Note: Descriptions are shown in the official language in which they were submitted.
CA 02525856 2005-11-14
WO 2004/113411 PCT/US2004/016459
POLYPHOSPHONATE FLAME RETARDANT CURING AGENT FOR EPOXY RESIN
This invention relates to a new use for the type of
polyphosphonate material that is described and claimed in
U.S. Patent Nos. 4,331,614 and 4,719,279, which are each
incorporated herein by reference. While U.S. patent No.
4,035,442 has described polyester filaments having improved
flame retardancy due to use of a poly(m-phenylene
cyclohexylphosphonate), the instant invention relates to
flame retarded epoxy compositions, as will be described in
greater detail below. The term "polyphosphonate°' as used
herein is to be construed as covering oligomeric phosphonate
materials as well as those of higher molecular weight.
Shouji Minegishi describes the reaction of epoxy
compounds with phosphonates in Journal of Polymer Science,
Part A, Polymer Chemistry, Vol. 37, 959-965:
O
*~O CH Hz R Ha C O P-~--
R2~0 P O ~ ~ R2 + ~-R---~ ~ ~ z CHZ R3
R3 O O O O
R2 R2
His goal was to prepare polymeric linear phosphonates with
little or no residual epoxy. Such polymers can be used as
additive type flame retardants.
The present invention relates to an epoxy resin
composition that can be used, for example, in printed wiring
boards for electronic applications: It represents a new
embodiment, for example, over the type of flame retarded
epoxy resin compositions described in PCT Patent Publication
1
CA 02525856 2005-11-14
WO 2004/113411 PCT/US2004/016459
No. WO 03/029258 where an epoxy resin, which contained the
type of polyphosphonate flame retardant additive that is
also used in the epoxy resin compositions of the present
invention, was cured in the presence of a methylimidazole
curing catalyst.
The composition of the present invention contains, as
one essential component, an epoxy resin. This component is
present at from about 50% to about 95%, by weight of the
total weight of the composition. This component can be a
non-halogen containing epoxy resin, such as a bisphenol A-
type of epoxy resin, or other resins of this general type
that have utility for the manufacture of printed wiring
boards or other electronic substrate materials of that type
(for example, bisphenol F epoxy, phenolic novolak epoxy,
cresol novolak epoxy, and/or bisphenol A novolak epoxy
resins). Compatible mixtures of any of these resins may be
employed, if desired.
The polyphosphonate flame retardant curing agent that
forms another essential additive of the compositions of the
present invention is generally present at from about 5% to
about 50%, by weight of the total weight of the composition,
for example, from about 10% to about 35%, by weight. In
regard to epoxy compositions of the present invention, and
unlike the invention described a.n PCT Patent Publication No.
WO 03/029258, this polyphosphonate functions as the
effective curing agent for the epoxy resin.
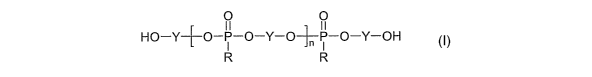
This curing agent is an polyphosphonate composition
having the formula:
2
CA 02525856 2005-11-14
WO 2004/113411 PCT/US2004/016459
HO-Y-~-O-P-O-Y-O-~-P-O-Y-OH
R R
where "Y" is an arylene and "n" can range from about 2 to
about 30. If desired, mixed arylenes diols and mixed
alkylphosphonates can be used to prepare mixed products.
This oligomeric or polymeric material has a phosphorus
content of greater than about 10%, by weight. The
polyphosphonate species in the composition comprise
oligomers or polymers of this type that can either contain -
OH end groups or not contain such end groups. The
individual polyphosphonate species that contain -OH end
groups can be monohydroxy or dihydroxy-substituted. The
concentration of polyphosphonate species in the composition
that contain hydroxy end groups can range from about 20% to
about 100%, based upon the total number of termination ends
("chain ends") that potentially could hold such end groups,
for example, from about 50% to about 100%. The number of OH
terminal groups can be controlled by the ratio of arylene to
phosphonate or by using end-capping agents such as triaryl
phosphates. In the former case, if excess arylene-
containing diol is used with the phosphonate reagent, for
example, in making the polyphosphonate, there will be a
higher hydroxyl content in the final product, and vice
versa. The preferred R group is methyl, but can be lower
alkyl.
By "Arylene" is meant any radical of a dihydric phenol.
The dihydric phenol can have its two hydroxy groups in non-
adjacent positions. Examples include the resorcinols;
hydroquinones; and bisphenols, such as bisphenol A,
bisphenol F, and 4,4'-biphenol, phenolphthalein, 4,4'-
3
CA 02525856 2005-11-14
WO 2004/113411 PCT/US2004/016459
thiodiphenol, or 4,4'-sulfonyldiphenol. A small amount of
polyhydric phenol, such as a novolac or phloroglucinol, with
three or more hydroxyl groups therein can be included to
increase the molecular weight. The Arylene group can be
1,3-phenylene, 1,4-phenylene, or a bisphenol diradical unit,
but it is preferably 1,3-phenylene.
The polyphosphonate curing agent for the epoxy resin
composition of this invention can be made by any of several
routes: (1) the reaction of an RPOC12 with HO-Aryl-OH or a
salt thereof, where R is lower alkyl, preferably methyl; (2)
the reaction of diphenyl alkylphosphonate, preferably
methylphosphonate, with HO-Arylene-OH under
transesterification conditions; (3) the reaction of an
oligomeric phosphate with repeating units of the structure -
OP(OR')-O-Arylene- with an Arbuzov rearrangement catalyst,
where R' is lower alkyl, preferably methyl; or,(4) the
reaction of an oligomeric phosphate with the repeating units
having the structure -OP(O-Ph)-O-Arylene with trimethyl
phosphate and an Arbuzov catalyst or With dimethyl
methylphosphonate with, optionally, an Arbuzov catalyst-.
The -OH end groups, a.f attached to Arylene can be produced
by having a controlled molar excess of the HO-Arylene-OH in
the reaction media. The -OH end groups, if acid type (P-
OH), can be formed by hydrolytic reactions. It is preferred
that the end groups of the oligomers be mainly -Arylene-OH
types .
The epoxy resin composition of the present invention
can contain optional additives like auxiliary flame
retardant additive as well including the following types of
materials: auxiliary curing catalysts, fiber and/or cloth
4
CA 02525856 2005-11-14
WO 2004/113411 PCT/US2004/016459
reinforcing additives; mineral fillers, such as Al(OH)3,
Mg(OH)2 or silica; release agents; colorants; and the like.
The present invention is further illustrated by the
Examples that follow.
Example 1
The aforementioned polyphosphonate curing agent (poly(m-
phenylene methylphosphonate)(29.1 g) was mixed with 70.1 g
of bisphenol A type epoxy (epoxy equivalent: 180) to prepare
a master batch. A 10 g sample was removed from the master
. batch and was placed in a nitrogen-purged oven at 179°C. A
sample was removed after ninety minutes and was cooled to
room temperature. The Barkol hardness was than measured
using G1 934-1 meter. The sample was uniformly cured with a
hardness of 28-29. The sample was submerged in acetone for
twelve hours. No swelling or dissolution was observed.
5
CA 02525856 2005-11-14
WO 2004/113411 PCT/US2004/016459
Comparative Example 2
A 10g sample of bisphenol A type epoxy (epoxy equivalent:
180) was placed the nitrogen-purged oven at 179°C for one
hundred minutes. Sample did not cure or gel. It was a
viscous liquid at room temperature and was completely
soluble a.n acetone.
Comparative Example 3
An oligomeric aliphatic phosphonate of the formula
O O
HO-Bu-~-O- IP-O-Bu-O~ IP-O-Bu-OH
R R
where "Bu" is C4H8, was combined with a bisphenol A di-epoxy
resin (epoxy equivalent of 180) to prepare a mixture
containing 70-wt% epoxy resin. The mixture was placed in an
oven at 150°C and was heated for one and one half hours. No
curing occurred. The resulting product was a viscous
liquid at room temperature.
Comparative Example 4
A polyphosphate, poly(ethyl ethyleneoxy) phosphate),
FYROL PNX brand from Akzo Nobel, was mixed with a bisphenol
A di-epoxy resin (epoxy equivalent of 180) to form a mixture
containing 70% of epoxy resin and 30% of FYROL PNX. Mixture
was placed in the oven at 150°C for one and one half hours.
It did not cure.
Comparative Example 5
A polyphosphonate, which is the reaction product of
dimethyl methylphosphonate, P205 and ethylene oxide (FYROL HP
brand from Akzo Nobel) with a hydroxyl number of 125mg KOH/g
6
CA 02525856 2005-11-14
WO 2004/113411 PCT/US2004/016459
was mixed with a bisphenol A di-epoxy resin (epoxy
equivalent 180) to form a mixture containing 70% of epoxy
resin and 30% of the polyphosphonate. The mixture was
placed in an oven at 150°C for one and one half hours. It
did not cure.
Example 6
A 30% by weight mixture of diphenyl methylphosphonate
and a bisphenol A di-epoxy resin (epoxy equivalent 180) was
heated at 150°C for one and one half hours. Reaction
product was a viscous liquid at room temperature. It was
analyzed by P31 NMR. It contained 20% of unreacted DPMP 56%
of a mono-inserted product and 23% of the di-inserted
product. The 13C NMR and FT IR spectra indicate presence of
a significant amount of unreacted epoxy groups essential to
incorporated the product in any epoxy composition.
Example 7
A 30% by weight mixture of the polyphosphonate used in
Example 1 and 70% by weight of a bisphenol A di-epoxy resin
(epoxy equivalent 180) were heated at 137°C for one hour.
The reaction product was a solid at room temperature but it
was completely soluble in chloroform or acetone. It was
analyzed by P3s NMR. About 52% of phosphorous remained
unchanged, and the rest was reacted with the epoxy
Example 8
The epoxy insertion product as described in Example 7
was placed in the oven at 150°C for one hour and then the
temperature was increased to 180°C over a period of one
7
CA 02525856 2005-11-14
WO 2004/113411 PCT/US2004/016459
hour. The resulting cured epoxy composition had a BARKOL
hardness of 32 (measured using Gl 934-1 meter).
Example 9
The epoxy insertion product as described in Example 7
was dissolved in acetone and mixed with ATH to make a blend
containing 62% ATH by weight and 38% of the epoxy
composition. Acetone was evaporated under reduced pressure
and resulting paste mixed well and cured at 150°C for one
hour followed by a gradual temperature increase to 160°C
over a period of thirty minutes. The resulting cured epoxy
composition had a BARKOL hardness of 52.
Example 10
The epoxy insertion product as described in Example 7
dissolved in acetone and combined with 1000 ppm of
methylimidazole and 1000 ppm of triphenyl phosphine.
Acetone was evaporated under reduced pressure and sample
cured at 138°C for one hour. The resulting cured epoxy
composition had a BARKOL hardness of 25.
8