Note: Descriptions are shown in the official language in which they were submitted.
CA 02516875 2005-08-24
1
METHOD FOR DIAGNOSIS OF RHEUMATOID ARTHRITIS
BACKGROUND OF THE INVENTION
Field of the Invention
The present invention relates to a method for the diagnosis
of rheumatoid arthritis. More specifically, the present
invention relates to a method for the diagnosis of rheumatoid
arthritis by analyzing a dipeptidyl peptidase and spinorphin,
which are expressed proteins in a sample collected from a subject .
Description of the Related Art
Rheumatoid arthritis (hereinafter abbreviated as RA) is
a disease characterized by chronic multijoint synovial
inflammation, and progressive destruction of cartilages and bone
tissues . It is known that increase in the concentration of many
protein-degrading enzymes (e. g., matrix metalloproteinase
(MMP),cathepsin,peptidase and the like)decompose proteoglycan
and collagen in the cartilage tissue (see Landewe R. and other
seven authors , Arthritis Rheum, 2004 , Vol . 50 , pp . 1390-1399 ,
Tchetverikov I. and other seven authors, An Rheum Dis, 2003,
Vol. 62, pp. 1094-1099). Furthermore, it is known that
interleukin-1(3 (IL-1(3), tumor necrosis factor-a (TNF-a),
various cytokines and enzyme inhibiting factors play important
roles in the etiology of RA (see Cross A. and other three authors,
Arthritis Rheum, 2004, Vol. 50, pp. 1430-1436, Raap T. and other
five authors, J Rheumatol, 2000, Vol. 27, pp. 2558-2565) . These
proteins relate to knee and joint intricately, and make worse
the condition from chronic inflammation to joint destruction.
CA 02516875 2005-08-24
2
For the medication of RA patients , non-steroid antiinflammatory
drugs(NSAIDs), disease-modifying antirheumatic drugs(DMARDs)
and biological drugs (anti-TNF receptors and IL-1 antagonists)
and the like are used (see Klimiuk PA. and other three authors,
J Rheumatol, 2004, Vol. 31, pp. 238-242, Dougados M, and other
six authors, J Rheumatol, 2003, Vol. 30, pp. 2572-2579, De A.
and other seven authors , J Rheumatol , 2002 , Vol . 29 , pp . 46-51 ) .
However, despite of intensive treatments, conditions of many
RA patients are not recovered, and the patients continue to feel
pain and to receive bone destruction. The pain of RA is
considered to be chronic and invasive, and is a signal that
stimulates various nociceptive receptors in peripheral regions
(knees and joints ) and is transferred to the cerebrum via the
spinal cord and perceived by the cerebrum.
As mentioned above, RA is a disease that can lead to serious
condition. However, current diagnosis method of RA is based
on the "ACR Revised Diagnosis Criteria" by the American College
of Rheumatology (ACR). The criteria of the diagnosis are 1)
morning stiffness (mainly in hands and fingers ) , lasting 1 hour
or more; 2 ) swelling of 3 or more joint areas ; 3 ) swelling in
handjoints(wristjoints,metacarpophalangealjoints,proximal
phalanx joints ) ; 4 ) swelling of symmetric joints ( the same joint
areas of the left and right hands ) ; 5 ) abnormal observation in
an X-ray photograph of hands; 6) subcutaneous nodule and 7)
positive response of RA in blood test . A patient shall be said
to have RA if he/she has satisfied with 4 or more criteria. In
the blood test , rheumatoid factor ( RF ) in serum is mainly measured .
RF is a self-antibody against Fc portion of IgG that appears
CA 02516875 2005-08-24
3
frequently in the serum of an RA patient . RF in a healthy person
is a polyactive antibody that reacts with various proteins other
than IgG, while a monoactive RF that reacts with only IgGFc exists
in RA patients besides the polyactive RF. The reactivity of
the monoactive RF to IgGFc is about 100-fold higher than that
of polyactive RF . Accordingly , said RF is used in the measurement
as an index for the determination of RA patients . However, there
are RA patients who are suffering from serum reaction-negative
RA in which RF is always not detected. In addition, it is known
that about 2~ of healthy persons can exhibit positive reaction.
Accordingly, a method for the determination of RA,
comprising subjecting urine collected from an RA patient to
liquid chromatography to detect the chromatogram peak of a
component specific to RA (see JP-A-1995-72133), a method for
the diagnosis of RA, comprising cleaving a sugar chain from a
sugar protein, labeling the sugar chain and purifying the labeled
sugar chain to prepare a sample for the analysis of the sugar
chain, analyzing the prepared sample by high performance liquid
chromatography using an ODS-silica column to give a composition
of the sugar chain in the sample, and diagnosing RA based on
the change in the composition of the sugar chain (see
JP-A-1996-228795) and the like have been suggested.
However, these methods are insufficient because the methods
show peaks higher than those observed in a healthy person, and
preparation of samples for the analysis in the methods are
complicated.
SUMMARY OF THE INVENTION
CA 02516875 2005-08-24
4
The present invention aims at providing a method for the
diagnosis of RA.
The pain of RA is considered to be chronic and invasive,
and is a signal that stimulates various nociceptive receptors
in peripheral regions (knees and joints) and is transferred to
the cerebrum via the spinal cord and perceived by the cerebrum.
A neuropeptide called as spinorphin (LV~TYPWT; sequence listing:
SEQ ID NO: 1 ) was isolated. Spinorphin is an endogenous peptide
derived from bovine spinal cord, which plays a role in the
activation of anti-inflammatory and anti-invasive properties .
It is known that spinorphin suppresses bradykinin (BK)-induced
nociceptive pain reaction in animal models, and that spinorphin
suppresses various functions of polymorphonuclear neutrophils
( PMNs ) , such as chemotaxis , 02 generation, exocytosis and the
like.
In order to elucidate the role of spinorphin in the control
of inflammation and pain, the present inventors have done various
studies with a focus on the change in activities of spinorphin
and metabolic enzymes in the cerebrospinal fluid ( CSF ) from RA
patients suffering from chronic pain and inflammatory condition ,
thereby to find that the amount of spinorphin, and the activation
of the dipeptidyl peptidase in CSF changed in RA patients.
Furthermore, the present inventors have compared the
activity of the dipeptidyl peptidase and the amount of
spinorphin using synovial fluid collected from joints of RA
patients and patients suffering from osteoarthritis
(hereinafter abbreviated to as OA) in order to investigate the
roles of the activity of the dipeptidyl peptidase and spinorphin
CA 02516875 2005-08-24
in inflammation and nociceptive pain reaction mechanism. As
a result, the inventors have found that the activity of the
dipeptidyl peptidase increases and the amount of spinorphin
decreases in an RA patient , and that a correlation exists between
5 the increase of the activity of the dipeptidyl peptidase and
the decrease of the amount of spinorphin, which resulted in the
completion of the present invention.
Namely, the present invention relates to:
(1) a method for the diagnosis of rheumatoid arthritis,
comprising measuring the activity of the dipeptidyl peptidase
in a sample collected from a mammal;
( 2 ) the method for the diagnosis according to the above-mentioned
(1), wherein the sample is at least one member selected from
joint synovial fluid, blood and cerebrospinal fluid;
( 3 ) the method for the diagnosis according to the above-mentioned
( 1 ) or ( 2 ) , further comprising measuring the amount of spinorphin
in the sample collected from a mammal;
( 4 ) the method for the diagnosis according to the above-mentioned
(3), wherein rheumatoid arthritis disease is estimated at a
spinorphin concentration of not more than 5 ng/mL in the measured
sample;
( 5 ) a method for measuring the activity of a dipeptidyl peptidase
for the diagnosis according to the above-mentioned (1),
comprising reacting a fluorescence substrate specific to a
dipeptidyl peptidase and an aminopeptidase inhibitor with a
sample in the presence or absence of a dipeptidyl peptidase
inhibitor, and measuring the fluorescence intensity of the
reaction solution after the reaction;
CA 02516875 2005-08-24
6
( 6 ) the measurement method according to the above-mentioned ( 5 ) ,
wherein the fluorescence substrate is a synthetic fluorescence
substrate having an Arg-Arg group;
( 7 ) the measurement method according to the above-mentioned ( 6 ) ,
wherein the synthetic fluorescence substrate is Arg-Arg-4
methylcoumaryl-7-amide;
( 8 ) the measurement method according to the above-mentioned ( 7 ) ,
comprising measuring the fluorescence intensity of
7-amino-4-methylcoumarin produced by the reaction;
(9) the measurement method according to any one of the
above-mentioned(5)to(8),wherein the aminopeptidase inhibitor
is bestatin;
(10) the measurement method according to any one of the
above-mentioned (5) to (9), wherein the dipeptidyl peptidase
inhibitor is tynorphin;
( 11 ) a kit for the diagnosis of rheumatoid arthritis, comprising
a fluorescence substrate specific to a dipeptidyl peptidase,
an aminopeptidase inhibitor and a dipeptidyl peptidase
inhibitor;
( 12 ) the kit according to the above-mentioned ( 11 ) , wherein the
fluorescence substrate is a synthetic fluorescence substrate
having an Arg-Arg group; and
( 13 ) The kit according to the above-mentioned ( 12 ) , wherein the
synthetic fluorescence substrate is Arg-Arg-4-methylcoumaryl-
7-amide.
According to the present invention, the activity of the
dipeptidyl peptidase in a sample, especially in the synovial
fluid collected from the joint of RA patients is specifically
CA 02516875 2005-08-24
7
higher than that of an OA patient . This means that the activity
of the dipeptidyl peptidase in a sample can be used as a criterion
for the diagnosis of RA. Accordingly, measurement of the
activity of the dipeptidyl peptidase in a sample for the
estimation and diagnosis of RA can be used as a novel method
of the diagnosis of RA.
Furthermore , the amount of spinorphin is low , i , a . , 5 ng/mL
in a sample, especially in the synovial fluid collected from
the joint of an RA patient. This means that the amount of
spinorphin in the sample can be used as a criterion of the diagnosis
of RA.
Accordingly, by measuring the amount of spinorphin besides
the above-mentioned measurement of the activity of the dipeptidyl
peptidase for the estimation and diagnosis of RA, the accuracy
of the diagnosis of RA can be improved, and said method can be
used as a novel method for the diagnosis of RA.
BRIEF DESCRIPTION OF THE DRAWINGS
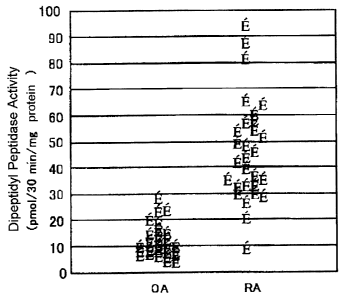
Fig. 1 shows the activity of the dipeptidyl peptidase in
the synovial fluid collected from OA patients and RA patients.
Fig. 2 shows the amount of spinorphin in the synovial fluid
collected from OA patients and RA patients.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
Hereinafter , the method for the diagnosis of RA of the present
invention is explained.
The sample that can be used for the present invention is
not specifically limited so long as it is collected from a mammal .
CA 02516875 2005-08-24
8
Examples of the sample may include samples from a living body
such as cerebrospinal fluid, joint synovial fluid, blood, plasma,
serum, saliva and urine, and various organs and tissues and the
like collected from animals such as humans , rats , mice , dogs ,
cattle, cats, rabbits, guinea pigs and the like. Among them,
joint synovial fluid, blood and cerebrospinal fluid are
pref erred .
The activity of the dipeptidyl peptidase in a sample can
be measured by reacting the sample with a reaction solution
comprising a fluorescence substrate specific to the dipeptidyl
peptidase and an aminopeptidase inhibitor in the presence or
absence of the dipeptidyl peptidase inhibitor, and measuring
the fluorescence intensity of the reaction solution after the
reaction.
Firstly, a reaction solution is prepared. There are
prepared a reaction solution obtained by adding a fluorescence
substrate and an aminopeptidase inhibitor to a buffer having
a pH suitable for the activity of the dipeptidyl peptidase,
preferably a pH of about 7 to 9 , and a reaction solution further
comprising a dipeptidyl peptidase inhibitor besides the
above-mentioned buffer, fluorescence substrate and
aminopeptidase inhibitor.
Examples of the buffer may include , for example , Tris
hydrochloric acid buffer, phosphate buffer and the like.
Examples of the fluorescence substrate may include, for
example, synthetic substrates having an Arg-Arg group such as
Arg-Arg-MCA, Boc-Gly-Arg-Arg-MCA, Suc-Ala-Ala-Phe-AMC and the
like. As used herein, MCA means 4-methylcoumaryl-7-amide, Boc
CA 02516875 2005-08-24
9
means t-butoxycarbonyl group, Suc means succinyl group and AMC
means 7-amino-4-methylcoumarin. Hereinafter MCA, Boc and AMC
have the same meanings as mentioned above.
The amount of the fluorescence substrate to be added is
preferably an amount sufficient to react with the dipeptidyl
peptidase in the sample, and is generally preferably about 0.2
t o 5 ~u,M .
Preferable examples of the aminopeptidase inhibitor may
include bestatin, leuhistin and the like. The aminopeptidase
inhibitor is added so that the reaction of aminopeptidase with
the above-mentioned substrate can be inhibited and that the
substrate specificity of the dipeptidyl peptidase to the
substrate can be increased. Thus, the enzyme activity of the
aminopeptidase in the sample can be inactivated. Therefore,
the amount of the aminopeptidase inhibitor is preferably an
amount sufficient for suppressing the aminopeptidase activity
in the sample, and is generally preferably about 50 to 250 ~,g/mL.
It is preferable that the synthetic fluorescence substrate
is preincubated with the aminopeptidase inhibitor at about 30
to 40°C, preferably about 37°C, for about 1 to 30 minutes.
It is preferable that the dipeptidyl peptidase inhibitor
is an inhibitor that specifically inhibits the dipeptidyl
peptidase to be used, and example thereof may include tynorphin
and the like . The amount of the dipeptidyl peptidase inhibitor
to be used is preferably an amount sufficient to inhibit the
activity of the dipeptidyl peptidase in the sample, and is
generally preferably about 50 to 200 ~,g/mL.
It is preferable that the reaction of the sample and the
CA 02516875 2005-08-24
above-mentioned reaction solution is incubated at about 20 to
40°C , preferably at about 37°C , for usually about 10 to 60
minutes ,
preferably about 20 to 40 minutes. By such incubation, for
example, in the case where Arg-Arg-MCA is used as a fluorescence
5 substrate, the fluorescence substrate liberates
7-amino-4-methylcoumarin (AMC) in a reaction solution by an
enzyme (dipeptidyl peptidase). This reaction can be
represented by the equation: Arg-Arg-MCA ~ Arg-Arg + AMC.
Secondly, it is preferable that the reaction is stopped
10 by the addition of a reaction stopping solution (e. g., acetic
acid, sodium acetate and the like) to the above-mentioned
reaction solution.
After the reaction is stopped, the fluorescence intensity
of the liberated reaction product is measured. The wavelength
for the measurement of the fluorescence intensity differs
depending on the reaction product. For example, in the case
where the reaction product is the above-mentioned AMC, it can
be measured at the excitation wavelength of 360 nm and the
fluorescence wavelength of 440 nm.
The activity of the dipeptidyl peptidase in the sample can
be explained by the following equation:
Activity = (Activity of substrate degradation in the absence
of a dipeptidyl peptidase inhibitor) - (Activity of substrate
degradation in the presence of a dipeptidyl peptidase inhibitor ) .
Such activity can be expressed in terms of the activity
per 1 mg of the protein relative to the total amount of the protein
in the sample, i.e. , the unit of pmol/incubation period (min)/mg
protein.
CA 02516875 2005-08-24
11
The total amount of the protein in the sample can be measured
by known methods for measuring proteins, e.g. , by the Bradford
method (Pierce Biotechnology, Inc. , Rockford, USA) and the like.
The above-mentioned activity of the substrate degradation
can be converted to a value per 1 mg of the protein in the sample
by depicting a standard curve based on the relationship between
the concentration of AMC and the value of fluorescence, and
calculating the value from the standard curve.
Although the activity of the dipeptidyl peptidase obtained
by the measurement based on the above-mentioned method varies
depending on the conditions of the measurement (reaction
temperature , reaction period and the like ) and the like , RA can
be diagnosed based on the measured activity value . For example,
using the synovial fluid collected from the joint of a subject
as a sample, RA can be diagnosed and estimated, for example,
by incubating synovial fluid ( 100 ~L) with Arg-Arg-MCA ( 1 ~u,M)
in a reaction mixture comprising 50 mM Tris hydrochloric acid
( pH 7 . 4 , 1 mL ) containing bestatin ( 30 ~ug/mL ) , in the absence
or presence of tynorphin at 37°C for 30 minutes and measuring
the fluorescence (excitation wavelength: 360 nm, fluorescence
wavelength: 440 nm) of the solution after incubation. In the
case where the activity of the dipeptidyl peptidase is not less
than about 20 pmol/30 min/mg protein, preferably about 30 to
200 pmol/30 min/mg protein, the patient can be diagnosed and
estimated as RA.
Examples of the dipeptidyl peptidase that can be measured
by the above-mentioned measurement of the activity of the
dipeptidyl peptidase may include, for example, dipeptidyl
CA 02516875 2005-08-24
12
peptidase III (DPPIII) of enkephalin-degrading enzyme,
dipeptidyl peptidase I (DPPI) of lysosome cystein protease,
dipeptidyl peptidaseII(DPPII),dipeptidyl peptidaseIV(DPPIV)
and the like, but are not limited thereto . Even if the dipeptidyl
peptidase used is any type, RA can be diagnosed and estimated
so long as the measured activity of the dipeptidyl peptidase
by the above-mentioned method is high.
Furthermore, the present invention also provides a method
for the diagnosis of RA, which comprises measuring the amount
of spinorphin in the sample in addition to the above-mentioned
measurement of the activity of the dipeptidyl peptidase.
The measurement of the amount of spinorphin in the sample
comprises the following steps (A) to (E):
(A) mixing a sample collected from a living body with
trichloroacetic acid to give a solution phase, and subjecting
said solution phase and a solvent to a reverse phase column
chromatography to elute spinorphin with said solvent;
(B) contacting the spinorphin eluted in the step (A) with
spinorphin immobilized on a carrier and a spinorphin antibody;
(C) removing the spinorphin antibody that has not been bound
to the spinorphin immobilized on the carrier;
(D) contacting the spinorphin antibody bound to the spinorphin
immobilized on the carrier obtained in the step ( C ) with a labeled
secondary antibody that specifically binds to said spinorphin
antibody so as to binding the spinorphin antibody bound to the
spinorphin immobilized on the carrier and the labeled secondary
antibody; and
(E) measuring the amount of label in the labeled secondary
CA 02516875 2005-08-24
13
antibody bound to the spinorphin antibody.
In the step (A) , a sample collected from a living body is
mixed with trichloroacetic acid to give a solution phase, and
said solution phase is then subjected to a reverse phase column
chromatography to elute spinorphin with a solvent.
Although the usage form of trichloroacetic acid varies
depending on the kind of the sample collected from a living body
to be measured, it is preferable to use an aqueous solution of
trichloroacetic acid comprising trichloroacetic acid of about
5 to 20% by mass. The amount of trichloroacetic acid is
preferably an amount sufficient to precipitate proteins in the
sample collected from a living body. It is preferable to add
the aqueous solution of trichloroacetic acid in an amount of
about 0.5 to 2 volume ratio relative to 1 volume of the solution
of the sample collected from a living body. The solution phase
can be separated by a known method such as filtration,
centrifugation or the like. Preferable examples of the column
for the reverse phase column chromatography may include an ODS
column and the like . The ODS column chromatography is carried
out using a column filled with a filler in which octadecylsilyl
groups ( ODS groups , C18 groups ) have been chemically bound to
a silica gel carrier. Examples of the ODS column may include
ODS-A 60-60/30 (manufactured by YMC Co., Ltd.), Inertsil ODS
(manufactured by GL Sciences, Inc. ) , L-column ODS (manufactured
by Chemicals Evaluation and Research Institute), Develosil ODS
UG-5 (manufactured by Nomura Chemical Co. , Ltd. ) , CAPCELL PAK
C18 MGII (manufactured by Shiseido Co. , Ltd. ) , ZORBAX XDB-C18
(manufactured by Yokogawa Analytical Systems Inc. ) , Symmetry
CA 02516875 2005-08-24
14
C18 (manufactured by Waters, Inc. ) , Nucleosil C18 (manufactured
by M. Nagel Co., Inc.) and the like.
Examples of the solvent to be used to elute spinorphin may
include alcohols having 1 to 5 carbon atom( s ) ( a . g . , methanol,
ethanol , propanol and the like ) and acetonitrile , a mixed solvent
of two or more kinds of these solvents, and a mixed solvent of
the above-mentioned solvent and water.
Secondly, in the step (B), the spinorphin eluted in the
step (A) is contacted with the spinorphin immobilized on a carrier
and a spinorphin antibody.
Examples of the carrier on which spinorphin is to be
immobilized may include a microtiter plate, a test tube, polymer
beads and the like. Since spinorphin is an oligopeptide, a
carrier having active functional groups such as amino groups ,
carboxyl groups and the like on the surface , and a carrier having
a coat slide of a hydrophobic polymer (e. g., Immuno Plate,
MaxiSorp (manufactured by Nalge Nunc International)) and the
like are preferred. It is preferable that spinorphin is
immobilized on a carrier by covalent bonding of spinorphin to
the surface of the carrier. It is preferable that said bonding
is carried out by dissolving spinorphin in a buffer such as
phosphate buffer, Tris-HC1 saline or the like, contacting said
solution with the carrier, and incubating the carrier at the
temperature of about 0 to 50°C, preferably at room temperature,
for at least not less than about 30 minutes , preferably about
to 120 minutes . It is preferable that the carrier on which
spinorphin has been immobilized is blocked with a blocking agent .
Examples of the blocking agent may include skim milk , Block Ace
CA 02516875 2005-08-24
(trade mark) and the like.
Spinorphin can be produced by a known method, for example,
the method described in JP-A-2000-95794.
As a preferable spinorphin antibody used for the present
5 invention, any antibody can be used so long as it specifically
binds to spinorphin. The spinorphin antibody can be prepared
easily by sensitizing an animal ( a . g . , rabbit , rat or the like )
using spinorphin as an antigen, and separating and purifying
the obtained antibody by a conventional method. It is preferable
10 to conjugate spinorphin with a carrier protein so as to increase
immunogenicity, because spinorphin is a peptide consisting of
seven amino acids and is generally too short to exhibit
immunogenicity. Examples of such carrier protein for
conjugation may include, for example, KLH (Keyhole Limpet
15 Hemocyanin), bovine serum albumin, ovalbumin and the like.
Examples of the method for conjugation may include a method
comprising activating the C-terminal carboxyl group of
spinorphin with a carbodiimide (e.g. 1-ethyl-3-(3-
dimethylaminopropyl)carbodiimide, etc.) and reacting the
activated spinorphin with a primary amine of a carrier protein
(EDC method) , a method comprising binding an amino group of a
protein and an SH group of a peptide (spinorphin) using MBS
(maleimide benzoyloxysuccinimide)-based crosslinking agent
(MBS method) and the like.
As the antibody prepared by the above-mentioned method,
either a polyclonal antibody or a monoclonal antibody can be
used.
It is preferable that the spinorphin antibody obtained as
CA 02516875 2005-08-24
16
above is specific to spinorphin. It is preferable that the
spinorphin antibody does not cross-react with spinorphin
analogues such as WYPWT ( sequence listing : SEQ ID NO: 2 ) , VYPWT
(sequence listing: SEQ ID NO: 3) , hemorphin-4 (YPWT; sequence
listing: SEQ ID NO: 4 ) , PWT, LWYPW ( sequence listing: SEQ ID
NO: 5 ) , tynorphin ( WYPW; sequence listing : SEQ ID NO: 6 ) , VYPW
( sequence listing: SEQ ID NO: 7 ) , YPW, LWYP ( sequence listing:
SEQ ID NO: 8 ) , WYP ( sequence listing: SEQ ID NO: 9 ) , VYP and
the like.
In the step (B), it is preferable that the contact of
spinorphin eluted in the step (A) with the spinorphin immobilized
on the carrier and the spinorphin antibody is performed by
contacting a solution wherein the above-mentioned spinorphin
rabbit antibody and the spinorphin eluted in the step (A) have
been dissolved or optionally diluted in a buffer (e. g., Tris
buffer saline, phosphate buffer and the like) , with a carrier
on which spinorphin has been immobilized. Such contact is
preferably carried out at usually 0°C to room temperature, while
allowing to stand or shaking slowly, for about 30 minutes to
2 hours, preferably about 30 minutes to 1 hour. During said
reaction, the spinorphin immobilized on a carrier and the
spinorphin eluted in the step (A) compete with the spinorphin
antibody so that the spinorphin immobilized on a carrier can
bind to the spinorphin antibody.
Secondly, in the step (C), the spinorphin antibody that
has not been bound to the spinorphin immobilized on a carrier
is removed.
I t is preferable that said removal is carried out , for example ,
CA 02516875 2005-08-24
17
by decanting the carrier to discard the solution contacting with
the carrier, or aspirating the solution using a pipette. It
is preferable that the carrier is further washed with the
above-mentioned buffer and the like at least 3 times , preferably
about 3 to 6 times.
Then, in the step (D), the spinorphin antibody bound to
the spinorphin immobilized on the carrier obtained in the step
(C) is contacted with a labeled secondary antibody that
specifically binds to said spinorphin antibody so as to binding
the spinorphin antibody bound to the spinorphin immobilized on
the carrier and the labeled secondary antibody.
Examples of the labeled secondary antibody may include
anti-rabbit IgG goat IgG antibody, goat anti-mouse antibody and
the like that are labeled with a label enzyme or a fluorescence
dye. Examples of the label enzyme may include peroxidase,
glucose oxidase, acidic phosphatase, alkaline phosphatase and
the like, preferably peroxidase, and especially preferably
horseradish peroxidase. Examples of the labeling fluorescence
dye may include MFP 488 , Alexa Fluor 488 , rhodamine, fluoresceine,
Cy2, Cy3 and the like.
The binding between the spinorphin antibody bound to the
spinorphin immobilized on a carrier and the labeled secondary
antibody is carried out usually at 0°C to room temperature, while
allowing to stand or shaking slowly, for about 30 minutes to
2 hours, preferably about 30 minutes to 1 hour.
In the step ( E ) , the amount of label in the labeled secondary
antibody bound to the spinorphin antibody in the step (D) is
measured.
CA 02516875 2005-08-24
18
In the case where a secondary antibody labeled with an enzyme ,
for example, is used, the amount of label in the labeled secondary
antibody bound to the spinorphin antibody bound to the
immobilized antigen ( spinorphin ) can be measured by measuring
the light absorbance at the wavelength corresponding to the color
tone of the color reaction between the label enzyme and the
substrate . The color reaction between the label enzyme and the
substrate can be carried out by contacting the substrate solution
with the enzyme-labeled secondary antibody. The substrate
differs depending on the label enzyme to be used. In the case
where the label enzyme is, for example, horseradish peroxidase,
examples of the preferable substrate to be used may include
o-phenylenediamine, 3,3',5,5'-tetramethylbenzidine and the
like. It is preferable that the light absorbance is measured
within about 10 minutes to 60 minutes after coloring.
In the case where fluorescence labeling is used, the
fluorescence intensity may be measured using a fluorometer.
In the case where the carrier is amicroplate, it is preferable
to use an autoreader or a microplate reader that can measure
lightabsorbancesuccessively and automatically in the case where
enzyme labeling is used, and it is preferable to use a fluorescence
microreader or the like in the case where fluorescence labeling
is used.
In the above-mentioned method, spinorphin can be measured
quantitatively in the concentration range of preferably about
10-1° to 10-' g/mL. Accordingly, in the case where a sample
comprising spinorphin at high concentration is used, it is
preferable to dilute the sample as appropriate with the
CA 02516875 2005-08-24
19
above-mentioned buffer and the like.
By using the method for the measurement of the present
invention, for example, in the case where the amount of spinorphin
in the synovial fluid collected from the joint of a subject is
measured to be about not more than 5 ng/mL, the subject can be
estimated to be suffering from rheumatoid arthritis.
Subsequently, the above-mentioned activity of the
dipeptidyl peptidase and the amount of spinorphin can be analyzed
statistically as follows. For example, the activity of the
dipeptidyl peptidase and the concentration of spinorphin in the
synovial fluid from an RA patient and an OA patient can be compared
by, for example, Mann Whitney U test . As a result of comparison,
in the case where the risk rate is not more than 5~, there may
be a significant difference. According to the present invention,
a negative correlation can be obtained by, for example, plotting
the activity of the dipeptidyl peptidase at the vertical axis
and the amount of spinorphin at the horizontal axis . From this
fact, RA can be diagnosed and estimated with high probability
by diagnosing and estimating from the characteristic values of
both the activity of the dipeptidyl peptidase and the amount
of spinorphin. Specifically, for example, in the case where
the synovial fluid in the joint of the subject is used as a sample
and the activity of the dipeptidyl peptidase in the synovial
fluid is not less than about 20 pmol/30 min/mg protein, preferably
about 30 to 200 pmol/30 min/mg protein, and the amount of
spinorphin is not more than 5 ng/mL, the probability of RA is
very high.
In the kit for diagnosis of RA of the present invention,
CA 02516875 2005-08-24
examples of the fluorescence substrate that is specific to the
dipeptidyl peptidase may include, for example, synthetic
fluorescence substrates having an Arg-Arg group such as
Arg-Arg-4-methylcoumaryl-7-amide, Boc-Gly-Arg-Arg-4-MCA and
5 the like.
Examples of the aminopeptidase inhibitor may include
bestatin, leuhistin and the like.
Examples of the dipeptidyl peptidase inhibitor may include
tynorphin and the like.
10 According to the kit for the diagnosis of RA of the present
invention, whether the person who provides a sample is suffering
from RA or not can be diagnosed readily and accurately within
a short period of time.
In the present invention, besides the above-mentioned kit
15 for diagnosis of RA, a kit comprising a spinorphin antibody,
an antigen for coating (spinorphin), a microtiter plate, a
labeled secondary antibody and other reagents may also be used
for the measurement of the concentration of spinorphin in a
sample.
20 For the kit for the measurement of the concentration of
spinorphin, examples of the spinorphin antibody may include a
spinorphin rabbit antibody and the like . Examples of the antigen
for coating may include a solution of spinorphin in a buffer.
Preferable examples of the microtiter plate may include a
microtiter plate having active functional groups such as amino
groups , carboxyl groups and the like on the surface , a microtiter
plate having a coat slide of a hydrophobic polymer.
Alternatively, a kit comprising a microtiter plate on which
CA 02516875 2005-08-24
21
spinorphin as an antigen has been immobilized, a spinorphin
antibody and a labeled secondary antibody instead of an antigen
for coating and a microtiter plate may be used. Examples of
the preferable labeled secondary antibody may include the
above-mentioned enzyme-labeled secondary antibody and
fluorescence-labeled secondary antibody. Further, in the case
where an enzyme-labeled secondary antibody is included in the
kit, other reagents may include, for example, the above-
mentioned substrate and the like, a washing liquid for plate
(such as a buffer added with a surfactant (e. g., Polysorbate
, etc . ) , etc . ) , a buffer ( a . g. , phosphate buffer , Tris buffer
saline) and the like.
According to the above-mentioned kit for the measurement
of the concentration of spinorphin, the concentration of
15 spinorphin in the sample can be measured readily and accurately
within a short period of time.
EXAMPLES
Hereinafter, the present invention is explained by way of
20 Examples, but the present invention is not limited thereto.
Reagent:
Spinorphin and tynorphin were obtained from the American
Peptide Company Inc., Sunnyvale, CA, USA.
Arg-Arg-MCA and bestatin were purchased from Sigma-Aldrich
Fine Chemical Co., St. Louis, MO, USA. HRP-F (ab')2 goat
anti-rabbit IgG (H+L) was purchased from Zymed Laboratories,
Inc. , Carlton Court, CA, USA. As the ODS column, ODS-A 60-60/30
(manufactured by YMC Co., Ltd., Kyoto, Japan) was used. All
CA 02516875 2005-08-24
22
of other reagents used were of high grade.
Collection of samples:
Synovial fluid was collected from OA patients ( 40 persons )
and RA patients ( 39 persons ) . A sample of the synovial fluid
was sucked from the knee joint of an outpatient, placed into
a plastic container and centrifuged at 4°C for 10 minutes at
1500xg. The supernatant was kept at -20°C until analysis.
Diagnosis of OA and RA was based on the "ACR Revised Diagnosis
Criteria" by the American College of Rheumatology (ACR).
The ages of OA patients were 74 to 88 years old (11 men
and 29 women ) , and their average morbid period was 6 years ( range
0 . 1 to 20 years ) . The ages of RA patients were 44 to 74 years
old ( 6 men , 32 women ) , and their average morbid period was 14 . 5
years ( range : 1 to 36 years , least erosive subset ( LES ) 3 persons ,
more erosive subset (MES ) 29 persons , multilating disease (MUD )
6 persons ) . All of the RA patients took several kinds of
medicines. The medicines were an anti-inflammatory agent, a
gold compound, methotrexate, sulfasalazine, a corticosteroid,
bucillamine and D-penicillamine. There was no person who had
received a corticosteroid or an intra-articular steroid at high
concentration before sucking of the synovial fluid. The
approval from each patient was reviewed by the Committee, and
individual informed concept was obtained from each patient.
The amount of protein in the synovial fluid was measured
by the Bradford method (Pierce Inc., Rockford, USA).
Statistical processing:
The activity of the dipeptidyl peptidase and the
concentration of spinorphin in the synovial fluid of RA patients
CA 02516875 2005-08-24
23
and OA patients were determined by the Mann Whitney U test , and
significant difference was recognized where the risk rate is
not more than 50.
EXAMPLES
Example 1
Dipeptidyl peptidase activity:
Dipeptidyl peptidase activity was measured by hydrolysis
of Arg-Arg-MCA on a specific synthetic fluorescence substrate.
Firstly, 50mM Tris-HCl buffer comprising bestatin (30 ~.g/mL)
and Arg-Arg-MCA ( 1 ~.vM) ( pH 7 . 4 , 1 mL ) was pre-incubated at 37°C
for 30 minutes. After the pre-incubation was completed, the
synovial fluid ( 100 ~.L) was added to the buffer and mixed, and
the mixture was incubated in the presence or absence of a
dipeptidyl peptidase inhibitor ( 100 ~ug/mL ) at 37°C for 30 minutes .
The reaction was stopped by adding 50~ (v/v) aqueous acetic acid.
The mixture was allowed to stand at 4°C for 10 minutes , and the
fluorescence intensity of the liberated AMC (which shows the
dipeptidyl peptidase activity) was measured using a fluorescence
spectrum spectrometer F-2000 (manufactured by Hitachi Co. Ltd,
Tokyo, Japan) at the excitation wavelength of 360 nm and the
fluorescence wavelength of 440 nm.
The dipeptidyl peptidase activity was calculated by the
following equation:
Activity = (Activity of substrate degradation in the absence
of a dipeptidyl peptidase inhibitor) - (Activity of substrate
degradation in the presence of a dipeptidyl peptidase inhibitor ) .
As the dipeptidyl peptidase inhibitor, tynorphin was used.
CA 02516875 2005-08-24
24
The above-mentioned activity of substrate degradation was
converted to a value per 1 mg of protein in the sample by depicting
a standard curve based on the relationship between the
concentration of AMC and the value of fluorescence, and
calculating the value from the standard curve.
Results:
The dipeptidyl peptidase activities in the synovial fluid
from RA patients and OA patients were 46.1~19.6 pmol/30 min/mg
protein ( n = 29 ) and 10 . 6~6 . 2 pmol/30 min/mg protein ( n = 33 ) ,
respectively (Fig. 1).
The dipeptidyl peptidase activity of the RA patients was
about 5-fold higher than that of the OA patients (p<0.001).
There was no correlation between the dipeptidyl peptidase
activity of RA and severity of the disease, the morbid period
or the values in the serum test such as C-reaction type protein
and blood sedimentation ratio and the like.
Example 2
Measurement of spinorphin
Synovial fluid sample ( 2 mL ) was mixed with the equivalent
amount of trichloroacetic acid (10% by mass), and the mixture
was allowed to stand at 4°C for 1 hour.
The mixture was centrifuged at 1500xg for 20 minutes , and
the supernatant was subjected to an ODS column. The column was
washed with water ( 10 volume-fold) , and spinorphin was eluted
with 800 (v/v) aqueous methanol solution. The solvent was
evaporated, and the eluate was dissolved in Tris buffer saline
(TBS, 0.5 mL) to prepare a sample.
CA 02516875 2005-08-24
Spinorphin ( 50 ng ) and TBS ( 100 ~,L ) were placed in a 96 -well
plate (Nunc-Immuno Plate, MaxiSorp Surface, Nalge Nunc
International, Denmark; hereinafter also referred to simply as
a plate ) and reacted at room temperature for 1 hour to immobilize
5 spinorphin on the plate . The plate on which spinorphin had been
immobilized was washed five times with 10 mM TBS (TBS-T)
comprising 0.1 ~ by mass of Tween 20. The plate on which
spinorphin had been immobilized was then blocked with TBS-T
comprising skim milk (10~ by mass) and sodium azide (0.1~ by
10 mass) at room temperature for 1 hour. Skim milk was removed
from the plate by washing. A spinorphin rabbit antibody (5
~,g/well ) was added to the plate , a diluted solution of the sample
(100 ~,L) was added thereto, and the mixture was subjected to
competitive reaction. The plate was reacted for 1 hour under
15 shaking slowly. The plate was then washed five times.
Horseradish peroxidase (HRP)-labeled F (ab')2goat anti-rabbit
IgG (H+L) (1000-fold dilution, 100 ~,L) was added to the plate
and reacted at room temperature for 1 hour. After the reaction
was completed, the plate was washed five times, and
20 o-phenylenediamine ( 2 . 2 E.iM) and citric acid buffer comprising
0 . 014 ( v/v ) aqueous hydrogen peroxide ( 0 . 1M, pH 5 , 100 ~,L ) were
then added to the plate. The plate was allowed to stand for
20 minutes, and the light absorbance of the solution in the wells
of the plate using an autoreader (Korona Electric Co.Ltd.,
25 Ibaragi, Japan) at 405 nm. The concentration of spinorphin in
the sample was calculated from ~B/Bo of the standard curve ( light
absorbance ratio; a value obtained by dividing the light
absorbance with the light absorbance at blank 0).
CA 02516875 2005-08-24
26
Results:
The amount of spinorphin in the synovial fluid from the
RA patients was 4. 2~3.4 ng/mL (n = 20) and that of the OA patients
was 10 . 1~7 . 1 ng/mL ( n = 23 ) , respectively ( Fig. 2 ) . The amount
of spinorphin in the RA patients was significantly lower ( P
0.01) than that in the OA patients.
In the 20 samples, the dipeptidyl peptidase activity and
the amount of spinorphin in the synovial fluid had a negative
correlation (r = -0.51) where both the dipeptidyl peptidase
activity and the amount of spinorphin could be measured.
Reference Example
Production of a spinorphin rabbit antibody
Spinorphin (obtained from American Peptide Company Inc.,
Sunnyvale , CA, USA) was bound to KLH ( keyhole limpet hemocyanin )
using EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide) to
give a KLH-bound spinorphin. The KLH-bound spinorphin was
injected subcutaneously to the backs of two rabbits (New Zealand
white ) . The KLH-bound spinorphin was immunized 8 times at the
intervals of 2 weeks . After the last immunization was completed,
blood was collected from the heart of the rabbit. Serum was
separated from the collected whole blood to give a spinorphin
rabbit antibody. The titer of the antibody was measured by
reacting a solid antigen ( 200 ng/well ) with diluted serum ( 100
~uL/well) , adding the secondary antibody after washing, finally
adding the substrate, and measuring the coloring. The serum
was diluted firstly 1000-fold, then every 3-fold and at the
maximum 81000-fold to prepare a diluted serum, and said diluted
CA 02516875 2005-08-24
27
serum was used for the reaction.
The cross reaction of anti-spinorphin exhibited by
spinorphin analogues [ WYPWT ( sequence listing: SEQ ID NO: 2 ) ,
VYPWT (sequence listing: SEQ ID NO: 3), hemorphin-4 (YPWT;
sequence listing: SEQ ID NO: 4) , PWT, LWYPW (sequence listing:
SEQ ID NO: 5 ) , tynorphin ( WYPW; sequence listing : SEQ ID NO:
6 ) , VYPW ( sequence listing: SEQ ID NO: 7 ) , YPW, LWYP ( sequence
listing : SEQ ID NO: 8 ) , WYP ( sequence listing : SEQ ID NO: 9 )
and VYP] was lower than 0.1% of that of cross reaction of
spinorphin. Therefore, a cross reaction was not recognized.
The spinorphin analogues were synthesized according to the method
disclosed in JP-A-2000-95794.
INDUSTRIAL APPLICABILITY
The present invention is useful as a novel method for the
diagnosis of RA.
CA 02516875 2005-08-24
SEQUENCE LISTING
Sequence Listing
<110~ Maruishi Pharmaceutical Co., Ltd.
<120~ METHOD FOR DIAGNOSIS OF RHEUMATOID ARTHRITIS
<130~ M15F1684(CA)P
<160~ 9
<210~ 1
<211~ 7
<212~ PRT
<213~ Artificial Sequence
<400~ 1
Leu Val Val Tyr Pro Trp Thr
1 5
<210) 2
<211) 6
<212~ PRT
<213~ Artificial Sequence
<400~ 2
Val Val Tyr Pro Trp Thr
1 5
<210~ 3
<211~ 5
<212~ PRT
<213~ Artificial Sequence
<400~ 3
Val Tyr Pro Trp Thr
1 5
<210~ 4
<211~ 4
<212~ PRT
<213~ Artificial Sequence
<400~ 4
Tyr Pro Trp Thr
1
<210~ 5
<211) 6
<212~ PRT
<213~ Artificial Sequence
<400~ 5
Leu Val Val Tyr Pro Trp
1 5
<210~ 6
<211~ 5
<212~ PRT
<213~ Artificial Sequence
<400~ 6
Val Val Tyr Pro Trp
1 5
<210~ 7
<211~ 4
<212~ PRT
<213~ Artificial Sequence
<400~ 7
Val Tyr Pro Trp
1
<210~ 8
<211) 5
<212~ PRT
<213~ Artificial Sequence
<400~ 8
Leu Val Val Tyr Pro
1 5
<210~ 9
<211~ 4
<212) PRT
<213~ Artificial Sequence
<400~ 9
Val Val Tyr Pro
1