Note: Descriptions are shown in the official language in which they were submitted.
CA 02505126 2005-05-05
WO 2004/043351 PCT/US2003/034549
SOLUBLE ORAL PATCH WITH COLLAGEN AND INTERLACED ACTIVE
INGREDIENTS
PRIORITY
This application claims priority from US Patent Application 101287,843 filed
05 November 2002 which, for the United States, is a CIP of 10/236,289 filed 04
September 2002 and claims priority from 60/344,577 filed 28 December 2001.
BACKGROUND
For treatment of health problems in the mouth or throat, people have for
centuries held in their mouths a composition containing herbal or other
medication for topical application. The oldest name for such a composition,
derived from Latin and previously from Greek, is "troche". A modern form of
troche is the cough drop, so named because it was formed by "dropping" hot,
viscous, sugar-based candy onto a sheet or into a mold where it cools to form
the
troche. Another modern form of troche is the throat "lozenge", so named
because it was in the shape of a diamond (like on playing cards), which is the
meaning of the word "lozenge". The structural characteristics of these types
of
troches are determined by their primary structural ingredients which are
typically
corn syrup or sugars, including sugar alcohols. These troches are only mildly
adherent to teeth and not significantly adherent to gums, cheeks, or lips.
To achieve higher concentrations of medication at a particular spot in the
mouth than troches can deliver, adherent oral patches have been developed. An
oral patch typically includes one or more flexible layers that do not dissolve
entirely such as invented by Anthony et al. and disclosed in US patent
5,713,852.
Another example of an oral patch is the DentiPatch which has one or more non-
soluble thermo-plastic layers and lidocaine, offered for sale by Noven
Pharmaceuticals, Inc. As used herein, the word "patch" does not include
preparations that move about the mouth rather than resting in one place, such
as
cough drops, throat lozenges, or other troches. Nor does it include
preparations
that do not hold together as a single item when held in the mouth such as
preparations of powder, liquid, paste, viscous liquid gel, or a tablet or
troche that
crumbles into a powder or paste when chewed or placed in saliva.
CA 02505126 2005-05-05
WO 2004/043351 PCT/US2003/034549
The most significant differences between an oral patch as used herein and
other forms of oral medicinal topical preparations such as troches are that an
oral
patch is designed to (1 ) release medication into the mouth over a relatively
long
period of time, such as 30 minutes or more, (2) be at least mildly adherent so
that
it can be placed in a preferred location and not be dislodged by gravity or
gentle
movement, and (3) remain in the mouth as a single item that will not spread to
be
in a plurality of locations in the mouth at one time.
There are many uses for preparations containing a medication to be
delivered topically in the mouth. In many treatment situations, it is
advantageous
to retain the preparation at one location in the mouth rather than allowing it
to
move in the mouth such as when talking. U. S. Patent 6,139,361 issued to Mark
Friedman surveys the known methods for adhering a slowly dissolving
medication to a location within the mouth. These methods include two forms of
adherent soluble patches, referred to by Friedman as "mucoadhesive erodible
tablets". These tablets are formed using polymers carboxymethylcellulose,
hydroxymethylcellulose, polyacrylic acid, and carbopol-934. None of these
polymers melts to a liquid at human mouth temperatures.
SUMMARY OR THE INVENTION
The invention is an adherent oral patch including a hydrophilic polymer
that is liquid at human mouth temperatures. The oral patch is made of two
primary components.
The first component is a porous molecular network formed as a unitary
solid structure that remains a solid at human mouth temperatures, in contrast
to
being crumbly or a paste. The network is preferably hydrophilic so that, even
when applied to a wet mucosal surface in the mouth, it will tend to adhere by
absorbing moisture from the mucosal surface. Preferably, the network slowly
dissolves in saliva so that the patch merely dissipates over time and the
patch
never has to be removed from the mouth.
The second component is a hydrophilic polymer that is liquid at human
mouth temperatures. The polymer is distributed throughout the pores of the
network. Because the polymer is hydrophilic and liquid at human mouth
2
CA 02505126 2005-05-05
WO 2004/043351 PCT/US2003/034549
temperatures, it will adhere very well to wet surfaces inside the mouth and is
quite soft which provides a soothing feeling to any sensitive tissue such as
canker sores. The second component is preferably partially hydrolyzed collagen
such as gelatin from animal protein.
The oral patch will adhere to teeth, gums, cheek, lips, or tongue without
the user first drying saliva from the tissue. If the patient merely places the
oral
patch in his or her mouth and presses it in the desired location for 10 to 40
seconds, it will adhere to the tissues that it has been touching without
movement,
even though those tissues are wet. This is far easier for patients to use than
requiring that the tissue first be dried with a towel before the adherent oral
patch
is placed. If the patient wants to use an oral patch in the lip or under the
tongue,
the oral patch can easily be removed for talking and then easily be replaced
without using a towel or a mirror.
A desired medication is also located within the pores of the network along
with the hydrophilic polymer.
The network component may be comprised of a thermo gel having a
melting temperature higher than human mouth temperatures. Preferably, the
thermo gel is elasto-plastic, such as formed by a mixture of the hydrogels
konjac
gum and xanthan gum dissolved in hot water and then cooled to form an elasto-
plastic gel. Alternatively, the network may be comprised of a complex
carbohydrate, such as cellulose, pectin, maltodextrin, or starch from potato,
rice,
corn, or wheat. Also, the network may be comprised of a hydrogel with a
melting
temperature higher than temperatures in the human mouth formed of amino
acids, such as peptides.
In preferred embodiments, the hydrophilic polymer gels to a solid at room
temperatures. This allows the oral patch to be removed from the mouth and
placed on a smooth surface, such as a plastic bag. Because the hydrophilic
polymer then gels, the oral patch again becomes handleable with the fingers to
return it to the mouth without being too sticky to handle,or leaving a residue
on
the fingers or on the plastic bag. In one such embodiment, the hydrophilic
polymer is protein gelatin (collagen) rendered from animal tissue, which
solidifies
3
CA 02505126 2005-05-05
WO 2004/043351 PCT/US2003/034549
at just below mouth temperatures and remains a solid even at clothes pocket
temperatures so it will not melt in a pocket.
In another aspect, the invention is a method for manufacturing an adherent
oral patch. In this method, ingredients for forming the porous network,
molecules
of the hydrophilic polymer, molecules of the medication, and water are mixed
together. The mixture is heated to dissolve all ingredients, either before the
ingredients are added together or after they are added together, and the
mixture
is then cooled, thereby causing the ingredients for forming a network to form
the
porous network as a unitary solid structure having the medication and the
hydrophilic polymer within its pores. Before it is cooled, the hot mixture may
be
deposited into a mold of a suitable shape to form the preferred unitary solid
structure. The mold may be formed in powdered starch, as is well known in the
candy making industry for forming gumdrops. Alternatively, the mold may be
formed of a rigid material such as metal or plastic. If the mold is thin
plastic or
aluminum, it may also serve as packaging for delivery of the oral patch to the
consumer.
BRIEF DESCRIPTION OF THE DRAWINGS
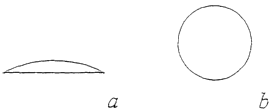
Figure 1 a shows a side view of a soft, adherent, soluble oral patch.
Figure 1 b shows a top view of the same oral patch.
Figure 2 shows, in representational form, the structure of the solid, porous
network, including the pores.
DETAILED DESCRIPTION
Figure 1 shows a preferred shape for the oral patch. It is made with slowly
dissolving hydrocolloids so that that it typically lasts in the mouth for at
least one
to six hours. The patch can be formed in the shape of a tablet or a lozenge or
a
wafer or any other desired shape. A preferred shape is a thin lentil as shown
in
Figure 1 a.
Figure 2 shows, in representational form, the structure of the solid, porous
network, including the pores. To practice this invention, a requirement for
the
network is that it remains a solid, rather than melting, at human mouth
4
CA 02505126 2005-05-05
WO 2004/043351 PCT/US2003/034549
temperatures. So that the oral patch will slowly erode, the network should be
made of a material with a low to moderate rate of disintegration in warm
saliva. If
the network does not erode fast enough, medication will be drawn out of the
network faster than the network erodes and, although a portion of the oral
patch
remains in the mouth, it will no longer be releasing medication. The
hydrophilic
polymer that, along with water and medication, fills pores of the network
helps to
slow the loss of medication from within pores of the network. Being a polymer,
its
molecules are long and they tend to be entangled by the network. The polymer
molecules, in turn, along with the network structure, tend to entangle
molecules
of the medication. To obtain greater entanglement, molecules of the medication
may be weakly chemically bound, such as by cross-linking, to molecules of the
hydrophilic polymer or the network or both.
To understand by analogy how the porous network filled with a polymer
that is liquid at mouth temperatures becomes very sticky without
disintegrating,
imagine a fish net bag filled with linguini Alfredo. When the linguini Alfredo
is
cold, such as when just removed from a refrigerator, the Alfredo sauce is
congealed and the entire structure is not very sticky. Imagine it is heated in
a
microwave oven. The Alfredo sauce melts and becomes quite sticky. By itself,
the fishnet bag is not sticky. But, the holes are large enough that strands of
linguini covered with sauce will bulge out of the holes. When warm, the entire
structure, if thrown against a wall, would probably stick, yet the bag keeps
it all
together as one piece. The strands of linguini are like the long molecules of
a
polymer that is liquid at mouth temperatures. Their length keeps them from
easily falling out of the fish net bag.
Many different compositions can be used to form the network. For ease of
manufacturing, it is convenient if the network is comprised of a thermo gel
having
a melting temperature higher than human mouth temperatures. This allows the
entire mixture to be a liquid at temperatures far above human mouth
temperatures and allows the network to be formed by cooling the mixture such
that the thermo gel forms the desired network by a gelation process. The
temperature at which the gel forms can be lower than human mouth
5
CA 02505126 2005-05-05
WO 2004/043351 PCT/US2003/034549
temperatures, provided the temperature at which it melts again is higher than
human mouth temperatures.
Readily available materials that form such a gel include agar, in various
forms, carrageenan, in most of its forms, particularly kappa carrageenan,
konjac
gum, locust bean gum, and xanthan gum. All of these materials form a thermo
gel that is sufficiently elastic or plastic or a combination thereof for the
network to
feel soft in the human mouth if it is adequately hydrated. If water is dried
out of
the network, it will become hard and will produce an unattractive feel when
placed in contact with sensitive tissues, such as canker sores. To prevent the
network from drying out, it may be packaged with a hermetic seal or a non-
evaporating plasticizes, such as glycerol (glycerin) may be added. However,
the
more glycerol is added the less adherent the oral patch will be.
Synthetic hydrogels may be used for either the network that does not melt
at mouth temperatures or the adherent, liquid polymer. Protein-based hydrogels
are usually prepared using proteins extracted from natural sources, but they
may
be synthesized, such as with diblock copolypeptide amphiphiles, as taught by
Nowak, et. al, "Rapidly Recovering Hydrogel Scaffolds From Self-Assembling
Diblock Copolypeptide Amphiphiles". Nowak, A. P.; Breedveld, V.; Pakstis, L.;
Ozbas, B.; Pine, D. J.; Pochan, D.; Deming, T. J. Nature , 2002, 417, 424-428.
The use of synthetic materials allows adjustment of copolymer chain length and
composition. Synthetic hydrogels may also be made from polysaccharides and
synthetic block copolymers which form thermoreversible gels and allow the
solubilisation of hydrophobic medications for controlled release, as taught by
Williams, PA, at the Centre for Water Soluble Polymers, North East Wales
Institute, Plas Coch, Mold Road, Wrexham, Wales.
Instead of forming the network with a true hydrogel, the network may be
formed with a complex carbohydrate, such as cellulose, pectin, starch,
maltodextrin or other polysaccharides. Forming of hydrated network structures
out of such materials is well known in the candy making industry for making
gummy candies. Or the network may be formed with a combination of a true
hydrogel and a complex carbohydrate.
6
CA 02505126 2005-05-05
WO 2004/043351 PCT/US2003/034549
The most crucial ingredient for the adherent oral patch is a hydrophilic
polymer that is liquid at human mouth temperatures located within pores of the
network. Collagen molecules, such as gelatin rendered from animal protein,
such
as from pork or cattle skin or from fish, serve very well as this ingredient.
Collagen molecules tend to adhere very well to the tissues of the mouth lining
which, themselves, are collagen molecules. The collagen molecules may be
partially hydrolyzed, making them shorter and lower in molecular weight, in
the
form of commercially available gelatin.
Hydrated hydrolyzed collagen assists with wound healing in human skin.
The inventor has found that, when applied in the mouth via an oral patch, it
reduces pain and assists with healing of wounds and other lesions in the
mouth.
Oral patches made according to this invention with no other active ingredient
were tested for short term pain reduction in aphthous ulcers with success
equal
to commercially available topical treatments having no anesthetic. Tests for
speeding of healing of oral wounds showed small but significant improvement.
Commercially available gelatins are graded according to "bloom strength"
which refers to the strength of the gel that is formed. Gelatin with a higher
bloom
strength (made with longer collagen molecules) is preferred for the adherent
oral
patch because it also has a higher viscosity in liquid form. The high
viscosity in
liquid form prevents the gelatin molecules from escaping the network
substantially faster than the network erodes, and the high viscosity better
retains
the medication for slow release. The highest commercially available bloom
strength, 250, is preferred.
The adherent oral patch is suitable for use with all of the medications
mentioned in U. S. Patent 6,139,861 issued to Friedman, including steroids,
such
as a glucocorticoid steroids, and non-steroidal anti-inflammatory drugs such
as
naproxen sodium, ibuprofen, acetaminophen, and ketaprofen. The medication
may also be an antimicobial, such as an anti-fungal for treatment of candida
organisms (thrush), such as nystatin, clotrimazole, miconazole, or
fluconazole.
The medication may be intended for treatment of canker sores (aphthous
ulcers),
including pharmaceutical antibiotics such as tetracycline, penicillin, or
amoxicillin,
7
CA 02505126 2005-05-05
WO 2004/043351 PCT/US2003/034549
or other canker sore treatment medications such as amlexanox or licorice root
(glycyrrhiza) extract in any of its forms, including deglycyrrhizinated (DGL)
or
enzymatically modified with glucuronidase enzyme.
If the network is formed of a hydrogel as described above, the oral patches
may be manufactured by processes well known in the candy making industry.
The process is to form a well-hydrated mixture at temperatures above the
activation temperature and below the boiling temperature of water so that
water
does not boil off and yet the hydrogels are fully activated for gelling when
the
product is cooled. In this process, the network can be formed of a combination
of
a true hydrogel such as xanthan gum with locust bean gum or with konjac gum
and a complex carbohydrate such as cellulose or pectin or starch. For the
medication licorice root extract, an effective ratio by weight is 56% water,
16%
gelatin, 11 % licorice extract, 10% cellulose, 4.8% glycerol, and 2.2% gums
such
as kappa carrageenan or xanthan gum plus locust bean gum or konjac gum
heated to between 130 and 200 degrees F. The water may be increased up to
75%, increasing the amount of subsequent drying required.
The hot mixture is poured or squirted into molds. The molds be may open
top molds, including a flat sheet, or closed molds. Open top molds may be
formed by pressing a plug into powdered starch such as cornstarch or may be
formed in a tray for packaging the products such as thermo formed PVC or PET
or a cold press laminate of aluminum and PVC with a thin layer of polyamide
for
strength. Closed molds may be used such as in an injection-molding machine.
The molds may be plastic lined, in which case the plastic becomes a part of
the
final packaging. A suitable size for each oral patch is 0.8 grams poured into
the
mold.
If the oral patches are deposited in powdered starch, the starch absorbs
some of the excess water and the oral patches are further dried in a drying
room
before being removed from the starch, packaged in a hermetic seal, and
sterilized with gamma radiation or heat and pressure in a retort.
If the oral patches are deposited in molds formed in a tray, the tray is
stored in a drying room until the oral patches lose a suitable amount of
moisture.
8
CA 02505126 2005-05-05
WO 2004/043351 PCT/US2003/034549
A suitable method of drying in trays is to expose them without convection to
room
temperature and humidity for 3 days or, with convection, for 24 hours. In the
drying process, the oral patches lose about 47% of their weight, so an oral
patch
that started at 0.8 grams poured into the mold becomes 0.42 grams. The trays
are then sealed with a film or foil lid that is adhered by conventional heat-
sealing
techniques and the entire package is sterilized with gamma radiation or heat
and
pressure in a retort.
For most applications, most users prefer that the oral patches be medium
dry to dry. With this starting dryness, the oral patches are more adherent and
have more integrity so they can be removed for talking or eating and then
replaced. The only draviiback to this dryness is that the oral patch becomes
hard
when it dries, giving the oral patch a less soothing feel. It is also less
conforming
and therefore does not stick well to hard surfaces such as guns and teeth.
When
the oral patch is placed in contact with delicate tissue, such as a large
canker
sore, most users prefer that the oral patch be moist and soft. Thus, it is
preferable to package the oral patches with a film that allows moisture to
pass so
moisture can easily be added to or removed from the oral patches without
removing them from the packaging. If the packaging film is a barrier to germs,
this allows the oral patches to remain sterile and not grow mold even when
they
are moist. Effective films are cellophane, polystyrene, poybutadiene,
polyamide,
Tyvek (matted polyethylene threads) and expanded films such as Goretex.
Polyamide with a thickness of .7 mil to 1.0 mil is effective. Allowing such a
package to sit for a day or two with a few drops of water on the package is
sufficient to hydrate the oral patch inside. Conversely, allowing the package
to sit
on a shelf in a dry room for one to three days is sufficient to dry out the
oral
patch.
While particular embodiments of the invention have been described above,
the scope of the invention should not be limited by the above descriptions but
rather limited only by the following claims.
9