereby releasing the closure device 68.
Shown in Figure 13 is the proximal end 100 of the delivery catheter 62.
The guiding mandrel 42 and the push tube 90 are configured coaxially within
the delivery catheter 62. The proximal end of the retrieval suture 96 is
routed
between the outside of the guiding mandrel 42 and the inside lumen of the
push tube 90 and is removably secured to the push tube 90. in a preferred
embodiment, the suture 96 can be removably secured to the push tube 90 by a
pressure fit cap that pinches and holds the suture to the outside diameter of
the
push tube. To complete the device delivery, the pressure fit cap is removed,
thereby freeing the suture 96 and allowing the suture to slip through the push
tube lumen and through the proximal eyelet of the helical formed wire, which
fully releases the device.
To allow the guiding of the delivery catheter through a defect without the
need of a guide wire, the distal end of the delivery catheter has an
articulated
tip.
This hooked tip facilitates "steering" of the delivery system through
passageways or "hooked" in a . much tike a bent tip on a guidewire
catheter. As shown in Figures 14 (A) through (C), the distal end of the
catheter
18
CA 02499304 1999-06-10
WO 00/12012 PCT/US99/13249
62 has an articulated tip 102. As shown in Figure 14 (A), the distal end of
the
catheter assumes a bend or angle 112 of approximately 60 to 90 degrees When
in the relaxed or unconstrained state. In this state, the distal end 104 of
the
device 68 is at the proximal position 106. Referring to Figure 14 (B), as the
distal end 104 of the device 68 is advanced towards the medial position 108,
the articulated tip 102 bends, approximating less of an angle: Shown is an .,
approximate starting angle of 90 degrees (Figure 14 (A)) transitioning to an
approximate angle of 45 degrees, as shown in Figure 14 (B). As the distal end
104 of the device 68 is further advanced to the distal position 110, the
articulated tip assumes an approximate linear or straight configuration. Thus
the distal tip is adapted to assume at least two different positions, a bent
guiding position as shown in Figure 14 (A), and a straightened device
deployment position as shown in Figure 14 (C). The distal tip actuates from
the
guiding position to the device deployment position when the defect closure
device is moved through the distal tip. It should be appreciated that the tip
is
able to assume a variety of bent configurations between these two extreme
positions simply by partially advancing or withdrawing the devices within the
tip.
The catheter tube comprises a flexible material adapted to flex so as to
straighten the hooked end as shown in Figure 14 (C) when a device is
deployed through the tube. In a prefer-ed embodiment, the delivery catheter is
constructed from a wire braiding support with approximately 40 crossovers per
inch, coated with hot melt Pebax, which can be procured from Elf Atochem
North American, Inc., Philadelphia, PA. Other stiffening materials may be
substituted for the Pebax, such as FEP or other suitable materials.
Alternatively, the catheter tube itself may be bowel from a material that will
accept a heat-set or molded or other formed bend. The dimensions of the wire
braiding are selected for the specific catheter size. Other materials may be
substituted for the wire braiding, for example polymer fibers or filaments may
be used for the catheter support. The catheter support can also be in the form
of a spiral or coil. The support may be eliminated providing the catheter has
adequate mechanical and flexural properties.
The pre-bent or hooked portion of the distal tip is formed by constraining
the distal tip in the bent or hooked configuration as the hot melt Pebax is
19
CA 02499304 1999-06-10
WO OO/1Z012 PCT/US99/13?.49
heated to its approximate melting or reflow temperture. The catheter is then
allowed to cool while remaining in the constrained state. The articulated
portion is, in a preferred embodiment, approximately 2.4" (61 mm) tong and
forms an approximate 90 degree angle. Radio-opaque rings, or tips may be
incorporated into the distal catheter tip by forming a mixture of
approximately
80% tungsten fine powder and 20% Pebax. The metal loaded mixture is then
melted and molded onto the distal catheter tip. To lower the surface friction
in
the push tube inner lumen, a densified, thin wall PTFE liner may be optionally
incorporated onto the inner lumen. In a preferred embodiment, the suture may
be affixed to the push tube by melting the hot melt Pebax around thesuture,
thereby embedding the suture into the push tube wall.
Figure 15 (A) shows a side view of a closure device 68, with a
longitudinal axis 120, in the deployed or large diameter state 124 having a
diameter d,.
Figure 15 (B) shows a side view of a closure device 68, with a
longitudinal axis 120, in the fully collapsed or small diameter state 122
having a
diameter d2, where d2 is less than d,, the ratio of d,:d2 being less than
about
50:1, depending on the final deployed diameter d, of the device. The ratio of
d,:dz should be between about 5:1 and about 50:1, with a ratio of about 5:1 to
about 50:1 being preferred (such as, 5:1, 7:1, 8:1, 9:1, 10:1, 12:1, 15:1,
20:1,
25:1, 30:1, 35:1, 40:1, 45:1.) Once in the collapsed state, the device can be
inserted along the longitudinal axis 120 inta a delivery tube or catheter 62.
Thus
the device has a compressed insertion configuration and an enlarged deployed
configuration.
Although, the present invention is preferred to close body defects like
atrial septal defects and ventricular septal defects, it can be used in other
applications where the undesired communication or passage in the body exists.
One specific example is Patent Ductus Arteriosis (PDA). PDA is a vessel
which shunts aorta and pulmonary artery. This shunt is supposed to close
immediately after childbirth. However, in certain congenital disease
conditions,
this vessel stays open after childbirth and hence leads to subsequent
complications. it is desired to have a catheter based or thoroscopic device to
block PDA. The present invention can be used for the PDA closure. Similarly,
CA 02499304 1999-06-10
WO 00/12012 PCT/US99/13249
it can be used to block the flow in any tubular structure in the body such as
fallopian tubes, arteriovenous fistula, etc. .
tn this respect, it should be appreciated that the present invention can
be introduced in a wide variety of manners, including by merely using a tube
("catheter"), through thoracoscopic delivery, or other means. For small
applications, it may be desirable to use pediatric sized catheters.
It should be appreciated from the foregoing description that an
important benefit of the present invention, particularly the helically
deployed
embodiment, is that it can be restrained to a very compact insertion diameter
and yet still fully expand to assume a full barrier to cover or seal a wall
defect.
This dramatic change in size is achieved by the ability of the elastic support
of
the present invention to assume a substantially elongated configuration in its
insertion configuration and then automatically bend into another periphery of
the closure device in its deployed configuration.
As the term "substantially elongated" is used herein, it is intended to
encompass any orientation of the elastic support that stretches the support
out
longitudinally within a delivery tube. Preferably a "substantially elongated"
support assumes nearly a straight line within the delivery tube; however, the
term is intended to encompass any longitudinally disposed orientation, such as
a stretched wire having one or more loops or kinks therein or even a support
that may include two or more lengths of wire along its length.
The advantage of this construction is that the closure device can be
compressed into very small tubes for delivery into tightly confined spaces.
For
instance, the closure device of the present invention will readily compact
into a
9 French (F) catheter tube, and even much smaller tubes such as 8F, 7.5F, 7F,
fi.SF, 6F, 5.5F, 5F, 4.5F, 4F, 3.5F, 3F, 2.5F, 2F and even smaller.
A further advantage of this construction of the closure device of the
present invention is that the device remains quite fiexibie in its compacted,
insertion configuration. This is particularly true where the elastic support
comprises only a single length of wire in its compacted state. This high
degree
of flexibility contributes to ease of manipulation of the device of the
present
invention, again assisting in deployment in tight confines.
21
CA 02499304 1999-06-10
WO 00/12012 PGT/US99/13249
Another way to express the advantages of the present invention is in
the length of the insertion configuration of the present invention relative to
the
total length of the periphery of the device in its deployed configuration.
This ratio is generally about 0.7 or more, and may include ratios of 0.8,
0.9, 1.0, 1.1, 1.2, 1.3, 1.4, 1.5 or more. Preferably the ratio is 0.7 or
more.
Although the invention has been described in conjunction with specific
embodiments; it is evident that many alternatives and variations will be
apparent to those skilled in the art in fight of the foregoing description and
annexed drawings. Accordingly, the invention is intended to embrace all of the
alternatives and variations that fait within the spirit and scope of the
appended
claims.
22