Note: Descriptions are shown in the official language in which they were submitted.
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
INDOLES USEFUL IN THE TREATMENT OF INFLAMMATION
Field of the Invention
This invention relates to.novel pharmaceutically-useful compounds, which
compounds are useful as inhibitors of microsomal prostaglandin E synthase-
1 (mPGES-1). The compounds are of potential utility in the treatment of
inflammatory diseases. The invention also relates to the use of such
compounds as medicaments, to pharmaceutical compositions containing
1 o them, and to synthetic routes for their production.
Background of the Invention
There are many diseases/disorders that are inflammatory in their nature.
Is One of the major problems associated with existing treatments of
inflammatory conditions is a lacy of efficacy and/or the prevalence of side
effects (real or perceived).
Inflammatory diseases that affect the population include asthma,
2o inflammatory bowel disease, rheumatoid arthritis, osteoarthritis, rhinitis,
conjunctivitis and dermatitis.
Inflammation is also a common cause of pain. Inflammatory pain may arise
for numerous reasons, such as infection, surgery or other trauma.
25 Moreover, several malignancies are known to have inflammatory
components adding to the symptomatology of the patients.
The cyclooxygenase (COX) enzyme exists in two forms, one that is
constitutively expressed in many cells and tissues (COX-1), and one that is
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
2
induced by pro-inflammatory stimuli, such as cytokines during an
inflammatory response (COX-2).
COXs metabolise arachidonic acid to the unstable intermediate
s prostaglandin HZ (PGH2). PGH2 is further metabolised to other
prostaglandins including PGEZ, PGF2a, PGD2, prostacyclin and
tllromboxane A2. These arachidonic acid metabolites are known to have
pronounced physiological and pathophysiological activity including pro-
inflammatory effects.
PGE2 in particular is known to be a strong pro-inflammatory mediator, and
is also known to induce fever and pain. Consequently, numerous drugs
have been developed with a view to inhibiting the formation of PGE2,
including "NSAIDs" (non-steroidal antiinflammatory drugs) and "coxibs"
1s (selective COX-2 inhibitors). These drugs act predominantly by inhibition
of COX-1 and/or COX-2, thereby reducing the formation of PGE2.
However, the inhibition of COXs has the disadvantage that it results in the
reduction of the formation of all metabolites of arachidonic acid, some of
2o which are known to have beneficial properties. In view of this, drugs which
act by inhibition of COXs are therefore known/suspected to cause adverse
biological effects. For example, the non-selective inhibition of COXs by
NSAIDs may give rise to gastrointestinal side-effects and affect platelet and
renal function. Even the selective inhibition of COX-2 by coxibs, whilst
2s reducing such gastrointestinal side-effects, is believed to give rise to
cardiovascular problems.
An alternative treatment of inflammatory diseases that does not give rise to
the above-mentioned side effects would thus be of real benefit in the clinic.
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
3
In particular, a drug that inhibits (preferably selectively) the
transformation
of PGH2 to the pro-inflammatory mediator PGE2 might be expected to
reduce the inflammatory response in the absence of a corresponding
reduction of the formation of other, beneficial arachidonic acid metabolites.
s Such inhibition would accordingly be expected to alleviate the undesirable
side-effects mentioned above.
PGH2 may be transformed to PGE2 by prostaglandin E synthases (PGES).
Two microsomal prostaglandin E synthases (mPGES-1 and mPGES-2), and
one cytosolic prostaglandin E synthase (cPGES) have been described.
mPGES-1 belongs to the membrane-associated proteins in the eicosanoi~d
and glutathione metabolism (MAPEG) family. Other members of this
family include the 5-Iipoxygenase-activating protein (FLAP), leukotriene C4
Is synthase and microsomal glutathione S-transferases (MGSTI, MGST2 and
MGST3).
Thus, agents that are capable of inhibiting the action of mPGESs and, in
particular, InPGES-l, and thus reducing the formation of the specific
2o arachidonic acid metabolite PGE2, are likely to be of benefit in the
treatment of inflammation.
Pri~r Art
2s Various indoles, and derivatives thereof, have been disclosed in
international patent applications WO 01/30343, WO 96/03377 and WO
99/33800, US patents Nos. S,I89,054 and 6,25,083 and European patent
application EP 483 881. However, none of these documents disclose or
suggest the use of the compounds disclosed therein in the treatment of
3o inflammation.
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
4
Structurally related indoles have been disclosed for potential use in the
treatment of inflammation in international patent application WO 99105104
and European patent application EP 985 666. However these documents do
s not disclose or suggest indoles that are substituted in the 1-position.
Other related indoles have been disclosed for potential use in the treatment
of inflammation in international patent application WO 94/13662, US patent
No. 5,081,145 and European patent application EF 535 924. However,
1o none of these documents disclose or suggest indoles that are directly
substituted at the benzenoid moiety of the indole ring with an aryl or
heteroaryl group.
US patent No. 5,081,138 and European patent application EP 166 591
Is disclose indoles for potential use in the treatment of inflammation.
However, neither of these documents disclose or suggest indoles that are
directly substituted at the 2-position of the indole ring with a carboxylic
acid group or a derivative thereof.
2o International patent applications WO 99/07351 and WO 99/07678 disclose
indoles for potential use in the treatment of inflammation but do not
disclose or suggest indoles that are substituted in the indolic 3-position by
an aryl, a heteroaryl or an amide group.
2s International patent applications WO 00/46198, WO 00/46197, WO
00/46195, WO 00/46199, WO 94/14434, WO 96/18393 and WO 02/30895
describe indole compounds for potential use in the treatment of
inflammation. However, there is no specific disclosure in any of these
documents of substitution at the benzenoid moiety of the indole ring with an
3o aryl or heteroaryl group.
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
Tnternational patent applications WO 98/08818 and WO 99/43672 also
disclose indoles for potential use in the treatment of inflammation.
However, there is no specific disclosure in either of these documents of
s indoles substituted in the 3-position by an aryl or heteroaryl group, or a
nitrogen derivative, such as an amine, amide or sulfonamide group attached
to the indole ring through the nitrogen atom.
Finally, international patent applications WO 99/43654 and WO 99/43651
1o describe indole compounds for potential use in the treatment of
inflammation. These documents do not specifically disclose indoles
substituted in the 3-position by an aryl or heteroaryl group, or a nitrogen
derivative, such as an amine, amide or sulfonamide group attached to the
indole ring through the nitrogen atom, and which are further substituted at
1s the ben~enoid moiety of the indole ring with an aryl or heteroaryl group.
1)iscIosure ~f the invention
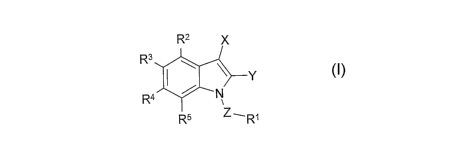
According to the invention there is provided a compound of formula I,
R2 )(
R3
\ ~ 1
Ra ~ N
R5
wherein
X represents:
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
6
i) an aryl group or a heteroaryl group, both of which groups are
optionally substituted by one or more substituents selected from A; or
ii) -N(R6)-E-R';
s E,represents a single bond, -C(O)- or -S(O)ri ;
Y represents -CH20H, -C(O)N(H)R8, -C(O)N(H)OR8 or -C(O)OR8;
Z represents a Cl_s all~ylene or a C2_8 heteroalkylene chain, both of which:
to (i) optionally contain one or more unsaturations (for example double or
triple bonds);
(ii) are optionally substituted by one or more substituents selected from
halo, -R8, -N(Rg)(R9), -ORg and =O; and/or
(iii) may form part of an additional 3- to 8-membered ring formed
Is between any one or more (e.g. one or two) members of the C1_g alkylene or
C2_8 heteroalkylene chain, which ring optionally contains 1 to 3 heteroatoms ,
and/or 1 to 3 unsaturations (for example double or triple bonds) and which
ring is itself optionally substituted by one or more substituents selected
from halo, -R8, hI(Rg)(R9), -OR8 and =O;
R1 represents an aryl or a heteroaryl group, both of which are optionally
substituted by one or more substituents selected from A;
one of the groups R~, R3, R4 and R5 represents an aryl group or a heteroaryl
2s group (both of which are optionally substituted by one or more substituents
selected from A) and:
a) the other groups are independently selected from hydrogen, Gl, an
aryl group, a heteroaryl group (which latter two groups are optionally
substituted by one or more substituents selected from A), C1_6 alkyl, C3_l~
(e.g. C3_g) cycloalkyl, C2_6 alkenyl, C~_6 alkynyl or C3_8 heterocycloall~yl
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
7
(which latter f ve groups are optionally substituted by one or more
substituents selected from Gl and/or Q1); and/or
b) any two other groups which are adjacent to each other are optionally
linked to form, along with two atoms of the essential benzene ring in the
compound of formula I, a 5- to 6-membered ring, optionally containing 1 or
more (e.g. 1 to 3) heteroatoms and/or 1 to 3 unsaturations (for example
double or triple bonds), which ring is itself optionally substituted by one or
more substituents selected from halo, -R8, -OR8 and =O;
1o A represents, on each occasion when mentioned above:
I) an aryl group or a heteroaryl group, both of which are optionally
substituted by one or more substituents selected from B;
II) a C~_6 alkyl, C3_to (e.g. C3_8) cycloalkyl, C~_6 alkenyl, C2_6 alkynyl or
C3_8 heterocycloalkyl group, all of which are optionally substituted by one
is or more substituents selected from Gl and/or Q1; or
III) a Gl group; or
IV) two adjacent A substituents may be linked together to form, along
with the essential atoms of the aryl or heteroaryl group to which the two A
substituents are attached, a further 5- to 6-membercd ring, optionally
2o containing 1 or more (e.g. 1 to 3) heteroatoms and/or 1 to 3 unsaturations
(for example double or triple bonds), which ring is itself optionally
substituted by one or more substituents selected from halo, -R8, -ORg and
=O;
2s Gl represents, on each occasion when mentioned above, halo, cyano, -N3,
NO2, -ONOa or Al-Rlo;
wherein AI represents a single bond or a spacer group selected from
-C(QZ)A2-, -S(O)nA3-, -N(Rll)A4-, -OA5- and -S-, in which:
A~ represents A6 or -S-;
3o A3 represents A6;
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
8
A4 represents A', -C(Q2)N(Rm)C(Qa)N(Rn)-, -C(Q2)N(Rm)C(Qa)O-,
-C(QZ)N(R11)S(0)nN(R11)-~ -C(QZ)S-~ -S(O)nN(Rn)C(Q2)N.(R11)-
-S(O)nN(R.l~)L,(Qz)~-~ -S(O)nN(Rll)S(O)nN(R11)- or -S(O)n0-;
AS represents A' or -S(O)n0-;
s A6 represents a single bond, -N(Rl1)- or -O-;
A' represents a single bond, -C(QZ)-, -C(QZ)N(Rll)-, -C(Q2)O-, -S(O)n or
-S(O)nN(Rn)~
Q1 and QZ independently represent, on each occasion when mentioned
above, =O, =S, NRl°, NN(Rl°)(Rli), NORI°,
NS(O)2N(Rz°)(Rll),
NCN, =C(H)N02 or =C(R~°)(R1)?
R6 and R' independently represent, on each occasion when mentioned
above:
is I) hydrogen;
II) an aryl group or a heteroaryl group, both of which are optionally
substituted by one or more substituents selected from B; or
III) a C1_6 alkyl, C3_lo (e.g. C3_g) cycloalkyl, CZ_6 alkenyl, C2_6 alkynyl or
C3_8 heterocycloalkyl group, all of which groups are optionally substituted
2o by one or more substituents selected from G2 and/or Q3; or
R6 and R~ may be linked together to form along with the N atom and
E- group to which R6 and R7 are respectively attached, a 5- to 8-membered
ring, optionally containing 1 to 3 heteroatoms and/or 1 to 3 unsaturations
(for example double or triple bonds), which ring is optionally substituted by
2s one or more substituents selected from GZ and/or Q3;
B represents, on each occasion when mentioned above:
I) an aryl group or a heteroaryl group, both of which are optionally
substituted by one or more substituents selected from G2 and/or wherein any
3o two adjacent atoms of the aryl or heteroaryl group may be linked together
to
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
9
form a further 5- to 6-membered ring, optionally containing 1 or more (e.g.
1 to 3) heteroatoms and/or 1 to 3 unsaturations (for example double or triple
bonds), which ring is itself optionally substituted by one or more
substituents selected from halo, -R8, -OR8 and =O;
s II) a C1_6 alkyl, C3_lo (e.g. C;_s) cycloalkyl, C2_6 alkenyl, C2_6 alkynyl
or
C3_g heterocycloalkyl group, all of which are optionally substituted by one
or more substituents selected from G2 and/or Q3; or
III) a GZ group; or
I~ two adjacent B substituents may be linked together to form, along
to with the essential atoms of the aryl or heteroaryl group to which the two B
substituents are attached, a further 5- to 6-membered ring, optionally
containing 1 or more (e.g. 1 to 3) heteroatoms and/or 1 to 3 unsaturations
(e.g. double or triple bonds), which ring is itself optionally substituted by
one or more substituents selected from halo, -R8, -ORg and =O;
1s
G2 represents, on each occasion when mentioned above, halo, cyano, -N3,
-NO2, -ONOZ or Ag_y2
wherein A8 represents a single bond or a spacer group selected from
-C(Q4)A9-, -S(C)nAlo-' -N(Rl3)Al l-' _OA12- and -S-, in which:
2o A9 represents Al3 or -S-;
Al° represents Als;
All represents A14, -C(Q4)N(R13)C(Q4)N(Rl3)-~ -C(Q4)N(R13)C(Q4)~-,
-C(Q4)N(R13)s(~)nN(Rl3)-~ -~'(Q4)'-~'-a -,f (~)nN(RI3)C(Q4)N(R13)-'
-S(~)nN(Rl3)C(Q4)~-~ -s(o)nN(Rl3)~(~)nN(Rl3)- pr -s(O)n~-
2s Ala represents Al4 or -S(O)n0-;
Al3 represents a single bond, -N(Rl3)- or -O-;
Al4 represents a single bond, -C(Q4)-, -C(Q4)N(Rl3)-, -C(Q4)O-, -S(O)ri or
-S(O)nN~l3)~
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
Q3 and Q4 independently represent, on each occasion when mentioned
above, =O, =S, NR12, NN(RiZ)(Rm)? NORtl, NS(O)2N(R'l)(Rl''),
NCN, =C(H)N02 or =C(R~2)(RI3);
s Rg, R9, Rr°, Rn, R12 and RI3 are independently selected from:
i) hydrogen;
ii) an aryl or a heteroaryl group, both of which are optionally substituted
by one or more substituents selected from G3 and/or wherein any two
adjacent atoms of the aryl or heteroaryl group may be linked together to
Io form a further 5- to 6-membered ring, optionally containing 1 or more (e.g.
1 to 3) heteroatoms, which ring is itself optionally substituted by one or
more substituents selected from halo, -R14, -ORI4 and =O; or
iii) a CI_6 alkyl, C3_lo (e.g. C3_8) cycloalkyl, C2_6 alkenyl, C2_6 alkynyl or
C3_8 heterocycloalkyl group, all of which are optionally substituted by one
Is or more substituents selected from G3 and/or Wl; or
any pair of Rg, R9, Rl°, Rli, R~2 and R13 may, for example when present
on
the same or on adjacent atoms, be linked together to form with those, or
other relevant, atoms, a further 5- to 8-membered ring, optionally
containing 1 to 3 heteroatoms and/or 1 to 3 unsaturations (for example
2o double or triple bonds), which ring is itself optionally substituted by one
or
more substituents selected from G3 and/or Wl;
G3 represents, on each occasion when mentioned above, halo, cyano, -N3,
-N02, -ON02 or AI5-R15;
2s wherein Als represents a single bond or a spacer group selected from
-C(,WZ)Ai6-, -S(O)nAm-, -N(Ri6)Ais-, -OAIg- and -S-, in which:
A16 represents A2° or -S-;
Al' represents AZO;
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
11
AIg represents A21, -C(W2)N(RI6)C(W2)N(R16)-, -C(WZ)N(R16)C(Wz)O-
-C(W2)N(R16)S(~)nN(Ri6)-~ -C(Wa)S-~ -S(O)nN(RI6)C(Wa)N(Rls)-
-S(O)nN(R16)C(Wz)O-~ -S(O)nN(RI6)S(O)nN(RI6)- or -S(O)n~-;
AI9 represents AZI or -S(O)nO-;
s A2° represents a single bond, -N(RI6)- or -O-;
A21 represents a single bond, -C(W2)-, -C(WZ)N(RI6)-, -C(WZ)O-, -S(O)ri or
-S(O)nN(R~6)~
WI and W2 independently represent, on each occasion when mentioned
to above, =O, =S, KRIS, NN(Rls)(R16), NORIS, I~TS(O)~N(Rls)(Rl6)~
NCN, =C(H)NO2 or =C(Rls)(R16);
Rla~ RIS ~d Rls ~.e independently selected from:
i) hydrogen;
is ii) an aryl or a heteroaryl group, both of which are optionally substituted
by one or more substituents selected from G4, methylenedioxy,
difluoromethylenedioxy and/or dimethylmethylenedioxy; or
iii) a CI_6 alkyl, C3_lo (e.g. C3_g) cycloalkyl, Ca_6 alkenyl, CZ_6 alkynyl or
C3_s heterocycloalkyl group, aI1 of which are optionally substituted by one
20 or more substituents selected fxom G4 and/or J; or
any pair of RI4, RIS and RI6 may, for example when present on the same or
on adjacent atoms, be linked together to form with those, or other relevant,
atoms, a further 5- to 7-membered ring, optionally containing 1 to 3
heteroatoms and/or 1 to 3 unsaturations (for example double or triple
2s bonds), which ring is itself optionally substituted by one or more
substituents selected from G4 and J;
G4 represents, on each occasion when mentioned above, halo, cyano, -N3,
-NO2, -ONOZ or A22-RI~;
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
12
wherein AZ2 represents a single bond or a spacer group selected from
-C(O)A23-, -S(O)nA24-' -N(Ria)Aas-~ _OA26- and -S-, in which:
A23 represents AZ' or -S-;
A24 represents A27;
s AZS represents A2g, -C(O)N(R~g)C(O)N(Rl$)-, -C(O)N(R1s)C(O)O-,
-C(O)N(Rls)S(O)"N(R~s)-, _C(O)S_, -S(O)nN(Rlg)C(O)N(Ri8)-,
-S(O)nN(Rls)C(O)O-~ -S(O)nN(Rl8)S(O)nN(R1s)- or -S(O)n0-
A26 represents A2g or -S(O)nO-;
AZ~ represents a single bond, -N(Rl8)- or -O-;
to A2s represents a single bond, -C(O)-, -C(O)N(Rlg)-, -C(O)O-, -S(O)ri or
-S(~)nN(RIg)~
J represents, on each occasion when mentioned above, =O, =S, =NRl',
NN(R17)(Rls), =NORM, =NS(O)2N(Rl~)(Rlg), ~TCN, =C(I-i)N02 or
is =C(Rl')(Rl8);
Rl~ and Rl8 are independently selected from hydrogen and C~_6 alkyl, which
latter group is optionally substituted by one or more substituents selected
from halo, -NH2, -N(H)Me, -N(H)Et, -N(H)i-Pr, -NMe2, -N(Me)Et,
20 -N(Me)i-Pr, -NEt2, -OH, -OMe, -OEt, -Oi-Pr and =O; and
n represents, on each occasion when mentioned above, 1 or 2,
or a pharmaceutically-acceptable salt thereof,
as
which compounds and salts are referred to hereinafter as "the compounds of
the invention".
Pharmaceutically-acceptable salts include acid addition salts and base
addition salts. Such salts may be foi~rned by conventional means, for
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
I3
example by reaction of a free acid or a free base form of a compound of the
invention with one or more equivalents of an appropriate acid or base,
optionally in a solvent, or in a medium in which the salt is insoluble,
followed by removal of said solvent, or said medium, using standard
s techniques (e.g. ifz vacu~, by freeze-drying or by filtration). Salts may
also
be prepared by exchanging a counter-ion of a compound of the invention in
the form of a salt with another counter-ion, for example using a suitable ion
exchange resin.
Io Compounds of the invention may contain double bonds and may thus exist
as E (entgegen) and Z (zuranzn2erz) geometric isomers about each individual
double bond. All such isomers and mixtures thereof are included within the
scope of the invention.
Is Compounds of the invention may also exhibit tautomerism. All tautomeric
forms and mixtures thereof are included within the scope of the invention.
Compounds of the invention may also contain one or more asymmetric
carbon atoms and may therefore exhibit optical and/or diastereoisomerism.
2o hiastereoisomers may be separated using conventional techniques, e.g.
chromatography or fractional crystallisation. The various stereoisomers
may be isolated by separation of a racemic or other mixture of the
compounds using conventional, e.g. fractional crystallisation or HPLC,
techniques. Alternatively the desired optical isomers may be made by
2s reaction of the appropriate optically active starting materials under
conditions which will not cause racemisation or epimerisation (i.e, a 'chiral
pool' method), by reaction of the appropriate starting material with a 'chiral
auxiliary' which can subsequently be removed at a suitable stage, by
derivatisation (i.e. a resolution, including a dynamic resolution), for
3o example with a homochiral acid followed by separation of the
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
14
diastereomeric derivatives by conventional means such as HPLC or
chromatography over silica, or by reaction with an appropriate chiral
reagent or chiral catalyst all under conditions known to the skilled person.
All stereoisomers and mixtures thereof are included within the scope of the
s invention.
Unless otherwise specified, Cr_q alkyl groups (where q is the upper limit of
the range) defined herein may be straight-chain or, when there is a sufficient
number (i.e. a minimum of three) of carbon atoms, be branched-chain.
to C1_6 alkyl groups that may be mentioned include methyl, ethyl, n-propyl,
isopropyl, ~-butyl, ,rec-butyl, isobutyl, tent-butyl, n-pentyl, isopentyl, jZ-
hexyl, and isohexyl.
CI_q alkylene chains (where q is the upper limit of the range) defined herein
is are straight-chain alkyl groups, which groups are attached at each terminal
end.
CV_q heteroalkylene chains (where v is the lower limit and q is the upper
limit of the range) defined herein are C~_q alkylene chains, wherein one or
2o more (e.g. 1 to q-1) carbon atoms have been replaced with a heteroatom.
C2_9 alkenyl groups (where q is the upper Limit of the range) defined herein
may be straight-chain or, when there is a sufficient number (i.e. a minimum
of three) of carbon atoms, be branched-chain. Such alkenyl groups may
2s contain one or more double bonds. C2_6 alkenyl groups that may be
mentioned include ethenyl (i.e, vinyl), 1-propenyl, 2-propenyl (i.e. allyl),
propadienyl, 1-butenyl, 2-butenyl, 3-butenyl, I,3-butadienyl, 1-pentenyl,
2-pentenyl, 4-pentenyl, and 5-hexenyl.
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
IS
C2_q alkynyl groups (where q is the upper limit of the range) defined herein
may be straight-chain or, when there is a sufficient number (i.e. a minimum
of four) of carbon atoms, be branched-chain. Such alkynyl groups may
contain one or more triple bonds. CZ_6 alkynyl groups that may be
s mentioned include ethynyl, 1-propynyl, 2-propynyl, 1-butynyl, 2-butynyl,
3-butynyl, 1-pentynyl, 2-pentynyl, 4-pentynyl, 1-hexynyl, 3-hexynyl, and 5-
hexynyl.
C3_q cycloalkyl groups (where q is the upper limit of the range) that may be
1o mentioned include monocyclic or bicyclic alkyl groups or fused ring
systems such as three fused cycloalkyl groups. Such cycloalkyl groups may
be saturated or unsaturated containing one or more double or triple bond
(forming for example a C3_q cycloalkenyl or a C3_q cycloalkynyl group).
C3_q (e.g. C3_In) cycloalkyl groups that may be mentioned include
I~ adamantyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl,
cyclooctyl, cyclopentenyl, cyclohexenyl, cycloheptenyl, cyclooctenyl,
cyclooctynyl, bicycloheptyl, bicyclooctyl, and bicyclooctenyl. Substituents
may be attached at any point on the cycloalkyl group. Further in the case
where the substituent is another cyclic compound, then the cyclic
2o substituent may be attached through a single atom on the cycloalkyl group,
forming a so-called "spiro"-compound.
C3_q heterocycloalkyl groups (where q is the upper limit of the range) that
may be mentioned include monocyclic or bicyclic alkyl groups in which at
2s Ieast one (e.g. one to four) of the atoms in the ring system is other than
carbon (i.e. a hetereoatom). Further, such heterocycloalkyl groups may be
saturated or unsaturated containing one or more double and/or triple bonds,
forming for example a C3_q heterocycloalkenyl or a C3_q heterocycloalkynyl
group. C3_q heterocycloalkyl groups that may be mentioned include
3o aziridinyl, azetidinyl, dihydropyranyl, dihydropyridyl, dihydropyrrolyl
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
I6
(including 2,5-dihydropyrrolyl), dioxolanyl (including 1,3-dioxolanyl),
dioxanyl (including 1,3-dioxanyl and 1,4-dioxanyl), dithianyl (including
1,4-dithianyl), dithiolanyl (including 1,3-dithiolanyl), imida~olidinyl,
imidazolinyl, morpholinyl, oxetanyl, oxiranyl, piperazinyl, piperidinyl,
s pyranyl, pyrazolidinyl, pyrrolidinonyl, pyrrolidinyl, pyrrolinyl,
quinuclidinyl, sulfolanyl, 3-sulfolenyl, tetrahydropyranyl, tetrahydrofuranyl,
tetrahydropyridyl, thietanyl, thiiranyl, thiolanyl, thiomorpholinyl,
trithianyl
(including 1,3,5-trithianyl), tropanyl and the like. Substituents on the
heterocycloalkyl groups may, where appropriate, be located on any atom in
to the ring system including a heteroatom. Further in the case where the
substituent is another cyclic compound, then the cyclic substituent may be
attached through a single atom on the heterocycloalkyl group, forming a so-
called "spiro"-compound. The point of attachment of a heterocycloalkyl
group may be via any atom in the ring system including (where appropriate)
1s a heteroatom, or an atom on any fused carbocyclic ring that may be present
as part of the ring system. Heterocycloalkyl groups may also be in the N or
S- oxidised form.
The term "halo", when used herein, includes fluoro, chloro, bromo and iodo.
~lry1 groups that may be mentioned include C6_i3 aryl (e.g. C6_lo) groups.
Such groups may be monocyclic, bicyclic or tricylic and have between 6
and 13 ring carbon atoms, in which at least one ring is aromatic. C6_13 aryl
groups include phenyl, naphthyl and the like, such as 1,2,3,4-tetrahydro-
2s naphthyl, indanyl, indenyl and fluorenyl. The point of attachment of aryl
groups may be via any atom of the ring system.
Heteroaryl groups that may be mentioned include those which have between
5 and 10 members. Such groups may be monocyclic, bicyclic or tricyclic,
3o in which at least one of the rings is aromatic and wherein at least one
(e.g.
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
I7
one to four) of the atoms in the ring system is other than carbon (i.e. a
heteroatom). Heteroaryl groups that may be mentioned include acridinyl,
benzimidazolyl, benzodioxanyl, benzodioxepinyl, benzodioxolyl (including
I,3-benzodioxolyl), benzofuranyl, benzofurazanyl, benzothiazolyl
s (including 2,1,3-benzothiazolyl), benzoxadiazolyl (including 2,1,3-
benzoxadiazolyl), benzoxazinyl (including 3,4-dihydro-21~ 1,4-
benzoxazinyl), benzoxazolyl, benzimidazolyl, benzomorpholinyl,
benzoselenadiazolyl (including 2,1,3-benzoselenadiazolyl), benzothienyl,
carbazolyl, chromanyl, cinnolinyl, furanyl, imidazolyl, imidazo[1,2-
Io a]pyridyl, indazolyl, indolinyl, indolyl, isobenzofuranyl, isochromanyl,
isoindolinyl, isoindolyl, isoquinolinyl, isothiazolyl, isoxazolyl,
naphthyridinyl (including 1,5-naphthyridinyl and 1,8-naphthyridinyl),
oxadiazolyl (including 1,2,3-oxadiazolyl, 1,2,4-oxadiazolyl and
1,3,4-oxadiazolyl), oxazolyl, phenazinyl, phenothiazinyl, phthalazinyl,
Is pteridinyl, purinyl, pyrazinyl, pyrazolyl, pyridazinyl, pyridyl,
pyrimidinyl,
pyrrolyl, quinazolinyl, quinolinyl, quinolizinyl, quinoxalinyl, tetrahydroiso-
quinolinyl (including 1,2,3,4-tetrahydroisoquinolinyl and 5,6,7,~-
tetrahydroisoquinolinyl), tetrahydroquinolinyl (including 1,2,3,4-
tetrahydroquinolinyl and 5,6,7,x-tetrahydroquinolinyl), tetrazolyl,
2o thiadiazolyl (including 1,2,3-thiadiazolyl, 1,2,4-thiadiazolyl and
1,3,4-thiadiazolyl), thiazolyl, thiochromanyl, thienyl, triazolyl (including
1,2,3-triazolyl, 1,2,4-triazolyl and 1,3,4-triazolyl) and the line. The point
of
attachment of heteroaryl groups may be via any atom in the ring system
including (Where appropriate) a heteroatom (such as a nitrogen atom), or an
2s atom on any fused carbocyclic ring that may be present as part of the ring
system. Heteroalyl groups may also be in the N or S'- oxidised form.
Heteroatoms that may be mentioned include oxygen, nitrogen, sulphur and
selenium.
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
I8
For the avoidance of doubt, optionally substituted methylenedioxy groups,
when attached to a ring system, are formed between any two adjacent atoms
of the ring system.
s For the avoidance of doubt, in cases in which the identity of two or more
substituents in a compound of the invention may be the same, the actual
identities of the respective substituents are not in any way interdependent.
For example, in the situation in which Rl and R3 are both aryl groups
substituted by one or more C1_6 alkyl groups, the alkyl groups in question
to may be the same or different. Similarly, when groups are substituted by
more than one substituent as defined herein, the identities of those
individual substituents are not to be regarded as being interdependent. For
example, when ~ and/or Rl represents e.g. an aryl group substituted by G1
in addition to, for example, Cl_6 alkyl, which latter group is substituted by
is Gl, the identities of the two Gl groups are not to be regarded as being
interdependent.
Compounds that may be mentioned include those in which:
E represents -C(O)-;
20 ~ when any two adjacent R2, R3, R4 or R5 groups, adjacent A substituents,
adjacent B substituents, and/or, when B represents aryl or heteroaryl,
adjacent atoms of those aryl or heteroaryl groups are linked to form a 5- to
6-membered ring, then that ring is fully saturated.
25 Preferred compounds of the invention include those in which:
Y represents -CH20H or, more preferably, -C(O)N(H)Rg, -C(O)N(H)OR8
or -C(O)ORg;
Ag represents a single bond, -C(Q4)A9-, -S(O)nAl°-, -N(R13)All- or -
OA12-;
A9 represents A13;
All represents A~4;
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
I9
Q' represents =O, =NN(R12)(R13), =N~Ri2, NS(O)2N(R12)(Rl'), =NCN or
=C(H)NO2;
Q4 represents =O;
A15 represents a single bond, -C(W2)A16-, -S(O)nAl~-, -N(R16)AIg- or
s -OA19-:
Al6 represents A2o;
Al8 represents A21;
WI represents =O, NN(Rls)(Rl6), =NORls, =NS(O)aN(Rls)(Rl~), NCN or
=C(H)NOZ;
to WZ represents =O;
when any pair of Rl~, Rls and R16 is linked together to form a further
optionally substituted 5- to 7-membered ring, then that ring optionally
contains only 1 unsaturation;
A22 represents a single bond, -C(O)A23-, -S(O)nA24-, -N(Rl8)A''5- or
15 -~A2~-;
A23 represents Az~;
A'S represents AZ&; and/or
J represents =O, 1VN(Rl')(Rig), I~TOR17, l~S(O)2N(Ri7)(RIa), NCN, or
-C(H)NO2;
More preferred compounds of the invention include those in which:
Y represents -CHZOH or, more preferably, -C(O)N(H)R8 or -C(O)ORg;
when any two members of the Cl_g alkylene or C2_8 heteroalkylene chain
that Z may represent form part of an additional optionally substituted 3- to
2s ~-membered ring, then that ring optionally contains 1 to 2 heteroatoms
andlor 1 unsaturation;
A1 represents -S- or, more preferably, a single bond, -C(Q2)A~-, -S(O)nA3-,
-N(Rii)A4- or _OAS-:
Aa represents A6;
A4 represents A~;
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
QI represents =O, =NORI°, NS(O)ZN(Rl°)(Rn), -NCN or
=C(H)N02;
Q 2 represents =O;
when two adjacent atoms of the optionally substituted aryl or heteroaryl
group that B may represent are linked together to form a further optionally
5 substituted ring, then the linking divalent substituent that forms part of
that
ring is selected from methylenedioxy, difluoromethylenedioxy and
dimethylmethylenedioxy;
Q3 represents =O, NORl2, NS(O)ZN(Rl')(Rl3), =NCN or =C(H)NO2;
when R~ and R' are linked to form an optionally substituted 5- to
1o membered ring, then that ring optionally contains only 1 unsaturation;
when two adjacent atoms of the optionally substituted aryl or heteroaryl
group that R8, R9, Rl°, Rll, Ri2 and/or R13 may represent are linked
together
to form a further optionally substituted ring, then the linking divalent
substituent that forms part of that ring is selected from methylenedioxy,
is difluoromethylenedioxy and dimethylmethylenedioxy;
Wl represents =O, =NORIS, NS(~)2N(R15)(R~6), -NCN or =C(H)N02;
and/or
RI4, Rls and R16 independently represent an aryl or heteroaryl group, both of
which groups are optionally substituted by one or more substituents selected
2o from halo, -NH2, -N(H)Me, -N(H)Et, -N(H)i-Pr, -NMe2, -N(Me)Et,
-N(Me)i-Pr, -NEt2, -OH, -OMe, -OEt, -Oi-Pr and =O or, R14, RIS and R16
are, more preferably, hydrogen or C1_6 alkyl, which latter group is optionally
substituted by one or more substituents selected from halo, -NH2, -N(H)Me,
-N(H)Et, -N(H)i-Pr, -NMe2, -N(Me)Et, -N(Me)i-Pr, -NEt2, -OH, -OMe,
2s -OEt, -Oi-Pr and =O;
More preferred compounds include those in which:
~ represents -CHZOH, -C(O)NHRg or, more preferably, -C(O)ORB;
when any one or more members of the C1_g alkylene or C2_8 heteroalkylene
3o chain that Z may represent form part of an additional ring, then that ring
is a
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
21
cyclopropyl group formed together with the same or adjacent carbon atoms
of that C1_8 alkylene or C2_s heteroalkylene chain;
when any adjacent pair of R2, R3, R4 or RS is linlced, to form an optionally
substituted ring, then the linking divalent substituent that forms part of
that
s ring is a methylenedioxy, difluoromethylenedioxy or dimethylmethylene
dioxy group;
when any two adjacent A substituents are linked to form an optionally
substituted ring, then the linking divalent substituent that forms part of
that
ring is selected from methylenedioxy, difluoromethylenedioxy and
to dimethylmethylenedioxy;
when any two adjacent B substituents axe linked to form an optionally
substituted ring, then the linking divalent substituent that forms part of
that
ring is selected from methylenedioxy, difluoromethylenedioxy and
dimethylmethylenedioxy;
is when R6 and R' are linked to form an optionally substituted ring, then that
ring is a 5- to 6-membered ring;
when any pair of R8, R9, Rl°, Rll, R12 and R13 is linked to form a
further
optionally substituted ring, then that ring optionally contains only 1
unsaturation; and/or
2o n represents 2;
Preferred compounds of the invention include those in which Rl, and (when
they represent an aryl or a heteroaryl group) X, R2, R3, R4 and/or RS
represent an optionally substituted fluorenyl, phenyl, naphthyl, pyrrolyl,
2s furanyl, thienyl, pyrazolyl, imidazolyl, oxazolyl, isoxazolyl, thiazolyl,
pyridyl (e.g. 2-pyridyl, 3-pyridyl or 4-pyridyl), indazolyl, indolyl,
indolinyl,
isoindolinyl, quinolinyl, 1,2,3,4-tetrahydroquinolinyl, isoquinolinyl, 1,2,3,4-
tetrahydroisoquinolinyl, quinolizinyl, benzofuranyl, isobenzofuranyl,
chromanyl, benzothienyl, pyridazinyl, pyrimidinyl, pyrazinyl, indazolyl,
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
22
benzimidazolyl, quinazolinyl, quinoxalinyl, 1,3-benzodioxolyl,
benzothiazolyl, and/or benzodioxanyl, group
Preferred values of Rl include optionally substituted fluorenyl (e.g. 2-
s fluorenyl) or pyridyl and, especially, optionally substituted phenyl.
Preferred values of X, when X represents an optionally substituted aryl or
heteroaryl group include optionally substituted phenyl, thienyl (e.g. 2-
thienyl), pyridyl (e.g. 3-pyridyl and 4-pyridyl), pyrazolyl, pyrazinyl or
1 o quinolinyl.
Preferred values of R2, R3, R4 and R5, when they represent an optionally
substituted aryl or heteroaryl group, include optionally substituted phenyl,
pyridyl (e.g. 3-pyridyl) or naphthyl (e.g. 1-naphthyl).
Is
Further preferred compound of the invention include those in which:
~ represents C1_6 alkylene, such as methylene, propylene or hexylene, in
which one of the carbon atoms in the chain may be replaced with a
heteroatom (e.g. oxygen) so forming, for example, an oxypentylene group;
2o R2 represents hydrogen or Gl;
R3 represents hydrogen, phenyl or pyridyl (e.g. 3-pyridyl), which latter two
groups are optionally substituted by one or more substituents selected from
A;
R4 represents hydrogen, phenyl or naphthyl, which Latter two groups are
2s optionally substituted by one or more substituents selected from A;
R5 represents hydrogen or phenyl, which latter group is optionally
substituted by one or more substituents selected from A or is preferably
unsubstituted;
when R2, R3, R4 or RS represents an optionally substituted phenyl, pyridyl or
3o naphthyl group, then the other substituents on the essential benzene ring
of
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
23
the indole of formula I, (i.e. R2, R3, R4 or R4 (as appropriate)) represent
hydrogen or Gl;
A represents Gl or any two adjacent A substituents may be linked to form a
further ring, wherein the linking divalent substituent that forms part of that
s ring is preferably methylenedioxy, which group is preferably unsubstituted;
G1 represents halo (such as chloro or fluoro), cyano, -N02 or Al-Rlo;
A2 represents A6;
A3 represents a single bond;
A4 represents A~;
AS represents A~ and, preferably, a single bond;
A~ represents a single bond, -C(Q2)- or -S(O)z-;
QZ represents =O;
R6 represents hydrogen or C1_3 alkyl group (such as methyl or ethyl), which
Iatter group is optionally substituted by G2;
Is R' represents an aryl group (such as phenyl) or a heteroaryl group (such as
pyridyl), which latter two groups are optionally substituted by one or more
substituents selected from B, or R' represents CI_4 alkyl (such as methyl,
ethyl, propyl, butyl (e.g. n-butyl or t-butyl)), C2.4 alkenyl (such as
ethenyl)
or CS_1o cycloallcyl (such as cyclohexyl or adamantyl), which latter three
2o groups are optionally substituted by one or more substituents selected from
G2; or
R6 and R~ are optionally linked to form a 5- to 6-membered ring optionally
substituted by =0;
B represents GZ;
2s G~ represents halo (such as chloro or fluoro), cyano, -N~2 or Ag-R12;
Ag represents a single bond, -N(R13)An- or -OA12-;
AI I and A12 independently represent A14;
Al4 represents a single bond;
R8 represents C1_3 alkyl (such as ethyl) or, preferably, hydrogen;
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
24
R1° represents hydrogen, aryl (such as phenyl), heteroaryl (such
as
tetrazolyl), C1_4 alkyl (such as methyl, ethyl, isopropyl or butyl (e.g. n-
butyl
or t-butyl)), C2_4 alkenyl (such as ethenyl or butenyl (e.g. but-3-enyl)) or
CS_6 cycloalkyl (such as cyclohexyl), which latter five groups are optionally
s substituted by one or more substituents selected from G3;
Rll represents hydrogen or C2_4 all~enyl (such as propenyl (e.g. propen-2-yl,
i.e. allyl));
R12 represents hydrogen, an aryl group (such as a phenyl group), a
heteroaryl group (such as a pyrrolyl group), C1_4 alkyl (such as methyl,
to isopropyl or butyl (e.g. n-butyl or t-butyl)) or CS_1° cycloalkyl
(such as
cyclohexyl or adamantyl) which latter four groups are optionally substituted
by one or more substituents selected from G3;
R13 represents hydrogen or C1_3 alkyl (such as methyl);
G3 represents halo (such as fluoro) or p,ls-Ris;
Is Als represents a single bond or-~A19-;
Alg represents a single bond;
Rls represents hydrogen, C1_3 alkyl (such as C1_2 alkyl (e.g. methyl)) or aryl
(such as phenyl).
20 (referred optional substituents on Rl, R6, R~ and (when they represent an
aryl or heteroaryl group) X, R~, R3, R4 and Rs groups are selected from:
halo (e.g. chloro, fluoro or bromo);
-NGz~
cyano;
2s methylenedioxy;
C1-s alkyl, which all~yl group may be linear or branched (e.g. C1_4 alkyl
(including methyl, ethyl, o-propyl, isopropyl, n-butyl, s-butyl, isobutyl or
t~
butyl), ja-pentyl, isopentyl, 3Z-hexyl or isohexyl), and which alkyl groups
are
optionally substituted by one or more substituents selected from a halo (e.g.
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
fluoro) group (so forming, for example, -CH2F, -CHF2 or -CF3), an aryl
group (such as phenyl) and OR19;
C2_6 alkenyl (e.g. ethenyl, 1-propenyl, 2-propenyl, 1-butenyl, 2-butenyl, 3-
butenyl, 1-pentenyl, 2-pentenyl, 4-pentenyl or 5-hexenyl);
s C;_1° (e.g. C3_g) cycloalkyl (e.g. cyclopropyl, cyclobutyl,
cyclopentyl,
cyclohexyl or cyclooctyl), optionally substituted with C1_6 alkyl (such as
methyl);
phenyl, optionally substituted with one or more substituents selected from
halo (e.g. fluoro or, especially, chloro) and ~R19;
1o a heteroaryl group selected from tetrazolyl and pyrrolyl, optionally
substituted by one or more C1_g alkyl group (such as methyl);
methylthio, methylsulfinyl, methylsulfonyl;
_~R19>
Zs -hT(Rl~)R2°;
-C(~)~R19;
-c(~)R19;
-C(~)~(R19)R20;
-~,(~)2~(RI9)R20; and/or
20 -N(R19)S(0)2R21;
wherein R19 and R2° independently represent, on each occasion when used
above, hydrogen, phenyl, C1_4 alkenyl (such as propenyl (e.g. propen-2-yl,
i.e. allyl) or butenyl (e.g. but-3-enyl)), C1_6 alkyl (such as methyl, ethyl,
f2-
propyl, isopropyl, h-butyl or t-butyl) which alkyl group is optionally
2s substituted by one or more fluoro atoms or a phenyl group;
R21 represents phenyl or C1_6 alkyl (such as methyl, ethyl, f~-propyl,
isopropyl, fz-butyl or t-butyl), which allcyl group is optionally substituted
by
one or more fluoro atoms.
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
26
When X represents an aryl or a heteroaryl group, then the substituents on
such groups are preferably selected from carboxy, acetyl, methoxy, -N02,
fluoro, methyl, chloro, hydroxymethyl, ethyl, isopropoxy, trifluoromethoxy
and methylthio.
s
Preferred optional substituents on Rl groups include phenoxy,
trifluoromethyl, vitro, fluoro, chloro, cyano, carbamoyl, trifluoromethoxy,
tetrazolyl (e.g. 2H tetrazol-5-yl) and methyl.
1o When they represent an aryl or heteroaryl group, preferred optional
substituents on R2, R3, R4 or RS groups include t-butyl, methylenedioxy,
benzyloxy, vitro, methoxy, acetyl, chloro, fluoro, N allyl-N
methanesulfonyl, cyano, trifluoromethyl, 2,2-dimethylpropionylamino,
lnethanesulfonylamino, amino, but-3-enylamino, isopropoxy, methylthio,
1s methylsulfonyl, ethenyl (i.e. vinyl), trifluoromethoxy, cyclohexyl, n-
butyl,
carboxy and hydroxymethyl.
Preferred optional substituents on Rg (when R6 does not represent hydrogen
and is not linlced to form a ring with R') include an optionally substituted
2o phenyl group. Optional substituents on such phenyl groups include halo
(especially fluoro) and Cl_3 alkoxy (such as methoxy), which substituents
are preferably in the 4-position of the phenyl ring.
Preferred optionally substituents on R' (when R' is not linked to form a ring
2s with R6) include chloro, methoxy, amino, methyl, dimethylamino, phenyl,
4-methoxyphenyl, adamantyl, cyclohexyl, 3,3,5,5-tetramethylcyclohexyl,
isopropoxy, trifluororriethyl, t-butyl, ~-butyl, isopropyl, trifluoromethoxy,
cyano, pyrrole (e.g. 2,5-dimethylpyrrole) and vitro.
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
27
Particularly preferred compounds of the invention include those of the
examples described hereinafter.
Compounds of the invention may be made in accordance with techniques
s that are well known to those skilled in the art, for example as described
hereinafter.
According to a further aspect of the invention there is provided a process for
the preparation of a compound of formula I, which process comprises:
(i) reaction of a compound of formula II,
R~
R3
R4 / H
R~
Is wherein ~, Y, RZ, R3, R4 and RS are as hereinbefore defined, with a
compound of formula III,
RrZLI III
2o wherein L1 represents a suitable leaving group, such as halo (e.g. chloro,
. bromo or iodo), a carboxylate group, a sulfonylate group (e.g. -OS(O}2CF3,
-OS(O)2CH3, -OS(O)ZPhMe or nonaflates), or an N imidazolyl group and Rl
and Z are as hereinbefore defined, for example at around 0°C to room
temperature, or at above room temperature (e.g. up to 40-180°C) in the
2s presence of a suitable base (e.g. sodium hydride, sodium bicarbonate,
potassium carbonate, pyrrolidinopyridine, pyridine, triethylamine,
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
28
tributylamine, trimethylamine, dimethylaminopyridine, diisopropylamine,
I,8-diazabicyclo[5.4.0]undec-7-ene, sodium hydroxide, potassium
hydroxide, N ethyl-diisopropylamine, N (methylpolystyrene)-4-
(methylamino)pyridine, lithiumdiisopropylamide or mixtures thereof) and
s an appropriate solvent (e.g. tetrahydrofuran, pyridine, toluene,
dichloromethane, chloroform, dimethylformamide, trifluoromethylbenzene,
dimethylsulfoxide or triethylamine). Preferred base/solvent systems include
sodium hydride/dimethylformamide, dimethylaminopyridine/pyridine,
sodium hydroxide/dichloromethane (optionally in the presence of a phase
1 o transfer catalyst (e.g. tetrabutylammonium hydrogensulfate)),
lithiumdiisopropylamide/tetrahydrofuran, potassium hydroxide/dimethyl-
sulfoxide. Other systems that may be mentioned include sodium/ammonia.
(ii) reaction of a compound of formula IV,
X
~z-~s
\ ~ m
~a
~~R~
wherein L4 represents L2 or L3, in which L2 represents a leaving group such
as halo (e.g. chloro, bromo or iodo), a sulfonylate group (e.g.
-OS(O)2CF3, -OS(O)ZCH3, -OS{O)2PhMe or nonaflates) or B(OH)~, L3
represents a leaving group such as B(OH)2,
-4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl, -Sn~u3, or a similar group
known to the skilled person, Lø is attached to one or more of the carbon
atoms of the benzenoid ring of the indole, and the remaining positions of the
benzenoid ring are substituted with 1 to 3 (depending on the number of L4
substituents) substituents R~ to RS as appropriate, and Z, X, Y, R~, R2, R3,
R4 and RS are as hereinbefore defined, with a compound of formula V,
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
29
Ra2Ls V
wherein R22 represents R2, R3, R4 or Rs (as appropriate), and Ls represents
s La (when L4 is L3) or L3 (when L4 is L2) as hereinbefore defined. The
skilled person will appreciate that L2 and L3 must be mutually compatible.
This reaction may be performed, for example in the presence of a suitable
catalyst system, e.g. a metal (or a salt or complex thereof) such as CuI,
Pd/C, Pd(OAc)2, Pd(Ph;P)2C12, Pd(Ph3P)4, Pd2(dba)3 or NiCl2 and a ligand
to such as t-Bu3P, (C6H~1)3P, Ph3P, P(o-Tol)3, 1,2-bis(diphenylphosphino)-
ethane, 2,2'-bis(di-tef°t-butylphosphino)-l,I'-biphenyl, 2,2'-
bis(diphenyl-
phosphino)-l,l'-binaphthyl, 1,1'-bis(diphenylphosphinoferrocene), 1,3-
bis(diphenylphosphino)propane or xantphos, together with a suitable base,
such as NazC03, K;P04, CszCO;, NaOH, KZC03, CsF, Et3N, (i-Pr)2NEt or
1s t-BuOK (or mixtures thereof) in a suitable solvent, such as dioxane,
toluene,
ethanol, dimethylformamide, ethylene glycol dimethyl ether, water,
dimethylsulfoxide, acetonitrile, dimethylacetamide, N methylpyrrolidinone
or mixtures thereof. The reaction may also be carried out for example at
room temperature or above (e.g, at a high temperature such as the reflux
2o temperature of the solvent system) or using microwave irradiation;
(iii) for compounds of formula I, wherein X represents an optionally
substituted aryl or heteroaryl group, reaction of a compound of formula VI,
R~ L2
R3
VI
R~ / N
25 R5 ~~R1
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
wherein Z, Y, L2, R1, R2, R3, R4 and R5 are as hereinbefore def ned, with a.
compound of formula VII,
XaL3 VII
5
wherein Xa represents an aryl or heteroaryl group, optionally substituted as
hereinbefore defined, and L3 is as hereinbefore defined, for example under
reaction conditions such as those hereinbefore described hereinbefore in
respect of process step (ii);
(iv) for compounds of formula I wherein X represents -N(R6)-E-R', reaction
of a compound of formula VI as hereinbefore defined, with a compound of
formula VIII,
is I~T(R6)-E-R' VIII
wherein E, R6 and R~ are as hereinbefore defined for example optionally in
the presence of an appropriate metal catalyst (or a salt or complex thereof)
such as Cu, Cu(~.l~c)2, CuI (or CuIldiamine complex), Pd(~Ac)2, Pd2(dba)3
or NiCh and an optional additive such as Ph3F, 2,2'-
bis(diphenylphosphino)-1,1'-binaphthyl or xantphos, in the presence of an
appropriate base such as Et3N, pyridine, N,N-dimethylethylenediamine,
Na2C03, I~3PO4, Cs2C0~ or t-BuOK (or mixtures thereof), in a suitable
solvent (e.g. dichloromethane, dioxane, toluene, ethanol,
2s dimethylfonnamide, ethylene glycol dimethyl ether, water,
dimethylsulfoxide, acetonitrile, dimethylacetamide, N methylpyrrolidinone
or mixtures thereof) or in the absence of an additional solvent when- the
reagent may itself act as a solvent (e.g. when Rl represents phenyl and L1
represents bromo, i.e. bromobenzene). This reaction may be carried out at
3o room temperature or above (e.g. at a high temperature, such as the reflux
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
3I
temperature of the solvent system that is employed) or using microwave
irradiati on;
(v) for compounds of formula I wherein X represents -N(R6)-E-R~, reaction
s of a compound of formula IX,
R6
R2 \NH
R3
N
W R1
wherein ~, Y, R1, R2, R3, R4, R$ and R6 are as hereinbefore defined, with a
compound of formula X,
R~_E_L1 X
wherein E, R~ and L1 are as hereinbefore defined, for example under
is reaction conditions such as those hereinbefore described in respect of
process step (i); or
(vi) for compounds of formula I wherein E represents a single bond and R~
is a C1_6 alkyl group, C3_6 alkenyl or a C3_6 alkynyl group, reduction of a
2o compound of formula I, wherein X represents -C(O)- and R~ represents H, a
CI_5 alkyl group, a C2_5 alkenyl or a CZ_5 alkynyl group, in the presence of a
suitable reducing agent. A suitable reducing agent may be an appropriate
reagent that reduces the amide group to the amine group in the presence of
other functional groups (for example an ester or a carboxylic acid). Suitable
2s reducing agents include borane and other reagents known to the skilled
person, under reaction conditions known to the skilled person.
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
32
Compounds of formula II may be prepared by:
(a) reaction of a compound of formula X.I,
X
~a_R5
\ ~ xi
N
H
wherein X, Y, L4, R2, R3, R4 and R5 are as hereinbefore defined with
a compound of formula V for example under conditions such as
to those described hereinbefore in respect of process step (ii);
(b) for compounds of formula II wherein X represents an optionally
substituted aryl or heteroaryl group, reaction of a compound of
formula XII,
zs
L2
~3
\ ~ x~l
R5
wherein Y, L2, R2, R3, R4 and RS are as hereinbefore defined, with a
compound of formula VII as hereinbefore defined, for example under
2o conditions such as those described hereinbefore in respect of process
step (iii);
(c) for compounds of formula II, wherein X represents N(R6)-E-R7,
reaction of a compound of formula XII as hereinbefore defined, with
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
33
a compound of formula VIII for example under conditions .such as
those described hereinbefore in respect of process step (iv);
(d) for compounds of formula II, wherein X represents N(R6)-E-R',
s reaction of a compound of formula XIII,
R6
R2 \NH
R3
\ ~ VIII
R~ / H
R5
wherein ~.', RZ, R3, R4, Rs and R6 are as hereinbefore defined with a
1o compound of formula X for example under conditions such as those
described hereinbefore in respect of process step (v);
Compounds of formula IV may be prepared by reaction of a compound of
formula XI as hereinbefore defined, with a compound of formula III as
Is hereinbefore defined for example under conditions such as those described
hereinbefore in respect of process step (i).
Compounds of formula IV in which L4 represents L3 may be prepared by
reaction of a compound of formula IV in which L4 represents L2, with an
2o appropriate reagent for the conversion of the LZ group to the L3 group.
This
conversion may be performed by methods blown to those skilled in the art,
for example, compounds of formula IV, in which L3 is 4,4,5,5-tetramethyl-
1,3,2-dioxaborolan-2-yl may be prepared by reaction of the reagent
bis(pinacolato)diboron with a compound of formula IV in which L4
2s represents L~', for example under reaction conditions such as those
described
hereinbefore in respect of process route (ii) above.
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
34
Compounds of formula VI may be prepared by:
(a) reaction of a compound of formula XII as hereinbefore defined with
s a compound of formula III as hereinbefore defined for example
under conditions such as those described hereinbefore in respect of
process step (i);
(b) for compounds of formula VI wherein L2 represents a sulfonylate
1o group, reaction of a compound of formula XIV,
R2
R3
\ ~ ~I~/
~5 ~ ~ R1
wherein Y, ~, Rl, I~2, R3, R4 and Ids are as hereinbefore defined, with
1s an appropriate reagent for the conversion of the hydroxyl group to
the sulfonylate group (e.g. tosyl chloride, mesyl chloride, triflic
anhydride and the like) under conditions known to those skilled in
the art.
2o Compounds of formula I~ may be prepared for example by reaction of a
compound of formula XITI as hereinbefore def ned with a compound of
formula III as hereinbefore defined for example under conditions such as
those described hereinbefore in respect ofprocess step (i).
2s Compounds of formula XII may be prepared by standard techniques. For
example:
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
(a) Compounds of formula XIT, wherein L2 represents halo may be
prepared by reaction of a compound of formula X~1,
R2
R3
\ ~ xv
R4 / H
5 R5
wherein R2, R3, R4, RS and Y are as hereinbefore defined, with a
reagent, or mixture of reagents known to be a source of halide ions.
For example, for bromide ions, N bromosuccinimide may be
1o employed, for iodide ions, iodine or a mixture of NaI and N
chlorosuccinimide may be employed, for chloride ions, N
chlorosuccinimide may be employed and for fluoride ions, 1-
(chloromethyl)-4-fluoro-1,4-diazoniabicyelo[2.2.2]octane bis(tetra-
fluoroborate) may be employed. This reaction may be carried out in
15 a suitable solvent (e.g. acetone or benzene) under conditions known
to the skilled person.
(b) by reaction of a compound of formula XVT,
L2
R~_Rs
\ y ?C1/I
L~. N
a,o
wherein Y, LZ, L4, Rz, R3, R4 and R5 are as hereinbefore defined with
a compound of formula V as hereinbefore defined, for example
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
36
under reaction conditions such as those described hereinbefore in
respect of process route (ii) above; or
(c) for compounds of formula XII wherein Ll represents a sulfonylate
s group, reaction of a compound of formula XVII,
R2 OH
R3
R4 /' H
R5
wherein Y, R2, R3, R4 and RS are as hereinbefore defined, with an
to appropriate reagent for the conversion of the hydroxyl group to a
sulfonylate group as described hereinbefore.
Compounds of formula XIII may be prepared by reaction of a compound of
formula XII as hareinbefore defined with a compound of formula XVIII,
Ha~TR6 XVIII
wherein R6 is as hereinbefore defined, for example under reaction
conditions such as those described in respect of process step (iv).
Compounds of formula XIII wherein R6 represents hydrogen may be
prepared by an aromatic nitration reaction carried out on a compound of
formula XV, as hereinbefore defined, followed by reduction of the nitro
group of the resultant intermediate to an amino group. both reactions may
2s be performed under conditions known to the skilled person.
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
37
Compounds of formulae III, V, VII, VIII, X, XVIII axe either commercially
available, are known in the literature, or may be obtained either by analogy
with the processes described herein, or by conventional synthetic
procedures, in accordance with standard techniques, from available starting
s materials using appropriate reagents and reaction conditions.
Indoles of formulae II, IV, VI, Ice, XI, XII, VIII, XIV, XV, XVI and XVTI
may also be prepared with reference to a standard heterocyclic chemistry
textbook (e.g. "Heterocyclic Chemistry" by J. A. Joule, K. Mills and G. F.
1o Smith, 3rd edition, published by Chapman ~ Hall) and/or made according to
the following general procedures.
For example compounds of formulae II, ~I, XIII and XV may be prepared
by reaction of a compound of formula SIX,
is
sU~ i xl~
H..H
H
wherein SUB represents the substitution pattern that is present in the
compound of formula II, XT, XIII or XV to be formed, (G) represents either
2o X (as required for formation of compounds of formulae II and XI), a
N(R6)H group (as required for formation of compounds of formula XIII)
or the (G) substituent is absent (as required for formation of compounds of
formula XV) and Y is as hereinbefore defined, under Fischer indole
synthesis conditions known to the person spilled in the art.
?s
Compounds of fornula XV may alternatively be prepared by reaction of a
compound of formula XX,
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
38
R2 O
3
R
~H
R4 /
R~
wherein R2, R3, R4 and Rs are as hereinbefore defined with a compound of
s formula ~I,
N3CHZY XXI
wherein Y is as hereinbefore defined, and preferably does not represent
-COON, under conditions known to the person skilled in the art (i.e.
conditions to induce a condensation reaction, followed by a thermally
induced cyclisation).
Compounds of formulae ~V and XVIT may be prepared by reaction of a
zs compound of formula III,
R2
Rs O~RX
X?CI i
N.~l
R5 Ry
wherein R" represents a C1_6 alkyl group, Ry represents either -Z-R1 as
2o hereinbefore defined (as required for formation of compounds of formula
XIV), hydrogen (as required for formation of compounds of formula XVII)
or a nitrogen-protected derivative thereof, and Y, R', R3, R4 and Rs are as
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
39
hereinbefore defined, under standard cyclisation conditions known to those
spilled in the art.
Compounds of formulae IX and XIII, wherein R6 represents H, may be
s prepared by reaction of a compound of formula XXIII,
R2
R3 ~ CN
~Cfii
R4 / N /y
R ~ Rv
wherein Y, R2, R3, R~, RS and Rv are as hereinbefore defined, for example
1o under intramolecular cyclisation conditions known to those skilled in the
art.
Compounds of formula II and ~I, wherein ~ represents aryl or heteroaryl,
may alternatively be prepared by reaction of a compound of formula HIV,
~CI~/
SIJ~
NH
I
wherein Q represents either -C(O)- or -CHZ-, ~ represents aryl or
heteroaryl, and SLTB and Y are as hereinbefore defined. When Q represents
-C(O)-, the intramolecular cyclisation may be induced by a reducing agent
such as TiCl3/CBK, TiCl4/Zn or SmI2 under conditions known to the skilled
person, for example, at room temperature in the presence of a polar aprotic
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
solvent (such as THF).. When Q represents -CH2-, the reaction may be
performed in the presence of base under intramolecular condensation
reaction conditions known to the skilled person.
s Compounds of formula XIX may be prepared by:
(a) reaction of a compound of formula XXV,
S U S ?C~V
N~NHz
H
to
wherein SLT~ is as hereinbefore defined with a compound of
formula XXVI,
(
\/I
is
wherein (G) and Y are as hereinbefore defined under
condensation conditions known to the skilled person; or
(b) reaction of a compound of formula ~~XVII,
\ X?C1/I
SUS
N+
z
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
41
wherein SUB is as hereinbefore defined with a compound of
formula XXVIII,
(G)
Rm
wherein Rm represents OH, O-C1_6 alkyl, C1_6 alkyl and (G)
and Y are as hereinbefore defined, for example under Japp-
I~lingemann conditions known to the skilled person.
to Compounds of formulae XX, XXI, XXII, XXIII, XIV, XV, XVI, XVH and
XVIII are either commercially available, are known in the literature, or may
be obtained either by analogy with the processes described herein, or by
conventional synthetic procedures, in accordance with standard techniques,
from available starting materials using appropriate reagents and reaction
is conditions.
The substituents X, Y, ~, Rl, Ra, R3, R4 and RS in final compounds of the
invention or relevant intermediates may be modified one or more times,
after or during the processes described above by way of methods that are
2o well known to those skilled in the art. Examples of such methods include
substitutions, reductions, oxidations, alkylations, hydrolyses,
esterifications,
and etherifications. The precursor groups can be changed to a different
such group, or to the groups defined in formula I, at any time during the
reaction sequence. For example, in cases where Y represent a carboxylic
2s acid ester functional group, the skilled person will appreciate that at any
stage during the synthesis (e.g. the final step), the relevant substituent may
be hydrolysed so forming for example a carboxylic acid functional group.
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
42
Tn cases where Y represents a carboxylic acid or carboxylic acid ester
functional group, the relevant substituent may be reduced, under suitable
conditions known to the skilled person (for example in the presence of other
potentially reducible functional groups), at any stage during the synthesis
s (e.g. the final step), so forming for example a hydroxymethyl substituent.
Compounds of the invention may be isolated from their reaction mixtures
using conventional techniques.
to It will be appreciated by those skilled in the art that, in the processes
described above and hereinafter, the functional groups of intermediate
compounds may need to be protected by protecting groups.
The protection and deprotection of functional groups may take place before
15 or after a reaction in the above-mentioned schemes.
Protecting groups may be removed in accordance with techniques that are
well known to those skilled in the art and as described hereinafter. For
example, protected compounds/intermediates described herein may be
2o converted chemically to unprotected compounds using standard
deprotection techniques.
The type of chemistry involved will dictate the need, and type, of protecting
groups as well as the sequence for accomplishing the synthesis.
The use of protecting groups is fully described in "Protective Groups in
Organic Chemistry", edited by J W F McOmie, Plenum Press (1973), and
"Protective Groups in Organic Synthesis", 3rd edition, T.W. Greene ~
P.G.M. Wutz, Wiley-Interscience (1999).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
43
Medical and Pharmaceutical Uses
Compounds of the invention are indicated as pharmaceuticals. According
to a further aspect of the invention there is provided a compound of the
s invention for use as a pharmaceutical.
Although compounds of the invention may possess pharmacological activity
as such, certain pharmaceutically-acceptable (e.g. "protected") derivatives
of compounds of the invention may exist or be prepared which may not
1o possess such activity, but may be administered parenterally or orally and
thereafter be metabolised in the body to form compounds of the invention.
Such compounds (which may possess some pharmacological activity,
provided that such activity is appreciably lower than that of the "active"
compounds to which they are metabolised) may therefore be described as
15 "prodrugs" of compounds of the invention.
~y "prodrug of a compound of the invention", we include compounds that
form a compound of the invention, in an experimentally-detectable amount,
within a predetermined time (e.g. about 1 hour), following oral or parenteral
2o administration. All prodrugs of the compounds of the invention are
included within the scope of the invention.
Furthermore, certain compounds of the invention (including, but not limited
to, compounds of formula I in which Y is -C(O)ORS and Rg is other than
2s hydrogen) may possess no or minimal pharmacological activity as such, but
may be administered parenterally or orally, and thereafter be metabolised in
the body to form compounds of the invention that possess pharmacological
activity as such (including, but not limited to, corresponding compounds of
formula I in which Y represents -COOH). Such compounds (which also
3o includes compounds that may possess some pharmacological activity, but that
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
44
activity is appreciably lower than that of the "active" compounds of the
invention to which they are metabolised), may also be described as
"prodrugs".
s Thus, the compounds of the invention are useful because they possess
pharmacological activity, and/or are metabolised in the body following oral
or parenteral administration to form compounds which possess
pharmacological activity.
1o Compounds of the invention are particularly useful because they may
inhibit (for example selectively) the activity of prostaglandin E synthases
(mPGESs) and particularly the activity of microsomal prostaglandin E
synthase-1 (ml'GES-1), i.e. they prevent the action of lnPGES-1 or a
complex of which the mPGES-1 enzyme forms a part, and/or may elicit a
15 mPGES-1 modulating effect, for example as may be demonstrated in the
test described below. Compounds of the invention may thus be useful in the
treatment of those conditions in which inhibition of a PGES, and
particularly mPGES-1, is required.
2o Compounds of the invention are thus expected to be useful in the treatment
of inflammation.
The term "inflammation" will be understood by those skilled in the art to
include any condition characterised by a localised or a systemic protective
2s response, which may be elicited by physical trauma, infection, chronic
diseases, such as those mentioned hereinbefore, and/or chemical and/or
physiological reactions to external stimuli (e.g. as part of an allergic
response). Any such response, which may serve to destroy, dilute or
sequester both the injurious agent and the injured tissue, may be manifest
3o by, for example, heat, swelling, pain, redness, dilation of blood vessels
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
and/or increased blood flow, invasion of the affected area by white blood
cells, loss of function and/or any other symptoms known to be associated
with inflammatory conditions.
s The term "inflammation" will thus also be understood to include any
inflammatory disease, disorder or condition pe~° se, any condition that
has
an inflammatory component associated with it, and/or any condition
characterised by inflammation as a symptom, including if2te~ alia acute,
chronic, ulcerative, specific, allergic and necrotic inflammation, and other
1 o forms of inflammation known to those skilled in the art. The term thus
also
includes, for the purposes of this invention, inflammatory pain, pain
generally andlor fever.
Accordingly, compounds of the invention may be useful in the treatment of
15 inflammatory bowel disease, irritable bowel syndrome, migraine, headache,
low back pain, fibromyalgia, myofascial disorders, viral infections (e.g~.
influenza, common cold, herpes zoster, and AIIaS), bacterial infections,
fungal infections, dysmenorrhea, burns, surgical or dental procedures,
malignancies (e.~. breast cancer, colon cancer, and prostate cancer),
2o atherosclerosis, gout, arthritis, osteoarthritis, juvenile arthritis,
rheumatoid
arthritis, rheumatic fever, ankylosing spondylitis, systemic Iupus
erythematosus, vasculitis, pancreatitis, nephritis, bursitis, conjunctivitis,
iritis, scleritis, uveitis, wound healing, dermatitis, eczema, psoriasis,
stroke,
diabetes , (e.g. diabetes mellitus), neurodegenerative disorders such as
2s Alzheimer's disease and multiple sclerosis, autoimmune diseases,
osteoporosis, asthma, chronic obstructive pulmonary disease, pulmonary
fibrosis, allergic disorders, rhinitis, ulcers, coronary heart disease,
sarcoidosis and any other disease with an inflarrunatory component.
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
46
Compounds of the invention are indicated both in the therapeutic and/or
prophylactic treatment of the above-mentioned conditions.
According to a further aspect of the present invention, there is provided a
s method of treatment of a disease which is associated with, and/or which can
be modulated by inhibition of, a PGES (such as a mPGES, e.g. mPGES-I),
and/or a method of treatment of a disease in which inhibition of the activity
of a FGES, and particularly mFGES-I, is desired and/or required (e.g.
inflammation), which method comprises administration of a therapeutically
1o effective amount of a compound of the invention, as hereinbefore defined,
to a patient suffering from, or susceptible to, such a condition.
"Patients" include mammalian (including human) patients.
15 The term "effective amount" refers to an amount of a compound, which
confers a therapeutic effect on the treated patient. The effect may be
objective (i.e. measurable by some test or marker) or subjective (i.e. the
subj ect gives an indication of or feels an effect).
2o Compounds of the invention will normally be administered orally,
intravenously, subcutaneously, buccally, rectally, dermally, nasally,
tracheally, bronchially, sublingually, intraperitoneally, topically (e.g.
ocularly), intramuscularly, intraspinally, epidurally, transdermally, by any
other parenteral route or via inhalation, in a pharmaceutically acceptable
2s dosage form.
Compounds of the invention may be administered alone, but are preferably
administered by way of known pharmaceutical formulations, including
tablets, capsules or elixirs fox oral, administration, suppositories for
rectal
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
47
administration, sterile solutions or suspensions for parenteral or
intramuscular administration, and the like.
Such formulations may be prepared in accordance with standard and/or
s accepted pharmaceutical practice.
According to a further aspect of the invention there is thus provided a
pharmaceutical formulation including a compound of the invention, as
hereinbefore~ defined, in admixture with a pharmaceutically acceptable
1o adjuvant, diluent or carrier.
Compounds of the invention may also be combined with other therapeutic
agents that are useful in the treatment of inflammation (e.g. NSAII~s and
coxibs).
According to a further aspect of the invention, there is provided a
combination product comprising: t
(A) a compound of the invention, as hereinbefore defined; and
(B) another therapeutic agent that is useful in the treatment of
2o inflammation,
wherein each of components (A) and (B) is formulated in admixture with a
pharmaceutically-acceptable adjuvant, diluent or carrier.
Such combination products provide for the administration of a compound of
the invention in conjunction with the other therapeutic agent, and may thus
be presented either as separate formulations, wherein at least one of those
formulations comprises a compound of the invention, and at least one
comprises the other therapeutic agent, or may be presented (i.e. formulated)
as a combined preparation (i.e. presented as a single formulation including a
3o compound of the invention and the other therapeutic agent).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
48
Thus, there is further provided:
(1) a pharmaceutical formulation including a compound of the invention, as
s hereinbefore defined, another therapeutic agent that is useful in the
treatment of inflammation, and a pharmaceutically-acceptable adjuvant,
diluent or carrier; and
(2) a kit of parts comprising components:
(a) a pharmaceutical formulation including a compound of the invention,
as hereinbefore defined, in admixture with a pharmaceutically-
acceptable adjuvant, diluent or carrier; and
(b) a pharmaceutical formulation including another therapeutic agent
that is useful in the treatment of inflammation in admi~rture with a
1s pharmaceutically-acceptable adjuvant, diluent or carrier,
which components (a) and (b) are each provided in a form that is suitable
for administration in conjunction with the other.
Compounds of the invention may be administered at varying doses. oral
2o dosages may range from between about 0.01 mg/kg of body weight per day
(mg/kg/day) to about 100 mg/kg/day, preferably about 0.01 to about IO
mg/kg/day, and more preferably about 0.1 to about 5.0 mg/kg/day. For oral
administration, the compositions typically contain between about 0.01 mg
to about 500 mg, and preferably between about 1 mg to about 100 mg, of
2s the active ingredient. Intravenously, the most preferred doses will range
from about 0.001 to about 10 mg/kg/hour during constant rate infusion.
Advantageously, compounds may be administered in a single daily dose, or
the total daily dosage may be administered in divided doses of two, three or
four times daily.
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
49
In any event, the physician, or the skilled person, will be able to determine
the actual dosage which will be most suitable for an individual patient,
which is likely to vary with the route of administration, the type and
severity of the condition that is to be treated, as well as the species, age,
s weight, sex, renal function, hepatic function and response of the particular
patient to be treated. The above-mentioned dosages are exemplary of the
average case; there can, of course, be individual instances where higher or
lower dosage ranges are merited, and such are within the scope of this
invention.
to
Compounds of the invention may have the advantage that they are effective,
and preferably selective, inhibitors of prostaglandin E synthases (PGES)
and particularly microsomal prostaglandin E synthase-I (mPGES-1). The
compounds of the invention may reduce the formation of the specific
1s arachidonic acid metabolite PGEZ without reducing the formation of other
arachidonic acid metabolites, and thus may not give rise to the associated
side-effects mentioned hereinbefore.
Compounds of the invention may also have the advantage that they may be
2o more efficacious than, be less toxic than, be longer acting than, be more
potent than, produce fewer side effects than, be more easily absorbed than,
and/or have a better pharmacokinetic profile (e.g. higher oral bioavailability
and/or lower clearance) than, and/or have other useful pharmacological,
physical, or chemical properties over, compounds known in the prior art,
2s whether for use in the above-stated indications or otherwise.
Biological Test
In the assay mPGES-1 catalyses the reaction where the substrate PGH2 is
3o converted to PGE2, mPGES-1 is expressed in E. coli and the membrane
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
fraction is dissolved in 20mM NaPi-buffer pH 8.0 and stored at -80 °C.
In
the assay lnPGES-1 is dissolved in O.1M KPi-buffer pH 7.35 with 2.SmM
glutathione. The stop solution consists of H2O / MeCN (7/3), containing
FeClz (25 mM) and HCl (0.15 M). The assay is performed at room
s temperature in 96-well plates. Analysis of the amount of PGE2 is
performed with reversed phase HPLC (Waters 2795 equipped with a 3.9 x
150 mm C18 column). The mobile phase consists of H2O / MeCN (7/3),
containing TFA (0.056%), and absorbance is measured at 195 nm with a
Waters 2487 LTV-detector.
to The following is added chronologically to each well:
1. 100 ~,L mPGES-1 in I~Pi-buffer with glutathione. Total protein
concentration: 0.02 mg/mL.
2. 1 ~L inhibitor in DMSO. Incubation of the plate at room temperature
for 25 minutes.
15 3. 4 uL of a 0.25mM PGH2 solution. Incubation of the plate at room
temperature for 60 seconds.
4. 100 ~I, stop solution.
I80 ~,L, per sample is analysed with HPLC.
2o Examples
The invention is illustrated by way of the following examples, in which the
following abbreviations may be employed:
BINAP 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl
2s dba dibenzylideneacetone
DMAP 4,4-dimethylaminopyridine
DME ethylene glycol dimethyl ether
DMF dimethylformamide
DMSO dimethylsulfoxide
3o EtOAc ethyl acetate
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
51
HPLC High Pressure Liquid Chromatography
MeCN acetonitrile
NBS N bromosuccinimide
NCS N chlorosuccinimide
s NMR nuclear magnetic resonance
TFA trifluoroacetic acid
THF tetrahydrofuran
TLC thin Layer chromatography
to Starting materials and chemical reagents specified in the syntheses
described below are commercially available from, e.g. Sigma-Aldrich Fine
Chemicals.
Example 1
is 6-(4-tee°t-~u lphen~)-1-(3-phenoxybenwl)-3-phenylindole-2-carbox lic
acid
(a) 6-(4-te~°t-~utylphen~l)indole-2-carboxylic acid ethyl ester
A mixture of 6-bromoindole-2-carboxylic acid ethyl ester (400 mg, 1.5
2o mmol), 4-tee°t-butylphenylboronic acid (400 mg, 2.25 mmol), I~3P04
(950
mg, 1.5 mmol), Pd(OAc)2 (18 mg, 0.075 mmol), 2,2'-bis(di-tef°t-
butylphosphino)-l,l'-biphenyl (45 mg, 0.15 mmol), and toluene (9 mL)
were stirred in an argon atmosphere for 30 min at room temperature, and at
100 °C for 40 min using microwave irradiation. The mixture was cooled
to
25 room temperature and poured into NaHC03 (aq., sat.). The mixture was
extracted with EtOAc and the combined extracts were washed with water,
brine and dried over Na2SO4. The organic phase was then concentrated and
the product purified by chromatography to give the sub-title compound (392
mg, ~l%).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
s2
(b) 6-(4-te~°t-But~phenyll-3-iodoindole-2-carboxylic acid ethyl ester
The reaction was performed with the exclusion of light. A solution of NaI
(300 mg, 2.0 mmol) in acetone (15 mL) was added dropwise to a stirred
solution of NCS (270 mg, 2.0 mmol) in acetone (4 mL), followed after 15
s min by the dropwise addition of 6-(4-ter°t-butylphenyl)indole-2-
carboxylic
acid ethyl ester (650 mg, 2.0 mmol; see step (a) above) in acetone (20 mL).
After 30 min at room temperature the mixture was poured into an aqueous
solution of Na2S203 (aq., 10%) and extracted with EtOAc. The combined
extracts were washed with water, brine and dried over Na2SO4. The organic
to phase was then concentrated and then purified by chromatography to give
the sub-title compound (743 mg, 82%).
(c) 6 ~4-tef~t-Buiylpheny~-3-iodo-1-(3-phenox~n~I)-indole-2-carboxylic
acid ethyl ester
is A solution of 6-(4-ter°t-butylphenyl)-3-iodoindole-2-carboxylic acid
ethyl
ester (743 rng, 1.66 mmol; see step (b) above) in DMF (10 mL) was added
carefully to a stirred suspension of NaH (41 mg, 1.69 mmol) in DMF (4
mL) at 0 °C. The mixture was stirred at room temperature for 25 min. A
solution of 3-phenoxyber~yl chloride (37~ mg, 1.69 mmol) in DMF (6 mL)
2o was then added in portions and the mixture was stirred at room temperature
for a further 24 h, then poured into water and extracted with t-BuOMe. The
combined extracts were washed with water, brine and dried over Na2SO4.
The organic phase was concentrated and the product purified by
chromatography and then crystallisation from EtOH to give the sub-title
25 compound (766 mg, 73 %).
(d) 6-~4-tef~t-Butylphenyl)-I-(3-phenoxYbenz~-3-phenylindole-2-carboxy-
lic acid eth 1
A mixture of 6-(4-tent-butylphenyl)-3-iodo-I-(3-phenoxybenzyl)-indole-2-
3o carboxylic acid ethyl ester (200 mg, 0.32 mmol; see step (c) above),
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
53
phenylboronic acid (59 mg, 0.48 mmol), I~3P04 (238 mg, 1.12 mmol),
Pd(OAc)2 (3.6 mg, 0.016 mmol) and toluene (3 mL) was stirred for 20 min
at room temperature and then for 4 h at 80 °C. The mixture was poured
into
NaHCO3 (aq., sat.) and extracted with EtOAc. The combined extracts were
s washed with water, brine and dried over Na2S04. The organic phase was
then concentrated and the product purified by column chromatography to
give the sub-title compound (163 mg, 88%).
6 ~4-ter°t-B ut~phenyl ~ l -(3 -phenoxyben~phenylin dol e-2-carb oxylic
to acid
A mixture of 6-(4-te~°t-butylphenyl)-1-(3-phenoxybenzyl)-3-
phenylindole-2-
carboxylic acid ethyl ester (163 mg, 0.281 nunol; see step (d)), aqueous
NaOH ( 1 M, 10 mL) and MeCN (40 mL) was heated at reflux for 4 h. The
mixture was then allowed to cool, acidified witl2 HCl (1M) to pH 2 and
1s extracted with EtOAc. The combined extracts were washed with water,
brine and dried over Na2SO4. The combined extracts were concentrated and
the product was purified by chromatography and recrystallisation firstly
from EtOH and then from MeCN to give the title compound (95 mg,
61°f°).
1H NMIZ (DMSO-d6, 200 MHz): ~ 7.6I-7.37 (12H, m), 7.29-7.17 (3H, m),
20 7.09-7.00 (1H, m), 6.97-6.90 (2H, m), 6.85 (1H, d, J=2.0 Hz), 6.83-6.77
(2H, m), 5.86 (2H, s), 1.37 (9H, s).
Examt~le 2
6-(4-tee°t-But~phenyl)-1-(3- hp enoxybenzyl)-3-(2-thienyl)indole-2-
carboxylic
25 acid
(a) 6 ~4-tee°t-Butylphenyl -~1-(3-phenoxybenzyl)-3-(2-thienyl~indole-2-
carb-
oxylic acid eth h~ester
2-(Tributylstannyl)thiophene (72 mg, 0.20 mmol) was added to a stirred
so mixture of 6-(4-tent-butylphenyl)-3-iodo-1-(3-phenoxybenzyl)-indole-2-
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
s4
carboxylic acid ethyl ester (150 mg, 0.24 mmol; see Example 1(d)), CuI (25
mg, 0.13 rmnol), Pd(PPh3)2C12 (18 mg, 0.026 mmol) and DMF (3 mL).
After 10 min at room temperature and 1 h at 90°C another portion
of
2-(tributylstannyl)thiophene (72 mg, 0.20 mmol) was added and the heating
s was continued for 3 h. The mixture was filtered through Celite~ and the
solids were washed with EtOAc. Concentration and purification by
chromatography gave the sub-title compound (125 mg, 90%).
(b) 6-(4-ter°t-Butyllahenyl~3-phenoxybenzyl)-3-(2-thienyl)indole-2-carb-
oxylic acid ethyl ester
A mixture of 6-(4-t~~°t-butylphenyl)-1-(3-phenoxybenzyl)-3-
phenylindole-2-
carboxylic acid ethyl ester (125 mg, 0.213 mmol; see step (a)), aqueous
KOH (2M, 2 mL) and MeCN (6 mL) was heated for 30 min at 130°C
using
microwave irradiation. The mixture was acidified with HCl (1M) to pH 2
is and exrtracted with EtOAc. The combined extracts were washed with water,
brine and dried over Na2S~~. Concentration and purification by
chromatography gave the title compound (91 mg, 77%). 1H I~1MR (DMS~-
d6, 200 MHz): b 7.75 (1H, d, ,I 8.4 Hz), 7.56-7.45 (6H, m), 7.44 (1H, dd,
J=4.0, 1.4 Hz), 7.30-7.15 (SH, m), 7.09-7.03 (1H, m), 6.98-6.90 (2H, m),
6.86-6.79 (3H, m), 5.86 (2H, s), 1.37 (9H, s).
Example 3
5-(3~4-Methylenedioxyphenyl)-3-phenyl-1-(3-phen~prola~)indole-2-carb-
oxy_lic acid
(a) N (2-Benzoyl-4-chlorolahen~)oxalamic acid eth liter
A mixture of 2-amino-5-chlorobenzophenone (I 1.6 g, 50 mmol), ethyl oxalyl-
chloride (6.8 g, 50 mmol) and toluene (70 mL) was heated at reflux for 1.5 h.
On cooling a yellow precipitate formed. EtOAc (250 mL) was added and the
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
solution was washed with NaHC03 (aq., 5%), H3P04 (aq., 5%), brine and
dried over Na2S0~. Concentration gave the sub-title compound (IS.Sg, 94%).
(b) S-Chloro-3-phenylindole-2-carboxylic acid ethyl ester
s TiCl4 in THF (0.25 M, 19.5 mL, 54.9 mmol) was added slowly to a stirred
mixture of N (2-benzoyl-4-chlorophenyl)oxalamic acid ethyl ester (8.88 g,
26.8 mmol; see step (a)), Zn (7.19 g, 110 mmol) and THF (60 mL) at room
temperature. After 2 h, silica gel was added and then after a further 30 min
the mixture was filtered through a pad of silica gel which was washed with
1o EtOAc. The combined filtrates were washed with NaHC03 (aq., 5°/~),
water,
brine and dried over Na2S04. Concentration and crystallisation of the residue
from CH2C12/petroleum ether gave the title compound (3.13g, 39°/~).
(c) 5-Chloro-3-phenyl-I ~3-phenylpropYl)indole-2-carboxylic acid ethyl ester
1s NaH (60 % dispersion in mineral oil, 0.25 g, 6.2 mmol) was washed with
hexane (2x1 1nL) and Et2~ (1 mL) and suspended in I~MF (1 mL). A
solution of S-chloro-3-phenylindole-2-carboxylic acid ethyl ester (1.55 g,
5.17 mmol; see step (b)) in I~MF (10 mL) was added carefully at 0°C and
the mixture was stirred for 20 min. A. solution of (3-bromopropyl)-benzene
20 (1.54 g, 7.75 mmol) in I~MF (3mL) was added carefully at 0°C. The
cooling bath was removed and the mixture was stirred at room temperature
for 16 h, poured into water, and extracted with EtOAc. The extract was
washed with water, brine and dried over Na2S~4. Concentration and
chromatography gave the title compound (1.66 g, 77%).
(d) S-(3,4-Meth~enedioxYphenyl)-3-phen 1-~1-(3-phenylpropyl~indole-2-car-
boxylic acid
The title compound was prepared in accordance with the procedure in
Example 1(a) from 5-chloro-3-phenyl-1-(3-phenylpropyl)indole-2
3o carboxylic acid ethyl ester (see step (c)) and 3,4
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
56
methylenedioxyphenylboronic acid, followed by hydrolysis in accordance
with the procedure described in Example 2(b).
1H NMR (CI~CI3, 200 MHz): 8 7.61-7.38 (8H, m), 7.36-7.I5 (SH, m), 7.04
(1H, s), 7.02 (1H, dd, J--8.5, 1.8 Hz), 6.85 (1H, d, J--8.5 Hz), 5.98 (2H, s),
s 4.67-4.55 (2H, m), 2.75 (2H, t, J--7.6 Hz), 2.31-2.12 (2H, m).
Example 4
3-Phenyl-1 ~3-phenylpropyl)-5-(3-pyridyl)indole-2-carboxylic acid
to (a) 3-Phenyl-1-(3-phenylpropyl)-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-
yllindole-2-carbox ~lic acid ethyl ester
A 0.01 M stock solution of a Pd/(C6Hlr)3P was prepared from Pd2(dba)3,
(0.457 g, 0.5 rnmol), tricyclohexylphosphine (0.841 g, 3 mmol) and dioxane
(100 mL). An aliquot of this stock solution (12.5 mL, O.I25 mmol Pd), 5-
1s chloro-3-phenyl-1-(3-phenylpropyl)indole-2-carboxylic acid ethyl ester
(1.05
g, 2.5 mmol; see Example 3(c)), bis(pinacolato)diboron (0.762 g, 3.0 mmol),
I~OAc (0.44 g, 4.5 mmol), and dioxane (25 mL) were heated at 80°C
for 16 h.
Another aliquot of the Pd/(C6H11);P reagent (2.5 mL, 0.025 lnmol Pd) was
added and the mixture was heated at 100°C for 24 h. The mixture was f
ltered
2o through Celite~, and the filtrate was concentrated and purified by
chromatography to give the sub-title compound (0.47 g, 37 %) together with
0.5 5 g recovered starting material.
(b) 3-Phenyl-1-(3-phen~propyl)-5-(3-p~yl)indole-2-carboxylic acid ethyl
25 ester
3-Phenyl-1-(3 -phenylpropyl)-5-(4, 4, 5, 5 -tetramethyl-1, 3 , 2-di oxab
orolan-2-
yl)indole-2-carboxylic acid ethyl ester (0.40 g, 91 mmol; see step (a)), 3-
iodopyridine (0.28 g, 1.37 mmol), aqueous Na2C03 (2M, 0.46 mL, 0.9I
mmol), Pd(PPh3)4 (53 mg, 46 ~mol), toluene (7.3 mL), and EtOH (I.8 mL)
so were heated at 80°C for I6 h. More 3-iodopyridine (0.19 g, 0.91
mrnol),
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
57
aqueous Na2CO3 (2M, 1.4 mL, 2.73 mmol), and Pd(PPh3)4 (23 mg, 20
~.mol) were added and the mixture was heated for a further 8 h. EtOAc (30
mL) and brine (30 mL) were added. The layers were separated and the
aqueous phase was washed with EtOAc. The combined organic phases
s were dried with brine and Na2S04. Concentration and chromatography gave
the sub-title compound (0.32 g, 76%).
(c) 3-Phenyl~l-(3-phenylpropyl)-5-(3-pyridyl)indole-2-carboxylic aeid
The title compound was prepared from 3-phenyl-1-(3-phenylpropyl)-5
lo (3-pyridyl)indole-2-carboxylic acid ethyl ester (see step (b)) in
accordance
with the procedure in Example 2(b).
1H NMR (CI~C13, 200 MHz): ~ 8.78 (1H, s), 8.52 (1H, d, ,I 4.2 Hz), 7.92
(1H, d, J 8.0 Hz)), 7.65-7.71 (1H, m), 7.59-7.13 (13H, m), 4.71-4.58 (2H,
m), 2.75 (2H, t, J 7.6 Hz), 2.32-2.17 (2H, m).
Is
Example 5
6-(4-Benzyloxypheny,-3-(3-carbox~yl)-1-(3-nitrobenzyl)indole-2-
carboxylic acid
The title compound was prepared in accordance with Example 1 from
20 4-benzyloxyphenylboronic acid, 3-nitrobenzylbromide and 3-carboxyphenyl-
boronic acid.
~H NMR (DMSO-d6, 200 MHz): d 13.02 (2H, s), 8.13-7.94 (5H, m), 7.75
( 1 H, ddd, .I--7. 6, 1. 5, 1. 5 Hz), 7. 63 ( 1 H, dd, J 7.6, 2.0 Hz), 7. 5 9-
7 .27 ( 12H,
m), 7.04-6.97 (1H, m), 6.12 (2H, s), 5.18 (2H, s).
as
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
58
Exam,~le 6
3-(3-Carboxyphenyl)-4-phenyl-1-[3-(trifluorometh 1)benzyllindole-2-
carboxylic acid
The title compound was prepared in accordance with Example 1 from
s 4-bromoindole-2-carboxylic acid ethyl ester, phenylboronic acid,
3-(trifluoromethyl)benzylbromide and 3-carboxyphenylboronic acid.
'H NMR (CI~Cl3, 200 MHz): ~ 7.79 (1H, dd, J 1.5, 1.5 Hz), 7.70 (1H, ddd,
J 7.7, 1.5, 1.5 Hz), 7.57-7.50 (2H, m), 7.47-7.34 (3H, m), 7.25-7.20 (1H, m),
7.06 (1H, dd, J 6.2, 1.8 Hz), 7.06-6.85 (7H, m), 5.93 (2H, s).
Example 7
6-(4-B enzyl oxyphenyl)-1-(3 -nitrob enzyl)-3 -(2-oxopyrroli din-1-~1~ indole-
2-
carbo~lic acid
Is (a) 6-(4-Eenzyloxy~henyl)-1-(3-nitrobenzyl)-3-(2-oxopyrrolidin-1-yl)indole-
2-carboxylic acid eth ly ester
A stock suspension of a CuI/MeNHCH2CH~NHMe complex was prepared by
heating CuI (95.2 mg, 0.5 lnmol), MeNHCH2CH2NHMe (213 ~,L, 2.0 mmol),
and dioxane (5 mL) at 100°C for 5 min using microwave irradiation. 1.5
mL
of this solution was added to 6-(4-benzyloxyphenyl)-3-iodo-1-(3-
nitrobenzyl)indole-2-carboxylic acid ethyl ester (630 mg, 1.0 mmol, prepared
in accordance with Example 1 from 6-bromoindole-2-carboxylic acid ethyl
ester, 4-benzyloxyphenylboronic acid, and 3-nitrobenzylbromide), I~3PO4
(530 mg, 2.5 mmol), and dioxane (5 mL). Pyrrolidinone (390 mg, 5.0 mmol)
2s was added and the mixture was stirred at 95°C for 24 h, cooled to
room
temperature, poured into aqueous HCl (O.1M) and extracted with EtOAc. The
combined extracts were dried with brine and NaZS04. Concentration and
chromatography gave the sub-title compound (538 mg, 91%).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
59
(b) 6-(4-Benzyloxyphenyl~I-(3-nitrobenzyl)-3-(2-oxopyrrolidin-1-yl)indole-
2-carboxylic acid
6-(4-Benzyloxyphenyl)-1-(3-nitrobenzyl)-3-(2-oxopyrrolidin-1-yl)indole-2
carboxylic acid ethyl ester (see step (a)) was hydrolyzed in accordance with
s Example 2(b), using dioxane as the solvent, to give the title compound.
1H NMR (DMSO, 200 MHz): ~ 13.37 (1H, s), 8.10-8.06 (2H, m), 7.96 (lI~,
s), 7.66-7.26 (12H, m), 7.00 (1H, d, J 7.8 Hz), 6.06 (2H, s), 5.17 (2H, s),
3.81-3.74 (2H, m), 2.46-2.38 (2H, m), 2.24-2.06 (2H, m).
1 o Example 8
3-(2-Oxop~olidin-1 yI)-I~3-phenox~enzyl)-5-phen~lindole-2-carbox lic
acid
The title compound was prepared in accordance with Example 7 from
5-bromoindole-2-carboxylic acid ethyl ester, phenylboronic acid,
Is 3-phenoxybenzylchloride and pyrrolidinone.
_ _ _ 1H NMR (CDCl3, 200 MHz): ~ 7.69-7.65 (1H, m), 7.63-7.52 (3H, m), 7.50-
7.15 (7H, m), 7.13-7.01 (1H, m), 7.00-6.91 (2H, m), 6.88-6.79 (3H, m), 5.74
(2H, s), 3.98 (2H, t, ,J 7.0 Hz), 2.72 (2H, t, .I 8.0 Hz), 2.41-2.34 (2H, m).
2o Example 9
I-(3 5-Difluorobenz~l)-4-methoxy-3-(2-oxopyrrolidin-1-~)-7-phenyl-
indole-2-carboxylic acid
(a) 2-Azido-3-(4-methoxybiphenyl-3-yacrylic acid ethyl ester
2s A solution of 4-methoxybiphenyl-3-carboxaldehyde (1.8 g, 8.48 mmol) and
azidoacetic acid ethyl ester (5.62 g, 44 mmol) in EtOH ( 15 mL) was added
dropwise to a solution of NaOEt (3.13 g, 46 nnnol) in EtOH (3 5 mL) at
-25°C. The mixture was stirred at that temperature for 10 min, kept in
the
freezer (-18°C) for 24 h and then poured whilst stirring vigorously to
a cooled
(0°C) solution of NH~CI (aq., sat.). The mixture was extracted with
EtOAc
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
and the combined extracts were washed with brine and dried over Na2S04.
Concentration and crystallisation from EtOH gave the title compound (1.~0 g,
66%).
s (b) 4-Methoxy-7-t~henylindole-2-carboxylic acid ethyl ester
A solution of 2-azido-3-(4-methoxybiphenyl-3-yl)aclylic acid ethyl ester
( 1.75 g, 5.40 mmol; see step (a)) in o-xylene (25 mL) was added dropwise
to boiling o-xylene (25 mL). The heating was continued for S min, then the
solution was allowed to cool to room temperature and lcept in the freezer
10 (-18°C) for 16 h. The precipitate was isolated by filtration, washed
with
petroleum ether and dried in vacuo to afford the sub-title compound (1.20 g,
74%).
(c) 1-(3 5-I~ifluoroben~,y1)-4-methox~3-(2-oxopyrrolidin-1-~)-7-phen~l-
Is indole-2-carboxylic acid
The title compound was prepared from 4-methoxy-7-phenylindole-2-
carboxylic acid ethyl ester (see step (b)) in accordance with Example 7.
1H NMR (L~MSO-d6, 200 MHz): S 7.34-7.21 (3H, m), 7.10-7.06 (2H, m),
7.01-6.91 (1H, m), 6.94 (1H, d, J 7.~ Hz), 6.67 (1H, d, ..T 8.1 Hz), 5.92-
zo 5.89 (2H, m), 5.51-5.28 (2H, m), 3.~9 (3H, s), 3.77-3.67 (2H, m), 2.37-2.17
(2H, m), 2.14-2.10 (2H, m).
Example 10
6-(3,4-Meth~lenedioxyphen~)-1-[3,5-bis(trifluoromethyl)benzyll-3-(4-
zs chlorobenzo l~ aminolindole-2-carboxylic acid
The title compound was prepared according in accordance with Example 7
from 6-bromoindole-2-carboxylic acid ethyl ester, 3,4-methylenedioxy-
phenylboronic acid, 3,5-bis(trifluoromethyl)benzylchloride and 4-chloro-
benzamide.
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
61
1H NMR (DMSO-d6, 200 MHz): 8 10.35 (1H, s), 8.09-8.03 (2H, m), 8.01-
7.99 (1H, m), 7.95-7.94 (1H, m), 7.81 (2H, s), 7.73 (1H, d, .I--8.6 Hz), 7.66-
7 . 5 9 (2H, m), 7.44 ( I H, dd, J 8 .6, 1.2 Hz), 7. 34 ( 1 H, d, J 1. 8 Hz),
7.22 ( 1 H,
dd, ,I 8.2, 1.8 Hz), 6.99 (1H, d, J 8.2 Hz), 6.08 (2H, s), 6.05 (2H, s).
Example 11
3- 3 5-Dimethox b~ylamino)-5-(4-nitrophenyl)-1-(3-phenoxy-
benzyl~indole-2-carboxylic acid
The title compound was prepared from 5-(4-nitrophenyl)indole-2-carboxylic
to acid ethyl ester (prepared from 5-(4,4,5,5-tetramethyl-I,3,2-dioxaborolan-2-
yl)indole-2-carboxylic acid ethyl ester (prepared from 5-bromoindole-2-
carboxylic acid ethyl ester) and 4-nitrobromobenzene), 3-phenoxy-
benzylchloride and 3,5-dimethoxybenzamide in accordance with Example 7.
1H NMl~ (DMSO-d6, 200 MHz): ~ 10.37 (1H, s), 8.32-8.24 (2H, m), 8.15
is (1H,~ s), B.OI-7.91 (2H, m), 7.76 (2H, s), 7.41-7.16 (~H, m), 7.15-7.05
(IH,
m), 6.99-6.90 (2H, m), 6.84-6.69 (4H, m), 5.90 (2H, s), 3.81 (6H, s).
Example 12
3-(3-Amino-4-methylbenzoylamino)-5-(4-tee~t-butylphenyl)-1-(3-c111oro-
2o benzyl)indole-2-carboxylic acid
The title compound was prepared in accordance with Example 7 from 5-
bromoindole-2-carboxylic acid ethyl ester, 4-tent-butylphenylboronic acid,
3-chlorobenzylchloride and 3-amino-4-methylbenzamide.
1H NMR (DMS~-d~, 200 MHz): 8 10.09 (1H, s), 8.00 (IH, s), 7.69-7.40 (6H,
2s m), 7.36-7.3I (3H, m), 7.19-6.94 (3H, m), 5.86 (2H, s), 4.3 (IH, br s), 3.3
(IH, br s), 2.11 (3H, s), 1.29 (9H, s).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
62
Example 13
5-(4-tee°t-Butylphen l~)-1-(3-chlorobenzyl)-3-[(pyridine-3-
carbonYl)amino]-
indole-2-carboxylic acid
The title compound was prepared in accordance with Example 7 from
s 5-bromoindole-2-carboxylic acid ethyl ester, 4-tee°t-
butylphenylboronic aeid,
3-chlorobenzylchloride and nicotinamide.
1H NMR (l~MSO-d6, 200 MHz): ~ 10.50 (1H, s), 9.20 (1H, s), 8.80-8.71 (1H,
m), 8.42-8.31 (1H, m), 7.91 (1H, s), 7.32-7.~2 (SH, m), 7.48-7.40 (2H, m),
7.34-7.27 (2H, m), 7.14 (1H, s), 7.06-6.97 (1H, m), 5.89 (2H, s), 1.29 (9H,
s).
Example 14
3-~4-(I~imethylamino)but~rylamino]-6-(3,4-meth~lenedioxyphenyl)-1-(3-
phenoxybenz~)indole-2-carboxylic acid
The title compound was prepared in accordance with Example 7 from
~s 6-bromoindole-2-carboxylic acid ethyl ester, 3,4-methylenedioxy-
phenylboronic acid, 3-phenoxybenzylchloride and 4-(dimethylamino)-
butyrylamide.
1H NMI~ (DMSO-d6, 200 MHz): b 12.3-11.2 (1H, br s), 8.22 (1H, d, J--8.6
Hz), 7.54 (1H, s), 7.37-7.05 (7H, m), 7.00-6.84 (SH, m), 6.71 (1H, dd,
2o J--8.2, 2.3 Hz), 6.14 (2H, s), 6.07 (2H, s), 2.81 (2H, t, J 7.5 Hz), 2.54
(6H,
s), 2.46 (2H, t, J 7.3 Hz), 1.94 (2H, dt, J 7.5, 7.3 Hz).
Example 15
1-(3-Cyanobenz,~l)-6-(3,4-methylenedioxyphenYl)-3-(3-phenylacrylo T~l-
2s amino)indole-2-carboxylic acid
The title compound was prepared in accordance with Example 7 from 6-
bromoindole-2-carboxylic acid ethyl ester, 3,4-methylenedioxy-
phenylboronic acid, 3-cyanobenzylchloride and cinnamamide.
1H NMR (DMSO-d6, 200 MHz): 8 11.95 (1H, br s), 8.28 (1H, d, J 8.6 Hz),
30 7.70-7.59 (6H, m), 7.54-7.37 (6H, m), 7.28-7.23 (2H, m), 7.15 (1H, dd,
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
63
.I--8.2, 1. 8 Hz), 6.96 ( 1 H, d, J--8.2 Hz), 6. 8 8 ( 1H, d, J--1 S . 8 Hz),
6.16 (2H,
s), 6.03 (2H, s).
Example 16
s 1-(3-Carbamo l~benzyl)-6-(3 4-methYlenedioxyphenYl)-3-(3-phenylacryloyl-
aminolindole-2-carboxylic acid
The title compound was isolated in the last synthetic step (the hydrolysis) in
the preparation of the title compound of Example 1 S.
1H NMR (DMSO-d6, 200 MHz): 8 11.58 (1H, br s), 8.17 (1H, d, .I 8.6 Hz),
l0 7.89 (IH, s), 7.74-7.55 (6H, m), 7.48-7.37 (3H, m), 7.34-7.23 (4H, m), 7.I6-
7.0 8 (2H, m), 6.95 ( 1 H, d, .I 8.2 Hz), 6.91 ( 1 H, d, J--15 .6 Hz), 6.13
(2H, s),
6.03 (2H, s).
Example 17
15 3-Acetylamino-S-(3 4-methylenedioxyphenyll-I-(S-phenoxypentyllindole-2-
carboxylic acid
The title compound was prepared in accordance with Example 7 from
5-bromoindole-2-carboxylic acid ethyl ester, 3,4-methylenedioxy-
phenylboronic acid, (5-bromopentyloxy)benzene, and acetamide.
20 1H NMR (DMSO-d6, 200 MHz): ~ 9.60 (1H, s), 7.71 (1H, s), 7.65-7.49 (2H,
m), 7.30-7.15 (3H, m), 7.09 (1H, dd, J 8.1, 1.4 Hz), 6.77 (IH, d, ~ 8.I Hz),
6.93-6.82 (3H, m), 6.04 (2H, s), 4.60-4.46 (2H, m), 3.90 (2H, t, .~=6.3 Hz),
2.09 (3H, s), 1.81-1.61 (4H, m), 1.48-1.32 (2H, m).
2s Exam~Ie 18
S-(3,4-Methylenedioxxphenyl~ 3-(2-oxot~iperidin-1-yl)-1T(S-phenoxy-
pentyl~indole-2-carboxylic acid
The title compound was prepared in accordance with Example 7 from 5-
bromoindole-2-carboxylic acid ethyl ester, 3,4-methylenedioxy-
3o phenylboronic acid, (5-bromopentyloxy)benzene, and 2-piperidone.
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
64
1H NMR (DMSO-d6, 200 MHz): 8 7.66-7.49 (3H, m), 7.30-7.18 (3H, m),
7.I3 (IH, dd, J--8.1, 1.8 Hz), 6.97 (IH, d, J--8.1 Hz), 6.92-6.83 (3H, m),
6.04 (2H, s), 4.65-4.50 (2H, m), 3.9I (2H, t, .J 6.4 Hz), 3.64-3.55 (2H, m),
2.44-2.28 (2H, m), 1.98-I.64 (8H, m), 1.52-1.33 (2H, m).
Example 19
3-(3-Acet~l~henyl)-5-(4-methoxyphenYl)-I-[3-(trifluorometh~l)ben~ll-
indole-2-carboxylic acid
to (a) 5-(4-Methoxyphenyllindole-2-carboxylic acid eth l
A mixture of 5-bromoindole-2-carboxylic acid ethyl ester (268 mg, 1.0
mmol), 4-methoxyphenylboronic acid (243 mg, 1.6 mmol), Pd(OAc)2 ( 11.2
mg, 50 nmol), 2-(di-t-butylphosphino)biphenyl (60 mg, 200 nmol), K3PO4
(425 mg, 2 mmol) and toluene (2 mL) was heated at 170°C for 10 min
using
1s microwave irradiation. The mixture was extracted with EOAc, and the
combined extracts washed with water, dried with Na2SO4, and concentrated.
The residue was crystallised from EtOH to yield the sub-title compound
(50%).
20 (b) 3-Iodo-5-(4-methoxyphenyl)indole-2-carboxylic acid ethyl ester
Na,I (566 mg, 3.8 mmol) in acetone ( 18 mL) was added dropwise to a
solution of NCS (458.5 mg, 3.4 mmol) in acetone (6 mL) at room
temperature. After 15 min, 5-(4-methoxyphenyl)indole-2-carboxylic acid
ethyl ester (1.01 g, 3.4 m~nol; see step (a)) in acetone (48 rnL) was added.
2s After 60 min Na2S203 (aq., I O%) was added and the mixture was extracted
with EtOAc, and the combined extracts washed with water, dried with
Na2SO4, and concentrated. The residue was crystallised from
EtOAc/heptane to yield the sub-title compound (958 mg, 66%)
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
(c) 3-Iodo-5-~4-methoxy~hen~l)-1-[3-(trifluoromethyl)benzyllindole-2-
carboxylic acid ethyl ester
A mixture of 3-iodo-5-(4-methoxyphenyl)indole-2-carboxylic acid ethyl
ester (200 mg, 475 nmol; see step (b)), 1-(bromomethyl)-3-
s (trifluoromethyl)benzene (182 ~L, 1.2 mmol), NaH (48 mg, 2 mmol) and
DMF (2.5 mL) was heated at 170°C for 2 min using microwave
irradiation.
The mixture was extracted with EOAc. The combined extracts were washed
with water, dried with Na2SO4 and concentrated. The residue was
crystallised from EtOH to yield the sub-title compound (several batches
to were combined and employed in subsequent steps).
(d) 3-(3-Acetylpheny?)-5-(4-methoxyphenyl)-1-(3-(trifluorometh 1)ben~~l1-
indole-2-carboxylic acid ethyl ester
A mixture of 3-iodo-5-(4-methoxyphenyl)-1-[3-(trifluoromethyl)benzyl]-
1s indole-2-carboxylic acid ethyl ester (100 mg, 173 ntnol; see step (c)), 3-
acetylphenylboronic acid (42.5 mg, 259 rimol), Na~C~; (27 mg, 259 nmol),
Pd(PPh;)2C12 (6.1 mg, 9 nmol) and DME/HZO/EtOH 7:3:2 (1 xnL) was
heated at 160°C for .10 minutes using microwave irradiation. The
reaction
mixture was filtered through Celite° and the filter cake was washed
with
2o Et2O. The combined filtrates were poured into NaHCO3 (aq., sat.) and
extracted with Et20. The combined extracts were dried over Na2S04 and
concentrated. Purification by chromatography yielded the sub-title
compound (74 mg, 71 %).
2s (e) 3-(3-Acetylphenyl)-5-(4-methoxyphen~)-1-f3-(trifluoromethyl)benzyl]-
indole-2-carbox ly is acid
A mixture of 3-(3-acetylphenyl)-5-(4-methoxyphenyl)-1-[3-(trifluoro-
methyl)benzyl]indole-2-carboxylic acid ethyl ester (59 mg, 103 rimol; see
step (d)), NaOH (2 M, 500 ~.L) and MeCN (2 mL) was heated at 120°C for
30 10 min using microwave irradiation. The mixture was acidified with HCl
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
66
(2IVI) and extracted with EOAc. The combined extracts were dried and
concentrated. Purification by chromatography yielded the title compound.
IH NMR (200 MHz, CDC13): 8 8.10 (m, 1H), 7.99 (m, 1H), 7.7I (m, 1H),
7.63-7.54 (m, 3H), 7.53-7.47 (m, 3H), 7.46 (m, 1H), 7.40 (m, 1H), 7.35 (d,
s J 8 Hz, 1H), 7.18 (d, J 8 Hz, 1H), 6.94 (m, 2H), 5.88 (s, 2H), 3.83 (s, 3H),
2.61 (s, 3H).
Example 20
5-(4-Methoxyt~hen l~)-3-phenyl-1-L3-(trifluoromethyl)benzyllindole-2-
to carboxylic acid
(a) 5 ~-Methoxyphen,~)-3-phenyl-1-j3-(trifluoromethyl)benzyllindole-2-
carboxylic acid eth ly ester
A mixture of 3-iodo-5-(4-methoxyphenyl)-1-[3-(trifluoromethyl)benzyl]-
is indole-2-carboxylic acid ethyl ester (12.9 mg, 22 nmol; see Example 19(c)),
phenylboronic acid (4.1 mg, 33 nmol), I~3PO4 (17 mg, 78 rimol), Pd(~Ac)2
(0.25 mg, 1.0 nmol) and toluene (500 ~.L) was heated at 170°C for 5
minutes using microwave irradiation. The mixture was filtered through
Celite n and the filter cake was washed with Et2~. The combined filtrates
2o were poured into NaHC43 (aq., sat.) and extracted with Et20. The
combined extracts were dried over Na2S04 and concentrated. Purification
by chromatography yielded the sub-title compound (60%).
(b) 5-(4-Methoxyphen 1~)-3-phenyl-1-[3-(trifluoromethyl)benzyllindole-2-
2s carboxylic acid
The title compound was prepared from 5-(4-methoxyphenyl)-3-phenyl-1-[3-
(trifluoromethyl)benzyl]indole-2-carboxylic acid ethyl ester (see step (a)) in
accordance with Example I9(e).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
67
Example 21
3,5-Bis(4-methoxyphen l~)-1-~3-(trifluoromethyl)ber~yllindole-2-
carboxylic acid
The title compound was prepared in accordance with Example 20 using
s 4-methoxyphenylboronic acid instead of phenylboronic acid.
1H NMR (200 MHz, CI~Cl3): 8 7.68 (m, 1H), 7.59 (dd, J 9, 2 Hz, IH),
7.52-7.46 (m, SH), 7.45 (m, 1H), 7.40-7.34 (m, 2H), 7.19 (d, .I--7 Hz, 1H),
7.02 (m, 2H), 6.95 (m, 2H), 5.87 (s, 2H), 3.89 (s, 3H), 3.84 (s, 3H).
to Example 22
5-(4-Methoxyphenyl)-3-(3-nitrophenylLl -f 3-(trifluoromethyl)benzyll-
indole-2-carboxylic acid
The title compound was prepared in accordance with Example 19 using
3-nitrophenylboronic acid and 2.5 mol% Pd(PPh;)ZCh.
is 1H NMR (200 MHz, CI~Cl3): d 8.39 (m, 1H), 8.26 (m, 1H), 7.83 (m, 1H),
7.65 (m, 1H), 7.62 (m, 1H), 7.58 (m, 1H), 7.54-7.34 (m, 6H), 7.19 (d, .I 7
Hz, IH), 6.95 (m, 2H), 5.89 (s, 2H), 3.83 (s, 3H).
Example 23
20 5-(4-Methoxyphe~l~-3-(~yridin-3-yl)-1-[3-(trifluorometh 1)~,benzyllindole-
2-carboxylic acid
The title compound was prepared in accordance with Example 19 using
pyridin-3-ylboronic acid and 5 mol% Pd(PPh;)2Cla.
1H NMR (200 MHz, DMS~-d6): b 8.76 (s, 1H), 8.50 (d, .I--4 Hz, IH), 8,00
2s (d, J--8 Hz, 1H), 7.72 (s, IH), 7.63-7.37 (m, 9H), 6.98 (m, 2H), 5.95 (s,
2H),
3.77 (s, 3H).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
68
Example 24
3-(4-Fluoro-3-methylphenyl)-5-(4-methox phenyl)-1-[3 ~trifluoromethyll
benzyllindole-2-carboxylic acid
The title compound was prepared in accordance with Example 19 using
4-fluoro-3-methylphenylboronic acid and 5 mol% Pd(PPh;)2C12.
1H NMR (200 MHz, DMSO-d6): 8 7.69-7.50 (m, SH), 7.42 (m, IH), 7.37-
7.28 (rn, 2H), 7.24-7.17 (m, 1H), 6.98 (m, 2H), 5.94 (s, 2H), 3.76 (s, 3H),
2.29 (d, J--2. Hz, 3H).
to Example 25
3-(3, 5-Dichlorophenyl)-5-(4-methoxyphenyl)-1-[3-(trifluoromethyl)-
benzyl~indole-2-carboxylic acid
The title compound was prepared in accordance with Example 19 using
3,5-dichlorophenylboronic acid and 3.5 mol°f° Pd(PPh;)2CI2.
Is 1H NMR (200 MHz, CI~Cl,): 8 7.63-7.5~ (m, 2H), 7.53-7.44 (m, 4H), 7.40-
7.34 (m, SH), 7.14 (d, J'--8 Hz, 1H), 6.97 (m, 2H), 5.~2 (s, 2H), 3.54 (s,
3H).
Example 26
5-(2-Methoxyphenyl)-3-(3-nitrophen l~)-1-[3-(trifluoromethyl)benzyll-
2o indole-2-carboxylic acid
(a) 5-(2-Methoxyphenyl)indole-2-carboxylic acid eth 1 ester
A mixture of 5-bromoindole-2-carboxylic acid ethyl ester (26~ mg, 1
mtnol), 2-methoxyphenylboronic acid (304 mg, 2 mmol), Pd(PPh3)2C12 (35
2s mg, 50 nmol), Na2CO3 (159 mg, 1.5 rnmol),and DMElH20/EtOH 7:3:2 (3
mL) was heated at 160°C for 8 minutes using microwave irradiation. The
mixture was poured into water and extracted with EOAc. The combined
extracts were dried over Na2S0~ and concentrated. The residue was
crystallised from EtOH/HZO to yield the sub-title compound (37%).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
69
(b) 5-(2-Methoxyphenyl)-3-(3-nitrophenyl)-1-[3-(trifluoromethyl)benzyll-
indole-2-carbox Tli~ c acid
The title compound was prepared in accordance with Example I9(b), 19(c)
and 19(d) using 3-nitrophenylboronic acid and 2.5 mol% Pd(PPh3)2Clz.
s 1H NMR (200 MHz, DMSO-d6): b 13.31 (br s, 1H), 8.32 (m, 1H), 8.23 (m,
IH), 7.98 (d, .I--8 Hz, 1H), 7.80-7.49 (m, 7H), 7.32-7.26 (m, 3H), 7.07 (d,
~I 8 Hz, 1H), 6.98 (t, J 8 Hz, IH), 6.01 (s, 2H), 3.72 (s, 3H).
Exam lp a 27
5-(2-Methoxyphenyl)-3-(4-methoxy~henyl~ 1-[3-(trifluoromethyl)benzyll-
indole-2-carboxylic acid
The title compound was prepared in accordance with Example 26 using
4-methoxyphenylboronic acid and 2.5 mol% Pd(PPh3)2C12.
1H NMR (200 MHz, DMSO-d6): 8 12.98 (br s, 1H), 7.66-7.60 (m, 3H),
1s 7.55 (t, J 8 Hz, 1H), 7.50-7.38 (m, 4H), 7.34-7.24 (m, 3H), 7.06 (d, .I--8
Hz,
IH), 7.04-6.95 (m, 3H), 5.95 (s, 2H), 3.81 (s, 3H), 3.7I (s, 3H).
Example 28
3-(3,5-Dichlorophenyl)-5-(2-methoxyphen~)-1-j3 ~trifluoromethyl)-
2o benzyl)indole-2-carboxylic acid
The title compound was prepared in accordance with Example 26 using
3,5-dichlorophenylboronic acid and 2.5 mol°f° Pd(PPh3)ZCl2.
IH NMR (200 MHz, DMSO-d6): 8 7.72 (s, 1H), 7.65-7.56 (m, SH), 7.54-
7.51 (m, 2H), 7.46 (m, 1H), 7.35-7.27 (m, 3H), 7.10 (m, 1H), 6.99 (m, 1H),
2s 5.96 (s, 2H), 3.73 (s, 3H).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
Example 29
5-~3-Acetylphenyl~ 3-(4-methoxyphen~)-1-(3-(trifluoromethyl)benzyll-
indole-2-carbox~Iic acid
5 (a) 5-(3-Acetylphenyl)indole-2-carboxylic acid ethyl ester
The sub-title compound was prepared in accordance with Example 26(a)
using 3-acetylphenylboronic acid and 2.5 mol% Pd(PPh3)2CI2 and heating at
160°C for 10 min.
10 (b) 5-(3-Acetylphen~)-3-~-methoxyphen~)-1-(3-(trifluoromethyl)benzyll-
indole-2-carboxylic acid
The title compound was prepared in accordance with Example 26(b) using
4-methoxyphenylboronic acid and 2.5 mol% Pd(PPh;)2C12.
IH I~IMR (200 MHz, DMSO-d6): ~ 13.06 (br s, 1H), 8.10 (s, 1H), 7.89 (m,
Is 2H), 7.80-7.67 (m, 3H), 7.65-7.54 (m, 4H), 7.44 (m, 2H), 7.28 (d, J 8 Hz,
1H), 7.03 (m, 2H), 5.98 (s, 2H), 3.82 (s, 3H), 2.63 (s, 3H).
Example 30
~,S-Bis~3-acetylphen~l)-1-~3-(trifluorometh~l)benzyllindole-2-carboxylic
2o acid
The title compound was prepared in accordance with Example 29 using
3-acetylphenylboronic acid and 2.5 mol% Pd(PPh3)2Clz
~H NMR (200 MHz, DMSO-d6): b 13.21 (br s, 1H), 8.10 (m, 2H), 7.98 (m,
1H), 7.89 (m, 2H), 7.84-7.78 (m, 2H), 7.74 (m, 1H), 7.70 (m, 1H), 7.67-
2s 7.52 (m, SH), 7.30 (d, J 8 Hz, 1H), 6.02 (s, 2H), 2.64 (s, 3H), 2.62 (s,
3H).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
71
Example 31
5-(3-Acetylphenyl)-3-phen~ j3-(trifluoromethyl)benzy~indole-2-
carboxylic acid
The title compound was prepared in accordance with Example 29 using
s phenylboronic acid and 2.5 mol% Pd(PPh3)2Ch.
1H NMR (200 MHz, DMSO-d6): ~ 13.14 (br s, 1H), 8.I0 (m, 1H), 7.89 (m,
2H), 7.82-7.67 (m, 3H), 7.65-7.44 (m, 8H), 7.38 (m, 1H), 7.30 (d, J--Hz,
1H), 5.99 (s, 2H), 2.62 (s, 3H).
to Exam lp a 32
5~3-Acetylbhenyl)-3-(4-p~ridyl)-I-[3-(trifluoromethyl)benzyl~indole-2-
carboxylic acid
The title compound was prepared in accordance with Example 29 using
4-pyridylboronic acid and 2.5 mol% Pd(PPh;)2C12.
1s 1H (200 MHz, DMS~-d6): ~ 8.65 (m, 2H), 8.13 (m, 1H), 7.91 (m,
2H), 7.85-7.80 (m, 1H), 7.77-7.73 (m, 2H), 7.66-7.51 (m, 6H), 7.30 (d, ..I--8
Hz, 1H), 6.01 (s, 2H), 2.63 (s, 3H).
Example 33
20 5-(3-Acetylphenyl)-3-(4-chlorophenyl)-I-[3-(trifluoromethyl)benzy~indole-
2-carbox lic acid
The title compound was prepared in accordance with Example 29 using
chlorophenylboronic acid and 2.5 moI% Pd(PPh3)2C12.
1H NMR (200 MHz, DMSO-d6): ~ 13.22 (br s, 1H), 8.I2 (m, 1H), 7.90 (m,
2s 2H), 7.83-7.67 (m, 3H), 7.65-7.50 (m, 8H), 7.29 (d, J 8 Hz, 1H), 6.00 (s,
2H), 2.63 (s, 3H).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
72
Example 34
~4-Chlorophenyl)-3-(3-p~rid~)-1-[3-(trifluorometh 1)benzyllindole-2-
carbox ly is acid
s (a) 5-(4-ChlorophenYl)indole-2-carboxylic acid eth 1 ester
The sub-title compound was prepared in accordance with Example 26(a)
using 4-chlorophenylboronic acid and 4.0 mol% Pd(PPh3)ZCI2 and heating
at 160°C for 10 min.
ro (b) 1-(3-(Trifluoromethyl)benzyl)-5-(4-chlorophen~)-3-(3-pyridyl)-1-[3-
(trifluoromethyl)benzz~Ilindole-2-carboxylic acid
The title compound was prepared in accordance with Example 26(b) using
3-pyridylboronic acid and 2.5 mol% Pd(PPh3)2C12.
is Example 35
5-(4-Chlorot~henyl)-3 -(4-(hydroxym eth~)phen~l)-1-[3 -(trifluoromethyl)-
benzyl]indole-2-carboxylic acid
The title compound was prepared in accordance with Example 34 using
4-(hydroxymethyl)phenylboronic acid and 5 mol% Pd(PPh;)?C12.
20 ~H NMR (200 MHz, DIMS~-d6): ~ 7.74 (m, 2H), 7.66-7.58 (m, 4H), 7.55-
7.26 (m, 9H), 5.95 (s, 2H), 5.29 (br s, 1H), 4.57 (s, 2H).
Example 36
5-(4-Chlorophenyl)-3-(4-fluoro-3-meth~phenyl)-1-j3-(trifluoromethyl)-
2s benzyl]indole-2-carboxylic acid
The title compound was prepared in accordance with Example 34.using
4-fluoro-3-methylphenylboronic acid and 5 mol% Pd(PPh;)aCl2.
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
73
Example 37
5-(4-Chlorophenyl)-3-(4-ethylphenyl)-1-[3-(trifluoromethyl benz~]indole-
2-carboxylic acid
The title compound was prepared in accordance with Example 34 using
s 4-ethylphenylboronic acid and 5 moI% Pd(PPhs)2C12.
1H NMIt (200 MHz, DMSO-d6): 8 7.76-7.70 (m, IH), 7.66-7.5~ (m, 5H),
7.57-7.50 (m, 2H), 7.4~-7.40 (m, 4H), 7.33-7.30 (m, 3H), 5.94 (s, 2H), 2.6~
(q, ~_~ Hz, 2H), 1.25 (t, .~ 8 Hz, 3H).
1 o Exam 1~ a 3 $
6-(3-Aminophenyl)-3-phenyl-1-((3-(trifluoromethoxy)benz~]indole-2-
carboxylic acid hydrochloride
(a) 6-(3-Aminophenyl)-3-~henyl-1-[(3-_ (trifluoromethox )benzyl~indole-2-
1s carboxylic acid ethyl ester hydrochloride
6-(3 -Nitrophenyl)-3 -phenyl-1-[(3 -(trifluoromethoxy)b errlzylJ-indole-2-
carboxylic acid ethyl ester, prepared in accordance with the procedure in
Example 1 (a)-(d) from 6-bromoindole-2-carboxylic acid ethyl ester, 3-nitro-
phenylboronic acid and 3-(trifluoromethoxy)benzyl chloride (1.55 g, 2.76
2o mmol), in Et~Ac (35 mL) was hydrogenated at ambient temperature and
pressure over Pd-C (10%, 440 mg) until all starting material was consumed
as judged by TLC. The mixture was filtered through Celite~ and the filtxate
concentrated. The residue was purified by chromatography, dissolved in
anhydrous EtzO, whereafter the sub-title compound was precipitated by the
2s addition of an excess of HCl (4M) in dioxane. Yield: 955 mg (73%).
(b) 6~3-A~.ninophenyl)-3-phenyl-1-f(3-(trifluoromethoxy)benzyl]indole-2-
carboxylic acid hydrochloride
The title compound was prepared by hydrolysis of 6-(3-aminophenyl)-3-
3o phenyl-1-[(3-(trifluoromethoxy)benzylJ-indole-2-carboxylic acid ethyl ester
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
74
in accordance with the procedure described in Example 2(b) (2M KOH
(aq.), dioxane, 100°C, 1 h), followed by precipitation from an ethereal
solution by addition of HCl (4M) in dioxane as described above.
1H NMR (200 MHz, DMSO-dg): 8 11.5-9.6 (3H, br s), 7.94 (1H, s), 7.67-
s 7.05 (15H, zn), 5.99 (2H, s), 4.5-3.0 (1H, br s).
Example 39
6-(3-(2,2-Dimethylpropion lamino)phenyl]-3-phenyl-1_[3-(trifluorometh-
ox ~benzy~indole-2-carboxylic acid
(a) 6-[3-(2 2-Dimeth~propion Iamino~ahenyl]-3-phen~-1-j3-(trifluoro
methoxy)benzyl]indole-2-carboxylic acid ethyl ester
Pivaloyl chloride ( 156 ~,L, 1.27 mmol) was added over 5 min to a stirred
solution of 6-(3-arninophenyl)-3-phenyl-1-[3-(trifluoromethoxy)ben~yl]-
Is indole-2-carboxylic acid ethyl ester hydrochloride (600 mg, 1.06 mmol; see
Example 38(a)), DMAP (65 mg, 0.53 mznol), Et3N (530 ~I,, 3.8 mmol), and
dry CH~Cl2 (10 mL). The mixture was stirred at room temperature overnight
whereafter another portion of pivaloyl chloride (0.156 ~L, 1.27 znmol) and
Et3N (530, ~,L, 3.8 mznol) was added. ~-l.fter 2 h at room temperature, the
2o mixture was diluted with CH2Ch and washed with HCl (aq., 1M), NaHCO3
(aq., sat.) and brine, and concentrated. The residue was treated with pentane
to give the title compound as a white solid (480 mg, 74%).
(b) 6-[3-(2 2-Dimeth lpro,~ionylamino~henyl~-3-phenyl-1-[3 (trifluoro
2s methoxY)benzyl]indole-2-carboxylic acid
The title compound was prepared by hydrolysis of 6-[3-(2,2-dimethyl-
propionylamino)phenyl]-3-phenyl-1-[3-(trifluoromethoxy)benzyl]indole-2-
carboxylic acid ethyl ester in accordance with the procedure described in
Example 1 (e) (2M KOH (aq.), dioxane, 120 °C, 2 h).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
7s
1H NMR (200 MHO, I?MSO-d6): 8 13.0 (IH, s), 9.29 (1H, s), 7.98-7.96 (1H,
m), 7.90 (1H, s), 7.72-7.66 (1H, m), 7.59-7.36 (IOH, m), 7.26-7.19 (2H, m),
7.11-7.07 (1H, m), 6.00 (2H, s), 1.24 (9H, s).
s Example 40
6-(3-(Methanesulfonylamino)phenyl)-3-phenyl-I-[3-(trifluoromethoxy)
benz~,l]indole-2-carboxylic acid
The title compound was prepared in accordance with the procedure in
Example 39 using methanesulfonyl chloride instead ofpivaloyl chloride.
to iH NMR (200 MI3z, DMSO-d6): 8 13.2-12.9 (1H, br s), 9.80 (IH, s), 7.88-
7.86 (1H, m), 7.56-7.35 (11H, m), 7.24-7.15 (3H, m), 7.10-7.05 (1H, m),
5.97 (2H, s), 3.00 (3H, s).
Example 41
Is 6-(3-hut-3-enylaminophenyl)-3-phenyl-1-[(3-(trifluoromethoxY ben~l~-
indole-2-carboxylic acid
(a) 6-(3-Eut-3-enylaminophenyl)-3-phenyl-1 j(3-(trifluoromethoxy)ben-
~,yl]'ir~dole-2-carboxylic acid eth, l ester
20 4-Bromo-1-butene (143 mg, 1.06 mmol) was added to a mixture of 6-(3-
aminophenyl)-3-phenyl-1-[3-(trifluoromethoxy)benzyl]indole-2-carboxylic
acid ethyl ester hydrochloride (400 mg, 0.71 mmol; see Example 38(a)),
NaI (3I7 mg, 2.12 mmol), I~2C03 (390 mg, 2.84 mmol), and dry DMF (3
~nL). The mixture was heated at 100°C for I2 h, allowed to cool and
poured
2s into water. The mixture was extracted with EtOAc and the combined
extracts were washed with water, brine, dried over Na2CO3 and
concentrated. The residue was purified by chromatography to give the title
compound (173 mg, 42%).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
76
(b) 6-(3-l3ut-3-enylaminophenyl~phenyl-1-f (3-(triftuoromethoxY)ben_
zyl]indole-2-carboxylic acid
The title compound was prepared by hydrolysis of 6-(3-but-3-enylamino-
phenyl)-3-phenyl-1-[(3-(trifluoromethoxy)benzyl]indole-2-carboxylic acid
s ethyl ester in accordance with the procedure described in Example 1 (e)
(NaOH (aq., 40%), DMF, room temperature, 2 h).
IH NMR (200 MHz, DMSO-d6): 8 7.79 (1H, s), 7.51-7.31 (8H, m), 7.24-
7.06 (4H, m), 6.84-6.80 (2H, m), 6.57-6.52 (IH, m), 5.97 (2H, s), 5.88 (IH,
ddt, .I--17.2, 10.3, 6.7 Hz), 5.15-4.98 (2H, m), 3.I1 (2H, t, J'--7.2 Hz) 2.30
to (2H, q, J 6.9 Hz).
Exam lt~ a 42
6-f3-(Allylmetllanesulfonylamino~phen ly_1-3-phenyl-1_C3-trifluoromethoxy
benzyl)indole-2-carboxylic acid
is Allyl iodide (54 ~.L, 0.59 mmol) was added to a mixture of 6-[3-(methane-
sulfonylamino)phenyl]-3 -phenyl-1-(3 -trifluoromethoxy-b enzyl) indole-2-
carboxylic acid ethyl ester (178 mg, 0.29 mmol; see Example 40), Cs2CO3
(333 mg, I.02 nnnol), and dry DIvlF (2.5 mL). The mixture was heated at
85°C for 30 min using microwave irradiation, allowed to cool and poured
2o into water. The mixture was extracted with EtOAc and the combined
extracts were washed with brine, dried over Na2CO3 and concentrated. The
residue was hydrolyzed in accordance with the procedure described in
Example 2(b) (2M I~OH (aq.), dioxane, l I0 °C, 55 min) to give the
title
compound (140 mg, 78%).
2s 1H NMR (200 MHz, DMSO-d6): 8 13.08 (1H, s), 7.94 (1H, s), 7.68-7.63
(2H, m), 7.58-7.34 (lOH, m), 7.25-7.20 (2H, m), 7.12-7.07 (1H, m), 6.OI
(2H, s), 5.79 (1H, ddt, J--17.1, 10.2, 5.9 Hz), S.I9 (1H, dd, J--17.1, 1.5
Hz),
5.07 (1H, dd, J I0.2, 1.5 Hz), 4.35 (2H, d, J=5.9 Hz), 3.05 (3H, s).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
77
Example 43
I-(3-Chlorobenzyl)-6-(3 5-difluorophenyl~[3-(4-methoxyphen
propion lamino]indole-2-carboxylic acid
s (a) 3-Arnino-1-(3-chlorobenzyl)-6~3 5-difluorophenyl)indole-2-carboxylic
acid ethyi ester
A mixture of 3-amino-6-bromo-1-(3-chlorobenzyl)indole-2-carboxylic acid
ethyl ester (1.04 g, 2.25 mmol, see Example 54(b)) Pd(OAc)Z (31 mg, O.I4
mmol), tri-o-tolylphosphine (84 mg, 0.28 mmol), K2C03 (I.33 g, 9.b4
to mmol) and toluene (30 mL) was stirred under argon at room temperature for
min whereafter 3,5-dimethoxyphenylboronic acid (0.78 g, 4.13 mmol)
and EtOH (10 mL) was added. The mixture was heated at reflux for 2.5 h
allowed to cool and filtered through Celite~'. The filter cake was washed
with EtOAc and the combined filtrates were washed with NaHCO~ (aq.,
zs sat.). The aqueous phase u~as extracted with EtOAc and the combined
organic phases were washed with water, brine and dried over NaZCO3.
Concentration and purification by chromatography gave the title compound
(1.28 g, 98%).
(b) 1-(3-Chlorobenzyl)-6-(3 5-difluorophenyl)-3-[~4-methox phen
propion lad]indole-2-carboxylic acid ethyl ester
DMAP (22.3 mg, 0.18 mmol) and Et3N (154 ~,L, l.I mmol) were added at
room temperature to a stirred mixture of 3-amino-1-(3-chlorobenzyl)-6-
(3,5-difluorophenyl)indole-2-carboxylic acid ethyl ester (150 mg, 0.37
2s mmol), 3-(4-methoxyphenyl)propionyl chloride (I09 mg, 0.55 mmol) and
MeCN (3.5 mL). The mixture was stirred at room temperature overnight,
poured into HCl (aq., IM) and extracted with EtOAc. The combined
extracts were washed with NaHC03 (aq., sat.), dried over Na2C03 and
concentrated. The sub-title compound was obtained by crystallisation of the
3o residue from EtOAc/benzene (I08 mg, 50 %).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
78
(c) 1-(3-Chloroben~yl~6-(3 5-difluorophenyl)-3-(3-(4-methoxyt~henyl)-
~ropionylaminolindole-2-carboxylic acid
The title compound was prepared by hydrolysis of 1-(3-chlorobenzyl)-6-
(3,5-difluorophenyl)-3-[3-(4-methoxyphenyl)-propionylamino]indole-2-
carboxylic acid ethyl ester (108 mg, 0.18 mmol) according to the procedure
described in Example 2(b) (KOH (aq., 2M), dioxane, 60 °C 50 min then
100
°C 30 min). 'Yield 75 mg (73 %).
1H NMR (200 MHz, I~MSO-d6): 8 13.6-13.2 (1H, br s), 9.73 (1H, s), 8.09-
l0 8.03 (1H, m), 7.57-6.86 (13H, m), 5.94 (2H, s), 3.73 (3H, s), 2.95-2.86
(2H,
m), 2.73-2.64 (2H, m).
The following Examples 44 to 53 were made in accordance With the
procedure in Example 43 using the appropriate acid chloride or sulfonyl
1s chloride.
Example 44
1-(3-Chlorobenzyl)-6-(3 5-difluorophenyl)-3-((3,5-dimethyladamantane-1-
carbonyl)aminolindole-2-carboxylic acid
20 1H NMR (200 MHz, I~MS~-d6): ~ 13.8-13.2 (1H, br s), 9.52 (1H, s), 8.04
(1H, s), 7.81 (1H, d, J 8.6 Hz), 7.60-7.48 (3H, m), 7.34-7.16 (3H, m), 7.11-
7.10 (1H, m), 6.98-6.90 (1H, m), 5.94 (2H, s), 2.16-2.11 (1H, m), 1.81-1.80
(2H, m), 1.68-1.53 (4H, m), 1.45-1.30 (4H, m), 1.23-1.14 (2H, m), 0.87
(6H, s).
Example 45
3-(2-Adamant-1-ylacetylamino~-1-(3-chlorobenzyl)-6-(3,5-difluorophenyl)-
indole-2-carboxylic acid
1H NMR (200 MHz, IJMSO-d6): 8 13.5-13.2 (1H, br s), 9.56 (1H, s), 8.04
(1H, s), 7.70 (1H, d, J 8.6 Hz), 7.59-7.49 (3H, m), 7.33-7.07 (4H, m), 6.94-
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
79
6.89 (1H, m), 5.90 (2H, s), 2.14 (2H, s), 1.96-1.92 (3H, m), 1.69-1.63 (12H,
m).
Example 46
s 1-(3-Chlorobenzyl)-6-(3,~-difluoro~hen~l)-3-(toluene-4-sulfonylamino)-
indole-2-carboxylic acid
1H NMI~ (200 MHz, l~MSO-d6): S 13.8-12.8 (1H, br s), 9.33 (1H, s), 8.02
(1H, s), 7.78 (1H, d, ,J 8.6 Hz), 7.58-7.52 (3H, m), 7.44-7.38 (2H, m),
7.34-7.I7 (4H, m), 6.99 (2H, s), 6.79-6.73 (1H, m), 5.84 (2H, s), 2.33 (3H,
to s).
Example 47
1-(3-Chlorobenz~l~ 3-(2-c~clohexylideneacetylamino)-6-(3,5-difluorophen-
yl)indole-2-carbox~ic acid
is 1H NMR (200 MHz, DMSO-d6): ~ 13.7-12.8 (1H, br s), 9.62 (1H, s), 8.03
{1H, s), 7.74 (1H, d, .I 8.6 Hz), 7.58-7.48 (3H, m), 7.33-7.23 (2H, m), 7.18
(1H, ddd, J=9.3, 2.3, 2.3 Hz), 7.09-7.07 (1H, m), 6.97-6.89 {1H, m), 5.94
(1H, s), 5.91 (2H, s), 2.89-2.83 (2H, m), 2.21-2.16 (2H, m), 1.65-1.52 (6H,
m).
Example 48
1;~3-Chlorobenzy~-6~3 5-difluorophenyl)-3-[2-(3 3,5 5-tetramethylcyclo-
hexylidene)acetylamino~indole-2-carboxylic acid
1H NMR (200 MHz, DMSO-d6): ~ 13.6-13.1 (1H, br s), 9.67 (1H, s), 8.06
2s (1H, s), 7.74 (1H, d, J--8.6 Hz), 7.61-7.51 (3H, m), 7.35-7.1b (3H, m),
7.11-
7.09 (1H, m), 6.99-6.91 {1H, m), 6.07 (1H, s), 5.94 (2H, s), 2.70 (2H, s),
1.99 (2H, s), 1.33 (2H, s), 0.97-0.96 (12H, m).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
Example 49
1-(3-Chlorobenzyl)-6-(3 5-difluorophen~L~r2-~3 3 5 5-tetramethylcyclo-
hexylidene)acetylamino]indole-2-carboxylic acid sodium salt
1H NMR (200 MHz, DMSO-d6): 8 12.13 (1H, s), 8.38 (1H, d, J= 8.6 Hz),
s 7.76 (1H, s), 7.48-7.42 (2H, m), 7.36-7.29 (1H, m), 7.25-7.15 (3H, m), 7.11
7.04 (2H, m), 6.17 (2H, s), 5.85 (1H, s), 2.74 (2H, s), 1.97 (2H, s), 1.31
(2H,
s), 0.97-0.92 (12H, rn).
Example 50
l0 1-(3-Chlorobenzyl)-6-(3,5-difluorophenyl)-~4-isopropox benzoylamino)
indole-2-carbox Iic acid
1H NMR (200 MHz, DMSO=d~): 8 13.6-13.2 (1H, br s), 10.13 (1H, s), 8.08
(1H, s), 8.03-7.96 (2H, m), 7.83 (1H, d, J=8.6 Hz), 7.62-7.51 (3H, m), 7.35-
7.12 (4H, m), 7.08-7.0I (2H, m), 6.99-6.93 (1H, m), 5.96 (2H, s), 4.74 (1H,
is septet, J=6.1 Hz), 1.29 (6H, d, J--6.I Hz).
Example 51
1-(3-Chlorobenzyl)-6-(3 5-difluorophen~)-3-~4-iso~ropoxybenzo lamino)
indole-2-carboxylic acid sodium salt
20 1H NMF~ (200 MHz, DMSO-d6): ~ 13.14 (IH, br s), 8.49 (1H, d, ..T--g.4 Hz),
7.98-7.91 (2H, m), 7.81 (1H, s), 7.51-7.44 (2H, m), 7.40-7.33 (1H, m), 7.25-
7.17 (3H, m), 7.16-6.99 (4H, m), 6.19 (2H, s), 4.72 (1H, septet, ,I--6.0 Hz),
1.28 (6H, d, J 6.0 Hz).
2s Example 52
1-(3-Chlorobenzyl)-6-(3,5-difluorophenyl)-3~4-(trifluoromethyl)benzoyl
amino]indole-2-carboxylic acid
1H NMR (200 MHz, DMSO-d6): ~ 13.7-13.1 (1H, br s), 10.49 (1H, s), 8.23
(2H, m), 8.11 (1H, s), 7.93 (2H, m), 7.77 (1H, d, J 8.6 Hz), 7.59-7.53 (3H,
3o m), 7.35-7.09 (4H, m), 6.99-6.94 (1H, m), 5.97 (2H, s).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
81
Example 53
1-(3-Chlorobenzyl)-3-[(6-chloropyridine-3-carbonyl)aminol-6-(3,5-di-
fluorophenyl)indole-2-carboxylic acid
s 1H NMR (200 Ml-Iz, DMSO-d6): d 14.21-14.16 (IH, br s), 8.99 (IH, d,
J 2 .2 Hz), 8 . 5 6 ( 1 H, d, J--8 . 6 Hz), 8 . 3 7 ( 1 H, dd, J--8 .4, 2 . 6
Hz), 7 . 8 3 -7 . 82
(1H, m), 7.74 (1H, d, J 8.2 Hz), 7.51-7.36 (3H, m), 7.30-7.06 (5H, m), 6.21
(2H, s).
to Example 54
3-(4-Chlorobenzo ly amino-1-(3-chlorobenzyl)-6-~4-(methylsulfonyl)-
phenylaindole-2-carboxylic acid
(a) 6-Bromo-1-(3-chlorobenzy~-3-nitroindole-2-carboxylic acid ethyl ester
Is Cu(NO;)2 X2.5 HZO (2.26 g, 9.74 mmol) was added to Ac~O (10 mL) at
°C. The mixture was stirred until a homogenous solution was formed
whereafter a solution 6-bromo-1-(3-chlorobenzyl)indole-2-carboxylic acid
ethyl ester, prepay ed from 6-bromoindole-2-carboxylic acid ethyl ester and
3-chlorobenzyl chloride in accordance with the procedure in Example 1 (c),
20 (4.72 g, 12.02 mmol), in Ac2O (20 mL) was added dropwise. The mixture
was allowed to come to room temperature and was stirred for I.5 h and
filtered. The filtrate was poured onto ice and was left to stir overnight. The
precipitate was collected and dried to give the sub-title compound (4.82 g,
92%).
(b) 3-Amino-6-bromo-1-(3-chlorobenz~l)indole-2-carboxylic acid ethyl
ester
A mixture of 6-bromo-1-(3-chlorobenzyl)-3-nitroindole-2-carboxylic acid
ethyl ester (4.82 g, 1I.01 mmol), Fe-powder (3.15 g, 56.3 mmol), NH4Cl
(aq., sat., 75 mL) and isopropanol (160 mL) was heated at reflux for 2 h,
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
82
whereafter additional portions of Fe-powder (3.15 g, 56.3 mmol) and
NHøCl (aq., sat., 75 mL) were added. The mixture was heated for an
additional 2 h, allowed to cool and filtered through Celite~. The filter cake
was washed with EtOAc and the combined filtrates were extracted with
s EtOAc. The combined extracts were washed with water, brine and dried
over NaZC03. Concentration and purif cation by chromatography gave the
title compound (3.99 g, 89%).
(c) 6-Bromo-3-(4-chlorobenzo~lamino)-1-(3-chlorobenzyl)indole-2-carbox-
ylic acid ethyl ester
A mixture of 3-amino-6-bromo-1-(3-chlorobenzyl)indole-2-carboxylic acid
ethyl ester (2.00 g, 4.91 mmol), 4-chlorobenzoyl chloride (1.72 g, 9.82
mmol), DMAP (300 mg, 2.46 mmol), Et3N (1.38 mL, 9.82 mmol) and
MeCN (50 mL) was stirred at room temperature for 24 h. The mixture was
1s poured into water and extracted with EtOAc. The combined extracts were
washed with water, brine and dried over Na~C03. Concentration,
crystallisation from EtOH/EtOAc ( 1:1 ) and chromatography gave the title
compound.
20 (d) 3-~4-Chlorobenzoylamino)-1-(3-chlorobenzyl)-6-f4-(methylsulfonyl)-
phen~lindole-2-carboxylic acid eth ly__ester
A mixture of 6-bromo-3-(4-chlorobenzoylamino)-1-(3-chloroben~yl)indole-
2-carboxylic acid ethyl ester (160 mg, 0.29 mmol), 4-(methanesulfonyl)-
phenylboronic acid (87.9 mg, 0.44 mmol), Pd(OAc)2 (3.4 mg, 0.015 mmol),
2s tri-o-tolylphosphine (8.8 mg, 0.029 mmol), K3PO4 (215 mg, 1.02 mmol),
toluene (3 mL) and EtOH (0.5 mL) was stirred at room temperature for 20
min and heated at 90 °C for 3h. The mixture was allowed to cool, poured
into NaHC03 (aq., sat.) and extracted with EtOAc. The combined extracts
were washed brine and dried over Na2C0;. Concentration and purification
3o by chromatography gave the title compound (120 mg, 67%).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
83
(e) 3-(4-Chlorobenzoylamino)-1-(3-chloroben~l)-6-f4 ~methylsulfonyl)-
phenyl]indole-2-carboxylic acid
The title compound was prepared by hydrolysis of 3-(4-chlorobenzoyl-
s amino)-I-(3-chlorobenzyl)-6-[4-(methylsulfonyl)phenyl]indole-2-carboxyl-
ic acid ethyl ester (120 mg, 0.19 mmol) according to the procedure
described in Example 1 (e). Yield 84 mg (74%).
1H hIMR (200 MHz, DMSO-d6): b 13.6-13.4 (IH, br s), 10.36 (1H, s), 8.12-
7.97 (7H, m), 7.83 (1H, d, J 8.4 Hz), 7.69-7.61 (2H, m), 7.57 (1H, d, .~=8.7
1o Hz), 7.38-7.25 (2H, m), 7.17-7.13 (1H, m), 7.03-6.96 (1H, m), 6.00 (2H, s),
3.26 (3H, s).
The following Examples 55 to 64 were made in accordance with the
procedure in Example 54 using the appropriately substituted phenylboronic
1s acids.
Exam Ip a 55
3-(4-Chlorobenzoylamino)-1-(3-chlorobenzyl)-6Tf4-(methylthio)phenyll
indole-2-carboxylic acid
20 1H MVII~ (200 MHz, DMSO-d~): d 13.7-13.1 (1H, br s), 10.35 (IH, s), 8.12-
8.04 (2H, m), 7.93 (IH, s), 7.77 (1H, d, .I--8.5 Hz), 7.74-7.61 (4H, m), 7.47
(1H, d, J--8.5 Hz), 7.39-7.25 (4H, m), 7.16-7.12 (1H, m), 7.04-6.97 (IH, m),
5.97 (2H, s), 2.51 (3H, s).
2s Exam lp a 56
3-(4-Chlorobenzoylamino)-I-(3-chlorobenzyl~6~'4-viny_lphenyl)indole 2
carboxylic acid
IH NMR (200 M~-Iz, DMSO-d6): d 13.6-13.2 (1H, br s), 10.35 (1H, s), 8.12-
8.04 (2H, m), 7.97 (1H, s), 7.78 (1H, d, J 8.4 Hz), 7.78-7.72 (2H, m), 7.69-
30 7.62 (2H, m), 7.61-7.54 (2H, m), 7.51 (IH, d, J--8.6 Hz), 7.38-7.25 (2H,
m),
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
84
7.18-7.13 (1H, m), 7.05-6.98 (1H, m), 6.79 (H, dd, J--17.8, 11.0 Hz), 5.98
(2H, s), 5.90 (1H, d, .l 17.8 Hz), 5.29 (1H, d, 11.0 Hz).
Example 57
s 3-(4-Chlorobenzoylamino~-1-(3-chlorobenzyl)-6-(4-isopropoxyphenyl)-
indole-2-carboxylic acid
1H .NMR (200 MHz, I~IvISO-d6): 8 13.5-13.3 (1H, br s), 10.35 (1H, s), 8.12-
8.03 (2H, m), 7.85 (1H, s), 7.75 (1H, d, J 8.5 Hz), 7.70-7.61 (4H, m), 7.43
(1H, d, ,I 8.7 Hz), 7.38-7.25 (2H, m), 7.15-7.11 (1H, m), 7.05-6.96 (3H, m),
5.96 (2H, s), 4.67 (1H, septet, J 6.0 Hz), 1.28 (6H, d, J 6.0 Hz).
Example 58
6-(4-te~°t-Butylphenyl)-3-(4-chlorobenzoylamino)-1-(3-
chlorobenzyl)indole-
2-caxbox~ic acid
Is 1H NMR (200 MHz, I~MS(~-d6): 8 13.5-13.3 (1H, br s), 10.34 (1H, s), 8.12-
8.03 (2H, m), 7.88 (1H, s), 7.76 (1H, d, J=8.4 Hz), 7.70-7.60 (4H, m), 7.52-
7.42 (3H, m), 7.38-7.25 (2H, m), 7.17-7.13 (1H, m), 7.05-6.98 (1H, m),
5.96 (2H, s), 1.31 (9H, s).
2o Example 59
3-(4-Chlorobenzoylamino,-1-(3-chlorobenzyl)-6-f 4-(trifluoromethyl)-
phenyl~indole-2-carboxylic acid
1H NMR (200 MHz, I3MS~-d6): ~ 13.6-13.3 (1H, br s), 10.43 (1H, s), 8.12
8.04 (3H, m), 8.03-7.95 (2H, m), 7.87-7.78 (3H, m), 7.69-7.61 (2H, m),
2s 7.54 (1H, dd, J 8.5, 1.0 Hz), 7.38-7.25 (2H, m), 7.16-7.12 (1H, m), 7.03
6.96 (1H, m), 6.00 (2H, s).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
8s
Example 60
3-(4-Chlorobenzoylamino)-1 ~3-chlorobenzyl)-6-f4-(trifluoromethoxy)-
phenyllindole-2-carboxylic acid
1H NMR (200 MHz, I~MS~-d6): 8 13.6-13.2 (1H, br s), I0.39 (1H, s), 8.10
s 8.02 (2H, m), 7.97 (1H, s), 7.89-7.82 (2H, m), 7.79 (IH, d, ,I--8.5 Hz),
7.67
7.59 (2H, m), 7.50-7.41 (3H, m), 7.36-7.23 (2H, m), 7.14-7.10 (1H, m),
7.02-6.95 (1H, m), 5.96 (2H, s).
Example 61
l0 3-(4-Chlorobenzo~lamino)-1-L-chlorobenzyl)-6-(4-cyclohexylphenyl)-
indole-2-carboxylic acid
1H NMI~ (200 MHz, acetone-d6): d 11.9-11.5 (1H, br s), 8.61-8.52 (1H, m),
8.24-8.15 (2H, m), 7.71 (1H, s), 7.66-7.58 (2H, m), 7.58-7.49 (2H, m), 7.45
7.37 (1H, m), 7.34-7.12 (6H, m), 6.16 (2H, s), 2.64-2.47 (1H, m), 1.93-1.68
is (SH, m), 1.60-1.22 (SH, m).
Example 62
6-(4-l3utylphenyl)-3-(4-chlorobenzoylamino~3-chlorobenzyl)indole-2-
carboxylic acid
20 1H MvII~ (200 MHz, I)MS~-d6): ~ 13.5-13.2 (1H, br s), 8.49 (IH, d, J--8.6
Hz), 8.12-8.00 (2H, m), 7.70-7.59 (4H, m), 7.57 (IH, s), 7.37-7.17 (6H, m),
7.16-7.08 (1H, m), 6.20 (2H, s), 2.61 (2H, t, J 7.4 Hz), 1.67-1.49 (2H, m),
I.42-I.22 (2H, m), 0.91 (3H, t, J 7.2 Hz).
2s Example 63
3-(4-Chlorobenzoylamino)-1-(3-chlorobenz~l)-6-(4-cyanophenyl)indole-2-
carboxylic acid
IH NMR (200 MHz, DMSO-d6): S I3.3-13.1 (1H, br s), 8.52 (1H, d, J 8.6
Hz), 8.I0-8.01 (2H, m), 7.98-7.84 (SH, m), 7.68-7.60 (2H, m), 7.43 (1H, dd,
3o J 8.6, 0.9 Hz), 7.34-7.I8 (3H, m), 7.14-7.07 (1H, m), 6.2I (2H, s).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
86
Example 64
3-(4-Chlorobenzo~amino~ 1-(3-chlorobenzyl)-6-(4-chlorophenyl)indole-2-
carboxylic acid
s 1H NMR (200 MHz, DMSO-d6): b 13.8-13.2 (1H, br s), 11.8-11.4 (1H, br
s), 8.14-8.02 (3H, m), 7.87 (IH, s), 7.81-7.73 (2H, m), 7.68-7.60 (2H, m),
7.56-7.48 {2H, m), 7.42 {1H, dd, J=8.7, 1.1 Hz), 7.36-7.22 (2H, m), 7.19-
7.14 (1H, m), 7.08-7.00 (1H, m), 6.07 (2H, s).
to The following Examples 65 to 109 were prepared by analogous techniques
to those described herein.
Example 65
1-(3-Chlorobenzyl)-6-~3 5-difluorophenyl)-3-(4-methoxybenzoylamino)-
is indole-2-carboxylic acid
1H MVIR (200 MHz, DMSO-d6): b 10.19 (1H, s), 8.14-8.02 (3H, m), 7.86
(1H, d, J 8.6 Hz), 7.64-7.53 (3H, m), 7.39-7.07 {6H, m), 7.03-6.96 (1H, m),
6.00 (2H, s), 3.87 (3H, s).
2o Example 66
1-(3-Chlorobenz~)-3-~4-methoxybenzoylamino)-6-naphth-1-ylindole-2-
carboxylic acid
1H NMR (200 MHz, DMSO-d6): b 10.21 (1H, s), 8.09-7.91 (4H, m), 7.86
{1H, d, J=8.4 Hz), 7.66-7.69 {2H, m), 7.62-7.51 (2H, m), 7.48 (1H, d, J 1.2
2s Hz), 7.46-7.29 (3H, m), 7.23 (1H, dd, J 8.4, 1.2 Hz), 7.17-7.14 (1H, m),
7.13-7.OS (2H, rn), 7.04-6.97 (1H, m) 5.91 (2H, s), 3.84 (3H, s).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
87
Example 67
6-Benzo~l 3~dioxol-5-yl-1-~3-cyanobenz~)-3-(3-phen~propionylamino)-
indole-2-carboxylic acid
1H NMR (200 MHz, DMSO-d6): & 9.7 (1H, s), 7.79 (1H, s), 7.70-7.64 (1H,
s m), 7.56-7.14 (12H, m), 6.97 (1H, d, .I 8.1 Hz), 6.03 (2H, s), 5.92 (2H, s),
3.00-2.90 (2H, m), 2.75-2.64 (2H, m).
Example 68
I-(3-Chlorobenzy~-6~naphth-1-~-3-pentanoylaminoindole-2-carboxylic
to acid
1H ,NMR (200 MHz, DMSO-d6): ~ 13.37 (1H, s), 9.67 (IH, s), 8.02-7.91
(2H, m), 7.74-7.65 (3H, m), 7.61-7.25 (6H, m), 7.22 (1H, dd, J--8.4, 0.9
Hz), 7.12-7.09 ( 1H, m), 7.02-6.93 ( 1H, m), 5.86 (2H, s), 2.41 (2H, t, J 7.2
Hz), 1.72-1.56 (2H, m), 1.49-I.29 (2H, m) , 0.93 (3H, t, ,I 7.3 Hz).
is
Example 69
1-(3-Chlorobenzyl)-6-(3,5-difluorophenyl)-3-pentanoylaminoindole-2-
carboxylic acid
1H NMI~ (200 MHz, DMS~-d6): ~ 13.4 (1H, br s), 9.67 (IH, s), 8.08 (1H,
2o s), 7.71 (1H, d, J 8.5 Hz), 7.62-7.51 (3H, m), 7.37-7.I8 (3H, m), 7.13-7.09
(IH, m), 7.00-6.92 (1H, m), 5.95 (2H, s), 2.42 (2H, t, J 7.3 Hz), 1.73-1.58
(2H, m), 1.49-1.31 (2H, m), 0.95 (3H, t, J 7.3 Hz).
Example 70
2s 3-[(Biphenyl-4-carbonyl)amino]-1~- 3-chlorobenzyl)-6-(3,5-difluorophenyl)-
indole-2-carboxylic acid
1H NMR (200 MHz, DMSO-d6): b 13.6-12.4 (1H, br s), 10.70 (1H, s), 8.21-
8.06 (3H, m), 7.96 (1H, d, J 8.6 Hz), 7.92-7.85 (2H, zn), 7.84-7.77 (2H, m),
7.63-7.17 (lOH, m), 7.06-6.99 (IH, m), 6.04 (2H, s).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
88
Example 71
1 ~'3-Chlorobenzyl)-6-(3 5-difluorophenyl)-3-[2 (4 methox henyl)acetyl
aminolindole-2-carboxylic acid
1H NMR (200 MHz, DMSO-d6): 8 9.81 (1H, s), 8.03 (1H, s), 7.63 (1H, d,
s J=8.6 Hz), 7.59-7.45 (3H, m), 7.35-7.13 (5H, m), 7.09-7.05 (1H, m), 6.95-
6.84 (3H, m), 5.91 (2H, s), 3.73 (3H, s), 3.66 (2H, s).
Examt~ie 72
1-(3-Chlorobenzyl)-6-(3 5-difluorophenyl)-3-phenylindole-2-carbox. lic
1o acid
iH NM1Z (200 MHz, DMSO-d6): ~ 13.10 (1H, s), 8.11 (1H, s), 7.60-7.43
(8H, m), 7.42-7.14 (5H, m), 7.03-6.95 (1H, m), 5.96 (2H, s).
Example 73
Is 6-Benzof 1,31dioxol-5-yl-1-(3-cyanobenz~)-3l4-(dimethylamino)butyryl
aminolindole-2-carboxylic acid hydrochloride
IH NMR (200 MHz, DMSO-d6): S 13.8-12.8 (1H, br s), 9.89 (1H, s), 7.87-
7.84 (1H, m), 7.75-7.67 (2H, m), 7.54 (IH, d, .~ 8.1 Hz), 7.51-7.39 (2H, rn),
7.36 (1H, d, J--I.7 Hz), 7.34-7.28 (1H, m), 7.23 (1H, dd, J 8.2, 1.7 Hz),
20 7.02 .(1H, d, J--8.I Hz), 6.08 (2H, s), 5.97 (2H, s), 3.19-3.08 (2H, m),
2.79
(6H, s), 2.58-2.48 (2H, m, overlapped With DMSO signal), 2.11-1.94 (2H,
m).
Example 74
as 6-Benzof 1 3ldioxol-5-yl-3-(3-phenylacrylo Iamino)-1-~3-(2H tetrazol 5
yI)benzyllindole-2-carboxylic acid
1H NMI~ (200 MHz, DMSO-d6): 8 9.95 (1H, s), 7.89-7.82 (3H, m), 7.71
(1H, d, J--8.6 Hz), 7.68-7.35 (8H, m), 7.33 (1H, d, J 1.7 Hz), 7.23-7.14
(2H, m), 7.06 (1H, d, J 15.7 Hz), 6.97 (1H, d, .J=8.2 Hz), 6.03 (2H, s), 6.01
so (2H, s).'
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
89
Example 7S
1-(3-Chlorobenzyl -S-(4-c~nophenyl)-3-(3,S-dimethoxybenzoylamino)-
indole-2-carboxylic acid
s 1H NMR (200 MHz, DMS~-d6): S 13.8-13.1 (1H, br s), 10.23 (1H, s), 8.08
(1H, s), 7.88 (4H, s), 7.75 (2H, s), 7.38-7.26 (2H, m), 7.24-7.17 (2H, m),
7.16-7.11 (1H, m), 7.05-6.95 (1H, m), 6.76-6.69 (1H, m), 5.90 (2H, s), 3.81
(6H, s).
to Example 76
1-(3-Chlorobenz~)-3-~entanoylamino-6-[4-(trifluoromethyl)phenyll-
indole-2-carboxylic acid
~H NMR (200 MHz, DMS~-d6): ~ 9.83 (1H, s), 7.96-7.73 (6H, m), 7.47
(1H, d, J 8.7 Hz), 7.33-7.21 (2H, m), 7.10-7.07 (1H, m), 6.97-6.92 (1H,
m), 5.93 (2H, s), 2.38 (2H, t, J 7.2 Hz), 1.69-1.55 (2H, m), 1.46-1.28 (2H,
m), 0.91 (3H, t, J=7.3 Hz).
Example 77
1-(3-Chlorobenzyl)-3-j~3 3 S,S-tetrameth ~~lcyclohexanecarbonyl)aminol-6-
20 ~4-(trifluoromethyl)phenyl)-indole-2-carboxylic acid
1H NMR (200 MHz, DMSO-d6): d 13.5-13.2 (1H, br s), 9.68 (1H, s), 8.03-
8.00 (1H, m), 7.99-7.94 (2H, m), 7.84-7.80 (2H, m), 7.70 (1H, d, ..T--8.S Hz),
7.51 (1H, d, J 8.S Hz), 7.35-7.25 (2H, m), 7.09 (1H, s), 7.01-6.94 (1H, m),
5.93 (2H, s), 2.85 (1H, t, J 12.4 Hz) 1.63 (2H, d, J 12.4 Hz), 1.34-1.21
2s (3H, m), 1.12-1.09 (1H, m), 1.06 (6H, s), 0.94 (6H, s).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
Example 78
3-Benzoylamino-1-(3-chlorobenzyl)-5-(4-c~phenyl)indole-2-carboxylic
acid
1H NMR (200 MHz, DMSO-d6): 8 10.33 (1H, s), 8.13-8.01 (3H, m), 7.88
s (4H, s), 7.77-7.73 (2H, m), 7.64-7.49 (3H, m), 7.3 8-7.26 (2H, m), 7. I 7-
7.12
(1H, m), 7.04-6.96 (1H, m), 5.90 (2H, s).
Example 79
5~(3-Chlorophenyl)-3-~hen~[(3-(trifluoromethoxY)benz~]indole-2-
1o carboxylic acid
1H NMR (200 MHz, DMSO-d6): ~ 13.2-13.0 (1H, br s), 7.78-7.70 (1H, m),
7.69-7.62 (3H, m), 7.60-7.30 (9H, m), 7.28-7.14 (2H, m), 7.10-7.02 (1H,
m), 5.92 (2H, s).
is Example 80
5-(~-Chlorophen~)-3-phenyl-1-[(3-~trifluoromethoxylbenzyllindole-2-
carbox~lic acid
IH NMR (200 MHz, DMSO-d6): 8 13.2-13.0 (1H, br s), 7.75-7.67 (1H, m),
7.56-7.19 (14H, m), 7.15-7.07 (1H, m), 5.93 (2H, s).
Example 81
5-(4-Chlorophenyl~~hen~[(3-(trifluoromethoxy)benzyl]indole-2-
carboxylic acid
1H NMR (200 MHz, I7MS0-d6): 8 13.2-13.0 (1H, br s), 7.77-7.56 (SH, m),
2s 7.54-7.30 (8H, m), 7.27-7.13 (2H, m), 7.I0-7.02 (IH, m), 5.92 (2H, s).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
91
Example 82
S-(2-Chlorophenyl)-3-pentanoylamino-1-[(3-(trifluoromethox )~benzyl~-
indole-2-carboxylic acid
1H NMR (200 MHz, I7MS0-d6): F~ 13.5-13.2 (1H, br s), 9.63 (1H, s), 7.69
s 7.58 (2H, m), 7.58-7.50 (1H, m), 7.48-7.31 (SH, m), 7.27-7.17 (IH, m),
7.13 (1H, s), 7.03 (1H, d, J--7.7 Hz), 5.87 (2H, s), 2.36 (2H, t, J--7.3 Hz),
1.67-1.47 (2H, m), 1.44-1.23 (2H, m), 0.88 (3H, t, J 7.2 Hz).
Example 83
l0 5-(3-Chlorophenyl)-3-pentanoylamino-1-[(3-(trifluoromethox )Y ben zYI]-
indole-2-carboxylic acid
1H NMR (200 MHz, DMSO-d6): 8 13.4-13.2 (1H, br s) 9.67 (1H, s) 7.86
(1H, s) 7.74-7.55 (4H, m) 7.53-7.33 (3H, m) 7.27-7.16 (1H, m) 7.09 (1H, s)
6.98 (1H, d, J=7.8 Hz) 5.87 (2H, s) 2.40 (2H, t, J=7.3 Hz) 1.72-1.50 (2H,
is m) 1.48-1.27 (2H, m) 0.91 (3H, t, J 7.2 Hz).
Example 84
5-(4-Chlorophenyl)-3-pentanoylamino-1-[(3-(trifluoromethox )y benzyl]-
indole-2-carboxylic acid
20 1H I~~MR (200 MHz, l~MSO-d6): b 13.4-13.1 (1H, br s), 9.65 (1H, s), 7.83
(1H, s), 7.72-7.56 (4H, m), 7.55-7.45 (2H, m), 7.40 (1H, dd, J--8.0 and 8.0
Hz), 7.25-7.16 (1H, m), 7.09 (1H, s), 6.97 (1H, d, J 8.0 Hz), S.S6 (2H, s),
2.40 (2H, t, .I 7.2 Hz), 1.70-1.50 (2H, m), 1.48-1.28 (2H, m), 0.91 (3H, t,
J=7.2 Hz).
Example 85
I-(3-Chlorobenzyl)-5-(4-chlorophenYl~phenylindole-2-carboxylic acid
IH NMR (200 MHz, DMSO-d6): b 13.2-13.0 (1H, br s), 7.77-7.57 (SH, m),
7.54-7.29 (9H, m), 7.22 (1H, s), 7.05-6.97 (1H, m), 5.87 (2H, s).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
92
Example 86
5-(4-Chlorophenyl)-1-(3 5-dimeth lbenz~l -3-phenylindole-2-carboxylic
acid
1H NMR (200 MHz, DMSO-d6): 8 13.I-13.0 (IH, br s), 7.73-7.57 (SH, m),
s 7.53-7.30 (7H, m), 6.85 (1H, s), 6.74 (2H, s), 5.77 (2H, s), 2.17 (6H, s).
Exam lp a 87
5-(4-Chlorophenyl)-1-(3 5-difluorobenz~l)-3-phenylindole-2-carboxylic
acid
ro 1H NMR (200 MHz, DMSO-d6): ~ 13.2-13.0 (1H, br s), 7.76-7.58 (5H, m),
7.55-7.31 (17H, m), 7.12 (IH, t, J--9.4, 2,5 Hz), 6.86-6.73 (2H, m), 5.89
(2H, s).
Example 88
1s 6->3enzof 1 3ldioxol-5-yl-3-pentanoylamino-I-(3-~henoxybenz5yl)indole 2
carboxylic acid
1H NMR (200 MHz, DMSO-d6): S 13.3 (1H, br s), 9.73 (IH, br s), 7.78 {1H,
s), 7.66 (1H, d, J--8.4 Hz), 7.42-7.I7 (6H, m), 7.16-7.07 (1H, m), 7.01 (1H,
d, J=8.2 Hz), 6.99-6.91 (2H, m), 6.84-6.76 (3H, m), 6.09 (2H, s), 5.93 (2H,
2o s), 2.41 (2H, t, J--7.0 Hz), 1.73-1.56 (2H, m), 1.50-1.30 (2H, m), 0.95
(3H,
t, .,T--7.2 Hz).
Exam lp a 89
6-Benzo~I,3ldioxol-5-yl-1-(9H fluoren-2-ylmeth~)-3-~entanoylamino
2s indole-2-carboxylic acid
1H NMR (200 MHz, DMSO-d6): ~ 13.2 (IH, br s), 10.16 (IH, br s), 7.85-
7.75 (4H, m), 7.53 (1H, d, J--7.4 Hz), 7.40-7.26 (5H, m), 7.25-7.13 (2H, m),
7.00 (1H, d, J 8.4 Hz), 6.06 (2H, s), 6.04 (2H, s), 3.83 (2H, s), 2.41 (2H, t,
J--7.4 Hz), 1.75-1.58 (2H, m), 1.51-1.30 (2H, m), 0.95 (3H, t, J--7.2 Hz).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
93
Example 90
1-(3-Chlorobenz~l)-6 ~3,5-difluorophenyl)-3-(3-phenylacryloylamino)-
indole-2-carboxylic acid
1H NMR (200 MHz, DMSO-d6): 8 13.52 (1H, br s), 10.1 (1H, s), 8.1.1 (1H,
s s), 7.79 (1H, d, J 8.5 Hz), 7.74-7.38 (9H, m), 7.36-7.18 (3H, m), 7.16-7.03
(2H, m), 7.01-6.94 (1H, m), 5.98 (2H, s).
Example 91
1-(3-Chlorobenzyl)-6-(3,5-difluorophenyl)-3-(2,2-dimethylpropion ~~l-
1o amino~indole-2-carboxylic acid
1H NMR (200 MHz, DMSO-d6): 8 I3.8-I3.2 (IH, s), 9.51 (IH, s), 8.06 (1H,
s), 7.81 (1H, d, .I--8.6 Hz), 7.63-7.51 (3H, m), 7.37-7.13 (4H, m), 7.01-6.93
(1H, m), 5.96 (2H, s), 1.31 (9H, s).
Is Exam,~le 92
3~4-Chlorobenzoylamino)-1-(3-chlorobenz~)-6-(3,5-difluorophenyl)-
indole-2-carboxylic acid
1H NNIR (200 MHz, DMSO-d6): ~ 10.3 (1H, s), 8.12~8.06 (3H, m), 7.80
(1H, d, J 8.6 Hz), 7.67-7.55 (5H, m), 7.37-7.14 (4H, m), 7.01-6.96 (1H, m),
20 5.99 (2H, s).
Example 93
I-(3-Chlorobenz~l)-6-(2~isopropoxylahen l~pentanoylaminoindole-2-
carboxylic acid
zs IH NMR (200 MHz, DMSO-d6): ~ I3.2-13.5 (IH, br s) 10.4 (IH, br s) 7.85
(1H, d, J--8.5 Hz) 7.56 (1H, s) 7.38-7.24 (4H, m) 7.21 (1H, d, J=8.4 Hz)
7.12-6.96 (4H, m) 5.93 (2H, s) 4.51 (1H, septet, J 6.1 Hz) 2.41 (2H, t,
J=7.3 Hz) 1.76-1.5 8 (2H, m) I .5 I -1.3 I (2H, m), 1. 05 (6H, d, J 6.1 Hz),
0.95 (3H, t, J 7.5 Hz).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
94
Example 94
1-(3-Chlorobenz~)-6 ~2-isopropoxyphenYl)-3-phenylindole-2-carbox~ic
acid
1H NMR (200 MHz, DMS~-d6): 8 12.99 (1H, br s), 7.71 (1H, s), 7.56-7.26
s (11H, m), 7.24-7.20 (1H, m), 7.13-6.97 (3H, m), 5.89 (2H, s), 4.54 (1H,
septet, J 6.0 Hz), 1.07 (6H, d, .I 6.0 Hz).
Example 95
3-((Biphenyl-4-carbon)amino]-1-(3-chlorobenzyl)-6-(2-isopropox hen-
lo y~indole-2-carboxylic acid
1H NMR (200 MHz, DMS~-d6): b l I.0-11.3 (IH, br s), 8.22-8.14 (2H, m),
7.97 (1H, d, ,I 8.4 Hz), 7.93-7.86 (2H, m), 7.84-7.78 (2H, m), 7.65 (1H, s),
7.60-7.44 (3H, m), 7.42-7.24 (SH, m), 7.16-6.98 (4H, m), 5.97 (2H, s), 4.53
(IH, septet, J 6.0 Hz), 1.07 (6H, d, J=6.0 Hz).
Example 96
1-(3-Chlorobenzyl)-6-~4-isopropoxyphen~l)-3-pentanoylaminoindole-2-
carboxylic acid
1H NMI~ (200 MHz, DMS~-d6): S 13.3 (1H, br s), 9.65 (IH, s), 7.81 (IH,
2o s), 7.70-7.92 (3H, m), 7.42 (1H, dd, J 8.5, 1.1 Hz), 7.37-7.25 (2H, m),
7.12
6.94 (4H, m), 5.92 (2H, s), 4.68 (1H, septet, .I--6.0 Hz), 2.42 (2H, t, J--7.4
Hz), 1.74-1.56 (2H, m), 1.51-1.29 (2H, m), 1.26 (6H, d, .I--6.0 Hz), 0.95
(3H, t, J--7.2 Hz).
Example 97
1-(3-Chlorobenzyl)-6-(4-isopropoxyphenyl~phenylindole-2-carboxylic
acid
1H NMR (200 MHz, DMSO-d6): 8 12.98 (1H, br s), 7.88 (1H, s), 7.70-7.61
(2H, m), 7.56-7.27 (9H, m), 7.26-7.22 (1H, m), 7.08-6.97 (3H, m), 5.97
(2H, s), 4.68 (1H, septet, J--6.0 Hz), 1.30 (6H, d, 6.0 Hz).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
Example 98
I-(3-Chlorobenzyl~-3,6-bis-(4-isopropoxyphenyl)indole-2-carboxylic acid
1H NMR (200 MHz, DMSO-d6): 8 12.95 (1H, br s), 7.85 (1H, s), 7.69-7.60
s (2H, m), 7.54 (1H, d, J 8.6 Hz), 7.47-7.27 (SH, m), 7.24-7.20 (1H, m),
7.06-6.96 (SH, m), S.9S (2H, s), 4.69 (1H, septet, .I 6.0 Hz), 4.68 (1H,
septet, J--6.0 Hz), 1.34 (6H, d, ~I--6.0 Hz) 1.30 (6H, d, .I--6.0 Hz).
Example 99
1o I-(3-Chlorobenz 1~)-6-~3-isopropoxyphen~)-3-phenylindole-2-carbox~lC
acid
1H NMR (200 MHz, DMSO-d6): 8 13.08 (1H, br s), 7.94 (1H, s), 7.58-7.31
(IOH, m), 7.30-7.21 (3H, m), 7.08-7.02 (IH, m), 6.97-6.89 (1H, m), 5.99
(2H, s), 4.73 (1H, septet, ,I--6.0 Hz), I.31 (6H, d, J 6.0 Hz).
is
Example 100
I-(3-Chlorobenzyl)-3-(3-isopropoxyphenyll-6-(4-isopropoxyphenyl)indole-
2-carboxylic acid
1H Mv» (200 IVI~Iz, DMSO-d6): ~ 7.81 (IH, s), 7.64-7.57 (2H, m), 7.5I
20 ( 1 H, d, J=8. S Hz), 7 .44-7.3 8 ( 1 H, m), 7.3 4 ( 1 H, dd, J--8 .0, 2. 0
Hz), 7. 3 0
7.24 (2H, m), 7.22-7.19 (1H, m), 7.05-6.93 (SH, m), 6.88 (1H, dd, ,l--8.0,
2.1 Hz), 5.89 (2H, s), 4.63 (1H, septet, .I--6.0 Hz), 4.61 (IH, septet, .I--
6.0
Hz), 1.28 (6H, d, J 6.0 Hz), 1.26 (6H, d, J--6.0 Hz).
2s Example I01
1-(3-Chlorobenz~l)-6-(3-isopropoxyphenYl)-3-t~entanoylaminoindole-2-
carboxylic acid
1H NMR (200 MHz, DMSO-d6): ~ I3.S-I3.1-(IH, br s), 9.64 (1H, br s),
7.84 (1H, s), 7.64 (1H, d, J 8.5 Hz), 7.41 (1H, d, .I--8.5 Hz), 7.36 (1H, d,
3o J--7.8 Hz), 7.31-7.17 (4H, m), 7.I2-7.08 (1H, m), 6.95-6.85 (2H, m), 5.91
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
96
(2H, s), 4.70 (1H, septet, J 6.0 Hz), 2.38 (2H, t, J--7.3 Hz), 1.70-1.53 (2H,
m), 1.47-I.27 (2H, m), 1.27 (6H, d, J--6.0 Hz), 0.91 (3H, t, J 7.2 Hz).
Example 102
s 1-(3-Chlorobenzyl)-6-(4-isopropoxyphen~I)-3-~2-isopropoxy henyl)indole-
2-carboxylic acid
1H NMR (200 MHz, DMSO-d6): ~ 12.67 (1H, br s), 7.81 (1H, s), 7.67-7.59
(2H, m), 7.43-7.26 (6H, m), 7.19-7.15 (1H, m), 7.13-6.97 (5H, m), 5.96
(2H, s), 4.67 (1H, septet, J 6.0 Hz), 4.46 (1H, septet, J 6.0 Hz), I.30 (6H,
d, ,I 6.0 Hz), I.I3 (6H, d, J--6.0 Hz).
Exam lp a 103
1-(3 -Chl orob enzyl)- 3 -» entanoylamino-S-[4-(trifluoromethyl)pheny~ indole-
2-carboxylic acid
Is 'H NMR (200 MHz, DMS~-d6): ~ I3.5-I3.3 (IH, br s), 9.7 (1H, br s), 8.93
(IH, s), 7.90-7.75 (4H, m), 7.73-7.63 (2H, m), 7.35-7.24 (2H, m), 7.I3-6.04
(1H, m), 7.00-6.92 (IH, m), 5.84 (2H, s), 2.40 (2H, t, ~ 7.5 Hz), 1.7I-1.51
(2H, m), 1.47-1.26 (2H, m), 0.9I (3H, t, ~ 7.2 Hz).
2o Example 104
1-(3-Chlorobenzyl)-5-[4~methylthio)phenyll-3-~entanoylaminoindole-2
carboxylic acid
~H NMR (200 MHz, DMS~-d6): 8 10.9-10.7 (IH, br s), 8.21 (IH, s), 7.59-
7.44 (4H, rn), 7.36-7.20 (4H, m), 7.12 (1H, s), 7.06-6.97 (1H, m), 5.96 (2H,
2s s), 2.48 (3H, s, overlapped With DMSO), 2.37 (2H, t, J 7.4 Hz), 1.71-1.51
(2H, m), 1.46-1.24 (2H, m), 0.90 (3H, t, J 7.2 Hz).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
97
Example 105
1-(3-Chlorobenzyl)-3-(4-methoxybenzoylamino)-5~~~4-meth lthio)phenyll
indole-2-carboxylic acid
1H NMR (200 MHz, DMSO-d6): ~ 12.0-1 I.8 (1H, br s), 8.47 (1H, s), 8.07-
s 7.96 (2H, m), 7.63-7.47 (4H, m), 7.36-7.22 (4H, m), 7.17 (1H, s), 7.I2-7.00
(3H, m), 6.03 (2H, s), 3.80 (3H, s), 2.48 (3H, s, overlapped with DMS~).
Example 106
3-((Biphenyl-4-carbonyl)aminol-5-(4-t~f°t-butylphenyl)-1-(3-
chlorobenzvll
to indole-2-carboxylic acid
1H NM~ (200 MHz, DMSO-d6): 8 10.4-10.3 (1H, br s), 8.19-8.09 (2H, m),
7.93 (1H, s), 7.89-7.8I (2H, m), 7.80-7.61 (4H, m), 7.62-7.35 (7H, m), 7.34-
7.25 (2H, m), 7.18-7.13 (1H, m), 7.05-6.97 (IH, m), 5.88 (2H, s), 1.28 (9H,
s).
Exam lp a I07
~3-Amino-4-methylbenzoylalnino)-1-(3-chlorobenzyl)-5-[4-~methylsul
fon~l)pheny~indole-2-carboxylic acid
1H (200 MHz, DMSO-d6): ~ 10.2-I0.1 (1H, br s), 8.16 (1H, s), 8.01-
7.86 (4H, m), 7.75-7.72 (2H, m), 7.37-7.24 (3H, m), 7.16-7.11 (2H, m),
7.09-6.93 (2H, m), 5.58 (2H, s), 3.22 (3H, s), 2.I 1 {3H, s).
Example 108
5-(4-te~°t-Butylphenyl)-1-(3-chlorobenzYl)-~4-isopropoxyphenyl)indole 2
2s carboxylic acid
1H NMR (200 MHz, DMSO-d6): 8 7.64 (IH, s), 7.60-7.34 (8H, m), 7.33-
7.22 (3H, m), 7.16-7.06 (1H, m), 6.99-6.89 {2H, m), 5.80 (2H, s), 4.62 (IH,
septet, J--6.0 Hz), 1.28 (6H, d, .J 6.0 Hz), 1.27 (9H, s).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
98
Example I09
1-(3-Chlorobenz~)-6-(4-isopropoxyphenyl)-3-(4-methoxybenzoylamino)-
indole-2-carboxylic acid
1H NMR (200 MHz, DMSO-d6): 8 I3.5-13.3 (IH, br s), 10.16 (IH, s), 8.09
s 8.00 (2H, m), 7.86-7.77 (2H, m), 7.70-7.62 (2H, m), 7.42 (IH, d, J--8.5 Hz),
7.38-7.24 (2H, m), 7.16-7.10 (2H, m), 7.07 (1H, s), 7.04-6.96 (3H, m), 5.95
(2H, s), 4.67 (1H, septet, J 6.1 Hz), 3.86 (3H, s), 1.28 (6H, d, J--6.1 Hz).
Example 110
1o N [1-(3-Chlorobenzyl)-2-hydroxymethyl-6-(4-isopropoxyphenyl)indol-3-
Y1Z 4-methoxybenzamide
LiAIH4 (6.4 mg, 0.17 mmol) was added to a solution of 1-(3-chlorobenzyl)-
6-(4-isopropoxyphenyl)-3-(4-methoxybenzoylamino)indole-2-carboxylic
acid ethyl ester, prepared in accordance with the procedure described in
Is Example 7(a), (100 mg, 0.17 mmol) in THF (5 mL) at 0°C. After
stirring
for 1 h another portion of LiAlH4 (6.4 mg, 0.17 mmol) was added and the
stirring was continued at room temperature for 1 h. The mixture was
acidified to pH 2 with HCl (aq., 4M), diluted with water and extracted with
EtOAc. The combined extracts were washed with water, brine, and dried
20 over NazSO~ and concentrated. Purification of the residue by chromato-
graphy gave the title compound (59 mg, 64%).
1H NMR (200 MHz, DMSO-d6): ~ 9.85 (1H, s), 8.08-7.99 (2H, m), 7.59
(1H, s), 7.57-7.50 (2H, m), 7.44 (1H, d, .I 8.4 Hz), 7.38-7.23 (3H, m), 7.21-
7.16 (1H, m), 7.14-7.02 (3H, m), 6.99-6.90 (2H, m), 5.63 (2H, s), 5.20 (1H,
2s t, J--5.1 Hz), 4.62 (1H, septet, J--6.0 Hz), 4.54 (2H, d, ,I--5.1 Hz), 3.83
(3H,
s), 1.25 (6H, d, J 6.0 Hz).
The following Examples I 11 to I30 were prepared by analogous techniques
to those described herein.
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
99
Exam Ip a 1 I 1
5-(4-te~°t-Butylphenyl)-1-(3-chlorobenzyl)-3-[4
~trifluoromethoxy)phenyll
indole-2-carboxylic acid
~H NMK (200 MHz, CDCl3): ~ 7.69-7.60 (2H, m), 7.58-7.30 (9H, m), 7.25-
s 7.12 (3H, m), 7.03-6.94 (1H, m), 5.83 (2H, s), 1.34 (9H, s).
Example 112
3-(4-Chlorobenzoylamino)-1-(3-ehloroben~l)-5~4 ~trifluoromethyl)-
phenyllindole-2-carboxylic acid
l0 1H NMR (200 MHz, I~MSO-d6): 8 I2.4-I2.2 (1H, br s), 8.53 (IH, s), 8.10-
7.99 (2H, m), 7.92-7.72 (4H, m), 7.64-7.~6 (4H, m), 7.34-7.I5 {3H, m),
7.10-7.01 (1H, m), 6.04 {2H, s).
Example 113
~s 5-(4-tei°t-Butylphenyl)-1-(3-chlorobenzyl)-3-[4-
(methylthio)phenyl)indole
2-carbox lic acid
1H NMP~ (200 MHz, CDCl;): ~ 7.73-7.69 (1H, m), 7.67-7.58 (1H, m),. 7.54-
7.30 (9H, m), 7.25-7.13 (3H, m), 7.02-6.94 (1H, m), 5.81 (2H, s), 2.56 (3H,
s), 1.35 (9H, s).
Example 124
1-(3-Chlorobenzyl)-6-(3 5-difluoro~henyl)-3 j4-(dimethylamino)benzo~
aminolindole-2-carboxXlic acid
1H NMR (200 MHz, DMSO-d6): ~ I3.6-13.4 (1H, br s), 10.08 (1H, br s),
as 8.05 (1H, s), 7.95-7.87 (3H, m), 7.59-7.48 (3H, m), 7.3I-7.I1 {4H, m), 6.97-
6.91 (1H, m), 6.82-6.74 (2H, m), 5.96 (2H, br s), 3.00 (6H, s).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
100
Example 1 I S
3-(4-te~°t-Butylbenzoylamino)-1-(3-chlorobenzyl)-6-(3,S-difluorophenyl)
indole-2-carboxylic acid
1H NMR (200 MHz, DMS~-d6): ~ 13.6-13.4 (1H, br s), 10.24 (1H, s), 8.11
s (1H, s), 8.03-7.96 (2H, m), 7.84 (1H, d, J 8.6 Hz), 7.62-7.54 (SH, m), 7.37-
7.13 (4H, m), 7.00-6.95 (1H, m), 5.99 (2H, s), 1.34 (9H, s).
Example I16
1-(3-Chlorobenzyl)-6-(3,S-difluorophen ly_)-3-(3 5-dimethoxybenzoyl
to amino)indole-2-carbox, lic acid
1H NMR (200 MHz, DMSO-d6): 8 13.6-13.3 (IH, br s), 10.26 (1H, s), 8.09
(1H, s), 7.82 (IH, d, ~=8.6 Hz), 7.60-7.49 (3H, m), 7.31-7.10 (6H, m), 7.00-
6.93 (1H, m), 6.73-6.69 (IH, m), 5.97 (2H, s), 3.81 (6H, s).
1 s Example 117
1-(3-Chlorobenzyl)-6-(3 S-difluorophenylL~[2-(3 3 S S-tetramethylcyclo
hex 1)acetylamino]-indole-2-carboxylic acid
1H NMR. (200 MHz, DMSO-d6): b 13.4-I3.3 (1H, br s), 9.68 (1H, s), 8.04
(1H, s), 7.67 (1H, d, J 8.5 Hz), 7.59-7.50 (3H, m), 7.30-7.14 (3H, rn), 7.I0
20 7.OS (1H, m), 6.96-6.89 (1H, m), 5.91 (2H, s), 2.25-2.19 (2H, m), 1.SS-1.45
(2H, m), 1.25-0.75 (SH, m), 0.99 (6H, s), 0.86 (6H, s).
Exam Ip a I I 8
1-C3-Chlorobenzyl)-6-(3 S-difluorophenyl)-3-[~3,3 S,S-tetramethylcyclo
2s hexyl)acetylaminolindole-2-carboxylic acid sodium salt
~H NMR (200 MHz, DMSO-d6): ~ 12.09 (1H, s) 7.35 (1H, d, J--8.S Hz)
7.78 (1H, s) 7.51-7.43 (2H, m) 7.34 (1H, d, ,I--8.S Hz) 7.27-7.16 (3H, m)
7. I S-7.07 (2H, m) 6. I7 (2H, s) 2.22-2. I 6 (2H, m) 1.54-1.44 (2H, m) 1.27-
1.18 (1H, m) I.06-0.73 (4H, m) 1.00 (6H, s), 0.87 (6H, s).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
I01
Example 119
1-(3-Chlorobenzyl)-3-(3-cyclohexylpropionylamino)-6-(3,5-difluorophen-
yl)indole-2-carboxylic acid
1H NMR (200 MHz, DMSO-d6): 8 13.4-13.3 (1H, br s), 9.63 (1H, s), 8.04
s (1H, s), 7.66 (1H, d, J=8.6 Hz), 7.58-7.48 (3H, m), 7.30-7.14 (3H, m), 7.08
7.05 (IH, m), 6.95-6.89 (IH, m), 5.91 (2H, s), 2.43-2.34 (2H, m), 1.79-1.48
(7H, m), 1.30-1.10 (4H, m), 0.98-0.81 (2H, m).
Example I20
Io 3-(4-Butylbenzoylamino~l-(3-chlorobenzyl)-6-(3,5-difluorophenyl)indole-
2-carboxylic acid
1H NMR (200 MHz, DMSO-d6): ~ 13.6-I3.3 (1H, br s), 10.21 (1H, s), 8.09
(1H, s), 7.98-7.92 (2H, m), 7.82 (1H, d, .J--8.~ Hz), 7.61-7.51 (3H, m), 7.38-
7.33 (2H, m), 7.31-7.11 (4H, m), 6.98-6.93 (1H, m), 5.96 (2H, s), 2.69-2.62
Is (2H, m), I.66-1.51 (2H, m), 1.40-1.22 (2H, m), 0.89 (3 H, t, ~=7.2 Hz).
Example 121
1-(3-Chlorobenz l,~)-6-~3 5-difluorophenyl)-3-(4-isopropylbenzoylamino)-
indole-2-carboxylic acid
20 1H NMR (200 MHz, DMSO-d6): d 13.6-13.3 (1H, br s), 10.26 (1H, br s),
8.08 (1H, m), 8.00-7.94 (2H, m), 7.83 (1H, d, .I--8.6 Hz), 7.60-7.50 (3H, m),
7.43-7.37 (2H, m), 7.32-7.11 (4H, m), 7.00-6.93 (IH, m), 5.97 (2H, s), 2.98
(1H, septet, J--7.4 Hz), 1.24 (6H, d, J 7.4 Hz).
2s Example 122
3-f ( 1-Adamantylcarbonyl)amino]- I-(3-chlorobenzyl)-6-(3,5-difluoro-
t~henyl)indole-2-carboxylic acid
1H NMR (200 MHz, DMSO-d6): 8 I3.8-13.2 (1H, br s), 9.45 (1H, s), 8.03
(IH, s), 7.79 (1H, d, .~=8.6 Hz), 7.58-7.46 (3H, m), 7.32-7.08 (4H, m), 6.96-
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
102
6.88 (1H, m), 5.92 (2H, s), 2.04-2.00 (3H, m), 1.96-1.95 (6H, m), 1.72-1.69
(6H, m).
Examlale I23
s 3-f(1-Adamantylcarbon~)amino]-I ~3-chlorobenzyl)-6 ~3 5-difluoro-
phen~)indole-2-carboxylic acid sodium salt
1H NMR (200 MHz, DMSO-d6): S 12.34 (1H, s), 8.37 (1H, d, .I--8.6 Hz),
7.74 (IH, s), 7.48-7.41 (2H, m), 7.3I-7.16 (4H, m), 7.14-7.04 (2H, m), 6.16
(2H, s), 2.05-1.99 (3H, m), 1.96-1.91 (6H, m), 1.73-1.68 (6H, m).
Io
Examlale I24
1-(3-Chlorobenzyl)-6-(3 5-difluoro~hen~)-3-[4-(trifluoromethoxY)benzoyl
amino]indole-2-carboxylic acid
1H Nl~~ (200 MHz, DMSO-d6): 8 13.6-I3.3 (1H, br s), 10.38 (1H, s), 8.19-
Is 8.09 (3H, m), 7.78 (1H, d, .I--8.6 Hz), 7.58-7.52 (5H, m), 7.32-7.11 (4H,
m),
6.99-6.94 (1H, m), 5.97 (2H, s).
Exam lp a 12 5
1-(3-Chlorobenzyl)-3-(4-cyanobenzo lamino)-6-(3 5-difluorophen~l)-
2o indole-2-carboxylic acid
1H NMR (200 MHz, DMSO-d6): ~ I3.5-13.3 (1H, br s), 10.50 (1H, s), 8.22-
B.IS (2H, m), 8.12-8.0I (3H, m), 7.77 (IH, d, J 8.6 Hz), 7.60-7.51 (3H, m),
7.35-7.I0 (4H, m), 7.00-6.94 (1H, m), 5.97 (2H, s).
2s Examlale 126
6-(4-Butylphenyl)-1-(3-chlorobenzyl)-3-[(3 5-dimethyladamant-1-
ylcarbonyl)amino]indole-2-carbox lic acid
1H NMR (200 MHz, DMSO-d6): ~ 13.5-13.4 (IH, br s), 9.51 (1H, s), 7.81
(1H, s), 7.77 (1H, d, ,T--8.8 Hz), 7.66-7.58 (2H, m), 7.42-7.36 (1H, m), 7.29-
30 7.23 (4H, m), 7.10-7.09 (1H, m), 6.96-6.91 (1H, m), 5.90 (2H, s), 2.60 (2H,
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
I03
t, J 7.2 Hz), 2.15-2.08 (IH, m), 1.81-1.78 (2H, m), 1.62-1.48 (6H, m), 1.40-
1.24 (6H, m), 1.19-1.16 (2H, m) 0.89 (3H, t, J 7.2 Hz), 0.85 (6H, s).
Example 127
s 6~- 4-Butylphenyl -~-(3-chlorobenz~)-3-[(3,5-dimethyladamant-1-
ylcarbonyl)amin~indole-2-carbox~ic acid sodium salt
1H NMR (200 MHz, DMSO-d6): ~ 12.30 (1H, s), 8.30 (1H, d, J--8.5 Hz),
7.57-7.50 (3H, m), 7.26-7.15 (6H, m), 7.11-7.05 (IH, m), 6.13 (2H, s), 2.57
(2H, t, .l 7.2 Hz), 2.15-2.08 (IH, m), 1.79-1.74 (2H, m), 1.59-1.47 (6H, m),
Io 1.39-1.25 (6H, m), 1.I9-1.15 (2H, m) 0.88 (3H, t, J--7.2 Hz), 0.85 (6H, s).
Example 128
6-( _4-~utvlphenvl)-1-(3-chl~robenzyl)-3-(4-(2,5-dimethylpyrrol-1-yI)benz-
oylaminolindole-2-carboxylic acid
Is IH NMR (200 MHz, DMSO-d6): b 13.4-13.2 (1H, br s), 10.48 (1H, br s),
8.20-8.12 (2H, m), 7.88 (1H, s), 7.81 (1H, d, J 8.5 Hz), 7.69-7.6I (2H, m),
7.48-7.42 (3H, m), 7.3 2-7.24 (4H, m), 7.16-7.13 ( 1 H, m), 7.04-6.9 8 ( 1 H,
m), 5.96 (2H, br s), 5.83 (2H, s), 2.60 (2H t, J=7.2 Hz), 2.01 (6H, s), 1.63-
1.49 (2H, m), 1.39-1.22 (2H, m), 0.89 (3H, t, J 7.2 Hz).
Exam lp a 129
6-(4-Bu lphenyl)-1-(3-chlorobenzyl)-3-[4-(trifluoromethoxy)benzoyl-
amino]indole-2-carboxylic acid
1H IvIMR (200 MHz, DMSO-d6): 8 13.5-13.3 (1H, br s), 10.37 (1H, s), 8.20
2s 8.14 (2H, m), 7.88 (1H, s), 7.76 (1H, d, J 7.6 Hz), 7.66-7.60 (2H, m), 7.59
7.53 (2H, m), 7.47-7.41 (1H, m), 7.32-7.23 (4H, m), 7.15-7.11 (1H, m),
7.02-6.96 (1H, m), 5.95 (2H, s), 2.60 (2H, t, J 7.2 Hz), 1.63-1.49 (2H, m),
1.39-1.21 (2H, m), 0.89 (3H, t, J--7.2 Hz).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
104
Example 130
6 (4-But~lphenyl)-I-(3-chlorobenzyl~3-f4-(trifluoromethoxy)benzoyl-
aminolindole-2-carbox~ic acid sodium salt
'H NMR (200 MHz, DMS~-d6): S 13.67 (1H, s), 8.51 (1H, d, J 7.6 Hz),
s 8.18-8.12 (2H, rn), 7.61-7.51 (5H, m), 7.31-7.I8 (6H, m), 7.14-7.08 (1H,
m), 6.19 (2H, s), 2.57 (2H, t, J 7.2 Hz), 1.62-1.47 (2H, m), 1.35-1.21 (2H,
m), 0.88 (3H, t, J 7.2 Hz).
Example 131
6 (4-Butylt~henyl)-1-(3-chlorobenz~)-3-(4-isopropoxybenzoylamino)-
indole-2-carboxylic acid
(a) 6-(4-B~utylphen~)-1-(3-chlorobenz~)-3-(4-isopropoxybenzoylamino)-
indole-2-carboxylic acid ethyl ester
Is A mixture of 3-amino-6-(4-butylphenyl)-1-(3-chlorobenzyl)indole-2-
carboxylic acid ethyl ester (250 mg, 540 nmol), 4-isopropoxybenzoyl
chloride (I62 mg, 810 nmol), DMAh (33 mg, 270 mnol), triethylamine (229
~,L, 1.63 mmol) and dry MeCN (2 mL) was stirred at room temperature
under argon for 18h and then heated at 80°C for IO min, at I00°C
for 5 min
2o and finally at 120°C for IO min using microwave irradiation. The
mixture
was poured into HCl (1M) and extracted with EOAc. The combined
extracts were washed with NaHCO3, dried with NazSO4 and concentrated.
The residue was crystallised from EOAclbenzene to yield the title
compound (150 mg, 44%).
2s
(b) 6-(4-ButylphenYl)-1-(3-chlorobenzyl)-3-(4-isopropoxybenzoylamino)-
indole-2-carboxylic acid
The title compound (45 mg, 32%) was prepared by hydrolysis of 6-(4-
butylphenyl)-1-(3-chlorobenzyl)-3-(4-isopropoxybenzoylamino)indole-2-
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
lOs
carboxylic acid ethyl ester (see Example 131(a)) under conditions as
hereinbefore described, for example at 90°C for 10 min in 1,4-dioxane.
1H NMR (200 MHz, DMSO-d6): d 13.5-13.3 (1H, br s), 10.14 (1H, s), 8.03
7.95 (2H, m), 7.86 (1H, s), 7.80 (1H, d, J 7.6 Hz), 7.67-7.60 (2H, m), 7.46
s 7.3 9 ( 1 H, m), 7.31-7 .23 (4H, m), 7.14-7.11 ( I H, m), 7.0 8-7 . 04 (2H,
m),
7.01-6.95 (1H, m), 5.94 (2H, s), 4.74 (1H, septet, J--6.0 Hz), 2.60 (2H, t,
J--7.2 Hz), 1.64-1.49 (2H, m), I.36-1.25 (2H, m), 1.30 (6H, d, J 6.0 Hz),
0.89 (3H, t, J=7.2 Hz).
to Example 132
6-(4-Butylphenyl)-1-(3-chlorobenz~l)-3-(3-isopropoxybenzoylamino~
indole-2-carboxylic acid
The title compound was prepared in accordance with the procedures
described herein.
is 1H NMR (200 MHz, DMSO-d6): 8 13.5-I3.3 (1H, br s), I0.25 (1H, s), 7.87
(1H, s), 7.75 (1H, d, .I--8.5 Hz), 7.67-7.54 (4H, m), 7.47-7.37 (2H, m), 7.34-
7.22 (4H, m), 7.16-7.10 (2H, m), 7.02-6.95 (1H, m), 5.94 (2H, s), 4.71 (1H,
septet, J=6.0 Hz), 2.60 (2H, t, J 7.2 Hz), 2.15-2.08 (2H, m), 1.36-1.21 (2H,
m), 1.29 (6H, d, J--6.0 Hz), 0.88 (3H, t, ,J=7.2 Hz).
Example 133
6-(4-Butylphenyl)-1-(3-chlorobenzyl)-3-(3-isopropox~jbenz~lamino)-
indole-2-carboxylic acid sodium salt
The title compound was prepared in accordance with the procedures
2s described herein.
~H NME~ (200 MHz, DMSQ-d6): b 13.58 (1H, s) 8.52 (1H, d, J=8.6 Hz)
7.60-7.51 (5H, m) 7.46-7.3 8 ( 1H, m) 7.29-7.17 (6H, m) 7.14-7.07 (2H, m)
6.18 (2H, s) 4.69 (1H, septet, J--6.0 Hz) 2.58 (2H, t, J--7.2 Hz) 1.63-1.49
(2H, m) 1.35-1.24 (2H, m) 1.30 (6H, d, ,.T--6.0 Hz), 0.88 (3H, t, J--7.2 Hz).
~o
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
106
Example 134
6-(4-Carboxyphenyl)-3-(4-chlorobenzo~amino)-1-(3-chlorobenzyl)indole-
2-carboxylic acid
The title compound was prepared in accordance with the procedures
s described herein.
1HNMR (200 MHz, I)MSO-d6): b 13.6-12.8 (2H, br s), 10.37 (1H, s), 8.13-
7.99 (SH, m), 7.94-7.86 (2H, m), 7.82 (1H, d, J 8.6 Hz), 7.68-7.61 (2H, m),
7.5 9-7.51 ( 1 H, m), 7.3 8-7.24 (2H, m), 7.17-7.11 ( 1 H, m), 7.04-6.95 ( 1
H,
m), 5.99 (2H, s).
Example 13 5
3-(4-Chlorobenzoylamino~-1-(3-chlorobenzyl)-6-(4-hydroxymethylphenyl)-
indole-2-carboxylic acid
I5 (a) 3-(4-Chlorobenzoylamino)-1-(3-chlorobenzyl)-6-(4-hydroxymethyl-
phenyl)indole-2-carboxylic acid ethyl ester
1M BH3xTHF (1M, 200~,L, 0.20 mmol) was added to a stirred solution of
6-(4-carboxyphenyl)-3-(4-chlorobenzoylamino)-1-(3-chlorobenzyl)indole-
2-carboxylic acid ethyl ester, prepared by analogous techniques to those
2o described hereinbefore, (120 mg, 0.20 mtnol) in THF (5 mL) at -5 °C.
The
temperature of the mixture was allowed to reach room temperature and
stirring was continued for 12 h whereafter another portion of 1M BH;xTHF
(1M, 200~,L, 0.20 mmol) was added. The mixture was stirred for 8 h at
room temperature and poured into AcOH (aq., 50%, 20 mL) and stirred for
2s 1 h. The mixture was extracted with EtOAc and the combined extracts were
washed with H2O and brine, dried over Na2S04 and concentrated.
Purification of the residue by chromatography gave the sub-title compound
(90 mg, 78 %).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
107
(b) 3-(4-Chlorobenzoylamino)-I-(3-chlorobenz~)-6-(4-h droxymethyl-
phen~)indole-2-carbox ly is acid
The title compound was prepared by hydrolysis of 3-(4-chlorobenzoyl-
amino)-1-(3-chlorobenzyl)-6-(4-hydroxymethylphenyl)indole-2-carboxylic
s acid ethyl ester in accordance with the procedure in Example 2(b) (NaOH
(1M), MeCN, 80 °C, 20 min).
1H NMR (200 MHz, I)MSO-d6): 8 13.5-13.2 (1H, br s), 10.35 (1H, s), 8.13
8.04 (2H, m), 7.92 (1H, s), 7.79 (IH, d, J 8.4 Hz), 7.70-7.57 (4H, m), 7.51
7.25 (SH, m), 7.17-7.12 (1H, m), 7.04-6.96 (1H, m), 5.98 (2H, s), 5.14-5.34
to (1H, m), 4.62-4.54 (2H, m).
Exam lp a 13 6
6-(4-Butylphenyl)-1-(3-chlorobenzyl)-3-[3 ~2 5-dimeth~~ rry ol-I-yl benz-
o lamino]indole-2-carboxylic acid
(a) 6-(4-Butylphenyl)-I-(3-chlorobenzyl)-3-(3-nitrobenzamido~indole-2-
carboxylic acid eth Iy ester
The sub-title compound was prepared in accordance with Example I31 (a)
using 3-nitrobenzoyl chloride (room temperature overnight, and then
90°C
2o for IO min, 110°C for 10 min and finally 130°C for 20 min).
(b) 3-(3-Aminobenzamido)-6-(4-buty~henyl)-1-(3-chlorobenz~ indole-2-
carboxylic acid ethyl ester
6-(4-B utylphenyl)-1-(3 -chlorob enzyl)-3 -(3 -nitrob enzamido)indol e-2-carb-
2s oxylic acid ethyl ester (23S mg, 0.39 mmol; see Example 136(a)) was
hydrogenated at ambient pressure and temperature using palladium on
charcoal (10%, 62 rng) in EtOAc for 6 h, then filtered through Celite°.
The
filtrate was concentrated and the residue was crystallised from
EtOAc/benzene to yield the sub-title compound (140 mg, 62%).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
108
(c) 6-(4-Butylphenyl)-1-(3-chlorobenz~)-3-(~2 5-dimethylpyrrol-1-yl)-
benzamido)indole-2-carboxylic acid ethyl ester
A mixture of 3-(3-aminobenzamido)-6-(4-butylphenyl)-I-(3-chlorobenzyl)-
indole-2-carboxylic acid ethyl ester ( 140 mg, 240 nmol; see Example
s 136(b)), hexane-2,5-dione (I89 ~.L, 1.2I mmol), p-toluenesulfonic acid (50
ng, 0.24 nmol) and toluene ( 1 mL) was stirred at room temperature for 1 h
and then heated at 70°C for 20 min using microwave irradiation. The
mixture was diluted with EOAc, washed with NaZC03 (aq., sat.), dried over
Na2SO4, and concentrated. The residue was crystallised from petroleum
to ether/benzene to yield the sub-title compound (97 mg, 61%).
(d) 6~4-Butylphenyl)-1-(3-chlorobenzyl)-3-[3-(2 5-dimethylpyrrol-1-yl)-
benzoylamino~Iindole-2-carboxylic acid
The title compound was prepared by hydrolysis of 6-(4-butylphenyl)-I-(3-
1s chlorobenzyl)-3-[3-(2,5-dimethylpyrrol-1-yl)benzoylamino]indole-2-
carboxylic acid ethyl ester (see Example 136(c)) in accordance with the
procedure described in Example 2(b) (2M T~CaH, dioxane, 70 °C, IO min,
then 80 °C 20 min and finally 90 °C 10 min).
~H IVTV~ (200 MHz, DMS~-d6): b I3.5-I3.3 (IH, br s), 10.8-10.4 (1H, br
2o s), 8.14-8.08 (1H, m), 7.93-7.80 (3H, m), 7.73-7.69 (1H, m), 7.66-7.60 (2H,
m), 7.55-7.49 (1H, m), 7.43 (1H, d, J 8.5 Hz), 7.31-7.23 (4H, m), 7.13-7.I 1
(1H, m), 7.02-6.96 (1H, m), 5.96 (2H, s), 5.83 (2H, s), 2.59 (2H, t, J--7.2
Hz), 2.00 (6H, s), 1.63-1.49 (2H, m), 1.39-1.21 (2H, m), 0.88 (3H, t, J--7.2
Hz).
Example 13 7
6-(4-Butylphenyl)-1-(3-chlorobenzyl)-3-[3-(2,5-dimeth~~yrrol-1-~l)benz-
o lamino]indole-2-carboxylic acid sodium salt
The title compound was prepared in accordance with the procedures
so described herein.
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
109
~H NMR (200 MHz, DMSO-d6): b 13.91 (IH, br s), 8.57 (1H, d, J--8.5 Hz),
8.11-8.07 (1H, m), 7.83-7.81 (1H, m), 7.74-7.66 (1H, m), 7.61-7.54 (3H,
m), 7.52-7.76 (1H, m), 7.31-7.19 (6H, m), 7.14-7.09 (1H, m), 6.19 (2H, s),
5.84 (2H, s), 2.59 (2H, t, J--7.2 Hz), 2.03 (6H, s), 1.64-1.49 (2H, m), 1.38
s 1.22 (2H, m), 0.89 (3H, t, J 7.2 Hz).
Ex_ amt~le 138
3-(4-Chlorobenzoylamino)-1-(4-chlorobenzyl)-5-[4-(trifluoromethyl)-
phenyll indole-2-carboxylic acid
to The title compound was prepared in accordance with the procedures
described herein.
1H NMR (200 MHz, DMSO-d6): b 13.5-13.1 (1H, br s), 10.6-10.4 (1H, br
s), 8.12-8.00 (3H, m), 7.93-7.84 (2H, m), 7.82-7.68 (4H, m), 7.68-7.57 (2H,
m), 7.41-7.30 (2H, m), 7.13-7.03 (2H, m), 5.89 (2H, m). . ,
Is
Example 139
3 -(Acetyl-(4-methoxyb enzyl) amino]-1-~3 -chlorobenz~I)-5~4-is oprop oxy-
phenyl)indole-2-carbox~Iic acid
20 (a) 3-E~romo-5-(4-isopropoxyphenyl)indole-2-carboxylic acid ethyl ester
A solution of NBS (0.904 5.082 mmol) in acetone (10 mL) was added
dropwise to a solution of 5-(4-isopropoxyphenyl)indole-2-carboxylic acid
ethyl ester, prepared in accordance with Example 1 (a) from 5-bromoindole-
carboxylic acid ethyl ester and 4-isopropoxyphenylboronic acid, (1.5 g, 4.62
zs mmol) in acetone (35 mL) at room temperature. After 2.5 h an additional
portion of NBS (164 mg, 0.92 rnmol) was added and temperature of the
mixture was increased to 45 °C. After 1.5 h the mixture was cooled to
room
temperature, poured into Na2S20~ (aq., 10%) and extracted with EtOAc.
The extract was washed with Na2S203 (aq., 10%), NaHCO; (aq., sat) and
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
110
brine, dried over Na2S04 and concentrated. The residue was crystallised
from EtOH to give the sub-title compound (1.63 g, 88 %).
(b) 1-(3-Chlorobenzyl)-5-(4-isopropoxyphenyl~indole-2-carboxylic acid
s ethyl ester
The sub-title compound was prepared in accordance with Example 1 (c)
from 3-bromo-5-(4-isopropoxyphenyl)indole-2-carboxylic acid ethyl ester
(1.0 g, 2.5 mmol, see step (a) above) and 3-chlorobenzylchloride (0.604 g,
3.75 mmol). Yield 0.784 g (60 ~/o).
to
(c) 1-(3-Chloroben~,yll-5-(4-isopropoxyphenyl)-3-f(4-methoxybenzyl)-
amino]indole-2-carboxylic acid ethyl ester
4-lVlethoxyben~ylamine (60 ~.L, 0.46 mmol) was added to a mixture of
1-(3-chlorobenzyl)-5-(4-isopropoxyphenyl)indole-2-carboxylic acid ethyl
is ester (200 mg, 0.38 mmol), Pd2(dba); (17.4 mg, 0.019 mmol), EINAP (35.5
mg, 0.057 m~nol), Cs~C03 (173 mg, 0.53 mmol) and anhydrous toluene (10
mL). The mixture was stirred at 120°C for 24 h, cooled to room
temperature
and diluted with Et20. The mixture was filtered through Celite~ and the
filter calve washed with Et20. The combined filtrates were concentrated and
2o the residue purified by chromatography to yield the sub-title compound
(0.213 g, 96 %).
(d) 3-~Acetyl-(4-methoxybenzyl)aminol-1-(3-chlorobenzyl)-5-(4-isoprop-
oxyphenyl)indole-2-carboxylic acid ethyl ester
25 Acetyl chloride (24 ~.L, 0.34 mmol) was added at room temperature to a
solution of 1-(3-chlorobenzyl)-5-(4-isopropoxyphenyl)-3-[(4-methoxybenz-
yl)amino]indole-2-carboxylic acid ethyl ester (200 mg, 0.34 mmol) in dry
toluene (2mL). The mixture was stirred at 70 °C for 1 h, allowed to
cool to
room temperature, diluted with EtOAc and washed with NaHCO; (aq., sat.),
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
111
brine and finally dried over Na2S04. Concentration and purification by
chromatography gave the sub-title compound (O.I36 g, 64 %).
(e) 3-(Acetyl-(4-methoxybenzyt)aminol-1-(3-chlorobenzyl)-5-(4-isoprop~
s oxyphenyl)indole-2-carboxylic acid
The title compound ivas prepared by hydrolysis of 3-[acetyl-(4-
methoxybenzyl)amino]-1-(3-chlorobenzyl)-5-(4-isopropoxyphenyl)indole-
2-carboxylic acid ethyl ester (136 mg, 0.217 mmol; see step (d) above), in
accordance with the procedure in Example 1(e) (NaOH (aq.), dioxane,
l0 80°C, I h). Yield: 93 mg (72 %).
1H NMI~ (200 MHz, DMSO-d6): 8 13.7-I3.3 (1H, br s), 7.55-7.40 (2H, m),
7.35-7.24 (4H, m), 7.15-7.06 (2H, m), 7.02-6.84 (SH, m), 6.80-6.71 (2H,
m), 5.86 (2H, s), 5.34 (1H, d, .I--14.0 Hz), 4.61 (1H, septet J=6.0 Hz), 4.21
(1H, d, .I--14.0 Hz), 3.64 (3H, s), 1.72 (3H, s), 1.26 (6H, d, ,I--6.0 Hz).
is
Example 140
1-(3-Chlorobenzyl)-3-; (4-chlorobutyryl)-[2-(4-fluoro~henyl)ethyllamino~-
5-(4-isopropoxyphenyl)indole-2-carboxylic acid
The title compound was prepared in accordance with the procedure in
2o Example 139(c)-(e) from 1-(3-chlorobenzyl)-5-(4-isopropoxyphenyl)indole-
2-carboxylic acid ethyl ester, 2-(4-fluorophenyl)ethylamine and 4-chloro-
butyryl chloride.
'H NMR (200 MHz, DMS~-d6): b 7.54-7.36 (SH, m), 7.34-6.87 (lOH, m),
6.05 (1H, d, J--16.0 Hz), 5.87 (IH, d, J--16.0 Hz), 4.60 (1H, septet, J 6.0
2s Hz), 4.19-3.96 (1H, m), 3.82-3.61 (1H, m), 3.22 (2H, t, .T--6.4 Hz); 2.85-
2.70 (2H, m), 2.37-2.10 (1H, m), 2.07-1.85 (1H, m), 1.67-1.44(2H, m), 1.25
(6H, d, J--6.0 Hz).
CA 02528626 2005-12-08
WO 2005/005415 PCT/GB2004/002996
112
Example 141
1-(3-Chlorobenzyl)-3-; (6-chloro~yridine-3-carbonyl)-f2-(4-fluorophenyl)-
ethyliamino~-5-(4-isopropoxyphenyl)indole-2-carboxylic acid
The title compound was prepared in accordance with the procedure in
Example 139(c)-(e) from 1-(3-chlorobenzyl)-5-(4-isopropoxyphenyl)indole
2-carboxylic acid ethyl ester, 2-(4-fluorophenyl)ethylamine and 6-chloro
nicotinoyl chloride.
1H NMR (200 MHz, I~MSO-d6): 8 8.31 (1H, d, J--1.9 Hz), 7.82 (1H, s),
7.75 (1H, dd, J 8.2, 1.9 Hz), 7.66-7.43 (4H, m), 7.30 (1H, d, .I--8.2 Hz),
l0 7.21-6.91 (9H, m), 6.38-6.29 (1H, m), 6.06 (1H, d, ,J 16.6 Hz), 5.60 (1H,
d,
J--16.6 Hz), 4.63 (1H, septet, J 6.1 Hz), 4.11-3.93 (2H, m), 3.09-2.75 (2H,
m), 1.27 (6H, d, J 6.1 Hz).
Example 142
Title compounds of the invention were tested in the biological test described
above and were found to exhibit 50% inhibition of mPGES-1 at a
concentration of 10M or below. For example the inhibition of mPGES-1 is
exemplified by the following compounds of the examples, as listed in the
following table:
2o Example 1: 96% inhibition at 1 OM
Example 2: 100% inhibition at lOM
Example 3: 85% inhibition at lOM
Example 4: 81 % inhibition at l OM
Example 5: 93% inhibition at lOM
Example 6: 100% inhibition at lOM