Note: Descriptions are shown in the official language in which they were submitted.
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
-1-
COMPOSITION GRADIENT CERMETS AND REACTIVE
HEAT TREATMENT PROCESS FOR PREPARING SAME
FIELD OF INVENTION
[0001] The present invention is broadly concerned with cermets, particularly
composition gradient cermets and reactive heat treatment process for preparing
same.
BACKGROUND OF INVENTION
[0002] Erosion resistant materials find use in many applications wherein
surfaces are subject to eroding forces. For example, refinery process vessel
internals exposed to aggressive fluids containing hard solid particles such as
catalyst particles in various chemical and petroleum environments are subject
to
both erosion and corrosion. The protection of these vessel internals against
erosion and corrosion induced material degradation especially at high tempera-
tures is a technological challenge. Refractory liners are used currently for
components requiring protection against the most severe erosion and corrosion
such as the inside walls of cyclones such as the internal cyclones in fluid
catalytic cracking units (FCCU). The life span of these refractory liners is
significantly limited by mechanical attrition of the liner, cracking and
spallation.
The state-of-the-art in erosion resistant materials is chemically bonded
castable
alumina refractories. These castable alumina refractories are applied to the
surfaces in need of protection and upon heat curing harden and adhere to the
surface via metal-anchors or metal-reinforcements. It also readily bonds to
other
refractory surfaces. ~'he typical chemical composition of one commercially
available refractory is 80.0% A1203, 7.2% Si02, 1.0% Fe~03, 4.8% MgO/CaO,
4.5% P205 in wt%.
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
-2-
[0003] Ceramic-metal composites are called cermets. Cermets of adequate
chemical stability can provide an order of magnitude higher erosion resistance
over refractory materials known in the art. Cermets are generally produced
using powder metallurgy techniques where metal and ceramic powders are
mixed, pressed and sintered at high temperatures. Since powder metallurgically
produced cermets usually have homogeneous microstructure and uniform
composition, sophisticated attachment methods are needed to attach cermets
onto the metallic surfaces wherein erosion resistance of the surface is
desired.
[0004] Composition gradient cermets are cermets wherein one surface of the
cermet is ceramic-rich and the unexposed surface is metal-rich. Tn a typical
composition gradient cermet there is a concentration gradient of the ceramic
in
the metal composition such that the concentration of the ceramic decreases
with
depth. These composition gradient cermets are desired and preferred for cost-
effective attachment of cermets directly onto metal or alloy surfaces using
methods such as welding due to the compatibility and ease of welding a sub-
stantially metallic object to another substantially metallic object.
Furthermore,
such composition gradient cermets can also exhibit superior durability particu-
larly under conditions wherein thermal fluctuations are present. However,
there
is a need for effective processes to prepare composition gradient cermets.
[0005] One object of the present invention is to provide a process for
preparation of cermets, particularly composition gradient cermets via reactive
heat treatment of a metal alloy.
[0006] Another object of the present invention is to provide a composition
gradient cermet product derived from the reactive heat treatment process.
[0007] These and other objects will become apparent from the description
that follows.
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
-3-
SUMMARY OF INVENTION
[0008] In one embodiment is a process for preparing a composition gradient
cermet material comprising the steps of:
- heating a metal alloy containing at least one of chromium and titanium at a
temperature in the range of about 600°C to about 1150°C to form
a heated
metal alloy;
- exposing said heated metal alloy to a reactive environment comprising at
least one member selected from the group consisting of reactive carbon,
reactive nitrogen, reactive boron, reactive oxygen and mixtures thereof in the
range of about 600°C to about 1150°C for a time sufficient to
provide a
reacted alloy; and
- cooling said reacted alloy to a temperature below about 40°C to
provide a
composition gradient cermet material.
[0009] Another embodiment is directed towards a composition gradient
cermet product obtained from the disclosed reactive heat treatment process.
BRIEF DESCRIPTION OF THE FIGURES
[0010] Figure 1 depicts carbon activity of an environment based on the
reaction CH4 ---> C + 2 H2 compared to austenitic stainless steels (a~ in
equilibrium with Fe3C). Also marked are the carbon activity values of gas
mixtures applicable to the instant invention.
[0011] Figure 2 depicts the mass gain due to carbon ingression (a measure of
cermet layer formation) of 304 stainless steel (74Fe: l8Cr:8Ni in wt%) as a
function of CH4 content in HZ at 1100°C for 3 hours.
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
-4-
[0012] Figure 3 depicts the thickness variation of surface cermet structure on
304 stainless steel as a function of temperature in 37.3 vol% CH4:62.7 vol% H2
environment for 3 hours.
[0013] Figure 4 depicts the thickness variation of surface cermet formed on
various Fe:Ni:Cr based high temperature alloys as a function of reaction times
at
1100°C in 37.3 vol% CH4:62.7 vol% Ha environments.
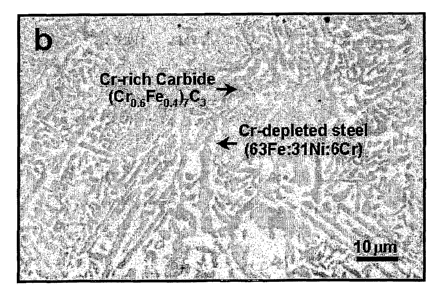
[0014] Figure 5 depicts scanning electron micrographs showing (a) surface
chromium carbide-metal cermet structure on 310 stainless steel (54Fe:21Ni:25Cr
in wt%) after reactive heat treatment at 1100°C for 3 hours in 37.3
vol%
CH4:62.7 vol% H2 environment and (b) enlarged area on the surface revealing
the Cr-rich carbide [(Cro,6Feo,4)~C3] and Cr-depleted steel (63Fe:31Ni:6Cr in
wt%) to produce a composite ceramic-metal two-phase structure. In this
scanning electron micrograph the Cr-rich carbides appear dark gray and the
metal appears recessed, because it has etched more deeply than the carbides.
These figures show the final product having the cermet surface, which is the
product of the process of the instant invention.
[0015] Figure 6 depicts optical micrographs showing M7C3 (M=Cr and Fe)
carbide-metal cermet structure on (a) 55Fe:35Cr:lONi (in wt%) alloy, (b)
45Fe:45Cri:lONi (in wt%) alloy and (c) 35Fe:55Cr:10Ni (in wt%) alloy after
reactive heat treatment at 1100°C for 24 hours in 10 vol% CH4:90 vol%
H2
environment.
[0016] Figure 7 depicts optical micrographs showing mixed TiC and lVhC3
(M=Cr and Fe) carbide-metal cermet structure on 60Fe:25Cr:10Ni:5Ti (in wt%)
alloy after reactive heat treatment at 1100°C for 24 hours in 10 vol%
CH~:90
vol% H2 environment.
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
-5-
DETAILED DESCRIPTION OF THE INVENTION
[0017] The first step of the process for preparing a composition gradient
cermet material comprises heating a metal alloy containing at least one of
chromium and titanium at a temperature in the range of about 600°C to
about
1150°C to form a heated metal alloy. The metal alloy containing at
least one of
chromium and titanium comprises from about 12 to 60 wt% chromium, from 0
to 10 wt% titanium, and from 30 to 88 wt% of metals selected from the group
consisting of iron, nickel, cobalt, silicon, aluminum, manganese, zirconium,
hafnium, vanadium, niobium, tantalum, molybdenum, tungsten, and mixtures
thereof. In a preferred embodiment the major mass constituent of the alloy is
iron. Thus, stainless steels such as type 304SS, 347SS, 321SS, 310SS and the
like and iron-nickel based alloys such as Incoloy 800H are particularly
suitable
for the instant process.
[0018] The second step of the process comprises exposing the heated metal
alloy to a reactive environment selected from the group consisting essentially
of
reactive carbon, reactive nitrogen, reactive boron, reactive oxygen and
mixtures
thereof in the range of about 600°C to about 1150°C for a time
period sufficient
to provide a reacted alloy.
[0019] When the reactive environment is a reactive carbon environment
carburization reactions are believed to occur. While not wishing to be bound
to
the mechanism of the reactive heat treatment process applicants believe that
the
carburization process leads to precipitation of chromium-rich and titanium
carbide phases for example Cr~C3, Cr23C6, (Cro.6Feo.a)~C3> (Cro.sFeo.a)a3Cs
and TiC
on the alloy surface and within the alloy matrix resulting in a cermet and
particularly a composition gradient carbide cermet.
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
-6-
[0020] A reactive carbon environment is defined as an environment in which
the thermodynamic activity of carbon (a~) in the environment is greater than
that
of the alloy.
(ac)environment > (ac)metal
The reactive carbon environment suitable for the instant invention comprises
at
least one of CO, CH4, C2H6 or C3H8. The reactive carbon environment may
optionally include H2. The reactive carbon environment may further comprise
02, CO2, and H20. The following reactions [1], [2] and [3] shown below are
some of the reactions that are believed to occur under the heat treatment
conditions to provide the reactive carbon. The carbon reacts with the metal
surface to form chromium-rich and titanium-rich carbide phases.
CO + H2 -----> C + H20 [ 1
2C0 -------> C + COZ [2]
CH4 -------> C + 2 HZ [3]
[0021] When heat treatment follows reaction [3], the carbon activity (a~) in
the environment is
ac = ~ G°/RT (PCH~~2~
where G° is the free energy of activation, R is the gas constant, T is
the tempera-
ture in Kelvin units and P is the partial pressure of the respective gases
methane
and hydrogen. Carbon activities as a function of (PoH4/P2H2) ~'e plotted in
Figure 1 wherein is indicated the preferred range of P~H4/P2H2 for the process
of
the instant invention.
[0022] When a mixture of methane and hydrogen are used to provide the
reactive carbon environment, the methane content in the gaseous mixture of
methane and hydrogen can range from about 1 vol% to about 99 vol%, prefer-
ably about 2 vol% to about 45 vol%. This is depicted in Figure 2, where the
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
mass gain due to carbon ingression (a measure of cermet layer formation) of
304
stainless steel (74Fe:18Cr:8Ni in wt%) at 1100°C exposed for 3 hours is
plotted
as a function of CH4 content in HZ. The preferred methane content in the
gaseous mixture of methane and hydrogen corresponds to the plateau region of
the curve. In this range, the reaction times are shorter to obtain a specific
thickness of cermet. Gas mixtures in which the methane content in the gaseous
mixture of methane and hydrogen is greater than 45 vol% can also be used.
However, in these ranges, solid carbon deposition on the alloy surface can be
encountered as indicated by the rapid increase of mass gain in Figure 2.
[0023] When a mixture of CO and hydrogen are used to provide the reactive
carbon environment, the CO content in the gaseous mixture of CO and hydrogen
can range from about 0.1 vol% to about 5 vol%, preferably about 0.1 vol% to
about 2 vol%.
[0024] When the reactive environment is a reactive nitrogen environment,
nitridation reactions are believed to occur. While not wishing to be bound to
the
mechanism of the reactive heat treatment process applicants believe that the
nitridation process leads to precipitation of chromium-rich and titanium
nitride
phases for example Cr2N and TiN on the alloy surface and within the alloy
matrix resulting in a cermet and particularly a composition gradient nitride
cermet.
[0025] A reactive nitrogen environment is defined as an environment in
which the thermodynamic activity of nitrogen (aN) in the environment is
greater
than that of the alloy.
~aN~environment ~ CaN~metal
Since molecular nitrogen is relatively inert in terms of nitridation of an
alloy,
ammonia-bearing atmospheres are preferred. Ammonia is metastable and
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
_g_
dissociates into molecular N2 and molecular H2 when heated to elevated
temperatures. The preferred composition of the reactive nitrogen environment
comprises at least one of air, ammonia and nitrogen. The composition can
further comprise H2, He, and Ar. In such a reactive nitrogen environment at
temperatures in the range of 600°C to 1150°C alloys containing
elements such as
Cr and Ti which have strong chemical affinities for nitrogen undergo rapid
nitridation reactions. In order to increase nitrogen absorption by the alloy,
molecular NH3 is preferred to dissociate on the alloy surface, thus allowing
dissociated atomic nitrogen to dissolve at the surface and diffuse into the
bulk
interior of the metal alloy. Similar to carburization process, nitridation can
lead
to the formation of surface nitrides, internal nitrides in the matrix and at
grain
boundaries near the alloy surface.
[0026] When a mixture of ammonia and hydrogen are used to provide the
reactive nitrogen environment, the ammonia content in the gaseous mixture of
ammonia and hydrogen can range from about 1 vol% to about 99 vol%,
preferably about 2 vol% to about 70 vol%.
[0027] The preferred temperature range for accomplishing the conversion of a
metal alloy containing chromium, titanium and mixtures thereof to a nitride
cermet is in the range of about 600°C to about 1150°C.
[0028] When the reactive environment comprises a mixture of reactive
carbon and reactive nitrogen a mixed composition gradient cermet comprising
carbide, nitride, carbonitride and mixtures thereof results. When the reactive
environment is a reactive carbon and nitxogen environment, carbonitridation
reactions are believed to occur. While not wishing to be bound to the
mechanism of the reactive heat treatment process applicants believe that the
carbonitridation process leads to precipitation of chromium-rich and titanium
carbonitride phases for example CrzCN and TiCN on the alloy surface and
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
within the alloy matrix resulting in a cermet and particularly a composition
gradient carbonitride cermet
[0029] A reactive carbon and nitrogen environment is defined as an environ-
ment in which the thermodynamic activity of carbon (a~) and nitrogen (aN) in
the
environment is greater than that of the alloy. The preferred composition of
the
reactive carbon and nitrogen environment comprises at least one of ammonia
and nitrogen and at least one of CO, CH4, C2H6 or C;HB. The composition can
further comprise H2, He, and Ar. In such a reactive carbon and nitrogen
environ-
ment at temperatures in the range of 600°C to 1150°C alloys
containing elements
such as Cr and Ti which have strong chemical affinities for carbon and
nitrogen
undergo rapid carbonitridation reactions. Similar to carburization or
nitridation
process, carbonitridation can lead to the formation of surface carbonitride,
internal carbonitride in the matrix and at grain boundaries near the alloy
surface.
[0030] When the reactive environment is a reactive boron environment,
boridation reactions are believed to occur. While not wishing to be bound to
the
mechanism of the reactive heat treatment process applicants believe that the
boridation process leads to precipitation of chromium-rich and titanium.
boride
phases for example Cr2B and TiB2 on the alloy surface and into the alloy
matrix
resulting in a cermet and particularly a composition gradient boride cermet.
[0031] A reactive boron environment is defined as an environment in which
the thermodynamic activity of boron (aB) in the environment is greater than
that
of the alloy. The preferred composition of the reactive boron environment
comprises for example at least one of diborane (B2H6), BC13, and BF3. The
composition can further comprise HZ, He, and Ar. In such a reactive boron
environment at temperatures in the range of 600°C to 1150°C
alloys containing
elements such as Cr and Ti which have strong chemical affinities for boron
undergo rapid boridation reactions. Similar to carburization or nitridation
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
-10-
process, boridation can lead to the formation of surface borides, internal
borides
in the matrix and at grain boundaries near the alloy surface.
[0032] When the reactive environment is a reactive oxygen environment,
oxidation reactions are believed to occur. While not wishing to be bound to
the
mechanism of the reactive heat treatment process applicants believe that the
oxidation process leads to precipitation of chromium-rich and titanium oxide
phases for example (Cr,Fe)20;, Cr203 and Ti02 on the alloy surface and within
the alloy matrix resulting in a cermet and particularly a composition gradient
oxide cermet.
[0033] A reactive oxygen environment is defined as an environment in which
the oxygen potential in the environment is greater than the oxygen partial
pressure in equilibrium with the oxide. The preferred composition of the
reactive oxygen environment comprises at least one of air, oxygen and C02. The
composition can further comprise H2, He, and Ar. In such a reactive, oxygen
environment at temperatures in the range of 600°C to 1150°C
alloys containing
elements such as Cr and Ti which have strong chemical affinities for oxygen
undergo rapid oxidation reactions. Similar to carburization or nitridation
process, oxidation can lead to the formation of surface oxides, internal
oxides in
the matrix and at grain boundaries near the alloy surface.
[0034] The third step of the process is cooling of the reacted alloy. The
cooling step can include a variety of cooling rates and/or an intermediate
temperature hold before cooling to below about 40°C. In one embodiment
the
cooling step comprises cooling the reacted alloy at a rate in the range of
0.5°C
per second to 25°C per second. In another embodiment the cooling step
com-
prises cooling said reacted alloy to a temperature in the range of
500°C to 100°C,
holding the temperature at any temperature in the range of 500°C to
100°C for a
time period between 5 minutes to 10 hours and thereafter cooling at a rate in
the
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
-11-
range of 0.5°C per second to 25°C per second to below about
40°C. Applicants
believe this preferred cooling profile has process and product advantages.
(0035] The exposure time (the time period the heated alloy is exposed to the
reactive environment) can vary in the range of about 1 hour to 800 hours to
achieve various thickness of the carbide, nitride, carbonitride, boride or
oxide
cermet on the surface the metal alloy. An example for carbide cermet is
depicted in Figure 4 where the thickness of the surface carbide cermet foamed
on
various Fe:Ni:Cr high temperature alloys is plotted as a function of exposure
time at conditions of 1100°C in 37.3 vol% CH4:62.7 vol% HZ environment.
Thus, this example shows that the process of the instant invention can be used
to
obtain any thickness of carbide cermet resulting in a composition gradient
carbide cermet. Alternately, the process can also be used to completely
convert
the entire bulk of the chromium, titanium or mixture of chromium and titanium
comprising alloy to a composition gradient cermet wherein the gradient
traverses
the entire thickness of the bulk alloy.
[0036] The thickness of cermet layers can be controlled by the composition of
the reactive environment, the temperature and the exposure time. Exposure
times can be determined experimentally as depicted in Figure 4 for a carbide
cermet. For thinner layers, the exposure time will be less, and for thicker
layers
the exposure time will be greater. Typical exposure times for a carbide cermet
can range from about 1 hour to about 500 hours, preferably from about 5 hours
to about 300 hours, and more preferably from about 10 hours to about 200
hours.
Thus, the exposure time and temperature are two variables that can provide a
desired thickness of cermet and a desired composition gradient cermet. For a
nitride cermet, typical exposure times can range from about 1 hour to about
800
hours, preferably from about 5 hours to about 500 hours, and more preferably
from about 10 hours to about 300 hours. Thus, the exposure time and tempera-
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
_Z~_
ture are two variables that can provide a desired thickness of nitride cermet
and a
desired composition gradient nitride cermet.
[0037] Typical layer or cermet structure thickness can range from at least
about 100 microns up to the thickness of the metal alloy being acted on,
preferably from about 5 mm to about 30 mm, more preferably from about 5 mm
to about 20 mm. Layer thickness can be determined by electron microscopy
techniques known to one of ordinary skill in the art of electron microscopy.
[0038] The instant invention is also applicable to an article consisting of an
amount of chromium-rich or titanium-rich carbide, nitride, carbonitride,
boride,
and oxide in combination with a chromium and titanium containing metal alloy.
[0039] The reactive heat treatment process of the instant invention results in
a
composition gradient cermet having erosion resistance superior to that of the
untreated alloy containing chromium, titanium and mixtures thereof as shown in
Example 4. This is because the erosion resistance of the alloy improves as the
cermet layer develops and provides hardening. In the instant invention, the
amount of reactive carbon, reactive nitrogen, reactive boron, reactive oxygen
diffusing into the metal alloy containing chromium, titanium and mixtures
thereof from the respective reactive environment is utilized to produce the
composition gradient cermet. The portion of the.alloy containing chromium,
titanium and mixtures thereof not converted to cermet, is unchanged and
maintains the physical properties it possessed prior to treatment in
accordance
with the instant invention. This composition gradient structure is
particularly
advantageous when one desires to use welding as an attachment method of the
carbide cermet to a surface. Furthermore, a composition gradient cermet can
have a superior thermal expansion match with the underlying metallic substrate
with superior durability under thermal fluctuations. Thus, the cermet layer
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
-13-
provides erosion resistance while retaining physical properties for the
attachment
and mechanical reliability of the alloy.
[0040] The composition gradient cermets produced by the process of instant
invention can be used in the temperature range of 300°C to 800°C
to protect any
steel or any other alloy surface exposed to severe erosion and abrasion. Some
non-limiting examples of these applications include protective linings, lining
tiles for fluid-solids separation cyclones as in the cyclone of Fluid
Catalytic
Cracking Unit used in refining industry, wear plates, nozzle and grid hole
inserts, turbine blades and components subject to erosion flow streams. In
these
applications composition gradient cermets prepared by the process of the
instant
invention offer a combination of erosion resistance and toughness as well as
an
optimization of thermal stresses within the component. Compared to conven-
tional cermets prepared via powder metallurgy method, it affords attachment
via
conventional welding techniques and a better matching of thermal expansion to
the base steel. It also could provide a superior method of protecting turbine
blades from both oxidation and erosion.
[0041] Another embodiment of the invention is directed to a composition
gradient cermet product prepared by the process comprising:
- heating a metal alloy containing at least one of chromium and titanium at a
temperature in the range of about 600°C to about 1150°C to form
a heated
metal alloy;
- exposing said heated metal alloy to a reactive environment comprising at
least one member selected from the group consisting of reactive carbon,
reactive nitrogen, reactive boron, reactive oxygen and mixtures thereof in the
range of about 600°C to about 1150°C for a time sufficient to
provide a
reacted alloy; and
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
-14-
- cooling said reacted alloy to a temperature below about 40°C.
[0042] The process of the instant invention can be applied to any surface. For
example the internal surface of any chemical or petroleum processing reactor
comprised of a metal selected from the group consisting essentially of
chromium, titanium and mixtures thereof at a temperature can be heated to a
temperature in the range of about 600°C to about 1150°C and then
exposed to a
reactive environment selected from the group consisting essentially of
reactive
carbon, reactive nitrogen, reactive boron, reactive oxygen and mixtures
thereof
in the range of about 600°C to about 1150°C for a time period
sufficient to
provide a reacted internal surface. Upon cooling to temperatures below about
40°C a composition gradient cermet material is formed on the internal
surface of
the reactor. The internal surface of the rector comprising the composition
gradient cermet can exhibit enhanced erosion resistance. One non-limiting
illustrative example of this use is the cyclone separator of a Fluid Catalyst
Cracking Unit in oil refining.
[0043] As another example, the surface of any object, for example the blades
of a turbine, can be made of a metal selected from the group consisting
essentially of chromium, titanium and mixtures thereof at a temperature,
heated
to a temperature in the range of about 600°C to about 1150°C and
then exposed
to a reactive environment selected from the group consisting essentially of
reactive carbon, reactive nitrogen, reactive boron, reactive oxygen and
mixtures
thereof in the range of about 600°C to about 1150°C for a time
period sufficient
to provide a heat treated object. Upon cooling to temperatures below about
40°C
a composition gradient cermet material is formed on the surface of the object
exposed to the reactive environment.
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
-15-
[0044) The cermet compositions prepared by the process of the instant
invention possess enhanced erosion and corrosion properties. The erosion rates
were determined by the Hot Erosion and Attrition Test (HEAT) as described in
the examples section of the disclosure. The erosion rate of the gradient
cermets
prepared by the process of the instant invention is less than 1.0x10-6 cclgram
of
SiC erodant. The corrosion rates were determined by thermogravimetric (TGA)
analyses as described in the examples section of the disclosure. The corrosion
rate of the gradient cermets prepared by the process of the instant invention
is
less than 1x10-lo g2~cm4sec.
[0045] The cermet compositions prepared by the process of the instant inven-
tion possess fracture toughness of greater than about 3 MPa~ml~2, preferably
greater than about 5 MPa~ml~2, and more preferably greater than about 10
MPa~ml~2. Fracture toughness is the ability to resist crack propagation in a
material under monotonic loading conditions. Fracture toughness is defined as
the critical stress intensity factor at which a crack propagates in an
unstable
manner in the material. Loading in three-point bend geometry with the pre-
crack in the tension side of the bend sample is preferably used to measure the
fracture toughness with fracture mechanics theory. The cermets of the instant
invention can be affixed to metal surfaces by mechanical means or by welding.
EXAMPLES
[0046] The following non-limiting examples are included to further illustrate
the invention.
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
-16-
EXAMPLE 1: Reactive Heat Treatment of Commercial Alloys
[0047] The reactive heat treatments were conducted on the selected
chromium containing commercial alloys, 304SS, 310SS, Haynes HR120 and
Inconel 353MA. The nominal compositions are given below.
TABLE 1
Compositions of Chromium Containing Commercial Alloys
Alloys UNS No. Composition (wt%)
304 Stainless 530400 Bal Fe:18.5Cr:9.6Ni:1.4Mn:0.6Si
Steel
310 Stainless 531000 Bal Fe:25.OCr:2I.ONi:l.5Si:2.OMn
Steel
Haynes HR120 N08120 Bal Fe:33.OCr:37.ONi:2.5Mo:2.5W:0.6Si
Inconel 353MA S353I5 Bal Fe:24.8Cr:34.8Ni:1.6Si: l.4Mn
[0048] The samples had rectangular geometry with dimensions of about 1.25
cm x 1.25 cm x 1 cm. The sample surfaces were ground to a 600 grit SiC finish
and cleaned ultrasonically in acetone. The procedure used in the invention was
to establish the kinetics of carburization of the selected alloys in a purely
carburizing environment (CH4-H2), which was determined thermogravimetric-
ally in a Cahn 1000 thermogravimetric unit. The investigations were carried
out
in the temperature range, 800°C to about 1160°C. A coupon was
heated to a
temperature of 1100°C in a hydrogen environment in a vertical quartz
reactor
tube and held at that temperature for approximately 5 minutes. Thereupon, the
environment was changed to 37.3 vol% CH4-62.7 vol% H2. After 3 hours of
exposure, lowering the furnace surrounding the quartz reactor cools the metal
sample. After the sample has attained room temperature, the surface
microstructure was examined by scanning electron microscopy. By "Bal" is
meant balance of metal in the constituent composition.
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
17-
[0049] Figure 5a reveals that a chromium carbide-metal cermet layer of 400
micron thickness has formed on 310 stainless steel (54Fe:21Ni:25Cr in wt%)
surface after reactive heat treatment at 1100°C for 3 hours in 37.3
vol%
CH4:62.7 vol% H2 environment. A magnified view of cermet microstructure,
revealing the Cr-rich carbide [(Cro.6Feo,ø)7C3] and Cr-depleted steel
(63Fe:31Ni:6Cr in wt%) to produce a composite ceramic-metal two-phase
structure, is depicted in Figure 5b. Cr-rich is meant that the metal Cr is of
a
higher proportion on a weight basis than the other constituent metals
comprising
M, where M is S4Fe:21Ni:25Cr in wt%. In this scanning electron micrograph
the Cr-rich carbides appear dark gray and the metal appears recessed, because
it
has etched more deeply than the carbides. These figures show the final product
having the cermet surface, which is produced in accordance with this
invention.
Changing the duration of exposure to the carbon gaseous environment changes
the thickness of the cementite layer. This is shown by the graph in Figure 4.
EXAMPLE 2: Reactive Heat Treatment of Commercial Alloys
[0050] The chromium containing alloys listed above were reactively heat
treated in a tube furnace for 24 hours at 1100°C in 10 vol% CH4:90 vol%
HZ
environment. Samples were heated to a temperature of 1100°C in a
hydrogen
environment and held at that temperature for approximately 5 minutes. After 24
hours of exposure, the alloy samples were cooled down. After the samples
reached room temperature (25°C), the surface microstructure and the
thickness
of cermet layer formed on various alloy surfaces were investigated by cross
sectional scanning electron microscopy. Chemical compositions of M~C3
carbide phase and Cr-depleted binder phase were investigated by semi-
quantitative energy dispersive x-ray spectroscopy. The tendencies of Fe and Ni
to partition between the metal matrix and the carbide precipitates are
expected to
be different. The thickness of cermet layers, Cr and Fe contents in M~C3
carbide
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
_18_
phase and composition of Cr-depleted metal matrix phase within cermet layers
are summarized below.
TABLE 2
The Thickness, Cr and Fe Contents in M7C3 Carbide Phase and
Composition of Cr-depleted Metal Matrix Phase within Cermet Layers after
Reactive Heat Treatment of Selected Chromium Containing: Commercial Alloys
ThicknessCr and Fe ContentsComposition of
Alloys of cermetin M7C3 CarbideCr-depleted metal
layer Phase (wt%) matrix base (wt%)
(mm)
304 Stainless 2.13 27.OCr:73.OFe 76.6Fe:3.6Cr:19.8Ni
Steel
310 Stainless 1.90 52.OCr:48.OFe 63.OFe:5.8Cr:30.2Ni
Steel
Haynes HR120 1.79 58.OCr:42.OFe 36.7Fe:4.4Cr:58.9Ni
Incone1353MA 1.50 58.OCr:42.OFe 37.4Fe:4.1Cr:58.5Ni
EXAMPLE 3: Reactive Heat Treatment of Custom-Made Alloys
[0051] Alloys containing different concentrations of Fe, Ni, Cr and Ti were
prepared by arc melting. The arc-melted alloy buttons were annealed at
1100°C
overnight in inert argon atmosphere and furnace-cooled to room temperature.
Cubical samples of about 1.25 cm x 1.25 cm x 0.75 cm were cut from the
buttons. The sample faces were polished to 600-grit finish and cleaned in
acetone. The specimens were exposed to a 10 vol% CH4:90 vol% H2 gaseous
environment at 1100°C for 24 hours.
[0052] Detailed electron microscopy and chemical analysis of the alloys after
exposure indicated that specific alloy compositions in the Fe-Ni-Cr system
generate cermet structure with M~C3 carbide and metal phase. The thickness of
cermet layers, Cr and Fe contents in M~C3 carbide phase and compositions of
Cr-depleted metal matrix phase within cermet layers are summarized in Table 3.
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
-19-
By contrast to the example of selected commercial alloys, relatively thick
cermet
layer was obtained and the concentration of Cr in metal matrix phase formed in
the Fe-Ni-Cr system was relatively enriched. Higher Cr concentration in metal
phase enhances oxidation resistance at higher temperatures. The optical
microscopic image shown in Figure 6 indicates the size and morphology of
M7C3 (M=Cr and Fe) carbide-metal cermet structure in the surface regions after
reactive heat treatment at 1100°C for 24 hours in 10 vol% CH4:90 vol%
H2.
[0053] An alloy of composition 60Fe:25Cr:10Ni:5Ti (in wt%) generates
cermet structure with mixed TiC and M~C3 carbide and metal phase. The
thickness of cermet layers, Cr and Fe contents in M7C3 carbide phase and
compositions of Cr-depleted metal matrix phase within cermet layers are
summarized in Table 3. The optical microscopic image shown in Figure 7
indicates the size and morphology of mixed TiC and M7C3 (M=Cr and Fe)
carbide-metal cermet structure in the surface regions after reactive heat
treatment
at 1100°C for 24 hours in 10 vol% CH4:90 vol% H2.
TABLE 3
The Thickness, Cr and Fe Contents in M7C3 Carbide Phase
and Composition of Cr-depleted Metal Matrix Phase within
Cermet Layers after Reactive Heat Treatment of Fe-Ni-Cr-Ti s sy tem
Thickness Cr and Fe ContentsComposition of
of cermet in M7C3 CarbideCr-depleted metal
lloys (wt%) layer (mm)Phase (wt%) matrix phase (wt%)
55Fe:35Cr:lONi 3.27 48.OCr:52.OFe 65.3Fe:7.9Cr:26.8Ni
45Fe:45Cr:lONi 3.35 77.1Cr:22.9Fe 67.6Fe:13.8Cr:18.6Ni
35Fe:55Cr:10Ni 1.00 79.OCr:2I.OFe 52.1Fe:7.OCr:40.9Ni
60Fe:25Cr:10Ni:5Ti2.50 66.3Cr:33.7Fe 74.5Fe:9.1Cr:16.4Ni
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
_20_
EXAMPLE 4: Erosion Testing
[0054] The reactive heat treatments were conducted on commercial 310SS to
prepare samples for Hot Erosion and Attrition Test (HEAT). The 310SS
samples had rectangular geometry with dimensions of about 2.0 inch x 2.0 inch
x 0.5 inch. One sample was reactively heat treated in a tube furnace for 138
hours at 1100°C in 10 vol% CH4:90 vol% H2 environment and named as
C310SS1100. The other sample was reactively heat treated in a tube furnace for
96 hours at 1150°C in 10 voI% CH4:90 vol% HZ environment and named as
C310SS1150.
[0055] Erosion Rate was measured as the volume of cermet, refractory, or
comparative material removed per unit mass of erodant particles of a defined
average size and shape entrained in a gas stream, and had units of cc/gram
(e.g.,
< 0.001 cc/1000 gram of SiC). Key defined erosion test conditions are erodant
material and size distribution, velocity, mass flux, angle of impact of the
erodant
as well as erosion test temperature and chemical environment.
[0056] Erosion Loss of Cermet was measured by the Hot Erosion and
Attrition Test (HEAT). The carrier gas and atmosphere, simulating the intended
use, but preferably air, were heated to the same temperature. HEAT tests were
preferably operated as follows. In the preferred operation of the HEAT test,
the
cermet specimen blocks (C310SS1100 and C310SS1150) of about 2 inch square
and about 0.5 inch thickness were weighed to an accuracy of ~0.01 mg. The
center of one side of the book was subjected to 1200g/min of SiC particles
entrained in an air jet exiting from a riser tube with a 0.5 inch diameter
where
the end of the riser tube was 1 inch from the target disk. The 58 ~,m angular
SiC
particles used as the erodant were 220 grit #1 Grade Black Silicon Carbide
(UI~
Abrasives, Inc., Northbrook, IL). The erodant velocity impinging on cermet
targets was 45.7 m/sec (I50 ftJsec) and the impingement angle of the gas-
erodant
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
-21-
stream on the target was 45°~5°, preferably 45°~2°
between the main axis of
the riser tube and the surface of the specimen disk. The carrier gas was air
for
all tests. The erosion tests in the HEAT unit were performed at 732°C
(1350°F)
for 7 hours. After testing the cermet specimen were again weighed to an
accuracy of ~0.01 mg, to determine the weight loss. The erosion rate was equal
to the volume of material removed per unit mass of erodant particles entrained
in
the gas stream, and has units of cc/gram. Improvement in Table 4 is the
reduction of weight loss due to erosion compared to a value of 1.0 for the
standard RESCOBONDTM AA-22S (Resco Products, Inc., Pittsburgh, PA).
AA-22S typically comprises at least 80.0% A1203, 7.2% Si02, 1.0°70
Fe20;, 4.8%
Mg0/CaO, 4.5% P205 in wt%. Micrographs of the eroded surface were electron
microscopically taken to determine damage mechanisms. Table 4 summarizes
the erosion loss of selected cermets as measured by the HEAT
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
- 22 -
~ b
a~ a~
o c~ o
O N 'd:
. .
s
.
O ~O d;
U
~r
W 00 t~
~
O O
V7
V7
U
~ ~~ o
w m
o aa~~
o
~a~ M N
~, , an
m d-
w~ ~ N N
~p
O
M,,
',,
b4 ~O
ue
N ~
''
d'
d
'
N N
;
O Oi
O
O O
m m
U U
CA 02523587 2005-10-25
WO 2004/104245 PCT/US2004/015552
-23-
[0057] The HEAT test measures very aggressive erodant particles. More
typical particles are softer and cause lower erosion rates. For example FCCU
catalysts are based on alumina silicates which are typically softer than
alulninas
which are typically much softer than SiC.
EXAMPLE 5: Corrosion Testing
[0058] Each of the cermets of Examples 4 was subjected to an oxidation test.
The procedure employed was as follows:
1 ) A specimen cermet of about 10 mm square and about 1 mm thick was
polished to 600 grit diamond finish and cleaned in acetone.
2) The specimen was then exposed to 100 cc/min air at 800°C in
thermogravimetric analyzer (TGA).
3) Step (2) was conducted for 65 hrs at 800°C.
4) After 65 hours the specimen was allowed to cool to ambient
temperature.
5) Thickness of oxide scale was determined by cross sectional
microscopy examination of the corrosion surface.
[0059] The thickness of oxide scale was ranging about 0.5 ~,m to about 1.5
~.m. The cermet compositions exhibited a corrosion rate less than about 1x10-1
g2/cm4~s or an average oxide scale of less than 30 ~.m thickness when subject
to
100 cc/min air at 800°C for at least 65 hours. These represent superior
corrosion
resistance.