Note: Descriptions are shown in the official language in which they were submitted.
CA 02514000 2005-07-27
FEXOFENADINE BASE POLYMORPHIC FORMS
FIELD OF THE INVENTION
The present invention relates to novel crystalline forms of fexofenadine
base, a process for the preparation thereof, and the use thereof in therapy.
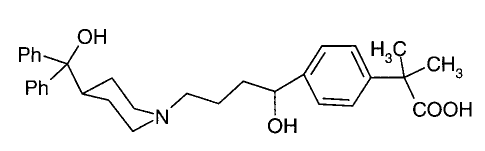
Fexofenadine, namely (4-[4-[4-(hydroxydiphenylmethyl)-1-
piperidinyl]-1-hydroxybutyl]-a,oc -dimethylbenzeneacetic acid)
OH
Ph ~ ~ H3C CH3
Ph
COOH
OH
is an antihistaminic, antiallergic and bronchodilator medicament,
marketed in the USA under the tradename Allegra~.
A number of processes for the preparation of fexofenadine are known,
for example those disclosed in US 4,254,129; US 5,750,703 and EP 1 260 505.
WO 02/066429, US 2003/002184 and WO 95/31437 disclose various
polymorphic forms of fexofenadine hydrochloride and WO 03/039482
discloses various polymorphic forms of fexofenadine base.
Different forms of biologically active compounds, such as polymorphs,
are known to have different bioavailability, release time and solubility,
which
allow, for example, dose reduction or prolonged administration intervals.
Moreover, the different physical properties that are often associated to
different physical forms of medicaments can be advantageously exploited in
the manufacture of pharmaceutical formulations.
There is therefore the need to provide novel polymorphic forms of
biologically active compounds with advantageous properties.
SUMMARY OF THE INVENTION
It has now been found that fexofenadine free base can exist, as well as
in the known crystalline forms cited above, also in three novel crystalline
CA 02514000 2005-07-27
2
forms stable at room temperature.
In a first aspect the invention relates to a novel crystalline form of
fexofenadine free base, herein referred to as Form a, and a process for its
preparation.
In a second aspect the invention relates to a novel crystalline form of
fexofenadine free base, herein referred to as Form 13, and a process for its
preparation.
In a further aspect the invention relates to a novel crystalline form of
fexofenadine free base, herein referred to as Form 'y, and a process for its
preparation.
Moreover, an object of the present invention is the use of the novel
crystalline forms of fexofenadine free base as intermediates in a process for
the purification of fexofenadine free base or fexofenadine hydrochloride,
useful to obtain fexofenadine free base or fexofenadine hydrochloride of
quality suitable for the therapeutical use.
Furthermore, the invention provides a pharmaceutical composition
containing an excipient and/or carrier and at least an active ingredient
consisting of fexofenadine free base Form a, 13 or'y.
BRIEF DISCLOSURE OF THE FIGURES
The three novel crystalline forms of Fexofenadine free base were
characterized with the XRPD technique (X-ray powder diffraction). The X-ray
diffraction spectra (XRPD) were registered with an APD 2000 8/8 automatic
diffractometer for powders and liquids (Ital-Structures), under the following
0
operative conditions: radiation CuKa (1.5418 A), scanning with a 0.03°C
angular step for an acquisition time of 1 sec.
Figure 1. XRPD spectrum of fexofenadine free base Form a.
Figure 2. XRPD spectrum of fexofenadine free base Form 13.
Figure 3. XRPD spectrum of fexofenadine free base Form y.
CA 02514000 2005-07-27
3
DETAILED DISCLOSURE OF THE INVENTION
As used herein, the expressions "fexofenadine free base" and
"fexofenadine base" are equivalents and relate to the same product.
As used in the following, the term precipitation (or "precipitate") has
the same meaning as crystallization (or "crystal"), and means the obtainment
of a solid form.
As first object, the present invention relates to a novel crystalline form
of fexofenadine base, herein referred to as Form a, having the XRPD
spectrum substantially as reported in Figure 1, wherein the most intense
diffraction peaks fall at 4.37; 8.75; 10.07; 13.16; 15.65; 16.22 and 23.57 in
28.
Said form a is prepared through a process comprising the following
steps:
- reaction of a fexofenadine addition salt with a basic organic agent in
an organic protic solvent; and
- separation of the precipitate.
A fexofenadine addition salt, used as the starting material for the
preparation of the novel Forms a, 13 and y of fexofenadine base as reported in
the following, can be a salt thereof, either anhydrous or hydrate, with a
suitable inorganic acid or organic, for example as known from WO 95/31437.
Preferred examples of inorganic acids are hydrochloric, hydrobromic, sulfuric,
phosphoric acids. Preferred examples of organic acids are both carboxylic
acids, such as acetic, propionic, glycolic, lactic, piruvic acids, or sulfonic
acids, such as methanesulfonic, ethanesulfonic and beta-
hydroxyethanesulfonic acids. Particularly preferred is fexofenadine
hydrochloride salt. As used herein, the expressions "fexofenadine addition
salt" and "fexofenadine salt" are equivalents and relate to the same product.
The preparation of said Form a can be carried out starting from a
dispersion, for example solution or suspension, of a fexofenadine addition
salt
CA 02514000 2005-07-27
4
in an organic protic solvent. Preferred examples of organic protic solvents,
according to the invention, are alcohols, preferably a C~-C6 alkanol, such as
methanol, ethanol, 1-propanol, 2-propanol or mixtures thereof, particularly
methanol. The concentration of the fexofenadine salt in the starting solution
or
suspension can approximately range from 5 to 50%, preferably from 15 to
30%. The temperature of the resulting solution or suspension can be
maintained between the room temperature and the reflux temperature,
preferably room temperature. Fexofenadine free base can be directly obtained
in situ by reacting the corresponding addition salt with a basic organic
agent.
A basic organic agent is typically ammonia; an amine, e.g. of formula
N(R,R2R~), wherein at least one of R,, R2 and R~ is a Cl-C6 alkyl or aryl
group, and the others are independently hydrogen or a C~-C6 alkyl or aryl
group; or a saturated or unsaturated 5- or 6- membered heterocyclic
compound, containing at least one nitrogen atom, for example pyridine,
piperidine or morpholine. A straight or branched C1-C6 alkyl group is
preferably a C~-C4 alkyl group, particularly methyl or ethyl. An aryl group is
preferably phenyl optionally substituted with one to three substituents
independently selected from hydroxy, halogen, cyano and amino. Examples of
specific basic organic agents are butylamine, triethylamine, tributylamine and
diisopropyl-ethylamine, particularly butylamine, triethylamine and
tributylamine. The molar ratio of basic agent to fexofenadine salt
approximately ranges from 0.8 to 1.2, preferably from 0.95 to 1.05. The
precipitated fexofenadine free base Form oc can be recovered with a
conventional technique, preferably by filtration, followed by washing with the
same solvent or mixtures of solvents as used in the reaction, then dried under
vacuum at a temperature depending on the solvent used. This temperature
approximately ranges from 0°C to the boiling temperature of the solvent
itself,
preferably drying is effected at room temperature.
CA 02514000 2005-07-27
In a second aspect, the invention relates to a novel crystalline form of
fexofenadine base, herein referred to as Form 13, having the XRPD spectrum
substantially as reported in Figure 2, wherein the most intense diffraction
peaks fall at 8.28; 10.68; 16.23; 16.80; 19.35 and 21.12 in 28.
5 Said Form 13 can be prepared by a process comprising the following
steps:
- reaction of a fexofenadine addition salt with a basic organic agent in
an organic erotic solvent;
- acidification of the solution with a carboxylic organic acid to
precipitate fexofenadine base Form 13; and
- separation of the precipitate.
The process can be carried out starting from a dispersion, such as a
solution or suspension, of a fexofenadine addition salt in an organic erotic
solvent as defined above, particularly methanol, ethanol, 1-propanol,
2-propanol or mixtures thereof; more preferably methanol. The temperature of
the resulting solution or suspension can be kept between the room temperature
and the reflux temperature, preferably at room temperature. The fexofenadine
free base can be directly obtained iu situ by reacting the corresponding
addition salt with a basic organic agent, as defined above. A particularly
preferred example of basic organic agent is triethylamine. The molar ratio of
basic agent to fexofenadine addition salt approximately ranges from 1.5 to
3.0,
preferably from 1.95 to 2.05. Fexofenadine free base Form 13 can be
precipitated by acidifying again the solution to pH approx. ranging from 4 to
7, preferably from 5 to 6, with a carboxylic organic acid. Said acid can be
for
example a mono-, bi- or tri- carboxylic acid, such as formic, acetic, oxalic,
malonic, malefic, fumaric, tartaric and citric acids. Preferred examples are
formic acid and acetic acid, more preferably acetic acid. The resulting solid
product can be recovered with conventional techniques, preferably by
CA 02514000 2005-07-27
6
filtration, followed by washing with the same solvent or mixtures of solvents
as used in the reaction, then dried under vacuum at a temperature depending
on the solvent used. This temperature approximately ranges from 0°C to
the
boiling temperature of the solvent, preferably room temperature.
As further object, the present invention relates to a novel crystalline
form of fexofenadine base, herein referred to as Form y, having the XRPD
spectrum substantially as reported in Figure 3, wherein the most intense
diffraction peaks fall at 5.63; 12.11; 15.83; 16.91; 20.39 and 24.44 in 28.
Said Form 'y can be prepared by a process comprising the following
steps:
- reaction of a fexofenadine addition salt with a basic organic agent in
an organic aprotic solvent; and
- separation of the precipitate.
The process can be carried out starting from a dispersion, such as a
solution or suspension, of a fexofenadine addition salt in an organic aprotic
solvent. Examples of organic aprotic solvents, according to the invention, are
acetone, diethyl ketone, methyl ethyl ketone, ethyl acetate, butyl acetate,
acetonitrile or mixtures thereof, preferably acetone or acetonitrile or
mixtures
thereof, particularly acetone or a mixture of acetone and acetonitrile wherein
the percentage in volume of acetone in the acetone/acetonitrile mixture
approximately ranges from 0.4 to 0.8, preferably approx. from 0.5 to 0.7. The
concentration of the fexofenadine salt in the starting solution or suspension
can approximately range from 5 to 50%, preferably from about 15 to 30%. The
temperature of the resulting solution or suspension can be kept at a
temperature ranging between room temperature and the reflux temperature,
preferably room temperature. Fexofenadine free base Form y can be directly
obtained i~ situ by reacting the corresponding addition salt with a basic
organic agent, as defined above. Preferred examples of basic organic agents
CA 02514000 2005-07-27
7
are butylamine, tributylamine and triethylamine. The molar ratio of basic
agent to fexofenadine salt approximately ranges from 0.8 to 1.2, preferably
from 0.95 to 1.05. The precipitated fexofenadine free base Form 'y can be
recovered with conventional techniques, preferably by filtration, followed by
washing with the same solvent or mixtures of solvents used in the reaction and
drying under vacuum at a temperature depending on the solvent used. This
temperature approximately ranges from 0°C to the boiling temperature of
the
solvent, preferably 50°C.
The skilled chemist will note that the conditions to obtain the dispersion
of a fexofenadine addition salt in a protic or aprotic solvent can be changed
without affecting the resulting polymorphic form. Likewise, the
crystallization
techniques can by slightly changed without affecting the resulting
polymorphic form. By way of example, crystallization may be made faster by
adding crystals previously formed.
The above recovery process to obtain the novel crystalline forms of the
invention allows to purify the final product from any impurities formed during
the fexofenadine synthetic process, derived from either parasitic reactions or
degradation of the product itself.
Therefore, a further object of the present invention is the use of
fexofenadine free base Form oc, Form 13 or Form y, as herein defined, in a
process for the preparation of fexofenadine free base, or a salt or
polymorphic
form thereof, having a purity degree suitable for the pharmaceutical or
veterinary use.
The novel Forms oc, 13 and y of fexofenadine base are useful for the
administration of fexofenadine in mammals in need of said treatment. Such
forms will be administered alone or as mixtures thereof or mixtures with one
or more of the polymorphic forms of fexofenadine base or salt already known,
particularly the hydrochloride. The content thereof in the mixtures depends on
CA 02514000 2005-07-27
8
their physical and biological characteristics and is left to the discretion of
those skilled in the art. Such forms can be formulated in a variety of
pharmaceutical compositions for the administration to humans or animals,
according to known techniques. The dosage of fexofenadine base in the
capsules, tablets, sugar-coated pills or other unit forms approximately ranges
from 20 to 200 mg; the exact dosage is left to the discretion of the
physician.
Therefore the invention also relates to a pharmaceutical composition
comprising a suitable carrier and/or excipient and, as active ingredient, at
least
one of fexofenadine base Form a, Form l3 or Form y, or mixtures thereof or
mixtures of at least one of them with one or more of the known fexofenadine
base polymorphic forms or a salt thereof. Said pharmaceutical composition
can optionally contain a therapeutically effective amount of pseudoephedrine.
The following examples illustrate the invention.
EXAMPLE 1
Preparation of fexofenadine free base Form a
A 100 ml round-bottom flask, equipped with magnetic stirrer, is loaded
with 10 g (18.6 mmoles) of fexofenadine hydrochloride and 50 ml of
methanol, to obtain a clear solution. Afterwards, 2.5 ml of triethylamine
( 1.81 g; 17.9 mmoles) are added. After about 2 minutes, a precipitate forms
which is filtered off, washed with methanol and dried at room temperature,
thereby affording 8.9 g (17.7 mmoles) of fexofenadine free base. XRPD
analysis of the product shows that it is a novel crystalline form of
fexofenadine free base, substantially characterised by the peaks as reported
in
Figure 1.
EXAMPLE 2
Preparation of fexofenadine free base Form 1~
A 100 ml round-bottom flask, equipped with magnetic stirrer, is loaded
with 10 g (18.6 mmoles) of fexofenadine hydrochloride and 50 ml of
CA 02514000 2005-07-27
9
methanol, to obtain a clear solution. Afterwards, 5 ml of triethylamine (3.63
g;
35.9 mmoles) are added thereto, then acetic acid is added to acidify again the
mixture. After about 2 minutes, a precipitate forms which is filtered off,
washed with methanol and dried under vacuum at room temperature, thereby
affording 7.9 g ( 15.8 mmoles) of fexofenadine free base. XRPD analysis of
the product shows that it is a novel crystalline form of fexofenadine free
base,
substantially characterised by the peaks as reported in Figure 2.
EXAMPLE 3
Preparation of fexofenadine free base Form y
A 100 ml round-bottom flask, equipped with magnetic stirrer, reflux
condenser and thermometer, is loaded with 10 g of fexofenadine
hydrochloride (18.6 mmoles), 30 ml of acetone and 20 ml of acetonitrile. The
mixture is warmed to a temperature of 60°C under stirring, to obtain a
suspension. Afterwards, 2.5 ml of triethylamine ( 1.81 g; 17.9 mmoles) are
added thereto. From the resulting clear solution, a precipitate immediately
forms which is filtered off and repeatedly washed first with the same
acetone-acetonitrile mixture as used in the preparation, then with only
acetone. Finally the solid product is dried in a static dryer under vacuum at
a
temperature of 50°C, thereby affording 8.1 g ( 16.2 mmoles) of
fexofenadine
free base. XRPD analysis of the product shows that it is a novel crystalline
form of fexofenadine free base, substantially characterised by the peaks as
reported in Figure 3.
EXAMPLE 4
Preparation of Fexofenadine free base form 'y
A 50 ml round-bottom flask, equipped with magnetic stirrer, reflux
condenser and thermometer, is loaded with 5 g of fexofenadine hydrochloride
(9.32 mmoles) and 25 ml of acetone. The mixture is refluxed under stirring, to
obtain a suspension. Afterwards, 0.9 ml of butylamine (0.68 g; 9.32 mmoles)
CA 02514000 2005-07-27
are added thereto. From the resulting clear solution, a precipitate
immediately
forms which is filtered off and repeatedly washed with acetone. Finally the
solid product is dried in a static dryer under vacuum at a temperature of
50°C,
thereby affording 4.1 g (8.21 mmoles) of Fexofenadine free base. XRPD
5 analysis of the product shows that it is a novel crystalline form of
fexofenadine free base, substantially characterised by the peaks as reported
in
Figure 3.
EXAMPLE 5
Preparation of Fexofenadine free base Form 'y
10 A 50 ml round-bottom flask, equipped with magnetic stirrer, reflux
condenser and thermometer, is loaded with 5 g of fexofenadine hydrochloride
(9.32 mmoles) and 25 ml of acetone. The mixture is refluxed under stirring, to
obtain a suspension. Afterwards, 2.22 ml of tributylamine (1.73 g;
9.32 mmoles) are added thereto. From the resulting clear solution, a
precipitate immediately forms which is filtered off and repeatedly washed
with acetone. Finally the solid product is dried in a static dryer under
vacuum
at a temperature of 50°C, thereby affording 3.5 g (7 mmoles) of
fexofenadine
free base. XRPD analysis of the product shows that it is a novel crystalline
form of fexofenadine free base, substantially characterised by the peaks as
reported in Figure one spectrum with peaks characteristics as substantially
reported in figure 3.