Note: Descriptions are shown in the official language in which they were submitted.
CA 02521817 2005-09-30
1
PROCESS FOR LEACHING LATERITIC ORE AT ATMOSPHERIC
PRESSURE
FIELD OF THE INVENTION
The present invention relates to a hydrometallurgical process to recover
nickel
and cobalt from nickeliferous Iaterite ores and, in particular, to the
atmospheric
leaching of both low magnesium fraction (eg limonite) and high magnesium
fraction (eg saprolite) ores with a mineral acid to dissolve nickel and
cobalt. In a
preferred form of the process, the process also includes a step in which
magnesium values in the leach liquor are recovered.
BACKGROUND OF THE INVENTION
The known reserves of nickel and cobalt in nickeliferous oxide ores, e.g.,
those
referred to as laterites comprising limonite and saprolite ore, are far
greater than
the corresponding reserves in sulfide ores. An important disadvantage when
processing laterite ores, however, is the inability to beneficiate these ores
by
conventional techniques.
A number of new hydrometallurgical processes are being developed for the
extraction of nickel and cobalt from nickeliferous laterite ores. Many of
these
processes require the dissolution of the metal values with sulfuric acid at
high
temperature (245°-270° C.) and pressure (525-785 psig),
followed by solid-liquid separation and neutralization of residual free acid
present at ambient pressure. This is the basic "Moa Bay Process", as described
by J. R. Boldt and P. E. Queneau in "The Winning of Nickel", Methuen, London,
1967. In this process, the nickeliferous ore is first made into a pulp having
a
solids content of about 40% before leaching at high temperature and pressure.
During pressure leaching most metals dissolve and iron and aluminum are
rejected by hydrolysis to hematite and alunite, respectively. After leaching,
the
pulp is cooled and washed by counter current decantation and the solids are
directed to tailing treatment. Excess acid is neutralized and the remaining
iron
and aluminum are precipitated as hydroxides with the addition of alkali.
Nickel
and cobalt are subsequently recovered via sulfide precipitation.
CA 02521817 2005-09-30
2
Several variations of the high-pressure acid leach (HPAL) method have been
devised with the aim of improving the process and economical aspects. For
example, U.S. Pat. No. 4,044,096 provides guidelines to optimize the high-
pressure acid leaching of nickeliferous lateritic ores through a combination
of
operational steps to improve the economics and efficiency of leaching. The
steps include scalping laterite ore to remove the coarse (high magnesium)
fraction and thus lower the acid consumption.
The HPAL process is most amenable for high iron ores containing 40 wt % iron
or higher. Lateritic ores with an iron content less than 40 wt % contain in
general a higher amount of acid consuming minerals and are therefore not
preferred for direct high pressure leaching. U.S. Pat. No. 3,804,613 teaches a
method of high-pressure acid leaching of saprolite ore at relatively low
acidlore
ratios by preconditioning the saprolite with leach liquor from the high-
pressure
leach step. No mention is made of concurrent limonite leaching.
U.S. Pat. No. 3,991,159 teaches the use of saprolite ore to neutralize acid
resulting from the high-pressure acid leach of limonite ore. Leaching of the
saprolite fraction is carried out at high temperature (150°-250°
C.) and pressure for effective iron and aluminum rejection, but with
relatively
low nickel extraction from the saprolite ore. In another process, U.S. Pat.
No.
4,097,575 teaches saprolite ore roasting at 500°-750° C. under
oxidizing conditions to increase its neutralization capacity before
neutralization
of HPAL liquors. This process suffers from the additional need for roasting
facilities.
While the prior art HPAL methods obtain a high extraction of nickel and
cobalt,
they require the use of expensive equipment and sophisticated materials of
construction to withstand the use of concentrated acid at the high
temperatures
needed (200°-300° C.). Several alternatives to the HPAL
process to recover nickel and cobalt from laterite ore have been proposed.
For example, U.S. Pat. No. 4,062,924 describes a method for leaching limonite
ores in acidic media at temperatures up to 110° C. and in the presence
CA 02521817 2005-09-30
3
of hydrogen sulfide gas to precipitate dissolved nickel and cobalt. Most
dissolved iron is also reduced to the divalent oxidation state however,
consuming very high amounts of the reducing gas in addition to high acid
consumption. U.S. Pat. No. 4,065,542 teaches a similar method. In this
process, ferrous iron produced by the method described above is used to leach
metal values from manganiferous sea nodules. U.S. Pat. No. 4,511,540
illustrates a way to recover nickel and cobalt from ores with a manganiferous
matrix by leaching with sulfuric acid in the presence of sulfur dioxide gas at
temperatures below the boiling point of the liquid solution. None of these
processes includes the treatment of saprolitic ores.
In the process of U.S. Pat. No. 3,793,432, limonite ore is leached with
sulfuric
acid at a pH below 1.5, while simultaneously adding alkaline iron-
precipitating
agents. The process is carried out at atmospheric pressures, but requires
leaching times in excess of 40 hours and usually from 60 to 100 hours for
efficient nickel extraction and iron precipitation. No use of saprolite is
made in
this process. U.S. Pat. No. 4,410,498 teaches a method to leach saproiite ore
with sulfuric acid at atmospheric pressure, while adding a reducing agent to
maintain the redox potential between 400 and 600 mV. In another process,
described in U.S. Pat. No. 5,571,308, nickel and cobalt are leached from
saprolite ore by contact with a mineral acid at room temperature or in the
temperature range of 60°-80° C. The leaching mode can be
conducted by heap, vat, or agitation leaching.
US Patent 6,261,527 also discloses a hydrometallurgical process for the
recovery of nickel and cobalt from both limonite and saprolite ores, however
in
that process, iron is rejected as jarosite.
There are environmental concerns with this iron removal process as the
jarosite
compounds are thermodynamically unstable. Jarosite may decompose slowly
to iron hydroxides releasing sulphuric acid. The released acid may redissolve
traces of precipitated heavy metals, such as Mn, Ni, Co, Cu and Zn, present in
the leach residue tailing, thereby mobilizing these metals into the ground or
surface water around the tailings deposit.
CA 02521817 2005-09-30
4
Another disadvantage of this process is that jarosite contains sulphate, and
this
increases the acid requirement for leaching significantly. Sulphuric acid is a
large input in acid leaching processing, so there is also an economic
disadvantage in the jarosite process.
The present invention aims to overcome or alleviate one or more of the
problems associated with prior art processes.
The discussion of documents, acts, materials, devices, articles and the like
is
included in this specification solely for the purpose of providing a context
for the
present invention. It is not suggested or represented that any or all of these
matters formed part of the prior art base or were common general knowledge in
the field relevant to the present invention as it existed before the priority
date of
each claim of this application.
SUMMARY OF THE INVENTION
According to the present invention, there is provided an atmospheric leaching
process in the recovery of nickel and cobalt from a lateritic ore, said
lateritic ore
including a low magnesium ore fraction and a high magnesium ore fraction, said
process including the steps of:
(a) forming an aqueous pulp of said lateritic ore,
(b) leaching said aqueous pulp with a concentrated mineral acid at
atmospheric pressure to produce a slurry containing a pregnant leach liquor
and
a leach residue,
(c) treating the pregnant leach liquor either separately or as part of said
slurry to recover dissolved nickel and cobalt therefrom, leaving a magnesium
containing barren solution,
(d) treating said magnesium containing solution to recover a magnesium
containing salt therefrom.
An advantage of the invention is the provision of an efficient and economical
method to leach both low magnesium (eg limonite) and high magnesium (eg
saprolite) ores in a single process stage at atmospheric pressure, to obtain
high
percent dissolution of nickel and cobalt. A further advantage of the method is
CA 02521817 2005-09-30
that it avoids the high capital costs associated with sophisticated
autoclaves.
Another advantage of a preferred form of the method is that it also avoids the
production of jarosite. An advantage of a preferred form of the invention, is
that
the magnesium containing barren solution produced from the leaching process
5 is treated to recover magnesium sulphate, which is then processed to give
MgO, Mg(OH)2 or MgC03 and S02. The S02 is advantageously used to
regenerate H2SO4. The Mg0 or MgC03 may be fed back into the leaching
process as a neutralising agent, disposed of as a stable residue, or sold as a
commercial product.
DETAILED DESCRIPTION OF THE INVENTION
The low magnesium containing ore traction includes the limonite fraction of
the
laterite ore (Mg wt % approximately less than 6). This fraction may also
include
low to medium level magnesium content smectite or nontronite ores which
generally have a magnesium content of about 4 wt % to 8 wt %. The high
magnesium containing ore fraction includes the saprolite fraction of the
laterite
ore (Mg wt % greater than approximately 8). This fraction may also include
smectite or nontronite ores. The formation in step (a) of an aqueous pulp of
both the low magnesium and high magnesium containing ore fractions is
generally carried out in sodium, alkali metal and ammonium free water at
solids
concentration from approximately 20 wt % and above, limited by slurry
rheology.
The ratio of acid to combined ore is typically at least 0.5. Preferably, the
ratio is
about 0.5 to 1.0, such as 0.5 to 0.7.
The aqueous pulp is subjected to a leaching step in step (b) utilising a
concentrated mineral acid at atmospheric pressure. Preferably leaching is
conducted whilst agitating leach reactants. Typically the leaching step is
carried
out at a temperature up to the boiling point of the leach reactants at
atmospheric pressure. Most preferably the reaction temperature is as high as
possible to achieve rapid leaching at atmospheric pressure. A preferred
leaching temperature is at least 60°C, more preferably at least
75°C. In a
preferred embodiment, leaching is carried out at around 80°C or higher,
such as
CA 02521817 2005-09-30
6
at least 85°C. In another preferred embodiment, leaching is conducted
at
around 95°C.
Preferably, the teaching of both the low and high magnesium fractions occurs
in
a single process stage, which may comprise a single step, in which the two
fractions are leached simultaneously, eg in the same tank or reactor.
Alternatively, the two fractions may be leached in sequential steps in the
single
process stage. In that case, preferably the low magnesium fraction (eg
limonite) is leached in a first step, and then the higher magnesium fraction
is
subsequently added to the slurry to be leached in a second step. The
sequential leaching of the low and high magnesium fractions may be in
accordance with the disclosure of WO 03/093517, the entire disclosure of which
is incorporated herein by reference.
Leaching is conducted for a period of time sufficient to release at least a
substantial portion of the nickel and cobalt from the laterite ore into
solution.
Typically leaching is conducted for up to 30 hours. However, preferably
leaching is conducted for up to 5 hours. More preferably, leaching is
conducted
for up to 4 hours. In a preferred embodiment, leaching is conducted for about
2
hours.
The leaching process typically also results in precipitation in at least some
of
the iron in the ore as one or more Fe containing compounds, such as a
sulphate, a hydroxide or an oxide.
The mineral acid used in the leaching process is preferably sulphuric acid,
more
preferably it is concentrated sulphuric acid. The concentration of sulphuric
acid
added to the ore pulp is preferably greater than 90 wt %. The dose of
sulphuric
acid is preferably 100 to 140 % of the stoichiometric amount required to
dissolve approximately over 90% of nickel, cobalt, iron, manganese and over 80
of the aluminum and magnesium in the ore.
The ratio of the high magnesium ore to low magnesium ore is ideally in a dry
ratio of from 0.5 to 1.3. Preferably, the ratio is from 1 to 1.30. However,
the
CA 02521817 2005-09-30
7
high/low magnesium ore ratio will largely depend on the laterite ore
composition.
The leaching of both the high and low magnesium fractions may optionally be
followed by a second leaching step. In the second leaching step, any unused
acid from the first leaching step may be reacted with additional high
magnesium
ore fraction, such as saprolite. Leaching conditions of temperature, time and
acid concentration are typically similar to those of the first leaching step.
Addition of saprolite can cause further precipitation of Fe containing
compounds.
Conditions of temperature, time and acid concentration may conveniently be
controlled to allow part or all of the iron and aluminum to be precipitated.
The
acidity may be conveniently controlled by the addition of saprolite, MgO,
Mg(OH)2, MgC03 or another alkali. For example, the leach slurry may be
treated in accordance with the method disclosed in WO 03/093517 (the entire
disclosure of which is incorporated herein by reference), in which saprolite
ore
is added to a leach slurry in order to precipitate goethite or other
relatively low
sulphate-containing forms of iron oxide or iron hydroxide. Alternatively, the
leach slurry may be treated in accordance with the method disclosed in US
6,261,527 (the entire disclosure of which is also incorporated herein by
reference) in which an iron precipitating agent selected from sodium,
potassium
ammonium ions and mixtures thereof is added to the slurry to precipitate
jarosite.
in a preferred embodiment, Mg0 is added to the slurry in order to precipitate
iron containing compounds. Preferably, the Mg0 addition results in an increase
of pH to a value of 3.0 or higher, causing iron precipitation.
The leached slurry is then treated to recover dissolved nickel and cobalt
values
therefrom. Such metal extraction treatment may be one or more of techniques
known to those working in the art. Examples of such metal extraction
techniques include ion exchange, resin-in-pulp, direct recovery by solvent
CA 02521817 2005-09-30
extraction, mixed hydroxide precipitation or mixed sulphide precipitation.
Preferably the recovered nickel and cobalt values are recovered as mixed
nickel/cobalt hydroxides or mixed nickel/cobalt sulphides.
Prior to or during recovery of nickel and cobalt from the leach liquor, the
solid
leach residue which usually includes precipitated iron compounds such as Fe
sulphates eg jarosite or Fe hydroxides, eg goethite, may be removed from
solution depending on the recovery process used. Alternatively, the solid
residue may be retained with the leach solution during subsequent removal of
residual Fe and/or AI.
Prior to or after recovery of nickel and cobalt from the leach solution, the
spent
leach solution is preferably treated to remove any residual Fe and/or AI in
solution. Typically, this step requires an increase in solution pH, such as by
adding a neutralising agent, such as MgO, Mg(OH)2 or MgC03, and preferably
addition of an oxidising agent such as air. Typically a sufficient quantity of
neutralising agent is added such that the solution pH is increased to around 3
or
above. A sufficient amount of the oxidising agent is also added to oxidise any
residual Fe2+ in solution to Fe3+, which then precipitates out as goethite.
After removal ofNi, Co, Fe and AI from the spent leach liquor, the supernatant
solution mainly contains dissolved magnesium, possibly together with a small
quantity of manganese. The supernatant solution is then treated in order to
recover the magnesium as magnesium salts. This is achieved typically by
evaporation until the magnesium salts crystalise out. Alternatively, reverse
osmosis or precipitation by a strong alkali such as caustic soda, soda ash or
lime, may be used.
The magnesium salt is typically a magnesium sulphate where the leaching acid
used was sulphuric acid. It has been the conventional practice to discard the
magnesium salts as waste, meaning that metal values in the salts are therefore
lost. Moreover, when the magnesium salt comprises magnesium sulphate, the
sulphate component is also lost, which increases the acid requirement for the
leaching process significantly. Sulphuric acid is usually an expensive input
in
CA 02521817 2005-09-30
9
acid leaching, so there is an economic disadvantage in simply discarding a
source of sulphate.
Accordingly, in a preferred embodiment of the process, the present invention
is
also concerned with treating the magnesium salt to recover magnesium
compounds. Where the magnesium salt comprises MgS04, the recovery
process also preferably includes a sulphate recovery stage. Preferably, the
magnesium is recovered as a magnesium oxide, magnesium hydroxide or
magnesium carbonate. More preferably, the magnesium is recovered as
magnesium oxide. The magnesium recovery process may comprise that
disclosed in co-pending Australian provisional patent application 2005900431
filed on 1 February 2005, the entire disclosure of which is incorporated
herein
by reference. Alternatively, the magnesium salt may be subjected to
calcination. Where the magnesium salt is magnesium sulphate, calcination
results in formation of Mg0 and/or MgC03 and S02 gas. The S02 gas may be
captured and fed to a sulphuric acid production process , in which sulphuric
acid
is regenerated according to the following process:
S02 + H2 O +'/2 02 = H2 S04.
The MgO, Mg(OH)2 or MgC03 produced from the magnesium salt is a good
source of alkaline compound, which can be fed back to the leach solution as a
neutralising agent to effect precipitation, separately or in combination,
metals
such as Ni, Co, AI, Fe, Mn and other elements as desired.
Example 1
A mixture of limonite and saprolite ore in a dry ratio of about 1 is formed
into an
aqueous pulp. The aqueous pulp is then mixed with concentrated sulphuric
acid, having a concentration of 93% H2S04, to form a leach slurry. The dose of
acid is greater than 100% of the stoichiometric amount required to dissolve
over
90% of the Ni and Co in the combined ore fractions. A first leaching process
is
conducted in a single reactor at a temperature of at least 80°C and for
at least 2
CA 02521817 2005-09-30
hours. During the first leaching process, iron compounds precipitate out of
solution.
Overflow from the leaching process is conveyed to a second reactor, where a
5 saprolite ore slurry is added to the mixture. A second leaching process is
then
conducted, also at a temperature of at least 80°C and for a time of
around 2
hours. During the second leaching process, further iron compounds precipitate
out from solution.
10 After completion of the second leaching process, the solid residue is
separated
from the leached slurry. The pregnant leach solution is then subjected to a
recovery process during which nickel and cobalt values are recovered.
The spent leach solution is also treated to remove any residual iron and
aluminium. This is effected by the addition of a neutralising agent comprising
Mg0 or MgC03. The pH of the barren solution is thereby increased, to a value
higher than 3. The iron is precipitated largely as hydroxides, such as
Fe(OH)3.
At this time, the barren leach solution contains mainly dissolved magnesium.
The spent leach solution is directed to an evaporation pond and excess water
evaporated therefrom, causing crystallisation of magnesium sulphate.
The magnesium sulphate is then subjected to a magnesium recovery process.
This comprises caicination to produce MgO, or MgC03, and S02 gas. The S02
gas is then used as a reactant in a sulphuric acid recovery process.
The following two Examples are concerned with the recovery of magnesium
from magnesium sulphate crystals. Example 2 is a Comparative Example
demonstrating calcination of MgS04.7H20 under non-reducing conditions,
which shows that MgS04 remains as the product. However, Example 3
demonstrates that calcination under reducing conditions achieves production of
Mg0 at moderate temperatures, that is, at temperatures significantly lower
than
those at which calcination is conventionally conducted.
CA 02521817 2005-09-30
11
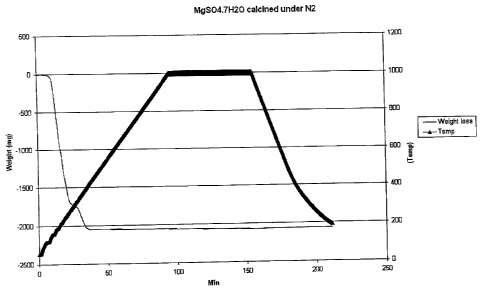
Example 2 - Comparative Example
A sample of magnesium sulphate heptahydrate (4.0353g) was placed in a small
crucible and calcined in a thermogravimetric analyser (TGA) under a flow of
dry
nitrogen (5L/min). The temperature in the TGA was raised by 10°C/min
from
room temperature to 1000°C. The sample exhibited a weight loss of
approximately 2.07g by 400°C and exhibited very little further weight
loss. The
resulting mass of the sample (1.9386g) corresponds closely with the formula
MgS04 (theoretical weight of 1.9706g). A graphical depiction of the TGA run is
shown in Figure 1.
Example 3
A sample of magnesium sulphate heptahydrate (4.0093g) was placed in a small
basket and calcined in the thermogravtmetric analyser (TGA) under a flow of
dry
hydrogen (5L/min). The temperature in the TGA was again raised by
10°C/min
from room temperature to 1000°C. The sample exhibited a weight loss of
approximately 2.038 by 350°C, corresponding to the loss of waters of
crystallisation. The weight then remained stable until 630°C at which a
further
weight loss of approximately 1.06g. Rapid weight loss slowed at a temperature
of 810°C. By the time 1000°C had been reached the total weight
toss was
approximately 3.29g. The remaining sample was carefully removed from the
container and weighed. The mass of the weighed sample (0.63g) corresponds
closely with the formula Mg0 (theoretical weight 0.655g). A graphical
depiction
of this run is shown in Figure 2.
Example 4
Example 4 is a Flowsheet, illustrated in Figure 3, which sets out the process
stages in an embodiment of the present invention. In this Example, separation
of the leach residue from the pregnant leach solution takes place prior to
removal of residual iron and aluminium and recovery of Ni and Co metal values.
The recovery of Ni and Co is effected using one of the techniques selected
from
mixed hydroxide precipitation, mixed sulphide precipitation, solvent
extraction or
ion exchange.
CA 02521817 2005-09-30
1~
Example 5
Example 5 is a Flowsheet, illustrated in Figure 4, setting out the process
stages
in a further embodiment of the present invention. In Example 5, the Ni and Co
metal values are recovered, using the Resin-in-Pulp (R-I-P) extraction
technique, prior to removal of residual iron and aluminum, subsequent
manganese precipitation and separation of leach residue from the barren
solution.
The above description of the invention is illustrative of the preferred
embodiments of the invention. Variations without departing from the spirit or
ambit of the invention described herein are to be considered to form part of
the
invention.