Note: Descriptions are shown in the official language in which they were submitted.
CA 02512380 2005-06-30
WO 2004/059310 PCT/IB2002/005680
CHOLESTEROL ENZYME ELECTRODE
Field of the invention
The present invention relates to a novel enzyme electrode useful for the
determination
of cholesterol in an aqueous solution. The present invention primarily also
provides a
process for the preparation of an enzyme electrode by coating an immobilized
cholesterol
oxidase (ChOx) and mediator on a silicate sol gel by microencapsulation.
Background of the invention
Cholesterol and its fatty acid esters are important compounds for human beings
as
they are components of nerve and brain cells and are precursors of other
biological
materials, such as bile acid and steroid hormones (P. L. Yeagle, Biology of
Cholesterol,
CRC Press: Its function and metabolism in biology and medicine: Plenum: New
York,
1972).
Cholesterol determination in blood is clinically important for the diagnosis
of heart
diseases since accumulation of cholesterol and its fatty acid esters in blood
due to excessive
ingestion can be fatal (D. Noble, Anal. Chem., 1993, 65, 1037A-41A). The
normal range of
blood serum values extends from 3to 6mm for total cholesterol while in the
hyperlipidamic
condition the level can increases to 1 OmM. It is therefore desired to develop
techniques that
allow convenient and rapid determination of cholesterol.
Various methods have been employed in the art for stabilization and
immobilization
of enzymes within carbon paste or covalently linking it to the surface of
glassy carbon
electrode or immobilizing it within a polymer film for the preparation of
enzyme electrode.
In recent years, enzyme immobilization with the retention of enzyme activity
within a sol-
gel matrix has become a potential tool for development of new biosensors.
Avnir et al
disclose the immobilization of organic compounds in inorganic supports by
introducing the
organic compound with a polymerization precursor [J. Phys. Chem., 88 (1984),
5969].
Sol-gel processed materials are known for their use in development of ceramic
films
for conductive, optical, mechanical and electro-optic applications [Brinker,
C. J., and
Scherer, G. W., Sol Gel Science, Academic Press, New York, (1989); Klein, L.
C., Annu.
Rev. Mater. Sci., 23 (1993) 437]. Braun et al report that alkaline phosphatase
retains its
activity when immobilized. in a sol-gel matrix [Mater. Lett.~ 10 (1990) 1].
There is disclosure in the art of the immobilization of enzymes including
glucose
oxidase within a sol-gel matrix [Yamanaka et al, Chem. Mater. 4 (1992) 495;
Shtelzer et al,
Biochem. Biotechnol., 19 (1994) 293; Narang et al, Anal. Chem., 66 (1994)
3139].
1
CA 02512380 2005-06-30
WO 2004/059310 PCT/IB2002/005680
Audebert and Sanchez report the construction of a ferrocene mediated sol-gel
biosensor using a two stage sol-gel preparation method based on TMOS and
commercial
colloidal silica of varying particle size [Chem. Mater. 5 (1993) 911].
According to this
literature reference, more than 80 % of the glucose oxidase retains its
activity in the gel and
the Faradic response of the electrode agrees with theoretical calculations
based on this
activity.
Lev et al disclose the use of sol-gel derived composite silica-carbon
electrodes and
claim the dual advantage of both the porosity and rigidity of the silica
matrix and the
electrical conductivity of the graphite [Anal. Chem,, 66 (1994) 1747]. In this
disclosure,
glucose oxidase is first adsorbed on the surface of the carbon powder and then
used for the
preparation of the sol-gel film on a glassy carbon electrode. Kurokawa et al
report a similar
method where fabricated glucose oxidase doped sol-gel composite is made of
various
composite fibers such as cellulose or titanium propoxide [Biotechnol. Bioeng.,
42 (1993)
394; Biotechnology 7 (1993) 5].
The co-immobilisation of cholesterol oxidase and horse radish peroxidase in a
sol
gel film is disclosed for example in Analytica Chimica Acta Vol 414, 23 pp,
2000. the
method of this disclosure comprises physical adsorption, physically entrapped
sandwich
and the use of microencapsulation technique for the immobilization of
cholesterol and
horse radish peroxidase on tetra ortho silicate derived sol gel films. The
response time for
cholesterol estimation is more than 100 minutes. A response time of 50 seconds
was
observed arnperometrically with a physically entrapped enzyme sandwich sol gel
film.
Further the enzyme electrode is reported to be stable for a period of 8 weeks
only.
Biosensors used in the art suffer from several drawbacks in terms of stability
and
shorter shelf life. Several have reported methods of immobilization of
biorecognition
elements for use in chemical sensing researchers jR. F. Taylor, Pratein
Immobilizing
Fundamentals and Applications: Marcel Dicker, New York (1975) Chapter 8, 263-
303 and
H. H. Weetall, Immobilized Enzyme; Antigen, Antibodies and Peptides
Preparation and
Characterization: Marcel Dicker, New York (1975) Chapter 6, 263-303]. The
methods
reported in literature can generally be classified into one of the following
categories (1)
physisorption (2) covalent attachment or (3) entrapment, among which
physisorption is the
simplest immobilization approach.
Several disadvantages arise with these methods of immobilization such as
problems
associated with the large size of the biorecognition elements (e.g. proteins
and enzymes).
Physisorption produces a range of biorecognition element orientations and
apparent biding
2
CA 02512380 2005-06-30
WO 2004/059310 PCT/IB2002/005680
affinities. Besides physisorption generally leads to a population of
biorecognizing elements
that is completely unresponsive to target analyte. The immobilized species is
completely
unresponsive to target analyte. The immobilized species will often
leach/desorb from
sensing interface because there are no covalent bonds. Covalent schemes
generally lead to
more stable and uniform (interim of biorecognition orientation) interface and
enzyme
leaching is minimized. Unfortunately covalent attachment can involve one or
more
chemical transformation and tends to be time consuming and can be costly.
US Patent 6,342,364 provides a sensor that electrochemically determines
cholesterol
in low density lipoprotein by only one feed of a sample. The sensor has: an
electrode
system that is mounted on an electrically insulating base plate and includes
at least a
working electrode and a counter electrode; an enzyme layer formed on the base
plate with
the electrode system; and a reagent layer that is arranged before the enzyme
layer in a
sample solution supply path to the electrode system. The enzyme layer includes
at least an
oxidoreductase and an electron mediator. The reagent layer includes a reagent
that
depresses reactivity of cholesterol in lipoproteins other than the low density
lipoprotein
with the oxidoreductase, for example, a reagent that attaches to lipoproteins
other than the
low density lipoprotein to form a water-soluble complex. However, the shelf
life of this
sensor is too low.
US Patent 6,214,612 discloses a cholesterol sensor for quantitative
determination of
cholesterol is provided containing an electrode system and a reaction reagent
system. The
electrode system contains a measuring electrode such as a carbon electrode and
a counter
electrode, and the reaction reagent system contains cholesterol dehydrogenase,
nicotinamide adenine dinucleotide and an oxidized electron mediator. Electron
mediators
include ferricyanide, 1,2-naphthoquinone-4-sulfonate, 2,6-dichlorophenol
indophenol,
dimethylbenzoquinone, 1-methoxy-5-methylphenazinium sulfate, methylene blue,
gallocyanine; thionine, phenazine methosulfate and Meldola's blue. Diaphorase,
cholesterol
esterase and a surfactant may also be present.
The electrode system above is on an insulating base plate, and the base plate
has a
covering member containing a groove that is a sample supplying channel which
extends
from an end -of the base plate - to -the electrode system. A reaction layer
containing the
reagent system in dry form and a layer of a hydrophilic polymer is provided on
the base
plate or the covering member, or on both the electrode system and covering
member so as
to be exposed to the sample supplying channel. During operation, the electron
mediator is
reduced in conjunction with oxidation of cholesterol in a sample by
cholesterol
3
CA 02512380 2005-06-30
WO 2004/059310 PCT/IB2002/005680
dehydrogenase, and an amount of current required to electrochemically re-
oxidize the
electron mediator is directly proportional to a quantity of cholesterol
present in the sample.
However, the sensor has a Iow shelf life and also potentially shows leaching
of both the
mediator and the enzyme.
US Patent 6,071,392 discloses a cholesterol sensor which comprises comprises
an
electrode system having a measuring electrode and a counter electrode formed
on an
electrically insulating base plate, an electrode coating layer for covering
the electrode
system and a reaction reagent layer formed on or in the vicinity of the
electrode coating
layer, wherein the reaction reagent layer comprises at least an enzyme for
catalyzing
cholesterol oxidation, an enzyme having a cholesterol ester hydrolyzing
activity and a
surfactant, the electrode coating layer comprises at least one selected from
the group
consisting of water-soluble cellulose derivatives and saccharides and is
contained at such a
concentration that imparts sufficient viscosity to a sample solution for
enabling it to hinder
invasion of said surfactant into said electrode system when said electrode
coating layer is
dissolved in said sample solution supplied to said sensor. The sensor of this
patent is aimed
at eliminating impairment of sensor response due to electrode degeneration
caused by
invading surfactant into the electrode system. While the response time is
stated to be low,
the shelf life is again not high due to potential enzymatic leaching.
US Patent 6,117,289 discloses a cholesterol sensor which comprises an
electrode
system composed of at least a measuring electrode and a counter electrode and
disposed on
an electrically insulating base plate and a reaction layer formed on or in the
vicinity of the
electrode system. The reaction layer contains cholesterol esterase for
catalyzing the
conversion of cholesterol ester into cholesterol, cholesterol oxidase and a
surfactant. The
response time was up to nine minutes. Additionally the presence of a
surfactant can result
in electrode degradation.
Electrochemically polymerised conducting polymers have also received
considerable
attention over the last two decades. The remarkable switching capacity of
these materials
between the conducting oxidised (doped) and the insulating reduced (undoped)
state is the
basis of many applications.
For example, polyconjugated conducting polymers have been proposed for
biosensing
applications because of advantageous characteristics such as direct and easy
deposition on
the sensor electrode by electrochemical oxidation of monomer, control of
thickness by
deposition of charge and redox conductivity and polyelectrolyte
characteristics of the
polymer useful for sensor applications.
4
CA 02512380 2005-06-30
WO 2004/059310 PCT/IB2002/005680
It is therefore highly desirable to develop biosensors that allow conventional
and
rapid determination of cholesterol.
Objects ~f the invention
The main object of the present invention is to provide a novel sol gel based
enzyme
electrode useful for the estimation of cholesterol in aqueous medium.
Another object of the invention is to provide a process for the preparation of
a novel
enzyme electrode, which allows an accurate and rapid estimation of cholesterol
in solution.
Yet another object of the present invention is to provide an enzymatic stable,
cost-
effective high sensitive enzyme electrode.
Still another object of the present invention is to provide an enzyme
electrode,
which provides an accurate measurement of cholesterol within a short time
period of 30
seconds.
It is yet another object of the invention to provide a novel sol gel based
enzyme
electrode which is reusable at least five times.
Summary of the invention
Accordingly the present invention relates to an enzyme electrode useful for
estimation of cholesterol in aqueous medium, said electrode comprising:
i. an electrically conductive base plate,
ii. a film of sol gel derived material deposited thereon,
said sol gel derived material of step b) being microencapsulated cholesterol
oxidase
with an electron mediator.
In one embodiment of the invention, the enzyme electrode showing zero leaching
of
the encapsulated enzyme and of the electron mediator, a response time of 30
seconds, being
reusable at least five times and a shelf life of six months.
In another embodiment of the invention the electrically conductive base plate
is
selected from indium tin oxide coated glass plate and silver coated non-
conducting polymer
surface.
In still another embodiment of the invention, the non-conducting polymer
surface is
selected from a film and a sheet.
In a further embodiment of the invention non-conducting polymer surface is
selected from the group consisting of polyacrylamide, polyvinyl chloride and
polyethylene.
In another embodiment of the present invention the sol material is silica sol.
In yet another embodiment of the invention, the silica sol is selected from
tetraethyl
orthosilicate and tetramethyl orthosilicate.
5
CA 02512380 2005-06-30
WO 2004/059310 PCT/IB2002/005680
In another embodiment of the invention the electron mediator is selected from
potassium ferricyanide, ferrocene and Prussian blue.
In a further embodiment of the invention the enzyme electrode has a
sensitivity of
0.4 volt.
In another embodiment of the invention the strength of cholesterol oxidase
used is
in the range of 3-5 IU per 1x1 cm2 of surface area. .
In a further embodiment of the invention the enzyme electrode works at a pH in
the
range of 6.5 -7.2.
The present invention also relates to a process for the preparation of enzyme
electrode useful for estimation of cholesterol in aqueous medium, which
comprises the
steps of
a. preparing a silicate solution by known methods,
b. immobilizing an enzyme cholesterol oxidase and an electron mediator by
slowly adding
simultaneously 0.05-0.1 M phosphate buffer containing 3-5 IU of cholesterol
oxidase and
about O.O1M of mediator to the above said silicate solution of step a),
c. allowing the above said resultant mixture to stand till the complete
encapsulation of
enzyme and mediator by observing turbidity,
d, spreading the resultant turbid mixture on a conductive base plate by
conventional
methods,
e. drying the conductive base plate with the spread mixture for at least 24
hrs at a
temperature in a range of 25-30°C to obtain the desired enzyme
electrode.
In an embodiment of the invention the silicate sol used is selected from
tetraethyl
orthosilicate and tetramethyl orthosilicate
In another embodiment of the invention, the phosphate buffer used has a pH in
a
range of 6.5-7.2.
In yet another embodiment of the invention the process of preparation of
enzyme
electrode is a single step process.
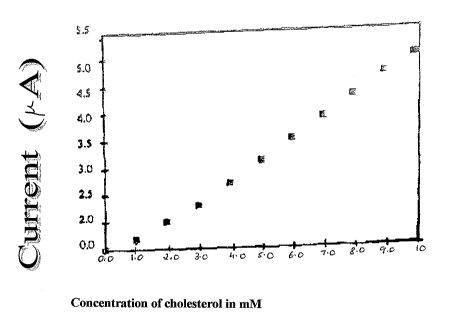
Brief description of the accompanying drawing
Figure 1 shows the response of the enzyme electrode as a function of the
concentration of cholesterol solution.
Detailed description of the invention
The present disclosure essentially involves the stages of preparation of sol
and
simultaneous addition of mediator in buffer solution added to sol along with
the cholesterol
oxidase enzyme. The mixture of sol and immobilized enzyme is allowed to stand
till the
6
CA 02512380 2005-06-30
WO 2004/059310 PCT/IB2002/005680
complete encapsulation of the enzyme is achieved. This stage is judged by
observing the
onset of turbidity of the mixture. Once the mixture turns turbid, it is usable
for deposition
on a substrate to prepare the desired electrode. The spread mixture when
allowed to dry for
long time of about 24 hours at a temperature of about 25- 30°C, results
in a thin film which
has the cholesterol sensing property.
The preparation of sol is accomplished by using preferably tetraethyl silicate
in pure
water and HCl. However tetramethyl silicate may also be used. The water used
in the
preparation of the sol is preferably pure water and more preferably a
deionized water of
more than lSMohms. The preparation of sol maybe accomplished by any
conventional
known means known to a person skilled in the art. For example, a stock sol-gel
solution is
prepared by mixing 4.5 ml of tetra ethyl orthosilicate (TEOS), 1.4 ml of H20
and 100u1 of
0.1 M HCl in a glass vial. The mixture was stirred regularly until a clean
solution was
obtained. This solution was used throughout the experiment and was diluted as
required.
Specific casting solution was prepared by mixing 0.5 m1 of the stock solution
with 0-0.2 ml
of deionized water.
The next critical step involves preparation of the sol geI containing the
immobilized
cholesterol oxidase enzyme. The speciality in this is the simultaneous
encapsulation and
immobilization of the enzyme while the mediator is being added gradually to
the sol with
the buffer containing the enzyme. The enzyme used is cholesterol oxidase of a
concentration in a range of 3-5 ILT per square cm of surface area. The
mediator used is
preferably potassium ferricyanide. For the immobilization of cholesterol
oxidase (ChOx)
80.1 of stock solution was added to 20.1 of 0.01M potassium ferricyanide
solution made in
0.1 M phosphate buffer (pH 7.0) containing 3U of ChOx for simultaneous
entrapment of
the enzyme and potassium ferricyanide as a mediator in the growing hydrolyzed
gel
forming silica network. The solution was kept aside until the enzyme and
mediator was
encapsulating completely with in the growing network.
Once the sol .gel containing the immobilized and microencapsulated enzyme is
prepared it is ready for use for deposition as a film on a conducting
substrate. The
conducting substrate may be a glass plate coated with a conducting film like
Indium Tin
Oxide (ITO) or may also be any other- substrate like a polymer film or a
sheet. These may
have a deposited silver film for use as a conducting surface for the
deposition of film of the
sol gel containing encapsulated enzyme. Prior to film casting indium tin oxide
(ITO) coated
glass plates were first treated with HNO3, for about 2 hrs and were
subsequently rinsed
thrice with Millipore water. The glass slides were finally washed with n-
propanol prior to
7
CA 02512380 2005-06-30
WO 2004/059310 PCT/IB2002/005680
film coating technique. The film may be prepared by any conventional means
known to a
person skilled in the art and is preferably kept in air for drying at a
temperature in a range of
25-30°C. Films of varying thickness doped with ChOx were then cast onto
the ITO glass
using the water sol-gel dilution scheme. The film was dried at 25°C and
was stored at 4°C.
A standard cholesterol solution was prepared by dissolving 3mg of Cholesterol
in
12.8 ml of propan-2-of and was mixed with 5.85 ml of Triton X -100 surfactant.
After
homogenization the volume was made up to 100m1 with O.1M phosphate buffer (pH
7.0)
and thermostat at 35°C. This standard solution was further diluted with
water to make
different cholesterol solutions.
The characteristics of the enzyme coated substrate are measured using
Amperometric
response studies using the standard cholesterol solution prepared above.
Amperometic
techniques are well known to a person skilled in the art. In this method,
essentially a three-
electrode cell configuration is used. The electrodes used are the working
electrode i.e. the
enzyme electrode of the present invention. Typically the enzyme electrode was
made on an
ITO coated glass. The second electrode is the reference electrode of Ag/AgCI.
In actual
measurement, cholesterol solution of strength varying between 0.5-lOmM in a
phosphate
buffer ofpH of 7.0 was used with the two electrodes as described above. The
current due to
enzymetrically produced H20~ was measured every 100 seconds. Typically a
response time
of seconds was measured for a concentration of
The reaction giving rise to current is due to the following scheme
Cholesterol +02 -~ 0-Cholesten-3-one + H202
HZOa ~ 02 + 2H+ + 2 e-
The results of the experiments are shown in Fig 1. In order to check if
addition of any
interfering agents in cholesterol, like glucose or ascorbic acid, will have
any deleterious
effect on the response of the enzyme electrode, the experiment was repeated
with
cholesterol solution mixed with the interfering agents. It was found that
these interfering
agents did not show any effect on the response to the enzyme electrode.
In an attempt to improve the shortcomings of the prior art disclosures of
cholesterol
measurement, the bio molecules are immobilized in sol-gel and have
comparatively
enhanced shelf lives. This is because of fact that (i) a variety of enzymes
may be
encapsulated in sol-gel matrices giving optical transparent glasses (ii) the
enzymes are
remarkable stable in such matrices (iii) these enzymes undergo characteristics
reversible
reaction in sol-gel glasses and (iv) spectroscopic changes occurring in sol-
gel glasses can
readily be quantified by optical spectroscopy. The sol-gel technique is
advantageous since
CA 02512380 2005-06-30
WO 2004/059310 PCT/IB2002/005680
little or no heating is required. Such enzyme molecules become entrapped in
the covalent
network rather then being chemically bound to the inorganic matrix as chemical
bonding of
the substrate may perturb the activity of the molecule. The fine pore network
in dried glass
(<l0nm) does not scatter visible radiation and allows the diffusion of small
molecules onto
the electrode surface. Porous inorganic xerogel such as tetra ethyl
orthosilicate (TEOS)
derived sol-gels are particularly attractive matrices for electrochemical
biosensors since
they combine physical rigidity, negligible swelling in aqueous solution,
chemical inertness
and thermal stability. These biosensors in principle have sensitivity and
rapid response time
and are also free from the problem of any detrimental effects on enzyme
activity.
Another significant advantage observed over the prior art enzyme electrodes is
that
there is zero leaching of the enzyme and the mediator. The electrode of the
invention also
has a reduced response time of 30 seconds and is reusable. It is also observed
that shelf life
of the electrode is enhanced and is about six months at ambient temperature of
25-30°C.
The inventive step of the present invention resides in the immobilization of
the
enzyme cholesterol oxidase (ChOx) and electron mediator in silicate sol-gel by
micro-
encapsulation technique and depositing the above said microencapsulated enzyme
and
mediator sol-gel film onto a conducting indium tin oxide (ITO) coated glass
plate for the
preparation of an enzyme electrode useful for the determination of cholesterol
in solution.
The following examples are given by the way of illustration and therefore
should not be constructed to limit the scope of the present invention in any
manner.
Example 1: Enzyme activity measurements:
A solution of 0.05 cm3 of 6mm cholesterol dissolved in propane-2-of and volume
of 3
cm3 of 0.1 M phosphate buffer (pH 7.0) were mixed and kept in a thermostat at
35°C. The
ChOx immobilized sol-gel film coated ITO glass plate was immersed and
incubated for 2
minute, the plate was removed and the absorbance of the solution was measured
at 240 nm
using a double beam spectrometer to determine the cholesterol produced by 'the
enzymatic
reaction. The apparent enzyme activity (Ucm3) was evaluated by the following
procedure
based on the difference in absorbance before and after incubation of the
enzyme
immobilized sol-gel glass plate.
~~ (Ucni 2) = AV/Ets
app
where A is a deference in absorbance before and after incubation, V is the
total
volume (3.OScm3), s is the millimolar extinction coefficient of cholesterol
(12.2), t is the
reaction time (min) and s is the surface area (cm3) of sol-gel film. One unit
of enzyme
9
CA 02512380 2005-06-30
WO 2004/059310 PCT/IB2002/005680
activity (U cm3) is defined as the activity that results in the production of
1 ul mol of
cholesterol per minute. The enzyme activity measurements were made on the
enzyme
(ChOx/HR.P) immobilized sol-gel film. No enzyme (ChOx/HRP) leaching was
observed
from the enzyme immobilized sol-gel film.
Example 2
Electrochemical estimation of cholesterol containing interfering reagents by
using
cholesterol oxidase immobilized sol-gel-ITO( ChOx/sol-gel/ITO ) electrode.
Cyclic Voltametery studies
When cholesterol comes in contact with enzyme electrode containing ChOx
immobilized in a TEOS derived sol-gel film the following enzymatic and
electrochemical
reaction occurs
Cholesterol +02 ~ ~-Cholesten-3-one + H2O2
H2O2 ~ OZ + 2H+ + 2 e-
The oxidation current for H202 is recorded as the sensor response in the
amperometric
biosensor. Owing to the direct immobilization of the enzyme, the sensor
properties such as
time and sensitivity are the reflection of the immobilized enzyme .The cyclic
voltammetry
experiments were carried out in 0.1 M phosphate buffer (pH 7.0) containing
different
concentration of cholesterol ( O.SmM to lOmM) using enzyme immobilized sol-gel
film
cast on ITO glass plate as a working electrode a Ag/AgCl reference electrode
and a Pt wire
as a counter electrode. The above experiment was conducted in the absence and
in the
presence of 0.1 xnM ascorbic acid and 0.5 mM glucose as interfering reagents.
The cyclic
voltametry shows an oxidation peak at 750 mV which keeps on increase in anodic
current
with an increase in concentration from 0.5 mM to 10 mM cholesterol. The rise
is attributed
to the direct oxidation of H~,O~ on the surface of the ITO coated glass plate.
However the
oxidation peak at 0.75V shifts anodically by 150mV to 0.9 V Vs Ag/AgCI with
increase in
anodic current in the presence of 0.1 mM ascorbic acid. The presence of 0.5 mM
glucose in
the cholesterol solution (1mM) also shows an increase in anodic current but
does not show
any significant effect on the oxidation potential of H202, thereby showing
that the presence
of both 0.1 mrn ascorbic acid 0.5 mm glucose in cholesterol have a significant
effect on the
observed anodic current.
Example 3: Amperometric response studies
A three electrode cell configuration similar to the one used in cyclic
voltameteric
experiment was used for the amperometric determination of cholesterol in
phosphate buffer
(pH 7.0). The working electrode (comprising cholesterol oxidase ChOx
immobilized sol-
CA 02512380 2005-06-30
WO 2004/059310 PCT/IB2002/005680
gel at ITO glass) was polarizing at 0.9V versus Ag/AgCI and amperometric
response to
cholesterol (0.5-lOmM) was measured by using amperometric calibration for
enzymematically produced H~02. The current was monitored every 100 sec after
dispensing
different concentration of cholesterol solution (2mM-IOmIVl) into the cell. A
maximum
current of 5.0 uA was obtained for 10-mM cholesterol above which no
significant change
in current could be observed .The response time to total cholesterol was found
to be 90 sec.
Example 4
Electrochemical estimation of cholesterol using cholesterol oxidase and
potassium
ferricyanide immobilized sol-gel indium tin oxide (ChOxlFe3+/sol-gel/ITO) as
electrode
and with influence of interfering reagents such as ascorbic acid (0.lmM) and
glucose
(O.SmM)
Cyclic Voltametery studies
The cyclic voltammetry experiments were carried out in 0.1 M phosphate buffer
(pH 7.0) containing different concentration of cholesterol using enzyme
cholesterol oxidase
and potassium ferricyanide immobilized sol-gel indium tin oxide (ChOx/Fe3+/sol-
gel/ITO)
film as a working electrode, a Ag/AgCI reference electrode and a Pt wire as a
counter
electrode. The following reactions occur
Cholesterol +ChOx -3 Cholestenone + ChOxr~
ChOxred + Fe3+ (ferricyanide) -~ ChOx + Fe2+ (ferrocyanide)
0.4V
Fe'+ (ferrocyanide) ~ Fe3+~ (ferricyanide) + e- (at electrode)
The oxidation current is recorded as the sensor response in the amperometric
biosensor. Owing to the direct immobilization of the enzyme, the sensor
properties such as
time and sensitivity are the reflection of the immobilized enzyme. An
oxidation peak
observed earlier in Example 2 at 0.9V vs. Ag/AgCl when enzyme immobilized sol-
gel film
without mediator was used as an electrode now shifts 300mV cathodically and is
observed
at 0.4V versus Ag/AgCI, which increases with increase in cholesterol
concentration (2 to
lOmM). The presence of 0.lmM ascorbic acid and O.SmM glucose in cholesterol
solution
does not show any significant efFect on the oxidation potential.
~ Example 5: Amperometric response studies
A three electrode cell configuration similar to the one used in cyclic
voltameteric
experiment has been used for the amperometric determination of cholesterol in
phosphate
buffer (pH 7.0). The working electrode (comprising cholesterol oxidase ChOx
immobilized
sol-gel at ITO glass) was polarized at 0.4V versus Ag/AgCl and amperometric
response to
11
CA 02512380 2005-06-30
WO 2004/059310 PCT/IB2002/005680
cholesterol of concentration varying from 2mM to lOmM was measured. The
current was
monitored every 100 sec after different concentration of cholesterol solution
(2mM to
lOmlVn into the cell (Figure 1). The anodic current measured in 6mM
cholesterol solution
(1mL) at ChOx/Fe3+/sol-gel/ITO polarized at 0.4 V yields the stead the state
in 30 seconds
and this response to cholesterol solution was reproducible to within 5%. The
lower
detection limit of cholesterol was found amperometrically to be O.SmM.
The main advantages of the present invention are:
1. Enzymatic electrodes prepared by the invention shows negligible enzyme
leaching.
2. The enzyme electrode prepared shows fast response to cholesterol in
solution
3. The enzyme electrode prepared is stable for a longer time.
4. The enzyme electrode prepared is highly sensitive to cholesterol.
20
12