Note: Descriptions are shown in the official language in which they were submitted.
CA 02509120 2005-06-02
APPARATUS AND METHODS FOR DISPENSING
PRE-FILLED CONTAINERS WITH PRECISELY-APPLIED PATIENT-
SPECIFIC INFORMATION
FIELD
The field relates in general to the dispensing of medication and like products
and, more specifically, to the application of patient-specific information to
containers
used to hold such medication and products.
BACKGROUND
The efficient and accurate dispensing of medication and like products by a
pharmacy is important to the process of fulfilling patient prescription
orders.
Fulfillment of a patient prescription order refers to the process of providing
medication and other articles and things to a patient (or care giver acting
for the
patient) responsive to a prescription. The prescription order may be fulfilled
by any
suitable pharmacy including, for example, retail pharmacies, mail order
outpatient
pharmacies, and hospital/extended care inpatient pharmacies.
A prescription order fulfilled by such pharmacies will typically comprise one
or more prescriptions for medication and may include other articles and
things, such
as nutriceuticals (e.g, nutritional supplements and vitamins), over-the-
counter
("OTC") medications, therapeutics, medication applicators, bandages, tape and
like
items. It is of the utmost importance to fulfill each prescription order such
that the
patient is provided with the correct medication, products, articles and things
of the
highest quality and to do so in a way which is as cost-effective as possible.
One way of fulfilling patient prescription orders for medication and like
items
has been to provide medication in a pre-filled container, such as a bottle,
vial or other
container type. The pre-filled containers are typically loaded by the
manufacturer,
repackager or other supplier. The pre-filled containers are delivered to the
pharmacy
responsible for fulfillment of the patient prescription orders whereupon they
are kept
in stock or inventory without special modification of the pre-filled
containers. The
:wsosoi -1-
i
CA 02509120 2005-06-02
pre-filled containers are a stock item.
The pre-filled containers are provided in various volumetric sizes, such as
75,
120 and 200 cubic centimeter containers. The pre-filled containers are
typically
loaded with a specific quantity of one or more medications or the like. The
medications and like products may be in any suitable form such as tablets,
powders or
liquids.
After loading, a removable safety seal, such as a film or foil seal, may be
applied across the container opening. A cap or other closure is then applied
across
the container opening. The closure can be any suitable type, such as a screw-
on cap
or a snap-fit cap. The closure is typically child-resistant and is replaceable
over the
opening, permitting the pre-filled container to be repeatably opened and
closed to
remove the medication. A tamper-evident neck seal may be applied to the cap.
The pre-filled containers are removed from inventory at the pharmacy when
needed to fulfill a patient prescription order. Typically, a label including
patient-
specific information as required by the prescription order is applied to the
container.
Depending on the resources of the pharmacy, the pre-filled containers may be
removed from inventory by means of an automatic product dispenser or may be
selected from inventory by a pharmacy worker who might retrieve the desired
pre-
filled container from a pharmacy storage location. Labels containing the
patient-
specific information may be applied by an automatic labeler or applied by
hand.
There are many advantages to using pre-filled containers for fulfillment of
patient prescription orders. One very important advantage is that the pre-
filled
containers are provided with detailed information, or indicia, which fully
identifies
the medication held in the pre-filled container. Such information is of
critical value to
pharmacy management, physicians, health care workers and, above all, the
patient.
The information permits proper and efficient handling of the pre-filled
container
throughout the entire distribution process, from point of manufacture (or
repackaging)
to delivery to the patient.
Information provided on the pre-filled containers may include the
manufacturer or supplier name, medication type, medication strength and
description,
lot number, expiration date and a National Drug Code ("NDC") identification
symbol.
Other important infomiation may be provided such as drug interaction notices
and a
2005-06A1 -2-
I
CA 02509120 2005-06-02
photograph, text or other representation or description of the appearance of
the
medication or product in the container. Information on the pre-filled
containers is
regulated by pharmacy and/or Food and Drug Administration regulations. As
regulations change over time, the information on the pre-filled containers
will change
to comply with the new regulations.
The information provided on or with the pre-filled containers is typically in
the form of both human-readable and machine-readable information. Bar codes
consisting of spaced-apart light and dark elements are one example of a form
of
machine-readable information which may be provided on the pre-filled
containers.
Radio Frequency Identification ("RFID") tags are another type of machine-
readable
information which may be associated with pre-filled containers. The
information
provided on or with the pre-filled containers enables pharmacy management to
immediately identify the medication contained in each pre-filled container and
to
easily manage an inventory of such pre-filled containers using commercially
available
inventory management tools.
Another advantage of pre-filled containers is that the use of such containers
enables pharmacy management to provide better, less expensive service to the
patient.
For example, pharmacy management can provide better control over the quality,
consistency and purity of the medication because the pre-filled container
remains
sealed from the point of manufacture or repackaging, up to and including
delivery to
the patient. And, errors associated with selecting an incorrect medication or
miscounting medication are reduced or avoided completely because manual hand
or
machine counting of individual tablets is unnecessary.
Avoidance of manual hand or machine counting of tablets frees the pharmacist
to consult with the patient, thereby providing a higher standard of care. By
reducing
the labor required to fulfill the order, pharmacy management is able to better
control
cost. And, use of pre-filled containers with clear markings and content
descriptions
provides an opportunity for improved control over valuable medication and
product
inventory.
However, there are disadvantages to existing methods of utilizing pre-filled
containers in the prescription order fulfillment process. In order to convert
one of
many identical pre-filled containers for use as a patient-specific container
for
2005.06-01 -3-
I
CA 02509120 2005-06-02
fulfillment of a patient prescription order, it is necessary to affix patient-
specific
information to the container. This is typically accomplished by affixing an
adhesive-
backed label to the pre-filled container. The labels are supplied on a release
liner
(such as wax paper or the like) and are removably affixed to the release
liner. The
infonnation may be affixed to the label in any suitable manner, such as by
printing
with any commercially-available printer. The patient-specific information
affixed to
the label will include important information such as the patient name,
medication
type, medication strength and description, physician information, signa,
instructions
for taking the medication and one or more types of machine-readable
information,
such as a bar code.
Disadvantageously, the use of known label applicators or hand application of
labels can result in indiscriminate application of the labels. Indiscriminate
application
of a label represents a problem to pharmacy management because some or all of
the
valuable information provided on the pre-filled containers may be covered and
obscured by the label. As a result, the information provided with the pre-
filled
container may be rendered unusable to pharmacy management, patient or others
in the
prescription order fulfillment chain.
As an example, pharmacy management may wish to match the contents of the
pre-filled container to the prescription order by utilizing both the
manufacturer-
applied bar code on the pre-filled container and the bar code printed on the
patient-
specific label. A match of the bar code information on the pre-filled
container and on
the label provides a powerful indication that the correct medication, at the
correct
strength, has been matched to the correct patient. However, if the bar code on
the
pre-filled container is covered by the label then this verification process is
not
possible.
By way of further example, pharmacy management may wish to read a lot
number or expiration date of the labeled container following application of
the
patient-specific label. Or, a patient who has received a pre-filled container
in
fulfillment of her prescription order may wish to read a drug interaction
notice or
other element of information provided by the manufacturer or repackager. None
of
this is possible if the patient-specific label covers or obscures some or all
of the
information provided on the pre-filled container.
2003.06.A1 -4-
CA 02509120 2007-09-13
One approach to dispensing pre-filled containers is described in U.S. Patent
No. 7,006,893. The system shown in the publication employs a laser to engrave
infonnation on a special label applied to the container. This approach, while
quite
effective for the intended use, requires that the containers be provided with
a special
label having a region for receiving the laser-engraved information. Containers
as
generally provided by the manufacturer, repackager or other supplier do not
include
such specialized labels.
It would be a significant advance in the art to provide an apparatus and
method
permitting phannacy management to automatically convert non-patient-specific
pre-
filled containers from stock into use as patient-specific containers and to do
so in a
way which would permit pharmacy management to fully utilize and optimize the
use
of information provided on the pre-filled container by the manufacturer,
repackager or
other supplier.
SUMMARY
The subject matter described herein represents an improvement to the process
of fulfilling patient prescription orders. A patient refers to any person
seeking
fulfillment of a prescription. More specifically, the subject matter described
herein
enables phannacy management to automatically convert non-patient-specific pre-
filled
containers of medication, nutriceuticals, OTC medications, therapeutics and
like
products into patient-specific containers as required by the patient's
prescription
order.
These pre-filled containers are standard "stock" containers because the pre-
filled containers are in a form as provided by the manufacturer, repackager or
other
supplier and utilized by the pharmacy without any requirement for modification
of the
pre-filled containers prior to conversion of each container to a patient-
specific
container. As can be readily appreciated, it is advantageous to utilize pre-
filled stock
containers because pharmacy management is not required to incur further costs
associated with modification of the containers before adapting them for use as
patient-
specific containers.
Conversion of such pre-filled containers to patient-specific pre-filled
containers is advantageously accomplished in a way which optimizes the use and
soowe-oi -5-
CA 02509120 2005-06-02
value of information already provided on each pre-filled container, thereby
providing
pharmacy management with greater control over fulfillment of the prescription
order
and over costs associated therewith.
This result is achieved through closely controlled and precise automatic
application of patient-specific indicia including patient-specific information
to each
pre-filled container. Put another way, application of the patient-specific
information
on the pre-filled container is not indiscriminate as is the case with known
automatic
information-application systems.
The improvements described herein enable placement of the patient-specific
information on the pre-filled container such that the patient-specific
information and a
selected or predetermined portion of information already on the pre-filled
container
can be observed and used by the pharmacy personnel. Without such control over
information placement, useful information on the pre-filled container would be
randomly covered, obscured or impaired by the patient-specific information and
thereby rendered unusable. And, this result is accomplished without having to
alter
the form of the standard or stock pre-filled stock container to ready such pre-
filled
stock container to receive the patient-specific information.
In a preferred embodiment, an information-application apparatus is utilized to
automatically and precisely apply patient-specific information to the pre-
filled stock
container. Suitable control apparatus is provided to control operation of the
information-application apparatus. The control apparatus may be any suitable
control
or combination of controls.
In certain embodiments, the infonmation-application apparatus is adapted to
precisely place a patient-specific label on the pre-filled stock container. A
preferred
information-application apparatus includes an output device, such as a printer
or print
engine, and a positioner. The preferred printer is a label printer which
generates a
label including second indicia including patient-specific information. The
printer
may apply information to the label in any suitable manner. The positioner
directs the
pre-filled container to a position for precisely receiving the leading edge of
the label
so that the label is in the desired position when fully affixed to the pre-
filled
container. The position in which the pre-filled container is precisely
positioned to
receive the label or other form of patient-specific indicia is referred to
herein as an
zoosos.m -6-
CA 02509120 2005-06-02
"indicia-receiving position." The patient-specific label is then applied to
the pre-
filled container. Application of the label is such that, after label
application, the
patient-specific infomiation and a predetermined portion of the information on
the
pre-filled container are observable and available for use.
The positioner component of the information-application apparatus preferably
comprises a container gripper and a drive mechanism. The drive mechanism is
operative to precisely orient a gripped pre-filled container to receive the
label. It is
preferred that the information-application apparatus is adapted for use with
pre-filled
containers that are generally in the form of a cylinder. Examples are bottles
and the
like. In such embodiments, the drive mechanism comprises a drive roller and a
motor
in power-transmission relationship with the drive roller. The gripper
comprises a pair
of idler rollers which are spaced apart from the drive roller. The idler
rollers are
positionable to urge a pre-filled container against the drive roller such that
rotation of
the drive roller moves the pre-filled container to the label-receiving
position. In this
embodiment, the pre-filled container is held in place for application of the
label by
three points of contact.
In certain embodiments, the information-application apparatus printer
comprises a label source, a print element adapted to print the information on
a label
and a feed mechanism adapted to supply label material to the print element.
The feed
mechanism, or a separate drive mechanism applies a printed label to a pre-
filled
container.
Most preferably, the drive roller and pre-filled container form a nip adjacent
the printer, and the feed mechanism feeds the printed label into the nip and
into
contact with the pre-filled container. Rotation of the pre-filled container by
the drive
roller draws the label into the nip. The adhesive-containing side of the label
is urged
against the pre-filled container to adhere the label to the pre-filled
container.
Preferably, each pre-filled container is identified to the information-
application apparatus by means of a reader apparatus. The reader apparatus may
comprise one reader or a plurality of readers. The reader reads container-
identification information associated with each pre-filled container and
communicates
that information to the control apparatus resulting in identification of the
pre-filled
container. Such a reader could be an optical reader adapted to scan a bar
code. The
200s.0b01 -7-
I
CA 02509120 2005-06-02
reader may also be adapted to identify an RFID transponder including a code
representing the pre-filled container contents. The reader apparatus could
include one
or both of these capabilities or could comprise separate optical and RFID
readers.
Following identification, the control apparatus obtains positioning
information
used to operate the information-application apparatus positioner to position
the pre-
filled container. The information is preferably in a database accessed by the
control
apparatus. In embodiments, the database includes positioning information for
each
pre-filled container enabling the positioner to move the pre-filled container
to the
label-receiving position. In embod'unents, the pre-filled container is
positioned
relative to a reference point associated with each pre-filled container. The
reference
point may be any suitable mark, structure (such as a key formed in the pre-
filled
container bottom surface) or thing. The position of the identified pre-filled
container
is identified by reference to the reference point. The control apparatus then
enables
operation of the positioner to position the pre-filled container in the
indicia-receiving
position.
In certain embodiments, the gripper may be adapted to accommodate a range
of different sizes of the pre-filled containers. For example, the gripper can
be
configured to accommodate pre-filled containers having different
circumferences. In
such embodiments, it is preferred that the gripper comprises a pair of idler
roller
supports each of which supports an idler roller. The supports for the idler
rollers are
movable toward and away from the other to accommodate different pre-filled
container circumferences. It is preferred that an actuator moves each support
under
control of the control apparatus. This arrangement permits the pre-filled
container to
be positioned so that the label can be received in the nip irrespective of the
container
circumference.
The control apparatus may be any suitable combination of controls and may
include apparatus such as a computer separately and/or in combination with
other
control components. The control apparatus preferably includes a set of
instructions
which operate the positioner to move the pre-filled container to the indicia-
receiving
position, operate the printer to print the second indicia and operate the
information-
application apparatus to apply the second indicia to the pre-filled container.
The information-application apparatus may be utilized in a variety of
2005-06-0I -8-
I
, ,
CA 02509120 2005-06-02
configurations including as part of a fully or partially automated
prescription
fulfillment system or as a stand-alone unit.
BRIEF DESCRIPTION OF THE DRAWINGS
The foregoing and other objects, features and advantages of the invention will
be apparent from the following more particular description of preferred
embodiments,
as illustrated in the accompanying drawings in which like reference characters
refer to
the same parts throughout the different views. The drawings are not
necessarily to
scale, emphasis instead being placed upon illustrating the principles of the
invention.
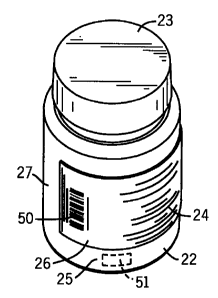
FIGURE 1 is a perspective view of an exemplary pre-filled stock container.
In the embodiment shown, indicia including information relating to the pre-
filled
container contents is provided on a label. The infonnation is in stylized
form.
FIGURE 2 illustrates the exemplary label of Figure 1 separate from the pre-
filled container. Information relating to the pre-filled container contents is
shown.
FIGURE 3 is an exemplary patient-specific label with indicia including
information relating to a patient's prescription order.
FIGURE 4 is a perspective view of the pre-filled stock container of Figure 1
with the patient-specific label of Figure 3 precisely positioned thereon. The
information is in stylized form.
FIGURE 5 illustrates the exemplary labels of Figure 4 but separate from the
pre-filled container. Information relating to the pre-filled container
contents and the
patient's prescription order is shown.
FIGURES 6A and 6B illustrate exemplary alternative forms of pre-filled stock
containers.
25. FIGURE 7 is a schematic illustration showing components of an exemplary
dispensing system including an exemplary information-application apparatus.
FIGURE 8 is a perspective view of an exemplary automatic pre-filled
container dispenser.
FIGURE 9 is a schematic illustration showing components of an exemplary
system including exemplary dispenser, container transport and information-
application apparatus.
FIGURE 10 is a perspective view of an exemplary information-application
2005-0"1 -9-
CA 02509120 2007-09-13
apparatus and a portion of a container transport mechanism. Components of a
positioner apparatus and a printer apparatus are shown. Certain parts are
shown in
dotted line or are not shown to facilitate understanding of the apparatus.
FIGURE 11 is a further perspective view of the information-application
apparatus of Figure 10.
FIGURE 12 is a further perspective view of the information-application
apparatus of Figure 10.
FIGURE 13A is a schematic illustration of the information-application
apparatus of Figure 10 shown in a position receiving a pre-filled container.
FIGURE 13B is a further schematic illustration of the information-application
apparatus of Figures 10 and 13A shown in a position identifying the pre-filled
container before application of patient-specific information thereto.
FIGURE 13C is a further schematic illustration of the information-application
apparatus of Figures 10 and 13A shown in an indicia-receiving position with
the pre-
filled container oriented to receive the leading edge of the label.
FIGURE 13D is a further schematic illustration of the information-application
apparatus of Figures 10 and 13A shown in a position receiving a precisely-
positioned
label with patient-specific information.
FIGURE 13E is a further schematic illustration of the information-application
apparatus of Figures 10 and 13A shown in a position verifying the pre-filled
container
following application of the patient-specific information and label.
FIGURE 13F is a further schematic illustration of the information-application
apparatus of Figures 10 and 13A shown in a position releasing the pre-filled
container.
FIGURE 13G is a further schematic illustration of the information-application
apparatus of Figures 10 and 13A but with a pre-filled container of a smaller
size than
the pre-filled container of Figures 13A-13F.
FIGURES 14A, 14B and 14C are a single logic flow diagram showing
exemplary process steps for operation of an exemplary information-application
apparatus.
FIGURE 15 is a perspective view of an alternative embodiment of an
exemplary information-application apparatus shown mounted to an automatic
dispenser. Certain parts are cut away to facilitate understanding of the
apparatus.
no&wol -10-
CA 02509120 2005-06-02
FIGURE 16 is a perspective view of a further alternative embodiment of an
exemplary information-application apparatus shown as a stand-alone apparatus.
Certain parts are cut away or not shown to facilitate understanding of the
apparatus.
FIGURE 17 is a perspective view of the embodiment of Figure 16. Certain
parts are cut away or not shown to facilitate understanding of the apparatus.
FIGURE 18A is a schematic illustration showing components of an exemplary
dispensing system including the information-application apparatus of Figure
15.
FIGURE 18B is a schematic illustration showing components of an exemplary
dispensing system including the information-application apparatus of Figures
16-17.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
Referring first to Figures 7-18B, exemplary automatic information-application
apparatus 10, 10 and 10 are shown. Information-application apparatus 10, 10
and
10 are adapted to generate a patient-specific label 56 and to precisely place
label 56
on pre-filled stock container 22. In general, information application
apparatus 10, 10
and 10 comprises an output device, preferably in the form of a label printer
(or print
engine) 12, together with positioner 14 and controller 16. Throughout the
detailed
description, like components for each apparatus 10, 10 and 10 are identified
by
common reference numbers. Positioner 14 preferably comprises container gripper
18,
drive mechanism 20 and related components. Gripper 18 and drive mechanism 20
are
operative to precisely orient a pre-filled container 22 to receive patient-
specific
information generated by printer 12.
Referring next to Figures 1, 4, 6A and 6B, there are shown representative pre-
filled containers 22. Reference number 22 will be associated with each such
pre-
filled container 22, and such reference number is used to refer to both single
pre-filled
containers 22 and plural pre-filled containers 22.
A pre-filled container 22 is a container loaded with a medicament,
nutriceutical, therapeutic agent or like product or thing. The pre-filled
containers 22
are "stock" articles meaning that such pre-filled containers 22 are capable of
being
used in the same form as provided by the manufacturer, repackager or other
supplier
without any requirement for modification of the pre-filled container 22 prior
to
application of patient-specific information. Each such pre-filled container 22
is
200S.06.01 -1 1-
~
i
CA 02509120 2005-06-02
referred to herein as a pre-filled stock container 22, a pre-filled container
22 or simply
as a container 22.
Pre-filled containers 22 are loaded, or pre-filled, with one or more articles
or
things at the point of manufacture or repackaging. The pre-filled container 22
contents may be in any form and quantity. For example, pre-filled container 22
contents may comprise bulk-form tablets such as capsules, caplets, ellipses,
ovals,
triangles, balls, multi-angles and the like. Other forms of pre-filled
container 22
contents, such as liquids and powders, may be loaded in pre-filled containers
22.
Pre-filled containers 22 typically vary in size and shape. For example, pre-
filled containers 22 may be provided in volumetric sizes, such as 75, 120, 200
cubic
centimeters. It is preferred that pre-filled containers 22 are provided in a
shape which
is generally cylindrical. Examples are the pre-filled containers 22 shown in
Figures 1,
4 and 6A. Exemplary apparatus represented by reference numbers 10, 10 and 10
are configured for use with pre-filled containers 22 having a shape which is
generally
cylindrical. However, apparatus 10, 10 and 10 may be configured to accommodate
other container 22 shapes such as the generally-rectangular shaped pre-filled
container 22 shown in Figure 6B. Containers 22 are typically, but not
exclusively,
made of plastic materials.
Pre-filled containers 22 include an opening (not shown) through which one or
more articles are loaded into each container 22. A removable safety seal (not
shown),
such as a film or foil seal, may be applied across such container opening
after
contents of pre-filled container 22 are loaded therein. A cap 23 or other
closure is
then applied across the pre-filled container opening. Cap 23 can be any
suitable type,
such as a screw-on cap (Figures 1, 4 and 6B) or a snap-fit cap (Figure 6A).
Cap 23 is
typically child-resistant and is replaceable over the opening of pre-filled
container 22
permitting pre-filled container 22 to be repeatably opened and closed to
remove the
container 22 contents. A tamper-evident neck seal (not shown) may be applied
to or
over cap 23.
Referring fiuther to Figures 1-2 and 4-6B, pre-filled containers 22 are
provided by the manufacturer, repackager or other supplier with indicia 24 to
describe
the pre-filled container 22 contents and to provide other information
important to the
pharmacy, care giver, patient and others involved in the distribution chain.
Indicia 24
20oS.06-01 -12-
CA 02509120 2005-06-02
are not limited to any particular type or form, and indicia 24 shown and
described
herein are merely illustrative. Indeed, the type and form of indicia 24 will
vary
considerably given the number of products and parties in the distribution
chain.
Indicia 24 may be applied to each pre-filled stock container 22 in any
suitable
manner. One manner of associating indicia 24 with pre-filled container 22 is
to apply
indicia 24 to an adhesive-backed labe126 affixed to pre-filled container 22.
Indicia
24 could be applied in other ways, such as by application directly to outer
surface 25.
Labe126 may be wrapped fully or partially around outer surface 25 of pre-
filled
container 22. The labe126 shown in Figure 1 is wrapped partially around pre-
filled
container 22 outer surface 25 such that a portion 27 of pre-filled container
22 outer
surface 25 is free of contact with labe126.
Figures 2 and 5 show detail of exemplary indicia 24 associated with the pre-
filled container 22 of Figures 1 and 4. In the embodiment, indicia 24 are
affixed to an
adhesive-backed labe126. Among the exemplary information comprising indicia 24
are: the name and address of the manufacturer 28 and/or repackager 30, the
type 32,
strength 34, description 36, lot number 38, expiration date 40 and quantity 44
of the
medication loaded in pre-filled container 22 and a National Drug Code
identifier
("NDC") 42. NDC 42 comprises a code which serves as a unique identifier of pre-
filled container 22 and its contents. Other information which may comprise
indicia
24 includes instructions for handling and storage 46 of pre-filled container
22 and
notices 48 relating to the container 22 contents. The U.S. Food and Drug
Administration, other regulatory agencies and pharmacies regulate the
information
provided as indicia 24. As regulations and other demands change over time,
indicia
24 associated with pre-filled containers 22 will change to comply with such
new
regulations and requirements.
Indicia 24 may be in the form of both human-readable information and
machine-readable information as shown in Figures 1-2 and 4-6B. Human-readable
information may include information such as described in connection with
reference
numbers 28-48. Machine-readable information may include any information or
data
storage device capable of being read by a machine.
An example of machine-readable information is a bar code 50. Bar code 50
comprises spaced apart light and dark elements. Typical bar code 50 formats
include
2005-06.01 -13-
CA 02509120 2005-06-02
UPC, Code 39, Interleaved 2 of 5 and Code 128. The elements comprising bar
code
50 can be read with a bar code scanning device (not shown) to generate a
signal
corresponding to a code. Representative scanning devices may include a laser
scanner or camera. As is known, a bar code 50 acts as an index to a record in
a
database. Recognition of bar code 50 accesses associated information in the
database.
In the example, bar code 50 includes a code corresponding to NDC 42.
Scanning of bar code 50, therefore, enables pharmacy management to rapidly and
accurately identify the unique signature of pre-filled container 22 and to use
that
information for any purpose, including to track pre-filled container 22 in
inventory
and to match pre-filled container 22 to a particular patient prescription
order. And,
scanning of bar code 50 may be used to control the process of applying patient-
specific information to pre-filled container 22 using apparatus 10, 10 and 10
as
described herein.
Machine-readable information in combination with, or in place of, bar code 50
can be associated with pre-filled container 22. For example, a two-dimensional
("2D") bar code (not shown) may be used. A bar code in 2D format links to
information in a database but also serve as a database including the
capability to
encode up to several thousand characters of machine-readable data. 2D symbol
formats which may be used are PDF417 and Data Matrix symbologies. PDF417
symbologies can be read by laser scanners, cameras, or other instruments using
optically sensitive devices such as charge-coupled devices ("CCDs'.'). Matrix
symbols are read by a camera or CCD reader.
A data storage device such as a Radio Frequency Identification ("RFID") tag
51 may be associated with each pre-filled container 22. As is known, an RFID
system
uses electromagnetic or electrostatic coupling in the radio frequency ("RF")
portion
of the electromagnetic spectrum to transmit signals. An RFID system comprises
a
transponder 51 (also referred to as a "tag"), an antenna and a transceiver.
Antenna
and transceiver may be included as part of reader 86 or as separate
components. Tag
51 is an integrated circuit containing RF circuitry and information to be
transmitted.
The antenna receives the RF. The transceiver reads the RF and transfers the
information to a processing device such as computer 104. Tag 51 may be affixed
to
2005.06-01 -14-
i
CA 02509120 2005-06-02
pre-filled container 22 at any location, not necessarily in a position which
can be
visually observed from the outside of pre-filled container 22. Tag 51 may be
encoded with any suitable information, such as NDC 42. It is expected that the
type
and form of machine-readable information will evolve over time. Future
improvements in such technology are intended to be within the scope of the
systems
and methods described herein.
Referring to Figures 2 and 5, a reference point 52 is provided. Information-
application apparatus 10, 10 and 10 utilizes reference point 52 to identify a
position
of pre-filled container 22 so that pre-filled container 22 can be oriented and
located in
the indicia-receiving position. Reference point 52 can be a point on labe126
which is
at a transition between a light and dark region of labe126, such as the
leading edge of
bar code 50 as shown in Figures 2 and 5 or another mark on pre-filled
container 22 or
label 26. Reference point 52 could be a mechanical element of pre-filled
container
22, such as a key-type indentation or protrusion (not shown) which mates with
a key
way (not shown) on positioner 14. The manner in which reference point 52 is
used to
orient pre-filled container 22 to the indicia-receiving position to receive
patient-
specific indicia 54 is fully described in connection with Figures 13C and 14A-
14C
below.
2005-06-01 -15-
.. . ..__....~.... . .. .. . . ._,. ,. . . .
CA 02509120 2005-06-02
As can be readily appreciated, indicia 24 or elements of indicia 24 are of
immense value to each party in the distribution chain. For example, persons
involved
in distribution of pre-filled containers 22 may use the information comprising
indicia
24 (particularly bar code 50 or tag 51) to track pre-filled container 22 in
inventory.
Persons involved in fulfillment of patient prescription orders may use the
information
comprising indicia 24 to select pre-filled container 22, to verify that the
medication
loaded in pre-filled container 22 is as called for by the prescription and to
process
payment for the container contents. And, the patient herself will use the
information
comprising indicia 24 for guidance in taking medication loaded in pre-filled
container
22. It is important that the information comprising indicia 24 be accessible
for those
involved in distribution and use of containers 22.
A generic pre-filled stock container 22 may be converted for use as a patient-
specific container as illustrated in Figures 3-5 by applying patient-specific
indicia 54
to the pre-filled container 22. The information associated with pre-filled
container 22
may be optimized by precise application of the patient-specific indicia 54 as
determined by pharmacy management such that indicia 24 are not impaired or
obscured and may be read by a human and/or a machine. As shown in the
embodiment of Figures 3-5, patient-specific indicia 54 are of the type which
is affixed
to an adhesive-backed labe156 for application over some or all of labe126 and
indicia
24 as determined by pharmacy management. Indicia 54 are preferably specific to
the
patient for whom container 22 is designated.
Among the exemplary information comprising indicia 54 are: the patient name
58, pharmacy name, street address, Internet address and telephone number 60,
the
type 62, strength 64, quantity 66 of the medication, the prescription number
68, fill
date 70, physician name 72, a text description or image of the appearance of
the
container contents 74 and refill information 76. And, indicia 54 may include
one or
more types of machine-readable information, such as a bar code 78 of the types
described herein. An RFID tag (not shown) of the type described in connection
with
tag 51 may be affixed to labe156 and associated with patient-specific indicia
54.
Such bar code 78 or other information may be used to uniquely associate pre-
filled
container 22 with a specific patient prescription order by computer 104 and
database
106. Pharmacy Boards regulate the information included in indicia 54. As
2005-0s-o1 -16-
CA 02509120 2007-09-13
regulations change in the future, the information comprising indicia 54 would
be
expected to change to comply with new regulations.
Referring next to Figures 7-13G, infonnation-application apparatus 10 will be
described in the context of an automated workflow management system ("WMS")
80.
As illustrated and described in connection with Figures 15-18B, information-
application apparatus 10 may be used in applications other than system 80. For
example, apparatus 10' is shown in combination with an automatic dispenser 226
(Figure 15 and 18A) while apparatus 10" is configured for use as a stand-alone
unit
(Figures 16-17 and 18B). Information-application apparatus 10' and 10" are
essentially identical to apparatus, particularly with respect to printer 12
and positioner
components 14 and it will be understood that the description of apparatus 10
also
applies to information-application apparatus 10' and 10".
System 80 illustrated schematically in Figure 7 represents an automated WMS
for use in processing patient prescription orders. System 80 comprises plural
automated components including container dispenser 82, container transport
mechanism 84, information-application apparatus 10 (including printer 12,
positioner
14 and reader 86), container collection and sortation apparatus 88, and
control
apparatus 16 for controlling operation of such components.
Referring more specifically to Figure 7, system 80 includes a container
dispenser 82, a container transport mechanism 84, information-application
apparatus 10 (including reader 86, positioner 14, and printer 12) and a
container
collection and sortation apparatus component 88. Control apparatus 16 controls
operation of components 82, 84, 10 and 88. Control apparatus 16 comprises
computer
104 and controllers 94, 95, 96 and 98. Interface 108 is provided for
transmission of
information between computer 104 and one or more of controllers 94, 95, 96 and
98
and components 82, 84, 10 and 88.
Computer 104 includes display I 10, keyboard 112 and mouse 114 to pennit
phannacy management to interact with system 80. Computer 104 may run a
prescription processing software program such as PPSTM from AutoMed
Technologies, Inc. of Vecnon Hills, Illinois. The PPS soRware residing on
computer
104 cnables operation of system 80 and provides information required to
initiate, for
each container 22 pertaining to a prescription order, the automatic process of
dispensing,
:~s-0"1 -17-
CA 02509120 2005-06-02
transporting, applying information and sorting and collecting. The PPS
software
manages operation of all components of the system 80 for fulfillment of
patient
prescription orders.
A database 106 identifying pending prescription orders and each pre-filled
container 22 used in system 80 resides on computer 104. Database 106 includes
information uniquely identifying each of the pre-filled containers 22 by means
of a
machine-readable code, such as bar code 50 or an RFID tag 51. Database 106
also
includes, for each pre-filled container 22, information necessary to precisely
position
container 22 for precise application of patient-specific indicia 54 to
container outer
surface 25 as described in full detail below. Database 106 may include other
information of use to pharmacy management, such as a complete record of all
prescription orders filled by system 80.
Controllers 94, 95, 96 and 98 are provided to enable operation of the
corresponding component or components 82, 84, 10 and 88 of system 80.
Controllers
94, 95, 96 and 98 may, for example, be programmable logic controllers ("PLC").
Representative PLCs for use as controllers 94, 95, 96, 98 are Allen-Bradley
Micrologix PLCs available from Rockwell Automation of Milwaukee, Wisconsin.
Controllers 94, 95, 96 and 98 may comprise other types of controls, for
example, one
or more microcontrollers (not shown) associated with the information-
application
apparatus 10 or other components 82, 84, 86, 88. The functions of controllers
94, 95,
96, 98 may be combined into fewer or more hardware elements as determined by
the
system manager.
System 80 may be scaled, configured and arranged as required by pharmacy
management. Such requirements may be based on the patient population served by
system 80, required prescription order processing throughput, available floor
space
and cost considerations.
As shown schematically in Figure 7, system 80 may optionally receive
prescription orders for fulfillment from a Pharmacy Information System ("PIS")
100.
If utilized, PIS 100 processes patient prescription orders and releases those
orders for
fulfillment by system 80 following completion of desired processes, such as
patient
data entry, drug utilization review and adjudication.
PIS 100 includes information-management software residing, for example, on
2005-0&01 -18-
,.. 4
CA 02509120 2005-06-02
computer 102. The information-management software residing on computer 102 may
be a standard, commercially-available database engine or other information-
storage
software which provides functions, such as administrative and accounting
functions.
A patient prescription order is initially presented to pharmacy personnel by a
patient or other person. Data pertaining to the prescription order is entered
into
computer 102 of PIS 100 using any suitable user interface, such as a client
computer
101, keyboard 103, mouse 105 and display 107. Data entered typically will
include,
for example, patient name, the type, strength, quantity of the medication,
prescriber
information (e.g., physician name), payor information, refill information and
instructions regarding the prescription. Computer 102 may automatically
associate
other information with the prescription order, such as the pharmacy name,
street
address, pharmacy Internet address and telephone number, prescription number,
fill
date and a text description or image of the appearance of the container
contents. PIS
100 computer 102 typically maintains a database of information on each
patient.
Following data entry, software residing on computer 102 is preferably utilized
to conduct a drug utilization review ("DUR") and to adjudicate any claim with
insurance or other third-party payor. Following any DUR and adjudication, the
patient prescription order is sent (for example as a data packet) from
computer 102 to
computer 104 via interface 109. Interface 109 may include any suitable
information-
transmission capability including modem, local area network ("LAN"), Internet
and
combinations thereof. PIS 100 and computer 102 may be co-located with system
80
and computer 104 at the same site or may be located at completely separate
sites as
required by pharmacy management.
Referring now to Figures 7-9, automatic dispenser 82 is provided to store and
to dispense one or more pre-filled stock containers 22 from a stock or
inventory of
pre-filled containers 22 as required to fulfill each patient prescription
order.
Referring to Figures 8 and 9, dispenser 82 includes a housing 116 enclosing a
plurality of storage shelves of which shelf 118 is representative. Housing 116
is
preferably fabricated from aluminum, stainless steel or plastic to be fully
compatible
with a pharmacy setting. Housing 116 may include transparent doors 120 which
open
and close to permit access to dispenser 82 for loading of pre-filled
containers 22 onto
shelves and to service the dispenser 82.
2005.0"1 -19-
CA 02509120 2005-06-02
Each shelf (e.g., 118) includes suitable vertically-oriented walls 122 which
organize pre-filled containers 22 into rows, such as row 124. Each row
contains one
type of pre-filled container 22. The shelves, such as shelf 118, may be
downwardly
angled such that gravity causes containers 22 in each row to slide toward the
front of
dispenser 82.
Each row (e.g., row 124) defmes a separate shelf location known to the PPS
software residing on computer 104 and identifiable to controller 94 and
dispenser 82
by a unique position in an x, y, z coordinate system. The unique position of
each
shelf location and the inventory of pre-filled containers 22 at each shelf
location is
stored in the PPS database 106 residing on computer 104. In addition, each
shelf
location may be indicated by a unique machine and/or human-readable code (not
shown) located proximate each row, such as row 124. The shelf-associated code
may
be a bar code, RFID tag or other suitable symbology. The code may be used to
track
pre-filled containers 22 to a specific row and shelf location to facilitate
inventory
replenishment and management or could be used to facilitate dispensing of the
correct
pre-filled container 22 from its row.
Dispenser 82 controller 94 receives a signal via interface 108 from computer
104. Controller 94 interprets the signal sent from computer 104 and enables
dispenser
82 to automatically operate to dispense a pre-filled container 22 as required
by the
patient prescription order. For example, controller 94 may enable operation of
a
dispenser transport mechanism (not shown) to transport the requested pre-
filled
container 22 from its shelf location to a position for discharge from the
dispenser 82.
Such a dispenser transport mechanism may be configured to move in an x, y, z
coordinate system between doors 120 and the shelves (e.g., 118) to the
appropriate
shelf location. A gripper (not shown) may be associated with the dispenser
transport
mechanism and such gripper may grip the lowermost pre-filled container 22 in
the
selected row. The dispenser transport mechanism then places pre-filled
container 22
through chute upper opening 126 into chute 128. Pre-filled container 22 falls
by
means of gravity down chute 128 and through chute lower opening 129 for
transport
by the container transport mechanism 84 to information-application apparatus
10.
As each pre-filled container 22 is dispensed, a signal is sent by dispenser 82
to
computer 104 via interface 108. Database 106 is updated to indicate that the
pre-
200546A1 -20-
_r
,
CA 02509120 2005-06-02
filled container 22 corresponding to each prescription order then being
fulfilled has
been dispensed and is in route to the information-application apparatus 10.
The
sequence in which pre-filled containers 22 are dispensed from dispenser 82 may
be
recorded in database 106 to facilitate this process of matching each pre-
filled
container 22 to a specific patient prescription order.
The size, capacity and type of automated dispenser 82 utilized will typically
vary depending on the throughput and other needs and requirements of pharmacy
management. An exemplary dispenser 82 suitable for use in system 80 is the
FastFind system available from AutoMed Technologies. A FastFind dispenser may
be modified to discharge pre-filled containers 22 stored therein simply by
affixing
chute 128 thereto and directing pre-filled containers 22 to the chute 128
upper
opening 126.
Figures 7-13G illustrate a container transport mechanism 84 for transporting
pre-filled containers 22 from dispenser 82 to information-application
apparatus 10.
Preferred transport mechanism 84 comprises an endless conveyor 130. Conveyor
link
chain 132 is powered by a motor drive (unshown) to transport pre-filled
containers 22
from or to components 82, 10 and 88. Drive unit is enabled for operation by
controller 95. Controller 95 receives a signal via interface 108 from computer
104
and interprets the signal to enable operation of the drive unit to power
conveyor link
chain 132 at a desired rate of movement. Guide rails 134, 136 are provided
adjacent
link chain to direct pre-filled containers 22 along the conveyor 130.
Conveyor 130 may be of any suitable type and may be arranged in any
suitable configuration to meet the requirements of the pharmacy management.
For
example, conveyor 130 may be a single-direction conveyor or a recirculating
conveyor. Conveyor 130 may include single or multiple lines amanged in series
or in
parallel. Container-transport mechanism 84 may be of types other than conveyor
130
and may comprise, for example, a walking beam conveyor or a robotic transport
apparatus configured to transport a single pre-filled container 22 to the
information-
application apparatus 10.
A representative conveyor 130 suitable for use as container transport
mechanism 84 is a Simpli-Flex modular conveyor system available from
Simplimatic Automation of Lynchburg, Virginia. The Simpli-Flex system is
useful,
20050", -21-
i
K.., ,.
CA 02509120 2005-06-02
at least in part, because it can be easily scaled and configured to meet the
specific
throughput and spacial requirements of pharmacy management and because it
includes a wide range of link chains 132 which can be adapted to transport
virtually
any type of pre-filled container 22.
Figures 9-13G illustrate information-application apparatus 10 and related
components. In the embodiment, information-application apparatus 10 is
configured
to precisely apply a patient-specific labe156 to the pre-filled container 22
and to do so
in a way which optimizes the value of indicia 24 provided with pre-filled
container
22.
Referring further to Figures 9-13G, information-application apparatus 10
preferably includes housing 135 (Figure 9), base 137 and an escapement device
138
secured to base 137. Escapement device 138 is enabled for operation by
controller 96
responsive to a signal from computer 104 via interface 108 to singulate
movement of
pre-filled containers 22 toward positioner 14. In the example, escapement
device 138
includes first 140 and second 142 stops and container sensor 144. Wedge-shaped
stop
ends 146, 148 enable the stops 140, 142 to slide easily between adjacent
containers 22
and to separate adjacent containers 22 from one another thereby facilitating
singulation.
A suitable actuator (not shown) enabled for operation by controller 96
alternately extends and retracts stops 140, 142 between the container-stop
position in
Figures 10-12 and the container-release position shown in Figure 9. The
escapement
device 138 actuator may comprise, for example, rack and pinion arrangement
(not
shown) in which a bi-directional motor powers a pinion gear meshed with racks
associated with each stop 140, 142. Rotation of the pinion gear in one
direction
retracts stop 140 and extends stop 142, while rotation in a second direction
extends
stop 140 and retracts stop 142.
Initially, stop 140 is retracted and stop 142 is extended as shown in Figures
10-12. Stop 140 blocks movement of all adjacent pre-filled containers 22 and
guide
rails 134, 136 constrain arrangement of the pre-filled containers 22 into the
form of a
single file row as shown in Figures 10-12. In the embodiment, conveyor 1301ink
chain 132 continues to move, sliding beneath pre-filled container 22 or
containers
stopped by escapement device 138. (see Figures 10-12).
2005.06.01 -22- ~ 4_
. . .. . .....{ , , . .........._.
CA 02509120 2005-06-02
Sensor 144 generates a signal for controller 96 and computer 104 when a held
container 22 is proximate sensor 144. Sensor 144 is preferably a photodetector
operatively connected to controller 96 and computer 104 via interface 108.
Preferably, each pre-filled container 22 held by escapement device 138 and
proximate
sensor 144 is known to computer 104 through updating of database 106 as pre-
filled
containers 22 are dispensed from dispenser 82 and are processed by information-
application apparatus 10.
A held pre-filled container 22 is released to positioner 14 of information-
application apparatus 10 when controller 96 generates a signal causing stop
140 to
extend and stop 142 to retract. Wedge-shaped end 146 easily slides between
adjacent
containers 22 when extended thereby enabling only one pre-filled container 22
to
move past. The process of extension and retraction of stops 140, 142 is
repeated for
each cycle such that each pre-filled container 22 is delivered one-by-one to
positioner
14.
Referring now to Figures 10-13G, pre-filled containers 22 are positioned for
receiving a patient-specific labe156 by positioner 14. Positioner 14 includes
a
container gripper 18, drive mechanism 20 and related components. Drive
mechanism
is operative to precisely orient a gripped, pre-filled container 22 to receive
patient-
specific labe156. Positioner 14, gripper 18 and drive mechanism 20 are enabled
for
20 operation by controller 96.
In the embodiment, information-application apparatus 10 is adapted to orient
pre-filled containers 22 having a generally cylindrical shape, such as that
shown in
Figures 1, 4 and 6A. Drive mechanism 20 includes a drive roller 150 and a
motor
152. Drive roller 150 is a generally cylindrically-shaped roller with a
frictional outer
surface 154 provided to positively engage and spin a pre-filled container 22
clamped
against drive roller 150 as described herein. Drive roller surface 154 may
comprise
any suitable material capable of positive engagement with outer surface 25 of
pre-
filled container 22. A surface 154 formed of a resilient, elastomeric material
is
preferred.
Drive roller 150 is mounted to a motor axle (not shown) and may be in a direct
drive relationship with motor 152. Motor controller 156 is operatively
connected to
controller 96 under control of the PPS residing on computer 104. As can be
2pp5,p6-0, -23-
1_
CA 02509120 2007-09-13
appreciated, motor 152 may be in any power-transmission relationship with
drive
roller 150. For example, motor 152 may power drive roller 150 through a
suitable
gear train (not shown).
As shown in Figures 9-13G, gripper 18 is configured to clamp a pre-filled
container 22 against drive roller 150. Rotation of drive roller 150 spins the
clamped
pre-filled container 22 for container identification and verification and for
precise
application of the patient-specific information 54 on pre-filled container 22.
Gripper 18 shown herein preferably includes a mechanical linkage that allows
it to accommodate a range of pre-filled containers 22 with diameters that
differ and to
position such pre-filled containers 22 in the proper indicia-receiving
position for
application of the patient-specific labe156 without requiring installation of
a separate
gripper 18 or gripper part. This feature allows pharmacy management to process
a
more diverse range of prescriptions through information-application apparatus
10.
Gripper 18 includes a gripper member 158, a pair of idler roller supports 160,
162 pivotally mounted on member 158 at joint 165 and a pair of unpowered idler
rollers 164, 166 journaled on a respective support 160, 162. Joint 165 enables
supports 160, 162 to pivot toward and away from each other and drive roller
150 to
capture and securely clamp a pre-filled container 22 against drive roller 150
with three
points of contact.
A dual-acting, air-powered linear actuator 168 extends and retracts member
158. Member 158 is mounted on guides 170, 172. Member 158 translates toward or
away from drive roller 150 along guides 170, 172 alternately in the directions
of dual-
headed arrow 174 (Figures 12, 13A, 13B).
Separate dual-acting, air-powered linear actuators 176, 178 extend and retract
a respective support 160, 162. The piston 180, 182 of respective actuator 176,
178 is
connected to a respective support 160, 162 at a pivotable linkage 184, 186. At
the
other end of each actuator 176, 178 a linkage 188, 190 pivotally joins each
actuator
176, 178 to member 158. This arrangement permits actuators 176, 178 to move
laterally with respect to member 158 enabling idler rollers 164, 166 on
supports 160,
162 to grip pre-filled containers 22 with three points of contact across a
range of
different pre-filled container 22 circumferences.
A compressed air source (not shown), tubing (not shown) and suitable valve
2005ob oi -24-
......y . .. ..... . . ., . ._ . . ...
CA 02509120 2005-06-02
apparatus (not shown) direct compressed air to actuators 168, 176, 178
enabling such
actuators to grip a pre-filled container 22 against drive roller 150 and to
release pre-
filled container 22. Controller 96 enables operation of the valve apparatus
and
compressed air source based on a signal received via interface 108 from
computer
104. Control of actuators 168, 176, 178 is well known to persons of skill in
the art.
When the actuators 168, 176, 178 are in an extended position as shown in
Figures 13B-13E and 13G, pre-filled container 22 is clamped against drive
roller 150.
Actuators 168, 176, 178 provide about five pounds/sq. inch of pressure to the
idler
rollers 164, 166. Actuators 168, 176, 178 extend member 158 and supports 160,
162
to the position shown in Figures 13B-13E and 13G until resistance to further
extension overpowers further movement of actuators 168, 176, 178. In this
manner,
supports 160, 162 and idler rollers 164, 166 are self-centering to position
each pre-
filled container 22 against the drive roller 150 as part of the process of
precisely
positioning pre-filled container 22 to receive patient specific information
54, for
example on label 56.
Rotation of drive roller 150 in the direction of arrow 192 causes the clamped
pre-filled container 22 to rotate in an opposite direction. Rotation of
clamped pre-
filled container 22 may be used to scan bar code 50 to identify pre-filled
container 22
to computer 104, to locate reference point 52, to rotate pre-filled container
22 to the
indicia-receiving position, to apply patient-specific labe156 to pre-filled
container 22
and for bar code scanning after labe156 is applied to verify to computer 104
that the
correct patient-specific labe156 has been applied to pre-filled container 22.
Clamping of pre-filled container 22 against drive roller 150 by idler rollers
164, 166 of gripper 14 results in formation of a nip 194 between outer surface
25 of
pre-filled container 22 and surface 154 of drive roller 150. Nip 194 is
utilized to
attach the patient-specific labe156 to pre-filled container 22.
Pre-filled container 22 is oriented by rotation to the indicia-receiving
position
shown in Figure 13C. Such position is the position enabling placement of the
patient-
specific information in the desired location on pre-filled container 22 as pre-
filled
container 22 is rotated. The indicia-receiving position will change depending
on the
specific location for placement of the patient-specific indicia 54 and the
size and
shape of pre-filled container 22.
soosa-m -25-
fi. .. ..._.,...
CA 02509120 2007-09-13
A patient-specific labe156 is fed along a path in the direction of arrow 196
(Figures 13C, 13D) and into nip 194 with an adhesive-containing side 198
facing
outer surface 25 of pre-filled container 22. The opposite rotation of drive
roller 150
and of pre-filled container 22 at the container-receiving position (Figure
13C) draws
labe156 into nip 194 and against outer surface 25 of pre-filled container 22.
Coaction
of idler rollers 164, 166 of gripper 18 and drive roiler 150 of drive
mechanism 20 urge
adhesive-containing side 198 of tabe156 against pre-filled container 22 to
affix label
56.
As is apparent from a comparison of Figures 13A-13F with Figure 13G,
gripper 18 can accommodate pre-filled containers 22 of different sizes without
changing a gripper or gripper part. This result is achieved by means of the
extensible
member 158 and supports 160, 162 which pivot at joint 165. The articulated
structure of gripper 18 enables supports 160, 162 and idler rollers 164, 166
to be
extended as far as necessary toward pre-filled container 22 for self-centering
movement to precisely position pre-filled container 22 in position to receive
labe156.
Information-application apparatus 10 further includes a reader 86 provided to
identify each pre-filled container 22 to computer 104 and PPS database 106.
Reader
86 may also identify the position of a gripped pre-filled container 22 so that
pre-filled
container 22 may be oriented for application of label 56. Reader 86 may also
be used
to verify that the correct patient-specific information has been applied to
the pre-filled
container 22. Reader 86 may comprise a single device as shown in Figures 9-12
or
may comprise a collection of devices, each adapted to read a type of
information.
Reader 86 is preferably configured to scan and read bar code 50 or other
machine-
readable indicia affixed to pre-filled container 22 by the manufacturer or
other
supplier. A typical optical-type reader 86 will include a laser emitter and a
detector.
As represented schematically in Figures 13B, 13E and 13G, energy beam 87
emitted
from the reader 86 is reflected from bar code 50. The detector detects the
pattern of
reflectance and reader 86 generates a signal corresponding to code 50 which is
transmitted to computer 104. Detection of bar code 50 results in generation of
a
signal corresponding to the unique NDC number 42 for pre-filled container 22.
The
signal is matched to information in the PPS database 106 on computer 104 to
ioos.a.oi -26-
CA 02509120 2007-09-13
uniquely identify pre-filled container 22 corresponding to the bar code 50.
Reader 86 preferably detects bar code 50 as a gripped pre-filled container 22
is
spun by the drive roller 150 with bar code 50 moving past reader 86. Reader 86
transmits a signal to computer 104 representative of information embedded in
the bar
code 50. Where an optically-detected reference point 52 is utilized, reader 86
may be
further adapted to detect reference point 52 and to communicate that
information to
computer 104. A representative scanner is a Microscan MS-820 industrial bar
code
scanner available from Microscan Systems, Inc. of Renton, Washington. Other
optical systems can be used as reader 86 including camera-based systems and
text-
reading systems.
Reader 86 may also include apparatus to identify pre-filled containers 22
which include machine-readable information other than bar code 50 and other
forms
of optically-read information. For example, reader 86 may include an RFID
transceiver adapted to receive information from RFID tag 51 affixed to pre-
filled
container 22. RFID tag 51 includes all information desired to identify pre-
filled
container 22 and infonnation obtained from RFID tag 51 is transmitted to
computer
104 and is used to identify pre-filled container 22 to system 80. The
infonmation may
include, for example, the NDC code 42 uniquely identifying pre-filled
container 22.
Once pre-filled container 22 is identified, it is matched to the corresponding
prescription order. Information in database 106 is accessed and a signal sent
for
controller 96 via interface 108. Controller 96 interprets the signal and
controls motor
152 to rotate pre-filled container 22 relative to reference point 52 and into
the indicia-
receiving position (Figure 13C) to receive the patient-specific information 54
for the
specific patient prescription order.
Referring to Figures 7 and 9-13G, information application apparatus 10 further
includes a printer (or print engine) 12. In the embodiment, printer 12 is
adapted to
generate a patient-specific labe156 for application to the pre-filled
container 22. An
exemplary labe156 is shown in Figures 3-5. Printer 12 may be a conventional
printer
which preferably includes the capability of generating indica 54 including
both
human-readable information and machine-readable information, such as bar code
78.
Printer 12 includes a print element 200 which prints information on label 56.
wos-oboi -27-
~.. . . .õ
CA 02509120 2005-06-02
Print element 200 may be capable of generating indicia 54 in any suitable
manner
such as by direct thermal, thermal transfer, laser or ink-jet printing on
label 56. A
suitable label printer 12 is a Zebra R110 PAX 3 Print Engine available from
Zebra
Technologies International of Vernon Hills, Illinois. As it is expected that
printer
technology will evolve over time, printers suitable for use as printer 12 and
which are
capable of affixing a wide range of information to a label 56 are expressly
intended to
be within the scope of the invention.
Labels 56 used in conjunction with print element 200 are preferably supplied
in the form of a supply roll 202. Roll-form labels 202 are well-suited for use
in a high
throughput information-application apparatus 10 used in a system such as
system 80.
Roll 202 includes a web 204 of release liner material (e.g., wax-coated paper)
with a plurality of labels 56 spaced apart along the web 204. Labels 56 may be
die
cut along web to form discrete labels. Adhesive-backed side 198 of labels 56
is
removably affixed to web 204 and an opposite side provided to receive
information
from print element 200. Labels 56 may have any suitable dimensions depending
on
the amount of coverage over container indicia 24 desired by pharmacy
management.
As shown in Figure 9, a feed mechanism 205 is provided to unroll web 204
from roll 202 and to supply labels 56 to print element 200 and to pre-filled
container
22. In the embodiment shown, feed mechanism 205 may include any suitable
apparatus for unwinding web 204 of label material from supply roll 202,
feeding web
204 to print element 200 and directing printed labels 56 onto a pre-filled
container 22.
In the example, roll 202 is preferably powered for unwinding by one or more
motors
(not shown). Powered rollers 206, 208, 210, 212, 214 and 216 pull web 204
through
printer 12 for take up by take-up roll reel 218.
Peel bar 219 separates labels 56 from release liner of web 204 by doubling the
web back progressively separating each label 56 from the web 204 starting with
the
label leading edge 220 and progressively moving toward the label trailing edge
222.
As label 56 is separated from web 204, skid plate 224 guides the printed
surface of
each label 56 toward pre-filled container 22. In the embodiment shown, printed
side
of label 56 faces skid plate 224 while adhesive-backed surface 198 faces away
from
skid plate 224 and toward a gripped pre-filled container 22 for application
thereto as
described in more detail below. Waste web material 204 is taken up on reel 218
and
2OV,0&01 -28-
_.~. _ _,..._
CA 02509120 2005-06-02
is discarded.
Application of the patient-specific indicia 54 may be verified by apparatus
10.
The pre-filled container 22 is rotated until the patient-specific bar code 78
is read by
reader 86 and the corresponding patient specific code signal is verified by
computer
104 to a pending prescription order by reference to database 106. (Figure 13E)
After application and verification of label 56, each pre-filled container 22
is
released to conveyor 130 of transport mechanism 84. Conveyor 130 directs the
pre-
filled containers 22 to an apparatus 88 structured to collect and sort pre-
filled
containers 22 shown schematically in Figure 7.
The collection and sortation apparatus shown schematically by reference
number 88 may comprise any suitable apparatus known to persons of skill in the
art
for collecting the pre-filled containers 22 and grouping or sorting such
containers 22
by patient prescription order. Pre-filled containers 22 for a common
prescription
order may be routed into a single lane, placed in a common tote, bag or other
container or otherwise segregated from other pre-filled containers 22. In this
way, all
pre-filled containers 22 according to a single prescription order are grouped
together
thereby facilitating verification by a pharmacist prior to providing the pre-
filled
containers 22 to the patient. The OptiFill brand robotic accumulator
available from
AutoMed Technologies is an example of a collecting and sorting apparatus 88
which
may be employed in system 80. A collection and sortation apparatus 88 is not a
requirement of system 80 as pre-filled containers 22 including patient-
specific indicia
54 may be grouped manually by a phannacy worker by other means. Articles other
than pre-filled container or containers 22 may be grouped with such collected
and
sorted pre-filled containers 22, for example by combining pre-filled
containers 22
with such further articles in a tote assigned to a particular patient
prescription order.
Referring again to Figure 7, information necessary for operation of system 80
resides on computer 104 or is accessible to computer 104. PPS software
residing on
computer 104 or other software controls operation of the system 80 and
components
10, 82, 84, 86, 88. Controllers 94, 95, 96 and 98 interpret signals generated
by
computer 104 to enable operation of the specific component 10, 82, 84, 86, 88.
The
instructions residing on computer 104 enable operation of positioner 14 to
move each
pre-filled container 22 to a label-receiving position, operate printer 12 to
print patient-
"OS~, -29-
.~
CA 02509120 2007-09-13
specific indicia 54 on, for example, labe156 and operate apparatus 10 to apply
indicia
54, for example on label 56, to pre-filled container 22. The instructions are
described
in more detail in connection with the process of operation and methods
described
below.
Database 106 identifying pending prescription orders and each pre-filled
container 22 used in the system resides on computer 104 or is accessible to
computer
104. Database 106 includes information uniquely identifying each pre-filled
container
22 by means of the NDC 42 or other code detected by reader 86. Computer 104
accesses database 106 and retrieves information corresponding to the
prescription
l0 order being filled and the corresponding pre-filled container 22. Pre-
filled container
22 is matched to a pending patient prescription order. In addition, database
106
includes, for each pre-filled container 22, positioning information enabling
positioner
14 to move each pre-filled container 22 to the proper indicia-receiving
position for
receiving a patient-specific label 56.
In the most highly preferred embodiments, database 106 includes infonnation
identifying each pre-filled container 22 and further identifying the time-
duration of
rotational displacement of pre-filled container 22 necessary to position pre-
filled
container 22 adjacent the skid plate 224 to receive label 56 in the desired
location.
This is the indicia-receiving position shown in Figure 13C. The time duration
may be
determined with reference to a time duration required for pre-filled container
22 to
rotate to a desired position once reference point 52 has been identified to
computer
104. Computer 104 through controller 96 triggers operation of printer 12 at
the
appropriate time to locate labe1561eading edge 220 exactly at the desired
position.
The result is precise placement of the labe156 as shown in Figures 4, 5 and
13C-13G.
Database 106 is programmed to generate the appropriate signal triggering
printing
based on the time duration for desired placement of indicia 54 on pre-filled
container
22.
For example, a representative drive roller 150 may have a 6 inch diameter and
be powered to rotate by motor 152 at 30 RPM. The circumference of roller 150
in the
example is 18.84 inches and one full rotation of the drive roller 150 is equal
to 18.84
inches. At 30 RPM, the drive roller 150 rotates 0.5 revolution/second.
Therefore
speed is 9.42 inches/second at 30 RPM (18.84 inches/rotation x 0.5
rotations/second).
2005.0a.o1 -30-
... .... . . .. ._....{..... _....... .. . ..._.,... .. .... .. . .
CA 02509120 2005-06-02
Table 1 sets forth the operational time for rotation of pre-filled container
22
following detection of reference point 52 based on the exemplary conditions
set forth
above.
Table 1
Pre-filled Spacial Distance Between Position of Time Duration of Rotation
Container Pre-fdled Container Relative to (Seconds)
Example Reference Point and Label Leading
Edge Placement (Inches)
1 1.00 0. 106 seconds (106 msec)
2 3.00 0.318 seconds (318 msec)
3 0.50 0.053 seconds (53 msec)
For a selected pre-filled container 22, it may be desired to place the leading
edge 220 of patient-specific label 56 on pre-filled container 22 one inch past
the
position of pre-filled container 22 when reference point 52 is identified to
computer
104 or controller 96. The time duration of rotation would be 0.106 seconds and
printer 12 would be triggered for operation such that the leading edge 220 is
inserted
into nip 194 0.106 seconds after identification of reference point 52 to
computer 104
or controller 96.
While labe156 and indicia 54 are shown applied to a sidewall of pre-filled
container 22, it should be noted that the such indicia 54 may be applied to
other
surfaces such as a bottom surface of pre-filled container 22.
Orientation processes other than by use of timing can be implemented. For
example, positioner 14 may orient pre-filled container 22 by means of
rotational
displacement relative to reference point 52. And, use of a key associated with
pre-
filled container 22 and mating key way can be used to ensure displacement of
pre-
filled container 22 to the desired position so that indicia 54 are precisely
applied to
pre-filled container 22.
Information-application apparatus 10 may be scaled as appropriate to
accommodate the requirements of a particular pharmacy. Figure 15 shows an
altemative information-application apparatus embodiment 10 mounted on an
exemplary automatic pre-filled container dispenser 226. Such embodiment is
2005-0&01 -31-
_ . .._. . a__
CA 02509120 2007-09-13
intended for use in a medium volume pharmacy environment as either a stand-
alone
component or part of an overall WMS system 80.
Figures 16 and 17 show a further alternative embodiment information-
application apparatus 10" in a stand-alone configuration. Such embodiment is
intended for use in a low volume pharmacy environment as a stand-alone
component.
Apparatus 10' and 10" share common parts and operational features with
embodiment 10 and like reference numbers are used to describe like parts of
apparatus
10, 10' and 10". The description of the structure and operation of apparatus
10 is
incorporated herein by reference with respect to apparatus 10' and 10".
Printer 12 is
not shown in Figures 15-17 to facilitate understanding of apparatus 10' and
10".
Referring to Figure 15, embodiment 10' includes a housing 135 (shown in a
partial cutaway view) secured to dispenser 226 and a base 137 secured to
housing 135.
Dispenser 226 automatically dispenses a pre-filled container 22 into chute 128
through top opening 126. Chute 128 directs pre-filled container 22 to base 227
adjacent positioner 14.
Referring to Figures 16 and 17, embodiment 10" includes housing 135 (shown
in a partial cutaway view) and a stand 228 with legs, such as leg 231. Stand
228 is not
required and housing 135 could be adapted to rest on a tabletop surface (not
shown).
A pharmacy worker drops a pre-filled container 22 into chute 236 through top
opening
238. Chute 236 directs pre-filled container 22 to base 227 adjacent positioner
14 as in
embodiment 10'.
Each embodiment 10' and 10" includes a reader 86, a positioner 14 including
a drive roller 150, motor 152, drive roller surface 154 and motor controller
156 as
described in connection with embodiment 10. Embodiments 10' and 10" also
include
a gripper 18 as described in connection with embodiment 10. Gripper 18
includes
gripper member 158, idler roller supports 160, 162, idler rollers 164, 166
actuators
168, 176, 178, pistons 180, 182 and linkages 184, 186, 188 and 190 as
described in
connection with embodiment 10. Member 158 translates on guides 170, 172 and
operates to position a pre-filled container 22 to form a label-receiving nip
194 in the
same manner as described in connection with embodiment 10. Embodiments 10' and
10" accommodate pre-filled containers 22 of different sizes as described in
:003.06A1 -32-
CA 02509120 2007-09-13
connection with embodiment 10.
Each of the information-application apparatus embodiments 10' and 10" is
adapted to operate with a printer (not shown) and printer feed mechanism (not
shown)
of a type as printer 12 and feed mechanism 205 shown and described in
connection
with embodiment 10. The printer is preferably utilized to supply a label 56
for precise
placement on pre-filled container 22. Feed mechanism 205 delivers a web 204 of
label material from a roll to a peel bar and skid plate 224 using powered
rollers and a
take-up reel as with embodiment 10.
Referring to Figures 18A and 18B, each embodiment 10' and 10" is provided
with control apparatus 16. Control apparatus 16 for apparatus 10' may comprise
computer 104 and controller 96 (such as a PLC) as described in connection with
information-application apparatus 10. Control apparatus 16 of embodiment 10"
may
comprise an on-board computer-type controller 104 as represented schematically
in
Figure 18B. Apparatus 10' and 10" may optionally interface with a PIS 100
through
any suitable means including computer 102 and 104. A database 106 residing on
computer 104 (Figure 18A) or computer-type controller 104 enables operation of
embodiments 10' and 10" to precisely apply information 54 as described in
connection with embodiment 10. A user interface 240, such as a touch screen
display,
is provided to permit a pharmacy worker to interface with stand alone
apparatus 10".
Optionally, each of embodiments 10' and 10" is provided with an ejection
mechanism in the form of a linear actuator 230. Piston (not shown) of actuator
230
extends to push a labeled pre-filled container 22 into discharge chute 234 for
manual
removal by a pharmacy worker.
Figures 14A, 14B and 14C are a diagram showing the software logic flow
from the point where a pre-filled container 22 arrives at positioner 14.
Accordingly,
such logic flow describes operation of each of apparatus 10, 10' and 10". The
logic
flow is described in connection with Figures 13A-13G which illustrate
operation of a
positioner 14 utilized in each of embodiments 10, 10' and 10".
As is apparent, the manner in which pre-filled container 22 arrives at
positioner 14 is not critical. For automated systems, any number of different
dispensers and types of container transport mechanisms 84 may be used. A
dispenser
(e.g., dispenser 82 or 226) and container transport mechanism 84 are not
required;
2005.Ob01 -33-
4...
CA 02509120 2005-06-02
pre-filled container 22 may be manually selected from inventory and manually
introduced to apparatus 10 as is noted with alternative embodiments 10 and 10
discussed herein.
The logic flow diagrams 14A, 14B and 14C describe operation of information-
application apparatus 10, 10 and 10 adapted to precisely apply patient-
specific
information 54 by means of labe156. Referring then to Figure 14A, a labeling
cycle
is initiated (point 300) upon delivery of pre-filled container 22 to
information-
application apparatus 10 as indicated in step 302. Once pre-filled container
22 is
delivered to information-application apparatus 10, 10 and 10 , positioner 14
will
capture pre-filled container 22 and position it to receive labe156 (step 304).
Capture of pre-filled container 22 is illustrated in Figures 13A and 13B.
Instructions residing on computer 104 and, optionally on controller 96,
control
operation of actuators 168, 176, 178 to manipulate gripper 18 to clamp pre-
filled
container 22 against drive roller 150. As shown in Figure 13A, actuator 176
partially
retracts idler roller support 162 to permit pre-filled container 22 to pass.
Actuator 178
partially extends idler roller support 160 and idler roller 166 across the
path of a
released container 22 moving on conveyor 130 to block further movement of pre-
filled container 22.
Next, and as shown in Figure 13B, gripper member 158 is moved toward drive
roller by means of actuator 168. Actuators 168, 176 and 178 further extend in
a self-
centering manner until rollers 164 and 166 clamp pre-filled container 22
firtnly
against drive roller 150. The preferred positioner 14 gripper mechanism 18
shown in
Figures 13A-13G will automatically adjust to accommodate multiple pre-filied
container 22 diameters to locate pre-filled containers 22 in the proper
position to
receive a patient-specific labe156. According to step 304, positioner 14
(through
motor 152 and drive roller 150) spins pre-filled container 22 clamped against
drive
roller 150.
In step 306, and as shown in Figure 13B, motor 152 powers drive roller 150 to
rotate counterclockwise in the direction of arrow 192. Spinning of pre-filled
container 22 triggers reader 86 to read machine-readable information 50
identifying
pre-filled container 22 to database 106 on computer 104. As noted elsewhere,
reader
86 is configured to read bar code 50 of indicia 24 or a data storage device 51
2005.A601 -34- . ... .~ ..._ . . . , . ......~ ... . _
CA 02509120 2005-06-02
associated with pre-filled containers 22 to identify container 22.
Reader 86 remains in operation until a determination is made (decision point
308) that the data has been read or a time out occurs at decision point 310.
If the data
is read, the data is transmitted to computer 104 in step 312.
In step 314, computer 104 performs a database query to determine if
information-application apparatus 10, 10 and 10 has the correct pre-filled
container
22 and to determine what time duration parameters to use to apply labe156. As
noted
elsewhere, database 106 can be a local database containing only the
information
required to operate information-application apparatus 10, 10 and 10 or a
remote
database containing information for operation of multiple areas of the
pharmacy.
Preferably, database 106 is integrated directly with the PPS software,
reducing the
amount of redundant data and communications.
In the event that pre-filled container 22 is incorrect or that a time out has
occurred because the container bar code 50 cannot be read (decision point
316),
positioner 14 will stop spinning pre-filled container 22 and release the un-
labeled pre-
filled container 22 at step 318. A reject station (not shown) will reject pre-
filled
container 22 into an error bin (not shown) ending the cycle (point 320).
If pre-filled container 22 is correct, computer 104 will communicate a signal
representing label offset data to the controller 96 and information-
application
apparatus 10, 10 and 10 (step 322). Such offset data may represent a time
duration
between which reference point 52 is identified and when the leading edge 220
of label
56 is to be affixed to pre-filled container 22. Drive roller 150 of positioner
14
preferably will continue to rotate pre-filled container 22. Computer 104
detects the
rotation and triggers reader 86 to read the position of reference point 52 on
pre-filled
container 22 (step 324). Reader 86 has the capability to read container
reference
point 52 component of indicia 24 (and a data storage device 51 if provided).
Reference point 52 and bar code 50 of indicia 24 can be the same component.
One
embodiment of this is to use bar code 50 containing the container
identification
information as both the identification component and reference point 52. As
illustrated in Figures 2 and 5, leading edge of bar code 50 could serve as
reference
point 52.
Referring next to Figure 14B, once reference point 52 is identified (decision
2005-0b01 -35-
f
CA 02509120 2005-06-02
point 326), positioner 14 continues to rotate pre-filled container 22 for a
time duration
based on information provided by computer 104 for the specific pre-filled
container
22 in process. Rotation continues until reference point 52 is in the proper
position in
relation to label printer 12 print element 200 (step 328). If reference point
52 is not
identified within a timeout period (decision point 330), positioner 14 will
stop
spinning pre-filled container 22 and release the unlabeled container 22 (step
332).
The reject station (not shown) will reject pre-filled container 22 into an
error bin
ending the cycle (point 334).
Next, a determination is made at point 336 regarding whether printer 12 is
ready to print patient-specific labe156. Printer 12 may be incapable of
printing due
to a mechanical failure, depletion of ink or shortage of label material 204.
If printer
12 is not ready to print labe156 (decision point 336), then positioner 14
stops rotating
pre-filled container 22 (step 338). Printer 12 is queried again to determine
whether
printer 12 is capable of printing label 56 at decision point 340. The query
continues
until a timeout determination is made at decision point 342. If a timeout
occurs,
positioner 14 releases unlabeled container 22 at step 344 ending the process
at point
346.
Referring again to Figure 14B and to Figures 13C, 13D, if printer 12 is ready,
the positioner 14 spins the pre-filled container 22 (step 348) to the proper
indicia-
receiving position (Figure 13C), label 56 is printed and is fed into nip 194
by feed
mechanism 205 of powered rollers 206-216 associated with printer 12. Drive
roller
150 will spin pre-filled container 22 and wrap printed labe156 around pre-
filled
container 22 (step 350). Figure 13D schematically illustrates a patient-
specific label
56 being pulled through nip 194 with the label adhesive backed side 198 facing
pre-
filled container 22. Figures 4, 5 and 13D-13F show an example in which patient-
specific labe156 is intentionally offset from the edge of the manufacturer's
labe126
based on positioning information in database 106 residing on computer 104.
Referring to Figures 14C and 13E, once patient-specific labe156 is wrapped, a
verification step 352 occurs to confirm that the correct patient-specific
label 56 has
been applied to pre-filled container 22. Reader 86 is triggered in step 352.
Figure
13E schematically illustrates the label verification process. Reader 86 scans
bar code
78 on patient-specific label 56 as pre-filled container 22 is rotated past
reader 86.
2005-0(~01 -36-
. . _ ~. ~....a _ .:.. . .. ~._.. __ _
.... . . . . . ..... ,_..,.{..._. . . ..._. ,___..,õ.. . ....._.. . .
CA 02509120 2005-06-02
A separate reader (not shown) could be provided to read the information on
patient-specific labe156 depending on the nature of that information. For
example,
printer 12 could imprint the label with an RFID transponder or with indicia
(for
example a 2D bar code) more suitable for detection with a camera. Whether
reader
86 comprises one or more devices, reader 86 must have the ability to read the
label
verification component of indicia 54 or data storage device.
At decision point 354, a determination is made regarding whether bar code 78
on labe156 has been properly read. If bar code 78 has not been read, reader 86
continues to attempt to read bar code 78 until a timeout occurs (decision
point 356).
If the bar code 78 has been properly read, the label data is compared in
database 106
against the expected data for a pending prescription order being fulfilled and
a
pass/fail determination is made (decision point 358).
If the timeout occurs or if the labe156 and bar code 78 fails the test at
decision
point 358, the positioner device 14 will stop spinning and release pre-filled
container
22 (step 360). The computer 104 will then record the container 22 as "bad" or
defective (step 362) and the cycle is ended at point 364.
If the label passes the test at decision point 358, then positioner device 14
will
stop spinning and release pre-filled container 22 (step 366). In this event,
computer
104 will record pre-filled container 22 as "good" at (step 368). Pre-filled
container 22
will be released for fulfillment of the patient prescription order ending the
labeling
cycle at point 364.
Figure 13F schematically illustrates the process wherein labeled pre-filled
container 22 is released. Actuator 168, retracts member 158 and actuators 168,
170
retract a respective support 160, 162. Labeled, pre-filled stock container 22
is now
free to move away from the information application apparatus 10 under the
power of
conveyor 130.
Optional additional verification steps not shown on Figures 14A- 14C may be
undertaken. For instance, conveyor 130 may transport labeled pre-filled
container 22
to reject station (not shown) where labe156 is again read by a reader (not
shown). If
the information in bar code 78 matches the expected data, pre-filled container
22 is
considered "good" and will travel beyond the reject station. If the
information in bar
code 78 does not match the expected data, or the if the data in bar code 78
was
20O3-0bo1 -37-
... _. .._._. ... .~_ ..~..,_.. . _ _
~., . .
_ . _ _ .. .. . . . . ..,.. ,# . ._..... . ..._:....,. . : . . ... .... . .
CA 02509120 2005-06-02
unreadable, pre-filled container 22 is considered "bad" and will be rejected
into an
error bin.
Referring to Figures 4 and 5, the output of information-application apparatus
10, 10 and 10 is a patient-specific pre-filled container 22 with information
specific
to the patient prescription order applied thereto. The pre-filled, non-
specific stock
container 22 has been converted to a patient-specific container 22 suitable
for
fulfillment of a patient prescription order without any requirement for
special
modification.
Advantageously, patient-specific labe156 is precisely positioned on pre-filled
container 22 so as to optimize the value of indicia 24 provided by the
manufacturer,
repackager or other supplier. Referring specifically to exemplary pre-filled
container
22 in Figures 4 and 5, it is immediately apparent that patient-specific
labe156 has
been precisely positioned in a way which leaves lot number 38, expiration date
40,
medication name 32, strength 34, quantity 44, bar code 50 and NDC 42
accessible for
use by pharmacy management and the patient. This valuable information has not
been covered and rendered inaccessible as would be the case with
indiscriminate
placement of the patient-specific labe156 on the pre-filled container 22.
A pharmacist or other person working in the pharmacy, therefore, may be able
to use both the information embodied in indicia 24 supplied with each pre-
filled
container 22 as well as information embodied in indicia 54 provided on patient-
specific labe156 to verify the suitability of the medication for the patient.
Bar code
50 supplied with pre-filled container 22 can be scanned together with the
patient-
specific bar code 78 to provide machine verification that the contents are
suitable for
the patient and are as called for by the prescription order. The ability to
match pre-
filled container 22 contents with patient labe156 automatically may remove the
need
to have a pharmacist verify the prescription order, thus creating a
significant
reduction in the cost associated with filling a prescription order. Also as
shown in
Figures 4 and 5, it is apparent that the patient may easily read indicia 24
supplied with
pre-filled container 22. This presents a further opportunity for the patient
to ensure
that the correct medication or other product has been provided.
* * *
While the principles of this invention have been described in connection with
2005-0", -38-
,.. .. .. _ .~_ __...._ _
_._. ....-.~__.. .
.. . . .~,. . . ._ . .
CA 02509120 2005-06-02
specific embodiments, it should be understood clearly that these descriptions
are
made only by way of example and are not intended to limit the scope of the
invention.
-39-
... . .......~.~ ... , _ .