Note: Descriptions are shown in the official language in which they were submitted.
CA 02528275 2005-11-29
1
METHOD FOR SURFACE TREATMENT OF ALUMINUM ALLOY
CROSS-REFERENCE TO RELATED APPLICATIONS
This application is based upon and claims the benefit
of priority from Japanese Patent application No. 2004-
347244 filed on November 30, 2004.
BACKGROUND OF THE INVENTION
Field of the Invention
The present invention relates to a method for surface
treatment of aluminum alloy. More particularly, it relates
to a method for surface treatment of aluminum alloy that
can form a zinc phosphate coating that is uniform, dense
and excellent in corrosion resistance.
Related Art
In the field of vehicle bodies and automobile parts,
building materials, and furniture, etc., metallic materials
such as steel plates, galvanized steel plates and aluminum
alloys have been used. These metallic materials are, after
molding, painted to be final products. Paint is applied to
improve the appearance of these metallic materials, and has
its principal object, to prevent corrosion of the material.
The material is then sent to surface treatment processes
such as degreasing, surface control, and chemical
conversion treatment in sequence.
In order to perform the following chemical conversion
treatment step, the surface must be adjusted to form a
NPF-062
CA 02528275 2005-11-29
2
uniform and highly dense chemical conversion treatment
coating rapidly over the surface of the metallic material.
Specifically, a surface control agent is brought into
contact with the surface of the material in order to have
the particles of the surface control agent adsorbed on the
surface of the material, therefore promoting the formation
of the chemical conversion treatment coating.
In vehicle body and other vehicle parts, aluminum
alloy is employed in part, and a member of the aluminum
alloy is in contact with a cold rolled steel plate
(hereinafter, referred to as a SPC) or an alloyed hot-dip
zinc-coated steel plate (hereinafter, referred to as a GA).
In these members wherein aluminum is a base metal, there
may be a case wherein aluminum is excessively dissolved in
the chemical conversion treatment agent to disturb a proper
formation of the chemical conversion coating.
To avoid this problem, a vehicle body can be
assembled with an insulating material sandwiched between
the aluminum alloy and SPC or aluminum alloy and GA. With
this configuration, the excess dissolution of aluminum in
the chemical conversion treatment agent is inhibited in the
chemical conversion treatment agent so that the formation
of excellent chemical conversion coating on the aluminum
alloy can be attained.
Use of insulating materials, however, disturbs
current flow of the aluminum alloy during the next electro-
deposition coating step, leading to failure of a proper
NPF-062
CA 02528275 2005-11-29
3
coating. For this reason, an additional connection to the
aluminum alloy via an electric cable, etc., is needed in
the electro-deposition coating step. Moreover,
retightening is further required as the insulating material
contracts during a baking step after the electro-deposition
coating step. In short, the case in which insulating
material requires more processes than usual operations and
production lines specific to these additional steps.
A larger content of copper in aluminum alloy reduces
its corrosion resistance property, in particular, filiform
rust resistance and water adherence resistance properties.
Degradation of these properties is caused by a large
potential difference existing between the aluminum and
copper that lead to a considerable amount of dissolution of
aluminum under the corrosive environment. In order to
assure excellent corrosion resistance property, contents of
copper in the aluminum alloy need to be reduced; however,
this solution poses a problem as it leads to a cost
increase.
It is therefore an urgent necessity for those with
ordinary skill in the art to develop a surface treatment
method that enables to form a uniform and highly dense
chemical conversion treatment coating with excellent
corrosion resistance property on the surface of aluminum
alloy. Research toward an improvement of chemical
conversion treatment agent has been carried out in order to
attain the method and is disclosed in the Japanese Patent
NPF-062
CA 02528275 2005-11-29
4
No. 3366826 (hereinafter Patent Document 1). The patent
document 1 proposed a water solution which mainly contains
0.1 g/1 to 2.0 g/1 of zinc ion, 0.1 g/1 to 4.0 g/1 of
nickel ion, 0.1 g/1 to 3.0 g/1 of manganese ion, 5 g/1 to
40 g/1 of phosphate ion, 0.1 g/1 to 15 g/1 of nitrate ion,
0.01 g/1 to 0.5 g/1 of nitrite ion, and as a fluoride, 0.5
g/1 to 1.0 g/1 of fluoride in a complex fluoride, 0.3 g/1
to 0.5 g/1 of fluoride in a simple fluoride, and further
proposes zinc phosphate treating agent that contains 0.025
g/1 to 0.45 g/1 of iron(Fe) in a chelating agent that can
chelate bond with iron ion. According to the zinc
phosphate treating agent, a uniform and highly dense zinc
phosphate coating with excellent corrosion resistance
property can be formed on the surface of an aluminum alloy
having a copper content of 0.1o by weight or less, or on
the surface treated by grinding thereof.
SUMMARY OF THE INVENTION
In order to solve the aforementioned problems, the
present invention offers an improvement in both a surface
adjustor and a chemical conversion treatment agent. It is
an object of the present invention to provide a method for
surface treatment of an aluminum alloy that can form a
uniform and highly dense zinc phosphate coating with
excellent corrosion resistance properties on the surface of
the aluminum alloy.
The present inventors have diligently studied to
NPF-062
CA 02528275 2005-11-29
solve the aforementioned problems. As a result, they
finally achieved a formation of a uniform and highly dense
zinc phosphate coating with excellent corrosion resistance
property on the surface of the aluminum alloy. The method
includes steps of surface adjusting the surface of the
aluminum alloy by way of a surface adjustor that contains a
determined amount of zinc phosphate particles, copolymer
containing carboxylic acid group, and natural hectorite
and/or synthetic hectorite, respectively, and then of
chemical treating of chelating agent that can chelate bond
with iron ion. More specific description will be given
below.
A first aspect of the present invention is a method
for surface treatment of aluminum alloy which includes
steps of degreasing and rinsing a surface of aluminum alloy,
surface adjusting the surface of the degreased and rinsed
aluminum alloy with a surface adjustor, chemical conversion
treating of the surface of the surface adjusted aluminum
alloy with a zinc phosphate chemical conversion treatment
agent, the surface adjustor having a pH of not less than 7
and not more than 12 and containing not less than 50 ppm
and not more than 2000 ppm of zinc phosphate particles
having not more than three micrometers of Dso, not less
than 2 ppm and not more than 200 ppm of a copolymer
containing carboxylic acid group that copolymerizes a
monomeric composition containing less than 50o by weight of
acrylic acid and more than 50o by weight of 2-acrylamide-2-
NPF-062
CA 02528275 2005-11-29
6
methylpropanesulfonic acid, and not less than 3 ppm and not
more than 200 ppm of natural hectorite and/or synthetic
hectorite, the zinc phosphate chemical conversion treatment
agent comprising an acid aqueous solution containing more
than 0.1 g/1 and less than 2.0 g/1 of zinc ion, more than
0.1 g/1 and less than 4.0 g/1 of nickel ion, more than 0.1
g/1 and less than 3.0 g/1 of manganese ion, 5 g/1 and less
than 40 g/1 of phosphate ion, more than 0.5 g/1 and less
than 1.0 g/1 of fluoride in a complex fluoride, more than
0.3/1 and less than 0.5 g/1 of fluoride in a simple
fluoride, and more than 0.025 g/1 and less than 0.45 g/1 of
iron(Fe) in a chelating agent that can chelate bond with
iron ion.
A second aspect of the present invention is a method
for surface treatment of aluminum alloy as in the first
aspect of the present invention in which the content of
copper in the aluminum alloy is not more than 0.2o by
weight.
According to the present invention, a method for
surface treatment of aluminum alloy can be provided that
can form a uniform and highly dense zinc phosphate coating
with excellent corrosion resistance properties on the
surface of the aluminum alloy.
BRIEF DESCRIPTION OF THE DRAWINGS
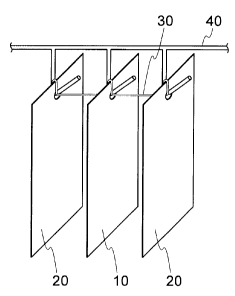
Fig. 1 shows an object material for treatment in
which aluminum alloy and SPC are contacted and conducted.
NPF-062
CA 02528275 2005-11-29
DETAILED DESCRIPTION OF THE INVENTION
Hereinafter, embodiments of the present invention
will be described in detail.
Surface adjustor
The surface adjustor employed in the present
invention contains a determined amount of zinc phosphate
particles, copolymer containing carboxylic acid group, and
natural hectorite and/or synthetic hectorite, respectively.
More specifically, the surface adjustor has a pH of not
less than 7 and not more than 12, and contains not less
than 50 ppm and not more than 2000 ppm of zinc phosphate
particles having not more than 3 micrometers of DSO, not
less than 2 ppm and not more than 200 ppm of a copolymer
containing carboxylic acid group that copolymerizes a
monomeric composition containing less than 50o by weight of
acrylic acid and more than 50o by weight of 2-acrylamide-2-
methylpropanesulfonic acid, and not less than 3 ppm and not
more than 200 ppm of natural hectorite and/or synthetic
hectorite. Conventionally known surface adjustor
containing bivalent or trivalent phosphate particles cannot
form a sufficient amount of chemical conversion coating on
the surface of aluminum alloy. On the other hand, the
surface adjustor employed in the present invention can form
a sufficient amount of chemical conversion coating on the
surface of aluminum alloy. Therefore a method for surface
treatment according to the present invention can provide a
NPF-062
CA 02528275 2005-11-29
8
sufficient amount of corrosion resistance property to the
aluminum alloy.
The zinc phosphate particle contained in the surface
adjustor employed in the present invention is not more than
3 micrometers of DSO, which is smaller than a zinc
phosphate particle employed in the conventional surface
adjustor. Because of this particle size, sedimentation of
zinc phosphate particle in the surface adjustor can be
inhibited and thus assures excellent stability. Moreover,
larger amounts of surface adjustor particles can be
adsorbed on the surface of the aluminum alloy and thus
promote formation of chemical conversion coating. In this
connection, it is not preferred that the zinc phosphate
particle is not more than 0.01 micrometers of DSO because
due to excessive dispersion it may lead to coagulation of
zinc phosphate particles, but it is preferred that the zinc
phosphate particle be not less than 0.05 micrometers and
not more than 1 micrometers of DSO.
The term "DSO" employed herein is called a "volume
50o median diameter" and means a particle diameter at a
point where accumulative curve exhibits 50o wherein the
curve is determined as the entire volume of the zinc
phosphate particle is assumed as 100% based upon the
particle size distribution in the dispersion. The DSO can
be measured by using for example a particle size measuring
device such as a laser Doppler type particle size analyzer
("Microtrac UPA150" manufactured by Nikkiso Co., Ltd).
NPF-062
CA 02528275 2005-11-29
9
The zinc phosphate particle can be obtained by using
zinc phosphate that is generally employed as a raw
material. Zinc phosphate includes tetrahydrate, dehydrate,
monohydrate, and anhydrate. The surface adjustor according
to the present invention can use any zinc phosphate
described above. Typically, a white colored tetrahydrate
powder which is readily available in general can be used
without being treated. Alternatively, these zinc phosphates
can be surface treated in various manners, for example,
surface treated with silane coupling agent, rosin, silicone
compound, or metallic alkoxide such as silicone alkoxide or
aluminum alkoxide. In addition, zinc phosphate are not
particularly limited in shape but can be any shape such as
a plate or a scale. These zinc phosphates are miniaturized
by conventionally known dispersion method using a bead
mill, a high-pressure homogenizer, or a supersonic
disperser to obtain a zinc phosphate having not more than 3
micrometers of D5o
The content of zinc phosphate particles are not less
than 50 ppm and not more than 2000 ppm, preferably, not
less than 60 ppm and not more than 1500 ppm. In the case in
which not more than 50 ppm of zinc phosphate particle is
contained, only a small amount of surface adjusting
particles are absorbed, leading to a failure of formation
of the chemical conversion coating. Also in the case in
which more than 2000 ppm of particles are contained, it is
not economical as the surface adjusting effect
NPF-062
CA 02528275 2005-11-29
corresponding to the increase in volume will not be
provided.
The surface adjustor employed in the present
invention contains a copolymer containing a carboxylic acid
group having monomeric compounds copolymerized. The
monomeric compounds contain less than 50o by weight of
acrylic acid and more than 50o by weight of 2-acrylamide-2-
methylpropanesulfonic acid. The copolymer containing a
carboxylic acid group acts as a dispersion agent and at the
same time have an effect of promoting a formation of
chemical conversion coating. With this property, uniform
and dense chemical conversion coating can be formed and
excellent corrosion resistance property can be provided
with aluminum alloy at the next chemical conversion step.
The copolymer containing a carboxylic acid group can
be obtained easily according to conventionally known
methods of copolymerization of monomeric compound under
catalysis of, for example, peroxide. The monomeric compound
contains not more than 50o by weight of acrylic acid and
more than 50o by weight of 2-acrylamide-2-
methylpropanesulfonic acid. When the contents of acrylic
acid are more than 50o by weight or the contents of 2-
acrylamide-2-methylpropanesulfonic acid are less than 500
by weight, excellent chemical conversion coating cannot be
formed on the surface of the aluminum alloy. It is
preferable that the lower limit of acrylic acid content be
20o by weight, and more preferably 25o by weight.
NPF-0 62
CA 02528275 2005-11-29
11
It is preferable that the higher limit of acrylic
acid content be 45o by weight, and more preferably 40o by
weight. It is preferable that the lower limit of the
content of 2-acrylamide-2
-methylpropanesulfonic acid be 55o by weight, and more
preferably 60% by weight. It is also preferable that the
higher limit of the content of 2-acrylamide-2-
methylpropanesulfonic acid be 80o by weight, and more
preferably 75o by weight.
The aforementioned monomeric compound may contain
other monomeric substances within a range that will not
impair the effect of the present invention. The other
monomeric substances cited herein include acrylic acid
methyl, acrylic acid ethyl, acrylic acid propyl, acrylic
acid butyl, acrylic acid pentyl, methacrylic acid methyl,
methacrylic acid ethyl, a methacrylic acid propyl,
methacrylic acid butyl, methacrylic acid pentyl, acrylic
acid hydroxy methyl, acrylic acid hydroxyethyl, an acrylic
acid hydroxypropyl, acrylic acid hydroxybutyl, acrylic acid
hydroxypentyl, methacrylic acid hydroxymethyl, methacrylic
acid hydroxyethyl, a methacrylic acid hydroxypropyl,
methacrylic acid hydroxybutyl, methacrylic acid
hydroxypentyl, acetic acid vinyl, etc,. These monomeric
substances can be independently mixed or two or more
substances may be mixed together in the above mentioned
monomeric compound.
The content of copolymer containing a carboxylic acid
NPF-062
CA 02528275 2005-11-29
12
group is not less than 2 ppm and not more than 200 ppm,
more preferably, not less than 4 ppm and not more than 100
ppm. In the case where not more than 2 ppm of copolymer
containing a carboxylic acid group are contained, the
diameter of zinc phosphate particles become larger due to
lack of dispersion properties, causing reduction of
stability of surface adjustor and sedimentation thereof.
Also, in the case in which more than 200 ppm of copolymer
containing a carboxylic acid group are contained, it is not
economical as the surface adjusting effect corresponding to
the increase in volume will not be provided.
Moreover, the surface adjustor employed in the
present invention contains natural hectorite and/or
synthetic hectorite. The natural hectorite and synthetic
hectorite respectively can be employed separately, or two
or more types of hectorite can be employed together. The
natural hectorite and/or synthetic hectorite is/are
contained in the surface adjustor so that the adjustor can
provide more excellent dispersion stability and can avoid
sedimentation of the zinc phosphate particles.
The natural hectorite is a trioctahedral type clay
mineral belonging to the montmorillonite group represented
in formula 1. The commercially available natural hectorite
includes, for example, BENTON EW, and BENTON AD;
manufactured by ELEMENTIS Co., Ltd.).
Formula 1
[Si8 (MgS. s41-io. ss) 020 OOH) 4M+o. ss'nH20~
NPF-062
CA 02528275 2005-11-29
13
The synthetic hectorite, represented in formula 2,
has a three crystalline layer structure, and is similar to
a hectorite that belongs to an unlimited layer expansion
type trioctahedral having an expansion lattice. The
commercially available synthetic hectorite includes, for
example, B, S, RD, RDS, XLG, XLS, etc., manufactured by
Laporte Industries Ltd. These hectorites are white colored
powders and can easily form sol or gel when water is added.
As other commercially available synthetic hectorite,
LUCENTITE SWN manufactured by Co-op Chemical Co Ltd can be
included.
Formula 2
[Si8 (MgaLib) 020 (OH) cF4_c~ X-MX+
In formula 2, 0<a~6, 0<b~6, 4<a+b<8, 0~=c<4, x=12-
2a-b, and M is almost Na. Synthetic hectorite contains
magnesium, silicone, and sodium as a principal component
and a small quantity of lithium and fluoride.
The content of natural hectorite and/or synthetic
hectorite is not less than 3 ppm and not more than 200 ppm,
more preferably, not less than 20 ppm and not more than 100
ppm. In the case in which less than 3 ppm of natural
hectorite and/or synthetic hectorite is contained,
sedimentation of phosphate zinc particles may not be
effectively avoided. Also, in the case in which more than
200 ppm of hectorite is contained, it is not economical as
the surface adjusting effect corresponding to the increase
in volume will not be provided.
NPF-062
CA 02528275 2005-11-29
14
The pH of surface adjustor employed in the present
invention is not less than 7 and not more than 12. When the
pH is less than 7, phosphate zinc particles cannot be
easily dissolved and can become unstable. On the other
hand, when the pH is more than 12, the pH of the chemical
conversion agent increases and may cause improper chemical
conversion.
The surface adjustor employed in the present
invention may further be mixed with other dispersion
agents, dispersing medium, or viscosity improver agents
within a range that will not impair the effects provided
according to the present invention. When a surface
adjustor prepared as such is used in order to surface
adjust the aluminum alloy, the surface adjustor is brought
into contact with the surface of aluminum alloy. Their
contacting method is not particularly limited, and
conventionally known methods such as dipping or spraying
can be used.
Zinc phosphate chemical conversion treatment agent
The zinc phosphate chemical conversion treatment
agent employed in the present invention may include an acid
aqueous solution containing not less than 0.1 g/1 and not
more than 2.0 g/1 of zinc ion, not less than 0.1 g/1 and
not more than 4.0 g/1 of nickel ion, not less than 0.1 g/1
and not more than 3.0 g/1 of manganese ion, not less than 5
g/1 and not more than 40 g/1 of phosphate ion, not less
than 0.5 g/1 and not more thanl.0 g/1 of fluoride in a
NPF-062
CA 02528275 2005-11-29
complex fluoride, not less than 0.3 g/1 and not more than
0.5 g/1 of fluoride in a simple fluoride, and not less than
0.025 g/1 and not more than 0.45 g/1 of iron(Fe) in a
chelating agent that can chelate bond with iron ion.
In the zinc phosphate chemical conversion treatment,
it is known that a uniform and dense zinc phosphate coating
can be formed on an iron based surface or a zinc based
surface by having bivalent or trivalent iron ion contained
in the chemical conversion treatment agent. The zinc
phosphate chemical conversion treatment agents employed in
the present invention have a chelating agent that can
chelate bond with iron ion. The chelating agent chelates an
eluted iron ion from the iron based surface. Because of
this, a constant amount of iron ion can be stably held
during the chemical conversion treatment. The effect of the
above mentioned iron ion can appear in the formation of the
zinc phosphate coating on the aluminum based surface.
Preferably, the concentration of zinc ions is not
less than 0.1 g/1 and not more than 2.0 g/1, and more
preferably not less than 0.3 g/1 and not more than 1.5 g/1.
In the case in which the concentration of zinc ion is less
than 0.1 g/1, a uniform zinc phosphate coating cannot be
formed on an aluminum based surface, and it causes lack of
hiding. In addition, formation of blue colored coatings is
observed partially where improper coating is applied. In
the case in which concentration of zinc ion is more than
2.0 g/1, zinc phosphate coating can be uniformly formed;
NPF-062
CA 02528275 2005-11-29
16
however, it is easily dissolved in alkalis. It is
particularly unfavorable for the coating as it is exposed
to an alkaline atmosphere during the next cationic electro-
deposition coating.
The concentration of nickel ion is not less than 0.1
g/1 and not more than 4.0 g/1, and more preferably not less
than 0.1 g/1 and not more than 2.0 g/1. In the case in
which the concentration of nickel ion is less than 0.1 g/1,
the corrosion resistance properties of iron are degraded,
and when the concentration of nickel ion is more than 4.0
g/1, the corrosion resistance properties of aluminum are
degraded.
The concentration of manganese ion is not less than
0.1 g/1 and not more than 3.0 g/1, and more preferably not
less than 0.6 g/1 and not more than 3.0 g/1. In the case
in which the concentration of manganese ion is less than
0.1 g/1, adhesion properties between the zinc based surface
and the coating as well as resistance properties to hot
salt water will not be sufficiently enhanced. Moreover,
when the concentration of manganese ion is more than 3.0
g/1, it is economically disadvantageous as the expected
effects corresponding to the increase in volume will not be
provided.
The concentration of phosphate ion is not less than 5
g/1 and not more than 40 g/1, and more preferably not less
than 10 g/1 and not more than 30 g/1. When the
concentration of phosphate ion is less than 5 g/1, it often
NPF-062
CA 02528275 2005-11-29
17
forms a non uniform zinc phosphate coating, and when the
concentration of phosphate ion is more than 40 g/1, it is
economically disadvantageous as the expected effects
corresponding to the increase in volume will not be
provided.
As for fluoride, the concentration of complex
fluoride is not less than 0.5 g/1 and not more than 1.0 g/1
of fluoride in a complex fluoride. In the case in which the
concentration of complex fluoride is less than 0.5 g/1 of
fluoride in a complex fluoride, uniform zinc phosphate
coating cannot be formed on the aluminum based surface and
thus fails to obtain excellent corrosion resistance
properties. Moreover, when the concentration of complex
fluoride is more than 1.0 g/1 of fluoride in a complex
fluoride, the iron based surface is excessively etched to
reduce the amount of zinc phosphate coating and thus also
fails to obtain excellent corrosion resistance properties.
On the other hand, the concentration of simple
fluoride is not less than 0.3 g/1 and not more than 0.5 g/1
of fluoride in a simple fluoride. In the case in which the
concentration of simple fluoride is less than 0.3 g/1 of
fluoride in a simple fluoride, uniform zinc phosphate
coating cannot be formed on the aluminum based surface and
thus fails to obtain excellent filiform rust resistance
properties. Moreover, when the concentration of simple
fluoride is more than 0.5 g/1 of fluoride in a simple
fluoride, the etching volume on the aluminum based surface
NPF-062
CA 02528275 2005-11-29
18
is increased to promote the formation of by-products having
Al, F, and Na as principal components on the aluminum based
surface and thus fails to obtain excellent resistance
properties to filiform rust and to water adhesion.
A source of supply for zinc ion includes, for
example, zinc oxide, carbonic acid zinc, nitric acid zinc,
etc. A source of supply for nickel ion includes, for
example, carbonic acid nickel, nitric-acid nickel, nickel
chloride, phosphate nickel, nickel hydroxide, etc. A
source of supply for manganese ion includes, for example,
manganese carbonate, manganese nitrate, manganese chloride,
manganese phosphate, etc. Moreover, a source of supply for
phosphate ion includes, for example, phosphate, zinc
phosphate, manganese phosphate, etc.
Furthermore, complex fluoride includes, for example,
SiF6, BF4, etc. , and a source of supply for SiF6 includes,
for example, hydrofluosilicic acid, hydrofluosilicic acid
nickel, hydrofluosilicic acid zinc, hydrofluosilicic acid
manganese, hydrofluosilicic acid iron, hydrofluosilicic
acid magnesium, hydrofluosilicic acid calcium, etc. As for
a source of supply for BF4, for example, boric hydrofluoric
acid, boric hydrofluoric acid nickel, boric hydrofluoric
acid zinc, boric hydrofluoric acid manganese, boric
hydrofluoric acid iron, boric hydrofluoric acid magnesium,
boric hydrofluoric acid calcium, etc., may be mentioned.
As for other fluoride, simple fluorides that supply
free fluorine ions include, for example, hydrofluoric acid,
NPF-062
CA 02528275 2005-11-29
19
potassium fluoride, sodium fluoride, ammonium fluoride,
acidic potassium fluoride, acidic sodium fluoride, ammonium
fluoride, acidic ammonium fluoride, etc. Aluminum ion
eluted from aluminum alloy binds to free fluorine ion in
the chemical conversion agent to form complex ions and
promote the formation of zinc phosphate coating.
The chemical conversion agent of zinc phosphate
according to the present invention includes not less than
0.025 g/1 and not more than 45 g/1 of iron(Fe) in a
chelating agent that can chelate (bind to) iron ion. As
such, an addition of a chelating agent that can chelate
bond with iron ion into chemical conversion agent of zinc
phosphate makes possible holding iron ions that eluded from
the iron based surface in the chemical conversion agent,
and thus form a uniform and dense zinc phosphate coating
with high coatability. Specific chelating agents include,
for example, citrate acid, tartaric acid, EDTA, gluconic
acid, succinic acid, digallic acid, malic acid, and their
compounds or derivatives.
In the case in which the content of iron in a
chelating agent is less than 0.025 g/1, the coatability of
zinc phosphate coating on the aluminum based surface is
degraded and fails to form a uniform and dense zinc
phosphate coating with high coatability. On the other hand,
when the content of iron in a chelating agent is more than
0.45 g/1, the amount of zinc phosphate coating is reduced
and thus fails to obtain excellent property of resistance
NPF-062
CA 02528275 2005-11-29
to filiform rust.
The pH of chemical conversion agent of zinc phosphate
is not less than 2.0 and not more than 5.0, and more
preferably, not less than 3.0 and not more than 4Ø The
pH can be adjusted by NaOH, ammonia aqueous solution,
nitric acid, etc. Aluminum alloy is chemical conversion
treated by having a chemical conversion agent of zinc
phosphate brought into contact with aluminum alloy. The
temperature of the contact is preferably not less than 40
degrees C and not more than 60 degrees C, and more
preferably, not less than 42 degrees C and not more than 48
degrees C. Examples of the method of the contact are
spraying and dipping. The time for spraying process and
dipping is not less than 1 minute and not more than 10
minutes, and more preferably, not less than 1.5 minutes and
not more than 3 minutes. Other methods for contacting zinc
phosphate with aluminum alloy may be conducted by flow
coating and roll coating. The chemical conversion treated
aluminum alloy is then advanced to rinse and drying
processes. The temperature for drying is not less than 80
degrees C and not more than 120 degrees C.
Aluminum alloy
The content of copper in aluminum alloy according to
the present invention is not more than 0.2% by weight. In
the case in which the content of copper in aluminum alloy
is large, because the corrosion resistance is reduced, the
content of copper needs to be not more than 0.1% by weight,
NPF-062
CA 02528275 2005-11-29
21
for example, in the Patent Document 1. On the other hand,
a surface treatment method according to the present
invention can provide excellent corrosion resistance
properties even when the content of copper is 0.2o by
weight.
EXAMPLES
The present invention will be further described in
detail based on examples; however, the invention is not
limited to these examples.
EXAMPLES 1 to 7 AND COMPARATUVE EXAMPLES 1 to 5
Target material for treatment
In any of examples 1 to 7 and comparative examples 1
to 5, a similar aluminum alloy is used as a target material
for treatment. Specifically, aluminum alloys containing
copper of not more than O.Olo by weight, of O.lo by weight,
and of 0.2o by weight were used. The size of the aluminum
alloys were 70 mmX150 mm and widths were 0.8 mm. These
aluminum alloys were ground on the entire surface by sand
paper No. 180 using a Double Action Sander (905B4D
manufactured by Compact Tool Co., Ltd.). Also, as a target
material for treatment, aluminum alloys having the entire
surface ground were brought into contact with and
conduction into a SPC having a similar size and a thickness
as with the aluminum alloys, i.e., the target materials for
treatment was three types of aluminum alloys having
NPF-062
CA 02528275 2005-11-29
22
different content of copper. On one hand of these aluminum
alloys were contacted with and conducted into SPC, and on
the other hand of these aluminum alloys were neither
contacted with nor conducted into SPC.
Fig. 1 shows a target material for treatment in which
aluminum alloy and SPC are in contact together and in
conduction with each other. The target materials for
treatment are prepared according to the following steps.
First, SPC 20 is disposed with an interval of approximately
20 mm at both ends of the aluminum alloy 10 and hung by a
hanger 40. Holes are made on the upper portion of the
aluminum alloys 10 and SPC 20. An aluminum wire 30
connects the aluminum alloy 10 and SPC 20 by connecting one
hole with another.
Treating process
In any of Examples 1 to 7 and Comparative Examples 1
to 5, the process were made in sequence of (a) degreasing,
(b) rinsing by water, (c) surface adjustment, (d) chemical
conversion, (e) rinsing by water, (f) rinsing by pure
water, (g) drying, and (h) electro-deposition coating.
During a rinsing process in (b) and (e), tap water was
sprayed on the target material for treatment for 15 seconds
at room temperature, and during a rinsing process (f) by
pure water, ion exchanged water was sprayed on the target
material for treatment for 15 seconds at room temperature.
During drying process (g), the target material for
treatment was dried at 80 degrees C for 5 minutes. For the
NPF-062
CA 02528275 2005-11-29
23
process of (a) degreasing, (c) surface adjustment, (d)
chemical and (h) electro-deposition coating, respectively,
details will be described below.
Degreasing
In any of Examples 1 to 7 and Comparative Examples 1
to 5, a similar degreasing treatment was carried out for
each of the above-mentioned target materials for treatment.
Specifically, in a water solution containing 1.5% by weight
of agent A and 0.9% by weight of agent B, these agents are
alkaline degreasing agent ("Surf Cleaner SD 250")
manufactured by Nippon Paint Co., Ltd., and the
aforementioned target material for treatment was immersed
and then degreased for two minutes at a temperature of 43
degrees C.
Surface adjustment
In the Examples 1 to 7, a surface adjustor
(hereinafter, referred to as surface adjustor 1 that
contains zinc phosphate particles, copolymer containing
carboxylic acid group, and natural hectorite and/or
synthetic hectorite) was used to adjust the surface.
Specifically, as an adjustment of surface adjustor 1, 0.3
part by mass of natural hectorite "BENTON EW" (manufactured
by ELEMENTIS Co., Ltd.) was added into 87.7 part by mass of
water, and stirred at 3000 rpm for 30 minutes by using a
dispersing machine to obtain a pregel. The resultant
pregel was added with 2 parts by mass of commercially
available "Aron A-6020" (a copolymer containing carboxylic
NPF-062
CA 02528275 2005-11-29
24
acid group containing 40o by weight of acrylic acid and 600
by weight of 2-acrylamide-2-methylpropanesulfon
manufactured by Toagosei Co., Ltd.) and 50 parts by mass of
zinc phosphate particles. The pH was adjusted by caustic
soda to obtain a surface adjustor (1500 ppm of
concentration of zinc phosphate particles, 60 ppm of
concentration of copolymer containing carboxylic acid
group, and 45 ppm of concentration of natural hectorite).
Then the target material for treatment was immersed into
the resultant surface adjustor 1 for 30 seconds at room
temperature to adjust the surface.
In Comparative Examples 1 to 5, not by using zinc
phosphate based surface adjustor as shown in Examples, but
by using titanium colloid based surface adjustor, surfaces
were adjusted. Specifically, in order to conduct a surface
treatment, the target material for treatment was immersed
into a water solution (hereinafter, referred to as a
surface adjustor 2) having a pH of 9.0 and containing O.lo
by weight of a surface adjustor ("Surf Fine 5N-10" for
initial make-up of electrolytic bath manufactured by Nippon
Paint Co., Ltd.) for 30 seconds at room temperature. As
for both surface adjustor 1 and surface adjustor 2, dry
solids contents are set almost equal.
As for Example 7 and Comparative Examples 5,
degreasing and rinsing were conducted on the target
materials for treatment and then the materials were
immersed for 60 seconds at a temperature of 43 degrees C
NPF-062
CA 02528275 2005-11-29
into a chemical conversion agent that contains no zinc
ions, nickel ions or manganese ions. In a case in which a
surface adjustor 1 was used for surface adjustment, even
though the chemical conversion in which aluminum material
and SPC were brought into contact and conduct to each
other, the reduction of zinc phosphate coating was rarely
observed. In order to determine the threshold limit value
that can hold the property at a side that has a small
amount of coating, the aforementioned operations were
conducted. The reason for immersing the target material
for treatment into a chemical conversion agent containing
no metal ion is that the amount of etching on the aluminum
material needs to be equal to the amount of chemical
conversion in which aluminum material and SPC were brought
into contact and conduction to each other. More
specifically, a treatment conducted within a short time
using a proper chemical conversion agent in order to keep a
small amount of coating can be performed with a small
amount of coating; however, this leads to an amount of
etching also becoming small. As this fails to attain
similar conditions of the contact and conduction made
between the aluminum material and the SPC, the target
material for treatment were immersed into a chemical
conversion agent containing no metal ion is used to control
the amount of etching. The target amount of etching was
0.5 g/m2.
Chemical conversion treatment
NPF-062
CA 02528275 2005-11-29
26
In any of Examples 1 to 7 and Comparative Examples 1
to 5, a similar chemical conversion treatment was carried
out by immersing each of above mentioned target materials
for two minutes at a temperature of 43 degrees C.
Specifically, a chemical conversion treatment agent was
used which includes more than 1.0 g/1 of zinc ion, 1.0 g/1
of nickel ion, 1.3 g/1 of manganese ion, 20 g/1 of
phosphate ion, 0.5 g/1 of fluoride in a complex fluoride e,
0.35 g/1 of fluoride in a simple fluoride, and further
including 0.025 g/1 of iron (Fe) in a chelating agent that
can chelate bond with iron ions. The overall acidity in
the chemical conversion agent was adjusted to 22.2 pt and
the free acidity in the agent was adjusted to 0.5 pt. As
for the example 7 and comparative example 5, as described
above, the time for chemical conversion treatment was made
shorter, specifically 10 seconds in Example 7 and 20
seconds in Comparative example 5.
Electrodeposition coating
In any of Examples 1 to 7 and Comparative Examples 1
to 5, a similar electro-deposition coating was carried out.
Specifically, cationic electro-deposition coating was
carried out using cationic electro-deposition coating
(~~Power Top V50 Gray" manufactured by Nippon Paint Co.,
Ltd.). As for the coating conditions, the coating
thickness after baking and drying is set as 25 micrometers.
After the electro-deposition coating, the material was
baked for 25 minutes at a temperature of 170 degrees C, and
NPF-062
CA 02528275 2005-11-29
27
cationic electro-deposition coating was formed on the
surface of the chemical conversion treated aluminum alloy.
Intercoating
On the electro-deposition coating, an intercoating
manufactured by Nippon Paint Co., Ltd. (Orga~~P-5A N-2.0")
was spray coated and baked for 20 minutes at a temperature
of 140 degrees C. The formed intercoating thickness after
baking was 35 micrometers.
Overcoating
On the intercoating, overcoating manufactured by
Nippon Paint Co., Ltd. (~~superlac M-95HB YR-511P") was
spray coated and baked for 20 minutes at a temperature of
140 degrees C. The formed overcoating thickness was 15
micrometers.
Evaluation
Filiform Rust Resistance
On the coating of the three coated plates, crosscuts
(length: 20cm) were made by using a sharp cutter, and salt
water was sprayed for 24 hours in conformance with JIS-
22371. The resultant plates were exposed under a humid
atmosphere having a relative humidity of 70 to 75o at a
temperature of 40 degrees C. The exposure of the plate for
240 hours was deemed to be one cycle. After four cycles,
blistering were visually checked from the crosscuts and
evaluated according to the criteria shown below.
In Table 1, NB means almost no blisters were
observed, MB means many blisters were observed.
NPF-062
CA 02528275 2005-11-29
28
Resistance to water adhesion
The test plate on which zinc phosphate coating and
cationic electrodeposition coating was formed was immersed
in hot water for 240 hours at a temperature of 40 degrees
C, and then exposed to cool to room temperature. A cross-
cut adhesion test was then performed in which degree of
peeling was visually checked to evaluate the test plate
according to the criteria shown below.
In Table 1, NP means almost no peeling was observed,
LP means little peeling was observed, and EP means
extensive peeling was observed.
Amount of chemical conversion coating
The chemical conversion treated test plate was
immersed into a water solution having 30o nitric acid for
one minute at room temperature to dissolve the chemical
conversion coating. The weight before/after the
dissolution was measured and calculated to determine the
amount of chemical conversion coating.
The results of evaluation of resistance properties
against filiform rust and water adhesion carried out for
Examples 1 to 7 and Comparative Examples 1 to 5 are shown
in Table 1 and 2.
Here is a summary of evaluation results in Table 1
and 2. First, when aluminum alloy and SPC were contacted
and conducted, excellent resistance property to corrosion
could not be obtained in the Comparative Examples, whereas
excellent resistance property to corrosion could be
NPF-062
CA 02528275 2005-11-29
29
obtained when the copper content in the aluminum alloy was
within a range of not more than 0.2o by weight in the
Examples. Secondly, the copper content in the aluminum
alloy was 0.2o by weight, excellent resistance property to
corrosion could not be obtained in the Comparative
Examples, whereas excellent resistance property to
corrosion could be obtained irrespective of whether
aluminum alloy and SPC were contacted and conducted in the
Examples. Thirdly, when the amount of coating of zinc
phosphate was as low as 0.3 g/m2, excellent resistance
property to corrosion could not be obtained in the
Comparative Examples, whereas excellent resistance property
to corrosion could be obtained in the Examples. Therefore,
it was recognized that a uniform and dense zinc phosphate
coating with high coatability could be formed on the
surface of aluminum alloy according to the present
invention.
While preferred embodiments of the present invention
have been described and illustrated above, it is to be
understood that they are exemplary of the invention and are
not to be considered to be limiting. Additions, omissions,
substitutions, and other modifications can be made thereto
without departing from the spirit or scope of the present
invention. Accordingly, the invention is not to be
considered to be limited by the foregoing description and
is only limited by the scope of the appended claims
NPF-062
CA 02528275 2005-11-29
~. ~ ~ ~ a~
F M O U7(C3O ~ ('7
~ O ~ O P:7 fl.i
w ~ o ~ I I a +-~o ~ o ~ z
0
M O N ,'~
y o I I P~ W
0 0~ o ~ ~ z z
0
('C o N ~ O
W m I I O CL1 W
O 61 O ~ N Z z
N
a,
ch o ~ ~ l~
~ I I rd C~l W
o ~ o ,~ r-I 2 z
a. N
m o
o as w
M o ~ I I o f~ ~ z z
a ~ns~ ~ U
c~ o cn~ o p ~-n
N .~ tU W w
c~ 0 0~I I a +~ o ,.~ ~ z z
~ ~ o
c~ o
a~,~ o as w
w .~ o ~ I I a +~ o
0 0
0 0 +~0 0
-rl ~I-~u7~+-~-r-I
~ ~ ~ m ~ m
~ ~ a~
~ , y
v ~ ~ ~ ~ d
U U -I tS
U 3 U n m
~I
N ~ O -U ~ w ~ 3
s~ '-Ci U -r~-I rid O
O -.i ~ 4..~~ -ri -r-I
U O ~ O
ov rtS~ G .~-~ U~
-n '~ c~ O rC3 O
~ f~N
pi s~ ~ ~ O U ~ N
N t5~ ~ O ~ T3
-i N +-~ +~ U _ ~ r~ -~i~ O is
U ~ U W +~ ~ .k-~U7>~ ''-It~
N ra O rtSU~.~ -r1U7 O -rl~-I.1,-rl
r-1 W U -IJ ~ +-~-.-1~-I~ N U +~
~-I ~ '~-r-IrIfv1 ~-I~ +~ N
rif ~ ~ O f~O O N O r1W r1O
H c/~ U U rti~ U fx U rariSO U
NPF-062
CA 02528275 2005-11-29
31
roro
a. ~ ~ ~ a~
o cnrtio ~ c~
m ~ o r.~ w
c~w ~ I a o ~ a +~ o ~ o ~ w
ro
y --I O N S~
O ~G C1~
U w ~ I I O 01O ~ N ~ t-l
N
roro
a,
o ~ ~ ,-.I
o r-~ a~
U W n'7I I O 61O ~ N
N
D
ro
m a, U~~ ,-iN
-I o u~rd o ~ t~
w N I i O ,~ r~ trl 0.~
o ~ a .t-~o ,~ o ~
.r.,
ro
~ o
-a o ~ ~ o
w ~ I I o m w
o o~a ~ o ~ N
0 0
w ~ o +~0 0
.o +~ ~ ~ .can
, ~ s~
N N ~ U U .~
x x O ~ N !~
U o U o ~-I ~ D rU ~-1r rd
,~ ,-I
0 3 o 3 s~ +~ O +-~
~ '~ '~ ~n 4-.Im I
U U , ~ 4-r U ''~-~I-~iN
N
N -~ ~ r-I O -.-i~ 3
~ rx w f~
-O
U - W d O
~ '~ U
O -r-I ~ y~ ~ -r-I -r-1
.h O O ~ O ~-I +~
u~ U .-I N -r-I
o\~ _ ft~.~.-~~',.I-~ u7
'r1 '~ N O cd O
~
Y-I
rd ~ N ~ O U ~ N
O b~ ~ O ~ "O
N O .1-~ +~ U _ tTr>~-~I~ O b~
U s~ U W .a-~~ +-~U7~ ~-I!
N cti O ra Ua.~ -rlu? O -rl~-I+.~-r-1
r1 W U +~ tT ~ -~ ~-I~ O
G 't~-rib u~ N ~ a--~O r~
rtf '~ ~ O ~''~ O ~ O r-~I4-Ir-iO
E-~ U7 U U rd~ U C~ U rd rcSN U
NPF-062