Note: Descriptions are shown in the official language in which they were submitted.
CA 02513130 2005-07-12
Case 1/1447-PCT
83060fft
Powder Inhaler
The invention relates to powder inhalers in which at least part of the inner
surface
coming into contact with the powder aerosol is microstructured. Preferably, it
is a
powder inhaler operating on the Bernoulli principle (a Bernoulli inhaler).
Prior art
A number of powder inhalers operating by various principles are known in the
literature. As the Bernoulli inhalers are preferred according to the
invention, these
will be discussed first. What Bernoulli inhalers have in common is that the
active
substance to be delivered is stored in a cylindrical capsule and this capsule
is
inserted in a capsule chamber of the inhaler. The capsule chamber is usually
cylindrical in shape, being somewhat longer and wider than the capsule so that
the
capsule is able to vibrate vertically (= axially), and also horizontally
(=radially), but
remains aligned substantially parallel to the chamber axis. The capsule
chamber has
an air inlet near one of its two ends and an air outlet opening in the region
of the
other end. The air outlet (air channel) leads to a mouthpiece. Within the
scope of
the present description of the invention, the direction running from the
capsule
chamber through the air channel to the mouthpiece defines the longitudinal
axis and
hence the axial direction. The direction perpendicular thereto defines the
vertical or
radial direction.
In order to deliver the active capsule contents, first of all the capsule is
opened,
normally at two places on its longitudinal casing. As a rule these openings
are
located close to the two longitudinal ends of the capsule. If an air stream is
now
generated from the air inlet towards the air outlet in the capsule chamber, it
runs
so along the longitudinal axis of the capsule and has two effects: on the one
hand the
capsule is moved mainly along its longitudinal axis by the air stream. It can
also
CA 02513130 2005-07-12
vibrate to a small degree. On the other hand, the air flowing along the two
capsule
openings produces a lower pressure in front of the capsule openings than
inside the
capsule, so that the powder contained in the capsule is picked up by the air
stream
and thereby nebulised.
The capsules normally used for inhalers of this kind consist of two cup-like
components which fit telescopically one inside the other. The outer shape of a
composite capsule of this kind is that of a closed cylinder with hemispherical
ends.
The cylinder has a longitudinal axis and a transverse axis. The longitudinal
axis is
the axis which runs parallel to the generatrix of the cylinder casing. The
longitudinal
axis is longer than the transverse axis with the result that the longitudinal
section of
the capsule has an oval geometry and the cross section has a circular
geometry.
Usually, the capsules for inhalable powders consist of hard gelatine but may
also
~5 consist of a plastic material. In connection with this reference is made to
EP
1100474.
DE 3345722 discloses an inhaler operating by the Bernoulli principle,
consisting of
two housing elements which are axially moveable towards each other, with a
single
2o capsule chamber. The inner surface of the hollow cylindrical capsule
chamber is
smooth.
WO 91 /02558 discloses another Bernoulli inhaler wherein instead of a single
capsule
chamber there are a plurality of capsule chambers arranged in a similar manner
to a
25 revolver magazine. The open ends of this magazine are delimited by walls,
the air
inlet or air outlet being located only at one point in these walls. This
magazine is
mounted to be rotatable so that a capsule chamber is only connected to the air
inlet,
the air outlet and the cutting elements required to open the capsule in a
certain
position.
CA 02513130 2005-07-12
EP 0911047 also discloses a Bernoulli inhaler. This consists of a) a cup-
shaped
lower part open upwardly, b) a plate which covers the opening in the lower
part and
perpendicular to which is formed a capsule chamber of the kind described
above,
whilst on the capsule chamber is provided a button which is moveable against a
spring which has two sharp spikes for opening the capsule, c) an upper part
with a
mouth tube which is connected to the capsule chamber, and capable of conveying
a
powder aerosol, and d) a lid. The elements a), b) c) and d) are joined
together by a
common hinge element so that they can be flipped relative to each other. In
addition,
this patent application describes a capsule holder wherein the capsule holder
may be
~o constructed as a hole in the plate b) and has ribs at the edge. The capsule
is
jammed into this capsule holder to hold it in readiness.
FR-A-2 146 202 describes a powder inhaler with a flat cylindrical chamber for
accommodating a capsule. The capsule opened at the ends rotates during the
inhaling process, driven by tangentially incoming air, about its transverse
axis.
US-A-4 069 819 describes a powder inhaler wherein the capsule is pierced
through
the base of the capsule chamber and during inhalation is set in motion by the
air
flowing in tangentially in the region of the base.
Other powder inhalers which do not operate by the Bernoulli principle include
for
example the inhalers disclosed in DE 3348370 and DE 3336486 which contain a
disc-shaped blister pack comprising a plurality of blisters arranged in a
circle. The
individual blisters each contain a dose of a powdered medicament intended for
inhalation. The blister pack contains the doses of the powdered medicament in
non-
encapsulated form. The blister pack is located in a chamber (storage chamber)
in
these inhalers and each of the blisters may be pierced at two opposite ends
perpendicular to the plane of the disc. An air channel connects the opened
blisters to
the mouthpiece. The inhaler is described in more detail in DE 3336486 by way
of
3o example. This comprises a housing in which there is a chamber (storage
chamber)
which has an air inlet and in which there is a disc-shaped round blister with
filled
CA 02513130 2005-07-12
pouches of medicament. The blister is loosely attached to a round rotatable
disc.
Around the disc there are holes which are in axial contact with the pouches of
medicament, i.e, the pouches and holes are located above and below one
another.
The chamber has an air outlet. The inhaler also comprises a piston which is
arranged so that it can open a pouch of medicament by piercing it, so that the
medicament is released into the chamber and can be breathed in through a
mouthpiece. Reference is made to the drawings in the patent application and US
patent specification.
DE 4106379 describes an inhaler into which a blister or the like for a
powdered
medicament can be placed. The blister consists of two strips of material which
can
be pulled away from each other, defining at least one container in which the
medicament is located. The inhaler is provided with a device which pulls the
two
strips of material apart at an opening station in order to open a container.
The user
~5 can inhale the powdered drug from the opened container through an outlet
member,
e.g. a mouthpiece connected to the opened container. One of the strips of
material
may also be a carrier strip with a plurality of pouches and the other strip of
material
may be a covering strip. Each pouch and the adjacent area of the covering
strip then
form a container. A drive device may be provided at the opening station for
pulling
2o the carrier strip and covering strip apart. This drive device may consist
for example
of two drive wheels (e.g. gear wheels) which hold the covering strip between
them in
driven engagement. In this case, too, each individual blister defines a kind
of storage
chamber in the inhaler, which is connected to the mouthpiece via an air
channel.
25 US 4524769 discloses a powder inhaler which comprises a nozzle (mouthpiece)
for
delivering the aerosol, an air channel and a storage chamber for the active
substance. A membrane may be moved back and forth between the air channel and
the storage chamber. This membrane has a plurality of means for receiving a
metered quantity of active substance. Preferably, the membrane is a conveying
so membrane which as a device preferably comprises holes or perforated
depressions
for transporting active substance from the storage chamber into the air
channel. In
4
CA 02513130 2005-07-12
each case at least one of the devices between the air channel and the storage
chamber is pushed back and forth. The device filled with a metered quantity of
active
substance is emptied in the air channel and then migrates back into the
storage
chamber to be filled there with another metered quantity of active substance
by a
s filling means. The powder inhaler accordingly comprises means for moving the
conveying membrane so that the filled devices containing the metered active
substance formulation are transported from the storage chamber to the place of
delivery of the active substance, the air channel, and from there the empty
device is
brought back to the storage chamber. A rota may also be provided in the
nozzle.
Numerous inhalers comprise impact plates and the like mounted approximately in
the
region of the flow path, through which the nebulised active substance
formulation is
conveyed for delivery. The task of these impact means, if any, is to break up
lumps.
In the development of any powder inhaler attention must be paid to ensuring
that
large accumulations of particles cannot be formed in the inhaler which would
jeopardise its re-use on grounds of pharmaceutical quality. Therefore, and in
order
to ensure optimum delivery of the powder formulation, the inner surfaces of
all the
parts which may come into contact with the cloud of aerosol, particularly the
storage
2o chamber and the mouthpiece, are particularly smooth in construction.
To prevent contamination of a new aerosol cloud with old deposited particles
it may
be advantageous if the powder inhaler is cleaned after one or more inhalation
processes, particularly those parts which come into contact with the powder
formulation. This cleaning is necessary in order to minimise contamination of
the
next aerosol cloud to be breathed in, i.e. in order to ensure the
pharmaceutical
quality of the next aerosol cloud to be administered.
Descr~tion of the Invention
5
CA 02513130 2005-07-12
Surprisingly, it has now been found that powder inhalers in which at least
part of the
inner surfaces which may come into contact with the powder aerosol are
provided
with a micro- or nano-structured surface, do not have worse delivery
characteristics
than powder inhalers with a smooth inner surface in these areas.
At the same time, surfaces of this kind are particularly suitable for washing
simply
with water, as the cleaning liquid simply rolls off the surface and carries
any
impurities with it.
~o No powder inhalers with a micro- or nano-structured surface are known from
the prior
art.
Therefore, it is an aim of the present invention to provide powder inhalers
which can
be cleaned more efficiently without affecting the delivery characteristics of
the
powder formulation.
A further aim is preferably to prepare Bernoulli inhalers of this kind, i.e.
inhalers
loaded with a capsule which contains the active substance formulation.
2o A further aim is to overcome the disadvantages known from the prior art.
Detailed Description of the Invention
The powder inhaler according to the invention essentially consists at least of
a) a
2s mouthpiece and b) an air channel opening into the mouthpiece which can be
filled
with the active substance formulation to be administered. Optionally, the
powder
inhaler contains a chamber, optionally equipped with an air inlet channel or
an air
opening, for receiving the active substance or the powdered, possibly
compressed
formulation containing the active substance, for receiving a capsule, a
blister pack
so containing active substance and/or a conveyor belt containing active
substance, each
of which contains the formulation holding the active substance. Means for
opening
CA 02513130 2005-07-12
the blisters or capsules are optionally provided. In the storage chamber or
air
channel the pharmaceutical composition is mixed with air and conveyed to the
user's
mouth through the mouthpiece.
In the case of Bernoulli inhalers, in particular, an air channel may connect
the
chamber to the mouthpiece. In this case the chamber may also comprise another
air
inlet channel or an opening. The chamber in the Bernoulli inhalers is
preferably a
chamber for holding a capsule (capsule chamber) which is provided according to
the
invention with means for opening the capsule at the side. The capsule chamber
is
constructed so that the reservoir capsule inserted can essentially only
perform a
movement in the longitudinal direction when an air current travelling
essentially
parallel to the longitudinal axis of the capsule passes through the capsule
chamber
and has only limited play along the transverse axis thereof. Such capsule
chambers
are typical of Bernoulli inhalers. Within the scope of the present invention
the
~5 chamber may also be designated a storage chamber or metering chamber.
The powder inhalers known from the prior art have a smooth, unstructured inner
surface. The same applies to other elements which may come into contact with
the
powder aerosol. According to the invention the structure of the inner surface
of these
2o parts having a critical inner surface is different from an unstructured or
smooth
surface. By unstructured or smooth is meant, for the purposes of the present
invention, surfaces which do not have a surface structure as described within
the
scope of the invention.
25 For the powder inhalers according to the invention any of the inhalers
described in
the "prior art" section hereinbefore may be used. To avoid repetition, the
features of
these inhalers mentioned above will not be discussed again at this point but
reference is made specifically to this section. The inhalers according to
DE3345722,
W091/02558 or EP0911047 are of particular interest.
7
CA 02513130 2005-07-12
The devices known by the brand names "TURBOHALER~ ", "EASYHALER~",
"DISCUS~" and "HANDIHALER~" may be mentioned by name, in particular.
The features mentioned in the prior art section hereinbefore for the powder
inhalers
generally described also apply to the powder inhaler according to the
invention, with
the exception of the parts which come into contact with the powder aerosol in
relation
to the configuration of the inner surface, and will therefore not be mentioned
again at
this point. In a preferred inhaler according to the invention the capsules
mentioned in
the same section may be used.
Within the scope of the present invention the inner surfaces of the parts
which come
into contact with the powder aerosol, i.e. the surfaces which are most in
contact with
the powder aerosol, are referred to as the critical surface. The critical
surfaces
specifically include the mouthpiece and the air channel opening into the
mouthpiece.
The inner surface of the lower part or the impact plate of an inhaler
according to
EP0911047 described hereinbefore may optionally also be provided with the
structure according to the invention.
In the case of Bernoulli inhalers (c.f. for example DE3345722, W091/02558 or
2o EP0911047), which are preferred according to the invention, the inner
surface of the
capsule chamber are also part of the critical inner surface.
In the case of inhalers according to DE3348370, DE3336486 and DE4106379 the
chamber for accommodating the blister pack may also be counted as a critical
surface.
In the case of an inhaler which uses the principle described for US4524769
hereinbefore, the storage chamber may also have a critical surface.
so In inhalers with a separator this may also be a critical surface. A
separator of this
kind is described for example in EP0633792.
s
CA 02513130 2005-07-12
According to the invention at least some of the critical surface of the powder
inhalers
is provided with a micro- or nano-structure. Surfaces with a micro-structure
having
self-cleaning properties are described in EP772514 or DE20114878U1, to which
reference is hereby made.
Preferably, at least 20% of the inner surface of the mouthpiece is micro- or
nano-
structured, more preferably 50% and most preferably at least 75%.
In the case of Bernoulli inhalers, preferably also or only the inner surface
of the
capsule chamber is micro- or nano-structured over at least 20% of its surface,
more
preferably at least 50% and most preferably at least 75%.
In the case of inhalers with blister packs or conveyor belts the inner surface
of the air
~5 channel opening into the mouthpiece is micro- or nano-structured over at
least 20%
of its surface, more preferably at least 50% and most preferably at least 75%.
The outer shape of the parts which have a critical inner surface is of no
significance
for the purposes of the present invention and may resemble the configuration
of the
2o devices known from the prior art. Thus, in Bernoulli inhalers, for example,
the outer
shape of the capsule chamber is determined by its position and any movements
thereof in the inhaler or the movements of other parts of the inhaler around
the
capsule chamber.
25 The structuring of the critical surface according to the invention is
achieved by
providing raised portions and depressions at least on areas of the critical
inner
surface. This produces the structural shapes according to the invention.
The raised portions and depressions may be in the form of peaks, spheres, flat
so surfaces, wedge shapes, hemispherical shapes, etc.
9
CA 02513130 2005-07-12
They may be randomly arranged or ordered, e.g. in rows, circles, in a zigzag,
meandering, etc.
The spacing between the raised portions on the surface structure is in the
range from
0.1 to 200 microns, preferably 0.1 to 100 microns. Distances of 0.1 to 1
micron are
more preferred. The spacings between the raised portions may differ from one
another.
To ensure optimum delivery of the particles, spacings of 1 to 25 microns
should be
1o avoided.
Accordingly, the preferred dimensions of the structure of the structural
shapes are
less than the diameter of the aerosol particles, which are typically 1 to 20
microns,
preferably 1 to 5 microns. The height of the raised portions or the depth of
the
depressions are in the range from 0.1 to 100 microns, preferably 0.1 to 50
microns.
Spacings of 0.1 to 10 microns are most preferred.
Preferably the raised portions of the surface structures are close enough
together to
ensure that hydrophilic drops of liquid, e.g. drops of water, roll of the
raised portions
2o without actually touching the underlying area. At the same time the raised
portions of
the surface structures should not be too close together or the depressions
should not
be too flat so as not to form a sealed surface, with respect to the droplet
size of the
liquid, in which the surface forces between the drops and the surface come
into effect
fully. It is desirable that the height of the raised portions from the base
should
increase as the distance between the raised portions increases. Preferably,
the
surfaces have raised portions measuring 0.1 to 50 microns wherein the spacing
between the raised portions is 0.1 to 100 microns.
The micro-structured surfaces preferably have at least two different kinds of
so structural shapes, the raised portions and/or depressions of which are
distinguished
from one another by different shapes, heights and/or intervals. Individual
examples
CA 02513130 2005-07-12
of the two different structural shapes may be at different spacings from their
neighbours. Details may be found in the prior art.
Preferably, the critical surfaces consist of hydrophobic materials or
materials which
have been given a durable hydrophobic finish or they are coated with such
materials
and the raised portions cannot be detached by water or water-containing
detergents.
The materials used may be plastics, metals, ceramics, glass, etc.
Preferred materials are glass and/or ceramics and/metals and/or plastics such
as
1o polyethylene, polypropylene, polycarbonate, polyacrylates, polyesters,
silanes, etc.
Plastics are preferred. If desired, a plastic of this kind may be provided
with a
coating of another plastic which carries the surface structure.
Structured surfaces of this kind may either be produced either by forming the
surface
structures during the manufacture from hydrophobic materials or by
subsequently
subtracting or adding material to the surfaces. These processes include
subsequent
stamping, etching, laser ablation, galvanic machining, adhesive bonding of a
structured film, adhesive bonding of a powder, spraying with suspensions,
depositing
sublimates.
Finally, it is possible to create self-cleaning surfaces of this kind on
objects by
subsequent provision of a durable hydrophobic surface on previously produced
surfaces with the desired structures.
One possible way of subsequently making a surface durably hydrophobic is by
subsequently silanising surfaces with the desired structures which have been
prepared beforehand. Silanising may be carried out on any materials which are
naturally hydrophilic but capable of reacting with the reactive groups of the
silanes so
that finally the surface consists of the hydrophobic groups of the silanes.
11
CA 02513130 2005-07-12
In order to produce the desired surface structures during the actual
manufacture from
hydrophobic polymers the objects may be produced in moulds which contain the
negative of the desired surface structure.
It is also possible to apply the hydrophobic polymers in the form of solutions
and/or
dispersions which produce the desired surface structures when dried and cured.
Such structures are formed for example from self-organising polymers or under
conditions as known in principle from the manufacture of matt paint surfaces.
If it is not possible or not desirable to create the desired surface
structures from the
outset, this may also be done subsequently, e.g. by subsequent stamping or
etching.
Stamping may be carried out, for example, using heated or heatable stamps. The
etching may be carried out using the known means for chemical etching or by
physical methods such as ion etching with oxygen or other irradiation which
leads to
roughening of the surface and a surface structure which can be used according
to
the invention.
The method in which a surface structure is produced depends on the material
used
2o and the desired micro-structure.
It has also been found that it is also possible to obtain the desired surface
structure
by adhesively bonding a powder of the hydrophobic polymers. Powders of
hydrophobic polymers with the desired particle size can be obtained. Optimum
2s results are only achieved, however, if powders with a relatively narrow
particle size
distribution are used.
As already described, the inner surface of the mouthpiece in all kinds of
powder
inhalers is one of the critical surfaces. The shape of the mouthpiece is
essentially
so defined by its function.
12
CA 02513130 2005-07-12
The mouthpiece, which is generally tubular and optionally somewhat flattened
may
be arranged axially or at an angle to the axis of the air channel connected to
it or
offset to one side of this axis.
In the case of the preferred Bernoulli inhalers, apart from the inner surface
of the
mouthpiece the inner surface of the capsule chamber is one of the critical
surfaces.
Therefore, these two elements will be discussed in more detail at this point.
In the
simplest case the mouthpiece is a tube which is connected to the capsule
chamber at
one end and is open at the other end.
It may be constructed in the form of a cap which is fitted on to a lower part
of the
inhaler which contains the capsule chamber. This cap may be hinged to the edge
of
the inhaler housing so as to be pivotable about an axis extending
perpendicularly to
the longitudinal axis of the inhaler. The mouthpiece and the lower part of the
inhaler
housing may, however, also be fixed to one another by a conventional push-fit
connection. In any case, access generally, to the capsule chamber and to the
cutting
device in the lower housing part, on the one hand, and to the inner components
such
2o as the perforated plate and the upper housing part (of the mouthpiece-cap)
is made
substantially easier by the removability or pivotability of the two
components.
In order to replace used capsules with fresh ones, in an embodiment of this
kind the
mouthpiece is flipped upwards or the push-fit connection between the
mouthpiece
and the lower housing part is undone. The capsule chamber is then freely
accessible, so that the emptied capsule can be removed and a full one
inserted. The
device is then flipped shut or pushed shut.
The inner shape of the capsule chamber is typically such that it comprises a
cavity
open on two sides for accommodating a disposable capsule for pharmaceutically
active inhalable substances. Preferably, these two openings are provided at
13
CA 02513130 2005-07-12
opposite ends or immediately adjacent these ends. The inner form may for
example
be a preferably uniform cylinder or cuboid. Preferably the inner configuration
resembles a cylinder.
The dimensions of the capsule chamber are matched to those of the capsule. The
cavity preferably has a diameter which is 1.1 to 2.5 times as great as the
capsule
diameter. Preferably, the cross section is 1.1 to 2.2 times, particularly 1.2
to 1.6
times as great as the capsule diameter.
The length of the inner cavity of the capsule chamber is 1.02 to 2 times as
great as
1o the length of the capsule, preferably 1.04 to 1.8, particularly 1.1 to 1.6
times as great
as the length of the capsule. The diameter of the chamber should be less than
the
length of the capsule, so that the capsule is held in the longitudinal
direction in the
chamber and cannot tilt to one side.
As an illustration some examples of typical capsule dimensions will now be
given, indicating the size of the capsule chamber.
Total length of the closed capsule: 26.1 ~0.3 mm; 23.3 ~0.3 mm; 24.2 ~0.3
mm;21.7~0.3mm; 19.4~0.3mm; 18.0+0.3mm; 15.9+0.3mm; 14.3+0.3
mm; 11.1 +0.3 mm.
2o Outer diameter of the capsule body: 9.55 mm; 8.18 mm; 7.36 mm; 7.34 mm;
6.63 mm; 6.07 mm; 5.57 mm; 5.05 mm; 4.68 mm.
External diameter of the capsule caps: 9.91 mm; 8.53 mm; 7.66 mm; 7.64
mm; 6.91 mm; 6.35 mm; 5.83 mm; 5.32 mm; 4.91 mm.
The standard commercial capsules are size 3, which is known at least in
Germany.
2~ In the telescopic capsules described the diameter of the upper part is 5.83
and the
diameter of the lower part is 5.57 mm.
The capsule chamber has two openings, an inlet for incoming air and an air
outlet.
The air inlet is smaller in cross section than the capsule chamber so that in
this
so region of the capsule chamber the flow velocity of the air is relatively
high and a
powder in the capsule is delivered by the Bernoulli effect. The air inlet
opening is
14
CA 02513130 2005-07-12
conveniently arranged centrally in the base of the chamber. On the air outlet
side
there may be a perforated plate or other device such as projecting components
to
prevent a capsule moving in the capsule chamber from blocking the air outlet
or any
capsule fragments formed from being sucked into the mouthpiece. The perforated
plate may for example be part of a funnel-shaped connecting member which can
be
fitted on to the start of the inhalation channel leading to the mouthpiece in
such a way
that the edge of the funnel with the perforated plate engages in a plate-
shaped insert
which forms the base of the mouthpiece. The perforated plate may, however,
also
be replaceably fixed by jamming it between the funnel edge of the connecting
1o member and a stop of the plate-shaped insert.
A plurality of openings may also be provided as the outlet opening. The cross
section available for the air to flow out of the capsule chamber is
conveniently greater
at every point than the air inlet opening so that the air charged with the
pharmaceutical composition can flow out unimpeded as far as possible. The air
outlet opening is expediently arranged centrally in the top of the chamber but
may
also be arranged to one side in the top region.
The provision of the two openings is intended to guide an air stream axially
through
2o the capsule chamber.
The capsule chamber has at at least one point along its longitudinal axis (in
relation
to the interior of the capsule chamber) an opening for or a connection to a
cutting
device which is provided with at least two sharp spikes or cutters for
piercing or
cutting open a capsule located in the capsule chamber. The cutting device is
moveable into the interior of the chamber counter to the pressure of the
spring and is
operated by means of a spring mounted actuating button. As the height of the
capsule chamber is determined by the length of the pharmaceutical capsules,
the
points or cutters of the cutting device are preferably located close to the
top or
so bottom end of the capsule chamber. The side wall of the capsule chamber may
have
radial bores or oblong slots in the region of its top and bottom end which
face the
CA 02513130 2005-07-12
spikes or cutting edges and serve to allow the spikes or cutters to pass
through. The
dimensions of these bores/slots are matched to the cross section of the spikes
or
cutting edges.
In a preferred embodiment of the Bernoulli inhaler according to the invention
the
guide for the spikes of the cutting device comprises a sealing plate. In this
way the
seal between the capsule chamber in the inhaling position and the cutting
device is
improved. For the spring mounting of the sealing plate it is possible to use
the spring
which resets the actuating button for the cutting device.
Finally, in another embodiment, a lever system is provided for actuating the
cutting
device. This lever system is preferably actuated by an actuating button
mounted on
the base or side of the housing of the inhaler. The lever system may consist
of a
rocker and a toggle lever, while the actuating button acts on one end of the
rocker
and the other end of the rocker presses on one end of the toggle lever, the
other end
of the toggle lever secured to the cutting device pushing the cutting device
forward.
The rocker and toggle lever are preferably mounted to be pivotable about axes
in
holders secured to the housing.
2o The capsule is supposed to be opened close to both its ends for the
inhalation
process. The hemispherical caps of the capsule should not be damaged thereby.
This is important because the capsule or caps of the capsule act as a sort of
valve.
Because of the pressure conditions the capsule is pulled towards the inlet
opening
counter to the inflowing air and closes it off. As the user continues to suck
on the
mouthpiece, suction is produced in the capsule chamber by which the capsule is
pulled towards the air outlet with the inflowing air. The suction now formed
at the air
inlet ensures that the capsule is pulled towards the inlet opening again. The
entire
process is repeated in rapid succession as long as the patient continues to
inhale
through the mouthpiece and sets the capsule vibrating strongly in the axial
direction.
16
CA 02513130 2005-07-12
Preferred Bernoulli inhalers are those described hereinbefore as embodiments
of DE
3345722, WO 91 /02558 or EP 0911047. Reference is hereby made once again to
the features mentioned in this section. The inhaler as described hereinbef~re
in
connection with EP 0911047 is particularly preferred.
In inhalers of this kind there can only be one capsule chamber according to
the
invention, in accordance with the remarks on DE 3345722 or EP 0911047.
However, the capsule chamber may also be part of a capsule chamber magazine as
described in WO 91/02558.
An inhaler of this kind has a revolver magazine with a plurality of usually
tubular
chambers each loaded with one capsule. The magazine is covered at each of its
two
open ends by a plate, one plate containing the air inlet opening and axially
thereto
the other plate containing the air outlet opening. As the magazine is
rotatably
mounted within these plates, one of the chambers can be pivoted into place
between
the two openings and thus form part of the continuous channel for the inhaled
air.
After an inhalation process has ended the revolver magazine is further rotated
until
the next chamber enters the air throughflow channel. One of the two plates may
be
separated from the magazine, for example, in order to remove used capsules
from
2o the chambers, or else the entire magazine can be removed for refilling, for
example.
According to this feature of the invention the revolver magazine is releasably
mounted in the inhaler housing. After the capsules in the revolver magazine
have
been used the entire revolver magazine can be replaced or refilled with
capsules.
The inhaler housing may have an eccentrically mounted pin on to which the
revolver
magazine can be fitted.
In order to fix the position of the revolver magazine it may be provided with
recesses
so associated with the capsule chambers for a spring-mounted locking bolt
arranged in
the inhaler housing. The recesses are arranged so that the locking bolt only
engages
17
CA 02513130 2005-07-12
therein when one of the capsule chambers is located precisely between the air
inlet
and outlet.
In this way it is possible to ensure that the revolver magazine does not move
during
the inhalation. The spring mounting of the locking bolt should be selected
with
regard to the spring constant so that accidental rotation of the revolver
magazine is
prevented by the locking but on the other hand if greater force is applied the
revolver
magazine can be rotated out of its locked position. Conical shapes for the
free end
of the locking bolt and suitably shaped recesses have a supporting effect.
The locking bolt is preferably arranged coaxially with the air throughflow
channel
underneath the capsule chamber and has a through-bore which simultaneously
forms the air inlet in the base. Preferably, the locking bolt is centrally
mounted in the
inhaler housing. According to another embodiment of the invention the locking
bolt is
1s acted upon by a spring the other end of which abuts on a stopper releasably
fixed in
the inhaler housing, which also has a central through-bore which is part of
the air
throughflow channel.
In a preferred embodiment the recesses for engagement of the locking bolt in
the
2o base are arranged in the base plate of the magazine, concentrically with
the air inlet
bores of the capsule chambers and like the casing constructed in the form of a
flat
truncated cone with its base facing outwards. Thus, these recesses are conical
or
funnel-shaped widenings of the air inlet bores, the widened area facing the
locking
bolt. The slopes produced by the widening correspond approximately to the
chamfers
2~ on the top of the locking bolt.
In a preferred embodiment these recesses have an encircling stop edge on the
base
of the casing of the truncated cone, but also in the base plate, which acts as
a
rotation preventer or stop for the head of the locking bolt when the latter
has engaged
3o in the corresponding recess. Because of this stop edge the magazine cannot
be
turned any further once the locking bolt has engaged.
18
CA 02513130 2005-07-12
According to another feature of this embodiment the said stop edge takes up
only
part or half of the periphery of the conical recess, i.e. the funnel-shaped
widening,
and is arranged so that when the locking bolt is engaged it prevents rotation
of the
magazine in one direction but allows it in the other direction, as the sloping
wall of the
funnel-shaped widening merges smoothly into the exterior of the base plate.
In another preferred embodiment only one of the recesses has a stop edge which
takes up the entire circumference of the recess so that when the Pocking pin
is
1o engaged it is impossible for the magazine to rotate in this recess. This
position is
then regarded as the end position of a magazine in which all the capsules have
been
used. In this embodiment, all the other recesses only have a rotation
preventer on
one side, i.e. effective in one direction, so that the magazine can only ever
be rotated
in the direction in which a capsule chamber containing an unused capsule is
brought
into play, until the end position described above in which locking is complete
is
reached. The user then knows that the magazine has to be loaded with fresh
capsules once this last capsule has been used.
In another preferred embodiment a tongue may be fixed to the locking bolt
which
2o extends as far as a stop on the inside of the operating button of the
cutting device
when the locking bolt assumes its upper stop position with the revolver
magazine
removed. In this position the said tongue acts as a barrier for the cutting
device.
When the magazine is inserted the locking bolt is pressed down again and in
this
way the barrier for the cutting device is removed.
The actuation of the cutting device may also be coupled to the rotary movement
of
the capsule magazine, so that at the press of a button first a capsule chamber
is
brought into the correct position and then the cutting device is engaged.
so If the revolver magazine and the part of the inhaler housing adjacent
thereto are
constructed with n angles, where n is a whole number indicating the number of
19
CA 02513130 2005-07-12
capsule chambers, the side surfaces of the inhaler housing part and of the
revolver
magazine may advantageously be aligned when the magazine is in the correct
position. It is then possible to determine immediately from outside whether
the
chamber is located in the air channel defined by the air inlet and the air
outlet.
In addition to the inhalers mentioned, the invention may also be used in
inhalers as
disclosed by DE 3336486 (US 4627432 , US 4778054), DE 3348370 (US 4627432,
US 540203), DE 4106379 (US 5590645, US 5860419, US 5873360, US 6032666,
US 20020053344, US 20020066451, US 6378519) or for example DE 3274065 (US
~ 0 4524769).
Characteristic features thereof may be found in the section on prior art.
The inhaler according to the invention makes it possible to deliver the
pharmaceutical
~5 composition more reliably than with devices known from the prior art, with
lower
standard deviations, and ensures good cleaning thereof.
It could not have been foreseen that the structured surface structures with
raised
portions and depressions in a powder inhaler would not have a detrimental
effect on
2o the characteristics of delivering a powdered formulation as an aerosol.
In particular, there was a fear that solid particles would adhere more
strongly to a
structured surface than to a smooth one and hence would lead to poorer
delivery of
the powder inhalers and/or contamination of the surtace.
However, it has been found that the powder particles suitable for
administration by
inhalation do not adhere any more strongly to a microstructured surface than
to a
smooth surface and any particles adhering can be removed without trace using
water. Drops of water landing on it force their way through the
microstructures and
so pick up any particles lodged therein.
CA 02513130 2005-07-12
Instead of or in addition to the remarks relating to powder inhalers, the
inside or
outside of the supply capsules may also be provided with the microstructured
surface
according to the invention. A capsule of this kind is preferably cylindrical
with
tapering ends. It consists of at least two partial elements fitting
telescopically into
one another. Preferably, these capsules have a longitudinal axis and a shorter
transverse axis. The longitudinal axis is the one running parallel to the
generatrices
of the cylindrical casing. The longitudinal axis is longer than the transverse
axis, so
that the longitudinal section of the capsule is oval while the cross section
is circular in
shape.
Preferably, the minimum of two partial elements are pushed into one another in
the
direction of the longitudinal axis.
Details of the capsule construction can be found in the section on prior art
and
elsewhere in this description.
In particular, details can be found in EP 1100474, to which reference is
hereby
expressly made.
2o Analogously thereto, the blister packs (DE 3348370, DE 3336486, DE
4106379) or conveyor belts (US 4524769) mentioned for the inhalers
described above may also be coated on their inside and outside with
microstructured surfaces of this kind.
Such blisters may have a blister bed with well-like depressions or cups
sealed off by an overlying film.
In these blisters, the cups may be arranged side by side as in a string of
pearls or may be arranged in rows. The blister beds may consist of a plastic
so or aluminium foil. The same is also true of the sealing films.
21
CA 02513130 2005-07-12
Materials which may be used are those disclosed in the prior art, such as
plastics, aluminium foils, etc.
The invention will be described in more detail hereinafter by means of
Examples and Figures.
22
CA 02513130 2005-07-12
Examples
Example 1
A smooth surface of plastics such as Resopal or polyethylene is provided with
a thin
even coating of an adhesive such as UHU PLUSs and then coated with a Teflon
powder such as Hosta flons TF 9205 (average particle size 7 microns). After
curing a
surface is obtained from which deposited particles such as soot and paint
powder
can be rinsed off with water.
Example 2
~o A smooth hydrophobic material such as PTFE is heated until it is
plastically
deformable. Then a high mesh screen from offset printing is pressed onto the
surface
and removed again.
After cooling, a surface is obtained with a regular arrangement of elevations
and
~5 depressions of similar height.
By using different screens of different mesh size and thickness, the
dimensions can
be altered and adjusted to an optimum. The properties of the surfaces thus
obtained
are optimum when the elevations have rounded tips. These surface structures
may,
20 of course, also be produced by heated stamping tools or rollers.
Corresponding films
may be adhered to a different smooth substrate.
Example 3
The method of adhering fenoterol to polyester films with a structured acrylic
layer is
25 investigated.
Film 1 bears structures in the region of 0.5 microns.
Film 2 bears structures in the region of 2 microns.
Film 3 bears structures in the region of 2 microns, with a 10 micron
superstructure.
A polyester film with an unstructured acrylic layer is used as reference.
3o The films of Example 3 were each stuck into the lid of a container. Defined
amounts
of powdered fenoterol were applied to the film sections using a cascade
impactor.
23
CA 02513130 2005-07-12
The impactor was operated at a flow rate of 39 I/min and the film sections
were
placed at precipitation stage 2. This test set-up makes it possible to deposit
on the
films defined amounts of powder with particles having an aerodynamic diarneter
of
about 4.3 to 5.2 microns.
The heaps of powder do not form a monolayer but rather an agglomerate of
particles
of powder. The adhesive forces between them differ from those between the
powder
and the film. The lid is placed on a container and put in a centrifuge. By
running it at
various speeds the powder is detached from the film by centrifugal force and
spun
~o into a collecting container. Then the difference between the mass of powder
in the
collecting container and on the film section in the lid is determined.
Results:
If the percentage of powder detached is plotted as a function of the ratio G
of
~5 centrifugal force to the weight force of the particles this function is
shown as
a sigmoid curve which rises sharply at first, becoming convergent at higher
G levels. The G value is also a measurement of the adhesion of a powder
particle to the surface of the film.
2o It is found that the curves plotted so not differ significantly for any of
the films
and no difference can be observed from the unstructured control material.
What is more or less common to all the curves is that the powder only begins
to be detached from the films upwards of G values of 200 to 800. At G values
of 57000 the percentage quantity of powder detached is from 47 to just 60%.
25 For
Film 1 the value G = 57000 is between about 49% and 56%,
Film 2: the value G = 57000 is between about 46% and 57%,
Film 3: the value G = 57000 is about 60%.
unstructured reference film: the value G = 57000 is about 47 to just 60%
24
CA 02513130 2005-07-12
The curves measured lead one to conclude that on structured surfaces the
characteristic structural size of which is less than the diameter of the
particles, the forces required to detach the particles from the surface are no
greater than on an unstructured surface, i.e. no higher adhesive forces occur
which would lead to heavier deposits.
Figures
Figure 1 shows a typical two-part capsule which may be provided with the
microstructured surface according to the invention.
Figure 2 shows an inhaler in which the capsule according to the invention
can be used.
Figures 3 a to d show a powder inhaler with a revolver magazine in which the
capsule according to the invention can be used.
Figure 4 shows a powder inhaler with an upper and lower part movable
relative to each other.
2o Figures 5 to 9 show examples of surtace structures.
Figure 1 shows a capsule (1 ) known from the prior art, consisting of a
capsule cap (2~
and a capsule bob (3). It is apparent that the outer diameter ofthe capsule
body is
smaller than that of the capsule cap over wide areas. This isparticularl~~
noticeable
in the region of the hemispherical end of the capsule body at the bottom.
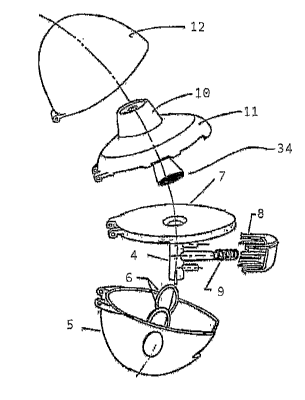
Figure 2 shows how an inhaler may be constructed in which a capsule
chamber according to the invention is integrated. Located in a lower part (5)
optionally with two windows (6) is a plate (7) connected to the capsule
so chamber (1 ). The capsules in the capsule chamber (1 ) are opened by means
of a button (8) provided with two specially sharpened spikes which is pressed
CA 02513130 2005-07-12
in counter to the pressure of the spring (9) and thereby cuts open or pierces
the capsule in the chamber in two places. As the user inhales through the
device using the mouthpiece (10) which is connected to the upper part (11 ),
the air enters the lower part (5) and from there goes into the capsule
chamber (4) at the lower end. The device is closed off by a lid (12), which is
hinged to the lower part (5), the plate (7) and the upper part (11 ), so that
when the lid is closed dust cannot enter the device. In the plate (7) there
may
optionally be capsule holders in the form of blind bores. Advantageously,
there is a perforated plate (34), which is fixed to the lower end of the mouth
~o tube (10) or of the inhalation channel leading to the opening of the
mouthpiece and, when the inhaler is in the closed position, covers the air
outlet opening of the capsule chamber (4). The drawings do not show
optional snap-fit hooks on the side of the mouth tube (10) or of the upper
part
(11 ) which is oriented towards the plate (7), which are capable of engaging
in
the plate (7). In this case the plate (7) has suitably complementary devices
(depressions or holes). Projections or snap fit hooks may also be provided
laterally on the plate (7), for example to enable the plate (7) to engage in
the
lower part. The above mentioned devices for engaging the mouthpiece (10)
or upper part (11 ) in the plate (7) or the plate (7) in the lower part (5)
are such
2o that the individual elements can easily be separated from one another
again.
In addition, a lug may be formed on the point on the lid (12) which is located
above the button (8) in the closed position so that this lug engages in a
depression on the top of the button (8) and blocks the button (8), so that the
button (8) cannot be pressed in the closed position. This prevents the
capsule from being accidentally perforated prematurely once it has been
inserted in the capsule chamber.
Figure 3: As can be seen from Figs. 3a, 3b and 3c, an inhaler with a revolver
magazine consists essentially of an inhaler housing (5) with a mouthpiece (10)
which
3o is hinged laterally to the upper edge of the inhaler housing (11 ) so as to
be pivotable
about an axis (13), and a revolver magazine (14) with the capsule chambers (4)
for
26
CA 02513130 2005-07-12
accommodating the capsules. The revolver magazine (14) can be fitted on to a
pin
(15) eccentrically mounted in the inhaler housing (5). After the revolver
magazine
(14) has been pushed on the mouthpiece (10) is moved into its normal position -
as a
cap on the housing; the inhaler is ready for use. A capsule (not shown) can
now be
pertorated by pressing the button (8). As can be seen from Figure 3c, the
revolver
magazine (14) in this case has 6 chambers (4) for accommodating the capsules
(not
shown). The base of each chamber (4) has an air inlet bore (16). In addition,
the
revolver magazine (14) has an axial guide (17) for the pin (15).
~o As may be seen from Figure 3d, the inhaler has, adjacent to the chamber (4)
mounted underneath the inhalation channel (18), the cutting device (19) which
is
operated by means of the button (8). This cutting device (19) has two spikes
(20)
which can be radially inserted into the upper and lower part, respectively, of
said
chamber (4), the outer wall of the revolver magazine having weakened or
frangible
~5 regions (21 ) at suitable points to assist the insertion of the spikes (2).
The spikes
(20) serve to open the capsule located in the chamber (4) close to the upper
and
lower ends thereof. The revolver magazine (14) also has, underneath the bores
(22),
conical recesses (23) in which a locking bolt (24) can engage as soon as the
corresponding chamber (4) is coaxial with the air inlet or inhalation channel
(18) of
2o the inhaler housing. The locking bolt is also conically formed at its end
engaging in
the recess (23). At the opposite end it is acted upon by a spring (26) which
bears on
a stopper (27) releasably fixed in the inhaler housing. This stopper, like the
locking
bolt, has a central through-bore which acts as an air inlet (25).
25 in order to prepare the inhaler, with the revolver magazine (14) in place,
this
magazine is rotated so that one of the chambers (4) is brought into a position
in
which the bore (22) in the base or the conical recess (23) is aligned
coaxially with the
air inlet opening (25). The positioning of the chamber (4) is made easier by
the
engagement of the locking bolt (24) in the recess (23). After the bolt has
engaged,
so the air inlet opening (25) and the base opening (22) in the chamber (4) are
in
alignment. The cap of the capsule is positioned on the base opening (22) and
closes
27
CA 02513130 2005-07-12
it off. By actuation of the button (18) counter to the force of a spring (9)
the cutting
edges (20) are moved radially towards the chamber (4), first piercing the
weakened
regions (21 ) or entering corresponding openings in the side wall of the
revolver
magazine and finally opening the capsule at the top and bottom close to its
ends.
The tapering caps of the capsules should not be destroyed as they are intended
to
act as a kind of valve.
When air is then sucked through the mouthpiece (10), the air flowing into the
chamber (4) from the base openings (28) in the housing (5) and the air inlet
(25) sets
~o the capsule vibrating violently, produces turbulence in the powder in the
capsule,
mixes with it and is finally inhaled. The mouthpiece (10) is generally tubular
in
construction but may also be adapted to the shape of the mouth and flattened.
Similarly, as an alternative to the embodiment shown, the mouthpiece may be
arranged axially or at an angle to the axis of the chamber or laterally offset
from the
~5 axis of the chamber.
At the base, the mouthpiece (10) may be provided with a plate-shaped insert
(29)
which is essentially solid. This plate-shaped insert (29) may also have
perforations,
however. Moreover, the start of the inhalation channel (18) may be covered
with a
2o screen which prevents the capsule or capsule fragments from being inhaled
into the
inhalation channel (18) in the mouthpiece. Alternatively, projections may be
provided
on the wall at this point to hold the capsule back. The perforated plate is
then
preferably arranged in the centre of the plate-shaped insert (29),
advantageously
clamped between a stop (30) on the plate (29) surrounding the air throughflow
and
25 the edge of a funnel-shaped connecting member (31 ), which is fitted on to
the
beginning (32) of the inhalation channel (19) in such a way that the edge of
the
funnel faces the plate-shaped insert (29) and engages therewith. The
alternatively
provided projections may also be arranged at this point.
so The embodiment of the inhaler according to the invention as shown in Figure
4
consists of the lower part (5) and the mouthpiece (10), which are fitted
together. The
28
CA 02513130 2005-07-12
lower part contains the air inlet channel (25) which is connected to the air
inlet into
the capsule chamber (1 ). The cutting device (19) is held in its normal
position by a
spring element (9). The mouthpiece (10) contains the capsule chamber (4).
Projections (33) which limit the play of the capsule project into the
extension of the
capsule chamber. A perforated plate (34) prevents fragments of capsule from
being
inhaled, for example. The inhaler may be axially compressed counter to the
pressure
of a spring element (35), the upper edge of the lower part reaching the
position (36).
In this position the blades or points (20) of the cutting device (19) may
penetrate
through the opening (21 ) into the capsule chamber (4) and open the capsule
secured
~o therein.
In order to use the inhaler according to Fig. 4 the lower part (5) and
mouthpiece (10)
are pulled apart, the capsule is inserted and the two parts of the inhaler are
fitted
together. After being pressed back into position (36) counter to the spring
element
~5 (35) the cutting device (19) is actuated and released again. Under the
pressure of
the spring element (35) the inhaler returns to the initial position shown in
Figure 4.
The active substance formulation from the capsule (not shown) can now be
inhaled
by breathing in through the mouthpiece (10).
2o Figures 5 to 9 show examples of surface structures, specifically the
surface
structures of the films according to Example 3.
Film 1 with structures in the region of 0.5 microns,
Film 2 with structures in the region of 2 microns,
Film 3 with structures in the region of 2 microns and 10 microns of
superstructure.
29