Note: Descriptions are shown in the official language in which they were submitted.
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
STRUCTURE BASED AND COMBINATORIALLY SELECTED OLIGONUCLEOSIDE
PHOSPHOROTHIOATE AND PHOSPHORODITHIOATE APTAMER TARGETING AP-1
TRANSCRIPTION FACTORS
TECHNICAL FIELD OF THE INVENTION
The present invention relates in general to the field of thioaptamers, and
more particularly, the use of
thioaptamers for screening, including high-throughput screening, of primary or
secondary target molecules
by using thioated aptamers bound to a substrate with specific targeting to the
AP-1 family of transcription
factors and for the treatment of viral infections, as well as, vaccines and
vaccine adjuvants that modify
host immune responses.
BACKGROUND OF THE INVENTION
This application claims priority to United States Provisional Patent
Application Serial No. 60/472,888,
filed May 23, 2003. This work was supported by the following United States
Government grants DARPA
(9624-107 FP), NIH (AI27744) and NIEHS (ES06676). Without limiting the scope
of the invention, its
background is described in connection with oligonucleotide agents and with
methods for the isolation and
generation thereof.
Virtually all organisms have nuclease enzymes that degrade rapidly foreign DNA
as an important in vivo
defense mechanism. The use, therefore, of normal oligonucleotides as
diagnostic or therapeutic agents in
the presence of most bodily fluids or tissue samples is generally precluded.
It has been shown, however,
that phosphoromonothioate or phosphorodithioate modifications of the DNA
backbone in oligonucleotides
can impart both nuclease resistance and enhance the affinity for target
molecules, such as for example the
transcriptional activating protein NF-xB.
Recent world events have heightened the awareness of possible bioterrorist
threats. Hemorrhagic fever
viruses (category A bioweapon agents) have reportedly been weaponized by the
former Soviet Union and
the United States (Borio et al., 2002; Hawley & Eitzen, 2001). Despite the
awareness of the potential of
Viral Hemorrhagic Fever viruses (Lassa, Junin), Encephalitic viruses (West
Nile, VEE) and other agents
both as bioweapons and as emerging viral diseases, few therapeutic options are
available to those infected.
Apart from supportive therapy, the only drug for treating Arenavirus
infections is Ribavirin and it is only
partially effective (McCormick et al, 1986a; Shulman, 1984; Enria et al.,
1987) while there are no
efficacious drugs to treat victims of West Nile infections (Peterson and
Marfin, 2002). There is an urgent
need to expand the current therapeutic armamentarium, which is hindered, at
least in part, by a lack of in-
depth knowledge concerning the mechanisms of Arenaviral pathogenesis (Peters &
Zaki, 2002).
1
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
Arenavirus pathogenesis stems from host immune response dysregulation and
endothelial dysfunction
(Peters & Zaki, 2002; Ignatyev et al., 2000; McCormick & Fisher-Hoch, 2002;
Walker et al., 1982;
McCormick et al., 1986b; Marta et al., 1999). West Nile pathogenesis is
associated with the inability of
host immune response to limit virus replication to levels below that required
for viral invasion of the CNS
(Solomon and Vaughn, 2002).
Lassa fever, a human arenavirus hemorrhagic fever virus endemic in West
Africa, affects up to 300,000
people annually and is responsible for up to 3000 deaths (McCormick, et al.,
1987). Lassa Fever virus is
difficult to study due to its hazardous nature (a BSL4 agent). Junin Virus is
the causative agent of
Argentine hemorrhagic fever (AHF). The annual incidence varies between 100-
4000 cases/yr. AHF has a
case fatality rate of 15-30% and is also a BSL4 agent. A well-established
animal model that resembles
Lassa Fever, using the non-pathogenic New World Arenavirus, Pichinde virus
(Jahrling et al., 1981) has
been used to study this class of pathogens. Serial passage of Pichinde virus
in guinea pigs was used to
develop a virulent variant that produces a disease in guinea pigs that mimics
human Lassa Fever in many
important respects including: viremia correlates with disease outcome (Johnson
et al., 1987; Aronson et
al., 1994), a relative paucity of pathologic findings in lethally infected
animals (Walker, et al., 1982;
Connolly, et al., 1993), terminal vascular leak syndrome (Katz & Starr, 1990)
and distribution of viral
antigens within the host (Connolly et al., 1993; Shieh et al., 1997; Aronson,
unpublished data).
Macrophage responses to the attenuated Pichinde virus, P2, with the virulent
Pichinde variant, P18 as well
as reassortants of the two variants (Zhang et al., 1999; Zhang et al., 2001;
Fennewald et al., 2002) may be
used to compare and modify the immune response to viral infection.
West Nile virus (Category B virus) is a mosquito-borne flavivirus that is a
neuropathogen in humans,
equines and avians (Solomon and Vaughn, 2002; Petersen and Martin, 2002).
Humans become infected
by the bite of an infected mosquito. The viruses are then thought to replicate
in the skin before being
transported to the local lymph nodes. West Nile may then spread via the blood
to other organs including
the liver, spleen, heart and kidney and eventually the brain. West Nile virus
may spread to the CNS via
either hematogenous spread or via the olfactory mucosa where there is no blood-
brain barrier. West Nile is
an emerging pathogen in the US, spreading across the country since it was
first identified in New York in
1999. As of October 3, 2002, the CDC has reported 2530 cases of West Nile
virus infection with 125
deaths in 32 states. West Nile is also responsible for major outbreaks in
other countries including Tunisia,
Romania, Algeria, Russia and Israel among others. Case fatality rates range
from 4-29%. Age is a risk
factor in the development of severe West Nile disease with many patients
exhibiting substantial morbidity.
Presently, treatment for West Nile is limited to supportive intervention.
There is no evidence that either
interferon or Ribavirin treatment is efficacious (Petersen and Marfin, 2002).
2
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
Arenavirus Hemorrhagic Fevers, such as Lassa fever, Junin, Argentine
hemorrhagic fever, Bolivian
hemorrhagic fever and Venezuelan hemorrhagic fever, have several features in
common with sepsis and
the systemic inflammatory response syndrome, including fulminant clinical
course, fever, shock, capillary
leak syndrome, decreased myocardial contractility, abnormalities of
coagulation and platelet function, and
elevated serum levels of TNFa (Aronson et al., 1994; Cummins, 1990).
Arenaviruses are non-cytopathic
viruses with a tropism for macrophages and other reticuloendothelial cells
(Cummins, 1990; Peters et al.,
1987); the pathogenesis of these diseases is believed to involve excessive
production of pro-inflammatory
cytokines (Aronson et al., 1995; Peters et al., 1987). Unpublished data
(Bausch et al., CDC) show
cytokines to be massively activated in human Lassa fever, and also confirm
that Lassa virus can directly
induce cytokine secretion by infecting human macrophages in vitro (Mahanty et
al., CDC, unpublished).
Alternatively, there is evidence that a swift elaboration of pro-inflammatory
cytokines and early
engagement of the (innate) immune response may help protect of the infected
host from lethal disease in
various hemorrhagic fever syndromes (Peters et al., 1987).
Endotoxic shock results from an innate, anaphylactic response to bacterial
lipopolysaccharide (LPS). The
NF-xB transcription factor, in conjunction with other cellular transcription
factors, plays a critical role in
gene activation, especially in acute phase and inflammatory responses
(Baeuerele, 1998; Barnes and
Karin, 1997), and in particular endotoxic shock, a complex pathophysiological
state which is considered to
be an exaggerated or dysregulated systemic acute inflammatory response
syndrome initiated by the
binding of bacterial LPS complexed with lipopolysaccharide binding protein
(LBP) to the CD14 receptor
on macrophages. A series of intracellular signaling events, in which NF-KB
activation figures importantly
leads to enhanced transcription of proinflammatory mediators, including TNFa,
IL-1 and inducible nitric
oxide synthase, ultimately promoting vasodilatation, capillary leakiness, and
myocardial suppression
(Murphy et al., 1998). In well-established mouse endotoxemia models, rapid
transient increases in NF-xB
DNA-binding activity can be detected in the nuclei of macrophages and other
cell types (Boher, et al.,
1997); similar observations have been made in human sepsis (Velasco et al.,
1997).
The AP-1 transcription factor family include the dimeric basic region leucine
zipper proteins that belong
to the Jun (c-Jun, Jung, JunD), Fos (c-Fos, FosB, Fra-1, Fra-2) Maf (c-Maf,
MafB, MafA, MafG/F/K, Nrl)
and ATF/CREB (CREB, CREBP-2, ATF1, ATF2, LRF1/ATF3, ATF4, ATFa, ATF6, B-ATF,
JDP1,
JDP2) subfamilies which recognize either 12-O-tetradecanoylphorbol-13-acetate
(TPA) response elements
(5'-TGAG/CTCA-3') or CAMP response elements (CRE, S'-TGACGTCA-3') (Chinenov
and Kerppola,
2001; Shaulian and Karin, 2002). These transcription factor binding sites are
elements in the promoters
and enhancers of numerous mammalian genes including IL-2, IL-3, IL-4, IL-5,
IFN(3, TNFa and GM-CSF
(Chineov and Kerppola, 2001). The c-Jun protein is the most potent
transcription factor. The c-Fos
proteins, which cannot homodimerize can form heterodimers with c-Jun and
thereby enhance their DNA
binding activities. The c-Fos, and FosB proteins contain transactivation
domains, however, Fral, Fra2 and
3
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
some splice variants of FosB do not. CREB and ATF1 can form homodimers and
heterodimers but do not
combine with other ATF proteins. ATF2, ATFa, CREBP-2, ATF3, ATF4 and ATF6
combine both with
themselves and with specific Jun and/or Fos family members. C-Fos and Fral can
heterodimerize with
ATF4, but not with ATF2 and ATF3.
There are numerous other possible homodimers and heterodimers possible among
this large group of BZIP
proteins. Jun, Fos and ATF family members can also bind to DNA upon
association with certain Maf,
C/EBP and non-bZIP member factors like NF-xB, NFAT and Smad. This can direct
AP-1 components to
promoter sequences that only slightly resemble consensus AP-1 and ATF motifs.
This variation in dimer
partner and DNA binding site specificity is assumed to provide AP1 subunits
with a high level of
flexibility in gene regulation. The regulation of AP-1 family of transcription
factor activity is complex but
briefly regulation occurs through: 1 ) changes in jun and fos gene
transcription and mRNA turnover, 2) Fos
and Jun protein turnover, 3) post-translational modifications of both Fos, Jun
other family proteins that
modulate their activities, and 4) interactions with other transcription
factors (Shaulian and Karin, 2001,
2002). AP-1 activity is induced by growth factors, cytokines,
neurotransmitters, polypeptide hormones,
cell/matrix interactions, bacterial and viral infections and a variety of
environmental stresses. These
activators stimulate a series of signaling events that involve a variety of
protein kinases including MAPKs,
ERKs and JNKs. Members of the Fos and Jun protein families participate in the
regulation of a variety of
cellular processes including cell proliferation, differentiation, apoptosis,
oncogenesis, inflammation, and
immunity (Chinenov and Kerppola, 2001 ).
SUMMARY OF THE INVENTION
The present invention demonstrates the use of "thioaptamersTM" to prevent
Arenavirus and Flavivirus
induced perturbations of the host response that lead to disease. Furthermore,
the present invention
provides for novel therapeutic interventions for the treatment of hemorrhagic
fevers, encephalitic viruses
and other viral infections, resulting from their use as bioweapons or as
emerging diseases. For example,
modified thioaptamers were used to demonstrate modulation of NF-xB and AP-1 to
increase the survival
of Arenavirus infected guinea pigs and mice infected with West Nile virus in a
well-established model
system. The present invention was also used to protect against viral infection
with a neuropathologic viral
infection. The modified thioaptamers of the invention were created and used to
protect mice challenged
with West Nile virus in a well-established model system.
The present invention is based on the recognition that thiomodified aptamers
may be designed, isolated
and used to manipulate transcription factors such as NFKB and AP-1 to
interdict the pathogenetic
sequence, or even boost early protective innate immune responses (Figure 1).
To demonstrate the
feasibility of using the modified thioaptamers disclosed herein at
physiological concentrations, animal
model systems were used that models both severe fatal disease and self limited
infection with mild
4
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
disease. For example, a well-recognized and widely used guinea pig model for
Lassa Fever uses the New
World arenavirus Pichinde (PIC) (Peters et al., 1987) was used and adapted to
study pathogenesis by
comparing an attenuated variant of PIC (P2) and a closely related virulent
variant derived by serial guinea
pig passage (P18) (Jahrling et al., 1981).
The present invention also uses the modified thio-aptamers to manipulate NF-xB
levels in vivo. For
example, the modified thioaptamers of the present invention were used to
modify toxic shock via IxBa
overexpression increased mouse survival after high dose LPS challenge. The
modified thioaptamers of the
present invention may be used to target the five NF-oB/Rel family proteins,
which combine to form 15
homo- and heterodimers. By targeting target one or more of the five NF-xB/Rel
family members, the
present invention is used to modify one or more of the signaling pathways that
regulate a specific
signaling function upon translocation across the cell nuclear membrane and
binding to a gene's promoter
region.
While it is recognized that the AP-1 and NF-xB transcription factor families
both play key roles in the
immune response and both represent appropriate targets for therapies for viral
infections, it has not been
possible to modify in a physiologic manner their activities. The present
invention allows for the
modification of transcription factor activities using modified thioaptamers
that act under physiological
conditions and at physiological levels to regulate transcriptional activation.
Such regulation may be used
to modify responses to diseases involving pathogenic or disfunctional
inflammatory responses such as
cancer, heart disease, inflammatory bowel disease, rheumatoid arthritis and
lupus.
The present invention was used to modulate induction of CREB, a transcription
factor regulated by cyclic
AMP (CAMP) signaling. The modified thioaptamers were used to modulate CREB
activity and were
demonstrated to modify virulent and attenuated Arenavirus infection. The CREB
protein is also a member
of the AP-1 family of transcription factors whose targeting by XBY-S2 has
provided protection for
animals infected with arenavirus and flavivirus. cAMP is a ubiquitous second
messenger (Antoni et al.,
2000) synthesized in cells by adenylyl cyclases in response to many extra-
cellular stimuli. Most cellular
effects of CAMP are mediated through the activation of cAMP dependent protein
kinases (PKA) (Sassone,
1995). PKA phosphorylation of substrates in all cellular compartments
regulates a large array of cellular
processes (Feliciello et al., 2001). Cyclic AMP induces changes in gene
expression that modulate
macrophage apoptosis (von Knethen & Briine, 2000) and could contribute to
pathogenic inflammatory
conditions and sepsis. There is also evidence for functional cross talk
between CAMP signaling and the
Jak/STAT pathway (Meloche et al., 2000). Agents that increase intracellular
concentrations of cAMP
inhibit IL-6 induced STAT activation in monocytes and interferon-(3 stimulated
phosphorylation of Jakl,
Tyk2, STAT1 and STAT2 in myeloma cells. Therefore, the modulation of the
Jak/STAT pathway by
5
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
cAMP is likely to play an important role in the regulation of immune and
inflammatory responses, which
may be regulated using the modified thiopatamers of the present invention.
The present invention provides a number of advantages due to the use of
modified thioaptamers and
combinatorial selection methods. The present invention provides very high
affinity - nM to sum-nM (>_
monoclonal IgMs and >non-substituted aptamers), target-specific aptamers,
demonstrating single protein
target binding within cellular extracts. The modified thioaptamers have
greater resistance to cellular or
serum nuclease degradation than normal backbone aptamers, or proteases towards
antibodies. Due to the
increased nuclease resistance, the aptamers disclosed herein may be packaged
to have indefinite shelf life,
ease of storage as lyophilized powders at room temperatures, unlike unmodified
RNA or antibodies and
are relatively inexpensive to produce. Furthermore, the methods and
compositions disclosed herein allow
for high reproducibility in quality control, unlike diasteromeric mixtures for
non-stereospecifically
produced monothiophosphate aptamers, or protein production of antibodies.
Finally, the use of bead-
based thioaptamer libraries or library of libraries provides large
combinatorial libraries readily selected by
multicolor flow cytometry at very high speeds (108/hr).
In one embodiment, the present invention is a system and method for
identifying both thioaptamer
sequences and binding one or more proteins that include the steps of,
incubating a thioaptamer library with
a sample suspected of including one or more proteins, e.g., target proteins.
The proteins that bind the
thioaptamers are selected from the one or more thioaptamers of the library to
which protein has bound, the
proteins are identified using mass spectrometry and/or the thioaptamer is
sequenced using, e.g., a method
that includes PCR amplifying the aptamer followed by, cloning and sequencing.
The thioaptamers may be
on beads, e.g., as part of a one-bead, one-thioaptamer library and may be
sequenced, e.g., directly on the
beads.
The system and method may also include the step of separating the protein into
fragments prior to
separation by liquid chromatography followed by mass spectrometry. In an
alternative method, the step of
identifying the protein by mass spectrometry (MS) may be, e.g., time-of flight
(TOF) MS. In one
example, prior to the step of identifying the protein, the protein may be
extracted and then separated by
liquid chromatography. The identification of the protein may be by surface
enhanced laser desorption
ionization (SELDI) or matrix assisted laser desorption ionization (MALDI)
prior to MS. The thioaptamers
may be attached to beads or a substrate, e.g., a semiconductor substrate.
Semiconductor substrates may be
used as arrays that permit detection of proteinahioaptamer binding and may
further include detectors that
are integral with the substrate (e.g., capacitance coupled devices) or even
surface metal for surface
plasmon resonance (SPR) detection. The thioaptamer library may even be a
microarray on a substrate that
does not include an integral detected, e.g., a glass slide on which a
thioaptamer library has been disposed
using, e.g., photolithography or digital optical chemistry. The location of
protein binding on such a
6
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
microarray may be detected using well known protein detection methods, e.g.,
fluorescence. The protein
for use with the invention may be protein from a crude extract or even
partially purified or isolated, e.g.,
one or more proteins isolated from a gel.
The system and method disclosed herein may further include the use of binding
the thioaptamers to beads
and sorting the beads to isolate and identify proteins that have specifically
bound to the thioaptamers. For
example, when using a thioaptamer library of beads, the beads may be sorted
based on protein binding,
e.g., based on fluorescence labeling of the aptamer and/or the protein using a
flow-cytometer. The protein
may be from a cell extract, which may even be a cell extract from a virally
infected or diseased cell.
Generally, the thioaptamers are attached to beads and the beads are
substantially protein-free. When using
a one-bead, one-thioaptamer (ODN) library or even a library of libraries the
thioaptamers may be one or
more beads that include an [S]-ODN and/or [Sz]-ODN combinatorial libraries.
The ODNs may be single
or double stranded and may include thin-modifications to one or both of the
strands
In one embodiment of the present invention the thioaptamet library includes,
or is designed to include,
sequence motifs for high affinity with cellular proteins selected from
proteins that are members of, e.g.,
the AP-1, RBP-Jx, NF-xB, NF IL-6, CREB and GRE protein families, and
combinations thereof. In
operation, the system and method may also include the step of comparing a
first and a second incubation
of one or more beads to a first and a second sample, respectively, wherein
differences in binding are used
to detect proteins that expressed differentially, e.g., proteins from a
virally-infected (or diseased) cell or
even a cancer cell. In an alternative embodiment, the method may also include
the steps of binding the
one or more thioaptamers to one or more beads, incubating the one or more
thioaptamer beads with a cell
extract from a cell wherein proteins from the cell extract are labeled with a
first dye; incubating the one or
more thioaptamers beads with a cell extract from a diseased-cell wherein
proteins from the diseased-cell
extract are labeled with a second dye, incubating the one or more thioaptamers
beads with a cell extract
from a diseased-cell pre-treated with thioaptamers or other drugs, wherein the
proteins of the diseased-cell
but drug-treated, are labeled with a third dye; and performing a three-color
flow cytometry that measured
the relative levels of the first, second and third dyes.
Another embodiment of the present invention is a complex combinatorial library
that includes one or more
concatenated thio-modified aptamers, wherein at least a portion of each of the
aptamers is partially thio-
modified. The one or more concatenated thioaptamers may be bound to a
substrate, e.g., one or more
beads, a semiconductor, a surface plasmon resonance surface (e.g., gold), a
multi-well plate and the like.
The concatenated aptamer may include two or more concatenated thio-modified
aptamers, wherein one or
more of the aptamers is partially thio-modified. In one example, the two or
more concatenated
thioaptamers may include nucleic acid sequences suspected of binding to
nuclear regulatory factors, and
may even be a library of thioaptamers. More particularly, the two or more
concatenated thioaptamers may
7
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
include nucleic acid sequences suspected of binding: NF-xB, RBP-Jx, AP-1, NF
IL-6, SP-1, GRE, SRE
and the like. In one example, one or more of the thioaptamers may be a library
of aptamers that binds to
one or more transcritption factors and includes sequences or sequence motifs
for transcription factor
binding, e.g., a NF-xB, a RBP-Jx, an AP-1, an NF IL-6, an SP-l, a GRE, an SRE
motif and/or mixtures
thereof.
The complex combinatorial library made by a method that includes the steps of
synthesizing an aptamer
bead library having a first thioaptamer and concatenating to each of the first
thioaptamers a second
aptamer or thioaptamer suspected of binding to, e.g., a nuclear regulatory
factor. In fact, the first and
second thioaptamers may even be suspected of binding the same nuclear
regulatory factor or a different
nuclear regulatory factor. Yet another embodiment of the present invention is
a method of identifying a
thio-modified therapeutic agent that includes mixing a sample suspected of
including a DNA binding
protein with a concatenated first and second thioaptamer under binding
conditions and isolating the one or
more DNA binding proteins that bind specifically to the concatenated aptamers.
Another embodiment of the present invention is a composition, adjuvant,
vaccine and method of
1 S modifying an immune response that includes providing a host cell with
aptamers that suppress the activity
of a nuclear regulatory factor critical for activation of an immune response.
The immune response may be
an innate immune response, a cytotoxic or a helper T cell immune response. In
one embodiment the
thioaptamer modified the immune response by shifting the helper 1-type (Thl)
to T helper 2-type (Th2)
ratio. The immune response that is modified may be to a virus, a bacteria, a
fungus, a cancer, a self
antigen, a heterologous antigen, a retrovirus, a hemorraghic virus or a
neuropathologic virus, e.g., West
Nile Virus. The immune response that is modified may be modified in vivo, in
vitro and/or ex vivo. The
modification of the immune response may be an increase or decrease of the
immune response as measured
by, e.g., antibody production, cytotoxic T cell activation, cytokine release,
apoptosis, cell proliferation,
cell killing, chromium release, nucleic or amino acid uptake or release and
other methods known to those
skilled in the immunological arts.
In one specific embodiment, the type of helper T cell response may be modified
by providing a host or
target cell with one or more thioaptamers that suppress the activity of a
nuclear regulatory factor critical
for activation of, e.g., a helper T cell response. The T cell immune response
may be to, e.g., a virus, a
bacteria, a fungus, a cancer, a self antigen, a heterologous antigen, a
retrovirus, a hemorraghic virus or
even a neuropathologic virus. The modification to the immune response may be
to a challenge to the
innate or the adaptive immune response. The helper T cell response may be a T
helper 1-type response or
a T helper 2-type response.
Another embodiment of the invention is a vaccine that includes an antigen and
a thioaptamer. The vaccine
may be to an antigen from, e.g., a virus, a bacteria, a fungus, a cancer, a
self antigen, a heterologous
8
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
antigen, a xenoantigen, a retrovirus, a hemorraghic virus or a neuropathologic
virus. The vaccine may be
provided in a lyophilized, a particulate or even a dissolved form and may even
include one or more
pharmaceutically acceptable salts, diluents, preservatives and the like. The
antigen may be, e.g., a live-
attenuated antigen or a heat-inactivated antigen. Examples of viral antigens
include: hemorrhagic fever
viruses, which include viruses from different viral families, e.g., Ebola,
Marburg, Lassa fever, New World
Arenavirus, Rift Valley Fever, yellow fever, Omsk hemorrhagic fever and
Kyasanur Forest Disease
viruses. Four viral families are generally implicated in hemorrhagic fever
infections, including: (1)
Arenaviridae (Lassa, Junin, Machupo, Guanarito, and Sabia viruses, which are
the causative agents of
Lassa fever and Argentine, Bolivian, Venezuelan, and Brazilian hemorrhagic
fevers, respectively); (2)
Filoviridae (Ebola and Marburg); (3) Flaviviridae (yellow fever, Omsk
hemorrhagic fever, and Kyasanur
Forest disease viruses); (4) Bunyaviridae (Rift Valley fever (RFV), Congo-
Crimean hemorrhagic fever.
Another target viral family includes Hantaviruses. Another antigen for
targeting includes neuropathologic
viruses, e.g., St. Louis encephalitis, Western equine encephalitis, Eastern
equine encephalitis, California
encephalitis serogroup (e.g., Lacrosse, Jamestown Canyon, Snowshoe Hare,
Trivittatus, Keystone, and
California encephalitis viruses), Powassan encephalitis, Venezuelan equine
virus, Argentine equine
encephalitis virus, Cache Valley virus and West Nile virus. Neuropathologic
viruses fall into various viral
families and are characterized by symptoms that include: fever of variable
severity associated with
neurologic symptoms ranging from headache to aseptic meningitis or
encephalitis, headache, confusion or
other alteration of the senses, nausea and vomiting. Signs may include fever,
meningismus, cranial nerve
palsies, paresis or paralysis, sensory deficits, altered reflexes,
convulsions, abnormal movements and
coma of varying degree.
The thioaptamers of the present invention may be an adjuvant that forms part
of a vaccine, such as a
composition that includes one or more partially thio-modified or even
concatenated aptamers that
modulate an immune response. When used as part of a vaccine, the thioaptamer
adjuvant may also
include at least one antigen. In addition to the examples of antigens listed
hereinabove, the antigens for
innate immune response activation may be a pathogen-associated molecular
pattern antigen, e.g., a CpG
molecule, a saccharide, a lectin, a polysaccharide and the like. As with the
thioaptamers described
hereinabove the adjuvant thioaptamer may include sequences for specific
recognition and binding to
nuclear regulatory factors, e.g., NF-AT, NF-KB, RBP-Jx, AP-1, NF IL-6, SP-1,
GRE and SRE. Examples
of partially thioaptamers include one or more of the aptamers of SEQ ID NOS.:
2, 3, 4, 5, 6, 7, 8 and 9.
The thioaptamer may an adjuvant that includes one or more partially
thioaptamers that bind to, e.g., a
DNA binding protein and modulate an immune response, e.g., an innate or an
adaptive immune response.
The adjuvant may be provided with a physiologically acceptable aqueous
vehicle, in a lyophilized, a
particulate or even a dissolved form with or without an antigen, e.g., the
antigen described hereinabove.
The thioaptamer may be specific for one or more downstream nuclear regulatory
factors that transduce a
9
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
intracellular signal from a Toll-Like receptor, e.g., a Toll-Like receptor 2,
a Toll-Like receptor 4 or a
pathogen-associated molecular pattern receptor. The adjuvant may be a
partially thioaptamer selected
from SEQ ID NOS.: 2, 3, 4, 5, 6, 7, 8, 9, 56 and/or 58. Another embodiment of
the present invention is a
T cell adjuvant that includes, e.g., a peptide antigen and an aptamer wherein
at least a portion of at least
S one nucleotide in the thioaptamer is thiophosphate-modified.
The present invention also includes a method of treating a hemorraghic viral
infection that includes the
steps of identifying a patient suspected of being infected with a hemorraghic
virus and providing the
patient with a therapeutic amount of a thioaptamer specific for a
transcription factor involved in viral
propagation or the immune cell response related to the virus. The
transcription factor may be, e.g., NF-
KB, RBP-Jx, AP-1, NF IL-6, SP-1, GRE, SRE, mixtures thereof and the like. The
thioaptamer will
generally bind specifically to a protein, e.g., a transcription factor and may
also include one or more of the
aptamers of SEQ ID NOS.: 2, 3, 4, 5, 6, 7, 8 and 9, e.g.,
XBY-6: 5'-CCAGGAGATsZTsiCCAC-3' SEQ ID NO. :
1
3'-GGsZTCCsiTCsiTAAGGsZTG-S'
XBY-S2: S'-CCAGTsZGACTsZCAGTszG-3'SEQ ID NO. :
2
3'-GGsiTCACszTGAGsZTCAC-S'
XBY-S1: 5'-TsZTszGCGCGCAACATsZG-3'SEQ ID NO. :
3
3'-AACGCGCGsZTsZTGszTAC-5'
XBY-C2: 5'-CCAGTGACTCAGTG-3' SEQ ID NO. :
4
3'-GGTCACTGAGTCAC-5'
XBY-C 1: 5'-TTGCGCGCAACATG-3' SEQ ID NO. :
5
3'-AACGCGCGTTGTAC-S'
S'-tGTGcAGGGACTgAtGaCGGt-3', SEQ ID NO.: 6
5'-CtGTGCatCGAaGTTtGCAtTt-3', SEQ ID NO.: 7
5'-AtGcAcAtCtCaGgAtGaCGGt-3', SEQ ID NO.: 8
5'-AGTTGcAGGtCaGgACCCAtTt- 3', SEQ ID NO.: 9
wherein the lowercase letters represent the thiophosphate 3' to the base. In
one examples, the method of
treatment may be directed to a neuropathologic viral infection and include the
steps of identifying a
patient suspected of being infected with a neuropathologic virus; and
providing the patient with a
therapeutic amount of a partially thioaptamer specific for transcription
factor involved in immune cell
activation. A thioaptamer for use in the method of treatment may be XBY-S2.
Yet another embodiment of the present invention is a method for modifying an
immune response that
includes administering a composition that includes an antigen and one or more
partially thio-modified
aptamers or thioaptamers. The modifications to the immune response include,
e.g., activation or
deactivation of the innate immune response and/or modifications to the type of
immune response mounted
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
(humoral versus cell-based) such as a change in the profile of helper T cell
involved with or "lead" the
immune response. The composition may also include cytokines, e.g., interleukin-
1 (IL-1), interleukin-2
(IL-2), interleukin-3 (IL-3), interleukin-4 (IL-4), interleukin-5 (IL-5),
interleukin-6 (IL-6), interleukin-7
(IL-7), interleukin-8 (IL-8), interleukin-10 (IL-10), interleukin-11 (IL-11),
interleukin-12 (IL-12),
interleukin-13 (IL-13), Type I Interferon, Type II Interferon, tumor necrosis
factor alpha (TNF-alpha),
transforming growth factor-beta (TGF-beta), lymphotoxin migration inhibition
factor, granulocyte-
macrophage colony-stimulating factor (GM-CSF), monocyte-macrophage CSF,
granulocyte CSF, vascular
epithelial growth factor (VEGF), angiogenin, transforming growth factor (TGF-
alpha) , fibroblast growth
factor, angiostatin, endostatin, mixtures or combinations thereof. The
composition may also include one
or more antigens, e.g., lipid A, phospholipase A2, endotoxins, staphylococcal
enterotoxin B, heat shock
proteins (HSPs), carbohydrates, Rh factors, DNA, nucleotides, RNA, mRNA, MART,
MAGE, BAGE,
GAGE, DAGE, mutant p53, tyrosinase, or a combination thereof. The aptamer may
stimulate specialized
antigen presenting cells (APCs), e.g., macrophages, dendritic cells and B
cells or non-specialized immune
or even non-immune cells. The aptamer may activate an innate immune response,
e.g., through Toll-Like
receptors that stimulate lymphocytes such as APCs, B cells and T cells. In one
example, the aptamer
activates an innate immune response that includes the simultaneous activation
of macrophages and
dendritic cells and of B cells and T cells. The aptamer may stimulate or
suppress the immune response.
In one specific embodiment, the present invention includes a method for
enhancing vaccine efficacy by
administering a composition that includes a partially thioaptamer specific for
a DNA binding protein and
an antigen to a subject animal. The aptamer may also include a carrier
molecule, e.g., liposomes,
microcapsules, microspheres, mixtures or combinations thereof. The target
immune response may be, e.g.,
to a cancer or a pathogenic infection. Alternatively, the target immune
response may be an anaphylactic
shock, allergic rhinitis, eczema, urticaria, anaphylaxis, transplant
rejection, systemic lupus erthymatosus,
rheumatoid arthritis, seronegative spondyloarthritides, Sjogren's syndrome,
systemic sclerosis,
polymyositis, dermatomyositis, Type I Diabetes Mellitus, Acquired Immune
Deficiency Syndrome,
Hashimoto's thyroiditis, Graves' disease, Addison's disease, polyendocrine
autoimmune disease, hepatitis,
sclerosing cholangitis, primary biliary cirrhosis, pernicious anemia, coeliac
disease, antibody-mediated
nephritis, glomerulonephritis, Wegener's granulomatosis, microscopic
polyarteritis, polyarteritis nodosa,
pemphigus, dermatitis herpetiformis, psoriasis, vitiligo, multiple sclerosis,
encephalomyelitis, Guillain-
Barre syndrome, Myasthenia Gravis, Lambent-Eaton syndrome, sclera, episclera,
uveitis, chronic
mucocutaneous candidiasis, Bruton's syndrome, transient hypogammaglobulinemia
of infancy, myeloma,
X-linked hyper IgM syndrome, Wiskott-Aldrich syndrome, ataxia telangiectasia,
autoimmune hemolytic
anemia, autoimmune thrombocytopenia, autoimmune neutropenia, Waldenstrom's
macroglobulinemia,
amyloidosis, chronic lymphocytic leukemia, or non-Hodgkin's lymphoma. The
partially thioaptamer may
be specific for a DNA binding protein, a cellular protein, a cell surface
protein, a saccharide or lipid or
11
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
combinations thereof. When provided in vaccine form, the thioaptamer
(thioaptamer) and an antigen may
be provided in dry form or even be disposed in a vehicle suitable for oral,
intramuscular, subcutaneous,
intravenous or parenteral administration, e.g., in a sterile saline solution.
The partially thioaptamer may
be specific for AP-1, NF-xB, NF IL-6, or combinations thereof.
BRIEF DESCRIPTION OF THE DRAWINGS
For a more complete understanding of the features and advantages of the
present invention, reference is
now made to the detailed description of the invention along with the
accompanying figures and in which:
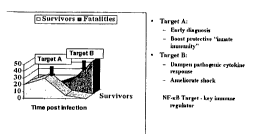
Figure 1 is a schematic representation for immune responses post infection, in
the left panel, Target A
represents immune response clearing virus with patient survival, in the right
panel Target B represents
cytopathogenic immune response resulting in shock;
Figure 2 is a graph that shows the production of TNF-a in P388D1 cells. Cells
were treated with polyI/C
(25 pg/ml) and media samples were taken at indicated times, the TNF- a levels
in the media were
determined using commercially available ELISA;
Figures 3A, 3B and 3C are bar graphs that show the production of by P388 cells
infected with P2 or P18
taken three days post-infection and assayed for TNF-a (3A), IL-6 (3B) and IL-
12 (3C);
Figure 4 is a gel that shows that the XBY-S2 aptamer binds specifically to
proteins in 70Z/3 cell nuclear
extracts and recombinant human AP-1;
Figure 5 is a gel that shows a supershift analysis using a variety of
antibodies specific for various members
of the AP-1 transcription factor family;
Figure 6 is a gel with a comparison of XBY-6 and IgK oligonucleotide binding
to proteins in 70ZJ3 cell
nuclear extracts in which multiple NF-xB dimers are shown to bind the IgK
oligonucleotide, with specific
binding of only p50 (or p105) containing dimers to XBY-6;
Figure 7 is a gel that shows that XBY-S2 eliminates AP1 DNA binding activities
in macrophages treated
with liposomes with and without the indicated aptamers for 24 hours, wherein
the nuclear extracts were
analyzed by electrophoretic mobility shift assay (EMSA) with the AP-1 and NF-
xB oligonucleotide
probes;
Figure 8 is a graph that shows the secretion of TNFa as measured by ELISA of
Mouse P388D1
macrophage cultures were treated with XBY-S2 for 12 hours followed by
stimulation with PolyI/C and
harvested at 24 hrs;
12
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
Figure 9 is a graph of IL-6 production assayed by ELISA of mouse P388D1
macrophage cultures treated
with XBY-S2 for 12 hours followed by stimulation with PolyI/C and harvested at
24 hrs;
Figure 10 is a graph that shows survival curves following Pichinde P18
infection. in guinea pigs treated
with the NF-rcB aptamer, XBY-6, the scrambled control, B92, or vehicle, MT, of
animals infected by
injection of 1000 pfu of Pichinde P18 at day 0, treatment consisted of
intraperitoneal injections at days 0,
1 and 2;
Figure 11 is a graph that shows survival curves of guinea pigs with
thioaptamers for infection by
arenavirus;
Figure 12 is a graph that shows survival curves following West Nile Virus
infection in guinea pigs treated
with the NF-xB aptamer XBY-6, the AP-1 aptamer XBY-S2, or the liposome vehicle
of animals infected
by injection with lethal doses of West Nile Virus;
Figure 13 are graphs that show SELDI detection of recombinant p50 using Epoxy-
activated ProteinChip
Arrays with XBY-6 (top), IgxB 22-mer duplex (middle) or control, poly (dLdC)
(bottom) covalently
linked to surfaces;
Figure 14 are graphs that show the detection of recombinant p50 on gel beads
using XBY-6. Top two
SELDI MS extract from beads spotted onto NP20 ProteinChip. Bottom two SELDI
spectra taken on beads
themselves, in which the control is no XBY-6 covalently attached to beads with
aminolinker; and
Figure 15 is a graph that shows the SELDI MS capture of endogenous p50 (p105)
from nuclear extracts on
Ciphergen PS20 Proteinchip Arrays, the topgraph shows covalently linked XBY-6
to array surface, in the
bottom, control no XBY-6 linked to surface.
DETAILED DESCRIPTION OF THE INVENTION
While the making and using of various embodiments of the present invention are
discussed in detail
below, it should be appreciated that the present invention provides many
applicable inventive concepts
that can be embodied in a wide variety of specific contexts. The specific
embodiments discussed herein
are merely illustrative of specific ways to make and use the invention and do
not delimit the scope of the
invention.
To facilitate the understanding of this invention, a number of terms are
defined below. Terms defined
herein have meanings as commonly understood by a person of ordinary skill in
the areas relevant to the
present invention. Terms such as "a", "an" and "the" are not intended to refer
to only a singular entity, but
include the general class of which a specific example may be used for
illustration. The terminology herein
13
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
is used to describe specific embodiments of the invention, but their usage
does not delimit the invention,
except as outlined in the claims.
As used herein, "synthesizing" of a random combinatorial library refers to
chemical methods known in the
art of generating a desired sequence of nucleotides including where the
desired sequence is random.
Typically in the art, such sequences are produced in automated DNA
synthesizers programmed to the
desired sequence. Such programming can include combinations of defined
sequences and random
nucleotides.
"Random combinatorial oligonucleotide library" means a large number of
oligonucleotides of different
sequence where the insertion of a given base at given place in the sequence is
random. "PCR primer
nucleotide sequence" refers to a defined sequence of nucleotides forming an
oligonucleotide which is used
to anneal to a homologous or closely related sequence in order form the double
strand required to initiate
elongation using a polymerase enzyme. "Amplifying" means duplicating a
sequence one or more times.
Relative to a library, amplifying refers to en masse duplication of at least a
majority of individual
members of the library.
As used herein, "thiophosphate" or "phosphorothioate" are used interchangeably
to refer analogues of
DNA or RNA having sulphur in place of one or more of the non bridging oxygens
bound to the
phosphorus. Monothiophosphates or phosphoromonothioates [aS] have only one
sulfur and are thus chiral
around the phosphorus center. Dithiophosphates are substituted at both oxygens
and are thus achiral.
Phosphoromonothioate nucleotides are commercially available or can be
synthesized by several different
methods known in the art. Chemistry for synthesis of the phosphorodithioates
has been developed by one
of the present inventors as set forth in U. S. Patent #5,218,088 (issued to
Gorenstein, D.G. and
Farschtschi, N., June 8, 1993 for a Process for Preparing Dithiophosphate
Oligonucleotide Analogs via
Nucleoside Thiophosphoramidite Intermediates), relevant portions incorporated
herein by reference.
As used herein, the terms "thio-modified aptamer" and "thioaptamer" are used
interchangeably to describe
oligonucleotides (ODNs) (or libraries of thioaptamers) in which one or more of
the four constituent
nucleotide bases of an oligonucleotide are analogues or esters of nucleotides
that normally form the DNA
or RNA backbones and wherein such modification confers increased nuclease
resistance. For example,
the modified nucleotide aptamer can include one or more phosphorothioate or
phosphordithioate linkages
selected from dATP(aS), dTTP(aS), dCTP(aS) and dGTP(aS), dATP(aS2), dTTP(aS2),
dCTP(aSz) and
dGTP(aS2). In another example, no more than three adjacent phosphate sites of
the modified nucleotide
aptamer are replaced with phosphorothioate groups. In yet another example, at
least a portion of non
adjacent dA, dC, dG, or dT phosphate sites of the modified nucleotide aptamer
are replaced with
phosphorothioate groups. In another example of a thioaptamer, all of the non-
adjacent dA, dC, dG, or dT
phosphate sites of the modified nucleotide aptamer are replaced with
phosphorothioate groups; all of the
14
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
non-adjacent dA, dC, dG, and dT phosphate sites of the modified nucleotide
aptamer are replaced with
phosphorothioate groups; or substantially all non-adjacent phosphate sites of
the modified nucleotide
aptamer are replaced with phosphorothioate groups. In still another embodiment
of the present invention,
no more than three adjacent phosphate sites of the modified nucleotide aptamer
are replaced with
phosphorodithioate groups. The thioaptamers may be obtained by adding bases
enzymatically using a mix
of four nucleotides, wherein one or more of the nucleotides is a mix of
unmodified and thiophosphate-
modified nucleotides, to form a partially thiophosphate-modified thioaptamer
library. In another example
of "thioaptamers" these are made by adding bases to an oligonucleotide wherein
a portion of the
phosphate groups are thiophosphate-modified nucleotides, and where no more
than three of the four
different nucleotides are substituted on the 5'-phosphate positions by 5'-
thiophosphates in each synthesized
oligonucleotide are thiophosphate-modified nucleotides.
Thiophosphate nucleotides are an example of modified nucleotides.
"Phosphodiester oligonucleotide"
means a chemically normal (unmodified) RNA or DNA oligonucleotide. Amplifying
"enzymatically"
refers to duplication of the oligonucleotide using a nucleotide polymerise
enzyme such as DNA or RNA
polymerise. Where amplification employs repetitive cycles of duplication such
as using the "polymerise
chain reaction", the polymerise may be, e.g., a heat stable polymerise, e.g.,
of Thermus aquaticus or other
such polymerises, whether heat stable or not.
"Contacting" in the context of target selection means incubating a
oligonucleotide library with target
molecules. "Target molecule" means any molecule to which specific aptamer
selection is desired.
"Essentially homologous" means containing at least either the identified
sequence or the identified
sequence with one nucleotide substitution. "Isolating" in the context of
target selection means separation
of oligonucleotide/target complexes, preferably DNA/protein complexes, under
conditions in which weak
binding oligonucleotides are eliminated.
By "split synthesis" it is meant that each unique member of the combinatorial
library is attached to a
separate support bead on a two (or more) column DNA synthesizer, a different
thiophosphoramidite or
phosphoramidite is first added onto both identical supports (at the
appropriate sequence position) on each
column. After the normal cycle of oxidation (or sulfurization) and blocking
(which introduces the
phosphate, monothiophosphate or dithiophosphate linkage at this position), the
support beads are removed
from the columns, mixed together and the mixture reintroduced into both
columns. Synthesis may
proceed with further iterations of mixing or with distinct nucleotide
addition.
Aptamers may be defined as nucleic acid molecules that have been selected from
random or unmodified
oligonucleotides ("ODN") libraries by their ability to bind to specific
targets or "ligands." An iterative
process of in vitro selection may be used to enrich the library for species
with high affinity to the target.
The iterative process involves repetitive cycles of incubation of the library
with a desired target,
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
separation of free oligonucleotides from those bound to the target and
amplification of the bound ODN
subset using the polymerase chain reaction ("PCR"). The penultimate result is
a sub-population of
sequences having high affinity for the target. The sub-population may then be
subcloned to sample and
preserve the selected DNA sequences. These "lead compounds" are studied in
further detail to elucidate
the mechanism of interaction with the target.
Dosage forms. A dosage unit for use of the aptamers and partially thioaptamers
of the present invention,
may be a single compound or mixtures thereof with other compounds, e.g., a
potentiator. The compounds
may be mixed together, form ionic or even covalent bonds. The aptamers and
partially thioaptamers of the
present invention may be administered in oral, intravenous (bolus or
infusion), intraperitoneal,
subcutaneous, or intramuscular form, all using dosage forms well known to
those of ordinary skill in the
pharmaceutical arts. Depending on the particular location or method of
delivery, different dosage forms,
e.g., tablets, capsules, pills, powders, granules, elixirs, tinctures,
suspensions, syrups, and emulsions may
be used to provide the aptamers and partially thioaptamers of the present
invention to a patient in need of
therapy that includes the aptamers and partially thioaptamers. The aptamers
and partially thioaptamers
may also be administered as any one of known salt forms.
Aptamers and partially thioaptamers is typically administered in admixture
with suitable pharmaceutical
salts, buffers, diluents, extenders, excipients and/or carriers (collectively
referred to herein as a
pharmaceutically acceptable carrier or carrier materials) selected based on
the intended form of
administration and as consistent with conventional pharmaceutical practices.
Depending on the best
location for administration, the aptamers and partially thioaptamers may be
formulated to provide, e.g.,
maximum and/or consistent dosing for the particular form for oral, rectal,
topical, intravenous injection or
parenteral administration. While the aptamers and partially thioaptamers may
be administered alone, it
will generally be provided in a stable salt form mixed with a pharmaceutically
acceptable carrier. The
carrier may be solid or liquid, depending on the type and/or location of
administration selected.
Techniques and compositions for making useful dosage forms using the present
invention are described in
one or more of the following references: Ansel, Introduction to Pharmaceutical
Dosage Forms 2nd Edition
(1976); Remington's Pharmaceutical Sciences, 17th ed. (Mack Publishing
Company, Easton, Pa., 1985);
Advances in Pharmaceutical Sciences (David Ganderton, Trevor Jones, Eds.,
1992); Advances in
Pharmaceutical Sciences Vol 7. (David Ganderton, Trevor Jones, James McGinity,
Eds., 1995); Aqueous
Polymeric Coatings for Pharmaceutical Dosage Forms (Drugs and the
Pharmaceutical Sciences, Series 36
(James McGinity, Ed., 1989); Pharmaceutical Particulate Carriers: Therapeutic
Applications: Drugs and
the Pharmaceutical Sciences, Vol 61 (Alain Rolland, Ed., 1993); Drug Delivery
to the Gastrointestinal
Tract (Elks Horwood Books in the Biological Sciences. Series in Pharmaceutical
Technology; J. G.
Hardy, S. S. Davis, Clive G. Wilson, Eds.); Modern Pharmaceutics Drugs and the
Pharmaceutical
16
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
Sciences, Vol 40 (Gilbert S. Banker, Christopher T. Rhodes, Eds.), and the
like, relevant portions
incorporated herein by reference.
For example, the aptamers and partially thioaptamers may be included in a
tablet. Tablets may contain,
e.g., suitable binders, lubricants, disintegrating agents, coloring agents,
flavoring agents, flow-inducing
agents and/or melting agents. For example, oral administration may be in a
dosage unit form of a tablet,
gelcap, caplet or capsule, the active drug component being combined with an
non-toxic, pharmaceutically
acceptable, inert carrier such as lactose, gelatin, agar, starch, sucrose,
glucose, methyl cellulose,
magnesium stearate, dicalcium phosphate, calcium sulfate, mannitol,
sorbitol,mixtures thereof, and the
like. Suitable binders for use with the present invention include: starch,
gelatin, natural sugars (e.g.,
glucose or beta-lactose), corn sweeteners, natural and synthetic gums (e.g.,
acacia, tragacanth or sodium
alginate), carboxymethylcellulose, polyethylene glycol, waxes, and the like.
Lubricants for use with the
invention may include: sodium oleate, sodium stearate, magnesium stearate,
sodium benzoate, sodium
acetate, sodium chloride, mixtures thereof, and the like. Disintegrators may
include: starch, methyl
cellulose, agar, bentonite, xanthan gum, mixtures thereof, and the like.
1 S The aptamers and partially thioaptamers may be administered in the form of
liposome delivery systems,
e.g., small unilamellar vesicles, large unilamallar vesicles, and
multilamellar vesicles, whether charged or
uncharged. Liposomes may include one or more: phospholipids (e.g.,
cholesterol), stearylamine and/or
phosphatidylcholines, mixtures thereof, and the like.
The aptamers and partially thioaptamers may also be coupled to one or more
soluble, biodegradable,
bioacceptable polymers as drug carriers or as a prodrug. Such polymers may
include:
polyvinylpyrrolidone, pyran copolymer, polyhydroxylpropylmethacrylamide-
phenol,
polyhydroxyethylasparta-midephenol, or polyethyleneoxide-polylysine
substituted with palmitoyl
residues, mixtures thereof, and the like. Furthermore, the aptamers and
partially thioaptamers may be
coupled one or more biodegradable polymers to achieve controlled release of
the aptamers and partially
thioaptamers, biodegradable polymers for use with the present invention
include: polylactic acid,
polyglycolic acid, copolymers of polylactic and polyglycolic acid, polyepsilon
caprolactone, polyhydroxy
butyric acid, polyorthoesters, polyacetals, polydihydropyrans,
polycyanoacylates, and crosslinked or
amphipathic block copolymers of hydrogels, mixtures thereof, and the like.
In one embodiment, gelatin capsules (gelcaps) may include the aptamers and
partially thioaptamers and
powdered carriers, such as lactose, starch, cellulose derivatives, magnesium
stearate, stearic acid, and the
like. Like diluents may be used to make compressed tablets. Both tablets and
capsules may be
manufactured as immediate-release, mixed-release or sustained-release
formulations to provide for a range
of release of medication over a period of minutes to hours. Compressed tablets
may be sugar coated or
17
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
film coated to mask any unpleasant taste and protect the tablet from the
atmosphere. An enteric coating
may be used to provide selective disintegration in, e.g., the gastrointestinal
tract.
For oral administration in a liquid dosage form, the oral drug components may
be combined with any oral,
non-toxic, pharmaceutically acceptable inert carrier such as ethanol,
glycerol, water, and the like.
Examples of suitable liquid dosage forms include solutions or suspensions in
water, pharmaceutically
acceptable fats and oils, alcohols or other organic solvents, including
esters, emulsions, syrups or elixirs,
suspensions, solutions and/or suspensions reconstituted from non-effervescent
granules and effervescent
preparations reconstituted from effervescent granules. Such liquid dosage
forms may contain, for
example, suitable solvents, preservatives, emulsifying agents, suspending
agents, diluents, sweeteners,
thickeners, and melting agents, mixtures thereof, and the like.
Liquid dosage forms for oral administration may also include coloring and
flavoring agents that increase
patient acceptance and therefore compliance with a dosing regimen. In general,
water, a suitable oil,
saline, aqueous dextrose (e.g., glucose, lactose and related sugar solutions)
and glycols (e.g., propylene
glycol or polyethylene glycols) may be used as suitable carriers for
parenteral solutions or even for
delivery via a suppository. Solutions for parenteral administration include
generally, a water soluble salt
of the active ingredient, suitable stabilizing agents, and if necessary,
buffering salts. Antioxidizing agents
such as sodium bisulfate, sodium sulfite and/or ascorbic acid, either alone or
in combination, are suitable
stabilizing agents. Citric acid and its salts and sodium EDTA may also be
included to increase stability.
In addition, parenteral solutions may include pharmaceutically acceptable
preservatives, e.g.,
benzalkonium chloride, methyl- or propyl-paraben, and/or chlorobutanol.
Suitable pharmaceutical carriers
are described in Remington's Pharmaceutical Sciences, Mack Publishing Company,
a standard reference
text in this field, relevant portions incorporated herein by reference.
Intranasal and Nasal. For direct delivery to the nasal passages, sinuses,
mouth, throat, esophagous, tachea,
lungs and alveoli, the aptamers and partially thioaptamers may also be
delivered as an intranasal form via
use of a suitable intranasal vehicle. For dermal and transdermal delivery, the
aptamers and partially
thioaptamers may be delivered using lotions, creams, oils, elixirs, serums,
transdermal skin patches and
the like, as are well known to those of ordinary skill in that art. Parenteral
and intravenous forms may also
include pharmaceutically acceptable salts and/or minerals and other materials
to make them compatible
with the type of injection or delivery system chosen, e.g., a buffered,
isotonic solution. Examples of
useful pharmaceutical dosage forms for administration of aptamers and
partially thioaptamers may include
the following forms.
Capsules. Capsules may be prepared by filling standard two-piece hard gelatin
capsules each with 10 to
500 milligrams of powdered active ingredient, 5 to 150 milligrams of lactose,
5 to 50 milligrams of
cellulose and 6 milligrams magnesium stearate.
18
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
Soft Gelatin Capsules. A mixture of active ingredient is dissolved in a
digestible oil such as soybean oil,
cottonseed oil or olive oil. The active ingredient is prepared and injected by
using a positive displacement
pump into gelatin to form soft gelatin capsules containing, e.g., 100-500
milligrams of the active
ingredient. The capsules are washed and dried.
S Tablets. A large number of tablets are prepared by conventional procedures
so that the dosage unit was
100-500 milligrams of active ingredient, 0.2 milligrams of colloidal silicon
dioxide, 5 milligrams of
magnesium stearate, 50-275 milligrams of microcrystalline cellulose, 11
milligrams of starch and 98.8
milligrams of lactose. Appropriate coatings may be applied to increase
palatability or delay absorption.
Effervescent tablets. To provide an effervescent tablet appropriate amounts
of, e.g., monosodium citrate
and sodium bicarbonate, are blended together and then roller compacted, in the
absence of water, to form
flakes that are then crushed to give granulates. The granulates are then
combined with the active
ingredient, drug and/or salt thereof, conventional beading or filling agents
and, optionally, sweeteners,
flavors and lubricants.
Injectable solution. A parenteral composition suitable for administration by
injection is prepared by
stirring 1.5% by weight of active ingredient in deionized water and mixed
with, e.g., up to 10% by
volume propylene glycol and water. The solution is made isotonic with sodium
chloride and sterilized
using, e.g., ultrafiltration. Parenteral and intravenous forms may also
include minerals and other materials
to make them compatible with the type of injection or delivery system chosen.
Suspension. An aqueous suspension is prepared for oral administration so that
each 5 ml contain 100 mg
of finely divided active ingredient, 200 mg of sodium carboxymethyl cellulose,
S mg of sodium benzoate,
1.0 g of sorbitol solution, U.S.P., and 0.025 ml of vanillin.
Mini-tabs. For mini-tablets, the active ingredient is compressed into a
hardness in the range 6 to 12 Kp.
The hardness of the final tablets is influenced by the linear roller
compaction strength used in preparing
the granulates, which are influenced by the particle size of, e.g., the
monosodium hydrogen carbonate and
sodium hydrogen carbonate. For smaller particle sizes, a linear roller
compaction strength of about 15 to
20 KN/cm may be used.
Kits. The present invention also includes pharmaceutical kits useful, for
example, for the treatment of
pathogenic infection or even a cancer. The kit will generally include one or
more containers containing a
pharmaceutical composition with a therapeutically effective amount of the
aptamers and/or partially
thioaptamers disclosed herein. Such kits may further include, one or more of
various conventional
pharmaceutical kit components, e.g., containers with one or more
pharmaceutically acceptable diluents, as
will be readily apparent to those skilled in the art. Printed instructions,
either as inserts or as labels,
19
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
indicating quantities of the components to be administered, guidelines for
mixture and/or administration,
may also be included in the kit.
The aptamers and partially thioaptamers and, optionally, one or more
potentiators may be mixed with a
pharmaceutically acceptable carrier. The carrier may be a solid or liquid and
the type is generally chosen
based on the type of administration being used. The active agent may be
coadministered in the form of a
tablet, capsule, liposome, as an agglomerated powder, in a liquid form or as a
suppository.
Vaccines. The present invention includes vaccines for both active and passive
immunization.
Immunogenic compositions, suitable for use as a vaccine, include the modified
thioaptamers of the present
invention. The thioaptamers are prepared in a manner disclosed herein. The
vaccines disclosed herein are
not the antigenic material, that is, they are not intended to cause an immune
response, but rather, are
include either alone or in combination with an antigen to "drive" or modify an
immune response by
altering the activity of nuclear binding proteins, including, e.g.: NF-ATs, AP-
ls, NF-IL6, NF-rcB, HIV
reverse transcriptase, Venezuelan Equine Encephalitis nucleocapsid (using an
RNA thioaptamer), HepC
IRES nucleic acid, proteins) involved in CpG-induced "innate immunity," and
the like. As known to
those in the immunological arts, the type of immunity, e.g., innate and/or
adaptive, that is activated (or
deactivated) is a critical step in the immune response. As such, the
thioaptamers may be under some
circumstances acting as an adjuvant but in others will actually be a direct
participant in the immune
response alone, that is, without addition of an antigen. The thioaptamers may
even be used to prime the
immune system prior a challenge.
In operation, the thioaptamer will generally be extensively dialyzed to remove
undesired small molecular
weight molecules and/or lyophilized for more ready formulation into a desired
vehicle. The preparation of
vaccines that include normal antigens are generally well understood in the
art, as exemplified by United
States Letters Patents 4,608,251; 4,601,903; 4,599,231; 4,599,230; 4,596,792;
and 4.578,770, relevant
portions of these incorporated herein by reference. Typically, such vaccines
are prepared as injectables.
Either as liquid solutions or suspensions: solid forms suitable for solution
in, or suspension in, liquid
prior to injection may also be prepared. The preparation may also be
emulsified. The active immunogenic
ingredient is often mixed with excipients that are pharmaceutically acceptable
and compatible with the
active ingredient. Suitable excipients are, for example, water, saline,
dextrose, glycerol, ethanol, or the
like and combinations thereof. In addition, if desired, the vaccine may
contain minor amounts of auxiliary
substances such as wetting or emulsifying agents, pH buffering agents, or
adjuvants which enhance the
effectiveness of the vaccines.
In vaccine form the thioaptamer may be administered, e.g., parenterally, by
injection, for example, either
subcutaneously, intraperitoneally, intranasally or into the lungs or even
intramuscularly. Additional
formulations that are suitable for other modes of administration include
suppositories and, in some cases,
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
oral formulations. For suppositories, traditional binders and carriers may
include, for example,
polyalkalene glycols or triglycerides: such suppositories may be formed from
mixtures containing the
active ingredient in the range of 0.5% to 10%, or even 1-2%. Oral formulations
include such normally
employed excipients as, for example, pharmaceutical grades of mannitol,
lactose, starch, magnesium
stearate, sodium saccharine, cellulose, magnesium carbonate, mixtures thereof
and the like. These
compositions take the form of solutions, suspensions, tablets, pills,
capsules, sustained release
formulations or powders and contain 10-95% of active ingredient, preferably 25-
70%.
The thioaptamers may be administered directly to the aerodigestive system (the
pulmonary system and/or
digestive tract) of a patient by an inhaled aerosol. Delivery of drugs or
other active ingredients directly to
a patient's lungs provides numerous advantages including: providing an
extensive surface area for drug
absorption; direct delivery of therapeutic agents to the disease site in the
case of regional drug therapy;
reducing the possibility of drug degradation in the patient's intestinal tract
(a risk associated with oral
administration); and eliminating the need for repeated subcutaneous
injections. Furthermore, delivery of
the thioaptamers to the pulmonary system via aerosol inhalation may be used to
deliver drugs
systemically, as well as for targeted local drug delivery for treatment of
respiratory ailments such as
pathogenic infections (viral, bacterial and fungal) or even lung cancer or
asthma. Aerosol devices for use
with the present invention in the clinical context include metered dose
inhalers, dry powder inhalers,
nebulizers and the like.
The thioaptamers may be formulated into the vaccine as neutral or salt forms.
Pharmaceutically
acceptable salts include those that are formed with inorganic acid, e.g.,
sodium, potassium, ammonium,
calcium, or ferric hydroxides, and such organic bases as isopropylamine,
trimethylamine, 2-ethylamino
ethanol, histidine, procaine, mixtures thereof and the like. The vaccines are
administered in a manner
compatible with the dosage formulation, and in such amount as will be
therapeutically effective. The
quantity to be administered depends on the subject to be treated, including,
e.g., the capacity of the
individual's immune system to activate an innate immune response, synthesize
antibodies or mount an
effective cytotoxic T cell response, and the degree of protection desired.
Precise amounts of active
ingredient required to be administered depend on the judgment of the
practitioner, however, suitable
dosage ranges are of the order of a few to several hundred micrograms active
ingredient per vaccination.
Suitable regimes for initial administration and booster shots are also
variable, but are typified by an initial
administration followed by subsequent inoculations or other administrations.
The manner of application
may be varied widely. Any of the conventional methods for administration of a
vaccine are applicable.
These are believed to include oral application on a solid physiologically
acceptable base or in a
physiologically acceptable dispersion, parenterally, by injection or the like.
The dosage of the vaccine
will depend on the route of administration and will vary according to the size
of the host.
21
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
Various methods of achieving an additional or complementary adjuvant effect
for the thioaptamer may
include, e.g., aluminum hydroxide or phosphate (alum), commonly used as 0.05
to 0.1 percent solution in
phosphate buffered saline, admixture with synthetic polymers of sugars
(Carbopol) used as 0.25 percent
solution. When provided with a antigenic protein, the thioaptamer may be
aggregated with the antigen
and other components of the vaccine by heat treatment with temperatures
ranging between 70° to 101 °C
for 30 second to 2 minute periods. Examples of aggregation include
reactivating with pepsin treated (Fab)
antibodies to albumin, mixture with bacterial cells such as C. parvum or
endotoxins or lipopolysaccharide
components of gram-negative bacteria, emulsion in physiologically acceptable
oil vehicles such as
mannide mono-oleate (Aracel A) or emulsion with 20 percent solution of a
perfluorocarbon (Fluosol-DA)
used as a block substitute may also be employed.
In many instances, it will be desirable to have multiple administrations of
the vaccine, usually not
exceeding six vaccinations, more usually not exceeding four vaccinations and
one or more, usually at least
about three vaccinations. The vaccinations will normally be at from two to
twelve week intervals, more
usually from three to five week intervals. Periodic boosters at intervals of 1-
5 years, usually three years,
will be desirable to maintain protective levels of the antibodies. The course
of the immunization may be
followed by assays for antibodies for the supernatant antigens. The assays may
be performed by labeling
with conventional labels, such as radionuclides, enzymes, fluorescers, and the
like. These techniques are
well known and may be found in a wide variety of patents, such as U.S. Patent
Nos. 3,791,932; 4,174,384
and 3,949,064, as illustrative of these types of assays.
The thioaptamers may be used as part of a vaccine to regulate the development
of Thl or Th2 subsets in a
subject or patient. In addition to in vivo modulation, the thioaptamers nay be
used ex vivo to modify cells
in vitro that are then administered to the subject. More particularly, the
thioaptamers disclosed herein may
be used to modulate the activity of a transcription factor (e.g., AP-1, NF-KB
or NF-AT family members)
that regulate innate or adaptive immune responses. In one example the
thioaptamer modulates the
development of Thl or Th2 cells in the subject is modulated.
The thioaptamer vaccine may include more that one thioaptamer in order to
modulate the activity of
additional transcription factors that contribute to regulating the expression
of Thl- or Th2-associated
cytokines. In one embodiment, a stimulatory method includes a first
thioaptamer that modulated the
activity of an AP-1 protein and a second agent that modulates the activity of
an NF-AT protein. The
second agent may be a thioaptamer or even an antigen.
The thioaptamer and the methods disclosed herein may be used to manipulate
Thl:Th2 ratios in a variety
of clinical situations. For example, a thioaptamer may be provided that
inhibits Th2 activation, which
may be useful in allergic diseases, malignancies and infectious diseases.
Conversely, the thioaptamer may
22
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
be used to enhance Th2 activation for treatment of autoimmune diseases and/or
to improve organ
transplantation.
The present inventors recognized that it is not possible to simply replace
thiophosphates in a sequence that
was selected for binding with a normal phosphate ester backbone
oligonucleotide. Simple substitution
was not practicable because the thiophosphates can significantly decrease (or
increase) the specificity
and/or affinity of the selected ligand for the target. It was also recognized
that thiosubstitution leads to a
dramatic change in the structure of the aptamer and hence alters its overall
binding affinity. The
sequences that were thioselected according to the present methodology, using
as examples of DNA
binding proteins AP-1, NF-IL6 and NF-xB, were different from those obtained by
normal phosphate ester
combinatorial selection.
The present invention takes advantage of the "stickiness" of thio- and dithio-
phosphate ODN agents to
enhance the affinity and specificity to a target molecule. In a significant
improvement over existing
technology, the method of selection concurrently controls and optimizes the
total number of thiolated
phosphates to decrease non-specific binding to non-target proteins and to
enhance only the specific
favorable interactions with the target. The present invention permits control
over phosphates that are to be
thio-substituted in a specific DNA sequence, thereby permitting the selective
development of aptamers
that have the combined attributes of affinity, specificity and nuclease
resistance.
In one embodiment of the present invention, a method of post-selection aptamer
modification is provided
in which the therapeutic potential of the aptamer is improved by selective
substitution of modified
nucleotides into the aptamer oligonucleotide sequence. An isolated and
purified target binding aptamer is
identified and the nucleotide base sequence determined. Modified achiral
nucleotides are substituted for
one or more selected nucleotides in the sequence. In one embodiment, the
substitution is obtained by
chemical synthesis using dithiophosphate nucleotides. The resulting aptamers
have the same nucleotide
base sequence as the original aptamer but, by virtue of the inclusion of
modified nucleotides into selected
locations in the sequences, improved nuclease resistance and affinity is
obtained.
RNA and DNA oligonucleotides (ODNs) can act as "aptamers," (i.e., as direct in
vivo inhibitors selected
from combinatorial libraries) for a number of proteins, including viral
proteins such as HIV RT (Burke et
al., 1996; Chen & Gold, 1994; Green et al., 1995; Schneider et al., 1995) and
transcription factors such as
human NF-xB (Bielinska et al., 1990; Lebruska & Maher, 1999; Lin et al., 1998;
Morishita et al., 1997;
Sharma et al., 1996). Decoy ODNs were developed to inhibit expression from CRE
and AP-1 directed
transcription in vivo and inhibit growth of cancer cells in vitro and in vivo
(Park et al., 1999). These
studies and others (Boccaccio et al., 1998; Cho-Chung, 1998; Eleouet et al.,
1998; Jin & Howe, 1997;
Mann, 1998; Morishita et al., 1995; Morishita et al., 1998; Osborne et al.,
1997; Tomita et al., 1997) have
demonstrated the potential of using specific decoy and aptamer ODNs to bind to
various proteins, serve as
23
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
therapeutic or diagnostic reagents, and to dissect the specific role of
particular transcription factors in
regulating the expression of various genes. In contrast to antisense agents,
duplex aptamers appear to
exhibit few if any non-specific effects.
Among a large variety of modifications, S-ODN and Sz-ODN render the agents
more nuclease resistant.
The first antisense therapeutic drug uses a modified S-ODN (CIBA Vision, A
Novartis Company). The
SZ-ODNs also show significant promise, however, the effect of substitution of
more nuclease-resistant
thiophosphates cannot be predicted, since the sulfur substitution can lead to
significantly decreased (or
increased) binding to a specific protein (Milligan, J.F. and Uhlenbeck, O.C.
(1989) and King et al., 2002
as well as structural perturbations (yolk et al., 2002) and thus it is not
possible to predict the effect of
backbone substitution on a combinatorially selected aptamer. Hence, the
present inventors recognized that
selection should be carried out simultaneously for both phosphate ester
backbone substitution and base
sequence.
Phosphorodithioate analogs have been synthesized to produce an important class
of sulfur-containing
oligonucleotides, the dithiophosphate SZ-ODNs. These dithioates include an
internucleotide
phosphodiester group with sulfur substituted for both nonlinking phosphoryl
oxygens, so they are both
isosteric and isopolar with the normal phosphodiester link, and are also
highly nuclease resistant. One
group showed highly effective protection of the dithioate against degradation
by endogenous nucleases
after 58% backbone modification. Significantly, the Sz-ODNs, in contrast to
the phosphoramidite-
synthesized monothiophosphate (S-ODNs), are achiral about the dithiophosphate
center, so problems
associated with diastereomeric mixtures (Lebedev & Wickstrom, 1996) are
completely avoided. The SZ-
ODNs and the S-ODNs, are taken up efficiently by cells, especially if
encapsulated in liposomes.
Thiophosphate aptamers or thioaptamers are capable of specifically and non-
specifically binding to
proteins. Importantly, it has been observed by the present inventors that
sulfurization of the phosphoryl
oxygens of oligonucleotides often leads to their enhanced binding to numerous
proteins (Gorenstein,
1994). The dithioate agents, for instance, appear to inhibit viral polymerases
at much lower
concentrations than do the monothiophosphates, which in turn are better than
the normal phosphates, with
Kd's for single strand aptamers in the nM to sub-nM range for HIV-1 RT
(Marshall & Caruthers, 1993)
and NF-xB (Yang et al., 2002, King et al. 2002). For HIV-1 RT, dithioates bind
28-600 times more tightly
than the normal aptamer oligonucleotide or the S-analogue. Sequence is also
important, as demonstrated
by the observation that a 14-nt dithioate based on the 3' terminal end of
human tRNAc'~
(CTGTTCGGGCGCCA)(SEQ ID NO.: 10) complementary to the HIV primer binding site
is a more
effective inhibitor (IDS° = 4.3 nM) than simply dithioate dC,4 (IDso =
62 nM) by an order of magnitude
(Marshall & Caruthers, 1993).
24
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
Oligonucleotides with high monothio- or dithiophosphate backbone substitutions
appear to be "stickier"
towards proteins than normal phosphate esters, an effect often attributed to
"non-specific interactions."
One explanation for the higher affinity of the thiosubstituted DNAs is the
poor cation coordination of the
polyanionic backbone (Cho et al., 1993, Volk et al., 2002) sulfur, being a
soft anion, does not coordinate
as well to hard canons like Na+, unlike the hard phosphate oxyanion. The
thiosubstituted phosphate esters
then act as "bare" anions, and since energy is not required to strip the
cations from the backbone, these
agents appear to bind even more tightly to proteins.
Even in specific protein-nucleic acid contacts, sulfurization of the
internucleotide linkages can lead to
enhanced binding (Marshall & Caruthers, 1993; Milligan & Uhlenbeck, 1989) (or
to decreased affinity).
The enhanced binding is very important, since most of the direct contacts
between DNA-binding proteins
and their binding sites are to the phosphate groups (Otwinowski et al., 1988)
(Chen et al., 1998;; Ghosh et
al., 1995; Muller et al., 1995). The present invention takes advantage of this
chemical "stickiness" to
enhance the specificity and affinity of thio- and dithiophosphate agents for a
protein target. It was
necessary, however, to optimize the total number of thioated phosphates to
decrease non-specific binding
to non-target proteins and thus enhance only the specific favorable
interactions with the target protein.
Also, thiosubstitution can also perturb the structure of the duplex (Cho et
al., 1993) (yolk et al, 2002)
although monothiophosphates substituted in the DNA strand of DNA/RNA hybrids
do not appear to have
dramatically altered duplex structures (Bachelin et al., 1998; Gonzalez et
al., 1995). The present invention
uses sequence-based, structure-based and combinatorial methods to identify
both sequences and
thiophosphate substitution patterns to develop thioaptamers that retained the
highest specificity and
affinity in binding to target proteins. The use of partial thiophosphate
substitution resulted in aptamer that
were more stable in vivo.
In vitro combinatorial selection of thiophosphate aptamers may be used with
the present invention. A
recent advance in combinatorial chemistry has been the ability to construct
and screen large random
sequence nucleic acid libraries for affinity to proteins or other targets
(Ekland et al., 1995; Gold et al.,
1997; Tian et al., 1995). The aptamer nucleic acid libraries are usually
selected by incubating the target
(protein, nucleic acid or small molecule) with the library and then separating
the non-binding species from
the bound. The bound fractions may then be amplified using the polymerase
chain reaction (PCR) and
subsequently reincubated with the target in a second round of screening. These
iterations are repeated
until the library is enhanced for sequences with high affinity for the target.
However, agents selected from
combinatorial RNA and DNA libraries have previously always had normal
phosphate ester backbones, and
so would generally be unsuitable as drugs or diagnostics agents that are
exposed to serum or cell
supernatants because of their nuclease susceptibility. The effect of
substitution of nuclease-resistant
thiophosphates cannot be predicted, since the sulfur substitution can lead to
significantly decreased (or
increased) binding to a specific protein (Milligan & Uhlenbeck, 1989).
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
The present invention have described the combinatorial selection of
phosphorothioate oligonucleotide
aptamers from random or high-sequence-diversity libraries, based on tight
binding to the target (e.g. a
protein or nucleic acid) of interest, relevant portions of which are
incorporated herein by reference. An in
vitro selection approach for RNA thioaptamers has also been described
Ellington and co-workers (Jhaveri
et al., 1998).
One approach used by the inventors is a hybrid monothiophosphate backbone.
Competition assay for
binding CK-1 42-mer aptamers were conducted. In standard competitive binding
assays, 32P -IgkB
promoter element ODN duplex was incubated with recombinant p50 or p65 and
competitor
oligonucleotide. The reactions were then run on a nondenaturing polyacrylamide
gel, and the amount of
radioactivity bound to protein and shifted in the gel was quantitated by
direct counting.
A combinatorial library was created by PCR, using an appropriate dNTP(aS) in
the Taq polymerization
step. A combinatorial thiophosphate duplex and single stranded (ss) libraries
was screened successfully
for binding to a number of different protein and nucleic acid targets,
including NF-IL6, NF-KB, HIV
reverse transcriptase, Venezuelan Equine Encephalitis nucleocapsid (using an
RNA thioaptamer), HepC
IRES nucleic acid, and others, including a protein involved in CpG-induced
"innate immunity." Briefly, a
filter binding method was used that was modified to minimize non-specific
binding of the S-ODNs to the
nitrocellulose filters. A column method may also be used in which the target
is covalently attached to a
column support for separation as well. The duplex, ssDNA and/or ssRNA S-ODN's
are eluted from the
filter under high salt and protein denaturing conditions. Subsequent ethanol
precipitation and for the
duplex DNA S-ODNs, another Taq polymerase PCR thiophosphate amplification
provided product pools
for additional rounds of selection (for RNA thioaptamers RT and T7 polymerase
were used). To increase
the binding stringency of the remaining pool of S-ODNs in the library and
select higher-affinity members,
the KCl concentration was increased and the amount of protein in subsequent
rounds was reduced as the
iteration number increased. After cloning, the remaining members of the
library were sequenced, which
allowed for "thioselect" T"' simultaneously for both higher affinity and more
nuclease-resistant,
"thioaptamer"T"' agents. The thioselect method has been used to isolate a
tight-binding thioaptamer for 7
of 7 targets tested.
NF-xB thioaptamers were created using thioselect for both in vitro
thioselection as well as rational design
of thioaptamers against NF-xB (Gorenstein et al., 1999a,b; 2001, 2002; King et
al., 2002). Sharma, et al.
demonstrated previously effective aptamer inhibition of NF-xB activity. They
further achieved inhibition
of NF-xB in cell culture using S-ODN duplex decoys with NF-~cB binding
consensus-like sequence
(GGGGACTTCC). The present inventors used the "CK-1" 42-mer duplex
oligonucleotide identified by
Shanna et al. (note: both the present inventors and Sharma et al.'s S-ODN
duplex was chemically
synthesized by sulfur oxidation with phosphoramidite chemistry and thus
contains in principle 282 or 1024
26
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
different stereoisomers!). The wild-type CK-1 duplex sequence contains 3
tandem repeats of a 14-mer
NF-xB consensus-like sequence (S'-CCA GGA GAT TCC ACC CAG GAG ATT CCA CCC AGG
AGA
TTC CAC 3') (SEQ ID NO.: 11).
S-ODN CK-1 monothioate aptamers were made because it was unlikely that the
phosphodiester form is
appropriate for therapeutics or diagnostics because of its short half life in
cells, cell extracts and serum.
The phosphorothioate and dithioate internucleoside modifications are therefore
needed. Using
recombinant protein homodimers of p50, p65, and c-Rel, the present inventors
confirmed that the CK-1
sequence could bind to and compete for binding to p65 homodimer, but not
p50/p50, in standard
electrophoretic mobility shift assays (EMSA)(data not shown). In contrast to
the fully substituted
phosphorothioate, the CK-1 aptamer inhibited p65/p65 and p50/p50 equally;
confirming that S-ODNs with
large numbers of phosphorothioate linkages are "sticky" and tend to bind
proteins non-specifically. The
present inventors also found that if the number of phosphorothioate linkages
is decreased to only 2-4,
specificity can be restored, but binding is not enhanced. Therefore, the
original publications described
only the specificity of the phosphodiester oligonucleotides and did not
address the problem of altered
specificity of the phosphorothioates.
Changing from purified recombinant proteins to cell culture and extracts, the
situation is further
complicated by the presence of the other cellular components, besides the
presence of other naturally
occurring NF-~cB homo- and heterodimers. When the present inventors attempted
to repeat the binding
inhibition studies of others using cell extracts, unexpected difficulties were
encountered. It was found that
the diester form of the CK-1 aptamer does not compete effectively for NF-xB
binding in cell extracts
derived from two different cell lines: the 70Z pre-B cell line and the RAW
264.7 mouse macrophage-like
line. The heterodimers in these cells either do not bind the CK-1 sequence
tightly enough, or it is bound
by other cellular components. Published reports describing CK-1 did not
present data using cell extracts,
perhaps due to similar difficulties (Sharma et al., 1996). Therefore, even
sequences with good binding
and specificity in the diester form, when fully thiophosphate-substituted,
lose their sequence specificity.
Thus, this stickiness makes the characterization of fully thioated aptamers in
vitro not necessarily
predictive of their activities in vivo.
TABLE 1. DNA Sequences from p50 Selection
Group 1 Sequences (n=16) Number
of Clones
CTG TGT TCT TGT GCC GTG TCC C 6/22 (SEQ 1D
NO.:
12)
CTG TGT TCT TGT GTC GTG TCC C 4/22 (SEQ m
NO.:
13)
CTG TGT TCT TGT GTC GTG CCC C 3/22 (SEQ ID
NO.:
14)
CCG TGT TCT TGT GCC GTG TCC C 2/22 (SEQ 1D
NO.:
15)
CCG TGT TCT TGT GTC GTG TCC C 1/22 (SEQ ID
NO.:
16)
27
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
TABLE 2. DNA Sequences from p65 Selection
Group 1 Sequences (n=8) Number of Clones
CGG GGT GTT GTC CTG TGC TCT CC 7/16 (SEQ ID NO.: 17)
CGG GGT GTT CTC CTG TGC TCT CC 1/16 (SEQ ID NO.: 18)
Group 2 Sequences (n=4)
CGG GGT GGT GTG GCG AGG CGG CC 2/16 (SEQ ID NO.: 19)
CGG GGT GGT GCG GCG AGG CGG CC 1/16 (SEQ ID NO.: 20)
CGG GGT GTG CTG CTG CGG GCG GC 1/16 (SEQ >D NO.: 21)
CGG GGT GTG CTG CTG CGG GCG GC 1/16 (SEQ ID NO.: 22)
Thioselection against NF-mB (p50:p50, p65:p65). As described in King, et al.
(2002) a unique
thiophosphate duplex library was screened for binding to the p50 homodimer.
Thioselection was repeated
through 15 rounds to enrich for sequences that bind to p50 with high affinity.
DNA sequences of multiple
clones were analyzed from the initial, 2nd, 6'", 10'x' and 1 S'" round
libraries. A striking convergence of the
DNA sequences was observed by round 15. Of the 22 clones analyzed, 16 had a
highly similar sequence
(Table 1). A thioaptamer representing this sequence was generated by PCR
amplification using a
biotinylated reverse primer. Binding studies were conducted using a
chemiluminescent EMSA, which
uses a biotinylated thioaptamer. The biotinylated thioaptamer binds tightly to
p50; the sequences are
different from those obtained for in vitro combinatorial selection against p65
homodimers (Table 2). The
chemically synthesized phosphorothioate aptamers are a diastereomeric mixture
of both Rp and Sp
configurations. The thioaptamers bind and compete for the same NF-xB site as
the known promoter
element IgkB (ICd = 78.9 t 1.9 nM for a Rel A-selected thioaptamer, and 19.6 t
1.25 nM for a p50-
selected thioaptamer). The normal phosphate ester backbone version of the Rel
A selected aptamer binds
Rel A with a ICa of 249.1 ~ 1.8 nM. The p50 dimer-selected chiral thioaptamer
binds to p50 with affinities
below 5 nM under conditions where no binding to p65 is observed. Similarly,
the p65 dimer-selected
chiral thioaptamer binds to p65 dimers with affinities below 5 nM under
conditions where no binding to
p50 is observed.
These EMSA binding studies demonstrated that the enhanced affinity can be
attributed to the presence of
sulfur. Collectively, these results further demonstrate the feasibility of the
thioaptamer selection
technology as a method for producing specific, high-affinity ligands to
proteins. It was also demonstrated
that the chemically synthesized (mixed diasteromer) thioaptamers bind tightly
in cell nuclear extracts to
both the p50:p65 heterodimer and p50:p50 homodimer. However, the enzymatically
synthesized, chiral
thioaptamer selected against the p50 homodimer only binds to p50:p50 in
nuclear extracts (Fennawald, et
al, unpublished; King, et al., 2002; Gorenstein, patents pending, 1999 a, b,
2001). Remarkably, for the
p50 homodimer the selection sequence appears to contain a pseudo-palindrome,
suggesting that 2 dimers
may be binding to the 22-mer sequence:
28
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
CTGTG PyT (CT) T G* T (G) TPy GTGTC CC (SEQ ID NO.: 23)
Dithiophosphate Aptamers Binding to Proteins. SZ-ODN CK-14 dithioate aptamers
were also isolated.
The CK-14 14-mer duplex was also synthesized with some strategically placed
dithioate linkages (both of
the non-bridging oxygens are replaced by sulfurs). As noted by the present
inventors, strategic dithioate
linkage ODNs have exhibit significant differences, as they have altered
binding specificity, and lack the
extreme "stickiness" of the fully thioated aptamer. With an increasing number
of dithioate substitutions in
the same sequence, binding by the SZ-ODN increases dramatically (data not
shown). One of the tightest-
binding dithioaptamer (XBY-6) contains 6 dithioate linkages on the two
strands. Significantly, the XBY-6
aptamer also binds to a single NF-oB dimer in cell extracts (data not shown),
while the standard
phosphodiester ODN shows no NF-xB-specific binding in extracts. Thus, the
present inventors succeeded
in synthesizing a thioate backbone modification which for the first time
increases the specific binding of
the oligonucleotide to NF-xB above that to other cellular proteins (Yang et
al., 1999). In standard
competitive binding assays, the'ZP-IgkB promoter element ODN was incubated
with recombinant p65 and
varying amounts of XBY decoy competitor. The relative binding ability of the
unlabeled ODNs was
determined by the concentration needed to compete effectively with the
standard labeled ODN. XBY1
through 6 correspond to CK-14 aptamers with with 1 though 6 dithiophosphate
substitutions, respectively
(Yang, et al., 1999).
ODN aptamer was incubated with 70Z/3 cell nuclear extract in the presence or
absence of anti-p50
antibody. Protein-bound ODN duplex was separated on a standard gel. XBY-6
shifts one complex in
nuclear extracts from a 70ZJ3 pre-B cell line. By using specific antibodies to
supershift the complex, p50
was identified as one component of the complex, which may be a complex that
include a p50 or p105
dimer, or a p50 (or p105)-containing heterodimer. Since XBY-6 binds more
tightly to p50/p50 than
p65/p65, the shifted band is likely to represent the p50 homodimer. The band
did not co-migrate with
either the p50/p50 or p50/p65 bands, but the change in the altered chemical
structure changes the mobility
of the ODN. Only one major band is seen, however, even though the lysate
contains at least two major
distinguishable NF-xB complexes (p50 homodimers and p50/p65 heterodimers).
These results demonstrate the use of aptamers having altered binding
specificity and affinity by
substituting only a limited number of internucleoside linkages, that is, a
portion of the internucleoside
linkages. The partially-modified aptamer was used to distinguish among various
NF-KB dimers within the
cell. The IgoB standard ODN does not show such specificity. Therefore, this
modified thioaptamer may
be used to bind to a single NF-KB dimer within cell supernatants and even
inactivate target dimers within
whole cells and animals. It was also found that when guinea pigs were injected
with LPS to induce
inflammatory response and XBY-6, an increase in the levels of TNF-a, was
observed above that when the
animals were injected with LPS alone. In animal macrophage extract studies, it
was found that XBY-6
29
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
eliminated a single p50 (or p105) dimer band on EMSAs. Since the p50 homodimer
appears to be a
transcriptional inhibitor of the immune response, these data demonstrate the
ability to target a single
protein within live animals, and the feasibility of altering the binding
specificity by substituting only a
limited number of internucleoside linkages (Gorenstein, et al. patents
pending, 1999 a, b; 2001, 2002).
Using the modified thioaptamer a I:1 binding stoichiometry of p65 to the 22mer
binding site known as
IgxB with a ICd near 4 nM. For one dithiophosphate aptamer, XBY-6, a binding
affinity to p65
homodimer of 1.4 nM vs. sub-nM to p50 was demonstrated.
Various thioaptamers have been made and isolated using the present invention
that can distinguish among
various NF-xB dimers within the cell. One of these decoys was able to bind to
a single NF-xB dimer in
cell extracts or within a cell in either cell culture or animal studies. These
results point to the importance
of using modified thiophosphate combinatorial selection methods to identify
minimally substituted
thioated oligonucleotides with high affinity, high binding specificity and
increased nuclease resistance in
vitro and in vivo.
Phosphorodithioate and phosphorothioate aptamers via split synthesis
combinatorial selection. The
identification of specific S-ODN and Sz-ODN thioaptamers that bind proteins
based upon in vitro
combinatorial selection methods is limited to substrates only accepted by
polymerises required for
reamplification of selected libraries by the polymerise chain reaction (PCR).
Another disadvantage of
using the polymerization of substituted nucleoside 5'-triphosphates into ODN
aptamers are the restrictions
on the choice of P-chirality by the enzymatic stereospecificity. For example,
it is known that [SP]-
diastereoisomers of dNTP(aS) in Taq-catalyzed polymerization solely yield [RP]-
phosphorothioate
stereoisomers (Eckstein, 1985). Therefore, using current methods it is not
possible to select [SP]-
phosphorothioate stereoisomers along with achiral Sz-ODN analogous since both
[Rp]-diastereoisomers of
dNTP(aS) and nucleoside dNTP(aSz) are not substrates of polymerises.
Additionally, these in vitro
combinatorial selection methods require many iterative cycles of selection and
reamplification of the
bound remaining members of the library by the PCR, which are quite time
consuming, although
automation of this in vitro selection is possible.
What is needed are methods that permit the isolation of, e.g., individual
aptamer:protein complexes
without the need for repeated iterative cycles of selection and
reamplification of likely binding targets.
Also needed are systems that permit the creation, isolation, sequencing and
characterization of making
[SP]-phosphorothioate stereoisomers along with achiral Sz-ODN analogs. To
overcome these limitations
of the in vitro combinatorial selection methods, the present inventors
developed a one-bead, one-
compound library made by using a split synthesis method to create an
alternative to in vitro combinatorial
selection methods. One-bead library systems have been used for organic
molecules (Felder, (1999)),
peptides (Lam, et al., 1991, 1995; Lam, 1995), and oligosaccharide libraries
(Zhu and Boom, 1998; Liang,
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
et al., 1996; Hilaire and Meldal, 2000). A one-bead one-oligonucleotide (one-
ODN) (e.g., O-ODN, S-
ODN, SZ-ODN, both DNA or RNA) may be used in conjunction with combinatorial
library selection
methodology used to identifying a specific oligonucleotide aptamer that binds
to specific proteins or other
molecules (Yang, et al., 2002; Gorenstein, et al., U.S. patent applied).
Furthermore, the method may use SZ-ODN reagents with sulfurs replacing both of
the non-bridging
phosphate oxygens that are isosteric and isopolar with the normal
phosphorodiester and are particularly
advantageous for binding and screening. Importantly, Sz-ODNs are achiral about
the dithiophosphate
center, which eliminated problems associated with diastereomeric mixtures
generally obtained for the
chemically synthesized S-ODN. The split synthesis approach disclosed herein
has been used for the
construction of O-ODN, S-ODN, Sz-ODN and RNA bead-based aptamer and
thioaptamer libraries
(Gorenstein et al, US Patents pending, 1999 a, b, 2001, 2002; awarded, 2002;
Yang et al., 2002). In this
procedure each unique member of the combinatorial library is attached to a
separate support bead. Targets
that bind tightly to only a few of the 104-108 different support beads can be
selected by binding the target
protein to the beads and then identifying which beads have bound target by
immunostaining techniques or
direct staining of the target or SELDI MS (see below). The present methodology
permits rapid screening
and identification of modified thioaptamers that bind to proteins such as NF-
xB using a novel PCR-based
identification tag of the selected bead.
To introduce many copies of a single, chemically pure S-ODN thioaptamer onto
each bead, a "mix and
separate" split synthesis method was used. A two-column DNA synthesizer was
used simultaneously for
construction of the library. The normal phosphate backbone linkages were
carried out using standard
phosphoramidite monomers via oxidation in column 1, while the phosphorothioate
linkages were carried
out using standard phosphoramidite monomers via sulfurization in column 2.
Dithioate are introduced by
using thiophosphoramites with sulfur oxidation. Two sequences of the same
length are programmed for
each column and are designed such that the bases are different at every equal
position not only for
diversifying base compositions but also for coding a phosphate,
phosphoromonothioate/dithioate.
For example, on an Expedite 8909 DNA synthesizer with dual columns, onto
column 1 a phosphoramidite
(for example: C) is coupled to the bead and after completion of oxidation, the
resulting product is
nucleotide (C) with a phosphotriester linkage. On column 2 a nucleoside
phosphorothioate is introduced
with a different base (T for example). The two columns are mixed and resplit
and in the second cycle,
additional phosphoramidites or phosphorothioamidites are introduced, followed
by oxidation and
sulfurization reactions individually in column 1 and column 2. After
additional coupling steps and after
split/pool synthesis is carried out, the end products comprise a combinatorial
library of thioaptamers with
varying monothioate, dithioate or normal phosphate ester linkages at varying
positions along the ODN
strand. On completion of the automated synthesis, the column is removed from
the synthesizer and dried
31
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
with argon. The bead bound fully protected ODNs are treated with 1 ml of
concentrated ammonia for 1h
at room temperature, incubated in a 55 °C oven for 15-16 h, removed
from the oven and cooled to room
temperature. Importantly, after deprotection, with this coupling scheme with a
non-cleavable
hexaethyleneglycol linkers. Linker attaching the first phosphoramidite (15 or
70 ~m beads provided by
ChemGenes), the thioaptamers are still covalently attached to the beads after
complete deprotection.
Thus, each bead contains a single sequence with a specified backbone
modification that is identified by
the base.
For example, this scheme was used to synthesize libraries of 4096 (2~z)
different thioaptamers attached to
beads, each bead containing a unique thioaptamer. This library consisted of a
22-nucleotide "random"
sequence (12 split/pool steps) flanked by IS nucleotide defined primer regions
at the 5' and 3' ends
(Yang, et al., 2002). A phosphorothioate linkage was introduced on every other
base in column 2,
following the "split and pool" approach. The single-stranded 52-mer S-ODN
random library was
converted to double-stranded DNA by Klenow DNA polymerase I (Promega) reaction
in the presence of
DNA polymerase buffer, dNTP mix and downstream primer. Therefore, the one
strand of the duplex
1 S potentially contained S-ODN modifications and the other complementary
strand were composed of ODN.
A duplex DNA library in which both strands contain S-ODN modifications could
also be generated using
a Klenow reaction with no more than three dNTP (a)S.
The dsDNA thioaptamer library beads were screened for the ability to bind the
NF-xB p50/p50 dimer
labeled with the Alexa Fluor 488 dye (Molecular Probes). After initial binding
of protein, the beads were
thoroughly washed with PBS with 0.1% Tween 20 to minimize nonspecific binding.
Typically, a few
positive beads were intensely stained when viewed by fluorescence, while the
majority of the beads
remained unstained as (data not shown). With the aid of a micropipette coupled
to a micromanipulator,
the intensely stained beads were retrieved. Only highly positive beads from
several thousand were found
using this method. As described below, multicolor flow cytometry and cell/bead
sorting was used to
automate the selection process to select the tightest binding thioaptamer-
protein complexes.
Sequencing may also be obtained directly from the bead. Each individually
selected bead was washed
thoroughly with 8 M urea (pH 7.2) to remove the protein and was directly used
for the "one-bead one-
PCR" amplification using the 5' and 3' end primers. The PCR product was cloned
using the TA Cloning
procedure (Invitrogen) and sequenced on an ABI Prism 310 Genetic Analyzer
(Applied Biosystems). The
four thioaptamers listed in Table 3 were obtained from the library. For
verification of these results, the S-
ODN, 5'-CtGTGAGtCGACTgAtGaCGGt-3' (SEQ ID NO.: 7) (small letters represent
location of 3'-
monothiophosphates), was synthesized independently on the non-cleavable linker
bead support, hybridized
with its complementary ODN and then mixed again with the p50/p50 protein
labeled with the Alexa Fluor
488 dye. The fluorescence intensity of all of the beads viewed under the
fluorescence microscope was
32
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
qualitatively similar to the intensity of the selected bead containing this
sequence within the combinatorial
library. These results demonstrate that the primer regions do not contribute
to the binding of p50/50.
Furthermore, it was found that not only normal monothio-ODN on the beads but
also dithio-modified
bead-bound sequences could be sequenced directly from the dithiophosphate
combinatorial library. Thus,
S the split synthesis has been used to create a "one-bead-one sequence" ODN
and that PCR can be used to
identify an S-ODN bound to a bead (Yang et al., 2002; Gorenstein et al, US &
Foreign Patents pending,
1999 a, b, 2001, 2002).
Bead-based thioaptamer library screen. Aliquots of S-ODN beads bound to NF-xB
p50/p50 homodimer
protein labeled with the Alexa Fluor 488 dye viewed under light microscopy.
The same beads viewed
under fluorescence microscopy, in which a positive green bead stained with
Alexa Fluor 488 dye were
easily identified in a background of many hundreds of nonreactive beads.
Single positive bead can easily
be retrieved with a handheld micropipette under fluorescence microscopy.
Although the beads were screened against a target protein labeled with a
fluorescent dye, the beads have
also been screened directly against cell extracts as well. The binding of the
NF-xB to a specific sequence
can be detected using a primary anti-NF-xB antibody such as anti-P50 (Rabbit
IgG antibody, Santa Cruz
Biotechnology, Inc.) followed by a secondary antibody conjugated with Alexa
Fluor 488 (goat anti-rabbit
IgG from Molecular Probes). Beads that included the XBY-6 oligonucleotide were
screened against WI-
38 VA13, an SV40 virus-transformed human fibroblastic cell line extract by
similar fluorescent
microscopy.
Other bead-based thioaptamer libraries. Combinatorial thioaptamer bead
libraries of over 106 different
sequences have also been readily prepared. The present inventors have
synthesized successfully a
monothio RNA library (2~5=32768) (Gorenstein, et al., patent pending, 2002).
Thus, standard
phosphoramidite (DNA and RNA) chemistry was used for the thioaptamer RNA
library. A 0.5 M 1H-
tetrazole in acetonitrile was used as DNA activator. A 0.5 M solution of DCI
(dicyanoimidazole) in
acetonitrile was used as RNA activator. The libraries were prepared on a 1
pmole scale of polystyrene
beads (66-70 pm). The downstream and upstream primers, 5'-d(GGATCCGGTGGTCTG)-
3' and 5'-
d(CCTACTCGCGAATTC)-3' were synthesized in parallel on a two-column DNA
synthesizer (Expedite
8909, Applied Biosystems). Following the S'-primer, the sequences programmed
on the synthesizer for
the combinatorial mono RNA library were S'-
r(GA*UC*CU*GA*AA*CU*GU*UU*UA*AG*GU*UG*GC*CG*AU*C)-3' (SEQ ID NO.: 24) on
column 1 and 5'-r(cU*aG*gA*cU*uG*gC*aC*aA*cC*gU*cA*cA*cU*gC*uA*u)-3' (SEQ ID
NO.: 25)
on column 2. The 3'-primer sequence completed the 61-mer programmed on the
synthesizer. A "split and
pool" occurred at each position indicated by an asterisk in order to
synthesize the combinatorial region for
the monothio RNA. The lower case letter indicates a 3'-thioate linkage, the
upper case letter indicates a
33
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
3'-phosphate linkage. The coupling yield was typically upwards of 98.5% as
determined by the
dimethoxytrityl cation assay (DNA couplings are typically >99%/nt).
Sulfurization chemistry utilized the
Beaucage reagent. The fully protected monothio RNA combinatorial library with
the non-cleavable linker
beads were treated with 4 ml of a mixture of 3:1 (v/v) (28%) NH3: EtOH at 39
°C for 21 hrs. The beads
were centrifuged, the supernatant was removed and the solid support was washed
with double-distilled
water. After lyophilization the solid support was treated with 2 ml of
triethylamine trihydrofluoride
(TEA-3HF) for 20 hrs at room temperature. Again, the beads were centrifuged,
the supernatant was
removed and the solid support was washed with double-distilled water. RT PCR
and TA cloning
confirmed the successful synthesis of the ssRNA thioaptamer library.
TABLE 3. Sequences of thioaptamers selected from split synthesis (small
letters indicate
thiophosphate 3' to base).
5'-tGTGcAGGGACTgAtGaCGGt-3' (SEQ ID NO.:
6)
5'-CtGTGCatCGAaGTTtGCAtTt-3' (SEQ ID NO.:
7)
5'-AtGcAcAtCtCaGgAtGaCGGt-3'(SEQ ID NO.:
8)
5'-AGTTGcAGGtCaGgACCCAtTt-3' (SEQ ID NO.:
9)
Flow cytometry sorting of thioaptamer bead-based library. The present
inventors have also demonstrated
the successful application of high throughput/multi-color flow cytometry and
bead sorting to screen
aptamer bead libraries for those beads which bind to, e.g., a target protein
(Gorenstein, et al., patent
pending, 2002). Modifications were made to a custom-built flow cytometer to
make it more amenable to
bead identification and isolation. For example, bead fluorescence and forward
scatter were the two
parameters chosen for real-time characterization of each aptamer bead passing
the first sort point of the
custom-built flow cytometer/sorter. Other scanning and sorting parameters may
be used to select, isolate,
view, designate, characeterize, etc. the beads through a flow cytometer.
In operation, "positive" beads (contain thioaptamer-bound target protein, the
target protein was
fluorescent-labelled with Alexa 488 dye) were easily sorted from negative
beads. Flow cytometry may be
used to replace, e.g., visual fluorescence microscope identification of beads
containing bound target
protein and the need to isolate the individual "positive" beads with the
micromanipulator described
previously. The flow-sorted "positive" beads can then be subjected to, e.g.,
one-bead PCR to identify the
thioaptamer that binds the target protein.
34
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
TABLE 4. Population Statistics for bead sorting, WinList analyses (all data
were color-compensated)
Sam 1e Total Re ion %Gate
i ure 6A: CONTROL.FCS
Rl : Autofluorescent 10000 9530 95.3
Beads
i ure 6B. FCS
R2: 50 Alexa 488 Positive10000 35 0.35
Beads
i ure 6C. FCS
R3: 65 PE Positive Beads20000 3488 17.44
i ure 6D. FCS
Rl: Autofl. Beads & 1000000963321 96.33
Carrier Beads
R2: 50 Alexa 488 Positive1000000354 0.04
Beads
R3: p65 PE Positive 1000000935 0.09
Beads ~
Fluorescence sorting was also used to demonstrate the use of the one-bead, one-
ODN:protein system using
dual color sorting. The IgxB dsDNA consensus sequences were immobilized onto
15-20 micron
polystyrene microspheres. The DNA bound beads were then incubated with
purified p50 and p65
proteins, respectively. DNA transcription factor complexes were detected with
primary antibodies
specific for the p50 and p65 proteins followed by an additional incubation
with Alexa 488- conjugated
secondary antibody for p50 and PE- conjugated secondary antibody for p65. The
beads were viewed by
fluorescent microscopy and then analyzed on the MCU's HiReCS system. A Control
Fluorescent Cell Sort
(CONTROL.FCS) shows the autofluorescent microspheres in the negative control
sample where the beads
were unbound. The majority of the beads in the "debris" population were the
0.8 micron carrier beads that
were used to bring up the volume of the samples since the beads were at a very
low dilution.
Innate Immunity Toll-Like Receptor Signaling. In another embodiment of this
invention, the present
inventors developed thioaptamers that enhance the innate immune response by
targeting the Toll-like
receptor (TLR) family in mammals, which is a family of transmembrane proteins
characterized by
multiple copies of leucine rich repeats in the extracellular domain and IL-1
receptor motif in the
cytoplasmic domain (Akira et al., 2001; Medzhitov, 2001). The TRL family is a
phylogenetically
conserved mediator of innate immunity that is essential for microbial
recognition. Ten human homologs
of TLRs (TLRl-10) have been described. By using a BLAST search, Hemmi et al.,
2000, have identified
and subsequently isolated a cDNA coding for TLR9. Gene knockout experiments
suggest that TRL9 acts
as a receptor for unmethylated CpG dinucleotides in the bacterial DNA. Human
and mouse TLR9 share
an overall amino-acid identity of 75.5%. TLR9 is highly expressed in spleen
(Krieg, 2002).
The immunostimulatory properties of bacterial DNA appears to be related to
short six base sequences
called CpG motifs that have the general structure of two 5' purines, an
unmethylated CpG motif, and two
3' pyrimidines (ICrieg, 2002). Though such sequences rarely appear in
mammalian DNA due to CpG
suppression and methylation of cytosine nucleotides, they are relatively
abundant in bacterial DNA,
occurring at the expected frequency (1 in 16) and in unmethylated form.
Indeed, studies have found
ODNs containing these sequence motifs to be strongly immunostimulatory,
resulting in the activation of B
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
cells, NK cells, and antigen-presenting cells, and in the induction of a
variety of cytokines including
interleukin-12 (IL-12), IL-6, and tumor necrosis factor-a. CpG ODNs have also
been found to be
effective as adjuvants in inducing antigen-specific T-helper-1-like responses,
and have been the focus of
much interest for their inclusion in anti-tumor vaccinations and use in other
therapeutic applications
(Klinman et al., 1999; Krieg et al, 1999). Adjuvants enhance nonspecifically
the immune response to an
antigen. For example, pathogenic Arenaviruses appear to block or modify
immunoregulatory cell
signaling pathways (Peters & Zaki, 2002, Solomon and Vaughn, 2002; Fennewald
et al., 2002). Using the
present invention it was possible to disrupt Arenavirus and Flavivirus cell
signals that contribute to
immune evasion and pathogenesis. Using thioaptamers it was demonstrated that
the thio-modified
aptamers of the present invention could be used to counteract viral induced
cellular perturbations and
protect the infected host.
Viral Strategies to manage the host. During the co-evolution of viruses and
their hosts, viruses have
developed ingenious strategies to counteract the host defenses that normally
control viral replication and
spread. Similarly, viral strategies modify the cellular environment to promote
viral macromolecular
synthesis and viral replication. This highly ordered interation often has the
unfortunate consequence of
inducing disease in the host. Viruses have evolved mechanisms to interfere
with major histocompatibility
complex antigen presentation, block apoptosis, disrupt complement cascades and
modulate multiple
cytokine networks (Lalani & McFadden, 1999; Ploegh, 1998). Viruses have
targeted cell-signaling
pathways involved in cytokine and chemokine signaling, the regulation of
apoptosis, and the cell cycle.
Studies have revealed a number of instances of direct viral intervention in
the receptor and receptor
proximal signaling, as well as direct interaction with signaling kinase
cascades and transcription factors
(McFadden et al., 1998; Ploegh, 1998; Hiscott, 2001; Hiscott et al., 2001).
Most examples have come
from large DNA viruses with sufficient coding capacity to encode viral
homologs of cellular proteins.
These viruses use molecular mimicry to exploit the cellular environment to
promote viral replication and
antagonize the immune response to sustain their survival in an immunocompetent
host (Cameron et al.,
1999; Willer et al., 1999; Hiscott et al., 2001). Influencing key
transcription factors that regulate pro or
anti-inflammatory cytokines is an efficient means by which viruses could
cripple multiple immune
responses (Powell et al., 1996; Tait et al., 2000). The strategies employed by
the smaller, less genetically
complex viruses are equally elegant, and often even more of an enigma.
Pichinde infection of guinea pigs is particularly suited to studies on the
immunomodulation by virus
infection. There are two virus variants with minimal genomic differences but
profoundly different effects
on the animal. Infection by the P2 variant of virus results in mild illness
from which the animal recovers.
Infection by the P18 variant results in death. These two virus variants were
used to distinguish an
effective immune response against the P2 virus, from an ineffective response
against the P18 virus.
36
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
Using the aptamers of the present invention, the differential effect of virus
infection was identified as
including a profound effect on the transcription factors NF-xB and RBP-Jx.
Data generated by the present
inventors (Fennewald et al., 2002) showed differential alterations in the
transcription factors NF-xB and
RBP-Jx in P2 and P18 virus-infected guinea pig peritoneal macrophages. The P2
variant shows less NF-
xB present and a higher mobility RBP-Jx complex. This observation was used in
an animal model of
arenavirus disease in which two virus variants differentially affect target
cell signaling pathways. NF-xB
and AP-1 (CREB) family members are key regulators of the immune response and
transcription factors
involved interferon response to virus infection all are differentially induced
in pathogenic Pichinde
infections. Using the aptamers of the present invention infected hosts
virulence was reduced by
modulating virus induced alterations in cellular signal transduction.
Many of the signaling pathways and transcription factors activated during
immune system activation lead
to the synthesis of the inflammatory cytokines. Certain pathways require the
expression of various
cytokines. The effect of the virus variants (and polyI/C) on the induction of
cytokines was determined.
Figure 2 is a graph that shows that polyI/C is an effective inducer of the
proinflammatory cytokine TNF-a.
Infection with P2 and P18 also alter the expression of this and other
inflammatory cytokines. In
particular, P2 and P18 induced equally cytokines such as IL-6; which are
moderately different in their
induction of TNF-a and substantially different in IL-12 induction (Figure 3).
Thus, differences in
signaling and inflammatory responses are associated with immune activation by
P2 virus and poor
activation by the P18 virus. For example, IL-12 is especially important in
directing the anti-viral immune
response to the effective Thl cytotoxic T cell response (Seow, 1998). In
addition to supporting the
association with the immune response, this data can be used to direct the
transcription factors to target.
For example, IL-6 induction is similar for both virus variants.
To target transcription factors key in regulating TNFa and IL12 and other key
mediators of the immune
response two thioaptamers were produced, XBY-6 (SEQ ID NO.: 1) targeting NF-xB
p50 homodimers and
XBY-S2 targeting AP-1, both with six dithio residues. In Figure 4, XBY-S2 (SEQ
ID NO.: 2) is
demonstrated to bind specifically to AP-1 proteins in pre-B cell nuclear
extracts (70ZJ3) and to human
recombinant c-jun protein dimers (AP-1). In Figure 5, supershift analyses
indicate that XBY-S2 binds to
several members of the AP-1 protein family including JunD, CREB and possibly
ATF2, and c-Jun. The
XBY-6 thioaptamer binds specifically to the NF-xB p50 (or p105) homodimer
(Figure 6). Macrophage
cultures were treated with XBY-S2 and XBY-6 and nuclear extracts were produced
to assay the effects of
these thioaptamers on the DNA binding activities of the transcription factors
to which they are targeted.
In Figure 7, macrophage cultures were treated with liposomes, and liposome
containing the indicated
thioaptamers overnight and nuclear extracts produced and assayed using the
indicated oligonucleotides.
37
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
The XBY-S2 thioaptamer efficiently eliminated transcription factor binding to
the AP-1 oligonucleotide.
In contrast, treatment with XBY-6 resulted in an increase in the NF-xB DNA
binding activity.
In order to determine the consequence of the elimination of AP-1 DNA binding
activity by XBY-S2,
stimulated macrophage cultures were incubated with the thioaptamer with
PolyI/C and measured the
elaboration of TNFa and IL-6 into culture media. The expression of both TNFa
and IL-6 are increased in
response to polyI/C (Figure 8 and 9). Pretreatment of cultures with XBY-S2
thioaptamer increases the
amount of both cytokines produced in response to poly I/C. These results
indicate that elimination of AP-
1 from cells by the XBY-S2 decoy thioaptamer increases the production of
cytokines.
It has been suggested that arenaviral and West Nile pathogenesis is the result
of viral perturbation of the
immune response resulting in the inappropriate expression of cytokines.
Therefore, modulation of cell
signaling by appropriate thioaptamers could reverse the inappropriate gene
expression and help to
alleviate the symptoms and perhaps prevent host death. Guinea pigs were
treated with the XBY-6
thioaptamer targeting NF-xB p50 homodimers at days 0, 1, and 2 day relative to
time of infection with a
lethal dose of Pichinde virus. Figure 10 demonstrates that the thioaptamer
prolongs the survival of
Arenavirus infected animals. A thioaptamer of the same base content but
scrambled in sequence and
containing CpG islands did not prolong survival (B92; Figure 10). Using the
XBY-S2 thioaptamer, 50-
80% protection of mice from a lethal West Nile virus infection was
demonstrated (Tables 5 and 6) as well
as prolongation of Pichinde virus survival similar to XBY-6 (data not shown).
TABLE 5. Female 3-4 week-old NIH Swiss mice were given aptamers at one day
before and 90 minutes
before administration of 10 LD50 WN virus strain USA99b by the ip route.
Group # surviving [%] AST (days~SD)
PBS only 0/5 [0] 7.2 f 0.4
Liposomes only 0/5 [0] 8.0 t 0.7
XBY-S2 4/5 [80] 9
XBY-6 4/5 [80] 11
Based on the preliminary results obtained with XBY-6 thioaptamer and Pichinde
virus, it was determined
if XBY-6 or XBY-S2 would have any antiviral activity against flaviviruses.
West Nile virus was selected
as a model system due to its high virulence in the mouse model. Mice were
challenged with a low dose of
virus (i.e., 30 pfu ~0 LDS°). The thioaptamers (10 pg) were delivered
IP in Tfx50 liposomes and
administered in two doses (one day before and 90 minutes before virus
challenge). Control mice given
PBS or liposomes succumbed to WN virus infection, while 80% of thioaptamer XBY-
S2 treated animals
survived challenge and remained healthy (Table 5). It was noted that both
thioaptamers had antiviral
activity. These results suggested that while the mechanism of protection may
involve binding of XBY-6
to NF-xB or XBY-S2 to AP-1.
38
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
In previous studies with West Nile virus the present inventors had observed
hat animals had a brief
viremia that peaked on day 3 pi prior to viral brain invasion. As such, three
animals from each test group
were sacrificed on days 3 and 6 post infection to determine viremias and virus
infectivity levels in the
brain. Accordingly, the protocol from the first study was repeated with
increased group sizes of 16 mice
(of which 6 would be sampled) and increasing the virus challenge to 100 LDso
virus. As shown in Table
6, the initial results were reproducible. Both control groups (PBS and
liposomes) succumbed to challenge
with WN virus while the thioaptamer-treated mice survived and remained
healthy. The proportion of mice
treated with XBY-S2 thioaptamer who survived challenge was the same in both
studies (80%) while
XBY-6 treatment protected SO% of mice in the second study as compared to 80%
of mice in the first
study. These differences were not statistically significant given the small
sample sizes.
To obtain fundamental information on the mechanism of protection, viremias and
brain infectivity titers
were measured in three mice sampled from each group on days 3 and 6 post
infection (Table 7). As
expected, viremias and brain infectivity titers in the control (PBS and
liposome) groups detected on day 3
prior to invasion of the brain and virus detectable in the brains on day 6
post infection. The thioaptamer
treated mice had reduced or undetectable viremias on day 3 post infection and
no detectable virus
infectivity in brains on day 6 post infection. These data indicate that the
thioaptamer causes a reduction in
the extraneuronal replication of the virus (as seen in the reduced viremias)
and that there is insufficient
virus to invade the central nervous system and cause encephalitic disease. The
difference between
virulent neuroinvasive strains of WN virus and poorly neuroinvasive attenuated
WN strains may be
explained by these results. Two mechanisms seem possible, although the
invention is in no way limited
by hypothesis: 1) first, the thioaptamer induces an immune response against WN
virus; or 2) the
thioaptamer blocks the WN virus replication. The thioaptamer may be inducing
localized interferon (or
other mediators of the innate immune response) that inhibits replication of
the virus since the thioaptamer
includes double-stranded DNA while double-stranded RNA is known to be an
efficient inducer of
interferon.
TABLE 6. Study 2: Female 3-4 week-old NIH Swiss mice were given aptamers at
one day before and 90
minutes before administration of 100 LDSO WN virus strain USA99b by the ip
route.
Group # surviving [%] AST(days~SD)
PBS only 0/10 [0) 8.3 t 0.8
Liposomes only 0/10 [0] 7.7 t I.1
XBY-S2 8/10 [80] 8.5~0.7
XBY-6 5/10 [50] 8.0t0.7
To investigate the activity of the modified thioaptamers and the antiviral
mechanism of action of the
thioaptamers, the susceptibility of thioaptamer-protected mice virus to
challenge was tested.
Thioaptamer-treated mice from the second study who survived WN virus infection
were challenged at 21
days post-infection with 100LDso of WN virus. All mice, including mock-
infected controls from study 2
39
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
succumbed to virus challenge. This result indicates that there was
insufficient virus replication in
thioaptamer-treated mice to induce an adaptive immune response. This would
suggest that the mechanism
of action of the thioaptamer is either innate immunity or direct antiviral
activity of the thioaptamer.
TABLE titers for Study 2 (see
7: Viremia Table 6)
and
brain
infectivity
Day 3 Day 6
Sample Serum titer BrainSerum titer grain titer
titer (pfu/brain)
(pfu/mL) (pfu/brain)(pfu/mL)
XBY-6 30,000 --' -- --
#1
XBY-6 -- -- -- --
#2
XBY-6 700 -- -- --
#3
XBY-S2 #1 100 -- -- --
XBY-S2 #2 -- -- -- --
XBY-S2 #3 -- -- -- --
Lipo #1 2,000 -- -- 500,000
Lipo #2 2,500 -- -- 6,500,000
Lipo #3 15,000 -- -- 3,500
PBS #1 25,000 -- 100 5,500,000
PBS #2 20,000 -- -- 180,000,000
PBS #3 4,500 -- -- 2,500,000
' -- indicates no virus detected; limits of detection were 50 pfu/ml of serum
and 25
pfu/brain
1. Liposomes + xbyc2 (10 ~g/well) 2. Liposomes + xbysl (10 pg/well)
3. Liposomes + XBY-S2 (S ~g/well) 4. Liposomes + XBY-S2 (10 ltg/well)
4. Liposomes only 5. Buffer only
Whether thioaptamers exhibited direct antiviral activity in cell culture was
also determined. The direct
antiviral activity of the thioaptamer was investigated in cell culture. Using
six-well dishes containing
Vero cells, duplicate wells were treated with one of the following samples:
Wells were incubated for 12 hours with the samples above and then challenged
with WN virus at a
multiplicity of infection (MOI) of 0.1. Samples were harvested from each well
at 0, 14, 24, 34 and 48
hours. No cytopathic effect was seen until 48 hours post virus infection. Each
well was assayed at each
time point by hemaggluttination (HA) assay to detect the presence of virus
particles. All samples showed
no detectable HA (i.e., < 4 HAU) except for the samples at 48 hours post virus
infection when all wells
had 32-64 HAUs. These results demonstrate that the thioaptamers have no direct
antiviral activity.
One potential explanation for the antiviral activity of thioaptamers is
induction of interferon. This
hypothesis was investigated by taking groups of four 3-4 week-old female NIH
Swiss and treat them with
either l0ug of XBY-S2 in liposomes, liposomes only, or buffer only on day 0
and day 1 post infection,
followed by sacrificing mice on day 2 post infection. Serum samples were
diluted 1 in 3 and run in
ELISAs to detect mouse interferon-al(3, interferon-y, or TNF-a. None of these
cytokines was detected in
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
the serum of any of the 12 mice sampled suggesting that interferon was not
involved in the antiviral
activity induced by thioaptamer XBY-S2.
Figure 10 and Tables 5 and 6 demonstrate that the survival of P18 virus
infected animals can be prolonged
using thioaptamers and thioaptamers can protect the majority of the animals
infected with West Nile virus.
These results demonstrate that modified thioaptamers alter the outcome of in
vivo viral infections by
Category A and B agents by the manipulation of transcription factors involved
in the immune response.
Figure 10 is a graph that shows survival curves following Pichinde P18
infection in guinea pigs treated
with the NF-xB aptamer, XBY-6, the scrambled control, B92, or vehicle, MT, of
animals infected by
injection of 1000 pfu of Pichinde P18 at day 0, treatment consisted of
intraperitoneal injections at days 0,
1 and 2;
Figure 11 is a graph that shows survival curves of guinea pigs with
thioaptamers for infection by
arenavirus. Figure 12 is a graph that shows survival curves following West
Nile Virus infection in guinea
pigs treated with the NF-xB aptamer XBY-6, the AP-1 aptamer XBY-S2, or the
liposome vehicle of
animals infected by injection with lethal doses of West Nile Virus.
SELDI MS Detection of NF-xB bound to Thioaptamer Surfaces and Beads. The
present inventors have
demonstrated that thioaptamers bind both purified, recombinant NF-xB p50 and
nuclear extracts on either
beads (or Ciphergen PBSII ProteinChip surfaces). Figures 13A-C are SELDI MS of
p50 binding to
various ProteinChips and beads. In Figure 13A, Ciphergen's SELDI mass
spectrometric methods were
used to detect recombinant p50 with using epoxy-activated ProteinChip Arrays.
Duplex aptamers with a
5'-amino terminus linked to a 12 carbon chain were synthesized. These duplex
aptamers were the
dithioate 14-mers XBY-6 (C12-XBY-6), the normal phosphate backbone 22-mer NF-
xB binding site with
the C12 5'-amino linker (C12-IgxB) or a non-specific, non-covalently linked
duplex (polydIdC) as a
control. These aptamers were spotted individually onto spots of a preactivated
ProteinChip Array (PS20)
in 2 p1 of 25 mM NaHC03 (pH 9) and incubated overnight at room temperature and
high humidity.
Following incubation, excess aptamer was removed by washing 2 times in 5 p1 25
mM PBS, 0.1% Triton
X-100 (pH 7.2) and the surface was blocked to limit non-specific binding with
1 p1 of 100 p.M bovine
serum albumin for 4 hrs. After blocking, excess BSA was washed away as above.
Next, 4.3 pmol
recombinant p50 was spiked into 100 pmol BSA in 5 p1 of optimized EMSA buffer
containing 20mM
DTT, 0.01 pM polydIdC and incubated on each of the aptamer/thioaptamer
surfaces for 2 hrs at room
temperature and high humidity. Following incubation, each spot was washed with
5 p1 of 50 mM Tris
buffer (pH 7.2), 0.1% CHAPS, 1 M urea, 0.5 M NaCI, followed by a water wash to
remove all non-
specific binding components. 0.8 p1 Sinapinic acid (saturated solution in 50%
acetonitrile, 0.5%
trifluoroacetic acid) was added to each spot, dried and the array analyzed in
the mass reader. As shown in
41
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
Figure 13, the p50 (MW ~ 46,200) on either the XBY-6 or IgoB bound surfaces
was detected, but not the
control. In other spectra with more stringent washing, the XBY-6 spot, but not
the IgxB spot, was shown
to retain the bound p50 (spectra not shown), confirming the tighter binding of
p50 to XBY-6 (sub-nM)
relative to IgoB (KD 4 nM).
Figure 14 shows that the XBY-6 thioaptamer can also capture recombinant p50
(MW ~ 46,200) on gel
beads to which the 5'amino-C12 linked XBY-6 is coupled to 20 u1 (1:1)
AminoLink~ Plus Coupling gel
(Pierce, Immunoprecipitation kit, cat # 45335). In this study, 3 ~g of C12-XBY-
6 was coupled overnight
at 4°C following the kit protocol. After quenching the gel, 6 ltg of
p50 in 1X EMSA buffer with polydIdC
was added to the gel and incubated for 2 hrs with shaking at room temperature.
The gel was washed to
remove nonspecifically bound proteins, followed by one quick rinse with water.
Protein bound to the gel
was extracted with S ~l of organic solvent (50% AcN and 0.01% TFA) with
shaking for 20 min. All of
the extracts were spotted onto NP20 ProteinChips, dried, followed by addition
of saturated SPA and read
on the Ciphergen PBSII MS (top two spectra). After extraction, 1 ~1 of the gel
was loaded onto NP20 chip
(bottom two spectra). Proteins still bound to the gel was analyzed using
saturated SPA on the PBSII.
Once again it was found that p50 can be identified by SELDI, both in the
extract and retained directly on
the beads.
Figure 15 shows the capture of nuclear extracts onto Ciphergen's PS20
ProteinChip Arrays: Either 0.5 ~g
of C12-XBY-6, 0.25 pm of C12-IgxB or 0.5 pg of poly dIdC were incubated on
PS20 chip overnight. The
chips were blocked with 7 mg/ml BSA in PBS/0.1% Tween-20. Following blocking,
49 pg of nuclear
extract in optimized EMSA buffer were incubated on each spot for 2 hr with
shaking. Each spot was
washed with PBS/0.1% Triton three times, followed by one quick wash with
water. Proteins bound on
each spot were analyzed using saturated SPA on the PBSII. These results
indicate that a protein was
bound with a MW 105,591, which may represent p105, the precursor to p50 or the
p50/p50 homodimer.
Bead-based phosphorodithioate and phosphorothioate thioaptamer combinatorial
libraries and high
throughput sorting against targeted proteins. The one-bead, one-aptamer split
synthesis method disclosed
herein was used to identify a specific ODN aptamer that targets proteins or
other biomolecules. In
combination with the split and pool synthesis combinatorial chemistry method
for creating a combinatorial
library of oligonucleotide agents (either phosphate, monothiophosphate or
dithiophosphate; Gorenstein et
al, U.S. Patent issued, 2002 and pending, 1999 a,b, 2001, 2002; Yang et al.,
2002, relevant portions
incorporated herein by reference) both monothiophosphate and dithiophosphate
combinatorial libraries
attached to individual support beads were shown to produce aptamers that
demonstrate target-specific
binding. Proteins that bind tightly to only a few of the 10'-10$ different
support beads may be selected by
binding either purified proteins, nuclear or cytoplasmic extracts or pools of
proteins to the beads and then
identifying which beads have bound target protein by immunostaining,
fluorescent staining techniques or
42
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
MS (SELDI). Thus, the methods and compositions created and isolated thereby
allow for rapid screening,
isolation and identification of specific thioaptamers that bind to proteins
such as NF-xB and AP-1 using
the PCR-based identification tag of the selected bead disclosed herein.
Preparation and composition of the thioaptamer libraries and libraries of
libraries. Depending on the
nature of the targeted protein, thioaptamer combinatorial libraries were
created that cover appropriate
sequence space relative to the targeted protein. For transcription factors
duplex thioaptamers were create
that have a significant population of sequences similar to the consensus
sequence. In the case of the in
vitro combinatorial selection approach disclosed herein, the complexity of the
library can be as large as
10'4 different sequences and thus can cover all sequence space for a small
(<22 nt) duplex. For the bead-
based thioaptamer libraries, complexity is limited to the number of different
beads - 106-108, depending
on their size.
To increase the complexity of the libraries one may also use a novel iterative
approach in which a bead-
based library of libraries of thioaptamers is made in which as many as 106
different thioaptamers are
attached to a single bead and thus have a total complexity of as many as 10'2-
10'4 sequences in the library
of library. For example, a library of libraries was prepared on a 1 mole scale
of polystyrene beads (60-70
Vim). The downstream and upstream primers, 5'-d(GGATCCGGTGGTCTG)-3' (SEQ ID
NO.: 26) and
S'-d(CCTACTCGCGAATTC)-3' (SEQ ID NO.: 27) were synthesized in parallel on a
two-column DNA
synthesizer (Expedite 8909, Applied Biosystems). Following the 5'-primer, the
sequences programmed on
the synthesizer for the combinatorial library were 5'-AT*GN*GA*AT*TT*NC*CA 3'
(SEQ ID NO.: 28)
on column 1 and 5'- GG*AG*NG*CN*CA*GG*AC -3' (SEQ ID NO.: 29) on column 2. The
3'-primer
sequence completed the 44-mer programmed on the synthesizer. A "split and
pool" was used at each
position indicated by an asterisk in order to synthesize the combinatorial
region for the library of libraries.
The letter N indicates a mixture of four bases (A, C, G and T). Five of the
beads were randomly selected
from the library and "one bead one PCR" was run, cloned and sequenced. The
results listed below
indicated the successful construction of the library of libraries.
E45-2-1: 5'-GG AG GA CT TT CC AC-3'(SEQ ID NO.:
30)
E45-2-2: 5'-GG AG GA CA TT GC AC-3'(SEQ ID NO. :
31)
E45-2-4: 5'-GG AG GA CC TT CC AC-3'
(SEQ ID NO. :
32)
E45-2-5: 5'-GG AG GA CC TT GC AC-3'(SEQ ID NO. :
33)
E45-2-11:5'-GG AG GA CN TT TC AC-3'(SEQ ID NO. :
34)
E45-2-12: S'-GG AG GA CC TT TC AC-3'(SEQ ID NO. :
35)
E45-3-1: S'-GG GA TG GT CA GG AC-3'(SEQ ID NO. :
36)
E45-3-3: 5'-GG GC GG AAT CA GG (SEQ ID NO. :
AC-3' 37)
E45-3-5: S'-GG GA AG AT CA GG AC-3'(SEQ ID NO. :
38)
E45-3-6: S'-GG GG TG AT CA GG AC-3'(SEQ ID NO. :
39)
E45-3-11: S'-GG AG TG CT CA GG CA-3'(SEQ ID NO. :
40)
E45-6-1: 5'-GG AG CG GT GT CC AC-3'(SEQ ID NO. :
41)
E45-6-2: 5'-GG GA GG GGA TT AC (SEQ ID NO. :
CA-3' 42)
E45-6-3: S'-GG AG CG GT TT GC CA-3'(SEQ ID NO. :
43)
43
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
E45-6-10: 5'-GG AG CG AT TT CC CA-3'(SEQ ID NO.:
44)
E45-6-11 5'-GG AG AG GT TT TC CA-3'(SEQ ID NO. :
45)
E45-7-1: 5'-AT AG GG CA CA GG AC-3'
(SEQ ID NO. :
46)
E45-7-2: 5'-AT AG NG CC CA GG AC-3'(SEQ ID NO. :
47)
E45-7-5: 5'-AT AG GG CG CA GG AC-3'(SEQ ID NO. :
48)
E45-8-1: 5'-GG AG GG CC CA GC AC-3'
(SEQ ID NO. :
49)
E45-8-2: 5'-GG AG AG CA CA TC AC-3'(SEQ ID NO. :
50)
E45-8-3: 5'-GG AG CG CG CA CC AC-3'(SEQ ID NO. :
51)
E45-8-4:5'-GG AG CG CG CA GC AC-3'
(SEQ ID NO. :
52)
E45-8-5: 5'-GG AG GG CT CA GC AC-3'
(SEQ ID NO. :
53)
E45-8-6: 5'-GG AG AG CA CA AC AC-3'(SEQ ID NO. :
54)
E45-8-10: 5'-GG AG CG CG CA TC AC-3'
(SEQ ID NO. :
55)
E45-8-11: 5'-GG AG AG CG CA CC AC-3'(SEQ ID NO. :
56)
For proteins in which there are no known sequence to design the library, the
user of the present invention
begins with a single-strand (ss) DNA or RNA thioaptamers with at least 30 nts
in the randomized or
combinatorial regions. Using the methodology created and developed by the
present inventors for creating
both duplex and ss DNA and RNA thioaptamer libraries by both enzymatic and
bead-based methods. One
such technique is the one-bead, one-ODN library ligation reaction in which
short (15 nucleotides) 5'- and
3'- sequences are sufficient to serve as primers for bead-based PCR (Yang et
al., 2002). To achieve even
longer combinatorial segments, it is possible to eliminate entirely one of the
primer segments. High
quality one-bead one-oligo libraries were contructed by join two pieces of DNA
based on an enzymatic
ligation reaction or using highly active phosphorothioate towards 5'-iodo
groups on the ODN. Standard
phosphoramidite chemistry was used for synthesis of 5' monophosphate ODN (5'-
~CCAGGAGATTCCAC-GGATCCGGTGGTCTGT-bead) (SEQ ID NO.: 57). The fully protected
ODN with the non-cleavable linker beads were treated with concentrated ammonia
at 37°C for 21 hours to
remove the protecting groups while allowing the ODN to remain attached to the
beads. A selected single
bead was mixed with the following components: 3 p1 of 40 pM 15 mer
oligonucleotide (5'-
CCTACTCGCGAATTC-3', (SEQ ID NO.: 58) 3 p1 of 10 X ligation buffer, 3 p1 of
DMSO, 2 ~1 of T4
RNA ligase and 19 p1 of ddHzO. The reaction was performed at 5 °C for
17 hrs. The supernatant was
removed carefully and washed with water. The single bead PCR reaction was run
under established
conditions. The PCR products were analyzed on a 15% native polyacrylamide gel.
The PCR product was
cloned using the TA Cloning procedure (Invitrogen) and sequenced on an ABI
Prism 310 Genetic
Analyzer (Applied Biosystems). The desired sequence (5'-CCTACTCGCGAATTC-
P o CCAGGAGATTCCAC-GGATCCGGTGGTCTGT-bead) (SEQ ID NO.: 58) was obtained.
These results show that the additional nucleic acid sequences may be added to
the one-bead, one-ODN
library with high quality and efficiency while maintaining the integrity of
the library. The ligation
reaction allows longer random regions of aptamers to be synthesized on the
beads with higher yield since a
primer region does not have to be stepwise synthesized onto the bead sequence.
The beads were screened
44
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
for the ability to bind the appropriate protein (such as the various NF-xB
dimers or AP1 dimers) labeled
with the Alexa Fluor 488 dye (Molecular Probes) or by binding fluorophor
labeled antibodies as
previously described. ABer thoroughly washing the protein-bound beads with PBS
and 0.1% Tween 20 to
minimize nonspecific binding, the beads are sorted using a multicolor flow
cytometry and cell/bead
sorting to visualize and sort the protein-bound thioaptamer beads and select
the tightest binding
thioaptamer-protein complexes as shown in Figure 6. The most intensely stained
beads will be retrieved.
Initially, the inventors concentrated on the NFxB and AP-1 dimers, but these
methods may be applied by
to other proteins involved in the immune response. Multicolor flow cytometry
was capable of sorting at
speeds of 10g beads per hour or viewed in terms of assays for thioaptamers
binding to target proteins, 10$
assays per hour.
High throughput sorting (HTS) of homo- and heterodimers to thioaptamers by
multi-color flow cytometry
using multi-color flow cytometry HTS may be used to select thioaptamers that
bind preferentially to
heterodimers of proteins. As described above, one monomer is tagged
fluorescently (A) with a dye (cy3
for example) and a different monomer (B) with another dye (cy5 for example).
Both proteins are mixed
together and allowed to bind to the bead thioaptamer library. Next, two-color
flow cytometry is used to
compare cy3/cy5 color levels of each bead. To select homodimers that have high
affinity for homodimer
A.A, beads that have high cy3 levels and low cy5 levels are selected.
Conversely, high cy5/low cy3
indicates a thioaptamer sequence with selectivity for the B.B dimer. For
heterodimers, beads are selected
for cy3/cy5 levels close to 1. SELDI MS may be used to determine which
proteins have been bound to
selected combinatory thioaptamer beads and also used with single bead PCR to
identify which beads) in
the combinatorial library have bound to protein(s).
More than 2 dyes and multi-color flow cytometry may be used to select various
multimers. Thus, for NF-
oB, at least 3 of the 5 different monomeric forms of the protein are combined,
each with a different
fluorphor and use 3-color flow cytometry to select thioaptamers that have high
affinity and selectivity to
homodimers A.A, B.B, C.C and various heterodimeric forms from the libraries.
In principle, there are few
limits to the number of detectable markers (e.g., fluorochromes) that may be
used with the present
invention, e.g., S-color flow cytometry may be used.
Sequencing may also be performed directly on the bead. Each individually
selected bead is washed
thoroughly with 8 M urea (pH 7.2) to remove the protein and directly used for
"one-bead one-PCR"
amplification using the 5' and 3' end primers (Yang, et al. 2002). The PCR
products are TA cloned and
sequenced as previously described to create hybrid thioaptamers with normal
phosphate,
monothiophosphate, and dithiophosphate mixed backbones as well, keeping the
total thiophosphate
backbone below 80% to minimize "non-specific" sticking.
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
The current approach demonstrated in the above examples requires a different
nucleotide sequence to
identify a backbone modification. Thioaptamer libraries were also created that
only differ in the position
of phosphate or dithioate but not in its base sequence. It has been shown that
the positions of
thiophosphates in a mixed backbone S-ODN can be determined by reaction of the
S-ODN with
iodoethanol followed by base catalyzed cleavage of the thiophosphate triester.
This approach was used to
identifying the location of monothio- and dithiophosphate linkages,
independent of base sequence.
Massively parallel, thioaptamer bead-based hts of the host and pathogen
proteome may be used with the
thioselection technology (both enzymatic [S]-ODN and synthetic [S]-ODN /[Sz]-
ODN) to develop
thioaptamers targeting very important proteins (e.g., NF-xB and AP-1) to
identify promising therapeutic
leads. Up to 1000's of different proteins in human and pathogen proteomes by
using a massively parallel,
thioaptamer bead-based HTS of the proteomes with specialized high-throughput
multicolor flow
cytometry/bead sorting in conjunction with SELDITM mass-spectrometric methods
to identify potential
new therapeutic targets both of proteins involved in the immune response to BT
viruses as well as viral
proteins. Thioaptamers may be identified to inhibit the differentially
expressed proteins in host-pathogen
interactions as well as underlying immune response processes and so ameliorate
cytopathological immune
responses resulting in shock or to enhance "innate immunity" to help mount a
more effective immune
response.
Mass spectrometric protein detection technology can be used to identify bound
proteins using HTS of
thioaptamer beads. This approach has significant advantages, since MS is more
sensitive than fluorescent
imaging and will be very useful for low-abundance proteins. In addition, if
more than one protein binds to
a given thioaptamer bead, then it will be possible to identify and quantify
these proteins by SELDI. This
is particularly helpful for identifying non-covalent dimers such as NF-xB or
AP-1 (there are 22 different
monomeric forn~s of AP-1 and thus in principle 100's of different combinations
of dimers possible).
Thioaptamer proteomic arrays were used to demonstrate the use of ProteinChip
array technology (e.g.,
Ciphergen) for protein identification of modified thioaptamer beads or
surfaces. SELDI MS combines the
well-established principles of solid-phase extraction and time-of flight mass
spectrometry in a process
known as surface enhanced laser desorption/ionization time-of flight mass
spectrometry. ProteinChip
Arrays may be customized by covalently attaching affinity reagents such as the
modified thioaptamers to
the spot surface. If the biological marker to be detected is known and
thioaptamer affinity reagents are
available, affinity surfaces can be designed to make use of this specific
thioaptamer-protein interaction.
Also, because SELDI uses mass spectrometric detection, several assays can be
multiplexed easily by
taking advantage of the unique masses of each bound protein.
High-throughput screening (HTS) of thioaptamer libraries by flow cytometry and
SELDI. Bead-based
methods were used to identify both thioaptamer sequences and binding proteins
in parallel, without the
46
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
need to select one thioaptamer for each purified protein. A number of [S]-ODN
or [SZ]-ODN
combinatorial libraries are synthesized, each containing 106 to 109 different,
but related members (or a
library of library with up to 10'4 sequences). The solid-phase split synthesis
described herein may be used
to create thioaptamer-bound bead libraries (one bead, one sequence or one
library) as above. Each library
can be sufficiently different to provide high affinity and selectivity to a
small number of cellular proteins
(such as AP-1 or NF-oB-type sequences). One or more of the thioaptamer library
beads are incubated
with cellular extracts, washed thoroughly to remove weakly bound proteins and
the bound proteins
visualized by direct fluorescent staining with cy3, cy5, SYPRO Ruby, or other
newer dyes for high
sensitivity (sub-nanogram). Fluorescently stained beads can be sorted in the
high-speed cell/bead sorter
for the top 102 or more beads which have the highest amount of bound protein.
The beads selected with
the greatest amount of protein bound will then be analyzed by SELDI MALDI-TOF
mass spectrometric
techniques determine which proteins are bound to each bead; even if more than
one protein binds to the
bead, the thioaptamer may be used to identify a select group of proteins in
cell extracts. The beads
selected are then analyzed by SELDI methods to identify if a fairly limited
number of different proteins
are bound to the specific bead. Alternatively, proteolysis of the proteins on
the bead with trypsin and
analysis of the peptide fragments by LC MS/MS QTOF2 can be used to identify
the proteins on each bead.
After removal of protein from the beads by detergent and urea, the thioaptamer
sequence on the bead can
be determined by the PCR "one bead sequencing" method disclosed herein. Thus,
a random library of
"sticky beads" is selected and an extract containing the complete proteome to
identify both the
thioaptamer sequence on the single beads and the proteins) bound.
HTS of combinatorial libraries to protein mixtures. Besides using cell
extracts, known mixtures of
hundreds of commercially available proteins (cytokines, transcription factors,
etc.) may be applied to the
mixture of thioaptamer bead libraries. HTS cell/bead sorting is used followed
by MS identification of
bound proteins. This involves direct SELDI determination of the protein or
peptide fragmentation
methods followed by MS identification of bound proteins. A major advantage in
using thioaptamers
rather than beads with proteins or monoclonal antibodies attached to them is
that proteolysis and MS
peptide identification is not complicated by proteolysis of bait proteins or
Mab's. This approach can be
used in parallel with other commercially available antibodies for virtually
any protein (particularly AP-1),
and serves as an alternative to the more general screening of the complete
proteome and identification by
SELDI MS methods alone. Once the sequences of the thioaptamers are identified,
these are synthesized in
larger quantities as reagents for diagnostics and therapeutics.
HTS of Thioaptamers Targeting Differentially Expressed Proteins in the
Proteome in virus infected cells.
The thioaptamer-based mufti-color flow cytometry HTS may also be used for
targeting differentially
expressed proteins within the host and pathogen proteomes, combined with MS
detection (SELDI). The
thioaptamer bead-based combinatorial library can be used in conjunction with
fluorescent tagging of
47
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
proteins followed by SELDI MS to identify proteins differentially expressed in
control vs. virus infected
cells. In this simple two-color assay, a combinatorial library (or a
combinatorial library of libraries) of
thioaptamer beads may be synthesized, each bead with a single thioaptamer
sequence (or a combinatorial
library of thioaptamer sequences on each bead). Up to 108 beads can be created
with a single thioaptamer
sequence on each bead. Cell extracts of a sample such as uninfected cells is
labeled fluorescently with a
dye (cy3 for example) as carried out previously and a virus-infected cell
extract is then labeled
fluorescently with another dye (cy5 for example). Both cell extracts are mixed
together and allowed to
bind to the bead thioaptamer library. Next, two-color flow cytometry is used
to compare cy3/cy5 color
levels of each bead. If cy3/cy5 level differs significantly (> 2-fold) from 1,
then the bead was captured.
To determine which proteins) have been bound to selected thioaptamer bead,
SELDI MS will be used to
characterize the bound target further. SELDI MS can be used to determine which
proteins have been
bound to selected combinatory thioaptamer libraries and also used with single
bead PCR to identify which
beads) in the combinatorial library have bound to protein(s). As shown above,
Ciphergen's ProteinChip
epoxy modified surfaces may be used to covalently attach 5'-amino-linker
thioaptamers to beads.
Ciphergen's ProteinChip array technology allows for solid-phase extraction to
desorb more weakly bound
proteins to thioaptamer surfaces, followed by surface enhanced laser
desorption/ionization time-of flight
mass spectrometry (SELDI-MS). Other diseases besides viral infections may be
similarly targeted using
the thioaptamers, systems and methods disclosed herein.
HTS of thioaptamers targeting differentially expressed proteins in the
proteome in virus infected cells
relative to treated cells ("High Throughput Pharmacoproteomics"). In this
embodiment, three-color
thioaptamer library bead sorting is used. In this three-color assay, a
combinatorial library (or a
combinatorial library of libraries) of thioaptamer beads is synthesized, each
bead with a single thioaptamer
sequence (or a combinatorial library of thioaptamer sequences on each bead).
Up to 10$ beads with a
single thioaptamer sequence on each bead (or 10'4 sequences on the library of
libraries) are made.
Uninfected cell extracts (or control extracts) are labeled fluorescently with
a cy3 for example. A virus-
infected cell extract (or any disease cell extract such as cancerous cells) is
labeled fluorescently with cy5,
and then a thioaptamer therapeutic treated, virus infected (or other disease)
cell culture is labeled with a
third dye. The three proteome cell extracts are mixed together in equal total
protein quantities and
allowed to bind to the bead thioaptamer library (or library of libraries).
Three-color flow cytometry is
used to compare cy3/cy5/dye 3 color levels of each bead. If cy3/cy5 level
differs from 1 (uninfected vs.
infected) and cy5/Sypro Ruby differs from 1 (infected vs. infected and
treated) differs from 1, then the
bead can be captured. Such a control assures that the thioaptamer drug
previously identified as a
promising lead does affect specific protein levels. To determine which
proteins) have been bound to
selected thioaptamer beads, SELDI MS can be used to characterize the proteins
bound to the target bead.
48
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
In one embodiment of the invention a complex of combinatorial libraries are
created in which multiple
transcription factor-like sequences with varying thiophosphate substitution
patterns are concatenated in a
single long sequence so that it can bind to multiple transcription factors
such as NF-xB, AP-1, SP-1, GRE,
SRE, etc., requiring a thioaptamer sequence of at least 20-40-mers. These
embodiments provide an
attractive approach to defining therapeutic strategies in which multiple
proteins can be targeted with
multiple thioaptamers. Such a combination (adjuvant) of drug therapeutics is
needed to improve immune
responses in cancer, AIDS, etc. Mammalian protein signaling pathways are often
redundant so that if one
pathway is affected, another can take over control. By perturbing multiple,
highly interwoven pathways, a
greater opportunity to modulate the immune response network is made available.
HT flow cytometry and bead selection. High-throughput screening (HTS) of
thioaptamer beads using
high-speed multicolor flow cytometry/cell sorting is used. In principle, more
than 10'° beads could be
screened within a single day, and specific bead subpopulations could be sorted
for subsequent proteomics
analysis. This group also has considerable experience in HTS of cells and
bacteria (as well as beads) for
subsequent molecular characterizations by PCR and gene expression microarray
analysis.
Advanced HTS technologies may be used for large library screening and
functional genomics. Single-cell
(or bead) sorting of rare subpopulations may be used to isolate single beads
from combinatorial libraries.
A special high speed sorter uses a unique two-stage signal processing system,
configured in hardware as a
single layer neural network, which allows for sophisticated cell or bead
classifications based on
multivariate statistics or learning through neural networks.
A 6-color high-speed flow cytometer/cell sorter is configured in hardware and
software as a single-layer
neural network that can also be used to generate real-time sort decisions on
the basis of multivariate
statistical classification functions. While it can perform the usual two-way
sorts it is commonly used in
"straight-ahead" sorting mode to allow for extremely high sort recovery and
purity at high throughput
rates or to efficiently sort single cells for cloning or for subsequent
molecular characterizations by PCR.
Multi-color flow cytometry as a quantitation and validation tool for
proteomics. These capabilities can
also be used to sort for thioaptamers that bind heterodimers or more complex
protein mixtures. By using
different fluorescently labeled dyes bound to specific proteins, beads are
sorted simultaneously that bind
homodimers and heterodimers. A covalently labeled p50 with Alexa-Fluor 488 dye
was isolated (data not
shown) and carried out 1- and 2-color thioaptamer bead sorting.
Production of large quantities of hybrid dithiophosphate aptamer. Using
chemistry developed
independently in both Caruthers' and Gorenstein's laboratory, the most
promising dithioate hybrid
backbone aptamers show good in vitro and in vivo binding to the targets will
be synthesized (Cho et al.,
49
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
1993; Farschtschi & Gorenstein, 1988; Gorenstein et al., 1990; Gorenstein et
al., 1992; Piotto et al., 1991)
on a 5-10 pmole scale and purified (Mono Q; Yang et al., 1999; 2002).
Preparation of nuclear and cytoplasmic extracts was conducted at various times
after virus infection, and
parallel uninfected control cultures of 5 x 10' cells are harvested and
collected by centrifugation. Cell
pellets are resuspended and washed in phosphate buffered saline (PBS). Next,
cells are lysed and the
cytoplasmic and nuclear fractions isolated. The nuclei are purified by
centrifugation through a cushion of
2M sucrose before protein extraction. The protein content in all fractions
will be determined by BCA
Assay according the manufacturer's directions (Pierce, Rockford, IL).
Mass spectrometric identification of bound proteins. As demonstrated above,
sorted "positive" beads can
be subjected to SELDI-MS analysis to confirm the identity of the proteins
bound to the thioaptamer beads
of the present invention (via MALDI MS molecular ion characterization). In
cases where the "positive"
bead's thioaptamer might have bound not only the target protein but other
proteins in a sample, e.g., a
secondary or even tertiary, etc. protein, SELDI-MS may be used to identify
this event through the
detection of multiple molecular ions.
Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS). For proteins which
cannot be
identified from the MI, proteolysis and multidimensional LC applying 2D
chromatographic separation of
peptides is used on-line with MS analysis (Link et al., 1999; Washburn et al.,
2001). This LC tandem MS
approach is carried out using strong cation exchange (SCX) chromatography
combined with reversed-
phase (RP) chromatography. Using a salt step gradient, tryptic peptides of
complexes are eluted from the
SCX column onto the RP column, and contaminants of salts and buffers are
washed to waste using a
diverter valve. Peptides are subsequently eluted from the RF column directly
into the MS, either for mass
fingerprinting, or for MS/MS sequence analysis. This LC tandem MS procedure is
very useful for small
amounts (femtomol) of complex. Yet another procedure is tandem LC/tandem MS.
The proteomes can be
either human, GP, hampster or mouse - human and mouse genome databases are
available.
LC or 2D SDS-PAGE and MS. These techniques are currently the major analytical
tools used to identify
proteins in the proteome. Thioaptamer bead libraries may be used to
differentially screen the proteomes,
using 2D gel analysis for differential analysis of protein expression. To
improve the comparative analysis
of gel imaging imaging software may be used to improve result resolution,
e.g., using Nonlinear USA, Inc.
(Progenesis). The automated imaging features of this 2D imaging software
reduce gel evaluation times
substantially and are an important step towards hands-free analysis.
2D gel electrophoresis. 2D PAGE can be conducted essentially as first
described by (O'Farrell, 1975).
High-throughput may be employed Pharmacia's IPGphor multiple sample IEF device
or the first
dimension, and Biorad's multiple gel SDS-PAGE systems (Protean Plus and
Criterion dodeca cells) for
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
the second. Gels will be stained with either SYPRO Ruby for high sensitivity
(sub-nanogram) or
Coomassie Blue when less sensitivity is required. Image analysis of gels will
be achieved with a Perkin
Elmer (PE) ProEXPRESS Proteomic Imaging System using Nonlinear's Progenesis
imaging software. A
Genomic Solutions' robotics recently purchased is utilized for protein spot
picking and for sample trypsin
S hydrolysis (Proteomic Protein Picker), and sample clean-up, and sample
application to MALDI plates
(ProPrep 4 Block System). Mass fingerprinting for protein identification may
use an Applied Biosystems
(AB) matrix-assisted laser desorption/ionization (MALDI) time-of flight (TOF)
Voyager DE STR MS.
Proteins will be identified with the Voyager's Prospector software. De novo
sequencing and analysis of
posttranslational modifications can be achieved by electrospray (ESI) MS/MS
(capillary LC nanoflow
option).
Isotope-coded affinity tags (ICAT). Some differential protein expression use
isotope-coded affinity tags
(ICATs) for quantitative analysis of complex protein mixtures (Gygi et al.,
1999). In this procedure, there
is an option to fractionate proteins before to proteolysis decreases the
complexity of proteins analyzed.
While this invention has been described in reference to illustrative
embodiments, this description is not
intended to be construed in a limiting sense. Various modifications and
combinations of the illustrative
embodiments, as well as other embodiments of the invention, will be apparent
to persons skilled in the art
upon reference to the description. It is therefore intended that the appended
claims encompass any such
modifications or embodiments.
51
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
Reference List
Aronson, J.F., Herzog, N.K., & Jerrells, T.R. (1994) "Pathological and
virological features of arenavirus
disease in guinea pigs: Comparison of two Pichinde virus strains,"
Am.J.Pathology 145, 228-235.
Aronson, J.F., Herzog, N.K., & Jerrells, T.R. (1995) "Tumor Necrosis Factor
(TNF) Plays a Role in the
Pathogenesis of Guinea Pig Arenavirus Disease," Amer.J.Trop.Med.Hygien 52, 262-
269.
Bachelin, M., Hessler, G., Kurz, G., Hacia, J.G., Dervan, P.B., & Kessler, H.
(1998) "Structure of a
stereoregular phosphorothioate DNA/RNA duplex," Nature Struct.Biol. S, 271-
276.
Berglund, J.A., Charpentier, B., & Rosbash, M. (1997) "A High Affinity Binding
Site for the HIV-1
Nucleocapsid Protein," Nucleic Acid Res. 25(5): 1042-1049.
Bielinska, A., Shivdasani, R.A., Zhang, L.Q., & Nabel, G.J. (1990) "Regulation
of Gene Expression with
Double-Stranded Phosphorothioate Oligonucleotides," Science 250, 997-1000.
Boccaccio, C., Ando, M., Tamagnone, L., Bardelli, A., Michieli, P.,
Battistini, C., & Comoglio, P.M.
(1998) "Induction of Epithelial Tubules by Growth Factor HGF Depends on the
STAT Pathway," Nature
391, 285-288.
Brill, W.K.D., Nielsen, J., & Caruthers, M.H. (1988) "Synthesis of
Dinucleoside Phosphorodithioates via
Thioamidites," Tetrahedron Lett. 29, 5517-5520.
Brill, W.K.D., Tang, J.Y., Ma, Y.X., & Caruthers, M.H. (1989) "Synthesis of
Oligodeoxynucleoside
Phosphorodithioates via Thioamidites," J.Am.Chem.Soc. 111, 2321-2322.
Burke, D.H., Scates, L., Andrews, K., & Gold, L. (1996) "Bent Pseudoknots and
Novel RNA Inhibitors of
Type 1 Human Immunodeficiency Virus (HIV-1) Reverse Transcriptase," J. Mol.
Biol. 269, 650-666.
Carteau, S., Batson, S.C., Poljak, L., Mouscadet, J.F., Derocquigny, H.,
Darlix, J.L., Kas, E., & Auclair, C.
(1997) "Human Immunodeficiency Virus Type 1 Nucleocapsid protein Specifically
Stimulates MG2+-
Dependent DNA Inegration in vitro," J. Virol. 71 , 6225-6229.
Chen, F.E., Huang, D.B., Chen, Y.Q., & Gosh, G. (1998) "Crystal Structure of
P50/P65 Heterodimer of
Transcription Factor NF-oB Bound to DNA," Nature S, 391-410.
Chen, H. & Gold, L. (1994) "Selection of high-affinity RNA ligands to reverse
transcriptase: Inhibition of
cDNA synthesis and RNase H activity," Biochemistry 33, 8746-8756.
Chen, Y.Q., Ghosh, S., & Ghosh, G. (1998) "A novel DNA Recognition Mode by the
NK-KB P65
Homodimer," Nat.Struct.Biol. 5, 67.
Cho-Chung, Y.S. (1998) "CRE-Palindrome Oligonucleotide as a Transcription
Factor Decoy and an
Inhibitor of Tumor Growth," Antisense & Nucleic Acid Drug Development 8, 167-
170.
Cho, Y.S., Zhu, F.C., Luxon, B.A., & Gorenstein, D.G. (1993) "2D H-1 and P-31
NMR Spectra and
Distorted A-DNA-Like Duplex Structure of a Phosphorodithioate
Oligonucleotide," J.Biomol.Struct.Dyn.
1l, 685-702.
Ciafre, S.A., Rinaldi, M., Gasparini, P., Seripa, D., Bisceglia, L., Zelante,
L., Farace, M.G., & Fazio, V.M.
(1995) "Stability and functional effectiveness of phosphorothioate modified
duplex DNA and synthetic
'mini-genes'," Nucleic Acids Research 23, 4134-4142.
Cummins, D. (1990) "Lassa fever.," Br. J. Hosp. Med. 43, 186-192.
52
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
Dyer, R.B. & Herzog, N.K. (1995) "Immunodepletion EMSA: A Novel Method to
Identify Proteins in a
Protein/DNA Complex," Nucleic Acids Res. 23, 3345-3346.
Dyer, R. B. and Herzog, N. K. (1995). Isolation of Intact Nuclei for Nuclear
Extract Preparation from
Fragile B-Lymphocyte Cell Lines. Biotechniques 19, 192-195.
Dyer, R. B., Collaco, C., Niesel, D. W., and Herzog, N. K. (1993). Shigella
flexneri invasion of HeLa cells
induces ~B DNA-binding activity. Infection and Immunity 61, 4427-4433.
Eckstein, F. (1985) "Nucleoside Phosphorothioates," Ann.Rev.Biochem. 54, 367-
402.
Ekland, E.H., Szostak, J.W., & Bartel, D.P. (1995) "Structurally complex and
Highly Active RNA Ligases
Derived from Random RNA Sequences," Science 269, 364-370.
Eleouet, J.F., Chilmonczyk, S., Besnardeau, L., & Laude, H. (1998)
"Transmissible Gastroenteritis
Coronavirus Induces Programmed Cell Death in Infected Cells through a Caspase-
Dependent Pathway,"
J. Virol. 72, 4918-4924.
Farschtschi, N. & Gorenstein, D.G. (1988) "Preparation of a Deoxynucleoside
Thiophosphoramidite
Intermediate in the Synthesis of Nucleoside Phosphorodithioates," Tetrahedron
Lett. 29, 6843-6846.
Felder, E.R. (1999) Resins, Linkers And Reactions For Solid-Phase Synthesis Of
Organic Libraries. In
Miertus, S. (ed.), In Combinatorial Chemistry and Technology, Principles,
Methods and Applications.
Marcel Dekker, Inc., NY, pp. 35-S 1.
Fennewald, S. M., Aronson, J. F., Zhang, L., and Herzog, N. K. (2002).
Alterations in NF-xB and RBP-Jx
by arenavirus infection of macrophages in vitro and in vivo. Journal of
Virology 76, 1154-1162.
Ghosh, G., Van Duyne, G., Ghosh, S., & Sigler, P.B. (1995) "Structure of NF-xB
P50 Homodimer Bound
to NF-KB Site," Nature 373, 303-310.
Gold, L., Brown, D., He, Y.Y., Shtatland, T., Singer, B.S., & Wu, Y. (1997)
"From Oligonucleotide
Shapes to Genomic Selex - Novel Biological Regulatory Loops,"
Proc.Natl.Acad.Sci. U.S.A. 94, 59-64.
Gold, L., Polisky, B., Uhlenbeck, O., & Yarus, M. (1995) "Diversity of
oligonucleotide functions,"
Annu.Rev.Biochem. 64, 763-797.
Goldfeld, A.E., Doyle, C., & Maniatis, T. (1990) "Human tumor necrosis factor
alpha gene regulation by
virus and lipopolysaccharide.," Proceedings of the National Academy of
Sciences of the USA 87, 9769-
9773.
Gonzalez, C., Stec, W., Reynolds, M.A., & James, T.L. (1995) "Structure and
dynamics of a DNA center
dot RNA hybrid duplex with a chiral phosphorothioate moiety: NMR and molecular
dynamics with
conventional and time-averaged restraints," Biochemistry 34, 4969-4982.
Gorenstein, D.G. (1994) "Conformation and Dynamics of DNA and Protein-DNA
Complexes by 31P
NMR," Chem.Rev. 94, 1315-1338.
Gorenstein, D.G. and Farschtschi, N., U. S. Patent #5,218,088, Process for
Preparing Dithiophosphate
Oligonucleotide Analogs via Nucleoside Thiophosphoramidite Intermediates
(Awarded, June 8, 1993;
Applied 1989).
Gorenstein, D.G., Fanni, T., Taira, K., & Farschtshi, N. (1990)
"Stereoelectronic Effects in the Hydrolysis
of Phosphonium Ions from Acyclic and Bicyclic Phosphates and
Phosphorothionates,"
Phosphorus,Sulfur,and Silicon 47, 93-104.
53
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
Gorenstein, D.G., Karslake, C., Granger, J.N., Cho, Y., & Piotto, M.E. (1992)
in Phosphorus Chemistry
(ACS Symposium Series) (Walsh, E.N., Griffith, E.J., Parry, R.W., & Quin,
L.D., Eds.) pp 202-217,
American Chemical Society, Washington, DC.
Gorenstein, D.G, King, D.J., Ventura, and D.A., Brasier, A.R. U. S. Patent
"Combinatorial Selection of
Oligonucleotide Aptamers" (Applied October, 1998).
Gorenstein, D.G., Herzog, N., Aronson, J., and Luxon, B., U. S. Patent
pending, "Thio-modified Aptamer
Synthetic Methods and Compositions" (Applied October, 1999).
Gorenstein, D.G., Herzog, N., Aronson, J., and Luxon, B., Foreign patent
pending, "Thin-modified
Aptamer Synthetic Methods and Compositions" (Applied October, 1999).
Gorenstein, D. G., Luxon, B., Herzog, N. and Yang, X.B., Patent application,
"Phosphoromonothioate and
Green, L., Waugh, S., Binkley, J.P., Hostomska, Z., Hostomsky, Z., & Tuerk, C.
(1995) "Comprehensive
chemical modification interference analysis of an RNA pseudoknot inhibitor to
HIV-1 reverse
transcriptase," J.Mol.Biol. 247, 60-68.
Hilaire, P.M.St. and Meldal, M. (2000) Glycopeptide and oligosaccharide
libraries. Angew. Chem. Int.
Ed., 39, 1162-1179.
Holland, J.H. (1994) in AnonymousMIT Press, Cambridge, MA.
Jahrling, P.B., Hesse, R.A., Rhoderick, J.B., Elwell, M.A., & Moe, J.B. (1981)
"Pathogenesis of
Pichinde virus strain adapted to produce lethal infections in guinea pigs,"
Infect.lmmun. 32, 872-880.
Jhaveri, S., Olwin, B., & Ellington, A.D. (1998) "In Vitro Selection of
Phosphorothiolated Aptamers,"
Bioorganic and Medicinal Chemistry Letters 8, 2285-2290.
Jin, G. & Howe, P.H. (1997) "Regulation of Clusterin Gene Expression by
Transforming Growth Factor
b," J.Biol.Chem. 272, 26620-26626.
King, D.J., Ventura, D.A., Brasier, A.R. and Gorenstein, D.G., "Novel
Combinatorial Selection of
Phosphorothioate Oligonucleotide Aptamers," Biochemistry, 37,16489-16493
(1998).
King, D.J., Bassett, S.E., Li, X., FermewaldS.A., Herzog, N.K., Luxon, B.A.,
Shope, R., & Gorenstein,
D.G. (2002) "Combinatorial selection and binding of phosphorothioate aptamers
targeting human NF
kappa B Rel A and p50.," Biochemistry, in press.
Lackey, D.B. & Patel, J. (1997) "Biomedical Synthesis of Chirally Pure RP
Oligonucleotide
Phosphorothioates," Biotechn.Lett. 19, 475-478.
Lam, K.S., et al., (1991) "A new type of synthetic peptide library for
identifying ligand-binding activity.
Nature, 354, 82-84.
Lam, K.S. (1995) Synthetic peptide libraries. In Molecular Biology and
Biotechnology: A Comprehensive
Desk Reference. Meyer, R.A. (ed.) p.880. VCH Publisher:NY.).
Leary JF, Schmidt DF, Gram JG, McLaughlin SR, Dalla TC, Burde S: High-speed
flow cytometric
analysis and sorting of human fetal cells from maternal blood for molecular
characterization. Annals of the
New York Academy of Sciences 1994, 731:138-141.
Leary, J.F., Corio, M.A., McLaughlin, S.R. System for High-Speed Measurement
and Sorting of Particles
U.S. Patents 5,204,884 (1993) and 5,804,143 (1998)
54
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
Leary, J.F. Strategies for Rare Cell Detection and Isolation In: Methods in
Cell BioloQV: Flow Cytometry
(Edited by Z. Darzynkiewicz, J.P. Robinson, H.A. Crissman) vol. 42: pp. 331-
358 (1994).
Leary, J.F., Schmidt, D., Gram, J.G., McLaughlin, S.R., DellaTorre, C., Ellis,
S.P. Isolation of Rare Cells
by High-Speed Flow Cytometry and High-Resolution Cell Sorting for Subsequent
Molecular
Characterization-Applications in Prenatal Diagnosis, Breast Cancer and
Autologous Bone Marrow
Transplantation In: Basic and Clinical Applications of Flow Cytometry. F.A.
Valeriote, A. Nakeef , M.
Valdivieso, Eds., Kluwer Academic Publishers, Boston, 1996, pp. 271-318.
Leary, J.F., McLaughlin, S.R., Kavanau, K. New Methods for Detection, Analysis
and Isolation of Rare
Cell Populations SPIE 2982: 240 - 253, 1996.
Leary, J.F., He, F., Reece, L.N. Detection and Isolation of Single Tumor Cells
Containing Mutated DNA
Sequences SPIE 3603: 93-101,1999.
Lebedev, A.V. & Wickstrom, E. (1996) "The Chirality Problem in P-Substituted
Oligonucleotides,"
Perspectives in Drug Discovery & Design 4, 17-40.
Lebruska, L.L. & Maher, LL.J. (1999) "Selection and Characterization of an RNA
Decoy for
Transcription Factor NF-xB.," Biochemistry 38, 3168-3174.
Liang, R., Yan, L., Loebach, J., Ge, M., Uozumi, Y., Sekanina, K., Horan, N.,
Gildersleeve, J., Thompson,
C., Smith, A., Biswas, K., Still, W.C., & Kahne, D. (1996) "Science," Science
274, 1520-1522.
Liang, R., et al., (1996) Parallel synthesis and screening of a solid phase
carbohydrate library. Science,
274, 1520-1522.
Lin, K.L, DiDonato, J.A., Hoffmann, A., Hardwick, J.M., & Ratan, R.R. (1998)
"Suppression of steady-
state, but not stimulus-induced NF-xB activity inhibits alphavirus-induced
apoptosis," J.Cell.Biol. 141,
1479-1487.
Mann, M.J. (1998) "E2F Decoy Oligonucleotide for Genetic Engineering of
Vascular Bypass Grafts,"
Antisense 8c Nucleic Acid Drug Development 8, 171-176.
Marshall, W.S. & Caruthers, M.H. (1993) "Phosphorodithioate DNA as a potential
therapeutic drug.
[Review]," Science 259, 1564-1570.
Milligan, J.F. & Uhlenbeck, O.C. (1989) "Determination of RNA-protein contacts
using thiophosphate
substitutions," Biochemistry 28, 2849-2855.
Morishita, R., Gibbons, G.H., Horiuchi, M., Ellison, K.E., Nakama, M., Zhang,
L., Kaneda, Y., Ogihara,
T., & Dzau, V.J. (1995) "A Gene Therapy Strategy Using a Transcription Factor
Decoy of the E2F
Binding Site Inhibits Smooth Muscle Proliferation in Vivo,"
Proc.Natl.Acad.Sci. USA. 92, 5855-5859.
Morishita, R., Higaki, J., Tomita, N., & Ogihara, T. (1998) "Application of
Transcription Factor "Decoy"
Strategy as means of Gene Therapy and Study of Gene Expression in
Cardiovascular Disease,"
Circulation Research 82, 1023-1028.
Morishita, R., Sugimoto, T., Aoki, M., Kida, L, Tomita, N., Moriguchi, A.,
Maeda, K., Sawa, Y., Kaneda,
Y., Higaki, J., & Ogihara, T. (1997) "In vivo Transfection of cis Element
"Decoy" against Nuclear Factor-
xB Binding Site Prevents Myocardial Infarction.," Nature Medicine 3, 894-899.
Muller, C.W., Rey, F.A., Sodeoka, M., Verdine, G.L., & Harrison, S.C. (1995)
"The NK-xB P50
Homodimer Bound to DNA," Nature 1995, 311-317.
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
Murphy, K., Haudek, S.B., Thompson, M., & Giroir, B.P. (1998) "Molecular
biology of septic shock,"
New Horizons 6, 181-193.
Nakamaye, K.L., Gish, G., Eckstein, F., & Vosberg, H.P. (1988) "Direct
sequencing of polymerase chain
reaction amplified DNA fragments through the incorporation of deoxynucleoside
alpha-
thiotriphosphates," Nucleic Acids Res. 16, 9947-9959.
Osborne, S.E., Matsumara, L, & Ellington, A.D. (1997) "Aptamers as Therapeutic
and Diagnostic
Reagents: Problems and Prospects," Current Opinion in Chem.Biol. I, 5-9.
Otwinowski, Z., Schevitz, R.W., Zhang, R.G., Lawson, C.L., Joachimiak, A.,
Marmorstein, R.Q., Luisi,
B.F., & Sigler, P.B. (1988) "Crystal structure of trp repressor/operator
complex at atomic resolution,"
Nature 335, 321.
Park, Y.G., Nesterova, M., Agrawal, S., & Cho-Chung, Y.S. (1999) "Dual
Blockade of Cyclic AMP
Response Element- (CRE) and AP-1 directed Transcription by CRE-transcription
Factor Decoy
Oligonucleotide," J.Biol.Chem. 274, 1573-1580.
Peters, C.J., Jahrling, P.B., Liu, C.T., Kenyon, R.H., McKee, Jr.K.T., &
Barrera-Oro, J.G. (1987)
"Experimental studies of arenavirus hemorrhagic fevers," Current Topics in
Microbiol.and Immunol. 134,
5-68.
Schneider, D.J., Feigon, J., Hostomsky, Z., & Gold, L. (1995) "High-affinity
ssDNA inhibitors of the
reverse transcriptase of type 1 human immunodeficiency virus," Biochemistry
34, 9599-9610.
Sharma, H.W., Perez, J.R., Higgins-Sochaski, K., Hsiao, R., & Narayanan, R.
(1996) "Transcription factor
decoy approach to decipher the role of NF-kappa B in oncogenesis," Anticancer
Res. 16, 61-69.
Stec, W.J., Grajkowski, A., Koziolkiewicz, M., & Uznanski, B. (1991) "Novel
Route to
Oligo(Deoxyribonucleoside Phosphorothioates) - Stereocontrolled Synthesis of P-
Chiral Oligo (Deoxy-
ribonucleoside Phosphorothioates)," Nucleic Acids Res. 19, 5883-5888.
Sterner, J.M. & Leary, J.F. (1989) "Use of biocarrier beads and flow cytometry
for single-cell studies of
fibronectin gene regulation in dibutyrl cyclic AMP reverse transformed CHO-K1
cells," Cell Biophysics
IS,
Tian, Y., Adya, N., Wagner, S., Giam, C.Z., Green, M.R., & Ellington, A.D.
(1995) "Dissecting
protein:protein interactions between transcription factors with an RNA
aptamer," RNA. 1, 317-326.
Tomita, N., Morishita, R., Higaki, J., & Ogihara, T. (1997) "A Novel Strategy
for Gene Therapy and Gene
Regulation Analysis Using Transcription Factor Decoy Oligonucleotides,"
Exp.Nephrol. S, 429-434.
Velasco, M., Diaz-Guerra, M.J.M., Martin-Sanz, P., Alvarez, A., & Bosca, L.
(1997) "Rapid up-regulation
of IxBb and abrogation of NF-xB activity in peritoneal macrophages stimulated
with lipopolysaccharide,"
J.Biol.Chem. 272, 23025-23030.
Volk, D., Yang, X.-B., Fennewald, S.M., Bassett, S., Venkitachalam, S.,
Herzog, N., Lluxon, B., &
Gorenstein, D. Bioorganic Chemistry (in press).
Wang, S., Lee, R. J., Cauchon, G., Gorenstein, D.G. and Low, P.S. "Delivery of
Antisense
Oligonucleotides against the Human Epidermal Growth Factor Receptor into
Cultured KB Cells with
Liposomes Conjugated to Folate via polyethylene glycol," Proc. Natl. Acad.
Sci., U.S.A., 92 3318-3322
( 1995).
Yang, X., Fennewald, S., Luxon, B.A., Aronson, J., Herzog, N.K., & Gorenstein,
D.G. (1999) " Aptamers
56
CA 02526853 2005-11-23
WO 2005/018537 PCT/US2004/016246
containing thymidine 3'-O-phosphorodithioates: synthesis and binding to
nuclear factor -xB," Bioorganic
& Med.Chem.Lett. 9, 3357-3362.
Yang, X., Bassett, S.E., Li, X., Luxon, B.A., Prow, R.K.T.W., Leary, J.F.,
Ellington, A., & Gorenstein,
D.G. (2002a) "Construction and selection of bead bound combinatorial
oligonucleoside phosphorothioate
and phosphorodithioate aptamer libraries designed for rapid PCR-based
sequencing, " Nucleic Acids Res.
30, 1-8.
Yang, X-B., Hodge, R., Luxon, B.A., Shope, R., Gorenstein, D. G. 2002b),
"Separation of Synthetic
Oligonucleotide Dithioates from Monothiophosphate Impurities by Anion-exchange
Chromotography on a
Mono Q Column, Analyt. Biochem., 306, 92-99.
Zhu, T., and Boom, G.J. (1998) A two-directional approach for the solid-phase
synthesis of trisaccharide
libraries. Angew. Chem. 1n1. Ed., 37, 1898-1900.
57