Note: Descriptions are shown in the official language in which they were submitted.
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
Description
USE OF THYROID-STIMULATING HORMONE
TO INDUCE LIPOLYSIS
FIELD OF THE INVENTION
The present invention relates to the treatment of obesity, the
complications associated with obesity, liver steatosis, insulin resistance,
and diabetes.
lVlore particularly, the invention relates to the use of thyroid-stimulating
hormone (TSH
or thyrotropin~ to stimulate lipolysis for the treatment of obesity,
complications
associated with obesity, liver steatosis, insulin resistance, and diabetes.
BACKGROUND OF THE INVENTION
~besity is a public health problem that is both serious and widespread.
Cane third of the population in industrialized countries has an excess weight
of at least
20% relative to the ideal weight. This phenomenon has spread to the developing
world,
particularly to the regions of the globe where economies are modernizing. As
of the year
2000, there were an estimated 300 million obese people worldwide.
Obesity considerably increases the risk of developing cardiovascular or
metabolic diseases. For an excess weight greater than 30%, the incidence of
coronary
diseases is doubled in subjects less than 50 years of age. Studies carried out
for other
diseases are equally revealing. For an excess weight of 20%, the risk of high
blood
pressure is doubled. For an excess weight of 30%, the risk of developing non-
insulin
dependent diabetes is tripled, and the incidence of dyslipidemia increased six
fold. The
list of additional diseases promoted by obesity is long; abnormalities in
hepatic function,
digestive pathologies, certain cancers, and psychological disorders are
prominent among
them.
Treatments for obesity include restriction of caloric intake, and increased
caloric expenditure through physical exercise. However, the treatment of
obesity by
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
2
dieting, although effective in the short-term, suffers from an extremely high
rate of
recidivism. Treatment with exercise has been shown to be relatively
ineffective when
applied in the absence of dieting. Other treatments include gastrointestinal
surgery or
agents that limit the absorption of dietary lipids. These strategies have been
largely
unsuccessful due to side effects of their use.
Current therapies for complications associated with obesity, including
type-2 diabetes, hyperlidpemia, and steatohepatitis, have been inadequate to
halt the
progression of these life-threatening pathologies in most instances.
Lipolytic agents have been investigated extensively and found to produce
striking improvements in adiposity, glucose sensitivity, and dyslipidemic
conditions.
These agents, agonists of sympathetic nervous system catecholamines have not
proven to
be successful therapeutics principally due to the inability thus far to create
specific
agents that target only adipose tissue without stimulating other tissues
responsive to
sympathetic innervation.
Clearly there remains a need for novel treatments that are useful for
reducing body weight and the deleterious effects associated with increased
adiposity in
humans. Therapies that can be administered to promote lipolysis and weight
loss would
help to control obesity and thereby alleviate many of the negative
consequences
associated with this condition.
BRIEF DESC1ZIPTI~l~ ~F' T>EIE DI2AWIN~-S
Figure 1. Dose response of TSH and isoproterenol-induced lipolysis in 3T3 Ll
adipocytes. Glycerol (upper panel) and free fatty acid (FFA; lower panel)
accumulations
were determined following a 4-hour treatment with TSH (solid squares) or
isoproterenol
(solid triangles) at the indicated concentrations.
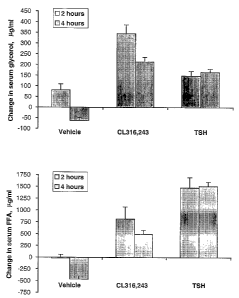
Figure 2. Stimulation of lipolysis in vivo by TSH. Male oblob mice (n=7-
8/group) were
injected with vehicle saline, TSH (300 ~~g/kg), or the (33-AR agonist CL
316,243
(lmg/lcg). Changes from baseline in serum glycerol (upper panel) and FFA
(lower
panel) at 2 and 4 hours post-injection were determined for each group as
described in
Example 3. Error bars are standard error of measurement.
Figure 3. Thyroid hormone levels in male oblob mice following 25 days of
treatment
with TSH. Mice (n=7-8/group) were injected daily with vehicle saline, TSH (267
~,g/lcg), (33-AR agonist CL 316,243 (lmg/lcg), or thyroxine (1-1.5 pg/mouse;
see example
4). The level of total circulating T4 in serum was determined by ELISA.
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
3
Figure 4. Serum glucose levels in male oblob mice following 28 days of
treatment with
TSH. Animals (n=4/group) were fasted for 4 hours immediately following the
dark
cycle, then blood was drawn, serum separated, and glucose levels determined by
enzymatic methods. Treatment groups are as described in Figure 3 and symbols
for
each group are shown in the figure legend.
Figure 5. Glucose challenge of animal groups described in Figure 4. Following
blood
sampling to measure basal glucose and insulin levels, glucose (1.5 g/kg), was
injected at
time zero and blood sampled again at 20, 40, and 120 minutes following
injection. Panel
A depicts blood glucose levels and Panel B, serum insulin levels.
SUMMARY OF THE INVENTION
In one aspect, the invention provides a method for inducing lipolysis in a
mammal comprising administering to the maanmal a pharmaceutically effective
amount
of a TSH polypeptide, wherein administration of the polypeptide results in a
clinically
significant decrease in the body weight of the mammal. In an embodiment, the
mammal
is obese. In another embodiment, the mammal has a body mass index greater than
25.
In a further embodiment, the body mass index is 26, between 26 and 50, or
greater than
50. In another embodiment, the decrease in body weight results fro lipolytic
stimulation
of adipose tissue.
In another aspect, the invention provides, a method for inducing weight
loss in a mammal, comprising administering to the mammal a pharmaceutically
effective
amount of a TSH polypeptide, wherein administration of the polypeptide results
in a
clinically significant decrease in body weight of the mammal. In an
embodiment, the
mammal is obese. In another embodiment, the mammal has a body mass index
greater
than 25. In a further embodiment, the body mass index is is 26, between 26 and
50, or
greater than 50.
In another aspect, the invention provides, a method for improving insulin
sensitivity in a mammal comprising administering to the mammal a
pharmaceutically
effective amount of a TSH polypeptide, wherein administration of the
polypeptide results
in increased sensitivity to insulin. In an embodiment, the blood glucose
levels in the
mammals are decreased. In another embodiment, the insulin levels are
decreased.
In another aspect, the invention provides, a method for treating, or a
method for preventing type-2 diabetes in a mammal, comprising administering to
the
mammal a pharmaceutically effective amount of a TSH polypeptide, wherein
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
4
administration of the polypeptide results in an improvement in the diabetic
state of the
mammal. In an embodiment the mammal is obese. In another embodiment,
administration of the polypeptide results in deceased serum glucose andlor
serum insulin
levels.
In another aspect, the invention provides, a method for treating
hyperlipidemia in a mammal comprising administering to the mammal a
pharmaceutically effective amount of a TSH polypeptide, wherein administration
of the
polypeptide results in decreased hyperlipidemia in the mammal. In an
embodiment, the
mammal is obese. In another embodiment, the mammal is type-2 diabetic. In
another
embodiment, the serum cholesterol and/or triglyceride levels of the mammal are
decreased.
In another aspect, the invention provides, a method for treating
steatohepatitis in a mammal, comprising administering to the mammal a
pharmaceutically effective amount of a TSH polypeptide, wherein administration
of the
polypeptide results in an improved steatohepatic state in the mammal. In an
embodiment, the mammal is obese. In another embodiment, the mammal is type-2
diabetic.
In another aspect, the invention provides, a method for preventing
steatohepatitis in a mammal with steatosis, comprising administering to the
mammal a
pharmaceutically effective amount of a TSH polypeptide, wherein administration
of the
polypeptide maintains or reduces the steatosis. In an embodiment, the mammal
is obese.
In another embodiment, the mammal is type-2 diabetic.
In another aspect, the invention provides, a method for lowering elevated
plasma cholesterol in a mammal, comprising administering a pharmaceutically
effective
amount of a TSH polypeptide to said mammal, wherein administration of the
polypeptide lowers the plasma cholesterol level in the mammal. In an
embodiment, the
mammal is type-2 diabetic and/or obese. In another embodiment, the mammal is
hypercholesterolemic.
In another aspect, the invention provides a method of lowering elevated
triglyceride levels in a mammal, comprising administering a pharmaceutically
effective
amount of a TSH polypeptide to said mammal, wherein administration of the
polypeptide lowers triglyceride levels in the mammal. In an embodiment, the
mammal is
type-2 diabetic. In another embodiment, the mammal is hypertriglyceridemic.
In another aspect, the invention provides a method for treating steatosis of
the liver in a mammal with steatosis, comprising administering to the mammal a
pharmaceutically effective amount of a TSH polypeptide, wherein administration
of the
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
polypeptide maintains or reduces the steatosis. In an embodiment, the mammal
is obese.
In another embodiment, the mammal is type-2 diabetic.
In another aspect, the invention provides a method for treating
atherosclerosis, comprising comprising administering to the mammal a
pharmaceutically
effective amount of a TSH polypeptide, wherein administration of the
polypeptide
improves the athersclotic state. In an embodiment, the mammal is
hyperlipidemic.
These and other aspects of the invention will become evident upon
reference to the following detailed description of the invention.
DETAILED DESCRIPTION OF THE INVENTION
The present invention fills the need for a novel therapy to promote weight
loss and/or treat the diabetic state frequently associated with obesity. The
present
invention comprises administering thyroid stimulating hormone (TSH) to an
individual
to promote lipolysis and thereby promote weight loss, reduce liver steatosis,
and/or
increase insulin sensitivity. The present invention further comprises a method
for
treating type-2 diabetes or a pre-diabetic condition in an individual
comprising
administering TSH to said individual. The invention further comprises a method
for
treating type-2 diabetes or a pre-diabetic condition in an individual
comprising
administering a pharmaceutically effective amount of TSH to the individual.
Additionally, the present invention comprises a method for improving insulin
sensitivity
in an individual comprising administering TSH to said individual without
disruption of
the thyroid axis. W an aspect, the individual is treated with a
therapeutically effective
amount of TSH. In another aspect, TSH is used to promote reversal of steatosis
or
steatohepatitis. In an aspect of the invention the individual is a mammal. In
an
embodiment the mammal is human.
The teachings of all of the references cited herein are incorporated in their
entirety herein by reference.
Herein we disclose methods that are useful for the treatment of obesity.
As described below, the ability to stimulate lipolysis in adipose tissue
provides a means
of intervening in a wide number of pathologies associated with obesity. In
particular, we
have discovered that TSH, when administered iya vitro or ifa vivo, potently
stimulates
lipolysis. As a consequence, metabolic rate is increased, leading to decreased
weight,
increased insulin sensitivity, and decreased serum hyperlipidemia. This
increase in
metabolism is independent of the activation of the thyroid axis. Further, we
have
discovered a method of administration of TSH that stimulates lipolysis
directly, without
chronic elevation of thyroid hormone levels.
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
6
When used to promote lipolysis, TSH can promote weight loss. The
invented methods are useful for treating conditions that include: obesity,
atherosclerosis
associated with obesity or dyslipidemia, diabetes, hypertension associated
with obesity or
diabetes, steatosis or steatohepatitis, or more generally the various
pathologies associated
with obesity.
In another aspect of the invention, TSH can be used for the maintenance
of weight loss in individuals who are treated with other medicaments that
induce weight
loss.
The invention is also useful for the treatment of non-insulin dependent
diabetes, especially that associated with obesity. In one embodiment, the use
of TSH to
treat non-insulin dependent diabetes is envisioned in non-obese individuals.
The invention is further useful for the treatment of dyslipidemias,
including hypercholesterolemia and hypertrglyceridemia.
Yet another aspect of the invention relates to the use of TSH to increase
resting metabolic rate in individuals. In one embodiment, individuals with low
resting
metabolic rate are adnunistered TSH to promote lipolysis and increase energy
utilization
while maintaining a euthyroid state.
Energy expenditure represents one side of the energy balance equation. In
order to maintain stable weight, energy expenditure should be in equilibuum
with energy
intake. Considerable efforts have been made to manipulate energy intake (i.e.,
diet and
appetite) as a means of maintaining or Losing weight; however, despite
enormous sums
of money devoted to these. approaches, they have been largely unsuccessful.
There h~.ve
also been efforts to increase energy expenditure pharmacologically as a means
of
managing weight control and treating obesity. Increasing energy metabolism is
an
attractive therapeutic approach because it has the potential of allowing
affected
individuals to maintain food intake at normal levels. Further, there is
evidence to
support the view that increases in energy expenditure due to pharmacological
means are
not fully counteracted by corresponding increases in energy intake and
appetite. See
Bray, G. A. (1991) Ann Rev Med 42, 205-216.
Energy expenditure can be stimulated pharmacologically by manipulation
of the central nervous system, by activation of the peripheral efferents of
the sympathetic
nervous system (SNS), or by increasing thyroid hormone levels.
Thyroid hol~none stimulates carbohydrate and lipid catabolism in most
cells of the body and increases the rate of protein synthesis. TSH stimulates
thyroid
hormone biosynthesis and secretion. The secretion of TSH from the thyrotrophs
of the
anterior pituitary is inhibited by circulating T4 and T3 and stimulated by
thyrotropin-
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
7
releasing hormone produced in the hypothalamus See Utiger, in Eiadocri~aology
ayad
Metabolis~a (Felig and Frohman, eds), pp. 261-347, McGraw-Hill, (2001).
As a result of the catabolism produced by thyroid hormone, heat is given
off and energy expenditure is increased. There has been an intense interest in
thyroid
hormone levels in obesity, due to the opportunity to increase basal energy
consumption
by increasing thyroid hormone levels. Studies have revealed that obese and
normal-
weight individuals have similar thyroid hormone profiles. An excess of thyroid
hormone
leads to various disorders, generally termed thyrotoxicosis. This condition is
characterized by an abnormally high metabolic rate, increased blood pressure,
high body
temperature, heat intolerance, irritability, and tremors of the fingers. Of
particular
concern in the obese state is the tendency to increased and more forceful
heartbeats.
Due to the adverse effects of elevated thyroid hormone levels, the use of
thyroid hormone to treat obesity has seen little success, other than in the
small fraction of
obese patients identified with hypothyroidism.
Much of the energy expended on a daily basis derives from resting
metabolic rate (RMR), which comprises 50-~0% of the total daily energy
expenditure.
For a review, see Astrup, A. (2000) End~crirae 13, 207-212. Noradrenaline
turnover
studies have shown that most of the variability in RMR that is unexplained by
body size
and composition is related to differences in SNS activity, suggesting that SNS
activity
does modulate RMR. See Snitlcer, S., et al. (2001) Obes. Rev. l, 5-15. Meal
ingestion is
accompanied by increased SNS activity, and studies have demonstrated that
increased
SNS activity in response to a meal accounts for at least part of meal-induced
thermogenesis.
The peripheral targets of the SNS involved in the regulation of energy
utilization are the (3-adrenoreceptors ((3-AR's). These receptors are coupled
to the
second messenger cyclic adenosine monophosphate (cAMP). Elevation of cAMP
levels
leads to activation of protein linase A (PKA), a multi-potent protein Icinase
and
transcuiption factor eliciting diverse cellular effects. See Bourne, H. R., et
al. (1991)
Nature 349, 117-127. Adipose tissue is highly innervated by the SNS, and
possesses
three known subtypes of (3-adrenoreceptors~ (31-, (32-, and X33-AR. Activation
of the SNS
stimulates energy expenditure via coupling of these receptors to lipolysis and
fat
oxidation. Increased serum free fatty acids (FFAs) produced by adipose tissue
and
released into the bloodstream stimulate energy expenditure and increase
thermogenesis.
For a review, see Astrup, A. (2000) E~zdocf-ifae 13, 207-212. In addition,
elevated PKA
levels increase energy utilization in fat by up-regulating uncoupling protein-
1 (UCP-1),
which creates a futile cycle in mitochondria, generating waste heat.
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
8
Over the past two decades, investigation of the physiological benefits of
SNS activation for the treatment of obesity and diabetes related to obesity
has centered
on pharmacological activation of the (33-AR. Expression of the (33-AR is
restricted to a
narrower range of tissues than the X31 or (32 isoforms, and is highly
expressed in rodent
adipose tissue compared to the other isoforms. Experimental work in rodents
treated
with (33-AR agonists has demonstrated that stimulation of lipolysis and fat
oxidation
produces increased energy expenditure, weight loss, and increased insulin
sensitivity.
See de Souza, C. J. and Burkey, B. F. (2001) Curr Pharm Des 7, 1433-1449.
However,
the potential benefits of the (33-AR agonists have not been realized, due to
their lack of
efficacy at the human [33-AR. Further, it has more recently been shown that
the levels of
(33-AR in rodent adipose tissue are much higher than in human adipose tissue.
In human
adipose tissue, the (31 and (32 isoforms represent the predominant
adrenoreceptor
isoforms. See Arch, J. R. (2002) Eur J Phar~rzac~l 440, 99-107. Thus, although
the
proof of-concept of stimulation of lipolysis for treatment of obesity has been
clearly
demonstrated in rodents, the mechanism for therapeutically producing the
corresponding
effects in humans is unrealized.
Strategies to promote lipid oxidation through lipolysis have demonstrated
improved insulin sensitivity at doses that do not promote weight loss, and
over time
periods that do not affect body weight. An insulin-sensitizing effect is more
readily
detectable than an anti-obesity effect. Stimulation of fat oxidation may
rapidly lower the
intracellular concentration of metabolites that modulate insulin signaling;
the anti-
obesity effect, in contrast, must develop gradually as large stoi°es of
fat are oxidized.
The present invention relates generally to methods that are useful for
stimulating lipolysis in adipose cells and/or tissue. Those having ordinary
skill in the art
will understand that lipolysis is the biochemical process by which stored fats
in the form
of triglycerides are released from fat cells as individual free fatty acids
into the
circulation. Stimulation of lipolysis has been clearly linked to increased
energy
expenditure in humans, and several strategies to promote lipolysis and
increase oxidation
of lipids have been investigated to promote weight loss and treat the diabetic
state
associated with obesity. These therapeutic efforts primarily focus on creating
compounds that stimulate the sympathetic nervous system (SNS) through its
peripheral
(3-adrenoreceptors. The discovery of potent TSH-promoted lipolysis in adipose
tissue,
both iu vivo and in vitro, presents a novel and specific method of treating
obesity, as well
as the insulin-resistant diabetic state associated with obesity.
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
9
As used herein, the term "brown fat" refers to adipose tissue depots that
contain high densities of mitochondria, and whose primary function is the
production of
heat through uncoupling of fat oxidation to ATP generation in the
mitochondria, (a
"futile" cycle). The teen "white fat" refers to the predominant form of
adipose tissue
and serves as the principal storage depot for fatty acids in the form of
triglycerides.
As used herein, the terms "obesity" and "obesity-related" are used to refer
to individuals having a body mass which is measurably greater than ideal for
their height
and frame. For example, these terms refer to individuals with body mass index
values of
greater than 25, equal to or greater than 30, equal to or greater than 35, and
greater than
40.
Thyroid-stimulating hormone (TSH), is a ~30 kDa glycoprotein
composed of two non-covalently linked peptide subunits, an alpha subunit and a
beta
subunit. The alpha subunit of TSH is the same as that of luteinizing hormone,
follicle-
stimulating hormone, and chorionic gonadotropin, and has the amino acid
sequence of:
mdyyrkyaaiflvtlsvflhvlhsapdvqdcpectlqenpffsqpgapilqcmgccfsrayptplrslektmlvqknvt
sest
ccvalcsynrvtvmggfkvenhtachcstcyyhles (SEQ lI? N~: 1). A polynucleotide
sequence for
SEQ ID NO:1 is shown in SEQ ID N0:2. Amino acids 25 to 116 of the alpha
subunit
comprise the mature protein (SEQ ID NO:3). The beta subunit of TSH is unique,
and
has the amino acid sequence of:
mtalflmsmlfglacgqamsfcipteytmhiewecaycltintticagymntrdinglclflpkyalsqd
vctyrdfiyrtveipgcplhvapyfsypvalsckcglccntdysdciheailctnyctlcpqlcsylvgfsv (SEQ
~ N~:
4) and determines the hormone's biological specificity. A polynucleotide
sequence for
SEQ 117 NO:4 is shown in SEQ >D NO:S. Amino acids 21 to 132 of the beta
subunit
comprise the mature protein (SEQ ~ NO:6). TSH may be produced by
biopharmaceutical methods using skills recognized in the art, or may be
obtained from
commercial sources, such as, for example, Genzyme Corporation (Cambridge, MA).
The thyroid gland produces two thyroid hormones, thyroxine (T4) and
triiodothyronine
(T3). The ratio of T~ to T3 in normal human serum is typically 100:1. Total
thyroid
hormone levels in a nornal human range from 5-11 ~ug/dl of serum; this range
is defined
as the euthyroid state. Excess levels of thyroid hormones (thyrotoxicosis)
result in a
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
hyperthyroid condition, and low levels of thyroid hormones in serum are
defined as a
hypothyroid state.
Steatosis is the accumulation of fat deposits in the liver. Steatosis of any
etiology can be associated with the development of fibrosis, so called
steatohepatitis, and
even cirrhosis of the liver.
TSH Promotes Elevation of cAMP in Adipose Tissue
TSH exerts its effects through interaction with the thyroid-stimulating
hormone (TSH) receptor. See Nalcabayashi, K., et al. (2002) J Clin lovest 109,
1445-
1452. The TSH receptor (TSHR) is a member of the G-protein coupled, seven-
transmembrane receptor superfamily. Activation of the TSH receptor leads to
coupling
with heterotrimeric G proteins, which evolve downstream cellular effects. The
TSH
receptor has been shown to interact with G proteins of subtypes GS, G9, G1~,
and Gi. In
particular, interaction with GS leads to activation of adenyl cyclase and
increased levels
of CAMP. See Laugwitz, I~. L., et al. (1996) Pf~~c Ncztl Acczel Sci U S A 93,
116-120.
Recent reports have documented the presence of TSHR in adipose tissue
of humans and rodents. See Bell, A., et al. (2000) Afn J Physiol Cell Physiol
279, 0335-
340, and Endo, T., et al. (1995) J Pi~l Claetu 270, 10833-10837.
Example 1 demonstrates the production of elevated CAMP by TSH in
cultured murine 3T3-Ll adipocytes and in primary human adipocytes. We have
discovered that TSH produces activation of a luciferase reporter gene
construct under the
control of cAMP response element (CRE) enhancer sequences. We typically
observe a
10- to 40-fold induction of the luciferase reporter gene in response to TSH
treatment,
indicating significant production of cAMP in adipocytes following activation
of the
TSHR. Thus, TSH could be an important physiological regulator of adipose
tissue
lipolysis, which is primarily controlled by intracellular CAMP levels. For a
review, see
Astntp, A. (2000) Endocrine 13, 207-212.
TSH Promotes Lipolysis in Adipocytes and Whole Animals
TSH was examined for its ability to activate lipolysis in cultured murine
3T3-Ll adipocytes as described in Example 2. Following treatment of adipocytes
with
test compounds for 4 hours, lipolysis was assessed by the accumulation of
glycerol and
free fatty acid (FFA) in the adipocyte culture medium. Treatment of adipocytes
with 10
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
11
nM recombinant human TSH produced near maximal levels of extracellular
glycerol and
FFA. Figure 1 compares the lipolytic activity of TSH to isoproterenol, a non-
specific (3-
adrenergic agonist. Maximal lipolysis achieved with TSH is approximately 50%
of that
produced by isoproterenol. Lipolysis is significantly stimulated by TSH at
concentrations of 1 nM, indicating that TSH is a potent regulator of lipolysis
in
adipocytes.
A significant aspect of the invention is the stimulation of lipolysis in vivo.
Intraperitoneal (IP) injection of TSH produces acute elevation of serum
glycerol and
FFA in whole animals. As described in Example 3, mice were fasted overnight
before IP
injection of TSH (300 p,g/kg), (33-AR agonist CL 316,243 (1 mg/kg), or vehicle
saline.
Serum was sampled before injection to establish baselines, then sampled again
at 2 and 4
hours post-injection. Although the vehicle controls show decreases in serum
glycerol
and FFA levels by four hours, the animals treated with TSH show significant
elevations
in both, indicating that TSH is a potent stimulator of lipolysis ita vivo. As
demonstrated
in Figure 2, the invention is a potent stimulator of increased serum FFA if2
vivo, showing
significant increases over the (33-AR agonist at the 4-hour time point. In one
embodiment of the invention, TSH is used to produce acute increases in plasma
FFA,
thus promoting increased basal metabolic rate.
Chronic Treatment of ~blob mice with TSH produces lipolysis without sustained
increases in thyroid hormone levels
~bese ~bl~b mice are frequently used as models of human obesity and
diabetes. To examine the effects of TSH-stimulated lipolysis in this model,
TSH, X33-AR
agonist CL 316,243, thyroxine, or vehicle saline were administered daily for 4
weeks by
1F injection as described in Example 4. A thyroxine group was included to
distinguish
the metabolic effects of thyroid hormone from the direct stimulation of
lipolysis in
adipocytes mediated by TSH.
An aspect of the instant invention is the discovery that TSH, when
introduced into the periphery by IP injection at doses that stimulate
lipolysis, does not
result in the creation of a chronic hyperthyroid state. As shown in Figure 3,
circulating
levels of thyroid hormone (T~) following one month of daily injections of TSH
do not
increase above the levels found in the vehicle controls. The TSH amounts
injected daily
are greater than 100 times the amount of TSH that would be expected to be
released in a
single day from the pituitary. The present invention thus provides for a
method of
introducing TSH without altering the thyroid hormone axis to produce a
profound
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
12
hyperthyroid state, while stimulating lipolysis to produce a therapeutic
effect for the
treatment of obesity and diabetes.
Following four weeks of treatment, injected animals were subjected to an
intraperitoneal glucose tolerance test (IPGTT) in order to evaluate the effect
of TSH
treatment on serum glucose levels and insulin sensitivity. The subject mice
were fasted
for four hours before blood sampling to obtain baseline glucose and insulin
levels, then
were injected with glucose to allow for measurement of insulin sensitivity to
a glucose
challenge. As shown in Figure 4, the fasted serum glucose levels in the TSH-
treated
animals were significantly lower than in the vehicle controls or the thyroxine-
treated
animals after 4 weeks of treatment. TSH is seen to be as effective as the
control (33-AR
agonist in reducing hyperglycemia in this model. Figure 4 demonstrates the
discovery
that peripheral administration of TSH does not act to reduce blood glucose via
thyroid
hormone activity, as increasing thyroid hormone levels by administration of
thyroxine
does not reduce fasting glucose levels compared to the vehicle control group.
An additional aspect of the invention is the improvement of insulin
sensitivity and reduced hyperglycemia in response to a glucose challenge, as
shown in
Figure 5. The glucose tolerance test is a typical diagnostic measure of
diabetes and
insulin sensitivity. See Defronzo R.A. et al. (1991) I~i.abetes Care 14, 173-
194. Panel A
demonstrates that the clearance of a glucose challenge is significantly
enhanced in the
TSH-treated group compared to vehicle- or thyroxine-treated groups. Panel B
shows that
insulin sensitivity in the. TSH-treated group is significantly improved
compared to
vehicle- or thyroxine-treated controls. The TSH and control (33-AR agonist
groups
exhibit enhanced glucose disposal with lower insulin levels compared to the
vehicle or
thyroxine treatment groups.
In a further aspect of the invention, stimulation of lipolysis by TSH
results in decreased serum lipid levels. Specifically, chronic treatment of
obl~b mice
with TSH leads to significant reductions in serum cholesterol and triglyceride
levels.
The invention comprises a method for lowering elevated plasma cholesterol and
triglyceride levels typically associated with obesity and type-2 diabetes. The
discovery
that TSH-stimulated lipolysis produces improvement in hyperlipidemia stands in
contrast
to the observation that sympathetic stimulation of lipolysis with (3-AR
agonists result in
no reduction in serum cholesterol levels, and typically result in unchanged or
slightly
increased serum triglyceride levels (e.g., serum lipid analysis data in
Example 4).
Further, the effects of TSH on lowering serum triglyceride and cholesterol
levels are not
due to increases in the circulating levels of thyroid hormone. As detailed in
Example 4,
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
13
chronic treatment with thyroid hormone resulted in elevated plasma
triglycerides and did
not reduce serum cholesterol levels.
The invention further provides a method for the treatment of obesity.
Stimulation of lipolysis can result in weight loss and reductions in fat mass
in animals
and humans. Increased lipolysis in fat through SNS stimulation by (3-AR
agonists
typically results in increases in scapular brown fat mass in rodents, and
decreases in
white fat tissue mass. The increased brown fat mass results in a higher
metabolic rate,
due to the oxidation of lipids for the production of heat in brown fat. Mice
treated with
TSH had increased brown fat mass over vehicle controls (Example 4). Further,
TSH-
treated mice had significant decreases in the mass of mesangial intra-
abdominal white
fat. In addition, body weight increases in the TSH group were reduced compared
to
controls (p=0.11). The TSH treatment group (n=8) contained the only
individuals (n=3)
that exhibited decreases from starting body weight after one month of
treatment. As
described above, the anti-obesity effect of treatment with TSH is expected to
develop
more slowly than the insulin-sensitizing effect.
In a further embodiment of the invention, TSH is useful for the treatment
of steatosis and steatohepatitis. Although liver disease is not a widely
appreciated
complication of obesity, epidemiologic evidence suggests that obesity
increases the risk
of cirrhosis. For example, in autopsy series, obesity was identified as the
only risk factor
for disease in l~% of cirrhotic subjects. See Yang, S.Q. et al. (1997) Proc
Natl Acad Sci
U S A 94, 2557-?562. Notably, cirrhosis is approximately six times more
prevalent in
obese individuals than in the general population. In the LJSA, the high
percentage of
overweight people in the general population partially explains the fact that
non-alcoholic
fatty liver disease (NAFLD) is the most common liver disease. Type-2 diabetes
is
present in 33% of these subjects. The degree of obesity correlates positively
with the
prevalence and severity of fatty liver (steatosis), and this in turn
correlates with
steatohepatitis. A current explanation of the pathogenesis of steatohepatitis
is the "two-
hits" hypothesis. See Day, C.F, and James, ~., Gastr-oeazterology 114, 842-
845. The
first "hit" is the depositing of fat in hepatocytes, leading to fatty
degeneration of the liver
or steatosis hepatitis. This fatty degeneration increases the organ's
sensitivity to the
second "hit", which can be any one of a variety of insults including diabetes,
lipid
peroxidation due to drug metabolism, or excess alcohol intake.
As detailed in Example 4, chronic treatment of oblob mice with TSH
significantly reverses steatosis in these subjects. The instant invention thus
produces a
method for reversing the first "hit" thought to be required for the
progression to
steatohepatitis and cirrhosis. Further, treatment with the invention of those
with
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
14
steatohepatitis, for whom no efficacious therapy is currently available, may
induce a
reversion to a normal (non-steatotic) hepatic state, preventing the
progression of pre-
cirrhotic hepatitis to cirrhosis.
Advantages of TSH as a Lipolysis-Stimulating A ent
The invention comprises a novel method of producing lipolysis and
increasing metabolic rate. Other strategies for therapeutically inducing
lipolysis
employed thus far have suffered from a lack of specificity, such as (3-AR
agonists in
general, or a lack of efficacy, as for the most specific of the (33-AR
agonists developed to
date. Most of the agents investigated for human use have not exhibited
sufficient
selectivity, and as a result have produced increased blood pressure and heart
rate due to
activation of sympathetic pathways in tissues other than adipose. See Arch, J.
R. (2002)
Ezzr J Phannacol 440, 99-107.
In spite of the emphasis on development of (33-AR-specific agonists,
recent human studies have implicated the (31- and (3~-adrenoreceptors as the
primary
mediators of sympathetically induced thennogenesis and energy expenditure.
Further,
studies in human obese populations suggest that the decreases in resting
metabolic rate
observed in these individuals are the result of impaired function of (3~-
adrenoreceptors in
adipose tissue. See Schiffelers, S. L., et al. (2001) J Clin Endocrinol Metab
86, 2191-
2199, and Blaalc, E. E., et al. (1993) Azn J Physiol 264, E11-17. Thus, a
novel
mechanism of increasing lipolysis without invoking sympathetic innervation
presents a
unique opportunity for the treatment of obesity.
Other studies in human lean and obese subjects have found that increases
in plasma FFA levels lead to increases in lipid oxidation and energy
expenditure. These
studies conclude that the accumulation of fat in obese subjects may be due to
a defect in
adipose tissue lipolysis rather than to defects in lipid utilization. See
Schiffelers, S. L., et
al. (2001) Int J ~bes Relat ~Vletab Disorel 25, 33-38.
Increased lipolysis in adipose tissue and the resulting decrease in
adipocyte size are negatively correlated with insulin resistance in human
cross-sectional
studies. See Weyer, C., et al. (2000) Diabetologia 43, 1498-1506. Thus a
method for
stimulating lipolysis and reducing adipocyte size is predicted to decrease the
insulin-
resistant diabetic state associated with obesity. The presence of significant
numbers of
TSH receptors in adipose tissue represents a novel method for the control of
lipolysis and
RMR in human obese populations.
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
Use of TSH to Treat Type-2 Diabetes
TSH can also be administered to treat type-2 diabetes mellitus (Type-2
DM). Type-2 DM is usually the type of diabetes that is diagnosed in patients
older than
30 years of age, but it also occurs in children and adolescents. Clinically,
it is
characterized by hyperglycemia and insulin resistance. Type-2 DM is commonly
associated with obesity, especially of the upper body (visceral/abdominal),
and often
occurs after weight gain.
Type-2 DM is a heterogeneous group of disorders in which
hyperglycemia results from both an impaired insulin secretory response to
glucose and a
decreased insulin effectiveness in stimulating glucose uptake by skeletal
muscle and in
restraining hepatic glucose production (insulin resistance). The resulting
hyperglycemia
may lead to other common conditions, such as obesity, hypertension,
hyperlipidemia,
and coronary artery disease.
TSH can be administered to an individual at dosages described below.
TSH can also be administered in conjunction with insulin, and other anti-
diabetic drugs
such as tolbutamide, chlorpropamide, etc.
Formulations and Administration of TSH
TSH can be administered to a human patient, alone or in pharmaceutical
compositions where it is mixed with suitable carriers or excipient(s) at
therapeutically
effective doses to treat or ameliorate diseases associated with obesity and
diabetes.
Treatment dosages of TSH should be titrated to optimize safety and efficacy.
Methods
for administration include intravenous, intraperitoneal, rectal, intranasal,
pulmonary,
subcutaneous, and intramuscular. Pharmaceutically acceptable carriers will
include
water, saline, and buffers, to name just a few. Dosage ranges would ordinarily
be
expected to be from O.lp,g to lmg per l~ilogram of body weight per day. A
useful dose to
try initially would be 25 ~,g/kg per day. However, the doses may be higher or
lower as
can be determined by a medical doctor with ordinary shill in the art. For a
complete
discussion of drug formulations and dosage ranges see Ref~aifagtoh's
Pha~~aaceutical
Sciences, 17th Ed., (Maclc Publishing Co., Easton, Penn., 1990), and Goodfnafa
a~ad
Gilrraafi's: Tl2e Phamaacological Basis of Therapeutics, 9th Ed. (Pergamon
Press 1996).
For pharmaceutical use, the proteins of the present invention can be
administered orally, rectally, parenterally (particularly intravenous or
subcutaneous),
intracisternally, intravaginally, intraperitoneally, topically (as powders,
ointments, drops
or transdermal patch) bucally, or as a pulmonary or nasal inhalant.
Intravenous
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
16
administration will be by bolus injection or infusion over a typical period of
one to
several hours. In general, pharmaceutical formulations will include a TSH
protein in
combination with a pharmaceutically acceptable vehicle, such as saline,
buffered saline,
5% dextrose in water or the like. Formulations may further include one or more
excipients, preservatives, solubilizers, buffering agents, albumin to prevent
protein loss
on vial surfaces, etc. Doses of TSH polypeptide will generally be administered
on a
daily to weel~ly schedule. Determination of dose is within the level of
ordinary skill in
the art. The proteins may be administered for acute or chronic treatment, over
several
days to several months or years. In general, a therapeutically effective
amount of TSH is
an amount sufficient to produce a clinically significant decrease in weight,
improvement
in the diabetic state associated with obesity, decrease in liver steatosis,
and /or increase
in insulin sensitivity.
The invention is further illustrated by the following non-limiting
examples.
Ex~rnple 1
TS~I Activation 0f 3T3 L1 AdipOCytes and I'~Iurnan AdipOCytes results in eAli~
Production
Su'naraccry
Differentiated marine 3T3 Ll adipocytes and primary human adipocytes
were used to study signal transduction of TSH. 3T3 Ll fibroblasts were
differentiated
into adipocytes and the cells were transduced with recombinant adenovirus
containing a
reporter construct, a firefly luciferase gene under the control of CAMP
response element
(CRE) enhances sequences. This assay system detects CAMP-mediated gene
induction
downstream of activation of GS-coupled G-protein coupled receptors (GPCR's).
Treatment of the differentiated 3T3 L1 cells with isoproterenol, a (3-
adrenoreceptor
agonist, resulted in elevation of CAMP levels and a 50-fold induction of
luciferase
expression. Treatment of differentiated 3T3 L1 cells with TSH also resulted in
elevated
cAMP levels and a 24-fold induction of luciferase expression. In a separate
experiment,
undifferentiated 3T3 L1 fibroblasts were transduced with the recombinant
adenovirus.
Treatment of the fibroblasts with TSH did not result in an increase in
reporter gene
induction. In another experiment, human primary adipocytes were also
transduced with
the recombinant adenovirus containing a reporter construct. Treatment of the
human
adipocytes with isoproterenol produced a 22-fold induction of luciferase
expression.
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
17
Treatment of the human adipocytes with TSH resulted in a 20-fold induction of
the
reporter gene. These results demonstrate TSH signaling through a GPCR in
murine
adipocytes and human adipocytes, and the production of cAMP levels similar to
those
achieved through (3-adrenoreceptor stimulation.
Experimental PYOCedure
3T3 L1 cells were obtained from the ATCC (CL-i73, Manasas, VA) and
cultured in growth medium as follows: the cells were propagated in DMEM high
glucose
(Life Technologies, cat. # 11965-092) containing 10% bovine calf serum (JRH
Biosciences, cat. # 12133-78P). Cells were cultured at 37°C in an 8%
COZ humidified
incubator. Cells were seeded in collagen-coated 96-well plates (Becton
Dickinson, cat. #
356407) at a density of 5,000 cells per well. Two days later, differentiation
medium was
added as follows: DMEM high glucose containing 10% fetal bovine serum
(Hyclone,
cat. # SH30071), 1 p.glml insulin, 1 ~,M dexamethasone, and 0.5 mM 3-isobutyl-
methyl
xanthine (ICIV, cat. #195262). The cells were incubated at 37°C in 8%
C02 for 4 days
and the medium replaced with DMEM high glucose containing 10% fetal bovine
serum
and 1 ~,g/ml insulin. The cells were incubated at 37°C in 8% C02 for 3
days, then the
medium was replaced with DMEM high glucose containing 10% fetal bovine serum.
The cells were incubated at 37°C in 8% C02 for 3 days, and the medium
was replaced
with DMEM low glucose (Life Technologies, cat. # 12387-015) containing 10%
fetal
bovine serum. The day before the assay, the cells were rinsed with F12 Ham
(Life
Technologies, cat. # 12396-016) containing 2 mM L-glutamine (Life
Technologies, cat.
# 25030-149), 0.5% bovine albumin fraction V (Life Technologies, cat. # 15260-
037), 1
mM MEM sodium pyruvate (Life Technologies, cat. # 11360-070), and 20 mM HEPES.
Cells were transduced with AV KZ55, an adenovixus vector containing I~55, a
CRE-
driven luciferase reporter cassette, at 5,000 particles per cell. Following
overnight
incubation, the cells were rinsed once with assay medium (F12 HAM containing
0.5°70
bovine albumin fraction V, 2 mM L-glutamine, 1 mM sodium pyruvate, and 20 mM
HEPES). 50 ~,1 of assay medium were added to each well followed by 50 ~,l of
2X
concentrated test protein. The plate was incubated at 37°C in 5% CO~
for 4 hours.
Medium was removed from the plate and the cells were lysed with 25 ~1 per well
of 1X
cell culture lysis reagent supplied in a luciferase assay kit (Promega, cat. #
E4530). The
cells were incubated at room temperature for 15 minutes. Luciferase activity
was
measured on a microplate luminometer (Perl~in Elmer Life Sciences, Inc., model
LB
96V2R) following automated injection of 40 ~.1 of luciferase assay substrate
into each
well. The method described above, with modifications, was also used to test
TSH and
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
18
isoproterenol on human adipocytes obtained from Stratagene (cat. # 937236)
seeded in
96-well plates. Human adipocytes were rinsed once with basal medium
(Stratagene, cat.
# 220002) containing 0.5% bovine albumin fraction V, then transduced with AV
KZ55
at 5,000 particles per cell. Following overnight incubation, the cells were
rinsed once
with assay medium comprised of basal medium containing 0.5070 bovine albumin
fraction
V and assayed as described above.
Example ~
TSH-Induced Lipolysis in 3T3 Ll Adipocytes
Sufrafnary
3T3 Ll adipocytes were treated with TSH and the non-specific (3-
adrenoreceptor agonist isoproterenol for 4 hours. Lipolysis was assessed by
the
accumulation of glycerol and FFAs in the conditioned medium. Figure 1 displays
dose-
response curves of TSH and isoproterenol for glycerol (upper panel) and FFA
(lower
panel). TSH potently stimulated lipolysis in the murine adipocytes, as shown
in Figure
1.
L~I~czsune~'aent ~f free fecttty eccids ifi c~faditz~faed r'zediez fa~~an
diffea-entiected 3T'3 Z,1 cells
Free fatty acids were measured using the Wako NEFA C kit (Walco
Chemicals CJmbH, lVeuss, C'rerrnany) for quantitative determination of non-
esterified (or
free) fatty acids with a modified protocol. Isoproterenol (ICl~), a lipolysis-
inducing
positive control, was diluted to a starting concentration of 2 ~.M in assay
medium (Life
Technologies low glucose DMEM, 1mM sodium pyruvate, 2 mM L-glutamine, 20 mM
HEPES, and 0.5°70 BSA). The isoproterenol was further diluted in half
log serial
dilutions. TSH was serially diluted down to 0.06 nM. The medium was removed
from
3T3 L1 adipocytes in 96-well plates. 50 ~.l of assay medium were added to each
well,
followed by 50 ~,l of TSH or isoproterenol to each well. The plates were
incubated for 4
hours at 37°C. 40 ~,l of conditioned medium were collected for glycerol
assay analysis,
and 40 ~.l of conditioned medium were collected for free fatty acid analysis.
Oleic acid
(Sigma) was dissolved in methanol and used as a reference for determining the
amount
of free fatty acids in the conditioned media. Waleo reagents A and B were
reconstituted
to 4X the recommended concentration. Conditioned media samples were assayed in
96-
well plates. 50 ~.1 of Walco reagent A were added to 5 ~,l of oleic acid
standard plus 40 q,l
of assay medium. 50 ~,l of Walco reagent A were added to 40 ~,1 of conditioned
medium
from differentiated 3T3 L1 cells and 5 ~,l of methanol. The 96-well plates
were
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
19
incubated at 37°C for 10 minutes. 100 ~,l of Walco reagent B were added
to each well.
The 96-well plates were incubated at 37°C for 10 minutes. The 96-well
plates were then
allowed to sit at room temperature for 5 minutes. The 96-well plates were
centrifuged in
a Beckman Coulter Allegra 6R centrifuge at 3250Xg for 5 minutes to remove air
bubbles. The absorbance at 530 nm was measured on the Wallac Victor2
Multilabel
counter.
Measurement of glycerol in coraditiorZed media frorn differentiated 3T3 LZ
cells
Glycerol was measured in conditioned media using the Sigma
Triglyceride (GPO-Trinder) kit with a modified protocol. Isoproterenol was
diluted to a
starting concentration of 2 ~,M. The isoproterenol was further diluted in half
log serial
dilutions. TSH was diluted to starting concentrations of 300 nM in assay
medium. TSH
was then serially diluted down to 0.06 nM. Medium was removed from 3T3 Ll
adipocytes in 96-well plates. 50 ~,1 of assay medium were added to each well,
followed
by 50 ~.ml of TSH or isoproterenol to each well. The plates were incubated for
4 hours
at 37°C. 40 ~,1 of conditioned medium were collected for glycerol assay
analysis, and 40
~l of conditioned medium were collected for free fatty acid analysis. The
glycerol
standard was diluted in water to a range from 200 nmols/10 ~l to 0.25 nmols/10
~,1.
Glycerol was used as a reference for determining the amount of glycerol in the
conditioned media. Sigma reagent A was reconstituted to the recommended
concentration. Conditioned media samples were assayed in 96-well plates. 150
~,1 of
Sigma reagent A were added to 10 ~,l of glycerol standard plus 40 ~,l of assay
medium.
150 p.Ll of Sigma reagent A were added to 40 ~,l of conditioned medium from
differentiated 3T3 L1 cells plus 10 ~.1 of water. The 96-well plates were
incubated for 15
minutes at room temperature. The 96-well plates were centrifuged in a Beckman
Coulter
Allegra 6R centrifuge at 3250Xg for 5 minutes to remove air bubbles. The
absorbance at
530 nm was measured on the Wallac Victor2 Multilabel counter.
Example 3
Stimulation of Lipolysis by TSH Ira Vivo
Snr~arnary
TSH, the (33-adrenoreceptor agonist CL 316,243 (CL), and saline vehicle
were examined for stimulation of lipolysis in mice following an overnight
fast. Mice
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
(n=7-8/group) were bled immediately before IP injection of TSH (300 ~g/kg), CL
(1
mg/lcg), or vehicle, and blood sampled by retro orbital draws at 2 and 4 hours
post-
injection. Lipolysis was assessed as the change in serum glycerol or FFA
compared to
baseline. Figure 2 shows the changes in levels of glycerol ( ~g/ml in serum,
upper panel)
and FFA (~,g/ml in serum, lower panel) for the treatment groups. TSH
administration
stimulated increased serum glycerol levels at 2 and 4 hours, compared to the
vehicle
controls (148 +/-23, (p=. 09) and 165 +/-15 ~,g/ml (p<. 001) versus 80+/-29
and -62 +l-
14). TSH increased serum FFA 1,477 +/-219 (p<. 001) and 1506+/-94 (p<. 001) at
2 and
4 hours compared to vehicle values -20+/-77 and -466+/-67, respectively (all
errors are
standard error of the mean). The control (3-AR agonist also significantly
elevated serum
glycerol and FFA levels.
Treatrraerzt Pr~t~col
C57BL/6 male oblob mice, age 10 weeks, were grouped to normalize
weight (n=7-8 for each treatment; average group weight = 37.8 g +/- 0.4 g).
Mice were
housed individually for 18 hours prior to treatment, at which time food was
withdi°awn,
with free access to water given. At approximately 8 a.m., the subjects were
anesthetized
with halothane and blood samples taken by retro-orbital eye bleeds. The blood
was
allowed to clot, and the serum was separated by centrifugation and frozen for
later
analysis. Test substances were administered by IP injection in a volume of 0.1
ml, and
the animals replaced in their cages for 2 hours v~ith free access to water. At
2 hours, the
mice were sacrificed and blood drawn by cardiac puncture.
llleczsu>"errzerzt of ~lycer-~Z arid FFA irz rraur~zrze serum
For measuring free fatty acids in serum, the method previously described
for measuring free fatty acids in conditioned medium was followed, with the
following
modifications. Walco reagents A and B were reconstituted to 2X the recommended
concentration. 75 ~1 of Wako reagent A were added to 5 ~,1 of oleic acid
standard plus 5
~,1 of water. 75 ~,1 of Wako reagent A were added to 5 ~.1 of serum plLlS 5
~,l of methanol
(to mirror the oleic acid standard conditions). The 96-well plates were
incubated at 37°C
for 10 minutes. 150 ~,l of Wako reagent B were added to each well. The 96-well
plates
were incubated at 37°C for 10 minutes. The 96-well plates were allowed
to sit at room
temperature for 5 minutes. The 96-well plates were centrifuged in a Beckman
Coulter
Allegra 6R centrifuge at 3250Xg for 5 minutes to remove air bubbles. The
absorbance at
530 nm was measured on the Wallac Victor2 Multilabel counter.
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
21
For measuring glycerol in serum, the method previously described for
measuring glycerol in conditioned medium was followed, with the modifications
described below.
Sigma reagent A was reconstituted to 0.5X the recommended
concentration. 200 ~,l of Sigma reagent A were added to 10 ~,1 of glycerol
standard. 200
l,~l of Sigma reagent A were added to 5 ~,1 of serum plus 5 ~,l of water. The
96-well
plates were incubated for 15 minutes at room temperature. The 96-well plates
were
centrifuged in a Beckman Coulter Allegra 6R centrifuge at 3250Xg for 5 minutes
to
remove air bubbles. The absorbance at 530 nm was measured on the Wallac
Victor2
Multilabel counter.
Example 4
Chronic Treatment ~f oblob Mice with TSH
.~1t1121y1a1~
TSH was administered daily for 28 days to obese male obfob mice. I?ata
was obtained for weight, food intake, glucose, insulin, lipid and thyroid
hormone. A
subset of the animal groups was subjected to a glucose tolerance test at the
end of the
study. At sacrifice, animals were examined for changes in adipose depot
weights, liver
pathology, and gross histology. As described below, TSH treatment resulted in
decreased resting glucose and insulin levels, and increased insulin
sensitivity in a glucose
tolerance test. Serum triglyceride and cholesterol levels were significantly
reduced
compared to controls, and thyroid hormone levels were not elevated above the
vehicle
group. Necropsy analysis of adipose tissues revealed substantial and
significant
increases in inter-scapular brown adipose tissue (IBAT), and significant
decreases in
intra-abdominal inesangial white fat. The TSH treatment group showed a strong
trend
toward decreased weight gain compared to controls and thyroxine-treated
animals.
Evaluation of liver histology sections was performed to examine the effect of
TSH-
mediated lipolysis on liver steatosis. Prominent liver steatosis typically
associated with
the oblob strain employed in these studies was significantly reversed by TSH
treatment,
exhibiting marked reduction in fat deposition in liver hepatocytes. Thyroid
hormone did
produce a change in the extent of steatosis.
Tr-eatllaent protocol
11-week old male obloh mice were individually caged and given a
standard lab chow (4% fat) with free access to food and water. Animals were
assigned to
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
22
a treatment group (n=7-8, average weight 54.3 +/- 0.3g per group), kept on a
12 hour
darlc cycle (6 PM to 6 AM), and injected each day between 7 and 9 AM. Chow
consumed by each animal was weighed twice weekly. All animals received
treatments
IP with an injection volume of 0.1 ml. TSH was administered at 267 ~,g/lcg,
and the (33-
AR agonist CL 316,243 at 0.75 mg/kg. Thyroid hormone (T4) was administered at
1.5
~,g/mouse for 4 days, reduced to 1 ~,g/mouse for 10 days, and returned to 1.5
E~g/mouse
for the next 14 days. The vehicle controls received sterile saline. TSH was
obtained
from Genzyme Pharmaceuticals, (Thyrogen~, catalog number 36778; Genzyme
Corporation, Cambridge, MA), CL316,243 from Sigma Biochemicals, and T4
obtained
from Calbiochem, Inc. All blood draws were performed by retro-orbital puncture
under
isoflurane anesthesia.
Body iveiglzt and food izatalze
Food intake did not differ significantly between groups (vehicle 5.9+/-.22,
CL16,243 6.3+/-.11, TSH 5.9 +/-.36, and thyroxine 6.1+/-.17 grams/day of
chow). Body
weight changes were assessed as the percentage increase in body weight from
the
beginning of the study. The weight in the vehicle group increased 8.8+/-.6%.
The
thyroxine group had a slightly greater increase in weight (9.4+/-.5%), and the
(33-AR
group a slightly lower increase in weight (7.7+/-4-1 %) compared to the
vehicle controls.
The TSH-treated group showed less weight gain than the vehicle controls (4.6+/-
2.4%,
p=0.11), with 3 of the 8 members of the group demonstrating an overall
decrease in
weight, the only animals in the study to do so.
Illeaszcz"ezzzeazt of se>"zzzza tdzyroxizze levels
After 25 days of treatment as described above, blood was sampled from
all treated animals (n=7-8/group), serum separated, and analyzed for total T4.
by a
commercially available hit (Biocheclc, Burlingame, CA). Figure 3 shows the
levels of
thyroxine determined for each group +/- standard error. After 25 days of
treatment, the
vehicle T4 levels were 5.14 +/-.08 ~,g/dl. The TSH-treated group had T4.
levels of 5.31
+/- .16, the (33-AR receptor group 5.14+/-.19, and the thyroxine-treated group
9.04+/-.47
~.g/dl. The (33-AR and TSH-treated groups had circulating levels of T4 that
were
significantly lower than controls (p<.02) and the thyroxine treatment group
had levels
significantly, higher than vehicle controls (p<.001).
Adipose tissue analysis
Four animals from each group were analyzed for evaluation of changes in
adipose tissue depot mass after 4 weeks of treatment. Following sacrifice,
brown
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
23
scapular fat (IBAT) and intra-abdominal fat were dissected and the tissues
weighed. All
3 treatment groups showed significant increases in brown scapular fat mass.
Thyroxine
showed the largest increase (I.26+/-.03 g, p<.001) vs. the vehicle control
(0.66+/-.07g).
Elevated thyroid hormone levels are known to act to stimulate 1BAT. TSH and CL
316,243 also increased IBAT mass (1.25+/-.13, p<.01 and 0.87+/-.03, p<.05,
respectively). These increases in 1BAT are associated with increased metabolic
rate as
described above. The mesangial fat depot was dissected and removed from the
colon for
weight determination. Mesangial fat is white adipose tissue and is only
readily
visualized and dissected in obese mice. Mesangial white fat was removed by
carefully
stripping the fat, associated matrix and vascular supporting bed from the
length of the
colon. The weight of the fat and matrix removed from the vehicle controls was
1.31+/-
.04g, and the material removed was quite white in appearance from the fat
cells in the
removed mass. The weights of depots removed from the CL 316,243, TSH, and T~
treatment groups ware 1.53+/-.10g (p=.08), 1.11+/-.03g (p<.02), and 1.18+/-
.11g
(p=.32), respectively. The appearance of the mesangial depots removed from the
thyroxine and particularly the TSH was much less white due to decreased fat
content in
the depot, and suggested that the relative loss in fat cell mass within the
depot was larger
than the change in weight of the removed structure suggested.
Liver steatosis
Liver sections were dissected from all treatment groups described above
and mounted in paraffin following fixation with 1~TBS-formalin. Sections were
mounted
and stained with hematotoxylin and eosin (H~zE) for visualization of hepatic
structural
changes. The extent of liver steatosis was evaluated on a four-point scale,
from 0 to 3,
with zero displaying no signs of liver steatotosis, and 4, representing
pronounced
macrovesicular and microvesicular steatosis. The averages of the groups (n=4)
showed
significant differences in the extent of steatosis as judged by the size of
the lipid
inclusions and the integrity of the hepatocyte structure visible in the
sections. Average
scoxes given to the groups were vehicle (4), thyroxine (3), TSH (2), and CL
316,243 (1).
IPGTT
Following 4 weeks of daily treatment as described above, mice
(n=4lgroup) were fasted for four hours immediately following the beginning of
the light
cycle, and a blood sample obtained before IP injection of a glucose solution
(1.5 g/lcg
body weight). Blood samples were obtained at 20, 4.0, and 120 minutes
following
injection to evaluate changes in serum glucose and insulin. Glucose
concentrations were
determined with a Freestyle blood glucose monitor (Therasense Corp.), and
insulin
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
24
concentrations were determined with a commercial ELISA kit (Alpco Diagnostics,
Windham NH). As shown in Figure 4, baseline blood glucose levels were
significantly
lower in the CL-treated animals (139 +/- 9, p<.05) and the TSH-treated animals
(114 +/-
7, p=.02), than in the vehicle controls (218 +/-25 mg/dl). The thyroxine-
treated animals
were not significantly different from the vehicle controls (252 +/- 34). As
shown in
Figure 5, the control (33-AR agonist and TSH-treated groups exhibited
increased insulin
sensitivity and increased clearance of the glucose load administered at time
zero
compared to vehicle controls. In particular, the thyroxine-treated group did
not exhibit
increased glucose clearance compared to vehicle-treated controls, providing
evidence
that the TSH-mediated effects are not mediated via the thyroid axis, but
through the
stimulation of lipolysis (serum glucose of TSH-treated and thyroxine-treated
groups at
120 minutes post glucose injection had values of 361+/-37 and 802+/-69 mg/dl,
respectively, p<.005).
Seruf~z lipid analysis
The study set used for the IP(iTT was treated an additional 7 days before
sacrifice (total treatment time of 5 weeks). Subject animals were fasted for 4
hours at the
beginning of the light cycle, and serum was obtained at sacrifice under
isoflurane
anesthesia. Triglyceride and total cholesterol levels were determined with the
Cholestech
LDX blood analyzer (Cholestech Corporation, Hayward CA). Serum triglyceride
levels
for the vehicle controls and (33-AR agonist CL 316,243 were 164+/-34 and 191+/-
9
mgldl, respectively. The serum triglycerides in the TSH-treated group were
lower (80+/-
mg/dl, p=.05), and the triglycerides in the thyroxine treated group higher
than the
vehicle controls (297+/-30 mg/dl, p=.05). Total cholesterol levels in the
vehicle-treated,
(33-AR agonist-treated, and thyroxine-treated groups were 220+/-16, 198+/-7,
and 231+/-
11 mg/dl, respectively. Total cholesterol in the TSH treatment group was
significantly
lower at 124+/-16 mg/dl, p<.01.
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
1
SEQUENCE LISTING
<110> ZymoGenetics, Inc.
<120> Use of Thyroid-Stimulating Hormone to
Induce Lipolysiis
<130> 03-O1PC
<150> 60/451.966
<151> 2003-03-05
<160> 6
<170> FastSEQ for Windows Version 4.0
<210>1
<211>351
<212>DNA
<213>Homo Sapiens
<220>
<221> CDS
<222> (1)...(351)
<400> 1
atg gat tac tac aga aaa tat gca get atc ttt ctg gtc aca ttg tcg 48
Met Asp Tyr Tyr Arg Lys Tyr Ala Ala Ile Phe Leu Ual Thr Leu Ser
1 5 10 15
gtg ttt ctg cat gtt ctc cat tcc get cct gat gtg cag gat tgc cca 96
Ual Phe Leu His Ual Leu His Ser Ala Pro Asp Ual Gln Asp Cys Pro
20 25 30
gaa tgc acg cta cag gaa aac cca ttc ttc tcc cag ccg ggt gcc cca 144
Glu Cys Thr Leu Gln Glu Asn Pro Phe Phe Ser Gln Pro Gly Ala Pro
35 40 45
ata ctt cag tgc atg ggc tgc tgc ttc tct aga gca tat ccc act cca 192
Ile Leu Gln Cys Met Gly Cys Cys Phe Ser Arg Ala Tyr Pro Thr Pro
50 55 60
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
2
cta agg tcc aag aag acg atg ttg gtc caa aag aac gtc acc tca gag 240
Leu Arg Ser Lys Lys Thr Met Leu Ual Gln Lys Asn Ual Thr Ser Glu
65 70 75 80
tcc act tgc tgt gta get aaa tca tat aac agg gtc aca gta atg ggg 288
Ser Thr Cys Cys Ual Ala Lys Ser Tyr Asn Arg Ual Thr Ual Met Gly
85 90 95
ggt ttc aaa gtg gag aac cac acg gcg tgc cac tgc agt act tgt tat 336
Gly Phe Lys Ual Glu Asn His Thr Ala Cys His Cys Ser Thr Cys Tyr
100 105 110
tat cac aaa tct taa 351
Tyr His Lys Ser
115
<210>2
<211>116
<212>PRT
<213>Homo Sapiens
<400> 2
Met Asp Tyr Tyr Arg Lys Tyr Ala Ala Ile Phe Leu Ual Thr Leu Ser
1 5 10 15
Ual Phe Leu His Ual Leu His Ser Ala Pro Asp Ual Gln Asp Cys Pro
20 25 30
Glu Cys Thr Leu Gln Glu Asn Pro Ph a Phe Ser Gln Pro Gly Ala Pro
35 40 45
Ile Leu Gln Cys Met Gly Cys Cys Phe Ser Arg Ala Tyr Pro Thr Pro
50 55 60
Leu Arg Ser Lys Lys Thr Met Leu Ual Gln Lys Asn Ual Thr Ser Glu
65 70 75 80
Ser Thr Cys Cys Ual Ala Lys Ser Tyr Asn Arg Ual Thr Ual Met Gly
85 90 95
Gly Phe Lys Ual Glu Asn His Thr Ala Cys His Cys Ser Thr Cys. Tyr
100 105 110
Tyr His Lys Ser
l15
<210>3
<211>92
<212>PRT
<213>Homo sapeins
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
3
<400> 3
Ala Pro Asp Ual Gln Asp Cys Pro Glu Cys Thr Leu Gln Glu Asn Pro
1 5 10 15
Phe Phe Ser Gln Pro Gly Ala Pro Ile Leu Gln Cys Met Gly Cys Cys
20 25 30
Phe Ser Arg Ala Tyr Pro Thr Pro Leu Arg Ser Lys Lys Thr Met Leu
35 40 45
Ual Gln Lys Asn Ual Thr Ser Glu Ser Thr Cys Cys Ual Ala Lys Ser
50 55 60
Tyr Asn Arg Ual Thr Ual Met Gly Gly Phe Lys Ual Glu Asn His Thr
65 70 75 80
Ala Cys His Cys Ser Thr Cys Tyr Tyr His Lys Ser
85 90
<210>4
<211>417
<212>DNA
<213>Homo sapiens
<220>
<221> CDS
<222> (1)...(417)
<400> 4
atg act get ctc ttt ctg atg tcc atg ctt ttt ggc ctt gca tgt ggg 48
Met Thr Ala Leu Phe Leu Met Ser Met Leu Phe Gly Leu Ala Cys Gly
1 5 10 15
caa gcg atg tct ttt tgt att cca act gag tat aca atg cac atc gaa 96
Gln Ala Met Ser Phe Cys Ile Pro Thr Glu Tyr Thr Met His Ile Glu
20 25 30
agg aga gag tgt get tat tgc cta acc atc aac acc acc dtc tgt get 144
Arg Arg Glu Cys Ala Tyr Cys Leu Thr Ile Asn Thr Thr Ile Cys Ala
35 40 45
gga tat tgt atg aca cgg gat atc aat ggc aaa ctg ttt ctt ccc aaa 192
Gly Tyr Cys Met Thr Arg Asp Ile Asn Gly Lys Leu Phe Leu Pro Lys
50 55 60
tat get ctg tcc cag gat gtt tgc aca tat aga gac ttc atc tac agg 240
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
4
Tyr Ala Leu Ser Gln Asp Ual Cys Thr Tyr Arg Asp Phe Ile Tyr Arg
65 70 75 80
act gta gaa ata cca gga tgc cca ctc cat gtt get ccc tat ttt tcc 288
Thr Ual Glu Ile Pro Gly Cys Pro Leu His Ual Ala Pro Tyr Phe Ser
85 90 95
tat cct gtt get tta agc tgt aag tgt ggc aag tgc aat act gac tat 336
Tyr Pro Ual Ala Leu Ser Cys Lys Cys Gly Lys Cys Asn Thr Asp Tyr
100 105 110
agt gac tgc ata eat gaa gcc atc aag aca aac tac tgt acc aaa cct 384
Ser Asp Cys Ile His Glu Ala Ile Lys Thr Asn Tyr Cys Thr Lys Pro
115 120 125
cag aag tct tat ctg gta gga ttt tct gtc taa 417
Gln Lys Ser Tyr Leu Ual Gly Phe Ser Ual
130 135
<210>5
<211>138
<212>PRT
<213>Homo Sapiens
<400> 5
Met Thr Ala Leu Phe Leu Met Ser Met Leu Phe Gly Leu Ala Cys Gly
1 5 10 15
Gln Ala Met Ser Phe Cys Ile Pro Thr Glu Tyr Thr Met His Ile Glu
20 25 30
Arg Arg Glu Cys Ala Tyr Cys Leu Thr Ile Asn Thr Thr Ile Cys Ala
35 40 45
Gly Tyr Cys Met Thr Arg Asp Ile Asn Gly Lys Leu Phe Leu Pro Lys
50 55 60
Tyr Ala Leu Ser Gln Asp Ual Cys Thr Tyr Arg Asp Phe Ile Tyr Arg
65 70 75 ~ 80
Thr Ual Glu Ile Pro Gly Cys Pro Leu His Ual Ala Pro Tyr Phe Ser
85 90 95
Tyr Pro Ual Ala Leu Ser Cys Lys Cys Gly Lys Cys Asn Thr Asp Tyr
100 105 110
Ser Asp Cys Ile His Glu Ala Ile Lys Thr Asn Tyr Cys Thr Lys Pro
115 120 125
CA 02517896 2005-09-O1
WO 2004/078947 PCT/US2004/006852
Gln Lys Ser Tyr Leu Ual Gly Phe Ser Ual
130 135
<210>6
<211>112
<212>PRT
<213>Homo sapiens
<400> 6
Phe Cys Ile Pro Thr Glu Tyr Thr Met His Ile Glu Arg Arg Glu Cys
1 5 10 15
Ala Tyr Cys Leu Thr Ile Asn Thr Thr Ile Cys Ala Gly Tyr Cys Met
20 25 30
Thr Arg Asp Ile Asn Gly Lys Leu Phe Leu Pro Lys Tyr Ala Leu Ser
35 40 45
Gln Asp Ual Cys Thr Tyr Arg Asp Phe Ile Tyr Arg Thr Ual Glu Ile
50 55 60
Pro Gly Cys Pro Leu His Ual Ala Pro Tyr Phe Ser Tyr Pro Ual Ala
65 70 75 80
Leu Ser Cys Lys Cys Gly Lys Cys Asn Thr Asp Tyr Ser Asp Cys Ile
85 90 95
His Glu Ala Ile Lys Thr Asn Tyr Cys Thr Lys Pro Gln Lys Ser Tyr
100 105 110