Note: Descriptions are shown in the official language in which they were submitted.
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
PYRAZOLE-AMIDES AND SULFONAMIpES
AS SODIUM CHANNEL MODULATORS
Technical Field
The present invention relates to certain pyra~ole-amide and pyra~,ole-
sulfonamide
compounds that modulate PN3 and are useful for treating neuropathic pain.
Background of the Invention
Sodium channel Mockers are effective in the treatment of various disease
states
including neuropathic pain. Neuropathic pain can be described as pain
associated with
damage or permanent alteration of the peripheral or central nervous system.
Clinical
manifestations of neuropathic pain include a sensation of burning or electric
shock, feelings
of bodily distortion, allodynia, and hyperalgesia.
Sodium channel-blocking agents selectively suppress abnormal ectopic neural
firing
in injured peripheral and central neurons. Alterations in either the level of
expression or
distribution of sodium channels within an injured nerve, therefore, have a
major influence on
the pathophysiology of pain associated with this type of trauma.
Navl.8 (also known as PN3) is a member of a family of voltage-gated sodium
channels. PN3-nulled mutant mice exhibit a pronounced analgesia to mechanical
noxious
stimuli. Selective "knock down" of PN3 protein in the rat dorsal root ganglion
with specific
antisense oligodeoxynucleotides prevents hyperalgesia and allodynia caused by
either chronic
nerve or tissue injury. In both human and animal models of neuropathic pain,
there is an
increased expression of PN3 at the site of peripheral nerve injury.
Patients with neuropathic pain do not respond to non-steroidal anti-
inflamatory drugs
and resistance or insensitivity to opiates is common. Gabapentin is the market
leading
treatment for neuropathic pain; its mechanism of action for pain is unknown.
As few as 30%
of patients respond to gabapentin treatment.
In view of the limited number of agents presently available and the low levels
of
efficacy of the available agents, there is a pressing need for compounds that
are potent,
specific inhibitors of ion channels implicated in neuropathic pain. The
present invention
provides such compounds, methods of using them, and compositions that include
the
compounds.
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
SUMMARY OF THE INVENTION
The present invention discloses pyrazole-amides and pyrazole-sulfonamides, a
method for modulating PN3 in mammals using these compounds, a method for
controlling
pain in mammals, and pharmaceutical compositions including those compounds.
More
particularly, the present invention is directed to compounds of formula (I)
N~R2
R1-N~~~ Rs
R4
(I),
or a pharmaceutically acceptable salt, amide, ester, or prodrug thereof,
wherein
RI is alkyl, aryl, arylalkyl, cycloalkyl, cycloalkylalkyl, haloalkyl,
heterocycle,
heterocyclealkyl, heteroaryl, or heteroarylalkyl;
RZ and R3 are independently hydrogen, alkenyl, alkoxy, alkoxyalkyl,
alkoxycarbonyl,
alkyl, alkylcarbonyl, alkylcarbonyloxy, alkylthio, alkynyl, aryl, arylalkyl,
carboxy,
cycloalkyl, cycloalkylalkyl, cyano, formyl, haloalkoxy, haloalkyl, halogen,
hydroxy,
hydroxyallcyl, mercapto, nitro, -NRARB, or (NRRRB)carbonyl;
RA and RB are independently hydrogen, alkyl, or alkylcarbonyl;
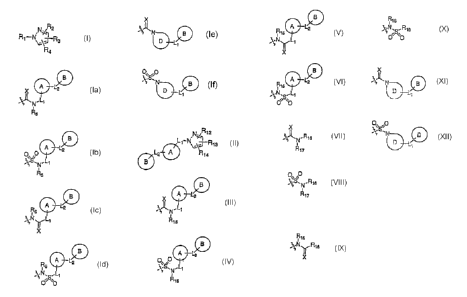
R4 is
B B
A L2 A L2 A L
A L
.L~ O~S~ .L~ .N5 LT R5 2
N -~ N :~ ~ ~N,S,.Li
is ~~
Rs . R5 , X . O O ,
X
II O~ ,O
~~N B ~S~N B
or
XisOorS;
RS is hydrogen, alkyl, alkylcarbonyl, allcylcarbonyloxy, or heterocycleallcyl;
LI is a bond or allcylene;
La is a bond or alkylene;
A is aryl, cycloalkyl, heteroaryl, or heterocycle;
B is aryl, cycloallcyl, heteroaryl, or heterocycle;
2
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
;, "..~ .. .
D is heterocycle wherein the heterocycle is azetidinyl, azepanyl, aziridinyl,
azocanyl,
1,1-dioxidothiomorpholinyl, morpholinyl, piperazinyl, piperidinyl,
pyrrolidinyl, or
thiomorpholinyl, wherein the heterocycle is optionally substituted with 1, 2,
3, or 4
substitutents independently selected from alkenyl, alkoxy, alkoxyalkyl,
alkoxycarbonyl,
alkoxysulfonyl, alkyl, alkylcarbonyl, alkylcarbonyloxy, alkylsulfonyl,
alkynyl, carboxy,
cyano, formyl, haloalkoxy, haloalkyl, halo, hydroxy, hydroxyalkyl, mercapto, -
NRARB,
(NRARB)carbonyl, (NRARB)sulfonyl.
In another embodiment, the present invention discloses compounds of formula
(II)
that modulate PN3 in mammals and are useful for controlling pain in mammals,
N~R12
Li-N~~~ Ris
L2-t' H ) R 14
or a pharmaceutically acceptable salt, amide, ester, or prodrug thereof,
wherein
R12 and R13 are independently hydrogen, alkenyl, alkoxy, alkoxyalkyl,
alkoxycarbonyl, alkyl, alkylcarbonyl, alkylcarbonyloxy, alkylthio, alkynyl,
aryl, arylalkyl,
carboxy, cycloalkyl, cycloalkylalkyl, cyano, formyl, haloalkoxy, haloalkyl,
halogen, hydroxy,
hydroxyalkyl, mercapto, nitro, -NRARB, or (NRARB)carbonyl;
RA and RB are independently hydrogen, alkyl, or alkylcarbonyl;
R14 is
B B
A L2 A L2 A L
A L
~~N~Li ~SO ~L1 .N15 L1 .N15 L 2
1
R15 , R15 , X , ~ 0 S ~ ,
II O R15 R15
,~~N.Ris ,~S~N.R16 ~N~Ri$
18
R17 , R17 , X ,
II ~~ s~
~~N B ~S~N B
~L1 ~L1
or
3
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
,. a.,.~. ,. ..
XisOorS;
Rrs is hydrogen, alkyl, alkylcarbonyl, alkylcarbonyloxy, or heterocyclealkyl;
R16 and RI~ are independently hydrogen, alkenyl, alkoxy, alkyl, aryl,
arylalkyl,
cycloalkyl, cycloalkylalkyl, heteroaryl, heteroarylalkyl, heterocycle, or
heterocyclealkyl;
Rr$ is alkyl, alkenyl, aryl, arylalkyl, cycloalkyl, cycloalkylalkyl,
heteroaryl,
heteroarylalkyl, heterocycle, heterocyclealkyl, -NRARB, or (NRARB)alkyl;
Ll is a bond or alkylene;
LZ is absent, a bond, or alkylene;
E1 is aryl, cycloalkyl, heteroaryl, or heterocycle;
B is absent, aryl, cycloalkyl, heteroaryl, or heterocycle;
D is heterocycle wherein the heterocycle is azetidinyl, azepanyl, aziridinyl,
azocanyl,
1,1-dioxidothiomorpholinyl, morpholinyl, piperazinyl, piperidinyl,
pyrrolidinyl, or
thiomorpholinyl, wherein the heterocycle is optionally substituted with 1, 2,
3, or 4
substitutents independently selected from alkenyl, alkoxy, alkoxyalkyl,
alkoxycarbonyl,
alkyl, alkylcarbonyl, alkylcarbonyloxy, alkynyl, carboxy, cyano, formyl,
haloallcoxy,
haloalkyl, halo, hydroxy, hydroxyalkyl, mercapto, -NRARB, or (NRARB)carbonyl.
In another embodiment, the pxesent invention discloses compounds that modulate
PN3 in mammals and are useful for controlling pain in mammals. These compounds
include:
1-(3-chlorophenyl)-N-[3-(methylsulfonyl)phenyl]-5-(trifluoromethyl)-1H-
pyrazole-4-
carboxamide;
1-(3-methylphenyl)-N-[3-(methylsulfonyl)phenyl]-5-(trifluoromethyl)-1H-
pyrazole-4-
carboxamide;
1-(4-methylphenyl)-N-[3-(methylsulfonyl)phenyl]-5-(trifluoromethyl)-1H-
pyrazole-4-
carboxamide;
1-(2-methoxyphenyl)-5-(trifluoromethyl)-N-[3-(trifluoromethyl)benzyl]-1H-
pyrazole-
4-carboxamide;
N-[2-(4-chlorophenyl)ethyl]-1-(2-methoxyphenyl)-5-(trifluoromethyl)-1H-
pyrazole-
4-carboxamide;
1-cyclohexyl-5-(trifluoromethyl)-N-[3-(trifluoromethyl)benzyl]-1H-pyrazole-4-
carboxamide;
4
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
.. .
1-cyclohexyl-N-[3-(methylsulfonyl)phenyl]-5-(trifluoromethyl)-1H-pyrazole-4-
carboxamide;
N-[2-(4-chlorophenyl)ethyl]-1-(7-chloroquinolin-4-yl)-5-(trifluoromethyl)-1H-
pyrazole-4-carboxamide;
1-(4-chlorophenyl)-5-methyl-N-[3-(trifluoromethyl)benzyl]-1H-pyrazole-4-
carboxamide;
1-(4-chlorophenyl)-N-[2-(4-chlorophenyl)ethyl]-5-methyl-1H-pyrazole-3-
carboxamide;
1-(4-chlorophenyl)-5-methyl-N-[3-(trifluoromethyl)benzyl]-1H-pyrazole-3-
carboxamide;
1-(4-chlorophenyl)-5-methyl-N-[3-(methylsulfonyl)phenyl]-1H-pyrazole-3-
carboxamide;
N-benzyl-1-(4-chlorophenyl)-5-hydroxy-1H-pyrazole-4-carboxamide;
1-(4-chlorophenyl)-5-hydroxy-N-[3-(methylsulfonyl)phenyl]-1H-pyrazole-4-
carboxamide;
1-(4-chlorophenyl)-5-cyano-N-[3-(methylsulfonyl)phenyl]-1H-pyrazole-4-
carboxamide;
1-(4-chlorophenyl)-N-[3-(methylsulfonyl)phenyl]-5-vinyl-1H-pyrazole-4-
carboxamide;
1-(4-chlorophenyl)-N-(3,4-dichlorobenzyl)-5-vinyl-1H-pyrazole-4-carboxamide;
5-acetyl-1-(4-chlorophenyl)-N-[3-(methylsulfonyl)phenyl]-1H-pyrazole-4-
carboxamide;
5-acetyl-1-(4-chlorophenyl)-N-(3,4-dichlorobenzyl)-1H-pyrazole-4-carboxamide;
1-(4-chlorophenyl)-N-(2-rnethoxybenzyl)-5-methyl-1H-pyrazole-3-carboxamide;
and
1-(4-chlorophenyl)-5-methyl-N-(2-methylbenzyl)-1H-pyrazole-3-carboxamide; or a
pharmaceutically acceptable salt, amide, ester, or prodrug thereof.
DETAILED DESCRIPTION OF THE PRESENT INVENTION
In one embodiment of the present invention, compounds of formula (I) are
disclosed
N~R2
R1~N~~,1 Rs
R4
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
(I),
or a pharmaceutically acceptable salt, amide, ester, or prodrug thereof,
wherein
Rl is alkyl, aryl, arylalkyl, cycloalkyl, cycloalkylalkyl, haloalkyl,
heterocycle,
heterocyelealkyl, heteroaryl, or heteroarylalkyl;
R2 and R3 are independently hydrogen, alkenyl, alkoxy, alkoxyalkyl,
alkoxycarbonyl,
alkyl, alkylcarbonyl, alkylcarbonyloxy, alkylthio, alkynyl, aryl, arylalkyl,
carboxy,
cycloalkyl, cycloalkylalkyl, cyano, formyl, haloalkoxy, haloalkyl, halogen,
hydroxy,
hydroxyalkyl, mercapto, vitro, -NRARB, or (NR,~RB)carbonyl;
R,~ and R~ are independently hydrogen, alkyl, or alkylcarbonyl;
R4 is
B
B
A L2 A L2 A L
A L2
X O O R5
II i Rs
,~~N.L1 ~S.N.Li ~N~Li ~N\S~Li
R5 , X . O O ,
X
II O~ ~O
~~N B ~S~N B
or
XisOorS;
R$ is hydrogen or alkyl;
LI is a bond or allcylene;
Lz is a bond or allcylene;
A is aryl, cycloallcyl, heteroaryl, or heterocycle;
B is aryl, cycloalkyl, heteroaryl, or heterocycle;
D is heterocycle wherein the heterocycle is azetidinyl, azepanyl, aziridinyl,
azocanyl,
1,1-dioxidothiomorpholinyl, morpholinyl, piperazinyl, piperidinyl,
pyrrolidinyl, or
thiomorpholinyl, wherein the heterocycle is optionally substituted with 1, 2,
3, or 4
substitutents independently selected from alkenyl, alkoxy, alkoxyalkyl,
alkoxycarbonyl,
alkyl, allcylcarbonyl, alkylcarbonyloxy, alkynyl, carboxy, cyano, formyl,
haloalkoxy,
haloallcyl, halo, hydroxy, hydroxyallcyl, mercapto, -NRARB, or
(NRARB)carbonyl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein Rl is aryl; R4 is
6
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
x
-~~ N B
~L1
and R2, R3, X, D, B, and Ll are as defined in formula (I).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein Rl is aryl; R~ is
X
-~~ N B
~L1
X is O; D is piperazmyl; Ll is a bond; B is aryl; and R2 and R3 are as
defined in formula (I).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein RI is aryl wherein the aryl is phenyl substituted with 1
halogen substituent
wherein a preferred halogen substituent is -Cl; R2 is hydrogen; R3 is
haloalkyl wherein a
preferred haloalkyl is trifluoromethyl; R~ is
X
~~ N B
~L1
X is O; D is piperazmyl; Ll is a bond; and B is aryl wherein the aryl is
phenyl substituted with 1 halogen substituent wherein a preferred halogen
substituent is -Cl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein Rl is aryl wherein the aryl is phenyl substituted with 1
halogen substituent
wherein a preferred halogen substituent is -Cl; R2 is hydrogen; R3 is
haloalkyl wherein a
preferred haloalkyl is trifluoromethyl; R4 is
X
~~~ N B
~L1
X is O; D is piperazmyl; Ll is a bond; and B is cycloalkyl wherein the
cycloalkyl is cyclohexyl.
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein Rl is aryl; R4 is
A L~
X
-~~N.Li
i
R5 ; and R2, R3, Rs, X, A, B, Li, and L2 are as defined in formula (I).
7
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
ii-.. ~i,..;, " . ..... .... . _ .....
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein Rl is aryl; Rø is
A L2
X
-~~NsLi
r
R5 ; X is O; Ll is a bond; A is piperidinyl; L2 is alkylene; B is aryl; and RS
is
as defined in formula (I).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein Rl is aryl wherein the aryl is phenyl substituted with 1
halogen substituent
wherein a preferred substituent is -Cl; R2 is hydrogen; R3 is alkyl; R4 is
A L2
X
-~~ N. L~
X is O; Ll is a bond; A is piperidinyl; L2 is alkylene; B is aryl wherein
the aryl is phenyl; and RS is as defined in formula (I).
In another embodiment of the present invention, compounds of formula (I) are
disclosed wherein Rl is aryl wherein the aryl is phenyl substituted with 1
halogen substituent
wherein a preferred substituent is -Cl; RZ is hydrogen; R3 is alkyl; R4 is
A L2
X
-~~N.L1
R5 ; X is O; Ll is a bond; A is piperidinyl; L2 is alkylene wherein -CH2- is
preferred; B is aryl wherein the aryl is phenyl; and RS is hydrogen.
In another embodiment of the present invention, compounds of formula (II) are
disclosed
N~Ria
Li-N~~~ Ris
L~-~ Ria
(u)
or a pharmaceutically acceptable salt, amide, ester, or prodrug thereof,
wherein
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
R12 and R13 are independently hydrogen, alkenyl, alkoxy, alkoxyalkyl,
alkoxycarbonyl, alkyl, alkylcarbonyl, alkylcarbonyloxy, alkylthio, alkynyl,
aryl, arylalkyl,
carboxy, cycloalkyl, cycloalkylalkyl, cyano, formyl, haloalkoxy, haloalkyl,
halogen, hydroxy,
hydroxyalkyl, mercapto, nitro, -NRARB, or (NRARB)carbonyl;
RA and RB are independently hydrogen, alkyl, or alkylcarbonyl;
R14 is
B B
A L A L
2 2 A L
X ~ A L2
~s i~ R15 R15
~.~N..L1 ~S~N.Li ~N~Li ~~\B~L1
R 15 ~ R 15 ~ IXl ,
X
O~ ~O R15 R
.~~N,R16 ~S~N~R16 ~N~Ri$ Nis R
' ~ 18
R17 , R1~ , X , ~O'~ O
X
II O~ ,O
'~~N B -~S~N B
~L1 ~L1
or
XisOorS;
R15 is hydrogen or alkyl;
RIB and Rl~ are independently hydrogen, alkenyl, alkoxy, alkyl, aryl,
arylallcyl,
cycloalkyl, cycloalkylalkyl, heteroaryl, heteroarylalkyl, heterocycle, or
heterocyclealkyl;
R18 is alkyl, alkenyl, aryl, arylalkyl, cycloalkyl, cycloalkylalkyl,
heteroaryl,
heteroarylallcyl, heterocycle, heterocyclealkyl, -NRARB, or (NRARB)alkyl;
Ll is a bond or allcylene;
L2 is absent, a bond, or allcylene;
A is aryl, cycloalkyl, heteroaryl, or heterocycle;
B is absent, aryl, cycloalkyl, heteroaryl, or heterocycle;
D is heterocycle wherein the heterocycle is azetidinyl, azepanyl, aziridinyl,
azocanyl,
1,1-dioxidothiomorpholinyl, morpholinyl, piperazinyl, piperidinyl,
pyrrolidinyl, or
thiomorpholinyl, wherein the heterocycle is optionally substituted with 1, 2,
3, or 4
substitutents independently selected from allcenyl, alkoxy, alkoxyalkyl,
alkoxycarbonyl,
allcyl, alkylcarbonyl, allcylcarbonyloxy, allcynyl, caxboxy, cyano, formyl,
haloallcoxy,
haloalkyl, halo, hydroxy, hydroxyalkyl, mercapto, -NRARB, or (NRARB)carbonyl.
9
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
In another embodiment of the present invention, compounds of formula (II) are
disclosed wherein Ll is a bond; A is heterocycle; L2 is alkylene; B is aryl;
R14 is
X
~~~~~R16
R1~ ; and Rh, R13, Rm and Rm are as defined in formula (II).
In another embodiment of the present invention, compounds of formula (II) are
disclosed wherein Ll is a bond; A is heterocycle wherein the heterocycle is
piperidinyl; L2 is
alkylene; B is aryl wherein the aryl is phenyl; R14 is
X
~~N.R16
R17 ; X is ~; Rr2 and R1~ are hydrogen; R13 is haloalkyl; and Rl~ is aryl
wherein the
aryl is phenyl substituted with 1 alkylsulfonyl substituent.
In another embodiment of the present invention, compounds of formula (II) are
disclosed wherein Ll is a bond; A is heterocycle; L~ and B are absent; and R14
is
XII
~~N.RIs
R1~ ; and RI2, R13, Ri6 and Rl~ are as defined in formula (II).
In another embodiment of the present invention, compounds of formula (II) are
disclosed wherein Ll is a bond; A is heterocycle wherein the heterocycle is
tetrahydropyran;
L2 and B are absent; Rr4 is
XII
~~N-R1s
I
R1~ ; and R12, RI3, Rm and Rl~ are as defined in formula (II).
In another embodiment of the present invention, compounds of formula (II) are
disclosed wherein LI is a bond; A is heterocycle wherein the heterocycle is
tetrahydropyran;
LZ and B are absent; Rr~ is
X
~~N.RIs
I
R1~ ; and R12 and R1G are hydrogen; R13 is alkyl or haloalkyl; and Ri~ is
aryl.
Another embodiment of the present invention relates to pharmaceutical
compositions
comprising a therapeutically effective amount of a compound of formula (I) or
a
pharmaceutically acceptable salt, amide, ester, or prodrug thereof.
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
;n.. .,. , " , ..... ..... .... ...
Another embodiment of the present invention relates to pharmaceutical
compositions
comprising a therapeutically effective amount of a compound of formula (II) or
a
pharmaceutically acceptable salt, amide, ester, or prodrug thereof.
Another embodiment of the present invention relates to a method for modulating
PN3
in a host mammal comprising administering a therapeutically effective amount
of a
compound of formula (1> or a pharmaceutically acceptable salt, amide, ester,
or prodrug
thereof.
Another embodiment of the present invention relates to a method for modulating
PN3
in a host mammal comprising administering a therapeutically effective amount
of a
compound of formula (II) or a pharmaceutically acceptable salt, amide, ester,
or prodrug
thereof.
Another embodiment of the present invention relates to a method for treating
pain, in
particular neuropathic pain, comprising administering a therapeutically
effective amount of a
compound of formula (I) or a pharmaceutically acceptable salt, amide, ester,
or prodrug
thereof.
Another embodiment of the present invention relates to a method for treating
pain, in
particular neuropathic pain, comprising administering a therapeutically
effective amount of a
compound of formula (II) or a pharmaceutically acceptable salt, amide, ester,
or prodrug
thereof.
Definition of Terms
As used throughout this specification and the appended claims, the following
terms
have the following meanings:
The term "alkenyl" as used herein, means a straight or branched chain
hydrocarbon
containing from 2 to 10 carbons and containing at least one carbon-carbon
double bond
formed by the removal of two hydrogens. Representative examples of alkenyl
include, but
are not limited to, ethenyl (vinyl), 2-propenyl, 2-methyl-2-propenyl, 3-
butenyl, 4-pentenyl, 5-
hexenyl, 2-heptenyl, 2-methyl-1-heptenyl, and 3-decenyl.
The term "alkoxy" as used herein, means an alkyl group, as defined herein,
appended
to the parent molecular moiety through an oxygen atom. Representative examples
of alkoxy
include, but are not limited to, methoxy, ethoxy, propoxy, 2-propoxy, butoxy,
tert-butoxy,
pentyloxy, and hexyloxy.
11
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
The term "alkoxyalkyl" as used herein, means an alkoxy group, as defined
herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of alkoxyalkyl include, but are not limited to, tent-
butoxymethyl, 2-
ethoxyethyl, 2-methoxyethyl, and methoxymethyl.
The term "alkoxycarbonyl" as used herein, means an alkoxy group, as defined
herein,
appended to the parent molecular moiety through a carbonyl group, as defined
herein.
Representative examples of alkoxycarbonyl include, but are not limited to,
methoxycarbonyl,
ethoxycarbonyl, and tent-butoxycarbonyl. ,
The term "alkoxysulfonyl" as used herein, means an alkoxy group, as defined
herein,
appended appended to the parent molecular moiety through a sulfonyl group, as
defined
herein. Representative examples of alkoxysulfonyl include, but are not limited
to,
methoxysulfonyl, ethoxysulfonyl and propoxysulfonyl.
The term "alkyl" as used herein, means a straight or branched chain
hydrocarbon
containing from 1 to 10 carbon atoms. Representative examples of alkyl
include, but are not
limited to, methyl, ethyl, n-propyl, iso-propyl, n-butyl, sec-butyl, iso-
butyl, tert-butyl, n-
pentyl, isopentyl, neopentyl, n-hexyl, 3-methylhexyl, 2,2-dimethylpentyl, 2,3-
dimethylpentyl,
n-heptyl, n-octyl, n-nonyl, and n-decyl.
The term "alkylcarbonyl" as used herein, means an alkyl group, as defined
herein,
appended to the parent molecular moiety through a carbonyl group, as defined
herein.
Representative examples of alkylcarbonyl include, but are not limited to,
acetyl, 1-oxopropyl,
2,2-dimethyl-1-oxopropyl, 1-oxobutyl, and 1-oxopentyl.
The term "alkylcarbonyloxy" as used herein, means an alkylcarbonyl group, as
defined herein, appended to the parent molecular moiety through an oxygen
atom.
Representative examples of alkylcarbonyloxy include, but are not limited to,
acetyloxy,
ethylcarbonyloxy, and tert-butylcarbonyloxy.
The term "alkylene" means a divalent group derived from a straight or branched
chain
hydrocarbon of from 1 to 10 carbon atoms. Representative examples of alkylene
include, but
are not limited to, -CH2-, -CH(CH3)-, -C(CH3)2-, -CH2CH2-, -CH2CHZCHz-,
-CH2CH2CH2CH2-, and -CH2CH(CH3)CHZ-.
The term "allcylsulfonyl" as used herein, means an alkyl group, as defined
herein,
appended to the parent molecular moiety through a sulfonyl group, as defined
herein.
12
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
Representative examples of alkylsulfonyl include, but are not limited to,
methylsulfonyl and
ethylsulfonyl.
The term "alkylthio" as used herein, means an alkyl group, as defined herein,
appended to the parent molecular moiety through a sulfur atom. Representative
examples of
alkylthio include, but are not limited, methylthio, ethylthio, tart-butylthio,
and hexylthio.
The term "alkynyl" as used herein, means a straight or branched chain
hydrocarbon
group containing from 2 to 10 carbon atoms and containing at least one carbon-
carbon triple
bond. Representative examples of alkynyl include, but are not limited, to
acetylenyl, 1-
propynyl, 2-propynyl, 3-butynyl, 2-pentynyl, and 1-butynyl.
The term "aryl" as used herein, means a phenyl group, or a bicyclic or a
tricyclic
fused ring system wherein one or more of the fused rings is a phenyl group.
Bicyclic fused
ring systems are exemplified by a phenyl group fused to a cycloalkyl group, as
defined
herein, or another phenyl group. Tricyclic fused ring systems are exemplified
by a bicyclic
fused ring system fused to a cycloalkyl group,~as defined herein, or another
phenyl group.
Representative examples of aryl include, but are not limited to, anthracenyl,
azulenyl,
fluorenyl, indanyl, indenyl, naphthyl, phenyl and tetrahydronaphthyl.
The aryl groups of this invention can be substituted with 1, 2, or 3
substituents
independently selected from alkenyl, alkoxy, alkoxyalkyl, alkoxycarbonyl,
alkoxysulfonyl,
alkyl, alkylcarbonyl, allcylcarbonyloxy, alkylsulfonyl, alkynyl, carboxy,
cyano, foimyl,
haloalkoxy, haloalkyl, halo, hydroxy, hydroxyalkyl, mercapto, -NRARB,
(NRARB)carbonyl, or
(NRARB)sulfonyl.
The term "arylalkyl" as used herein, means an aryl group, as defined herein,
appended
to the parent molecular moiety through an alkyl group, as defined herein.
Representative
examples of arylalkyl include, but are not limited to, benzyl, 2-phenylethyl,
3-phenylpropyl,
and 2-naphth-2-ylethyl. ,
The term "carbonyl" as used herein, means a -C(O)- group.
The term "carboxy" as used herein, means a -COZH group.
The term "cyano" as used herein, means a -CN group.
The term "cycloalkyl" as used herein, means a saturated cyclic hydrocarbon
group
containing from 3 to 8 carbons. Examples of cycloalkyl include cyclopropyl,
cyclobutyl,
cyclopentyl, cyclohexyl, cycloheptyl, and cyclooctyl.
13
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
The cycoalkyl groups of the present invention are optionally substituted with
1, 2, 3,
or 4 substituents selected from
The term "cycloalkylalkyl" as used herein, means a cycloalkyl group, as
defined
herein, appended to the parent molecular moiety through an alkyl group, as
defined herein.
Representative examples of cycloalkylalkyl include, but are not limited to,
cyclopropylmethyl, 2-cyclobutylethyl, cyclopentylmethyl, cyclohexylmethyl, and
4-cycloheptylbutyl.
The term "formyl" as used herein, means a -C(O)H group.
The term "halo" or "halogen" as used herein, means -Cl, -Br, -I or -F.
The term "haloalkoxy" as used herein, means at least one halogen, as defined
herein,
appended to the parent molecular moiety through an allcoxy group, as defined
herein.
Representative examples of haloallcoxy include, but are not limited to,
chloromethoxy, 2-
fluoroethoxy, trifluoromethoxy, and pentafluoroethoxy.
The term "haloalkyl" as used herein, means at least one halogen, as defined
herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of haloalkyl include, but are not limited to,
chloromethyl, 2-
fluoroethyl, trifluoromethyl, pentafluoroethyl, and 2-chloro-3-fluoropentyl.
The term "heteroaryl," as used herein, refers to an aromatic five- or six-
membered
ring wherein 1, 2, 3, or 4 heteroatoms axe independently selected from N, O,
or S. The five
membered rings have two double bonds and the six membered rings have three
double bonds.
The heteroaryl groups are connected to the parent molecular moiety through a
carbon or
nitrogen atom. The term "heteroaryl" also includes bicyclic systems where a
heteroaryl ring
is fused to a phenyl group, a monocyclic cycloalkyl group, as defined herein,
a heterocycle
group, as defined herein, or an additional heteroaryl group; and tricyclic
systems where a
bicyclic system is fused to a phenyl group, a monocyclic cycloalkyl group, as
defined herein,
a heterocycle group, as defined herein, or an additional heteroaryl group.
Representative
examples of heteroaryl include, but are not limited to, benzothienyl,
benzoxadiazolyl,
cinnolinyl, dibenzofuranyl, furopyridinyl, furyl, irnidazolyl, indazolyl,
indolyl, isoxazolyl,
isoquinolinyl, isothiazolyl, naphthyridinyl, oxadiazolyl, oxazolyl, pyridinyl,
pyridazinyl,
pyrimidinyl, pyrazinyl, pyrazolyl, pyrrolyl, quinolinyl, tetrazolyl,
thiadiazolyl, thiazolyl,
thienopyridinyl, thienyl, triazolyl, and triazinyl.
The heteroaryl groups of the present invention are substituted with 0, 1, 2,
3, or 4
14
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
substituents independently selected from alkenyl, alkoxy, alkoxyalkyl,
alkoxycarbonyl,
alkoxysulfonyl, alkyl, alkylcarbonyl, alkylcarbonyloxy, alkylsulfonyl,
alkynyl, carboxy,
cyano, formyl, haloalkoxy, haloalkyl, halo, hydroxy, hydroxyalkyl, mercapto, -
NRARB,
(NRARB)carbonyl, or (NRARB)sulfonyl.
The term "heteroarylalkyl" as used herein, means a heteroaryl, as defined
herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of heteroarylalkyl include, but are not limited to,
pyridin-3-ylmethyl
and 2-pyrimidin-2-ylpropyl.
The term "heterocycle," as used herein, refers to a three, four, five, six,
seven or eight
membered ring containing one, two, or three heteroatoms independently selected
from the
group consisting of nitrogen, oxygen, and sulfur. The three membered ring has
zero double
bonds. The four and five membered ring has zero or one double bonds. The six
membered
ring has zero, one, or two double bonds. The seven and eight membered rings
have zero, one,
two, or three double bonds. The heterocycle groups of the present invention
can be attached
to the parent molecular moiety through a carbon atom or a nitrogen atom.
Representative
examples of heterocycle include, but are not limited to, azetidinyl, azepanyl,
aziridinyl,
azocanyl, morpholinyl, piperazinyl, piperidinyl, pyrrolidinyl,
tetrahydropyranyl, and
thiomorpholinyl.
The heterocycles of the present invention are substituted with 0, l, 2, 3, or
4
substituents independently selected from allcenyl, alkoxy, alkoxyalkyl,
alkoxycarbonyl,
alkoxysulfonyl, alkyl, alkylcarbonyl, alkylcarbonyloxy, allcylsulfonyl,
alkynyl, carboxy,
cyano, formyl, haloalkoxy, haloalkyl, halo, hydroxy, hydroxyalkyl, mercapto,
oxo, -NRARB,
(NRARB)carbonyl, or (NRARB)sulfonyl.
The term "heterocyclealkyl" as used herein, means a heterocycle, as defined
herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of heterocyclealkyl include, but are not limited to,
pyridin-3-
ylmethyl and 2-pyrimidin-2-ylpropyl.
The term "hydroxy" as used herein, means an -OH group.
The term "hydroxyalkyl" as used herein, means at least one hydroxy group, as
defined
herein, is appended to the parent molecular moiety through an alkyl group, as
defined herein.
Representative examples of hydroxyallcyl include, but are not limited to,
hydroxymethyl, 2-
hydroxyethyl, 3-hydroxypropyl, 2,3-dihydroxypentyl, and 2-ethyl-4-
hydroxyheptyl.
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
The term "mercapto" as used herein, means a -SH group.
The term "nitro" as used herein, means a -N02 group.
The term "-NRARB" as used herein, means two groups, Rl and R2, which are
appended to the parent molecular moiety through a nitrogen atom. R1 and R2 are
each
independently hydrogen, alkyl, or alkylcarbonyl. Representative examples of -
NR,~R~
include, but are not limited to, amino, methylamino, acetylamino, and
acetylmethylamino.
The term "(NRARB)carbonyl" as used herein, means a -NRARB group, as defined
herein, appended to the parent molecular moiety through a carbonyl group, as
defined herein.
Representative examples of (NRARn)carbonyl include, but are not limited to,
aminocarbonyl,
(methylamino)carbonyl, (dimethylamino)carbonyl, and
(ethylmethylamino)carbonyl.
The term "(NRARB)sulfonyl" as used herein, means a -NRARB group, as defined
herein, appended to the parent molecular moiety through a sulfonyl group, as
defined herein.
Representative examples of (NRARB)sulfonyl include, but are not limited to,
aminosulfonyl,
(methylamino)sulfonyl, (dimethylamino)sulfonyl, and
(ethylmethylamino)sulfonyl.
The term "oxo" as used herein, means a =O moiety.
The term "sulfonyl" as used herein, means a -SO2- group.
Compounds of the present invention can exist as stereoisomers, wherein
asymmetric
or chiral centers are present. Stereoisomers are designated (R) or (S),
depending on the
configuration of substituents around the chiral carbon atom. The terms (R) and
(S) used
herein are configurations as defined in ILTPAC 1974 Recommendations for
Section E,
Fundamental Stereochemistry, Pure Appl. Chem., (1976), 45: I3-30. The present
invention
contemplates various stereoisomers and mixtures thereof and are specifically
included within
the scope of this invention. Stereoisomers include enantiomers, diastereomers,
and mixtures
of enantiomers or diastereomers. Individual stereoisomers of compounds of the
present
invention may be prepared synthetically from commercially available starting
materials
which contain asymmetric or chiral centers or by preparation of racemic
mixtures followed
by resolution, a technique well-known to those of ordinary shill in the art.
These methods of
resolution are exemplified by (I) attachment of a mixture of enantiomers to a
chiral auxiliary,
separation of the resulting mixture of diastereomers by recrystallization or
chromatography
and liberation of the optically pure product from the auxiliary, (2) direct
separation of the
mixture of optical enantiomers on chiral chromatographic columns, or (3)
formation of a
diastereomeric salt followed by selective recrystallization of one of the
diastereomeric salts.
16
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
Abbreviations
Abbreviations which have been used in the descriptions of the Schemes and the
Examples that follow are: Ac for acetyl, DMAP for IV,l~T-
dimethylaminopyridine, Et for ethyl
Preparation of Compounds of the Present Invention
The compounds and processes of the present invention will be better understood
in
connection with the following synthetic Schemes and Examples which illustrate
a means by
which the compounds of the present invention can be prepared. Further, all
citations herein
are incorporated by reference.
Scheme 1
~O~ DMAP O O O pyridine
+ aq. (CHs)2NH ~N~R + ~ ~ CH2CI2
O O 2 R2 O R~ -
~ II (2)
R ~O~R (~)
2 2
(1)
O O p
Rz R2
~N ~ R 1) RfNHNH2; \ OH (COCI)2 ~ CI
--~ R1-N -~ Ri-N
O R2 2) aq. KOH ~Nr CH2CI2
(g) (5) (6)
B
6
( ) + A L2 Et3N A L2
HN'Li CH3CN R2 O ,1-1
'N
Rs Ri-N ~ R
,N,- 5
(7) (s)
B
A L2 A L2
R2 O EDCI R O
R1-N ~ OH + HN~Li Et3N 2 N~-j
~N~ R5 ~H~~ R1-N, , R
N 5
(5) (7) (8)
17
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
Pyrazoles of general formula .(8), wherein Rl, R$, Li, A, LZ, and B are as
defined in
formula (I) and R2 is alkenyl, alkyl, alkynyl, aryl, arylalkyl, cycloalkyl,
cycloalkylalkyl,
haloalkoxy, or haloalkyl, can be prepared as described in Scheme 1. Ethyl
vinyl ether can be
treated with an anhydride of general formula (1), aqueous methylamine, and
1V,IV-
dimethylaminopyridine to provide enaminones of general formula (2) as
described in l~ellor,
et. al., Tetrahedron, 56:7255-7267 (2000). Enaminones of general formula (2)
can be treated
with an anhydride of general formula (1) and pyridine to provide compounds of
general
formula (3). Compounds of general formula (3) can be treated with hydrazines
of general
formula (4) to provide pyrazoles which can be treated with aqueous base
including, but not
limited to, potassium hydroxide or sodium hydroxide to provide acids of
general formula (5).
Acids of general formula (5) can be treated with oxalyl chloride to provide
acid chlorides of
general formula (6). Acid chlorides of general formula (6) can be treated with
amines of
general formula (7) and triethylamine or diisopropylethylamine to provide
pyrazoles of
general formula (8).
Acids of general formula (5) can also be treated with an amine of general
formula (7),
a carbodiimide including, but not limited to, 1-(3-dimethylaminopropyl)-3-
ethylcarbodiimide
hydrochloride (EDCI) or dicyclohexycarbodiimide (DCC), and triethylamine to
provide
pyrazoles of general formula (8).
Scheme 2
OMe O R2
O O ~ RiNHNH2 R2 O
Me2N OMe Et0 O (4) OEt
Et0 R2 ~ ~ Ri_N
(1 ~) p-TsOH H NMe2 ,N,
(11) (12)
A L2
R2 O R2 O 1
N
aq. KOH ~ ~OH Scheme 1 1 ,
(12) -~ Ri-N~ , R N~N~ R5
N
(5) (8)
Pyrazoles of general formula (8), wherein R1, Rs, L1, A, Lz, and B are as
defined in
formula (I) and R2 is alkenyl, alkyl, alkynyl, aryl, arylallcyl, cycloalkyl,
cycloalkylalkyl,
haloallcoxy, or haloalkyl, can be prepared as described in Scheme 2. Keto
esters of general
18
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
formula (10) can be treated with N-(dimethoxymethyl)-N,N-dimethylamine and an
acid
including, but not limited to, para-toluenesulfonic acid to provide compounds
of general
formula (11). Compounds of general formula (I1) can be treated with hydrazines
of general
formula (4) to provide esters of genexal formula (12) as described in Menozzi,
et. al., J. Het.
Cheixi., 24:1669 (1987). Esters of general formula (12) can be treated with
aqueous base
including, but not limited to, potassium hydroxide or sodium hydroxide to
provide acids of
general formula (5). Acids of general formula (S) can be processed as
described in Scheme 1
to provide pyrazoles of general formula (8).
Scheme 3
R2 R2
O O RiNHNH2 1 ~ 1
R2~~~OEt ~ R -N,N~OEt aqy R N~N OH
(14) O (15) O (16) O
R R2
2
(COCI)2 1 ~ A L2 Et3N R1-N ~ H
(16) ~. R -N' , - , ~ N
CH2CI2 N CI + HN'Li CH3CN N
(17) O RS A L~
(7) (18) a
Pyrazoles of general formula (18), wherein Rl, R$, Ll, A, L2, and B are as
defined in
formula (I) and RZ is alkenyl, alkyl, alkynyl, aryl, arylallcyl, cycloalkyl,
cycloalkylalkyl,
haloallcoxy, or haloallcyl, can be prepared as described in Scheme 3. Keto
esters of general
formula (14) can be treated with hydrazines of general formula (4) to provide
esters of
general formula (15) as described in Kordik, et. al., Bio. Med. Chem. Let.,
11:2287-2290
(2001). Esters of general formula (15) can be treated with aqueous base
including, but not
limited to, potassium hydroxide or sodium hydroxide to provide acids of
general formula
(16). Acids of general formula (16) can be treated with oxalyl chloride to
provide acid
chlorides of general formula (17). Acid chlorides of general formula (17) can
be treated with
amines of general formula (7) and triethylamine or diisopropylethylamine to
provide
pyrazoles of general formula (18).
19
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
Scheme 4
I O HO O
OEt aq~ KOH, ~ OH (COCI)2
RyN ~ R1-N -
'N' EtOH 'N~ CH2CI2
(~0) (21
O
HO
A L2 Et3N ~ L2
Ri-N ~ ~CI + ~ HO O
~N~ HN~Li CH3CN ~1
~N
(22) R5 R1'N \ Rs
,N,
(~)
(23)
Pyrazoles of general formula (23), wherein Rl, R5, Ll, A, LZ, and B are as
defined in
formula (I), can be prepared as described in Scheme 4. Esters of general
formula (20),
prepared using the procedure described in Beck, et, al., J. Het. Chem.,
267:267-270 (1987),
can be treated with aqueous base including, but not limited to, potassium
hydroxide or
sodium hydroxide to provide acids of general formula (21). Acids of general
formula (21)
can be treated with oxalyl chloride to provide acid chlorides of general
formula (22). Acid
chlorides of general formula (22) can be treated with amines of general
formula (7) and
triethylarnine or diisopropylethylamine to provide pyrazoles of general
formula (23).
Scheme 5
CI O NC O NC O
~OEt KCN ~ OEt aq. KOH ~ OH
Ri-N > R1-N -~ Ri_N
'N 18-crown-6, 'N EtOH 'NJ
(25) CH3CN (26) (27)
O
NC
(COCI)2 ~ CI A L2 Et3N
(27) ~ R1~N ~ + ~ NC O
CH~CI2 'Nr HN'L1 CHsCN
'N
(28) R5 R1-N, ~ R
N 5
(7) (29)
Pyrazoles of general formula (29), wherein Rl, R5, Ll, A, L2, and B are as
defined in
formula (I), can be prepared as described in Scheme 5. Esters of general
formula (25) can be
treated with potassium cyanide or sodium cyanide to provide esters of general
formula (26).
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
Esters of general formula (26) can be treated with aqueous base including, but
not limited to,
potassium hydroxide or sodium hydroxide to provide acids of general formula
(27). Acids of
general formula (27) can be treated with oxalyl chloride to provide acid
chlorides of general
formula (28). Acid chlorides of general formula (28) can be treated with
amines of general
formula (7) and triethylamine or diisopropylethylamine to provide pyrazoles of
general
formula (29).
Scheme 6
O R~SnBu3 R~ ~ R~ O
OEt (33) ~ OEt aq. KOH, w OH
R1-N Ri-N --~ Ri-N
~Nr PdCl2(PPh3)2, ~Nr EtOH ~N'
(2p) PhCH3 (31 ) (32)
R2 O A Lz A L2
(COCI)2 ~ CI EtsN R O
(32) CH~ R~-N --.~ z ~1..
.N~ + HN~L1 CH3CN ~ N
(33) Rs R~-N, ~ Rs
N
(7) (~)
Pyrazoles of general formula (8), wherein Rl, R5, Ll, A, L2, and B are as
defined in
formula (I) and R2 is allcenyl, alkyl, alkylcarbonyl, alkynyl, or aryl, can be
prepared as
described in Scheme 6. Esters of general formula (20) can be treated with a
palladium
catalyst including, but not limited to, dichlorobis[tri(o-
tolyl)phosphine]palladium(II) and a tin
compound of general formula (30) wherein RZ is alkenyl, alkyl, alkynyl, aryl,
or a vinyl ether
including, but not limited to, ethoxyvinyl to provide esters of general
formula (31). Esters of
general formula (31) can be treated with aqueous base including, but not
limited to,
potassium hydroxide or sodium hydroxide to provide acids of general formula
(32). Acids of
general formula (32) can be treated with oxalyl chloride to provide acid
chlorides of general
formula (33). Acid chlorides of general formula (33) can be treated with
amines of general
formula (7) and triethylamine or diisopropylethylamine to provide pyrazoles of
general
formula (8) wherein Rz is allcenyl, alkyl, alkylcarbonyl, alkynyl, or aryl.
It is to be understood that the order of the reactions in the synthesis
exemplified in
Scheme 6 can be rearranged. For example, the tin coupling reaction can be
executed as the
21
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
last step to provide pyrazoles of general formula (8) wherein RZ is alkenyl,
alkyl,
alkylcarbonyl, alkynyl, or aryl.
Example 1
1-(3-chloro~he~l)-4-~ f 1-(4-chlorophenxl)-5-(trifluoromethyl)-1H-pyrazol-4-
yllcarbon~pi~erazine hydrochloride
Example 1A
4-Dimethylamino-1,1,1-trifluoro-but-3-en-2-one
Trifluoroacetic anhydride (2.0 g, 9.5 mmol.) was dissolved in dichloromethane
(20
mL) and the mixture was cooled to 0 °C with an ice bath. Ethyl vinyl
ether (0.69 g, 9.5
mmol) and a catalytic amount of DMAP were added and the mixture was stirred
for 30 min.
The ice bath was removed and the mixture was warmed to ambient temperature and
stirred
for an additional 2 h. The mixture was cooled to -5 °C with an
ice/brine bath and 40% (w/v)
aqueous dimethylannine (3.5 mL) was added. The mixture was stirred at -5
°C for 10 rnzn
then diluted with dichloromethane (20 mL). The organic phase was washed with
brine (20
mL), dried over Na2SO4, and filtered through a 1/2" plug of silica gel. The
silica gel plug
was washed with EtOAc (150 mL) and the mixture was concentrated under reduced
pressure
and recrystallized from cold EtzO/hexanes (1:50) to provide 1.0 g of the
desired product. M5
(DCIlNH3) m/z 168 (M+H)+.
Example 1B
3-Dimethylaminomethylene-1,1,1,5,5,5-hexafluoro-pentane-2,4-dione
The product from Example 1A (1.0 g, 6.0 mmol) was dissolved in dichloromethane
(7
mL) and cooled to -5 °C with an ice/brine bath. Pyridine (0.61 g, 7.7
mmol) and
trifluoroacetic anhydride (1.6 g, 7.7 mmol) were added and the mixture was
stirred at -5 °C
for 10 min. The mixture was warmed to ambient temperature, diluted with water
(75 mL)
and extracted twice with dichloromethane (75 mL). The combined extracts were
concentrated under reduced pressure to provide 1.3 g of the desired product.
MS (DCI/NH3)
m/z 264 (M+H)+.
Example 1C
22
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
1-(4-Chlorophe~l~-5-trifluoromethyl-1H-pyrazole-4-carboxylic acid
4-Chlorophenylhydrazine hydrochloride, the product from Example 1B, and
triethylamine were dissolved in acetonitrile (12 mL) and stirred at ambient
temperature for 16
h. The solvent was removed under reduced pressure and the crude material was
dissolved in
EtOAc/hexanes (1:1) and filtered through a 1/2" silica gel frit. The solvent
was removed
under reduced pressure and the crude material was dissolved in 1,4-dioxane (15
mL).
Aqueous I~OH was added and the mixture was heated at reflux for 30 min. The
mixture was
cooled to ambient temperature diluted with 2N HCl (6 mL), and extracted twice
with EtOAc
(20 mL). The combined extracts were washed with brine, dried over Na2S0~, and
concentrated under reduced pressure. The crude material was triturated with
hexanes to
provide the desired product.
MS (DCI/NH3) m/z 291 (M+H)+.
Example 1D
1-(3-chlorophenyl)-4-1 ~ 1-(4-chloro~henyl)-5-(trifluoromethyl)-1H-pyrazol-4-
yllcarbon~pi~erazine hydrochloride
A solution of the product from Example 1C in dichloromethane (15 mL) was
treated
with oxalyl chloride and a catalytic amount of DMF. The mixture was allowed to
stir at
ambient temperature for 1 h and the solvent and excess oxalyl chloride were
removed under
reduced pressure. The material was re-dissolved in dichloromethane (15 mL) and
treated
with 1-(3-chlorophenyl)piperazine and triethylamine and allowed to stir at
ambient
temperature for 1 h. The mixture was diluted with NaHC03 (10 mL) and extracted
twice
with EtOAc (15 mL). The combined extracts were dried and concentrated under
reduced
pressure and the material was purified by silica gel chromatography. The
purified material
was dissolved in EtzO and treated with ethanolic HCl to obtain the salt. MS
(DCI/NH3) m/z
469 (M-HCl)~". 1H NMR (DMSO-d~) S 8.09 (d, 1H, J=0.7 Hz), 7.69 (d, 2H, J=9.2
Hz), 7.63
(d, 2H, J=8.8 Hz), 7.24 (t, 1H, J=8.1 Hz), 7.00 (t, 1H, J=2.2 Hz), 6.93 (dd,
1H, J=8.5, 0.7
Hz), 6.83 (dd, 1H, J=7.8, 0.7 Hz), 3.77 (br s, 2H), 3.53 (br s, 2H), 3.26 (br
s, 2H), 3.19 (br s,
2H).
Example 2
23
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
1-~ f 1-(4-chloro~henxlLS-(trifluorometh 1y )-IH-~yrazol-4-~lcarbon ly~l-4-
c~clohexylp~erazine
A solution of the product from Example 1C and N-cyclohexylpiperazine were
processed as described in Example 1D to provide the desired product. MS
(DCI/NH3) m/z
441 (M-HCl)+. 1H NIatlI~ (DMSO-d~) cS 8.03 (d, 1H, J=0.7 Hz), 7.68 (d, 2H,
J=8.8 Hz), 7.61
(d, 2H, J=8.8 Hz), 3.60 (br s, 2H), 3.34 (br s, 2H), 2.51 (br s, 2H), 2.45 (br
s, 2H), 2.27 (br s,
2H), 1.79-1.68 (m, 4H), 1.61-1.52 (m, 1H), 1.26-1.00 (m, 5H).
Example 3
1-~-chlorophenyl)-N- f 3-(methylsulfonyl)phenyll-5-(trifluoromethyl)-1H-
pyrazole-4-
carboxamide
Example 3A
1-(3-Chlorophenyl)-5-trifluorometh~rl-1H-pyrazole-4-carbox lyid
3-Chlorophenylhydrazine hydrochloride, the product from Example 1B, and
triethylamine were processed as described in Example 1C to provide the desired
product.
MS (ESI-) m/z 289 (M-H)-.
Example 3B
1-(3-chlorophenyl)-N- f 3-(methylsulfonyl)phenxll-5-(trifluoromethyl)-1H-
pyrazole-4-
carboxamide
A solution of the product from Example 3A and 3-(methanesulfonyl)aniline were
processed as described in Example 1D to provide the desired product. MS (ESI+)
m/z 444
(M+H)+; 1H NMR (DMSO-d~) ~ 10.89 (s, 1H), 8.40 (d, 1H, J=0.7 Hz), 8.37-8.34
(rn, 1H),
8.02 (dt, 1H, T=7.1, 2.2 Hz), 7.76-7.62 (m, 5H), 7.56 (dt, 1H, J=7.8, 1.5 Hz),
3.23 (s, 3H).
Example 4
1-(3-methylphenYl)-N- f 3-(methylsulfon~phenyll-5-(trifluorometh~l?-1H-
pyrazole-4-
carboxamide
Example 4A
24
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
1-(3-Meth ly_phenyl)-5-trifluorometh 1-~1H-~yrazole-4-carbox, lic acid
3-Methylphenylhydrazine hydrochloride, the product from Example 1B, and
triethylamine were processed as described in Example 1C to provide the desired
product. MS
(ESI-) m/z 269 (M-H)'.
Example 4B
1- 3-methylphenyl)-N-f 3-(methylsulfonyl)phen l~~trifluoromethyl)-1H-p~razole-
4-
carboxamide
A solution of the product from Example 4A and 3-(methanesulfonyl)aniline were
processed as described in Example 1D to provide the desired product. MS (ESI+)
m/z 441
(M+NH4)+. 1H NMR (DMSO-dG) ~ 10.88 (s, 1H), 8.38-8.34 (m, 2H), 8.01 (dt, 1H,
J=6.8,
2.4 Hz), 7.72-7.63 (m, 2H), 7.53-7.41 (m, 2H), 7.38-7.30 (m, 2H), 3.23 (s,
3H), 2.42 (s, 3H).
Example 5
1-(4-meth~phenyl)-N-f 3-(meth lsulfon~phenyll-5-(trifluoromethyl)-1H-~yrazole-
4-
carboxamide
Example 5A
1-(4-Methylphenyl)-5-trifluoromethyl-1H-~yrazole-4-carboxylic acid
4-Methylphenylhydrazine hydrochloride, the product from Example 1B, and
triethylamine were processed as described in Example 1C to provide the desired
product.
MS (ESI-) m/z 269 (M-H)'.
Exam 1p a 5B
1-(4-methylphenyl)-N-f3-(meth lsulfo~l)phenyll-5-(trifluorometh 1y )1H-
pyrazole-4-
carboxamide
A solution of the product from Example 5A and 3-(methanesulfonyl)aniline were
processed as described in Example 1D to provide the desired product. MS (ESI+)
m/z 441
(M+NH4)+. 1H NMR (DMSO-d~) b 10.88 (s, 1H), 8.37-8.34 (m, 1H), 8.33 (d, 1H,
J=0.7 Hz),
8.00 (dt, 1H, J=7.1, 2.2 Hz), 7.71-7.64 (m, 2H), 7.43-7.40 (m, 4H), 3.23 (s,
3H), 2.42 (s, 3H).
Example 6
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
1~2-methoxyuhenyl)-5-(trifluorometh 1 -N-f3- trifluoromethyl)benz I~H-pyrazole-
4-
carboxamide
Example 6A
1~2-Methoxyphenyl)-5-trifluoromethyl-1H-~yrazole-4-carboxylic acid
2-Methoxyphenylhydrazine hydrochloride, the product from Example 1B, and
triethylamine were processed as described in Example 1C to provide the desired
product.
MS (EST-) m/z 285 (M-H)'.
Example 6B
1~2-methoxyphenyl)-5-(trifluorometh 1)-~trifluoromethyl)benz Iy 1-1H-pyrazole-
4-
carboxamide
A solution of the product from Example 6A and 3-trifluoromethylbenzyl amine
were
processed as described in Example 1D to provide the desired product. MS (ESI+)
m/z 444
(M+H)+. 1H NMR (DMSO-d~) 8 9.16 (t, 1H, J=5.9 Hz), 8.19 (d, 1H, J=0.7 Hz),
7.69-7.52
(m, 5H), 7.42 (dd, 1H, J=7.8, 1.7 Hz), 7.26 (dd, 1H, J=8.5, 1.0 Hz), 7.10 (td,
1H, J=7.8, 1.4
Hz), 4.54 (d, 2H, J=5.8 Hz), 3.76 (s, 3H).
Example 7
N-f2-(4-chlorophen I~yli-1-(2-methoxyphenyl)-5-(trifluoromethyl)-1H-pyrazole-4-
carboxamide
A solution of the product from Example 6A and 2-(4-chlorophenyl)ethylamine
were
processed as described in Example 1D to provide the desired product. MS (ESI+)
m/z 424
(M+H)+; 1H NMR (DMSO-d~) S 8.58 (t, 1H, J=5.8 Hz), 8.04 (d, 1H, J=0.7 Hz),
7.56 (ddd,
1H, J=9.2, 7.5, 1.7 Hz), 7.40 (dd, 1H, J=7.8, 1.7 Hz), 7.36 (d, 2H, J=8.5 Hz),
7.31-7.23 (m,
3H), 7.10 (td, 1H, J=7.5, 1.0 Hz), 3.76 (s, 3H), 3.45 (q, 2H, J=6.7 Hz), 2.82
(t, 2H, J=7.1
Hz).
Example 8
1-cyclohexyl-5-(trifluoromethyl)-N-f 3-(trifluoromethyl)benzyll-1H-pyrazole-4-
carboxamide
Example 8A
26
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
1-C clohexyl-5-trifluorometl~l-1H-pyrazole-4-carboxylic acid
Cyclohexylhydrazine hydrochloride, the product from Example 1B, and
triethylamine
were processed as described in Example 1C to provide the desired product. MS
(ESI+) m/z
281 (M+NH4)+.
Example 8B
1-cyclohexyl-5-(trifluoromethxl)-N-f 3-(trifluoromethyl)benz l~ 1-1H-pyrazole-
4-carboxamide
A solution of the product from Example 8A and 3-trifluoromethylbenzyl amine
were
processed as described in Example 1D to provide the desired product.
MS (ESI+) m/z 420 (M+H)~; 1H NMR (DMSO-d6) b 9.06 (t, 1H, J=5.9 Hz), 7.91 (s,
1H),
7.66-7.53 (m, 4H), 4.49 (d, 2H, J=6.1 Hz), 4.33-4.I8 (m, 1H), 1.97-1.77 (m,
6H), 1.73-1.60
(m, 1H), 1:51-1.3I (m, 2H), 1.29-1.13 (m, 1H).
Example 9
1-cyclohexyl-N- f 3-(methylsulfon~phenyll-5-(trifluoromethyl)-1H-pyrazole-4-
carboxamide
A solution of the product from Example 8A and 3-(methanesulfonyl)aniline were
processed as described in Example 1D to provide the desired product. MS
(DCI/NH3) m/z
433 (M+NH4)+; ~H NMR (DMSO-d~) 8 10.80 (s, 1H), 8.33 (s, 1H), 8.08 (s, 1H),
7.94 (dt, 1H,
J=7.1, 2.0 Hz), 7.69-7.62 (m, 2H), 4.37-4.24 (m, 1H), 3.21 (s, 3H), 1.99-1.80
(m, 6H), 1.75-
1.63 (m, 1H), 1.53-1.34 (m, ZH), I.32-1.17 (m, 1H).
Example 10
N-f2-(4-chlorophen 1y )ethyll-1-(7-chloroquinolin-4-yl)-5-(trifluorometh l~H-
pyrazole-4-
carboxamide
Example 10A
1-(7-Chloro-quinolin-4-yl)-5-trifluoromethyl-1H-p~razole-4-carboxylic acid
(7-Chloroquinolin-4-yI)-hydrazine hydrochloride, the product from Example 1B,
and
triethylamine were processed as described in Example 1C to provide the desired
product. MS
(ESI+) m/z 342 (M+H)+.
Example lOB
27
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
N-f 2-(4-chlorophenyl)ethyll-1-(7-chloroquinolin-4-yl)-5-(trifluoromethyl)-1H-
~yrazole-4
carboxamide
A solution of the product from Example 10A and 2-(4-chlorophenyl)ethylamine
were
processed as described in Example 1D to provide the desired product.
MS (ESI+) m/z 479 (M)+; iH NMZZ (DMS~-d6) S 9.18 (d, 1H, J=4.7 Hz), 8.74 (t,
1H, J=5.8
Hz), 8.32 (d, 1H, J=2.0 Hz), 8.30 (s, 1H), 7.86 (d, 1H, J=4.4 Hz), 7.78 (dd,
1H, J=8.8, 2.0
Hz), 7.41-7.28 (m, 5H), 3.50 (q, 2H, J=6.8 Hz), 2.85 (t, 2H, J=7.3 Hz).
Example 11
1-(1-benz~piperidin-4-yl)-N-f3-(methylsulfonyl)phenyll-5-(trifluoromethyl)-_
1H-pyrazole-4-
carboxamide
Example 11A
1-(1-Benz~piperidin-4-yl)-5-trifluoromethyl-1H-pyrazole-4-carboxylic acid
(1-Benzylpiperidin-4-yl)-hydrazine dihydrochloride, the product from Example
1B,
and triethylamine were processed as described in Example 1C to provide the
desired product.
MS (ESI+) m/z 354 (M+H)~".
Example 11B
1-(1-benzylpiperidin-4-yl)-N-f3-(methylsulfonyl)phen~ll-5-(trifluorometh~l)-1H-
pyrazole-4-
carboxamide
A solution of the product from Example 11A and 3-(methylsulfonyl)aniline were
processed as described in Example 1D to provide the desired product. MS (ESI+)
m/z 506
(M)+; 1H NMR (DMSO-d~) 8 10.84 (br s, 1H), 10.38 (br s, 1H), 8.32 (br s, 1H),
8.16 (br s,
1H), 7.94 (br d, 1H, J=7.6 Hz), 7.67-7.55 (m, 4H), 7.52-7.46 (m, 2H), 4.76 (m,
1H), 4.32 (m,
2H), 3.52-3.33 (m, 4H), 3.21 (s, 3H), 2.20-2.12 (m, 4H).
Example 12
1-(4-chlorophenyl)-5-methyl-N-f 3-(trifluorometh 1)benz l~-1H-~yrazole-4-
carboxamide
Example 12A
28
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
Ethyl 2-acetyl-3-(dimethylamino)acrylate
A mixture of ethyl acetoacetate(3.9 g, 30 mmol), p-toluenesulfonic acid
monohydrate
(catalytic amount) and N,N-dimethylformamide dimethyl acetal (5.3 g, 45 mmol)
was stirred
at 100 °C for 1.5 h. The mixture was distilled under vacuum (10 TO1T).
The distillate
collected between 145 °C and 165 °C afforded 3.1 g of the
desired compound as a yellowish
oil. MS (ESI) m/z 186 (M+H)+; 1H NMl2 (CDCl3) S 7.67 (s, 1H), 4.23 (q, 2H,
J=7.1 Hz),
3.04 (s, 6H), 2.33 (s, 3H), 1.32 (t, 3H, J=7.1 Hz).
Example 12B
1-(4-Chlorophenyl)-5-methyl-1H-~yrazole-4-carboxylic acid ethyl ester
An acetonitrile solution of the product from Example 12A (0.87 g, 4.7 mmol)
and
triethylamine (650 ~,L, 4.70 mmol) was added to a suspension of 4-
chlorophenylhydrazine
hydrochloride (0.84 g, 4.7 mmol) in acetonitrile (20 mL). The reaction mixture
was stirred at
25° C for 10 h. The solution was concentrated and the residue was
purified by silica gel flash
column chromatography (elution with 14% ethyl acetate/hexanes) to afford 1.22
g of the
desired product as a brown oil. MS (ESI) m/z 265 (M+H)+; 1H NMR (CDC13) ~ 8.02
(s, 1H),
7.51-7.46 (m, 2H), 7.40 7.35 (m, 2H),, 4.33 (q, 2H, J=7.lHz), 2.57 (s, 3H),
1.38 (t, 3H,
J=7.1Hz).
Example 12C
1-(4-Chlorophenyl)-5-metal-1H-pxrazole-4-carboxylic acid
The product from Example 12B (1.2 g, 4.5 mmol) was dissolved in methanol (10
mL)
and treated with a solvent mixture of THF (12 mL), 20% KOH (12 mL) and
methanol (12
mL). The solution was stirred at 25 °C for 10 h. The reaction mixture
was then diluted with
ethyl acetate (100 mL) and was partitioned between ethyl acetate (150 mL) and
water (300
mL). The aqueous layer was acidified to pH 2 and repartitioned between
dichloromethane
(200 mL) and water (250 mL). The organic layer was dried (sodium sulfate) and
concentrated
in vacuo to afford 1.1 g of the desired product as a white solid. MS (DCIlNH3)
m/z 237
(M+H)+; 1H NMl~ (CDC13) 8 8.10 (s, 1H), 7.52-7.47 (m, 2H), 7.42-7.37 (m, 2H),
4.78 (s,
1H), 2.59 (s, 3H).
Example 12D
29
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
1-(4-Chlorophenyl)-5-methyl-1H-~yrazole-4-carbonyl chloride
The product from Example 12C (570 mg, 2.40 mmol) in dichloromethane (30 mL)
was treated with oxalyl chloride (230 ~.L, 2.60 mmol), catalyzed by one drop
of DMF. The
reaction mixture was stirred at 25 °C for 3 h and the solvent was
evaporated to afford 610 mg
of the title coa~npou~nd as a white solid.
Exam lt~ a 12E
1-(4-chlorophenyl)-5-methyl-N-f3-(trifluorometh 1)benzyll-1H-~yrazole-4-
carboxamide
A solution of the product from Example 12D and 4-cyclohexylpiperidine were
processed as described in Example 1D to provide the desired product. MS (ESI)
m/z 394
(M+H)+;1H NMR (CDCl3) & 8.77 (t, 1H, J=6.1 Hz), 8.17 (s, 1H), 7.69-7.54 (m,
8H), 4.53 (d,
2H, J=5.8 Hz), 2.52 (s, 3H).
Example 13
N-(1-benzylniperidin-4-yl)-1-(4-chlorophenyl)-5-methyl-1H ~yrazole-4-
carboxamide
A solution of the product from Example 12D and 1-benzylpiperidin-4-ylamine
were
processed as described in Example 1D to provide the desired product. MS (ESI)
m/z 409
(M+H)+; 1H NMR (DMSO-d~) 8 8.13 (s, 1H), 7.85 (d, 1H, J=7.8 Hz), 7.63-7.52 (m,
4H),
7.36-7.21 (m, 5H), 3.81-3.68 (m, 1H), 3.47 (s, 2H), 2.50 (s, 3H), 2.81 (d, 2H,
J=11.5 Hz),
2.01 (t, 2H, J=11.5 Hz), 1.77 (d, 2H, J=12.1 Hz), 1.54 (q, 2H, J=11.7 Hz).
Example 14
1-(4-chlorophenyl)-N-f 2-(4-chlorophen_yl)ethyl)-5-methyl-1H-pyrazole-3-
carboxamide
Example 14A
1-(4-Chlorophenyl)-5-meth 1-~yrazole-3-carboxylic acid eth l~ ester
To an acetonitrile solution (25 mL) of 4-chlorophenylhydrazine hydrochloride
(1.0 g,
5.6 mmol)and triethylamine (780 ~,L, 5.60 mmol) was added ethyl acetopyruvate
(820 ~.L,
5.60 mmol). The solution was stirred at 25 °C for 12 h. The reaction
mixture was diluted with
dichloromethane (200 mL) and washed with water (250 mL). The organic Iayer was
dried
(sodium sulfate) and concentrated, and the residue was purified by silica gel
flash column
chromatography (elution with 25% ethyl acetate/hexanes) to afford 0.67 g of
the title
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
r.
compound as a light yellow oil. MS (ESI) m/z 265 (M+H)~; 1H NMR (DMSO-d6) 8
7.62 (s,
4H), 6.77 (s, IH), 4.29 (q, 2H, J=7.1 Hz), 2.34 (s, 3H), 1.30 (t, 3H, J=7.1
Hz).
Example 14B
1-(4-Chlorophenyl)-5-methyl-1H-~yrazole-3-carboxylic acid
A solution of the product from Example 14A were processed as described in
Example
12C to provide the desired product. MS (ESI) m/z 237 (M+H)+.
Example 14~C
1-(4-Chlorophenyl)-5-methyl-1H-pyrazole-3-carbonyl chloride
A solution of the product from Example 14B were processed as described in
Example
12D to provide the desired product.
Example 14D
1-(4-chlorophenyl)-N-f 2-(4-chlorophenyl)eth~ll-5-methyl-1H-pyrazole-3-
carboxamide
A solution of the product from Example 14D and 4-chlorophenethylamine were
processed as described in Example 1D to provide the desired product. MS (ESI)
m/z 390
(M+H)+; 1H NMR (DMSO-d~) 8 10.23 (s, 1H), 8.40-8.36 (m, 2H), 8.13-8.06 (m,
1H), 7.68-
7.58 (m, 6H), 3.22 (s, 3H), 2.58 (s, 3H).
Example 15
1~4-chlorophenyl)-5-methyl-N-f 3-(trifluorometh~lLenzyll-1H-pyrazole-3-
carboxamide
A solution of the product from Example 14D and 3-(trifluoromethyl)benzylamine
were processed as described in Example 1D to provide the desired product. MS
(ESI) mlz
394 (M+H)+; 1H NMR (DMSO-d~) 8 8.93 (t, 1H, J = 6.3 Hz), 7.68-7.52 (m, 8H),
6.68 (d; 1H,
J = 1.0 Hz), 4.50 (d, 2H, J = 6.4 Hz), 2.34 (d, 3H, J = 0.7 Hz).
Example I6
1-(4-chlorophenyl)-5-meth-N-f3-(methylsulfonyl)phen 1y 1-1H-pyrazole-3-
carboxamide
A solution of the product from Example 14D and 3-(methanesulfonyl)aniline were
processed as described in Example 1D to provide the desired product. MS (ESI)
m/z 407
(M+NH4)+; IH NMR (300MHz, DMSO-d~) ~ 10.50 (s, 1H), 8.49 (q, 1H, J=I.2 Hz),
8.18-
31
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
8.10 (m, 1H), 7.76-7.58 (m, 6H), 6.84 (d, 1H, J=1.0 Hz), 3.20 (d, 3H, J=1.0
Hz), 2.39 (d, 3H,
J-0.7 Hz).
Example 17
N-benzy~4-chlorophen l~ydroxy-1H-pyrazole-4-carboxamide
Example 17A,
1-(4-Chlorophenyl)-5-hydrox -y 1H-pyrazole-4-carboxylic acid
1-(4-Chloro-phenyl)-5-iodo-1H-pyrazole-4-carboxylic acid ethyl ester (0.5 g,
1.3
mmol) (J. Heterocycl. Chem. 1987, 267, 267-270) in EtOH (40 mL) was treated
with 20%
I~OH (10 mL) for 1 h at room temperature. The reaction mixture was
concentrated and the
residue was partitioned in EtOAc/H20. The aqueous layer was acidified to pH S
and the
resulting precipitate was filtered off to give 0.2 g of a (1:3) mixture of 1-
(4-chloro-phenyl)-5-
iodo-1H-pyrazole-4-carboxylic acid and 1-(4-chloro-phenyl)-5-hydroxy-1H-
pyrazole-4-
carboxylic acid. MS (ESI+) m/z 238 (M)+.
Exam 1p a 17B
1-(4-Chlorophenyl)-5-hydroxy-1H-pyrazole-4-carbonyl chloride
Example 17A (0.06 g, 0.3 mmol) in CH2C12 (5 mT~) was treated with oxalyl
chloride
(0.I mL), catalyzed by the addition of a few drops of DMF. After the reaction
mixture was
stirred at room temperature for 1 h the solvent was evaporated to give the
acid chloride,
Example 17C
N-benzyl-1-(4-chlorophen l~-5-hydroxy-1H-py razole-4-carboxamide
Example 17B was dissolved in THF (3 mL) and reacted with benzylamine (0.50 g,
0.45 mmol) in the presence of triethylamine (0.1 mL) and a catalytic amount of
DMAP. The
reaction mixture was stirred at ambient temperature for 16 h, the solvents
were evaporated in
vacuo and the remaining residue was purified by silica gel chromatography
(elution with 50%
EtOAc/hexandes) to yield the title compound. MS (DCI/NH3) m/z 329 (M+H)*; IH
NMR
(300 MHz, DMSO-d~) 8 4.47 (d, 2H), 7.30 (m, 5H), 7.60 (d, 2H), 7.88 (d, 2H),
8.20 (s, 1H),
8.76 (t, 1H), 8.98 (s, 1H).
Example 18
32
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
1-(4-chlorophenyl)-5-hydroxX N-f3-(methylsulfonyl) henvll-IH-pyrazole-4-
carboxamide
A solution of Example 17B was treated with 3-(methylsulfonyl)aniline
hydrochloride
in the presence of triethylamine and a catalytic amount of DMAP as described
in Example
17C to yield the title compound. MS (DCI/NH3) m/z 393 (M+H)+; ~H NMR (DMSO-d6)
S
3.20 (s, 3H), 7.62 (m, 4.H), 7.95 (d, 2H), 8.15 (m, 1H), 8.32 (s, IH), 8.36
(s, 1H), 9.16 (s, IH)
11.80 (s, 1H).
Example 19
1-(4-chlorophenyl)-5-c~no-N-f3-(methylsulfonyl)phen l~p~razole-4-carboxamide
Example 19A
~4-Chlorophenyl)-5-cyano-1H-pyrazole-4-carboxylic acid eth 1y ester
Ethyl 5-chloro-1-(4-chlorophenyl)-1H-pyrazole-4-carboxylate (J. Heterocycl.
Chem.
1987, 267, 267-270) (0.24 g, 0.80 mmol) in acetonitrile (30 mL) was stirred at
reflux for 16 h
with potassium cyanide (0.12 g, 1.8 mmol) and 18-crown-6 (0.5 g). The solvents
were
evaporated in vacuo and the residue was chromatographed on silica gel eluting
with 2:1
hexane-ether to yield 0.2 g of the title compound. MS (DCI/NH3) m/z 292 (M+H)
+; ~H
NMR (CDC13) S I.40 (t, 3), 4.40 (q, 2H), 7.54 (d, 2H), 7.67 (d, 2H), 8.18 (s,
1H).
Example 19B
1-(4-Chlorophenyl)-5-cyano-1H-pyrazole-4-carboxylic acid
Example 19A (0.2 g, 0.7 mmol) was stirred at ambient temperature in EtOH (10
mL)
and 20 % I~OH (5 mL) for 1 h. Ethanol was evaporated in vacuo and the
remaining residue
was dissolved in water and acidified to yield 0.12 g of the title compound as
a tan solid. MS
(DCIlNH3) m/z 265 (M+NH4)+; 1H NMR (CDC13) 7.56 (d, 2H), 7.70 (d, 2H), 8.23
(s, 1H),
12.80 (br s, 1H).
Example 19C
1-(4-chlorophenyl)-5-cyano-N-f 3-(methylsulfon~phenyll-1H-pyrazole-4-
carboxamide
Example 19B (0.1 g, 0.4 mmol) in CH2C12 was treated with oxalyl chloride (0.1
mL),
catalyzed by the addition of DMF. The reaction mixture was stirred at ambient
temperature
for 2 h, the solvents were removed in vacuo and the obtained acid chloride was
dissolved in
33
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
THF (3 mL) and reacted with 3-methylsulphonylaniline hydrochloride (0.I g, 0.5
mmol) in
the presence of triethylamine (0.14 mL, 1.0 mmol) and a catalytic amount of
DMAP. The
reaction mixture was stirred at ambient temperature for 16 h, evaporated and
purified by
chromatography to yield the title compound as a tan solid. MS (DCI/IVH3) m/z
418 (M+1VH4.)
+;1H NM~ (DMSO-d6) S 3.22 (s, 3H), 7.72 (m, 4H), 7.88 (d, 2H), 8.12 (m, 1H),
8.36 (s, 1H),
8.69 (s, 1H), 9.16 (s, 1H), 10.73 (s, 1H).
Example 20
1-(4-chlorophenyl)-1V-[3-(methylsulfonyl)phenyll-5-vinxl-1H-~yrazole-4-
carboxamide
Example 20A
1-(4-Chlorophenyl)-5-vin 1-~H-pyrazole-4-carboxylic acid eth, l
1-(4-Chloro-phenyl)-5-iodo-1H-pyrazole-4-carboxylic acid ethyl ester (0.76 g,
2
mmol) ( J. Heterocycl. Chem. 1987, 267, 267-270) was heated for 5 h at reflux
in toluene (10
mL) with tributylvinyltin (0.95 g, 3.0 mmol) and dichlorobis[tri(o-tolyl)
phosphine]palladium(II) (16 mg). The reaction mixture was evaporated in vacuo
and the
obtained residue was chromatographed on silica gel (elution with 10%
EtOAclhexanes) to
yield 0.60 g of the title compound. MS (DCI/NH3) m/z 277 (M+NHø)~.
Example 20B
1-(4-Chlorophenyl)-5-vin 1-~~yrazole-4-carboxylic acid
Example 20A (0.6 g, 2.2 mrnol) in EtOH (5 mL) was treated with 20 % KOH (2 mL)
fox 2 h at ambient temperature. Ethanol was evaporated and the aqueous layer
was acidified
to yield 0.4 g of the title compound as a tan solid. MS (DCI/NH3) mlz 249
(M+H)+; 1H NMR
(DMSO-d6) 8 5.60 (d, 2H) 6.83 (dd, 1H), 7.52 (d, 2H), 7.62 (d, 2H), 8.02 (s,
1H), 12.63 (br s,
1H).
Example ZOC
1-(4-Chlorophenyl)-5-vin~lH-pYrazole-4-carbonyl chloride
The product from Example 20B (0.4 g, 1. 6 mmol) in CHaCl2 (5 mL) was treated
with
oxalyl chloride (0.5 mL) in the presence of a catalytic amount of DMF. The
reaction mixture
34
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
was stirred at ambient temperature for 2 h upon which the solvent was
evaporated to yield the
title compound.
Example 201
1-(4-chlor~henyl)-N-f 3-(meth ls~ ulfon~phen 11-5-vinyl-1H-pxrazole-4-
carboxamide
Example 20C (0.054 g, 0.20 mmol) in THF (3 mL) was stirred for 16 h at ambient
temperature with 3-methylsulphonylaniline hydrochloride (0.052 g, 0.25 mmol)
in the
presence of triethylamine (0.7 ml,, 0.5 mmol) and a catalytic amount of DMAP.
The reaction
mixture was evaporated in vacuo and the residue was chromatographed to yield
the title
compound as a solid. MS (DCI/NH3) m/z 419 (M+NH4)~; 1H NMl2 (DMS~-d~) ~ 3.22
(s,
3H), 5.52 (s, 1H), 5.58 (d, 1H), 6.92 (dd, 1H), 7.55 (d, 2H), 7.65 (m, 4H),
8.05 (m 1H), 8.34
(s, 1H), 8.37 (s, 1H), 10.41 (s, 1H).
Example 21
1-(4-chlorophenyl)-N-(3,4-dichlorobenzyl)-5-vinyl-1H-pyrazole-4-carboxamide
The product from the Example 20C (0.054 g, 0.20 mmol) in THF (3 mL) was
stirred
for 16 h at ambient temperature with 3,4-dichlorobenzylamine (0.044 g, 0.25
mmol) in the
presence of triethylamine (0.35 mL, 0.25 mmol) and a catalytic amount of DMAP.
The
reaction mixture was evaporated in vacuo and the residue was chromatographed
to yield the
title compound as a solid. MS (DCI/NH3) m/z 406 (M+H)+; 1H NMR (DMSO-d~) 8
4.42 (d,
1H), 5.47 (s, 1H), 5.52 (d, 2H), 6.92 (dd, 1H), 7.31 (dd, 1H), 7.5 (d, 2H),
7.62 (m, 4H), 8.18
(s, 1H),8.82 (t, 1H).
Example 22
5-acetyl-1-(4-chlorophenyl)-N-f 3-(meth lsulfonyl)phenyl-1H-p~razole-4-
carboxamide
Example 22A
5-Acetyl-1-(4-chlorophen Iy )-1H-~yrazole-4-carboxylic acid ethyl ester
1-(4-Chlorophenyl)-5-iodo-1H-pyrazole-4-carboxylic acid ethyl ester (0.76 g, 2
mmol) (J. Heterocycl. Chem. 1987, 267, 267-270) was heated for 2 h at reflux
in toluene (10
mL) with tributyl(1-ethoxyvinyl)tin (1.0 mL, 3.0 mmol) and dichlorobis[tri(o-
tolyl)phosphine]palladium(II) (I6 mg). The reaction mixture was evaporated in
vacuo and the
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
obtained residue was stirred at ambient temperature for I6 h in 1:1 THF-2N HCl
(5 mL). The
reaction mixture was evaporated and the obtained residue was chromatographed
on silica gel
(elution with 10% EtOAclhexanes) to yield 0.20 g of the title compound. MS
(DCI/NH3) m/z
293 (M+H)~; 1H NMR (DMSO-ds) b 1.28 (t, 3H), 2.67 (s, 3H), 4.28 (q, 2H), 7.50
(d, 2H),
7.61 (d, 2H), 8.16 (s, 1H).
Example 22B
5-Acetyl-1-(4-chlorophenyl)-1H-pyrazole-4-carboxylic acid
Example 22A (0.2 g, 0.7 mmol) in EtOH (5 mL) was treated with 20 % KOH (2 mL)
for 2 h at ambient temperature. Ethanol was evaporated and the aqueous layer
was acidified
to yield 0.14 g of the title compound as a tan solid. MS (DCI/NH3) m/z
266(M+H)+; 1H NMR
(DMSO-d~) 8 2.67 (s, 3H), 7.50 (d, 2H), 7.60 (d, 2H), 8.10 (s, 1h), 12.80 (br
s, 1H).
Example 22C
5-Acet~4-chlorophenyl)-1H-pyrazole-4-carbonyl chloride
Example 22B (0.2 g, 0.8 mmol) in CH2C12 (5 mL) was treated with oxalyl
chloride
(0.4 mL) in the presence of a catalytic amount of DMF. The reaction mixture
was stirred at
ambient temperature for 2 h upon which the solvent was evaporated to yield the
tiltle
compound.
Example 22D
5-acetyl-1-(4-chlorophenyl)-N- f 3-(methylsulfonyl)phenyll-1H-pyrazole-4-
carboxamide
Example 22C (0.048 g, 0.17 mmol) in THF (3 mL) was stirred fox 16 h at ambient
temperature with 3-(methylsulfonyl)aniline hydrochloride (0.043 g, 0.21 mmol)
in the
presence of triethylamine (0.7 mL, 0.5 mmol) and a catalytic amount of DMAP.
The reaction
mixture was evaporated in vacuo and the residue was chromatographed to yield
the title
compound as a solid. MS (DCI/NH3) m/z 435 (M+NH4)+; 1H NMR (DMSO-d~) 8 2.61
(s,
3H), 3.22 (s, 3H), 7.51 (d, 2H), 7.61 (d,2H), 7.66 (d, 2H), 8.08 (s, 1H), 8.33
(s, 1H), 8.39 (s,
1H), 10.7 (s, 1H).
Exam 1p a 23
5-acetyl-1-(4-chlorophenyl)-N-(3,4--dichlorobenz l~-IH~yrazole-4-caxboxamide
36
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
Example 22C (0.048 g, 0.17 mmol) in THF (3 mL) was stirred fox 16 h at ambient
temperature with 3,4-dichlorobenzylamine (0.035 g, 0.20 mmol) in the presence
of
triethylamine (0.35 mL, 0.25 mmol) and a catalytic amount of DMAP. The
reaction mixture
dues evaporated in vacuo and the residue was chromatographed to yield the
title compound as
a solid. MS (DCI/1~1H3) m/z 423 (M+H)+; 1H 1~MZ (DMSO-d6) S 2.6 (s, 3H), 4.43
(d, 2H),
7.32 (dd, 1H), 7.48 (d, 2H), 7.58 (d, 2H), 7.62 (d, 2H), 8.2 (s, 1H), 9.12 (t,
1H).
Example 24
1-(4-chlorophen~l)-N-(2-methox b~ enzyl)-5-methyl-lH~yrazole-3-carboxamide
A solution of the product from Example 14D and 2-methoxybenzylamine were
processed as described in Example 1D to provide the desired product. MS (ESI)
mlz 356
(M+H)+; 1H NMR (300MHz, DMSO-d~) ~ 8.48 (t, 1H, J = 6.1 Hz), 7.60-7.69 (m,
4H), 7.22
(ddd, 1H, J = 8.1, 7.5, 1.7 Hz), 7.14 (dd, 1H, J = 7.5, 1.7 Hz), 6.98 (dd, 1H,
J = 8.5, 1.0 Hz),
6.89 (t, 1H, J = 7.5, 1.0 Hz), 6.68 (d, 1H, J = 0.7 Hz), 4.40 (d, 2H, J = 6.1
Hz), 3.82 (s, 3H),
2.35 (s, 3H).
Exam 1p a 25
1-(4-chlorophenyl)-5-methyl-N-(2-meth lbenzyl)-1H-~~razole-3-carboxamide
A solution of the product from Example 14D and 2-methylbenzylamine were
processed as described in Example 1D to provide the desired product. MS (ESI)
m/z 340.
(M+H)+; 1H NMR (300MHz, DMSO-d~) ~ 8.60 (t, 1H, J = 6.1 Hz), 7.96-7.59 (m,
4H), 7.26-
7.19 (m, 2H), 7.17-7.10 (m, 2H), 6.68 (d, 1H, J = 0.7 Hz), 4.41 (d, 2H, J =
6.1 Hz), 2.35 (s,
3H), 2.31 (s, 3H).
Functional Studies on Tetxodotoxin Resistant (TTX-R) Currents in
Neuropathic Pain Model
Abnormal activity of sodium channels in the peripheral nervous system plays a
role in
the pathophysiology of chronic pain. Sodium channels are critical elements in
the
transduction of action potentials in excitable tissues such as nerve and
muscle, and as such,
participate in many physiological processes. The recent identification of
sensory neuron-
specific sodium channels such as Navl.B, the observation that their expression
is altered in
chronic pain states, and the demonstration that Navl.B antisense attenuates
pain in animal
37
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
models suggest that these channels are attractive targets for drug discovery.
Given the
restricted expression pattern of these channels, selective blockers could, in
principle, be
effective analgesics without undesirable effects observed with nonselective
sodium channel
Mockers. Although excitability of sensory neurons can be modulated by various
receptors
and ion cha~.nnel processes, sodium channels directly regulate neuronal
excitability.
Spontaneously ectopic action potential firing in dorsal root ganglion (DRG)
neurons
is believed to be the underlying mechanism that evokes neuropathic pain
following nerve
injury. It has been recognized for some time that tetrodotoxin-resistant (TTX-
R) current
increases in chronic pain, and several studies have implicated Navl.8 as the
primary channel
responsible for this increased current.
To examine functional effects, TTX-R sodium currents were studied in dorsal
root
ganglion (DRG) neurons from rats 14 days following spinal nerve ligation
(SNL). Small size
neurons (C-fiber neurons < 25 Vim) from L4 and L5 DRG were dissociated from
rats, and Na+
currents were measured in the presence of 100 nM TTX by whole-cell current
recording. The
total Na+ current density was reduced by 4.2% in L5 DRG neurons from SNL rats
compared
to L5 DRG neurons from sham operated rats. This reduction in current density
was
attributable to a significant reduction in TTX-resistant, but not TTX-
sensitive currents.
Moreover, this decrease in TTX-R current was observed only in the L5 injured
region,
whereas there was a significant increase in TTX-R currents in the L4 uninjured
region.
Although TTX-R currents were significantly decreased in L5 ganglia, the
increased
TTX-R currents in L4 ganglia may be responsible for the firing and sensation
of neuropathic
pain. A Nav 1.8 channel inhibitor may attenuate neuropathic pain by blocking
currents in L4
DRG neurons, as well as by blocking currents generated at the nociceptive
peripheral
terminals. Representative compounds of the present invention demonstrated
ICsos from about
500 nM to about 3 p.M.
Compounds of the present invention inhibit the PN3 sodium channel and are
therefore
useful as analgesics for neuropathic pain.
The present invention also provides pharmaceutical compositions that comprise
compounds of the present invention. The pharmaceutical compositions comprise
compounds
of the present invention formulated together with one or more non-toxic
pharmaceutically
acceptable carriers.
38
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
The pharmaceutical compositions of this invention can be administered to
humans
and other mammals orally, rectally, parenterally , intracisternally,
intravaginally, topically (as
by powders, ointments or drops), bucally or as an oral or nasal spray. The
term
"parenterally," as used herein, refers to modes of administration which
include intravenous,
intramuscular, intraperitoneal, intrasternal, subcutaneous and intraarticular
injection and
infusion.
The term "pharmaceutically acceptable carrier," as used herein, means a non-
toxic,
inert solid, semi-solid or liquid filler, diluent, encapsulating material or
formulation auxiliary
of any type. Some examples of materials which can serve as pharmaceutically
acceptable
carriers are sugars such as, but not limited to, lactose, glucose and sucrose;
starches such as,
but not limited to, corn starch and potato starch; cellulose and its
derivatives such as, but not
linnited to, sodium carboxymethyl cellulose, ethyl cellulose and cellulose
acetate; powdered
tragacanth; malt; gelatin; talc; excipients such as, but not limited to, cocoa
butter and
suppository waxes; oils such as, but not limited to, peanut oil, cottonseed
oil, safflower oil,
sesame oil, olive oil, corn oil and soybean oil; glycols; such as propylene
glycol; esters such
as, but not limited to, ethyl oleate and ethyl laurate; agar; buffering agents
such as, but not
linnited to, magnesium hydroxide and aluminum hydroxide; alginic acid; pyrogen-
free water;
isotonic saline; Ringer's solution; ethyl alcohol, and phosphate buffer
solutions, as well as
other non-toxic compatible lubricants such as, but not limited to, sodium
lauryl sulfate and
magnesium stearate, as well as coloring agents, releasing agents, coating
agents, sweetening,
flavoring and perfuming agents, preservatives and antioxidants can also be
present in the
composition, according to the judgment of the formulator.
Pharmaceutical compositions of this invention for parenteral injection
comprise
pharmaceutically acceptable sterile aqueous or nonaqueous solutions,
dispersions,
suspensions or emulsions as well as sterile powders for reconstitution into
sterile injectable
solutions or dispersions just prior to use. Examples of suitable aqueous and
nonaqueous
carriers, diluents, solvents or vehicles include water, ethanol, polyols (such
as glycerol,
propylene glycol, polyethylene glycol and the like), vegetable oils (such as
olive oil),
injectable organic esters (such as ethyl oleate) and suitable mixtures
thereof. Proper fluidity
can be maintained, for example, by the use of coating materials such as
lecithin, by the
maintenance of the required particle size in the case of dispersions and by
the use of
surfactants.
39
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
These compositions may also contain adjuvants such as preservatives, wetting
agents,
emulsifying agents and dispersing agents. Prevention of the action of
microorganisms can be
ensured by the inclusion of various antibacterial and antifungal agents, fox
example, paraben,
chlorobutanol, phenol sorbic acid and the like. It may also be desirable to
include isotonic
agents such as sugars, sodium chloride and the like. Prolonged absorption of
the injectable
pharmaceutical form can be brought about by the inclusion of agents which
delay absorption
such as aluminum monostearate and gelatin.
In some cases, in order to prolong the effect of the drug, it is desirable to
slow the
absorption of the drug from subcutaneous or intramuscular injection. This can
be
accomplished by the use of a liquid suspension of crystalline or amorphous
material with
poor water solubility. The rate of absorption of the drug then depends upon
its rate of
dissolution which, in turn, may depend upon crystal size and crystalline form:
Alternatively,
delayed absorption of a parenterally administered drug form is accomplished by
dissolving or
suspending the drug in an oil vehicle.
Injectable depot forms are made by forming microencapsule matrices of the drug
in
biodegradable polymers such as polylactide-polyglycolide. Depending upon the
ratio of drug
to polymer and the nature of the particular polymer employed, the rate of drug
release can be
controlled. Examples of other biodegradable polymers include poly(orthoesters)
and
poly(anhydrides). Depot injectable formulations are also prepared by
entrapping the drug in
liposomes or microemulsions which are compatible with body tissues.
The injectable formulations can be sterilized, for example, by filtration
through a
bacterial-retaining filter or by incorporating sterilizing agents in the form
of sterile solid
compositions which can be dissolved or dispersed in sterile water or other
sterile injectable
medium just prior to use.
Solid dosage forms for oral administration include capsules, tablets, pills,
powders
and granules. In such solid dosage forms, the active compound may be mixed
with at least
one inert, pharmaceutically acceptable carrier or excipient, such as sodium
citrate or
dicalcium phosphate and/or a) fillers or extenders such as starches, lactose,
sucrose, glucose,
mannitol and silicic acid; b) binders such as carboxymethylcellulose,
alginates, gelatin,
polyvinylpyrrolidone, sucrose and acacia; c) humectants such as glycerol; d)
disintegrating
agents such as agar-agar, calcium carbonate, potato or tapioca starch, alginic
acid, certain
silicates and sodium carbonate; e) solution retarding agents such as paraffin;
f) absorption
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
accelerators such as quaternary ammonium compounds; g) wetting agents such as
cetyl
alcohol and glycerol monostearate; h) absorbents such as kaolin and bentonite
clay and i)
lubricants such as talc, calcium stearate, magnesium stearate, solid
polyethylene glycols,
sodium lauryl sulfate and mixtures thereof. In the case of capsules, tablets
and pills, the
dosage form may also comprise buffering agents.
Solid compositions of a similar type may also be employed as fillers in soft
and hard-
filled gelatin capsules using such carriers as lactose or milk sugar as well
as high molecular
weight polyethylene glycols and the like.
The solid dosage forms of tablets, dragees, capsules, pills and granules can
be
prepared with coatings and shells such as enteric coatings and other coatings
well-known in
the pharmaceutical formulating art. They may optionally contain opacifying
agents and may
also be of a composition such that they release the active ingredients) only,
or preferentially,
in a certain part of the intestinal tract, optionally, in a delayed manner.
Examples of
7
embedding compositions which can be used include polymeric substances and
waxes.
The active compounds can also be in micro-encapsulated form, if appropriate,
with
one or more of the above-mentioned carriers.
Liquid dosage forms for oral administration include pharmaceutically
acceptable
emulsions, solutions, suspensions, syrups and elixirs. In addition to the
active compounds,
the liquid dosage forms may contain inert diluents commonly used in the art
such as, fox
example, water or other solvents, solubilizing agents and emulsifiers such as
ethyl alcohol,
isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl
benzoate, propylene
glycol, 1,3-butylene glycol, dimethyl formamide, oils (in particular,
cottonseed, groundnut,
corn, germ, olive, castor and sesame oils), glycerol, tetrahydrofurfuryl
alcohol, polyethylene
glycols and fatty acid esters of sorbitan and mixtures thereof.
Besides inert diluents, the oral compositions may also include adjuvants such
as
wetting agents, emulsifying and suspending agents, sweetening, flavoring and
perfuming
agents.
Suspensions, in addition to the active compounds, may contain suspending
agents as,
for example, ethoxylated isostearyl alcohols, polyoxyethylene sorbitol and
sorbitan esters,
microcrystalline cellulose, aluminum metahydroxide, bentonite, agar-agar,
tragacanth and
mixtures thereof.
Compositions for rectal or vaginal administration are preferably suppositories
which
41
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
can be prepared by mixing the compounds of this invention with suitable non-
irritating
carriers or carriers such as cocoa butter, polyethylene glycol or a
suppository wax which are
solid at room temperature but liquid at body temperature and therefore melt in
the rectum or
vaginal cavity and release the active compound.
Compounds of the present invention can also be administered in the form of
liposomes. As is known in the art, liposomes are generally derived from
phospholipids or
other lipid substances. Liposomes are formed by mono- or mufti-lamellar
hydrated liquid
crystals which are dispersed in an aqueous medium. Any non-toxic,
physiologically
acceptable and metabolizable lipid capable of forming liposomes can be used.
The present
compositions in liposome form can contain, in addition to a compound of the
present
invention, stabilizers, preservatives, excipients and the like. The preferred
lipids are natural
and synthetic phospholipids and phosphatidyl cholines (lecithins) used
separately or together.
Methods to form liposomes are known in the art. See, for example, Prescott,
Ed.,
Methods in Cell Biology, Volume XIV, Academic Press, New York, N.Y. (1976), p.
33 et
seq.
Dosage forms for topical administration of a compound of this invention
include
powders, sprays, ointments and inhalants. The active compound may be mixed
under sterile
conditions with a pharmaceutically acceptable carrier and any needed
preservatives, buffers
or propellants which may be required. Opthalmic formulations, eye ointments,
powders and
solutions are also contemplated as being within the scope of this invention.
Actual dosage levels of active ingredients in the pharmaceutical compositions
of this
invention can be varied so as to obtain an amount of the active compounds)
which is
effective to achieve the desired therapeutic response for a particular
patient, compositions and
mode of administration. The selected dosage level will depend upon the
activity of the
particular compound, the route of administration, the severity of the
condition being treated
and the condition and prior medical history of the patient being treated.
When used in the above or other treatments, a therapeutically effective amount
of one
of the compounds of the present invention can be employed in pure form or,
where such
forms exist, in pharmaceutically acceptable salt, ester or prodrug form. The
phrase
"therapeutically effective amount" of the compound of the invention means a
sufficient
amount of the compound to treat disorders, at a reasonable benefit/rislc ratio
applicable to any
medical treatment. It will be understood, however, that the total daily usage
of the
42
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
compounds and compositions of the present invention will be decided by the
attending
physician within the scope of sound medical judgement. The specific
therapeutically
effective dose level for any particular patient will depend upon a variety of
factors including
the disorder being treated and the severity of the disorder; activity of the
specific compound
employed; the specific composition employed; the age, body weight, general
health, sex and
diet of the patient; the time of administration, route of administration, and
rate of excretion of
the specific compound employed; the duration of the treatment; drugs used in
combination or
coincidental with the specific compound employed; and like factors well known
in the
medical arts.
The term "pharmaceutically acceptable salt," as used herein, means salts
derived from
inorganic or organic acids. The salts can be prepared in situ during the final
isolation and
purification of compounds of the present invention or separately by reacting
the free base of a
compound of a compound of the present invention with an inorganic or organic
acid.
Representative acid addition salts include, but are not limited to, acetate,
adipate, alginate,
citrate, aspartate, benzoate, benzenesulfonate, bisulfate, butyrate,
camphorate,
camphorsufonate, digluconate, glycerophosphate, hemisulfate, heptanoate,
hexanoate,
fumarate, hydrochloride, dihydrochloride, hydrobromide, hydroiodide, 2-
hydroxyethansulfonate (isethionate), lactate, maleate, fumarate,
methanesulfonate, nicotinate,
2-naphthalenesulfonate, oxalate, pamoate, pectinate, persulfate, 3-
phenylpropionate, picrate,
pivalate, propionate, succinate, sulfate, (L) tartrate, (D) tartrate, (DL)
tartrate, thiocyanatea,
phosphate, glutamate, bicarbonate, p-toluenesulfonate, and undecanoate.
Representative
examples include, but are not limited to N-(1-benzylpiperidin-4-yl)-5-methyl-1-
phenyl-1H-
pyrazole-4-carboxamide hydrochloride, N-(1-benzylpiperidin-4-yl)-5-methyl-1-
phenyl-1H-
pyrazole-4-carboxamide (L) tartrate, or N-(1-benzylpiperidin-4-yl)-5-methyl-1-
phenyl-1H-
pyrazole-4-carboxamide benzene sulfonate.
The term "pharmaceutically acceptable ester," as used herein, means esters of
compounds of the present invention which hydrolyze in vivo and include those
that break
down readily in the human body to leave the parent compound or a salt thereof.
Examples of
pharmaceutically acceptable, non-toxic esters of the present invention include
C1-to-C~ alkyl
esters and C5-to-C7 cycloalkyl esters, although C1-to-C~ alkyl esters are
preferred. Esters of
the compounds of the present invention may be prepared according to
conventional methods.
Representative examples include, but are not limited to, ethyl 4-(4-{ [(1-
benzylpiperidin-4-
43
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
yl)amino]carbonyl}-5-methyl-1H-pyrazol-1-yl)benzoate or methyl 4-(4-{[(1-
benzylpiperidin-
4-yl)amino]carbonyl }-5-methyl-1H-pyrazol-1-yl)benzoate.
The term "pharmaceutically acceptable amide," as used herein, means to non-
toxic
amides of the present invention derived from ammonia, primary Cl-to-CG alkyl
amines and
secondary C1-to-C6 dialkyl amines. In the case of secondary amines, the amine
may also be
in the form of a 5- or 6-membered heterocycle containing one nitrogen atom.
Amides
derived from ammonia, C1-to-C3 alkyl primary amides and C1-to-C2 dialkyl
secondary
amides are preferred. Amides of the compounds of the present invention may be
prepared
according to conventional methods. Representative examples include, but are
not limited to,
1-[4-(aminocarbonyl)phenyl]-N (1-benzylpiperidin-4-yl)-5-methyl-1H-pyrazole-4-
carboxamide or N-(1-benzylpiperidin-4-yl)-1-{4-
[(dimethylamino)carbonyl]phenyl}-5-
methyl-1H-pyrazole-4-carboxamide.
The term "pharmaceutically acceptable prodrug" or "prodrug,"as used herein,
represents those prodrugs of the compounds of the present invention which are,
within the
scope of sound medical judgement, suitable for use in contact with the tissues
of humans and
lower animals without undue toxicity, irritation, allergic response, and the
like. Prodrugs of
the present invention may be rapidly transformed in vivo to compounds of the
present
invention, for example, by hydrolysis in blood. Representative examples
include, but are°not
limited to, N-acetyl-N-(1-benzylpiperidin-4-yl)-5-methyl-1-phenyl-1H-pyrazole-
4-
carboxarnide, N-(1-benzylpiperidin-4-yl)-N-(2,2-dimethylpropanoyl)-5-methyl-1-
phenyl-1H-
pyrazole-4-carboxamide, or ethyl 1-benzylpiperidin-4-yl[(5-methyl-1-phenyl-1H-
pyrazol=4-
yl)carbonyl]carbamate.
The present invention contemplates compounds of the present invention formed
by
synthetic means or formed by in vivo biotransformation.
The compounds of the invention can exist in unsolvated as well as solvated
forms,
including hydrated forms, such as hemi-hydrates. In general, the solvated
forms, with
pharmaceutically acceptable solvents such as water and ethanol among others
are equivalent
to the unsolvated forms for the purposes of the invention.
The total daily dose of the compounds of this invention administered to a
human or
lower animal may range from about 0.01 to about 125 mg/kg/day. For purposes of
oral
administration, more preferable doses can be in the range of from about 0.1 to
about 150
mg/lcg/day. If desired, the effective daily dose can be divided into multiple
doses for
44
CA 02525325 2005-11-O1
WO 2004/099154 PCT/US2004/013530
purposes of administration; consequently, single dose compositions may contain
such
amounts or submultiples thereof to make up the daily dose.