Note: Descriptions are shown in the official language in which they were submitted.
CA 02501253 2005-03-18
AppIicstion number. num~ro d~ d~:nards: ~ ~ a 3
Fi'rures :~
Pa~_es: ~ ~,
Unscannable items
re~ei~Ted with this application
(Request orz~inal documents in File Prep. Section on the 10th Floor)
Dowaments recus avec cette demande ne pouvant ~tre balayes
(Commander les documents ori~inau~ Bans 1a section de preparation des dossiers
au
l0ie:ne etasej
. r
CA 02501253 2005-03-18
Means and methods for individualized drug therapy and
for predicting adverse drug reaction
Field of the invention
The present invention relates to the individualization of therapy. More
specifically, the
present invention relates to systems and the use of genotyping aiming at the
individualization
of therapy and/or individualization of drug dosing. with a therapeutic agent
or a class of
therapeutic agents in accordance with a novel method, which is particularly
designed for the
identification and medication of populations of patients who have a genetic
predisposition to
cardiac disorders. In particular, the present invention relates to a method of
determining the
genetic predisposition of a subject to be at risk for a cardiac disease or
dysfunction
comprising assaying a sample of a subject for the presence of a variant allele
of at least one
gene selected from the group consisting of genes involved in (a) the
generation of reactive
oxygen species (ROS) or (b) drug transmembrane transport; wherein the presence
of the
variant allele is considered indicative of a higher risk for cardiotoxicity
compared to a control.
The present invention further concerns methods for the treatment of a disease
comprising
administering to a subject in need of such treatment a non-cardiotoxic amount
of a drug or a
non-cardiotoxic analog or substitute thereof, wherein said subject carries at
least one of said
variant alleles.
Background of the invention
Anthracyclines are well established as highly efficacious antineoplastic
agents for various
haematopoietic and solid tumors. A dose-response relationship has been
demonstrated for
anthracyclines and some tumors, with lower doses resulting in decreased
survival and
remission rates (Shan et al., Ann. Intern. Med. 125 (1996), 47-58). On the
other hand, dose
escalation results in a dose-dependent cardiotoxicity (Von Hoff et al., Ann.
Intern. Med. 91
(1979), 710-717). Two distinct types of anthracycline-induced cardiotoxicity
(ACT) have
been defined. Acute ACT occurs during the treatment, often immediately after
the first dose,
and manifests itself predominantly in the form of arrhythmias, rarely also as
pericarditismyocarditis or acute left ventricular failure. Chronic ACT
presents within one year
after anthracycline administration as congestive heart failure. In addition,
recent sri~dies
postulate a distinct late-onset cardiotoxicity, which develops after years or
even decades of
asymptomatic survival. This form may well be the most frequent one, which,
however,
CA 02501253 2005-03-18
2
remains to be confirmed in additional studies (Shan et al., Ann. Intern. Med.
125 (199G),
47-58). The causal relationship between the individual ACT forms is unclear.
The incidence of ACT depends on the cumulative dose of the drug. For
doxorubicin in bolus-
injected patients it is 0.14 % at doses below 400 mg/m2 and raises from 7 % at
550 mg/m2 to
18 % at 700 mg/m2 (Von Hoff et al., Ann. Intern. Med. 91 (1979), 710-717). The
steep
increase in cardiotoxicity at doses higher than 550 mg/m2 has led to the
commonly applied
upper dosing limit of S50 mg/m2. Switching from bolus to prolonged intravenous
infusion
(Legha et al., Ann. Intern. Med. 96 (1982), 133-139) has reduced the incidence
of
doxorubicin-induced cardiotoxicity (Zucchi and Danesi, Curr. Med. Chem. Anti-
Canc. Agents
3 (2003), 151-171).
Among many factors proposed, only cumulative anthracycline dose and previous
irradiation
with cardiac involvement have been confirmed as independent risk factors for
ACT (Torti et
al., Ann. Intern. Med. 99 (1983), ?45-749). On the other hand, there is
increasing evidence
that the genetic makeup is a major determinant of drug response and toxicity.
Regarding
chronic ACT, there is ample evidence from transgenic mouse models to support
this
hypothesis. For example, overexpression of the multiple drug resistance gene
MDR1 protects
the heart from the toxic effect of doxorubicin (Dell' Acqua et al., Hum. Gene
Ther. 10 ( 1999),
1269-1279). In humans, the presence of a genetic component is suggested by the
wide
variation in the individual sensitivity to anthracyclines. Thus, doxorubicin
doses exceeding
1000 mg/m2 are tolerated by some patients (Henderson et al., J. Clin. Oncol. 7
(1989),
560-571 ), whereas others develop ACT after less than 200 mg/m2. However,
nothing is
known about the identity of genes and variants underlying this variability.
This knowledge
may not only help to develop individualized therapies, but may also help to
understand drug
induced cardiotoxicity and the pathophysiology of acute and chronic congestive
heart failure
in general.
Thus, there is a need for diagnostic tests which would allow the detection and
pre-selection of
patients who are at high risk for cardiotoxicity, in particular in response to
drug therapy and
generally therapeutic intervention.
The solution to said technical problem is achieved by providing the
embodiments
characterized in the claims, and described further below.
CA 02501253 2005-03-18
3
Summary of the invention
The present invention is directed to a system aiming at the individualization
of therapy and/or
individualization of drug dosing method including determining the genetic
predisposition of a
subject to be at risk for a cardiac disease or dysfunction. In particular, the
method of the
present invention comprises assaying a sample of a subject for the presence of
a variant allele
of at least one gene selected from the group consisting of genes involved in
(a) the generation
of reactive oxygen species (ROS) or (b) drug transmembrane transport; wherein
the presence
of the variant allele is considered indicative of a higher risk for
cardiotoxicity compared to a
control.
Furthermore, the present invention relates to a method for the treatment of
cancer comprising
administering to a subject in need of such treatment a non-cardiotoxic amount
of an
anthracycline or a non-cardiotoxic analog or substitute thereof, wherein said
subject carnes at
1 S least one variant allele in a gene involved in the NAD(P)H oxidase mufti-
enzyme complex or
encoding multidrug resistance-associated protein 1 (MRP1) or multidrug
resistance-associated
protein 2 (MRP2) as well as to a method for the treatment of cancer comprising
administering
to a subject in need of such treatment an anthracycline, wherein said subject
does not carry
any one of said variant alleles. Preferably, a sample of the subject to be
treated has been
assayed in accordance with the method of the present invention. The use of the
anthracycline
in accordance with the method of the present invention may be accompanied by
the use of
further therapeutic agents such as cardioprotective and/or other
antineoplastic agents
It is thus also an object of the present invention to provide a pharmaceutical
composition
comprising an anthracycline and an agonisbactivator of a gene or gene product
involved in
the NAD(P)H oxidase mufti-enzyme complex or transmembrane transport of
multidrug
resistance-associated protein 1 (MRP1) or multidrug resistance-associated
protein 2 (MRP2).
It is another object of the present invention to provide oligonucleotides,
e.g. in form of a
primer or probe, for use in determining the presence of variant allele in a
gene involved in the
NAD(P)H oxidase mufti-enzyme complex or encoding multidrug resistance-
associated
protein 1 (MRP1) or multidrug resistance-associated protein 2 (MRP2) for
diagnosing a
higher risk for cardiotoxicity, especially anthracycline induced
cardiotoxicity. Usually, said
oligonucleotides comprise at least 10, preferably at least 15 nucleotides,
more preferably at
CA 02501253 2005-03-18
4
least 20 nucleotides in length while a hybridizing polynucleotide to be used
as a probe usually
comprises at least 50, preferably at least 100, more preferably at least 200,
or most preferably
at least 500 nucleotides in length. Also comprised in accordance with the
method of the
invention are hybridizing oligo- and polynucleotides which are useful for
analyzing DNA-
protein interactions via, e.g., electrophoretic mobility shift analysis
(EMSA).
The present invention also concerns a kit for use in a method of the present
invention
comprising poly- or oligonucleotides, a chip or array, reference samples,
amplification and/or
sequencing means, buffer, detergents, biochemical regents, detection means, or
the like, and
optionally a reformulated drug with a drug dose which is tailored to a patent
carrying a variant
allele as defined herein.
For the purpose of the present invention the following terms are defined
below.
1 S The term "individualization" as it appears herein with respect to therapy
is intended to mean a
therapy having specificity to at least an individual's phenotype as calculated
according to a
predetermined formula on an individual basis.
The term "sample" is intended to mean a sample usually obtained from a
biological entity and
includes, but is not to be limited to, any one of the following: tissue,
cerebrospinal fluid,
plasma, serum, saliva, blood, cells like peripheral blood lymphocytes, nasal
mucosa, urine,
synovial fluid, tumor sample, microcapillary, microdialysis and breath.
The term "therapeutic agent" or "drug" are used interchangeably herein and are
intended to
mean an agent (s) and/or medicine (s) used to treat the symptoms of a disease,
physical or
mental condition, injury or infection.
The term "treatment" is intended to mean any administration of a
pharmaceutical compound
to an individual to treat, cure, alleviate, improve, diminish or inhibit a
disease, physical or
mental condition, injury or infection in the individual.
The term "individual treated" is intended to mean any individual being
subjected to the
administration of (i) a pharmaceutical compound, for treating, curing,
alleviating, improving,
CA 02501253 2005-03-18
diminishing or inhibiting a disease, physical or mental condition, injury or
infection, or (ii) a
probe substrate for determining multi-determinant metabolic phenotype.
The term "dosage" includes the size, frequency, formulation, co-medication and
number of
doses of at least one therapy to be given to a patient. This also includes
newly prescribed
therapies and/or therapies, both singly and in combination and is irrespective
of the way of
administration.
The term "patient" or "subject" includes any organism, particularly a human or
other
mammal, suffering from a disease, in need or desire of treatment for a
disease, or in need of
testing or screening for a disease. A patient includes any mammal, including
farm animals or
pets, and includes humans of any age or state of development.
The term "therapeutic agent regime" or "dosage regimen" is the course of
action or use of a
therapeutic agent or drug, or combination of therapeutic agents or drugs in
treating a patient
including, for example, at least one of dosage, schedule of administration,
choice and/or
combination of therapeutic agents.
As used herein, the terms "nucleic acid", "polynucleotide", or "nucleic acid
sequence" refer to
a polymer of deoxyribonucleotides or ribonucleotides, in the form of a
separate fragment or as
a component of a larger construct. Polynucleotide or nucleic acid sequences of
the invention
include DNA, RNA, including mRNA and cDNA sequences. The polynucleotides of
the
sample of the present invention are typically genomic DNA or RNA.
As used herein, the terms "polypeptide" and "protein" are used interchangeably
herein and
refer to a polymer of amino acid residues in the form of a separate fragment
or component of
a larger construct. A polypeptide may encode for a functional protein or
fragments of a
protein.
In the context of the present invention the term "variant allele" of the given
target gene
including, nucleotide and amino acid sequences, respectively, as used herein
means that the
nucleotide, and optionally amino acid sequence of said variant allele differs
from the wild
type nucleotide and amino acid sequences by way of nucleotide, and optionally
amino acid
substitution(s), additions) and/or deletion(s). Genomic, cDNA and marker
sequences of
CA 02501253 2005-03-18
6
various target genes including those specifically described herein can be
retrieved from, for
example Genbank, http://www.ncbi.nim.nih.gov/Genbank/GenbankOverview.html and
EMBL Bioinformatics. Reference or wild type sequences for the NCF4, CYBA,
RAC2,
MRP1 and MRP2 polynucleotides are for example the GeneBank accession Nos.
given in
Table 2.
Unless stated otherwise the term "variant allele" and "allelic variant" are
used interchangeably
herein. Furthermore, in the following, said genes involved in (a) the
generation of reactive
oxygen species (ROS) or (b) drug transmembrane transport may also be
designated "target
genes". Likewise, any one of said genes in the generation of ROS or drug
transmembrane
transport may be designated "target gene". At some places the terms "target
sequence" or
"target protein" may be used synonymously.
The term "corresponding" as used herein means that a position is not only
determined by the
number of the preceding nucleotides and amino acids, respectively. The
position of a given
nucleotide or amino acid in accordance with the use of the present invention
which may be
deleted, substituted or comprise one or more additional nucleotides) may vary
due to
deletions or additional nucleotides or amino acids elsewhere in the gene or
the polypeptide.
Thus, under a "corresponding position" in accordance with the present
invention is to be
understood that nucleotides or amino acids may differ in the indicated number
but may still
have similar neighboring nucleotides or amino acids. Said nucleotides or amino
acids which
may be exchanged, deleted or comprise additional nucleotides or amino acids
are also
comprised by the term "corresponding position". Said nucleotides or amino
acids may for
instance together with their neighbors form sequences which may be involved in
the
regulation of gene expression, stability of the corresponding RNA or RNA
editing, as well as
encode functional domains or motifs of the protein of the invention.
The term "pharmaceutical composition" as used herein comprises the substances
of the
present invention, and optionally one or more pharmaceutically acceptable
earners.
Brief description of the drawing
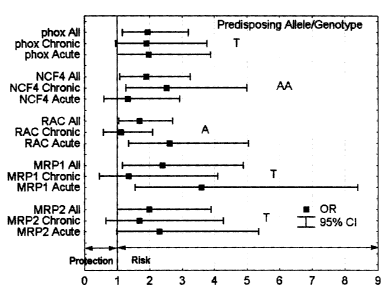
Fig. 1: Odds ratios for the development of ACT (chronic cases, acute cases,
all cases)
conferred by the predisposing alleles identified in this study.
CA 02501253 2005-03-18
7
Detailed description of the invention
The present invention is based on a method which involves the genotyping of
the patients
prior to administering a drug so that its dosage is tailored to the patient's
genetic profile. The
method also relates to pharmaceutical compositions and the preparation of
them, which are
tailored to the genetic profile of these patients. Thus, in a first aspect the
present invention
relates to a method of determining the genetic predisposition of a subject to
be at risk for a
cardiac disease or dysfunction comprising assaying a sample of a subject for
the presence of a
variant allele of at least one gene selected from the group consisting of
genes involved in (a)
the generation of reactive oxygen species (ROS) or (b) drug transmembrane
transport;
wherein the presence of the variant allele is considered indicative of a
higher risk for
cardiotoxicity compared to a control.
Genotyping is performed by analyzing the genetic sequence of a gene coding for
a specific
enzyme often by a polymerase chain reaction assay (PCR) or a PCR with a
restriction
fragment length polymorphism assay (PCR-RFLP) or a PCR and dideoxysequencing.
Preferably genotyping methods are employed, which are amenable to high-
throughput
detection of multiple genetic polymorphisms, for example with mismatch primers
in allele-
specific polymerase chain reaction as described in Ishiguro et al., Anal.
Biochem. 337 (2005),
256-261. The genes are examined for the presence of genetic mutations that can
be linked to
increased or decreased enzyme levels or activity, which in turn result in a
specific phenotype,
i.e. a higher susceptibility to cardiac disorders. The genotype is a
theoretical measurement of
what an individual's phenotype should be. In principle, the genetic mutation
or allele may
present in any region of the gene including the coding region, promoter,
enhancer sequences,
intron, exon, intron/exon junction and vice versa, poly signal and the like,
but also including
polymorphisms which are not located within the gene encoding the metabolic
factor but
which nevertheless display a tight linkage to the observed phenotype.
The present invention is based on observations made in experiments aiming at
investigating
the role of the individual genetic makeup in doxorubicin-induced
cardiotoxicity. The analysis
was conducted in patients enrolled in the prospective, multicenter randomized
phase III trial
called NHL-B, conducted between 1993 and 2000 by the German High-Grade non-
Hodgkin's
Lymphoma Study Group (DSHNHL) (Pfreundschuh et al., Blood 104 (2004), 634-641;
Pfreundschuh et al., Blood 104 (2004), 626-633). Genes and polymorphisms were
selected
CA 02501253 2005-03-18
8
using a candidate approach. The experiments performed in accordance with the
present
invention suggest that among the various mechanisms suggested to mediate the
cardiotoxicity,
increased sensitivity to anthracyclines -derived reactive oxygen species (ROS)
is involved in
this disease. Furthermore, genotyping of variants in proteins implicated in
the transport of
anthracyclines revealed that this class of proteins and genes is involved as
well.
Without intending to be bound by theory it is believed that genetic variants
in anthracycline,
e.g., doxorubicin transport and free radical metabolism modulate the
individual risk to
develop cardiotoxicity (ACT), mainly presenting as arrhythmias (acute ACT) or
congestive
heart failure (chronic ACT). In particular, significant associations ,
including polymorphisms
of the NAD(P)H oxidase and doxorubicin efflux transporters could be
determined.
Thus, the present invention provides a method of determining the genetic
predisposition of a
subject to be at risk for a cardiac disease or dysfunction comprising assaying
a sample of a
subject for the presence of a variant allele of at least one gene selected
from the group
consisting of genes involved in (a) the generation of reactive oxygen species
(ROS) or (b)
drug transmembrane transport; wherein the presence of the variant allele is
considered
indicative of a higher risk for cardiotoxicity compared to a control.
The test sample may be obtained using any technique known in the art including
biopsy,
blood sample, sample of bodily fluid (e.g., urine, lymph, ascites, cerebral
spinal fluid, pleural
effusion, sputum, stool, tears, sweat, pus, etc.), surgical excisions. needle
biopsy, scraping,
etc.; see also the Examples.
A sample, e.g. nucleic acid sample may be obtained from the test sample using
any techniques
known in the art (Ausubel et al. Current Protocols in Molecular Biology (John
Wiley & Sons,
Inc., New York, 1999); Molecular Cloning: A Laboratory Manual, 2nd Ed., ed. by
Sambrook,
Fritsch, and Maniatis (Cold Spring Harbor Laboratory Press: 1989); Nucleic
Acid
Hybridization (B. D. Hames & S. J. Higgins eds. 1984); the treatise, Methods
in Enzymology
(Academic Press, Inc., N.Y.); each of which is incorporated herein by
reference). The nucleic
acid may be purified from whole cells using DNA or RNA purification
techniques. The
sample may also be amplified using PCR or in vivo techniques requiring
subcloning. In a
preferred embodiment, the sample is obtained by isolating mRNA from the cells
of the test
CA 02501253 2005-03-18
9
sample and reverse transcribing the RNA into DNA in order to create cDNA (Khan
et al.,
Biochem. Biophys. Acta 1423 (1999), 17-28; incorporated herein by reference).
Methods for assaying a sample of a subject for the presence of a variant
allele of a target gene
utilize the principle that specific sequence differences can be translated
into reagents for allele
differentiation. These reagents provide the necessary backbone for the
development of
diagnostic tests. Examples for such reagents include, but are not limited to
oligonucleotides
that deviate from the wild type target gene sequence in the alterations) in
the nucleotide
sequence or which are capable of priming the synthesis of a cDNA or cRNA
through said
alterations) in the template gene or transcripts derived therefrom.
Frequently, the principles
of diagnostic tests for the determination of the individual target gene status
include, but are
not limited to differences in the hybridization efficiencies of such reagents
to the various
variant alleles. In addition, differences in efficacy of such reagents in, or
as different
substrates for, enzymatic reactions, e.g. ligases or polymerases or
restriction enzymes can be
applied. The principles of these are well known to experts of the field.
Examples are PCR and
LCR techniques, Chip-hybridizations or MALDI-TOF analyses. Such techniques are
described in the prior art, e.g., PCR technique: Newton, (1994) PCR, BIOS
Scientific
Publishers, Oxford; LCR-technique: Shimer, Ligase chain reaction. Methods Mol.
Biol. 46
(1995), 269-278; Chip hybridization: Ramsay, DNA chips: State-of the art.
Nature
Biotechnology 16 (1998), 40-44; and MALDI-TOF analysis: Ross, High level
multiplex
genotyping by MALDI-TOF mass spectrometry, Nature Biotechnology 16 (1998),
1347-
1351. Other test principles are based on the application of reagents that
recognize specifically
or that are being specifically recognized by the allelic variant as translated
expressed protein.
Examples are allele-specific antibodies, peptides, substrate analogs,
inhibitors, or other
substances which bind to (and in some instances may also modify the action of)
the various
protein forms that are encoded by the variant alleles. In addition,
conventional functional
biochemical assays or cell-based in vitro assays may be used.
Additionally, the presence of a variant allele can be monitored by using a
primer pair that
specifically hybridizes to a region of the target gene and by carrying out an
amplification
reaction according to standard procedures. Specific hybridization of the above
mentioned
probes or primers preferably occurs at stringent hybridization conditions. The
term "stringent
hybridization conditions" is well known in the art; see, for example, Sambrook
et al.,
"Molecular Cloning, A Laboratory Manual" second ed., CSH Press, Cold Spring
Harbor,
CA 02501253 2005-03-18
1989; "Nucleic Acid Hybridisation, A Practical Approach", Hames and Higgins
eds., IRL
Press, Oxford, 1985. Furthermore, the genomic DNA obtained from the subject
may be
sequenced to identify mutations which may be characteristic fingerprints of
mutations in the
target gene. The present invention further comprises methods wherein such a
fingerprint may
5 be generated by RFLPs of DNA obtained from the subject, optionally the DNA
may be
amplified prior to analysis, the methods of which are well known in the art.
For example, the
sample nucleic acid, e.g., amplified or cloned fragment, may be sequenced by
dideoxy or
other methods. Hybridization with the variant sequence may also be used to
determine its
presence, by Southern blots, dot blots, etc. The hybridization pattern of a
control and variant
10 sequence to an array of oligonucleotide probes immobilized on a solid
support, as described
in US patent no. 5,445,934, or in international application W095/35505, may
also be used as
a means of detecting the presence of variant sequences. Single stxand
conformational
polymorphism (SSCP) analysis, denaturing gradient gel electrophoresis (DGGE),
mismatch
cleavage detection, and heteroduplex analysis in gel matrices are used to
detect
conformational changes created by DNA sequence variation as alterations in
electrophoretic
mobility. Alternatively, where a polymorphism creates or destroys a
recognition site for a
restriction endonuclease (restriction fragment length polymorphism, RFLP), the
sample is
digested with that endonuclease, and the products size fractionated to
determine whether the
fragment was digested. Fractionation is performed by gel or capillary
electrophoresis,
particularly acrylamide or agarose gels. Corresponding methods may be applied
on basis of
the expressed proteins, for example using proteases which cleavage site is
destroyed in the
variant allele.
In a preferred embodiment of the method of the present invention the genetic
predisposition is
determined by genotyping. It is a well known fact that genomic DNA of
individuals, which
harbor the individual genetic makeup of all genes, including any one of the
target genes
described herein, can easily be purified from individual blood samples. These
individual DNA
samples are then used for the analysis of the sequence composition of the
alleles of the target
gene that are present in the individual which provided the blood sample. The
sequence
analysis can be carried out by PCR amplification of relevant regions of said
genes, subsequent
purification of the PCR products, followed by automated DNA sequencing with
established
methods (e.g. ABI dyeterminator cycle sequencing); see also supra and Example
1.
CA 02501253 2005-03-18
11
Depending on the nature of the allele, i.e. whether it said allele is dominant
or recessive one
important parameter that has to be considered in the attempt to determine the
individual
genotype by direct DNA-sequencing of PCR-products from human blood genomic DNA
is
the fact that each human harbors (usually, with very few abnormal exceptions)
two gene
copies of each autosomal gene (diploidy). Because of that, care has to be
taken in the
evaluation of the sequences to be able to identify unambiguously not only
homozygous alleles
but also heterozygous alleles.
It is well known in the art that genes comprise structural elements which
encode an amino
acid sequence as well as regulatory elements which are involved in the
regulation of the
expression of said genes. Structural elements are represented by exons which
may either
encode an amino acid sequence or which may encode for RNA which is not
encoding an
amino acid sequence but is nevertheless involved in RNA function, e.g. by
regulating the
stability of the RNA or the nuclear export of the RNA.
Regulatory elements of a gene may comprise promoter elements or enhancer
elements both of
which could be involved in transcriptional control of gene expression. It is
very well known in
the art that a promoter is to be found upstream of the structural elements of
a gene. Regulatory
elements such as enhancer elements, however, can be found distributed over the
entire locus
of a gene. Said elements could be reside, e.g., in introns, regions of genomic
DNA which
separate the exons of a gene. Promoter or enhancer elements correspond to
polynucleotide
fragments which are capable of attracting or binding polypeptides involved in
the regulation
of the gene comprising said promoter or enhancer elements. For example,
polypeptides
involved in regulation of said gene comprise the so called transcription
factors.
Said introns may comprise further regulatory elements which are required for
proper gene
expression. Introns are usually transcribed together with the exons of a gene
resulting in a
nascent RNA transcript which contains both, exon and intron sequences. The
intron encoded
RNA sequences are usually removed by a process known as RNA splicing. However,
said
process also requires regulatory sequences present on a RNA transcript said
regulatory
sequences may be encoded by the introns.
In addition, besides their function in transcriptional control and control of
proper RNA
processing andlor stability, regulatory elements of a gene could be also
involved in the control
of genetic stability of a gene locus. Said elements control, e.g.,
recombination events or serve
to maintain a certain structure of the DNA or the arrangement of DNA in a
chromosome.
CA 02501253 2005-03-18
12
Therefore, single nucleotide polymorphisms can occur in exons of a gene which
encode an
amino acid sequence as discussed supra as well as in regulatory regions which
are involved in
the above discussed process. The analysis of the nucleotide sequence of a gene
locus in its
entirety including, e.g., introns is in light of the above desirable. The
polymorphisms
comprised by the polynucleotides of the present invention can influence the
expression level
of protein activity via mechanisms involving enhanced or reduced transcription
of the target
gene, stabilization of the gene's RNA transcripts and alteration of the
processing of the
primary RNA transcripts.
Usually, said variant allele results from a nucleotide deletion, addition
and/or substitution
compared to the corresponding wild type gene. In one embodiment of the method
of the
invention a nucleotide deletion, addition and/or substitution results in
altered expression of
the variant gene compared to the corresponding wild type gene. Preferably,
said nucleotide
substitution(s), additions) or deletions) refer ed to in accordance with the
method of the
present invention results) in one or more changes of the corresponding amino
acids) of the
proteins encoded by the target genes.
Without intending be bound by theory it is believed that said variant allele
generally results in
lower amount of protein activity compared to a control, for example because of
reduced
expression of the target gene or altered enzymatic activity. The decreased
amount or level of
protein activity as referred to herein includes in addition to a significantly
decreased amount
of transcripts encoding a functional gene product also a normal or even
elevated amount of
transcripts encoding a gene product which has no activity or a significantly
decreased activity.
The term "lower amount of protein activity" means that the level of activity
of the gene
products of the mentioned target genes is determined and the level is compared
to a reference
standard. As a reference standard, preferably a sample is obtained from cells
or tissues of a
subject having the wild type allele of the respective target genes in its
genome. Preferably,
said subject is homozygous or hemizygous with respect to the selected target
gene.
As discussed supra, the variant alleles which correspond to coding regions of
the target gene
effect the amino acid sequences of the polypeptides encoded by said variant
alleles. The
variant polypeptides preferably, but not necessarily exhibit altered
biological and/or
immunological properties when compared to their corresponding wild type
counterpart.
Preferred variant polypeptides in accordance with the use of the invention are
those, which
exhibit an altered biological activity, i.e. altered enzymatic function
resulting in reduced,
CA 02501253 2005-03-18
13
enhanced or complete loss of catalytic activity or altered transport function
resulting in
reduced, enhanced or complete loss of transport activity or altered binding to
receptors or
other drug targets resulting in altered activation of signal transduction
pathways or altered
inhibition of transporter or enzyme function. It might be necessary to obtain
a sample
comprising biological material such as isolated cells or tissue from the
subject prior to
perform said methods for determination of the activities of the wild type and
the variant
polypeptides, respectively. Whether a variant polypeptide has an altered
activity or level of
expression compared to its wild type corresponding counterpart can be
determined by
standard techniques well known in the art. Such standard techniques may
comprise, e.g.,
ELISA based assays, RIA based assays, HPLC-based assays, mass spectroscopy-
based
assays, western blot analysis or assays which are known in the art; see also
the references
cited herein.
As described in the examples, the method of the present invention has been
established inter
alia on the basis of specific alleles of genes involved in the generation of
ROS, i.e. encoding
proteins of the NAD(P)H oxidase mufti-enzyme complex, i.e. NCF4, CYBA and
RAC2. In
particular, chronic ACT associated with a variant of the NAD(P)H oxidase
subunit NCF4 (-
212A>G, OR: 2.5, 95 % CI: 1.3 - 5.0). Acute ACT was associated with the
His72Tyr
polymorphism in the p22phox subunit (OR: 2.0, 95 % CI: 1.0 - 3.9) and with the
variant
7508T>A (OR: 2.6, 95 % CI: 1.3 - 5.1 ) of the RAC2 subunit of the same enzyme.
Therefore,
in a preferred embodiment of the present invention said variant allele to be
determined is a
polymorphism corresponding to the C242T (His72Tyr) polymorphism of the CYBA
gene, a
polymorphism corresponding to the 212A>G polymorphism in the NCF4 gene or a
polymorphism corresponding to the 7508T>A polymorphism in the RAC2 gene.
In addition, it could be shown that genes involved in drug transmembrane
transport, i.e. genes
encoding mufti-drug resistance protein, especially multidrug resistance-
associated protein 1
(MRPI) or multidrug resistance-associated protein 2 (MRP2) associate with
anthracycline
cardiotoxicity. In particular, acute ACT was associated with the G1y671 Val
variant of the
doxorubicin efflux transporter MRP1 (OR: 3.6, 95 % CI: 1.6 - 8.4) and with the
Val l 188G1u-
Cys1515Tyr haplotype of the functionally similar MRP2 (OR: 2.3, 95 % CI: 1.0 -
5.4).
Therefore, in another and additional preferred embodiment of the present
invention,
respectively, said variant allele to be determined is a polymorphism
corresponding to the
G1y671 Val polymorphism in the MRP1 gene or a polymorphism corresponding to
the
CA 02501253 2005-03-18
14
Vall 188G1u or Cysl515Tyr polymorphism in the MRP2 gene. Further,
polymorphisms in the
human gene for the multidrug resistance-associated protein 1 (MRP-1) and means
and
methods for their diagnosis are described, for example in international
application
W002/059142, the disclosure content of which is incorporated herein by
reference. In the
background section W002/059142 also provides a review over the human multidrug
resistance-associated protein (MRP) family, a subfamily of the ATP-binding
cassette (ABC)
protein superfamily, said information is likewise incorporated herein.
A subject may not suffer from cardiotoxicity at first place but only after
medication with a
given drug. For example, as explained above and in the examples, a significant
number of
patients treated with anthracyclines develop cardiotoxicity (ACT), mainly
presenting as
arrhythmias (acute ACT) or congestive heart failure (chronic ACT).
Accordingly, the method
of the present invention is preferably applied in cases, wherein said cardiac
disease or
dysfunction the risk of which a subject is to be diagnosed for is induced by a
drug. In this case
the method of the present invention is particularly useful for selecting
patients who have to
undergo chemotherapy and who have been diagnosed to be at high risk of
developing
cardiotoxicity to the intended drug in order to provide an adapted such low
dose and/or
combination therapy, or an alternative treatment.
Preferably, said drug is an antineoplastic agent, most preferably said drug is
an anthracycline.
Anthracyclines belong to a member of a family of chemotherapy drugs that are
also
antibiotics and that originally come from the fungus Streptococcus peucetius.
The
anthracyclines act to prevent cell division by disrupting the structure of the
DNA and
terminate its function. They do so in two ways: (1) they intercalate into the
base pairs in the
DNA minor grooves; and (2) they cause free radical damage of the ribose in the
DNA. The
anthracyclines are frequently used in leukemia therapy. The anthracyclines
include
daunorubicin (Cerubidine), doxorubicin (DOX, Adriamycin, Rubex), epirubicin
(4'epi-
doxorubicin, Ellence, Pharmorubicin), and idarubicin (4-demethoxy-
daunorubicin, Idamycin),
and as well as their isomers and analogs, for example morpholino-and
cyanomorpholino-
doxorubicin (morpholino-DOX and cyanomorpholino DOX). Anthracyclines, for
example
doxorubicin and epirubicin are widely used in cancer therapy.
In a preferred embodiment of the present invention the methods and therapeutic
uses
described herein are applied to cancer treatment with doxorubicin.
CA 02501253 2005-03-18
As summarized in the background section and in the examples there is a lower
incidence of
heart damage following doxorubicin infusion as opposed to bolus injection
suggesting a role
for the drug's peak levels in drug-induced cardiotoxicity. Accordingly,
besides the initial dose
also the route of administration should be taken into account. For example, in
case a patient is
5 diagnosed positive for risk of cardiotoxicity in accordance with a method of
the present
invention it is recommended to administer the drug by intravenous infusion
rather than by
bolus injection.
With respect to the particular cardiac disease or dysfunction these usually
comprise
10 arrhythmia and/or congestive heart failure. For example, patients treated
with anthracyclines
develop cardiotoxicity (ACT), mainly presenting as arrhythmias (acute ACT) or
congestive
heart failure (chronic ACT). Accordingly, in one embodiment said cardiac
disease or
dysfunction a subject may be at risk for comprises anthracycline-induced
cardiotoxicity
(ACT). Said ACT may be acute ACT or chronic ACT.
As is evident from the above, a prerequisite for selecting a suitable therapy
is the knowledge
of the presence or absence of a variant allele referred to in accordance with
the method of the
present invention. Therefore, the method of the present invention encompasses
the
determination of the presence or absence of said variant alleles in a sample
which has been
obtained from said subject. The sample which is obtained by the subject
comprises biological
material which is suitable for the determination of the presence or absence of
said variant
alleles, such as isolated cells or tissue. For example, the said sample can be
a plasma sample,
a blood sample, a saliva sample, a tumor sample, a tissue sample or bodily
fluid sample.
Additionally, the presence or expression of variant target gene can be
monitored by using a
primer pair that specifically hybridizes to either of the corresponding
nucleic acid sequences
and by carrying out a PCR reaction according to standard procedures. Specific
hybridization
of the above mentioned probes or primers preferably occurs at stringent
hybridization
conditions. The term "stringent hybridization conditions" is well known in the
art; see, for
example, Sambrook et al., "Molecular Cloning, A Laboratory Manual" second ed.,
CSH
Press, Cold Spring Harbor, 1989; "Nucleic Acid Hybridisation, A Practical
Approach",
Hames and Higgins eds., IRL Press, Oxford, 1985. Furthermore, the mRNA, cRNA,
cDNA or
genomic DNA obtained from the subject may be sequenced to identify mutations
which may
be characteristic fingerprints of mutations in the polynucleotide or the gene
of the invention.
CA 02501253 2005-03-18
16
The present invention further comprises methods wherein such a fingerprint may
be generated
by RFLPs of DNA or RNA obtained from the subject, optionally the DNA or RNA
may be
amplified prior to analysis, the methods of which are well known in the art.
RNA fingerprints
may be performed by, for example, digesting an RNA sample obtained from the
subject with
a suitable RNA-Enzyme, for example RNase Tl, RNase T2 or the like or a
ribozyme and, for
example, electrophoretically separating and detecting the RNA fragments as
described above.
Further modifications of the above-mentioned embodiment of the invention can
be easily
devised by the person skilled in the art, without any undue experimentation
from this
disclosure; see, e.g., the examples. An additional embodiment of the present
invention relates
to a method wherein said determination is effected by employing an antibody of
the invention
or fragment thereof. The antibody used in the method of the invention may be
labeled with
detectable tags such as a histidine flags or a biotin molecule.
In a preferred embodiment of the present invention, the above described
methods comprise
PCR, ligase chain reaction, restriction digestion, direct sequencing, nucleic
acid amplification
techniques, hybridisation techniques, immunodiagnostic methods or biological
or enzymatic
activity assays. Diagnostic screening may be performed for polymorphisms
through the use of
microsatellite markers or single nucleotide polymorphisms (SNP). The
microsatellite or SNP
polymorphism itself may not phenotypically expressed, but is linked to
sequences that result
in altered activity or expression. Two polymorphic variants may be in linkage
disequilibrium,
i.e. where alleles show non-random associations between genes even though
individual loci
are in Hardy-Weinberg equilibrium. Linkage analysis may be performed alone, or
in
combination with direct detection of phenotypically evident polymorphisms. The
use of
microsatellite markers for genotyping is well documented. For examples, see
Mansfield et al.,
Genomics 24 (1994), 225-233; and Ziegle et al., Genomics 14 (1992), 1026-1031.
The use of
SNPs for genotyping is illustrated in Golevieva, Am, J. Hum. Genet. 59 (1996),
570-578; and
in Underhill et al., Proc. Natl. Acad. Sci. USA 93 (1996), 196-200.
Hence, the variant alleles of the target genes of the present invention are
preferably
determined by genotyping. Further means and methods for genotyping and
phenotyping,
respectively, which can be used in and/or adapted to methods of the present
invention are
described in international application WO01/55432.
The foregoing methods are preferably conducted at least twice on a given
sample using, for
example, at least one different primer pair specific for particular alleles.
Following detection,
CA 02501253 2005-03-18
17
one may compare the results seen in a given patient with a statistically
significant reference
group of normal subjects and a patient to be treated. In this way, it is
possible to correlate the
number and kind of variant alleles with various clinical states or disease
prognosis. The
method of the present invention can include the combination of two, three or
all of the alleles,
weighted according to F-power in a multivariate analysis, to increase the
accuracy of the
method. One of ordinary skill in the art recognizes that Genbank accession
numbers refer to
numbers used to identify nucleotide sequences available in Genbank (Benson et
al.,
"GenBank", Nucleic Acids. Res. 30 (2002), 17-20).
In addition, the present invention relates to the use of an oligonucleotide
for determining the
presence of variant allele in accordance with the method of the present
invention. Preferably,
said oligo- or polynucleotide is about 15 to 50, preferably 20 to 40, more
preferably 20 to 30
nucleotides in length and comprises the nucleotide sequence of any one of the
primer or target
sequences disclosed in Table 2, infra, or a complementary sequence.
Hence, in a still further embodiment, the present invention relates to a
primer or probe
consisting of an oligonucleotide as defined above. In this context, the term
"consisting of
means that the nucleotide sequence described above and employed for the primer
or probe of
the invention does not have any further nucleotide sequences of the target
gene involved in
the generation of ROS or drug transmembrane transport immediately adjacent at
its 5' and/or
3' end. However, other moieties such as labels, e.g., biotin molecules,
histidin flags, antibody
fragments, colloidal gold, etc. as well as nucleotide sequences which do not
correspond to the
particular target gene may be present in the primer and probes of the present
invention.
Furthermore, it is also possible to use particular nucleotide sequences of the
respective target
gene and to combine them with other nucleotide sequences derived from the
target gene,
wherein these additional nucleotide sequences are interspersed with moieties
other than
nucleic acids or wherein the intervening nucleic acid does not correspond to
nucleotide
sequences of the target gene.
Furthermore, it is evident to the person skilled in the art that the
oligonucleotide can be
modified, for example, by thin-phosphate-backbones and/or base analogs well
known in the
art (Flanagan, Proc. Natl. Acad. Sci. USA 96 (1999), 3513-8; Witters, Breast
Cancer Res.
Treat. 53 ( 1999), 41-50; Hawley, Antisense Nucleic Acid Drug Dev. 9 ( 1999),
61-9; Peng Ho,
Brain Res. Mol. Brain Res. 62 (1998), 1-11; Spiller, Antisense Nucleic Acid
Drug Dev. 8
CA 02501253 2005-03-18
18
(1998), 281-93; Zhang, J. Pharmacol. Exp. Ther. 278 (1996), 971-9; Shoji,
Antimicrob.
Agents Chemother. 40 (1996), 1670-5; Crooke, J. Pharmacol. Exp. Ther. 277
(1996), 923-37).
In one embodiment of the invention, an array and chip, respectively, of
oligonucleotides are
provided, where discrete positions on a solid support of the the array or chip
are
complementary to one or more of the provided polymorphic sequences, e.g.
oligonucleotides
of at least 12 nt., frequently 20 nt. or larger, and including the sequence
flanking the
polymorphic position. Such an array may comprise a series of oligonucleotides,
each of which
can specifically hybridize to a different polymorphism. For examples of
arrays, see Hacia et
al., Nature Genetics 14 (1996), 441-447; Lockhart et al., Nature Biotechnol.
14 (1996), 1675-
1680; and De Risi et al., Nature Genetics 14 (1996), 457-460.
The term "solid support" as used herein refers to a flexible or non-flexible
support that is
suitable for carrying said immobilized targets. Said solid support may be
homogenous or
inhomogeneous. For example, said solid support may consist of different
materials having the
same or different properties with respect to flexibility and immobilization,
for instance, or
said solid support may consist of one material exhibiting a plurality of
properties also
comprising flexibility and immobilization properties. Said solid support may
comprise glass-,
polypropylene- or silicon-chips, membranes oligonucleotide-conjugated beads or
bead arrays.
Thanks to the present invention the particular drug selection, dosage regimen
and
corresponding patients to be treated can be determined in accordance with the
present
invention. The dosing recommendations will be indicated in product labeling by
allowing the
prescriber to anticipate dose adjustments depending on the considered patient
group, with
information that avoids prescribing the wrong drug to the wrong patients at
the wrong dose.
Furthermore, the present invention relates to a kit useful for performing the
method of the
present invention, said kit comprising polynucleotides, oligonucleotides, a
chip or array,
reference samples, amplification and/or sequencing means, buffer, detergents,
biochemical
regents, detection means, or the like, and optionally a reformulated drug with
a drug dose
which is tailored to a patent carrying a variant allele as defined
hereinabove, and optionally
instructions for carrying out a method of the invention.
The kit of the invention may contain further ingredients such as selection
markers and
components for selective media suitable for the generation of transgenic cells
and animals.
The kit of the invention can be used for carrying out a method of the
invention and could be,
CA 02501253 2005-03-18
19
inter alia, employed in a variety of applications, e.g., in the diagnostic
field or as research
tool. The parts of the kit of the invention can be packaged individually in
vials or other
appropriate means depending on the respective ingredient or in combination in
suitable
containers or multicontainer units. Manufacture of the kit follows preferably
standard
procedures which are known to the person skilled in the art. The kit may be
used for methods
for detecting expression of a mutant form of the polypeptides, genes or
polynucleotides in
accordance with any one of the above-described methods of the invention,
employing, for
example, immunoassay techniques such as radioimmunoassay or enzymeimrnunoassay
or
preferably nucleic acid hybridization and/or amplification techniques such as
those described
herein before and in the Examples.
In a further aspect the present invention relates to the use of a drug or
prodrug for the
preparation of a pharmaceutical composition for the treatment or prevention of
a disorder of a
subject diagnosed by the method described hereinbefore.
In one embodiment, a given drug as defined herein is used for the preparation
of a
pharmaceutical composition for the treatment of a disease which is amenable to
a
corresponding therapy with said drug, wherein said pharmaceutical composition
is designed
for administration to a patient who carnes at least one variant allele as
defined hereinbefore,
wherein the drug dose is lower than compared to the use for administering to a
patient without
carrying said variant allele. By "wherein the drug dose is lower than compared
to the use for
administering to a patient without carrying said variant allele" a standard
dose is meant which
is routinely administered to patients in need thereof without regarding the
genotype. Such a
general population of patients is considered as having the normal genotype,
i.e. wild type
genotype.
The methods of the invention may also provide, for example, the optimization
of therapy for a
disease such as cancer. The invention also provides a method of designing a
therapy for a
patient, and a method of prescribing a therapy for a patient, including making
recommendations for drugs and/or combinations of drugs not yet prescribed for
that patient.
Accordingly, in a further embodiment the present invention relates to a method
for the
preparation of an medicament individual subject or subpopulation of subjects
comprising
reformulation of the drug or pro-drug, wherein said individualized dosage is
calculated from
the dose usually applied and recommended for in-patients, without taking gene
CA 02501253 2005-03-18
polymorphisms into consideration, as taken from manufacturers' recommendations
factored
against the metabolic quotient determined in accordance with the
pharmacokinetic model of
the present invention. The definitions and explanations of the terms made
above apply mutatis
mutandis to all of the methods described herein. The term "suitable therapy"
as used herein
5 means that a substance according to the invention is selected and said
substance is
administered in a certain dosage to a subject, wherein said substance and said
dosage are
selected based on the knowledge of the presence or absence of at least one,
preferably at least
two, more preferably at least three and most preferably at least four variant
alleles referred to
in accordance with the use of the invention. Said substance and said dosage of
the substance
10 are selected in a way that they are most effective on one hand and on the
other hand they do
not cause toxic or undesirable side effects.
In one embodiment, the present invention relates to a method for the treatment
of a disease
comprising administering to a subject in need of such treatment a non-
cardiotoxic amount of a
15 drug as defined herein or a non-cardiotoxic analog or substitute thereof,
wherein said subject
carnes at least one variant allele as defined above. For example, a drug as
defined above may
be used for the preparation of a pharmaceutical composition for the treatment
of a disease
which is amenable to a corresponding therapy with said drug, wherein said
pharmaceutical
composition is designed for administration to a patient who carries at least
one variant allele
20 as defined herein, wherein the drug dose is lower than compared to the use
for administering
to a patient without carrying said variant allele.
In addition, or alternatively suitable analogs and derivatives of drugs such
as anthracyclines,
which are less cardiotoxic may be used; see, e.g., Danesi et al., Toxicology
70 (1991), 243-
253, which describes cardiotoxicity and cytotoxicity of the anthracycline
analog 4'-deoxy-4'-
iodo-doxorubicin. Sugar-modified derivatives of antitumor anthracycline,
daunorubicin, are
described in Pawlowska et al., Oncol. Res. 14 (2004), 469-474, and
characterization of
anthracenediones and their photoaffinity analogs is described in Chou et al.,
Biochem.
Phanmacol. 63 (2002), 1143-1147. Pouna et al., Cancer Chemother. Pharmacol. 35
(1995),
257-261, describe evaluation of anthracycline cardiotoxicity with the model of
isolated,
perfused rat heart exemplified by a comparison of new analogues versus
doxorubicin.
Furthermore, reduced toxicity by a novel disaccharide analogue is reported by
Minotti et al. in
Chem. Res. Toxicol. 13 (2000), 1336-1341 and Br. J. Pharmacol. 134 (2001),
1271-1278.
CA 02501253 2005-03-18
21
In addition, or alternatively the drug may substituted, for example an
anthracycline-based
chemotherapy may be changed to a non-anthracyline-based therapy.
In another embodiment, the present invention relates to a method for the
treatment of a
disease comprising administering to a subject in need of such treatment a drug
as defined
herein, wherein said subject does not carry any one of said variant alleles as
described above.
Put in other words, a drug such as an antineoplastic drug may be used for the
preparation of a
pharmaceutical composition for the treatment of a disease which is amenable to
a
corresponding therapy with said drug, wherein said pharmaceutical composition
is designed
for administration to a patient who does not carry any one of said variant
alleles of, for
example MRP1 and MRP2.
Hence, a particular object of the present invention concerns dnag/pro-drug
selection and
formulation of pharmaceutical compositions for the treatment of diseases which
are amenable
to chemotherapy taking into account the polymorphism of the variant forms) of
genes
involved in (a) the generation of reactive oxygen species (ROS) or (b) drug
transmembrane
transport, which co-segregate with the affected phenotype of the patient to be
treated. This
allows the safe and economic application of drugs which for example were
hitherto
considered not appropriate for therapy of, e.g., cancer due to either their
side effects in some
patients, i.e. cardiotoxicity. The means and methods described herein can be
used, for
example, to improve dosing recommendations and allows the prescriber to
anticipate
necessary dose adjustments depending on the considered patient group.
Accordingly, prior to
treatment a sample of the patient has preferably been assayed in accordance
with the method
of the present invention as described above.
In a particular preferred embodiment of the present invention said drug to be
used is an
antineoplastic agent as defined hereinbefore said disease is cancer.
Chemotherapy for cancer is varied, because there are so many different forms
of this disease.
Treatment may rely on a single anticancer medication - that is, single agent
chemotherapy - or
it may involve combination chemotherapy with a number of different anticancer
drugs. Such
drugs destroy cancer cells by preventing them from growing and dividing
rapidly. Drugs and
chemotherapy regimens which may be improved in accordance with the present
invention
include but are not limited to:
CA 02501253 2005-03-18
22
ABVD: Doxarubicin (Adriamycin~), bleomycin, vinblastine, and dacarbazine
ACVBP: Doxorubicin, cyclophosphamide, vindesine, bleomycin, prednisone,
intrathecal
methotrexate
ASHAP-mBACOS-MINE: Doxorubicin, methylprednisolone, cytarabine, platinum,
methotrexate, bleomycin, cyclophosphamide, vincristine
BEP: BCNU (carmustine), etoposide, procarbazine
CEOP-B: Cyclophosphamide, epirubicin, vincristine, methylprednisolone,
bleomycin
CEOP-Bleo: Cyclophosphamide, epirubicin, vincristine, prednisone, bleomycin
CEP: Cyclophosphamide, etoposide, prednisolone
CEVOP: Cyclophosphamide, epirubicin, etoposide, vincristine, prednisone
CIOP-B: Cyclophosphamide, idarubicin, vincristine, prednisone, bleomycin
CHOP: Cyclophosphamide, doxorubicin, vincristine, prednisone
CHOP-B: Cyclophosphamide, doxorubicin, vincristine, methylprednisolone,
bleomycin
CHOP-R: Cyclophosphamide, doxorubicin, vincristine, prednisone, rituximab
1 S CHOEP: Cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone
CNOP: Cyclophosphamide, mitoxantrone, vincristine, prednisone
CTVP: Cyclophosphamide, pirarubicin, teniposide, prednisone
CVP: Cyclophosphamide, teniposide, prednisone
ChIVPP: Chlorambucil (Leukeran~), vinblastine sulfate (Velban~), procarbazine
hydrohloride (Matulane~), and prednisone;
CVP: Cyclophosphamide, vindesine, prednisolone
EVA: Etoposide, vinblastine, and doxorubicin
EVAP: Etoposide (VP-16; VePesid~), vinblastine sulfate (Velban~), doxorubicin
(Adriamycin~), and prednisone
ISHAP-mBICOS-MINE: Idarubicin, methylprednisolone, cytarabine, platinum,
methotrexate,
bleomycin, cyclophosphamide, vincristine
LOPP: Chlorambucil (Leukeran~), vincristine sulfate (Oncovin~), procarbazine
hydrochloride (Matulane~), and prednisone
MACOP-B: Methotrexate, doxorubicin, cyclophosphamide, vincristine, bleomycin,
prednisone
m-BACOD: Methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine,
prednisone
MEP: Mitoxantrone, etoposide, procarbazine
MiCEP: Mitoxantrone, etoposide, cyclophosphamide, prednisone
CA 02501253 2005-03-18
23
MOPP: Mechlorethamine, vincristine (Oncovin~), procarbazine, and prednisone;
NOVP: Mitoxantrone (Novantrone~), vincristine (Oncovin~), vinblastine, and
prednisone
PAdriCEBO: Prednisolone, doxorubicin, cyclophosphamide, etoposide, bleomycin,
vincristine
PMitCEBO: Prednisolone, mitoxantrone, cyclophosphamide, etoposide, bleomycin,
vincristine
ProMACE-CytaBOM: Cyclophosphamide, doxorubicin, etoposide, cytarabine,
bleomycin,
vincristine, methotrexate, prednisone
ProMECE-CytaBOM: Cyclophosphamide, epirubicin, etoposide, cytarabine,
bleomycin,
vincristine, methotrexate, prednisone
PVABEC: Etoposide, doxorubicin, cyclophosphamide, vincristine, bleomycin,
prednisone
P-VEBEC: Epirubicin, cyclophosphamide, etoposide, vinblastine, bleomycin,
prednisone
T-COP, THP-COP: Pirarubicin, cyclophosphamide, vincristine, prednisone
THP-COPE: Pirarubicin, cyclophosphamide, vincristine, prednisone, etoposide
VMP: Etoposide, mitoxantrone, prednimustine
VNCOP-B: Cyclophosphamide, mitoxantrone, vincristine, etoposide, bleomycin,
prednisone
VEEP: Vincristine, epirubicin, etoposide, and prednisolone;
Preferably, method and therapeutic use of the present invention, respectively,
involves any
one of the aforementioned drugs or drug regimen wherein said drug or at least
one of said
drugs belongs to class of anthracyclines; see also supra.
The pharmaceutical compositions and formulations referred to herein are
administered at least
once in accordance with the use of the present invention. However, the said
pharmaceutical
compositions and formulations may be administered more than one time, for
example once
weekly every other week up to a non-limited number of weeks.
Thanks to the method of the present invention, it is possible to efficiently
select a suitable
therapy for a subject, preferably a human, suffering, for example, from
lymphoma, Hodgkin's
and Non-Hodgkin's Disease, respectively, colorectal cancer, cervical cancer,
gastric cancer,
lung cancer, malignant glioma, ovarian cancer, and pancreatic cancer. Thereby,
mistreatment
of patients based on wrong medications and the results thereof, such as
development of
resistance towards cancer therapy, and subsequent increased costs in health
care, can be
efficiently avoided. Furthermore, patients that are at high risk can be
excluded from therapy
prior to the first dose andlor dosage can be adjusted according to the
individual's genetic
CA 02501253 2005-03-18
24
makeup prior to the onset of drug therapy. Also, agonists/activators for the
mentioned target
genes (e.g. MRP 1 ) can be applied in genetically defined patient
subpopulations. Thus, adverse
effects can be avoided and the optimal drug level can be reached faster
without time-
consuming and expensive drug monitoring-based dose finding. This can reduce
costs of
S medical treatment and indirect costs of disease (e.g. shorter time and less
frequent
hospitalization of patients).
Accordingly, the present invention also relates to combination preparations,
i.e.
pharmaceutical compositions comprising an antineopIastic agent and an
agonist/activator of a
protein involved in the generation of reactive oxygen species (ROS) or drug
transmembrane
transport.
Agonists and activators of NAD(P)H oxidase activity are well known in the art
and include,
for example, hormones such as Endothelin-1 and angiotensin II. Furthermore,
thrombin,
interleukin-1 (IL-1), platelet-derived growth factor (PDGF), tumor necrosis
factor-oc (TNF-a),
and lactosylceramide also stimulate NAD(P)H oxidase activity. In addition,
activation of the
oxidase can be mediated by intracellular second messengers, including calcium.
For review of
NAD(P)H oxidase system and regulation see Griendling et al., Circulation
Research 86
(2000), 494, and references cited therein. Furthermore, methods and materials
involved in
identifying agonists NADPH oxidase activity are described for example in
international
application W003/095667.
Agonists and activators of drug transmembrane transport are also known in the
art. For
example, Worm et al. (Biol. Chem. 276 (2001 ), 39990-40000) describe up-
regulation of P-
glycoprotein in response to S-aza-2'-deoxycytidine and S-aza-2'-deoxycytidine-
induced up-
regulation of multidrug resistance-associated proteins. In addition, methods
and compositions
for regulating MRP2 are described in international application W003/042400.
Hence,
preferably said protein involved in the generation of ROS is derived from the
NAD(P)H
oxidase mufti-enzyme complex and the protein involved in transmembrane
transport is MRP 1
or MRP2.
As already explained for the preceding embodiments the antineoplastic agent to
be used in the
methods of the present invention is preferably an anthracycline, and most
preferably
doxorubicin; see also infra.
CA 02501253 2005-03-18
The appropriate concentration of the therapeutic agent might be dependent on
the particular
agent. The therapeutically effective dose has to be compared with the toxic
concentrations;
the clearance rate as well as the metabolic products play a role as do the
solubility and the
formulation. Therapeutic efficacy and toxicity of compounds can be determined
by standard
5 pharmaceutical procedures in cell cultures or experimental animals, e.g.,
ED50 (the dose
therapeutically effective in 50 % of the population) and LD50 (the dose lethal
to 50 % of the
population). The dose ratio between therapeutic and toxic effects is the
therapeutic index, and
it can be expressed as the ratio, LD50/ED50.
10 The term "pharmaceutical composition" as used herein comprises the
substances of the
present invention and optionally one or more pharmaceutically acceptable
carriers. The
substances of the present invention may be formulated as pharmaceutically
acceptable salts.
Acceptable salts comprise acetate, methylester, HCI, sulfate, chloride and the
like. The
pharmaceutical compositions can be conveniently administered by any of the
routes
15 conventionally used for drug administration, for instance, orally,
topically, or by inhalation.
The substances may be administered only after the genotype of the patient has
been
determined. Depending on a patient's genotype three different dosage forms
which are suited
to treat patients who have three (ultrafast metabolizers), one (intermediate
metabolizers) or no
wild type gene (poor metabolizers) are given the patient. These dosage forms
are prepared by
20 combining the drugs with standard pharmaceutical carriers according to
conventional
procedures. These procedures may involve mixing, granulating and compressing
or dissolving
the ingredients as appropriate to the desired preparation. It will be
appreciated that the form
and character of the pharmaceutically acceptable character or diluents is
dictated by the
amount of active ingredient with which it is to be combined, the route of
administration and
25 other well-known variables. The carner(s) must be "acceptable" in the sense
of being
compatible with the other ingredients of the formulation and not deleterious
to the recipient
thereof. The pharmaceutical carrier employed may be, for example, either a
solid or a liquid.
Exemplary of solid carriers are lactose, terra alba, sucrose, talc, gelatin,
agar, pectin, acacia,
magnesium stearate, stearic acid and the like. Exemplary of liquid carriers
are phosphate
buffered saline solution, syrup, oil such as peanut oil and olive oil, water,
emulsions, various
types of wetting agents, sterile solutions and the like. Similarly, the
carrier or diluents may
include time delay material well known to the art, such as glyceryl mono-
stearate or glyceryl
distearate alone or with a wax. The substance according to the present
invention can be
administered in various manners to achieve the desired effect. Said substance
can be
CA 02501253 2005-03-18
26
administered either alone or in the formulated as pharmaceutical preparations
to the subject
being treated either orally, topically or by inhalation. Moreover, the
substance can be
administered in combination with other substances either in a common
pharmaceutical
composition or as separated pharmaceutical compositions.
The diluents are selected so as not to affect the biological activity of the
combination.
Examples of such diluents are distilled water, physiological saline, Ringer's
solutions,
dextrose solution, and Hank's solution. In addition, the pharmaceutical
composition or
formulation may also include other carriers, adjuvants, or nontoxic,
nontherapeutic,
nonimmunogenic stabilizers and the like. A therapeutically effective dose
refers to that
amount of the substance according to the invention, which ameliorate the
symptoms or
condition. Therapeutic efficacy and toxicity of such compounds can be
determined by
standard pharmaceutical procedures in cell cultures or experimental animals,
e.g., ED50 (the
dose therapeutically effective in 50 % of the population) and LD50 (the dose
lethal to 50 % of
the population). The dose ratio between therapeutic and toxic effects is the
therapeutic index,
and it can be expressed as the ratio, LD50/ED50.
The dosage regimen will be determined by the attending physician and other
clinical factors;
preferably in accordance with any one of the above described methods. As is
well known in
the medical arts, dosages for any one patient depends upon many factors,
including the
patient's weigth, body surface area, age, the particular compound to be
administered, sex, time
and route of administration, general health, and other drugs being
administered concurrently.
Progress can be monitored by periodic assessment.
The pharmaceutical compositions and formulations referred to herein are
administered at least
once in accordance with the use of the present invention. However, the said
pharmaceutical
compositions and formulations may be administered more than one time, for
example from
one to four times daily up to a non-limited number of days.
Specific formulations of the substance according to the invention are prepared
in a manner
well known in the pharmaceutical art and usually comprise at least one active
substance
referred to herein above in admixture or otherwise associated with a
pharmaceutically
acceptable Garner or diluent thereof. For making those formulations the active
substances)
will usually be mixed with a carrier or diluted by a diluent, or enclosed or
encapsulated in a
CA 02501253 2005-03-18
27
capsule, sachet, cachet, paper or other suitable containers or vehicles. A
carrier may be solid,
semisolid, gel-based or liquid material which serves as a vehicle, recipient
or medium for the
active ingredients. Said suitable carnets comprise those mentioned above and
others well
known in the art, see, e.g., Remington's Pharmaceutical Sciences, Mack
Publishing Company,
Euston, Pennsylvania. The formulations can be adopted to the mode of
administration
comprising the forms of tablets, capsules, suppositories, solutions,
suspensions or the like.
The dosing recommendations will depend on the genotype of the patient and will
be indicated
in the product labeling. This will allow the doctor to select the drug
formulation that contains
the drug concentration which is suited to the specific genotype of a patient.
It is a prerequisite
that the gene for the drug metabolizing enzyme is first analyzed before the
specific drug
formulation is prescribed. This will avoid that the wrong drug is prescribing
the wrong
patients at the wrong dose.
'The present invention also encompasses all embodiments described in
connection with
pharmaceutical compositions in US patents: 4,695,578; 4,753,789; 5,578,628;
5,955,488;
6,063,802; 4,886,808; 6,294,548; 4,906,755.
The kit of the present invention may be tailored for phenotypic and/or
genotypic screening.
According to one embodiment, the assay system and kit preferably employ
antibodies specific
to a plurality of metabolites on a suitable substrate allowing for detection
of the preferred
metabolites in a biological sample of an individual after consumption of a
corresponding
probe substrate. The assay systems of the present invention may be provided in
a plurality of
forms including but not limited to a high-throughput assay system or a
dipstick based assay.
These and other embodiments are disclosed and encompassed by the description
and
examples of the present invention. Further literature concerning any one of
the materials,
methods, uses and compounds to be employed in accordance with the present
invention may
be retrieved from public libraries and databases, using for example electronic
devices. For
example the public database "Medline" may be utilized, which is hosted by the
National
Center for Biotechnology Information and/or the National Library of Medicine
at the National
Institutes of Health. Further databases and web addresses, such as those of
the European
Bioinformatics Institute (EBI), which is part of the European Molecular
Biology Laboratory
(EMBL) are known to the person skilled in the art and can also be obtained
using Internet
CA 02501253 2005-03-18
28
search engines. An overview of patent information in biotechnology and a
survey of relevant
sources of patent information useful for retrospective searching and for
current awareness is
given in Berks, TIBTECH 12 (1994), 352-364.
S The above disclosure generally describes the present invention. Several
documents are cited
throughout the text of this specification. Full bibliographic citations may be
found at the end
of the specification immediately preceding the claims. The contents of all
cited references
(including literature references, issued patents, published patent
applications as cited
throughout this application and manufacturer's specifications, instructions,
etc) are hereby
expressly incorporated by reference; however, there is no admission that any
document cited
is indeed prior art as to the present invention.
The above disclosure generally describes the present invention. A more
complete under-
standing can be obtained by reference to the following specific examples which
are provided
herein for purposes of illustration only and are not intended to limit the
scope of the
invention.
EXAMPLES
The examples which follow further illustrate the invention, but should not be
construed to
limit the scope of the invention in any way. Detailed descriptions of
conventional methods,
such as those employed herein can be found in the cited literature; see also
"The Merck
Manual of Diagnosis and Therapy" Seventeenth Ed. ed by Beers and Berkow (Merck
& Co.,
Inc. 2003).
The practice of the present invention will employ, unless otherwise indicated,
conventional
techniques of cell biology, cell culture, molecular biology, transgenic
biology, microbiology,
recombinant DNA, and immunology, which are within the skill of the art.
Methods in molecular genetics and genetic engineering are described generally
in the current
editions of Molecular Cloning: A Laboratory Manual, (Sambrook et al., (1989)
Molecular
Cloning: A Laboratory Manual, 2nd ed., Cold Spring Harbor Laboratory Press);
DNA
Cloning, Volumes I and II (Glover ed., 1985); Oligonucleotide Synthesis (Gait
ed., 1984);
Nucleic Acid Hybridization (Harnes and Higgins eds. 1984); Transcription And
Translation
(Hames and Higgins eds. 1984); Culture Of Animal Cells (Freshney and Alan,
Liss, Inc.,
CA 02501253 2005-03-18
29
1987); Gene Transfer Vectors for Mammalian Cells (Miller and Calos, eds.);
Current
Protocols in Molecular Biology and Short Protocols in Molecular Biology, 3rd
Edition
(Ausubel et al., eds.); and Recombinant DNA Methodology (Wu, ed., Academic
Press). Gene
Transfer Vectors For Mammalian Cells (Miller and Calos, eds., 1987, Cold
Spring Harbor
Laboratory); Methods In Enzymology, Vols. 154 and 155 (Wu et al., eds.);
Immobilized Cells
And Enzymes (IRL Press, 1986); Perbal, A Practical Guide To Molecular Cloning
(1984); the
treatise, Methods In Enzymology (Academic Press, Inc., N.Y.); Immunochemical
Methods In
Cell And Molecular Biology (Mayer and Walker, eds., Academic Press, London,
1987);
Handbook Of Experimental Immunology, Volumes I-IV (Weir and Blackwell, eds.,
1986).
Reagents, cloning vectors, and kits for genetic manipulation referred to in
this disclosure are
available from commercial vendors such as BioRad, Stratagene, Invitrogen, and
Clontech.
General techniques in cell culture and media collection are outlined in Large
Scale
Mammalian Cell Culture (Hu et al., Curr. Opin. Biotechnol. 8 (1997), 148);
Serum-free
Media (Kitano, Biotechnology 17 (1991), 73); Large Scale Mammalian Cell
Culture (Curr.
Opin. Biotechnol. 2 (1991), 375); and Suspension Culture of Mammalian Cells
(Birch et al.,
Bioprocess Technol. 19 (1990), 251); Extracting information from cDNA arrays,
Herzel et al.,
CHAOS 11, (2001), 98-107.
Example 1: Patients and methods for analysis of the genetic predisposition to
a cardiac
disorder
Studv design and data mans eg ment
Study design and the demographic characteristics of the entire study
population have been
described in detail elsewhere (Pfreundschuh et al., Blood 104 (2004), 634-641;
Pfreundschuh
et al., Blood 104 (2004), 626-633). The present pharmacogenomic analysis was
designed as a
nested case-control study including all cases and matched controls of the
cohort. The study
protocol was approved by the relevant institutional review boards and ethics
committees and
all participants gave written informed consent. The trial included patients
from 162 centers
with normal lactate dehydrogenase (LDH) aged =60 years (NHL-B 1 ) and patients
aged 61-75
years (NHL-B2) with normal and elevated LDH levels. Young patients with
elevated LDH
levels were recruited into a different trial addressing the role of high dose
chemotherapy with
stem cell transplantation (Kaiser et al., J. Clin. Oncol. 20 (2002), 4413-
4419). Since no DNA
was available from patients included in this latter trial, the data from these
young high-risk
patients could not be included in the present analysis.
CA 02501253 2005-03-18
Altogether, 1697 patients aged 18 to 75 years with aggressive NHL (mainly:
diffuse large B
cell NHL) were recruited and eligible patients were randomized into the four
arms of the
study. The arms were designed to compare the standard CHOP-21 chemotherapy
with three
variants including either an addition of etoposide (CHOEP-21: 100 mg/mz on
days 1-3), the
5 shortening to 2-week intervals using recombinant human granulocyte colony-
stimulating
factor (rhG-CSF; CHOP-14) or both (CHOEP-14). All patients received the
standard CHOP
scheme defined as cyclophosphamide (750 mg/m2 i.v.), doxorubicin (50 mg/m2
i.v.),
vincristine (2 mg i.v.) all given on day 1, and prednisone (100 mg/ day per
os) given on days
1-5. All patients were planned to receive 6 cycles of chemotherapy. Acute
toxicities were
10 classified according to the WHO Handbook for Reporting Results of Cancer
Treatment.
During treatment, electrocardiography was conducted in patients complaining of
symptoms
suggestive of impaired heart function. Follow-up examinations were conducted
every 3
months in the first 2 years and every 6 months thereafter. The follow -up
examinations
relevant to cardiotoxicity detection were scheduled at 1, 2 and 5 years after
the therapy and
15 included electrocardiography and echocardiography as well as physical
examination. The two
relevant diagnoses reported to the study center were arrhythmia and heart
failure. Detailed
information was then obtained for the individual patients from the reporting
physicians.
Treatment-related cardiotoxicity cases were defined based on the following
criteria: Cases of
arrhythmia (in the absence of arrhythmia in the patient history prior to
treatment), of
20 myocarditis-pericarditis and of acute heart failure until the end of the
third cycle, were defined
as acute ACT. Cases recorded after the third cycles were defined as chronic
heart failure cases
in the absence of heart failure prior to chemotherapy. A reduction of the
ejection fraction (EF)
below 50 % or of the fractional shortening below 25 % was classified as
chronic ACT. The
evaluation and classification of cases was performed by two independent teams
of physicians,
25 and a consensus was established during a third, joint evaluation.
DNA isolation and eenotypinQ
Peripheral blood lymphocytes were collected before therapy with patients'
consent. The
genotyping studies were conducted after authorization by the ethics committee
of the
30 University of Goettingen. Genotyping was performed either by
pyrosequencingTM on the
PSQTM HS 96A System, or or on 7900 HT Sequence Detection System using pre-
developed
assays by Applied Biosystems (SNP Assays-on-Demand). Genetic variants
genotyped by
the latter, commercially available assays; see Example 2, infra. Pximer
sequences for the other
assays can be obtained upon request. Several controls were included to exclude
mix-ups and
CA 02501253 2005-03-18
31
other errors during genotyping. Thus, each plate contained a well with DNA-
free reaction mix
to detect contamination with DNA. Another well contained a dedicated DNA,
which was
expected to yield identical genotypes for all plates genotyped for a given
genetic variant.
Furthermore, seven DNAs were genotyped twice for all variants. No genotyping
errors were
detected using these controls.
Statistical analysis
The deviation of the genotype distributions from Hardy-Weinberg equilibrium
(HWE) was
tested with Pearson's goodness-of fit Chi-square test. The lack of deviation
of the genotype
distribution among controls from HWE was necessary for the subsequent
association testing.
The latter was performed using the procedure by Freidlin (Freidlin et al.,
Hum. Hered. 53
(2002), 146-152), which is a modified Cochran-Armitage trend test. Pearson's
(Von Hoff et
al., Ann. Intern. Med. 91 (1979), 710-717) test was used to validate the
results of Freidlin's
test especially for genotypes where no or few homozygotes of the variant
allele were detected.
Wherever necessary due to small cell counts, Fisher's exact test was used to
validate results.
Results were also validated using multiple logistic regression for each
genetic variant
individually, adjusting for age, gender, total dose received, and for dosing
interval (14 versus
21 days). In addition multiple logistic regression was used to investigate
combinations of
genetic variants that were individually significant. The significance level
was set at 5 % , as
appropriate for screening purposes. Therefore, no adjustment for multiple
testing was made.
Example 2: Polymorphisms in the genes involved in the generation of reactive
oxygen
species (ROS) and drug transmembrane transport are indicative for
cardiac disorders
Of the 1697 patients enrolled, 147 were reported to the study center because
of cardiac
problems during or after chemotherapy treatment. Of those, based on a detailed
source data
review, 38 were excluded from further analysis since there was evidence of pre-
existing
cardiac disease or the cardiac dysfunction could not be substantiated. Of the
remaining 109
patients, 55 developed an acute and 54 a chronic ACT (cumulative incidence of
either form
3.2 %). No DNA samples were available for 22 of them, leaving 87 patients (44
with an acute
and 43 with a chronic ACT) who were subjected to genetic analysis presented in
the
following. A detailed review of the 44 acute ACT patient files revealed 12
cases of atrial
fibrillation, 2 cases of myocarditis-pericarditis, in 1 case myocardial
infarction and S patients
showed clinical signs of acute heart failure. The 43 chronic cases showed a
reduction of the
ejection fraction (EF) below 50 % or fractional shortening values below 25 %.
The median
CA 02501253 2005-03-18
32
time between the therapy onset and first report of cardiotoxicity was 6
months, with the
interquartile range of 4 to 15 months.
The ACT cases were then matched for age, gender and weight with 363 patients
free of any
clinical symptoms of arrhythmia, heart failure or other cardiac symptoms
possibly related to
cardiotoxicity at any time point of the study. T'he data on cases and controls
are given in Table
1.
._~
fi,.w.. F
.
s,
.
.
~~
~ 1 ~>
d~
~
~~
~__~
\
7/16 3/21 0/37 12/151
3% 2% 7% 8%
2.717.9 1.2f13.2 2.0110.9 1.3111.0
4.2f13.8 4.1115.5 4.2114.6 4.9113.7
[186.5] 00 [121.3] 04 40 [90.0]
[160.5]
HOP-14 10 HOP-14 12 HOP-14 22 HOP-14 103
HOEP-14 8 HOEP-1413 HOEP-14 21 HOEP-14 93
HOP-21 11 HOP-21 8 HOP-21 19 HOP-21 76
HOEP-21 14 HOEP-2111 HOEP-21 25 HOEP-21 91
Table 1: Gender, age, weight, received doxorubicin dose and distribution among
the
therapy arms of cases and controls. Age given as mean +/ - SD. Dose given as
10 median. Interquartile differences are given in brackets.
In each group, patients were distributed approximately equal among the four
arms of the
therapy. The cumulative total dose of doxorubicin was by about 7 % lower in
cases compared
to controls, due to the necessity to abandon treatment in some patients due to
arrhythmias. Six
15 out of 87 cases (7 %) and 15 out of 363 controls (4 %) received irradiation
of the mediastinal
region (p=0.26, Fisher's exact test). A total of 210 polymorphisms in 82 genes
with
conceivable role in ACT were analyzed in controls. The genes play a role in
the metabolism
of reactive oxygen species, in drug transport and metabolism, DNA repair,
endothelial
physiology, the renin angiotensin aldosteron-system, muscle contraction and
structure,
inflammation and cell cycle. Furthermore, variants of adrenergic receptors
were analyzed. All
CA 02501253 2005-03-18
33
but 17 variants were confirmed as biallelic markers. The Ser893A1a variant of
MDR1 and
rs746578 TTN/Titin proved to be triallelic, whereas 15 other variants were
monomorphic in
the DNA samples screened. The genotypes of all but 14 biallelic markers were
in Hardy-
Weinberg equilibrium among controls.
Table 2: Primer and target sequences of the MRP1, MRP2, , CYBA, RAC2, and NCF4
genes.
Entrez
Gene Gene mRNA Target Sequence context rs number
GeneiDsequence
MRP1 4363 -
NM 004996TCTCCATCCCYGAAG (G/T] TGCTTTGGTGGCCGT
MRP2 1244 NM 0 3 TGAAACACAATGAGG [T/A] GAGGATTGACACCAA8187694
2.1
MRP2 12~ NM 000392.1GGAAGATTATAGAGT [G/A] CGGCAGCCCTGAAGA8187710
CYBA 1535 NM 000101.1CCCAGGGGACAGAAG [C/T] ACATGACCGCCGTGG4673
RAC2 5880 13058338
282188.2 ACGGAGGAAGGATGG [A/T] GCATTCAAGGAACCC
NCF4 4689 AL008637.1AAGACACCCTGATG [A/G] CTGGGACCCCATCTC1883112
Gene Effect Sequence Forward PrlmerSequence Reverse Primer
MRP1 GIy671Va1BIOTIN- TTGTCCATCTCAGCCAAGAG
CTGAGCCAGGTGTGTTGTG
MRP2 Va11188G1uGCAGCGATTTCTGAAACACA BIOTIN-CCTCCCACCGCTAATATCAA
MRP2 Cys1515TyrTGGTCCTAGACAACGGGAAG BIOTIN-CTAACCCATGGGGCCTTCT
CYBA His72TyrGTTTTGTGGGAGGAAAGAGG BIOTIN -CGGCCCGAACATAGTAATTC
RAC2 intron qssa ID: C 11744093
2 1
NCF4 Promoter
region Assa ID: C 11521119
1
Gene Sequence Sequencing PCR Fra ( pent Method Assay
Primer length
MRP1 CGGCCACCAAAGCA 132 Pyro. reverse
MRP2 TTCTGAAACACAATGAGG 155 Pyro. forward
MRP2 CAACGGGAAGATTATAGAG 147 Pyro. forward
CYBA CCCCAGGGGACAG 184 Pyro. forward
RAC2 Cetera RefSNP TaqMan
ID
hCV11744093
NCF4 Cetera RefSNP TaqMan
ID
hCV11521119
CA 02501253 2005-03-18
34
As shown in Table 3, six variants showed statistically significant (p<0.05)
associations with
ACT in either association test.
Table 3: Distribution of genotypes and p values for polymorphisms showing
associations with ACT.
Chronic ACT showed an association with the -212A>G variant of the NAD(P)H
oxidase
30 subunit NCF4, which is responsible for down-regulation of theenzyme. The
predisposing
NCF4 genotype is AA and it conferred an OR of 2.5 (95 % CI: 1.3 - 5.0) (Fig. 1
).
CA 02501253 2005-03-18
Acute ACT was associated with two SNPs in two further subunits of the same
enzyme:
p22phox and RAC2. The predisposing allele T at position 242 (242C>T) in the
gene CYBA,
coding for p22phox, leads to the missense mutation His72Tyr. The distribution
of the
genotypes in cases deviated from HWE (p<0.001). The homozygous earners of T
and C
5 alleles were underrepresented (in acute ACT 3 instead of 7 expected for TT
and 13 instead of
17 for CC, whereas the heterozygotes were overrepresented (28 instead of
expected 21 ). The
group of homozygous and heterozygous carriers of the T allele had an increased
risk of acute
ACT characterized by an odds ratio of 2.0 (95 % CI: 1. 0 - 3.9, p=0.048). The
almost identical
increase in the odds ratio for chronic cases of 1.9 (95 % CI: 1.0 - 3.8) was
of borderline
10 statistical significance (p=0.062). The other association with acute ACT
was found for the
intron 2 variant (7508T~A) of the regulatory subunit of the enzyme RAC2, which
conferred
an OR of 2.6 (95 % CI: 1.3 - 5.1) for carrier of the A allele.
In addition, acute ACT was associated with three polymorphisms in the
transmembrane efflux
15 transporters of anthracyclines, MRP1 and MRP2 (Table 3). The predisposing
allele A of the
MRP1 variant G1y671Va1 conferred an odds ratio of 3.6 (95 % CI: 1.6 - 8.4).
The two
missense mutations in MRP2, Vall 188G1u and Cys1515Tyr, yielded identical
frequencies and
relationships with acute ACT, as characterized by an odds ratio of 2.3 (95 %
CI: 1.0 - 5.4).
An inspection of the individual genotypes revealed a 100 % linkage
disequilibrium between
20 these variants in the samples genotyped.
For all six variants, the effects of the genotypes on the risk of cardiac
disease were
statistically significant when all cases were taken together (Table 3). A
consistent, albeit not
statistically significant trend towards increased risk was observed for all
other combinations
25 of these six variants and phenotypes (Fig. 1). The exclusion of mediastinum-
in-adiated
patients had no major effect on the associations, with all p values <_ 0.051.
Results were validated by using multiple logistic regression analysis for each
genetic variant
individually, adjusting for age, gender, cumulative dose administered until
development of
30 ATC, and for dosing interval (14 versus 21 days). This analysis confirmed
the results of the
monovariate analysis with odds ratios of 1.9 (p=0.010) for the p22phox 242T
allele, of 2.0
(p=0.016) for NCF4, of 1.7 (p=0.025) for the RAC2 "A" allele. With respect to
the drug
transporters, an odds ratio of 2.5 (p=0.016) for the MRP 1 allele and an odds
ratio of 1.9
(p=0.071) for the two linked MRP2 amino acid substitutions were confirmed by
logistic
CA 02501253 2005-03-18
36
regression analysis. A two-locus analysis for the various pairs of the
associating CYBA,
NCF4, RAC2, MRP1, and MRP2 polymorphisms revealed no interactions between the
genes
involved.
S Example 3: Implications for the findings in Example 2
This is the first analysis of the genetic predisposition to ACT in humans. The
cumulative
incidence of ACT in this study (6.4 %) is slightly higher than recent
estimates of 3 to 5
(Shan et al., Ann. Intern. Med. 125 (1996), 47-58). This was primarily caused
by a much
higher prevalence of acute ACT, which accounted for every second ACT case
detected. The
prevalence of acute ACT is usually estimated at 1 % (Zucchi and Danesi, Curr.
Med. Chem.
Anti-Canc. Agents 3 (2003), 151-171). The much higher incidence of acute ACT
in this study
may have been caused by a higher detection rate, in part reflecting the
increased detection of
arrhythmias through improved monitoring as well as the enhanced awareness of
ACT.
Furthermore, all cardiac events occurring during the initial three months as
acute ACT were
counted. There is no established time cut -off between acute and chronic ACT.
Classification
based on symptoms rather than time-points is inaccurate. Arrhythmias, usually
classified as
acute cardiotoxicity, may be a first and only sign of cardiac dysfunction many
years after the
treatment. On the other hand, congestive heart failure has been observed in
patients within
hours to days after a first doxorubicin administration (Shan et al., Ann.
Intern. Med. 125
(1996), 47-58). In animal models, congestive heart failure develops within
several weeks, i.e.
prior to completion of many typical doxorubicin treatments (Forrest et al.,
Cancer Res. 60
(2000), 5158-5164). The arbitrary cut -off between acute and chronic ACT
established in
accordance with the present invention is believed to be a fair compromise
between these
divergent definitions and observations.
In addition, it cannot be excluded that in some patients arrhythmia might have
been caused by
other factors such as fluid overload and may therefore have not been related
to doxorubicin.
Finally, the increased frequency of acute (although not chronic) ACT may also
have been
increased by the co-medication with cyclophosphamide, which itself may be
cardiotoxic
(Gharib and Burnett, Eur. J. Heart Fail. 4 (2002), 235-242). Mediastinal
irradiation,
previously reported to have a borderline effect on cardiotoxicity (Torti et
al., Ann. Intern.
Med. 99 (1983), 745-749), was not significantly different between cases and
controls and
exclusion of irradiated patients had no effect on associations found. In some
patients,
cardiotoxicity may have been enhanced by co-medications, which were, however,
not among
CA 02501253 2005-03-18
37
the mandatory data collected. Dosing interval applied (14 versus 21 days) had
no effect on
cardiotoxicity.
Heart failure has been previously associated with variants of adrenergic
receptors and of the
angiotensin-converting enzyme (Small et al., N. Engl. J. Med. 347 (2002), 1135-
1142;
Borjesson et al., Eur. Heart J. 21 (2000), 1853-1858; Kaye et al.,
Pharmacogenetics 13 (2003),
379-382; Andersson and Sylven, J. Am. Coll. Cardiol. 28 (1996), 162-167). In
the present
experiments no associations between any of these variants and ACT have been
detected.
Likewise, no interaction between the ADRA2C GlyAlaGlyPro322-325de1 and the
ADRB1G1y389Arg variant (Small et al., N. Engl. J. Med. 347 (2002), 1135-1142)
has been
fould, altogether suggesting a different underlying pathophysiology. On the
other side, ACT
was asso ciated with variants of proteins implicated in two distinct
processes, the generation
of reactive oxygen species and drug, i.e. anthracycline transmembrane
transport. The
NAD(P)H oxidase mufti-enzyme complex catalyzes the 1-electron reduction of
oxygen using
either NADH or NAD(P)H as the electron donor. Best investigated in endothelium
and
macrophages, the enzyme has been recently demonstrated in the myocardium,
where it may
be a major source of reactive oxygen species (Heymes et al., J. Am. Coll.
Cardiol. 41 (2003),
2164-2171). The C242T (His72Tyr) polymorphism of the CYBA gene coding for
p22phox
affects a heme-binding site thought to be essential for the stability of the
protein. Under ex
vivo conditions, the tyrosine variant was originally reported to confer a 20-
40 % reduction in
basal activity in vessel samples (Guzik et al., Circulation 102 (2000), 1744-
2747). This
finding has been in part confirmed by Wyche et al. (Hypertension 43 (2004),
1246-1251),
who reported a gene-dose dependent reduction of phorbol ester-stimulated,
although not of
basal, NAD(P)H oxidase activity in T allele carriers. Association of reduced
activity to
generate ROS with ACT seems to be opposite to what would be expected. One may
speculate
that under in vivo conditions, inherited reduced activity of NAD(P)H oxidase
may result in
impaired ROS defence capacity and therefore in increased ROS levels under
anthracycline
exposure. On the other hand, Shimo-Nakanishi et al. (Shimo-Nakanishi et al.,
Atherosclerosis
175 (2004), 109-115) have recently reported an T allele-conferred increase in
NAD(P)H
oxidase activity both in human probands and in cells transfected with CYBA
expression
constructs. Similarly conflicting are the reported associations between the T
allele and
coronary artery disease, where an increased (Cai et al., Eur. J. Clin. Invest.
29 ( 1999),
744-748; Cahilly et al., Circ. Res. 86 (2000), 391-395), decreased (moue et
al., Circulation 97
(1998), 135-137), as well as no change (Li et al., Am. J. Med. Genet. 86
(1999), 57-61;
CA 02501253 2005-03-18
38
Gardemann et al., Atherosclerosis 145 (1999), 315-323) in risk have been
described.
Altogether, the final verdict on the functional consequences of the C242T
allele is still out.
The NCF4 gene encodes the p40phox subunit of the NAD(P)H oxidase, which is
responsible
for the downregulation of the enzyme (Lopes et al., Biochemistry 43 (2004),
3723-3730). The
S NCF4 variant predisposing to ACT is located in the putative promoter of the
gene, but its
functional consequences are at present unknown.
The NAD(P)H oxidase requires for its activity also binding of the small GTPase
RAC2,
which may in addition induce the enzyme complex assembly (Price et al., J.
Biol. Chem. 277
(2002), 19220-19228), possibly by activation of cytosolic protein kinases
(Shalom-Barak and
Knaus, J. Biol. Chem. 277 (2002), 40659-40665). A single nucleotide
polymorphism located
in intron 2 of RAC2 may serve as a marker for a functional, at present unknown
variant.
Alternatively, it could itself be functional and affect e.g. splicing or
transcription of RAC2.
Altogether, the association with variants encoding three proteins of the of
NAD(P)H oxidase
complex is a strong evidence for the role of this enzyme in anthracycline-
induced heart
failure.
The propensity to ACT is also increased in patients carrying variant alleles
of the multidrug
resistance proteins MRP1 and MRP2. Both genes belong to subfamily C of ATP -
binding
cassette (ABC) transporters and act as cellular efflux pumps for numerous endo-
and
exogenous substrates. The human MRP1 confers resistance to anthracyclines, and
was in fact
originally cloned from a doxorubicin-selected cancer cell line (Cole et al.,
Science 258
(1992), 1650-1654). MRP1 is expressed in human (Flens et al., Am. J. Pathol.
148 (1996),
1237-1247) and murine (Wijnholds et al., Nat. Med. 3 (1997), 1275-1279)
myocardium. A
targeted deletion of MRP1 in the mouse increases the accumulation of etoposide
in the heart
(Wijnholds et al., J. Clin. Invest. 105 (2000), 279-285), but no data are
available on the
accumulation of doxorubicin. As opposed to polarized cells, in the
cardiomyocytes MRP1 is
found in the cytoplasm in addition to plasma membrane (Flens et al., Am. J.
Pathol. 148
(1996), 1237-1247). It has been postulated that such an expression may permit
sequestration
of doxonabicin in lysosomes, i.e. away from its target the nucleus (Rajagopal
and Simon, Mol.
Biol. Cell 14 (2003), 3389-3399). A low-frequency protein variant of MRP1
(Arg433Ser) has
been shown to affect resistance to anthracyclines in vitro, but has not been
determined in the
present study (Conrad et al., Pharmacogenetics 12 (2002), 321-330). The effect
on
doxorubicin resistance or transport of the variant 671 Val, which shows an
association with
CA 02501253 2005-03-18
39
acute ACT in this study, has not been investigated (Conrad et al., J. Hum.
Genet. 46 (2001 ),
656-663).
MRP2 has a substrate spectrum similar to that of MRPl and increases the
resistance to
doxorubicin when overexpressed in HEK 293 cells (Cui et al., Mol. Pharmacol.
55 (1999),
929-937). Physiologically, the MRP2 protein is expressed in the apical
membrane of
polarized cells in the liver and in the kidney and there is no evidence for
its expression in the
heart. The observed association could be caused by a reduced biliary
elimination of
doxorubicin, which normally accounts for 50 % of its disposition. In support
of this model, an
inhibition of MRP2 expression by a bacterial toxin decreased biliary clearance
of doxorubicin
and increased its plasma concentration (Hidemura et al., Antimicrob. Agents
Chemother. 47
(2003), 1636-1642). The lower incidence of heart damage following doxorubicin
infusion as
opposed to bolus injection suggests a role for the drug's peak levels in
doxorubicin-induced
cardiotoxicity (Hortobagyi et al., Cancer 63 (1989), 37-45). Significant inter-
individual
differences in doxorubicin pharmacokinetics have been reported previously
(Jacquet et al.,
Cancer Chemother. Pharmacol. 27 (1990), 219-225; Piscitelli et al., Clin.
Pharmacol. Ther. 53
(1993), 555-561) and they could result from genetic variants in transporters
such as MRP2.
The two missense variants associating with ACT, Va11188G1u and Cys1515Tyr,
were initially
described in the Japanese (Itoda et al., Drug Metab. Dispos. 30 (2002), 363-
364). No data
regarding their functional significance have been published and the specific
variant relevant to
doxorubicin treatment cannot be inferred from our results, due to the 100 %
linkage
disequilibrium between the two missense mutations in our cohort.
Additional support for the associations found can be derived from the
functional context of
the findings of the present invention. In the case of ACT, this support is
provided by the
functional similarity between the proteins. Both MRP1 and MItP2 transport
doxorubicin.
Therefore, an association of variants of either gene with ACT can be regarded
as an additional
evidence for the hue character of this association. Similarly supporting is
our finding of the
association of variants of CYBA, NCF4 and RAC2, which are members of the same
signalling complex.
The relationship between the acute and chronic ACT is unclear. Acute ACT is
usually
attributed to damage caused by reactive oxygen species arising from one-
electron reduction of
anthracyclines. The chronic form has been proposed to result , at least in
part, from the effects
CA 02501253 2005-03-18
of antracycline alcohols, which are generated by their two-electron reduction
by enzymes
such as aldo-keto reductases and carbonyl reductases (Mordente et al.,
Biochem. Pharmacol.
66 (2003), 989-998). However, there is increasing evidence for a "unifying
hypothesis" of
ACT (Mordente et al., IUBMB Life 52 (2001 ), 83-88). Reactive oxygen species
have been
5 found to induce the expression of these enzymes (Spycher et al, Faseb J. 11
(1997), 181-188)
and may thus facilitate the formation of the toxic alcohol metabolites and
chronic ACT. The
present data appear to support a common pathophysiology of acute and chronic
ACT. Indeed,
variants of the NAD(P)H oxidase subunits and the two MRP genes exhibit a
consistent trend
to associate with either ACT form, although not all of these associations
reach the set level of
10 significance. The generally lower number of significant associations for
chronic cases
considered separately could be caused by the relatively short follow-up
period. Indeed,
abnormal cardiac parameters have been described in 18 % of patients after 4-10
years
(Steinherz et al, Jama 266 (1991), 1672-1677) and in 65 % of patients after 15
years
(Lipshultz et al., N. Engl. Med. 324 (1991), 808-815). Therefore, it is likely
that the controls
15 used contain a significant number of patients which will develop
cardiotoxicity in the future
and their genetic makeup diminishes the strength of the associations. On the
other hand, all
six variants show statistically significant associations when acute and
chronic cases are taken
together.
20 The ultimate goal of studies such as this is to develop a system of mo
lecular-genetic
diagnostic tests which would help to detect persons at high risk for
cardiotoxicity. The
population-based attributable risk (PAR) estimates the proportion of ACT cases
caused by the
Garner-status of a given genetic variant in relation to all cases of ACT. The
PAR values are
between 7 % for MRP2 and 29 % for CYBA. These values suggest that up to 29 %
of the
25 possible ACT cases could be explained by the carrier status. The
sensitivity varies between
15 % (MRP1) and 70 % (CYBA), indicating that a substantial portion of at risk-
individuals
could be detected.