Note: Descriptions are shown in the official language in which they were submitted.
CA 02527025 2011-11-14
-1-
COOLING A FINAL PRODUCT TO HEAT A FURNACE GAS
The present invention relates to a process in which a starting material is
converted into a
final product by reaction of a gas at elevated temperature. The process is
particularly
concerned with using the thermal energy of the final product to raise the
temperature of the
gas prior to the reaction of the gas with the starting material. The intention
here is to
enhance the thermal efficiency of the system. The invention also relates to an
apparatus
suitable for carrying out the process.
The present invention will be described with particular reference to the
reduction of nickel
oxide by reaction with hydrogen at elevated temperature to form nickel metal.
However, it
is to be appreciated that the principle underlying the invention is more
generally applicable
and may be employed with other reaction systems that rely on the same basic
tenets.
It is conventional in the art to reduce nickel oxide to nickel in a furnace at
elevated
temperature using hydrogen as the reducing agent. Hydrogen is also used to
maintain a
reducing atmosphere for the product when formed in order to prevent re-
oxidation of the
metal at elevated temperature. Furthermore, hydrogen is used in the cooling
system
(cooler) employed to lower the temperature of the product prior to discharge
to the air.
In such cooling systems hydrogen is passed over the hot product (nickel)
thereby achieving
rapid cooling. The hydrogen also provides a reducing atmosphere and this
prevents re-
oxidation of the product. Heat is removed from the hydrogen by thermal
exchange with a
suitable medium, such as water, which is then piped away for heat removal
before being
re-circulated to extract further heat from the hydrogen. The cooled hydrogen
is re-
circulated and re-used to provide rapid cooling of hot product. The use of
hydrogen as
coolant means that the rate of cooling is greatly accelerated when compared
with quiescent
cooling. In turn, this means that a more compact (shorter) cooling system can
be employed
when hydrogen is used.
Hydrogen which is used in the cooling process is used upstream in the furnace
for the
CA 02527025 2005-11-25
PCT/AU20041000837
P.'WPEINaU21666l Mwmdmaudw,31MNS = - Received 31 March 2005
-2-
reduction reaction. However, as this hydrogen exits the cooling system at a
temperature of
about 150 C, it is necessary to increase the temperature of it significantly
before it is
delivered to the furnace, and here heaters may be used to pre-heat the
hydrogen.
Alternatively, the temperature of the hydrogen may be increased using heat
energy from
the furnace, resulting in a drop in temperature of the furnace. Additional
energy is
invariably also supplied to the furnace to counteract the chilling effect
caused by
introduction of hydrogen at a temperature relatively lower than the operating
temperature
of the furnace. This leads to an increase in the cost of constructing a
reduction reactor due
to the need to employ additional equipment (heaters for the hydrogen) and in
operating the
reactor due to higher energy demands.
The present invention seeks to overcome these disadvantages by using thermal
energy of
the final product to heat the gas reactant (hydrogen in the case illustrated)
to a temperature
higher than that achieved using the conventional technique described above.
The invention
also seeks to provide an apparatus which includes specific structural
components to
achieve this end. These structural components may add to the overall cost of
the apparatus
but this expense would be at least off-set by the savings in energy and/or the
reduction in
other furnace hardware associated with the invention.
Accordingly, in one aspect the present invention provides a process for
converting a
starting material into a final product by reaction of the starting material
with a gas in a
furnace at elevated temperature followed by cooling of the final product using
a cooler,
wherein, prior to introduction of the gas into the furnace the temperature of
the gas is
elevated by use of a thermal exchange means which is provided between the
furnace and
the cooler and which facilitates the transfer of thermal energy associated
with the final
product to the gas and wherein the thermal exchange means comprises a series
of
individual baffle plates.
Central to the present invention is the use of a thermal exchange means to
transfer thermal
energy from the final product to the gas used to effect conversion of the
starting material.
The thermal exchange means is intended to absorb radiant heat from the final
product and
convey this heat to the gas by convection and conduction. As will be explained
in more
Amended Sheet
IPEA/AU
CA 02527025 2005-11-24
WO 2005/001362 PCT/AU2004/000837
-3-
detail below, the thermal exchange means may be configured and positioned to
maximise
heat transfer to the gas.
In the process of the present invention the conversion of starting material to
final product
takes place at elevated temperature. The resultant final product exits the
furnace at
elevated temperature. The present invention resides in using thermal energy
associated
with the final product to heat the gas which is used in the conversion
reaction. This heat
transfer takes place upstream of the cooler which is used to cool the final
product.
The gas being heated by use of the thermal exchange means is the same gas as
used to
effect conversion of the starting material to the final product in the
furnace. Contacting the
final product with this gas therefore also prevents any reverse reaction that
would
otherwise lead to reformation of the starting material. Thus, if the
conversion is the
reduction of nickel oxide using hydrogen, the use of hydrogen in the thermal
exchange
means maintains a reducing atmosphere that prevents oxidation of the nickel
product. In
this way the gas used in the thermal exchange means provides cooling of the
final product
under conditions that will prevent reformation of the starting material.
Hydrogen is also
used during annealing of steel and stainless steel continuous strip to prevent
oxidation that
would otherwise occur. Cooling of the final product by use of the thermal
exchange means
will also reduce the load on the cooler to which the final product is
subsequently delivered.
The present invention may be applied to a variety of chemical conversions in
which a
starting material is reacted with a gas at elevated temperature to yield a
final product which
is also at elevated temperature. For instance, the conversion may be a
reduction in which a
metal oxide is reacted with a reducing gas, to yield the metal. One such
conversion is that
of nickel oxide to nickel using hydrogen. This usually takes place at a
temperature of
about 800 to about 1200 C, for example, about 900 to about 1000 C. Usually,
the starting
material and final product are both solids, although this is not essential.
The invention is
especially useful in processes that require the same gas for effecting the
chemical reaction
and for contributing to cooling of the final product. As explained above,
hydrogen is often
used as process gas and coolant. The invention may also be useful for
processes in which
CA 02527025 2005-11-24
WO 2005/001362 PCT/AU2004/000837
-4-
impurities are removed from a starting material by reaction with a gas at
elevated
temperature. For example, hydrogen may be used to remove sulphur from metal
(e.g.
nickel) briquettes.
It will be appreciated that the present invention relies on the existence of a
final product at
elevated temperature in order to heat the gas used for the necessary chemical
conversion.
It follows from this that the present invention is not useful on start-up.
However, the
chemical conversions to which the present invention is applicable tend to be
run
continuously on an industrial scale. Thus, once start-up has been effected the
process of
the present invention may also be run continuously. Typically, when the
process of the
invention is up and running, all of the gas delivered to the furnace will have
been heated in
accordance with the present invention without requiring the addition of energy
from an
external source.
An important aspect of the present invention is the thermal exchange means
which is used
to transfer heat energy of the final product to the gas before the gas is
introduced into the
furnace where the required chemical conversion takes place. Preferably, the
thermal
exchange means is configured to optimise the amount of heat energy transferred
from the
final product to the gas. This will enhance the overall thermal efficiency of
the system.
Typically, the final product will cool at a slower rate than the gas
temperature increases
because the final product has a greater thermal mass.
Heat transfer to the gas may also be optimised by providing the thermal
exchange means
immediately adjacent the furnace. This is because it is preferred from the
viewpoint of
maximising radiant heat transfer that the temperature of the final product be
as high as
possible as it is introduced into the thermal exchange means from the furnace.
If the
temperature of the final product cools to any significant degree before
entering the thermal
exchange means, the effectiveness of radiant heat transfer to the gas via the
thermal
exchange means will be diminished. This effect can be minimised by positioning
the
thermal exchange means immediately adjacent the furnace so that the final
product from
the furnace undergoes minimal cooling before being processed by the thermal
exchange
1 +
CA 02527025 2005-11-25
p.i0 cdiaesswoso,mmammaoo-3i/Mos PCT/AU2004/000837
Received 31 March 2005
-5-
means. This principle can be applied to optimise the amount of heat
transferred from the
final product to the gas. It is also desirable to use a gas having high
conductivity and
specific heat, such as hydrogen, although this will obviously depend upon the
chemical
conversion being undertaken.
The thermal exchange means comprises a series of individual baffle plates.
Preferably, the
baffle plates are parallel to each other and inclined at an angle of from 30
to 60 ,
preferably 45 , to the horizontal. The plates are inclined in the direction of
intended gas
flow. The number, size, geometry, inclination and spacing of the plates are
intended to
cause turbulence in the gas thereby optimising heat transfer by ensuring
intimate contact
between incoming gas and the surfaces of the plates. Desirably, to maximise
heat transfer,
the flow of incoming gas will contact a large surface area of the plates
before exiting the
thermal exchange means. The spacing between the baffle plates should be
adjusted to
ensure suitable upflow of gas between adjacent plates. If the spacing is too
small,
increased flow resistance occurs which reduces upflow.
To assist in elevating the temperature of the baffle plates, at least on start-
up, the material
from which they are made preferably has high thermal conductivity. However, it
will be
appreciated that once thermal equilibrium has been reached and the plates have
attained a
steady temperature, the amount of energy withdrawn from the hot product will
substantially equal the amount of energy conveyed to the gas passed over the
plates. In this
case the conductivity of the plates is not particularly relevant (nor is their
weight or
thickness.) Usually, the plates are made of steel (such as stainless steel)
and are blackened
to maximise radiant heat transfer. This blackening may be done by techniques
known in
the at, such as chemical oxidation. However, here it will be necessary to take
into account
the conversion that is to take place in the furnace. If the conversion
involves reduction of a
starting material, it will probably not be appropriate to use oxidation-
blackened plates
since the reduction may reverse the blackening process. Obviously, the
material from
which the plates are formed should have sufficient structural integrity at the
temperatures
30, likely to be encountered in operation.
Amended sheet
IPEA/AU
CA 02527025 2005-11-24
WO 2005/001362 PCT/AU2004/000837
-6-
To ensure a high degree of heat uptake by the baffle plates they are located
in close
proximity to the (hot) final product. Usually, the baffle plates are
positioned above (and
adjacent) the product as the product is transported through (and out of) the
thermal
exchange means, the spacing between the trailing (lower) edge of the plates
and the
product being as little as possible. It has been found that a significant
portion of gas can
pass beneath the plates in the space immediately above the product. Reducing
the
clearance between the baffle plate adjacent the inlet and the product as it is
being
transported can help to prevent this and thus enhance performance. In an
attempt to
minimise the spacing between the trailing edge of the baffle plates and the
hot product, the
trailing edge of each plate may be provided with a flap which is easily
displaced by contact
with the product. Use of such an arrangement allows the trailing edge of the
each baffle
plate to be in as close thermal proximity to the product as possible but
enables variations in
product height to be taken into account. The flap will usually be formed of
the same
material as the baffle plate and be attached (loosely) to the plate by a hinge
permitting the
flap to move relative to the baffle plate. After being displaced by product
being
transported the flap will swing back into a resting position. One function of
the flaps is to
encourage gas flow over the baffle plates.
The fact that the baffle plates are located adjacent the final product being
transported
means that, at thermal equilibrium, there is an essentially constant rate of
radiant heat
transfer from the product to the plates (and conductive heat transfer from the
plates to the
circulating gas in contact with the plates). The gas is passed over the plates
generally in a
direction counter to that of the final product as it is being transported.
In one embodiment the final product is transported through the thermal
exchange means on
a conveyor belt. In this case the trailing edge of each baffle plate will be
arranged so as to
be in close thermal proximity to the product as it is being transported. It is
likely that the
starting material enters and moves through the furnace on the same conveyor
belt. The
baffle plates will be positioned at a location within the thermal exchange
means such that
they are adjacent the final product as it enters the thermal exchange means
and is
transported through it.
CA 02527025 2005-11-24
WO 2005/001362 PCT/AU2004/000837
-7-
In another embodiment no conveyor belt is used and here the product may be
transported
over a series of mandrels or rollers. This would be the case for example in a
continuous
strip furnace. Similar considerations will then apply as described above with
respect to use
of a conveyor belt. It will be appreciated that the exact manner in which the
final product
is transported through the thermal exchange means is not especially critical
provided that
the intended thermal relationship between the final product and the thermal
exchange
means is preserved.
In an embodiment of the invention the efficacy of the thermal exchange means
may be
enhanced by positioning the baffle plates below and adjacent the final product
as it is
transported. The reason for this is that gas (for the conversion) entering the
thermal
exchange means is cooler, and thus heavier, than gas already present. The
natural
tendency of this "fresh" gas is to flow downwards. Positioning the thermal
exchange
means below the final product being transported is likely to mean that more of
this "fresh"
gas will contact the thermal exchange means. It is of course possible to have
thermal
exchange means above and below the final product as it is transported.
In one embodiment the baffle plates are corrugated to impart greater
turbulence and offer
increased surface area for gas contact. The thermal exchange means includes a
suitable
housing in order to ensure flow of gas over (and between) the baffle plates as
required.
This housing will be provided over the hot product as it is transported after
exiting the
furnace.
The thermal exchange means includes an inlet for "cold" gas and an outlet for
"heated"
gas. The inlet and outlet are provided in close proximity to the hot product
being
transported. Thus, with respect to the thermal exchange means, the gas outlet
corresponds
to the inlet for the final product, and vice versa. This is the simplest
arrangement although,
in principle, the gas inlet and outlet may be entirely distinct from the
product inlet and
outlet. Gas delivered to the thermal exchange means gas may already have been
used
repeatedly (by means of recirculation and instream cooling) downstream of the
thermal
CA 02527025 2005-11-24
WO 2005/001362 PCT/AU2004/000837
-8-
exchange means. The outlet delivers gas at an elevated temperature to the
furnace.
In one embodiment in order to generate turbulence over the plates, the gas is
delivered
under pressure. This is desirable since impingement of the gas with the final
product
and/or with heat exchange surfaces within the thermal exchange means may
enhance the
rate of heat uptake by the gas. In one embodiment, the gas may be directed
from a
pressure chamber of the cooler downstream of the furnace (and thermal exchange
means).
In another embodiment gas is delivered to the thermal exchange means via an
inlet
provided close to the roof thereof. This has also been found to assist in
creating gas
turbulence in the thermal exchange means. Alternatively, or additionally, a
circulating fan
may be provided to enhance the rate of convection heat transfer between the
baffle plates
and the gas.
In one embodiment the thermal exchange means also includes a guide means which
is
provided adjacent the outlet of the thermal exchange means and which is
configured to
gather and funnel heated gas towards the outlet. In one embodiment this guide
means
takes the form of a plate which extends across the height of the thermal
exchange means
and which is inclined in the opposite direction to the other plates. Gas
impinging upon this
plate is deflected towards the outlet of the thermal exchange means. It has
been found that
the presence of this guide (exit) plate contributes to the overall performance
of the thermal
heat exchanger.
In one embodiment the guide plate extends above the height of the baffle
plates. The
effect of this is to divert more of the upper-level flow in the thermal
exchange means close
to the guide plate.
The position of the guide plate adjacent the gas outlet may also influence the
velocity of
gas flowing across this plate. This may cause some of the gas flowing parallel
to and
adjacent the final product to be diverted upwards thereby increasing the net
flow over the
top of the guide plate.
CA 02527025 2005-11-24
WO 2005/001362 PCT/AU2004/000837
-9-
The baffle and guide plates are usually attached to the housing at their
edges. Typically,
the plates are supported at one edge only. The supporting structures should be
configured
to minimise flow disruption and distortion on expansion of the plates. In
practice, both
sides of the plates may be supported.
Preferably, by use of the present invention, the gas to be used in the furnace
is heated to
70-90%, more preferably 80-90% of the furnace temperature. Prior to the
present
invention considerable energy input would otherwise have been required to
achieve a
corresponding temperature increase. This is especially so using hydrogen
because of the
high specific heat of hydrogen and the elevation of temperature required. The
present
invention thus affords a significant energy saving. Furthermore, in accordance
with the
present invention the gas temperature is raised to a value which is relatively
close to the
operating temperature of the furnace. This greatly reduces the temperature
slump that
would occur in the furnace if gas were introduced unheated or at a temperature
significantly lower than that of the furnace operating temperature. This also
means that the
chemical reaction in the furnace is likely to progress more immediately since
little or no
additional thermal energy needs to be supplied.
The gas which is used in the process of the present invention will obviously
vary
depending upon the chemical conversion being undertaken. Mixtures of two or
more
suitable gases may be used. In reduction reactions, such as the conversion of
nickel oxide
to nickel, hydrogen, or a mixture of hydrogen and nitrogen at a volume ratio
of 75:25
respectively, are typically used. Hydrogen is especially useful as it has a
high specific heat
and high thermal conductivity.
Once delivered to the furnace the gas effects conversion of the starting
material to the final
product. Exhaust process gases from the furnace are removed and handled in a
conventional manner. The final product is removed from the furnace, typically
on a
conveyor belt as mentioned, and after passage through the thermal exchange
means is
delivered to a cooler where the product is cooled. Conventional coolers may be
used in
this respect.
CA 02527025 2005-11-24
WO 2005/001362 PCT/AU2004/000837
-10-
The present invention also provides an apparatus suitable for carrying out the
process
described herein. The apparatus comprises a furnace provided upstream of a
thermal
exchange means as described, the thermal exchange means being provided
upstream of a
(product) cooler. In this context, the term "upstream" is intended to denote
the relative
position of these components of the apparatus, the starting material being
introduced at the
upstream end and the final product cooled at the downstream end. The furnace
and
thermal exchange means are in communication with each other in that the final
product
exiting the furnace is delivered to the thermal exchange means for heat uptake
by the gas.
These two components are also in communication in order to allow hot gas from
the
thermal exchange means to be delivered to the furnace. As noted above, the gas
outlet is
also the final product inlet to the thermal exchange means, and vice versa.
The cooler
receives final product which has been "processed" by the thermal exchange
means.
Usually, the same conveyor belt or mandrels/rollers will do this. When at
least a portion of
(pressurised) gas is fed from the cooler to the inlet of the thermal exchange
means for the
purpose of increasing turbulence, suitably arranged piping will generally be
used.
The accompanying non-limiting figure illustrates a thermal exchange means
which may be
used in accordance with the present invention.
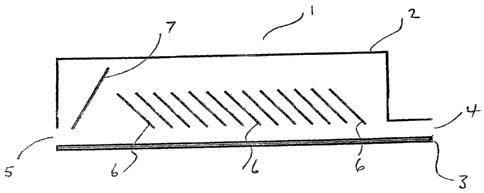
The figure shows a thermal exchange means (1) which includes a housing (2)
provided
over a conveyor belt (3) upon which final product is transported after leaving
the furnace
(not shown). The housing includes an inlet (4) for feed gas at relatively low
temperature
and an outlet (5) for delivery of heated gas to the furnace. The thermal
exchange means
(1) includes a series of baffle plates (6) which are provided at about 45 to
the horizontal.
These plates (6) are inclined in the direction of intended gas flow from inlet
(4) to outlet
(5). The plates (6) are in close proximity to the conveyor belt (3) and in use
will be heated
by hot product transported on the conveyor belt (3) (from left to right in the
figure and thus
counter to the direction of gas flow through the thermal exchange means (1)).
Gas flow
within the thermal exchange means (1) is turbulent resulting in gas flowing
across and
between the baffle plates (6). This causes the temperature of the gas to
increase, the baffle
CA 02527025 2005-11-24
WO 2005/001362 PCT/AU2004/000837
-11-
plates (6) communicating thermal energy from the final product on the conveyor
belt (3) to
the gas. The thermal exchange means is also provided with a guide plate (7)
which is
provided at the outlet (5) end of the thermal exchange means (1). This guide
plate (7)
extends across the height of the housing (2) and is inclined in the opposite
direction to the
baffle plates (6). The function of the guide plate (7) is to channel hot gas
towards the
outlet (5).
Embodiments of the present invention will now be illustrated by the following
non-
limiting example.
Example
Thermal exchange means - dimensions and characteristics
Housing: stainless steel.
Length: 2640mm.
Height (roof to conveyor belt): 650mm.
Inlet/outlet dimension: 150mm, provided at opposite ends of the thermal
exchange means
100mm above the product on the conveyor belt. Practically, this dimension will
be
dependant on the product thickness, and it is desirable to minimise the
distance between
the product and the bottom of the plates in order to discourage laminar flow
over the
product as this can by-pass the plates.
A stainless steel plate separates the thermal exchange means from the adjacent
compartment of the apparatus.
Baffle plates
13 Stainless steel plates, 2mm thick, first plate (inlet end) 368mm in length
remaining
plates, 354mm in length inclined at 45 to the horizontal. The leading edge of
the last plate
is 665mm from outlet of thermal exchange means.
Plate spacing: 125mm (measured along the horizontal).
CA 02527025 2005-11-24
WO 2005/001362 PCT/AU2004/000837
-12-
Clearance from conveyor belt: 140mm (first plate, inlet end); 150mm (remaining
12
plates).
Clearance from roof: 250mm
Guide plate
Stainless steel, 2mm thick, 500mm in length inclined at 60 to the horizontal
in opposing
direction to baffle plates, located 100mm from outlet.
Clearance from conveyor belt: 140mm
It will be appreciated that the accompanying figure illustrates this thermal
exchange means
schematically.
Nickel powder is transported on the conveyor belt at lm/min at a temperature
of 980 C. A
mixture of nitrogen and hydrogen at a volume ratio N2:H2 of 25:75 was
introduced at
150 C at an entry rate of 500m3/hr. The gas exit temperature was found to be
about
700 C. The surface temperature of the nickel powder was found to decrease by
on average
about 150 C during transit through the thermal exchange means. It was also
found that the
highest gas velocity occurred at the outlet where the heated and expanded gas
is forced
through a relatively small exit aperture.
It will be appreciated that there are numerous variables associated with the
present
invention. In practice testing will be employed to determine the most
efficient way in
which to put the invention into effect for a given system. This would be
within the
abilities of one skilled in the art.
Throughout this specification and the claims which follow, unless the context
requires
otherwise, the word "comprise", and variations such as "comprises" and
"comprising", will
be understood to imply the inclusion of a stated integer or step or group of
integers or steps
but not the exclusion of any other integer or step or group of integers or
steps.