Note: Descriptions are shown in the official language in which they were submitted.
CA 02504559 2005-04-20
DYE SOLUBILIZATION BINDING ASSAY
FIELD OF THE INVENTION
The present invention relates to binding assays, in particular
immunoassays, where a target analyte is bound to a capture agent via
specific forces. The colourimetric and fluorometric assays disclosed herein
employ the solubilization of bound dye particles for the amplification of the
detected optical absorbance or fluorescence signal and the control of the dye
particle radius for optimization of sensitivity and dynamic range.
BACKGROUND OF THE INVENTION
Binding assays have found widespread use in the detection and
quantification of analytes in a multitude of industries. The success of
binding
assays over alternative assay methodologies is owed to their ability to
provide
rapid, selective, sensitive and quantitative detection of a wide array of
target
species, ranging from small molecules to complex cellular antigens.
The most commonly used binding assay is the immunoassay, in which
antibodies are employed to bind and immobilize a target analyte. Antibodies
are protein molecules that are produced by an organism for the purpose of
identifying and isolating an antigen that may pose a danger to the host
organism. Antibodies have unique chemical and spatial structures designed
to bind to target antigens with high affinity and specificity. This property
of an
antibody can be exploited to produce a highly specific and sensitive assay for
a target analyte. For example, antibodies adsorbed onto a surface will
CA 02504559 2005-04-20
capture and immobilize target analyte present in a sample. Although
polyclonal and monoclonal antibodies provide an excellent means of
capturing a target analyte, alternative binding assay receptors exist. These
capture agents include oligonucleotides, phage display of antibody fragments,
bacterial display of peptides and proteins, molecular imprinted polymers (MIP)
and aptamers.
Regardless of the type of receptor used in a binding assay, a reporter
or label agent is required to detect and measure the presence of the bound
analyte. A wide variety of label technologies have been applied to
immunoassays, enabling detection via optical, electronic, chemical or physical
phenomena.
Although radioactive tracer labels were initially used for
immunoassays, the hazardous nature of the radioactive isotopes hampered
their adoption in widespread rapid testing. Enzymes have enjoyed
remarkable success as immunoassay reporter molecules due to their ability to
catalyze chemical reactions that produce large measurable signals. Since an
enzyme is not consumed in a chemical reaction, it is capable of producing
numerous reaction products from a single binding event. This multiplicative
feature of enzyme-based immunoassays provides amplification and offers
increased sensitivity. Following the catalysis of a chemical reaction in the
presence of a bound enzyme, optical phenomena including chromogenesis
(wavelength-dependent absorption), chemiluminescence, or fluorescence can
occur. The most commonly used enzymes are horseradish peroxidase or
alkaline phosphatase. Fluorescent molecules (fluorophores) are also often
2
CA 02504559 2005-04-20
used as reporter molecules in immunoassays. Although fluorescence offers
the ability to measure very low analyte concentrations with wide dynamic
range, results are often compromised by a large background signal caused by
autofluorescence (endogenous sample fluorescence), light-scattering effects,
and non-specific binding.
The flexibility provided by the different labeling technologies has led to
the development of many immunoassay formats for rapid screening and
clinical testing. The most widely used immunoassay is the enzyme-linked
immunosorbent assay (ELISA), which is typically a two-site binding or
"sandwich" assay. Antibodies bound to a solid surface capture and
immobilize antigens, which then capture a second antibody that is labeled
with an enzyme. The enzyme catalyzes a reaction with a chromogenic
substrate that leads to wavelength-dependent absorption, producing a colour
change. Alternatively, the enzyme can catalyze a chemiluminescent reaction.
ELISA offers inexpensive assays incorporating amplification, but with the
disadvantages of requiring multiple wash steps, temperature dependence,
poor repeatability owing to its multi-step nature, problems with reagent
consistency, demanding storage requirements, and long incubation times.
Other assay formats involving fluorescence include phase-resolved
fluorescence (PRF), time-resolved fluorescence (TRF), and fluorescence
polarization (FP). The first two of these methods exploit fluorophores with
very long spontaneous emission lifetimes. For example, in time-resolved
fluorescence, the sample is subjected to a pulsed light source and the
fluorescence signal is integrated only after waiting for the autofluorescence
3
CA 02504559 2005-04-20
signal to decay. This provides a means of isolating the primary fluorescence
(autofluorescence) from the desired secondary fluorescence signal,
increasing the signal-to-noise ratio. Typical fluorophores used in such assays
are lanthanide chelates such as europium, samarium, terbium and
dysprosium. Although time- and phase-resolved fluorescence assays provide
enhanced sensitivity and shorter acquisition time relative to ELISA, existing
methods do not incorporate a means of amplification. Unlike temporally
sensitive fluorescence assays, fluorescence polarization assays detect the
polarization of light emitted by fluorophore labels. If a labeled molecule is
bound through an antigen-antibody interaction, it is unlikely to undergo
rotation upon the absorption of excitation light. This causes the emitted
fluorescence to be polarized, allowing for the discrimination of bound and
unbound fluorophores based on the degree of polarization of the collected
fluorescence. Unfortunately, FP assays, which do not provide amplification,
are only suitable for small analyte molecules since large molecules in
solution
are less likely to rotate and act as a source of polarized background
fluorescence.
Each of the above immunoassay technologies possesses deficiencies
related to one or more of the following effects: long and laborious process
steps, inconsistency in producing reagents, poor signal amplification, large
background, difficulty in storage, complexity in instrumentation, poor
accuracy
and long incubation or acquisition times. What is required is an assay format
that provides an amplified detection scheme without the drawbacks listed
above. A step towards this goal was recently achieved by Trau and
4
CA 02504559 2005-04-20
coworkers, who described a novel immunoassay format employing
nanoencapsulated microcrystalline particles for large amplification in a
fluorescence assay (D. Trau et al., DE10042023 (2003), D. Trau et al.,
"Nanoencapsulated Microcrystalline Particles for Superamplified Biochemical
Assays", Anal. Chem. 74, 5480 (2002)). Their method involves a sandwich
assay using antibody-coated nanoencapsulated crystalline fluorogenic
precursor particles as labels. Such fluorogenic precursor label particles are
capable of producing a very large number of dye particles upon solubilization,
dramatically amplifying the measured fluorescence signal relative to that of
an
assay with a directly labeled fluorophore. The method offers an improvement
over prior attempts at fluorophore amplification that were plagued by
experimental difficulties and high cost, such as liposome encapsulation (H. A.
Rongen et al., "Liposomes and Immunoassays", J. Immunol. Methods 204,
105 (1997)) and perylene microparticles prepared via precipitation in an
antibody-rich solution (A. Kamyshny and S. Magdassi, "Chemiluminescence
Immunoassay in Microemulsions", Colloids Surf. B 11, 249 (1998)).
Unfortunately, the method requires that the microcrystalline particles are
coated using a complex and labor-intensive "layer-by-layer" procedure for
sufficient encapsulation and colloidal stabilization. Furthermore, the
microparticles exist in a wide distribution of sizes, ranging from ~ 100 nm to
1.5 wm, producing non-optimal binding, washing and amplification. Finally,
the solubilization of the dye is done in dimethyl sulfoxide, a hazardous
solvent
that may limit the usefulness of the approach.
5
CA 02504559 2005-04-20
A simpler and more effective approach to amplification through
solubilization involves the use of colloidal dyes. Such dyes, also known as
textile dyes, are non-toxic, inexpensive and widely available. Their detailed
chemistry is well known and may be tailored for the attachment of a wide
variety of antigens, antibodies and aptamers. Furthermore, they have
excellent optical properties including strong visible absorption and efficient
fluorescence (upon solubilization). Many different dyes exist with a broad
range of colours for assay multiplexing. The use of colloidal dyes in
immunoassays was pioneered by Gribnau and coworkers (T. C. J. Gribnau et
al., US4373932 (1983), T. Gribnau et al., "The Application of Colloidal Dye
Particles as Labels in Immunoassays: Dispersed) Dye Immunoassays
("DIA")", in T. C. J. Gribnau, J. Visser and R. J. F. Nivard (Eds.), Affinity
Chromatograph and Related Techniques. Elsevier, Amsterdam, 411 (1982),
and T. Gribnau, A. van Sommeren and F. van Dinther, "DIA - Disperse Dye
Immunoassay", in I. M. Chaiken, M. Wilchek and I. Parikh (Eds.), Affinity
Chromatography and Biological Recognition, Academic Press, Orlando, FL,
375 (1983)). The dispersed dye immunoassay (DIA) described in US patent
4373932 (now in the public domain) involves the use of water-dispersible
hydrophobic dye particles, which are coated with antibodies, as labels in a
heterogeneous sandwich immunoassay. Such dyes can be drawn from a
wide variety of water-dispersible classes, including disperse dyes, transfer
dyes, fat dyes (solvent dyes), vat dyes, organic pigments, sulfuric dyes,
mordant dyes, solubilized (leuco) vat dyes, solubilized (leuco) sulphur dyes,
azoic dyes, oxidation bases and ingrain dyes. Various methods can be used
6
CA 02504559 2005-04-20
to successfully bind antibodies to the surface of the dye particles without
reducing their effective immunochemical activity.
Most importantly, as taught by US patent 4373932, solubilization of
the dye particles in an appropriate buffer (e.g. an organic solvent)
dramatically intensifies the measured absorbance. This prior art, however,
does not disclose a method of conducting an assay in which the fluorescence
of solubilized dye is measured. The solubilization of dye is critical, because
when in colloidal form, the close proximity of dye molecules leads to rapid
non-radiative decay. This process severely quenches the fluorescence of any
excited dye molecules and dramatically lowers the fluorescent signal.
Conversely, the solubilization of dye particles into a dye solution physically
separates adjacent molecules and enables efficient and strong fluorescence.
Furthermore, the pH of the dye solution must be accurately controlled in order
to enable efficient fluorescence. The enhanced absorption and efficiency of
fluorescence of dye in a solubilized form therefore leads to a large
enhancement of the fluorescence signal and thus the sensitivity of the assay.
Despite this potential for a superior immunoassay based on the
solubilization of bound dye particles, the use of colloidal dyes in
immunoassays has been primarily restricted to lateral flow assays. Such
assays, also known as dipstick assays, are highly useful in field applications
where spectrophotometric equipment, refrigeration and trained personnel are
not available. Such assays were initially described by Snowden and Hommel
(K. Snowden and M. Hommel, "Antigen Detection Immunoassay Using
Dipsticks and Colloidal Dyes", J. Immunol. Methods 140, 57 (1991 )), using a
7
CA 02504559 2005-04-20
procedure known as the dipstick colloidal dye immunoassay. Instead of
solubilizing bound dye particles and measuring absorbance as proposed by
Gribnau and coworkers, the dipstick colloidal dye immunoassay uses a
nitrocellulose membrane coated with antibodies that is exposed to analyte in
the sample. The membrane is then incubated in a colloidal solution of dye
particles coated with antibodies, which are bound by the analyte. After
washing the membrane in water, the unbound dye particles are removed and
the presence of bound dye particles causes a visible colour change.
Although such assays are ideal for field applications, they do not answer the
need of clinical settings that require a quantitative and sensitive assay.
Therefore, the use of colloidal dyes in immunoassays has to date been
rather limited and considerable opportunities remain for their usage in highly
sensitive immunoassays with amplification. As disclosed in this invention, an
improvement over the prior art method, involving measuring fluorescence
from solubilized dye and controlling the dye particle radius, leads to a
dramatic improvement in the sensitivity, dynamic range, detection limit,
selectivity and accuracy of the assay.
8
CA 02504559 2005-04-20
SUMMARY OF THE INVENTION
Accordingly, the present invention provides a method of conducting an
assay for the detection of a target analyte with enhanced sensitivity, dynamic
range, detection limit, selectivity and accuracy using a sandwich assay
format. A liquid sample is first brought into contact with a solid phase,
where
the solid phase is coated with receptors that have a high affinity for an
analyte
that may be present in the sample. After an incubation period in which the
analyte binds to the receptors, and is thereby immobilized onto the solid
phase, a colloidal solution of dye particles is introduced. The dye particles
are coated with a second type of receptor that also has a high affinity for
the
analyte, but a low affinity for the first receptor and also a low affinity for
the
solid phase. The dye particles therefore bind to the analyte and become
immobilized onto the solid phase. The solid phase is then separated from the
liquid phase, which in tum separates the bound dye particles from the
unbound dye particles. A solubilization buffer, maintained at an appropriate
pH, is then added to solubilize the bound dye particles, creating a dye
solution. The fluorescence of the resulting dye solution is measured, wherein
the solubilized dye molecules strongly absorb excitation light and emit light
with high efficiency, and the concentration of the analyte is determined using
a pre-determined standard curve. Thus, the present invention provides an
assay for a target analyte comprising the steps of:
a) contacting a solid-phase coated with first receptors having a high
affinity for the target analyte with a known sample volume so that any target
9
CA 02504559 2005-04-20
analyte present in said sample volume binds with said first receptors so that
said target analyte is bound to said solid phase;
b) adding a solution containing colloidal dye particles coated with
second receptors having high affinity for the target analyte, but low affinity
for
the solid-phase and the first receptors, so that said coated colloidal dye
particles bind to any of the immobilized target analyte present forming bound
coated colloidal dye particle-target analyte complexes on the solid-phase;
c) separating said coated colloidal dye particles not bound to said solid
phase from the bound coated colloidal dye particle-target analyte complexes
on the solid-phase;
d) forming a dye solution by solubilizing dye particles of the bound
coated colloidal dye particle-target analyte complexes into a solubilization
buffer which is maintained in a pre-selected pH range;
e) measuring fluorescence upon optically exciting said dye solution
with excitation light at an appropriate wavelength; and
f) relating said measured fluorescence to a concentration of said target
analyte in said known sample volume using a pre-established standard curve.
The present invention also provides a method of conducting an assay
for the detection of a target analyte using a competitive assay format,
permitting the measurement of analytes with low molecular weights. A liquid
sample is first brought into contact with a solid phase, where the solid phase
is coated with receptors that have a high affinity for an analyte that may be
present in the sample. Immediately after introducing the liquid sample, a
colloidal solution of dye particles is also introduced. The dye particles are
CA 02504559 2005-04-20
coated with the target analyte. The dye particles therefore compete with the
analyte for the available binding sites of the immobilized receptors on the
solid phase. The solid phase is then separated from the liquid phase, which
in turn separates the bound dye particles from the unbound dye particles. A
solubilization buffer, maintained at an appropriate pH, is then added to
solubilize the bound dye particles, creating a dye solution. The fluorescence
of the resulting dye solution is measured, wherein the solubilized dye
molecules strongly absorb excitation light and emit light with high
efficiency,
and the concentration of the analyte is determined using a pre-determined
standard curve.
Thus, in another aspect of the invention there is provided an assay for
a target analyte, comprising:
a) contacting a solid-phase coated with first receptors having a high
affinity for the target analyte with a known sample volume so that any target
analyte present in said sample volume binds with said first receptors so that
said target analyte is bound to said solid phase;
b) adding a colloidal solution containing colloidal dye particles coated
with the target analyte, so that the colloidal dye particles compete for
binding
sites of the immobilized receptors on the solid phase;
c) separating said coated colloidal dye particles not bound to said solid
phase from the bound coated colloidal dye particle-target analyte complexes
on the solid-phase;
11
CA 02504559 2005-04-20
d) forming a dye solution by solubilizing the dye particles not bound to
said solid phase into a solubilization buffer which is maintained in a pre-
selected pH range;
e) measuring fluorescence upon optically exciting said dye solution
with excitation light at an appropriate wavelength;
f) relating said measured fluorescence to a concentration of said target
analyte in said known sample volume using a pre-established standard curve.
In a preferred embodiment of the invention, the radius of the dye
particles is chosen to lie within a narrow range in order to optimize the
sensitivity of the assay via amplification and to optimize the dynamic range
of
the assay via the control over washing and binding forces.
In another preferred embodiment of the invention, the receptors are
immobilized on the surface of magnetic beads. The use of magnetic beads
allows for optimization of assay parameters and can be utilized to a)
compensate for variations in dye properties, and b) increase receptor surface
area and thereby improve signal to noise. The use of receptor-coupled
magnetic beads also allows for easier washing and separation steps.
In another preferred embodiment of the invention, multiple assays are
multiplexed using different dye colours. In this embodiment, multiple mobile
solid supports, or regions on a single solid support, are prepared with
surface
chemistries having specific affinities for the different analytes. Each
distinct
surface captures a distinct analyte in the sample with high affinity. Dye
particles of multiple colours, each colour having a distinct analyte-specific
surface chemistry, are bound by their respective immobilized target analytes.
12
CA 02504559 2005-04-20
The radius of each type of dye particle can be varied in order to obtain an
optimized sensitivity for each individual assay. After solubilization of the
captured or unbound dye, the concentration of each dye and hence each
analyte is obtained through spectral analysis of the fluorescent signal.
The present invention also provides a method for the detection of a
target analyte, comprising the steps of:
a) contacting a solid-phase coated with receptors having a high affinity
for the target analyte with a known volume of a liquid sample being tested for
a presence or absence of the target analyte, the liquid sample containing a
known amount of colloidal dye particles having the target analyte bound
thereto, wherein in the absence of target analytes in the liquid sample target
analytes bound to the colloidal dye particles bind to the receptors to form
colloidal dye particle-target analyte-receptor complex, and in the presence of
target analytes in the liquid sample the target analytes preferentially bind
to
the receptors to form target analyte-receptor complexes;
b) removing the solid phase from contact with said liquid sample and
forming a dye solution by exposing the solid phase to a solubilizing solvent
for
solubilizing any dye particles of the colloidal dye particle-target analyte-
receptor complexes into a solubilization buffer;
c) measuring fluorescence upon optically exciting said dye solution
with excitation light at an appropriate wavelength; and
d) relating said measurted fluorescence to a concentration of said
target analyte in said known sample volume using a pre-established standard
curve.
13
CA 02504559 2005-04-20
The present invention also provides a method for the detection of a
target analyte, comprising the steps of:
a) contacting a first solid-phase coated with receptors having a high
affinity for the target analyte with a known volume of a liquid sample being
tested for a presence or absence of the target analyte, the liquid sample
containing a known amount of colloidal dye particles having the target analyte
bound thereto, wherein in the absence of target analytes in the liquid sample
target analytes bound to the colloidal dye particles bind to the receptors to
form colloidal dye particle-target analyte-receptor complex, and in the
presence of target analytes in the liquid sample the target analytes
preferentially bind to the receptors to form target analyte-receptor
complexes;
b) separating said coated colloidal dye particles not bound to said solid
phase from the bound coated colloidal dye particle-target analyte complexes
on the solid-phase;
c) forming a dye solution by solubilizing the dye particles not bound to
said solid phase into a solubiGzation buffer which is maintained in a pre-
selected pH range;
d) measuring fluorescence upon optically exciting said dye solution
with excitation light at an appropriate wavelength; and
e) relating said measured fluorescence to a concentration of said
target analyte in said known sample volume using a pre-established standard
curve.
14
CA 02504559 2005-04-20
A further understanding of the functional and advantageous aspects of
the invention can be realized by reference to the following detailed
description
and drawings.
BRIEI: DESCRIPTION OF THE DRAWINGS
The invention will now be described, by way of non-limiting examples
only, reference being had to the accompanying drawings, in which:
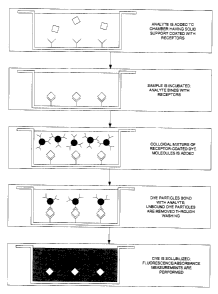
FIG. 1 is a flow chart illustrating the steps involved in the assay if a
solid phase was used.
FIG. 2 shows the process steps in a competitive assay where the
receptors have been immobilized on magnetic beads.
FIG. 3 shows the dose-response curve of a competitive assay for
morphine where the dependence of the fluorescence of the bound dye on the
anaiyte concentration is plotted.
FIG. 4 illustrates the response of a competitive assay for morphine
where the dependence of the fluorescence of the unbound dye on the analyte
concentration is plotted.
FIG. 5 is a schematic of an optical detection cell optimized for
absorbance measurements with a long path length.
FIG. 6 plots an example of the probability distributions of the contact
and washing forces, p~(F) and pW(F).
FIG. 7 plots an example of the probability distributions of the net
binding force for dye particles bound by one, two and three bonds.
CA 02504559 2005-04-20
FIG. 8 plots the number of solubilized dye molecules as a function of
the number of analyte molecules bound on to the solid support for different
dye particle radii in a simulated assay, demonstrating the existence of an
optimal dye particle radius.
FIG. 9 plots the number of solubilized dye molecules as a function of
the number of analyte molecules bound on to the solid support for different
dye particle radii, demonstrating the sensitivity of the assay to cross-talk
phenomena.
FIG. 10 plots the number of solubilized dye molecules as a function of
the number of analyte molecules bound on to the solid support for different
dye particle radii, demonstrating the sensitivity of the assay to variations
in
the binding force.
DETAILED DESCRIPTION OF THE INVENTION
The present invention provides a novel immunoassay for the detection
of analytes using dye particles. More specifically, the present invention
describes a method of performing a heterogeneous or homogeneous binding
assay using dye particles as chromatic labels that are detected via
absorbance or fluorescence following their solubilization.
The term "receptor", as used herein, means antibodies, antigens, DNA,
RNA, nucleic acids, aptamers, enzymes, or any other molecular species
capable of exhibiting a specific binding affinity for the analyte.
16
CA 02504559 2005-04-20
The term "analyte", as used herein, means antibodies, antigens,
nucleic acids, aptamers, enzymes, molecules, proteins, viruses, bacteria,
ions, or any species whose presence or concentration in a sample is sought.
The term "solid support", as used herein, means a surface onto which
the first receptor molecules can be coated, adsorbed or bound. For example,
the solid support may be a microwell, the walls of a capillary tube, or a
microsphere.
The term "colloidal dye", as used herein, means disperse dyes,
transfer dyes, fat dyes (solvent dyes), vat dyes, organic pigments, sulfuric
dyes, mordant dyes, solubilized (leuco) vat dyes, solubilized (leuco) sulphur
dyes, azoic dyes, anthraquinine dyes, coumarin dyes, oxidation bases and
ingrain dyes.
The term "solubilization buffer", as used herein, means any buffer
capable of entirely or nearly entirely solubilizing the dye particles, e.g, an
alkaline solution or organic solvent.
Figure 1 shows a flow chart describing a two-site sandwich
immunoassay representative of an embodiment of the present invention. A
solid support is coated with a receptor having a high affinity for the analyte
under consideration. The sample is introduced and analyte molecules are
bound to the solid support with one or more receptor molecules via specific
chemical bonding following a brief incubation period. A colloidal solution of
dye particles coated with a second type of receptor (also having a high
affinity
for the analyte), is then introduced. During incubation, the dye particles
bind
to the analyte via the attached receptor molecules and are thus immobilized
17
CA 02504559 2005-04-20
on the solid support. The unbound dye particles are washed using a liquid
washing buffer, leaving only the dye particles bound by the analyte. A
solubilization buffer is added and the bound dye particles are solubilized,
releasing their colour into solution. The concentration of the dye is measured
via a fluorometric measurement and the analyte concentration is obtained
using a pre-established standard curve relating the measured signal to the
analyte concentration.
An alternative assay format is shown in Figure 2, where a flow chart of
a competitive assay is described. As in Figure 1, a solid support is coated
with a receptor having a high affinity for the target analyte. The sample is
introduced and analyte molecules are bound to the solid support via specific
chemical bonding. A colloidal solution of dye particles coated with the
analyte
is then introduced. During incubation, the analyte-coated dye particles
compete with the analyte from the sample volume for binding sites of the
receptors on the solid phase. The unbound dye particles are washed using a
liquid washing buffer, leaving only the dye particles bound by the analyte. A
solubilization buffer is added and the bound dye particles are solubilized,
releasing their colour into solution. The concentration of the dye is measured
via a fluorometric measurement and the analyte concentration is obtained
using a pre-established standard curve relating the measured signal to the
analyte concentration.
Figure 3 shows an exemplary dose-response curve of the assay in
competitive format where the target analyte is morphine. As can be
18
CA 02504559 2005-04-20
appreciated from this graph, the signal contrast is more than a factor of ten
in
arbitrary fluorescing units.
Instead of following the process steps as highlighted in Figures 1 and 2
in which the analyte concentration is deduced from the fluorescence of
solubilized bound dye, it is possible to infer the analyte concentration from
a
measurement of the unbound dye. This is achieved by solubilizing the
unbound dye particles after the separation step and measuring the
fluorescence of the unbound solubilized dye. This approach has the added
benefit of eliminating the wash steps that can prolong the assay time. The
major drawback of this assay format is its limited dynamic range relative to
the assay format in which the bound dye is measured. Figure 4 shows an
exemplary dose-response curve of this assay format, in which the target
analyte is morphine.
The method of amplification via solubilization disclosed in US Patent
No. 4,373,932 was described primarily in the context of increasing the signal
produced by absorbance of the bound dye. In particular, measurements of
the increase in absorbance before and after solubilization were provided,
demonstrating this effect. However, the process of solubilization can lead to
even greater benefits for assays based on fluorometric measurements, and
the prior art fails to teach a method to realize this benefit. In addition to
the
amplification of the fluorescence signal due to a better penetration of
excitation light and more homogeneous excitation of the dye molecules, the
quantum yield will be markedly improved as a result of inhibited quenching.
When an excited dye molecule is in colloidal form, the close proximity of
other
19
CA 02504559 2005-04-20
molecules allows the non-radiative transfer of energy in a process known as
self quenching. This process can cause a large reduction in the quantum
yield, significantly reducing the sensitivity and accuracy of an assay.
However, upon solubilization, molecules can be efficiently excited and lack
the self quenching decay channel, allowing for a very high quantum yield. It
is also noted that both fluorescence and absorbance measurements may be
carried out using a cell that is optimally designed for high absorption and
the
efficient excitation and collection of fluorescence, in order to provide a
potentially more sensitive measurement.
In a preferred embodiment of the invention, magnetic beads are used
as a mobile solid phase for the separation and extraction of bound dye
particles. The separation of the magnetic beads from the surface is
performed using one of many known methods in the prior art, all of which use
a magnetic field to spatially isolate the magnetic beads. Magnetic beads
enable a significant enhancement in the repeatability and ultimately the
precision of the assay. This enhancement is possible because the number of
magnetic beads in the assay, and hence the amount of surface area for
immobilizing dye particles, can be accurately controlled. This is particularly
important for assays employing colloidal dye particles, since variations can
exist in the affinity of bound receptors, and the smoothness, size and
geometry of the dye particles. Therefore, the control over surface area
provided by the number of magnetic beads used in an assay offers a means
of accurately compensating for batch-to-batch variations the properties of dye
particles. Furthermore, magnetic beads, by their very nature as a mobile
CA 02504559 2005-04-20
solid phase evenly distributed within a liquid phase, allows for a more
uniform
reaction between the dye particles, analyte, and receptors, which reduces the
time required for the assay incubation. Finally, the use of magnetic beads
allows for many convenient and easily automated methods of extraction that
are known in the prior art.
The colloidal solution can be prepared using the methods taught in
United States Patent No. 4,373,932 by Gribnau et al., in which numerous
techniques are disclosed for the preparation of dye sots coated with
antibodies, which patent is incorporated herein in its entirety. These methods
can be generalized to the preparation of dye particles coated with other
receptor capture agents, including aptamers. A preservative such as
thimersol can be added to the colloidal solution to provide a long and stable
shelf life.
In the prior art, the means of optical detection of the solubilized dye
has focused almost exclusively on colourimetry. Although the absorbance of
the dye can indeed be amplified by solubilization, the degree of amplification
depends critically on the geometry of the optical cell used for absorbance.
Unfortunately, no consideration of this important element of the assay design
has been given in the prior art. A dramatic enhancement in the degree of
amplification can be obtained by a careful choice of the optical cell used to
measure the absorbance of the solubilized dye. In particular, transferring the
solubilized dye solution into a long and narrow capillary cell allows for a
large
increase in the optical path length of an absorbance measurement.
Furthermore, the use of a low index cladding material, such as Teflon, offers
21
CA 02504559 2005-04-20
the ability to provide a liquid waveguide for the optical beam used in an
absorbance measurement, with path lengths limited only by the volume of the
dye solution.
Figure 5 shows an example illustrating the concept of amplification
through soiubilization and path length enhancement. A small volume of
solubilization buffer is chosen to wet the solid surface upon which dye
particles are bound during incubation. The dye solution formed following
solubilization is placed in a capillary tube, either by pipetting, centrifugal
force
or capillary action, and the dye solution fills the capillary. The capillary
may
be a single capillary tube or a capillary housed in a cartridge containing
single
or multiple capillaries. An optical beam is directed along the axis of the
capillary tube and propagates through the capillary tube before encountering
the optical detector.
The optical beam may be produced either by a laser or an incoherent
source such as a light emitting diode or lamp. If the beam is sufficiently
collimated that it does not encounter the walls of the capillary tube during
propagation, the capillary tube need not be a waveguide with Teflon cladding.
If a monochromatic or narrow-spectrum source is used, no filtering element is
needed prior to detection. However, if a broadband source is used, a filtering
element placed either before or after the measurement cell is required. In a
preferred embodiment, a polychromatic light source is used in order provide
one measurement of the transmission through the cell within the bandwidth of
the dye's absorbance, and another measurement of the transmission through
the cell outside of the dye's absorbance bandwidth. This second
22
CA 02504559 2005-04-20
measurement facilitates the subtraction of background broadband losses due
to poor coupling and scattering. Finally, if multiple assays are multiplexed
in
the same incubation chamber, with a different coloured dye particle for each
assay, additional beams or filtering elements can be added in order to
spectrally resolve and quantify the absorbance of each dye. If a detailed
measurement of the absorbance spectrum is obtained (i.e. absorbance
measurements at multiple spectral points), curve fitting methods can be used
to extract the individual contributions of different dye particles with
partially
overlapping spectra, increasing the number of dyes (assays) that can be
multiplexed and also increasing the sensitivity of each individual
measurement.
Although the sensitivity of the assay can be significantly improved by
controlling the geometry of the optical measurement cell and using the
fluorescence of solubilized dye to avoid self quenching, it can be further
improved by controlling the dye particle radius to obtain optimal specific
binding and minimal non-specific binding following a washing step. The
sensitivity of a binding assay is highly dependent on the detailed chemical
nature and forces present during the binding process. For example, in a
sandwich assay employing single-molecule dye labeling (rather than a large
dye particle), it is often very difficult to remove dye-labeled receptor
molecules
that bond non-specifically to the surface. If such molecules cannot be
removed, the sensitivity of the assay will be degraded by a large background
signal. Although the term "washing" is commonly applied to the process of
removing unbound and non-specifically-bound dye-labeled receptor
23
CA 02504559 2005-04-20
molecules, the washing process in this case is not characterized by an
applied fluidic force. Indeed, due to the presence of the boundary layer, a
moving fluid is unable to effectively remove non-specifically bound molecules.
Instead, the washing process uses a probabilistic thermal escape process to
induce the release and removal of non-specifically bound molecules. The
probability of escape of a bound molecule with a binding energy of Eb at a
temperature T is proportional to a E~kT, where k is Boltzman's constant. If
the
binding energy is not too much larger than kT (26 meV at room temperature),
then there can be a high probability that the molecule will escape over a
given
time interval. A certain percentage of non-specifically bound dye particles
can then escape and be removed simply by incubating the solution at a given
temperature. Although the binding energy of a typical specific bond is on the
order of 0.3-0.8 eV, the binding energy of a non-specific bond can take on a
wide range of values, depending on the affinity of the interaction. It follows
that this process is very inefficient and will inevitably lead to inefficient
washing and a significant background signal from unwashed non-specifically
bound dye-labeled molecules. In contrast to the single-molecule washing
method, washing forces can be applied to larger microscopic particles.
Therefore, the assay embodied by the present invention offers the potential to
optimize these forces for the efficient removal of non-specifically bound dye
particles without disturbing the specifically bound particles. Unlike other
assays utilizing the optical identification and enumeration of large micron-
sized microspheres that require that the particle size be sufficiently large
for
optical resolution in a microscope, the assay of the present invention
provides
24
CA 02504559 2005-04-20
the flexibility to optimize over a very wide range of nanoscopic to
microscopic
particle sizes.
In order to quantify this concept further, it is useful to consider a simple
model of the forces involved during the specific and non-specific binding of a
dye particle in the inventive assay. We consider first a solid support with an
area of A$ that is uniformly coated with receptor molecules. Given a
spherical dye particle with radius R , the effective contact area between the
dye particle and the surface can be approximated by
A~" =0.181R (1)
(see K. Cooper et al., "Simulation of the Adhesion of Particles to Surfaces",
J.
Colloid. interface Sciences 234, 284 (2001 )), where R is in units of pm and
A~e" is in units of pmt. The number of individual contact area elements of
area A~e" on the entire solid support area AS is given by
As - 5.53As
Noel! _ - ~ (2)
A~err R
Following the incubation of a sample containing a given concentration
of analyte molecules, we assume that Na analyte molecules bind to the
surface via attachment to receptor molecules so that the average number of
analyte molecules per contact area element is
~ = Na . (3)
N~~~
For a given particle radius, the parameter ,u is linearly proportional to the
concentration of analyte molecules in the sample volume, and is used
henceforth as a measure of analyte concentration.
CA 02504559 2005-04-20
Following the incubation of dye particles coated with receptor
molecules, a total of Nb dye particles specifically bind to the surface. A
further N~ dye particles bind non-specifically to the surface through
frictional
contact forces. Since a single dye particle can be bound by more than one
analyte-receptor bond, it follows that Nb < NQ , and the number of bound dye
particles must be calculated using statistical methods. Although the average
number of analyte particles per contact area element is ~c , the value of ~c
will
typically be much less than unity. One must therefore calculate Nb by
considering the probability distribution of ,u , which can be shown to be a
Poissonian distribution:
_ fake-,~
pk - k~ ,
where pk is the probability of that a given contact area element will have k
analyte molecules, and therefore k bonds to a single dye particle. This
relation allows one to calculate N~ , the number of dye particles that will be
bound with k bonds, by writing Nbk = pkN~err , with the total number of bound
dye particles given by
Nb - Ncell ~ p k ' ~ 5
k=1
it is important to note that in the limit of a large number of dye particles
binding, the surface coverage will be saturated and the above expression will
no longer be valid. The surface coverage is assumed to be saturated when
Nb ~ N~,r l 2 , where
26
CA 02504559 2005-04-20
AS AS
Nsar = A =
sphere
Although equation (5) provides an expression for the number of bound
dye particles, the model is incomplete because it has not yet considered the
effect of washing on the number of bound particles. In order to do so, one
must consider the forces acting on a dye particle during the washing process.
These forces include the binding force due to the analyte-receptor bonds Fb ,
the contact force between a dye particle and the solid support due to
frictional
(van der Waals) forces F~, and the washing force FW . Reported values for
Fb have ranged from low tens of pN to approximately 250 pN, depending on
the affinity of the analyte-receptor interaction. However, the contact force
is
by definition statistical in nature, since small variations in the surface
roughness of the dye particle or solid support can lead to large differences
in
the contact force. It is therefore appropriate to consider the contact force
as a
force probability density function p~ (F) , which peaks at the average contact
force F~ . A similar argument can be applied to the washing force (the applied
force), which can vary due to geometrical effects, turbulence and
orientational
effects, and is described by a second probability density function pw(F) that
peaks at F", . Figure 6 shows an example of the relationship between the
probability density functions p~(F) and pw(F) . In this example, the non-
specifically bound particles (bound via the contact force) are efficiently
washed due to the fact that Fw > F~ .
27
CA 02504559 2005-04-20
If the binding force Fb is small relative to Fw , then dye particles bound
by one or two analyke-receptor bonds will also be washed away, decreasing
the sensitivity of the assay. This will decrease the value of Nb relative to
that
obtained in equation (5). The effect of the washing force on dye particles
with
multiple analyte-receptor bonds is illustrated in Figure 7 (assuming a binding
force of 50 pN). The net force binding a dye particle is given as the sum of
the contact force and k times the binding force. Since the contact force is
described by a probability distribution function, the net binding force for a
dye
particle bound by one bond (and the contact force) is given by the probability
distribution p~(F-Fb) . Similarly, the binding force for a dye particle bound
by
k bonds (and the contact force) is given by the probability distribution
p~(F-kF'b) . A given dye particle will only remain bound after washing if
some or all of p~(F-kFb) lies beyond pw(F). In the case of Figure 7, it is
clear that most of the dye particles bound by single and double bonds will be
removed by washing. The probability pk that a given bead with k bond will
remain intact after washing can be calculated as follows:
F
Pk = JP~~F-~'b~f Pw~F'~F'dF. (6)
0 0
Having considered the effect of washing forces, it is now possible to
calculate the total number of bound beads Nb' that remain after washing.
This is done by rewriting equation (5) and including the probability pk from
equation (6):
28
CA 02504559 2005-04-20
s
Nb =Nm Pk~~Pk~P~~PW~Fb~
k=1
This expression clearly establishes the link between the measured optical
signal from the dye (which is proportional to Nb') and the analyte
concentration p and force parameters p~ , pw and Fb .
The above model provides a quantitative relationship between the
sensitivity of the assay and the relevant physical parameters. However, the
essential observation to be made is that many of the parameters depend
critically on the dye particle radius R . These parameters include the number
of individual contact area elements N~" a R-' , the contact force F~ and its
distribution width, which increase with R , and the washing force Fw and its
distribution width, which also increase with R . Finally, the optical signal
generated via absorbance or fluorescence is proportional to Nb', which is
itself proportional to R3
The various dependencies of the assay parameters on R clearly
indicate that a trade off will exist between efficient washing and having a
large
number of bound particles (low R regime) and amplification via solubilization
(high R regime). This fact is illustrated in Figure 8 for a simulated assay
with
efficient washing and a binding force of 50 pN and a solid support area of AS
=
4 mm2. In this figure, the number of solubilized dye molecules is plotted
against number of analyte molecules bound on to the solid support, assuming
a molar mass of 331 g/M and a specific gravity of unity for each dye molecule.
29
CA 02504559 2005-04-20
One readily observes that an intermediate value of R ~ 400 nm provides
optimal sensitivity.
In addition to an enhancement in sensitivity, the optimal assay also
provides a vast increase in dynamic range. This is apparent in Figure 8,
where it can be seen that the dynamic range of the non-optimized assay is
only approximately two orders of magnitude, while the optimized assay has a
dynamic range in excess of six orders of magnitude. This dramatic increase
in the dynamic range is produced by two effects. Firstly, the minimal
detectable analyte concentration is determined by the analyte concentration
where only a single particle is bound prior to solubilization.
In the case of large, non-optimized particles, the requirement of
multiple bonds per particle (i.e. many bonds are required to survive the large
washing force) severely limits the number of bound particles after washing.
However, in an optimized assay with smaller particles, one or very few bonds
are required to survive washing and the analyte concentration at which a
single bead is bound is many orders of magnitude lower than that of a large
particle assay. Secondly, as mentioned above, the maximum analyte
concentration is estimated by the concentration where the projected surface
area of the bound particles (prior to washing) is equal to half the total
support
area. Clearly, the number of bound particles will be inversely proportional to
the particle radius. Indeed, for very large particles far from the optimal
radius,
the maximum number of analyte particles (i.e. the analyte concentration) is
much lower than that of the optimized radius.
CA 02504559 2005-04-20
Furthermore, since a large percentage of the bound particles of the
non-optimized assay are removed by washing (since many bonds are needed
to survive the large washing force), the number of solubilized molecules is
very low. The optimized assay, however, allows many more particles to bond
to the surface at saturation. Since the washing force is sufficiently small to
cause minimal removal of bound particles, the number of dye molecules after
solubiiization is very high. However, it is important to note that if the
particles
are too small, then the amplification will be very low and the washing force
will
be too small to eliminate non-specifically bound particles. It is therefore
apparent that the optimal assay, with an intermediate radius, provides both
high sensitivity and large dynamic range.
Although the preceding discussion demonstrates that the sensitivity
and dynamic range of the dye solubilization assay may be optimized by
controlling the particle radius, it can also be shown that this optimization
procedure leads to enhanced specificity. The specificity is determined by the
sensitivity of the assay to non-specific binding events. These events, in most
cases, will have binding forces significantly lower than the primary specific
analyte bond. However, if the washing process is inefficient, some of these
weaker bonds may remain, causing the assay noise floor to rise. However, if
the particle size is optimized so that the washing force and contact force are
of similar magnitude to the binding force, then a situation can occur in which
a
single specific bond will not be broken by washing, while there is a high
probability that a weaker non-specific bond will be broken. In such a case,
the washing process improves the specificity of the assay. The effect of
31
CA 02504559 2005-04-20
"specific washing" is demonstrated in Figure 9, where the number of
solubilized dye molecules is plotted as a function of the number of analyte
molecules bound on to the solid support for two different radii - one near the
optimization point ( R ~ 357 nm) and one much larger ( R ~ 1500 nm). An
analyte with a binding force of 50 pN is simulated and a second cross-
reacting species with a binding force of 20 pN is also assumed to be present.
The signal due to the additional cross-reacting species is indicated on the
figure as "noise", and the concentration of the cross-reacting species is
assumed to be 100 times that of the analyte at a given analyte concentration.
As clearly shown in the figure, the noise exceeds the signal for the non-
optimized assay. However, for the optimized assay, the noise signal is
always almost an order of magnitude less than the signal. This illustrates
that
optimization provides the additional benefit of the lowest background due to
non-specific binding events.
An additional benefit beyond sensitivity, dynamic range and specificity
is insensitivity to variations in affinity. If the assay is optimized in such
a way
that the binding force is of similar magnitude to the washing and contact
forces, then, as describe above, a single bond can survive the washing step.
In this case, any additional affinity (bond strength) will have a negligible
effect
on the number of bound particles after washing. However, if the assay is not
optimized and multiple bonds are required, the assay will be very sensitive to
subtle changes in affinity. Such affinity variations are often present when
antibodies are used as receptors in an immunoassay. This principle is
illustrated in Figure 10, where number of solubilized dye molecules is again
32
CA 02504559 2005-04-20
plotted as a function of the number of analyte molecules bound on to the solid
support for two different radii (optimized and non-optimized). The non-
optimized assay is very sensitive to the binding force, with an increase in
the
binding force of only 25 pN producing a change of an order of magnitude in
the number of solubilized dye particles. In contrast, the number of
solubilized
dye molecules in the optimized assay is nearly independent of the increase in
binding force. Therefore, the optimized assay provides the additional benefit
of insensitivity to variations in analyte-receptor bond affinity.
The present invention, describing improvements to the dispersed dye
immunoassay, incorporates this optimization step into the design of the
assay. This optimization process may be conducted empirically by
determining the dependence of sensitivity and dynamic range on particle
radius. Since the binding force for different analytes will vary in strength,
the
radius of the dye particle should be optimized uniquely for each analyte. This
enables the design of a multiplexed assay (using different colours) for
several
different analytes, with each individual assay having an optimized sensitivity
and dynamic range.
Finally, as previously mentioned, it should be apparent to those skilled
in art that the receptor molecules attached to the dye particles can include
nucleic acid oligonucleotides for the detection of DNA or RNA via a
hybridization reaction. Furthermore, the receptor molecules attached to the
solid support in a sandwich assay may also be oligonucleotides, facilitating
the formation of a DNA sandwich assay. Apatmers, which are also formed
33
CA 02504559 2005-04-20
out of nucleic acids, may be used for the detection of a wide range of
antigens.
As an example, the coumarin family of disperse textile dyes provides
an excellent chemistry for the attachment of nucleic acid receptors. In
particular, the commerically available dye Luminous Red G possesses unique
surface chemical and structural characteristics that could allow it to be
efficiently used as a hetero-functional solid support with mild surface
modification or no modification at all. Indeed, this dye has functional groups
capable of reacting with two or more chemically distinct functional linkers,
e.g.
amines, thiols and carboxy groups. The linkers could serve two purposes: to
covalently bind two distinct chemical entities which otherwise would remain
non-reactive toward each other and as a physical spacer that provides
greater accessibility and freedom to each of the linked bio-molecules such as
thiol-modified DNA oligomers or amino-modified oligos. In addition, as a
result
of the reactive nature of the dye hetero-functional groups, the covalent
linkage to bio-molecules is highly stable, eliminating the possibility of
leakage
from the dye surface. Such resilience leads to enhanced sensitivity and
dynamic range of the assay.
The immobilization of oligo-receptor molecules onto the surface of the
dye particle can be performed using either covalent or non-covalent bonding.
An example of the steps involved in covalent bonding is provided below, in
which a 5'amino linker covalently binds to a disperse dye:
1. Wash 100 mg disperse dye beads 3 times for 5 minutes each, in
reagent grade water with centrifugation.
34
CA 02504559 2005-04-20
2. Air dry the beads for 10 minutes and add 1 ml of 200g/L EDAC (1-
ethyl-3-(3-dimethylaminopropyl carbodiimide), and mix for 15 min.
3. Rinse 2 times in H20 and dry at room temperature for 10 minutes.
4. Dilute Oligo (0.5-10 pmol) in Buffer (0.5 M NaHC03, pH; 8.4, and 0.1
v/v Tween 20)
5. Add 100 mg of the washed (steps 1-3) dye beads to 1 ml of the
solution of step 4.
6. Mix the resulting solution with agitation for 1 hour.
7. Wash the mixture 3 times, each time for 5 min in reagent grade water
with centrifugation
8. Add 0.1 N NaOH and agitate for 20 minutes in order to quench
remaining active group.
9. Wash 3 times for 5 min. each in reagent grade water with
centrifugation and Air dry
10. Store desiccated at 4° C
The immobilization of oligonucleotides onto a disperse dye can be
performed using non-covalent bonding. Examples of non-covalent
immobilization are provided in the three examples below:
A) EDC protocol:
1. Wash the dye beads through the steps 1-3 of the preceding example
2. Add 50 mL of a 10 mM EDC containing 10 pmol of oligo to 50 mg to
the washed beads
3. Incubate overnight at around 37° C with agitation
4. Wash with TNTw sol.
CA 02504559 2005-04-20
5. Store at 4° C (can be kept for over period of time)
B) CTAB protocol
1. Add 50 pL of a 0.03 mM CTAB containing 10 pmol of oligo to washed
dye
2. Incubate overnight at around 37° C with agitation
3. Wash with TNTw sol.
4. Store at 4° C (can be kept for over period of time)
C) NaCL protocol
Version I:
1. Add 50 p,L of a 0.2 nmole/ml oligo solution in 500 mM NaCL to washed
dye
2. Incubate Incubate overnight at around 37° C with agitation
3. Wash with TNTw sol.
4. Store at 4° C (can be kept for over period of time)
Version II:
1. Add 50 ~.L of a 0.2 nmole/ml oligo in 3X PBS (0.15 M phosphate 0.45
M NaCI, pH:7 to washed dye and Incubate 2 hours at 37° C
2. Wash 3X with 1X PBS containing 0.05% Tween 20 (PBST)
3. Block with 1 % skimmed milk or BSA in 1 X PBS for 1 hour at 37° C
4. Store at 4° C (can be kept for over period of time)
As will be clear to those possessing the ordinary skill of the art, many
variations and modifications of the present invention are possible that do not
diverge from its scope and spirit. It is therefore to be understood that,
within
36
CA 02504559 2005-04-20
the scope of the preceding disclosure, the invention may be practiced
otherwise than as specifically claimed.
As used herein, the terms "comprises", "comprising", "including" and
"includes" are to be construed as being inclusive and open ended, and not
exclusive. Specifically, when used in this specification including claims, the
terms "comprises", "comprising", "including" and "includes" and variations
thereof mean the specified features, steps or components are included.
These terms are not to be interpreted to exclude the presence of other
features, steps or components.
The foregoing description of the preferred embodiments of the
invention has been presented to illustrate the principles of the invention and
not to limit the invention to the particular embodiment illustrated. It is
intended
that the scope of the invention be defined by all of the embodiments
encompassed within the following claims and their equivalents.
37
CA 02504559 2005-04-20
REFERENCES CITED
PATENT DOCUMENTS
1. D. Trau et al., DE10042023 (2003).
2. T. C. J. Gribnau et al., US4373932 (1983).
OTHER PUBLICATIONS
1. Trau et al., "Nanoencapsulated Microcrystalline Particles for
Superamplified Biochemical Assays", Anal. Chem. 74, 5480 (2002).
2. H. A. Rongen et al., "Liposomes and Immunoassays", J. Immunol.
Methods 204, 105 (1997).
3. A. Kamyshny and S. Magdassi, "Chemiluminescence Immunoassay in
Microemulsions", Colloids Surf. B 11, 249 (1998).
4. T. Gribnau et al., "The Application of Colloidal Dye Particles as Labels
in Immunoassays: Dispersed) Dye Immunoassays ("DIA")", in T. C. J.
G~ibnau, J. Visser and R. J. F. Nivard (Eds.), Affinity Chromatograph
and Related Techniques, Elsevier, Amsterdam, 411 (1982).
5. Gribnau, A. van Sommeren and F. van Dinther, "DIA - Disperse Dye
Immunoassay", in I. M. Chaiken, M. Wilchek and I. Parikh (Eds.),
Amity Chromatography and Biological Recognition, Academic Press,
Orlando, FL, 375 (1983).
6. K. Snowden and M. Hommel, "Antigen Detection Immunoassay Using
Dipsticks and Colloidal Dyes", J. Immunol. Methods 140, 57 (1991 ).
38
CA 02504559 2005-04-20
7. K. Cooper ef al., "Simulation of the Adhesion of Particles to Surfaces",
J. Colloid. Interface Sciences 234, 284 (2001 ).
39