Note: Descriptions are shown in the official language in which they were submitted.
CA 02501204 2005-04-05
WO 2004/033301 PCT/US2003/029477
PROCESS FOR MAKING WATER-SOLUBLE POUCHES
Technical field
The present invention is in the field of pouch manufacture, in particular it
relates to fluid-
containing water-soluble pouches and their use for detergency applications.
Background of the Invention
The use of water-soluble pouches for different applications, especially for
cleaning
applications, has become increasingly popular. Among many other advantages
pouches
avoid the contact of the user with the cleaning composition which may contain
bleach
and/or other irritant substances.
Often the geometry and size of the pouch is determined by its application, for
example in
the case of pouches for use in automatic dishwashing machines, the geometry
and size of
the pouch may be determined among other things by the shape of the detergent
dispenser
compartment.
One of the most efficient ways of producing pouches is using a horizontal-
forming
process. One of the drawbacks of pouches made according to this process,
especially
fluid-containing pouches, is that the pouch inevitably contains certain amount
of air. This
air takes part of the space that otherwise would be occupied by active fluid.
The problem
is more acute in the case of shallow pouches, i.e., pouches with large base to
height ratio
and in the case of pouches sealed by heat sealing. In view of this discussion,
there is the
need of minimizing the amount of air entrained in horizontal-formed fluid-
containing
pouches, especially in shallow heat sealed pouches.
The heat-sealing process requires maintenance of the sealing area in a dry
condition.
Contamination of the sealing area can translate into a weak sealing.
Therefore, a
requirement during the sealing process is to maintain the fluid level in the
open pouch at a
certain distance below the sealing area of the pouch. This requirement gives
rise to
pouches having a considerable volume occupied by air as compared with the
volume
CA 02501204 2005-04-05
WO 2004/033301 PCT/US2003/029477
2
occupied by active fluid. As discussed above, this phenomenon is more acute in
case of
shallow pouches.
Summary of the Invention
Applicants have now found that the volume of air contained in a fluid-
containing pouch
of a given shape can be reduced by making the pouch by means of a vacuum-
assisted
process in a mould comprising a cavity and flange, wherein the effective fluid
surface
area during the pouch making process is smaller than the area of the region
defined by the
sealing lines during the pouch making process. The desired final shape of the
pouch can
be achieved by controlling the size of the area created by the sealing lines
and the
strechability of the film material.
Thus according to the first aspect of the invention, there is provided a
vacuum-assisted
process for making a fluid-containing water-soluble pouch comprising the steps
of:
a) placing a first sheet of elastic film material over a horizontal mould
comprising a cavity and a flange;
b) drawing the film material into the cavity with vacuum assistance while
maintaining the film under tension to form an open pouch;
c) filling the open pouch with a volume of fluid to define an effective fluid
surface area Af;
d) closing and sealing the open pouch with a second sheet of film material
along one or more sealing lines, the region bounded by the sealing lines
having an area As; and
e) releasing the vacuum applied to the pouch;
and wherein the volume of fluid relative to that of the cavity and the
position of the
sealing lines relative to the flange are such that Af and AS are in a ratio of
from about
1:1.2 to about 1:5, preferably from about 1:1.6 to about 1:4 and more
preferably from
about 1:1.8 to about 1:3.
The process of the invention requires the assistance of vacuum for drawing the
film into
the mould and stretching the film. The drawing of the film can be additionally
helped by
CA 02501204 2005-04-05
WO 2004/033301 PCT/US2003/029477
3
heating of the film, either outside or inside the mould. Heating seems to
reduce the
formation of wrinkles in the film.
The flange of the mould is considered to be the peripheral horizontal region
surrounding
the cavity in the region of its rim. The "effective fluid surface area, Af" is
the calculated
area of the surface of the fluid in the open pouch under static conditions.
The area of the
region defined by the sealing lines AS is considered to be the area of an
imaginary plane
resting horizontally on the mould flange wherein the sealing lines delimit the
perimeter of
the plane.
In preferred embodiments the first sheet of film material is subjected to a
deformation of
from about 20% to about 55%, preferably from about 30% to about 40% in at
least one
direction during steps b) to d) of the process. Preferably, the first and
second sheets of
film material are similar in size (e.g., their dimensions do not differ more
than 10%,
preferably S%) under static conditions, e.g., when they are not under tension.
When the
pouch is being formed, the first sheet is stretched but preferably the second
one is not or
is deformed to a lesser extent. Therefore, after the pouch is formed and the
vacuum
released, the elastic forces acting on the first sheet are balanced between
the sheets and
equilibrium is established. This process gives rise to pouches having
different geometry
to that of the cavity in which they are formed. The final shape of the pouch
is mainly
determined by the deformation of the first sheet during the pouch making
process, the
shape of the mould cavity and the shape of the sealing lines.
The deformation being defined herein as: (x;/ xf)x 100; wherein x; is the
length of the film
between two opposing points of the sealing lines before the film has been
subjected to the
vacuum and xf is the length of the film between the same two opposing points
of the
sealing lines after the film has been subjected to the vacuum and before the
vacuum has
been released. '
The deformation of the first sheet of film material under vacuum can be
determined by
for example, drawing a grid on the sheet, subjecting the sheet to the vacuum
conditions of
the process and then heating and cooling the sheet. The heating step is such
as to
CA 02501204 2005-04-05
WO 2004/033301 PCT/US2003/029477
4
transform the elastic deformation into plastic deformation and after cooling
the sheet will
maintain the structure. Thus, the deformation of the sheet can be measured by
comparing
the size of the initial grid with that of the final grid.
In order to achieve a robust sealing, especially in the cases in which the
sealing is
performed by means of heat sealing, contamination of the sealing area by the
fluid should
be avoided. On the other hand, the level of the fluid below the flange of the
mould should
be as small as possible in order to reduce the volume of air entrapped into
the pouch. It
has been found that an optimum sealing robustness/volume of entrapped air is
reached
when the open pouch is filled to a height of about 3 mm, preferably about 2 mm
and more
preferably about 1 mm below the flange of the mould.
In a preferred embodiment the mould cavity has cylindrical geometry and the
region
defined by the sealing lines has a parallelepiped geometry, preferably the
footprint (when
the pouch is placed into the dispenser) of the resulting pouch is
substantially rectangular
or square. The majority of dishwashing dispensers have a rectangular or square
geometry, therefore pouches having those shapes are better suited for
dispenser fit.
In a preferred embodiment the pouch includes a plurality of sealing lines and
sealing of
the pouch is intermittently performed, preferably by means of heat sealing, by
for
example sealing firstly two opposite sides of the pouch-to-be followed by
sealing two
other opposite sides. The second sheet is usually bowed to a certain extent
during the
sealing step. Preferably an anti-bowing step takes place during the sealing,
this further
reduces the amount of air entrained in the pouch. The anti-bowing can be
performed by
for example, air means, i.e., a current of air blowing downwards towards the
pouch, or by
weight means, e.g., a bar pushing the second sheet downwards. This bar should
have an
appropriate shape, usually matching the shape of the aperture of the cavity
but slightly
smaller, in order to maximize the amount of air evacuated from the pouch
without
establishing contact between the fluid and the film. Alternatively, the
sealing can be
carried out by means of solvent sealing.
CA 02501204 2005-04-05
WO 2004/033301 PCT/US2003/029477
The fluid contained in the pouches made according to the process of the
invention can be
in the form of a liquid, gel or paste. The fluid can also comprises a solid or
a multitude of
solid inserts, such as for example micro-beads, noodles or one or more
pearlized balls.
According to a second aspect of the invention, there are provided pouches made
by the
process of the invention. In a preferred embodiment there is provided a
horizontally-
formed fluid-containing water-soluble pouch having a first and a second sheet
sealed
together (preferably by means of heat sealing) and a base length to height
ratio of from
about 50:1 to about 2:1, preferably from about 20:1 to about 3:1 and more
preferably
from about 10:1 to about 4:1 characterised in that both sheets are stretched
and under
tension and the volume of fluid and the volume of air contained in the pouch
are in a ratio
of from about 1.7:1 to about 8:1, preferably from about 2:1 to about 6:1.
These pouches
are characterised by having a volume of entrapped air smaller than that of
traditional
horizontal formed pouches while presenting a very robust sealing.
The height of the pouch is defined as the maximum longitudinal dimension,
perpendicular
to one of the pouch bases, when the pouch is lying on one of the bases which
has the
maximum footprint under a static load of about 2 Kg. The base length is
defined as the
maximum width of the pouch in a plane perpendicular to the longitudinal
direction under
the same conditions.
In another aspect of the invention, there is also provided a horizontally-
formed fluid-
containing water-soluble pouch having a first and a second sheet sealed
together and a
base length to height ratio of from about 50:1 to about 2:1, preferably from
about 20:1 to
about 3:1 and more preferably from about 10:1 to about 4:1 characterised in
that the
pouch is formed by a process including the step of subjecting the second sheet
to an anti-
bowing step during the sealing of the pouch for purposes of reducing air
entrainment and
the volume of fluid and the volume of air contained in the pouch are in a
ratio of from
about 1.7:1 to about 8:1, preferably from about 2:1 to about 6:1.
The pouches of the invention are also suitable for making mufti-compartment
pouches,
either fluid/fluid or solid/fluid mufti-compartment pouches. The process of
the invention
CA 02501204 2005-04-05
WO 2004/033301 PCT/US2003/029477
6
can be used to make only one compartment or alternatively it can be used for
making the
whole mufti-compartment pouch. Mufti-compartment pouches are preferably made
by
forming and filling a first open pouch and closing this open pouch with a
second pre-
formed pouch. This process is described in WO 02/42408. Especially suitable
for use in
dishwashing are powder/liquid pouches.
There are also provided methods for using the pouches of the invention for
laundry and
dishwashing. The pouches can be placed either in the detergent dispenser or in
the
interior of the machine. In the case of dishwashing, the pouches are
preferably placed
into the dispenser.
Detailed descn~tion of the invention
The present invention envisages a horizontal process for making fluid-
containing water-
soluble pouches. The pouches produced according to the process of the
invention contain
an air volume lower than that contained in pouches made according to
traditional
processes. This is especially advantageous in pouches having a high base
length to height
ratio, i.e., shallow pouches. The present invention also envisages the use of
these
pouches for dishwashing and laundry.
The pouches of the invention can comprise any liquid composition compatible
with the
pouch material. These pouches are especially useful in the field of
detergency.
The pouch is preferably made of a material which is soluble or dispersible in
water, and
has a water-solubility of at least 50%, preferably at least 75% or even at
least 95%, as
measured by the method set out here after using a glass-filter with a maximum
pore size
of 20 microns.
50 grams ~ 0.1 gram of capsule or pouch material is added in a pre-weighed 400
ml
beaker and 245m1 ~ lml of distilled water is added. This is stirred vigorously
on a
magnetic stirrer set at 600 rpm, for 30 minutes. Then, the mixture is filtered
through a
qualitative sintered-glass filter with a pore size as defined above (max. 20
micron). The
water is dried off from the collected filtrate by any conventional method, and
the weight
CA 02501204 2005-04-05
WO 2004/033301 PCT/US2003/029477
7
of the remaining material is determined (which is the dissolved or dispersed
fraction).
Then, the % solubility or dispersability can be calculated.
Preferred pouch materials are polymeric materials, preferably polymers which
are formed
into a film or sheet. The pouch material can, for example, be obtained by
casting, blow-
moulding, extrusion or blown extrusion of the polymeric material, as known in
the art.
Preferred polymers, copolymers or derivatives thereof suitable for use as
pouch material
are selected from polyvinyl alcohols, polyvinyl pyrrolidone, polyalkylene
oxides,
acrylamide, acrylic acid, cellulose, cellulose ethers, cellulose esters,
cellulose amides,
polyvinyl acetates, polycarboxylic acids and salts, polyaminoacids or
peptides,
polyamides, polyacrylamide, copolymers of maleic/acrylic acids,
polysaccharides
including starch and gelatine, natural gums such as xanthum and carragum. More
preferred polymers are selected from polyacrylates and water-soluble acrylate
copolymers, methylcellulose, carboxymethylcellulose sodium, dextrin,
ethylcellulose,
hydroxyethyl cellulose, hydroxypropyl methylcellulose, maltodextrin,
polymethacrylates,
and most preferably selected from polyvinyl alcohols, polyvinyl alcohol
copolymers and
hydroxypropyl methyl cellulose (HPMC), and combinations thereof. Preferably,
the level
of polymer in the pouch material, for example a PVA polymer, is at least 60%.
Mixtures of polymers can also be used. This may in particular be beneficial to
control the
mechanical andJor dissolution properties of the compartment or pouch,
depending on the
application thereof and the required needs. For example, it may be preferred
that a
mixture of polymers is present in the material of the compartment, whereby one
polymer
material has a higher water-solubility than another polymer material, and/or
one polymer
material has a higher mechanical strength than another polymer material. It
may be
preferred that a mixture of polymers is used, having different weight average
molecular
weights, for example a mixture of PVA or a copolymer thereof of a weight
average
molecular weight of 10,000-40,000, preferably around 20,000, and of PVA or
copolymer
thereof, with a weight average molecular weight of about 100,000 to 300,000,
preferably
around 150,000.
CA 02501204 2005-04-05
WO 2004/033301 PCT/US2003/029477
8
Also useful are polymer blend compositions, for example comprising
hydrolytically
degradable and water-soluble polymer blend such as polylactide and polyvinyl
alcohol,
achieved by the mixing of polylactide and polyvinyl alcohol, typically
comprising 1-35%
by weight polylactide and approximately from 65% to 99% by weight polyvinyl
alcohol,
if the material is to be water-dispersible, or water-soluble. It may be
preferred that the
PVA present in the film is from 60-98% hydrolysed, preferably 80% to 90%, to
improve
the dissolution of the material.
Most preferred pouch and wrap materials are P~VA films known under the trade
reference
Monosol M8630, as sold by Chris-Craft Industrial Products of Gary, Indiana,
US, and
PVA films of corresponding solubility and deformability characteristics. Other
films
suitable for use herein include films known under the trade reference PT film
or the K-
series of films supplied by Aicello, or VF-HP film supplied by Kuraray.
The water-soluble film herein may comprise other additive ingredients than the
polymer
or polymer material. For example, it may be beneficial to add plasticisers,
for example
glycerol, ethylene glycol, diethyleneglycol, propylene glycol, sorbitol and
mixtures
thereof, additional water, disintegrating aids. It may be useful that the
pouch or water-
soluble film itself comprises a detergent additive to be delivered to the wash
water, for
example organic polymeric soil release agents, dispersants, dye transfer
inhibitors.
The pouches of the invention preferably comprise detergent auxiliaries or
compositions.
These detergent auxiliaries or compositions can comprise traditional
detergency
components and can also comprise organic solvents having a cleaning function
and
organic solvents having a carrier or diluent function or some other
specialised function.
The compositions will generally be built and comprise one or more detergent
active
components which may be selected from bleaching agents, surfactants,
alkalinity sources,
enzymes, thickeners (in the case of liquid, paste, cream or gel compositions)
and anti-
corrosion agents (e.g. sodium silicate). Highly preferred detergent components
include a
builder compound, an alkalinity source, a surfactant, an enzyme and a
bleaching agent.
CA 02501204 2005-04-05
WO 2004/033301 PCT/US2003/029477
9
The organic solvents should he selected so as to be compatible with the
tableware/cookware as well as with the different parts of an automatic
dishwashing
machine. Furthermore, the solvent system should be effective and safe to use
having a
volatile organic content above 1 mm Hg (and preferably above 0.1 mm Hg) of
less than
about 50%, preferably less than about 30%, more preferably less than about 10%
by
weight of the solvent system. Also they should have very mild pleasant odours.
The
individual organic solvents used herein generally have a boiling point above
about 150°C,
flash point above about 100°C and vapor pressure below about 1 mm Hg,
preferably
below 0.1 mm Hg at 25°C and atmospheric pressure.
Solvents that can be used herein include: i) alcohols, such as benzyl alcohol,
1,4-
cyclohexanedimethanol, 2-ethyl-1-hexanol, furfuryl alcohol, 1,2-hexanediol and
other
similar materials; ii) amines, such as alkanolamines (e.g. primary
alkanolamines:
monoethanolamine, monoisopropanolamine, diethylethanolamine, ethyl
diethanolamine;
secondary alkanolamines: diethanolamine, diisopropanolamine, 2-
(methylamino)ethanol;
ternary alkanolamines: triethanolamine, triisopropanolamine); alkylamines
(e.g. primary
alkylamines: monomethylamine, monoethylamine, monopropylarnine,
monobutylamine,
monopentylamine, cyclohexylamine), secondary alkylamines: (dimethylamine),
alkylene
amines (primary alkylene amines: ethylenediamine, propylenediamine) and other
similar
materials; iii) esters, such as ethyl lactate, methyl ester, ethyl
acetoacetate, ethylene glycol
monobutyl ether acetate, diethylene glycol monoethyl ether acetate, diethylene
glycol
monobutyl ether acetate and other similar materials; iv) glycol ethers, such
as ethylene
glycol monobutyl ether, diethylene glycol monobutyl ether, ethylene glycol
monomethyl
ether, ethylene glycol monoethyl ether, diethylene glycol monomethyl ether,
diethylene
glycol monoethyl ether, propylene glycol butyl ether and other similar
materials; v)
glycols, such as propylene glycol, diethylene glycol, hexylene glycol (2-
methyl-2, 4
pentanediol), triethylene glycol, composition and dipropylene glycol and other
similar
materials; and mixtures thereof.
Surfactant
Surfactants suitable herein include anionic surfactants such as alkyl
sulfates, alkyl ether
sulfates, alkyl benzene sulfonates, alkyl glyceryl sulfonates, alkyl and
alkenyl
CA 02501204 2005-04-05
WO 2004/033301 PCT/US2003/029477
sulphonates, alkyl ethoxy carboxylates, N-acyl sarcosinates, N-acyl taurates
and alkyl
succinates and sulfosuccinates, wherein the alkyl, alkenyl or acyl moiety is
C5-C20,
preferably C l 0-C 1 g linear or branched; cationic surfactants such as
chlorine esters (US-
A-4228042, US-A-4239660 and US-A-4260529) and mono C6-C16 N-alkyl or alkenyl
ammonium surfactants wherein the remaining N positions are substituted by
methyl,
hydroxyethyl or hydroxypropyl groups; low and high cloud point nonionic
surfactants
and mixtures thereof including nonionic alkoxylated surfactants (especially
ethoxylates
derived from C6-Clg primary alcohols), ethoxylated-propoxylated alcohols
(e.g., BASF
Poly-Tergent~ SLF18), epoxy-capped poly(oxyalkylated) alcohols (e.g., BASF
Poly-
Tergent~ SLF18B - see WO-A-94/22800), ether-capped poly(oxyalkylated) alcohol
surfactants, and block polyoxyethylene-polyoxypropylene polymeric compounds
such as
PLURONIC~, REVERSED PLURONIC~, and TETRONIC~ by the BASF-Wyandotte
Corp., Wyandotte, Michigan; amphoteric surfactants such as the C1z-CZO alkyl
amine
oxides (preferred amine oxides for use herein include C~Z lauryldimethyl amine
oxide, C~4
and C,6 hexadecyl dimethyl amine oxide), and alkyl amphocarboxylic surfactants
such as
MiranolTM C2M; and zwitterionic surfactants such as the betaines and
sultaines; and
mixtures thereof. Surfactants suitable herein are disclosed, for example, in
US-A-
3,929,678 , US-A- 4,259,217, EP-A-0414 549, WO-A-93/08876 and WO-A-93/08874.
Surfactants are typically present at a level of from about 0.2% to about 30%
by weight,
more preferably from about 0.5% to about 10% by weight, most preferably from
about
1% to about 5% by weight of composition. Preferred surfactant for use herein
are low
foaming and include low cloud point nonionic surfactants and mixtures of
higher foaming
surfactants with low cloud point nonionic surfactants which act as suds
suppresser
therefor.
Builder
Builders suitable for use herein include water-soluble builders such as
citrates, carbonates
and polyphosphates e.g. sodium tripolyphosphate and sodium tripolyphosphate
hexahydrate, potassium tripolyphosphate and mixed sodium and potassium
tripolyphosphate salts; and partially water-soluble or insoluble builders such
as crystalline
layered silicates (EP-A-0164514 and EP-A-0293640) and aluminosilicates
inclusive of
CA 02501204 2005-04-05
WO 2004/033301 PCT/US2003/029477
11
Zeolites A, B, P, X, HS and MAP. The builder is typically present at a level
of from
about 1 % to about 80% by weight, preferably from about 10% to about 70% by
weight,
most preferably from about 20% to about 60% by weight of composition.
Amorphous sodium silicates having an Si02:Na20 ratio of from 1.8 to 3.0,
preferably
from 1.8 to 2.4, most preferably 2.0 can also be used herein although highly
preferred
from the viewpoint of long term storage stability are compositions containing
less than
about 22%, preferably less than about 15% total (amorphous and crystalline)
silicate.
Enzyme
Enzymes suitable herein include bacterial and fungal cellulases such as
Carezyme and
Celluzyme (Novo Nordisk A/S); peroxidases; lipases such as Amano-P (Amano
Pharmaceutical Co.), M1 LipaseR and LipomaxR (Gist-Brocades) and LipolaseR and
Lipolase UltraR (Novo); cutinases; proteases such as EsperaseR, AlcalaseR,
DurazymR and
SavinaseR (Novo) and MaxataseR, MaxacalR, ProperaseR and MaxapemR (Gist-
Brocades);
a and (3 amylases such as Purafect Ox AmR (Genencor) and TermamylR, Bang,
FungamylR, DuramylR, and NatalaseR (Novo); pectinases; and mixtures thereof.
Enzymes
are preferably added herein as prills, granulates, or cogranulates at levels
typically in the
range from about 0.0001 % to about 2% pure enzyme by weight of composition.
Bleaching agent
Bleaching agents suitable herein include chlorine and oxygen bleaches,
especially
inorganic perhydrate salts such as sodium perborate mono-and tetrahydrates and
sodium
percarbonate optionally coated to provide controlled rate of release (see, for
example,
GB-A-1466799 on sulfate/carbonate coatings), preformed organic peroxyacids and
mixtures thereof with organic peroxyacid bleach precursors and/or transition
metal-
containing bleach catalysts (especially manganese or cobalt). Inorganic
perhydrate salts
are typically incorporated at levels in the range from about 1 % to about 40%
by weight,
preferably from about 2% to about 30% by weight and more preferably from abut
5% to
about 25% by weight of composition. Peroxyacid bleach precursors preferred for
use
herein include precursors of perbenzoic acid and substituted perbenzoic acid;
cationic
peroxyacid precursors; peracetic acid precursors such as TAED, sodium
acetoxybenzene
CA 02501204 2005-04-05
WO 2004/033301 PCT/US2003/029477
12
sulfonate and pentaacetylglucose; pernonanoic acid precursors such as sodium
3,5,5-
trimethylhexanoyloxybenzene sulfonate (iso-NOBS) and sodium nonanoyloxybenzene
sulfonate (HOBS); amide substituted alkyl peroxyacid precursors (EP-A-
0170386); and
benzoxazin peroxyacid precursors (EP-A-0332294 and EP-A-0482807). Bleach
precursors are typically incorporated at levels in the range from about 0.5%
to about 25%,
preferably from about 1 % to about 10% by weight of composition while the
preformed
organic peroxyacids themselves are typically incorporated at levels in the
range from
0.5% to 25% by weight, more preferably from 1% to 10% by weight of
composition.
Bleach catalysts preferred for use herein include the manganese
triazacyclononane and
related complexes (US-A-4246612, US-A-5227084); Co, Cu, Mn and Fe
bispyridylamine
and related complexes (US-A-5114611); and pentamine acetate cobalt(III) and
related
complexes(US-A-4810410).
Low cloud point non-ionic surfactants and suds suppressers
The suds suppressers suitable for use herein include nonionic surfactants
having a low
cloud point. "Cloud point", as used herein, is a well known property of
nonionic
surfactants which is the result of the surfactant becoming less soluble with
increasing
temperature, the temperature at which the appearance of a second phase is
observable is
referred to as the "cloud point" (See Kirk Othmer, pp. 360-362). As used
herein, a "low
cloud point" nonionic surfactant is defined as a nonionic surfactant system
ingredient
having a cloud point of less than 30° C., preferably less than about
20° C., and even more
preferably less than about 10° C., and most preferably less than about
7.5° C. Typical
low cloud point nonionic surfactants include nonionic alkoxylated surfactants,
especially
ethoxylates derived from primary alcohol, and
polyoxypropylene/polyoxyethylene/polyoxypropylene (PO/EO/PO) reverse block
polymers. Also, such low cloud point nonionic surfactants include, for
example,
ethoxylated-propoxylated alcohol (e.g., BASF Poly-Tergent~ SLF18) and epoxy-
capped
poly(oxyalkylated) alcohols (e.g., BASF Poly-Tergent~ SLF18B series of
nonionics, as
described, for example, in US-A-5,576,281).
Preferred low cloud point surfactants are the ether-capped poly(oxyalkylated)
suds
suppresser having the formula:
CA 02501204 2005-04-05
WO 2004/033301 PCT/US2003/029477
13
R~ ~-~CHZ - i H -O)X - ~CH2 -CH2 -O~ - ~CH2 - CH'-O)Z-H
R2 R3
wherein R' is a linear, alkyl hydrocarbon having an average of from about 7 to
about 12
carbon atoms, Rz is a linear, alkyl hydrocarbon of about 1 to about 4 carbon
atoms, R3 is a
linear, alkyl hydrocarbon of about 1 to about 4 carbon atoms, x is an integer
of about 1 to
about 6, y is an integer of about 4 to about 15, and z is an integer of about
4 to about 25.
Other low cloud point nonionic surfactants are the ether-capped
poly(oxyalkylated)
having the formula:
RIO(RIIO)nCH(CH3)ORIII
wherein, RI is selected from the group consisting of linear or branched,
saturated or
unsaturated, substituted or unsubstituted, aliphatic or aromatic hydrocarbon
radicals
having from about 7 to about 12 carbon atoms; RII may be the same or
different, and is
independently selected from the group consisting of branched or linear CZ to
C~ alkylene
in any given molecule; n is a number from 1 to about 30; and RI,~ is selected
from the
group consisting of:
(i) a 4 to 8 membered substituted, or unsubstituted heterocyclic ring
containing
from 1 to 3 hetero atoms; and
(ii) linear or branched, saturated or unsaturated, substituted or
unsubstituted,
cyclic or acyclic, aliphatic or aromatic hydrocarbon radicals having from
about 1 to about 30 carbon atoms;
(b) provided that when RZ is (ii) then either: (A) at least one of RI is other
than Cz
to C3 alkylene; or (B) RZ has from 6 to 30 carbon atoms, and with the further
proviso that when RZ has from 8 to 18 carbon atoms, R is other than C1 to CS
alkyl.
Other suitable components herein include organic polymers having dispersant,
anti-
redeposition, soil release or other detergency properties invention in levels
of from about
CA 02501204 2005-04-05
WO 2004/033301 PCT/US2003/029477
14
0.1% to about 30%, preferably from about 0.5% to about 15%, most preferably
from
about 1% to about 10% by weight of composition. Preferred anti-redeposition
polymers
herein include acrylic acid containing polymers such as Sokalan PA30, PA20,
PA15,
PA10 and Sokalan CP10 (BASF GmbH), Acusol 45N, 480N, 460N (Rohm and Haas),
acrylic acid/maleic acid copolymers such as Sokalan CPS and
acrylic/methacrylic
copolymers. Preferred soil release polymers herein include alkyl and
hydroxyalkyl
celluloses (US-A-4,000,093), polyoxyethylenes, polyoxypropylenes and
copolymers
thereof, and nonionic and anionic polymers based on terephthalate esters of
ethylene
glycol, propylene glycol and mixtures thereof.
Heavy metal sequestrants and crystal growth inhibitors are suitable for use
herein in
levels generally from about 0.005% to about 20%, preferably from about 0.1% to
about
10%, more preferably from about 0.25% to about 7.5% and most preferably from
about
0.5% to about 5% by weight of composition, for example diethylenetriamine
penta
(methylene phosphonate), ethylenediamine tetra(methylene phosphonate)
hexamethylenediamine tetra(methylene phosphonate), ethylene diphosphonate,
hydroxy-
ethylene-l,l-diphosphonate, nitrilotriacetate, ethylenediaminotetracetate,
ethylenediamine-N,N'-disuccinate in their salt and free acid forms.
The compositions herein can contain a corrosion inhibitor such as organic
silver coating
agents in levels of from about 0.05% to about 10%, preferably from about 0.1%
to about
5% by weight of composition (especially paraffins such as Winog 70 sold by
Wintershall,
Salzbergen, Germany), nitrogen-containing corrosion inhibitor compounds (for
example
benzotriazole and benzimadazole - see GB-A-1137741) and Mn(II) compounds,
particularly Mn(II) salts of organic ligands in levels of from about 0.005% to
about 5%,
preferably from about 0.01 % to about 1 %, more preferably from about 0.02% to
about
0.4% by weight of the composition.
Other suitable components herein include colorants, water-soluble bismuth
compounds
such as bismuth acetate and bismuth citrate at levels of from about 0.01% to
about 5%,
enzyme stabilizers such as calcium ion, boric acid, propylene glycol and
chlorine bleach
scavengers at levels of from about 0.01 % to about 6%, lime soap dispersants
(see WO-A-
CA 02501204 2005-04-05
WO 2004/033301 PCT/US2003/029477
93/08877), suds suppressors (see WO-93/08876 and EP-A-0705324), polymeric dye
transfer inhibiting agents, optical brighteners, perfumes, fillers and clay.
Liquid detergent compositions suitable for use in the pouches of the invention
can also
containe low quantities of low molecular weight primary or secondary alcohols
such as
methanol, ethanol, propanol and isopropanol. Other suitable carrier solvents
used in low
quantities includes glycerol, propylene glycol, ethylene glycol, 1,2-
propanediol, sorbitol
and mixtures thereof.
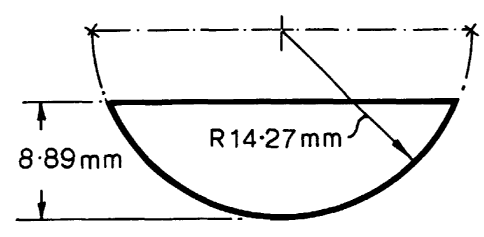
Example 1
Monosol M8630 (76 p.m thick), PVA film, supplied by Chris Craft Inc, Gary,
Indiana,
USA, is used for making the pouch. The film is delivered to the mould and
anchored by
means of a belt. The mould comprises a hemi-spherical cavity cut with a
spherical drill
of 14.27 mm radius to a depth of 8.89 mm (as shown in Figure 1). The film is
drawn into
the mould by means of vacuum. The vacuum is applied to the film in two stages.
Initially a high vacuum of approximately 800 mbar is applied to the film to
form the open
pouch followed by the application of a low vacuum of approximately 300 mbar to
hold
the film in the cavity. The deformation of the film under these conditions is
28%. 1.9 ml
of detergent product is dosed into the open pouch leaving 0.9 ml of head
space. A second
sheet is placed over the open pouch and sealed in two steps by means of two
parallel seal
bars (2 mm wide). The first step is performed by seal bars having a centre-to-
centre pitch
of 38 mm and the second step is performed by seal bars perpendicular to the
bars of the
first step and having a centre-to-centre pitch of 34 mm.
Example 2
Example 1 is repeated with the addition of an anti-bowing step during the
second sealing
step. During this step a finger located between the seal bars pushes out some
of the air in
the head space before closing the pouch. This contributes to a further
reduction of the
volume of air entrapped into the pouch.