Note: Descriptions are shown in the official language in which they were submitted.
CA 02503455 2005-04-22
Boehringer Ingelheim Pharma GmbH & Co. KG Case 1/1402
D-55216 Ingelheim/Rhein PCT text
82041 pct.211
Selected CGRP antagonists, processes for preparing them
and their use as pharmaceutical compositions
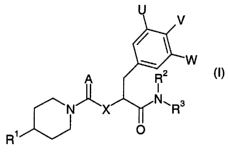
The present invention relates to CGRP antagonists of general formula
U
V
'W
A R2
I
N X N~Rs
R'
(I)
the tautomers, diastereomers, enantiomers, hydrates, mixtures thereof and
the salts thereof as well as the hydrates of the salts, particularly the
physiologically acceptable salts thereof with inorganic or organic acids,
pharmaceutical compositions containing these compounds, the use thereof
and processes for the preparation thereof.
In the above general formula (I) in a first embodiment
A denotes an oxygen or sulphur atom, a phenylsulphonylimino or cyanimino
group,
X denotes an oxygen or sulphur atom, an imino group optionally substituted
by a C~~-alkyl group or a methylene group optionally substituted by a
C,_6-alkyl group,
U denotes a C~_6-alkyl, C2_6-alkenyl or C2~-alkynyl group wherein each
methylene group may be substituted by up to 2 fluorine atoms and each
methyl group may be substituted by up to 3 fluorine atoms,
CA 02503455 2005-04-22
V denotes a chlorine or bromine atom, an amino, methylamino or hydroxy
group,
W denotes a hydrogen, fluorine, chlorine, bromine or iodine atom, a difluoro-
or trifluoromethyl group,
R' denotes a saturated, mono- or diunsaturated 5- to 7-membered aza, diaza,
triaza, oxaza, thiaza, thiadiaza or S,S-dioxido-thiadiaza heterocyclic group,
in which the abovementioned heterocycles are linked via a carbon or
nitrogen atom,
contain one or two carbonyl or thiocarbonyl groups adjacent to a nitrogen
atom,
may be substituted at one of the nitrogen atoms by an alkyl group,
may be substituted at one or at two carbon atoms by an alkyl group, by a
phenyl, phenylmethyl, naphthyl, biphenylyl, pyridinyl, diazinyl, furyl,
thienyl, pyrrolyl, 1,3-oxazolyl, 1,3-thiazolyl, isoxazolyl, pyrazolyl,
1-methylpyrazolyl, imidazolyl or 1-methylimidazolyl group, while the
substituents may be identical or different, and
while an olefinic double bond of one of the abovementioned unsaturated
heterocycles may be fused to a phenyl, naphthyl, pyridine, diazine,
1,3-oxazole, thienyl, furan, thiazole, pyrrole, N-methylpyrrole or quinoline
ring, to a 1 H-quinolin-2-one ring optionally substituted at the nitrogen
atom by an alkyl group or to an imidazole or N-methylimidazole ring or
also two olefinic double bonds of one of the abovementioned unsaturated
heterocycles may each be fused to a phenyl ring,
while the phenyl, pyridinyl, diazinyl, furyl, thienyl, pyrrolyl, 1,3-
oxazolyl, 1,3-thiazolyl, isoxazolyl, pyrazolyl, 1-methylpyrazolyl,
CA 02503455 2005-04-22
3
imidazolyl or 1-methylimidazolyl groups contained in R' as well as
benzo-, thieno-, pyrido- and diazino-fused heterocycles in the carbon
skeleton may additionally be mono-, di- or trisubstituted by fluorine,
chlorine, bromine or iodine atoms, by alkyl, alkoxy, nitro, alkylthio,
aikylsufphinyl, alkylsuiphonyl, alkyisulphonyiamino, phenyl,
difluoromethyl, trifluoromethyl, alkoxycarbonyl, carboxy, hydroxy,
amino, alkylamino, dialkylamino, acetyl, acetylamino, propionylamino,
aminocarbonyl, alkylaminocarbonyl, dialkylaminocarbonyl,
(4-morpholinyl)carbonyl, (1-pyrrolidinyl)carbonyl,
(1-piperidinyl)carbonyl, (hexahydro-1-azepinyl)carbonyl, (4-methyl-1-
piperazinyl)carbonyl, methylenedioxy, aminocarbonylamino, alkanoyl,
cyano, difluoromethoxy, trifluoromethoxy, trifluoromethylthio,
trifluoromethylsulphinyl or trifluoromethylsulphonyl groups, while the
substituents may be identical or different,
R2 denotes the hydrogen atom,
a phenylmethyl group or a C2_~-alkyl group which may be substituted in the w
position by a cyclohexyl, phenyl, pyridinyl, diazinyl, hydroxy, amino,
alkylamino, dialkylamino, carboxy, alkoxycarbonyl, aminocarbonyl,
aminocarbonylamino, acetylamino, 1-pyrrolidinyl, 1-piperidinyl,
4-(1-piperidinyl)-1-piperidinyl, 4-morpholinyl, hexahydro-1H-1-azepinyl, [bis-
(2-hydroxyethyi)]amino, 4-alkyl-1-piperazinyl or 4-(c~-hydroxy-C2_~-alkyl)-1-
piperazinyl group,
a phenyl or pyridinyl group,
while the abovementioned heterocyclic groups and phenyl groups may
additionally be mono- di- or trisubstituted in the carbon skeleton by
fluorine, chlorine, bromine or iodine atoms, by methyl, alkoxy,
difluoromethyl, trifluoromethyl, hydroxy, amino, C,_3-alkylamino, di-
(C,_3-alkyl)-amino, acetylamino, aminocarbonyl, cyano,
methylsulphonyloxy, difluoramethoxy, trifluoromethoxy, trifluoromethylthio,
CA 02503455 2005-04-22
4
trifluoromethylsulphinyl, trifluoromethylsulphonyl, amino-C~_3-alkyl,
C,_3-alkylamino-C,_3-alkyl or di-(C~_3-alkyl)-amino-C~_3-alkyl groups and the
substituents may be identical or different,
R3 denotes the hydrogen atom or a C,_3-alkyl group optionally substituted by a
phenyl or pyridinyl group,
while the C~_3-alkyl group may be linked to an alkyl group present in R2 or
a phenyl or pyridyl ring present in R2 and the nitrogen atom to which they
are bound, forming a ring, or
R2 and R3 together with the enclosed nitrogen atom denote a group of general
formula
R5
(CR\R9)q
Y'
CR6R
~ (CR8R9)~ ~ / R4
N CR6R~ , (ll)
wherein
Y' denotes the carbon atom or, if R5 is a pair of free electrons, it may also
denote the nitrogen atom,
q and r, if Y' denotes the carbon atom, represent the numbers 0, 1 or 2,
or
q and r, if Y' denotes the nitrogen atom, represent the numbers 1 or 2,
R4 denotes the hydrogen atom, an amino, alkylamino, cycloalkylamino,
dialkylamino, N-(cycloalkyl)-alkylamino, dicycloalkylamino, hydroxy, alkyl,
cycloalkyl, amino-C2_~-alkyl, alkylamino-C2_~-alkyl, dialkylamino-C2_~-alkyl,
CA 02503455 2005-04-22
5
aminoiminomethyl, alkylcarbonyl, alkylsulphonyl, alkylcarbonylamino,
alkylsulphonylamino, N-alkylcarbonyl-N-alkylamino, N-alkylsulphonyl-
N-alkylamino, aminocarbonylamino, alkyiaminocarbonylamino,
diaikylaminocarbonylamino, cycioalkyfaminocarbonylamino,
dicycloalkylaminocarbonylamino, phenylaminocarbonylamino,
aminocarbonylalkyl, alkylaminocarbonylalkyl, dialkylaminocarbonylalkyl,
aminocarbonylaminoalkyl, alkoxycarbonyl, alkoxycarbonylalkyl or
carboxyalkyl group,
or, if Y' does not denote the nitrogen atom, the carboxy, aminomethyl,
alkylaminomethyl or dialkylaminomethyl group,
a phenyl, phenyl-C,_3-alkyl, pyridinyl, diazinyl, 1-naphthyl, 2-naphthyl,
pyridinylcarbonyl or phenylcarbonyl group which may each be mono-, di-
or trisubstituted in the carbon skeleton by fluorine, chlorine, bromine or
iodine atoms, by alkyl, alkoxy, methylsulphonyloxy, difluoromethyl,
trifluoromethyl, hydroxy, amino, acetylamino, aminocarbonyl,
aminocarbonylamino, aminocarbonylaminomethyl, cyano, carboxy,
alkoxycarbonyl, carboxyalkyl, alkoxycarbonylalkyl, alkanoyl,
w-(dialkylamino)alkanoyl, cu-(dialkylamino)alkyl,
w-(dialkylamino)hydroxyalkyl, c~-(carboxy)alkanoyl, difluoromethoxy,
trifluoromethoxy, trifluoromethylthio, trifluoromethylsulphinyl or
trifluoromethylsulphonyl groups, while the substituents may be identical or
different,
a saturated or mono- or polyunsaturated 4- to 10-membered azacycloalkyl
group, a 5- to 10-membered oxaza-, thiaza, diaza- or triazacycloalkyl
group, a 6- to 10-membered azabicyclo- or diazabicycloalkyl group, a
1-alkyl-4-piperidinylcarbonyl or 4-alkyl-1-piperazinylcarbonyl, a 1-alkyl-
4-piperidinylamino, 1-alkyl-4-piperidinylaminocarbonyl or 1-alkyl-
4-piperidinylaminosulphonyl group,
CA 02503455 2005-04-22
6
while the abovementioned mono- and bicyclic heterocycles are
bound via a nitrogen or carbon atom,
a methylene group in the abovementioned mono- and bicyclic
heterocycles may be replaced by a carbonyl or sulphonyl group,
in the abovementioned mono- and bicyclic heterocycles any
methylene group not directly bound to a nitrogen, oxygen or sulphur
atom may be substituted by one or two fluorine atoms,
the abovementioned mono- and bicyclic heterocycles as well as the
1-alkyl-4-piperidinylcarbonyl- and 4-alkyl-1-piperazinylcarbonyl group
in the ring may be mono- or polysubstituted by a C~_~-alkyl group
and/or
monosubstituted by a benzyl, alkanoyl, dialkylamino, phenylcarbonyl,
pyridinylcarbonyl, carboxy, carboxyalkanoyl, carboxyalkyl,
alkoxycarbonylalkyl, alkoxycarbonyl, aminocarbonyl, alkylamino-
carbonyl, alkylsulphonyl, cycloalkyl or cycloalkylalkyl group, or
substituted by a cycloalkylcarbonyl, azacycloalkylcarbonyl,
diazacycloalkyicarbonyl or oxazacycloalkylcarbonyl group optionally
alkyl-substituted in the ring,
while the alicyclic moieties contained in these substituents each
comprise 3 to 10 ring members and the heteroalicyclic moieties
each comprise 4 to 10 ring members and
the phenyl and pyridinyl groups contained in the abovementioned
groups may in turn be mono-, di- or trisubstituted by fluorine,
chlorine, bromine or iodine atoms, by alkyl, alkoxy,
methylsulphonyloxy, difluoromethyl, trifluoromethyl, hydroxy,
amino, acetylamino, aminocarbonyl, aminocarbonylamino,
aminocarbonylaminomethyl, cyano, carboxy, alkoxycarbonyl,
carboxyalkyl, alkoxycarbonylalkyl, alkanoyl, c~-
CA 02503455 2005-04-22
7
(dialkylamino)alkanoyl, w-(carboxy)alkanoyl, difluoromethaxy,
trifluoromethoxy, trifluoromethylthio, trifluoromethylsulphinyl or
trifluoromethylsulphonyl groups, while the substituents may be
identical or different,
R5 denotes a hydrogen atom,
a C~_4-alkyl group , while an unbranched alkyl group may be substituted
in the w position by a phenyl, pyridinyl, diazinyl, amino, alkylamino,
dialkylamino, 1-pyrrolidinyl, 1-piperidinyl, 4-methyl-1-piperazinyl,
4-morpholinyl or hexahydro-1H-1-azepinyl group,
an alkoxycarbonyl, the cyano or aminocarbonyl group or also, if Y'
denotes a nitrogen atom, a pair of free electrons,
or, if Y' does not denote a nitrogen atom, also the fluorine atom, or
R4 and R5 together, if Y' denotes the carbon atom, denote a 4- to 7-
membered cycloaliphatic ring in which one or two methylene groups may
be replaced by an -NH- or -N(alkyl)- group and one or two additional
methylene groups may be replaced by carbonyl groups,
while a hydrogen atom bound to a nitrogen atom within the
abovementioned group R4 may be replaced by a protecting group,
R6 and R', which may be identical or different, in each case denote a
hydrogen atom, a C~_3-alkyl or dialkylamino group or also, if Y' does not
denote a nitrogen atom, the fluorine atom and
R$ and R9, which may be identical or different, each denote a hydrogen
atom or a C,_3-alkyl, carboxy or C~_3-alkoxycarbonyl group,
while, unless otherwise stated, all the abovementioned alkyl and alkoxy
CA 02503455 2005-04-22
groups as well as the alkyl groups present within the other groups specified
comprise 1 to 7 carbon atoms and may be straight-chain or branched, while
each methylene group may be substituted by up to 2 fluorine atoms and each
methyl group may be substituted by up to 3 fluorine atoms,
all the abovementioned cycloalkyl groups as well as the cycloalkyl groups
present within the other groups specified, unless otherwise stated, may
comprise 3 to 10 carbon atoms, while each methylene group may be
substituted by up to 2 fluorine atoms,
all the abovementioned aromatic and heteroaromatic groups may additionally
be mono- di- or trisubstituted by fluorine, chlorine or bromine atoms, by
cyano
or hydroxy groups and the substituents may be identical or different and
by the protective groups mentioned in the foregoing and subsequent
definitions are meant the protective groups familiar from peptide chemistry,
particularly
a phenylalkoxycarbonyl group with 1 to 3 carbon atoms in the alkoxy moiety
optionally substituted in the phenyl nucleus by a halogen atom, by a nitro or
phenyl group or by one or two methoxy groups,
for example the benzyloxycarbonyl, 2-vitro-benzyloxycarbonyl,
4-vitro-benzyloxycarbonyl, 4-methoxy-benzyloxycarbonyl, 2-chloro-
benzyloxycarbonyl, 3-chloro-benzyloxycarbonyl, 4-chloro-
benzyloxycarbonyl, 4-biphenylyl-a,a-dimethyl-benzyloxycarbonyl or
3,5-dimethoxy-a,a-dimethyl-benzyloxycarbonyl group,
an alkoxycarbonyl group with a total of 1 to 5 carbon atoms in the alkyl
moiety,
for example the methoxycarbonyl, ethoxycarbonyl, n-propoxycarbonyl,
isopropoxycarbonyl, n-butoxycarbonyl, 1-methylpropoxycarbonyl,
CA 02503455 2005-04-22
9
2-methylpropoxy-carbonyl or tert.butyloxycarbonyl group,
the allyloxycarbonyl, 2,2,2-trichloro-(1,1-dimethylethoxy)carbonyl or 9-
fluorenylmethoxycarbonyl group or
the formyl, acetyl or trifluoroacetyl group.
A second embodiment of the present invention comprises the compounds of
the above general formula (I), wherein
A, U, V, W, X, R2 and R3 are defined as mentioned in the first embodiment
hereinbefore and
R' denotes a mono- or diunsaturated 5- to 7-membered aza, diaza, triaza or
thiaza heterocyclic group,
in which the abovementioned heterocycles are linked via a carbon or
nitrogen atom,
contain one or two carbonyl groups adjacent to a nitrogen atom,
may be substituted at a carbon atom by a phenyl, pyridinyl, diazinyl,
thienyl, pyrrolyl, 1,3-thiazolyl, isoxazolyl, pyrazolyl or 1-methylpyrazolyl
group and
an olefinic double bond of one of the abovementioned unsaturated
heterocycles may be fused to a phenyl, naphthyl, pyridine, diazine,
thienyl or quinoline ring or to a 1H-quinolin-2-one ring optionally
substituted at the nitrogen atom by a methyl group,
while the phenyl, pyridinyl, diazinyl, thienyl, pyrrolyl, 1,3-thiazolyl,
isoxazolyl, pyrazolyl or 1-methylpyrazolyl groups contained in R' as
well as the benzo-, pyrido- and diazino-fused heterocycles in the
carbon skeleton may additionally be mono-, di- or trisubstituted by
CA 02503455 2005-04-22
fluorine, chlorine, bromine or iodine atoms, by alkyl, alkoxy, nitro,
difluoromethyl, trifluoromethyl, hydroxy, amino, alkylamino, dialkyl-
amino, acetylamino, acetyl, cyano, difluoromethoxy or
trifluoromethoxy groups, while the substituents may be identical or
different,
while the abovementioned alkyl groups or the alkyl groups contained in the
abovementioned groups, unless otherwise stated, contain 1 to 7 carbon
atoms and may be branched or unbranched, while each methylene group may
be substituted by up to 2 fluorine atoms and each methyl group may be
substituted by up to 3 fluorine atoms, and
the abovementioned aromatic and heteroaromatic groups may additionally be
mono-, di- or trisubstituted by fluorine, chlorine or bromine atoms or by
cyano
or hydroxy groups and the substituents may be identical or different.
A third embodiment of the present invention comprises the compounds of the
above general formula fl), wherein
A, U, V, W, X, R2 and R3 are defined as mentioned in the first embodiment
and
R' denotes a monounsaturated 5- to 7-membered diaza or triaza heterocyclic
group,
while the abovementioned heterocycles are linked via a nitrogen atom,
contain a carbonyl group adjacent to a nitrogen atom and
may additionally be substituted at a carbon atom by a phenyl group,
and while an olefinic double bond of one of the abovementioned
unsaturated heterocycles may be fused to a phenyl, thienyl or quinoline
ring,
CA 02503455 2005-04-22
ll
while the phenyl groups contained in R' as well as benzo-fused
heterocycles in the carbon skeleton may additionally be mono-, di- or
trisubstituted by fluorine, chlorine, bromine or iodine atoms, by methyl,
methoxy, nitro, difluoromethyl, trifluoromethyl, hydroxy, amino,
alkylamino, dialkylamino, acetylamino, acetyl, cyano, difluoromethoxy
or trifluoromethoxy groups, while the substituents may be identical or
different, but are preferably unsubstituted, or monosubstituted by a
fluorine, chlorine or bromine atom or by a methyl or methoxy group,
white, unless otherwise stated, all the abovementioned alkyl groups as well as
the alkyl groups present within the other groups comprise 1 to 7 carbon
atoms and may be straight-chain or branched and the abovementioned
aromatic and heteroaromatic groups may additionally be mono- di- or
trisubstituted by fluorine, chlorine or bromine atoms or by cyano or hydroxy
groups and the substituents may be identical or different.
A fourth embodiment of the present invention comprises the compounds of
the above general formula (I), wherein
A, U, V, W, X, R2 and R3 are defined as mentioned in the first embodiment
and
R' denotes a 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
yl, 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl, 4-(5-oxo-3-phenyl-
4,5-dihydro-1,2,4-triazol-1-yl)-piperidin-1-yl, 4-(2-oxo-1,2-dihydro-
imidazo[4,5-
c]quinolin-3-yl)-piperidin-1-yl, 4-(2-oxo-1,2-dihydro-4H-thieno[3,4-
d]pyrimidin-
3-yl)-piperidin-1-yl, 4-(2-oxo-1,4-dihydro-2H-thieno[3,2-d]pyrimidin-3-yl)-
piperidin-1-yl, 4-(5-oxo-4,5,7,8-tetrahydro-2-this-4,6-diaza-azulen-6-yl)-
piperidin-1-yl, 4-(2-oxo-1,2,4,5-tetrahydro-thieno[3,2-d]-1,3-diazepin-3-yl)-
piperidin-1-yl, 4-(2-oxo-1,2,4,5-tetrahydro-thieno[2,3-d]-1,3-diazepin-3-yl)-
piperidin-1-yl or 4-(2-oxo-1,4-dihydro-2H-thieno[2,3-d]pyrimidin-3-yl)-
piperidin-
1-yl group,
CA 02503455 2005-04-22
12
while the abovementioned mono- and bicyclic heterocycles in the carbon
skeleton may additionally be monosubstituted by a methoxy group,
while the abovementioned aromatic and heteroaromatic groups by fluorine,
chlorine or bromine atoms, by cyano or hydroxy groups may additionally be
mono- di- or trisubstituted and the substituents may be identical or
different.
A fifth embodiment of the present invention comprises the compounds of the
above general formula (I), wherein
A, U, V, W, X and R' are defined as mentioned in the first embodiment and
R2 denotes the hydrogen atom or
a phenylmethyl group or a C2_~-alkyl group which may be substituted in the w
position by a phenyl, pyridinyl, hydroxy, amino, alkylamino, dialkylamino,
carboxy, alkoxycarbonyl, aminocarbonyl, aminocarbonylamino, acetylamino,
1-pyrrolidinyl, 1-piperidinyl, 4-morpholinyl, [bis-(2-hydroxyethyl)]amino
group
while the abovementioned heterocyclic groups and phenyl groups may
additionally be mono-, di- or trisubstituted in the carbon skeleton by
fluorine, chlorine, bromine or iodine atoms, by methyl, alkoxy,
difluoromethyl, trifluoromethyl, hydroxy, amino, C,_3-alkylamino, di-
(C~_3-alkyl)-amino, acetylamino, aminocarbonyl, cyano, difluoromethoxy,
trifluoromethoxy, amino-C~_3-alkyl, C~_3-alkylamino-C~_3-alkyl or
di-(C~_3-alkyl)-amino-C~_3-alkyl groups and the substituents may be
identical or different,
R3 denotes the hydrogen atom or a C~_3-alkyl group,
while the C~_3-alkyl group may be linked to an alkyl group present in R2
or a phenyl or pyridyl ring present in R2 and the nitrogen atom to which
they are bound, forming a 5- to 7-membered ring, or
CA 02503455 2005-04-22
13
R2 and R3 together with the enclosed nitrogen atom denote a group of general
formula
R5
(CRS R9)q
Y'
CR6R
N ~ (CR8R9)~ ~CR R Ra
6 7 ~ (ll)
wherein
Y' denotes the carbon atom or, if R5 denotes a pair of free electrons, it
may also denote the nitrogen atom,
q and r, if Y' denotes the carbon atom, represent the numbers 0 or 1 or
q and r, if Y' denotes the nitrogen atom, represent the numbers 1 or 2,
R4 denotes the hydrogen atom, a hydroxy, amino, alkylamino, C3.~-cyclo-
alkyfamino, N-(C3_6-cycloalkyl)-alkylamino or dialkylamino, an alkyl,
trifluoromethyl, C3_6-cycloalkyl, dialkylamino-C2_~-alkyl, carboxyalkyl,
alkoxycarbonylalkyl, alkylsulphonyl, alkylsulphonylamino or
N-(alkylsulphonyl)-alkylamino group,
or, if Y' does not denote the nitrogen atom, it denotes the carboxy or
dialkylaminomethyl group,
a phenyl, phenyl-C~_3-alkyl, pyridinyl or diazinyl group each of which may
be substituted by a fluorine, chlorine or bromine atom or by a
trifluoromethylcarbonyl, methyl or methoxy group,
a saturated or mono- or polyunsaturated 4- to 7-membered azacycloalkyl
CA 02503455 2005-04-22
74
group, a 5- to 7-membered oxaza-, diaza or triazacycloalkyl group, a 7- to
9-membered azabicyclo or diazabicycloalkyl group, a 1-alkyl-
4-piperidinylamino or 1-alkyl-4-piperidinylaminosulphonyl group,
while the abovementioned mono- and bicyclic heterocycles are
bound via a nitrogen or carbon atom,
a methylene group of the abovementioned mono- and bicyclic
heterocycles may be replaced by a carbonyl or sulphonyl group,
in the abovementioned mono- and bicyclic heterocycles any
methylene group not directly bound to a nitrogen, oxygen or sulphur
atom may be substituted by one or two fluorine atoms,
the abovementioned mono- and bicyclic heterocycles may be
substituted by one or two C~_3-alkyl groups wherein each methyiene
group may be substituted by up to 2 fluorine atoms and each methyl
group may be substituted by up to 3 fluorine atoms, and/or
by a C3_6-cycloalkyl-C,_3-alkyl, benzyl, C~_4-alkanoyl, di-(C~_3-alkyl)-
amino or C~_3-alkylsulphonyl, by an alkoxycarbonyl,
benzyloxycarbonyl, aikoxycarbonylalkyl, carboxy or carboxyalkyl
group,
R5 denotes a hydrogen atom, a C~_3-alkyl or alkoxycarbonyl group or,
if Y' denotes a nitrogen atom, it may also denote a pair of free electrons,
Or
R4 and R5 together, if Y' denotes the carbon atom, represent a 5- to 6-
membered cycloaliphatic ring in which one or two methylene groups may
be replaced by a -NH or -N(methyl) group and one or two further
methylene groups may be replaced by carbonyl groups,
CA 02503455 2005-04-22
R6 and R', which may be identical or different, in each case denote a
hydrogen atom or a C,_3-alkyl or di-(C,_3-alkyl)-amino group and
R$ and R9, which may be identical or different, in each case denote a
hydrogen atom or a C,_3-alkyl, carboxy or C~_3-alkoxycarbonyl group,
while, unless otherwise stated, all the abovementioned alkyl groups as well as
the alkyl groups present within the other groups comprise 1 to 7 carbon
atoms and may be straight-chain or branched and the abovementioned
aromatic and heteroaromatic groups may additionally be mono-, di- or
trisubstituted by fluorine, chlorine or bromine atoms or by cyano or hydroxy
groups and the substituents may be identical or different.
A sixth embodiment of the present invention comprises the compounds of the
above general formula (I), wherein
A, U, V, W, X and R' are defined as mentioned in the first embodiment and
R2 denotes a phenylmethyl group or a C2_~-alkyl group which may be
substituted in the w position by a phenyl, amino, alkylamino or dialkylamino
group,
while the abovementioned phenyl group may be substituted by an amino-
C,_3-alkyl, C,_3-alkylamino-C~_3alkyl or di-(C~_3-alkyl)-amino-C,_3-alkyl
group, or
R3 denotes the hydrogen atom or a C~_3-alkyl group,
R2 and R3 together with the nitrogen atom to which they are bound denote a
7-dimethylaminomethyl-1,2,4,5-tetrahydro-3-benzazepin-3-yl or 2-amino-
4,5,7,8-tetrahydro-thiazolo[4,5-d]azepin-6-yl- group or
R2 and R3 together with the enclosed nitrogen atom denote a group of general
CA 02503455 2005-04-22
l6
formula
R5
~CR~9)9
Y'
CR6R
N ~ ~CR8R9~~'CR R Ra
s ~ , yl)
wherein
Y' denotes the carbon atom or, if R5 denotes a pair of free electrons, it
may also denote the nitrogen atom,
q and r, if Y' denotes the carbon atom, represent the numbers 0 or 1 or
q and r, if Y' denotes the nitrogen atom, represent the numbers 1 or 2,
R4 denotes the hydrogen atom,
a phenyl, benzyl or pyridinyl group which may be substituted in each case
by a fluorine, chlorine or bromine atom, by a trifluoromethylcarbonyl,
methyl or methoxy group,
a hydroxy, carboxy, methyl, trifluoromethyl, n-propyi, phenyl, p-tolyl,
p-trifluoromethylcarbonyl-phenyl, p-(3-dimethylaminopropyl)-phenyl,
amino, benzyl, tent-butylamino, dimethylamino, diethylamino,
diethylaminomethyl, 2-dimethylaminoethyl, 2-diethylaminoethyl, 5-
aminopentyl, methoxycarbonyl, methoxycarbonylmethyl, perhydro-azepin-
1-yl, 4-methyl-perhydro-1,4-diazepin-1-yl, 1-methyl-1-piperidinyl-4-yl, 4-
piperazin-1-yl, 4-acetyl-piperazin-1-yl, 4-cyclopropylmethyl-piperazin-1-yl,
pyrrolidin-1-yl, 4-ethyl-piperazin-1-yl, 4-isopropyl-piperazin-1-yl, piperidin-
1-yl, piperidin-4-yl, morpholin-4-yl, 4,4-difluoro-1-piperidin-1-yl, 1-methyl-
1-aza-bicyclo[3.2.1]oct-4-yl or 4-methyl-piperazin-1-yl, 4-ethylpiperazin-
CA 02503455 2005-04-22
17
1-yl, 1-methyl-piperidin-1-yl, 4-carboxymethyl-piperazin-1-yl,
1-carboxymethyl-piperidin-4-yl, 4-benzyloxycarbonyl-piperazin-1-yl,
1-ethoxycarbonylmethyl-piperidin-4-yl, azetidin-1-yl, 5-methyl-2,5-diaza-
bicyclo[2.2.1]hept-2-yl, 1-benzyl-piperidin-4-yl, 4-benzyl-piperazin-1-yl,
4-dimethylaminomethyl-1-phenyl, 2,2,2-trifluoroethyl-piperazin-1-yl,
1-methylsulphonyl-piperidin-4-yl, piperidin-1-yl-methyl, 1-methyl-piperidin-
4-yl-amino, methylsulphonylamino, N-methylsulphonyl-N-methylamino,
N-(cyclopentyl)-methylamino, 1,1-dioxo-~,6-isothiazolidin-2-yl, 2-oxo-
perhydro-1,3-oxazin-3-yl, cyclohexyl, 2-oxo-imidazolidin-1-yl, 2-methyl-
imidazol-1-yl, 4-methyl-imidazol-1-yl, 4-thiazol-2-yl, 2,4-dimethyl-imidazol-
1-yl, 4-imidazol-1-yl, 1,2,4-triazol-1-yl, 1-aza-bicyclo[2.2.2]oct-3-yl,
1-methyl-piperidin-4-yl-methylsulphonyl, 1H-imidazol-4-yl,
4-ethoxycarbonylmethyl-piperazin-1-yl, 1-ethoxycarbonyl-piperidin-4-yl,
4-tert-butoxycarbonylmethyl-piperazin-1-yl, 1-(2,2,2-trifluoroethyl)-
piperidin-4-yl, 4-methylsulphonyl-piperazin-1-yl, 2-carboxy-4-methyl-
piperazin-1-yl, 3-carboxy-4-methyl-piperazin-1-yl, 2-ethoxycarbonyl-4-
methyl-piperazin-1-yl, 3-ethoxycarbonyl-4-methyl-piperazin-1-yl or
4-(2,2,2-trifluoroethyl)-piperazin-1-yl- group,
R5 denotes a hydrogen atom, a methyl group or, if Y' denotes a nitrogen
atom, it may also denote a pair of free electrons, or
R4 and R5 together, if Y' denotes the carbon atom, denote a 1-methyl-
piperidin-4-ylidene, cyclohexylidene or imidazolidin-2,4-dion-5-ylidene
group,
Rs and R' in each case denote a hydrogen atom or a dimethylamino
group and
R8 and R9 in each case denote the hydrogen atom, a carboxy or
ethoxycarbonyl group,
while, unless otherwise stated, all the abovementioned alkyl groups as well as
CA 02503455 2005-04-22
18
the alkyl groups present within the other groups comprise 1 to 7 carbon
atoms and may be straight-chain or branched and the abovementioned
aromatic and heteroaromatic groups may additionally be mono-, di- or
trisubstituted by fluorine, chlorine or bromine atoms, by cyano or hydroxy
groups and the substituents may be identical or different,
while in all the embodiments mentioned above those compounds wherein
(i) A denotes an oxygen atom, a cyanoimino or phenylsulphonylimino
group,
X denotes an oxygen atom, an imino or methylene group,
U denotes an unbranched C,~-alkyl, C2_4-alkenyl or C2_4-alkynyl group
wherein each methylene group may be substituted by up to 2 fluorine
atoms and the methyl group may be substituted by up to 3 fluorine
atoms,
V denotes an amino or hydroxy group and
W denotes a hydrogen, chlorine or bromine atom or a trifluoromethyl
group,
are of exceptional importance,
those compounds wherein
(ii) A denotes an oxygen atom,
X denotes an oxygen atom, an imino or methylene group,
U denotes a methyl, ethyl, C2.~-alkenyl or C2_4-alkynyl group wherein
the methylene group may be substituted by up to 2 fluorine atoms and
the methyl group may be substituted by up to 3 fluorine atoms,
CA 02503455 2005-04-22
19
V denotes an amino or hydroxy group and
W denotes a hydrogen, chlorine or bromine atom or a trifluoromethyl
group,
are of particularly outstanding importance and
those compounds wherein
(iii) A denotes an oxygen atom,
X denotes an oxygen atom, an imino or methyfene group,
U denotes a trifluoromethyl or pentafluoroethyl group,
V denotes an amino or hydroxy group and
W denotes a hydrogen, chlorine or bromine atom or a trifluoromethyl
group,
are of most particularly outstanding importance.
A seventh embodiment of the present invention comprises the compounds of
the above general formula (I) wherein
A denotes an oxygen atom, a cyanoimino or phenylsulphonylimino group,
X denotes an oxygen atom, an imino or methylene group,
U denotes an unbranched C,~-alkyl, Cz_6-alkenyl or C2_6-alkynyl group
wherein each methylene group may be substituted by up to 2 fluorine atoms
and the methyl group may be substituted by up to 3 fluorine atoms,
CA 02503455 2005-04-22
V denotes an amino or hydroxy group,
W denotes a hydrogen, chlorine or bromine atom or a trifluoromethyl group,
R' denotes a monounsaturated 5- to 7-membered diaza or triaza heterocyclic
group,
while the abovementioned heterocycles are linked via a nitrogen atom,
contain a carbonyl group adjacent to a nitrogen atom,
may additionally be substituted at a carbon atom by a phenyl group and
an olefinic double bond of one of the abovementioned unsaturated
heterocycles may be fused to a phenyl, thienyl or quinoline ring,
while the phenyl groups contained in R~ as well as benzo-fused
heterocycles in the carbon skeleton may additionally be mono-, di- or
trisubstituted by fluorine, chlorine, bromine or iodine atoms, by methyl,
methoxy, nitro, difluoromethyl, trifluoromethyl, hydroxy, amino,
alkylamino, dialkylamino, acetylamino, acetyl, cyano, difluoromethoxy
or trifluoromethoxy groups, while the substituents may be identical or
different, but are preferably unsubstituted or are monosubstituted by a
fluorine, chlorine or bromine atom or by a methyl or methoxy group,
R2 denotes the hydrogen atom or
a phenylmethyl group or a C2_~-alkyl group which may be substituted in the w
position by a phenyl, pyridinyl, hydroxy, amino, alkylamino, dialkylamino,
alkoxycarbonyl, carboxy, aminocarbonyl, aminocarbonylamino, acetylamino,
1-pyrrolidinyl, 1-piperidinyl, 4-morpholinyl or [bis-(2-hydroxyethyl)Jamino
group,
CA 02503455 2005-04-22
21
while the abovementioned heterocyclic groups and phenyl groups may
additionally be mono-, di- or trisubstituted in the carbon skeleton by
fluorine, chlorine, bromine or iodine atoms, by methyl, alkoxy,
difluoromethyl, trifluoromethyl, hydroxy, amino, C~_3-alkylamino, di-
(C~_3-alkyl)-amino, acetylamino, aminocarbonyl, cyano, difluoromethoxy,
trifluoromethoxy, amino-C,_3-alkyl, C,_3-alkylamino-C,_3-alkyl or
di-(C~_3-alkyl)-amino-C~_3-alkyl groups and the substituents may be
identical or different,
R3 denotes the hydrogen atom or a C~_3-alkyl group,
while the C,-3-alkyl group may be linked to an alkyl group present in R2 or
a phenyl or pyridyl ring present in R2 and the nitrogen atom to which they
are bound, forming a 5- to 7-membered ring, or
R2 and R3 together with the enclosed nitrogen atom denote a group of general
formula
R5
(CR 9)q
Y'
CR6R
N,(CR8R9)~'CR R Ra
s ~ , (II)
wherein
Y' denotes the carbon atom or, if R5 denotes a pair of free electrons, it
may also denote the nitrogen atom,
q and r, if Y' denotes the carbon atom, represent the numbers 0 or 1 or
q and r, if Y' denotes the nitrogen atom, represent the numbers 1 or 2,
CA 02503455 2005-04-22
22
R4 denotes the hydrogen atom, a hydroxy, amino, alkylamino, C3_6-cyclo-
alkylamino, N-(C3_6-cycloalkyl)-alkylamino or dialkylamino, an alkyl,
trifluoromethyl, C3_6-cycloalkyl, dialkylamino-C2_7-alkyl, carboxyalkyl,
alkoxycarbonylalkyl, alkylsulphonyl, alkylsulphonylamino or
N-(alkylsulphonyl)-alkylamino group,
or, if Y' does not denote the nitrogen atom, it denotes the carboxy or
dialkylaminomethyl group,
a phenyl, phenyl-C~_3-alkyl, pyridinyl or diazinyl group which may be
substituted in each case by a fluorine, chlorine or bromine atom, by a
trifluoromethylcarbonyl, methyl or methoxy group,
a saturated or mono- or polyunsaturated 4- to 7-membered azacycloalkyl
group, a 5- to 7-membered oxaza-, diaza- or triazacycloalkyl group, a 7-
to 9-membered azabicyclo- or diazabicycloalkyl group, a 1-alkyl-
4-piperidinylamino or 1-alkyl-4-piperidinylaminosulphonyl group,
while the abovementioned mono- and bicyclic heterocycles are
bound via a nitrogen or carbon atom,
a methylene group of the abovementioned mono- and bicyclic
heterocycles may be replaced by a carbonyl or sulphonyl group,
in the abovementioned mono- and bicyclic heterocycles any
methylene group not directly bound to a nitrogen, oxygen or sulphur
atom may be substituted by one or two fluorine atoms,
the abovementioned mono- and bicyclic heterocycles may be
substituted by one or two C~_3-alkyl groups, wherein each methylene
group may be substituted by up to 2 fluorine atoms and each methyl
group may be substituted by up to 3 fluorine atoms, and/or
may be substituted by a C3_6-cycloalkyl-C~_3-alkyl, benzyl,
CA 02503455 2005-04-22
23
C~_4-alkanoyl, di-(C~_3-alkyl)-amino or C~,3 alkyisulphonyl, by an
alkoxycarbonyl, benzyloxycarbonyl, alkoxycarbonylalkyl, carboxy or
carboxyalkyl group,
R5 denotes a hydrogen atom, a C~_3-alkyl or alkoxycarbonyl group or,
if Y' denotes a nitrogen atom, it may also denote a pair of free electrons,
or
R4 and R5 together, if Y' denotes the carbon atom, denote a 5- to 6-
membered cycloaliphatic ring wherein one or two methylene groups may
be replaced by a -NH or -N(methyl) group and one or two further
methylene groups may be replaced by one or two carbonyl groups,
R6 and R', which may be identical or different, in each case denote the
hydrogen atom or a C~_3-alkyl or di-(C,_3-alkyl)-amino group and
R$ and R9, which may be identical or different, in each case denote the
hydrogen atom or a C,_3-alkyl, carboxy or C~_3-alkoxycarbonyl group,
while, unless otherwise stated, the abovementioned alkyl groups or the alkyl
groups contained in the abovementioned groups contain 1 to 7 carbon atoms
and may be branched or unbranched and the abovementioned aromatic and
heteroaromatic groups may additionally be mono-, di- or trisubstituted by
fluorine, chlorine or bromine atoms, by cyano or hydroxy groups and the
substituents may be identical or different.
An eighth embodiment of the present invention comprises the compounds of
the above general formula (I), wherein
A denotes an oxygen atom,
X denotes an oxygen atom, an imino or methylene group,
CA 02503455 2005-04-22
24
U denotes a methyl, ethyl, C2_4-alkenyl or C2_4-alkynyl group wherein the
methylene group may be substituted by up to 2 fluorine atoms and the methyl
group may be substituted by up to 3 fluorine atoms,
V denotes an amino or hydroxy group,
W denotes a hydrogen, chlorine or bromine atom or a trifluoromethyl group,
R' denotes a 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
yl, 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl, 4-(5-oxo-3-phenyl-
4,5-dihydro-1,2,4-triazol-1-yl)-piperidin-1-yl, 4-(2-oxo-1,2-dihydro-
imidazo[4,5-
cJquinolin-3-yl)-piperidin-1-yl, 4-(2-oxo-1,2-dihydro-4H-thieno[3,4-
d]pyrimidin-
3-yl)-piperidin-1-yl, 4-(2-oxo-1,4-dihydro-2H-thieno[3,2-d]pyrimidin-3-yl)-
piperidin-1-yl, 4-(5-oxo-4,5,7,8-tetrahydro-2-this-4,6-diaza-azulen-6-yl)-
piperidin-1-yl, 4-(2-oxo-1,2,4,5-tetrahydro-thieno[3,2-d]-1,3-diazepin-3-yl)-
piperidin-1-yl, 4-(2-oxo-1,2,4,5-tetrahydro-thieno[2,3-d]-1,3-diazepin-3-yl)-
piperidin-1-yl or 4-(2-oxo-1,4-dihydro-2H-thieno[2,3-dJpyrimidin-3-yl)-
piperidin-
1-yl group,
while the abovementioned mono- and bicyclic heterocycles in the carbon
skeleton may additionally be monosubstituted by a methoxy group,
R2 denotes a phenylmethyl group or a C2_~-alkyl group which may be
substituted in the c~ position by a phenyl, amino, alkylamino or dialkylamino
group,
while the abovementioned phenyl group may be substituted by an amino-
C~_3-alkyl, C~_3-alkylamino-C,_3-alkyl or di-(C~_3-alkyl)-amino-C,_3-alkyl
group, or
R3 denotes the hydrogen atom or a C~_3-alkyl group,
CA 02503455 2005-04-22
R2 and R3 together with the nitrogen atom to which they are bound denote a
7-dimethylaminomethyl-1,2,4,5-tetrahydro-3-benzazepin-3-yl or 2-amino-
4,5,7,8-tetrahydro-thiazolo[4,5-d]azepin-6-yl- group or
R2 and R3 together with the enclosed nitrogen atom denote a group of general
formula
R5
(CR 9)a
Y'
CRsR
N ~ (CR8R9)~ ~CR R Ra
(III)
wherein
Y' represents the carbon atom or, if R5 denotes a pair of free electrons, it
may also denote the nitrogen atom,
q and r, if Y' denotes the carbon atom, represent the numbers 0 or 1 or
q and r, if Y' denotes the nitrogen atom, represent the numbers 1 or 2,
R4 denotes the hydrogen atom,
a phenyl, benzyl or pyridinyl group which may be substituted in each case
by a fluorine, chlorine or bromine atom, by a trifluoromethylcarbonyi,
methyl or methoxy group,
a hydroxy, carboxy, methyl, trifluoromethyl, n-propyl, phenyl, p-tolyl,
p-trifluoromethylcarbonyl-phenyl, p-(3-dimethylaminopropyl)-phenyl,
amino, benzyl, tent-butylamino, dimethylamino, diethylamino,
diethyfaminomethyl, 2-dimethylaminoethyi, 2-diethylaminoethyl, 5-
aminopentyl, methoxycarbonyl, methoxycarbonylmethyl, perhydro-azepin-
CA 02503455 2005-04-22
26
1-yl, 4-methyl-perhydro-1,4-diazepin-1-yl, 1-methyl-1-piperidinyl-4-yl, 4-
piperazin-1-yl, 4-acetyl-piperazin-1-yl, 4-cyclopropylmethyl-piperazin-1-yl,
pyrrolidin-1-yl, 4-ethyl-piperazin-1-yl, 4-isopropyl-piperazin-1-yl, piperidin-
1-yl, piperidin-4-yl, morpholin-4-yl, 4,4-difluoro-1-piperidin-1-yl, 1-methyl-
1-aza-bicyclo[3.2.1]oct-4-yl, 4-methyl-piperazin-1-yl, 4-ethylpiperazin-1-yl,
1-methyl-piperidin-1-yi, 4-carboxymethyl-piperazin-1-yl, 1-carboxymethyl-
piperidin-4-yl, 4-benzyloxycarbonyl-piperazin-1-yl,
1-ethoxycarbonylmethyl-piperidin-4-yl, azetidin-1-yl, 5-methyl-2,5-diaza-
bicyclo[2.2.1]hept-2-yl, 1-benzyl-piperidin-4-yl, 4-benzyl-piperazin-1-yl,
4-dimethylaminomethyl-1-phenyl, 2,2,2-trifluoroethyl-piperazin-1-yl,
1-methylsulphonyl-piperidin-4-yl, piperidin-1-yl-methyl, 1-methyl-piperidin-
4-yl-amino, methylsulphonylamino, N-methylsulphonyl-N-methylamino,
N-(cyclopentyl)-methylamino, 1,1-dioxo-~.6-isothiazolidin-2-yl, 2-oxo-
perhydro-1,3-oxazin-3-yl, cyclohexyl, 2-oxo-imidazolidin-1-yl, 2-methyl-
imidazol-1-yl, 4-methyl-imidazol-1-yl, 4-thiazol-2-yl, 2,4-dimethyl-imidazol-
1-yl, 4-imidazol-1-yl, 1,2,4-triazol-1-yl, 1-aza-bicyclo[2.2.2]oct-3-yl,
1-methyl-piperidin-4-yl-methylsulphonyl, 1 H-imidazol-4-yi,
4-ethoxycarbonylmethyl-piperazin-1-yl, 1-ethoxycarbonyl-piperidin-4-yl,
4-tert-butoxycarbonylmethyl-piperazin-1-yl, 1-(2,2,2-trifluoroethyl)-
piperidin-4-yl, 4-methylsulphonyl-piperazin-1-yl, 2-carboxy-4-methyl-
piperazin-1-yl, 3-carboxy-4-methyl-piperazin-1-yl, 2-ethoxycarbonyl-4-
methyl-piperazin-1-yl, 3-ethoxycarbonyl-4-methyl-piperazin-1-yl or
4-(2,2,2-trifluoroethyl)-piperazin-1-yl group,
RS denotes a hydrogen atom, a methyl group or, if Y' denotes a nitrogen
atom, it may also denote a pair of free electrons, or
R4 and R5 together, if Y' denotes the carbon atom, denote a 1-methyl-
piperidin-4-yiidene, cyclohexylidene or imidazolidin-2,4-dion-5-ylidene
group,
R6 and R' in each case denote a hydrogen atom or a dimethylamino
group and
CA 02503455 2005-04-22
27
R8 and R9 in each case denote the hydrogen atom, a carboxy or
ethoxycarbonyl group,
while, unless otherwise stated, all the abovementioned alkyl groups as well as
the alkyl groups present within the other groups comprise 1 to 7 carbon
atoms and may be straight-chain or branched and the abovementioned
aromatic and heteroaromatic groups may additionally be mono-, di- or
trisubstituted by fluorine, chlorine or bromine atoms or by cyano or hydroxy
groups and the substituents may be identical or different.
The following are mentioned as examples of most particularly preferred
compounds of the above general formula (I):
Structure f Name
LCl_7_/d_aminn-~_rhlnrn_~,_
-. - , _ _
\ , trifluoromethyl-benzyl)-1-[4-(4-
NF
_ methyl-piperazin-1-yl)-piperidin-1-
(1)
~
;
~~N~N~N~N~H,
N yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-
"
1, 3-benzodiazepin-3-yl)-piperidin-1-
yl]-butan-1,4-dione
F
HzN 2-(4-amino-3-chloro-5-
~
F
trifiuoromethyl-benzyl)-1-(
1'-methyl-
'
[4,4
]bipiperidinyl-1-yl)-4-[4-(2-oxo-
~' 1,4-dihydro-2H-quinazolin-3-yl)-
N~
piperidin-1-yl]-butan-1,4-dione
;__', 2-(4-amino-3-chloro-5-
' trifluoromethyl-benzyl)-1-[4-(4-
methyl-piperazin-1-yl)-piperidin-1-
(3)
'
yl)-4-[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
F-?~
2-(4-a m i n o-3-ch l o
~~a ro-5-
~ trifluoromethyl-benzyl)-1-
' '
'
(4) ~,~~~ [1,4
~ ]bipiperidinyl-1
-yl-4-[4-(2-oxo-
1,4-dihydro-2H-quinazolin-3-yl)-
piperidin-1-yl]-butan-1,4-dione
CA 02503455 2005-04-22
28
Structure Name
~N
rN~ 2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl)-4-[4-(2-oxo-
(5) ~N ~ ; F 1,4-dihydro-2H-quinazolin-3-yl)-
" NH.F ' piperidin-1-yl]-1-(4-pyridin-4-yl-
piperazin-1-yl)-butan-1,4-dione
N~ 2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl)-1-[4-(1-
methyl-piperidin-4-ylamino)-
6 N'G
( ) " ~
~
'
U
' piperidin-1-yl]-4-[4-(2-oxo-1,4-
~
dihydro-2H-quinazolin-3-yl)-
piperidin-1-yl]-butan-1,4-dione
FF NH.
F , ~ ~G [4-(1-{2-(4-amino-3-chloro-5-
H
~N~ trifluoromethyl-benzyl)-4-oxo-4-[4-
~ ~NJ
N
~" (2-oxo-1,4-dihydro-2H-quinazolin-3-
I , N yl)-piperidin-1-yl]-butyryl}-piperidin-
H
4-yl)-piperazin-1-yl]-acetic
acid
F F NH,
F , ~ _~ methyl (1'-{2-(4-amino-3-chloro-5-
,
N~ trifluoromethyl-benzyl)-4-oxo-4-[4-
'",
~
N N (2-oxo-1,4-dihydro-2H-quinazolin-3-
' l
~
~ N y
)-piperidin-1-yl]-butyryl}-
"
[4,4']bipiperidinyl-1-yl)-acetate
F F NH,
F , ~ ~ , (1'-{2-(4-amino-3-chloro-5-
N~ H
trifluoromethyl-benzyl)-4-oxo-4-[4-
~
N N (2-oxo-1,4-dihydro-2H-quinazolin-3-
~ ~ N~~ yl)-piperidin-1-yl]-butyryl}-
" [4,4']bipiperidinyl-1-yl)-acetic
acid
~N FFF ~s". 4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-
~
carbox lic acid- R -1- 4-amino-3-
(10) N~ Y [( ) (
~ ; chloro-5-trifluorometh
~N~H-~N~' l
b
l
2
N~ y
-
enzy
)-
-
" 1,4'-bipiperidinyl-1'-yl-2-oxo-ethyl]-
amide
"N FFF '~~~ 4-(2-oxo-1,2,4,5-tetrahydro-1,3-
'
benzodiazepin-3-yl)-piperidin-1-
s N~' carbox lic acid- R -1- 4-amino-3-
11 H, Y [( ) (
( ) ~ ~ N~-CN~H'~N~ chloro-5-trifluoromethyl-benzyl)-2-
" (4-dimethylamino-piperidin-1-yl)-2-
oxo-ethyl)-amide
CA 02503455 2005-04-22
29
Structure Name j
F F ~ 4-(2-oxo-1,2,4,5-tetrahydro-1,3-
F
benzodiazepin-3-yl)-piperidin-1-
11 carbox lic acid- R -1-
(12) 4-amino-3-
~
~N \ I
I ~ N~N chloro 5-trifluoromethyl-benzyl)-2-
~~
~
~
" oxo-2-(4-pyridin-4-yl-piperazin-1-yl)-
~
~
ethyl]-amide
F 4-(2-oxo-1,2,4,5-tetrahydro-1,3-
F '~f
n
benzodiazepin-3-yl)-piperidin-1-
~~H~
N carbox lic acid- R -1-
4-amino-3-
(13) Y [( ) (
~
~
, ; N~ ~N chloro-5-trifluoromethyl-benzyl)-2-
H-~N
H ~ (1'-methyl-4,4'-bipiperidinyl-1-yl)-2-
oxo-ethyl]-amide
benzyl4-[1-((R)-3-(4-amino-3-
i
""_ F 'F '~ chloro-5-trifluoromethyl-phenyl)-2-
{[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
"
"-C"'~
C
~1
b
"~
(14) benzodiazepin-3-yl)-piperidin-1-
H
~
;
"
carbonyl]-amino}-propionyl)-
piperidin-4-yl]-piperazin-1-
'
carbox late
I
F ethyl [1'-((R)-3-(4-amino-3-chloro-5-
"~' ~ i
trifluoromethyl-phenyl)-2-{[4-(2-oxo-
"
-
~;
~- 1,2,4,5-tetrahydro-1,3-benzodi-
o ~ C ;
(15) ~ ~, ~ ~
~"
" ~~
"
-~o - azepin-3-yl)-piperidin-1-carbonyl]-
~
amino}-propionyl)-4,4'-bipiperidinyl-
~
1-yl]-acetate
F ~~. 4-(2-oxo-1,2,4,5-tetrahydro-1,3-
H?N FF
n~
benzodiazepin-3-yl)-piperidin-1-
.cH
rN carbox lic acid- R -1-
4-amino-3-
(16) Y {( ) (
"
~
~ ; N~~N chloro-5-trifluoromethyl-benzyl)-2-
~"'j; N
" ~ ~ [4-(4-methyl-piperazin-1-yl)-
piperidin-1-yl]-2-oxo-ethyl}-amide
F . 4-(2-oxo-1,2,4,5-tetrahydro-1,3-
H.N FF
n
benzodiazepin-3-yl)-piperidin-1-
N cH~
carbox lic acid- R -1-
~ ~N-G 4-amino-3-
' Y {( ) (
NJ
-~N
H chloro-5-trifluoromethyl-benzyl)-2-
~
N~
H ~ ~ [4-(1-methyl-piperidin-4-yl)-
piperazin-1-yl]-2-oxo-ethyl}-amide
4-(2-oxo-1,2,4,5-tetrahydro-1,3-
'
benzodiazepin-3-yl)-piperidin-1-
~
o carbox lic acid- R -1-
N 4-amino-3-
( 1$) y\ > ~ Y [( ) ( I
chloro-5-trifluorometh
V-benz
l)-2-
y
y
" ~ ~ ~ (4-azetidin-1-yl-piperidin-1-yl)-2-
oxo-ethyl]-amide
CA 02503455 2005-04-22
Structure Name
4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-
H.N F FF ~~ benzodiazepin-3-yl)-piperidin-1-
N N.cH' n carboxylic acid- (R -1- 4-amino-3-
(
(19) ~ ; N~ ~"'~H-~N~ chloro-5-trifluoromethyl-benzyl)-2-
" ~ ~ [4-(5-methyl-2,5-diaza-
bicyclo[2.2.1 ]hept-2-yl)-piperidin-1-
I -2-oxo-eth I -amide
~N FFF °°~~. 4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-
carbox lic acid- R -1- 4-amino-3-
y [( ) (
(2~) ' ~ H~ ~N~H'~N~ chloro-5-trifluoromethyl-benzyl)-2-
oxo-2-(4-piperazin-1-yl-piperidin-1-
yl)-ethyl]-amide
[1'-((R)-3-(4-amino-3-chloro-5-
H.N 'FF q~ trifluoromethyl-phenyl)-2-t[4-(2-oxo-
"~OH n
(21) ~ ~ II 1,2,4,5-tetrahydro-1,3-benzodi-
~"~"~ air"~ ~ azepin-3-yl)-piperidin-1-carbonyl]-
amino}-propionyl)-4,4'-bipiperidinyl-
1-yl]-acetic acid
11,N F FF 4-(2-oxo-1,2,4,5-tetrahydro-1,3
benzodiazepin-3-yl)-piperidin-1
(22) ~ N~ carboxylic acid-[1-(4-amino-3
~ ; N~ -~N H N~ chloro-5-trifluoromethyl-benzyl)-2-
1,4'-bipiperidinyl-1'-yl-2-oxo-ethyl]-
amide
(S)-2-(4-amino-3-bromo-5-
trifluoromethyl-benzyl)-1-(4-(4-
"~N ~" cH n meth f- i erazin-1- I - i eridin-1-
(23) ~o "J Y P P Y ) P P
~N~ yl]-4-[4-(2-oxo-1, 2, 4, 5-tetra hyd ro-
1,3-benzodiazepin-3-yl)-piperidin-1-
yl]-butan-1,4-dione
F F NK ~~~ (S)-2-(4-amino-3-bromo-5-
~ ; B' trifluoromethyl-benzyl)-4-[4-(2-oxo-
NON ~ ' ,N 1,2,4,5-tetrah dro-1,3-
(24) ~ ~~~~ y
~N benzodiazepin-3-yl)-piperidin-1-yl]-
1-(4-pyridi n-4-yl-piperazin-1-yl)-
butan-1,4-dione
F F NK ~..~ (S)-2-(4-amino-3-bromo-5-
i ; B' trifluoromethyl-benzyl)-1-1,4'
(25) '~"~N~ ~ N~ bipiperidinyl-1'-yl-4-[4-(2-oxo
~N~ 1,2,4,5-tetrahydro-1,3
benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
CA 02503455 2005-04-22
31
Structure Name
F F "H~ ~~, (S)-2-(4-amino-3-bromo-5-
trifl uoromethyl-benzyl)-1-[4-( 1-
(26) '' "-~"~" ~" methyl-piperidin-4-yl)-piperazin-1-
" ° ~~" yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-
1,3-benzodiazepin-3-yl)-piperidin-1-
yl]-butan-1,4-dione
F F "H, «,. (S)-2-(4-amino-3-bromo-5-
trifluoromethyl-benzyl)-1-( 1'-methyl-
(2~) ~o-C" ~" 4,4'-bipiperidinyl-1-yl)-4-[4-(2-oxo-
~" 1,2,4,5-tetrahydro-1, 3-benzodiaze-
pin-3-yl)-piperidin-1-yl)-butan-1,4-
dione
F F ""; ~". (S)-2-(4-amino-3-trifluoromethyl-
°H3 benzyl)-1-[4-(4-methyl-piperazin-1-
(2g) ~ -C" ~" yl)-piperidin-1-ylJ-4-[4-(2-oxo-
1,2,4,5-tetrahydro-1, 3-benzodiaze-
pin-3-yl)-piperidin-1-yl]-butan-1,4-
dione
(S)-2-(4-amino-3-chloro-5-
~ "'~ trifluoromethyl-benzyl)-1-(4-
° dimethylamino-piperidin-1-yl)-4-[4-
(29) ~ H-~~"~ °"~"'"' (2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
(S)-2-(4-amino-3-chloro-5-
a ; ~ "F' trifluoromethyl-benzyl)-1-(3-
(30) I \ N~N~"~ dimethylamino-piperidin-1-yl)-4-[4-
/ H~O ~/ O,°i"~C~ (2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-ylj-
butan-1,4-dione
I °~ "~ ~ (S)-2-(4-amino-3-chloro-5-
\ " "~ ' FF' trifluoromethyl-benzyl)-4-[4-(2-oxo-
(31) ~ ~ °"~ 1,2,4,5-tetrahydro-1,3
/ " ° " benzodiazepin-3-yl)-piperidin-1-yl)-
1-(4-pyrrolidin-1-yl-piperidin-1-yl)-
butan-1,4-dione
°~ "~_ ~°" (S)-2-(4-amino-3-chloro-5-
" "~" FF trifluoromethyl-benzyl)-1-(1'-methyl-
(32) ~ ~ ° 4,4'-bipiperidinyl-1-yl)-4-[4-(2-oxo-
1,2,4, 5-tetrahydro-1, 3-benzodiaze-
~°"= pin-3-yl)-piperidin-1-yl)-butan-1,4-
dione
CA 02503455 2005-04-22
32
Structure Name
( S)-2-(4-a m i no-3-ch
l o ro-5-
trifluoromethyl-benzyl)-1-[4-(4-
~
~
" cyclopropylmethyl-piperazin-1-yl)-
(33) ~ ~
~
~-~"
piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1, 3-benzodiazepin-3-yl)-
piperidin-1-yl]-butan-1,4-dione
'". (S)-2-(4-amino-3-chloro-5-
- FF trifluoromethyl-benzyl)-1-(4-
~ ~ ~
I ~
(34) N morpholine-4-yl-piperidin-1-yl)-4-[4-
H ~
(2-oxo-1, 2,4, 5-tetra
hyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
(S)-2-(4-amino-3-chloro-5-
- trifluoromethyl-benzyl)-4-[4-(2-oxo-
~N~ N~ 1
~ ~ 2
4
t
t
5
h
1
d
(35) N ,
H~ ,
,
ra
ro-
-
e
y
,3-
benzodiazepin-3-yl)-piperidin-1-yl]-
1-(4-perhydro-azepin-1-yl-piperidin-
1-yl)-butan-1,4-dione
( S)-2-(4-ami no-3-chloro-5-
trifluoromethyl-benzyl)-1-[4-(4-
meth I- erh dro-1 4
di
i
1
I
(3g) ~ o N~ -
aze
n-
-
Y P Y P Y )
piperid in-1-yl]-4-[4-(2-oxo-1,2,4,
' 5-
tetrahydro-1,3-benzodiazepin-3-yl)-
~
piperidin-1-yl]-butan-1,4-dione
(S)-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl)-1-[4-(4-
~
(37) ~ ~~ isopropyl-piperazin-1-yl)-piperidin-
~
"~~K 1-yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-
1,3-benzodiazepin-3-yl)-piperidin-1-
yl]-butan-1,4-dione
( S)-2-(4-amino-3-chloro-5-
- FF trifluoromethyl-benzyl)-1-1,4'-
~ ~ '
~ ~N~ ~
(38) p- bipiperidiny!-1
N -yl-4-[4-(2-oxo-
1, 2,4, 5-tetra hyd ro-1,
3-
benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
(S)-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl)-1-[4-(
1-
th
l
i
idi
4
l
h
me
y
-p
per
n-
-y
)-per
ydro-1,4-
diazepin-1-ylJ-4-[4-(2-oxo-1,2,4,
5-
'" tetrahydro-1,3-benzodiazepin-3-yl)-
piperidin-1-yl]-butan-1,4-dione
CA 02503455 2005-04-22
33
i
Structure Name
° «, (S)-2-(4-amino-3-chloro-5-
\ , "F trifluoromethyl-benzyl)-1-[3-(4-
(40) ~ ; ~-C"~"~N,~ methyl-piperazin-1-yl)-azetidin-1-yl]-
p ° ~"-°~ 4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
°, ~~, (S)-2-(4-amino-3-chloro-5-
\ , "F trifluoromethyl-benzyl)-4-[4-(2-oxo-
° FF 1,2,4,5-tetrahydro-1,3-
(41) i ~ "~-~~~N~N~ benzodiazepin-3-yl)-piperidin-1-ylj-
1-(3-pyrrolidin-1-yi-azetidin-1-yl)-
butan-1,4-dione
°, ~. (S)-2-(4-amino-3-chloro-5-
\ , ""~ trifluoromethyl-benzyl)-4-[4-(2-oxo-
° FF 1,2,4,5-tetrahydra-1,3-
(42) i ~ "~-~ ;N~"~N~ penzodiazepin-3-yl)-piperidin-1-yl]-
1-(3-piperidin-1-yl-azetidin-1-yl)-
butan-1,4-dione
(S)-2-(4-amino-3-chloro-5-
° _ ; ~ "~ trifluoromethyl-benzyl)-1-(3-
43 ~ ~ N N'~-" F F diethylamino-azetidin-1-yl)-4-[4-(2-
( ) ~ ~ ° ~ >~'~ oxo-1,2,4,5-tetrahydro-1,3-
"'~ benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
G ~" (S)-2-(4-amino-3-chloro-5-
\ , "~ trifluoromethyl-benzyl)-1-(4-
° FF azetidin-1-yl-piperidin-1-yl)-4-[4-(2-
(44) i ~ "~-~N~"~N~ oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
-~~ (S)-1-[4-(4-acetyl-piperazin-1-yl)-
~"~"'~"~ piperidin-1-yl]-2-(4-amino-3-chloro-
(45) " G ~~ 5-trifluoromethyl-benzyl)-4-[4-(2-
"~,° oxo-1,2,4,5-tetrahydro-1,3-
"~= benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
(S)-2-(4-amino-3-chloro-5-
G n.
trifluoromethyl-benzyl)-1-(4-
(46) ~"~"~" F~~ diethylaminomethyl-piperidin-1-yl)-
4-[4-(2-oxo-1, 2, 4, 5-tetra hyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
CA 02503455 2005-04-22
34
Structure Name
(S)-2-(4-amino-3-chloro-5-
a-,N~N~N,~ trifluoromethyl-benzyl)-1-[4-(4-ethyl-
~
(4~) H- piperazin-1-yl)-piperidin-1-yl]-4-[4-
y-~~
JN (2-oxo-1,2,4,5-tetrahydro-1,3-
\"'"' benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
(S)-2-(4-amino-3-chloro-5-
/~/~'~A~ trifluoromethyl-benzyl)-1-[4-(
~N~N~N~N 1-ethyl-
~
(48) " piperidin-4-yl)-piperazin-1-ylJ-4-[4-
~~
N (2-oxo-1,2,4,5-tetrahydro-1,3-
~'"' benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
N~a
(S)-2-(4-amino-3-chloro-5-
I trifiuoromethyl-benzyl)-4-[4-(2-oxo-
I ~ 1
~~N 2
N 4
5
h
H- ,
(49) ,
~ ,
-tetra
ydro-1,3-
/ benzodiazepin-3-yl)-piperidin-1-yl]-
1-(3, 4, 5, 6-tetra hydro-2H-4,
4'-
bipyridinyl-1-yl)-butan-1,4-dione
"~, '"' (S)-2-(4-amino-3-chloro-5-
I i trifluoromethyl-benzyl)-4-[4-(2-oxo-
~N~ ~N 1
2
4
5
N~ ,
(50) " ,
~ ,
-tetrahydro-1,3-
/ benzodiazepin-3-yl)-piperidin-1-yl]-
'" 1-(4-pyridin-4-yl-piperazin-1-yl)-
butan-1,4-dione
, ~, (S)-2-(4-amino-3-chloro-5-
" '
\ , trifluoromethyl-benzyl)-4-[4-(2-oxo-
~
FF 1,2,4,5-tetrahydro-1,3-
(51 i ~
) ~ ~"~N~
H benzodiazepin-3-yl)-piperidin-1-ylJ-
N~
1-(3-perhydro-azepin-1-yl-azetidin-
1-yl)-butan-1,4-dione
(S)-2-(4-amino-3-chloro-5-
N
~
~' trifluoromethyl-benzyl)-1-[4-(1-
' b
~N~ l
~ i
idi
4
l
i
(52) ' N-~ enzy
LN~N -p
per
-y
n-
)-p
perazin-1-
" , ~ yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-
1,3-benzodiazepin-3-yl)-piperidin-1-
yl]-butan-1,4-dione
,~. NH.
(S)-2-(4-amino-3-chloro-5-
~F trifluoromethyl-benzyl)-1-[4-(4-
~ ~ ~N~ benz
l-
i
erazin-1-
l)-
i
eridin
1
(53) -N N p
" p
' y
y
p
p
-
-
yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-
' b
1,3-benzodiazepin-3-yl)-piperidin-1-
yl]-butan-1,4-dione
CA 02503455 2005-04-22
3S
Structure Name
a, (S)-2-(4-amino-3-chloro-5-
', "F triffuoromethyf-benzyl)-1-(7-
~~ ~, ~" 'F dimethyfaminomethyl-1,2,4,5-
~
" '
'
~ ~
H tetrahydro-3-benzazepin-3-yl)-4-[4-
o
'
,"-H
(54)
",' (2-oxo-1,2,4
5-tetrahydro-1,3-
,
benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
,~'F P"' (S)-2-(4-amino-3-chloro-5-
"'~ t
Q ifl
~ "~ l
b
l
1
4
th
4
~ r
~ uorome
- -
enzy
)-
-[
-(
y
-
~ dimethylaminomethyl-phenyl)-
(55) <- >
piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-
~~"J tetrahydro-1, 3-benzodiazepin-3-yl)-
piperidin-1-yl]-butan-1,4-dione
~E '-' (S)-2-(4-amino-3-chloro-5-
~~ trifluoromethyl-benzyl)-4-[4-(2-oxo-
~ ~o ~G ""
o 1, 2,4, 5-tetra hyd ro-1,
~-", 3-
(56) ~ benzodiazepin-3-yl)-piperidin-1-yl]-
<;
' "-. 1-{4-[4-(2,2,2-trifluoro-ethyl)-
piperazin-1-yl]-piperidin-1-yl}-butan-
1,4-dione
',~F " (S)-2-(4-amino-3-chloro-5-
Cart-~:" ~--~-""- trifluoromethyl-benzyl)-1-(1'-
methanesulphonyl-4,4'-
~ N,
(57) ~'J bipiperidinyl-1-yl)-4-[4-(2-oxo-
~b=s
"-~ 1, 2,4, 5-tetrahydro-1,
b 3-
benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
'F '~"' (S)-2-(4-amino-3-chloro-5-
-~ -C"~o ~ trifluoromethyl-benzyl)-1-(9-methyl-
~ ~
"H:
H 3,9-diaza-spiro[5.5]undec-3-yl)-4-[4-
G
(5$)
(2-oxo-1, 2,4, 5-tetra
hyd ro-1, 3-
H;~" benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
FF ''~' (S)-2-(4-amino-3-chloro-5-
~ ; N~ -~" ~ , "~ trifluoromethyl-benzyl)-4-[4-(2-oxo-
~ 1,2,4,5-tetrahydro-1,3-
(59)
benzodiazepin-3-yl)-piperidin-1-yl]-
~
~" 1-(4-piperidin-1-ylmethyl-piperidin-
1-yl)-butan-1,4-dione
F 'F ~- (S)-2-(4-amino-3-chloro-5-
"~ -~" ~ ~ "" trifluoromethyl-benzyl)-1-[4-(2-
" " dimethylamino-ethyl)-piperidin-1-yl]-
(6~)
4-[4-(2-oxo-1, 2, 4, 5-tetrahydro-1,
3-
"'-" benzodiazepin-3-yl)-piperidin-1-y1]-
butan-1,4-dione
CA 02503455 2005-04-22
36
Structure Name
'' ~~ (S)-2-(4-amino-3-chloro-5-
~-C"~-~""~ trifluoromethyl-benzyl)-N-methyl-N-
".~- [2-( 1-methyl-piperidin-4-yl)-ethyl]-4-
(61
)
oxo-4-[4-(2-oxo-1, 2,4,
5-tetrahydro-
"~". 1,3-benzodiazepin-3-yl)-piperidin-1-
yl]-butyramide
F FF '"~ (S)-2-(4-amino-3-chloro-5-
~ ; "~-C"~ s, "": trifluoromethyl-benzyl)-N-methyl-N-
(62)
" G (1-methyl-piperidin-4-ylmethyl)-4-
oxo-4-[4-(2-oxo-1,2,4,5-tetrahydro-
"~" 1, 3-benzodiazepin-3-yl)-piperidin-1-
yl]-butyramide
F F c~~a
(S)-2-(4-amino-3-chloro-5-
N-( N O ~~,-NH? trifluoromethyl-benzyl)-4-[4-(2-oxo-
~.J
~
(63) H 1,2,4,5-tetrahydro-1,3-
~
~~ ~
benzodiazepin-3-yl)-piperidin-1-yl]-
1-piperidin-1-yl-butan-1,4-dione
F ' (S)-2-(4-amino-3-chloro-5-
~ trifluoromethyl-benzyl)-4-[4-(2-oxo-
N N ~ , NH;
1,2,4, 5-tetrahyd ro-1,
(64) 3-
benzodiazepin-3-yl)-piperidin-1-yl]-
1-(4-propyl-piperidin-1-yl)-butan-
1,4-dione
F c cna
F (S)-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl)-1-(4-benzyl-
(65) H ~ piperidin-1-yl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1, 3-benzodiazepin-3-yl)-
piperidin-1-yl)-butan-1,4-dione
F 'F ~" (S)-2-(4-amino-3-chloro-5-
"~-C"~ ~ , NH. trifluoromethyf-benzyl)-1-[4-(2-
" ~ . diethylamino-ethyl)-piperidin-1-yl]-4-
(66) %
~ [4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
F FF "~ (S)-2-(4-amino-3-chloro-5-
~~, N~, trifluoromethyf-benzyl)-1-(3-aza-
(67) H . spiro[5.5]undec-3-yl)-4-[4-(2-oxo-
i
l
1, 2,4, 5-tetrahyd ro-1,
3-
benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
CA 02503455 2005-04-22
37
Structure Name
''F ~'M N-(1-{(S)-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl)-4-oxo-4-[4-
~
" (2-oxo-1,2,4,5-tetrahydro-1,3-
(6$) N .
~~
benzodiazepin-3-yl)-piperidin-1-yl]-
"=os- butyryl}-piperidin-4-yl)-N-methyl-
methanesulphonamide
''F '~"' N-(1-{(S)-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl)-4-oxo-4-[4-
" " (2-oxo-1,2,4,5-tetrahydro-1,3-
~
" benzodiazepin-3-yl)-piperidin-1-yf]-
_ butyryl}-piperidin-4-yl)-
methanesulphonamide
''F '~- (S)-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl)-1-[4-
70
" G (cyclopentyl-methyl-amino)-
( ) ~
~- piperidin-1-yl]-4-(4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-
piperidin-1-yl]-butan-1,4-dione
F c~.~i
(S)-2-(4-amino-3-chloro-5-
~ , ""- ifl
tr
~ uoromethyl-benzyl)-1-(4-methyl-
(71) ' pipendm-1-yl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1, 3-benzodiazepin-3-yl)-
piperidin-1-yl]-butan-1,4-dione
methyl (1-{{S)-2-(4-amino-3-chloro-
" ~ 5-trifluoromethyl-benzyl)-4-oxo-4-[4-
(72) (2-oxo-1,2,4, 5-tetrahydro-1,
3-
benzodiazepin-3-yl)-piperidin-1-yl]-
"''- butyryl}-piperidin-4-yl)-acetate
FFF ''~ (S)-2-(4-amino-3-chloro-5-
/ N N ~ , NH_ trifluoromethyl-benzyl)-1-(4-
~ C ~
(73) hydroxy-piperidin-1-yl)-4-[4-(2-oxo-
1, 2, 4, 5-tetra hyd ro-1,
3-
benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
F 'F '~' (S)-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl)-4-(4-(2-oxo-
~~ 1,2,4,5-tetrahydro-1,3-
{74) j
~ benzodiazepin-3-yl)-piperidin-1-yl]-
'F F 1-(4-trifluoromethyl-piperidin-1-yl)-
butan-1,4-dione
CA 02503455 2005-04-22
38
Structure Name
F 'f ''' (S)-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyf)-1-[4-(
1,1-
(75) H ~ ~ dioxo-~,6-isothiazolidin-2-yl)-
i
i
4
2
1
2
4
piper
n-1-yl]-4-[
-(
-oxo-
,
,
, 5-
d
tetrahydro-1, 3-benzodiazepin-3-yl)-
piperidin-1-yl]-butan-1,4-dione
''F "'~ (S)-2-(4-amino-3-chloro-5-
~~~ NH: trifluoromethyl-benzyl)-1-[4-(2-oxo-
" '~ perhydro-1,3-oxazin-3-yl)-piperidin-
1-yl]-4-[4-(2-oxo-1, 2,4,
5-tetra hyd ro-
C ~ 1,3-benzodiazepin-3-yl)-piperidin-1-
yl]-butan-1,4-dione
~F '~n methyl 1-{(S)-2-(4-amino-3-chloro-
5-trifluoromethyl-benzyl)-4-oxo-4-[4-
H '~
(77) (2-oxo-1,2,4,5-tetrahydro-1,3-
"" benzodiazepin-3-yl)-piperidin-1-yl]-
butyryl}-piperidin-4-carboxylate
F 'F '~'"' (S)-2-(4-amino-3-chloro-5-
l
~N~ ~ , NH, t rifluoromethyl-benzyl)-1-(4-
" N ~ cyclohexyl-piperidin-1-yl)-4-[4-(2-
oxo-1, 2,4, 5-tetrahydro-1,
3-
benzodiazepin-3-yl)-piperidin-1-yl]-
i
butan-1,4-dione
F 'F ',~ (S)-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl)-1-(4-tert-
H O
butylammo-p~pendm-1-yl)-4-[4-(2-
H, ~ oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
''~ ''~ (S)-2-(4-amino-3-chloro-5-
I
s~ NH, trifluoromethyl-benzyl)-4-[4-(2-oxo-
I
" '~ 1,2,4,5-tetrahydro-1,3-
i
benzodiazepin-3-yl)-piperidin-1-yl]-
1-(4-phenyl-piperidin-1-yl)-butan-
I
1,4-dione i
"F ~~ (S)-2-(4-amino-3-chloro-5-
~ o~H~ O ! trifluoromethyl-benzyl)-4-[4-(2-oxo-
NH
1) C 1,2,4,5-tetrahydro-1,3-
N, ~
benzodiazepin-3-yl)-piperidin-1-yl]-
i
1-(4-p-tolyl-pi peridin-1-yi)-butan-
1,4-dione '
CA 02503455 2005-04-22
39
Structure Name
F 'F "'"' 8-{(S)-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl)-4-oxo-4-[4-
H O O CI (2-oxo-1,2,4,5-tetrahydro-1,3-
(82)
benzodiazepin-3-yl)-piperidin-1-yl]-
H
butyryl}-1, 3, 8-triaza-
spiro[4.5]decan-2,4-dione
F FF a- (S)-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyf)-1-[4-(2-oxo-
~
(83) ~ ~ '~ imidazolidin-1-yl)-piperidin-1-yl]-4-
"
[4-(2-oxo-1, 2, 4, 5-tetrahyd
ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
(S)-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl)-1-(4-amino-
~
(84) 4-methyl-piperidin-1-yl)-4-[4-(2-oxo-
"
"
1,2,4, 5-tetrahydro-1, 3-benzodiaze-
pin-3-yl)-piperidin-1-yl]-butan-1,4-
dione
(S)-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl)-1-[4-(
' ~ 1-
~~"J'~'Nf" methyl-piperidin-4-yl)-piperazin-1-
(85) >
~
yl]-4-[4-(2-oxo-1, 2,4,
5-tetrahydro-
~", 1,3-benzodiazepin-3-yl)-piperidin-1-
yl]-butan-1,4-dione
(S)-2-(4-amino-3-chloro-5-
F
, trifluoromethyl-benzyl)-1-(2-amino-
NH.
(86)
~"~5~"" 4 5,7,8-tetrahydro-thiazolo[4,5-
1
4
4
2
5
f
2
~~-CN ,
,
-(
-oxo-
,
-
)-4-[
d]azepin-6-y
" tetrahydro-1,3-benzodiazepin-3-yl)-
piperidin-1-yl]-butan-1,4-dione
(S)-2-(4-amino-3-chloro-5-
"H trifluoromethyl-benzyl)-1-[4-(2-
~ ~ "' meth I-fmidazol-1- I - i
(87) eridin-1- I -
-C ~"~".~
Y Y) PP Y]
4-(4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
(S)-2-(4-amino-3-chloro-5-
N"_ trifluoromethyl-benzyl)-1-(4-(4-
O CI
( methyl-imidazol-1-yl)-piperidin-1-yl]-
) ~j 4
"~"~ t
d
1
3
4
1
2
5
t
h
2
~N-CN ,
lf ra
", ro-
,
-
-(
-oxo-
,
,
-
e
y
4-[
" benzodiazepin-3-yl)-piperidin-1-yl]-
- butan-1,4-dione
CA 02503455 2005-04-22
Structure Name
F F °°-~ (S)-2-(4-amino-3-chloro-5-
~NH: trifluoromethyl-benzyl)-4-[4-(2-oxo-
o ~ ~ ~~ 1,2,4,5-tetrahydro-1,3-
N N ~"~N' benzodiazepin-3-yl)-piperidin-1-yl]-
( ) ~ ~ ~ ~ 1-(4-thiazol-2-yl-piperazin-1-yl)-
butan-1,4-dione
(S)-2-(4-amino-3-chloro-5-
,F "H: trifluoromethyl-benzyl)-1-[4-(2,4-
dimeth I-imidazol-1- I - i eridin-1-
90 N "~N Y Y) PP
( ) ~ -CN~ ~ .-~~H3 yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-
1,3-benzodiazepin-3-yl)-piperidin-1-
yl]-butan-1,4-dione
F F ~n (S)-2-(4-amino-3-chloro-5-
NH; trifluoromethyl-benzyl)-1-(4-
o ~ ~ ~~ imidazol-1- I- i eridin-1-yl)-4-[4-(2-
(91 ) ,,N Y P P
W.~ oxo-1,2,4,5-tetrahydro-1,3-
~ ~N N benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
F F ~~~~ (S)-2-(4-amino-3-chloro-5-
~NH: trifluoromethyl-benzyl)-4-[4-(2-oxo-
1, 2, 4, 5-tetrahyd ro-1, 3-
92 ~ N N ~ N" benzodiazepin-3-yl)-piperidin-1-yl]-
( ) ~~~ ~ " J 1-(4-1,2,4-triazol-1-yl-piperidin-1-
yl)-butan-1,4-dione
F F ~°° (S)-2-(4-amino-3-chloro-5-
NH~ trifiuoromethyl-benzyl)-1-[4-(1-aza
bic cfo 2.2.2 oct-3- 1 i erazin-1
o G y )-p p
Y [ 1
N O ~.J
(93) ; -C ~, " "~ yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-
1,3-benzodiazepin-3-yl)-piperidin-1-
yl]-butan-1,4-dione
F F
F NH (S)-2-(4-amino-3-chloro-5-
i ~ ~ trifluoromethyl-benzyl)-4-[4-(2-oxo-
(94) ~ ° N~NH 1,2,4,5-tetrahydro-1,3-
' - N~ ~"~ '-' benzodiazepin-3-yl)-piperidin-1-yl]-
" ° 1-piperazin-1-yl-butan-1,4-dione
4-{(S)-2-(4-amino-3-chloro-5-
'FF m
N" trifluoromethyl-benzyl)-4-oxo-4-[4-
g5 ~ ~ ~~ ~~N 5_N ~-~N (2-oxo-1,2,4,5-tetrahydro-1,3-
( ) ~ ~ ~ ~ o ~~ ~ benzodiazepin-3-yl)-piperidin-1-yl]-
Ho
butyryl}-piperazin-1-sulphonic acid- '
(1-methyl-piperidin-4-yl)-amide
CA 02503455 2005-04-22
41
Structure Name
- , "'~ (S)-2-(4-amino-3-chloro-5-
~ F F trifluoromethyl-benzyl)-N-(5-amino-
~ '
~"~ "~
(96) p-~ pentyl)-4-oxo-4-[4-(2-oxo-1,2,4,5-
~NH. tetrahydro-1,3-benzodiazepin-3-yl)-
piperidin-1-yl]-butyramide
, '~, (S)-2-(4-amino-3-chloro-5-
"
\ , trifluoromethyl-benzyl)-N-(3-
~
(97) ~ ~ aminomethyl-benzyl)-4-oxo-4-[4-(2-
~ ~"~N~ F ' NH
" oxo-1,2,4,5-tetrahydro-1,3-
.
benzodiazepin-3-yl)-piperidin-1-yl]-
butyramide
1-{(S)-2-(4-amino-3-chloro-5-
~
~ ~" " "~'
~ "- trifluoromethyl-benzyl)-4-oxo-4-[4-
H ~
(98) (2-oxo-1,2,4,5-tetrahydro-1,3-
H benzodiazepin-3-yl)-piperidin-1-yl]-
butyryl}-piperidine-4-carboxylic
acid
N " ' I NtS
( 1-{(S)-2-(4-amino-3-chloro-5-
-~ , trifluoromethyl-benzyl)-4-oxo-4-[4-
(99) (2-oxo-1, 2,4, 5-tetra
hyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-
" butyryl}-piperidin-4-yl)-acetic
acid
-"' (S)-2-(4-amino-3-chloro-5-
~"..C" o",~N trifluoromethyl-benzyl)-1-[4-(8-
~
(100)" methyl-8-aza-bicyclo[3.2.1]oct-3-yl)-
piperazin-1-yl]-4-[4-(2-oxo-1,2,4,5-
~ i
'"3 tetrahydro-1,3-benzodiazepin-3-yl)-
i
piperidin-1-yl]-butan-1,4-dione
", (S)-2-(4-amino-3-chloro-5-
" '
\ , trifluoromethyl-benzyl)-4-[4-(2-oxo-
F
FF 1,2,4,5-tetrahydro-1,3-
(101 ~ ;
) ~ ~"'~' ~ N~
H enzodiazepin-3-yl)-piperidin-1-yl]-
~"H b
1-(4-piperazin-1-yl-piperidin-1-yl)-
butan-1,4-dione
' 'F '~'~' (S)-2-(4-amino-3-chloro-5-
N~~"~ ~ ~ NH: trifluoromethyl-benzyl)-1-[4-(1H-
(102)H . imidazol-4-yl)-piperidin-1-yl]-4-[4-(2-
oxo-1,2,4,5-tetrahydro-1,3-
HN~," benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
CA 02503455 2005-04-22
4?
Structure Name
(S)-2-(4-amino-3-chloro-5-
'~ "" °~ trifluoromethyl-benzyl)-4-[4-(2-oxo-
O FF~F
° 1,2,4,5-tetrahydro-1,3-
(103) ~ ~ p-~~"~"~"''~~ F~F benzodiazepin-3-yl)-piperidin-1-yl]-
1-{4-[4-(2, 2, 2-trifluoro-acetyl)-
phenyl]-piperazin-1-yl}-butan-1,4-
dione
,~" G ~.. (S)-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl)-1-[4-(4-
methyl-piperazin-1-yl)-piperidin-1-
(104) ~ ~"~~N~"~NVN-°". yl]_4_(4-(2-oxo-1,4-dihydro-2H
" ° quinazolin-3-yl)-piperidin-1-yl]
butan-1,4-dione
"" °~ ~~~~ (S)-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl)-1-[4-(4-
(105) ""~N-CND-"~"v"-°"~ methyl-piperazin-1-yl)-piperidin-1-
yl]-4-[4-(5-oxo-3-phenyl-4, 5-
dihydro-1,2,4-triazol-1-yl)-piperidin-
1-yl]-butan-1,4-dione
".N °~ ~°n (S)-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl)-1-(4-(4-
"N~" N " ",--,"-°" methyl-piperazin-1-yl)-piperidin-1-
(106) ~ -C -~ ~ " yl]_4-(4-(2-oxo-1,2-dihydro-
1 / N
imidazo[4,5-c]quinolin-3-yl)-
piperidin-1-yl]-butan-1,4-dione
".N °~ ~~~ (S)-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl)-1-[4-(4-
s ~ ~ methyl-piperazin-1-yl)-piperidin-1-
(107) ~N-CND-N~-N~N-°", yl]-4-[4-(2-oxo-1,2-dihydro-4H-
° I
thieno[3,4-d]pyrimidin-3-yl)-
piperidin-1-yl]-butan-1,4-dione
F (S)-2-(4-amino-3-chloro-5-
"." F F ~~ trifluoromethyl-benzyl)-1-[4-(5-
"~"~'"' n methyl-2,5-diaza-bicyclo[2.2.1]hept-
(108) ~ ; "~-C"'~-~"~ 2-yl)-piperidin-1-yl]-4-[4-(2-oxo-
" ° ° 1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
FF F U (S)-2-(4-amino-3,5-bis-
"" F trifluoromethyl-benzyl)-1-[4-(4-
(109) ; ~ ~"~- ~ -~" methyl-perhydro-1,4-diazepin-1-yl)- ~
' "~ " O piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-
" ° tetrahydro-1,3-benzodiazepin-3-yl)-
piperidin-1-yl]-butan-1,4-dione
CA 02503455 2005-04-22
43
Structure Name
( S)-2-(4-ami no-3, 5-bis-
""
, trifluoromethyl-benzyl)-1-[4-(1-
;
F' methyl-piperidin-4-yl)-perhydro-1,4-
(110) ,; diazepin-1-yl]-4-[4-(2-oxo-1,2,4,5-
~~"~.~-~",~,
" tetrahydro-1,3-benzodiazepin-3-yl)-
"
piperidin-1-yl]-butan-1,4-dione
F F <" (S)-2-(4-amino-3,5-bis-
F
"
, ~ trifluoromethyl-benzyl)-1-[4-(1-
"F
methyl-piperidin-4-yl)-piperazin-1-
F' ~"-H~
(111) , yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-
, ~ "~"~ ~"
1,3-benzodiazepin-3-yl)-piperidin-1-
yl]-butan-1,4-dione
( S)-2-(4-a m i no-3, 5-bi
s-
trifiuoromethyl-benzyl)-1-[4-(
1-aza-
F bicyclo[2.2.2]oct-3-yl)-piperazin-1-
(112) yl]-4-[4-(2-oxo-1, 2,4,
5-tetrahydro-
1,3-benzodiazepin-3-yl)-piperidin-1-
yl]-butan-1,4-dione
(S)-2-(4-amino-3,5-bis-
""
n
. trifluoromethyl-benzyl)-1-[4-(4-
F
~
FF ~"-" meth I- i erazin-1-yl)-piperidin-1-
(113) Y PP
~ dro-
~"~'"J 5-tetrah
4
1
2
o
4
2
4
l
, y
"~" ,
/ y
O ,
,
-ox
-
-(
-[
]-
H O 1,3-benzodiazepin-3-yl)-piperidin-1-
yl]-butan-1,4-dione
(S)-2-(4-amino-3,5-bis-
NH
' F trifluoromethyl-benzyl)-4-[4-(2-oxo-
, FF ~ 1,2,4,5-tetrahydro-1,3-
(114) , ~ "~N~N~' ~--~ benzodiazepin-3-yl)-piperidin-1-yl]-
1-(4-perhydro-azepin-1-yl-piperidin-
1-yl)-butan-1,4-dione
(S)-2-(4-a m i no-3, 5-bis-
trifluoromethyl-benzyl)-1-1,4'-
(115) ~ F N bipiperidinyl-1'-yl-4-[4-(2-oxo-
~ ; 1,2,4,5-tetrahydro-1,3-
~-CN~jlr"~ 'J
H benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
(S)-2-(4-a m i no-3, 5-bis-
F
NF: trifluoromethyl-benzyl)-4-[4-(2-oxo-
\
"~ 1,2,4,5-tetrahydro-1,3-
(116) ~ ~ benzodiazepin-3-yl)-piperidin-1-yl]-
~
N
~
H 1-(4-piperazin-1-yl-piperidin-1-yl)-
H
~
butan-1,4-dione
CA 02503455 2005-04-22
44
Structure Name
~, 4-(2-oxo-1,2,4,5-tetrahydro-1,3-
FF benzodiazepin-3-yl)-piperidin-1-
F
F ""
F ~ carboxylic
117 x acid-{(R)-1-(4-amino-3,5-
=
N~-"
N-~3
) H bis-trifluoromethyl-benzyl)-2-[4-(4-
( ~
~,
, ; "~-CN
methyl-piperazin-1-yl)-piperidin-1-
yl]-2-oxo-ethyl}-amide
a, 4-(2-oxo-1,2,4,5-tetrahydro-1,3-
FF benzodiazepin-3-yl)-piperidin-1-
F
"H
o ,~ carboxylic
(118) x acid-{(R)-1-(4-amino-3,5-
~"
"~;"-",
p bis-trifluoromethyl-benzyl)-2-[4-(1-
~,
~"-CN
methyl-piperidin-4-yl)-piperazin-1-
yl]-2-oxo-ethyl}-amide
4-(2-oxo-1,
2,4,
5-tetrahyd
ro-1,
3-
" ~ benzodiazepin-3-yl)-piperidin-1-
_ ~' F carbox
lic
acid-[(R)-1-(4-amino-3,5-
Y
(119) ~ ' bis-trifluoromethyl-benzyl)-2-1,4'-
~ ; "~ ~"~~ "~-"~
" bipiperidinyl-1'-yl-2-oxo-ethyl]-
amide
F F , 4-(2-oxo-1,2,4,5-tetrahydro-1,3-
F
F: benzodiazepin-3-yl)-piperidin-1-
~
"
~
/ carboxylic
(120) ~ - acid-[(R)-1-(4-amino-3,5-
F l)-2-oxo-2-
~ l-benz
~" H o ~ eth
ifl
bi
t
, y
~ y
~"H uorom
r
s-
H (4-piperazin-1-yl-piperidin-1-yl)-
ethyl]-amide
, 4-(2-oxo-1,2,4,5-tetrahydro-1,3-
FFF benzodiazepin-3-yl)-piperidin-1-
NN-
/ carboxylic acid-(R)-1-(4-amino-3-
(121) ~~ l-benz
~ ; l)-2-
-~"~o~ eth
ifl
t
5
hl
~ y
"~"v"-H, y
uorom
r
oro-
-
c
H [4-(4-methyl-piperazin-1-yl)-
piperidin-1-yl]-2-oxo-ethyl
ester
4-(2-oxo-1,4-dihydro-2H-quinazolin-
o ~ ~, 3-yl)-piperidin-1-carboxylic
" acid-[2-
-~ '
~ '
-~
~ - I-1- 4-bromo-
(122) ~ bi i eridin I-1
' N 1 4
N [ , 1 P P Y Y (
"
, ~ N~ H p
3-methyl-benzyl)-2-oxo-ethyl]-
amide
4-(2-oxo-1, 4-d ihyd ro-2H-q
ui nazol i n-
~~ H_ 3-yl)-piperidin-1-carboxylic
'~ acid-[1-
~' '
~~
' ~ " -
(123) " 4-bromo-3-meth I-benzyl)-2-(1
H ( Y
'~'"
~.~ H
- methyl-[4,4']bipiperidinyl-1-yl)-2-
oxo-ethyl]-amide
CA 02503455 2005-04-22
Structure Name
4-(2-oxo-1,4-dihydro-2H-quinazolin-
G". 3-yl)-piperidin-1-carboxylic
~ acid-{1-
~
~
124 ' ~ " 4-bromo-3-meth I-Benz I
( ) " -2- 4- 4-
H ( Y Y ) [ (
~"% "
C~.~
", methyl-piperazin-1-yl)-piperidin-1-
"
yl]-2-oxo-ethyl}-amide
4-(2-oxo-1,4-dihydro-2H-quinazolin-
~
~ 3-yl)-piperidin-1-carboxylic
G"~ acid-{1-
~
~
~
125 ' , " 4-bromo-3-meth
" I-benz
H I
'~"'~" -2-
C~k 4-
1-
Y
Y
)
[
" methyl-piperidin-4-yl)-piperazin-1-
"
yl]-2-oxo-ethyl}-amide
ethyl {4-[1-(3-(4-bromo-3-methyl-
phenyl)-2-{[4-(2-oxo-1,4-dihydro-
" ~
(126) ~.~ 2H-quinazolin-3-yl)-piperidin-1-
~~
~ ;
" carbonyl]-amino}-propionyl)-
" ~
piperidin-4-yl]-piperazin-1-yl}-
acetate
N ~ "> ethyl [1'-(3-(4-bromo-3-methyl-
" phenyl)-2-{[4-(2-oxo-1,4-dihydro-
~ ~
(127) ~ ~ "~ 2H-quinazolin-3-yl)-piperidin-1-
"
"
~
",G~ carbonyl]-amino}-propionyl)-
[4,4']bipiperidinyl-1-yl]-acetate
ethyl {4-[4-(3-(4-bromo-3-methyl-
"~p~N"' phenyl)-2-{[4-(2-oxo-1,4-dihydro-
2H-quinazolin-3-yl)-piperidin-1-
(128) ~ ' "-~~ ~
~
carbon I]-amino - ro ion
" I -
Y } P P Y)
"~ piperazin-1-yl]-piperidin-1-yl}-
acetate
~ K
{4-[ 1-(3-(4-bromo-3-methyl-phenyl)-
2
4
2
1
4
dih
d
2H
-{[
H ~ -(
-oxo-
,
-
y
ro-
-
(129) ~ ; ~ quinazolin-3-yl)-piperidin-1-carbo-
~
~~ nyl]-amino}-propionyl)-piperidin-4-
yl]-piperazin-1-yl}-acetic
acid
~ Br
[1'-(3-(4-bromo-3-methyl-phenyl)-2-
~
N O "~CFL
l~ H { [4-( 2-oxo-1, 4-d i h
~ yd ro-2 H-
(130) ~ ~ ~ quinazolin-3-yl)-piperidin-1-
"
HO~O carbonyl]-amino}-propionyl)-
[4,4']bipiperidinyl-1-yl]-acetic
acid
CA 02503455 2005-04-22
46
Structure Name
Br
'
{4-[4-(3-(4-bromo-3-methyl-phenyl)-
2-{[4-(2-oxo-1,4-dih dro-2H-
(131) y
i ; ~ ~ ° ~N~N quinazolin-3-yl)-piperidin-1-carbo-
° ~~° nyl]-amino-propionyl)-piperazin-1-
yl]-piperidin-1-y!~-acetic acid
8- ~" 2-(4-bromo-3-methyl-benzyl)-1-[4
~ (4-methyl-piperazin-1-yl)-piperidin
132 ~ , N'~N~-N~",°H _
( ) ~~° ° 1-yl]-4-[4 (2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
2-(4-bromo-3-methyl-benzyl)-1-( 1'-
(133) ~ , N~'~N ° ° NON-°H~ methyl-[4,4']bipiperidinyl-1-
yl)-4-[4-
' " (2-oxo-1,4-dihydro-2H-quinazolin-3-
yl)-piperidin-1-yl]-butan-1,4-dione
1-[1,4']Bipiperidinyl-1'-yl-2-(4-
(134) ~ ~ ~~~N ° ° N~N~ bromo-3-methyl-benzy!)-4-[4-(2-
' ° oxo-1,4-dihydro-2H-quinazolin-3-
yl)-piperidin-1-yl]-buts n-1, 4-dione
H~~'°H' 2-(4-bromo-3-methyl-benzyl)-1-{4-
[4-(3-dimethylamino-propyl)-
135
( ) ~ ~ N-~~.N.~ phenyl]-piperazin-1-yl}-4-[4- 2-oxo-
(
1,4-dihydro-2H-quinazolin-3-yl)-
piperidin-1-yl]-butan-1,4-dione
[4-( 1-{2-(4-bromo-3-methyl-benzyl)-
° ~ 4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
(136) ' N~,N ° N quinazolin-3-yl)-piperidin-1-yl]-
butyryl}-piperidin-4-yl)-piperazin-1-
yl]-acetic acid
e,
~N~°_°", methyl (1'-{2-(4-bromo-3-methyl-
° ° benzyl)-4-oxo-4-[4-(2-oxo-1,4-
(137) , N~" ° N dihydro-2H-quinazolin-3-yl)-
piperidin-1-yl]-butyryl}-
[4,4']bipiperidinyl-1-yl)-acetate
CA 02503455 2005-04-22
47
i
Structure Name
B, (1'-{2-(4-bromo-3-methyl-benzyl)-4-
",G
"~OH
oxo-4-[4-(2-oxo-1
4-dihydro-2H-
(138)~" " ,
quinazolin-3-yl)-piperidin-1-yl]-
\ " butyryl}-[4,4']bipiperidinyl-1-yl)-
~ ~ ".~
H acetic acid
4-(2-oxo-1,4-dihydro-2H-quinazolin-
~ G". 3-yl)-piperidin-1-carboxylic
~ acid{1-
~
~
139 " 4-
( ' ~ " ( chloro-3-methyl-benzyl)-2-[4-(4-
) ~"% "
H
~.~ O G
~ methyl-piperazi n-1-yl)-piperidi
" n-1-
yl]-2-oxo-ethyl}-amide
4-(2-oxo-1,4-dihydro-2H-quinazolin-
N~N~"-~~N 3-yl)-piperidin-1-carboxylic
(140)~~ " O ~ acid-[2-
'
'
[1,4
H O ]bipiperidinyl-1
-yl-1-(4-chloro-3-
methyl-benzyl)-2-oxo-ethyl]-amide
4-(2-oxo-1,4-dihydro-2H-quinazolin-
~ G"3 3-yl)-piperidin-1-carboxylic
" acid-{1-
~
~
141 .
( '~'"-~'" ~ (4-chloro-3-methyl-benzyl)-2-[4-(1-
) ' ~ " " H
~
G"
" ~ methyl-piperidin-4-yl)-piperazin-1-
'
yl]-2-oxo-ethyl}-amide
4-(2-oxo-1,4-dihydro-2H-quinazolin-
", '
3-yl)-piperidin-1-carboxylic
acid-[1-
(142); ~ ~ " N ~'" '
N O (4-chloro-3-methyl-benzyl)-2-(1'-
H
Q
G ,methyl-[4,4']bipiperidinyl-1-yl)-2-
"
ioxo-ethyl]-amide
~I ethyl [1'-(3-(4-chloro-3-methyl-
~
N'
GH.
~ H phenyl)-2-{[4-(2-oxo-1,4-dihydro-
~
(143)\ " 2H-quinazolin-3-yl)-piperidin-1-
' - ".~
"
"
~
" carbonyl]-amino}-propionyl)-
G~
, [4,4']bipiperidinyl-1-yl]-acetate
tert-butyl{4-[ 1-(3-(4-chloro-3-methyl-
N~" "~~ phenyl)-2-{[4-(2-oxo-1,4-dihydro-
~ H "1
" 2H-quinazolin-3-yl)-piperidin-1-
(144)' -
-~
~'
" carbonyl]-amino}-propionyl)-
o~
"
~~~GH: piperidin-4-yl]-piperazin-1-yl}-
acetate
CA 02503455 2005-04-22
48
Structure Name
~ , 'H, 4-(2-oxo-1,4-dihydro-2H-quinazolin-
"~" 3-yl)-piperidin-1-carboxylic acid-{1-
(145) , ".~ " ° '~~"-~F (4-chloro-3-methyl-benzyl)-2-oxo-2-
[1'-(2, 2, 2-trifluoro-ethyl )-
[4,4']bipiperidinyl-1-yl]-ethyl}-amide
°~ 4-(2-oxo-1,4-dihydro-2H-quinazolin-
~' '"' 3-yl)-piperidin-1-carboxylic acid-(1-
"~"~~.".~ F 4-chloro-3-met
(146) ~ -~F ( hyl-benzyl)-2-oxo-2-
H o ~"
{4-[4-(2, 2, 2-trifluoro-ethyl)-
piperazin-1-yl]-piperidin-1-yl)-ethyl)-
amide
' (1'-(3-(4-chloro-3-methyl-phenyl)-2
{[4-(2-oxo-1, 4-di hyd ro-2H
(147) ~ ~ N~~ H ° ~N quinazolin-3-yl)-piperidin-1
H ,~~° carbonyl]-amino)-propionyl)
[4,4']bipiperidinyl-1-yl]-acetic acid
'H° 2-(4-chloro-3-methyl-benzyl)-1-[4-
°
(4-ethyl-piperazin-1-yl)-piperidin-1-
{148) ~, "~'~" °"~"~" yl]-4-[4-(2-oxo-1,4-dihydro-2H-
H
""' quinazolin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
'H~
2-(4-chloro-3-methyl-benzyl)-1-(1'
(149) ~ ~ "~~" ° ° "~"-'". methyl-[4,4']bipiperidinyl-1-yl)-4-[4
H (2-oxo-1,4-dihydro-2H-quinazoiin-3
yl)-piperidin-1-yl]-butan-1,4-dione
CHI
1-[1,4']bipiperidinyl-1'-yl-2-(4-chloro-
150 ~ "~N ° " " 3-methyl-benzyl)-4-[4-(2-oxo-1,4-
( ) ~ ~ "-~° ° ~ ~ dihydro-2H-quinazolin-3-yl)-
H
piperidin-1-yl]-butan-1,4-dione
'"° 2-(4-chloro-3-methyl-benzyl)-1-[4-
(4-methyl-piperazin-1-yl)-piperidin-
(151 ) \ , "~'~" ° ° "~-",~ "-'", 1-yl]-4-(4-(2-oxo-1,4-dihydro-
2H-
" quinazolin-3-yl)-piperidin-1-ylj-
butan-1,4-dione
CA 02503455 2005-04-22
49
Structure Name
"- 2-(4-chloro-3-methyl-benzyl
)-1-[4-
o' ( 1-methyl-pi perid i n-4-yl
)-pi perazi n-
(152) ~ , ~'~N N~N~N-'H, 1-yl]-4-[4-(2-oxo-1,4-dihydro-2H-
H quinazolin-3-yl)-piperidin-1-yl]-
butan-1,4-dione
H' 2-(4-chloro-3-methyl-benzyl)-1-[4-
(4-methanesulphonyl-piperazin-1-
(153) ; , ~N N~-"~N yl)-piperidin-1-yl]-4-[4-(2-oxo-1,4-di-
,
" hydro-2H-quinazolin-3-yl)-piperidin-
o ~'"~
1-yl]-butan-1,4-dione
,
'H' 2-(4-chloro-3-methyl-benzyl)-1-[4-
(4-isopropyl-piperazin-1-yl)-
j
(154) ~(' ~N N~-".~N piperidin-1-yl]-4-[4-(2-oxo-1,4-
H
dihydro-2H-quinazolin-3-yl)-
H,
r
'
piperidin-1-yl]-butan-1,4-dione
H ethyl 1-{2-(4-chloro-3-methyl-
benzyl)-4-oxo-4-[4-(2-oxo-1,4-
(155) ~" ~N~N'H' dihydro-2H-quinazolin-3-yl)-
~H3 piperidin-1-yl]-butyryl}-4-(1-methyl-
piperidin-4-yl)-piperazin-2-
carboxylate
'H, '~ ethyl 1-( 1-{2-(4-ch loro-3-methyl-
~
.~/~ ~ benzyl)-4-oxo-4-[4-(2-oxo-1
156 , \ ~~N~~N~N.'H3 4-
' f
( - H ~ dihydro-2H-quinazolin-3-yl)-
)
~''H piperidin-1-yl]-butyryl}-piperidin-4-
yl)-4-methyl-piperazin-2-carboxylate
'H ethyl 4-( 1-{2-(4-ch loro-3-methyl-
benzyl)-4-oxo-4-[4-(2-oxo-1,4-
15 ~''"'
"
N
( ,~.N'H, dihydro-2H-quinazolin-3-yl)-
7) ~~~N r
~-
piperidin-1-yl]-butyryl}-piperidin-4-
yl)-1-methyl-piperazin-2-carboxylate
ethyl 4-{2-(4-chloro-3-methyl-
' - benzyl)-4-oxo-4-[4-(2-oxo-1,4-
N N~N'~"H, dihydro-2H-quinazolin-3-yl)-
(158)
~ ~
~ ; N~ piperidin-1-yl]-butyryl}-1-(1-methyl-
yH_
piperidin-4-yl)-piperazin-2-
carboxylate
CA 02503455 2005-04-22
Structure Name
G
" 2-(4-chloro-3-methyl-benzyl)-4-[4-
(2-oxo-1,4-dihydro-2H-quinazolin-3-
"
(159) ".G" yl)-piperidin-1-yl]-1-[1'-(2,2,2-
'~~"~F '
trifluoro-ethyl)-[4,4
]bipiperidinyl-1-
yl]-butan-1,4-dione
G
2-(4-chloro-3-methyl-benzyl)-4-[4-
(2-oxo-1 4-dihydro-2H-quinazolin-3-
"
(160) , ".G" yl)-piperidin-1-yl]-1-{4-[4-(2,2,2-
~' ~% "~F F
trifluoro-ethyl)-piperazin-1-yl]-
piperidin-1-yl}-butan-1,4-dione
G
[4-(1-{2-(4-chloro-3-methyl-benzyl)-
J
"~ 4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
(161) ~ "~" O quinazolin-3-yl)-piperidin-1-yl]-
butyryl}-piperidin-4-yl)-piperazin-1-
yl]-acetic acid I
G
", "-~,", methyl (1'-{2-(4-chloro-3-methyl-
" benzyl)-4-oxo-4-[4-(2-oxo-1,4-
(162) , "~" dihydro-2H-quinazolin-3-yl)-
piperidin-1-yl)-butyryl}-
[4,4']bipiperidinyl-1-yl)-acetate
( 1'-{2-(4-chloro-3-methyl-benzyl
)-4-
" oxo-4-[4-(2-oxo-1,4-dihydro-2H-
(163) , "~" quinazolin-3-yl)-piperidin-1-yl]-
'
butyryl}-[4,4
]bipiperidinyl-1-yl)-
acetic acid
GH,
1-{2-(4-chloro-3-methyl-benzyl)-4-
" "~,"~NK oxo-4-[4-(2-oxo-1,4-dihydro-2H-
(164) , "~ o~ quinazolin-3-yl)-piperidin-1-yl]-
butyryl}-4-(1-methyl-piperidin-4-yl)-
piperazin-2-carboxylic
acid
" 1-( 1-{2-(4-chloro-3-methyl-benzyl)-
G
~' 4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
165 ~ ; ~~"~~
"~'
_
- uinazolin-
( ) " q 3-yl)-pipendin-1-yl]-
~
H ~
H butyryl}-piperidin-4-yl)-4-methyl-
piperazin-2-carboxylic
acid
CA 02503455 2005-04-22
51
Structure Name
"~ 4-( 1-{2-(4-chloro-3-methyl-benzyl
)-
4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
(166)"~-"~~"3 quinazolin-3-yl)-piperidin-1-yl]-
~ ~ ~~'"
" butyryl}-piperidin-4-yl)-1-methyl-
piperazin-2-carboxylic
acid
;"~ , 2-(4-chloro-3-methyl-benzyl)-1-[4-
(1-methyl-piperidin-4-yl)-piperazin-
(167)~ ~ ~~" ~ .~" 1-yl]-4-[4-(2-oxo-1,2 4,5-tetrahydro-
", 1,3-benzodiazepin-3-yl)-piperidin-1-
yl]-butan-1,4-dione
;"~ , 2-(4-chloro-3-methyl-benzyl)-1-[4-
(4-methyl-piperazin-1-yl)-piperidin-
(168), ~ ~~" "~"~ 1-yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-
" "
"3 1,3-benzodiazepin-3-yl)-piperidin-1-
yl]-butan-1,4-dione
G
H,C [4-(1-{2-(4-chloro-3-methyl-benzyl)-
~
-~H
'
J 4-oxo-4-[4-(5-oxo-3-phenyl-4,5-
N
~
(169)HN~~~ dihydro-1,2,4-triazol-1-yl)-piperidin-
1-yl]-butyryl}-piperidin-4-yl)-
piperazin-1-yl]-acetic
acid
G
H,C (methyl 1'-{2-(4-chloro-3-methyl-
N~~H,
~;
~ benzyl)-4-oxo-4-[4-(5-oxo-3-phenyl-
N N
(170)~~~' 4,5-dihydro-1,2,4-triazol-1-yl)-
HN"N
piperidi n-1-yl]-butyryl}-4,4'-
bipiperidinyl-1-yl)-acetate
G
N. (1'-{2-(4-chloro-3-methyl-benzyl)-4-
N-~H
'
oxo-4-[4-(5-oxo-3-phenyl-4,5-
N
N~
(171 ~ dihydro-1,2,4-triazol-1-yl)-piperidin-
) ~~
H 1-yl]-butyryl}-4,4'-bipiperidinyl-1-yl)-
acetic acid
'" 2-(3-bromo-4-chloro-5-methyl-
~' B, benzyl)-1-[4-(4-methyl-piperazin-1-
(172)a'~'~" "~-" ~" yl)-piperidin-1-yl]-4-[4-(2-oxo-1,4-
"~ dihydro-2H-quinazolin-3-yl)-piperi-
din-1-yl]-butan-1,4-dione
CA 02503455 2005-04-22
52
Structure Name
;' , 2-(3-bromo-4-chloro-5-methyl-
~' w benzyl)-1-(1'-methyl-
(173)~ r ~~N "~N [4,4']bipiperidinyl-1-yl)-4-[4-(2-oxo-
"
1,4-dihydro-2H-quinazolin-3-yl)-
piperidin-1-yl]-butan-1,4-dione
2-(3-bromo-4-chloro-5-methyl-
/'~ benzyl)-4-[4-(2-oxo-1,4-dihydro-2H-
O \ CHI (~ 1
N
(174)~j N' " quinazolin-3-yl)-piperidin-1-yl]-1-(4-
\ , ~~N
pyridin-4-yl-piperazin-1-yl)-butan-
1,4-dione
;' , 2-(3-bromo-4-chloro-5-methyl-
~' H, benzyl)-1-[4-(1-methyl-piperidin-4-
(175); ~ ~~N "~%"-~"-=H. yl)-piperazin-1-yl]-4-[4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperi-
din-1-yl]-butan-1,4-dione
N,C Br
Ci ~N~H [4-(1-{2-(3-bromo-4-chloro-5-
~"J methyl-benzyl)-4-oxo-4-[4-(2-oxo-
"
(176)I ~ N~" O 1,4-dihydro-2H-quinazolin-3-yl)-
piperidin-1-yl]-butyryl}-piperidin-4-
yl)-piperazin-1-yl]-acetic
acid
H~~_~ 'C, "~~ = methyl (1'-{2-(3-bromo-4-chloro-5-
methyl-benzyl)-4-oxo-4-[4-(2-oxo-
"~
177 " 1 4-dih dro-2H- uinazolin-3-
N~ I -
' r Y Y )
- H~ piperidin-1-yl]-butyryl}-
[4,4']bipiperidinyl-1-yl)-acetate
H,C Br
( 1'-{2-(3-bromo-4-chloro-5-methyV-
N benzyl)-4-oxo-4-[4-(2-oxo-1,4-di-
(178), N.GN hydro-2H-qmnazolin-3-yl)-piperidin-
'
]bipiperidinyl-1-yl)-
H 1-yl]-butyryl}-[4,4
acetic acid
B' , 4-(2-oxo-1,4-dihydro-2H-quinazolin-
' N~ ~"~CH, 3-yl)-piperidin-1-carboxylic
acid{1-
4
l
h
l
5
-ch
(17g)~ oro-
-met
y
-
(3-bromo-
~ ~ benzyl)-2-[4-(4-methyl-piperazin-1-
~ ~'
H yl)-pspendm-1-yl]-2-oxo-ethyl}-
amide '
CA 02503455 2005-04-22
53
Structure Name
4-(2-oxo-1,4-dihydro-2H-quinazolin-
CHj
3-yl)-piperidin-1-carboxylic
~ acid-[2-
N
(180) , N~" [1,4']bipiperidinyl-1'-yl-1-(3-bromo-
H 4-chloro-5
~"~ l-benz
l
meth
2-o
-
y
)-
-
y
xo
H ethyl]-amide
4-(2-oxo-1,4-dihydro-2H-quinazolin-
'
3-yl)-piperidin-1-carboxylic
' H: acid-{1-
~ hl
-~ l
b
h
3
N,~ , romo-4-c
H -
81 ~" oro-5-met
(1 ) y
J -
~' (
; ~ ~ ' benzyl)-2-[4-(1-methyl-piperidin-4-
N
yl)-piperazin-1-yl]-2-oxo-ethyl}-
amide
, 4-( 2-oxo-1, 4-d i hyd
ro-2 H-q a i n azol i
n- '
3-yl)-piperidin-1-carboxylic
acid-[1-
(3-bromo-4-chloro-5-methyl-
(182)
~ " ~
~
\ ~ benzyl)-2-(1'-methyl-
N-
[4,4']bipiperidinyl-1-yl)-2-oxo-ethyl]-
amide
e' Ci
H 4-(2-oxo-1,4-dihydro-2H-quinazolin-
3 3-yi)-piperidin-1-carboxylic
acid-[1-
(183) ~ N~N H N~/N (3-bromo-4-chloro-5-methyl-
~ ," (4
ridi
4
b
l)
2
xo
2
l
-py
-y
enzy
-
-o
-
-
n-
-
piperazin-1-yl)-ethyl]-amide
/ 2-(4-chloro-3-trifluoromethyl-
N~N N~N-CH,
N~ benzyl)-1-(1'-methyl-
H
(184) ~ ~ [4,4']bipiperidinyl-1-yl)-4-[4-(2-oxo-
1,4-dihydro-2H-quinazolin-3-yl)-
piperidin-1-yl]-butan-1,4-dione
F
2-(4-chloro-3-trifluoromethyl-
~ benzyl)-1-[4-(4-methyl-piperazin-1-
N~-N
N-'",
O yl)-piperidin-1-yf]-4-[4-(2-oxo-1,4-di-
(185) , N~"
hydro-2H-quinazolin-3-yl)-piperidin-
" 1-yl]-butan-1,4-dione
F
F F
1-[1,4']bipiperidinyl-1'-yl-2-(4-chloro-
(186) ~'N 3-trifluoromethyl-benzyl)-4-[4-(2-
oxo-1,4-dihydro-2H-quinazolin-3-
H yl)-piperidin-1-yl]-butan-1,4-dione
CA 02503455 2005-04-22
54
Structure Name
F F CI
F , ~ [4-(1-{2-(4-chloro-3-trifluoromethyl-
"
~"~ benzyl)-4-oxo-4-[4-(2-oxo-1,4-di-
".~
"~
(187) ~" hydro-2H-quinazolin-3-yl)-piperidin-
~ ~ "~ 1-yl]-butyryl}-piperidin-4-yl)-pipera-
" zin-1-yl]-acetic acid
'~'~ methyl (1'-{2-(4-chloro-3-
G.
"~ trifluoromethyl-benzyl)-4-oxo-4-[4-
H3
(188) ~; " (2-oxo-1,4-dihydro-2H-quinazolin-3-
' - H~ yl)-piperidin-1-yl]-butyryl}-
[4,4']bipiperidinyl-1-yl)-acetate
FF G
~, (1'-{2-(4-chloro-3-trifluoromethyl-
"~ benzyl)-4-oxo-4-[4-(2-oxo-1,4-di-
(189) ~" " hydro-2H-quinazolin-3-yl)-piperidin-
~ - "~ 1-yl]-butyryl}-[4,4']bipiperidinyl-1-yl)-
acetic acid
~ 2-(4-chloro-3-trifluoromethyl-
~
N~N N~N-CH,
~
1 ~
J
~ benzyl)-1-(1 -methyl-
-
.
Fi
(190) r ~ [4,4']bipiperidinyl-1-yl)-4-[4-(2-oxo-
1,4-dihydro-2H-quinazolin-3-yl)-
piperidin-1-yl]-butan-1,4-dione
i
the enantiomers, the diastereomers and the salts thereof, while the
compounds
CA 02503455 2005-04-22
(14) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-{(R)-1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-[4-
( 1-methyl-piperidin-4-yl )-piperazin-1-yl]-2-oxo-ethyl)-amide,
(15) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-[(R)-1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-(4-
azetidin-1-yl-piperidin-1-yl )-2-oxo-ethyl]-amide,
(16) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-{(R)-1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-[4-
( 5-methyl-2, 5-d iaza-bicyclo[2.2.1 ]hept-2-yl)-pi peridin-1-yl]-2-oxo-ethyl}-
amide,
(17) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-[(R)-1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-
oxo-2-(4-piperazin-1-yl-piperidin-1-yl)-ethyl]-amide,
(18) [1'-((R)-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-2-{[4-(2-oxo-
1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-carbonyl]-
amino)-propionyl)-4,4'-bipiperidinyl-1-yl]-acetic acid,
(19) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-[1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-1,4'-
bipiperidinyl-1'-yl-2-oxo-ethyl]-amide,
(20) (S)-2-(4-amino-3-bromo-5-trifluoromethyl-benzyl)-1-[4-(4-methyl-
piperazin-1-yl )-piperidin-1-yl]-4-[4-(2-oxo-1, 2,4, 5-tetrahyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(21 ) (S)-2-(4-amino-3-bromo-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1, 3-benzodiazepin-3-yl )-piperidin-1-yl]-1-(4-pyridi n-4-yl-
piperazin-1-yl)-butan-1,4-dione,
(22) (S)-2-(4-amino-3-bromo-5-trifluoromethyl-benzyl)-1-1,4'-bipiperidinyl-1'-
CA 02503455 2005-04-22
56
yl-4-[4-(2-oxo-1, 2,4, 5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione,
(23) (S)-2-(4-amino-3-bromo-5-trifluoromethyl-benzyl)-1-[4-(1-methyl-
piperidin-4-yl)-piperazin-1-yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl )-piperidin-1-yl]-butan-1,4-dione,
(24) (S)-2-(4-amino-3-bromo-5-trifluoromethyl-benzyl)-1-(1'-methyl-4,4'-
bipiperidinyl-1-yl)-4-[4-(2-oxo-1,2,4, 5-tetrahydro-1,3-benzodiazepin-3-
yl)-piperidin-1-yl]-butan-1,4-dione,
(25) (S)-2-(4-amino-3-trifluoromethyl-benzyl)-1-[4-(4-methyl-piperazin-1-yl)-
piperidin-1-yl]-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-benzod iazepi n-3-yl )-
piperidin-1-yl]-butan-1,4-dione,
(26) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-(4-dimethylamino-
piperidin-1-yl)-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-
piperidin-1-yl]-butan-1,4-dione,
(27) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-1-(4-pyrrolidin-1-yl-
piperidin-1-yl)-butan-1,4-dione,
(28) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-(1'-methyl-4,4'-
bipiperid inyl-1-yl )-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-benzodiazepi n-3-
yl)-piperidin-1-yl]-butan-1,4-dione,
(29) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-
cyclopropylmethyl-piperazin-1-yl )-piperidin-1-yl]-4-[4-(2-oxo-1,2,4, 5
tetrahydro-1, 3-benzodiazepin-3-yl)-piperidin-1-ylJ-butan-1,4-dione,
(30) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-(4-morpholine-4-yl-
piperidin-1-yl)-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-
piperidin-1-yl]-butan-1,4-dione,
CA 02503455 2005-04-22
57
(31) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1, 3-benzodiazepin-3-yl)-piperidin-1-ylJ-1-(4-perhydro-azepin-
1-yl-piperidin-1-yl)-butan-1,4-dione,
(32) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-methyl-
perhydro-1,4-diazepin-1-yl)-piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yi]-butan-1,4-dione,
(33) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-isopropyl-
piperazin-1-yl )-piperidin-1-yl]-4-[4-( 2-oxo-1, 2,4, 5-tetrahydro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(34) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-1,4'-bipiperidinyl-1'-
yl-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione,
(35) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(1-methyl-
pi peridi n-4-yl )-perhyd ro-1, 4-diazepi n-1-yl]-4-[4-(2-oxo-1,2,4, 5-
tetrahydro-1, 3-benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(36) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[3-(4-methyl-
piperazin-1-yl )-azetidi n-1-yl]-4-[4-(2-oxo-1, 2,4, 5-tetrahyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(37) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-1-(3-pyrrolidin-1-yl-
azetidin-1-yl)-butan-1,4-dione,
(38) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-1-(3-piperidin-1-yl-
azetidi n-1-yl )-butan-1, 4-dione,
(39) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-(4-azetidin-1-yl-
CA 02503455 2005-04-22
58
piperidi n-1-yl )-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-benzodiazepi n-3-yl
)-
piperidin-1-yl]-butan-1,4-dione,
(40) (S)-1-[4-(4-acetyl-piperazin-1-yl)-piperidin-1-yl]-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl )-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(41) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-(4-
diethylaminomethyl-piperidin-1-yl)-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl )-piperidin-1-yl]-butan-1,4-dione,
(42) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-ethyl-
piperazin-1-yl )-piperidi n-1-yl]-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-
benzodiazepin-3-yl )-piperidin-1-yl]-butan-1,4-dione,
(43) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(1-ethyl-
piperidin-4-yl)-piperazin-1-yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(44) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahyd ro-1, 3-benzodiazepi n-3-yl )-pi perid in-1-yl]-1-(3, 4, 5, 6-tetra
hyd ro-
2H-4,4'-bipyridinyl-1-yl)-butan-1,4-dione,
(45) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahyd ro-1, 3-benzodiazepi n-3-yl )-piperidin-1-yl]-1-(4-pyrid in-4-yl-
piperazin-1-yl)-butan-1,4-dione,
(46) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-1-(3-perhydro-azepin-
1-yl-azetid in-1-yl )-butan-1, 4-d lone,
(47) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(1-benzyl-
piperidi n-4-yl )-pi perazi n-1-yl]-4-[4-(2-oxo-1, 2, 4, 5-tetra hyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
CA 02503455 2005-04-22
59
(48) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-benzyl-
piperazin-1-yl)-piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepi n-3-yl )-piperid i n-1-yl]-butan-1,4-dione,
(49) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-(7-dimethyl-
ami nomethyl-1,2, 4, 5-tetrahyd ro-3-benzazepin-3-yl )-4-[4-(2-oxo-1,2, 4, 5-
tetrahydro-1, 3-benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(50) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-
dimethylaminomethyl-phenyl)-piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(51) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1, 3-benzodiazepin-3-yl)-piperidin-1-yl]-1-{4-[4-(2,2,2-
trifluoro-ethyl)-piperazin-1-yl]-piperidin-1-yl}-butan-1,4-dione,
(52) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-(1'-
methanesulphonyl-4,4'-bipiperidinyl-1-yl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1, 3-benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(53) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-(9-methyl-3,9-
d iaza-spi ro[5.5]u ndec-3-yl )-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(54) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-1-(4-piperidin-1-yl-
methyl-piperidin-1-yl)-butan-1,4-dione,
(55) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(2-
dimethylamino-ethyl)-piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
CA 02503455 2005-04-22
(56) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-N-methyl-N-[2-(1-
methyl-piperidin-4-yl)-ethylJ-4-oxo-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-ylJ-butyramide,
(57) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-N-methyl-N-(1-
methyl-piperidin-4-ylm ethyl )-4-oxo-4-[4-(2-oxo-1, 2,4, 5-tetrahydro-1, 3-
benzodiazepin-3-yl)-piperidin-1-ylJ-butyramide,
(58) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(2-diethylamino-
ethyl )-piperid in-1-yIJ-4-[4-(2-oxo-1, 2, 4, 5-tetra hydro-1, 3-benzod
iazepin-
3-yl)-piperidin-1-yl]-butan-1,4-dione,
(59) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(cyclopentyl-
methyl-ami no)-piperidi n-1-ylJ-4-[4-(2-oxo-1, 2,4, 5-tetra hyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(60) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(1,1-dioxo-1,6-
isothiazolidin-2-yl)-piperidin-1-ylJ-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-ylJ-butan-1,4-dione,
(61) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(2-oxo-perhydro
1, 3-ox azi n-3-yl )-p i peri d i n-1-yl J-4-[4-( 2-oxo-1, 2, 4, 5-tetra h yd
ro-1, 3
benzodiazepin-3-yl)-piperidin-1-ylJ-butan-1,4-dione,
(62) methyl1-{(S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4
(2-oxo-1, 2, 4, 5-tetra hydro-1, 3-benzod iazepi n-3-yl )-piperidi n-1-yl]
butyryl}-piperidin-4-carboxylate,
(63) 8-{(S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-
1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-ylJ-butyryl}-1,3,8-
triaza-spiro[4.5Jdecan-2,4-dione,
CA 02503455 2005-04-22
61
(64) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(2-oxo-
imidazolidin-1-yl )-piperidi n-1-yl]-4-[4-(2-oxo-1, 2,4, 5-tetrahydro-1, 3-
benzodiazepin-3-yl )-piperidin-1-yl]-butan-1,4-dione,
(65) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(1-methyl-
pi peridi n-4-yl )-piperazi n-1-yl]-4-[4-(2-oxo-1, 2, 4, 5-tetrahydro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(66) (S)-2-(4-amino-3-chloro-5-trifluoromethyi-benzyl)-1-(2-amino-4,5,7,8-
tetrahydro-thiazolo[4,5-d]azepin-6-yl)-4-[4-(2-oxo-1,2,4,5-tetrahydro-
1,3-benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(67) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(2-methyl-
imidazol-1-yl )-piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl )-piperid in-1-yl]-butan-1,4-dione,
(68) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-methyl-
imidazol-1-yl)-piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(69) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1, 3-benzodiazepin-3-yl)-piperidin-1-yl]-1-(4-thiazol-2-yl-
piperazin-1-yl)-butan-1,4-dione,
(70) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(2,4-dimethyl
imidazol-1-yl)-piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(71 ) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-(4-imidazoi-1-yl-
piperidin-1-yl)-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-
piperidin-1-yl]-butan-1,4-dione,
CA 02503455 2005-04-22
62
(72) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-1-(4-1,2,4-triazol-1-yl-
piperidin-1-yl)-butan-1,4-dione,
(73) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(1-aza-
bicycio[2.2.2]oct-3-yl )-piperazi n-1-yl]-4-[4-( 2-oxo-1, 2,4, 5-tetrahyd ro-
1, 3-
benzodiazepin-3-y1)-piperidin- 1-yi]-butan-1,4-dione,
(74) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-N-(5-amino-pentyl)-4-
oxo-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl~piperidin-1-
yl]-butyramide,
(75) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-N-(3-aminomethyl-
benzyl )-4-oxo-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-benzodiazepi n-3-yl )-
piperidin-1-yl]-butyramide,
(76) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(8-methyl-8-aza-
bicyclo[3.2.1 ]oct-3-yl)-piperazin-1-yl]-4-[4-(2-oxo-1, 2,4, 5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(77) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetra hyd ro-1, 3-benzod iazepi n-3-yl )-piperidi n-1-yl]-1-(4-piperazi n-1-yl-
piperidin-1-yl)-butan-1,4-dione,
(78) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(1H-imidazol-4-
yl)-piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-
yl )-piperidin-1-yl]-butan-1, 4-dione,
(79) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl~1-[4-(4-methyl-
piperazin-1-yl)-piperidin-1-yl]-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-
yl)-piperidin-1-yl]-butan-1,4-dione,
CA 02503455 2005-04-22
63
(80) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyi)-1-[4-(4-methyl-
piperazin-1-yl)-piperidin-1-yl]-4-[4-( 5-oxo-3-phenyl-4,5-dihydro-1,2,4-
triazol-1-yl )-piperidin-1-yl]-butan-1, 4-dione,
(81) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-methyl-
piperazin-1-yl)-piperidin-1-yl]-4-[4-(2-oxo-1,2-dihydro-imidazo[4,5-
c]quinolin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(82) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-methyl-
pi perazin-1-yl )-pi peridin-1-yl]-4-[4-(2-oxo-1, 2-dihydro-4H-thieno[3,4-
d]pyrimidin-3-yl )-piperidin-1-yl]-butan-1,4-dione,
(83) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(5-methyl-2,5-
d iaza-bicyclo[2.2.1 ] hept-2-yl )-piperid in-1-yl]-4-[4-(2-oxo-1, 2, 4, 5-
tetrahydro-1, 3-benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(84) (S)-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-1-[4-(4-methyl-perhydro-
1,4-diazepin-1-yl)-piperidin-1-ylJ-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(85) (S)-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-1-[4-(1-methyl-piperidin-
4-yl )-perhyd ro-1, 4-diazepin-1-yl]-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(86) (S)-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-1-[4-(1-methyl-piperidin-
4-yl )-piperazi n-1-yl]-4-[4-(2-oxo-1, 2, 4, 5-tetrahydro-1, 3-benzodiazepi n-
3-yl)-piperidin-1-yl]-butan-1,4-dione,
(87) (S)-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-1-[4-(1-aza-
bicyclo[2.2.2]oct-3-yl )-piperazi n-1-yl]-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-
1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
CA 02503455 2005-04-22
64
(88) (S)-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-1-[4-(4-methyl-piperazin-
1-yl)-piperidin-1-yl]-4-[4-( 2-oxo-1, 2, 4, 5-tetrahydro-1, 3-benzodiazepin-3-
yl)-piperidin-1-yl]-butan-1,4-dione,
(89) (S)-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahyd ro-1, 3-benzodiazepi n-3-yl )-piperid in-1-yl]-1-(4-perhyd ro-azepin-
1-yl-piperidin-1-yl)-butan-1,4-dione,
(90) (S)-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-1-1,4'-bipiperidinyl-1'-yl-
4-[4-( 2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-benzod iazepi n-3-yl )-piperidi n-1-
yl]-
butan-1,4-dione,
(91) (S)-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-1-(4-piperazin-1-yl-
piperidin-1-yl)-butan-1,4-dione,
(92) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-{(R)-1-(4-amino-3,5-bis-trifluoromethyl-benzyl)-2-[4-(4-
methyl-piperazin-1-yl)-piperidin-1-yl]-2-oxo-ethyl}-amide,
(93) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-{(R)-1-(4-amino-3,5-bis-trifluoromethyl-benzyl)-2-[4-(1-
methyl-piperidin-4-yl )-piperazin-1-yl]-2-oxo-ethyl}-amide,
(94) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-[(R)-1-(4-amino-3,5-bis-trifluoromethyl-benzyl)-2-1,4'-
bipiperidinyl-1'-yl-2-oxo-ethyl]-amide,
(95) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-[(R)-1-(4-amino-3,5-bis-trifluoromethyl-benzyl)-2-oxo-2-
(4-piperazin-1-yl-piperidin-1-yl)-ethyl]-amide,
CA 02503455 2005-04-22
b5
(96) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-(R)-1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-[4-
(4-methyl-piperazin-1-yl)-piperidin-1-yl]-2-oxo-ethyl ester,
(97) 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-carboxylic acid-[1-
(4-bromo-3-methyl-benzyl)-2-( 1'-methyl-[4,4']bipiperidinyl-1-yl)-2-oxo-
ethyl]-amide,
(98) 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-carboxylic acid-{1-
(4-bromo-3-methyl-benzyl )-2-[4-(4-methyl-pi perazin-1-yl )-pi peridin-1-
yl]-2-oxo-ethyl}-amide,
(99) 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-carboxylic acid-{1-
(4-bromo-3-methyl-benzyl )-2-[4-( 1-methyl-piperidi n-4-yl )-piperazin-1-
yl]-2-oxo-ethyl}-amide,
(100) 2-(4-bromo-3-methyl-benzyl)-1-(1'-methyl-[4,4']bipiperidinyl-1-yl)-4-[4-
(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(101) 1-[1,4']Bipiperidinyl-1'-yl-2-(4-bromo-3-methyl-benzyl)-4-[4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidin-1-y1]-butan-1,4-dione,
(102) 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-carboxylic acid{1-
(4-chloro-3-methyl-benzyl)-2-[4-(4-methyl-piperazin-1-yl)-piperidin-1-yl]-
2-oxo-ethyl}-amide,
(103) 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-carboxylic acid-{1-
(4-chloro-3-methyl-benzyl )-2-[4-( 1-methyl-piperidin-4-yl )-piperazin-1-yl]-
2-oxo-ethyl}-amide,
(104) 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-carboxylic acid-[1-
(4-chloro-3-methyl-benzyl )-2-( 1'-methyl-[4,4']bipiperidinyl-1-yl)-2-oxo-
ethyl]-amide,
CA 02503455 2005-04-22
66
(105) 2-(4-chloro-3-methyl-benzyl)-1-(4-(4-methyl-piperazin-1-yl)-piperidin-1-
yl]-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butan-1,4-
dione,
(106) 2-(4-chloro-3-methyl-benzyl)-1-[4-(1-methyl-piperidin-4-yl)-piperazin-1-
yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
yl]-butan-1,4-dione,
(107) 2-(4-chloro-3-methyl-benzyl)-1-[4-(4-methyl-piperazin-1-yl)-piperidin-1-
yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
yl]-butan-1,4-dione,
(108) 2-(3-bromo-4-chloro-5-methyl-benzyl)-1-[4-(4-methyl-piperazin-1-yl)-
piperidin-1-yl]-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-
yl]-butan-1, 4-dione,
(109) 2-(3-bromo-4-chloro-5-methyl-benzyl)-1-(1'-methyl-[4,4']bipiperidinyl-1-
yl)-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butan-1,4-
dione,
(110) 2-(3-bromo-4-chloro-5-methyl-benzyl)-1-[4-(1-methyl-piperidin-4-yl)-
piperazin-1-yl]-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-
y1]-butan-1,4-dione,
(111) 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-carboxylic acid{1-
(3-bromo-4-chloro-5-methyl-benzyl )-2-[4-(4-methyl-pi perazin-1-yl )-
piperidin-1-yl]-2-oxo-ethyl}-amide,
(112) 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl~piperidin-1-carboxylic acid-{1
(3-bromo-4-chloro-5-methyl-benzyl)-2-[4-( 1-methyl-piperidin-4-yl)
piperazin-1-yl]-2-oxo-ethyl}-amide,
CA 02503455 2005-04-22
b7
(113) 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-carboxylic acid-[1-
(3-bromo-4-chloro-5-methyl-benzyl)-2-(1'-methyl-[4,4']bipiperidinyl-1-yl)-
2-oxo-ethyl]-amide,
(114) 2-(4-chloro-3-trifluoromethyl-benzyl)-1-[4-(4-methyl-piperazin-1-yl)-
piperidin-1-yl]-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-
yl]-butan-1,4-dione,
the enantiomers, the diastereomers and the salts thereof are of particular
importance and the compounds
(1) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-methyl-
pi perazin-1-yl )-pi peridi n-1-yl]-4-[4-(2-oxo-1, 2, 4, 5-tetrahydro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(2) 2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-(1'-methyl-
[4, 4']bipiperidi nyl-1-yl)-4-[4-(2-oxo-1, 4-d ihydro-2H-q ui nazoli n-3-yl)-
piperidin-1-yl]-butan-1,4-dione,
(3) 2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-methyl-piperazin-
1-yl )-p i peri d i n-1-yl]-4-[4-( 2-oxo-1, 4-d i h yd ro-2 H-q a i nazol i n-
3-yl )-
piperidin-1-yl]-butan-1,4-dione,
(4) 2-(4-amino-3-chloro-5-trifluoromethyl-benzylr1-[1,4']bipiperidinyl-1'-yl-
4-[4-(2-oxo-1,4-dihydro-2H-q ui nazoii n-3-yl )-pi perid in-1-yl]-butan-1, 4-
dione,
(5) 2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,4-dihydro-
2H-quinazolin-3-y1)-piperidin-1-yl]-1-(4-pyridin-4-yl-piperazin-1-yl)-
butan-1,4-dione,
(6) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-[(R)-1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-
1,4'-bipiperidinyl-1'-yl-2-oxo-ethyl]-amide,
CA 02503455 2005-04-22
68
(7) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-[(R)-1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-(4-
dimethylamino-piperidin-1-yl)-2-oxo-ethyl]-amide,
(8) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-[(R)-1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-
oxo-2-(4-pyridin-4-yl-piperazin-1-yl)-ethyl]-amide,
(9) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-[(R)-1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-(1'-
methyl-4,4'-bipiperidinyl-1-yl)-2-oxo-ethyl]-amide,
(10) ethyl [1'-((R)-3-(4-amino-3-chloro-5-trifluoromethyi-phenyl)-2-{[4-(2-oxo-
1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-carbonyl]-
amino)-propionyl)-4,4'-bipiperidinyl-1-ylJ-acetate,
(11) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-{(R)-1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-[4-
(4-methyl-piperazin-1-yl)-piperidin-1-yIJ-2-oxo-ethyl}-amide,
(12) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzadiazepin-3-yl)-piperidin-1-
carboxylic acid-{(R)-1-(4-amino-3-chloro-5-trifiuoromethyl-benzyl)-2-[4-
( 1-methyl-pi perid in-4-yl )-piperazin-1-yl)-2-oxo-ethyl}-amide,
(13) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-[(R)-1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-(4-
azetidin-1-yl-piperidin-1-yl)-2-oxo-ethyl]-amide,
(14) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-{(R)-1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-(4-
( 5-methyl-2, 5-diaza-bicyclo[2.2.1 )hept-2-yl)-pi peridin-1-yl]-2-oxo-ethyl)-
amide,
CA 02503455 2005-04-22
69
(15) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-[(R)-1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-
oxo-2-(4-piperazi n-1-yl-piperidin-1-yl )-ethyl]-amide,
(16) [1'-((R)-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-2-{[4-(2-oxo-
1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-carbonyl]-
amino}-propionyl)-4,4'-bipiperidinyl-1-yl]-acetic acid,
(17) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-[1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-1,4'-
bipiperidinyl-1'-yl-2-oxo-ethyl]-amide,
(18) (S)-2-(4-amino-3-bromo-5-trifluoromethyl-benzyl)-1-[4-(4-methyl-
piperazin-1-yl)-piperidin-1-yl]-4-[4-(2-oxo-1, 2,4, 5-tetrahyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(19) (S)-2-(4-amino-3-bromo-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1, 3-benzodiazepi n-3-yl )-piperidin-1-yl]-1-(4-pyrid in-4-yl-
piperazin-1-yl)-butan-1,4-dione,
(20) (S)-2-(4-amino-3-bromo-5-trifluoromethyl-benzyl)-1-1,4'-bipiperidinyl-1'-
yl-4-[4-( 2-oxo-1, 2,4, 5-tetrahyd ro-1, 3-benzodiazepin-3-yl)-piperidin-1-yl]-
butan-1,4-dione,
(21) (S)-2-(4-amino-3-bromo-5-trifluoromethyi-benzyl)-1-[4-(1-methyl-
pi peridi n-4-yl )-piperazin-1-yl]-4-[4-( 2-oxo-1, 2,4, 5-tetrahydro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(22) (S)-2-(4-amino-3-bromo-5-trifluoromethyl-benzyl)-1-(1'-methyl-4,4'-
bipiperidinyl-1-yl)-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-
yl)-piperidin-1-yl]-butan-1,4-dione,
(23) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-1-(4-pyrrolidin-1-yl-
CA 02503455 2005-04-22
piperidin-1-yl)-butan-1,4-dione,
(24) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-(1'-methyl-4,4'-
bipiperidinyl-1-yl)-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-
yl)-piperidin-1-yl]-butan-1,4-dione,
(25) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-
cyclopropylmethyl-piperazin-1-yl)-piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(26) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-1-(4-perhydro-azepin-
1-yl-pi perid in-1-yl )-butan-1, 4-d lone,
(27) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-methyl-
perhydro-1,4-diazepin-1-yl)-piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-
tetrahyd ro-1, 3-benzodiazepin-3-yl )-piperidin-1-yl]-butan-1,4-dione,
(28) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-1,4'-bipiperidinyl-1'-
yl-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-benzodiazepi n-3-yl )-piperidi n-1-
yl]-
butan-1,4-dione,
(29) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(1-methyl-
piperidin-4-yl)-perhydro-1,4-diazepin-1-yl]-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1, 3-benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(30) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[3-(4-methyl-
pi perazi n-1-yl )-azetidi n-1-yl]-4-[4-( 2-oxo-1, 2,4, 5-tetrahydro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(31 ) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-(4-azetidin-1-yl-
pi peridi n-1-yl )-4-[4-( 2-oxo-1, 2, 4, 5-tetra hyd ro-1, 3-benzodiazepi n-3-
yl )-
piperidin-1-yl]-butan-1,4-dione,
CA 02503455 2005-04-22
71
(32) (S)-1-[4-(4-acetyl-piperazin-1-yl)-piperidin-1-yl]-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(33) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-(4-
diethylaminomethyl-piperidin-1-yl)-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(34) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-ethyl-
piperazi n-1-yl )-piperidi n-1-yl]-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-
benzodiazepin-3-yi)-piperidin-1-yl]-butan-1,4-dione,
(35) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(1-ethyl-
pi peridi n-4-yl )-piperazi n-1-yl]-4-[4-(2-oxo-1, 2,4, 5-tetrahyd ro-1, 3-
benzodiazepin-3-yl )-piperidin-1-yl]-butan-1,4-dione,
(36) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-1-(3,4,5,6-tetrahydro-
2H-4,4'-bipyridinyl-1-yl)-butan-1,4-dione,
(37) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yi)-piperidin-1-yl]-1-(4-pyridin-4-yl-
piperazin-1-yl)-butan-1,4-dione,
(38) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(1-benzyl-
piperidin-4-yl )-piperazin-1-yl]-4-[4-(2-oxo-1, 2,4, 5-tetrahydro-1, 3-
benzodiazepin-3-yl )-piperid in-1-yl]-butan-1,4-dione,
(39) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-(1'-
methanesulphonyl-4,4'-bipiperidinyl-1-yl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1, 3-benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
CA 02503455 2005-04-22
72
(40) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-(9-methyl-3,9-
diaza-spiro[5.5]undec-3-yl)-4-[4-(2-oxo-1,2,4,5-tetrahydro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(41) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahyd ro-1, 3-benzodiazepi n-3-yl )-pi perid in-1-yl]-1-( 4-piperid i n-1-
yl-
methyl-piperidin-1-y1)-butan-1,4-dione,
(42) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(2-
dimethylamino-ethyl )-piperidin-1-y1)-4-[4-( 2-oxo-1, 2, 4, 5-tetrahydro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(43) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-N-methyl-N-[2-(1-
methyl-piperidin-4-yl)-ethyl]-4-oxo-4-[4-(2-oxo-1,2,4, 5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-butyramide,
(44) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-N-methyl-N-(1-
methyl-piperidin-4-ylmethyl)-4-oxo-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl)-butyramide,
(45) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(2-diethylamino-
ethyl)-piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-
3-yl )-piperid i n-1-yl]-butan-1, 4-d lone,
(46) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(2-oxo-perhydro-
1, 3-oxazin-3-y1)-piperid in-1-yl]-4-[4-( 2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(47) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(2-oxo-
imidazolidin-1-yl)-piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
CA 02503455 2005-04-22
73
(48) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(1-methyl-
piperidin-4-yl )-piperazin-1-yl]-4-[4-( 2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl)-butan-1,4-dione,
(49) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(2-methyl-
imidazol-1-yl)-piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(50) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-methyl-
imidazol-1-yl)-piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl)-butan-1,4-dione,
(51) (S)-2-(4-amino-3-chloro-5-trifiuoromethyl-benzyl)-1-[4-(2,4-dimethyl
imidazol-1-yl)-piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(52) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-(4-imidazol-1-yl-
piperidi n-1-yl )-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-benzodiazepi n-3-yl
)-
piperidin-1-yl]-butan-1,4-dione,
(53) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl)-1-(4-1,2,4-triazol-1-yl-
piperidin-1-yi)-butan-1,4-dione,
(54) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(1-aza-
bicyclo[2.2.2]oct-3-yl )-piperazin-1-yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl)-butan-1,4-dione,
(55) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(8-methyl-8-aza-
bicyclo[3.2.1 )oct-3-yl )-piperazi n-1-yl)-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-
1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
CA 02503455 2005-04-22
74
(56) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-1-(4-piperazin-1-yl-
piperidin-1-yl)-butan-1,4-dione,
(57) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(1H-imidazol-4-
yl )-piperid i n-1-yl)-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-benzodiazepi n-
3-
yl)-piperidin-1-yl]-butan-1,4-dione,
(58) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-methyl-
piperazin-1-yl)-piperidin-1-yl]-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-
yl)-piperidin-1-yl]-butan-1,4-dione,
(59) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-methyl-
piperazin-1-yl)-piperidin-1-yl]-4-[4-(5-oxo-3-phenyl-4,5-dihydro-1,2,4-
triazol-1-yl)-piperidin-1-yl]-butan-1,4-dione,
(60) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl~1-[4-(4-methyl-
piperazin-1-yl)-piperidin-1-yl]-4-[4-(2-oxo-1,2-dihydro-imidazo[4,5-
c]quinolin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(61) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-(4-(4-methyl-
piperazin-1-yl)-piperidin-1-yl]-4-[4-(2-oxo-1,2-dihydro-4H-thieno[3,4-
d]pyrimidin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(62) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(5-methyl-2,5-
diaza-bicyclo[2.2.1 ]hept-2-yl)-piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-y1]-butan-1,4-dione,
(63) (S)-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-1-[4-(4-methyl-perhydro-
1, 4-d iazepi n-1-yl )-piperid i n-1-yl]-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1,
3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
CA 02503455 2005-04-22
(64) (S)-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-1-[4-(1-methyl-piperidin-
4-yl )-perhydro-1, 4-diazepin-1-yl]-4-[4-( 2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(65) (S)-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-1-[4-(1-methyl-piperidin-
4-yl )-piperazi n-1-yl]-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-benzodiazepi n-
3-yl)-piperidin-1-yl]-butan-1,4-dione,
(66) (S)-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-1-[4-(1-aza-
bicyclo[2.2.2]oct-3-yl )-piperazi n-1-yl]-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-
1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione,
(67) (S)-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-1-[4-(4-methyl-piperazin-
1-yl)-piperidin-1-yl]-4-[4-(2-oxo-1, 2,4, 5-tetrahydro-1, 3-benzodiazepin-3-
yl)-piperidin-1-yl]-butan-1,4-dione,
(68) (S)-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-1-(4-perhydro-azepin-
1-yl-pi perid i n-1-yl )-butan-1,4-d ione,
(69) (S)-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-1-1,4'-bipiperidinyl-1'-yl-
4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yi)-piperidin-1-yl]-
butan-1,4-dione,
(70) (S)-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-1-(4-piperazin-1-yl-
piperidin-1-yl)-butan-1,4-dione,
(71) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxyiic acid-{(R)-1-(4-amino-3,5-bis-trifluoromethyl-benzyl)-2-[4-(4-
methyl-piperazin-1-yl)-piperidin-1-yl]-2-oxo-ethyl}-amide,
CA 02503455 2005-04-22
76
(72) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-{(R)-1-(4-amino-3,5-bis-trifluoromethyl-benzyl)-2-[4-(1-
methyl-piperidin-4-yl)-piperazin-1-yl]-2-oxo-ethyl}-amide,
(73) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-[(R)-1-(4-amino-3,5-bis-trifluoromethyl-benzyl)-2-1,4'-
bipiperidinyl-1'-yl-2-oxo-ethyl]-amide,
(74) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-[(R)-1-(4-amino-3,5-bis-trifluoromethyl-benzyl)-2-oxo-2-
(4-piperazin-1-yl-piperidin-1-yl)-ethyl]-amide,
(75) 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-(R)-1-(4-amino-3-chloro-5-trifiuoromethyl-benzyl)-2-[4-
(4-methyl-piperazin-1-yl)-piperidin-1-yl]-2-oxo-ethyl ester,
(76) 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-carboxylic acid-[1-
(4-bromo-3-methyl-benzyl )-2-( 1'-methyl-[4, 4']bipiperidinyl-1-yl )-2-oxo-
ethyl]-amide,
(77) 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-carboxylic acid-{1-
(4-bromo-3-methyl-benzyl )-2-[4-(4-methyl-pi perazin-1-yl )-piperidi n-1-
yl]-2-oxo-ethyl}-amide,
(78) 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-carboxylic acid-{1-
(4-bromo-3-methyl-benzyl)-2-[4-( 1-methyl-piperidin-4-yl)-piperazin-1-
yl]-2-oxo-ethyl}-amide,
(79) 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-carboxylic acid{1-
(4-chloro-3-methyl-benzyl)-2-[4-(4-methyl-piperazin-1-yl)-piperidin-1-yl]-
2-oxo-ethyl}-amide,
CA 02503455 2005-04-22
77
(80) 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-carboxylic acid-{1-
(4-chloro-3-methyl-benzyl )-2-[4-( 1-methyl-pi perid in-4-yl )-pi perazin-1-
yl]-
2-oxo-ethyl}-amide,
(81) 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-carboxylic acid{1-
(3-bromo-4-chloro-5-methyl-benzyl )-2-[4-(4-methyl-piperazin-1-yl )-
pi peridi n-1-yl]-2-oxo-ethyl)-amide,
(82) 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-carboxylic acid-{1
(3-bromo-4-chloro-5-methyl-benzyl)-2-[4-( 1-methyl-piperidin-4-yl)
piperazin-1-yl]-2-oxo-ethyl-amide,
the enantiomers, the diastereomers and the salts thereof are of exceptional
importance.
The compounds of general formula (I) are prepared by methods known in
principle. The following methods have proved particularly satisfactory for
preparing the compounds of general formula (I) according to the invention:
(a) In order to prepare compounds of general formula (I) wherein X
denotes an oxygen atom or the NH group and R' to R3 are as hereinbefore
defined, with the proviso that these groups R2 and R3 do not contain any free
carboxylic acid function:
Reacting piperidines of general formula
R'
''-~~NH , (III)
wherein R' is as hereinbefore defined,
(i) with carbonic acid derivatives of general formula
CA 02503455 2005-04-22
78
A
G- _G
(IV)
wherein A is as hereinbefore defined and G denotes a nucleofugic group,
preferably the phenoxy, 1H-imidazol-1-yl, 1H-1,2,4-triazol-1-yl,
trichloromethoxy or the 2,5-dioxopyrrolidin-1-yloxy group, with the proviso
that X denotes the NH group, or
(ii) with carbonic acid derivatives of general formula
A
G- 'G
wherein A denotes the oxygen atom and G denotes a nucleofugic group
which may be identical or different, preferably the chlorine atom or the p-
nitrophenoxy or trichloromethoxy group, with the proviso that X denotes the
oxygen atom,
and with compounds of general formula
V
R2
I
I"~~N N.Rs
H O ,(V)
wherein X denotes the oxygen atom or an -NH group and U, V, W, R2 and R3
are as hereinbefore defined, with the proviso that R2 and R3 do not contain
any free carboxylic acid and/or any other free primary or secondary aliphatic
amino function or any other free hydroxy functions.
CA 02503455 2005-04-22
79
The fundamentally two-step reactions are normally carried out as one-pot
processes, in which, preferably, in the first step, one of the two components
(III) or (V) is reacted with equimolar amounts of the carbonic acid derivative
of
general formula (IV) in a suitable solvent at Lower temperature, then at feast
equimolar amounts of the other component (III) or (V) are added and the
reaction is completed at a higher temperature. The reactions with bis-
(trichloromethyl)-carbonate are preferably carried out in the presence of at
least 2 equivalents (based on bis-(trichloromethyl~-carbonate) of a tertiary
base, for example triethylamine, N-ethyldiisopropylamine, pyridine, 1,5-diaza-
bicyclo-[4.3.0]-non-5-ene, 1,4-diazabicyclo[2.2.2Joctane or 1,8-diazabicyclo-
[5.4.0]-undec-7-ene. The solvents used, which should be anhydrous, may be
for example tetrahydrofuran, dioxane, dimethylformamide, dimethylacetamide,
N-methyl-2-pyrrolidone, 1,3-dimethyl-2-imidazolidinone or acetonitrile, while
if
bis-(trichloromethyl)-carbonate is used as the carbonyl component anhydrous
chlorohydrocarbons, for example dichloromethane, 1,2-dichloroethane or
trichloroethylene are preferred. The reaction temperatures for the first
reaction
step are between -30°C and +25°C, preferably -5°C and
+10°C, for the
second reaction step between +15°C and the boiling temperature of the
solvent used, preferably between +20°C and +70°C (cf. also: H.
A. Staab and
W. Rohr, "Synthesen mit heterocyclischen Amiden (Azoliden)", Neuere
Methoden der Praparativen Organischen Chemie, Volume V, p. 53-93, Verlag
Chemie, Weinheim/Bergstr., 1967; P. Majer and R.S. Randad, J. Org. Chem.
59, p. 1937-1938 (1994); K. Takeda, Y. Akagi, A. Saiki, T. Sukahara and H.
Ogura, Tetrahedron Letters 24 (42), 4569-4572 (1983)); S.R. Sandier and W.
Karo in "Organic Functional Group Preparations", Vol. II, S. 223-245,
Academis Press, New York 1971 ).
(b) In order to prepare compounds of general formula (I) wherein X
denotes the methylene group and R' to R3 are as hereinbefore defined, with
the proviso that these groups do not contain any free carboxylic acid and/or
other free primary or secondary aliphatic amino function:
Coupling a carboxylic acid of general formula
CA 02503455 2005-04-22
V
O R2
HO N~Rs
O ~ (VI)
wherein U, V, W, R2 and R3 are as hereinbefore defined, to a piperidine of
general formula
R'
''I~~ N H , ( I I I )
wherein R' has the meanings given hereinbefore.
The coupling is preferably carried out using methods known from peptide
chemistry (cf. e.g. Houben-Weyl, Methoden der Organischen Chemie, Vol.
15/2), for example using carbodiimides such as e.g. dicyclohexylcarbodiimide
(DCC), diisopropyl carbodiimide (DIC) or ethyl-(3-dimethylaminopropyl)-
carbodiimide, O-(1H-benzotriazol-1-yl)- N,N-N',N'-tetramethyluronium
hexafluorophosphate (HBTU) or tetrafluoroborate (TBTU) or
1H-benzotriazol-1-yl-oxy-tris-(dimethylamino)-phosphonium hexa-
fluorophosphate (BOP). By adding 1-hydroxybenzotriazole (HOBt) or
3-hydroxy-4-oxo-3,4-dihydro-1,2,3-benzotriazine (HOObt) the reaction speed
can be increased. The couplings are normally carried out with equimolar
amounts of the coupling components as well as the coupling reagent in
solvents such as dichloromethane, tetrahydrofuran, acetonitrile, dimethyl
formamide (DMF), dimethyl acetamide (DMA), N-methylpyrrolidone (NMP) or
mixtures thereof and at temperatures between -30 and +30°C, preferably -
20
and +25°C. If necessary, N-ethyl-diisopropylamine (DIEA) (Hianig base)
is
preferably used as an additional auxiliary base.
CA 02503455 2005-04-22
81
The so-called anhydride process is used as a further coupling method for
synthesising compounds of general formula (I) (cf. also: M. Bodanszky,
"Peptide Chemistry", Springer-Verlag 1988, p. 58-59; M. Bodanszky,
"Principles of Peptide Synthesis", Springer-Verlag 1984, p. 21-27). The
Vaughan variant of the mixed anhydride process is preferred (J.R. Vaughan
Jr., J. Amer. Chem.Soc. 73, 3547 (1951)), in which the mixed anhydride of the
carboxylic acid of general formula (VI) which is to be coupled and
monoisobutyl carbonate is obtained, using isobutyl chlorocarbonate in the
presence of bases such as 4-methyl-morpholine or 4-ethylmorpholine. The
preparation of this mixed anhydride and the coupling with amines are carried
out in a one-pot process, using the abovementioned solvents and at
temperatures between -20 and +25°C, preferably 0°C and
+25°C.
(c) In order to prepare compounds of general formula (I) wherein X
denotes the methylene group and R2 and R3 are as hereinbefore defined, with
the proviso that these groups do not contain any free primary or secondary
amine:
Coupling a compound of general formula
V
'W
O Rz
I
Nu N~R3
O , (VII)
wherein U, V, W, R2 and R3 are as hereinbefore defined, with the proviso that
R2 and R3 do not contain any free primary or secondary amine, and Nu
denotes a leaving group, for example a halogen atom, such as the chlorine,
bromine or iodine atom, an alkylsulphonyloxy group with 1 to 10 carbon atoms
in the alkyl moiety, a phenylsulphonyloxy or naphthylsulphonyloxy group
optionally mono-, di- or trisubstituted by chlorine or bromine atoms or by
CA 02503455 2005-04-22
82
methyl or nitro groups, while the substituents may be identical or different,
a
1 H-imidazol-1-yl, a 1 H-pyrazol-1-yl optionally substituted by one or two
methyl
groups in the carbon skeleton, a 1 H-1,2,4-triazol-1-yl, 1 H-1,2,3-triazol-1-
yl,
1H-1,2,3,4-tetrazol-1-yl, a vinyl, propargyl, p-nitrophenyl, 2,4-
dinitrophenyl,
trichlorophenyl, pentachlorophenyl, pentafluorophenyl, pyranyl or pyridinyl, a
dimethylaminyloxy, 2(1H)-oxopyridin-1-yl-oxy, 2,5-dioxopyrrolidin-1-yloxy,
phthalimidyloxy, 1H-benzo-triazol-1-yloxy or azide group,
with a piperidine of general formula
R'
'/'~~NH , (III)
wherein R' is as hereinbefore defined.
The reaction is carried out under Schotten-Baumann or Einhorn conditions,
i.e. the components are reacted in the presence of at least one equivalent of
an auxiliary base at temperatures between -50°C and +120°C,
preferably
-10°C and +30°C, and optionally in the presence of solvents. The
auxiliary
bases used are preferably alkali metal and alkaline earth metal hydroxides,
e.g. sodium hydroxide, potassium hydroxide or barium hydroxide, alkali metal
carbonates, e.g. sodium carbonate, potassium carbonate or caesium
carbonate, alkali metal acetates, e.g. sodium or potassium acetate, as well as
tertiary amines, e.g. pyridine, 2,4,6-trimethylpyridine, quinoline,
triethylamine,
N-ethyl-diisopropylamine, N-ethyl-dicyclohexylamine,
1,4-diazabicyclo[2.2.2]octane or 1,8-diazabicyclo[5.4.0]undec-7-ene, the
solvents used may be, for example, dichloromethane, tetrahydrofuran,
1,4-dioxane, acetonitrile, dimethyl formamide, dimethyl acetamide,
N-methyl-pyrrolidone or mixtures thereof; if alkali metal or alkaline earth
metal
hydroxides, alkali metal carbonates or acetates are used as the auxiliary
bases, water may also be added to the reaction mixture as cosolvent.
(d) In order to prepare compounds of general formula (I) wherein all the
groups are as hereinbefore defined:
CA 02503455 2005-04-22
83
Coupling a carboxylic acid of general formula
A
N- 'X
1
R , (VIII)
wherein all the groups are as hereinbefore defined, with an amine of general
formula HNR2R3, wherein R2 and R3 are as hereinbefore defined, with the
proviso that it does not contain any free carboxylic acid and/or other free
primary or secondary aliphatic amino function.
The coupling is preferably carried out using methods known from peptide
chemistry (cf. e.g. Houben-Weyl, Methoden der Organischen Chemie, Vol.
15/2), for example using carbodiimides such as e.g. dicyclohexylcarbodiimide
(DCC), diisopropyl carbodiimide (DIC) or ethyl-(3-dimethylaminopropyl)-
carbodiimide, O-(1 H-benzotriazol-1-yl)- N,N-N',N'-tetramethyluronium
hexafluorophosphate (HBTU) or tetrafluoroborate (TBTU) or
1 H-benzotriazol-1-yl-oxy-tris-(dimethylamino)-phosphonium hexa-
fluorophosphate (BOP). By adding 1-hydroxybenzotriazole (HOBt) or
3-hydroxy-4-oxo-3,4-dihydro-1,2,3-benzotriazine (HOObt) the reaction speed
can be increased. The couplings are normally carried out with equimolar
amounts of the coupling components as well as the coupling reagent in
solvents such as dichloromethane, tetrahydrofuran, acetonitrile, dimethyl
formamide (DMF), dimethyl acetamide (DMA), N-methylpyrrolidone (NMP) or
mixtures thereof and at temperatures between -30 and +30°C, preferably -
20
and +25°C. If necessary, N-ethyl-diisopropylamine (DIEA) (Hunig base)
is
preferably used as an additional auxiliary base.
CA 02503455 2005-04-22
84
The so-called anhydride process is used as a further coupling method for
synthesising compounds of general formula (I) (cf. also: M. Bodanszky,
"Peptide Chemistry", Springer-Verlag 1988, p. 58-59; M. Bodanszky,
"Principles of Peptide Synthesis", Springer-Verlag 1984, p. 21-27). The
Vaughan variant of the mixed anhydride process is preferred (J.R. Vaughan
Jr., J. Amer. Chem.Soc. 73, 3547 (1951)), in which the mixed anhydride of the
carboxylic acid of general formula (VI) which is to be coupled and
monoisobutyl carbonate is obtained, using isobutyl chlorocarbonate in the
presence of bases such as 4-methyl-morpholine or 4-ethylmorpholine. The
preparation of this mixed anhydride and the coupling with amines are carried
out in a one-pot process, using the abovementioned solvents and at
temperatures between -20 and +25°C, preferably 0°C and
+25°C.
(e) In order to prepare compounds of general formula (I) wherein R' is as
hereinbefore defined, with the proviso that no free primary or secondary
amine is present:
Coupling a compound of general formula
U
V
"W
A
~ Nu
N_ 'X
1
R ~ (1X)
wherein all the groups are as hereinbefore defined and Nu denotes a leaving
group, for example a halogen atom, such as the chlorine, bromine or iodine
atom, an alkylsulphonyioxy group with 1 to 10 carbon atoms in the alkyl
moiety, a phenylsulphonyloxy or naphthylsulphonyloxy group optionally mono-
di- or trisubstituted by chlorine or bromine atoms, by methyl or vitro groups,
while the substituents may be identical or different, a 1 H-imidazol-1-yl, a 1
H-
CA 02503455 2005-04-22
RS
pyrazol-1-yl optionally substituted by one or two methyl groups in the carbon
skeleton, a 1H-1,2,4-triazol-1-yl, 1H-1,2,3-triazol-1-yl, 1H-1,2,3,4-tetrazol-
1-yl,
a vinyl, propargyl, p-nitrophenyl, 2,4-dinitrophenyl, trichlorophenyl,
pentachlorophenyl, pentafluorophenyl, pyranyl or pyridinyl, a
dimethylaminyloxy, 2(1H)-oxopyridin-1-yl-oxy, 2,5-dioxopyrrolidin-1-yloxy,
phthalimidyloxy, 1H-benzo-triazol-1-yloxy or azide group,
with an amine of general formula HNR2R3, wherein R2 and R3 are as
hereinbefore defined, with the proviso that no free carboxylic acid and/or
other
free primary or secondary aliphatic amino function is present.
The reaction is carried out under Schotten-Baumann or Einhorn conditions,
i.e. the components are reacted in the presence of at least one equivalent of
an auxiliary base at temperatures between -50°C and +120°C,
preferably
-10°C and +30°C, and optionally in the presence of solvents. The
auxiliary
bases used are preferably alkali metal and alkaline earth metal hydroxides,
e.g. sodium hydroxide, potassium hydroxide or barium hydroxide, alkali metal
carbonates, e.g. sodium carbonate, potassium carbonate or caesium
carbonate, alkali metal acetates, e.g. sodium or potassium acetate, as well as
tertiary amines, e.g. pyridine, 2,4,6-trimethylpyridine, quinoline,
triethylamine,
N-ethyl-diisopropylamine, N-ethyl-dicyclohexylamine,
1,4-diazabicyclo[2.2.2]octane or 1,8-diazabicyclo[5.4.0]undec-7-ene, the
solvents used may be, for example, dichloromethane, tetrahydrofuran,
1,4-dioxane, acetonitrile, dimethyl formamide, dimethyl acetamide,
N-methyl-pyrrolidone or mixtures thereof; if alkali metal or alkaline earth
metal
hydroxides, alkali metal carbonates or acetates are used as the auxiliary
bases, water may also be added to the reaction mixture as cosolvent.
The new compounds of general formula (I) according to the invention contain
one or more chiral centres. If for example there are two chiral centres the
compounds may occur in the form of two pairs of diastereomeric antipodes.
The invention covers the individual isomers as well as the mixtures thereof.
CA 02503455 2005-04-22
86
The diastereomers may be separated on the basis of their different physico-
chemical properties, e.g. by fractional crystallisation from suitable
solvents, by
high pressure liquid or column chromatography, using chiral or preferably
non-chiral stationary phases.
Racemates covered by general formula (I) may be separated for example by
HPLC on suitable chiral stationary phases (e.g. Chiral AGP, Chiralpak AD).
Racemates which contain a basic or acidic function can also be separated via
the diastereomeric, optically active salts which are produced on reacting with
an optically active acid, for example (+) or (-)-tartaric acid, (+) or (-)-
diacetyl
tartaric acid, (+) or (-)-monomethyl tartrate or (+)-camphorsuiphonic acid, or
an optically active base, for example with (R)-(+)-1-phenylethylamine, (S)-(-)-
1-phenyfethyfamine or (S)-brucine.
According to a conventional method of separating isomers, the racemate of a
compound of general formula (I) is reacted with one of the abovementioned
optically active acids or bases in equimolar amounts in a solvent and the
resulting crystalline, diastereomeric, optically active salts thereof are
separated using their different soiubilities. This reaction may be carried out
in
any type of solvent provided that it is sufficiently different in terms of the
solubility of the salts. Preferably, methanol, ethanol or mixtures thereof,
for
example in a ratio by volume of 50:50, are used. Then each of the optically
active salts is dissolved in water, carefully neutralised with a base such as
sodium carbonate or potassium carbonate, or with a suitable acid, e.g. dilute
hydrochloric acid or aqueous methanesulphonic acid, and in this way the
corresponding free compound is obtained in the (+) or (-) form.
The (R) or (S) enantiomer alone or a mixture of two optically active
diastereomeric compounds covered by general formula I may also be
obtained by performing the syntheses described above with a suitable
reaction component in the (R) or (S) configuration.
The starting compounds of general formula (141) may be obtained, if they are
not known from the literature or even commercially available, according to the
CA 02503455 2005-04-22
g7
processes described in WO 98/11128 and DE 199 52 146. The starting
compounds of general formula (IV) are commercially available. Compounds of
general formula (V) may be obtained by methods familiar to the peptide
chemist from protected phenylalanines and amines of general formula
HNR2R3.
The phenyalanine derivatives needed to prepare the optically pure
compounds of general formula (V) may be prepared from the compounds of
general formula
U
V
,W
H~N OR
I
H O ,(X)
wherein U, V and W are as hereinbefore defined and R denotes an
unbranched alkyl group, preferably the methyl or ethyl group, by racemate
cleavage.
This racemate cleavage can be carried out using enzymatic methods, while
only one enantiomer of the racemate is transformed and the mixture produced
is then separated using physicochemical methods, preferably using
chromatographic methods. A suitable enzyme system for this step consists of
the enzyme Alcaiase 2.4 L FG (Novozymes A/S; DK 2880 Bagsvaerd). The
compounds of general formula (X) can then be converted into the
enantiomerically pure compounds of general formula (V) by methods familiar
to the peptide chemist.
If the group X in compounds of general formula (V) denotes the oxygen atom,
the hydroxycarboxylic acids needed for the synthesis, of general formula
CA 02503455 2005-04-22
88
U
V
-, W
H~O OH
O ~ (XI)
wherein U, V and W are as hereinbefore defined,
may be prepared from compounds of general formula (X), with the proviso
that R denotes the hydrogen atom.
With the proviso that V does not denote the amino or methylamino group, the
compounds of general formula (XI) may be obtained by diazotisation of
compounds of general formula (X) with a suitable diazotising reagent,
preferably sodium nitrite in an acidic medium. If enantiomerically pure
compounds are used, the corresponding enantiomerically pure
hydroxycarboxylic acid compounds are obtained, and the configuration is
retained during the reaction.
Another method of obtaining compounds of general formula (XI) wherein U, V
and W are as hereinbefore defined comprises alkylating the compound
O O
O N~O
U
(XII)
with correspondingly substituted benzyl chlorides, benzyl bromides or benzyl
iodides of general formula (X111)
CA 02503455 2005-04-22
89
U
V
~W
X , (X111)
wherein U, V and W are as hereinbefore defined and X denotes a chlorine,
bromine or iodine atom, analogously to methods known from the literature
(Michael T. Grimmins, Kyle A. Emmitte and Jason D. Katz, Org. Lett. 2, 2165-
2167 [2000]).
The diastereomeric products obtained can then be separated by physico-
chemical methods, preferably using chromatographic methods. The hydrolytic
cleaving of the chiral auxiliary, coupling with amines of general formula
HNR2R3 and cleaving the benzyl protecting group also provides access to
enantiomerically pure hydroxycarboxylic acid compounds of general formula
(V).
The starting compounds of general formula (VI) are obtained for example by
reacting amines of general formula HNR2R3 with 2-(alkoxycarbonylmethyl)-3-
aryl-propanoic acids and subsequently hydrolytically cleaving the alkyl group.
The 2-(alkoxycarbonylmethyl)-3-aryl-propanoic acids required may be
prepared analogously to methods known from the literature (David A. Evans,
Leester D. Wu, John J. M. Wiener, Jeffrey S. Johnson, David H. B. Ripin and
Jason S. Tedrow, J. Org.Chem 64, 6411-6417 [1999]; Saul G. Cohen and
Aleksander Milovanovic, J. Am. Chem. Soc. 90, 3495-3502 [1968]; Hiroyuki
Kawano, Youichi Ishii, Takao Ikariya, Masahiko Saburi, Sadao Yoshikawa,
Yasuzo Uchida and Hidenori Kumobayashi, Tetrahedron Letters 28, 1905-
1908 [1987]). Carboxylic acids of general formula (VIII) may be prepared from
generally available starting materials in accordance with the processes
described in WO 98/11128.
CA 02503455 2005-04-22
The compounds of general formula I obtained may, if they contain suitable
basic functions, be converted, particularly for pharmaceutical use, into their
physiologically acceptable salts with inorganic or organic acids. Suitable
acids
include for example hydrochloric acid, hydrobromic acid, phosphoric acid,
nitric acid, sulphuric acid, methanesulphonic acid, ethanesulphonic acid,
benzenesulphonic acid, p-toluenesulphonic acid, acetic acid, fumaric acid,
succinic acid, lactic acid, mandelic acid, malic acid, citric acid, tartaric
acid or
malefic acid.
Moreover, the new compounds of formula (I), if they contain a carboxylic acid
function, may if desired be converted into the addition salts thereof with
inorganic or organic bases, particularly for pharmaceutical use into the
physiologically acceptable addition salts thereof. Suitable bases for this
include, for example, sodium hydroxide, potassium hydroxide, ammonia,
cyclohexylamine, dicyclohexylamine, ethanolamine, diethanolamine and
triethanolamine.
The present invention relates to racemates if the compounds of general
formula (I) have only one chiral element. However, the application also
includes the individual diastereomeric pairs of antipodes or mixtures thereof
which are obtained if there is more than one chiral element in the compounds
of general formula (I), as well as the individual optically active enantiomers
of
which the abovementioned racemates are made up.
Also included in the subject matter of this invention are the compounds
according to the invention, including the salts thereof, in which one or more
hydrogen atoms are replaced by deuterium.
The new compounds of general formula (I) and the physiologically acceptable
salts thereof have valuable pharmacological properties, based on their
selective CGRP-antagonistic properties. The invention further relates to
pharmaceutical compositions containing these compounds, their use and the
preparation thereof.
CA 02503455 2005-04-22
91
The new compounds of general formula I and the physiologically acceptable
salts thereof have CGRP-antagonistic properties and exhibit good affinities in
CGRP receptor binding studies. The compounds display CGRP-antagonistic
properties in the pharmacological test systems described hereinafter.
The following experiments were carried out to demonstrate the affinity of the
abovementioned compounds for human CGRP-receptors and their
antagonistic properties:
CA 02503455 2005-04-22
92
A. Binding studies with SK-N-MC cells (expressing the human CGRP
receptor)
SK-N-MC cells are cultivated in "Dulbecco's modified Eagle medium". The
medium is removed from confluent cultures. The cells are washed twice with
PBS buffer (Gibco 041-04190 M), detached by the addition of PBS buffer
mixed with 0.02% EDTA, and isolated by centrifuging. After resuspension in
20 ml of "Balanced Salts Solution" [BSS (in mM): NaCI 120, KCI 5.4, NaHC03
16.2, MgS04 0.8, NaHP04 1.0, CaCf2 1.8, D-glucose 5.5, HEPES 30, pH
7.40] the cells are centrifuged twice at 100 x g and resuspended in BSS. After
the number of cells has been determined, the cells are homogenised using an
Ultra-Turrax and centrifuged for 10 minutes at 3000 x g. The supernatant is
discarded and the pellet is recentrifuged in Tris buffer (10 mM Tris, 50 mM
NaCI, 5 mM MgCl2, 1 mM EDTA, pH 7.40) enriched with 1 % bovine serum
albumin and 0.1 % bacitracin, and resuspended ( 1 ml / 1000000 cells). The
homogenised product is frozen at -80°C. The membrane preparations are
stable for more than 6 weeks under these conditions.
After thawing, the homogenised product is diluted 1:10 with assay buffer (50
mM Tris, 150 mM NaCI, 5 mM MgCl2, 1 mM EDTA, pH 7.40) and
homogenised for 30 seconds with an Ultra-Turrax. 230 pl of the homogenised
product are incubated for 180 minutes at ambient temperature with 50 pM'25I
iodotyrosyl-Calcitonin-Gene-Related Peptide (Amersham) and increasing
concentrations of the test substances in a total volume of 250 NI. The
incubation is ended by rapid filtration through GF/B-glass fibre filters
treated
with polyethyleneimine (0.1 %) using a cell harvester. The protein-bound
radioactivity is measured using a gamma counter. Non-specific binding is
defined as the bound radioactivity in the presence of 1 NM human CGRP-
alpha during incubation.
The concentration binding curves are analysed using computer-aided non-
linear curve matching.
CA 02503455 2005-04-22
93
The compounds mentioned hereinbefore show ICSO values s 10000 nM in the
test described.
B. CGRP Antagonism in SK-N-MC cells
SK-N-MC cells (1 million cells) are washed twice with 250 NI incubation buffer
(Hanks' HEPES, 1 mM 3-isobutyl-1-methylxanthine, 1% BSA, pH 7.4) and
pre-incubated at 37°C for 15 minutes. After the addition of CGRP (10
NI) as
agonist in increasing concentrations (10-" to 10-6 M), or additionally the
substance in 3 to 4 different concentrations, the mixture is incubated for
another 15 minutes.
Intracellular cAMP is then extracted by the addition of 20 NI of 1 M HCI and
centrifugation (2000 x g, 4°C, for 15 minutes). The supernatants are
frozen in
liquid nitrogen and stored at -20°C.
The cAMP contents of the samples are determined by radioimmunoassay
(Messrs. Amersham) and the pA2 values of antagonistically acting substances
are determined graphically.
The compounds of genera! formula I exhibit CGRP-antagonistic properties in
the in vitro test model described, in a dosage range between 10-'2 and 10-5 M.
In view of their pharmacological properties the compounds of general formula
I and the salts thereof With physiologically acceptable acids are thus
suitable
for the acute and prophylactic treatment of headaches, particularly migraine
or
cluster headaches, tension headaches and chronic headache. The
compounds according to the invention are also suitable for preventing
migraine headache during the prodrome period or for preventing and treating
migraine headache which occurs prior to or during menstruation.
Moreover, the compounds of general formula I also have a positive effect on
the following diseases: non-insulin-dependent diabetes mellitus ("NIDDM"),
cardiovascular diseases, morphine tolerance, diarrhoea caused by clostridium
CA 02503455 2005-04-22
94
toxin, skin diseases, particularly thermal and radiation-induced skin damage
including sunburn, inflammatory diseases, e.g. inflammatory diseases of the
joints (arthritis), neurogenic inflammation of the oral mucosa, inflammatory
lung diseases, allergic rhinitis, asthma, diseases accompanied by excessive
vasodilatation and resultant reduced blood supply to the tissues, e.g. shock
and sepsis or erythema. In addition, the compounds according to the
invention have a general pain-relieving effect, particularly in cases of
neuropathic pain, as in, for example, complex regional pain syndrome
(CRPS), in neuropathic pain within the framework of systemic neurotoxic
diseases such as, for example, diabetes mellitus, and in pain caused by
inflammatory processes. The symptoms of menopausal hot flushes caused
by vasodilatation and increased blood flow in oestrogen-deficient women and
hormone-treated patients with prostate carcinoma are favourably affected by
the CGRP-antagonists of the present application in a preventive and acute-
therapeutic capacity, this therapeutic approach being distinguished from
hormone replacement by the absence of side effects.
The dosage required to achieve a corresponding effect is conveniently 0.0001
to 3 mg/kg of body weight, preferably 0.01 to 1 mg/kg of body weight, when
administered intravenously or subcutaneously and 0.01 to 10 mg/kg of body
weight, preferably 0.1 to 10 mg/kg of body weight when administered orally,
nasally or by inhalation, 1 to 3 x a day in each case.
If the treatment with CGRP antagonists and/or CGRP release inhibitors is
given as a supplement to conventional hormone substitution, it is advisable to
reduce the doses specified above, in which case the dosage may be from 1/5
of the lower limits mentioned above up to 1/1 of the upper limits specified.
The compounds prepared according to the invention may be administered
either on their own or optionally in combination with other active substances
for the treatment of migraine by intravenous, subcutaneous, intramuscular,
intrarectal, intranasal route, by inhalation, transdermally or orally, while
aerosol formulations are particularly suitable for inhalation. The
combinations
may be administered either simultaneously or sequentially.
CA 02503455 2005-04-22
Categories of active substance which may be used in the combination include
e.g. antiemetics, prokinetics, neuroleptics, antidepressants, neurokinine
antagonists, anticonvulsants, histamine-H1 receptor antagonists,
antimuscarinics, a-blockers, a-agonists and a-antagonists, ergot alkaloids,
mild analgesics, non-steroidal antiinflammatories, corticosteroids, calcium
antagonists, 5-HT~B,~p agonists or other anti-migraine agents, which may be
formulated together with one or more inert conventional carriers and/or
diluents, e.g. with corn starch, lactose, glucose, microcrystalline cellulose,
magnesium stearate, polyvinyl pyrrolidone, citric acid, tartaric acid, water,
water/ethanol, water/glycerol, water/sorbitol, water/polyethylene glycol,
propylene glycol, cetylstearyl alcohol, carboxymethylcellulose or fatty
substances such as hard fat or suitable mixtures thereof, into conventional
galenic preparations such as plain or coated tablets, capsules, powders,
suspensions, solutions, metered dose aerosols or suppositories.
Thus other active substances which may be used for the combinations
mentioned above include for example the non-steroidal antiinflammatories
aceclofenac, acemetacin, acetylsalicylic acid, azathioprine, diclofenac,
diflunisal, fenbufen, fenoprofen, flurbiprofen, ibuprofen, indometacin,
ketoprofen, leflunomide, lornoxicam, mefenamic acid, naproxen,
phenylbutazone, piroxicam, sulphasalazine, zomepirac or the
pharmaceutically acceptable salts thereof as well as meloxicam and other
selective COX2-inhibitors, such as for example rofecoxib and celecoxib.
It is also possible to use ergotamine, dihydroergotamine, metoclopramide,
domperidone, diphenhydramine, cyclizine, promethazine, chlorpromazine,
vigabatrin, timolol, isometheptene, pizotifen, botox, gabapentin, topiramate,
riboflavin, montelukast, lisinopril, prochloroperazine, dexamethasone,
flunarizine, dextropropoxyphene, meperidine, metoprolol, propranolol, nadolol,
atenolol, clonidine, indoramin, carbamazepine, phenytoin, valproate, ami-
tryptiline, lidocaine or diltiazem and other 5-HT~B,~p-agonists such as, for
example, almotriptan, avitriptan, eletriptan, frovatriptan, naratriptan,
rizatriptan, sumatriptan and zolmitriptan.
CA 02503455 2005-04-22
96
The dosage of these active substances is expediently 1/5 of the lowest
recommended dose to 1/1 of the normally recommended dose, i.e. for
example 20 to 100 mg of sumatriptan.
The invention further relates to the use of the compounds according to the
invention as valuable adjuvants for the production and purification (by
affinity
chromatography) of antibodies as well as in RIA and ELISA assays, after
suitable radioactive labelling, for example by tritiation of suitable
precursors,
for example by catalytic hydrogenation with tritium or replacing halogen atoms
with tritium, and as a diagnostic or analytical adjuvant in neurotransmitter
research.
Experimental section
As a rule, IR, 'H-NMR and/or mass spectra have been obtained for the
compounds prepared. Unless otherwise stated, Rf values were obtained using
ready-made silica gel TLC plates 60 F254 (E. Merck, Darmstadt, Item no.
1.05714) without chamber saturation. The Rf values obtained under the name
Alox were obtained using ready-made aluminium oxide 60 F254 TLC plates
(E. Merck, Darmstadt, item no. 1.05713) without chamber saturation. The
ratios given for the eluants relate to units by volume of the solvent in
question.
The units by volume given for NH3 are based on a concentrated solution of
NH3 in water.
Unless otherwise stated the acid, base and saline solutions used for working
up the reaction solutions are aqueous solutions having the concentrations
specified.
For chromatographic purification, silica gel made by Millipore (MATREXT"', 35-
70 pm) was used. For chromatographic purification Alox (E. Merck,
Darmstadt, standardised aluminium oxide 90, 63-200 pm, Article no.
1.01097.9050) is used.
The HPLC data provided are measured using the parameters specified below:
Analytical column: Zorbax column (Agilent Technologies), SB (Stable Bond) -
C18; 3.5 Nm; 4.6 x 75 mm; column temperature: 30°C; flow: 0.8 mL /
min;
97
injection volume: 5 µL; detection at 254 nm
CA 02503455 2005-04-22
98
Method A:
time (min)percent by volume of percent by volume of acetonitrile
water (with 0.1% formic acid)
(with 0.1% formic acid)
0 90 10
9 10 90
10 90
11 90 10
In preparative HPLC purifications as a rule the same gradients are used as
were used to raise the analytical HPLC data.
The products are collected under mass control and the fractions containing
the product are combined and freeze-dried.
If no detailed information is given as to the configuration, it is not clear
whether it is a pure enantiomer or whether partial or even complete
racemisation has occurred.
The following abbreviations are used in the description of the experiments:
abs. absolute
Boc tert.-butoxycarbonyl
CDI N,N'-carbonyldiimidazole
CDT 1,1'-carbonyldi-( 1,2,4-triazofe)
Cyc cyclohexane
DCM dichloromethane
DMF N,N-dimethylformamide
EtOAc ethyl acetate
EtOH ethanol
semiconc.semiconcentrated
HCI hydrochloric acid
HOAc acetic acid
HOBt 1-hydroxybenzotriazole-hydrate
i. vac. in vacuo (in a vacuum)
CA 02503455 2005-04-22
99
KOH potassium hydroxide
conc. concentrated
MeOH methanol
NaCI sodium chloride
NaOH sodium hydroxide
org. organic
PE petroleum ether
RT room temperature
TBTU 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium-
tetrafluoroborate
TFA trifluoroacetic acid
THF tetrahydrofuran
Instructions for preparing the amines used (starting comaounds)
Examele A1
2-methyl-5-piperidin-4-yl-2,5-diaza-bicyclo[2.2.1]heptane (Examples 7.9 and
10.79)
H ~ N\~ N\~N ~
A1a 2-(1-benzyl-piperidin-4-yl)-5-methyl-2,5-diaza-
bicyclo[2.2.1 ]heptane
0.73 mL (12.7 mmol) acetic acid were added to a solution of 2.38 mL (12.7
mmol) 1-benzyl-piperidin-4-one and 3.5 g (12.77 mmol) of 2-methyl-2,5-diaza-
bicyclo[2.2.1]heptane in 100 mL MeOH and the reaction mixture was stirred
for 3 h at RT. Under a nitrogen current 0.99 g (15.0 mmol) NaBH3CN were
added and the reaction solution was stirred overnight at RT. It was acidified
with 7 mL of conc. HCI, stirred for 1 h at RT and evaporated down i.vac. The
residue was combined with 200 mL of 15% K2C03 solution, extracted twice
with 200 mL DCM in each case and the combined organic phases were dried
over Na2S04. After the desiccant and solvent had been eliminated the residue
was purified by chromatography (silica gel, gradient: DCM to DCM/MeOH/NH3
CA 02503455 2005-04-22
100
10:85:5).
Yield: 2.1 g (58% of theory)
ESI-MS: (M+H)+ = 286
Rf = 0.15 (silica gel, DCM/MeOH/NH3 80:20:2)
A1 b 2-methyl-5-piperidin-4-yl-2,5-diaza-bicyclo[2.2.1 ]heptane
A solution of 2.1 g (7.36 mmol) 2-(1-benzyl-piperidin-4-yl)-5-methyl-2,5-diaza-
bicyclo[2.2.1]heptane in 100 mL MeOH was combined with 500 mg of 10%
Pd/C and hydrogenated at RT and 50 psi H2 for 3 h. The catalyst was suction
filtered and the solvent eliminated i.vac. The product was used for further
reactions without being purified.
Yield: 1.4 g (97% of theory)
ESI-MS: (M+H)+ = 196
Rf = 0.10 (silica gel, DCM/MeOH/NH3 80:20:2)
Examale A2
1-cyclopropylmethyl-4-piperidin-4-yl-piperazine (Example 10.4)
H ~ N\.~N~N
A2a tert. butyl 4-(4-cyclopropylmethyl-piperazin-1-yl)-piperidine-1-
carboxylate
1.26 g (20.0 mmol) NaBH3CN were added in 4 batches at RT to a solution of
1.71 g (5.0 mmol) tert. butyl 4-piperazin-1-yl-piperidine-1-carboxylate and
0.75
mL (10.0 mmol) cyclopropanecarbaldehyde in 100 mL of EtOH and the
reaction mixture was stirred overnight at RT. The mixture was evaporated
down i.vac., the residue was taken up in saturated NaHC03 solution,
extracted exhaustively with EtOAc and the organic phase was dried over
Na2SOa. After the desiccant and solvent had been eliminated the residue was
purified by chromatography (silica gel, EtOAc/MeOH/NH3 90:10:0.5).
Yield: 1.36 g (84% of theory)
CA 02503455 2005-04-22
101
EI: (M)+ = 323
A2b 1-cyclopropylmethyl-4-piperidin-4-yl-piperazine
mL TFA were added to a solution of 1.36 g (4.2 mmol) tert. butyl 4-(4-
cyclopropylmethyl-piperazin-1-yl)-piperidine-1-carboxylate in 30 mL DCM and
the reaction mixture was stirred for 4 h at RT. The reaction solution was
evaporated down i.vac., the residue combined with diethyl ether, the
precipitate was suction filtered and dried. The product was precipitated as
the
tris-trifluoroacetate salt.
Yield: 1.86 g (78% of theory)
EI: (M)+ = 223
Exam~~le A3
4-azetidin-1-yl-piperidine (Example 10.15)
H
~N~~N~
A3a 4-azetidin-1-yl-1-benzyl-piperidine
1.0 mL (17.49 mmol) acetic acid was added to a solution of 3.0 mL (16.45
mmol) of 1-benzyl-piperidin-4-one and 1.0 g (17.51 mmol) azetidine in 100 mL
DCM and the reaction mixture was stirred for 1 h at RT. 6.0 g (39.55 mmol) of
NaBH(OAc)3 were added in 4 batches within 1 h while cooling with ice and the
reaction solution was stirred overnight at RT. 15% K2C03 solution was added
and stirring was continued for another hour. 200 mL EtOAc were added, the
organic phase was separated off and dried over MgS04. After the desiccant
and solvent had been eliminated the residue was purified by chromatography
(silica gel, gradient: DCM to MeOH/NH3 9:1 ).
Yield: 3.2 g (84% of theory)
EI: (M)+ = 230
Rr = 0.57 (silica gel, DCM/MeOH/cyc/NH3 70:15:15:2)
CA 02503455 2005-04-22
102
A3b 4-azetidin-1-yl-piperidine
A solution of 3.2 g (13.89 mmol) 4-azetidin-1-yl-1-benzyl-piperidine in 50 mL
MeOH was combined with 500 mg 10% Pd/C and hydrogenated for 7.5 h at
RT and 3 bar H2.The catalyst was suction filtered and the solvent eliminated
i.vac. The product was used for further reactions without being purified.
Yield: 1.9 g (98% of theory)
ESI-MS: (M+H)+ = 141
Rf = 0.19 (silica gel, DCM/MeOH/NH3 70:25:5)
Example A4
1-azetidin-3-yl-perhydro-azepine (Example 10.22)
H-N~N
A4a 1-(1-benzhydryl-azetidin-3-yl)-perhydro-azepine
Under a nitrogen atmosphere 31.7 g (320 mmol) of perhydro-azepine were
added to a solution of 31.7 g (100 mmol) 1,1-dibenzyl-azetidin-3-yl
methanesulphonate in 200 mL of DMF and the mixture was stirred for 7 days
at 50°C. The reaction solution was combined with 1 L water, the aqueous
phase was extracted twice with EtOAc, the combined organic phases were
washed twice with water and dried over Na2S04. After the desiccant and
solvent had been eliminated the residue was twice purified by
chromatography (silica gel, 1st column: DCM/MeOH/NH3 19:1:0.025 and 2nd
column: tert-butylmethylether).
Yield: 22.2 g (69% of theory)
Rf = 0.82 (silica gel, DCM/MeOH/NH3 19:1:0.025)
A4b 1-azetidin-3-yl-perhydro-azepine
g 10% Pd/C were added to a solution of 22.0 g (68.6 mmol) of 1-(1-
benzhydryl-azetidin-3-yl)-perhydro-azepine in 400 mL MeOH and 69 mL of 2
N HCI and the reaction mixture was hydrogenated for 3 h at 45°C
until the
CA 02503455 2005-04-22
103
theoretical uptake of H2 had been achieved. After filtration the solvent was
eliminated i.vac. and the product, which was obtained as the bis-
hydrochloride, was further reacted without being purified.
Yield: 15.5 g ( 100% of theory)
melting point: 205-220°C
Rf = 0.08 (silica gel, DCM/MeOH/NH3 9:1:0.1 )
Example A5
1-benzyl-4-piperidin-4-yl-piperazine (Example 10.24)
H~N\~~N~N I \
A5a tert. butyl 4-(4-benzyl-piperazin-1-yl)-piperidine-1-carboxylate
A solution of 66.7 mL (381 mmol) 1-benzyl-piperazine and 75.8 g (380 mmol)
tert. butyl 4-oxo-piperidine-1-carboxylate in 1 L THF was adjusted to pH 5
with
glacial acetic acid and then 100 g (448 mmol) NaBH(OAc)3 was added
batchwise within 2 h while cooling with ice and the reaction mixture was
stirred overnight at RT. The reaction solution was carefully made alkaline
with
2.2 M K2C03 solution, stirred for 1 h, exhaustively extracted with EtOAc and
the combined organic phases were dried over Na2S04. After the desiccant
and solvent had been eliminated the residue was further reacted without
being purified.
Yield: 114 g (83% of theory)
ESI-MS: (M+Na)+ = 382
Rr = 0.74 (silica gel, DCM/MeOH/cyc/NH3 70:15:15:2)
A5b 1-benzyl-4-piperidin-4-yl-piperazine
20 mL TFA were added to a solution of 5 g (13.91 mmol) tert. butyl 4-(4-
benzyl-piperazin-1-yl)-piperidine-1-carboxylate in 200 mL DCM and the
reaction mixture was stirred overnight at RT. The mixture was evaporated
down i. vac., the residue was combined with 200 mL 15% K2C03 solution,
CA 02503455 2005-04-22
104
extracted three times with 100 mL DCM in each case and the combined
organic phases were dried over MgS04. After the desiccant and solvent had
been eliminated the desired product was obtained, which was further reacted
without being purified.
Yield: 2.9 g (80% of theory)
ESI-MS: (M+H)+ = 260
Rf = 0.58 (silica gel, DCM/MeOH/NH3 70:25:5)
Example A6
Dimethyl-(2,3,4,5-tetrahydro-1H-3-benzazepin-7-ylmethyl)-amine (Example
10.25)
H-N I /
A6a 1-(7-dimethylaminomethyl-1,2,4,5-tetrahydro-3-benzazepin-3-yl)-
2,2,2-trifluoro-ethanone
11.7 mL (23.4 mmol) of a 2 M dimethylamine solution in THF were added to a
solution of 4.5 g (16.59 mmol) 3-(2,2,2-trifluoro-acetyl)-2,3,4,5-tetrahydro-
1H-
3-benzazepin-7-carbaldehyde in 150 mL THF and the solution was adjusted
to pH 5 with 1 mL glacial acetic acid. After 30 min 4.62 g (21.79 mmol)
NaBH(OAc)3 were added and the reaction mixture was stirred overnight at
RT. The reaction solution was carefully combined with saturated NaHC03
solution, stirred for 30 min, exhaustively extracted with EtOAc, the organic
phase was separated off and dried over Na2S04. After the desiccant and
solvent had been eliminated the desired product was obtained, which was
further reacted without being purified.
Yield: 4.5 g (90% of theory)
ESI-MS: (M+H)+ = 301
Rr = 0.76 (silica gel, DCM/MeOH/cyc/NH3 70:15:15:2)
A6b dimethyl-(2,3,4,5-tetrahydro-1H-3-benzazepin-7-ylmethyl)-amine
CA 02503455 2005-04-22
105
50 mL water and 8.5 g (61.51 mmol) K2C03 were added to a solution of 4.5 g
(14.98 mmol) 1-(7-dimethylaminomethyl-1,2,4,5-tetrahydro-3-benzazepin-3-
yl)-2,2,2-trifluoro-ethanone in 50 mL MeOH and the reaction mixture was
stirred for 72 h at RT. The reaction solution was evaporated down i.vac., the
residue combined with DCM, filtered to remove insoluble constituents and
evaporated down i.vac. The desired product was obtained in the form of a
light brown oil.
Yield: 2.9 g (95% of theory)
ESI-MS: (M+H)+ = 205
Rf = 0.39 (silica gel, DCM/MeOH/cyc/NH3 70:15:15:2)
Example A7
1-piperidin-4-yl-4-(2,2,2-trifluoro-ethyl)-piperazine (Example 10.27)
H~N\~~N~N~CF3
A7a 1-[4-(1-benzyl-piperidin-4-yl)-piperazin-1-yl]-2,2,2-trifluoro-
ethanone
A solution of 8.21 mL (57.83 mmol) trifluoroacetic anhydride in 40 mL DCM
was added dropwise to a solution, cooled to 0°C, of 15.0 g (57.83 mmol)
1-(1-
benzyl-piperidin-4-yl)-piperazine and 20.1 mL (145 mmol) triethylamine in 200
DCM and the reaction mixture was stirred for 5 h at RT. Water was added, the
organic phase was separated off and dried over Na2S04. After the desiccant
and solvent had been eliminated the crude product was further reacted
without being purified.
Yield: 8.3 g (40% of theory)
EI: (M)+ = 205
Rr = 0.48 (silica gel, DCM/MeOH/NH3 90:10:1 )
A7b 1-(1-benzyl-piperidin-4-yl)-4-(2,2,2-trifluoro-ethyl)-piperazine
0.53 g (14.07 mmol) NaBH4 were added to a solution of 1.0 g (2.81 mmol) 1-
[4-(1-benzyl-piperidin-4-yl)-piperazin-1-yl]-2,2,2-trifluoro-ethanone in 9 mL
of
CA 02503455 2005-04-22
106
1,4-dioxane and 1 mL of THF and then a solution of 1.08 mL (14.07 mmol)
TFA in 10 mL 1,4-dioxane was added dropwise to the resulting suspension
within 10 min. The reaction solution was refluxed for 5 h, after cooling it
was
decomposed with water and evaporated down i.vac. The residue obtained
was purified by chromatography (silica gel, EtOAc/MeOH/NH3 95:5:0.5).
Yield: 0.41 g (43% of theory)
ESI-MS: (M+H)+ = 342
Rf = 0.45 (silica gel, EtOAc/MeOH/NH3 80:20:2)
A7c 1-piperidin-4-yl-4-(2,2,2-trifluoro-ethyl)-piperazine
50 mg of 10% Pd/C were added to a solution of 0.41 g (1.20 mmol) of 1-(1-
benzyl-piperidin-4-yl)-4-(2,2,2-trifluoro-ethyl)-piperazine in 30 mL MeOH and
the reaction mixture was hydrogenated for 2.5 h at RT and 3 bar H2. To
complete the reaction a spatula tip of Pd(OH)2 was added and hydrogenation
was continued for a further 1.5 h. After the catalyst had been removed by
suction filtering and the solvent had been eliminated the desired product was
obtained.
Yield: 0.28 g (94% of theory)
ESI-MS: (M+H)+ = 252
Rr = 0.05 (silica gel, EtOAc/MeOH/NH3 70:30:3)
Example A8
Methyl-[2-(1-methyl-piperidin-4-yl)-ethyl]-amine (Example 10.32)
Ni
A8a (N,N-dimethyl-2-{1-methyl-piperidin-4-yl)-acetamide
A solution of 9.3 g (50 mmol) ethyl N-methylpiperidinyl-4-acetate in 50 mL
40% aqueous methylamine solution was stirred for 15 h at 80°C in a bomb
tube. The solvent was eliminated i.vac. and the residue dissolved in EtOAc.
The organic phase was dried over MgS04 and the solvent was eliminated
CA 02503455 2005-04-22
107
i.vac.
Yield: 4.7 g (55% of theory)
Rf = 0.53 (silica gel, DCM/MeOH 21:1 )
A8b methyl-[2-(1-methyl-piperidin-4-yl)-ethyl]-amine
3.1 g (20 mmol) N,N-dimethyl-2-(1-methyl-piperidin-4-yl)-acetamide in 40 mL
THF were added dropwise to a solution of 1.1 g (30 mmol) lithium aluminium
hydride in 50 mL diethyl ether. The mixture was refluxed for 2 h and then
stirred overnight at RT. After the addition of water, 6 N NaOH and more water
the solution was stirred for 30 min. After filtration the solvent was
eliminated
i.vac. The residue was dissolved in diethyl ether, dried over MgS04 and the
solvent was eliminated i.vac.
Yield: 2.5 g (80% of theory)
Rf = 0.29 (Alox, DCM/MeOH 21:1 )
Example A9
Cyclopentyl-methyl-piperidin-4-yl-amine (Example 10.41 )
~N\~~N
H
A9a (1-benzyl-piperidin-4-yl)-cyclopentyl-methyl-amine
6.0 mL (105 mmol) glacial acetic acid were added to a solution of 6.5 g (31.8
mmol) (1-benzyl-piperidin-4-yl)-methyl-amine and 2.7 g (32.0 mmol)
cyclopentanone in 200 mL THF and the reaction mixture was heated to
55°C
for 10 min. After cooling to 15°C 10.6 g (50.0 mmol) NaBH(OAc)3 were
added
batchwise and the reaction solution was stirred overnight at RT. To complete
the reaction another 3.0 g (14.2 mmol) NaBH(OAc)3 were added and the
mixture was stirred for a further 5 h at RT. 100 mL of water were carefully
added, the pH was made alkaline using Na2C03, the mixture was extracted
exhaustively with diethyl ether, the combined organic phases were washed
with water and evaporaoted down i.vac. After elimination of the solvent the
CA 02503455 2005-04-22
108
residue was purified by chromatography (silica gel, EtOAc/MeOH/NH3
9:1:0.3).
Yield: 5.0 g (58% of theory)
EI: (M)+ = 272
Rf = 0.6 (silica gel, EtOAcIMeOH/NH3 9:1:0.3)
A9b cyclopentyl-methyl-piperidin-4-yl-amine
1.0 g 10% Pd/C were added to a solution of 5.0 g (18.35 mmol) (1-benzyl-
piperidin-4-yl)-cyclopentyl-methyl-amine in 100 mL MeOH and the reaction
mixture was hydrogenated for 3 h at RT and 5 bar H2. After the catalyst had
been removed by suction filtering and the solvent had been eliminated the
desired product was obtained, which was further reacted without being
purified.
Yield: 3.0 g (90% of theory)
Rf = 0.05 (silica gel, EtOAc/MeOH/NH3 9:1:0.4)
Example A10
4-(1,1-dioxo-1~,6-isothiazolidin-2-yl)-piperidine (Example 10.46)
~N\~~N
~O
A10a 1-benzyl-4-(1,1-dioxo-1~,6-isothiazolidin-2-yl)-piperidine
3-chloro-propan-1-sulphonyl chloride was added dropwise at RT to a solution
of 19.0 g (100 mmol) 1-benzyl-piperidin-4-ylamine and 27.2 g (197 mmol)
K2C03 in 200 mL acetonitrile. The reaction solution was left to stand
overnight. After filtration the solvent was distilled off. The residue was
taken
up in 120 mL EtOH and combined with 6.2 g (111 mmol) KOH. The mixture
was refluxed for 1 h and after cooling acidified with HCI. The precipitate was
suction filtered and dried.
Yield: 15.4 g (46% of theory)
melting point: 255-257°C
CA 02503455 2005-04-22
109
A10b 4-(1,1-dioxo-1~,6-isothiazolidin-2-yl)-piperidine
Analogously to Example 17d the product was prepared from 16.5 g (56.1
mmol) 1-benzyl-4-{1,1-dioxo-1~,6-isothiazolidin-2-yl)-piperidine.
Yield: 8.5 g (74% of theory)
melting point: 89-92°C
Rf = 0.13 (silica gel, DCM/MeOH 8:2)
Example A11
tert-butyl-piperidin-4-yl-amine (Example 10.50)
H
H ~N~/~ N
A11a (1-benzyl-piperidin-4-yl)-tert-butyl-amine
Under an argon atmosphere a solution of 8.6 mL (78 mmol) TiCl4 in 100 mL
toluene was added dropwise to a solution, cooled to 0°C, of 24.1 mL
(130
mmol) 1-benzyl-piperidin-4-one and 55 mL (519 mmol) of tent-butylamine in
200 mL toluene in such a way that the internal temperature did not exceed
15°C. The reaction mixture was stirred overnight at RT, the precipitate
formed
was suction filtered and the solution remaining after the addition of 65 mg of
platinum oxide was hydrogenated until the theoretical uptake of H2 had been
achieved. After hydrogenation had ended, 160 mL of 2 N NaOH solution
were added to the suspension, it was filtered, the organic phase was
separated off, the aqueous solution was extracted three times with toluene
and the combined organic phases were dried over Na2SOa. After the
desiccant and solvent had been eliminated the product was further reacted
without being purified.
Yield: 13.9 g (43% of theory)
A11 b tent-butyl-piperidin-4-yl-amine
1.5 g 10% Pd/C were added to a solution of 13.9 g (56.0 mmol) (1-benzyl-
CA 02503455 2005-04-22
110
piperidin-4-yl)-tert-butyl-amine in 140 mL MeOH and the reaction mixture was
hydrogenated at RT until the theoretical uptake of H2 had been achieved (2 h).
After the catalyst had been removed by suction filtering and the solvent was
eliminated, the desired product was obtained, which was further reacted
without being purified.
Yield: 8.3 g (95% of theory)
Example A12
4-(2-methyl-imidazol-1-yl)-piperidine (Example 10.58)
N
~J
H
A12a tert. butyl 4-(2-methyl-imidazol-1-yl)-piperidine-1-carboxylate
Under a nitrogen atmosphere 1.0 g (22.92 mmol) NaH (55% in mineral oil)
were added batchwise to a solution of 2.0 g (21.92 mmol) 2-methyl-1 H-
imidazole in 20 mL DMF at RT within 20 min and the reaction mixture was
stirred for 30 min at this temperature. Then a solution of 4.0 g (14.32 mmol)
tert. butyl 4-methanesulphonyloxy-piperidine-1-carboxylate in 50 mL DMF was
slowly added dropwise and the reaction solution was then stirred for 2.5 h at
100°C. The mixture was evaporated down i. vac., the residue was taken
up in
150 mL DCM, the organic phase was washed twice with 50 mL water in each
case and dried over MgS04. After the desiccant and solvent had been
eliminated the residue was further reacted without being purified.
Yield: 0.65 g (17% of theory)
ESI-MS: (M+H)+ = 266
Rr = 0.56 (silica gel, DCM/MeOHlcyc/NH3 70:15:15:2)
A12b 4-(2-methyl-imidazol-1-yl)-piperidine
A solution of 650 mg (2.45 mmol) tert. butyl 4-(2-methyl-imidazol-1-yl)-
piperidine-1-carboxylate was dissolved in 10 mL of 4 M HCI in 1,4-dioxane
CA 02503455 2005-04-22
111
and the reaction mixture was stirred for 2 h at RT. The mixture was
evaporated down i. vac., the residue was combined with diisopropylether and
a little isopropanol, the precipitate was removed by suction filtering and
dried
in the circulating air dryer. The desired product was obtained as the
dihydrochloride.
Yield: 430 mg (74% of theory)
ESI-MS: (M+H)+ = 166
R, = 0.54 (silica gel, DCM/MeOH/NH3 75:25:5)
Example A13
4-(2,4-dimethyl-imidazol-1-yI)-piperidine (Example 10.61 )
N
,N
H
A13a tert. butyl 4-(2,4-dimethyl-imidazol-1-yl)-piperidine-1-carboxyiate
Prepared analogously to Example A12a from 2.2 g (22.20 mmvl) of 2,4-
dimethyl-1H-imidazole and 4.0 g (14.32 mmol) of tert. butyl 4-
methanesulphonyloxy-piperidine-1-carboxylate.
Yield: 0.45 g (11% of theory)
ESI-MS: (M+H)+ = 280
Rf = 0.51 (silica gel, DCM/MeOH/cyc/NH3 70:15:15:2)
A13b 4-(2,4-dimethyl-imidazol-1-y1)-piperidine
Prepared analogously to Example A12b from 450 mg (1.61 mmol) of tert.
butyl 4-(2,4-dimethyl-imidazol-1-yl)-piperidine-1-carboxylate. The desired
product was obtained as the dihydrochloride.
Yield: 300 mg (74% of theory)
ESI-MS: (M+H)+ = 180
Rf = 0.63 (silica gel, DCM/MeOH/NH3 75:25:5)
Example A14
CA 02503455 2005-04-22
112
piperazin-1-sulphonic acid-(1-methyl-piperidin-4-yl)-amide (Example 10.66)
o~~o
H~N.~/~ ~S~
~N N~~~N~
H
A14a 4-benzyl-piperazin-1-sulphonic acid-(1-methyl-piperidin-4-yl)-
amide
1.89 g (11.0 mmol) 1,3,2-benzodioxathiole-2,2-dioxide were added to a
solution of 1.0 g (8.76 mmol) 1-methyl-piperidin-4-ylamine and 1.25 mL (9.0
mmol) triethylamine in 50 mL of DCM cooled to 0°C, the cooling bath was
removed and the reaction mixture was stirred overnight at RT. The mixture
was evaporated down i. vac., the residue was stirred with diethyl
ether/diisopropylether, filtered and the crude product was dried.
Under a nitrogen atmosphere this crude product (2.5 g) and 3.16 g (17.4
mmol) 1-benzyl-piperazine were dissolved in 100 mL 1,4-dioxane and the
reaction mixture was refluxed for 2 h. The mixture was evaporated down i.
vac. and the residue was purified by chromatography (silica gel,
DCMIMeOH/NH3 90:10:1 ).
Yield: 1.1 g (36% of theory)
ESI-MS: (M+H)+ = 353
Rr = 0.50 (silica gel, DCM/MeOH/NH3 80:20:1 )
A14b piperazin-1-sulphonic acid-(1-methyl-piperidin-4-yl)-amide
500 mg of 10% Pd/C were added to a solution of 1.1 g (3.12 mmol) of 4-
benzyl-piperazin-1-sulphonic acid-(1-methyl-piperidin-4-yl)-amide in 100 mL
MeOH and the reaction mixture was hydrogenated at RT until the theoretical
uptake of H2 had been achieved (2.5 h). After the catalyst had been removed
by suction filtering and the solvent had been eliminated the desired product
was obtained, which was further reacted without being purified.
Yield: 0.82 g (100% of theory)
ESI-MS: (M+H)+ = 263
Rr = 0.05 (silica gel, EtOAc/MeOHlNH3 90:10:1 )
CA 02503455 2005-04-22
113
Example A15
ethyl 1-( 1-methyl-piperidin-4-yl)-piperazin-2-carboxylate
H ~N.~/~
N~
O
A15a 1-tert-butyl, 3-ethyl 4-(1-methyl-piperidin-4-yl)-piperazin-1,3-
dicarboxylate
A solution of 1.0 g (3.87 mmol) 1-tent-butyl, 3-ethyl piperazin-1,3-
dicarboxylate
and 0.45 mL (3.87 mmol) of 1-methyl-piperidin-4-one in 25 mL THF was
adjusted to a pH of between 5 and 6 with glacial acetic acid and then 1.0 g
(4.48 mmol) NaBH(OAc)3 were added batchwise and the reaction mixture was
stirred overnight at RT. To complete the reaction another 1 mL (8.6 mmol) of
1-methyl-piperidin-4-one and 0.2 g (0.9 mmol) of NaBH(OAc)3 were added
and the mixture was stirred for a further 2 h at RT. The excess NaBH(OAc)3
was destroyed by the addition of a little water, the mixture was saturated
with
K2C03 and stirred. The K2C03 was filtered, the organic phase was
evaporated down and purified by chromatography (silica gel,
EtOAc/MeOH/NH3 90:10:1 ).
Yield: 0.78 g (57% of theory)
ESI-MS: (M+H)+ = 356
Rf = 0.46 (silica gel, EtOAc/MeOH/NH3 80:20:2)
A15b ethyl1-(1-methyl-piperidin-4-yl)-piperazin-2-carboxylate
2.0 mL TFA were added to a solution of 0.78 g (2.20 mmol) 1-tert-butyl, 3-
ethyl 4-(1-methyl-piperidin-4-yl)-piperazin-1,3-dicarboxylate in 30 mL DCM
while cooling with ice and the reaction mixture was stirred for 3 h at RT. The
mixture was evaporated down i. vac. and the product, which was obtained as
the tris-trifluoroacetate salt, was further reacted without any purification.
Yield: quantitative
EI: (M)+ = 255
CA 02503455 2005-04-22
114
Rf = 0.11 (silica gel, EtOAc/MeOH/NH3 70:30:3)
Example A16
ethyl 4-( 1-methyl-pi peridin-4-yl)-piperazin-2-carboxylate
H~N.~/~ ~"
~N N/
O
O
A16a 1-tent-butyl, 3-ethyl 4-benzyl-piperazin-1,3-dicarboxylate
A solution of 5.0 mL (42.10 mmol) benzylbromide in 50 mL THF was added
dropwise to a solution of 10.69 g (41.39 mmol) 1-tert-butyl, 3-ethyl
piperazine-
1,3-dicarboxylate and 7.32 mL (42 mmol) of ethyl diisopropylamine in 150 mL
of THF, the mixture was stirred for 2 h at RT and then refluxed for 3 h. To
complete the reaction another 0.5 mL (4.21 mmol) of benzylbromide were
added and the mixture was refluxed for a further 3 h. After cooling the
reaction
solution was filtered, the filtrate was evaporated down i.vac. and the residue
was purified by chromatography (silica gel, cyclEtOAc 8:2).
Yield: 13.04 g (90% of theory)
ESI-MS: (M+H)+ = 349
R, = 0.51 (silica gel, cyc/EtOAc 8:2)
A16b ethyl1-benzyl-piperazin-2-carboxylate
35 mL TFA were added to a solution of 13.04 g (37.42 mmol) 1-tert-butyl, 3-
ethyl 4-benzyl-piperazin-1,3-dicarboxylate in 200 mL DCM while cooling with
ice and the reaction mixture was stirred for 5 h at RT. The mixture was
evaporated down i. vac. and the product, which was obtained as the bis-
trifluoroacetate salt, was further reacted without any purification.
Yield: quantitative
ESI-MS: (M+H)+ = 249
A16c ethyl1-benzyl-4-(1-methyl-piperidin-4-yl)-piperazin-2-carboxylate
CA 02503455 2005-04-22
115
4.0 g (17.93 mmol) NaBH(OAc)3 were added to a solution of 8.15 g (17.11
mmol) ethyl 1-benzyl-piperazin-2-carboxylate (used as the bis-trifluoroacetate
salt) and 2.05 mL (17.22 mmol) of 1-methyl-piperidin-4-one in 200 mL of THF
and the reaction mixture was stirred overnight at RT. The mixture was
evaporated down i. vac. and the residue was purified by chromatography
(silica gel, gradient: EtOAc to EtOAc/MeOH/NH3 50:50:2).
Yield: 5.91 g (100% of theory)
ESI-MS: (M+H)+ = 346
R~ = 0.53 (silica gel, EtOAc/MeOH/NH3 80:20:2)
A16d ethyl4-(1-methyl-piperidin-4-yl)-piperazin-2-carboxylate
0.5 g Pd(OH)2 were added to a solution of 5.91 g (17.11 mmol) ethyl 1-benzyl-
4-(1-methyl-piperidin-4-yl)-piperazin-2-carboxylate in 150 mL EtOH and the
reaction mixture was hydrogenated for 3.5 h at RT and 3 bar H2. After the
catalyst had been removed by suction filtering and the solvent had been
eliminated the desired product was obtained, which was further reacted
without being purified.
Yield: 4.44 g (100% of theory)
ESI-MS: (M+H)+ = 256
Rs = 0.24 (silica gel, EtOAc/MeOH/NH3 70:30:3)
Example A17
ethyl 1-methyl-4-piperidin-4-yl-piperazin-2-carboxylate
H'N~~~N~i
~ ~N
O O
A17a 1-tent-butyl, 3-ethyl 4-methyl-piperazin-1,3-dicarboxylate
At RT a solution of 1.25 mL (19.90 mmol) of iodomethane in 20 mL of THF
was slowly added dropwise to a solution of 5.04 g (19.51 mmol) of 1-tert-
butyl,
3-ethyl piperazine-1,3-dicarboxylate and 3.4 mL (19.52 mmol) of
ethyldiisopropylamine in 100 mL THF, the reaction mixture was stirred for 20
CA 02503455 2005-04-22
116
min and then heated to 60°C for 3 h. To complete the reaction a further
0.2
mL (3.18 mmol) of iodomethane were added and the mixture was heated to
75°C for a further 3 h. After cooling the mixture was filtered to
remove
insoluble constituents, the filtrate was evaporated down and the residue was
purified by chromatography (silica gel, EtOAc).
Yield: 4.2 g (79% of theory)
ESi-MS: (M+H)+ = 273
Rf = 0.58 (silica gel, EtOAc)
A17b ethyl1-methyl-piperazin-2-carboxylate
20 mL TFA were added to a solution of 4.20 g (15.42 mmol) 1-tent-butyl, 3-
ethyl 4-methyl-piperazin-1,3-dicarboxylate in 80 mL of DCM while cooling with
ice and the reaction mixture was stirred for 1 h at RT. The mixture was
evaporated down i. vac. and the product, which was obtained as the bis-
trifluoroacetate salt, was further reacted without being purified.
Yield: quantitative
ESI-MS: (M+H)+ = 173
Rf = 0.16 (silica gel, EtOAc/MeOH/NH3 70:30:3)
A17c ethyl4-(1-benzyl-piperidin-4-yl)-1-methyl-piperazin-2-carboxylate
4.5 g (20.17 mmol) NaBH(OAc)3 were added batchwise to a solution of 6.17 g
(15.41 mmol) ethyl 1-methyl-piperazin-2-carboxylate (used as the bis-
trifluoroacetate salt) and 3.77 mL (19.93 mmol) 1-benzyl-piperidin-4-one in 80
mL THF and the reaction mixture was stirred for 2 h at RT. Excess
NaBH(OAc)3 was destroyed by the addition of a little water, the reaction
solution was evaporated down i.vac. and the residue was purified by
chromatography (silica gel, gradient: EtOAc/MeOH/NH3 90:10:1 to
EtOAclMeOH/NH3 80:20:2).
Yield: quantitative
ESI-MS: (M+H)+ = 345
R, = 0.41 (silica gel, EtOAc/MeOH/NH3 80:20:2)
A17d ethyl1-methyl-4-piperidin-4-yl-piperazin-2-carboxylate
1 g 10% Pd/C was added to a solution of the crude product from A17c in 200
CA 02503455 2005-04-22
117
mL EtOH and the reaction mixture was hydrogenated for 17.5 h at RT and 3
bar H2. After the catalyst had been removed by suction filtering and the
solvent had been eliminated the desired product was obtained, which was
further reacted without being purified.
Yield: 5.32 g (100% of theory, based on A17a)
ESI-MS: (M+H)+ = 256
Rf = 0.1 (silica gel, EtOAc/MeOH/NH3 50:50:5)
Example A18
ethyl 4-methyl-1-piperidin-4-yl-piperazin-2-carboxylate
H~N~~~N~ ,
~N
O O
A18a ethyl1-benzyl-4-methyl-piperazin-2-carboxylate
A solution of 1.4 mL (22.29 mmol) iodomethane in 50 mL of THF was slowly
added dropwise to a solution of 10.3 g (21.64 mmol) of ethyl 1-benzyl-
piperazin-2-carboxylate (see Example A16b, used as the bis-trifluoroacetate
salt) and 12 mL (68.89 mmol) of ethyldiisopropylarriine in 200 mL THF and the
reaction mixture was stirred overnight at RT. To complete the reaction another
0.2 mL (3.18 mmol) of iodomethane were added and the mixture was stirred
for another 2 h at RT. The precipitate formed was filtered, the filtrate was
evaporated down i.vac. and the residue was purified by chromatography
(silica gel, EtOAc/MeOH/NH3 90:10:1 ).
Yield: 4.1 g (72% of theory)
ESI-MS: (M+Na)+ = 285
Rr = 0.83 (silica gel, EtOAc/MeOH/NH3 90:10:1 )
Al8b ethyl4-methyl-piperazin-2-carboxylate
450 mg 10°!° Pd/C were added to a solution of 4.1 g (15.63 mmol)
ethyl 1-
benzyl-4-methyl-piperazine-2-carboxylate in 100 mL EtOH and the reaction
CA 02503455 2005-04-22
118
mixture was hydrogenated for 11 h at RT and 5 bar H2. To complete the
reaction 450 mg Pd(OH)2 were added and the reaction mixture was
hydrogenated for a further 2.5 h at 50°C and 3 bar H2.The catalyst was
suction filtered, the filtrate was evaporated down and the residue was
purified
by chromatography (silica gel, EtOAc/MeOH/NH3 80:20:2).
Yield: 2.18 g (81 % of theory)
ESI-MS: (M+H)+ = 173
Rf = 0.56 (silica gel, EtOAc/MeOH/NH3 80:20:2)
A18c ethyl1-(1-benzyl-piperidin-4-yl)-4-methyl-piperazin-2-carboxylate
A solution of 1.58 g (9.17 mmol) ethyl 4-methyl-piperazin-2-carboxylate and
1.68 mL (9.2 mmol) 1-benzyl-piperidin-4-one in 30 mL THF was adjusted to a
pH of 5 with glacial acetic acid and then 2.2 g (9.86 mmol) NaBH(OAc)3 were
added batchwise and the reaction mixture was stirred overnight at RT. Excess
NaBH(OAc)3 was destroyed by the addition of a little water and the reaction
solution was dried over K2CO3. The supernatant solution was decanted off,
evaporated down i.vac. and the residue was purified by chromatography
(silica gel, gradient: EtOAc to EtOAc/MeOH/NH3 90:9:1 ).
Yield: 0.71 g (22% of theory)
ESI-MS: (M+H)+ = 346
Rf = 0.84 (silica gel, EtOAc/MeOH/NH3 70:30:3)
A18d ethyl4-methyl-1-piperidin-4-yl-piperazin-2-carboxylate
200 mg Pd(OH)2 were added to a solution of 2.28 g (6.6 mmol) ethyl 1-(1-
benzyl-piperidin-4-yl)-4-methyl-piperazin-2-carboxylate in 100 mL of EtOH
and the reaction mixture was hydrogenated for 9.5 h at RT and 3 bar H2. To
complete the reaction another 100 mg Pd(OH)2 were added and the reaction
mixture was hydrogenated for a further 6 h. The catalyst was suction filtered,
the filtrate was evaporated down and the residue was further reacted without
being purified.
Yield: 1.7 g (100% of theory)
ESI-MS: (M+H)+ = 256
Rr = 0.21 (silica gel, EtOAc/MeOH/NH3 60:40:4)
CA 02503455 2005-04-22
119
Example A19
tert-butyl (4-piperidin-4-yl-piperazin-1-yl)-acetate
H /N\/~N\/\'N
O
A19a tert. butyl [4-(1-benzyl-piperidin-4-yl)-piperazin-1-yl]-acetate
100 g (709 mmol) K2C03 were added to a solution of 74 g (123 mmol) 1-(1-
benzyl-piperidin-4-yl)-piperazine (used as the tris-trifluoroacetate salt) in
1 L
acetonitrile and the suspension was stirred for 10 min at RT. Then 20 mL (133
mmol) of tert. butyl bromoacetate were added. The reaction mixture was
stirred for 3 h at RT and combined with MgS04 to dry it. The insoluble
constituents were filtered off, the solvent was evaporated down i.vac., the
residue was combined with water, the precipitate formed was suction filtered
and dried at 50°C in a circulating air dryer.
Yield: 30 g (65% of theory)
ESI-MS: (M+H)+ = 374
Rf = 0.51 (silica gel, DCM/MeOH/cyc/NH3 70:15:15:2)
A19b tert. butyl (4-piperidin-4-yl-piperazin-1-yl)-acetate
6 g 10% Pd/C were added to a solution of 30 g (80.3 mmol) tert. butyl [4-(1-
benzyl-piperidin-4-yl)-piperazin-1-yl]-acetate in 300 mL THF and the reaction
mixture was hydrogenated at 50°C and 3 bar H2 until the theoretical
uptake of
H2 had been achieved. After the catalyst had been removed by suction
filtering and the solvent had been eliminated the desired product was
obtained, which was further reacted without being purified.
Yield: 22.3 g (98% of theory)
ESI-MS: (M+H)+ = 284
R, = 0.17 (silica gel, DCM/MeOH/cyc/NH3 70:15:15:2)
Example A20
CA 02503455 2005-04-22
120
ethyl (4-piperidin-4-yl-piperazin-1-yl)-acetate
H~N\~~N~N~O~
O
A20a tert. butyl 4-(4-ethoxycarbonylmethyl-piperazin-1-yl)-piperidine-1-
carboxylate
A solution of 1.42 mL ( 13.3 mmol) ethyl chloroacetate in 10 mL acetonitrile
was added to a solution of 4.0 g (13.08 mmol) tert. butyl 4-piperazin-1-yl-
piperidine-1-carboxylate (used as the hydrochloride) and 6.54 mL (39.23
mmol) ethyldiisopropylamine in 50 mL acetonitrile, cooled to 0°C. After
the
cooling bath had been removed a spatula tip of Nal was added and the
reaction mixture was stirred overnight at RT. The mixture was combined with
saturated NaHC03 solution, extracted exhaustively with DCM and the organic
phase was dried over Na2S04. After the desiccant and solvent had been
eliminated the residue was further reacted without being purified.
Yield: 4.25 g (91 % of theory)
ESI-MS: (M+H)+ = 356
Rf = 0.67 (silica gel, DCMIMeOH 9:1 )
A20b ethyl (4-piperidin-4-yl-piperazin-1-yl)-acetate
6 mL TFA were added to a solution of 4.25 g (11.96 mmol) tert. butyl 4-(4-
ethoxycarbonylmethyl-piperazin-1-yl)-piperidine-1-carboxylate in 80 mL DCM,
cooled to 0°C, and the reaction mixture was stirred overnight at RT.
The
mixture was evaporated down i. vac., the residue was stirred with tert-
butylmethylether, the precipitate was suction filtered and the product, which
was obtained as the tris-trifluoroacetate salt, was dried.
Yield: 6.7 g (94% of theory)
ESI-MS: (M+H)+ = 256
Rf = 0.38 (silica gel, DCM/MeOH/NH3 75:25:5)
Example A21
CA 02503455 2005-04-22
121
ethyl (4,4']bipiperidinyl-1-yl-acetate
H~N
I IO
A2la tert. butyl 1'-ethoxycarbonylmethyl-[4,4')bipiperidinyl-1-
carboxylate
The product was obtained analogously to Example A19a from 8.40 g (31.1
mmol) of tert. butyl [4,4']bipiperidinyl-1-carboxylate and 3.53 mL (31.1 mmol)
of ethyl bromoacetate.
Yield: 9.4 g (85°I° of theory)
EI-MS: (M)~ = 355
Rf = 0.64 (silica gel, EtOAc/MeOH 9:1 )
A21 b ethyl_[4,4']bipiperidinyl-1-yl-acetate
The product was obtained as the bis-trifluoroacetate salt analogously to
Example 20b from 7.50 g (21.2 mmol) tert. butyl 1'-ethoxycarbonylmethyl-
[4,4']bipiperidinyl-1-carboxylate.
Yield: 10.1 g (99% of theory)
ESI-MS: (M+H)+ = 255
Rf = 0.15 (silica gel, DCM)
Example A22
ethyl (4-piperazin-1-yl-piperidin-1-yl)-acetate
0
H
'IO
A22a tert. butyl 4-(4-benzyl-piperazin-1-yl)-piperidine-1-carboxylate
A solution of 66.7 mL (381 mmol) of 1-benzylpiperazine and 75.6 g (380
mmol) tert. butyl 4-oxo-piperidine-1-carboxylate was adjusted to pH 5 with
glacial acetic acid. 100 g (380 mmol) of NaBH(OAc)3 were added over 2 h
CA 02503455 2005-04-22
122
while cooling with ice and the mixture was stirred overnight at RT. The
reaction mixture was made alkaline with K2C03 solution (300 g/L), stirred for
one hour at RT and extracted three times with EtOAc. The organic phase was
dried over MgS04 and the solvent was eliminated i.vac.
Yield: 114 g (83°I° of theory)
ESI-MS: (M+H)' = 369
Rf = 0.74 (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2)
A22b 1-benzyl-4-piperidin-4-yl-piperazine
The product was obtained as the tris-trifluoroacetate salt analogously to
Example 20b from 40.0 g (111 mmol) tert. butyl 4-(4-benzyl-piperazin-1-yi)-
piperidine-1-carboxylate.
Yield: 54.8 g (82% of theory)
ESI-MS: (M+H)+ = 260
Rf = 0.18 (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2)
A22c ethyl [4-(4-benzyl-piperazin-1-yl)-piperidin-1-yl]-acetate
The product was obtained analogously to Example A19a from 51.8 g (86
mmol) of 1-benzyl-4-piperidin-4-yl-piperazine (used as the tris-
trifluoroacetate)
and 10.3 mL (91 mmol) of ethyl bromoacetate.
Yield: 25.3 g (85% of theory)
EI-MS: (M)+ = 346
Rf = 0.58 (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2)
A22d ethyl (4-piperazin-1-yl-piperidin-1-yl)-acetate
The product was obtained analogously to Example A19b from 25.3 g (73.3
mmol) ethyl [4-(4-benzyl-piperazin-1-yl)-piperidin-1-yl]-acetate. The product
contains 59% methyl (4-piperazin-1-yl-piperidin-1-yl)-acetate.
Yield: 17.4 g (93% of theory)
ESI-MS: (M+H)' = 256 and for the methyl ester: (M+H)+ = 242
Rf = 0.15 (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2)
Example A23
CA 02503455 2005-04-22
123
1-(2,2,2-trifluoro-ethyl)-[4,4'Jbipiperidinyl
H~N ~ /F
~\N
F
F
A23a tert. butyl 1'-(2,2,2-trifluoro-acetyl)-[4,4'Jbipiperidinyl-1-
carboxylate
The product was prepared analogously to Example A7a from 15.0 g (55.9
mmol) of tert. butyl [4,4']bipiperidinyl-1-carboxylate.
Yield: 19.0 g (93% of theory)
ESI-MS: (M+Na)+ = 387
A23b tert. butyl 1'-(2,2,2-trifluoro-ethyl)-[4,4'Jbipiperidinyl-1-carboxylate
The product was prepared analogously to Example A7b from 20.7 g (56.7
mmol) tert. butyl 1'-(2,2,2-trifluoro-acetyl)-[4,4')bipiperidinyl-1-
carboxylate.
Yield: 19.9 g ( 100% of theory)
ESI-MS: (M+H)+ = 351
Rf = 0.78 (silica gel, PE/EtOAc 1:1 )
A23c 1-(2,2,2-trifluoro-ethyl)-[4,4'Jbipiperidinyl
The product was obtained in the form of the bis-trifluoroacetate analogously
to
Example A20b from 21.4 g (61.1 mmol) tert. butyl 1'-(2,2,2-trifluoro-ethyl)-
[4,4'Jbipiperidinyl-1-carboxylate.
Yield: 26.8 g (92% of theory)
ESI-MS: (M+H)+ = 251
Rf = 0.17 (silica gel, DCM/MeOHlNH3 9:1:0.1 )
Example A24
dimethyl-[3-(4-piperazin-1-yl-phenyl)-propylJ-amine
H~N~N \
CA 02503455 2005-04-22
124
A24a 1-[4-(4-benzyl-piperazin-1-yl)-phenyl]-ethanone
A solution of 35.3 g (200 mmol) benzylpiperazine and 13.8 g (100 mmol) of 4-
fluoroacetophenone in 34 mL (200 mmol) ethyldiisopropylamine was refluxed
for 2 days. After cooling the residue was stirred with tent-butylmethylether,
suction filtered and dried in the air. The product was further reacted without
being purified.
Yield: 12.2 g (42% of theory)
EI-MS: (M)+ = 294
R, = 0.53 (silica gel, PE/EtOAc 3:2)
A24b 1-[4-(4-benzyl-piperazin-1-yl)-phenyl]-3-dimethylamino-propan-1-one
1.40 g paraformaldehyde were added to a solution of 6.9 g (23.4 mmol) of 1-
[4-(4-benzyl-piperazin-1-yl)-phenyl]-ethanone and 2.90 g (35.1 mmol)
dimethylamine hydrochloride in 100 mL EtOH and 10 mL conc. HCI. The
reaction mixture was refluxed for 20 h. The solvent was eliminated i.vac. and
the residue combined with acetonitrile. The precipitate was suction filtered
and dried at 30°C in the circulating air dryer.
Yield: 4.4 g (48% of theory as the hydrochloride)
EI-MS: (M)+ = 351
R, = 0.35 (silica gel, DCM/EtOAc/cyc/MeOH/NH3 60:16:5:5:0.6)
A24c dimethyl-[3-(4-piperazin-1-yl-phenyl)-propyl]-amine
2 g 10% Pd/C were added to a solution of 8.00 g (20.6 mmol) 1-[4-(4-benzyl-
piperazin-1-yl)-phenyl]-3-dimethylamino-propan-1-one in 6.7 mL of conc. HCI
and 300 mL of MeOH. The reaction mixture was stirred at 3 bar H2 and
50°C
for 3 h. After filtration the filtrate was evaporated to dryness. The residue
was
combined with EtOH and EtOAc and stirred overnight. The precipitate was
suction filtered under nitrogen and dried at 20°C in the circulating
air dryer.
Yield: 5.7 g (86% of theory as the bis-hydrochloride)
EI-MS: (M)+ = 247
Rr = 0.35 (DCM/cyc/MeOH/NH3 70:15:15:2)
The other amines used in the preparation of the final compounds are either
CA 02503455 2005-04-22
125
commercially obtainable or were prepared by methods known from the
literature.
CA 02503455 2005-04-22
l26
Preparation of the final compounds
Example 1
( S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-(4-(4-methyl-piperazin-1-
yl )-piperidin-1-yl]-4-(4-(2-oxo-1,2, 4, 5-tetrahydro-1, 3-benzod iazepin-3-yl
)-
piperidin-1-yl]-butan-1,4-dione
F3C NHZ
CI
O
N%~N~~~N~N~CH3
O
N
N~O
1a (4-amino-3-chloro-5-trifluoromethyl-phenyl)-methanol
69.56 g (0.43 mol) CDI were added to a solution of 93.4 g (0.39 mol) 4-amino-
3-chloro-5-trifluoromethyl-benzoic acid (described in Arzneim.-Forsch. 1984,
34(11A), 1612-1624) in 1 L THF and the mixture was stirred for 1 h at
40°C.
The reaction mixture was then carefully added to a solution of 51.4 g (1.36
mol) NaBH4 in 450 mL of water at RT under a nitrogen atmosphere and with
cooling. The mixture was stirred for 2 h at RT, combined with 500 mL water
and 300 mL semiconc. HCI, stirred for another hour and then exhaustively
extracted with EtOAc. The combined org. phases were dried over Na2S04 and
evaporated down i. vac. The oil remaining was combined with 500 mL PE and
stirred while cooling with ice. The precipitate was suction filtered, washed
with
PE and dried. 29.7 g of the desired product were obtained.
The mother liquor was evaporated down again, combined with PE and cooled.
The precipitate obtained was again washed with PE and dried. A further 21.8
g of the desired product were obtained.
CA 02503455 2005-04-22
127
Yield: 51.5 g (59% of theory) of a white solid
Rf = 0.73 (silica gel: PE/EtOAc = 1:1 )
1 b 4-amino-3-chloro-5-trifluoromethyl-benzaldehyde
A mixture of 17.0 g (75.4 mmol) of (4-amino-3-chloro-5-trifluoromethyl-
phenyl)-methanol, 100 g (1.15 mol) of manganese dioxide and 300 mL of
DCM was stirred overnight at RT. The precipitate was suction filtered and the
solution evaporated down i. vac. The desired product was obtained as a white
solid.
Yield: 16.0 g (95% of theory)
ESI-MS: (M+H)+ = 224/226 (CI)
1 c diethyl (2-((R)-4-benzyl-2-oxo-oxazolidin-3-yl)-2-oxo-ethyl]-
phosphonate
A solution of 168.0 g (0.56 mol) (R)-4-benzyl-3-(2-bromo-acetyl)-oxazolidin-2-
one and 188.6 mL (1.1 mol) of triethylphosphite was stirred for 1.5 h at
60°C,
while the ethylbromide formed was distilled off. The reaction mixture was
concentrated by evaporation i. vac. and the residue remaining was purified by
chromatography on silica gel. The desired product was obtained in the form of
a yellowish-brown oil.
Yield: 130 g (65 % of theory)
ESI-MS: (M+H)+ = 356
1d (R)-3-[(E)-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-acryloylj-4-
benzyl-oxazolidin-2-one
Under a nitrogen atmosphere 3.93 g (90.0 mmol) of NaH (55% in mineral oil)
were added batchwise to a solution of 31.98 g (90.0 mmol) of diethyl [2-((R)-
4-benzyl-2-oxo-oxazolidin-3-yl)-2-oxo-ethyl]-phosphonate in 400 mL of THF.
The reaction mixture was stirred for 30 min at RT and for a further 35 min at
CA 02503455 2005-04-22
128
35°C. After the development of gas had ended, 16.0 g (71.5 mmol) of 4-
amino-3-chloro-5-triffuoromethyl-benzaldehyde, dissolved in 50 mL THF, were
added dropwise and stirred for a further 12 h at RT. The reaction solution was
combined with saturated NH4C1 solution, the mixture was extracted
exhaustively with EtOAc and the combined extracts were dried and
evaporated down i. vac. The residue remaining was purified by
chromatography on silica gel. The desired product was obtained in the form of
a yellow oil.
Yield: 38.2 g (62% of theory)
ESI-MS: (M+H)+ = 425/427 (CI)
Rf = 0.55 (silica gel: PE/EtOAc = 2:1 )
1e (R)-3-[3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-propionyl)-4-
benzyl-oxazolidin-2-one
A mixture of 23.7 g (55.8 mmoi) of (R)-3-[(E)-3-(4-amino-3-chloro-5-
trifluoromethyl-phenyl)-acryloyl]-4-benzyl-oxazolidin-2-one, 400 mL of MeOH
and 5.0 g of Raney nickel was shaken for 2 h at RT and 3 bar of H2 in a Parr
autoclave. The catalyst was suction filtered and the solvent removed i. vac.
The desired product was obtained in the form of a yellow oil.
Yield: 22.5 g (95% of theory)
ESI-MS: (M+H)+ = 427/429 (CI)
1f terf.-butyl (S)-3-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-((R)-4-
benzyl-2-oxo-oxazolidin-3-yl)-4-oxo-butanoate
Under an argon atmosphere 63.24 mL (63.24 mmol) of a sodium-
bis(trimethylsilyl)-amide solution (1 M in THF) was added dropwise to a
solution of 22.5 g (52.71 mmol) of (R)-3-[3-(4-amino-3-chloro-5-
trifluoromethyl-phenyl)-propionyl)-4-benzyl-oxazolidin-2-one in 105 mL THF
which had been cooled to -78°C and the mixture was stirred for 2 h at -
78°C.
38.9 mL (263.5 mmol) of tert.butyl bromoacetate were added dropwise to the
CA 02503455 2005-04-22
129
reaction mixture at -78°C, this was stirred for a further 24 h at -
78°C and then
heated to RT. After the addition of 200 mL of a saturated NHQCI solution the
mixture was extracted twice with 300 mL of EtOAc, the combined org. phases
were dried over Na2S04 and evaporated down i. vac. The residue remaining
was purified by chromatography on silica gel. The desired product was
obtained in the form of a yellow oil.
Yield: 15.6 g (55% of theory)
ESI-MS: (M+H)+ = 541/543 (CI)
Rf = 0.35 (silica gel, PE/EtOAc = 8:2)
1g 4-tert.-butyl (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-succinate
11.75 ml (115.1 mmol) H202 (35% in water) were added to a solution of 2.51 g
(57.6 mmol) lithium hydroxide hydrate in 150 mL of water. This mixture was
then added dropwise to an ice-cooled solution of 15.6 g (28.8 mmol) of tert.-
butyl (S)-3-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-((R)-4-benzyl-2-oxo-
oxazolidin-3-yl)-4-oxo-butanoate in 600 mL of THF and the reaction mixture
was stirred for a further 2 h while cooling with ice. Then 150 mL of saturated
sodium sulphite solution were added to the reaction mixture and acidified with
citric acid solution. The org, phase was separated off, dried and evaporated
down i. vac. 15.6 g of a viscous yellow oil were obtained.
The aqueous phase was exhaustively extracted with EtOAc, the combined
org. phases were washed with water, dried and evaporated down i. vac. A
further 5.5 g of a yellow oil were obtained.
The crude product, which still contained (R)-4-benzyl-oxazoiidin-2-one, was
further reacted without purification.
Yield: 21.1 g crude product
ESI-MS: (M+H)+ = 380/382 (CI)
CA 02503455 2005-04-22
130
1g tert, butyl (S)-3-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(4-
methyl-piperazin-1-yl)-piperidin-1-yl]-4-oxo-butanoate
A mixture of 15.4 g (40.3 mmol) of 4-tert. butyl (S)-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl)-succinate, 7.4 g (40.3 mmol) of (1-methyl-4-piperidin-
4-
yl)-piperazine, 5.45 g (40.3 mmol) of HOBt, 12.94 g (40.3 mmol) of TBTU,
11.77 mL (85.0 mmol) of triethylamine and 400 mL of THF was stirred for 12 h
at RT. Then the mixture was evaporated down i. vac. and the residue
remaining was distributed between EtOAc and NaHC03 solution. The org.
phase was separated off, dried and evaporated down i. vac. The residue
obtained was purified by chromatography on aluminium oxide. The desired
product was obtained in the form of a yellow oil.
Yield: 11.0 g (50% of theory)
ESI-MS: (M+H)+ = 547/549 (CI)
Rf = 0.35 (Alox; EtOAc/CH2C12 = 6:4)
1h (S)-3-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(4-methyl-
piperazin-1-yl)-piperidin-1-yl]-4-oxo-butanoic acid
5.75 g (38.4 mmol) of Nal, 3 mL of anisol and 4.92 mL (38.4 mmol) of
trimethylsilylchloride were added to the solution of 7.0 g (12.8 mmol) of
tert.
butyl (S)-3-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(4-methyl-
piperazin-1-yl)-piperidin-1-yl]-4-oxo-butanoate in 375 mL acetonitrile. The
reaction mixture was stirred for 90 min at 40°C, combined with another
5.75 g
(38.4 mmol) of Nal and 4.92 mL (38.4 mmol) of trimethylsilylchloride and
stirred for a further 2 h at 40°C. The mixture was evaporated down i.
vac. and
further reacted as the crude product.
1 i (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-methyl-
piperazin-1-yl)-piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione
A mixture of 6.3 g (12.8 mmol) of (S)-3-(4-amino-3-chloro-5-trifluoromethyl-
CA 02503455 2005-04-22
131
benzyl)-4-[4-(4-methyl-piperazin-1-yl)-piperidin-1-yIJ-4-oxo-butanoic acid,
3.16
g (12.9 mmol) of 3-piperidin-4-yl-1,3,4,5-tetrahydro-1,3-benzodiazepin-2-one,
4.66 g (14.5 mmol) of TBTU, 1.96 g (14.5 mmol) of HOBt, 9.45 mL (68 mmol)
of triethylamine and 300 mL DMF was stirred for 12 h at RT. The reaction
mixture was evaporated down i. vac., the residue was distributed between
EtOAc and saturated NaHC03 solution. The org. phase was separated off,
dried and evaporated down i. vac. The residue was purified by
chromatography on silica gel. The yellow oil obtained was triturated with
ether
and suction filtered. The desired product was obtained in the form of a white
solid.
Yield: 3.8 g (39% of theory)
ESI-MS: (M+H)+ = 718/20 (CI)
Rf = 0.22 (silica gel, EtOAc/MeOH/conc. aqueous NH3 = 70:30:3)
Example 2
2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-(1'-methyl-[4,4'Jbipiperidinyl-
1-
yl)-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butan-1,4-
dione
~CH3
N O
H
2a 2-chloro-4-chloromethyl-6-trifluoromethyl-phenylamine
0.94 mL (13.00 mmol) of SOCI2 was added at RT to a solution of 1.00 g (4.43
mmol) of (4-amino-3-chloro-5-trifluoromethyl-phenyl)-methanol in 50 mL DCM
and the mixture was stirred for 3 h at RT. The reaction mixture was poured
onto ice and the aqueous phase was exhaustively extracted with DCM. The
CA 02503455 2005-04-22
132
combined org. phases were washed with ice-cold NaHC03 solution, dried
over Na2S04, filtered through activated charcoal and evaporated down i. vac.
The crude product was used in the following reaction step without any further
purification.
Yield: 1.08 g (quantitative yield)
EI-MS: M+ = 243/245/247 (CI2)
Rr = 0.81 (silica gel, PE/EtOAc = 2:1 )
2b tert.butyl4-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-3,3-bis-
ethoxycarbonyl-butyrate
193 mg (4.43 mmol) of NaH (55% in mineral oil) were added batchwise to a
solution of 1.20 g (4.43 mmol) of 1-tert.-butyl-4-ethyl 3-ethoxycarbonyl-
succinate in 50 mL of abs. THF under a nitrogen atmosphere and while
cooling with ice and the mixture was stirred for 1 h at RT. 1.1 g (4.43 mmol)
of
2-chloro-4-chloromethyl-6-trifluoromethyl-phenylamine, dissolved in 10 mL
abs. THF, was added dropwise and the mixture was stirred for 16 h at RT.
The reaction mixture was diluted with water and the aqueous phase extracted
with EtOAc. The org. phase was dried over MgS04 and evaporated down i.
vac. The crude product was used in the next reaction step without any further
purification.
Yield: 2.1 g (98% of theory)
ESI-MS: (M+H)+ = 482!484 (CI)
Rr = 0.48 (silica gel, PE/EtOAc = 4:1 )
2c 4-(4-amino-3-chloro-5-trilluoromethyl-phenyl)-3,3-bis-ethoxycarbonyl-
butanioc acid
20 mL of TFA was added to a solution of 30.0 g (62.25 mmol) of tert.butyl 4-
(4-amino-3-chloro-5-trifluoromethyl-phenyl)-3,3-bis-ethoxycarbonyl-butanoate
in 200 mL DCM while cooling with ice and the mixture was stirred for 16 h at
RT. The reaction mixture was evaporated down i. vac. and the residue
CA 02503455 2005-04-22
133
recrystallised from PE. The precipitate was filtered off, washed with PE and
dried.
Yield: 23.6 g (89% of the yield)
ESI-MS: (M-H)~ = 424/426 (CI)
2d diethyl2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-{2-oxo-2-[4-(2-
oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-ethyl}-malonate
3.2 mL (23.0 mmol) of triethylamine were added dropwise to a solution of 8.00
g (19.0 mmol) of 4-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-3,3-bis-
ethoxycarbonyl-butanoic acid, 4.39 g (19.0 mmol) of 3-piperidin-4-yl-3,4-
dihydro-1H-quinazolin-2-one, 6.00 g (18.0 mmol) of TBTU and 2.75 g (18.0
mmol) of HOBT in 100 mL of THF and the mixture was stirred for 16 h at RT.
The solid formed was filtered off, washed with diethyl ether and dried i. vac.
Yield: 10.45 g (87% of theory)
2e 2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butanoic acid
3.13 g (78.25 mmol) of NaOH, dissolved in 300 mL water, were added to a
solution of 10.00 g (15.65 mmol) of diethyl 2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl )-2-{2-oxo-2-[4-(2-oxo-1, 4-di hyd ro-2H-qui nazolin-3-
yl )-
piperidin-1-yl]-ethyl}-malonate in 600 mL EtOH and the mixture was refluxed
for 4 h. EtOH was evaporated off i. vac., the reaction mixture was acidified
to
pH 1 with conc. HCI and 1 hour at RT. The precipitate formed was filtered off,
washed with water and dried i. vac.
Yield: 8.01 g (95% of theory)
CA 02503455 2005-04-22
134
2f 2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-(1'-methyl-
[4, 4']bipi perid i nyl-1-yl )-4-[4-(2-oxo-1,4-di hydro-2H-q ui nazol i n-3-yl
)-
piperidin-1-yl]-butan-1,4-dione
2.0 mL triethylamine were added to a solution of 0.80 g (1.48 mmol) of 2-(4-
amino-3-chloro-5-trifluoromethyV-benzyl~4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
quinazoiin-3-yi)-piperidin-1-yl]-butanoic acid, 0.28 g (1.50 mmol) of 1-methyl-
[4,4')bipiperidinyl, 0.49 g (1.50 mmol) of TBTU and 0.23 g (1.50 mmol) of
HOBT in 100 mL of THF and the mixture was stirred for 16 h at RT. The
reaction mixture was evaporated down i. vac., the residue was combined with
saturated NaHC03 solution and the mixture was exhaustively extracted with
EtOAc. The combined org. extracts were dried over MgS04 and evaporated
down i. vac. The residue was purified by column chromatography (silica gel,
gradient : EtOAc/MeOH/ NH3 94!5/1 to 70/25/5).
Yield: 253 mg (24°l0 of theory)
ESI-MS: (M+H)+ = 703/705 (CI)
Rf = 0.66 (silica gel, DCM/cyc/MeOH/NH3 70/15/15/2)
Example 3
2-(4-amino-3-chloro-5-trifluoromethyl-benzyl )-1-[4-(4-methyl-piperazin-1-yl )-
piperidin-1-yl)-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl)-
butan-1,4-dione
N HZ
CI
N ~N~N~CH3
O
N O
H
The product was obtained analogously to Example 2f starting from 0.80 g
CA 02503455 2005-04-22
135
(1.48 mmol) of 2-(4-amino-3-chloro-5-trifluoromethyl-benzyi)-4-oxo-4-[4-(2-
oxo-1,4-dihydro-2H-quinazolin-3-y1)-piperidin-1-y1]-butanoic acid and 0.30 g
(1.50 mmol) of 1-methyl-4-piperidin-4-yl-piperazine.
Yield: 600 mg (57% of theory)
EI-MS: M+ = 703/705 (CI)
Rf = 0.56 (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2)
Example 4
2-(4-amino-3-chloro-5-trifluoromethyl-benzyl )-1-[1,4']bipiperidinyl-1'-yl-4-
[4-(2-
oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butan-1,4-dione
3
0
/~N
N\~~N\~
O
N O
H
The product was obtained analogously to Example 2f starting from 0.80 g
(1.48 mmol) of 2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-
oxo-1,4-dihydro-2H-quinazofin-3-y1)-piperidin-1-yl]-butanoic acid and 0.27 g
(1.50 mmol) of [1,4']bipiperidinyl.
Yield: 240 mg (24°!° of theory)
ESI-MS: (M+H)+ = 689/691 (CI)
Rf = 0.59 (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2)
CA 02503455 2005-04-22
136
Examale 5
2-(4-amino-3-chloro-5-trifluorom ethyl-benzyf )-4-[4-(2-oxo-1, 4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-yl]-1-(4-pyridin-4-yl-piperazin-1-yl)-butan-1,4-
dione
/~N
N O
H
The product was obtained analogously to Example 2f starting from 0.80 g
(1.48 mmol) of 2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-
oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butanoic acid and 0.24 g
(1.48 mmol) of 1-pyridin-4-yl-piperazine.
Yield: 500 mg (50% of theory)
Ei-MS: M+ = 683/685 (CI)
Rf = 0.35 (silica gel, MeOH)
Example 6
2-(4-amino-3-chloro-5-trifluoromethyl-benzyl )-1-[4-( 1-methyl-piperidi n-4-
ylamino)-piperidin-1-yl]-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-
1-yl]-butan-1,4-dione
H
N N~~~N~CH
3
~4
N O
H
CA 02503455 2005-04-22
137
6a (1-benzyl-piperidin-4-yl)-(1-methyl-piperidin-4-yl)-amine
A solution of 15.0 g (78.8 mmol) of 1-benzyl-piperidin-4-ylamine and 10 mL
(78.8 mmol) of 1-methyl-piperidin-4-one in 300 mL of THF was acidified with
HOAc to pH 5 and stirred for 1 h at RT. 19.0 g (90.0 mmol) of NaBH(OAc)3
was added and the mixture was stirred for 16 h. The mixture was evaporated
down i. vac., the residue was dissolved in MeOH and precipitated by the
addition of HCI in MeOH. The precipitate formed was filtered off, washed with
MeOH and dried i. vac.
Yield: 21.8 g (70% of theory)
Rf = 0.30 (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2)
6b (1-methyl-piperidin-4-yl)-piperidin-4-yl-amine-trihydrochloride
A solution of 10.0 g (25.3 mmol) of (1-benzyl-piperidin-4-yl)-(1-methyl-
piperidin-4-yl)-amine in 120 mL MeOH was added to a suspension of 5 g of
10% Pd/C in 80 mL water and the mixture was hydrogenated for 2 h at 50
°C
under 3 bar H2. The reaction mixture was filtered and the filtrate was
evaporated down i. vac. The residue was combined with EtOH, the precipitate
formed was filtered off, washed with EtOH and ether and dried i. vac.
Yield: 7.75 g (quantitative yield)
6c 2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(1-methyl-piperidin-
4-ylamino)-piperidin-1-yiJ-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidin-1-yl]-butan-1,4-dione
The product was obtained analogously to Example 2f starting from 0.80 g
(1.48 mmol) of 2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-
oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butanoic acid and 0.24 g
(1.48 mmol) of (1-methyl-piperidin-4-yl)-piperidin-4-yl-amine-
trihydrochloride.
CA 02503455 2005-04-22
138
Yield: 300 mg (25% of theory)
EI-MS: M+ = 717/719 (CI)
Rf = 0.20 (silica gel, MeOH)
Example 6.1
[4-( 1-{2-(4-amino-3-chloro-5-trifluoromethyl-benzyl )-4-oxo-4-[4-( 2-oxo-1, 4-
d ihyd ro-2H-qui nazoli n-3-yl )-piperid i n-1-yl]-butyryl}-pi peridin-4-yl )-
piperazi n-1-
yl]-acetic acid
N ~N~N~O~H
O
N O
I
H
Prepared analogously to Example 16.4 from 108 mg (0.20 mmol) 2-(4-amino-
3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-ylrpiperidin-1-yl]-butanoic acid and 51 mg (0.20 mmol) ethyl (4-
piperidi n-4-yl-piperazi n-1-yl )-acetate.
Yield: 16 mg ( 10% of theory)
ESI-MS: (M+H)+ = 748/750(CI)
Example 6.2
Methyl (1'-{2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-
1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-ylJ-butyryl}-[4,4')bipiperidinyl-1-
yl )-
acetate
CA 02503455 2005-04-22
139
CF3
/ NHZ
O \ CI
N N N~Ow
/ ~~ O O
N O
I
H
Prepared analogously to Example 16.5 from 216 mg (0.4 mmol) 2-(4-amino-3-
chloro-5-trifluoromethyl-benzyl )-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-
3-yl)-piperidin-1-yl]-butanoic acid and 102 mg (0.2 mmol) ethyl
[4,4']bipiperidinyl-1-yl-acetate.
Yield: 22 mg (22% of theory)
ESI-MS: (M+H)+ = 761/763(CI)
Rf = 0.33 (silica gel, DCM/MeOH 9:1 )
Example 6.3
( 1'-{2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butyryl}-[4,4']bipiperidinyl-1-yl)-
acetic acid
NHZ
CI
N ~\~'~~~~N ~O ~H
/ ~ O O
N O
I
H
Prepared analogously to Example 16.6 from 201 mg (0.26 mmol) methyl (1'-
{2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-
2H quinazolin-3-yl)-piperidin-1-yl]-butyryl}-[4,4']bipiperidinyl-1-yl)-
acetate.
Yield: 22 mg (22% of theory)
ESI-MS: (M+H)' = 761/763(CI)
CA 02503455 2005-04-22
l40
Rf = 0.21 (silica gel, DCM/MeOH 9:1 )
Exam~~le 7
4-(2-oxo-1,2,4, 5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-carboxylic
acid-[(R)-1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-1,4'-bipiperidinyl-
1'-
yl-2-oxo-ethyl]-amide
CF3
NHZ
O \ CI
N ~N ~N\~~N~~
H
N O
N~O
H
7a (R)-2-amino-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-N-((1S,2S)-
2-hydroxy-1-methyl-2-phenyl-ethyl)-N-methyl-propionamide
The alkylation was carried out in accordance with the general procedure
described by A. G. Myers et al. (J. Org. Chem. 1999, 64, 3322-3327.) starting
from 31.72 g (132 mmol) of 2-amino-N-((1S,2S)-2-hydroxy-1-methyl-2-phenyl-
ethyl)-N methyl-acetamide-monohydrate and 33.8 g (138 mmol) of 2-chloro-4-
chloromethyl-6-trifluoromethyl-phenylamine. The crude product was purified
by column chromatography (silica gel, DCM/ cyc/MeOH/NH3 70:15:15:2)
purified.
Yield: 10.0 g (18% of theory)
ESI-MS: (M+H)* = 430/432 (CI)
Rf = 0.48 (silica gel, DCM/ cyc/MeOH/NH3 70:15:15:2)
7b (R)-2-amino-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-propionic
acid
The hydrolysis was carried out in accordance with the general procedure
described by A. G. Myers et al. (J. Org. Chem. 1999, 64, 3322-3327.) starting
CA 02503455 2005-04-22
141
from 10.0 g (23.0 mmol) of (R)-2-amino-3-(4-amino-3-chloro-5-trifluoromethyl-
phenyl)-N-((1 S,2S)-2-hydroxy-1-methyl-2-phenyl-ethyl)-N-methyl-
propionamide. The crude product was used in the next synthesis step without
any further purification.
7c (R)-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-2-tert.-
butoxycarbonylamino-propionic acid
A solution of 3.71 g (35.0 mmol) of NaHC03 in 100 mL water was added to a
solution of 6.5 g (23.0 mmol) of (R)-2-amino-3-(4-amino-3-chloro-5-
trifluoromethyl-phenyl)-propionic acid in 140 mL of THF. 15.28 g (70.0 mmol)
of Boc-anhydride was added and the mixture was stirred for 3 h at RT. THF
was evaporated off i. vac., the aqueous phase was washed with EtOAc and
acidified with 10% citric acid solution. The aqueous phase was exhaustively
extracted with EtOAc, the combined org. extracts were dried over Na2S04 and
evaporated down i. vac. The crude product was used in the next reaction step
without any further purification.
Yield: 2.00 g (15% of theory)
ESI-MS: (M-H)- = 381/383 (CI)
7d tert.-butyl [(R)-1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-
[1,4']bipiperidinyl-1'-yl-2-oxo-ethyl]-carbaminate
1.53 mL (11.00 mmol) of triethylamine were added to a solution of 2.00 g
(5.22 mmol) of (R)-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-2-tert.-
butoxycarbonylamino-propionic acid, 0.99 g (5.30 mmol) of [1,4')bipiperidinyl,
1.77 g (5.50 mmol) of TBTU and 0.74 g (5.50 mmol) of HOBt in 150 mL of
THF and the mixture was stirred for 16 h at RT. The reaction mixture was
evaporated down i. vac., the residue was combined with saturated NaHC03
solution and the mixture was exhaustively extracted with EtOAc. The
combined org. extracts were dried over MgS04 and evaporated down i. vac.
The residue was purified by column chromatography (aluminium oxide
(neutral, activity III), DCM/MeOH 99:1 ).
CA 02503455 2005-04-22
142
Yield: 500 mg ( 18% of theory)
7e (R)-2-amino-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-1-
[1,4']bipiperidinyl-1'-yl-propan-1-one-dihydrochloride
mL HCI (12 M in EtOH) were added at RT to a solution of 500 mg (0.75
mmol) of tert. butyl [(R)-1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-
[1,4']bipiperidinyl-1'-yl-2-oxo-ethyl]-carbaminate in 50 mL EtOH and the
mixture was stirred for 3 h and then evaporated down i. vac. The crude
product was used in the next reaction step without any further purification.
Yield: 380 mg (quantitative yield)
7f 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-[(R)-1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-
1,4'-bipiperidinyl-1'-yl-2-oxo-ethyl]-amide
180 mg (1.10 mmol) of CDT were added at 0 °C to a solution of 380 mg
(0.75
mmol) of (R)-2-amino-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-1-
[1,4']bipiperidinyl-1'-yl-propan-1-one-dihydrochloride in 50 mL of DMF and
0.56 mL (4.00 mmol) of triethylamine and the mixture was stirred for 1.5 h at
0°C. 242 mg (0.99 mmol) of 3-piperidin-4-yl-1,3,4,5-tetrahydro-1,3-
benzodiazepin-2-one were added and the reaction mixture was stirred for 1.5
h at 100 °C. DMF was evaporated off i, vac. and the residue was
purified by
column chromatography (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2) followed
by HPLC.
Yield: 140 mg (20% of theory)
ESI-MS: (M+H)+ = 704/706 (CI)
Rr = 0.58 (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2)
Example 7.1
CA 02503455 2005-04-22
143
4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-benzodiazepi n-3-yl )-piperidin-1-
carboxylic
acid-[(R)-1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-(4-dimethylamino-
piperid i n-1-yl )-2-oxo-ethyl]-amide
CF3
/ NHz
Ct
N N~N\~~N~
H O
N
~ N~O
H
7.1 a ethyl (R)-2-amino-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-
propionate
A solution of 3.5 g (10.97 mmol) (R)-2-amino-3-(4-amino-3-chloro-5-
trifluoromethyl-phenyl)-propionic acid in 100 mL EtOH and 70 mL ethanolic
HCI (11.5 M) was stirred overnight at RT. The mixture was evaporated down
i.vac., the residue was taken up in 150 mL water, combined with 30 mL of
15% K2C03 solution, extracted with 150 mL EtOAc, the organic phase was
separated off and dried over Na2S04. After removal of the desiccant and
solvent the desired product was obtained.
Yield: 3.5 g (92% of theory)
ESI-MS: (M+H)' = 311/313 (CI)
7.1 b ethyl (R)-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-2-{[4-(2-
oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carbonyl]-amino}-propionate
1.8 g (11.0 mmol) CDT were added to a solution of 3.2 g (10.2 mmol) of ethyl
(R)-2-amino-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-propionate and 1.8
mL (10.3 mmol) ethyldiisopropylamine in 150 mL THF cooled to 0°C and
the
reaction mixture was stirred for 45 min at this temperature and after removal
of the ice bath stirred for a further 30 min. Then 2.5 g (10.2 mmol) 3-
piperidin-
4-yl-1,3,4,5-tetrahydro-1,3-benzodiazepin-2-one suspended in 50 mL THF
were added. 40 mL DMF were added to the reaction solution and this was
CA 02503455 2005-04-22
144
stirred for 2 h at 80°C. It was evaporated down i.vac., combined with
200 mL
EtOAc and 200 mL 10% citric acid solution, the organic phase was separated
off, extracted with 150 mL NaHC03 solution and dried over Na2S04. After
elimination of the desiccant and solvent the desired product was obtained.
Yield: 5.9 g (100% of theory)
ESI-MS: (M+H)+ = 582/584 (CI)
Rr = 0.4 (silica gel, EtOAc)
7.1 c (R)-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-2-{[4-(2-oxo-
1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-carbonyl]-
amino}-propionic acid
To a suspension of 6.0 g (10.31 mmol) ethyl (R)-3-(4-amino-3-chloro-5-
trifl uoromethyl-phenyl )-2-{[4-( 2-oxo-1, 2, 4, 5-tetra hydro-1, 3-benzod
iazepi n-3-
yl)-piperidin-1-carbonyl]-amino}-propionate in 50 mL THF was added a
solution of 0.64 g (15 mmol) lithium hydroxide hydrate in 100 mL of water. A
further 100 mL each of water and THF were added to this suspension, and
after 5 min a solution was formed. It was stirred for 1 h at RT, the THF was
eliminated i.vac., diluted with 100 mL of water and 1 M HCI was added
dropwise while cooling with ice until an acidic reaction was obtained. The
substance precipitated was filtered, washed with water and dried in the air.
Yield: 5.5 g (96% of theory)
ESI-MS: (M+H)+ = 554/556 (CI)
7.1d 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-[(R)-1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-(4-
dimethylamino-piperidin-1-yl)-2-oxo-ethyl]-amide
241 mg (0.75 mmol) TBTU, 0.21 mL (1.5 mmol) triethylamine and 103 mg (0.8
mmol) dimethyl-piperidin-4-yl-amine were added to a solution of 400 mg (0.72
mmol) of (R)-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-2-{[4-(2-oxo-
1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-carbonyl]-amino}-
propionic acid in 10 mL DMF and the reaction mixture was stirred overnight at
RT. The reaction solution was poured onto 150 mL of 15% K2C03 solution,
stirred for 10 min at RT, the substance precipitated was suction filtered,
washed with 30 mL water and dried overnight in the air. The crude product
CA 02503455 2005-04-22
145
was suspended in isopropanol, stirred overnight at RT, suction filtered and
dried at 40°C.
Yield: 350 mg (73% of theory)
ESI-MS: (M+H)+ = 664/666 (CI)
Retention time (HPLC): 6.0 min (method A)
The following compounds were prepared analogously from (R)-3-(4-amino-3-
chloro-5-trifluoromethyl-phenyl)-2-{[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-carbonyl]-amino}-prapionic acid and the
corresponding amount of amine:
CF3
/ NHZ
O \ ~ CI
~ R
N~N
H O
N
N~O
H
ExampleR Yield Mass Retention
time
(%) spectrum HPLC
(method)
7.2 ~ ~ N 44 699/701 6.1 min
N
I
~N [M+HJ (A)
7.3 ' 27 718/720 6.2 min
N
/',N~ [M+HJ+ (A)
7.4 ~ ~ 79 839/841 7.1 min
w
N~ ~ [M+HJ+ (A)
~
N ~
o
The following compounds were prepared analogously from (R)-3-(4-amino-3-
chloro-5-trifluoromethyl-phenyl)-2-{[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
CA 02503455 2005-04-22
146
benzodiazepin-3-yl)-piperidin-1-carbonyl]-amino}-propionic acid and the
corresponding amount of amine, the crude product being purified by
chromatography (silica gel, gradient: DCM to MeOH/NH3 9:1 within 45 min):
ExampleR Yield (%) Mass Retention
time
spectrum HPLC
(method)
7.5 ' ~ 53 790/792 6.6 min
1
N
N~o [M+Hl+ (A)
0
7.6 ' , 84 719/721 5.7 min
N\~N N/ +
[M+H~ (A)
7~~ ', 43 719/721 5.0 min
~N +
~
N
N [M+H~ (A)
The following compounds were prepared analogously from (R)-3-(4-amino-3-
chloro-5-trifluoromethyl-phenyl)-2-{[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-carbonyl]-amino)-propionic acid and the
corresponding amount of amine, while the crude product was purified directly
by HPLC:
ExampleR Yield (%) Mass Retention
time
spectrum HPLC
(method)
7~$ ', 17 6761f78 6.2 min
N~~N
I [M+H~+ (A)
7.9 ' ~ 52 731/733 5.5 min
N
~~N
N' M+H + A
[ 1 ()
CA 02503455 2005-04-22
147
Example 7.10
4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-benzodiazepi n-3-yl )-pi perid in-1-
carboxylic
acid-[(R)-1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-oxo-2-(4-piperazin-1-
yl-piperidin-1-yl)-ethyl]-amide
CF3
NH2
O \ CI
N~N~N~J~N~N~H
H ,IO
N
N~O
H
A solution of 600 mg (0.72 mmol) benzyl 4-[1-((R)-3-(4-amino-3-chloro-5-
trifluoromethyl-phenyl)-2-{[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-
yl)-piperidin-1-carbonyl]-amino)-propionyl)-piperidin-4-yl]-piperazin-1-
carboxylate (Example 7.4) and 200 mg Raney nickel in 50 mL MeOH were
hydrogenated at RT and 50 psi of H2 for 12 h. The catalyst was filtered off,
the
solvent evaporated down i.vac. and the residue purified by HPLC.
Yield: 160 mg (32% of theory)
ESI-MS: (M+H)+ = 705/707 (CI)
Retention time (HPLC): 5.5 min (method A)
CA 02503455 2005-04-22
148
Example 7.11
[1'-((R)-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-2-{[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-carbonyl]-amino}-propionyl)-
4,4'-bipiperidinyl-1-yl]-acetic acid
C F3
NHZ
O \ CI
N~N~N ~ O
N~ H
H O I fO
N
N~O
H
A solution of 20.1 mg (0.47 mmol) lithium hydroxide hydrate in 10 mL water
was added to a solution of 250 mg (0.32 mmol) ethyl [1'-((R)-3-(4-amino-3-
chloro-5-trifluoromethyl-phenyl)-2-{[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-carbonyl]-amino}-propionyl)-4,4'-bipiperidinyl-
1-yl]-acetate (Example 7.5) in 5 mL THF and the reaction mixture was stirred
for 2 h at RT. 0.5 mL 1 M HCI were added, the substance precipitated was
filtered off and dried at 50°C. The crude product was purified by HPLC.
Yield: 85 mg (35% of theory)
ESI-MS: (M+H)+ = 762/764 (CI)
Retention time (HPLC): 6.2 min (method A)
The following Examples may be prepared analogously:
Example Structure
7.12
~NHz
~
O ~
CI
N~N
~N~N~
H O
N
~ N~O
H
CA 02503455 2005-04-22
149
T.13 GIF
~NHs
~~f
GI
O
~ ~N~ ,H
N N if ~/'~N~N
~~I
H 0
N
N~O
H
T.14 G'F,
~NH~
\ II
CI
O~~
~N~~~~~~~ .H
~N~N~ - V N
H O
N
N~p
H
Example 8
4-(2-oxo-1, 2, 4, 5-tetrahydro-1, 3-benzodiazepin-3-yl )-piperidin-1-
carboxylic
acid-[1-(4-amino-3-chloro-5-trifluoromethyl-benzyi)-2-1,4'-bipiperidinyl-1'-yl-
2-
oxo-ethyl]-amide
0
N' _N
H
N O
N~O
H
8a diethyl2-acetylamino-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-
malonate
24.11 g (0.11 mol) diethyl 2-acetyiamino-malonate was added to a freshly
prepared solution of 2.55 g (0.11 mol) sodium in 200 mL abs. EtOH under a
nitrogen atmosphere and the mixture was stirred for 15 min at RT. A solution
of 27.00 g (0.11 mol) 2-chloro-4-chloromethyl-6-trifluoromethyl-phenyiamine
(Example 2a) in 100 mL of 1,4-dioxane was rapidly added dropwise and the
mixture was stirred for 4 h at RT. 500 mL of water were added and the
CA 02503455 2005-04-22
150
mixture was stirred for a further 16 h. The precipitate formed was filtered
off,
washed with water and dried i. vac.
Yield: 40.0 g (84% of theory)
Rf = 0.14 (silica gel, PE/EtOAc = 2/1 )
8b 2-amino-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-propionic acid-
hydrochloride
50 mL conc. HCI were added to a solution of 40.0 g (94.16 mmol) of diethyl 2-
acetylamino-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-malonate in 110
mL AcOH and 150 mL of water and the reaction mixture was heated to 140
°C
for 4 h. The precipitate formed was filtered off and discarded. The filtrate
was
evaporated down i. vac., combined with 100 mL of EtOH and stirred for 15
min at RT. The precipitate formed was filtered off, washed with EtOH and
dried i. vac. The crude product was used in the next reaction step without any
further purification.
Yield: 16 g (53% of theory)
ESI-MS: (M-H)- = 281/283 (CI)
8c ethyl2-amino-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-propionate
16 g (50.14 mmol) of 2-amino-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-
propionic acid-hydrochloride were dissolved in 350 mL of HCI (12 M in EtOH)
and stirred for 5 h at RT. The reaction mixture was evaporated down to 100
mL i. vac. and combined with 200 mL diethylether. The precipitate formed was
filtered off, washed with diethylether and dried i. vac.
Yield: 12.2 g (70% of theory)
ESI-MS: (M+H)+ = 311/313 (CI)
8d ethyl3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-2-t[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-carbonyl]-amino}-
CA 02503455 2005-04-22
I51
propionate
4.15 g (23.04 mmol) of CDT were added at 0 °C to a suspension of 8.00 g
(23.04 mmol) of ethyl 2-amino-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-
propionate and 16.0 mL (115.00 mmol) of triethylamine in 100 mL of DMF and
the mixture was stirred for 1.5 h at 0 °C. A solution of 5.64 g (23.00
mmol) of
3-piperidin-4-yl-1,3,4,5-tetrahydro-1,3-benzodiazepin-2-one in 200 mL of DMF
was added and the mixture was heated to 100 °C for 2 h. The reaction
mixture was cooled to RT, diluted with 1.5 L water and stirred for a further
10
min. The precipitate formed was filtered off, washed with water and dried i.
vac.
Yield: 13.0 g (97% of theory)
ESI-MS: (M+H)+ = 582/584 (CI)
8e 3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-2-{[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-carbonyl)-amino}-
propionic acid
45 mL of 1 M NaOH were added to a solution of 13.00 g (22.34 mmol) of
ethyl 3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-2-{[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-carbonyl]-amino}-propionate in
100 mL EtOH and the mixture was stirred for 16 h at RT. EtOH was
evaporated off i. vac., 45 mL of 1 M HCI were added and the mixture was
stirred for 15 min. The precipitate formed was filtered off, washed with water
and dried i. vac. at 75 °C.
Yield: 10.5 g (85°!° of theory)
ESI-MS: (M-H)- = 552/554 (CI)
8f 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-[1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-1,4'-
bipiperidinyl-1'-yl-2-oxo-ethyl]-amide
CA 02503455 2005-04-22
l52
0.69 mL (5.00 mmol) of triethylamine were added to a solution of 1.00 g (1.81
mmol) of 3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-2-{[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-carbonyl]-amino}-propionic
acid, 0.34 g (1.81 mmol) of [1,4']bipiperidinyl and 0.64 g (2.00 mmol) of TBTU
in 150 rnL of THF and the mixture was stirred for 16 h at RT. The reaction
mixture was evaporated down i. vac., the residue was combined with
saturated NaHC03 solution and the mixture was exhaustively extracted with
EtOAc. The combined org. extracts were dried over MgS04 and evaporated
down i. vac. The residue was purified by column chromatography (silica gel,
EtOAc/MeOH/ NH3 = 75:25:2.5).
Yield: 350 mg (28°l0 of theory)
ESI-MS: (M+H)+ = 704/706 (CI)
R, = 0.58 (silica gel, DCM/cyc/MeOH/NH3 = 70/15/15/2)
Example 9
( S)-2-(4-am i no-3-bromo-5-trifluoromethyl-benzyl )-1-[4-(4-methyl-pi perazin-
1-
yl )-piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-
piperidin-1-yl]-butan-1,4-dione
CF3
/ NHz
O \ I Br
N ~~N\~~N~/~'N i
O
N
/ \ N~p
H
9a ethyl4-amino-3-trifluoromethyl-benzoate
A solution of 150 g (0.66 mol) N-(4-cyano-2-trifluoromethyl-phenyl)-acetamide
in 360 mL dry EtOH and 540 mL 10 M ethanolic HCL was heated to 70°C for
2 h 45 min in a pressure apparatus. After the solution had cooled the
precipitate formed was suction filtered, washed with EtOH and the filtrate was
CA 02503455 2005-04-22
153
evaporated down i.vac. The residue was combined with 300 mL water and
EtOH in each case, vigorously stirred, suction filtered and washed with
water/EtOH 1:1.
The crude product was further reacted without purification.
Yield: 153 g (100% of theory)
Rf = 0.4 (silica gel, PE/EtOAc 1:1 )
9b 4-amino-3-trifluoromethyl-benzoic acid
At RT a solution of 153 g (0.66 mol) ethyl 4-amino-3-trifluoromethyl-benzoate
in 350 mL EtOH was added to a solution of 100 g (2.5 mol) NaOH in 250 mL
water and the reaction mixture was stirred for 2 h at 45°C. EtOH was
eliminated i. vac., the remaining aqueous solution was acidified with conc.
HCI, the precipitated product was suction filtered, washed with water and
dried in the air.
Yield: 129 g (96% of theory)
ESi-MS: (M-H)- = 204
9c 4-amino-3-bromo-5-trifluoromethyl-benzoic acid
A solution of 6 mL (117 mmol) bromine in 50 mL acetic acid was slowly added
dropwise to a solution of 21.0 g (102 mmol) 4-amino-3-trifluoromethyl-benzoic
acid in 250 mL acetic acid and then heated to 60°C for 2 h. After
cooling it
was combined with 1 L water and the precipitate was suction filtered. The
residue was dissolved in DCM, the organic phase made alkaline with NaOH
solution, the aqueous phase was separated off and acidified with conc. HCI.
The precipitate was suction filtered and dried at 60°C.
Yield: 18 g (62% of theory)
ESI-MS: (M-H)- = 282/284 (Br)
Rf = 0.6 (silica gel, PE/EtOAc/AcOH 50:50:1 )
9d (4-amino-3-bromo-5-trifluoromethyl-phenyl)-methanol
12 g (74 mmol) CDI were added to a solution of 18 g (63.4 mmol) 4-amino-3-
bromo-5-trifluoromethyl-benzoic acid in 400 mL THF, stirred for 1 h at RT and
heated to 40°C for 1 h. The activated acid was then added dropwise to a
solution of 8.0 g (212 mmol) NaBH4 in 200 mL water, while the temperature
CA 02503455 2005-04-22
l54
should not exceed 40°C. The reaction mixture was stirred for 2.5 h at
RT,
combined with 300 mL of semiconc. HCI, stirred for 1 h and exhaustively
extracted with EtOAc. The organic phase was washed with 15% K2C03
solution and dried over Na2S04. After the desiccant and solvent had been
eliminated the residue was dissolved in isopropanol at 50°C; after
cooling the
precipitate was suction filtered, taken up in PE and suction filtered again.
Yield: 12.5 g (73% of theory)
El: (M)+ = 269/271 (Br)
Rf = 0.9 (silica gel, MeOH)
9e 4-amino-3-bromo-5-trifluoromethyl-benzaldehyde
53 g (0.61 mol) Mn02 were added to a solution of 12.5 g (46.3 mmol) (4-
amino-3-bromo-5-trifluoromethyl-phenyl)-methanol in 150 mL DCM and the
reaction mixture was stirred overnight at RT. The Mn02 was suction filtered,
washed with DCM, the solvent was eliminated i, vac. and the residue stirred
with PE. The precipitate was suction filtered, washed with a little PE and
dried.
Yield: 9.5 g (77% of theory)
ESI-MS: (M+H)+ = 268/270 (Br)
Rf = 0.6 (silica gel, PE/EtOAc 2:1 )
9f 1-methyl2-[1-(4-amino-3-bromo-5-trifluoromethyl-phenyl)-meth-(E)-
ylidene]-succinate
27.9 g (71.0 mmol) 1-methyl 2-(triphenyl-~,5-phosphanylidene)-succinate were
added to a solution of 9.5 g (35.4 mmol) 4-amino-3-bromo-5-trifluoromethyl-
benzaldehyde in 80 mL THF and the reaction mixture was heated to 40°C
for
120 h. The precipitate was suction filtered, the filtrate evaporated down
i.vac.,
the residue combined with water and EtOAc, the organic phase was
separated off, washed three times with water and extracted three times with
5% K2C03 solution. The aqueous phase was acidified with conc. HCI, the
precipitate formed was separated off, washed with water and dried at
60°C.
Yield: 5.9 g (44% of theory)
ESI-MS: (M+H)+ = 382/384 (Br)
CA 02503455 2005-04-22
155
9g 1-methyl (S)-2-(4-amino-3-bromo-5-trifluoromethyl-benzyl)-succinate
Under an argon atmosphere 130 mg (+)-1,2-bis{(2R,5R)-2,5-
diethylphospholano)benzol(cyclooctadiene)rhodium(I)tetrafluoroborate were
added to a solution of 5.9 g (15.44 mmol) 1-methyl 2-[1-(4-amino-3-bromo-5-
trifluoromethyl-phenyl)-meth-(E)-ylidene]-succinate in 50 mL degassed MeOH
and 5.9 mL triethylamine and the reaction mixture was hydrogenated at 50
psi H2 for 4 h. Then the reaction solution was evaporated down i.vac., the
residue was dissolved in 100 mL EtOAc, washed twice with 2 M HCI and
exhaustively extracted with 5% K2C03 solution. The aqueous phase was
acidified with conc. HCI, exhaustively extracted with EtOAc and the organic
phase was dried over Na2S04. After the desiccant and solvent had been
eliminated the desired product was obtained.
Yield: 5.8 g (98% of theory)
ESI-MS: (M-H)- = 382/384 (Br)
9h methyl (S)-2-(4-amino-3-bromo-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-
oxo-1, 2, 4, 5-tetrahyd ro-1, 3-benzod iazepin-3-yl )-pi peridin-1-yl]-
butanoate
3.70 g (15.1 mmol) 3-piperidin-4-yl-1,3,4,5-tetrahydro-1,3-benzodiazepin-2-
one were added to a solution of 5.80 g {15.1 mmol) 1-methyl (S)-2-{4-amino-
3-bromo-5-trifluoromethyl-benzyl)-succinate, 4.98 g (15.1 mmol) TBTU, 2.04 g
(15.1 mmol) HOBt and 4.87 mL (35 mmol) triethylamine in 200 mL THF and
the reaction mixture was stirred overnight at RT. The reaction solution was
evaporated down i.vac., combined with EtOAc and 20% citric acid solution,
the organic phase was separated off, washed with saturated NaHC03 solution
and dried over Na2S04. After the desiccant and solvent had been eliminated
the desired product was obtained.
Yield: 9.2 g (100% of theory)
ESI-MS: (M+H)+ = 6111613 (Br)
9i (S)-2-(4-amino-3-bromo-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-
1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-butanoic acid
A solution of 1.04 g (24.30 mmol) lithium hydroxide hydrate in 30 mL water
CA 02503455 2005-04-22
l56
was added at RT to a solution of 9.2 g (15.05 mmol) methyl (S)-2-(4-amino-3-
bromo-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-butanoate in 70 mL THF and the reaction
mixture was stirred for 3 h at RT. The THF was eliminated i.vac., the aqueous
solution was acidified with conc. HCI, exhaustively extracted with EtOAc, the
organic phase was separated off and dried over Na2S04. After the desiccant
and solvent had been eliminated the desired product was obtained.
Yield: 7.8 g (87% of theory)
ESI-MS: (M+H)+ = 597/599 (Br)
Analogously to the sequence described in 9f to 9i, 1-methyl (S)-2-(4-amino-3-
chloro-5-trifluoromethyl-benzyl)-succinate and (S)-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-butanoic acid were able to be obtained
from 4-amino-3-chloro-5-trifluoromethyl-benzaldehyde (see Example 1 b).
9k (S)-2-(4-amino-3-bromo-5-trifluoromethyl-benzyl)-1-[4-(4-methyl-
piperazi n-1-yl )-piperidi n-1-yl]-4-[4-(2-oxo-1, 2,4, 5-tetrahyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione
154 mg (0.84 mmol) 1-methyl-4-piperidin-4-yl-piperazine were added to a
solution of 500 mg (0.84 mmol) of (S)-2-(4-amino-3-bromo-5-trifluoromethyl-
benzyl )-4-oxo-4-[4-(2-oxo-1,2,4, 5-tetrahydro-1, 3-benzodiazepin-3-yl)-
piperidin-1-yl]-butanoic acid, 289 mg (0.9 mmol) TBTU, 122 mg (0.9 mmol)
HOBt and 0.35 mL (2.5 mmol) triethylamine in 40 mL THF and 5 mL DMF and
the reaction mixture was stirred overnight at RT. The reaction solution was
evaporated down i.vac., combined with EtOAc and saturated NaHC03
solution, the organic phase was separated off and dried over Na2S04. After
the desiccant and solvent had been eliminated the residue was purified by
chromatography (silica gel, gradient: DCM to MeOH/NH3 95:5).
Yield: 423 mg (66% of theory)
ESI-MS: (M+H)+ = 762/764 (Br)
Retention time (HPLC): 5.9 min (method A)
The following compounds were prepared analogously from in each case 500
CA 02503455 2005-04-22
157
mg (S)-2-(4-amino-3-bromo-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-
1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl)-butanoic acid and
the corresponding amount of amine:
CF3
/ NHZ
O ~ Br
N~~R
~O
N
N~O
H
ExampleR Yield (%) Mass Retention
time
spectrum HPLC
(method)
9.1 N 50 742/744 6.4 min
/N~
N
[M+Hl+ (A)
9.2 ~N~~N\~ 48 747/749 6.5 min
[M+Hl+ (A)
9.3
N 61 762/764 5.4 min
\ ~ ' ~
~CH3
~N ~~~
N
[M+Hl+ (A)
9.4 ~N 34 761/763 6.3 min
N~CH3
[M+Hl+ (A)
The following Examples may be prepared analogously:
Example ~ Structure
CA 02503455 2005-04-22
1J8
9.5 °F
~NH~
O \ I Br I
N N Nw
O
NI
NRO
H
9.6 'F'
~NHz
O \ I Br
N N N
O
N
N~O
H
9.7 ~
/.»NHz
O \ '' Br
N~~N~N~H
~/ '''N
O
N
N~O
H
9.8 'IF
~NH,
O \ II Br
N~N~N.H
O
NI
NRO
H
9.9 'IF.
/\ /NH,
O \~~ Br
N N
~N~~~~~ ~H
O
N
N~O
H
Exam,~le 9.10
( S)-2-(4-amino-3-trifluoromethyl-benzyl )-1-[4-(4-methyl-piperazin-1-yl )-
piperid i n-1-ylJ-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-benzodiazepi n-3-yl
)-
piperidin-1-yIJ-butan-1,4-dione
CA 02503455 2005-04-22
159
CF3
/ NHZ
OI
N~~~ \~~ ~Ni
(~O
N
/ \ N~p
H
100 mg 10°l° Pd/C were added to a solution of 150 mg (0.2 mmol)
(S)-2-(4-
am ino-3-bromo-5-trifl uoromethyl-benzyl )-1-[4-(4-methyl-pi perazin-1-yl )-
piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-
piperidin-1-yl]-butan-1,4-dione in 20 mL MeOH and the reaction mixture was
hydrogenated at 50 psi H2 for 3 h at RT. The catalyst was suction filtered,
the
solvent was evaporated down i.vac., the residue was combined with 5%
K2C03 solution and EtOAc, the organic phase was separated off and
evaporated down i.vac. The residue was triturated with diisopropylether and
suction filtered.
Yield: 134 mg (100% of theory)
ESI-MS: (M+H)+ = 684
Retention time (HPLC): 5.5 min (method A)
Example 10
(S)-2-{4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-(4-dimethylamino-
piperidin-1-yl )-4-[4-( 2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-benzodiazepi n-3-yl)-
piperidin-1-yl]-butan-1,4-dione
CA 02503455 2005-04-22
160
CF3
/ NH2
O \ CI CHs
N~~N\~~N~CH3
O
N
N~O
H
80.3 mg (0.25 mmol) TBTU, 0.21 mL (1.2 mmol) ethyldiisopropylamine and
38.5 mg (0.3 mmol) dimethyl-piperidin-4-yl-amine were added to a solution of
130 mg (0.24 mmol) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzy4r4-oxo-
4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-
butanoic
acid in 10 mL DMF and the reaction mixture was stirred overnight at RT. The
reaction solution was poured into 80 mL 15% K2C03 solution, stirred for 10
min at RT, the precipitated substance was suction filtered, washed with 5 mL
water and dried in the air over the weekend. Then the product was purified by
chromatography using HPLC.
Yield: 82 mg (53% of theory)
ESI-MS: (M+H)+ = 663/665 (CI)
Retention time (HPLC): 6.1 min (method A)
The following compounds were prepared analogously:
CF3
NHz
O \ CI
N~R
~O
N
N~O
H
CA 02503455 2005-04-22
161
ExampleR Yield (%) Mass Retention
time
spectrum HPLC
(method)
10.1 ' , 55 663/665 6.3 min
N
~~ +
cH3 [M+H] 6.5 min
H3C
(A)
10.2 ' , 51 689/691 6.4 min
N
N i
[M+Hl+ (A)
10.3 ' , 56 717/719 6.2 min
N
N~cH3 [M+H]+ (A)
10.4 " , 49 758/760 6.0 min
[M+H]+ (A)
10.5 ' , 45 705/707 6.2 min
N~ o +
[M+H] (A)
10.6 ' , N 58 717/719 6.6 min
~~
N [M+Hl+ (A)
10.7 ' . 54 732/734 5.4 min
N
~ ~N-cH3
N
~ [M+H]+ (A)
10.8 ', 50 746/748 5.9 min
N\~N
N~ [M+H]+ (A)
10.9 ' , 67 703/705 6.4 min
N\~ N
+
[M+H] (A)
10.10 ~N,~~ ,cH 53 732/734 5.5 min
3
.
[M+H]+ (A)
CA 02503455 2005-04-22
162
Example R Yield (%) Mass Retention
time
spectrum HPLC
(method)
10.11 ~N N,~H3 25 690/692 6.0 min
~/N
fM+Hl+ (A)
10.12 ~ 48 661/663 6.3 min
N
~ IM+Hl+ (A)
N
10.13 ~N~~ 56 675!677 6.5 min
IN
~ IM+Hl+ CA)
10.14 ~ 53 663/665 6.4 min
~N~
+
. N IM+Hl (A)
10.15 ' ., 52 675/677 6.3 min
~
N~~ N
IM+H1+ ~A)
10.16 ' , 36 746/748 6.1 min
_ +
N~~N
N
IM+H) CA)
10.17 ' , 38 705/707 6.4 min
N~~%~ N ~
IM+H)+ (A)
10.18 ' , 52 7321734 5.8 min
~
~N~ IM+H)+ (A)
~.
10.19 ' , 40 732!734 5.4 min
~ +
N IM+Hl (A)
10.20 ' , ~ N 53 1 697/699 6.3 min
N
IM+Hl+ CA)
CA 02503455 2005-04-22
163
ExampleR Yield (%) Mass Retention
time
spectrum HP~C
(method)
10.21 ' , ~ N 60 698!700 6.3 min
N~ \ I
'
~/ [M+H]+ (A)
~N
10.22 ~ ~ 53 689/691 6.7 min
N
N~ [M+Hl+ (A)
10.23 ' , 58 794/796 5.7 min
~N~'~ [M+H]+ (A)
N I \
10.24 ' , 21 794/796 6.8 min
N\~N +
N I \ [M+H] (A)
10.25 47 739!741 6.6 min
i
/ N-CH3
i N HaC [M+H]+ (A)
Example 10.26
( S)-2-(4-ami no-3-chloro-5-trifluoromethyl-benzyl )-1-[4-(4-
dimethylaminomethyl-phenyl )-piperidin-1-y4]-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd
ro-
1,3-benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione
CF3
/ NHz
O \ C~
~~N
'' ~N
N//~~//
N~p
H
CA 02503455 2005-04-22
164
80 mg (0.25 mmol) TBTU and 87 NL (0.5 mmol) ethyldiisopropylamine were
added to a solution of 130 mg (0.24 mmol) (S)-2-(4-amino-3-chloro-5-
trifl uoromethyl-benzyl )-4-oxo-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-butanoic acid in 2 mL DMF and the mixture
was stirred for 30 min at RT. Then 80 mg (0.31 mmol) dimethyl-(4-piperidin-4-
yl-benzyl)-amine (used as the hydrochloride) were added and the reaction
mixture was stirred overnight at RT. The reaction solution was filtered
through
an injection filter and purified directly by chromatography using HPLC.
Yield: 80 mg (45% of theory)
ESI-MS: (M+H)+ = 753/755 (CI)
Retention time (HPLC): 6.6 min (method A)
The following compounds were prepared analogously from in each case 130
mg (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-
1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-butanoic acid and
the corresponding amount of amine (with 1.07 eq. of ethyldiisopropylamine in
the case of the free amines and the additional amount of base required when
using amine salts):
CF3
/ NH2
O CI
N~~R
~O
N
N~O
H
ExampleR Yield (%) Mass Retention
time
spectrum HPLC
(method)
CA 02503455 2005-04-22
165
10.27 ' , 51 786/788 6.9 min
N N~~cF M+H + A
10.28 ', 41 781/783 8.1 min
N O~ O
N~S' IM+H]+ (A)
10.29 ' , 61 703/705 6.2 min
N
~ IM+H]+ (A)
N
10.30 ' , ~ 48 717/719 6.4 min
N
~~%~N\~ ~ IM+H]t (A)
10.31 ', c"3 31 691/693 6.2 min
N 3
~~%~N~c" IM+H]+ (A)
10.32 ~ , 31 691/693 6.3 min
N'~~'J'/~N~CH3
c"3 [M+H]+ (A)
10.33 ~"3 19 677/679 6.3 min
' ~
N~.'~C~ ~CH3
N
IM+HI~ (A)
Example 10.34
(S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-[4-(2-oxo-1,2,4,5-
tetra hydro-1, 3-benzodiazepi n-3-yl)-pi peridi n-1-yl]-1-pi peridi n-1-yl-
butan-1, 4-
dione
C F3
/ NHZ
O \ CV
N~~N\~
O
N
N~O
H
62 mg (0.19 mmol) TBTU and 34 NL (0.2 mmoi) ethyldiisopropylamine were
CA 02503455 2005-04-22
166
added to a solution of 100 mg (0.18 mmol) (S)-2-(4-amino-3-chioro-5-
trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-butanoic acid in 2 mL DMF and this was
stirred for 30 min at RT. Then 24 NL (0.24 mmol) piperidine were added and
the reaction mixture was stirred for 64 h at RT. The reaction solution was
purified directly by chromatography using HPLC.
Yield: 54 mg (48% of theory)
ESI-MS: (M+H)+ = 620/622 (CI)
Retention time (HPLC): 8.4 min (method A)
The following compounds were prepared analogously from in each case 100
mg (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-
1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-butanoic acid and
the corresponding amount of amine:
CF3
/ NH2
O \ I CI
N~~R
~O
N
N~O
H
ExampleR Yield (%) Mass Retention
time
spectrum HPLC
(method)
10.35 ' , 46 662/664 9.6 min
N
[M+Hl+ (A)
10.36 ' ~ 47 ~ 710/712 9.6 min
N i
f ~ [M+H]+ (A)
CA 02503455 2005-04-22
167
ExampleR Yield (%) Mass Retention
time
spectrum HPI-C
(method)
10.37 ' , ~ 23 719/721 f.4 min
N
~~%'~N ~
IM+H~ ~A)
10.38 ' , ~ 53 688/690 9.9 min
N
CM+Hl+ tA)
10.39 ' , ~H 51 727/729 7.7 min
s
N ~
N
+ H1+ CA)
oso CM
10.40 " , H 50 713/715 7.4 min
N I
~~N
~
;s IM+Hl+ CA)
0
0
10.41 ' , N ~H 39 717/719 6.6 min
3
~N
~ CM+H)+ (A)
10.42 ' , 56 634/636 8.7 min
N\v
/
CM+H)+ CA)
10.43 ' , 48 692/694 8.2 m i n
N~y,~~~0 ~
+
~ IM+Hl CA)
~
o
10.44 " , 52 ~ 636/638 7.0 min
N\~O\ i
H (M+Hl+ (A)
10.45 ' , 58 688/690 8.7 min
N~~CFs
[M+H~ CA)
10.46 ' , 50 739/741 7.6 min
N
~N
~ tM+i".jl+ (A)
OSo
CA 02503455 2005-04-22
168
Example R Yield (%) Mass Retention
time
spectrum HPLC
(method)
10.47 ' , ~ 41 719/721 7.2 min
N
[M+HJ (A)
10.48 ' , 0 23 678/680 8.0 min
N~~~~o
[M+H) CA)
10.49 ' , 24 702/704 10.5 min
N
[M+Hl+ (A)
10.50 ', H 32 691/693 6.6 min
N
~N
\ [M+Hl+ (A)
10.51 ' , N ~ I 44 696/698 9.2 min
[M+H1+ CA)
10.52 " , ~ 44 710/712 9.6 m i
N n
[M+Hl+ (A)
10.53 ' , 0 43 ~ 704!706 7.0 min
N
~~~-~ , H
N N [M+Hl (A)
H
10.54 ' , (~ 51 704/706 7.0 rnin
N
'
,N-H i
~~ ,
N~
o [M+Hl CA)
10.55 ' , H 54 649/651 6.2 min
N~~~N~H
+
[M+H) CA)
10.56 ' , 68 719/721 5.4 min
_ +
N/~~
/
N [M+H1 CA)
Example 10.57
CA 02503455 2005-04-22
169
( S)-2-(4-amino-3-chloro-5-trifl uoromethyl-benzyl )-1-(2-ami no-4, 5, 7, 8-
tetrahydro-thiazolo[4,5-djazepin-6-yl)-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione
CF3
NH2
O \ I CI
N~~N
- O~ ~ N
N I
S~NHZ
N O
H
A solution of 300 mg (0.54 mmol) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-
benzyl)-4-oxo-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-
piperidin-1-yl]-butanoic acid and 190 mg (0.59 mmol) TBTU in 0.4 mL (2.27
mmol) ethyldiisopropylamine and 15 mL DMF was stirred for 1 h at RT. Then
160 mg (0.64 mmol) 5,6,7,8-tetrahydro-4H-thiazolo[4,5-djazepin-2-yfamine
was added (used as the hydrobromide) and the reaction solution was stirred
for a further 3 h at RT. The reaction mixture was poured into 50 mL 15%
K2C03 solution, the precipitated product was suction filtered and purified by
chromatography (silica gel, gradient: DCM to DCM/MeOH/NH310:9:1 ).
Yield: 100 mg (26°!° of theory)
ESI-MS: (M+H)+ = 704/706 (CI)
Retention time (HPLC): 6.1 min (method A)
The following compounds were prepared analogously from in each case 300
mg (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-
1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-butanoic acid and
the corresponding amount of amine (used as the free amine or as the amine-
hydrochloride):
CA 02503455 2005-04-22
170
CF3
/ NH2
O \ CI
~~ R
~N
O
N
N~p
H
ExampleR Yield (%) Mass Retention
time
spectrum HPLC
(method)
10.58 ' , ~N 40 700/702 6.4 min
N
N
(M+Hl+ CA)
10.59 N 42 700/702 6.4 min
' ,
~
N~~N
fM+Hl+ CA)
10.60 ' , ~ 50 704/706 7.5 min
"N
fM+Hl+ CA)
10.61 " , ~N 31 714/716 6.5 min
NN
fM+Hl+ CA)
10.62 " , N 62 686/688 6.3 min
N
N~~
J +
LM+Hl tA)
10.63 ' , N 62 ~ 687/689 7.2 min
j
~~
IM+H) CA)
10.64 ' , 15 730/732 6.0 min
N
~N N LM+i.,i)+ (A)
10.65 ' , 18 621/623 6.1 min
N N~H
IM+H)+ CA)
The compounds of Examples 10.64 and 10.65 could both be isolated from
CA 02503455 2005-04-22
171
one reaction mixture as the 3-piperazin-1-yl-1-aza-bicyclo[2.2.2]octane used
was contaminated with piperazine.
Examale 10.66
4-{( S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl )-4-oxo-4-[4-(2-oxo-1, 2,
4, 5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-butyryl}-piperazin-1-
sulphonic acid (1-methyl-piperidin-4-yl)-amide
CF3
NH2
OII CI p O
~~~N~ vSi
N ~~ ~N~ ~IV~~C~N~
O H
N
~ N~p
H
A solution of 500 mg (0.90 mmol) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-
benzyl )-4-oxo-4-[4-(2-oxo-1, 2, 4, 5-tetra hydro-1, 3-benzodiazepin-3-yl )-
piperidin-1-yl]-butanoic acid and 320 mg (1.00 mmol) TBTU in 0.2 mL (1.14
mmol) ethyldiisopropylamine and 50 mL THF was stirred for 1 h at RT. Then
270 mg (1.03 mmol) piperazin-1-sulphonic acid-(1-methyl-piperidin-4-yl)-
amide and 5 mL DMF were added. The reaction solution was stirred overnight
at RT. The reaction mixture was diluted with 50 mL EtOAc, extracted with 30
mL 15°!° K2C03 solution and the organic phase was dried over
Na2S04. After
the desiccant and solvent had been eliminated the residue was purified by
chromatography (silica gel, gradient: DCM to DCM/MeOH/NH3 10:9:1 ).
Yield: 170 mg (24% of theory)
ESI-MS: (M+H)' = 797/799 (CI)
Retention time (HPLC): 6.4 min (method A)
Example 10.67
( S)-2-(4-amino-3-chloro-5-trifl uoromethyl-benzyl )-N-(5-ami no-pentyl )-4-
oxo-4
CA 02503455 2005-04-22
172
[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-
butyramide
CF3
NH2
O \ CI H
/~ H t
N N N~H
O
N
N~O
H
61 mg (0.3 mmol) of tert-butyl (5-amino-pentyl)-carbaminate were added to a
solution of 260 mg (0.47 mmol) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-
benzyl)-4-oxo-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-
piperidin-1-yl]-butanoic acid and 161 mg (0.50 mmol) TBTU in 0.43 mL (2.50
mmol) ethyldiisopropylamine and 10 mL DMF and the reaction mixture was
stirred overnight at RT. 80 mL of 15% K2C03 solution were added, the
resulting mixture was stirred for 10 min, the precipitated substance was
suction filtered; it was then washed with water and dried in the air. The
crude
product was dissolved in 20 mL DCM, combined with 2 mL TFA and stirred for
2 h at RT. The reaction mixture was neutralised with 15% K2C03 solution, the
organic phase was separated off and evaporated down. The crude product
thus obtained was purified directly by HPLC.
Yield: 110 mg (37% of theory)
ESI-MS: (M+H)+ = 637!639 (CI)
Retention time (HPLC): 6.0 min (method A)
The following compound was prepared analogously from 260 mg (0.47 mmol)
( S)-2-(4-ami no-3-chloro-5-trifluoromethyl-benzyl )-4-oxo-4-[4-( 2-oxo-1, 2,
4, 5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-ylj-butanoic acid and 142 mg
(0.6 mmol) tert. butyl (2-aminomethyl-benzyl)-carbaminate:
~Example~ R TYield (%)Mass Retention time
CA 02503455 2005-04-22
173
---._- Spectrum HPLC
(method)
10.68 H ~ ~ H 47 671/673 6.4 min
N \ N..H
[M+H] (A)
Exam~~le 10.69
1-{(S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-butyryl}-piperidine-4-
carboxylic acid
CF3
NHZ
O \ CI O
~N O
~~~N~~~~~ ,H
O
N
N~O
H
2 mg (0.05 mmol) lithium hydroxide hydrate, dissolved in a little water, were
added to a solution of 15 mg (0.02 mmol) methyl 1-{(S)-2-(4-amino-3-chloro-
5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-butyryl}-piperidin-4-carboxylate (Example
10.48) in 5 mL THF and the reaction mixture was stirred for 3 h at RT. The
solvent was eliminated i. vac., the residue was taken up in water and
acetonitrile and lyophilised.
Yield: 14 mg (96% of theory)
ESI-MS: (M+H)+ = 664/666 (CI)
Retention time (HPLC): 7.2 min (method A)
The following compound was prepared analogously from 20 mg (0.03 mmol)
methyl (1-{(S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-
oxo-1,2, 4, 5-tetrahydro-1, 3-benzod iazepin-3-y1 )-pi peridi n-1-yl]-butyryl}-
CA 02503455 2005-04-22
174
piperidin-4-yl)-acetate (Example 10.43):
ExampleR Yield (J) Mass Retention
time
spectrum HPLC
(method)
10.70 ' , 96 678/680 7.3 min
N
~~~~~\" [M+Hl
Example 10.71
( S)-2-(4-am i no-3-chloro-5-trifluoromethyl-benzyl )-1-[4-(8-methyl-8-aza-
bicyclo[3.2.1 ]oct-3-yl)-piperazin-1-yl]-4-[4-(2-oxo-1,2,4,5-tetrahydra-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione
CF3
NH2
O \ CI
N%~N~N Ni
O
N
N~O
H
850 mg (2.4 mmol) 8-methyl-3-piperazin-1-yl-8-aza-bicyclo[3.2.1]octan were
added to a solution of 1.04 g (1.88 mmol) (S)-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyi )-4-oxo-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butanoic acid, 642 mg (2.0 mmol) TBTU
and 1.64 mL (9.6 mmol) ethyldiisopropylamine in 20 mL DMF and the reaction
mixture was stirred overnight at RT. The reaction solution was combined with
15% K2C03 solution, stirred for 10 min at RT, the precipitated substance was
suction filtered, washed with 50 mL water and dried in the air and purified by
chromatography (silica gel, gradient: DCM to DCM/MeOH/NH3 10:85:5).
Yield: 1.07 g (77% of theory)
CA 02503455 2005-04-22
175
ESI-MS: (M+H)+ = 744/746 (CI)
Retention time (HPLC): 5.4 min (method A)
Example 10.72
( S)-2-(4-ami no-3-chloro-5-trifluoromethyl-benzyl )-4-[4-(2-oxo-1, 2, 4, 5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-1-(4-piperazin-1-yl-
piperidin-
1-yl)-butan-1,4-dione
CF3
/ NHZ
O \ I CI
N~N~~~N~N~H
I IO
N
N~O
H
10.72a benzyl4-(1-{(S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-
oxo-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-
piperidin-1-yl]-butyryl}-piperidin-4-yl )-piperazin-1-carboxylate
241 mg (0.75 mmol) TBTU, 0.62 mL (3.6 mmol) ethyldiisopropylamine and
215 mg (0.71 mmol) benzyl 4-piperidin-4-yl-piperazin-1-carboxylate were
added to a solution of 390 mg (0.71 mmol) (S)-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl )-4-oxo-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butanoic acid in 10 mL DMF and the
reaction mixture was stirred overnight at RT. The reaction solution was
poured into 80 mL 15% K2C03 solution, stirred for 10 min at RT, the
precipitated substance was suction filtered, washed with 5 mL water and dried
in the air over the weekend.
Yield: 580 mg (98% of theory)
ESI-MS: (M+H)+ = 838!840 (CI)
10.72b (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzylr4-[4-(2-oxo-
1, 2, 4, 5-tetrahyd ro-1, 3-benzodiazepi n-3-yl )-pi perid in-1-yl]-1-(4-
CA 02503455 2005-04-22
176
piperazin-1-yl-piperidin-1-yl)-butan-1,4-dione
100 mg Raney nickel were added to a solution of 250 mg (0.30 mmol) benzyl
4-( 1-{(S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-
1, 2, 4, 5-tetrahyd ro-1, 3-benzodiazepi n-3-yl )-pi perid i n-1-yl]-butyryl}-
pi perid in-4-
yl)-piperazin-1-carboxylate in 30 mL MeOH and the reaction mixture was
stirred for 5 h at RT and 50 psi H2. To complete the reaction another 100 mg
of Raney nickel were added and the mixture was stirred for a further 10 h at
RT. The catalyst was suction filtered, the solvent eliminated i. vac. and the
residue purified by HPLC.
Yield: 88 mg (42% of theory)
ESI-MS: (M+H)+ = 704/706 (CI)
Retention time (HPLC): 5.6 min (method A)
Examale 10.73
(S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(1 H-imidazol-4-yl)-
piperidin-1-yl)-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-
piperidi n-1-yl]-butan-1, 4-dione
CF3
/ NH2
O \ CI N
~~N ~ N_H
'' ~N
// O
N
N~p
H
400 mg (1.25 mmol) TBTU and 0.65 mL (3.73 mmol) ethyldiisopropylamine
were added to a solution of 650 mg (1.18 mmol) (S)-2-(4-amino-3-chloro-5-
trifl uoromethyl-benzyf )-4-oxo-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butanoic acid in 20 mL DMF and this was
stirred for 30 min at RT. Then 340 mg (1.52 mmol) 4-(1H-imidazol-4-yl)-
piperidin (used as the bis-hydrochloride) were added and the reaction mixture
was stirred overnight at RT. It was evaporated down i.vac., combined with 30
CA 02503455 2005-04-22
177
mL of 15% K2C03 solution, extracted twice with in each case 15 mL of DCM
and the organic phase was dried with MgS04. After the desiccant and solvent
had been eliminated the residue was purified by chromatography (silica gel,
gradient: DCM to DCM/MeOH/NH3 20:75:5).
Yield: 460 mg (57% of theory)
ESI-MS: (M+H)+ = 686/688 (CI)
Rf = 0.35 (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2)
Example 10.74
( S)-2-(4-am i no-3-ch1 oro-5-trifl uorom ethyl-benzyl )-4-[4-(2-oxo-1, 2, 4,
5-
tetrahyd ro-1, 3-benzodiazepi n-3-yl )-piperidi n-1-yl]-1-{4-[4-(2, 2, 2-
trifluoro-
acetyl)-phenyl]-piperazin-1-yl}-butan-1,4-dione
CF3
/ NH2
O
O \ CI / F
I~F
N ~~ N\/~N \ I F
O
N
\ N~p
H
10.74a tert. butyl 4-(4-bromo-phenyl)-piperazine-1-carboxylate
Boc-anhydride was added batchwise to a suspension of 10.0 g (36 mmol) 4-
(4-bromo-phenyl)-piperazine (used as the hydrochloride) and 15 mL (108
mmol) triethylamine in 150 mL THF and the reaction mixture was heated to
60°C for 3 h. After cooling it was poured onto water, the precipitate
was
extracted with EtOAc, the organic phase was washed with water and dried
over Na2S04. After the desiccant and solvent had been eliminated the desired
product was obtained.
Yield: 12.0 g (98% of theory)
Rr = 0.6 (silica gel, cyc/EtOAc 2:1 )
10.74b tert. butyl 4-[4-(2,2,2-trifluoro-acetyl)-phenyl]-piperazin-1-
CA 02503455 2005-04-22
178
carboxylate
Under a nitrogen atmosphere a solution of 1.02 g (3.0 mmol) tert. butyl 4-(4-
bromo-phenyl)-piperazine-1-carboxylate in 20 mL THF was slowly added
dropwise to a solution of 2.06 mL (3.3 mmol) n-butyllithium (1.6 M in n-
hexane) in 40 mL dry THF cooled to -78°C and this was then stirred for
15
min at this temperature. Then a solution of 0.43 mL (3.0 mmol) of N,N-
diethyl-2,2,2-trifluoroacetamide in 10 mL of THF was slowly added dropwise.
After the addition had ended the reaction mixture was kept for 2 h at -
78°C,
then poured onto 100 mL water, extracted twice with in each case 50 mL
EtOAc, the organic phase was suction filtered through Na2S04, evaporated
down i.vac. and purified by chromatography (silica gel, cyc/EtOAc 3:1 ).
Yield: 267 mg (25% of theory)
EI: (M)+ = 358
Rf = 0.37 (silica gel, cyclEtOAc 3:1 )
10.74c 2,2,2-trifluoro-1-(4-piperazin-1-yl-phenyl)-ethanone
2.0 mL TFA were added to a solution of 267 mg (0.75 mmol) tert. butyl 4-[4-
(2,2,2-trifluoro-acetyl)-phenyl)-piperazin-1-carboxylate in 30 mL DCM cooled
to 0°C and the reaction mixture was stirred for 24 h, while warming up
to RT.
It was evaporated down i.vac.; the crude product was further reacted without
purification.
ESI-MS: (M+H)+ = 259
10.74d (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyi)-4-[4-(2-oxo-
1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-1-{4-[4-
(2,2,2-trifluoro-acetyl)-phenylj-piperazin-1-yl}-butan-1,4-dione
The crude product obtained in 10.74c was added to a solution of 234 mg
(0.42 mmol) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-
oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-butanoic acid,
136 mg (0.42 mmol) TBTU, 57 mg (0.42 mmol) HOBt and 0.14 mL (1.0 mmol)
triethylamine in 20 mL THF and 2 mL DMF and the reaction mixture was
stirred for 2 h at RT. The reaction solution was combined with semisaturated
NaHC03 solution and extracted with 30 mL EtOAc. The organic phase was
suction filtered through Na2S04, the filtrate was evaporated down i.vac. and
CA 02503455 2005-04-22
179
the residue was purified by chromatography (silica gel, EtOAc/MeOH 95:5).
Yield: 246 mg (73% of theory)
ESI-MS: (M+H)+ = 793/795 (CI)
Rf = 0.27 (silica gel, EtOAc)
Example 10.75
( S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl )-1-[4-(4-methyl-piperazin-1-
yf )-piperidin-1-yl]-4-[4-( 2-oxo-1, 4-dihyd ro-2H-quinazolin-3-yl )-piperidin-
1-yl]-
butan-1,4-dione
CF3
/ NHZ
O \ I CI
N~~N\ ~N~
~N~
N O
N O
I
H
10.75a methyl (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-
4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-
butanoate
A solution of 3.0 g (8.83 mmol) 1-methyl (S)-2-(4-amino-3-chloro-5-
trifluoromethyl-benzyl)-succinate and 3.05 g (9.5 mmol) TBTU, 1.28 g (9.47
mmol) HOBt in 1.7 mL (9.76 mmol) ethyldiisopropylamine and 100 mL DMF
was stirred for 1 h at RT. Then 2.2 g (9.51 mmol) 3-piperidin-4-yl-3,4-dihydro-
1 H-quinazolin-2-one were added and the reaction solution was stirred
overnight at RT. The reaction mixture was evaporated down i.vac., the
residue was taken up in DCM, washed with 10% citric acid solution and 15%
K2C03 solution and dried over Na2S04. The desiccant was eliminated by
filtering through activated charcoal; after elimination of the solvent the
desired
product was obtained.
Yield: 4.8 g (98% of theory)
CA 02503455 2005-04-22
180
ESI-MS: {M+H)+ = 553/555 (CI)
Rf = 0.71 (silica gel, DCM/cyclMeOH/NH3 70:15:15:2)
10.75b (Sr2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-
oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butanoic acid
A solution of 558 mg (13.02 mmol) lithium hydroxide hydrate in 12 mL water
was added to a solution of 4.8 g (8.68 mmol) methyl (S)-2-(4-amino-3-chloro-
5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidin-1-yl]-butanoate in 28 mL THF and the reaction mixture was stirred
for 7 h at RT. ft was evaporated down i. vac., combined with 100 mL water,
acidified with 1 M HCI and the precipitate formed was suction filtered. The
residue was dissolved in EtOAc, extracted with 15% K2C03 solution and the
aqueous phase was again acidified with 1 M HCI. The precipitate formed was
suction filtered and dried.
Yield: 4.2 g (90% of theory)
ESI-MS: (M+H)+ = 539/541 (CI)
R, = 0.09 (silica gei, DCM/cyclMeOH/NH3 70:15:15:2)
10.75c (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-methyl-
piperazi n-1-yl )-piperidin-1-yl]-4-[4-(2-oxo-1, 4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-yl]-butan-1,4-dione
The crude product was obtained analogously to 10.75a from 500 mg (0.93
mmol) (S)-2-{4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-
1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butanoic acid and 180 mg (0.98
mmol) 1-methyl-4-piperidin-4-yl-piperazine. After being worked up as
described it was purified first by chromatography (silica gel, gradient: DCM
to
DCM/MeOH/NH3 70:27:3) and then by HPLC.
Yield: 120 mg (18% of theory)
ESI-MS: (M+H)+ = 704/706 (CI)
Rf = 0.43 (silica gel, DCM/cyclMeOH/NH3 70:15:15:2)
Retention time (HPLC): 5.6 min (method A)
Example 10.76
CA 02503455 2005-04-22
181
( S)-2-(4-ami no-3-chloro-5-trifluoromethyl-benzyl )-1-[4-(4-methyl-piperazin-
1-
yl)-piperidin-1-yl]-4-[4-(5-oxo-3-phenyl-4,5-dihydro-1,2,4-triazol-1-yl)-
piperidin-
1-yl]-butan-1,4-dione
CF3
/ NHZ
O \ ' CI
N ~N\~~N\/~'N i
N1 ~ IIO
/\
N O
H
10.76a methyl (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-
4-[4-(5-oxo-3-phenyl-4,5-dihydro-1,2,4-triazol-1-yl)-piperidin-1-
yl]-butanoate
The desired product was obtained analogously to 10.75a from 3.0 g (8.31
mmol) 1-methyl (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-succinate
and 3.55 g (9.45 mmol) 5-phenyl-2-piperidin-4-yl-2,4-dihydro-1,2,4-triazol-3-
one.
Yield: 2.5 g (50% of theory)
ESI-MS: (M+H)+ = 566!568 (CI)
Rf = 0.67 (silica gel, DCMlcyc/MeOHlNH3 70:15:15:2)
10.76b (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(5-
oxo-3-phenyl-4, 5-dihyd ro-1,2, 4-triazol-1-yl )-piperid i n-1-yl]-
butanoic acid
The desired product was obtained analogously to 10.75b from 2.5 g (4.42
mmol) methyl (S)-2-(4-amino-3-ehloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(5-
oxo-3-phenyl-4,5-dihydro-1,2,4-triazol-1-yl)-piperidin-1-yl)-butanoate.
Yield: 2.5 g (50% of theory)
ESI-MS: (M+H)+ = 552/554 (CI)
Rf = 0.14 (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2)
10.76c (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-methyl-
CA 02503455 2005-04-22
182
piperazin-1-yl)-piperidin-1-yl]-4-[4-(5-oxo-3-phenyl-4,5-dihydro-
1,2,4-triazol-1-yl)-piperidin-1-yl]-butan-1,4-dione
The crude product was obtained analogously to 10.75a from 500 mg (0.91
mmol) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(5-oxo-3-
phenyl-4,5-dihydro-1,2,4-triazol-1-yl)-piperidin-1-yl]-butanoic acid and 180
mg
(0.98 mmol) 1-methyl-4-piperidin-4-yl-piperazine. After being worked up as
described it was purified by chromatography (silica gel, gradient: DCM to
DCM/MeOH/NH3 70:27:3).
Yield: 350 mg (54% of theory)
ESI-MS: (M+H)' = 717/719 (CI)
Rf = 0.44 (silica gel, DCMlcyclMeOHINH3 70:15:15:2)
Retention time (HPLC): 5.6 min (method A)
Example 10.77
( S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl )-1-[4-(4-methyl-piperazin-1-
yl)-piperidin-1-yl]-4-[4-(2-oxo-1,2-dihydro-imidazo[4,5-c]quinoline-3-yl)-
piperidin-1-yl]-butan-1,4-dione
CF3
/ NH2
O \ CI
N_ N~~N\~~N~N~
O
\/
N O
H
10.77a methyl (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-
4-[4-(2-oxo-1,2-dihydro-imidazo[4,5-cJquinolin-3-yl)-piperidin-1-
yl]-butanoate
The desired product was obtained analogously to 10.75a from 3.0 g (8.31
mmol) 1-methyl (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-succinate
and 2.55 g (9.40 mmol) 3-piperidin-4-yl-1,3-dihydro-imidazo[4,5-cJquinolin-2-
one.
CA 02503455 2005-04-22
183
Yield: 5.2 g (100% of theory)
ESI-MS: (M+H)+ = 590/592 (CI)
Rf = 0.66 (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2)
10.77b (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-
oxo-1,2-dihydro-imidazo[4,5-c]quinolin-3-yl)-piperidin-1-yl]-
butanoic acid
The desired product was obtained analogously to 10.75b from 5.2 g (8.81
mmol) methyl (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-
oxo-1,2-dihydro-imidazo[4,5-c]quinolin-3-yl)-piperidin-1-yl]-butanoate.
Yield: 2.75 g (54% of theory)
ESI-MS: (M+H)+ = 576/578 (CI)
Rf = 0.09 (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2)
10.77c (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-methyl-
piperazin-1-yl)-piperidin-1-yl]-4-[4-(2-oxo-1,2-dihydro-
imidazo[4,5-c]quinolin-3-yl)-piperidin-1-yl]-butan-1,4-dione
The crude product was obtained analogously to 10.75a from 500 mg (0.87
mmol) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-
1,2-dihydro-imidazo[4,5-c]quinolin-3-yl)-piperidin-1-yl]-butanoic acid and 170
mg (0.93 mmol) 1-methyl-4-piperidin-4-yl-piperazine. After being worked up
as described the residue was combined with diisopropylether and treated in
an ultrasound bath, the product was suction filtered and dried.
Yield: 520 mg (81% of theory)
ESi-MS: (M+H)+ = 741/743 (CI)
Rf = 0.40 (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2)
Retention time (HPLC): 4.5 min (method A)
Example 10.78
( S)-2-(4-ami no-3-chloro-5-trifluoromethyl-benzyl )-1-[4-(4-methyl-piperazin-
1-
yl)-piperidin-1-yl]-4-[4-(2-oxo-1,2-dihydro-4H-thieno[3,4-dJpyrimidin-3-yl)-
piperidin-1-yl]-butan-1,4-dione
CA 02503455 2005-04-22
184
CF3
/ NH2
W
0 CI
N~~N\~~N~N~
_ ~ O
S ~
N O
I
H
10.78a methyl (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-
4-[4-(2-oxo-1,2-dihydro-4H-thieno[3,4-dJpyrimidin-3-yl)-piperidin-
1-yl)-butanoate
The desired product was obtained analogously to 10.75a from 3.0 g (8.31
mmol) 1-methyl (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-succinate
and 3.34 g (9.51 mmol) 3-piperidin-4-yl-3,4-dihydro-1H-thieno[3,4-djpyrimidin-
2-one.
Yield: 2.2 g (45°I° of theory)
ESI-MS: (M+H)+ = 559/561 (CI)
Rf = 0.56 (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2)
10.78b (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-
oxo-1,2-dihydro-4H-thieno[3,4-dJpyrimidin-3-yl)-piperidin-1-yl)-
butanoic acid
The desired product was obtained analogously to 10.75b from 2.2 g (3.94
mmol) methyl (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-
oxo-1,2-dihydro-4H-thieno[3,4-djpyrimidin-3-yl)-piperidin-1-yl)-butanoate.
Yield: 1.10 g (51 % of theory)
ESI-MS: (M+H)+ = 545/547 (CI)
Rr = 0.24 (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2)
10.78c (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-methyl-
piperazin-1-yl)-piperidin-1-yl)-4-[4-(2-oxo-1,2-dihydro-4H-
thieno[3,4-dJpyrimid in-3-yl)-piperidin-1-yl)-butan-1,4-dione
The crude product was obtained analogously to 10.75a from 500 mg (0.92
CA 02503455 2005-04-22
185
mmol) (S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-
1,2-dihydro-4H-thieno[3,4-c~pyrimidin-3-yl)-piperidin-1-yl]-butanoic acid and
180 mg (0.98 mmol) 1-methyl-4-piperidin-4-yl-piperazine. After being worked
up as described it was purified by chromatography (silica gel, gradient: DCM
to DCM/MeOH/NH3 70:27:3).
Yield: 100 mg (81 % of theory)
ESI-MS: (M+H)+ = 710/712 (CI)
Rf = 0.43 (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2)
Retention time (HPLC): 5.5 min (method A)
Example 10.79
( S)-2-(4-ami no-3-chloro-5-trifl uoromethyl-benzyl )-1-[4-(5-methyl-2, 5-d
iaza-
bicyclo[2.2.1 ]hept-2-yl)-piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione
CF3
/ NHZ
O \ I CI
N ~~ N\~~N~~N ~
O
N
N~O
H
Prepared analogously to Example 10.26 from 100 mg (0.18 mmol) (S)-2-(4-
amino-3-chloro-5-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-1,2,4,5-tetrahydro-
1,3-benzodiazepin-3-yl)-piperidin-1-yl]-butanoic acid and 39.1 mg (0.2 mmol)
2-methyl-5-piperidin-4-yl-2,5-diaza-bicyclo[2.2.1]heptane using triethylamine
as the base.
Yield: 83 mg (63% of theory)
ESI-MS: (M+H)+ = 730/732 (CI)
Retention time (HPLC): 5.6 min (method A)
The following compounds may be prepared analogously to the methods
CA 02503455 2005-04-22
186
described:
Example Structure
1 U.BU ~F,
~NHz
~
GI
0II \
~N~
N ' ~ ~N N.H
0
N
~ N~0
H
10.81
~NHz
~
CI
0 \
N N~~~~-~ H
N
O
N
N~p
H
Example 11
( S)-2-(4-amino-3, 5-bis-trifluoromethyl-benzyl )-1-[4-( 4-methyl-perhydro-1,
4-
diazepi n-1-yl)-piperidi n-1-yl]-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione
CF3
/ NH2
O CF3 ( 'N-
N ~~N\~~'N
O
N
N~O
H
11a 4-amino-3,5-bis-trifluoromethyl-benzaldehyde
A solution of 5 g (19.67 mmol) 4-amino-3,5-bis-trifluoromethyl-benzonitrile in
30 mL formic acid was shaken in 10 equal portions in pressurised containers
for 20 h at 110°C. The individual portions were combined, filtered,
washed
with formic acid and evaporated down i.vac. The residue was purified by
chromatography (silica gel, PE/EtOAc 9:1 ).
CA 02503455 2005-04-22
187
Yield: 3.8 g (75% of theory)
ESI-MS: (M-H)- = 256
11b 1-methyl2-[1-(4-amino-3,5-bis-trifluoromethyl-phenyl)-meth-(E)-
ylidene]-succinate
12.79 g (32.6 mmol) 1-methyl 2-(triphenyl-~.5-phosphanylidene)- succinate
were added to a solution of 4.2 g (16.33 mmol) 4-amino-3,5-bis-
trifluoromethyl-benzaldehyde in 80 mL THF and the reaction mixture was
heated to 40°C for 120 h. It was evaporated down i, vac., the residue
was
combined with water and EtOAc, the organic phase was separated off,
washed with water and extracted three times with in each case 80 mL 5%
K2C03 solution. The combined aqueous phases were acidified with conc. HCI,
the oily precipitate was extracted twice with in each case 100 mL EtOAc and
dried over Na2S04. After the desiccant and solvent had been eliminated the
desired product was obtained.
Yield: 5.9 g (97% of theory)
EI: (M)+ = 371
11c 1-methyl (S)-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-succinate
Prepared analogously to Example 9g from 5.9 g 1-methyl 2-[1-(4-amino-3,5-
bis-trifluoromethyl-phenyl)-meth-(E)-ylidene]-succinate.
Yield: 5.9 g (97% of theory)
ESI-MS: (M+H)+ = 374
11d methyl (S)-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-
1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-butanoate
Prepared analogously to Example 9h from 4.40 g (11.79 mmol) 1-methyl (S)-
2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-succinate and 2.89 g (11.78 mmol)
3-piperidin-4-yl-1, 3,4, 5-tetrahydro-1, 3-benzodiazepin-2-one.
Yield: 6.75 g (95% of theory)
ESI-MS: (M+H)+ = 601
R, = 0.13 (silica gel, PE/EtOAc 1:1 )
CA 02503455 2005-04-22
188
11e (S)-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-
1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-butanoic acid
A solution of 0.72 g ( 16.75 mmol) lithium hydroxide hydrate in 30 mL water
was added to a solution of 6.7 g (11.16 mmol) methyl (S)-2-(4-amino-3,5-bis-
trifl uoromethyl-benzyl )-4-oxo-4-(4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butanoate in 50 mL THF at RT and the
reaction mixture was stirred for 5 h at RT. The THF was eliminated i, vac.,
the
aqueous solution cooled to 10°C and adjusted to pH 1 with conc. HCI,
during
which time the product was precipitated. This was suction filtered and dried
at
65°C. The dried substance was combined with 300 mL diisopropylether,
stirred overnight, suction filtered, washed with diisopropylether and dried.
Yield: 5.6 g (86% of theory)
ESI-MS: (M+H)+ = 587
11f (S)-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-1-[4-(4-methyl-perhydro-
1,4-d iazepi n-1-yl )-piperidi n-1-yl]-4-[4-(2-oxo-1, 2,4, 5-tetrahyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-dione
197 mg (1.0 mmol) 1-methyl-4-piperidin-4-yl-perhydro-1,4-diazepine were
added to a solution of 400 mg (0.68 mmol) (S)-2-(4-amino-3,5-bis-
trifluoromethyl-benzyl )-4-oxo-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butanoic acid, 241 mg (0.75 mmol) TBTU
and 0.25 mL (1.8 mmol) triethylamine in 5 mL DMF and the reaction mixture
was stirred overnight at RT. The reaction solution was slowly poured into 150
mL of 15% K2C03 solution, the precipitated product was suction filtered and
dried in the air. The crude product was purified by chromatography (silica
gel,
gradient: DCM to MeOH/NH3 95:5).
Yield: 400 mg (77% of theory)
ESI-MS: (M+H)+ = 767
Rf = 0.2 (silica gel, DCM/MeOH/NH3 85:15:1.5)
Retention time (HPLC): 5.6 min (method A)
The following compounds were prepared analogously from in each case 400
mg (Example 11.7: 387 mg) (S)-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-4-
oxo-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-
CA 02503455 2005-04-22
189
butanoic acid and the corresponding amount of amine:
CF3
NHZ
O \ CF3
N~~R
~O
N
N~p
H
ExampleR Yield Mass Retention
(%) time
spectrum HPLC
(method)
11.1 ~N 52 766 5.7 min
N~ ~N~
[M+Hl+ (A)
11.2 ' ~ 64 752 5.6 min
N~~~N~ [M+Hl+ (A)
11.3 ' ~ 21 764 6.2 min
N
'
~N N [M+H]+ CA)
11.4 ' 74 752 6.0 min
~ +
~i~ ~
~
N [M+Hl (A)
11.5 ' ~ N 55 751 6.8 min
~~ [M+H)+ CA)
N
11.6 ' ~ 62 737 6.7 min
N~~N
+
[M+H1 (A)
11.7 ' ~ 100 872
0
N
~
~. [M+H~
'~NJ~ I ~
CA 02503455 2005-04-22
J90
Example 11.7 was further reacted without purification.
Example 11.8
( S)-2-(4-amino-3, 5-bis-trifluoromethyl-benzyl )-4-[4-(2-oxo-1, 2, 4, 5-
tetrahydro-
1,3-benzodiazepin-3-yl)-piperidin-1-yl]-1-(4-piperazin-1-yl-piperidin-1-yl)-
butan-1,4-dione
CF3
/ NH2
O \ CF3
N~N~ J~N~N~H
O
N
/ \ N~o
H
A solution of 560 mg (0.64 mmol) benzyl 4-(1-{(S)-2-(4-amino-3,5-bis-
trifluoromethyl-benzyl )-4-oxo-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-
benzodiazepin-3-yl)-piperidin-1-yl]-butyryl}-piperidin-4-yl)-piperazin-1-
carboxylate (crude product from Example 11.7) in 50 mL MeOH was
combined with 200 mg 10% Pd/C and the reaction mixture was hydrogenated
for 3 h at RT and 3 bar H2. The catalyst was suction filtered, the solution
evaporated down i.vac. and the residue purified by chromatography (silica gel,
gradient: DCM to DCM/MeOH/NH310:85:5).
Yield: 230 mg (49% of theory)
ESI-MS: (M+H)+ = 738
Rf = 0.27 (silica gel, DCM/MeOH/NH3 50:50:5)
The following compounds may be prepared analogously:
Example ~ Structure
CA 02503455 2005-04-22
191
Example Structure
CF
11.9 ~NHa
O \ CFA
N N N\
N 0
N~p
H
11.10 GF
~NHz
0 \ I CF
N N N
N 0
N~p
H
11.11 GF
~NHz
O \ ~ CFA
N
N N
N~ 0
~ N~0
H
11.12 GF
~NHz
O \ I CFA
N~N~N \ I
N ~ ~ l~~lO
N~Q
H
11.13 GF
~NHz
O \ ~ CFA
~N~N~ ~H
N
N O
N~O
H
11.14 GF
~NHz
O \ I CF3
N N~.~~-~ .H
N
N O
N~O
H
Example 12
CA 02503455 2005-04-22
192
4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-carboxylic
acid-{( R)-1-(4-amino-3, 5-bis-trifluoromethyl-benzyl )-2-[4-(4-methyl-pi
perazin-
1-yl)-piperidin-1-yl]-2-oxo-ethyl}-amide
CF3
NH2
O \ CF3
N~N~N\~~N~N/
H O
N
~ N~O
H
12a (4-amino-3,5-bis-trifluoromethyl-phenyl)-methanol
Under a nitrogen atmosphere 1.06 g (28 mmol) NaBH4 were added batchwise
to a solution of 7.2 g (28.0 mmol) 4-amino-3,5-bis-trifluoromethyl-
benzaldehyde (Example 11a) in 100 mL MeOH and the reaction mixture was
stirred for 2 h at RT. The reaction solution was acidified With 1 M HCI,
evaporated down i.vac., the residue was combined with 150 mL water and
150 mL EtOAc, the organic phase was separated off and dried over Na2SOa.
After the desiccant and solvent had been eliminated the residue was purified
by chromatography (silica gel, PE/EtOAc 9:1 ).
Yield: 5.1 g (70% of theory)
ESI-MS: (M-H)- = 258
Rr = 0.15 (silica gel, PE/EtOAc 9:1 )
12b 4-chloromethyl-2,6-bis-trifluoromethyl-phenylamine
4.35 mL (60 mmol) thionyl chloride were added to a solution of 5.1 g (19.68
mmol) (4-amino-3,5-bis-trifluoromethyl-phenyl)-methanol in 80 mL DCM at RT
and the reaction mixture was stirred for 3 h at RT. The reaction solution was
poured onto ice and ice-cold NaHC03 solution, the organic phase was
separated off and dried over Na2S04. After the desiccant and solvent had
been eliminated the residue was further reacted without purification.
Yield: 5.4 g (99% of theory)
Rr = 0.55 (silica gel, PE/EtOAc 4:1 )
CA 02503455 2005-04-22
193
12c diethyl2-acetylamino-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-
malonate
Under a nitrogen atmosphere 4.34 g (20.0 mmol) diethyl 2-acetylamino-
malonate were added to a solution of sodium ethoxide (prepared by reacting
0.46 g (20.0 mmol) sodium with EtOH) in 50 mL dry EtOH and the reaction
mixture was stirred for 15 min at RT. Then a solution of 5.4 g (19.45 mmol) of
4-chloromethyl-2,6-bis-trifluoromethyl-phenylamine in 100 mL 1,4-dioxane
was added dropwise within 5 min, the reaction solution was stirred for a
further 4 h at RT, combined with 1 L water and stirred overnight . The
precipitate formed was filtered off, washed with water and dried in the air.
Yield: 5.2 g (57% of theory)
ESI-MS: (M+H)+ = 459
Rf = 0.65 (silica gel, PE/EtOAc 2:1 )
12d monoethyl2-acetylamino-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-
malonate
2.0 mL 6 M NaOH solution were added to a solution of 5.1 g (11.13 mmol)
diethyl 2-acetylamino-2-(4-amino-3,5-bis-trifluoromethyl-benzyl)-malonate in
80 mL dry EtOH and the reaction mixture was stirred overnight at RT. it was
evaporated down i. vac., the residue was taken up in 150 mL water, acidified
with 1 M HCI, the aqueous phase was extracted with 150 mL EtOAc and the
organic phase was dried over Na2S04. After the desiccant and solvent had
been eliminated the residue was further reacted without purification.
Yield: 4.3 g (90% of theory)
ESI-MS: (M+H)+ = 431
Rf = 0.1 (silica gel, PE/EtOAc 2:1 )
12e ethyl2-acetylamino-3-(4-amino-3,5-bis-trifluoromethyl-phenyl)-
propionate
A solution of 4.3 g (10.0 mmol) monoethyl 2-acetylamino-2-(4-amino-3,5-bis-
trifluoromethyl-benzyl)-malonate in 200 mL isopropanol and 80 mL toluene
was heated to 100°C for 15 h. It was evaporated down i. vac. and the
residue
was further reacted without being purified.
CA 02503455 2005-04-22
l94
Yield: 3.8 g (98% of theory)
Rr = 0.60 (silica gel, PE/EtOAc 1:1 )
12f ethyl (R)-2-acetylamino-3-(4-amino-3,5-bis-trifluoromethyl-phenyl)-
propionate
4 mL Alcalase 2.4 L FG (Novozymes A/S; DK 2880 Bagsvaerd) was added to
a solution of 3.65 g (20.5 rnmol) Na2HP04 dihydrate in 130 mL water warmed
to 37 °C and the pH was adjusted to 7.5 by the addition of NaH2P04
dihydrate. Then a solution of 3.8 g (9.84 mmol) ethyl 2-acetylamino-3-(4-
amino-3,5-bis-trifluoromethyl-phenyl)-propionate in 40 mL acetone was added
dropwise at 37 °C with stirring. The pH value of the reaction mixture
was
constantly kept in the range from 7.4-7.6 by the addition of 1 M NaOH. After
the addition had ended the mixture was stirred for 4 h at 37 °C. After
cooling
to RT the reaction mixture was combined with 300 mL DCM, 300 mL 15°to
K2C03 solution and 200 mL water. The organic phase was separated off,
washed with 7% K2C03 solution and dried over Na2S04. After the desiccant
and solvent had been eliminated the crude product (2.2 g) was further reacted
without purification.
ESI-MS: (M+H)+ 387
Rf = 0.60 (silica gel, PE/EtOAc 1:1 )
12g ethyl (R)-2-amino-3-(4-amino-3,5-bis-trifluoromethyl-phenyl)-
propionate
A solution of 2.2 g of the above crude product was refluxed in 4 M HCI for 1.5
h. It was evaporated down i. vac., the residue was taken up in 50 mL EtOH
and 50 mL ethanolic HCI (11.5 M) and the reaction mixture was stirred
overnight at RT. It was evaporated down again i.vac., combined with 50 mL
15% K2C03 solution, extracted with 200 mL EtOAc, the organic phase was
separated off and evaporated down i.vac. The crude product (1.8 g) was
further reacted without purification.
ESI-MS: (M+H)' 345
Rf = 0.50 (silica gel, DCM/MeOH/NH3 90:10:1 )
12h ethyl (R)-3-(4-amino-3,5-bis-trifluoramethyl-phenyl)-2-{[4-(2-oxo-
CA 02503455 2005-04-22
195
1,2,4,5-tetrahydro-1, 3-benzodiazepin-3-yl)-piperidin-1-carbonyl]-
amino)-propionate
0.94 g (5.7 mmol) CDT were added to a solution of 1.8 g of the above crude
product in 50 mL THF cooled to -5°C and the reaction mixture was
stirred for
45 min at this temperature and after removal of the ice bath stirred for a
further 30 min. Then a solution of 1.28 g (5.2 mmol) 3-piperidin-4-yl-1,3,4,5-
tetrahydro-1,3-benzodiazepin-2-one in 50 mL DMF was added. The reaction
solution was heated to 80°C for 2 h, after cooling it was evaporated
down
i.vac., the residue was combined with 150 mL EtOAc and 150 mL 10% citric
acid solution, the organic phase was separated off, washed with 150 mL
saturated NaHC03 solution and dried over Na2SOa. After the desiccant and
solvent had been eliminated the crude product (3.7 g) was further reacted
without purification.
ESI-MS: (M+H)+ 616
Rf = 0.25 (silica gel, EtOAc)
12i (R)-3-(4-amino-3,5-bis-trifluoromethyl-phenyl)-2-{[4-(2-oxo-1,2,4,5-
tetrahydro-1, 3-benzodiazepin-3-yl)-piperidi n-1-carbonyl]-a mino}-
propionic acid
A solution of 0.4 g (9.5 mmol) lithium hydroxide hydrate in 50 mL water was
added to a solution of 3.7 g of the above crude product in 50 mL THF and the
reaction mixture was stirred overnight at RT . The THF was eliminated i.vac.,
mixed with 100 mL water and acidified with 1 M HCI. The precipitated product
was suction filtered, washed with 50 mL water and dried in the drying
cupboard at 60°C.
Yield: 2.6 g (90% of theory based on 12f)
ESI-MS: (M-H)- = 586
Retention time (HPLC): 7.1 min (method A)
12k 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carboxylic acid-{(R)-1-(4-amino-3,5-bis-trifluoromethyi-benzyl)-2-[4-(4-
methyl-pi perazi n-1-yl )-piperidin-1-yl]-2-oxo-ethyl}-amide
155 mg (0.85 mmol) 1-methyl-4-piperidin-4-yl-piperazine were added to a
solution of 500 mg (0.85 mmol) (R)-3-(4-amino-3,5-bis-trifluoromethyl-phenyl)-
CA 02503455 2005-04-22
196
2-{[4-(2-oxo-1, 2, 4, 5-tetrahydro-1, 3-benzod iazepi n-3-yl )-pi perid in-1-
carbonyl]-
amino}-propionic acid, 289 mg (0.9 mmol) TBTU and 0.28 mL (2.0 mmol)
triethylamine in 50 mL THF and the reaction mixture was stirred overnight at
RT. It was evaporated down i. vac., the residue was taken up in 100 mL
EtOAc and 100 mL 10% citric acid solution, the organic phase was separated
off and the solvent was eliminated i.vac. Then the residue was purified by
chromatography (silica gel, gradient: DCM to DCM/MeOH/NH3 10:85:5).
Yield: 570 mg (89% of theory)
ESI-MS: (M+H)+ = 753
R, = 0.5 (silica gel, DCM/MeOH/NH3 85:15:1.5)
The following compounds were prepared analogously from (R)-3-(4-amino-
3,5-bis-trifluoromethyl-phenyl)-2-{[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-carbonyl]-amino}-propionic acid and the
corresponding amount of amine:
CF3
/ NHz
O \ I CF3
~.L~ R
N~N
H O
N
N~O
H
ExampleR Yield (%) Mass R, value
on
spectrum silica gel
(eluant)
12.1 ' ~ 89 753 0.4
N
~N/~~N~ [M+H]+ (DCM/MeOH/
NH3
85:15:1.5)
CA 02503455 2005-04-22
197
Example R Yield (%) Mass Rf value
spectrum on
silica gel
(eluant)
12.2 ' ~ 64 738 0.5
[M+H]+ (DCMtMeOHI
NH3
85:15:1.5)
12.3 ' ~ 80 873
0
N~~N~ +
~
o I ~ [M+H]
N
Example 12.3 was further reacted without purification.
Example 12.4
4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-carboxylic
acid-[(R)-1-(4-amino-3, 5-bis-trifluoromethyl-benzyl r2-oxo-2-(4-piperazin-1-
yl-
piperidin-1-yl)-ethyl]-amide
CF3
NHZ
O CF3
N~~~N~ H
N~N~ ~N~
H O
N
~ N~O
H
Prepared analogously to Example 11.8 from 450 mg (0.52 mmol) benzyl 4-[1-
((R)-3-(4-amino-3,5-bis-trifluoromethyl-phenyl)-2-{[4-(2-oxo-1,2,4,5-
tetrahydro-
1,3-benzodiazepin-3-yl)-piperidin-1-carbonyl]-amino}-propionyl)-piperidin-4-
yIJ-piperazin-1-carboxylate (crude product from Example 12.3).
Yield: 200 mg (53% of theory)
CA 02503455 2005-04-22
198
ESI-MS: (M+H)+ = 739
Rf = 0.3 (silica gel, DCM/MeOHINH3 50:50:5)
The following compounds may be prepared analogously:
ExampleStructure
12.5 QIF
~1NHz
~
CF
0 \ '
N N i
N
H O
N
N~O
H
12.6 'F
~NHi
I
O \
CF
I~ ~N~%'~~.~.~ ~H
~N~N~ - V N
H O
I ~ N~p
H
12.7 'F
~NHi
I
CF,
O \
N N~N~N~H
H O
N
.~ N~O
H
12.8 'F=
~NH2
I
CFA
O \
N N N Ny
H O
N
N~Q
H
12.9 'F
~NHz
I
CFA
O \
N N N N
H O
N
N~O
H
CA 02503455 2005-04-22
199
ExampleStructure
12.10 ~F
~NHz
I
O \
CF,~
~N~N~N~'N~~I\\\,,~~I
N H 0
~ N~0
H
Example 13
( R)-1-(4-amino-3-chloro-5-trifl uoromethyl-benzyl )-2-[4-(4-methyl-pi perazin-
1-
yl)-piperidin-1-yl]-2-oxo-ethyl 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-
3-yl)-piperidi n-1-carboxylate
CF3
NHZ
O ~ CI
N~O~N\~~N~Ni
O
N
N~p
H
13a (S)-3-[(R)-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-2-benzyloxy-
propionyl]-4-benzyl-oxazolidin-2-one
Under a nitrogen atmosphere a solution of 1.33 g (4.1 mmol) (S)-4-benzyl-3-
(2-benzyloxy-acetyl)-oxazolidin-2-one in 15 mL THF, cooled to -60°C,
was
added dropwise within 10 min to a solution of 5.1 mL (5.1 mmol, 1 M in THF)
sodium-bis-trimethylsilylamide in 4 mL THF cooled to -60°C and the
reaction
mixture was stirred for for 1 h at this temperature. Then it was cooled to
-70°C and a solution of 2.0 g (8.2 mmol) 2-chloro-4-chloromethyl-6-
trifiuoromethyl-phenylamine (Example 2a) in 15 mL of THF was slowly added
dropwise. The reaction solution was kept for 1 h at -70°C and then
allowed to
warm up to RT within 2 h. 50 mL saturated NH4CI solution were added, the
mixture was extracted with 50 mL EtOAc, the organic phase was separated
off, the aqueous phase was extracted again with 50 mL EtOAc, the combined
CA 02503455 2005-04-22
200
organic phases were washed with 100 mL saturated NaCI and 1 M KHS04
solution and dried over Na2SOa. After the desiccant and solvent had been
eliminated the residue was purified by chromatography (silica gel, PE/EtOAc
4:1 ).
Yield: 2.1 g (96°l0 of theory)
ESI-MS: (M+H)+ = 533/535
Rr = 0.15 (silica gel, PEIEtOAc 4:1 )
13b (R)-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-2-benzyloxy-
propionic acid
A solution of 0.34 g (8.0 mmol) lithium hydroxide hydrate and 1.38 mL (16
mmol, 35% in water) H202 in 25 mL water was added to a solution, cooled to
0°C, of 2.1 g (3.94 mmol) (S)-3-[(R)-3-(4-amino-3-chloro-5-
trifluoromethyl-
phenyl)-2-benzyloxy-propionyl]-4-benzyl-oxazolidin-2-one in 50 mL THF and
the reaction mixture was stirred for 2 h at 0°C. 5 mL of saturated
Na2S03
solution and 5 mL of saturated NaHC03 solution were added, the mixture was
stirred for another 30 min and then the THF was eliminated i.vac. The
aqueous residue was extracted twice with 50 mL EtOAc in each case and the
combined organic phases were dried over MgS04. After the desiccant and
solvent had been eliminated the crude product was further reacted without
purification.
13c (R)-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-2-benzyloxy-1-[4-(4-
methyl-piperazin-1-yl)-piperidin-1-yl]-propan-1-one
1.35 g (4.20 mmol) TBTU, 0.70 mL (5.0 mmol) triethylamine and 0.75 g (4.01
mmol) 1-methyl-4-piperidin-4-yl-piperazine were added to a solution of 1.5 g
(4.01 mmol) (R)-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-2-benzyloxy-
propionic acid in 50 mL THF and the reaction mixture was stirred overnight at
RT. The reaction solution was evaporated down i.vac., the residue was
combined with 200 mL EtOAc and 200 mL saturated NaHC03 solution, the
organic phase was separated off and extracted with 100 mL of 5% citric acid
solution. The citric acid extract was made alkaline with K2C03 and extracted
twice with 100 mL EtOAc in each case. The combined organic phases were
evaporated down i.vac. and the residue further reacted without purification.
CA 02503455 2005-04-22
201
Yield: 1.75 g (81 % of theory)
ESI-MS: (M+H)+ = 539/541 (CI)
Retention time (HPLC): 5.9 min (method A)
13d (R)-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-2-hydroxy-1-[4-(4-
methyl-piperazin-1-yl)-piperidin-1-ylJ-propan-1-one
0.32 mL (2.5 mmol) chloro-trimethyl-silane were added to a suspension of 450
mg (0.84 mmol) (R)-3-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-2-
benzyloxy-1-[4-(4-methyl-piperazin-1-yl)-piperidin-1-yl]-propan-1-one and 380
mg (2.5 mmol) Nal in 30 mL acetonitrile and the reaction mixture was stirred
for 7 h at 80°C. 30 mL EtOH and 20 mL isopropanol were added, the
mixture
was stirred for 30 min at RT, 15 mL of NH3 solution were added and the
mixture was stirred for a further 30 min. It was evaporated down i. vac., the
residue was combined with 100 mL 15% K2C03 solution, extracted with 100
mL EtOAc, the organic phase was separated off, washed with 3% Na2S03
solution and trocknete over Na2S04. After the desiccant and solvent had been
eliminated the residue was further reacted without purification.
Yield: 300 mg (80% of theory)
Retention time (HPLC): 3.7 min (method A)
13e 4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-
carbonyl chloride
6 g (12.1 mmol) of phosgene (20 wt.% in toluene) were added to a solution
cooled to 0°C of 2.5 g (10.2 mmol) 3-piperidin-4-yl-1,3,4,5-tetrahydro-
1,3-
benzodiazepin-2-one and 2.6 mL (14.9 mmol) ethyldiisopropylamine in 75 mL
DCM and the reaction mixture was stirred for 30 min at this temperature. It
was allowed to warm up to RT, evaporated down i.vac. to approx. 50 mL and
filtered through silica gel, washed with 200 mL DCM/EtOAc (1:1 ) and the
combined filtrates were evaporated down again i.vac. The residue was stirred
with diisopropylether, suction filtered and dried i.vac.
Yield: 2.42 g (77% of theory)
Rr = 0.43 (silica gel, DCM/EtOAc 1:1 )
13f (R)-1-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-2-[4-(4-methyl-
CA 02503455 2005-04-22
202
piperazin-1-yl)-piperidin-1-yl]-2-oxo-ethyl 4-(2-oxo-1,2,4,5-tetrahydro-
1,3-benzodiazepin-3-yl)-piperidin-1-carboxylate
Under a nitrogen atmosphere 31 mg (0.7 mmol) NaH (55% in mineral oil)
were added to a solution, cooled to 0°C, of 300 mg (0.67 mmol) (R)-3-(4-
amino-3-chloro-5-trifluoromethyl-phenyl)-2-hydroxy-1-[4-(4-methyl-piperazin-
1-yl)-piperidin-1-yl]-propan-1-one in 30 mL THF and the reaction mixture was
stirred for 30 min at this temperature. Then 246 mg (0.8 mmol) 4-(2-oxo-
1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-carbonylchloride were
added batchwise and after the removal of the cooling bath the reaction
solution was stirred for 3 h at RT. It was evaporated down i. vac., the
residue
was combined with 4 mL acetonitrile and purified by HPLC-MS.
Yield: 88 mg ( 15% of theory)
ESI-MS: (M+H)+ = 720/722 (CI)
Retention time (HPLC): 6.0 min (method A)
The following compounds can be prepared from (R)-3-(4-amino-3-chloro-5-
trifluoromethyl-phenyl)-2-benzyloxy-propionic acid and the appropriate amines
analogously to Example 13c, 13d and 13f:
ExampleStructure
13.1 ~F~
~NHz
' ~
0
CI
N 0 N N
O
N
I ~ N~p
H
F
13.2 NH,
CI
N~
N O~ ~N~N~
0
N
~ N~0
H
CA 02503455 2005-04-22
203
Example Structure
13.3 F
~NH?
0 (''~I CI
N O N~%'"~N~
N~ O
~ N~0
H
13.4 F=
~NHz
OI ('' II CI
N~O N~
~N~N~H
N O
N~O
H
13.5 F
~NHz
('' II CI
N 0 N~N~N'H
NL O
~ N~0
H
13.6 F=
~NH~
('' II CI
~N~O~N~N\
NL I~/I 0
~ N~O
H
F
13.7 NH,
CI
N~ N
~N O
NL ~1~I 0
~ N~p
H
F
13.8 ~NHz
' CI ~N
/\ II
N 0 N~
~N
N O
N~O
H
CA 02503455 2005-04-22
204
Example Structure
13.9 pF'
~NHz
I
CI
O \
N p N\~%~~ ,H
N
O
N
N~p
H
Example 14
4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-carboxylic acid (2-
(1,4']bipiperidinyl-1'-yl-1-(4-bromo-3-methyl-benzyl)-2-oxo-ethyl]-amide
0
N"N
H O
N O
I
H
14a ethyl 2-amino-3-(4-bromo-3-methyl-phenyl)-propionate hydrochloride
The mixture of 31.4 g ( 115 mmol) N-(diphenylmethylene)-glycinethylester,
28.5 g (108 mmol) (4-bromo-3-methylphenyl)-methylbromide, 3.55 g (11.0
mmol) tetrabutylammonium bromide, 116 g (550 mmol) K2C03 and 400 mL
acetonitrile was refluxed for 4 h. The solid was filtered off, the mother
liquor
was concentrated by evaporation in vacuo. The residue was taken up in 500
mL tert-butylmethylether and after the addition of 200 mL 10% HCI it was
stirred overnight at RT. The organic phase was separated off, the aqueous
phase was washed twice more with 50 mL tert -butylmethylether, then
neutralised with 10% Na2C03 solution while being externally cooled with ice
and exhaustively extracted with DCM. The combined organic phases were
washed twice more with 50 mL water, dried over MgS04, filtered through
activated charcoal and evaporated down in vacuo. The oily residue remaining
was dissolved in 50 mL anhydrous EtOH, combined with ethereal HCI solution
CA 02503455 2005-04-22
205
and then diluted with tert-butylmethylether to give a total volume of 500 mL.
After 20 minutes' stirring a colourless crystalline precipitate was formed
which
was suction filtered and dried in the air.
Yield: 17.6 g (47% of theory)
Rf = 0.45 (silica gel, DCM/MeOH/NH3 9:1;0.1 )
14b ethyl3-(4-bromo-3-methyl-phenyl)-2-{[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-carbonyl]-amino}-propionate
3.28 g (20.0 mmol) CDT and 2.77 mL (20.0 mmol) triethylamine were added
to an ice-cooled suspension of 6.45 g (20.0 mmol) ethyl 2-amino-3-(4-bromo-
3-methyl-phenyl)-propionate hydrochloride in 50 mL DMF. The reaction
mixture was then stirred for 1 h at 0 °C and 1 hour at RT, then
combined with
the suspension of 4.63 g (20.0 mmol) 3-piperidin-4-yl-3,4-dihydro-1 H-
quinazoline-2-one in 50 mL DMF. The mixture was heated to 80 °C for 1.5
h
and then stirred into in 500 mL water. The precipitate which solidified after
some time was ground up using an Ultra-Turrax stirrer, washed thoroughly
with water, suction filtered and dried at 50 °C in the circulating air
dryer.
Yield: 11.9 g (97% of theory; contains 1.0 eq. DMF)
Rf = 0.40 (silica gel, DCM/MeOH/NH3 9:1:0.1 )
14c 3-(4-bromo-3-methyl-phenyl)-2-{[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-
yl)-piperidin-1-carbonyl]-amino}-propionic acid
60 mL of 1 M NaOH were added to a solution of 10.9 g (20 mmol) ethyl 3-(4-
bromo-3-methyl-phenyl)-2-{[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidin-1-carbonyl]-amino}-propionate in 60 mL EtOH and the mixture was
then refluxed for 2 h. After cooling it was diluted with 50 mL water and
acidified with 20% citric acid solution. The precipitate obtained was suction
filtered, washed thoroughly with water and dried at 50 °C in the
circulating air
dryer.
Yield: 9.6 g (93% of theory)
ESI-MS: (M-H)~ = 513/515 (Br)
Rr = 0.10 (silica gel, DCM/MeOH 9:1 )
14d 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-carboxylic acid [2-
CA 02503455 2005-04-22
206
[ 1, 4']bi pi peridi nyl-1'-yl-1-(4-bromo-3-methyl-benzyl )-2-oxo-ethyl]-amide
The product was obtained analogously to Example 2f from 515 mg (1.00
mmol) 3-(4-bromo-3-methyl-phenyl)-2-{[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-
yl)-piperidin-1-carbonyl]-amino}-propionic acid and 177 mg (1.00 mmol)
[1,4']bipiperidinyl.
Yield: 320 mg (48% of theory)
ESI-MS: (M+H)+ = 665/667 (Br)
Rf = 0.33 (silica gel, DCM/MeOH/NH3 9:1:0.1 )
The following compounds were prepared analogously from 3-(4-bromo-3-
methyl-phenyl)-2-{(4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-
carbonyl]-amino}-propionic acid and the corresponding amount of amine:
/ Br
~ R
N- _N
/ ~~ H O
N O
I
H
Exampl R Yield Mass Rf
(%)
spectrum(silica gel)
14.1 ' ~ 52 679/681 0.28
N
N'c"3 [M+H]+ (DCM/MeOH/NH3 9:1:0.1)
14.2 ' ~ 50 0.24
N~ ~
~~N
N~CH3 (DCM/MeOH/NH3 9:1:0.1)
14.3 ' ~ 25 680/682 0.19
N
~N~~~N~c"3 [M+H]' (DCM/MeOH/NH3 9:1:0.1)
CA 02503455 2005-04-22
207
ExamplR Yield Mass R,
(1)
a spectrum (silica gel)
14.4 " 25 774/776 0.45
~ +
~~N
~N~o~ [M+Na] (DCM/MeOH 9:1
)
0
14.5 " ~ 40 751 /753 0.48
N
N~~ [M+H]+ (DCM/MeOH 9:1)
0
14.6 ' 28 774/776 0.39
~
N [M+Na)+ (DCM/MeOH 9:1)
~N~~~N~o~
0
Example 15
{4-[1-(3-(4-bromo-3-methyl-phenyl)-2-{[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-
yl)-piperidin-1-carbonyl]-amino)-propionyl)-piperidin-4-yl]-piperazin-1-yl}-
acetic
acid
Br
0
N~N N\ ~N~/'N~O~H
H O p
N O
I
H
1.0 mL (1.00 mmol) 1 M NaOH were added to a solution of 80 mg (0.11
mmol) ethyl {4-[1-(3-(4-brorno-3-methyl-phenyl)-2-{[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-carbonyl]-amino}-propionyl)-piperidin-4-yl]-
piperazin-1-yl}-acetate (Example 14.4) in 4 mL THF. The reaction mixture was
was stirred overnight at RT and the solvent was eliminated i. vac. 1 mL 1 M
CA 02503455 2005-04-22
208
HCI was added to the residue and it was evaporated to dryness again. The
residue was taken up in EtOH and after filtration the mother liquor was
concentrated by evaporation i.vac. The residue was triturated with
diisopropylether and after filtration dried in the air.
Yield: 80 mg ( 100% of theory)
Retention time (HPLC): 5.9 min (method A)
The following compounds were prepared analogously from the respective
ethyl esters (Examples 14.5 and 14.6):
Br
R
N~N
/ ~~ H O
~N
''~~~..N"O
I
H
Example R Yield Mass R, (silica gel)
(%) spectrum or
Retention time
HPLC
15.1 ' 59 745/747 0.12
~
N [M+Na]+ (DCM/MeOH 9:1)
N~o'H
0
15.2 ' ~ 81 7221724 5.5 min
~N~N~o~H [M-Hl
0
Example 16
2-(4-bromo-3-methyl-benzyl)-1-[4-(4-methyl-piperazin-1-yl)-piperidin-1-yl]-4-
[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butan-1,4-dione
CA 02503455 2005-04-22
209
~N
N O
I
H
16a 4-tent-butyl, 1-ethyl 2-(4-bromo-3-methyl-benzyl)-2-ethoxycarbonyl-
succinicate
The product was prepared analogously to Example 2b from 11.4 g (41.7
mmol) 4-tert-butyl, 1-ethyl 2-ethoxycarbonyl-succinate and 11.0 g (41.7 mmol)
1-bromo-4-bromomethyl-2-methyl-benzene.
Yield: 21.3 g (100°!° of theory)
Rf = 0.64 (silica gel, PE/EtOAc 8:2)
16b ethyl2-(4-bromo-3-methyl-benzyl)-2-ethoxycarbonyl-succinicate
The product was prepared analogously to Example 2c from 21.3 g (41.7
mmol) 4-tert-butyl, 1-ethyl 2-(4-bromo-3-methyl-benzyl)-2-ethoxycarbonyl-
succinate.
Yield: 7.8 g (47% of theory)
Rf = 0.26 (silica gel, PEIEtOAc 8:2)
16c diethyl2-(4-bromo-3-methyl-benzyl)-2-{2-oxo-2-[4-(2-oxo-1,4-dihydro-
2H-quinazolin-3-yl)-piperidin-1-yl]-ethyl}-malonate
The product was prepared analogously to Example 2d from 7.80 g (19.4
mmol) ethyl 2-(4-bromo-3-methyl-benzyl)-2-ethoxycarbonyl-succinate and
4.50 g (19.4 mmol) 3-piperidin-4-yl-3,4-dihydro-1H-quinazolin-2-one .
Yield: 8.30 g (70°l0 of theory)
EI-MS: (M)+ = 613/615 (Br)
Rf = 0.80 (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2)
16d 2-(4-bromo-3-methyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-yl]-butanoic acid
CA 02503455 2005-04-22
210
The product was prepared analogously to Example 2e from 8.30 g ( 13.5
mmol) diethyl 2-(4-bromo-3-methyl-benzyl)-2-{2-oxo-2-[4-(2-oxo-1,4-dihydro-
2H-quinazolin-3-yl)-piperidin-1-yl]-ethyl}-malonate.
Yield: 5.10 g (74% of theory)
EI-MS: (M)+ = 513/515 (Br)
Rf = 0.20 (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2)
16e 2-(4-bromo-3-methyl-benzyl)-1-[4-(4-methyl-piperazin-1-yl)-piperidin-1-
yl]-4-[4-(2-oxo-1, 4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yIJ-butan-1,4-
dione
The product was prepared analogously to Example 2f from 0.51 g (1.00
mmol) 2-(4-bromo-3-methyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-yl]-butanoic acid and 0.18 g (1.00 mmol) 1-methyl-
4-piperidin-4-yl-piperazine.
Yield: 250 mg (37% of theory)
EI-MS: (M)+ = 678/680 (Br)
Rf = 0.50 (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2)
The following compounds were prepared analogously from 2-(4-bromo-3-
methyl-benzyl )-4-oxo-4-[4-(2-oxo-1, 4-di hydro-2H-quinazolin-3-yl)-pi peridi
n-1-
yl]-butanoic acid and the corresponding amount of amine:
/~N
O
N O
l
H
ExampleR Yield Mass R,
(%)
spectrum(silica gel)
CA 02503455 2005-04-22
211
ExampleR Yield Mass Rf
(%)
spectrum (silica gel)
16.1 ' ~ 44 677/679 0.50
N
N~~"3 [M]+ (DCM/cyc/MeOH/NH3
70:15:15:2)
16.2 46 663/665 0.52
,.
N
~~N~~ [M]' (DCM/cyc/MeOH/NH3
70:15:15:2)
16.3 ' ~ ~ N~ 27 7421744 0.56
N I
~
~N [M]' (DCM/cyc/MeOH/NH3
70:15:15:2)
Example 16.4
[4-( 1-{2-(4-bromo-3-methyl-benzyl )-4-oxo-4-[4-(2-oxo-1, 4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-yl]-butyryl)-piperidin-4-yl)-piperazin-1-yl]-
acetic
acid
%~N ~O~H
~., o
N O
I
H
This synthesis was carried out by the Chemspeed ASW2000 synthesising
robot (Chemspeed Ltd., Rheinstraf3e 32, CH-4302 Augst, Switzerland).
Mixture:
AGV 1: 102 mg (0.20 mmol) 2-(4-bromo-3-methyl-benzyl)-4-oxo-4-[4-(2-
oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butanoic acid
in 3 mL THF;
CA 02503455 2005-04-22
212
AGV 2: 51 mg (0.20 mmol) ethyl (4-piperidin-4-yl-piperazin-1-yl)-acetate
in 2 mL THF;
AGV 3: 64 mg (0.20 mmol) TBTU in 2 mL DMF;
AGV 4: 0.14 mL (1.00 mmol) triethylamine;
AGV 5: 1.00 mL 4 M NaOH;
AGV 6: 1.00 mL 4 M HCI;
AGV 7: 6 mL THF.
The AGV's 1 to 4 were positioned accordingly, then pipetted together by the
robot and shaken for 8 h at RT. The reaction mixtures were concentrated by
evaporation, combined with 7 mL EtOAc, the resulting solutions were each
washed with 10 mL of 10% K2C03 solution and 6 mL water and again freed
from solvent. The residues were each dissolved in AGV 7 and after the
addition of AGV 5 stirred for 6 h at RT. The reaction mixtures were each
neutralised by the addition of AGV 6, then concentrated by evaporation. The
residue obtained was dissolved in 1.9 mL DMF and added to a microtitre
plate. The samples were separated using an HPLC-MS apparatus (Agilent
Technologies, Agilent 1100 Series Modules and Systems for HPLC and
LC/MS), the product was collected under mass control. The end product was
freeze-dried.
Yield: 4 mg (3 % of theory).
ESI-MS: (M-H)- = 721/723 (Br)
(M+H)+ = 723/725 (Br)
Example 16.5
methyl (1'-{2-(4-bromo-3-methyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-yl]-butyryl}-[4,4'~bipiperidinyl-1-yl)-acetate
CA 02503455 2005-04-22
213
~N N~Ow
O O
N O
I
H
This synthesis was carried out by the Chemspeed ASW2000 synthesising
robot (Chemspeed Ltd., Rheinstraf3e 32, CH-4302 Augst, Switzerland).
Mixture:
AGV 1: 206 mg (0.40 mmol) 2-(4-bromo-3-methyl-benzyl)-4-oxo-4-[4-(2-
oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butanoic acid
in 3 mL THF;
AGV 2: 102 mg (0.40 mmol) ethyl [4,4']bipiperidinyl-1-yl-acetate in 4 mL
THF;
AGV 3: 128 mg (0.40 mmol) TBTU in 4 mL DMF;
AGV 4: 0.14 mL (1.00 mmol) triethylamine;
The AGV's 1 to 4 were positioned accordingly, then pipetted together by the
robot and shaken for 8 h at RT. The reaction mixtures were concentrated by
evaporation, combined with 7 mL EtOAc and 6 mL 10% K2C03 solution,
shaken vigorously, the aqueous phase was removed and discarded. The
organic phase was concentrated by evaporation and dissolved in 6 mL of
MeOH. One third of this solution was taken and added to a microtitre plate.
The samples were separated using an HPLC-MS apparatus (Agilent
Technologies, Agiient 1100 Series Modules and Systems for HPLC and
LC/MS), the product was collected under mass control. The end product was
freeze-d ri ed .
Yield: 9 mg (9 % of theory).
EI-MS: (M)+ = 735/737 (Br)
Rf = 0.38 (silica gel, DCM/MeOH 9:1 )
The remaining 2/3 of the MeOH solution were concentrated by evaporation
CA 02503455 2005-04-22
214
and the crude product (195 mg) was further reacted in Example 16.6.
Example 16.6
(1'-{2-(4-bromo-3-methyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-
3-yl)-piperidin-1-yl]-butyryl}-[4,4']bipiperidinyl-1-yl)-acetic acid
/ Br
0
N
N ~ N~O~H
O O
N O
I
H
This synthesis was carried out by the Chemspeed ASW2000 synthesising
robot (Chemspeed Ltd., Rheinstra(3e 32, CH-4302 Augst, Switzerland).
Mixture:
AGV 1: 195 mg (0.26 mmol) methyl (1'-{2-(4-bromo-3-methyl-benzyl)-4-
oxo-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-
butyryl}-[4,4']bipiperidinyl-1-yl)-acetate;
AGV 2: 5 mL MeOH
AGV 3: 1.00 mL 4 M NaOH;
AGV 4: 1.00 mL 4 M HCI;
AGV 1 was dissolved in AGV 2 and then AGV 3 was added. The mixture was
shaken for 5 h at 20°C and then neutralised with AGV 4. The reaction
mixture
was concentrated by evaporation and dissolved in 2 mL ~MF. The samples
were separated using an HPLC-MS apparatus (Agilent Technologies, Agilent
1100 Series Modules and Systems for HPLC and LC/MS), the product was
collected under mass control. The end product was freeze-dried.
Yield: 22 mg (11 % of theory).
ESI-MS: (M+H)+ = 722/724 (Br)
CA 02503455 2005-04-22
215
Rf = 0.22 (silica gel, DCM/Me4H 9:1 )
Examgle 17
4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-carboxylic acid{1-(4-
chloro-3-methyl-benzyl)-2-[4-(4-methyl-piperazin-1-yl)-piperidin-1-yl]-2-oxo-
ethyl}-amide
~N
N O
I
H
17a ethyl 2-amino-3-(4-chloro-3-methyl-phenyl)-propionate hydrochloride
The product was prepared analogously to Example 14a from 31.4 g (115
mmol) N (diphenylmethylene)-glycinethylester and 25.2 g (115 mmol) of 4-
bromomethyl-1-chloro-2-methyl-benzene.
Yield: 20.4 g (64% of theory)
ESI-MS: (M+H)+ = 241/243 (CI)
Rf = 0.35 (silica gel, DCM/MeOH/NH3 9:1:0.1 )
17b ethyl3-(4-chloro-3-methyl-phenyl)-2-{[4-(2-oxo-1,4-dihydro-2H
quinazolin-3-yl)-piperidin-1-carbonyl]-amino}-propionate
The product was obtained analogously to Example 14b from 5.56 g (20.0
mmol) ethyl 2-amino-3-(4-chloro-3-methyl-phenyl)-propionate hydrochloride
and 4.63 (20.0 mmol) 3-piperidin-4-yl-3,4-dihydro-1H-quinazolin-2-one.
Yield: 9.50 g (95% of theory)
ESI-MS: (M-H)- = 497/499 (CI)
17c 3-(4-chloro-3-methyl-phenyl)-2-{[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-
yl)-piperidin-1-carbonyl]-amino}-propionic acid
CA 02503455 2005-04-22
?16
The product was obtained analogously to Example 14c from 9.50 g (19.0
mural) ethyl 3-(4-chloro-3-methyl-phenyl)-2-{[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-carbonyl]-amino}-propionate.
Yield: 8.90 g (99 % of theory)
ESI-MS: (M-H)- = 469!471 (CI)
R, = 0.10 (silica gel, DCM/MeOH 9:1 )
17d 4-(2-oxo-1,4-dihydro-2H quinazolin-3-yl)-piperidin-1-carboxylic acid{1-(4-
chloro-3-methyl-benzyl )-2-[4-(4-methyl-piperazin-1-yl )-piperidi n-1-yl]-2-
oxo-ethyl}-amide
The product was prepared analogously to Example 2f from 706 mg (1.50
mmol) of 3-(4-chloro-3-methyl-phenyl)-2-{[4-(2-oxo-1,4-dihydro-2H-quinazolin-
3-yl)-piperidin-1-carbonyl]-amino}-propionic acid and 275 g (1.50 mmol) of
1-methyl-4-piperidin-4-yl-piperazine.
Yield: 250 mg (26% of theory)
ESI-MS: (M+H)+ = 636/638 (CI)
Rf = 0.17 (silica gel, DCM/MeOH/NH3 9:1:0.1 )
The following compounds were prepared analogously from 3-(4-chloro-3-
methyl-phenyl)-2-{[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-
carbonyl]-amino}-propionic acid and the corresponding amount of amine:
ci
O
N~N R
/ ~~ H O
N O
I
H
Example R Yiefd Mass R,
(%)
spectrum (silica gel)
CA 02503455 2005-04-22
21?
Example R Yield Mass R,
(%)
spectrum (silica gel)
17.1 54 621!623 0.33
N
~~N~ [M+H]+ (DCM/MeOH/NH3
9:1:0.1)
17.2 35 636/638 0.18
.
.
N\ ~ [M+H]' (DCM/MeOH/NH3
~N~~~
~CHa
N
9:1:0.1)
17.3 27 635/637 0.26
..
N
~N~CHs [M+H]+ (DCM/MeOH/NH3
9:1:0.1)
17.a ~ 24 707/709 0.49
~
N
N~o~ [M+Na]+ (DCM/MeOH 9:1
)
0
17.5 37 736/738 0.39
..
o [M+Na]+ (DCM/MeOH 9:1
)
0
17.6 ~ ~ 37 703/705 0.36
N
N
[M+Na]' (DCMIMeOH/NH3
F F 9:1:0.1)
17.7 ~ ~ 49 704!706 0.40
~~N
~~F [M+Na]' (DCM/MeOH/NH3
9:1:0.1)
Example 18
[1'-(3-(4-chioro-3-methyl-phenyl)-2-{(4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidin-1-carbonylJ-amino)-propionyl)-[4,4'Jbipiperidinyl-1-yl]-acetic acid
CA 02503455 2005-04-22
238
O
N~N ~~O~H
/ %~ H I IO
N O
I
H
The product was prepared analogously to Example 15 from 220 mg (0.28
mmol) ethyl [1'-(3-(4-chloro-3-methyl-phenyl)-2-{[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-carbonyl]-amino}-propionyl)-[4,4']bipiperidinyl-1-
yl]-
acetate.
Yield: 190 mg (99 % of theory)
ESI-MS: (M+H)+ = 679/681 (CI)
Rf = 0.13 (silica gel, DCMIMeOH 9:1 )
Example 19
2-(4-chloro-3-methyl-benzyl )-1-[4-(4-ethyl-pi perazin-1-yl )-piperidin-1-yl]-
4-[4-
(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butan-1,4-dione
N N~N~
/ ~~ O
N O
I
H
19a 4-tert-butyl, 1-ethyl 2-(4-chloro-3-methyl-benzyl)-2-ethoxycarbonyl-
succinate
The product was prepared analogously to Example 2b from 19.5 g (71.0
mmol) 4-tent-butyl, 1-ethyl 2-ethoxycarbonyl-succinate and 15.5 g (71.0 mmol)
4-bromomethyl-1-chloro-2-methyl-benzene.
CA 02503455 2005-04-22
219
Yield: 25.7 g (88% of theory)
Rf = 0.74 (silica gel, DCM)
19b ethyl2-(4-chloro-3-methyl-benzyl)-2-ethoxycarbonyl-succinate
The product was prepared analogously to Example 2c from 21.3 g (41.7
mmol) 4-tent-butyl, 1-ethyl 2-(4-chloro-3-methyl-benzyl)-2-ethoxycarbonyl-
succinate.
Yield: 22.2 g (100% of theory)
Rf = 0.18 (silica gel, DCM)
19c diethyl2-(4-chloro-3-methyl-benzyl)-2-{2-oxo-2-[4-(2-oxo-1,4-dihydro-
2H-quinazolin-3-yl)-piperidin-1-yl]-ethyl}-malonate
The product was prepared analogously to Example 2d from 8.00 g (22.4
mmol) ethyl 2-(4-chloro-3-methyl-benzyl)-2-ethoxycarbonyl-succinate and
5.18 g (22.4 mmol) 3-piperidin-4-yl-3,4-dihydro-1 H-quinazolin-2-one.
Yield: 9.20 g (72% of theory)
EI-MS: (M)+ = 569/570 (CI)
Rf = 0.64 (silica gel, DCM/MeOH/NH3 9:1:0.1 )
19d 2-(4-chloro-3-methyl-benzyl}-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-ylj-butanoic acid
The product was prepared analogously to Example 2e from 9.20 g (16.2
mmol) diethyl 2-(4-chloro-3-methyl-benzyl)-2-{2-oxo-2-[4-(2-oxo-1,4-dihydro-
2H-quinazolin-3-yi)-piperidin-1-ylj-ethyl}-malonate.
Yield: 7.20 g (95% of theory)
ESI-MS: (M-H)~ = 468/470 (CI)
19e 2-(4-chloro-3-methyl-benzyl)-1-[4-(4-ethyl-piperazin-1-yl)-piperidin-1-ylj-
4-[4-(2-oxo-1,4-dihyd ro-2H-quinazoli n-3-yl )-pi perid in-1-ylj-butan-1,4-
dione
The product was prepared analogously to Example 2f from 470 mg (1.00
mmol) 2-(4-chloro-3-methyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-ylj-butanoic acid and 593 mg (1.10 mmol) 1-ethyl-
4-piperidin-4-yl-piperazine tris-trifluoroacetate.
220
CA 02503455 2005-04-22
220
Yield: 280 mg (43% of theory)
EI-MS: (M)+ = 648/650 (CI)
Rf = 0.47 (silica gel, DCM/MeOH/NH3 9:1:0.1 )
The following compounds were prepared analogously from 2-(4-chloro-3-
methyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-
yl]-butanoic acid and the corresponding amount of amine:
~N
//
N O
I
H
ExampleR Yield Mass R,
(%)
spectrum (silica gel)
19.1 ' ~ 48 634/636 0.49
N
N'~"3 (M]' (DCM/cyc/MeOH/NH3
70:15:15:2)
19.2 32 620/622 0.54
N~~ [M+H]' (DCM/cyc/MeOH/NH3
70:15:15:2)
19.3 ' 44 635/637 0.50
~ +
~N [M+H] (DCM/cyc/MeOH/NH3
70:15:15:2)
19.4 ' ~ 39 6351637 0.49
N
~N N~ [M+H]+ (DCM/cyc/MeOH/NH3
70:15:15:2)
CA 02503455 2005-04-22
221
ExampleR Yield Mass Rf
(%)
spectrum (silica gel)
19.5 ' ~ 57 698/700 0.54
N
M D
'
[ (
] CM/MeOH/NH3
9:1:0.1 )
19.6 ' ~ 50 662/664 0.48
~ [M]+ ~ (DCM/M
~ OH/NH
N e
3
9:1:0.1 )
19.7 ' ~ 1 707/709 0.76
N
N~~~N~ [M+H]' (DCM/MeOH/NH3
o~ 8:2:0.2)
19.8 ' ~ 30 707/709 0.77
~~ ~ +
N~ [M+H] (DCMIMeOH/NH3
~
8:2:0.2)
0
19.9 ~ 11 707/709 0.56
1
o ,
0
N N [M+H]+ (DCM/MeOH/NH3
~N~ 8:2:0.2)
19.10 0 ~ 8 707/709 0.56
o
~ [M+HJ+ (EtOAc/MeOH/NH3
N~
~N'~N~ 8:2:0.2)
19.11 ' ~ 30 702/704 0.36
N F
N F [M+H]a (DCM/MeOH/NH3
F 9:1:0.1 )
19.12 ' ~ 41 703/705 0.72
'
F [M+H] (DCM/MeOH/NH3
F 8:2:0.1 )
Example 19.13
CA 02503455 2005-04-22
222
[4-( 1-{2-(4-chloro-3-methyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-yl]-butyryl)-piperidin-4-yl)-piperazin-1-yl]-
acetic
acid
~N I I O.H
. o
N O
I
H
The product was obtained analogously to Example 16.4 from 94 mg (0.20
mmol) 2-(4-chloro-3-methyl-benzyl)-4-oxo-4-(4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-yl]-butanoic acid and 51 mg (0.20 mmol) ethyl (4-
piperidin-4-yl-piperazi n-1-yl )-acetate.
Yield: 27 mg (19 % of theory)
EI-MS: (M)+ = 679/681 (CI)
Retention time (HPLC): 5.9 min (method A)
Example 19.14
methyl (1'-{2-(4-chloro-3-methyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-yl]-butyryl)-[4,4']bipiperidinyl-1-yl)-acetate
cl
N . ~~,~~~~N ~Ow
/ O
I
N O
I
H
The product was obtained analogously to Example 16.5 from 188 mg (0.40
CA 02503455 2005-04-22
Z~J
mmol) 2-(4-chloro-3-methyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-yl]-butanoic acid and 102 mg (0.40 mmol) ethyl
[4, 4']bipi perid i nyl-1-yl-acetate.
Yield: 27 mg (30 % of theory)
ESI-MS: (M+H)+ = 692/694 (CI)
Rf = 0.36 (silica gel, DCMIMeOH 9:1 )
Example 19.15
( 1'-{2-(4-chloro-3-methyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-
3-yl)-piperidin-1-yl]-butyryl}-[4,4']bipiperidinyl-1-yl)-acetic acid
N ~O~H
/ O
N O
I
H
The product was obtained analogously to Example 16.6 from 184 mg (0.26
mmol) methyl (1'-{2-(4-chloro-3-methyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-
2H-quinazolin-3-yl)-piperidin-1-yl]-butyryl}-[4,4']bipiperidinyl-1-yl)-
acetate.
Yield: 30 mg (16 % of theory)
ESI-MS: (M+H)+ = 678/680 (CI)
Rs = 0.21 (silica gel, DCM/MeOH 9:1 )
The following compounds were prepared from the relevant ethyl esters
(Examples 19.7 to 19.9) analogously to Example 15:
CA 02503455 2005-04-22
224
Br
O
N- _N
~~ H O
N O
I
H
ExampleR Yield Mass R,
(%) spectrum (silica gel)
19.16 = ~ 18 679/681 0.05
N
N~~~N~ [M+H]+ (EtOAc/MeOH/NH3
0 0 6:4:0.4)
H
19.17 ~ ~ 50 679/681 0.21
'
[M+H] (DCM/MeOH/NH3
~
8:2:0.2)
0
H
19.18 0 " 48 679/681 0.14
N\~N\ ~ [M+H]+ (EtOAc/MeOH/NH3
~
N
6:4:0.4)
Example 20
2-(4-chloro-3-methyl-benzyl )-1-[4-( 1-methyl-piperidin-4-yl )-pi perazin-1-
yl]-4-[4-
(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-
dione
CA 02503455 2005-04-22
225
'N ~~C~% N ~
N
H
20a diethyl2-(4-chloro-3-methyl-benzyl)-2-{2-oxo-2-[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-ethyl}-malonate
The product was prepared analogously to Example 2d from 1.43 g (4.00
mmoi) ethyl 2-(4-chloro-3-methyl-benzyi)-2-ethoxycarbonyl-succinate and 981
mg (4.00 mmol) 3-(1-methyl-piperidin-4-yl)-1,3,4,5-tetrahydro-1,3-
benzodiazepin-2-one.
Yield: 2.10 g (90% of theory)
Rf = 0.69 (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2)
20b 2-(4-chloro-3-methyl-benzyl)-4-oxo-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-butanoic acid
The product was prepared analogously to Example 2e from 2.10 g (3.60
mmol) diethyl 2-(4-chloro-3-methyl-benzyl)-2-{2-oxo-2-[4-(2-oxo-1,2,4,5-
tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-ethyl}-malonate. The
product was further reacted without purification.
Yield: 1.20 g (69 % of theory)
20c 2-(4-chloro-3-methyl-benzyl)-1-[4-(1-methyl-piperidin-4-yl)-piperazin-1-
yl]-4-[4-(2-oxo-1, 2, 4, 5-tetrahyd ro-1, 3-benzod iazepi n-3-yl )-piperidi n-
1-
yl]-butan-1,4-dione
The product was prepared analogously to Example 2f from 800 mg (1.65
mmol) 2-(4-chloro-3-methyl-benzyl)-4-oxo-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-butanoic acid and 302 mg (1.65 mmol) 1-
( 1-methyl-piperidin-4-yl )-pi perazine.
Yield: 400 mg (37% of theory)
CA 02503455 2005-04-22
226
EI-MS: (M)+ = 648/650 (CI)
Rr = 0.50 (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2)
Example 20.1
2-(4-chloro-3-methyl-benzyl )-1-[4-(4-methyl-piperazin-1-yl )-piperidin-1-yl]-
4-[4-
(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-piperidin-1-yl]-butan-1,4-
dione
ci
N ~N~Ni
O
N
N~p
H
The product was prepared analogously to Example 2f from 400 mg (0.83
mmol) 2-(4-chloro-3-methyl-benzyl)-4-oxo-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-
benzodiazepin-3-yl)-piperidin-1-yl]-butanoic acid and 152 mg (0.83 mmol) 1-
methyl-4-piperidin-4-yl-piperazine.
Yield: 200 mg (37% of theory)
EI-MS: (M)+ = 648/650 (CI)
Rf = 0.51 (silica gel, DCM/cyc/MeOH/NH3 70:15:15:2)
Example 21
[4-( 1-{2-(4-chloro-3-methyl-benzyl)-4-oxo-4-[4-( 5-oxo-3-phenyl-4,5-di hydro-
1,2,4-triazol-1-yl )-piperidi n-1-yl]-butyryl}-piperidin-4-yl )-piperazin-1-
yl]-acetic
acid
CA 02503455 2005-04-22
227
O N ~N~N~O~H
O
li-N N
I
'N
21a diethyl2-(4-chloro-3-methyl-benzyl)-2-{2-oxo-2-[4-(5-oxo-3-phenyl-4,5-
di hyd ro-1, 2, 4-triazol-1-yl )-piperid in-1-yl]-ethyl}-malonate
The product was prepared analogously to Example 2d from 5.00 g (14.0
mmol) ethyl 2-(4-chloro-3-methyl-benzyl)-2-ethoxycarbonyl-succinate and
3.42 g (14.0 mmol) 5-phenyl-2-piperidin-4-y1-2,4-dihydro-1,2,4-triazol-3-one.
Yield: 5.50 g (67 % of theory)
Rf = 0.50 (silica gel, DCM/MeOH/NH3 9:1:0.1 )
21 b 2-(4-chloro-3-methyl-benzyl)-4-oxo-4-[4-(5-oxo-3-phenyl-4,5-dihydro-
1,2,4-triazol-1-yl)-piperidin-1-yl]-butanoic acid
The product was prepared analogously to Example 2e from 5.50 g (9.43
mmol) diethyl 2-(4-chloro-3-methyl-benzyl)-2-{2-oxo-2-[4-(5-oxo-3-phenyl-4,5-
dihydro-1,2,4-triazol-1-y1)-piperidin-1-y1]-ethyl}-malonate.
Yield: 2.80 g (62 % of theory)
ESI-MS: (M-H)~ = 481/483 (CI)
21c [4-(1-{2-(4-chloro-3-methyl-benzyl)-4-oxo-4-[4-(5-oxo-3-phenyl-4,5-
dihydro-1,2,4-triazol-1-yl)-piperidin-1-yl]-butyryl}-piperidin-4-yl)-
piperazin-1-yl]-acetic acid
The product was obtained analogously to Example 16.4 from 96 mg (0.20
mmol) 2-(4-chloro-3-methyl-benzyl)-4-oxo-4-[4-(5-oxo-3-phenyl-4,5-dihydro-
1,2,4-triazol-1-yl)-piperidin-1-yl]-butanoic acid and 51 mg (0.20 mmol) ethyl
(4-
piperidi n-4-yl-pi perazi n-1-yl )-acetate.
Yield: 3 mg (2 % of theory)
ESI-MS: (M+H)+ = 692/694 (CI)
CA 02503455 2005-04-22
228
Example 21.1
methyl (1'-{2-(4-chloro-3-methyl-benzyl)-4-oxo-4-[4-(5-oxo-3-phenyl-4,5-
dihydro-1,2,4-triazol-1-yl)-piperidin-1-yl]-butyryl}-4,4'-bipiperidinyl-1-yl)-
acetate
cl
w
o ~-
N ~ /O
%~N N
O O
Fi-N N
I
~N
The product was obtained analogously to Example 16.5 from 193 mg (0.40
mmol) 2-(4-chloro-3-methyl-benzyl)-4-oxo-4-[4-(5-oxo-3-phenyl-4,5-dihydro-
1,2,4-triazol-1-yl)-piperidin-1-yl]-butanoic acid and 102 mg (0.40 mmol) ethyl
[4,4']bi pi perid inyl-1-yl-acetate.
Yield: 14 mg (15 % of theory)
ESI-MS: (M+H)+ = 705/707 (CI)
Rf = 0.32 (silica gel, DCM/MeOH 9:1 )
Examel,e 21.2
( 1'-{2-(4-chloro-3-methyl-benzyl )-4-oxo-4-[4-( 5-oxo-3-phenyl-4, 5-dihydro-
1,2,4-triazol-1-yl)-piperidin-1-yl]-butyryl}-4,4'-bipiperidinyl-1-yl)-acetic
acid
CA 02503455 2005-04-22
229
~N ~~O\H
O O
H_N N
I
'N
The product was obtained analogously to Example 16.6 from 187 mg (0.2fi
mmol) methyl (1'-{2-(4-chloro-3-methyl-benzyl)-4-oxo-4-[4-(5-oxo-3-phenyl-
4,5-dihydro-1,2,4-triazol-1-yl)-piperidin-1-yl]-butyryl}-4,4'-bipiperidinyl-1-
yl)-
acetate.
Yield: 11 mg (6 % of theory)
ESI-MS: (M+H)+ = 691/693 (CI)
Rf = 0.21 {silica gel, DCM/MeOH 9:1 )
Example 22
2-( 3-bromo-4-chloro-5-methyl-benzyl )-1-[4-(4-methyl-piperazi n-1-yl )-
piperid i n-
1-yl]-4-[4-(2-oxo-1,4-d ihyd ro-2H-qui nazolin-3-yl )-piperidin-1-yl]-butan-
1,4-
dione
N ~ N~/'N i
N O
I
H
22a 1-(3-bromo-4-chloro-5-methyl-phenyl)-ethanone
25.0 g (148 mmol) 1-(4-chloro-3-methyl-phenyl)-ethanone were added
dropwise to 59.2 g (444 mmol) of aluminium trichloride. The temperature rose
CA 02503455 2005-04-22
230
to 70°C. The mixture was stirred for 30 min at 80°C and then at
this
temperature 10.7 mL (170 mmol) bromine were added dropwise. The reaction
solution was stirred for 1 h at 80°C and then added to ice. The aqueous
phase
was extracted with diethyl ether and the combined organic extracts were
washed with saturated NaHC03 solution. The organic phase was dried over
Na2S04 and the solvent was eliminated i. vac. Purification was carried out by
column chromatography on silica gel (toluene).
Yield: 14.0 g (38% of theory)
EI-MS: (M)+ = 246/248/250 (Br, CI)
Rf = 0.28 (silica gel, toluene)
22b 3-bromo-4-chloro-5-methyl-benzoic acid
8.7 mL (171 mmol) bromine was added dropwise at 0°C to a solution of
22.8 g
(570 mmol) NaOH in 114 mL water so that the temperature did not exceed
10°C. 14.0 g (57.0 mmol) 1-(3-bromo-4-chloro-5-methyl-phenyl)-ethanone
in
57 mL 1,4-dioxane was added dropwise at 10°C and the mixture was
stirred
for 2 h at RT. The reaction mixture was diluted with water and the bromine
form obtained was separated off. The aqueous phase was acidified with
semiconc. HCI, the precipitate was suction filtered and washed with water.
Yield: 11.0 g (78 % of theory)
EI-MS: (M)+ = 248/250/252 (Br, CI)
melting point: 207-209°C
22c (3-bromo-4-chloro-5-methyl-phenyl)-methanol
8.1 g (50 mmol) CDI were added to a solution of 11.0 g (44 mmol) 3-bromo-4-
chloro-5-methyl-benzoic acid in 285 mL THF at RT. The reaction mixture was
for stirred for 1 h at 40°C. This solution was added to a solution of
5.38 g
(142 mmol) NaBH4 in 47.5 mL water. The mixture was stirred for 3 h at RT,
then diluted with 300 mL water and acidified with semiconc. HCI. The
aqueous phase was extracted with EtOAc and the organic phase was washed
successively with water and saturated NaHC03 solution. The organic phase
was dried over Na2S04 and the solvent was eliminated i. vac.
Yield: 9.00 g (87% of theory)
ESI-MS: (M-H)~ = 233/235/237 (Br, CI)
CA 02503455 2005-04-22
231
R, = 0.62 (silica gel, PE/EtOAc 1:1 )
22d 1-bromo-5-bromomethyl-2-chloro-3-methyl-benzene
5.2 mL (19 mmol) phosphorus tribromide was added dropwise to a solution of
9.00 g (38 mmol) (3-bromo-4-chloro-5-methyl-phenyl)-methanol in 250 mL
diethyl ether at RT and refluxed for 1 h. The reaction mixture was added to
saturated NaHC03 solution, the organic phase was separated off, washed
with water and dried over Na2S04. After the desiccant and solvent had been
eliminated the desired product was obtained.
Yield: 10.7 g (94% of theory)
EI-MS: (M)+ = 296/298/300/302 (2Br, CI)
Rf = 0.89 (silica gel, PE/EtOAc 1:1 )
22e 4-tent-butyl, 1-ethyl 2-(3-bromo-4-chloro-5-methyl-benzyl)-2-
ethoxycarbonyl-succinate
The product was prepared analogously to Example 2b from 9.86 g (36 mmol)
4-tent-butyl, 1-ethyl 2-ethoxycarbonyl-succinate and 10.7 g (36 mmol) 1-
bromo-5-bromomethyl-2-chloro-3-methyl-benzene.
Yield: 17.5 g (99% of theory)
ESI-MS: (M+H)+ = 513/515/517 (Br, CI)
Rf = 0.57 (silica gel, DCM)
22f 1-ethyl2-(3-bromo-4-chloro-5-methyl-benzyl)-2-ethoxycarbonyl-
succinate
The product was prepared analogously to Example 2c from 18.0 g (37 mmol)
4-tert-butyl, 1-ethyl 2-(3-bromo-4-chloro-5-methyl-benzyl)-2-ethoxycarbonyl-
succinate. The crude product which still contained TFA was further reacted
without purification.
ESL-MS: (M-H)- = 4331435/437 (Br, C1)
22g diethyl2-(3-bromo-4-chloro-5-methyl-benzyl)-2-{2-oxo-2-[4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-ethyl}-malonate
The product was prepared analogously to Example 2d from 18.2 g (42 mmol)
1-ethyl 2-(3-bromo-4-chloro-5-methyl-benzyl)-2-ethoxycarbonyl-succinate and
CA 02503455 2005-04-22
232
9.60 g (42 mmol) 3-piperidin-4-yl-3,4-dihydro-1H-quinazolin-2-one .
Yield: 15.0 g (56% of theory)
EI-MS: (M)+ = 647/649/651(Br, CI)
Rf = 0.60 (silica gel, DCM/MeOHlNH3 9:1:0.1 )
22h 2-(3-bromo-4-chloro-5-methyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-
2H-quinazolin-3-y1)-piperidin-1-yl]-butanoic acid
The product was prepared analogously to Example 2e from 15.0 g (23 mmol)
diethyl 2-( 3-bromo-4-chloro-5-methyl-benzyl )-2-{2-oxo-2-[4-( 2-oxo-1, 4-
dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-ethyl}-malonate.
Yield: 11.8 g (93% of theory)
Rf = 0.20 (silica gel, EtOAc/MeOH/acetic acid 8:2:0.1 )
22i 2-(3-bromo-4-chloro-5-methyl-benzyl)-1-[4-(4-methyl-piperazin-1-yl)-
piperidin-1-yl]-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-
yl]-butan-1,4-dione
The product was prepared analogously to Example 2f from 1.09 g (2.00
mmol) 2-(3-bromo-4-chloro-5-methyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-
2H-quinazolin-3-yl)-piperidin-1-yl]-butanoic acid and 367 mg (2.00 mmol) 1-
methyl-4-piperidin-4-yl-piperazine.
Yield: 773 mg (54% of theory)
EI-MS: (M)+ = 712/714/716 (Br, CI)
Rf = 0.24 (silica gel, DCM/MeOH/NH3 9:1:0.1 )
The following compounds were prepared analogously from 2-(3-bromo-4-
chloro-5-methyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidin-1-yl]-butanoic acid and the corresponding amount of amine:
CA 02503455 2005-04-22
233
~N
O
N O
I
H
ExampleR Yield Mass R,
(t)
spectrum (silica gel)
22.1 ' ~ 74 711/13/150.63
N
N~~"3 [M]+ (DCM/MeOH/NH3
8:2:0.1)
22.2 ~ ~ ~ N 56 692/94/960.26
N
~ I
ON [M]+ (DCM/MeOH/NH3
9:1:0.1)
22.3 ' ~ 13 713/15/170.43
N
N~~~N~ [M+H]+ (DCM/MeOH/NH3
8:2:0.1)
Example 22.4
[4-( 1- f 2-(3-bromo-4-chloro-5-methyl-benzyl )-4-oxo-4-[4-(2-oxo-1,4-dihyd ro-
2H-quinazolin-3-yl)-piperidin-1-yl)-butyryl}-piperidin-4-yl)-piperazin-1-yl)-
acetic
acid
CA 02503455 2005-04-22
234
N ~N~N~O~H
O
N O
I
H
The product was obtained analogously to Example 16.4 from 109 mg (0.20
mmol) 2-(3-bromo-4-chloro-5-methyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-
2H-quinazolin-3-yl)-piperidin-1-yl]-butanoic acid and 51 mg (0.20 mmol) ethyl
(4-pi perid in-4-yl-piperazin-1-yl )-acetate.
Yield: 10 mg (6% of theory)
ESI-MS: (M-H)' = 755/757/759 (Br, CI)
Rf = 0.21 (silica gel, DCM/MeOH 9:1 )
Example 22.5
methyl (1'-{2-(3-bromo-4-chloro-5-methyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butyryl}-[4,4']bipiperidinyl-1-yl)-
acetate
cl
N~N N~Ow
%~ IOI I IO
N O
t
H
The product was obtained analogously to Example 16.5 from 220 mg (0.40
mmol) 2-(3-bromo-4-chloro-5-methyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-
2H-quinazolin-3-yl)-piperidin-1-yl]-butanoic acid and 102 mg (0.40 mmol) ethyl
[4,4']bipiperidinyl-1-yl-acetate.
CA 02503455 2005-04-22
235
Yield: 19 mg (18 % of theory)
ESI-MS: (M+H)+ = 770/772/774 (Br, CI)
Rf = 0.36 (silica gel, DCM/MeOH 9:1 )
Example 22.6
( 1'-{2-(3-bromo-4-chloro-5-methyl-benzyl )-4-oxo-4-[4-(2-oxo-1,4-di hydro-2H-
quinazolin-3-yl)-piperidin-1-yl]-butyryl}-[4,4']bipiperidinyl-1-yf)-acetic
acid
%~N ~O~H
0
N O
I
H
The product was obtained analogously to Example 16.6 from 204 mg (0.26
mmol) methyl (1'-{2-(3-bromo-4-chloro-5-methyl-benzyl)-4-oxo-4-[4-(2-oxo-
1,4-di hyd ro-2H-q ui nazoli n-3-yl )-pi peridi n-1-yl]-butyryl}-[4, 4'] bipi
perid inyl-1-yl )-
acetate.
Yield: 25 mg (12 % of theory)
ESI-MS: (M+H)+ = 756/758/760 (Br, CI)
Rf = 0.23 (silica gel, DCM/MeOH 9:1 )
Example 23
4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-carboxylic acid{1-(3-
bromo-4-chloro-5-methyl-benzyl )-2-[4-(4-methyl-piperazin-1-yl )-pi peridin-1-
yl]-
2-oxo-ethyl}-amide
CA 02503455 2005-04-22
236
CI
O \ Br
N~N N~~~N~/~
~N~
N H O
N O
I
H
23a ethyl2-amino-3-(3-bromo-4-chloro-5-methyl-phenyl)-propionate
hydrochloride
The product was prepared analogously to Example 14a from 6.06 g (22.2
mmol) N-(diphenylmethylen)-giycinethylester and 6.30 g (115 mmol) 1-bromo-
5-bromomethyl-2-chloro-3-methyl-benzene.
Yield: 5.82 g (77% of theory)
ESI-MS: (M+H)+ = 320/322/324
Rf = 0.70 (silica gel, DCM/MeOH/NH3 9:1:0.1 )
23b ethyl3-(3-bromo-4-chloro-5-methyl-phenyl)-2-{[4-(2-oxo-1,4-dihydro-
2H-quinazolin-3-yl)-piperidin-1-carbonyl]-amino}-propionate
The product was obtained analogously to Example 14b from 5.82 g (16.3
mmol) ethyl 2-amino-3-(3-bromo-4-chloro-5-methyl-phenyl)-propionate
hydrochloride and 3.77 (16.3 mmol) 3-piperidin-4-yl-3,4-dihydro-1H-
quinazolin-2-one.
Yield: 7.60 g (81 % of theory)
Rf = 0.52 (silica gel, DCM/MeOH/NH3 9:1:0.1 )
23c 3-(3-bromo-4-chloro-5-methyl-phenyl)-2-{[4-(2-oxo-1,4-dihydro-2H
quinazolin-3-yl)-piperidin-1-carbonyl]-amino}-propionic acid
The product was obtained analogously to Example 14c from 7.60 g (13.1
mmol) ethyl 3-(3-bromo-4-chloro-5-methyl-phenyl)-2-{(4-(2-oxo-1,4-dihydro-
2H-quinazolin-3-yl)-piperidin-1-carbonyl]-amino)-propionate.
Yield: 6.70 g (93 % of theory)
ESI-MS: (M-H)- = 547/549/551 (Br, CI)
Rr = 0.05 (silica gel, DCM/MeOH/NH3 9:1:0.1 )
CA 02503455 2005-04-22
237
23d 4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-carboxylic acid{1-(3-
bromo-4-chloro-5-methyl-benzyl )-2-[4-(4-methyl-piperazin-1-yl )-
piperidin-1-yl]-2-oxo-ethyl}-amide
The product was prepared analogously to Example 2f from 1.35 g (2.45
mmol) 3-(3-bromo-4-chloro-5-methyl-phenyl)-2-{[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-carbonyl]-amino}-propionic acid and 449 g (2.45
mmol) 1-methyl-4-piperidin-4-yl-piperazine.
Yield: 1.10 g (63% of theory)
ESI-MS: (M+H)+ = 714/716/718 (Br, CI)
R, = 0.41 (silica gel, DCM/MeOH/NH3 9:1:0.1 )
The following compounds were prepared analogously from 3-(3-bromo-4-
chloro-5-methyl-phenyl )-2-{[4-( 2-vxo-1, 4-d ihydro-2H-qui nazolin-3-yl)-pi
peridin-
1-carbonyl]-amino}-propionic acid and the corresponding amount of amine:
/ GI
-Br
R
N
I
H O
H
ExampleR Yield Mass R,
(%) spectrum (silica gel)
23.1 ~ 44 699/701/7030.40
~
N [M+H]+ (DCM/MeOH/NH3
~~N~,~
9:1:0.1 )
23.2 ' ~ 55 714!716/7180.24
N~ ~
~N~~~N~CH3 [M+H]+ (DCM/MeOH/NH3
9:1:0.1 )
CA 02503455 2005-04-22
238
ExampleR Yield (%) Mass R,
spectrum (silica get)
23.3 ' ~ 53 713/715/7170.37
N
N~~"3 [M+H]+ (DCM/MeOH/NH3
9:1:0.1)
23.4 ~ ~ ~ N 39 694!696/6980.49
N
~ I
N [M+H]' (DCM/MeOH/NH~
9;1:0.1 )
Example 24
2-(4-chloro-3-trifluoromethyl-benzyl )-1-( 1'-methyl-[4,4']bipiperidinyl-1-yl)-
4-[4-
(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butan-1,4-dione
/~N
''
N O
I
H
24a 1-methyl2-[1-(4-chloro-3-trifluoromethyl-phenyl)-meth-(E)-ylidene]-
succinate
20.7 mL (158 mmol) dimethyl succinate were added to a freshly prepared
sodium methoxide solution (prepared by dissolving 3.64 g (158 mmol) sodium
im MeOH) in 300 mL MeOH and the reaction mixture was stirred for 1 h at
RT. Then 30 g (144 mmol) 4-chioro-3-trifluoromethyl-benzaldehyde were
added and the reaction solution was refluxed for 6 h. It was evaporated down
i. vac., the residue was taken up in water, acidified with 20% citric acid
solution and extracted exhaustively with EtOAc. The organic phase was
extracted five times, each time with 200 mL of 3% NH3 solution, the combined
CA 02503455 2005-04-22
239
aqueous phases were acidified with citric acid solution, exhaustively
extracted
with EtOAc and dried over Na2S04. After the desiccant and solvent had been
eliminated the desired product was obtained in the form of a yellow oil.
Yield: 12 g (26% of theory)
Rf = 4.33 (silica gel, PE/EtOAc/AcOH 75:25:5)
24b 1-methyl2-(4-chloro-3-trifluoromethyl-benzyl)-succinate
200 mg 10°!° Pt/C were added to a solution of 2.0 g (6.2 mmof) 1-
methyl 2-[1-
(4-chloro-3-trifluoromethyl-phenyl)-meth-(E)-ylidene]-succinate in 20 mL
MeOH and the reaction mixture was hydrogenated at RT and 3 bar H2 for 3
h. The catalyst was filtered off and the solvent was evaporated down i.vac.
The crude product was further reacted without purification.
Yield: 1.85 g (92% of theory)
Rf = 0.38 (silica gel, PE/EtOAc/AcOH 75:25:5)
24c methyl2-(4-chloro-3-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-
dihydro-2H-quinazolin-3-yf )-piperidin-1-yl]-butanoate
A solution of 1.5 g (4.6 mmol) 1-methyl 2-(4-chloro-3-trifluoromethyl-benzyl)-
succinate, 1.64 g (5.1 mmol) TBTU, 0.69 g (5.0 mmol) HOBt and 1.32 mL (7.5
mmol) ethyldiisopropylamine in 100 mL of a THF/water mixture (9:1 ) was
stirred for 10 min at RT and then combined with 1.2 g (5.0 mmol) of 3-
piperidin-4-yl-3,4-dihydro-1 H-quinazolin-2-one. The reaction mixture was
stirred for 2 h at RT, evaporated down i. vac., the residue was combined with
saturated NaHC03 solution, exhaustively extracted with EtOAc and the
combined organic extracts were dried over MgS04. After the desiccant and
solvent had been eliminated the desired product was obtained, which was
further reacted without purification.
Yield: 2.2 g (89% of theory)
Rr = 0.6 (silica gel, DCM/MeOHlcyclNH3 70:15:15:2)
24d 2-(4-chloro-3-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-yi]-butanoic acid
16 mL 1 M NaOH solution were added to a solution of 2.2 g (4.1 mmol) methyl
2-(4-chloro-3-trifluoromethyl-benzyl )-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
CA 02503455 2005-04-22
240
quinazolin-3-yl)-piperidin-1-yl]-butanoate in 20 rnL MeOH and the reaction
mixture was stirred for 5 h at 50°C. It was diluted with 170 mL water,
extracted twice with 30 mL tert-butylmethylether, the aqueous phase was
combined with 16 mL 1 M HCI, extracted three times with 70 mL EtOAc and
the combined organic phases were dried over Na2S04. After the desiccant
and solvent had been eliminated the residue was triturated with
diisopropylether, suction filtered and dried in the air.
Yield: 1.1 g (51% of theory)
Rf = 0.25 (silica gel, DCM/MeOH/cyc/NH3 70:15:15:2)
24e 2-(4-chloro-3-trifluoromethyl-benzyl)-1-(1'-methyl-[4,4']bipiperidinyl-1-
yl)-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butan-1,4-
dione
Prepared analogously to 24c from 790 mg (1.5 mmol) 2-(4-chloro-3-
trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidin-1-yl]-butanoic acid and 370 mg (1.6 mmol) 1-methyl-
[4,4']bipiperidinyl.
Yield: 530 mg (51% of theory)
EI: (M)+ = 687/689 (CI)
Rf = 0.6 (silica gel, DCM/MeOH/cyc/NH3 70:15:15:2)
Example 24.1
2-(4-chf oro-3-trifluorom ethyl-benzyl )-1-[4-(4-methyl-pi perazin-1-yl)-pi
peridin-1-
yl]-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butan-1,4-
dione
~N N~Ni
N O
I
H
CA 02503455 2005-04-22
241
280 mg (1.53 mmol) 1-methyl-4-piperidin-4-yl-piperazine were added to a
solution of 800 mg (1.53 mmol) 2-(4-chioro-3-trifluoromethyl-benzyl)-4-oxo-4-
[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butanoic acid, 544
mg
(1.7 mmol) TBTU, 206 mg (1.53 mmol) HOBt and 0.69 mL (4.94 mmol)
triethylamine in 100 mL THF and the reaction mixture was stirred for 2.5 h at
RT. It was evaporated down i. vac., the residue was taken up in DCM, the
organic phase was washed twice with saturated NaHC03 solution and dried
over MgS04. After the desiccant and solvent had been eliminated the residue
was purified by chromatography (silica gel, MeOH).
Yield: 400 mg (38% of theory)
EI: (M)+ = 688/690 (CI)
Rf = 0.25 (silica gel, MeOH)
Example 24.2
1-[1,4']bipiperidinyl-1'-yl-2-(4-chloro-3-trifluoromethyl-benzyl)-4-[4-(2-oxo-
1,4-
dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butan-1,4-dione
N ~ N\~
O
N O
I
H
Prepared analogously to Example 24.1 from 800 mg (1.53 mmol) 2-(4-chloro-
3-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidin-1-yl]-butanoic acid and 271 mg (1.61 mmol) [1,4')bipiperidinyl.
Yield: 470 mg (46% of theory)
EI: (M)+ = 673/675 (CI)
Rf = 0.28 (silica gel, MeOH)
CA 02503455 2005-04-22
242
Example 24.3
[4-( 1-{2-(4-ch loro-3-trifluoromethyl-benzyl )-4-oxo-4-[4-(2-oxo-1,4-di hyd
ro-2H
quinazolin-3-yl)-piperidin-1-yl]-butyryl}-piperidin-4-yl)-piperazin-1-yl]-
acetic
acid
N ~O~H
O
N O
I
H
Prepared analogously to Example 16.4 from 105 mg (0.2 mmol) 2-(4-chloro-3-
trifluoromethyl-benzyl )-4-oxo-4-[4-(2-oxo-1, 4-di hyd ro-2H-q ui nazolin-3-yl
)-
piperidin-1-yl]-butanoic acid and 51 mg (0.2 mmol) ethyl (4-piperidin-4-yl-
piperazin-1-yl)-acetate.
Yield: 13 mg (8% of theory)
ESI-MS: (M+H)+ = 733/735 (CI)
Retention time (HPLC): 6.3 min (method A)
Example 24.4
methyl (1'-{2-(4-chloro-3-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-
dihydro-
2H-quinazolin-3-yl)-piperidin-1-yl]-butyryl}-[4,4']bipiperidinyl-1-yl)-acetate
CA 02503455 2005-04-22
243
CI
N ~\~'~~'C~N ~O ~
O
N O
I
H
Prepared analogously to Example 16.5 from 209 mg (0.4 mmol) 2-(4-chloro-3-
trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidin-1-yl]-butanoic acid and 102 mg (0.4 mmol) ethyl [4,4']bipiperidinyl-
1-
yl-acetate.
Yield: 17 mg (17% of theory)
ESI-MS: (M+H)+ = 746/748 (CI)
Rf = 0.44 (silica gel, DCM/MeOH 9:1 )
Example 24.5
( 1'-{2-(4-ch loro-3-trifluoromethyl-benzyl )-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-yl]-butyryl)-[4,4']bipiperidinyl-1-yl)-acetic
acid
~N ~O~H
O O
N O
I
H
Prepared analogously to Example 16.6 from 198 mg (0.26 mmol) methyl (1'-
{2-(4-chloro-3-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-
quinazolin-3-yl)-piperidin-1-yl]-butyryl)-[4,4']bipiperidinyl-1-yl)-acetate.
Yield: 19 mg (9% of theory)
CA 02503455 2005-04-22
244
ESI-MS: (M+H)+ = 732/734(CI)
Rr = 0.22 (silica gel, DCM/MeOH 9:1 )
Example 24.6
2-(4-chloro-3-trifluoromethyl-benzyl)-1-( 1'-methyl-[4,4']bipiperidinyl-1-yl)-
4-[4-
(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin-1-yl]-butan-1,4-dione
/~N ~~~~~N i
//
N O
I
H
Prepared analogously to Example 24.1 from 800 mg (1.53 mmol) 2-(4-chloro-
3-trifluoromethyl-benzyl)-4-oxo-4-[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-
piperidin-1-ylJ-butanoic acid and 279 mg (1.53 mmol) 1-methyl-
[4,4'Jbipiperidinyl.
Yield: 320 mg (30% of theory)
EI: (M)+ = 687/689 (CI)
Rr = 0.20 (silica gel, MeOH)
The following compounds may also be prepared by the processes described
hereinbefore:
Example Structure
25.1 ~F
~NHz
('
II
CFA
C
\
N ~ N
N
N~p
H
CA 02503455 2005-04-22
245
Example Structure
25.2 cF
~NHz
I
0 \
CF
N~N~
O V v ~Ni
0
N
~ N~p
H
25.3 pF,
~NHz
I
0 \
CFA
N O~N~N~N~
O
N
~ N~p
H
25.4 cF
~NHz
I
0 \
CFa
~p~N~~~.~~ i
N
O
N
I ~ Nk0
H
25.5 cF
~NHz
I
O \
CF
N O N~N~ ,H
~N
O
N
N~p
H
25.6 cF
~NHz
I
OII \
CFA
~O~N../~
~N~ 'H
N
0
N
N~p
H
25.7 pF=
~NHz
~ ~I pF' I
~N~O~N~Nw
' 0
//I
~~//
N
I ~ Nk0
H
CA 02503455 2005-04-22
246
ExampleStructure
25.8 cF
~NHz
('
I
\~
O
CFA
N O N N
NL
~ N~0
H
25.9 cF
~NHz
('
II
\
O
CFo~N
N O N~
~N
0
NL
~ N~0
H
25.10 cIF,
~NHz
('
II
O
\
CF
N 0 N\~:%~~N'H
N 0
N~O
H
ExampleStructure
26.1 ~F
~NH?
('
I
\~
O
Br
N 0 N N
N
N~p
H
26.2 cF,
~NHz
r'
I
\~
0
Br
N O N~N~N~
O
NL
~ N~O
H
26.3 ~F
~NHz
('
I
\~
0
Br
N O~N~N~Ni
I'
0
N
N~p
H
CA 02503455 2005-04-22
247
Example Structure
26.4 cF
~NHz
\ I
Br
0
N O N~%'~~~N~
O
NL
I ~ N~0
H
26.5 cF,
~NHz
\ I
O
Br
N O N~N~N~H
0
N
I ~ NkO
H
26.6 cv
~NHz
\ l
OII
Br
N~O~N
~N~ 'H
N
0
N
I ~ Nko
H
26.7
~NH~
\ I
0II
Br I
~N~O~N~NW
I
I 0
~/
N
I ~ N~p
H
26.8 cIF
~NHz
\ II
O
8r
N 0 N N
O
N
I ~ N~0
H
26.9 cF,
~NHz
~
O \
Br
N O~N~N \ I
''O
N
I ~ N~p
H
CA 02503455 2005-04-22
248
Example Structure
26.10 cIF
~NHz
II
O \
8r
N 0 N~~:%~ 'H
N
0
N
/ v Nk0
H
Example Structure
27.1 cF
~NHz
~ I
O
Br
N N N N
H 0
N
~ N~p
H
27.2 cF
~NHz
\ I
O
Br
N N N~N~N~
I
H O
N
N~0
H
27.3 cF
~NHz
~ I
0 _
Br
N../~ ~./~
N N~ ~/'~N~N~
H O
N
N~0
H
27.4 cF
~NHz
(/\ II
O
Br
N N \~.%~"~N i
H 0
N
~ N~p
H
27.5 cIF
~NH2
('~~ ~
O
Br
N N N~N~N'H
H O
N
N~p
H
CA 02503455 2005-04-22
249
Example Structure
27.6
~NHi
\ I
Br
O
N~
N N ~N~N~H
H 0
N
r ~ Nk0
H
27.7 c'F
~NHz
\ II
Br I
OII
~N~N~NW
H 0
N
r v Nkp
H
27.8 cF
~NHz
\ I
Br
0
N N N N
H O
N
r ~ N~O
H
27.9 c~F
~NHz
II
0 \
Br ~N
N N~N~N \ I
H IIO
N
r ~ Nko
H
27.10 pF
~NHz
\ I
o
Br
N~%'~~~ ~H
N . N
H 0
N
r ~ N~p
H
Example Structure
28.1 pF>
~NHMe
I
O \
Ci
N N N
H O
N
r ~ NCO
H
CA 02503455 2005-04-22
250
Example Structure
28.2 ~~F
/."NHMe
II
CI
p~~ \
~N~N~N~.'Ni
H II0
N
~ N~O
H
2s.s ~F
~NHMe
I
pI //''~~~
CI
~N~N~N~N~N~
I H O
/
Nf
N~O
H
28.4 cF
~NHMe
I
CI
p ~
N ~~
~N~ \~!'~~Ni
H 0
N
I ~ N~O
N
28.5 cF,
~NHMe
I
CI
OII _ ~
~N~N~N~H
~N
H O
NI
I~~ N~O
H
28.6 'F
~NHMe
0 ~ I CI
~N~N~N~N~NH
I
I H O
~/
N
I ~ N~O
H
28.7 cF
~NHMe
'
0I //''\~~
CI
//~~.. x' N~N~
~N~N ~/'~~
H 0
N
~ N~O
H
CA 02503455 2005-04-22
251
Example Structure
28.8 C~F
/.NHMe
I~
OII \
CI
NV "' NV
~N~N~
I I
I
H O
N
~/
I ~ Nip
H
28.9 cF
~NHMe
O ~ I CI ~N
N N~N~
~
N
H O
N
I ~ Nko
H
28.10 CF
~NHMe
I
0 \
CI
N N N\~~%'~ iH
I N
N H O
/ ~ N~0
H
Example Structure
29.1 pF
~NHMe
O \ ~ CI
N N
N O
N~p
H
29.2 pF3
~NHMe
O ~ ~ CI
~N~N~N~
N O
/ ~ N~p
H
29.3
~NHMe
OI //~~\//~~~CI
~N~N.~
~1(~l, ' ~ ~N ~N
N O
/ v Nk0
H
CA 02503455 2005-04-22
252
Example Structure
29.4 cF
~NHMe
0 \ I CI
N /
N N
N O
/ ~ N~p
H
29.5 cIF
~NHMe
0 \ II CI
N N~N~N'H
N 0
/ v Nk0
H
CF
29.6 ~NHMe
/
0 \ CI
N~N~N~ 'H
N
N O
/ ~ N~p
H
29.7 cIF
~NHMe
0 \ II CI I
~N~N N\
N 0
/ ~ N~O
H
CF
29. ~ ~ NHMe
0 \ CI
N N N
N O
/ ~ N~p
H
29.9 cF
~NHMe
0 \ I CI ~N
N~H~N//\ SS
N
/ ~ N~0
H
CA 02503455 2005-04-22
2SJ
Example Structure
29.10 CIF
~NHMe
0 //II(~\~~I CI
N N~~~~-~ ,H
N
N Q
N~O
H
Example Structure
30.1 cF
~NHMe
~
O \
CI
N O N N
O
Nj
N~O
H
30.2 CF
~NHMe
I
O \
CI
N O N NON
O
N
N~O
H
30.3 cIF
~NHMe
I~
CI
OI' \
N~Q~N~
~N~N~
0
N
N~0
H
30.4 cF
~NHMe
~
O'I \
CI
u ~N~~~~.~ i
~N~O~ N
0
N'
~ N~p
H
30.5 CF
~NHMe
~
O \
CI
N 0 N N../~ ,H
~N
N O
I ~ N~0
H
CA 02503455 2005-04-22
254
Example Structure
30.6 cF
~NHMe
I
CI
0 \
~~.... N~ ~ H
~N~O~ ~N N
I
' 0
ce/
N
I ~ N~O
H
30.7 cF
~NHMe
~
CI
0 \
~N~O~N~Nw
I
~ I I0
~/
N
N~O
H
30.8 cF,
~NNMe
~
CI
0 \
N O N N
0
N
r v Nko
H
30.9
~NHMe
O _ \ ' CI
N O~N~
r
N
I
' 0
ce/
N
I ~ N~0
H
30.10 cIF
~NHMe
II
O \
CI
N~%'~.~ ~H
O . N
N 0
I ~ Nko
H
The following compounds may be obtained using 4-amino-3-chloro-5-methyl-
benzoic acid as starting material:
Example ~ Structure
CA 02503455 2005-04-22
255
Example Structure
31.1
~NH~
0 ~ ~ CI
N N N
N[ O
N~p
N
31.2
~NHz
0 ~ I CI
N N~N~N/
N 0
r v N.~Q
H
31.3
~NHz
OI' ~ I CI
~N~ /~.~
N '' ~ ~N~N/
N O
~ N~O
H
31.4
~'NHz
O f'\ I~ CI
N /
N N
N O
~ N~0
H
31.5
~NHz
O \ ~ CI
N~N~N.H
N
N O
~ N~p
H
31.6
~NHz
OII ~ I CI
~N~
N ~N~N~H
N VO
r v Nko
H
CA 02503455 2005-04-22
256
Example Structure
31.7
~NH?
0 I'\~~ CI I
~N~N Nw
N O
N~p
H
31.8
~NHz
O /'\ II CI
N N N
Nl
~ N~p
H
31.9
~NHz
O I'\~I CI ~N
N~N~N \ I
NL - v ~O
~ N~p
H
31.10
~NHz
O /'\ I~ CI
N N~%'~~~N'H
N O
N~p
H
Example Structure
32.1
NHz
\ I
CI
0II
~N~H~N~N~
N
N~O
H
32.2
~NHz
\ I
CI
O _
N~N,..~
N N ~Ni
H O
N
I ~ N~p
H
CA 02503455 2005-04-22
25 7
Example Structure
32.3 ~NHz
/
~
O ~
CI
N N~N~N~N/
~~I
H 0
N
N~O
H
32.4
~NHz
I~
CI
I IN N ll ~\r/~N/
H O
N
I ~ N~0
H
32.5
~NH~
/'\~I
CI
0
N N N~N~N~H
H O
N
~ N~0
H
32.6 ~NHz
II
~
O _
CI
~ ~N~ ,H
~N N 1f ~N~N
H ~~O
N
N~0
H
32.7
~NHz
I'\~I
OI
CI
~N ~N ~N~N W
H ~ ~0
N
N~0
H
32.8
~NH~
I'\~I
0
CI
N N
N
~ N~O
H
CA 02503455 2005-04-22
258
Example Structure
32.9 ~NHz
CI / N
~ ~N./~ ~ I
N N if ~N
H ~~O
N
~ N~0
H
32.10
~NH7
~
O \
CI
N N N\.:%~~ ,H
N
H 0
N
N~0
H
Example Structure
33.1
~NHz
I'~~I
p
CI
N O N N
O
N
I ~ Nko
H
33.2 NH,
~ I
o
c.
N O N~N~N~
O
N
N~0
N
33.3 ~NHz
CI
-~~'N~
N 0 11 ~N~Ni
O
N
N~p
H
33.4
~NHz
I-\~I
0
CI
N 0 N~%'~~~N~
O
N(
N~0
H
CA 02503455 2005-04-22
259
Example Structure
33.5
~NHz
I
CI
O'I \
~, N~N~N'H
~N~O
I 0
//I
~~//
N
I ~ N~O
H
33.6
~NHz
~
CI
O~~ \
N~
~N~O~ \/~'N NiH
)) 'l0
1
1
~
..//
N ~
I ~ Nko
H
33.7
~NHz
'
CI
OI' \
~N~O~N~N\
I
/I
~
N
/ v Nk0
H
33.8
~NHz
I
CI
O \
N 0 N
O
N
L-~ N~O
H
33.9
~NHz
~
CI
0 \
N O~N~N \ I
0
N
~~ N~0
H
33.10
~NH7
I
CI
0 \
N~%'~~~ ~H
O N
O
N
I~~ N~0
H
The following compounds may be obtained using 2-chloro-6-trifluoromethyl-
phenol as starting material, if necessary blocking the phenolic hydroxy
CA 02503455 2005-04-22
260
function using a suitable protective group:
Example Structure
34.1 cF
~OH
O \ ~ CI
~N~N~
O
N
/ v Nk0
H
34.2 cF
~OH
O \ ~ CI
~N~N~N/
NJ O
~ N~O
H
34.3 cF
~OH
CI
~N~N~N/
O
N[~
~ N~p
H
34.4
~OH
o ~ I a
N
N
0
N
.~ N~O
H
34.5 cF
~OH
o ('~~I ~ci
N
~N~N~N~H
0
N(~
N~p
H
34.6 cF
~OH
O \ ~ CI
N~N~N~H
N O
N~p
H
CA 02503455 2005-04-22
261
Example Structure
34.7
~OH
0 \ ~ CI I
N N Ny
N O
/ \ Nk0
H
34.8 cIF
/\ 'OH
0 \ I~ CI
N N N
N( 0
/ \ N~0
H
34.9 F>
~OH
O \ I CI ~N
\
N~N~N
N O
/ \ Nk0
H
34.10 I
~OH
O \ I~ CI
N N~~~~~~ ~H
N
N O
/ ~ N~O
H
Example Structure
35.1 cIF
~OH
('
II
OI
\
CI
~, I N~N
~N~N~
H O
N
/ \ N~0
H
35.2
~OH
('\ ''
O
CI
N~ N "/~
N N ~N
I
H O
N
\ N~O
H
CA 02503455 2005-04-22
262
Example Structure
35.3 C'F
~OH
\ I~
CI
0
N N~N~N~N~
H 0
N
I ~ N~p
H
35.4 pF
~OH
' ~
CI
0~~
// ~N~.~~.i.~ i
~N~N~ N
H O
N
~ N~0
H
35. 5 CF,
~OH
\ ~
O
CI
N~N~N~N~N
H O
N
/ v Nk0
H
35.6 CIF
~OH
\ I~
O'I _
CI
N~N N~./~
~N~N~H
I
H O
N
I ~ N~p
H
35.7
~OH
~ ~
OII
CI
N~Nw
~N~N~
H O
N
r v Nkp
H
35.8 cF
~OH
r'~~~
0
CI ~
N~N
~N~N~
H O
N
N~0
H
CA 02503455 2005-04-22
263
ExampleStructure
35.9 cF,
~oH
I'
O _
\ II CI ~N
N ~N~ ~ I
I II ~N
H O
N
N~p
H
35.10 ~F3
~OH
['
O~
\ II CI
[/ ~N~%'~~~ ~H
N/\N II N
I
H O
N
V
N~0
H
Example Structure
36.1 CIF,
~OH
CI
~N O~N~N
I
~l
N
~
N ~
p
H
36.2 CF,
~OH
CI
N O~N~N~Ni
O
'
V
N
I ~ Nk0
H
36.3 cIF,
~OH
('
\~I CI
0
N O~N~N~Ni
~ ~
NL
O
N~O
H
36.4 CF3
~OH
r'
\~I CI
O
N O N~''~~N ~
O
N
N~p
H
CA 02503455 2005-04-22
264
Example Structure
36.5 p
~OH
('\ I~
O
CI
N O N~N~N'N
O
Nl
N~p
H
36.6 pF,
~OH
('~~~
O
CI
N ,H
N O ~N~N
0
NL
~ N~p
H
36.7 cF,
~OH
r'~~~
OI
CI
~N~O~N~N W
I O
~1
~
N
r v Nkp
H
36.8 cIF
~OH
['~~I
O
CI
N O N N
NL
~ N~p
H
36.9 pF
~oH
I'\~I
pI
C~ ~N
~N~O~N~N
I O
~1
~
NL
N~p
H
36.10 pF,
~oH
I'~~I
o
a
N O N\~~%~~N'H
O
N
r v N.~p
H
Example 37
CA 02503455 2005-04-22
265
(S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-methyl-piperazin-1-
yl )-pi peridin-1-yl]-4-[4-( 2-oxo-1,4-d ihyd ro-2H-thieno[3, 2-d] pyri midi n-
3-yl )-
piperidin-1-yl]-butan-1,4-dione
C F3
NHZ
O \ CI
%~N ~/ N
N \r~ ~j N,CH3
S ~~ O
N O
H
Example 37.1
( S)-2-(4-am i no-3-chloro-5-trifl uoromethyl-benzyl )-1-[4-(4-methyl-
piperazin-1-
yl )-pi peridin-1-yl)-4-[4-( 5-oxo-4, 5, 7, 8-tetrahyd ro-2-this-4, 6-d iaza-
azu len-6-yl )-
piperidin-1-yl]-butan-1,4-dione
CF3
NHZ
O \ CI
~~~N\~~N
~N ~N.CH3
/I O
N
\O
S / N
H
Examale 37.2
(S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl )-1-[4-(4-methyl-piperazin-1-
yl)-piperidin-1-yl]-4-[4-(2-oxo-1,2,4,5-tetrahydro-thieno[3,2-d)-1,3-diazepin-
3-
yl )-piperidin-1-yl]-butan-1, 4-d lone
CA 02503455 2005-04-22
266
CF3
NHz
O CI
%~N\ ~N~
~N ~-N,CH3
O
N
S ~ N~O
H
Example 37.3
( S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl)-1-[4-(4-methyl-piperazin-1-
yl )-pi perid i n-1-yl]-4-[4-(2-oxo-1,2,4, 5-tetrahyd ro-th ieno[2, 3-d]-1, 3-
diazepin-3-
yl)-piperidin-1-yl]-butan-1,4-dione
CF3
NHZ
O \ CI
!~N~~~N\~~
~~N _ ~ ~N.,CH3
O
N
N~o
S H
Example 37.4
( S)-2-(4-amino-3-chloro-5-trifluoromethyl-benzyl )-1-[4-(4-methyl-piperazin-1-
yl)-piperidin-1-yl]-4-[4-(2-oxo-1,4-dihydro-2H-thieno[2,3-d]pyrimidin-3-yl)-
piperidin-1-yl]-butan-1,4-dione
CA 02503455 2005-04-22
267
CF3
NHZ
O \ CI
/~N ~N.CH3
~N~~~N~
/J O
N O
H
Exam Ip a 37.5
( S)-2-(4-ami no-3-chloro-5-trifl uoromethyl-benzyl )-1-(4, 4-difluoro-1, 4'-
bipiperidinyl-1'-yl)-4-[4-(2-oxo-1,2,4,5-tetrahydro-1,3-benzodiazepin-3-yl)-
piperidin-1-ylJ-butan-1,4-dione
CF3
NHZ
O CI
N~~N\~~N F
O
~N F
N~O
H
CA 02503455 2005-04-22
268
The following Examples describe the preparation of pharmaceutical
formulations which contain as active substance any desired compound of
general formula (I):
Example I
Capsules for Qowder inhalation containing 1 ma of active ingredient
Composition:
1 capsule for powder inhalation contains:
active ingredient 1.0 mg
lactose 20.0 mg
hard gelatine capsules 50.0 ma
71.0 mg
Method of preparation:
The active ingredient is ground to the particle size required for inhaled
substances. The ground active ingredient is homogeneously mixed with the
lactose. The mixture is transferred into hard gelatine capsules.
Example II
Inhaiable solution for Respimat~ containing 1 ma of active ingredient
Composition:
1 puff contains:
active ingredient 1.0 mg
benzalkonium chloride 0.002 mg
disodium edetate 0.0075 mg
purified water ad 15.0 NI
Method of preparation:
CA 02503455 2005-04-22
269
The active ingredient and benzalkonium chloride are dissolved in water and
transferred into Respimat~ cartridges.
Example III
Inhalable solution for nebulisers containing 1 mct of active ingredient
Composition:
1 vial contains:
active ingredient 0.1 g
sodium chloride 0.18 g
benzalkonium chloride 0.002 g
purified water ad 20.0 ml
Method of preparation:
The active ingredient, sodium chloride and benzalkonium chloride are
dissolved in water.
CA 02503455 2005-04-22
270
Example IV
Propellant ctas-operated metering aerosol containing 1 ma of active ingredient
Composition:
1 puff contains:
active ingredient 1.0 mg
lecithin 0.1
propellant gas ad 50.0 NI
Method of preparation:
The micronised active ingredient is homogeneously suspended in the mixture
of lecithin and propellant gas. The suspension is transferred into a
pressurised container with a metering valve.
Example V
Nasal sgray containing 1 mg of active in reQ dient
Composition:
active ingredient 1.0 mg
sodium chloride 0.9 mg
benzalkonium chloride 0.025 mg
disodium edetate 0.05 mg
purified water ad 0.1 ml
Method of preparation:
The active ingredient and the excipients are dissolved in water and
transferred into a suitable container.
CA 02503455 2005-04-22
271
Example VI
Injectable solution containing 5 ma of active substance per 5 ml
Composition:
active substance 5 mg
glucose 250 mg
human serum albumin 10 mg
glycofurol 250 mg
water for injections 5 ml
ad
Preparation:
Giycofurol and glucose are dissolved in water for injections (Wfl); human
serum albumin is added; active ingredient is dissolved with heating; made up
to specified volume with Wfl; transferred into ampoules under nitrogen gas.
Example VII
Inlectable solution containing 100 mg of active substance per 20 ml
Composition:
active substance 100 mg
monopotassium dihydrogen phosphate
= KH2POq, 12 mg
disodium hydrogen phosphate
= Na2HP04~2H20 2 mg
sodium chloride 180 mg
human serum albumin 50 mg
Polysorbate 80 20 mg
water for injections ad 10 ml
CA 02503455 2005-04-22
272
Preparation:
Polysorbate 80, sodium chloride, monopotassium dihydrogen phosphate and
disodium hydrogen phosphate are dissolved in water for injections (Wfl);
human serum albumin is added; active ingredient is dissolved with heating;
made up to specified volume with Wfl; transferred into ampoules.
Example VIII
Lyophilisate containing 10 mc~of active substance
Composition:
Active substance 10 mg
Mannitol 300 mg
human serum albumin 20 mg
water for injections ad 2 ml
Preparation:
Mannitol is dissolved in water for injections (Wfl); human serum albumin is
added; active ingredient is dissolved with heating; made up to specified
volume with Wfl; transferred into vials; freeze-dried.
Solvent for iyophiiisate:
Polysorbate 80 = Tween 80 20 mg
mannitol 200 mg
water for injections ad 10 ml
Preparation:
Polysorbate 80 and mannitol are dissolved in water for injections (Wfl);
transferred into ampoules.
CA 02503455 2005-04-22
273
Example IX
Tablets containing 20 mg of active substance
Composition:
active substance20 mg
lactose 120
mg
maize starch 40 mg
magnesium stearate2 mg
Povidone K 25 18 mg
Preparation:
Active substance, lactose and maize starch are homogeneously mixed;
granulated with an aqueous solution of Povidone; mixed with magnesium
stearate; compressed in a tablet press; weight of tablet 200 mg.
Example X
Capsules containing 20 mg active substance
Composition:
active substance 20 mg
maize starch 80 mg
highly dispersed silica 5 mg
magnesium stearate 2.5 mg
Preparation:
Active substance, maize starch and silica are homogeneously mixed; mixed
with magnesium stearate; the mixture is packed into size 3 hard gelatine
capsules in a capsule filling machine.
CA 02503455 2005-04-22
274
Example XI
Suppositories containing 50 mg of active substance
Composition:
active substance 50 mg
hard fat (Adeps solidus) q.s. ad 1700 mg
Preparation:
Hard fat is melted at about 38°C; ground active substance is
homogeneously
dispersed in the molten hard fat; after cooling to about 35°C it is
poured into
chilled moulds.
Example XII
Iniectable solution containing 10 mq of active substance per 1 ml
Composition:
active substance 10 mg
mannitol 50 mg
human serum albumin 10 mg
water for injections ad 1 ml
Preparation:
Mannitol is dissolved in water for injections (Wfi); human serum albumin is
added; active ingredient is dissolved with heating; made up to specified
volume with Wfl; transferred into ampoules under nitrogen gas.