Note: Descriptions are shown in the official language in which they were submitted.
CA 02507656 2005-05-27
WO 2004/050093 1 PCT/EP2003/012913
82498pct
TIOTROPIUM-CONTAINING MEDICAMENT COMBINATIONS USED FOR INHALING
The present invention relates to new pharmaceutical compositions for
inhalation
containing one or more, preferably one, tiotropium salt combined with one or
more
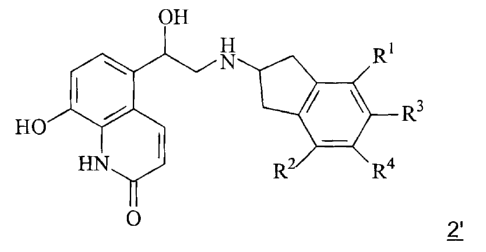
pharmacologically acceptable acid addition salts of a compound of formula 2',
N R'
R3
HO
R2 R4
2'
wherein the groups R', R2, R3 and R4 may have the meanings given in the claims
and in the specification, processes for preparing them and their use in the
treatment of respiratory complaints.
Background to the invention
The compound tiotropium bromide, a salt of tiotropium, is known from European
Patent Application EP 418 716 A1 and has the following chemical structure:
Me~ ,Me
N
O _
~H Br
O
~S 0
OH
This compound may also be referred to by the chemical name (1a,2(3,4(3,5a,7[3)-
7-
[(hydroxydi-2-thienylacetyl)oxy]-9,9-dimethyl-3-oxa-9-
azoniatricyclo[3.3.1.02'4]
nonane-bromide and has valuable pharmacological properties. The name
CA 02507656 2005-05-27
WO 2004!050093 2 PCTlEP20031012913
tiotropium is to be interpreted within the scope of the present invention as a
reference to the free cation 1'.
Tiotropium bromide, and other salts of tiotropium, are highly active
anticholinergics
and may therefore provide a therapeutic benefit in the treatment of asthma or
COPD (chronic obstructive pulmonary disease).
Tiotropium salts are preferably administered by inhalation. Suitable inhalable
powders packed into appropriate capsules (inhalettes) and administered by
suitable powder inhalers may be used. Alternatively, it may be administered by
the use of suitable inhalable aerosols. These also include powdered inhalable
aerosols which contain, for example, HFA134a, HFA227 or mixtures thereof as
propellant gas. They may also be administered by inhalation using suitable
solutions of the tiotropium salt.
Detailed description of the invention
Surprisingly, an unexpectedly beneficial therapeutic effect, particularly a
synergistic effect can be observed in the treatment of inflammatory or
obstructive
respiratory complaints if one or more, preferably one, tiotropium salt 1 is
used in
conjunction with pharmacologically acceptable salts of a betamimetic of
formula 2'
OH
R~
Rs
HO
R2 Ra
0 2~
wherein the groups R', R2, R3 and R4 may be defined as hereinafter.
This significantly reduces undesirable side effects, for example, which are
frequently observed when a-mimetics are administered to humans. Examples of
central side effects of a-mimetics include, for example, general malaise,
agitation,
insomnia, anxiety, trembling fingers, sweating and headaches.
CA 02507656 2005-05-27
WO 2004/050093 3 PCT/EP2003/012913
Accordingly, the present invention relates to combinations of pharmaceutical
compositions characterised in that that contain one or more, preferably one,
tiotropium salt 1 in combination with a pharmacologically acceptable salt of a
compound of formula_2'
OH
N R'
Rs
HO
H
R2 R4
O 2'
wherein
R' and R2 which may be identical or different denote hydrogen or C~-C4-alkyl;
R3 and R4 which may be identical or different denote hydrogen, C~-C4-alkyl,
-O-C~-C4-alkyl, - C~-C4-alkylene-O-C~-C4-alkyl or
R3 and R4 together denote one of the bridging groups
- C~-C4-alkylene- or -O-C~-C4-alkylene-O-.
Preferably in the combinations according to the invention salts of the
compounds
of formula_2' are used wherein
R~ and R2 which may be identical or different denote hydrogen, methyl or
ethyl;
R3 and R4 which may be identical or different denote hydrogen, methyl, ethyl,
propyl, butyl, methoxy, ethoxy, methyoxymethyl, or methoxyethyl, or
R3 and R4 together denote one of the bridging groups
propylene, butylene, -O-ethylene-O- or -O-propylene-O-.
More preferably in the combinations according to the invention salts of the
compounds of formula_2' are used wherein
R' and RZ which may be identical or different denote hydrogen or ethyl,
preferably hydrogen;
R3 and R4 which may be identical or different denote hydrogen, methyl, ethyl,
propyl, butyl or methyoxymethyl or
R3 and R4 together denote one of the bridging groups
butylene or -O-ethylene-O-.
CA 02507656 2005-05-27
WO 2004/050093 4 PCT/EP2003/012913
Particularly preferably according to the invention, in the combinations
according to
the invention salts of the compounds of formula 2' are used wherein
a) R' and R2 denote hydrogen and R3 and R4 denote ethyl; or
b) R' and R2 denote hydrogen and R3 and R4 denote methyl; or
c) R' and R2 denote ethyl and R3 and R4 denote hydrogen; or
d) R' and R2 denote hydrogen and R3 and R4 together denote butylene;
or
e) R' and R2 denote hydrogen and R3 and R4 together denote -O-
ethylene-O-; or
f) R' and R2 denote hydrogen and R3 and R4 denote tert.-butyl or
g) R' and R2 denote hydrogen and R3 and R4 denote iso-propyl; or
h) R' and R2 denote hydrogen and R3 and R4 denote methoxymethyl.
Of the compounds mentioned above, the structure defined under a) wherein R'
and R2 denote hydrogen and R3 and R4 denote ethyl is of outstanding importance
in the pharmaceutical combinations according to the invention. The acid
addition
salts of this compound are hereinafter also referred to as compounds 2a while
any reference to the free base of this compound is characterised by the name
2a'
according to the following formula
OH
HO ~ ~ Ethyl
yl
2a.
The salts 2 of the compounds of formula 2' may be used in the pharmaceutical
combinations according to the invention in the form of their racemates,
enantiomers or mixtures thereof. The separation of enantiomers from the
racemates may be carried out using methods known in the art (e.g. by
chromatography on chiral phases, etc.). If the salts of the compounds of
formula
2' are used in the form of their enantiomers, the enantiomers in R
configuration at
the C-OH- group are particularly preferred.
CA 02507656 2005-05-27
WO 2004/050093 5 PCT/EP2003/012913
The alkyl groups used, unless otherwise stated, are branched and unbranched
alkyl groups having 1 to 4 carbon atoms. Examples include: methyl, ethyl,
propyl
or butyl. The groups methyl, ethyl, propyl or butyl may optionally also be
referred
to by the abbreviations Me, Et, Prop or Bu. Unless otherwise stated, the
definitions
propyl and butyl also include all possible isomeric forms of the groups in
question.
Thus, for example, propyl includes n-propyl and iso-propyl, butyl includes iso-
butyl,
sec. butyl and tert.-butyl, etc.
The alkylene groups used, unless otherwise stated, are branched and unbranched
double-bonded alkyl bridges with 1 to 4 carbon atoms. Examples include:
methylene, ethylene, propylene or butylene.
The alkyloxy groups (also referred to as -O-C~-C4-alkyl groups) used, unless
otherwise stated, are branched and unbranched alkyl groups having 1 to 4
carbon
atoms which are linked via an oxygen atom. The following may be mentioned, for
example: methyloxy, ethyloxy, propyloxy or butyloxy. The groups methyloxy,
ethyloxy, propyloxy or butyloxy may optionally also be referred to by the
abbreviations MeO, EtO, PropO or BuO. Unless otherwise stated, the definitions
propyloxy and butyloxy also include all possible isomeric forms of the groups
in
question. Thus, for example, propyloxy includes n-propyloxy and iso-propyloxy,
butyloxy includes iso-butyloxy, sec. butyloxy and tert.-butyloxy, etc. The
word
alkoxy may also possibly be used within the scope of the present invention
instead
of the word alkyloxy. The groups methyloxy, ethyloxy, propyloxy or butyloxy
may
optionally also be referred to as methoxy, ethoxy, propoxy or butoxy.
The term alkylene-alkyloxy groups, unless otherwise stated, denotes branched
and unbranched alkyl bridges having 1 to 4 carbon atoms which are mono-, di-
or
trisubstituted, preferably monosubstituted by an alkyloxy group.
Within the scope of the present invention any reference to compounds 2 is to
be
understood as being a reference to physiologically acceptable acid addition
salts.
By physiologically acceptable acid addition salts 2 are meant according to the
invention pharmaceutically acceptable salts which are selected from the salts
of
hydrochloric acid, hydrobromic acid, sulphuric acid, phosphoric acid,
methanesulphonic acid, acetic acid, fumaric acid, succinic acid, lactic acid,
citric
CA 02507656 2005-05-27
WO 2004/050093 6 PCT/EP2003/012913
acid, tartaric acid or malefic acid. If desired, mixtures of the
abovementioned acids
may be used to prepare the salts 2.
According to the invention the salts 2 are preferably selected from among the
hydrochloride, hydrobromide, sulphate, phosphate, fumarate, methanesulphonate
and maleate. Particularly preferably, the salts 2 in the case of the compound
2a'
are selected from the hydrochloride and maleate, of which the maleate is
particularly preferred.
If, within the scope of the present invention, there is a reference to
compounds of
formula 2' which are not in the salt form, this is indicated by the
designation 2' ,
whereas a reference to 2 should be regarded as a reference to the acid
addition
salts of a compound of formula 2'. The compound of formula 2' and 2 and
processes for the preparation thereof are known from WO 00/75114, to which
reference is hereby made.
The term tiotropium within the scope of the present invention is to be
regarded as
a reference to the free cation (1')
+ Me
Me~N,
O
\\ , H
~'O
,S Y-O
/f~OH
~ 1'.
By the tiotropium salts 1 which may be used within the scope of the present
invention are meant the compounds which contain, in addition to tiotropium as
counter-ion (anion), chloride, bromide, iodide, methanesulphonate,
para-toluenesulphonate or methylsulphate. Within the scope of the present
invention, the methanesulphonate, chloride, bromide and iodide are preferred
of all
the tiotropium salts 1, the methanesulphonate and bromide being of particular
CA 02507656 2005-05-27
WO 2004/050093 7 PCT/EP2003/012913
importance. Of outstanding importance according to the invention is tiotropium
bromide. The tiotropium salts 1 may optionally be used in the form of the
solvates
and hydrates thereof. Particularly preferably, the hydrates are used. Of all
the
hydrates of the tiotropium salts 1 which may be used according to the
invention it
is particularly preferable within the scope of the present invention to use
the
crystalline tiotropium bromide monohydrate described in WO 02/30928. Reference
is hereby made to the entire contents of this International Patent
Application. This
crystalline tiotropium bromide monohydrate is characterised by an endothermic
maximum at 230 ~ 5°C at a heating rate of 10K/min, when thermally
analysed by
DSC. It is also characterised in that in the IR spectrum it has bands inter
alia at
wavelengths 3570, 3410, 3105, 1730, 1260, 1035 and 720 cm-'. Finally, this
crystalline tiotropium bromide monohydrate has a simple monoclinic cell with
the
following dimensions: a =18.0774 A, b = 11.9711 A, c = 9.9321 A, (3 =102.691
°, V
= 2096.96 A3 as determined by monocrystalline X-ray structural analysis.
The active substance combinations according to the invention are surprisingly
characterised both by a rapid onset of activity and by their long-lasting
effect. This
is very important to the patient as on the one hand they will rapidly
experience an
improvement in their condition after taking the combination and on the other
hand
because of the long-lasting effect it is sufficient to take the drug once a
day.
The abovementioned effects are observed both when the two active substances
are taken simultaneously in a single active substance formulation and when
they
are administered successively in separate formulations. It is preferable
according
to the invention to administer the two active substance ingredients
simultaneously
in a single formulation.
In one aspect the present invention relates to a pharmaceutical composition
which
contains one or more tiotropium salts 1 and salts 2 of a compound of formula
2'
optionally in the form of their solvates or hydrates. The active substances
may
either be combined in a single preparation or contained in two separate
formulations. Pharmaceutical compositions which contain the active substances
1
and 2 in a single preparation are preferred according to the invention.
CA 02507656 2005-05-27
WO 2004/050093 8 PCT/EP2003/012913
In another aspect the present invention relates to a pharmaceutical
composition
which contains, in addition to therapeutically effective quantities of 1 and
2, a
pharmaceutically acceptable excipient. In one aspect the present invention
relates
to a pharmaceutical composition which does not contain any pharmaceutically
acceptable excipient in addition to therapeutically effective quantities of 1
and 2.
The present invention also relates to the use of 1 and 2 for preparing a
pharmaceutical composition containing therapeutically effective quantities of
1 and
2 for treating inflammatory or obstructive diseases of the respiratory tract,
particularly asthma and/or COPD, by simultaneous or successive administration.
The present invention is also directed to the simultaneous or successive use
of
therapeutically effective doses of the combination of the above pharmaceutical
compositions 1 and 2 for treating inflammatory or obstructive diseases of the
respiratory tract, particularly asthma or COPD.
The proportions in which the two active substances 1 and 2 may be used in the
active substance combinations according to the invention are variable. Active
substances 1 and 2 may possibly be present in the form of their solvates or
hydrates. Depending on the choice of the salts 1 and 2, the weight ratios
which
may be used within the scope of the present invention vary on the basis of the
different molecular weights of the various salt forms. The proportions by
weight
specified below were therefore based on tiotropium 1' and the free bases 2'.
The active substance combinations according to the invention may contain 1'
and
2' in proportions by weight in a range from 1:300 to 30:1, preferably from
1:230 to
20:1, more preferably from 1:150 to 1:1, still more preferably from 1: 50 to
5:1,
more preferably from 1:35 to 2:1.
For example, without restricting the scope of the invention thereto, preferred
combinations of 1 and 2 may contain tiotropium 1' and the particularly
preferred
compound 2a' in the following weight ratios: 1:40; 1:39; 1:38; 1:37; 1:36;
1:35;
1:34; 1:33; 1:32; 1:31; 1:30; 1:29; 1:28; 1:27; 1:26; 1:25; 1:24; 1:23; 1:22;
1:21;
1:20; 1:19; 1:18; 1:17; 1:16; 1:15; 1:14; 1:13; 1:12; 1:11; 1:10; 1: 9; 1:8;
1:7; 1:6;
1:5; 1:4; 1:3; 1:2; 1:1; 2:1; 3:1; 4:1; 5:1.
CA 02507656 2005-05-27
WO 2004/050093 9 PCT/EP2003/012913
The pharmaceutical compositions according to the invention containing the
combinations of 1 and 2 are usually administered by giving tiotropium 1' and
2'
together in doses from 0.01 to 1 OOOONg, preferably from 0.1 to 2000Ng, more
preferably from 10 to 1000Ng, still more preferably from 15 to 500pg per
single
dose.
For example, combinations of 1 and 2 according to the invention contain an
amount of tiotropium 1' and the particularly preferred compound 2a' such that
the
total dose per single dose is 15Ng, 20Ng, 25Ng, 30Ng, 35~rg, 45Ng, 50Ng, 55Ng,
60Ng, 65Ng, 70Ng, 75pg, 80pg, 85Ng, 90Ng, 95Ng, 100Ng, 105Ng, 110Ng, 115Ng,
120Ng, 125Ng, 130pg, 135Ng, 140pg, 145pg, 150Ng, 155Ng, 160Ng, 165Ng,
170Ng, 175Ng,180Ng, 185pg, 190Ng, 195pg, 200pg, 205pg, 21 Opg, 21 SNg, 220Ng,
225Ng, 230Ng, 235Ng, 240Ng, 245Ng, 250Ng, 255Ng, 260Ng, 265Ng, 270pg,
275Ng, 280Ng, 285Ng, 290pg, 295Ng, 300Ng, 305pg, 310pg, 315Ng, 320Ng,
325Ng, 330Ng, 335pg, 340pg, 345Ng, 350Ng, 355pg, 360pg, 365Ng, 370Ng,
375Ng, 380Ng, 385Ng, 390pg, 395pg, 400pg, 405Ng, 410Ng, 415Ng, 420pg,
425pg, 430pg, 435Ng, 440Ng, 445pg, 450pg, 455pg, 460Ng, 465Ng, 470pg,
475Ng, 480Ng, 485Ng, 490Ng, 495~rg, 500Ng, 505Ng, or the like. In these dosage
ranges the active substances 1' and 2a' are present in the ratios by weight
described above.
For example, without restricting the scope of the invention thereto, the
combinations of 1 and 2 according to the invention may contain the following
amounts of tiotropium 1' and 2a' for example: 5Ng of 1' and 12.5Ng of 2a', 5pg
of
1' and 25pg of 2a', 5pg of 1' and 50Ng of 2a', 5Ng of 1' and 75Ng of 2a', 5pg
of 1'
and 100pg of 2a', 5pg of 1' and 200Ng of 2a', 10Ng of 1' and 12.5pg of 2a',
10pg
of 1' and 25Ng of 2a', 1 ONg of 1' and 50Ng of 2a', 1 ONg of 1' and 75Ng of
2a',1 ONg
of 1' and 100Ng of 2a', 10pg of 1' and 200pg of 2a', 18Ng of 1' and 12.5pg of
2a',
18Ng of 1' and 25Ng of 2a' , 18pg of 1' and 50pg of 2a', 18Ng of 1' and 75Ng
of 2a',
18pg of 1' and 100Ng of 2a', 18Ng of 1' and 200Ng of 2a', 20Ng of 1' and12.5pg
of 2a', 20pg of 1' and 25Ng of 2a', 20pg of 1' and 50Ng of 2a', 20pg of 1' and
75Ng
of 2a', 20pg of 1' and 100Ng of 2a', 20pg of 1' and 200pg of 2a', 36pg of 1'
and
12.5Ng of 2a', 36pg of 1' and 25Ng of 2a', 36Ng of 1' and 50Ng of 2a', 36pg of
1'
and 75Ng of 2a', 36pg of 1' and 100Ng of 2a', 36pg of 1' and 200pg of 2a',
40Ng
of 1' and 12.5pg of 2a', 40Ng of 1' and 25Ng of 2a', 40pg of 1' and 50pg of
2a',
CA 02507656 2005-05-27
WO 2004/050093 10 PCT/EP2003/012913
40Ng of 1' and 75Ng of 2a', 40pg of 1' and 100pg of 2a' or 40Ng of 1' and
200Ng of
2a'.
If the active substance combination in which 1 denotes tiotropium bromide and
2
denotes the particularly preferred salt 2a, the maleate salt of the compound
2a', is
used as the preferred combination of 1 and 2 according to the invention, the
quantities of active substance 1' and 2a' administered per single dose
mentioned
by way of example correspond to the following quantities of 1 and 2a
administered
per single dose: 6pg of 1 and 16.2pg of 2a, 6pg of 1 and 32.4Ng of 2a, 6pg of
1
and 64.8Ng of 2a, 6Ng of 1 and 97.2Ng of 2a, 6Ng of 1 and 129.6Ng of 2a, 6Ng
of
1 and 259.2pg of 2a, 12Ng of 1 and 16.2Ng of 2a, 12pg of 1 and 32.4Ng of 2a,
12Ng of 1 and 64.8Ng of 2a, 12Ng of 1 and 97.2pg of 2a, 12pg of 1 and 129.6Ng
of
2a, 12Ng of 1 and 259.2pg of 2a, 21.7Ng of 1 and 16.2Ng of 2a, 21.7pg of 1 and
32.4Ng of 2a, 21.7pg of 1 and 64.8Ng of 2a, 21.7Ng of 1 and 97.2Ng of 2a,
21.7pg
of 1 and 129.6Ng of 2a, 21.7pg of 1 and 259.2pg of 2a, 24.1 pg of 1 and 12.5Ng
of
2a, 24.1 Ng of 1 and 32.4Ng of 2a, 24.1 pg of 1 and 64.8Ng of 2a, 24.1 Ng of 1
and
97.2Ng of 2a, 24.1 Ng of 1 and 129.6pg of 2a, 24.1 pg of 1 and 259.2Ng of 2a,
43.3Ng of 1 and 16.2Ng of 2a, 43.3pg of 1 and 32.4Ng of 2a, 43.3Ng of 1 and
64.8Ng of 2a, 43.3Ng of 1 and 97.2Ng of 2a, 43.3pg of 1 and 129.6pg of 2a,
43.3pg
of 1 and 259.2~rg of 2a, 48.1 pg of 1 and 16.2Ng of 2a, 48.1 Ng of 1 and
32.4pg of
2a, 48.1 Ng of 1 and 64.8Ng of 2a, 48.1 Ng of 1 and 97.2pg of 2a or 48.1 Ng of
1 and
129.6pg of 2a, 48.1 Ng of 1 and 259.2Ng of 2a.
If the active substance combination of 1 and 2 used according to the invention
is
the combination in which 1 denotes the crystalline tiotropium bromide
monohydrate which is particularly preferred according to the invention and 2
denotes the particularly preferred salt 2a, the maleate salt of the compound
the quantities of active substance 1' and 2a' administered per single dose
mentioned by way of example correspond to the following quantities of 1 and 2a
administered per single dose: 6.2pg of 1 and 16.2pg of 2a, 6.2Ng of 1 and
32.4Ng
of 2a, 6.2pg of 1 and 64.8pg of 2a, 6.2pg of 1 and 97.2pg of 2a, 6.2Ng of 1
and
129.6Ng of 2a, 6.2Ng of 1 and 259.2pg of 2a, 12.5Ng of 1 and 16.2Ng of 2a,
12.5Ng
of 1 and 32.4trg of 2a, 12.5Ng of 1 and 64.8Ng of 2a, 12.5pg of 1 and 97.2pg
of 2a,
12.5~g of 1 and 129.6Ng of 2a, 12.5Ng of 1 and 259.2pg of 2a, 22.5Ng oft and
16.2pg of 2a, 22.5Ng of 1 and 32.4Ng of 2a, 22.5pg of 1 and 64.8pg of 2a,
22.5Ng
CA 02507656 2005-05-27
' _ WO 2004/050093 11 PCT/EP2003/012913
of 1 and 97.2Ng of 2a, 22.5Ng of 1 and 129.6pg of 2a, 22.5Ng of 1 and 259.2pg
of
2a, 25Ng of 1 and 12.5pg of 2a, 25Ng of 1 and 32.4pg of 2a, 25pg of 1 and
64.8pg
of 2a, 25Ng of 1 and 97.2Ng of 2a, 25Ng of 1 and 129.6pg of 2a, 25Ng of 1 and
259.2Ng of 2a, 45Ng of 1 and 16.2Ng of 2a, 45Ng of 1 and 32.4pg of 2a, 45Ng of
1
and 64.8pg of 2a; 45Ng of 1 and 97.2Ng of 2a, 45Ng of 1 and 129.6Ng of 2a,
45Ng
of 1 and 259.2Ng of 2a, 50pg of 1 and 16.2Ng of 2a, 50Ng of 1 and 32.4Ng of
2a,
50Ng of 1 and 64.8Ng of 2a, 50Ng of 1 and 97.2Ng of 2a or 50Ng of 1 and
129.6Ng
of 2a, 50pg of 1 and 259.2pg of 2a.
The active substance combinations of 1 and 2 according to the invention are
preferably administered by inhalation or by nasal application. For this
purpose,
ingredients 1 and 2 have to be made available in inhalable forms. Inhalable
preparations include, in particular, inhalable powders. Inhalable powders
according to the invention containing the combination of active substances 1
and 2
may consist of the active substances on their own or of a mixture of the
active
substances with physiologically acceptable excipients. The preparations
according to the invention may contain the combination of active substances 1
and 2 either together in one formulation or in two separate formulations.
These
formulations which may be used within the scope of the present invention are
described in more detail in the next part of the specification.
A) Inhalable powders containing the combinations of active substances 1
and 2 according to the invention:
The inhalable powders according to the invention may contain 1 and 2 either on
their own or in admixture with suitable physiologically acceptable excipients.
If the active substances 1 and 2 are present in admixture with physiologically
acceptable excipients, the following physiologically acceptable excipients may
be
used to prepare these inhalable powders according to the invention:
monosaccharides (e.g. glucose or arabinose), disaccharides (e.g. lactose,
saccharose, maltose, trehalose), oligo- and polysaccharides (e.g. dextrane),
polyalcohols (e.g. sorbitol, mannitol, xylitol), salts {e.g. sodium chloride,
calcium
carbonate) or mixtures of these excipients with one another. Preferably, mono-
or
disaccharides are used, while the use of lactose or glucose is preferred,
particularly, but not exclusively, in the form of their hydrates. For the
purposes of
CA 02507656 2005-05-27
WO 2004/050093 12 PCT/EP2003/012913
the invention, lactose is the particularly preferred excipient, while lactose
monohydrate is most particularly preferred.
Within the scope of the inhalable powders according to the invention the
excipients
have a maximum average particle size of up to 250pm, preferably between 10 and
150pm, most preferably between 15 and 80Nm. It may sometimes seem
appropriate to add finer excipient fractions with an average particle size of
1 to
9pm to the excipients mentioned above. These finer excipients are also
selected
from the group of possible excipients listed hereinbefore. In particularly
preferred
inhalable powders the excipient is characterised by an average particle size
of 12
to 35~,m, more preferably from 13 to 30~m. Also particularly preferred are
inhalable powders in which the 10% fine content is about 1 to 4 Nm, preferably
about 1.5 to 3 Nm.
By the average particle size is meant here the 50% value of the volume
distribution measured using a laser diffractometer by the dry dispersion
method.
Analogously, the 10% fine content in this instance refers to the 10% value of
the
volume distribution measured using a laser diffractometer.
Preferably, excipients of high crystallinity are used for the powder
formulations
according to the invention. This crystallinity can be assessed by means of the
enthalpy released as the excipient is dissolved (solution enthalpy). In the
case of
the excipient lactose monohydrate, which is most preferably used acording to
the
invention, it is preferable to use lactose which is characterised by a
solution
enthalpy of > 45 J/g, preferably > 50 J/g, particularly preferably > 52 J/g.
Finally, in order to prepare the inhalable powders according to the invention,
micronised active substance 1 and 2, preferably with an average particle size
of
0.5 to 1 Opm, more preferably from 1 to 5pm, are added to the excipient
mixture.
If the active substance 1 used is the crystalline tiotropium bromide
monohydrate
disclosed by WO 02/30928 which is particularly preferred according to the
invention the following procedure has proved particularly suitable for
micronising
this crystalline active substance modification. The process may be carried out
using conventional mills. Preferably, the micronisation is carried out with
the
CA 02507656 2005-05-27
._ WO 2004/050093 13 PCT/EP20031012913
exclusion of moisture, more preferably, using a corresponding inert gas such
as
nitrogen, for example. It has proved particularly preferable to use air jet
mills in
which the material is comminuted by the impact of the particles on one another
and on the walls of the grinding container. According to the invention,
nitrogen is
preferably used as the grinding gas. The material for grinding is conveyed by
the
grinding gas under specific pressures (grinding pressure). Within the scope of
the
present invention, the grinding pressure is usually set to a value between
about 2
and 8 bar, preferably between about 3 and 7 bar, most preferably between about
3.5 and 6.5 bar. The material for grinding is fed into the air jet mill by
means of the
feed gas under specific pressures (feed pressure). Within the scope of the
present invention a feed pressure of between about 2 and 8 bar, preferably
between about 3 and 7 bar and most preferably between about 3.5 and 6 bar has
proved satisfactory. The feed gas used is also preferably an inert gas, most
preferably nitrogen again. The material to be ground (crystalline tiotropium
bromide monohydrate) maybe fed in at a rate of about 5 - 35 g/min, preferably
at
about 10-30 g/min.
For example, without restricting the subject of the invention thereto, the
following
apparatus has proved suitable as a possible embodiment of an air jet mill: a 2-
inch
Microniser with grinding ring, 0.8 mm bore, made by Messrs Sturtevant Inc.,
348
Circuit Street, Hanover, MA 02239, USA. Using this apparatus, the grinding
process is preferably carried out with the following grinding parameters:
grinding
pressure: about 4.5 - 6.5 bar; feed pressure: about 4.5 - 6.5 bar; supply of
grinding
material: about 17 - 21 g/min.
The ground material thus obtained is then further processed under the
following
specific conditions. The micronisate is exposed to water vapour at a relative
humidity of at least 40% at a temperature of 15-40°C, preferably 20-
35°C, most
preferably 25-30°C . Preferably, the humidity is set to a value of 50 -
95% r. h.,
preferably 60 - 90% r.h., most preferably 70 - 80% r.h. By relative humidity
(r.h.) is
meant the quotient of the partial steam pressure and the steam pressure of the
water at the temperature in question. Preferably, the micronisate obtained
from the
grinding process described above is subjected to the chamber conditions
mentioned above for a period of at least 6 hours. Preferably, however, the
micronisate is subjected to the chamber conditions mentioned above for about
12
CA 02507656 2005-05-27
WO 2004/050093 14 PCT/EP2003/012913
to 48 hours, preferably about 18 to 36 hours, more preferably about 20 to 28
hours.
The micronisate of tiotropium bromide obtainable by the above method has a
characteristic particle size of between 1.0 Nm and 3.5 pm, preferably between
1.1 Nm and 3.3 Nm, most preferably between 1.2 Nm and 3.ONm and Q~s.s> of more
than 60%, preferably more than 70 %, most preferably more than 80%. The
characteristic value Q~5.8~ indicates the quantity of particles below 5.8 Nm,
based
on the volume distribution of the particles. The particle sizes were
determined
within the scope of the present invention by laser diffraction (Fraunhofer
diffraction). More detailed information on this subject can be found in the
experimental descriptions of the invention.
Also characteristic of the tiotropium micronisate according to the invention
which
was prepared by the above process are Specific Surface Area values in the
range
between 2 m2/g and 5 m2/g, more particularly between 2.5 m2/g and 4.5 m2/g and
most outstandingly between 3.0 m2/g and 4.0 m2/g.
After the starting materials have been weighed in the inhalable powders are
prepared from the excipient and the active substances 1 and 2 using methods
known in the art. Reference may be made to the disclosure of WO 02/30390, for
example.
The inhalable powders according to the invention may be prepared and
administered either in the form of a single powder mixture which contains both
1
and 2 or in the form of separate inhalable powders which contain only 1 or 2.
The inhalable powders according to the invention may be administered using
inhalers known from the prior art.
Inhalable powders according to the invention which contain a physiologically
acceptable excipient in addition to 1 and 2 may be administered, for example,
by
means of inhalers which deliver a single dose from a supply using a measuring
chamber as described in US 4570630A, or by other means as described in
DE 36 25 685 A. Preferably, the inhalable powders according to the invention
CA 02507656 2005-05-27
WO 2004/050093 15 PCT/EP2003/012913
which contain physiologically acceptable excipient in addition to 1 and 2 are
packed into capsules (to produce so-called inhalettes) which are used in
inhalers
as described, for example, in WO 94128958.
A particularly preferred inhaler for administering the pharmaceutical
combination
according to the invention in inhalettes is shown in Figure 1.
This inhaler (Handyhaler) for inhaling powdered pharmaceutical compositions
from
capsules is characterised by a housing 1 containing two windows 2, a deck 3 in
which there are air inlet ports and which is provided with a screen 5 secured
via a
screen housing 4, an inhalation chamber 6 connected to the deck 3 on which
there
is a push button 8 provided with two sharpened pins 7 and movable counter to a
spring 8, and a mouthpiece 12 which is connected to the housing 1, the deck 3
and a cover 11 via a spindle 10 to enable it to be flipped open or shut, and
air
holes 13 for adjusting the flow resistance.
For administering the inhalable powders according to the invention containing
1
and 2 using powder-filled capsules it is particularly preferred to use
capsules the
material of which is selected from among the synthetic plastics, most
preferably
selected from among polyethylene, polycarbonate, polyester, polypropylene and
polyethylene terephthalate. Particularly preferred synthetic plastic materials
are
polyethylene, polycarbonate or polyethylene terephthalate. If polyethylene is
used
as one of the capsule materials which is particularly preferred according to
the
invention, it is preferable to use polyethylene with a density of between 900
and
1000 kg/m3, preferably 940 - 980 kg/m3 , more preferably about 960 - 970 kg/m3
(high density polyethylene).
The synthetic plastics according to the invention may be processed in various
ways using manufacturing methods known in the art. Injection moulding of the
plastics is preferred according to the invention. Injection moulding without
the use
of mould release agents is particularly preferred. This method of production
is well
defined and is characterised by being particularly reproducible.
In another aspect the present invention relates to the abovementioned capsules
which contain the abovementioned inhalable powders containing 1 and 2
CA 02507656 2005-05-27
WO 20041050093 16 PCT/EP2003/012913
according to the invention. If the inhalable powders according to the
invention are
intended to be packed into capsules (inhalettes) for the preferred use
described
above, fill amounts of from 1 to 30mg, preferably from 3 to 20mg, preferably 5
to
mg of inhalable powder per capsule are recommended. These contain,
5 according to the invention, either together or separately, the
abovementioned
dosages of 1 and 2 per single dose. As already mentioned, the present
invention
also relates to a kit consisting of two capsules each of which contains the
active
substances 1 and 2 optionally combined with one of the abovementioned
physiologically acceptable excipients.
The present invention also relates to an inhalation kit consisting of one or
more of
the above capsules characterised by a content of inhalable powder containing 1
and 2 according to the invention in conjunction with the inhaler according to
Figure 1.
The present invention also relates to the use of the abovementioned capsules,
characterised by a content of inhalable powder containing 1 and 2 according to
the
invention, for preparing a pharmaceutical composition for treating respiratory
complaints, especially for treating COPD and/or asthma.
Filled capsules which contain the inhalable powders according to the invention
are
produced by methods known in the art, by filling the empty capsules with the
inhalable powders according to the invention.
B) Propellant gas-driven inhalation aerosols containing the combinations of
active substances 1 and 2
Inhalation aerosols containing propellant gas according to the invention may
contain substances 1 and 2 dissolved in the propellant gas or in dispersed
form.
1 and 2 may be present in separate formulations or in a single preparation, in
which 1 and 2 are either each dissolved, dispersed or only one or two of the
components is or are dissolved and the other or others is or are dispersed.
The
propellant gases which may be used to prepare the inhalation aerosols
according
to the invention are known from the prior art. Suitable propellant gases are
selected from among hydrocarbons such as n-propane, n-butane or isobutane and
halohydrocarbons such as fluorinated derivatives of methane, ethane, propane,
CA 02507656 2005-05-27
WO 2004/050093 17 PCTlEP20031012913
butane, cyclopropane or cyclobutane. The propellant gases mentioned above
may be used on their own or in mixtures thereof. Particularly preferred
propellant
gases are halogenated alkane derivatives selected from TG134a, TG227 and
mixtures thereof.
The propellant-driven inhalation aerosols according to the invention may also
contain other ingredients such as co-solvents, stabilisers, surtactants,
antioxidants, lubricants and pH adjusters. All these ingredients are known in
the
art.
The inhalation aerosols containing propellant gas according to the invention
may
contain up to 5 wt.-% of active substance 1 and/or 2. Aerosols according to
the
invention contain, for example, 0.002 to 5 wt.-%, 0.01 to 3 wt.-%, 0.015 to 2
wt.-%,
0.1 to 2 wt.-%, 0.5 to 2 wt.-% or 0.5 to 1 wt.-% of active substance 1 and/or
2.
If the active substances 1 and/or 2 are present in dispersed form, the
particles of
active substance preferably have an average particle size of up to10~m,
preferably
from 0.1 to 5~,m, more preferably from 1 to 5~,m. If 1 is to be used in the
form of its
crystalline tiotropium bromide monohydrate this may optionally be used in the
form
of the micronisate described in more detail in the previous section.
The propellant-driven inhalation aerosols according to the invention mentioned
above may be administered using inhalers known in the art (MDIs = metered dose
inhalers). Accordingly, in another aspect, the present invention relates to
pharmaceutical compositions in the form of propellant-driven aerosols as
hereinbefore described combined with one or more inhalers suitable for
administering these aerosols. In addition, the present invention relates to
inhalers
which are characterised in that they contain the propellant gas-containing
aerosols
described above according to the invention.
The present invention also relates to cartridges which are fitted with a
suitable
valve and can be used in a suitable inhaler and which contain one of the
above-mentioned propellant gas-containing inhalation aerosols according to the
invention. Suitable cartridges and methods of filling these cartridges with
the
CA 02507656 2005-05-27
WO 2004/050093 18 PCT/EP2003/012913
inhalable aerosols containing propellant gas according to the invention are
known
from the prior art.
C) Propellant-free inhalable solutions or suspensions containing the
combinations of active substances 1 and 2 according to the invention:
It is particularly preferred to use the active substance combination according
to the
invention in the form of propellant free inhalable solutions and suspensions.
The
solvent used may be an aqueous or alcoholic, preferably an ethanolic solution.
The solvent may be water on its own or a mixture of water and ethanol. The
relative proportion of ethanol compared with water is not limited but the
maximum
is up to 70 percent by volume, more particularly up to 60 percent by volume
and
most preferably up to 30 percent by volume. The remainder of the volume is
made up of water. The solutions or suspensions containing 1 and 2, separately
or
together, are adjusted to a pH of 2 to 7, preferably 2 to 5, using suitable
acids.
The pH may be adjusted using acids selected from inorganic or organic acids.
Examples of suitable inorganic acids include hydrochloric acid, hydrobromic
acid,
nitric acid, sulphuric acid and/or phosphoric acid. Examples of particularly
suitable
organic acids include ascorbic acid, citric acid, malic acid, tartaric acid,
malefic
acid, succinic acid, fumaric acid, acetic acid, formic acid and/or propionic
acid etc.
Preferred inorganic acids are hydrochloric and sulphuric acids. It is also
possible
to use the acids which have already formed an acid addition salt with one of
the
active substances. Of the organic acids, ascorbic acid, fumaric acid and
citric acid
are preferred. If desired, mixtures of the above acids may be used,
particularly in
the case of acids which have other properties in addition to their acidifying
qualities, e.g. as flavourings, antioxidants or complexing agents, such as
citric acid
or ascorbic acid, for example. According to the invention, it is particularly
preferred to use hydrochloric acid to adjust the pH.
According to the invention, the addition of editic acid (EDTA) or ane of the
known
salts thereof, sodium edetate, as stabiliser or complexing agent is
unnecessary in
the present formulation. Other embodiments may contain this compound or these
compounds. In a preferred embodiment the content based on sodium edetate is
less than 100 mg/100m1, preferably less than 50mg/100m1, more preferably less
than 20mg/100m1. Generally, inhalable solutions in which the content of sodium
edetate is from 0 to 10mg/100m1 are preferred.
CA 02507656 2005-05-27
WO 2004/050093 19 PCT/EP2003/012913
Co-solvents and/or other excipients may be added to the propellant-free
inhalable
solutions according to the invention. Preferred co-solvents are those which
contain hydroxyl groups or other polar groups, e.g. alcohols - particularly
isopropyl
alcohol, glycols - particularly propyleneglycol, polyethyleneglycol,
polypropyleneglycol, glycolether, glycerol, polyoxyethylene alcohols and
polyoxyethylene fatty acid esters. The terms excipients and additives in this
context denote any pharmacologically acceptable substance which is not an
active
substance but which can be formulated with the active substance or substances
in
the physiologically suitable solvent in order to improve the qualitative
properties of
the active substance formulation. Preferably, these substances have no
pharmacological effect or, in connection with the desired therapy, no
appreciable
or at least no undesirable pharmacological effect. The excipients and
additives
include, for example, surfactants such as soya lecithin, oleic acid, sorbitan
esters,
such as polysorbates, polyvinylpyrrolidone, other stabilisers, complexing
agents,
antioxidants and/or preservatives which guarantee or prolong the shelf life of
the
finished pharmaceutical formulation, flavourings, vitamins and/or other
additives
known in the art. The additives also include physiologically acceptable salts
such
as sodium chloride as isotonic agents.
The preferred excipients include antioxidants such as ascorbic acid, for
example,
provided that it has not already been used to adjust the pH, vitamin A,
vitamin E,
tocopherols and similar vitamins and provitamins occurring in the human body.
Preservatives may be used to protect the formulation from contamination with
pathogens. Suitable preservatives are those which are known in the art,
particularly cetyl pyridinium chloride, benzalkonium chloride or benzoic acid
or
benzoates such as sodium benzoate in the concentration known from the prior
art.
The preservatives mentioned above are preferably present in concentrations of
up
to 50mg/100m1, more preferably between 5 and 20mg/100m1.
Preferred formulations contain, in addition to the solvent water and the
combination of active substances 1 and 2, only benzalkonium chloride and
sodium
edetate. In another preferred embodiment, no sodium edetate is present.
CA 02507656 2005-05-27
WO 2004!050093 20 PCT/EP2003/012913
The propellant-free inhalable solutions according to the invention are
administered
in particular using inhalers of the kind which are capable of nebulising a
small
amount of a liquid formulation in the required therapeutic dose within a few
seconds to produce an aerosol suitable for therapeutic inhalation. Within the
scope of the present invention, preferred nebulisers are those in which a
quantity
of less than 100pL, preferably less than 50p,L, more preferably between 20 and
30~,L of active substance solution can be nebulised in preferably one spray
action
to form an aerosol with an average particle size of less than 20~m, preferably
less
than 10~m, in such a way that the inhalable part of the aerosol corresponds to
the
therapeutically effective quantity.
An apparatus of this kind for propellant-free delivery of a metered quantity
of a
liquid pharmaceutical composition for inhalation is described for example in
International Patent Application WO 91114468 and also in WO 97112687 (cf. in
particular Figures 6a and 6b). The nebulisers (devices) described therein are
known by the name Respimat0.
This nebuliser (Respimat~) can advantageously be used to produce the inhalable
aerosols according to the invention containing the combination of active
substances 1 and 2. Because of its cylindrical shape and handy size of less
than
9 to 15 cm long and 2 to 4 cm wide, this device can be carried at all times by
the
patient. The nebuliser sprays a defined volume of pharmaceutical formulation
using high pressures through small nozzles so as to produce inhalable
aerosols.
The preferred atomiser essentially consists of an upper housing part, a pump
housing, a nozzle, a locking mechanism, a spring housing, a spring and a
storage
container, characterised by
- a pump housing which is secured in the upper housing part and which
comprises at one end a nozzle body with the nozzle or nozzle arrangement,
- a hollow plunger with valve body,
- a power takeoff flange in which the hollow plunger is secured and which is
located in the upper housing part,
- a locking mechanism situated in the upper housing part,
- a spring housing with the spring contained therein, which is rotatably
mounted on the upper housing part by means of a rotary bearing,
CA 02507656 2005-05-27
'_ WO 2004/050093 21 PCT/EP2003/012913
- a lower housing part which is fitted onto the spring housing in the axial
direction.
The hollow plunger with valve body corresponds to a device disclosed in
WO 97/12687. It projects partially into the cylinder of the pump housing and
is
axially movable within the cylinder. Reference is made in particular to
Figures 1 to
4, especially Figure 3, and the relevant parts of the description. The hollow
plunger with valve body exerts a pressure of 5 to 60 Mpa (about 50 to 600
bar),
preferably 10 to 60 Mpa (about 100 to 600 bar) on the fluid, the measured
amount
of active substance solution, at its high pressure end at the moment when the
spring is actuated. Volumes of 10 to 50 microlitres are preferred, while
volumes of
10 to 20 microlitres are particularly preferred and a volume of 15 microlitres
per
spray is most particularly preferred.
The valve body is preferably mounted at the end of the hollow plunger facing
the
valve body.
The nozzle in the nozzle body is preferably microstructured, i.e. produced by
microtechnology. Microstructured valve bodies are disclosed for example in
WO-94107607; reference is hereby made to the contents of this specification,
particularly Figure 1 therein and the associated description.
The nozzle body consists for example of two sheets of glass and/or silicon
firmly
joined together, at feast one of which has one or more microstructured
channels
which connect the nozzle inlet end to the nozzle outlet end. At the nozzle
outlet
end there is at least one round or non-round opening 2 to 10 microns deep and
5
to 15 microns wide, the depth preferably being 4.5 to 6.5 microns while the
length
is preferably 7 to 9 microns.
In the case of a plurality of nozzle openings, preferably two, the directions
of
spraying of the nozzles in the nozzle body may extend parallel to one another
or
may be inclined relative to one another in the direction of the nozzle
opening. In a
nozzle body with at least two nozzle openings at the outlet end the directions
of
spraying may be at an angle of 20 to 160° to one another, preferably 60
to 150°,
most preferably 80 to 100°. The nozzle openings are preferably arranged
at a
spacing of 10 to 200 microns, more preferably at a spacing of 10 to 100
microns,
CA 02507656 2005-05-27
WO 2004/050093 22 PCT/EP2003/012913
most preferably 30 to 70 microns. Spacings of 50 microns are most preferred.
The directions of spraying will therefore meet in the vicinity of the nozzle
openings.
The liquid pharmaceutical preparation strikes the nozzle body with an entry
pressure of up to 600 bar, preferably 200 to 300 bar, and is atomised into an
inhalable aerosol through the nozzle openings. The preferred particle or
droplet
sizes of the aerosol are up to 20 microns, preferably 3 to 10 microns.
The locking mechanism contains a spring, preferably a cylindrical helical
compression spring, as a store for the mechanical energy. The spring acts on
the
power takeoff flange as an actuating member the movement of which is
determined by the position of a locking member. The travel of the power
takeoff
flange is precisely limited by an upper and lower stop. The spring is
preferably
biased, via a power step-up gear, e.g. a helical thrust gear, by an external
torque
which is generated when the upper housing part is rotated counter to the
spring
housing in the lower housing part. In this case, the upper housing part and
the
power takeoff flange have a single or multiple V-shaped gear.
The locking member with engaging locking surfaces is arranged in a ring around
the power takeoff flange. It consists, for example, of a ring of plastic or
metal
which is inherently radially elastically deformable. The ring is arranged in a
plane
at right angles to the atomiser axis. After the biasing of the spring, the
locking
surfaces of the locking member move into the path of the power takeoff flange
and
prevent the spring from relaxing. The locking member is actuated by means of a
button. The actuating button is connected or coupled to the locking member. In
order to actuate the locking mechanism, the actuating button is moved parallel
to
the annular plane, preferably into the atomiser; this causes the deformable
ring to
deform in the annular plane. Details of the construction of the locking
mechanism
are given in WO 97/20590.
The lower housing part is pushed axially over the spring housing and covers
the
mounting, the drive of the spindle and the storage container for the fluid.
CA 02507656 2005-05-27
' ~ WO 2004/050093 23 PCT/EP2003/012913
When the atomiser is actuated the upper housing part is rotated relative to
the
lower housing part, the lower housing part taking the spring housing with it.
The
spring is thereby compressed and biased by means of the helical thrust gear
and
the locking mechanism engages automatically. The angle of rotation is
preferably
a whole-number fraction of 360 degrees, e.g. 180 degrees. At the same time as
the spring is biased, the power takeoff part in the upper housing part is
moved
along by a given distance, the hollow plunger is withdrawn inside the cylinder
in
the pump housing, as a result of which some of the fluid is sucked out of the
storage container and into the high pressure chamber in front of the nozzle.
If desired, a number of exchangeable storage containers which contain the
fluid to
be atomised may be pushed into the atomiser one after another and used in
succession. The storage container contains the aqueous aerosol preparation
according to the invention.
The atomising process is initiated by pressing gently on the actuating button.
As a
result, the locking mechanism opens up the path for the power takeoff member.
The biased spring pushes the plunger into the cylinder of the pump housing.
The
fluid leaves the nozzle of the atomiser in atomised form.
Further details of construction are disclosed in PCT Applications WO 97/12683
and WO 97/20590, to which reference is hereby made.
The components of the atomiser (nebuliser) are made of a material which is
suitable for its purpose. The housing of the atomiser and, if its operation
permits,
other parts as well, are preferably made of plastics, e.g. by injection
moulding. For
medicinal purposes, physiologically safe materials are used.
Figures 2a/b attached to this patent application, which are identical to
Figures 6a/b
of WO 97/12687, show the nebuliser (Respimat~) which can advantageously be
used for inhaling the aqueous aerosol preparations according to the invention.
Figure 2a shows a longitudinal section through the atomiser with the spring
biased
while Figure 2b shows a longitudinal section through the atomiser with the
spring
relaxed.
CA 02507656 2005-05-27
WO 2004/050093 24 PCT/EP2003/012913
The upper housing part (51 ) contains the pump housing (52) on the end of
which
is mounted the holder (53) for the atomiser nozzle. In the holder is the
nozzle
body (54) and a filter (55). The hollow plunger (57) fixed in the power
takeoff
flange (56) of the locking mechanism projects partially into the cylinder of
the
pump housing. At its end the hollow plunger carries the valve body (58). The
hollow plunger is sealed off by means of the seal (59). Inside the upper
housing
part is the stop (60) on which the power takeoff flange abuts when the spring
is
relaxed. On the power takeoff flange is the stop (61 ) on which the power
takeoff
flange abuts when the spring is biased. After the biasing of the spring the
locking
member (62) moves between the stop (61 ) and a support (63) in the upper
housing part. The actuating button (64) is connected to the locking member.
The
upper housing part ends in the mouthpiece (65) and is sealed off by means of
the
protective cover (66) which can be placed thereon.
The spring housing (67) with compression spring (68) is rotatably mounted on
the
upper housing part by means of the snap-in lugs (69) and rotary bearing. The
lower housing part (70) is pushed over the spring housing. Inside the spring
housing is the exchangeable storage container (71 ) for the fluid (72) which
is to be
atomised. The storage container is sealed off by the stopper (73) through
which
the hollow plunger projects into the storage container and is immersed at its
end in
the fluid (supply of active substance solution).
The spindle (74) for the mechanical counter is mounted in the covering of the
spring housing. At the end of the spindle facing the upper housing part is the
drive
pinion (75). The slider (76) sits on the spindle.
The nebuliser described above is suitable for nebulising the aerosol
preparations
according to the invention to produce an aerosol suitable for inhalation.
If the formulation according to the invention is nebulised using the method
described above (Respimat~) the quantity delivered should correspond to a
defined quantity with a tolerance of not more than 25%, preferably 20% of this
amount in at least 97°!°, preferably at least
98°!° of all operations of the inhaler
(spray actuations). Preferably, between 5 and 30 mg of formulation, most
CA 02507656 2005-05-27
WO 2004/050093 25 PCT/EP2003/012913
preferably between 5 and 20 mg of formulation are delivered as a defined mass
on
each actuation.
However, the formulation according to the invention may also be nebulised by
means of inhalers other than those described above, e.g. jet stream inhalers.
Accordingly, in a further aspect, the invention relates to pham~aceutical
formulations in the form of propellant-free inhalable solutions or suspensions
as
described above combined with a device suitable for administering these
formulations, preferably in conjunction with the Respimat~. Preferably, the
invention relates to propellant-free inhalable solutions or suspensions
characterised by the combination of active substances 1 and 2 according to the
invention in conjunction with the device known by the name Respimat~. In
addition, the present invention relates to the above-mentioned devices for
inhalation, preferably the Respimat~, characterised in that they contain the
propellant-free inhalable solutions or suspensions according to the invention
as
described hereinbefore.
The propellant-free inhalable solutions or suspensions according to the
invention
may take the form of concentrates or sterile inhalable solutions or
suspensions
ready for use, as well as the above-mentioned solutions and suspensions
designed for use in a Respimat~. Formulations ready for use may be produced
from the concentrates, for example, by the addition of isotonic saline
solutions.
Sterile formulations ready for use may be administered using energy-operated
fixed or portable nebulisers which produce inhalable aerosols by means of
ultrasound or compressed air by the Venturi principle or other principles.
Accordingly, in another aspect, the present invention relates to
pharmaceutical
compositions in the form of propellant-free inhalable solutions or suspensions
as
described hereinbefore which take the form of concentrates or sterile
formulations
ready for use, combined with a device suitable for administering these
solutions,
characterised in that the device is an energy-operated free-standing or
portable
nebuliser which produces inhalable aerosols by means of ultrasound or
compressed air by the Venturi principle or other methods.
CA 02507656 2005-05-27
WO 2004!050093 26 PCT/EP2003/012913
The Examples which follow serve to illustrate the present invention in more
detail
without restricting the scope of the invention to the following embodiments by
way
of example.
Starting materials
I) Micronising crystalline tiotropium bromide monohydrate:
The tiotropium bromide monohydrate obtainable by the process according to WO
02/30928 is micronised with an air jet mill of the 2-inch microniser type with
grinding ring, 0.8 mm bore, made by Messrs Sturtevant Inc., 348 Circuit
Street,
Hanover, MA 02239, USA. Using nitrogen as the grinding gas the following
grinding parameters are set, for example:
grinding pressure: 5.5 bar; feed pressure: 5.5 bar; supply (of crystalline
monohydrate) or flow speed: 19 g/min.
The ground material obtained is then spread out on sheet metal racks in a
layer
thickness of about 1 cm and subjected to the following climatic conditions for
24 -
24.5 hours: temperature: 25 - 30 °C; relative humidity: 70-80%.
Measuring methods:
I) Determining the particle size of micronised tiotropium monohydrate:
Measuring equipment and settings:
The equipment is operated according to the manufacturer's instructions.
Measuring equipment: HELOS Laser-diffraction spectrometer, (SympaTec)
Dispersing unit: RODOS dry dispenser with suction funnel,
(SympaTec)
Sample quantity: 200 mg ~ 150 mg
Product feed: Vibri Vibrating channel, Messrs. Sympatec
Frequency of vibrating channel: rising to 100
Duration of sample feed: 15 to 25 sec. (in the case of 200 mg)
Focal length: 100 mm (measuring range: 0.9 - 175 pm)
CA 02507656 2005-05-27
WO 2004/050093 27 PCTlEP20031012913
Measuring time: about 15 s (in the case of 200 mg)
Cycle time: 20 ms
Start/stop at: 1 % on channel 28
Dispersing gas: compressed air
Pressure: 3 bar
Vacuum: maximum
Evaluation method: HRLD
Sample preparation /product feed:
About 200 mg of the test substance are weighed onto a piece of card.
Using another piece of card all the larger lumps are broken up. The powder is
then
sprinkled finely over the front half of the vibrating channel (starting about
1 cm
from the front edge). After the start of the measurement the frequency of the
vibrating channel is varied so that the sample is fed in as continuously as
possible,
However, the quantity of product should not be too great either, so as to
ensure
adequate dispersal.
II) Determining the particle size of the excipient (in this case lactose):
Measuring eguipment and settincts:
The equipment is operated according to the manufacturer's instructions.
Measuring equipment: HELOS Laser-diffraction spectrometer, (SympaTec)
Dispersing unit: RODOS dry disperser with suction funnel,
(SympaTec)
Sample quantity: 200 mg 100 mg
Product feed: Vibri Vibrating channel, Messrs. Sympatec
Frequency of vibratingchannel: 100 % rising
Focal length: 200 mm (measuring range: 1.8 - 350 Nm)
Measuring time: about 10 s (in the case of 200 mg)
Cycle time: 10 ms
Start/stop at: 1 % on channel 28
Dispersing gas: compressed air
Pressure: 3 bar
CA 02507656 2005-05-27
WO 2004/050093 28 PCT/EP2003/012913
Vacuum: maximum
Evaluation method: HRLD
Sample preparation /product feed:
About 200 mg of the test substance are weighed onto a piece of card.
Using another piece of card all the larger lumps are broken up. The powder is
transferred into the vibrating channel. A gap of 1.2 to 1.4 mm is set between
the
vibrating channel and funnel. After the start of the measurement the frequency
of
the vibrating channel is increased as continuously as possible to 100 %
towards
the end of the measurement.
III) Determining the specific surface area of tiotropium bromide
monohydrate, micronised (1-point BET method):
Method:
The specific surface is determined by exposing the powder sample to a
nitrogen/helium atmosphere at different pressures. Cooling the sample causes
the
nitrogen molecules to be condensed on the surtace of the particles. The
quantity
of condensed nitrogen is determined by means of the change in the thermal heat
conductivity of the nitrogen/helium mixture and the surface of the sample is
calculated by means of the surtace nitrogen requirement. Using this value and
the weight of the sample, the specific surface is calculated.
Eauipment and materials:
Measuring equipment: Monosorb, Messrs Quantachrome
Heater: Monotektor, Messrs Quantachrome
Measuring and drying gas: nitrogen (5.0) / helium (4.6) 70/30, Messer
Griesheim
Adsorbate: 30% nitrogen in helium
Coolant: liquid nitrogen
Measuring cell: with capillary tube, Messrs. W. Pabisch
GmbH&Co. KG
Calibration peak; 1000 NI, Messrs. Precision Sampling Corp.
Analytical scale: R 160 P, Messrs. Satorius
CA 02507656 2005-05-27
WO 2004!050093 29 PCT/EP2003/012913
Calculating the specific surface:
The measured values are indicated by the equipment in [m2] and are usually
converted into [cm2/g] on weighing (dry mass):
Aspez = specific surface [cm2/g]
Aspez = ~ ~ 10000 MW = Measured value [m2]
mtr = dry mass [g]
10000 = conversion factor [cm2/m2]
IV) Determining the heat of solution of lactose (enthalpy of solution) E~:
The solution enthalpy is determined using a solution calorimeter 2225
Precision
Solution Calorimeter made by Messrs. Thermometric.
The heat of solution is calculated by means of the change in temperature
occurring (as a result of the dissolving process) and the system-related
change in
temperature calculated from the base line:
Before and after the ampoule is broken, electrical calibration is carried out
with an
integrated heating resistor of a precisely known power. A known heat output is
delivered to the system over a set period and the jump in temperature is
determined.
Method and eguipment parameters:
Solution calorimeter: 2225 Precision Solution Calorimeter,
Messrs Thermometric
Reaction cell: 100 ml
Thermistor resistance: 30.0 kSZ (at 25 °C)
Speed of stirrer: 500 U/min
Thermostat: Thermostat of 2277 Thermal Activity Monitor TAM, Messrs
Thermometric
Temperature: 25 °C ~ 0.0001 °C (over 24h)
Measuring ampoules: Crushing ampoules 1 ml, Messrs Thermometric
Seal: Silicon stopper and beeswax, Messrs. Thermometric
Weight: 40 to 50 mg
Solvent: Chemically pure water
CA 02507656 2005-05-27
WO 2004/050093 30 PCT/EP2003/012913
Volume of solvent: 100 ml
Bath temperature: 25°C
Temperature resolution: High
Starting temperature: -40mK (~ 10mK) temperature-offset
Interface: 2280-002 TAM accessory intertace 50 Hz,
Messrs Thermometric
Software: SoICaI V1.1 for WINDOWS
Evaluation: Automatic evaluation with Menu point CALCULATION/
ANALYSE EXPERIMENT. (Dynamics of base line;
calibration after breakage of ampoule).
Electrical calibration:
The electrical calibration takes place during the measurement, once before and
once after the breakage of the ampoule. The calibration after the breakage of
the
ampoule is used for the evaluation.
Amount of heat: 2.5 J
Heating power: 500 mW
Heating time: 10 s
Duration of base lines: 5 min (before and after heating)
CA 02507656 2005-05-27
WO 2004/050093 31 PCT/EP2003/012913
V~ Examples of formulations
A) Inhalable powders:
1)
Ingredients Ng per capsule
tiotropium bromide monohydrate10.8
2a'-hydrochloride 35
lactose 4954.2
Total 5000
2)
Ingredients Ng per capsule
tiotropium bromide monohydrate21.7
2a'-maleate salt 75
lactose 4903.3
Total 5000
3)
Ingredients Ng per capsule
tiotropium bromide monohydrate 22.5
2a'-maleate salt 80.5
lactose 4897
Total 5000
4)
Ingredients Ng per capsule
tiotropium bromide x 22.5
H20
2a'-maleate salt 95.5
lactose 4828
Total 5000
CA 02507656 2005-05-27
WO 2004/050093 32 PCT/EP2003/012913
B) Propellant-driven inhalable aerosols:
1)
Ingredients % by weight
tiotropium bromide monohydrate 0.015
2a'-hydrochloride 0.066
soya lecithin 0.2
TG134a : TG227 = 2:3 ad 100
2)
Ingredients % by weight
tiotropium bromide 0.029
compound 2a'-maleate salt 0.033
absolute ethanol 0.5
isopropyl myristate 0.1
TG 227 ad 100