Note: Descriptions are shown in the official language in which they were submitted.
CA 02501807 2005-04-08
WO 2004/032953 PCT/DK2003/000691
TREATMENT OF ALLERGIC CONDITIONS BY USE OF IL 21
FIELD OF THE INVENTION
The invention relates to management, treatment and prevention of allergic
reactions,
allergic diseases and allergic conditions by administation of IL-21. Further,
the invention
relates to treatment of parasitic diseases by administering IL-21 to patients
infected with
parasites.
BACKGROUND OF THE INVENTION
IL-21, which has also been termed Zalpha11, is a cytokine, which was shown to
be
produced by activated CD4+ T lymphocytes after stimulation with anti-CD3,
phorbol ester
plus ionomycin (Parrish-Novak et al., Nature 408, 57 - 63 (2000)).
Numerous studies have shown that eosinophils play a central in the development
of
asthma and other allergic diseases (reviewed in Foster et al., TRENDS in
Molecular
Medicine 8, 162 -167 (2002)). Eosinophils are produced from stem cells in the
bone marrow
('eosinopoiesis') in response to IL-5 and eotaxin, and the eosinophils migrate
via the blood to
the lungs where they are activated. Such activated eosinophils release
proinflammatory
molecules and granular proteins, which can damage the lung tissue and induce
airways
hyperresponsiveness. It is widely held that so-called T helper 2 ('TH2')
lymphocytes in the
lungs orchestrate most cellular reactions via their release of 'TH2 cytokines'
(i.e. IL-4, IL-5, IL-
9, IL-10 and IL-13). According to this view, eosinophils and basophils act as
effector cells
only (reviewed in Wills-Karp et al. Annu. Rev. Immunol. 77, 255 (1999)).
Surprisingly, we
have now discovered that IL-21 is produced by, stored in and released by human
eosinophilic granulocytes (hereinafter 'eosinophils'). IL-21 has been shown to
act as a co-
stimulatory cytokine for proliferation of T and B lymphocytes (Parrish-Novak
et al., supra).
Thus, eosinophils may in addition to their role as primary effector cells,
which damage the
respiratory epithelium, also play another - to this point unrecognized -
protective effect by
releasing IL-21. Released IL-21 from eosinophils (hereinafter 'eosinophilic IL-
21') may in turn
either directly, or in concert with other cytokines, prevent differentiation
and activation of
cells involved in allergic reactions and thereby contribute to a less severe
allergic reaction.
According to this view, eosinophils may play a dual role in allergic
reactions. On one hand
the release granular proteins from eosinophils can damage tissues, but on the
other hand
release of eosinophilic IL-21 may reduce the allergic reactions via an effect
on other cells.
Asthma has been increasing in prevalence, morbidity, and mortality over the
last two
SUBSTITUTE SHEET (RULE 26)
CA 02501807 2005-04-08
WO 2004/032953 PCT/DK2003/000691
2
decades, and there is a strong need for improved treatment of asthma and other
allergic
diseases.
The above brief description of the central role of eosinophils in asthma is
believed to
apply to allergic reactions in general. A list of allergic conditions or
diseases, which is not
intended in anyway to limit the scope of the invention, include asthma,
anaphylaxis, drug
reactions, food allergy, insect venom allergy, allergic.rhinitis, urticaria,
eczema, atopic
dermatitis, allergic contact allergy, allergic conjunctivitis. Such allergic
reaction, allergic
diseases or allergic conditions may be managed, treated or prevented by
administering
I L-21.
Eosinophils are further important for combating parasitic diseases including
but not
limited to helminthic infections.
The present invention relates to a method for treating, preventing and/or
managing
' allergic diseases or conditions by administration of iL-21.
SUMMARY OF THE INVENTION
It has been found that eosinophils synthesize, store and/or secrete IL-21.
Eosinophils may be involved in the protective response to allergic reactions,
diseases and
conditions. The invention relates to managing, treating and preventing
allergic reactions,
conditions and diseases by administration of IL-21.
DEFINITIONS
, A "polypeptide" is a polymer of amino acid residues linked by peptide bonds,
and
. may be produced naturally or synthetically. Polypeptides of less than about
10 amino acid
residues are commonly referred to as "peptides".
A "protein" is a macromolecule comprising one or more polypeptide chains,
which
may be produced naturally or synthetically. A protein may also comprise non-
peptidic
components, such as carbohydrate groups or other non-peptidic substituents.
Carbohydrates
and other non-peptidic substituents may be added to a protein by the cell in
which the protein
is produced, and will vary with the type of cell. Carbohydrates and other non-
peptidic
substituents may also be added synthetically after the cell-based production
of the protein.
Proteins are defined herein in terms of their amino acid backbone structures;
substituents
such as carbohydrate groups or other non-peptidic substituents are generally
not specified,
but may be present nonetheless.
International Patent Application No. PCT/US06067, publication no. WO 00/53761,
published September 14, 2000, which is hereby incorporated in this application
in its entirety,
CA 02501807 2005-04-08
WO 2004/032953 PCT/DK2003/000691
3
discloses IL-21 (as "cytokine zalpha11 ligand") as SEQ ID No. 2, which is
hereby
incorporated in this application in its entirety, and which is also shown as
SEQ ID No. 2 in
this application, as well as methods for producing it and antibodies thereto
and a
polynucleotide sequence encoding 1L-21 as SEQ ID No. 1. The present invention
also
contemplates the use of IL-21 polypeptides which as used herein should be
taken to mean
polypeptides with a sequence identity to the polypeptide of SEQ ID No: 2, or
their orthologs
comprising at least 70%, at least 80%, at least 90%, at least 95%, or greater
than 95%. The
present invention also includes the use of polypeptides that comprise an amino
acid
sequence having at least 70%, at least 80%, at least 90%, at least 95% or
greater than 95%
10: sequence identity to the sequence of amino acid residues 1 to 162,
residues 30 to 162, or
residues 33 to 162 of SEQ ID No: 2. Methods far determining percent identity
are described
below. The IL-21 polypeptides of the present invention have retained all or
some of the
biological activity of IL-21 which makes IL-21 useful for treating allergic
reactions, allergic
diseases and allergic conditions. Some of the polypeptides may also have a
biological
activity which is higher than the biological activity of IL-21.
"Eosinophilic cells", "eosinophilic granulocytes" or "eosinophils" are defined
as
leukocytes that contain red-staining eosinophil granules (e.g. with Wright's
or Hematoxylin &
Eosin stain). Eosinophils are involved both in normal physiological 'reactions
and in disease
processes. Included in the definition, but not limited to this definition, are
(i) eosinophils in the
bone marrow, (ii) eosinophils circulating in peripheral blood, (iii)
eosinophils involved allergic
reactions (including but not limited to asthma, anaphylaxis, drug reactions,
food allergy;
insect venom allergy, allergic rhinitis, urticaria, eczema, atopic dermatitis,
allergic contact
allergy, allergic conjunctivitis), (iv) eosinophils involved in malignant
diseases (with Hodgkin's
lymphoma, mycosis fungoides, chronic myelogenous leukemia, and cancer of the
lung,
stomach, pancreas, ovary or uterus as non-limiting examples), (v) eosinophils
involved in
autoimmune reactions, (vi) eosinophils involved in collagen vascular diseases
(with
rheumatoid arthritis and periarteritis as non-limiting examples), (vii)
eosinophils involved in
. helminthic infections, and/or (vii) eosinophils involved in iatrogenic
disorders. Also included
are eosinophils in Loeffler's syndrome, eosinophilia-myalgia syndrome and
idiopathic
hypereosinophilic syndromes.
"Eosinophific IL-21", as used herein, should be taken to mean LL-21 that is
produced
by, stored in and/or secreted by eosinophilic granulocytes.
The term "treatment" and "treating" as used herein means the management and
care of a patient for the purpose of combating a condition, such as a disease
or a disorder.
The term is intended to include the full spectrum of treatments for a given
condition from
CA 02501807 2005-04-08
WO 2004/032953 PCT/DK2003/000691
4
which the patient is suffering, such as prevention of the condition, the
delaying of the
progression of the disease, disorder or condition, the alleviation or relief
of symptoms and
complications, and/or the cure or elimination of the disease, disorder or
condition. The
patient to be treated is preferably a mammal, in particular a human being.
DESCRIPTION OF THE DRAWINGS
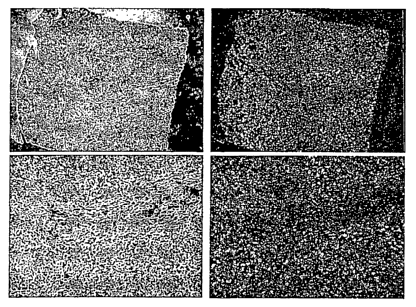
Figure 1a
In situ hybridization showing expression of IL-21 mRNA in a specimen of
Hodgkin's
Lymphoma with eosinophilic infiltration. Images of the same field are shown as
brightfield in
the left panels and as darkfield in the right panels. The signal for IL-21
mRNA is seen as
bright spots in the darkfield images.
Figure 1 b
In situ hybridization showing expression of iL-21 mRNA in eosinophils in a
specimen
of Hodgkin's Lymphoma with eosinophilic infiltration. In the high
magnification picture at right
the signal for IL-21 mRNA is visible as black silver grains over eosinophils
(arrows).
Figure 2
In situ hybridization showing,expression of IL-21 mRNA in a specimen of
Hodgkin's
Lymphoma with lymphocytic predominance. Images of the same field are shown as
brightfield in the left panel and as darkfield in the right panel.-The signal
for IL-21 mRNA is
seen over lymphocytes as bright spots surrounding the Reed-Sternberg cell
(arrow) in the
darkfield image.
Figure 3
In situ hybridization showing weak expression of IL-21 receptor mRNA in
specimens
of Hodgkin's Lymphoma with eosinophilic infiltration (upper panels) or with
lymphocytic
predominance (lower panels). Images of the same field are shown as brightfield
in the left
panels and as darkfield in the right panels. The weak signal for IL-21 R mRNA
is seen
diffusely all over the tissue as bright spots in the darkfield images.
Figure 4
SEQ ID NO. 1: DNA sequence encoding IL-21.
CA 02501807 2005-04-08
WO 2004/032953 PCT/DK2003/000691
Figure 5
SEQ ID NO. 2: amino acid sequence of IL-21.
DESCRIPTION OF THE INVENTION
It has been found that eosinophils synthesize, store and/or secrete IL-21.
5 Eosinophils may be involved in the protective response to allergic
reactions, diseases and
conditions.
In one embodiment, the invention relates to treating diseases or conditions
where
eosinophils are involved in a protective response, including but not limited
to allergic
reactions or conditions, by administration of IL-21 to a subject in need
thereof.
In one embodiment, the invention relates to treating allergic reactions,
conditions
and diseases by administration of IL-21 to a subject in need thereof.
In one embodiment, the invention relates to treating diseases or conditions
where
eosinophils are involved in a protective response, including but not limited
to allergic
reactions or conditions, by administration of IL-21 polypeptides that have a
sequence identity
to the polypeptide of SEQ ID No: 2, or their orthologs of at least 70%; at
least 80%, at least
90%, at least 95%, or greater than 95%. The present invention also includes
the use of
polypeptides that comprise an amino acid sequence having at least 70%, at
least 80%, at
least 90%, at least 95% or greater than 95% sequence identity to the sequence
of amino
acid residues 1 to 162, residues 30 to 162, or residues 33 to 162 of SEQ
IDvNo: 2. Methods
for determining percent identity are described below. The: polypeptides of the
present
invention having a sequence identity to the polypeptide of SEQ ID No: 2, or
their orthologs as
described above have retained all or some of the biological activity of IL-21
which makes
IL-21 useful for treating allergic reactions, allergic diseases and allergic
conditions.
In one embodiment, the invention relates to treating allergic reactions,
conditions
and diseases by administration of IL-21 polypeptides that have a sequence
identity to the
polypeptide of SEQ ID No: 2, or their orthologs of at least 70%, at least 80%,
at least 90%, at
least 95%, or greater than 95%. The present invention also. includes the use
of polypeptides
that comprise an amino acid sequence having at least 70%, at least 80%, at
least 90%, at .
least 95% or greater than 95% sequence identity to the sequence of amino acid'
residues 1 to
162, residues 30 to 162, or residues 33 to 162 of SEQ ID No: 2. Methods for
determining
percent identity are described below. The polypeptides of the present
invention having a
sequence identity to the polypeptide of SEQ ID No: 2, or their orthologs as
described above
have retained all or some of the biological activity of IL-21 which makes IL-
21 useful for
treating allergic reactions, allergic diseases and allergic conditions.
CA 02501807 2005-04-08
WO 2004/032953 PCT/DK2003/000691
6
In one embodiment, the invention relates to treating diseases or conditions
where
eosinophils are involved, including but not limited to allergic reactions or
conditions, by
administration of an IL-21 mimetic, that is a compound which is not an IL-21
polypeptide as
described above, but which has the biological activity of 1L-21. An IL-21
mimetic maybe a
peptide, such as a polypeptide or an oligopeptide or may be non-proteins; such
as a smaller
organic molecule.
In one embodiment, the invention relates to treating diseases or conditions
where
eosinophils are involved in a protective response, including but not limited
to allergic
reactions or conditions, by administration of an IL-21 mimetic, that is a
compound which is
not an IL-21 polypeptide as described above, but which has the biological
activity of IL-21.
An IL-21 mimetic may be a peptide, such as a polypeptide or an oligopeptide or
may be non-
proteins, such as a smaller organic molecule.
In one embodiment, the invention relates to treating diseases or conditions
where
eosinophils are involved in a protective response, including but not limited
to allergic
reactions or conditions, by administration of a polynucleotide encoding IL-21
or a IL-21
polypeptide that have a sequence identity to the polypeptide of SEQ ID No: 2,
or their
orthologs, of at least 70%, at least 80%, at least 90%, at least 95%, or
greater than 95%. In a
further embodiment, the present invention relates to managing, treating and
preventing
allergic reactions, conditions and diseases by administration of a
polynucleotide encoding
polypeptides that comprise an amino acid sequence having at (east 70%, at
least 80%, at
least 90%, at least 95% or greater than 95% sequence identity to the sequence
of amino
acid residues 1 to 162, residues 30 to 162, or residues 33 to 162 of SEQ ID
No: 2. An
example, of such a polynucleotide is shown as SEQ ID No. 1 coding for a
polypeptide with a
sequence as shown in SEQ ID No. 2.
. In one embodiment, the invention relates to treating allergic reactions,
conditions
and diseases by administration of a polynucleotide encoding IL-21 or a IL-21
polypeptide that
have a sequence identity to the polypeptide of SEQ ID No: 2, or their
orthologs, of at least
70%, at least 80%, at least 90%, at least 95%, or greater than 95%. In a
further embodiment,
the present invention relates to managing, treating and preventing allergic
reactions,
conditions and diseases by administration of a polynucleotide encoding
polypeptides that
comprise an amino acid sequence having at least 70°!°, at least
80%, at least 90%, at least
95% or greater than 95% sequence identity to the sequence of amino acid
residues 1 to 162,
residues 30 to 162, or residues 33 to 162 of SEQ ID No: 2.
Percentage sequence identity between two amino acid sequences is determined by
a Needelman-Wunsch alignment, useful for both protein and DNA alignments. For
protein
CA 02501807 2005-04-08
WO 2004/032953 PCT/DK2003/000691
7
alignments the default scoring matrix used is BLOSUM50, and the penalty for
the first
residue in a gap is -12, while the penalty for additional residues in a gap is
-2. The alignment
may be made with the Align software from the FASTA package version v20u6
(W:R:.Pearson
and D.J. Lipman (1988), "Improved Tools for Biological Sequence Analysis",
PNAS 85:2444-
2448; and W.R. Pearson (1990) "Rapid and Sensitive Sequence Comparison with
FASTP
and FASTA", Methods in Enzymology, 183:63-98).
IL-21 or other IL-21 polypeptides for use in treating asthma or other allergic
conditions according to the present invention may be administered alone or in
combination
with other established therapies such as X32-agonists, cromolyn, leukotriene
receptor
antagonists, corticosteroidcytokines, cytokine antagonists, interleukins and
interleukin
antagonists, which are only provided as non-limiting examples.
PHARMACEUTICAL COMPOSITIONS
IL-21 or other IL-21 polypeptides for use in treating asthma or other allergic
conditions according to the present invention may be administered alone or in
combination
with pharmaceutically acceptable carriers or excipients, in either single or
multiple doses.
The pharmaceutical compositions comprising IL-21 or other IL-21 polypeptides
for use in
treating asthma or other allergic conditions according to the present
invention may be
formulated with pharmaceutically acceptable carriers or diluents as well as
any other known
adjuvants and excipients in accordance with conventional techniques such as
those .
disclosed in Remington: The Science and Practice of Pharmacy, 19th Edition,
Gennaro, Ed.,
Mack Publishing Co., Easton, PA, 1995. The' compositions may appear in
conventional
forms, for example capsules, tablets, aerosols, solutions or suspensions
The pharmaceutical compositions may be specifically formulated for
administration
by any suitable route such as the oral, rectal, nasal, pulmonary, topical
(including buccal and
sublingual), transdermal, intracisternal, intraperitoneal, vaginal and
parenteral (including
subcutaneous, intramuscular, intrathecal, intravenous and intradermal) route.
It will be
appreciated that the preferred route will depend on the general condition and
age of the
subject to be treated, the nature of the condition to be treated and the
active ingredient
chosen. The route of administration may be any route, which effectively
transports the active
compound to the appropriate or desired site of action.
Pharmaceutical compositions for oral administration include solid dosage forms
such as hard or soft capsules, tablets, troches, dragees, pills, lozenges,
powders and
granules. Where appropriate, they can be prepared with coatings such as
enteric coatings or
CA 02501807 2005-04-08
WO 2004/032953 PCT/DK2003/000691
8
they can be formulated so as to provide controlled release of the active
ingredient such as
sustained or prolonged release according to methods well known in the art.
Liquid dosage forms for oral administration include solutions, emulsions,
aqueous or
oily suspensions, syrups and. elixirs.
~ Pharmaceutical compositions for parenteral administration include sterile
aqueous
and non-aqueous injectable solutions, dispersions, suspensions or emulsions as
well as
sterile powders to be reconstituted in sterile injectable solutions or
dispersions prior to use.
Depot injectable formulations are also contemplated as being within the scope
of the present.
invention,
, Other suitable administration forms include. suppositories, sprays,
ointments,
cremes, gets, inhalants, dermal patches, implants. etc.
A typical oral dosage is in the range of from about 0.001 to about 100 mg/kg
body
weight per day, such as from about 0.01 to about 50 mglkg body weight per day,
for example
from about 0.05 to about 10 mg/kg body weight per day administered in one or
more
dosages such as 1 to 3 dosages. The exact dosage will depend upon the nature
of the IL-21
polypeptide chosen, the frequency and mode of administration, the sex, age,
weight and
general condition of the subject treated, the nature and severity of the
condition treated and
any concomitant diseases to be treated and other factors evident to those
skilled in the art.
The formulations may conveniently be presented in. unit dosage form by methods
.known to those skilled in the art. A typical unit dosage form for oral
administration one or
more times per day such as 1 to 3 times per day may contain from 0.05 to about
1000 mg,
for.exampie from about 0.1 to about 500.mg, such as from about 0.5 mg to about
200 mg.
For parenteral routes such as intravenous, intrathecal, intramuscular and
similar
administration,. typically doses are in the order of about half the dose
employed for orai
administration.
Non-protein IL-21 mimetics for use in treating asthma or other allergic
conditions
according to the present invention are generally utilized as the free
substance or as a
pharmaceutically acceptable salt thereof. Examples are an acid addition salt
of a compound
having the utility of a free base and a base addition salt of a compound
having the utility of a
free acid. The term "pharmaceutically acceptable salts" refers to non-toxic
salts of such
compounds which are generally prepared by reacting the free base with a
suitable organic or
inorganic acid or by reacting the acid with a suitable organic or inorganic
base. When such a
compound contains a free base such salts are prepared in a conventional manner
by treating
a solution or suspension of the compound with a chemical equivalent of a
pharmaceutically
acceptable acid. When such a compound contains a free acid such salts are
prepared in a
CA 02501807 2005-04-08
WO 2004/032953 PCT/DK2003/000691
9
conventional manner by treating a solution or suspension of the compound with
a chemical
equivalent of a pharmaceutically acceptable base. Physiologically acceptable
salts of a
compound with a hydroxy group include the anion of said.compound in
combination with a
suitable cation such as sodium or ammonium ion. Other salts which are not
pharmaceutically
acceptable~may be useful in the preparation of compounds of the invention and
these form a
further aspect of the invention.
Salts of IL-21 polypeptides are especially relevant when the protein is in
solid or
crystalline form
For parenteral administration, solutions of the IL-21 polypeptides or IL-21
mimetics
1,0: in sterile aqueous solution, aqueous propylene glycol or sesame or peanut
oil may be
employed. Such aqueous solutions should be suitably buffered if necessary and
the liquid
diluent first rendered isotonic with;sufficient saline or glucose. The aqueous
solutions are
particularly suitable for intravenous, intramuscular, subcutaneous and
intraperitoneal
administration. The sterile aqueous media employed are all readily available
by standard
15. techniques known to those skilled in the art.
Suitable pharmaceutical carriers include inert solid diluents or fillers,
sterile aqueous
solution and various organic solvents. Examples of solid carriers are lactose,
terra alba;
sucrose, cyclodextrin, talc, gelatine, agar, pectin, acacia, magnesium
stearate, stearic acid
and lower alkyl ethers of cellulose. Examples of liquid carriers are syrup,
peanut oil, olive oil,
20 phospholipids, fatty acids, fatty acid amines, polyoxyethylene and water.
Similarly, the carrier
or diluent may include any sustained release material known in he art, such as
glyceryl
monostearate or gfyceryl distearate, alone or mixed with a wax. The
pharmaceutical
compositions formed by combining a IL-21 polypeptide or IL-21 mimetic for use
in treating
asthma or other allergic conditions according to the present invention and the
25 pharmaceutically acceptable carriers are then readily administered in a
variety of dosage
forms suitable for the disclosed routes of administration. The formulations
may conveniently
be presented in unit dosage form by methods known in the art of pharmacy.
For nasal administration, the preparation may contain a IL-21 polypeptide or
IL-21
mimetic dissolved or suspended in a liquid carrier, in particular an aqueous
carrier, for
30 aerosol application. The carrier may contain additives such as solubilizing
agents, e.g.
propylene glycol, surfactants, absorption enhancers such as lecithin
(phosphatidylcholine) or
cyclodextrin, or preservatives such as parabenes.
Formulations of IL-21 polypeptides or IL-21 mimetics for use in treating
asthma or
other allergic conditions according to the present invention suitable for oral
administration
35 may be presented as discrete units such as capsules or tablets, each
containing a
CA 02501807 2005-04-08
WO 2004/032953 PCT/DK2003/000691
predetermined amount of the active ingredient, and which may include a
suitable excipient.
Furthermore, the orally available formulations may be in the form of a powder
or granules, a
solution or suspension in an aqueous or non-aqueous liquid, or an oil-in-water
or water-in-oil
liquid emulsion.
5 Compositions intended for oral use may be prepared according to any known
method, and such compositions may contain one or more agents selected from the
group
consisting of sweetening agents, flavouring agents, colouring agents, and
preserving agents
in order to provide pharmaceutically elegant and palatable preparations.
Tablets maycontain
the active ingredient in admixture with non-toxic pharmaceutically-acceptable
excipients
10 which are suitable for the manufacture of tablets. These excipients may be
for example, inert
diluents, such as calcium carbonate, sodium carbonate, lactose, calcium
phosphate or
sodium phosphate; granulating and disintegrating agents, for example corn
starch or alginic
acid; binding agents, for example, starch, gelatine or acacia; and lubricating
agents; for
example magnesium stearate, stearic acid or talc. The tablets may be uncoated
or they may
be coated by known techniques to delay disintegration and absorption in the
gastrointestinal
tract and thereby provide a sustained action over a longer period. For
example, a time delay
material such as glyceryl monostearate or glyceryl distearate may be employed.
They may
also be coated by the techniques described in U.S. Patent Nos. 4,356,108;
4,166,452; and
4,265,874, incorporated herein by reference, to form osmotic therapeutic
tablets for
controlled release.
Formulations for oral use may also be presented as hard gelatine capsules
where
the active ingredient is mixed. with an inert solid diluent, for example,
calcium carbonate,
calcium phosphate or kaolin, or.a soft gelatine capsules wherein the active
ingredient is
mixed with water or an oil medium, for example peanut oil, liquid paraffin, or
olive oil.
Aqueous suspensions may contain the IL-21 polypeptides or IL-21 mimetics in
admixture with excipients suitable for the manufacture of aqueous suspensions.
Such
excipients are suspending agents, for example sodium carboxymethylcellulose,
methylcellulose, hydroxypropylmethylcellulose, sodium alginate,
polyvinylpyrrolidone, gum
tragacanth and gum acacia; dispersing or wetting agents may be a naturally-
occurring
phosphatide such as lecithin, or condensation products of an alkylene oxide
with fatty acids,
for example polyoxyethylene stearate, or condensation products of ethylene
oxide with long
chain aliphatic alcohols, for example, heptadecaethyl-eneoxycetanol, or
condensation
products of ethylene oxide with partial esters derived from fatty acids and a
hexitol such as
polyoxyethylene sorbitol monooleate, or condensation products of ethylene
oxide with partial
esters derived from fatty acids and hexitol anhydrides, for example
polyethylene sorbitan
CA 02501807 2005-04-08
WO 2004/032953 PCT/DK2003/000691
11
monooleate. The aqueous suspensions may also contain one or more colouring
agents, one
or more flavouring agents, and one or more sweetening agents, such as sucrose
or
saccharin.
Oily suspensions may be formulated by suspending the active ingredient in a
vegetable oil, for example arachis oil, olive oil, sesame oil or coconut oil,
or in a mineral oil
such as a liquid paraffin. The oily suspensions may contain a thickening
agent, for example
beeswax, hard paraffin or cetyl alcohol. Sweetening agents such as those set
forth above,
and flavouring agents may be added to provide a palatable oral preparation.
These
compositions may be preserved by the addition of an anti-oxidant such as
ascorbic acid.
10. ~ . Dispersible powders and granules suitable for preparation of an
aqueous
suspension by the addition of water provide the active compound in admixture
with a
dispersirig or wetting agent, suspending agent and one or more preservatives.
Suitable
dispersing or wetting agents and suspending agents are exemplified by those
already
mentioned above. Additional excipients, for example,~sweetening, flavourings
and colouring
agents may also be present.
The pharmaceutical compositions of IL-21 polypeptides or IL-21 mimetics for
use in
treating asthma or other allergic conditions according to the present
invention may also be in
the form of oil-in-water emulsions. The oily phase may be a vegetable oil, for
example, olive
oil.or arachis oil, or a mineral oil, for example a liquid paraffin, or a
mixture thereof. Suitable
emulsifying agents may be naturally-occurring gums, for example gum acacia or
gum
tragacanth, naturally-occurring phosphatides, for example soy bean, lecithin,
and esters or
partial esters derived from fatty acids and hexitol anhydrides, for example
sorbitan
monooleate, and condensation products of said partial esters with ethylene
oxide, for
example polyoxyethylene sorbitan monooleate. The emulsions may also contain
sweetening
and flavouring agents.
Syrups and elixirs may be formulated with sweetening agents, for example
glycerol,
propylene glycol, sorbitol or sucrose. Such formulations may also contain a
demulcent,
. preservatives and flavouring and colouring agents. The pharmaceutical
compositions may be
in the form of a sterile injectible aqueous or oleaginous suspension. This
suspension may be
formulated according to the known methods using suitable dispersing or wetting
agents and
suspending agents described above. The sterile injectable preparation may also
be a sterile
injectable solution or suspension in a non-toxic parenterally-acceptable
diluent or solvent, for
example as a solution in 1,3-butanediol. Among the acceptable vehicles and
solvents that
may be employed are water, Ringer's solution, and isotonic sodium chloride
solution. In
addition, sterile, fixed oils are conveniently employed as solvent or
suspending medium. For
CA 02501807 2005-04-08
WO 2004/032953 PCT/DK2003/000691
12
this purpose, any bland fixed oil may be employed using synthetic mono- or
diglycerides. In
addition, fatty acids such as oleic acid find use in the preparation of
injectables.
The compositions may also be in the form of suppositories for rectal
administration
of the compounds of the invention. These compositions can be prepared by
mixing the drug
- with a suitable non-irritating excipient which is solid at ordinary
temperatures but liquid at the
rectal temperature and will thus melt in the rectum to release the drug. Such
materials
include, cocoa butter and polyethylene glycols, for example.
For topical use, creams, ointments, jellies, solutions of suspensions, etc.,
containing
the compounds of the invention are contemplated. For the purpose of this
application! topical
applications shall include mouth washes and gargles.
The IL-21 polypeptides or IL-21 mimetics for use in treating asthma or other.
allergic
conditions according to the present invention may also be administered in the
form of
liposome delivery systems, such as.small unilameliar vesicles, large
unilamellar vesicles, and
multilamellar vesicles. Liposomes may be formed from a variety of
phospholipids, such as
cholesterol, stearylamine, or phosphatidylcholines.
In addition, some of the IL-21 polypeptides or IL-21 mimetics for.use in
treating
asthma or other allergic conditions according to the present invention may
form solvates with
water or common organic solvents. Such solvates are also encompassed within
the scope of
the invention.
If a solid carrier is used for oral administration, the preparation may be
tablettedj
placed in a hard gelatine capsule in powder or pellet form or it can be in the
form of a troche
or lozenge. The amount of solid carrier wilt vary widely but will usually
be:from about 25 mg ..
to about 1 g. If a liquid carrier is used, the preparation may be in the form
of a syrup,
emulsion, soft gelatine capsule or sterile injectable liquid such as an
aqueous or
non-aqueous liquid suspension or solution.
The IL-21 polypeptides or IL-21 mimetics for use in treating asthma or other
allergic
conditions according to the present invention may be administered to a mammal,
especially a
human, in need of such treatment.. Such mammals include also animals, both
domestic
animals, e.g. household pets, and non-domestic animals such as wildlife.
Pharmaceutical compositions containing a compound according to the invention
may be administered one or more times per day or week, conveniently
administered at
mealtimes. An effective amount of such a pharmaceutical composition is the
amount that
provides a clinically significant effect. Such amounts will depend, in part,
on the particular
condition to be treated, age, weight, and general health of the patient, and
other factors
evident to those skilled in the art.
CA 02501807 2005-04-08
WO 2004/032953 PCT/DK2003/000691
13
EXAMPLES
Expression of IL-21 and IL-21 R mRNA in Hodgkin Lymphoma
In situ hybridization analysis of tissue micro array slides (DAKO tumor
multislide,
DAKO cat: No. T1064) has revealed that IL-21 and IL-21R mRNA are expressed in
Hodgkin
Lymphoma specimens. The signal is seen in several specimens of Hodgkins
disease derived
from different patients. All other types of cancers that are represented on
the multislide
(approximately 20 different types) show no expression of IL-21 or IL-21 R
mRNA.
Probe preparation, hybridization conditions and Stringency washing:
Probe preparation:
In situ hybridization is performed with a probe generated by in vitro
transcription
~'~ ~ from a plasmid containing the following cDNA fragment: A PCR fragment of
human IL-21
containing basepair 344 to basepair 833 (acc:no.: AF254069 (se Table' A))
containing a
single mismatch compared to the published sequence was cloned into the
transcription
vector pCRBlunt II (Invitrogen).
Hybridization:
The specific activity of the S-35 labelled probe was 80000 cpm/ml in the
firial
hybridization mixture (consisting of 10 x SALTS, deionised Formamid, 50%
dextransulphate,
t-RNA (10 mg/ml), 1.0 M DTT). The sections were hybridized overnight at
47°C.
Strinaency wash:
The sections were washed in 50% formamide, 1x SALTS (300 mM NaCI, 10 mM
Tris (pH 6.8), 10 mM NaP04, 5 mM EDTA, 0.02% Ficoll 400, 0.02%
polyvinylpyrolidone; and
0.02% BSA) and 10 mM dithiothreitiol for 1 hour at 57°C and 1 hour at
62°C. After RNAse A
treatment (20 pg/ml RNase A) in 0.5 M NaCI, 10 mM Tris-CI (pH 7.2), 1 mM EDTA)
at 37°C
for 30 min., the sections were washed for 30 min. in 0.1 x SSC at room
temperature. The
sections were dipped in autoradiographic emulsion and exposed for 21 days.
Results:
In the specimens belonging to the histomorphological defined subgroup of
Hodgkins
lymphoma with eosinophilic infiltration, IL-21 mRNA is expressed predominantly
in
eosinophilic granulocytes (Figure 1a). Following in situ hybridization the
sections are
counterstained with hematoxylin and eosin (H&E) whereby it is possible to
morphologically
CA 02501807 2005-04-08
WO 2004/032953 PCT/DK2003/000691
14
identify the IL-21 mRNA positive cells as eosinophils (arrows in figure 1 b).
In specimens with
lymphocytic predominance expression of IL-21 mRNA is found in small clusters
of
lymphocytes surrounding Reed-Sternberg cells (figure 2)
IL-21 R mRNA is diffusely expressed in lymphocytes in both the eosinophilic
and
lymphocytic types of histopathology (figure 3)
Conclusion:
The expression of IL-21 in. eosinophils may indicate that said eosinophilic IL-
21
exerts a protective role. According to this view, eosinophilic infiltration or
increased number
of eosinophils in body fluids and/or tissues in certain disorders or disease
states or
conditions may indicate that said eosinophils play a protective role by
releasing IL-21, which
in turn reduces an allergic reaction or allergic disease. Thus, diseases where
eosinophils are
involved in a protective role, including but not limited to allergic diseases
may be treated by
administration of IL-21 polypeptides or IL-21 mimetics.
IL-21 and the IL-21 receptor may play a role in the patho-physiology of
Hodgkin's
Lymphoma, and IL-21 signalling may therefore be a target for pharmacological
intervention in
Hodgkin's disease.
Particularly the distinct expression of IL-21 in lymphocytes immediately
surrounding
the malignant Reed-Sternberg cells indicate that the IL-21 mRNA expression is
involved in a
cross-talk between Reed-Sternberg cells and surrounding lymphocytes and the IL-
21
expression may even be induced by the Reed-Sternberg cells.
A method to demonstrate IL-21 and 1L-21R expression in an eosinophii-like cell-
fine, HL-60:
HI-60. cells are cultured in the presence of butyric acid to induce an
eosinophil~like'pheno
type, and are subsequently cultured with cytokines or growth. factors, non
limiting examples
are GM-CSF, eotaxin, IL-4, IL-5 and IFN-y, and harvested after 1-72 hrs
culture and analysed
for IL-21 and IL-21 R mRNA expression by RT-PCR and for IL-21 protein
expression by
ELISA and IL-21 and lL-21 R protein expression by flow cytometry
A method to demonstrate IL-21 and IL-21R expression in eosinophils:
Eosinophils are iso-
lated from human blood and cultured with cytokines or growth factors as
described in Woerly
et al., J.Leukoc.8iol. 72:769-779, 2002; Schmid-Grendelmeier et al., J Immunol
169:1021-
1027, 2002, non limiting examples are GM-CSF, eotaxin, IL-4, IL-5 and IFN-y,
and harvested.
CA 02501807 2005-04-08
WO 2004/032953 PCT/DK2003/000691
after 1-72 hrs culture and analysed for IL-21 and IL-21 R mRNA expression by
RT-PCR and
for IL-21 protein expression by ELISA and IL-21 and IL-21 R protein expression
by flow cy-
tometry.
5 A method to demonstrate abnormal expression of IL-21 and IL-21 R in
eosinophils from pa-
tients with allergic diseases: Eosinophils are isolated from the blood from
patients with aller-
gic diseases and from healthy donors and analysed directly ex vivo for IL-21
and IL-21 R ex-
pression by RT-PCR, ELISA or flow cytometry, or stimulated with growth factors
or cytokines
before analysis of IL-21 and IL-21 R expression as described above.
A method to demonstrate abnormal expression of IL-21 and IL-21 R in
eosinophils from pa-
tients with allergic diseases: Biopsies from patients with allergic diseases
and from healthy
donors challenged with relevant antigens are sectioned are stained by
immunohistochemistry
for IL-21 and IL-21 R expression together with a relevant marker to define the
cell-type, for
example EG=2 as an eosinophil marker.
A method to demonstrate that absence of IL-21 leads to increased disease
severity in a
mouse model of asthma: IL-21 deficient and wild type mice are immunized with
ovalbumin
and subsequently challenged by intranasal instillation of ovalbumin, and the
airway respon-
siveness, the cellular composition of the bronchoalveolar lavage, and
cytokine, chemokine
and IgE levels are measured as described in Hogan et al., J Immunol 171:2644-
2651 (2003)
or Medoff et al., J Immunol 168:5278-5286, 2002 or Denzler et al., J Immunol
165:5509-5517
(2000) or Ishimitsu et al., J Immunol 166:1991-2001 (2001 ). . An increased
disease severity
in IL-21 deficient mice indicates that IL-21 may be useful to treat asthma. .
A method to demonstrate that neutralization of IL-21 leads to increased
disease severity in a
mouse model of asthma: Mice are immunized with ovalbumin and subsequently
challenged
by intranasal instillation of ovalbumin. Before, after or together with
ovalbumin challenge, the
mice are treated with neutralizing IL-21 antibody. The airway responsiveness,
the cellular
composition of the bronchoalveolar lavage, and cytokine, chemokine and IgE
levels are
measured as described in Hogan et al. J Immunol 171:2644-2651 (2003) or Medoff
et al., J
Immunol 168:5278-5286 (2002) or Denzler et al., J Immunol 165:5509~5517 (2000)
or Ishi-
CA 02501807 2005-04-08
WO 2004/032953 PCT/DK2003/000691
16
mitsu et al., J Immunol 166:1991-2001 (2001 ). An increased disease severity
in mice treated
with neutralizing IL-21 antibody indicates that IL-21 may be useful to treat
asthma.
A method to demonstrate that IL-21 treatment leads to diminished disease
severity in a
. mouse model of asthma: Mice are immunized with ovalbumin and subsequently
challenged
by intranasal instillation of ovalbumin. Before, after or together with
ovalbumin challenge, the
mice are treated with IL-21 protein, a plasmid encoding IL-21 or buffer or
control plasmid.
The airway responsiveness, the cellular composition of the bronchoalveolar
lavage, and cy-
tokine, chemokine and IgE levels are measured as described in Hogan et al., J
Immunol
171:2644-2651 (2003) or Medoff et al., J Immunol 168:5278-5286 (2002) or
Denzler et al., J
Immunol 165:5509-5517 (2000) or Ishimitsu et al., J Immunol 166:1991-2001
(2001).
A method to demonstrate that absence of IL-21 leads to increased disease
severity in
helminthic infections: IL-21 deficient and wild type mice are immunized with
killed (frozen)
microfilariae followed by i.v. injection with live microfilariae, and the
allergic response is
measured as described above and in Hall et al., Infect.lmmun. 66:4425-4430
(1998)
A method to demonstrate that lL-21 may be used to treat helminthic infections:
mice are im-
munized with killed (frozen) microfilariae followed by i.v. injection with
live microfilariae. Be-
~ fore, after or together with injection of live microfilariae, the mice are
treated with IL-21 pro-
tein, a plasmid encoding IL-21 or buffer or control plasmid. The allergic
response is meas-
ured as described above and in Hall et al. as above.
CA 02501807 2005-04-08
WO 2004/032953 PCT/DK2003/000691
17
Table A
Human IL-21 cDNA fra ment aeneration:
Interleukin 21 (IL-21 ) protein accession no. Q9HBE4.
Interleukin 21 (IL-21 ) original DNA accession no. AF254069.
Probe seauence 489 bp:
ATGAGATCCAGTCCTGGCAACATGGAGAGGATTGTCATCTGTCTGATGGTCATCTTCTT
GGGGACACTGGTCCACAAATCAAGCTCCCAAGGTCAAGATCGCCACATGATTAGAATGC
~GTCAACTTATAGATATTGTTGATCAGCTGAAAAATTATGTGAATGACTTGGTCCCTGAATT
' TCTACCAGCTCCAGAAGATGTAGAGACAAACTGTGAGTGGTCAGCTfTTTCCTGTTTTCA
GAAGGCCCAACTAAAGTCAGCAAATACAGGAAACAATGAAAGGATAP.TCAATGTATCAAT
TAAAAAGCTGAAGAGGAAACCACCTTCCACAAATGCAGGGAGAAGACAGAAACACAGAC
TAACATGCCCTTCATGTGATTCTTATGAGAAAAAACCACCCAAAGAATTCCTAGAAAGAT
TCAAATCACTTCTCCAAAAGATGATTCATCAGCATCTGTCCTCTAGAACACACGGAAGTG
AAGATTCCTGA
emb11af2540691af254069 Homo Sapiens interleukin 21 (IL-21) mRNA,
complete cds.
Length = 642
Score = 961 bits (485), Expect = 0.0
Identities = 488/489 (99~)
Strand = Plus / Plus
Query: 1 atgagatccagtcctggcaacatggagaggattgtcatctgtctgatggtcatcttcttg 60
IIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIII
Sbjct: 47 atgagatccagtcctggcaacatggagaggattgtcatctgtctgatggtcatcttcttg 106
~JO Query:61 gggacactggtccacaaatcaagctcccaaggtcaagatcgccacatgattagaatgcgt120
IIIIIIilllllllillllllllllllllllllllllllllllllllllillllllll
Sb II 166
t tccacaaatcaagctcccaaggtcaagatcgccacatgattagaatgcgt
acact
107
: gg
jc ggg
Query:121 caacttatagatattgttgatcagctgaaaaattatgtgaatgacttggtccctgaattt180
IIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIilllllllllll
Sbjct: 167 caacttatagatattgttgatcagctgaaaaattatgtgaatgacttggtccctgaattt226
Query: 181 ctaccagctccagaagatgtagagacaaactgtgagtggtcagctttttcctgttttcag240
IIIIIIIIIIIIIIIIIIillllllllllllllllllllllllllllllllllllll
Sb II 286
agacaaactgtgagtggtcagctttttcctgttttcag
at
ta
ctcca
aa
cca
227
t
jct: g
g
g
g
g
c
g
Query: 241 aaggcccaactaaagtcagcaaatacaggaaacaatgaaaggataatcaatgtatcaatt300
IIIIIIIIIIIIIIIIIIIIilllllllllllilllllllllllllllllllllll
Sbjct: IIII 346
287 aaggcccaactaaagtcagcaaatacaggaaacaatgaaaggataatcaatgtatcaatt
Query: 301 aaaaagctgaagaggaaaccaccttccacaaatgcagggagaagacagaaacacagacta360
IIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIillllllll
b aaaccaccttccacaaatgcagggagaagacagaaacacagacta406
aa
a
aaa
ct
347
S g
jct: gg
g
g
aa
Query:361 acatgcccttcatgtgattcttatgagaaaaaaccacccaaagaattcctagaaagattc420
IIIIIIIIIIIIIIIIIIIIIIIIIIIIIilllllllllliillllllllllllllll
b II 466
attcttatgagaaaaaaccacccaaagaattcctagaaagattc
cccttcat
t
at
407
jct: g
S g
g
ac
Query:421 aaatcacttctccaaaagatgattcatcagcatctgtcctctagaacacacggaagtgaa480
IIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIilllllllllllllllllllllll
CA 02501807 2005-04-08
WO 2004/032953 PCT/DK2003/000691
18
Sbjet: 467 aaatcacttctccaaaagatgattcatcagcatctgtcctctagaacacacggaagtgaa 526
Query: 481 gattcctga 489
Sbjct: 527 gattcctga 535
CA 02501807 2005-04-08
WO 2004/032953 PCT/DK2003/000691
19
TABLE B
Human IL-21 amino acid seauence protein accession no. Q9HBE4, also shown as
SEQ ID
No. 2, including the signal peptide comprising residues 1 to 29:
1...MRSSPGNMERIVICLMVIFLGTLVHKSSSQGQDRHMIRMRQLIDIVDQLK...50
51..NYVNDLVPEFLPAPEDVETNCEWSAFSCFQKAQLKSANTGNNERIINVSI...100
101.KKLKRKPPSTNAGRRQKHRLTCPSCDSYEKKPPKEFLERFKSLLQKMIHQ...150
151 HLSSRTHGSEDS