Note: Descriptions are shown in the official language in which they were submitted.
CA 02525256 2011-07-28
Title: Oxidation Process Using Microchannel Technology and Novel
Catalyst Useful in Same
Technical Field
This invention relates to an oxidation process using microchannel technology
and a
novel catalyst useful in the oxidation process.
Background of the Invention
Complete combustion of methane and higher order hydrocarbons is difficult to
achieve under fuel-rich conditions in microchannel reactors with relatively
short contact
times. The combustion is incomplete and this leads to undesirable levels of
carbon
monoxide and carbon deposits. The problem therefore is to find a way in which
to conduct a
complete combustion reaction in a microchannel reactor. This invention
provides a solution
to this problem.
Partial oxidation reactions typically involve reacting a hydrocarbon with
oxygen in the
presence of a catalyst to form hydrogen and carbon monoxide. Examples include
the
conversion of methane to hydrogen and carbon monoxide. A problem with these
reactions
is that they are exothermic and are typically conducted in fixed bed reactors
where hot
spots tend to form. The formation of these hot spots increases the tendency of
the catalyst
to deactivate. This invention provides a solution to this problem.
This invention relates to a process wherein a partial oxidation reaction or a
partial
oxidation reaction coupled with combustion reaction is conducted in a
microchannel reactor
wherein the tendency to form hot spots is reduced and selectivity to the
desired product is
enhanced. Reductions in these hot spots with the inventive process is believed
to be due at
least in part to the fact that the microchannel reactor provides enhanced heat
transfer
characteristics and more precise control of residence times. In one
embodiment, a novel,
stable and highly active partial oxidation catalyst is used in the inventive
process.
With the inventive process it is possible to obtain relatively high heat and
mass
transfer rates and shorter contact times as compared to prior art processes
wherein
microchannel reactors are not used. This provides for more precise temperature
control as
compared to such prior art. This, in turn, leads to an increase in catalyst
durability and a
reduction in the formation of undesired by-products. With this process, it is
possible to
CA 02525256 2011-07-28
2
obtain relatively high levels of conversion of the hydrocarbon reactant and
high levels of
selectivity to the desired product as compared to such prior art.
Summary of the Invention
This invention relates to a process for converting a hydrocarbon reactant to a
partial
oxidation reaction product comprising CO and H2 in a microchannel reactor, the
microchannel reactor comprising at least one process microchannel and at least
one partial
oxidation reaction catalyst in the at least one process microchannel, the
process
comprising:
(A) mixing a
reactant composition comprising the hydrocarbon reactant
and oxygen or a source of oxygen in the at least one process microchannel and
flowing the
resulting mixture in the at least one process microchannel in contact with the
at least one
partial oxidation reaction catalyst under partial oxidation reaction
conditions to form the
product, the hydrocarbon reactant comprising methane, the contact time for the
reactant
composition within the at least one process microchannel being up to about 500
milliseconds, the temperature of the reactant composition and product within
the at least
one process microchannel being up to about 1150 C, the conversion of the
hydrocarbon
reactant being at least about 50%.
In one embodiment of the invention, the catalyst used in step (A) is a partial
oxidation catalyst, the product formed in step (A) is an intermediate product,
and the
process further comprises the following additional step subsequent to step
(A):
(B) flowing the intermediate product formed in step (A)
through a
microchannel reactor in contact with a combustion catalyst under reaction
conditions to form
a final product comprising CO2 and H20.
In one embodiment, the reactant composition further comprises H20 and the
product
comprises H2, CO and CO2.
In one embodiment, the invention relates to a catalyst comprising a
composition
represented by the formula
mia .2b
m Mc Ald Ox
wherein
M1 is Rh, Ni, Pd, Pt, Ru, Co or a mixture of two or more thereof;
M2 is Ce, Pr, Tb or a mixture of two or more thereof;
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
3
M3 is La, Ba, Zr, Mg, Ca or a mixture of two or more thereof;
a is a number in the range of about 0.0001 to about 1;
b is a number in the range of zero to about 0.9999;
c is a number in the range of about 0.0001 to about 0.9999;
d is a number in the range of about 0.0001 to about 0.9999; and
x is the number of oxygens needed to fulfill the valency requirements of the
elements present;
the catalyst being coated on a substrate or supported on a foam, felt, wad or
fin.
In one embodiment, the invention relates to a process for making a supported
catalyst, comprising:
(A) applying a layer of A1203 over at least part of a support structure;
(B) calcining the treated support structure formed in step (A);
(C) applying a promoter or stabilizer to the surface of the calcined
support
structure formed in step (B), the promoter or stabilizer comprising La, Ba,
Zr, Mg,
Ca, or an oxide or nitrate thereof, or a mixture of two or more thereof;
(D) calcining the treated support structure formed in step (C);
(E) applying a catalytic metal or oxide or nitrate thereof to the surface
of
the calcined support structure formed in step (D), the catalytic metal
comprising Rh,
Ni, Pd, Pt, Ru, Co or a mixture of two or more thereof; and
(F) calcining the treated support structure formed in step (E) to form the
supported catalyst.
Brief Description of the Drawings
In the annexed drawings, like parts and features have like designations.
Fig. 1 is a schematic flow sheet illustrating the inventive partial oxidation
process in a particular form wherein a hydrocarbon reactant and oxygen or a
source
of oxygen contact the inventive catalyst in a microchannel reactor and react
to form
a product comprising hydrogen and a carbon oxide.
Fig. 2 is a schematic flow sheet illustrating the operation of a particular
form
of a microchannel reactor used with the inventive partial oxidation process.
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
4
Fig. 3 is a schematic illustration of a process microchannel used with the
inventive partial oxidation process, the process microchannel containing a
catalyst
having a flow-by configuration.
Fig. 4 is a schematic illustration of a process microchannel used with the
inventive partial oxidation process, the process microchannel containing a
catalyst
having a flow-through configuration.
Fig. 5 is a schematic illustration of a process microchannel used in the
inventive partial oxidation process, the process microchannel containing a fin
assembly comprising a plurality of fins, the inventive catalyst being
supported by the
fins.
Fig. 6 illustrates an alternate embodiment of the process microchannel and
fin assembly illustrated in Fig. 5.
Fig. 7 illustrates an alternate embodiment of the fin assembly illustrated in
Fig. 5.
Fig. 8 is a plot of process performance versus time for the tests disclosed in
Example 2.
Fig. 9 is a plot of process performance versus time for the tests disclosed in
Example 7.
Fig. 10 illustrates the channel arrangement for the microchannel reactor used
in the tests disclosed in Example 8.
Fig. 11 illustrates the fin assembly for the microchannel reactor used in
Example 9.
Detailed Description of the Invention
The term "microchannel" refers to a channel having at least one internal
dimension of height or width of up to about 10 millimeters (mm), and in one
embodiment up to about 5 mm, and in one embodiment up to about 2 mm, and in
one embodiment up to about 1 mm. In one embodiment, the height or width is in
the range of about 0.05 to about 10 mm, and in one embodiment about 0.05 to
about 5 mm, and in one embodiment about 0.05 to about 2 mm, and in one
embodiment about 0.05 to about 1.5 mm, and in one embodiment about 0.05 to
about 1 mm, and in one embodiment about 0.05 to about 0.75 mm, and in one
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
embodiment about 0.05 to about 0.5 mm. Both height and width are perpendicular
to the direction of flow through the microchannel.
The term "adjacent" when referring to the position of one channel relative to
the position of another channel means directly adjacent such that a wall
separates
5
the two channels. This wall may vary in thickness. However, "adjacent"
channels
are not separated by an intervening channel that would interfere with heat
transfer
between the channels.
The term "fluid" refers to a gas, a liquid, or a gas or a liquid containing
dispersed solids, or a mixture thereof. The fluid may be in the form of a gas
containing dispersed liquid droplets.
The term "contact time" refers to the volume of the reaction zone within the
microchannel reactor divided by the volumetric feed flow rate of the reactant
composition at a temperature of 0 C and a pressure of one atmosphere.
The term "residence time" refers to the internal volume of a space (e.g., the
reaction zone within a microchannel reactor) occupied by a fluid flowing
through the
space divided by the average volumetric flowrate for the fluid flowing through
the
space at the temperature and pressure being used.
The term "reaction zone" refers to the space within the microchannel reactor
wherein the reactants contact the catalyst.
The term "conversion of hydrocarbon reactant" refers to the hydrocarbon
reactant mole change between the reactant composition and the product divided
by
the moles of the hydrocarbon reactant in the reactant composition.
The term "selectivity to desired product" refers to the moles of the desired
oxygenate or nitrile produced divided by the moles of the desired oxygenate or
nitrile
produced plus moles of other products (e.g., CO, CO2) produced multiplied by
their
respective stoichiometric factors. For example, for the oxidation of ethylene
to
ethylene oxide with carbon dioxide as an unwanted side product, the production
of
one mole of ethylene oxide and one mole of carbon dioxide would correspond to
a
selectivity of 100 x (1/(1 + 0.5)) = 67%.
The term "hydrocarbon" denotes a compound having a hydrocarbon or
predominantly hydrocarbon character. These hydrocarbon compounds include the
following:
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
6
(1) Purely hydrocarbon compounds; that is, aliphatic compounds, (e.g.,
alkane or alkylene), alicyclic compounds (e.g., cycloalkane, cycloalkylene),
aromatic
compounds, aliphatic- and alicyclic-substituted aromatic compounds, aromatic-
substituted aliphatic compounds and aromatic-substituted alicyclic compounds,
and
the like. Examples include methane, ethane, ethylene, propane, propylene,
ethyl
cyclohexane, toluene, the xylenes, ethyl benzene, styrene, etc.
(2) Substituted hydrocarbon compounds; that is, hydrocarbon compound
containing non-hydrocarbon substituents which do not alter the predominantly
hydrocarbon character of the compound. Examples of the non-hydrocarbon
substituents include hydroxy, acyl, nitro, etc.
(3) Hetero substituted hydrocarbon compounds; that is, hydrocarbon
compounds which, while predominantly hydrocarbon in character, contain atoms
other than carbon in a chain or ring otherwise composed of carbon atoms.
Suitable
hetero atoms include, for example, nitrogen, oxygen and sulfur.
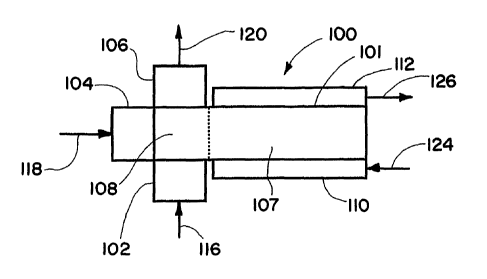
The inventive process may be conducted as illustrated in Figs. 1 and 2.
Referring to Fig. 1, the process is operated using microchannel reactor 100
which
includes microchannel reactor core 101, reactant header 102, oxidant header
104,
product footer 106, heat exchange header 110 and heat exchange footer 112. The
microchannel reactor core 101 includes reactor zone 107, and manifold and
recuperator 108. The reactant composition comprising the hydrocarbon reactant
flows into the microchannel reactor 100 through the reactant header 102, as
indicated by directional arrow 116. The oxygen or source of oxygen flows into
the
microchannel reactor 100 through the oxidant header 104 as indicated by
directional
arrow 118. The hydrocarbon reactant and oxygen or source of oxygen flow into
and
through the manifold and recuperator 108 into the reactor zone 107 wherein
they
contact the catalyst and react to form the desired product. The product flows
from
the reactor zone 107 through the manifold and recuperator 108 to product
footer
106, and out of product footer 106 as indicated by directional arrow 120. A
heat
exchange fluid may flow into heat exchange header 110, as indicated by
directional
arrow 124, and from heat exchange header 110 through microchannel reactor core
101 to heat exchange footer 112, and out of heat exchange footer 112, as
indicated
by directional arrow 126. The reactants may be preheated prior to entering the
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
7
reactor zone. The hydrocarbon reactant and the oxygen or source of oxygen may
be mixed prior to entering the reactor zone, or they may be mixed in the
reactor
zone.
Within the microchannel reactor core 101, the oxygen or source of oxygen
may be added to the hydrocarbon reactant using staged addition. This is shown
in
Fig. 2 which illustrates repeating unit 130, which is used in the microchannel
reactor
100 illustrated in Fig. 1. Repeating unit 130 is housed within housing unit
131 and
includes process microchannels 140 and 150, oxidant microchannel 160, orifices
170, and heat exchange microchannels 180 and 190. The hydrocarbon reactant
flows through process microchannels 140 and 150, as indicated by the
directional
arrows 141 and 151, respectively. Oxygen or a source of oxygen flows through
oxidant microchannel 160 into orifices 170, as indicated by directional arrows
161.
The oxygen or oxygen source mixes with the hydrocarbon reactant in the process
microchannels 140 and 150. The process microchannels 140 and 150 have
reaction zones 142 and 152, respectively, wherein the catalyst is present and
the
reactants contact the catalyst and undergo reaction to form the desired
product, and
channel zones 143 and 153, respectively, wherein further contact with the
foregoing
catalyst or a different catalyst may be effected, or product cooling and/or
quenching
may be effected. The catalyst positioned in the reaction zone is a partial
oxidation
catalyst. In one embodiment, a combustion catalyst may be positioned
downstream
of the partial oxidation catalyst in the reaction zones 142 and 152 and/or in
the
channel zones 143 and 153. The product exits the process microchannels 140 and
150, as indicated by the directional arrows 144 and 154, respectively. The
product
exiting the process microchannels 140 and 150 flows to the manifold and
recuperator 108, and from the manifold and recuperator 108 through the product
footer 106 as indicated by directional arrow 120. Heat exchange fluid flows
from
header 110 through heat exchange channels 180 and 190, as indicated by
directional arrows 181, and 191 and 192, respectively, to heat exchange footer
112.
The heat exchange channels 180 and 190 are aligned to provide a flow in a
cross-
current direction relative to the process microchannels 140 and 150 as
indicated by
arrows 181, 191 and 192. The process microchannels 140 and 150 transfer heat
to the heat exchange channels. The heat exchange fluid may be recirculated
using
known techniques. Alternatively, the heat exchange channels may be oriented to
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
8
provide for flow of the heat exchange fluid in a cocurrent or counter current
direction
relative to the direction of the flow of fluid through the process
microchannels 140
and 150. The repeating unit 130 illustrated in Fig. 2 may occur once within
the
microchannel reactor 100 or it may be repeated any number of times, for
example,
two, three, four, five, ten, twenty, fifty, one hundred, hundreds, one
thousand,
thousands, ten thousand, tens of thousands, one hundred thousand, hundreds of
thousands or millions of times. The staged oxygen addition provided for in
this
process provides the advantage of lowering local oxygen pressure and favoring
desired lower-order partial oxidation reactions over higher-order competing
and
undesired combustion reactions.
Each of the process microchannels 140 and 150 and the oxidant
microchannel 160 may have at least one internal dimension of height or width
of up
to about 10 mm, and in one embodiment from about 0.05 to about 10 mm, and in
one embodiment about 0.05 to about 5 mm, and in one embodiment about 0.05 to
about 2 mm, and in one embodiment about 0.05 to about 1.5 mm, and in one
embodiment about 0.05 to about 1 mm, and in one embodiment about 0.05 to about
0.5 mm. The other internal dimension of height or width may be of any value,
for
example, it may range from about 0.1 cm to about 100 cm, and in one embodiment
from about 0.1 to about 75 cm, and in one embodiment from about 0.1 to about
50
cm, and in one embodiment about 0.2 cm to about 25 cm. The length of each of
the
process microchannels 140 and 250, and the oxidant microchannel 160, may be of
any value, for example, the lengths may range from about 1 cm to about 500 cm,
and in one embodiment 1 cm to about 250 cm, and in one embodiment 1 cm to
about 100 cm, and in one embodiment 1 cm to about 50 cm, and in one
embodiment about 2 to about 25 cm.
Each of the heat exchange channels 180 and 190 may have at least one
internal dimension of height or width of up to about 10 mm, and in one
embodiment
about 0.05 to about 10 mm, and in one embodiment about 0.05 to about 5 mm, and
in one embodiment from about 0.05 to about 2 mm, and in one embodiment from
about 0.5 to about 1 mm. The other internal dimension may range from about 1
mm
to about 1 m, and in one embodiment about 1 mm to about 0.5 m, and in one
embodiment about 2 mm to about 10 cm. The length of the heat exchange
channels may range from about 1 mm to about 1 m, and in one embodiment about
CA 02525256 2011-07-28
9
1 cm to about 0.5 m. These heat exchange channels may be microchannels. The
separation between each process microchannel 140 or 150 and the next adjacent
heat
exchange channel 180 or 190 may range from about 0.05 mm to about 5 mm, and in
one
embodiment about 0.2 mm to about 2 mm.
The microchannel reactor 100 may be made using known techniques. These include
laminating interleaved shims, where shims designed for the process
microchannels, oxidant
microchannels and heat exchange channels are interleaved.
The housing 131, process microchannels 140 and 150, oxidant microchannel 160,
and heat exchange channels 180 and 190 may be made of any material that
provides
sufficient strength, dimensional stability and heat transfer characteristics
to permit operation
of the inventive process. These materials include steel (e.g., stainless
steel, carbon steel,
and the like); monel; inconel; aluminum; titanium; nickel; platinum; rhodium;
copper;
chromium; brass; alloys of any of the foregoing metals; polymers (e.g.,
thermoset resins);
ceramics; glass; composites comprising one or more polymers (e.g., thermoset
resins) and
fiberglass; quartz; silicon; or a combination of two or more thereof.
Alternatively, the staged addition of the oxygen or source of oxygen to the
microchannel reactor may be effected using separate devices, through the use
of small
orifices or jets within one device, or from a microporous membrane or
alternate sparging
sheet. The staged addition of oxygen to partial oxidation reactions, and
specifically oxidative
dehydrogenation reactions, is disclosed in Tonkovich, Zilka, Jimenz, Roberts,
and Cox,
1996, "Experimental Investigations of Inorganic Membrane Reactors: a
Distributed Feed
Approach for Partial Oxidation Reactions," Chemical Engineering Science,
51(5), 789-806).
In one embodiment, the process microchannels 140 and 150 may contain a bulk
flow
path. The term "bulk flow path" refers to an open path (contiguous bulk flow
region) within
the process microchannels. A contiguous bulk flow region allows rapid fluid
flow through the
microchannels without large pressure drops. In one embodiment, the flow of
fluid in the bulk
flow region is laminar. Bulk flow regions within each process microchannel may
have a
cross-sectional area of about 0.05 to about 10,000 mm2, and in one embodiment
about 0.05
to about 5000 mm2, and in one embodiment about 0.1 to about 2500 mm2, and in
one
embodiment about 0.2
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
to about 1000 mm2, and in one embodiment about 0.3 to about 500 mm2, and in
one
embodiment about 0.4 to about 250 mm2, and in one embodiment about 0.5 to
about 125 mm2. The bulk flow regions may comprise from about 5% to about 95%,
and in one embodiment about 30% to about 80% of the cross-section of the
process
5 microchannels 140 and 150.
The reactant composition may be in the form of a fluid. This fluid may be a
liquid or a gas, and in one embodiment it is in the form of a gas. This fluid
may be
in the form of a gas containing dispersed liquid droplets. The reactant
composition
comprises methane and may further comprise one or more additional hydrocarbon
10 reactants. The concentration of methane in the mixture of methane and
one or
more additional hydrocarbon reactants may range up to about 100% methane, and
in one embodiment from about 10 to about 90% by volume methane, and in one
embodiment about 50 to about 90% by volume methane.
The purity of the reactant composition is not critical, though it is desirable
to
avoid the presence of compounds which may poison the catalyst. As a result,
the
reactant composition may further comprise impurities such as air, carbon
dioxide,
and the like.
The reactant composition may include a diluent material. Examples of such
diluents include nitrogen, helium, carbon dioxide, liquid water, steam, and
the like.
The volume ratio of diluent to hydrocarbon reactant in the reactant
composition may
range from zero to about 80% by volume, and in one embodiment from zero to
about 50% by volume. However, an advantage of at least one embodiment of the
invention is that it is possible to conduct the inventive process without the
use of
such diluents, thus a more efficient and compact process may be provided.
The hydrocarbon reactant comprises methane and may further comprise one
or more additional hydrocarbon compounds that are capable of undergoing an
oxidation reaction, and are a fluid (and in one embodiment a vapor) at the
temperature and pressure used within the process microchannels. Examples
include saturated aliphatic compounds (e.g., alkanes), unsaturated aliphatic
compounds (e.g., monoenes, polyenes, and the like), alkyl substituted aromatic
compounds, alkylene substituted aromatic compounds, oils, normally liquid
fuels,
and the like.
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
11
The saturated aliphatic compounds include alkanes containing 2 to about 20
carbon atoms per molecule, and in one embodiment 2 to about 18 carbon atoms,
and in one embodiment 2 to about 16 carbon atoms, and in one embodiment 2 to
about 14 carbon atoms, and in one embodiment 2 to about 12 carbon atoms, and
in one embodiment 2 to about 10 carbon atoms, and in one embodiment 2 to about
8 carbon atoms, and in one embodiment 2 to about 6 carbon atoms, and in one
embodiment 2 to about 4 carbon atoms. These include ethane, propane,
isopropane, butane, isobutane, the pentanes, the hexanes, the heptanes, the
octanes, the nonanes, the decanes, and the like.
The unsaturated aliphatic compounds include alkenes or alkylenes containing
2 to about 20 carbon atoms, and in one embodiment 2 to about 18 carbon atoms,
and in one embodiment 2 to about 16 carbon atoms, and in one embodiment 2 to
about 14 carbon atoms, and in one embodiment 2 to about 12 carbon atoms, and
in one embodiment 2 to about 10 carbon atoms, and in one embodiment 2 to about
8 carbon atoms, and in one embodiment 2 to about 6 carbon atoms per molecule,
and in one embodiment 2 to about 4 carbon atoms. These include ethylene;
propylene; 1-butene; 2-butene; isobutylene; 1-pentene;2-pentene; 3-methy1-1-
butene; 2-methyl-2-butene; 1-hexene; 2,3-dimethy1-2-butene; 1-heptene; 1-
octene;
1-nonene; 1-decene; and the like.
The unsaturated aliphatic compounds may comprise polyenes. These
include dienes, trienes, and the like. These compounds may contain 3 to about
20
carbon atoms per molecule, and in one embodiment 3 to about 18 carbon atoms,
and in one embodiment 3 to about 16 carbon atoms, and in one embodiment 3 to
about 14 carbon atoms, and in one embodiment 3 to about 12 carbon atoms, and
in one embodiment 3 to about 10 carbon atoms, and in one embodiment about 4 to
about 8 carbon atoms, and in one embodiment about 4 to about 6 carbon atoms.
Examples include 1,2-propadiene (also known as allene); 1,3-butadiene; 2-
methyl-
1,3-butadiene (also known as isoprene); 1,3-pentadiene; 1,4-pentadiene; 1,5-
hexadiene; 2,4-hexadiene; 2,3-dirnethy1-1,3-butadiene; and the like.
The alkyl or alkylene substituted aromatic compounds may contain one or
more alkyl or alkylene substituents. These compounds may be monocyclic (e.g.,
phenyl) or a polycyclic (e.g., naphthyl). These compounds include alkyl
substituted
aromatic compounds containing one or more alkyl groups containing 1 to about
20
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
12
carbon atoms, and in one embodiment 1 to about 18 carbon atoms, and in one
embodiment 1 to about 16 carbon atoms, and in one embodiment 1 to about 14
carbon atoms, and in one embodiment 1 to about 12 carbon atoms, and in one
embodiment 1 to about 10 carbon atoms, and in one embodiment 1 to about 8
carbon atoms, and in one embodiment about 2 to about 6 carbon atoms, and in
one
embodiment about 2 to about 4 carbon atoms. These also include the akylene
substituted aromatic compounds containing one or more alkylene groups
containing
2 to about 20 carbon atoms, and in one embodiment 2 to about 18 carbon atoms,
and in one embodiment 2 to about 16 carbon atoms, and in one embodiment 2 to
about 14 carbon atoms, and in one embodiment 2 to about 12 carbon atoms, and
in one embodiment 2 to about 10 carbon atoms, and in one embodiment 2 to about
8 carbon atoms, and in one embodiment about 2 to about 6 carbon atoms, and in
one embodiment about 2 to about 4 carbon atoms. Examples include toluene, o-
xylene, m-xylene, p-xylene, hemimellitene, pseudocumene, mesitylene,
prehnitene,
isodurene, durene, pentamethylbenzene, hexamethylbenzene, ethylbenzene, n-
propylbenzene, cumene, n-butylbenzene, isobutylbenzene, sec-butylbenzene, tert-
butylbenzene, p-cymene, styrene, and the like.
The hydrocarbon reactant may further comprise a natural oil, synthetic oil or
mixture thereof. The natural oils include animal oils and vegetable oils
(e.g., castor
oil, lard oil) as well as mineral oils such as liquid petroleum oils. Oils
derived from
coal or shale may be used. Synthetic oils include hydrocarbon oils such as
polymerized and interpolymerized olefins, polyphenyls, alkylated diphenyl
esters,
alkylated diphenyl sulfides, and the like. Alkylene oxide polymers and
interpolymers
and derivatives thereof where the thermal hydroxyl groups have been modified
by
esterification, etherification, etc., constitute another class of known
synthetic oils that
can be used as the hydrocarbon reactant. The synthetic oils that are useful as
the
hydrocarbon reactant include the esters of dicarboxylic acids with a variety
of
alcohols. The hydrocarbon reactant may comprise a poly-alpha-olefin. The
hydrocarbon reactant may comprise a Fischer-Tropsch synthesized hydrocarbon.
The hydrocarbon reactant may be obtained from a process stream generated
during
oil refining, chemical synthesis, and the like.
The hydrocarbon reactant may further comprise a normally liquid
hydrocarbon fuel. These include distillate fuels such as motor gasoline,
diesel fuel
CA 02525256 2011-07-28
13
or fuel oil. Hydrocarbon reactants derived from vegetable sources, mineral
sources, and
mixtures thereof may be used. These include hydrocarbon reactants derived from
soybean, rapeseed, palm, shale, coal, tar sands, and the like.
The oxygen or oxygen source may comprise molecular oxygen, air or other
oxidants, such as nitrogen oxides, which can function as a source of oxygen.
The
oxygen source may be carbon dioxide, carbon monoxide or a peroxide (e.g.,
hydrogen
peroxide). Gaseous mixtures containing oxygen, such as mixtures of oxygen and
air, or
mixtures of oxygen and an inert gas (e.g., helium, argon, etc.) or a diluent
gas (e.g.,
carbon dioxide, water vapor, etc.) may be used.
The mole ratio of carbon in the hydrocarbon reactant to oxygen may range from
about 10:1 to about 1:1, and in one embodiment about 4:1 to about 1:1, and in
one
embodiment about 2.4:1 to about 1.6:1.
The heat exchange fluid may be any fluid. These include air, steam, liquid
water,
gaseous nitrogen, liquid nitrogen, other gases including inert gases, carbon
monoxide,
molten salt, oils such as mineral oil, and heat exchange fluids such as
Dowtherm A and
Therminol which are available from Dow-Union Carbide.
The heat exchange fluid may comprise one or more of the reactant streams.
This can provide process pre-heat and increase overall thermal efficiency of
the
process.
In one embodiment, the heat exchange channels comprise process channels
wherein an endothermic reaction is conducted. These heat exchange process
channels
may be microchannels. Examples of endothermic reactions that may be conducted
in
the heat exchange channels include steam reforming and dehydrogenation
reactions. A
typical heat flux for convective cooling in a microchannel reactor is on the
order of about
1 to about 10 W/cm2. The incorporation of a simultaneous endothermic reaction
to
provide an improved heat sink may enable a typical heat flux of roughly an
order of
magnitude above the convective cooling heat flux. The use of simultaneous
exothermic
and endothermic reactions to exchange heat in a microchannel reactor is
disclosed in
U.S. Patent Application Serial No. 10/222,196, filed August 15, 2002.
In one embodiment, the heat exchange fluid undergoes a phase change as it
flows through the heat exchange channels. This phase change provides
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
14
additional heat removal from the process microchannels beyond that provided by
convective cooling. For a liquid heat exchange fluid being vaporized, the
additional
heat being transferred from the process microchannels would result from the
latent
heat of vaporization required by the heat exchange fluid. An example of such a
phase change would be an oil or water that undergoes boiling.
The cooling of the process microchannels 140 and 150 during the inventive
process, in one embodiment, is advantageous for controlling selectivity
towards the
main or desired product due to the fact that such added cooling reduces or
eliminates the formation of undesired by-products from undesired parallel
reactions
with higher activation energies. As a result of this cooling, in one
embodiment, the
temperature of the reactant composition at the entrance to the process
microchannels 140 and 150 may be within about 200 C, and in one embodiment
within about 150 C, and in one embodiment within about 100 C, and in one
embodiment within about 50 C, and in one embodiment within about 25 C, and in
one embodiment within about 10 C, of the temperature of the product (or
mixture
of product and unreacted reactants) at the exit of the process microchannels.
The catalyst used in a microchannel reactor may have any size and
geometric configuration that fits within the process microchannels. The
catalyst
may be in the form of particulate solids (e.g., pellets, powder, fibers, and
the like)
having a median particle diameter of about 1 to about 1000 pm, and in one
embodiment about 10 to about 500 pm, and in one embodiment about 25 to about
250 pm. The catalyst may be supported in a porous structure such as a foam,
felt,
wad or a combination thereof. The term "foam" is used herein to refer to a
structure
with continuous walls defining pores throughout the structure. The term "felt"
is
used herein to refer to a structure of fibers with interstitial spaces
therebetween.
The term "wad" is used herein to refer to a structure of tangled strands, like
steel
wool. The catalyst may be supported on a honeycomb structure.
The catalyst may be in the form of a flow-by structure such as a felt with an
adjacent gap, a foam with an adjacent gap, a fin structure with gaps, a
washcoat on
any inserted substrate, or a gauze that is parallel to the flow direction with
a
corresponding gap for flow. An example of a flow-by structure is illustrated
in Fig. 3.
In Fig. 3, the catalyst 300 is contained within process microchannel 302. An
open
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
passage way 304 permits the flow of fluid through the process microchannel 302
in
contact with the catalyst 300 as indicated by arrows 306 and 308.
The catalyst may be in the form of a flow-through structure such as a foam,
wad, pellet, powder, or gauze. An example of a flow-through structure is
illustrated
5 in Fig. 4. In Fig. 4, the flow-through catalyst 400 is contained within
process
microchannel 402 and the fluid flows through the catalyst 400 as indicated by
arrows
404 and 406.
The catalyst may be directly washcoated on the interior walls of the process
rnicrochannels, grown on the walls from solution, or coated in situ on a fin
structure.
10 The catalyst may be in the form of a single piece of porous contiguous
material, or
many pieces in physical contact. In one embodiment, the catalyst is comprised
of
a contiguous material and has a contiguous porosity such that molecules can
diffuse
through the catalyst. In this embodiment, the fluids flow through the catalyst
rather
than around it. In one embodiment, the cross-sectional area of the catalyst
15 occupies about Ito about 99%, and in one embodiment about 10 to about
95% of
the cross-sectional area of the process microchannels. The catalyst may have a
surface area, as measured by BET, of greater than about 0.5 m2/g, and in one
embodiment greater than about 2 m2/g.
The catalyst may comprise a porous support, an interfacial layer on the
porous support, and a catalyst material on the interfacial layer. The
interfacial layer
may be solution deposited on the support or it may be deposited by chemical
vapor
deposition or physical vapor deposition. In one embodiment the catalyst has a
porous support, a buffer layer, an interfacial layer, and a catalyst material.
Any of
the foregoing layers may be continuous or discontinuous as in the form of
spots or
dots, or in the form of a layer with gaps or holes.
The porous support may have a porosity of at least about 5% as measured
by mercury poroshetry and an average pore size (sum of pore diameters divided
by number of pores) of about 1 to about 1000 pm. The porous support may be a
porous ceramic or a metal foam. Other porous supports that may be used include
carbides, nitrides, and composite materials. The porous support may have a
porosity of about 30% to about 99%, and in one embodiment about 60% to about
98%. The porous support may be in the form of a foam, felt, wad, or a
combination
thereof. The open cells of the metal foam may range from about 20 pores per
inch
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
16
(ppi) to about 3000 ppi, and in one embodiment about 20 to about 1000 ppi, and
in
one embodiment about 40 to about 120 ppi. The term "ppi" refers to the largest
number of pores per inch (in isotropic materials the direction of the
measurement
is irrelevant; however, in anisotropic materials, the measurement is done in
the
direction that maximizes pore number).
The buffer layer, when present, may have a different composition and/or
density than both the porous support and the interfacial layers, and in one
embodiment has a coefficient of thermal expansion that is intermediate the
thermal
expansion coefficients of the porous support and the interfacial layer. The
buffer
layer may be a metal oxide or metal carbide. The buffer layer may be comprised
of
A1203, T102, Si02, Zr02, or combination thereof. The A1203 may be a-A1203, y-
A1203
or a combination thereof. a-A1203 provides the advantage of excellent
resistance
to oxygen diffusion. The buffer layer may be formed of two or more
compositionally
different sublayers. For example, when the porous support is metal, for
example a
stainless steel foam, a buffer layer formed of two compositionally different
sub-layers may be used. The first sublayer (in contact with the porous
support) may
be Ti02. The second sublayer may be a-A1203 which is placed upon the Ti02. In
one embodiment, the a-A1203 sublayer is a dense layer that provides protection
of
the underlying metal surface. A less dense, high surface area interfacial
layer such
as alumina may then be deposited as support for a catalytically active layer.
The porous support may have a thermal coefficient of expansion different
from that of the interfacial layer. In such a case a buffer layer may be
needed to
transition between the two coefficients of thermal expansion. The thermal
expansion
coefficient of the buffer layer can be tailored by controlling its composition
to obtain
an expansion coefficient that is compatible with the expansion coefficients of
the
porous support and interfacial layers. The buffer layer should be free of
openings
and pin holes to provide superior protection of the underlying support. The
buffer
layer may be nonporous. The buffer layer may have a thickness that is less
than one
half of the average pore size of the porous support. The buffer layer may have
a
thickness of about 0.05 to about 10 pm, and in one embodiment about 0.05 to
about
5 pm.
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
17
In one embodiment of the invention, adequate adhesion and chemical
stability may be obtained without a buffer layer. In this embodiment the
buffer layer
may be omitted.
The interfacial layer may comprise nitrides, carbides, sulfides, halides,
metal
oxides, carbon, or a combination thereof. The interfacial layer provides high
surface
area and/or provides a desirable catalyst-support interaction for supported
catalysts.
The interfacial layer may be comprised of any material that is conventionally
used
as a catalyst support. The interfacial layer may be comprised of a metal
oxide.
Examples of metal oxides that may be used include y-A1203, Si02, Zr02, T102,
tungsten oxide, magnesium oxide, vanadium oxide, chromium oxide, manganese
oxide, iron oxide, nickel oxide, cobalt oxide, copper oxide, zinc oxide,
molybdenum
oxide, tin oxide, calcium oxide, aluminum oxide, lanthanum series oxide(s),
zeolite(s) and combinations thereof. The interfacial layer may serve as a
catalytically
active layer without any further catalytically active material deposited
thereon.
Usually, however, the interfacial layer is used in combination with a
catalytically
active layer. The interfacial layer may also be formed of two or more
compositionally different sublayers. The interfacial layer may have a
thickness that
is less than one half of the average pore size of the porous support. The
interfacial
layer thickness may range from about 0.5 to about 100 pm, and in one
embodiment
from about 1 to about 50 pm. The interfacial layer may be either crystalline
or
amorphous. The interfacial layer may have a BET surface area of at least about
1
m2/g.
The catalyst may be deposited on the interfacial layer. Alternatively, the
catalyst material may be simultaneously deposited with the interfacial layer.
The
catalyst layer may be intimately dispersed on the interfacial layer. That the
catalyst
layer is"dispersed on" or "deposited on" the interfacial layer includes the
conventional understanding that microscopic catalyst particles are dispersed:
on the
support layer (i. e., interfacial layer) surface, in crevices in the support
layer, and in
open pores in the support layer.
The catalyst may be supported on an assembly of one or more fins which
may be positioned within each of the process microchannels. Examples are
illustrated in Figs. 5-7. Referring to Fig. 5, fin assembly 500 includes fins
502 which
are mounted on fin support 504 which overlies base wall 506 of process
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
18
microchannel 508. The fins 502 project from the fin support 504 into the
interior of
the process microchannel 508. The fins 502 extend to and contact the interior
surface of upper wall 510 of process microchannel 508. The fin channels 512
between the fins 502 provide passage ways for fluid to flow through the
process
microchannel 508 parallel to its length. Each of the fins 502 has an exterior
surface
on each of its sides, this exterior surface provides a support base for a
catalyst.
With the inventive process, the reactant composition flows through the fin
channels
512, contact the catalyst supported on the exterior surface of the fins 502,
and react
to form a product. The fin assembly 500a illustrated in Fig. 6 is similar to
the fin
assembly 500 illustrated in Fig. 5 except that the fins 502a do not extend all
the way
to the interior surface of the upper wall 510 of the microchannel 508. The fin
assembly 500b illustrated in Fig. 7 is similar to the fin assembly 500
illustrated in Fig.
5 except that the fins 502b in the fin assembly 500b have cross sectional
shapes in
the form of trapezoids. Each of the fins may have a height ranging from about
0.02
mm up to the height of the process microchannel 508, and in one embodiment
from
about 0.02 to about 10 mm, and in one embodiment from about 0.02 to about 5
mm,
and in one embodiment from about 0.02 to about 2 mm. The width of each fin may
range from about 0.02 to about 5 mm, and in one embodiment from about 0.02 to
about 2 mm and in one embodiment about 0.02 to about 1 mm. The length of each
fin may be of any length up to the length of the process microchannel 508, and
in
one embodiment from about 5 mm to about 500 cm, and in one embodiment about
1 cm to about 250 cm, and in one embodiment about 1 cm to about 100 cm, and in
one embodiment about 2 cm to about 25 cm. The gap between each of the fins
may be of any value and may range from about 0.02 to about 5 mm, and in one
embodiment from about 0.02 to about 2 mm, and in one embodiment from about
0.02 to about 1 mm. The number of fins in the process microchannel 508 may
range from about 1 to about 50 fins per centimeter of width of the process
microchannel 508, and in one embodiment from about 1 to about 30 fins per
centimeter, and in one embodiment from about 1 to about 10 fins per
centimeter,
and in one embodiment from about 1 to about 5 fins per centimeter, and in one
embodiment from about 1 to about 3 fins per centimeter. Each of the fins may
have
a cross-section in the form of a rectangle or square as illustrated in Figs. 5
and 6,
or a trapezoid as illustrated in Fig. 7. When viewed along its length, each
fin may
CA 02525256 2011-07-28
19
be straight, tapered or have a serpentine configuration. The fins may be made
of any
material that provides sufficient strength, dimensional stability and heat
transfer
characteristics to permit operation for which the process microchannel is
intended.
These materials include: steel (e.g., stainless steel, carbon steel, and the
like); monel;
inconel; aluminum; titanium; nickel; platinum; rhodium; copper; chromium;
brass; alloys
of any of the foregoing metals; polymers (e.g., thermoset resins); ceramics;
glass;
composites comprising one or more polymers (e.g., thermoset resins) and
fiberglass;
quartz; silicon; or a combination of two or more thereof. The fin may be made
of an
A1203 forming material such as an alloy comprising Fe, Cr, Al and Y, or a
Cr203 forming
material such as an alloy of Ni, Cr and Fe.
The catalyst may comprise Rh, Pt, Ni, Cr, Ru, Pd, Os, Ir, or an oxide thereof,
or a
mixture of two or more thereof. Partial oxidation catalysts based on one or
more of the
foregoing are disclosed in U.S. Patents 5,648,582 and 6,409,940 Bl; U.S.
Patent
Application Publications 2002/0004450 Al, 2002/0012624 Al and 2002/0115730 Al;
PCT International Publication Nos. WO 99/48805, WO 01/80992 A2 and WO
02/066403
Al; and European Patent Application Publication Nos. EP 0640561 Al, EP 0725038
Al
and EP 0741107 Al. These catalysts may be in any of the forms or supported on
any of
the support structures discussed above.
The partial oxidation catalyst may comprise platinum or an oxide thereof
deposited on a ceramic support as disclosed in U.S. Patent 5,648,582.
The partial oxidation catalyst may comprise nickel and rhodium, or oxides
thereof, deposited on a support structure made of a spine!, a perovskite,
magnesium
oxide, a pyrochlore, a brownmillerite, zirconium phosphate, magnesium
stabilized
zirconia, zirconia stabilized alumina, silicon carbide, yttrium stabilized
zirconia, calcium
stabilized zirconia, yttrium aluminum garnet, alumina, cordierite, Zr02,
MgA120, Si02 or
T102. These are disclosed in U.S. Patent 6,409,940 B1.
The partial oxidation catalyst may comprise a lanthanide-promoted rhodium
catalyst as disclosed in U.S. Patent Publication No. 2002/0115730 Al.
The partial oxidation catalyst may comprise a Ni-Cr, Ni-Co-Cr or Ni-Rh alloy
as
disclosed in U.S. Patent Publication No. 2002/0012624 Al.
CA 02525256 2011-07-28
The partial oxidation catalyst may comprise rhodium, nickel, chromium, or a
combination thereof supported on ceramic oxide fiber as disclosed in U. S.
Patent
Publication No. 2002/0004450 Al.
The partial oxidation catalyst may comprise rhodium supported on a refractory
5 oxide support as disclosed in PCT International Publication No. WO
99/48805.
The partial oxidation catalyst may comprise a rhodium gauze or rhodium felt as
disclosed in PCT International Publication No. WO 01/80992 A2.
The partial oxidation catalyst may comprise a rhodium-spinel catalyst as
disclosed in PCT International Publication No. WO 02/066403 Al.
10 The partial oxidation catalyst may comprise a Group VIII metal (e.g.,
Ru, Rh, Pd,
as, Ir, Pt) supported on a refractory oxide having at least two cations as
disclosed in
EP 0640561 Al.
The partial oxidation catalyst may comprise rhodium and/or ruthenium having a
layered hydrotalcite type structure as disclosed in EP 0725038 Al.
15 The partial oxidation catalyst may comprise a nickel-based catalyst or
ruthenium
based catalyst as disclosed in EP 0741107 A2.
The partial oxidation catalyst may comprise a composition represented by the
formula
Mia M2b M3c Ala Ox (I)
wherein in formula (I): M1 is Rh, Ni, Pd, Pt, Ru, Co or a mixture of two or
more thereof;
M2 is Ce, Pr, Tb or a mixture of two or more thereof; M3 is La, Ba, Zr, Mg, Ca
or a
mixture of two or more thereof; a is a number in the range of about 0.0001 to
about 1,
and in one embodiment 0.01 to about 1; b is a number in the range of zero to
about
0.9999, and in one embodiment zero to about 0.2; c is a number in the range of
about
0.0001 to about 0.9999, and in one embodiment about 0.01 to about
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
21
0.2; d is a number in the range of about 0.0001 to about 0.9999, and in one
embodiment about 0.1 to about 0.9; and x is the number of oxygens needed to
fulfill
the valency requirements of the elements present; the catalyst being coated on
a
substrate or supported on a foam, felt, wad or fin. In one embodiment M1 is Rh
or
Ni, and in one embodiment it is Rh. In one embodiment M3 is La or Mg, and in
one
embodiment it is La. In one embodiment the catalyst may be represented by the
formula Rh/LaAl11018 or Rh/LaA103.
In one embodiment, the process for making the catalyst represented by
formula (I) comprises the steps of: (A) applying a layer of A1203over the
native oxide
layer to form a treated support structure; (B) calcining the treated support
structure
formed in step (A); (C) applying a promoter or stabilizer to the surface of
the
calcined support structure formed in step (B), the promoter or stabilizer
comprising
La, Ba, Zr, Mg, Ca, or an oxide or nitrate thereof, or a mixture of two or
more
thereof; (D) calcining the treated support structure formed in step (C); (E)
applying
a catalytic metal, or oxide or nitrate thereof, to the surface of the calcined
support
structure formed in step (D), the catalytic metal comprising Rh, Ni, Pd, Pt,
Ru, Co,
or a mixture of two or more thereof; and (F) calcining the treated support
structure
formed in step (F) to form the supported catalyst. In one embodiment, the
catalyst
formed in step (F) may be reduced in hydrogen.
The support structure may be made of a material comprising: steel;
aluminum; titanium; iron; nickel; platinum; rhodium; copper; chromium; brass;
an
alloy of any of the foregoing metals; a polymer; ceramics; glass; a composite
comprising polymer and fiberglass; quartz; silicon; or a combination of two or
more
thereof. In one embodiment the support structure may be made of an alloy
comprising Fe, Cr, Al and Y, and the native oxide layer may comprise A1203. In
one
embodiment, the support structure may be made of an alloy comprising Ni, Cr
and
Fe, and the native oxide layer may comprise Cr203. In one embodiment, the
promoter or stabilizer may be La or Mg, and in one embodiment it is La. In one
embodiment, the catalytic metal is Rh or Ni, and in one embodiment it is Rh.
The support structure may be heated prior to step (A) to a temperature in the
range of about 300 C to about 1400 C, and in one embodiment about 700 to about
1200 C, for about 0.1 to about 1000 hours, and in one embodiment about 1 to
about
10 hours. When the support structure is made of metal, this heat treating step
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
22
advantageously provides a layer of native oxide on the surface of the support
structure.
During step (A) a slurry comprising A1203 or a colloidal dispersion (i.e., a
sol)
comprising A1203 may be applied over the native oxide layer. The slurry may
comprise about 1 to about 50% by weight A1203, up to about 20% by weight Zr02,
up to about 25% by weight La (NO3).6H20, with the remainder being water. The
slurry coating may have a thickness of about 10 to about 100 microns. The
colloidal
dispersion may contain about 1% to about 30% by weight A1203 with the
remainder
being water. The colloidal dispersion coating may have a thickness of about 1
to
about 50 microns.
During step (B) the treated support structure may be calcined in air at a
temperature in the range of about 150 C to about 1200 C, and in one embodiment
about 300 to about 700 C, for about 0.1 to about 1000 hours, and in one
embodiment about 1 to about 10 hours.
During step (C) a solution comprising La (NO3)3 may be applied to the surface
of the calcined support structure.
During step (D) the treated support structure may be calcined in air at a
temperature in the range of about 150 C to about 1200 C, and in one embodiment
about 500 to about 1100 C, for about 0.1 to about 1000 hours, and in one
embodiment about 1 to about 10 hours.
During step (E) a composition comprising Rh (NO3)3 may be applied to the
surface of the calcined support structure.
During step (F) the treated support structure may be calcined in air at a
temperature in the range of about 150 C to about 1200 C, and in one embodiment
about 400 C to about 1100 C, for about 0.1 to about 1000 hours, and in one
embodiment about Ito about 10 hours.
The combustion catalyst may comprise any combustion catalyst. These
include, for example, noble metals such as Pt, Rh, Pd, Co, Cu, Mn, Fe, Ni;
oxides
of any of these metals; perovskites and aluminates. In one embodiment, the
combustion catalyst is accompanied by an activity-enhancing promoter such as
Ce,
Tb or Pr, their oxides, and combinations thereof. In one embodiment, a
promoter
element is present in at least about 1:1 molar ratio as compared to the active
catalyst element or elements, and in one embodiment a promoter element is
present
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
23
in the range of about 0.5:1 to about 10:1 molar ratio as compared to an active
catalyst element (moles promoter(s): moles active catalyst element(s)). These
catalysts may be in any of the forms or supported on any of the support
structures
discussed above.
The contact time of the reactants and/or products with the catalyst within the
process microchannels may range up to about 500 milliseconds (ms), and in one
embodiment from about 0.1 ms to about 500 ms, and in one embodiment about 0.1
ms to about 400 ms, and in one embodiment about 0.1 ms to about 300 ms, and in
one embodiment about 0.1 ms to about 200 ms, and in one embodiment about 0.1
ms to about 100 ms, and in one embodiment from about 1 ms to about 75 ms, and
in one embodiment about 1 ms to about 50 ms, and in one embodiment about 1 ms
to about 25 ms, and in one embodiment about 1 ms to about 10 ms, and in one
embodiment about 1 ms to about 5 ms.
The space velocity (or gas hourly space velocity) for the flow of the reactant
composition and product through the process microchannels may be at least
about
100 hr-1 (normal liters of hydrocarbon/hour/liter of reaction chamber) or at
least
about 100 ml feed/(g catalyst) (hr). The space velocity may range from about
100
to about 2,000,000 hr-1 based on the volume of the process microchannels, or
from
about 100 to about 2,000,000 ml feed/(g catalyst) (hr). In one embodiment, the
space velocity may range from about 500 to about 1,000,000 hr-1, or about 500
to
about 1,000,000 ml feed/(g catalyst) (hr), and in one embodiment from about
1000
to about 1,000,000 hr-1, or from about 1000 to about 1,000,000 ml feed/(g
catalyst)
(hr).
The temperature of the reactant composition entering the process
microchannels may range from about 200 C to about 1000 C, and in one
embodiment about 150 C to about 700 C, and in one embodiment about 150 C to
about 600 C, and in one embodiment about 200 C to about 600 C. In one
embodiment the temperature may be in the range of about 150 C to about 500 C,
and in one embodiment about 150 C to about 400 C, and in one embodiment about
200 C to about 300 C. In one embodiment, the temperature may be in the range
of about 335 C to about 1000 C.
The temperature of the reactant composition and product within the process
microchannel may range up to about 1150 C, and in one embodiment up to about
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
24
1100 C, and in one embodiment up to about 1050 C, and in one embodiment up
to about 1000 C, and in one embodiment up to about 950 C, and in one
embodiment up to about 900 C, and in one embodiment up to about 850 C, and in
one embodiment up to about 800 C, and in one embodiment up to about 750 C,
and in one embodiment up to about 700 C.
The reactant composition entering the process microchannels may be at a
pressure of at least about 0.1 atmosphere, and in one embodiment at least
about
0.5 atmosphere. In one embodiment the pressure may range from about 0.1 to
about 100 atmospheres, and in one embodiment from about 0.5 to about 50
atmospheres, and in one embodiment about 1 to about 40 atmospheres, and in one
embodiment from about 1 to about 35 atmospheres.
The pressure drop of the reactants and/or products as they flow through the
process microchannels may range up to about 2 atmospheres per meter of length
of the process microchannel (atm/m), and in one embodiment up to about 1
atm/rn,
and in one embodiment up to about 0.5 atm/m, and in one embodiment up to about
0.2 atm/m.
The flow of the reactants and/or products through the process microchannels
may be laminar or in transition, and in one embodiment it is laminar. The
Reynolds
Numberfor the flow of reactants and/or products through the process
microchannels
may be up to about 4000, and in one embodiment up to about 2300, and in one
embodiment in the range of about 10 to about 2000, and in one embodiment about
100 to about 1500.
The heat exchange fluid entering the heat exchange channels may have a
temperature of about -70 C to about 650 C, and in one embodiment about 0 C to
about 500 C, and in one embodiment about 100 C to about 300 C. The heat
exchange fluid exiting the heat exchange channels may have a temperature in
the
range of about -60 C to about 630 C, and in one embodiment about 10 C to about
490 C. The residence time of the heat exchange fluid in the heat exchange
channels may range from about 1 to about 1000 ms, and in one embodiment about
1 to about 500 ms, and in one embodiment from 1 to about 100 ms. The pressure
drop for the heat exchange fluid as it flows through the heat exchange
channels may
range from about 0.05 to about 50 psi/ft, and in one embodiment from about 1
to
about 25 psi/ft. The flow of the heat exchange fluid through the heat exchange
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
channels may be laminar or in transition, and in one embodiment it is laminar.
The
Reynolds Number for the flow of heat exchange fluid flowing through the heat
exchange channels may be up to about 4000, and in one embodiment up to about
2300, and in one embodiment in the range of about 10 to about 2000, and in one
5 embodiment about 10 to about 1500.
The product exiting the microchannel reactor may be at a temperature in the
range of about 100 C to about 1000 C, and in one embodiment about 200 C to
about 800 C, and in one embodiment about 300 C to about 600 C. The product
may be cooled to a temperature in the range of about 50 C to about 300 C, and
in
10 one embodiment about 50 C to about 200 C, and in one embodiment about 50
C
to 150 C, and in one embodiment about 50 C to about 100 C, in about 5 to about
100 ms, and in one embodiment about 5 to about 75 ms, and in one embodiment
about 5 to about 50 ms, and in one embodiment about 10 to about 50 ms.
Advantages of the inventive process include: maximization of contact
15 between the hydrocarbon reactant, oxygen or source of oxygen, and the
catalyst;
and minimization of undesired reactions.
Advantages of the inventive process include the possibility of process
intensification. Conventional processes of the prior art often operate under
conditions of reactant dilution to prevent runaway reactions, while the
inventive
20 process may be operated, if desired, under more intensive conditions
leading to
greater throughput. By combining catalytic microchannel processing with heat
exchange it is possible to operate at hydrocarbon feed/oxygen ratios that
would
conventionally lead to high temperatures and loss of selectivity, but by
removing
heat rapidly through heat exchange, the temperature in the process
microchannels
25 may be maintained relatively low, for example, below about 700 C, and in
one
embodiment below about 600 C, and in one embodiment below about 500 C, thus
maximizing selectivity to desired products.
Advantages of the inventive process include the enhancement of reaction
selectivity due to the dimensions of the microchannel reactor. In reactors of
conventional dimension, reactions propagated homogeneously in the in the
gaseous
phase make a significant contribution to the overall make-up of the product.
These
reactions tend to be indiscriminate and often result in the production of
undesirable
by-products such as CO and CO2 or hydrocarbon pyrolysis products. For example,
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
26
if the reactant mixture contains propane, full and partial oxidation can take
place as
well as pyrolysis leading to the production of ethane and methane.
The level of conversion of the hydrocarbon reactant may be about 50% or
higher, and in one embodiment about 60% or higher, and in one embodiment about
70% or higher, and in one embodiment about 80% or higher.
The level of selectivity of the desired product may be about 30% or higher,
and in one embodiment about 50% or higher, and in one embodiment about 60%
or higher, and in one embodiment about 70% or higher, and in one embodiment
about 80% or higher, and in one embodiment about 85% or higher, and in one
embodiment about 90% or higher, and in one embodiment about 95% or higher. In
one embodiment, the level of selectivity to the desired product may be in the
range
of about 50% to about 95% , and in one embodiment about 75% to about 95%.
The yield of the desired product may be about 9% or higher per cycle, and
in one embodiment about 20% or higher, and in one embodiment about 40% or
higher, and in one embodiment about 50% or higher per cycle, and in one
embodiment about 70% or higher, and in one embodiment 80% or higher, and in
one embodiment about 90% or higher per cycle. The term "cycle" is used herein
to
refer to a single pass of the reactants through the process nnicrochannels.
In one embodiment, the level of conversion of the hydrocarbon reactant is at
least about 30%, the level of selectivity of the desired product is at least
about 30%,
and the yield of the desired product is at least about 9% per cycle.
In one embodiment, the process is conducted in a reactor containing a
plurality of heat exchange channels operating in parallel, the total pressure
drop for
the heat exchange fluid flowing through the heat exchange channels is up to
about
10 atmospheres, and in one embodiment up to about 5 atmospheres, and in one
embodiment up to about 2 atmospheres.
Example 1
La203 stabilized A1203 is synthesized by using a sol-gel technique as follows.
24.7 g of aluminum butoxide are dissolved into 74.5 g of 2-butanol in a beaker
with
constant stirring. In another beaker, 4.0 g of La(NO3)3.6H20 are dissolved
into 59.7
g of ethanol with constant stirring. The two solutions are mixed and stirred
for 15
min. Subsequently 4.4 g of deionized H20 are added slowly into the mixture.
The
obtained solution is heated to 80-100 C and kept it at this temperature for 2
hours.
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
27
The alcohols are vaporized during this time. The resulting solid is dried at
120 C
overnight and calcined at 1000 C for 24 hours in air at a heating and cooling
rate of
4 C/min. The resulting material has 22 wt.% La203 and 78 wt.% A1203. Its BET
surface area and pore volume are 64 m2/g and 0.35 cm3/g, respectively. The
solid
is crushed and 88-150 microns particles are chosen as catalyst support.
Rh/La203-A1203 catalyst is prepared by incipient wetness impregnation as
follows. 0.96 g of 10 wt.% Rh(NO3)3 solution are dropped onto 0.8 g of the
La203-
A1203 particles. After drying at 120 C for 1 hour, the sample is calcined at
500 C for
1 hour in air at a heating and cooling rate of 3.5 C/min. This impregnation
process
is repeated once. The catalyst is calcined at 800 C for 1 hour. The Rh loading
is
8.0 wt.%.
30 mg of catalyst are loaded in a tube reactor for testing partial oxidation
of
methane activity. The catalyst is reduced with H2 at 450 C for 30 min before
use.
The feed gas composition contains 29.6% of CH4 and 70.4% of air (CH4/02 =
2/1),
with 3.4 standard liters per minute (SLPM). The gas hourly space velocity
(GHSV)
is 5.8 x 106h-1. CH4 conversion is calculated by the difference in methane
flow rates
before the reaction and after the reaction. CO selectivity is obtained by
[CON[CO]
+ [CO2]) and H2 selectivity is calculated by [H2]/([H21 + [H20]). At a tube
skin
temperature of 700 C, 88% of CH4 conversion, 97% of CO selectivity and 91% of
H2 selectivity are obtained. 02 conversion is 100%.
Example 2
La203 stabilized A1203 was synthesized by a sol-gel technique as follows.
24.7 g of aluminum butoxide are dissolved into 74.5 g of 2-butanol in a beaker
with
stirring. In another beaker, 4.0 g of La(NO3)3.6H20 are dissolved into 59.7 g
of
ethanol with stirring. The two solutions are mixed and stirred for 15 min.
Subsequently 4.4 g of deionized H20 are added slowly into the mixture. The
obtained solution is heated to 80-100 C and maintained at this temperature for
2
hours. The alcohols are vaporized during this time. The resulting solid is
dried at
120 C overnight and calcined at 1000 C for 24 hours in air at a heating and
cooling
rate of 4 C/min. The resulting material contains 22 wt.% La203 and 78 wt.%
A1203.
Its BET surface area and pore volume are 64 m2/g and 0.35 cm3/g, respectively.
The solid is crushed and 88-150 microns particles are chosen as catalyst
support.
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
28
Rh/La203-A1203 catalyst is prepared by incipient wetness impregnation as
follows. 0.96 g of 10 wt.% Rh(NO3)3 solution are dropped onto 0.8 g of the
La203-
, A1203 particles. After drying at 120 C for 1 hour, the sample is
calcined at 500 C for
1 hour in air at a heating and cooling rate of 3.5 C/min. This impregnation
process
is repeated once. The catalyst is calcined at 1000 C for 1 hour. The Rh
loading is
8.0 wt. /0.
30 mg of catalyst are loaded in a tube reactor for testing partial oxidation
of
methane activity. The catalyst is reduced with H2 at 450 C for 30 min before
use.
The feed gas compositions are 29.6% of CH4 and 70.4% of air (CH4/02 = 2/1),
with
3.4 SLPM. GHSV is 5.8 x 106 h-1. The tube skin temperature is 700 C. 90% of
CH4
conversion, 97% of CO selectivity and 87% of H2 selectivity are obtained. 02
conversion is 100%. The process is conducted for 260 hours. The results are
disclosed in Fig. 8.
These tests results indicate that this catalyst is very stable. As shown in
Fig.
8, CH4 conversion, CO selectivity and H2 selectivity are substantially
unchanged
during 260 hours time-on-stream. These results demonstrate that the Rh/La203-
A1203 catalyst is highly active for the partial oxidation of methane to CO and
H2 at an
extremely high space velocity.
Example 3
Fig. 7 shows the geometry of a fin that is useful for conducting a partial
oxidation reaction process in a process microchannel. The trapezoidal shape of
the
fins provides mechanical rigidity at the base of fins. All the fins are
supported on
rectangular base to enhance heat transfer characteristics of the fin. The fin
is
fabricated from FeCrAlY using the Wire EDM method. The following table
summarizes dimensions of the fin:
Dimension (in)
Fin thickness
At base 0.005
At top 0.002
Fin spacing
At base 0.012
At top 0.017
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
29
Fin height 0.029
Rectangular base 0.020
height
Overall width 0.180
Overall Height 0.049
Overall length 1.500
An A1203 slurry is prepared by mixing 7.2 g of gamma A1203 powder, 12 g of
deionized H2O and 42 g A1203 beads with 3 mm diameter. The pH value is
adjusted
to 3.5-4 using nitric acid. The A1203 is acidic gamma A1203 which is ground to
powder smaller than 150 micrometers. The mixture is ball-milled for 8 hours.
0.8
g of 25 wt.% A1203 sol (Sasol 14N4-25) is added to 4.2 g of the slurry with
stirring.
The FeCrAlY fin is cleaned in iso-propanol for 20 min with sonication. After
drying at 100 C for 1 h and cooling to room temperature, the fin is cleaned in
20
wt.% HNO3 solution for 20 min with sonication. The fin is then rinsed with
deionized
water until the pH value is 7. After drying at 120 C for 1 hour, the fin is
heated to
1000 C in air at a heating rate of 3.5 C/rnin and calcined at 1000 C for 8
hours in air.
A dense A1203 layer is generated after the calcination. The A1203 layer
functions as
a protection scale and also improves the adhesion between the coating and the
fin.
The A1203 slurry is washcoated onto the fin by dipping. The excess slurry is
removed by jetting air over the coated surface. The fin is dried at 120 C for
1 hour
and then calcined at 450 C for 4 hours at a heating and cooling rate of 3.5
C/min.
A 7.5 wt.% La(NO3)3solution is impregnated onto the fin by dipping. The fin is
dried
at 120 C for 1 hour and then calcined at 1000 C for 4 hours in air at a
heating and
cooling rate of 3.5 C/min. The La203 on the surface stabilizes the A1203. The
slurry
loading is 25.4 mg per fin. A 10 wt.% Rh(NO3)3 solution is dropped onto the
fin and
the excess solution is blown out by compressed air. The resulting fin
supported
catalyst is dried at 120 C for 1 hour and then calcined at 1000 C for 1 h in
air. The
Rh loading is 4.8 mg per fin.
The fin supported catalyst is tested for partial oxidation of methane to
syngas
at 1 atmosphere in a pellet. The pellet is a cylindrical metal rod having a
diameter
of 0.5 inch and a length of 2 inches. The pellet has a rectangular
microchannel cut-
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
away in its center. The cut-away extends through the rod along its interior
axis. The
cut-away has a height of 0.05 inch and a width of 0.18 inch. The fin supported
catalyst is placed in the cut-away for testing. Gas tight connections are made
on
each side of the cut-away. The reactants flow through tubing to the cut-away,
and
5 through the cut-away in contact with the fin supported catalyst. The
pellet is placed
in a furnace. The temperature of the furnace is increased to keep the pellet
outside
skin temperature at mid-length at 850 C. The temperature of the feed stream at
the
inlet of the furnace is at room temperature and is preheated before entering
the
pellet. The length of the tubing from the entrance of the furnace to the
pellet is 10
10 feet. The outlet pressure of the product stream is atmospheric pressure.
The
pressure drop in the pellet is measured using a Capsuhelic differential
pressure
gauge. The composition of the product is analyzed with a two-column Gas
Chromatograph. The performance of the fin supported catalyst is measured in
terms of CH4 conversion, H2 selectivity and CO selectivity.
15 CH4 Conversion (%) = (VcH4, in ¨VcH4, out)/(VcH4, in) x 100
H2 Selectivity (%) = (VH2, out, actual)/(VH2, out, theoretical) x 100
CO Selectivity (%) = (Vco, out)/(V0, out
+ V02, out) x 100
The catalyst is reduced with H2 at 400 C for 30 min before use. The feed gas
compositions are 29.6% of CH4 and 70.4% of air (CH4/02 = 2/1), with 2030
ml/min
20 of total flow rate (standard conditions). The contact time is 3.3 ms.
The contact time
is defined as the ratio of flow volume in the pellet without the fin to the
volumetric
flow rate. The following table summarizes the fin supported catalyst
performance
after 157 hours of operation.
Parameter Value
25 Coating Type Powder slurry
wash-coat
Fuel composition 29.6% CH4,
70.4% air
Fuel contact time 3.3 ms
CH4 Conversion (at 850 C) 85%
H2 Selectivity (at 850 C) 92%
30 CO Selectivity (at 850 C) 95%
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
31
Pressure drop 5.6 psi
Example 4
An alternate fin for use in a partial oxidation reaction process provides the
advantage of reduced pressure drop. The flow area is increased by reducing
number of fins. There are five fins projecting up from the fin support. The
fins have
a trapezoidal cross section as indicated in Fig. 7. The thickness of the fin
along with
trapezoidal shape of the fins provides mechanical rigidity at the base of the
fins.
The fins are supported on rectangular support or base to enhance heat transfer
characteristics of the fin. The fin is made from FeCrAlY. The fin is
fabricated by
the wire EDM method. The following table summarizes dimensions of the fin:
Dimension (in)
Fin Thickness
At base 0.020"
At top 0.010"
Fin spacing
At base 0.012"
At top 0.022"
Fin height 0.033"
Rectangular base height 0.020"
Overall width 0.180"
Overall height 0.053"
Overall length 1.500"
An A1203 slurry is prepared by mixing 7.2 g of gamma A1203 powder, 12 g of
deionized H2O and 42 g A1203 beads with 3 mm diameter. The pH value is
adjusted
to 3.5-4 using nitric acid. The A1203 is acidic gamma A1203 and is ground to
powder
smaller than 150 micrometers. The mixture is then ball-milled for 8 hours. 0.8
g of
25 wt.% A1203 sol (Sasol 14N4-25) is added to 4.2 g of the slurry with
stirring.
The FeCrAlY fin is cleaned in iso-propanol for 20 min with sonication. After
drying at 100 C for 1 hour and cooling to room temperature, the fin is cleaned
in 20
wt.% HNO3 solution for 20 min with sonication. The fin is rinsed with
deionized
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
32
water until the pH value is 7. After drying at 120 C for 1 hour, the fin is
heated to
1000 C in air at a heating rate of 3.5 C/min and calcined at 1000 C for 8
hours in
air. The A1203 slurry is washcoated onto the fin by dipping. The excess slurry
is
removed by jetting air over the coated surface. The fin is dried at 120 C for
1 hour
and then calcined at 450 C for 4 hours at a heating and cooling rate of 3.5
C/min.
A 7.5 wt.% La(NO3)3 solution is impregnated onto the slurry-coated fin by
dipping.
The fin is dried at 120 C for 1 hour and calcined at 1000 C for 4 hours in air
at a
heating and cooling rate of 3.5 C/min. The slurry loading is 6.0 mg per fin. A
10
wt.% Rh(NO3)3 solution is dropped onto the fin and the excess solution is
blown out
by compressed air. The fin is dried at 120 C for 1 hour and then calcined at
1000 C
for 1 hour in air. The Rh loading is 1.0 mg per fin.
The resulting fin supported catalyst is tested for partial oxidation of
methane
to syngas at 1 atmosphere in the pellet described in Example 3. The pellet is
placed
in a furnace. The temperature of the furnace is adjusted to keep the pellet
skin
temperature at mid-length at 805 C. The temperature of the feed stream at the
inlet
of furnace is at room temperature. The feed stream is preheated before
entering
the pellet. The length of tubing from the entrance of furnace to the pellet is
10 feet.
The outlet pressure of the product stream is atmospheric pressure. The
pressure
drop in the pellet is the difference between the inlet and the outlet
pressures. The
composition of product is analyzed with a two-column Gas Chromatograph. The
performance of the fin is measured in terms of CH4 conversion, H2 selectivity
and
CO selectivity. The following table summarizes catalyst performance for the
fin after
115 hours of operation.
Parameter Value
Coating Type Powder slurry wash-coat
Fuel composition 29.6% CH4, 70.4% air
Fuel contact time 3.3 ms
CH4 Conversion (at 850 C) 78%
H2 Selectivity (at 850 C) 93%
CO Selectivity (at 850 C) 93%
Pressure drop 2.8 psi
Example 5
An FeCrAlY fin is fabricated with saw-cut method and tested for catalyst
performance. The following table summarizes dimensions of the fin:
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
33
Dimension (in)
Fin Thickness
At base 0.010"
At top 0.005"
Fin spacing
At base 0.017"
At top 0.022"
Fin height 0.033"
Rectangular base height 0.020"
Overall width 0.180"
Overall height 0.053"
Overall length 1.500"
An A1203 slurry is prepared by mixing 7.2 g of gamma A1203 powder, 12 g of
deionized H2O and 42 g A1203 beads with 3 mm diameter. The pH value was
adjusted to 3.5-4 using nitric acid. The A1203 is acidic gamma A1203, is
ground to
powder smaller than 150 micrometers. The mixture is then ball-milled for 8
hours.
0.8 g of 25 wt.% A1203 sol (Sasol 14N4-25) is added to 4.2 g of the slurry
with
stirring.
The FeCrAlY fin is cleaned in iso-propanol for 20 min with sonication. After
drying at 100 C for 1 hour and cooling to room temperature, the fin is cleaned
in 20
wt.% HNO3 solution for 20 min with sonication. The fin is then rinsed with
deionized
water until pH value reaches 7. After drying at 120 C for 1 hour, the fin is
heated to
1000 C in air at a heating rate of 3.5 C/min and calcined at 1000 C for 8
hours in air.
The A1203 slurry is washcoated onto the fin by dipping. The excess slurry is
removed by jetting air over the coated surface. The fin is dried at 120 C for
1 hour
and then calcined at 450 C for 4 hours at a heating and cooling rate of 3.5
C/min.
7.5 wt.% La(NO3)3 solution is impregnated onto the slurry-coated fin by
dipping. The
fin is dried at 120 C for 1 hour and calcined at 1000 C for 4 hours in air at
a heating
and cooling rate of 3.5 C/min. The slurry loading is 18.7 mg per fin. 10 wt.%
Rh(NO3)3 solution is dropped onto the fin and the excess solution is blown out
by
compressed air. The fin is dried at 120 C for 1 hour and calcined at 1000 C
for 4
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
34
hours in air. The Rh loading is 3.2 mg per fin.
The resulting fin supported catalyst is tested for partial oxidation of
methane
at 1 atmosphere in the pellet described in Example 3. The pellet is placed in
a
furnace. The catalyst is reduced with H2 at 400 C for 30 min before use. The
feed
gas compositions are 29.6% of CH4 and 70.4% of air (CH4/02 = 2/1), with 2372
ml/min of total flow rate (standard conditions). The contact time is 3.3. The
temperature of the furnace is adjusted to keep the pellet skin temperature at
mid-
length at 850 C. The temperature of the feed stream at the inlet of furnace is
at
room temperature. The feed stream is preheated before entering pellet. The
length
of tubing from the entrance of furnace to the pellet is 10 feet. The outlet
pressure
of the product stream is atmospheric pressure. The pressure drop in the pellet
is
measured by a capsuhelic differential pressure gauge. The composition of
product
is analyzed with a two-column Gas Chromatograph. The performance of the fin is
measured in terms of CH4 conversion, H2 selectivity and CO selectivity. The
following table summarizes the fin supported catalyst performance after 400
hours
of operation.
Parameter Value
Coating Type Powder slurry wash-coat
Fuel composition 29.6% CH4, 70.4% air _
Fuel contact time 3.3 ms
CH4 Conversion (at 850 C) 75%
H2 Selectivity (at 850 C) 72%
CO Selectivity (at 850 C) 91%
Pressure drop 2.1 psi
Example 6
A fin having the same dimensions as the fin in Example 5 is cleaned in iso-
propanol for 20 min with sonication. After drying at 100 C for 1 hour and
cooling to
room temperature, the fin is cleaned in 20 wt.% HNO3 solution for 20 min with
sonication. The fin is rinsed with deionized water until the pH value reaches
7. After
drying at 120 C for 1 hour, the fin is heated to 1000 C in air at a heating
rate of
3.5 C/min and calcined at 1000 C for 8 hours in air. A dense A1203 layer is
generated after calcination. The A1203 layer functions as a protection scale
and also
improves the adhesion between the coating and the fin. A1203 sol (25 wt.%,
Sasol
14N4-25) is coated onto the fin by dipping. The excess sol is removed by
jetting air
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
over the coated surface. The fin is dried at 120 C for 1 hour and calcined at
450 C
for 4 hours at a heating and cooling rate of 3.5 C/min. The sot coating
process is
repeated 3 to 4 times until 17 mg of A1203 loading per fin is achieved. 7.5
wt.%
La(NO3)3 solution is impregnated onto the fin by dipping. The fin is dried at
120 C
5
for 1 hour and calcined at 1000 C for 4 hours in air at a heating and cooling
rate of
3.5 C/min. 10 wt.% Rh(NO3)3 solution is dropped onto the fin and the excess
solution is blown out by compressed air. The fin is dried at 120 C for 1 hour
and
calcined at 500 C for 1 hour in air. The Rh(NO3)3 solution coating is repeated
once
and the fin is calcined at 1000 C for 4 hours. The Rh loading is 5.2 mg per
fin.
10
The resulting fin supported catalyst is tested for partial oxidation of
methane
to syngas at 1 atmosphere using the pellet described in Example 3. The pellet
is
placed in a furnace. The catalyst is reduced with H2 at 450 C for 30 min
before use.
The feed gas compositions were 29.6% of CH4 and 70.4% of air (CH4/02= 2/1),
with
2361 ml/min of total flow rate (standard conditions). The contact time is 3.3
ms. The
15
temperature of the furnace is adjusted to keep the pellet skin temperature at
mid-
length at 800 C. The temperature of the feed stream at the inlet of the
furnace is
at room temperature. The feed stream is preheated before entering the pellet.
The
length of tubing from the entrance of furnace to the pellet is ten feet. The
outlet
pressure of the product stream is atmospheric pressure. The pressure drop in
the
20
pellet is measured by capsuhelic differential pressure gauge. The composition
of
product is analyzed with two-column Gas Chromatograph. The performance of the
fin is measured in terms of CH4 conversion, H2 selectivity and CO selectivity.
The
performance of the fin supported catalyst after 600 hours of steady-state
operation
is indicated below.
Parameter Value
Coating Type Sol wash-coat
Fuel composition 29.6% CH4, 70.4% air
Fuel contact time 3.3 ms
CH4 Conversion (at 800 C) 71%
H, Selectivity (at 800 C) 70%
CO Selectivity (at 800 C) 87%
Pressure drop 1.4 psi
The foregoing fin supported catalyst is tested with an n-butane and CH4 fuel
mixture. The feed gas contains 7.2% CH4, 7.2% n-butane and 85.6% air with a
total
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
36
flow rate of 2091 ml/min. A four column gas chrornatograph is used to analyze
the
outlet gas composition. The temperature of the furnace is adjusted to keep
pellet
skin temperature at mid-length at 800 C. The performance of the fin supported
catalyst after 300 hours of operation is summarized below.
Parameter Value
CoatingType Powder slurry
wash-coat
7.5% CH, 7.5% n-butane,
Fuel composition
85% air
Fuel contact time 3.3 ms
CH4 Conversion (at 800 C) 60%
n-butane conversion (at 800 C) 76%
H2 Selectivity (at 800 C) 77%
CO Selectivity (at 800 C) 82%
Pressure drop 1.0 psi
Example 7
A fin having the same dimensions as the fin in Example 3 is cleaned in iso-
propanol for 20 min with sonication. After drying at 100 C for 1 hour and
cooling to
room temperature, the fin is cleaned in 20 wt.% HNO3 solution for 20 min with
sonication. The fin is rinsed with deionized water until the pH value reaches
7. After
drying at 120 C for 1 hour, the fin is heated to 1000 C in air at a heating
rate of
3.5 C/min and calcined at 1000 C for 8 hours in air. A dense A1203 layer is
generated after calcination. The A1203 layer functions as a protection scale
and also
improves the adhesion between the coating and the fin. A1203 sol (25 wt.%,
Sasol
14N4-25) is coated onto the fin by dipping. The excess sol is removed by
jetting air
over the coated surface. The fin is dried at 120 C for 1 hour and calcined at
450 C
for 4 hours at a heating and cooling rate of 3.5 C/min. The sol coating
process is
repeated 4 to 5 times until 22 mg of A1203 loading per fin is achieved. 7.5
wt. /0
La(NO3)3 solution is impregnated onto the fin by dipping. The fin is dried at
120 C
for 1 hour and calcined at 1000 C for 4 hours in air at a heating and cooling
rate of
3.5 C/min. 10 wt.% Rh(NO3)3 solution is dropped onto the fin and the excess
solution is blown out by compressed air. The fin is dried at 120 C for 1 hour
and
calcined at 1000 C for 1 hour in air. The Rh loading is 1.5 mg per fin.
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
37
The resulting fin supported catalyst is tested for partial oxidation of
methane
to CO and H2 at 1 atmosphere using the pellet described in Example 3. The
pellet
is placed in a furnace. The catalyst is reduced with H2 at 450 C for 30 min
before
use. The feed gas composition contains 29.6% of CH4 and 70.4% of air (CH4/02 =
2/1), with 2030 nril/min of total flow rate (standard conditions). The
temperature of
the furnace is adjusted to keep the pellet skin temperature at mid-length at
850 C.
The temperature of the feed stream at the inlet of the furnace is at room
temperature. The feed stream is preheated before entering the pellet. The
length
of tubing from the entrance of furnace to the pellet is ten feet. The outlet
pressure
of the product stream is atmospheric pressure. The contact time is 3.3 ms. The
pressure drop in the pellet, which is measured by capsuhelic differential
pressure
gauge, is 3.7 psi. The composition of product is analyzed with two-column Gas
Chromatograph. The performance of the fin is measured in terms of CH4
conversion, H2 selectivity and CO selectivity. The results are shown in Fig.
9.
The test results indicate that this catalyst is stable. As shown in Fig. 9,
CH4
conversion, CO selectivity and H2 selectivityare substantially unchanged
during 840
hours time-on-stream.
Example 8
A welded Inconel reactor is fabricated to test methane combustion
performance. The reactor includes two parallel channels for the combustion.
Each
channel is 0.160" wide and 0.025" tall. The length of reactor is 7.00". The
channels
are separated by 0.060" rib between them. On one side of the combustion
channels,
an identical pair of channels (referred as air channels) is placed to flow air
required
for combustion of fuel. The combustion and air channels are separated by an
orifice
plate with 12 circular orifices (0.012" diameter) spaced along the reactor
length to
distribute air into the fuel. The orifices are non-uniformly spaced to
distribute air in
the combustion channel. The first orifice is placed at the beginning of the
reactor.
The subsequent orifices are placed at distances of 0.252", 0.555", 0.905",
1.304",
1.751", 2.248", 2.794", 3.393", 4.047", 4.760", and 5.528" from the first
orifice. On
the other side of the combustion channel, a single heat exchange channel is
placed
to carry fluid which acts as a sink for combustion heat. The channel is 0.380"
wide
and 0.012" tall. The length of the channel is the same as the combustion
channel
length. The arrangement of different channels is shown in Fig. 10.
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
38
The combustion channels are coated with a combustion catalyst with solution
coating. The device is first calcined in air at 1000 C for 1 hour to generate
a
chromia layer on the surface. The heating and cooling rate is 3.5 C/min.
Subsequently, a solution containing 5.7 wt.% of Pd(NO3)2 and 43 wt.% of
Ce(NO3)3.6H20 is doped onto the channels. The excess solution is blown out by
compressed air. The coated channels are then dried at 100 C for 1 hour. The Pd
coating process is repeated twice. The coated channels are then calcined in
air at
850 C for 1 hour.
The fuel used for the combustion is methane. The total flow rate of methane
in the combustion channels is 1.0 standard liters per minute (SLPM). The total
air
flow rate in the air channels is 11.5 SLPM. The air is preheated to reactor
temperature before mixing it in fuel. The heat sink is provided by a steam
methane
reforming reaction. The sink channel (referred as SMR channel) is coated with
a
steam methane reforming (SMR) catalyst. A mixture of 1.09 SLPM and 2.63 cc of
water vapors are flowed through SMR channel. The inlet temperature of flow in
SMR
channel is between 800 C and 850 C. The average temperature of the combustion
channel is between 850 C and 925 C. Based on the volume of combustion channel,
the contact time is 4.4 ms. The methane conversion in the combustion channel
is
calculated as:
CH4 Conversion (%) (VcH4, in ¨\/cH4, out)/(VcH4, in) x 100
For the combustion catalyst, the methane conversion is 30.6% at an average
temperature of 862 C. For this device an average of 9.3 W/cm2 is transferred
to the
SMR reaction. The pressure drop in the combustion channel is between 2.5 and
5.0
psi.
Example 9
Another Inconel reactor device is fabricated to test combustion performance
using a supported partial oxidation catalyst. The device has same combustion
and
air channel dimensions as microchannel reactor used in Example 8 except for
the
total partial oxidation and combustion channel length. A serrated metal sheet
is used
as a fin as shown in Fig. 11. Two fins are introduced at the beginning of the
two
partial oxidation and combustion channels. The total length of the partial
oxidation
and combustion channel is 8.5" to accommodate the fin, where the fin is 1.5"
long
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
39
and the subsequent combustion channel is 7" long. The fin is made of FeCrAIY.
The dimensions of the fin are summarized in the table below.
Dimensions
Overall 1.500"
Length
Overall width 0.180"
Overall height 0.022"
Fin thickness 0.002"
Fins per inch 60
The fins are coated with a partial oxidation catalyst to convert methane to CO
and H2 before combustion. The fins are cleaned in iso-propanol for 20 min with
sonication. After drying at 100 C for 1 hour and cooling to room temperature,
the
fins are cleaned in 20 wt.% HNO3 solution for 20 min with sonication. The fins
are
then rinsed with deionized water until pH value is 7. After drying at 120 C
for 1 hour,
the fins are heated to 1000 C in air at a heating rate of 3.5 C/min and
calcined at
1000 C for 8 h in air. A dense A1203 layer is generated after the calcination
and the
A1203 layer functions as a protection scale and also improves the adhesion
between
the coating and the fins. Also, an A1203 and ZrO2 containing slurry is
prepared for
coating. 10 g ZrO2 powder, 55 g of deionized H2O, 1.2 ml of concentrated
nitric acid
and 200 g A1203 beads with 3 mm diameter are mixed in a container. The mixture
is then ball-milled for 2 days. After that, 2.0 g of ZrO2 slurry, 0.54 g of
gama-A1203
powder, 0.46 g of La(NO3)3.6H20 and 0.5 g of H2O are mixed with stirring. The
A1203, which is acidic gamma A1203, is ground to a powder smaller than 53
microns.
Subsequently, the above A1203¨ZrO2 slurry is washcoated onto the fins by
dipping.
The slurry-coated fins are dried at 120 C for 1 hour and then calcined at 1000
C for
1 hour at a heating and cooling rate of 3.5 C/min. The slurry loading is 6.4
mg per
fin. After that, 10 wt. /0 Rh(NO3)3 solution is dropped onto the fins and the
excess
solution is blown out by compressed air. Finally the slurry-coated catalysts
are dried
at 120 C for 1 hour and then calcined at 500 C for 1 hour in air. The Rh
loading is
around 0.6 mg per fin.
CA 02525256 2005-11-08
WO 2004/103549
PCT/US2004/010611
In the combustion channels, the orifice plate for distributing air into the
fuel
is modified by increasing the number of orifices to 17 and introducing non-
circular
orifices. The first orifice is placed in the combustion channel at a distance
between
0.01" and 0.20" after the partial oxidation zone. The first orifice consists
of
5 rectangular slots with semi-circular ends of diameter 0.012". The
longest length of
the slot is in the direction of flow. The second orifice is equilateral
triangular in shape
with 0.012" side length and is placed at a distance of 0.133" from first
orifice. The
third & fourth orifices are of 0.012" diameter holes placed 0.267" from first
orifice.
The fifth orifice is again a triangular slot placed 0.386" from the first
orifice. Orifice
10 six to fifteen are circular holes with diameter 0.012" and are placed
at 0.594",
0.769", 0.969", 1.168", 1.615", 2.112", 2.658", 3.257", 3.257", 3.857", 4.624"
from
the first orifice. Orifice sixteen and seventeen are 0.012" diameter holes
place
5.392" from first orifice. This pattern of orifices provides an ideal oxygen
equivalence ratio of 0.5, defined as:
Y02
15 4.02 = v
"L 02 + Y02,stoic
Where Y02 is the mole fraction of oxygen and Y02,st01, is stoichiometric
oxygen mole
fraction necessary for complete combustion.
The combustion channels are coated with combustion catalyst. The device
is first calcined in air at 1000 C for 3 h to generate a chromia layer on the
surface.
20 The heating and cooling rate is 3.5 C/min. 10 wt.% Rh(NO3)3 solution is
then
dropped onto the combustion channels and the excess solution is blown out by
compressed air. After drying at 100 C for 1 hour, the coated channels are
calcined
at 800 C for 1 hour in air. Subsequently, a solution containing 5.7 wt.% of
Pd(NO3)2
and 43 wt.% of Ce(NO3)3.6H20 is doped onto the channels. The excess solution
is
25 blown out by compressed air. The coated channels are then dried at 100
C for 1
hour. The Pd coating process is repeated once. The coated channels are then
calcined at 1000 C for 1 h. Finally, 10 wt.% Pt(NH3)4(NO3)2 solution is
dropped onto
the channels. After drying at 100 C for 1 hour, the coated channels are
calcined at
900 C for 1 hour in air.
30 The reactor performance with integrated partial oxidation and
combustion
reaction is tested. The total flow rate of methane in the two combustion
channels is
1.33 SLPM. The methane is premixed with air to have CH4:02 ratio of 2:1 in the
CA 02525256 2012-12-14
41
partial oxidation channels. The total flow rate of air in the air channels is
10.9 SLPM.
The air is preheated to the reactor temperature before mixing into fuel. The
heat
sink is provided by a steam methane reforming reaction. The sink channel
(referred
as SMR channel) is coated with steam methane reforming (SMR) catalyst. A
mixture
of 2.18 SLPM and 5.27 cc of water vapors flow through the SMR channel. The
inlet
temperature of flow in SMR channel is between 800 C and 850 C. The average
partial oxidation zone temperature is between 750 C and 800 C and average
combustion zone temperature is between 850 C and 925 C. Based on the volume
of combustion channels, the contact time in combustion channels is 4.5 ms. The
total CH4 conversion is 92.2%, an increase of 61.6% as compared to that
without
partial oxidation catalyst. This demonstrates that partial oxidation assists
methane
combustion significantly. For this device an average of 18.8 IN/cm2 is
transferred to
the SMR reaction. The pressure drop in the combustion channel is between 2.5
and
5.0 psi.