Note: Descriptions are shown in the official language in which they were submitted.
. h
CA 02505713 2000-O1-21
s.
WO 00/42948 PCT/US00101557
' TITLE OF THE INVENTION
LOW PROFILE STENT AND GRAFT COMBINATION
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates to endoprosthetic devices, such as stents
and stent-grafts; that are used to repair and>or treat diseased or damaged
vessels and other structures within a body, and particularly fo such devices
that
can be introduced at small delivery profiles and then enlarged in place.
2. Description of Related Art
Stems and stent-grafts are used in the treatment of vascular and other
disease. They are particularly useful for treatment of vascular or arterial
occlusion or stenosis typically associated with vessels narrowed by disease.
Intraluminal stents and stent-grafts function to hold these vessels open
mechanically. In some instances, they may be used subsequent to, or as an
adjunct to, a balloon angioplasty procedure. Stent-grafts, which include a
graft
cover, are also particularly useful for the treatment of aneurysms. An
aneurysm may be characterized as a sac fom~ed by the dilatation of a wall or
an artery, vein, or vessel. Typically the aneurysm is filled with fluid or
clotted
blood. The stent-graft provides a graft liner to reestablish a flow lumen
through
the aneurysm as well as a stent structure to support the graft and to resist
occlusion or stenosis.
Treatment of a bifurcation site afflicted with such defects as an
occlusion, stenosis, or aneurysm i~ a particularly demanding application for
either scents or stent-grafts. A bifurcation site is generally where a single
lumen
or artery (often called the "trunk°) splits into two lumens or arteries
(often called
"branches°), such as in a "Y" configuration. For example, one such
bifurcation
site. is found within the human body at the location where the abdominal aorta
branches into the left arid right (or ipsilateral and contralateral,
respectively)
iliac arteries.
When a defect, such as an aneurysm; is located very close to the
bifurcation of a trunk into two branches, treatment becomes especially
difficult.
One reason for this difficulty is that neither the trunk nor either of the
branches
provides a sufficient portion of healthy bessel wall proximal and distal to
the
defect to which a straight section of single lumen stent or stent-graft can be
CA 02505713 2000-O1-21
~ ~ WO 00/42948 PCTNSOOl01557
secured. The stem or stent-graft must span the bifurcation site and yet allow
relatively undisturbed flow through each of the branches and trunk.
Stents and stent-grafts pffer considerable advantages over conventional
surgery. Because they are comparatively less invasive, reduced mortality and
morbidity, combined with shorter hospital stay, are the more significant
advantages of stent and stent-graft therapies. Low profile endoprostheses
(that is, endoprostheses that can be compacted into a small size for delivery)
continue to be developed that enable the introduction of such devices 'through
progressively smaller holes cut or punched through vessel wails. These tow
profile devices reduce blood loss and procedural morbidity compared to higher
profile devices. Preferably, low profile devices should also be more flexible
in
the compacted delivery state. Devices that are more flexible during delivery
better enable passage through tortuous vessels en route to the desired
delivery
site. Furthermore, thinner viialled devices may cause less flow disturbance at
the inlet and outlet to the graft.
The preferred device is one that can be introduced "percutaneously,"
that is through a small transcutaneous incision or puncture 12 Bench (Fj (4.0
mm) or less in diameter. Percutaneous delivery of a stent or stmt-graft can
often be done on an out-patient basis, and is typically associated with
decreased patient morbidity, deceased time to patient ambulation, decreased
procedural time, and potential reduction in health care delivery cost compaced
to surgical delivery of endoprostheses.
A "stent-graft" is formed by providing a covering on either the inside,
outside, or both surfaces of the stent. These covered devices can provide a
number of improvements over conventional uncovered stents. First, the cover
may provide a fluid barrier (that is, either liquid or gas or both).,
prohibiting
transmural fluid leakage from the inside, to the outside of the device, or
inhibiting transmural infiltration of fluids into the lumen of the device, or
both.
Second, covered devices can: also limit tissue encroachment into the device
over time. Third, it is believed that a covered device may provide an improved
flow surface, which may aid in longer ane more effective operating life for
the
device.
While covered stent devices have many benefits, unfortunately current
covered-stents used for the treatment of disease of large vessels (e.g.,
thoracic
or abdominal aortic vessels) generally require a surgical incision to provide
a
2
CA 02505713 2000-O1-21 '~,
WO 00/42948 PCTNS00/01557
Large enough access site to~deliver such devices. Virtually all such devices
currently are too large for less-invasive percutaneous delivery.
The current~standard procedure for stent and stent-graft delivery is '
outlined below. The stent or-stent-graft device is reduced in diameter
("compacted") to enable it to be introduced through small incisions or
punctures
via a traps-catheter approach. "Self-expanding" devices inherently increase in
diameter once a restraining mechanism is removed. "Restraining mechanisms"
typically fit over part or aft of the outer surface of 'compacted self-
expanding
devices to constrain them in a reduced diameter on the delivery catheter until
deployment. "Deployment" is the term liven to increasing the diameter of
these intrafuminal devices and subsequent detachment of the device from the
delivery catheter. "Deployed inner diameter" as used herein is the device
inner
diameter measured immediately subsequent to releasing the device from its
restraining mechanism in a 36 - 40 °C water bath and pressurizing the
device
to 1 Atm with an appropriately sized balloon dilatation catheter. An
appropriately sized balloon wiN transmit the 1 Atm pressure to the device. For
'~
devices that cannot withstand a 1 Atm pressure, the deployed inner diameter
correspands to the size of the device immediately prior to rupture. For
devices
that require balloon expansion, the applied pressure is that pressure required
to
fully deploy the device to its intended dimensions.
Once self-expanding devices are properly -positioned within the body,
the restraining mechanism is removed, thereby deploying and anchoring the
device. "Balloon-expandable" devices require the use of a balloon catheter or
other means of dilatation within the recipient luminal structure for
deployment
and anchoring. Such devices are typically mounted~and delivered on top of a
balloon, which inherently increases their delivery profile.
As has been noted, percutaneous delivery is almost always preferred
but is difficult or impossible to achieve for larger devices. A device
(including
restraining mechanism, if any) with a maximum outer dimension of no more.
than 10 French (F} (3.3 mm) can almost always be delivered percutaneously.
More skilled physicians may opt to place devices percutaneousiy with
dimensions of 12 F (4.D mm); 13 F (4:3 mm), 14 F (4.7 mm), or more; although
bleeding .and other complications increase markedly with increasing access
site
size. Generafiy herein,.a ".percutaneous" device is considered to be a device
that has an outer diameter in a delivered state of no more than 12 F.
3
CA 02505713 2000-O1-21
WO 00/42948 PCT/US00/01557
Devices are generally placed .into the body through percutaneously or
surgically placed introducer sheaths that are sized according to their inner
diameter. The wall thicknesses of these sheaths typically adds about 2 F to
the.
size of the device. That is, a 12 F introducer sheath has about a 14 F outer
diameter. "French" measurements used herein define the size of a hole
through which a device will pass. For example, device with a measurement of
"10 French" will pass through a 10 French hole (which has a diameter of 3.3
mm). A device need not have a circular cross-section in order to pass through
a circular 10 French hole so long as the hole is large enough to accommodate
the widest cross-section dimension of the device. The delivery size of an
intraluminal stent-graft device is a function of stent geometry, stent-graft
compacting efficiency, volume of the stmt, volume of the stent cover,
thickness
of the restraining r~iechanisrn (if any), and the outer diameter of any
guidewire
or catheter within the lumen of the compacted device.
There are many problems encountered in attempting to compact a
device into its smallest deliverable dimensions. First, the maferial of the
stent
element itself takes up a certain volume: If a graft component is added, this
further increases the bulk of the device. Accordingly, there are absolute
limits
to compaction based strictly on the volume of the component parts.
Second, all known stent element designs provide the stent-with crush-
resistance (which is required if the stmt is to have any struckurai value in
holding open a vessel). This resistance to crushing further confounds attempts
to tightly compact the device - with the risk that over-compacting the stent
may
damage its crush-resistance (and thus its structural value .as a stent). On
the
other hand, less resilient stent devices might be more receptive.to
compaction,
but are less effective in holding open the vessel once deployed.
Third, the graft material is also at risk of damage during compacting.
Since the stent and the graft are compacted together, the stent element must
be designed and compacted in such a way that it will not damage the graft
when the two are compressed together.
Fourth, any compaction of a stent or stent-graft will likely tend to redube
the flexibility of the compacted device. Extreme compaction may produce a
compacted device that is so inflexible that it will not negotiate tortuous
paths in
the body.
Fifth, as has been noted, currently available delivery devices and
techniques (e.g., introducer sheaths, guidewires, delivery catheters, etc.)
also
4
CA 02505713 2000-O1-21
' WO 00/42948 PCT/USOOI015S7
' add bulk to the device - generally adding about 2 to 3 F (0.67 to 1 mm) or
m4re
to the profile of the apparatus that must be delivered through the vascular
access site.
Sixth, there are covered stents available today that can be compressed
into small delivery profiles, but these devices undergo extreme elongation in
their compressed state, with extreme foreshortening when transitioned to their
deployed dimensions. These extremes in device length between compact and
deployed dimensions make these devices difficult to properly position and
deploy. Additionally, these devices tend to have less resilient stent
structures.
Finally, perhaps the greatest deficiency of these devices is that they must -
be
covered with a material that can likewise undergo extreme elongation and
contraction .to match. the longitudinal behavior of the stent. element. As' a
result,
preferred biocompatible graft materials such as polytetrafuoroethyiene (PTFE)
and woven DACRONC~7 polyester are not readily used on these devices since
neither is capable of extre(ne stretching and rebounding.
Results have been reported that a braided stent graft with a highly porous
elastomeric covering allows the stent, when compacted for delivery, to be
substantially elongated. Distributing the stent cross-sectional mass over a
longer length (up to 40% length change) allows percutaneous delivery of a
large device. Although these devices can fit through smaller delivery sites of
8
to 10 F, exact placement is often difhcult'because of the significant
longitudinal
retraction o~ recoil of the-stent graft as it reaches its deployed size. The
design
of this stent relies on extreme elongation to achieve its compaction, hence
undesirable foreshortening of the device naturally occurs during deployment.
As a result, this design cannot accommodate a longitudinal strength member
that would resist elongation during compaction of the device: In order to
allow
the stent frame to undergo extreme changes in length, elastomeric coverings
are employed to permit the cover.to expand and. contract along with the stent
frame. The coverings primarily serve as a barrier to the passage of blood
andfor tissue or other elements in use; although their stretch and recovery
requirements severely limit they types of materials that can be used in this
device. Thickness and porosity is also a design limitation for these
elastomeric stent coverings. To reduce porosity the coverings often must be
thicker (often about 0.05 mm or greater), which can adversely effect delivery
profile.
5
CA 02505713 2000-O1-21
CVO 00142948 PCTNS00/01557
It should be evident from the above description that it is very desirable
to provide an endoprosthetic device that can be delivered percutaneousiy. This
is especially true for an endoprosthesis that combines the benebts of both a
stent and a graft. However, there currently are a number of serious
constraints
limiting the ability to compact endoprosthetic devices, and particularly large
vessel endoprosthetic devices (for instance, for treatment of aortic diseases
and trauma}, into their smallest possible delivery profiles.
SUMMARY OF THE INVENTION
The present invention substantially reduces the delivery profle of
endovascular devices and substantially increases the ratio of the deployed.
inner diameter and compacted outer diameter of such devices.
To achieve extremely small delivery profiles, low profile devices of the
present invention are constructed using the following techniques:
exceptionally
thin and strong covers (e.g:, expanded polytetrafluoroethylene and/or
polyester
mesh); small gauge (diameter) wire (e.g., nitinol wire) to cons#ruct stent
frames
or thin-walled cut tubing; scent geometry that enables a high packing
efficiency;
low mass means of attaching covers to stent frames; and improved methods
for compacting stent-grafts. Furthermore; a low profile means of restraining
the
device in the non-distended (i.e., compacted} state, ready for delivery, are
also
utilized in the design. .Finally, improved delivery techniques that enable the
use
of lower profiile devices are also incorporated.
An important purpose of the present invention is to provide
percutaneously deliverable, large diameter stent-graft devices for the
treatment
of large vessel (e.g., aortic) disease. An endoprosthetic device of the
present
invention can achieve extremely high compaction effrciencies, containing a
stmt-graft with a deployed inner diameter of greater than 23 mm into a
compacted dimension with a diameter of less than 12 F. This .can be achieved
with minimal longitudinal length change in the device between its compacted
and deployed dimensions.
Further advantages of the present invention are to provide a very low
profile covered-stent, and to provide a covered-stent with the broadest
deployment range, from the delivery size~to the fully extended (i.e.,
deployed)
size. Still another advantage of the present invention is the ability to
create
very small implantable devices that are capable of being delivered in
extremely
6
CA 02505713 2000-O1-21
.
WO 00/42948 PCT/US00/01557
small compacted dimensions. These and other benefits of the present
invention will be appredated from review of the following description.
~p~SCRIPTION OP THE DRAWINGS
The operation of the present invention should become apparent from the
following description when considered in 'conjunction with the accompanying
drawings, in which:
Figure 1 is three-quarter side elevation view of a large diameter thoracic
aortic stent-graft of the' present invention shown in its fully deployed
dimension;
Figure 2 is a three-quarter side elevation view of a large diameter stent-
graft of the present invention shown in its compacted dimension and mounted
on a delivery catheter beneath a sleeve-like restraining mechanism;
Figure 3 is a side elevation view of one embodiment of deployment
apparatus for use with the present invention;
Figure 4 is a three-quarter side elevation view of a two-part, modular,
large diameter bifurcated stent-graft of the present invention shown in its
fully
deployed dimension;
Figure 5 is a top view of the stent-graft of Figure 4;
Figure 6 is a side elevation view of the stent-graft of Figure 4 shown in
its deployed orientation (i.e., with the modular components of Figure 4 joined
together);
Figure 7 is a top plan view of a stent pattern used in a thoracic aortic
stem-graft shown in Figure 1: The stent-graft is cylindrical but is
represented in
this "flat plan configuration" by making a longitudinal cut along the length
of the
endoprosthesis and the uncoiling of the endoprosthesis along this cut into a
flat
sheet;
Figure 8 is a flat pattern configuration of the stent element that may be
used to form a straight stent-graft of the present invention;
Figure 9 is a flat pattern configuration of the stent element used to form
the trunk component of the modular bifurcated stent-graft of Figures 4 through
6;
Figures 10 through 13 are side cross-section views of the steps of
deploying. a straight slant-graft of the present invention within a vessel
hewing
an aneurysm, the stent-graft being deployed .from a fully compacted dimension
in Figure10 to a fully deployed dimension in Figure 13;
7
CA 02505713 2000-O1-21 '~
' WO 00/42948 ~ PCT/US00/01557
Figure 14 is a top plan view of one embodiment of a tapered die used to
compact the stent-graft of the .present avention;
Figure 1,5 is a side cross-section view of the tapered die of Figure 14
along section line 15-15;
Figure 16a is a three-quarter isometric view of another embodiment of a
tapered die used to establish a pleated compacted endoprosthesis of the
present invention, this tapered die including fluting (i.e., longitudinal
ridges) to
assist in compaction by forming folded pleats in the device during compaCtion;
Figure 16b is a side cross-section view of the tapered die of Figure 16a
along line 16b-16b;
Figure ~16c is a top plan view of the tapered die of Figures 16a arad 16b
showing a circular stent-frame being drawn through tt~e tapered die using
tether
lines;
Figure 17A is a side elevation view of a segment of a straight stent-graft
of the present invention shown in its fully deployed dimension;
Figure 17B is a side elevation. view_of the segment of straight stent-graft
of Figure 17A shown in its compacted dimension having been folded into
pleats;
Figure 18 is a section view of the pleated stent-graft of Figure 17B along
section line 18-18;
Figure 19 is a side elevation view of a partially covered stent-graft of the
present invention in a fully deployed dimension having tether tines attached
to it
for compaction through a tapered die, the tether lines being aligned with
scent
undulations aft facing in the same direction;
Figures 20 through 22 are side elevation views of a compression fixture
used to compact a stent-graft of the present invention through a tapered die
and into.a restraining sleeve, a straight stent-graft of the present invention
being illustrated in the sequential cocnpacction steps;
Figure 23 is a flat pattern configuration of another embodiment of a
~ restraining sleeve used to constrain the stent-graft of the, present
invention;
Figure 24 is side elevation view of a restraining sleeve incorporating the
pattern of Figure 23, with the sleeve shown partially removed via an integral,
multi~filarnent deployment line;
figure 25 is a side elevation view of another embodiment of a stent-
graft of the p~esertt invention being deployed in an aneurysmal, bifurcated
blood vessel shown in cross-section;
8
CA 02505713 2000-O1-21
WO OOI42948 PC'T/US00f01557
Figure 26 is longitudinal cross-section view of the stent-graft
embodiment shown in Figure 25 illustrating a pusher mechanism for stent-graft
deployment directly from a delivery catheter without use of a guidewire or a
separate restraining sleeve on the stent-graft;
figure 27 is a side elevation view of another embodiment of a stent-
graft of the present invention being deployed in an aneurysmal, bifurcated
blood vessel shown in cross-secttion;
Figure 28 is a flat pattern configuration of a stent element of the present
invention cut from a metal tube; and
Figure 29 is a side elevation view of another embodiment of apparatus
used to compact stent-grafts of the present invention, this embodiment
employing a two-stage tapered die.
DETAILED DESCRIPTION OF THE INVENTION
The present invention.is an improved endoprosthetic device, partiaularly~
such a device for use in large diameter vessels, that is capable of being
compacted into very small delivery dimensions. For instance, stent-graft
endoprosthetic devices of the present invention 'nay be formed in large
deployed dimensions for thoracic aortic vessels (with diameters of 26, 28, 30
mm or more) or-for bifurcated abdominal aortic vessels (with diameters of 23,
25, 27 mm or more) that can be delivered at very small compacted dimensions
of '14 F (4.7 mm) or less. In fact, the present invention can even produce
large
vessel stmt-graft devices that can be delivered percutaneously at less than or
equal to 12 F (4.0 mm).
Additionally, the compacting technology of the present invention also
permits construction of extremely small .devices; on the order of 4 mm or less
in
deployed diameter that can be delivered in a compacted dimension of less than
3 or 2 F (1 or 0.7 mm).
Figure 1 illustrates one embodiment of an endoprosthesis 10 of the
present invention at its deployed dimension. This endoprosthesis 10 co'nprises
a stent element 12 and a cover 14.attached together to form a stent-graft
combination. This particular endoprosthesis 10 is designed-forlining a
thoracic
aortic vessel, for Instance to treatwad aneurysm therein. Typically this
.requires
the device to have a cross-sectional diameter "a" in its deployed dimension of
about 26'mm to 40 mm or more.
9
CA 02505713 2000-O1-21
WO OOI42948 ~ ~ PCT/US00/O~SS7
Current commercially available stmt-graft devices used to treat thoracic
aortic aneurysms of this .type are delivered in compacted dimensions of about
18 F (6.0 mm) to' 27 F (9.0 mm) or more. These compacted dimensions 'are so
large, that percutaneous delivery of these devices is difficult or impossible.
Typically delivery of these large vessel endoprostheses requires a surgical
cut-
down to access deeper but larger blood vessels for device insertion.
Alternatively, some large vessel devices available today can be delivered at
small delivery profiles, but these devices must undergo.extreme and highly
undesirable changes in longitudinal length between compacted and deployed
dimensions.
By contrast, by employing the materials and compaction techniques of
the present invention, as described below; the present invention can. be
reduced to compacted dimensions small enough for percutaneous delivery.
Moreover, such extreme compaction. can be.achieved with minimal elongation
or foreshortening of the device between compacted and deployed dimensions.
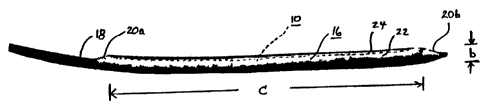
Figure 2 illustrates a scent-graft endoprosthesis 10 of the present invention
presented in compacted dimensions for delivery. lath a self expanding stent-
graft 10, the device is contained within a restraining device 16 and mounted
on
delivery catheter 18. Olives 20a, 20b are provided at the proximal and distal
ends of the device as mounted on the delivery catheter to assist in holding
the
stent-graft in place on the catheter and to aid in guiding the device through
smaller vessels.
This embodiment of the present invention employs a restraining device
16 comprising a membrane 22 of material wrapped around the, stent-graft 10
and sewn in.place with a deployment line 24. Removal of the deployment line
24 will cause the stent-graft device to self-expand to its deployed dimension,
such as that illustrated in Figure 1.
In the compacted dimension shown in Figure 2, the device as mounted
on the catheter has a cross-sectional diameter' of "b." With the present
invehtion diameter b comprises 12 F (4.0 rnm}, 11 F (3.7 mm), 10 F (3.3 mm),
9 F (3.0 mm), 8 F (2.7 mm), 7 F (2.3 mm), 6 F (2.0 mm) or. less. Preferably
the
device is compacted to a diameter of 12 F or less, so that conventional
delivery
apparatus can be .employed and percutaneous delivery can stilt be performed
through an introducing sheath of 12 f or less. More preferably, the stent-
graft
10 is compacted to under 9 or 8 F, allowing percutaneous.delivery through a 9
F delivery apparatus. ft should be noted that while the dimension "diameter"
is
CA 02505713 2000-O1-21
" ,
WO 00/42948 iPGT/US00/01557
used herein, it should be understood that this dimension is intended to define
the effective cross-sectional dimension of the device and is not intended ~to
limit
the present invention to devices with circular cross-sectional shapes.
With respect to device length; the thoracic device illustrated in Figures 1~
and. 2 undergoes ~essentialiy no .change between its compacted longitudinal
length' "c" and its ~depioyed longitudinal length "d ~ In this ihstance, the
.ratio of
c:d is significantly less than 1.25 (i.e., gignifcantly less than a 20% change
in
length). The endoprostheses of the present invention sh9uk! undergo less than
.a 25% change in longitudinal length between its compacted dirnertsion and its
deployed dimension. Preferably, the device will undergo less than a
20°~
change in length, and even more preferably it will. undergo less than a 15% or
10% change in length, Most preferably, an ertdoprosthesis of the. present
invention wilt experience less than a 5°!o change in longitudinal
length between
.its fully compacted ~d'rmension and its fully deployed dimension. In this
respect,
the entire device as deployed .is maintained compacted within 25% or less of
its deployed longitudinal dimensions, without the need for excessive
elongation
of the device in its compacted state or the splitting of the device into
multiple
parts in order to:achieve low profile delivery-.
In order.to achieve the small compacted dimension of the present
invention, the first important design element is to employ unique love=profile
materials. Cover-14 comprises a thin but strong material that is
biocompatible,
sufficiently flexible to undergo extreme compaction while returning undamaged
to a .fully deployed state, and sufficiently shong so as to provide proper
support
of the vessel walls once deployed. The preferred material comprises
polytetrafluoroethyiene (PTFE), and especially an expanded PTFE.rilaterial.
This .expanded PTFE material is described. in United States Patents 3;953,566,
3,962,153, 4,096,227, 4,187,390, 4,902,423, and 5,476,589. Polyester material,
such as woven DACRON~ polyester, may also be suitable.
The preferred expanded PTFE material for use in the present invention
comprises a material having: a thickness of less than about 0.03 mm, and more
preferably less than about 0.00~'mm; and a longitudinal matrix tensile
strength
of mare than about fi50 M~Pa, and more preferably more than about 800 MPa.
Layers of this material are used to create the stent cover. Note .that the
thickness of the cover may be less than the sum of the thicknesses of the
individual layers because the film tends to decrease.in thickness during the
11
- ~ CA 02505713 2000-O1-21
_ _ . _._
_ . .
WO 00/42948 ~ PCT/U~00/01557
heat bonding process used to attach the film to the stent frame. "Thickness"
can be measured.with a snap gage or an optical comparitor, or by the use of a
scanning' electron -rnicrograph. "Longitudinal matrix tensile strength" refers
to
the matrix tensile strength of.the material that in the direction that is
'parallel to
the predominant orientation of the fibrils, which corresponds,to the higher
strength direction of the material. Tensile strength may be determined using
an INSTRON tensile tester. For a porous polytetrafluoroethylene (PTFE)
material; such as expanded f~TFE, matrix tensile stcehgth is detemlined as the
tensile strength of the material multiplied by the quotient of the density of
the
'i0 PTFE polymer and the bulk density of the expanded.PTFE material. For the
~pu~pose .of calculating matrix tensile strength, 2:2 glcc is used as the
value for
the PTFE polymer density. Bulk density takes into account any porosity of. the
expanded PTFE materiat.
For blood conduit applications,. the cover should resist the passage of
liquids under pressures of about 150 mrn Hg or more. For applications
requiring that the covey provide exceptional liquid or gas ,permeation
resistance
(for example, a cover that may be required to resist bile permeati.on), a
permeability. as quantified by a Gurley Number of greater than about 60
seconds for 1 Cm3 of material for 1,00 cc of air is.preferred, and even more
preferably a Gurley Numtrer in excess of .100 seconds for 1 cm2 of material
for
100 cc of air; a thickness .of about 0.05 to 0.25 mm, with a thickness of
about
0.10 to 0.20 mrn preferred; a water entry pressure of about 34 to 102 kPa or
more, with 48 to 62 kP3 or more preferred.
The stent element 12'and the cover 14 of .the present invention are .
adhered together (for instance, by an adhesive andlor by ~ wrap of an adhered
film or by bonding the stent element between layers or to layers of the cover)
to
maintain position of the covet 14 on the eniioprosthesis 10. .The attachment
of
the cover 14 to the stent element 12 also restricts the stent element 12 from
excessively longitudinally elongating v~hen longitudinal tension is applied to
the
endoprosthesis 10. It is believed preferable that the cover 14 line the
interior
of the stent element 12, as shown, but acceptable results, may also be
achieved
with the cover 14 placed on the Qutside of the stent element 12, with the
cover
being placed .both inside and outside of the stent element 12, or with.the
stent
element 12 being embedded within the cover 14. As such, the term "cover" as
used herein is intended to include, any generally continuous material that is
.
12
CA 02505713 2000-O1-21
W0 00/42948 . IPCT/US00/01557
placed aside of andlor placed outside of and/or mounted integrally with the
stent element 12 of the. pwesent invention.
In order to achieve the lowest possible profile for the device of the
present invention, it is very desirable that the cover 14 be attached ~to
the~stent~
element 12 in such a way that the device does not become significantly less
flexible and the device does not have significantly more mater<al added to it:
Although attaching the covet using a ribbon of adhered film can produce
acceptable results, it is nat believed to be_preferred since the film may
increase
the rigidity of the device, impart undesirable undulations or corrilgations to
the
luminal surface during bonding, and the film also adds volurpe to the device.
Accordingly, the preferred attachment method for joining the stem. element 7 2
and the.,cover 14 is to apply layers of the cover tci both the inside
and~outside of
the. stem frame; then bonding the layers together at a temperature.above the
crystalline melt temperature of PTFE (327°C}. The luminaf surface is
~ preferably fomled to be as smooth as possible (i.e., a smooth surface devoid
of .
as much corrugation as possible). Alternatively, attachment may tie pertormed"
by coating the stent frame with an adhesive or applying an adhesive to the
cover material, then bonding the 'cover to the frame. One suitable adhesive
material for these applications is fluorinated ethylene propylene (FOP.).
The stent element 12 is preferably formed from a super elastic material
that will.withstand extreme compaction~yet vivid readily return to its
original
dimensions without damage when unconstrained. Additionally, if the stent
element 12 is formed from a material that will self-expand in place, rrtaximum
compaction can be achieved since a means of dilatation or expansion (e.g.,
employing a baltoon catheter) need not be delivered withimthe device in its
cornpacted_ dimensions. Suitable materials include alloys of stainless steel,
nickel-titanium alloys .(nitinol}, tantalum, platinum, and titanium and rigid
polymers.
The preferred material comprises a nickel-titanium alloy (nitinol) metal
wire having a diameter of about 0..4 mm or less, and more preferably; a
diameter of about 0.2 mm or less. For extremely small stent-grafts, a wire
with
a diameter of about 0.1 mm or less may be preferred. The preferred nitinol
wire comprises a nickel content of about 51 % and a titanium content of about
49% (for example, SE 508 nitinol wire available from Nitinol De~iices &
Components, F~emont, CA, USA). Additional properties that the wire may
beneficially have include: a tensile strength of about 1200 MPa or more;- cold
13
_ ~ CA 02505713 2000-O1-21 _- .
_~~ ._
' WO 00142948 " . PCTIUS00/OX557
working of about 40=45%; a tensile modulus of approximately 35 to 70 x 108
kPa; and an electropoiished finish.
A self-expanding stent element may .be formed from this material
using a pin jig and following conventional procedures, such as those taught in
U.S. Patent Nos. 8,042,605; 6,361,637; and 6,520,986 to Martin et al. The
' preferred cross-sectional shape of the stent structure is not necessarily
circular.
It is possible that wire having an oval-shaped cross-section or a nitinol
ribbon
may be configured into an acceptable device. Likewise, with laser-cut tubes,
there is a great deal of flexibility with cutting and polishing to achieve,non-
circular cross-sectional geometries.
Since the elastic properties of the scent material, the moment of inertia
of,stent cross-sectional geometry and the design of the overall stent
structure
cornbiiie to dictate the physical characteristics of, the stent, the specific
strut
material, cross-sectional geometry, and stent design maybe integrally
iinked'to
~. given clinical applications.
By forming a device using the preferred materials. of about 0.003 mm
thick expanded PTFE membrane and about 0.3.mm diameter.nitinol metal wire
that are adhered together by~heat bonding, a 40 mm diameter thoracic aortis
stent-graft device, as shown in Figure 1, can be readily. compacted down to a
3.33 mm or less diameter delivery profile, as~shown in.Figure.2. As is
explained in greater detail below, .even further.profixe reduction can be
achieved
by employing unique folding pnd restraining advances of the present invention.
A delivery apparatus that may be used to deliver an endoprosthesis 10
of the present invention is iAustrated in figure 3. This deployment apparatus
26 comprises: an infroducer sleeve 28;,a restraining device l6; a distal shaft
30; a proximal shaft.32;~ a strain relief 34; a deployment port 36;
a.deployrnent
knob 38 mounted within the deployment port 36 that is connected to a ' '
deployment line 24 attached to a restraining device 16 surrounding, the
endoprosthesis 10; a side ann adapter 4D; a filushing port 42; and ~a
guidewire
port 44. A radiopaque marker 46 may be provided on the distal shaft 30 to aid
in the remote positioning of the endoprosthesis 10. The operation of the
deployment apparatus 26 is explained in detail below with reference to Figures
10 through 13.
. Low profile delivery of a bifurcated- device, such.as that employed in
repairing an abdominal aortic aneurysm (AAA),.is an even more challenging
14
'~ CA 02505713 2000-O1-21
~WO 00/42948 PCT/US00/01557
application of the present invention. The challenge in these applications is
that
an aneurysm will, commonly form at the junction of the common iliac arteries
in
the ~abdominel aorta. ~In order to repair this defect, a device ideally
comprises a
bifurcated structure that has one large opening at one (proximal) end that
splits
into two smaller legs at the other (distal) end. In this manner, the device
can
attach to the host artery above the aneurysm and below the aneurysm in each
of the iliac arteries individually, thereby excluding the aneurysmal lesion
from
the blood stream.
Although a bifurcated device is preferred for treating AAA, such devices
have a number of inherent problems. : First, the fact that a bifurcated device
has two tegs presents a placement problem when the device is to be delivered
by way of one of the iliac arteries. While the upper proximal end and one leg
sari be easily positioned properly around the aneurysm; the ability to then
direct
the other leg through the ather iliac artery can be a challenge for medical
personnel. Second, the complexity of the bifurcated device necessarily adds a
substantial amount of bulk to the device when compacted.
Numerous proposals have been made to address the first of these
problems. One common approach is illustrated in Figures 4 through 6. In this
embodiment, a bifurcated endoprosthesis 48 is provided that includes a trunk
segment 50 having a long ipsilateral leg 52 and a short contralateral leg 54.
The trunk segment 50 is.delivered through the ipsilateral iliac artery and
positioned and deployed in place. A separate contralateral leg stent-graft
segment 56~is then delivered through the contralateral iliac artery and
deployed
to join o the short contralateral leg 54 on the trunk segment 50 to complete
the
bifurcated device 48. The completed device is illustrated in Figure 6.
Even with separation of 'the bifurcated device into two separately
deployable segments 50, 56, the trunk segment 50 t~nnot be compacted into
small enough dimensions for percutaneous delivery (although the contralateral
leg segment 56 typically can be compacted using~conventional methods from
deployed dimensions of about 8 to 76 mm in diameter down to compacted
dimensions of about 4 to 5 mm in diameter). - Typically the trunk segment 50
will have a deployed large proximal opening 58 measuring.about 20 to 36 mm
in diameter ,(°a") and a small distal opening 60 on the ipsilateral leg
52
measuring about 8 to 16 mm~in diameter. Curren8y, this trunk segment 50 is
delivered at a diameter of about 18 F (6.0 mm) - entirely too large for
percutaneous delivery.
CA 02505713 2000-O1-21 '_~
' WO 40/42948 ~ PCT/US00/p1557
However, the device 48 may be constructed from a stent frame 62
employing the 0.3 mm nitinol wire previously described, with a cover 64
constructed ftom the 0.003 mm thick expanded PTFE membrane previously
described. Joining the .stent frame and cover together via heat bonding
produces a, tow profile-steht-graft-of .the present invention. .Employing the
low
profile materials previously described, the trunk segment 50, having a
proximal
opening 38 of about 31 mm in diameter and a distal opening 60 of about 13
mm in diameter, can be reduced to a compacted dimension of 10'F (3.33 mm)
or less. The total cover thickness, formed from multiple film layers, is
preferably less than about 0.02 mrrm and more preferably about 0:013 mm or
less.
The preferred winding patterns for the various start elements of the
present invention ace iAustrated in Figures 7 through 9. Figure 7 illustrates
(in
flat orientation) a winding pattern 66 for a thoracic aortic endoprasthesis
shown
in Figure 1. 1n this instance the slant element 12 comprises an undulated wire
having a series of forvvard facing apices 68 and rearward facing apices 70. As
will be appreciated following review of the compaction techniques discussed
below, it is preferred that the apices 68, 70 of each cow are in phase with
the
apices in neighboring rows. For instance, the forward facing apices 68 in row
72a are directly in phase with the forward facing apices 68 of rows 72b and
72c. This winding pattern includes two longitudinal struts 74a, 74b to aid in
maintaining the longitudinal length and column stiffness of the
endoprosthesis.
Figure 8 illustrates (again in flat ,orientation) a winding pattern 76 for a
start element used in a straight dimensioned endoprosthesis. Again, forward
facing.apices 78 and rearward facing apices 80 are in~phase with. neighboring
forward and rearward facing apices.
Figure 9 illustrates (again in flat orientation} a winding pattern 82 for the
proximal end of the trunk segment 50 of the bifurcated graft shown in Figures
4
through 6. Again, within the constraints of this more complicated winding
<.
pattern, the forvvard facing apices 84 and rearward facing apices 86 are
essentially in phase with neighboring fornrard and rearward facing apices.
While these winding patterns are preferred for the vario~rs described
orientations, it should be appreciated that the exact pattern used may be
application and material specific. Accordingly, the present invention is not
intended to be limited to the winding patterns illustrated.
16
CA 02505713 2000-O1-21
~WO 00/42948 PCTIUS00/01557
The process for deploying an endoprosthesis of the present invention is
iAustrated in Figures 10 through 13. In this, instance a straight tube
endoprosthesis, similar to the one illustrated in Figures f through 3, is
being
deployed in a vessel 88 having an aneurysm 90 therein.
initially, a small incision is fom~ed through the patient's skin at a site
remote from the aneurysm, for instance to expose arid access the femoral
artery at the patient's groin. Using the deployment apparatus illustrated in
Figure 3, the delivery catheter 18 is passed through the patient's skin into
the
femoral artery via an indwelling introducer sheath. The introducer sheath is
left
in place through the skin and arterial wail to hold open this access site and
provide a conduit into and out of the patient for insertion. and withdrawal of
the
endoprostheses and other tools of the physician. Ultimately, it is the outer
diameter of the introducer sheath that determines whether the procedure can
be performed percutaneously. Most commercially available introducer sheaths
have.a wall thickness of about 1 F (0.33 mm), adding about 2 F (0.67 mm) to
the diameter of the access site. Accordingly, a 10 F compacted endoprosthesis
will require about a 12 F access site for introduction using conventional
introduces sheaths.
The endoprosthesis 10, confined in restraining device 16 and -mounted
on the delivery catheter shaft 18, can be negotiated through the various blood
vessels until it is positioned within the aneurysm 90, as~illustrated in
Figure 10.
Positioning 'of the device 10 in the vessel 88 can be directed using a
fluoroscope or similar device. Radiopaque marker 46 can be used to aid in
precise positioning of the device.
Once properly positioned, the restraining device 16 can be removed by
actuating deployment line 24. This will allow the self expanding device 10 to
progressively enlarge in place, as shown in figures 11 and 12. ' Once the
restraining device 16 is completely opened or removed., the endoprosthesis 10
will be fully deployed, completely sparining the aneurysm 90, as is
illustrated in
Figure 13. The delivery cafheter 18 can then be removed. At this stage the
device 10 can be further enlarged using a balloon catheter (which may be used
to assure proper anchorage and ;smooth any wrlnkles that may have formed
during deployment). Following any subsequent procedures; all tools and
delivery apparatus, including the introduces sheath, are removed and the
access site is sealed.
17
CA 02505713 2000-O1-21
' WO 00/42948 ~ PCTNS00101557
It is preferred to compact the endoprostheses of the present invention
through a funnel-shaped tapered die 92, such as that illustrated in Figures 14
and 15. The die 92 has a large opening 94 at one end and an internal. taper 96
leading to a much smaller operiing_ 98 at the opposite end. Preferably the
large
opening 94 is sized to be target than the deployed dimension of the
endoprosthesis. The taper 96 is preferably set at an angle 10'0 of
approximately 5 to 45°. The smaller opening should be approximately the
final .
desired compacted dimension of the endoprosthesis. The process for
compacting an endoprosthesis through such a die 94 is explained in greater
detail below in reference to I=igures 19 through 22.
Compacting through a smooth tapered die 92, such as that illustrated in
Figures 14 and 15; provides very good results. However, compacting in this
manner tends to produce random folds within the compacted device.
Moreover, the orientation of the forvvard-facing and rearward-facing, apices
of
the stent tends to be random and disorganized. The present inventors have
determined that far more effective and extensive compaction can be achieved if
the process of folding the endopr~sthesis into its compacted.dimension is more
carefully controlled. In particular, it has been determined that optimal
compaction of some endoprostheses can be achieved by folding into evenly
spaced pleats.
Figures 16a through 16c illustrate a modified tapered die 102 that is
designed to, provide pleated folds into an endoprosthesis. This die 102 again
includes a large opening 94 at one end and an internal taper 96, and a small
opening 98 at its opposite end. However, in this die 102 a number of raised
flutes (or ridges) 104 are provided within the tapered die separated by
grooves
106. The raised flutes 104 and/or the grooves 106 may be formed by molding
or machining the shapes into the die 102. Alternatively, as is illustrated,
the
flutes 104 may be formed by forming evenly spaced bands 108 wound around
the tapered die 102, such as by using nylon filament with a diameter of about
0.38 mm. Regardless of how the flutes 104 are formed, each raised flute 104
preferably corresponds to,one desired pleat to be formed in the
endoprosthesis. Additionally, the flutes maybe configured to be flee-floating
within the lumen of the tapered die so as to allow lateral movement of the
flute
as an endoprosthesis is drawn through it. This, for example, may be acfiieved
by fixing the radial position of. the flutes at the inlet to the die 94, but
not
restricting the radial position of the flute through the remainder of the die
18
CA 02505713 2000-O1-21
WO 00142948 ' PCT/US00/01557
lumen. Alternatively, lines may be attached to the endoprosthesis prior to
drawing and removed subsequent to compaction.
A stent or stent-graft is pulled through the tapered die by tying a series
of tether, lines around the circumference of the scent. When using a fluted,
tapered die 102 precise pleat placement can be achieved by tying the tether
lines to the portions of the stent that are to be folded outward. When pulled
through the fluted, tapered die, the tether lines will self align with the
grooves in
the die {thus folding outwardly) while the untethered portions of the stent
will
pass over the flutes (thus folding inwardly).
This process is illustrated in the single stent ring 110 being drawn
through the tapered die 102 by tether lines 112 in Figure 16c. As can be seen,
the tether lines 112 have aligned with grooves 106, folding the stent ring 110
outwardly, white the untethered portions of the stent Ping 110 are drawn over
the flutes104 and are being folded inwardly.
As is illustrated in Figures 17A, 17B, and 18, a scent-graft imptantable
device 114 formed in this manner will pass from a deployed dimension
°a" to a v'
pleated compacted dimension °b." Since the tether lines can direct
where the
folds will occur, this folding technique can be used to direct all of the
forward
facing apices 116a in the stent frame to fold inwardly. In the folded
orientation
of Figure 17B, all the forward facing apices 116a have been folded beneath the
outer surface of the compacted device while the rearward facing apices 116b
have been folded to the outer surface of the compacted device into pleats 118.
This kind of control of folding is believed to be very beneficial to maximize
folding efficiencies by increasing the density of the compacted
endoprosthesis.
Additionally, it has been found that it is sometimes beneficial to have
exposed
apices of a stmt all facing in only one direction (that is, only the rearward
facing
apices 116b are exposed in Figure 17B). In this way, the folded device is less
likely to catch on biological structures (such as plaque and side branches),
restraining sleeves, deployment lines, and other devices that may be pulled
over the compacted stent.
The advantage of forming a stent frame with all of the forward aid
rearward facing apices in phase with one another should now be evident from
the above description. By keeping the apices in phase, .pleats can be formed
that will direct all of the apices of one orientation into or out of the
compacted
devices. Additionally, by employing in-phase apices, greater compaction is
achievable (since all of the apices will fold and compact in the same
direction).
19
CA 02505713 2000-O1-21
'WO 00142948 ~ PCT/~JS00/01557
It should be appreciated that the pleated folding methods described
herein can be used to direct apices into a wide variety of folded patterns. As
such, the terms "forvvard" and "rearward" facing apices are used only for
convenience to describe sets of apices that face in one direction or an
opposite
direction, without regard to the actual direction the device may ultimately be
deployed.
Figure 19 illustrates a partially covered stent-graft 120 being prepared
for compacting through a tapered die. .ln this instance the stent-graft 120
comprises a device with covered segment 122 and an uncovered segment 124.
The tether lines 112 are attached to either end of the stent frame 126 in an
evenly spaced manner. In this instance the tether lines 112 are aligned with
rearward facing apices 128a, 128b, 128c (which are intended to remain
exposed}. The tether lines '112 may be formed from thin wires, .polymer
fibers,
or other suitable materials. The tether lines 112 are joined together to form
a
termination such as a knot (or cuff) 130.
One apparatus 132 suitable for compacting endoprostheses through a .
tapered die is illustrated in Figures 20 through 22. The apparatus 132
comprises a jig 134 for holding a tapered die 92 and a restraining device 16,
and an actuation mechanism 136, in this example a screw drive 138 actuated
by a motor 140.
A stent or stent-graft device 142, with tether lines 112 attached, is then
oriented by large opening 94: Tether lines 11'2 ale then passed through the
die
92 and the restraining device 16, and attached to the actuation mechanism 136
at post 144, as is shown in Figure 21. Once attached, the actuation
mechanism is used to draw the stent-graft 142 through the tapered die 94 and
into the restraining device 16 using a constant rate of translation, or,
alternatively, a constant tensile force applied to the tether lines. The.
device is
preferably pulled through the die at a fow rate, such as 200 mm/min or slower.
After the stem-graft device 142 has been compacted into the restraining device
16, the tether tines 112 can be removed and the compacted device can then be
mounted on a catheter and otherwise packaged and prepared for delivery.
Alternatively, the device can be compacted directly onto a catheter.
It has been found that significantly smaller compacted dimensions can
be achieved if the endoprosthesis undergoes repeated compressions through a
series of progressively smaller tapered dies. It is believed that an
additional
reduction in compacted size can be achieved simply bypassing the
CA 02505713 2000-O1-21
WO 00142948 PCT/I1S00/01557
endoprosthesis through a series of 2 or mope, preferably 3 to 6, tapered dies
of
progressively smaller dimensions. As long as.excessive compaction is' not
attempted, this process does not appear to damage the endoprostheses.
Dra~ivin~g the ~endoprosthesis repeatedly through a same-sized die can also
enable the device to be subsequently drawn through an even smaller die. This'
technique can reduce the profile by '1 to 2 F ~r more. .
The restraining device 16 used to contain the self-expanding
endoprostheses of the present invention may also be reduced in profile to aid
in
reducing the ultimate compacted dirhension of the present invention. With
respect~to the membrane.res#raining device previouslydiscussed'and
illustrated in Figure.2, the overaN thickness of the membrane may be reduced
to its absolute minimurio dimensions. For example, the preferred restraining
means will have a thickness of 0.07 mm or less, and more preferably 0.025 mm
or less. .
Another approarti is to ~ernploy a ~ releasable thread as the restraining
device '! 6. For instance, Figures 23 and' 24 illustrate a series of threads
146a,
146b, 146c, and 146d that are formed into a warp knit 14$ around a device :.
150. This form of containment device is disclosed in United States Patent No.
6,224,627 issued May 1, 2001, to Armstrong et al. By releasing one thread of
the warp knit 148 at one end of the device (for example, thread 146a), the
entire restraining device will unravel and separate as a cohesive deployment
line 152, as is shown in Figure 24. This form of restraining device has proven
very effective at both containing a self-expanding stent element and releasing
it as an entire unit. Moreover, this form of restraining device adds minimal
profile to the compacted device. Although not preferred, another device
employing threads to contain an endoprosthesis with minimal profile increase
is disclosed in United States Patent No. 5,405,378 to Strecker.
Still another embodiment of a device for containing 'the compacted stent
is illustrated in Figures 25 through 27. When deploying therapeutic devices
into
the vessels of the human body conventional techniques entail starting the
procedure with a'standard guidewire to traverse tortuous bends and or
obstructions. Once the guidewire "~s directed to the desired destination in
the
vessel; a catheter such as a~guiding catheter or iritroducer sheath is
coaxially
inserted over the guidewire and advanced to the treatmenYsite. At this point
in
the procedure, as.-depicted in Figure 25'; the Ginlcian could remove the
,~ 21
- ~ CA 02505713 2000-O1-21 _ _~ '
WQ 00/42948 ~~~ . fCTIUS00/01557
.guidewire and deploy'a device through the catheter, advance a
device.~ielivery
catheter through the indwelling catheter or replace 'the initial guidewire
with a
smaller guidewi~e.~ The smaller. guidevvire is 'frequently used in order to
traverse
a small vessel side branch or obstructive .lesion and deliver therapeutic
devices. The use of smaller guidewires has the added beneft of allowing the '
use of even lower profile devices since the lumen of the stent or ~stent-graft
can
be reduced further during packing.-
it is maintained that even smaller profile devices can be introduced
should the need for a guidewire be .obviated. Such is the case should the
following procedure be_fc~llowed: iritroduce a guidewire past the site to be
treated, coaxially position a long introduces sheath ~of catheter to the enci
of the
guidewire, removewtlie guidewire, advance the compacted stent or scent-graft
beyond the.end flf the introduces sheath by pushing it with mews such as a
wire, and deploy the stent or scent-graft. This procedure affords the ability
to
compact stents.or stmt-grafts.to the extent that no appreciable lumen exists
in
the compacted state. This further ~eductio~ in profile, although m'inimal,'can
bes .
enough,to convert a surgical. procedure to a percutaneous procedure. .
One device; allowing for such a procedure is illustrated in ~iguresv25 and
26. in this embodiment an endoprosthesis 154 is compacted di~ectiy into a
~ long introduces sheath 156,. with. the introduces sheath 156 serving both as
a
means of directing the endoprosthesis to, the .treatment site and as the
restraining dev'~ce used to hold the endoprosthesis in its compacted dimension
until deployment. As is shown in Fig4re 26; a pusher mechanism 158 may be
directed through the introduces sheath 156 to push the endoprosthesis .out of
the tube and deploy. it in place. This form of deployment apparatus can save
significant.compacted profile by eliminating the need for a guidewire andlor a
separate restraining sleeve on the stent or stent-graft. ..
Figure 27 demonstrates that the same constraint mechanism shown in
f=figures 25 and 26 can be combined with "other restraining devices 160, 'such
as
the knitted restraining ~tevice illustrated in Figures 23 and 24, to provide
for
delivery in distinct phases. In this instance a first segment 162 of the .
endoprosthesis 154_ deploys when .pushed from the introduces sheath 156 while
a second segment 164 remains contained by restraining device 160.
Restraining device 160 can be separately removed when desired by actuating
deployment line 166..
22
CA 02505713 2000-O1-21
' WO 00142348 PCT/US00/01557
'' By way of summary, the present invention employs a series of
techniques that combine to reduce the delivery profiles of stent-graft
.devices.
These techniques include:
Thin. s>'~ncLcQyerina~
Thin, strong expanded PTFE andlor polyester materials are employed
to deduce the mass and volume of ttae stent covering. In the case of the use
of
expanded PTFE films, the~profile is significantly reduced by creating a
circumferentially and longitudinally strong cover by applying. very high
strength
films directly to the stent frame. Other thin, strong biomaterials may also be
used in the present invention; including but not limited to fluoropolymer
elastomeric materials and polyurethanes.
Thini~trong stent~frames (wires and cut framesl:
Nitinol is used because of its superior strength, super elasticity, and
biocompatibilty. Alternative materials. including, but not limited.to,
tantalum,
titanium and stainless steel may also be used.
i-~ic~acking efflq!encYstent framg,desian:
Nitinol wire stent-frames are formed utilizirig a construction that enables
a very high degree of cornpaction because of: nesting of in-phase apices;
sliding of the apices over top of one another upon compaction to ease the
compaction process; and facilitating folding efficiency of the material.
Imaroved method of attaching cover material to stent frame:
Bonding of the graft covering to the stent is accomplished using as little
additional material as possible. In many cases, the expanded PTFE material is
simply .heat bonded together. For examples in. which the stent frame is
covered
vi~ith, but not encapsulated by, expanded PTFE material, the stent frame is f
rst
prepared by applying a very thin coating of FEP powder. Other bonding
techniques may etnpioy coating the scent frame by dipping it in FEP
dispersion,
using expanded PTFE film containing either a continuous or discontinuous
layer of FEP, or using another suitable bonding agent,
(mowed stent-qraftpackjna techniaues:
23
CA 02505713 2000-O1-21 ~~: ,
wo oo,4i94s ~ , rc~rmsooioiss7 '
' For a given .scent-graft design; it was unexpectedly learned that
repeated pulls of the devices through the same sized smooth. dies enabled a
further reduction in compacted profile. Furthermore, a fluted tapered die
enables even greater compaction by producing an effiaent stent-graft folding
pattern.
Low profile retraining methods: w
The delivery profile is further reduced by drawing down a delivery tuba
to obtain a strong, thin-walled means of restraining the stem-graft in the
compacted state. Alternatively, a delivery tube constructed from knitted
threads that unravel when pulled from a line extending outside the body can be
used as a low profile restraining cover.
Deliv~rv techniaugs: '
Delivery techniques, such as using an introducer sheath and a pusher
mechanism, can be employed to further reduce the profile of cornpatted
devices to be introduced.
Individually, each of these techniques results in a measurable decrease
in profile when applied to stent-grafts: The combination of these properties
provides dramatic improvements in delivery profiles. Referring again. to
Figures
1 and 2, an endoprosthesis of the present invention having a deployed
dimension of "a" in cross-section diameter and a compacted dimension of "b" in
cross-section diameter is capable of achieving dramatic ratios of expansion.
For example, a conventional 40 mm aortic stent-graft with limited
foreshortening might achieve a ratio of a:b of 3.5:1 to 5:1. By contrast, a 40
mm stent-graft endoprosthesis of the present invention can achieve ratios of
a:b ofi at least 7;1 up through 8:1,. 9:1, 10:1, 11:1, 12:1, 13:1 and 14:1 or
mope.
As has been explained; this can -lead to a device that is capable of
achieving a deployed dimension 'of 23 mm or rivore (and preferably 26, 28, 30,
32, 34, 36, 38, 40, 42 mm or more) in cross-sectional diameter that can be
reduced to a compacted dimension of 12 F or less (and preferably less than 11
F, 10 F, 9 F, 8 F, 7 F, 6 F, or less).
As has further been explained, the compacting technology of the
present invention also permits construction of extremely small devices, on the
order of 4 mm. or less in deployed diameter that can be delivered in a
24
CA 02505713 2000-O1-21
1 v .
WO OOI42948 _ PCTNS00/01557
. _
compacted dimension of less than 3 or 2 F (1 or 0.7 mm). These very small
devices possess a~:b ratios of 2:1, 3:1,. 4:1, 4.5:1, and 5:1, or more.
Equally important the stent-grafts of the present invention achieve
substantial compaction.with minimal change in length betuveen the enlarged
deployed dimension and the compacted dimension. As a result, the device can
be accuTatety positioned and deployed. Additionally, the lack of significant
foreshortening of the stent element atiows more preferreii cover materials to
be
used, such as expanded PTFE .and woven polyester, that are not capable ~of
undergoing substantial elongation arid.contraction. As has been noted, the
endoprostheses pf the present inveritibn should undergo less than a 25%
change in longitudinal. length between its compacted dimension arid its
deployed diniension. Progressively desirable the device will undgrgo less than
a 20%, 15°~,~10°~0, 5%, 4%, 3%, 2%, 1% or tess change in
longitudinal.length
betweeil its fully compacted dimensicjn and its fully deployed dimension.
The.conslstent length of the present invention is achieved through the
combination of materials and structures defined herein. Among the highiy
effective methods of preventirig elongation or foreshortening of the device
during compaction or deployment are:.to employ stent element pattern s that
will
~ naturally resist change in longitudinal length when compacted; to use
relatively
inelastic covey material; and to employ longitudinal structural elemenfs, such
as
struts 74 shown in Figure 7 or longitudinally applied (relatively inelastic)
tapes
or similar structures, to resist longitudinal changes in device length.
.25 . Without Intending to limit the scope of the present invention, tfie
following examples illustrate how the present invention can be made and
practiced:
E~cample 1
A 40 mm innei diameter thoracic aortic stmt-graft is created. The stent
portion is built using 0.30 mm, diameter, 40-45% cold worked NiTi (n.itinol)
wire
(SE 508; Nitinol bevices &.~Components, Fremant, CA) formed using a mandrel
with protr~ud.ing pins. The stent is constructed using a-single wire, creating
an
undulating, helical, tubular stent member by winding the wire on a pin fixture
as
described in the~above,mentioned published PCT patent application. See
Figures 7 through 9:
CA 02505713 2000-O1-21 =___ y )
_ __ i, _ . ii .
WO 00142948 ~ PC'TlUS00101~557
Once the wire is formed on the pin fixture, it is, heat treated in a
convection oven set at 450aC for-15 minutes. After. removal from the oven and
quenching in a water bath, the wire frame is unwound from the fixture creating
~~
a freestanding tubular stmt frame.
The stent cover is constructed from a strong, thin film. ~A suitable.film
comprises expanded PTF!= (ePTFE) film made in accordance with the
teachings of United States Patent 5,476,589 to Bacino. This expanded PTFE
"cover film° material is chosen for its biocompatibility, strength, and
thinness.
The preferred material possesses a matrix tensile strength of about 900 MPa in
its high strength (longitudinal) direction a thickness of about 0.003 mm, and
a
density of less than about 0.8 g/cc and more preferably between about 0.15 to
0.4 g/cc. Matrix tensile strength is determined with an INSTRON tensile
testing
machine, using pneumatic cord and yarn grip jaws, a 25.4 mm wide sample, a
102 mm paw separation distance, and a crosshead speed of 200 mm/minute.
A 28 mrn inner diameter ePTFE tube possessing a wall thickness of '"~
about 0.10 mm and a density of about 0.5 glcc is stretched over a 40 mm outer
diameter mandrel. This tube serves as a cushion to aid in the subsequent
lamination of the ePTFE material t0 the, stent, frame and is not part of the
final
device. Suitable expanded PTFE tubes for this use are commercially available.
A "sacrificial film". is also used to.facilitate the construction of the
inventive device, serving as a release:layer to aid in removal of the stent-
graft .
from the cushion tube ar!d m8ndret and providing a radial force to aid in
bonding the ePTFE to the stent. The sacrificial film. is preferably one with
high
strength (or "retraction force") that~will withstand the.processing
conditions. A
suitable film is one made in. accordance with United States. Patent 3,953,566,
incorporated by reference, that has been sintered to maintain its dimensions
during processing. This film is 25.4 mm and 50.8 mm wide, approximately-
0:093 rnm thick, and possesses a matrix tensile strength of about690 Mpa in
, its high strength (longitudinal) direction, 'tested as described above.
lthas a~
density of about 0.2 - 0.3 glcc. This film is not a part of the final device.
It
~shoutd be noted that the PTFE films used in all-the examples have all been
subjected-to temperatures_exceeding the crystalline. melt temperature of PTFE
("sintered"). .One layer of this 25.4 mm wide film is~ helically wrapped on
top of
the cushion tube with about a 10% overlap; creating .a continuous layer. The
tail end of this film is left exposed at both~ends of the rriandrel.
26
CA 02505713 2000-O1-21
WO 00/42948 PCTNS00/01557
Helical wrapping facilitates later removal of this film. This film layer is
unraveled from under the device at the end of the process by pulling an this
tail,
in order to facilitate the removal of the stent-graft from the cushion tube
and
mandrel.
Next, two layers of cover film are applied in a cigarette wrap fashion
such that the high strength direction, of the fiim is oriented along the
longitudinal
axis of the tube, thereby creating a seam oriented along the entire length of
the
tube. One layer of the same cover film is then circumferentially
applied...Triat
is, the film is rolled an fop of the previous layers such that the.high
strength
direction of the film is oriented perpendicularly to the longitudinal axis of
the
tube. This procedure also produces a seam oriented along the entire length of
the tube, but is not transferred to the luminal surface. The stent frame is
then
placed over the covered mandrel in such a way that the undulations are aligned
in phase. Next, an additional circumferential layer'of the cover ~Im is
applied,
followed by two layers of the cover film applied longitudinally:' Finally,
eight
layers of 50.8 mm wide film of the same type described above are applied in
an up and .back helical pattern. The cushion tube is secured to the mandrel
with bands of wire to prevent longitudinal shrinkage during subsequent
heating.
The sequence of preparing the device and the number and orientation of film
24 ~. layers for this 'and other examples appear in Table 1. This table also
describes
properties of the stent-grafts.
27
CA 02505713 2000-O1-21 Jj
WO 00!42948 PCT/iJS00/01557
' TABLE 1
Exam le~ Exam le 3 Exam le 4 Exam le 6
1
De to ID 40 mm ~ 26 mm 31 x 13 mm 23 x 13 mm
~
1116ire 0.30 ri~tm 0.20 mm 0.30 mm 0.20 mm
Diameter
Number of 8 8 top of trunk:top of trunk:
8 8
A ices 1e : 4 le : 4
Stent Frame 15 minutes 15 minutes 15 minutes .15 minutes
@ @ @ @
Treatment 450C 450C 450C 450C
FEP Heat Na Na n/a Na
Treatment
Cushion OD _ 40 mm OD = 26 mm OD -= 31 mm OD = 31 mrn
Tube/ OD = 13 mm ~ Op = 13 mm
Mandrel
Inner 1 layer ~ ! layer !.layer 1 layer
Release f
iim
Inner i.arig.2 layers 2' layers 2 layers 2 layers
Film
inner Circum.i layer 1 layer 1 layer 1 layer
Film
Stent Frame wire wire wire wire
Outer 1 Layers 1 layers . 1 layers 1 layers
Circum. Film
Outer Long. 2 layers 2 layers 2 layers 2 layers
Fiim
Outer Comp. yes yes yes ~ yes
Fiim .
Heat 40 minutes 20 minutes 30 minutes 20 minutes
@ @ @ @
Bondin 380C 380C 380C 380C
Delivery 10F; 3.2813.336 F: 1.9612.011OF: 3.28/3.336 F: 1.9612.01
Tube 9 F: 2.92 9 F: 2.92
/3.00 /3.00
Dimensions
(in mm)
ID/OD
Guidewire 0.89 mm/10 0.89 mm/6 0.89 mm/10 0.89 mml6 F
F F F
Diameter! no wire/9 no wire/9
F F
Delivery
Profile
a:b 12.2:1 13.3:1 9.E1 11.7:1
Ratio 13.7:1 10.6:1
Key: "n/a" indicates not applicable; "ID" indicates inner diarn~ter; "OD"
indicates outer diameter; "Number of apices" indicates the number of exposed
apices at an end of a graft; "Long." indicates longitudinal; °Circum."
indicates
circumferential; "Comp." indicates compression; "Delivery profile" indicates
smallest sized hole through which the compacted device plus delivery tube can
fit; "a" indicates deployed dimension in .mm; "b" indicates compacted
dimension in mm.
28
' ' ~ CA 02505713 2000-O1-21
'' ~ WO 00/42948 PCTNS00/01557
The entire assembly is placed in an oven set to' 380°C for 40 minutes.
The heat-induced retraction of .the sacrifsaal film provides compressive
bonding
forces, thereby heat bonding the cover films, providing an integral stent-
graft.
The assembly is removed from the oven and allowed to coot. The eight outer
layers of sacrificial flm are removed, then the single inner layer the
sacrificial
tilm .is removed. Next, the-device and cushion tube are removed from the
mandrel, and. the stent-graft and cushion tube are separated.
Expanded PTFE sewing thread (RASTEX~ Expanded PTFE Thread,
1200 'denier, available from W.L. Gore 8~ Associates, Inc., Elkton, MD) is
tied to
one end of the device in order to facilitate pulling the device through a
30°
included angle, polymeric, smooth, tapered fixture (funnel) in order to reduce
the diameter. The device is successively pulled through longer funnels
possessing the same inlet diameters (therefore, possessing smaller diameter
outlets), thereby reducing its compacted diameter. The device is compacted to
its minimum diameter using a fixture in which the small end of the funnel is
mated with a capture tube that houses a thin-walled (approximately 0.025 to
0.038 mm wall thickness) polyester tube. This polyester tube is constructed by
elongating a heated polyester shrink tube (item ~urriber.210100CST, available
from Advanced Polymers, Inc., Salem, NH). The polyester tube is employed to
maintain the stent-graft in the non-distended state and serve as a delivery
housing tube for the stent-graft. The diametric reduction is facilitated by
chilling
the nitinol-based device with a refrigerant spray (Freeze Mist, GC Thorsen,
Inc., Rockford, IL) during draw-down through the tapered die. The final
constrained device plus polyester delivery tube fit through a 10 F hole. The
thickness of the bonded ePTFE covering is approximately 0.013 mm. The
device is pulled from the polyester tube. Upon release from the tube, the
stent-
graft is warmed to about physiologic body temperature (35 - 40 °C);to
deploy it.
The device is fadialiy compressed once again after a 0:89 mm wire is inserted
in the lumen of the device in order to simulate the presence of a guidewire.
The
use of the term °guidewire° in the examples and tables refers to
such a.spacer
wire.
The device is once again captured inside a tube. The device plus a
polyester constraining tube fit through a 10 F hole. Once deployed, the device
self-expands in a 36 °C water bath to a 38 mm inner diameter. Gently
pulling
the stmt-graft over a tapered mandrel increases its inner diameter to 40 mm.
Note that blood pressure applies a radial force in vivo and self-expanding
29
CA 02505713 2000-O1-21
' 'VI~O 00142948 PCT/US00l0155T
devices are typically subjected to balloon dilatation once they are deployed.
(Note that the stent-grafts in all examples self-expand to the deployed
diameters presented in Tables 1 and 2, unless otherwise noted.)
The graft is once more compacted without a guidewire. This time the
delivered profile of the captured device is 9 F (that is, its dimension inside
a
delivery tube). The stent-graft deploys (self expands) to a 39 mm inner
diameter in a 40 °C water bath. Gently pulling it over a tapered
mandrel
increases its inner diameter to 40 mm.
The a:b ratio for this device ranges from 12.2:1 to 13.7:1 and increased
with successive pull-downs.
Foreshortening is a percentage defined as the change in length from the
captured state to the deployed state divided by the length of the device in
the
captured state, where the diameter of the device is at the minimum. The
device is once again captured inside a 9F tube without a guid~wire, as
described above. The length of the device is measured in the captured state at
7.68 cm. The device is deployed in a 36 °C water bath. It deploys to a
40 mm.
The length of the device is measured in the deployed state at 7.62 cm. The
device foreshortened 1 %.
Example 2
Another 40 mm inner diameter thoracic aortic stent-graft is constructed
using polyester as the.stent covering material. The stent:portion is
constructed
using 0.20 mm diameter nitinol wire (SE 508; 40-45% cold worked; Nitinol
Devices & Components, Fremont, CA). Yellow polyester film (PES 30I25~
available from Saatitech, Inc., Somers, NY) is employed as the stent covering.
The polyester material is approximately 0.046 mm thick. The stent member is
formed and heat treated in the manner described above in Example 1. The
stent covering is attached to the inner surface of the scent frame with CV-8
Sutures (available from W.L. Gore & Associates, Inc., Flagstaff, AZ), using a
running stitch and tying the ends of the of the sutures together.
As in Example 1, the device is pulled down into a tapered fixture and
contained within a capture tube containing a removable ppiyester inner liner.
A
0.89 mm guidewire is inserted inside the stent-graft prior to compaction. A
long
tail of the polyester fabric material is used to pull the stent-graft through
the
fixture. The inner and outer diameters of the delivery tube are approximately
' CA 02505713 2000-O1-21 . ~ ,
' ' WO 00!42948 PCTNS00/01557
3.94 and 4.01 mm, respectively. The 40 mm stent-graft over a guidewire plus
polyester tube (delivery tube) fit within a 12 F hole. The stent-graft deploys
to
39 rrim in ~a 36 °C water bath: It increases in inner diameter to 40
riim when
gently pulled over a tapered, mandrel.
The a:b ratio for this device is 10.2:1.
!Example 3
A 26 ~mm inner diameter thoracic aortic stent-graft is constructed using -
0.20 mm nitinol wire~(SE 508; 40-45% cold worked; Nitinol Devices &
Components, Frernont, CA) and ePTFE film. This stent-graft is made in the .
same manner, with tlae same materials, as described in example 1, following
the steps outlined in fiable~ 1. .The dev9ce. is~drawn~ down over a 0.89 iiim
diameter.wire to simulate the presence of a guidewire. The stent-graft and
wire
aye pulled into a polyester tube (fabricated as described in Example 1 ). The
~ stent-graft.plus polyester tube fits within a 6 F hole. .The stent-graft
deploys to
24 mm in a 36 °C water bath. Gently pulling the stent-graft over a
tapered
mandrel deploys the device to 26 mm. . '
The a:b ratio fog this device is 13.3:1.
Example 4
The bifurcated stent-graft of the present invention consists of a modular
design as described in U.S. Patent No. 6,551,350 to Thornton et al. This
design
incorporates a main body (i.e., trunk) component that incorporates the trunk,
one
leg, and a portion of the contralateral leg, as is illustrated in Figure 4.
The
contralateral leg constitutes the other component. These two components are
independently introduce into the vessels. The contralateral leg is positioned
inside the contralateral leg portion of the main body component. The geometry,
hence volume, of the main body component mandates that its delivery profile'is
always larger than that of the leg component. Achieving a percutaneously
deliverable main body component ensures that the entire device can be
percutaneously delivered. Consequently, only main body components are
constructed for the purposes of this and other bifurcated stent-graft
examples.
The main body component of a 31 mm (trunk inner diameter) by 13 mm
(limb inner diameters) bifurcated stent-graft.designed for the treatment of
abdominal aortic aneurysm disease is constructed using 0.3p mm nitinol wire
31
~CA 02505713 2000-O1-21
- ~ ,
WO O.OI42948 ~ PCT/US00/01557
and an expanded PTFE film.: The stent portion is built using 0:30 mm diameter,
40-45% cold worked' NiTi (nitinol) wire (SE 508; Nitinol Devices & Components,
Fremont, CA) formed using a inandret with protruding pins as previously
described. The stent is constructed~usirig a single wire; creating ari
undulating,
helical, tubutar, bifurcated stent member by winding the wire on a pin fixture
as
previously described. fihis pattern includes in-phase nested apices that aid
in
compaction.
With,the exception.of steps required to accommodate the bifurcated
shape, of ttie stent-graft, this stent-graft component is i~nade in the same
. manner, vuith the same rriaterials, as described m Example 1 following the
steps outlined in Table 1. .
The. bifurcated section is constructed as follows. Y-shaped pin fixtures
are used to construct.tiie free standing stent frames and Y~shaped mandrel
tppling is used to construct the stent-graft devices. . As in other. examples,
a
cushion tube is employeii as a construction aid. .One large. ePTFE tube is
sutured to two small.e~ ePTFE tubes to form a bifurcated cushion tube. Once
placed on the mandrel the cushion tube is wrapped with sabrificial film with .
each leg of the construction wrapped individually and an additional layer
covering the trunk. Subsequent layers of cover.~lm are.applied.;over the
entire
construction bridging over the gap between the individual legs of the v
bffurcation. The cover film covering the two legs is applied loosely to allow
.
seam sealing of the cover film between the legs to form the smaller tubes of
the
bifurcation. The seam is sealed by hand uvith a solderitlg iron set at 400
°C. As
before, more sacr'~cial film ~Is applied for compression heat bonding of the
assembly. To apply the necessary compressiveforces between and around-
.the legs of the bifurcation; scraps of cushion tube material formed into two
wedges and ,Covered with pol~rimide sheeting (0.03 mm thickness, 12.7 mm
wide, #TKH-100, available from Fralock Corp., Canoga Park, CA) are.ptaced
on both sides, between the legs; and under ttie, sacrificial film bonding
layer.
Retraction forces of the sacrificial film during heat bonding forced the
wedges
into the space between the legs_thereby facilitating bonding the stent frame
and
graft covering material together. After heat bonding, the sacrifcial .film and
the
wedges are removed.
The stent-graft is compacted in the same manner as described in
Example 1. The trunk portion is pulled into the die first. The stent-graft
plus
0.89 mm guidewire plus capture. tube fit within a 10 F delivery tube. The
stent
32
CA 02505713 2000-O1-21
' ~ wo ooi4z9as ~ pc~rrosooroiss~
graft deploys, it self-expands, to a 31 mm inner diameter in a 36 °C
water-bath.
The device is compacted and restrained within a polyester tube again, this
time
without a guidewire. The delivery profile is reduced to 9 F. The stent-graft
deploys (self-expands) to a 30 mrn inner diameter in a 40 °C water
bath.
Gently pulling it over a tapered mandrel increases its inner diameter to 31
mm.
The a:b ratip of this device ranges from 9.5:1 to 10.6:1.
Example 5
Another main body component of a 31 mm (trunk inner diameter) by 12
mm (limb inner diameters) bifurcated.sterit-graft is constructed using
polyester
material as the stent covering material. The stent portion is constructed
using
0.20 mm, 40-45% Gold worked NiTi (nitinoi) wire (SE 508; Nitinoi Devices &
Components, F~emont, CA). Yellow polyester.film (PES 30/25, available from
S.aatitech, Inc., Somers, NY) is employed as the stent covering. The polyester
material is approximately 0.046 mm thick. The stent member is formed and
heat treated in the manner described above in Example 4. The stent covering
is attached to the inner surface of the stent frame with CV-8 Sutures
(available
from W.L. Gore 8 Associates, Inc., Flagstaff, AZ), using a running stitch and
tying the ends of the of the sutures together.
As in Example 4; the device is pulled down into a tapered fixture and
contained within a capture tube containing a removable polyester inner liner.
A
long tail of the polyester fabric material is used to pull the stent-graft
through
the fixture.
The delivery tube inner and outer diameter dimensions are 3.28 mm
y , and 3.33 mm, respectively. The stent-graft main body component plus 0.89
mm wire plus polyester tube (delivery tube) fit within a 10 F hole. The stent-
graft deploys to a 31 mm inner diameter in a 40 °C water bath.
The a:b~ ratio is 9.5:1.
The stent-graft is then compacted again without a guidewire. The stent-
graft is pulled into a delivery tube possessing inner and outer diameter
dimensions of 2.91 mm and 2.97 mrn, respectively. The stent~raft plus
delivery tube fit within a 9 F hole and deployed (self expanded) of 36
°C to 30.5
mm. Gently pulling it over a tapered mandrel increases its diameter to 31 mm.
The a:b rafro is 10.6:1.
Example 6
33
' CA 02505713 2000-O1-21
~WO 00/42948 PCTlUS00/01557
A 23.mm (trunk inner diameter) by 13 mm (limb inner diameters)
bifurcated stent-graft main body component is. constructed using 0.20 mm
diameter, 40-45°!° cold worked NiTi (nitinol) wire (SE 508;
Nitinol Devices &
Components, Fremont, CA) and ePTFE as the stent covering material. The
stent member is formed and heat treated in the manner described above in
Example 4. This stmt-graft component is made.in the same manner, with the
same materials, as described in Example 4 following the steps outlined in
Table
1.
As in Example 4, the device is pulled down into a tapered fixture and
contained within a capture tube containing a removable polyester inner liner.
The scent-graft main body component plus 0.89 mm guidewire plus polyester
tube (delivery tube) fit within a 6 F hole. The device 'is deplt~yed; allowed
to self-
expand, in a 36 °C water bath. The trunk deploys to an inner diameter
of ,21
mm. .Gently pulling the stent-graft over a tapered mandrel increases its~inher
diameter to 23 rnm.
The a:b ratio for this device is 11.7:1.
Example 7
A 3.2 mm inner diameter stent-graft is created using ePTFE film and
0.10 mm diameter, 40-45% cold worked NiTi (nitinol) wife. (SE 508; Nitinol
Devices & Components, Fremont, CA). With the exceptiors of the means of
attaching the film to the stent frame and the. use. of 6.35 mm wide as opposed
to wider sacrificial film, this stent-graft is made in the same manner, with
the
same materials, as described in Example 1 following the steps outlined in
Table
2. This table also describes properties of the stent-grafts of this and other
examples.
The stem frame is powder coated with fluorinated ethylene propylene
(FEP) powder (NC1500, available from Daikin industries, Ltd., Osaka, Japan).
FEP powder is placed and stirred in a kitchen blender to Create a fine fag of
FEP dust. The wire stent frame is cooled with a refrigerant spray, then placed
in the fog, thereby coating the FEP to the wire. The FEP is then heat bonded
to the wire 'by placing the coated stent frame into. a convection oven set at
320°C for 3 minutes. The FEP coating enhanced later bonding of the
cover film
to the stem frame:
The device is pulled down into a tapered fixture and contained within a
capture tube containing a removable polyester inner lines as described in
34
CA 02505713 2000-O1-21
WO 00/42948 ~ PCT/USOOI01557
Example 1. The stent-graft plus 0.46 mm guidewire plus delivery tube fit
within
a 3 F hole. A 36°C water bath is used to deploy the stent-graft: The
device is
deployed, Then compacted again without a guidewire: This time the device plus
Capture tube fit within a 2.3 F Note. The stent-graft is deployed again in the
36°C water bath.
The stent-graft exhibits an a:b ratio ranging from 3.4:1 to 4.5:1.
' CA 02505713 2000-O1-21
WO 00142948 PCTNS00/0155?
TABLE 2
Exam 1e 7 - ~ ~ Exam .le 8 Exam le 9
De to ed- 3.2 mm 2 mm 3 mm
ID
Number of 4 3 8
A ices
Wire 0.10 mm 0.10 mm [tube thickness
=
Diameter 0.11 mm
Stent Frame6 minutes @ 450.G6 minutes.'@ 6 minutes @ 450C
450C
Treatment
FEP Heat 3 minutes 'aL7 3 minutes @ 320C3 minutes @ 320C
320C
Treatment
Cushion OD = 3.2 mm OD = 2 mm ~ OD = 3 mrn
Tubel
Mandrel
. Inner 1 layer 1 layer 1 layer
Release -
Film
inner Long.2 layers . 2 layers 3 layers
Film
Inner Circum.nla wla 1 layer
Film
Stent Framewire wire cut tube
T Outer 1 layer ~ 1 layer n1a
Circum.
Film
Outer Long.1 layer 1 layer Na
Film
Outer Comp.yes yes Na
Film
heat bonding5 minutes 380C 4 minutes 380G 5 minutes 380C
Delivery 3 F: 0:94/1.00 2 F: 0.61/0.66 n/a
Tube 2.3 F: 0.71 /0.762.5 F: 0.76f0.81
Dimensions
(in mm)
ID/OD '
Guidewire 0.46 mml3 F no wirel2 F no wirel4.2 F
Diameter/ no wire/2.3 F 0.30 mm/2.5 F
Delivery
Profile
a:b 3.4:1 3.3:1 2.1:1
Ratio 4.5:1 2.6:1
Key: °n/a" indicates not applicable; "ID" indicates inner diameter;
"c?D"
indicates outer diameter; °Number of apices" indicates the number of
exposed
apices at an end of a graft; °Long." indicates longitudinal;
°Circum " indicates
~ circumferential; "Comp." indicates compression; "Delivery profile" indicates
smallest sized hole through which the compacted device plus delivery tube can
fat; "a" indicates deployed dimension in mm; "b" indicates 'compacted
dimension in mm.
Example 8
3fi
CA 02505713 2000-O1-21
WO 00/42948 PGTNS00/01557
A 2 mm inner diameter stent-graft is Constructed using 0.10 mm nitinol
wire (SE 508; 40-45% cold worked; Nitinol Devices & Components, Fremont,
CA) and an expanded PTFE film. This stent-graft is made in the same manner,
with the same materials, as described in Example 7 following the steps
outlined
in Table 2. FEP is coated onto the stent frame in the same manner as
described in Example 7, as well.
The device i~ pulled down into a tapered fixture and contained within a
capture tube containing a removable polyester inner liner as described in
Example 7 and Table 2. _ The stent-graft without a guidewire plus delivery
tube
fit within a 2 F fiole. The .device is deployed in a 36 °C water bath,
then
compacted again, with a 0.30 mm guidewire. This time the device plus
capture tube fits within a 2.5 F hole. It deploys (self expands) to a 2 mm
inner
diameter in a 40 °C water bath.
The a:b ratio for this stent-graft ranges from 2.6:1 to 3.3:1.
Example 8 .
For this example a section of nitinol tubing (0.11.mm wall thickness, 1.30
mm outer diameter, available from Nitinol Devices & Components, Fremont,
CA.) is machined into a configuration similar to the helical pattern of
serpentine
bends of wire in the other examples. The stent element 170 is illustrated in
Figure 28. . The stent pattern is cut from the nitinol tube using a NdYag
laser
(available from Laserage, Waukegan, IL). The laser removes material from the
tubing to leave only a framework skeleton that serves as the stent frame. The
laser machined tube is then chilled with a refrigerant spray and stretched up
on
_ a taperEd. mandrel to achieve a 3.0 mm inner diameter.
The stent frame is next subjected to the stent frame (heat) treatment
described in Table 2, then quenched.i~ water, in order to set the stent frame
at
this larger, deployed state, diameter. The stent frame is then FEP powder
coated in the same manner as described in Example 7. The graft is then
fabricated and attached to the scent frame in the same manner, with the same
materials, as described in Example 7 following the steps outlined in Table 2.
This self expanding stent-graft device is pulled down into a tapered
fixture and capture tube as described in Example 7 and Table 2, without a
liner
inside the capture tube. No guidewire is inserted into the lumen pr'ror to
compaction. The stent-graft is pulled by grasping a portion of untrimmed
covering material extending beyond the end of the stent frame. The stent-graft
37
CA 02505713 2000-O1-21
WO 00/42948 PCT/US00/01557
without a delivery tube fit within a 1.4 mm hole. .The stmt-graft is deployed
from the capture tube at ambient temperature and expands to a 3.0 mm inner
d iameter.
The a:b ratio for this stent-graft is 2.1:1.
Example 10
Commercially available 26 mm and 40 mm inner diameter thoracic
aortic stent-grafts (Thoraac EXCLUDER' Endovascular Prosthesis, W. L.
Gore and Associates, Inc., Flagstaff, AZ) are also subjected to some of the
inventive compaction techniques to determine if their delivery profiles could
be
reduced beyond current compacted .dimensions. Presently, the manufacturer
suggests that lntroducer sheaths sized 22 F and 24 F be used with these 26
mm and 40 mm devices, respectively. .
The. outer sealing cuffs are removed from these devices prior to any
testing. A 26 mm device without any wire iriserted into its lumen, is pulled
through a funnel-shaped tapered die into a capture tube as described in
Example 1, except that a two part tapered die is used and and no delivery tube
liner is present inside the capture tube.
As is illustrated i~ Figure 29, a two part die 172 is employed having a
first stage funnel section 174 and a second stage, funnel section 176. The
first
stage section 174 has an included angle of 12°, the second stage
section 176
has an included .angle of 12:4°. The device is pulled into successively
smaller
capture tubes 178. Containment within a 3.43 mm capture tube indicates that it
will fit within an 11 F hole. A 36 °C water bath is used to deploy the
stent-graft.
The graft is,deplqyes! to 24_mm,:increasing to 26 mm when,gently pulled
over a tapered mandrel.
Another 26 mm device, also without a guidewire, is pulled through a ,
fluted tapered die, thereby fitting into a 3.18 mm capture tube, hence it fit
within
a 10 F hole. The second stage section 176 is modified to create flutes in the
manner previously described with reference to Figures 16a through 16c. Eight
(8) flutes are created, matching the number of apices of the device.
Using the fluted compaction techniques previously described, the
delivery profile of the 26 mm devices is reduced by 1 F. A 36 °C water
bath is
used to deploy the stent-graft. The 'graft is deployed to 24 mm, increasing to
26 mm when gently pulled over a tapered mandrel.
The use of the fluted die increases the a:b ratio from 7.6:1 to 8.2:1.
38
CA 02505713 2000-O1-21
WO 00142948 PCTNS00/01557
The 40 mm device without a guidewire is subjected to the same
compaction process using a tvvo part smooth tapered die as described in.Figure
29. The first stage section 174 and second stage section 'f76 have included
angles of 12° and 7.2°, respectively. The device is successively
pulled into
capture tubes 178 possessing inner diameters of 6.35 min, 6.fl0 mm, and 5.33
mm. The size of the.device precluded it from being drawn into a 5.00 mm inner
diameter captura Tube, fihis 40 rtim device is subsequently pulled through the
5.33 mm capture tube a total of five times to prepare it for pulling into a
smaller
capture tube. The force required to pull the device. through this capture tube
9 0 decreases from 27 kg for the first pull to 18 kg for the last pull. The
decrease iri
force suggests that the device could be pulled into a smaller capture tube
without damaging the device. The device is then successfully pulled into a
5.00
mm capture tube. This step of repeatedly pulling the stent-graft through a ,
same sized .capture tube enables it to be ~cbmpacted further.
The a:b ratio for this stent-graft~is 8:1.
While particular embodiments of the present invention have taesn
illustrated and described herein, the .present inverttion should not be
limited to
. such itlustratior~s and descriptions. It should be apparent that changes and
modifications may be~iacorporated and embodied as part of. the present
invention within the scope~of the following claims.
39