Note: Descriptions are shown in the official language in which they were submitted.
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
PEPTIDE MEDIATED SYNTHESIS OF METALLIC AND MAGNETIC MATERIALS
RELATED APPLICATIONS
This application claims benefit of provisional patent
application serial no. 60/411,804 filed September 18, 2002 to
Belcher et al., which is hereby incorporated by reference in
its entirety.
STATEMENT OF GOVERNMENT SUPPORT
The research carried out in the subject application was
supported in part by grants from the Army Research Office,
Grant No. DADD19-99-0155, the government may own certain
rights.
In addition, a nucleotide and/or amino acid sequence
listing is incorporated by reference of the material on
computer readable form.
TECHNICAL FIELD OF THE INVENTION
The present invention is directed to organic materials
capable of binding to inorganic materials, and specifically,
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
toward specific peptide sequences that tightly and directly
bind to metal materials including magnetic materials.
BACKGROUND OF THE INVENTION
In biological systems, organic molecules exert a
remarkable level of control over the nucleation and mineral
phase of inorganic materials such as calcium carbonate and
silica, and over the assembly of building blocks into complex
structures required for biological function.
Materials produced by biological processes are typically
soft, and consist of a surprisingly simple collection of
molecular building blocks (i.e., lipids, peptides, and nucleic
acids) arranged in astoundingly complex architectures. Unlike
the semiconductor industry, which relies on a serial
lithographic processing approach for constructing the smallest
features on an integrated circuit, living organisms execute
their architectural "blueprints" using mostly non-covalent
forces acting simultaneously upon many molecular components.
Furthermore, these structures can often elegantly rearrange
between two or more usable forms without changing any of the
molecular constituents.
The use of "biological" materials to process the next
generation of microelectronic devices provides a possible
solution to resolving the limitations of traditional
processing methods. The critical factors in this approach are
identifying the appropriate compatibilities and combinations
2
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
of biological-inorganic materials, and the synthesis of the
appropriate building blocks.
SUMMARY OF THE INVENTION
The present inventors have designed constructs and
produced biological materials that direct and control the
assembly of inorganic materials, including metallic and
magnetic materials, into controlled and sophisticated
structures. Of particular interest are ferromagnetic
materials, and particulate materials including nanoparticulate
materials. The use of biological materials to create and
design materials that have interesting electrical, magnetic or
optical properties may be used to decrease the size of
features and improve the control of, e.g., the opto-electical
properties of the material, as well as control of material
fabrication. For example, room temperature methods have been
developed in the present invention for preparing materials
which formerly involved high temperature preparation methods.
A combinatorial peptide phage display library expressing
a large collection of bacterial phage that expresses millions
of different peptide sequences on their surfaces was combined
with biopanning techniques to select specific peptide
sequences that tightly and directly bind to metal materials
including magnetic materials (e. g., Co, Copt SmCo5, or FePt).
The present inventors have found that these metal and magnetic
material binding molecules, including peptides, can be used to
control the nucleation of inorganic materials, as has been
3
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
demonstrated in nature and with II-VI semiconductors. If
proteins can be used to control the nucleation of metal,
including magnetic, materials, then magnetic nanoparticles and
their applications could be prepared much cheaper and easier
than using traditional methods. The nanomolecular metals,
including magnets and magnetic material, may be used, e.g.,
for micro or nanomachines, dynamos, generators, magnetic
storage or any other applications for materials that are
magnetic or may be magnetized. Another use for these
materials is to modify the surface of metal, including
magnetic, materials. The peptides can act as linkers for
attaching over materials to the surface of the magnetic
material, allowing the self-assembly of complex
nanostructures, which could form the basis of novel electronic
devices.
The present inventors have recognized that this approach
of selecting binding peptides (using combinatorial peptide
libraries and panning techniques) may also be used to form and
control the nucleation of metal materials, including magnetic
materials. Other techniques being researched to synthesize
metal particles, including magnetic nanoparticles, are based
on a high temperature synthesis that must be performed in an
inert atmosphere using expensive reagents and often require
further processing and purification after synthesis to
fabricate particles, including nanoparticles, with the desired
shape and crystallinity. The result is that preparing
magnetic nanoparticles in the traditional fashion is expensive
and not conducive to large scale and/or volume production.
The approach presented herein is generally performed at room
temperatures using inexpensive reagents yielding nanoparticles
with controlled crystallinity, reducing the cost for the
4
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
synthesis of metal particles, including magnetic
nanoparticles, with controlled crystal structure and
orientation.
Peptide-mediated synthesis of metal materials, including
magnetic materials, provides a much cheaper and
environmentally friendly approach to the synthesis of metal
materials, including magnetic nanoparticles. Current
protocols for preparing metal nanoparticles, including
magnetic nanoparticles, are time consuming, expensive and
yield nanoparticles coated with organic surfactants. These
surfactants are not amicable to further modification of the
nanoparticles. Advances in the field of molecular biology
enable the functionalization of peptides, therefore, particles
and nanoparticles grown from peptides will also be easily
functionalized. Peptide functionalization facilitates their
incorporation into electronic devices and integration into
magnetic memory devices.
One form of the present invention is a method for using
self-assembling biological molecules, e.g., bacteriophage,
that are genetically engineered to bind to metals,
nanoparticles-, and magnetic or other materials and to
organize well-ordered structures. These structures may be,
e.g., nanoscale arrays of particles and nanoparticles. Using
bacteriophage as an example, self-assembling biological
materials can be selected for specific binding properties to
particular surfaces (e.g., semiconductor), and thus, the
modified bacteriophage and the methods taught herein may be
used to create well-ordered structures of the materials
selected.
5
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
More particularly, the present invention includes
compositions and methods for creating metal materials,
including magnetic materials, particles, and nanoparticles.
One embodiment is a method of making a metal particle,
including magnetic particle, including the steps of; providing
a molecule comprising a portion that binds specifically to a
metal surface, including a magnetic surf ace, and contacting
one or more metal material precurosrs, including magnetic
material precursors, with the molecule under conditions that
permit formation of the metal material, including the magnetic
particle. The molecule may be, e.g., a biological molecule
such as an amino acid oligomer or peptide. The oligomer may
be, for example, between about 7 and about 100 amino acids
long, and more particularly, between about 7 and about 30
amino acids long, and more particularly about 7 and about 20
amino acids long, and may form part of a combinatorial library
and/or include a chimeric molecule.
The types of metal materials, including magnetic
particles, that are disclosed herein may be formed from, e.g.,
Co, CoPt, SmCo5, and/or Feet. Another method of the present
invention includes a method for identifying molecules that
bind through non-magnetic interactions with a magnetic
material including the steps of contacting an amino acid
oligomer library with a magnetic material to select oligomers
that bind specifically to the magnetic material and eluting
those oligomers that bind specifically to the magnetic
material. The oligomer library may be a library of self-
assembling molecules, e.g., a phage library such as an M13
phage library. The library may even be contained in a
bacterium and may be assembled externally.
6
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
A method of making a magnetic particle may also include
the step of contacting a molecule that initiates magnetic
molecule formation with magnetic material precursors and a
reducing agent. The molecule that initiates magnetic molecule
formation with magnetic material precursors may be contacted
at, e.g., room temperature or below a temperature of, e.g.,
100, 200 or even 300 degrees centigrade. The molecule may be
an amino acid oligomer of, e.g., between about 7 and 20 amino
acids long. The magnetic particle may be a Co, CoPt, SmCo5,
or Feet magnetic particle in the form of a magnetic quantum
dot or even a film. The skilled artisan will recognize that
combinations or one or more of the magnetic particles
disclosed herein may be positioned in a wide assortment of
one-, two- and three-dimensional locations, shapes, and the
like for particular uses.
The present invention also includes magnetic particles,
e.g., nanoparticles made by the methods disclosed herein.
These magnetic particles may form a portion of an integrated
circuit made by fixing a magnetic material binding peptide to
a substrate; contacting one or more magnetic material
precursors with the magnetic material binding peptide under
conditions that form a magnetic particle; and forming a
magnetic crystal on the substrate. The magnetic material
binding peptide may be linked chemically to a substrate, e.g.,
silicon or other semiconductor substrate. The magnetic
particles of the present invention may be used to make memory,
short- or long-term storage, identification systems or any use
that the skilled artisan will recognize may be made of these
particles. Examples of other used for the magnetic micro-,
nano- and femto-particles of the present invention include,
micro or nano-motors, dynamos and the like.
7
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
Another form of the present invention is a method of
creating nanoparticles that have specific alignment
properties. This is accomplished by creating, e.g., an M13
bacteriophage that has specific binding properties, amplifying
the bacteriophage to high concentrations (e.g., incubation of
phage library with bacterial host culture to allow infection,
replication, and subsequent purification of virus), and
resuspending the phage.
This same method may be used to create bacteriophage that
have three liquid crystalline phases, a directional order in
the nemetic phase, a twisted nemetic structure in the
cholesteric phase, and both directional and positional order
in smectic phase. In one aspect the present invention is a
method of making a polymer, e.g., a film, comprising the steps
of, amplifying a self-assembling biological molecule
comprising a portion that binds a specific semiconductor
surfaces to high concentrations and contacting one or more
semiconductor material precursors with the self-assembling
biological molecule to form or direct the formation of a
crystal.
Another form of the present invention is method for
creating nanoparticles that have differing cholesteric pitches
by using, e.g., an M13 bacteriophage that has been selected to
bind to semiconductor surfaces and resuspending the phage to
various concentrations. Another form of the present invention
is a method of preparing a casting film with aligned
nanoparticles by using, e.g., genetically engineered M13
bacteriophage and re suspending the bacteriophage.
Still another form of the present invention is a method
of preparing a nanoparticle film comprising the steps of
8
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
adding a solution of nanoparticles to a surface, evaporating
the solution of nanoparticles on the surface, and annealing
the nanoparticles to the surface, where the nanoparticles are
magnetic molecules. The surface may include any
microfabricated solid surface to which molecules may attach
through either covalent or non-covalent bonds, such Langmuir-
Bodgett films, glass, functionalized glass, germanium,
silicon, PTFE, polystyrene, gallium arsenide, gold, silver, or
any materials comprising amino, carboxyl, thiol or hydroxyl
functional groups incorporated onto a surface. Annealing
generally occurs by high temperatures under an inert gas
(e.g., nitrogen). Another form of the present invention is a
nanoparticle film prepared by the method just described.
BRIEF DESCRIPTION OF THE FIGURES
For a more complete understanding of the features and
advantages of the present invention, reference is now made to
the detailed description of the invention along with the
accompanying FIGURES in which corresponding numerals in the
different FIGURES refer to corresponding parts and in which:
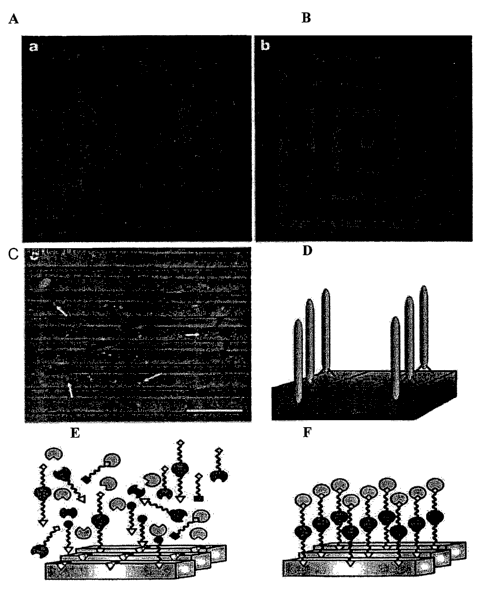
FIGURE 1 are X-ray photoelectron spectroscopy (XPS) elemental
composition determination of phage-substrate interactions
through the intensity of a gold 4f-electron signal (A-C),
model of phage discrimination for semiconductor
heterostructures (D), and examples of bivalent synthetic
peptides with two-component recognition attachments (E-F);
FIGURE 2 depicts schematic diagrams of the smectic alignment
of M13 phages in accordance with the present invention;
9
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
FIGURE 3 include images of the A7-ZnS suspensions using (A-B)
POM, (C) AFM, (D) SEM, (E) TEM, and (F) TEM image with
electron diffraction insert;
FIGURE 4 include images of the M13 bacteriophage nanoparticle
as (A) photograph of the film, (B) schematic diagram of the
film structure, (C) AFM image, (D) SEM image, (E-F) TEM images
along the x-z and z-y planes;
FIGURE 5 is (A) TEM image of annealed SmCo5 nanoparticles, (B)
TEM image with the selected area electron diffraction pattern
and (C) STEM image of annealed SmCo5 nanoparticles;
FIGURE 6 are examples of binding assays illustrating (A) the
specificity of the Co-specific phage for Co and (B) an
isotherm of the Co-specific phage on Co in accordance with the
present invention;
FIGURE 7 includes a series of high resolution TEM images of
Copt nanoparticles prepared using (A) phage that express the
7-constrained peptide that selectively binds to Copt, (B)
phage that express a random peptide, and (C) wild-type phage;
FIGURE 8 is (A) high resolution TEM image of Co nanoparticles
that have been grown using a l2mer peptide that selectively
bind to Co and (B) the corresponding electron diffraction
pattern;
FIGURE 9 are (A) high resolution TEM image of Feet
nanoparticles that have been grown using phage that express a
l2mer peptide and are selective for Feet, wherein (B) shows
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
the electron diffraction pattern both of which are compared to
(C) Feet nanoparticles grown using wild-type phage;
FIGURE 10 is (A) high resolution TEM image of SmCo5
nanoparticles grown using a l2mer that selectively binds SmCo5
as a template, (B) an electron diffraction pattern of a
selected area of (A) and (C) SmCoS nanoparticles grown using
wild-type phage as a control;
FIGURE 11 is (A) an AFM image of Co-specific phage with Co
nanoparticles bound to its P3 protein and (B) the
corresponding MFM image;
FIGURE 12 is (A) a hysteresis loop of biologically prepared
Feet nanoparticles and (B) a higher resolution scan of the
central portion of the loop to clarify the coercivity;
FIGURE 13 is (A) a hysteresis loop of biologically prepared
SmCo5 nanoparticles and (B) the central portion of the loop
plotted on a smaller axis to clarify the coercivity; and
FIGURE 14 include (A) TEM of Copt nanoparticles grown using a
phage that has been genetically engineered to express a Copt
specific l2mer sequence on their P8 proteins, (B) higher
resolution TEM image of the same Copt nanoparticles, (C) the
corresponding electron diffraction pattern, (D) STEM image of
similarly prepared particles, (E) STEM mapping for Pt, and (F)
STEM mapping for Co in accordance with the present invention.
11
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
DETAILED DESCRIPTION OF THE INVENTION
This application claims benefit of provisional patent
application serial no. 60/411,804 filed September 18, 2002 to
Belcher et al., which is hereby incorporated by reference in
its entirety including the figures, summary, detailed
description, working examples, claims, and sequence listing.
Although making and using various embodiments of the
present invention are discussed in detail below, it should be
appreciated that the present invention provides many
applicable inventive concepts that can be embodied in a wide
variety of specific contexts. The specific embodiments
discussed herein are merely illustrative of specific ways to
make and use the invention, and do not delimit the scope of
the invention.
To facilitate the understanding of this invention, a
number of terms are described further below. As used herein,
"metal material" can be, for example, a substance that
encompasses, but is not limited to, metal alloys, metal
oxides, and pure metals, that may or may not have the magnetic
and/or ferromagnetic properties, may be crystalline,
polycrystalline or amorphous. Metal materials may also exist
in several spatial forms, including particles, patterned
surfaces or layered films. The term "particle" can refer to
the size and shape of said materials, and includes but is not
limited to micron-scaled particles, nano-scaled particles
(called nanoparticles), single molecule of metal materials and
other sizes and shapes here unsaid but controlled by the
described biological methods.
12
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
The term binding molecule is hereby defined as a molecule
that binds, recognizes or directs the growth of a metal
material. Examples of binding molecules includes but are not
limited to peptides, amino acid oligomers, and nucleic acid
oligomers. These binding molecules may be selected from
combinatorial library screening, or synthesized, conjugated or
formulated independently from such libraries. These binding
molecules may be coupled to a substrate, i.e. conjugated to a
surface or to scaffolds, such as M13 viruses where the binding
molecules are displayed on viral coats or various binding
molecule-conjugated structures.
The inventors have previously shown that peptides can
bind to semiconductor materials. In the present invention,
the inventors demonstrate that binding molecules, including
peptides, can specifically bind to metal materials, including
magnetic materials. These peptides have been further
developed into a way of nucleating nanoparticles and directing
their self-assembly. The main features of the peptides are
their ability to recognize and bind technologically important
materials with face specificity, to nucleate size-constrained
crystalline semiconductor materials, and to control the
crystallographic phase of nucleated nanoparticles. The
peptides can also control the aspect ratio of the
nanoparticles and therefore, the optical properties.
Briefly, the facility with which biological systems
assemble immensely complicated structure on an exceedingly
minute scale has motivated a great deal of interest in the
desire to identify non-biological systems that can behave in a
similar fashion. Of particular value would be methods that
could be applied to materials with interesting electronic or
13
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
optical properties, but of which natural evolution has not
selected for interactions between biomolecules and such
materials.
The present invention is based on recognition that
biological systems efficiently and accurately assemble
nanoscale building blocks into complex and functionally
sophisticated structures with high perfection, controlled size
and compositional uniformity.
Peptide Sequence Selection
One method of providing a random organic polymer pool is
using a Phage-display library, based on a combinatorial
library of random peptides containing between 7 and 12 amino
acids fused to the pIII coat protein of M13 bacteriophage,
providing different peptides that were reacted with
crystalline semiconductor structures. Five copies of the pIII
coat protein are located on one end of the phage particle,
accounting for 10-16 nm of the particle. The phage-display
approach provided a physical linkage between the peptide
substrate interaction and the DNA that encodes that
interaction. The examples described here used as examples,
five different single-crystal semiconductors: GaAs (100), GaAs
(111)A, GaAs(111)B, InP(100) and Si(100) . These substrates
allowed for systematic evaluation of the peptide substrate
interactions and confirmation of the general utility of the
methodology of the present invention for different crystalline
structures.
Protein sequences that successfully bound to the specific
crystal were eluted from the surface, amplified by, e.g., a
million-fold, and reacted against the substrate under more
14
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
stringent conditions. This procedure was repeated five times
to select the phage in the library with the most specific
binding. After, e.g., the third, fourth and fifth rounds of
phage selection, crystal-specific phage were isolated and
their DNA sequenced. Peptide binding has been identified that
is selective for the crystal composition (for example, binding
to GaAs but not to Si) and crystalline face (for example,
binding to (100) GaAs, but not to (111)B GaAs).
Twenty clones selected from GaAs(100) were analyzed to
determine epitope binding domains to the GaAs surface. The
partial peptide sequences of the modified pIII or pVIII
protein are shown in TABLE 1, revealing similar amino-acid
sequences among peptides exposed to GaAs.
TABLE 1. Partial peptide sequences of modified pIII or
pVIII proteins.
With increasing number of exposures to a GaAs surface,
the number of uncharged polar and Lewis-base functional groups
increased. Phage clones from third, fourth and fifth round
sequencing contained on average 300, 40% and 44% polar
functional groups, respectively, while the fraction of Lewis-
base functional groups increased at the same time from 41% to
48% to 55°s. The observed increase in Lewis bases, which should
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
constitute only 34~ of the functional groups in random 12-mer
peptides from our library, suggests that interactions between
Lewis bases on the peptides and Lewis-acid sites on the GaAs
surface may mediate the selective binding exhibited by these
clones.
The expected structure of the modified 12-mers selected
from the library may be an extended conformation, which seems
likely for small peptides, making the peptide much longer than
the unit cell (5.65 A°) of GaAs. Therefore, only small binding
domains would be necessary for the peptide to recognize a GaAs
crystal. These short peptide domains, highlighted in TABLE l,
contain serine- and threonine-rich regions in addition to the
presence of amine Lewis bases, such as asparagine and
glutamine. To determine the exact binding sequence, the
surfaces have been screened with shorter libraries, including
7-mer and disulphide constrained 7-mer libraries. Using these
shorter libraries that reduce the size and flexibility of the
binding domain, fewer peptide-surface interactions are
allowed, yielding the expected increase in the strength of
interactions between generations of selection.
Phage, tagged with streptavidin-labeled 20-nm colloidal
gold particles bound to the phage through a biotinylated
antibody to the M13 coat protein, were used for quantitative
assessment of specific binding. X-ray photoelectron
spectroscopy (XPS) elemental composition determination was
performed, monitoring the phage substrate interaction through
the intensity of the gold 4f-electron signal (FIGURES lA-C).
Without the presence of the G1-3 phage, the antibody and the
gold streptavidin did not bind to the GaAs(100)substrate. The
gold-streptavidin binding was, therefore, specific to the
16
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
phage and an indicator of the phage binding to the substrate.
Using XPS it was also found that the G1-3 clone isolated from
GaAs(100) bound specifically to GaAs(100) but not to
Si(100)(see FIGURE 1A). In complementary fashion the S1
clone, screened against the (100) Si surface, showed poor
binding to the (100) GaAs surface.
Some GaAs clones also bound the surface of InP (100),
another zinc-blende structure. The basis of the selective
binding, whether it is chemical, structural or electronic, is
still under investigation. In addition, the presence of
native oxide on the substrate surface may alter the
selectivity of peptide binding.
The preferential specific binding of the G1-3 clone to
GaAs(100), over the (111)A (gallium terminated) or (111)B
(arsenic terminated) face of GaAs was demonstrated (FIGURE 1B,
C). The G1-3 clone surface concentration was greater on the
(100) surface, which was used for its selection, than on the
gallium-rich (111)A or arsenic-rich (111)B surfaces. These
different surfaces are known to exhibit different chemical
reactivities, and it is not surprising that there is
selectivity demonstrated in the phage binding to the various
crystal faces. Although the bulk termination of both 111
surfaces give the same geometric structure, the differences
between having Ga or As atoms outermost in the surface bilayer
become more apparent when comparing surface reconstructions.
The composition of the oxides of the various GaAs surfaces is
also expected to be different, and this in turn may affect the
nature of the peptide binding.
The intensity of Ga 2p electrons against the binding
energy from substrates that were exposed to the G1-3 phage
17
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
clone is plotted in FIGURE 1C. As expected from the results
in FIGURE 1B, the Ga 2p intensities observed on the GaAs
(100), (111)A and (111)B surfaces are inversely proportional
to the gold concentrations. The decrease in Ga 2p intensity
on surfaces with higher gold-streptavidin concentrations was
due to the increase in surface coverage by the phage. XPS is
a surface technique with a sampling depth of approximately 30
angstroms; therefore, as the thickness of the organic layer
increases, the signal from the inorganic substrate decreases.
This observation was used to confirm that the intensity of
gold-streptavidin was indeed due to the presence of phage
containing a crystal specific bonding sequence on the surface
of GaAs. Binding studies were performed that correlate with
the XPS data, where equal numbers of specific phage clones
were exposed to various semiconductor substrates with equal
surface areas. Wild-type clones (no random peptide insert)
did not bind to GaAs (no plaques were detected). For the G1-3
clone, the eluted phage population was 12 times greater from
GaAs ( 100 ) than f rom the GaAs ( 111 ) A surface .
The G1-3, G12-3 and G7-4 clones bound to GaAs(100) and
InP(100) were imaged using atomic force microscopy (AFM). The
InP crystal has a zinc-blende structure, isostructural with
GaAs, although the In-P bond has greater ionic character than
the GaAs bond. The 10-nm width and 900-nm length of the
observed phage in AFM matches the dimensions of the M13 phage
observed by transmission electron microscopy (TEM), and the
gold spheres bound to M13 antibodies were observed bound to
the phage (data not shown). The InP surface has a high
concentration of phage. These data suggest that many factors
are involved in substrate recognition, including atom size,
charge, polarity and crystal structure.
18
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
The G1-3 clone (negatively stained) is seen bound to a
GaAs crystalline wafer in the TEM image (not shown). The data
confirms that binding was directed by the modified pIII
protein of G1-3, not through non-specific interactions with
the major coat protein. Therefore, peptides of the present
invention may be used to direct specific peptide-semiconductor
interactions in assembling nanostructures and heterostructures
(FIGURE lE).
X-ray fluorescence microscopy was used to demonstrate the
preferential attachment of phage to a zinc-blende surface in
close proximity to a surface of differing chemical and
structural composition. A nested square pattern was etched
into a GaAs wafer; this pattern contained 1-~.m lines of GaAs,
and 4-~m Si02 spacing in between each line (FIGURES 1A-1B).
The G12-3 clones were interacted with the GaAs/Si02 patterned
substrate, washed to reduce non-specific binding, and tagged
with an immuno-fluorescent probe, tetramethyl rhodamine (TMR).
The tagged phage were found as the three lighter lines (red,
if in color) and the center dot, in FIGURE 1B, corresponding
to G12-3 binding only to GaAs. The Si02 regions of the
pattern remain unbound by phage and are dark in color. This
result was not observed on a control that was not exposed to
phage, but was exposed to the primary antibody and TMR (FIGURE
1A). The same result was obtained using non-phage bound G12-3
peptide.
The GaAs clone G12-3 was observed to be substrate-
specific for GaAs over AlGaAs (FIGURE 1C). AlAs and GaAs have
essentially identical lattice constraints at room temperature,
5.66 A° and 5.65 A°, respectively, and thus ternary alloys of
AlxGa1-xAs can be epitaxially grown on GaAs substrates. GaAs
19
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
and AlGaAs have zinc-blende crystal structures, but the G12-3
clone exhibited selectivity in binding only to GaAs. A
multilayer substrate was used, consisting of alternating
layers of GaAs and of A1o.98Gao.ozAs. The substrate material was
cleaved and subsequently reacted with the G12-3 clone.
The G12-3 clones were labeled with 20-nm gold-
streptavidin nanoparticles. Examination by scanning electron
microscopy (SEM) shows the alternating layers of GaAs and
A1o,98Gao.ozAs within the heterostructure (FIGURE 1C) . X-ray
elemental analysis of gallium and aluminum was used to map the
gold-streptavidin particles exclusively to the GaAs layers of
the heterostructure, demonstrating the high degree of binding
specificity for chemical composition. In FIGURE 1D, a model
is depicted for the discrimination of phage for semiconductor
heterostructures, as seen in the fluorescence and SEM images
(FIGURES lA-C).
The present invention demonstrates the powerful use of
phage-display libraries to identify, develop and amplify
binding between organic peptide sequences and inorganic
semiconductor substrates. This peptide recognition and
specificity of inorganic crystals has been extended to other
substrates, including GaN, ZnS, CdS, Fe304, Fez03, CdSe, ZnSe
and CaC03 using peptide libraries.
Bivalent synthetic peptides with two-component
recognition (FIGURES lE-F) are currently being designed; such
peptides have the potential to direct nanoparticles to
specific locations on a semiconductor structure. These
organic and inorganic pairs should provide powerful building
blocks for the fabrication of a new generation of complex,
sophisticated electronic structures.
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
Metallic and Magnetic Materials
In the present invention, specific binding and
recognition of binding molecules is extended in unexpected
ways to metal materials including but not limited to magnetic
and ferromagnetic materials, including particles and
nanoparticles. A combinatorial peptide phage display library
expressing a large collection of bacteriophage that expresses
millions of different peptide sequences on their surfaces was
combined with biopanning techniques to select specific peptide
sequences that tightly and directly bind to and recognize
metal materials, including magnetic materials, (e.g., Co,
SmCo5, Copt and FePt). The present inventors have found that
these magnetic material binding peptides can be used to
control the nucleation of inorganic materials, as has been
demonstrated in nature and in the III-V and II-VI
semiconductors. If proteins can be used to control the
nucleation of magnetic materials, then magnetic nanoparticles
could be prepared much cheaper and easier than using
traditional methods. The nanomolecular magnets and magnetic
material may be used, e.g., for micro or nanomachines,
dynamos, generators, magnetic storage or any other
applications for material that are magnetic or may be
magnetized. Another use for these materials is to modify the
surface of magnetic materials. The peptides can act as
linkers for attaching other materials to the surface of the
magnetic material, allowing the self-assembly of complex
nanostructures, which could form the basis of novel electronic
devices.
The present inventors have recognized that this approach
of selecting binding peptides (using combinatorial peptide
21
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
libraries and panning techniques)'has not been used with
magnetic materials, and peptides have never been used to
control the nucleation of magnetic materials. There are
currently many other techniques being researched to synthesize
magnetic nanoparticles. All of these efforts are based on a
high temperature synthesis that must be performed in an inert
atmosphere using expensive reagents and often require further
processing and purification after synthesis to fabricate
nanoparticles with the desired shape and crystallinity. The
result is that preparing magnetic nanoparticles in the
traditional fashion is very expensive and not conducive to
scale up. The approach presented herein can be performed at
room temperatures using inexpensive reagents yielding
nanoparticles with controlled crystallinity, making it a much
cheaper approach to the synthesis of magnetic nanoparticles.
This approach may also be used to control crystal structure
and crystal orientation.
Peptide-mediated synthesis of magnetic materials provides
a much cheaper and environmentally friendly approach to the
synthesis of magnetic nanoparticles. The current protocol
for preparing magnetic nanoparticles is both time-consuming
and expensive. In addition, the current protocol yields
nanoparticles that are coated with organic surfactants. These
surfactants are not amicable to further modification of the
nanoparticle. Advances in the field of molecular biology have
enabled the functionalization of peptides, suggesting that
nanoparticles grown from peptides will also be easily
functionalized, which facilitates their incorporation into
electronic devices and integration into magnetic memory
devices.
22
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
Current techniques for preparing magnetic nanoparticles
are expensive and time consuming requiring high temperatures,
inert atmospheres, expensive reagents, cumbersome
purifications, and post synthetic modifications. This new
technique for preparing magnetic nanoparticles using peptides
to mediate particle formation alleviates all of these concerns
allowing much more rapid and inexpensive particle synthesis.
In addition, better control of crystal structure and
orientation is achievable.
Known techniques may be used to produce enough peptide to
prepare large quantities of nanoparticles. Genetically
designed organisms may be used to produce the peptide or
peptides of interest. The peptides) may be manufactured in
one of the coat proteins of, e.g., M13 bacteriophage. The
bacteriophage may be further designed or engineered to express
the protein in additional coat proteins. Furthermore,
bacteria, such as E. coli, may be engineered to express the
peptides of interest in one or more designs or at locations of
interest. One distinct advantage of using peptides for
localizing or positioning the magnetic materials made herein
is that they do not have the limitations inherent in
semiconductor processing, which is generally limited to two
dimensions, e.g., using photolithography. The peptides) of
the present invention may be used in or about a matrix that
permits the three-dimensional positioning or synthesis of the
peptides. These peptides may then be formed as a film, in
lines or striations, layers, dots, in grooves, on the surface,
sides or bottom of an opening and the like.
Magnetic nanostructures have a variety of applications,
including memory devices, sensors, ferrofluids, etc. The
23
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
materials, particles, and nanoparticles described herein are
applicable to all of these fields.
Still further, the metallic and magnetic materials of the
invention can be used in methods of use in applications which
include the following. Additional applications include
therapeutics, diagnostics, engineering, chemical engineering
processing of reactions, cellular, and environmental
applications. For example, magnetic separations can be
carried out (including bulk separations in large scale
processing of reaction processes). Other applications include
purifications, therapeutics, biocompatibility, drug delivery,
imaging contrast agents, localization (in vivo) of magnetics
which are externally addressable. Drugs delivery can include
the coupling of particles to drugs or chemotherapeutics
followed by localization in the body by magnetic fields.
Proper particle design can yield cellular penetration.
Another application is blood-urine detection. In engineering
applications, display devices can be made with controlled
aspect ratio magnetic particles coupled to optoactive
materials including fluorescent and birefringent materials.
Sensor devises can be made wherein binding events change the
moment of inertia for magnetic particles coupled to binding
elements. The moment of inertia change can be detected
through polarization decay, including use of a coupled
optically active agent. Another application is in storage.
For example, memory can be made wherein the readout involves
response to time varying magnetic field. The writing step may
involve binding of a specific moiety to a specific address.
Cellular applications include cell modifications and cell
triggering. In cellular modification, the size of the
magnetic particle can be adjusted to allow penetration into
24
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
the cell, wherein the particle is coupled with a reagent.
Magnetic fields can be used as a motive force for penetration.
This can be useful for transfection procedures. In cellular
triggering, the reagent coupled with the magnetic particle can
enter the cell and then time varying magnetic fields can be
used to trigger a reponse in the cell.
Examples of magnetic separation include classical
affinity based separations in-vitro and localization of
reagents in-vivo. In affinity based separation, the magnetic
nanoparticles can have an advantage because of the smaller
size and large aspect ratio, and good control over size and
shape distribution. Another advantage is if the particles
have high magnetic permitivity. The particle can be long and
can rotate in the magnetic field, thus generating additional
forces from the shape effect. More powerful separation forces
can be achieved per mg of reagent. In localization of
reagents in vivo, magnetic particles can be injected or
ingested coupled with reagents. External, spatially varying
field can be applied to a subject causing particles to collect
in the region of highest gradient B. Small size of particle
plus reagent can allow for reagent to access tissues or even
penetrate cells.
More particularly, the present inventors have used
combinatorial peptide phage display libraries (i.e., large
collections of bacterial phage that express millions of
different peptide sequences on their surfaces) and biopanning
techniques to select specific peptide sequences that tightly
bind directly to magnetic materials (s-Co, CoPt, FePt). By
selecting and identifying specific peptide sequences that
interact with high affinity to magnetic materials, one can
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
quickly and easily identify peptides that can potentially be
used to control the nucleation of magnetic nanostructures.
Using peptides to control the nucleation of magnetic
nanoparticles enables the synthesis of magnetic nanostructures
under ambient conditions. The traditional protocols for
preparing magnetic nanoparticles often require elaborate
synthetic schemes and extensive purification, implying that
peptide-mediated nucleation would provide a much cheaper
alternative to nanoparticle synthesis.
One of the special advantages of the present invention is
that the peptides selected by this approach permit peptides to
be selected to bind specifically and directly to magnetic
materials. These peptides have demonstrated an ability to
nucleate selectively magnetic nanostructures with controlled
crystallinity. To date, Co nanoparticles have been prepared
of hexagonally close packed Co, and Copt and Feet
nanoparticles have been prepared with the layered
crystallinity traditionally associated with the Invar alloys.
These crystal structures exhibit the largest magnetic
susceptibility of their respective materials, and that these
materials retain their desirable magnetic properties at the
nanometer length scale. These properties make these materials
excellent candidates for the fabrication of next generation
magnetic memory devices. Currently memory devices are
prepared using a CoCr alloy with a density of 16.3 Gb/in2.
The smaller size of these nanoparticles conceivably allows the
construction of memory devices with a density in the
terabit/in2 range. With the present invention, SmCo5
nanoparticles are prepared that possess HCP P6/mm
crystallinity.
26
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
Using peptides to control the nucleation of the
nanoparticles also facilitates further functionalization of
the nanoparticles. Nanoparticles prepared in the traditional
fashion are often coated with hydrophobic surfactants making
further functionalization (activity or active group
attachments) a laborious process. Nanoparticles prepared as
disclosed herein may be coated with peptides, which are
relatively easy to functionalize using a variety of chemical
and biological techniques, as known to those of skill in the
art. Further functionalization of these nanoparticles allows
their self-assembly into complex architectures and memory
devices.
The particles and nanoparticles prepared using peptides
to control their crystallinity possess the ability to
revolutionize the magnetic recording industry due to their
small size, high magnetic susceptibility and ease of
preparation.
Example I. Peptide Preparation, Isolation, Selection
and Characterization
Peptide selection. The phage display or peptide library
was contacted with the semiconductor, or other, crystals in
Tris-buffered saline (TBS) containing 0.1a TWEEN-20, to reduce
phage-phage interactions on the surface. After rocking for 1
hour at room temperature, the surfaces were washed with 10
exposures to Tris-buffered saline, pH 7.5, and increasing
TWEEN-20 concentrations from 0.1°s to 0.5%(v/v). The phage were
eluted from the surface by the addition of glycine-HC1 (pH
2.2) 10 minute, transferred to a fresh tube and then
27
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
neutralized with Tris-HC1 (pH 9.1). The eluted phage were
titered and binding efficiency was compared.
The phage eluted after third-round substrate exposure
were mixed with their Escherichia coli (E. coli) ER2537 host
and plated on LB XGal/IPTG plates. Since the library phage
were derived from the vector M13mp19, which carries the lacZa
gene, phage plaques were blue in color when plated on media
containing Xgal (5-bromo-4-chloro-3-indoyl-(3-D-galactoside)
and IPTG (isopropyl-(3-D-thiogalactoside). Blue/white screening
was used to select phage plaques with the random peptide
insert. Plaques were picked and DNA sequenced from these
plates.
Substrate preparation. Substrate orientations were
confirmed by X-ray diffraction, and native oxides were removed
by appropriate chemical specific etching. The following etches
were tested on GaAs and InP surfaces: NH40H:H20 (1:10), HCl:HzO
( 1 : 10 ) , H3P04 : H20z : H20 ( 3 : 1 : 50 ) at 1 minute and 10 minute etch
times. The best element ratio and least oxide formation
(using XPS)for GaAs and InP etched surfaces was achieved using
HCl: H20 for 1 minute followed by a deionized water rinse for
1 minute. However, since an ammonium hydroxide etch was used
for GaAs in the initial screening of the library, this etch
was used for all other GaAs substrate examples. Si(100) wafers
were etched in a solution of HF:H20 1:40 for one minute,
followed by a deionized water rinse. All surfaces were taken
directly from the rinse solution and immediately introduced to
the phage library. Surfaces of control substrates, not exposed
to phage, were characterized and mapped for effectiveness of
the etching process and morphology of surfaces by AFM and XPS.
28
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
Multilayer substrates of GaAs and of A1o,98Gao.oz As were
grown by molecular beam epitaxy onto GaAs(100). The
epitaxially grown layers were Si-doped (n-type) at a level of
x 101' cm 3.
5 Antibody and Gold Labeling. For the XPS, SEM and AFM
examples, substrates were exposed to phage for 1 hour in Tris-
buffered saline then introduced to an anti-fd bacteriophage-
biotin conjugate, an antibody to the pIII protein of fd phage,
(1:500 in phosphate buffer, Sigma) for 30 minutes and then
rinsed in phosphate buffer. A streptavidin-20-nm colloidal
gold label (1:200) in phosphate-buffered saline (PBS, Sigma)
was attached to the biotin-conjugated phage through a biotin-
streptavidin interaction; the surfaces were exposed to the
label for 30 minutes and then rinsed several times with PBS.
X-ray Photoelectron Spectroscopy (XPS). The following
controls were done for the XPS examples to ensure that the
gold signal seen in XPS was from gold bound to the phage and
not non-specific antibody interaction with the GaAs surface.
The prepared GaAs(100) surface was exposed to three
conditions: (1) antibody and the streptavidin-gold label, but
without phage; (2) G1-3 phage and streptavidin-gold label, but
without the antibody; and (3) streptavidin-gold label, without
either G1-3 phage or antibody.
The XPS instrument used was a Physical Electronics Phi
ESCA 5700 with an aluminum anode producing monochromatic
1,487-eV X-rays. All samples were introduced to the chamber
immediately after gold-tagging the phage (as described above)
to limit oxidation of the GaAs surfaces, and then pumped
overnight at high vacuum to reduce sample outgassing in the
XPS chamber.
29
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
Atomic Force Microscopy (AFM). The AFM used was a
Digital Instruments Bioscope mounted on a Zeiss Axiovert 100s-
2tv, operating in tip scanning mode with a G scanner. The
images were taken in air using tapping mode. The AFM probes
were etched silicon with 125-mm cantilevers and spring
constants of 20~100 Nrril driven near their resonant frequency
of 200+400 kHz. Scan rates were of the order of 1+5 mms-1.
Images were leveled using a first-order plane to remove sample
tilt.
Transmission Electron Microscopy (TEM). TEM images were
taken using a Philips EM208 at 60 kV. The Gl-3 phage (diluted
1:100 in TBS) were incubated with GaAs pieces (500 mm) for 30
minutes, centrifuged to separate particles from unbound phage,
rinsed with TBS, and resuspended in TBS. Samples were stained
with 2% uranyl acetate.
Scanning Electron Microscopy (SEM). The G12-3 phage
(diluted 1:100 in TBS) were incubated with a freshly cleaved
hetero-structure surface for 30 minutes and rinsed with TBS.
The G12-3 phage were tagged with 20 nm colloidal gold. SEM and
elemental mapping images were collected using the Norian
detection system mounted on a Hitachi 4700 field emission
scanning electron microscope at 5 kV.
Example II. Biofilms
The present inventors have recognized that organic-
inorganic hybrid materials offer new routes for novel
materials and devices. Size controlled nanostructures give
optically and electrically tunable properties of semiconductor
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
materials and organic additives modify the inorganic
morphology, phase, and nucleation direction. The
monodispersed nature of biological materials makes the system
compatible for highly ordered smectic-ordering structure.
Using the methods of the present invention, highly ordered
nanometer scale as well as multi-length scale alignment of II-
VI semiconductor material using genetically engineered, self-
assembling, biological molecules, e.g., M13 bacteriophage that
have a recognition moiety of specific semiconductor surfaces
were created.
Using the compositions and methods of the present
invention nano- and multi-length scale alignment of
semiconductor materials was achieved using the recognition and
self-ordering system described herein. The recognition and
self-ordering of semiconductors may be used to enhance micro
fabrication of electronic devices that surpass current
photolithographic capabilities. Application of these
materials include: optoelectronic devices such as light
emitting displays, optical detectors and lasers; fast
interconnects; and nano-meter scale computer components and
biological sensors. Other uses of the biofilms created using
the present invention include well-ordered liquid crystal
displays and organic-inorganic display technology.
The films, fibers and other structures may even include
high-density sensors for detection of small molecules
including biological toxins. Other uses include optical
coatings and optical switches. Optionally, scaffoldings for
medical implants or even bone implants; may be constructed
using one or more of the materials disclosed herein, in single
or multiple layers or even in striations or combinations of
31
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
any of these, as will be apparent to those of skill in the
art.
Other uses for the present invention include electrical
and magnetic interfaces, or even the organization of 3D
electronic nanostructures for high-density storage, e.g., for
use in quantum computing. Alternatively, high density and
stable storage of viruses for medical application that can be
reconstituted, e.g., biologically compatible vaccines,
adjuvants and vaccine containers may be created with the films
and or matrices created with the present invention.
Information storage based on quantum dot patterns for
identification, e.g., department of defense friend or foe
identification in fabric of armor or coding. The present
nanofibers may even be used to code and identify money.
Building well-ordered, well-controlled, two and three
dimensional structure at the nanolength scale is the major
goal of building next generation optical, electronic and
magnetic materials and devices. Current methods of making
specific nanoparticles are limited in terms of both length
scale and the types of materials. The present invention
exploits the properties of self-assembling organic or
biological molecules or particles, e.g., M13 bacteriophage to
expand the alignment, size, and scale of the nanoparticles as
well as the range of semiconductor materials that can be used.
The present inventors have recognized that monodisperse
biomaterials having anisotropic shapes are an alternative way
to build well-ordered structures. Nano- and multi-length
scale alignment of II-VI semiconductor material were
accomplished using genetically engineered M13 bacteriophage
32
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
that possess a recognition moiety (a peptide or amino acid
oligomer) for specific semiconductor surfaces.
Seth and coworkers have characterized Fd virus smectic
ordering structures that have both a positional and
directional order. The smectic structure of Fd virus has
potential application in both multi-scale and nanoscale
ordering of structures to build 2-dimensional and 3-
dimensional alignment of nanoparticles. Bacteriophage M13 was
used because it can be genetically modified, has been
successfully selected to have a shape identical to the Fd
virus, and has specific binding affinities for II-VI
semiconductor surfaces. Therefore, M13 is an ideal source for
smectic structure that can serve in mufti-scale and nanoscale
ordering of nanoparticles.
The present inventors have used combinatorial screening
methods to find M13 bacteriophage containing peptide inserts
that are capable of binding to semiconductor surfaces. These
semiconductor surfaces included materials such as zinc
sulfide, cadmium sulfide and iron sulfide. Using the
techniques of molecular biology, bacteriophage combinatorial
library clones that bind specific semi-conductor materials and
material surfaces were cloned and amplified up to
concentrations high enough for liquid crystal formation.
The filamentous bacteriophage, Fd, has a long rod shape
(length: 880 nm; diameter: 6.6 nm) and monodisperse molecular
weight (molecular weight: 1.64 x 10'). These properties
result in the bacteriophage's lyotropic liquid crystalline
behavior in highly concentrated solutions. The anisotrophic
shape of bacteriophage was exploited as a method to build
well-ordered nanoparticle layers by use of biological
33
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
selectivity and self-assembly. Monodisperse bacteriophage
were prepared through standard amplification methods. In the
present invention, M13, a similar filamentous bacteriophage,
was genetically modified to bind nanoparticles such as zinc
sulfide, cadmium sulfide and iron sulfide.
Mesoscale ordering of bacteriophage has been demonstrated
to form nanoscale arrays of nanoparticles. These
nanoparticles are further organized into micron domains and
into centimeter length scales. The semiconductor
nanoparticles show quantum confinement effects, and can be
synthesized and ordered within the liquid crystal.
Bacteriophage M13 suspension containing specific peptide
inserts were made and characterized using AFM, TEM, and SEM.
Uniform 2D and 3D ordering of nanoparticles was observed
throughout the samples.
AFM. Includes Digital Instruments Bioscope mounted on a
Zeiss Axiovert 100s-2tv,operating in tip scanning mode with a
G scanner. The images were taken in air using tapping mode.
The AFM probes were etched silicon with 125 mm cantilevers and
spring constants of 20~100 Nm-1 driven near their resonant
frequency of 200~400 kHz. Scan rates were of the order of 1~5
mms-1. Images were leveled using a first-order plane to remove
sample tilt. FIGURES 2A and 2B are schematic diagrams of the
smectic alignment of M13 phages observed using AFM.
TEM. TEM images were taken using a Philips EM208 at 60
kV. The G1-3 phage (diluted 1:100 in TBS) were incubated with
semiconductor material for 30 minutes, centrifuged to separate
particles from unbound phage, rinsed with TBS, and resuspended
in TBS. Samples were stained with 2% uranyl acetate.
34
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
SEM. The phage (diluted 1:100 in TBS) were incubated
with a freshly cleaved hetero-structure surface for 30 minutes
and rinsed with TBS. The G12-3 phage were tagged with 20 nm
colloidal gold. SEM and elemental mapping images were
collected using the Norian detection system mounted on a
Hitachi 4700 field emission scanning electron microscope at 5
kV.
Genetically engineered M13 bacteriophage that had
specific binding properties to semiconductor surfaces was
amplified and purified using standard molecular biological
techniques. 3.2 mL of bacteriophage suspension
(concentration: -10' phages/~L) and 4 mL of overnight culture
were added to 400 mL LB medium for mass amplification. After
amplification, ~30 mg of pellet was precipitated. The
suspensions were prepared by adding Na2S solutions to ZnCl2
doped A7 phage suspensions at room temperature. The highest
concentration of A7-phage suspension was prepared by adding 20
~L of 1 mM ZnCl2 and Na2S solutions, respectively into the ~30
mg of phage pellet. The concentration was measured using
extinction coefficient of 3.84 mg/mL at 269 nm.
As the concentration of the isotropic suspension is
increased, nemetic phase that has directional order,
cholesteric phase that has twisted nemetic structure, and
smectic phase that has directional and positional orders as
well, are observed. These phases had been observed in Fd
viruses that did not have nanoparticles.
Polarized optical microscopy (POM). M13 phage
suspensions were characterized by polarized optical
microscope. Each suspension was filled to glass capillary
tube of 0.7 mm diameter. The highly concentrated suspension
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
(127 mg/mL) exhibited iridescent color [5] under the
paralleled polarized light and showed smectic texture under
the cross-polarized light (FIGURE 3A). The cholesteric
pitches in FIGURE 3B can be controlled by varying the
concentration of suspension as shown in TABLE 2. The pitch
length was measured and the micrographs were taken after 24
hours later from the preparation of samples.
TABLE 2. Cholesteric pitch and concentration relationship.
Concentration Pitch length
(mg/ml) (um)
76.30 31.9
71.22 51.6
56.38 84.8
50.52 101.9
43.16 163.7
37.04 176.1
27 . 54 -- I- - 259 . 7
AFM. For AFM observation, 5 ~L of M13 suspension
(concentration: 30 mg/mL) of M13 bacteriophage suspension was
dried for 24 hours on the 8 mm x 8 mm mica substrate that was
silated by 3-amino propyl triethyl silane for 4 hours in the
dessicator. Images were taken in air using tapping mode.
Self-assembled ordering structures were observed due to the
anisotropic shape of M13 bacteriophage, 880 nm in length and
6.6 nm in width. In FIGURE 3C, M13 phage lie in the plane of
the photo and form smectic alignment.
SEM. For SEM observation, the critical point drying
samples of bacteriophage and ZnS nanoparticles smectic
suspension (concentration of bacteriophage suspension 127
mg/mL) were prepared. In FIGURE 3D, nanoparticles rich areas
and bacteriophage rich areas were observed. The length of the
36
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
separation between nanoparticles and bacteriophage correspond
to the length of bacteriophage. The ZnS wurzite crystal
structure was confirmed by electron diffraction pattern using
dilution sample of the smectic suspension with TEM (FIGURES 3E
and 3F) .
Preparation of the biofilm. Bacteriophage pellets were
suspended with 400 ~L of Tris-buffered saline (TBS, pH 7.5)
and 200 ~,L of 1 mM ZnClz to which 1mM NaZS was added. After
rocking for 24 hours at room temperature, the suspension
(contained in a 1 mL eppindorff tube) was slowly dried in a
dessicator for one week. A semi-transparent film ~15 ~m thick
was formed on the inside of the tube. This film, shown in
FIGURE 4A, was carefully taken using a tweezers. A schematic
diagram of the biofilm is shown in FIGURE 4B.
SEM observation of biofilm. Nanoscale bacteriophage
alignment of the A7-ZnS film were observed using SEM. In
order to carry out SEM analysis the film was cut then coated
via vacuum deposition with 2 nm of chromium in an argon
atmosphere. Highly close-packed structures, FIGURE 4D were
observed throughout the sample. The average length of
individual phage, 895 nm is reasonable analogous to that of
phage, 880 nm. The film showed the smectic like A- or C-like
lamellar morphologies that exhibited periodicity between the
nanoparticle and bacteriophage layers. The length of
periodicity corresponded to that of the bacteriophage. The
average size of nanoparticle is ~20nm analogous to the TEM
observation of individual particles.
TEM observation of biofilm. ZnS nanoparticle alignment
was investigated by embedding the film in epoxy resin (LR
37
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
white) for one day and polymerized by adding 10 ~l of
accelerator. After curing, the resin was thin sectioned using
a Leica Ultramicrotome. These ~50 nm sections were floated on
distilled water, and picked up on blank gold grids. Parallel-
aligned nanoparticles in a low, which corresponded to x-z
plane in the schematic diagram, were observed, FIGURE 4 E-F.
Since each bacteriophage had 5 copies of the A7 moieties, each
A7 recognize one nanoparticle (2--3 nm size) and aligned
approximately 20 nm in a width and extended to more than two
micrometers in length. The two micrometers by 20 nm bands
formed in parallel each band separated by 700 nm. This
discrepancy may come from the tilted smectic alignment of the
phage layers with respect to observation in the TEM, which is
reported by Marvin group. A y-z axis like nanoparticle layer
plane was also observed similar to that shown in FIGURE 1F.
The SAED patterns of the aligned particles showed that the ZnS
particles have the wurzite hexagonal structure.
AFM observation of biofilm: The surface orientation of
the viral film was investigated using AFM. In FIGURE 4C,
phage were shown to have formed an parallel aligned
herringbone pattern that have almost right angle between the
adjacent director normal (bacteriophage axis) on most of
surface that is named as smectic O. The film showed long
range ordering of normal director that is persistent to the
tens of micrometers. In some of areas where two domain
layers meet each other, two or three multi-length scale of
bacteriophage aligned paralleled and persistent to the smectic
C ordering structure.
Nano and multi-length scale alignment of semiconductor
materials using the recognition and as well as self-ordering
38
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
system enhances the future microfabrication of electronic
devices. These devices have the potential to surpass current
photolithographic capabilities. Other potential applications
of these materials include optoelectronic devices such as
light-emitting displays, optical detectors, and lasers, fast
interconnects, nano-meter scale computer component and
biological sensors.
EXAMPLE III. Formation of Metallic and Magnetic
Materials
A phage display technique was used to discover novel
peptides that bind selectively to magnetic materials. In
these particular studies, films of the magnetic materials were
prepared by first synthesizing colloidal dispersions of the
magnetic materials. These colloidal solutions were then drop
coated onto Si wafers and annealed under Nz to generate the
r
desired crystal structure. Phage display was then performed
on these films (E-Co, CoPt, and FePt), and peptides were
discovered that bind selectively to each substrate. These
peptides were then used to nucleate unique nanoparticles by
mixing the phage expressing the peptide of interest, the metal
salt, and a reducing agent.
The synthesis of nanoparticles with controlled size and
composition is of fundamental and technological interest. In
the last few years there has been a flurry of papers
describing the synthesis of nanoparticles composed of metals
and semiconductors with remarkable control over the size and
shape of the resulting nanoparticles. Recently it has been
shown that peptides identified via phage display can bind
selectively to inorganic surfaces and can be used to control
the nucleation of semiconducting nanoparticles. In this case,
39
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
the peptides can control the size, shape, composition, and
even the crystallinity of the resulting nanoparticles. Due to
the success of peptides in controlling the synthesis of
semiconducting nanoparticles, there is a great deal of
interest in applying the technology to other materials of
interest.
One particularly interesting and commercially useful
class of materials is ferromagnets, including particles and
nanoparticles. Ferromagnetic materials are the cornerstone of
the billion dollar per year magnetic recording industry.
Current devices use a CoCr alloy for data storage because of
the high magnetic susceptibility and ease of preparation.
Other materials are currently in development. One such
material is metallic Co, which has a magnetic anisotropy in
the range of 10' ergs/cm3. This high magnetic anisotropy
suggests that particles as small as 10 nm in diameter, can act
as single domains and function as memory elements. Current
technology uses memory elements with a domain size that is in
the range of hundreds of nanometers, so generating Co
nanoparticles in the 10 nm size range would be a dramatic
improvement that would lead to much denser memory devices.
More interesting ferromagnetic materials are the magnetic
alloys of Pt, specifically Feet and Copt. These materials
have very large magnetic anisotropies (108 ergs/cm3), due to
the Invar effect, in which perturbations in the lattice
constant caused by the layering of Fe and Pt atoms causes the
Pt to develop a magnetic state. The large anisotropy
possessed by these systems suggests that nanoparticles as
small as 2 nm can act as ferromagnets at room temperature,
implying that they can be used in the development of very
high-density memory devices.
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
Due to the large magnetic anisotropies of these systems,
a great deal of effort has been invested in the synthesis of
particles and nanoparticles composed of these materials.
Several different synthetic protocols have been developed for
E-Co, FePt and Copt and they all possess the same fundamental
weaknesses. All of these synthetic strategies rely on the
restricted precipitation of nanoparticles in the presence of
surfactants at elevated temperatures. All of these
nanoparticle preparations must be performed in an inert
atmosphere with expensive reagents, making them very expensive
and not amicable to scale up. Furthermore, these preparations
often require further modifications of the particles,
including high temperature annealing to attain the desired
crystallinity, and size selective precipitation to acquire
monodisperse populations of particles. These extra synthetic
steps increase the cost of these synthetic strategies.
Since these materials are commercially important, a novel
synthetic strategy was desired. Applying the principle of
peptide-mediated synthesis to magnetic materials provides such
an alternative. In these studies phage display selection was
performed on the magnetic materials of interest (Co, CoPt,
SmCo5, and Feet) to identify peptides that specifically bind
to the magnetic materials with high affinity. After
characterization, these peptides were then used to control the
nucleation of magnetic nanoparticles. In these studies, phage
expressing the peptides of interest were mixed with the
metallic salts of the metals of interest. A reducing agent
(NaBH4) was then added to generate the nanoparticles. The
nanoparticles were formed and characterized using TEM. The
synthesis of the present invention was performed under ambient
41
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
conditions to provide a much cheaper alternative to existing
synthetic strategies for generating magnetic nanoparticles.
X-Ray Diffraction Analysis of Magnetic Nanoparticles
Magnetic surfaces had to be generated to use as
substrates in the phage display. To accomplish this, magnetic
nanoparticles were prepared in the traditional fashion, and
drop coated onto Si wafers. Before the phage display studies
were begun, the surfaces were characterized with x-ray
diffraction (XRD) to ensure the material possessed the
appropriate crystallinity.
The XRD pattern obtained for s-Co correlated well with
patterns obtained from the literature, displaying a triplet of
peaks between 45 degrees and 50 degrees that are particularly
distinctive because they correspond to the (221), (310), and
(311) crystal planes of s-Co. The Feet and Copt patterns also
agreed with the literature spectra for FePtll with peaks
corresponding to the (001), (110), (111), (200), (002), (210),
(112), and (202) planes of Feet and Copt. The XRD on SmCo5
agreed with literature values for HCP SmCo5 with peaks
representing the (101), (110), and (111) facets. This is the
first reported synthesis of HCP SmCo5 nanoparticles. FIGURE
5A is a high resolution TEM image of a SmCo5 nanoparticle and
FIGURE 5B is a selected area of the TEM image showing the
electron diffraction pattern. Several spots in the
diffraction pattern correlate well with the known facets of
HCP SmCo5 (FIGURE 51B). FIGURE 5C is a STEM image of the
annealed SmCo5 nanoparticles and illustrates their size,
shape, and overall morphology.
42
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
Sequence Analysis and Binding Assays of Binding Phage
TABLE 3 lists all of the peptides that were selected
using phage display for their ability to bind to the magnetic
materials of interest.
TABLE 3. Selected clones with magnetic binding properties.
Material 7-Constrained Sequence l2mer Sequence
E-Co * ALSPHSAPLTLY (SEQ ID .:15)
N0
Copt NAGDHAN (SEQ ID N0.:12) SVSVGMKPSPRP (SEQ ID .:16)
N0
Feet SKNSNIL (SEQ ID N0.:13) HNKHLPSTQPLA (SEQ ID .:17)
N0
SmCo5 TKPSWQ (SEQ ID N0.:14) WDPYSHLLQHPQ (SEQ ID .:18)
N0
*No consensus sequence was obtained for the 7-constrained library on
s-Co .
All of the selected sequences appear to be valid
sequences that should possess high affinity for the metallic
surfaces. Histidine residues appear in several of the
sequences. Due to its imidazole side group, histidine is an
excellent ligand for metals, so its presence in these
sequences is expected. With the exception of the 7-
constrained sequence on Copt, all of the sequences isolated
for the Pt alloys contain a lysine residue. Lysine-Pt
interactions are believed to be important in the function of
cisplatin, an important anticancer drug. The Lysine-Pt
interaction suggests that these sequences bind selectively to
these materials, however, the present invention is not limited
to any mechanism of interaction, known or unknown.
Specific Binding Assays. To determine the affinity of
the isolated phage for the magnetic substrate, two studies
were performed. In the first study several different peptide-
containing phage were exposed to a Co surface including our Co
specific phage, a random phage, and wild type phage.
43
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
Additionally, the Co-specific phage was exposed to several
different material surfaces. The results are depicted in
FIGURE 6. The Co-specific phage possessed a relative higher
affinity for Co than either the wild-type phage or a random
phage library sequence (FIGURE 6A). Additionally, the Co-
specific phage displayed a greater affinity for Co than for
Si, suggesting they bound preferentially to the Co surface.
In the second study a Co surface was immersed into a
solution of the Co-specific phage. This study was repeated at
several different concentrations of phage. Plotting the
amount of adsorbed phage vs. the concentration of phage
(FIGURE 6B) indicated that the adsorption of phage onto the Co
surface followed the Langmuir model for adsorption of analytes
on a surface. Since the adsorption is Langmuirian, generating
a reciprocal plot revealed a linear correlation between the
adsorbed phage and the concentration (not shown). The slope
of this line is equal to the binding constant, and in the case
of Co, the phage possessed a kaas of 2 x 10-12 M. This is the
first measurement of the thermodynamic properties associated
with the binding between a phage and an inorganic surface,
making it difficult to interpret, but the magnitude of this
binding constant is comparable to several other biological
interactions. This approach may be used for the Copt and Feet
systems.
Both studies showed that the peptides selected using
phage display screening possessed specific binding towards Co
and not towards other materials. It is this specificity that
can be used to direct metal materials formation, including
magnetic materials.
44
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
TEM Analysis of Nanoparticles Prepared Through Peptide-
Mediated Nucleation
In one embodiment of the present invention, nanoparticles
were prepared using peptides to modify and/or control
crystallinity. High resolution TEM images of Copt
nanoparticles grown using the 7-constrained sequence are shown
in TABLE 3 were also taken (not shown). These nanoparticles
had lattice spacings of 0.19 and 0.22 nm, which correlates
with the lattice spacing of L10 Copt.
High resolution TEM images of nanoparticles grown using
wild type phage were also taken as were images of Copt
nanoparticle grown using phage with a random peptide insert
(not depicted). In both control studies, nanoparticles still
form, but they lacked the crystallinity that the particles
grown with the Copt selective peptide possess. Nanoparticles
grown in the absence of phage aggregate and precipitate out of
solution, making TEM imaging nearly impossible.
High-resolution TEM images were also taken of Feet
nanoparticles grown using the phage that expresses the l2mer
peptide, which is selective for Feet (not depicted). These
nanoparticles exhibited similar lattice spacing to the Copt
nanoparticles suggesting they are composed of L10 Feet.
Electron diffraction patterns were taken of these same
particles, e.g., FePt nanoparticles grown in the presence of
wild type phage (not depicted). Again, these nanoparticles
lack the crystallinity of the nanoparticles grown with the
Feet selective phage. Also, nanoparticles grown in the
absence of phage aggregate and precipitate out of solution
before they could be imaged.
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
High resolution TEM images of Copt nanoparticles grown
using the 7-constrained sequence from Table 1 are shown in
FIGURE 7. The lattice spacing in these nanoparticles is at or
about 0.22 nm and correlating well with literature values for
HCP Co of approximately 0.19 nm (FIGURE 7A) and with the
lattice spacing of Llo Copt. A selected area was also used to
observe the electron diffraction pattern of the nanoparticles
(not shown). Several bands were present in the diffraction
pattern that correlate with the facets of HCP Co and indicate
that the nanoparticles were, in fact, composed of HCP Co. In
control experiments with either wild-type phage (FIGURE 7C),
nonspecific phage (FIGURE 7B), nanoparticles still form, but
lack the crystallinity that the particles grown with the Copt
selective peptide possess. Nanoparticles grown in the absence
of phage aggregate and precipitate out of solution, making TEM
imaging nearly impossible.
FIGURE 8 shows high resolution TEM images of Co
nanoparticles grown using the phage that expressed the l2mer
peptide that binds specifically to Co (FIGURE 8A). The
lattice spacing in these particles is 0.2 nm, which correlates
well with the literature values for HCP Co (0.19 nm). A
selected area is chosen for electron diffraction pattern for
these nanoparticles (FIGURE 8B). Several bands are present in
the diffraction pattern that correlate with the facets of HCP
Co, indicating that the nanoparticles are composed of HCP Co.
In control experiments involving either wild-type phage,
nonspecific phage, or no phage, Co particles aggregate and
sediment out of solution (not shown).
FIGURE 9A shows a high resolution TEM image of Feet
nanoparticles grown using phage that expressed a l2mer peptide
46
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
selective for Feet. These nanoparticles exhibit similar
lattice spacing to the Copt nanoparticles and were likely
composed of Llo Feet. FIGURE 9B is the corresponding electron
diffraction pattern, and FIGURE 9C an image of Feet
nanoparticles grown in the presence of wild type phage. In
the absence of wild-type phage, nanoparticles lacked the
crystallinity of the nanopaticles grown with the FePt-
selective phage. In addition, nanoparticles grown in the
absence of phage aggregated and precipitated out of solution
before they can be imaged.
High resolution TEM images were also taken of SmCo5
nanoparticles grown using phage that expresses the l2mer
peptide that is specific to SmCo5 (FIGURE l0A). A selected
area was used to observe the electron diffraction pattern
(FIGURE lOB). Again, the diffraction pattern showed several
bands that correlated with the facets of HCP SmCo5. Control
experiments performed with the SmCo5 system yielded results
similar to that observed for the Co system, such that
nanoparticles aggregated and/or precipitated out of solution
when nonspecific phage were used. TEM images of such
particles showed some crystalline domains, but the majority of
the material was amorphous.
MFM Characterization of Nanoparticles
Magnetic Force Microscopy (MFM) was used to characterize
the magnetic properties of the nanoparticles. Atomic force
images of phage that were used to nucleate Co nanoparticles
were first taken (FIGURE 11A). A large aggregate of
nanoparticles was evident at the end of the phage, indicating
that the P3 proteins were controlling the nucleation of the
nanoparticles as expected. Corresponding MFM image was taken
47
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
to confirm these results (FIGURE 11B)). Here, the phage could
not be seen because they were non-magnetic, but the aggregate
of nanoparticles was still clearly visible, indicating the
nanoparticles possess a high degree of magnetic anisotropy.
SQUID. In one embodiment of the present invention, the
magnetic properties of the nanoparticles may be quantified
using a Superconducting Quantum Interference Device (SQUID)
magnetometer. SQUID magnetrometry was used to further
characterize the particles. With SQUID, a room temperature
hysteresis loop for Feet nanoparticles grown using the l2mer
peptide expressed on phage was taken (FIGURE 12A). A high-
resolution hysteresis loop of the central portion of the scan
was also taken to clarify the presence of the coercivity
(FIGURE 12B). These samples possessed relatively low
coercivity (approximately 50 Oe). The data represents the
first example of ferromagnetic nanoparticles grown under
ambient conditions. Hysteresis loops were also measured on
biologically prepared SmCos nanoparticles (FIGURE 13). The
hystersis was much larger for these nanoparticles (400 Oe).
This result was expected since macroscopic samples of SmCos
typically display higher coercivity values than Feet.
Magnetic-Specific Peptides on P8 Coat Proteins
In one embodiment of the present invention, nanoparticles
with magnetic behaviors are prepared using the material-
specific phage that were expressed on the p3 protein of M13
bacteriophage. The p3 protein is only present on one end of
the rod-shaped phage and is present in limited numbers (3-5
copies per phage). Alternatively, the p8 coat protein is
expressed along the length of the phage, and there are
hundreds of copies per phage. For this reason, the p8 protein
48
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
was engineered to express a Copt-specific peptides, and Copt
nanoparticles were nucleated along the length of the phage.
One example of the material preparation is presented below.
Other methodologies apparent to those of ordinary skill in the
art of material and biologic sciences may be used without
undue experimentation.
Upon nucleation of magnetic materials, including magnetic
particles and nanoparticles, the peptides, with or without
phage, can be heated to sufficiently high temperatures to burn
off and eliminate the binding molecules associated with the
scaffold in a high temperature annealing process. For
example, heating to 500°C or 1,000°C can be carried out for
times which provide optimum burn off and elimination. The
temperatures can be also in the range for metal annealing,
whereby polycrystalline domains can fuse into single
crystalline domains.
Methodology.
Materials. Samarium (III) Chloride, Platinum (II)
Acetylacetonate (Pt(Acac)2, Dihydrogen Hexachloroplatinate
(H2PtC16) , and Cobalt Octacarbonyl (Co2 (CO) 8) were purchased
from Alfa Aesar. Iron Pentacarbonyl (Fe (CO) 5) , Cobalt (II)
Chloride (CoCl2), Iron (II) Chloride (FeClz), Trioctylphosphine
oxide (TOPO), Sodium Borohydride (NaBH4), oleyl amine, and
oleic acid were purchased from Aldrich.
Nanoparticle Synthesis of s-Co. Co nanoparticles were
prepared by first dissolving 0.6 g of Co2(CO)8 in 5 mL of o-
dichlorobenzene. This mixture was stirred for one hour to
dissolve the Co and 20 mL of o-dichlorobenzene, 0.416 g of
TOPO, and 0.2 mL of oleic acid were mixed in a 500 mL three-
49
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
necked reaction vessel under Ar. This mixture was then heated
to 100 degrees Centigrade. The mixture was then exposed to
vacuum for 5 minutes to remove any dissolved O2 and H20. The
mixture was then heated to boiling (180 degrees Centigrade),
and Co solution was added. The mixture turned black and
generated a cloud of CO gas. After 20 minutes of refluxing,
the reaction was cooled to room temperature. To purify the
particles, 3 mL of Co nanoparticle solution was mixed with 3
mL of ethanol. After 1 hour, the mixture was centrifuged at
10,000 rpm for 5 minutes. The precipitant was resuspended in
3 mL of CHZC12 followed by 3m1 of ethanol and the
centrifugation step was repeated. The precipitant was then
resuspended in 3 mL of CHZCIz .
Nanoparticle Synthesis of Feet. 20 mL of phenyl ether,
0.205 g of Pt(Acac)2, and 0.358 g of 1,2-tetradecanediol were
mixed and heated to 100 degrees Centigrade under Ar after
which 0.16 mL of oleic acid, 0.17 mL of oleyl amine, and 0.13
mL of Fe(CO)5 were added. The mixture was heated to 300
degrees Centigrade and refluxed for 30 minutes and allowed to
cool to room temperature. Feet nanoparticles were purified in
a similar fashion to the Co nanoparticles
Nanoparticle Synthesis of Copt. Preparation was
identical to Feet, except 0.16 g of Coz(CO)$ was substituted
for 0 . 13 mL of Fe (CO) 5.
Nanoparticle Synthesis of SmCoS. An arrested
precipitation approach was taken to prepare nanoparticles of
SmCo5. This technique was adapted from previous efforts at
preparing nanoparticles. 38.75 mg of CoClz was mixed with
16.0 mg of SmCl3 and dissolved in 20 mL of phenyl ether.
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
0.357 mL of oleic acid was then added to the mixture, which
was then heated to 100 degrees Centigrade under Ar. 1.35 mL
of trioctylphosphine was then added. The mixture was then
exposed to vacuum for ten minutes to remove any remaining
dissolved Oz or H20 from solution. After purging the solution
with vacuum, it was heated to a 290 degrees Centigrade to boil
the phenyl ether. 1 mL of superhydride solution was then
added. The solution turns from blue to black immediately.
The black mixture was then refluxed for 20 minutes and allowed
to cool to room temperature
Film Formation. To prepare films for phage display
selection, a colloidal solution of nanoparticles was drop
coated onto a Si slide. The solvent was allowed to evaporate.
In the case of Feet and Copt, the slides were then annealed at
700 degrees Centigrade for 30 minutes under NZ to form the L10
phase. XRD analysis was performed on all of these slides to
ensure they were the proper material.
Peptide Selection. The use of a phage display library
technique was used to find peptides that bind exclusively to
s-Co, and the L10-phase of Copt and Feet. Specifically, the
Ph.D.-12(tm) and Ph.D.-7 CTM Phage Display Peptide Library
Kits were used beginning with 1 ~L (or an initial amount) of
phage display library to initiate selection against the
magnetic substrates (in 1 mL of TBS). For E-Co, selections
were performed in a 10 mM solution of NaBH4 in TBST. After
five rounds of panning, peptides and DNA of the peptides were
isolated and sequences were obtained from the University of
Texas DNA Core Facility. These sequences, which correspond to
the peptides displayed on the bacteriophage, underwent
analysis to determine consensus sequences. Analysis of the
51
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
DNA sequences consisted of percent abundance of amino acid per
position. Because of the possibility of non-specific binding
in the first two rounds, analysis was only performed on the
last three rounds of panning.
Binding Affinity. To determine that the peptides bind
specifically to E-Co, CoPt, and Feet, binding affinity was
determined. Titer counts were obtained from consensus peptide
panning studies and compared to titer counts of WT and random
peptides not raised to s-Co, CoPt, and Feet. Panning studies
were then performed using varying concentrations of phage to
determine the binding constant of the phage to the metallic
surf ace of interest.
Peptide-Mediated Nucleation of Co. Approximately 880 ul
of Hz0 were mixed with 100 ~L of 1 mM CoCl2 and 20 ~L of phage
solution (pfu = 1011). The mixture was gently agitated for 30
minutes, and then 100 ~L of 100 mM NaBH4 was added. The
solution was vortexed, and allowed to incubate for another 5
minutes. 100 mL of a solution of TOPO and oleic acid
dissolved in CHzCl2 was then added. The mixture was vortexed
and gently agitated for 1 hour. Over this time period the
CHZC12 layer changed to dark grey. This was repeated with
several different phage, including Co-1, Co-2, wild type
phage, and a TBS solution containing no phage.
Peptide-Mediated Nucleation of Copt. For nucleation, 50
~L of 1 mM CoCl2 solution was mixed with 50 ~L of 1 mM HZPtCI6
solution. 10 ml of phage solution was then added (pfu =
1011). The mixture was agitated gently for 30 min, and 20 ~L
of 100 mM NaBH4 was then added. The solution was immediately
52
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
vortexed and placed on a tumbler for 30 min. The final
solution was yellow in color.
Peptide-Mediated Nucleation of Feet. Feet was prepared in
a similar fashion to Copt, except a FeCl2 solution was used in
place of CoCl2.
Peptide-Mediated Nucleation of SmCo5. Identical to Co
synthesis except 100 ~L of 1 mM CoCl2 was replaced with 16.7
~L of 1 mM SmCl3 and 83 ul of 1 mM CoClz.
P8 Expression of Peptides. Genetically modified E. coli
were amplified overnight in 20 mL LB media, diluted 1:100 and
then grown to O.D. - 0.6. Tetracycline-HC1 (1000x) and 100mM
IPTG was added to a final concentration of lmM. The IPTG
triggers the production of the modified p8 protein within the
cell for their incorporation into the viral coat during
assembly. The mixture is allowed to rest for 1 hour without
shaking. Infection by the helper phage after 1 hour is then
followed by shaking overnight at 39 degrees Centigrade. Phage
are then separated and purified by centrifugation and PEG
precipitation. The amplified phage pellet is resuspended into
10 mL of TBS (pH 7.5) and dialyzed in 18 MW water. 0.5 mL of
both 5mM CoCl2 and 5mM HZPtCI6 is added to 1 mL of amplified
phage stock which has been spun down and the supernatant
removed. This is allowed to shake for 60 minutes, after which
0.5 mL of 100mM NaBH4 is added as a reducing agent.
TEM images of the nanoparticles were taken along with the
selected area electron diffraction pattern that showed many
bands corresponding to the expected values for the Copt
facets. An STEM image of one of these phage with Copt
nanoparticle grown along its P8 proteins was also taken. The
53
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
length of this structure correlates to the length of a phage
(800 nm). FIGURE 14A depicts the TEM image of the
nanoparticles, and FIGURE 14B the resolution image with the
selected area electron diffraction pattern (FIGURE 14C)
showing many bands corresponding to the expected values for
the Copt facets. The STEM image of one of these phage with
Copt nanoparticles grown along its P8 proteins is shown in
FIGURE 14D. The EDS mapping for Pt (FIGURE 14E) and Co
(FIGURE 14F) indicate that Co and Pt are both found along the
length of the structure in equal concentrations.
The present invention illustrates phage display may be
used to identify peptides that bind to magnetic materials. The
identification is rapid and cost-effective and requires few
additional materials. These peptides may then be used to
control the nucleation of magnetic nanoparticles, granting the
user control over the size, composition, and crystallinity of
the resulting nanoparticles. These peptides allow the
synthesis of nanoparticles under ambient conditions, making
them a desirable alternative to current synthetic strategies.
Phage display libraries and experimental methods for
using them in biopanning are further described, for example,
in the following U.S. patent publications to Belcher et al.:
(1) "Biological Control of Nanoparticle Nucleation, Shape, and
Crystal Phase"; 2003/0068900 published April 10, 2003; (2)
"Nanoscale Ordering of Hybrid Materials Using Genetically
Engineered Mesoscale Virus"; 2003/0073104 published April 17,
2003; (3) "Biological Control of Nanoparticles"; 2003/0113714
published June 19, 2003; and (4) "Molecular Recognition of
Materials"; 2003/0148380 published August 7, 2003.
54
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
Applications of the present invention, including methods
of use, are described in the following references. Use of
superparamagnetic materials in magnetic resonance imaging is
described in, for example, U.S. Patent No. 5,262,176 to
Palmacci et al. (Nov. 16, 1993), including use of colloids and
superparamagnetic metal oxide covered with a polymer, which is
hereby incorporated by reference in its entirety.
Superparamagnetic materials are also described in, for
example, Lee Josephson et al., Bioconjugate Chem., 1999, 10,
186-191, including biocompatible dextran coated
superparamagnetic iron oxide particles derivatized with a
peptide sequence, and is hereby incorporated by reference in
its entirety. Applications include magnetic resonance imaging
and magnetic separations. J. Manuel Perez et al., J. Am.
Chem. Soc., 2003, 125, 10192-10193, describes viral-induced
self-assembly of magnetic nanoparticles for use in magnetic
nanosensors, including MRI, capable of detecting a variety of
targets including nucleic acids and proteins. This reference
is incorporated by reference in its entirety.
Finally, surfaces can be patterned by a variety of
methods known in the art including microlithography and
nanolithography and use of resists and self-assembled
monolayers, including functionalized self-assembled
monolayers.
Although making and using various embodiments of the
present invention are discussed in detail below, it will be
appreciated that the present invention provides many
applicable inventive concepts that can be embodied in a wide
variety of specific contexts. The specific embodiments
discussed herein are merely illustrative of specific ways to
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
make and use the invention, and do not delimit the scope of
the invention.
56
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
SEQUENCE LISTING
<110> Belcher, Angela M.
Reiss, Brian D.
Mao, Chuanbin
Solis, Daniel J.
Aggarwal, Anuj
Sweeney, Roz
<120> PEPTIDE MEDIATED SYNTHESIS OF MAGNETIC NANOPARTICLES
<130> 119927-1060
<140> N/A
<141> 2002-09-18
<150> 60/326,583 '
<151> 2001-10-02
<160> 18
<170> PatentIn version 3.1
<210> 1
<211> 12
<212> PRT
<213> artificial sequence
<220>
<223> peptide
<400> 1
Ala Met Ala Gly Thr Thr Ser Asp Pro Ser Thr Val
1 5 10
<210> 2
<211> 12
<212> PRT
<213> artificial sequence
<220>
<223> peptide
<900> 2
Ala Ala Ser Pro Thr Gln Ser Met Ser Gln Ala Pro
1 5 10
<210> 3
<211> 12
<212> PRT
<213> artificial sequence
<220>
1/5
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
<223> peptide
<900> 3
His Thr His Thr Asn Asn Asp Ser Pro Asn Gln Ala
1 5 10
<210> . 9
<211> 12
<212> PRT
<213> artificial sequence
<220>
<223> peptide
<900> 9
Asp Thr Gln Gly Phe His Ser Arg Ser Ser Ser Ala
1 5 10
<210> 5
<211> 12
<212> PRT
<213> artificial sequence
<220>
<223> peptide
<900> 5
Thr Ser Ser Ser Ala Leu Gln Pro Ala His Ala Trp
1 5 10
<210> 6
<211> 12
<212> PRT '
<213> artificial sequence
<220>
<223> peptide
<900> 6
Ser Glu Ser Ser Pro Ile Ser Leu Asp Tyr Arg Ala
1 5 10
<210> 7
<211> 12
<212> PRT
<213> artificial sequence
<220>
<223> peptide
2/5
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
<900> 7
Ser Thr His Asn Tyr Gln Ile Pro Arg Pro Pro Thr
1 5 10
<210> 8
<211> 12
<212> PRT
<213> artificial sequence
<220>
<223> peptide
<900> 8
His Pro Phe Ser Asn Glu Pro Leu Gln Leu Ser Ser
1 5 10
<210> 9
<211> 12
<212> PRT
<213> artificial sequence
<220>
<223> peptide
<900> 9
Gly Thr Leu Ala Asn Gln Gln Ile Phe Leu Ser Ser
1 5 10
<210> 10
<211> 12
<212> PRT
<213> artificial sequence
<220>
<223> peptide
<400> 10
His Gly Asn Pro Leu Pro Met Thr Pro Phe Pro Gly
1 5 10
<210> 11
<211> 12
<212> PRT
<213> artificial sequence
<220>
<223> peptide
3/5
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
<400> 11
Arg Leu Glu Leu Ala Ile Pro Leu Gln Gly Ser Gly
1 5 10
<210>. 12
<211> 7
<212> PRT
<213> artificial sequence
<220>
<223> 7-Constrained Sequence
<900> 12
Asn Ala Gly Asp His Ala Asn
1 5
<210> 13
<211> 7
<212> PRT
<213> artificial sequence
<220>
<223> 7-Constrained Sequence
<900> 13
Ser Lys Asn Ser Asn Ile Leu
1 5
<210> 14
<211> 7
<212> PRT
<213> artificial sequence
<220>
<223> 7-Constrained Sequence
<400> 19
Thr Lys Pro Ser Val Val Gln
1 5
<210> 15
<211> 12
<212> PRT
<213> artificial sequence
<220>
<223> l2mer Sequence
<900> 15
4/5
CA 02499318 2005-03-17
WO 2004/033488 PCT/US2003/029555
Ala Leu Ser Pro His Ser Ala Pro Leu Thr Leu Tyr
1 5 10
<210> 16
<211> 12
<212> PRT
<213> artificial sequence
<220>
<223> l2mer Sequence
<400> 16
Ser Val Ser Val Gly Met Lys Pro Ser Pro Arg Pro
1 5 10
<210> 17
<211> 12
<212> PRT
<213> artificial sequence
<220>
<223> l2mer Sequence
<900> 17
His Asn Lys His Leu Pro Ser Thr Gln Pro Leu Ala
1 5 10
<210> 18
<211> 12
<212> PRT
<213> artificial sequence
<220>
<223> l2mer Sequence
<400> 18
Trp Asp Pro Tyr Ser His Leu Leu Gln His Pro Gln
1 5 10
5/5