Note: Descriptions are shown in the official language in which they were submitted.
CA 02507690 2005-05-18
-1-
Title: MASK ASSEMBLY WITH INTEGRATED SENSORS
Field of the invention
[0001] This invention relates to a mask assembly that is used for the
detection of physiological information and the treatment of medical
conditions.
More particularly, this invention relates to a mask assembly with integrated
sensors for sensing the efficacy of treatment of medical conditions.
Background of the invention
[0002] Obstructive Sleep Apnea (OSA) is a life-threatening condition
characterized by frequent episodes in which an individual stops breathing or
breathes less efficiently during sleep. OSA is caused by a blockage of the
airway typically resulting from the collapse and closure of the soft tissue in
the
rear of the throat during sleep. Wth each apnea event, the brain arouses the
individual in order for the individual to resume breathing, but consequently
sleep is extremely fragmented and of poor quality.
[0003] According to the National Institute of Health, OSA currently
affects more than twelve million Americans (4% of men and 2% of women),
making this disorder as common as adult diabetes. Further, the disrupted
and/or poor quality sleep that is associated with OSA may lead to serious
health issues including hypertension, heart disease, diabetes, and stroke.
Moreover, untreated sleep apnea may be responsible for job impairment and
motor vehicle crashes. For example, the Department of Transport in the UK
estimates that 20% of road accidents leading to death and serious injury are
caused by drowsiness or sleep disorders.
[0004] When an individual is diagnosed with OSA, the individual may
be prescribed a therapeutic regime involving the use of a Continuous Positive
Airway Pressure (CPAP) device. The CPAP device works by delivering a
steady flow of air through a soft, pliable mask worn over the individual's
nose.
The CPAP device essentially pressurizes the throat of the individual thereby
preventing the collapse of the soft tissue and keeping the airways open and
allowing the individual to breathe uninterrupted during sleep.
CA 02507690 2005-05-18
-2-
[0005] The CPAP device is both loud and uncomfortable and has met
with various non-compliance issues. However, it is possible to augment the
CPAP device to control gas delivery to the individual according to changes in
the physiological state of the wearer. These changes can be seen in
brainwave patterns, blood oxygen saturation and breathing patterns. One or
more of the individual's EEG, EOG, EMG, position, breathing and blood
oxygen levels can be monitored by a monitor unit associated with the CPAP
device. In some instances, the monitoring unit may be part of the CPAP
device. The EEG is used to observe brain activities during sleep. The EMG
is used to observe muscle tone during sleep. The EOG is used to observe
eye movement during sleep. The three physiological signals (i.e. EEG, EOG,
and EMG) may be used together to score sleep stages.
[0006] Arousals due to upper airway resistance may be detected from a
shift in frequency of the patient's EEG and/or EOG as well as a decrease in
blood oxygen levels. The monitoring unit includes an algorithm that detects
the arousals and sleep stages, and uses the physiological information to
automatically adjust the delivered respiratory gas pressure to the CPAP
device wearer based on the physiological information. The algorithm can also
use the physiological information to determine the effectiveness of the
treatment.
[0007] In order to measure the physiological information, various
sensors are attached to the CPAP patient. For instance, to measure EEG,
EOG and EMG, several electrodes may be applied to the patient's head and
face. To measure blood oxygen level, a blood oximetry probe may be applied
to the patient's finger or earlobe. To measure body position, an
accelerometer-based sensor may be placed on the patient's chest. These
sensors are connected to the monitoring unit with appropriate electrical
wires.
Once the sensors are applied, the CPAP device wearer puts on the mask
assembly. The mask assembly includes a nasal mask (or a nasal/oral mask)
and a harness that maintains the position of the nasal mask on the face of the
wearer.
CA 02507690 2005-05-18
-3-
[0008] However, the application of electrodes by the CPAP device
wearer is not an easy process; it is difficult, time consuming and prone to
errors. The process involves preparing the site to reduce impedance,
attaching one electrode at a time with tape and/or adhesives, and then
individually wiring each electrode to the monitoring unit. In a clinical
setting,
the electrodes are typically positioned by a polysomnography technician
according to standard positions, or as directed by a physician. In a non-
clinical setting, such as at a patient's home, the patient or another
untrained
person may be required to position the electrodes on the patient. Such
untrained persons may have difficulty placing the electrodes in the correct
location or correctly wiring the electrode to the monitoring unit. Even in a
clinical setting, a trained technician may position an electrode incorrectly.
[0009] Furthermore, once the electrodes and the other sensors are
applied to the wearer and connected to the monitoring unit, the arrangement
results in many wires emanating from different locations on the wearer to the
monitoring unit. As a result, the arrangement is uncomfortable for the wearer
and interferes with the wearer's natural movements, which makes it difficult
for the wearer to sleep. Consequently, the wearer may find it difficult to
find a
comfortable sleep position. In addition, the wearer may move during sleep
such that the sensors become disconnected from the monitoring unit. Also,
motion of the wires connecting the electrodes to the monitoring unit
introduces
electrical artifacts that hides the underlying physiological information.
These
technical difficulties also prevent sleep studies from being conducted at a
patient's home. Further, the unfamiliar environment in the sleep lab may
dramatically affect the patient's sleep which in turn may lead to an
inaccurate
diagnosis.
[0010] It is desired to address or ameliorate one or more of the
shortcomings, disadvantages or problems associated with prior systems or
devices, or to at least provide a useful alternative thereto.
Summary of the invention
CA 02507690 2005-05-18
-4-
[0011] The invention relates to a mask assembly with integrated
sensors for sensing the efficacy of treatment of medical conditions, such as
obstructive sleep apnea during treatment with continuous positive airway
pressure.
[0012] In one aspect, the invention provides a mask assembly adapted
to be worn by a wearer for treatment of a medical condition. The mask
assembly comprises a mask shaped to fit over at least the nose of the person,
the mask including a gas inlet for providing pressurized gas to the wearer; a
harness assembly attached to the mask, the harness assembly including a
plurality of straps for securing the mask assembly to the head of the wearer;
and, sensors located on the mask assembly for providing physiological
information about the person for determining efficacy of treatment and for
varying operational parameters of the treatment, the sensors being located on
at least one of the mask and the harness assembly.
[0013] In one embodiment, the sensors include electrodes. Preferably,
the sensors include three electrodes arranged in a triangular configuration
with at least a portion of the configuration being disposed on a forehead of
the
wearer.
[0014] In another embodiment, the mask includes a vertical mounting
plate extending upwardly from the top of the mask and wherein a first
electrode is located at the mask at the nasion of the person and a second
electrode is located at the vertical mounting plate at the central forehead
region of the person.
[0015] In another embodiment, the mask includes a vertical mounting
plate extending upwardly from the top of the mask and a forehead support
member attached horizontally therewith, the forehead support member having
vertically elongated apertures at either end, and wherein the harness
assembly includes right and left upper straps that engage the corresponding
elongated aperture on the forehead support member, wherein a first electrode
is located at the mask at the nasion of the person, a second electrode is
located at the upper right strap horizontally offset with respect to the
center of
CA 02507690 2005-05-18
-5-
the right eye of the wearer, and a third electrode is located at the upper
left
strap horizontally offset with respect to the center of the left eye of the
wearer.
[0016] In another embodiment, the mask includes a vertical mounting
plate extending upwardly from the top of the mask and a forehead support
member attached horizontally therewith, the forehead support member having
vertically elongated apertures at either end, and wherein the harness
assembly includes right and left upper straps that engage the corresponding
elongated aperture on the forehead support member, right and left lower
straps that engage elongated apertures at the bottom of the mask, a right
vertical strap behind the right ear of the wearer that connects the right
upper
strap to the right lower strap and a left vertical strap behind the left ear
of the
wearer that connects the left upper strap to the left lower strap, the right
and
left vertical straps located proximally to the right and left mastoids of the
wearer, wherein a first electrode is located at the upper right strap
horizontally
offset with respect to the center of the right eye of the wearer, a second
electrode is located at the upper left strap horizontally offset with respect
to
the center of the left eye of the wearer, and a third electrode is located at
one
of the right and left vertical straps proximally to the corresponding mastoid
of
the wearer.
[0017] In another embodiment, the mask includes a vertical mounting
plate extending upwardly from the top of the mask and a forehead support
member attached horizontally therewith, the forehead support member having
vertically elongated apertures at either end, and wherein the harness
assembly includes right and left upper straps that engage the corresponding
elongated aperture on the forehead support member, right and left lower
straps that engage elongated apertures at either side near the bottom of the
mask, a right vertical strap behind the right ear of the wearer that connects
the
right upper strap to the right lower strap and a left vertical strap behind
the left
ear of the wearer that connects the let upper strap to the left lower strap,
the
right and left vertical straps located proximally to the right and left
mastoids of
the wearer, wherein a first electrode is located at the vertical mounting
plate at
CA 02507690 2005-05-18
-6-
the central forehead region of the wearer and a second electrode is located at
one of the right and left vertical straps proximally to the corresponding
mastoid of the wearer.
(0018] In a further embodiment, the mask includes a forehead support
member extending vertically from the top of the mask, the forehead support
member having right and left horizontal ends that extends above the
eyebrows of the wearer, wherein a first electrode is located at the mask at
the
nasion of the wearer, a second electrode is located at the right horizontal
end
of the forehead support member above the right eyebrow of the wearer and
horizontally offset with respect to the center of the right eye of the wearer,
and
a third electrode is located at the left horizontal end of the forehead
support
member above the left eyebrow of the wearer and horizontally offset with
respect to the center of the left eye of the wearer.
(0019] In another embodiment, the sensors further include a blood
oximeter sensor. The blood oximeter sensor may be located at the forehead
support member in close proximity to the forehead of the wearer. The
sensors may further include a pressure transducer sensor disposed within the
mask.
(0020] In another embodiment, the sensors further include a position
sensor. Preferably, the mask includes a forehead support member extending
vertically therefrom, the position sensor being located at the forehead
support
member. Preferably, the sensors further include at least two of a blood
oximeter sensor, a pressure transducer and a position sensor.
(0021] In another embodiment, the mask assembly further includes a
remote processing unit connected to the sensors for processing the
physiological information. The remote processing unit may include a sleep
efficacy algorithm for processing the physiological information and generating
a sleep information profile for the wearer. Preferably, the remote processing
unit includes a wireless transceiver for wirelessly transmitting signals
related
to the physiological information, and a battery for providing power to the
remote processing unit.
CA 02507690 2005-05-18
_7_
[0022] In another aspect, the invention relates to a mask assembly
adapted to be worn by a wearer for treatment of a medical condition. The
mask assembly comprises a mask shaped to fit over at least the nose of the
person, the mask including a gas inlet for providing pressurized gas to the
wearer; a harness assembly attached to the mask, the harness assembly
including a plurality of straps for securing the mask assembly to the head of
the wearer; and, at least two electrodes located on the inside of the mask
assembly and being spaced with regards to one another for sensing
physiological information including at least one of the EEG, EMG and EOG of
the wearer whereby the physiological information is used to monitor the
efficacy of treatment or to vary operational parameters of the treatment, the
at
least two electrodes being located on at least one of the mask and the
harness assembly.
[0023] In yet another aspect, the invention relates to a mask assembly
adapted to be worn by a wearer for treatment of a medical condition, the mask
assembly including sensors located on the mask assembly for sensing
physiological information from the wearer and a remote processing unit
located on the mask assembly and connected to the sensors for processing
the physiological information.
[0024] In a further aspect, the invention relates to a mask assembly for
wearing by a wearer for treatment of a medical condition. The mask
assembly comprises a nasal interface for providing pressurized gas to the
wearer, the nasal interface comprising a gas inlet for receiving a source of
gas
and a gas outlet for providing gas directly to the naves of the wearer. The
mask assembly further comprises at least one strap or other harness means
connected to the nasal interface for securing the mask assembly to the head
of the wearer. The mask assembly also has sensors located on the mask
assembly for measuring physiological signals of the wearer during treatment
of the medical condition to determine efficacy of the treatment and to vary
operational parameters of the treatment. The sensors are located on at least
one of the strap or forehead member of the mask assembly.
CA 02507690 2005-05-18
_g_
(0025] The forehead member preferably comprises at least two
electrodes disposed so as to contact the skin of the forehead of the wearer of
the mask assembly during treatment so that the electrodes can pick up the
physiological signals. Such physiological signals may be electromyography
(EMG), electroencephalography (EEG) or eletroocularography (EOG) signals.
(0026] Preferably, the nasal interface comprises a gas supply tube in
fluid communication with the gas source and passing between the mouth and
the nose of the wearer. The nasal interface comprises two neris feed portions
for feeding gas into the nostrils of the wearer and substantially occluding
the
nostrils against airflow other than through the nasal interface.
(0027] In a still further aspect, the invention relates to an electrode
placement assembly for locating electrodes on the forehead of a wearer of the
electrode placement assembly. The electrode placement assembly
comprises a forehead placement assembly, at least one strap and at least two
electrodes. The forehead placement assembly is dimensioned to extend
laterally across a forehead of the wearer and has a lower portion for at least
partly overlying a nasion area of the wearer. The at least one strap is
connected to the forehead placement assembly for securing the electrode
placement assembly to the wearer. The at least two electrodes are positioned
on at least one of the forehead placement assembly and the at least one strap
so that the at least two electrodes contact the skin of the wearer.
(0028] The electrode placement assembly preferably comprises
attachment means for attaching a nasal interface to the electrode placement
assembly so that the nasal interface is positioned to provide gas to the neres
of the wearer. The attachment means may comprise flexible attachment
members for attaching the nasal interface to the at least one strap.
Alternatively, the attachment means may comprise a connector member
positioned on the forehead placement assembly for connecting the nasal
interface tot he forehead placement assembly.
(0029] In one embodiment, the forehead placement assembly may be
formed of a unitary flexible plate. Alternatively, the forehead placement
CA 02507690 2005-05-18
_g_
assembly may be comprised of separate interconnected members.
Preferably, one of the at least two electrodes is positioned on the lower
portion of the forehead placement assembly for at least partly overlying the
nasion area. Another of the at least two electrodes may be positioned on the
forehead placement assembly away from the one electrode for overlying an
area of the forehead vertically displaced from the nasion area. This other
electrode may be positioned centrally above the forehead or on either lateral
side above, or extending laterally of, the pupils of the wearer.
[0030] In one particular embodiment, the electrode placement
assembly comprises four electrodes. Three of these electrodes are located
on the forehead placement assembly for positioning laterally across the
forehead of the wearer. A fourth electrode is located on the lower portion of
the forehead placement for at least partly overlying the nasion area.
[0031] Advantageously, the electrode placement assembly can be used
with different nasal interfaces, according to the particular nasal interface
design preferred by the wearer. For example, some wearers may prefer a
mask which covers the entire nose while other wearers may prefer less
obtrusive tubing to lie across the upper lip and beneath the nose and having
gas outlets feeding directly into the neres of the wearer.
Brief description of the drawings
[0032] For a better understanding of the invention and to show more
clearly how it may be carried into effect, reference will now be made, by way
of example only, to the accompanying drawings which show exemplary
embodiments of the invention and in which:
Figure 1 is a general block diagram of a CPAP system including
a mask assembly with integrated sensors in accordance with an embodiment
of the invention;
Figure 2a is a front view of a mask assembly with integrated
sensors in accordance with another embodiment of the invention;
Figure 2b is a side view of the mask assembly of Figure 2a;
CA 02507690 2005-05-18
- 10-
Figure 3 is a front view of mask assembly with integrated
sensors according to an alternate embodiment;
Figure 4 is a front view of a mask assembly with integrated
sensors according to another embodiment;
Figure 5a is a front view of a mask assembly with integrated
sensors and a remote processing unit according to another embodiment;
Figure 5b is a block diagram of the remote processing unit of
Figure 5a;
Figure 5c is a block diagram of the remote processing unit
incorporating a wireless communication according to another embodiment;
Figure 6 is a block diagram of the monitoring unit of Figure 1 in
accordance with another embodiment.
Figure 7 is a front view of a mask assembly according to another
embodiment; and
Figure 8 is a front view of another mask assembly according to
yet another embodiment.
Detailed description of embodiments of the invention
[0033 It will be appreciated that for simplicity and clarity of illustration,
elements shown in the figures have not necessarily been drawn to scale. For
example, the dimensions of some of the elements may be exaggerated
relative to other elements for clarity. Further, where considered appropriate,
reference numerals may be repeated among the figures to indicate
corresponding or analogous elements. In addition, numerous specific details
are set forth in order to provide a thorough understanding of the invention.
However, it will be understood by those of ordinary skill in the art that the
invention may be practiced without these specific details. In other instances,
well-known methods, procedures and components have not been described in
detail so as not to obscure the invention.
CA 02507690 2005-05-18
-11-
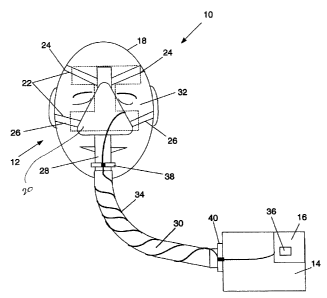
[0034] Referring now to Figure 1, shown therein is a block diagram of a
CPAP system 10 including a mask assembly 12 and a CPAP device 14 with
an associated monitoring unit 16 for use by a CPAP device wearer 18. The
monitoring unit 16 is shown as being an integral part of the CPAP device 14.
Other configurations are possible in which the monitoring unit 16 is separate
from the CPAP device 14. The mask assembly 12 includes a nasal interface
or mask 20 and a harness 22. Harness 22 includes upper straps 24 and
lower straps 26. The mask assembly 12 also includes a gas inlet 28 for
receiving air, or another suitable gas such as pure oxygen, from the CPAP
device 14 via a hose 30. The nasal mask 20 can be made from polystyrene
or some other suitable material. The nasal mask 20 may also incorporate a
cushion for providing a comfortable and tight fit with the face of the wearer
18.
[0035] Nasal mask 20 is one form of nasal interface that can be used
with embodiments of the invention. Other forms of nasal interface are shown
and described in relation to Figures 7 and 8.
(0036] In other embodiments, the harness may include one strap or
more than two straps. Further, although the mask assembly 12 is shown
having the nasal mask 20, it should be understood that the invention is also
applicable to mask assemblies having a nasal/oral mask which covers both
the nose and mouth of the CPAP wearer ("user"). Accordingly, the use of the
word mask herein refers to both nasal masks and nasal/oral masks. The
mask assembly 12 further includes sensors 32 positioned on the nasal mask
12, the straps 24 and 26 of the harness 22 or on both the nasal mask 12 and
the straps 24 and 26. In this exemplary embodiment, the sensors 32 are
connected to the monitoring unit 16 via a cable 34. However, the sensors 32
may also be wirelessly coupled to the monitoring unit 16. The sensors 32
include electrodes for detecting one or more of the EEG, EMG or EOG of the
CPAP wearer 18. The sensors 32 may further include at least one of a blood
oximetry sensor, a body position sensor and a pressure sensor as is
described in further detail below.
CA 02507690 2005-05-18
-12-
[0037] The physiological information provided by the sensors 32 is pre-
processed by the monitoring unit 16 to improve signal quality and then
processed according to a sleep efficacy algorithm 36. The sleep efficacy
algorithm 36 monitors the quality of sleep for the wearer 18, This can include
determining how long the wearer 18 is in a given sleep state, how many
different sleep states the wearer 18 has experienced during sleep, the
fragmentation of their sleep states and how many arousals the wearer 18 has
experienced. Accordingly, the sleep efficacy control algorithm 36 can
generate sleep profile information for the wearer 18 (the sleep profile
information may include data, such as a test score, related to efficacy and
compliance). The sleep efficacy algorithm 36 further generates a control
signal to control the operational parameters of the CPAP device 14 such as
activating or deactivating the CPAP device 14 or altering the amount of
pressure that is provided to the gas inlet 28 to improve the quality of sleep
of
the wearer 18. The sleep efficacy algorithm 36 may use standard techniques,
as is commonly known to those skilled in the art, to process the physiological
signals, determine the quality of sleep and generate the control signal. The
sleep efficacy algorithm 36 may identify sleep stages and generate the sleep
profile information based on the physiological information sensed from the
wearer 18.
[0038] The sensors 32 are integrated directly on the inner surface of
the mask assembly 12 rather than being separately attached as is done with
conventional CPAP devices. Since the sensors 32 are integrated into the
mask assembly 12, the sensors 32 do not have to be separately attached by
the wearer 18. This ensures that the sensors 32 are repeatedly applied to the
same location on the wearer's face and head every time the wearer 18 wears
the mask assembly 12. In addition, the mask assembly 12 can be positioned
by the wearer 18, along with the sensors 32, without the aid of a medical
professional. Furthermore, since the sensors 32 are already in place, the
preparation time prior to going to sleep is reduced for the wearer 18.
CA 02507690 2005-05-18
-13-
[0039] The wiring associated with the sensors 32 is integrated into a
cable 34 that runs along the length of the hose 30. The cable 34 may run
along the inside or outside of the hose 30. In one embodiment, the cable 34
runs along the inside of the hose 30. In another embodiment, the cable 34 is
wound around the outside of the hose 30, as illustrated in Figure 1. In both
instances, the wiring is constricted to the mask assembly 12 instead of
hanging loosely on the body of the wearer 18 as is done with conventional
CPAP devices. Further, the cable is shielded to reduce the possibility of
receiving electromagnetic interference. Connectors 38 and 40 are also
provided at either end of the hose 30 so that the hose 30 can be disconnected
from the mask assembly 12 and the CPAP device 14 when the mask
assembly 12 or the hose 30 requires replacement. This wiring arrangement
of the invention provides the wearer 18 with increased mobility and less
discomfort. Accordingly, the wearer 18 will enjoy a better quality of sleep.
The mask assembly 12 with the integrated sensors 32 is also easy to use and
the sensors 32 are automatically engaged when the wearer 18 puts on the
mask assembly 12.
[0040] In the present embodiment, the electrodes that are used as
sensors 32 are preferably removably attachable to the mask assembly 12 and
configured for placement against the skin of the wearer 18 for sensing the
physiological signals. Accordingly, the mask assembly 12 includes
attachment means (not shown) for holding the electrodes in place and
providing an electrical connection with the cable 34. The implementation of
the attachment means depends on the particular type of electrode that is
used. For one type of electrode, the attachment means may be circular
apertures, or a cutout portion, with an inner metallic contact, which may be a
metallic ring. The apertures are sized to receive cylindrical electrodes which
have a plastic portion, a solid conductive gel portion and a metallic
conductor
disposed there between. The plastic portion is placed in the aperture so that
the conductive gel portion is placed against the skin of the wearer 18 when
the mask assembly 12 is worn. One example of such an electrode is the
HydrodotT"" biosensor, available from Physiometrix Inc. of N. Billerica, MA,
CA 02507690 2005-05-18
-14-
USA. These electrodes are preferable in that the electrodes require minimal
preparation of the skin of the CPAP wearer 18 and the electrodes can be
used for several nights before having to be replaced.
[0041] Many other types of electrodes may also be used. For instance,
metal electrodes can be used which are directly integrated into the mask
assembly 12 and do not have to be replaced. In this instance, the wearer 18
may be required to apply a conductive gel to each metallic electrode prior to
use. The metallic electrodes may be permanently attached to the mask
assembly 12 and would require cleaning each night to remove the old
conductive paste before new paste is applied. Saline electrodes may also be
used. Saline electrodes have a reservoir that contains saline. Over the
course of the night, the reservoir empties. Accordingly, the CPAP wearer 18
must refill the reservoir prior to use of the mask assembly 12. Disk
electrodes
that are made from gold, silver or carbon may also be used. In addition, peel
and stick electrodes that have a layer of silver-silver chloride may also be
used. The peel and stick electrodes are likely to need replacement each
night. One side of a peel and stick electrode has silver-silver chloride for
attachment to the skin, and the other side has a conductive metallic surface.
The peel and stick electrode may be held in place by a fastener that ensures
that the metallic backing makes electrical contact with a corresponding wire
in
the mask assembly 12.
[0042] It should be noted that the type of electrodes used as the
sensors 32 does not limit the invention. Further, it should be understood that
regardless of the electrodes used for the sensors 32, it may still be
beneficial
for the wearer 18 to prepare the skin locations which will receive the
electrodes when the wearer 18 wears the mask assembly 12. Accordingly,
the wearer 18 may cleanse and slightly abrade their skin with an appropriate
cleanser such as NuPrepT"" cleanser, available from Weaver & Co. of Aurora
CO, USA. In some instances, the wearer 18 may also apply a conductive
paste, such as EC2T"" cream for example, to lower the impedance of their skin
in order to obtain better physiological signals. EC2T"" cream is available
from
CA 02507690 2005-05-18
-15-
Astro-Med Inc. of West Warwick, RI, USA. The harness 22 of the mask
assembly 12 may be adjusted to apply sufficient pressure to ensure that the
electrodes make a good physical contact with the wearer 18.
[0043] The electrodes are preferably located at predetermined
locations on the face and head of the wearer 18 in order to obtain good signal
quality and different types of physiological data with a minimal number of
electrodes. Due to the fewer number of electrodes, the mask assembly 12 is
easier and more comfortable to wear. The inventors have been able to obtain
good physiological data from as little as two electrodes which can provide
EEG, EOG or EMG data. This is in contrast to standard sleep staging systems
which make use of up to eleven surface electrodes located on the ears,
central and occipital lobes, and besides the eyes and on the chin of the
wearer. In one embodiment, the inventors have found that one set of
preferred locations for the electrodes are on the nasion and approximately 4
cm higher on the forehead just above FpZ. The physiological signals
obtained from the forehead at these locations provide data related to the
CPAP wearer's brainwaves, facial muscle tone and eye movements. Another
preferred combination includes three electrodes in which one electrode is
located at the nasion, another electrode is located just above and to the left
of
Fp1 and another electrode is located just above and to the right of Fp2.
However, other locations, and other combinations of electrodes, may also be
suitable as described below.
[0044] Referring now to Figures 2a and 2b, shown therein are front and
side views, respectively, of an embodiment of the mask assembly 112 with
integrated sensors in accordance with this embodiment of the invention. The
nasal mask 20 of the mask assembly 112 includes a vertical mounting plate
114 that is connected to a forehead support member 116. The forehead
support member 116 has finro elongated apertures 118 for receiving the straps
24. The mask assembly 112 also includes a flexible seal 120 that rests
against the face of the CPAP wearer 18. The seal 120 can be made from an
elastomer, urethane foam, rubber or other suitable material and is glued or
CA 02507690 2005-05-18
-16-
press fit against the rear of the nasal mask 20. The harness assembly 22
includes vertical straps 122, on either side of the head of the wearer 18,
which
connect the upper and lower straps 24 and 26 just behind the ear of the
wearer 18. The harness assembly 22 also includes a crown strap 124 that
crosses over the crown or vertex of the wearer 18 to connect the upper straps
24 to one another. There are also two elongated apertures 122 disposed at
either side near the bottom of the nasal mask 20. The elongated apertures
122 are engaged by the lower straps 26 of the harness assembly 22.
[0045] In this embodiment, exemplary locations are shown for
electrodes E1, E2, E3, E4 and E5. Electrodes E1 and E2 are located on the
nasal mask 20 and the vertical mounting plate 114 that correspond to the
nasion and central forehead regions, respectively, of the CPAP wearer 18.
Electrodes E3 and E4 are located on the right and left upper straps 24.
Electrode E5 is located on the mastoid of the CPAP wearer 18. The electrode
E5 may be placed anywhere behind the right or left ear of the wearer 18. The
wires from the electrodes E1, E2, E3, E4 and E5 are not shown. However, it
should be understood that separate wires from each electrode E1, E2, E3, E4
and E5 are bundled together into the cable 34 and run along the hose 30 of
the CPAP system 10 as described above.
[0046] The electrode E1 is located at, or approximately 1 cm above, the
nasion, which is the depression at the root of the nose of the wearer 18, and
is roughly between the eyebrows of the wearer 18. The electrodes E3 and E4
are located just below the hairline and spaced apart lining up between the
centerline and the outside of the eyes of the wearer 18. The horizontal and
vertical displacements of electrodes E3 and E4 are important for detecting
certain EEG information as described below. For instance, if the electrodes
E3 and E4 are too close together, then they will not be able to distinguish
signals that originate from the deeper structures of the brain. The electrode
E5 on the mastoid can help to detect alpha waves in the EEG of the wearer
18 since the electrode E5 is close to the occipital region of the wearer 18.
Physiological information from the electrode E5 may be necessary if sufficient
CA 02507690 2005-05-18
-17-
information cannot be detected from the frontal electrodes (this depends on
the quality of sleep staging performed by the efficacy monitoring algorithm
36).
[0047] Electrode locations other than those shown for electrodes E1,
E2, E3, E4 and E5 are also possible. For instance it is possible to place one
electrode below and beside one eye of the CPAP wearer 18 and the other
electrode above and beside the other eye of the CPAP wearer 18. This is the
traditional location of EOG electrodes which maximally detect horizontal and
vertical eye movements. In addition, it may be possible to vertically flip the
location of the electrode E1 with respect to the electrodes E3 and E4.
Therefore, rather than forming an upside triangle pattern, as shown in Figure
2a, the electrodes E1, E3 and E4 can be oriented in a right side up triangle
pattern. This may involve elongating the vertical mounting plate 114.
[0048] It is to be noted that each of these electrode locations are on
exposed skin surfaces (i.e. not on top of hair) in order to provide a good
skin-
electrode contact as well as to provide minimal discomfort to the wearer 18.
Further, the electrodes are preferably not placed on any large muscles to
prevent having the physiological data contaminated with undesirable muscular
artifacts. Further, the degree to which the locations of the electrodes E1,
E2,
E3, E4 and E5 can vary depends on the nature of the efficacy monitoring
algorithm 36. Small changes on the order of +/- 1 cm have little effect.
However, it is important to maintain a certain amount of vertical displacement
between electrode E1 and the other frontal electrodes E2, E3 and E4. A
vertical displacement of as much as 6 cm may be used.
[0049] Various subsets of the electrodes may be used in particular
embodiments of the invention. One combination may be electrodes E1 and
E2. Another combination may be electrodes E1, E3 and E4. Another
combination may be electrodes E3, E4 and E5. Another combination may be
electrodes E2 and E5. In each of these combinations, there is no reference
electrode since one of the electrodes is used to provide both ground and
reference signals. This results in a sight reduction in signal quality but the
CA 02507690 2005-05-18
-18-
benefit is a reduced number of electrodes. Alternatively, it may be possible
to
use one of fihe electrodes as a ground electrode and another of the electrodes
as a reference electrode, if necessary. For example, in one combination,
electrode E2 may be used to provide a ground signal and electrode E1 may
be used to provide the reference signal.
[0050] A single channel of physiological information can be derived
from two frontal electrodes. However, there is a reduction in the amount of
physiological information that is available to determine the sleep stages when
only a single channel is used. For instance, with a single channel, detection
of eye movements is limited, and EMG information is weak. Also standard
EEG features such as sawtooth waveforms, spindles, K-complex, alpha and
delta waveforms may be changed. Furthermore, it is difficult to resolve K-
complex signals and spindles from one another using only the electrodes E4
and E3. These signals are more difficult to detect because they do not
originate in the frontal lobes of the brain. However, they are useful since
they
can be used to differentiate between some of the sleep stages. Accordingly, it
is preferable, and more robust, although not necessary, to use a subset of
electrodes that contains at least three electrodes. However, in some cases it
may be possible to use only two electrodes.
[0051] The combination of electrodes E1, E3 and E4 provides three
channels of physiological data which have a sufficient content of EEG, EMG
and EOG information to perform frontal sleep staging (the term "front" is used
since the physiological data is obtained from the front/face of the wearer
18).
One of the three channels is obtained from electrode pair E3 and E1, another
of the other channels is obtained from electrode pair E4 and E1 and another
of the channels is obtained from electrode pair E3 and E4. The data provided
by electrode pairs E3 and E1, and E4 and E1 may be used to detect EEG and
EOG signals while the data provided by electrode pair E3 and E4 may be
used to detect EMG signals. Accordingly, the electrode configuration of
electrodes E1, E3 and E4 may be used to detect both horizontal and vertically
oriented potentials which is desirable for detecting horizontal and vertical
eye
CA 02507690 2005-05-18
-19-
movements. Also, dipoles in the brain generate EEG spindles that have
different orientations. These EEG spindles, which are helpful for sleep
staging, can be detected with electrodes that detect horizontal and vertically
oriented potentials. Two channels are also better than a single channel in
distinguishing eye blinks from other EEG waveforms such as K-complex delta
activity that is usually less symmetric. With this electrode configuration,
eye
blinks and rapid eye movements can be used to assist in the detection of
wake and REM states since alpha frequencies, which also indicate sleep
arousal, originate in the occipital lobe at the rear of the head of the wearer
18
and this is difficult to detect with frontal electrodes. Arousals are also
determined by an abrupt increase in alpha and beta band activity of the EEG
signals which is evident on the frontal channels. Arousals are important for
determining the quality of sleep and the efficacy of therapy.
[0052] Referring now to Figure 3, shown therein is a front view of an
alternate embodiment of a mask assembly 212 with integrated sensors in
accordance with the invention. In this embodiment, the nasal mask 20
includes a contoured forehead support member 214 with horizontal sides that
extend over the eyebrows of the wearer 18. The electrodes E1, E4 and E3
are all integrated onto the forehead support member 214 of the nasal mask 20
rather than the left and right straps 24. In particular, the electrode E1 is
preferably located at the nasion of the wearer 18, the electrode E3 is located
near the right horizontal end of the forehead support member 214 horizontally
offset with respect to the center of the right eye of the wearer 18, and the
electrode E4 is located near the left horizontal end of the forehead support
member 214 horizontally offset with respect to the center of the left eye of
the
wearer 18. Electrodes E3 and E4 preferably rest just below the hairline of the
wearer 18.
[0053] Referring now to Figure 4, shown therein is a front view of
another alternate embodiment of a mask assembly 312 with additional
integrated sensors in accordance with the invention. The mask assembly 312
includes an oximeter sensor 314, a pressure transducer 316 and a position
CA 02507690 2005-05-18
-zo-
sensor 318. It should be noted that not all three additional sensors 314, 316
and 318 are needed and that additional embodiments are possible in which
various subsets of these additional sensors are integrated into the mask
assembly 312. The oximeter sensor 314 is preferably located at the forehead
support member 214 in close proximity with the forehead of the wearer 18.
Alternatively, the oximeter sensor 314 may be located on an ear clip or
inserted into the ear canal and a wire run from the oximeter sensor 314 along
one of the straps 24 or 26 and along the nasal mask 20 at which point the
wire is integrated within the cable 34. The pressure transducer 316 is
disposed within the nasal mask 20 preferably in close proximity to the gas
inlet 28. The position sensor 318 is also preferably located on the forehead
support member 214. However, the position sensor 318 may be located
within the nasal mask 20; no contact with the skin is required and so the
location of the position sensor 318 may be whatever is best suits the
ergonomics and manufacturability of the mask assembly 1Z.
(0054] The oximeter sensor 314 may be used to help detect sleep
apnea since it provides physiological information from which desaturation and
resaturation events in oxygen saturation of the arterial blood of the wearer
18
can be identified. During sleep apnea, there is no air movement into the chest
of the wearer 18 and the wearer 18 becomes progressively more hypoxic and
hypercarbic. Consequently, OSA may be detected by looking at the rate of
change of oxygen desaturations measured during sleep. The oximeter sensor
314 includes light emitting diodes that emit near infrared light at the
forehead
skin of the wearer 18. The light gets scattered and a portion of the light is
reflected to the oximeter sensor 314. The amount of light that gets reflected
is
related to the spectral absorption of the underlying tissue from which the
average oxygenation of the tissue can be derived. Conventional forehead
reflectance oximeters may be used, such as the one by Masimo of Irving, CA,
USA to measure peripheral blood oxygenation. Also, the INVOST"" cerebral
oximeter made by Somanetics of Troy, MI, USA may be used as the oximeter
sensor 314 to measure oxygenation of the brain.
CA 02507690 2005-05-18
-21 -
[0055] The pressure transducer 316 is used to detect the pressure
within the cavity of the nasal mask 20 from which the breathing rate of the
wearer 18 can be derived. The breathing rate of the wearer 18 can provide
an indication of apnea and hypopnea events. Any suitable pressure
transducer with an appropriate size may be used.
[0056] The position sensor 318 is used to detect the position of the
head of the CPAP wearer 18. This is important since the occlusion that
occurs during sleep apnea happens mainly when the wearer 18 is lying on
their back since the soft tissue in the back of the throat collapsing due to
gravity. In addition, when the wearer 18 is in the supine position, more
effort
is required to breathe and consequently additional pressure from the CPAP
device 14 is needed. The position of the head relates closely to that of the
throat. Accordingly, locating the position sensor 318 on the mask assembly
12 is advantageous. In an alternative, it may be possible to locate a position
sensor on the chest of the wearer 18 and run the corresponding wire up to the
mask assembly 12 where it is integrated into the cable 34.
[0057] Referring now to Figure 5a, shown therein is a front view of
another embodiment of a mask assembly 412 with integrated sensors and a
remote processing unit 414 in accordance with the invention. The electrodes
E1, E3 and E4, the oximeter sensor 314, the pressure transducer 316 and the
position sensor 318 are connected to the remote processing unit 414 which
processes the signals provided by these sensors prior to transmitting the
signals to the monitoring unit 16 via the cable 34. This results in better
quality
signals with reduced noise and less contamination caused by motion and
electromagnetic interference. The cable 34 may include a power supply
connection to provide power to the remote processing unit 414. Alternatively,
the remote processing unit 414 may be battery powered. It should be
understood that for this embodiment there can be various combinations of the
sensors since the oximeter sensor 314, the pressure transducer 316 and the
position sensor 318 are optional.
CA 02507690 2005-05-18
-22-
[0058] Referring now to Figure 5b, shown therein is a block diagram of
the remote processing unit 414. The remote processing unit 414 includes
several interfaces for providing an electrical connection with the integrated
sensors on the mask assembly 412. The remote processing unit 414 includes
a head position sensor interface 416, an oximeter interface 418, an electrode
interface 420 and a pressure transducer interface 422 for electrical interface
with the appropriate sensor. As mentioned previously, some of the sensors
are optional. Accordingly, the remote processing unit 414 may not require
each of the interfaces shown in Figure 5b.
[0059] The remote processing unit 414 further includes an oximeter
signal processor 424 that is connected to the oximeter interface 418 and a
control unit 426. The control unit 426 directs the activity of the remote
processing unit 414 and processes each of the signals provided by the
sensors. The control unit 426 may be a digital signal processor. It should be
noted that the oximeter signal processor 424 is optional and the processing
performed by the oximeter signal processor 424 may be done by the control
unit 426.
[0060] The remote processing unit 414 further includes a pre-
processing unit 428 that is connected to the electrode interface 420 and an
analog-to-digital converter (ADC) 430 that is connected between the pre-
processing unit 428 and the control unit 426. It is well known to those
skilled
in the art that the EEG, EMG and EOG signals are very small amplitude
signals (on the order of micro-volts) and that pre-processing is required to
remove noise from these signals and amplify these signals. Accordingly, the
pre-processing block 428 includes a high-pass filter stage with a cutoff
frequency of 0.1 to 1 Hz for removing large DC contact potentials and an
amplification stage with a gain on the order of 1,000 VN for amplifying the
electrode signals.
(OOfi1] The remote processing unit 414 further includes a memory unit
432 connected to the control unit 426 for storing the measured signals. The
memory unit 432 may also be used for storing operational parameters for the
CA 02507690 2005-05-18
-23-
remote processing unit 414 as well as programs that are used to process the
measured signals. The memory unit 432 is non-volatile and can be a flash
memory unit, and the like.
[0062] The remote processing unit 414 also includes a host
communications unit 434 and a power supply unit 436 connected to the
control unit 426. The communications unit 434 directs communication
between the remote processing unit 414 and the monitoring unit 16. The
communications unit 434 may be a high speed, synchronous serial port such
as a UART and the like. The power supply unit 434 is connected to the power
wire provided by the cable 34 and processes the power supply signal for use
by the remote processing unit 414. The processed power supply signal is
provided to the control unit 426 to power the control unit 426 and for
distribution to the remaining components of the remote processing unit 426.
[0063] It should be noted that the remote processing unit 414 is
optional and that all of the signal processing that is done by the remote
processing unit 414 may be done by the monitoring unit 16. In this case, the
monitoring unit 16 has similar components as those shown in Figure 5b.
[0064] In use, the head position intertace 416 receives a position signal
438 that is provided by the position interface sensor 318 (position sensors
based on mercury switches provide digital signals). The oximeter intertace
418 receives a raw oximetry signal 440 from the oximeter sensor 314. The
oximeter signal processor 424 processes the raw oximeter signal 440 and
provides a processed oximetry signal 442. The electrode interface 420
receives raw electrode signals 444 from the electrodes E1, E3 and E4 and the
pressure transducer interface 450 receives a raw pressure signal 446. Both
of these raw signals are sent to the pre-processing unit 428 which generates
pre-processed signals 448. The pre-processed signals 448 are then digitized
by the ADC 430 resulting in digital pre-processed signals 450. The position
signal 438, processed oximetry signal 442 and digital pre-processed signals
450 are then sent to the control unit 426.
CA 02507690 2005-05-18
-24-
[0065] In an alternative, the remote processing unit 414 may also
perform the sleep efficacy algorithm 36 which can be stored on the memory
unit 432. Accordingly, the remote processing unit 414 may determine the
sleep profile information for the wearer 18, generate the control signal to
improve the sleep quality experienced by the wearer 18 and send the control
signal to the CPAP device 14 to augment the pressure that is delivered to the
nasal mask 20. In addition, the sleep profile information may be transmitted
to a caregiver through a wire connection to a computer. Wireless
transmission may also be used as discussed below. The sleep efficacy
algorithm 26 may employ a frontal staging algorithm to calculate the sleep
profile of the CPAP wearer 18.
[0066] Referring now to Figure 5c, shown therein is a block diagram of
an alternate embodiment of a remote processing unit 514 incorporating a
wireless communications unit 516 and an antenna 518 in accordance with the
invention. The wireless communication unit 516 runs a suitable wireless
protocol such as the BLUETOOTHT"'~ protocol which is suitable for short-range
communication. For longer-range communication, the wireless
communication unit 516 may employ another communications protocol such
as CDMA. The remote processing unit 514 also includes a battery 520 that is
connected to the power supply unit 436. Accordingly, in this case, there is no
need for the cable 34.
[0067] For the remote processing units 412 and 512, noise is dealt with
by selecting amplifiers with a high CMRR, by having low capacitance isolation
of the power supply unit 436 and having a low impedance connection from the
electrodes to the skin of the wearer 18. It should be understood that the
embodiments for remote processing unit 412 and 512 are exemplary and that
some of the components may be combined. For instance, the memory unit
432 and the communications unit 434 may be integrated into the control unit
426.
[0068] Referring now to Figure 6, shown therein is a block diagram of
an exemplary embodiment of the monitoring unit 16 of Figure 1. The
CA 02507690 2005-05-18
-25-
monitoring unit 16 may be directly integrated within the CPAP device 14 or it
may be separate and works alongside the CPAP device 14. The monitoring
unit 16 includes a control unit 600, a mask interface 602, a remote
communications unit 604, a removable non-volatile memory 606, a memory
unit 608, a power supply unit 610 and an external communications unit 612
connected as shown in Figure 6.
[0069] The control unit 600 controls the operation of the monitoring unit
16 and may be a digital signal processor, a controller or the like. The mask
intertace 602 is an interface between the monitoring unit 16 and the sensors
on the mask assembly. Accordingly, the mask interface 602 may be an
electrical interface with appropriate terminals for receiving the cable 34.
Alternatively, in the instances in which the mask assembly includes a wireless
remote processing unit, the mask interface 602 may be an antenna. The
remote communications unit 604 directs the transmission of data between the
mask assembly and the monitoring unit 16. In the instance in which the mask
assembly includes a wireless remote processing unit, the remote
communications unit 604 employs an appropriate communications protocol
such as the BLUETOOTHT"" protocol. In the case of a wired connection to the
mask, the remote communications unit 604 may be a high speed,
synchronous serial port such as a UART and the like.
[0070] The control unit 600 receives the data transmitted from the mask
assembly. In one embodiment, the control unit 600 may execute the sleep
efficacy algorithm 36, which is stored in the memory unit 608, and generate a
control signal for the CPAP device 14. In another embodiment, the remote
processing unit may perform the sleep efficacy algorithm 36, generate the
control signal and send the control signal, as well as the sleep profile
information, to the control unit 600. In both cases, the control unit 600
sends
the control signal to the CPAP device 14 via the external communications unit
612. The external communications unit 612 may also be used to connect to
an external computer or network for transfer of the sleep profile information.
Accordingly, besides having a connection to the CPAP device 14, the external
CA 02507690 2005-05-18
-26-
communications unit 612 may include an Ethernet device, a USB device, a
telephone or wireless modem and the like for connection to an external
computing device or network.
(0071] The removable non-volatile memory 606 may store the sleep
profile information that includes data, such as test scores, related to the
compliance and efficacy of CPAP therapy on the wearer 18. The removable
non-volatile memory 606 may also store raw data obtained from the sensors
on the mask assembly for inspection by a qualified health professional. The
removable non-volatile memory 606 is optional and all of this data may be
stored on the memory unit 608.
(0072] As mentioned previously, the sleep efficacy algorithm 36 may
use a frontal staging algorithm, based on at least the electrode signals, to
determine which stage of sleep the wearer 18 is in. The information provided
by the electrodes is important since it is well known that some sleep apnea
events occur more frequently in some of the sleep stages rather than others.
The sleep stages include sleep stages 1, 2, 3 and 4 and REM sleep. In some
individuals, sleep apnea may be more prevalent in the REM stage. In stage 1
sleep, the EEG is characterized by low voltage, mixed frequency activity,
without rapid eye movement and usually with relatively high EMG activity.
Stage 2 sleep is characterized by sleep spindles, which are bursts of
distinctive waves of 12 to 14 Hz predominantly seen in the central vertex
region, as well as K complexes which are delineated, negative, sharp waves
immediately followed by positive components lasting more than 0.5 seconds.
The K complexes predominantly appear in the central vertex region. REM
sleep is characterized by low voltage, mixed frequency EEG activity with the
lowest EMG activity and sawtooth waves that appear in the frontal regions of
the brain usually in conjunction with bursts of rapid eye movements. Muscle
atonia occurs during REM sleep which can affect airway patency and result in
increased sleep apnea. In addition, sleep onset can be determined by the
alpha EEG waveform as well as eye blinks (i.e. the lack thereof). Sleep
stages 3 and 4 are known as deep sleep states. They are characterized by
CA 02507690 2005-05-18
-27-
the dominance of high amplitude (for example, greater than 75 wV) and low
frequency (for example, 0.5 to 2 Hz) slow delta activities. Delta activities
are
predominantly seen in the frontal region. Accordingly, electrode position is
important for detecting each of these types of signals.
[0073] In one instance, the sleep efficacy algorithm 36 activates the
CPAP device 14 only once the CPAP wearer 18 falls asleep, thereby easing
the transition from wake to sleep, making the therapy more comfortable and
improving compliance. The sleep efficacy algorithm 36 may also use the
sleep profile information to vary the CPAP titration pressure depending on the
sleep stage. For example, more pressure may be delivered in the REM sleep
stage in which the incidence of sleep apnea increases due to the relaxation of
the throat muscles.
[0074] When combined with an automated sleep efficacy algorithm that
performs sleep staging and pressure control, the mask assembly of the
invention provides a quick, convenient means for monitoring and improving
the sleep profile of the wearer 18. The sleep profile information can be used
by physicians to improve the quality of care and allow them to objectively
assess the efficacy of treatment and monitor changes to therapy. The
efficacy of therapy can be used by employers or law enforcement personnel
to prevent hazardous equipment such as cars, airplanes and industrial
machines from being operated by individuals who are impaired due to
inadequate sleep. The efficacy of therapy can also be used by insurers to
determine the need for continued treatment in order to save costs. Further,
the sleep profile information can be used to control CPAP therapy.
[0075] Referring now to Figure 7, there is shown an alternative mask
assembly 712. The mask assembly 712 comprises a flexible forehead plate
714 for holding electrodes E1, E2, E3 and E4 in position on the forehead of
the wearer 18 and a strap 724 for securing the forehead plate 714 to the
wearer 18. Mask assembly 712 also comprises a nasal interface 720
connected to forehead plate 714 via connector member 732. Nasal interface
720 receives pressurized gas through a gas supply tube 730 for feeding the
CA 02507690 2005-05-18
-28-
gas directly into the nostrils of the wearer 18 through gas outlet ports 726
of
nasal interface 720.
[0076] Nasal interface 720 is shown in Figure 7 as being shaped as a
loop extending downwardly over the wearer's face from the forehead and
diverging around the nose. The divergent limbs of the loop of nasal interface
720 are joined at the bottom by a lip portion 725 which is designed to
generally overlie the upper lip of the wearer 18 and be held upwardly against
the wearer's nose so that outlet ports 726 feed directly into the nostrils of
the
wearer 18. Outlet ports 726 may be formed as tubular extensions which
extend well into the wearers nostrils or may be formed so as to otherwise
substantially occlude the wearer's nostrils so that relatively little of the
gas
supplied through gas outlets 726 leaks out of the nostrils.
[0077] Forehead plate 714 is formed roughly in a T-shape when viewed
from the front while worn by the wearer 18. A lower portion 736 projects
downwardly from forehead plate 714 and houses electrode E1 so as to
generally, or at least partly, overlie the nasion area of the wearer 18.
Electrodes E2, E3 and E4 are spaced laterally across forehead plate 714 in a
similar manner to the arrangement shown in Figure 2A. Electrode E2 acts as
a ground electrode relative to the measured signals from electrodes E1, E3
and E4. Electrode E3 and E4 are positioned in laterally extending wings 734
of forehead plate 714 so as to overlie a central part of the forehead on each
lateral side in a similar manner to the arrangements shown and described in
relation to Figure 2A.
[0078] Nasal interface 720 is connected to forehead plate 714 by
connector 732 at a tubing portion 731 which extends between the nasal loop
of nasal interface 720 and gas supply tube 730. The connection achieved by
connector 732 may be mechanical or chemical, for example by snap fitting or
adhesion. Other forms of removable or non-removable connection may be
provided by connector 732.
[0079] Strap 724 is connected to forehead plate 714 at each lateral
wing 734 by any suitable attachment mechanism. As shown in Figure 7, strap
CA 02507690 2005-05-18
-29-
724 is attached to forehead plate 714 by looping through a suitably shaped
slot 718 in each lateral wing 734. Strap 724 passes around the head of
wearer 18 and attaches to itself to form a loop snugly fitting around the
wearer's head. The attachment of the parts of strap 724 together may be
achieved by any suitable attachment mechanism.
[0080] Although not specifically shown in Figure 7, forehead plate 714
may comprise additional sensors, such as those shown and described in
relation to other embodiments. Additionally, the features of mask assembly
712 may be combined with, or substituted for, other features of other mask
assembly embodiments shown and described herein, where such
combination or substitution would result in a workable mask assembly.
Features described in relation to other embodiments may be used instead of,
or in addition to, the features of mask assembly 712, where such addition or
substitution of features would result in a workable mask assembly.
[0081] As with other embodiments of the mask assembly, electrodes
E1, E2, E3 and E4, as well as any other sensors located on forehead plate
714, may employ a wireless communication module to communicate with
monitoring unit 16. Such a wireless communication arrangement is shown
and described in co-pending United States Patent Application Serial No.
, filed on May 17, 2005 and entitled "Wireless Physiological
Monitoring System" the entire contents of which is hereby incorporated by
reference. Alternatively, dedicated conductors maybe connected to each
such electrode or sensor and wired back to monitoring unit 16, for example
along gas supply tube 730.
[0082] Referring now to Figure 8, there is shown a mask assembly 812
according to another embodiment. Mask assembly 812 is similar to mask
assembly 712, except that it uses an alternative nasal interface 820. Mask
assembly 812 has a flexible forehead plate 814 and strap 824, which are the
same as forehead plate 714 and 712, respectively. As mask assembly 812 is
substantially similar to mask assembly 712, the same reference numerals are
used to indicate the same features and functions as between the
CA 02507690 2005-05-18
-30-
embodiments, except that the reference numerals in Figure 8 all begin with an
"8" in the hundreds column, as compared to the "7" in the hundreds column
shown in Figure 7. Because of these similarities, and in order to avoid
repetition, we will only describe the features of the embodiment shown in
Figure 8 that are different to the features of the embodiment shown in Figure
7.
[0083] Nasal interface 820 is of a slightly different form then nasal
interface 720, whereby the gas supply tube (not shown) feeds into nasal
interface 820 via a tubing loop that extends across the cheeks of the wearer
and around to the back of the head or neck, rather than looping upwardly
around the nose (as in Figure 7). Nasal interface 820 is connected to
forehead plate 814 via strap 824 using flexible connectors 832. Flexible
connectors 832 serve to maintain lip portion 825 and gas outlets 826 in place
against the wearer's nose by pulling up the tubing so that it passes above the
ears or across the top of the ears of the wearer as it passes around to the
back of the wearers head.
[0084] Forehead plates 714 and 814 are preferably formed using
printed circuit sensors and electrodes, such as those supplied by Vermed, Inc.
of Vermont, USA, under the trade name Pc-Sensor. Other forms of flexible
printed circuit devices which may be used to form forehead plate 714 to 814
are made by Conductive Technologies, Inc. of York, Pennsylvania, USA.
Alternatively, more conventional electrodes may be used within a flexible
forehead plate formed of molded plastic, such as a polyvinyl chloride (PVC)
plastic. Preferably, the plastic is relatively thin and flexible to
accommodate
the contours of the wearer's forehead, while having sufficient structural
integrity and rigidity to maintain the electrodes in their respective
positions
and to enable suitable attachment of the straps 724, 824.
[0085] The nasal interface 720, which loops around the wearers nose,
from across the central forehead, maybe of a form similar to that supplied by
AEIOMed, Inc. of Minnesota, USA, based on its aura interface. A nasal
interface of a kind similar to nasal interface 820 may be obtained from
CA 02507690 2005-05-18
-31 -
InnoMed Technologies, Inc., of Florida, USA based on their Nasal-AireT""
product line. It should be noted that, while Figures 7 and 8 show only one
strap for securing the mask assembly to the wearer's head, additional straps
may be used in a manner similar to the straps shown and described in relation
to Figures 1, 2A, 2B, 3, 4 and 5A. Also, if desired, one or more of the
electrodes of mask assembly 712, 812 may be located on a part of the strap
724, 824 or on additional straps not shown.
[0086] It should be understood that features shown and described in
relation to each of the embodiments may be used in combination or
substitution with any features of the other described embodiments, where
such a combination or substitution would not result in an unworkable
arrangement or configuration. Accordingly, the present invention is
contemplated to encompass all such combinations or substitutions resulting in
operative embodiments.
[0087] It should be understood that various modifications can be made
to the embodiments described and illustrated herein, without departing from
the invention. For instance, the invention is applicable to other types of gas
delivery devices such as variable positive air pressure devices, demand
positive pressure devices and other variations of such devices. The invention
can also be used in other instances where an individual wears a mask as well
as sensors for gathering physiological data such as in critical care units. In
addition, it should be understood that the particular masks shown herein are
shown as examples and that the invention is applicable to other mask
designs.