Note: Descriptions are shown in the official language in which they were submitted.
CA 02514689 2005-08-05
Sleeve and Micro-Encapsulated Topical Analgesic for Pain Relief
FIELD OF THE INVENTION
The present invention relates to a topical preparation used to reduce or treat
pain,
and a new sleeve used for application of topical preparations. The active
ingredients of
the topical preparation- are micro-encapsulated to provide extended release of
the active
ingredients and improved feel and working characteristics of the preparation.
The micro-
encapsulated active ingredients are especially useful in connection with
preparations
applied via sleeves or patches, however, the encapsulated topical analgesic
may be
administered in the form of a cream, ointment, balm, stick, patch, sleeve or
other forms
used for topical application.
BACKGROUND OF THE INVENTION
Topical preparations in the form of analgesics are widely used to relieve
pain.
They are typically used by consumers with muscle or joint pains, soreness,
sprains or
strains, and are used by consumers unable to tolerate oral analgesics, for
example users
who suffer from ulcers or who are pregnant, or consumers desiring topical
analgesia as an
adjunct to oral medication. Topical analgesics typically come in the form of
ointments,
lotions, solutions and creams that are applied to the affected area. They
relieve the pain
and stiffness of arthritis, rheumatism, muscle and joint soreness, back pain,
bursitis,
tendonitis, muscle strains and sprains, bruises and cramps.- In more recent
years, topical
analgesics have been administered via adhesive patches, particularly in cases
of back
aches and soreness.
Although topical analgesics are effective in providing prompt pain relief,
their
effect is not always long lasting. Some topical analgesics must be reapplied
often because
the effect wears off quickly. Topical analgesics may also be rubbed off during
daily
activities or wiped off by the rubbing of clothes against the affected area.
Recently,
patches consisting of topical analgesics have been used to realize longer
lasting pain
CA 02514689 2005-08-05
2
relief, such as described in U.S. Pat. No. 6,277,401, which teaches a patch
for drug
delivery through a foam layer. Many other topical analgesic patches are simply
a layer of
adhesive impregnated with a topical analgesic formulation and a layer of gauze
or other
fabric. These patches apply the active ingredients in the form of a semi-
flexible patch that
holds the analgesic and protects it from being worn off during daily
activities. Although
usually having longer e~cacy than topical application of creams and ointments,
the
analgesic patches only minimally extend release of the active ingredients and
so the
effectiveness of the analgesic wears off quickly. Moreover, the patches have a
number of
draw backs. Consumers do not like the feel of the patches because they feel
wet, often
have strong menthol odors, and do not adhere well to joints. The application
of the
patches can also be difficult. Additionally, the patches do not stay in place,
except when
used on flat body areas such as the back, which are not subject to substantial
movements
or flexing actions. Finally, use of the patches on hirsute body areas may
result in
additional pain due to unintended hair extraction when the patch is removed.
Despite the existence of topical analgesic creams, lotions and sticks and
topical
analgesics in the form of patches in the prior art, there is room for
improvement. None of
the prior art discloses employing a micro-vesicle system for administration of
the active
ingredients in order to provide for extended release of the active ingredient
resulting in
more effective treatment. Use of the micro-vesicle technology may also reduce
the wet
and soggy feeling associated with the application of analgesic patches,
because less
ointment is required to achieve the desired effect. Moreover, the prior art
does not
disclose the use of topical analgesics in the form of sleeves or joint-
specific applications
so that the topical preparation stays in place through the full range of
motion and repeated
bending of a joint, and without the requirement of an adhesive to hold the
patch in place.
A variety of methods are currently used to administer topical analgesics for
pain
relief. The methods typically involve the topical administration of analgesics
to the
affected area. Some products are administered in the form of creams, lotions,
ointments
and sticks. Although these products provide faster and more localized pain
relief than the
oral administration of drugs, the relief may be short lived if the analgesic
is not long
CA 02514689 2005-08-05
3
lasting or wears off quickly. Other products include patches impregnated with
topical or
external analgesics that are applied to the target area. Although these
products provide
longer lasting pain relief by holding the active ingredients, patches still do
not provide
substantially extended release of active ingredients and do not suitably
adhere to joint
areas. Removal of adhesive patches may also produce consumer discomfort. A
more
effective and long lasting method of administering these products is needed.
The current invention consists of a product for topical administration of
extended
release substances for relieving pain. The invention comprises micro-
encapsulation of
one or more active ingredients to provide extended release of the active
ingredient in the
form of a cream, lotion, ointment or stick. Alternatively, the invention may
be in the form
of a flexible patch or sleeve, or joint specific application, providing an
extended release
active formulation.
SUMMARY OF THE INVENTION
The present invention recognizes and addresses the foregoing disadvantages,
and
others, of prior art compositions and methods.
Briefly, the present invention is a novel topical preparation used to treat
pain and
improve joint function. The topical preparation consists of micro-encapsulated
active
ingredients to provide extended release of the active ingredients. The micro-
encapsulated
topical preparation may be administered in the form of a cream, lotion,
solution,
ointment, balm, stick, patch or other forms used for topical application. The
present
invention may also be used in connection with a sleeve so that the topical
preparation is
easy to apply and stays in place during the full range of motion and repeated
bending of a
joint, and without the requirement of an adhesive.
One novel aspect of the topical preparation of the present invention is the
use of
micro-encapsulated active ingredients for extended release of the active
ingredients and
long lasting relief.
CA 02514689 2005-08-05
4
Another novel aspect of the invention is the use of micro-vesicle technology
to
reduce the unpleasant wet and soggy feelings associated with the application
of analgesic
patches.
Another novel aspect of the present invention is the use of topical analgesics
in
the form of sleeves so that the topical analgesic is suitably positioned at
joint areas and
stays in place.
Another novel aspect of the present invention is the use of topical analgesics
carried in the form of sleeves so that the topical analgesic carrier may be
removed
without producing the consumer discomfort of an adhesive carrier.
More particularly, it is an object of the present invention to provide a
topical
preparation with micro-encapsulated active ingredients in the form of a sleeve
which is
long lasting, easy to apply, and securely positioned at joint areas.
Additional objects and advantages of the invention will be set forth in part
in the
following description, or may be obvious from the description, or may be
learned through
the practice of the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
Certain aspects of the invention may be better appreciated with reference to
the
following illustrations:
FIG. 1 is a top plan view of a sleeve according to the present invention with
patch
area adapted to receive a topical analgesic preparation in the form.
FIG. 2 is a side plan view of the sleeve of FIG. 1.
CA 02514689 2005-08-05
FIGS. 3a and 3b show the sequential application of the sleeve to the wrist.
FIGS. 4a, 4b and 4c show the sequential application of the sleeve to the
elbow.
FIG. 5 illustrates a flattened knit sleeve with an insert.
DETAILED DESCRIPTION OF THE INVENTION
This invention teaches a topical preparation for pain relief in humans and a
flexible sleeve for application of topical preparations. The topical
preparation contains a
micro-encapsulated active ingredient formulation to provide extended release
of the
active ingredients for long lasting pain relief and improved touch and feel of
the
preparation to the consumer. The topical preparation may be in the form of a
stick, cream,
lotion, solution or ointment applied to the affected area. Alternately, the
topical
preparation may be administered in the form of an impregnated patch or sleeve.
The
micro-encapsulation of the active ingredient of the topical preparation
provides
immediate, and long lasting pain relief. In addition, the use of micro-
encapsulated active
ingredients tends to improve the feel of the entire preparation. The topical
preparation
may be used to reduce or treat pain, soreness or stiffness associated with
arthritis,
rheumatism, muscle and joint soreness, back pain, bursitis tendonitis, muscle
strains and
sprains, bruises and cramps.
A variety of topical analgesics may be used in connection with the present
invention. The most common topical analgesics are local anesthetics and anti-
inflammatories such as salicylates or NSAIDS, and counter-irritants including
capsaicin
and aromatic compounds. Anesthetics such as lidocaine, that act on local
sensory
afferents, are intended to totally block pain receptors and numb the area of
application.
Salicylates and NSAIDS such as ibuprofen, are anti-inflammatory compounds that
inhibit
pain and inflammation and are generally taken internally. Counter-irritants
and aromatics,
especially terpenes, are substances such as menthol, oil of wintergreen,
camphor,
eucalyptus, mustard plasters and turpentine oil, that mask sensations of pain
by
CA 02514689 2005-08-05
6
stimulating local pain afferents and thereby creating a feeling of cold or
heat over the
affected area. Capsaicin is a natural ingredient found in cayenne peppers.
Capsaicin is
believed to operate in an anesthetic fashion by depleting Substance-P from
sensory
afferents and thereby suppressing transmission of pain to the brain. Menthol
is a
compound obtained from peppermint oils, or other mint oils, or made
synthetically.
Menthol has local anesthetic and counterirritant qualities.
The preferred active ingredient of the present invention is menthol or
menthol/menthyl derivatives, such as 1-menthol. Effective amounts of menthol
range
from 1.25% to 16% by weight of the preparation. Alternately, an effective
amount of
menthyl lactate may be used, usually between about 0.5% to 20% by weight of
the
preparation. Capsaicin, or other capsaicinoids, vanilloids, or vanillyl butyl
ether, may also
be used. The effective amount of capsaicin ranges from 0.025% to 0.25% by
weight of
the preparation.
The base formula can be any suitable carrier, anhydrous, water-based, an
emulsion of either oil-in-water or water-in-oil, or alcohol. Additional or
alternative
ingredients may be used in the composition of the present invention,
including, the
external analgesic ingredients or combinations recognized in the Food and Drug
Administration's External Analgesic Drug Products for Over-The-Counter Human
Use;
Tentative Final Monograph, published at 48 Federal Register, pp.5852-5869,
Feb. 8,
1983.
The form of furnishing the topical analgesic is novel to the field because it
provides the active ingredient in a micro-encapsulated form which provides for
extended
release of the active ingredient and reduces the soggy wet feeling when the
topical
analgesic is held in the form of a patch. Micro-encapsulation can be achieved
through
liposome or NOVASOMES~. brand technology. The preferred technology for micro-
encapsulation is liposome based technology, without the use of phospholipids.
This
technology is used with products supplied by Novavax Inc. of Buena, New Jersey
under
the NOVASOMES ~. brand. However, other methods of micro-encapsulation may be
CA 02514689 2005-08-05
7
used. NOVASOMES~. based technology generally uses non-phospholipid vesicles
made
from amphiphiles using a manufacturing process, in which drugs can be
encapsulated for
oral or topical delivery into the body. Amphiphiles include fatty alcohols and
acids,
ethoxylated fatty alcohols and acids, glycol esters of fatty acids, glycerol
fatty acid
anmono and diesters, ethoxylated glycerol fatty acid esters, glyceryl ethers,
fatty acid
diethanolamides and dimethyl amides, fatty acyl sarcosinates, alkyds and
phospholipids.
NOVASOMES~. brand vesicles are preferred over traditional phospholipid based
liposomes because they are more stable. NOVASOMES~. brand vesicles are shaped
like
tiny balloons and formed by approximately 5-7 concentric membrane-like outer
walls.
They are typically 0.3-0.7 microns in diameter. They are stable in the
presence of
electrolytes and within a pH range of 2-10.
The topical preparation of the present invention may be provided in the form
of a
cream, lotion, solution, ointment, stick, patch or sleeve. The micro-
encapsulation of the
active ingredients in a preparation administered using a patch provides for
longer lasting
relief which does not feel as wet as typical topical analgesic patches.
Optionally, the
topical preparation may be administered in the form of a sleeve. As such, the
sleeve may
be used on joints such as the knees or wrist and other areas where a patch is
difficult to
keep in place. The material such as foam, hydrogel, nonwoven fabric, knitting
or weaving
of the patch or sleeve can be of any type, such as those commonly used in the
art in
connection with analgesic patches commonly available under the ICY HOT~ brand
from
Chattem, Inc., under the DURA PATCHTM brand from U.S. Dermatologics, Inc.,
under
the THERMO PATCHTM brand from LecTec Corporation, as the MENTHOLATUM
PAIN PATCH~ from the Mentholatum Co., or as the TIGER BALM PATCHTM from
Haw Par Healthcare, Ltd. Preferably, the sleeve material is light and
flexible, or non-
occulsive. In addition to holding the topical analgesic in place, another
advantage of this
invention is the added benefit of joint-specific applications such as joint-
specific sleeves
that provide support of a joint so that the pain is reduced as a result of
compression,
restricted range of motion, or mechanical support.
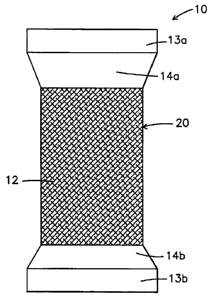
Referring now to the drawings, FIG. 1 is a top plan view of a preferred
CA 02514689 2005-08-05
g
embodiment of the analgesic sleeve 10 according to the present invention. The
sleeve 10
comprises an interior side 11 (shown in FIG. 4a); an exterior side 12; first
and second
welts 13a, 13b forming first and second end sections; first and second
transition areas
14a, 14b interior of the end sections; and a body 20 having a therapeutic
section such as
patch 15 (shown in FIG. 4a) containing the active ingredients located on the
interior side
11 of the sleeve 10. In a preferred use, the sleeve 10 essentially defines a
lumen, having a
wall formed of a flexible and elastic material with a patch area 15 that holds
the micro-
encapsulated topical analgesic. This sleeve 10 may be advantageously used on
joints,
such as the elbows, knees, ankles or wrists. The sleeve 10 is preferably knit
from a
combination of conventional yarns and specialty moisture management yarns.
Fibers
such as Sorbtek from Unifi, Technofine from Gelanots, and 4DG from Fiber
Innovative
Technology, Inc. may be employed for moisture management. Typically,
approximately
one half of the circumference of a sleeve 10 will be composed of moisture
management
yarns and dosed or impregnated with the preparation comprising one or more
topical
analgesics, preferably in micro-encapsulated form.
FIG. 2 is a side plan end view of a preferred embodiment of an analgesic
sleeve
according to the present invention. As in FIG. 1, the sleeve 10 defines a
lumen, with a
wall formed of a flexible material having a patch 15 that holds the micro-
encapsulated
topical analgesic. However, this sleeve 10 also has a thumb cavity 16 in first
transition
area 14a to secure the sleeve 10 in place over the hand and wrist. This
configuration is
particularly well-suited for the management of carpal tunnel syndrome, or
repetitive
motion injury of the forearm and wrist. The transition areas 14a, 14b and
welts 13a, 13b
at each end of the sleeve 10 are not generally comprised of moisture
management yarns
impregnated with analgesic preparations. The sleeves 10 illustrated are
particularly sized
for application to a wrist or small elbow, and additional sizes maybe
appropriately
manufactured for knees, larger elbows, and-to cover the usual range of
dimensions of the
human body. Thus, in a stretched or extended state, the lumen of the sleeves
should
accommodate a human extremity, such as an arm or leg. Furthermore, the
moisture
management system need not be yarn based, but may comprise a hydrogel, foam or
other
material held in place by a sleeve 10 of any suitable material including
nonwovens, and
CA 02514689 2005-08-05
9
especially stretchable non-woven fabrics.
The sleeve 10 according to the present invention should fit snuggly against
the
skin so that the patch 15 is in sufficient contact with the affected area to
be effective. If
the sleeve 10 is too loose, the active ingredients of the patch 15 may not
have sufficient
contact with the affected area to be effective, and so in a relaxed state, the
sleeve should
effectively contact the patch to the affected area of the human extremity
received within
the sleeve. However, if the sleeve 10 is too tight it will cause discomfort to
the affected
area. For user comfort and support, a joint specific sleeve 10, when
appropriately sized,
may optionally be designed to provide a compression of about 3-40 mmHG and
preferably about 7-30 mmHG. The presently preferred compression of the sleeve
10 is
10-15 mm Hg. The wearer may receive pain relief or improved joint function
through
action of analgesic preparations or compression, or both.
The sleeve 10 is preferably manufactured in various sizes to accommodate the
widest range of human limb circumferences while holding the patch 15 of the
sleeve 10
in contact with the skin. For example, a large sleeve 10 that expands to fit
8" to 24" in
circumference is best used for knees, large ankles and elbows. A small sleeve
10 that
expands to fit 5" to 12" in circumference is best used for wrists, small
ankles and elbows.
A preferred embodiment of the sleeve 10 is formed from a continuously knit
fabric bandage roughly in the form of a tube sock. The dimensions of a sleeve
10
according to this invention will typically provide a patch 15 area of at least
about four (4)
inches by two (2) inches in the relaxed condition or about five (5) inches by
four (4)
inches in the stretched condition. The longer welts 13a, 13b at each end of
the sleeve 10
keep the garment from rolling down, slipping or moving when subjected to
typical ranges
of joint motion. For this purpose, the welts 13a, 13b have incorporated in
them coated or
uncoated spandex yarns or other similar elastic yarns.
The thumbhole cavities 16 allow the user to apply the sleeve 10 to the hand or
the
portion of the wrist closest to the hand by cutting the sleeve 10 as
designated on the
CA 02514689 2005-08-05
thumbhole cutout 18. The user may then orient the sleeve with her/his thumb
through the
thumb cavity 16. Two thumbhole cutouts 18 positioned 180 degrees from one
another are
provided on each sleeve 10 to allow the user to apply the sleeve 10 to either
hand and
orient the patch 15 to either the top or bottom portion of the hand or wrist.
Alternatively,
the sleeve 10 may be manufactured with two pre-cut reinforced thumb cavities
16 that
may be used as a thumb hole. The reinforced thumb cavities 16 are preferably
provided
180 degrees from one another to allow the user to apply the sleeve 10 to
either hand and
orient the patch 15 to either the top or bottom portion of the hand or wrist.
When made of knit fabric, the sleeve 10 is preferably constructed of 72% nylon
and 10% spandex/lycra and 18% SORBTEKTM brand synthetic fiber. The absorbent
fiber
is placed in the patch area 15 for maximum efficacy. This results in a
flexible, breathable
sleeve 10 that is comfortable for the user. The patch area 15 is centered
longitudinally on
the sleeve 10. The welts 13a, 13b are preferably knit with nylon and spandex
yarns at the
ends of the sleeve 10. Each welt 13 is preferably 22-28 mm long in its relaxed
state. The
two thumbhole cut outs 18 are preferably knit into the first transition area
14a of each
sleeve 10 in a reinforced area which is approximately 20 mm long×25 mm
wide.
Preferably, black or colored yarn is stitched in the center of each thumbhole
cut out 18 to
guide the consumer to slit the sleeve 10 if a thumbhole is necessary.
The illustrated sleeve 10 may be knit on a 400 needle circular knitting
machine.
The knitting pattern on the body 20 of the sleeve 10 is a 3×2 rib
pattern with three
raised stitches and two recessed stitches forming longitudinal ribs in the
sleeve. The patch
area 15 contains the absorbent yarn, preferably SORBTEKTM. color PMS 30054
(blue)/Unifi color 4895H Sorbtek Blue, plated with nylon. The knitting pattern
of the
patch area 15 is an 8×7 rib pattern. The welts 13a, 13b are knit with
nylon and 210
denier Spandex yarns at each end of the sleeve 10. Each welt 13a, 13b is about
an inch
long in the relaxed state. Welts 13a, 13b are knit in a l×l ribbed
pattern. In the
preferred embodiment of a smaller sleeve for use on wrists and small elbow
joints, there
are two thumbhole cut outs 18 knit into the transition areas 14 of each sleeve
10 in a
reinforced area approximately 20mm long×25mm wide. A line of knitting of
CA 02514689 2005-08-05
11
colored or black yarn is put in the center of each thumbhole cut out 18 to
show the
consumer where to slit the sleeve 10 if the thumbhole is desired. The line of
knitting is
about 15 mm long in the transverse direction across the sleeve 10, and is
centered
longitudinally and transversely within the reinforced thumbhole cut outs 18.
The top edge
of the thumbhole cut outs 18 is 33 mm from the edge of the sleeve 10 including
the welt
13. Both thumbhole cut outs 18 are positioned at the same end of the sleeve
10. The
longitudinal centerline of the thumbhole cut outs 18 is aligned with the
longitudinal edge
of the patch 15 on each side of the patch 15. Thumbhole cut out 18 knitting
pattern is a
positive stitch.
After a knit sleeve 10 has been manufactured, the sleeve 10 is preferably
positioned with its interior 11 facing outward, and the exterior surface 12
being flatly
collapsed against itself. An insert 22, preferably made from about 10 ml PVC,
is placed
between the upper and lower portions of the exterior surface 12 to provide
shape to the
flattened and inside-out sleeve 10. When positioned in this fashion, the patch
area 15
should be substantially on the top side of the PVC insert 22, and the portion
of the body
20 without the absorbent yarn forming the patch area should be positioned
beneath the
PVC insert 22. The PVC insert 22 is positioned in this fashion beneath the
patch area 15
of the body 20 of the sleeve 10 to facilitate the dispensing of the topical
analgesic, in
liposome form, onto the patch, and to some extent to keep the topical
analgesic contained
and concentrated. Even so, there is generally some migration of the topical
analgesic into
yarns other than those forming the patch area 15. A typical dosing of about
two grams of
topical analgesic in liposome form is applied onto the patch area 15. The
particular
formulation of the topical analgesic and materials used for the patch area 15
may affect
the preferred dosage amounts. For a smaller sleeve, the PVC insert 22 is
preferably about
10" long and 2 1/2" wide at its center and tapered at both ends to a 1 3/4"
straight end
perpendicular to the sides. The PVC insert 22 may be perforated or otherwise
scored
across the center to allow for easier folding. This permits the sleeve 10 to
be readily
folded transversely across the center along the perforations in the PVC insert
22, with the
patch area 15 treated with topical analgesic forming the inside of the fold.
When folded,
the ends of the sleeve 10 should match, and the patch area 15 should be folded
upon
CA 02514689 2005-08-05
12
itself. The sleeve 10 is then inserted into a pouch and sealed to maintain the
active
ingredients. The pouch is preferably formed of a plastic and foil laminate
film for this
purpose, and for consumer convenience.
Referring now to FIGS. 3a, 3b, 4a, 4b and 4c in the preferred embodiment, the
sleeve 10 is packaged and reaches the consumer both folded and "inside out."
Thus, when
the sleeve 10 is removed from its pouch and unfolded, the portion of the
sleeve 10 that
contains the patch 15 treated with analgesic is inside out on the "interior"
side 11 of the
sleeve 10 and visible to the consumer. When applying a sleeve 10 to the wrist,
the
consumer first removes the PVC insert 22, and then cuts a slit on a thumbhole
cut out 18
as shown in FIG. 3a, thereby creating a thumb hole 16. The consumer then
applies the
sleeve 10 by pulling the thumb hole 16 over the thumb and the sleeve 10 over
the
affected area while turning the sleeve 10 inside out so that the patch area 15
is positioned
on the area of the wrist where the analgesic is desired.
As shown in FIGS. 4a,4b and 4c, when applying the sleeve 10 to the arm, elbow,
leg, knee or ankle, the consumer first pulls the sleeve 10 adjacent to the
affected area
while the portion of the sleeve 10 that contains the analgesic treated patch
area 15 is
inside out on the "interior" side 11 of the sleeve 10 and visible to the
consumer. The first
welt 13a of the sleeve is positioned next to the affected area, and the
remainder of the
sleeve 10 is opposite the first welt 13a from the affected area as shown in
FIG. 4a. Once
the sleeve 10 is in position, the consumer rolls the second welt 13b of the
sleeve 10 over,
leaving the first welt 13a very nearly in the same position while the inside
out sleeve is
reversed so that the portion of the sleeve 10 that contains the analgesic
patch 15 is left on
the interior side 11 of the sleeve 10 and is placed over the affected area
coming into
contact with the skin as shown in FIGS. 4b and 4c. It is anticipated that in
many cases the
affected area will be in proximity to joints such as wrists, elbows, knees and
ankles.
The present sleeve design may also be utilized in wound care to apply
medication
to a wound, particularly on the arms and legs of a patient.
CA 02514689 2005-08-05
13
The topical analgesics of the invention are further illustrated by means of
the
following illustrative embodiments, which are provided for the purpose of
illustration
only and are not meant to limit the invention to the particular components and
amounts
disclosed. The following examples show composition of preferred embodiments of
topical analgesic formulations.
Example 1 below discloses a micro-encapsulated topical preparation comprising
16% menthol Novasomes solution. The solution is a white, smooth solution with
the
following specifications, stability data and ingredients:
EXAMPLE 1
Specifications
pH=5.0-8.0
Visc=1000-3000 cps
Assay Menthol=15.7% - 16.8%
Spec gravity=0.95000-0.9900
Ingredients Percent By Weight
Deionized Water 48.0-50.0
Menthol USP 15.0-17.0
Diisopropyl Adipate 12.0-14.0
Glyceryl Monostearate 8.0-10.0
Diethylene Glycol mono 4.0-6.0
ethyl ether
Polysorbate 80 2.0-3.0
Glyceryl Dilaurate 2.0-3.0
Soya sterol 1.0-3.0
PEG-150 stearate 0.5-1.5
Cetyl Alcohol 0.5-1.5
Phenoxyethanol 0.3-0.5
Methylparaben 0.1-0.3
Disodium EDTA 0.05-0.15
Xanthan Gum 0.005-0.01
CA 02514689 2005-08-05
14
Example 2 below discloses a micro-encapsulated topical analgesic concentrate
comprising 32% menthol Novasomes solution. The solution is white, smooth
solution
with the following specifications, stability data and ingredients:
EXAMPLE 2
Specifications
pH=6.5-8.5
Visc=5000-8000 cps
Assay Menthol=30.4% -33.86%
Spec gravity=0.9555-0.9755
Ingredients Percent By Weight
Deionized Water 25.0-27.0
Menthol USP 31.0-33.0
Diisopropyl Adipate 15.0-17.0
Diethylene Glycol mono 4.0-6.0
ethyl ether
Glyceryl Monostearate 2.5-3.5
Polysorbate 80 2.0-3.0
Glyceryl Dilaurate 2.0-3.0
PEG-150 stearate .5-1.5
Cetyl Alcohol .5-1.5
Soya sterol .4-.6
Phenoxyethanol .3-.5
Methylparaben .1-.3
Disodium EDTA .OS-.15
Xanthan Gum 0.005-.O1
This concentrate is intended to be diluted to the level set by the FDA
Tentative
Monograph described above.
CA 02514689 2005-08-05
Yet another similar Novasomes formulation is disclosed in Example 3 below with
both menthol and menthyl lactate:
Ingredients Percent By Weight
Deionized Water 38.0-42.0
Menthol USP 15.0-17.0
Menthyl Lactate 15.0-17.0
Diisopropyl Adipate 12.0-17.0
Glyceryl Monostearate 2.5-7.0
Diethylene Glycol mono 2.5-5.0
ethyl ether
Glyceryl Dilaurate 2.0-3.0
Polysorbate 80 1.5-2.5
PEG-150 stearate 0.5-1.5
Soya sterol 0.4-0.6
Cetyl Alcohol 0.4-1.5
Phenoxyethanol 0.3-0.5
Methylparaben 0.1-0.3
Disodium EDTA 0.05-0.15
Xanthan Gum 0.005-0.1
Glycerin 0.1-0.25 Citric Acid (optional)
about 0.05
While preferred embodiments of the invention have been described above, it is
to
be understood that any and all equivalent realizations of the present
invention are
included within the scope and spirit thereof. Thus, the embodiments depicted
are
presented by way of example only and are not intended as limitations upon the
present
invention. While particular embodiments of the invention have been described
and
shown, it will be understood by those skilled in the art that the present
invention is not
limited thereto since many modifications can be made.