Note: Descriptions are shown in the official language in which they were submitted.
CA 02509953 1998-02-12
1
PROCESS FOR MAKING
GLYPHOSATE BY OXIDIZING N-SUBSTITUTED GLYPHOSATES
This application is a divisional of Canadian Patent
Application S.N. 2,275,866, filed February 12, 1998.
BACKGROUND OF THE INVENTION
This invention generally relates to a process
for converting N-substituted N-(phosphonomethyl)glycines
(sometimes referred to as "N-substituted glyphosate"), as
well as esters and salts thereof, to N-
(phosphonomethyl)glycine (sometimes referred to as
"glyphosate"), as well as esters and salts thereof, via a
noble-metal catalyzed oxidation.reaction. This invention
is particularly directed to converting N-substituted
glyphosates, as well as esters and salts thereof, having a
single N-carboxymethyl functionality.
Glyphosate is described by Franz in U.S. Patent
No. 3,799,758 and has the following formula:
1= 0~~~ N P O H
~
Glyphosate and its salts conveniently are applied as a
post-emergent herbicide in an aqueous formulation. It is
a highly effective and commercially important broad-
spectrum herbicide useful in controlling the growth of
germinating seeds, emerging seedlings, maturing and
established woody and herbaceous vegetation, and aquatic
plants.
Various methods for making glyphosate from N-
substituted glyphosates are known in the art. For
example, in U.S. Patent No. 3,956,370, Parry et al. teach
that N-benzylglycine may be phosphonomethylated to N-
benzyl glyphosate, and then reacted with hydrobromic or
hydroiodic acid to cleave the benzyl group and thereby
produce glyphosate. In U.S. Patent No. 3,927,080,
Gaertner teaches that N-t-butylglycine may be
phosphonomethylated to form N-t-butyl glyphosate, and then
CA 02509953 1998-02-12
2
converted to glyphosate via acid hydrolysis. Glyphosate
also may be produced from N-benzyl glyphosate via
hydrogenolysis, as described, for example, in European
Patent Application No. 55,695 and Maier, L. Phosphorus,
Sulfur and Silicon, 61, 65-7 (1991). These processes are
problematic in that they produce undesirable byproducts
such as isobutylene and toluene which create difficulties
due to their potential toxicities. Moreover, acid
hydrolysis and hydrogenation of N-substituted glyphosates
has been demonstrated only for alkyl groups such as
tertiary butyl and benzyl groups which are known to be
susceptible to such reactions. Dealkylation of N-methyl,
N-isopropyl, and other N-substituted glyphosates which are
not readily susceptible to acid hydrolysis or catalytic
hydrogenation has not been demonstrated.
Other methods for making glyphosate are directed
to oxidatively cleaving N-(phosphonomethyl)iminodiacetic
acid (sometimes referred to as "PMIDA"):
HOzC03H2
COZH
PMIDA may be synthesized from phosphorus trichloride,
formaldehyde, and an aqueous solution of the disodium salt
of iminodiacetic acid, as described by Gentilcore in U.S.
Patent No. 4,77S,498:
N0 C HpC
NH . PC I 3 - 3H2 0 NH( ~) + H3P03 - 2NcC (+ ) + r= I
~.dOz: HUZ(,.. /
CH.O HO2C~ P0Hz
-' N~
CG2
PM1 DA
It is well-known in the art that PMIDA may be converted
into glyphosate by heterogeneous oxidation over carbon
CA 02509953 1998-02-12
3
catalysts as described, for example, in U.S. Patent No.
3,950,402 to Franz and U.S. Patent No. 4,654,429 to
Balthazor et al.; by homogenous catalytic oxidatiori as
described, for example, in Riley et al. J. Amer. Chem.
Soc. 113, 3371-78 (1991) and Riley et al. Inorg. Chem. 30,
4191-97 (1991); and by electrochemical oxidation using
carbon electrodes as described, for example, in U.S.
Patent No. 3,835,000 to Frazier et al. These oxidation
methods, however, have been reported to be useful only for
preparing glyphosate from PMIDA, an N-substituted
glyphosate having two N-carboxymethyl functionalities.
None of these prior art oxidation methods have been
reported to be useful for preparing glyphosate from N-
substituted glyphosate compounds having only one N-
carboxymethyl functionality, i.e., where R' in the
following formula is other than -CHZCOzH:
II Il~u+
HJ-_-~rl -fti-CH.'
I ~UH
F
To the contrary, many prior art references suggest that if
R' is a functionality other than a-CHZCOzH group, the
prior art methods will cleave the -CH2CO2H group rather
than R', and will therefore fail to produce glyphosate.
This is particularly true for the prior art which is
directed to heterogenous catalytic oxidations over carbon
and electrochemical oxidations using carbon electrodes.
The mechanisms for these oxidations are well known in the
art, particularly for electrochemical oxidations where it
is known as the Kolbe reaction, described in various
organic electrochemistry books, e.g., S. Torii and H.
Tanaka, Organic Electrochemistry 535-80 (H. Lund and M.M.
Baizer eds., Marcel Dekker, 3rd ed. 1991). Both
mechanisms involve the oxidative degradation of carboxylic
acid to a carbon radical and carbon dioxide:
CA 02509953 2006-05-16
4
HOZC--~ PO3HZ
~N-/ \N____/03HZ
C02C -e HO2C
PMIDA + COZ + H+
carbon catalyst
or carbon
electrode
-e +HZO
H
I
HOZCN~ /PO3H2 + CH2O + H+
glyphosate .v
There is no suggestion that these mechanisms could be used
to cleave any other functionality besides -CHzCO2H.
Thus, a more general method for oxidizing N-
substituted glyphosates to glyphosates is therefore
desirable. Such a method would allow a wider range of N-
substituted glycines to be used as raw materials for the
production of glyphosate. Such a method also could be used
to make glyphosate from N-methylglyphosate (sometimes
referred to as " NMG "), an undesirable byproduct from the
carbon-catalyzed oxidation of PMIDA.
SUMMARY OF THE INVENTION
Among the aspects of the invention, therefore,
is to provide a process for making glyphosate (as well as
its salts and esters) by oxidizing N-substituted
glyphosates (as well as salts and esters thereof). More
particularly, it is an aspect of this invention to provide
a process for making glyphosate (as well as its salts and
esters) by oxidizing N-substituted glyphosates (as well as
salts and esters thereof) having a single N-carboxymethy'l
functionality. For example, it is an aspect of this
invention to provide a process for making glyphosate by
oxidizing NMG.
Briefly, therefore, the present invention is
directed to a novel process for making a composition
having the formula (I):
CA 02509953 2006-05-16
o II ~
OFf
l I
R~P~C-CNj-i''GM2-p~,
oR~
(I~
In this formula, R', R', and R' are i.ndependently hydregen,
substituted or unsubstituted hydrocarbyl, or an
S agronor;i-cally acceptable catiorn. This invention coa:prises
contActir.g a solution with a noble metal catalyst and
introducing oxygen into the solutior.. The solutien
contains an N-substituted glyphosate havir.g the formula
('_'I)
I 0
I _
RC,CH~ i CFi, ~p"
ORs
R
R2
(II)
In formula (II) , RI and R' are independently hydrogen,
halogen, -PO3H2, -SO3H, -Noõ or subatituted or
unsubstituted hydrocarbyl other than -CO2F:. R', R', and R'
are as defined above for formula (1) above.
In another embodiment of this invention, the
composition (i.e., formula (I)) to be prepared +s
glyphosate or a salt therecE, and the N-substituted
glyphosate (i.e., formula (II)) is NM57 or a salt thereof.
During the process, a solution having a temperature of
from about 125 to about 150 C and containing NMG or a salt
thoreoE is contacted with a noble metal catalyst
comprising platinum. Also during the process, 2,2,6,6-
tetramethyl piperidine N-oxide is added to tae eolutior..
Further, oxygen is introduced into the solution at a rate
which imparts a finite dissrolved oxygen concentration in
the solution that is no greater than 2.0 ppm.
In accordance with a preferred aspect of the present:
invention, there is provided an oxidation catalyst comprising a
noble metal having an electroactive molecular species with an
oxidation potential of at least about 0.3 volts vs. saturated
calomel electrode (SCE) adsorbed thereon.
CA 02509953 2006-05-16
5a
In accordance with the above, the
preferred noble metal is selected from the group
consisting of platinum, palladium, rhodium, iridium,
osmium, gold and combinations thereof. Preferably the
noble metal is selected from the group consisting of
platinum and palladium.
In a further preferred embodiment of the
present invention, there is provided an oxidation
catalyst where the concentration of noble metal on the
surface of the support is from about 3 to about 7.5
wt.% based on the total mass of the catalyst.
CA 02509953 1998-02-12
6
BRIEF DESCRIPTION OF THE DRAWINGS
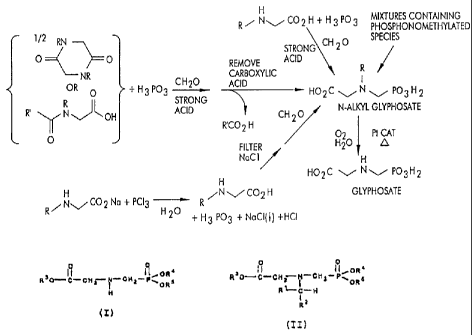
Figure 1 shows the chemical steps that may be
taken to produce glyphosate in accordance with this
invention using various N-substituted glycine precursors.
Figure 2 summarizes various compounds that may
be produced during the oxidation of NMG.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention provides a novel and
useful method for manufacturing glyphosate, its salts, and
its esters, in an aqueous medium wherein an N-substituted
glyphosate or a salt or ester thereof (collectively
referred to as "N-substituted glyphosate reactant") is
oxidatively cleaved with oxygen over a noble metal
catalyst. Advantages of preparing glyphosate from N-
substituted glyphosates using this method include the
simplicity of the procedure, the low cost of the oxidant
(e.g., air or molecular oxygen), and the durability of the
catalyst (i.e., little or no deactivation of the catalyst
over several cycles).
Unlike the prior art methods for oxidatively
cleaving N-substituted glyphosates to make glyphosate,
this method is not limited to the oxidation of PMIDA
(which has two N-carboxymethyl functionalities). Instead,
this method also may be used to make glyphosate by
oxidatively cleaving N-substituted glyphosates having only
one N-carboxymethyl functionality. This invention,
therefore, significantly widens the range of N-substituted
glyphosates that may be oxidized to make glyphosate.
This, in turn, significantly widens the range of N-
substituted glycines (a precursor to many N-substituted
glyphosates) which may serve as the raw material to
prepare glyphosate. This invention also is valuable
because it provides a method to prepare glyphosate from
NMG, an undesirable byproduct from the carbon-catalyzed
oxidation of PMIDA.
CA 02509953 1998-02-12
7
T'ne N-substiituted glyphcsate reactants of the
present invention have the following for:nula r
0
.
RsO--c-CFi= i-OHa 'P~oR'
Rt-C~'H
t2
R
wherein preferably Ri and 8I are independently hydrogen,
halogen, -P01H=, -SO3H, -Na3, or a subst;.tuted. or
unsubstituted hydrocarbyl other than -CO,H; and R', R', and
Rs aze independently hydrogen, a subatituted or
unaubstituted hydrocarbyl, or ar, agronomically acceptable
cation.
As ueed herein, the term hydrocarbyln is
defined as a radical consisting exclusively of carbon and
hydrogen. The hydrecarbyl may be branched or urtbranched,
may be saturated or uneaturated, and may contain one or
more rings. Suitable hydrocarbyl moieties include alkyl,
alkenyl, alkyny?, and aryl moieties. They also include
alkyl, alkenyl, alkynyl, and aryl moieties substituted
with other aliphatic or cyclic hydrocarbyl groups, guch as
alkaryl, alkena.ryl and alkynaryl.
The term Bsubstituted hydrocarbyl" is defined as
a hydrocarbyl wherein at leaat one hydrogen atom has been
substituted with an atom other than hydrogen. For
example, the hydrogen atom may be replaced by a halogen
atom, such as a chlorine or fluorine atom. The hydrogen
atom alternatively may be substituted by an oxygen atom to
form, for example, a hydroxy gxoup, an eeher, an ester, an
anhydride, an aldehlyde, a ketone, or a carboxylic acid
~except that neither Rt nor R' may be a carboxy group,
i.e., -COHj . The hydrogen atom also may be replaced by a
nitrogen atom to form an amide or a nitro functionality,
although substitution by nitrogen to form an amine or a
nitrile functionality preferably shoul8 be avoided. :n
addition, the hydrogen atom may be replaced with a sulfur
CA 02509953 1998-02-12
B
atont to form, for example, -SO,H, although substitution by
sulfur to form a thiol should be avoided.
It should be recognized that R1 and R' may
together form a ring. This ring may be either a
hydrocarbon ring or a hetarocycle, and at least one
hydrogen on the ring may be substituted as described above
for substituted hydrocarbyl functionalitiea.
Xn a preferred embodiment, Rl, R', R', and Rs are
each hydrogen, and R1 is a linear, branched, or cyclic
hydrocarbyl containing up to about 19 carbon atoms. In a
more preferred embodiment, R', lt', and R are each
hydxogen, and -CHR1R' is methyl ( i. e., Rl and R= are
hydrogen), i9opropyl (i.e., R1 and Z2 are -CH,), benzyl
(i.e., R' is hydrogen and R2 is phenyl), or n-pentyl (i.e.,
R= ia hydrogen and R' is a 4-carbon, straight-chain
hydrocarbyl).
Many N-substituted glyphosate reactants may be
prepared by phosphonomethy7.ating the corresponding iJ-
substituted glycines, their salts, or their amides, for
example, by the following reaction:
/NH . CMiQ + HIPO3
R' R'
Phoaphonomethylation of secondary amines is well-knowri in
the art, and discussed at length in Redmore, D. Tooics in
DhosBho ous Chemistry, Vol. 8, 515-95 (E.G. Griffith & M.
Grayson eds., Johr. Wiley & Sona 1976); and in a chapter
entitled "a-substituted Phosphonates" in Mastalerz, P.
Handbook of OrganQnhosphorus Chg=tstKy 277-375 (Robert
F=ngel ed., Marcel Dekker 1992).
Several methods may be used to prapare Ny-
substituted glycinca and their salts and amidQe. In one
embodiment of this invention, the N-substituted glycine is
prepared by the condensation of hydrogen cyanide,
CA 02509953 1998-02-12
9
formaldehyde, and N-substituted amines, followed by
hydrolysis to N-substituted glycine or a salt thereof:
H O
, N H C H C = H C N N
2 2 H2(~ q~ '~H
This reaction is known as the Strecker synthesis. The
Strecker synthesis is well-known in the art and is
described in Dyker, G. Angewandte Chimie Int'l Ed. in
English, Vol. 36, No. 16, 1700-2 (1997). The resulting N-
substituted glycine may be converted to an N-substituted
glyphosate by reacting it with formaldehyde and
phosphorous acid (H3P03) in the presence of a strong acid.
In a different embodiment of this invention, the
N-substituted glycine is prepared by dehydrogenation of N-
substituted ethanolamine in the presence of a base
(preferably sodium hydroxide) to form salts of N-
substituted glycines:
Cu CaC31v~t H 0
71 Ff . NdCH zH z
Ori
H
This reaction is described by Franczyk in U.S. Patent Nos.
5,292,936 and 5,367,112, and by Ebner et al. in U.S.
Patent No. 5,627,125. The N-substituted ethanolamine
precursor may be prepared in at least two ways. First,
ketones may be condensed with monoethanolamine in the
presence of hydrogen, a solvent, and a noble metal
catalyst. This reaction is described in Cope, A.C. and
Hancock, E.M. J. Am. Chem. Soc., 64, 1503-6 (1942). N-
substituted ethanolamines also may be prepared by reacting
a mono-substituted amine (such as methylamine) with
ethylene oxide to form the mono-substituted ethanolamine.
This reaction is described by Y. Yoshida in Japanese
Patent Application No. 95-141575. The resulting N-
substituted glycine salt may be converted to N-substituted
CA 02509953 1998-02-12
glyphosate by reacting it with phosphorus trichloride
(PC13) in water, and then filtering out the salt and adding
formaldehyde.
In an alternative embodiment of this invention,
5 N-substituted glycine is prepared by condensation of N-
substituted amides, formaldehyde, and carbon monoxide in
the presence of a catalyst:
q 0 H 0
p Co ca;aiys: ~ ~
c H G ~~ N
A 2 -'-~
N
OH F1~0 i G/ =i~
H ~}
This reaction (i.e., carboxymethylation) is described by
10 Beller et al. in European Patent Application No. 0680948;
and Knifton, J.F. Applied Homogeneous Catalysis 159-68 (B.
Cornils et al. eds., VCH, Weinheim, Germany 1996). The
product of this reaction is the N-acetyl of the N-
substituted glycine which may be hydrolyzed to the N-
substituted glycine. The N-substituted glycine then may
be converted into the corresponding N-substituted
glyphosate by reacting it with phosphorous acid and
formaldehyde in the presence of a strong acid, and then
removing the carboxylic acid by methods generally known in
the art, such as distillation or membrane separation.
In a further embodiment of this invention, the
N-substituted glycine is prepared by the reductive
alkylation of glycine achieved by reacting carbonyl
compounds with glycine and hydrogen in the presence of a
catalyst:
H 0
o Hz ~
HzN~ . -i R~N
Gcatalyst H H 20
~~
R
This reaction is described by Sartori et al. in U.S.
Patent No. 4,525,294. The N-substituted glycine may be
CA 02509953 1998-02-12
11
converted to N-substituted glyphosate by reacting it with
formaldehyde and phosphorous acid in the presence of a
strong acid.
This inveintion also provides a new and useful
method for conversion of N-substituted glyphosates which
are not derived from the phosphonomethylation of N-
substituted glycines. For example, this method is
particularly useful for making glyphosate from NMG, an
undesirable byproduct from the carbon-catalyzed oxidation
of PMIDA.
Figure 1 summarizes the methods for preparing
glyphosate from the materials discussed above. The
symbols used in Figure 1 have the usual meanings familiar
to those skilled in the art.
To oxidize the N-substituted glyphosate
reactant, it preferably is first mixed with water and then
fed into a reactor along with an oxygen-containing gas or
a liquid containing dissolved oxygen. In the presence of
a noble metal catalyst, the N-substituted glyphosate
reactant is oxidatively converted into glyphosate and
various byproducts:
0 G
II II~'pa O. Hzo II Il~oa'
F3G-C-C1z-N-P~ brPr:,:ljcts
1 I -~ Noble metdi oN '~ Cat isc ~
I v
P'
wherein R1, R2, R3, R', and RS are defined as above. In a
preferred embodiment, the catalyst subsequently is
separated by filtration and the glyphosate then is
isolated by precipitation, for example, by evaporation of
a portion of the water and cooling.
The amount of N-substituted glyphosate reactant
in the aqueous medium is typically from about 1 to about
80 wtA ([mass of N-substituted glyphosate reactant =
total reaction mass] x 100%). More preferably, the amount
of N-substituted glyphosate reactant is from about 5 to
CA 02509953 1998-02-12
12
about 50 wt.%, and most preferably from about 20 to about
40 wt.%.
Preferably, the reaction is conducted at'a
temperature of from about 50 to about 200 C. More
preferably, the reaction is conducted at a temperature of
from about 70 to about 150 C, and most preferably from
about 125 to about 150 C.
The pressure in the reactor during the oxidation
generally depends on the temperature used. Preferably,
the pressure is sufficient to prevent the reaction mixture
from boiling. If an oxygen-containing gas is used as the
oxygen source, the pressure also preferably is adequate to
cause the oxygen to dissolve into the reaction mixture at
a rate sufficient to sustain the desired rate of reaction.
The pressure preferably is at least equal to atmospheric
pressure. Preferably, the pressure is from about 30 to
200 psig. More preferably, when the temperature is in the
most preferred range of from about 125 to about 150 C, the
pressure is from about 40 to about 100 psig.
The oxygen source for the oxidation reaction may
be any oxygen-containing gas or a liquid containing
dissolved oxygen. Preferably, the oxygen source is an
oxygen-containing gas. As used herein, an "oxygen-
containing gas" is any gaseous mixture containing
molecular oxygen which optionally may contain one or more
diluents which are non-reactive with the oxygen or the
reactant or product under the reaction conditions.
Examples of such gases are air, pure molecular oxygen, or
molecular oxygen diluted with helium, argon, neon,
nitrogen, or other non-molecular oxygen-containing gases.
Preferably, at least about 20% by volume of the oxygen-
containing gas is molecular oxygen, and more preferably,
at least about 50% of the oxygen-containing gas is
molecular oxygen.
The oxygen may be introduced by any conventional
means into the reaction medium in a manner which maintains
CA 02509953 1998-02-12
13
the dissolved oxygen concentration in the reaction mixture
at the desired level. If an oxygen-containing gas is
used, it preferably is introduced into the reactiori medium
in a manner which maximizes the gas' contact with the
reaction solution. Such contact may be obtained, for
example, by dispersing the gas through a diffuser such as
a porous glass frit or by sintering, shaking, or otrier
methods known to those skilled in the art.
The oxygen preferably is fed to the reaction
mixture at a rate which is sufficient to maintain the
dissolved oxygen concentration at a finite level. More
preferably, the oxygen is fed at a rate sufficient to
maintain the dissolved oxygen concentration at no greater
than about 2.0 ppm, but at a high enough concentration to
sustain the desired reaction rate. It should be noted
that the partial pressure of the oxygen in the reactor
affects the rate at which oxygen is delivered to the
reaction mixture and preferably is from about 0.5 to about
10 atm.
The catalyst used in this invention comprises a
noble metal, preferably platinum (Pt), palladium (Pd),
rhodium (Rh), iridium (Ir), osmium (Os), or gold (Au). In
general, platinum and palladium are more preferred, and
platinum is most preferred. Because platinum is presently
most preferred, much of the following discussion will be
directed to use of platinum. It should be understood,
however, that the same discussion is generally applicable
to the other noble metals and combinations thereof.
The noble metal catalyst may be unsupported,
e.g., platinum black, commercially available from various
sources such as Aldrich Chemical Co., Inc., Milwaukee, WI;
Engelhard Corp., Iselin, NJ; and Degussa Corp., Ridgefield
Park, NJ. Alternatively, the noble metal catalyst may be
deposited onto the surface of a support, such as carbon,
alumina (A1Z03) , silica (SiO2) , titania (TiO2) , zirconia
(ZrOZ), siloxane, or barium sulfate (BaSO4), preferably
CA 02509953 1998-02-12
14
silica, titania, or barium sulfate. Supported metals are
common in the art and may be commercially obtained from
various sources, e.g., 5% platinum on activated carbon,
Aldrich Catalogue No. 20,593-1; platinum on alumina
powder, Aldrich Catalogue No. 31,132-4; palladium on
barium sulfate (reduced), Aldrich Catalogue No. 27,799-1;
and 5% Palladium on activated carbon, Aldrich Catalogue
No. 20,568-0. As to carbon supports, graphitic supports
generally are preferred because such supports tend to have
greater glyphosate selectivity.
The concentration of the noble metal catalyst on
a support's surface may vary within wide limits.
Preferably it is in the range of from about 0.5 to about
wt.% ([mass of noble metal = total mass of catalyst] x
15 100%), more preferably from about 2.5 to about 10 wt.%,
and most preferably from about 3 to about 7.5 wt.%. At
concentrations greater than about 20 wt.%, layers and
clumps of noble metal tend to form. Thus, there are fewer
surface noble metal atoms per total amount of noble metal
20 used. This tends to reduce the catalyst's activity and is
an uneconomical use of the costly noble metal.
The weight ratio of the noble metal to the N-
substituted glyphosate reactant in the reaction mixture
preferably is from about 1:500 to about 1:5. More
preferably, the ratio is from about 1:200 to about 1:10,
and most preferably from about 1:50 to about 1:10.
In a preferred embodiment, a molecular
electroactive species (i.e., a molecular species that may
be reversibly oxidized or reduced by electron transfer) is
adsorbed to the noble metal catalyst. It has been
discovered in accordance with this invention that
selectivity and/or conversion of the noble metal catalyst
may be improved by the presence of the electroactive
molecular species, particularly where the catalyst is
being used to effect the oxidation of NMG to form
glyphosate. In this instance, the electroactive molecular
CA 02509953 1998-02-12
species preferably is hydrophobic and has an oxidation
potential (E,) of at least about 0.3 volts vs. SCE
(saturated calomel electrode). Many such oxidatiori
potentials may be found in the literature. A compilation
5 showing the oxidation potential and reversibility for a
large number of electroactive molecular species may be
found in Encyclopedia of Electrochemistry of the Elements
(A. Bard and H. Lund eds., Marcel Dekker, New York,
publication dates vary between volumes). Specific
10 references showing the oxidation potentials for
electroactive molecular species are: for
triphenylmethane, Perichon, J., Herlem, M., Bobilliart,
F., and Thiebault, A. Encyclopedia of Electrochemistry of
the Elements vol. 11, p. 163 (A. Bard and H. Lund eds.,
15 Marcel Dekker, New York, NY 1978); for N-
hydroxyphthalimide, Masui, M., Ueshima, T. Ozaki, S.
J.Chem. Soc. Chem. Commun. 479-80 (1983); for tris(4-
bromophenyl)amine, Dapperheld, S., Steckhan, E.,
Brinkhaus, K. Chem. Ber., 124, 2557-67 (1991); for
2,2,6,6-tetramethyl piperidine N-oxide, Semmelhack, M.,
Chou, C., and Cortes, D. J. Am. Chem. Soc., 105, 4492-4
(1983); for 5,10,15,20-tetrakis(pentafluorophenyl)-
21H,23H-porphine iron (III) chloride, Dolphin, D.,
Traylor, T., and Xie, L. Acc. Chem. Res., 30, 251-9
(1997); and for various porphyrins, Fuhrhop, J.H.
Porphyrins and Metalloporphyrins 593 (K. Smith, ed.,
Elsevier, New York, 1975).
Electroactive molecular species also are useful
in the context of the oxidation of N-isopropyl glyphosate
to form glyphosate. In that context, an electroactive
molecular species preferably is adsorbed to a noble metal
catalyst on a graphitic carbon support. In the presence
of the graphitic carbon support, the electroactive
molecular species has been found to increase the noble
metal catalyst's glyphosate selectivity.
CA 02509953 1998-02-12
16
Examples of generally suitable electroactive
molecular species include triphenylmethane; N-
hydroxyphthalimide; 5,10,15,20-
tetrakis(pentafluorophenyl)-21H,23H-porphine iron (III)
chloride (abbreviated "Fe(III)TPFPP chloride"); 2,4,7-
trichlorofluorene; tris(4-bromophenyl)amine; 2,2,6,6-
tetramethyl piperidine N-oxide (sometimes referred to as
"TEMPO"); 5,10,15,20-tetraphenyl-21H,23H-porphine
iron(III) chloride (sometimes referred to as "Fe(III)TPP
chloride"); 4,4'-difluorobenzophenone; 5,10,15,20-
tetraphenyl-21H,23H porphine nickel(II) (sometimes
referred to as (Ni(II) TPP"); and phenothiazine. When the
noble metal catalyst is being used to catalyze the
oxidation of NMG to glyphosate, the most preferred
electroactive molecular species include N-
hydroxyphthalimide; tris(4-bromophenyl)amine; TEMPO;
Fe(III)TPP chloride; and Ni(II) TPP.
Electroactive molecular species may be adsorbed
to the noble metal catalyst using various methods
generally known in the art. The electroactive molecular
species may be added directly to the oxidation reaction
mixture separately from the noble metal catalyst. For
example, 2,2,6,6-tetramethyl piperidine N-oxide ("TEMPO")
may be added to the reaction mixture without first being
adsorbed to the noble metal catalyst, as illustrated in
Example 13. Using this method, the electroactive
molecular species adsorbs to the noble metal catalyst
while in the reaction mixture. Alternatively, the
electroactive molecular species is adsorbed to the noble
metal catalyst before being added to the oxidation
reaction mixture. Generally, the electroactive molecular
species may be adsorbed to the catalyst using, for
example, liquid phase deposition or gas phase deposition.
Example 8 illustrates using liquid phase deposition to
adsorb the electroactive molecular species.
CA 02509953 1998-02-12
17
The oxidation reaction preferably is carried out
in a batch reactor so that the reaction may be contained
until the conversion to glyphosate is complete. However,
other types of reactors (e.g., continuous stirred tank
reactors) also may be used, although preferably: (1) there
should be sufficient contact between the oxygen, N-,
substituted glyphosate reactant, and the catalyst; and (2)
there should be adequate retention time for substantial
conversion of the N-substituted glyphosate reactant to
glyphosate.
It should be noted that this invention has the
ability to oxidize N-substituted glyphosates in the
presence of other chemical species which may arise in the
course of previously known methods for preparing
glyphosate. For example, this invention has the ability
to oxidize NMG in the presence of phosphoric acid or
phosphonomethylated species which are byproducts of the
carbon-catalyzed oxidation of PMIDA, such as
aminomethylphosphonic acid ("AMPA"), N-methyl-
aminomethylphosphonic acid ("MAMPA"), and glyphosate.
It should be further recognized that this
reaction process may be conducted where a sub-
stoichiometric amount (i.e., less than one equivalent) of
base is present in the reaction mixture. The presence of
the base, however, may be deleterious to selectivity under
some reaction conditions.
EXAMPLES
General
Most of the examples below describe the
oxidation of NMG to form glyphosate. In addition to
glyphosate, MAMPA and phosphoric acid (H3PO4) also may
form. Further, the glyphosate product may further oxidize
to form AMPA. This is summarized in Figure 2.
CA 02509953 1998-02-12
18
High pressure liquid chromatography ("HPLC ) was
used to analyze the products formed during the reactions
digcussed in the following examples. An fon exchange
separation was used, and the analytes were detected using
W/visible detection following post-column reaction to
for<a a phoaphomolybdate complex. This method can
distinguieh between, A'MG, glyphosate, and phosphoric acid,
but AMPA and MAMPA coelute. 8ecause AMPA and tr1AIMPA have
the same response factor, on a molar basis, the aum of the
AMPA and MAMPA concentrations can be reliably determined.
This value is reported as (M)AMPA in the examples below.
SxAMPLE 1.
This example illustrates a typical synthesis of
NMG. Approximately 69.9 g sarcosine (1.00 mole), 82,0 g
phosphorous acid (1.0 mole), and iio g concantXatied
hydxachloric acid weza mixed and refluxed in a 130 C oil
bath. Next, 89.3 g of 37!k forrnalin (1.1 mole) was added
dropwise over 20 minutes and the reaction was continued
for an additional 85 minutes. Rt tnis point, NMR revealed
the fvllowing product distribution (on a molar basis):
89.94 lrYG, 2.1% phosphorous acid, 1.9ir phosphoric acid,
0.4-t hydroxymethyl phosphorous acid, and 5.71 of an
unknown product (1v7M: triplet, 8.59 ppm). After cooling
to room temperature, 40 g sodium hydroxide was added,
followed by 250 g water. This led to the formation of a
white precipitate which subsequently was recovered by
filtration and assayed by HPLC. The total recovered yield
of N'MG was 70,54 based on the ameunt of sarcosine and
phosphorous acid used.
Other N-a;kyl glyphosates also may be ir.ade in a
similar manne_.
E7LAMPLE 2.
Thia exa:mple illustrates the conversion of RMG
to glyphoeate using a Pt catalyst and oxygen.
CA 02509953 1998-02-12
19
Approximately 10.0 g NMG, 140 g water, and 1 g
platinum black (Aldrich Chemical Co., Inc., Milwaukee, WI)
were combined in a round bottom flask equipped with a
water-cooled reflux condenser immersed in a 150 C oil
bath. Oxygen was bubbled through for four hours as the
solution was stirred. At the end of this period, HPLC
analysis revealed the following product distributions (on
a molar basis): 86.4% glyphosate, 8.7% NMG, 2.2% (M)AMPA,
and 2.7% phosphoric acid. Glyphosate precipitated from
the solution after cooling to room temperature.
In a second experiment, a mixture of 10.0 g NMG,
2.0 g platinum black, and sufficient water to bring the
total volume of the mixture to 200 ml, was stirred for 2
hours and 40 minutes at a temperature of 80 C while oxygen
at a pressure of one atmosphere was bubbled through.
Analysis of the reaction mixture indicated the following
product distribution in molar terms: 85.4% glyphosate,
8.1% phosphoric acid, and 6.5% unknown components. No NMG
was detected.
EXAMPLE 3.
This example illustrates the conversion of N-
isopropyl glyphosate to glyphosate using a Pt catalyst and
oxygen. Approximately 1.0 g N-isopropyl glyphosate, 10 g
water, and 0.3 g platinum black (Aldrich Chemical Co.,
Inc., Milwaukee, WI) were combined in a round bottom flask
(equipped with a water-cooled reflux condenser) and
immersed in a 80 C oil bath. A stream of oxygen was
introduced at the reaction surface for 18 hours as the
solution was stirred. At the end of this period, 31P NMR
revealed the following product distributions (on a molar
basis): 91% glyphosate, 1% amino phosphonic acid, 6%
phosphoric acid, and 2% unknown product (15.0 ppm),
Glyphosate precipitated from solution after cooling to
room temperature.
CA 02509953 1998-02-12
EXAMPLE 4.
Various N-alkyl glyphosates were used under the
same conditions as described in Example 3 to produde
glyphosate. In other words, the only parameter which was
5 varied was R' in the following formula:
0 0
-io- I C I - C C H 2- I p I oH
r 2 ri-~
'OH
Table 1 shows the alkyl group (i.e., R') used, as well as
the conversion and glyphosate selectivity.
TABLE 1
10 Use of Various N-Alkyl Glyphosates to Prepare Glyphosate
Alkyl Group Conversion Glyphosate
(~) Selectivity
(~)
methyl 91 95
isopropyl 79 98
isopropyl 100 91
15 n-pentyl 62 82
benzyl 81 89
cyclohexyl 66 11
EXAMPLE 5.
This example illustrates the conversion of NMG
20 to glyphosate using unsupported platinum and a variety of
catalysts in which platinum is dispersed on a non-
carbonaceous support.
Approximately 1.0 g NMG, 10 g water, and 2.0 g
of 5% platinum on barium sulfate were combined in a round
bottom flask (equipped with a water-cooled reflux
condenser) and immersed in a 95 C oil bath. Oxygen was
bubbled through the reaction for 23 hours as the solution
was stirred. At the end of this period, HPLC analysis
CA 02509953 1998-02-12
21
revealed the following product distributions (on a molar
basis): 78.2% glyphosate, 2.4% NMG, 9.4% (M)AMPA, and
10.0% phosphoric acid. Glyphosate precipitated from
solution after cooling to room temperature.
In a separate experiment, the data in Table 2
was obtained by heating to reflux a mixture containing 1 g
of NMG, 20 ml water, and sufficient catalyst to contain 5
mg of platinum metal in a magnetically-stirred, round-
bottom flask equipped with a reflux condenser. Oxygen was
bubbled through for 5 hours using a needle. The catalyst
was then removed by filtration and the filtrate analyzed
by HPLC.
As Table 2 indicates, two platinum black
catalysts were tested. The Engelhard V2001 (Engelhard
Corp., Iselin, NJ) catalyst has a much smaller surface
area than the Aldrich platinum black catalyst (Aldrich
Chemical Co., Inc., Milwaukee, WI). As Table 2 shows, the
Engelhard V2001 catalyst, with its lower surface area, had
lower selectivity and conversion, even though 30 times
more of the Engelhard catalyst (i.e., 150 mg) was used
compared to the Aldrich catalyst (i.e., 5 mg).
CA 02509953 1998-02-12
~
0
=,4
=,-~
SS .==i
O O> -- r N c- w m
-r-t oto ' ' . . r~
ao M ~ oo 1~ ao
z a~
~
a,
~4 ~
~
>
-,1 -~ O kD r1 ao 00 l- m
s4 Quo~ .
p m ~ N I~ N N r-I N
4-4
O ~.
ri
~ O~ M O [~ l!1 N ~O O~ 01
-r-1 Ic: 1i 0%0 lf1 0 CO Q1 N o0 r-I a0
N~ a0 I~ O~ OD 6~ QO Ol 00
4J
N a f~ 0 U]
N ~
O O
aJ Ul N O -T L~
11 S4 = = = = = = =
0 ,y v cr N ~ (') [- M
rl r-1 H cn m N
~
0
~
tJ
a
~
ro
41 2 a U (1d
=rl .~ rl
ro '~ 41 CT1 ~
Q1 E N
~4 Q W O a U
0 'o O 0 41
N N
U v r-+ 0 0 U) C!] .~'. 0 0
r~ N (d M a) -4 -A
~ U U u~ N W W~A E-+ CIl
a)
~D ~ ,~ ~ 4J ~ 4-1 .u w ~ 1-3
A A o a a a a w a a
,,.4 0 =,-a
0 1J l.) N oW oW oW o'P =o cN ow
a a,7 Ln Lo Ln u, - ln lfl
v
m
Ln 0
~
CA 02509953 1998-02-12
23
A third experiment was conducted which
illustrztes that aluminum oxide and ailoxanes (Deloxan;
Degussa Corp., Ridgefield Park, NJ) may be used as
supports for the metal catalyst. The following
experiments were conducted overnight at 95 C and 1 atnt
uring sufficient catalyst to be equivalent to 0.1 g
platinum metal, 1 g NMO, and 10 ml of water. Oxygen wns
introduced through a needle at 50 sccm (i.e., standard cm'
per min.). The resulting solution wae filtered and
analyzed by BPLC and the dissolved platinum concentration
was analyzed by inductively-coupled plasmafmaes
spectrometry. The data is shown"in Table 3.
*Trade-mark
CA 02509953 1998-02-12
O
.~
~ >1
ro
'CS 'i
-,~
X Q-r-1 .--~ (V r-4 QO al N N 0 kD 01 OD O
0 W 11 o10 . . . . ' . . . .
U~ 00 %0 M lf1 a0 N CO lf1 lw
ri =
z a,
~
-H
~4
::I
a~., ,-~ in .- ~ N N ln V~ ri lD
m
~1 ~ V p ko '-I rl O N Ol ~0 ~ O ri
~4 H H H rri N v in in
0 ~- i
v
ul
m v >'
41
O m [- M H 10 c-1 Il) OJ 01 l- O% 1w
t ~ 0 .~ w u N 61 f=1 o c+1 N o lD L~
.'~ V v 4) OD 00 l- CU CO a0 l11 l, v ['M eM
N t"I U c) Ul
r~ 'b
y ~
s4 0
0 -r-I
U] l!1 I~ r ~D M v r oo t!1 N
74 " =
N ~ a0 [, N 10 l~ rl N l~ M a0 Ln
CI~ > 0) CO 01 41 10 Ln Ul M V' Lf1
41 0
a u
b
b 41 41 41
4-3 s~ m w m
a >4 >1
ro ro
ai N ~4
ro ~~ ro ro ro ro ro
4 >1
0 X X ~ =~~! U .~+ U
tC V N ~ 0 N O O G G+J E.0
4j 0 0 F: U) 0 ,-i r+ ~% :j s:'. ~s F-:
~ U -.-i =ri Q) 0 =ri =.i -r-I ri rl 4) ri N
U u C/) Cn 3-4 fQ H M U2 ct N S a M 3 i
~D r+ -i Ln a.-> Q w .u 4.) iJ i~ ~ -LJ 44 .tJ w
.Q A O a a w a a, a a a a u-+ a 4-4
44 0 =.Ai -li -ri
0 J-) 4.3 ri ar w 10 aP OP aP ar ar+ w 't7 ow ro
a a Cl] tf) U1 - tf) Ul M tf) L11 U7 - tn -
a~
m
Ln 0 Ln
~ ~
CA 02509953 1998-02-12
a1cAMPLg 6.
This example illustrates the use of palladium
instead of platinum as a catalyst for the oxidation of NMt3
to glltphosate. A solution consisting of 3.0 g of NMG, 0.3
5 g of palladium black, and 57 g of water was refluxed in
air over a weekend under a water-cooled reflux condenser.
NMR ana+ysis indicated the following product distributiona
97.2* NMG, 2.6% glyphosate, and 0.05t phosphoric acid.
N]CAMPLB 7.
10 This example demonstrates that catalysta
consisting of graphitic carbon supports impregnated with
platinum have greater glyphasate selectivity relative to
catalysts consisting of non-graphitic carbon supporta
impregnated with platinum. Also, this exasrple
i5 der.tornstraLes that less b.AMPA ar_d A14PA are formed when
catalysts consisting of graphitic carbon supports
impregnated with platinum are used.
The following example describes the results of
oxidizing NMa using catalysts consisting of platinum
20 dispersed on a commercially available carbon support.
F106 carbon and the platinumJFio6 carbon catalyst are
available from pegussa Corp. (Ridgefield Park, N3).
Sibunit carbon is ananufactured as described in by
Surovikin at al. in U.S. Patent 4,978,649, and may be
:25 purchased from the Boreskov Ynstitute of Catalysis in
Novosibirsk, Russia as can platinum catalystg supported on
Sibunit carbon. However, the catalyst uaed ir. this
example was prepared from the carbon itself by
impregnation with,platinum salts followed by reduction
3.0 with sodium borohydride which is a standard for the
preparation of supported platinum catalysts, The general
preparatioa of platinum or, a carbon support is well-known
in the art and is deacribed, for example, in stilea, A.B.
talyst SvmiRcrts snd SupmojtgdCata3=ygts, Theoreticalard
AbcliCd Concents (Butterworths, Boston, MA 1987) ; and in
CA 02509953 1998-02-12
26
a chapter by R.L. Moss in Exnerimental Methods in
Catalytic Research, Vol. 2, Ch. 2, pp. 43-94 (R.B.Anderson
& P.T. Dawson, eds., Academic Press, New York, NY 1976).
The 20% Pt/Vulcan XC-72R*carbon catalyst is manufactured
by Johnson-Matthey and may be purchased through Alfa/Aesar
(Ward Hill, MA). These three carbons are respectively not
graphitic, somewhat graphitic, and almost completely
graphitic.
Approximately 100 mg of the catalyst (except as
noted), 10 ml of water, and 1 g of NMG were refluxed for
five hours while oxygen was bubbled through via a needle.
The reaction was then filtered and analyzed by HPLC.
Table 4 shows the results.
*Trade-mark
CA 02509953 1998-02-12
>1
4-)
=,~
L: a'~ ow
~ -- ao co ~o
0
O .
rt
U O
x
X a >
z r-i o
0\' a~ co 0
(; U v N r-i rl
A 4-)
Sa cd =.a
ffS UI >
rI
U ,~ ow N m
U p, U v lD r o0
~r =,~ ?~
i' -1
N
Ei ~-+ ~
C7 0
.ri
~ y4
r1 a) CO M M
(n > O) lf) l!l
=r-I G~
0
E
0
U
41
~+
O O N
0 Q t-
Ln N i
0
~ 1' Q
v
m U >C
~ ro
S
w
U
O U o 3 A ?
v)
O r,
.u r~ ,1 a o
a ~s a ~
oW ~-1
oW .1-) oW O tI3
Ul (1) ch N U
lf)
CA 02509953 1998-02-12
28
EXAMPLE 8.
This example illustrates the improved
selectivities which may be achieved when an electrdactive
molecular species is adsorbed to a noble metal catalyst.
All of the electroactive molecular species adsorbed to
platinum black in this example undergo oxidation and
reduction by electron transfer. Thus, the treatment of
platinum-containing catalysts by both electroactive
molecular species and their oxidative precursors is
exemplified herein.
This experiment was conducted by heating to
reflux a mixture containing 1 g of NMG, 20 ml water, and
50 mg of platinum metal in a magnetically-stirred, round-
bottom flask equipped with a reflux condenser. Oxygen was
bubbled through for 5 hours using a needle. The catalyst
was then removed by filtration and the filtrate analyzed
by HPLC.
To prepare the organic-treated catalysts, 0.5 g
of platinum black (Aldrich Chemical Co., Inc., Milwaukee,
WI) was added to a solution of 25 mg of the poison (i.e.,
the electroactive molecular species) in 50 ml of anhydrous
acetonitrile. The mixture sat capped in an Erlenmeyer
flask for four days, except that the 4,4'-
difluorobenzophenone catalyst only was exposed to solution
for one day. The catalyst subsequently was recovered by
filtration, rinsed with acetonitrile and diethyl ether,
and air-dried overnight.
The 2,4,7-trichlorofluorene catalyst was
prepared using 0.3 g of Pt black and 30 ml of a solution
consisting of 834.5 ppm 2,4,7-trichlorofluorene in
acetonitrile/1% CH2C12 solution (used to facilitate
dissolution of the electroactive molecular species) which
was allowed to evaporate at room temperature. The
catalyst subsequently was washed with ethanol and air-
dried.
CA 02509953 1998-02-12
29
The inorganic-treated catalysts were prepared by
combining 0.50 g of Pt black, 50 tnl of tetrahydrofuran,
and either 25 or 1o0 mg of the inorganic electroactive
molecular speciea, and stirring overnight at room
temperature in a sealed 125 ml Erlenmeyer flask. The
catalyst was recovered by filtration, washed with diethyl
ether, and air-dried overnight.
The inorganic species used, all of whicY, are
available from Aldrich Chemical (Milwaukee, WI), wereo
1. 5,10.15,20-tatrakis(pentafluorophenyi)-21H,23H-
porphine iron (III) chloride (abbreviated
"Fe(IIi)TPFPP chloride in Table 5). Approximately
25 mg was usad to prepare the catalyst.
2. 5,10,15,20-tetraphenyl-21H,23H-porphine iron (III)
chloride (abbreviated Fe(IiY) TPP chlorwde" in Table
5), Approximately 25 mg was uaed to prepare the
catalyst.
3. 5,10,15,20-tetraphenyl-21H,23H-porphine nickel (II)
(abbreviated as "Ni(II) TPP" in Table 5).
Approximately 25 mg was used to prepare the catalyst.
4. Ruthenium-tris(2,21-bipyridine) dichloride
(abbreviated as " [Ru(bpy),) C13N in Table 5) .
Approximately 100 mg was used to prepare the
catalyst.
S. Ferrocesne, Approximately 100 mg was used to prepare
the catalyst.
Where available, literature data on the
oxidation potential (E:j,) of the electroactive molecular
species is reported in Table S. This example ill-astrates
that electroactive mclecular species being relatively
CA 02509953 1998-02-12
soluble in water (e.g., fQrrocane and (Iiu4Ib&y),JC1,) are
lgss effective at enhancing glyphosate selectivity. This
example also demonstrates that hydrophobic electroactive
molecular species increase the catalyst's selectivity.
5 FlectroacLive tr.olecular species having oxidation
potentials more negative than about +0.3 V vg SCE
generally decrease conversion. Thus, the preferred
electroactive molecular species for enhancing=the
selectivity and conversion of MG oxidation may be either
10 organic or inorganic, but should be hydrophobic and have
oxidation potentials more positive than about 0.3 volts
vs. SCE.
CA 02509953 1998-02-12
ow
~ o ~ 0 ~ W N cn rn co '! w
aI
a 0 m .r m 'IV o 0 0 ~o o) o
O ~ ~j . . . .
U T N N N N t0
t~ N
RJ r-~
'b U
r I CJ)
X
0
~ J ) oW
z [!1 rl u) N t11 01 d~ ~ l~ ri M Ol ~D
O ~
ri Q1 . 1 O c0 N
O i () M M M M N m
~ N OD Ol 01 Q1 Q1 O~ 0~ W Ol Q1 O a0
r-1
(n r-i Q)
Q) C7 cl)
U
4)
w O~ r~ QO Ql 00
Ln Cf) Q1 m M N H
~4 \o Lf1 N l0 L(1 -1 N N Ql M [~ O
H ~ R1 v ~I' lf) ltl M h N N w U1 m [-
M W ~ U
U
QJ
,--i
O
U [r Ul r1 Lfl N ~
QJ ul 1 ~ O N O H r-I M ~
n
~ ~ . . O . . . .
' ~ n.
.,-{ W> I ri rl + O O ~-i rl ri O
41 + + + + + + + +
U ,
(a
0
~4
tJ
U
N ~
0
44 -rl C:
O S i N rt3 'U
Q) o b -- a -~+ v
rn N O ~
-~ S-a rtf c~ A U r-+
~ rv
O 0 4 Cl+ .~ 0
E N 0 Oa. U
0 :j rs. a
ri ~-1 ,-, .-, LL 04 a 4)
~ x ~ H E-+ a
0 - 7. N
[
~4 '31 0 d i~-+ A O
~ 04 -a ~
~, W~ - aU a) =,-4 a a)
z N z4-1 H41 -,zv w w Z w
Ln o Ln
r I H
CA 02509953 1998-02-12
32
EXAMPLE 9.
This example illustrates the effect of
electroactive molecular species on the platinum-catalyzed
oxidation of N-isopropyl glyphosate using the commercially
available catalyst 20% Pt on Vulcan XC-72R carbon
(manufactured by Johnson-Matthey and is available from
Alfa/Aesar (Ward Hill, MA)). The commercial catalyst was
tested along with a catalyst which had been impregnated
with two electroactive molecular species: N-
hydroxyphthalimide and triphenylmethane.
These catalysts were used to oxidize N-isopropyl
glyphosate by the method described in the previous
example. Approximately 1 g of N-isopropyl glyphosate was
substituted for the NMG. The results shown in Table 6
demonstrate that electroactive molecular species improve
the selectivity of platinum on carbon catalysts for this
reaction. Modifiers with less positive oxidation
potentials such as triphenylmethane appear to be more
effective than those with more positive oxidation
potentials, such as N-hydroxyphthalimide. This example
also demonstrates that the use of graphitic supports for
platinum is less effective in suppressing undesired side
reactions in N-isopropyl glyphosate oxidations than is the
case for NMG.
CA 02509953 1998-02-12
O v M d N Ln
(0 a
U) ~
O [ ~D N
x N
N
~
C7
r-+
O
N L!~ N
f4 hcx;
O m r+
H N
~
z
44
0
ro~
~ m ao un kO
Ci arn o r+ o
-~ =~ ~
4-1 a a, r, N ff, ~n
ro ~r--l
~+ c7 ua
x
0
"o 0 (1) N N
rn ~4 \ l- r-I r-i O
M ~ Q U r- ao ko
H w
O
.r{
U
~ U
~ ~ r-
m \ O v N
}4 W; O rl O
~ + + +
>
U
N ~
-4
.~ .C
O 0
~ ~ b~ ~ t71
~ U 3 E 3 ~ ~
> zj w 'b b
N(1) N N ro!!1
i' (N 41iJ 0~l
U
ro r~ ai ai r' rn
~ ~4 N E
11 ri fi1 1-) .
U ~
,~ RS ro~ Rf 1J M (lS 41 N
W U ~ U'd U.~ U (1) rn
A 1 - + Q1 r-+ f . 1 tn ri E-
0 > \ 0 0) \ Q, ~
E
a E
m Q ~.~2s ~ ~,
rt3 aa in okO.~ (d N aa -.i in
r-I 0 N 0 ~ 0 Ul 0 f-1 O
a N'-' N Z r-I ::J N J-1 M
Ln 0
r--i
CA 02509953 1998-02-12
34
EXAMPLE 10.
This example demonstrates that both selectivity
and conversion may be improved by minimizing the dissolved
oxygen concentration.
In a 300 mg 316 stainless steel autoclave
reactor, 4.4 gram NMG were combined with 1 gram platinum
black in 145 g deionized water. The reaction mixture was
heated to 70 C at 60 psig, and a nitrogen/oxygen mixture
was bubbled through with vigorous mixing for 4 hours. The
dissolved oxygen concentration was measured using an
Orbisphere dissolved oxygen probe, calibrated to read 26.4
ppm O2 at 70 C/60psig air saturation, and controlled by
varying the Nz/OZ blend. Two runs were conducted with the
dissolved O, concentration being maintained at 2-3 ppm and
10 ppm. HPLC analysis of the reaction mixture at 2 hrs
and 4 hrs gave the results shown in Table 7.
TABLE 7
Minimizing Dissolved Oxygen Concentration
During NMG Oxidation
Dissolved Time Conv. Glyphosate MAMPA H3PO4
Oxygen (hr) (%) Select (%) Select Select
Concentration (%) (%)
(ppm)
2.75 2 66% 75.96 5.48 18.56
2.75 4 82% 76.16 5.95 17.89
10.4 2 60% 70.70 14.97 14.33
10.2 4 76% 69.83 16.21 13.97
11 EXAMPLE 11.
This example illustrates the platinum-catalyzed
oxidation of N-substituted glyphosates in which the
substituent on the nitrogen atom contains atoms other than
carbon or hydrogen. In particular, it describes the
oxidation of glyphosine (-HOZCCHZN(CH2PO,H2)2) and N-
hydroxyethyl glyphosate, which are prepared by the
CA 02509953 1998-02-12
phosphonomethylation of glycine and N-hydroxyethyl glycine
respectively by reacting with formaldehyde and phosphorous
acid in the presence of heat and a strong acid, as
generally illustrated in Redmore, D. Topics in Phosphorous
5 Chemistry Vol. 8, 515-85 (E.G. Griffith & M. Grayson eds.,
John Wiley & Sons 1976); and in a chapter entitled "-a-
substituted Phosphonates" in Mastalerz, P. Handbook of
Organophosphorus Chemistry 277-375 (Robert Engel ed.,
Marcel Dekker 1992). Approximately 1 g of the substrate,
10 20 ml of water, and 50 mg of platinum black were combined
in a round-bottom flask. The oxidation was conducted by
the same procedure used for the oxidation of NMG in
Example 8. The product distribution was analyzed via 31P
NMR. 74.9% of the glyphosine was oxidized with a
15 glyphosate selectivity of 50.2%. The other major product
was bis (phosphonomethyl) amine (-HN(CH2P03H,) 2) which
accounted for 39.1% of the oxidized glyphosine. Small
quantities of AMPA and of unidentified products also were
detected. The use of the platinum black catalyst treated
20 with tris(4-bromophenyl) amine described in Example 8 led
to an increase in conversion to 86.8%, but no change in
selectivity.
Oxidation of N-hydroxyethyl glyphosate resulted
in 46.7% oxidation of the substrate and a product
25 distribution of 61.2% glyphosate, 22.4% N-hydroxyethyl-
aminomethylphosphonic acid, and 16.3% phosphoric acid.
EXAMPLE 12.
This example illustrates the rates and
selectivities achievable by conducting the oxidation of
30 NMG over platinum black at elevated temperature and the
fact that no deactivation of the catalyst is detectable
over seven cycles.
A 300 ml glass pressure bottle was equipped with
a thermocouple and two fritted filters. One of the
35 filters was located about half an inch above the center of
CA 02509953 1998-02-12
36
the bottom of the bottle was used for gas dispersion. The
second filter, located about an inch from the bottom and
not centered, was used for the withdrawal of liquids. A
gas exit line leading to a back pressure regulator was set
to maintain the pressure at 50 psig also was provided.
Approximately 60 g of NMG was loaded into the vessel along
with 3 g of platinum black from Aldrich Chemical
(Milwaukee, WI) and 180 ml of water, along with a stir
bar. The bottle was immersed in an oil bath, magnetically
stirred and heated under a slow nitrogen flow until the
internal temperature reached 125 C, giving a homogeneous
solution. Oxygen and nitrogen were then bubbled through
the reaction mixture at rates of 1.5 and 0.5 slpm (i.e.,
standard liters per min.), respectively for 30 minutes
followed by a further 30 minutes of reaction at a flow
rate of 1 slpm each for oxygen and nitrogen, followed by a
final 30 minutes with a nitrogen flow rate of 1.5 slpm and
an oxygen flow rate of 0.5 slpm. Stirring was continued
and the mixture remained homogeneous throughout the entire
90 minute period. A slow nitrogen flow was then
established to maintain the pressure. The contents of the
bottle were withdrawn through the liquid withdrawal frit,
leaving the catalyst in the bottle. About 100 ml of water
was injected through the frit and them withdrawn to remove
residues from the reaction. The bottle was then allowed
to cool. Again, 60 g of NMG and 180 ml of water was added
and the cycle repeated. Seven such cycles were conducted
with the results shown in Table 8.
Platinum concentrations in solution at the end
of the run varied from 0.3 to 1.1 ppm after the first
cycle as determined by inductively-coupled plasma mass
spectrometry. Although a higher amount of platinum
leached into solution during the first cycle (i.e., the
concentration of dissolved platinum was 4.2 ppm), it is
believed that most of the lost platinum was primarily
unreduced platinum on the platinum black's surface.
CA 02509953 1998-02-12
37
TABLE 8
Repeated Oxidation of NMG over Pt Black at 125 C
Run Conversion Glyphosate (M)AMPA H3POI
no. (%) Selectivity Selectivity Selectivity
1 89.8 82.4 5.6 12.0
2 80.9 87.1 3.6 9.2
3 84.7 79.0 8.5 12.5
4 66.7 83.4 5.6 11.0
5 79.1 81.8 7.6 10.6
6 75.6 79.5 7.3 13.2
7 78.1 79.4 9.0 11.6
EXAMPLE 13.
This example demonstrates the selectivities that
may be achieved when N-alkyl glyphosates are oxidized at
low rates of oxygen delivery and moderate conversion if an
electroactive molecular species such as TEMPO (i.e.,
2,2,6,6-tetramethyl piperidine N-oxide) is added to the
reaction mixture. No pretreatment of the catalyst is
required. This example further demonstrates that the
conversion improves over the first few cycles when the
electroactive molecular species is added to the mixture.
Finally, this example demonstrates that the electroactive
molecular species reduces the amount of noble metal loss.
Approximately 60 g of NMG, 180 ml of water, 3 g
of platinum black (Aldrich Chemical, Milwaukee, WI), and
40 mg of TEMPO dissolved in 1 ml of acetonitrile were
combined in the pressure reactor described in Example 12.
The mixture was heated to 125 C while stirring under a 50
psig nitrogen atmosphere, forming a homogeneous mixture.
A nitrogen/oxygen mixture (75% nitrogen, 25% oxygen by
volume) was bubbled through for 90 minutes at a flow rate
of 1 slpm while the pressure was maintained at 50 psig.
The reaction mixture then was withdrawn through a fritted
CA 02509953 1998-02-12
38
filter, leaving the catalyst behind. Another 60 g of NMG,
180 ml of water, and 40 mg of TEMPO in 1 ml of
acetonitrile subsequently was added to the flask and the
cycle repeated. Four cycles in all were performed. In
all cases, (M)AMPA concentrations were below the
quantifiable limits, although traces were detected. The
only quantifiable byproduct detected was phosphoric acid.
The conversions and selectivities at the end of each of
the four cycles are shown in Table 9.
As in Example 12, the concentration of dissolved
platinum was determined at the end of each run by
inductively-coupled plasma mass spectrometry. This
dissolved platinum concentration was less than 0.1 ppm in
cycles 2, 3, and 4. This is lower than the leaching
observed in Example 12. As with Example 12, a higher
amount of platinum leached into solution during the first
cycle (i.e., the concentration of dissolved platinum was
8.3 ppm); however, it is believed that most of the lost
platinum was primarily unreduced platinum on the platinum
black's surface.
TABLE 9
Oxidation of NMG
in the Presence of TEMPO at 125 C for 90 Min.
Cycle Conversion Glyphosate H3PO4
Number (%) Selectivity (%) Selectivity (~)
1 32.6 98.3 1.7
2 38.0 98.1 1.9
3 43.3 98.1 1.9
4 46.2 97.3 2.7
EXAMPLE 14.
These examples illustrate the selectivity
achievable if NMG is prepared via the direct
phosphonomethylation of sarcosine amides, such as N-acetyl
CA 02509953 1998-02-12
39
and N-propionyl sarcosine or sarcosine anhydride rather
than sarcosine itself.
Approximately 20.0 g N-acetyl sarcosine (152.5
mmole), 12.5 g phosphorous acid (152.4 mmole), and 37.6 g
concentrated hydrochloric acid were mixed and refluxed in
a 120 C oil bath. Approximately 13.6 g of 37% formalin
(167.6 mmol) was added dropwise over 20 minutes. The
reaction was continued for an additional 19 hours. HPLC
analysis revealed a 99% yield of NMG based on moles of
charges.
Under the same conditions, 20.0 g N-
propionylsarcosine (137.8 mmole) was converted into NMG
using 11.3 g phosphorous acid (137.8 mmole), 10.0 g
concentrated hydrochloric acid, and 12.3 g of 37% formalin
(152.1 mmole). HPLC analysis revealed a 96.6% yield of
NMG based on moles of N-propionylsarcosine charged.
Also under the same conditions, 2.06 g sarcosine
anhydride (14.50 mmole) was converted into NMG using 2.38
g phosphorous acid (29.02 mmole), 5.7 g concentrated
hydrochloric acid, and 2.6 g of 37% formalin (32.02
mmole). HPLC analysis revealed a 97.2% yield of NMG based
on moles of sarcosine anhydride charged.
In an additional experiment, 2.0 g N-acetyl
sarcosine (15.3 mmole) and 1.25 g phosphorous acid (15.3
mmole) were mixed with 3.1 g concentrated sulfuric acid
and 1.7 g water and then refluxed in a 120 C oil bath.
Approximately 1.4 g of 37% formalin (16.7 mmol) was added
dropwise over 20 minutes. The reaction was continued for
an additional 18 hours. 31P NMR analysis revealed a 98%
yield of NMG based on moles of N-acetyl sarcosine charged.
EXAMPLE 15.
This example demonstrates oxidizing NMG under
conditions very similar to those of Example 12, except
that a sub-stoichiometric base is present in the reaction
mixture.
CA 02509953 1998-02-12
Approximately 60 g NMG, 9.6 g of 28-30% ammonium
hydroxide (0.25 equivalents), and 170 ml water were
combined in the apparatus described in Example 12 and
stirred for one hour at an internal temperature of 125 C
5 while 0.75 slpm of pure oxygen was bubbled through at a
pressure of 50 psig. HPLC analysis of the reaction-
mixture indicated that 23.5% of the NMG had been oxidized
with a selectivity to glyphosate of 65.7%. The
selectivities of (M)AMPA and H3PO4 were 21.1% and 13.2%,
10 respectively.
As the results indicate, the NNIG oxidation
proceeds, although conversion and selectivity were lower
compared to a reaction conducted in the absence of base.
EXAMPLE 16.
15 This example demonstrates that NMG may be
oxidized selectively to glyphosate in the presence of
glyphosate and similar compounds. One gram of platinum
black was combined with 300 g of a solution containing
about 6% NMG and lesser quantities of glyphosate, AMPA,
20 MAMPA, formaldehyde, formic acid, and sodium chloride.
The mixture was heated to 150 C for 4 hours while oxygen
was passed through the reactor at a pressure of 70 psig.
At the conclusion of the reaction, NMR and HPLC analysis
indicated that most of the NMG had been converted to
25 glyphosate.
* * * * * * * * *
The above description of the preferred
embodiment is intended only to acquaint others skilled in
the art with the invention, its principles, and its
30 practical application, so that others skilled in the art
may adapt and apply the invention in its numerous forms,
as may be best suited to the requirements of a particular
use. The present invention, therefore, is not limited to
the above embodiments and may be variously modified.