Note: Descriptions are shown in the official language in which they were submitted.
CA 02508964 2000-03-27
DESCRIPTION
BATTERY AND EQUIPMENT OR DEVICE HAVING BATTERY AS PART OF ITS
STRUCTURE, AND LOCALLY-DISTRIBUTED POWER GENERATION
METHOD AND POWER GENERATION DEVICE THEREFOR
This is a division of co-pending Canadian Patent Application Serial No.
2,363,401
filed on March 27, 2000.
[Technical Field]
The present invention relates to a battery and equipment or device having the
battery
as part of its structure, and a locally-distributed power generation method
and a power
generation device therefor. More particularly, the present invention relates
to a battery of a
three-dimensional structure comprising powdered active materials and capable
of storing a
large power, and equipment or device having the battery as part of its
structure, an alkali
primary battery and an alkali secondary battery of long lives in which
discharge voltages are
less likely to be reduced, and a locally-distributed power generation method
which utilizes a
power of transfer and transport means such as a power-driven two-wheeled
vehicle, a power-
driven three-wheeled vehicle, a power-driven four-wheeled vehicle, ship, or
the like and a
power generation device therefor.
[Background Art]
The present invention relates to a battery. In view of the prior arts,
objectives to be
achieved by the present invention are broadly classified into five objectives
as follows.
The first objective is to provide a battery which obviates drawbacks of the
conventional battery having a structure in which a plate-shaped, solid-
cylindrical, or hollow-
cylindrical active material that has a certain volume
CA 02508964 2000-03-27
2
is immersed in an electrolytic solution. The second objective is to provide a
three-dimensional battery of a large power capacity which has been unfulfilled
in
the conventional battery. The third objective is to provide practical use of
the
battery of the three-dimensional structure as means for achieving the first or
second objective. The fourth objective is to provide an alkali primary battery
or
an alkali secondary battery of long lives in which discharge voltages are less
likely to be reduced. The fifth objective is to provide a locally-distributed
power
generation method utilizing the battery of the three-dimensional structure and
a
power generation device therefor. Hereinbelow, the first to fifth objectives
will
be described according to comparison with the prior arts.
1. Prior Art and First Objective
Conventionally, the battery is structured such that the plate-shaped, the
solid-cylindrical, or the hollow-cylindrical active material is immersed in
the
electrolytic solution. The battery has a layered structure with an
electrolytic
plate sandwiched between a cathode and an anode.
For example, Japanese Laid-Open Patent Publication No. Hei. ? - 169513
discloses a method and device that thermally or chemically recovers a battery
material after discharge to continuously generate a power by utilizing a
combustion heat of a fossil fuel.
However, the conventional battery has the following problems.
(1) Scale up is impossible.
A current flowing in a battery is directly proportional to an area of a
membrane. For example, in case of the battery having a membrane area of 1
m2 and a power of 1 W, an area of one billon m2 is required to obtain one
million
kW. This corresponds to a square of approximately 32 kilometer square, and
CA 02508964 2000-03-27
3
cannot be formed into a flange. Even if the number of membranes is increased
as a solution to this, the scale up is unfulfilled.
(2) Degradation of active materials or a catalyst cannot be dealt with.
In the conventional battery, since the active materials and the catalyst are
used as components of the battery, the entire battery must be replaced when
degraded. In actuality, the replacement is impossible and the degraded battery
is discarded.
(3) A heat transmitter for heat generation and heat absorption in association
with charge and discharge cannot be provided.
In view of a battery characteristic in which exothermic reaction or
endothermic reaction is conducted in association with charge and discharge of
the battery, a power conversion efficiency is reduced with an increase in
temperature and a reaction speed decreases with a decrease in temperature, it
is
necessary to provide a heat transmitter in the battery for adjustment so as to
obtain appropriate temperature. However, since the conventional battery is
complex in structure, the heat transmitter is not provided. Besides, since the
battery is small and a battery surface area with respect to its output is
small, it is
naturally cooled or heat-absorbed. In some cases, the upper limit temperature
is set by using a temperature fuse but any temperature control device is not
provided for the battery.
(4) An energy density is low.
In the conventional battery, the current is directly proportional to the area
of the membrane. For example, in case of the battery having a membrane area
of 1 m~ and a power of 1 W, one million membrane batteries each having a
membrane area of 1 m2 and a width of 0.1 m are required and therefore have a
CA 02508964 2000-03-27
4
volume of 100000 m3 to create a battery of 1000 kW . Consequently, the
energy density cannot be increased.
The first invention has been developed in view of the above-described
problems, and the first objective to be achieved by the first invention is to
provide
a battery comprising powdered active materials in vessels, in which scale up
can
be achieved, degraded active materials and catalyst can be recovered and
replaced, the heat transmitter can be provided in the battery, and the energy
density can be increased.
2. Prior Art and Second Objective
Conventionally, the battery is structured such that the active materials
are formed to have a predetermined shape such as a solid cylinder or a hollow
cylinder and immersed in the electrolytic solution, and the electrolyte plate
is
sandwiched between a cathode and an anode to have a layered structure.
Specifically, as shown in Fig. 49, a nickel hydrogen battery is layered by
adhering a current collector 431, a cathode 432, a separator 433, an anode
434,
and a current collector 435 in this order. This example is disclosed in
Japanese
Laid-Open Patent Publication No. Hei. 9 - 298067. The battery disclosed in
this
publication is structured such that a plurality of element batteries (unit
batteries) each comprising a cathode mainly composed of nickel hydroxide, an
anode mainly composed of hydrogen-occluding alloy, a separator formed of a
polymer non-woven fabric cloth, and an electrolytic solution composed of an
alkali aqueous solution, are connected in series and stored in a metallic
square
vessel and an opening thereof is sealed by a sealing plate having a reversible
vent.
The conventional battery 430 has a membrane structure (two
CA 02508964 2000-03-27
dimensional), including the above-described structure. To obtain the battery
430 of a large capacity, it is extended to make it thinner as shown in.Fig. 50
or
wound, or the unit batteries 430 are connected in parallel as shown in Fig.
51.
Or otherwise, as shown in Fig. 12, a plurality of electrode plates 436 are
interposed in a number of unit batteries 430 and wirings 437 connected to the
respective electrode plates 436 are pulled out of the batteries to allow these
electrodes to be connected to electrode plates 438 of another unit batteries
that
have different polarity, thereby obtaining a layered structure.
However, the conventional batteries of Figs. 49 - 52, the following
problems arise.
(1) Scale up is limited.
The conventional battery has a membrane structure (two-dimensional),
and the current flowing in the battery is directly proportional to the area of
the
membrane. Therefore, for example, if 1W power is generated in lm2 area, then
(100 X 100)m2 area is required to generate 10 kW power. Accordingly, the
number of membranes may be increased or the membrane may be enlarged and
wound. In either case, the battery becomes extremely large and is difficult to
practice. Consequently, the batteries must be connected in parallel, and
thereby, the whole structure becomes complex.
(2) A production cost of a battery is extremely high due to a large capacity.
In case of the battery of the membrane structure, if an attempt is made to
obtain the Iarge capacity, the area of the membrane must be correspondingly
increased, and the production cost becomes higher with an increase in the
battery capacity. For this reason, the scale up results in no advantage in
production cost.
CA 02508964 2000-03-27
6
(3) Degradation of the battery cannot be dealt with.
Since the active materials have a fixed shape such as the plate or
cylinder as components of the battery, the whole battery must be replaced when
these materials are degraded, because it is impossible to replace only the
active
materials.
(4) When the batteries are connected in series, a device cost is high and a
resistance energy loss in a connected portion is large. For example, when a
plurality of batteries of 1.6V - 2.OV per battery are connected to obtain a
voltage
as high as 1 OOV, they must be connected by means of wirings. The working
cost therefore becomes high and the loss of heat generated due to the current
passing through the connected portion causes an energy loss.
The second invention has been developed in view of the above-described
problems. The second objective to be achieved by the second invention is to
provide a layered-type three-dimensional battery that is three-dimensionally
structured to allow a capacity of the battery to be increased by increasing a
volume (cell) of the battery and gives a number of advantages associated with
scale up.
3. Prior Art and Third Objective
In general, in various equipment or devices, spaces therein are not
efficiently utilized, as described in embodiments below.
Accordingly, the third objective to be achieved by the third invention is to
provide practical and effective use of the three-dimensional battery in which
the
battery of the three-dimensional structure according to the first or second
invention constitutes part of the various equipment or devices.
4. Prior Art and Fourth Objective
CA 02508964 2000-03-27
7
The practical battery can be broadly classified into a primary battery
incapable of repeating charge/discharge, a secondary battery capable of
repeating charge/discharge, a special battery comprising a physical battery
(for
example, solar battery) and a biological battery (for example, erizyrne
battery),
and a fuel battery.
The fourth objective is to obviate drawbacks of the alkali primary battery
and the alkali secondary battery among these practical batteries.
The battery is composed of an anode, a cathode, and an electrolyte as
three main components. During discharge, the anode discharges an electron to
an external circuit by an electrochemical reaction and the anode itself is
oxidized,
while the cathode receives the electron from the external circLtit by the
electrochenvcal reaction and the cathode itself is reduced, and the
electrolyte
serves as an ion transmission medium between the anode and the cathode in
the electrochemical reaction because it is ion-transmissible. Thus, the
oxidation occurs in the anode and the reduction occurs in the cathode during
discharge, and reduced materials (non-oxidized materials) such as
hydrogen-occluding alloy, cadmium, iron, zinc, lead, and the like are used as
anode materials and oxidized materials are used as cathode materials.
For example, an alkali manganese battery as a type of the alkali primary
battery generally uses manganese dioxides and carbon as cathode active
materials, zinc as an anode active material, and a potassium hydroxide
solution
or a sodium hydroxide solution as an electrolytic solution. In this alkali
manganese battery, the reaction progresses as follows:
(Anode) Zn + 40H- --> Zn (OH)4 2- + 2e-
(Cathode) Mn02 + H2 O + e- --j MnOOH + OH'
CA 02508964 2000-03-27
A nickel-cadmium accumulator battery as a typical alkali secondary
battery generally uses nickel hydroxide and carbon as the cathode active
material, cadmium as the anode active material, and a potassium hydroxide
solution as the electrolytic solution. In the nickel-cadmium accumulator
battery, the reaction progresses as follows:
(Anode] Cd + 20H- f Cd (OH)2 + 2e-
(Cathode] Ni00H + H2 O + e' ~ Ni (OH)2 + OH-
(Whole Battery] Cd + 2Ni00H + 2H2 O~ 2Ni (OH)2 + Cd (OH) 2
In the above reaction formula, an arrow pointing right indicates a
discharge reaction and an arrow pointing left indicates a charge reaction. As
can be seen from the formula, the discharge reaction in the anode produces
hydroxide such as zinc hydroxide or cadmium hydroxide. It is important that
the electrodes have a certain mechanical strength or are corrosion-resistant
in a
potential region and it is particularly important that the electrodes have
superior
conductivity.
Since metal oxide or metal hydroxide have generally high specific
resistance and low conductivity, a mixture of conductive materials such as
carbon, zinc, and cobalt as conduction promoter is conventionally used as
cathode materials comprising metal oxides. However, since a metal is used to
promote the oxidation as the anode active material, the discharge causes the
metal to be chemically changed into a metal oxide or a metal hydroxide,
thereby
resulting in reduced conductivity. Accordingly, to increase the conductivity,
there has been proposed use of a pellet material in which a conductivity
material
such as powdered carbon, powdered nickel, or powdered cobalt is mixed into the
metal such as zinc as the anode active material, or use of an anode current
CA 02508964 2000-03-27
9
collector comprising a metal such as zinc to which the conductivity material
is
pressed to be strongly stuck.
However, the above-described pressure-application process or granulating
process for obtaining the pellet material is complex and increases the
production
cost.
The fourth invention has been developed in view of the above problems
and the fourth objective to be achieved by the fourth invention is to provide
an
alkali primary battery and an alkali secondary battery that show a preferable
discharging characteristic during discharge (in which a discharge voltage is
less
likely to be reduced), have long lives, and low production cost.
5. Prior Art and Fifth Objective
The conventional locally-distributed power generation equipment is a
fixed-type cogeneration equipment for generating warm air, cool air, warm
water,
and steam by using heat energy generated secondarily by power generation and
supplying a steam energy and a heat energy. Also, in the locally-distributed
cogeneration equipment, solar power generation, wind power generation, or the
like is utilized.
As the prior art, it is known that a solar battery installed in a house is
utilized to charge a battery of an electric automobile.
Japanese Laid-Open Patent Publication No. Hei. 6 -225406 discloses a
technique for charging a battery of an electric automobile by using a
commercial
power supply and a fuel battery power generation equipment systematically
operated with the power supply .
To generalize the locally-distributed cogeneration equipment, it is necessary
CA 02508964 2000-03-27
l
to install power generation equipment in houses or offices. However, the power
generation equipment is expensive and requires a long time period to obtain an
economic effect due to difference between a purchasing price of the power
generation equipment and a price of power when purchased as the home power
generation equipment. Thus, since the power generation equipment for houses
and offices has a high equipment cost and is unpayable unless it is used for a
long time period, it is difficult to generalize the locally-distributed
cogeneration
equipment. To facilitate the generalization of the solar power generation, the
state tried to pay half of the equipment cost, which was economically
unsuccessful, and a great deal of budget was surplus.
The fifth invention has been developed in view of the above-described
problems, and the fifth objective to be achieved by the fifth invention is to
provide
a locally-distributed power generation method capable of utilizing a power
generation system installed in automobile or the like originally used as
transfer
and transport means for houses and offices instead of installing only the
fixed-type power generation equipment for houses or offices, to allow
transport
equipment and private power generation equipment to be utilized as common
equipment, thereby significantly reducing the equipment cost, and capable of
performing the cogeneration without the power generation equipment in houses
or offices.
A technique of utilizing the fixed-type power generation equipment such as
the solar power generation for charging the transfer and transport means such
as automobiles is known but a technique of utivzing a power generated by the
transfer and transport means such as automobile for the fixed-type power
generation equipment for houses or the like is not known.
CA 02508964 2000-03-27
11
[Disclosure of Invention]
In accordance with one aspect of the present invention there is provided an
alkali
secondary battery comprising a cathode current collector, a cathode active
material and an
electrolytic solution, a separator that permits passage of an ion but does not
permit passage of
an electron, an anode active material and an electrolytic solution, and an
anode current
collector which are placed in this order, wherein metal carbide or a mixture
of metal carbide
and the metal is used as the anode active material.
CA 02508964 2000-03-27
IZ
BRIEF DESCRIPTION OF DRAWINGS
Fig. 1 (a) is a schematic cross-sectional view showing a structure of a
battery according to a first embodiment of a first invention and Fig. 1 (b) is
a view
showing an example of a discharge curve of the battery of the first invention;
Fig. 2 is a schematic cross-sectional view showing a structure of a
battery according to a second embodiment of the first invention;
Fig. 3 is a schematic cross-sectional view showing an example of a
structure of a battery according to a third embodiment of the first invention;
Fig. 4 is a schematic cross-sectional view showing another example of
the battery according to the third embodiment of the first invention;
Fig. 5 is a schematic cross-sectional view showing an example of a
structure of a battery according to a fourth embodiment of the first
invention;
Fig. 6 is a schematic cross-sectional view showing another example of
the structure of the battery according to the fourth embodiment of the first
invention;
Fig. 7 is a schematic cross-sectional view showing a structure of a
battery according to a fifth embodiment of the first invention;
Fig. 8 is a schematic cross-sectional view showing an example of a
structure of a battery according to a sixth embodiment of the first invention;
Fig. 9 is a schematic cross-sectional view showing another example of
CA 02508964 2000-03-27
13
the structure of the battery according to the sixth embodiment of the first
invention;
Fig. 10 is a schematic cross-sectional view showing an example of a
structure of a battery according to a seventh embodiment of the first
invention;
Fig. 11 is a schematic cross-sectional view showing another example of
the structure of the battery according to the seventh embodiment of the first
invention;
Fig. 12 is a schematic cross-sectional view showing a structure of a
battery according to an eighth embodiment of the first invention;
Fig. 13(a) is a perspective view showing an example of a verification tester
of a layered-type three-dimensional battery of the second invention and Fig.
13(b) is a central longitudinal sectional view schematically showing the
battery of
Fig. I3(a);
Fig. 14 is a perspective view showing a portion of main components prior
to assembling (in a disassembled state) of the verification tester of the
layered-type three-dimensional battery of Figs. 13(a), 13(b);
Fig. 15 is a central longitudinal sectional view schematically showing a
layered-type three-dimensional battery according to a second embodiment of the
second invention;
Fig. 16 is a central longitudinal sectional view schematically showing a
layered-type three-dimensional battery according to a third embodiment of the
second invention;
Fig. 17 is a central longitudinal sectional view schematically showing a
layered-type three-dimensional battery according to a fourth embodiment of the
second invention;
CA 02508964 2000-03-27
14
Fig. 18 is a central longitudinal sectional view schematically showing a
layered-type three-dimensional battery according to a fifth embodiment of the
second invention;
Fig. 19 is a central longitudinal sectional view schematically showing a
layered-type three-dimensional battery according to a sixth embodiment of the
second invention;
Fig. 20 is a longitudinal sectional view of a door having a
chargeable/dischargeable three-dimensional battery in an inner space thereof;
Fig. 21 is a longitudinal sectional view of a bridge piller having a
chargeable/dischargeable three-dimensional battery in an inner space thereof;
Fig. 22 is a perspective view of a dam having a chargeable/dischargeable
three-dimensional battery in an inner space thereof;
Fig. 23 is a schematic view showing a structure of a radiator as a power
storage;
Fig. 24 is a longitudinal sectional view showing a house having a
chargeable/dischargeable three-dimensional battery in a ceiling portion;
Fig. 25 is a cross-sectional view showing part of a bonnet having a
chargeable/dischargeable three-dimensional battery on an inner surface side;
Fig. 26 is a cross-sectional view showing a vicinity of a ground surface in
which a chargeable/dischargeable three-dimensional battery is formed;
Fig. 27 is a longitudinal sectional view of a tableware having a
chargeable/dischargeable three-dimensional battery in a side portion thereof;
Fig. 28 is a cross-sectional view showing a floor of a house having a
chargeable/dischargeable three-dimensional battery ;
Fig. 29 is a side view showing a trailer in which a
CA 02508964 2000-03-27
chargeable/dischargeable three-dimensional battery is mounted;
Fig. 30(a) is a longitudinal sectional view showing an electric motor in
which a chargeable/dischargeable three-dimensional battery is built in a
casing
and Fig. 30(b) is a longitudinal sectional view showing an electric motor in
which
a chargeable/dischargeable three-dimensional battery is built in a base
portion
thereof;
Fig. 31 is a longitudinal sectional view showing a turbo engine in which a
chargeable/dischargeable three-dimensional battery is built in a casing;
Fig. 32 is a perspective view showing part of dual-structured ship in
which a chargeable/dischargeable three-dimensional battery is built;
Fig. 33 is a longitudinal sectional view showing part of ship in the
longitudinal direction in which a chargeable/dischargeable three-dimensional
battery is built;
Fig. 34 is a cross-sectional view showing a wing of an airplane in which a
chargeable/dischargeable three-dimensional battery is built;
Fig. 35 is a cross-sectional view showing a tire of a road roller in which a
chargeable/dischargeable three-dimensional battery is built;
Fig. 36 is a schematic view showing a structure of a
chargeable/dischargeable three-dimensional battery installed in a bottom
pox-tion of a vehicle body of an electric train;
Fig. 37(a) is a cross-sectional view showing an electric locomotive having
a chargeable/dischargeable three-dimensional battery and Fig. 3?(b) is a
schematic view showing an example of a mechanism for driving an electric motor
by means of a chargeable/dischargeable three-dimensional battery from an
electric generator when applied to the turbo engine;
CA 02508964 2000-03-27
16
Fig. 38(a) is a cross-sectional view showing an electric locomotive to
which a power vehicle is connected and Fig. 38(b) is a schematic view showing
an example of power storage equipment from an electric generator to the
chargeable/dischargeable three-dimensional battery when applied to the turbo
engine;
Fig. 39 is a cross-sectional view showing a low-noise electric train having
a chargeable/dischargeable three-dimensional battery;
Fig. 40(a) is a cross-sectional view showing a normal power line, Fig.
40(b) is a cross-sectional view showing a power line in which the
chargeable/dischargeable three-dimensional battery is built, and Fig. 40(c) is
a
schematic flow diagram showing an example in which power is supplied from
the power line in which the chargeable/dischargeable three-dimensional battery
is built, to a terminal device;
Fig. 41 is a cross-sectional view showing an electric pole in which the
chargeable/dischargeable three-dimensional battery is built;
Fig. 42 is a cross-sectional view showing a battery in which the
chargeable/dischargeable three-dimensional battery is built;
Fig. 43 is a cross-sectional view showing a flashlight in which the
chargeable/dischargeable three-dimensional battery is built;
Fig. 44(a) is a longitudinal cross-sectional view showing the
chargeable/dischargeable three-dimensional battery formed in the vicinity of
the
surface ground and Fig. 44(b) is a schematic view showing an example of a
structure of a metal bullet shooting device using a rail gun;
Fig. 45 is a schematic view showing a structure of an alkali primary
battery according to a first embodiment of a fourth invention;
CA 02508964 2000-03-27
17
Fig. 46 is a schematic view showing a structure of an alkali secondary
battery according to a second embodiment of the fourth invention;
Fig. 47 is a view showing an example of a discharge curve of the alkali
secondary battery of the fourth invention;
Fig. 48 is a schematic explanatory view systematically showing a device
that carries out a locally-distributed power generation method according to a
first embodiment of the fifth invention;
Fig. 49 is a central longitudinal sectional view schematically showing the
conventional battery having a general membrane structure;
Fig. 50 is a central longitudinal sectional view schematically showing the
conventional long-type battery having a general membrane structure;
Fig. 51 is a central longitudinal sectional view schematically showing the
state in which the conventional batteries having a general membrane structure
are connected in parallel; and
Fig. 52 is a central longitudinal sectional view schematically showing the
state in which the conventional batteries having a general membrane structure
are connected in series.
[Best Mode for Carrying Out the Invention]
Hereinafter, embodiments of the present invention will be described.
The present invention is not limited to the embodiments described below but
may be suitably altered and carried out.
1. Embodiments of the First Invention
(First Embodiment)
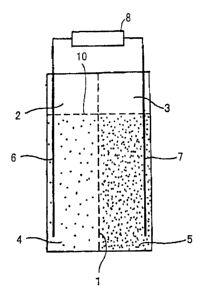
Fig. 1 (a) shows a battery according to a first embodiment of a first
CA 02508964 2000-03-27
1g
invention. As shown in Fig. 1 (a), an anode cell 2 and a cathode cell 3 are
provided with a separator 1 interposed therebetween. The anode cell 2 is
filled
with an anode powdered active material and an electrolytic solution 4 and the
cathode cell 3 is filled with a cathode powdered active material and an
electrolytic solution 5. Examples of a combination of the powdered active
materials for the anode and the cathode are hydrogen-occluding alloy and
nickel
hydroxide, cadmium and nickel hydroxide, or the like. An example of the
hydrogen-occluding alloy is La o.s (Ce, Nd) o.is Zr. o.os Ni s.s Co 0.8 A1
o.s. ~ As the
electrolytic solution, for example, a KOH aqueous solution may be used. The
separator 1 serves as an ion-passing membrane and does not serve as a
powder-passing membrane. As the separator 1, for example, an unglazed
pottery, an ion exchange resin membrane, metal fibers, or the like may be
used.
An anode current collector 6 comprising a conductor and a cathode
.current collector 7 comprising a conductor are respectively provided in the
anode cell 2 and the cathode cell 3. The current collectors 6, 7 are connected
to
a load means (for discharge) or a power generation means 8 (for charge).
Reference numeral 10 denotes an electrolytic solution interface.
Subsequently, charge and discharge of the battery of this embodiment
will be described in detail.
(Charge)
When the battery is connected to the power generation means 8, an
electron is discharged from the power generation means 8 and reaches the
anode current collector 6. The electron reacts with the anode powdered
material immediately on the anode current collector 6 or while traveling
through
the anode powdered material. An anion produced by the reception of the
CA 02508964 2000-03-27
19
electron by the anode powdered active material passes through the separator 1
and enters the cathode cell 3, where it reacts with the cathode powdered
active
material and discharges the electron. The electron moves to the cathode
current collector 7 immediately or through the powdered active material and is
supplied to the power generation means 8.
(Discharge)
When the battery is connected to the load means 8, the anode current
collector 6 discharges the electron to an external circuit. The discharged
electron travels through the load means 8 and reaches the cathode current
collector 7. The electron reacts with the cathode active powdered material
immediately on the cathode current collector 7 or while traveling through the
powdered active material. An anion produced by the reception of the electron
by the cathode powdered material passes through the separator 1 and enters the
anode cell 2, where it reacts with the anode powdered active material and
discharges the electron. The electron moves to the anode current collector 6
immediately or through the powdered active material and is supplied to the
load
means 8.
Fig 1 (b) is a view showing comparison in discharge curves between the
battery according to the present invention and the conventional battery each
having a nominal capacity of 5 Ah. In Fig. 1 (b), a black circle (~) indicates
the
discharge curve of the battery of the present invention and a white circle (~)
indicates the discharge curve of the conventional battery. The battery of the
present invention is a battery of a three-dimensional structure in which the
cathode cell is filled with the powdered nickel hydroxide and the electrolytic
solution and the anode cell is filled with the powdered hydrogen-occluding
alloy
CA 02508964 2000-03-27
and the electrolytic solution (see Fig. 1 (a)). The conventional battery is a
battery
of a two-dimensional structure in which a plate electrode comprising nickel
hydrogen is used as the cathode, a plate electrode comprising
hydrogen-occluding alloy is used as the anode, and these electrodes are
immersed in the electrolytic solution. In Fig. 1 (b), a longitudinal axis
indicates a
terminal voltage (~ and a lateral axis indicates a discharge capacity (Ah).
Since
the change in the voltage during discharge is subjected to the influence of
concentration polarization due to the change in concentration of the
electrolytic
solution (in this comparative experiment, potassium hydroxide solution), the
concentration of the electrolytic solution of the battery of the present
invention
and the concentration of the electrolytic solution of the conventional battery
are
adjusted so as to be equal during discharge. In discharge of the battery, the
continuation of discharge to an extent that the voltage is below a fixed
voltage is
undesirable in view of degradation of the electrode, or the like, and
therefore,
there exists a discharge ternlination voltage at which the discharge should be
terminated. The lower the discharge termination voltage is, the longer the
discharge time is. With this regard, since the battery of the present
invention
has the three-dimensional structure with the electrode active material
powdered,
an energy density is greatly improved without fluidizing the powdered
material;
and the discharge voltage does not rapidly decrease as can be seen from "1" of
Fig. 1 (b), as compared to the conventional battery of the two-dimensional
structure using the plate electrodes.
On the other hand, as can be seen from "O" of Fig. ~ (b), in the
conventional battery, the discharge voltage rapidly decreases in approximately
4.5 h. Therefore, assuming that the discharge termination voltage is 1.OV, the
CA 02508964 2000-03-27
21
discharge must be terminated in approximately 4 h in the conventional battery
for protection of battery equipment, whereas the discharge can continue for
approximately 5 h in the battery of the present invention.
(Second Embodiment)
Fig. 2 shows a battery according to a second embodiment of the first
invention. Herein, to increase efficiency of contact between powdered
materials
or between the powdered materials and the current collectors 6, 7, a fluid
fluidizing and dispersing means 9 using a gas or liquid is adapted to fluidize
(agitate) the powdered materials in the respective cells 2, 3. Such
fluidization
increases the efficiency of contact between the powdered materials, reduces
contact resistance because of preferable contact between the powdered active
materials and the current collectors, increases conductivity between the
powdered active materials and the current collectors or between the powdered
active materials, and increases a diffLision speed of ions in the electrolytic
solution. As a result, a large current flows and a large power can be obtained
as
compared to a case where the powdered materials are not fluidized.
Instead of or along with the fluid fluidi2ang and dispersing means 9,
agitating means such as vane-like agitators may be provided in the respective
cells 2, 3 to fluidize (agitate) the powdered materials. As the fluid
fluidizing and
dispersing means 9, a device such as a dispersion plate and a spray nozzle for
uniformly dispersing the gas or the liquid in a horizontal cross section in
the cell
can be cased, although this is not shown in the Fig. 2 for the sake of
simplicity.
As the gas (or liquid) introduced into the fluid fluidizing and dispersing
means
9, for example, nitrogen, argon (or an electrolytic solution such as potassium
hydroxide solution) or the like may be used. When the powdered materials are
CA 02508964 2000-03-27
22
fluidized by the gas, the gas introduced into the fluid fluidizing and
dispersing
means 9 is discharged out of upper portions of the respective cells 2, 3. When
the powdered materials are fluidized by the liquid, the liquid introduced into
the
fluid fluidizing and dispersing means 9 is discharged out of bottom portions
of
the cells 2, 3.
Except the addition of the fluidizing means, the other constitution and
function are similar to those of the first embodiment.
(Third Embodiment)
Figs. 3, 4 show batteries according to a third embodiment of the first
invention. Referring to Fig. 3, to increase the efficiency of contact between
the
current collectors and the powdered active materials, a plate-shaped anode
current collector 11 and a plate-shaped cathode current collector 12 are
respectively used as the anode current collector and the cathode current
collector for larger contact areas. Referring to Fig. 4, to increase the
efficiency of
contact between the current collectors and the powdered active materials, a
tubular anode current collector 13 and a tubular cathode current collector 14
are respectively used as the anode current collector and the cathode current
collector for larger contact areas. The shapes other than plate and tube can
be
adopted so long as they can increase surface areas of the current collectors.
The other constitution and function are identical to those of the second
embodiment.
[Fourth Embodiment]
Figs. 5, 6 show batteries according to a fourth embodiment of the first
invention. Referring to Fig. 5, fluid fluidizing and dispersing units using a
liquid
or a gas are respectively used as the anode current collector and the cathode
CA 02508964 2000-03-27
23
current collector. Referring to Fig. 6, agitators rotatably driven by motors
or the
like (not shown) are respectively used as the anode current collector and the
cathode current collector.
As shown in Fig. 5, an anode current collector and dispersing unit 15
and a cathode current collector and dispersing unit 16 are devices such as the
dispersion plate or the spray nozzle for uniformly dispersing the gas or
liquid in
the horizontal cross sections of the respective cells 2, 3. Also, the
agitating
means such as the vane-like agitators or the like may be provided in the
respective cells 2, 3.
As shown in Fig. 6, an anode current collector and agitator 1? and a
cathode current collector and agitator 18 serve to agitate (fluidize) the
powdered
active materials and directly make contact with the powdered materials. The
vane-like agitators or the like rotatably driven by motors or the like (not
shown)
may be used as the anode current collector and agitator 17 and the cathode
current collector and agitator 18 but the constitution~of the agitating means
is
not limited. As shown in Fig. 6, although fluid fluidizing and dispersing
units
19 using a liquid or a gas are also used, these may be omitted.
The other constitution and function are identical to those of the second
embodiments.
[Fifth Embodiment]
Fig. 7 shows a battery according to a fifth embodiment of the first
invention. In this embodiment, as powdered active materials,
hydrogen-occluding alloy is used on an anode side and nickel hydroxide is used
on a cathode side. As shown in Fig. 7, an anode cell 2 is filled with the
powdered hydrogen-occluding alloy and an electrolytic solution 20 and a
CA 02508964 2000-03-27
24
cathode cell 3 is filled with a powdered nickel hydroxide and an electrolytic
solution 21. As the hydrogen-occluding.alloy, for example, La.o.s (Ce, Nd)
o.ls
Zr o.os Ni s.s Co 0.8 Al o.s, or the like is used . As the electrolytic
solution, for
example, 6 normal KOH aqueous solution or the like may be used.
Charge and discharge of the battery of this embodiment will be described
in detail.
(Charge)
When the battery is connected to the power generation means 8, an
electron is discharged from the power generation means 8 and reaches the
anode current collector 6. The electron reacts with the powdered
hydrogen-occluding alloy immediately on the anode current collector 6 or while
traveling through the powdered hydrogen-occluding alloy as follows. M denotes
the hydrogen-occluding alloy and MHx denotes metal hydride.
M + xH20 + xe- ~ MHx + xOH'
The hydroxyl ion generated by the reaction passes throug~-i the separator
1 and enters the cathode cell 3, where it reacts with the powdered nickel
hydroxide, and discharges the electron as represented by the following
reaction.
Ni (OH)2 + OH- --~ Ni00H + H2 O + e-
The generated electron moves to the cathode current collector 7
immediately or through the powdered nickel oxyhydroxide or powdered nickel
hydroxide and is supplied to the power generation means 8.
(Discharge)
When the battery is connected to the load means 8, the anode current
collector 6 discharges the electron to an external circuit. The discharged
electron travels through the load means 8 and reaches the cathode current
CA 02508964 2000-03-27
collector 7. The electron moves to the powdered nickel oxyhydroxide from the
cathode current collector 7. The electron reacts with water immediately or
through the powdered nickel oxyhydroxide to produce nickel hydroxide and
hydroxyl. The hydroxyl passes through the separator 1 and is introduced to the
anode cell 2, where it reacts with metal hydride and discharges the electron.
The electron moves to the anode current collector 6 immediately or through the
powdered hydrogen-occluding alloy and is supplied to the Ioad means 8.
The other constitutions and functions are similar to those of the second
embodiment. The battery of this embodiment can be carried out in the
constitutions of the third and fourth embodiments and in constitutions of
sixth
and seventh embodiments.
(Sixth Embodiment)
Figs. 8, 9 show batteries according to a sixth embodiment of the fu-st
invention. In this embodiment, a heat transmitter is installed in the battery
and functions as a current collector. It should be noted that the heat
transmitter and the current collector can be independently provided. Referring
to Fig. 8, an anode current collector and heat transmission tube 22 is
provided in
the anode cell 2 and a cathode current collector and heat transmission tube 23
is provided in the cathode cell 3. Referring to Fig. 9, an anode current
collector
and heat transmission plate 24 is provided in the anode cell 2 and a cathode
current collector.and heat transmission plate 24 is provided in the cathode
cell
3.
With reference to Fig. 8, charge and discharge of the battery of this
embodiment will be described in detail.
(Charge)
CA 02508964 2000-03-27
26
When the battery is connected to the power generation means 8, an
electron is discharged from the power generation means 8 and reaches the
anode current collector 22. The electron reacts with the anode powdered active
material immediately on the anode current collector 22 or while traveling
through the powdered active material. An anion generated by the fact that the
anode powdered active material has received the electron passes through the
separator 1 and enters the cathode cell 3, where it reacts with the cathode
powdered active material and discharges an electron. The electron moves to
the cathode current collector 23 immediately or through the powdered active
material and is supplied to the power generation means 8.
As described above, the current collectors serve as heat transmission
tubes on the both cathode and anode sides to simultaneously transmit the
electron and heat by contact with the powdered active materials. A heat
medium such as water and air is flowed through the anode current collector and
heat transmission tube 22 and the cathode current collector and heat
transmission tube 23, for collecting/supplying heat.
[Discharge]
When the battery is connected to the load means 8, the anode current
collector 22 discharges an electron to an external circuit. The discharged
electron travels through the load means 8 and reaches the cathode current
collector 23. The electron reacts with the cathode powdered active material
immediately on the cathode collector 23 or while traveling through the
powdered
active material. An anion generated by the fact that the cathode powdered
active material has received the electron passes through the separator 1 and
enters the anode cell 2, where it reacts with the anode powdered active
material
CA 02508964 2000-03-27
27
and discharges an electron. The electron moves to the anode current collector
22 immediately or through the powdered active material and is supplied to the
load means 8.
The current collectors of Fig. 9 on the both anode and cathode sides
serve as hollow heat transmission plates to transmit the electron and heat
simultaneously by the contact with the powdered material. The heat medium
such as water and air is flowed through an anode current collector and heat
transmission plate 24 and a cathode current collector and heat transmission
plate 25, for collecting/ supplying heat. The details of the charge and
discharge are similar to those of Fig. 8. The shape of the heat transmitters
is
not limited to tube or plate but another shape may be adopted.
The other constitutions and functions are similar to those of the second
embodiment. It should be noted that the constitution of this embodiment can
be combined into the constitutions of the third and fourth embodiments and a
constitution of a seventh embodiment mentioned later.
[Seventh Embodiment)
Figs. 10. I 1 show batteries according to a seventh embodiment of the
first invention. In this embodiment, there are provided a discharging device
for
discharging the powdered active material from vessels and a supply device for
supplying the powdered active material to the vessels. In addition, there are
provided a device for recovering the discharged powdered materials, a device
for
making up (refilling) the powdered materials, and a device for charging the
discharged powdered material by a thermal or chemical reaction.
First of all, charge and discharge of the battery of this embodiment will
be explained in detail.
CA 02508964 2000-03-27
28
[Charge]
When the battery is connected to the power generation means 8, an
electron is discharged from the power generation means 8 and reaches the
anode current collector 6. The electron reacts with the anode powdered
material immediately on the anode current collector 6 or while traveling
through
the anode powdered active material. An anion generated by the fact that the
anode powdered active material has received the electron passes through the
separator 1 and enters the cathode cell 3, where it reacts with the cathode
powdered active material and discharges an electron. The electron moves to
the cathode current collector 7 immediately or through the powdered active
material, and is supplied to the power generation means 8.
(Discharge)
When the battery is connected to the load means 8, the anode current
collector 6 discharges an electron to an external circuit. The discharged
electron travels through the load means 8 and reaches the cathode current
collector 7. The electron reacts with the cathode powdered active material
immediately on the cathode current collector 7 or while traveling through the
cathode powdered active material. An anion generated by the fact that the .
cathode powdered active material has received the electron passes through the
separator 1 and enters the anode cell 2, where it reacts with the anode
powdered
active material and discharges the electron. The electron moves to the anode
current collector 6 immediately or through the powdered active material and is
supplied to the load means 8.
The other constitutions and functions are similar to those of the second
embodiment.
CA 02508964 2000-03-27
29
(Recovery and Makeup of Active Materials)
Referring to Fig. 10, recovery and makeup of an active material (catalyst)
of the battery of this embodiment will be described in detail. In Fig. 10,
although only the constitution on the anode side is illustrated, the same
device
or the like is provided on the cathode side.
As shown in Fig. 10, the powdered active material degraded as the result
of the charge/discharge is discharged from the anode cell 2 as a slurry
together
with an electrolytic solution (electrolytic liquid) and part of or all of the
powdered
active material is discarded by a separator 26 as necessary. The electrolytic
solution is separated and the powdered material is supplied from the separator
26 to a recovery unit 27, where the powdered material is subjected to
acidizing
such as cleansing using hydrochloric acid. The powdered material recovered by
the recovery unit 27 is supplied to a mixer 28 to which new powdered material
equal in amount to the powdered material discarded by the separator 26 is
supplied from a makeup powdered material hopper 2. The recovered and made
up powdered material is re-mixed with the electrolytic solution by the mixer
28
and supplied as the slurry from a slurry pump (not shown) to the anode cell 2.
The constitution for separating and mixing the electrolytic solution is not
illustrated.
With reference to Fig. 11, recovery and makeup in association with the
reaction of the battery of this embodiment will be described. In Fig. 11,
although only the constitution on the anode side-is illustrated, the same
device
or the like is provided on the cathode side.
As shown in Fig. 11, the powdered material generated by the charge and
discharge is discharged from the anode cell 2 as the slurry together with the
CA 02508964 2000-03-27
electrolytic solution, and part or all of the powdered active material is
discarded
by the separator 26 as necessary. The electrolytic solution is separated and
the
powdered material is supplied from the separator 26 to a reactor 30, where it
reacts with a fuel supplied from a fuel supply tube 31 and is changed into a
re-dischargeable active material. The charged powdered material in the reactor
30 is supplied to the mixer 28, to which new powdered material equal in amount
to the powdered material discarded by the separator 26 is supplied from the
makeup powdered material hopper 29. The recovered and made up powdered
material is re-mixed with the electrolytic solution by the mixer 28 and
supplied
as the slurry from a slurry pump (not shown) to the anode cell 2. The
constitution for separating and mixing the electrolytic solution is not
illustrated.
In the reactor 30, in case of a nickel hydrogen battery, the following
reaction is conducted:
M + (x / 2) H2 -~ MHx
As a result, an active material identical to MHx generated in the following
reaction during charge is produced:
M + xH20 + xe' -~ MHX + xOH'
In the reactor on the cathode side, in case of the nickel hydrogen battery,
the following reaction by oxygen or air is conducted as follows:
Ni (OH)2 + ( 1 / 4) 02 --~ Ni00H + 1 / 2H2 O
As a result, an active material identical to Ni00H generated in the
following reaction during charge is produced:
Ni (OH)2 + OH- --> Ni00H + H2 O + e'
The constitution of this embodiment can be suitably combined into the
constitutions of the third, fourth and sixth embodiments.
CA 02508964 2000-03-27
31
(Eighth Embodiment)
Fig. 12 shows a battery according to an eighth embodiment of the first
invention. In this embodiment, the hydrogen-occluding alloy is used as the
anode powdered active material, hydrogen and hydrogen-containing gas or
hydrogen carbide gas or an alcohol-like material or an ether-like materia3 is
used
as anode agitating (Iluidizing) gas, nickel hydroxide is used as the cathode
powdered active material, and oxygen or air is used as cathode agitating
(fluidizing) gas. As shown in Fig. 12, the anode cell 2 is filled with the
powdered
hydrogen-occluding alloy and an electrolytic solution 20 and the cathode cell
3 is
filled with the powdered nickel hydroxide and an electrolytic solution 21. The
fluid fluidizing and dispersing means 9 serves to supply hydrogen to the anode
cell 2 and supply oxygen or air to the cathode cell 3. An example of the
hydrogen-occluding alloy, La o.a (Ce, Nd) o.i s Zr o.os Ni s.a Co o.s A1 o.s
or the like
is used. As the electrolytic solution, for example, a KOH aqueous solution or
the like may be used.
In the anode cell 2, hydrogen is fed to the powdered hydrogen-occluding
alloy and the electrolytic solution 20 and the following reaction is
conducted:
M + (x /2) H2 ~ MHx
When the battery is connected to the load means 8, hydrogen occluded
in the hydrogen-occluding alloy reacts with a hydroxyl in the electrolytic
solution
as follows and discharges an electron and water:
M~ + xOH- --~ M + xH20 + xe-
The discharged electron moves to the anode current collector 6
immediately or through the powdered hydrogen-occluding alloy. The electron
travels from the anode current collector 6, through the load means 8, and to
the
CA 02508964 2000-03-27
32
cathode current collector 7. The electron moves to the powdered nickel
oxyhydroxide from the cathode current collector 7. The electron moves
immediately or through the powdered nickel oxyhydroxide and reacts according
to the following reaction to produce nickel hydroxide and hydroxyl. The
hydroxyl passes through the separator 1, and is introduced to the anode cell
2,
where it reacts with metal hydride.
Ni00H + H20 + e- --+ Ni (OH)2 + OH-
In the cathode cell 3, in case of the nickel hydrogen battery, the following
reaction by using oxygen or air is conducted.
Ni (OH)2 + (1/4) 02 -~ Ni00H + (1/2) H20
As a result, an active material identical to Ni00H generated in the
following reaction during charge is generated as follows:
Ni (OH)2 + OH- --> Ni00H + H20 + e-
The other constitution and function are similar to those of the second
embodiment. The battery off' this embodiment may be carried out by the
constitutions of the third, fourth, sixth, and seventh embodiments.
2. Embodiments of the Second Invention
(First Embodiment)
Fig. 13 is a perspective view and a schematic cross-sectional view
showing an example of a verification tester of a layered-type three-
dimensional
battery according to a first embodiment of the second invention and Fig. 14 is
a
perspective view showing a portion of main components prior to assembling (in
a
disassembled state) of the verification tester of Figs. 13. As shown in Fig.
13, a
layered-type three-dimensional battery 41 is nickel-hydrogen battery.
As shown in Fig. 14, the battery is structured to have a pair of two cell
CA 02508964 2000-03-27
33
(vessel) members 42 each having a square central opening 42a penetrating
therethrough in a thickness direction thereof. In the example in Figs. 13, two
pairs (four in total) cell members 42 are provided. As shown in Fig. 14, a
shallow (in this example, 0.5mm deep) concave portion 42b is formed annularly
at a periphery of the opening 42a of each of the cell members 42. A
substantially-square and alkali resistant ion-permeable separator (in this
example Tenors separator) 43 is fitted into the concave portion 42b between
the
cell members 42. The separator 43 is a membrane which permits only ions to
pass therethrough but does not permit powdered electrode n, h or electricity
to
pass therethrough. In addition to the above, an unglazed pottery, an ion
exchange resin membrane, glass, or the like is used. Two injection ports 42c
are formed in an upper surface of each of the cell members 42 such that they
vertically penetrate toward the opening 42a and are spaced apart from each
other in the width direction thereof. Rubber plugs 44 are removably attached
to
the respective injection ports 42c.
A substantially-square, alkali-resistant, conductive, and plate-shaped
current collecting member (in this example, nickel plate) 45 is fitted into
the
concave portion 42b between the cell members 42 in each pair. Alkali-resistant
and conductive current collectors (in this example, nickel plate) 46, 47 are
provided on opposite sides of the two pairs of the cell members 42. Rubber
packings 48 are respectively interposed between the cell members 42, between
the cell member 42 and the current collector 46, and the cell member 42 and
the
current collector 47. The rubber packings 48 have openings 48a shaped
identically to the openings 42a in central portions thereof and have outer
shapes
identical to those of the cell members 42. A plurality of insertion holes 42d,
48d,
CA 02508964 2000-03-27
34
46d, 47d are sequentially formed at peripheries of the openings 42a, 48a in
the
cell members 42, the packings 48, and the current collectors 46, 47 such that
these holes penetrate in the thickness directions thereof and are spaced in
peripheral directions thereof. Non-conductive bolts 49 are inserted through
the plurality of insertion holes 42d, 48d, 46d, 47d and nuts (not shown) are
securely screwed to tip screw portions 49a of the bolts 49. Small holes 46e
and
small holes 47e are respectively formed at upper end portions of the left-end
(cathode) and right-end (anode) current collectors 46, 4? such that these
holes
are spaced in the width directions thereof. In this example, cathode terminals
50 and anode terminals 51 are respectively fitted to the small holes 46e of
the
left-end current collector 46 and the small holes 47e of the right-end
cttirent
collector 47 and one end portions of wirings 52, 53 are connected to these
terminals.
A potassium hydroxide solution k as the electrolytic solution is injected
into each of the cell members 42 through the injection ports 42c. Powdered
nickel hydroxide n as the cathode powdered active material, powdered
hydrogen-occluding alloy h as the anode powdered active material, the powdered
nickel hydroxide n as the cathode powdered active material, the powdered
hydrogen-occluding alloy h as the anode powdered active material are put into
the potassium hydrogen aqueous solution k sequentially from the left end cell
member 42 of Fig. 13(b) and suspended. As a result, from the left end to the
right end of Fig. 13(b), a cathode cell 54, an anode cell 55,.the cathode cell
54,
and the anode cell 55 are sequentially formed.
The layered-type three-dimensional battery 41 is thus constituted. The
battery 41 of this example is structured such that two nickel hydrogen unit
CA 02508964 2000-03-27
batteries (secondary batteries) 56 are connected in series to generate a
voltage of
approximately 2.4v. Load means 5? such as 2.4v electric bulb is connected
between the cathode terminal 50 and the anode terminal 51 of the battery 41 by
means of wirings 52, 53. During discharge of the charged battery 41, the
powdered nickel oxyhydroxide n in the cathode cell 54 which is in contact with
the cathode current collector 46 of a left-side first unit battery 56 provided
with
the cathode terminals 50 receives an electron (e-) from the cathode current
collector 46 together with a hydrogen ion to be formed into nickel hydroxide.
In
the anode 55, the powdered hydrogen-occluding alloy h discharges the electron
(e-) and the hydrogen ion (H+), and the hydrogen ion passes through the
ion-permeable separator 43 and travels to the cathode cell. In summary, the
following reaction in the cathode cell 54 is conducted:
Ni00H + H+ + e- --~ Ni (OIi)2
On the other hand, the following reaction in the anode cell 55 is
conducted:
MHx --~ M + x H+ + xe- (M: (powdered) hydrogen-occluding
alloy)
Following this, the electron (e-) discharged from the powdered
hydrogen-occluding alloy h in the anode cell 55 is collected to the current
collecting member 45 forming a separating wall between the anode cell 55 and
the cathode cell 54 of the right-side second unit battery 56 while moving
through
the powdered hydrogen-occluding alloy h, and the powdered nickel
oxyhydroxide n in the cathode cell 54 of the second unit battery receives the
electron (e-) from the current collecting member 45. The electron (e') and the
hydrogen ion are fed to the powdered nickel oxyhydroxide n to be formed into
CA 02508964 2000-03-27
36
nickel hydroxide. In the anode cell 55 of the right-side second unit battery
56,
the powdered hydrogen-occluding alloy h discharges the electron (e-) and the
hydrogen ion (H+), and the hydrogen ion passes through the ion-permeable
separator 43 and travels to the cathode cell 54. The electron (e-) discharged
in
the anode cell 55 is collected to the anode current collector 47 and moves
from
the anode terminal 51, through the wiring 53, and to the load means 57, and
moves to the cathode current collector 46 through the wining 52. Thereby, a
current slows from the cathode terminal 50 of the cathode current collector
46,
through the load means 57, and to the anode terminal 51 of the anode current
collector 47. In this way, a voltage of 1.2V X 2 (2.4V) is generated
(discharge is
performed).
On the other hand, the three-dimensional battery 41 is charged in the
following manner. A charger 58 applies a predetermined voltage to the battery
41 to cause the electron (e-) to be fed from the anode terminal 51 of the
anode
current collector 47 to the anode cell 55 of the right-side second unit
battery 56.
The electron (e ) moves in the powdered hydrogen-occluding alloy h, thereby
causing the following reaction to be conducted to generate a hydroxyl ion.
M + xH20 + xew-~ MHx + xOH- (M: (powdered
hydrogen-occluding alloy)
The hydroxyl ion (OH-) generated in the anode cell 55 passes through the
ion-permeable separator 43 and moves into the cathode cell 54 on the left
side,
where it reacts with the powdered nickel hydroxide n according to the
following
formula and discharges the electron (e-). .
Ni(OH)2 + OH' --> Ni00H + H20 + e-
The electron (e-) discharged in the cathode cell 54 is collected to the
CA 02508964 2000-03-27
37
current collecting member 45 and moves to the powdered hydrogen-occluding
alloy h in the anode cell 55 on the left side. Thereby, the reaction
represented
by the above formula is conducted and a hydroxyl ion is generated. The
hydroxyl ion (OH-) generated in the anode cell 55 passes through the
ion-permeable separator 43 and moves into the cathode cell 54 of the first
unit
battery 56 on the Ieft side, where it reacts with the powdered nickel
hydroxide n
according to the above formula and discharges the electron (e-) . The electron
(e ) is collected to the cathode terminal 50 of the cathode current collector
46 and
sent to the charger 58.
(Second Embodiment)
Fig. 15 is a central longitudinal sectional view schematically showing a
layered-type three-dimensional battery according to a second embodiment of the
second invention.
As shown in Fig. 15, a three-dimensional battery 41-1 of this
embodiment is a lead storage battery structured such that 6 pairs of unit lead
batteries 46 are connected in series. The unit lead storage battery 56
comprises
a cathode cell 54 and an anode cell 55 which are separated by an acid-
resistant
and ion-permeable separator 43 provided in a middle portion thereof. A
leftmost wall of the cathode cell 54 of a leftmost (first pair) unit battery
56 and~a
rightmost wall of the anode cell 55 of a rightmost (sixth pair) unit battery
56 are
respectively constituted by a side wall of acid-resistant conductor (platinous
plate or lead plate) as a current collector 46 and a side wall of acid-
resistant
conductor (platinous plate or lead plate) as a current collector 47. A right
side
wall of the anode cell 55 of the unit battery 56 of the first pair and a left
side wall
of the cathode cell 54 of the unit battery 56 of the sixth pair are
respectively
CA 02508964 2000-03-27
38
constituted by side walls of acid-resistant conductors (platinous plate or
lead
plate) as current collecting members 45. A four pairs of unit batteries 56
situated at an intermediate position are connected in series by means of the
acid-resistant conductors (platinous plate or lead plate) as the current
collecting
members 45 serving as separating walls defining the unit batteries 56 in
respective pairs. The leftmost (first pair) unit battery 56 and the rightmost
(sixth pair) unit battery 56 are connected in series by means of the acid-
resistant
conductor side walls (platinous plates or lead plates) as the current
collecting
members 45.
In this example, each of the cells 54, 55 is filled with a dilute sulfuric
acid
solution (sulfuric acid aqueous solution) r as a common electrolytic solution.
Powdered lead dioxide (Pb02) A is put into the dilute sulfuric acid solution
in the
cathode cell 54 and suspended. Powdered metallic lead (Pb) B is put in the
dilute sulfuric acid solution in the anode cell 55 and suspended.
The three-dimensional battery 41-1 according to the second embodiment
as described above discharges as follows. Specifically, the cathode cell 54 in
contact with the left-end cathode current collector 46 receives an electron
from
the current collector 46 and the electron (e-) is fed to the powdered lead
dioxide A,
which is converted into lead sulfate (PbS04) and an ion is genrated according
to
the following formula:
Pb02 + 4H+ + SO42- + 2e- ~ PbS04 + 2H20
Then,.an anion in the cathode cell 54 moves through the ion-permeable
separator 43 and into the cathode cell. 55, where it reacts with powdered
metallic
lead B and discharges the electron(e-] and the metallic lead is oxidized to be
generated into powdered lead sulfate according to the following formula:
CA 02508964 2000-03-27
39
Pb + SO42- -~ PbS04 + 2e'
The electron in the anode cell 55 is collected to the current collecting
member 45 and is fed from the current collecting member 45 to the powdered
lead dioxide A in the cathode cell 54 on the right side, and the reaction is
conducted according to the above formula to generate lead dioxide (PbS04) and
the ion. The anion in the cathode cell 54 moves through the ion-permeable
separator 43 into the anode cell 55, where it reacts with the powdered
metallic
lead B and discharges the electron and powdered lead sulfate is generated
according to the above formula. The electron is collected to the current
collecting member 45. This reaction is sequentially repeated in the respective
unit batteries 56. The electron moves from the right-end anode current
collector.47, through load means (not shown), and to the left-end cathode
current collector 46. Conversely, a current flows from the cathode current
collector 46, through the load means (not shown), and to the right-end current
collector 47. In this example, a voltage of approximately 13.6V is generated.
It should be noted that any acid-resistant conductors may be used as the
current collectors or electrodes. For example, carbon or conductive polymer
may be used.
(Third Embodiment)
Fig. 16 is a central longitudinal sectional view schematically showing a
layered-type three-dimensional battery according to a third embodiment of the
second invention.
As shown in Fig. 16, a three-dimensional battery 41-2 of this
embodiment is a lead storage battery similarly to that of the second
embodiment
of Fig. 15. A rotational shaft 59 is rotatably provided in the battery 41-2
such
CA 02508964 2000-03-27
that it penetrates through the battery 41-2 in the axial direction thereof,
and is
rotated manually or by a rotation drive device (not shown). A plurality of
agitation vanes 59a are provided at positions corresponding to the cells 54,
55 on
the rotational shaft 59 such that they are protruded in the direction
orthogonal
to the rotational shaft 59 and are adapted to agitate the dilute sulfuric
solutions
r and suspended powdered lead dioxide A or powdered metallic lead B in the
respective cells 54, 55 by rotation of the rotational shaft. This constitution
differs from that of the battery 41-1 of the second embodiment.
According to the three-dimensional battery 41-2 of this embodiment, the
powdered lead dioxide A and the powdered metallic lead B as powdered
electrodes are agitated to provide preferable contact between the powdered
. electrode A and the current collector 46, between the powdered electrode B
and
the current collecting members 47, or between the powdered electrodes A, B and
the current collecting members 45. Therefore, a capacity'of each of the cells
54,
55 (cell member 42: see Fig. 13) can be increased and a power can be
correspondingly increased. In addition, since the agitation of the powdered
lead
dioxide A and the powdered metallic lead B as the powdered electrodes can
prevent the adhesion of lead sulfate particulars to the current collectors or
the
current collecting members, lead plates can be employed as the current
collectors 46, 47 and the current collecting members 45. Since the battery 41-
2
is identical to the battery 41-1 of the second embodiment except the agitating
means 59, the corresponding parts are referenced to by the same reference
numerals and description thereof is omitted.
(Fourth Embodiment)
Fig. 17 is a central longitudinal sectional view schematically showing a
CA 02508964 2000-03-27
41
layered-type three-dimensional battery according to a fourth embodiment of the
second invention.
As shown in Fig. 17, a three-dimensional battery 41-3 of this
embodiment is a lead storage battery having a structure similar to the
structure
of the third embodiment of Fig. 16 and provided with agitating means different
from that of the battery 41-2 of the third embodiment. Specifically, the
agitating means of this embodiment comprises agitating means 60 for the
cathode cell 54 and agitating means 61 for the anode cell 55. The respective
agitating means 60, 61 respectively comprise circulation pumps 62, 63.
Dispersion nozzles 66, 67 are respectively attached to injection ports of
circulation tubes 64, 65 of the sulfuric acid aqueous solution r and filters
68, 69
.. for the powdered electrodes A, B are attached to suction ports of these
tubes.
With this constitution, the sulfwic acid aqueous solution r is circulated. In
the
battery 41-3 of this embodiment, the sulfuric acid aqueous solution r is
ejected
through the dispersion nozzle 66 or 67 to the cathode cell 54 or the anode
cell 55
to allow the powdered electrode A or B to be agitated. A trap or the like is
used
to insulate the pump from the electrolytic solution.
According to the three-dimensional battery 41-3 of this embodiment, the
powdered lead dioxide A and the powdered metallic lead B as powdered
electrodes are also agitated, thereby obtaining preferable contact between the
powdered electrode A and the cun-ent collector 46, between the powdered
electrode B and the current collector 47, or between the powdered electrodes
A,
B and the current collecting members 45. Therefore, a capacity of each of the
cells 54, 55 (cell member 42: see Fig. 13) can be increased and a power sari
be
correspondingly increased. In addition, the adhesion of lead sulfate
particulars
CA 02508964 2000-03-27
42
to the current collectors or the current collecting members can be prevented,
lead plates can be employed as the current collectors 46, 47 and the current
collecting members 45. Since the battery 41-3 is identical to the battery 41-2
of
the third embodiment except the agitating means, the corresponding parts are
referenced to by the same reference numerals and description thereof is
omitted.
[Fifth Embodiment]
Fig. 18 is a central longitudinal sectional view schematically showing a
layered-type three-dimensional battery according to a fifth embodiment of the
second invention.
As shown in Fig. 18, a three-dimensional battery 41-4 of this
embodiment is a lead storage battery having a structure similar to the
structure
of the fourth embodiment and provided with agitating means different from that
of the battery 41-3,of the fourth embodiment. Specifically, the agitating
means
is adapted to feed an inert gas such as nitrogen and argon to the cathode cell
54
and the anode cell 55 such that the inert gas, is fed from inert gas sources
70,
through pipings 73, 74, blowers 71, 72, and dispersion nozzles ?5, 76, and to
a
potassium hydroxide aqueous solution k, thereby agitating and fluidizing the
powdered electrodes n, h. The inert gas such as nitrogen and argon fed to the
cathode cell 54 and the anode cell 55 travel through pipings 77, ?8 and
filters 79,
80 and are opened in atmosphere and discharged.
The three-dimensional battery 41-4 of this embodiment is a nickel
hydrogen three-dimensional secondary battery in which powdered nickel
hydroxide n and powdered hydrogen-occluding alloy h are respectively put into
the cathode cell 54 and the anode cell 55 and are suspended in the potassium
hydroxide aqueous solution k as an electrolytic solution. Oxygen or air is
CA 02508964 2000-03-27
43
employed as an agitating and fluidizing gas of the cathode cell 54 and
hydrogen
is employed as an agitating and fluidizing gas of the anode cell 55. Thereby,
the
following reaction is conducted. In the anode cell 55, hydrogen reacts with
the
hydrogen-occluding alloy h according to the following formula:
M + (x/2)H2 -~ MHx
At this time, when the battery is connected to the load means 5?(see
Fig.13), the hydrogen occluded in the powdered hydrogen-occluding alloy h
reacts with a hydroxyl ion in the electrolytic solution k to discharge an
electron
and water as follows:
MHx + xOH- ~ M + xH20 + xe'
The discharged electron is collected to the anode current collector 47 and
moves through the load means 57 (see Fig. 13) and to the cathode current
collector 46. The electron moves to the powdered nickel oxyhydroxide n in the
cathode cell 46, and reacts with water to be converted into nickel hydroxide
and
a hydroxyl ion according to the following formula:
Ni00H + H20 + e' -~ Ni(OH)2 + OH'
The hydroxyl ion permeates the separator 43 and moves to the anode cell
55, where it reacts with metal hydride and discharges an electron and water.
On the other hand, in the cathode cell 54, oxygen or air is fed and the
following reaction is converted:
Ni(OH)2 + ( 1 / 4) 02 --> Ni00H + 1 / 2 H20
As a result, the reaction being conducted during charge according to the
following formula generates Ni00H and power is generated:
Ni(OH)2 + OH' -~ Ni00H + H20 + e-
[Sixth Embodiment]
CA 02508964 2000-03-27
44
Fig. 19 is a central longitudinal sectional view schematically showing a
layered-type three-dimensional battery according to a sixth embodiment of the
second invention.
As shown in Fig. 19, a three-dimensional battery 41-5 of this
embodiment is constituted by a nickel hydrogen secondary battery similarly to
the first embodiment of Fig. 13. In the battery 41-5, capacities of the
cathode
cell 54 and the anode cell 55 are significantly increased. A number of studs
81,
82, 83 are provided protrusively from the current collectors 46, 47 and the
current collecting member 45 toward the inside of the cathode cell 54 and the
inside of the anode cell 55 such that these studs are spaced apart from one
another. In this embodiment, since nickel plates are used as the current
collectors 46, 47 and the current collecting member 45, the studs 81, 82, 83
integral with the current collectors and current collecting member are also
constituted by the nickel plates. While in the battery 41-5 of this
embodiment,
the capacities of the cells 54, 55 are significantly increased, electricity
(electrons
and current) can be satisfactorily transmitted because the powdered electrodes
n, h are reliably in contact with the current collectors 46, 47 and the
current
collecting member 45. The agitating means 59 or 60, 61 of the third
embodiment or the fourth embodiment may be combined into the battery 41-5 of
this embodiment.
(Alternative Embodiment)
In addition to the embodiments~of the three-dimensional battery of the
second invention, the battery can be also embodied as described below.
1) Nickel hydroxide and cadmium, or nickel hydroxide and iron hydroxide
may be used as the cathode powdered active material and the powdered anode
CA 02508964 2000-03-27
active material instead of the above materials.
2) Two to six unit secondary batteries 56 are connected in series by means
of the conductive (acid-resistant or alkali-resistant) conductive member 45 in
the
above-described embodiments, but any number of unit batteries may be
connected in series according to a required voltage.
3). The capacity of the battery can be adjusted by increasing the capacities
pf
the cell members 42 according to a required power capacity and providing the
agitating means or studs as necessary.
3. Embodiments of Third Invention
Subsequently, with regard to the embodiments of the third invention,
equipment or device having a battery of three-dimensional structure
(three-dimensional battery) as part of its structure and a function of
chargeable/dischargeable power storage equipment, rotary equipment using a
power stored in the three-dimensional battery as a power source, a mobile body
using a power stored in the three-dimensional battery as a power source, power
conveying means for supplying the power stored, in the three-dimensional
battery to another equipment, and equipment for converting the power stored in
the three-dimensional battery into photo energy, kinetic energy or thermal
energy, will be described below in detail.
(Equipment or Device Having Three-Dimensional Battery as Part of Its Structure
and Fl~nction as Chargeable/Dischargeable Power Storage Equipment).
(Door)
In many cases, a door such as a door of a building or a door of
automobile has a dual structure for thermal insulation and strength
improvement but an inner space thereof is not e~ciently utilized.
CA 02508964 2000-03-27
46
Accordingly, the inner space of the door is utilized as cells of a
chargeable/dischargeable three-dimensional battery.
Specifically, the three-dimensional battery is charged with the
above-described mechanism and the inner space of the door is utilized as a
power storage.
When this embodiment is applied to the door of the building, the power
stored in the three-dimensional battery in the door can be utilized as an
emergency power supply if power supply is stopped due to a trouble caused by
electric power failure of a commercial power supply. Also, when this
embodiment is applied to the door of automobile, it is not necessary to
additionally mount accumulator battery. Besides, a battery active matxrial is
mainly composed of metallic particulars and is therefore resistant to impact
generated by collision in automobile accident. Further, the active material
has
sound absorbing ability and is sound-proof.
Fig. 20 is a longitudinal sectional view of a door having a
chargeable/dischargeable three-dimensional battery in an inner space thereof.
In Fig. 20, reference numeral 91 denotes a door housing, reference numeral 92
denotes a cathode terminal utilizing a hinge, reference numeral 93 denotes an
anode terminal utilizing a hinge, and reference numeral 94 denotes conductive
current collecting members. A plurality of cells are defined by the current
collecting members 94 and non-conductive separators 95. Each of the cells is
divided into two parts by an ion-permeable separator 96. One cell of the
divided
cells is filled with the cathode powdered active material and an electrolytic
solution 97 and the other cell of the divided cells is filled with the anode
powdered active material and an electrolytic solution 98. Reference numeral 99
CA 02508964 2000-03-27
47
denotes a key device and reference numeral 100 denotes a knob.
(Bridge Pier)
In general, bridge piers are made of steel or concrete, and the bridge piers
made of steel have a hollow structure. However, hollow inner spaces thereof
are
not efficiently utilized.
Accordingly, the inner space of the hollow and steel-made bridge pier is
utilized as cells of a chargeable/dischargeable three-dimensional battery.
Specifically, the three-dimensional battery is charged with the
above-described mechanism and the inner space of the bridge pier is utilized
as
a power storage.
A bridge pier hollow portion is filled with powdered iron as an active
material so as to be resistant to buckling breakdown. For example, in a case
where there is ocean near the bridge pier, a power generated by utilizing
ocean
temperature difference or a power generated by utilizing tidal current can be
stored, or a power generated by utilizing wind power can be stored.
Fig. 21 is a longitudinal sectional view of a bridge pier having a
chargeable/dischargeable three-dimensional battery in an inner space thereof.
In Fig. 21, reference numeral 101 denotes a bridge pier block, reference
numeral
102 denotes branch flanges, reference numeral 103 denotes a conductive
current collecting member. Each cell defined by the current collecting member
103 is divided into two parts by an ion-permeable separator 104. One cell of
the
divided cells is filled with the cathode powdered active material and an
electrolytic solution 105 and the other cell of the divided cells is filled
with the
anode powdered active material and an electrolytic solution 106.
For example, consider the following case. A bridge grider is constituted
CA 02508964 2000-03-27
48
by four bridge piers having cumulated 80 blocks of 20m square and 5m height.
A bridge pier block 101 is made of iron alloy and the inside thereof is
nickel-plated. The separator 104 is made of a material having non-conductivity
and high strength such as metal oxide sinter. An active material of a mixture
of
powdered nickel hydroxide and powdered metallic nickel is used as the cathode
powdered active material, an active material of a mia~ure of powdered iron
hydroxide and powdered metallic nickel is used as the anode powdered active
material, and a 6 normal potassium hydroxide solution is used as an
electrolytic
solution. Under the above-described conditions, a power of 70 billion kWhr can
be stored: This power is equivalent to a commercial power for about one month
which is used in Japan.
(Dam)
In general, a dam is a huge structure having a filling structure and made
of concrete. Nevertheless, in actuality, its enormous volume is utilized
exclusively as means for converting a positional energy of water into a power.
Accordingly, an outer shell of the dam is employed as a steel-made dam
and an inner space thereof is employed as huge cells of the
chargeable/dischargeable three-dimensional battery.
In other words, in addition to utilization as equipment for covering the
positional energy of water into the power, the dam is utilized in such a
manner
that the three-dimensional battery is charged with the above-described
mechanism and its inner space of the dam is a power storage.
As a result, a power storage efficiency becomes as high as 95% although
a hoisting water power generation efficiency is 60%.
Fig. 22 is a perspective view of a dam having a chargeable/dischargeable
CA 02508964 2000-03-27
49
,three-dimensional battery in an inner space thereof. In Fig.22, reference
numeral 111 denotes a cathode current collector, reference numeral 112
denotes an anode current collector, and reference numeral 113 denotes
conductive current collecting members. Each cell defined by the current
collecting members 113 is divided into two parts by an ion-permeable separator
114. A cell portion of the divided cells and close to the cathode current
collector
is filled with the cathode powdered active material and an
electrolytic.solution
115 and a cell portion of the divided cells and close to the anode current
collector
as filled with the anode powdered active material and an electrolytic solution
116.
(Radiator)
In a liquid-cooling type radiator, water or oil is used as a cooling medium.
It is difficult to convert the cooling medium into a fuel or the like, and the
cooling
medium is employed exclusively as a coolant.
Accordingly, the radiator is constituted by a chargeable/dischargeable
three-dimensional battery and the electrolytic solution is used as the cooling
medium.
Specifically, heat necessary for charge/discharge of the battery is
received via the electrolytic solution and the radiator is used as the power
storage.
As a result, it becomes unnecessary to mount the accumulator battery in
automobile and power storage efficiency of the battery is improved. In
particular, the reaction speed of the battery at a low ambient temperature is
accelerated by heating the electrolytic solution.
Fig. 23 is a view schematically showing a structure of the radiator as a
power storage. In Fig. 23, reference numeral 121 denotes a radiator body and
CA 02508964 2000-03-27
reference numeral 122 denotes fins. The radiator body 121 is divided into two
parts by an ion-permeable separator 123. One side of the divided radiator is
filled with the cathode powdered active material and an electrolytic solution
124
and the other side of the divided radiator is filled with the anode powdered
active
material and an electrolytic solution 125. Reference numeFal 126 denotes a
cathode current collector and reference numeral 127 denotes an anode current
collector. Reference numerals 128a, 128b denote active material separation
filters for recovering the active material and the active material separation
filter
128b is connected to a heat source. Heat is transmitted to the radiator body
121 from the heat source.
Roof tiles, thatches, ceramics, or the like, which are heat insulative and
water repellent are employed in roofs of general houses. The roof itself has
no
energy conversion function and a large space between the roof and a ceiling is
wasted.
Accordingly, the space between the roof and the ceiling is utilized to form
a chargeable/dischargeable three-dimensional battery.
Specifically, instead of soil filled into an attic as a heat insulating
material
and weight, powdered active materials of the three-dimensional battery are
filled
into the attic to be utilized as a power storage.
For example, if a power generated by a solar battery cell installed on the
roof or by wind power generation is stored in the three-dimensional battery
and
the three-dimensional battery is conf gored to have a heat exchange function,
then, in summer, an indoor warm air is suctioned to be utilized in a battery
reaction of the three-dimensional battery, and in winter, heat generated as
the
CA 02508964 2000-03-27
51
result of the battery reaction of the three-dimensional battery is discharged
indoors. Thereby, in summer, it is cool indoors and in winter, it is warm
indoors. The three-dimensional battery can be used as air-conditioning
equipment as well as a power storage. In addition, when the three-dimensional
battery having the heat exchange function is installed in a ceiling portion of
automobile, the same air-conditioning effect is obtained.
Fig. 24 is a longitudinal sectional view showing a house having a
chargeable/dischargeable three-dimensional battery in a ceiling portion. In
Fig.
24, reference numeral 131 denotes a roof, reference numerals 132a, 132b
denote walls. A plurality of current collecting members 134 are placed from
the
one wall 132a to the other wall 132b in the ceiling portion surrounded by the
roof 131, the walls 132a, 132b, and a beam 133. Each cell defined by the
current collecting members 134 is divided into two parts by an ion-permeable
separator 135. A cell portion of the divided cells and close to a cathode
current
collector 136 is filled with the cathode powdered active material and an
electrolytic solution 137 and a cell portion of the divided cells and close to
an
anode current collector 138 is filled with the anode powdered active material
and
an electrolytic solution 139.
jAutomobile Bonnet and Trunk Cover]
A bonnet and a trunk cover of automobile are used as a cover for an
engine and other components and a reinforcement member, but its inner
surface portion is not utilized.
Accordingly, the bonnet or the trunk cover is utilized as a casing of the
three-dimensional battery and a chargeable/dischargeable~three-dimensional
battery is formed on an inner surface side of the bonnet or the trunk cover.
CA 02508964 2000-03-27
52
Specifically, the bonnet or the trunk cover is configured to have a battery
function.
As a result, the accumulator battery mounted in the bonnet becomes
unnecessary. Farther, the three-dimensional battery functions as the
reinforcement member and the strength of the bonnet or the trunk cover is
increased.
Fig. 25 is a cross-sectional view showing part of the bonnet having a
chargeable/dischargeable three-dimensional battery on the inner surface side.
In Fig. 25, reference numeral 141 denotes a bonnet and reference numeral 142
denotes conductive current collecting members. Each cell defined by the
current collecting members 142 is divided into two parts by an ion-permeable
separator 143. One of the divided cells is filled with the cathode powdered
active material and an electrolytic solution 144 and the other of the divided
cells
is filled with the anode powdered active material and an electrolytic solution
145.
(Road)
In general, a road is constructed of an underlayer roadbed, an
upperlayer roadbed on the underlayer roadbed, and a surface layer portion
paved with asphalt. In actuality, the roadbeds are employed exclusively as a
base of the road.
Accordingly, a material of the roadbed generally used is replaced by
powdered active materials and a chargeable/dischargeable three-dimensional
battery is formed around a ground surface.
Specifically, the three-dimensional battery is charged with the
above-described mechanism and a great amount of power is stored in the road.
As a result, freezing of the road can be prevented by heat generated
CA 02508964 2000-03-27
53
resulting from a battery reaction. In addition, the recovery of the powdered
active materials makes the material of the roadbed recyclable.
Fig. 26 is a cross-sectional view showing a vicinity of a ground surface in
which the chargeable/dischargeable three-dimensional battery is formed. In
Fig. 26, reference numeral 151 denotes an asphalt pavement, reference numeral
152 denotes a cathode current collector, reference numeral 153 denotes an
anode current collector, and reference numeral 154 denotes the conductive
current collecting members. Each cell defined by the current collecting
members 154 is divided into two parts by an ion-permeable separator 155. A
cell portion of the divided cells and close to the cathode current collector
is filled
with the cathode powdered active material and an electrolytic solution 156 and
a
cell portion of the divided cells and close to the anode current collector is
filled
with the anode powdered active material and an electrolytic solution 157.
(Tableware)
In general, heat-insulating pottery or metallic tableware of a dual
structure is used as heat-retentive tableware. However, because the tableware
is highly heat-insulating and has a large heat capacity, it is necessary to
heat or
cool the tableware according to temperature of food therein for preferable
heat
retention before the food is put in the tableware.
Accordingly, the tableware is configured to have a bottom or side portion
of a dual structure, and an inner space of the dual structure is utilized to
form a
chargeable/dischargeable three-dimensional battery and a heat generating
element or a cooling element is embedded in the inner space.
Specifically, by using a power stored in the three-dimensional battery as
a power supply, the heat generating element or the cooling element is
activated,
CA 02508964 2000-03-27
54
thereby allowing warm hood to be kept heated and cold food to be kept cooled.
As a result, it is not necessary to heat the tableware before the warm food
is put therein and therefore, the food does not become cold. Likewise, it is
not
necessary to cool the tableware before the cold food is put therein and,
therefore,
the food does not become warm.
Fig. 27 is a longitudinal sectional view of a tableware having a
chargeable/dischargeable three-dimensional battery in a side portion thereof.
In Fig. 27, reference numeral 161 denotes a handle of the tableware. A
tableware body 162 is dual-structiu-ed and has an inner space. The inner
space of the side portion of the tableware body 162 is divided into two parts
by
an ion-permeable separator 163. One of the divided spaces is filled with the
cathode powdered active material and an electrolytic solution 164 and the
other
of the divided spaces is filled with the anode powdered active material and an
electrolytic solution 165. A heat generating element (or cooling element) 166
is
embedded in the bottom portion of the tableware. Reference numeral 167
denotes a power supply switch and reference numeral 168 denotes a charging
jack. The above-structured three-dimensional battery of the tableware side
portion is charged from the charging jack 168, and the power supply switch 167
is turned on when the food is put in the tableware to cause the heat
generating
element (or cooling element) 166 to be activated by power charged in the
three-dimensional battery of the side portion. Thereby, the food in the
tableware is kept heated or cooled.
[Balance weight]
A hoisting machine such as a power shovel, forklift, and a crane is
generally provided with a balance weight as an essential attachment for the
CA 02508964 2000-03-27
purpose of keeping the balance between the machine and heavy load to be
handled. The balance weight is chunk of metal and is exclusively utivzed to
balance the weight.
Accordingly, a cathode current collector and an anode current collector
are provided in the balance weight. An ion-permeable separator is interposed
between the cathode current collector and the anode current collector.
A chargeable/dischargeable three-dimensional battery is formed in such a
manner that a cathode powdered active material and an electrolytic solution
are
filled between the cathode current collector and the ion-permeable separator
and
an anode powdered active material and an electrolytic solution are filled
between
the anode current collector and the ion-permeable separator.
That is, the balance weight is utilized not only as a weight but as a power
storage.
As a result, the power of the three-dimensional battery built in the
balance weight can be utilized as an activation power supply of the hoisting
machine such as the power shovel, the forklift, and the crane.
(Floor)
In some houses, underfloor spaces are utilized as indoor heating sources
by flowing high-temperature combustion exhaust gas thereunder or installing
electric heaters thereunder. However, the resulting heat is difficult to
utilize for
cooling, and therefore, a space under the floor is not efficiently utilized.
Accordingly, a chargeable/dischargeable three-dimensional battery is
formed in the undei-floor space.
Specifically, the underfloor space serves as a power storage and one of
electrodes releases heat and the other electrode absorbs heat during
CA 02508964 2000-03-27
56
charge/discharge, which is utilized for indoor heating/cooling.
Thus, the released/absorbed heat of the battery is directly utilized as the
power supply for cooling/heating. Consequently, an energy conversion
efficiency is improved as compared to general air-conditioning equipment that
utilizes evaporation heat or radiation heat associated with
expansion/compression of a compressive heat transmission medium.
Fig. 28 is a cross-sectional view showing a floor of a house having a
chargeable/dischargeable three dimensional battery. In Fig. 28, reference
numeral 171 denotes a floor, reference numeral 172 denotes a cathode,
reference numeral 173 denotes an anode, and reference numeral 174 denotes
conductive current collecting members. Each cell defined by current collecting
members 174 provided from the cathode toward the anode is divided into two
parts by an ion-permeable separator 175. A cell portion of the divided cells
and
close to the cathode is filled with the cathode powdered active material and
an
electrolytic solution 176 and a cell portion of the divided cells and close to
the
anode is filled with the anode powdered active material and an electrolytic
solution 177. Reference numeral 178 denotes a heat medium supply
cooling/heating switching device and reference numeral 179 denotes a heat
medium collecting cooling/heating switching device. The heat medium flowing
through a heat medium circulation space 180 under the floor from the heat
medium supply cooling/heating switching device 178 is collected into the heat
medium collecting cooling/heating switching device 179 and is supplied to the.
cathode cell inner heat exchanger 182 through a cathode heat exchanger heat
medium supply pipe 181. Then, the heat medium flows through the cathode
heat exchanger heat medium discharge pipe 183 and reaches the heat medium
CA 02508964 2000-03-27
57
supply cooling/heating switching device 178. The heat medium collected into
the heat medium collecting cooling/heating switching device 179 is supplied to
an anode cell inner heat exchanger 185 through an anode heat exchanger heat
medium supply pipe 184. Then, the heat medium flows through an anode heat
exchanger heat medium discharge pipe I86 and reaches the heat medium
supply cooling/heating switching device 178. The heat meditun supply
cooling/heating switching device 178 and the heat medium collecting
cooling/heating switching device 179 are switched to cooling/heating, to allow
chemical reaction heat resulting from the battery reaction during
charge/discharge to be utilized as a cooling source or a heating source.
(Bed)
In general, beds are heat insulating, and are warm in winter but are hot
in summer.
Accordingly, a chargeable/dischargeable three-dimensional battery is
formed in the bed by utilizing a portion under a bed surface into which
elasticity
means such as a spring body is provided.
Specifically, the bed serves as a power storage. Since one electrode
releases heat and the other electrode absorbs heat during charge/discharge,
the
releasing reaction is utilized for heating and the absorbing reaction is
utilized for
cooling.
Thus, the released/absorbed heat of the battery is directly utilized as the
power supply for cooling/heating. Consequently, an energy conversion
efficiency is improved as compared to general air-conditioning equipment that
utilizes evaporation heat or radiation heat associated with
expansion/compression of a compressive heat transmitter medium.
CA 02508964 2000-03-27
5g
A specific illustration is similar to that of Fig. 28, and is therefore
omitted
(the floor 171 rnay be assumed to be a bed surface).
(Construction Power Supply)
In a place where a commercial power supply is unavailable, an engine
electric generator is employed as a type of construction power supply, but
environmental pollution such as noises or exhaust gases arise.
Accordingly, a chargeable/dischargeable three-dimensional battery is
mounted in a vehicle and installed in a construction site. Under construction,
the power is supplied from the three-dimensional battery when necessary.
Thus, power supply means that makes little noises and exhausts little
gases can be provided. This is very effective particularly when the
construction
power supply is required in a closed space such as a house-packed place or a
tunnel.
Fig. 29 is a side view showing a trailer in which a
chargeable/dischargeable three-dimensional battery is mounted. In Fig. 29,
reference numeral 191 denotes a power car and reference numeral 192 denotes
a trailer in which the three-dimensional battery is mounted.
(Rotary Equipment Using Power Stored in Three-Dimensional Battery as Power
Source]
(Electric Motor)
In general, an electric motor has a drawback that the electric motor is not
activated unless the power is supplied from an external power supply and a
current more than a rated value flows when being activated.
Accordingly, a chargeable/dischargeable three-dimensional battery is
formed by using a casing or a seat of the electric motor as a battery housing.
CA 02508964 2000-03-27
59
Specifically, a power storage device is included in the electric motor.
Thereby, the electric motor can be activated without supplying the power from
the external power supply.
Thus, incorporating of the battery into the electric motor can reduce a
volume of the whole device. At activation, since the power is supplied from
the
three-dimensional battery as well as the external power supply; large feeding
equipment becomes unnecessary and a usage amount of the external power can
be suppressed. In a normal drive state of the electric motor, the external
power
can be dispensed with by using only the thee-dimensional battery to supply the
power, while at power electric failure, the electric motor is activated by
using the
battery.
Fig. 30(a) is a longitudinal sectional view showing an electric motor in
which the chargeable/dischargeable three-dimensional battery is built in the
casing. In Fig. 30(a), reference numeral 201 denotes a rotational shaft,
reference numeral 202 denotes a rotator, reference numeral 203 denotes a
magnetic coil, reference numeral 204 denotes a cathode current collector,
reference numeral 205 denotes an ion-permeable separator, and reference
numeral 206 denotes an anode current collector. A cathode powdered active
material and an electrolytic solution 207 are filled between the cathode
current
collector 204 and the ion-permeable separator 205 and an anode powdered
active material and an electrolytic solution-208 are filled between the anode
current collector 206 and the ion-permeable separator 205. While one battery
is illustrated in Fig. 30(a), a high voltage can be obtained by laminating
batteries
in the circumferential direction thereof or in the longitudinal direction of
its axis.
The anode current collector 206 is circular, but if rectangular current
collectors
CA 02508964 2000-03-27
are laminated in the longitudinal direction of its axis, then a volume
efficiency of
the electric motor can be improved.
Fig. 30(b) is a longitudinal sectional view showing an electric motor in
which a chargeable/dischargeable three-dimensional battery is built in a base
portion thereof. In Fig. 30(b), reference numeral 209 denotes a base portion
of
an electric motor 215; reference numeral 210 denotes a cathode current
collector, reference numeral 211 denotes an ion-permeable separator, and
reference numeral 212 denotes an anode current collector. A cathode
powdered active material and an electrolytic solution 21-3 are filled in a
portion
between the cathode current collector 210 and the separator 211 and an anode
powdered active material and an electrolytic solution 214 are filled between
the
anode current collector 212 and the separator 211.
If the three-dimensional battery of the present invention is adopted in
appliance activated by a small-sized electric motor, for example, a portable
tape
recorder, then a space of a battery currently used can be saved, and the
electric
motor is made slightly larger. Therefore, the entire portable tape recorder
can
be made small. If the three-dimensional battery of the present invention is
employed in a large-sized electric motor, then a large current required at
activation of the electric motor can be also supplied from the three-
dimensional
battery. Consequently, a voluminous power supply device required only at the
activation can be dispensed with, and the amount of usage of an external power
can be significantly reduced.
(Engine)
In general, a jacket for circulating a cooling medium is provided in a
casing of an engine such as a reciprocal engine or a turbo engine. An electric
CA 02508964 2000-03-27
61
motor is necessary to start the engine and a power must be supplied from an
external power supply to activate the electric motor.
Accordingly, a chargeable/dischargeable three-dimensional battery is
formed by utilizing a casing of the engine as a battery housing.
Specifically, the casing seining as the battery absorbs heat of the engine
and efficiently converts the heat into a power and the power is stored in
outside
of the engine casing.
Since the engine thus has a storing function, the external power supply
can be dispensed with. In addition, since the heat of the engine is utilized
to
store the power, the heat energy which has been conventionally discarded
externally can be converted into the electric energy and stored. Consequently,
the energy efficiency can be improved.
Fig. 31 is a longitudinal sectional view showing a turbo engine in which a
chargeable/dischargeable three-dimensional battery is built in a casing. In
Fig.
31, reference numeral 221 denotes a rotational shaft, reference numeral 222
denotes a turbine, reference numeral 223 denotes a casing, reference numeral
224 denotes a cathode current collector, reference numeral 225 denotes an
ion-permeable separator, and reference numeral 226 denotes an anode current
collector. A cathode powdered active material and an electrolytic solution 22?
are filled between the cathode current collector 224 and the separator 225 and
an anode powdered active material and an electrolytic solution 228 are filled
between the anode current collector 226 and the separator 225.
It is preferable that the battery of Fig. 31 adopts a structure of a battery
(e.g., molten-carbonate type fuel battery using a carbonate such as lithium
carbonate and potassium carbonate as electrolytes and activated at a high
CA 02508964 2000-03-27
62
temperature of approximately 650 '~) activated at a relatively high
temperature
according to an activated temperature of the engine and the casing 223 is used
as an electrode that absorbs the heat by charge. Fig. 31 shows the turbo
engine.
In case of the reciprocal engine, a cooling double jacket on an outer
periphery of
a cylinder can be used as the casing the battery.
[Mobile Body Using Power Stored in Three-dimensional Battery as Power
Source ]
(Dual-Structured Ship)
In many cases, ship for transporting a liquid which would pollute sear
water if leaked, such as a tanker, has 'a dual-structure to prevent the leak
of the
liquid into the sea caused by accident or the like. In actuality, the
dual-structured portion is not efficiently utilized.
Accordingly, a chargeable/dischargeable three-dimensional battery
using the sea water and alkali as an electrolytic solution is formed in the
dual-structured portion.
Specif cally, the dual-structured portion of the ship can be utilized as the
power storage.
As a result, the stored power can be utilized as a power source for the
cruising ship.
Fig. 32 is a perspective view showing part of the dual-structured ship in
which the chargeable/dischargeable three-dimensional battery is built. In Fig.
32, reference numeral 231 denotes a tank wall corresponding to a cathode
current collector, reference numeral 232 denotes an ion-permeable separator,
and reference numeral 233 denotes a ship outer wall corresponding to an anode
current collector. A cathode powdered active material and an electrolytic
CA 02508964 2000-03-27
63
solution 234 are filled between the cathode current collector 23I and the
separator 232 and an anode powdered active material and an electrolytic
solution 235 are filled between the anode current collector 233 and the
separator
232. In this embodiment, the sea water can be also utilized as the
electrolytic
solution. If the dual-structured portion of the dual-structured ship is thus
efficiently utilized as the three-dimensional battery, for example, 5% of the
weight of the 1 million ton tanker is utilized as the battery, then the ship
is
capable of cruising for about 60 hours with an engine power of one hundred
thousands horse power.
(Ship)
A great quantity of petroleum, natural gases, nuclear fuels, coil and so
forth as an energy source are transported by enormous ship of large
displacement capacity for the purpose of reducing a transport cost, but there
has been no means for directly transporting the power.
Accordingly, part of or all of a ship belly is used as a
chargeable/dischargeable thee-dimensional battery.
Specifically, the ship belly is utilized as a power storage.
As a result, the stored power can be utilized as the power source of the
cruising ship.
Fig. 33 is a partially longitudinal sectional view showing part of ship in
which a chargeable/dischargeable three-dimensional battery is built. In Fig.
33,
reference numeral 241 denotes a ship separating wall corresponding too a
cathode current collector and reference numeral 242 denotes a ship outer. wall
corresponding to an anode current collector. A plurality of conductive current
collecting members 243 serving as the separating walls are interposed between
CA 02508964 2000-03-27
the cathode current collector 241 and the anode current collector 242 and each
cell defined by the current collecting members 243 is divided into two parts
by an
ion-permeable separator 244. A cell portion of the divided cells and close to
the
cathode current collector is filled with the cathode powdered active material
and
an electrolytic solution 245 and a cell portion of the divided cells and close
to the
anode current collector is filled with an anode powdered active material and
an
electrolytic solution 246.
Assuming that the three-dimensional battery is created in the ship
having displacement capacity of one million tons, a power of 100 million kWhr
can be stored. If the power costs 10 yen per 1 kWhr, then the power that costs
1 billion yen can be transported, and this is preferable because the
efficiency in
tr-ansporting the natural gases or coil is improved.
(Airplane)
A body of an airplane has a dual structure so as to be pressure-resistant
and a wing thereof has a dual structure to obtain strength. Part of an inner
space of the wing is filled with a fuel but the remaining inner space is not
efficiently utilized.
Accordingly, the inner space of the wing is utilized to form cells of a
chargeable/dischargeable three-dimensional battery.
Specifically, the power stored in the three-dimensional battery in the
wing is utilized as a power at activation of an engine of the airplane and a
power
source inside the flying airplane.
As a result, since a power gas turbine and a dedicated battery becomes
unnecessary, a lightweight airplane is achieved.
Fig. 34 is a cross-sectional view of a wing of an airplane in which a
CA 02508964 2000-03-27
chargeable/dischargeable three-dimensional battery is built In Fig. 34,
reference numeral 251 denotes an inner wing separating wall corresponding to a
cathode current collector and reference numeral 252 denotes an outer wing
separating wall corresponding to an anode current collector. A plurality of
conductive current collecting members 253 serving as separating walls are
interposed between the cathode current collector 251 and the anode current
collector 252 and each cell defined by the current collecting members 253 is
divided into two parts by an ion-permeable separator 254. A cell portion of
the
divided cells and close to the cathode current collector is filled with a
cathode
powdered active material and an electrolytic solution 255 and a cell portion
of
the divided cells and close to the anode current collector is filled with an
anode
powdered active material and an electrolytic solution 256.
(Road Roller)
A road roller is generally provided with large and heavy tires and the tires
serve as weights. Metallic masses are filled in the inside of the tires and
the
filled materials are not efficiently utilized. - -
Accordingly, the metallic masses inside of the tires of the road roller may
be replaced by powdered active materials to form a chargeable/dischargeable
three-dimensional battery.
Specifically, the tires of the road roller are utilized as a mobile power
supply.
As a result, the tires can be efficiently utilized as the mobile power as well
as the weights.
Fig. 35 is a cross-sectional view showing a tire of a road roller in which a
chargeable/dischargeable three-dimensional battery is built. In Fig. 35,
CA 02508964 2000-03-27
66
reference numeral 261 denotes a rotational shaft corresponding to a cathode
current collector and reference numeral 262 denotes an outer wall
corresponding to an anode current collector. A conductive current collecting
member 263 serving as a separating wall is interposed between the cathode
current collector 261 and the anode current collector 262 and each cell
defined
by the current collecting member 263 is divided into two parts.by an
ion-permeable separator 264. A cell portion of the divided cells and close to
the
cathode current collector is filled with a cathode powdered active material
and
an electrolytic solution 265 and a cell portion of the divided cells and close
to the
anode current collector is filled with an anode powdered active material and
an
electrolytic solution 266.
(Electric Train)
In general, a power is supplied from a power line through a pantograph.
In actuality, building of a wire is costly and time-consuming. Also, the
friction
between the pantograph and the power line causes noises.
Accordingly, a bottom portion of a vehicle body of the electric train is
used as cells of chargeable/dischargeable three-dimensional battery.
Specifically, the power of the three-dimensional battery is stored in the
bottom portion of the vehicle body to be used as a power for traveling. .
As a result, the building of the wire becomes unnecessary.
Fig. 36 is a schematic view showing a structure of a
chargeable/dischargeable three-dimensional battery installed in the bottom
portion of the vehicle body of the electric train. In Fig. 36, reference
numeral
271 denotes a cathode current collector and reference numeral 272 denotes an
anode current collector. A plurality of conductive current collecting members
CA 02508964 2000-03-27
67
273 serving as the separating walls are interposed between the cathode current
collector 271 and the anode current collector 272 and each cell defined by the
current collecting members 273 is divided into two parts by an ion-permeable
separator 274. A cell portion of the divided cells and close to the cathode
current collector is filled with a cathode powdered active material and an
electrolytic solution 275 and a cell portion of the divided cells and close to
the
anode current collector is filled with an anode powdered active material and
an
electrolytic solution 276.
For example, if 1 ton three-dimensional battery is created, then a 100
kWhr power can be stored, and an electric train traveling around the city can
travel for several tens minutes and can be charged in a short time (several
minutes) while the train is not moving. I~owever, to travel 16 vehicles of a
bullet
train, the maximum power of 15000 kW is required, and the bullet train cannot
travel for 2 hours without mounting 4 ton three-dimensional battery in each
vehicle. It is therefore preferable that the three-dimensional battery having
a
capacity as small as about 2 ton is mounted together with an engine electric
generator, a fuel battery or the like.
jElectric Locomotive)
An electric locomotive is adapted to travel by driving an electric motor by
a power generated by an engine electric generator. Since the response to
variation of a load is slow, fly wheels are mounted to the
electric~locomotive.
However, the -energy stored in the engine electric generator is little and a
traveling performance is adversely affected by the variation of angular
momentum.
Accordingly, a chargeable/dischargeable three-dimensional battery is
CA 02508964 2000-03-27
68
installed between the electric generator and the electric motor.
Specifically, the power stored in the three-dimensional battery is used to
drive the electric motor and utilized as the power for traveling.
As a result, the response to variation of the load is improved and an
efficiency of the engine is improved, thereby increasing the maximum engine
power. Simultaneously, the emissions of polluted substances can be
advantageously reduced.
Fig. 37(a) is a cross-sectional view showing an electric locomotive having
a chargeable/dischargeable three-dimensional battery. In Fig. 37(a), reference
numeral 281 denotes a driver's seat, reference numeral 282 denotes an engine
electric generator, reference numeral 283 denotes a three-dirnensional
battery,
reference numeral 284 denotes an electric motor, reference numeral 285
denotes a control device, and reference numeral 286 denotes driving wheels.
Fig. 37(b) is a view schematically showing an example of a mechanism for
driving
an electric motor via a chargeable/dischargeable three-dimensional battery
from
an electric generator when applied to the turbo engine. In Fig 37(b),
reference
numeral 287 denotes a compressor, reference numeral 288 denotes a fuel tank,
and reference numeral 289 denotes a combustion chamber. An air 290
externally introduced is compressed by a compressor 287, and the resulting
high-pressure air and a fuel in the fuel tank 288 are combusted by the
combustion chamber 289 to generate a high-temperature and high-pressure gas.
A ldnetic energy of the high-temperature and high-pressure gas is supplied to
the three-dimensional battery 293 through an expander 291 'and an electric
generator 292 and converted into a power to be stored therein. The power is
supplied to the electric motor 295 through the control device 294.
CA 02508964 2000-03-27
69
(Power Vehicle)
In general, a power is supplied to an electric locomotive and an electric
train from a power line through a pantograph. The train cannot travel on
non-electrified Line and during electric power failure. Accordingly, a power
vehicle constituted by vehicles in which an electric generator and a
chargeable/dischargeable three-dimensional battery or only the
chargeable/dischargeable three-dimensional battery is mounted is connected to
the electric locomotive or electric train.
Specifically, the power of the power vehicle is used to drive the electric
motor and is utilized as the power for traveling of the electric locomotive or
the
electric train.
As a result, the electric locomotive or the electric train can travel on the
non-electrified line.
Fig. 38(a) is a cross-sectional view showing an electric locomotive to
which a power vehicle is connected and Fig. 38(b) is a view schematically
showing an example of power storage equipment from an electric generator to .
the chargeable/dischargeable three-dimensional battery when applied to the
turbo engine. In Fig. 38(a), reference numeral 301 denotes an electric
locomotive and reference numeral 302 denotes a power vehicle. The
components identical t those of Fig. 37 are referenced to by the same
reference
numerals and description thereof is omitted. The difference between Fig. 38(b)
and Fig. 37(b) is that Fig. 37(b) includes the control device 294 and the
electric
generator 295 but Fig. 38(b) does not.
(how-Noise Electric Train)
In general, a power is supplied from a power line through a pantograph,
CA 02508964 2000-03-27
and therefore, a friction between the pantograph and the power line causes a
noise. For this reason, the electric train travels at a low speed to lessen
the
noise when traveling in a house-packed place. However, low-speed traveling of
the electric train as a high-speed transport means causes a severe time loss
and
the train cannot reach destination at a desired timing.
Accordingly, a power vehicle constituted by vehicles in which an electric
generator and a chargeable/dischargeable three-dimensional battery or only the
chargeable/dischargeable three-dimensional battery is mounted is connected to
the train as a power supply, and the three-dimensional battery is mounted in
each vehicle.
Specifically, during high-speed traveling, the pantograph is stored and
the train travels with the power stored in the three-dimensional battery.
As a result, the noise during the high-speed traveling can be lessened.
Fig. 39 is a cross-sectional view showing a low-noise electric train having
a chargeable/dischargeable three-dimensional battery and differs from Fig.
38(a)
in that a pantograph 311 is added to the electric locomotive 301 of Fig.
38(a).
[Power Conveying Means for Supplying Power Stored in Three-Dimensional
Battery to Another Equipment)
(Electric Wire)
Conventionally, a coaxial cable is employed for high-frequency power
transport and a parallel-type cable is employed for low-frequency power
transport. If a power source stops~power supply for a moment, or a short-time
power electric failure occurs, the power supply stops, which might lead to a
serious accident in equipment which does not permit momentary inactivation.
Accordingly, the power line is used as a current collector, and powdered
CA 02508964 2000-03-27
71
active materials are filled around the power line. Thereby, the power line can
have a function of the chargeable/dischargeable three-dimensional battery.
Specifically, the three-dimensional battery is formed in conformity to a
voltage of equipment requiring a power, and the .power stored in the
three-dimensional battery is supplied for a short time.
As a result, in the equipment activated with a DC of a relatively small
voltage, a required power can be supplied from the three-dimensional battery
when the power source stops the supply of power for a moment, and
consequently, the electric equipment continues to be activated when a
commercial power~source stops supply of the power for a moment, the power
source is switched, or a power source pug is discharged. In particular,
electric
troubles in equipment activated with a small power such as a personal computer
or an electric watch can be satisfactorily dealt with.
Fig. 40(a) is a cross-sectional view showing a normal power line, Fig. 40(b)
is
a cross-sectional view of the power line in which the chargeable/dischargeable
three-dimensional battery is built, and Fig. 40(c) is a schematic flow diagram
showing an example in which a power is supplied to a terminal device from the
power line in which the chargeable/dischargeable three-dimensional battery is
built.
In Fig. 40(a), reference numerals 321, 322 denote power lines. In Fig.
40(b), reference numeral 323 denotes a power line corresponding to a cathode
current collector and reference numeral 324 denotes an electric line
corresponding to an anode current collector. A plurality of conductive current
collecting members 325 are interposed between the cathode current collector
323 and the anode current collector 324 to form a plurality of cells. Each
cell
CA 02508964 2000-03-27
72
is divided into two parts by an ion-permeable separator 326. A cell portion of
the divided cells and close to the cathode current collector is filled with a
cathode
powdered active material and an electrolytic solution 327 and a cell portion
of
the divided cells and close to the anode current collector is filled with an
anode
powdered active material and an electrolytic solution 328.
In Fig. 40(c), reference numeral 329 denotes a AC I00 V power supply;
reference numeral 330 denotes an AC 100 V power line, reference numeral 331
denotes a rectifier, reference numeral 332 denotes a power line in which the
three-dimensional battery is built, and reference numerals 333 denotes a
personal computer. For example, if a powdered active material of 10 gr is
filled
in the power line 332, a nickel hydrogen battery is capable of feeding a DC
current at 7.2V and lA for 400 seconds.
(Electric Pole)
Cables are provided in an upper portion of an electric pole for the
purpose of carrying a power. However, a structure itself of the electric pole
is
not efficiently utilized.
Accordingly, the electric pole is configured to have a structure of a
chargeable/dischargeable three-dimensional battery.
Specifically, the power is supplied from a commercial power supply
during a normal state and from the three-dimensional battery during electric
power failure.
As a result, the power can be supplied without interruption during the
electric power failure of the commercial power supply.
Fig. 41 is a cross-sectional view showing an electric pole in which the
chargeable/dischargeable there-dimensional battery is built. In Fig. 41,
CA 02508964 2000-03-27
73
reference numeral 341 denotes a ground surface, reference numeral 342
denotes a cathode, and reference numeral 343 denotes an anode. A plurality of
current collecting members 344 are interposed between the cathode and anode.
Each cell defined by the current collecting members 344 is divided into two
parts
by an ion-permeable separator 345. A cell portion of the divided cells and
close
to the cathode current collector is filled with the cathode powdered active
material and an electrolytic solution 346 and a cell portion of the divided
cells
and close to the anode is filled with the anode powdered active material and
an
electrolytic solution 347.
(Equipment for Converting Power Stored in Three-Dimensional Battery into
Photo Energy, Kinetic Energy, or Heat Energy)
(Electric Bulb)
In general, an electric bulb is adapted to be lighted in such a manner
that a glass case including a filament therein is connected to a metallic
case, and
a power is supplied to the filament via the metallic case. As should be
understood, to light the electric bulb, a external power supply is required.
Accordingly, powdered active materials are filled in the metallic case of
the electric bullet to form a chargeable/dischargeable three-dimensional
battery.
Specifically, a terminal of the three-dimensional battery and a filament
terminal of the electric bulb are shorted, thereby lighting the electric bulb.
As a result, the electric bulb can be lighted without the use of the
external power supply.
Fig. 42 is a cross-sectional view showing an electric bulb in which the
chargeable/dischargeable thl-ee-dimensional battery is built. In Fig. 42,
reference numeral 351 denotes a cathode current collector, 352 denote an anode
CA 02508964 2000-03-27
74
current collector, and reference numeral 353 denotes an ion-permeable
separator. A cathode powdered active material and an electrolytic solution 354
are filled between the cathode current collector 351 and the separator 353 and
an anode powdered active material and an electrolytic solution 355 are filled
between the anode current collector 352 and the separator 353. Reference
numeral 356 denotes a filament, reference numeral 357 denotes a filament
terminal, reference numeral 358.denotes a battery cathode terminal, and
reference numeral 359 denotes a charging jack. Since one end of the filament
356 is internally connected to the anode current collector 352 of the battery,
the
filament terminal 357 and the battery cathode terminal 358 are shorted,
thereby
lighting the electric bulb.
(Flashlight)
In general, in a flashlight, a battery is put in a tubular case with a power
switch to light an electric bulb: Since the flashlight has a dual case
structure in
which a battery case is put into the case of the flashlight, it is voluminous
and
heavyweight.
Accordingly, the case of the flashlight is utilized as a current collector
and powdered active materials and electrolytic solutions are filled in the
case, to
form a chargeable/dischargeable three-dimensional battery.
Specifically, the case of the flashlight is utilized as a housing of the
three-dimensional battery.
As a result, the battery put in the conventional flashlight can be
dispensed with and therefore, a~lightweight and small-sized flashlight is
achieved.
Fig. 43 is a cross-sectional view showing a flashlight in which the
CA 02508964 2000-03-27
chargeable/dischargeabke three-dimensional battery is built. In Fig. 43,
reference numeral 36I denotes an electric bulb, reference numeral 362 denotes
a switch, reference numeral 363 denotes a cathode current collector, reference
numeral 364 denotes an anode current collector, and reference numeral 365
denotes an ion-permeable separator. A cathode powdered active material and
an electrolytic solution 366 are filled between the cathode current collector
363
and the separator 365 and an anode powdered active material and an
electrolytic solution 367 are filled between the anode current collector 364
and
the separator 365.
(Huge Meteor Orbit Changing Device)
There has been proposed a method for shooting a metallic bullet placed
in two rails into a huge meteor by using a power of a lead battery as an
energy
to change an orbit of the meteor, as a device for changing the orbit of the
huge
meteor. The energy for shooting the bullet is actually short.
Accordingly, a chargeable/dischargeable three-dimensional battery with
a large current is formed around a ground surface.
Specifically, the large current stored in the three-dimensional battery is
changed into a kinetic energy and an energy with which the metallic bullet is
shot from a rail gun into the meteor can be significantly increased.
Fig. 44(a) is a longitudinal cross-sectional view showing the
chargeable/dischargeable three-dimensional battery formed around the ground
surface. In Fig. 44(a), reference numeral 371 denotes. a ground surface,
reference numeral 372 denotes a cathode, and reference numeral 373 denotes
an anode. A plurality of conductive current collecting members 374 are
interposed between the cathode 371 and the anode 372. F~ach cell defined by
CA 02508964 2000-03-27
76
current collecting members 374 is divided into two parts by an ion-permeable
separator 375. A cell portion of the divided cells and close to the cathode is
filled with the cathode powdered active material and an electrolytic solution
376
and a cell portion of the divided cells and close to the anode is filled with
the
anode powdered active material and an electrolytic solution 377.
Fig. 44(b) is a schematic view showing an example of a structure of a
metal bullet shooting device using a rail gun. In Fig. 44(b), reference
numeral
378 denotes a chargeable/dischargeable three-dimensional battery, reference
numeral 379 denotes a metallic bullet, reference numeral 380 denotes an H-type
steel brush corresponding to a cathode and reference numeral 381 denotes an
H-type steel brush corresponding to an anode. For example, if the
three-dimensional battery having the structure of Fig. 44(a) is formed over a
region of 10 km, square, then a power of ( 105 V X 1013 A) can be stored. With
this power, a magnetic field of (0. 5 X 1018 W) is formed from an airy region
to a
ground surface and an electromagnetic power is given to the metallic bullet.
Specifically, a force of 1035 N 'is applied to the rail composed of the
brushes 380,
381 and having a width of l Om and a bullet made of nickel having a diameter
of
50m and a length of 100m can be shot at an accelerated speed approximately
1 / 10000 time as high as a velocity of light. Consequently, almost all the
meteors can be shot down.
(Melting Device)
A melting furnace in which various materials are melted is provided with
a large-power supplying equipment which costs a lot.
Accordingly, a chargeable/dischargeable three-dimensional battery with
a high output and a small capacity is provided in the melting furnace.
CA 02508964 2000-03-27
77
Specifically, the three-dimensional battery is charged by appropriate
power generating means and a high-output and small-capacity power stored in
the three-dimensional battery is supplied to the melting furnace when a
material
is melted. An electric energy of the power is converted into a heat energy to
be
used for melting the material.
Thus, the material can be melted in a relatively small power supplying
equipment.
3. Embodiments of Fourth Invention
(First Embodiment)
Fig. 45 is a schematic view showing a structure of an alkali primary
battery according to a first embodiment of the fourth embodiment As shown in
Fig. 45, an anode cell 392 and a cathode cell 393 are provided with an
ion-permeable separator 391 interposed therebetween. An anode powdered
active material and an electrolytic solution 394 are filled in the anode cell
392
and a cathode powdered active material and an electrolytic solution 395 axe
filled
in the cathode cell 393. Powdered iron carbide is used as powdered anode
material and may be replaced by a powdered mi~rtwe of iron carbide and iron.
The iron carbide refers to an iron carbide product at least partially having a
chemical composition of Fe3C. The iron carbide can be produced by a method
disclosed in Japanese Laid-Open Patent Publication No. Hei. 9 -48604 filed by
the applicant, but when an iron-containing material is reduced and carburized
to produce the iron carbide, it is not necessary to use the iron carbide
product
with all components of the iron-containing mateiial converted into the iron
carbide. This is because the more a carburized portion contained in the iron
carbide is, the higher conductivity is obtained, but a producing cost of the
iron
CA 02508964 2000-03-27
78
carbide product including much carburized portion with high conversion rate is
high. With this regard, when Fe3C composition of the iron carbide product is
more than 5 atom %, required conductivity as an anode powdered material can
be ensured. In addition, the producing cost can be relatively low.
A powdered mixture of manganese dioxide and carbon is used as the
cathode powdered active material. A potassium hydride aqueous solution, is
used as the electrolytic solution in the anode cell 392 and the cathode cell
393.
The separator 391 serves as an ion-permeable membrane and does not
serve as a powder-passing membrane. As the separator 391, for example, an
unglazed pottery, an ion exchange resin membrane, metal fibers, a non-woven
fabric cloth, or the like may be used. An anode current collector 396
comprising a conductor and a cathode current collector 397 comprising .a
conductor are respectively provided in the anode cell 392 and the cathode cell
393. The current collectors 396, 397 are connected to load means 398. The
current collectors 396, 397 are preferably made of metal which is not corroded
in
an alkali solution, and for example, a plate comprising carbon steel plated
with
nickel can be used.
Subsequently, discharge of an alkali primary battery according to the
first embodiment of the fourth invention will be described in detail.
When the battery is connected to the load means 398, the anode current
collector 396 discharges an electron to an external circuit. The discharged
electron travels from the anode current collector 396, through the load means
398, and to the cathode current collector 397. The electron reacts with the
cathode powdered active material immediately on the cathode current collector
397 or while traveling through the powdered material. An anion generated by
CA 02508964 2000-03-27
79
the fact that the cathode powdered active material has received the electron
passes through the separator 391 and enters the anode cell 392, where it
reacts
with the anode powdered active material and discharges the electron. The
electron travels to the anode current collector 396 immediately or through the
powdered material and is supplied to the load means 398. This cycle is
repeated.
The above-described discharge reaction is represented by a chemical
reaction formulae for an anode side and a cathode side as follows:
(Anode) Fe + 20H- -~ Fe (OH)2 + 2e'
(Cathode) Mn02 + H20 + e- --> MnOOH + OH-
Fig. 45 only illustrates a schematic structure of the alkali primary
battery and may adopt a variety of structures such as a cylindrical or layered
structure.
(Second Embodiment)
Fig. 46 is a schematic view showing a structure of an alkali secondary
battery according to a second embodiment of the fourth invention. The
components identical to those of Fig. 45 are referenced to by the same
reference
numerals, and is not described in detail. The difference between the
constitution of Fig. 45 and the constitution of Fig. 46 is that a powdered
mixture
of nickel hydroxide and carbon is used as a cathode powdered active material
and fluid fluidizing and dispersing means 399, 400 are used in the
constitution
of Fig. 46. In addition to this, in Fig. 46, the load means 398 is replaced by
load
means (for discharge) or power generation means (for charge) 401.
Herein, to increase efficiency of contact between powdered materials or
between the powdered materials and the current collectors 396, 397 in the
CA 02508964 2000-03-27
anode cell 392 and the cathode cell 393, a gas or liquid is supplied into the
respective cells 392, 393 from the fluid fluidizing and dispersing means 399,
400.
Instead of or along with the fluid fluidizing and dispersing means 399, 400,
agitating means such as vane-like agitators may be provided in the respective
cells 392, 393 to fluidize the powdered materials.
Subsequently, charge of an alkali secondary battery according to the
second embodiment of the fourth invention is described but discharge thereof
is
not described because the discharge is identical to that of the alkali primary
battery.
When the battery is connected to the power generation means 401, an
electron is discharged from the power generation means 401 and reaches the
anode current collector 396. The electron reacts with the anode powdered
active material immediately on the anode current collector 396 or while
traveling
through the anode powdered active material. An anion generated by the fact
that the anode powdered active material has received the electron passes
through the separator 391 and enters the cathode cell 393, where it reacts
with
the cathode powdered active material and discharges the electron. The
electron moves to the cathode current collector 397 immediately or through the
powdered material and is supplied to the power generation means 401. This
cycle is repeated.
The above-described charge and discharge reactions are represented 'by
chemical reaction formulae for an anode side and a cathode side as follows:
(Anode) Fe + 20H' -~~ Fe (OH)2 + 2e'
(Cathode) Ni00H + H20 + e' -~ N i (OH)2 + OH'
(Whole Battery) Fe + 2Ni00H + 2H20 --~ 2Ni (OH)2 + Fe (OH)2
CA 02508964 2000-03-27
gI
In the above formulae, an arrow indicating right represents a discharge
reaction and an arrow indicating left represents a charge reaction.
Fig. 46 only illustrates a schematic structure of the alkali secondary
battery and may adopt a variety of structures such as a cylindrical or layered
structure.
( Discharge Curve )
Fig. 47 is a view showing an eXample of a discharge curve of the alkali
secondary battery (nominal capacity: 3Ah) of the fourth invention. In Fig. 47,
a
longitudinal axis indicates a terminal voltage (~ and a lateral axis indicates
a
discharge capaaty (Ah). In the alkali secondary battery, powdered iron carbide
(about 30 atom % of the ion-containing material is iron carbide) is used as an
anode active material and a powdered mixture of nickel hydroxide and carbon is
used as a cathode active material. In this case, nitrogen is introduced into
the
cells by the fluid fluidizing and dispersing means 399, 400. As can be clearly
seen from Fig. 47, preferable discharge characteristic is shown without rapid
decrease of the discharge voltage.
4. Embodiments of Fifth Invention
Fig. 48 schematically shows a device that carries out a locally-distributed
power generation method according to a first embodiment of the fifth
invention.
In Fig. 48, an automobile 411 comprises an engine 412 such as a gasoline
engine, a diesel engine, and a gas turbine, an electric generator 413,
traveling-source battery (battery) 414 for power storage, and an electric
motor
(motor) 415. The automobile 411 uses the engine 412 to cause the electric
generator 413 to be activated in order to generate a power, which is stored in
the
traveling-source battery 414. The automobile 411 is adapted to travel by the
CA 02508964 2000-03-27
82
engine 412 and by the electric motor 415 driven by the power from the battery
414 during traveling as its original purpose and only by the electric motor
415
when traveling load is less.
In the method and device of the fifth invention, the automobile or the Iike
constituted as described above is utilized as fixed.power generation equipment
for houses and offices when it is not moving. It should be noted that it is
possible to use an automobile with a device that generates a power by using a
fuel battery instead of the device that uses the engine to activate the
electric
generator to generate the power. A power-driven two-wheeled vehicle,
power-driven three-wheeled vehicle, ship or the like, as well as a power-
driven
four-wheeled vehicle may be employed so long as it has a similar function.
As shown in Fig. 48, when automobile 411 is put in a car barn of a house
416, a fixed battery (battery) 418 installed in the house 416 is connected to
the
traveling-source battery 414 mounted in the automobile 411 by means of a
connecter 417. Thereby, the power generated by rotation of the power
generator 413 using the engine 412 is supplied to the fixed battery 418 and
charged therein. The power from the fixed battery 418 is converted into AC and
its voltage is adjusted by an inverter 419 and used in the loads 420. A
commercial power supply (not shown) is connected between the inverter 419 and
the loads 420. Or otherwise, the commercial power.supply may be directly
connected between the fixed battery 418 and DC load and used.
When the battery capacity of the traveling-source battery 414 is reduced,
the engine 412 is activated and the electric generator 413 is rotated for
charge.
In this case, to lower the noise of an engine emission, a silencer may be
outerly
provided on an exhaust tube of the automobile 411.
CA 02508964 2000-03-27
83
As shown in Fig. 48, when wind power generation equipment or solar
power generation equipment is installed in the house 416, that is, the power
generated by a wind power generator 421 or a solar battery 422 is supplied to
the
fixed battery 418, the power can be used in the load 420 together with the
power
from the traveling-source battery 414. When the wind power generation
equipment and the solar power generation equipment are installed in the house
independently or in combination, a large-capacity battery (battery) becomes
necessary, and equipment cost is increased. On-the other hand, the power is
supplied from the battery mounted in the automobile, or the like, the battery
(fixed battery 418) to be installed in the house becomes. small. Consequently,
the equipment cost can be significantly reduced.
When the battery capacity of the traveling-source battery 414 is small
and the power generated by the wind electric generator 421 or the solar
battery
422 is greater than the power consumed by the loads 420, the traveling-source
battery 414 can be charged with the power stored in the fixed battery 418.
In this embodiment, the wind power generation equipment or the solar
power generation equipment is installed in the house 416. A wind power and
solar light are optionally utilized, and the wind power electric generator
421, the
solar battery 422 and the fixed battery 418 can be dispensed with. The
installment of at least the inverter 419 is satisfactory. The power of the
automobile can be used in houses by connecting the inverter 419 to the
traveling-source battery 414 by means of the connector 417 or the like.
In this embodiment, onty power equipment is explained. A heat energy
generated in air-conditioning equipment, a radiator, or the like of the
automobile
or the like is utilized in the house to perform cogeneration. For example,
warm
CA 02508964 2000-03-27
84
air, cool air, or the like can be supplied from the air-conditioning
equipment, the
radiator, or the like of the automobile or the like, through a duct, and to
the
house, and utilized for air-conditioning in the house. The heat energy
generated in air-conditioning equipment, a radiator, or the like of the
automobile
or the like can be utilized in a tent or cottage outside, which is irrelevant
to the
cogeneration.
As mentioned previously, the conventional house cogeneration
equipment is costly and is unpayable if not used for a long time period.
Although the state tried to pay half of the equipment cost of the solar power
generation, which was economically unsuccessful, and a great deal of budget
was surplus. Accordingly, by utilizing the power energy generated from the
automobile or the like as transfer and transport means for the house instead
of
installing the conventional cogeneration equipment independently, house
equipment cost can be significantly reduced and the distributed-type power
generation can be developed.
In the automobile or the like in which a battery for power storage is
mounted together with the device that uses the engine to activate the electric
generator to generate the power, or the device that generates the power by the
fuel.battery, the power amount of the battery is several tens kW hr; and~is
sufficient as the power consumed in one house. When people go outside, they
often use automobiles. In such cases, power supply is performed depending on
whether or not the automobile is moving, by selectively using the
traveling-source power or the fixed-type power.
For example, if 3 million yen is paid to purchase private power generation
equipment, this is uneconomical in view of difference between 3 million yen
and
CA 02508964 2000-03-27
g5
a purchasing price of the power. However, if 3 million yen is paid to purchase
an automobile, this is economical because the automobile can be used as
transfer and transport means as its original purpose as. well as the power
generation equipment.
The traveling-source battery 414 and the fixed battery 418 may be
batteries of the three-dimensional structure in which powdered active
materials
are used on the cathode side and the anode side as shown in Figs. 1 to 12.
Thus, the three-dimensional battery is preferable because, when part of or all
of
the degraded powdered materials is discarded, and the degraded powdered
material is recovered by the recovery unit 27, and new powdered materials
equivalent in amount to the discarded powdered materials are supplied to a
vessel as shown in Fig. 10 according to the seventh embodiment of the first
invention, charging can be started immediately.
While this embodiment has been described with regard to houses, the
same is the case with othces.
CA 02508964 2000-03-27
86
The battery of improved charging/discharging characteristic without fluidizing
the
powdered active materials or without equipment for fluidizing the powered
active materials.
The improvements thereof are as follows:
( 1 ) Scale up is achieved.
The current flowing through the battery is directly proportional to the
surface area of a
reacting material. Accordingly, by using the powdered active materials, the
battery
comprising the powdered materials in the vessels can be created. The battery
is three-
dimensionally structured by using the powdered active materials. For example,
in case of the
battery having a volume of 1 liter and a power of 1W, if it is scaled up to
1m3, lOm3, and
100m3, the corresponding powers are respectively lkW, 1000kW, and 1 million
kW.
In addition, when the powdered active materials are used to create the
battery, scale
up becomes advantageous. For example, if the conventional battery of 1kW costs
100
thousand yen, then, one million batteries are required to obtain 1 million kW
and costs 100
billion yen. On the other hand, in the battery of the present invention, the
scale up results in
advantages, i.e., a reduced production cost of about 100 million yen.
(2) The degraded active material and catalyst can be recovered and replaced.
When the powdered active materials and catalyst are degraded, they are
discharged,
and recovered or replaced by new active materials and catalyst, or
CA 02508964 2000-03-27
g7
otherwise, they are re-charged by thermal reaction or chemical reaction, to be
re-supplied. For example, the powdered active material and catalyst are
discharged as a slurry together with the electrolytic solution through a pipe
from
the vessel. Then, the powdered active material is separated from the
electrolytic
solution and re-mixed with the electrolytic solution after recovery or
addition of
new materials, to. be created into the slurry, which is supplied to the
battery by a
slurry pump.
For example, the conventional small-sized battery is capable of charging
and discharging about 500 times, and the conventional large-sized battery is
activated for about 8000 consecutive hours. On the other hand, since in the
battery of the present invention, the active material and the catalyst are
kept in
best conditions by circulation and recovery or make up of the active material
and
catalyst, the life of the battery, and hence, the life of the battery
equipment can
be prolonged 50 to 100 times.
(3) Heat transmitters can be provided in the battery.
The battery has a simple structure in which the powdered active material
and catalyst are suspended in the electrolytic solution. By utilizing a
battery
characteristic in which a heat transmitter is easy to provide therein, heat
transmitted through the heat transmitter provided in the battery can keep
reaction temperature in the battery constant, and power conversion efficiency
is
reduced with an increase in temperature, whereas reaction speed is reduced
with a decrease in temperature, the temperature in the battery can be
appropriately adjusted. Besides, since high-temperature substances and
Iow-temperature substances collected through the heat transmitter can be
utilized for air-conditioning or power generation, energy generation
efficiency
CA 02508964 2000-03-27
g8
and energy usage efficiency can be increased.
(4) Energy density can be increased.
The current flowing through the battery is directly proportional to the
surface area of the reaction material. Accordingly, the powdered active
materials are used to create the battery. The creation of the battery using
the
powdered active materials increases the surface area. For example, the
powdered material of 1 m3 has a surface area of 300000 m2 and has an
increased energy density. Also, if the conventional battery has a membrane
area of 1 m2 and a power of 1 W, then 3 million membrane batteries each having
an area of 1 m2 and a width of O.lm are required to create a battery of 3000
kW
and has a volume of 300000 m3. If the battery of the present invention uses a
powdered material having a particle diameter of 1 a m to obtain the same
power,
then it has a volume of about 10 m3 and has an energy density made 30000
times higher. Thus, the energy density can be significantly increased.
CA 02508964 2000-03-27
89
In the three-dimensional battery, the capacity (power) of the battery can be
increased
by increasing capacities of the respective cells of the pair of cells.
Assuming
that a capacity of 1 liter generates a power of 1W, then power of 1kW can be
obtained by increasing the capacity to 1 m3 and a power of l OkW can be
obtained by increasing the capacity to 10 m3. The scale up results in
advantages in the production cost. Spec~cally, if the conventional battery of
lOW costs I O thousand yen, then the battery of l OkW costs ZO million yen. On
the other hand, since the production cost of the battery of the present
invention
is reduced with the scale up, the battery cost of the present invention of
about 1
million yen equals about 1 / 10 of the conventional battery.
On the other hand, the voltage is determined depending on the type of
powdered active materials (corresponding to the conventional general
electrodes)
filled in the pair' of cells. For example, when powdered metallic lead and
powdered lead oxide are used, approximately 2.4V voltage is generated. So, it
is
necessary to connect 5 to 6 unit batteries in series to obtain 12V or more. .
However, according to the second invention, unit batteries situated at
intermediate position (except opposite end positions) can use current
collecting
members made of the same material on the anode side and on the cathode side.
Since the cathode and anode electrodes need not be provided differently from
the
conventional battery, separating walls defining a pair of cells (unit battery)
are
constituted by conductive current collecting members to enable structural and
CA 02508964 2000-03-27
electrical series connection. The separating wall is configured to have a
considerably small thickness (e.g., 0.5mm) and a large area (e.g., 127 mm X
127 mm). In addition, the current flows in the thickness direction of the
separating wall. Therefore, a large current flows with little resistance and a
power loss is very little. F~u-ther, since the two pairs of unit batteries can
be
directly connected by means of the separating walls, plural pairs of unit
batteries
can be connected in series and in layers. Thereby, the whole battery is
configured to have a minimum capacity and made small.
Furthermore, in the three-dimensional battery ,
the powdered active materials function as a membrane (battery body) of the
conventional battery of a membrane structure and the current flowing through
the battery is directly proportional to the surface area of the active
materials.
Since the powdered active materials are suspended in the electrolytic solution
and occupy most of the volume of the battery casing, the energy density can be
greatly increased. Also, since the powdered active materials are put into the
electrolytic solution (dilute sulfuric acid for lead storage battery), and are
mixed
and suspended therein, the powdered active materials are separated from the
electrolytic solutions or replaced together with the electrolytic solutions
for
recovery when degraded. The life of the battery can be significantly
(approximately 50 to 100 times) prolonged.
It is preferable in the three-dimensional battery
that agitating means would be provided in each of the cells to fluidize the
powdered active material suspended in the electrolytic solution when a large
power is required. The agitating rizeans includes means for mechanically
agitating the powdered active materials using a rotational shaft with
agitating
CA 02508964 2000-03-27
91
vanes that is rotatably provided in the cells by a drive unit such as a motor
or
means for dispersing and fluidizing the powdered materials in the electrolytic
solution by supplying or circulating a liquid or a gas into the electrolytic
solution
by means of a pump or a blower. In the three-dimensional battery, the
agitating
means agitates the powdered material in the electrolytic solution to be
dispersed
therein, thereby improving efficiency of contact between the active materials,
reducing contact resistance because of preferable contact between the powdered
- ~ materials and the current collecting members or the current collectors ,
increasing conductivity, and increasing ion dispersion speed in the
electrolytic
solution. Consequently, a large current flows and a large power can be
obtained. In addition, a width of each cell (spacing in a series direction)
can be
increased and the capacity of the battery can be increased.
In the three-dimensional battery , conductive
studs may be provided integrally with and protrusively from the current
collecting members or the current collectors toward inside of the respective
cells.
In this three-dimensional battery, since contact areas between the current
collecting members or the current collectors and the powdered materials are
greatly increased, and the contact resistance is reduced, the width of each
cell
(spacing in the series direction) can be enlarged, and~the capacity of the
battery
can be greatly increased.
It is preferable that in the three-dimensional battery of the second
invention, a function for stopping fluidization of the powdered active
material to
reduce amount of a power supplied from the battery would be added to the
agitating means. By addition of the function to stop fluidization of the
powdered materials to the agitating means like. this three-dimensional
battery,
CA 02508964 2000-03-27
92
the fluidization of the powdered materials can be arbitrarily stopped, and,
consequently, ~e amount of a power from the battery can be reduced.
It is preferable that in the three-dimensional battery, the powdered active
material that discharges the electron would be hydrogen-occluding alloy,
cadmium, iron, zinc or lead, because these materials are inexpensive and
practicable. Farther, it is preferable that in the three-dimensional battery
of the
second invention, the active material that absorbs the electron would be
nickel
oxyhydroxide, lead dioxide, or manganese dioxide, because these materials are
inexpensive and practical.
CA 02508964 2000-03-27
93
In the alkali primary battery and the alkali secondary battery, since carbon
is a good
conductor of electricity, preferable electricity conductivity can be ensured,
and degradation of
a discharging characteristic (reduction of a discharge voltage) can be
suppressed even if
metal of the anode active material is chemically changed into oxide or
hydroxide. By a
simple method that uses metal carbide or the mixture of the metal carbide and
this metal as
the anode active material, expensive conduction promoter such as high-purity
carbon and a
special treatment for adding conductivity to the anode become unnecessary and
a production
cost can be suppressed.
It is preferable that the cathode active material and the anode active
material would be
powdered. The reason is that since the battery structure becomes three-
dimensional, the scale
up results in advantages (scale up reduces a production cost), the degraded
active material
can be recovered and replaced, and heat transmitters can be provided in the
battery, the
operation according to the battery characteristic becomes possible and the
energy power
generation efficiency can be improved. In addition, the surface area is
increased and the
energy density is increased.
Furthermore, it is preferable that the iron carbide is used as the metal
carbide. The
metal carbide is an inexpensive material. As disclosed in Japanese Laid-Open
Patent
Publication No. Hei. 9-48604 filed by the applicant, the iron carbide is
produced in such a
manner that iron-containing material is partially reduced using a reducing
gas, and then the
partially reduced material is further reduced and carburized using reducing
and carburizing
gases. This method is particularly preferable because the iron carbide can be
produced
promptly and economically.
Since the battery is structured to have powdered active materials put in the
vessels, it
has a three-dimensional structure and can be scaled up. By creating the
battery using the
powdered active materials, the scale up advantageously reduces the production
cost.
When the powdered active material and catalyst are degraded, they are
discharged and
recovered or replaced by new active materials and catalyst. Or otherwise, they
are re-charged
by thermal reaction or chemical reaction to be re-supplied. Thereby, since the
active material
and catalyst are always kept in best condition, the life of the battery, and
hence the life of the
battery equipment can be significantly prolonged.
CA 02508964 2000-03-27
94
By utilizing a battery characteristic in which a heat transmitter can be
provided, the heat transmitter provided in the battery can keep reaction
temperature in the battery constant, and power conversion efficiency is
reduced
with an increase in temperature, whereas a reaction speed is reduced with a
decrease in temperature, the temperature in the battery can be appropriately
adjusted. Besides, since the collected high-temperature substances and
low-temperature substances can be utilized for air-conditioning or power
generation, energy generation efficiency and energy usage efficiency can be
increased.
Since the battery is created by using the powdered active materials, the
surface area of the reacting material is increased and the energy density is
significantly increased.
Since at least one of fluid fluidizing and dispersing means and agitating
means using a liquid or a gas for fluidizing the powdered active materials in
the
electrolytic solutions in the two vessels may be connected to the two vessels
or
provided in the two vessels to provide efficient contact between the powdered
active materials and between the powdered active materials and the current
collectors. With this constitution, efficiency of contact between the active
materials is improved, contact resistance is reduced because of preferable
contact between the powdered active materials and the current collectors, and
conductivity between the active materials and the current collecto~r~s or
between
the active materials is increased, and the ion dispersion speed in the
electrolytic
solution is increased. Consequently, a large current flows and a large power
can be obtained as compared to the battery comprising the unfluidized
powdered active materials.
CA 02508964 2000-03-27
Since the capacity (power) of the battery can be increased by increasing the
capacities of the respective cells of a pair of cells, the scale up results in
advantages in the production cost. The voltage is determined depending on the
type (material) of the powdered active materials filled in the pair of cells.
It is
necessary to connect a plurality of unit batteries in series when a large
voltage is
required. Since the current collecting members on the anode side and the
cathode side of the unit battery are made of the same material, and anode and
cathode electrodes are not formed unlike the conventional battery, separating
walls defining the pair of cells (unit battery) may be constituted by the
conductive current collecting members. Thereby, the batteries can be
connected in series structurally and electrically and the thickness thereof
can be
made small. As a result, the whole battery can be made compact and
small-sized. In addition, since the current flows in the thiclmess
direction,'a
large current flows with little resistance.
The powdered active materials function as a membrane (battery body) of
the conventional battery of the membrane structure and the.current flowing in
the battery is directly proportional to the surface area of the active
materials.
The powdered materials are suspended in the electrolytic solutions and the
total
surface area_of the total powdered materials is several thousands to several
tens
thousands times as large as that of the conventional battery of the membrane
structure. So, the energy density is made several thousands to several ten
thousands higher. Also, the powdered active materials are mixed in and
suspended in the electrolytic solutions (dilute sulfuric acid for lead storage
battery). When the powdered active materials are degraded, ~ the powdered
CA 02508964 2000-03-27
96
active materials together with the electrolytic solutions can be changed and
the
powdered active materials can be recovered. Consequently, the life of the
battery can be significantly prolonged.
By providing agitating means for fluidizing the powdered materials
suspended in the electrolytic solutions in the respective cells to agitate the
powdered materials in the electrolytic solutions, the powdered materials as
electrodes are prevented from falling down due to its weight, and diffused in
the
electrolytic solutions. As a result, contact efficiency between powdered
materials is improved and preferable contact between the powdered materials
and the current collecting members or the current collectors is obtained,
resulting in reduced contact resistance and an increased power. l~trther,
width of each cell (spacing in the series direction) is increased and the
capacity of
the battery can be increased.
By providing conductive studs integrally with and protrusively from the
current collectors or the current collecting members toward the inside of the
cell,
the contact areas of the current collecting members and the powdered materials
or the contact areas of the current collectors and the powdered.materials are
significantly increased and contact resistance is reduced. Therefore, the
width of
each cell (spacing in the series direction) can be increased and the capacity
of the
battery can be significantly increased.
By addition of the function to stop fluidization of the powdered materials to
the agitating means to reduce the amount of power supplied from the battery,
the fluidization of the powdered materials can be arbitrarily stopped,
resulting in
a reduced amount of the power fi-om the battery.
CA 02508964 2000-03-27
97
It is possible to provide practical and effective use of the three-dimensional
battery as part of various equipment or devices. Specifically, by adding the
function of the chargeable/dischargeable power storage' equipment in addition
to the original function of the equipment or device, a free space is utilized
to store
a large power and the power storage efficiency can be greatly increased.
Fl~rther, the absorbed/released heat associated with the battery reaction can
be
utilized for air-conditioning, or heating, cooling or the like of the
materials.
In the three-dimensional battery comprising two vessels provided with
conductive current collectors in contact with the powdered active materials
suspended in the electrolytic solutions, at least one of fluid fluidizing and
dispersing means and agitating means using a liquid or a gas for fluidizing
the
powdered active materials in the electrolytic solutions in the two vessels may
be
connected to the two vessels or provided in the two vessels. Thereby,
preferable
contact between the powdered active materials and the current collectors is
provided and contact resistance is thereby reduced, resulting in improved
conductivity and increased ion diffusion speed in the electrolytic solutions.
Consequently, a Iarge current flows and a Iarge power can be stored.
Furthermore, the power stored in the three-dimensional battery is
conveyed by power conveying means to be utilised as rotation power of rotary
equipment, power of a mobile body, or photo energy, kinetic energy or heat
energy.
Without adding expensive conduction promoter such as high-purity carbon
to the anode active materials and.a special treatment for adding conductivity
to
the anode, it is possible to provide the alkali primary battery and the alkali
CA 02508964 2000-03-27
98
secondary battery which have discharge voltages less likely to be reduced,
have
long lives, and are produced at a low cost.
When the cathode active material and the anode active material are
powdered, the battery structure becomes three-dimensional, the scale up
results
in advantages (scale up reduces a production cost), the degraded active
material
can be recovered and replaced, and heat transmitters can be provided in the
battery. Therefore, the operation according to the electric characteristic
becomes possible and the energy power generation efficiency can be improved.
In addition, the surface area is increased and the energy density is
increased.
Iron carbide as metal carbide is inexpensive and is particularly preferable
as the anode active material.
By utilizing a power generation system provided in automobile or the like
originally used as transfer and transport means for houses or offices, the
equipment cost can be significantly reduced and cogeneration can be carried
out
without the power generation equipment in the houses or the offices.
Since the power generation equipment cost is significantly reduced and the
power generation equipment is economical, the locally-distributed cogeneration
equipment can be generalized.
Since the locally-distributed cogeneration equipment becomes inexpensive
and is generalized, the effective use of the energy is facilitated. As a
result,
economical effect is obtained and generation of carbon dioxide can be reduced.
In particular, since the battery mounted in the transfer means and
transport means and the battery fixed to the houses or the o~ces are
constituted
by the battery comprising the powdered active materials on the cathode side
and
CA 02508964 2000-03-27
99
the anode side, part or all of degraded powdered active materials are
discarded, the degraded
powdered materials are recovered, and new powdered materials equal in amount
to the
discarded powdered materials are supplied. As a result, charge can be started
immediately.
(Industrial Applicability)
The present invention is constituted as described above, and is therefore
suitable as a
battery of a three-dimensional structure comprising powdered active materials
and capable of
storing a large power, and equipment or device having the battery as part of
its structure, and
an alkali primary battery and an alkali secondary battery of long lives in
which discharge
voltages are less likely to be reduced, and a locally-distributed power
generation device
which utilizes a power of transfer and transport means such as a power-driven
two-wheeled
vehicle, a power-driven three-wheeled vehicle, a battery-wheeled four-wheeled
vehicle, ship,
or the like.
CA 02508964 2000-03-27
1~
1, 43, 96, 104, 114, 123, 135, 143, 155, 163, 175, 205, 211, 225, 232, 244,
254, 264, 274, 326, 345, 353, 365, 375, 391...ion-permeable separator, 2,
55, 392...anode cell, 3, 54, 393...cathode cell, 4, 9, 8, 106, 116, 125, 139,
145, 157, 165, 177, 208, 214, 228, 235, 246, 256, 266, 276, 328, 347, 355,
367, 377, 394...anode powdered active material and electrolytic solution, 5,
97, 105, 115, 124, 137, 144, 156, 164, 176, 207, 213, 227, 234, 245, 255,
265, 275, 327, 346, 354, 366, 376, 395...cathode powdered active material
and electrolytic solution, 6, 396... anode current collector, 7, 397.. .
cathode
current collector, 8...load means or power generation means, 9, 399,
400...fluid fluidizing and dispersing means, 10...electrolytic solution
interface, 1 l...plate-shaped anode current collector, 12...plate-shaped
cathode current collector, 13...tubular anode current collector,
14...tubular cathode current collector, 15...anode current collector and
dispersing unit, 16...cathode current collector and dispersing unit,
17...anode current collector and agitator, 18...cathode current collector and
agitator, 19...fluid fluidizing and dispersing unit, 20...powdered
hydrogen-occluding alloy and electrolytic solution, 21...powdered nickel
hydroxide and electrolytic solution, 22... anode current collector and heat
transmission tube, 23...cathode current collector and heat transmission
tube, 24...anode current collector and heat transmission plate,
25...cathode current collector and heat transmission plate, 26...separator,
27...recovery unit, 28...mixer, 29...make-up powdered material hopper,
30...reactor, 31...fue1 supply tube, 41, 41-1 to 41-5...layered-type
three-dimensional battery, 42...cel1 member, 45, 94, 103, 113, 134, 142,
154, 156, 174, 243, 253, 263, 273, 325, 344, 374...current collecting
CA 02508964 2000-03-27
101
member, 46, 111, 126, 136, 152, 204, 210, 224, 231, 241, 251, 261, 271,
323, 351, 363...cathode current collector, 47, 112, 127, 138, 153, 206, 212,
226, 233, 242, 252, 262, 272, 324, 352, 364...anode current collector,
48...packing, 49...bolt, 56...unit battery, 57, 398...load means,
58...charger, 59, 60, 61...agitating means, n, h, A, B...powdered material
(active material), k, r...electrolytic solution, 71, 72...blower, 81, 82,
83...stud, 91...door housing, 92...cathode terminal, 93...anode terminal,
lOl...bridge pier block, 121...radiator body, 131...roof, I4l...bonnet,
151...asphalt pavement, 162...tableware body, 166...heat generating
element (or cooling element), 171...floor, 172, 342, 372, 380...cathode,
173, 343, 373, 381...anode, 182...cathode cell inner heat exchanger,
185...anode cell inner heat exchanger, 192...trailer, 282...engine electric
generator, 283, 293, 378...three-dimensional battery, 284, 295...electric
motor, 292...electric generator, 301...electric locomotive, 302...power
vehicle, 311...pantograph, 321, 322...power line, 332...power line having
built-in three-dimensional battery, 341...ground surface, 356...filament,
357...filament terminal, 358...battery cathode terminal, 36I...electric
bulb, 379...metallic bullet, 398...load means, 401...power generation
means, 411...automobile, 4I2...engine, 413...electric generator,
414...traveling-source battery, 415...electric motor, 416...house,
417...connector, 418...fixed battery, 419...inverter, 420...load,
421...wind power generator, 422...solar battery