Note: Descriptions are shown in the official language in which they were submitted.
CA 02523911 2005-10-27
WO 2004/096725 PCT/US2004/012986
I
~I~~d~LE-~f~LLE~ ~~~I~~4~~ i~~l~P~~TU~E-~E1~~4~iIC ~~4w~I~~~ITES
A~I~ f1111ETH~~S ~F IJSE
STATE11~IIEf~T REGARDIf~G FEDERALLY SP~NS~RED RESEARCH ~R DE!/EL~PIViEiVT
[0001] blot Applicable.
Background of the Invention
[0002] This invention is related to the field of carbon nanotubes, and more
particularly, but not
by way of limitation, to composites and products comprising single-walled
carbon nanotubes.
[0003] Carbon nanotubes (also referred to as carbon fibrils) are seamless
tubes of graphite
sheets with full fullerene caps which were first discovered as multi-layer
concentric tubes or
multi-walled carbon nanotubes and subsequently as single-walled carbon
nanotubes in the
presence of transition metal catalysts. Carbon nanotubes have shown promising
applications
including nanoscaie electronic devices, high strength materials, electron
field emission, tips for
scanning probe microscopy, and gas storage.
[0004] Generally, single-walled carbon nanotubes are preferred over multi-
walled carbon
nanotubes for use in these applications because they have fewer defects and
are therefore
stronger and more conductive than multi-walled carbon nanotubes of similar
diameter. Defects
are less likely to occur in single-walled carbon nanotubes than in multi-
walled carbon nanotubes
because multi-walled carbon nanotubes can survive occasional defects by
forming bridges
between unsaturated carbon valances, while single-walled carbon nanotubes have
no
neighboring walls to compensate for defects.
[0005] Single-walled carbon nanotubes exhibit exceptional chemical and
physical properties
that have opened a vast number'of potential applications.
[0006] However, the availability of these new single-walled carbon nanotubes
in quantities and
forms necessary for practical technology is still problematic. Large scale
processes for the
production of high quality single-walled carbon nanotubes are still needed,
and suitable forms
of the single-walled carbon nanotubes for application to various technologies
are still needed.
It is to satisfying these needs that the present invention is directed.
CA 02523911 2005-10-27
WO 2004/096725 PCT/US2004/012986
2
Description of the Drawings
[0007] Figure 1 shows a schematic drawing of several reactors which can be
used to produce
the products of the present invention.
[~~~~] Figure ~ is a graph showing the concentration of CO~ downstream of
reactor S~ as a
function of reacfiion time fortwo Co:Mo(1:3)/silica catalysts (2% metal
loading) with two different
silica compositions (silica gel-60 and Hi-Sil~-210). Reaction run at
850°C with a space velocity
of 67,000 h-'.
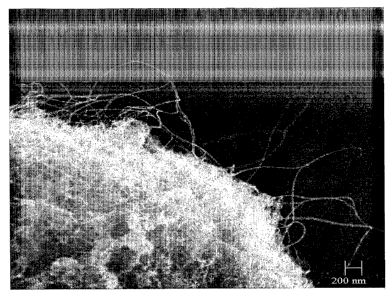
[0009] Figure 3 is a scanning electron micrograph of a nanotube-ceramic
composite product
showing bundles of SWNTs which remain intercalated among silica particles.
[0010] Figure 4. is an I vs. V graph showing curves for composites prepared at
different
temperatures and H2 concentrations.
[0011] Figure 5 is a TEM image of prepared MCM-41 support material.
(0012] Figure 6 is an XRD spectrum of MCM-41 prepared as in Figure 5.
[0013] Figure 7 is an I vs. V graph showing curves for nanotube-ceramic
composites prepared
using various silica supports.
[0014] Figure 8 is an I vs. V graph showing curves for Aerosil 380 nanotube-
ceramic
composites synthesized at 750°C and 850°C.
[0015] Figure 9 is an I vs. V graph showing curves for two nanotube-ceramic
composites with
different metal loadings (2% and 6%).
[0016] Figure 10 is an I vs. V graph showing curves for composites and SWNTs
after different
purification treatments.
Description of the Invention
[0017] The present invention contemplates composites of single-walled carbon
nanotubes
(SWNTs) and a ceramic support (e.g., silica) comprising a small amount of
catalytic metal, e.g.,
cobalt and molybdenum. The particle comprising the metal and ceramic support
is used as the
catalyst for the production of the single-walled carbon nanotubes. The
nanotube-ceramic
composite thus produced can be used "as prepared" without further purification
providing
significant cost advantages. The nanotube-ceramic composite has also been
shown to have
improved properties versus those of purified carbon nanotubes in certain
application such as
field emission.
[0018] Furthermore, with adjustment of the structure of the ceramic component,
e.g., by using
a silica support without microporosity, such as precipitated and fumed
silicas, an important
increase in the quality of the SWNTs produced can result. Other nanotube-
ceramic composites
may be produced based on support materials comprising AI203, La-stabilized
aluminas, MgO
CA 02523911 2005-10-27
WO 2004/096725 PCT/US2004/012986
3
and Zr02, for example, which are suitable for a large variety of applications.
When incorporated
in polymeric matrices, these nanotube-ceramic composites may impart improved
properties to
the polymer. These properties include thermal conductivity, thermal stability
(tolerance to
degradation), electrical conductivity, modification of crystallization
leinetics, strength, elasticity
modulus, fracture toughness, and other mechanical properties. These, and other
characteristics and properties of the present invention are described in
further detail below.
[0019] The catalysts which provide the ceramic c~mponent of the nanotube-
ceramic composite
of the present invention are prepared in one embodiment by impregnating the
support
component (e.g., silica) with different metal solutions of specific
concentrations. For example,
the Co:Mo/Si02 catalysts are prepared by impregnating various silica supports
with aqueous
solutions of cobalt nitrate and ammonium heptamolybdate to obtain the
bimetallic catalysts of
the chosen compositions (see U.S. Patent 6,333,016, the entirety of which is
hereby expressly
incorporated by reference herein). The liquid/solid ratio is kept at incipient-
wetness conditions,
which is different for each support. The total metal loading is preferably
from 0.1 %-20% by
weight. After impregnation, the catalysts are prefrably first dried in air at
room temperature,
then in an oven at 120°C, and finally calcined in flowing air at
500°C.
[0020] SWNTs can be produced on these catalysts in different reactors known in
the art such
as fixed bed reactors, moving bed reactors or fluidized bed reactors. The
fluidized bed reactor
can be operated in both batch mode and continuous mode for example.
[0021] The present work has used four lab-scale reactors to study and optimize
the reaction
conditions for the Co:Mo/Si02 series (Figure 1). The first reactor (A)
consisted of a horizontal
quartz tube of 1 inch in diameter, in which a ceramic boat with 0.5 g of
calcined catalyst was
placed. This is a typical reactor configuration commonly found in the
literature about carbon
nanotube synthesis. The second and third reactors (B1 and B2) were typical
quartz fixed-bed
reactors of 1/8 and 1/4 inch in diameter, respectively. Reactor B1 is loaded
with 0.05 g of
catalyst and it is considered a differential reactor when it is operated with
a space velocity of
400,000 h-'. Reactor B2 contained 0.5 g of the catalyst and was run with a
space velocity of
67,000 h-'. Finally, the fourth reactor (C) is a fluidized bed reactor.
[0022] In all cases, the catalyst is pre-reduced (e.g., by exposure to H2 at
500°C) before the
catalyst is exposed to reaction conditions. Prior to exposure to a carbon
containing gas (e.g.,
CO), the catalysfi is heated in He up to the reaction temperature
(700°C- 1050°C).
Subsequently, a carbon-containing gas or gasified liquid is introduced. After
a given reaction
period that ranged from 1 to 600 min, the reactor was purged with He and
cooled down to room
temperature.
[0023] For a continuous or semi-continuous system, the pretreatment of the
catalyst may be
CA 02523911 2005-10-27
WO 2004/096725 PCT/US2004/012986
4
done in a separate reactor, for example, for pretreatment of much larger
amounts of catalyst
whereby the catalyst can be stored for later use in the SWNT production unit.
With this new
i~nethodology, a flaidized bed reactor can be kept operating continuously at
the reaction
temperature, thus eliminating the preliminary heating and cooling steps from
the reaction
process.
[0024] By varying the reaction conditions, the catalyst selectively produces
SWNTs by the
disproportionation of CO (decomposition into C and CO~) in a preferred
temperature range of
700-950°C (see U.S. Serial No. 10/115,534., which is hereby expressly
incorporated by
reference herein in its entirety). A synergism between Co and Mo is critical
for the performance
of this catalyst [4]. Separately, these metals are not effective; they are
either inactive (Mo
alone) or unselective (Co alone). The catalyst is only effective when both
metals are
simultaneously present on the silica support with an intimate Co-Mo
interaction. The basis for
selectivity of the catalyst has been studied.
[0025] Without wishing to be constrained by theory, it is believed that the
selectivity towards
SWNT production strongly depends on the stabilization of Coa+ species by Mo
oxide species
as explained below. We found that the extent of the Co-Mo interaction is a
function of the
Co:Mo.ratio in the catalyst and has different forms during the different
stages of the catalyst life
j4]. In the calcined state, Mo is in the form of a well-dispersed Mos+ oxide.
The state of Co
strongly depends on the Co:Mo ratio. At low Co:Mo ratios, it interacts with Mo
in a superficial
Co molybdate-like structure. At high Co:Mo ratios, it forms a non-interacting
Co304 phase.
During the subsequent reduction treatment in hydrogen, the non-interacting Co
phase is
reduced to metallic Co, while the Co molybdate-like species remain as well-
dispersed Co2+ ions.
This Co-Mo interaction inhibits the Co sintering that typically occurs at the
high temperatures
required for the formation of carbon nanotubes. When large Co particles are
present less
desirable forms of carbon (mostly graphitic nanofibers) are produced. By
contrast, when the
Co clusters are so small that they are only composed by a few atoms, only
SWNTs are
produced [2, 4]. When metal atoms begin to agglomerate in the presence of
gaseous CO,
there is a nucleation period over which there is no growth of nanotubes. This
nucleation
involves the disruption of Co atoms from its interaction with Mo oxide when
the latter becomes
carbidic. This disruption is followed by surface migration leading to
agglomeration into mobile
clusters that continue to grow under the bombardment of CO molecules. Some of
these
molecules decompose and begin to rearrange (nucleate) until a favorable
configuration
(embryo) is reached, which triggers the formation of the nanotube. When this
embryo is
formed, the subsequent incorporation of carbon and SWNT formation would
proceed at a fast
rate, perhaps only controlled by mass transfer. As a result, one may conclude
that the growth
CA 02523911 2005-10-27
WO 2004/096725 PCT/US2004/012986
S
of each tube is limited by nucleation, and after nucleation is completed, it
is controlled by mass
transfer. For this reason, we have observed that the deposition of carbon on a
solid catalyst
continues for hours, although the growth of a single tube only takes
milliseconds. The diameter
of the tube is determined by the size of the embryo, therefore, control of
nanotube diameter is
possible by control of the size of the metal cluster under reaction
conditions.
[0026] Impr~~ement ~f ~WfVT ~electivit~ b~ using n~n-micr~p~r~~a~ silica as
supp~rt
material
[0027] In systematic studies of SWNT growth under different reaction
conditions, it has been
demonstrated that mass transfer limitations are important in determining
quality and yield of
SWNTs. External mass transfer limitations can be minimized by adjusting the
reaction
conditions and modifying the reactor configuration. On the other hand, to
minimize internal
diffusion problems, the pore structure and particle size parameters of the
catalyst particle can
be adjusted. In general, small particles with larger pore sizes, orsmall non-
porous particles can
be used to reduce internal mass transfer limitations. However, the size of
particles cannot be
made much smaller without modifying the reactor design. Due to the high space
velocity
needed to keep the CO conversion low and the high surface velocity needed to
minimize the
external mass transfer limitation, excessively reducing the particle size of
the catalyst would
excessively increase the pressure drop in a fixed-bed reaction system. For
this reason, a
fluidized-bed reaction system is a preferred alternative. In such a reactor
much finer particles
can be used than in a fixed bed reactor. In some cases, particles as fine as
powder can be
used. In those cases, agglomeration and sticking to the walls and between the
particles can
be avoided by well-established techniques such as stirring and vibration,
which break
interpaticle bonds and improve fluidizability. Preferably the particle size of
the powders to be
used fall under the type A category of the Geldart classification.
[0028] Another method that could be used to minimize diffusional limitations
that may occur
during the growth of carbon nanotubes is the in-situ fragmenfiation of
catalyst particles that
expose a higher surface area to the gas phase as the reaction proceeds. This
is a typical
method used in polymerization processes to improve and modify the reaction
kinetics [25]. The
in-situ fragmentation of the catalyst is obtained using a special support
which might or might
not need the use of special binders. This type of catalysts could be used in
two ways. For
example, as the nanotubes grow, the particles break exposing new surface and
therefore
increasing the total carbon yield obtained with such catalyst. Alternatively,
a binder used in the
support is disintegrated under the reaction conditions and a finer powder is
generated in the
reactor. Again the use of a finer powder may increase the final carbon yield.
CA 02523911 2005-10-27
WO 2004/096725 PCT/US2004/012986
6
[0029] We have observed that the microporosity of he silica support was
responsible in part
for the production of undesired forms of carbon in the resulting catalytic
product. Mass transfer
limitations inside these microspores together with a physical impediment for
the growth of
SWi~Ts inside pores, may be responsible forthe reduction in nanotube quality.
This hypothesis
was verified by studying the influence of the maximum temperature reached
during the catalyst
preheating step. Two reactions were run at the same temperature (750°C)
for 2 hours using
a Co:Mo(1:3)lsilica-gel 60 (2°/~ metal loading) catalyst. In one case,
the usual procedure was
used and the catalyst was preheated to 750°C in He. In the second case,
the catalyst was first
preheated to 950°C (thereby decreasing microporosity) and then cooled
down to 750°C. The
latest pretreatment resulted in a much better product with a quality parameter
c of 0.83 while
for the first case c was only 0.62 (the quality parameter c increases as the
quantity of
amorphous carbon in the product decreases). However, no differences were
observed in the
diameter distribution of the SWNT produced and the carbon yield.
[0030] The structure of the silica is compromised at a temperature as high as
950°C and
therefore the micropores of the support tend to collapse. The average pre-
treatment pore
diameter of the silica-gel 60 is 6 nm. Single-walled nanotubes are not able to
grow in pores that
are much smaller than that and therefore those pores would lead to the
formation of amorphous
carbon. When the smallest pores collapse due to the preheating at
950°C, the production of
amorphous carbon decreases and the quality of the material increases.
[0031] In order to verify that hypothesis and improve the perFormance of the
catalyst, a different
silica support with a different pore structure was studied. The new Si02 used
was a precipitated
silica "Hi-Sil~-210" (commercially available from PPG) which lacks
microporosity.
[0032) A catalyst comprising Co:Mo(1:3) (2% metal loading) was prepared with
the Hi-Sil~-210
silica and three experiments were conducted running the Boudouard reaction for
2 hours at
750°C , 850°C and 950°C using the same procedure as
previously described. A fourth reaction
was run also at 750°C but used catalyst which had been pretreated with
heating at 950°C. The
results obtained for the quality parameter c and the carbon yield are
summarized in Table 1 and
were somewhat different than the results obtained for the silica-gel 60. No
significant increase
was observed in either c orfihe carbon yield when the pre-heating and reaction
temperature was
750°C or 850°C, while when the preheating and reaction
temperatures were 950°C there was
an abrupt decrease of both parameters (to .80 and 2.0°/~,
respectively). The second
remarkable observation is that the quality of the SWNTs produced at
750°C and 850°C (c =
0.97) was much higherfihan that one obtained using silica gel-60 even under
the best operating
conditions (c = 0.83) (see previous discussion re: silica gel-60).
CA 02523911 2005-10-27
WO 2004/096725 PCT/US2004/012986
7
Preheating Reaction Yield
Catalyst Temperature (°C) Temperature (°C) 1-DlG (Wt%)
Co:M~(1:3)/SiO~ - Hi-Sil° (2% metal loading) 750 750
0.97
9.3%
Co:Mo(1:3)/SiO~ - Hi-Sil° (2% metal loading) 850 850 0.97
10.0%
Co:Mo(1:3)/SiO~ - Hi-Sil~ (2% metal loading) 950 950 0.80
2.0%
Co:Mo(1:3)/SiO~ - Hi-Sil~ (2% metal loading) 950 750 0.97
11.4%
Table 1 - Yield and quality of SWNT obtained using a Co:Mo(1:3(/SiO2 - Hi-Sil~
catalyst in
Reactor B2. Reaction run for 2 hours at 5.8 atm.
[0033] The results of the preheating treatmenfi were also important. The great
increase in c
reported before using the silica gel-60 as the catalyst support when the
catalyst was preheated
to 950°C was not observed when the silica with low microporosity (Hi-
Sil~ silica) was used.
[0034] These results indicate that the microporosity of the silica gel-60 was
responsible at least
in part for the formation of amorphous carbon that lowered the selectivity
towards SWNTs (i.e.,
decreased c). The increase in the quality parameter c when the reaction
temperature increased
was related to the collapse of micropores due to the higher temperatures. A
similar quality
improvement observed when the catalyst was preheated to 950°C and the
disappearance of
this temperature effect when Hi-Sil~-210 silica (with low microporosity) is
used, strongly
supports this hypothesis.
[0035] Interestingly, another difference observed with the Hi-Sil~-210 silica
was that the carbon
yield obtained at a reaction temperature of 950°C was very low Qust 2
wt%). Moreover, the
quality (i.e., selectivity) (c = 0.8) was also much lower than the c obtained
at 750°C and 850°C.
These observations indicate a higher rate of deactivation of the catalyst due
to sintering. The
lower surface area of this support probably makes the catalyst more exposed to
the sintering
effect.
[0036] It is important to note that when the reaction was run at 750°C
and 850°C for 2 hours,
the carbon yield was slightly higher than when silica gel-60 was used.
However, similar yields
had been obtained with Hi-Sil~-210 for longer reaction times, showing that
indeed the overall
reaction rates are different in both cases. Moreover, when the CO~ produced
was f~Ilowed by
online mass spectroscopy (see Figure 2), it was observed that the reaction
rate using Hi-Sil~-
210 was at least twice as fast during the first 30 minutes of reaction than
when silica gel-60 was
used. Afterwards, the production of C02 slows down sharply and becomes lower
than for the
CA 02523911 2005-10-27
WO 2004/096725 PCT/US2004/012986
case with the silica gel-60 catalyst. This observation indicates that the
primary period of the
production of SWNTs is during the first 30 minutes of reaction.
[0037] These observations provide strong evidence that the internal diffusion
is limiting the
overall reaction rate for the production of SW~~Ts. Since, as mentioned
before, the growth of
the SWNTs themselves occurs in milliseconds, the nucleation step of the
nanotubes is the one
that is being limited by the internal diffusion. Among the different phenomena
That the
nucleation step involves, the one that may be more likely affected is the
release of the cobalt
clusters.
[0038] Katura plots and Raman spectra were used to study the relationship
between diamefier
distribution and reaction temperature for production of single-walled carbon
nanotubes. Raman
spectra were obtained using 633 nm and 514 nm lasers. Reactions ran for two
hours in reactor
B2 using the Co:Mo (1:3)/Hi-Sil~ silica with 2% metal loading. The reaction
was run at 5.8 atm
and at 750°C, 850°C, and 950°C When silica gel-60 is used
as a support, as the reaction
temperature is increased, the SWNTs produced have larger diameters and the
diameter
distribution becomes broader. For instance, the average diameter for SWNTs
produced at a
reaction temperature of 750°C is about 0.9 nm, while SWNTs produced at
reaction
temperatures of 850°C and 950°C have diameters of about 1.1 nm
and about 1.4 nm,
respectively.
[0039] Finally, it was observed that similar results are obtained when other
non-porous silicas
(e.g., fumed silicas Aerosil~ 380 and Aerosil~ 90 (commercially available from
Degussa Corp.)
and Cab-o-sil~ (commercially available from Cabot Corp.)) are used as the
catalyst support.
[0040] The nanotube-ceramic composites described herein may be formed from
support
materials comprising fumed silica nanoparticles (e.g.,10-20 nm in diameter),
precipitated silica,
silicas including silica gel, alumina (AI203), La-stabilized aluminas, Mg0
(magnesium oxide),
mesoporous silica materials including SBA-15 and Mobil Crystalline Materials
(including MCM-
41 ), zeolites (including Y, beta, KL and mordenite), and Zr02 (zirconium
dioxide). The catalysts
in one embodiment, comprise cobalt and molybdenum (or other catalytic metals)
and make up,
preferably up to 20% wt of the ceramic catalyst particle. The ceramic catalyst
may further
comprise chromium, for example, or other metals including Fe, Ni, or W, or
others as listed in
U.S. Patent No. 6,333,016, or 6,413,487 or in U.S. Serial No. 60/529,665, each
of which is
hereby expressly incorporated herein in its entirety. Each nanotube-ceramic
composite
preferably comprises up to 50% carbon by weight, for example, 1 to 10% of the
total weight of
the composite. Preferably at least 50°/~ of the SWNTs have outer
diameters of 0.7 nm to 1.0
nm, more preferably at least 70%, and still more preferably at least 90%. In
another
embodiment at least 50% of the SWNTs have outer diameters of 1.0 nm to 1.2 nm,
more
CA 02523911 2005-10-27
WO 2004/096725 PCT/US2004/012986
9
preferably at least 70%, and most preferably at least 90%. In yet another
embodiment, at least
50% of the SWNTs have outer diameters of 1.2 nm~to 1.5 nm, more preferably at
least 70%,
and most preferably at leasfi 90°/~.
[~~a.~] The support materials upon which the catalytic metals are disposed to
form the metallic
catalytic particles are not carbon nanotubes. The carbon nanotubes are
produced only after
the metallic catalyfiic particles are exposed to reaction conditions.
[0042] lJt. ility
[~04.~~ The carbon nanotube-catalyst support compositions produced herein can
be used, for
example as, electron field emitters, fillers of polymers to modify mechanical
and electrical
properties of the polymers, fillers of coatings to modify mechanical and
electrical properties of
the coatings, fillers for ceramic materials, and/or components of fuel-cell
electrodes. These of
course are merely examples of how the compositions of the invention can be
used and use is
not limited to them. The present used are described in further detail below.
[0044 Uses in field-emission displays
[0045] Single-walled carbon nanotubes have attracted considerable attention as
field emitter
materials due to their superioremission characteristics, high chemical
stability, and outstanding
mechanical strength. Even though a great deal of effort is being done around
the world to bring
nanotube applications to fruition, only a few have shown real potential. Among
them, field-
emission displays (FEDs) will be one of the first commercial applications.
FEDs are
characterized by superior display performances such as fast response time,
wide viewing
angles, wide operation temperatures, cathode ray tube (CRT) like colors, ultra-
slim features,
low-cost and low-power consumption. FED technology is one of the most
promising
approaches for direct view displays larger than 60" diagonal [5]. At present,
there are no well-
developed technologies for growing in-situ vertically aligned nanotubes over a
large area of
glass substrates at low temperatures. An alternative technology is the use of
nanotubes
produced separately and later deposited on the cathode by techniques such as
the screen-
printing method. The deposition of a mixture of nanotubes and dielectric
nanoparticles (DNPs)
leads to much improved emission characteristics [e.g., see Ref. 6 and U.S.
Patents 6,664,722
and 6,479,939]. This development makes a perfect combination with the high
quality nanotube-
ceramic composites described herein. The nanotube-ceramic composites are
particularly
suitable for this application since the Si~z is in the form of (dielectric)
nanoparticles and have
shown excellent results in this regard (see Example I).
[0046 The nanotube-ceramic composites produced herein are shown in one
embodiment in
Figure 3. The nanosized particles of the silica support are physically spacing
the bundles of
CA 02523911 2005-10-27
WO 2004/096725 PCT/US2004/012986
nanotubes apart, which might be beneficial for field emission applications.
The nanotube-
ceramic composites of the present invention have at least two advantages over
a purely
physical mixture of purified nanotubes and Si~~.
(00~.~~ In particular, the efficiency with which the silica particles space
the nanotube bundles
apart is much higher, and the cost of the presently described composites is
orders of magnitude
lower than purified single-walled carbon nanotubes.
(0043 Uses as filler to modify mechanical and electrical properties of
polymers
(0049] Thermoplastic and thermosetting materials have been filled with
particulate
reinforcements such as SiO~ to improve mechanical, thermal and chemical
properties. When
this reinforcement material is in the nanoscale size, the enhancement of such
properties is
noticeable higher. For this reason, fumed silica, which is available with 10-
20 nm particle size,
is commonly used as a reinforcement of PVC, silicones, acrylics [7 -11 ] and
vulcanized rubbers
[12]. It is also used as component material for dental filling [13],
electronic packaging [14], and
thickeners of paints and coatings [15].
(0050] Single wall carbon nanotubes show unmatched electrical and mechanical
properties,
which make them good candidates to be incorporated into polymer matrices in
order to obtain
high-strength, conductive polymers. However, to~ capitalize on the properties
of carbon
nanotubes, a good dispersion of the nanotubes in the polymer matrix is needed.
Ideally, this
dispersion should contain individual nanotubes embedded in fihe polymer
matrix. However,
although many scientists are working in this area, no technique developed
until this moment
has been entirely successful in achieving this order of dispersion.
(0051] Use of the nanotube-ceramic compositions described herein as polymer
filler provides
the advantages of both nano-sized silica and SWNTs. In addition, the
dispersion techniques
that have been developed for the incorporation of SiOz into different polymer
matrices can still
be applied to the nanotube-ceramic composition, therefore increasing, at the
same time, the
dispersion of the SWNTs. This dispersion can be carried out either in the
molten state of the
polymer or in solutions of the dissolved polymer in solvents of varying
reactivity. Reactive
solvents can be low-molecularweight thermo-setting resins which blend with the
matrix polymer
and may improve the processing conditions (e.g., blend viscosity and
processing temperature).
ii~loreover, the surface chemistry of the Si~~ can be easily changed for its
incorporation in a
specific polymer matrix by generating grafting sites, which can be used as
anchoring sites for
enhancement of polymer-filler adhesion and/or sites for starting in-situ
polymerization.
(0052] Uses as catalysts for in-situ polymerization
(0053] A novel technique that we have invented for use in maximizing the
dispersion of SWNT
CA 02523911 2005-10-27
WO 2004/096725 PCT/US2004/012986
11
in polymer matrices is "in-situ-polymerization" (see U.S. Serial No.
10/464,041, the entirety of
which is hereby expressly incorporated by reference herein in its entirety).
We have shown that
the properties of the SWNT-polymer composites obtained by this technique are
much better
than those obtained for merely a physical mi3zture of the same polymer and the
nanotubes [18,
17]. A method that we used to incorporate and disperse SWNT in polymers was a
technique
called mini-emulsion polymerization, a well-established method for producing
polymer particles
with very narrow size distributions. This process has the advantage of
requiring substantially
less surfactant to stabilize the reacting hydrophobic droplets inside the
aqueous medium than
in conventional emulsion polymerization. It also eliminates the complicated
kinetics of monomer
transfer into micelles that takes place in the conventional emulsion
polymerization. SWNT-filled
polystyrene (SWNT-PS) and styrene-isoprene composites prepared by this method
showed
distinctive physical features such as: uniform black coloration; high
solubility in toluene as well
as in tetrahydrofuran (THF); and semiconductor to ohmic electrical behavior.
[0054 In-situ-polymerization techniques can also be used to obtain good
dispersions of the
presently claimed nanotube-ceramic composites in different matrices. Moreover,
these
nanotube-ceramic composites can be selectively tailored for in-situ-
polymerization of specific
polymers by adding an active agent to either the composite or the bare
catalyst before the
nanotubes are produced. As an example we have developed a SWNT/SiO~ composite
which
has been doped with chromium to make it effective in in-situ-polymerization of
ethylene.
Polyethylene produced using Phillips Cr/SiO2 catalysts represents 20% of the
worldwide
production of polyethyfenes [18]. Since this catalyst needs to be activated
under CO at high
temperatures to be effective for polymerization[19], the present nanotube-
ceramic composites
doped with chromium can be already active for ethylene polymerization after
the growth of the
nanotubes by CO disproportionation. In fact, during the growth of SWNT, the
catalyst is treated
under pure CO at high temperatures. The chromium-doped nanotube-ceramic
composite
comprises an effective polymerization catalyst.
[0055] Uses as filler for ceramic materials
[0056] Ceramics are traditionally hard but easy to break materials. Carbon
nanotubes added
to a ceramic material can greatly enhance its resistance to fracturing as well
as increasing
thermal and electrical conductivity of the ceramic. These new materials could
eventually
replace conventional ceramics or even metals in countless products. For
example, scientists
have mixed alumina powder wifih single-wall carbon nanotubes and then forced
the particles
together with a combination of heat, pressure, and pulses of electric current.
Called spark-
plasma sintering, the method operates at lower temperatures than the
conventional sintering
CA 02523911 2005-10-27
WO 2004/096725 PCT/US2004/012986
12
technique used in previous attempts to make nanotube-reinforced composites.
When the
researchers made a ceramic with nanotubes as 5.7% of its material, the
product's fracture
toughness increased to more than twice fihat of a pure-alumina ceramic. With
carbon
nanotubes afi 10°4° of the volume, the ceramic's toughness
nearly tripled.
[0057] Due to the high-price of single-wall carbon nanotubes, it has been
thought that the
earliest uses of ceramics made with these materials would probably be
applications in which
cost is a secondary concern, such as in space vehicles and medical devices.
However, the
nanotube-ceramic composites described herein may be easily used to reinforce
these ceramics
and further, because of their low cost, may make possible their use in wider
range of
applications.
[0058] Uses in fuel cell electrodes
[0059] The current drive to reduce the use of fossil fuels due to their
environmental and
geopolitical impact have given fuel cells an extraordinary push as alluring
alternatives to
combustion engines. The basic parts of a fuel cell are an ion conducting
electrolyte, a cathode,
and an anode. A fuel such as hydrogen (or methanol) is brought into the anode
compartment
where it releases electrons and forms protons, which diffuse to the cathode
compartment,
where they react with oxygen and consume the electrons. The electrolyte acts
as a barrier for
gas diffusion, but allows ion transport.
[0060] Among different types of fuel cells, polymer electrolyte membrane (PEM)
fuel cells are
generally preferred for most portable systems. They operate by transporting
hydronium ions
through hydrated regions of a sulfonated polymer. Due to the high conductivity
of the
membranes they can operate at low temperatures (< 100°C). Moreover,
recent progress has
allowed the use of proton-conducting membranes such as Nafion (an ionomer) +
silica + PW
(a heteropolyacid based on phosphorus tungsten), which can operate 'water-
free' and at low
temperatures. In parallel with the development of electrolyte membranes, great
attention is
being paid worldwide to the development of improved electrodes to enhance
reaction kinetics,
decrease Pt loadings, and increase the tolerance to CO poisoning.
[0061] The CO poisoning of the anode is a serious problem in PEM fuel cells.
Some promising
results have been obtained by alloying Pt with Ru, Mo, Sn, or W~x. Several
substrates have
been investigated to maximise the dispersion of Pt (an electrocatalyst) and
the effectiveness
of the electrodes. For example, Bessel et al. [20] have investigated graphite
nanofibers as
support for platinum particles fuel cell electrodes. They compared various
types of graphite
nanofibers with Vulcan carbon (XC-72). Catalysts consisting of 5 wt.% platinum
supported on
graphite nanofibers were found to exhibit activities comparable to that
displayed by about 25
CA 02523911 2005-10-27
WO 2004/096725 PCT/US2004/012986
13
wt.% platinum on Vulcan carbon. Furthermore, the graphite nanofiber supported
metal particles
were observed to be significantly less susceptible to CO poisoning than the
traditional catalysts.
This impr~vement in performance was ascribed to specific crystallographic
orienfiations that Pt
would adopt when dispersed on the graphite nanofibers. Similarly, Ra]esha et
al. [21] have
found that a combination of Pt and W supported on multi-walled carbon
nanotubes results in
much more efficienfi elecfirodes for mefihanol fuel cells than fihose
supported on Vulcan carbon,
which was attributed to a much higher dispersion of the Pt metal.
~0062~ All fihese resulfis indicate that our single-walled carbon nanotubes of
the nanotube-
ceramic composite described herein (or the SWNT alone), with a much higher
surFace area,
and more perfect structure than multi-walled carbon nanofiubes, or graphite
nanofibers should
be even more efficient. Also, the higher electrical conductivity of SWNT
compared to other
forms of carbon will be a favorable characteristic in the final electrode.
[0063 Uses in solar cells
[0064] Researchers from Cambridge University's engineering department [22]
have developed
photovoltaic devices that, when doped with single-wall carbon nanotubes,
perform better than
undoped photovoltaic devices. The nanotube diodes were made by depositing
organic films
containing SWNTs on to glass substrates coated with indium-tin oxide (ITO).
Aluminium
electrodes were then thermally evaporated under a vacuum to form a sandwich
configuration.
The interaction of the carbon nanotubes with the polymer poly(3-
octylfihiophene) (P30T) allows
excitons generated by light in the polymer to dissociate into their separate
charges and travel
more easily.
(0065] The operating principle of this device is that the interaction of the
carbon nanofiubes with
the polymer allows charge separation of the photogenerated excitons in the
polymer and
efficient electron transport to the electrode through the nanotubes. The
electrons travel through
the nanotube length and then hop or tunnel to the next nanotube. This results
in an increase
in the electron mobility and balances the charge carrier transport to the
electrodes. In addition,
the researchers found that the composite's conductivity is increased by a
factor of 10, indicating
percolation paths within the material. This doping of P30T polymer diodes with
SWNTs also
improves the device's photovoltaic performance, increasing the photocurrent by
more than two
orders ~f magnifiude and doubling the open-circuit voltage.
[0066] The presently-described nanotube-ceramic composifies can be very useful
for this
application since a more controlled film preparation and polymer doping is
required for further
improvements in the performance of these devices. In particular, the presently-
described
nanotube-ceramic composites can help in achieving the required dispersion of
SWNT in the
CA 02523911 2005-10-27
WO 2004/096725 PCT/US2004/012986
14
polymer matrix used in this type of device.
(0067] Further, the cost advantage of the present compositions make their use
in solar cells
economically favorable.
(008] Example
(0069] Workwas conducted to determine the nanotube diameterdistribution and
quantity which
optimized the performance of the nanotube-ceramic composites in field emission
devices.
[0070] SWNTs obtained at higher temperatures shows broader diameter
distribution centered
at large diameters but bundles of smaller size [2].
(0071] A similar increase in diameter is observed when Hz is added in small
concentrations to
the carbon source fed to the reactor. However, if the concentration of HZ is
too high, carbon
nanofibers start to form and the process loses selectivity toward SWNT. For
example, with
pure CO, SWNTs of small diameter (0.8 nm OD) are produced; with 3% HZ in CO
the diameter
increases (1.3 nm OD); with 10% H2 in CO mostly multi-walled nanotubes (19 nm
OD) are
produced.
(0072] In parallel, the field emission characteristics of this series of
samples were studied to
determine the effect of SWNT diameter distribution and quality of the SWNT
material. The I
vs. V curves for the corresponding nanotube-ceramic composites of the three
samples are
shown in Figure 4. For the best performance of a field emission device, it is
obvious that higher
current densities at lower electric field are desired. With this concept in
mind, it is clear that the
composite with the best performance was that obtained at 850°C and 3%
HZ. The sample
produced at 850°C with no H2 followed this one in performance, followed
by the sample
produced at 750°C.
(0073] In all cases, the samples showed good stability, meaning little
deterioration in the
sample after reaching a current density of almost 5 mA/cmz. This is observed
by the low
hysteresis of the I vs. V curves
(0074] The influence of the dielectric structure on the field emission
emission characteristics
of the nanotube-ceramic composites was also studied. For this purpose a series
of different
composites was prepared using different silica supports for the catalyst
particle. The silicas
include a silica gel 60 with an average pore diameter of 60 A, a Hi-Sil~-210
silica with no
microporosity and a surface area of 250 m2/g, and two different aerosols
(Aerosil~ 90 and
Aerosil~ 380) with specific surface areas of 90 and 380 ma/g and an average
particle size of 20
and 7 nm, respectively. A series of MCM-41 were also specially synthesized to
try to improve
the field emission. Due to the highly ordered pore structure but the lower
selectivity towards
SWNT during the reaction process that this material showed, the composites
provided a poorer
field emission performance.
CA 02523911 2005-10-27
WO 2004/096725 PCT/US2004/012986
[0075] The MCM-41 silicas were prepared by mixing 100 g of CTAOH with 50 g of
tetramethylammonium silicate and stirred for 30 minutes. Then 12.5 g of Hi-
Sil~-x was added
to the solution, stirred for five minutes, and poured into an autoclave. The
autoclave was
placed in oven at 150°C for 4~8 hours. Upon removal, the autoclave was
allowed to cool to room
temperature. The solid was vacuum filtered with a Biachner funnel, washed with
nanopure
water, and dried under ambient condifiions. The predried solid was calcined in
air by heating
from room temperature to 540°C over a twenty-hour period then soaked
for two hours. The
calcined samples were designated as MCM-41-210, MCM-41-233, and MCM-41-915
indicating
the different Hi-Sil~ silicas used to start with. Figure 5 shows a TEM picture
of the synthesized
MCMs. The picture shows regular hexagonal array of uniform channels, which is
typical for
MCM-41. The average pore diameter in all the samples is about 40 A.
[0076] We also characterized the MCM samples using X-ray Diffraction Spectra
(XRD). The
XRD patterns (Figure 6) indicate that the samples exhibited hexagonal
structures with a high
degree of structural ordering, since all of the spectra featured three of (hkn
interplanar spacing
associated with hexagonal lattice structure. The peaks seen in the spectra are
narrow (100)
peaks and well separated (110) and (200) reflections. The cylindrical unit
cell parameter (ao)
is equivalent to the interplanar spacing of d,oo, and the hexagonal unit cell
parameter (ao) is
equivalent to the interplanar spacing of d~oo (2/J3). From the interplanar
spacing, the pore
diameter of the samples was determined to be around 45 A, which is in good
agreement with
the TEM data.
[0077] For the study of the structure of the support, the same Co:Mo catalyst
was prepared
using the different supports and the nanotube-ceramic composites were prepared
under
reaction conditions at a temperature of 850°C. In this case, no
hydrogen was included in the
feed. The I vs. V curves for these samples are observed in Figure 7. In this
case, the samples
with the best field emission performance were those with the Aerosil~ silicas,
which are fumed
silicas with an average particle size in the nano-scale range. The Aerosil~ 90
sample, which
showed a slightly better performance than the Aerosil~ 380, has an average
particle diameter
of 20 nm, while the Aerosil~ 380 has an average particle size of 7 nm. The
small difference in
the field emission characteristics of these two samples appears to indicate
that the average
particle size of the fumed silica always in the 7 - 20 nm range is much less
important than the
general structure of the support. The sample made with the Hi-Sil~-210 silica
needed an
electric field of 1.6 V/pm more (4.02 V/um against 2.41 V/pm) than the
Aerosil~ 380 sample to
achieve the same (4.75 mA/cm~) current density. In this case, the structure of
this silica is
completely different since Hi-Sil~-210 is a precipitated silica with a
specific surface area of 150
m2/g. One important characteristic of the Hi-Sil~-210 silica is its absence of
microporosity. On
CA 02523911 2005-10-27
WO 2004/096725 PCT/US2004/012986
16
the other hand, the silica gel 60 is highly microporous. The nanotube-ceramic
composite
prepared using this silica, had poor field emission performance and did not
achieve current
densities higher than 0.12 mPJcm~. Similarly, the fVlCNls prepared, which have
pore diameters
in the order ofi ~.0 ~ sh~wed the same poor behavior. The I~vuser selectivity
towards SWNT ofi
these samples as observed by the lower quality parameter (1-~/G) obtained from
Raman
spectra, appears to be the reason for this phenomenon.
[0078] The Aerosil~ composites showed excellent performance achieving the
targeted current
density at very low electric field. To verify the correspondence of field
emission with synthesis
temperature described above using the Hi-Sil~-210 silica, another Aerosil~
composite was
prepared, and the nanotube-ceramic composite was synthesized at 750°C.
The comparison
with that obtained at 850°C is showed in Figure 8. Again, the same
trend is observed (better
performance with higher synthesis temperatures). The composite produced at
850°C had a
much better performance than that produced at 750°C.
[0079] Another aspect of the Aerosil~ composite synthesized at 850°C
that is important to note
is the extremely low hysteresis observed in its 1 vs. V curve. No other
material tested herein
had shown such performance, with almost no deterioration of the sample after
achieving current
densities of almost 5 mA/cm2.
[0080 Finally, the effect of the carbon content in the SWNT composite on the
field emission
performance of the material was studied. To accomplish this, different methods
of changing
the carbon/silica ratio were used. The first one was to increase the carbon
yield during the
synthesis of the SWNT of the nanotube-ceramic composite. This was achieved by
increasing
the metal loading on the original catalyst particle from 2% to 6%. With this,
two composites,
one containing 10% SWNT and the other 20% SWNT were compared. Although earlier
studies
showed that an optimal performance was achieved by a 50% SWNT/50% dielectric
material
mixture, the I vs. V curves of these two samples (Figure 9) showed that the
material with 16%
SWNT behaved worse than that the material with only 10 % SWNT. The yield does
not
increase linearly with the metal content of the catalyst and therefore the
efficiency of the metal
decreases as shown in Table II. For instance, for two samples with 2 wt% and
10 wt% metal,
the metal efficiencies were 500wt% and 200wt%, respectively. Although even in
the best case
the effiiciency is low and only 147 moles of carbon are produced per mol ofi
Co that is the active
species, the efficiencies obtained using the present method of synthesis are
much higher than
those obtained by any other method. For example, the highest efficiency
reported by Ci, et al.
[23] using the filoating catalyst method with acetylene as the carbon source
and Fe as the active
catalyst was 3.25 moles of carbon per mol of Fe. Similarly, the C/Fe ratio in
the HipCO~
method is 10/1 [24].
CA 02523911 2005-10-27
WO 2004/096725 PCT/US2004/012986
I7
[0081] Table II also shows the quality parameter X (1-D/G) obtained from Raman
spectra (514
nm laser) of the product obtained using the different catalysts (2 wt%, 6 wt%
and 10 wt% metal
loading). Although there is a clear trend where the quality parameter
decreases as the metal
loading is increased, it is important to remark that the quality of the SWi~T
does not differ mach
in this metal loading range and should not be a factor for the difference in
field emission.
T~tal petal ~i-~IG Field (wt~/~) Efficiency
i~~dlllf~ (~c~rbon/~mefi~l~(P11~ICavb~n/t'll~IC~)
(fit/~)
2% 0.947 10% 500% 147
6% 0.946 16% 272% 80
10% 0.940 19% 192% 56
Table I1- Quality parameter 1-D/G, carbon yield and Metal efficiency as
function of metal
loading on a Co:Mo(1:3)/SiO2 Hi-Sil~ catalyst series. Reaction run at
750°C and 5.8 atm.
[0082] In conclusion, the increase in metal/SWNT ratio produced a decrease in
the field
emission performance and therefore, the preferred nanotube-ceramic composite
is that one
with only 10% SWNT but the maximum SWNT/metal ratio.
[0083] Although working with the nanotube-ceramic composite produced as shown
herein
(without purification) has an important cost advantage, other post-treatments
to increase the
SWNT content were explored. The post-treatment consisted in the removal of the
metals by an
acid attack with concentrated HCI and the partial removal of the silica
support by both basic
attacks with a NaOH solution and an acid attack with an HF solution.
[0084] The NaOH treated sample increased the concentration of SWNT to 80% but
resulted
in a product with no field emission at all. The samples purified with HF
reduced the amount of
silica even more resulting in a material with only traces of silica. This
material was tested in two
different forms. One, in a gel form as resulted from the purification process
containing mainly
1 % SWNT and 99% water, and a second one in dried form that resulted from the
lyophilization
of the gel. Figure 10 shows a comparison of these new samples with the
nanotube-ceramic
composite, in the form of I vs. V curves. Again these purification methods did
not result in any
improvement in the field emission of the nanotubes but it rather considerably
decreased the
performance of the material. Ultimately, the best field emission material has
been the
nanotube-ceramic composite with 10% SWNT content.
[0085] Changes may be made in the construction and the operation of the
various components,
elements and assemblies described herein or in the steps or the sequence of
steps of the
methods described herein without departing from the scope of the invention as
defined in the
following claims.
CA 02523911 2005-10-27
WO 2004/096725 PCT/US2004/012986
I8
Literature Cited
[0086] 1. "Mefihod of Producing Nanofiubes" D. E. Resasco, B. ~ifiiyanan, J.
H. Harwell, W.
Alvarez. U.S. Patent i~o. 5,333,016 (2001 ). "i~'iethod and Apparatus for
Producing i~anotubes"
D. E Resasco, L. Balzano, W. Alvarez, B. Kitiyanan, US Patent 6,413,487 (2002)
[0087] 2. "Characfierization of single-walled carbon nanofiubes (SWNT)
produced by CO
disproportionafiion on Co-Mo catalysts" W. E. Alvarez, F. Pompeo, J. E.
Herrera, L. Balzano,
and D. E. Resasco. Chemistry of IIVllaterials 14 (2002) 1853-1858
(0088] 3. "Synergism of Co and Mo in the catalytic producfiion of single-wall
carbon
nanotubes by decomposition of CO" W. E. Alvarez, B. Kitiyanan, A. Borgna, and
D. E. Resasco,
Carbon, 39 (2001 ) 547-558 ,
[0089] 4. "Relationship Between the Structure/Composition of Co-Mo Catalysts
and their
Ability to Produce Single-Walled Carbon Nanotubes by GO Disproportionation"
Jose E. Herrera,
Leandro Balzano, Armando Borgna, Walter E. Alvarez, Daniel E. Resasco, Journal
ofCatalysis
204 (2001 ) 129
(0090] 5. "Large screen home FEDs for advanced digital broadcasting", F. Sato
and M.
Seki, Proc. of Asia DisplaylIDW '07, Nagoya, Japan (2001 ) 1153
[0091] 6. "New CNT Composites for FEDs That Do Not Require Activation" D. S.
Mao, R.
L. Fink, G. Monty, L. Thuesen, and Z. Yaniv, Proc. 9th Int. Display
IlVorkshops lIDVIl'02,
Hiroshima, Japan (2002) 1415
[0092] 7. "Transmittance and mechanical properties of PMMA-fumed silicas
composites"
B. Abramoff, J. Covino, J. Appl. Poly. Sci. 46 (1989)
[0093] 8. "Study of the effect of the effect of fumed silica on rigid PVC
properties" S.
Fellahi, S. Boukobbal, F. Boudjenana, J. Vinyl. Tech. 15 (1993) 17-21
(0094] 9. "Influence of fumed silica properties on the processing, curing and
reinforcement
properties of silicone rubber" H Cochrane, C.S. Lin, Rubber Chem. Technol. 66
(1993) 48-60
[0095] 10. "Rheological and mechanical properties of filled rubber: silica-
silicone" M. I.
Aranguren, E. Mora, C.W. Macosko, J. Saam, Rubber Chem. Technol. 67 (1994) 820-
33
[0096] 11. "Compounding fumed silicas infio polydimefihylsiloxane: bound
rubber and final
aggregate size" M. I. Aranguren, E. Mora, C.W. Macosko, J. Saam, J. Colloid
Interface Sci..
195 (1997) 329-37
[0097,12. "Effect of polymer-filler and filler-filler interacfiions on dynamic
properties of filled
vulcanizates" M.J. Wang, Rubber Che. Technol. 71 (1998) 520-89
[0098] 13. "Dental material with inorganic filler particles coated, with
polymerizable binder"
CA 02523911 2005-10-27
WO 2004/096725 PCT/US2004/012986
19
H. Rentsch, W. Mackert, Eur. Pat. Appl., EP 732099 A2 19960918 (1996)
(0099] 14. "Thermal conductivity, elastic modulus, and coefficient of thermal
expansion of
polymer composites filled with ceramic particles for electronic packaging."
C.P.. Wong,
B~Ilampally, S. Raja, J. Appl. P~lym. Bci. 7q. (1999) 3396-403
(0100] 15. "Role of rheological additives in protective coatings" R. E. Van
Dorem, D. N.
Nash, A. Smifih, J. Protective Coatings Linings 6 (1989) 47-52
(0101] 16. "SWNT-filled thermoplastic and elastomeric composites prepared by
miniemulsion polymerization" H. Barraza, F. Pompeo, E. O'Rear, D. E. Resasco,
fVan~ Letters
2 (2002) 797-802
(0102] 17. "Nucleation of Polypropylene Crystallization by Single-Walled
Carbon
Nanotubes", B. P. Grady, F. Pompeo, R. L. Shambaugh, and D. E. Resasco,
Journal of
Physical Chemistry B 106 (2002) 5852-5858
[0103] 18. A. Razavi, Chemistry 3 (2000) 615
(0104] 19. "The influence of Cr precursors in the ethylene polymerization on
Cr/SiO2
catalysts", A.B. Gaspar, L.C. Dieguez, Applied Catalysis A: General 227 (2002)
241-254
(0105] 20. "Graphite Nanofibers as an Electrode for Fuel Cell Applications",
Bessel, Carol
A.; Laubernds, Kate; Rodriguez, Nelly M.; Baker, R. Terry K., J. Phys. Chem. B
(2001 ), 105,
1089-5647 '
[0106] 21. "Pt-W03 supported on carbon nanotubes as possible anodes for direct
methanol fuel cells", B. Rajesha, V. Karthik, S. Karthikeyan, K.
Ravindranathan Thampi, J.-M.
Bonard, B. Viswanathan Fuel81 (2002) 2177-2190
[0107] 22. "Single-wall carbon nanotube/conjugated polymer photovoltaic
devices",
Kymakis, E.; Amaratunga, G. A. J., Applied Physics Letters (2002), 80(1), 112-
114
[0108] 23. Ci L., Xie S., Tang D., Yan X., Li Y., Liu Z., Zou X., Zhou W.,
Wang G., Chem.
Phys. Lett., 349(3,4) (2001 ) 191
(0109] 24. Nikolaev P., Bronikowski M.J., Bradley R.K., Rohmund F., Colbert
D.T., Smith
K.A., Smalley R.E., Chem. Phys. Lett., 313 (1999) 91
(0110] 25. Laurence, R.L., and M.G. Chiovetta, "Heat and Mass Transfer During
Olefin
Polymerization from the Gas Phase," Polymer Reaction Engineering: Influence of
Reaction
Engineering on Polymer Properties, K.H. Reichert and W. Geisler, eds., Hanser,
Munich (1983)