Note: Descriptions are shown in the official language in which they were submitted.
CA 02514271 2005-07-25
SPECIFICTION
METHOD FOR FORMING ANODIC OXIDE LAYER ON SURFACE OF
ALUMINUM OR ALUMINUM ALLOY
TECHNICAL FIELD
The present invention relates to an improvement of a method for
forming an anodic oxide layer on a surface of aluminum or an aluminum
alloy.
BACKGROUND ART
A method for forming a corrosion-resistant oxide layer by anodizing
aluminum or an alloy thereof in an electrolytic solution such as an aqueous
solution of nitric acid, sulphuric acid or chromic acid for the purpose of
mainly improving corrosion resistance thereof is known as an alumite
treatment. Articles subjected to the alumite treatment are widely utilized
in various fields with a central focus on daily commodities such as a pan and
a teakettle.
However, since an upper layer of an alumite layer is ordinarily
porous, corrosion resistance is not sufficient and abrasion resistance and
coloring easiness are not satisfactory. In order to solve these problems, a
technique for forming a composite layer of an aluminum oxide layer and an
acrylic resin composition, a technique for forming a dense composite layer in
a short period of time regardless of a shape or the like of an article to be
treated, and a technique for improving a coloring property are disclosed in:
1
CA 02514271 2005-07-25
Patent Document 1: JP-B No. O1-019479~
Patent Document 2: JP-A No. 02-097698 and
Patent Document 3: JP-B No. 05-014033.
However, there is a problem in that, in the case of any one of these
surface treatment methods and other known alumite treatment methods,
although the anodic oxide layer can easily be formed on an Al-Mn alloy, it is
impossible to perform the treatment on duralumin or a die cast alloy and it
is difficult to perform the treatment on other aluminum alloys.
The layer to be formed by a conventional method is restricted to
have a comparably small thickness of about 30 to about 50 Vim, a low
hardness and the like and, accordingly, there is a given limitation upon
applications thereof.
The present invention has been achieved in order to solve these
problems and an object of the present invention is to provide a method for
treating a surface of aluminum or an aluminum alloy which can treat
various types of aluminum alloys involving not only aluminum itself, but
also duralumin and a die cast alloy, can apply a thick layer of 300 to 500 hum
and has a number of advantages such that the layer to be obtained has a
high surface hardness, an excellent heat resistance, an antibiotic action and
the like and can produce various types of aluminum materials which can be
utilized in a far wide field compared with a conventional one.
The above-described object according to the present invention can be
attained by performing an anodic oxidation treatment by using a bath liquid,
which involves an aqueous solution containing 250 gr/1 to 350 gr/1 of sulfuric
acid and 15 gr/1 to 25 gr/1 of nickel sulfate under the following conditions:
2
CA 02514271 2005-07-25
(a) Bath liquid temperature: -10°C to +25°C~
(b) Voltage: DC 100 V to 200 V~ and
(c) Current density: 0.5 A/dm2 to 20 A/dm2.
For the sake of convenience of explanation, the above-described
treatment according to the present invention is referred to as "the present
treatment (1)" and a product to be obtained thereby is referred to as
"present product (1)".
The object according to the present invention can be performed more
favorably by using a bath liquid in which the bath liquid to be used in the
present treatment (1) is further added with a low polymerization acrylic
resin composition in the range of from 280 gr/1 to 320 gr/1.
For the sake of convenience of explanation, the above-described
treatment according to the present invention is referred to as "the present
treatment (2)" and a product to be obtained thereby is referred to as
"present product (2)".
In the present treatment (2), in order to prevent a so-called "burning
(YAKE in Japanese)", it is recommended to use a bath liquid further added
with tartaric acid in the range of from 5 gr/1 to 15 gr/1.
When the method according to the present invention is applied to an
aluminum alloy selected from the group consisting of duralumin, an
aluminum alloy for die cast and an aluminum alloy without containing Mn
which are difficult to be treated by a conventional surface treatment method,
it is desirable to perform an anodic oxidation treatment by using any one of
the above-described bath liquids under the following conditions:
(d) Bath liquid temperature: -10°C to -5°C~
3
CA 02514271 2005-07-25
(e) Voltage: DC 130 V to 170 V~ and
(f) Current density: 8 A/dm2 to 12 A/dm2.
When the anodic oxidation treatment is performed on a surface of an
aluminum alloy containing Mn, it is desirable to perform the treatment by
using any one of the above-described bath liquids under the following
conditions:
(g) Bath liquid temperature: +15°C to +18°C~
(h) Voltage: DC 130 V to 170 V~ and
(i) Current density: 8 A/dm2 to 12 A/dm2.
In a further desirable aspect of the present invention, after the
anodic oxide layer is formed on the surface of aluminum or the aluminum
alloy by any one of the above-described various types of treating methods, it
is recommended to impregnate silver in the anodic oxide layer by
performing a treatment using a bath liquid which involves an aqueous
solution further containing 10 gr/1 to 30 gr/1 of silver sulfate or silver
nitrate,
15 gr/1 to 20 gr/1 of boric acid and 1 gr/1 to 2 gr/1 of nickel sulfate under
the
following conditions:
(j) Bath liquid temperature: +10°C to +20°C~
(k) voltage: AC 10 V to 15 V
(1) current density: 1 A/dm2 to 2 A/dm2~ and
(m) Current applying period: 2 minutes to 3 minutes.
For the sake of convenience of explanation, the above-described
treatment according to the present invention is referred to as "the present
treatment (3)" and a product to be obtained thereby is referred to as
"present product (3)".
4
CA 02514271 2005-07-25
The above-described object according to the present invention can be
attained by a method for forming an anodic oxide layer on a surface of
aluminum or an aluminum alloy which is characterized in that an anodic
oxide layer having a thickness of 300 ~m to 600 ~tm is formed on a surface of
aluminum or an aluminum alloy by any one of the above-described various
treating methods and, after the above-described silver impregnation is
performed on the layer, a surface layer was removed by polishing by a
thickness of 50 ~m to 100 ,um and, then, an ultra-hard flat surface is
obtained.
BRIEF DESCRIPTION OF DRAWINGS
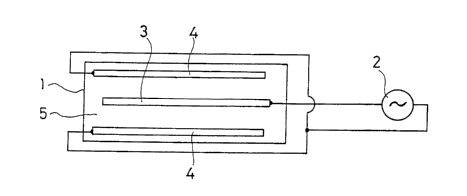
FIG. 1 is an explanatory view showing an embodiment of a device
for carrying out a method for forming an anodic oxide layer on a surface of
aluminum or an aluminum alloy according to the present invention and
FIG. 2 is an enlarged cross-sectional view showing a layer portion of
aluminum or an aluminum alloy subjected to the present treatment (2).
BEST MODE FOR CARRYING OUT THE INVENTION
Hereinafter, the present invention is specifically described with
reference to drawings.
In FIG. 1, reference numeral 1 denotes an electrolyte bath reference
numeral 2 denotes an AC power supply reference numeral 3 denotes an
aluminum or aluminum alloy member to be treated by the method
according to the present invention reference numeral 4 denotes a
non-consumable electrode such as carbon or graphite and reference
CA 02514271 2005-07-25
numeral 5 denotes a bath liquid involving a predetermined electrolytic
solution.
The present treatment (1) performs an anodic oxidation treatment
by using a device as shown in FIG. 1 and an aqueous solution containing
250 gr/1 to 350 gr/1 of sulfuric acid, 15 gr/I to 25 gr/1 of nickel sulfate as
a
bath liquid under the following conditions:
(a) Bath liquid temperature: -10°C to +25°C~
(b) Voltage: DC 100 V to 200 V~ and
(c) Current density: 0.5 A/dm2 to 20 A/dm2.
As described above, the present invention is entirely different from
the conventional method in the point that the anodic oxidation treatment is
performed under the treating conditions of a high sulfuric acid ion
concentration, a low temperature and a high current density.
When a value of each of the above-described treating conditions is
less than a lower limit thereof, a treating efficiency is aggravated, while,
when it is more than an upper limit thereof, hardness of the layer is
decreased and a desired layer can not be obtained these are problematic.
Nickel sulfate is added for the purpose of enhancing hardness of the
layer to be formed.
As for a raw material of aluminum or an aluminum alloy to form an
anodic oxide layer thereon by a treatment method according to the present
invention, those as described in Table 1 are mentioned.
6
CA 02514271 2005-07-25
TABLE 1
Highly pure aluminum 99.9% Type
or
more
99.7% A1070
aluminum
P
ure Industrially pure aluminum gg,5% A1050
99.0% A1100
A1-Cu type (containing 3.5 to 6.8%of Cu) A2000
A1-Mn type (containing 1.0 to 1.5%of Mn) A3000
A1-Si type (containing Si to a greatextent) A4000
llo
i
l
y
num a A1-Mg type (containing 0.5 to 5.0%of Mg) A5000
um
A
A1-Mg-Si type (containing about1.0% Mg and Si) A6000
of
A1-Zn type (containing 5.0 to 6.0%of Zn) A7000
Further, when the method according to the present invention is
applied to a hard-treating aluminum alloy which has been difficult to be
treated by the conventional method such as duralumin, an aluminum alloy
for die cast or an aluminum alloy without containing Mn, or any one of other
aluminum alloys, a desired anodic oxide layer can be formed by using any
one of above-described bath liquids and adopting the following conditions:
(d) Bath liquid temperature: -10°C to -5°C~
(e) Voltage: DC 130 V to 170 V, and
(f) current density: 8 A/dm2 to 12 A/dm2.
On the other hand, when the anodic oxidation treatment is
performed on a surface of an aluminum alloy containing Mn, a desired
anodic oxide layer can be formed by adopting the following conditions:
(g) Bath liquid temperature: +15°C to +18°C~
(h) Voltage: DC 130 V to 170 V~ and
7
CA 02514271 2005-07-25
(i) Current density: 8 A/dm2 to 12 A/dm2.
When the present invention to be constituted as described above is
compared with a conventional method, the present invention has such
advantages as described below.
(1) In the conventional method, although an anodic oxide layer can
easily be formed in an Al-Mn type alloy, it is impossible to treat duralumin
and a die cast alloy and it is difficult to treat other alloys.
On the other hand, according to the method of the present invention,
duralumin, a die cast alloy and all types of other aluminum alloys can be
treated.
(2) In the conventional method, a layer having a thickness of about
30 to about 50 ~m and, even at a maximum, about 100 ~m can be formed.
On the other hand, according to the method of the present invention, a layer
having a thickness as large as 300 to 500 ~m can easily be formed.
(3) As for the layer to be formed according to the conventional
method, although a surface layer thereof is hard (Vicker's hardness: 400 or
less), an inside thereof is porous and hardness thereof is low.
On the other hand, as for the layer to be formed by the method
according to the present invention, a surface layer thereof is hard and a
Vickers hardness is about 450 to about 500. Particularly, a lower layer is
denser and harder than a surface. When a thickness of 50 to 150 ~m is
removed from a surface, hardness becomes 800 to 1000 in terms of Vickers
hardness.
(4) As for the layer to be formed by the method according to the
present invention, heat conductivity thereof is high and is comparable with
8
CA 02514271 2005-07-25
that of copper.
(5) As for the layer to be formed by the method according to the
present invention, surface thermopenetration resistance is low.
Then, when ice is placed on a tray made of aluminum or an alloy
material thereof having the layer formed by the method according to the
present invention, ice is melted at twice the speed of ice on an untreated
tray. Therefore, for example, the tray can favorably be utilized as a tray
for unfreezing a frozen food. Further, when a container made of aluminum
to be heated for popcorn is subjected to the treatment according to the
method of the present invention, a time period from the time of starting
heating to the time of explosion of the popcorn is reduced from conventional
6 minutes to 3 minutes.
(6) As for the layer to be formed by the method according to the
present invention, heat resistance is as high as about 800°C.
(7) The layer to be formed by the method according to the present
invention has an antibiotic action.
Therefore, an aluminum material or an aluminum alloy material on
which an anodic oxide layer is formed by the method according to the
present invention can favorably be utilized in a wide field, for example, as a
tray for ice-making or unfreezing, a rice cooker, a pan, a kettle, a teakettle
and other cooking devices for heating, an instantaneous hot-water heater, a
heat exchanger, an air-conditioner, a freezer, a refrigerator, an oil heater,
a
radiator, a cooling fin, an air- or water-cooled engine (acceleration of heat
release), a wing of an airplane (de-icing), a heat sink for a semiconductor, a
semiconductor package, a heat pipe, a bearing, various types of sliding
9
CA 02514271 2005-07-25
members, a brake shoe, a manufacturing apparatus for popcorn or
ice-cream, a chassis for an electric apparatus, a casing for a motor, an
electric transformer or the like.
These articles utilize a property of the present product which
conducts heat efficiently.
Next, the present treatment (2) is described.
When the present treatment (2) is performed, it is characterized
that the anodic oxidation treatment is performed by using a bath liquid in
which the bath liquid used in the above-described present treatment (1) is
further added with a low polymerization acrylic resin composition in the
range of from 280 gr/1 to 320 gr/1.
As for the low polymerization acrylic resin composition to be added,
for example, an article containing, based on percentages, 68% of
hydroxypropyl methacrylate, 10% of neopentyl glycol dimethacrylate, 19.5%
of polypropylene glycol methacrylate, 1% of 1,6-hexanediol diglycidyl ether,
1% of butyl peroxyoctoate, 500 ppm of hydroquinone monmethyl ether and
0.3% of dicyandiamide is favorably used.
For the purpose of prevention of "burning", it is recommended to
further add tartaric acid to the above-described bath liquid in the range of
from 5 gr/1 to 15 gr/1.
By such present treatment (2) as described above, an oxide layer
which is a composite of aluminum oxide and the acrylic resin composition is
formed. Namely, a metallurgical porous oxide layer and the acrylic resin
composition are acid-ionized and polymerized therebetween and, then, form
a tough and dense composite layer, to thereby enhance corrosion resistance
CA 02514271 2005-07-25
and abrasion resistance to a great extent. Further, since the layer is
formed while drawing out a gas in a pinhole portion, the layer has
characteristics such that pinholes are small in number and, further, since
the oxide layer is slowly formed at a low temperature, it is excellent in
density and, since the layer is hard to be peeled off, it can be subjected to
machining and a surface roughness thereof remains unchanged.
The anodic oxide layer obtained by the present treatment (2) is now
explained with reference to an enlarged cross-sectional view showing a
layer portion of FIG. 2.
In FIG. 2, reference numeral 21 denotes an aluminum material or
aluminum alloy material as a base metal reference numeral 22 denotes an
anodic oxide layer reference numeral 23 denotes a barrier layer reference
numeral 24 denotes a porous layer portion reference numeral 25 denotes an
acrylic resin composition layer portion.
The anodic oxide layer 22 involves a barrier layer 23 formed on the
aluminum material or aluminum alloy material 22, a porous layer portion
24 formed thereon and an acrylic resin composition layer portion 25 which
is impregnated inside the porous layer and fixed therein. By these two
layer portions 24, 25, a tough and dense composite layer is formed. As for
the composite layer, as a portion thereof is closer to the barrier layer 23,
the
portion comes to have a higher hardness and becomes denser and, as
described below, by removing a region close to a surface by machining, the
surface having a further higher hardness can be obtained.
Next, the present treatment (3) is described.
When the present treatment (3) is performed, after the anodic oxide
11
CA 02514271 2005-07-25
layer is formed on a surface of an aluminum or aluminum alloy by any one
of the above-described various types of treating methods, it is characterized
in that silver is impregnated in the anodic oxide layer by performing an
anodic oxidation treatment using a bath liquid which involves an aqueous
solution further containing 10 gr/1 to 30 gr/1 of silver sulfate or silver
nitrate,
15 gr/1 to 20 gr/1 of boric acid and 1 gr/1 to 2 gr/1 of nickel sulfate under
the
following conditions:
(j) Bath liquid temperature: +10°C to +20°C~
(k) Voltage: AC 10 V to 15 V
(1) Current density: 1 A/dm2 to 2 A/dm2~ and
(m) Current applying period: 2 minutes to 3 minutes.
Decrease of a silver ion concentration along a progress of treatment
is covered by replenishment of silver sulfate or silver nitrate.
Boric acid is added mainly for adjustment of electric conductivity of
the electrolytic solution.
When the voltage is less than 10 V, a treatment efficiency becomes
deteriorated, while, when it is over 15 V, deposition of silver is unduly
rapidly performed and the oxide layer can not sufficiently be impregnated
inside the porous layer and uneven coloring, peeling or the like tends to be
generated.
In a same manner, when the temperature of the electrolytic solution
is less than +10°C, the treatment efficiency is aggravated, while, when
it is
more than +20°C, uneven coloring tends to be generated.
By such present treatment (3) as described above, a silver ion is
deeply impregnated inside the porous anodic oxide layer (electrolytically
12
CA 02514271 2005-07-25
impregnated by alternating voltage) and, then, combines with aluminum
oxide, to thereby form a tough dense composite layer. For this account, the
surface layer excellent in the heat conductance, the corrosion resistance, the
abrasion resistance, the antibiotic action and the like can be formed.
Further, the surface layer has the electric conductance and has a small
coefficient of friction and a small color change in time. Further, the layer
has effects such as far-infrared emission, removal of static electricity and
the like.
Such present treatment (3) can be performed on all types of
aluminum material and aluminum alloy material and can form a thick layer
having various types of excellent characteristics as described above on the
surface thereof.
In the present invention, further, an anodic oxide layer having a
thickness of 300 ~m to 600 ~m is formed on a surface of aluminum or an
aluminum alloy by the above-described various types of treating methods
and, then, further, the above-described silver impregnation is performed
and, thereafter, a surface layer is removed by polishing in a depth of from 50
to 100 ~um from the surface and, subsequently, an aluminum material or
aluminum alloy material having a ultra-hard smooth surface can be
provided.
Namely, as for the layer to be formed by the method according to the
present invention, a surface hardness is high and is about 450 to about 500
in terms of Vickers hardness. Particularly, a lower layer is denser than
the surface and is higher in hardness. Then, when 50 to 150 ,um from the
surface is removed, the aluminum material or aluminum alloy material
13
CA 02514271 2005-07-25
having an ultra-hardness of 800 to 1000 in terms of Vickers hardness and a
smooth face can be obtained.
Hereinafter, various types of characteristics of the present product
are shown.
In Table 2, characteristics of products on which the present
treatment has been applied are shown by materials.
14
CA 02514271 2005-07-25
TABLE
2
Pr
sent
roduct
MaterialConventional
e
type alumite TicknessSilver Heat Antibiotic
HardnessInsuration Slipperiness
a impregnation conductionproperty
Substantioally
AL00 ** 60 Possible 450 Possessing2.5
increased
The same The same
AL10 ** 60 Possible 450 as as 5
2
above. above. .
The same The same
AL20 * 60 Possible 450 as as 5
2
above. above. .
The same The same
AL30 ** 60 Possible 450 as as 2
5
above. above. .
The same The same
AL40 ** 60 Possible 450 as as 5
2
above. above. .
The same The same
AL50 *** 100 Possible 450 as as 5
2
above. above. .
The same The same
AL60 *** 100 Possible 450 as as 5
2
above. above. .
The same The same
AL70 ** 60 Possible 450 as as 5
2
above. above. .
The same The same
** 60 Possible 450 as as 5
2
above. above. .
The same The same
AC2 ** 60 Possible 370 as as
above. above.
AC3 60 Possible 370 The same The same
as as
** above. above.
The same The same
AC4 ** 60 Possible 370 as as
above. above.
AC7 The same The same
** 60 Possible 370 as as
above. above.
ADC1 * 30 Possible 370 The same The same
as as
above. above.
ADC2 The same The same
* 30 Possible 370 as as
above. above.
ADC3 * 30 Possible 370 The same The same
as as
above. above.
ADC4 The same The same
* 30 Possible 370 as as
above. above.
Conventional alumite marks:
*; Impossible to be machined
**: Difficult to be machined
***: Easy to be machined
CA 02514271 2005-07-25
As for the heat conductivity, when that of silver is taken as 1, that of
the present product is 0.9~ that of copper is 0.94 and that of aluminum is
0.53. Therefore, the heat conductivity of the present product is higher
than that of aluminum as a base metal and is comparable with that of
copper.
This property shows that the present product is excellent as a raw
material for various types of heat transfer members, diathermal members,
heat releasing members.
As for the hardness (Hv), that of aluminum is 80, that of stainless
steel is 200, that of the present product is 450. Accordingly, the hardness
of the present product is more than twice that of stainless steal.
By making use of this property, various types of parts which require
abrasion resistance such as a gear, a roller, a guide rail, a shaft, a
bearing, a
brake shoe, a cylinder liner and piston, a valve, a piston pump and a screw
pump can be produced.
As for the upper temperature limit (°C), that of polytetrafluoro-
ethylene is 260°C~ that of aluminum is 660°C~ and that of the
surface layer
of the present product is 800°C.
By making use of this property, the present product can provide a
flame-retardant shutter, heat-resistant wall material and the like.
When the abrasion resistance test was performed, it has been found
that an abrasion amount of the present product was one tenth the abrasion
amount of ordinary hard-type alumite.
Namely, the abrasion test was conducted by arranging a test piece to
be in a rotating side and a resin-type oil-less bearing material in a fixing
16
CA 02514271 2005-07-25
side. Testing conditions were as follows: vibration speed: lm/s~ face
pressure: 20 kgf/cm2~ and test duration: 3 hours. As a result, the abrasion
amount of the hard-type alumite was 2.5 pm while that of the present
product was 0.25 ~tm.
When a burning-down test was conducted, the surface pressure of
the burning-down of the present product was twice that of the ordinary
hard-type alumite.
Namely, as for the burning-down test, a wear coefficient was
measured by arranging the test piece to be in a rotating side and a
resin-type oil-less bearing material in a fixing side. A load at the time the
wear coefficient showed an abrupt increase was evaluated as a critical load
of the burning-down. The critical load of the ordinary hard-type alumite
was 160 kgf/cm2, while that of the present product was 320 kgf/cm2.
When a progress of a crack was measured by a high-temperature
test, the present product was small in the number of initial cracks and also
small in the number of cracks increased by heating, compared with
TUFRAM (trade name: a product prepared by subjecting hard-type alumite
to a sintering treatment and, then, impregnating the resultant alumite with
polytetrafluoroethylene).
Namely, when the number of cracks in a measuring area of 16.4
mm2 in a flat portion was measured, those of the present product were 0
before heating and 12 after heating, while those of the TUFRAM were 263
before heating and 321 after heating.
An antibiotic activity test was conducted. Details thereof are
described below.
17
CA 02514271 2005-07-25
(a) Specimen:
Specimen l: aluminum: a surface-treated article subjected to a
silver-impregnating treatment according to the present invention (surface
layer thickness: 25 pm)
Specimen 2: aluminum: non-treated article
(b) Purpose of test
An antibiotic force test is performed on the specimens.
(c) Outline of test
Specimens (hereinafter, referred to also as "samples") were
inoculated with Escherichia coli, Staphyloccocus aureus, Vibrio
parahaemolyticus and Salmonella enteritidis by means of dropping
respective bacterial liquids thereon and, after stored for 24 hours at
35°C,
the number of viable bacteria in each of the samples was counted.
(d) Testing method
(i) Bacteria provided for testing
Escherichia coli IFO 3301
Staphylococcus aureus IFO 12732
Vibrio parahaemolyticus RIMD 2210100 and
Salmonella enteritidis IFO 3313.
(ii) Culture medium
NA culture medium: ordinary agar culture medium
NB culture medium: ordinary bouillon culture medium added with
0.2% of meat extract and
SA culture medium: reference agar culture medium.
(iii) Adjustment of bacterial liquid
18
CA 02514271 2005-07-25
Bacteria used in the test were inoculated in an NA culture medium
at 35°C and incubated for 16 to 24 hours and, thereafter, again
inoculated in
an NA culture medium at 35°C and incubated for 16 to 20 hours. After
such incubation, the resultant fungus bodies of bacteria used in the test
were dispersed in a 1/200 concentration NB culture medium and
appropriately diluted in the 1/200 concentration NB culture medium such
that the number of the fungus bodies come to be 105 to 10~, to thereby
prepare a bacterial liquid. On this occasion, the NA culture medium and
the 1/200 concentration NB culture medium which have been added with
3% of table salt were used for Vibrio parahaemolyticus RIMD 2210100.
(iv) Adjustment of sample
A testing face of the sample was lightly wiped with absorbent cotton
containing 99.9% (v/v) ethanol and, then, sufficiently dried.
(v) Testing operation
0.5 ml of a bacterial liquid was dropped on a sample and, then, after
attached with a polyethylene layer, stored for 24 hours at 35°C and,
thereafter, the number of viable bacteria was counted. Further, 0.5 ml of
the bacterial liquid was dropped in a plastic petri dish and, then, after
attached with a polyethylene layer, allowed to be a reference sample and,
thereafter, subjected to testing in a same manner as in the above. A
parallel measurement was conducted three times.
iv) Measurement of number of viable bacteria
Viable bacteria were washed out of each sample by using 9.5 ml of a
SCDLP culture medium (available from Nihon Pharmaceutical Co., Ltd.).
The resultant washed-out liquid was subjected to a measurement of the
19
CA 02514271 2005-07-25
number of viable bacteria by a pour plate culture method (incubated for 48
hours at 35°C) using an SA culture medium, to thereby determine the
number thereof per sample. On this occasion, the SCDLP culture medium
and the SA culture medium which have been added with 3% of table salt
were used for Vibrio parahaemolyticus.
(e) Test result
The measuring results of the number of viable bacteria of the
bacteria used in the test which has been dropped on the sample are as
shown below in Table 3.
CA 02514271 2005-07-25
TABLE 3
Number of
viable
bacteria
(per sample)
Bacteria used
in
*1 Storage Sample
time
test Test-1 Test-2 Test-3
At *2 5 5 5
-up
~l~e Reference 2.2 x 10 2.5 x 10 1.5 x 10
Specimen <10 *3 <10 ~ <10
1)
l Aft
t
d
i er s
Escherichia ore
co
for 24 Specimen 5.6 x 106 1.0 x 107 1.9 x 104
hrs. at 2)
35
C
Reference*' 1.8 x 10' 1.7 x 107 2.4 x 10'
At *2 5 6 5
-up
~l~e Reference 4.6 x 10 3.4 x 10 4.4 x 10
Specimen 3.5 x 103 <10 <10
lococcus 1)
Sta
h
p ester
y to
d
s
re
aureus for 24 Specimen 1.1 x 104 1.2 x 103 1.0 x 105
hrs. at 2)
35
C
Reference*2 3.0 x 106 6.2 x 106 4.6 x 105
At *Z 5 5 5
-up
~l~e Reference 2.8 x 10 3.5 x 10 3.4 x 10
Specimen < 10 20 2.6 x 102
Vibrio 1)
est
t
d
parahaemolyticuser s Specimen 7.5 x 106 3.5 x 107 4.2 x 107
ore 2)
for 24
hrs. at
35
C
Reference*Z 4.9 x 105 6.5 x 106 4.7 x 105
At *2 5 5 5
-up
~i~e Reference 2.5 x 10 2.2 x 10 2.4 x 10
Specimen <10 <10 <10
Salmonella 1)
ester
to
d
enteritidis s Specimen 80 3.1 x 102 3.2 x 102
re 2)
for 24
hrs. at
35
C
Reference*Z 1.1 x 107 1.7 x 107 8.1 x 105
21
CA 02514271 2005-07-25
In Table 4 below, performances of treated articles (Tough-coat: trade
name) according to the above-described patent documents 1 and 2 and
another treated article (Metal-coat: trade name) according to the
above-described patent document 3 are shown as comparable examples.
These performances are by far excellent compared with known articles but
are inferior to the above-described present product.
22
CA 02514271 2005-07-25
TABLE 4 1/2
Observation Treated
of film
crack thickness
a
Color
g~.face
Sample exhibiting Outer Inner
No surface surface
u'regularityFlat Curved
state evaluation
portionportion Flat CurvedFlat Curved
portionportionportionportion
No.l Although
there is
Hard-type a fine dent Few
of 1 &
Silver Many gad 25.0 22.7 22.5 22.5
l l
i it
20
a mm or narrow
um ess,
te is
a flat. Ex.
No.2 SlightlyThe same Few
as &
Tough-coatdark above Few Excellent26.7 22.8 21.5 22.6
silver
20 a . narrow
No.3 The same SomewhatFew
as but
Tough-coatDark Not 44 37 37 42
silver Ex. 7 8 5 4
20 ~ above. many wide . . . .
No.4 Dark
The same Few
Metal-coatbrownish-as None & Excellent17 16 14 15
5 3 5 5
silver red above. narrow . . . .
25 a
No.5 Light
The same SomewhatFew
Metal-coatbrownish-as & Not 44 38 33 38
Ex 2 4 6 4
silver red above. many narrowl. . . . .
50 a
No.6
Dark The same Few
brass as &
.Metal-coat None Excellent14 13 13 13
8 9 6 8
copper Yellow above. narrow . . . .
25 a
No.7
Light The same SomewhatFew
brass as but
Metal-coat Not 50.0 42.1 42.5 45.0
Yellow above man wide Ex.
copper . y
50 a
Large projections
No.B of about
10 mm
ElectrolessSlightlycan be foundNone None Excellent5 6 5 5
in a 5 5 6 7
nickel dark . . . .
silver plurality
a of places.
Not Ex.
No.9 There are
many
Degreasing projections
and
sever None None Excellent- - - -
treatment recesses
in
only streaks.
Not Ex.
23
CA 02514271 2005-07-25
TABLE 4 212
Hardness Progress
Sample of DischargeComprehensiv
No number d gas a evaluation
of
cracks
HardnessEvaluation150C 200 250 AverageEvaluation
C C
No.l 261 267 260 263 D
Hard-type339 C D ExtremelyD
alumite 23 45 105 58 D small
20
a
18 ? 7 11 B
2 The
N same
o.
Tough-coat483 A B asthe B
20 a 22 13 38 24 B above
37 52 53 47 C
N The
3 same
o.
Tough-coat445 B C asthe C
20 a 37 72 90 66 D above
0 0 0 0 A
No The
4 same
.
Metal-coat464 A A as the A
silver 2 13 20 12 B above
25 a
39 49 28 39 B
No The
same
.
Metal-coat473 A C as the C
silver 35 75 47 52 D above
50 a
0 0 0 0 A
No The
6 same
.
,Metal-coat483 A as the A
copper 17 7 21 16 B above
25 a
33 43 50 42 C
No The
7 same
.
Metal-coat483 A C as the C
copper 35 74 79 63 D above
50 a
No The
8 same
.
Electroless302 C as the C
nickel above
5 a
No.9
The
same
Degreasing78.5 D as the C
treatment
only above
Upper
column:
The
number
of
cracks
at
an
initial
stage
Viper
16.4
dm2)
Lower
column:
Increment
of
cracks
generated
by
the
test
A:0~10 B:11~30C:31~50D:51
24
CA 02514271 2005-07-25
INDUSTRIAL APPLICABILITY
Since the present invention is constituted as described above,
according to the present invention, such action effects as described below
can be achieved.
(1) In the conventional method, although an anodic oxide layer can
easily be formed in an Al-Mn type alloy, it is impossible to treat duralumin
and a die cast alloy and it is difficult to treat other alloys.
On the other hand, according to the method of the present invention,
duralumin, a die cast alloy and all types of other aluminum alloys can be
treated.
(2) In the conventional method, a layer having a thickness of about
30 to about 50 ~m and, even at a maximum, about 100 ~m can be formed.
On the other hand, according to the method of the present invention, a layer
having a thickness as large as 300 to 500 ~m can easily be formed.
(3) As for the layer to be formed according to the conventional
method, although a surface layer thereof is hard (Vicker's hardness: 400 or
less), an inside thereof is porous and hardness thereof is low.
On the other hand, as for the layer to be formed by the method
according to the present invention, a surface layer thereof is hard and a
Vickers hardness is about 450 to about 500. Particularly, a lower layer is
denser and harder than a surface. When a thickness of 50 to 150 ~m is
removed from a surface, hardness becomes 800 to 1000 in terms of Vickers
hardness.
(4) As for the layer to be formed by the method according to the
present invention, heat conductivity thereof is high and is comparable with
CA 02514271 2005-07-25
that of copper.
(5) As for the layer to be formed by the method according to the
present invention, surface thermopenetration resistance is low.
Then, when ice is placed on a tray made of aluminum or an alloy
material thereof having the layer formed by the method according to the
present invention, ice is melted at twice the speed of ice on an untreated
tray. Therefore, for example, the tray can favorably be utilized as a tray
for unfreezing a frozen food. Further, when a container made of aluminum
to be heated for popcorn is subjected to the treatment according to the
method of the present invention, a time period from the time of starting
heating to the time of explosion of the popcorn is reduced from conventional
6 minutes to 3 minutes.
(6) As for the layer to be formed by the method according to the
present invention, heat resistance is as high as about 800°C.
(7) The layer to be formed by the method according to the present
invention has an antibiotic action.
Therefore, an aluminum material or an aluminum alloy material on
which an anodic oxide layer is formed by the method according to the
present invention can favorably be utilized in a wide field, for example, as a
tray for ice-making or unfreezing, a rice cooker, a pan, a kettle, a teakettle
and other cooking devices for heating, an instantaneous hot-water heater, a
heat exchanger, an air-conditioner, a freezer, a refrigerator, an oil heater,
a
radiator, a cooling fin, an air- or water-cooled engine (acceleration of heat
release), a wing of an airplane (de-icing), a heat sink for a semiconductor, a
semiconductor package, a heat pipe, a bearing, various types of sliding
26
CA 02514271 2005-07-25
members, a brake shoe, a manufacturing apparatus for popcorn or
ice-cream, a chassis for an electric apparatus, a casing for a motor, an
electric transformer or the like.
27