Note: Descriptions are shown in the official language in which they were submitted.
CA 02502460 1997-05-01
I
WO 97/41228 PCT/US97/07688'
-1-
USE OF THE GREEN FLUORESCENT PROTEIN AS A SCREENABLE
MARKER FOR PLANT TRANSFORMATION
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates to a method of plant
transformation in which a DNA construct carrying a gene
encoding the green fluorescent protein (GFP) is
introduced into plant cells which are then screened for
the presence of the protein and transformed cells are-
regenerated into transgenic plants. In particular, the
present invention provides methods for circumventing the
cellular toxicity of the GFP by regulating expression of
the gene encoding the protein or directing the protein to
a subcellular compartment where it is not toxic to the
cell. The present invention provides DNA constructs for
cell transformation in which expression of a gene
encoding the GFP is placed under the control of an
inducible, constitutive or tissue-specific promoter. In
addition, DNA constructs are provided in which a
Zo nucleotide sequence encoding the GFP is operably linked
to a signal or targeting sequence which directs the
expressed protein to a subcellular compartment where the
protein is not toxic to the cell. Moreover, the present
invention provides a nucleotide sequence encoding GFP
that. is optimized for expression of the GFP gene in
CA 02502460 1997-05-01
WO 97/41228 PCTIUS97/07688
-2-
plants and to GFP-encoding nucleotide sequences that code
for light-shifted versions of GFP. The present invention
also provides a method for selecting plant cells
transformed with a gene encoding a screenable marker
flanked on the 5-prime and 3-prime ends with a
recombinase-specific target sequence, and introducing a
gene encoding a site specific recombinase into the
transformed plant cells and selecting transformed plant
cells that no longer express the screenable marker. In
addition, the present invention provides a method of
reducing GFP toxicity by transforming plant cells with a
gene encoding the GFP together with a gene encoding an
oxygen scavenger such as superoxidase dismutase.
2. Background
Expression vectors include at least one genetic
marker that allows transformed cells to" be either
recovered by negative selection, i.e. inhibiting growth
of cells that do not contain the selectable marker gene,
or by screening for product encoded by the genetic
marker. Many of the commonly used selectable marker
genes for plant transformation were isolated from
bacteria and code for enzymes that metabolically detoxify
a selective chemical agent which may be an antibiotic or
a herbicide. Other selectable marker genes encode an
altered target which'is insensitive to the inhibitor.
The most commonly used selectable marker gene for
plant transformation is the neomycin phosphotransferase
II (nptil) gene, isolated from TnS, which when placed
under the control of plant regulatory signals confers
resistance to kanamycin. Fraley et al., Proc. Natl.
Acad. Sci. U.S.A., 80: 4803 (1983). Another commonly
used selectable marker gene is the hygromycin
phosphotransferase gene which confers resistance to the
antibiotic hygromycin. Vanden Elzen et al., Plant Mot.
Biol. , ,k: 299 (1985).
CA 02502460 1997-05-01
WO 97/41228 PCT1US97107688
-3-
Additional =selectable marker genes of bacterial
origin that confer resistance to antibiotics include
gentamycin acetyl transferase, streptomycin
phosphotransferase, aminoglycoside-3'-adenvl transferase,
the bleomycin resistance determinant. Hayford et al.,
Plant Physiol. 86: 1216 (1988), Jones et al., Mol. Gen.
Genet., 2..Q.: 86 (1987), Svab et al., Plant Mol. Biol. I :
197 (1990), Hille et al., Plant Mol. Biol. 7: 171 (1986).
Other selectable marker genes confer resistance to
herbicides such as glyphosate, glufosinate or broxynil.
Comai et al., Nature 317: 741-744 (1985), Gordon-Kamm et
al., Plant Cell 2: 603-618 (1990) and Stalker et al.,
Science 242: 419-423 (1988).
Other selectable marker genes for plant
transformation are not of bacterial origin. These genes
include, for example, mouse dihydrofolate reductase,
plant 5-enolpyruvylshikimate-3-phosphate synthase and
plant acetolactate synthase. Eichholtz et al., Somatic
Cell Mol. Genet. ;,3,: 67 (1987), Shah et al., Science 2m:
478 (1986), Charest et al., Plant Cell Rep. 8: 643
(1990).
Although many of these markers have been used for
selecting transformed plant tissue, these selection
systems involving toxic -chemical agents can have
disadvantages or limitations. One disadvantage is that
it may be difficult to recover normal, viable transformed
plants directly from chemical selection. Everett et al.,
Bio/Technology5: 1201-1204 (1987). Another disadvantage
is that not all selectable marker systems work for all
tissues, in all plant species, due in part to differences
in sensitivity of a particular tissue or plant species to
the selective agent. The success of any given marker for
transformation of a 'given plant species is not easily
predicted. Moreover, potential regulatory issues
surrounding the use of antibiotic resistance genes and
the use of herbicide resistance genes, for plant species
capable of outcrossing with weedy species are additional
disadvantages of these markers.
CA 02502460 1997-05-01
WO 97/41228 PCTAJS97/07688
-4-
Another -class of marker genes for plant
transformation require screening of presumptively
transformed plant cells rather than direct genetic
selection of transformed cells for resistance to a toxic
substance such as an antibiotic. These genes are
particularly useful to quantify or visualize the spatial
pattern of expression of a gene in specific tissues and
are frequently referred to as reporter genes because they
can be fused to a gene or gene regulatory sequence for
the investigation of gene expression. Commonly used
genes for screening presumptively transformed cells
include Q-glucuronidase (GUS)--;- 13-galactosidase,
luciferase, and chloramphenicol acetyltransferase
Jefferson, R.A., Plant Mol. Biol. Rep. 5-: 387 (1987).,
Teeri et al., EMBO J. k: 343 (1989), Koncz et al., Proc. Natl. Acad. Sci.
U.S.A. 84: 131 (1987), De Block et al.,
ElBO J. a: 1681 (1984). Another approach to the
identification of relatively rare transformation events
has been use. of a gene that encodes a dominant
constitutive regulator of the Zea mays anthocyanin
pigmentation pathway. Ludwig et al., Science 2,47: 449
(1990).
Although chemical selection of plant cells
transformed with selectable marker genes has been
successful with plant species and varieties that are
easily cultured in vitro, the choice of selectable marker
systems that have been shown to be successful for cereals
and many other agronomically important plant species is
very limited. In general, plant species that ter.: toward
organogenesis and/or shoot propagation have been
difficult to transform by means of chemical selection.
The success rate with these plant species continues to
improve, however, as evidenced by recent advances in Type
I selection of maize inbreds and small grain cereals such
as barley. Koziel et al., Bio/Technology 11: 194-200
(1993) and Mendel et al. In: Transformation of Plants and
Soil Microorganisms, Wang et. al. eds., Cambridge Press
(1995).
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-5-
Likewise, there has been little success in using
visual screening methods for primary identification of
transformed cells. The GUS gene was used to investigate
germline transmission. McCabe et al., Plant Physiol.,
87(3): 671 (1988) and McCabe et al., Plant Cell Tissue
organ Cult. 33(3): 227 (1993). Histochemical staining
for GUS activity was used to locate transgenic sectors in
cotton and soybean transformants that ultimately produced
transgenic seeds. Since histochemical analysis for GUS
activity requires destruction of portions of the
presumptively transformed plant tissue, this method is
labor intensive and impractical for routine production of
transgenic plants. This method is particularly
unsuitable for plant species such as maize and other
cereals in which transformants are recovered, even under
optimum conditions, at low frequency. Recovery of
transformed progeny was reported once in barley using GUS
expression as a screening tool, but the method was found
to be very labor-intensive. Ritala et al., Plant Mol.
Biol. 2A: 317-325 (1994). There have been no reports at
all of success with GUS or other screenable markers with
maize.
More recently, in vivo methods for visualizing GUS
activity that do not require destruction of plant tissue
have been made available. Molecular Probes Publication
2908, Imagene Green'", p. 1-4 (1993) and Naleway et al.,
J. Cell Biol. =: 151a (1991). However, these in vivo
methods for visualizing GUS activity have not proven
useful for recovery of transformed cells because of low
sensitivity and high fluorescent backgrounds.
Despite the fact that luciferase genes have been
available for many years, this strategy for visualizing
transformed cells has also not been successfully adapted
for routine recovery of plant transformants. The
luciferase-based screening methods are limited by the
fact that most of these systems require the presence of
luciferin, a compatible luciferase and an exogenously
supplied cofactor. In the absence of the substrate,
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-6-
enzyme or cof actor, the system does not bioluminesce.
Cells transformed with a luciferase gene must have cell
walls and plasma membranes that are permeable, or
rendered permeable, to a compatible luciferin in order to
detect bioluminescence. For example, tobacco plants
regenerated from cells transformed with a firefly
luciferase gene and exposed to a liquid medium containing
firefly luciferin exhibited bioluminescence primarily
along their major veins. Ow et al., Science ,: 856
(1986). Accordingly, a screening method has not been
successfully developed for routine plant transformation
that does not involve chemical selection or assays, often
labor-intensive, that , require the sa'cr:i ice or
destruction of tissue samples for analysis.
A gene encoding GFP has been utilized as a marker
for gene expression in prokaryotic and eukaryotic cells.
Chalfie et al., Science ,',: 802 (1994) Many cnidarians
utilize GFPs as energy-transfer acceptors in
bioluminescence.. A gene encoding GFP isolated from a
cnidarian and expressed in a heterologous prokaryotic or
eukaryotic host produces a protein capable of
fluorescence. 'A cDNA encoding the Aequorea victoria GFP
produced a fluorescent product when expressed in
Escherichia coli or Caenorhabditis elegans cells. Green
fluorescence was detected in transformed cells upon
illumination with a long-wave ultraviolet (UV) source
without having to supply substrates or cofactors. In
addition, fluorescence was stable for at least 10 min
when illuminated with 450 to 490 nm light.
Transformation of plant cells with a gene encoding GFP
and detection of fluorescence has been reported.
Haseloff et al., TIG 11: 328-329 (1995). Transformed
Arabidopsis cells could be regenerated into whole plants.
However, the regenerated plants expressing GFP exhibited
signs of mild to moderate toxicity in the light compared
to plants not expressing GFP. The strongest GFP
expressors proved more difficult to regenerate.
Likewise, in a recent report describing GFP expression in
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-7-
kanamycin-selected tobacco transformants, Chiu et al.,
Current Biol. k: 325-330 (1996) note that high GFP
expression levels inhibited regeneration of t=ransgenic
plants.
A need therefore exists for a cell transformation._
method which does not rely, or does not rely solely on,
selection of cells carrying a gene that confers
resistance to a toxic substance. A need exists for a
method for efficiently and easily identifying transformed
plant cells with a visual screenable marker. A need also
exists for a method of cell transformation that does not
require destruction of presumptively transformed tissue
to assay for the presence of a selectable marker gene.
A need exists for a method for cell transformation that
combines a selectable marker gene and a screenable marker
gene. In addition, a need exists for a method of cell
transformation which does not require exogenous supply of
a substrate or cofactor for detection of the polypeptide
encoded by a selectable marker gene. Yet another need
exists for a method of cell transformation that
circumvents the cellular toxicity of the GFP.
SUMMARY OF THE' INVENTION
Accordingly, it is an object of the present
invention to provide a method for cell transformation
which does not depend on selection of cells carrying a
gene that confers resistance to a toxic substance.
It is another object. of the present invention to
provide a method for cell transformation which combines
the selection of cells carrying a gene that confers
resistance. to a toxic substance with screening cells for
the presence of a substance that renders transformed
cells-identifiable.
It is a further object of the present invention to
provide a method for cell transformation which does not
require destruction of presumptively transformed tissue
to assay for the presence of a selectable marker gene.
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688' .
Yet another object of the present invention is to
provide a method for cell transformation which does not
require that an exogenous substrate or cofactor be
provided to assay for a polypeptide encoded by a
selectable marker gene.
It is another object of the present invention to
provide a method for cell transformation that circumvents
the cellular toxicity of the GFP.
These and other objects are achieved, in accordance
with one embodiment of the present invention, by
providing an isolated DNA molecule comprising a
nucleotide sequence encoding the GFP operably linked to
an inducible promoter. The inducible promoter can be
selected from the group consisting of the estrogen-
inducible promoter, the estradiol-inducible promoter, the
ACE1 promoter, the IN2 promoter and the tetracycline
repressor promoter.
Also provided is an isolated DNA molecule comprising
as nucleotide sequence encoding the GFP wherein the
nucleotide sequence is operably linked to. a targeting
sequence for subcellular localization which directs a
protein to a subcellular compartment.
An isolated DNA molecule is provided comprising a
nucleotide sequence selected from the group consisting of
(a) SEQ ID NO: 1; (b) a nucleotide sequence that has
substantial sequence similarity with SEQ ID NO: 1; and
(c) a functional fragment of (a) or (b), wherein said DNA
molecule encodes a GFP. The nucleotide sequence encoding
a GFP may be operably linked to a nucleotide sequence
encoding a targeting sequence for subcellular
localization which directs a protein to a subcellular
compartment. The nucleotide sequence encoding a GFP and
a targeting sequence may further comprise a promoter
operably linked to said nucleotide sequence.
Also provided are expression vectors comprising DNA
molecules encoding a GFP, optionally operably linked to
an inducible promoter and/or targeting sequence. The
expression vector of the instant invention may carry a
CA 02502460 1997-05-01
WO 97/41228 PCTIUS97/07688
-9-
nucleotide sequence encoding a foreign protein operably
linked to a second promoter.
Also provided is a method of using the expression
vectors of the instant invention to produce a transformed
plant, comprising the steps of introducing an expression
carrying a gene encoding GFP into regenerable plant cells
and selecting cells containing the GFP for regeneration.
The method of the instant invention may include the step
of inducing GFP expression where the nucleotide sequence
encoding GFP is operably linked to an inducible promoter.
The regenerable plant cells utilized in the method of the
instant invention are selected from the group consisting
of Zea, Brassica and Helianthus cells.
Also provided are transgenic plants expressing the
isolated DNA molecule encoding GFP. In addition,
transgenic plants comprising a vector carrying a
nucleotide sequence encoding GFP are provided.
A method for producing a transgenic plant is
provided comprising the steps of (a) constructing an
expression vector comprising (i) a first promoter which
is an inducible promoter, operably linked to a nucleotide
sequence encoding a GFP, and (ii) a second promoter
operably linked to a foreign gene; (b) introducing said
expression vector into regenerable plant cells; (c)
inducing expression of the gene encoding the GFP and
selecting transformed plant. cells containing said
protein; and (d) regenerating transformed plants from
said selected transformed plant cells. The inducible
promoter may be selected from the group including the
estrogen-inducible promoter, the estradiol-inducible
promoter, the ACE1 promoter, the IN2 promoter and the
tetracycline repressor promoter.
Also provided is a method-for producing a transgenic
plant, comprising the steps: (a) constructing an
expression vector comprising (i) a first promoter
operably linked to a nucleotide sequence encoding a
sequence for subcellular localization which directs a
protein to a subcellular compartment which is operably
CA 02502460 1997-05-01
WO 97/41228 PCTIUS91/07688
-10-
linked to a nucleotide sequence encoding a GFP, and (ii)
a second promoter operably linked to a foreign gene;, (b)
introducing said expression vector into regenerable plant
cells; (c) selecting transformed plant cells containing
said GFP; and (d) regenerating transformed plants from_
said selected transformed plant cells. The targeting
sequence for subcellular localization directs the GFP to
the mitochondria, chloroplasts, peroxisomes, vacuole,
endoplasmic reticulum, cell wall or for secretion
generally into the apoplast. This method may include a
nucleotide sequence encoding a GFP selected from the
group consisting of: (a) SEQ ID NO: 1; (b) a nucleotide
sequence that has substantial sequence similarity with
SEQ ID NO: 1; and (c) a functional fragment of (a) or
(b) .
Also provided. is a method for producing -a transgenic
plant, comprising the steps: (a) constructing an
expression vector comprising (i) a first promoter
operably linked to a nucleotide sequence encoding a
screenable marker flanked on the 5-prime and 3-prime ends
with a recombinase-specific target sequence, and (ii) a.
second promoter operably linked to a foreign gene; (b)
introducing said expression vector into regenerable plant
cells; (c) selecting transformed plant cells containing
said screenable marker; (d) transforming the plant cells
with a second expression vector containing a gene
encoding a site-specific recombinase; (e) selecting plant
cells that no longer express the screenable marker; (f)
regenerating transformed plants from said selected
transformed plant cells; and (g) isolating said foreign
protein. The site-specific recombinase may be selected
from the group consisting of the FLPtFRT, Ac/DS and
cre/lox systems. In addition, the screenable marker may
be the GFP.
An isolated DNA molecule comprising a nucleotide
sequence encoding the GFP fused in frame to a nucleotide
sequence encoding superoxide dismutase is also provided.
Cells. transformed with the DNA molecule contain a fusion
CA 02502460 2011-03-04
WO 97/41228 PCT1US97/07688 -
-11-
protein that (i) produces green fluorescence in the
presence of UV to blue light and (ii) displays superoxide
dismutase activity.
Also provided is a method for producing a transgenic
plant comprising: (a) constructing an expression vector
comprising (i) a first promoter operably linked to a
nucleotide sequence encoding a GFP, and (ii) a second
promoter operably linked to a gene encoding an enzyme
that is an oxygen scavenger, and (iii) a third promoter
operably linked to a foreign gene; (b) introducing said
expression vector into regenerable plant cells; (c)
selecting transformed plant cells containing said GFP;
and (d) regenerating transformed plants from said
selected transformed plant cells. The oxygen scavenger
enzyme may be superoxide dismutase.
Alternatively, a method for producing a transgenic
plant is provided comprising: (a) constructing an
expression vector comprising (i) a first promoter
operably linked to a nucleotide sequence encoding a
fusion protein comprising the GFPand an oxygen. scavenger
enzyme fused in frame, and (ii) a second promoter
operably linked to a foreign gene; (b) introducing said
expression vector into regenerable plant cells; (c)
.selecting transformed plant-cells containing said the GFP
and oxygen scavenger enzyme activity; and (d)
regenerating transformed plants from said selected
transformed plant cells. The oxygen scavenger enzyme may
be superoxide dismutase.
CA 02502460 2011-03-04
WO 97/41228 PCT/US97/07688
- Ila -
An aspect of the invention is to provide an isolated DNA
molecule comprising a nucleotide sequence, wherein the
nucleotide sequence is: (a) SEQ ID NO: 1, wherein said
sequence encodes a modified green fluorescent protein
(GFPm); (b) SEQ ID NO: 1, wherein the nucleotides TCC at
positions 193-195 are changed to ACG, and wherein said
sequence encodes a red shifted green fluorescent protein
(GFPr); (c) SEQ ID NO: 1, wherein nucleotides TAC at
positions 196-198 are changed to CAC, and wherein said
sequence encodes a blue fluorescent protein (BFP); or
(d) SEQ ID NO: 1, wherein nucleotides TTC at positions
295-297 are changed to AGC, nucleotides ATG at positions
457-459 are changed to ACC, and nucleotides GTG at
positions 487-489 are changed to GCC, and wherein said
sequence encodes a soluble green fluorescent protein
(GFPs). The isolated DNA molecule can be operably linked
to a nucleotide sequence encoding a signal sequence for
subcellular localization which directs the protein
encoded by said DNA molecule to a subcellular
compartment. The isolated DNA molecule can be operably
linked to a nucleotide sequence encoding a sequence for
mitochondrial localization which directs the expression
of said green fluorescent protein to the mitochondria.
Another aspect of the invention is to provide a method
for producing a transgenic plant, wherein said
transgenic plant is produced by positive selection, by
screening for a transformed cell without selection of a
cell carrying a gene that confers resistance to a toxic
substance, comprising the steps: (a) providing at least
one expression vector comprising (i)a first promoter,
operably linked to the isolated DNA molecule described
CA 02502460 2011-03-04
WO 97/41228 PCT/US97/07688
- lib -
above, and (ii) a second promoter operably linked to a
foreign gene; (b) introducing said expression vector
into regenerable plant cells; (c) screening for a
transformed plant cell containing said green fluorescent
protein, wherein said screening does not depend on
negative selection of cells carrying a gene that confers
resistance to a toxic substance; and (d) regenerating
a transformed plant from the transformed plant cell
identified by said screening.
Another aspect of the invention is to provide expression
vectors comprising the DNA molecules described above.
Another aspect of the invention is to provide a method
of using the expression vector described above to
produce a transformed plant, comprising the steps of
introducing said expression vector into regenerable
plant cells, screening for cells containing said GFPm,
GFPr, BFP or GFPs, and regenerating a transformed plant
from cells identified by said screening as containing
GFPm, GFPr, BFP or GFPs. The regenerable plant cells
can be Zea, Brassica or Helianthus cells.
Another aspect of the invention is to provide the
expression vector described above wherein the DNA
molecule is operably linked to a first promoter and the
expression vector optionally comprises a second promoter
linked to a foreign gene. The first promoter can be an
inducible, constitutive or tissue-specific promoter.
The first promoter can be an estrogen-inducible
promoter, an estradiol-inducible promoter, an ACE1
promoter, an IN2 promoter or a tetracycline repressor
promoter. The first promoter can be a CaMV, actin,
CA 02502460 2011-03-04
WO 97/41228 PCT/US97/07688
- llc -
ubiquitin, pEMU, MAS or histone promoter. The
expression vector can further comprise a nucleotide
sequence for subcellular localization that directs the
protein encoded by the DNA molecule to a subcellular
compartment. The subcellular localization sequence can
direct expression to the mitochondria, chloroplast,
cytoplasm, peroxisome, endoplasmic reticulum, cell wall,
apoplast or nucleus. The DNA molecule can be operably
linked to a mitochondrial promoter and the subcellular
localization sequence can direct expression of the
protein to the mitochondria. The subcellular
localization sequence can direct expression to the
chloroplast. The expression vector can further comprise
a recombinase-specific target sequence wherein the
target sequence flanks the 5-prime and 3-prime ends of
the first promoter, the DNA molecule or both. The
recombinase-specific target sequence can comprise
FLP/FRT, Ac/DS or cre/lox.
Another aspect of the invention is to provide a
transgenic plant cell containing the DNA molecules
described above. The plant cell can be from a plant of
the genera Zea, Brassica, or Helianthus. Also provided
is a transgenic cell from a transgenic seed of a plant
regenerated from the plant cell. The plant cell can be
isolated.
Another aspect of the invention is to provide a
transgenic Zea mays plant cell containing the DNA
molecule described above. The plant cell can be
isolated.
CA 02502460 2011-03-04
WO 97/41228 PCT/US97/07688
- lid -
Another aspect of the invention is to provide a
transgenic plant cell comprising the expression vector
described above. Also provided is a transgenic plant
cell from a plant regenerated from the plant cell. The
plant cell can be a monocot or a dicot plant cell. The
monocot plant cell can be of the genera Zea. The dicot
plant cell can be of the genera Brassica or Helianthus.
The plant cell can be isolated.
Another aspect of the invention is to provide the use of
the DNA molecule described above to transform a plant
cell.
Another aspect of the invention is to provide the use of
the expression vector described above to transform a
plant cell.
BRIEF DESCRIPTION OF THE DRAWINGS
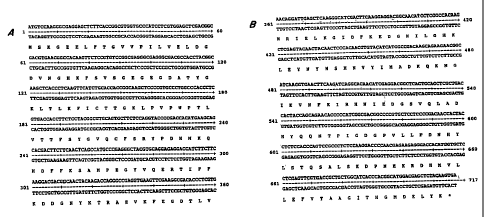
Figure 1 presents the nucleotide sequence [SEQ ID
NO:l] encoding a GFP with its corresponding amino acid
sequence [SEQ ID NO: 2].
Figure 2 shows the Plasmid Map of PHP167.
Figure 3 shows the Plasmid Map of PHP762.
Figure 4 shows the Plasmid Map of PHP7921.
Figure 5 shows the Plasmid Map of PHP8144.
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688'
-12-
DETAILED DESCRIPTION
1. Definitions
In the description that follows, a number of terms
are used extensively. The following definitions are
provided to facilitate understanding of the invention.
A structural gene is a DNA sequence that is
transcribed into messenger RNA (mRNA) which is 'then
translated into a sequence of amino acids characteristic
of a specific polypeptide.
A promoter is. a DNA sequence that erects the
transcription of a structural gene. Typically, a
is promoter is located in the 5-prime region of a gene,
proximal to the transcriptional start site of a
structural gene. If a promoter is an inducible promoter,
then the rate of transcription increases in response to
an inducing agent. In contrast, the rate of
transcription is not regulated by an inducing agent if
the promoter is a constitutive promoter. For example, a
promoter may. be regulated in a tissue-specific or t ssue-
preferred manner such that it is only active in
transcribing the associated coding region in a specific
tissue type(s) such as leaves, roots or meristem.
A cytoplasmically localized protein is a protein
coded for by a gene which does not include any specific
signal for targeting of the protein into any subcellular
organelle or compartment or any signals for secretion of
the protein. For example, the GFP structural gene
operably linked 5-prime to a promoter and to an
appropriate 3-prime sequence encodes for a protein
compartmentalized in the cytoplasm.
A sicnal or tarcetinc sequence is a structural
peptide domain required for targeting of a given
polypeptide to a subcellular organelle, subcellular
compartment or secretion from the cell. As used herein,
the phrase sequence for subcellular localization is
CA 02502460 1997-05-01
WO 97/41228 PCTIUS97/07688
-13-
intended to refer collectively to any form of signal,
targeting or retention sequence as defined herein. Signal
sequences for a polypeptide directed to the chloroplast
or mitochondrion are largely localized to the amino-
terminus of the polypeptide. Signal sequences for a
polypeptide directed to the glyoxysomes and peroxisomes
are largely localized to the carboxy-terminal domains of
the polypeptide. Accordingly, targeted transport of the
GFP protein is accomplished by means of chemically
joining in proper reading frame, or operably linking, the
nucleotide sequence encoding a signal sequence to the 5-
prime and/or 3-prime region of the GFP structural gene.
A mitochondrial targeting sequence facilitates the
transport of a protein to a mitochondria) compartment.
Typically, the mitochondrial targeting sequence is
located at the amino-terminus of a polypeptide.
A secretion targeting sequence targets a polypeptide
for export into the extracellular-space through the ER.
For example, operably linking a nucleotide sequence
encoding the barley alpha amylase 1 (BAA) secretory
targeting sequence to the 5-prime end of a structural
gene targets the encoded protein for export into the
extracellular space.
A cell wall taraetinc sequence targets a polypeptide'
for export. from the cell but the polypeptide is
specifically localized to the cell wall. For example,
cell wall localization of a polypeptide is accomplished
by operably linking a nucleotide sequence encoding BAA 5-
prime, and operably linking a nucleotide sequence
encoding a portion of the maize hydroxyproline-rich
glycoprotein 3-prime to a gene encoding the polypeptide.
Steifel et al., Plant Cell Z: 785-793 (1990).
A vacuolar signal sequence facilitates the transport
of a protein to the vacuole. For example, vacuolar
targeting is accomplished by fusing the BAA secretory
signal sequence at the amino-terminus of the protein and
a sequence encoding a vacuolar signal sequence to the
carboxy-terminus. Transport of a polypeptide to the
CA 02502460 1997-05-01
WO 97/41228 PCT1US97/07688
-14-
vacuole is therefore accomplished by means operably
linking a nucleotide sequence encoding BAA 5-prime, and
a nucleotide sequence encoding a vacuolar signal sequence
3-prime to a gene encoding a polypeptide. Alternatively,
vacuolar targeting is accomplished by constructing a
nucleotide sequence comprising in the 5-prime to 3-prime
direction nucleotide sequences encoding a vacuole signal
sequence, BAA and a polypeptide.
An endoplasmic reticulum retention sequence targets
a polypeptide for localization in the lumen of the
endoplasmic reticulum. For example, a polypeptide is
targeted for retention in the endoplasmic reticulum
through the addition of the BAA sequence on the amino-
terminus and an endoplasmic reticulum signal sequence on
the carboxy-terminus of the polypeptide.
A nuclear targeting sequence facilitates transport
of a polypeptide to the nucleus. Typically, the nuclear
signal sequence is located at the amino-terminus of a
polypeptide. In order to retain the nuclear targeted
protein in the nucleus, it may be necessary to increase
the molecular weight of the protein by means of fusing an
unrelated protein to the'carboxy-terminus of the targeted
protein. For example, GFP was retained in the nucleus by
operably linking a nucleotide sequence encoding a nuclear
signal sequence 5-prime and a nucleotide sequence
encoding maize acetolactate synthase 3-prime to a gene
encoding a polypeptide.
A..peroxisomal targeting sequence facilitates the
transport of a polypeptide into the pero.cisome.
Typically, the peroxisomal signal sequence is a
tripeptide located at the carboxy-terminus of a
polypeptide.
A chlorcnlaat targetina sequence facilitates the
transport of a nuclear encoded protein to a chloroplast
compartment. Typically, the chloroplast signal sequence
is located at the amino-terminus of a polypeptide.
Accordingly, transport of a polypeptide to a chloroplast
compartment is accomplished by means of operably linking
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-15-
the nucleotide sequence encoding a chloroplast signal
sequence to the 5-prime region of a gene encoding a
polypeptide.
An isolated DNA molecule is a fragment of DNA that
is not integrated in the genomic DNA of an organism.
Complementary DNA (cDNA) is a single-stranded DNA
molecule that is formed from an mRNA template by the
enzyme reverse transcriptase. Typically, a primer
complementary to portions of mRNA is employed for the
initiation of reverse transcription. Those skilled in
the art also use the term "cDNA" to refer to a double-
stranded DNA molecule., consisting of such a single-
stranded DNA molecule and its complementary DNA strand.
The term expression refers to the biosynthesis of a
gene product. For example, in the case of.a structural
gene, expression involves transcription of the structural
gene into mRNA and the translation of mRNA into one or
more polypeptides.
A cloning vector is a DNA molecule, such as a
plasmid, cosmid, or bacteriophage, that has the
capability of replicating autonomously in a host cell.
Cloning vectors typically contain one or a small number
of restriction endonuclease recognition sites at which
foreign DNA sequences can be inserted in a determinable
fashion without loss of an essential biological function
of the vector, as well as a marker gene that is suitable
for use in the identification and selection of cells
transformed with the cloning vector. Marker genes
typically include, for example, genes that provide
tetracycline resistance, ampicillin resistance or
kanamycin resistance.
An expression vector is a DNA molecule comprising a
gene that is expressed in a host cell. Typically, gene
expression is placed under the control of certain
regulatory elements, including constitutive or inducible
promoters, tissue-specific regulatory elements, and
enhancers. Such a gene is said to be operably linked to
the regulatory elements.
CA 02502460 1997-05-01
WO 97/41228 PCT1US97/07688
-16-
Transformation includes introduction of genetic
material into plant cells resulting in chromosomal
integration and stable heritability through meiosis.
Transformation also includes introduction of genetic
material into plant cells in the form of plant viral
vectors involving epichromosomal replication and gene
expression which may exhibit variable properties with
respect to meiotic stability.
A foreign gene refers in the present description to
a DNA sequence that is operably linked to at least one
heterologous regulatory element.
A recombinant host may be any prokaryotic or
eukaryotic cell that contains either a cloning vector or
expression vector. This term also includes those
prokaryotic or eukaryotic cells that have been -
genetically engineered to contain the cloned gene(s) in
the chromosome or genome of the host cell.
A transaenic pant is a plant having one or more
plant cells that contain an expression vector.
A green fluorescent protein (GFP), or a functional
fragment of a GFP, is capable of producing a green
fluorescence. GFP absorbs in the UV to blue range with
a peak at 395 nm and emits in the.green with.a peak at
510 nm. The "red-shifted"-version of GFP is a modified
version of GFP which absorbs between 480 to 490 nm and
emits in the green with a peak at 510nm. Red-shifted GFP
is abbreviated as GFPr. The.."blue fluorescent protein"
is a modified'version of GFP which absorbs around 380nm
and emits in the blue with a peak at around 445nm. Blue
fluorescent protein is abbreviated as BFP. GFP. is a
nucleotide sequence coding for GFP in which the DNA
sequence has been modified based on codon preference in
maize (Fig. 1).
Two nucleic acid molecules are considered to have a
substantial sequence similarity if their nucleotide
sequences share a similarity of at least 50%. Sequence-
similarity determinations can be performed, for example,
using the FASTA program (Genetics Computer Group;
CA 02502460 1997-05-01
WO 97/41228 PCTIUS97/07688
-17-
Madison, WI) Alternatively, sequence similarity
determinations can be performed using BLAST (Basic Local
Alignment Search Tool) of the Experimental GENIFO(R)
BLAST Network Service. See Altschul et al., J. Mol.
Biol. 3: 403 (1990). Also, see Pasternak et al.,
"Sequence Similarity Searches, Multiple Sequence
Alignments, and Molecular Tree Building," in METHODS IN
PLANT MOLECULAR BIOLOGY AND BIOTECHNOLOGY, Glick et al.
(eds.), pages 251-267 (CRC Press 1993).
2. Reducing GYP Toxicity in Transformed Plants
Oxidative stress associated with GFP -Ã uor-escence
has been suggested to cause cellular toxicity. Haseloff
et al., TIG 11: 328-329 (1995). If GFP is to be useful
as a screenable marker for plant transformation, as well
as a reporter gene for detection of gene expression,
strategies for reducing GFP toxicity must'be available.
The gene encoding GFP can be operably linked to an
inducible promoter so that GFP can be transiently
expressed and transformed cells identified.
Alternatively, the gene encoding GFP can be operably
linked to a signal sequence for targeting to an organelle
or subcellular compartment or for secretion to the
apoplast where GFP is not toxic to the cell.
Oxidative stress can be ameliorated by transforming
cells containing GFP with an enzyme that serves as an
oxygen scavenger. Such enzymes are well known in the
art.' For example, genes encoding the enzyme supe_oxide
dismutase may protect against the primary oxidative
stresses associated with GFP fluorescence. Balzan et
al., Proc. Nat Acad. Sci. 21: 4219-4223 (199S). Other
enzymes involved in the oxidative stress response, such
as ascorbate peroxidase or glutathione S-transferase,
could help mitigate secondary effects in the cell.
Conklin, Plant Physiol. la: 203-212..(1995).
More specifically, oxidative stress can be
ameliorated by transforming cells with a DNA construct
CA 02502460 1997-05-01
WO 97/41228 PCTIUS97/07698
-18-
carrying genes encoding GFP and SOD. Alternatively, the
gene encoding GFP can be fused in frame to the gene
encoding SOD. Cells transformed with this DNA-construct
produce a fusion protein that cause the cells to
fluoresce in the presence of UV-blue light and express
SOD activity.
The toxicity of GFP in transformed plants can be
eliminated by excising the screenable marker gene
following detection of transformed cells or sectors. The
FLP/FRT system is used in conjunction with GFPm as a
visible viable marker for FRT excision. The FLP/FRT
system has been demonstrated in maize suspension cells
using GUS expression as an indicator of FRT excision.
Lysnik et al., NAR 2,1: 969-975 (1993). For example,
plant cells are bombarded with a DNA construct containing
the' GFP gene flanked by FRT sequences as well as a
foreign or agronomic gene of interest. The GFP gene may
be operably linked to a constitutive promoter or an
inducible promoter. In addition, the GFP gene may be
operably linked to a signal sequence. Stable
transformants -are detected by means of screening for GFP.
Transgenic callus pieces are spread on medium and
bombarded a second time with a FLP recombinase construct.
Callus is monitored periodically under UV to blue
illumination to detect cells that no longer express GFP.
Callus pieces that no longer express GFP are. regenerated
and analyzed for expression of the foreign. or agronomic
gene. Agronomically useful transgenic plants are thereby
produced that do not contain a marker gene.
The sensitivity of the screening method can be
further increased by placing two markers genes between
the FRT sequences. For example, plant cells are
bombarded with a DNA construct containing the PAT and GFP
genes flanked by FRT sequences and a foreign or agronomic
gene. Stable transformants are recovered on bialaphos-
containing medium and positive GFP-expression is
confirmed. The transformed callus is then bombarded with
FLP. . The callus is then grown for 2-6 weeks with no
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-19-
selection until 'clear GFP-null sectors can be identified.
These sectors can be transferred onto
bialaphos/chlorophenol red multiwell test plates to
confirm bialaphos sensitivity (i.e. within 3-5 days).
Callus pieces that no longer express GFP or PAT are
regenerated and. analyzed for expression of the foreign or
agronomic gene. This permits recovery of agronomically
useful transformants without any marker genes in the
final product.
Likewise, the Ac/Ds system of maize can also be used
in transgenic plants to excise the screenable marker gene
that is transformed together with a foreign or agronomic
gene. Mobilization of Ac and/or Ds has been demonstrated
in diverse plants such as tomato, tobacco and
Arabidopsis. Yoder et al., In Tomato Technology, Alan R.
Liss, Inc. pp 189-198 (1987); Yoder et al., U.S. Patent
No. 5,225,341; Baker et al., EMBO J J: 1547-1554 (1987)
and Lawson et al. Mol. Gen. Genet., ;z: 608-615 (1994).
Likewise, the cre/lox recombinase system from
bacteriophage P1 could also be used in conjunction with
GFP. Excision of transgenes in plants using the cre/lox
system was first demonstrated in tobacco. Odell et al.,
Mol. Gen. Genet., Z: 369-378 (1990) and Dale and Ow,
Proc. Natl. Acad. Sci. USA, La: 10558-10562 (1991)=.
Similar to the FLP and Ac systems described above, GFP
expression provides an efficient, easily scorable
phenotype for monitoring excision.
3. Isolation of Nucleotide Sequences Encoding GFP
Peptide sequences from purified GFPs are used to
design degenerate oligonucleotide primers for polymerase
chain reactions and gene cloning. Protein extracts can
be prepared from cnidarian cells by standard methods
known to the art. See, for example, Harlow and Lane,
Antibodies, A Laboratory Manual, Cold Spring Harbor
Press, (1988). in a preferred embodiment, cnidarian
cells are extracted into a buffer, and the extracts
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-20-
separated into membrane and soluble fractions. Each
fraction is tested for green fluorescence activity in the
presence of blue light.
Fractions which contain GFP activity are then
purified further to determine which components are
responsible for the activity. Purification of the active
fractions can be carried out by methods known in the art.
See, for example, PROTEIN PURIFICATION METHODS - A
PRACTICAL APPROACH, Harris et al., Eds. (IRL Press,
Oxford, 1989). Extracts prepared as described above are
purified by sequential size exclusion chromatography
isoelectric focusing, HPLC size exclusion chromatography,
and chromatography on an affinity column. Fractions
which display GFP activity can be analyzed further by
SDS-PAGE analysis to determine the approximate molecular
mass of the active component.
Purified GFPs prepared by the methods described
above can be sequenced using methods well known in the
art, for example using a gas phase peptide sequencer
(Applied Biosystems, Foster City, CA). To determine as
much of the peptide sequence as possible, it is preferred
that the proteins of the present invention be cleaved
into smaller fragments more suitable for 'gas-phase
sequence analysis. This can be achieved by treatment of
a purified GFP with selective peptidases, and in a
particularly preferred embodiment, with endoproteinase
lys-C (Boehringer). The fragments so produced can be
separated by reversed-phase HPLC chromatography.
The peptide sequences of the proteins determined as
above can be used to determine the DNA sequence encoding
the protein. Methods for carrying out this determination
are well known in the art. See, for example Sambrook
et al., MOLECULAR CLONING: A LABORATORY MANUAL, Second
Edition, (Cold Spring Harbor Laboratory Press, Cold
Spring Harbor, NY 1989).
In a preferred embodiment of the present invention,
the peptide sequences are used to design degenerate
oligonucleotide primers for polymerise chain reactions.
CA 02502460 1997-05-01
WO 97/41228 PCTIUS97/07688' -
-21-
Each degenerate primer set will preferably contain every
possible DNA sequence encoding the corresponding peptide
sequences. Primer sets are prepared in both the sense
and antisense orientation. Suitable oligonucleotide
primers can be synthesized using commercial synthesizers,
such as those supplied by Applied Biosystems (Foster
City, CA). In a particularly preferred embodiment,, the
primers include additional nucleotide sequences
containing restriction endonuclease cleavage sites. The
presence of such sites allows for the directional cloning
of PCR products into suitable cloning vectors after
treatment with anappropriate restriction enzyme. See
Finney, "Molecular Cloning of PCR Products" in CURRENT.
PROTOCOLS IN MOLECULAR BIOLOGY, Ausubel et al. Eds. (John
Wiley & Sons, New York, 1987) p. 15.7.1.
Template DNA for the PCR can be prepared from
appropriate cnidarian cells or tissues using methods-well
known in the art. See Sambrook et al., supra. In a
preferred embodiment, host cells are crushed under liquid
nitrogen and mRNA is extracted using a commercially
available' kit (Pharmacia, Piscataway, NJ).
The mRNA preparation can then be used as a template
for cDNA synthesis using poly(dT) or random hexamer
primers by standard techniques. See Sambrook et al.,
supra. in a particularly preferred embodiment, cDNA-
synthesis is carried out using a commercially available
kit (Pharmacia).
The cDNA can then be used directly for PCR using the
method of Saiki et al., Science Z :. 487 (1988). The
cDNA also is used to prepare a cDNA library by. standard
methods. See Sambrook et al., supra. In a particularly
preferred embodiment, the cDNA is packaged into
bacteriophage particles using a commercially available
kit (Promega, Madison, WI). The packaged cDNA is then
transfected into E. calf to produce a cDNA library.
In an alternative preferred embodiment, genomic DNA
from cnidarian cells or tissue can be used as the
template DNA for the PCR. Genomic DNA can be prepared by
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-22-
standard methods and PCR can then be used to prepare
double stranded DNA molecules to probe the cDNA library
and the genomic DNA for the gene(s) encoding GFP. In a
preferred embodiment, degenerate primers are prepared
S corresponding to the termini of the longest peptide-
sequence determined by peptide sequencing. In a
particularly preferred embodiment, primers are used in a
PCR with first strand cDNA as template to amplify the DNA
encoding the peptide. PCR is carried out under standard
conditions. See Sakai et al., supra.
PCR amplification products are analyzed by
polyacrylamide gel electrophoresis using standard
methods. If an amplification product of the expected
size (based on the peptide sequence) is found, the
product is digested with appropriate restriction enzymes,
ligated into a cloning vector and cloned by standard
methods. See Sambrook et al, supra. In a preferred
embodiment, clones are sequenced to verify that sequences
according to the. expected peptide sequence are present.
Once the DNA sequence encoding the peptide is known,
it can be used to prepare non-degenerate primers
corresponding' to that sequence, again containing
restriction enzyme recognition sequences to aid in
cloning of DNA products. _ These primers are used in
combination with degenerate primers corresponding to
other peptide sequences to generate PCR amplification
products which can be cloned and then analyzed as above.
By these means, fragments-of the gene sequence of the
protein can be determined. Alternative methods for
carrying out this PCR analysis include use of the 5' or
3' RACE methods using commercially available kits, such
as those manufactured by Life Technologies .(Gaithersburg,
MD) orClontech (Palo Alto, CA). Primers for this method
are selected according to the manufacturer's directions.
Gene fragments prepared as above are excised from
the cloning vector by restriction enzyme digestion,
labeled with 32P by conventional methods and-used as
probes .to identify the complete gene encoding the
CA 02502460 1997-05-01
WO 97/4122$ PCT/US97/07688
-23-
protein from within a cDNA or genomic library. In a
preferred embodiment, the probe is chosen such that it-is
long enough to ensure hybridization specificity, while
remaining short enough to allow reasonable rates of
hybridization to the target gene.
A cnidarian genomic DNA library can be prepared by
means well-known in the art. See, for example, Slightom
et al. "Construction of A. Clone Banks," in METHODS IN
PLANT MOLECULAR BIOLOGY AND BIOTECHNOLOGY, Glick et al.
(eds.), pages 121-146 (CRC Press, 1993). Genomic DNA can
be isolated from cnidarian tissue, for example, by lysing
plant tissue with the detergent. Sarkosyl'", digesting' the
lysate with proteinase K, clearing insoluble debris from
the lysate by centrifugation, precipitating nucleic acid
from the lysate using. isopropanol. and purifying
resuspended DNA on a cesium chloride density gradient.
See, for example,- Ausubel et al. (eds.), CURRENT
PROTOCOLS IN MOLECULAR BIOLOGY, pages 2.3.1-2.3.3 (Wiley
Interscience 1990) ["Ausubel").
DNA fragments that are suitable for the production
of a genomic library can be obtained by the random
shearing of genomic DNA or by the partial digestion of
genomic DNA with restriction endonucleases. See, for
example, Ausubel at pages 5.3.2-5.4.4, and Slightom et
al., supra. Genomic DNA fragments can be inserted into
c vector, such as a bacteriophage or cosmid vector, in
accordance with conventional techniques, such as the use
of restriction enzyme digestion to provide appropriate
termini, the use of alkaline phosphatase treatment to
avoid undesirable joining of DNA molecules, and ligation
with appropriate ligases. Techniques for such
manipulation are disclosed by Slightom et al., supra, and
are well-known in the art. Also see Ausubel at pages
3Ø5-3.17.5.
Screening of the cDNA or genomic library is carried
out by conventional methods. See Sambrook et al, supra.
Clones which hybridize to the probe are purified and
their sequences determined. To facilitate sequencing,
CA 02502460 1997-05-01
nested deletions in the clones can be created using
standard protocols, or by commercially available kits
TM
such as Erase-abase (Promega, Madison, WI) or The
'rm
Deletion Factory (Life Technologies, Gaithersburg, MD)-,
following the manufacturer's instructions. The sequences
obtained are analyzed for the presence of open reading
frames by conventional methods and to check if the entire
gene sequence has been found. In a preferred embodiment,
cDNA libraries are prepared by both random hexamer and
poly (dT) priming from samples, and are used to maximize*
the chances of finding the complete coding sequence of
the desired gene.
Once the entire coding sequence of the e=ne-=for GFP
has been determined, the gene can be inserted into an
appropriate expression system. The gene can be expressed
in any number of different recombinant DNA expression
systems to generate large amounts of protein, which can
then be purified.
4. Chemical Synthesis of Genes Encoding GYP
Once the amino acid sequence of a GFP is known, a
gene encoding this GFP can be synthesized. In addition,
the nucleotide sequence of . synthetic gene encoding GFP
can be. optimized for expression in plants by modifying
the codon usage to include plant preferred codons. See,
for example, Murray et al., MAR 17: 477 (1989). Even
more specifically, the nucleotide sequence of a synthetic
gene encoding GFP can be optimized for express_on in
monocotyledonous or dicotyledonous plants. See, for
example, Campbell et al., Plant Physiol. 92: 1 (1990).
Genes encoding GFP can be obtained, for example, by
synthesizing the genes with mutually priming long
oligonucleotides. See, for example. Ausubel at pages
8.2.8 to 8.2.13. Also, see Wosnick et al., Gene 60:115
(1987). Moreover, current techniques using the
polymerase chain reaction provide the ability to
synthesize genes as large as 1.8 kilobases in length.
CA 02502460 1997-05-01
WO 97/41228 PC IUS97/07688
-25-
Adang et al., Plant Molec. Biol. 21: 1131 (1993) ; Bambot
et al., PCR Methods and Applications a: 266 (1993).
5. Identification of Functional Fragments of the GYP
DNA clones can be analyzed using a variety of
techniques such as restriction analysis, Southern
analysis, primer extension analysis, and DNA sequence
analysis. Primer extension analysis or Si nuclease
protection analysis, for example, can be used to localize
the putative start site of transcription of the cloned
gene. Ausubel at pages 4.8.1-4.8.5; Walmsley et al.,
"Quantitative and Qualitative Analysis of Exogenous Gene
Expression by the Si Nuclease Protection Assay," in
METHODS IN MOLECULAR BIOLOGY, VOL. 7: GENE TRANSFER AND
EXPRESSION PROTOCOLS, Murray (ed.), pages 271-281 (Humana
Press Inc. 1991). Functional fragments of the GFP
protein are identified .by production of green
20= fluorescence in the presence of blue UV light.
The general approach of such functional analysis
involves subcloning DNA fragments of a genomic clone,
cDNA clone or synthetic gene-sequence into-an expression
vector, introducing the expression vector into a
heterologous host, and pcreening to detect green
fluorescence in the presence of UV to blue light.
Methods for generating fragments of a cDNA or genomic
clone are well-known. Variants of an isolated DNA
encoding GFP can be produced by deleting, adding and/or
substituting nucleotides for the isolated nucleotides,
for example, the nucleotide sequence of SEQ ID NO: 1.
Such variants. can be obtained, for example, by
oligonucleotide-directed mutagenesis, linker-scanning
mutagenesis, mutagenesis using the polymerase chain
reaction, and the like. See, for example, Ausubel, pages
8Ø3-8.5.9. Also see generally, McPherson (ed.),
DIRECTED MUTAGENESIS: A PRACTICAL APPROACH,¨ (IRL Press
1991). Thus,. the present invention also encompasses DNA
molecules comprising nucleotide sequences that have
CA 02502460 1997-05-01 ,
WO 97/41228 PCT/US97/07688
-26-
substantial sequence similarity with SEQ ID NO: 1 and
encode a GFP.
6. Methods for Plant Transformation
The GFP-based screening method for plant
transformation of the instant application can be used in
conjunction with any method of plant transformation and
regeneration. Numerous methods for plant transformation
have been developed, including biological and physical,
plant transformation protocols. See, for example, Miki
et al., "Procedures for. Introducing Foreign DNA into
Plants" in Methods in Plant Molecular Biology and
Biotechnology, Glick, B.R. and Thompson, J.E. Eds. (CRC
Press, Inc., Boca Raton, 1993) pages 67-88. In addition,
expression vectors and in vitro culture methods for plant
cell or tissue transformation and regeneration of plants
are available.. See, for example, Gruber et al., "Vectors
for Plant Transformation" in Methods in Plant Molecular
Biology and Biotechnology, Glick, B.R. and Thompson, J.E.
Eds. (CRC Press, Inc., Boca Raton, 1993) pages 89-119.
A. Agrobacterium-mediated Transformation
.The most widely utilized method for introducing an
expression vector into plants is based on the natural
transformation system of Agrobacterium. See, for
example, Horsch et al., Science X7,:1229 (1985). A.
tumefaciens and A. rhizogenes are plant pathogenic. soil
bacteria which genetically transform plant cells. The Ti
and Ri plasmids of A. tumefaciens and A. rhizogenes,,
respectively, carry genes responsible for .genetic
transformation of the plant. See, for example, Kado,
C.I., Crit. Rev. Plant. Sci. 12: 1 (1991). Descriptions
of Agrobacterium vector systems and methods for
Agrobacterium-mediated gene transfer are provided by
Gruber et al., supra, Miki et al., supra, and Moloney et
al., Plant Cell Reports 8: 238 (1989).
CA 02502460 1997-05-01
WO 97/41228 PCTIUS97/07688
-27-
B. Direct-Gene Transfer
Despite the fact the host range for Agrobacteriiun-
mediated transformation is broad, some major cereal crop
species and gymnosperms have generally been recalcitrant
to this mode of gene transfer, even though some success
has recently been achieved in rice. Hiei et al., The
Plant Journal 6: 271-282 (1994) Several methods of
plant transformation, collectively referred to as direct
gene transfer, have been developed as an alternative to
Agrobacterium-mediated transformation.
A generally applicable method of plant
transformation ismicroprojectile-mediated transformation
wherein DNA is carried on the surface of microprojectiles..
measuring 1 to4 m. The expression vector is introduced
into plant tissues with a biolistic device that
accelerates the microprojectiles to speeds of 300 to 600
m/s which is sufficient to penetrate plant cell walls and
membranes. Sanford et al., Part. Sci. Technol. 1: 27
(1987), Sanford,. J.C., Trends Biotech. k: 299 (1988)
Sanford, J.C., Physiol. Plant 779: 206 (1990), Klein et
al., Biotechnology IQ: 268 (1992).
Another method for physical delivery of DNA to
plants is sonication of target cells. Zhang et al.,
Bio/Technology.2: 996 (1991)_. Alternatively, liposome or
spheroplast fusion have been used to introduce expression
vectors into plants. Deshayes et al., EMBO J., g: 2731
(1985), Christou et al., Proc Natl. Acad. Sci. U.S.A. Q4:
3962 (1987).' Direct uptake-of DNA into protoplasts using
CaClz precipitation, polyvinyl alcohol or rely-L-
ornithine have also been reported. Hain et al., Mol.
Gen. Genet. =: 161 (1985) and Draper et al., Plant Cell
Physiol. 11: 451 (1982). Electroporation of protoplasts
and whole cells and tissues have also been described.
Donn et al., in Abstracts of VIIth International Congress
on Plant Cell and Tissue Culture IAPTC, A2-38, p 53
(1990); D'Halluin et al., Plant Cell j: 1495-1505 (.1992)
and Spencer et al., Plant Mot. Biol. 21: 51-61 1.1994).
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-28-
A preferred method is microprojectile-mediated
bombardment of immature embryos. The embryos can be
bombarded on the embryo axis side to target the meristem
at a very early stage of development or bombarded on the
scutellar side to target cells that typically form callus
and somatic embryos. Targeting of the scutellum using
projectile bombardment is well known to those in the art
of cereal tissue culture. Klein et al., Bio/Technol., 1:
559-563 (1988) ; Kartha et al., Plant Cell Rep. Q: 429-432
(1989); Sautter et al., Bio/Technol., 1: 1080-1085
(1991); Chibbar et al., 34: 43.5-460 (1991). The
scutellar origin of regenerable callus from cereals is
well known. Green et al., Crop Sci., : 417-421 (1975);
Lu et al., TAG 62: 109-112 (1982); and Thomas and Scott,
J. Plant 121: 159-169 (1985). Targeting the
scutellum and then using chemical selection to recover
transgenic plants is well established in cereals.
D/Halluin et al., Plant Cell 1: 1495-1505 (1992) ; Perl et
al., MGG A: 279-284 (1992); Cristou et al.,
Bio/Technol. a: 957-962 (1991).. This literature reports
DNA targeting of the scutellum and recovery of transgenic
callus, plants and progeny based on a chemical selection
regime. None of these references teach successful plant
transformation wherin transformed cells are: visualized'
with a screenable marker such as GUS.
A preferred transformation method involves
bombardment of the scutellar surface of immature embryos
to introduce a fGP expression cassette and any other
cotransformed genes. Embryos can be pretreated for 1 to
48 hours with a high osmoticum medium or left on a high-
osmoticum medium for 24-48 hours after bombardment to
improve cell survival and transformation frequencies.
Immature embryos are then cultured on typical callus-
inducing medium with no selective agent. At each
subculture transfer, i.e., every two weeks, the culture
is monitored using UV-blue light for GFP fluorescence.
Fluorescing calli are separated from non-fluorescing
CA 02502460 1997-05-01
WO 97141228 PCT1US97107688'
= -29-
callus, and grown to the point where plants can be
regenerated through standard media progressions.
Many exemplary variations, in terms of target cells
and tissue culture routes are known in the art that could
$ be used with GFP in this fashion. Some of these
variations include introduction of GFP expression into
protoplasts, suspension cells, type II callus, and type
I callus, as in Morocz et al., Theor. Appl. Genet. "-
721-726 (1990), Gordon-Kamm et al., Plant Cell 2: 603-618.
(1990), Fromm et al., Bio/Technol..e: 833-839 (1990) and
D'Halluin et al., Plant Cell 4: '1495-1505 (1992).
Microspores and microspore-derived embryogenic callus
also represent a feasible alternative target-,/.culturing
route for using GFP screening to recover transformants.
See Sun et al., Plant Cell Rep.. 8: 313-316 .(1989) and
Mitchell et al., J. Plant Phys.iol. 37: 530-536 (1991).
Furthermore, it is expected that the method of the
present invention is also be applicable to any dicot
species that is known to have a callus stage, for
example, tobacco, canola or soybean.
In the case of the-maize meristem target, the method
entails -selective enlargement of transgenic sectors,
toward genetic homogeneity, in cell layers that
contribute to germline. transmission. This method is
described in U.S. Patent No: 5',736,369,
and
comprises the steps of (A) introducing foreign DNA into
a meristem that is not enclosed by sheathing leaves; (B)
inducing reorganization of the meristem to increase
transgenic sector size, whereby the likelihood that a
transgenic sector will contribute to germline
transmission is increased; and (C) exposing the meristem
to conditions under which it differentiates to form a
plantlet, wherein the plantlet contains the transgenic
sector or is homogeneously transformed by the foreign
DNA, such that the plantlet can be grown into a
transformed cereal plant that will transmit the foreign
DNA to progeny. The foreign DNA can be introduced into
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-30-
a plurality of, meristems, at least some of which
differentiate in step (C) to form a plurality of
plantlets.
In one preferred embodiment, reorganization is
effected through at least one manipulation selected from
the group consisting of (i) imposition of a nonlethal
selective pressure on the meristems, (ii) mechanically-
induced meristem reorganization, and (iii) hormonally
induced shoot multiplication. In another preferred
embodiment the conditions in step (C) are such that the
meristems undergo maturation and plant differentiation to
form shoot apices, and the method further comprises
effecting reorganization of meristem tissue in the shoot
apices to enlarge transformed sectors or to produce
periclinal L2 chimeras. The reorganization in this
regard can be effected, for example, by exposing the
shoot apices to nonlethal selection pressure such that
transformed cells have a competitive growth advantage
over nontransformed cells in the shoot apices, and the
proportion of transformed cells in the shoot apices is
increased. In yet another preferred embodiment, the
method further, comprises a step before step (B) , e.g.,
before step (A), of wounding the apical dome selectively.'
A method of the present invention also can comprise the
further steps of (i) dissecting out an axillary bud from
above the base of a leaf of a plantlet when a chimeric
sector is observed in a substantial portion of the leaf,
and then (ii) germinating the axillary bud into a whole
plant or subjecting the axillary bud to shoot
multiplication.
Likewise, the introduction of GFP-encoding DNA into
dicot meristems provides an efficient method of mapping
transgenic sectors and following these up into a germline
and into progeny. Using mature imbibed seeds as the
starting material, the meristem is exposed by. removal of
the cotyledons, - and DNA is introdiced into meristem
cells. A preferred method for introducing DNA into
meristem cells is Agrobacterium-mediated delivery, but
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-31-
other DNA delivery methods can be used with this explant
such as bombardment with DNA-coated particles, or silicon
carbide fiber-mediated delivery. Kaeppler et al., Plant
Cell Rep. 9: 415-418 (1990). After DNA introduction, the
meristem explants are cultured.
Plants can-be manipulated, for example, by removal
of the apical meristem, to stimulate axillary or
secondary buds which can exhibit larger transgenic
sectors relative to the primary shoot. Flowers above
transgenic shoots are pollinated and the progeny are
analyzed for transgene presence and expression. A
variety of starting explants can regenerate shoots in
sunflower, and thus represent alternative targets for
GFP-encoding DNA delivery and transmission to progeny.
These include the seedling meristem (as above), also the
seedling hypocotyl, the mature cotyledon, the immature
cotyledon, zygotic immature embryos, somatic embryos, and
primary leaflets. See for example, respectively, Greco
et al., Plant Sci. Lett. 21: 73-77 (1984); Krauter et
al., Helia U: 117-122 (1991) .; Power Am. J. Bot. 74: 497-
503 (1987); Krauter et al., Theor. Appl. Genet. 82: 521-
525 (1991); Finer, Plant Cell Rep. ¾: 372-374 (1987) , and
Greco et al., Plant Sci. Lett. U: 73-77 (1984).
In similar fashion, GFP expression could be used in
other dicot species, for example, soybean, to map sectors
and/or identify transformants and follow the transgenes
through to progeny. A GFP-encoding cassette and other
cotransformed genes can be introduced into soybean
cotyledonary node cells using Agrobacterium. The
explants are cultured on Gamborg (B5) medium with or
without kanamycin selection. With no selection, GFP
expression is used to identify transgenic chimeric or
homogeneously transformed plantlets, which are rooted,
grown to maturity and pollinated in the greenhouse.
Progeny seed are collected for analysis. The use of,
cotyledonary nodes and Agrobacterium is presented by way
of example, but other target cells and culturing. methods
could, be used with GFP for soybean. In addition to the
CA 02502460 1997-05-01
WO 97/41228 PCTIUS97/07688
-32-
cotyledonary node cells, mature cotyledons and immature
cotyledons have both been used for transformation. See
Hinchee et al., Bio/Technol. 6: 915-922 (1988) and
Parrott et al., Plant Cell Rep. 2: 615-617 (1989).
Meristems from immature soybean seed embryonic axes have
also been used for bombardment-mediated soybean
transformation. See Christou et al., Plant Physiol. 87:
671-674 (1988) and McCabe et al., Bio/Technol'. 1: 923-926
(1988) .
7. Promoters
A. Inducible Promoters
An inducible promoter is operably linked to a
nucleotide sequence encoding a GFP. Optionally, the
inducible promoter is operably linked to a nucleotide
sequence encoding a signal sequence which is operably
linked to a nucleotide sequence encoding GFP. With an
inducible promoter the rate of transcription increases in
response to an inducing agent.
Any inducible promoter can be used in the instant
invention. See ward et al. Plant Mot. Biol. : 361-366
(1993). Exemplary inducible promoters include that from
the ACE1 system which responds to copper (Metz et al.
PNAS U: 4567-4571 (1993)); 1n2 gene from maize which
responds to benzenesulfonamide herbicide safeners
(Hershey et al., Mol. Gen. Genetics 22,7: 229-237 (1991)
and Gatz et al., Mo1. Gen. Genetics 2ga: 32-38 (1994)) or
Tet- repressor from Tn10 (Gatz et al., Mol. Gen. Genet.
227: 229-237 (1991). A particularly preferred inducible
promoter is a promoter that responds to an inducing agent
to which plants do not normally respond., An exemplary
inducible promoter is the inducible promoter from a
steroid hormone gene the transcriptional activity of
which is induced by a glucocorticosteroid hormone.
Schena et al., Proc. Natl. Acad. Sci. U.S.A. 10421
(1991).
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-33-
The expression vector comprises an inducible
promoter operably linked to a nucleotide sequence
encoding GFP. The expression vector is introduced into
plant cells and presumptively transformed cells are
exposed to an inducer of the inducible promoter. The_
cells are screened for the presence of GFP protein by
means of illuminating the cells with W to blue, light and
screening for the presence of green fluorescence.
B. Tissue-specific or Tissue Preferred Promoters
A tissue-specific promoter is operably linked to a
nucleotide sequence encoding a GFP. Optionally, the
tissue-specific promoter is operably linked to a
nucleotide sequence encoding a signal sequence which is
operably linked to a nucleotide sequence encoding GFP.
Plants transformed with a gene encoding GFP operably
linked to a tissue-specific promoter produce the GFP
protein exclusively, or preferentially, in a specific
tissue.
Any tissue-specific or tissue-preferred promoter can
be utilized in the instant invention. Exemplary tissue
specific or tissue-preferred promoters include a root-
preferred promoter such as that from the phaseolin gene
(Murai et al., Science 21: 476-482 (1983) and Sengupta-
Gopalan.et al., Proc. Natl. Acad. Sci. USA j: 3320-3324
(1985)); a leaf-specific and light-induced promoter such
as that from cab or rubisco (Simpson et al., F.'0 J.
4(11): 2723-2729 (1985) and, Timko et al., Nature
579-582 (1985)); an anther-specific promoter such as that
from LAT52 (Twell et al., Mol. Gen. Genet. ZL7 : 240-245
(1989)); a pollen-specific promoter such as that from
Zm13 (Guerrero et al., Mol. Gen. Genet-.: 161-168
(1993))= or a microspore-preferred promoter such as-.that
from apg (Twell et al., Sex. Plant Reprod. 6: 217-224
(1993).
The expression vector comprises a tissue-specific or
tissue-preferred promoter operably linked to a nucleotide
sequence encoding GFP. The expression vector is
WO 97141228 CA 02502460 1997-05-01 PCT/1JS97/07688
-34-
introduced into plant cells. The cells are screened for
the presence of GFP protein by means of illuminating the
cells with UV to blue light and screening for the
presence of green fluorescence.
C. Constitutive Promoters
A constitutive promoter is operably linked to a
nucleotide sequence encoding a GFP or the constitutive
promoter is operably linked to a nucleotide sequence
encoding a signal sequence which is operably linked to a
nucleotide sequence encoding GFP.
Many different constitutive promoters can be
utilized in the instant invention. Exemplary
constitutive promoters include the promoters from plant
viruses such as the 35S promoter rom CaMV (Odell et al.,
Nature 313: 810-812 (1985) and the promoters from such
genes as rice actin (McElroy et al., Plant Cell 2: 163-
171 (1990)); ubiquitin (Christensen et al., Plant =Mol.
Biol. 12: 619-632 (1989) and Christensen et al., Plant
Mol. Biol. 18: 675-689 (1992)); pEMU (Last et al., Theor.
Appl. Genet_ 81: 581-588 (1991)); MAS (Velten et al.,
EIMO J. 3: 2723-2730 (1984)) and maize H3 histone
(Lepetit et al., Mol. Gen. Genet. 2 1: 276-285 (1992) and
Atanassova et a l . , Plant Jqurnal 2 3 : 291-300 (1992)).
The ALS promoter, a XbaI/NcoI fragment 5-prime to
the Brassica napus ALS3 structural gene (or a nucleotide
sequence that has substantial sequence similarity to said
XbaI/NcoI fragment) , represents a particularly useful
constitutive promoter,.- Pioneer Hi-Bred
International US Patent No: 5,659,026.
The expression vector comprises a constitutive
promoter operably linked to a nucleotide sequence
encoding GFP. The expression vector is. introduced into
plant cells and presumptively transformed cells are
screened for the presence of GFP protein by means of
illuminating the cells with UV to blue light and
screening for the presence of green fluorescence.
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-35-
8. Signal Sequences For Targeting Proteins to
Subcellular Compartments
Transport of GFP to a subcellular compartment such
as the chloroplast, vacuole, peroxisome, glyoxysome, cell
wall or mitochondrion, or for secretion into the
apoplast, is accomplished by means of operably linking
the nucleotide sequence encoding a signal sequence to the
5-prime and/or 3-prime region of a gene encoding GFP.
Targeting sequences at the 5-prime and/or 3-prime end of
the structural gene may determine, during protein
synthesis and processing, where the encoded protein is
ultimately compartmentalized. The presence of_a.signal
sequence directs a polypeptide to either an intracellular
organelle or subcellular compartment or for secretion to
the apoplast.
In addition to the targeting sequences described
above, the addition of amino acids to the encoded protein
(fusing GFP to either another protein, a protein
fragment, or a peptide) can further influence the fate of
GFP. For example, a nuclear localization signal (NLS)
alone is not sufficient to accumulate GFP in the nucleus.
The GFP protein is sufficiently small that it freely
diffuses from the nucleus into the cytoplasm, probably
through the nuclear pores. The fusion of GFP to a
sufficiently large protein permits accumulation in the
nucleus if the GFP protein also contains an NLS at the
amino-terminus. GFP can also be targeted to the nucleus
by fusing GFP to proteins that are normally targeted to
the nucleus.
The following exemplary signal sequences were
utilized for targeting of GFP. However, the invention
encompasses use of any signal sequence, or combination of
signal sequences, to facilitate transport of GFP to a.
subcellular compartment or the apoplast where the protein
is not toxic to the cell but can be detected.
More specifically, GFP is expressed in plant cells
transformed with a expression vector carrying a
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-36-
nucleotide sequence encoding a signal sequence(s), which
directs a protein to a subcellular compartment or to the
apoplast, operably linked to a gene encoding GFP. This
GFP gene construct can be expressed from a constitutive,
tissue-specific, tissue-preferred or inducible promoter.
GFP is transported to a subcellular compartment or the
apoplast where it is not toxic to the plant cell. Cells
illuminated with UV to blue light can be detected because
they emit green fluorescence. The transformed cells are
selected for regeneration. If the expression vector also
carries a gene encoding a foreign protein or
agronomically useful protein, the regenerated plant can
be used for production of this protein.
A. PHP8080 - Ubi::CTP-GFPm
A chloroplast signal sequence from the maize
cab-m7 has been characterized and the nucleotide
sequence is shown below. Becker et al., Plant Mol.
Biol. 22: 49 (1992). The signal sequence includes
21 amino acids of the cab-m7 protein that extends
beyond the processing site (marked by *).
ATO GCG TCC TCC ACG ATG GCC CTC TCC TCC ACC GCC TTC GCC
M A S S T M A L S S T A F A
-
GGC AAG GCC GM AAC GTG CCG TCG TCG TCC GCC TTC GAG
G K A V N V P S S S A F B
GCC CGC GTG ACC ATO AGG AAG ACG GCG GCC AAG GCC AM
A R V T ON R K T A A K A K K
CCA OCT GCG GCG TCC WA AGC CCG TOG TAC GGC CCC ATG
P A A A S G S P W Y G P N
B. PHP8087 - CPN60::GFPm
The mitochondria) signal sequence from
the maize chaperonin 60 gene is shown.below.
Close, P.S., Master's Themes, Iowa State
University (1993). The signal sequence
includes intron 1 of cpn60II (marked by -) and
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-37-
includes 2- amino acids from the cpn6OII
protein beyond the-processing site (marked by
ATG TAC CCC CCG GCC GCt AGC CTC CCC TCC AAC CCC CGC CAA GCC CCC AGC
M Y R A A A S L A S K A R Q A G S
AGC TCC GCC CCT CGC CAG-gtgagagcagaetcgtgtttatacgegtgtgatgggtetgatg
S S A A R 0
gaggatcccgccetcagatttggtaatgttggcctggggttctagtagttctgegtgcagggetgtg
ggtttgetgcatggtgctgtttattttggtggegatctgceggaatetgtagttegctegegcaaaa
tetaagctagctcgctaatggegtactggegtggggtttcacattaatctacggtggtgaact.egte
aetaccgtcetccagttagctgttagaeaeegaataeaetgattggcagttgagaaacattgatetg
atccagcagaaatcgatgtcttgtgaaattegttattttattgtcgtgtaacettggggeatggeag
tctctaattgatcacgcactcacctctgttgtgtgtgatgctttateg-C" GGA ACC AGG CD
V G S R L
GCC TGG AGC AGO AAC TAT OCT GCC ATC
A W S R N Y =A A M
C. PHP8144 -_ Ubi::BAA-GFPm - Cloned sequence
includes 1 amino acid beyond processing site
(marked by *, lower case bases show linker.,
sequence).
The barley alpha amylase 1 signal
sequence is shown below. Knox, C., et al.,
"Structure- and organization of Two Divergent
Alpha-Amylase Genes From Barley", Plant Mol.
Biol. 1:3-17 '(1987). The cloned sequence
includes sequence only up to processing site.
ATG CCC AAC LAO CAC CTG ACC CTC TCC CTC Trc CTC CM CTC CTC GGC CTC TCC
M A N K H L S L S L F L V L L G L S
CCC TCC CTC GCC TCC CCA tec ATO
A S L A S =0 S N
D. PHP8757 - Ubi::BAA-GFPm-BLVT - Signal
sequence starts at *, lower case bases show
linker sequence.
The barley lectin vacuole signal sequence
is shown below. Lerner et al., Plant Physiol.
124-129 (1989).
ARC ate tac GTG TTC CCC GAG CCC ATC CCC CCC AAC TCC ACC LTC CTG GCC GAG
K I Y =V F A 9 A I A A N S T L V A E
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-38-
E. PHP8758 - Ubi::BAA-GFPm-HDEL - Signal
sequence starts at *, lower case bases show
linker sequence.
The HDEL endoplasmic reticulum retention signal
from maize b-70 is shown below. Fontes et al.,
Plant Cell 1: 483-496 (1991).
AAG ate tac GAC GGC GGC GTG GAC GAC GAC CAC GAC GAG CTC
K I Y *D G G V D D D H 0 E L
F. PHP8759 - Ubi::BAA-SVT-GFPm - Marked by
lower case bases show linker sequence.
The sweet potato sporamin vacuole signal
sequence is shown below. Matsuoka et al.,
Proc. Natl. Acad. Sci. 88: 834 (1991). The
sequence shown includes 4 additional sporamin
amino acids beyond the processing site (marked
by *) .
ATG GCC AAC AAG CAC CTG AGC CTC TCC CTC TTC CTC GTG CTC CTC GGC CTC TCC
M A N K N L S L S L F L V L L G L S
GCC TCC CTC GCC TCC GGA CAC AGC AGG TTC AAC CCC ATC AGG CTG CCC ACC ACC
A S L A S G 'H S R F N P I R L P T T
CAC GAG CCC GCC AGC AGC GAG ACC gga tcc ATG
H E P A *S S E T G S M
G. PHP8882 - Ubi::GFPm-SKL - (marked by *)
The consensus peroxisome signal sequence
designated SLK is shown below. Gould et al.,
J. Cell Biol 108: 1657 (1989).
AAG TCC AAG CTT
K *S K L
H.' Ubi::GR-GFPm
The pea glutathione reductase
mitochondrial and chloroplast signal sequence
is shown below. Creissen et al., Plant J. Z:
129 (1991). The sequence shown includes 20
additional amino acids beyond the putative
processing site (marked by *).
CA 02502460 1997-05-01
WO 97/41228 PCT/US97107688
-39-
ATG AAC CAG GCG ATG GCC ACC CCG CTU TCC CTG TCC TOC TGC TCC CCU ACC CTG
N N Q A N A T P L S L S C C S P T L
ACC AGO TCC ACC CTG TTC TTC ACC MG ACC TTC CCU TTC TCC CGC TCC TTC TCC.
T R S T L F F T K T F P F S R S F S
Sj ACC CCU CTG CCU CTG TCC ACC AAG ACC CTC ATC TCC CTG TCC CCU CCU CAC AGG
T P L P L S T K T L I S L S P P H R
ACC TTC GCC GTG AGO GCT GAG TCC CAG AAC CGC GCC GAC CCU GCC AGO CAG TAC
E S Q N G A D P A R Q Y D F D L F T
GAC TTC GAG CTG TTC ACC ATC UGC
I G T F A V =R A
I. PHP9053 - Ubi::NLS-GFPm-MALS
The nuclear localization signal from simian
virus 40 (SV40) was fused to the N terminus of GFPm.
In order to retain the protein in the nucleus the
molecular weight of NLS-GFPm was increased by making
a carboxy terminal addition of the large unrelated
protein maize acetolactate synthase (ALS) (lower
case bases show linker sequence).Kalderon, D.,
Robers, B., Richardson, W., and Smith A., "A short
amino acid sequence able to specify nuclear
location", Cell ,: 499-509 (1984).
CCG CCC AAG AAU AAO CGC AAG GTG ccc ATG ...
P P K K' K A K V P M
AM atc cac ATO
K I H M
J. Ubi::GFPm-HRGP
A carboxy terminal fusion of a portion of the
maize HRGP coding sequence to GFPm was used to
anchor GFP to the cell wall. Sequence encoding
amino acids 177 to 328 was used. Stiefel, V., Ruiz-
Avila, L.,_ Raz R., Valles M., Gomez J., Pages M.,
Martinez-Izquierdo J., Ludevid M., Landale J.,
Nelson T., and Puigdomenech P., "Expression of a
maize cell wall hydroxiproline -rich glycoprotein
gene in early leaf and root vascular
differentiation", Plant Cell 2: 785-793 (1990).
TAC ACT CCA AOC CCT MC CCA CCG OCT ACC AAG CCT CCC UCG
Y T P S P K P P A T K P P T
CA 02502460 1997-05-01
WO 97/41228 PCT/US97107688
-40-
CCC AAG CCU ACC=CCG CCA ACG TAC ACC CCT TCG CCA AAG CCT
P K P T P P T Y T P S P K P
CCG ACA CCC AAG CCC ACC CCG CCG ACC TAC ACC CCT TCT CCC
P T P K P T P P T Y T P S P
AAG CCT CCC ACG CCC AAG CCG ACC CCC CCC ACC TAC ACT CCA
K P P T P K P T P P T Y T P
AGC CCC AAG CCT CCC ACA CAC CCC ACC CCC MG CCG ACC CCA _
S P K P P T H P T P K P T P
CCG ACG TAC ACC CCT TCC CCA AAG CCT CCG ACG CCC AM CCG
P T Y T P S P K P P T P K P
ACC CCA CCG ACG TAC ACC CCT TCC CCA AAG CCT CCG ACA CCC
T P P T Y T P S P K P P T P
AAG CCG ACC CCA CCG ACG TAC ACC CCT TCC CCA MG CCT CCG
K P T P P T Y T P S P K P P
ACA CCC AM CCG ACC CCA CCG ACO TAC ACT CCC ACA CCG AAG
T P K P T P P T Y T P T P K
CCG CCG GCC ACC AAG CCG CCC ACC TAC ACT CCG ACG CCG CCG
P P A T K P P T Y T P T P P
GTG TCT CAC ACC CCC AGC CCG CCG CCA CCA TAC TAC
V S H T P S P P P P Y Y
K. Ubi::GFPm-SOD
Superoxide dismutase coding sequence fused to
GFPm. Alcendor D., Chapman. G.,. and Beaman B.,
"Isolation, sequencing and expression of the
superoxide dismutase-encoding gene (sod) of Nocardia
asteroides strain GUH-2", Gene ]j,: 143-147 (1995).
GTG GCT GAG TAC ACC CTG CCG GAT CTG GAT SAC GAC TAC AGC
V A E Y' T L P D L D Y D Y S
GCC TGG AAC CCC ACA TCT CCG GCC AGA TCA ACG AGC TGA CAC
A L E P H I S G 0 I N E L H
CAT TCC AAG CAC CAC GCC CCC TAC CTC GCC GOT GCC AAC ACG
H S K H H A A Y V A G A N T
GCA CTG GAG AM CTG GM GCC GCC COT GAG GCC GGC CAT CAC
A L E K L E A A R E A G D H
AGC GCG ATC TTC CTG CAC GAG MG AAC CTC GCC TTC CAC CTC
S A I F L H E K N L A F H L
GGC GGA CAC GTC AAC CAC TCC ATC TOO TOO AAG AAC CTG TCC
G G H V N H S I W W K N L S
CCC AAC GOT GCC GAC AAG CCG OTC GGC GAG CTO GCC GCC GCC
P N G G D K P V G E L A A A
ATC CAC CAC CAG TTC GOT TCG TTC GAC AAG TTC CGC GCC CAG
I D 0 Q F G S F D K F R A .Q
TIC ACC GCC GCC GCC AAC GCC CTG CAG GCC TCG GCC TOG GCG
F T A A A N G L Q G S G W A
GIG CTC GOT TAC GAC ACC CTC GCC CAG AAG CTO CTG ACC TTC
V L 0 Y 0 T L G Q K L L T P
CAG CTC TAC GAC CAC CAG GCC AAC GIG CCG CTO CAC ATC ATC
Q L Y D Q Q A N V P L G I I
CCG CTO CTC CAG OTC GAC ATG TOO GAG CAC OCC TTC TAC CTG
P L L Q V D H W E H A F Y L
CAG TAC AAG AAC OTC AAG GCC GAC TAC GIG ACC GCO TTC TOG
Q Y K H V K A 0 Y V T A F W
CA 02502460 1997-05-01
WO 97141228 -4l- PCTAJS97/07688
AAC GTC OTC AAC TGG 0CC GAC GTG C?G GAC CGC rTC GGC AAG
$ V V N N. A D V Q D A F G K
GCC GTC AAC CAG 0GC AN GGC CTT ATC TTC 000
A V N 0 0 K 0 L I F 0
9. Detection of GFP in Transformed Plant Celle
GFP is detected in transformed plant cells by
conventional spectrophometric methods. The transformed
cells or tissue are screened for the presence of GFP
protein by means of the illuminating the cells with UV to
blue light and screening for the presence of green
fluorescence.
Compound and dissecting microscopes were fitted with
appropriate filter combinations for fluorescent protein
excitation. Illumination with UV-blue light (exciting
around the absorption maximum of 395 nm or around the
minor peak at approximately 47S nm for GFP; around 480-
490 nm for the red-shifted version and around 380 nm for
the blue fluorescent protein) is required for
visualization. A hand-held lamp for benchtop work also
permits good visualization. Cut-off filters or bandpass
filters between the fluorescing tissue and the viewer
(i.e. around the intermediate objective or the eyepieces
of the microscope, or hand-held in front of the eyes if
working on the benchtop) greatly reduced background
autofluorescence from the tissue. For GFP and the red-
shifted GFP this cut-off or bandpass filter permitted
light between 500-550 nm to reach the viewer. For the
blue fluorescent protein, this filter permitted the
passage of 420 to 450 nm light. Useful filters and
wavelengths are not restricted to those described above,
and further generic description of the optical
characteristics of this system are available. Heim at
al., Currently Biology 6: 178-182 (1996).
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-42-
10. 'Foreign Protein Genes and Agronomic Genes
with transgenic plants according to the present
invention, a foreign protein can be produced in
commercial quantities. Thus, the selection and
propagation techniques described above yield a plurality
of transgenic plants which are harvested in a
conventional manner, and a foreign protein then is
extracted from a tissue of interest or from total
biomass. Protein extraction from plant biomass can be
accomplished by known methods which are discussed, for
example, by Heney and Orr, Anal.-Biochem. lj: 92-6
(1981).
According to a preferred embodiment, the transgenic
plant provided for commercial production of foreign
protein is maize. In another preferred embodiment, the
biomass of interest is seed. For the relatively. small
number of transgenic plants that show higher levels of.
expression, a genetic map can be generated, primarily via
conventional RFLP and PCR analysis, which identifies the
approximate chromosomal location of the integrated DNA
molecule. For exemplary methodologies in this regard,
see Glick and Thompson, METHODS IN PLANT MOLECULAR
BIOLOGY AND BIOTECHNOLOGY 2 9-284 (CRC Press, 1993). Map
information concerning chromosomal location is useful for
proprietary protection of a subject transgenic plant. If
unauthorized propagation is undertaken and crosses made
with other germplasm, the map of the integration region
can be compared to similar-maps for suspect plants, to
determine if the latter have a common parentage with the
subject plant. Map comparisons would involve
hybridizations, RFLP, PCR and sequencing, all of which
are conventional techniques.-
Likewise, by means of the present invention,
agronomic genes can be expressed in transformed plants.
More particularly, plants can be genetically engineered
to express various phenotypes of agronomic interest. The.
CA 02502460 1997-05-01
wO 97/412. PCTAJS97/07688
-43-
genes implicated in this regard include, but are not
limited to, those categorized below.
1. Genes That Confer Resistance To Pests or Disease
And That Encode:
(A) Plant disease resistance genes. Plant
defenses are often activated by specific interaction
between the product of a disease resistance gene (R) in
the plant and the product of a corresponding avirulence
(Avr) gene in the pathogen. A plant variety can be
transformed with cloned resistance gene to engineer
plants that are resistant to specific pathogen strains.
See, for example Jones et al., Science 266: 789 (1994)
(cloning of the tomato Cf-9 gene for resistance to
Cladosporium fulvum) ; Martin et al., Science 262: 1432
(1993) (tomato Pto gene for resistance to Pseudomonas
syringae pv. tomato encodes a protein kinase); Mindrinos
et al., Cell 78:1089 (1994) (Arabidopsis RSP2 gene for
resistance to Pseudomonas syringae).
(B) A Bacillus thuringiensis protein., a derivative
-thereof or a synthetic polypeptide modeled thereon. See,
for example, Geiser et al., Gene jI: 109 (1986), who
disclose the cloning and nucleotide sequence of a Bt 6-
endotoxin gene. Moreover, DNA molecules encoding 6-
endotoxin genes can be purchased from American Type
Culture Collection (Rockville, MD), under ATCC accession
Nos. 40098, 67136, 31995 and 31998.
(C) A lectin. See, for example, the disclosure
by. Van Damme et al., Plant Molec. Bio1.'.j: 825 (1994),
who disclose the nucleotide se3quences of several C1i via
miniata mannose-binding lectin genes.
(D) A vitamin-binding protein such as avidin.
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-44-
The application' teaches the use of avidin and avidin
homologues as larvicides against insect pests.
(E) An enzyme inhibitor, for example, a protease
inhibitor or an amylase inhibitor. See, for example, Abe
et al., J. Biol. Chem. 2.U: 16793 (1987) (nucleotide
sequence of rice cysteine proteinase inhibitor), Huub et
al., Plant Molec. Biol. 2,1: 985 (1993) (nucleotide
sequence of cDNA encoding tobacco proteinase. inhibitor
I), and Sumitani et al., Biosci. Biotech. Biochem. 577:
1243 (1993) (nucleotide sequence of Streptomyces
nitrosporeus a-amylase inhibitor).
(F) An-insect-specific hormone or pheromone such
as an ecdysteroid and juvenile hormone, a variant
thereof, a mimetic based thereon, or an antagonist. or
agonist thereof. See, for example, the disclosure by
Hammock et al.., Nature lgg,: 458 (1990), of baculovirus
expression of cloned juvenile hormone esterase, an
inactivator of juvenile hormone.
(G) An, insect-specific peptide or neuropeptide
which, upon expression, disrupts the physiology of the
affected pest. For example, see the disclosures of
Regan, J. Biol. Chem. 22: 9 (1994) (expression cloning.
yields DNA coding for insect diuretic hormone receptor),
and Pratt et al., Biochem. Biophys. Res. Comm. Ifil: 1243
(1989) (an allostatin is identified in Diplopcera
puntata). See also U.S. patent No. 5,266,317 to Tomalski
et al.,. who disclose genes encoding insect-specific,
paralytic neurotoxins.
(H) An insect-specific venom produced in nature
by a snake, a wasp, etc. For example, see Pang et al.,
Gene 1": 165 (1992), for disclosure of heterologous
expression in plants of a gene coding for a scorpion
insectotoxic peptide.
CA 02502460 1997-05-01
WO 97141118 PCT/US97107688 ,
-45-
(I) An enzyme responsible for an hyperaccumulation
of a monterpene. a sesquicerpene, a steroid, hydroxamic
acid, a phenylpropanoid derivative or another non-protein
molecule with insecticidal activity.
(J) An enzyme involved in the modification,
including the post-translational modification, of a
biologically active molecule; for example, a glycolytic
enzyme, a proteolytic enzyme, a lipolytic enzyme, a
nuclease, a cyclase, a transaminase. an esterase, a
hydrolase, a phosphatase, a kinase, a'phosphorylase, a
polymerase, an elastase, a chitinase and'a glucanase.
whether natural or synthetic. See PCT application WO
93/02197 in the name of Scott et al.. which discloses the
nucleotide sequence of a callase gene. DNA molecules
which. contain chitinase-encoding sequences can be
obtained, for example, from the ATCC under accession Nos.
39637 and 67152. See also Kramer ec al.. Insect Biochem.
Molec. Biel. 23: 691 11993), who teach the nucleotide
sequence of a cDNA encoding tobacco hookworm chitinase.
and Kawalleck ec al., Plant Malec. Biol. 21: 673 (1993).
who provide the nucleotide sequence of the parsley ubi4-2
polyubiquitin gene.
M. A molecule that stimulates signal
transduction. For example, see the disclosure by Botella
ec al., Plant Molec. Biol. 24: 757 (1994), of nucleotide
sequences for mung bean calmodulin cDPIA clones, and
Griess ec al.. Plant Physioi. 104: 1467 (1994), who
provide the nucleotide sequence of a maize calmodulin
cDNA clone.
(L) A hydrophobic moment peptide. See U.S. patent
No:- 5,580,852 (disclosure of peptide
derivatives of Tachyplesin which inhibit fungal plant
pathogens) and U.S. Patent No: 5,607,914 (teaches synthetic
antimicrobial peptides that confer disease resistance).
WO 97/41228 CA 02502460 1997-05-01 PCT/US97/0768$
-46-
(M) A membrane permease, a channel former or a
channel blocker. For example, see the disclosure by
Jaynes et a1 Plant Sci. 2: 43 (1993), of heterologous
expression of a. cecropin-p lytic peptide analog to render
transgenic tobacco' plants resistant to Pseudomonas
solanacearum.
(N) A viral-invasive protein or a complex toxin
derived therefrom. For example, the accumulation of
viral coat proteins in transformed plant cells imparts
resistance to viral infection and/or disease development
effected by the virus from which the coat protein gene is
derived, as well as by related viruses. See Beachy et
al., Ann. Rev. Phytopathol. 28: 451 (1990). Coat
protein-mediated resistance has been conferred upon
transformed plants against alfalfa mosaic virus, cucumber
mosaic virus, tobacco streak virus, potato virus X,
potato virus Y, tobacco etch virus, tobacco rattle virus
and tobacco mosaic virus. Id.
(0) An insect-specific antibody or an immunotoxin
25, derived therefrom. Thus, an antibody targeted to a
critical metabolic function in the insect gut would
inactivate an affected enzyme, killing the insect. Cf.'-
Taylor et al., Abstract #497, SEVENTH INT'L SYMPOSIUM ON
MOLECULAR PLANT-MICROBE-INTERACTIONS (1994) (enzymatic
inactivation in transgenic tobacco via production of
single-chain antibody fragments).
(P) A virus-specific antibody. See, for.example,
Tavladoraki et al., Nature 3 j6: 469 (1993), who show-that
transgenic plants expressing recombinant antibody genes
are protected from virus attack.
CA 02502460 1997-05-01
WO 97/41228 PCT/US.97107688
-47-
(Q) A devel opmental -arrest ive protein produced in
nature by a pathogen or a parasite. Thus, fungal endo a-
1,4-D-polygalacturonases facilitate fungal colonization
and plant nutrient release by solubilizing plant cell
wall homo-a-1,4-D-galacturonase. See Lamb et al.,
Bio/Technology 12: 1436 (1992). The cloning and
characterization of a gene which encodes a bean
endopolygalacturonase-inhibiting protein is described by
Toubart et al., Plant J. j: 367 (1992).
(R) A developmental-arrestive protein produced in
nature by a plant. For example, Logemann et al.,
Bio/Technology.lQ: 305, (1992) , have shown thit=ransgenic
plants expressing the barley ribosome-inactivating gene
have an increased resistance to fungal disease.
2. Genes That Confer Resistance To A Herbicide. For
Examflls:
(A) A herbicide that inhibits the growing point
or meristem, such as an imidazalinone or a sulfonylurea.
Exemplary genes in this category code for mutant ALS and
AHAS enzyme as described, for example, by Lee et al.,
EMBO J. 7: 1241 (1988), and Miki et'al., Theor. Appl.
'25 Genet. 80: 449 (1990), respectively.
(B) Glyphosate (resistance imparted by mutant EPSP
synthase and aroA genes, respectively) and other
phosphono compounds such as glufosinate (PAT ~i.nd bar
genes), and pyridinoxy or phenoxy proprionic acids and
cycloshexones (ACCase inhibitor-encoding, genes). See,
for example, U.S. patent No. 4,940,835 to Shah et al.,
which discloses the nucleotide sequence of a form of EPSP
which can confer glyphosate resistance. A DNA molecule
encoding a mutant aroA gene can be obtained under ATCC
accession No. 39256, and the nucleotide sequence of the
mutant gene is disclosed in U.S. patent No. 4,769,061 to
Comai. European patent application No. 0 333 033 to
CA 02502460 1997-05-01
WO 97/41228 PCT/US97107688
-48-
Kumada et al. and U.S. patent No. 4,975,374 to Goodman et
al. disclose nucleotide sequences of glutamine synthetase
genes which confer resistance to herbicides such as L-
phosphinothricin.' The nucleotide sequence of a
phosphinothricin-acetyl-transferase gene is provided in
European application No. 0 242 246 to Leemans et al. De
Greef et al., Bio/Technology 2: 61 (1989), describe the
production of transgenic plants that express chimeric bar
genes coding for phosphinothricin acetyl transferase
activity. Exemplary of genes conferring resistance to
phenoxy proprionic acids and cycloshexones, such as
sethoxydim and haloxyfap, are the Accl-S1, Accl-S2 and
Accl-S3 genes described by Marshall et al., Theor. Appl.
Genet. Q3: 435 (1992).
(C) A herbicide that inhibits photosynthesis, such
as a triazine (psbA and gs+ genes) and a benzonitrile
(nitrilase gene). Przibilla et al., Plant Cell .: 169
(1991), describe the transformation of Chlamydomonas with
plasmids encoding mutant psbA genes. Nucleotide
sequences for nitrilase genes are disclosed in U.S.
patent No. 4,810,648 to Stalker, and DNA molecules
containing these genes are available under ATCC accession
Nos. 53435, 67441 and 67442. Cloning and expression of
DNA coding for a glutathione S-transferase is described
by Hayes et al., Biochem. J. .: 173 (1992).
3. Genes That Confer Or Contribute To A Value-
Added Trait. Such As:_
(A) Modified fatty acid metabolism, for example,
by transforming a plant with an antisense gene of
stearoyl-ACP desaturase to increase stearic acid content
of the plant. See Knultzon et al., Proc. Nat'l Acad Sci.
USA Q,: 2624 (1992)
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688'
-49-
(B) Decreased phytate content
(1) Introduction of a phytase-encoding gene
would enhance breakdown of phytate, adding more
free phosphate to the transformed plant. For
example, see Van Hartingsveldt et al., Gene 12,7:
87 (1993), for a disclosure of the nucleotide
sequence of an Aspergillus niger phytase gene.
(2) A gene could be introduced that reduces
phytate content. In maize, this, for example,
!0 could be accomplished, by cloning and then re-
introducing DNA associated with the single allele
which is responsible for maize mutants
characterized by low levels of phytic acid. See
Raboy et al., Maydica }j: 383 (1990).
i5
(C) Modified carbohydrate composition effected,
for example, by transforming plants with, a gene coding
for an enzyme .that alters the branching pattern of
starch. See Shiroza et al., J. Bacteriol. 170: 810
20 (1988) (nucleotide sequence of Streptococcus mutans
fructosyltransferase gene), Steinmetz et al., Mol. Gen.
Genet. 200: 220 (1985) (nucleotide sequence of Bacillus
subtilis levansucrase gene), Pen et al., Bio/Technology
35: 2.92 (1992) (production of transgenic plants that
25 express Bacillus licheniformis cr-amylase), Elliot et al.,
Plant Molec. Biol. U: 515 (1993) (nucleotide sequences
of tomato invertase genes), Segaard et al., J. Biol.
Chem. 268: 22480 (1993) (site-directed mutagenesis of
barley amylase gene), and Fisher et al., Plant Physiol.
102: 1045 (1993) (maize endosperm starch branching enzyme
II) .
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-50-
Example 1
Construction of Genes and Vectors
The GFPm nucleotide sequence was derived from
a back translation of the protein sequence using maize
preferred codons. Sequence analysis was performed using
the Wisconsin Sequence Analysis Package from Genetics
Computer Group, Madison, WI. The nucleotide sequence was
assembled from a series of synthetic oligonucleotides.
Cloning sites include a 5-prime flanking BamHI
restriction site, an Af1III site at the start codon, a 3-
prime flanking HpaI site or a Bg1II site converting the
stop codon to an isoleucine (Fig. 1).
ZS Amino terminal fusions to GFPm were created using
synthetic oligonucleotides encoding the signal sequence
flanked by a 5-prime BamHI or Bg1II restriction site and
3-prime NcoI site. The synthetic signal sequences were
cloned into PHP7921 (Fig. 4) using the 5-prime flanking
BamHI and AfIIII restriction sites of GFPm to create in-
frame fusions. Sequences cloned in this manner include
the maize chloroplast signal sequence, barley alpha-
amylase signal sequence, pea glutathione reductase signal
sequence, and SV40 nuclear localization signal. The
sporamin vacuole, signal sequence was synthesized with a
5-prime BspEI and 3-prime BamHI for insertion between the
BAA pre-sequence and GFPm coding sequence of pHP8144
(Fig. 5). The CPN60 promoter site, created by site-
directed mutagenesis, was used with the GFPm AfIIII site
to make the fusion sequence. Each of plasmids PHP7921
and PHP8144 are based on pUC18.
Carboxy terminal sequences were synthesized with
a 5-prime BglII and 3-prime HpaI site, and cloned into
pHP7921 or pHP8144 using the 3-prime Bf1II and HpaI sites
of GFPm. The barley lectin vacuole target, HDEL ER
retention signal, SKL peroxisome signal, ALS coding
sequence and HRGP coding sequence fusions were made using
this strategy.
CA 02502460 1997-05-01
WO 97/41228 PCT/US97ro7688
-51-
C C C G
0
ta 44 -4 7 go o .4 v -A fa
p ~N1 A
4I = L u"4 v U w =
O U y tyJ a 04 mi d m p V r 4
y b r+ r4 a Of
G fpr, w m w m ,0 G
1" 0 P-4
(d x P3 Ai u 14 .9
V M H M M w
H = - H H r
= A a a a a
U,
=
= y N
= r
O C7 t9 C7 C,
n
= ^ N
p=p = y
E R
O H
a
Q a I a m a A 4
fir 5-4 - - -
a oa g o o g
w1 U v U u 0
u
C =
.~ u
dr 1 1 f I 1 1
A =
H U)
U
C i as
=
14 44 =
Aj 41
44 -4 ,4 "4
a
Ai 4) 9 10
04 "4 -.4 -P4
~ ~Np rN4 nNN NNg ~Np
F V m 8 m E E E
a ,g= m on w 0% m
a 01 %D Of o
t` r t- co m co
CA 02502460 1997-05-01
WO 97141228 PCT/US97/07688
-52-
u :4 o 1k o r4 i a o +'
N N c U' N u a a K N ro c a
=p =p w a ~ w a a =+ ro m E a aEi .-~i w A O E '4 ro u
y O .=d -4i O V ..I H w Q 'rol V .i V O, M a c V
v~ u e ro" d c a >. v a u o n x a '' re N 41 o u a'
0 C 0. N IM E > rn C 'O V d 01 3 W N
>
ro =~ ro u "4 A ro N .q O c O N =.~
H E w u N 15 14 oa- m a a ul
0. a a a a
E
1k w w w 1k
t9 C9 t7 t9 C9
to
=4~ u
N .d =.4 E
to to
"4 4
O Y g ro 7 0
re -4 O
N a
v > VO 41 >
m q
-ro
E a
1'A =~7fyi
0
u
o aj it it aJ
.r =44 =.i =.1
N N N N N
=.1 rl =.1 =.1 .4
t- r, ~+ Ip 01
451 %n Y1 in
r4 1~ f. I-
m m m co to
CA 02502460 1997-05-01
WO 97/41228 PCT/US97107688
-53-
.~ u C 0 0 ()
0 u C u d b tL u U
41 E >' ~+ L U W L 01 R
c .4
u 0 v c o lcu o c a
a- M R A '"~ I-I .=I ,N 41 JJ 0> U W d dt i4 0 w
t0 K 0 14 tJ U P4 0 Q
al u C7 H M V
C 0 1 d y U y Z V V Q a w 0 O w m
a +a - u 4)
u
M H M FI I.I
I-I 1.4 M M 1.1
.N M ..1 M M
04 a a a 0.
I I I I
1 1 I 1 I
4
00 In,
4) 4)
14 G R G >.
a
I I I I
I I 1 ~
N
I 1
N
h I E+ I~1 I I
H H
4 R G c G
+1 M M +~ M
u iJ a LI 41
=.4 =.I ..I .,4 M
7 ~ 7 7
N N~NgNp 1N~
E E E E
N ~p en r O
IO m in - IA N
44 0% O O O
O m 0% 0% 0%
CA 02502460 1997-05-01
WO 97/41228 - PCT/US97/07688-
-54-
of " r 1.1
c'j
w 0 0 W a f0) o w =l o o M? =M ,u 4 vOi
m == y I 10
a n m u ~' w v C to A' x 'a ++ ro ~ of "-r ~r A 11.1 p w at
0 o b A v c> 0 v u -+ > ~+ a w w ./ ~C
u C. w C u b X .G O a .14 " w
w 'o 'd r
w m x E GO two A Y C A O a a tmi.
1p 7 L)
M H IM 1=4 ~ 1~1 M
.4 "4
a a a a s a a
U, b
A 1
b to 'o
v a s n A a w
N h. W Cw7 0
.i
F' 'd N
14 V Q
E E E E
> A a V a A
- O. AI O. GI OI
41 Ai 41 aj Aj
U U U U U
1-4
.=1
1 1 O
1 1 1 $4
N y
1 1 1 1 1 1
1 1 / 1 1 1
=~
= 1 -.4 INA -.4 =N = i
1+1 f=1
x aa
V O V V V V
N N nN~ 1N~ N 1Np
E E E E
= rl a '4 m m
m e= 14 m
m 40 m m m co
CA 02502460 1997-05-01
WO 97141228 PCT/US97/07688
-55-
-ro 0
0 S. 1) 01
o
b'd 0 "4 of
I ~' EE G NE a O
r c w X y mar
W b 41 u W M
C7 E~ A
H H H H H H H
i
C C C q C
a as aaaa
of
a
W A V; H In H
to -
V hI Lr. f' ~I f'
m y O Z zZ
O O
E E E
b '4 a
0-4
g g
0
4) 4j u
u u u
1 1 1 ' 1
I ' 1 1 1
- 4) y 4) m
In .+ ul In
"4 N N
0 0
N N fd w
"4 '4 ~ 3 a 3
In r .a
P O r1 in
.I o 0 v
4D to 40 m
,CA 02502460 1997-05-01
WO 97/41228 PCT1US97/07688
-56-
The ubiquitin promoter is described in
Christensen et al., Plant Mol. Biol. 18: 675-689
(1992). The 4xERE promoter is-described in Klein-
Hitpass et al., cell 46: 1053-1061 (1986). The CPN60;
2x35S and ALS.promoters are described in Close P. Ph.D.
Dissertation, Iowa State University; Gardner et al.,
Nucl. Acid Res. 9: 2871-2888 (1981) and U.S. Patent
No: 5,659,026, respectively. The Omega .prime
(O') 5-prime sequence is described by Gallie ec al.,
Nucl. Acids Res. 15: 3257-3273 (1987) while the Adh 1
intron I is described by Dennis et al., Nucl. Acids
Res. 12: 3983-3990 (1984). The 3-prime sequence pinll
is described in An et al., Plant Cell 1: 115-122
(1989). Structural genes used in the construction of
the vectors include the GFP gene (native jellyfish)
described by Prasher et al., Gene 111: 229-233 (1992);
the maize ALS gene described by Fromm et al.,
Bio/Technol. 8: 883 (1990) and the maize HPRG described
by Steifel et al.. Plant Mol. Biol. Il: 483-493 (1988) .
The FLP/FRT system contained in plasmids PHP8674 and
PHP8007 is described in O'Gorman et al., Science 251:
1351-1355 (1991) and Jayaram M., Proc. Natl. Acad. Sci.
USA 82:.5875-5879 (1985) The Ac/Ds system is
described by Muller-NeumanrL ec al.. Mol. Gen. Genet_
i2.: 19-24 (1984)
For-Agrobacceriurn-mediated transformation of
sunflower, the backbone of PHP8011 is a bin 19-based
vector. Beven, Nucl. Acids Res. l.: 8711-8721 (1984).
The NPT-II cassette was removed from bin 19 and the
following cloned between the left and right border
sequences; the double 35S promoter, omega', the GFPm
coding sequence and the pinll 3' region-(all described
above) were cloned downstream of the left border
sequence, followed in a head-to-tail orientation by the
BnALS3 promoter. the NPT-II coding sequence and the
pinll 3' region. Beven, "Binary Agrobacterium Vectors
for Plant Transformation", Nucl. Acids Res. 12: 8711-
8721 (1984).
CA 02502460 1997-05-01
WO 97/41228 - PCTIUS97/07698
-57-
GFPr, BFP and GFPs (soluble GFP) were
constructed by means of oligonucleotide-directed
mutagenesis of the GFPm nucleotide sequence shown in
Figure 1 as described by Ausubel, supra. With regard
to GFPr, TCC was changed to ACG resulting in the S65T
substitution in the GFP amino acid sequence. With
regard to BFP, TAC was changed to CAC, resulting in the
Y66H substitution in the GFP amino acid sequence.
With regard to GFPs, the following sequence
changes in GFPm were made. TCC was changed to AGC,
resulting in a F99S substitution in the GFP amino acid
sequence. The oligonucleotide used to introduce this
mutation also caused a silent mutation at R96 (AGC
changed to CGG), thereby, adding a BsrBI restriction
site. ATG was changed to ACC, resulting in an M153T
substitution in the GFP amino acid sequence. GTG was
changed to GCC, resulting in a V163A substitution in
the GFP amino acid sequence. A single oligonucleotide
was used to change the amino acids at 153 and 163.
This oligonucleotide also caused a silent mutation at
A154 (GCC changed-to GCG), which added a SacII
restriction site. As reported by Crameri et al.,
supra, these three amino acid changes did not alter the
absorption/emission characteristics of GFP. These
three amino acid substitutions, however, increased the
solubility of GFP. Maize cells transformed with the
GFPs gene fluoresce in blue to W light based on
transient assays.
CA 02502460 1997-05-01
WO 97/41228 PCTIUS97107688
-58-
Example 2
Maize Transformation with GFP Screening or Bialaphos
Selection
Maize Hi-II immature embryos were co-transformed
via microprojectile bombardment with a mixture of
PNP7814 (ubi :: PAT :: pinll) and a plasmid comprising the GFP gene. Plasmid
PNP7814 carries the PAT gene encoding resistance to bialaphos
operably linked to the ubiquitin promoter and the
terminator from the pinlI gene. Standard
CaCl2/spermidine precipiation of DNA onto tungsten
microprojectiles was used to prepare the DNA-particles.
Prior to bombardment, the embryos of approximately 1.5-
2.0 mm in length were cultured on 560P medium including
N6 salts, Erikkson's vitamins, 0.69 mg/L proline, 2
mg/L 2,4-D, and 3% sucrose. After 4-5 days of
incubation in the dark at 28 C, embryos were-removed
from 560P medium and cultured, coleorhizal end up, on
560L medium which is equivalent to 560P except that it
contains 12`c sucrose. Embryos were allowed to
acclimate to this medium for 3 h prior to
transformation.
Embryos were transformed using the PDS-1000
Helium Gun from Bio-Rad at one shot per sample using
650 PSI rupture disks. On average approximately 0.0067
pg of DNA was delivered per shot. For each treatment
(pat gene with bialaphos selection or GFP gene with
screening for GFP), 447 embryos were used. For the
bialaphos-selection treatment; following bombardment,
the embryos were maintained on 560L medium for 48 h
before transfer to 560R selection medium containing N6
salts, Erikkson's vitamins, 2 mg/L 2,4-D, 3% sucrose
and 3mg/L bialaphos. Plates were maintained at 28 C in
the dark and were observed for colony recovery.
Transfer of colonies to fresh medium occurred every two
weeks. Transgenic colony recovery under bialaphos
CA 02502460 1997-05-01
WO 97/41228 PCTIUS97/07688
-59-
selection was accomplished by picking the healthy,
growing transgenic calli- from amongst the inhibited
background of non-transgenic tissue as the material was
progressively transferred to fresh media. For the GFP
screening treatment, 560P media was used after
bombardment and the two-day recovery on 560L. Calli
were transfered to fresh non-selective 560P medium
every two weeks. Screening for.GFP expression was
carried out at each transfer using a Xenon and/or
Mercury light source with the appropriate filters for
GFP visualization.
Table 2
Recovery of Transgenic Events in Maize Hi-II Callus
Using
Either GFP Visualization or Bialaphos Selection
Number of Events Recovered
Selection Method Initial Events Events Capable
of Regeneration
Bialaphos 16 12
selection
Screened using 9 8
GFP
Transgenic events were recovered from each
treatment. The largest number of transgenic events
were consistently obtained by means of bialaphos
selection. However, the frequency of recovery of
transgenic events with GFP screening was approximately
one-half the rate of recovery with bialaphos selection
which waq surprisingly high considering that GFP
screening does not rely on selection of transgenic
events in the presence of a cellular toxin.
Once GFP expressing colonies were identified
they were monitored regularly for new growth and
expression using the Xenon light source. Plant cells
containing GFP were regenerated by transferring the
callus to 288 medium containing MS salts, 1 mg/L IAA,
CA 02502460 1997-05-01
WO 97/41228 PCTIUS97/07688
-60-
0.5 mg/L zeatin and 4% sucrose. The callus was placed
in the light. As plantlets developed they were
transferred to tubes containing 272K, hormone-free MS
medium and 3% sucrose. The percentage of green
fluorescent colonies that regenerated into whole plants
was determined.
Leaf samples were collected from TO transgenic
plants from events recovered by means of bialaphos
selection, or for events recovered by means of GFP
visualization (non-selected). Genomic DNA was
extracted from all leaf samples and Southern analysis
was performed using the PAT and GFP genes as
hybridization probes. Hybridization for the PAT gene
and GFP gene were performed sequentially on the same
blots, by stripping and reprobing for the other
transgene. Bands hybridizing for the PAT gene and for
the GFP gene were observed in all 8 GFP-screened events
tested and in all 12 of the bialaphos selected events
tested. Copy numbers and integration patterns for both
treatments appeared similar, and were consistent with
previous experiments by the inventors using bialaphos
selection of callus. Copy numbers ranged from low
(single or two copies for each gene) to numerous (i.e.,
>5 copies per gene). Integration events were
predominantly at single integration sites but in some
cases integration at multiple locations in the genome
was observed.
Bialaphos is the most efficient chemical
selective agent for maize. Consequently, the high
frequency with which transgenic events were obtained
with GFP screening, compared to bialaphos selection,
was unexpected. An advantage of GFP screening over
bialaphos selection is that non-transformed tissue is
not killed. Accordingly, transgenic sectors containing
the GFP gene develop in a healthier environment for
growth and development.
CA 02502460 1997-05-01
WO 97141228 PCT1US97/07688
-61-
Example 3
The GFP Marker in Maize Cytoplasm, Chloroplast and
Mitochondria
Maize A188 x B73-derived cells were transformed
with plasmids PHP8080, PHP7921 and PHP8087. Each of
these plasmids is described in Example 1 and Table 1.
Plasmid PHP8080 carries the GFPm gene operably linked
to the ubiquitin promoter and a chloroplast signal
sequence. GFP is directed to the chloroplasts of cells
transformed with PHP80BO. Plasmid PHP7921 carries the
GFPm gene operably linked to ubiquitin promoter. GFP
remains in the cytoplasm of cells transformed with
pPGP7921. Plasmid PHP8087 carries the GFPm gene
operably lined to the CPN60 promoter and CPN60 signal
sequence. GFP is directed to mitochondria of cells
transformed with pPGP8087.
. The cells were transformed by culturing maize
embryos approximately 1.5 to 2 mm in length onto 560P
medium containing N6 salts, Erikkson's vitamins, 0.69
g/L Proline, 2 mg/L 2,4-D and 3% sucrose. After 4-5
days of incubation in the dark at 28 C, embryos were
removed from 560P medium and cultured, coleorhizal end
up, onto 560L medium which is equivalent to 560P but
contains 12% sucrose. Embryos were allowed to
acclimate to this medium for 3 h prior to
transformation. Embryos were transformed using the
PDS-1000 Helium Gun from Bio-Rad at one shot per sample
using 650PSI rupture disks. DNA delivered per shot
averaged at 0.0667 g. Following bombardment, all
embryos were maintained on 560L medium for 48 hours
before transfer to 3% sucrose and 3 mg/L bialaphos.
Plates were maintained at 28 C in the dark and were
observed for colony recovery with transfers to fresh
medium occurring every two weeks. Transgenic colony
recovery was noted initially as growing callus tissue
with a healthy phenotype on selection. Screening for
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-62-
GFP expression was completed with each transfer using a
Xenon light source with UV filter attachments.
GFP localization was confirmed by analysis of TO
callus. Samples of calli from the targeted GFP
transformations were fixed in FAA and then examined
using an inverted microscopes with UV filters to
visualize GFP. For each targeted construct GFP
expression was localized,to the specified organelle.
Once GFP expressing colonies were identified
they were monitored regularly, for new growth and
expression using the Xenon light source. Plant cells
containing GFP were regenerated by transferring the
callus to 288 medium containing MS salts, 1 mg/L IAA,
0.5 mg/L zeatin and 4% sucrose. The callus was placed
in the light. As plantlets developed they were
transferred to tubes containing 272K,.hormone-free MS
medium and 3% sucrose. The percentage of green
fluorescent colonies that regenerated into whole plants
was determined.
Regeneration of plants from callus transformed
with PHP8080, PHP7921, and PHP8087 appeared to vary
between constructs; plants were successfully
regenerated from independent transformed calli at
respective frequencies of 35, 54 and 86%.
Transformation was confirmed by Southern blot analyses
using both the BAR and GFPm genes as hybridization
probes. GFP localization in various subcellular
compartments was confirmed in stable transgenic maize
cells using epifluorescent microscopy and image
enhancement software.
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-63-
Table 3
Regeneration Frequency Of Maize Transformed With GFP
Directed to Different Subcellular Compartments
Chloroplast
Exp 4 Total Green Regenerate Percentage Ave.
Colonies Plant
Event
..4.01 32 23 4 17 6
.4.03 0 - - -
..4.06 13 11 8 73 3
Totals 45 34 12 35=
cytoplasm
Exp N Total Green Regenerate Percentage Ave.
Colonies PlantsE
vent
...4.01 7 6 2 33 4
..4.03 7 5 4 s0 5
..4.06 8 7 6 86 4
..4.08 9 9 3 33 2
..4.11 3 3 1 33 4
Totals 34 30 16 54=
Mitocbondrial
Exp N Total Green Regenerate Percentage Ave.
Colonies Plants
Event
..4.03 5 3 1 33 9
..4.06 13 11 11 100 4
Totals 18 14 12 86=
= This is not a mean regeneration frequency, but instead is calculated
from the totals for all treatments.
Transformed plants containing GFP in the
mitochondrial subcellular compartment grew, more
vigorously than plants containing GFP in the cytoplasm
or chloroplast. The transformed plants containing GFP
in the mitochondria were equivalent to the controls in
terms of growth rate and appearance.,
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-64-
In contrast, transformed plants containing GFP
in the chloroplast were difficult to regenerate. This
callus produced a preponderance of roots with few
shoots. Almost all the plants containing GFP in the
chloroplast that were regenerated and reached maturity
clearly exhibited stress with narrow and somewhat
flaccid leaves, reduced stature, yellow leaves
(presumably caused by reduced chlorophyll levels) and
male sterility.
Germination of Ti embryos have been tested in
the following manner. Aliquots of seed from a number
of independent transformahts were imbibed and the
embryos were excised and placed on hormone-free MS
medium with 3% sucrose 'for germination (this in vitro
test made it easier to visualize the seedling). All
embryos were germinated in the dark and then scored for
visual GFP expression using UV-blue excitation under
the dissecting microscope.
For each event tested aliquots of 5 or 10
seeds/event were imbibed, excised and scored for
germination and fluorescence. The germination
frequency for excised progeny produced on-plants
transformed with PHP7921 and PHP8080 was good. The
germination frequency for excised progeny produced on
plants transformed with PHP8087 was good for 2 events
(10/10), low for one event (2/10). No detectable
germination was observed for 2 events. This lowered
germination frequency could be due to mitochondrially-
expressed GFP expression per se, but it could also be
the result of event-to-event variation sometimes
observed in transgenic lines.
Germination of a single event from plants
transformed with PHP7921 was tested differently. Seed
were screened using a W-blue light source, and 10 seed
with GFP-expressing seed (embryos and endosperm) were
selected. Five of these seed were planted in soil, and
in the other 5 the embryos were excised and germinated
on medium. None of the seed in soil germinated, while
CA 02502460 1997-05-01
WO 97/41228 PCTIUS97/07688
-65-
all excised embryos germinated, grew and the seedlings
continued to fluoresce. For events where germination
appears to be negatively impacted, this could be due to
increased GFP concentrations during seed maturation and
dry-down. High GFP concentrations and aggregation of
the protein has been found to be toxic in E. coli.
Crameri et al., Nature Biotechnol. 14: 315-319 (1995).
CA 02502460 1997-05-01
WO 97/41228 PCT/US97107688
-66-
Table 4
Ti Progeny Analysis
Plant Germination and GFPm Expression
Plasmid Even N of TI N of TI I of TI
Tested Seed Tested Seed Plants
Germinated Expressing
Per Event GFPm
PHP8087: 1 10 0 0
CPN60::mit-GFPm-PinD
PHP8087: 2 t0 0 0
C PN60:: m it-G FPm-P iaf
PHP8087: 3 10 10 1
C PN60:: m it-G FPm-PinD
PHP8087: 4 10 2 0
C PN60:: mir-GFPm-PinD
PHP8087: 5 10 10 2
CPN60::mii-GFPm-PinD
PHP7921: 1 10 10 4
Ubi::Ubi intron-Mm-PinD
PHP8080: 1 5 5 0
Ubi::Ubi intron-CTP-Mm-PinD
PHP8080: 2 5 4 0
Ubi::Ubi intron-CTP-Mm-PinD
PHP8080: 3 5 4 0
Ubi::Ubi intron-CTP-Mm-PinD
PHP4810: I 5 5 0
Ubi::Ubi intron-BAR-PinD
=PHP7921: 2 10 5 5
Ubi::Ubi intron-GFPm-PinD 5 germinated Germination Seed
in vitro and occurred planted in
S germinated only from soil also
in soil in vim were
cultures GFPm+
* For all seed except those designated in the
PHP7921 transformation, embryos were excised from
imbibed seed and germinated on MS medium containing no
hormones and 3% sucrose. All embryos were germinated
in the dark to allow for visualization of GFPm
expression. One event from PHP7921 was tested by
planting one half of the seed in soil and one half with
embryos excised from endosperm with culture in vitro.
All seed planted in soil did not germinate. The
germination rate for embryos excised from the endosperm
and cultured in vitro was 100%. All plants expressed
GFPm.
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-67-
Example 4
Sunflower Transformation with GFP Screening, GOS
Screening and Kanamycin Selection
Experiments were undertaken to test GFP, GUS and
NPTII as marker genes for sunflower transformation.
Two different kinds of meristem systems (split
meristem; intact meristem) and two different kinds of
particle systems (FG, focusing gun: He, helium gun)
were used. The screenable markers GFP and GUS were
tested with the intact meristem.system under conditions
involving no chemical selection but rather selection of
transformants based on visual (GFP) or assayable (GUS)
expression in transformed sectors. The NPTII marker
gene was tested with the split meristem method using
kanamycin for chemical selection of transformants and
assays for NPTII expression to identify transformed
sectors. In some cases, the experiments were carried
only so far as identifying transformed sectors while
other experiments were carried out all the way through
to test for seed transmission of the marker gene to
progeny. Transmission to progeny was confirmed by
NPTII ELISA.
Regardless of the exact meristem system or type
of particle gun used, the basic transformation protocol
involved a combination of wounding by particle
bombardment, followed by use of Agrobacterium for DNA
delivery, as described by-Bidney et al., Plant Mol.
Biol. 18: 301-3,13 (1992). The plasmids used for giant
transformation in these experiments were PHP167
containing GUS as the screenable marker, PHP8011
containing GFP as the screenable marker as well as the
NPTII gene, and PHP762 containing NPTII as the
selectable marker. Both PH-P167 and PHP762 are binary
vectors derived from pGA473. See An et al., EMBO J. 1:
277-284 (1985). The binary backbone for PHP8011 is bin
19. See Bevan, NAR la: 8711-8721 (1984).
CA 02502460 1997-05-01
WO 97/41228 - 6 8 - PCT/US97/07688
Procedures for preparation of Agrobacterium and
preparation of particles for all of the experiments are
described in Bidney et al., supra. The Pioneer
sunflower line SMF3 was used in all experiments. See
Burrus et al., Plant Ce11 Rep. 12: 161-166 (1991) for
information concerning SMF3. The Agrobacterium strain
EHA101 is described in Bidney et al. supra and strain
EHA105 is described in Hood et al., Transgen. Res..,3,:
208-218 (1993). Procedures for use of the helium gun,
intact meristem preparation, tissue culture and co-
cultivation conditions, and transgenic plant recovery
for the intact meristem experiments are also described
in Bidney et al., supra. Procedures for the use of the
focusing gun, i.e., as in the intact meristem
experiments, are described in Sautter et al.,
Bio/Technol. J: 1080-1085 (1991). Procedures for use
of the helium gun, split meristem preparation, tissue
culture, co-cultivation, kanamycin selection and
transgenic plant.recovery for the split meristem
experiments are described in Malone-Schoneberg et al.,
Plant Sci. In: 199-207 (1994).
Parameters and general use of the focusing gun
were similar to those 1.4 m described in Sautter et
al., supra. Gold particleq measuring at a
concentration of 0.5x106/ l and 80 bars of nitrogen
were typically used although different sized particles
(0.25 to 2.5 m) and different nitrogen pressures (40-
160 bar) were tested. For the Dupont PDS-1000 helium
driven particle bombardment device, the meristems were
bombarded twice at the highest shelf with 600 psi
rupture discs and a vacuum of 26 inches Hg and 1.8 m
tungsten particles. The intact meristem transformation
method-involved imbibing seed for 24 hours in the dark,
removing the cotyledons and root radical, followed by
culturing of the meristem explants. Twenty-four hours
later, the primary leaves were removed to expose the
apical.meristem. The explants were placed apical dome
side up and bombarded twice with particles, followed by
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688'
-69-
co-cultivation with Agrobacterium. To start the co-
cultivation for-intact meristems a O.5 1 droplet of
OD600 4.0 Agrobacterium was placed on the meristem.
After a 3-day co-cultivation the meristems were
transferred to culture medium with 250 g/ml cefotaxime
(+ 100 mg/l kanamycin for the NPTII selection
experiments). Selection has also been done using 50
gm/l kanamycin. The split meristem method involved
imbibing seed, breaking of the cotyledons to produce a
clean fracture at the plane of the embryonic axis,
excising the root tip and then bisecting the explants
longitudinally between the primordial leaves. The two
halves were placed cut surface up on the medium then
bombarded twice with particles, followed by co-
cultivation with Agrobacterium. For split meristems,
after bombardment the meristems were placed in an OD6
0.6 Agrobacterium suspension for 30 minutes. They were
then removed from the suspension onto solid culture
medium for 3-day co-cultivation. After this period,
the meristems were transferred to fresh medium with 250
g/ml cefotaxime (+ 100mg/i kanamycin for selection).
Selection has also been done using 50 gm/l kanamycin.
Table 5 summarizes the results from experiments
looking at the frequency of. transformed sectors in
primary shoots and secondary branches. Table 6
summarizes the results from experiments carried through
to progeny for analysis of seed transmission.
Transformants were identified in either seed or plants
by assaying for marker gene expression. For GUS
expression, activity was determined using either
histochemical staining or the fluorometric assay, see
Jefferson et al., SCZ O J. ,: 3901-3907 (1987) .
Inheritance of transgene expression was assessed using
an NPTII ELISA.
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-70-
TABLE 5
Overall Transformed Sector Frequencies
for Sunflower Transformation
PLASMID MERISTEM AVE. REPS
MARKER PEP # GUN PREPARATION SECTORS (N)
Screening:
GUS 167 FG Intact 22.0 15
GUS 167 FG Intact 15.0 3
GUS 167 He Intact 13.6 12
GFP 8011 He Intact 7.5 1
Selection:
NPTII/Xan 762 FG Intact 12.7 3
_
TABLE 6
Overall Sector and Seed' Transmission
for Sunflower Transformation
PLASMID MERISTEM AVE. % REPS SEED
MARKER PHP N GUN PREPAR- SECTORS (N) TRANS-
ATION MISSION
Screening:
GUS 167 FG Intact 15.0 3 0/8
GFP 8011 He Intact 7.5 1 9/10
8.lection;
NPTII/KAN 762 FG Intact 12.7 4 0/2
NPTII/KAN 8011 He Split 1.6 4 6/8
For evaluation of initial sector frequencies,
GUS seemed to be the most effective marker gene (Tables
5 and 6). However, based on screening alone (no
selection) the observed GUS sectors did not
subsequently contribute to the germline and thus did
not transmit to progeny (Table 6). It was unlikely
that this was due to the use of the focusing gun
because, both quantitatively and qualitatively, mapping
of GUS sectors after use of each type of gun was
comparable. GFP was significantly better than NPTII
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-71-
(7.5% vs. 1.8%, 'See Table 6) for both sector frequency
and seed transmission when compared using the helium
gun. Progeny from GFP-expressing (no chemical
selection but instead visually selecting using GFP) TO
plants were analyzed for NPTII expression (Table 6)
Progeny from 10 plants were tested and 9 showed
inheritance of NPTII activity. From kanamycin-selected
TO plants, 6 out of 8 tested showed transmission of
NPTII expression to the progeny. Not only has GFP
"visual selection" worked unexpectedly well for
recovering the primary transformants (i.e. compare
Tables 3 and 4), but cotransformation and inheritance
of transgene expression was also very efficient.
CA 02502460 1997-05-01
WO 91/41228 PCT/US97/07688-
-72-
Table 7: Progeny Analysis of Transformation Experiments of SMF3
with GFP (PHP8OI1: CaMV-GFPm, AIL-NP11I)
Treatment SID No. of TO NMI Maximum Transmissi
(Comments) Seeds Map NPT!I on
Screened Range Efficiency
281976 246 12.6--> 30 9.9
281977 139 16.3-> 11.9
281978 127 > 30 > 30
281979 175 9-27 6
Topdown Non-selected
281980 74 > 30 4
281983 15 3-21.3 6.5 9/10
(from I experiment. 319454 124 4.1-18.7 5.9
120 explants in total)
319453 118 + + 8.6
281982 47 > 30 6.2
281981 3 > 30 0
248112 6 ++ 2.3
248113 24 > 30 7.8
Split selected 248111 41 >30 >30
248107 23 17 2.7
248114 67 > 30 1.2
(from 4 experiments. 248110 8 4.1 0 6/8
955 cxplants in total)
248109 6 10.7 0
248115 86 ++ 5.5
Parallel experiments comparing GFP screening,
GUS screening and NPTII/kanamycin selection can be
performed using intact meristems, the Agroba cteri um
strain EHA105 and the helium gun, in side-by-side
comparisons. Plasmid .PHP6011 can be used for GFP/NPTII
comparisons. An identical plasmid substituting the GUS
structural gene for GFP can be used for GUS/NPTII
comparisons, and both plasmids can be used in side-by-
side experiments for GUS/GFP comparisons. Sector
frequency in primary shoots and secondary shoots can be
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-73-
assayed and calculated as described above. Inheritance
and expression of transgenes can also be analyzed in
these experiments.
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-74-
Example 5
GFP As A FLP-Mediated Excision Marker In Maize
Immature embryos of the Hi-II genotype were
bombarded with PHP8674 which contains a GFPm reporter
gene that is activated upon FLP recombinase-mediated
FRT excision. Plasmid PHP8674 also carries a
constitutively expressed gene for bialaphos resistance.
Stably transformed callus was selected on bialaphos and
examined under UV-blue light for GFP expression. No
GFP expression was observed in these primary
transformants. Pieces of callus transformed with
PHP8674 were then bombarded with PHP8007. Plasmid
PHP8007 contains the FLP recombinase gene operably
linked to the ubiquitin promoter. Callus sectors
expressing GFP were first observed ten days after the
second bombardment. These GFP-expressing sectors
continued to grow and express the transgene after one
month of culture. As a negative control the FRT/GFPm
stably transformed callus was also bombarded with a
non-FLP containing plasmid. This treatment did not
produce GFP expressing sectors.
As a second control for this experiment the
consecutive treatments were done in opposite order.
Hi-II immature embryos were bombarded with PHPB007
which carries the FLP recombinase gene and a bialaphos
resistance gene. Stable transformants were recovered
following selection on medium containing bialaphos.
These calli were re-bombarded with PHP8674. GFP
expression was observed in the callus but this faded
over 5-10 days until it could no longer be detected.
The stably transformed callus expresses FLP
recombinase. Introduction of PHP8674 likely provided
the substrate for excision (the FRT sites along with
spacer DNA between the sites) which activated the GFP
cassette while still on the plasmid.' This "activated
CA 02502460 1997-05-01
WO 97/41228 PCTIOS97/07688
-75-
expression" was only transient and disappeared as the
plasmid was degraded.
CA 02502460 1997-05-01
WO 97/41228 PCT/US97/07688
-76-
Example 6
GPP Targeting to the Nucleus as a Fusion Protein
Plasmid PHP9053 was constructed in which the GFP
protein was fused to the ALS enzyme (designated MALS.
for maize-ALS). The ALS gene, which encodes a.protein
normally found in the cytoplasm, is typically used to
confer herbicide resistance. Fang et al., Plant Mol.
Biol. 11: 1185-1187 (1992). A nuclear localization
signal from simian virus 40 was fused to the N terminus
of GFPm as described in Example 1 (Kalderon et al.,
Cell 39: 499-509 (1984)--with the ALS enzyme fused to
the C terminus. The sequence encoding this nuclear
targeted fusion protein (in construct PHP9053) was
introduced into maize cells using particle bombardment
as described previously. Once gene expression was
confirmed through GFP fluorescence under the
microscope, tissue was fixed as described earlier and
examined to determine subcellular localization of GFP.
Fluorescence was localized to the nucleus. Plant
regeneration and assessment of toxicity for this
experiment has not yet been done.
GFP was targeted to the nucleus by fusing the
protein to another polypeptide that is normally
compartmentalized in the nucleus. For example, the
GFPZm-RAD51 fusion found in plasmid PHP8744 contains
RAD51, a protein required for mitotic and meiotic
recombination and double-stranded break repair, fused
to GFP. The gene encoding RAD51 was cloned from yeast.
Haaf et al., Proc. Nat Acad. Sci. JZ: 2298-2302 .(1995).
Degenerate primers based on the nucleotide sequence of
the RAD$1 gene cloned--from yeast were used to identify
and isolate clones containing the RAD51 gene from
maize. The carboxy terminus of GFPm was fused to the
amino terminus of ZmRAD51a. Maize cells were
transformed with the GFPm-ZmRAD51 fusion and
fluorescence was localized in the nucleus. Plant*
WO 97141228 CA 02502460 1997-05-01 PCT/US97107688
-77-
regeneration and assessment of toxicity for this
experiment has not yet been done.
Although the foregoing refers to particular
preferred embodiments, it will be understood that the
present invention is not so limited. It will occur to
those of ordinary skill in the art that various
modifications may be made to the disclosed embodiments
and that such modifications are intended to be within
the scope of the present invention, which is defined by
the following claims.
All publications and patent applications
mentioned in this specification are indicative of the
level of skill of those in the art to which the
invention pertains.
20
CA 02502460 2008-09-04
SEQUENCE LISTING
GENERAL INFORMATION:
APPLICANT: PIONEER HI-BRED INTERNATIONAL, INC.
TITLE OF INVENTION: USE OF THE GREEN FLUORESCENT PROTEIN AS A SCREENABLE
MARKER FOR PLANT TRANSFORMATION
NUMBER OF SEQUENCES: 24
CORRESPONDENCE ADDRESS: 800 Capital Square, 400 Locust Street, Des
Moines,, IA 50309, U.S.A.
COMPUTER READABLE FORM:
COMPUTER: IBM PC compatible
OPERATING SYSTEM: PC-DOS/MS-DOS
CURRENT APPLICATION DATA:
APPLICATION NUMBER: CA 2,502,460
FILING DATE: 01-MAY-1997
CLASSIFICATION: C12N - 15/12
PRIOR APPLICATION DATA:
APPLICATION NUMBER: US 60/016,345
FILING DATE: 01-MAY-1996
PATENT AGENT INFORMATION:
NAME: TORYS LLP
REFERENCE NUMBER: 31539-2197
INFORMATION FOR SEQ ID NO.:1:
SEQUENCE CHARACTERISTICS:
LENGTH: 717 base pairs
TYPE: nucleic acid
STRANDEDNESS: single
TOPOLOGY: linear
FEATURE:
NAME/KEY: CDS
LOCATION: 1..714
FEATURE:
NAME/KEY: mat-peptide
LOCATION: 1..714
SEQUENCE DESCRIPTION: SEQ ID NO.:1:
ATG TCC AAG GGC GAG GAG CTC TTC ACC GGC GTG GTG CCC ATC CTC GTG 48
Met Ser Lys Gly Glu Glu Leu Phe Thr Gly Val Val Pro Ile Leu Val
78
CA 02502460 2008-09-04
1 5 10 15
GAG CTC GAC GGC GAC GTG AAC GGC CAC AAG TTC TCC GTG TCC GGC GAG 96
Glu Leu Asp Gly Asp Val Asn Gly His Lys Phe Ser Val Ser Gly Glu
20 25 30
GGC GAG GGC GAC GCC ACC TAC GGC AAG CTC ACC CTC AAG TTC ATC TGC 144
Gly Glu Gly Asp Ala Thr Tyr Gly Lys Leu Thr Leu Lys Phe Ile Cys
35 40 45
ACC ACC GGC AAG CTC CCC GTG CCC TGG CCC ACC CTC GTG ACC ACC TTC 192
Thr Thr Gly Lys Leu Pro Val Pro Trp Pro Thr Leu Val Thr Thr Phe
50 55 60
TCC TAC GGC GTG CAG TGC TTC TCC AGG TAC CCC GAC CAC ATG AAG CAG 240
Ser Tyr Gly Val Gln Cys Phe Ser Arg Tyr Pro Asp His Met Lys Gln
65 70 75 80
CAC GAC TTC TTC AAG TCA GCC ATG CCC GAG GGC TAC GTG CAG GAG AGG 288
His Asp Phe Phe Lys Ser Ala Met Pro Glu Gly Tyr Val Gln Glu Arg
85 90 95
ACC ATC TTC TTC AAG GAC GAC GGC AAC TAC AAG ACC AGG GCC GAG GTG 336
Thr Ile Phe Phe Lys Asp Asp Gly Asn Tyr Lys Thr Arg Ala Glu Val
100 105 110
AAG TTC GAA GGC GAC ACC CTC GTG AAC AGG ATT GAG CTC AAG GGC ATC 384
Lys Phe Glu Gly Asp Thr Leu Val Asn Arg Ile Glu Leu Lys Gly Ile
115 120 125
GAC TTC AAG GAG GAC GGC AAC ATC CTC GGC CAC AAG CTC GAG TAC AAC 432
Asp Phe Lys Glu Asp Gly Asn Ile Leu Gly His Lys Leu Glu Tyr Asn
130 135 140
TAC AAC TCC CAC AAC GTG TAC ATC ATG GCC GAC AAG CAG AAG AAC GGC 480
Tyr Asn Ser His Asn Val Tyr Ile Met Ala Asp Lys Gln Lys Asn Gly
145 150 155 160
ATC AAG GTG AAC TTC AAG ATC AGG CAC AAC ATC GAG GAC GGC TCA GTG 528
Ile Lys Val Asn Phe Lys Ile Arg His Asn Ile Glu Asp Gly Ser Val
165 170 175
CAG CTC GCT GAC CAC TAC CAG CAG AAC ACC CCC ATC GGC GAC GGC CCC 576
Gln Leu Ala Asp His Tyr Gln Gln Asn Thr Pro Ile Gly Asp Gly Pro
180 185 190
GTG CTC CTC CCC GAC AAC CAC TAC CTC TCC ACC CAG TCC GCC CTC TCC 624
Val Leu Leu Pro Asp Asn His Tyr Leu Ser Thr Gln Ser Ala Leu Ser
195 200 205
AAG GAC CCC AAC GAG AAG AGG GAC CAC ATG GTG CTC CTC GAG TTC GTG 672
Lys Asp Pro Asn Glu Lys Arg Asp His Met Val Leu Leu Giu Phe Val
79
CA 02502460 2008-09-04
210 215 220
ACC GCT GCT GGC ATC ACC CAC GGC ATG GAC GAG CTC TAC AAG 714
Thr Ala Ala Gly Ile Thr His Gly Met Asp Glu Leu Tyr Lys
225 230 235
TGA 717
INFORMATION FOR SEQ ID NO.:2:
SEQUENCE CHARACTERISTICS:
LENGTH: 238 amino acids
TYPE: amino acid
TOPOLOGY: linear
MOLECULE TYPE: protein
SEQUENCE DESCRIPTION: SEQ ID NO.:2:
Met Ser Lys Gly Glu Glu Leu Phe Thr Gly Val Val Pro Ile Leu Val
1 5 10 15
Glu Leu Asp Gly Asp Val Asn Gly His Lys Phe Ser Val Ser Gly Glu
20 25 30
Gly Glu Gly Asp Ala Thr Tyr Gly Lys Leu,Thr Leu Lys Phe Ile Cys
35 40 45
Thr Thr Gly Lys Leu Pro Val Pro Trp Pro Thr Leu Val Thr Thr Phe
50 55 60
Ser Tyr Gly Val Gln Cys Phe Ser Arg Tyr Pro Asp His Met Lys Gln
65 70 75 80
His Asp Phe Phe Lys Ser Ala Met Pro Glu Gly Tyr Val Gln Glu Arg
85 90 95
Thr Ile Phe Phe Lys Asp Asp Gly Asn Tyr Lys Thr Arg Ala Glu Val
100 105 110
Lys Phe Glu Gly Asp Thr Leu Val Asn Arg Ile Glu Leu Lys Gly Ile
115 120 125
Asp Phe Lys Glu Asp Gly Asn Ile Leu Gly His Lys Leu Glu Tyr Asn
130 135 140
Tyr Asn Ser His Asn Val Tyr Ile Met Ala Asp Lys Gln Lys Asn Gly
145 150 155 160
Ile Lys Val Asn Phe Lys Ile Arg His Asn Ile Gl,u Asp Gly Ser Val
165 170 175
CA 02502460 2008-09-04
Gln Leu Ala Asp His Tyr Gln Gln Asn Thr Pro Ile Gly Asp Gly Pro
180 185 190
Val Leu Leu Pro Asp Asn His Tyr Leu Ser Thr Gln Ser Ala Leu Ser
195 200 205
Lys Asp Pro Asn Glu Lys Arg Asp His Met Val Leu Leu Glu Phe Val
210 215 220
Thr Ala Ala Gly Ile Thr His Gly Met Asp Glu Leu Tyr Lys
225 230 235
INFORMATION FOR SEQ ID NO.:3:
SEQUENCE CHARACTERISTICS:
LENGTH: 159 base pairs
TYPE: nucleic acid
STRANDEDNESS: single
TOPOLOGY: linear
FEATURE:
NAME/KEY: CDS
LOCATION: 1..159
FEATURE:
NAME/KEY: mat_peptide
LOCATION: 1..159
SEQUENCE DESCRIPTION: SEQ ID NO.:3:
ATG GCG TCC TCC ACG ATG GCC CTC TCC TCC ACC GCC TTC GCC GGC AAG 48
Met Ala Ser Ser Thr Met Ala Leu Ser Ser Thr Ala Phe Ala Gly Lys
1 5 10 15
GCC GTG AAC GTG CCG TCG TCG TCC GCC TTC GAG GCC CGC GTG ACC ATG 96
Ala Val Asn Val Pro Ser Ser Ser Ala Phe Glu Ala Arg Val Thr Met
20 25 30
AGG AAG ACG GCG GCG AAG GCC AAG CCA GCT GCG GCG TCC GGG AGC CCG 144
Arg Lys Thr Ala Ala Lys Ala Lys Pro Ala Ala Ala Ser Gly Ser Pro
35 40 45
TGG TAC GGC CCC ATG 159
Trp Tyr Gly Pro Met
INFORMATION FOR SEQ ID NO.:4:
SEQUENCE CHARACTERISTICS:
81
CA 02502460 2008-09-04
LENGTH: 53 amino acids
TYPE: amino acid
TOPOLOGY: linear
MOLECULE TYPE: protein
SEQUENCE DESCRIPTION: SEQ ID NO.:4:
Met Ala Ser Ser Thr Met Ala Leu Ser Ser Thr Ala Phe Ala Gly Lys
1 5 10 15
Ala Val Asn Val Pro Ser Ser Ser Ala Phe Glu Ala Arg Val Thr Met
20 25 30
Arg Lys Thr Ala Ala Lys Ala Lys Pro Ala Ala Ala Ser Gly Ser Pro
35 40 45
Trp Tyr Gly Pro Met
NFORMATION FOR SEQ ID NO.:5:
SEQUENCE CHARACTERISTICS:
LENGTH: 537 base pairs
TYPE: nucleic acid
STRANDEDNESS: single
TOPOLOGY: linear
FEATURE:
NAME/KEY: CDS
LOCATION: join(l..69, 496..537)
FEATURE:
NAME/KEY: mat_peptide
LOCATION: join(1..69, 496..537)
SEQUENCE DESCRIPTION: SEQ ID NO.:5:
ATG TAC CGC GCG GCC GCT AGC CTC GCC TCC AAG GCG CGG CAA GCC GGG 48
Met Tyr Arg Ala Ala Ala Ser Leu Ala Ser Lys Ala Arg Gln Ala Gly
1 5 10 15
AGC AGC TCC GCC GCT CGC CAG GTGAGAGCAG ACTCGTGTTT ATACGCGTGT 99
Ser Ser Ser Ala Ala Arg Gln
GATGGGTCTG ATGGAGGATC CCGCCCTCAG ATTTGGTAAT GTTGGCCTGG GGTTCTAGTA 159
GTTCTGCGTG CAGGGCTGTG GGTTTGCTGC ATGGTGCTGT TTATTTTGGT GGCGATCTGC 219
CGGAATCTGT AGTTCGCTCG CGCAAAATCT AAGCTAGCTC GCTAATGGCG TACTGGCGTG 279
82
CA 02502460 2008-09-04
GGGTTTCACA TTAATCTACG GTGGTGAACT CGTCACTACC GTCCTCCAGT TAGCTGTTAG 339
ACACCGAATA CACTGATTGG CAGTTGAGAA ACATTGATCT GATCCAGCAG AAATCGATGT 399
CTTGTGAAAT TCGTTATTTT ATTGTCGTGT AACCTTGGGG CATGGCAGTC TCTAATTGAT 459
CACGCACTCA CCTCTGTTGT GTGTGATGCT TTATAG GTT GGA AGC AGG CTT GCC 513
Val Gly Ser Arg Leu Ala
TGG AGC AGG AAC TAT GCT GCC ATG 537
Trp Ser Arg Asn Tyr Ala Ala Met
35
INFORMATION FOR SEQ ID NO.:6:
SEQUENCE CHARACTERISTICS:
LENGTH: 37 amino acids
TYPE: amino acid
TOPOLOGY: linear
MOLECULE TYPE: protein
SEQUENCE DESCRIPTION: SEQ ID NO.:6:
Met Tyr Arg Ala Ala Ala Ser Leu Ala Ser Lys Ala Arg Gln Ala Gly
1 5 10 15
Ser Ser Ser Ala Ala Arg Gln Val Gly Ser Arg Leu Ala Trp Ser Arg
20 25 30
Asn Tyr Ala Ala Met
INFORMATION FOR SEQ ID NO.:7:
SEQUENCE CHARACTERISTICS:
LENGTH: 72 base pairs
TYPE: nucleic acid
STRANDEDNESS: single
TOPOLOGY: linear
FEATURE:
NAME/KEY: CDS
LOCATION: 1..72
FEATURE:
NAME/KEY: mat-peptide
LOCATION: 1..72
83
CA 02502460 2008-09-04
SEQUENCE DESCRIPTION: SEQ ID NO.:7:
ATG GCC AAC AAG CAC CTG AGC CTC TCC CTC TTC CTC GTG CTC CTC GGC 48
Met Ala Asn Lys His Leu Ser Leu Ser Leu Phe Leu Val Leu Leu Gly
1 5 10 15
CTC TCC GCC TCC CTC GCC TCC ATG 72
Leu Ser Ala Ser Leu Ala Ser Met
INFORMATION FOR SEQ ID NO.:8:
SEQUENCE CHARACTERISTICS:
LENGTH: 24 amino acids
TYPE: amino acid
TOPOLOGY: linear
MOLECULE TYPE: protein
SEQUENCE DESCRIPTION: SEQ ID NO.:8:
Met Ala Asn Lys His Leu Ser Leu Ser Leu Phe Leu Val Leu Leu Gly
1 5 10 15
Leu Ser Ala Ser Leu Ala Ser Met
INFORMATION FOR SEQ ID NO.:9:
SEQUENCE CHARACTERISTICS:
LENGTH: 45 base pairs
TYPE: nucleic acid
STRANDEDNESS: single
TOPOLOGY: linear
FEATURE:
NAME/KEY: CDS
LOCATION: 1..45
FEATURE:
NAME/KEY: mat peptide
LOCATION: 1..45
SEQUENCE DESCRIPTION: SEQ ID NO.:9:
GTG TTC GCC GAG GCC ATC GCC GCC AAC TCC ACC CTC GTG GCC GAG 45
Val Phe Ala Glu Ala Ile Ala Ala Asn Ser Thr Leu Val Ala Glu
1 5 10 15
84
CA 02502460 2008-09-04
INFORMATION FOR SEQ ID NO.:10:
SEQUENCE CHARACTERISTICS:
LENGTH: 15 amino acids
TYPE: amino acid
TOPOLOGY: linear
MOLECULE TYPE: protein
SEQUENCE DESCRIPTION: SEQ ID NO.:10:
Val Phe Ala Glu Ala Ile Ala Ala Asn Ser Thr Leu Val Ala Glu
1 5 10 15
INFORMATION FOR SEQ ID NO.:11:
SEQUENCE CHARACTERISTICS:
LENGTH: 33 base pairs
TYPE: nucleic acid
STRANDEDNESS: single
TOPOLOGY: linear
FEATURE:
NAME/KEY: CDS
LOCATION: 1..33
FEATURE:
NAME/KEY: mat peptide
LOCATION: 1..33
SEQUENCE DESCRIPTION: SEQ ID NO.:11:
GAC GGC GGC GTG GAC GAC GAC CAC GAC GAG CTC 33
Asp Gly'Gly Val Asp Asp Asp His Asp Glu Leu
1 5 10
INFORMATION FOR SEQ ID NO.:12:
SEQUENCE CHARACTERISTICS:
LENGTH: 11 amino acids
TYPE: amino acid
TOPOLOGY: linear
MOLECULE TYPE: protein
SEQUENCE DESCRIPTION: SEQ ID NO.:12:
Asp Gly Gly Val Asp Asp Asp His Asp Glu Leu
1 5 10
CA 02502460 2008-09-04
INFORMATION FOR SEQ ID NO.:13:
SEQUENCE CHARACTERISTICS:
LENGTH: 141 base pairs
TYPE: nucleic acid
STRANDEDNESS: single
TOPOLOGY: linear
FEATURE:
NAME/KEY: CDS
LOCATION: 1..141
FEATURE:
NAME/KEY: mat peptide
LOCATION: 1..141
SEQUENCE DESCRIPTION: SEQ ID NO.:13:
ATG GCC AAC AAG CAC CTG AGC CTC TCC CTC TTC CTC GTG CTC CTC GGC 48
Met Ala Asn Lys His Leu Ser Leu Ser Leu Phe Leu Val Leu Leu Gly
1 5 10 15
CTC TCC GCC TCC CTC GCC TCC GGA CAC AGC AGG TTC AAC CCC ATC AGG 96
Leu Ser Ala Ser Leu Ala Ser Gly His Ser Arg Phe Asn Pro Ile Arg
20 25 30
CTG CCC ACC ACC CAC GAG CCC GCC AGC AGC GAG ACC GGA TCC ATG 141
Leu Pro Thr Thr His Glu Pro Ala Ser Ser Glu Thr Gly Ser Met
35 40 45
INFORMATION FOR SEQ ID NO.:14:
SEQUENCE CHARACTERISTICS:
LENGTH: 47 amino acids
TYPE: amino acid
TOPOLOGY: linear
MOLECULE TYPE: protein
SEQUENCE DESCRIPTION: SEQ ID NO.:14:
Met Ala Asn Lys His Leu Ser Leu Ser Leu Phe Leu Val Leu Leu Gly
1 5 10 15
Leu Ser Ala, Ser Leu Ala Ser Gly His Ser Arg Phe Asn Pro Ile Arg
20 25 30
Leu Pro Thr Thr His Glu Pro Ala Ser Ser Glu Thr Gly Ser Met
35 40 45
86
CA 02502460 2008-09-04
INFORMATION FOR SEQ ID NO.:15:
SEQUENCE CHARACTERISTICS:
LENGTH: 12 base pairs
TYPE: nucleic acid
STRANDEDNESS: single
TOPOLOGY: linear
FEATURE:
NAME/KEY: CDS
LOCATION: 1..12
FEATURE:
NAME/KEY: mat peptide
LOCATION: 1..12
SEQUENCE DESCRIPTION: SEQ ID NO.:15:
AAG TCG AAG CTT 12
Lys Ser Lys Leu
1
INFORMATION FOR SEQ ID NO.:16:
SEQUENCE CHARACTERISTICS:
LENGTH: 4 amino acids
TYPE: amino acid
TOPOLOGY: linear
MOLECULE TYPE: protein
SEQUENCE DESCRIPTION: SEQ ID NO.:16:
Lys Ser Lys Leu
1
INFORMATION FOR SEQ ID NO.:17:
SEQUENCE CHARACTERISTICS:
LENGTH: 240 base pairs
TYPE: nucleic acid
STRANDEDNESS: single
TOPOLOGY: linear
FEATURE:
NAME/KEY: CDS
LOCATION: 1..240
87
CA 02502460 2008-09-04
FEATURE:
NAME/KEY: mat-peptide
LOCATION: 1..240
SEQUENCE DESCRIPTION: SEQ ID NO.:17:
ATG AAC CAG GCG ATG GCC ACC CCG CTG TCC CTG TCC TGC TGC TCC CCG 48
Met Asn Gln Ala Met Ala Thr Pro Leu Ser Leu Ser Cys Cys Ser Pro
1 5 10 15
ACC CTG ACC AGG TCC ACC CTG TTC TTC ACC AAG ACC TTC CCG TTC TCC 96
Thr Leu Thr Arg Ser Thr Leu Phe Phe Thr Lys Thr Phe Pro Phe Ser
20 25 30
CGC TCC TTC TCC ACC CCG CTG CCG CTG TCC ACC AAG ACC CTG ATC TCC 144
Arg Ser Phe Ser Thr Pro Leu Pro Leu Ser Thr Lys Thr Leu Ile Ser
35 40 45
CTG TCC CCG CCG CAC AGG ACC TTC GCC GTG AGG GCT GAG TCC CAG AAC 192
Leu Ser Pro Pro His Arg Thr Phe Ala Val Arg Ala Glu Ser Gln Asn
50 55 60
GGC GCG GAC CCG GCC AGG CAG TAC GAC TTC GAC CTG TTC ACC ATC GGC 240
Gly Ala Asp Pro Ala Arg Gln Tyr Asp Phe Asp Leu Phe Thr Ile Gly
65 70 75 80
INFORMATION FOR SEQ ID NO.:18:
SEQUENCE CHARACTERISTICS:
LENGTH: 80 amino acids
TYPE: amino acid
TOPOLOGY: linear
MOLECULE TYPE: protein
SEQUENCE DESCRIPTION: SEQ ID NO.:18:
Met Asn Gln Ala Met Ala Thr Pro Leu Ser Leu Ser Cys Cys Ser Pro
1 5 10 15
Thr Leu Thr Arg Ser Thr Leu Phe Phe Thr Lys Thr Phe Pro Phe Ser
20 25 30
Arg Ser Phe Ser Thr Pro Leu Pro Leu Ser Thr Lys Thr Leu Ile Ser
35 40 45
Leu Ser Pro Pro His Arg Thr Phe Ala Val Arg Ala Glu Ser Gln Asn
50 55 60
Gly Ala Asp Pro Ala Arg Gln Tyr Asp Phe Asp Leu Phe Thr Ile Gly
65 70 75 80
88
CA 02502460 2008-09-04
INFORMATION FOR SEQ ID NO.:19:
SEQUENCE CHARACTERISTICS:
LENGTH: 42 base pairs
TYPE: nucleic acid
STRANDEDNESS: single
TOPOLOGY: linear
FEATURE:
NAME/KEY: CDS
LOCATION: 1..42
FEATURE:
NAME/KEY: mat_peptide
LOCATION: 1..42
SEQUENCE DESCRIPTION: SEQ ID NO.:19:
CCG CCC AAG AAG AAG CGC AAG GTG CCC ATG AAG ATC CAC ATG 42
Pro Pro Lys Lys Lys Arg Lys Val Pro Met Lys Ile His Met
1 5 10
INFORMATION FOR SEQ ID NO.:20:
SEQUENCE CHARACTERISTICS:
LENGTH: 14 amino acids
TYPE: amino acid
TOPOLOGY: linear
MOLECULE TYPE: protein
SEQUENCE DESCRIPTION: SEQ ID NO.:20:
Pro Pro Lys Lys Lys Arg Lys Val Pro Met Lys Ile His Met
1 5 10
INFORMATION FOR SEQ ID NO.:21:
SEQUENCE CHARACTERISTICS:
LENGTH: 456 base pairs
TYPE: nucleic acid
STRANDEDNESS: single
TOPOLOGY: linear
FEATURE:
NAME/KEY: CDS
LOCATION: 1..456
89
CA 02502460 2008-09-04
FEATURE:
NAME/KEY: mat-peptide
LOCATION: 1..456
SEQUENCE DESCRIPTION: SEQ ID NO.:21:
TAC ACT CCA AGC CCT AAG CCA CCG GCT ACC AAG CCT CCC ACG CCC AAG 48
Tyr Thr Pro Ser Pro Lys Pro Pro Ala Thr Lys Pro Pro Thr Pro Lys
1 5 10 15
CCG ACC CCG CCA ACG TAC ACC CCT TCG CCA AAG CCT CCG ACA CCC AAG 96
Pro Thr Pro Pro Thr Tyr Thr Pro Ser Pro Lys Pro Pro Thr Pro Lys
20 25 30
CCG ACC CCG CCG ACG TAC ACC CCT TCT CCC AAG CCT CCG ACG CCC AAG 144
Pro Thr Pro Pro Thr Tyr Thr Pro Ser Pro Lys Pro Pro Thr Pro Lys
35 40 45
CCG ACC CCG CCG ACG TAC ACT CCA AGC CCC AAG CCT CCC ACA CAC CCG 192
Pro Thr Pro Pro Thr Tyr Thr Pro Ser Pro Lys Pro Pro Thr His Pro
50 55 60
ACG CCC AAG CCG ACC CCA CCG ACG TAC ACC CCT TCC CCA AAG CCT CCG 240
Thr Pro Lys Pro Thr Pro Pro Thr Tyr Thr Pro Ser Pro Lys Pro Pro
65 70 75 80
ACG CCC AAG CCG ACC CCA CCG ACG TAC ACC CCT TCC CCA AAG CCT CCG 288
Thr Pro Lys Pro Thr Pro Pro Thr Tyr Thr Pro Ser Pro Lys Pro Pro
85 90 95
ACA CCC AAG CCG ACC CCA CCG ACG TAC ACC CCT TCC CCA AAG CCT CCG 336
Thr Pro Lys Pro Thr Pro Pro Thr Tyr Thr Pro Ser Pro Lys Pro Pro
100 105 110
ACA CCC AAG CCG ACC CCA CCG ACG TAC ACT CCC ACA CCG AAG CCG CCG 384
Thr Pro Lys Pro Thr Pro Pro Thr Tyr Thr Pro Thr Pro Lys Pro Pro
115 120 125
GCC ACC AAG CCG CCC ACC TAC ACT CCG ACG CCG CCG GTG TCT CAC ACC 432
Ala Thr Lys Pro Pro Thr Tyr Thr Pro Thr Pro Pro Val Ser His Thr
130 135 140
CCC AGC CCG CCG CCA CCA TAC TAC 456
Pro Ser Pro Pro Pro Pro Tyr Tyr
145 150
INFORMATION FOR SEQ ID NO.:22:
SEQUENCE CHARACTERISTICS:
LENGTH: 152 amino acids
TYPE: amino acid
CA 02502460 2008-09-04
TOPOLOGY: linear
MOLECULE TYPE: protein
SEQUENCE DESCRIPTION: SEQ ID NO.:22:
Tyr Thr Pro Ser Pro Lys Pro Pro Ala Thr Lys Pro Pro Thr Pro Lys
1 5 10 15
Pro Thr Pro Pro Thr Tyr Thr Pro Ser Pro Lys Pro Pro Thr Pro Lys
20 25 30
Pro Thr Pro Pro Thr Tyr Thr Pro Ser Pro Lys Pro Pro Thr Pro Lys
35 40 45
Pro Thr Pro Pro Thr Tyr Thr Pro Ser Pro Lys Pro Pro Thr His Pro
50 55 60
Thr Pro Lys Pro Thr Pro Pro Thr Tyr Thr Pro Ser Pro Lys Pro Pro
65 70 75 80
Thr Pro Lys Pro Thr Pro Pro Thr Tyr Thr Pro Ser Pro Lys Pro Pro
85 90 95
Thr Pro Lys Pro Thr Pro Pro Thr Tyr Thr Pro Ser Pro Lys Pro Pro
100 105 110
Thr Pro Lys Pro Thr Pro Pro Thr Tyr Thr Pro Thr Pro Lys Pro Pro
115 120 125
Ala Thr Lys Pro Pro Thr Tyr Thr Pro Thr Pro Pro Val Ser His Thr
130 135 140
Pro Ser Pro Pro Pro Pro Tyr Tyr
145 150
INFORMATION FOR SEQ ID NO.:23:
SEQUENCE CHARACTERISTICS:
LENGTH: 621 base pairs
TYPE: nucleic acid
STRANDEDNESS: single
TOPOLOGY: linear
FEATURE:
NAME/KEY: CDS
LOCATION: 1..621
FEATURE:
NAME/KEY: mat peptide
LOCATION: 1..621
91
CA 02502460 2008-09-04
SEQUENCE DESCRIPTION: SEQ ID NO.:23:
GTG GCT GAG TAC ACG CTG CCG GAT CTG GAT TAC GAC TAC AGC GCC CTG 48
Val Ala Glu Tyr Thr Leu Pro Asp Leu Asp Tyr Asp Tyr Ser Ala Leu
1 5 10 15
GAA CCC CAC ATC TCC GGG CAG ATC AAC GAG CTG CAC CAT TCC AAG CAC 96
Glu Pro His Ile Ser Gly Gln Ile Asn Glu Leu His His Ser Lys His
20 25 30
CAC GCC GCC TAC GTC GCC GGT GCC AAC ACG GCA CTG GAG AAG CTG GAA 144
His Ala Ala Tyr Val Ala Gly Ala Asn Thr Ala Leu Glu Lys Leu Glu
35 40 45
GCC GCC CGT GAG GCC GGC GAT CAC AGC GCG ATC TTC CTG CAC GAG AAG 192
Ala Ala Arg Glu Ala Gly Asp His Ser Ala Ile Phe Leu His Glu Lys
50 55 60
AAC CTC GCG TTC CAC CTC GGC GGA CAC GTC AAC CAC TCC ATC TGG TGG 240
Asn Leu Ala Phe His Leu Gly Gly His Val Asn His Ser Ile Trp Trp
65 70 75 80
AAG AAC CTG TCC CCC AAC GGT GGC GAC AAG CCG GTC GGC GAG CTG GCC 288
Lys Asn Leu Ser Pro Asn Gly Gly Asp Lys Pro Val Gly Glu Leu Ala
85 90 95
GCG GCC ATC GAC GAC CAG TTC GGT TCG TTC GAC AAG TTC CGC GCG CAG 336
Ala Ala Ile Asp Asp Gln Phe Gly Ser Phe Asp Lys Phe Arg Ala Gln
100 105 110
TTC ACC GCC GCG GCC AAC GGC CTG CAG GGC TCG GGC TGG GCG GTG CTC 384
Phe Thr Ala Ala Ala Asn Gly Leu Gln Gly Ser Gly Trp Ala Val Leu
115 120 125
GGT TAC GAC ACC CTC GGC CAG AAG CTG CTG ACC TTC CAG CTC TAC GAC 432
Gly Tyr Asp Thr Leu Gly Gln Lys Leu Leu Thr Phe Gln Leu Tyr Asp
130 135 140
CAG CAG GCC AAC GTG CCG CTG GGC ATC ATC CCG CTG CTC CAG GTC GAC 480
Gln Gln Ala Asn Val Pro Leu Gly Ile Ile Pro Leu Leu Gln Val Asp
145 150 155 160
ATG TGG GAG CAC GCC TTC TAC CTG CAG TAC AAG AAC GTC AAG GCC GAC 528
Met Trp Glu His Ala Phe Tyr Leu Gln Tyr Lys Asn Val Lys Ala Asp
165 170 175
TAC GTG ACC GCG TTC TGG AAC GTC GTC AAC TGG GCC GAC GTG CAG GAC 576
Tyr Val Thr Ala Phe Trp Asn Val Val Asn Trp Ala Asp Val Gln Asp
180 185 190
CGC TTC GGC AAG GCC GTC AAC CAG GGC AAG GGC CTT ATC TTC GGG 621
92
CA 02502460 2008-09-04
Arg Phe Gly Lys Ala Val Asn Gln Gly Lys Gly Leu Ile Phe Gly
195 200 205
INFORMATION FOR SEQ ID NO.:24:
SEQUENCE CHARACTERISTICS:
LENGTH: 207 amino acids
TYPE: amino acid
TOPOLOGY: linear
MOLECULE TYPE: protein
SEQUENCE DESCRIPTION: SEQ ID NO.:24:
Val Ala Glu Tyr Thr Leu Pro Asp Leu Asp Tyr Asp Tyr Ser Ala Leu
1 5 10 15
Glu Pro His Ile Ser Gly Gln Ile Asn Glu Leu His His Ser Lys His
20 25 30
His Ala Ala Tyr Val Ala Gly Ala Asn Thr Ala Leu Glu Lys Leu Glu
35 40 45
Ala Ala Arg Glu Ala Gly Asp His Ser Ala Ile Phe Leu His Glu Lys
50 55 60
Asn Leu Ala Phe His Leu Gly Gly His Val Asn His Ser Ile Trp Trp
65 70 75 80
Lys Asn Leu Ser Pro Asn Gly Gly Asp Lys Pro Val Gly Glu Leu Ala
85 90 95
Ala Ala Ile Asp Asp Gln Phe Gly Ser Phe Asp Lys Phe Arg Ala Gln
100 105 110
Phe Thr Ala Ala Ala Asn Gly Leu Gln Gly Ser Gly Trp Ala Val Leu
115 120 125
Gly Tyr Asp Thr Leu Gly Gln Lys Leu Leu Thr Phe Gln Leu Tyr Asp
130 135 140
Gln Gln Ala Asn Val Pro Leu Gly Ile Ile Pro Leu Leu Gln Val Asp
145 150 155 160
Met Trp Glu His Ala Phe Tyr Leu Gln Tyr Lys Asn Val Lys Ala Asp
165 170 175
Tyr Val Thr Ala Phe Trp Asn Val Val Asn Trp Ala Asp Val Gln Asp
180 185 190
Arg Phe Gly Lys Ala Val Asn Gln Gly Lys Gly Leu Ile Phe Gly
93
CA 02502460 2008-09-04
195 200 205
94