Note: Descriptions are shown in the official language in which they were submitted.
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
METHOD AND APPARATUS FOR THE ELECTROCHEMICAL
TREATMENT OF CONTAMINATED AQUEOUS MEDIA
BACKGROUND OF THE INVENTION
Field of the Invention
The present invention relates to an apparatus and a method for
purifying contaminated liquids and, more particularly, to a method and, an
apparatus for the electrochemical treatment of contaminated liquids, such as
livestock and industrial wastewater.
Description of the Prior Art
It is known to remove contaminants from wastewater by flowing
the contaminated water through an electrochemical reactor, such as an
electrofloatation reactor or an electrocoagulation reactor.
One problem with known electrochemical reactors is that the
electrodes thereof, which may be soluble or insoluble, often get fouled by
pollutants and oxidation products during the electrolysis process. This lowers
capacity and reduces efficiency. Attempts have been made to overcome this
problem.
For instance, United States Patent No. 4,338,178 issued on July 6,
1982 to Efimov et al. discloses a vertical electrocoagulation cell mounted
directly
under a horizontal electrofloatation cell. A "pure" electrolyte is injected
under the
electrocoagulation cell to provide for the separation of the effluent and the
sacrificed consumable electrodes of the electrocoagulation cell in order to
prevent
fouling of the consumable electrodes. The unit disclosed in this patent has
the
disadvantage of requiring a great amount of "clean" water to operate under
normal condition. Furthermore, the application is limited to small process
unit.
United States Patent No. 5,558,755 issued on September 24, 1996
to Gardner-Clayson et al. discloses a tubular electrochemical cell with non-
consumable electrodes. The apparatus includes a fluidized bed of metallic
particles to promote turbulence inside the electrochemical cell and, thus,
improve
current efficiency. The tubular cells will be most lilcely difficult to
replace and
-1-
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
repair. Furthermore, such an apparatus is not suitable for treatment of
effluent
having a high concentration of organic br colloid matters. Indeed, the
metallic
particles will suffer from thin film depositions to its surface. Such a
phenomenon
may impair the coagulation to proceed successfully.
. European Patent number EP 0794157 issued to Ming Shing
discloses an electrochemical cell in which the effluent to be treated flows
upwardly through a tortuous S-shaped path. Because high pressure of water is
required to prevent clogging, the electrode blades must be tightly sealed. It
is
obvious that electrode maintenance and replacement are time consuming.
Moreover, negative pressure occurs in the dead zone of the flow path. This can
result in an excessive gas buildup.
A common shortcoming of all the foregoing prior art apparatus is
the buildup of metal oxide layers on the anode surfaces. These layers may also
lead to an excessive increase of the ohmic resistance of the electrochemical
cell
and therefore to a frequent interruption of the electrochemical process. On
the
other hand, if the treatment lasts over an optimal point, the solubilization
of
phosphate raises, and therefore the yield of the electrochemical treatment
falls.
The foregoing disadvantages are overcome by the present process
and apparatus. Additionally, the proposed method and apparatus achieve other
advantages discussed more fully below.
SUMMARY OF THE INVENTION
It is therefore an aim of the present invention to prevent the
electrodes of an electrochemical reactor from fouling.
It is also an aim of the present invention to provide an
electrochemical apparatus which is adapted to efficiently remove suspended
solids, colloids, organic matters, pathogens, particulate and soluble
phosphorus
that are present in wastewater.
It is a further aim of the present invention to provide an
electrochemical reactor which is simple of construction and which is provided
with easy removable electrode plates for maintenance and cleaning.
-2-
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
It is a further aim of the present invention to provide a new
electrochemical reactor with high specific surface area and low investment and
operation costs.
It is a still further aim of the present invention to provide an
electrochemical cell which is simple to manufacture, install and connect to an
external source of electricity.
Waste water generally contains negatively charged colloids organic
particles which are not easy to settle and thereafter to remove. It is a
further
object of one embodiment of the present invention to provide reaction chambers
to precede either simultaneity or separately with charge neutralization,
coagulation, flocculation and sedimentation in compact equipment. The charge
net carried by each particles are neutralized-with the anions released in the
anode.
Once charge neutralization takes place several particles come together which
will
result into coagulation. Flocculation is the stage whereby the destabilized
particles are induced to collect into larger aggregates. It is followed by
rapid
settling in a sedimentation chamber.
It is also a further obj ect of the present invention to provide an
electrochemical apparatus which can operate in a small room area.
It is also a further obj ect of the present invention to provide a
suitable arrangement of electrofloatation reactor, electrocoagulation reactor,
settling chamber, collecting device for relatively cleaned water and a back
recirculation of this cleaned water to electrochemical cell reactors in order
to cope
electrodes fouling.
It is also a further object of the present invention to provide a
method and apparatus of obtaining feedbaclcs on the electrode condition during
the electrolysis process.
Therefore, in accordance with the present invention, there is
provided an apparatus for electrochemical purification of a contaminated
aqueous
medium, comprising at least one electrochemical reactor and a re-circulation
system for causing at least a portion of the aqueous medium to flow baclc into
the
electrochemical reactor after the contaminated aqueous medium has been
treated.
-3-
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
In accordance with a further general aspect of the present
invention, there is provided a method for separating contaminants from a
contaminated effluent, comprising the steps of: feeding the contaminated
effluent
to be treated into an apparatus comprising at least one electrochemical
reactor and
a re-circulation system, submitting the contaminated effluent to a succession
of
electrochemical treatments by re-circulating the contaminated effluent through
the
apparatus for a period of time sufficient to obtain an agglomeration of the
contaminants contained in the effluent, and evacuating the agglomerated
contaminants.
In accordance with a still further general aspect of the present
invention, there is provided an electrochemical cell, comprising a set of
electrode
plates mounted between a pair of opposed non-conductive walls defining on
respective inner faces thereof a series of longitudinally spaced-apart
parallel slots
for receiving opposed longitudinal edges of the electrode plates, thereby
holding
the electrode plates in a parallel spaced-apart relationship to one another.
According to a further general aspect of the present invention, the
contaminated liquid is submitted to a succession of electrochemical treatments
and the contaminants are separated from the liquid by floatation and by
gravity
settling. The liquid to be treated is re-circulated through the apparatus mtil
the
level of contaminants contained in the liquid. reaches a predetermined level.
Following the application of .the electrochemical treatments, a portion of the
contaminants is carried up by floatation and is extracted in the form of foam.
Another portion of the contaminants is agglomerated (i.e. flocculated and
coagulated) in the form of sludge. The sludge is preferably separated from the
liquid by a gravity settling process.
The present invention provides for the reduction of the
concentration of phosphorous in the contaminated liquid to a level as low as 1
mg/1, thereby allowing the purified liquid to be discharged back into the
environment. The present invention also provides for the oxidation of the non-
biodegradable organic residual loads, and the destruction of pathogenic germs.
-4-
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
The present treatment method is particularly effective for effluents
which have gone through a preliminary biological purification treatment.
According to a further general aspect of the present invention,
there is provided an apparatus for performing the above-described purification
~ treatment method. The apparatus may comprise a main tanlc equipped with
electrofloatation and electrocoagulation reactors and defining a gravity
settling
chamber, and a system for re-circulating the liquid to be treated through the
apparatus. The re-circulation of purified liquid through the reactors greatly
contributes to prevent the electrodes of the reactors from fouling. The
recirculation of the liquid is also advantageous in that the size of the
apparatus
can be kept to a minimum and the manufacturing costs thereof can be reduced.
Finally, by having more than one passage of the effluent to be treated through
the
apparatus, it has been found that economic efficiency and the purification
rate of
the process can be increased.
The present invention provides for the application of a series of
complex electrochemical treatments based on the combined effect of
electrofloatation, electrocoagulation and electrofloculation by ensuring an
optimal
re-circulation period of the contaminated liquid to be treated as a function
of its
characteristics and the degree of purification to be obtained. By controlling
the
interaction, the duration and the intensity of each step of the treatment and
by re-
circulating the contaminated liquid, it becomes possible to obtain a
significant
improvement of the agglomeration effects on the contaminants, thereby
facilitating removal thereof through the use of floatation and settling
techniques.
It also becomes possible to take advantage of the effect of the various
electrochemical reactions to promote the -oxidation of the organic residual
and
non-biodegradable loads and the destruction of the pathogenic germs.
According to a more specific aspect of the present invention, there
is provided a method for treating a contaminated aqueous effluent, comprising
the
steps of feeding the contaminated aqueous effluent to a treatment apparatus
comprising at least one electrochemical reactor, a settling chamber and an
effluent re-circulating system, submitting the effluent to a succession of
-5-
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
electrochemical treatments by re-circulating the effluent through the
apparatus for
a predetermined number of cycles so as to obtain a separation asld an
agglomeration of the contaminants in the form of foam and sludge, removing
from the effluent the foam produced in the electrochemical reactor and the
sludge
settled in the settling chamber, and collecting the clean effluent into a
reservoir.
According to another feature of the present invention, the
apparatus is provided with a programmable control system for controlling the
circulation of the effluent through the apparatus, the number of passes
thereof
through the various treatment sections of the apparatus as well as the
intensity of
the electric field to be generated in the electrochemical reactors of the
apparatus
as a function of the degree of contamination of the effluent.
According to a more specific construction of the present invention,
the electrochemical reactors are realized by a combination of the two
following
variants:
An electrochemical reactor with insoluble electrodes, such as
stainless steel grids or plates, titanium grids or plates or any other
corrosion-proof
conducting plates. The connection of the electrodes to a source of electric
current
results in the generation of hydrogen and oxygen bubbles. The main role of
this
type of reactor is to create a floatation reaction with the formation of foam
at the
surface of the liquid to be treated. A great portion of the contaminants
present in
the liquid to be treated will be contained in the formed foam. It is also
possible to
take benefit from the oxidation phenomenon of the organic matter in order to
improve the efficiency of the overall purification process. For amplifying the
flocculation process and the oxidation effects, an injection of fme air
bubbles can
be added to the flow of contaminated liquid passing through the reactor.
' An electrochemical reactor with soluble electrodes (sacrificial
consiunable electrodes), such as steel, aluminum or iron electrode plates. In
addition of generating hydrogen and oxygen bubbles, the connection of the
soluble electrodes to a source of electric current is associated with the
production
of electrode dissolution products with which the contaminated liquid is mixed
as
it flows through the reactor. The main role of this type of reactor is to
produce
-6-
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
metallic cations (Fe2+, Al3+), which contribute to destabilize negatively
charged
colloidal suspension. Furthermore, in a lightly alkaline reactional
environment
(pH comprised between 7.1 and 8.5), these cations are immediately transformed
into their corresponding hydroxides which by their reactivity provide for a co-
y precipitation of the soluble hydroxides and phosphorous particles. The
injection
of fine air bubbles in the stream of the contaminated liquid which flows
through
the reactor is associated with an agitation of the liquid which contributes to
prevent fouling of the electrodes and to oxidize ferrous ions to ferric ions.
According to a more specific construction of the present invention,
the settling chamber of the apparatus comprises a vertical reservoir having a
generally rectangular cross-section and at least a pair of sidewalls which are
inclined at about 60 degrees. The settling chamber may further include a set
of
lamellar plates which are also inclined at about 60 degrees to facilitate the
decantation.
According to a more specific construction of the present invention,
the re-circulation system of the apparatus comprises a pump mounted in a re-
circulation line. A series of weir plates are preferably provided between the
various sections of the main tank to control the flow of the effluent through
the
apparatus.
In accordance with another aspect of the present invention, a
mechanical separator, such as a filtration membrane is integrated into an
electrochemical treatment device. A re-circulation circuit is provided for
causing
at least a portion of the effluent leaving the reactor to be redirected
therein. A
switching mechanism is preferably provided for selectively isolating the
mechanical separator from the re-circulation circuit, thereby allowing the
effluent
to be only re-circulated through the reactor. At the beginning of the
purification
process, the electrochemical reactor will most lilcely be used alone, whereas
at the
end of the process both the electrochemical reactor and the mechaucal
separator
will be used to treat the effluent.
In accordance with a still further general aspect of the present
invention, there is provided an electrochemical treatment device for treatment
of a
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
contaminated effluent, comprising a recirculation circuit for circulating said
effluent through a closed loop, an electrochemical reactor and a mechanical
separator integrated to said closed loop for gradually purifying the effluent
each
time the effluent is circulated therethrough.
BRIEF DESCRIPTION OF THE DRAWINGS
Having thus generally described the nature of the invention,
reference will now be made to the accompanying drawings, showing by way of
illustration a preferred embodiment thereof, and in which:
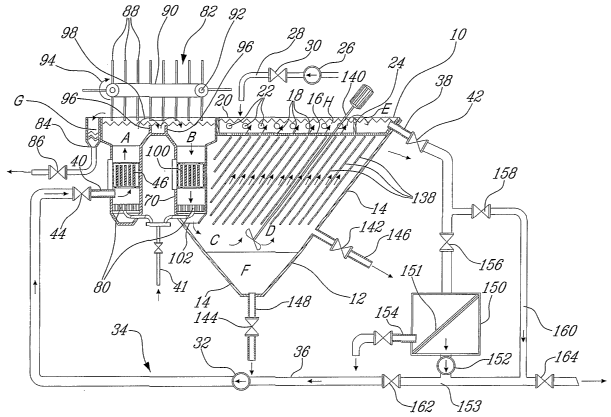
Fig. 1 is a schematic vertical cross-section view of an apparatus for
the electrochemical treatment of contaminated liquids in accordance with a
preferred embodiment of the present invention;
Fig. 2 is a top plan view of the apparatus shown in Fig. 1;
Fig. 3 is a perspective view of one half of the apparatus shown in
Fig. 1;
Figs. 4a to 4c are perspective view of an electrocoagulation cell in
accordance with a preferred embodiment of the present invention;
Fig. 5 is a perspective view of an electrocoagulation cell in
accordance with a second embodiment of the present invention;
Fig. 6 is a perspective view of an electrofloatation cell in
accordance with a preferred embodiment of the present invention;
Figs. 7a and 7b are perspective view of an electrofloatation cell in
accordance with a second embodiment of the present invention;
Figs. 8a and 8b are graphic representations of the evolution of the
residual phosphorus during the purification process of synthetic water;
Fig. 8c is a graphic representation of the evolution of the pH of the
synthetic water during the purification process thereof; and
Fig. 9 is an example of a graphic that can be generated by the
controlled system of the apparatus to determine the condition of the
electrodes.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
Fig. 1 illustrates an electrochemical treatment apparatus 10 suited
for separating contaminants from a contaminated aqueous medium, such as
_g_
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
livestock wastewater (e.g. liquid manure) and industrial wastewater. The
apparatus 10 is particularly suited for purifying wastewater from livestock or
agricultural industries.
As shown in Figs. 1 to 3, the apparatus 10 comprises a top open
ended main tanlc 12 divided into a plurality of compartments or chambers
interconnected in fluid flow communication. More specifically, the main tank
10
.defines a first reactor chamber A, a second reactor chamber B, a gravity
settling.
chamber D having a bottom contaminant settling zone F, a clear liquid
collection
chamber H provided at the top of the gravity settling chamber D for receiving
clear liquid therefrom, a clear liquid disposal chamber E connected in fluid
flow
communication to the collection chamber H for receiving cleax liquid
therefrom,
and a foam receiving chamber G for receiving the foam resulting from the
passage of the contaminated aqueous medium through the first and second
reactor
chambers A and B. The main tank 12 is preferably formed from metal sheets and
the reactor chambers A and B are preferably lined with an electric insulating
material, such as a plastic material or from metal sheets with plasticization.
The
main tank 12 can be also made of concrete.
As shown in Figs. 1 and 3, the settling chamber D is provided with
a pair of sidewalls 14 inclined at an angle comprised between 45 and 60
degrees.
The collection chamber H has an open bottom wall 16 for allowing liquid to
pass
from the settling chamber D into the collection chamber H. According to a
preferred embodiment of the present invention, the open bottom wall 16 is
provided in the form of a perforated plate covering part of the settling
chamber D
and defining a plurality of inlet openings 1 ~. The collection chamber H
further
includes a pair of sidewalk 20 defining a series of spaced-apart openings 22
adapted to be located under the level of liquid for ensuring an intake of
clear
liquid and prevent the presence of foam in the disposal chamber E, as will be
seen
hereinafter. The collection chamber H is provided at one end thereof with a
weir
plate 24 for allowing the clear liquid to flow at a given rate from the
collection
chamber H into the disposal chamber E.
-9-
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
As shown in Fig. 1, a pump 26 is provided for feeding the aqueous
medium to be treated into the main tank 12 via a delivery pipe 28. A valve 30
is
provided for adjusting the flow of contaminated aqueous medium through the
delivery pipe 28. Once the main tanlc 12 has been filled up so that the level
of
contaminated aqueous medium rises above the openings 22 in the sidewalk 20 of
the collection chamber H, as shown in Fig. 1, the pump 26 is shut down and a
pump 32 forming part of a re-circulation system 34 is activated to circulate
the
aqueous medium through the apparatus 10.
As shown in Fig. l, the pump 32 of the re-circulation system 34 is
mounted in a re-circulation line 36 having an inlet pipe 38 connected in fluid
flow
communication with the disposal chamber E and an outlet pipe 40 comiected in
fluid flow communication with a bottom end portion of the first reactor
chamber
A. First and second valves 42 and 44 are provided upstream and downstream of
the re-circulation pump 32 for controlling the flow of the aqueous medium
through the re-circulation line 36.
The aqueous medium to be treated is first supplied to the bottom of
the first reactor chamber A. According to a preferred embodiment of the
present
invention, the aqueous medium is caused to flow upwardly through a non-
consumable electrofloatation cell 46 removably mounted in the first reactor
chamber A. The first reactor chamber A and the electrofloatation cell 46 form
an
electrofloatation reactor in which a portion of the contaminants contained in
the
aqueous medium to be treated is transformed into foam, as will be seen
hereinafter.
As shown in Fig. 6, the electrofloatation cell 46 includes a pair of
horizontal electrode plates 48, each of which is sandwiched between a base
plate
50 and a clamping plate 52 defining a central rectangular opening. The base
plate
50 and the clamping plate 52 of each set are drawn one towards the other by
four
clamping screws 54. Four ~ plastic cap screws 56 and four plastic nuts 58 are
provided for holding the two sets of clamping and base plates 50 and 52
together.
Four plastic washers 60 are provided between the two sets of plates about the
cap
screws 56 to isolate the electrode plates 48 from one another. Electric
connectors
- 10-
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
(not shown) are provided for allowing the so assembled electrofloatation cell
to
be fed with do current or alternating current. Each base plate 50 is provided
with a
mounting ann 62 by which the electrofloatation cell can be releasably secured
to
the sidewall of the first reactor chamber A. Each electrode plates 48 are
preferably provided in the form of a stainless steel screen.
Alternatively, as shown in Figs. 7a and 7b, the electrofloatation
cell 46 can take the form of a series of parallel and vertically oriented
spaced-
apart monopolar plates 64 depending from a pair of laterally spaced-apart
support
members 66 removably mounted to an inner surface of a closure plate 68 adapted
to be removably bolted to a sidewall of the first reaction chamber A to close
an
opening defined therein. In this way, the electrofloatation cell 46 can be
readily
removed as a unit from the first reactor chamber A through the opening
normally
closed by the closure plate 68. The supports 66 are made of an electrically
conducting material and are connected at respective proximal ends thereof to
electric connectors 70 mounted to the closure plate 68. As shown in Fig. 7b,
each
monopolar plate 64 is provided at an upper edge thereof with a pair of
laterally
spaced-apart internally threaded cylinders 72 for threaded engagement with
corresponding threaded pins 74. As shown in Fig. 7a, the pins 74 extend
through
longitudinally extending slots 76 defined in the support members 66 and are
provided with respective head portions 78 for allowing the pins 74 to hang
from
the support members 66 once inserted in the slots 76 thereof. It is understood
that
the pins 74 and the cylinders 72 are made of electrically conducting material
for
allowing current to be fed to the plates 64 via the support members 66.
As shown in Fig. 1, an air diffuser 80 is provided in the bottom
portion of the first reactor chamber A underneath the electrofloatation cell
46 for
injecting compressed air in the form of fine bubbles into the first reaction
chamber A. By feeding the electrofloatation cell 46 with do current or
alternating
current, oxygen and hydrogen micro-bubbles are generated. (diameter of about
10
~,m) The oxygen and hydrogen bubbles, lilce the injected air bubbles, rise to
the
surface bringing with them the suspended and colloidal particles contained in
the
aqueous medium, which flows in the same direction (co-current flow) as the
-11-
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
bubbles generated in the first reactor chamber A. The suspended and colloidal
particles are agglomerated and gradually transformed into foam at the surface
of
the aqueous medium. As shown in Fig. 1, a foam remover 82 is provided for
directing the foam formed at the surface of the aqueous medium into the foam
receiving chamber G, wherein the foam is drained via a drain 84 operated by a
valve 86. The foam remover 82 comprises a plurality of fins 88 extending
outwardly at right angles from an endless belt 90 extending over a pair of
rollers
92, one of the rollers 92 being driven in rotation for driving the belt 90 in
the
direction indicated by arrow 94.
The removal of the suspended and colloidal particles allows the
aqueous medium to progressively become clearer. In addition of trapping the
particles and bringing them to the surface, the oxygen bubbles act as an
oxidant
and allows the organic molecules to split up, thereby facilitating the
oxidation
thereof. The hydrogen formed at the cathode of the electrofloatation cell 46
is a
reducer which provides for the hydrogenation of the organic molecules, thereby
rendering the organic molecules less resistant to oxidation.
The air diffuser 80 could be replaced by a commercially available
ultrasound generator mounted at the bottom of the reactor chamber A to emit
sound vibrations to cause dirt accumulated on the electrode plates to fall
therefrom, thereby preventing fouling of the electrodes.
As shown in Figs. 1 to 3, the aqueous medium is directed from the
top open end of the first reactor chamber A ixlto the top open end of the
second
reactor chamber B. The flow of aqueous medimn between the first and second
reactor chambers A and B is controlled by a pair of weir plates 96 over which
the
aqueous medium has to flow to pass from the first reactor chamber A into the
second reactor chamber B. As indicated by arrows 98 in Figs. 1 and 3, the
aqueous medimn is circulated downwardly through the second reactor chamber B,
i.e. in a direction opposite to the flow of aqueous medium in the first
reactor
chamber A. As shown in Figs. 1 and 2, the second reactor chamber B houses an
electrocoagulation cell 100 through which the aqueous medium is passed
-12-
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
vertically downwardly before flowing into the gravity settling chamber D via
an
outlet opening 102 defined at the bottom of the second reactor chamber B.
As shown in Figs. 4a, 4b and 4c, the electrocoagulation cell 100
comprises a plurality of side-by-side soluble electrode plates, including
monopolar steel plates 104 and bipolar steel plates 106 housed in spaced-apart
relationship in an open ended casing 108 comprising a back wall 110, a pair of
sidewalls 112 and a front wall 114. The front and back walls 110 and 114 are
provided on respective inner surfaces thereof with a plurality of laterally-
spaced
apart vertical slots 116 for respectively receiving the front and rear
longitudinal
edges of the electrode plates. The back wall 110, the front wall 114 and the
sidewalk 112 are made of an insulating material, such as plastic, and are
glued,
screwed or otherwise secured together in a box-like configuration. The front
plate
114 defines a series of through holes 115 for receiving a corresponding series
of
threaded connectors 117 extending forwardly from the supply bars 119 secured
to
the front longitudinal edges of the monopolar plates 104. As shown in Fig. 4c,
each supply bar 119 is secured to an associated monopolar plate 104 by means
of
screws 121. The supply bars 119 are preferably made of brass or bronze. A
front
cover plate 118 provided with a series of sealing plugs 120 is adapted to be
removably mounted to the sidewall of the second reaction chamber B to enable
ready access to the electrocoagulation when need be. The threaded connectors
117 are adapted to extend through the sealing plugs 120 for allowing the
electrode plates to be connected to a source of current external to the second
reactor chamber B.
Fig. 5 illustrates a second embodiment of an electrocoagulation
cell which includes a set of soluble electrode plates, including monopolar
steel
plates 122 and bipolar steel plates 124, received between a pair of plastic
plates
126 having a series of slots 128 defined in respective inner facing side
thereof for
receiving opposed longitudinal edges of the soluble electrode plates. The
monopolar plates 122 are electrically connected in pairs by engagement of iron
bars 130 with the hoolcs 132 provided at respective upper edges of the
monopolar
plates 122. Each ,iron bar 130 is bolted at one end thereof to an associated
-13-
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
connector 134, which is in turn, connected to a source of current (not
shoran). The
plastic plates 126 are beveled at the bottom end thereof for allowing the
electrocoagulation cell illustrated in Fig. 5 to be lowered into the second
reaction
chamber B and seated in position therein by engagement of the plastic plates
126
with the inner surface of the second reactor chamber A. In this way, the
electrocoagultion cell can be easily removed from and installed in the second
reactor chamber B via the top open end thereof and that without the use of any
tool.
As shown in Fig. 1, pressurized air is fed into the bottom of the
second reactor chamber B via the air diffuser 80. The aqueous medium flows in
counter-current to the ascending movement of the air bubbles and the oxygen
and
hydrogen bubbles formed in the second reactor chamber B. Indeed, by feeding do
current or alternating current to the electrocoagulation cell 100, oxygen and
hydrogen micro-bubbles (diameter of about 10 ~,m) are generated and these
micro-bubbles, while rising to the surface in counter-current to the flow of
contaminated aqueous medium, bring with them some of the suspended and the
colloidal particles which are still present in the aqueous medium. The
suspended
and colloidal particles are agglomerated at the surface~of the aqueous medium
and
gradually transformed into foam. As for the foam generated in the first
electrochemical reactor, the foam generated in the second electrochemical
reactor
is removed by the foam remover 82.
The electrocoagulation cell 100 produces major coagulation and
flocculation effects on the contaminants contained in the aqueous medium. At
the
electrocoagulation cell 100, anodic and cathodic reactions as well as
homogenous
phase reactions occur simultaneously. At the anode where the electrons are
directed, the oxidation reactions occur at the boundary reactional layer. The
electrons from the boundary layer are picked up by the anode and directed to
an
external circuit (not shown). In the case of an iron electrode, the following
reactions occur:
Fe -~ Fe2+ +2e (1)
and Had -~ 2H++OZ (g)+2e
(2)
-14-
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
The first equation (1) is the reaction of the dissolution of the anode
(iron material) and is the main source of ferrous ions in the second reactor.
This
reaction is observed when the solution in the reactor turns to green. The
other
equation (2) is the parasite reaction of the evolution of the oxygen due to
the
electrolysis of the water. At the cathode, where the electrons are supplied,
the
reduction reactions occur at the boundary layer of the electrodes. The third
reaction (3), namely the evolution of the hydrogen due to the electrolysis of
the
water is defined as follows:
2Hz0+2e -~HZ(g) + 20H- (3)
The experimental observation of the slow evolution.of the pH at
the beginning of the reaction shows that another cathodic reaction takes place
in
parallel. This other reaction corresponds to the dissolution reaction of the
oxides
or of the iron hydroxides on the electrode plates. This reaction would be
defined
as follows:
Fe(OH)z + 2H+ + 2e -~ Fe + 2Hz0 (4a)
or under the form of:
Fe203 + 6H''- + 6e ~ Fe, Fez+ + 3H20 (q,b)
Hydrolysis reactions of Fe2+ ions (reaction 5) also occur in the
homogenous phase. The Fe2+ ions are oxidised by the dissolved oxygen (reaction
6) and the phosphates are subj ect to a precipitation reaction:
Fe2++ 2H20 ~Fe(OH)Z+ 2H~
Fe2+ +1~4 OZ + 20H- + l~2Hz0 -~ Fe(OH)3 (6)
-15-
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
~~ r ~ c~A " =~ i) 2 0 U 5
Reaction (5) occurs when the pH of the solution reaches the pH of
CH+ C KsP
precipitation, i.e. : CFe2+ where Ksp is the product of solubility of
Fe(OH)Z and C Fe2+ is the concentration of Fe2+.
Reaction (6) represents the oxidation of the ferrous ions by
dissolved oxygen in the form of ferric hydroxide precipitate. The change of
colour of the solution from green to brown or light amber is indicative of the
presence of this reaction:
The soluble phosphates contain in the aqueous medium to be
treated are mainly in the form of orthophosphates (HP04~- and P043-). The
phosphates are eliminated by the adsorption thereof on the iron hydroxides.
The main hypothesis for the removal of phosphorus in the presence
of an electric field is the adsorption of the phosphorus on the hydroxide
surface
followed by a surface reaction to form a complex Fe-P. This reaction can be
defined as follows:
HP04 + --- FeOH -~ --- FePOø + Hz0
In the event of increased pH, the formed Fe-P complex will be
destabilised by the competitive adsorption of the OH- ions and the phosphorus
will be rejected back in the solution: Therefore, the treatment must be
stopped
when the optimal pH is reached in order to prevent the phosphorus from being
released back into the solution to be decontaminated. Corresponding optimal pH
and reaction time are calculated from a kinetic model where electrochemical,
the
initial pH, the electric conductivity, the phosphate concentration, and the
concentration of suspending particles are previously known. Optimal values of
the time reaction and pH are settled in the automatic system.
The passage of the electric current in the electrocoagulation cell
100 also generates Fe2+is+ rations. These rations destabilise the negative
charges
of the colloids, thereby providing for a better flocculation of the colloids.
-16-
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
__ ~ ~, ys J
Furthermore, by working at an optimal pH, the canons form some hydroxide flocs
Fe (OH) " (n = 2 or 3), which precipitate, adsorb and attract the soluble and
organic phosphorus contained in the aqueous medium to be treated.
It is pointed out that according to one feature of the present
invention, the electrofloatation cell 46 and the electrocoagulation cell 100
can be
interchangeably mounted in either one of the first and the second reactor
chambers A and B. In this way, the electrofloatation treatment could be done
with
the contaminated aqueous medium flowing in counter-current to the oxygen and
hydrogen bubbles and .the electrocoagulation treatment could be done with the
contaminated aqueous medium flowing in co-current to the oxygen and hydrogen
bubbles.
It is also pointed out that the settling or sedimentation chamber D
could be placed between the first and second reactor chambers A and B. Also,
the
settling chamber could be provided in the form of an individual concrete
sedimentation tank.
At its exit from the second reactor chamber B, the aqueous
medium flows in the form of a j et through a turbulence zone C in the settling
chamber D. As shown in Figs. 1 and 3, a set of spaced-apart parallel smooth
plates 138 inclined at approximately 60 degrees is preferably removably
mounted
in the settling chamber D. The plates 138 act as a mechanical separator. The
aqueous medium loaded with iron hydroxide particles and other flocs is subject
to
decantation while rising to the surface of the settling chamber between the
plates
138. A mechanical agitator 140 is provided to ensure an optimal mixing ratio
in
the flocculation zone of the settling chamber D. Progressively, the particles
will
settle to the bottom F of the settling chamber D in the form of sludge and the
clear liquid will rise to the surface where the collection chamber H ensures
its
discharge into the disposal chamber E. The openings 22 in the sidewalls of the
collection chamber H will prevent the foam formed during the purification
process from being discharged into the disposal chamber E.
It is noted that the plates 138 could be replaced by a standard
ultrafiltration unit.
-17~
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
As shown in Fig. 1, first and second valves 142 and 144 are
respectively connected to first and second outlet conduits 146 and 148 for
respectively discharging the clear liquid and the sludge from the settling
chamber
D.
A mechanical separation system 150 is preferably selectively
combined to the electrochemical reactors A and B. The separation system can be
integrated in the re-circulation system 34 or, alternatively, into the
settling
chamber D. The separation system 150 can be used as an alternative to the
plates
138 or in addition thereof.
Indeed, as shown in Fig. l, the treated liquid collected in the
disposal chamber E is selectively directed into the separation system 150 or
into a
bypass conduit 160. At the beginning of the treatment process the filtration
liquid
is typically bypassed by closing valve 156 and opening valve 158. Later one
during the treatment process, when the concentration of solid matter in the
liquid
falls under a predetermined threshold, the valve 156 is opened to allow the
liquid
to flow through the separation system 150 before being either pumped back into
reactor A by pump 32 or directed to an outlet of the apparatusl0 through valve
164. Both valves 156 and 158 can be opened at the same time so that only a
portion of the total flow is processed through the supplemental separation
system
1-50.
The separation system 150 can be provided in the form a
separation system, such as a hydrocyclone, a particle filtration unit, a
membrane
filtration unit, etc. The separation system, 150 may comprise a membrane
module
151, a suction pump 151, and a concentrate outlet line 154 that can be
connected
to a backwash module (not shown) when it is desired to wash the separation
system 150. The membrane module 151 preferably has an average pore size
between 0.0002~,m and SO~,m and more preferably have an average pore size
between O.OOOS~,m and 1 Vim. Membranes manufactured by Hydranautics under
the Trade Mark ESFA 1 to 5 series, HydraCap 40-60 series may be used. As an
option, immersed membranes may replace the plates 138 in the settling chamber
-18-
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
D, suitable membranes include for example those manufactured by Zenon
Envirornnental Inc (ZeeWeed° 500 series), by Mitsubishi or by
Kubota.
The suction pump 152 creates a transmembrane pressure across the
membranes of the module 151 resulting in the rejection of bacteria or
suspended
solids. The concentrate is evacuated through line 154. The liquid pumped
through
the membranes by the suction pump 152 permeate to the permeate line 153. If it
is desired to evacuate the filtered liquid, valve 162 is closed and valve 164
is
opened. However, if it is desired to re-circulate the filtered liquid through
the
reactors A and B and the settling chamber D in a closed loop circuit until the
level
of contaminant contained in the aqueous medium reaches an acceptable level,
valve 162 is opened and valve 164 is closed.
The recirculation of "clear" liquid through the reactor chambers A
and B advantageously prevents fouling of the electrode plates and clogging of
the
inter-electrode spaces by the impurities contained in the contaminated aqueous
medium. The injection of pressurize air below the electrochemical cells 46 and
100 also contributes prevent fouling of the electrode plates. Pressurized air
could
also be injected into the settling chamber D for allowing oxidation of ferric
and .
ferrous ions.
Once the desired level of purification has been reached, the
recirculation pump 32 is shut down and the valves 142 and 144 are opened. The
purified liquid is then discharged via outlet conduit 146 and the sludge
formed
during the purification process is discharged via outlet conduit 148.
The operation of the apparatus 10 is controlled by an automated
control unit (not shown), In this way, the operation of the recirculation pump
32,
the suction pump 152, the valves 42, 44, 142, 144 156, 158, 162 and 164 and
the
electrochemical cells 46 and 100 can be automatically controlled to obtain the
desired level of decontamination. More specifically, the control unit is
adapted to
control the circulation of the effluent through the apparatus 10, the number
of
passes thereof through the various treatment sections of the apparatus as well
as
the intensity of the electric field to be generated in the electrochemical
reactors as
a function of the degree of contamination of the effluent.
-19-
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
Experimental trials have been conducted in two steps:
1) Electrochemical tests on a synthetic effluent
2) Electrochemical tests on a secondary effluent originating
from biological treatments
1. Electrochemical tests on a synthetic effluent:
These tests were conducted for establishing the basics of the
phenomenologic of the reduction of the amount of the phosphorous by
electrochemical reactions through the use of soluble anodes. Synthetic water
(tap
water with phosphorous salt and sodium salt) was used to carry on the
experimentation.
The experimental conditions are summarized in Table 1. The
results are presented in Figs. 8a, 8b and 8c. Fig. 8c illustrates the pH
evolution
and Figs. 8a and 8b illustrate the evolution of phosphorus in function of the
treatment time. These results show that the virtual removal model of
phosphorus
is representative of the reality. On the other hand, the model can be used to
predict the optimum reaction time within which residual phosphorous is at its
minimal value (_< 1 mg-P/L).
2. Tests on effluents originating from biological treatments:
Table 2 shows the results at the optimal rates of phosphorus
removal. These experimental results are in accordance with the phenomenologic
model (not shown) to predict the performance according to the operating
conditions (current .intensity, reaction time and the electric conductivity)
and the
configuration of the electrochemical cell. The global purification tendency
can be
predicted with the proposed phenomenologic model. One of the major advantages
of the present invention is the safe scale-up of the process.
-20-
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
From table 2, it can be observed that the removal efficiency for the
following elements can reach:
- suspended solids: up to 9$%
- total phosphorous: up to 98%
- total nitrogen: up to 60%
- total colifonns: up to 99%
The condition of the electrodes can be defined by the following
relation:
c~ (t)= "ate of reaction of the protofa at the elect~~odes surface at t
~~ate of ~eactiofa of the proton at the electrodes su~~face at t = 0
Physically, the parameter ~ represents the surface of an active site
available on an electrode (namely the cathode) expressed in a non-dimensional
number for an electrochemical reaction. The higher the non-dimensional number
~ is, the more the active sites are plentiful. In other words, the electrode
is in a
very clean state. On the other hand, a low value of ~ (below 0.5) provides an
indication that less and less number of active sites are available, thereby
indicating that the electrode has to be cleaned or changed. A ~ value
comprised
between 0.5 and 0.8 indicates a normal operating condition of the electrodes.
Fig. 9 describes the evolution of the condition of the electrodes ~
as a function of time. At the beginning, the electrode is in a very clean
condition.
Despite the various variations of the composition at the inlet of the reactor,
notably the important fluctuations of the suspended matters, the condition of
the
electrodes remains in a normal operating condition (points 21-4.6). ~n the
other
hand, when the suspended solids reach a critical threshold (>_ 3000-10000
mg/L),
the electrode is in a worn out state (point 51). Despite everything, the
condition of
the electrodes is continuously renewed as a result of the re-circulation
process of
the purified water which provides for a progressive removal of the dirt from
the
electrodes (points 51 -92).
-21 -
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
The condition of the electrodes can be monitored and cleaned as
required by at least one of the following modes:
1) A rapid increase of the current intensity at the beginning of the
reaction by the control unit and the return to a normal point of
operation after a little while (after a few seconds or a few minutes);
2) by ultrasound;
3) by mechanical means; and
4) by changing the polarity.
Table 1: Experimental Conditions for trials in synthetic water
Block No Current Electric Conduct. Temp
Density (A/m2) Field (S/cm) (oC)
(V/m)
1 93 645 2.135 19.1
2 93 590 2.15 20.5
3 93 496 2.2 20.2
4 93 512 2.04 19.7
5 186 961.5 2.18 21.4
6 279 14200 2.23 23.2
7 232 11420 2.26 22.8
8 279 14600 2.12 21.6
_22_
CA 02510467 2005-06-16
WO 2004/056711 PCT/CA2003/002005
Table 2:
Total Total Suspended
phosphorus nitrogen solids
(mg/L) m /L m /L
# IN OUT 1N OUT ~ IN OUT
1 57.0 5.75 52.5 40 634 20
2 135.0 4.2 360 235 2750 60
3 89.5 1.9 50.0 42.5 225 10
4 74.0 2.75 70 27.5 167 10
84.3 5.75 67.5 42.5 300 40
6 77.0 1.63 73 50 133 40
7 73.0 1.25 87.5 65.0 200 40
8 143 5.25 200.0 55.0 2600 100
9 86.0 3.0 n.m. n.m 120 10
107.0 3.0 95.0 80. 632 200
11 83.0 4.9 117.5 77.3 800 150
12 92.5 5.5 90.0 65. 800 75
13 61.5 5.3 75.0 67.5 450 38
14 69.1 3.1 97.5 90. 150 133
69.0 3.0 87.5 80.0 650 20
16 65.5 3.5 72.5 50. 300 40.
17 88.0 4.3 80.0 65.0 400. 33
18 143.5 5.3 132.5 65.0 1500 125
19 42.0 4.3 70.0 60.0 300 33
Average 86.3 3.9 104.3 69.9 690.1 61.9
Minimum 42.0 1.25 50.0 27.5 120 10
Maximum 143.5 5.75 360.0 235.0 2750 200
- 23 -