Note: Descriptions are shown in the official language in which they were submitted.
CA 02526663 2005-11-22
WO 2004/108702 PCT/EP2004/005593
- 1 -
Indole derivatives with apoptosis-inducing effect
The present invention relates to novel indole
derivatives which have a better biological effect,
which are better tolerated, which exhibit better oral
bioavailability and which are employed as drugs for
treating tumor diseases, in particular when drug
resistance exists against other active compounds and
when a carcinoma is metastasizing.
The treatment of cancer diseases is of great importance
in medicine. There is a worldwide need for effective
cancer therapies in order to achieve a treatment which
is appropriate to a patient and is target-orientated.
This can be seen in the large number of scientific
studies which have recently appeared in the fields of
applied oncology and fundamental research relating to
cancer therapy.
The effects of tumor inhibitors are due to a very wide
variety of mechanisms, only some of which are known. It
is not unusual for known tumor drugs to be found to
have new mechanisms of action. This is also to be
expected in the case of the compounds according to the
invention. Many tumor drugs act by way of mechanisms
such as blockading the mechanism of cell division in
the cell, preventing the tumor from being supplied with
nutrients and oxygen (antiangiogenesis), preventing
metastasis, preventing the reception and the onward
transmission of growth signals to the tumor cell or
forcing the tumor cell into programed cell death
(apoptosis).
Because they have different mechanisms of action,
including interacting with different intracellular
targets, the clinically relevant cytostatic agents are
frequently administered in combination in order to
achieve a synergistic therapeutic effect.
CA 02526663 2005-11-22
2 -
Indole derivatives are used in a great variety of ways
as pharmacodynamically active compounds and as building
blocks for synthesis in pharmaceutical chemistry.
Documents WO 99/51224 Al and WO 01/22954 Al describe
indol-3-yl derivatives which have an antineoplastic
effect and which can be substituted by a large number
of groups, including by 2-, 3-, 4- and 8-quinoline
radicals or 2-, 3-, 4-, 5- and 6-pyridine radicals. A
2-methyl-8-quinolinyl group is mentioned in Example 60
as being a substituent on the amide group. However, no
biological properties are mentioned.
WO 99/55696 Al describes substituted hydroxyindoles as
being inhibitors of phosphodiesterase 4. However, the
compounds according to the invention are not reported
to have any antineoplastic activity, nor is it
suggested that they might have this activity.
WO 02/08225 Al describes 2-(1H-indol-3-yl)-2-oxo-
acetamide derivatives which have an antineoplastic
effect in relation to solid tumors. However, there is
no mention of specific implementation examples
containing quinoline, pyridopyrazine or indazolyl
radicals.
Patent specification WO 00/67802 describes indole-3-
glyoxylamides which are substituted by relatively long-
chain fatty acids as being potential antineoplastic
agents. However, there is no mention of specific
implementation examples containing quinoline,
pyridopyrazine or indazolyl radicals. Nor are any
biological data given with regard to such
implementation examples.
The publication by W.-T. Li et al. (J. Med. Chem. 2003,
46, 1706 ff.) describes N-heterocyclic indolyl-
glyoxylamides as being orally active compounds which
possess antineoplastic activity. However, no
CA 02526663 2011-02-25
3 -
information is provided as regards their mechanism of
action.
Patent application WO 03/022280 A2 describes
3-glyoxylamideindoles and their use as drugs for
antineoplastic treatment. Their general formula also
includes 6-quinoline derivatives. In addition, two
examples containing a 6-quinoline radical are mentioned
as implementation examples and verified by means of
biological results. However, there is no mention of
specific implementation examples containing
pyridopyrazine or indazolyl radicals.
US patent application publication US 2003/018482 Al describes novel
indolylglyoxylamides. In this case, the compounds
according to the invention are described as being
antineoplastic agents possessing cytotoxic activity and
as being angiogenesis inhibitors. In addition to this,
a 6-quinoline derivative is shown as an implementation
example (compound 3; p. 10) and verified by means of
antiproliferative data (see p. 19; Tables la and lb)
and antiangiogenic properties (see p. 20). However,
there is no mention of specific implementation examples
containing pyridopyrazine or indazolyl radicals.
The Applicant's WO 02/10152. A2 already describes a
second class of indole derivatives for treating tumors.
In this document, the active compound N-(2-methyl-6-
quinolyl)-[1-(4-chlorobenzyl)indol-3-yl]glyoxylamide,
inter alia, was tested for its antiproliferative effect
on a variety of tumor cell lines.
Clinically tested compounds which either bind to the
microtubules (paclitaxel and vincristine) or inhibit
topoisomerase II (doxorubicin, etoposide and
mitoxantrone) are at present being successfully
employed in cancer therapy against, inter alia, breast
cancer, ovarian cancer, stomach cancer and lung cancer,
and in Kaposi's sarcoma and in leukemias. However,
CA 02526663 2005-11-22
- 4 -
their use is limited by the appearance of drug
resistances and also by serious neurological,
gastrointestinal, cardiovascular and hepatic side
effects.
An object underlying the invention is now to make
available cytotoxic substances which possess combined
mechanisms of action and which are suitable for
treating a large number of tumors, in particular when
active compound resistances exist against other drugs
and when carcinomas are metastasizing.
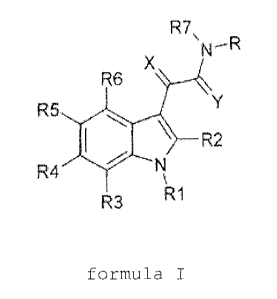
This object is achieved by indole derivatives of the
general formula I
R7\
X N-R
R6
R5 Y
R2
R4 N
R3 R1
formula I
in which
R: is a saturated, unsaturated or aromatic,
substituted or unsubstituted (C2-C19)-
heterocycle which contains one or more
heteroatoms selected from the group N, 0 and S
and which is directly linked to the amide
nitrogen, with the heterocycle preferably being
(i) unsubstituted or substituted 5-, 6-,
7-quinolyl,
(ii) unsubstituted or substituted 2-, 3-, 6-,
7- and 8-pyridopyrazinyl,
CA 02526663 2005-11-22
- 5 -
(iii) unsubstituted or substituted 3-, 4-, 5-,
6- and 7-indazolyl,
(iv) unsubstituted or substituted 2-, 3-, 4-,
5- and 6-pyridyl,
(v) unsubstituted or substituted 3-, 4- and
5-isoxazolyl,
(vi) unsubstituted or substituted 3-, 4- and
5-isothiazolyl,
Rl: is unsubstituted or substituted alkyl-aryl,
R2: is (i) hydrogen,
(ii) unsubstituted or substituted (C1-C6)-
alkyl,
R3-R6: are
(i) hydrogen
(ii) unsubstituted or substituted (C1-C6)-
alkyl,
(iii) unsubstituted or substituted (C3-C7)-
cycloalkyl,
(iv) amino, mono-(C1-C4)-alkylamino, di-
(C1-C4) -al kyl amino,
(v) halogen,
(vi) (C1-C4)-alkyl which is substituted by one
or more fluorine atoms, preferably
trifluoromethyl group,
(vii) cyano, straight-chain or branched cyano-
(C1-C6) -alkyl,
(viii) (C1-C6)-alkylcarbonyl,
(ix) carboxyl, (C1-C4)-alkoxycarbonyl,
carboxy- (C1-C6) -alkyl or (C1-C6) -
alkoxycarbonyl- (C1-C6) -alkyl,
(x) hydroxyl,
(xi) - (C1-C6) -alkoxy,
(xii) aryl- (C1-C4)-alkoxy, preferably benzyl-
oxy,
(xiii) (C1-C6)-alkoxycarbonylamino, (C1-C6)-
alkoxycarbonylamino- (C1-C6)-alkyl,
CA 02526663 2005-11-22
- 6 -
R7: is
(C1-C6) -alkylcarbonyl, preferably acetyl or
propionyl,
(C1-C6)-alkoxycarbonyl, preferably methoxy-
carbonyl, ethoxycarbonyl or propoxycarbonyl,
and
X, Y: are oxygen or sulfur,
the tautomers and stereoisomers, including the
diastereomers and enantiomers, thereof, and also the
physiologically tolerated salts thereof.
When R is an unsubstituted or substituted 2-, 3-, 4-,
5- or 6-pyridyl group and Rl-R6 have the abovementioned
meaning, R7 must not, in this case, be an acetyl
radical or a tert-butyloxycarbonyl group.
The invention furthermore relates to indole derivatives
of the formula I in which
R7\
N-R
X
R6
R5 Y
R2
R4 N
3 R1
formula I
R: is, directly linked to the amide nitrogen,
(i) substituted 6-quinolyl, unsubstituted or
substituted 7-quinolyl, where 2-methyl-
6-quinolyl is excluded and where, when X
is a sulfur atom, R can also be
unsubstituted 6-quinolyl.
CA 02526663 2005-11-22
7 -
(ii) unsubstituted or substituted 2-, 3-, 6-,
7- and 8-pyridopyrazinyl,
(iii) unsubstituted or substituted 3-, 4-, 5-,
6- and 7-indazolyl,
R1: is unsubstituted or substituted alkyl-aryl,
R2: is hydrogen
R3-R6: are
(xiv) hydrogen
(xv) unsubstituted or substituted (C1-C6)-
alkyl,
(xvi) unsubstituted or substituted (C3-C7)-
cycloalkyl,
(xvii) amino, mono-(C1-C4)-alkylamino, di-
(C1-C4)-alkylamino,
(xviii) halogen,
(xix) (C1-C4)-alkyl which is substituted by
one or more fluorine atoms, preferably
trifluoromethyl group,
(xx) cyano, straight-chain or branched
cyano- (C1-C6) -alkyl,
(xxi) (C1-C6) -alkylcarbonyl,
(xxii) carboxyl, (C1-C4)-alkoxycarbonyl, car-
boxy- (C1-C6) -alkyl or (C1-C6) -alkoxy-
carbonyl- (C1-C6)-alkyl,
(xxiii) - (C1-C6) -alkoxy,
(xxiv) aryl-(C1-C4)-alkoxy, preferably benzyl-
oxy,
(xxv) (C1-C6) -alkoxycarbonylamino, (C1-C6) -
alkoxycarbonylamino- (C1-06) -alkyl,
and
R7: hydrogen
X, Y: are oxygen or sulfur,
CA 02526663 2005-11-22
8 -
the tautomers and stereoisomers, including the
diastereomers and enantiomers, thereof, and also the
physiologically tolerated salts thereof.
The present invention is a further development of the
invention which is described in WO 02/10152. It was
observed that the indole derivatives which were
obtained by replacing the 2-methyl-6-quinolyl group
with unsubstituted or substituted 2-, 3-, 6-, 7- and
8-pyridopyrazinyl or unsubstituted or substituted 3-,
4-, 5-, 6- and 7-indazolyl exhibit a superior
antiproliferative effect on a variety of tumor cell
lines.
If was furthermore observed that the compounds
according to the invention exert a powerful cytotoxic
effect which can be due to a very wide variety of
different mechanisms. One mechanism of the compounds
according to the invention, which is demonstrated in
the invention, is based on inhibiting tubulin
polymerization and on inhibiting topoisomerase II. This
leads to the arrest of tumorigenic cells in the G2M
phase. In addition to this, the compounds according to
the invention induce apoptosis.
It was furthermore observed that the compounds
according to the invention have a superior solubility
in water and consequently also a superior oral
bioavailability.
In addition, it was demonstrated that introducing an
acetyl radical as the R7 radical resulted in the
compounds according to the invention having superior
in-vivo activity while at the same time being better
tolerated.
The substance class which is described in the invention
should open up the possibility of obtaining
antineoplastic medication which is lower, longer
CA 02526663 2005-11-22
- 9 -
lasting and better tolerated than can be achieved using
the conventional cytostatic agents. In particular, it
should be possible to circumvent the disadvantageous
development of resistance, as is known to occur in the
case of many antineoplastic agents. The effect
augmentation which is achieved using the indole
derivatives according to the invention should make drug
usage more efficient. In addition to this, it ought to
be possible to extend the treatment to cases which are
resistant to therapy.
In a preferred embodiment, R1 is 4-chlorobenzyl, R2-R6
are hydrogen, R is heterocycle and R7 is alkylcarbonyl
or alkoxycarbonyl in the indole derivative of the
formula I.
In another preferred embodiment, R is unsubstituted
5-quinolyl, unsubstituted 6-quinolyl or unsubstituted
7-quinolyl and R7 is acetyl or propionyl in the indole
derivative of the formula I.
In another preferred embodiment, R is unsubstituted
5-quinolyl, unsubstituted 6-quinolyl or unsubstituted
7-quinolyl and R7 is methoxycarbonyl, ethoxycarbonyl or
propionoxycarbonyl in the indole derivative of the
formula I.
Some terms which are used in the description and the
patent claims are defined below.
In connection with "heterocycle", the term is
understood as meaning, insofar as not explicitly
mentioned above, pyrrole, furan, thiophene, pyrazole,
thiazole, indole, oxazole, imidazole, isothiazole,
isoxazole, 1,2,3-triazole, 1,2,4-triazole,
1,2,4-oxadiazole, 1,3,4-oxadiazole, 1,2,5-thiadiazole,
1,3,4-thiadiazole, tetrazole, pyridine, pyrimidine,
pyridazine, pyrazine, benzofuran, indazole, carbazole,
benzoxazole, benzimidazole, benzothiazole,
CA 02526663 2005-11-22
- 10 -
benzotriazole, quinoline, cinnoline, quinoxaline,
quinazoline, phthalazine, pyridopyrazine,
1,2,3-triazine, 1,2,4-triazine, 1,3,5-triazine, purine,
pteridine, acridine and phenanthridine.
Within the meaning of this invention, the expression
"alkyl" encompasses acyclic saturated or unsaturated
hydrocarbons which may be straight-chain or branched.
In connection with "alkyl", the term "substituted" is
understood as being, within the meaning of this
invention and insofar as not explicitly defined above,
the replacement of a hydrogen radical with F, Cl, Br,
I, CN, NH2, NH-alkyl, NH-cycloalkyl, OH or 0-alkyl,
where polysubstituted radicals are to be understood as
meaning those which are substituted more than once,
e.g. twice or three times, either at .different atoms or
at identical atoms, for example three times at the same
C atom, as in the case of -CF3 and -CH2CF3, or at
different sites, as in the case of
-CH(OH)-CH2-CH2-CHC12. The polysubstitution can be
effected using the same substituents or different
substituents.
The expression "alkyl-aryl" means (C1-C6)-alkyl-(C6-C14)-
aryl and preferably (C1-C6)-alkyl-C6-aryl.
In regard to "alkyl-aryl" and to "cycloalkyl",
"substituted once or more than once" is understood,
within the meaning of this invention and insofar as not
explicitly mentioned above, as meaning the single or
multiple, for example twofold, threefold or fourfold
replacement of one or more hydrogen atoms in the ring
system with F, Cl, Br, I, CN, NH2, NH-alkyl, OH, 0-
alkyl, CF3, alkyl, (C6-C10) -aryl, (C6-C10) -aryl- (C1-C6) -
alkyl and/or heterocyclyl at one or, where appropriate,
different atoms (with it being possible for a
substituent, for its part, to be substituted, where
appropriate). In this connection, the multiple
replacement is effected using the same substituent or
using different substituents.
CA 02526663 2005-11-22
- 11 -
With regard to "heterocycle", "substituted once or more
than once" is understood, within the meaning of this
invention and insofar as not explicitly mentioned
above, as being the single or multiple, e.g. twofold,
threefold or fourfold, replacement of one or more
hydrogen atoms in the ring system with F, Cl, Br, I,
nitro, amino, C1-C6-alkyl, preferably methyl, mono-
(C1-C6) -alkylamino, di- (C1-C6) -alkylamino, hydroxyl,
C1-C6-alkoxy, benzyloxy, carboxyl, (C1-C6)-alkoxycar-
bonyl, (C1-C6) -aIkoxycarbonylamino or (C1-C6) -alkyl
which is substituted, once or more than once, by
fluorine, preferably trifluoromethyl, (C6-Cj()) -aryl
and/or (C6-C10) -aryl- (C1-C6) -alkyl at one or, where
appropriate, different atoms (with it being possible
for a substituent for its parts to be substituted,
where appropriate). In this connection, the multiple
replacement is effected using the same substituents or
using different substituents.
Provided the compounds according to the invention of
the general formula I possess at least one center of
asymmetry, they can be present in the form of their
racemates, in the form of the pure enantiomers and/or
diastereomers or in the form of mixtures of these
enantiomers and/or diastereomers. The stereoisomers can
be present in the mixtures in any arbitrary
proportions. Provided this is possible, the compounds
according to the invention can be present in the form
of the tautomers.
Thus, methods which are known per se can be used, for
example, to separate the compounds according to the
invention of the general formula I which possess one or
more chiral centers and occur as racemates into their
optical isomers, that is enantiomers or diastereomers.
The separation can be effected by means of column
separation on chiral phases or by means of
recrystallization from an optically active solvent or
using an optically active acid or base or by means of
CA 02526663 2005-11-22
- 12 -
derivatizing with an optically active reagent, such as
an optically active alcohol, and subsequently cleaving
off the residue.
If they contain a sufficiently acidic group, such as
the carboxyl group, the compounds according to the
invention of the general formula I can be converted
into their physiologically tolerated salts using
inorganic and/or organic bases. Examples of suitable
inorganic bases are sodium hydroxide, potassium
hydroxide and calcium hydroxide while examples of
suitable organic bases are ethanolamine, diethanol-
amine, triethanolamine, cyclohexylamine, dibenzylethyl-
enediamine and lysine. In this connection, the
stoichiometry of the salts of the compounds according
to the invention which are formed can be either an
integral or a nonintegral multiple of one.
If they possess a sufficiently basic group, such as a
secondary or tertiary amine, the compounds according to
the invention of the general formula I can be converted
into salts using inorganic and organic acids. The
pharmaceutically acceptable salts of the compounds
according to the invention in accordance with the
general structure I are preferably formed with
hydrochloric acid, hydrobromic acid, sulfuric acid,
phosphoric acid, methanesulfonic acid, p-toluene-
sulfonic acid, carbonic acid, formic acid, acetic acid,
trifluoroacetic acid, oxalic acid, malonic acid, maleic
acid, succinic acid, tartaric acid, pyruvic acid, malic
acid, embonic acid, mandelic acid, fumaric acid, lactic
acid, citric acid, glutamic acid or aspartic acid. The
salts which are formed are, inter alia, hydrochlorides,
hydrobromides, sulfates, phosphates, methanesulfonates,
sulfoacetic acid, tosylates, carbonates, hydrogen
carbonates, formates, acetates, triflates, oxalates,
malonates, maleates, succinates, tartrates, malates,
embonates, mandelates, fumarates, lactates, citrates
and glutaminates. In this connection, the stoichiometry
CA 02526663 2005-11-22
- 13 -
of the salts of the compounds according to the
invention which are formed can be an integral or
nonintegral multiple of one.
Preference is likewise given to solvates and, in
particular, hydrates of the compounds I according to
the invention which can be obtained, for example, by
crystallization from a solvent or from aqueous
solution. In this connection, one, two, three or any
arbitrary number of solvate or water molecules can
combine with the compounds according to the invention
to form solvates and hydrates.
It is known that chemical substances form solids which
are present in different states of order which are
termed polymorphic forms or modifications. The
different modifications of a polymorphic substance can
differ greatly in their physical properties. The
compounds according to the invention of the general
formula I can be present in different polymorphic
forms, with it being possible for particular
modifications to be metastable.
Both the compounds of the formula I and their salts are
biologically active. The compounds of the formula I can
be administered in free form or as salts with
physiologically tolerated acids or bases.
The compounds of the general formula can be
administered orally, rectally, via the buccal route
(e.g. sublingually), parenterally (e.g. subcutaneously,
intramuscularly, intradermally or intravenously),
topically or transdermally.
The invention furthermore relates to drugs having a
content of at least one of the compounds of the formula
I, or their salts with physiologically tolerated
inorganic or organic acids, and, where appropriate,
CA 02526663 2005-11-22
- 14 -
pharmaceutically utilizable carrier substances and/or
diluents or auxiliary substances.
These drugs are used for treating tumor diseases, in
particular for treatment in connection with tumor
diseases involving drug resistance against other active
compounds and/or in connection with tumor diseases
involving a metastasizing carcinoma.
Examples of suitable administration forms are tablets,
sugar-coated tablets, capsules, solutions for infusion
or ampoules, suppositories, plasters, powder
preparations which can be used for inhalation,
suspensions, creams and ointments.
The compounds according to the invention can also be
dispersed in a microparticulate, e.g. nanoparticulate,
composition.
In detail, the therapeutically valuable properties
which have been found relate to the following
advantages:
= the compounds according to the invention are
characterized by powerful antiproliferative
properties;
= the compounds according to the invention inhibit
tubulin polymerization;
= the compounds according to the invention inhibit
topoisomerase II;
= the compounds according to the invention arrest
dividing cells in the G2/M phase;
= the compounds according to the invention induce
apoptosis;
= the compounds according to the invention are
characterized by powerful antineoplastic
activities in vivo while also being better
tolerated;
CA 02526663 2005-11-22
- 15 -
+ the compounds according to the invention of the
formula I are active in vitro on mdr-resistant
cell lines, in contrast to paclitaxel,
vincristine, doxorubicin or etoposide.
Greatest preference is given to compounds according to
the general formula I which are included in the
following selection:
2-[1-(4-chlorobenzyl)-1H-indol-3-yl]-2-oxo-N-
pyrido[2,3-b]pyrazin-7-ylacetamide (1)
2-[l-(4-chlorobenzyl)-1H-indol-3-yl]-N-(1H-
indazol-5-yl)-2-oxoacetamide (4)
N-{2-[1-(4-chlorobenzyl)-1H-indol-3-yl]-2-
oxoacetyl}-N-quinolin-6-ylacetamide (2)
methyl {2-[1-(4-chlorobenzyl)-1H-indol-3-yl]-2-
oxoacetyl}quinolin-6-ylcarbamate (3)
ethyl {2-[1-(4-chlorobenzyl)-1H-indol-3-yl]-2-
oxoacetyl}quinolin-6-ylcarbamate (5)
propyl {2-[1-(4-chlorobenzyl)-1H-indol-3-yl]-2-
oxoacetyl}quinolin-6-ylcarbamate (6)
N-{2-[1-(4-chlorobenzyl)-1H-indol-3-yl]-2-
oxoacetyl}-N-quinolin-6-ylpropionamide (7)
ethyl {2-[l-(4-chlorobenzyl)-1H-indol-3-yl]-2-
oxoacetyl}pyridin-4-ylcarbamate (8)
2-[1-(4-chlorobenzyl)-1H-indol-3-yl]-N-quinolin-6-
yl-2-thioxoacetamide (11)
Compounds (1), (4) and (11) are compounds in which the
radical R7 is hydrogen. Compounds (2), (3), (5) and (6)
CA 02526663 2005-11-22
- 16 -
to (8) contain an alkylcarbonyl group of an
alkoxycarbonyl group as the group R7.
The following compounds (9), (10), (12), (13), (14) and
(15) are compounds which were also investigated for the
purposes of comparison. Compounds (9), (10), (14) and
(15) are known from the prior art. Compound (9) is
described in the Applicant's WO 02/10152, compound (10)
is described in WO 03/022280, compound (13) is covered
by the claims in WO 02/08225 Al, and compounds (12),
(14) and (15) are covered by the claims in WO 99/51224
Al and WO 01/22954.
2-[l-(4-chlorobenzyl)-1H-indol-3-yl]-N-(2-
methylquinolin-6-yl)-2-oxoacetamide (9)
2-[l-(4-chlorobenzyl)-1H-indol-3-yl]-2-oxo-N-
quinolin-6-ylacetamide (10)
2-[1-(4-chlorobenzyl)-1H-indol-3-yl]-2-oxo-N-
quinolin-8-ylacetamide (12)
2-[1-(4-chlorobenzyl)-1H-indol-3-yl]-N-
isoquinolin-5-yl-2-oxoacetamide (13)
2-[1-(4-chlorobenzyl)-1H-indol-3-yl]-2-oxo-N-
pyridin-4-ylacetamide (14)
2-[1-(4-fluorobenzyl)-1H-indol-3-yl]-N-(2-
methylquinolin-8-yl)-2-oxoacetamide (15)
Compounds of the general formulae Ia and Ib of the
scheme can be obtained in accordance with the following
Scheme 1:
CA 02526663 2005-11-22
- 17 -
Scheme 1
1st step
\ ~ I NaH, DMSO
N N
H 1I CI R
if R
III IV
2nd step
1. (COCI)2
2. H2N-Het
0 0\ /R 3rd step (a) 0 H
N, RC(O)CI N, HET
\ I I 0 HET NaH ( 0
N or N
/ (RCO)20 /
R D MAP, R
TEA \
Ib la
The compounds of the general formula Ic, in which X =
S, can be prepared in accordance with Scheme 2:
Scheme 2
3rd step (b)
0 I H
o P`S;P o H
HET S' 01~ N,HET
\ N O N 0
Toluene
R
a
la
Ic
Compounds of the general formula Ic, in which Y = S,
can be obtained using methods known from the literature
CA 02526663 2011-02-25
- 18 -
(W.-D. Malmberg et al. Liebigs Ann. Chem. 10, 1983;
1649-1711).
The starting compounds II, III and IV can either be
obtained commercially or prepared using procedures
which are known per se. The starting compounds II, III
and IV are valuable intermediates for preparing the
indole derivatives according to the invention of the
formula I.
For the preparation of the starting compounds and
target compounds, reference may be made, for example,
to the following standard works of organic synthesis:
= Houben-Weyl, Volume E 7a (Part 1) pp. 290-492, pp.
571-740
= Houben-Weyl, Volume E 7a (Part 2) pp. 119-156, pp.
205-686, pp. 157-204
= The monograph "Heterocyclic Compounds"
(Elderfield),
Volume 1, pp. 119-207, pp. 397-616
Volume 3, pp. 1-274
Volume 6, pp. 101-135, pp. 234-323
= The monograph "Comprehensive Organic Chemistry"
(S.D. Barton, W.D. Ollis)
Volume 4, pp. 155-204, pp. 205-232, pp. 493-
564
The skilled person is familiar, on account of his
specialist knowledge, with the solvents and auxiliary
agents, and reaction parameters, such as reaction
temperature and reaction duration, which are to be
used, where appropriate.
The following compounds, whose inclusion in the survey
below is evident from their respective chemical
CA 02526663 2005-11-22
- 19 -
designations, were synthesized in accordance with these
general directions for steps 1, 2 and 3, as based on
synthesis Schemes 1 and 2. The compounds according to
the invention were characterized analytically by means
of their melting points and/or by means of 1H NMR
spectroscopy and/or mass spectroscopy.
The chemicals and solvents employed were either
obtained commercially from the customary suppliers
(Acros, Avocado, Aldrich, Fluka, Lancaster, Maybridge,
Merck, Sigma, TCI, etc.) or synthesized.
The invention will be explained in more detail with the
aid of the following examples without being restricted
to them.
Examples
Example 1 (Reaction in accordance with Scheme 1, 1st
step):
Preparation of 1-(4-chlorobenzyl)indole
A solution of 5.86 g (0.05 mol) of indole in 25 ml of
DMSO is added to a mixture of 1.32 g of sodium hydride
(0.055 mol, mineral oil suspension) in 50 ml of
dimethyl sulfoxide. The resultant mixture is heated at
60 C for 1.3 hours; after that, it is allowed to cool
down and 17.7 g (0.11 mol) of 4-chlorobenzyl chloride
are added dropwise. The solution is heated to 60 C and
allowed to stand overnight; it is then poured into
200 ml of water while stirring. This mixture is
extracted several times with a total of 75 ml of CH2C12r
after which the organic phase is dried with anhydrous
sodium sulfate and filtered and the filtrate is
evaporated in vacuo.
Yield: 11.5 g (95% of theory)
CA 02526663 2005-11-22
- 20 -
Example 2 (Reaction in accordance with the 2nd step of
Scheme 1):
2-[1-(4-Chlorobenzyl)-1H-indol-3-yl]-2-oxo-N-pyrido-
[2,3-b]pyrazin-7-ylacetamide (1)
A solution of 10.2 g (10.7 mMol) of 1-(4-chlorobenzyl)-
indole in 200 ml of ether is added dropwise, at 0 C and
under nitrogen, to a solution of 1.12 ml of oxalyl
chloride in 50 ml of ether. The mixture is heated to
reflux for 2 hrs. and the solvent is subsequently
evaporated off. 30 ml of DMF are then added to the
residue, after which 1.93 g (13.9 mMol) of potassium
carbonate are added and the suspension is cooled down
to 0 C; a solution of 1.57 g (10.7 mMol) of amino
component in 10 ml of DMF is then added dropwise. The
reaction mixture is left to stir overnight at room
temperature. It is finally stirred into ice water and
the resulting precipitate is filtered off with suction.
The crude product which is obtained is chromatographed
on 100 g of silica gel using n-heptane/ethyl acetate =
4:1
Yield: 3.23 g (68.0%)
m.p.: 250 C
1H-NMR (DMSO-D6) S = 11.56 (s, 1H), 9.53 (d, 1H), 9.12
(s, 1H), 9.09 (d, 1H), 9.04 (s, 1H), 8.32 (d, 1H), 7.6
(d, 1H), 7.40 (d, 2H), 7.35 (m, 3H), 7.32 (m, 2H), 5.64
(s, 2H) ppm
Example 3 (Reaction in accordance with the 3rd step (a)
of Scheme 1):
N-{2-[1-(4-Chlorobenzyl)-1H-indol-3-yl]-2-oxoacetyl}-N-
quinolin-6-ylacetamide (2)
CA 02526663 2005-11-22
- 21 -
0.833 g (6.82 mMol) of DMAP, 1.38 g (13.6 mMol) of
triethylamine and 13.9 g (136 mMol) of acetic anhydride
are added, under nitrogen, to a stirred solution of
6.0 g (13.6 mMol) of 2-[1-(4-chlorobenzyl)-1H-indol-3-
yl]-2-oxo-N-quinolin-6-ylacetamide in 60 ml of DMF. The
reaction mixture is stirred at room temperature for 10
minutes and, after that, poured into 200 ml of ethyl
acetate. After 300 ml of water have been added, the
mixture is shaken in a separating funnel after which
the two phase separate. Precipitation begins after 20
minutes. The pale yellow crystals are filtered off and
dried in vacuo at 60 C.
Yield: 4.04 g (61.5%)
m.p.: 122.9 C
1H-NMR (600 MHz, DMSO-d6) 6 = 9.02 (d, 1H), 8.54 (s,
1H), 8.44 (d, 1H), 8.21 (d, 1H), 8.17 (d, 1H), 8.10 (m,
1H), 7.88 (m, 1H), 7.65 (m, 1H), 7.58 (m, 1H), 7.44 (d,
2H) , 7.33 (d, 2H) , 7.28 (m, 2H) , 5.60 (s, 2H) , 2.15 (s,
3H).
MS(ESI) m/z 482.1 (MH+), (theor. 481.94)
Example 4 (Reaction in accordance with the 3rd step (a)
of Scheme 1):
Methyl {2-[1-(4-chlorobenzyl)-1H-indol-3-yl]-2-oxo-
acetyl}quinolin-6-ylcarbamate (3)
930.2 mg (27.3 mMol) of NaH (as a 60% strength
dispersion in mineral oil) are added, under nitrogen,
to a cooled, stirred solution of 10.0 g (22.7 mMol) of
2-[1-(4-chlorobenzyl)-1H-indol-3-yl]-2-oxo-N-quinolin-
6-ylacetamide in 500 ml of dry THF. The solution is
stirred at 0 C until a yellow precipitate separates out
and, after that, stirred for a further 15 minutes.
After that, 2.58 g (27.3 mMol) of methyl chloroformate
CA 02526663 2005-11-22
- 22 -
are added dropwise at a temperature below +5 C. The
reaction is monitored by thin layer chromatography
(eluent: n-heptane/ethyl acetate 1/1 RF = 0.11) . The
reaction mixture is poured into water and the resulting
mixture is extracted with ethyl acetate; the organic
phase is washed with a saturated solution of sodium
chloride and dried over anhydrous MgSO4. Evaporating
off the solvent yields a crude product, which is
purified by column chromatography (n-heptane/acetone
2/1) in order to give 3. Thin layer chromatography
shows that 3 still contains slight impurities, which
can be removed by stirring the crude 3 with acetone for
1 h. Filtration yields 3 as pale yellow crystals.
Yield: 3.0 g (26.5%)
m.p.: 178.5 C
1H-NMR (600 MHz, DMSO-d6) 6 = 9.02 (d, 1H), 8.58 (s,
1H), 8.47 (d, 1H), 8.17 (m, 3H), 7.84 (m, 1H), 7.63 (m,
2H) , 7.44 (d, 2H) , 7.34 (m, 4H) , 5.60 (s, 2H) , 3 .65 (s,
3H).
MS(ESI) m/z 498.2 (MH+), (theor. 497.94)
Example 5 (Reaction in accordance with the 3rd step (b)
of Scheme 2):
Preparing 2-[1-(4-chlorobenzyl)-1H-indol-3-yl]-N-
quinolin-6-yl-2-thioxoacetamide (11)
3.68 g (9.1 mMol) of 2,4-bis(4-methoxyphenyl)-1,3-
dithia-2,4-diphosphetane-2,4-disulfide are added, under
nitrogen, to a suspension of 4.00 g (9.1 mMol) of 2-[1-
(4-chlorobenzyl)-1H-indol-3-yl]-2-oxo-N-quinolin-6-
ylacetamide in 200 ml of toluene, after which the
mixture is heated at 75 C for 3 h. The residue which
has formed is filtered off in the hot from the reaction
solution and subsequently washed with 100 ml of
CA 02526663 2005-11-22
- 23 -
methylene chloride. The filtrate is concentrated in
vacuo and the residue is chromatographed on flash
silica gel (eluent: methylene chloride/methanol 99:1).
The product fractions are filtered on flash silica gel
(eluent: n-heptane/ethyl acetate 1:1) after the solvent
has been removed once more.
Yield: 0.46 g (11% of theory)
ESI-MS: We = 456.1 (MH+), (theor. 455.97)
1H-NMR (DMSO-D6) 8 = 10.89 (s, 1H), 8.8 (s, 1H), 8.75
(s, 1H), 8.55 (s, 1H), 8.12 (d, 1H), 8.35 (d, 1H), 8.0
(d, 1H), 7.93 (d, 1H), 7.63 (d, 1H), 7.50 (m, 1H), 7.4
(m, 3H), 7.3,(m, 3H), 5.6 (s, 2H) ppm.
The following compounds of the formula I were
simplified in analogy with the synthesis route in
Scheme 1 and in accordance with Examples 2 and 3.
Example 6:
2-[1-(4-Chlorobenzyl)-1H-indol-3-yl]-N-(1H-indazol-5-
yl)-2-oxoacetamide (4)
m.p.: 203 C
1H-NMR (DMSO-D6) S = 13.02 (s, 1H), 10.7 (s, 1H), 9.04
(s, 1H), 8.48 (s, 1H), 8.42 (d, 1H), 8.06 (s, 1H), 7.73
(d, 1H), 7.6 (d, 1H), 7.55 (d, 1H), 7.40 (d, 2H), 7.28-
7.35 (m, 4H), 5.63 (s, 2H) ppm
Example 7:
Ethyl {2-[1-(4-chlorobenzyl)-1H-indol-3-yl]-2-oxo-
acetyl}quinolin-6-ylcarbamate (5)
m.p.: 199 C
CA 02526663 2005-11-22
- 24 -
'H NMR (600 MHz, DMSO-d6) 6 = 9.02 (m, 1H), 8.60 (s,
1H), 8.48 (d, 1H), 8.15 (m, 3H), 7.83 (m, 1H), 7.63 (m,
sH), 7.43 (d, 2H), 7.32 (m, 4H), 5.60 (s, 2H), 4.15 (q,
2H), 0.95 (t, 3H).
MS (ESI) m/z 514.2, 512.1 (MH+), (theor. 511.97)
Example 8:
Propyl {2-[1-(4-chlorobenzyl)-1H-indol-3-yl]-2-oxo-
acetyl}quinolin-6-ylcarbamate (6)
m.p.: 164 C
'H-NMR (600 MHz, DMSO-d6) 6 = 9.02 (m, 1H), 8.60 (s,
1H), 8.48 (d, 1H), 8.17 (m, 3H), 7.84 (m, 1H), 7.63 (m,
2H), 7.43 (d, 2H), 7.33 (m, 4H), 5.61 (s, 2H), 4.03 (t,
2H), 1.32 (m, 2H), 0.56 (t, 3H).
Example 9:
N-{2-[1-(4-chlorobenzyl)-1H-indol-3-yl]-2-oxoacetyl}-N-
quinolin-6-ylpropionamide (7)
1H NMR (600 MHz, DMSO-d6) 9.03 (m, 1H), 8.52 (s,
1H), 8.45 (d, 1H), 8.23 (d, 2H), 8.18 (d, 1H), 8.13 (m,
1H), 7.88 (m, 1H), 7.65 (m, 1H), 7.58 (m, 1H), 7.45 (d,
2H), 7.30 (m, 4H), 5.59 (s, 2H), 2.61 (q, 3H), 0.88 (t,
3H).
Example 10:
Ethyl {2-[1-(4-chlorobenzyl)-1H-indol-3-yl]-2-oxo-
acetyl}pyridin-4-ylcarbamate (8)
m.p.: 62 C
1H NMR (500 MHz, DMSO-d6) 5 = 8.74 (m, 2H), 8.52 (s,
1H), 8.12 (m, 1H) , 7. 60 (m, 1H), 7.55 (m, 2H) , 7.40 (m,
CA 02526663 2005-11-22
- 25 -
2H), 7.30 (m, 4H), 5.57 (s, 2H), 4.10 (q, 2H), 0.95 (t,
3H).
Example 11 (Comparison substance):
Preparation of 2-[1-(4-chlorobenzyl)-1H-indol-3-yl]-N-
(2-methylquinolin-6-yl)-2-oxoacetamide (9)
Yield: 14.8 g (77.3% of theory)
m.p.: 182-185 C
1H-NMR (CDC13) 6 = 9.58 (s, 1H), 9.12 (s, 1H), 8.5 (s,
1H) , 8 . 4 1 ( s , 1H) , 8.05 ( t , 2H) , 7.78 (d, 1H) , 7 . 4 (dd,
1H), 7.32 (m, 4H), 7.26 (s, 1H), 7.15 (d, 1H), 5.38 (s,
2H), 2.73 (s, 3H) ppm
Example 12 (Comparison substance):
2-[1-(4-Chlorobenzyl)-1H-indol-3-yl]-2-oxo-N-quinolin-
6-ylacetamide (10)
m.p.: 200 C
'H-NMR (DMSO-D6) 6 = 11.5 (s, 1H), 9.05 (s, 1H), 8.85
(s, 1H), 8.66 (s, 1H), 8.32 (d, 2H), 8.12 (d, 1H), 8.03
(d, 1H), 7.63 (d, 1H), 7.53 (dd, 1H), 7.42 (d, 2H),
7.30-7.38 (m, 4H), 5.63 (s, 2H) ppm
Example 13 (Comparison substance):
2-[1-(4-Chlorobenzyl)-1H-indol-3-yl]-2-oxo-N-quinolin-
8-ylacetamide (12)
m.p.: 178 C
CA 02526663 2005-11-22
- 26 -
Example 14 (Comparison substance):
2-[1-(4-Chlorobenzyl)-1H-indol-3-yl]-N-isoquinolin-5-
yl-2-oxoacetamide (13)
m.p.: 239-241 C
Example 15 (Comparison substance):
2-[l-(4-Chlorobenzyl)-1H-indol-3-yl]-2-oxo-N-pyridin-4-
ylacetamide (14)
m.p.: 264 C
Example 16 (Comparison substance):
2-[1-(4-Fluorobenzyl)-1H-indol-3-yl]-N-(2-methylquino-
lin-8-y1)-2-oxoacetamide (15)
m.p.: 200-202 C
Biological effects of the compounds according to the
invention
Carrying out in-vitro and in-vivo tests on selected
tumor models showed the presence of the following
pharmacological activities.
Example 17: Antiproliferative effect on various tumor
cell lines
The antiproliferative activity of substances 1, 2, 4,
9, 11, 12, 13 and 15 was investigated in a
proliferation test performed on established tumor cell
lines (D.A. Scuderio et al. Cancer Res. 1988, 48, 4827-
4833). The test which is used determines the cellular
dehydrogenase activity and makes it possible to
determine cell viability and determine cell number
CA 02526663 2005-11-22
- 27 -
indirectly. The cell lines which are used are the human
cervical carcinoma cell line KB/HeLa (ATCC CCL17), the
ovarian adenocarcinoma cell line SKOV-3 (ATCC HTB77),
the human glioblastoma cell line SF-268 (NCI 503138)
and the lung carcinoma cell line NCI-H460 (NCI 503473).
XTT proliferation assay, EC50 in g/ml
Example KB/HeLa SKOV3 SF-268 NCI-H460
1 0.045 0.029 0.042 0.046
2 0.202 0.123 0.166 0.168
4 0.335 0.144 >3.16 0.233
11 0.036 0.029 0.036 0.057
9 (C) 0.183 0.174 0.261 0.344
12 (C) >3.16 >3.16 >3.16 >3.16
13 (C) >3.16 >3.16 >3.16 >3.16
(C) >3.16 n.d. >3.16 n.d.
C = Comparison substance; n.d.: not determined
10 Table 1: Ability of the substances according to the
invention to inhibit proliferation in the XTT
cytotoxicity test carried out on human tumor
cell lines
15 The results show that implementation examples 1, 2, 4
and 11 are very potent inhibitors of the proliferation
of selected tumor cell lines.
Example 18: Antiproliferative effect on MDR tumor cell
lines
For further characterization, substances 1, 2, 4 and 11
were investigated with regard to their effect on
multidrug-resistance cell lines as compared with that
on nonresistant wild-type cell lines.
The cell lines which were investigated are the murine
cell line L1210, the acute myeloid leukemia cell line
LT12 and the resistant lines L1210/mdr and LT12/mdr.
CA 02526663 2005-11-22
- 28 -
The murine cell line P388 (methyl cholanthrene-induced
lymphoid neoplasm) and doxorubicin-resistant P388 were
also included as test systems.
The results are summarized in Table 2 below:
XTT proliferation assay, EC50 in g/ml
LT12 LT12mdr L1210 L1210VCR P388 P388ADR
Example
1 0.015 0.017 0.018 0.021 0.012 0.019
2 0.225 0.272 0.206 0.558 0.224 0.215
4 0.084 0.093 0.246 0.241 0.175 0.231
11 0.023 0.054 0.052 0.067 0.018 0.051
Paclitaxel 0.005 0.34 0.048 >3.16 0.035 >3.16
Vincristine 0.002 0.134 0.015 >3.16 0.004 0.93
Doxorubicin 0.029 >3.16 0.269 >3.16 0.204 >3.16
Mitoxantrone 0.006 3.1 0.09 2.1 0.053 0.608
Etoposide 0.094 >3.6 0.269 >3.16 0.202 >3.16
C = Comparison example
Table 2: Inhibitory effect. of the substances on human
tumor cell lines in the XTT proliferation
test.
Substances 1, 2, 4 and 11 exhibit a very potent
inhibitory effect on all the cell lines tested, while
the classic substances which have a tubulin-inhibiting
effect, such as paclitaxel or vincristine, and the
topoisomerase II inhibitors (doxorubicin, mitoxantrone
and etoposide) can be seen to have an effect on the
MDR1-resistant cell lines which is at least greatly
reduced.
CA 02526663 2005-11-22
- 29 -
Example 19: Inhibition of tubulin polymerization
Substances 1, 4, 9, 11, 12, 13 and 15 were tested for
their ability to inhibit the polymerization of bovine
tubulin in an in-vitro test (D.M. Bollag et al. Cancer
Res. 1995, 55, 2325-2333). This test uses tubulin which
has been purified by cycles of polymerization and
depolymerization and which is caused to polymerize by
adding GTP and heating it. Table 3 gives the EC50 values
causing inhibition of the polymerization of tubulin
containing 30% associated proteins (MPAs).
Example Inhibition of tubulin polymerization,
EC50 in g/ml
1 0.71
4 1.26
11 0.97
9 (C) 1.16
12 (C) >10 M
13 (C) >10 M
(C) >10 M
Vincristine 0.35
C = Comparison example
Table 3: Inhibition of tubulin polymerization. Mean
values of two independent experiments.
The results (see Table 3) show that substances 1, 4, 9
and 11 have a very potent inhibitory effect on tubulin
polymerization while compounds 12, 13 and 15 do not
exert any effect.
Example 20: Inhibition of topoisomerase II
The ability of substance 1 to inhibit topoisomerase II
was examined in two different in-vitro tests.
CA 02526663 2011-02-25
30 -
= kDNA assay for testing topoisomerase II activity:
In this assay, which was described by P. Arimondo
(Anti-Cancer Drug Design 2000, 15(6), 413-421), kDNA is
treated with human DNA topoisomerase II in the absence
or presence of the test compounds. In the assay,
compound 1 according to the invention was tested at
three different concentrations (100, 31.6 and 10 M). A
positive control and the reference compounds
m-amsacrine (m-amsa), paclitaxel (Taxo1T"') and
vincristine, with the concentration in each case being
100 M, were used for comparison.
Implementation of the assay:
2 }J.L of lOx assay buffer, 1 jiL of kDNA (200 ng), 0.5 L
of human topoisomerase II (1 unit) and 15.5 L of H2O
are added by pipette to 1 p.L of initially introduced
test substance (20 times concentrated in 100% DMSO) and
the reagents are mixed.
The reaction assay samples are placed in a heating
block which has been preheated to 37 C and incubated at
37 C for 10 min. The incubation is stopped after adding
4 L of 5x Stop buffer and the substance is
subsequently extracted with CIA. After that, 20 L of
the supernatant are loaded onto a 1% agarose gel
containing 0.25 g of ethidium bromide/mL and separated
at 100 V for 1 h. Finally, the gel is photographed
under UV excitation (see Figure 1). The inhibition of
the decatenation of kDNA is quantified using the
Ge1Pro Analyzer Software (see Figure 2).
= pRYG relaxation assay for testing topoisomerase II
activity:
This relaxation assay was used to further demonstrate
the inhibitory properties of the compounds according to
the invention on topoisomerase II. In the assay, the
CA 02526663 2005-11-22
- 31 -
compound 1 according to the invention was tested at
three different concentrations (100, 31.6 and 10 M).
The reference compounds m-amsacrine, paclitaxel (Taxol)
and vincristine were used, at concentrations of 316 and
100 M, for comparison.
The assay is carried out as follows:
2 L of lOx assay buffer, 0.5 L of pRYG DNA (125 ng),
0.5 L of human topoisomerase II (1 unit) and 16 L of
H2O are added by pipette to 1 L of initially
introduced test substance (20 times concentrated in
100% DMSO) and the reagents are mixed. The reaction
assay samples are placed in a heating block which has
been preheated to 37 C and incubated at 37 C for 30
min. The incubation is stopped after adding 4 L of 5x
Stop buffer. After that, 10 L of the assay sample are
loaded onto a 1.2% agarose gel containing 0.25 g of
ethidium bromide/mL and separated at 100 V for 2.5 h.
Finally, the gel is photographed under UV excitation
(see Figure 3). The inhibition of pRYG relaxation is
quantified using the GelPro Analyzer Software (see
Figure 4).
Taken overall, it can be stated that compound 1
according to the invention was shown to significantly
inhibit topoisomerase II in both assays. The results
obtained with compound 1 are comparable with the
inhibition values obtained with the topoisomerase II
inhibitor m-amsacrine. As expected, neither paclitaxel
nor vincristine was observed to have any inhibitory
effects in the two assays.
Example 21: Cell cycle analysis
The cell cycle comprises the progress of the cell from
one cell generation to the next.
CA 02526663 2005-11-22
- 32 -
During the resting phase (GO) and presynthetic phase
(G1), the cell possesses a diploid set of chromosomes
(2c) . In the synthetic phase (S), the quantity of DNA
is increased by replication. The S phase ends when the
premitotic phase (G2M), in which the cell possesses a
reduplicated complement of chromosomes (4c) and a
doubled content of DNA, is reached. In the subsequent
mitotic phase (M), which is of short duration, the
reduplicated chromosomes are uniformly apportioned
between two daughter cells, which then in each case
once again possess a diploid content of DNA and are in
the G0l phase, which means that the cell cycle can
begin afresh.
For the cell cycle analysis, KB/HeLa cells were treated
with different concentrations of the test substances
(0.1-1000 nM) at 37 C for 24 hours.
The percentage of cells arrested in the G2/M phase of
the cell cycle after having been treated with reference
substances or selected test substances is shown in
Table 4 below. The results were analyzed using special
analytical software (ModFitTM)
Example EC50 in nM (50% of cells in G2/M)
1 25.2
2 125.3
4 252
11 41.8
14 (C) >1000
paclitaxel 26.9
mitoxantrone 25.3
Table 4: Concentration required for inhibiting 50% of
the cells in the G2/M phase.
Compounds 1, 2, 4 and 11 according to the invention
exhibit activities which are comparable to those of the
reference compounds paclitaxel and mitoxantrone.
CA 02526663 2005-11-22
- 33 -
Example 22: Demonstration of apoptosis
CDDP1ns nucleosome ELISA test:
Nuclear fragmentation is a late consequence of
apoptotic processes. The changes which can be observed
in this connection can be attributed to DNA strands
being cleaved by endonucleases and the fragmentation
into nucleosome particles which results therefrom.
The CDDP1as nucleosome ELISA test described by Roche
Molecular Biochemicals was used for demonstrating the
nucleosome particles.
For this, the effects of compounds 1 and 2 on the U-937
cell line were investigated at different concentrations
(1 nM-10 M; 24 h of treatment). (See Figure 5 and
Figure 6).
In this test, it was possible to observe a
concentration-dependent increase of nucleosomes in the
cell lysate for compounds 1 and 2. It was not possible
to demonstrate any significant increase in the cell
culture supernatant, which is evidence in support of
apoptotic cell death occurring after treatment with 1
and 2.
Example 23: Demonstration of the saturation solubility
in water of the compounds according to the invention
The saturation solubility in water of compounds 1, 2,
10 and 14 was determined as described below. A maximum
of 1% DMSO was added for the purpose of solubilizing
the substances and improving the wetting of the
samples. An HPLC-UV method was used for checking the
content. The results are summarized in Table 5 below:
CA 02526663 2005-11-22
- 34 -
Name of compound Saturation solubility in water
[ g/ml]
1 25.0
2 28.5
(C) 0.038
14 (C) 0.35
Table 5: Saturation solubilities of compounds 1, 2, 10
and 14
5
Compounds 1 and 2 according to the invention differ
from compounds 10 and 14 in being more soluble in
water.
10 Example 24: In-vivo activity
The in-vivo activity and tolerability of compound 2
according to the invention, as compared with those of
substances 10 and 14, were examined in a human
xenograft model (melanoma, MEXF-462) . The results are
summarized in Table 6 below:
in-vivo activities of compounds 2, 10 and 14 (melanoma;
MEXF462)
Compound Dose Adminis- Deaths Optimum T/C% (day)
[mg/kg] tration nl
2 80 P.O. 0/6 mice 0.0% (18)
complete remission
in the case of all
6 animals
10 (C) 70 P.O. 5/6 mice 2.3% (7)
dead
10 (C) 55 P.O. 2/6 mice 0.8% (14)
dead
14 (C) 32 P.O. 3/5 mice 14.6% (7)
dead
CA 02526663 2005-11-22
- 35 -
14 (C) 16 P.O. 3/5 mice 0.7% (18)
dead
1) Number of dead animals as compared with the total
number
Table 6: In-vivo activities of compounds 2, 10 and 14
(melanoma; MEXF462)
In this xenograft model, compound 2 was observed to
produce complete remission of the tumors in the treated
animals while also being very well tolerated (no
deaths). While comparable antineoplastic effects were
observed in the case of compounds 10 and 14, these
latter compounds were less well tolerated.
Example 25: In-vivo activity
The in-vivo activity and tolerability of compound 2
according to the invention, as compared with those of
substance 10, were examined in another human xenograft
model (mammary gland, MAXF857).
The results are shown in the following table:
Effect of 2 and 10 on the mammary cancer MAXF857
Compound Dose Adminis- Deaths Optimum T/C
[mg/kg] tration nl % (day)
2 80 P.O. 0/6 mice 9.6% (10)
10 (C) 40 P.O. 2/6 mice dead 6.5% (10)
1) Number of dead animals as compared with the total
number
Table 7: In-vivo activities of compounds 2 and 10
(mammary gland; MAXF857)
CA 02526663 2005-11-22
- 36 -
While compounds 2 and 10 were observed to have
comparable antineoplastic effects, substance 10 (2/6
mice dead) is substantially less well tolerated than 2.