Note: Descriptions are shown in the official language in which they were submitted.
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
MECHANICAL APPARATUS AND METHOD FOR
DILATING AND DELIVERING A THERAPEUTIC
AGENT
BACKGROUND OF THE INVENTION
Cardiovascular disease is commonly accepted as being
one of the most serious health risks facing our society
today. Diseased and obstructed coronary arteries can
restrict the flow of blood and cause tissue ischemia and
necrosis. While the exact etiology of sclerotic
cardiovascular disease is still in question, the
treatment of narrowed coronary arteries is more defined.
Surgical construction of coronary artery bypass grafts
(CABG) is often the method of choice when there are
several diseased segments in one or multiple arteries.
Open heart surgery is, of course, very traumatic for
patients. In many cases, less traumatic, alternative
methods are available for treating cardiovascular disease
percutaneously. These alternate treatment methods
generally employ various types of percutaneous
transluminal angioplasty (PTCA) balloons or excising
devices (atherectomy) to remodel or debulk diseased
vessel segments. A further alternative treatment method
involves percutaneous, intraluminal installation of
expandable, tubular stents or prostheses in sclerotic
lesions.
A recurrent problem with the previous devices and
PTCA procedures is their failure to maintain patency due
to the growth of injured vascular tissue. This is known
as "restenosis" and may be a result of the original
injury to the vessel wall occurring during the
angioplasty procedure. Pathologically restenosis
represents a neointimal proliferative response
characterized by smooth muscle cell hyperplasia that
1
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
results in reblockage of the vessel lumen necessitating
repeat PTCA procedures up to 35-50~ of all cases. It has
been generally accepted that a certain therapeutic agents
or medicaments may be capable of selectively inhibiting
the growth of these hyperproliferating smooth muscle
cells and thereby reduce the rate of restenosis after the
primary interventional procedure.
Heretofore, various devices have been disclosed
which may be used to deliver a therapeutic agent or
medicament to a blood vessel while undergoing
angioplasty. Balloon angioplasty catheters have been
used to place and deliver a various therapeutic agents or
medicaments within human vessels. For example, in U.S.
Patent Nos. 5112,305, 5,746,716, 5,681,281, 5,873,852,
5,713,863 and 6,102,904 disclose and claim a balloon
catheter system with various injector plates mounted on
the balloon for delivering a drug into an arterial
segment.
Alternatively a standard angioplasty balloon may be
coated with a polymeric material which is then used to
bond certain medicaments or theraputic agents. These
agents are then delivered to the desired therapeutic site
by inflation of the balloon and diffusion of the
medicatment or therpeutic agent into the vessel wall.
Only limited quantities of therapeutic agents can be
delivered because of "wash-out" of the drug into the
circulation during balloon placement and due to the
limited time the inflated balloon can be left in place
due to ischemia caused by the balloon.
In addition, previously disclosed methods of
delivering drug to a site of treatment are described
which utilize iontophoretic or electrophoretic means as
disclosed in Patent No. 5,499,971. Using these
iontophoretic or electroporetic means passive diffusion
2
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
of the drug or medicament is enhanced by placing the
medicament or theraputic agent in close proximity to the
site of treatment and then using electrically to augment
delivery of the drug into the tissues or cells. These
methods generally place the drug inside a balloon mounted
distally on a catheter whereby the balloon is composed of
a semi-porous material through which the drug can
diffuse .
Alternatively the electrodes themselves may be used
as a method for iontophoretic or electroporetic drug
delivery. One such method is disclosed in Patent No.
6,219,577 which describes coating the surface of band-
like electrodes with a polymer which bonds the drug and
delivers it to the site of treatment. This method has the
disadvantage of not have the capability to dilate the
obstruction prior or concurrent to the delivery of a
drug. Additionally the surface area of contact of the
electrode bands with the vessel wall are limited to only
the central portion of the arc shaped bands. This limits
the contact surface area of the drug coated electrodes.
This method also has the inherent disadvantage that since
the site of therapy is intravascular, most of the drug
will be washed off or dissolved off the electrodes into
the circulating blood stream before it is advanced
through the vascular system from its percutaneous entry
and to the distal site of treatment. This again limits
the amount of the drug delivered to the site and also
potentially subjects the patient to harmful or toxic
systemic exposure.
Additional devices have been disclosed which attempt
to improve the depth of penetration into tissue by
pressure driving a solution of the drug into the vessel
wall through small orifices in the balloon material.
There is, however, some evidence that high pressure
"jetting" of a drug solution out of small pores close to
3
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
the vessel lumen can in fact cause vessel wall injury.
The development of double skinned, microporous (or
weeping) balloons obviated this "jetting" effect to some
extent, but diffusion of the drug into the vessel wall is
still slow, and much of the drug can be lost through
subsequent "washout effects". This method leads to
limited amounts of drugs or therapeutics agents delivered
to the tissues or cells. Furthermore, in all of these
methods the balloon must be expanded and thereby
restricts blood flow to the distal arterial segments
while the balloon is in the expanded configuration thus
limiting the time the drug delivering balloon can be
clinically utilized.
There are also several disadvantages using either a
stent or balloon catheter to delivery a therapeutic agent
or medicament to a vascular segment. Regarding the
therapeutic agent eluting stents, once the stent a.s
deployed, there is no means outside of invasive surgical
excision, to remove the eluting stent from the vascular
segment. Therefore, stents or implanted prostheses with
therapeutic agent eluting properties must be precisely
calibrated to deliver an exact quantity of the
therapeutic agent or medicament to the vascular segment
upon stent deployment. Balloon catheters employed to
delivery a therapeutic agent or medicament to a vascular
segment have limitations including potential balloon
rupture and ischemia due to balloon inflation limiting
distal blood flow to the artery. This leads to tissue
ischemia and potential necrosis. Even "perfusion" type
angioplasty balloons used to delivery a therapeutic agent
or medicament to the affected artery provide far less
than physiological blood flow during balloon inflation
and dwell times are limited by ischemia and tissue
necrosis.
4
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
Recent studies have demonstrated the effectiveness
of a number of agents (e. g., paclitaxel, rapamycin,
Actinomycin D) on the prevention of unwanted cellular
proliferation. These agents have proven efficacy in the
treatment of cancer and transplant rejection. A major
advantage of these agents is the high lipid solubility.
that causes tissue levels to be high for an extended
period of time since they cannot be rapidly cleared.
However, this advantage is also a disadvantage because
the delivery of these medicaments must generally pass
hydrophilic boundaries.
Thus, it can be seen that there is a need for a new
and improved device to selectively delivery a therapeutic
agent or medicament. to an arterial segment and which
overcomes these disadvantages.
In general, it is an object of this present
invention to provide a mechanical dilatation device and
method which is capable of dilating an obstruction within
a vascular segment while delivering, either passively or
by an electrically active means, a therapeutic agent or
medicament to the vessel segment.
Another object of the invention i.s to provide a
method to deliver high concentrations of agents that are
poorly soluble or insoluble in aqueous media to selected
sites in the body including arteries, veins or other
tubular structures, prosthetic devices such as grafts,
and tissues such as, but not limited to, brain,
myocardium, colon, liver, breast and lung.
Another object of the invention is to provide a
percutaneous device and method of the above character
which can be used for prolonged periods in exposing or
delivering a therapeutic agent or medicament to a
5
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
vascular segment while allowing continuous perfusion of
blood into the vessel distal to the treatment area.
Another object of the invention is to provide a
device that can control the release or diffusion of a
medicament or therapeutic agent to minimize potential
systemic affects and maximize the diffusion or delivery
of the medicament or therapeutic agent to the site of
treatment.
Another object of the invention is to provide a
device that is not susceptible to structural damage
(balloon rupture) and subsequent release of therapeutic
agents or drug materials into the vasculature.
SUN~1ARY Of THE INVENTION
It is known that therapeutic agent therapy can
reduce the proliferation of rapidly growing cells. The
present invention employs various means of delivery with
a mechanical dilatation device for enlarging a flow
passage of a vessel by dilating and delivering a liposome
or micelle or micelle-encapsulated therapeutic agent or
medicament to an obstruction a.n a vessel. Since the
therapeutic agent or medicament is capable of selectively
inhibiting the growth of proliferating cells, the present
invention not only achieves acute patency of a vessel but
employs medical therapy to maintain chronic patency
through the prevention of restenosis.The present
invention comprised a substantially cylindrically shaped
expansion member and includes a means engaged to the
expansion member for altering the distance between the
proximal end and the distal end of the expansion member
thereby transforming the expansion member between a
diametrically contracted configuration and a
diametrically expanded configuration. A liposome or
6
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
micelle-encapsulated therapeutic agent or medicament can
be coated directly on the expansion member or
alternatively, the therapeutic agent or medicament can be
incorporated into a polymer or other substrate coated on
the expansion mesh. If desired, the same or another
therapeutic agent or medicament can be coated on the
marker bands mounted on the catheter located within the
expansion mesh or injected through a delivery lumen which
has a distal port located inside the expansion member.
Due to its unique design, the present invention has
significant perfusion capability which allows the
catheter and its distal expansion member or mesh to be in
a expanded configuration and engaged to the vessel wall
for proloned periods. This allows sufficient time for
passive or electrically active migration of the
therapeutic agent or medicament to the vessel or organ
without causing ischemic related events. The catheter
also comprises either an over-the-wire or rapid exchange
designs.
The present invention also can include a conduction
means that provides electrical communication from a
connector on the proximal end of the catheter to the
distal conductive flexible elongate elements thereby
providing the distal expandable mesh with a means to
control or facilitate the release or delivery of a
medicament or therapeutic agent to ~a treatment site. In
this embodiment, the invention relates to catheter-based
devices which provide an electrical driving force that
can increase the rate of migration of liposome or
micelle-encapsulated medicaments and other therapeutic
agents from the expansion member and into body tissues
and cells using iontophoresis only, electroporation only,
or combined iontophoresis and electroporation. In
addition, a charge can be applied to the expansion member
that is opposite the liposome or micelle-encapsulated
therapeutic agent or medicament, or to the substrate that
7
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
incorporates the therapeutic agent or medicament in order
to create a significant bond between the therapeutic
agent and the expandable mesh.
The invention also takes advantage of the prior body
of knowledge that has demonstrated the enhanced
solubility and delivery of agents after they have been
incorporated into liposome or micelles or micelles.
Since liposome or micelles and micelles possess both
lipophilic and hydrophilic regions, they can be used to
solubilize compounds that are insoluble in water. If
charged liposome or micelles are used, these charged
molecules can move in an electrical field.
This disclosure demonstrates the delivery of
uncharged, lipophilic medicaments or agents by
incorporating them into charged liposome or micelles and
then delivering them to the target site by
electrophoresis.
The present method also comprises the steps of
advancing the catheter and expansion member to the
obstruction in a vessel and applying opposed forces on
said expansion member in an axial direction to move the
expansion member to an expanded configuration wherein the
expansion member dilates the obstruction and the
catheter/expansion member assembly actively (or
passively) delivers the liposome or micelle-encapsulated
therapeutic agent or medicament to the obstruction.
One preferable approach may be to 1) energize the
3$ catheter to create a bond between the therapeutic agent
and expansion mesh and then advance the system to the
treatment segment, 2) expand the expansion member to
8
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
dilate the segment, 3) allow perfusion to passively
transfer the therapeutic agent into the tissues.
Another preferable approach may be to 1) energize
the catheter to create a bond between the liposome or
micelle enclosed therapeutic agent and expansion mesh and
then advance the system to the treatment segment, 2)
expand the expansion member to dilate the segment while
allowing perfusion, 3) apply electrical energy to cause
iontophoresis of the therapeutic agent into the tissues
and/or 4) apply electrical energy for electroporation to
be applied to permeabilize the cells. Preferably, the
catheter is able to perform steps 2, 3 and 4 sequentially
without repositioning of the catheter. Even more
preferably, the catheter is designed to maintain a high
concentration of drug in the tissue extracellular spaces
(e. g. by iontophoresis) such that the subsequent creation
of transient pores in cell surface membranes by
electroporation pulses results in greatly improved
intracellular delivery of the medicament or therapeutic
agent.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a side-elevational view partially in
section of a mechanical dilatation and medicament
delivery device incorporating the present invention.
Figure 2 is a cross-sectional view taken along the
line 2-2 of Figure 1.
Figure 2a is a cross-sectional view taken along the
line 2-2 of Figure 1 also demonstrating the electrical
connection means.
9
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
Figure 3 is a cross-sectional view taken along the
line 3-3 of Figure 1.
Figure 3a is a cross-sectional view taken along the
line 3-3 of Figure 1 also demonstrating the electrical
connection means.
Figure 4 is a cross-sectional view taken along the
line 4-4 of Figure 1.
Figure 5 is a cross-sectional view taken along the
line 5-5 of Figure 1.
Figure 5a is a cross-sectional view taken along the
l5 line 5-5 of Figure 1 also demonstrating the electrical
connection means.
Figure 6 is a cross-sectional view taken along the
line 6-6 of Figure 1.
Figure 6a is a cross-sectional view taken along the
line 6-6 of Figure 1 also demonstrating the electrical
connection means.
Figure 7 is a greatly enlarged view of a portion of
the dilatation and medicament delivery device in a
partially expanded state.
Figures 8a-8f depict a variety of electric waveforms
for use in iontophoresis and electrophoresis with the
catheter and distal mesh of the present invention.
Figure 9 is a partial side-elevational view of
another embodiment of a mechanical dilatation and
medicament delivery device incorporating the present
invention that can be utilized in conjunction with a
rapid exchange technique.
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
Figure 9a is an enlarged side-elevational view of
the rapid exchanged embodiment of the mechanical
dilatation and medicament delivery device demonstrating
S the guidewire entry ports in the inner and outer
elongated tubular members.
Figure 10 a.s a side-elevational view of the distal
extremity of the device shown in Figures 1-9 showing the
distal extremity with the expansion member in an expanded
condition.
Figure 11 is a cross sectional view of the flexible
elongated elements demonstrating the passive or
electrically active dispensing of the liposome or
micelle-encapsulated therapeutic agent or medicament into
the vessel wall.
Figure 12 is a cross sectional view demonstrating
the dispensing of a liposome or micelle-encapsulated
therapeutic agent or medicament from bands affixed to the
inner tubular member located within the expandable mesh.
Figure 13 is a cross sectional view of the one
flexible elongate elements of the expandable mesh
demonstrating the passive or electrically active
dispensing of a liposome or micelle-encapsulated
therapeutic agent or medicament from the elongate
element.
Figure 14 is a cross sectional view of one of the
flexible elongate elements of the expandable mesh
demonstrating the dispensing of the liposome or micelle
encapsulated therapeutic agent or medicament incorporated
within a substrate coating over the elongate element.
11
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
Figure 15 is a cross sectional view of one of the
flexible elongate elements of the expandable mesh
demonstrating the dispensing of a liposome or micelle
encapsulated therapeutic agent or medicament with the aid
of electrical current.
Figure 16 is a cross sectional side view of the
flexible elongated elements demonstrating the passive or
electrically active dispensing of the liposome or
micelle-encapsulated therapeutic agent or medicament into
the vessel wall.
Figure 17 is a cross section side view of a typical
liposome or micelle encapsulating a generic medicament.
DETAIIrED DESCRIPTION OF THE DRAWINGS
In general, the present invention relates generally
to devices that are used to dilate and dispense a
medicament or therapeutic agent to an obstruction within
a stenotic segment of a vessel. The device is comprised
of an cylindrical expansion member to be disposed in an
obstruction in a vessel carrying flowing blood. The
cylindrical expansion member has first and second ends
and an intermediate portion between the first and second
ends. The cylindrical expansion member also has a flow
passage extending therethrough with a diameter and a
longitudinal central axis. The diameter of the flow
passage is a variable with movement of the first and
second ends relative to each other along the longitudinal
central axis from a diametrically contracted position to
a diametrically expanded condition. The cylindrical
expansion member is comprised of a plurality of flexible
elongate elements each of which extends helically about
the longitudinal extending central axis. The flexible
elongate elements are coated with one or more liposome or
12
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
micelle-encapsulated medicaments, therapeutic agents,
drugs, pharmaceuticals, plasmids, genes or other agents.
For the purposes of this application, the terms used,
liposome or micelle-encapsulated medicaments and
therapeutic agents, will be used to encompass all the
particular agents described herein. It is also
contemplated that the liposome or micelle-encapsulated
medicament or therapeutic agent may be incorporated with
a non-medicament ,substrate that has been previously or
simultaneously coated on the flexible elongate elements.
Furthermore, an electrical means can be incorporated into
the catheter system to cause 1) electrical bonding of the
therapeutic agent to the mesh and/or 2) active
migration/dispersion of the agent into the
vessel/tissues. In addition, the present invention can
include coating one or more of the bands secured to the
central catheter element within the expansion mesh With
one or more therapeutic agents.
The plurality of the flexible elongate elements of
the expansion mesh have a first common direction of
rotation are axially displaced relative to each other and
cross a further plurality of the flexible elongate
elements also axially displaced relative to each other
but having a second common direction opposite to that of
the first direction of rotation to form a braided
cylindrical expansion member. The crossing of the
flexible elongate elements occurs in an area of contact
between the flexible elongate elements.
First and second means is provided respectively
engaging the first and second ends of said cylindrical
expansion member for retaining said first and second ends
in contracted positions. Means is provided for causing
relative axial movement of the first and second ends
towards each other to cause the intermediate cylindrical
portion of the expansion member to contract
13
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
longitudinally and to expand diametrically by causing the
flexible elongate elements in the intermediate portion of
the cylindrical member to move closer to each other
expanding the diametric dimensions of the cylindrical
expansion member thereby allowing it to contact the
vessel wall and enable it to dilate an obstruction within
the vessel. Flexible elongate elements at the first and
second ends of the cylindrical expansion member remain
contracted around and within first and second means and
are thereby prevented from moving closer which maintains
spacing between the flexible elongate members so that
blood in the vessel can continue to flow through the
first and second ends and through the flow passage in the
cylindrical expansion member while the cylindrical
expansion member is in engagement with vessel wall and
dilating an obstruction within the vessel.
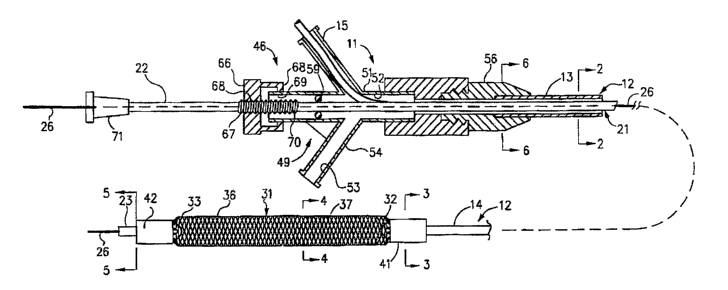
More in particular as shown in Figures 1-7 of the
drawings, the mechanical dilatation and medicament
delivery device 11 shown therein consists of a first or
outer flexible elongate tubular member 12 having proximal
and distal extremities 13 and 14 with the flow passage 16
extending from the proximal extremity 13 to the distal
extremity 14. Figures 2a, 3a, 5a, and 6a are provided to
represent the embodiment that includes an electrical
conduction means extending from the proximal connector
and engaged to the distal expansion member 31. A second
or inner flexible tubular member 21 is coaxially and
slidably disposed within the flow passage 16 of the first
or outer flexible elongate tubular member 12 and is
provided With proximal and distal extremities 22 and 23
with a flow passage 24 extending from the proximal
extremity 22 to the distal extremity 23. If the flexible
elongate elements of the dilating member are made of a
metallic material such as stainless steel, elgiloy or
other conductive material, an electrical lead can be
connected to the mesh to make it part of the circuit.
14
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
The electrical lead can either run along or within one of
the lumens of the catheter or can be in the form of a
braid that is made of a conductive material and have
generally functions to provide reinforcement to the
catheter shaft. . A second electrode could be placed on
the distal tip of the catheter via a small band with its
electrical lead running down one of the lumens to the
proximal end of the catheter. Alternatively, the
electircal lead could be engaged to the patient's skin or
could be the guidewire over which the catheter is
routinely advanced.
The flexible elongate elements of the catheter could
be coated with a polymeric material or similar substrate
onto which the liposome or micelle-encapsulated
medicament or theraputic agent could adsorb. Synthetic
polymers or natural polymers can be used, such as amino
acid polymers or polysaccharides. The polymer is
selected depending on the therapeutic agent required, the
polymer's compatibility with a patient and the ultimate
pharmacologic effect desired. These polymers could
include hydrophilic polymers used for their absorptive
properties of aqueous solutions. The flexible elongate
elements, either coated or uncoated, could then be
submerged in a solution of a liposome or micelle-
encapsulated therapeutic agents or medicaments with a
specific charge and an electrical charge could be applied
to render the flexible elongate members opposite in
charge to that of the liposome or micelle-encapsulated
therapeutic agent or medicament. This would create a
significant bonding of the liposome or micelle-
encapsulated agent or medicament to the flexible elongate
elements. Typically, the flexible elongate elements of
the mesh will be charged with the attached liposome or
micelle-encapsulated therapeutic agent or medicament just
prior to advancing the catheter through the patient's
vasculature to the site of dilatation and therapy without
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
significant loss of the drug in the bloodstream. Once
the site of obstruction or treatment is reached, the
charge on the mesh could be reversed using the same
electrodes thus driving the liposome or micelle-
s encapsulated therapeutic agent or medicament into the
target tissue. In this case, the electrode placed on the
skin of the patient would be used to cause active
diffusion or iontophoresis of the therapeutic agent or
medicament into the target tissues. As shown in Figures
8a-8f, the present invention can employ flow of
electrical current in the from of various waveforms to
perform the iontophoresis and/or electroporation
procedures. Possible waveforms contemplated for the
present invention include square waves, rectangular
waves, saw-toothed waves, sinusoidal waves that do not
reverse polarity, rectified sinusoidal waves, and other
waveform shapes which may reverse polarity but provide a
net flow of current in the desired direction.
Electrical current could also be coordinated with
the patient's elctrocardiogram ~ such that electrical
current is provided to the mesh only during certain
phases of cardiac depolarization. This "gating" of the
electrical current would avoid the potential danger of
discharging electrical current to the heart during
vunerable phases of depolarization which may lead to
cardiac arrhythmias.
Iontophoretically enhanced delivery requires that
the therapeutic agent carry a net charge under
physiological conditions whereas electroporation alone
would be used for delivering treatment agents that are
not sufficiently ionized to iontophorese well into
tissues. Electroporation may also be the preferred
strategy for enhancing localized cellular targeting of a
systemically administered therapeutic agent.
16
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
As used herein, the term "iontophoresis" means the
migration of ionizable molecules through a medium driven
by an applied low-level electrical potential. This
electrically mediated movement of molecules into tissues
is superimposed upon concentration gradient dependent
diffusion processes. If the medium or tissue through
which the molecules travel also carries a charge, some
electro-osmotic flow occurs. However, generally, the rate
of migration of molecules with a net negative charge
towards the positive electrode and vice versa is
determined by the net charge on the moving molecules and
the applied electrical potential. The driving force may
also be considered as electrostatic repulsion.
Iontophoresis usually requires relatively low constant DC
current in the range of from about 2-10 mA. In a well
established application of iontophoresis, that of
enhancing drug delivery through the skin (transdermal
iontophoresis), one electrode is positioned over the
treatment area and the second electrode is located at a
remote site, usually somewhere else on the skin. With the
present invention the return electrode may be similarly
positioned on the skin. Alternatively the tip of the
guide wire emerging from the distal end of the support
catheter may serve as the return electrode.
As used herein, the term "electroporation" means the
temporary creation of holes or aqueous pores in the
surface of a cell membrane by an applied electrical
potential and through which therapeutic agents may pass
into the cell. Electroporation is now widely used in
biology, particularly for transfection studies, where
plasmids, DNA fragments and other genetic material are
introduced into living cells. During electroporation
pulsing, molecules that are not normally membrane
permeant are able to pass from the extracellular
environment into the cells during the period of induced
17
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
reversible membrane permeabilization. The permeabilized
state is caused by the generation of an electrical field
in the cell suspension or tissue of sufficient field
strength to perturb the cell surface membrane's
proteolipid structure. This perturbation (sometimes
referred to as dielectric breakdown) is believed to be
due to both a constituent charge separation and the
effect of viscoelastic compression forces within the
membrane and it's sub-adjacent cytoskeletal structures.
The result is a localized membrane thinning. At a
critical external field strength, pores or small domains
of increased permeability are formed in the membrane
proteolipid bi-layer.
A guide wire 26 of a conventional type is adapted to
be introduced through the flow passage 24 in the inner
flexible elongate tubular member for use in guiding the
mechanical dilatation and medicament delivery device 11
as a over-the-wire design as hereinafter described. The
guide wire 26 can be of a suitable size as for example
0.010"-0.035" and can have a suitable length ranging from
150 to 300 centimeters. For example, the first or outer
flexible elongate tubular member 12 can have an outside
diameter of 0.6-3 millimeters with a wall thickness of
0.12 millimeters to provide a flow passage of 0.75
millimeters in diameter. Similarly, the second or inner
flexible elongate tubular member 21 can have a suitable
outside diameter as for example 0.6 millimeters With a
wall thickness of 0.12 millimeters and a flow passage 24
of 0.45 millimeters in diameter. The flexible elongate
tubular members 12 and 21 can be formed of a suitable
plastic as for example a polyimide, polyethylene, Nylon
or polybutylterphalate (PBT).
In accordance with the present invention an
essentially cylindrically shaped expansion member 31 is
provided which has a first or proximal end 32 and a
18
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
second or distal end 33 with a central or inner flow
passage 34 extending from the proximal end 32 to the
distal end 33 along a longitudinally extending central
axis and has a diameter which is a variable as
hereinafter described. The cylindrically shaped
expansion member 31 is comprised of a plurality of
flexible elongate elements or filaments 36 each of which
extends helically about the longitudinally extending
central axis. The flexible elongate elements 36 are
formed of suitable materials which can be utilized in the
human blood as for example stainless steel, Nitinol,
AermetTM, ElgiloyTM or certain other plastic fibers . The
flexible elongate elements 36 can have a suitable
diameter as for example 0.001 to 0.010 inches or can be
configured as a round, elliptical, flat or triangular
wire ribbon. A plurality of the flexible elongate
elements 36 have a first common direction of rotation
about the central axis as shown in Figures 1 and 7 are
axially displaced relative to each other and cross a
further plurality of the flexible elongate elements 36
also axially displaced relative to each other but having
a second common direction of rotation opposite to that of
the first direction of rotation to form a double helix or
braided or mesh-lake cylindrical expansion member with
the crossing of flexible elongate elements 36 occurring
in the area of contact between the flexible elongate
elements to form openings or interstices 37 therebetween.
Thus the flexible elongate elements 36 form an expansion
member 31 which provides a central or inner flow passage
34 which is variable in diameter upon movement of the
first and second ends of the expansion member 31 relative
to each other along the longitudinally extending central
axis.
Means is provided for constraining the first and
second or proximal and distal ends 32 and 33 of the
expansion member 31 and consists of a first or proximal
19
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
collar 41 and a second or distal collar 42. The first
and second collars 41 and 42 are formed of a suitable
material such as a polyimide. The first or proximal
collar 41 has a suitable length as for example 1.0 to 5.0
millimeters and a.s sized so that it can fit over the
first or proximal end 32 of the expansion member 31 when
it is in a contracted position and over the distal
extremity 14 of the first or outer flexible elongate
member 12. In order to ensure that elongate elements or
filaments 36 of the first or proximal extremity 32 are
firmly secured to the distal extremity 14 of the first or
outer flexible elongate member 12,' an adhesive can be
provided bonding the first or proximal end 32 to the
collar 41 and to the distal extremity 14 of the first or
outer flexible elongate tubular member 12. The second or
distal collar 42 can be of a suitable size and typically
may be slightly smaller in diameter because it need
merely secure the elongate element or filaments 36 of the
distal end 33 of the expansion member 31 to the distal
extremity 23 of the second or inner flexible elongate
tubular member 21. An adhesive (not shown) is provided
to firmly secure the second or distal end 33 of the
expansion member 31 between the second or distal collar
42 and the distal extremity of the inner flexible
elongate tubular member 21. In this manner it can be
seen that the cylindrical expansion member 31 has its
proximal end curved conically inward toward and secured
to the distal extremity of the outer flexible elongate
tubular member 12 and the second or distal end 33 of the
expansion member 31 also curves conically inward toward
and is secured to the distal extremity of the second or
inner flexible elongate tubular member 21.
Typically the distance between the first and second
collars 41 and 42 can range from between 5 to 150
millimeters. Typically the distal end 23 of the second or
inner flexible elongate tubular member 21 extends
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
approximately 5-170 millimeters beyond the distal
extremity 14 of the first or outer flexible elongate
tubular member 12.
It can be seen that by moving the first or outer
flexible elongate tubular member 12 and the second inner
flexible elongate tubular member 21 axially with respect
to each other, the first and second ends of the expansion
member 31 are moved towards each other causing the
elongate elements or filaments 36 of an intermediate
portion of the cylindrical expansion member between the
first and second ends to move closer to each other to
cause these flexible elongate elements to move into
apposition with each other and to expand in a first
radial direction the intermediate portion of the
cylindrical expansion member 31 (Figure 7) and to cause
the diameter of the central flow passage 34 to increase.
The portions of the expansion member 31 immediately
adjacent the first and second collars 41 and 42 remain
restrained by the collars 41 and 42 causing the flexible
elongate elements 36 immediately adjacent to the collars
41 and 42 to curve sonically toward, and remain crossed
and unable to come into close apposition and thereby
provide openings or interstices 37 therebetween, which
remain relatively constant in shape and size so that
blood can flow from the first and second ends 32 and 33
through the central or inner flow passage 34 as
hereinafter described.
The essentially cylindrical shape of the expansion
member when expanded in a radial directon provides an
enlarged surface of contact between the expansion member
and the vessel wall or obstruction. This enlarged surface
of contact enables the cylindrical expansion member to
deliver an amount of medicament or therapeutic agent
Which is present on the surface of the flexible elongate
elements that comprise the expansion member. This
21
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
delivery of medicament or therpeutic agent may be by the
various well known means previously described such as
passive or electrically active diffusion, pressure,
iontophoresis or electroporesis.
One example of the means provided in the mechanical
dilatation and medicament delivery device 11 for causing
relative movement between the first or outer flexible
elongate tubular member 12 and the second or inner
flexible elongate tubular member 21 and consists of a
linear movement mechanism 46. The linear movement
mechanism 46 includes a Y-adapter 49 that is provided
with a central arm 51 having a lumen 52 through which the
second or inner flexible elongate tubular member 21
extends. The,lumen or flow passage 52 is in
communication with the lumen 16 of outer flexible
elongate tubular member 12 and with a flow passage 53 in
a side arm 54 which is adapted to receive a syringe (not
shown) so that saline, radiocontrast liquid or a
medicament/therapeutic agent can be introduced through
the side arm 54 and into the flow passage 52 in the Y-
adapter 49 and thence into lumen 16 of outer member 12.
The distal end of screw mechanism 46 is provided with a
fitting 56 with inner lumen 57 into which the proximal
end 13 of flexible elongate tubular member 12 is seated
and held in place by an adhesive 58 at the distal end of
fitting 56. Lumen 57 is thereby in communication with
flow passage 52 of central arm 51 and with flow passage
53 of side arm 54. An O-ring 59 that is adapted to form
a fluid-tight seal with respect to the second or inner
flexible tubular member 21 is disposed in the lumen 52 of
the central arm 51. An interiorly threaded knurled knob
66 is threaded onto an exteriorly threaded member 67
which is secured to and surrounds the proximal extremity
22 of inner flexible elongate tubular member 21. The
knob 66 is provided with an inwardly extending flange 68
which seats in an annular recess 69 in the central arm
22
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
51. Thus, rotation of the knob 66 causes advancement or
retraction of threaded member 67 and the second or inner
flexible elongate tubular member 21 with respect to the
fitting 56. Indicia 68 in the form of longitudinally
spaced-apart rings 70 are provided on the member 67 and
serve to indicate the distance that the second or inner
flexible elongate tubular member 21 has been advanced and
retracted with respect to the first or outer flexible
elongate member 12.
A Luer-type fitting 71 is mounted on the proximal
extremity 22 of the inner elongate flexible tubular
member 21 and is adapted to be engaged by a finger of the
hand. The guide Wire 26 extends through the fitting 71
and into the lumen 24 of inner elongate flexible tubular
member 21.
It should be appreciated that even though one
particular linear movement mechanism 46 has been provided
for advancing and retracting the flexible elongate
members 12 and 21 with respect to each other, other
mechanisms also can be utilized if desired to provide
such relative movement. Other possible designs that
could be employed are scissors-jack, racket-type or
straight slide mechanisms.
Another embodiment of a dilatation and medicament
delivery device incorporating the present invention is
shown in Figures 9 and 9a. ~ As shown therein, the rapid
exchange designed mechanical dilatation and medicament
delivery device 101 is constructed in a manner similar to
the mechanical dilatation and medicament delivery device
11 with the exception that it is provided with rapid
exchange capabilities. This is accomplished by providing
an.outer flexible elongate tubular member 102 having a
lumen 103 therein and an inner flexible elongate tubular
member 106 having a lumen 107 which have the expansion
23
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
member 31 secured thereto by the proximal and distal
collars 41 and 42. The outer flexible elongate tubular
member 102 is provided with a port or opening 111 into
the corresponding lumen 103 and which is 13-60
centimeters from the distal extremity 32 of the expansion
member 31. A corresponding port or opening 112 into
corresponding lumen 107 is provided within the inner
flexible elongate tubular member 106. These ports 111
and 112 are positioned so that When the expansion member
31 is in its expanded position with the distal
extremities of the members 102 and 106 being in closest
proximity to each other, the openings 111 and 112 are in
registration with each other. In this position, the
mechanical dilatation and medicament delivery device 101
can be loaded onto the guide wire 16 by advancing the
most proximal extremity of guide wire 26 first into lumen
107 of the distal extremity of the inner flexible
elongate member 106 and then back through port or opening
112 and port 111 which are in registration and out of the
flexible elongate tubular member 102. The expansion
member 31 is next contracted from its diametrically
expanded condition to a contracted condition by moving
the distal extremities of outer and inner flexible
elongate tubular members 102 and 106 further apart by
operation of screw mechanism 46. This procedure is
performed while maintaining a stable position of the
external position of guide wire 26 in a constant position
in relation to port 111. As the distal extremity of
flexible tubular member 106 is moved further from the
distal extremity of flexible elongate tubular member 102,
port 112 will move out of registration with port 111
While maintaining guide wire 26 within lumen 107 and
advancing the distal extremity of the flexible elongate
tubular member 106 along the guide wire 26. In this
diametrically contracted state of the expansion member
31, the mechanical dilatation and medicament delivery
device 101 may be advanced along guide wire 26 through
24
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
the region of stenosis in the blood vessel and
enlargement of expansion member 31 may occur using screw
mechanism 46 in the manner previously described. Once
dilatation and medicament delivery has been completed,
expansion member 31 can be diametrically contracted and
the mechanical dilatation and medicament delivery device
101 may be removed from the blood vessel and the guiding
catheter by maintaining a stable position of guide wire
26 in relation to the blood vessel and retracting device
101 along guide wire 26 until the distal extremity of
inner flexible member 106 exits the patient's body. The
mechanical dilatation and medicament delivery device 101
may now be rapidly exchanged with another mechanical
device 101 as for example, one having an expansion member
31 which can be increased to a larger diameter over a
standard 175 to 185 centimeter length guide wire 26.
The expansion member 31 is comprised of 16-64
individual elements formed of 0.001 to 0.005 inch
diameter wire of a suitable metal such as stainless steel
helically wound around a longitudinal central axis. The
helices are Wound in opposite directions. Stretching or
elongation of the cylindrical expansion member 31 results
in a reduction in diameter of the expansion member 31.
Mechanical fixation of the proximal and distal
extremities 22 and 23 of the expansion member 31 holds
these extremities in reduced diameter configurations.
The positions of the elements 21 in these extremities
cannot change in relation to each other. Therefore, the
crossing angles of the elements 36 remain constant.
Shortening of the cylindrical expansion member 31 with
the ends fixed results in the formation of a cylindrical
center section of great rigidity with the elements 36 in
close apposition to each other. The tapered proximal and
distal extremities of the expansion member 31 causes the
stresses on the individual elements 36 to be balanced.
Since the proximal and distal extremities 22 and 23 are
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
held in constant tapered positions, the interstices
between the elements are maintained allowing blood to
flow into and out of the cylindrical center section when
the expansion member 31 is shortened as shown a.n Figure
10. Shortening of the expansion member 31 results in a
significant increase in the metal density per unit length
in the center portion of the expansion member 31 while
the metal density at the ends is relatively constant.
This increase in metal density in the center section
results in significant radial force generation as the
elements 36 are compressed in a longitudinal direction.
As seen in Figure 11 the flexible elongated elements
36 are designed to either passively or electrically cause
the therapeutic agent or medicament 40 to dispense or
migrate into the vessel wall 17. Figure 13 demonstrates
in a cross sectional view a more detailed view of one of
the flexible elongate elements 36 of the expandable mesh
31 designed to either passively or electrically dispense
the therapeutic agent or medicament 40 from the elongate
element 36. Figure 12 shows a cross sectional view
demonstrating the dispensing of a therapeutic agent or
medicament from bands 62 affixed to the inner tubular
member located within the expandable mesh 31.
Figure 14 is another cross sectional view of one of
the flexible elongate elements 36 of the expandable mesh
31 demonstrating the dispensing of the therapeutic agent
or medicament 40 that is incorporated within a substrate
43 over the elongate element. The substrate 43 can
function to better adhere the medicament 40 to the
surface of the flexible elongate element 36, time the
release of the medicament into the vessel wall 17, be an
agent for transferring the medicament 40 across the cell
membrane boundaries either by passive or pressure
mediated transfer or actively byiontophoresis or
electroporation, or any combination of the services.
26
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
Figure 15 is another cross sectional view of one of the
flexible elongate elements 36 of the expandable mesh 31
demonstrating the dispensing of a therapeutic agent or
medicament 40 with the aid of electrical current applied
to the flexible elongate elements.
Figure 16 is a cross sectional side view of the
flexible elongated elements 36 demonstrating the passive
or electrically active dispensing of the therapeutic
agent or medicament 40 into the vessel wall 17.
To perform as a liposome or micelle-encapsulated
therapeutic agent or medicament source,40 for the present
invention, the flexible elongate elements 36 themselves
can be coated as described in more detail below.
A liposome or micelle-encapsulated therapeutic agent
or medicament 40 can be coated on (or incorporated into a
polymer or other substrate 43 and coated on the expansion
mesh 31 and/or specific bands 62 mounted on the catheter
located within the expansion mesh. One particular
therapeutic agent or medicament 40a can be coated upon
any one of the components described above, for example
the expansion mesh and another therapeutic agent or
medicament 40b can be coated upon another component, for
example, the marker bands. Alternately, a therapeutic
agent delivery lumen that has a distal port located
inside the expansion member can be used to selectively
release and deliver a particular therapeutic agent or
medicament.
The liposome or micelle-encapsulated therapeutic
agent 40 can be an anticoagulant, such as D-Phe-Pro-Arg
chloromethyl ketone, an RGD peptide-containing compound,
heparin, an antithrombin compound, a platelet receptor
antagonist, an anti-thrombin antibody, an anti-platelet
27
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
receptor antibody, aspirin, a prostaglandin inhibitor, a
platelet inhibitor or a tick anti-platelet peptide.
The liposome or micelle-encapsulated therapeutic
agent 40 can be a promoter of vascular cell growth, such
as a growth factor stimulator, a growth factor receptor
agonist, a transcriptional activator, and a translational
promoter. Alternatively, the therapeutic agent 40 can be
an inhibitor of vascular cell growth, such as a growth
factor inhibitor, a growth factor receptor antagonist, a
transcriptional repressor, a translational repressor, an
antisense DNA, an antisense RNA, a replication inhibitor,
an inhibitory antibody, an antibody directed against
growth factors, a bifunctional molecule consisting of a
growth factor and a oytotoxin, or a bifunctional molecule
consisting of an antibody and a cytotoxin.
The liposome or micelle-encapsulated therapeutic
agent 40 can be a cholesterol-lowering agent, a
vasodilating agent, or other agents that interfere with
endogenous vasoactive mechanisms. Additionally, the
therapeutic agent 40 can be a smooth muscle inhibitor,
such as: an agent that modulates intracellular calcium
binding proteins; a receptor blocker for contractile
agonists; an inhibitor of the sodium/ hydrogen
antiporter; a protease inhibitor; a nitrovasodilator; a
phosphodiesterase inhibitor; a phenothiazine; a growth
factor receptor agonist; an anti-mitotic agent; an
immunosuppressive agent; or a protein kinase inhibitor.
Alternatively, the liposome or micelle-encapsulated
therapeutic agent 40 may be disposed on or within a
28
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
substrate or polymer 43, which can be biodegradable and
adapted for slow release of the liposome or micelle-
encapsulated therapeutic agent 40. A substrate or
polymer 43 laden with one or more therapeutic agents 40
can be positioned on the bands, or coated on the flexible
elongate elements 36.
A biodegradable substrate or polymer 43 such as
polylactide, polyanhydride, polyorthoester or
polyglycolide, for example can be used. In addition to
synthetic polymers, natural polymers can be used, such as
amino acid polymers or polysaccharides. The polymer 50
is selected depending on the therapeutic agent required,
the polymer's 43 compatibility with a patient and the
ultimate pharmacologic effect desired. For example, if
the effect need only last a short period, a thin polymer
43 can be used with a limited amount of therapeutic agent
capable of diffusing from the polymer 50 into the
arterial wall or lumen of the vesicle. Alternatively,
only the layer closest to the body fluid would contain
the liposome or micelle-encapsulated therapeutic agent
40. Another alternative would be to use a polymer 43
which is biodegradable over a long period of time.
Naturally, the opposite characteristics would be selected
for a desired prolonged release.
Generally, the substrate or polymer 43 has a
liposome or micelle-encapsulated therapeutic agent 40
release rate of between about 0.001 pg/em2-min and about
100 pg/cm2-min, especially between about 0.01 pg/cmz-min
and 10 ug/cm2-min. In addition, the substrate or polymer
43 generally has a thickness of between about 0.01 mm and
29
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
mm, especially between about 0.1 mm and 1.0 mm. As
can be appreciated, the device 10 can be comprised of two
or more different therapeutic agents 40 or two or more
different polymers 43 to obtain a desired effect and
5 release rate. In addition, the polymers 43 can have
different solubilities or diffusion characteristics to
accomplish non-uniform therapeutic agent 40 release.
10 The methodology for coating of a polymer and/or a
therapeutic agent or medicament onto the bands or
flexible elongate elements of the expansion member is
well known to those skilled art or can be determined by
reference to standard references. In addition, the
characteristics of the particular substrate or polymer 43
for these purposes'is well known to the skilled artisan
or can be determined by reference to standard references,
e.g., Biodegradable Polymers as Therapeutic agent
Delivery Systems, R. Langer and M. Chasin, Eds., Marcel
Dekker Inc., New York, NY, USA (1990); Engleberg and
Kohn, "Physico- mechanical properties of degradable
polymers used in medical applications: a comparative
study," Bionuzterials 12:292-304 (1991); Controlled
Release Delivery Systems, T. J. Roseman and S. D.
Mansdorf, Eds., Marcel Dekker Inc., New York, NY , USA
(1983); and "Controlled Release Technology,
Pharmaceutical Applications, ACS Symposium Series, Vol.
348, P. I. Lee and W. R. Good, Eds., American Chemical
Society, Washington, D.C., USA (1987).
Operation and use of the mechanical dilatation and
medicament delivery device 11 may now be briefly
described as follows. Let it be assumed that the patient
which the medical procedure is to be performed utilizing
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
the mechanical dilatation and medicament delivery device
11 has one or more stenoses which at least partially
occlude one or more arterial~vessels supplying blood to
the heart and that it is desired to enlarge the flow
passages through these stenoses. Typically the
mechanical dilatation and medicament delivery device 11
would be supplied by the manufacturer with the
cylindrical expansion member 31 in its most contracted
position to provide the lowest possible configuration in
terms of diameter and so that the diameter approximates
the diameter of the outer flexible elongate tubular
member 12 and previously coated with a therapeutic agent
or medicament 40. Alternatively, the mechanical
dilatation and medicament delivery device will be
supplied either uncoated or coated only with the bonding
polymer present on the dilatation member and without any
liposome or micelle-encapsulated therapeutic agent or
medicament 40 on the expansion mesh. In this example, a
container having a solution of the liposome or micelle-
encapsulated therapeutic agent 40 can be separately
supplied whereby sometime prior to inserting the
mechanical dilatation and medicament delivery device into
the patient, the expansion mesh 31 is immersed or dipped
into the container in order to coat the flexible elongate
members 36. Appropriate time and/or temperatures will be
allowed for the medicament solution to adsorb, dry and
adhere to the polymer coated expansion mesh, or
alternately, a charge can be applied to facilitate
bonding of the medicament or therapeutic agent to the
polymer coated expansion member.
Preferably, the coated expansion member 35 should
have a diameter that is only slightly greater than the
tubular member 12, as for example by 1.0 - 2.3
millimeters. The first and second collars 41 and 42 also
have been sized so they only have a diameter that is
slightly greater than the outer diameter of the outer
31
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
flexible elongate tubular member 12. To bring the
cylindrical expansion member 31 to its lowest
configuration, the linear movement mechanism 46 has been
adjusted so that there is a maximum spacing between the
distal extremity 23 of the inner flexible elongate
tubular member 21 and the distal extremity 14 of the
outer flexible elongate tubular member 12. In this
position of the expansion member 31, the flexible
elongate elements 36 cross each other at nearly right
angles so that the interstices or openings 37
therebetween are elongated with respect to the
longitudinal axis.
If applicable, the present invention has the
flexible elongate elements of the catheter coated with a
liposome or micelle-encapsulated medicament or
therapeutic agent that can be subjected to an electrical
current that renders the flexible elongate members to
have a charge opposite to that of the therapeutic agent
or medicament. Applicable liposome or micelle-
encapsulated therapeutic agents or medicaments will have
inherent charge potentials that when opposite charges are
applied to the expansion member, an electrical bond is
established between the surface of the expansion member
and the liposome or micelle-encapsulated therapeutic
agent or medicament. Electrical energy or current may be
applied from an electrical connector located on the
proximal end of the catheter, through the leads 45 and to
the coated expansion member 35. This would create a
significant bonding of the liposome or micelle-
encapsulated therapeutic agent or medicament 40 to the
flexible elongate elements 36. The continuously charged
mesh with the attached liposome or micelle-encapsulated
therapeutic agent or medicament 40 could then be advanced
through the patient's vasculature , to the site of
dilatation and therapy without significant loss of the
medicament in the bloodstream.
32
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
The mechanical dilatation and medicament delivery
device 11 is then inserted into a guiding catheter (not
shown) typically used in such a procedure and introduced
into the femoral artery and having its distal extremity
in engagement with the ostium of the selected coronary
artery.
Thereafter, the guide wire 26 can be inserted
independently of the mechanical dilatation and medicament
delivery device 11. If desired the guide wire 26 can be
inserted along with the mechanical dilatation and
medicament delivery device 11 with its distal extremity
extending beyond the distal extremity of device 11. The
guide wire 26 is then advanced in a conventional manner
by the physician undertaking the procedure and is
advanced into the vessel containing a stenosis. The
progress of the distal extremity of the guide wire 26 is
observed fluoroscopically and is advanced until its
distal extremity extends distally of the stenosis. With
the expansion member 31 in its diametrically contracted
position and the liposome or micelle-encapsulated
medicament or therpeutic agent coated thereon, the
mechanical dilatation and medicament delivery device 11
is advanced over the guide wire 26. The distal extremity
23 of the second or inner flexible elongate tubular
member 21 is advanced through the stenosis over the guide
wire 26 until it is distal to the stenosis and so that
the distal extremity 14 of the first or outer flexible
elongate tubular member 12 is just proximal of the
stenosis.
After the expansiori member 31 is in a desired
position in the stenosis, the expansion member 31 is
expanded from its diametrically contracted position to an
expanded position by moving the distal extremities 14 and
23 closer to each other by operation of the screw
33
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
mechanism 46. This can be accomplished by holding one
distal extremity stationary and moving the other distal
extremity towards it or by moving both distal extremities
closer to each other simultaneously. This movement of
the distal extremities 14 and 23 causes collars 41 and 42
to move closer to each other and to cause the central
flexible elongate elements 36 forming the double helix
mesh of the intermediate portion 31a of the flexible
cylindrical expansion member 31 to move relative to each
other to progressively decrease the vertical crossing
angle of the double helically wound flexible elongate
elements 36 from approximately 140° to 170° in its
extended state to 5° to 20° in its axially contracted
state and to progressively change the interstices or
openings 37 from diamond-shaped openings with long axes
parallel to the central longitudinal axis of the catheter
a.n its extended state .to substantially square-shaped
openings in its intermediately contracted state to
elongate diamond-shaped interstices or openings with the
longitudinal axes extending in directions perpendicular
to the central longitudinal axis With the flexible
elongate elements 36 coming into close apposition to each
other while at the same time causing radial expansion of
the expansion member and to progressively increase the
diameter of the central flow passage 34. The enlargement
of expansion member 31 in addition to being viewed
fluoroscopically can also be ascertained by the indicia
68 carried by the threaded member 67.
The intermediate portion 31a of the cylindrical
expansion member 31 when fully expanded is almost a solid
tubular mass which has significant radial strength to
fully expand a stenosis or alternatively a stent or
prosthesis. In addition, because of spring-like
properties of the enlarged expansion member being
comprised of helically wound flexible elongate elements
36, the expansion member 31 can conform to a curve within
34
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
the blood vessel while still exerting significant radial
force to the stenosis or alternatively a stent or
prosthesis and to make possible compression of the
stenosis without tending to straighten the curve in the
vessel which typically occurs with standard straight
angioplasty balloon systems. Since the expansion member
or alternatively a stent or prosthesis is coated with a
therapeutic agent or medicament one or more therapeutic
agents or medicaments can be delivered to the vessel
during the time of device expansion while blood is
permitted to flow unobstructed to the distal vessel (see
Figs . 11-16) .
Additionally an electrical charge can be provided to
the dilatation member or mesh that is opposite in charge
to that used to bind the liposome or micelle-encapsulated
medicament to the mesh or expansion member. This charge
will then tend to drive the liposome or micelle-
encapsulated medicament or therapeutic agent into the
tissue through iontophoretic means. The iontophoretic
process is known to facilitate or assist the transport of
the liposome or micelle-encapsulated medicament or
therapeutic agent across the selectively permeable
membranes and enhance tissue penetration. Since the
present invention involves the use of electrical energy,
there are many possible waveforms contemplated for use.
As depicted in Figs 8a-8f, square waves 61, rectangular
waves 63 , saw toothed waves 64 , sinusoidal waves that do
not reverse polarity 65, rectified sinusoidal waves, 72
and modified rectangular or other waves 73. The primary
characteristic of the preferred waveforms is that they
all provide a net flow of current to the coated expansion
member 35. It must be appreciated by those skilled in
the art, that the waveforms with frequencies and duty
cycles must be capable of delivering the desired current
under varying impedances encountered by the expansion
member 35 and the surrounding vessel wall 17 and fluids.
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
After a predetermine time, the, electrical current
can be altered to achieve another purpose or terminated.
Since blood flows continuously through the dilatation and
medicament delivery device 11 during the dilatation and
medicament delivery procedure, there is minimal danger of
ischemia occurring. This makes it possible to maintain
dilatation and medicament delivery 11 of the obstruction
over extended periods of time when desired. One.
particularly advantage for the mechanical dilatation and
medicament delivery device 11 is that it could be used
with patients which have obstructions of a critical
nature that cannot even tolerate relatively short periods
of balloon dilatation without leading to ischemia and ,
creating permanent damage or shock to the patient.
Another advantage of the present invention is the
increased contact area of the cylindrical expansion
member with the vessel wall can lead to increased
adsorption of the medicament or therapeutic agent by the
tissues.
.After dilatation and medicament delivery of the
lesion has been carried out for an appropriate length of
time, the expansion member 31 can be moved from its
expanded position to a contracted position by, for
example, operation of the screw mechanism 46 in a reverse
direction to cause separation of the distal extremities
14 and 23 to thereby cause elongation of the expansion
member 31 with a concurrent reduction in diameter.
After the expansion member 31 has been reduced to
its contracted or minimum diameter, the mechanical
dilatation and medicament delivery device 11 can be
removed along with the guide wire 26 after which the
guiding catheter (not shown) can be removed and the
36
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
puncture site leading to the femoral artery closed in a
conventional manner.
Describe below are some examples of experiments
conducted using the present invention.
Example 1. Local delivery of 7-Amino Actinomycin D
7-Amino Actinomycin D is a fluorescent (emits at 610 nm,
bred]) analog of Actinomycin D, a potent inhibitor of
cellular proliferation. Tt is very lipophilic and poorly
soluble in water. Liposome or micelles were prepared by
mixing 3.0 mg of phosphatidylcholine, 3.0 mg of
cholesterol and 0.3 mg of phosphatidylserine in a test
tube. Chloroform (200 microliters) was added and the
solution was evaporated to dryness in a test tube. 7-
Amino Actinomycin D (500 mg) was dissolved in 8 mM CaCl2
for a final concentration of 0.5 mgjml. The 7-Amino
Actinomycin D solution Was added to the lipid mixture in
small aliquots with constant stirring. The hydrogel-
coated metal mesh catheter was placed in the 7-amino
Actinomycin D / liposome or micelle mixture and then used
for drug delivery in the following manner: The hydrogel-
coated metal mesh catheter was placed in the 7-Amino
Actinomycin D / liposome or micelle mixture and then
removed. In some cases, the hydrogel-coated mesh portion
of the catheter was covered with a retractable sheath to
prevent loss of the compound during the transport of the
catheter from the arterial access site to the target
site. When the catheter was positioned at the target
Site the sheath was retracted and the mesh was expanded
against the arterial wall. Iontophoersis was performed
by applying an electrical current to the mesh. The
37
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
circuit was completed by pacing a patch on the skin that
was connected to the circuit and had an opposite charge
than the mesh. In this example the iontophoresis
parameters were 5 mA, and 8 V, applied for 10 minutes.
The results also show 7-Amino Actinomycin D throughout
the vessel wall and in the outer layer of the vessel.
There is also evidence of localization of the 7-Amino
Actinomycin D in the nuclei of the cells.
Example 2. Local Delivery of Paclitaxel
Paclitaxel is one of the most potent inhibitors of
cellular proliferation in clinical use and has been shown
to be efficacious in a large number of cancers.
Paclitaxel is very lipophilic and essentially insoluble
in water. Liposome or micelles were prepared by mixing
0.72 mg phosphatidylcholine and 0.8 mg of
phosphatidylserine in a test tube with 800 microliters of
chloroform: The solution was evaporated to dryness.
Paclitaxel labeled with a fluorescent probe (Oregon
Green) was dissolved in methanol to obtain a 20 1 mg / 1
ml solution. Twenty-five microliters of this solution
was combined with 975 microliters of 8 mM CaCl2. The
paclitaxel solution was added to the dried lipid mixture
in small aliquots with constant stirring. The hydrogel-
coated metal mesh catheter was placed in the paclitaxel /
liposome or micelle mixture and then removed. In some
cases, the hydrogel-coated mesh portion of the catheter
is covered with a retractable sheath to prevent loss of
the compound during the transport of the catheter from
the arterial access site to the target site. T~Then the
catheter was positioned at the target site the sheath was
38
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
retracted and the mesh was expanded against the arterial
wall. Iontophoersis was performed by applying an
electrical current to the mesh. The circuit was
completed by pacing a patch on the skin that was
connected to the circuit and had an opposite charge than
the mesh. In this example the iontophoresis parameters
were 7 mA and 8 V, applied for 20 minutes. The results
showed the paclitaxel throughout the vessel wall and in
the outer layer of the vessel.
Although, the procedure hereinbefore described was
for treatment of a single stenosis, a.t should be
appreciated that if desired during the same time that the
mechanical dilatation and medicament delivery device 11
is within the guiding catheter, other vessels of the
patient having stenoses therein can be treated in a
similar manner merely by retracting the distal extremity
of the mechanical dilatation and medicament delivery
device 11 from the stenosis being treated, placing
another prosthesis over the expansion member, and then
advancing it into another stenosis in another vessel in a
similar manner.
2$ The advantages of using the present invention is the
ab111ty t0 deliver a liposome or micelle-encapsulated
therapeutic agent or medicament to a vascular segment for
prolonged periods while allowing continuous perfusion of
blood into the distal to the treatment area.
35
39
CA 02526508 2005-10-31
WO 2004/098697 PCT/US2003/013180
From the foregoing, it can be seen that there has
been provided a mechanical dilatation and medicament
delivery device which can be used in a similar manner to
a balloon catheter in dilating a vessel segment or
deploying a stent during an interventional procedure with
the outstanding advantage that blood can continue to flow
to the distal blood vessel during the procedure while
delivery of a liposome or micelle-encapsulated medicament
or therapeutic agent is also accomplished. This permits
a longer vessel dilatation and medicament delivery
without tissue ischemia. Furthermore, the dilatation and
medicament delivery device provides either passive or
active delivery of a medicament or therapeutic agent to
the affected vessel walls via the coated expansion member
or via a stent or prostheis coated with such an agent..
Furthermore, the mechanical dilatation and medicament
delivery device also provides the advantages of known
expanded non-compliant diameter and therefore exact
sizing.
25
35