Note: Descriptions are shown in the official language in which they were submitted.
CA 02527193 2005-11-25
WO 2004/096314 PCT/US2004/010788
INTRACRANIAL CATHETER ASSEMBLY FOR
PRECISE TREATMENT OF BRAIN TISSUE
FIELD OF INVENTION
The present invention relates to the intracranial transfer of fluids and, in
particular, to devices for affecting such transfer.
BACKGROUND OF THE INVENTION
Movement disorders such as epilepsy and Parkinson's disease have been
estimated to affect some 1-2% of the developed world's population and up to
10% of
people in underdeveloped countries. Currently, approximately 75% of those who
suffer from movement disorders are responsive in some degree to drugs.
Electrical stimulation has also been utilized to treat some movement
disorders.
In the treatment of epilepsy, studies have been performed in which awake
patients
undergoing temporal lobe surgery underwent cortical stimulation. Such
stimulation of
the visual and hearing areas of the brain reproducibly caused the patients to
experience
visual and auditory phenomena. This discovery was made possible by the
identification
that certain brain subregions served specific functions, such as sight,
hearing, touch
and movement of the extremities and proved that direct electrical stimulation
of the
brain regions could cause partial reproduction or suppression of the
functions.
As suggested by these results, it is known that certain types of treatment of
specific portions of the brain are able to suppress certain unwanted behavior
which
results from movement disorders. This behavior may include seizures such as
those
suffered by epileptics. However, the studies faced a major problem in that
there was
an inability to precisely electrically stimulate very small volumes of the
brain.
The advent of needle-shaped penetrating depth electrodes helped to overcome
this obstacle faced by electrical stimulation. Depth electrodes can be placed
within the
brain tissue itself, enabling optimal surface contact with elements of the
brain that are
targeted for stimulation. This allowed for safe, chronic electrical
stimulation of very
small discrete volumes of brain.
-1-
CA 02527193 2005-11-25
WO 2004/096314 PCT/US2004/010788
In treatment, electrical stimulation has been used with the recording and
analysis of changes in brain activity to predict the occurrence of epileptic
seizures. The
time of onset of such seizures is often predictable by neural discharge
monitoring, even
when the exact causal nature of precipitating dysfunction is not understood.
Electrodes have been used to obtain signals representative of current brain
activity
along with a signal processor for continuous monitoring and analysis of these
electrical
signals in order to identify important changes or the appearance of precursors
predictive of an impending change.
While the electrical stimulation of brain tissue has been somewhat effective
in
the treatment of migraines, epilepsy and other neurological problems, patients
often
experience diminishing returns with such treatment. Furthermore, because each
patient
reacts differently to electrical stimulation, substantial time must be spent
to determine
the specific amplitude, frequency, pulse width, stimulation duration, etc.
which may
result in effective treatment. In addition, such parameters often require
continual
adjustment in order to remain effective.
The combination of drug delivery and electrical stimulation and/or monitoring
has been shown to be more effective in some intracranial treatments. Such drug
delivery and stimulation or monitoring is typically performed by instruments
which are
inserted into the brain at different locations or along different tracks.
Other systems
employ a single device which must be removed and reinserted to provide for
delivery
of multiple drugs or use of different electrical devices.
Since the introduction of probes or other similar devices into the brain is
common in many surgical procedures today, there are a variety of probes
available.
Such probes typically include ports for drug delivery or electrical, chemical,
electrochemical, temperature and/or pressure contacts which enable the
observation
and analysis of the brain state or contacts providing stimulation. These ports
and
contacts must typically be positioned at specific points or regions in the
brain.
Probes used in intracranial penetration are typically fabricated so that their
introduction to the brain is as minimally traumatic as possible. In addition
to being
minimally traumatic during insertion, certain inserted probes must also be
able to
remain implanted without causing injury through unintended movement. In some
uses,
-2-
CA 02527193 2005-11-25
WO 2004/096314 PCT/US2004/010788
a probe may be implanted and remain in the patient's brain for weeks or
longer.
Changes in the positioning of the probe often occur during placement or during
such
extended periods. Therefore, the probe must be capable of precise placement
and as
biocompatible as possible. In response to these requirements, state of the art
intracranial probes are typically thin, flexible pieces with smooth surfaces
to minimize
the amount of brain tissue contacted and to minimize damage to contacted brain
tissue.
While such thin, flexible probes are sufficiently biocompatible, they are
delicate
and often difficult to insert along specific trajectories or lines of
insertion. During
typical implantation, a surgeon feeds the probe into the brain through an
aperture in the
skull. In this process, the surgeon has very little control over the distal
end of the
probe. In order to provide more rigidity to the probe to overcome this
problem, a
removable stylet may be inserted into the probe before implantation. Still,
veering
from the intended line of insertion is not altogether prevented by
introduction of a
stylet to the probe.
While typical intracranial probes have smooth surfaces so as to not cut any
contacted tissue, many such probes are made of elastomers or other such
materials
which, although smooth, do not easily slide through brain tissue. The drag
encountered by these types of probes can result in injury to the contacted
brain tissue.
Therefore, there is a continuing significant need in the field of intracranial
treatment, particularly with insertion of probes into the interior of the
brain, for
improvements in accuracy of insertion and avoidance of injury, while retaining
efficiency and ease of use.
In addition, there is a need in the field of intracranial treatment to
minimize the
d
invasiveness of intracranial treatment and to reduce the number of instruments
which
penetrate brain tissue or the number of times a single instrument must
penetrate brain
tissue.
Furthermore, there is a need in the field of intracranial treatment to provide
the
ability to precisely locate the position of a probe during insertion to ensure
proper
positioning.
-3-
CA 02527193 2005-11-25
WO 2004/096314 PCT/US2004/010788
OBJECTS OF THE INVENTION
It is an object of the invention to provide an improved intracranial insertion
device which prevents injury to the patient.
Another object of the invention is to provide a catheter assembly which is
simple in structure and operation in order to facilitate intracranial
procedures.
Another object of the invention is to provide a catheter assembly which allows
for the precise insertion of drug delivery ports or contacts in the brain
while avoiding
extensive trauma to and scarring of brain tissue.
Another object of the invention is to provide an outer catheter which includes
contacts for stimulation and/or monitoring the brain and which receives and
guides a
drug delivery catheter to the targeted brain tissue for drug delivery.
Another object of the invention is to provide an outer catheter which includes
contacts for stimulation and/or monitoring the brain and which receives and
guides a
cerebral spinal fluid recovery catheter to the targeted brain tissue for
sampling cerebral
spinal fluid through a dialysis membrane.
Another object of the invention is to provide an outer catheter which includes
location markers for allowing the positioning of the outer catheter to be
determined
during or after insertion of the catheter into the brain and which receives
and guides an
inner catheter for delivering or removing fluid from the targeted brain
tissue.
Another object of the invention is to provide a depth electrode which receives
and guides an inner catheter for delivering or removing fluid from the
targeted brain
tissue and remains in position when the inner catheter is removed, allowing
for further
insertions of the inner catheter without further extended contact with brain
tissue
during insertion.
Another object of the invention is to provide an outer catheter including an
inflatable balloon for sealing any insertion tract to permit effective drug
delivery to the
targeted brain tissue region.
Still another object of the invention is to provide a method of safely
inserting,
through use of an outer catheter, a catheter in a patient's brain which
provides for drug
delivery and/or cerebral spinal fluid withdrawal as well as stimulation and/or
monitoring of brain activity.
-4-
CA 02527193 2010-06-28
Yet another object of the invention is to provide a trajectory catheter which
can
be mounted to the patient's skull and connected to an inner catheter such that
the inner
catheter is positioned and held at the targeted brain tissue region.
These and other objects of the invention will be apparent from the following
descriptions and from the drawings.
BRIEF SAY OF THE INVENTION
In accordance with the present invention, an intracranial catheter assembly is
provided for precise treatment of brain tissue. The catheter assembly of this
invention
addresses certain problems and shortcomings of the prior art, including those
noted
above, and provides a unique structure satisfying a number of specific
intracranial
treatment needs.
In one aspect, the present invention provides a catheter assembly for
intracranial treatment of a patient, comprising an outer catheter and an inner
catheter.
The outer catheter has an exterior surface and a distal end, an inflatable
balloon
mounted upon the exterior surface, at least one element distal to the balloon,
at least
one aperture and a lumen. The element is adapted to monitor electrical changes
in
brain activity within the tissue region to electronically stimulate the tissue
region or to
provide information on a precise position of the element when the element is
located
entirely within the brain and being mounted proximal to the distal end of the
outer
catheter upon an exterior surface of a distal portion of the outer catheter.
The inner
catheter is sized to be received within the lumen and has a passageway
extending
between a proximal end and at least one port, the lumen being adapted to guide
the
inner catheter to the tissue region to deliver treatment agents.
-5-
CA 02527193 2010-06-28
In certain embodiments, the aperture is-preferably-axially aligned with the
inner
lumen In such embodiments, the inner catheter can extend through the aperture
when
the inner catheter is received within the lumen.
In other embodiments, the outer catheter has a closed end which blocks
passage of the inner catheter therethrough. In such embodiments the aperture
is
located along a side of the outer catheter. The outer catheter can include
more than
one aperture which are preferably radially and axially spaced about the outer
catheter.
In still other embodiments, the outer catheter has an aperture axially aligned
with the
inner lumen and other apertures which are located along a side which may be
radially
or axially spaced.
20
-5a-
CA 02527193 2005-11-25
WO 2004/096314 PCT/US2004/010788
In certain preferred embodiments, the outer catheter further includes a
conduit
extending from the proximal end to an inflatable balloon which is adapted to
be inflated
to seal a tract created during insertion of the assembly into the brain. The
inflatable
balloon may be inflated with a fluid which does not come in contact with brain
tissue;
however, in some embodiments, the balloon is inflated with a fluid which is
then
introduced to the tissue region surrounding the balloon. Such a fluid-can be
any type
of medicament, and will be referred to herein as a "drug," such term including
other
types of medicaments. Introduction of the fluid can occur at a slow or fast
rate as fluid
permeates through or otherwise leaves the balloon and can occur before,
simultaneous
with, or after transfer of fluid through the lumen or passageway such that
multiple
fluids may be administered separately through the catheter assembly.
The inner catheter preferably has at least one port. In some embodiments, the
ports are designed to be in communication with the apertures of the outer
catheter
when the inner catheter is inserted into the lumen to a preferred position. In
other
embodiments, the position of the ports do not correspond with the apertures -
particularly when an aperture is axially aligned with the lumen and the distal
portion of
inner catheter passes through the aperture. In certain preferred embodiments
there are
at least two ports which are axially spaced on a side of the inner catheter
along a line
parallel to the passageway. In other embodiments, the ports are radially and
axially
spaced about the inner catheter. In yet other embodiments, the inner catheter
includes
a port axially aligned with passageway and at least one port positioned on its
side.
The preferred port is adapted to deliver or remove fluids from the surrounding
brain tissue. In certain embodiments, the port includes a dialysis membrane
adapted to
receive cerebral spinal fluid.
In some embodiments, the outer catheter and inner catheter preferably have
proximal ends with one of the proximal ends including a luer fitting and the
other of
the proximal ends configured for connection to the luer fitting. In such
embodiments,
the outer catheter is inserted into the patient's brain to a desired position,
then the
inner catheter is inserted into the lumen and the catheters are connected to
one another
via the luer fitting such that the inner catheter is secured at the desired
position in the
brain.
-6-
CA 02527193 2005-11-25
WO 2004/096314 PCT/US2004/010788
In some embodiments, the outer catheter is preferably adapted to connect to
the patient's skull when inserted to the targeted portion. In such
embodiments, the
inner catheter is preferably adapted to connect to the outer catheter when
inserted to
the targeted portion. Therefore, during use, the outer catheter may be
inserted to the
targeted position and secured to the skull while the inner catheter is
inserted into the
lumen and then secured to the outer catheter. Such connections are preferably
performed by screwing the outer catheter to the skull and the inner catheter
to the
outer catheter, i.e., via threads on each of the catheters.
In certain embodiments, the at least one element is a contact which monitors
activity in the brain. In such embodiments, the outer catheter can be
considered as a
depth electrode. The contact may be of the type which senses electrical,
chemical or
electrochemical activity in the brain. The contact preferably senses brain
function in
the tissue region. In other embodiments the contact provides electrical
stimulation to
the tissue region. The contact may be a collar circumscribing the outer
catheter, a
micro contact, a macro contact, or other types of contacts. The depth
electrode
preferably includes an electrical lead corresponding to each contact.
In other preferred embodiments, the element is a location marker for allowing
the position of the outer catheter to be located within the brain. The
location marker is
preferably adapted to be located by magnetic resonance imaging or computerized
x-ray
tomography such that such imaging devices can be used to locate the position
of the
catheter in the patient's brain during or after insertion or implantation.
In yet other preferred embodiments, multiple elements on the outer catheter
include both at least one contact and at least one marker.
The invention also includes a method of treating a tissue region in the brain
of a
patient comprising (a) inserting into the brain an outer catheter having at
least one
element, at least one aperture and a lumen, (b) inserting into the lumen an
inner
catheter having a passageway extending between a proximal end and at least one
port,
and (c) transferring fluids between the tissue region and the proximal end
through the
passageway. In the inventive method the outer catheter guides the inner
catheter to
the tissue region. Therefore, the inner catheter may be non-rigid or otherwise
ill-
designed to be precisely inserted into a patient's brain and still be
precisely positioned
-7-
CA 02527193 2005-11-25
WO 2004/096314 PCT/US2004/010788
at the desired position in the brain. The method preferably includes
positioning a stylet
in the lumen during insertion of the outer catheter into the brain and
removing the
stylet from the outer catheter before the inner catheter is inserted into the
lumen. The
method also preferably includes connecting the outer catheter to the patient's
skull and
connecting the inner catheter to the outer catheter. The catheters may be
connected by
screwing reciprocal threaded parts together, by use of a luer fitting or other
connecting
members.
In certain embodiments, the element is a location marker and the method
further includes locating the position of the location marker in the brain.
The location
marker is preferably located by magnetic resonance imaging or by computerized
x-ray
tomography.
In other embodiments, the element is a contact and the method further
comprises monitoring the tissue region via the contact. The contact preferably
provides electrical stimulation to the tissue region, senses brain activity in
the tissue
region, or multiple contacts allow for performance of each of these functions.
The
preferred monitoring action includes sensing chemical changes at the tissue
region
and/or sensing electrical changes at the tissue region. The contact is
preferably a
collar-type contact, a micro contact for monitoring cells or neurons, a macro
contact
for monitoring tissue, or a fiber optic contact.
In preferred methods, the inner catheter has a distal end which passes through
the aperture into the tissue region beyond the outer catheter during the
insertion of the
inner catheter into the lumen. In other preferred embodiments, the inner
catheter has a
distal end and the outer catheter has a closed lumen so that the distal end is
contained
within the outer catheter when the inner catheter is inserted into the lumen.
The transferring action preferably includes delivering drugs to the tissue
region
through the at least one port and/or withdrawing cerebral spinal fluid through
the at
least one port. In certain embodiments, the inner catheter includes a micro-
dialysis
membrane at the at least one port and cerebral spinal fluid passes through the
membrane during the withdrawing action.
In certain preferred embodiments, the outer catheter includes an inflatable
balloon and the method further comprises inflating the balloon to seal a tract
created
-8-
CA 02527193 2005-11-25
WO 2004/096314 PCT/US2004/010788
during insertion of the assembly into the brain. The balloon is preferably
inflated with
a drug which is delivered to the tissue region through the balloon. The
preferred
method further comprises delivering a second drug to the tissue region through
the at
least one port.
Some preferred embodiments of the invention include a first inner catheter
having a first length for delivering fluids to a first tissue region and a
second inner
catheter having a second length greater than the first length for delivering
fluids to a
second tissue region within the patient's brain. More preferably, the assembly
comprises a third inner catheter having a third length greater than the second
length for
delivering fluids to a third tissue region within the patient's brain. As is
understood the
assembly can include many inner catheters of different lengths and having
different
arrangements of ports to provide for treatment of specific desired tissue
regions in the
brain.
Each different inner catheter is preferably introduced to the brain through a
single outer catheter such that an instrument penetrates a large portion of
the brain
tissue between the skull and the desired tissue regions only once. Such an
assembly
allows for inserting the outer catheter through the intervening brain tissue,
precisely
locating the outer catheter relative to a specific tissue region, inserting
corresponding
inner catheter (the inner catheter which would position a port at the tissue
region when
inserted into the lumen) in the lumen, treating the tissue region, withdrawing
the used
inner catheter from the lumen, inserting another inner catheter corresponding
to
another desired specific tissue region, treating that region, etc.
BRIEF DESCRIPTION OF THE DRAWINGS
The drawings furnished herewith illustrate a preferred construction of the
present invention in which the above advantages and features are clearly
disclosed as
well as others which will be readily understood from the following description
of the
illustrated embodiment. In the drawings:
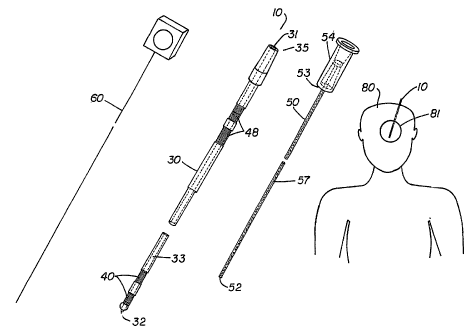
FIGURE 1 is a perspective view of a catheter assembly and patient, shown with
dashed lines representing otherwise unseen internal features, in accordance
with the
invention.
-9-
CA 02527193 2005-11-25
WO 2004/096314 PCT/US2004/010788
FIGURES 2a and 2b are perspective views of an alternate outer catheter
having a closed end, shown with dashed lines representing otherwise unseen
internal
features, in accordance with the invention.
FIGURE 3 is a perspective view of an outer catheter when receiving a stylet,
shown with dashed lines representing otherwise unseen internal features, in
accordance
with the invention.
FIGURE 4 is a perspective view of an outer catheter when receiving an inner
catheter, shown with dashed lines representing otherwise unseen internal
features, in
accordance with the invention.
FIGURES 5a, 5b, 5c and 5d are perspective views of alternate inner catheters,
shown with dashed lines representing otherwise unseen internal features, in
accordance
with the invention.
FIGURE 6 is a perspective view of an outer catheter having a balloon shown
both deflated and inflated, with dashed lines representing otherwise unseen
internal
features, in accordance with the invention.
FIGURE 7 is a perspective view of an alternate version of the outer catheter
shown in FIGURE 6, shown with dashed lines representing otherwise unseen
internal
features, in accordance with the invention.
FIGURE 8 is a perspective view of an alternate version of the outer catheter
shown in FIGURES 6 and 7, shown with dashed lines representing otherwise
unseen
internal features, in accordance with the invention.
FIGURES 9a, 9b, 9c and 9d are perspective views of alternate versions of the
outer catheters shown in FIGURES 6-8, shown with dashed lines representing
otherwise unseen internal features, in accordance with the invention.
FIGURE 10 is a perspective view of a preferred outer catheter having a balloon
and contacts with an electrical lead, shown with dashed lines representing
otherwise
unseen internal features, in accordance with the invention.
FIGURE 11 is a perspective view of an alternate version of the outer catheter
shown in FIGURE 10, shown with dashed lines representing otherwise unseen
internal
features, in accordance with the invention.
-10-
CA 02527193 2005-11-25
WO 2004/096314 PCT/US2004/010788
FIGURE 12 is a perspective view of an alternate version of the outer catheter
shown in FIGURES 10 and 11, shown with dashed lines representing otherwise
unseen
internal features, in accordance with the invention.
FIGURE 13 is a perspective view of an alternate version of the outer catheter
shown in FIGURES 10-12, shown with dashed lines representing otherwise unseen
internal features, in accordance with the invention.
FIGURES 14a, 14b, 14c and 14d are perspective views of alternate versions of
the outer catheters shown in FIGURES 10-13, shown with dashed lines
representing
otherwise unseen internal features, in accordance, with the invention.
FIGURES 15 and 16 are perspective views of a preferred catheter assembly in
which the inner catheter includes a dialysis membrane, shown with dashed lines
representing otherwise unseen internal features, in accordance with the
invention.
FIGURES 17a and 17b are perspective views of a preferred catheter assembly
which provides for connection between the outer catheter and the patient's
skull and
includes a location marker and including a set of inner catheters, shown with
dashed
lines representing otherwise unseen internal features, in accordance with the
invention.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
Referring to FIGURE 1, a catheter assembly in accordance with the present
invention is generally designated by the reference numeral 10. Catheter
assembly 10
allows intracranial treatment of a patient by providing an outer catheter 30
and inner
catheter 50 which cooperate to transfer fluids between a tissue region 81 in
the
patient's brain 80 and an external receptacle or device. Also shown with
catheter
assembly 10 is stylet 60 which can be received by outer catheter 30 to prevent
the
entrance of brain tissue into outer catheter 30 during insertion into the
brain.
Outer catheter 30 is preferably between about 0.6 and 1.5 millimeters, most
preferably about 1.0 millimeter and is comprised of polyurethane, silicone,
polyimede,
or other biocompatible material. Outer catheter 30 includes a lumen 33 which
extends
from proximal opening 31 to aperture 32. Outer catheter 30 also includes
elements 40
which may provide for monitoring of brain tissue or for providing a location
marker for
determining the precise position of outer catheter 30 within the brain. As
shown,
-11-
CA 02527193 2010-06-28
elements 40 include distal contacts 41 which can sense brain activity in
tissue region 81
via electrical, electrochemical, chemical or pressure changes within the
brain.
Preferred contacts 41 are platinum, platinum iridium or other biocompatible
conductive
material. For pressure sensing, contact 41 is a miniature pressure-sensing
contact
which is preferably a miniature optical pressure transducer less than about 2
millimeters
long. Brain activity sensed by distal contacts 41 is transmitted to an
external
connector through proximal contacts 48 and then to a computer or instrument
which
records and/or analyzes such activity. During insertion or implantation
proximal
contacts 48 remain outside of the patient and allow for connection to such an
instrument. Proximal contacts 48 are preferably stainless steel or other
alloys or
materials which are noncorrosive conductors which can endure the sterilization
process.
Inner catheter 50 is preferably polyimede, polyimede-coated glass or other
similar material and includes a passageway 51 which extends from proximal end
53 to
port 52. Passageway 51 has an inner diameter which may vary depending on the
desired flow rate of fluid therethrough but is preferably between about 25
microns and
0.5 millimeters. As shown, port 52 is axially aligned with passageway 51 (as
is
aperture 32 with lumen 33) such that fluids may be transferred to or from the
tissue
region 81 at port 52, e.g, drugs may be administered to tissue region 81,
cerebral
spinal fluid may be withdrawn, or both. Inner catheter 50 is shown as
including a luer
fitting 54 which provides for connection with outer catheter 30.
FIGURES 2a and 2b depict alternate embodiments of outer catheter 30 in
which lumen 33 has a closed end 34. In such embodiments, apertures 32 are
positioned along the side or sides of the outer catheter. For instance, FIGURE
2a
shows apertures 32 axially spaced along a line parallel to lumen 33. FIGURE 2b
shows apertures 32 axially and radially spaced about outer catheter 30. In
addition,
outer catheter 30 is shown as including a luer fitting 36 providing for
connection with
inner catheter 50.
-12-
CA 02527193 2005-11-25
WO 2004/096314 PCT/US2004/010788
FIGURE 3 shows stylet 60 received within lumen 33 for insertion into the
patient's brain. Stylet 60 prevents brain tissue from entering lumen 33 during
insertion
and may provide rigidity to outer catheter 30 if outer catheter 30 is not
rigid.
FIGURE 4 shows inner catheter 50 received within lumen 33. As shown, distal
portion 56 of inner catheter 50 extends through aperture 32 to reach the
desired region
in the brain. Port 52 is shown axially aligned with passageway 51 although
additional
ports 52 can be positioned along the sides of distal portion 56.
FIGURES 5a shows inner catheter 50 while FIGURES 5b, 5c and 5d show
alternate embodiments of distal end 56 of inner catheter 50 in which port 52
is axially
aligned with passageway 51 (FIGURE 5b), multiple ports 52 are axially spaced
along a
line parallel to passageway 51 (FIGURE 5c), and multiple ports 52 are axially
and
radially spaced about inner catheter 50 (FIGURE 5d). It is understood that an
inner
catheter 50 can include both an axially aligned port 52 and ports 52
positioned along
its side.
FIGURES 6-14d pertain a preferred embodiment of the catheter assembly 10 in
which outer catheter 30 includes an inflatable balloon 38. As shown in FIGURE
6, a
conduit 37 leads to balloon 38 to provide for the introduction of a fluid to
inflate
balloon 38 and, if necessary to withdraw fluid from balloon 38 to cause
deflation (in
certain embodiments fluid permeates through balloon 38 to treat the tissue
region
surrounding balloon 38). As shown, conduit 37 terminates at a plug which can
be
connected to another device to receive or dispense fluid. Conduit 37 runs
alongside
lumen 33 and terminates at balloon 38. FIGURES 6-8 show the alternate
embodiments in which apertures 32 are variously positioned as discussed above.
In
each of FIGURES 6-8 elements 40 are distal contacts 41, and more specifically
are
macro contacts of the collar-type which circumscribe outer catheter 30.
Proximal
contacts 3 8 connect to distal contacts 41 to communication brain activity
from distal
contacts 41 to a recording or analysis instrument. Proximal contacts 38 do not
enter
the patient's brain, instead they provide connection to such an instrument.
Balloon 38 can be inflated to block any insertion tract created when catheter
assembly 10 is inserted into the brain such that any drug administered to the
brain
cannot migrate through any tract. In addition, balloon 38 may be inflated with
a drug
-13-
CA 02527193 2005-11-25
WO 2004/096314 PCT/US2004/010788
or other fluid which is intended to be administered to the brain. In this
manner, fluids
may be transferred between the brain and the apertures 32 at the same time
fluids are
introduced to the brain through balloon 38 through permeation. Balloon 38 is
particularly adept at administering fluids to the brain slowly over a period
of time
which may allow for effective introduction of the fluid to the brain.
FIGURES 9a, 9b, 9c and 9d differ from FIGURES 6-8 in that outer catheter
30 includes a distal portion which has a reduced diameter. Such an embodiment
provides for minimized invasiveness at the targeted tissue region. FIGURES 9b,
9c
and 9d are enlarged views of the distal portion about which apertures 32 may
be
variously positioned.
FIGURES 10-14d depict an outer catheter 30 which includes micro contacts
44 and/or macro contacts 45 and a lead 42 which communicates brain activity
through
connector 46. As shown, lead 42 runs alongside conduit 37 and lumen 33 to
distal
contacts 41 (micro contacts 44 and/or macro contacts 45). It is noted that
sensing
contacts 41 may be positioned on both the distal and proximal sides of balloon
38.
Such a design allows for monitoring of brain tissue which is being treated
with drugs
simultaneous with monitoring of brain tissue which is not being treated. Micro
contacts 44 and apertures 32 are shown variously positioned in FIGURES 10-14d
as
apertures 32 were shown and discussed as being variously positioned above. For
example, FIGURES 14b, 14c and 14d show the distal portions of outer catheter
30
and have an axially aligned aperture 32 and axially spaced micro contacts 44
in a line
parallel to lumen 33 (FIGURE 14b), micro contacts 44 and apertures 32 axially
spaced
in a line parallel to lumen 33 (FIGURE 14c) and radially and axially spaced
apertures
32 and axially spaced micro contacts 44 (FIGURE 14d).
FIGURES 15 and 16 depict a catheter assembly 10 which includes a inner
catheter 50 with a port 52 which is a micro dialysis membrane 55. In such an
embodiment, outer catheter 30 includes apertures 32 which allow cerebral
spinal fluid
to reach membrane 55. Fluid moves through membrane 55 and is transferred
through
passageway 51 to external receptacles or analysis devices. In FIGURE 15,
proximal
opening 31 is axially aligned with outer catheter 30 such that lumen 33 passes
through
proximal contacts 48. In FIGURE 16, lumen 33 branches off of outer catheter 30
-14-
CA 02527193 2005-11-25
WO 2004/096314 PCT/US2004/010788
through a flexible tubing before reaching proximal contacts 48 (proximal
contacts 48
are connected to distal contacts 41 by an unshown connection). Inner catheter
is
received in lumen 33 and moves into outer catheter 30 to ports 32. In such an
embodiment, inner catheter is sufficiently flexible to navigate lumen 33.
FIGURE 17a shows catheter assembly 10 including an outer catheter 30 which
has a location marker 43 as element 40. Location marker 43 is preferably
comprised
of a material which contains a mobile phase suitable for MRI imaging by
commercial
machines, and which is sufficiently X-Ray-opaque for adequate imaging on CT or
X-
ray. Catheters 30,50 also include threads 39,58 which provide for attachment
to the
patient's brain and between the catheters 30,50. Such an outer catheter 30 can
be
called a trajectory catheter when used in this manner. In a preferred method
of use,
trajectory catheter 30 is inserted into the brain and positioned at a desired
location in
the brain by using marker 43. Outer catheter 30 is then connected to the
patient's skull
by screwing threads 39 into the skull. Then inner catheter 50 is inserted
through lumen
33 and connected to outer catheter 30 by threads 58.
FIGURE 17b shows the distal portions 56 of a set 59 of inner catheters
including ports 52 which are variously positioned on distal portions 56 as
shown. In
certain preferred embodiments, inner catheters 50 of different lengths, such
as those
shown, are supplied with an outer catheter 30 such that, after inserting outer
catheter
30 into the patient's brain, an inner catheter 50 of a specific length is
selected to treat a
desired tissue region at a known location beyond outer catheter 30. After
treatment at
that location, the inner catheter 50 can be removed and another inner catheter
50 of a
different length and/or different port arrangement can be inserted into the
patient's
brain to treat a different desired tissue region. For instance, an inner
catheter 50 which
extends 0.5 cm beyond outer catheter 30 may be used to treat the tissue region
0.5 cm
beyond outer catheter 30 and then removed from lumen 33 before another inner
catheter 50 which extends 2.0 cm beyond outer catheter 30 is inserted through
lumen
33 and used to treat the tissue region 2.0 cm beyond outer catheter 30. A set
of inner
catheters 50 is preferably provided with an outer catheter 30 such that a
physician may
select specific inner catheters 50 to treat the desired tissue regions. Such a
set allows
for specific treatment of different tissue regions, such as those found in and
around
-15-
CA 02527193 2005-11-25
WO 2004/096314 PCT/US2004/010788
tumors, with the same or different drugs without requiring multiple insertions
through
the intervening brain tissue.
In some embodiments, outer catheter has apertures on its side (not shown)
which correspond to ports 52. In other embodiments, outer catheter has only a
open
ended lumen 33 such that the aperture is aligned with lumen 33, and inner
catheter 50
includes ports 52 on its distal portion which extends out of lumen 33 when
inserted
into the patient's brain. Outer catheter 30 can further include contacts 41 as
disclosed
in the prior figures.
While the invention has been described with respect to specific embodiments by
way of illustration, many modifications and changes will occur to those
skilled in the
art. It is, therefore, to be understood that the appended claims are intended
to cover
all such modifications and changes as fall within the true scope and spirit of
the
invention.
-16-