Note: Descriptions are shown in the official language in which they were submitted.
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-1-
BENZAMIDE INHIBITORS OF THE P2X~ RECEPTOR
The present invention relates to novel benzamide inhibitors of the P2X~
receptor,
processes for their preparation, intermediates useful in their preparation,
pharmaceutical
compositions containing them, and their use in therapy. The active compounds
of the present
invention are useful in the treatment of inflammatory diseases such as
osteoarthritis and
rheumatoid arthritis; allergies, asthma, COPD, cancer, reperfusion or ischemia
in stroke or
heart attack, autoimmune diseases and other disorders. The active compounds
are also
antagonists of the P2X~ receptor.
The P2X~ receptor (previously known as P2Z receptor), which is a ligand-gated
ion
channel, is present on a variety of cell types, largely those known to be
involved in
inflammatory/immune process, specifically, macrophages, mast cells and
lymphocytes (T and
B). Activation of the P2X~ receptor by extracellular nucleotides, in
particular adenosine
triphosphate, leads to the release of interleukin-1 ~3 (IL-1 (i) and giant
cell formation
(macrophages/microglial cells), degranulation (mast cells) and proliferation
(T cells),
apoptosis, and L-selectin shedding (lymphocytes). P2X~ receptors are also
located on
antigen-presenting cells (APC), keratinocytes, salivary acinar cells (parotid
cells), hepatocytes
and mesangial cells.
P2X~ antagonists are known in the art, such as those described in
International
Patent Publications WO 01/46200, WO 01/42194, WO 01/44213, W099/29660, WO
00/61569, WO 99/29661, WO 99/29686, WO 00/71529, and WO 01/44170, as well as
in
USSN (attorney docket number PC23106A, filed November 12, 2002).
Benzamides, heteroarylamides and reverse amides for uses other than inhibition
of
the P2X~ receptor are described in various publications, such as International
Patent
Publications WO 97/22600, EP 138,527, WO 00/71509, WO 98/28269, WO 99/17777
and
WO 01/58883.
SUMMARY OF THE INVENTION
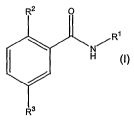
The present invention relates to a compound of the formula
R~
N/
H
(I)
wherein R' is (C~-C6)alkyl, optionally substituted by (C3-C~o)cycloalkyl, (C6-
C~o)aryl,
(C~-C~o)heterocyclyl, or (C~-C~o)heteroaryl, wherein each of said (C~-
C6)alkyl, (C3-
C~o)cycloalkyl, (Cs-C~o)aryl, (C~-C~o)heterocyclyl, or (C~-C~o)heteroaryl are
optionally
substituted by one to three suitable moieties independently selected from the
group consisting
CONFIRMATION COPI~
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-2-
of hydroxy, halogen, CN-, (C~-C6)alkyl, HO(C~-C6)alkyl, (C~-Cs)alkyl-NH(C=O)-,
NHZ(C=O)-,
(C~-C6)alkoxy, or (C3-C~o)cycloalkyl, wherein said (C3-C~o)cycloalkyl is
optionally. substituted
by one or more moieties selected from halogen, or (C~-C6)alkyl-;
RZ is hydrogen, halogen, -CN, and (C~-C6)alkyl, wherein said (C~-C6)alkyl is
optionally
substituted by one to three suitable moieties, independently selected from the
group
consisting of halo, hydroxy, amino, -CN, (C~-C6)alkyl, (C~-C6)alkoxy, -CF3,
CF30-,
(C~-C6)alkyl-NH-, [(C~-C6)alkyl]Z-N-, (C~-C6)alkyl-S-, (C~-C6)alkyl-(S=O)-,
(C~-C6)alkyl-(SOz)-,
(C~-C6)alkyl-O-(C=O)-, formyl, (C~-C6)alkyl-(C=O)-, and (C3-C6)cycloalkyl; and
R3 is a suitably substituted nitrogen linked (C~-C~o)heterocyclyl of the
formula:
N "N N
or
O N/ O N
II III
or the pharmaceutically acceptable salts or solvates or prodrugs thereof.
The present invention relates to a compound of the formula
R~
N/
H
(I)
wherein R' is (C~-Cs)alkyl, optionally substituted by (C3-C~o)cycloalkyl, (C6-
C~o)aryl,
(C~-C~o)heterocyclyl, or (C~-C~o)heteroaryl, wherein each of said (C~-
C6)alkyl, (C3-
C~o)cycloalkyl, (C6-C~o)aryl, (C~-C~o)heterocyclyl, or (C~-C~o)heteroaryl are
optionally
substituted by one to three suitable moieties independently selected from the
group consisting
of hydroxy, halogen, CN-, (C~-C6)alkyl, HO(C~-Cs)alkyl, (C~-Cs)alkyl-NH(C=O)-,
NH2(C=O)-,
(C~-C6)alkoxy, or (C3-C~o)cycloalkyl, wherein said (C3-C~o)cycloalkyl is
optionally substituted
by one or more moieties selected from halogen, or (C~-C6)alkyl-;
RZ is hydrogen, halogen, -CN, and (C~-C6)alkyl, wherein said (C~-C6)alkyl is
optionally
substituted by one to three suitable moieties, independently selected from
,the group
consisting of halo, hydroxy, amino, -CN, (C~-C6)alkyl, (C~-Cg)alkoxy, -CFA,
CF30-,
(C~-C6)alkyl-NH-, [(C~-C6)alkyl]Z-N-, (C~-C6)alkyl-S-, (C~-C6)alkyl-(S=O)-,
(C~-Cs)alkyl-(SOz)-,
(C~-C6)alkyl-O-(C=O)-, formyl, (C~-C6)alkyl-(C=O)-, and (C3-Cs)cycloalkyl;
R3 is a nitrogen linked (C~-C~o)heterocyclyl of the formula:
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-3-
R~ Ra
N
,~ ~ N or
O N R5
'~- .ww
IV V
wherein R4 and R5 are independently selected from the group of suitable
substituents,
such as hydrogen, halo, hydroxy, -CN, HO-(C~-Cs)alkyl, (C~-C6)alkyl, wherein
said (C~-
C6)alkyl is optionally substituted with one to three fluoro, (C~-C6)alkoxy
optionally substituted
with one to three fluoro, HOaC-, (C~-Cs)alkyl-O-(C=O)-, R6R8N(OZS)-, (C~-
C6)alkyl-(OAS)-NH-,
R6R8N C=O - R6R8N CHZ)m , C6-C~o)aryl,
(C~-C6)alkyl-OZS-[(C~-C6)alkyl-N]-, ( ) , ( (
(C3-C8)cycloalkyl, (C~-C~o)heteroaryl, (C~-C~o)heterocyclyl, (C6-C~o)aryl-O-,
(C3-C8)cycloalkyl-O-, (C~-C~o)heteroaryl-O- and (C~-C~o)heterocyclyl-O-; and
E R' is independently selected from the group of suitable substituents such as
hydrogen and (C~-C6)alkyl optionally substituted with one to three halogens,
hydroxy, -CN,
(Ci-C6)alkoxy-, (C2-C6)alkenoxy, (C~-C6)alkyl-SO~-, NH2-, ((C~-C6)alkyl)~ N-,
((C~-C6)alkenyl)~
N-, ((CZ-C6)alkynyl)~ N-, NH~(C=O)-, (C~-C6)alkyl-(C=O)N-, ((C~-Cs)alkyl)~ N-
(C=O)-, (CZ
C6)alkenyl-(C=0)N-, ((CZ-C6)alkenyl)n N-(C=O)-, (C~-C6)alkynyl-(C=O)N-, ((CZ-
C6)alkynyl)~ N
(C=O)-, (C~-C6)alkyl-(C=O)-, (CZ-C6)alkenyl-(C=O)-, (CZ-C6)alkynyl-(C=O)-, (C3-
C~o)cycloalkyl
' (C=O)-, ((C~-C~o)heterocyclyl-(C=O)-, (C6-C~o)aryl-(C=O), (C~-C~o)heteroaryl-
(C=O), (C~-
C6)alkyl-(C=O)O-, (C~-C6)alkenyl-(C=O)O-, (C2-C6)alkynyl-(C=O)O-, (C~-C6)alkyl-
O(C=0)-,
(C~-C6)alkenyl-O-(C=O)-, (C2-C6)alkynyl-O-(C=O)-, (C3-C~o)cycloalkyl, (C6-
C~o)aryl,
(C~-C~o)heterocyclyl, and (C~-C~o)heteroaryl;
wherein R4, R5 and R' may each be optionally substituted on any aliphatic or
aromatic
carbon atom by one to three suitable moieties, independently selected from the
group
consisting of halo, hydroxy, amino, -CN, (C~-C6)alkyl, (C~-C6)alkoxy, -CF3,
CF30-,
(Ci-C6)alkyl-NH-, [(C~-C6)alkyl]Z-N-, (C~-C6)alkyl-S-, (C~-C6)alkyl-(S=O)-,
(C~-C6)alkyl-(SOZ)-,
(C~-C6)alkyl-O-(C=O)-, formyl, (C~-C6)alkyl-(C=O)-, and (C3-C6)cycloalkyl;
R6 and R$ are each independently selected from the group consisting of
hydrogen,
(C~-C6)alkyl, HO-(CZ-C6)alkyl and (C3-C8)cycloalkyl, or R6 and R8 may
optionally be taken
together with the nitrogen atom to which they are attached to form a 3 to 8
membered
heterocycle;
n is an integer from zero to two; and
m is an integer from one to two;
or the pharmaceutically acceptable salts or solvates or prodrugs thereof.
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-4-
The present invention also relates to the pharmaceutically acceptable acid
addition
salts of compounds of the formula I. The acids which are used to prepare the
pharmaceutically
acceptable acid addition salts of the aforementioned base compounds of this
invention are
those which form non-toxic acid addition salts, i.e., salts containing
pharmacologically
acceptable anions, such as the chloride, bromide, iodide, nitrate, sulfate,
bisulfate, phosphate,
acid phosphate, acetate, lactate, citrate, acid citrate, tartrate, bitartrate,
succinate, maleate,
fumarate, gluconate, saccharate, benzoate, methanesulfonate, ethanesulfonate,
benzenesulfonate, p-toluenesulfonate and pamoate [i.e., 1,1'-methylene-bis-(2-
hydroxy-3-
naphthoate)]salts.
The invention also relates to base addition salts of formula I. The chemical
bases that
may be used as reagents to prepare pharmaceutically acceptable base salts of
those
compounds of formula I that are acidic in nature are those that form non-toxic
base salts with
such compounds. Such non-toxic base salts include, but are not limited to
those derived from
such pharmacologically acceptable cations such as alkali metal cations (e.g.,
potassium and
sodium) and alkaline earth metal cations (e.g., calcium and magnesium),
ammonium or water-
soluble amine addition salts such as N-methylglucamine-(meglumine), and the
lower
alkanolammonium and other base salts of pharmaceutically acceptable organic
amines.
This invention also encompasses pharmaceutical compositions containing
prodrugs of
compounds of the formula I. Compounds of formula I having free amino, amido,
hydroxy or
carboxylic groups can be converted into prodrugs. Prodrugs include compounds
wherein an
amino acid residue, or a polypeptide chain of two or more (e.g., two, three or
four) amino acid
residues which are covalently joined through peptide bonds to free amino,
hydroxy or carboxylic
acid groups of compounds of formula I. The amino acid residues include the 20
naturally
occurring amino acids commonly designated by three letter symbols and also
include,
4-hydroxyproline, hydroxylysine, demosine, isodemosine, 3-methylhistidine,
norvalin, beta-
alanine, gamma-aminobutyric acid, citrulline, homocysteine, homoserine,
ornithine and
methionine sulfone. Prodrugs also include compounds wherein carbonates,
carbamates,
amides and alkyl esters which are covalently bonded to the above substituents
of formula I
through the carbonyl carbon prodrug sidechain.
This invention also encompasses compounds of formula I containing protective
groups. One skilled in the art will also appreciate that compounds of the
invention can also
be prepared with certain protecting groups that are useful for purification or
storage and can
be removed before administration to a patient. The protection and deprotection
of functional
groups is described in "Protective Groups in Organic Chemistry", edited by
J.W.F. McOmie,
Plenum Press (1973) and "Protective Groups in Organic Synthesis", 3rd edition,
T.W. Greene
and P.G.M. Wuts, Wiley-Interscience (1999).
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-5-
The compounds of this invention include all stereoisomers (e.g., cis and traps
isomers)
and all optical isomers of compounds of the formula I (e.g., R and S
enantiomers), as well as
racemic, diastereomeric and other mixtures of such isomers.
The compounds, salts and prodrugs of the present invention can exist in
several
tautomeric forms, including the enol and imine form, and the fceto and enamine
form and
geometric isomers and mixtures thereof. All such tautomeric forms are included
within the
scope of the present invention. Tautomers exist as mixtures of a tautomeric
set in solution.
In solid form, usually one tautomer predominates. Even though one tautomer may
be
described, the present invention includes all tautomers of the present
compounds. One
example of a tautomeric structure is when R3 is a group of the formula
O N~N
One skilled in the art will appreciate that this group can also be drawn as
its tautomer
O N~N
The present invention also includes atropisomers of the present invention.
Atropisomers refer to compounds of formula I that can be separated into
rotationally restricted
isomers.
The compounds of this invention may contain olefin-like double bonds. When
such
bonds are present, the compounds of the invention exist as cis and traps
configurations and as
mixtures thereof.
A "suitable substituent" is intended to mean a chemically and pharmaceutically
acceptable functional group i.e., a moiety that does not negate the biological
activity of the
inventive compounds. Such suitable substituents may be routinely selected by
those skilled in
the art. Illustrative examples of suitable substituents include, but are not
limited to halo groups,
perFluoroalkyl groups, perfluoroalkoxy groups, alkyl groups, alkenyl groups,
alkynyl groups,
hydroxy groups, oxo groups, mercapto groups, alkylthio groups, alkoxy groups,
aryl or
heteroaryl groups, aryloxy or heteroaryloxy groups, aralkyl or heteroaralkyl
groups, aralkoxy or
heteroaralkoxy groups, HO-(C=O)- groups, amino groups, alkyl- and dialkylamino
groups,
carbamoyl groups, alkylcarbonyl groups, alkoxycarbonyl groups,
alkylaminocarbonyl groups
dialkylamino carbonyl groups, arylcarbonyl groups, aryloxycarbonyl groups,
alkylsulfonyl
groups, arylsulfonyl groups and the like. Those skilled in the art will
appreciate that many
substituents can be substituted by additional substituents. Further examples
of suitable
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-6-
substituents include those recited in the definition of compounds of Formula
I, including R~
through R', as defined hereinabove.
As used herein, the term "spiro" refers to a connection between two groups,
substituents etc., wherein the connection can be depicted according to the
following formula
\ /
As used herein, the term "alkyl," as well as the alkyl moieties of other
groups referred
to herein (e.g., alkoxy), may be linear or branched (such as methyl, ethyl, n-
propyl, isopropyl,
n-butyl, iso-butyl, secondary-butyl, terfiary-butyl); optionally substituted
by 1 to 3 suitable
substituents as defined above such as fluoro, chloro, trifluoromethyl, (C~-
C6)alkoxy,
(C6-C~o)aryloxy, trifluoromethoxy, difluoromethoxy or (C~-C6)alkyl. The phrase
"each of said
alkyl" as used herein refers to any of the preceding alkyl moieties within a
group such alkoxy,
alkenyl or alkylamino. Preferred alkyls include (C~-C6)alkyl, more preferred
are (C~-C4)alkyl, and
most preferred are methyl and ethyl.
As used herein, the term "cycloalkyl" refers to a mono, bicyclic or tricyclic
carbocyclic
ring (e.g., cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl,
cyclooctyl, cyclononyl,
cyclopentenyl, cyclohexenyl, bicyclo[2.2.1]heptanyl, bicyclo[3.2.1]octanyl and
bicyclo[5.2.0]nonanyl, etc.); optionally containing 1 or 2 double bonds and
optionally substituted
by 1 to 3 suitable substituents as defined above such as fluoro, chloro,
trifluoromethyl,
(C~-Cs)alkoxy, (C6-C~o)aryloxy, trifluoromethoxy, difluoromethoxy or (C,-
C6)alkyl.
As used herein, the term "halogen" includes fluoro, chloro, bromo or iodo or
fluoride,
chloride, bromide or iodide.
As used herein, the term "alkenyl" means straight or branched chain
unsaturated
radicals of 2 to 6 carbon atoms, including, but not limited to ethenyl, 1-
propenyl, 2-propenyl
(allyl), iso-propenyl, 2-methyl-1-propenyl, 1-butenyl, 2-butenyl, and the
like; optionally
substituted by 1 to 3 suitable substituents as defined above such as fluoro,
chloro,
trifluoromethyl, (C~-C6)alkoxy, (C6-C~o)aryloxy, trifluoromethoxy,
difluoromethoxy or (C~-Cs)alkyl.
As used herein, the term "alkynyl" is used herein to mean straight or branched
hydrocarbon chain radicals having one triple bond including, but not limited
to, ethynyl,
propynyl, butynyl, and the like; optionally substituted by 1 to 3 suitable
substituents as defined
above such as tluoro, chloro, trifluoromethyl, (C~-Cs)alkoxy, (C6-C~o)aryloxy,
trifluoromethoxy,
difluoromethoxy or (C~-Cs)alkyl.
As used herein, the term "carbonyl" or "(C=O)" (as used in phrases such as
alkylcarbonyl, alkyl-(C=O)- or alkoxycarbonyl) refers to the joinder of the
>C=O moiety to a
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-7-
second moiety such as an alkyl or amino group (i.e. an amido group).
Alkoxycarbonylamino
(i.e. alkoxy(C=O)-NH-) refers to an alkyl carbamate group. The carbonyl group
is also
equivalently defined herein as (C=O). Alkylcarbonylamino refers to groups such
as
acetamide.
As used herein, the term "oxo" is used herein to mean a double bonded oxygen
(=O)
radical wherein the bond partner is a carbon atom. Such a radical can also be
thought as a
carbonyl group.
As used herein, the term "(C~-C4)alkyl-OZS-[(C~-C4)alkyl-N]" is used herein to
mean a
radical of the formula
alkyl-S\
N-
alkyl
As used herein, the term "aryl" means aromatic radicals such as phenyl,
naphthyl,
tetrahydronaphthyl, indanyl and the like; optionally substituted by 1 to 3
suitable substituents as
defined above such as fluoro, chloro, trifluoromethyl, (C~-C6)alkoxy, (C6-
C~o)aryloxy,
trifluoromethoxy, difluoromethoxy or (C~-C6)alkyl.
As used herein, the term "heteroaryl" refers to an aromatic heterocyclic group
usually
with one heteroatom selected from O, S and N in the ring. In addition to said
heteroatom, the
aromatic group may optionally have up to four N atoms in the ring. For
example, heteroaryl
group includes pyridyl, pyrazinyl, pyrimidinyl, pyridazinyl, thienyl, furyl,
imidazolyl, pyrrolyl,
oxazolyl (e.g., 1,3-oxazolyl, 1,2-oxazolyl), thiazolyl (e.g., 1,2-thiazolyl,
1,3-thiazolyl), pyrazolyl,
tetrazolyl, triazolyl (e.g., 1,2,3-triazolyl, 1,2,4-triazolyl), oxadiazolyl
(e.g., 1,2,3-oxadiazolyl),
thiadiazolyl (e.g., 1,3,4-thiadiazolyl), quinolyl, isoquinolyl, benzothienyl,
benzofuryl, indolyl,
and the like; optionally substituted by 1 to 3 suitable substituents as
defined above such as
fluoro, chloro, trifluoromethyl, (C~-Cs)alkoxy, (C6-C~o)aryloxy,
trifluoromethoxy, difluoromethoxy
or (C~-Cs)alkyl. Particularly preferred heteroaryl groups include oxazolyl,
imidazolyl, pyridyl,
thienyl, furyl, thiazolyl and pyrazolyl.
The term "heterocyclic" as used herein refers to a cyclic group containing 1-9
carbon
atoms and 1 to 4 hetero atoms selected from N, O, S(O)S or NR. Examples of
such rings
include azetidinyl, tetrahydrofuranyl, imidazolidinyl, pyrrolidinyl,
piperidinyl, piperazinyl,
oxazolidinyl, thiazolidinyl, pyrazolidinyl, thiomorpholinyl,
tetrahydrothiazinyl, tetrahydro-
thiadiazinyl, morpholinyl, oxetanyl, tetrahydrodiazinyl, oxazinyl,
oxathiazinyl, indolinyl,
isoindolinyl, quinuclidinyl, chromanyl, isochromanyl, benzoxazinyl, and the
like. Examples of
said monocyclic saturated or partially saturated ring systems are
tetrahydrofuran-2-yl,
tetrahydrofuran-3-yl, imidazolidin-1-yl, imidazolidin-2-yl, imidazolidin-4-yl,
pyrrolidin-1-yl,
pyrrolidin-2-yl, pyrrolidin-3-yl, piperidin-1-yl, piperidin-2-yl, piperidin-3-
yl, piperazin-1-yl,
piperazin-2-yl, piperazin-3-yl, 1,3-oxazolidin-3-yl, isothiazolidine, 1,3-
thiazolidin-3-yl,
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
_g_
1,2-pyrazolidin-2-yl, 1,3-pyrazolidin-1-yl, thiomorpholin-yl, 1,2-
tetrahydrothiazin-2-yl,
1,3-tetrahydrothiazin-3-yl, tetrahydrothiadiazin-yl, morpholin-yl, 1,2-
tetrahydrodiazin-2-yl,
1,3-tetrahydrodiazin-1-yl, 1,4-oxazin-2-yl, 1,2,5-oxathiazin-4-yl and the
like; optionally
containing 1 or 2 double bonds and optionally substituted by 1 to 3 suitable
substituents as
defined above such as fluoro, chloro, trifluoromethyl, (C~-C6)alkoxy, (C6-
C~o)aryloxy,
trifluoromethoxy, difluoromethoxy or (C~-C6)alkyl. Preferred heterocyclics
include
tetrahydrofuranyl, pyrrolidinyl, piperidinyl, piperazinyl and morpholinyl.
Nitrogen heteroatoms as used herein refers to N=, >N and -NH; wherein -N=
refers to
a nitrogen double bond; >N refers to a nitrogen containing two bond
connections and -N refers
to a nitrogen containing one bond.
"Embodiment" as used herein refers to specific groupings of compounds or uses
into
discrete subgenera. Such subgenera may be cognizable according to one
particular
substituent such as a specific R' or R3 group. Other subgenera are cognizable
according to
combinations of various substituents, such as all compounds wherein R2 is
chloro and R' is
(C~-C4)alkyl, optionally substituted by (C3-C~o)cycloalkyl. The phrase "in
combination with
each of the aforementioned embodiments" refers to combinations of the
identified
embodiment with each embodiment previously identified in the specification.
Thus an
embodiment of compounds wherein R' is (C~-C4)alkyl, optionally substituted by
(C3-
C~o)cycloalkyl "in combination with each of the aforementioned embodiments"
refers to
additional embodiments comprising combinations with each embodiment previously
identified
in the specification.
Thus, the invention also provides compounds in which R' is (C~-C4)alkyl,
optionally
substituted by (C3-C~o)cycloalkyl, wherein said (Ci-C4)alkyl or (C3-
C~o)cycloalkyl are optionally
substituted by one to three suitable moieties independently selected from the
group consisting
of hydroxy, halogen, CN-, (C~-C6)alkyl, HO(C~-C6)alkyl, (C~-C6)alkyl-NH(C=O)-,
NHz(C=O)-,
(C~-C6)alkoxy, or (C3-C~o)cycloalkyl, wherein said (C3-C~o)cycloalkyl is
optionally substituted
by one or more moieties selected from halogen, or (C~-C6)alkyl-.
. The invention further provides compounds in which R~ is (C~-C4)alkyl,
optionally
substituted by (C6-C~o)aryl, wherein said (C~-C4)alkyl or (C6-C1o)aryl are
optionally substituted
by one to three suitable moieties independently selected from the group
consisting hydroxy,
halogen, CN-, (C~-C6)alkyl, HO(C~-C6)alkyl, (C~-Cs)alkyl-NH(C=O)-, NHZ(C=O)-,
(C~-
C6)alkoxy, or (C3-C~o)cycloalkyl, wherein said (C3-C~o)cycloalkyl is
optionally substituted by
one or more moieties selected from halogen, or (C~-C6)alkyl-.
' Moreover, the invention contemplates compounds in which RZ is halogen and
(Ci-
Cs)alkyl, and preferably compounds in which RZ is chloro, methyl or ethyl.
In one embodiment of the invention, R3 is a nitrogen linked (C~-
C~o)heterocyclyl of
formula (IV), wherein R4 and R5 are independently hydrogen or (C~-C4)alkyl,
preferably
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
_g_
hydrogen or methyl, and R' is independently selected from the group of
suitable substituents
such as hydrogen and (C~-C6)alkyl optionally substituted with one to three
substituents
independently selected from halogens, hydroxy, -CN, (C~-C6)alkoxy-, (CZ-
C6)alkenoxy,
(C~-C6)alkyl-SOZ-, NHZ-, (C~-C6)alkyl)~ N-, ((CZ-C6)alkenyl)~ N-, ((CZ-
C6)alkynyl)~ N-,
NH2(C=O)-, (C~-C6)alkyl-(C=O)N-, ((C1-C6)alkyl)~ N-(C=O)-, (CZ-C6)alkenyl-
(C=O)N-, ((C2-
C6)alkenyl)~ N-(C=O)-, (CZ-C6)alkynyl-(C=O)N-, ((Ca-C6)alkynyl)~ N-(C=O)-, (C~-
C6)alkyl-
(C=O)-, (C2-Cs)alkenyl-(C=O)-, (CZ-C6)alkynyl-(C=O)-, (C3-C~o)cycloalkyl-(C=O)-
, ((C~-
C~o)heterocyclyl-(C=O)-, (C6-C~o)aryl-(C=O), (C~-C~o)heteroaryl-(C=O), (C1-
C6)alkyl-(C=O)O-,
(CZ-C6)alkenyl-(C=O)O-, (C2-C6)alkynyl-(C=O)O-, (C~-Cs)alkyl-O(C=O)-, (C~-
C6)alkenyl-O-
(C=O)-, (Ca-C6)alkynyl-O-(C=O)-, (C3-C~o)cycloalkyl, (C6-C~o)aryl, (C~-
C~o)heterocyclyl, and
(C~-C~o)heteroaryl; wherein R' may optionally be substituted on any ring
aliphatic or aromatic
carbon atom by one to three suitable moieties, independently selected from the
group
consisting of halo, hydroxy, amino, -CN, (C~-Cq)alkyl, (C~-Cq)alkoxy, -CF3,
CF30-,
(C~-Cq)alkyl-NH-, [(C~-Cq)alkyl]2-N-, (Ci-Cq)alkyl-S-, (C~-Cq)alkyl-(S=O)-,
(C~-Cq)alkyl-(SOZ)-,
(C~-Cq)alkyl-O-(C=O)-, formyl, (C~-Cq)alkyl-(C=O)-, and (C3-C6)cycloalkyl.
Another embodiment of the invention are compounds in which R3 is a nitrogen
linked
(C~-C~o)heterocyclyl of formula (IV), wherein Rq and R5 are independently
hydrogen or (C~-
Cq)alkyl, preferably hydrogen or methyl, and R' is hydrogen.
A further embodiment of the invention are compounds in which R~ is a nitrogen
linked
(C~-C,o)heterocyclyl of formula (IV), wherein Rq and R5 are independently
hydrogen or (C~
Cq)alkyl, preferably hydrogen or methyl, and R' is (C~-Cq)alkyl optionally
substituted with one
to three substituents independently selected from halogens, hydroxy, -CN, (C~-
Cq)alkoxy-,
(CZ-Cq)alkenoxy, and (C~-Cq)alkyl-SOZ-. Preferably, R' is (C~-Cq)alkyl
optionally substituted
with one to three substituents independently selected from halogens, hydroxy, -
CN, or (C~
Cq)alkoxy-.
Still further, the invention provides compounds in which R3 is a nitrogen
linked (C~-
C~o)heterocyclyl of formula (IV), wherein Rq and R5 are independently hydrogen
or (C~-
Cq)alkyl, preferably hydrogen or methyl, and R' is (C~-Cq)alkyl optionally
substituted with one
to three substituents independently selected from NHZ-, (C~-Cq)alkyl)~ N-,
((CZ-Cq)alkenyl)n N-,
((C2-Cq)alkynyl)~ N-, NHZ(C=O)-, (C~-Cq)alkyl-(C=0)N-, ((C~-Cq)alkyl)~ N-(C=O)-
, (CZ-
Cq)alkenyl-(C=0)N-, ((CZ-Cq)alkenyl)~ N-(C=O)-, (CZ-Cq)alkynyl-(C=O)N-, and
((CZ-
Cq)alkynyl)~ N-(C=O)-. Preferably, R' is (C~-Cq)alkyl optionally substituted
with one to three
substituents independently selected from NHZ-, (C~-Cq)alkyl)~ N-, NHZ(C=O)-,
(C~-Cq)alkyl-
(C=O)N-, and ((C~-Cq)alkyl)~ N-(C=O)-.
° The invention also provides compounds in which R3 is a nitrogen
linked (Ci-
C~o)heterocyclyl of formula (IV), wherein Rq and R5 are independently hydrogen
or (C~-
Cq)alkyl, preferably hydrogen or methyl, and R' is (C~-Cq)alkyl optionally
substituted with one
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-10-
to three substituents independently selected from (C~-C4)alkyl-(C=O)-, (CZ-
C4)alkenyl-(C=O)-,
(CZ-C4)alkynyl-(C=O)-, (C3-C~o)cycloalkyl-(C=O)-, ((C~-C~o)heterocyclyl-(C=O)-
, (C6-C~o)aryl-
(C=O), and (C~-C~o)heteroaryl-(C=O), and preferably, R' is (C~-C4)alkyl
optionally substituted
with one to three substituents independently selected from (C~-C4)alkyl-(C=O)-
, (C3-
C~o)cycloalkyl-(C=O)-, ((C~-C~o)heterocyclyl-(C=O)-, (Cs-C~o)aryl-(C=O), and
(Ci-
C~o)heteroaryl-(C=O).
Another embodiment of the invention are compounds in which R3 is a nitrogen
linked
(C~-C~o)heterocyclyl of formula (IV), wherein R4 and R5 are independently
hydrogen or (C~-
C4)alkyl, preferably hydrogen or methyl, and R' is (C~-C4)alkyl optionally
substituted with one
to three substituents independently selected from (C~-C4)alkyl-(C=O)O-, (C~-
C4)alkenyl-
(C=O)O-, (Cz-C4)alkynyl-(C=O)O-, (C~-C4)alkyl-O(C=O)-, (CZ-C4)alkenyl-O-(C=O)-
, and (CZ-
C4)alkynyl-O-(C=O)-. Preferably, R' is (C~-C4)alkyl optionally substituted
with one to three
substituents independently selected from (C~-C4)alkyl-(C=O)O- and (C~-C4)alkyl-
O(C=O)-.
Furthermore, the invention provides compounds in which R3 is a nitrogen linked
(C~
C~o)heterocyclyl of formula (IV), wherein R4 and R5 are independently hydrogen
or (C~
C4)alkyl, preferably hydrogen or methyl, and R' is (C~-C4)alkyl optionally
substituted with one
to three substituents independently selected from (C3-C~o)cycloalkyl-, (C6-
C~o)aryl-,
(C~-C~o)heterocyclyl-, and (C~-C~o)heteroaryl-.
In one embodiment of the invention, R~ is a nitrogen linked (C~-
C~o)heterocyclyl of
formula (V), wherein R4 and R5 are independently hydrogen or (C~-CQ)alkyl,
preferably
hydrogen or methyl, and R' is independently selected from the group of
suitable substituents
such as hydrogen and (C~-C6)alkyl optionally substituted with one to three
substituents
independently selected from halogens, hydroxy, -CN, (C~-C6)alkoxy-, (CZ-
C6)alkenoxy,
(C~-C6)alkyl-SO2-, NH2-, (C~-C6)alkyl)~ N-, ((CZ-C6)alkenyl)n N-, ((C2-
C6)alkynyl)~ N-,
NH2(C=O)-, (C~-C6)alkyl-(C=O)N-, ((C~-C6)alkyl)~ N-(C=O)-, (CZ-C6)alkenyl-
(C=O)N-, ((C2-
C6)alkenyl)~ N-(C=O)-, (Cz-C6)alkynyl-(C=O)N-, ((Cz-C6)alkynyl)~ N-(C=O)-, (C~-
Cs)alkyl-
(C=O)-, (Cz-C6)alkenyl-(C=O)-, (C2-C6)alkynyl-(C=O)-, (C3-C~o)cycloalkyl-(C=O)-
, ((C~-
C~o)heterocyclyl-(C=O)-, (C6-C~o)aryl-(C=O), (C~-C~o)heteroaryl-(C=O), (C~-
Cs)alkyl-(C=O)O-,
(CZ-Cs)alkenyl-(C=O)O-, (CZ-C6)alkynyl-(C=O)O-, (C~-C6)alkyl-O(C=O)-, (C2-
C6)alkenyl-O-
(C=O)-, (C2-C6)alkynyl-O-(C=O)-, (C3-C~o)cycloalkyl, (C6-C~o)aryl, (C~-
C~o)heterocyclyl, and
(C~-C~o)heteroaryl; wherein R' may optionally be substituted on any ring
aliphatic or aromatic
carbon atom by one to three suitable moieties, independently selected from the
group
consisting of halo, hydroxy, amino, -CN, (C~-C4)alkyl, (Ci-C4)alkoxy, -CF3,
CF30-,
(C~-C4)alkyl-NH-, [(C~-C4)alkyl]2-N-, (C~-C4)alkyl-S-, (C~-C4)alkyl-(S=O)-,
(C~-C4)alkyl-(S02)-,
(C~-C4)alkyl-O-(C=O)-, formyl, (C~-C4)alkyl-(C=O)-, and (C3-Cs)cycloalkyl.
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-11-
Another embodiment of the invention are compounds in which R3 is a nitrogen
linked
(C~-C~o)heterocyclyl of formula (V), wherein R4 and R5 are independently
hydrogen or (C~-
C4)alkyl, preferably hydrogen or methyl, and R' is hydrogen.
A further embodiment of the invention are compounds in which R3 is a nitrogen
linked
(C~-C~o)heterocyclyl of formula (V), wherein R4 and R5 are independently
hydrogen or (C~
C4)alkyl, preferably hydrogen or methyl, and R' is (C~-C4)alkyl optionally
substituted with one
to three substituents independently selected from halogens, hydroxy, -CN, (C~-
C4)alkoxy-,
(C2-C4)alkenoxy, and (C~-C4)alkyl-SOa-. Preferably, R' is (C~-C4)alkyl
optionally substituted
with one to three substituents independently selected from halogens, hydroxy, -
CN, or (C~
C4)alkoxy-.
Still further, the invention provides compounds in which R3 is a nitrogen
linked (C~-
C~o)heterocyclyl of formula (V), wherein R4 and R5 are independently hydrogen
or (C~-
C4)alkyl, preferably hydrogen or methyl, and R' is (C~-C4)alkyl optionally
substituted with one
to three substituents independently selected from NHS-, (C~-C4)alkyl)"N-, ((CZ-
C4)alkenyl)~ N-,
((C~-C4)alkynyl)~ N-, NH2(C=O)-, (C~-C~)alkyl-(C=O)N-, ((C~-C4)alkyl)~ N-(C=O)-
, (CZ-
C4)alkenyl-(C=O)N-, ((C~-C4)alkenyl)~ N-(C=O)-, (C2-C4)alkynyl-(C=O)N-, and
((CZ-
C4)alkynyl)~ N-(C=O)-. Preferably, R' is (C~-C4)alkyl optionally substituted
with one to three
substituents independently selected from NHZ-, (C1-C4)alkyl)~ N-, NH2(C=O)-,
(C~-C4)alkyl-
(C=O)N-, and ((C~-C4)alkyl)~ N-(C=O)-.
The invention also provides compounds in which R3 is a nitrogen linked (C,-
C~o)heterocyclyl of formula (V), wherein R4 and R5 are independently hydrogen
or (C~-
C4)alkyl, preferably hydrogen or methyl, and R' is (C~-C4)alkyl optionally
substituted with one
to three substituents independently selected from (C~-C4)alkyl-(C=O)-, (CZ-
C4)alkenyl-(C=O)-,
(C~-C4)alkynyl-(C=O)-, (C3-C~o)cycloalkyl-(C=O)-, ((C~-C~o)heterocyclyl-(C=O)-
, (C6-C~o)aryl-
(C=O), and (C~-C~o)heteroaryl-(C=O), and preferably, R' is (C~-C4)alkyl
optionally substituted
with one to three substituents independently selected from (C~-C4)alkyl-(C=O)-
, (C3-
C~o)cycloalkyl-(C=O)-, ((C~-C~o)heterocyclyl-(C=O)-, (C6-C~o)aryl-(C=O), and
(C~-
C~o)heteroaryl-(C=O).
Another embodiment of the invention are compounds in which R3 is a nitrogen
linked
(C~-C~o)heterocyclyl of formula (V), wherein R4 and R5 are independently
hydrogen or (C~
C4)alkyl, preferably hydrogen or methyl, and R' is (C~-C4)alkyl optionally
substituted with one
to three substituents independently selected from (C~-C4)alkyl-(C=O)O-, (CZ-
Cø)alkenyl
(C=O)O-, (CZ-C4)alkynyl-(C=O)O-, (C~-C4)alkyl-O(C=O)-, (CZ-C4)alkenyl-O-(C=O)-
, and (CZ
C4)alkynyl-O-(C=O)-. Preferably, R' is (C~-Cd)alkyl optionally substituted
with one to three
substituents independently selected from (C~-C4)alkyl-(C=O)O- and (Ci-C4)alkyl-
O(C=O)-.
Furthermore, the invention provides compounds in which R3 is a nitrogen linked
(C~-
C~o)heterocyclyl of formula (V), wherein R4 and R5 are independently hydrogen
or (C~-
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-12-
C4)alkyl, preferably hydrogen or methyl, and R' is (C~-C4)alkyl optionally
substituted with one
to three substituents independently selected from (C3-C~o)cycloalkyl-, (C6-
C~o)aryl-,
(C1-C~o)heterocyclyl-, and (C~-C~o)heteroaryl-.
The present invention also provides compounds of formula (I) wherein R3 is a
nitrogen linked (C~-C~o)heterocyclyl of formula (IV):
Ra
N
N
O N/
(IV)
wherein R4 is hydrogen or methyl,
and R' is selected from the group consisting of:
HO
HO ~ \~ ~ Hp ,
HO
OH OH
HO , ~ , HO~~~ , and
OH O ~ \H
OH
HO
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (IV), R4 is hydrogen or methyl, and R' is
The present invention also contemplates compounds of formula (I) wherein R3 is
a
nitrogen linked (Ci-C~o)heterocyclyl of formula (IV), R4 is hydrogen or
methyl, and R' is
selected from the group consisting of:
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-13-
H3C0 ' H300 ,
OH OH
H
HsCO
OH
H3C~0 ~ , O ~ , and
OH OH
O
OH
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (IV), R4 is hydrogen or methyl, and R' is
0
ICI '0
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (Ci-
C~o)heterocyclyl of formula (IV), R4 is hydrogen or methyl, and R' is selected
from:
~N~~ > HZN'~~ '
~ HZN'
HZN~//1\\~~ , and ~ \
OH
Finally, the invention further provides compounds of formula (I) wherein R3 is
a nitrogen
linked (C~-C~o)heterocyclyl of formula (IV), R4 is hydrogen or methyl, and R'
is selected from:
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-14-
I I I I
w ~ , w ~ .w ~ ~ w ~ ,
OH OH OH
N
, ~N N
N
O 0
N
N ~ > > ~N
and
0
O
The present invention also provides compounds of formula (I) wherein R3 is a
nitrogen linked (C~-C~o)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is selected from the group consisting of:
HO
HO ~ \~ ~ Hp ,
HO
OH OH
HO , ~ , HO~~~ , and
OH O ~ \H
OH
HO
and R~ is (C~-C4)alkyl, optionally substituted by (C3-Cio)cycloalkyl, wherein
said (C~-
C4)alkyl or (C3-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting hydroxy, halogen, CN-, (C~-
C6)alkyl, HO(C~-
C6)alkyl, (C~-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-C6)alkoxy, or (C3-
C~o)cycloalkyl, wherein
said (C3-C~o)cycloalkyl is optionally substituted by one or more moieties
selected from
halogen, or (C~-C6)alkyl-.
The present invention also provides compounds of formula (I) wherein R3 is a
nitrogen linked (C~-C~o)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is selected from the group consisting of:
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-15-
HO
HO ~ \~ ~ HO
HO
OH OH
HO
HO ~ ~ , ~~ , and
OH OH
OH
HO
and R' is (C~-C4)alkyl, optionally substituted by (Cs-C~o)aryl, wherein said
(C~-C4)alkyl
or (Cs-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting hydroxy, halogen, CN-, (C~-Cs)alkyl, HO(C~-
C6)alkyl, (C~-
C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-C6)alkoxy, or (C3-C~o)cycloalkyl, wherein
said (C3-
C~o)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (C~-
Cs)alkyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C,o)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is
o-/\~
and R~ is (C~-C4)alkyl, optionally substituted by (C3-C~o)cycloalkyl, wherein
said (C~-
C4)alkyl or (C3-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting hydroxy, halogen, CN-, (C~-
C6)alkyl, HO(C~-
C6)alkyl, (C~-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-C6)alkoxy, or (C3-
C~o)cycloalkyl, wherein
said (C3-C~o)cycloalkyl is optionally substituted by one or more moieties
selected from
halogen, or (C~-C6)alkyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-16-
and R~ is (C~-C4)alkyl, optionally substituted by (C6-C~o)aryl, wherein said
(C~-C4)alkyl
or (C6-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting of hydroxy, halogen, CN-, (C~-C6)alkyl,
HO(C~-C6)alkyl,
(C~-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-Cs)alkoxy, or (C3-C~o)cycloalkyl,
wherein said (C3-
C~o)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (C~-
C6)alkyl-.
The present invention also contemplates compounds of formula (I) wherein R3 is
a
nitrogen linked (C~-C~o)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is selected from the group consisting of:
H3C0
H3C0
OH ' OH
H3C0
OH
H3C~0 ~ , 0 ~ , and
OH OH
0
OH
and R' is (C~-C4)alkyl, optionally substituted by (C3-C~o)cycloalkyl, wherein
said (C~-
C4)alkyl or (C3-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting hydroxy, halogen, CN-, (C~-
Cs)alkyl, HO(C~-
C6)alkyl, (C~-C6)alkyl-NH(C=O)-, NH2(C=O)-, (C~-Cs)alkoxy, or (C3-
C~o)cycloalkyl, wherein
said (C3-C~o)cycloalkyl is optionally substituted by one or more moieties
selected from
halogen, or (C~-Cs)alkyl-.
The present invention also contemplates compounds of formula (I) wherein R3 is
a
nitrogen linked (C~-C~o)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is selected from the group consisting of:
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-17-
H3C0 ~ H3C0
OH OH
H3CO > >
OH
H3C~o ~ , 0 ~ , and
OH OH
O
OH
and R' is (C~-C4)alkyl, optionally substituted by (Co-C~o)aryl, wherein said
(C~-C4)alkyl
or (Cs-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting of hydroxy, halogen, CN-, (Ci-C6)alkyl,
HO(C~-C6)alkyl,
(C~-C6)alkyl-NH(C=O)-, NH2(C=O)-, (C~-C6)alkoxy, or (C3-C~o)cycloalkyl,
wherein said (C3-
C~o)cYcloalkyl is optionally substituted by one or more moieties selected from
halogen, or (C~-
C6)alkyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C,-
C~o)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is
and R' is (C~-C4)alkyl, optionally substituted by (C3-C~o)cycloalkyl, wherein
said (C~-
C4)alkyl or (C3-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting of hydroxy, halogen, CN-, (C~-
C6)alkyl,
HO(C~-C6)alkyl, (Ci-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-Cs)alkoxy, or (C3-
C~o)cycloalkyl,
wherein said (C3-C~o)cycloalkyl is optionally substituted by one or more
moieties selected
from halogen, or (C~-C6)alkyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-18-
and R~ is (C~-C4)alkyl, optionally substituted by (C6-C~o)aryl, wherein said
(C~-C4)alkyl
or (C6-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting of hydroxy, halogen, CN-, (C~-C6)alkyl,
HO(C~-C6)alkyl,
(C~-C6)alkyl-NH(C=O)-, NH2(C=O)-, (C~-C6)alkoxy, or (C3-C~o)cycloalkyl,
wherein said (C3-
C~o)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (C~-
C6)alkyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is selected from:
~N~ ~ H N~~ '
~ HZN\
HZN~//~\\~~ ,an ~ \d
OH
and R' is (C~-C4)alkyl, optionally substituted by (C3-C~o)cycloalkyl, wherein
said (C~-
C4)alkyl or (C3-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting of hydroxy, halogen, CN-, (C~-
C6)alkyl,
HO(C~-C6)alkyl, (C~-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-C6)alkoxy, or (C3-
C~o)cycloalkyl,
wherein said (C3-C~o)cycloalkyl is optionally substituted by one or more
moieties selected
from halogen, or (C~-C6)alkyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is selected from:
N ~ ~
HZN'~~ '
HZN'
HzN~//1\\~~ , and ~ \ .
OH
and R~ is (C~-C4)alkyl, optionally substituted by (Cs-C~o)aryl, wherein said
(C~-C4)alkyl
or (C6-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting of hydroxy, halogen, CN-, (C~-C6)alkyl,
HO(C~-C6)alkyl,
(Ci-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-C6)alkoxy, or (C3-C~o)cycloalkyl,
wherein said (C3-
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-19-
C~o)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (Ci-
C6)alkyl-.
The invention further provides compounds of formula (I) wherein R3 is a
nitrogen linked
(C~-C~o)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is selected from:
/ / / /
\ ~ > \ , \ , \ ,
OH OH OH
/I / ~
' , N N
N
O O
/ ' N
N ~ ~ ~N
\ ~ ~ and
O
a
and R' is (Ci-C4)alkyl, optionally substituted by (C3-C~o)cycloalkyl, wherein
said (C~
C4)alkyl or (C3-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting of hydroxy, halogen, CN-, (C~-
C6)alkyl,
HO(C~-C6)alkyl, (C~-Cs)alkyl-NH(C=O)-, NH2(C=O)-, (Ci-C6)alkoxy, or (C3-
C~o)cycloalkyl,
wherein said (C3-C~o)cycloalkyl is optionally substituted by one or more
moieties selected
from halogen, or (C~-C6)alkyl-.
Finally, the invention further provides compounds of formula (I) wherein R3 is
a nitrogen
linked (C~-C~o)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is selected from:
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-20-
\ ~ > \ ~ \ ~ , \
OH OH OH
' , N , N
\ ,
N
O 0
N \ ~ ~~N
> N/
\ ,and
O
O
and R' is (C~-C4)alkyl, optionally substituted by (C6-C~o)aryl, wherein said
(C~-C4)alkyl
or (C6-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting of hydroxy, halogen, CN-, (C~-Cs)alkyl,
HO(C~-C6)alkyl,
(Ci-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-C6)alkoxy, or (C3-C~o)cycloalkyl,
wherein said (C3-
C~o)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (C~-
C6)alkyl-.
The present invention also provides compounds of formula (I) wherein R3 is a
nitrogen linked (C~-C~o)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is selected from the group consisting of:
HO
HO ~ \~ ~ Hp ,
HO
OH OH
HO , ~ , HO~~ , and
OH O ~ \H
OH
..
HO
R~ is chloro, methyl or ethyl;
and R' is (C~-C~)alkyl, optionally substituted by (C3-Cio)cycloalkyl, wherein
said (C~-
C4)alkyl or (C3-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-21-
independently selected from the group consisting of hydroxy, halogen, CN-, (C~-
C6)alkyl,
HO(C~-C6)alkyl, (C~-C6)alkyl-NH(C=O)-, NH~(C=O)-, (C~-C6)alkoxy, or (C3-
C~o)cycloalkyl,
wherein said (C3-C~o)cycloalkyl is optionally substituted by one or more
moieties selected
from halogen, or (C~-C6)alkyl-.
The present invention also provides compounds of formula (I) wherein R3 is a
nitrogen linked (C~-Cio)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is selected from the group consisting of:
HO
HO ~ \~ ~ HO
HO
OH OH
HO , ~ , HO~~~ , and
OH O ~ \H
OH
HO
RZ is chloro, methyl or ethyl;
and R' is (C~-C4)alkyl, optionally substituted by (C6-C~o)aryl, wherein said
(C~-C4)alkyl
or (C6-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting of hydroxy, halogen, CN-, (C~-C6)alkyl,
HO(Ci-C6)alkyl,
(C~-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-C6)alkoxy, or (C3-C~o)cycloalkyl,
wherein said (C3-
C,o)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (C~-
C6)alkyl-.
Also provided are compounds of formula (I) wherein R~ is a nitrogen linked (C~-
C~o)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is
RZ is chloro, methyl or ethyl;
and R~ is (C~-C4)alkyl, optionally substituted by (C3-C~o)cycloalkyl, wherein
said (C~
C4)alkyl or (C3-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting of hydroxy, halogen, CN-, (C~-
Cs)alkyl,
HO(C~-C6)alkyl, (C~-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-C6)alkoxy, or (C3-
C~o)cycloalkyl,
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-22-
wherein said (C3-C~o)cycloalkyl is optionally substituted by one or more
moieties selected
from halogen, or (C~-Cs)alkyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is
RZ is chloro, methyl or ethyl;
and R' is (C~-C4)alkyl, optionally substituted by (C6-C~o)aryl, wherein said
(C~-C4)alkyl
or (C6-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting of hydroxy, halogen, CN-, (C~-C6)alkyl,
HO(C~-C6)alkyl,
(C~-C6)alkyl-NH(C=O)-, NH~(C=O)-, (C~-C6)alkoxy, or (C3-C~o)cycloalkyl,
wherein said (C3
C~o)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (C~
Cs)alkyl-.
The present invention also contemplates compounds of formula (I) wherein R3 is
a
nitrogen linked (C~-C~o)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is selected from the group consisting of:
H3C0 ~ H3C0
OH OH
H3C
H3C0 > ~ \ >
OH
Ha0~0 , O ~ , and
OH OH
O
OH
RZ is chloro, methyl or ethyl;
and R~ is (Ci-C4)alkyl, optionally substituted by (C3-C~o)cycloalkyl, wherein
said (Ci-
C4)alkyl or (C3-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting of hydroxy, halogen, CN-, (C~-
C6)alkyl,
HO(C~-C6)alkyl, (C~-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-C6)alkoxy, or (C3-
C~o)cycloalkyl,
wherein said (C3-C~o)cycloalkyl is optionally substituted by one or more
moieties selected
from halogen, or (C~-C6)alkyl-.
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-23-
The present invention also contemplates compounds of formula (I) wherein R3 is
a
nitrogen linked (C~-C~o)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is selected from the group consisting of:
H3C0 ~ H3C0
OH OH
H3C0 H C
OH
H3C~0 , 0 ~ , and
OH OH
O
off
R2 is chloro, methyl or ethyl;
and R' is (C~-C4)alkyl, optionally substituted by (Cs-C~a)aryl, wherein said
(C~-C4)alkyl
or (C6-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting of hydroxy, halogen, CN-, (C~-C6)alkyl,
HO(C~-Cs)alkyl,
(C~-Cs)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-Cs)alkoxy, or (C3-C~o)cycloalkyl,
wherein said (C3-
C~o)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (C~-
C6)alkyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is
R2 is chloro, methyl or ethyl;
and R' is (C~-C4)alkyl, optionally substituted by (C3-C~o)cycloalkyl, wherein
said (C~
C4)alkyl or (C3-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting of hydroxy, halogen, CN-, (C~-
C6)alkyl,
HO(C~-C6)alkyl, (Ci-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-C6)alkoxy, or (C3-
C~o)cycloalkyl,
wherein said (C3-C~o)cycloalkyl is optionally substituted by one or more
moieties selected
from halogen, or (C~-C6)alkyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (IV),
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-24-
R4 is hydrogen or methyl,
R' is
~'1~~
RZ is chloro, methyl or ethyl;
and R' is (C~-C4)alkyl, optionally substituted by (C6-C~o)aryl, wherein said
(C~-C4)alkyl
or (C6-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting of hydroxy, halogen, CN-, (C~-C6)alkyl,
HO(C~-C6)alkyl,
(C~-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-C6)alkoxy, or (C3-C~o)cycloalkyl,
wherein said (C3
C~o)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (C~
C6)alkyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C,-
C~o)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is selected from:
~N ~ HZN'~~ '
H2N
HaN~//~\\~~ ,and
off
R~ is chloro, methyl or ethyl;
and R' is (C~-C4)alkyl, optionally substituted by (C~-C~o)cycloalkyl, wherein
said (C~-
C4)alkyl or (C3-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting of hydroxy, halogen, CN-, (C,-
C6)alkyl,
HO(C~-C6)alkyl, (C~-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-C6)alkoxy, or (C3-
C~o)cycloalkyl,
wherein said (C3-C~o)cycloalkyl is optionally substituted by one or more
moieties selected
from halogen, or (C~-C6)alkyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (Ci-
C~o)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is selected from:
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-25-
~N~ ~ H N'
HaN'
HZN'~/~\~~ ,an ~ \d
OH
RZ is chloro, methyl or ethyl;
and R' is (C~-C4)alkyl, optionally substituted by (C6-C~o)aryl, wherein said
(C~-C4)alkyl
or (C6-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting of hydroxy, halogen, CN-, (C~-C6)alkyl,
HO(C~-C6)alkyl,
(C~-C6)alkyl-NH(C=O)-, NH~(C=O)-, (C~-C6)alkoxy, or (C3-C~o)cycloalkyl,
wherein said (C3-
C~o)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (C~-
C6)alkyl-.
The invention further provides compounds of formula (I) wherein R3 is a
nitrogen linked
(C~-C~o)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is selected from:
/ / / /
\ , \ , \ , \ ,
OH OH OH
/ /
> > ~N . N
N
O O
/ N
N\\~ \
,and
O
O
RZ is chloro, methyl or ethyl;
and R~ is (C~-C4)alkyl, optionally substituted by (C3-Cio)cycloalkyl, wherein
said (C~
C4)alkyl or (C3-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting of hydroxy, halogen, CN-, (C~-
C6)alkyl,
HO(C~-C6)alkyl, (C~-C6)alkyl-NH(C=O)-, NH2(C=O)-, (C~-C6)alkoxy, or (C3-
Cio)cycloalkyl,
wherein said (C3-C~o)cycloalkyl is optionally substituted by one or more
moieties selected
from halogen, or (C~-Cs)alkyl-.
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-26-
Finally, the invention further provides compounds of formula (I) wherein R3 is
a nitrogen
linked (C~-C~o)heterocyclyl of formula (IV),
R4 is hydrogen or methyl,
R' is selected from:
\ ~ \ ° \ ~ \ ~ >
OH OH OH
> > ~N ~ N
\ ~ \
N
O O
N \ ~ ~~N
' N
\ ,and
O
0
R~ is chloro, methyl or ethyl;
and R' is (C~-C4)alkyl, optionally substituted by (C6-C~o)aryl, wherein said
(C~-C4)alkyl
or (C6-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting of hydroxy, halogen, CN-, (C~-C6)alkyl,
HO(C~-C6)alkyl,
(C~-C6)alkyl-NH(C=O)-, NH~(C=O)-, (C~-C6)alkoxy, or (C3-C~o)cycloalkyl,
wherein said (C3-
C~o)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (C~-
C6)alkyl-.
The present invention also provides compounds of formula (I) wherein R3 is a
nitrogen linked (C~-C~o)heterocyclyl of formula (V):
R5
,n.w (V)
R4 and R5 are independently hydrogen or methyl,
and R' is selected from the group consisting of:
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-27-
HO
HO ~ \~ ~ Hp
HO
OH OH
HO , ~ , HO.~~ , and
OH O ~ \H
OH
HO
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C,-
C~o)heterocyclyl of formula (V), R4 and R5 are independently hydrogen or
methyl, and R' is
~1
The present invention also contemplates compounds of formula (I) wherein R3 is
a
nitrogen linked (C~-C~o)heterocyclyl of formula (V), R4 and R5 are
independently hydrogen or
methyl, and R' is selected from the group consisting of:
H3C0 ~ H3CO
OH OH
H3CC
H3C0 > ~ \ >
OH
H3C~0 ~ , O ~ , and
OH OH
0
OH
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (V), R4 and R5 are independently hydrogen or
methyl, and R' is
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (V), R4 and R5 are independently hydrogen or
methyl, and R' is
selected from:
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-28-
~N~~ ~ HZN''
HZN'
HZN~//~\\~~ , an ~ \d
OH
Finally, the invention further provides compounds of formula (I) wherein R3 is
a nitrogen
linked (C~-C~o)heterocyclyl of formula (V), R4 and R5 are independently
hydrogen or methyl,
and R' is selected from:
\ ~ \ ~ \ ~ > \ ,
OH OH OH
> > V N ~ N
\
N
O O
' N
N~ \ ~ > N
,and
O
0
The present invention also provides compounds of formula (I) wherein R3 is a
nitrogen linked (C~-Cio)heterocyclyl of formula (V),
R4 and R5 are independently hydrogen or methyl,
R' is selected from the group consisting of:
HO
\ ~ HO ~ \~ , HO ,
HO
OH OH
HO , ~ , HO~~~ , and
OH O ~ \H
OH
HO
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-29-
and R' is (C~-C4)alkyl, optionally substituted by (C3-C~o)cycloalkyl, wherein
said (C~-
C4)alkyl or (C3-C,o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting hydroxy, halogen, CN-, (C~-
Cs)alkyl, HO(C~-
C6)alkyl, (C~-C6)alkyl-NH(C=O)-, NHa(C=O)-, (C~-C6)alkoxy, or (C3-
C~o)cycloalkyl, wherein
said (C3-C~o)cycloalkyl is optionally substituted by one or more moieties
selected from
halogen, or (C~-C6)alkyl-.
The present invention also provides compounds of formula (I) wherein R3 is a
nitrogen linked (C~-C~o)heterocyclyl of formula (V),
R4 and R5 are independently hydrogen or methyl,
R' is selected from the group consisting of:
HO
HO ~ \~ ~ Hp
HO
OH OH
HO
HO ~ ~ , ~ , and
OH OH
' OH
HO
and R' is (C~-C4)alkyl, optionally substituted by (C6-C~o)aryl, wherein said
(C~-C4)alkyl
or (C6-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting hydroxy, halogen, CN-, (C~-C6)alkyl, HO(C~-
C6)alkyl, (C~-
C6)alkyl-NH(C=O)-, NH~(C=O)-, (C~-C6)alkoxy, or (C3-C~o)cycloalkyl, wherein
said (C3-
C~o)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (C~-
C6)alkyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (V),
R4 and R5 are independently hydrogen or methyl,
R' is
and R' is (C~-C4)alkyl, optionally substituted by (C3-C~o)cycloalkyl, wherein
said (C1-
C4)alkyl or (C3-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting hydroxy, halogen, CN-, (C~-
Cs)alkyl, HO(C~-
Cs)alkyl, (C~-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-Cs)alkoxy, or (C3-
C~o)cycloalkyl, wherein
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-30-
said (C3-C~o)cycloalkyl is optionally substituted by one or more moieties
selected from
halogen, or (C~-C6)alkyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (V),
R4 and R5 are independently hydrogen or methyl,
R~ is
and R' is (C~-C4)alkyl, optionally substituted by (C6-C~o)aryl, wherein said
(C~-C4)alkyl
or (C6-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting of hydroxy, halogen, CN-, (C~-C6)alkyl,
HO(C~-C6)alkyl,
(C~-Cs)alkyl-NH(C=O)-, NH2(C=O)-, (C~-Cs)alkoxy, or (C~-C~o)cycloalkyl,
wherein said (C3-
C~o)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (C~-
C6)alkyl-.
The present invention also contemplates compounds of formula (I) wherein R3 is
a
nitrogen linked (C~-C~o)heterocyclyl of formula (V),
R4 and R5 are independently hydrogen or methyl,
R' is selected from the group consisting of:
H3CO ~ >
H3C0
OH OH
H3C
H3C0 ~ ~ \~ >
OH
H3C~0 ~ , O ~ , and
OH OH
O
OH
and R~ is (C~-C4)alkyl, optionally substituted by (C3-C~o)cycloalkyl, wherein
said (C~-
C4)alkyl or (C3-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting hydroxy, halogen, CN-, (C~-
C6)alkyl, HO(C~-
C6)alkyl, (C~-C6)alkyl-NH(C=O)-, NH2(C=O)-, (Ci-Cs)alkoxy, or (C3-
C~o)cycloalkyl, wherein
said (C3-C~o)cycloalkyl is optionally substituted by one or more moieties
selected from
halogen, or (C~-C6)alkyl-.
The present invention also contemplates compounds of formula (I) wherein R3 is
a
nitrogen linked (C~-C~o)heterocyclyl of formula (V),
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-31-
R4 and R5 are independently hydrogen or methyl,
R' is selected from the group consisting of:
H3C0
H3C0
OH OH
H3C0 H
OH
H3C~0 ~ , p ~ , and
OH OH
O
OH
and R~ is (C~-C4)alkyl, optionally substituted by (C6-C~o)aryl, wherein said
(C~-C4)alkyl
or (C6-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting of hydroxy, halogen, CN-, (C~-C6)alkyl,
HO(C~-C6)alkyl,
(C~-C6)alkyl-NH(C=O)-, NH2(C=O)-, (C~-C6)alkoxy, or (C3-C~o)cycloalkyl,
wherein said (C3-
C~o)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (C~-
C6)al kyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (V),
R4 and R5 are independently hydrogen or methyl,
R' is
and R' is (C~-C4)alkyl, optionally substituted by (C~-C~o)cycloalkyl, wherein
said (C~-
C4)alkyl or (C~-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting of hydroxy, halogen, CN-, (C~-
C6)alkyl,
HO(C~-C6)alkyl, (C~-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-C6)alkoxy, or (C3-
C~o)cycloalkyl,
wherein said (C3-C~o)cycloalkyl is optionally substituted by one or more
moieties selected
from halogen, or (C~-C6)alkyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (V),
R4 and R5 are independently hydrogen or methyl,
R' is
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-32-
and R~ is (C~-C4)alkyl, optionally substituted by (C6-C1o)aryl, wherein said
(C~-C4)alkyl
or (C6-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting of hydroxy, halogen, CN-, (C~-C6)alkyl,
HO(C~-C6)alkyl,
(C~-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-C6)alkoxy, or (C3-C~o)cycloalkyl,
wherein said (C3-
C~o)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (C~-
Cs)alkyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (V),
R4 and R5 are independently hydrogen or methyl,
R' is selected from:
~N ~ H N
HzN'
HzN~//1\\~~ , an ~ \d
O CCH
and R' is (C~-C4)alkyl, optionally substituted by (C3-C~o)cYcloalkyl, wherein
said (C~-
C4)alkyl or (C3-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting of hydroxy, halogen, CN-, (C~-
C6)alkyl,
HO(C~-C6)alkyl, (C~-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-C6)alkoxy, or (C3-
C~o)cycloalkyl,
wherein said (C3-C~o)cycloalkyl is optionally substituted by one or more
moieties selected
from halogen, or (C~-C6)alkyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (V),
R4 and R5 are independently hydrogen or methyl,
R' is selected from:
~N~~ > HZN~~~ '
HZN'
HzN~//~\\~~ ,an ~ \d
OH
and R~ is (C~-C4)alkyl, optionally substituted by (C6-C~o)aryl, wherein said
(C~-C4)alkyl
or (C6-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting of hydroxy, halogen, CN-, (C~-C6)alkyl,
HO(C~-C6)alkyl,
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-33-
(C~-Cs)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-Cs)alkoxy, or (C3-C~o)cycloalkyl,
wherein said (C3-
C~o)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (C~-
Cs)alkyl-.
The invention further provides compounds of formula (I) wherein R3 is a
nitrogen linked
(C~-C~o)heterocyclyl of formula (V),
R4 and R5 are independently hydrogen or methyl,
R' is selected from:
\ , \ ~ > \ , \ ,
OH OH OH
\ ' I , ~N N
,
N ,
O O
N/
N\ , \ , N
,and
O
O
and R' is (C~-C4)alkyl, optionally substituted by (C3-C~o)cycloalkyl, wherein
said (C~-
C4)alkyl or (C3-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting of hydroxy, halogen, CN-, (C~-
Cs)alkyl,
HO(C~-Cs)alkyl, (C~-Cs)alkyl-NH(C=O)-, NH2(C=O)-, (C~-Cs)alkoxy, or (C3-
C~o)cycloalkyl,
wherein said (C3-C~o)cycloalkyl is optionally substituted by one or more
moieties selected
from halogen, or (C~-Cs)alkyl-.
Finally, the invention further provides compounds of formula (I) wherein R3 is
a nitrogen
linked (C~-C~o)heterocyclyl of formula (V),
R4 and R5 are independently hydrogen or methyl,
R' is selected from:
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-34-
\ , \ , \ , \ ,
OH OH OH
> > V N . N
N
O 0
' N
N \ \ ~ N
1
0
0
and R' is (C~-C4)alkyl, optionally substituted by (C6-C~o)aryl, wherein said
(C~-C4)alkyl
or (C6-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting of hydroxy, halogen, CN-, (C,-C6)alkyl,
HO(C~-C6)alkyl,
(C~-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-C6)alkoxy, or (C3-C~o)cycloalkyl,
wherein said (C3-
Cio)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (C~-
C6)alkyl-. .
The present invention also provides compounds of formula (I) wherein R3 is a
nitrogen linked (C~-C~o)heterocyclyl of formula (V),
R4 and R5 are independently hydrogen or methyl,
R' is selected from the group consisting of:
HO
HO ~ \~ ~ HO ,
HO
OH OH
HO , ~ , HO~~~ , and
OH O ~ \H
OH
HO
RZ is chloro, methyl or ethyl;
and R' is (C~-C4)alkyl, optionally substituted by (C3-C~o)cycloalkyl, wherein
said (C~-
C4)alkyl or (C3-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting of hydroxy, halogen, CN-, (C~-
C6)alkyl,
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-35-
HO(C~-C6)alkyl, (C~-C6)alkyl-NH(C=O)-, NH~(C=O)-, (Ci-C6)alkoxy, or (C3-
C~o)cycloalkyl,
wherein said (C3-C~o)cycloalkyl is optionally substituted by one or more
moieties selected
from halogen, or (C~-C6)alkyl-.
The present invention also provides compounds of formula (I) wherein R3 is a
nitrogen linked (C~-C~o)heterocyclyl of formula (V),
R4 and R5 are independently hydrogen or methyl,
R' is selected from the group consisting of:
HO
HO ~ \~ ~ HO
HO
OH OH
HO , ~ , HO~~~ , and
OH O ~ \H
OH
HO
R~ is chloro, methyl or ethyl;
and R' is (C~-C4)alkyl, optionally substituted by (C6-C~o)aryl, wherein said
(C~-C4)alkyl
or (C6-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting of hydroxy, halogen, CN-, (C~-C6)alkyl,
HO(C~-C6)alkyl,
(C~-C6)alkyl-NH(C=O)-, NH~(C=O)-, (C~-C6)alkoxy, or (C3-C~o)cycloalkyl,
wherein said (C3
C~o)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (C~
C6)alkyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (V),
R4 and R5 are independently hydrogen or methyl,
R' is
R2 is chloro, methyl or ethyl;
and R~ is (C~-C4)alkyl, optionally substituted by (C3-G~o)cycloalkyl, wherein
said (C~-
C4)alkyl or (C3-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting of hydroxy, halogen, CN-, (C~-
C6)alkyl,
HO(C~-C6)alkyl, (C~-C6)alkyl-NH(C=O)-, NH2(C=O)-, (C~-C6)alkoxy, or (C3-
C~o)cycloalkyl,
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-36-
wherein said (C3-C~o)cycloalkyl is optionally substituted by one or more
moieties selected
from halogen, or (C~-C6)alkyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (V),
R4 and R5 are independently hydrogen or methyl,
R' is
RZ is chloro, methyl or ethyl;
and R' is (C~-C4)alkyl, optionally substituted by (C6-C~o)aryl, wherein said
(C~-C4)alkyl
or (Cs-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting of hydroxy, halogen, CN-, (C~-C6)alkyl,
HO(C~-C6)alkyl,
(C~-C6)alkyl-NH(C=O)-, NH~(C=O)-, (C~-Cs)alkoxy, or (C3-C~o)cycloalkyl,
wherein said (C3-
C~o)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (Ci-
Cs)alkyl-.
The present invention also contemplates compounds of formula (I) wherein R3 is
a
nitrogen linked (C~-C~o)heterocyclyl of formula (V),
R4 and R5 are independently hydrogen or methyl,
R' is selected from the group consisting of:
H3C0 ~ H3C0
r
OH OH
H3CC
H3C0 > ~ \ >
OH
HsC~O , O ~ , and
OH OH
O
OH
RZ is chloro, methyl or ethyl;
and R~ is (C~-C4)alkyl, optionally substituted by (C3-C~o)cycloalkyl, wherein
said (C~-
C4)alkyl or (C3-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting of hydroxy, halogen, CN-, (Ci-
C6)alkyl,
HO(C~-C6)alkyl, (C~-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-C6)alkoxy, or (C3-
C~o)cycloalkyl,
wherein said (C3-C~o)cycloalkyl is optionally substituted by one or more
moieties selected
from halogen, or (C~-Cs)alkyl-.
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-37-
The present invention also contemplates compounds of formula (I) wherein R~ is
a
nitrogen linked (C~-C~o)heterocyclyl of formula (V),
R'' and R5 are independently hydrogen or methyl,
R' is selected from the group consisting of:
H3C0 ~ H3C0
OH OH
H3C
H3C0
OH
H3C~0 ~ , O ~ , and
OH OH
O
off
Rz is chloro, methyl or ethyl;
and R~ is (C~-C4)alkyl, optionally substituted by (C6-C~o)aryl, wherein said
(C~-C4)alkyl
or (C6-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting of hydroxy, halogen, CN-, (C~-C6)alkyl,
HO(C~-C6)alkyl,
(C~-C6)alkyl-NH(C=O)-, NH2(C=O)-, (C~-C6)alkoxy, or (C3-C~o)cycloalkyl,
wherein said (C3-
C~o)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (C~-
C6)alkyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (V),
R4 and R5 are independently hydrogen or methyl,
R' is
RZ is chloro, methyl or ethyl;
and R' is (C~-C4)alkyl, optionally substituted by (C3-C~o)cycloalkyl, wherein
said (C~
C4)alkyl or (C3-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting of hydroxy, halogen, CN-, (C~-
C6)alkyl,
HO(C~-C6)alkyl, (C~-C6)alkyl-NH(C=O)-, NH2(C=O)-, (C~-C6)alkoxy, or (C3-
C~o)cycloalkyl,
wherein said (C3-C~o)cycloalkyl is optionally substituted by one or more
moieties selected
from halogen, or (C~-C6)alkyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (V),
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-38-
R4 and R5 are independently hydrogen or methyl,
R' is
R2 is chloro, methyl or ethyl;
and R' is (C~-C4)alkyl, optionally substituted by (C6-C~o)aryl, wherein said
(C~-C4)alkyl
or (C6-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting of hydroxy, halogen, CN-, (C~-C6)alkyl,
HO(C~-C6)alkyl,
(C~-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-C6)alkoxy, or (C3-C~o)cycloalkyl,
wherein said (C3
C~o)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (C~
C6)alkyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (V),
R4 and R5 are independently hydrogen or methyl,
R' is selected from:
N
, HZN
HZN\
HzN~//~\\\~~ ,an ~ \d
oN
RZ is chloro, methyl or ethyl;
and R~ is (C~-C4)alkyl, optionally substituted by (C3-C~o)cycloalkyl, wherein
said (C~-
C4)alkyl or (C3-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting of hydroxy, halogen, CN-, (C~-
C6)alkyl,
HO(C~-C6)alkyl, (C1-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-C6)alkoxy, or (C3-
C~o)cycloalkyl,
wherein said (C3-C~o)cycloalkyl is optionally substituted by one or more
moieties selected
from halogen, or (C~-C6)alkyl-.
Also provided are compounds of formula (I) wherein R3 is a nitrogen linked (C~-
C~o)heterocyclyl of formula (V),
R4 and R5 are independently hydrogen or methyl,
R' is selected from:
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-39-
N . HZN'~~ '
HZN
HzN~//~\\~~~ ,and
OH
RZ is chloro, methyl or ethyl;
and R' is (Ci-C4)alkyl, optionally substituted by (C6-C~o)aryl, wherein said
(C~-C4)alkyl
or (C6-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting of hydroxy, halogen, CN-, (C~-C6)alkyl,
HO(C~-C6)alkyl,
(C~-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-C6)alkoxy, or (C3-C~o)cycloalkyl,
wherein said (C3-
C~o)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (C~-
C6)alkyl-.
The invention further provides compounds of formula (I) wherein R3 is a
nitrogen linked
(C~-C~o)heterocyclyl of formula (V),
R4 and R5 are independently hydrogen or methyl,
R' is selected from:
\ , \ , \ , \ ,
OH OH OH
~ I ~ ~ N~
' I , ~ , N
\ ~ \ ~~ .
N
O O
> N/
N\ \ ~ N
,and
O
O
RZ is chloro, methyl or ethyl;
and R~ is (C~-C4)alkyl, optionally substituted by (C3-C~o)cycloalkyl, wherein
said (C~
C4)alkyl or (C3-C~o)cycloalkyl are optionally substituted by one to three
suitable moieties
independently selected from the group consisting of hydrvxy, halogen, CN-, (C~-
C6)alkyl,
HO(C~-Cs)alkyl, (Ci-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-Cs)alkoxy, or (C3-
C~o)cycloalkyl,
wherein said (C3-C~o)cycloalkyl is optionally substituted by one or more
moieties selected
from halogen, or (Ci-C6)alkyl-.
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-40-
Finally, the invention further provides compounds of formula (I) wherein R3 is
a nitrogen
linked (C~-C~o)heterocyclyl of formula (V),
R4 and R5 are independently hydrogen or methyl,
R' is selected from:
\ , \ ~ > \ ~ , \
OH OH OH
I
\ ' , ~N N
N
p O
N
N\ \ ~ N
and
O
0
RZ is chloro, methyl or ethyl;
and R' is (C~-C4)alkyl, optionally substituted by (C6-C~o)aryl, wherein said
(C~-C4)alkyl
or (C6-C~o)aryl are optionally substituted by one to three suitable moieties
independently
selected from the group consisting of hydroxy, halogen, CN-, (C~-C6)alkyl,
HO(C~-C6)alkyl,
(C~-C6)alkyl-NH(C=O)-, NHZ(C=O)-, (C~-C6)alkoxy, or (C3-C~o)cycloalkyl,
wherein said (C3-
C~o)cycloalkyl is optionally substituted by one or more moieties selected from
halogen, or (C~-
C6)alkyl-.
Examples of other compounds of formula I are the following:
2-Chloro-5-(3-ethyl-5-oxo-4,5-d ihydro-[1,2,4]triazol-1-yl)-N-(1-hydroxy-
cycloheptylmethyl)-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-(3-methoxymethyl-5-oxo-4,5-d ihydro-
[1,2,4]triazol-1-yl)-benzamide
2-Chloro-5-(3-cyclopropyl-5-oxo-4,5-dihydro-[1,2,4]triazol-1-yl)-N-(1-hydroxy-
cycloheptylmethyl)-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[3-(2-hydroxy-ethyl)-5-oxo-4,5-
dihydro-
[1,2,4]triazol-1-yl]-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-(5-oxo-3-phenyl-4,5-dihydro-
[1,2,4]triazol-1-yl)-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-(5-oxo-3-pyridin-4-yl-4,5-dihydro-
[1,2,4]triazol-1-yl)-benzamide
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-41-
2-Chloro-N-(1-hydroxy-cyclohexylmethyl)-5-(3-methyl-5-oxo-4,5-dihydro-
[1,2,4]triazol-
1-yl)-benzamide
2-Chloro-N-[2-(2-chloro-phenyl)-ethyl]-5-(3-methyl-5-oxo-4,5-dihydro-
[1,2,4]triazol-1-
yl)-benzamide
2-Chloro-5-(3-methyl-5-oxo-4,5-dihydro-[1,2,4]triazol-1-yl)-N-(1-p-tolyl-
cyclohexylmethyl)-benzamide
2-Chloro-5-(3-methyl-5-oxo-4,5-dihydro-[1,2,4]triazol-1-yl )-N-(1-p-tolyl-
cyclohexylmethyl)-benzamide
2-Chloro-5-[4-(2,3-d ihydroxy-propyl)-3-methyl-5-oxo-4,5-dihydro-
[1,2,4]triazol-1-yl]-N-
(1-hydroxy-cycloheptylmethyl)-benzamide
2-Chloro-5-{4-[(cyclopropylmethyl-carbamoyl)-methyl]-3-methyl-5-oxo-4,5-
dihydro-
[1,2,4]triazol-1-yl}-N-(1-hydroxy-cycloheptylmethyl)-benzamide
2-Chloro-5-(4-dimethylcarbamoylmethyl-3-methyl-5-oxo-4,5-dihydro-
[1,2,4]triazol-1-
yl)-N-(1-hydroxy-cycloheptylmethyl)-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-(3-methyl-4-methylcarbamoylmethyl-5-
oxo-4,5-d ihydro-[1,2,4]triazol-1-yl)-benzam ide
2-Chloro-5-(4-ethylcarbamoylmethyl-3-methyl-5-oxo-4,5-dihydro-[1,2,4]triazol-1-
yl)-N-
(1-hydroxy-cycloheptylmethyl)-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-(3-methyl-4-{[(oxetan-3-ylmethyl)-
carbamoyl]-methyl]-5-oxo-4,5-dihydro-[1,2,4]triazol-1-yl)-benzamide
5-[4-(Azetidin-3-ylcarbamoylmethyl)-3-methyl-5-oxo-4,5-dihydro-[1,2,4]triazol-
1-yl]-2-
chloro-N-(1-hydroxy-cycloheptylmethyl)-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[3-methyl-5-oxo-4-(pyrrolidin-3-
ylcarbamoylmethyl)-4,5-dihydro-[1,2,4]triazol-1-yl]-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-(3-methyl-5-oxo-4-(piperidin-4-
ylcarbamoylmethyl)-4,5-dihyd ro-[1,2,4]triazol-1-yl]-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-{3-methyl-5-oxo-4-[(tetrahydro-
pyran-4-
ylcarbamoyl)-methyl]-4,5-dihydro-[1,2,4]triazol-1-yl}-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-(3-methyl-5-oxo-4-[(tetrahydro-
furan-3-
ylcarbamoyl)-methyl]-4,5-dihydro-[1,2,4]triazol-1-yl}-benzamide
2-Chloro-5-[4-(3-dimethylamino-2-hydroxy-propyl)-3-methyl-5-oxo-4,5-dihydro-
[1,2,4]triazol-1-yl]-N-(1-hydroxy-cycloheptylmethyl)-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[4-(2-hydroxy-3-pyrrolidin-1-yl-
propyl)-3-
methyl-5-oxo-4,5-dihydro-[1,2,4]triazol-1-yl]-benzamide
5-[4-(3-Amino-2-hydroxy-propyl)-3-methyl-5-oxo-4,5-dihydro-[1,2,4]triazol-1-
yl]-2-
chloro-N-(1-hydroxy-cycloheptylmethyl)-benzamide
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-42-
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-{4-[2-hydroxy-3-(oxetan-3-yloxy)-
propyl]-
3-methyl-5-oxo-4,5-dihydro-[1,2,4]triazol-1-yl}-benzam ide
5-{4-[3-(Azetidin-3-yloxy)-2-hydroxy-propyl]-3-methyl-5-oxo-4,5-dihydro-
[1,2,4]triazol-
1-yl}-2-chloro-N-(1-hydroxy-cycloheptylmethyl)-benzamide
5-{4-[3-(Azetid in-3-yloxy)-propyl]-3-methyl-5-oxo-4, 5-d ihyd ro-
[1,2,4]triazol-1-yl}-2-
chloro-N-(1-hydroxy-cycloheptylmethyl)-benzam ide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-{4-[2-hydroxy-3-(pyrrolidin-3-
yloxy)-
propyl]-3-methyl-5-oxo-4,5-dihydro-[1,2,4]triazol-1-yl}-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-{3-methyl-5-oxo-4-[3-(pyrrolidin-3-
yloxy)-
propyl]-4,5-dihydro-[1,2,4]triazol-1-yl}-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-{4-[2-hydroxy-3-(piperidin-4-yloxy)-
propyl]-3-methyl-5-oxo-4,5-dihydro-[1,2,4]triazol-1-yl}-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-{3-methyl-5-oxo-4-[3-(piperidin-4-
yloxy)-
propyl]-4,5-dihydro-[1,2,4]triazol-1-yl}-benzamide
2-Chloro-5-[4-(3,4-dihydroxy-butyl)-3-methyl-5-oxo-4,5-dihydro-[1,2,4]triazol-
1-yl]-N-
(1-hydroxy-cycloheptylmethyl)-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[3-methyl-5-oxo-4-(2-
trifluoromethoxy-
ethyl)-4,5-dihydro-[1,2,4]triazol-1-yl]-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[4-(2-methoxy-ethyl)-5-oxo-4,5-
dihydro-
[1,2,4]triazol-1-yl]-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-(5-oxo-4,5-dihydro-[1,2,4]triazol-1-
yl)-
benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-(3-methyl-5-oxo-4,5-dihydro-
[1,2,4]triazol-1-yl)-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[4-(2-hydroxy-ethyl)-5-oxo-4,5-
dihydro-
[1,2,4]triazol-1-yl]-benzamide
2-Chloro-5-(4-cyanomethyl-5-oxo-4,5-dihydro-[1,2,4]triazol-1-yl)-N-(1-hydroxy-
cycloheptylmethyl)-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[4-(2-methoxy-ethyl)-3-methyl-5-oxo-
4,5-
dihydro-[1,2,4]triazol-1-yl]-benzamide
2-Chloro-5-(4-cyanomethyl-3-methyl-5-oxo-4,5-dihydro-[1,2,4]triazol-1-yl)-N-(1-
hydroxy-cycloheptylmethyl)-benzamide
2-Chloro-N-(1-hydroxy-3,3-dimethyl-cyclohexylmethyl)-5-(3-methyl-5-oxo-4,5-
dihydro-
[1,2,4]triazol-1-yl)-benzamide
5-(4-Carbamoylmethyl-3-methyl-5-oxo-4,5-dihydro-[1,2,4]triazol-1-yl)-2-chloro-
N-(1-
hydroxy-cycloheptylmethyl)-benzamide
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-43-
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[4-(2-hydroxy-ethyl)-3-methyl-5-oxo-
4,5-
dihydro-[1,2,4]triazol-1-yl]-benzamide
5-[4-(2-Am ino-ethyl)-3-methyl-5-oxo-4,5-dihydro-[1,2,4]triazol-1-yl]-2-chloro-
N-(1-
hydroxy-cycloheptylmethyl)-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[4-(2-hydroxy-3-methoxy-propyl)-3-
methyl-5-oxo-4,5-dihydro-[1,2,4]triazol-1-yl]-benzam ide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[4-(2-hydroxy-3-methoxy-propyl)-3-
methyl-5-oxo-4,5-d ihydro-[1,2,4]triazol-1-yl]-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[4-(2-hydroxy-2-methyl-propyl)-3-
methyl-
5-oxo-4,5-dihydro-[1,2,4]triazol-1-yl]-benzamide
2-Chloro-N-(1-hydroxy-cyclohexylmethyl)-5-(4-methyl-2-oxo-2,3-dihydro-im
idazol-1-
yl)-benzamide
2-Chloro-N-(1-hydroxy-cyclohexylmethyl)-5-(2-oxo-2,3-dihydro-im idazol-1-yl)-
benzamide
2-Chloro-5-(4-methyl-2-oxo-2,3-dihydro-imidazol-1-yl)-N-(1-p-tolyl-
cyclohexylmethyl)-
benzamide
2-Chloro-5-(2-oxo-2,3-dihydro-im idazol-1-yl)-N-(1-p-tolyl-cyclohexylmethyl)-
benzamide
2-Chloro-N-(4,4-difluoro-1-p-tolyl-cyclohexylmethyl)-5-(4-methyl-2-oxo-2,3-
dihydro-
imidazol-1-yl)-benzamide
2-Chloro-N-(4,4-difluoro-1-p-tolyl-cyclohexylmethyl)-5-(2-oxo-2,3-dihydro-
imidazol-1-
yl)-benzamide
2-Chloro-5-[3-(2,3-dihydroxy-propyl)-4-methyl-2-oxo-2,3-dihydro-im idazol-1-
yl]-N-(1-
hydroxy-cycloheptylmethyl)-benzamide
2-Chloro-5-[3-(2,3-dihydroxy-propyl)-2-oxo-2,3-dihydro-imidazol-1-yl]-N-(1-
hydroxy-
cycloheptylmethyl)-benzamide
2-Chloro-5-{3-[(cyclopropylmethyl-carbamoyl)-methyl]-4-methyl-2-oxo-2,3-
dihydro-
imidazol-1-yl}-N-(1-hydroxy-cycloheptylmethyl)-benzamide
2-Chloro-5-{3-[(cyclopropylmethyl-carbamoyl)-methyl]-2-oxo-2,3-dihydro-
imidazol-1-
yl}-N-(1-hydroxy-cycloheptylmethyl)-benzamide
2-Chloro-5-(3-dimethylcarbamoylmethyl-4-methyl-2-oxo-2,3-dihydro-imidazol-1-yl
)-N-
(1-hydroxy-cycloheptylmethyl)-benzamide
2-Chloro-5-(3-dimethylcarbamoylmethyl-2-oxo-2,3-dihydro-imidazol-1-yl)-N-(1-
hydroxy-cycloheptylmethyl)-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-(4-methyl-3-methylcarbamoylmethyl-2-
oxo-2,3-dihydro-imidazol-1-yl)-benzamide
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
2-Chloro-5-(3-dimethylcarbamoylmethyl-2.-oxo-2,3-dihydro-imidazol-1-yl)-N-(1-
hydroxy-cycloheptylmethyl)-benzamide
2-Chloro-5-(3-ethylcarbamoylmethyl-4-methyl-2-oxo-2,3-dihydro-im idazol-1-yl)-
N-(1-
hydroxy-cycloheptylmethyl)-benzamide
2-Chloro-5-(3-ethylcarbamoylmethyl-2-oxo-2,3-d ihydro-imidazol-1-yl)-N-(1-
hydroxy-
cycloheptylmethyl)-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-(4-methyl-3-{[(oxetan-3-ylmethyl)-
carbamol]-methyl}-2-oxo-2,3-dihydro-imidazol-1-yl )-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-(3-{[(oxetan-3-ylmethyl)-carbamoyl]-
methyl}-2-oxo-2,3-dihydro-imidazol-1-yl)-benzamide
5-[3-(Azetidin-3-ylcarbamoylmethyl)-4-methyl-2-oxo-2,3-dihydro-imidazol-1-yl]-
2-
chloro-N-(1-hydroxy-cycloheptylmethyl)-benzamide
5-[3-(Azetidin-3-ylcarbamoylmethyl)-2-oxo-2,3-d ihydro-im idazol-1-yl]-2-
chloro-N-(1-
hydroxy-cycloheptylmethyl)-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[4-methyl-2-oxo-3-(pyrrolidin-3-
ylcarbamoylmethyl)-2,3-dihydro-imidazol-1-yl]-benzamide
2-Chloro-N-(1-llydroxy-cycloheptylmethyl)-5-[2-oxo-3-(pyrrolidin-3-
ylcarbamoylmethyl)-2,3-dihydro-imidazol-1-yl]-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[4-methyl-2-oxo-3-(piperidin-4-
ylcarbamoylmethyl)-2,3-dihydro-imidazol-1-yl]-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[2-oxo-3-(piperidin-4-
ylcarbamoylmethyl)-2,3-dihydro-imidazol-1-yl]-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-{4-methyl-2-oxo-3-[(tetrahydro-
pyran-4-
ylcarbamoyl)-methyl]-2,3-dihydro-imidazol-1-yl}-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-{2-oxo-3-[(tetrahydro-pyran-4-
ylcarbamoyl)-methyl]-2,3-dihydro-imidazol-1-yl}-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-{4-methyl-2-oxo-3-[(tetrahydro-
furan-3-
ylcarbamoyl)-methyl]-2,3-dihydro-imidazol-1-yl}-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-{2-oxo-3-[(tetrahydro-furan-3-
ylcarbamoyl)-methyl]-2,3-dihydro-imidazol-1-yl}-benzamide
2-Chloro-5-[3-(3-dimethylamino-2-hydroxy-propyl)-4-methyl-2-oxo-2,3-dihydro-
imidazol-1-yl]-N-(1-hydroxy-cycloheptylmethyl)-benzamide
2-Chloro-5-[3-(3-dimethylamino-2-hydroxy-propyl)-2-oxo-2,3-dihydro-imidazol-1-
yl]-N-
(1-hydroxy-cycloheptylmethyl)-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[3-(2-hydroxy-3-pyrrolidin-1-yl-
propyl)-4-
methyl-2-oxo-2,3-dihydro-imidazol-1-yl]-benzamide
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
' 45
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[3-(2-hydroxy-3-pyrrolid in-1-yl-
propyl)-2-
oxo-2,3-dihydro-imidazol-1-yl]-benzamide
5-[3-(3-Amino-2-hydroxy-propyl)-4-methyl-2-oxo-2,3-dihydro-imidazol-1-yl]-2-
chloro-
N-(1-hydroxy-cycloheptylmethyl)-benzamide
5-[3-(3-Amino-2-hydroxy-propyl)-2-oxo-2,3-dihydro-imidazol-1-yl]-2-chloro-N-(1-
hydroxy-cycloheptylmethyl)-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-{3-[2-hydroxy-3-(oxetan-3-yloxy)-
propyl]-
4-methyl-2-oxo-2,3-dihydro-imidazol-1-yl}-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-{3-[2-hydroxy-3-(oxetan-3-yloxy)-
propyl]-
2-oxo-2,3-dihydro-imidazol-1-yl}-benzamide
5-{3-[3-(Azetidin-3-yloxy)-2-hydroxy-propyl]-4-methyl-2-oxo-2,3-dihydro-
imidazol-1-
yl}-2-chloro-N-(1-hydroxy-cycloheptylmethyl)-benzamide
5-{3-[3-(Azetidin-3-yloxy)-2-hydroxy-propyl]-2-oxo-2,3-dihydro-im idazol-1-yl}-
2-chloro-
N-(1-hydroxy-cycloheptylmethyl)-benzamide
5-{3-[3-(Azetidin-3-yloxy)-propyl]-4-methyl-2-oxo-2,3-dihydro-imidazol-1-yl}-2-
chloro-
N-(1-hydroxy-cycloheptylmethyl)-benzamide
5-{3-[3-(Azetidin-3-yloxy)-propyl]-2-oxo-2,3-dihydro-imidazol-1-yl}-2-chloro-N-
(1-
hydroxy-cycloheptylmethyl)-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-{3-[2-hydroxy-3-(pyrrolidin-3-
yloxy)-
propyl]-4-methyl-2-oxo-2,3-dihydro-imidazol-1-yl}-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-{3-[2-hydroxy-3-(pyrrolidin-3-
yloxy)-
propyl]-2-oxo-2,3-dihydro-im idazol-1-yl}-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-{4-methyl-2-oxo-3-[3-(pyrrolidin-3-
yloxy)-
propyl]-2,3-dihydro-imidazol-1-yl}-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-{2-oxo-3-[3-(pyrrolidin-3-yloxy)-
propyl]-
2,3-dihydro-imidazol-1-yl}-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-{3-[2-hydroxy-3-(piperidin-4-yloxy)-
propyl]-4-methyl-2-oxo-2,3-dihydro-imidazol-1-yl}-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-{3-[2-hydroxy-3-(piperidin-4-yloxy)-
propyl]-2-oxo-2,3-dihydro-imidazol-1-yl}-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-{4-methyl-2-oxo-3-[3-(piperidin-4-
yloxy)-
propyl]-2,3-dihydro-imidazol-1-yl}-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-{2-oxo-3-[3-(piperidin-4-yloxy)-
propyl]-
2,3-dihydro-imidazol-1-yl}-benzamide
2-Chloro-5-[3-(3,4-dihydroxy-butyl)-4-methyl-2-oxo-2,3-dihydro-imidazol-1-yl]-
N-(1-
hydroxy-cycloheptylmethyl)-benzamide
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-46-
2-Chloro-5-[3-(3,4-dihydroxy-butyl)-2-oxo-2,3-dihydro-imidazol-1-yl]-N-(1-
hydroxy-
cycloheptylmethyl)-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[4-methyl-2-oxo-3-(2-
trifluoromethoxy-
ethyl)-2,3-dihydro-imidazol-1-yl]-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[2-oxo-3-(2-trifluoromethoxy-ethyl)-
2,3-
dihydro-imidazol-1-yIJ-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-(4-methyl-2-oxo-2,3-dihydro-
imidazol-1-
yl)-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-(2-oxo-2,3-dihydro-im idazol-1-yl)-
benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[3-(2-hydroxy-ethyl)-4-methyl-2-oxo-
2,3-
dihydro-imidazol-1-yl]-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[3-(2-hyd roxy-ethyl)-2-oxo-2,3-
dihydro-
imidazol-1-yl]-benzamide
2-Chloro-5-(3-cyanomethyl-4-methyl-2-oxo-2,3-dihydro-imidazol-1-yl)-N-(1-
hydroxy-
cycloheptylmethyl)-benzamide
2-Chloro-5-(3-cyanomethyl-2-oxo-2,3-d ihydro-imidazol-1-yl)-N-(1-hydroxy-
cycloheptylmethyl)-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[3-(2-methoxy-ethyl)-4-methyl-2-oxo-
2,3-
dihydro-imidazol-1-yl]-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[3-(2-methoxy-ethyl)-2-oxo-2,3-
dihydro-
imidazol-1-yl]-benzamide
5-(3-Carbamoylmethyl-4-methyl-2-oxo-2,3-dihydro-im idazol-1-yl)-2-chloro-N-(1-
hydroxy-cycloheptylmethyl)-benzamide
5-(3-Carbamoylmethyl-2-oxo-2,3-dihydro-imidazol-1-yl)-2-chloro-N-(1-hydroxy-
cycloheptylmethyl)-benzamide
5-[3-(2-Am ino-ethyl)-4-methyl-2-oxo-2,3-dihydro-imidazol-1-yl]-2-chloro-N-(1-
hydroxy-
cycloheptylmethyl)-benzamide
5-[3-(2-Amino-ethyl)-2-oxo-2,3-dihydro-imidazol-1-yl]-2-chloro-N-(1-hydroxy-
cycloheptylmethyl)-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[3-(2-hydroxy-3-methoxy-propyl)-4-
methyl-2-oxo-2,3-dihydro-imidazol-1-yl]-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[3-(2-hydroxy-3-methoxy-propyl)-2-
oxo-
2,3-dihydro-imidazol-1-yl]-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[3-(2-hydroxy-2-methyl-propyl)-4-
methyl-
2-oxo-2,3-dihydro-imidazol-1-yl]-benzamide
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-47-
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[3-(2-hydroxy-2-methyl-propyl)-2-
oxo-
2,3-dihydro-imidazol-1-yl]-benzamide.
Certain specific compounds of the invention are those compounds identified in
Examples 1-14, which are incorporated herein by reference.
The present invention also includes isotopically-labeled compounds, which are
identical to those recited in Formula I, but for the fact that one or more
atoms are replaced by
an atom having an atomic mass or mass number different from the atomic mass or
mass
number usually found in nature. Examples of isotopes that can be incorporated
into
compounds of the invention include isotopes of hydrogen, carbon, nitrogen,
oxygen,
phosphorous, fluorine and chlorine, such as ~H, 3H,'3C, ~4C, ESN, 18~~ ~y~
saP, szP~ 355, ~sF,
and 36CI, respectively. Compounds of the present invention, prodrugs thereof,
and
pharmaceutically acceptable salts of said compounds or of said prodrugs which
contain the
aforementioned isotopes and/or other isotopes of other atoms are within the
scope of this
invention. Certain isotopically-labelled compounds of the present invention,
for example
those into which radioactive isotopes such as 3H and '4C are incorporated, are
useful in drug
and/or substrate tissue distribution assays. Tritiated, i.e., 3H, and carbon-
14, i.e., '4C,
isotopes are particularly preferred for their ease of preparation and
detectability. Further,
substitution with heavier isotopes such as deuterium, i.e., ZH, can afford
certain therapeutic
advantages resulting from greater metabolic stability, for example 'increased
in vivo half-life or
reduced dosage requirements and, hence, may be preferred in some
circumstances.
Isotopically-labelled compounds of Formula I of this invention and prodrugs
thereof can
generally be prepared by carrying out the procedures disclosed in the Schemes
and/or in the
Examples and Preparations below, by substituting a readily available
isotopically-labelled
reagent for a non-isotopically-labelled reagent.
The compounds of Formula I or a pharmaceutically acceptable salt thereof can
be
used in the manufacture of a medicament for the prophylactic or therapeutic
treatment of any
disease state in a human, or other mammal, which is exacerbated or caused by
excessive or
unregulated cytokine production by such mammal's cells, such as but not
limited to
monocytes and/or macrophages.
The present invention relates to a method for treating an IL-1 mediated
disease in a
mammal in need thereof, which comprises administering to said mammal an
effective amount
of a compound of formula I.
The present invention also relates to a method for treating an IL-1 mediated
condition.
As defined herein, an "IL-1 mediated condition" includes but is not limited to
a disease or
disorder selected from the group consisting of arthritis (including psoriatic
arthritis, Reiter's
syndrome, rheumatoid arthritis, gout, traumatic arthritis, rubella arthritis,
rheumatoid
spondylitis, osteoarthritis, gouty arthritis and acute synovitis),
inflammatory bowel disease,
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-48-
Crohn's disease, emphysema, acute respiratory distress syndrome, adult
respiratory distress
syndrome, asthma, bronchitis chronic obstructive pulmonary disease, chronic
pulmonary
inflammatory disease, silicosis, pulmonary sarcoidosis, allergic reactions,
allergic contact
hypersensitivity, eczema, contact dermatitis, psoriasis, sunburn, cancer,
tissue ulceration,
restenosis, periodontal disease, epidermolysis bullosa, osteoporosis, bone
resorption disease,
loosening of artificial joint implants, atherosclerosis, aortic aneurysm,
congestive heart failure,
myocardial infarction, stroke, cerebral ischemia, head trauma, neurotrauma,
spinal cord injury,
neuro-degenerative disorders, Alzheimer's disease, Parkinson's disease,
migraine, depression,
peripheral neuropathy, pain, cerebral amyloid angiopathy, nootropic or
cognition enhancement,
amyotrophic lateral sclerosis, multiple sclerosis, ocular angiogenesis,
corneal injury, macular
degeneration, corneal scarring, scleritis, abnormal wound healing, burns,
autoimmune
disorders, Huntington's disease, diabetes, AIDS, cachexia, sepsis, septic
shock, endotoxic
shock, conjunctivitis shock, gram negative sepsis, toxic shock syndrome,
cerebral malaria,
cardiac and renal repen'usion injury, thrombosis, glomerularonephritis, graft
vs. host reaction,
allograft rejection, organ transplant toxicity, ulcerative colitis, or muscle
degeneration, in a
mammal, including a human, comprising administering to said mammal an amount
of a
compound to formula I, effective in treating such a condition.
The present invention relates to a pharmaceutical composition for the
treatment of an
IL-1 mediated disease in a mammal which comprises an effective amount of a
compound
according of formula I and a pharmaceutically acceptable carrier.
The present invention relates to a pharmaceutical composition for the
treatment of an
IL-1 mediated condition in a mammal, including a human, comprising an amount
of a compound
of formula I, effective in treating such a condition and a pharmaceutically
acceptable carrier.
Preferably, the compounds of the invention are useful for the treatment of
rheumatoid
arthritis, osteoarthritis, psoriasis, allergic dermatitis, asthma, chronic
obstructive pulmonary
disease (COPD), hyperresponsiveness of the airway, septic shock,
glomerulonephritis,
irritable bowel disease, Crohn's disease, ulcerative colitis, atherosclerosis,
growth and
metastases of malignant cells, myoblastic leukemia, diabetes, Alzheimer's
disease,
meningitis, osteoporosis, burn injury, ischemic heart disease, stroke and
varicose veins.
~ The present invention also provides a compound of formula (I), or a
pharmaceutically
acceptable salt or solvate thereof, as hereinbefore defined for use in
therapy.
In another aspect, the invention provides the use of a compound of formula
(I), or a
pharmaceutically acceptable salt or solvate thereof, as hereinbefore defined
in the
manufacture of a medicament for use in therapy.
The invention further provides a method of treating osteoarthritis which
comprises
administering a therapeutically effective amount of a compound of formula (I),
or a
pharmaceutically acceptable salt or solvate thereof, as hereinbefore defined
to a patient.
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-49-
The invention further provides a method of effecting immunosuppression (e.g.
in the
treatment of rheumatoid arthritis, irritable bowel disease, atherosclerosis or
psoriasis) which
comprises administering a therapeutically effective amount of a compound of
formula (I), or a
pharmaceutically acceptable salt or solvate thereof, as hereinbefore defined
to a patient.
The invention also provides a method of treating an obstructive airways
disease (e.g.
asthma or COPD) which comprises administering to a patient a therapeutically
effective
amount of a compound of formula (I), or a pharmaceutically acceptable salt or
solvate thereof,
as hereinbefore defined to a patient.
The term "treating", as used herein, refers to reversing, alleviating,
inhibiting the
progress of, or preventing the disorder or condition to which such term
applies, or one or more
symptoms of such disorder or condition. The term "treatment", as used herein,
refers to the act
of treating, as "treating" is defined immediately above.
The present invention also provides a pharmaceutical composition comprising a
compound of formula (I), or a pharmaceutically acceptable salt or solvate
thereof, as
hereinbefore defined in association with a pharmaceutically acceptable
adjuvant, diluent or
carrier.
The invention further provides a process for the preparation of a
pharmaceutical
composition of the invention which comprises mixing a compound of formula (I),
or a
pharmaceutically acceptable salt or solvate thereof, as hereinbefore defined
with a
pharmaceutically acceptable adjuvant, diluent or carrier.
For the above-mentioned therapeutic uses the dosage administered will, of
course,
vary with the compound employed, the mode of administration, the treatment
desired and the
disorder indicated. The daily dosage of the compound of formula
(I)/salt/solvate (active
ingredient) may be in the range from 1 mg to 1 gram, preferably 1 mg to 250
mg, more
preferably 10 mg to 100 mg.
The present invention also encompasses sustained release compositions.
The present invention also relates to processes of preparing the compounds of
formula I and intermediates used in such processes.
One embodiment of the processes of the invention relates to the preparation of
compounds of formula I,which may be carried out by one or more of the
synthetic methods
outliried in Schemes I-VIII, detailed below. The present invention also
provides methods and
intermediates useful in the synthesis of compounds of formula (I), and
identified in Schemes I
VIII below.
One of ordinary skill in the art will appreciate that the compounds of the
invention are
useful in treating a diverse array of diseases. One of ordinary skill in the
art will also
appreciate that when using the compounds of the invention in the treatment of
a specific
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-50_
disease that the compounds of the invention may be combined with various
existing
therapeutic agents used for that disease.
For the treatment of rheumatoid arthritis, the compounds of the invention may
be
combined with agents such as TNF-a inhibitors such as anti-TNF monoclonal
antibodies
(such as Remicade, CDP-870 and DZE~) and TNF receptor immunoglobulin molecules
(such
as Enbrel~), COX-2 inhibitors (such as meloxicam, celecoxib , rofecoxib,
valdecoxib,
paracoxib, and etoricoxib) low dose methotrexate, lefunomide; ciclesonide;
hydroxychloroquine, d-penicillamine, auranofin or parenteral or oral gold.
The present invention still further relates to the combination of a compound
of the
invention together with a leukotriene biosynthesis inhibitor, 5-lipoxygenase
(5-LO) inhibitor or
5-lipoxygenase activating protein (FLAP) antagonist selected from the group
consisting of
zileuton; ABT-761; fenleuton; tepoxalin; Abbott-79175; Abbott-85761; N-(5-
substituted)
thiophene-2-alkylsulfonamides; 2,6-di-terf-butylphenol hydrazones;
methoxytetrahydropyrans
such as Zeneca ZD-2138; the compound SB-210661; pyridinyl-substituted
2-cyanonaphthalene compounds such as L-739,010; 2-cyanoquinoline compounds
such as
L-746,530; indole and quinoline compounds such as MK-591, MK-886, and BAY x
1005.
The present invention still further relates to the combination of a compound
of the
invention together with a receptor antagonists for leukotrienes LTB4, LTC4,
LTD4, and LTE4
selected from the group consisting of the phenothiazin-3-ones such as L-
651,392; amidino
compounds such as CGS-25019c; benzoxalamines such . as ontazolast;
benzenecarboximidamides such as BIIL 284/260; and compounds such as
zafirlukast,
ablukast, montelukast, pranlukast, verlukast (MK-679), RG-12525, Ro-245913,
iralukast
(CGP 45715A), and BAY x 7195.
The present invention still further relates to the combination of a compound
of the
invention together with a PDE4 inhibitor including inhibitors of the isoform
PDE4D.
The present invention still further relates to the combination of a compound
of the
invention together with a antihistaminic H~ receptor antagonists including
cetirizine,
loratadine, desloratadine, fexofenadine, astemizole, azelastine, and
chlorpheniramine.
The present invention still further relates to the combination of a compound
of the
invention together with a gastroprotective HZ receptor antagonist.
The present invention still further relates to the combination of a compound
of the
invention together with an a~- and a2-adrenoceptor agonist vasoconstrictor
sympathomimetic
agent, including propylhexedrine, phenylephrine, phenylpropanolamine,
pseudoephedrine,
naphazoline hydrochloride, oxymetazoline hydrochloride, tetrahydrozoline
hydrochloride,
xylometazoline hydrochloride, and ethylnorepinephrine hydrochloride.
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-51-
The present invention still further relates to the. combination of a compound
of the
invention together with anticholinergic agents including ipratropium bromide;
tiotropium
bromide; oxitropium bromide; pirenzepine; and telenzepine.
The present invention still further relates to the combination of a compound
of the
invention together with a ~i~- to (34-adrenoceptor agonists including
metaproterenol,
isoproterenol, isoprenaline, albuterol, salbutamol, formoterol, salmeterol,
terbutaline,
orciprenaline, bitolterol mesylate, and pirbuterol; or methylxanthanines
including theophylline
and aminophylline; sodium cromoglycate; or muscarinic receptor (M1, M2, and
M3)
antagonist.
The present invention, still further relates to the combination of a compound
of the
invention together with an insulin-like growth factor type I (IGF-1 ) mimetic.
The present invention still further relates to the combination of a compound
of the
invention together with an inhaled glucocorticoid with reduced systemic side
effects, including
prednisone, prednisolone, flunisolide, triamcinolone acetonide, beclomethasone
dipropionate,
budesonide, fluticasone propionate, and mometasone furoate.
The present invention still further relates to the combination of a compound
of the
invention together with (a) tryptase inhibitors; (b) platelet activating
factor (PAF) antagonists;
(c) interleukin converting enzyme (ICE) inhibitors; (d) IMPDH inhibitors; (e)
adhesion molecule
inhibitors including VLA-4 antagonists; (f) cathepsins; (g) MAP kinase
inhibitors; (h) glucose-6
phosphate dehydrogenase inhibitors; (i) kinin-B~ - and BZ -receptor
antagonists; Q) anti-gout
agents, e.g., colchicine; (k) xanthine oxidase inhibitors, e.g., allopurinol;
(I) uricosuric agents,
e.g., probenecid, sulfinpyrazone, and benzbromarone; (m) growth hormone
secretagogues;
(n) transforming growth factor (TGFp); (o) platelet-derived growth factor
(PDGF); (p) fibroblast
growth factor, e.g., basic fibroblast growth factor (bFGF); (q) granulocyte
macrophage colony
stimulating factor (GM-CSF); (r) capsaicin cream; (s) Tachykinin NK~ and NK3
receptor
antagonists selected from the group consisting of NKP-608C; SB-233412
(talnetant); and
D-4418; and (t) elastase inhibitors selected from the group consisting of UT-
77 and ZD-0892.
The present invention still further relates to the combination of a compound
of the
invention together with an inhibitor of matrix metalloproteases (MMPs), i.e.,
the stromelysins,
the collagenases, and the gelatinases, as well as aggrecanase; especially
collagenase-1
(MMP-1), collagenase-2 (MMP-8), collagenase-3 (MMP-13), stromelysin-1 (MMP-3),
stromelysin-2 (MMP-10), and stromelysin-3 (MMP-1,1 ).
The compounds of the invention can also be used in combination with existing
therapeutic agents for the treatment of osteoarthritis. Suitable agents to be
used in
combination include standard non-steroidal anti-inflammatory agents
(hereinafter NSAID's)
such as piroxicam, diclofenac, propionic acids such as naproxen, flubiprofen,
fenoprofen,
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-52-
ketoprofen and ibuprofen, fenamates such as mefenamic acid, indomethacin,
sulindac,
apazone, pyrazolones such as phenylbutazone, salicylates such as aspirin, COX-
2 inhibitors
such ~ as celecoxib, valdecoxib, rofecoxib and etoricoxib, analgesics and
intraarticular
therapies such as corticosteroids and hyaluronic acids such as hyalgan and
synvisc.
The compounds of the present invention may also be used in combination with
anticancer agents such as endostatin and angiostatin or cytotoxic drugs such
as adriamycin,
daunomycin, cis-platinum, etoposide, taxol, taxotere and farnesyl transferase
inhibitors, VegF
inhibitors, COX-2 inhibitors and antimetabolites such as methotrexate
antineoplastic agents,
especially antimitotic drugs including the vinca alkaloids such as vinblastine
and vincristine;.
The compounds of the invention may also be used in combination with antiviral
agents such as Viracept, AZT, aciclovir and famciclovir, and antisepsis
compounds such as
Valant.
The compounds of the present invention may also be used in combination with
cardiovascular agents such as calcium channel blockers, lipid lowering agents
such as
statins, fibrates, beta-blockers, Ace inhibitors, Angiotensin-2 receptor
antagonists and platelet
aggregation inhibitors.
The compounds of the present invention may also be used in combination with
CNS
agents such as antidepressants (such as sertraline), anti-Parkinsonian drugs
(such as
deprenyl, L-dopa, Requip, Mirapex, MAOB inhibitors such as selegine and
rasagiline, come
inhibitors such as Tasmar, A-2 inhibitors, dopamine reuptake inhibitors, NMDA
antagonists,
Nicotine agonists, Dopamine agonists and inhibitors of neuronal nitric oxide
synthase), and
anti-Alzheimer's drugs such as donepezil, tacrine, COX-2 inhibitors,
propentofylline or
metryfonate.
The compounds of the present invention may also be used in combination with
osteoporosis agents such as roloxifene, droloxifene, lasofoxifene or fosomax
and
immunosuppressant agents such as FI<-506, rapamycin, cyclosporine,
azathioprine, and
methotrexate.
DETAILED DESCRIPTION OF THE INVENTION
Compounds of the formula I may be prepared according to the following reaction
schemes and discussion. Unless otherwise indicated R' through R' in the
reaction schemes
and discussion that follows are as defined above.
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-53-
Scheme 1
R'
RZ O L-R7 ~ N
\ N~R~ VII _ 0 N
H
/ I \
H
R3 / N~R~
R2 O
I O VI
VIII
Scheme 2
O NON O N~N
\ \
OH I H
/ N~Ri
Rz O Rz O
IX
O NON
/ C1
R2 O
X
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
o -54-
Scheme 3
O
O Ra
HO
a OH
HN'NH2 R N'N
O
XIII
R2 O RZ O
XI I XIV
Ra
O N
/ OH
RZ O
IX
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-55-
Scheme 4
NHZ
NHZ
\ \
O
/ OH
R2 ~o R2 O
XV XVI
HN~NHZ
R~ O
XII
Scheme 5
P, R \
L-R~
VII o N
H
1 ~ NwR~
R'~ R2 O
I
VIII o XVII
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-56-
Scheme 6
Ra Ra
N~ 5 HZN_R,
O~N~R
/ O
R2 O
XVIII XIX
Scheme 7
Ra
O'
O\
a N
O R ~ Rs
O O N
Ph~O~NH HzN O
R5 ~ \
\ XXI ~ ~ 0\
O\ Rz
RZ 0
XX XXII
Ra
O N R5
/ O\
RZ O
XVIII
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-57- t , , , . v . ~ _ _ _
Scheme 8
O
NHz Ph~O~NH
\ ~ \
/ O\ / O\
R~ O R~ O
XVI XX
Scheme 1 refers to the preparation of compounds of the formula VI. Compounds
of
the formula VI can be prepared from compounds of formula I by reaction with a
compound of
the formula VII: L-R', in the presence of base, wherein L is a suitable
leaving group, such as
chloro, bromo, iodo tosylate or mesylate. Suitable bases include, but are not
limited to,
triethylamine, polymer supported BEMP, cesium carbonate, potassium carbonate,
and
sodium hydride, where cesium carbonate is preferred. The aforesaid reaction
can be
performed at temperatures ranging from 0 °C to 100 °C in the
presence of a polar solvent
including but not limited to dimethylsulfoxide, dimethylformamide, equal
amounts of
dimethylsulfoxide and acetone, or equal amounts of dimethylformamide and
acetone,
generally for a period of 2 hours to 72 hours, where the preferred conditions
are
dimethylsulfoxide at ambient temperature for 18 hours.
Compounds of the formula VI may also be prepared from compounds of the formula
I
by reaction of an appropriately substituted epoxide of the formula VIII either
neat or in the
presence of a polar solvent including but not limited to dimethylformamide,
dimethylsulfoxide,
and tetrahydrofuran. The aforesaid reaction can be performed at temperatures
ranging from
0 °C to 100 °C for a period of 2 to 72 hours, where the
preferred conditions are
dimethylforamide at 60 °C for 24 hours.
Scheme 2 refers to the preparation of compounds of the formula I. Compounds of
the formula I can be prepared from compounds of formula IX by reacting with a
compound of
formula XI, HZN-R~, in the presence of a coupling reagent such as 1-[3-
(dimethylamino)propyl]-3-ethylcarbodiimide (EDCI), dicyclohexylcarbodiimide
(DCC), 1,1'-
carbonyldiimidazole (CDI) and a base such as dimethylaminopyridine (DMAP) or
triethylamine in an aprotic solvent, such as methylene chloride,
dimethylformamide, or
dimethylsulfoxide, preferably 1-[3-(dimethylamino)propyl]-3-ethylcarbodiimide
and
dimethylaminopyridine in dimethyl formamide. The aforesaid reaction may be run
at a
temperature from 22 °C to 60 °C, for a period of 1 hour to 20
hours, preferably 22 °C for 18
hours.
Compounds of the formula I may also be prepared from compounds of the formula
X
by reaction with a compound of formula XI in the presence of a base including
but not limited
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-58-
to dimethylaminopyridine (DMAP), triethylamine, aqueous sodium hydroxide or
aqueous
potassium hydroxide in an aprotic solvent, such as methylene chloride, ethyl
acetate,
dichloroethane, dimethylformamide, or dimethylsulfoxide, preferably aqueous
sodium
hydroxide and dichloroethane. The aforesaid reaction may be run at a
temperature from 22
°C to 60 °C, for a period of 1 hour to 24 hours, preferably at
ambient temperature for 3 hours.
Compound X can be prepared from compound IX by reaction with a reagent capable
of
generating an acid chloride such as thionyl chloride or oxalyl chloride in the
presence of a
polar aprotic solvent such as ethyl acetate, methylene chloride, or
dichloroethane at a
temperature of 22 °C to 60 °C, for a period of 1 hour to 24
hours, preferably oxalyl chloride in
methylene chloride at ambient temperature for 16 hours.
Scheme 3 refers to the preparation of compounds of the formula XIV and IX.
Compounds of the formula IX can be converted into compounds of the formula I
by the
methods described in Scheme 2. A compound of the formula IX can be prepared
from a
compound of the formula XIV by reaction with diphenylphosphoryl azide and a
base such as
triethylamine, pyridine, or diisopropyl ethylamine, preferably triethylamine,
at a temperature
between 80 and 120 °C for a period of 2 to 16 hours, preferably 120
°C for 2 hours.
Compounds of the formula XIV can be prepared from compounds of the formula XII
by reaction with a compound of the formula XIII, HOZC(CO)R4, in the presence
of 10%
hydrochloric acid at a temperature between 20 and 80 °C for a period of
1 to 24 hours,
preferably 1.5 hours at ambient temperature.
Scheme 4 refers to the preparation of compounds of the formula XVI and XII.
Compounds of the formula XII can be converted into compounds of the formula
XIV by the
methods described in Scheme 3. A compound of the formula XII can be prepared
from a
compound of the formula XVI by reaction with an acid such as hydrochloric acid
and/or glacial
acetic acid, followed by treatment with sodium nitrite in a solvent such as
water at a
temperature from 0 °C to 25 °C, and the reaction is generally
run from a period of 30 minutes
to about 2 hours, preferably 0 °C for 30 minutes. This intermediate is
then treated with
tin(II)chloride dihydrate in a solvent such as acid, preferably hydrochloric
acid at a
temperature from 0 to 25 °C for a period of 2 to 8 hours, preferably 0
°C for 30 minutes.
A compound of the formula XVI can be prepared from a compound of the formula
XV
by reaction with methanol in the presence of an acid such as sulfuric acid at
a temperature
between ambient temperature and reflux for a period of 4 to 24 hours,
preferably at reflux for
4 hours.
Scheme 5 refers to the preparation of compounds of the formula XVII. Compounds
of
the formula XVII can be prepared from compounds of formula I by reaction with
a compound
of the formula VII, in the presence of base, wherein L is a suitable leaving
group, such as
chloro, bromo, iodo tosylate or mesylate. Suitable bases include, but are not
limited to,
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-59-
triethylamine, polymer supported BEMP, cesium carbonate, potassium carbonate,
and
sodium hydride, where cesium carbonate is preferred. The aforesaid reaction
can be
performed at temperatures ranging from 0 °C to 100 °C in the
presence of a polar solvent
including but not limited to dimethylsulfoxide, dimethylformamide, equal
amounts of
dimethylsulfoxide and acetone, or equal amounts of dimethylformamide and
acetone,
generally for a period of 2 hours to 72 hours, where the preferred conditions
are
dimethylsulfoxide at ambient temperature for 18 hours.
Compounds of the formula XVII may also be prepared from compounds of the
formula I by reaction of an appropriately substituted epoxide of the formula
VIII either neat or
in the presence of a polar solvent including but not limited to
dimethylformamide,
dimethylsulfoxide, and tetrahydrofuran. The aforesaid reaction can be
performed at
temperatures ranging from 0 °C to 100 °C for a period of 2 to 72
hours, where the preferred
conditions are dimethylforamide at 60 °C for 24 hours.
Scheme 6 refers to the preparation of compounds of the formula XIX. Compounds
of
the formula XIX can be prepared from compounds of formula XVIII by reacting
with a base
such as NaOH, followed by reaction with a compound of formula XI, in the
presence of a
coupling reagent such as 1-[3-(dimethylamino)propyl]-3-ethylcarbodiimide
(EDCI),
dicyclohexylcarbodiimide (DCC), 1,1'-carbonyldiimidazole (CDI) and a base such
as
dimethylaminopyridine (DMAP) or triethylamine in an aprotic solvent, such as
methylene
chloride, dimethylformamide, or dimethylsulfoxide, preferably 1-[3-
(dimethylamino)propyl]-3-
ethylcarbodiimide and dimethylaminopyridine in dimethyl formamide. The
aforesaid reaction
may be run at a temperature from 22 °C to 60 °C, for a period of
1 hour to 20 hours,
preferably 22 °C for 18 hours.
Scheme 7 refers to the preparation of compounds of the formula XXII and XVIII.
A
compound of the formula XIX can be prepared from a compound of the formula
XXII by
reaction with an acid such as dilute sulfuric acid or concentrated
hydrochloric acid at a
temperature from ambient to reflux for 2 to 16 hours. Compounds of the formula
XXII can be
prepared from compounds of the formula XX by reaction with a compound of the
formula XXI,
HZNCH(R4)CR5(OCH3)z, in the presence of an organic base such as triethylamine,
diisopropylethylamine, or pyridine in a solvent such a dimethyl sulfoxide at a
temperature from
ambient temperature to 60 °C for 2 to 24 hours.
Scheme 8 refers to the preparation of compounds of the formula XX. Compounds
of
the formula XX can be converted into compounds of the formula XXII and XVIII
by the
methods described in Scheme 7. A compound of the formula XX can be prepared
from a
compound of the formula XVI by reaction with phenyl chloroformate with an
organic base
such as pyridine in a solvent such as tetrahydrofuran at a temperature from 0
°C to ambient
temperature for 2 to 24 hours, preferably ambient for 12 hours.
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-60-
The activity of the compounds of the invention for the various disorders
described
above can be determined according to one or more of the following assays. All
of the
compounds of the invention that were tested had an ICSO of less than 10 pM in
the in vitro
assay described below.
Preferably, the compounds of the invention have an ICSO in the in vitro assays
described below of less than 100 nM, more preferably less than 50 nM, and most
preferably
less than 10 nM. Still further, the compounds of the invention preferably have
an ICSO in the
range of 0.01 nM -100 nM, more preferably between 0.05 nM - 50 nM, and most
preferably
between 0.10 nM -10 nM.
PHARMACOLOGICAL ANALYSIS
Certain compounds such as benzoylbenzoyl adenosine triphosphate (bbATP) are
known to be agonists of the P2X~ receptor, effecting the formation of pores in
the plasma
membrane (Drug Development Research (1996), 37 3 , p. 126). Consequently, when
the
receptor is activated using bbATP in the presence of ethidium bromide (a
fluorescent DNA
probe), an increase in the fluorescence of intracellular DNA-bound ethidium
bromide is
observed. Alternatively, the propidium dye YOPRO-1 can be substituted for
ethidium bromide
so as to detect uptake of the dye. The increase in fluorescence can be used as
a measure of
P2X~ receptor activation and therefore to quantify the effect of a compound on
the P2X~
receptor.
In this manner, the compounds of the invention can be tested for antagonist
activity at
the P2X~ receptor. 96-Well flat bottomed microtitre plates are filled with 250
pl of test solution
comprising 200 pl of a suspension of THP-1 cells (2.5 x 106 cellsiml, more
preferably
prestimulated as described in the literature with a combination of LPS and TNF
to promote
receptor expression) containing 10~M ethidium bromide, 25 pl of a high
potassium, low
sodium buffer solution (10mM Hepes, 150 mM I<CI, 5 mM D-glucose and 1.0% FBS
at pH
7.5) containing 10-5M bbATP, and 25 pl of the high potassium buffer solution
containing 3 x
10~M test compound (more preferably 5 x 10'~M, more preferably 1 x 10-4M.more
preferably 1
x 10-3M). The plate is covered with a plastic sheet and incubated at
37°C for one hour. The
plate~is then read in a Perkin-Elmer fluorescent plate reader, excitation 520
nm, emission 595
nm, slit widths: Ex 15 nm, Em 20 nm. For the purposes of comparison, bbATP (a
P2X~
receptor agonist) and pyridoxal 5-phosphate (a P2X~ receptor antagonist) can
be used
separately in the test as controls. From the readings obtained, a pICSO figure
can be
calculated for each test compound, this figure being the negative logarithm of
the
concentration of test compound necessary to reduce the bbATP agonist activity
by 50%.
° In like manner, the compounds of the invention can be tested for
antagonist activity at
the P2X~ receptor using the cytokine IL-1 ~3 as the readout. Blood collected
from normal
volunteers in the presence of heparin is fractionated using lymphocyte
separation medium
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-61-
obtained from Organon Technica (Westchester, PA). The region of the resulting
gradient
containing banded mononuclear cells is harvested, diluted with 10 ml of
Maintenance Medium
(RPMI 1640, 5% FBS, 25 mM Hepes, pH 7.2, 1% penicillin/streptomycin), and
cells are
collected by centrifugation. The resulting cell pellet was suspended in 10 ml
of Maintenance
Medium and a cell count was performed. In an average experiment, 2 x 105
mononuclear
cells are seeded into each well of 96-well plates in a total volume of 0.1 ml.
Monocytes are
allowed to adhere for 2 hours, after which the supernatants are discarded and
the attached
cells are rinsed twice and then incubated in Maintenance Medium overnight at
37°C in a 5%
C02 environment.
The cultured monocytes can be activated with 10 ng/ml LPS (E. coli serotype
055:B5;
Sigma Chemicals, St. Louis, MO). Following a 2-hour incubation, the activation
medium is
removed, the cells are rinsed twice with 0.1 ml of Chase Medium (RPMI 1640, 1
% FBS, 20
mM Hepes, 5 mM NaHC03, pH 6.9), and then 0.1 ml of Chase Medium containing a
test
agent is added and the plate is incubated for 30 minutes; each test agent
concentration can
be evaluated in triplicate wells. ATP then is introduced (from a 100 mM stock
solution, pH 7)
to achieve a final concentration of 2 mM and the plate is incubated at
37°C for an additional 3
hours. Media were harvested and clarified by centrifugation, and their IL-1 a
content was
determined by ELISA (R&D Systems; Minneapolis, MN).
The compositions of the present invention may be formulated in a conventional
manner using one or more pharmaceutically acceptable carriers. Thus, the
active
compounds of the invention may be formulated for oral, buccal, intranasal,
parenteral (e.g.,
intravenous, intramuscular or subcutaneous), topical or rectal administration
or in a form
suitable for administration by inhalation or insufflation.
For oral administration, the pharmaceutical compositions may take the form of,
for
example, tablets or capsules prepared by conventional means with
pharmaceutically
acceptable excipients such as binding agents (e.g., pregelatinized maize
starch,
polyvinylpyrrolidone or hydroxypropyl methylcellulose); fillers (e.g.,
lactose, microcrystalline
cellulose or calcium phosphate); lubricants (e.g., magnesium stearate, talc or
silica);
disintegrants (e.g., potato starch or sodium starch glycolate); or wetting
agents (e.g., sodium
lauryl sulphate). The tablets may be coated by methods well known in the art.
Liquid
preparations for oral administration may take the form of, for example,
solutions, syrups or
suspensions, or they may be presented as a dry product for constitution with
water or other
suitable vehicle before use. Such liquid preparations may be prepared by
conventional
means with pharmaceutically acceptable additives such as suspending agents
(e.g., sorbitol
syrup, methyl cellulose or hydrogenated edible fats); emulsifying agents
(e.g., lecithin or
acacia); non-aqueous vehicles (e.g., almond oil, oily esters or ethyl
alcohol); and
preservatives (e.g., methyl or propyl p-hydroxybenzoates or sorbic acid).
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-62-
For buccal administration, the composition may take the form of tablets or
lozenges
formulated in conventional manner.
The compounds of formula I can also be formulated for sustained delivery
according
to methods well known to those of ordinary skill in the art. Examples of such
formulations can
be found in United States Patents 3,538,214, 4,060,598, 4,173,626, 3,119,742,
and
3,492,397, which are herein incorporated by reference in their entirety.
The active compounds of the invention may be formulated for parenteral
administration by injection, including using conventional catheterization
techniques or
infusion. Formulations for injection may be presented in unit dosage form,
e.g., in ampules or
in multi-dose containers, with an added preservative. The compositions may
take such forms
as suspensions, solutions or emulsions in oily or aqueous vehicles, and may
contain
formulating agents such as suspending, stabilizing and/or dispersing agents.
Alternatively,
the active ingredient may be in powder form for reconstitution with a suitable
vehicle, e.g.,
sterile pyrogen-free water, before use.
The active compounds of the invention may also be formulated in rectal
compositions
such as suppositories or retention enemas, e.g., containing conventional
suppository bases
such as cocoa butter or other glycerides.
For intranasal administration or administration by inhalation, the active
compounds of
the invention are conveniently delivered in the form of a solution, dry powder
formulation or
suspension from a pump spray container that is squeezed or pumped by the
patient or as an
aerosol spray presentation from a pressurized container or a nebulizer, with
the use of a
suitable propellant, e.g., dichlorodifluoromethane, trichlorofluoromethane,
dichlorotetrafluoroethane, heptafluoroalkanes, carbon dioxide or other
suitable gas. In the
case of a pressurized aerosol, the dosage unit may be determined by providing
a valve to
deliver a metered amount. The pressurized container or nebulizer may contain a
solution or
suspension of the active compound. Capsules and cartridges (made, for example,
from
gelatin) for use in an inhaler or insufflator may be formulated containing a
powder mix of a
compound of the invention and a suitable powder base such as lactose or
starch.
A proposed dose of the active compounds of the invention for oral, parenteral
or
buccal administration to the average adult human for the treatment of the
conditions referred
to above (inflammation) is 0.1 to 200 mg of the active ingredient per unit
dose which could be
administered, for example, 1 to 4 times per day.
The compound of formula (I) and pharmaceutically acceptable salts and solvates
thereof may be used on their own but will generally be administered in the
form of a
pharmaceutical composition in which the formula (I) compound/salt/solvate
(active ingredient)
is in association with a pharmaceutically acceptable adjuvant, diluent or
carrier. Depending
on the mode of administration, the pharmaceutical composition will preferably
comprise from
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-63-
0.05 to 99% w (percent by weight), more preferably from 0.10 to 70% w, of
active ingredient,
and, from 1 to 99.95% w, more preferably from 30 to 99.90% w, of a
pharmaceutically
acceptable adjuvant, diluent or carrier, all percentages by weight being based
on total
composition.
Aerosol formulations for treatment of the conditions referred to above in the
average
adult human are preferably arranged so that each metered dose or "puff" of
aerosol contains
20pg to 1000pg of the compound of the invention. The overall daily dose with
an aerosol will
be within the range 100pg to 10 mg. Administration may be several times daily,
for example
2, 3, 4 or 8 times, giving for example, 1, 2 or 3 doses each time.
Aerosol combination formulations for treatment of the conditions referred to
above
(e.g., adult respiratory distress syndrome) in the average adult human are
preferably
arranged so that each metered dose or "puff" of aerosol contains from about 1
pg to 1000 pg
of the compound of the invention. The overall daily dose with an aerosol will
be within the
range 100 pg to 10 mg. Administration may be several times daily, for example
2, 3, 4 or 8
times, giving for example, 1, 2 or 3 doses each time.
Aerosol formulations for treatment of the conditions referred to above (e.g.,
adult
respiratory distress syndrome) in the average adult human are preferably
arranged so that
each metered dose or "puff' of aerosol contains from about 20 pg to 1000 pg of
the
compound of the invention. The overall daily dose with an aerosol will be
within the range
100 pg to 10 mg of the P2X~ receptor inhibitor. Administration may be several
times daily, for
example 2, 3, 4 or 8 times, giving for example, 1, 2 or 3 doses each time.
This invention also encompasses pharmaceutical compositions containing and
methods of treating or preventing comprising administering prodrugs of
compounds of the
formula I. Compounds of formula I having free amino, amido, hydroxy or
carboxylic groups can
be converted into prodrugs. Prodrugs include compounds wherein an amino acid
residue, or a
polypeptide chain of two or more (e.g., two, three or four) amino acid
residues which are
covalently joined through peptide bonds to free amino, hydroxy or carboxylic
acid groups of
compounds of formula I. The amino acid residues include the 20 naturally
occurring amino
acids commonly designated by three letter symbols and also include, 4-
hydroxyproline,
hydroxylysine, demosine, isodemosine, 3-methylhistidine, norvalin, beta-
alanine, gamma-
aminobutyric acid, citrulline homocysteine, homoserine, ornithine and
methionine sulfone.
Prodrugs also include compounds wherein carbonates, carbamates, amides and
alkyl esters
which are covalently bonded to the above substituents of formula I through the
carbonyl carbon
prodrug sidechain.
The following Examples illustrate the preparation of the compounds of the
present
invention. Melting points are uncorrected. NMR data are reported in parts per
million (d) and
are referenced to the deuterium lock signal from the sample solvent
(deuteriochloroform
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-64-
unless otherwise specified). Mass Spectral data were obtained using a
Micromass ZMD
APCI Mass Spectrometer equipped with a Gilson gradient high performance liquid
chromatograph. The following solvents and gradients were used for the
analysis. Solvent A;
98% water/2% acetonirile/0.01 % formic acid and solvent B; acetonitrile
containing 0.005%
formic acid. Typically, a gradient was run over a period of about 4 minutes
starting at 95%
solvent A and ending with 100% solvent B. The mass spectrum of the major
eluting
component was then, obtained in positive or negative ion mode scanning a
molecular weight
range from 165 AMU to 1100 AMU. Specific rotations were measured at room
temperature
using the sodium D line (589 nm). Commercial reagents were utilized without
further
purification. THF refers to tetrahydrofuran. DMF refers to N,N-
dimethylformamide.
Chromatography refers to column chromatography performed using 32-63 mm silica
gel and
executed under nitrogen pressure (flash chromatography) conditions. Room or
ambient
temperature refers to 20-25°C. All non-aqueous reactions were run under
a nitrogen
atmosphere for convenience and to maximize yields. Concentration at reduced
pressure
means that a rotary evaporator was used.
One of ordinary skill in the art will appreciate that in some cases protecting
groups
may be required during preparation. After the target molecule is made, the
protecting group
can be removed by methods well known to those of ordinary skill in the art,
such as described
in Greene and Wuts, "Protective Groups in Organic Synthesis" (3rd Ed, John
Wiley & Sons
1999).
EXAMPLE 1
2-Chloro-N-(1-hyd roxy-cyclohentylmethyl)-5-f4-(2-methoxy-ethyl)-5-oxo-4.5-
dihydro
(1,2,41triazol-1-yll-benzamide
,CH3
O
~N~
O~N~N
H HO
N
CI O
(A) 5-Amino-2-chloro-benzoic acid methyl ester
To a slurry of 5-amino-2-chloro-benzoic acid (20.Og, 0.12 mol) in methanol
(150 mL)
was added concentrated sulfuric acid (25 mL). The resulting reaction was
heated at reflux for
4 hours, cooled, and concentrated in vacuo. The residual was taken up in water
and
neutralized with Na2C03. The aqueous was extracted with CHZCI2 (3x). The
organics were
dried over sodium sulfate and concentrated in vacuo to yield the above named
compound
(14.0 g).
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-65-
(B) 2-Chloro-5-hydrazino-benzoic acid methyl ester hydrochloride salt
To 5-amino-2-chloro-benzoic acid methyl ester (14.0 g, 75.0 mmol) in water
(100 mL)
was added concentrated HCI (100 mL), followed by glacial acetic acid (100 mL).
The solution
was stirred at ambient temperature for 15 minutes. The reaction was then
cooled to 0 °C, and
a solution of sodium nitrite ( 5.69, 82.5 mmol) in water (15 mL) was added
dropwise over 30
minutes, maintaining the temperature at 0 °C. The resulting reaction
was stirred for 30
minutes at 0 °C, then a solution of SnC12~2H20 (50.8 g, 225 mmol) in
concentrated HCI (100
mL) was added over 30 minutes, maintaining the temperature at 0 °C. The
slurry was stirred
at 0 °C for 30 minutes, and the resulting precipitate collected by
filtration, washed with water,
followed by hexane. The solid was dried in a vacuum oven at 50 °C to
give the title
compound (16.3 g).
(C) 5-(N'-Carboxymethylene-hydrazino)-2-chloro-benzoic acid methyl ester
2-Chloro-5-hydrazino-benzoic acid methyl ester hydrochloride salt (2.0 g, 8.5
mmol)
and oxoacetic acid (1.0 g, 10 mmol) were taken up in 10% HCI and stirred at
ambient
temperature for 1.5 hours. The resulting orange precipitate was collected by
filtration. The
filter cake was taken up in EtOAc, washed with brine, and dried over sodium
sulfate.
Concentration in vacuo yielded the above named compound (1.2 g).
(D) 2-Chloro-5-(5-oxo-4,5-dihydro-[1,2,4]triazol-1-yl)-benzoic acid methyl
ester
5-(N-Carboxymethylene-hydrazino)-2-chloro-benzoic acid methyl ester (514.0 mg,
2.0
mmol), diphenylphosphoryl azide (605.0 mg, 2.2 mmol), and triethylamine (0.3
mL, 2.2 mmol)
in toluene (15 mL) were heated at 120 °C for 1 hour under N2. The
reaction was cooled then
1.0 M NaOH (20 mL) added, and the resulting solution stirred for 10 minutes.
The layers
were separated, and the organic phase acidified to pH 1 using 1.0 N HCI. The
resulting
precipitate was collected by filtration, washed with hexane, and dried at 50
°C in vacuum oven
for 12 hours to give the title compound (0.24 g).
(E) 2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-(5-oxo-4,5-dihydro-
[1,2,4]triazol-1-yl)-benzamide
To 2-chloro-5-(5-oxo-4,5-dihydro-[1,2,4]triazol-1-yl)-benzoic acid methyl
ester (48.0
mg, 0.2 mmol) in DMF (3 mL) was added 1-aminomethyl-cycloheptanol HCI (54.0
mg, 0.3
mmol), polymer-supported CDI (660 mg, 0.6 mmol), polymer-supported DMAP (280
mg, 0.20
mmo~), and HOBt (40.0 mg, 0.3 mmol). The resulting reaction was shaken for 3
hours at
ambient temperature. The reaction was filtered, washed with methanol, and the
filtrate
concentrated in vacuo. The residue was purified by reverse phase HPLC using a
(5-100%
gradient of 0.1 % formic acid in acetonitrile and 0.1 % formic acid in water)
to yield 20 mg of
the above named compound.
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-66-
(F) 2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[4-(2-methoxy-ethyl)-5-oxo-
4,5-dihydro-[1,2,4]triazol-1-yl]-benzamide
A slurry of 2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-(5-oxo-4,5-dihydro
[1,2,4]triazol-1-yl)-benzamide (44.4 mg, 0.122 mmol) and CsZC03 (66.0 mg,
0.202 mmol)
were stirred in DMSO (0.813 mL) at ambient temperature for 20 minutes. 2-
Bromoethyl
methyl ether (17.0 mg, 0.122 mmol) was added and the reaction stirred at
ambient
temperature for 48 hours. The reaction was diluted with water (15-fold) and
the aqueous
extracted with CHZCh (3x). The organics were dried over sodium sulfate, and
concentrated in
vacuo. The crude was purified by reverse phase HPLC .reverse phase HPLC using
a (25-
65% gradient of 0.1% formic acid in acetonitrile and 0.1% formic acid in
water) (to give the
title compound (18 mg). LCMS (m/z) 423.5 M+1.
The compounds of Examples 2-14, identified in Table 1 below, can be prepared
according to the method of Example 1.
TABLE 1
EXAMPLE MOLSTRUCTURE IUPAC NAME DATA
#
LCMS M/Z
2 HN-~ 2-Chloro-N-(1- 365.2
N
~
N' hydroxy-cycloheptylmethyl)-
o
/ H~ 5-(5-oxo-4,5-dihydro-
N [1,2,4]triazol-1-yl)-
CI o Ho benzamide
3 CH3 2-Chloro-N-(1- 379.5
~
hydroxy-cycloheptylmethyl)-
N
O N' 5-(3-methyl-5-oxo-4,5-
dihydro-[1,2,4]triazol-1-yl)-
HO benzamide
CI o
4 Hod 2-Chloro-N-(1- 409.5
hydroxy-cycloheptylmethyl)-
5-[4-(2-hydroxy-ethyl)-5-
oxo-4,5-dihydro-
ci o [1,2,4]triazol-1-yl]-
benzamide
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
67
EXAMPLE # MOLSTRUCTURE IUPAC NAME DATA I
LCMS M/Z
N~~~ 2-Chloro-5-(4- 404.5
o N~'\N cyanomethyl-5-oxo-4,5-
dihydro-[1,2,4]triazol-1-yl)-
N Ho N-(1-hydroxy-
o H cycloheptylmethyl)-
cl
benzamide
o cH3 cH3 2-Chloro-N-(1- 437.6
hydroxy-cycloheptylmethyl)-
o N'N 5 [4 (2-methoxy-ethyl)-3-
Ho methyl-5-oxo-4,5-dihydro-
[1,2,4]triazol-1-yl]-
ci o
benzamide
7 CH3 2-Chloro-5-(4- 418.5
N~N~ cyanomethyl-3-methyl-5-
o~N'N oxo-4,5-dihydro-
Ho [1,2,4]triazol-1-yl)-N-(1-
o H hydroxy-cycloheptyl
CI methyl)-benzamide
8 H CH3 2-Chloro-N-(1- 393.5
hydroxy-3,3-dimethyl-
O N'N cyclohexylmethyl)-5-(3-
CH
N HO CH3 methyl-5-oxo-4,5-dihydro-
[1,2,4Jtriazol-
CI
1-yl)-benzamide
~/ CH3 5-(4- -436.4
N
HzN p~~ Carbamoylmethyl-3-methyl-
5-oxo-4,5-dihydro-
i
[1,2,4]triazol-1-yl)-2-chloro-
ci o Ho N-(1-hydroxy-
cycloheptylmethyl)-
I benzamide
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
-68-
EXAMPLE MOLSTRUCTURE IUPAC NAME DATA
# LCMS M/Z
I
90 H~ cH3 2-Chloro-N-(1- 423.5
N~
hydroxy-cycloheptylmethyl)-
~N.N
i
5-[4-(2-hydroxy-ethyl)-3-
methyl-5-oxo-4,5-dihydro-
c~ o H [1,2,4]triazol-1-yl]-
benzamide
11 HZN~ CH3 5-[4-(2-Amino- 422.4
ethyl)-3-methyl-5-oxo-4,5-
dihydro-[1,2,4]triazol-1-yl]-
2-chloro-N-(1-hydroxy-
ci o H cycloheptylmethyl)-
benzamide
EXAMPLE 12
2-Chloro-N-(1-hydroxy-cycloheatylmethyl)-5-f4-(2-hydroxy-3-methoxy-proayl)-3-
methyl
5-oxo-4,5-dihydro-~1.2,41triazol-1-yl1-benzamide
CH3
H3C,0 H,,,, N II
O O~N.N
H HO
. I / N
I I
CI O
(A) 2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-[4-(2-hydroxy-3-methoxy-
propyl)-3-methyl-5-oxo-4,5-dihydro-[1,2,4]triazol-1-yl]-benzamide
2-Chloro-N-(1-hydroxy-cycloheptylmethyl)-5-(5-oxo-4,5-dihydro-[1,2,4]triazol-1-
yl)-
benzamide (38 mg, 0.1 mmol) and R-(-)-glycidyl methyl ether (0.3 mL) in DMF
(0.1 mL) were
heated at 80 °C for 2 hours. The reaction was concentrated in vacuo,
then purified by reverse
phase HPLC using a (25-50% gradient of 0.1 % formic acid in acetonitrile and
0.1 % formic
acid in water) to give the title compound (20 mg). LCMS (mlz) 467.6 M+1.
CA 02511189 2005-06-20
WO 2004/058731 PCT/IB2003/006232
.. -69-
The compounds of Examples 13-14, identified in Table 2 below, can be prepared
according to the method of Example 12.
TABLE 2
EXAMPLE MOLSTRUCTURE IUPAC NAME DATA
# LCMS M/Z
13 oH3 2-Chloro-N-(1- 467.6
o ~~ hydroxy-cycloheptylmethyl)-
O N
5-[4-(2-hydroxy-3-methoxy-
propyl)-3-methyl-5-oxo-4,5-
~'
~ dihydro-[1,2,4]triazol-1-yl]-
ci o
benzamide
14 H 2-Chloro-N-(1- 451.6
~
CH3
3 hydroxy-cycloheptylmethyl)-
~N~
~ ~II/
~
N~N
O ~ 5-[4-(2-hydroxy-2-methyl-
H HO propyl)-3-methyl-5-oxo-4,5-
N~ dihydro-[1,2,4]triazol-1-yl]-
c~ o
benzamide
The present invention is not to be limited in scope by the specific
embodiments
described herein. Indeed, various modifications of the invention in addition
to those described
herein will become apparent to those skilled in the art from the foregoing
description and the
accompanying figures. Such modifications are intended to fall within the scope
of the
appended claims.
All patents, applications, publications, test methods, literature, and other
materials
cited herein are hereby incorporated herein by reference in their entireties.