Note: Descriptions are shown in the official language in which they were submitted.
CA 02527330 2005-11-28
WO 2004/108257 PCT/US2004/017117
MASS SPECTROMETER AND RELATED IONIZER AND METHODS
BACKGROUND OF THE INVENTION
1. Field of the Invention
This invention relates to so called crossed field mass spectrometric analyzers
and ionizers which separate ionized gas particles according to their mass to
charge
ratio by motion in an electric field and a magnetic field perpendicular to
each other.
2. Description of the Prior Art
The use of mass spectrometers in determining the identity and
quantity of constituent materials in a gaseous, liquid or solid specimen has
long
been known. It has been known, in connection with such systems, to analyze the
specimen under vacuum through conversion of the molecules or atoms into an
ionic
form, separating the ions by mass to charge ratio, and pennitting the ions to
bombard a detector. See, generally, U.S. Patent Nos. 2,882,410; 3,070,951;
3,590,243; and 4,298,795. See, also, U.S. Patent No.'s 4,882,485 and
4,952,802.
In general, mass spectrometers contain an ionizer inlet assembly
- wherein the specimen to be analyzed is received, a high vacuum chamber which
cooperates with the ionizer inlet, an analyzer assembly which is disposed
within the
:high vacuum chamber and is adapted to receive ions from the ionizer. Detector
means are employed in making a determination as to the constituent components
of
the specimen employing mass to charge ratio as a distinguishing
characteristic. By
one of many known means, the molecules or atoms of the gaseous specimen
contained in the ionizer are converted into ions, which are analyzed by such
equipment.
It has been known with prior art cycloidal mass spectrometers to use
a fixed collector and ramped electric field in looking at only one mass to
charge
ratio at a time In many prior art mass spectrometer systems, regardless of
whether
they were of the cycloidal type or not, the ionizers were quite large and, as
a result,
dominated the design and specifications of the systems to be employed
therewith.
U. S. Patent 5,304,799 discloses a cycloidal mass spectrometer
having a housing defining an ion trajectory volume, an electric field
generator for
establishing an electric field within the ion trajectory volume and an ionizer
for
receiving gaseous specimens to be analyzed and converting the same into ions,
which travel through orthogonal electric and magnetic fields and subsequently
impinge on a collector. This spectrometer was designed to have a plurality of
different ions' mass to charge ratios impinging on the collector depending on
the
CA 02527330 2012-03-30
71548-282
strength of the fields. It was stated that the cycloidal mass spectrometer and
ionizer
may be miniaturized to as provide a small, readily portable instrument.
It has been known to employ crossed fields mass spectrometry in two
types of analytical problems. It has been employed in the identification of
molecules
with high molecular weight. It has also been employed in precise measurement
of
the relative abundance of isotopes.
It has also been known to employ mass spectrometers in connection
with situations involving low mass to charge ratio such as in helium leak
detectors
and hydrogen analyzers. Mass spectrometry has been employed in such situations
as it is nearly free of interference within the mass range and because of its
sensitivity.
The analyzers typically employed in helium leak detectors, for example, are
generally
smaller copies of larger analyzers such as sector field mass spectrometers
which are
easier to manufacture but provide a lower performance level and tend to be
relatively
expensive.
Quadrupole analyzers are small and less expensive than magnetic
separators, but their filter quality decreases when approaching the lower end
of the
mass scale. The so-called "zero blast" represents the contribution of the
particles the
quadropole is not tuned on as a result of the weak filter characteristics. For
a helium
leak detector, for example, the zero blast portion of hydrogen interferes with
the
helium signal at about 4 amu.
The present invention focuses on field structures of a cycloidal mass
spectrometer wherein a circular motion is imposed by a linear motion.
SUMMARY OF THE INVENTION
According to an aspect of the present invention, there is provided a
mass spectrometer comprising a first generally planar electrode, a second
generally
planar electrode disposed generally parallel to and spaced from said first
electrode,
said first and second electrodes structured to create an electric field
therebetween, a
magnetic field generator structured to create a magnetic field oriented
generally
2
CA 02527330 2012-11-02
71548-282
perpendicular to said electric field, said first and second electrodes each
having a base and
walls projecting generally toward the other to cooperate in defining an ion
generating
chamber, an ion exit permitting certain ions to exit said chamber, and an ion
collector
disposed exteriorly of and adjacent to said ion exit for receiving said ions
passing
therethrough.
According to another aspect of the present invention, there is provided an
ionizer comprising a first generally planar electrode, a second generally
planar electrode
disposed generally parallel to and spaced from said first electrode, said
first and second
electrodes structured to create an electric field therebetween, a magnetic
field generator
structured to create a magnetic field oriented generally perpendicular to said
electric field,
said first and second electrodes each having a base and walls projecting
generally toward the
other to cooperate in defining an ion generating chamber, and an ion exit
permitting certain
ions to exit said chamber.
According to another aspect of the present invention, there is provided a
method of analyzing a gas comprising providing first and second generally
planar electrodes
which are oriented generally parallel to and spaced from each other with walls
projecting
therefrom to define an ion generating chamber, imposing an electric field on
said chamber,
imposing a magnetic field oriented generally perpendicular to said electric
field on said
chamber, establishing separation of a plurality of ions according to mass to
charge ratio, and
causing certain of said ions to exit said chamber on the basis of mass to
charge ratio.
According to another aspect of the present invention, there is provided a
method of generating ions comprising providing first and second generally
planar electrodes
which are oriented generally parallel to and spaced from each other with walls
projecting
therefrom to define an ion generating chamber, imposing an electric field on
said chamber,
imposing a magnetic field oriented generally perpendicular to said electric
field on said
chamber, establishing separation of said ions according to mass to charge
ratio, and causing
certain ions to exit said chamber on the basis of mass to charge ratio.
3
CA 02527330 2012-03-30
71548-282
In one embodiment of the invention which may function as a mass
spectrometer or analyzer, first and second planar, generally parallel
electrodes which
generate an electrical field therebetween and having projecting walls which
cooperate
with the base of the general planar electrodes to define an ion generating
chamber.
Electric fields generated by the electrodes are oriented perpendicular with
respect to
a magnetic field which may be generated by permanent magnets or electromagnets
in a manner well known to those skilled in the art. Certain ion beams based
upon
mass to charge ratio exit through an ion exit with the other separated ion
beams
being separated on the mass to charge basis being retained within the ion
generating
chamber. An ion collector operatively associated with the ion exit is
positioned
adjacent thereto and may cooperate with processing means well known to those
skilled in the art in determining the identity of the molecule or atom.
Employing the
same apparatus without the ion collector can result in the device functioning
as a
mass selective ion generator. Related methods are also disclosed.
The apparatus and method of this embodiment are particularly
structured to be employed with low mass materials which may be 20 amu or less.
Some embodiments may provide a mass spectrometer which is
particularly suited to the special requirements of the low mass range.
Some embodiments may provide such a mass spectrometer wherein
the electric field while not necessarily uniform serves to separate the
trajectories of
ions of different mass number at the low end of the mass scale, rather than
providing
high resolving power at the upper end of the mass scale.
Some embodiments may provide such a mass spectrometer wherein
the real focusing properties can be replaced by approximation achieved by
designing
special field profiles in three spatial dimensions.
Some embodiments may provide such a mass spectrometer wherein
the electrodes would be of small dimension, of simple structure and
inexpensive to
manufacture.
3a
CA 02527330 2012-03-30
71548-282
Some embodiments may provide an analyzer or ionizer which will be
robust against imperfections in the magnetic field without materially
interfering with
the desired results and thereby allowing the use of small, inexpensive
magnets.
Examples of embodiments of the invention will now be described with
reference to the illustrations appended hereto.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure us a representation of the trajectory of a positively charged
particle in an electric field perpendicular to a magnetic field.
Figure 2 shows representations of the trajectory of four ions having
different mass to charge ratios.
Figure 3 shows the trajectory of ions of different mass to charge ratios
and associated collectors at positions before complete cycloids are flown by
the ions.
Figure 4 shows ions with different starting velocities and their
trajectories as related to an adjacent pair of electrodes.
3b
CA 02527330 2012-03-30
71548-282
Figure 5 is a representation of equal potential lines in an electric field
generated by a pair of spaced electrodes.
Figure 6 is a perspective view of a pair of spaced electrodes of
an embodiment of the present invention.
Figure 7 is a perspective view of the electrodes of Figure 27 in a
relative closed position.
Figure 8 is a partially schematic representation of an analyzer of
an embodiment of the present invention.
Figures 9 through 11, respectively, show the analyzer with the ion
starting point varied in the x-position (Figure 9), the y-position (Figure
10), and the
z-position (Figure 11).
Figure 12 illustrates the confinement capabilities in the z-direction.
Figure 13 illustrates a cross-section of the analyzer with ion beams of
four different low mass to charge ratios.
Figure 14 is similar to Figure 13, but shows the trajectories.for the
voltage being doubled in Figure 14 as compared with Figure 13.
Figure 15 shows a plot of trajectories for twenty ions having different
mass to charge ratios.
Figure 16 illustrates a group of trajectories of ions starting at the
same point and having different mass to charge ratios.
Figure 17 shows a plot of intensity versus mass to charge ratio of a
test gas mixture as measured by an analyzer of an embodiment of the present
invention.
Figures 18(a) and (b) show, respectively, equipotential lines in a
virtual plane taken through the analyzer.
Figures 18(c)(d) and 18(e)(f) show, respectively such equipotential
linesTor virtual planes positioned at different levels.
Figures 19(a)(b) show equipotential lines taken at a first virtual plane
location and Figures 19(c)(d) and Figures 19(e)(f) show, respectively,
equipotential
lines taken at the.shown virtual plane position.
Figures 20(a),(b),(c),(d),(e),(f) show, respectively, the analyzer with
virtual cutting planes at three locations and the corresponding equipotential
lines of
the electric field.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
In traditional mass spectrometer, emphasis is frequently placed on
features such as focusing, the use of "perfect" electric and magnetic fields
and the
4
CA 02527330 2005-11-28
WO 2004/108257 PCT/US2004/017117
linear relationship between the mass to charge ratio and the extent of
separation,
e.g., length or angle. An advantageous feature of the additional embodiments
is
that useful and reliable information can be obtained without requiring the
ultimate in
these hereinbefore described characteristics particularly with respect to
molecules
having a low mass which is generally on the order of less than 20 amu such as
hydrogen and helium or double ionized nitrogen, for example.
The development of crossed-fields mass spectrometry was mainly
driven by two types of analytical problems, i.e., identification of molecules
with
high molecular weight and precise measurement of the relative abundance of
isotopes. While both of these problems come from different applications, in
terms
of physics they may be considered to be equivalent. To solve these problems
required instruments with high resolution which can be designed properly if
the
equations of motion can be established and the ion trajectories are
predictable. As
mathematical difficulties were anticipated with arbitrary fields, the
development.
started with clearly defined boundary conditions such as uniform electric and.
magnetic fields, which were preferably with perfectly straight field lines.
Magnets
and the devices to create the electric field were the most costly components
in many
mass spectrometers. With increasing demands in precision, cost, weight and
dimensions were increased as well.
Among the other areas where mass spectrometers have established
usefulness are where only low mass to charge ratios were involved. Examples of
such usage are the helium leak detector and hydrogen analyzers. An advantage
of
mass spectrometry in this context is its being nearly free of interference in
this mass
range and because of its sensitivity. The analyzers used in helium leak
detectors,
for example, are mostly smaller copies of larger analyzers, e.g., sector field
mass
spectrometers which are easier to manufacture, but show a lower performance.
Nevertheless, these analyzers are relatively expensive.
Quadrupole analyzers are small and less expensive than magnetic
separators, but their filter quality decreases on approaching the lower end of
the
mass scale. The so-called zero blast represents the contribution of particles
that the
quadrupole is not tuned on as a result of weak filter characteristics. For a
helium
leak detector this does mean that the zero blast portion of the hydrogen from
the
one, two and three atomic mass units interferes with the helium signal at four
atomic mass units
=
5
CA 02527330 2005-11-28
WO 2004/108257 PCT/US2004/017117
The analytical technique of mass spectrometry generates mass spectra
regardless of the sample-introduction technique, the method of ion formation,
or the
way ions are separated. When a molecule is ionized, a characteristic ion,
representing the intact molecule and/or a group of ions of different masses
that
represent fragments of the ionized molecule, is formed. When these ions are
separated, the plot of their relative abundance versus the mass to charge
ratio (m/z)
of each ion constitutes a mass spectrum. Learning to identify a molecule from
its
mass spectrum is much easier than using any other type of spectral
information.
The mass spectrum shows the mass of the molecule and the masses of its pieces.
Mass spectrometry offers more information about an analyte from less sample
than
any other technique. Mass spectrometry is also the most accurate technique for
the
detellnination of mass. The only disadvantage of mass spectrometry compared to
other techniques is that, usually, the sample is consumed; however, so little
sample
is required, it is inconsequential.
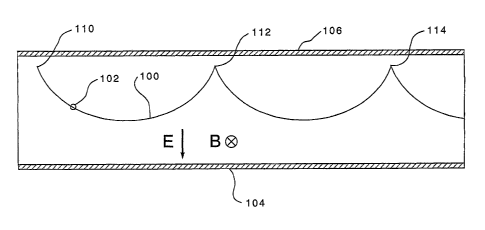
Figure 1 shows the trajectory 100 of a positively charged particle 102
in a uniform electric field E created by the generally parallel electrodes
104,106,
while a uniform magnetic Field B is acting perpendicular to the electric field
in a
direction into the page. The magnetic field can be created by permanent
magnets or
electromagnets. In Figure 1 and all following figures the magnet parts which
will
be well known to those skilled in the art are not shown. Instead of this the
direction
of B is marked by a symbol. According to the symbol the magnetic north pole is
always above the drawing plane, while the south pole is located beneath it. If
the
particle starting at the starting point 110 does not have initial energy, the
trajectory
is a cycloid. The electrodes 104, 106 are assumed to form a perfect capacitor
to
create a homogeneous field. This is related to the electrode area 104, 106 and
relatively large spacing therebetween.
This principle is used in cycloidal mass spectrometers, which are
double focusing instruments. See generally U.S. Patents 2,882,410; 3,070,951;
3,590,243; and 4,298,795. An analyzer is said to be double focusing, if
locations
like 112 and 114, where the starting point's conditions are reproduced
periodically,
do not depend on the starting energy, nor the starting angle of the particle.
In
Figure 1 and all following discussions the starting energy to be zero if not
expressed
differently.
Figure 2 shows four trajectories 120, 122, 124, 126, flown by ions
with mass to charge ratios of 1, 2, 3, and 4, respectively, starting at the
starting
6
CA 02527330 2005-11-28
WO 2004/108257 PCT/US2004/017117
point 112 between electrodes 114, 116 is assumed. The pitch of one cycloid,
which
is the distance between two points that are reproduced periodically, is
proportional
to the mass to charge ratio, m/z. Thus the pitch of an ion with m/z=4 equals
four
times the pitch of an ion with m/z=1. This is the physical separation effect
in
cycloidal mass analyzers. In Figure 2 the ions impinge, respectively, after
one
cycloid on the collectors 130, 132, 134, 136, where they are discharged. The
resulting current into the collector is the measure for the number of ions
hitting the
collector per time unit.
The separation of ions with different m/z ratios does not require
flying a complete cycloid which is what is necessary to achieve the double
focusing
properties. In Figure 2 the separation starts right with the start of the
motion. In
Figure 3 the collectors 136, 137, 138 and 139 are positioned between
electrodes
140, 144 as to trajectories 150, 152, 154 and 156 in a way that does not allow
full
cycloids for the ions with m/z=2, m/z=3, and m/z= 4. The particles fly inside
a
perfect capacitor with a uniform electric field. The obvious advantage of the
arrangement in Figure 3 is the shrunken size of the analyzer. A negative is
the loss
in resolution because of the short trajectory, and a widened ion beam because
the
collectors 137, 138 and 139 are outside the focus. If ions of the same rn/z
ratio
start with different energies and different angles they hit the collector
plane at
different positions. This effect is demonstrated in Figure 4 wherein 10 ions
have
m/z=4 but different starting energies at the starting point 170 fly on
different
trajectories that result in a widening ion beam which hits the collector 188
only
partially. As a result there would be an additional loss in resolving power
and a
decrease in sensitivity.
In Figure 4 the ions with higher starting velocity at point 170
between generally parallel electrodes 172, 174 in direction of the motion are
more
deflected to the outer side of the cycloid, 180, correspondingly ions with
lower or
negative starting velocity fly trajectories closer to the inside 182.
Intuitively it can
be seen that an electric field, which varies in an appropriate way should
compensate
the deflection of the ions depending on their starting energy. Qualitatively
it is
evident that the field has to become stronger in areas where the ions with
higher
starting energy fly. Then this field will focus the ions regardless of their
starting
energy on the collector 188 or as hereinafter described, on a collector slit.
Figures 1 through 4 were produced by the ion flight simulation
program SIMION 3D V6.0 from the Idaho National Engineering Laboratory and
7
CA 02527330 2005-11-28
WO 2004/108257 PCT/US2004/017117
the Lockheed Idaho Technology Company. Unifoi __ In electric fields can be
simulated easily when the edges of the electrodes defined extend to the
boundaries
of the three-dimensional working space. Then the program assumes the extension
of the electrodes as infinite. SIMION was used then to find electrode
structures to
generate fields, that can approximate the ideal focusing field for a fragment
of a
cycloidal trajectory.
The simplest structure found is a "non-perfect" capacitor consisting
of two parallel plates. "Non-perfect" means that the gap between the
electrodes is
large compared to their area. Those capacitors produce fringing fields which
leads
to increasing field strength near the edges of the electrodes.
In Figure 5 the electrodes 190, 192, now with finite dimensions, are
connected to a voltage source as marked with + and -. The field generated is
presented as equipotential lines. In the circled area 300 the radius of the
curvature
is increased which corresponds to an increase in field strength. For a group
of ions
having the same rn/z ratio, but different starting energies at the starting
point 302 a
voltage can be chosen in a way that the ions fly half a cycloid 304 until they
reach
the physical end of the electrode area.
The simple modeling experiment showed already significantly
focusing tendency. Not acceptable was the wrong direction of the field
curvature in
z-direction (perpendicular to the planes the ion trajectories are located in).
This
accelerates the ions away from the intended path and they are lost for the
detection
process.
Starting with the plain capacitor the electrodes' shapes were refined
by successive approximation in the simulation process. Replacing the flat
plate
electrodes by U-shaped electrodes corrected the curvature in z-direction,
adding two
facepla-tes to each electrode improved the focusing proper-ties. Eventually
all
dimensions were varied in steps to find the optimum set. The properties of the
field
created by these electrodes are discussed hereinafter.
The final shape of a preferred form of the electrodes is seen in Figure
6. They can be made of sheet metal, made of coated ceramics or machined from
bulk metal. The overall structure consists of a first generally U-shaped part
310
having a base 312 and two parallel sidewalls 314, 316, for example, and a
faceplate
320, which is connected to the U-shaped part 310. The second generally U-
shaped
electrode 326 has a base 328 and two parallel sidewalls 330, 332. A faceplate
336
is also connected to generally U-shaped electrode 326. One electrode, 326, has
two
8
CA 02527330 2005-11-28
WO 2004/108257 PCT/US2004/017117
orifices 340 and 344 that will become entrance and exit apertures for an
electron
beam to ionize gas molecules or gas atoms. The other electrode, 310, has slit
350
in the faceplate 320 that will become the exit aperture for the ions before
they reach
the collector. After assembling the electrodes 310 and 326 to a suitable
holder with
interposed electrical insulation (not shown in the drawings) or which leaves
an air
gap between electrodes 310, 326 in the final position with respect to each
other is
shown in Figure 7. In Figure 7, the electrodes are insulated, the electrode
parts of
310 are separated from the adjacent parts of electrode 326 by small gaps 370,
372,
374, 376, and 378.
In this configuration the electrodes form a cuboid. Typical
dimensions of the first prototypes are 14 mm in the x direction, 8 mm in the y-
direction, and 7mm in the z-direction. There is no limited range within which
the
dimensions have to fit. Another prototype for instance was made with
dimensions 7
mm x 4nun x 3.5 mm. Because the ratio of each dimension to another one has not
changed, the principal function is not affected. The voltage applied to the
electrodes has to be decreased by the square of the reduction factor to obtain
qualitatively the same analyzer operation. However, in a real application the
influence of thermal motion, imperfections in manufacturing, and
electromagnetic
distortions becomes more serious with decreasing field strength.
The configuration to complete an analyzer is shown in Figure 8. The
magnet and vacuum chamber which are well known to those skilled in the art are
not shown in this drawing. The way to ionize the gas molecules has no
influence on
the separator. However, this analyzer is particularly suitable for electron
impact
ionization. A current source (not shown) connected to the filament terminals
500,
502 heats the filament 504, which then emits electrons by thermal emission.
Provided the potential of the filament 504, relative to the electrode, is
negative the
electrons are accelerated to the entrance aperture 340. The electron beam 510
created remains narrow while crossing the inner volume of the cuboid because
the
electrons fly parallel to the magnetic field lines. The electron beam 510
exits
through the aperture 344 and impinges on the anode 520, which is connected by
its
terminal 526 to a potential, which is positive compared to the electrode 326
(not
shown).
If the potential difference between the filament 504 and the electrode
326 is larger than the ionization energy for an atom or a molecule ionization
takes
9
CA 02527330 2005-11-28
WO 2004/108257 PCT/US2004/017117
place. The commonly used ionization energy is 70 eV where most gases show a
maximum in ionization efficiency.
Ions created by the electron beam fly on trajectories 530, which are
alike, half a cycloid. The ions fly along a mathematically correct cycloid if
the
electric and magnetic fields are uniform and perpendicular to each other. If
the m/z
ratio of the ions matches the conditions provided by the applied fields the
ions are
focused into the exit slit 350 and impinge on the collector 534 which will
responsively emit a signal to an associated microprocessor (not shown). Ions
with
lower or higher m/z ratio miss the slit.
The ionizer, as described, does not have a physical aperture to
release the ions. Instead the whole area where ions are created - this is the
volume
of the electron beam inside the electrodes - contributes to the ion beam
leaving the
ion source. Imaging properties of the electric field reduce the influence of
the
beam's diameter on the resolving power. This is shown in Figures 9 through 13,
which show simulations for the analyzer shown in Figure 8 with the dimensions
14
mm (x), 7 mm (y) and 4 mm (z). For 10 ions with miz=4 the starting point is
varied in x-position (Figure 9), y-position (Figure 10), and z-position
(Figure 11).
In Figure 9 the ion trajectories 540 converge to a point 544, in Figure 10
with ion
trajectories the initial spread in y is reduced by about 30%, Figure 11 shows
that
the influence of the distribution of the trajectories in z is minimal.
Figure 12 demonstrates the confinement capabilities in z-direction.
The drawing shows the projection of the ion beam 560 from Figure 11 into the y-
z-
plane. 30 ions starting from different locations 566 are deflected to the x-y-
plane in
the center of the analyzer before they reach the collector slit position 568.
In Figure 13 the separation characteristic for low m/z ratios is
simulated. Four ion beams 570, 572, 574, 576 with m/z ratios 1 through 4 start
at
an area 584. For a certain voltage applied to the electrodes 310, 326 (Fig. 8)
only
ions with the appropriate in/z ratio - in Figure 13 ions with m/z-=4 - can
penetrate
the collector slit 586 and impinge on the collector 590.
The analyzer can be tuned to other m/z ratios by using the fact that
the reciprocal m/z ratios z/m are proportional to the voltages needed to hit
the
collector slit. Doubling the voltage that tuned the analyzer to m/z=4 in
Figure 13
tunes the system to m/z=2, which is shown in Figure 14 starting at 592 and
having
trajectories 594, 596, 598, 700.
CA 02527330 2005-11-28
WO 2004/108257 PCT/US2004/017117
The trajectories of 20 ions from m/z=1 to 20 are plotted in Figure
15 from 704 through 706. The analyzer is tuned to miz=4. It can be seen that
the
separation in the plane 702 is no longer a linear function of the m/z ratio.
The field
profile widens the spread at the lower m/z scale.
For this analyzer with the dimensions listed above and a collector slit
width of 5mm the usable mass range ends by m/z=18, which is shown in Figure
16. The ions 720, 722 and 724 with m/z ratios 17, 18, and 19 start at the same
point 728. If the ionizer is tuned to m/z=-17 as shown in this simulated plot
and the
ion with m/z =17 exits through the middle of the collector slit 586, the ions
with
in/z=-16 and m/z 18 cannot reach the collector 590.
Figure 17 shows a mass spectrum 4m/z=1 amu to m/z---40 amu for
a test gas mixture containing hydrogen, helium, oxygen, nitrogen and argon. At
low m/z ratios the analyzer provides sufficient resolving power to use it for
hydrogen analyzers or helium leak detectors, for example.
Figures 18(a)-(f), 19(a)-(f) and 20(a)-(f) show equipotential lines in
different planes inside the analyzer. The figures to the right of the
potential
diagram indicates where the virtual planes are located.
From Figure 18 we conclude that the field 730 around the center
approaches in a rough approximation a uniform field which explains the
similarity
of the trajectories shown before with cycloids. The increase in curvature in
the
vicinity of the collector 732 is introduced to compensate the loss of energy
focusing.
Figure 19 suggests that ions starting at extreme z positions 734, 736
will experience a strongly spoiled electric field. The trajectories presented
above
are obtained when the ions starting positions are located in a restricted
central area
740 where the field approximates the uniform structure.
The area 742 in Figure 20(c) confirms that the electric field near the
center approaches uniformity. For the confining properties to be seen in
Figure
20(a) the potential is curved as shown by the circle 744 in Figure 20(a).
Because of its simple structure and the small physical dimensions the
analyzer described herein can act as a low cost, but high performance ion
source to
introduce ions into a mass spectrometer. The same arrangement without the
collector provides all capabilities for an ion source. If it is tuned properly
to the m/z
position the mass spectrometer is tuned at, its mass selectivity holds back
most of
the unwanted ions from the mass spectrometer's analyzer. This improves the
11
CA 02527330 2012-03-30
71548-282
resolving power with any mass spectrometer. In connection with a quadrupole
filter
the zero blast can be suppressed efficiently.
Another advantage of the cuboid design is the chance to use it as a
closed ion source. Closed ion sources are connected to the analyzer's vacuum
with
low molecular flow conductance. As a result, the pressure in the ion source
can be
higher than in the analyzer which increases the sensitivity. For this purpose
the
gaps shown in Figure 8 should be very narrow or even sealed with an insulator.
The gaseous sample then can be introduced by a thin tube at nearly any
position of
the electrodes.
It will be appreciated that in the embodiment of Figures 1 through 20
the miniaturized mass spectrometer or analyzer or ionizer provides an
effective
means employing mass to charge ratio as controlled by adjusting the electric
field in
the presence of a magnetic field to determine what mass charge to ratio ion
beam
will be emitted or permitted to exit through the exit opening and which will
not.
Among numerous uses of this separation apparatus and method are in leak
detection
as, for example, in helium or hydrogen uses. Further, the system is designed
to
function effectively with partial cycloid ion beams. It is particularly suited
to
making determination with respect to materials having low mass such as, for
example, on the order of 20 amu or less. All of this can be accomplished while
employing a very small housing.
Whereas particular embodiments have been described hereinabove,
for purposes of illustration, it will be evident to those skilled in the art
that
numerous variations of the details may be made.
12