Note: Descriptions are shown in the official language in which they were submitted.
CA 02529141 2005-12-21
Description
A Process for Sterile Packa~in~ of Containers with Drop-Dispensers, and
Means for Actuating the Process.
Technical Field
The invention relates to a process for sterile packing of containers with drop
dispensers and means for actuating the process.
Background Art
The prior art teaches containers with drop-counting dispensers closed by caps,
often having a security strip, which containers must be packaged in sterile
surroundings given the nature of their contents. These containers are used,
for
example, in the pharmaceutical field, and in particular in the field of
ophthalmology, for containing eye-drops, for sterile cleaning liquids, and the
like.
Generally these containers comprise a flagon, made of glass or a plastic
material,
which is provided with a drop dispenser and closed by a cap made of plastic.
The
invention relates in particular to the latter type of container, with drop-
dispenser
and plastic cap.
At present the sterile packaging of these containers which obviously takes
place
in sterile surroundings, is performed in the following way.
The packaging companies, which are generally pharmaceutical industries,
receive
the various pieces making up the container in closed packages containing a
high
number of units; in particular, packages containing the flagons, the drop-
dispensers and the caps are separately delivered.
The packages arrive at the pharmaceutical packagers with their internal parts
already sterile, or they can be sterilised after arriving; the sterilisation
thereof is
CA 02529141 2005-12-21
_Z_
done, with the packages closed, by gamma ray bombardment which sterilises the
insides of the packages without any need to open them.
Using various lines and systems, of known type, these packages are introduced
into a sterile environment and are opened while there, so that the sterility
thereof
is not compromised. The various sterile parts which exit are sent on to
machines,
located in the sterile environment, which position and predispose the various
pieces for the filling and closing stages of the containers.
During this packaging stage, which as has been mentioned is done in a sterile
environment and in general using continuous automatic machines, the t7agon is
filled with the product in a work station; in a following work station, the
drop-
dispenser is inserted on the flagon by press-fit; and in a further work
station the
flagon-drop dispenser assembly is completed by screwing on the cap, at which
point the container exits the sterile environment and is ready to be put up
for sale.
This packaging process involves considerable expense, primarily for
sterilisation
of the parts of the container; gamma ray sterilisation includes costs that are
calculated in terms of "occupied volume", i.e. the number of objects to be
sterilised, which, in the described process, is a very high number even though
the
volume consists mostly of empty space, being the inside of the containers, the
drop-dispensers and the closure caps which are hollow elements. Secondly, the
high costs are due to the presence, internally of the sterile machines where
the
packaging takes place, of a high number of infeeding lines of the single
components and a large number of work stations, which, apart from the cost of
these specific lines and stations, lead to the need to keep a fairly large
space
under sterile conditions.
A further drawback in the known processes is the difficulty of regulating the
torque in the cap-dispenser coupling.
The main aim of the present invention is to provide a process for sterile
CA 02529141 2005-12-21
-3-
packaging of containers having drop-dispensing mechanisms, and the means for
realising the process, which process and means reduce the drawbacks in the
prior
art, and in particular reduce the costs and wastage during the packaging
process.
An advantage of the invention is that container packaging times are reduced.
A further advantage of the invention is that containers are obtained which are
safer and easier for a consumer to use.
These aims and advantages are achieved by the invention, as it is
characterised
in the appended claims.
Disclosure of Invention
Further characteristics and advantages of the present invention will better
emerge
from the detailed description that follows of a possible actuation of the
process,
and a possible embodiment of the means for realising the process, illustrated
purely by way of non-limiting example in the accompanying figure l, which
shows a section in vertical elevation of a flagon group, drop-dispenser and
cap
which make up the means for actuating the process of the invention.
The sterile packaging process is applied on containers used in particular in
the
pharmaceutical field for containing products such as medicinal drops, sterile
cleaning liquids and the like, which containers comprise a flagon 1, made
preferably of a plastic material, on which after filling with the product they
are
destined to contain, a drop-dispenser 2 and a closure cap 3 are to be fitted,
the cap
3 closing the package but which can be removed to enable use of the product,
and
replaced to close the flagon before further use; both the drop-dispenser 2 and
the
cap 3 are made of a plastic material, and preferably by injection moulding.
The process includes, as with known processes, a sterilisation stage of the
flagon,
the drop-dispenser and the closure cap.
While the sterilisation of the flagon is done using known processes, for
sterilising
the drop-dispenser and the cap the process of the invention includes a
preparation
CA 02529141 2005-12-21
-4-
stage of removably anchoring the closure cap on the drop-dispenser; this
anchoring stage is done under conditions of perfect cleanliness but not
necessarily sterility, generally by the manufacturer of the drop-dispenser and
the
cap; during this stage the cap and the drop-dispenser are solidly constrained
to
one another and a pre-assembled group is obtained, which is already configured
to be directly connected to the flagon. This anchoring stage, however,
produces
a connection between the drop-dispenser and the cap which can be resolved at
moment of use of the package; for this purpose the anchoring stage of the cap
on
the drop-dispenser is achieved by a threaded coupling between an outside of
the
drop-dispenser and an inside of the cap, which easily enables, at moment of
use,
the anchored state between the drop-dispenser and the cap to be removed and
replaced, i.e. the container can easily be opened and closed.
Once assembled, the drop-dispenser-cap groups are packed in a packaging, which
contains a considerable number of units, which is sent on to a successive
stage
of sterilisation of the pre-assembled groups; this sterilisation stage is
performed,
with the packagings closed, by bombardment of gamma rays which, in a known
process, sterilise the inside of the packaging and the contents thereof with
no
need for opening the packaging.
With respect to known processes, this stage of sterilisation, the economic
cost of
which depends on the volume of the packaging, is much less expensive because
the volume occupied by the drop-dispenser-cap groups is much smaller (with the
drop-dispenser-cap groups proposed for actuating the process of the invention,
as shall be seen herein below, the volume is about half) than the totality of
the
volumes occupied by the drop-dispenser and cap when considered separately; in
other words, with the sterilisation of the volume represented, in known
processes,
by the packaging containing the caps, sterilisation can be performed of both
the
cap and the drop-dispenser.
CA 02529141 2005-12-21
-5-
This stage of sterilisation can be perfornled by the packaging company or, as
frequently occurs, by an external company, which sends the packaging company
the packages containing the drop-dispenser-cap groups already sterilised.
The drop-dispenser-cap groups are then placed in an aseptic environment
represented by the plant performing the filling and closing of the packages;
the
flagons are also placed in the aseptic environment. The flagons are then
filled in
this aseptic environment, following known processes.
Once the flagon is filled the pre-assembled drop-dispenser-cap group is
inserted,
so that in a single operation at a single work-station the package is provided
with
the drop-dispenser and the cap destined to close the container.
The insertion stage of the pre-assembled drop-dispenser-cap group is performed
by a simple pressure-insertion of the drop-dispenser-cap group on the neck of
the
flagon. This stage is much simpler and more reliable than known processes,
which include a pressure insertion of the drop-dispenser on the flagon
followed
by the screwing-on of the cap on the neck of the flagon. The screwing-on of
the
cap is indeed rather a delicate operation and must be done most carefully in
order
not to subject the cap to erroneous torques which might lead to an imperfect
insertion of the cap, or even a breakage of the cap. In the process of the
invention,
the screw-on stage, which is generally done after the realisation of the drop
dispenser and the cap, is made especially easy because the screw-coupling is
effected partly on the cap and partly on the drop-dispenser, which are both
obtainable by injection moulding and thus with a very high forming precision:
also, the assembly of the drop-dispenser-cap group is performed in much easier
conditions, as although the realisation and manipulation of the various
components is always done in perfectly clean conditions (hygienic ambience)
the
assembly thereof does not necessarily have to be done in a sterile
environment.
The above-described process also offers considerable advantages for transport
CA 02529141 2005-12-21
-6-
and storage of the components of the package (the overall shipping volume of
the
drop-dispenser and the caps is halved). Control is also easier, as only one
drop
dispenser-cap group control is needed instead of the two required when the
components are separate.
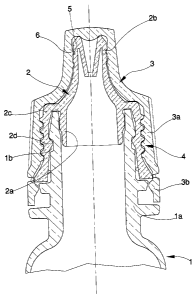
To actuate the above-described process means are used which comprise a
container which in turn comprises a flagon l, of which only the upper part is
shown in the figure of the drawing, which flagon 1 is destined to contain the
product to be packaged; an annular end 2a of a drop-dispenser 2 is press-
fitted on
the mouth la of the flagon l, the drop-dispenser 2 being superiorly provided
with
an appendix 2b which doses the contents and which projects externally of the
flagon 1 when the drop-dispenser is inserted in the mouth la. The drop-
dispenser
comprises a skirt 2c which is arranged externally of and concentrically to the
annular end 2a. An annular cavity is defined between the skirt 2c and the end
2a,
in which cavity the mouth la of the flagon is jointed when the drop-dispenser
2
is press-inserted on the flagon 1. To guarantee a solid joint of the drop-
dispenser
2 on the flagon l, there is an annular relief 2d internally on the skirt 2c,
which
lodges in a corresponding annular cavity 1b on the mouth of the flagon 1.
The means for realising the process further comprise a closure cap 3 having
the
function of closing the container and enabling it to be re-opened, so that the
product in the container can be dosed by the drop-dispenser, and then closed.
The
cap 3 comprises a bell-shaped zone 3a which covers the skirt 2c of the drop-
dispenser 2, and imitates the shape thereof, when the cap 3 is placed on the
container to close it. Preferably the cap 3 also comprises a security strip
arranged
inferiorly of the cap and connected thereto, in a known way, by easy-break
ribs.
The strip is to evidence when a container has been opened. Also included are
means for fastening which enable the cap 3 to be fastened removably to the
drop-
dispenser 2. The means for fastening comprise a screw-coupling 4 which is
partly
CA 02529141 2005-12-21
made on the external surface of the skirt 2c of the drop-dispenser 2 and
partly on
the internal surface of the bell 3a of the cap 3.
The screw-coupling 4 enables an easy and efficacious coupling between the drop-
dispenser and the cap, usable for realising the previously-described process;
also,
thanks to the conformation of the drop-dispenser and the cap, and by means of
the said coupling, a drop-dispenser-cap group is obtained which exhibits a
volume equal to that of the cap alone, inasmuch as once the coupling is
achieved
the drop-dispenser is entirely contained inside the cap.
Preferably the appendix 2b for dosing on the drop-dispenser comprises, on an
external surface thereof, one or two (as shown in the figure) annular cavities
5,
in each of which, when the cap 3 is connected to the drop-dispenser 2, an
annular
relief 6 is inserted which annular relief 6 is made on the internal surface of
the
cap 3. This guarantees an excellent seal of the closed container.
The flagon l, the drop-dispenser and the cap described define together means
for
actuating the process of the invention in a way which is easy and rapid. The
process could however be actuated with different drop-dispensers and caps from
those described, such as ones using a press-fitting, or having seal rings
instead
of the described annular cavities 5 and the annular reliefs 6; or even having
different systems for identifying when the container has been first opened.