Note: Descriptions are shown in the official language in which they were submitted.
CA 02513667 2005-07-15
WO 2004/067133 PCT/US2004/001119
CYCLIC MEMBRANE SEPARATION PROCESS
FIELD OF THE INVENTION
This invention relates to a cyclic process using a selectively gas permeable
membrane to separate a component from a gas mixture. More specifically, it
relates to a
membrane separation process useful for recovery of volatile organic compounds
emitted
from storage tanks utilizing a membrane comprising a selectively gas permeable
membrane polymer. The process includes repetitively cycling between flow and
non-
flow of gas through the membrane.
BACKGROUND OF THE INVENTION
Liquid volatile organic compounds ("VOC") are stored and dispensed from tanks.
A very common example is in the field of distribution of combustion engine
fuel such as
gasoline for fueling automobile and aircraft engines. The storage tanks
usually have large
capacities, receive'bulk shipments of fuel from a supply source and dispense
smaller
amounts in multiple events, e.g., filling individual automobile tanks at
service stations.
The gas space above the liquid in the tank is sometimes called the "ullage" of
the tanlc.
Usually there is a high concentration of VOC in the ullage of fuel storage
tanks.
Prior to the time that air pollution by VOC emissions became an environmental
protection concern, emission controls on storage tanks were directed mainly to
preventing
fire and explosion hazards. Few controls were aimed at curbing fugitive
emissions such
as emissions of VOC incidental to dispensing fuel from bulk storage tanks and
to storing
the fuel in the tanks.
More recently heightened awareness has developed of the need to reduce
fugitive
emissions resulting from storage and dispensing of VOC. As a result vapor
recovery
systems for VOC with increasing degrees of sophistication have been deployed.
For
example, to reduce enviromnental emissions of VOC vapor during automotive and
other
types of fuel fill-ups, fuel suppliers and distributors have begun to install
vapor recovery
systems at fuel dispensing stations. Such systems usually have suction
equipment that
draws VOC vapors and air present during fuel transfer at the fuel dispensing
nozzle back
to the ullage of a bulk storage tank. The returning gas mixture enters the
void in the tank
created when the dispensed liquid leaves.
1
CA 02513667 2005-07-15
WO 2004/067133 PCT/US2004/001119
Traditionally storage tanks merely had P/V valves (Pressure-Vacuum valves)
intended to maintain the tank within a range of slight positive and negative
pressure, i.e.,
a few inches of water pressure. The returning gas mixture from dispensing
operations, as
well as other factors, caused pressure to build up in the ullage over time. Of
course, when
tank pressure exceeded the upper limit of the P/V valve, excess gas containing
VOC was
discharged to the environment.
Certain advanced VOC fugitive emission control systems are designed to operate
with a slight negative pressure in the ullage of the bulk storage tank. That
is the tank is
under a vacuum relative to ambient atmosphere. Such systems offer the
advantage that
any leaks that occur will cause outside air to flow into the vapor recovery
systems, rather
than allow vapor to escape to the atmosphere. In addition to the gas buildup
mentioned
earlier, air in-leakage contributes to pressure increase in the tank. The
liquid fuel
evaporates into the incoming fresh air and the mass of the vaporized fuel plus
the mass of
air within the fixed ullage volume increases the pressure. Negative pressure
thus can only
be maintained if gas is exhausted to the environment from time to time.
However, it is
necessary to strip all or a portion of the VOC from the exhausted gas.
Otherwise, the
VOC in the discharged gas defeats the purpose of the pollution control system.
Various techniques have been proposed to remove VOC emissions from bulk
storage tanks operating at subatmospheric pressure. A method gaining
commercial
acceptance uses a selectively gas permeable membrane to separate the VOC
component
from the benign air component of the ullage mixture. The non-VOC component,
composed primarily of nitrogen and oxygen, is preferentially permeable through
the
membrane and is emitted to atmosphere substantially free of the VOC component.
VOC
is less permeable, largely does not pass through the membrane and is returned
to the
storage tank.
The membrane separation vapor recovery system is contemplated to operate
cyclically and emit to atmosphere discontinuously. Emissions occur only when
the tank
pressure exceeds a pre-selected high pressure limit. At other times, flow
through the
membrane is stopped. For example, tank pressure descends below the high
pressure limit
as a consequence of discharging primarily non-VOC component gas to the ambient
atmosphere. At a preselected low pressure limit, discharge stops., At these
times, the
vapor is stagnant in the separation membrane module and in the gas transfer
lines
immediately upstream and downstream of the module.
2
CA 02513667 2008-09-15
Although the separation membrane selectively permeates oxygen and nitrogen, it
does not absolutely reject VOC compounds. Consequently, the gas that permeates
the
membrane and is vented to the environment includes some VOC vapor, albeit less
than
that which would vent had the membrane not been utilized. It has been
discovered that a
very high concentration pulse of VOC vapor emits from the membrane module at
the start
of a venting cycle, i.e., directly after rising tank pressure initiates flow
through the
membrane and venting commences at the end of a stagnant period. After a while,
the
concentration of VOC in the permeate/exhaust gas decreases to a steady state
value in the
expected manner. A significant quantity of VOC vapor is released to the
atmosphere by
the time the gas venting portion of the cycle stops. As a result, the time-
averaged quantity
of VOC compounds discharged to the air is still unacceptably high.
It is desirable to reduce overall emissions of VOC compounds below that which
results from conventional separation membrane-based, fuel tank vapor recovery
systems.
SUMMARY OF DRAWINGS
Embodiments of the invention will now be described with reference to the
accompanying drawings, in which:
Fig. 1 is a schematic diagram of a conventional apparatus for carrying out a
cyclic
gas separation process;
Fig. 2 is a typical plot of VOC concentration in gas emitted vs. time for
single
cycles of operation of a conventional vapor recovery system as shown in Fig. 1
and of a
vapor recovery system according to an embodiment of the novel process;
Fig. 3 is a schematic diagram showing an embodiment of the vapor recovery
system according to the present invention in which the membrane is a glassy
polymer;
Fig. 4 is a schematic diagram showing another embodiment of the vapor recovery
system according to the present invention in which the membrane is a rubbery
polymer;
and
Fig. 5 is a schematic diagram showing another embodiment of the vapor recovery
system according to the present invention.
DETAILED DESCRIPTION
With reference to the schematic flow diagram of Fig. 1 it is seen that a
traditional
liquid fuel dispensing system includes a bulk fuel storage tank 1 that
contains an inventory
3
CA 02513667 2008-09-15
of liquid fuel 2. The volume of the tank above the liquid level 3 is known as
the ullage 4.
The liquid fuel is typically a highly volatile organic compound ("VOC") and
therefore, the
ullage is occupied by a gas composition which is highly concentrated in VOC
vapor. In a
typical fuel dispensing operation adapted to refuel automobile tanks with
gasoline, the
liquid gasoline is withdrawn from the storage tank via a transfer line 5 that
leads to a
pumping station 6. The gasoline is dispensed by fuel dispensing pump 7 via a
hose 8
through nozzle 9 into the filler tube 11 and mobile tank in automobile 10.
Modern conventional fuel dispensing systems also typically include a vapor
capturing apparatus 12. Typically, the vapor capturing apparatus is a part of
the fuel
filling nozzle. The capturing apparatus 12 is designed to draw into vapor
transfer line 13
fugitive VOC vapor emitted as the fuel pours into the filler tube. This vapor
is generated
by a small amount of the volatile liquid fuel vaporizing while going into the
tank and by
VOC vapor in the automobile tank that is displaced by the incoming liquid
fuel. The
capturing apparatus may also draw in some air that leaks through gaps in the
seal between
the capturing apparatus and the filler tube.
3a
CA 02513667 2005-07-15
WO 2004/067133 PCT/US2004/001119
Preferably the ullage of the bulk storage tank and the vapor transfer line 13
are
maintained at subatmospheric pressure so that any leaks draw vapor into the
tank. This
prevents pollution of the environment that might occur by VOC vapor leaking
out if the
pressure was positive relative to atmospheric pressure. Some dispensing system
designs
utilize a vacuum pump in vapor transfer line 13 (not shown). This supplements
the
vacuum driving force provided by the low pressure at the storage tank to
capture fugitive
emissions at the nozzle. Many independently operating dispensing stations can
be
connected to the bulk storage tank although only one is illustrated. In
installations with
multiple storage tanks, a common vapor transfer line is often used.
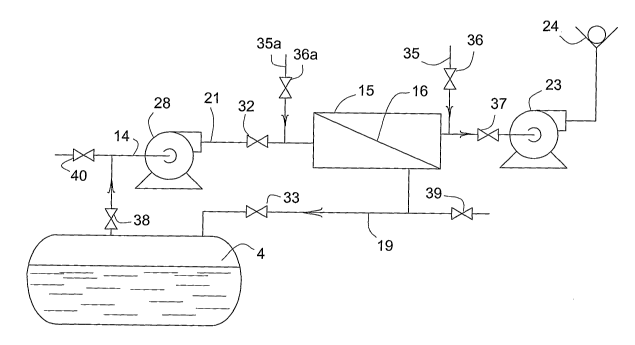
The bulk storage tank is equipped with a vapor recovery system 20 which
includes, among others, elements 28, 15, 23, 24 and connecting transfer lines.
Recovery
system 20 functions in large part to generate the subatmospheric pressure
within the
ullage 4. Gas in the ullage is drawn out by gas conveying device 28 and is
blown through
membrane module 15. Then the gas is exhausted to atmosphere through vent line
25. A
second gas conveying device 23 additionally helps remove the vented gas.
Operation of the vapor recovery system 20 takes place in repetitive cycles
that
each have two sequential segments. Customarily, the system operation cycles
between
segments as needed to maintain pressure in the ullage at a vacuum between a
lower
vacuum limit and an upper vacuum limit. As fuel is dispensed at station 6
vapor
continues to feed into the ullage 4 through line 13 and pressure in the ullage
can increase
or decrease depending on the volume ratios of fuel dispensed to the gas
returned to the
ullage, but it usually increases. Inward leakage will cause the pressure
inside the tank to
rise gradually. Pressure in the storage tank can also increase as a
consequence of refilling
of the tank itself, temperature changes, or evaporation of the liquid over
time. Upon the
pressure elevating to the upper vacuum limit, the first segment begins and the
gas
conveying devices 28 and 23 start running. As this first segment of operation
proceeds,
gas is expelled from the vent by continued operation of the gas conveying
devices and
pressure in the tank drops. When pressure drops below the lower pressure
limit, the
second segment starts and gas conveying devices shut down. This causes
movement of
the gases through the vapor recovery system 20 to cease. Pressure buildup then
resumes
and the cycle repeats.
In further detail, the vapor recovery system includes a vapor take off line 14
that
leads into a membrane module 15 which contains a selectively gas permeable
separation
4
CA 02513667 2005-07-15
WO 2004/067133 PCT/US2004/001119
membrane 16. The membrane divides the interior of the module into a feed-
retentate
chamber 17 and a permeate chamber 18 which are each in contact witli opposite
sides of
the membrane. The membrane material has the ability to pass certain components
of the
gas mixture taken from the ullage and to reject other components. Typically,
oxygen,
nitrogen and other minor concentration, low molecular weight gaseous
components
present in air, e.g., argon, ozone, carbon dioxide and the like, permeate
through the
membrane. VOC vapor molecules permeate very slowly compared to the other
permeating components. Consequently, the feed-retentate chamber becomes
enriched
with VOC which are returned to the bulk storage tank 1 througli return line
19. An air
conveying device 28, such as a vacuum pump, blower, fan or similar mechanism
forces
the VOC-enriched gas through the return line 19.
As a result of selective permeation, gas in the permeate chamber 18 has a
lower
concentration of VOC than the gas in the ullage of the tank. However, it may,
and
usually does, contain some small amount of VOC. Ordinarily, the cleaned air
with
reduced VOC contamination is drawn from the permeate chamber through line 22
utilizing air conveying device 23. This air can then be discharged to
atmosphere. A
check valve 24 or similar conventional flow control device can be employed to
prevent
ambient air from flowing baclcward through the vapor recovery system into the
storage
tank and raising the pressure in the tank. The main objective of the vapor
recovery
system is to discharge to the environment as little VOC as practicable.
The process and apparatus of this invention differ from conventional
technology
for vapor recovery of VOC primarily in that for at least a portion of the
second segment
of the cyclic process a quantity of diluent gas is charged into the membrane
module 15.
While the diluent enters, the valves in the vapor recovery system are adjusted
to direct the
flowing diluent in a manner that will be explained more thorougllly below. The
diluent
gas can be any gaseous composition that is free of the components rejected by
the
membrane, i.e., VOC. The diluent gas also should not be reactive with VOC at
conditions existing in the vapor recovery system. Examples of suitable diluent
gas
compositions include air, carbon dioxide, hydrogen, helium, nitrogen and
mixtures
thereof. Preferably, the diluent gas is air.
As a beneficial consequence of introducing diluent gas into the membrane
module
during the second segment of each cycle the amount of VOC emitted per cycle
from the
vapor recovery system to the environment is reduced. The precise reason for
the
5
CA 02513667 2005-07-15
WO 2004/067133 PCT/US2004/001119
reduction of VOC emissions discovered to occur when air is deliberately
charged to the
module during the second segment of each cycle is not presently understood.
Without
wishing to be bound by a particular theory, it is thought that the reduction
is based on two
phenomena. Firstly, during operation of a conventional vapor recovery system
as
depicted in Fig. 1, VOC are present in the module at the end of each first
segment. The
gas concentration in the feed chamber is the same as in the ullage of the
storage tank. The
second segment of each cycle typically lasts about 30 minutes and is much
longer than
the first segment. During this period the concentration of VOC on the two
sides of the
membrane equilibrates. Thus a comparatively large quantity of VOC migrates
into the
permeate chamber. At begimiing of the first segment of the next cycle the
quantity of
VOC residing in the permeate chamber flows forward to the vent transfer line
and
ultimately to the ambient environment. In accord with the novel process,
however, a
significant portion of the VOC present in the module at the end of the first
segment is
displaced to the storage tank by diluent gas before the next cycle first
segment starts.
Therefore less VOC is available to surge through the module and to exhaust
througli the
vent line at each first segment start-up.
Secondly, the diluent gas tends to purge the free volume of the selectively
gas
permeable membrane. By comparison, in the conventional process exposure of the
membrane to high concentration of VOC during the second segment causes VOC
present
in the module to occupy to a high degree the free volume of the membrane
composition.
The term "plasticizing" (of the membrane) is used to refer to this condition.
The
plasticized membrane is not in optimum condition to selectively permeate the
non-VOC
components during the first segment of each cycle. More specifically, a
membrane
plasticized with VOC would be expected to permeate a higher amount of VOC than
one
which is not plasticized. Under the novel process, the diluent gas sweeps past
or through
the membrane. This draws at least some VOC out of the free volume and thereby
places
the membrane in better condition to selectively permeate the gas mixture
components
during the first segment portion of the next cycle.
The novel process and system are thus adapted to remove preferably at least
about
5 % of the VOC that would otherwise be present at first segment start-up. More
preferably the amount removed is at least about 10 %, and most preferably at
least about
25 %. The VOC emissions will be less than would occur had the diluent gas not
been
6
CA 02513667 2005-07-15
WO 2004/067133 PCT/US2004/001119
added to the module during the second segment. Preferably, the VOC emissions
will be
reduced by more than 10%.
The effectiveness of the novel vapor recovery system can be explained with
reference to Fig. 2. Curve A represents typical performance expected during
one cycle
for a bulk fuel storage tank using a conventional vapor recovery system such
as that
shown in Fig. 1. It is a plot of the concentration in vent line 25 of VOC
content as
volume percent of the gas emitting from a hypothetical system. The
accumulation of gas
returning to the storage tank 1 from fuel dispensing operations and inward
leakage
increase the pressure within the storage tank to a pressure above the high
vacuum limit.
This triggers activation of the vapor recovery system. First segment operation
begins
with gas conveying devices 28 and 23 starting up (point Al). Detection of an
increase in
VOC concentration by a sensor in the vent line 25 occurs a short time, usually
about
several seconds later (point A2). As first segment operation of the recovery
system
continues ullage gas selectively permeates the membrane and displaces the
initially high
VOC concentration gas in the permeate chamber to the vent. This lowers the
pressure in
the storage tank and also causes the VOC concentration in the vent line to
climb steeply
(point A3). The membrane operates to reject VOC, and consequently, the
concentration
of VOC in the emitted gas peaks and begins to drop (point A4). Thereafter, the
VOC
concentration reduces gradually (point A5) and begins to approach a steady
state value.
The elapsed time between points Al and A6 is relatively brief, and usually
extends from
about 30 seconds to about 3 minutes. When a sufficient quantity of gas has
been vented
from the system to reduce the pressure in the storage tank below the lower
vacuum limit,
(point A6) an automatic control system causes the gas conveying devices 28 and
23 to
stop operating as the second segment begins. The second segment usually lasts
for a long
time as compared to the first segment. Not uncommonly, the duration is at
least about 15
minutes and can be in the range of about 30 minutes to about 1-3 hours or
still longer,
depending upon the sizes of the storage tank and the ullage gas volume and the
rate of in-
leakage. Breaks in the abscissa and curves in Fig. 2 indicate the extended
passage of
time. Because the sensor in the vent line 25 is normally distant from the
module and
3o because flow is stopped during the second segment, the concentration of VOC
remains at
a value VOCo. This value is well above zero because some VOC do permeate the
membrane during the first segment and are present in the vented gas. The first
segment of
the next cycle begins at point A1'.
7
CA 02513667 2008-09-15
Fig. 2 also shows curve B for the VOC concentration vs. time performance of a
similar VOC fuel dispensing unit in which the vapor recovery system is
operated
according to this invention. The events in the cycle occur at the same times
as just
described. That is, first segment occurs in the period between points B 1 and
B6. The
second segment period is from point B6 to point B 1'. However, some ambient
air is
charged into the membrane module during the second segment of operation. Due
to the
charging of air the peak concentration (point B4) is lower than the peak
concentration of
the conventional process. Also, because the module is purged with diluent gas
that is free
of VOC, the VOC concentration of gas in the vent line ultimately reduces to
near zero at
commencement of the next first segment (point B 1'). The rate of reduction of
VOC
concentration in the second segment (i.e., between points B6 and B1') depends
upon the
geometry of any particular system and the location of the sensor relative to
the position
where the diluent gas is introduced. In sum, the cumulative area under curve B
during
first segment (i.e., between points B 1 and B6) representing the total amount
of VOC
discharged to the environment per cycle, is less then occurs conventionally.
Various embodiments of the novel process and system will now be described with
reference to Fig. 3. Like parts in different drawing figures have common
reference
numbers.
In an aspect of the invention, the diluent gas is charged to the membrane
module at
a position in fluid communication with the permeate chamber of the module.
Hence in
one embodiment of the present invention the vapor recovery system is modified
to
incorporate automatic block valve 36 in diluent gas feed line 35. In
operation, valve 36 is
opened for a period of time during the second segment of the cycle. This
allows a supply
of diluent gas, preferably ambient air, to enter the permeate chamber. Under
the driving
force of pressure due to the vacuum condition then existing in ullage 4, the
air permeates
backward through the membrane and into the feed-retentate chamber. The fresh
air thus
dilutes the VOC in the permeate chamber and is thought to purge the
plasticizing VOC
from the free volume of the membrane material. At a time defined by a preset
time span, a
sensed VOC concentration in the system or a pressure in the system, backward
flow into
the ullage is stopped. This prevents further rise of the pressure in the
storage tank.
Stopping backward flow can be accomplished by closing valves 32 and 33, or
alternatively
closing valve 36. Before the first segment of the next vapor recovery cycle
starts, valves
32, 33, and 36 are reset respectively to open, open and closed conditions.
8
CA 02513667 2008-09-15
The position of valve 32 is not critical. It can be placed anywhere in feed
gas
transfer line 14, 21 between ullage 4 and the feed-retentate chamber. Valve
placement
proximate to the entrance of the feed-retentate chamber is preferred because
this reduces
the volume of dead space which the dilution gas encounters as it travels
toward the ullage.
Likewise, valve 33 may be placed anywhere in the retentate line between the
storage tank
and membrane module, but it is preferred to be proximate to the feed-retentate
chamber.
Similarly, diluent air feed line 35 can be placed downstream of gas conveying
device 23.
Naturally, this presumes that gas conveying device 23 and all other
intervening equipment
in the permeate discharge line from the air introduction point to the permeate
chamber of
the module do not significantly impede the backward flow of gas. It is
recalled that the
diluent air is charged during the second segment of vapor recovery operation
during which
gas conveying devices 28 and 23 are shut down.
Other variations of the above mentioned embodiment are also contemplated. For
example, line 35 can feed directly into the permeate chamber. Alternatively,
if air can
pass backwards through conveying device 23 when the device is stopped, the
function of
valve 36 can be replaced by valve 37 or a bidirectional valve replacing check
valve 24.
For example, check valve 24 may be replaced by a pressure-vacuum ("P/V")
valve, which,
during the second segment, will admit diluent air into the permeate chamber
until the
pressure gradient across the P/V valve drops below a preselected minimum
value.
In another aspect, the diluent gas is charged to the membrane module at a
position
in fluid communication with the feed-retentate chamber of the module. For
example,
diluent air would be charged through lines 35a, and controlled by valve 36a.
In operation,
at appropriate time, block valve 32 is closed while air flows in through valve
36a.
Because the ullage is at negative pressure relative to the incoming diluent
gas, the gas will
travel through the feed-retentate chamber and return to the ullage via line
19.
For better results, i.e., lower VOC emissions, the diluent gas should sweep
across
the feed-retentate surface of the membrane 16. Care should be exercised to
configure the
position of the incoming diluent gas line 35a to assure that a significant
portion of the gas
does not bypass contact with the membrane surface. This often can be
accomplished by
placing the feed line 35a and return line 19 at opposite ends of the feed-
retentate chamber.
9
CA 02513667 2005-07-15
WO 2004/067133 PCT/US2004/001119
Other contemplated variations are those in which the diluent gas feed line is
placed at a different position in the module feed line. For example, line 35a
can be
positioned in transfer line 14 upstream of gas conveying device 28. With line
35a in this
position, valve 38 should be closed during the second segment and gas
conveying device
28 can optionally be used to push air through the feed-retentate chamber.
Unless check
valve 24 is functional to prevent the escape of possible outflow, it is
recommended to
include an optional block valve 37 in the permeate discharge line for this
purpose.
In another possible variation, line 35a can be positioned in line 19 between
valve
33 and the feed/retentate chamber.
As mentioned, a major objective of the novel vapor recovery system is to
enable
reduced environmental emissions of VOC vapor while operating the vapor system
in fluid
conmiunication with the ullage of the storage tank at a pressure below ambient
atmospheric pressure. Lower emissions occur if a diluent gas is added to the
membrane
module during at least a portion of the second segment of each vapor recovery
operation
cycle. Certain control protocols are contemplated to achieve this.
According to one such protocol the diluent gas is charged to the module during
times that the pressure at a preselected position in the system is within a
predetermined
pressure range. Again, it is recognized that the start of the second segment
of the vapor
recovery cycle is characterized by a low pressure in the vapor handling
system. Charging
diluent gas to the module will cause the system pressure to increase. This
control
protocol provides that the diluent gas flows into the module until the system
pressure rises
to a predetermined upper pressure limit. This protocol can be implemented in
various
ways. For example, an electronic pressure sensor can be used to trigger
control of
admission of diluent gas. In another representative example, control can be
effected
mechanically, for instance by using a P/V valve at position 24, (Fig. 4) as
mentioned
previously.
A different operating protocol calls for charging diluent gas for a duration
effective to obtain a specified concentration at a position in the module or
connected
piping. That is, a sensor for a VOC concentration analyzer can be placed in
fluid
communication with the feed-retentate chamber or the permeate chamber. The
analyzer
should be capable of providing real-time analyses of VOC concentration and
generating a
signal for input to an automatic control system. The control system is adapted
to
CA 02513667 2005-07-15
WO 2004/067133 PCT/US2004/001119
manipulate valve 36 or 36a in response to the input signal. Such analyzers and
control
systems are well known in the art. An example of such a control system is the
C-series of
programmable logic controllers (PLC's) available from Omron Electronics LLC,
One
East Cominerce Drive, Schaumburg, IL, 60173. An example of an appropriate
analyzer
is the model 317WP nondispersive infrared hydrocarbon sensor manufactured by
Nova
Analytical Systems, LTD., 270 Sherman Ave. N., Hamilton, ON, CA, L8L 6N5. The
vapor in the module or piping at commencement of the second seginent will have
an
initial, relatively high, concentration of VOC. Upon introduction of the
diluent gas, the
VOC concentration will reduce. This protocol provides that the diluent gas
flows into the
module until the VOC concentration at the sensor position descends below a
predetermined lower concentration limit.
In another operating protocol the charging of diluent gas occurs for a
duration of
predetermined length of time. That is, at some time after start of the second
segment, the
gas dilution feed valve is opened to allow diluent gas to enter the module.
The valve
remains open only for a length of time selected in advance. At expiration of
the time
limit, the diluent gas feed valve is closed. Preferably, the diluent gas feed
period begins
simultaneously with commencement of the second segment. Flow rate of diluent
gas is
another parameter that can be adjusted to optimize the reduction of VOC
emissions. For
example, during the predetermined duration for gas charging, the flow rate can
be
maintained at a fixed value. Emission results can be observed for one or more
successive
cycles. Either the diluent gas flow rate, the diluent gas feed duration or a
combination of
rate and duration can be changed for different cycles to determine which
settings provide
optimum emission performance. In another contemplated embodiment, the flow
rate of
diluent gas can be throttled according to a predetermined program. That is,
while the
diluent gas valve is open the flow rate can be increased, decreased or
otherwise adjusted
for best results.
It should be understood that any combination of more than one of the above
mentioned control protocols can also be implemented. One of ordinary skill in
the art
given the teaching of this disclosure will be able to manipulate the control
variables to
achieve lowest VOC emissions without undue experimentation.
The novel process and system incorporates a module that comprises a
selectively
gas permeable membrane. Any membrane composition that has good selectivity for
components of air relative to VOC can be used. Usually the membrane is a
polymer
11
CA 02513667 2008-09-15
composition. VOC are known to be solvents for many polymers. Thus the membrane
composition should be inert to VOC.
Preferably the membrane should include a thin layer of selectively permeable,
high free volume, non-porous polymer. The non-porous layer can be supported on
a
porous substrate, such as a microporous hollow fiber. Representative polymers
include
polytrimethylsilylpropyne, polyperfluoro(allyl vinyl ether), copolymers of
2,2,4-trifluoro-
5-trifluoromethoxy-1,3-dioxole and tetrafluoroethylene (TFE), and certain
amorphous
copolymers of perfluoro-2,2-dimethyl-1,3-dioxole ("PDD"). Copolymers of PDD
are
particularly preferred in that they have a unique combination of superior
permeability and
selectivity for a variety of gas mixtures. Especially preferred are copolymers
of PDD with
fluoromonomers such as TFE, vinylidene fluoride, perfluoromethyl vinyl ether,
hexafluoropropylene, chlorotrifluoroethylene and mixtures thereof. Gas
separation
membranes comprising PDD are disclosed in US 5,051,114 (Nemser et al.).
The structure of the membrane module is not critical. Flat, pleated, spiral
wound,
ribbon tube and hollow fiber membranes can be used. Hollow fiber membranes are
preferred. Hollow fiber membranes can be assembled in large numbers within a
so-called
hollow fiber membrane module. The structure and method of hollow fiber
membrane
modules is well known in the art. For example, see US 3,339,341 (Maxwell et
al.) and US
5,985,002 (Grantham).
The disclosure above is directed largely to embodiments of this invention in
which
the polymer component of the selectively gas permeable membrane is utilized in
a so-
called glassy polymeric state. It is well understood in the field of polymer
physical
chemistry that amorphous polymers and amorphous regions of crystalline
polymers
undergo second order phase transitions defined by a glass transition
temperature ("Tg").
At temperatures well below Tg these polymers are hard, stiff and glassy
although not
necessarily brittle. In a temperature zone near Tg the polymers are leathery
and at
temperatures well above Tg they are rubbery.
The performance of selectively gas permeable polymer membranes is affected by
whether the polymer is glassy or rubbery. For example, the selectivity between
the
atmospheric gas species that are usually present in ambient air, i.e., oxygen,
nitrogen,
12
CA 02513667 2008-09-15
argon, carbon dioxide and the like, and vapor species of VOC is such that
glassy selective
polymers preferentially permeate atmospheric gas species relative to the VOC
species.
Conversely, rubbery selective polymers are preferentially permeable to VOC and
less
preferentially permeable to the atmospheric gases. The vapor recovery systems
shown in
Figs. 1 and 3 are designed to emit to atmosphere the permeate compositions.
Therefore
these systems utilize glassy polymer membranes which separate ullage gas into
a permeate
enriched in air and depleted of VOC vapor. The VOC vapor enriched retentate is
returned
to the storage tank.
It is contemplated that the novel process for reducing emissions of VOC can be
applied to a vapor recovery system that utilizes a rubbery polymer. Very
basically stated,
such a system differs from a glassy polymer membrane system in that the
retentate gas
composition of the former is emitted to atmosphere and the permeate gas
composition is
returned to the ullage of the storage tank. A gas recovery system of this type
is disclosed
in US 5,571,310 of Nanaji.
Fig. 4 illustrates a schematic flow diagram for the novel vapor recovery
system of
this invention that utilizes a rubbery polymer gas selective membrane 16r.
During the first
segment of cyclic operation, ullage gas flows from ullage 4 through transfer
lines 14 and
21 into the feed-retentate chamber 17 of module 15. Feed gas conveying device
28
pressurizes the feed to facilitate separation by membrane 16r and to force the
benign
retentate through the exhaust transfer line to vent valve 24. VOC components
preferentially transfer through the membrane into permeate chamber 18 and this
VOC
enriched composition is returned to the ullage via transfer line 19. A vacuum
pump 42
assists in drawing the permeate through the membrane.
At a suitable time as described above, the second segment of operation begins.
Vacuum pump 42 and gas conveying device 28 are stopped and valve 33 is closed.
Within
the second segment of operation, valve 36 is opened to admit a diluent gas,
preferably air,
from line 35 into the feed-retentate chamber 17. Line 35 can be configured to
introduce
the diluent gas directly into chamber 17 or indirectly via transfer line 25 as
shown. Valves
32 and 38 and other system elements in lines 14 and 21 are adapted to allow
flow of
purged gas from the feed-retentate chamber backward into the ullage 4.
Alternatively, an
optional bypass return line 46 is provided. Thus when any system
13
CA 02513667 2005-07-15
WO 2004/067133 PCT/US2004/001119
elements such as device 28 prevent backflow, valve 44 can be opened and purged
gas
from feed-retentate chamber 17 can flow into ullage 4 through bypass return
46.
The diluent gas should be introduced to the feed-retentate chamber such that
the
chamber 17 is adequately purged of VOC species prior to starting the first
segment of the
next cycle. Preferably the diluent gas should flow through the chamber to
maximize the
purging effect. Therefore, introduction of diluent gas via line 35a upstream
of the
module, e.g., into transfer line 21 is less preferred.
The feed-retentate chamber 17 should not be purged by introducing the diluent
gas
into the permeate chamber 18 of the module. While not wishing to be bound by a
10particular theory, it is believed that VOC species preferentially migrate
through the
rubbery polymer selective membrane by passing through a polymer in which VOC
are
liighly absorbed. Should diluent gas flow backward from the permeate chamber,
through
the rubbery polymer membrane so as to purge the feed-retentate chamber it is
expected
that the membrane polymer would also be purged of VOC. This would render the
meinbrane less effective to permeate VOC immediately upon starting the next
cycle first
segment.
Theoretically, one might select a membrane of a particular amorphous polymer
and choose to operate at temperatures above Tg where the polyiner is rubbery
or below
the Tg where the polymer is glassy. Then the appropriate configuration, i.e.,
either that of
Fig. 3 or Fig. 4, would be selected for the vapor recovery system. In
practice, however,
one is expected to choose a polymer which has an optimum combination of
performance,
mechanical and physical property characteristics. That is, the selectivity and
permeance
respecting the substances being separated as well as the ability to fabricate
the polymer
into a durable membrane in desired form and to operate the membrane at a
temperature
compatible with the vapor recovery process should all be considered. The
totality of
these factors will determine whether the polymer is glassy or rubbery at
separation
conditions and which flow configuration should be used.
Returning to consideration of the novel vapor recovery system in which the
selectively gas permeable membrane comprises a glassy polymer, an additional
embodiment of the invention, for convenience sometimes referred to herein as
the
"vacuum technique" will now be discussed. This embodiment can be understood
with
reference to Fig. 5.
14
CA 02513667 2005-07-15
WO 2004/067133 PCT/US2004/001119
A major distinction of the vacuum technique for reducing VOC emissions
relative
to the embodiments disclosed above is that no diluent gas is charged into the
membrane
during the second segment of the cycle. Instead, increased suction, i.e.,
lower absolute
pressure, is imposed on the penneate chamber of the membrane module to purge
VOC
components. In general, the process is operated as earlier described, however,
during the
second cycle, valves 32 and 53 are closed and a suction is drawn on the
permeate or
feed/retentate chambers. The suction can be provided by an additional vacuum
pump (not
shown) having its suction port in fluid communication with the membrane
module. In a
preferred embodiment of the vacuum technique illustrated in Fig. 5, second gas
conveying device 23 operates to evacuate the contents of the membrane module.
This not
only removes the contents of the permeate chamber but also draws gas from the
feed-
retentate chamber through the membrane. In one mode of operation, the vapor
from the
module is permitted to exhaust to atmosphere through the vent at check valve
24 by
opening valve 54. In a more preferred mode of operation, a vacuum return
transfer line
50 is provided with block valve 52. With valve 33 open (and valve 54 closed),
the gas
discharging from second air conveying device 23 can return to the ullage 4.
Before
starting the first segment of the next cycle, valve 52 is closed.
In either of the above-described modes of operating the vacuunl technique, the
suction generating device (vacuum pump or device 23) can be permitted to
operate
continuously for the duration of the second segment. Alternatively, valve 37
can be
closed and the suction generating device can be stopped before the end of the
second
segment according to a predetermined control protocol. For example, stopping
the
suction can occur after a preselected duration, after the pressure in the
membrane module
has decreased to a preselected vacuuin limit, or after the concentration of
VOC at a
reference location in the membrane module has attained a preselected value.
Preferably,
the suction is applied such that the absolute pressure in the module reduces
to less than
about 0.5 atmosphere. It is thus seen that the vacuum technique advantageously
captures
the VOCs resident in the membrane module at conclusion of the first segment of
the cycle
without adding a volume of diluent gas to the system.
3o EXAMPLES
This invention is now illustrated by examples of certain representative
embodiments thereof, wherein all parts, proportions and percentages are by
volume
CA 02513667 2005-07-15
WO 2004/067133 PCT/US2004/001119
unless otherwise indicated. All units of weight and measure not originally
obtained in SI
units have been converted to SI units.
Examples 1- 5 and Comparative Examples 1- 5
Fixed Duration Protocol Vapor Recovery System Operation
Experiments were performed at the site of an operating retail gasoline service
station using a vapor processor system with the configuration illustrated in
Figure 3. The
service station had three underground gasoline storage tanks with ullage
spaces in fluid
communication. Together, they held 50327 liters of liquid gasoline at a
temperature of 15
C, and a total of 58901 liters of ullage volume. VOC levels were measured in
the vent
stream with a Nova Analytical Systems Model 7204FS hydrocarbon analyzer
modified to
operate on the NDIR (nondispersive infrared) principal.
A series of vapor recovery cycles was operated in the apparatus described
above.
During the second segment portion of the test cycles ambient air feed was
either not
admitted to the membrane module, admitted to the module on the permeate
chamber side
of the membrane, or admitted to the module on the feed retentate chamber side
of the
module. As applicable, the air was admitted at beginning of the second
segment.
Settings of the valves in the system during the second segment were as
illustrated in
Table I below:
Table I
Valve # No Air Admission Feed Air Admission Permeate Air Admission
32 Open Open Open
33 Open Closed Open
36 Closed Closed Open
36a Closed Closed Closed
37 Open Open Open
38 Open Closed Open
39 Closed Open Closed
40 Closed Open Closed
2o During Feed Air Admission, Gas Conveying Device 28, which was a blower, was
activated so as to move air through the feed retentate chainber at a rate of
820 liters per
minute.
16
CA 02513667 2005-07-15
WO 2004/067133 PCT/US2004/001119
VOC concentration (VOC %) of the gas leaving the vent stack was measured
during the immediately subsequent first segment portion. Three concentration
values
were determined for each cycle. These were (i) the initial VOC concentration
at start of
first segment, i.e., (point A2, Fig. 2) when membrane separation started, (ii)
peak VOC
concentration, i.e., (point A4, Fig. 2) the maximum vented concentration, and
(iii) final
VOC concentration (point A6, Fig. 2) when membrane separation stopped. From
these
determinations, the difference between the peak height and the value at the
end of the
cycle (P-E) was calculated. Air admission conditions and analytical results
are presented
in Table II.
Table II
Second Segment Air Feed A2 A4 A6 A4-A6
Sample Initial Peak End P-E
No. Place Duration VOC % VOC % VOC % VOC %
Comp. Ex. 1 0939 - None 1.9 9.3 4.9 4.4
Comp. Ex. 2 0959 - None 1.4 9.1 5.1 4.0
Comp. Ex. 3 1025 - None 1.7 9.1 5.0 4.1
Ex. 1 1040 Permeate 10 min. 2.9 4.9 3.8 1.1
Ex. 2 1051 Permeate ca. 1 sec. 2.4 7.3 4.5 2.8
Comp. Ex. 4 1105 - None 2.0 9.2 5.3 3.9
Ex. 3 1117 Feed 10 sec. 3.4 4.7 4.1 0.6
Ex. 4 1125 Feed 3 sec. 2.8 5.7 4.4 1.3
Ex. 5 1135 Permeate 5 min. 3.3 5.0 4.2 0.8
Comp. Ex. 5 1146 - None 1.7 9.5 5.4 4.1
The data reveals that when air was added to the module, peak concentrations
were
substantially lower according to the novel process (4.7-7.3 % vs. 9.1-9.5 %).
End VOC
values (representing the steady state running condition) for the invention
were also lower
than the controls (3.8-4.5 % vs. 4.9-5.4 %). P-E values for the conventional
process were
consistently about 4 percentage units while the operative examples did not
exceed 2.8
percentage units. Generally, longer admission times for the air produced lower
overall
VOC concentrations of air emitted during the cycle. Altllough Ex. 2
demonstrated the
effectiveness of the invention, the amount of VOC emission reduction was
intermediate
because of the mere momentary duration of the air feeding. When Ex. 2 results
are
excluded, the operative examples dramatically point to the fact that both the
peak and
steady state VOC emissions are much improved by practice of this invention.
17
CA 02513667 2005-07-15
WO 2004/067133 PCT/US2004/001119
Although specific forms of the invention have been selected for illustration
in the
drawings and the preceding description is drawn in specific terms for the
purpose of
describing these forms of the invention fully and amply for one of average
skill in the
pertinent art, it should be understood that various substitutions and
modifications which
bring about substantially equivalent or superior results and/or performance
are deemed to
be within the scope and spirit of the following claims.
18